Abstract
The two main proteolytic machineries of eukaryotic cells, lysosomes and proteasomes, receive substrates by different routes. Polyubiquitination targets proteins for proteasomal degradation, whereas autophagy delivers intracellular material for lysosomal hydrolysis. The importance of autophagy for cell survival has long been appreciated, but more recently, its essential role in both innate and adaptive immunity has been characterized. Autophagy is now recognized to restrict viral infections and replication of intracellular bacteria and parasites. Additionally, this pathway delivers cytoplasmic antigens for MHC class II presentation to the adaptive immune system, which then in turn is able to regulate autophagy. At the same time, autophagy plays a role in the survival and the cell death of T cells. Thus, the immune system utilizes autophagic degradation of cytoplasmic material, to both restrict intracellular pathogens and regulate adaptive immunity.
Main Text
Introduction
Eukaryotic cells degrade proteins with two main hydrolytic machineries, proteasomes and lysosomes. In both systems, catabolic activities are physically separated from intracellular substrates—in proteasomes, by localization in a barrel-shaped multiprotein complex and in lysosomes, by its vesicular membrane. Therefore, access to the hydrolytic activities within these structures determines whether a protein is degraded. For proteasomes, polyubiquitination allows substrates to gain access to the catalytic chamber for proteolysis (Ciechanover et al., 1984). For lysosomes, autophagy delivers intracellular constituents for degradation, but how autophagosome cargo is selected remains unclear (De Duve and Wattiaux, 1966). Although proteasomes degrade soluble short-lived proteins, autophagy targets cell organelles and aggregates of long-lived proteins for degradation (Henell et al., 1987).
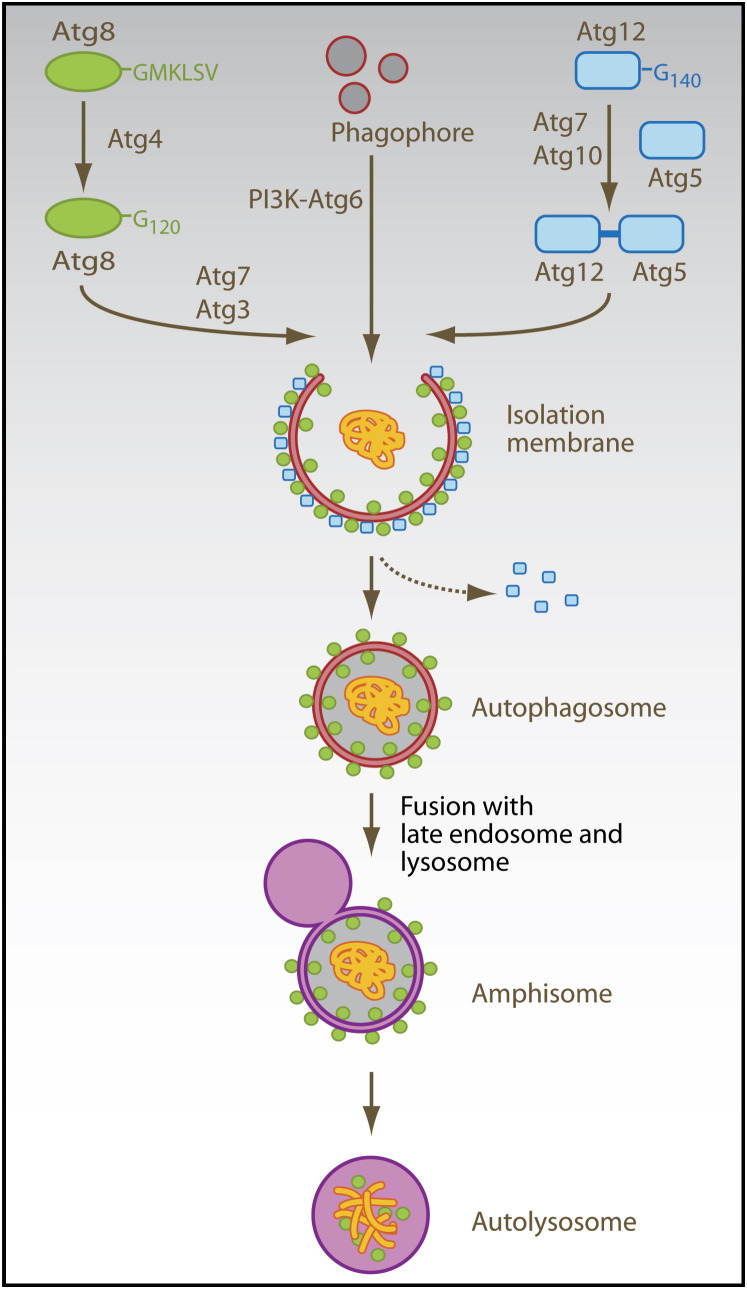
Autophagy is composed of at least three distinct pathways: microautophagy, chaperone-mediated autophagy, and macroautophagy (Mizushima and Klionsky, 2007). Microautophagy involves budding of small cytosol-containing vesicles, and this budding occurs directly into the lysosomal lumen. During chaperone-mediated autophagy, proteins are directly imported into lysosomes via the LAMP-2a transporter (Cuervo and Dice, 1996, Cuervo and Dice, 2000) assisted by cytosolic (Chiang et al., 1989) and lysosomal (Agarraberes et al., 1997) HSC70 chaperones. Substrates of chaperone-mediated autophagy carry signal peptides for sorting into lysosomes, similar to other protein-transport mechanisms across membranes (Agarraberes and Dice, 2001). Finally, during macroautophagy, a cup-shaped isolation membrane forms de novo from sources that are still debated (Juhasz and Neufeld, 2006). In this process, cytosolic constituents are enclosed in a double-membrane vesicle, called autophagosome (Fengsrud et al., 2000a, Stromhaug et al., 1998), which then fuses with lysosomes and late endosomes for degradation of the inner autophagosomal membrane and its cargo (Ohsumi, 2001) ( Figure 1). The class III phosphatidylinositol 3-kinase (PI3K) and its binding partner, the autophagy-related gene (Atg) 6 (also known as Beclin-1), is required for the initiation of the isolation membrane (Kihara et al., 2001, Zeng et al., 2006). In addition, two ubiquitin-like systems are essential for the formation of autophagosomes: In one of them, the five C-terminal amino acids of the ubiquitin-like protein Atg8 (also known as light chain [LC] 3) are cleaved off by the Atg4 protease to liberate a glycine residue (G120). This C-terminal residue then gets transferred to phosphatidylethanolamine in the forming isolation membrane by the E1- and E2-like enzymes Atg7 and Atg3. Although Atg8 (LC3) gets recycled from the outer autophagosomal membrane by deconjugation from the phospholipid, it remains attached to the inner autophagosomal membrane, and this proportion is degraded with the inner autophagosomal membrane in lysosomes and late endosomes after fusion with these vesicles (Ichimura et al., 2000, Kabeya et al., 2000). In the other ubiquitin-like system, Atg12 gets coupled via its C-terminal glycine residue (G140) to a lysine residue of Atg5 by the E1- and E2-like enzymes Atg7 and Atg10. The Atg12-Atg5 complex associates with Atg16 and then binds to the outer surface of the isolation membrane. Upon completion of the autophagosome, the Atg5-Atg12-Atg16 complex dissociates from the outer autophagosomal membrane (Mizushima et al., 1998).
Figure 1.
Molecular Machinery of Macroautophagy
Atg6 (Beclin-1) is part of the type III PI3K complex that initiates autophagosome formation. Two ubiquitin-like systems are required for formation of the isolation membrane and couple Atg8 (LC3) and Atg12 to phosphatidylethanolamine (PE) and Atg5, respectively. The five C-terminal amino acids of Atg8 (LC3) are cleaved of by Atg4 to reveal glycine 120 (G120), which is required to link the protein after activation by Atg7 and ligation by Atg3 to PE in the autophagosomal membrane (green circles). Similarly, glycine 140 (G140) is used by Atg7 and Atg10 to couple Atg12 to Atg5. This complex then localizes to the outer membrane of the forming autophagosome (blue squares). Upon autophagosome completion, the Atg12-Atg5 complex recycles from the outer membrane, and only Atg8 (LC3) remains associated with the completed autophagosome. Autophagosomes then fuse with late endosomes and lysosomes for degradation of their cargo and their intravesicular membranes.
The exact functions of the components of the molecular machinery for macroautophagy are not well understood to date. Because the Atg5-Atg12-Atg16 complex localizes to the convex surface of the isolation membrane, it may determine the curvature of the forming autophagosome through a protein lattice, not unlike what clathrin coating does for endosomes (Trombetta and Mellman, 2005). Furthermore, because Atg8 (LC3) is primarily localized to the inner autophagosomal membrane, especially after autophagosome completion, it was proposed that this molecule could serve as an anchor for autophagy substrates. Two proteins, Alfy and p62 (also known as SQSTM1), have been suggested for the targeting of proteins to autophagosomes (Bjorkoy et al., 2005, Simonsen et al., 2004). Alfy has been found in proximity to autophagic membranes and colocalizing with protein aggregates under conditions of starvation and proteasome inhibition, which are known to upregulate macroautophagy (Mizushima and Klionsky, 2007, Simonsen et al., 2004). Similarly, p62 (SQSTM1) has been found to localize to protein aggregates, is degraded by macroautophagy, and could be coimmunoprecipitated with Atg8 (LC3) (Bjorkoy et al., 2005). Both proteins were suggested to target protein aggregates to autophagosomes, in the case of p62 (SQSTM1) by binding to Atg8 (LC3).
These studies on the molecular mechanisms of autophagy and its importance in protein metabolism have set the stage to analyze its importance during immune responses. In the following sections, we will discuss autophagy during innate immune responses to viruses, bacteria, and parasites and highlight its role in the initiation and execution of adaptive immune responses.
Escape from Autophagy by Viruses
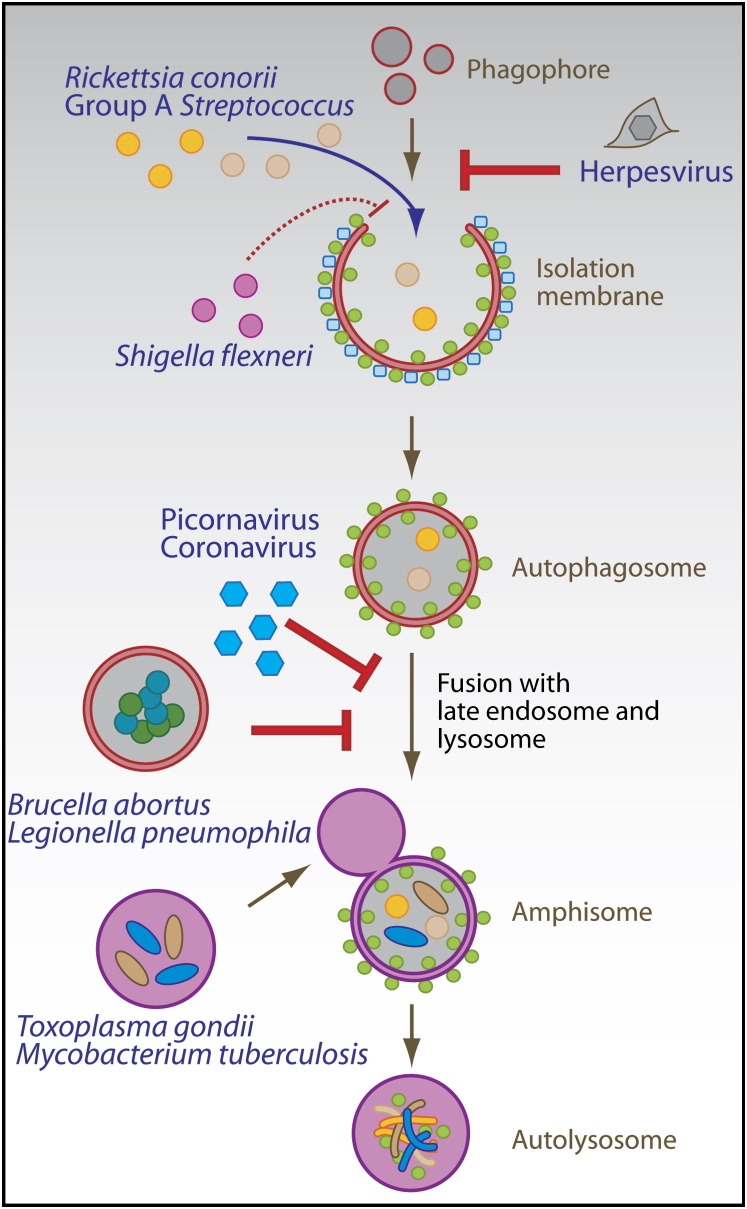
Viral immune escape from proteasomal degradation has been characterized in great detail (Tortorella et al., 2000). In contrast, the interaction between viruses and the autophagic machinery is just beginning to be elucidated. The three following main outcomes of these interactions have been noted: Macroautophagy successfully limits viral replication, viruses inhibit macroautophagy to avoid restriction of their replication, or viruses use accumulated autophagosomes for their replication ( Figure 2).
Figure 2.
Innate Immune Control by Macroautophagy
Viruses, bacteria, and parasites get cleared or interfere with their destruction by macroautophagy. Herpesviruses encode inhibitors of autophagosome formation, whereas picornaviruses and coronaviruses inhibit fusion of autophagosmes with lysosomes in order to replicate on autophagosomal membranes. Rickettsia conorii and group A Streptococcus, examples of bacterial pathogens that escape the endosome, are degraded by macroautophagy, but Shigella flexneri has developed an escape strategy that avoids its import into autophagosomes. Brucella abortus and Legionella pneumophila block autophagosome-lysosome fusion and replicate in autophagic vesicles. Pathogens such as Toxoplasma gondii and Mycobacterium tuberculosis condition phagosomes as replication niches, which can be cleared by macroautophagy after immune activation of the host cell.
Only two studies have demonstrated so far that macroautophagy can successfully limit viral replication. One example comes from plant infection by the tobacco mosaic virus (TMV). This single-stranded RNA virus of the Tobamovirus family replicates more efficiently when Atg6 (Beclin-1) or Atg7 are silenced in Nicotiana benthamiana plants (Liu et al., 2005). The second example is a single-stranded RNA virus of the Togavirus family. Sindbis virus of this virus family causes encephalitis, which can be ameliorated by overexpression of Atg6 (Beclin-1) in mice (Liang et al., 1998). After virus-driven Atg6 (Beclin-1) overexpression, less Sindbis virus RNA-positive cells, decreased brain pathology, and lower viral titers are detected in mice. Although the role of constitutive macroautophagy in Sindbis-virus-mediated encephalitis has not been elucidated so far, enhanced macroautophagy seems to confer protection against viral replication. Another protective role that macroautophagy seems to play during viral infections is to deliver pathogen constituents for detection by endosomal receptors that initiate immune responses, such as toll-like receptors (TLRs) (Lee et al., 2007). One member of this family, TLR7, detects single-stranded RNA. In the case of vesicular stomatitis virus (VSV) and Sendai virus (SV) infection, TLR7-dependent activation of plasmacytoid dendritic cells (pDCs) and their type I IFN secretion seem to rely on macroautophagy because Atg5-deficient pDCs are unable to secrete IFN-α in response to SV and VSV infection. However, further studies are required to ensure that pDC function is not generally compromised in the absence of macroautophagy and to analyze to what extent this pathway contributes to the detection of viral infections in vivo.
In contrast to these examples of protection from viral infection via macroautophagy, two double-stranded DNA viruses of the herpesvirus family, herpes simplex virus-1 (HSV-1) and Kaposi's sarcoma-associated herpesvirus (KSHV), escape the fate of restriction or detection by macroautophagy via expression of inhibitors of this pathway. Both viruses target Atg6 (Beclin-1) for this purpose. HSV-1 expresses the early-antigen, infected cell protein 34.5 (ICP34.5), which inhibits macroautophagy and thereby allows increased viral replication in mouse embryonic fibroblasts (Tallóczy et al., 2002). Interestingly, recombinant HSV-1 with a mutant ICP34.5 protein that can no longer interact with Atg6 (Beclin-1) and thereby fails to inhibit macroautophagy is severely attenuated with respect to neurovirulence in mice (Orvedahl et al., 2007). This suggests that macroautophagy inhibition is essential for efficient replication of HSV-1 in the central nervous system of mice in vivo. Atg6 (Beclin-1) is also the target of macroautophagy inhibition by KSHV. Its Bcl-2 homolog binds to Atg6 (Beclin-1) and thus inhibits macroautophagy (Pattingre et al., 2005), but it remains unclear whether this function is important for KSHV replication.
An even more dramatic subversion of macroautophagy is practiced by several single-stranded RNA viruses, which replicate in the cytoplasm. Picornaviruses (poliovirus and rhinoviruses), coronaviruses (mouse hepatitis virus and severe acute respiratory syndrome coronavirus), and one arterivirus (equine arteritis virus) replicate on the surface of autophagosomal membranes (Dales et al., 1965, Jackson et al., 2005, Pedersen et al., 1999, Prentice et al., 2004a, Prentice et al., 2004b). These viruses probably block the fusion of autophagosomes, which carry their replication complexes, with lysosomes. In the case of poliovirus and rhinoviruses, this macroautophagy regulation seems to be brought about by the 2BC and 3A proteins, which are sufficient to induce the accumulation of autophagosomes (Jackson et al., 2005). Generation of autophagic membranes as scaffolds for the replication machinery is essential because inhibition of macroautophagy by siRNA-mediated silencing of Atg12 or Atg8 (LC3) inhibits viral replication (Jackson et al., 2005). Therefore, a number of single-stranded RNA viruses interfere with autophagosome maturation in order to replicate on the surfaces of these vesicles. Similarly, to their benefit, the double-stranded RNA virus rotavirus of the reovirus family and the single-stranded DNA virus B19 parvovirus both induce macroautophagy, with rotavirus mediating this effect via its NSP4 protein (Berkova et al., 2006, Nakashima et al., 2006). Rotavirus seems to replicate in close proximity to autophagic membranes, and B19 parvovirus might prolong the survival of infected cells via macroautophagy. However, the exact function of macroautophagy induction by these two viruses is not entirely understood.
Although all viruses listed above regulate macroautophagy to promote their own replication, two other viruses use the molecular macroautophagy machinery differently for their benefit. Human immunodeficiency virus 1 (HIV-1) induces autophagy-dependent apoptosis or autophagic cell death in bystander CD4+ T cells when the envelope protein of HIV-1 interacts with the chemokine receptor CXCR4 on the cell surface of CD4+ T cells (Espert et al., 2006). By these means, HIV-1 might deplete the CD4+ T cell compartment beyond destruction through viral replication. Another way in which a virus can benefit from the macroautophagy machinery is demonstrated by the single-stranded RNA virus bovine viral diarrhea virus (BVDV) of the flavivirus family. This virus has incorporated part of the Atg8 (LC3) sequence for specific proteolytic processing of its polyprotein (Meyers et al., 1998), presumably via the Atg4 protease, which liberates G120 of Atg8 (LC3) for coupling to autophagosomal membranes (Figure 1). In this fashion, the virus uses a specific proteolytic event of the macroautophagy machinery to process its protein products.
Thus, both viruses and their hosts have evolved various ways to utilize or subvert macroautophagy for their benefit. The many pathways by which viruses target this catabolic process suggest that it is important for innate immunity against these pathogens.
Autophagic Defense against Intracellular Bacteria and Parasites
In contrast to viruses, a number of microbial pathogens have been described to be successfully targeted by macroautophagy during innate immune responses. However, most of these studies have been performed in vitro, and it remains to be established whether macroautophagy can restrict bacteria and parasites in vivo.
Bacteria and parasites have been found to be susceptible to macroautophagy in two cellular compartments after entering their host cells. Free bacteria in the cytosol can fall prey to macroautophagy, and pathogen-conditioned phagosomes can either fuse with or get engulfed by autophagosomes (Figure 2). Group A Streptococci multiply freely in atg5 −/− mouse embryonic fibroblasts after escaping from endosomes (Nakagawa et al., 2004). In contrast in wild-type cells, cytosolic group A Streptococci become enveloped by autophagosomes and are delivered for lysosomal hydrolysis. Similarly, the cytoplasmic bacterium Rickettsia conorii was observed in autophagosomal structures in endothelial cells by electron microscopy (Walker et al., 1997). Proinflammatory cytokines lead to increased macroautophagy and Rickettsia clearance from the cytoplasm. However, it has not been conclusively shown that the protective cytokines required autophagy for restriction of Rickettsia. Furthermore, metabolic inhibition of Listeria monocytogenes after phagosome lysis renders these bacteria susceptible for macroautophagy and lysosomal clearance (Rich et al., 2003). Engulfment of Listeria can be blocked by pharmacological autophagy inhibitors and accelerated by starvation. In addition to eliminating pathogens in the cytosol, macroautophagy can also target phagosomes that have been conditioned by bacteria or parasites to avoid fusion with lysosomes. The best-studied examples for these are Mycobacterium tuberculosis and Toxoplasma gondii (Andrade et al., 2006, Gutierrez et al., 2004, Ling et al., 2006). M. tuberculosis was shown to be cleared from mouse macrophages and a human macrophage cell line by macroautophagy (Gutierrez et al., 2004, Singh et al., 2006). Starvation-induced clearance of the M. tuberculosis variant bovis BCG is inhibited by pharmacological inhibitors of macroautophagy. In addition to starvation, lipopolysaccharide (LPS)-induced autophagic clearance of M. tuberculosis-containing phagosomes via TLR4-TRIF-RIP1-p38MAPK signaling (Xu et al., 2007 [this issue of Immunity]) has been observed. An autophagy-dependent protective mechanism is the lysosomal generation of bactericidal peptides from ubiquitin after autophagic delivery of ubiquitinylated protein aggregates (Alonso et al., 2007). The induction of autophagy by starvation resulted in enhanced delivery of ubiquitinated proteins to microbacteria-harboring phagosomes and increased the bactericidal activity of lysosomes. Similarly, T. gondii appears to be susceptible to macroautophagic degradation in mouse and human macrophages (Andrade et al., 2006, Ling et al., 2006). Clearance of T. gondii tachyzoites upon macrophage activation could be inhibited by pharmacological inhibitors of macroautophagy and siRNA silencing of Atg6 (Beclin-1). Less well characterized is the macroautophagic degradation of Salmonella enterica and uropathogenic Escherichia coli-containing vacuoles (Amer et al., 2005, Birmingham et al., 2006). However, S. enterica at least seems to stimulate macroautophagy via injection of its SipB protein into the cytosol through the bacterial type III secretion system (Hernandez et al., 2003).
As summarized above for viruses, bacteria have also developed counteracting mechanisms against innate restriction by macroautophagy. Again, both autophagosome generation and maturation into autolysosomes are targeted (Figure 2). However, unlike viruses, these mechanisms do not seem to generally inhibit macroautophagy of the host cell in trans but rather prevent autophagic clearance of the particular bacterial pathogens in cis, without affecting the degradation of other autophagy substrates. Shigella flexneri inhibits macroautophagy, which is induced by the binding of its VirG protein to Atg5 (Ogawa et al., 2005). This specific interaction of the autophagic machinery with a bacterial protein is disrupted by Shigella's IcsB protein, and loss of IcsB leads to autophagic clearance of these bacteria from the cytosol. A number of other bacterial and parasitic pathogens trigger macroautophagy for their efficient replication. Instead of using autophagosomal membranes as scaffolds for replication, as discussed above for single-stranded RNA viruses, they replicate most effectively inside autophagosomes and autolysosomes. Pathogens of this category include Francisella tularensis, Brucella abortus, Porphyromonas gingivalis, Leishmania mexicana, Chlamydia trachomatis, Coxiella burnetii, and Legionella pneumophila (Al-Younes et al., 2004, Amer and Swanson, 2005, Celli et al., 2003, Checroun et al., 2006, Dorn et al., 2001, Gutierrez et al., 2005, Pizarro-Cerda et al., 1998, Schaible et al., 1999). At least some of these bacteria use their type III and type IV secretion systems to trigger autophagy and also inhibit fusion of autophagosomes with lysosomes to generate their replication niche. For example, 10–30 kDa components of Legionella type IV secretion substrates are able to stimulate macroautophagy in mouse macrophages (Amer and Swanson, 2005). In contrast, the type IV secretion system of Brucella is required to prevent fusion of the vesicular Brucella replication compartment with lysosomes (Celli et al., 2003). Establishment of this compartment can be inhibited by pharmacological inhibitors of macroautophagy (Pizarro-Cerda et al., 1998). Thus, by using their elaborate secretion systems, successful bacterial pathogens can subvert macroautophagy for their benefit, and therefore avoid autolysosomal degradation in their host cells.
Apart from pathogen clearance, macroautophagy provides another type of protection from bacterial pathogenesis during Vibrio cholerae infection. This noninvasive intestinal pathogen secretes the hemolytic exotoxin cytolysin, but gut epithelial cell lines resist cell death induced by this toxin through the induction of macroautophagy (Gutierrez et al., 2007). Atg5-deficient cells on the contrary displayed poor survival upon exposure to Vibrio cytolysin.
Thus, bacterial pathogens, both after escaping from endosomes into the cytosol and in phagosomes, can become substrates for macroautophagy. The efficiency of this innate immune defense mechanism most probably forced successful bacterial pathogens to develop escape mechanisms against autophagic degradation.
Antigen Presentation via Autophagy
In addition to limiting pathogen replication in host cells, macroautophagy also delivers viral, parasitic, and bacterial antigens to late endosomal compartments, where macroautophagy substrates are then degraded by lysosomal hydrolases. The fusion vesicles between autophagosomes and late endosomes, the so-called amphisomes, have been isolated and ultrastructurally characterized by electron microscopy (Berg et al., 1998, Liou et al., 1997). In these studies, double-membrane-enveloped autophagosomes receive colloidal gold after endocytosis, and the resulting amphisomes could be stabilized by inhibition of lysosomal degradation. The amphisomes display multivesicular and multilamellar morphology. Exactly the same characteristics have been reported for major histocompatibility complex (MHC) class II loading compartments (MIICs) (Zwart et al., 2005), which are now considered to be conventional late endosomes that are equipped with the molecular machinery to load antigenic fragments onto MHC class II molecules for presentation to CD4+ T cells.
We have recently shown that GFP-Atg8 (GFP-LC3)-positive autophagosomes fuse frequently with MIICs, as identified by the presence of MHC class II, the lysosomal membrane protein LAMP-2 and the chaperone HLA-DM, which is involved in peptide loading onto MHC class II molecules (Schmid et al., 2007). We observe 50%–80% overlap between Atg8 (LC3) and MHC class II in professional antigen-presenting cells, such as dendritic cells and B cell lines, as well as epithelial cell lines, in which MHC class II expression was induced by proinflammatory cytokines. This cell-biological evidence suggests that macroautophagy frequently delivers autophagosome content, including pathogen-derived proteins, to MIICs ( Figure 3).
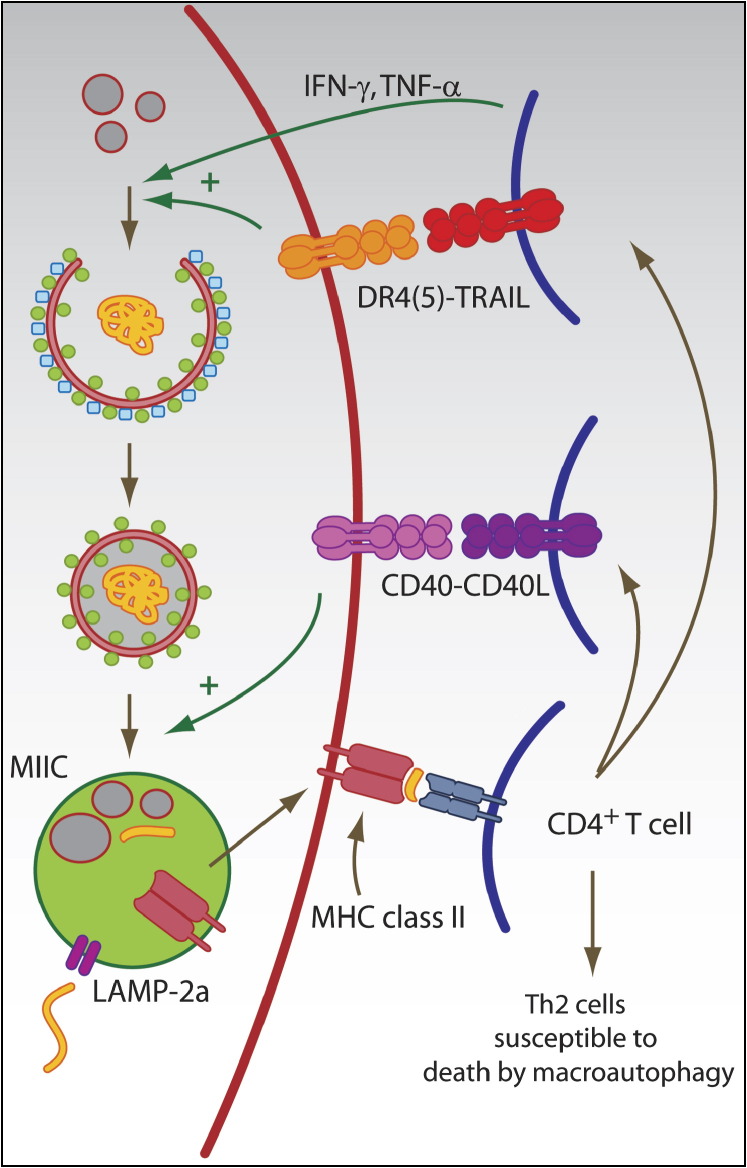
Figure 3.
Role of Macroautophagy in Adaptive Immune Responses
Autophagic pathways can deliver antigens for MHC class II presentation. Autophagosomes and LAMP-2a, the transporter associated with chaperone-mediated autophagy, can transport antigens into the MHC-class-II-loading compartment (MIIC). In MIICs, the antigen is processed and loaded onto MHC class II molecules for CD4+ T cell stimulation. Activated CD4+ T cells can then in turn enhance macroautophagy and autophagosome-lysosome fusion via type II IFN and TNF family members (IFN-γ, TNF, TRAIL, and CD40L). In addition, Th2-polarized CD4+ T cells are susceptible to cell death by macroautophagy.
Biochemical isolation of natural MHC class II ligands reveals that up to 20% of eluted peptides originate from cytosolic and nuclear proteins (Chicz et al., 1993, Dengjel et al., 2005, Dongre et al., 2001, Rammensee et al., 1999). Among them, two fragments of Atg8 (LC3) (MAP1LC3B93-109 and MAP1LC3B93-110) have been isolated from HLA-DR molecules of Epstein Barr virus (EBV)-transformed B cell lines (Dengjel et al., 2005). Other sources of natural MHC class II ligands also fit the characteristics of autophagy substrates. Some long-lived proteins, which appear to be preferentially degraded by autophagy (Henell et al., 1987), have been frequently found to give rise to MHC class II peptide cargo. For example, glyceraldehyde-3-phosphate dehydrogenase (GAPDH) with the extraordinarily long half-life of 130 hr (Dice and Goldberg, 1975) has been described as a substrate of chaperone-mediated autophagy (Aniento et al., 1993) and has been isolated from autophagosomes (Fengsrud et al., 2000b). In good agreement with autophagic delivery of antigens for MHC class II loading, GAPDH-derived peptides have been isolated from four different MHC class II molecules, but no natural MHC class I ligands have been described from this protein so far (Rammensee et al., 1999). In contrast, cyclins with half-lives in the order of minutes are one of the most frequent sources of MHC class I peptides and have been eluted from 17 different MHC class I molecules, but only one MHC class II ligand from this group of proteins has been described so far (Rammensee et al., 1999). Short and long half-life correlate to some extent with the degradation route of proteins. For example, the nuclear antigen 1 of EBV (EBNA1) contains a glycine alanine (GA)-repeat domain, which inhibits its degradation via the proteasome and presentation on MHC class I (Hoyt et al., 2006, Lee et al., 2004, Levitskaya et al., 1995, Levitskaya et al., 1997). EBNA1's half-life is very long (Levitskaya et al., 1997, Tellam et al., 2001) but is shortened considerably upon deletion of the GA repeat (Levitskaya et al., 1997). GA-deleted EBNA1 is degraded by the proteasome and efficiently presented on MHC class I (Lee et al., 2004, Levitskaya et al., 1997). In contrast, long-lived wild-type EBNA1 is presented on MHC class II after macroautophagy (Paludan et al., 2005). Similarly, influenza matrix protein 1 (MP1) is intracellularly processed onto MHC class II (Jaraquemada et al., 1990), but when the half-life of MP1 is shortened by N-end-rule modification with enhanced proteasomal degradation, endogenous MHC class II presentation is lost (Gueguen and Long, 1996).
Further evidence, consistent with the hypothesis that proteins with long half-lives are preferentially processed for MHC class II presentation, comes from analysis of the RAD23 protein. Not unlike EBNA1, this protein contains a domain that prevents proteasomal proteolysis (Heessen et al., 2005). Deletion of this UBA2 domain shortens RAD23's half-life and makes it more susceptible to proteasomal degradation. Interestingly, when autophagy is induced by starvation and then natural HLA-DR ligands are eluted, peptides derived from cytosolic and nuclear antigens are upregulated, whereas natural ligands from membrane and secreted proteins are not affected (Dengjel et al., 2005). A protein whose MHC class II presentation is strongly upregulated after 24 hr of starvation is RAD23. Taken together, all these examples suggest that long-lived autophagy substrates are efficiently processed for MHC class II presentation to CD4+ T cells.
CD4+ T Cell Stimulation after Autophagy
Direct involvement of the molecular machinery for autophagy in intracellular antigen presentation on MHC class II has been documented only for a few antigens so far. The first study implicating macroautophagy in antigen processing onto MHC class II demonstrated that pharmacological inhibition of autophagosome formation decreased MHC class II presentation of the overexpressed complement C5 protein in mouse macrophage and B cell lines (Brazil et al., 1997). In this study and many follow-up studies, inhibitors of the class III PI3 kinase complex, including 3-methyladenine (Seglen and Gordon, 1982), have been used to inhibit macroautophagy. However, 3-methyladenine has additional effects on membrane trafficking such as endocytosis, on lysosomal acidification, on phosphorylation of some signal transduction molecules, and on mitochondrial permeability (Mizushima, 2004). Therefore, pharmacological inhibition of macroautophagy can be used as a first indication that this pathway is involved, but this finding should be confirmed by RNA interference or in knockout cells. With these caveats of pharmacological inhibitors in mind, two additional studies have used those to implicate macroautophagy in intracellular MHC class II antigen processing of the tumor antigen Mucin gene 1 (MUC1) product (Dörfel et al., 2005) and the model antigen neomycin phosphotransferease II (NeoR) (Nimmerjahn et al., 2003). Of these, the latter study controlled, as carefully as possible, for macroautophagy-independent side effects of the pharmacological inhibitors by demonstrating that CD8+ T cell recognition of the tumor antigen tyrosinase and MHC class II antigen processing of extracellular NeoR were not influenced by 3-methyladenine. In addition, NeoR accumulated intracellularly and was excluded from delivery to lysosomes by this treatment. Therefore, the authors provided good evidence that NeoR is delivered by macroautophagy for MHC class II presentation in EBV-transformed B cells and in renal cell carcinoma cells.
In addition to these studies on self, tumor, and model antigens, we have demonstrated that the viral antigen EBNA1 is processed intracellularly for MHC class II presentation to CD4+ T cells via macroautophagy in EBV-transformed B cells and EBNA1-transfected EBV-negative Hodgkin's lymphoma cells (Münz et al., 2000, Paludan et al., 2005). Upon inhibition of lysosomal degradation, we have found EBNA1 accumulating in autophagosomes, visualized as multiple membrane-enveloped vesicles or isolation membranes in electron microscopy. Furthermore, intracellular EBNA1 processing for MHC class II presentation is inhibited by siRNA-mediated silencing of the essential autophagy gene Atg12. According to these data, EBNA1 is the first antigen for which physiological amounts have been demonstrated to be processed onto MHC class II after macroautophagy. More recently, we have shown that targeting of influenza MP1 to autophagosomes by fusing this antigen to Atg8 (LC3) enhanced MHC class II presentation to MP1-specific CD4+ T cell clones 5- to 20-fold, whereas MHC class I presentation to MP1-specific CD8+ T cell clones was not affected by attachment to Atg8 (LC3) (Schmid et al., 2007). We have observed increased MP1-Atg8 (MP1-LC3) presentation on MHC class II in epithelial cells, EBV-transformed B lymphoblastoid cells, and dendritic cells. Targeting of the fusion antigen for MHC class II presentation is dependent on coupling of MP1-Atg8 (LC3) to the autophagosomal membrane because a mutant protein lacking the glycine residue (Gly120) used for the ubiquitin-like conjugation reaction does not show enhanced MHC class II presentation. Furthermore, inhibition of macroautophagy by RNA silencing of Atg12 inhibited MP1-Atg8 (LC3) transport to vesicles that are MHC class II positive. Taken together, these data suggest that several antigens can enter the MIIC via macroautophagy and that this pathway leads to efficient MHC class II presentation to CD4+ T cells.
In addition to macroautophagy, chaperone-mediated autophagy has also been implicated in delivering cytosolic antigens for MHC class II presentation (Figure 3). The two autoantigens glutamate decarboxylase 65 (GAD65) and the mutant human immunoglobulin κ chain SMA showed enhanced processing onto MHC class II after overexpression of LAMP-2a, the transporter of chaperone-mediated autophagy (Zhou et al., 2005). Because MHC class II processing of these antigens also requires proteasomal and calpain-mediated degradation (Lich et al., 2000), Zhou and colleagues suggested that these autoantigens are processed into peptides in the cytosol and then are imported into MIICs by LAMP-2a. Thus, both macroautophagy and chaperone-mediated autophagy can deliver cytosolic and nuclear antigens into MIICs for efficient MHC class II presentation to CD4+ T cells. Future studies now need to address to which extent these pathways contribute to CD4+ T cell responses in vivo.
Role of Autophagy in CD4+ T Cell Immunity and Tolerance
CD4+ T cell stimulation after autophagy-mediated antigen processing could be crucial both during steady-state tolerance induction and immune control of pathogens and tumors. Self-antigen presentation on MHC class II of both thymic epithelial cells and dendritic cells could lead to central and peripheral tolerance maintenance in the CD4+ T cell compartment. In agreement with this hypothesis, thymic epithelia display great amounts of autophagosomes in the GFP-LC3 transgenic macroautophagy reporter mouse in the steady state (Mizushima et al., 2004). Interestingly, more autophagosomes could be found in this tissue in newborns than in adult mice. These findings correlate with T cell selection and central tolerance induction for CD4+ T cells being most active at a young age (Starr et al., 2003). In addition, we find considerable autophagy in immature dendritic cells (Schmid et al., 2007), which have been implicated in peripheral tolerance induction (Steinman et al., 2003). Some of these tolerance mechanisms might be compromised in patients with mutations in Atg16 and Crohn disease (Hampe et al., 2007, Rioux et al., 2007). This inflammatory bowel disease occurs in individuals with genetic predisposition and is probably triggered by the enteric microflora. Therefore, decreased macroautophagy due to Atg16 mutations might either impair innate resistance to invading bacteria in intestinal epithelial cells and thereby trigger inflammation as a result of increased antigenic load or lead to insufficient tolerance induction against commensals in the gut and trigger Crohn disease. On the basis of these findings, it is tempting to speculate that macroautophagy might contribute to self-antigen presentation during central and peripheral tolerance maintenance for CD4+ T cells.
CD4+ T cells are crucial orchestrators of adaptive immunity. Their activation is required for both B cell differentiation and immunoglobulin maturation during humoral immunity, as well as efficient noninflammatory CD8+ T cell priming and maintenance of CD8+ T cell function during cell-mediated immunity (Bevan, 2004, McHeyzer-Williams and McHeyzer-Williams, 2005). In addition, CD4+ T cells can have direct effector functions against infected and transformed cells (Heller et al., 2006). Therefore, autophagic delivery of intracellular antigens for MHC class II presentation could lead to efficient CD4+ T cell priming by infected professional antigen-presenting cells, such as dendritic cells. This mechanism could also contribute to CD4+ T cell immune surveillance of inflamed tissues, which upregulate MHC class II upon exposure to proinflammatory cytokines (Reith and Mach, 2001), as we observed for epithelial cells (Schmid et al., 2007).
Taken together, the discussed findings show that intracellular antigen processing via autophagy broadens the immunological functions and usefulness of MHC class II presentation. This pathway expands the traditional view that MHC class II presentation is restricted to professional antigen-presenting cells with considerable endocytic activity to the new paradigm that MHC class II molecules also enable immune surveillance and tolerance induction for intracellular antigens during immune activation and in the steady state. Therefore, MHC class II might play a much broader role, similar to MHC class I, at sites of inflammation and MHC class II upregulation.
Autophagy Regulation as an Effector Mechanism of the Adaptive and Innate Immune System
In addition to macroautophagy as an innate immune mechanism in infected host cells, this pathway is also induced as an effector mechanism of innate and adaptive lymphocytes via interferons (IFNs) and members of the tumor necrosis factor (TNF) family (Figure 3).
Both type I and II IFNs have been reported to modulate macroautophagy. Restriction of HSV-1 infection by macroautophagy in vitro and in vivo was found to be dependent on the type I IFN-inducible double-stranded-RNA-dependent protein kinase R (PKR) (Orvedahl et al., 2007, Tallóczy et al., 2002). In order to develop neurovirulence, HSV-1 carries the ICP34.5 protein, which inhibits PKR-dependent macroautophagy induction by binding to Atg6 (Beclin-1) (Orvedahl et al., 2007). Type II IFN has been reported to enhance Mycobacterium tuberculosis and Ricksettia conorii degradation by macroautophagy in infected cells (Gutierrez et al., 2004, Singh et al., 2006, Walker et al., 1997). Mouse macrophages seem to be most susceptible to IFN-γ-induced upregulation of macroautophagy, whereas in human tissues, this effect is more difficult to demonstrate and probably proceeds with slower kinetics (Pyo et al., 2005). Type II IFN seems to induce macroautophagy and mycobacterial clearance through immunity-related GTPases (IRGs), also known as p47 GTPases, including the mouse LRG-47 and the human IRGM proteins. Mouse tissues are probably more susceptible to this macroautophagy regulation mechanism because their IRGs are IFN-γ inducible, whereas the human IRG is not. In addition, IFN-γ might also induce macroautophagy via death-associated protein kinases (DAPk). Overexpression of DAPk and its close family member DAPk-related protein kinase (DRP)-1 has been shown to increase macroautophagy in human epithelial cell lines (Inbal et al., 2002). Irrespective of the exact signaling pathways, these studies suggest that not only infected cells can upregulate macroautophagy via IFN-α and IFN-β but also potent type I IFN producers such as pDCs, as well as IFN-γ-secreting natural killer (NK) and T cells, might induce clearance of intracellular pathogens and enhance MHC class II presentation in infected tissues via this pathway.
Macroautophagy induction via TNF family members has been primarily investigated in cell-death research. The interplay between autophagy and apoptosis underlying these studies has been recently reviewed (Kroemer and Jaattela, 2005). First, TNF-α itself was found to upregulate macroautophagy in cells lacking NF-κB activation (Djavaheri-Mergny et al., 2006). Second, TNF-related apoptosis-inducing ligand (TRAIL) was described to induce autophagy in human epithelial cells (Mills et al., 2004). Consistent with this, inactivation of Fas-associated death domain (FADD), the signaling adaptor protein of the TRAIL receptor, decreases autophagy induction by TRAIL (Thorburn et al., 2005). As a third TNF family member, CD40L has been demonstrated to induce macroautophagy-mediated fusion of Toxoplasma gondii-containing phagosomes with lysosomes via CD40 signaling on mouse and human macrophages (Andrade et al., 2006). RNA silencing of Atg6 (Beclin-1) inhibited CD40-mediated clearance of T. gondii. Various cell types of the innate and adaptive immune system could potentially use this macroautophagy induction that is TNF family member mediated, including TNF-α-secreting dendritic cells, TRAIL-expressing NK and T cells, and CD40L-presenting CD4+ T cells. However, future studies are required to investigate whether macroautophagy induction during immune responses contributes substantially to resistance against infections in vivo.
Regulation of T Cell Survival and Death by Macroautophagy
Dendritic cells and NK and T cells might induce macroautophagy in infected cells to fight infections, but at the same time the fate of these immunological effectors seems to be determined by their own capacity to perform macroautophagy. The extent to which this process contributes to cell survival and cell death has so far primarily been investigated in T cells. Although the relationship between autophagy and apoptosis is still not entirely clear, a picture is emerging that autophagy often precedes apoptosis, often as a last rescue attempt before cell death (Kroemer and Jaattela, 2005). In some instances, however, macroautophagy is required for cell death or can even execute it, especially when the apoptosis machinery is compromised. These different modulations of cell death by macroautophagy are also reflected in studies on T cells (Figure 3). Atg5 deficiency leads to decreased T and B cell numbers in mice, and both CD4+ and CD8+ T cells fail to undergo efficient proliferation upon T cell receptor stimulation (Pua et al., 2007). These findings were interpreted as macroautophagy playing a role in T cell maintenance during the steady state and after activation. Furthermore, Th2 polarized CD4+ T cells, which are thought to support humoral immune responses most efficiently, display more GFP-Atg8 (GFP-LC3)-positive autophagosomes than Th1 cells, which have been shown to support cell-mediated immunity better (Li et al., 2006). In this study, the Th2 cell line D10 was more resistant to cell death upon growth-factor withdrawal when macroautophagy was inhibited by RNA silencing of Atg7 or Atg6 (Beclin-1), whereas steady-state survival and proliferation were not affected by macroautophagy inhibition. These studies suggest that Th2-polarized CD4+ T cells either die by macroautophagy or need the autophagy machinery to execute apoptotic cell death upon growth-factor deprivation. The latter interpretation is more likely because HIV-1 envelope protein induces CD4+ T cell apoptosis via macroautophagy and is inhibited upon RNA silencing of Atg7 or Atg6 (Beclin-1) (Espert et al., 2006). According to these studies, macroautophagy seems to be more important for CD8+ than CD4+ T cell survival, and Th2-polarized CD4+ T cells are more sensitive to cell death via macroautophagy than Th1-polarized cells. These data suggest that when manipulating macroautophagy for enhanced pathogen clearance, one should keep in mind that cellular components of the immune system, such as Th2 cells, might be sensitive to cell-death induction via macroautophagy. Therefore, any benefit gained by innate clearance of pathogens in infected cells via macroautophagy enhancement might be lost by compromising another arm of immune control, including Th2-supported humoral immunity.
Conclusions and Future Perspectives
Previous studies on autophagy in innate and adaptive immune responses have revealed interesting roles for this pathway in innate and adaptive immunity. However, these now need to be investigated in vivo. With the demonstration that macroautophagy inhibition is required for neurovirulence of HSV-1 in mice (Orvedahl et al., 2007), a step into this direction has been taken, but more such in vivo studies need to be performed. In vivo characterizations of the role of autophagy during tolerance induction and in dendritic cells for the initiation of immune responses are of special interest. With conditional knockout mice for essential macroautophagy genes (Hara et al., 2006, Komatsu et al., 2006), siRNAs for silencing Atg genes (Jackson et al., 2005, Schmid et al., 2007), and viral inhibitors of macroautophagy (Orvedahl et al., 2007, Pattingre et al., 2005), these studies become feasible in mice. A second important area is the investigation of substrate characteristics for pathogen and antigen import into autophagosomes. Although ubiquitination has been proposed as an import signal for macroautophagy (Bjorkoy et al., 2005), no firm evidence for this hypothesis has been presented. Finally, starvation-independent regulation of autophagy, especially during infections, is just beginning to become unraveled. A better understanding of this process, however, is required to evaluate it for therapeutic potential, especially because macroautophagy can not only support cell survival and pathogen clearance but also cell death. For manipulations of this pathway in patients, one has to walk a fine line between evoking its beneficial effects and avoiding tissue damage.
Acknowledgments
D.S. is a recipient of a predoctoral fellowship from the Schering Foundation. C.M. is supported by the Arnold and Mabel Beckman Foundation, the Alexandrine and Alexander Sinsheimer Foundation, the Burroughs Wellcome Fund, the Dana Foundation's Neuroimmunology program, the National Cancer Institute (R01CA108609 and R01CA101741), the National Institute of Allergy and Infectious diseases (RFP-NIH-NIAID-DAIDS-BAA-06-19), the Foundation for the National Institutes of Health (Grand Challenges in Global Health), and an Institutional Clinical and Translational Science Award (to the Rockefeller University Hospital).
References
- Agarraberes F.A., Dice J.F. Protein translocation across membranes. Biochim. Biophys. Acta. 2001;1513:1–24. doi: 10.1016/s0304-4157(01)00005-3. [DOI] [PubMed] [Google Scholar]
- Agarraberes F.A., Terlecky S.R., Dice J.F. An intralysosomal hsp70 is required for a selective pathway of lysosomal protein degradation. J. Cell Biol. 1997;137:825–834. doi: 10.1083/jcb.137.4.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonso S., Pethe K., Russell D.G., Purdy G.E. Lysosomal killing of Mycobacterium mediated by ubiquitin-derived peptides is enhanced by autophagy. Proc. Natl. Acad. Sci. USA. 2007;104:6031–6036. doi: 10.1073/pnas.0700036104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Younes H.M., Brinkmann V., Meyer T.F. Interaction of Chlamydia trachomatis serovar L2 with the host autophagic pathway. Infect. Immun. 2004;72:4751–4762. doi: 10.1128/IAI.72.8.4751-4762.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amer A.O., Byrne B.G., Swanson M.S. Macrophages rapidly transfer pathogens from lipid raft vacuoles to autophagosomes. Autophagy. 2005;1:53–58. doi: 10.4161/auto.1.1.1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amer A.O., Swanson M.S. Autophagy is an immediate macrophage response to Legionella pneumophila. Cell. Microbiol. 2005;7:765–778. doi: 10.1111/j.1462-5822.2005.00509.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrade R.M., Wessendarp M., Gubbels M.J., Striepen B., Subauste C.S. CD40 induces macrophage anti-Toxoplasma gondii activity by triggering autophagy-dependent fusion of pathogen-containing vacuoles and lysosomes. J. Clin. Invest. 2006;116:2366–2377. doi: 10.1172/JCI28796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aniento F., Roche E., Cuervo A.M., Knecht E. Uptake and degradation of glyceraldehyde-3-phosphate dehydrogenase by rat liver lysosomes. J. Biol. Chem. 1993;268:10463–10470. [PubMed] [Google Scholar]
- Berg T.O., Fengsrud M., Stromhaug P.E., Berg T., Seglen P.O. Isolation and characterization of rat liver amphisomes. Evidence for fusion of autophagosomes with both early and late endosomes. J. Biol. Chem. 1998;273:21883–21892. doi: 10.1074/jbc.273.34.21883. [DOI] [PubMed] [Google Scholar]
- Berkova Z., Crawford S.E., Trugnan G., Yoshimori T., Morris A.P., Estes M.K. Rotavirus NSP4 induces a novel vesicular compartment regulated by calcium and associated with viroplasms. J. Virol. 2006;80:6061–6071. doi: 10.1128/JVI.02167-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevan M.J. Helping the CD8+ T-cell response. Nat. Rev. Immunol. 2004;4:595–602. doi: 10.1038/nri1413. [DOI] [PubMed] [Google Scholar]
- Birmingham C.L., Smith A.C., Bakowski M.A., Yoshimori T., Brumell J.H. Autophagy controls Salmonella infection in response to damage to the Salmonella-containing vacuole. J. Biol. Chem. 2006;281:11374–11383. doi: 10.1074/jbc.M509157200. [DOI] [PubMed] [Google Scholar]
- Bjorkoy G., Lamark T., Brech A., Outzen H., Perander M., Overvatn A., Stenmark H., Johansen T. p62/SQSTM1 forms protein aggregates degraded by autophagy and has a protective effect on huntingtin-induced cell death. J. Cell Biol. 2005;171:603–614. doi: 10.1083/jcb.200507002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brazil M.I., Weiss S., Stockinger B. Excessive degradation of intracellular protein in macrophages prevents presentation in the context of major histocompatibility complex class II molecules. Eur. J. Immunol. 1997;27:1506–1514. doi: 10.1002/eji.1830270629. [DOI] [PubMed] [Google Scholar]
- Celli J., de Chastellier C., Franchini D.M., Pizarro-Cerda J., Moreno E., Gorvel J.P. Brucella evades macrophage killing via VirB-dependent sustained interactions with the endoplasmic reticulum. J. Exp. Med. 2003;198:545–556. doi: 10.1084/jem.20030088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Checroun C., Wehrly T.D., Fischer E.R., Hayes S.F., Celli J. Autophagy-mediated reentry of Francisella tularensis into the endocytic compartment after cytoplasmic replication. Proc. Natl. Acad. Sci. USA. 2006;103:14578–14583. doi: 10.1073/pnas.0601838103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang H.L., Terlecky S.R., Plant C.P., Dice J.F. A role for a 70-kilodalton heat shock protein in lysosomal degradation of intracellular proteins. Science. 1989;246:382–385. doi: 10.1126/science.2799391. [DOI] [PubMed] [Google Scholar]
- Chicz R.M., Urban R.G., Gorga J.C., Vignali D.A., Lane W.S., Strominger J.L. Specificity and promiscuity among naturally processed peptides bound to HLA-DR alleles. J. Exp. Med. 1993;178:27–47. doi: 10.1084/jem.178.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciechanover A., Finley D., Varshavsky A. Ubiquitin dependence of selective protein degradation demonstrated in the mammalian cell cycle mutant ts85. Cell. 1984;37:57–66. doi: 10.1016/0092-8674(84)90300-3. [DOI] [PubMed] [Google Scholar]
- Cuervo A.M., Dice J.F. A receptor for the selective uptake and degradation of proteins by lysosomes. Science. 1996;273:501–503. doi: 10.1126/science.273.5274.501. [DOI] [PubMed] [Google Scholar]
- Cuervo A.M., Dice J.F. Unique properties of lamp2a compared to other lamp2 isoforms. J. Cell Sci. 2000;113:4441–4450. doi: 10.1242/jcs.113.24.4441. [DOI] [PubMed] [Google Scholar]
- Dales S., Eggers H.J., Tamm I., Palade G.E. Electron microscopic study of the formation of poliovirus. Virology. 1965;26:379–389. doi: 10.1016/0042-6822(65)90001-2. [DOI] [PubMed] [Google Scholar]
- De Duve C., Wattiaux R. Functions of lysosomes. Annu. Rev. Physiol. 1966;28:435–492. doi: 10.1146/annurev.ph.28.030166.002251. [DOI] [PubMed] [Google Scholar]
- Dengjel J., Schoor O., Fischer R., Reich M., Kraus M., Muller M., Kreymborg K., Altenberend F., Brandenburg J., Kalbacher H., et al. Autophagy promotes MHC class II presentation of peptides from intracellular source proteins. Proc. Natl. Acad. Sci. USA. 2005;102:7922–7927. doi: 10.1073/pnas.0501190102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dice J.F., Goldberg A.L. A statistical analysis of the relationship between degradative rates and molecular weights of proteins. Arch. Biochem. Biophys. 1975;170:213–219. doi: 10.1016/0003-9861(75)90112-5. [DOI] [PubMed] [Google Scholar]
- Djavaheri-Mergny M., Amelotti M., Mathieu J., Besancon F., Bauvy C., Souquere S., Pierron G., Codogno P. NF-kappaB activation represses tumor necrosis factor-alpha-induced autophagy. J. Biol. Chem. 2006;281:30373–30382. doi: 10.1074/jbc.M602097200. [DOI] [PubMed] [Google Scholar]
- Dongre A.R., Kovats S., deRoos P., McCormack A.L., Nakagawa T., Paharkova-Vatchkova V., Eng J., Caldwell H., Yates J.R., 3rd, Rudensky A.Y. In vivo MHC class II presentation of cytosolic proteins revealed by rapid automated tandem mass spectrometry and functional analyses. Eur. J. Immunol. 2001;31:1485–1494. doi: 10.1002/1521-4141(200105)31:5<1485::AID-IMMU1485>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Dörfel D., Appel S., Grunebach F., Weck M.M., Muller M.R., Heine A., Brossart P. Processing and presentation of HLA class I and II epitopes by dendritic cells after transfection with in vitro transcribed MUC1 RNA. Blood. 2005;105:3199–3205. doi: 10.1182/blood-2004-09-3556. [DOI] [PubMed] [Google Scholar]
- Dorn B.R., Dunn W.A., Jr., Progulske-Fox A. Porphyromonas gingivalis traffics to autophagosomes in human coronary artery endothelial cells. Infect. Immun. 2001;69:5698–5708. doi: 10.1128/IAI.69.9.5698-5708.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espert L., Denizot M., Grimaldi M., Robert-Hebmann V., Gay B., Varbanov M., Codogno P., Biard-Piechaczyk M. Autophagy is involved in T cell death after binding of HIV-1 envelope proteins to CXCR4. J. Clin. Invest. 2006;116:2161–2172. doi: 10.1172/JCI26185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fengsrud M., Erichsen E.S., Berg T.O., Raiborg C., Seglen P.O. Ultrastructural characterization of the delimiting membranes of isolated autophagosomes and amphisomes by freeze-fracture electron microscopy. Eur. J. Cell Biol. 2000;79:871–882. doi: 10.1078/0171-9335-00125. [DOI] [PubMed] [Google Scholar]
- Fengsrud M., Raiborg C., Berg T.O., Stromhaug P.E., Ueno T., Erichsen E.S., Seglen P.O. Autophagosome-associated variant isoforms of cytosolic enzymes. Biochem. J. 2000;352:773–781. [PMC free article] [PubMed] [Google Scholar]
- Gueguen M., Long E.O. Presentation of a cytosolic antigen by major histocompatibility complex class II molecules requires a long-lived form of the antigen. Proc. Natl. Acad. Sci. USA. 1996;93:14692–14697. doi: 10.1073/pnas.93.25.14692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierrez M.G., Master S.S., Singh S.B., Taylor G.A., Colombo M.I., Deretic V. Autophagy is a defense mechanism Inhibiting BCG and mycobacterium tuberculosis survival in infected macrophages. Cell. 2004;119:753–766. doi: 10.1016/j.cell.2004.11.038. [DOI] [PubMed] [Google Scholar]
- Gutierrez M.G., Saka H.A., Chinen I., Zoppino F.C., Yoshimori T., Bocco J.L., Colombo M.I. Protective role of autophagy against Vibrio cholerae cytolysin, a pore-forming toxin from V. cholerae. Proc. Natl. Acad. Sci. USA. 2007;104:1829–1834. doi: 10.1073/pnas.0601437104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierrez M.G., Vazquez C.L., Munafo D.B., Zoppino F.C., Beron W., Rabinovitch M., Colombo M.I. Autophagy induction favours the generation and maturation of the Coxiella-replicative vacuoles. Cell. Microbiol. 2005;7:981–993. doi: 10.1111/j.1462-5822.2005.00527.x. [DOI] [PubMed] [Google Scholar]
- Hampe J., Franke A., Rosenstiel P., Till A., Teuber M., Huse K., Albrecht M., Mayr G., De La Vega F.M., Briggs J., et al. A genome-wide association scan of nonsynonymous SNPs identifies a susceptibility variant for Crohn disease in ATG16L1. Nat. Genet. 2007;39:207–211. doi: 10.1038/ng1954. [DOI] [PubMed] [Google Scholar]
- Hara T., Nakamura K., Matsui M., Yamamoto A., Nakahara Y., Suzuki-Migishima R., Yokoyama M., Mishima K., Saito I., Okano H., Mizushima N. Suppression of basal autophagy in neural cells causes neurodegenerative disease in mice. Nature. 2006;441:885–889. doi: 10.1038/nature04724. [DOI] [PubMed] [Google Scholar]
- Heessen S., Masucci M.G., Dantuma N.P. The UBA2 domain functions as an intrinsic stabilization signal that protects Rad23 from proteasomal degradation. Mol. Cell. 2005;18:225–235. doi: 10.1016/j.molcel.2005.03.015. [DOI] [PubMed] [Google Scholar]
- Heller K.N., Gurer C., Münz C. Virus-specific CD4+ T cells: Ready for direct attack. J. Exp. Med. 2006;203:805–808. doi: 10.1084/jem.20060215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henell F., Berkenstam A., Ahlberg J., Glaumann H. Degradation of short- and long-lived proteins in perfused liver and in isolated autophagic vacuoles–lysosomes. Exp. Mol. Pathol. 1987;46:1–14. doi: 10.1016/0014-4800(87)90026-8. [DOI] [PubMed] [Google Scholar]
- Hernandez L.D., Pypaert M., Flavell R.A., Galan J.E. A Salmonella protein causes macrophage cell death by inducing autophagy. J. Cell Biol. 2003;163:1123–1131. doi: 10.1083/jcb.200309161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyt M.A., Zich J., Takeuchi J., Zhang M., Govaerts C., Coffino P. Glycine-alanine repeats impair proper substrate unfolding by the proteasome. EMBO J. 2006;25:1720–1729. doi: 10.1038/sj.emboj.7601058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichimura Y., Kirisako T., Takao T., Satomi Y., Shimonishi Y., Ishihara N., Mizushima N., Tanida I., Kominami E., Ohsumi M., et al. A ubiquitin-like system mediates protein lipidation. Nature. 2000;408:488–492. doi: 10.1038/35044114. [DOI] [PubMed] [Google Scholar]
- Inbal B., Bialik S., Sabanay I., Shani G., Kimchi A. DAP kinase and DRP-1 mediate membrane blebbing and the formation of autophagic vesicles during programmed cell death. J. Cell Biol. 2002;157:455–468. doi: 10.1083/jcb.200109094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson W.T., Giddings T.H., Jr., Taylor M.P., Mulinyawe S., Rabinovitch M., Kopito R.R., Kirkegaard K. Subversion of cellular autophagosomal machinery by RNA viruses. PLoS Biol. 2005;3:e156. doi: 10.1371/journal.pbio.0030156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaraquemada D., Marti M., Long E.O. An endogenous processing pathway in vaccinia virus-infected cells for presentation of cytoplasmic antigens to class II-restricted T cells. J. Exp. Med. 1990;172:947–954. doi: 10.1084/jem.172.3.947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juhasz G., Neufeld T.P. Autophagy: A forty-year search for a missing membrane source. PLoS Biol. 2006;4:e36. doi: 10.1371/journal.pbio.0040036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabeya Y., Mizushima N., Ueno T., Yamamoto A., Kirisako T., Noda T., Kominami E., Ohsumi Y., Yoshimori T. LC3, a mammalian homologue of yeast Apg8p, is localized in autophagosome membranes after processing. EMBO J. 2000;19:5720–5728. doi: 10.1093/emboj/19.21.5720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kihara A., Kabeya Y., Ohsumi Y., Yoshimori T. Beclin-phosphatidylinositol 3-kinase complex functions at the trans-Golgi network. EMBO Rep. 2001;2:330–335. doi: 10.1093/embo-reports/kve061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komatsu M., Waguri S., Chiba T., Murata S., Iwata J., Tanida I., Ueno T., Koike M., Uchiyama Y., Kominami E., Tanaka K. Loss of autophagy in the central nervous system causes neurodegeneration in mice. Nature. 2006;441:880–884. doi: 10.1038/nature04723. [DOI] [PubMed] [Google Scholar]
- Kroemer G., Jaattela M. Lysosomes and autophagy in cell death control. Nat. Rev. Cancer. 2005;5:886–897. doi: 10.1038/nrc1738. [DOI] [PubMed] [Google Scholar]
- Lee H.K., Lund J.M., Ramanathan B., Mizushima N., Iwasaki A. Autophagy-dependent viral recognition by plasmacytoid dendritic cells. Science. 2007;315:1398–1401. doi: 10.1126/science.1136880. [DOI] [PubMed] [Google Scholar]
- Lee S.P., Brooks J.M., Al-Jarrah H., Thomas W.A., Haigh T.A., Taylor G.S., Humme S., Schepers A., Hammerschmidt W., Yates J.L., et al. CD8 T cell recognition of endogenously expressed Epstein-Barr virus nuclear antigen 1. J. Exp. Med. 2004;199:1409–1420. doi: 10.1084/jem.20040121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levitskaya J., Coram M., Levitsky V., Imreh S., Steigerwald-Mullen P.M., Klein G., Kurilla M.G., Masucci M.G. Inhibition of antigen processing by the internal repeat region of the Epstein-Barr virus nuclear antigen-1. Nature. 1995;375:685–688. doi: 10.1038/375685a0. [DOI] [PubMed] [Google Scholar]
- Levitskaya J., Sharipo A., Leonchiks A., Ciechanover A., Masucci M.G. Inhibition of ubiquitin/proteasome-dependent protein degradation by the Gly-Ala repeat domain of the Epstein-Barr virus nuclear antigen 1. Proc. Natl. Acad. Sci. USA. 1997;94:12616–12621. doi: 10.1073/pnas.94.23.12616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C., Capan E., Zhao Y., Zhao J., Stolz D., Watkins S.C., Jin S., Lu B. Autophagy is induced in CD4+ T cells and important for the growth factor-withdrawal cell death. J. Immunol. 2006;177:5163–5168. doi: 10.4049/jimmunol.177.8.5163. [DOI] [PubMed] [Google Scholar]
- Liang X.H., Kleeman L.K., Jiang H.H., Gordon G., Goldman J.E., Berry G., Herman B., Levine B. Protection against fatal Sindbis virus encephalitis by beclin, a novel Bcl-2-interacting protein. J. Virol. 1998;72:8586–8596. doi: 10.1128/jvi.72.11.8586-8596.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lich J.D., Elliott J.F., Blum J.S. Cytoplasmic processing is a prerequisite for presentation of an endogenous antigen by major histocompatibility complex class II proteins. J. Exp. Med. 2000;191:1513–1524. doi: 10.1084/jem.191.9.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling Y.M., Shaw M.H., Ayala C., Coppens I., Taylor G.A., Ferguson D.J., Yap G.S. Vacuolar and plasma membrane stripping and autophagic elimination of Toxoplasma gondii in primed effector macrophages. J. Exp. Med. 2006;203:2063–2071. doi: 10.1084/jem.20061318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liou W., Geuze H.J., Geelen M.J., Slot J.W. The autophagic and endocytic pathways converge at the nascent autophagic vacuoles. J. Cell Biol. 1997;136:61–70. doi: 10.1083/jcb.136.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Schiff M., Czymmek K., Talloczy Z., Levine B., Dinesh-Kumar S.P. Autophagy regulates programmed cell death during the plant innate immune response. Cell. 2005;121:567–577. doi: 10.1016/j.cell.2005.03.007. [DOI] [PubMed] [Google Scholar]
- McHeyzer-Williams L.J., McHeyzer-Williams M.G. Antigen-specific memory B cell development. Annu. Rev. Immunol. 2005;23:487–513. doi: 10.1146/annurev.immunol.23.021704.115732. [DOI] [PubMed] [Google Scholar]
- Meyers G., Stoll D., Gunn M. Insertion of a sequence encoding light chain 3 of microtubule-associated proteins 1A and 1B in a pestivirus genome: Connection with virus cytopathogenicity and induction of lethal disease in cattle. J. Virol. 1998;72:4139–4148. doi: 10.1128/jvi.72.5.4139-4148.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills K.R., Reginato M., Debnath J., Queenan B., Brugge J.S. Tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) is required for induction of autophagy during lumen formation in vitro. Proc. Natl. Acad. Sci. USA. 2004;101:3438–3443. doi: 10.1073/pnas.0400443101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizushima N. Methods for monitoring autophagy. Int. J. Biochem. Cell Biol. 2004;36:2491–2502. doi: 10.1016/j.biocel.2004.02.005. [DOI] [PubMed] [Google Scholar]
- Mizushima N., Klionsky D.J. Protein turnover via autophagy: Implications for metabolism. Annu. Rev. Nutr. 2007 doi: 10.1146/annurev.nutr.27.061406.093749. in press. Published online February 20, 2007. [DOI] [PubMed] [Google Scholar]
- Mizushima N., Noda T., Yoshimori T., Tanaka Y., Ishii T., George M.D., Klionsky D.J., Ohsumi M., Ohsumi Y. A protein conjugation system essential for autophagy. Nature. 1998;395:395–398. doi: 10.1038/26506. [DOI] [PubMed] [Google Scholar]
- Mizushima N., Yamamoto A., Matsui M., Yoshimori T., Ohsumi Y. In vivo analysis of autophagy in response to nutrient starvation using transgenic mice expressing a fluorescent autophagosome marker. Mol. Biol. Cell. 2004;15:1101–1111. doi: 10.1091/mbc.E03-09-0704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Münz C., Bickham K.L., Subklewe M., Tsang M.L., Chahroudi A., Kurilla M.G., Zhang D., O'Donnell M., Steinman R.M. Human CD4+ T lymphocytes consistently respond to the latent Epstein-Barr virus nuclear antigen EBNA1. J. Exp. Med. 2000;191:1649–1660. doi: 10.1084/jem.191.10.1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa I., Amano A., Mizushima N., Yamamoto A., Yamaguchi H., Kamimoto T., Nara A., Funao J., Nakata M., Tsuda K., et al. Autophagy defends cells against invading group A Streptococcus. Science. 2004;306:1037–1040. doi: 10.1126/science.1103966. [DOI] [PubMed] [Google Scholar]
- Nakashima A., Tanaka N., Tamai K., Kyuuma M., Ishikawa Y., Sato H., Yoshimori T., Saito S., Sugamura K. Survival of parvovirus B19-infected cells by cellular autophagy. Virology. 2006;349:254–263. doi: 10.1016/j.virol.2006.03.029. [DOI] [PubMed] [Google Scholar]
- Nimmerjahn F., Milosevic S., Behrends U., Jaffee E.M., Pardoll D.M., Bornkamm G.W., Mautner J. Major histocompatibility complex class II-restricted presentation of a cytosolic antigen by autophagy. Eur. J. Immunol. 2003;33:1250–1259. doi: 10.1002/eji.200323730. [DOI] [PubMed] [Google Scholar]
- Ogawa M., Yoshimori T., Suzuki T., Sagara H., Mizushima N., Sasakawa C. Escape of intracellular shigella from autophagy. Science. 2005;307:727–731. doi: 10.1126/science.1106036. [DOI] [PubMed] [Google Scholar]
- Ohsumi Y. Molecular dissection of autophagy: Two ubiquitin-like systems. Nat. Rev. Mol. Cell Biol. 2001;2:211–216. doi: 10.1038/35056522. [DOI] [PubMed] [Google Scholar]
- Orvedahl A., Alexander D., Talloczy Z., Sun Q., Wei Y., Zhang W., Burns D., Leib D., Levine B. HSV-1 ICP34.5 confers neurovirulence by targeting the Beclin 1 autophagy protein. Cell Host Microbe. 2007;1:23–35. doi: 10.1016/j.chom.2006.12.001. [DOI] [PubMed] [Google Scholar]
- Paludan C., Schmid D., Landthaler M., Vockerodt M., Kube D., Tuschl T., Münz C. Endogenous MHC class II processing of a viral nuclear antigen after autophagy. Science. 2005;307:593–596. doi: 10.1126/science.1104904. [DOI] [PubMed] [Google Scholar]
- Pattingre S., Tassa A., Qu X., Garuti R., Liang X.H., Mizushima N., Packer M., Schneider M.D., Levine B. Bcl-2 antiapoptotic proteins inhibit Beclin 1-dependent autophagy. Cell. 2005;122:927–939. doi: 10.1016/j.cell.2005.07.002. [DOI] [PubMed] [Google Scholar]
- Pedersen K.W., van der Meer Y., Roos N., Snijder E.J. Open reading frame 1a-encoded subunits of the arterivirus replicase induce endoplasmic reticulum-derived double-membrane vesicles which carry the viral replication complex. J. Virol. 1999;73:2016–2026. doi: 10.1128/jvi.73.3.2016-2026.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizarro-Cerda J., Moreno E., Sanguedolce V., Mege J.L., Gorvel J.P. Virulent Brucella abortus prevents lysosome fusion and is distributed within autophagosome-like compartments. Infect. Immun. 1998;66:2387–2392. doi: 10.1128/iai.66.5.2387-2392.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prentice E., Jerome W.G., Yoshimori T., Mizushima N., Denison M.R. Coronavirus replication complex formation utilizes components of cellular autophagy. J. Biol. Chem. 2004;279:10136–10141. doi: 10.1074/jbc.M306124200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prentice E., McAuliffe J., Lu X., Subbarao K., Denison M.R. Identification and characterization of severe acute respiratory syndrome coronavirus replicase proteins. J. Virol. 2004;78:9977–9986. doi: 10.1128/JVI.78.18.9977-9986.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pua H.H., Dzhagalov I., Chuck M., Mizushima N., He Y.W. A critical role for the autophagy gene Atg5 in T cell survival and proliferation. J. Exp. Med. 2007;204:25–31. doi: 10.1084/jem.20061303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyo J.O., Jang M.H., Kwon Y.K., Lee H.J., Jun J.I., Woo H.N., Cho D.H., Choi B., Lee H., Kim J.H., et al. Essential roles of Atg5 and FADD in autophagic cell death: Dissection of autophagic cell death into vacuole formation and cell death. J. Biol. Chem. 2005;280:20722–20729. doi: 10.1074/jbc.M413934200. [DOI] [PubMed] [Google Scholar]
- Rammensee H., Bachmann J., Emmerich N.P., Bachor O.A., Stevanovic S. SYFPEITHI: Database for MHC ligands and peptide motifs. Immunogenetics. 1999;50:213–219. doi: 10.1007/s002510050595. [DOI] [PubMed] [Google Scholar]
- Reith W., Mach B. The bare lymphocyte syndrome and the regulation of MHC expression. Annu. Rev. Immunol. 2001;19:331–373. doi: 10.1146/annurev.immunol.19.1.331. [DOI] [PubMed] [Google Scholar]
- Rich K.A., Burkett C., Webster P. Cytoplasmic bacteria can be targets for autophagy. Cell. Microbiol. 2003;5:455–468. doi: 10.1046/j.1462-5822.2003.00292.x. [DOI] [PubMed] [Google Scholar]
- Rioux J.D., Xavier R.J., Taylor K.D., Silverberg M.S., Goyette P., Huett A., Green T., Kuballa P., Barmada M.M., Datta L.W., et al. Genome-wide association study identifies new susceptibility loci for Crohn disease and implicates autophagy in disease pathogenesis. Nat. Genet. 2007;39:596–604. doi: 10.1038/ng2032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaible U.E., Schlesinger P.H., Steinberg T.H., Mangel W.F., Kobayashi T., Russell D.G. Parasitophorous vacuoles of Leishmania mexicana acquire macromolecules from the host cell cytosol via two independent routes. J. Cell Sci. 1999;112:681–693. doi: 10.1242/jcs.112.5.681. [DOI] [PubMed] [Google Scholar]
- Schmid D., Pypaert M., Münz C. MHC class II antigen loading compartments continuously receive input from autophagosomes. Immunity. 2007;26:79–92. doi: 10.1016/j.immuni.2006.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seglen P.O., Gordon P.B. 3-Methyladenine: Specific inhibitor of autophagic/lysosomal protein degradation in isolated rat hepatocytes. Proc. Natl. Acad. Sci. USA. 1982;79:1889–1892. doi: 10.1073/pnas.79.6.1889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonsen A., Birkeland H.C., Gillooly D.J., Mizushima N., Kuma A., Yoshimori T., Slagsvold T., Brech A., Stenmark H. Alfy, a novel FYVE-domain-containing protein associated with protein granules and autophagic membranes. J. Cell Sci. 2004;117:4239–4251. doi: 10.1242/jcs.01287. [DOI] [PubMed] [Google Scholar]
- Singh S.B., Davis A.S., Taylor G.A., Deretic V. Human IRGM induces autophagy to eliminate intracellular mycobacteria. Science. 2006;313:1438–1441. doi: 10.1126/science.1129577. [DOI] [PubMed] [Google Scholar]
- Starr T.K., Jameson S.C., Hogquist K.A. Positive and negative selection of T cells. Annu. Rev. Immunol. 2003;21:139–176. doi: 10.1146/annurev.immunol.21.120601.141107. [DOI] [PubMed] [Google Scholar]
- Steinman R.M., Hawiger D., Nussenzweig M.C. Tolerogenic dendritic cells. Annu. Rev. Immunol. 2003;21:685–711. doi: 10.1146/annurev.immunol.21.120601.141040. [DOI] [PubMed] [Google Scholar]
- Stromhaug P.E., Berg T.O., Fengsrud M., Seglen P.O. Purification and characterization of autophagosomes from rat hepatocytes. Biochem. J. 1998;335:217–224. doi: 10.1042/bj3350217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tallóczy Z., Jiang W., Virgin H.W., Leib D.A., Scheuner D., Kaufman R.J., Eskelinen E.L., Levine B. Regulation of starvation- and virus-induced autophagy by the eIF2alpha kinase signaling pathway. Proc. Natl. Acad. Sci. USA. 2002;99:190–195. doi: 10.1073/pnas.012485299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tellam J., Sherritt M., Thomson S., Tellam R., Moss D.J., Burrows S.R., Wiertz E., Khanna R. Targeting of EBNA1 for rapid intracellular degradation overrides the inhibitory effects of the Gly-Ala repeat domain and restores CD8+ T cell recognition. J. Biol. Chem. 2001;276:33353–33360. doi: 10.1074/jbc.M104535200. [DOI] [PubMed] [Google Scholar]
- Thorburn J., Moore F., Rao A., Barclay W.W., Thomas L.R., Grant K.W., Cramer S.D., Thorburn A. Selective inactivation of a Fas-associated death domain protein (FADD)-dependent apoptosis and autophagy pathway in immortal epithelial cells. Mol. Biol. Cell. 2005;16:1189–1199. doi: 10.1091/mbc.E04-10-0906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tortorella D., Gewurz B.E., Furman M.H., Schust D.J., Ploegh H.L. Viral subversion of the immune system. Annu. Rev. Immunol. 2000;18:861–926. doi: 10.1146/annurev.immunol.18.1.861. [DOI] [PubMed] [Google Scholar]
- Trombetta E.S., Mellman I. Cell biology of antigen processing in vitro and in vivo. Annu. Rev. Immunol. 2005;23:975–1028. doi: 10.1146/annurev.immunol.22.012703.104538. [DOI] [PubMed] [Google Scholar]
- Walker D.H., Popov V.L., Crocquet-Valdes P.A., Welsh C.J., Feng H.M. Cytokine-induced, nitric oxide-dependent, intracellular antirickettsial activity of mouse endothelial cells. Lab. Invest. 1997;76:129–138. [PubMed] [Google Scholar]
- Xu Y., Jagannath C., Liu X.D., Sharafkhaneh A., Kolodziejska K.E., Eissa N.T. Toll-like receptor 4 is a sensor for autophagy associated with innate immunity. Immunity. 2007;27:135–144. doi: 10.1016/j.immuni.2007.05.022. this issue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng X., Overmeyer J.H., Maltese W.A. Functional specificity of the mammalian Beclin-Vps34 PI 3-kinase complex in macroautophagy versus endocytosis and lysosomal enzyme trafficking. J. Cell Sci. 2006;119:259–270. doi: 10.1242/jcs.02735. [DOI] [PubMed] [Google Scholar]
- Zhou D., Li P., Lott J.M., Hislop A., Canaday D.H., Brutkiewicz R.R., Blum J.S. Lamp-2a facilitates MHC class II presentation of cytoplasmic antigens. Immunity. 2005;22:571–581. doi: 10.1016/j.immuni.2005.03.009. [DOI] [PubMed] [Google Scholar]
- Zwart W., Griekspoor A., Kuijl C., Marsman M., van Rheenen J., Janssen H., Calafat J., van Ham M., Janssen L., van Lith M., et al. Spatial separation of HLA-DM/HLA-DR interactions within MIIC and phagosome-induced immune escape. Immunity. 2005;22:221–233. doi: 10.1016/j.immuni.2005.01.006. [DOI] [PubMed] [Google Scholar]