With the continually growing popularity of ferrets, practitioners will encounter individuals that suffer from viral disease more frequently. Ferrets have been used extensively as animal models to study a variety of viral diseases, many of which only occur following experimental infection. This article provides a review of viral diseases that occur naturally in ferrets ( Table 1). The etiology, transmission, and pathogenesis of each virus is discussed. There is special emphasis on clinical signs, diagnostic tests, and methods to control and prevent these conditions.
Table 1.
List of naturally occurring viruses of domestic ferrets
| Viruses | Family | Nucleic acid | Envelope | Size (nm) |
|---|---|---|---|---|
| Distemper virus | Paramyxoviridae | ssRNA | yes | 150–300 |
| Coronavirus | Coronaviridae | ssRNA | yes | 60–220 |
| Influenza virus | Orthomyxoviridae | ssRNA | yes | 90–120 |
| Rabies virus | Rhabdoviridae | ssRNA | yes | 70–85 × 130–380 |
| Rotavirus | Reoviridae | dsRNA | no | 80–130 |
| Parvovirus | Parvoviridae | ssDNA | no | 18–26 |
| Infectious bovine rhinotracheitis virus | Herpesviridae | dsDNA | yes | 46–48 |
Abbreviations: ds, double stranded; ss, single stranded.
Distemper
Distemper is caused by an RNA virus of the genus Morbillivirus in the Paramyxoviridae family. There is one serotype of canine distemper virus (CDV) with several strains that produce different clinical presentations [1]. Distemper probably is the most serious infectious disease of ferrets; mortality rates approach 100%. Infection of ferrets with CDV is uncommon because of the availability of effective vaccines and client education on the importance of vaccination. Unvaccinated dogs and wild Canidae, Mustelidae and Procyonidae may serve as reservoirs of the disease [1]. A CDV epizootic was reported in a black-footed ferret (Mustela nigripes) colony in the fall of 1985 in Wyoming. The source of infection was not identified, but several badgers and coyotes in the area had CDV-neutralizing antibodies [2].
Pathogenesis
CDV is transmitted by aerosol exposure to infected body fluids and by direct contact with infected animals and contaminated fomites. The virus is shed in ocular and nasal secretions, saliva, urine, and feces [1]. Viral shedding begins approximately 7 days postinfection [3]. The primary site of viral replication is the respiratory epithelium and lymphoid tissue of the nasopharynx [1], [4]. The virus disseminates by way of peripheral blood leukocytes to the liver, kidneys, gastrointestinal tract, urinary bladder, and brain. Viremia has been documented 2 days postinoculation or infection and persists until the virus is neutralized by antibodies or the animal dies [5]. Gastric hypochlorhydria has been associated with CDV infection in ferrets [6]. The lack of gastric acid was hypothesized to be due to direct action of the virus on gastric mucosa or was secondary to the viral effect on the central nervous system.
Clinical signs
Infected ferrets become symptomatic after an incubation period of 7 to 10 days [1], [7], [8]. In ferrets, natural CDV infection most often consists of a catarrhal phase followed by a fatal neurotropic phase [9]. The initial phase is characterized by anorexia, pyrexia, conjunctivitis, and serous nasal discharge. An erythematous, pruritic rash appears on the chin ( Fig. 1) and eventually spreads to the inguinal area [1], [9]. Hyperkeratosis of the footpads ( Fig. 2) occurs inconsistently in ferrets [1]. Melena may be observed early in the course of the disease [1], [9]. Some ferrets die during the catarrhal phase secondary to bacterial infections, such as pneumonia. Clinical signs that are seen in the neurotropic phase include hyperexcitability, muscle tremors, hypersalivation, seizures, and coma. Ferrets die 12 to 16 days after being infected with ferret-adapted CDV [1], [8]. The disease has a longer course when ferrets are infected with a wild canine strain; death occurs 21 to 35 days postinfection [1]. Sometimes a decrease in temperature is observed by the time the animals become moribund [4].
Fig. 1.
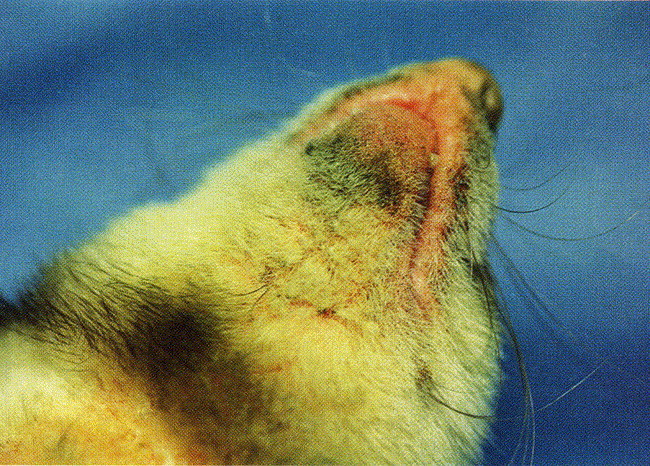
An erythematous, cutaneous rash on the chin of a ferret with canine distemper virus infection. (From Blair EM, Chambers MA, King HA. Treating distemper in a young ferret. Vet Med 1998;98(7):656; with permission.)
Fig. 2.
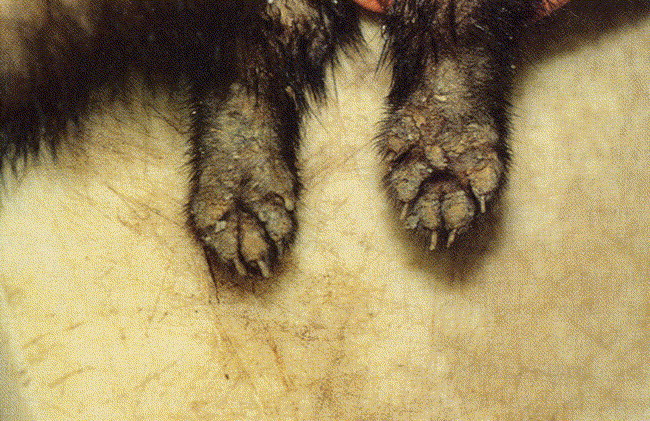
Hyperkeratosis on the footpads of a ferret with canine distemper virus infection. (From Blair EM, Chambers MA, King HA. Treating distemper in a young ferret. Vet Med 1998;98(7):657; with permission.)
Diagnosis
A tentative diagnosis of canine distemper is based on the presence of typical clinical signs, severe leukopenia, a history of potential exposure to the virus, and questionable vaccination. A study demonstrated that virulent and attenuated CDV strains caused a severe leukopenia 1 week after infection [4]. Leukocyte counts from animals that were infected with virulent strains remained low, whereas those of animals that received attenuated strains progressively increased to reach preinfection, or near preinfection, values 35 days postinfection.
The diagnosis of canine distemper is confirmed routinely by an immunofluorescence test on peripheral blood smear, buffy coat, or conjunctival scrapings in live animals. In experimentally-infected ferrets, a reverse transcriptase–polymerase chain reaction (RT-PCR) has been used to detect the virus in peripheral blood [10]. Recently, nested-PCR was found to be a sensitive and specific method for diagnosis of CDV infection [11].
On postmortem examination, the gross lesions correspond to the aforementioned clinical signs. On hematoxylin and eosin–stained sections, round eosinophilic intracytoplasmic and occasional intranuclear inclusion bodies are present in epithelial cells of the trachea, bronchi, and urinary tract [1]. Other tissues, such as skin, gastrointestinal tract, salivary and adrenal glands, spleen, lymph nodes and brain, have been reported to contain inclusions [1].
Treatment and prevention
Ferrets that have signs that are suggestive of canine distemper infection should be placed in isolation. Supportive care, including fluid therapy, systemic and ophthalmic antibiotics, gavage feeding, and bathing with antipruritic shampoo, may be initiated. Administration of immune anti-CDV serum also may be considered. Thorough daily disinfection of the environment is indicated. In all cases, prognosis is grave. The virus is inactivated by heat, visible light, and commonly used disinfectants (0.75% phenol, 0.2% roccal, 2%–5% sodium hydroxide, and 0.1% formalin) [1], [3]. Some investigators reported that out of more than 1000 cases of distemper, not a single ferret survived the infection [8]. Blair et al [12] treated distemper in a 7-month-old ferret while in the catarrhal phase. The ferret's life span and quality of life were improved with treatments, but the neurotropic phase could not be prevented and the animal was euthanized. When faced with an outbreak of canine distemper in a ferret colony, euthanasia and repopulation following disinfection is recommended.
Distemper is prevented best by vaccination. Two vaccines are approved for use in ferrets in North America—Fervac-D Canine Distemper Vaccine (United Vaccines Inc., Madison, Wisconsin) and Purevax Ferret Distemper Vaccine (Merial, Athens, Georgia and Montréal, Quebec, Canada). Fervac-D is a modified-live virus vaccine of chick cell origin. A 5.9% incidence of anaphylactic reaction has been reported with this vaccine [13]. Adverse events developed within 25 minutes and were characterized by hyperemia, hypersalivation, and vomiting. Purevax is a lyophilized vaccine of a recombinant canary pox vector that expresses the HA and F glycoproteins of CDV. The manufacturer uses the term “ferret distemper” vaccine on the product label to prevent confusion with their canine products; however, there is no such thing as a “ferret distemper virus” and ferrets are infected by CDV. The absence of adjuvant or entire distemper virus decreased postvaccination risks and the manufacturer reports an incidence of 0.3% reversible anaphylactic reactions in its field safety trials. Another modified-live canine distemper vaccine attenuated in primate cell line (Galaxy D, Schering Plough Animal Health, Omaha, Nebraska) has been studied in ferrets. This vaccine was effective in preventing canine distemper in young ferrets that were challenged with virulent CDV after two vaccine inoculations [14]; however, the duration of immunity and the incidence of vaccine reactions are unknown. Clinical use of this vaccine is extra label (not approved for use in ferrets by USDA) and requires informed owner consent. Postvaccinal distemper infections were reported in black-footed ferrets that were vaccinated with chicken embryo–tissue culture–origin CDV vaccine and in domestic ferrets that were vaccinated with canine cell products [15], [16]. Therefore, vaccination of ferrets with these products must not be performed.
Most ferrets that are obtained from pet stores at a young age received only one dose of CDV vaccine before leaving the breeding facility. Repeated inoculations are required because maternal antibodies may interfere with proper immune response to CDV vaccine antigens. Ferret kits should be vaccinated at 8 weeks of age and every 3 weeks for a total of 3 vaccinations [17], [18]. Yearly booster vaccines are recommended [17]. Observation of the ferret for 25 minutes following vaccination is advisable. In the event that a vaccine reaction occurs, administration of fluids, oxygen, antihistamine, and epinephrine may be indicated.
Parvovirus
Parvovirus strains of varying virulence and immunogenicity cause Aleutian disease. The disease was first reported in mink in the 1940s and got its name because mink that were homozygous for the Aleutian (blue) gene were affected most severely [1]. The infection was documented in ferrets in the late 1960s [19]. At least three separate strains of Aleutian disease virus (ADV)—distinct from the mink strains—have been identified in ferrets [20], [21]. The ferret strains are believed to be mutant strains of the mink parvovirus; the hypervariable capsid region of the ferret strains of ADV is similar to that of the mink parvovirus [22]. Ferrets can be infected with the mink virus [20], [21] and ferret ADV can infect mink; however, the virulence is lower compared with mink that are infected with mink strains [21], [23], [24].
Pathogenesis
Transmission of the virus may occur by aerosolization; by direct contact with urine, feces, saliva, and blood; or by contact with fomites [1], [24]. Vertical transmission of ADV occurs in mink [1], [25]. The lesions that are caused by ADV infection are immune mediated, but the mechanism by which ADV interferes with the immune system is unknown. The severity of disease depends on the origin (mink or ferret) of the ADV strain that is involved as well as the immune status and genotype of the infected individual [25].
Minks that are infected with mink ADV strains deposit immune complexes in various organs that result in glomerulonephritis, bile duct proliferation, and arteritis [26]. Mink kits from antibody-free jills die from acute interstitial pneumonia when infected with virulent ADV [27]. Affected individuals are immunosuppressed, and therefore, are more susceptible to influenza, viral enteritis, and distemper [1], [26]. Ferrets that are inoculated with ferret-adapted ADV exhibit marked, persistent hyperglobulinemia and periportal lymphocytic infiltrates of the liver [21]; however, immunocompetent adult ferrets that are infected experimentally can develop a persistent infection without clinical disease [21]. Mink strains cause milder lesions and only a moderate elevation in gamma globulins in ferrets [1].
Clinical signs
Most ferrets that show clinical signs are between 2 and 4 years old. Ferrets can be infected for years before clinical symptoms are noted [26]. Any situation that leads to immunosuppression may play a role in the development of clinical disease. The clinical presentation of infected ferrets varies. Some ferrets that are infected with ADV die without clinical signs in good body condition [24]. Generally, ferrets show signs of a chronic wasting disease with progressive weight loss, malaise, and melena [1]. Acute dyspnea was described in one report [28]. Central nervous system signs, such as tremors, ataxia, paralysis, and convulsions, also have been reported [25], [29], [30], [31], [32]. Affected animal also may present with fecal and urinary incontinence [25], [29].
Diagnosis
A presumptive diagnosis can be made based on a high gamma globulin concentration, the history, and clinical signs. Although hypergammaglobulinemia is considered to be pathognomonic in mink, this feature is not always present in infected ferrets [28], [29]. Serum protein electrophoresis often shows that gamma globulins account for more than 20% of the total protein concentration [1], [21].
Other clinical pathologic findings of infected ferrets are variable. Anemia, possibly attributable to hemolysis and decreased erythrocyte production, may be present [25]. Biochemical abnormalities, such as azotemia and elevated liver enzymes, may be seen according to damage that results from immune complex deposition. Proteinuria and urinary casts that are secondary to kidney damage also may be observed [28].
Diagnosis of Aleutian disease is confirmed antemortem with a positive serum titer coupled with hypergammaglobulinemia or lymphoplasmacytic inflammation in tissue biopsy samples. Two serologic tests are available for ADV testing—counterimmunoelectrophoresis (CEP or CIEP) (United Vaccines, Madison, Wisconsin) and ELISA (Avecon Diagnostics, Bath, Pennsylvania). The specificity and sensitivity of the ELISA test has not been investigated in ferrets. Therefore, results must be interpreted with caution. The CEP test is used routinely in ferrets and is an effective method for identifying ferrets that have ADV antibodies [25], [30], [31], [32], [33]. Presence of antibodies without clinical disease for extended periods is possible [34]. Ferrets probably develop persistent and nonpersistent, nonprogressive forms of ADV infection similar to mink [25], [35].
Detection of ADV DNA by in situ hybridization has been performed, but this method is not practical to screen for this condition [36]. Recently, polymerase chain reaction amplification of part of the capsid gene that is specific to ADV and restriction fragment length polymorphism to distinguish the ferret types of ADV from the mink types of ADV are valuable, time-saving assets for diagnosis of this infection in ferrets [20].
At necropsy, infected ferrets may have few or no gross lesions. Hepatosplenomegaly, splenomegaly, and mesenteric lymphadenopathy have been reported [23], [24]. The most consistent histologic findings of ADV infection in ferrets are periportal infiltration of the liver by plasma cells, lymphocytes, and macrophages with stimulated lymphoid tissues [23], [24]. Bile duct hyperplasia, periportal fibrosis, and membranous glomerulonephrosis have been documented [23], [24], [28]. In individuals that present with neurologic signs, perivascular lymphocytic cuffing in the brain and spinal cord ( Fig. 3) and lymphoplasmacytic meningitis may be observed [25], [30], [31].
Fig. 3.
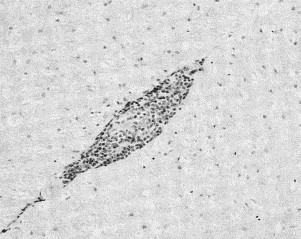
Severe perivascular cuffing with mononuclear cells in the spinal cord of a ferret with Aleutian disease. (From Welchman D de B, Oxenham M, Done SH. Aleutian disease in domestic ferrets: diagnostic findings and survey results. Vet Rec 1993;132:481; with permission.)
Treatment and control
There is no definitive treatment of Aleutian disease in ferrets. Symptomatic ferrets may benefit from use of anti-inflammatory medication or immunosuppressive drugs, such as prednisone and cyclophosphamide. In mink, treatment with cyclophosphamide has been used to control infections for up to 16 weeks, but virus titers did not decrease [37]. Administration of gamma globulin–containing ADV antibody may be considered because it contributed to decreased mortality rates in mink kits [38].
Control of Aleutian disease is dependent on testing, cessation of breeding, and isolation of seropositive ferrets. All seropositive ferrets should be considered to be potential sources of ADV and should be isolated from seronegative ferrets [1]. In mink farms, testing by CIEP and removing all individuals that have ADV antibodies has been an efficient method to eradicate the disease [39]. This approach, coupled with thorough disinfection, should be considered in any facility with a large number of ferrets. Formalin, sodium hydroxide, and a phenolic disinfectant were efficacious against ADV in the presence of organic material [40].
There is no vaccine to prevent Aleutian disease and vaccination probably is contraindicated because of the immune-mediated nature of this condition. In mink, vaccination exacerbated the severity of Aleutian disease [41].
Coronavirus
In the late 1980s, a novel diarrheal disease that affected domestic ferrets was reported by pet owners and ferret breeders in the mid-Atlantic area of the United States. Since that time, this condition has been diagnosed throughout North America and in several other countries. The disease was named epizootic catarrhal enteritis (ECE) on the basis of similarities to the epizootic catarrhal gastroenteritis of mink [42]. ECE of mink is caused by a coronavirus that is related to transmissible gastroenteritis virus of pigs [43]. Research strongly implicates a coronavirus as the causative agent of ECE because: (1) microscopic lesions that were consistent with intestinal coronavirus infection were detected consistently in diseased ferrets; (2) coronavirus particles were identified in the feces and enterocytes, but no other viruses could be identified; and (3) immunohistochemical staining of jejunum showed coronavirus antigens in affected ferrets, but not in healthy individuals [44].
Clinical presentation
The disease is characterized by high transmissibility, high morbidity, and a low mortality rate. Ferrets show signs of lethargy and anorexia within 48 to 72 hours postinfection. Vomiting is the first sign of gastrointestinal disease in most ferrets, but it may go unnoticed by some owners because it subsides within hours [44]. Subsequently, profuse green, bile-tinged diarrhea with a variable amount of mucus develops ( Fig. 4). The stool's appearance is responsible for the term “green slime disease” that also is used to describe this condition [45].
Fig. 4.
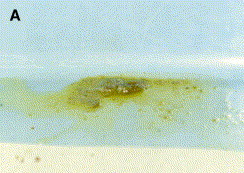

(A, B) A. Profuse green bile-tinged diarrhea from the young ferret with epizootic catarrhal enteritis shown in Fig. B. (Courtesy of C. Greenacre, DVM, Knoxville, Tennessee.)
The severity of clinical signs is highly variable; older ferrets that have concurrent diseases, such as insulinoma, long-standing infection with Helicobacter mustelae, or adrenal neoplasia, are prone to develop severe clinical signs. Ulcerations of the intestinal wall may occur which leads to the presence of blood in the feces. Young ferrets tend to have mild or subclinical infection. The hypersecretory phase of uncomplicated ECE often resolves within 5 to 7 days in healthy young animals. In some ferrets, this phase may be followed by a period of maldigestion or malabsorption of variable duration secondary to persistent lymphocytic inflammation of the intestinal wall. The feces become yellowish in color and contain grainy material that resembles bird seed.
Diagnosis
ECE often can be diagnosed solely on the basis of characteristic historical findings and clinical signs [44]. Thorough collection of the history data often reveals exposure to an asymptomatic ferret 48 to 72 hours before the onset of clinical signs. Generally, clinicopathologic findings are nonspecific. Inanition may cause increased serum activity of alanine aminotransferase and alkaline phosphatase secondary to mobilization of peripheral fat stores to the liver, with resultant hepatocellular swelling [1], [44], [45]. Hypoalbuminemia may develop as a result of enteritis and malabsorption in chronically affected individuals. Leukocytosis may be present in ferrets that have concurrent bacterial infection or gastric ulcers. Definitive diagnosis of coronavirus infection often is difficult. Coronavirus-like particles may be identified in the feces by electron microscopy during the acute phase of the disease process. Characteristic histologic lesions that are seen in intestinal coronavirus infection, such as lymphocytic enteritis with villus atrophy, fusion, blunting and vacuolar degeneration or necrosis of the apical epithelium, may be identified on intestinal biopsy or necropsy specimens [44].
Treatment
Ferrets may become dehydrated rapidly during the hypersecretory phase of infection. Dehydration and electrolyte imbalances need to be addressed. Fluids that are supplemented with dextrose and electrolytes may be administered orally, subcutaneously, or intravenously, according to the degree of dehydration. Antibiotic may be indicated to prevent secondary bacterial infection, particularly in cases with suspected intestinal ulcerations. In these cases, administration of sucralfate and an H2 antagonist (eg, cimetidine) also are beneficial. Sucralfate requires an acidic environment to be effective, so it should be given at least 30 minutes before an H2 antagonist. Syringe feeding with a highly digestible diet (Science Diet A/D, Hill's Prescription Diet) may be indicated if anorexia persists. If clinical signs that are suggestive of maldigestion develop, oral administration of prednisone may be indicated. Use of an anti-inflammatory dose of prednisone for 1 to 4 weeks, was followed by gradual tapering of the dose successfully.
Influenza virus
Influenza viruses belong to the class Orthomyxoviridae. Human influenza types A and B are pathogenic to ferrets [1]. Ferrets also are susceptible to avian, seal, equine, and swine influenza A viruses, although only human, avian, and swine strains induce clinical signs [46], [47], [48], [49], [50]. Infection with influenza B virus less frequently results in illness and is associated with a milder clinical course [1]. Transmission of influenza virus from human to ferrets and from ferrets to humans was documented in the 1930s [51], [52]. Ferrets are used extensively as an animal model for influenza virus pathogenesis and immunity studies because their biologic response to influenza infection is similar to that of humans [53], [54].
Pathogenesis and clinical signs
Influenza virus is transmitted by aerosol droplets from an infected individual, either to a human or a ferret. After intranasal inoculation, the virus localizes and replicates in great numbers within the nasal mucosa [55]. Following a short incubation period, the body temperature increases and then decreases approximately 48 hours later [56]. Transmission of the virus begins at the height of pyrexia and continues for 3 to 4 days [53]. As in humans, the disease is characterized by upper respiratory signs. Clinical signs appear 48 hours postinfection and include anorexia, malaise, fever, sneezing, and serous nasal discharge [1], [56]. Infection usually is mild in adult ferrets compared with neonates who can be severely ill [57]. Conjunctivitis, photosensitivity, and unilateral otitis also may be seen [1], [58].
Influenza infection may involve the lower respiratory tract in some susceptible animals [1], [59]. Usually, influenza virus is confined to the bronchial and bronchiolar tissues [53], [60]. The disease may be fatal in 1- to 2-day-old ferret kits secondary to bronchiolitis, pneumonia, and aspiration of material from the upper respiratory tract [57], [61], [62]. Lancefield group C hemolytic streptococci have been involved in secondary bacterial pneumonia [56].
Influenza virus may infect the intestinal epithelium and cause limited enteritis [50], [63]. Hepatic dysfunction also has been reported in ferrets that were infected experimentally with influenza [64]. Neurologic symptoms, including ataxia, hind-limb paresis, and torticollis, were reported in ferrets that were infected experimentally with avian influenza A (H5N1) viruses that were isolated from the 1997 outbreaks of disease in domestic poultry markets in Hong Kong [50], [65].
Diagnosis
Generally, the diagnosis of influenza infection is based on the presence of compatible clinical signs, a history of exposure to infected individuals, and recovery from illness within 7 to 10 days. The differential diagnosis of any ferrets that has upper respiratory signs should include canine distemper. The usually mild and brief nature of influenza infections help to distinguish it from distemper. The use of virus isolation or hemagglutinin-inhibiting antibody titers on acute and convalescent serum samples rarely is needed for a diagnosis [1]. An enzyme-linked immunosorbent assay has been used to detect antibodies against influenza A and may be used to obtain a diagnosis rapidly [66].
Clinical pathologic findings may present abnormalities. Studies demonstrated an elevation in the neutrophil:lymphocyte ratio in the peripheral blood [50], [67]. Transient lymphopenia, with a loss of 60% to 65% of peripheral blood lymphocytes 3 days postinfection was reported experimentally with avian influenza A (H5N1) [50]. Plasma biochemical values generally are within reference ranges, but increases in concentrations of creatinine, blood urea nitrogen, potassium, albumin, and alanine aminotransferase were reported in some infected ferrets [64].
Treatment
In most cases, infected ferrets can be treated at home. Owners should be instructed to let their ferret rest until fully recovered. Offering affected animals their favorite diet, highly palatable food (chicken baby food, beef baby food), or a highly energetic diet (Science Diet A/D, Hill's Prescription Diet) is indicated. Force feeding and offering water by syringe can be performed as needed. Treatments to relieve clinical symptoms should be done on a case by case basis. If coughing is persistent, a pediatric cough suppressant without alcohol (at the pediatric dosage on a per weight basis) has been used [59]. To alleviate nasal congestion, the use of an antihistamine, such as diphenhydramine (2–4 mg/kg, by mouth, every 8 to 12 hours) [8], [59], or intranasal delivery of phenylephrine may help [68]. Antibiotics may be indicated to control secondary bacterial infections of the respiratory tract. Neonates typically succumb to secondary bacterial infections. Therefore, antibiotics may be useful in these patients to reduce mortality [69].
The use of non steroidal anti-inflammatory drugs to control fever is of questionable benefit because fever seems to be important in restricting the severity of infection [70]. Experimentally, ferrets who received aspirin had cooler body temperature, but they shed more virus and their viral levels decreased less rapidly compared with ferrets that were not treated with an antipyretic.
Administration of antiviral medication has been studied in ferrets. Amantadine hydrochloride (6 mg/kg, by mouth, every 12 hours) (Symmetrel, Bristol-Myers Squibb Canada, Montreal, Quebec, Canada) has been effective in treating ferrets that have influenza [71]. Zanamivir (12.5 mg/kg) (Relenza, Glaxo Wellcome, Mississauga, Ontario, Canada), given as a one-time intranasal dose was able to prevent influenza infection [72]. Administration of amantadine in ferrets rapidly produces antiviral resistance, but use of zanamivir does not [73].
Prevention and control
Vaccination of ferrets against influenza virus generally is not recommended because it is usually a mild disease and the antigenic variation of the virus complicates vaccination [1], [59]. Experimentally, ferrets who recovered from influenza infection remained resistant to infection with the same strain for 5 weeks following initial infection [74]. Ferret kits are protected from disease by milk-derived antibodies in immunized females [75].
Controlling influenza infection resides in avoiding exposure of susceptible ferrets to infected individuals, either ferret or human. Owners should be advised to minimize contact with their ferrets if they have a respiratory infection and should be sure to wash their hands thoroughly before changing the animal's cage, food, and water. Veterinarians who have respiratory infections may consider wearing a mask and gloves.
Rhabdovirus
Rhabdovirus causes rabies, an acute and almost invariably fatal disease that affects many mammals and humans. Over the past several years, there have been numerous reports of ferret bite injuries, including unprovoked attacks on infants and small children [76], [77], [78]. These reports brought great controversy over the acceptability of keeping ferrets as pets with regard to the potential risk for rabies. This was because the period of viral shedding in the animal's saliva—before the onset of recognizable signs—was unknown at that time [1], [79], [80]. To the author's knowledge, there is no reported case of human rabies secondary to a ferret bite. Since 1958, less than 30 cases of rabies in domestic ferrets have been reported to the U.S. Centers for Disease Control [81], [82]. One of theses cases was attributed to vaccinating a ferret with modified-live virus rabies vaccine [1].
Pathogenesis
Rabies virus must contact nerve endings and enter nerve fibers before infection occurs. Exposure to rabies virus does not always lead to productive infection [81], [82], [83]. Host response to rabies virus is influenced by the rabies virus variant, the viral dose, the route of transmission, the host species, and individual variations [84]. Infection occurs primarily by contact of nerve endings with infected saliva from a rabid animal as a result of a bite wound. Contact with the conjunctiva or olfactory mucosa also can result in transmission [84]. Transmission of rabies through ingestion of an infected mouse was unsuccessful [85].
The pathogenesis of rabies in ferrets has been studied using European red fox rabies variant, North Central skunk rabies variant, and raccoon rabies variant [81], [82], [83]. The mean incubation period is approximately 1 month [81], [83]. Clinical signs reported include ascending paralysis, ataxia, tremors, paresthesia, hyperactivity, anorexia, cachexia, bladder atony, constipation, fever, and hypothermia [81], [82], [83]. Two of 19 rabid ferrets that were inoculated with raccoon rabies variant showed aggressive behavior [83]. Signs were reported to be mild in ferrets that were inoculated with the European red fox rabies variant [82]. Mean morbidity period was 4 to 5 days [81], [83]. Mortality in ferrets that were inoculated with the European red fox variant was dose dependent [82]. In the study that used skunk variant, ferret susceptibility was dose dependent and the incubation period was inversely proportional to dose [81]. In contrast, ferrets that were inoculated with raccoon variant were only moderately susceptible—regardless of dose—and there was no correlation found between viral dose and incubation period [83]. Ferrets who survived experimental infection remained clinically normal except for one ferret that was given skunk rabies variant and had severe paralytic sequelae [81], [82], [83].
The immunologic response of ferrets to rabies virus infection seems to vary depending on the rabies virus variant that was inoculated. Virus neutralizing antibodies were demonstrated in 14 of 33 (42.4%) rabid ferrets that were inoculated with skunk rabies variant compared with only 2 of 19 (10.5%) rabid ferrets that were given raccoon rabies variant [81], [83]. Also, among ferrets that survived the infection, the proportion of seropositive cases was greater in ferrets that were given skunk rabies variant [81], [83].
Ferrets may or may not excrete rabies virus in their saliva depending on the virus variant to which they have been exposed. Viral shedding was not documented in the saliva 120 days postinoculation with European red fox variant [82]. Rabies virus was not detected in the saliva of any ferrets that were given skunk rabies variant, but it was isolated from the submaxillary salivary gland of one rabid ferret that was euthanized [81]. Shedding of rabies virus in the saliva was documented in ferrets that were inoculated with the raccoon rabies variant [83]. Rabies virus was isolated from the salivary glands of 63% of rabid ferrets and 47% shed rabies virus in their saliva. Initial viral excretion ranged from 2 days before the onset of clinical signs to 6 days after the onset. Viral shedding also was documented in 1 of 23 ferrets that were inoculated with a rodent strain of rabies virus [86].
Diagnosis
Rabies should be included in the differential diagnoses of any ferret that has an unexpected onset of paralysis or acute personality change, especially if the ferret is unvaccinated, has access to outdoors, or lives in an area that is experiencing an epizootic of rabies. A ferret that is suspected of having rabies should be euthanized and its head should be submitted to the appropriate laboratory. Generally, the diagnosis is based on direct immunofluorescent antibody testing of brain tissue [1], [81], [83]. Confirmation may be established by intracerebral inoculation of suckling mice or inoculation of tissue culture with homogenized brain tissue [1].
Prevention and control
The protective efficacy of killed rabies vaccine was demonstrated in ferrets that were vaccinated once subcutaneously with an inactivated rabies virus and were challenged 1 year later with street virus of fox origin. Vaccinated ferrets had a survival rate of 89% compared with less than 6% for unvaccinated controls [87]. Domestic ferrets that were vaccinated with a commercially inactivated rabies vaccine showed rapid induction of virus-neutralizing antibody—a seroconversion that persisted at least 7 months [88]. Based on these studies, killed rabies vaccines were approved for immunizing ferrets [89], [90] ( Table 2). Ferrets should be vaccinated once at 3 months of age or older and then annually thereafter [87]. An animal is considered to be immunized if the primary vaccination was administered at least 28 days previously [89]. Because a rapid anamnestic response is expected, an animal is considered to be vaccinated currently immediately after booster vaccination [89].
Table 2.
Killed rabies vaccines licensed for use in ferrets in the United States and Canada
| Trade name | Country where licensed | Manufacturer | Dosage | Route | Recommended schedule |
|---|---|---|---|---|---|
| Imrab 3 TF | Canada United States | Merial | 1 mL | Subcutaneous | 3 months, then annually |
| Imrab 3 | Canada United States | Merial | 1 mL | Subcutaneous | 3 months, then annually |
| Prorab | Canada | Intervet Canada | 1 mL | Subcutaneous | 3 months, then annually |
Data from National Association of State Public Health Veterinarians. Compendium of animal rabies prevention and control, 2003. Morb Mortal Wkly Rep 2003;52(RR-5):14; Rabies Vaccines licensed in Canada. Canadian Food Inspection Agency, Animal Health and Production Division, Veterinary Biologics Section. Available at: http://www.inspection.gc.ca/english/anima/vetbio/prod/rabrage.shtml. Accessed October 2004.
Local injection site reactions were reported to develop frequently with rabies vaccine [91]. Studies in cats showed that rabies vaccines more consistently produce granulomatous inflammation at vaccine sites, although leukemia virus vaccines are incriminated more often in the pathogenesis of vaccine-associated sarcomas [92], [93], [94]. There is only one report of vaccine-associated sarcoma in a ferret [91]. The ferret had been vaccinated for distemper and rabies on an annual basis in the dorsal area of the neck or interscapular area, so it is impossible to determine from which vaccine the tumor arose. Practitioners should consider establishing a protocol in regard to the site of administration of rabies and distemper vaccines. This would allow the determination of which vaccine may be involved in the event that a tumor develops.
A study demonstrated that the cellular response to the canary pox–vectored rabies vaccine in ferrets was much milder than to the adjuvanted rabies vaccines [95]. Consequently, its future use may be associated with a decreasing number of local vaccine reactions. Also, because persistence of lymphocytes and macrophages has been suggested to play a role in the pathogenesis of vaccine-associated sarcomas, the canary pox–vectored vaccine would be less likely to be involved in the oncogenesis of these tumors. Although it shows great promise, practitioners should remember that canary pox–vectored rabies vaccine is not approved for use in ferrets in North America at this time.
To the author's knowledge, there is only one report of anaphylactic reaction in a ferret following administration of an inactivated rabies vaccine [13], [96]. This ferret previously had an anaphylactic reaction after receiving a distemper and rabies vaccine simultaneously. Observation of ferrets for 25 minutes following rabies vaccination is advisable.
Unvaccinated ferrets that are exposed to a rabid animal should be euthanized immediately [89], [97]. If the owner refuses, the animal should be quarantined strictly for 6 months and vaccinated 1 month before being released [89], [97]. Ferrets that are vaccinated currently should be revaccinated immediately [89], [97].
If a healthy ferret bites a person, it should be confined and observed for 10 days [89], [97]. The animal needs to be evaluated by a veterinarian at the first sign of illness during confinement. If signs that are suggestive of rabies develop, the animal should be euthanized and tested for rabies [89], [97].
Rotavirus
Rotavirus belongs to the family Reoviridae. All rotaviruses are unique because they possess double-stranded RNA genome. Generally, these viruses cause diarrhea in young animals and children, but they also can occur in older individuals [98]. An atypical rotavirus was isolated from neonatal ferrets (Mustela putorius furo) that had diarrhea at a large commercial farm in the United States. The disease was recognized at the ferret farm for several years and was referred as “ferret kit disease” [1]. Partial characterization identified this virus as an atypical rotavirus, based on the lack of rotavirus group A common antigen and on its distinct double-stranded RNA electropherotype pattern in polyacrylamide gels. In Finland, a rotavirus outbreak in ferret kits had a mortality rate that approached 100% [1].
Clinical presentation
Clinical signs of rotavirus infection occur in 2- to 6-week-old ferrets. Soft yellow to green diarrhea is present with associated fecal staining or matted hair on their bodies. Erythema of the anus and perineum also is reported [98]. The disease was prevalent throughout the year at the American commercial farm, with a increased incidence in colder months. Morbidity among primiparous jills was high (up to 90%); the morbidity rate decreased with each gestation to range between 10% to 25% in multiparous jills [98]. The condition could be reproduced in 2- to 3-week-old ferret kits that were inoculated orally with viral preparations that were obtained from diarrheic ferrets; however, mortalities nor histologic lesions were observed in individuals that were infected experimentally [1], [98].
Diagnosis
Antemortem diagnosis is difficult. Viral particles may be detected by electron microscopy in clarified, ultracentrifuged fecal suspensions, following negative staining in symptomatic ferrets. In a study, 58% of diarrheic ferrets that were infected naturally were positive for rotavirus particles in their feces. The ferret atypical rotavirus does not react with the Rotazyme test (Abbott Laboratory, Chicago, Illinois), a commercially available enzyme immunoassay [1], [98]. The prevalence of this viral infection is unknown in ferrets because of the absence of reliable serologic tests. On postmortem examination, gross lesions are limited to the gastrointestinal tract. Subtle histologic lesions that consist of mild blunting of the tips of the intestinal villi of the small intestine with enterocyte metaplasia to cuboidal cells may be observed.
Treatment/prevention
Secondary bacterial infections may be a significant factor in the severity of the diarrhea. Therefore, antibiotics are indicated and may contribute to accelerated recovery and decreased mortality rates. Additional supportive care, including appropriate fluid therapy and force feeding, are indicated in most cases.
In piglets, colostral antibodies play a key role in the protection against rotavirus [1]. This also may be true for ferrets. Ferret kits acquire most of their passive immune globulins from their mothers by intestinal transmucosal absorption from colostrum and milk; they acquire all of their IgA from their mother's milk [99]. The observed decrease in morbidity from ferrets of primiparous jills to ferrets of multiparous jills may be secondary to build-up of colony immunity with increasing age and exposure to the virus [98].
Oral vaccination of primiparous jills was attempted at a ferret breeding farm; however, this procedure was ineffective. Atypical rotaviruses have not been cultivated successfully in cell culture [1]. The ability to propagate the virus in cell culture in sufficient numbers will play a key role in the development of a vaccine.
Infectious bovine rhinotracheitis virus
The infectious bovine rhinotracheitis virus (IBR) belongs to the family Herpesviridae. There is only one published case report of spontaneous IBR infection in a ferret [100]. The virus was isolated from the liver, the spleen, and the lungs of a clinically normal ferret. Its diet consisted of 5% raw beef by-products; it was hypothesized that virus-laden raw beef was the source of infection. The pathogenesis by which the virus disseminated to the liver, spleen, and lung tissue has not been elucidated. In contrast to naturally-occurring infection, experimental infection of ferrets with IBR virus by intranasal and intraperitoneal inoculations induced acute and chronic respiratory disease [101]. Considering that IBR virus can cause pathology in ferrets, these animals should not be fed raw meat or meat products [1].
Summary
Distemper and rabies vaccination are highly recommended because of the almost invariable fatal outcome of these conditions. Vaccination should constitute an important part of a ferret's preventative medicine program. With the current and anticipated development and licensing of new vaccines, practitioners are invited to gain awareness of the latest vaccine information. Establishment of a practice vaccination protocol with regards to the site of administration of rabies and distemper vaccines is paramount to document any future abnormal tissue reactions.
Influenza is the most common zoonotic disease that is seen in ferrets. Although it generally is benign in most ferrets, veterinarians must take this condition seriously. The characteristic continuous antigenic variation of this virus may lead to more virulent strains; the recent emergence of avian influenza virus outbreaks; and the increased susceptibility of elderly, young, and immunosuppressed individuals.
References
- 1.Fox J.G., Pearson R.C., Gorham J.R. Viral diseases. In: Fox J.G., editor. Biology and diseases of the ferrets. 2nd edition. Lippincott Williams & Wilkins; Philadelphia: 1998. pp. 355–374. [Google Scholar]
- 2.Williams E.S., Thorne E.T., Appel M.J.G. Canine distemper in black-footed ferrets (Mustela nigripes) from Wyoming. J Wild Dis. 1988;24(3):385–398. doi: 10.7589/0090-3558-24.3.385. [DOI] [PubMed] [Google Scholar]
- 3.Appel M.J.G., Summers B.A. Pathogenicity of morbilliviruses for terrestrial carnivores. Vet Microbiol. 1995;44:187–191. doi: 10.1016/0378-1135(95)00011-x. [DOI] [PubMed] [Google Scholar]
- 4.Von Messling V., Springfeld C., Devaux P. A ferret model of canine distemper virus virulence and immunosuppression. J Virol. 2003;77(23):12579–12591. doi: 10.1128/JVI.77.23.12579-12591.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Crook E., Gorham J.R., McNutt S.H. Experimental distemper in mink and ferrets. I. Pathogenesis. Am J Vet Res. 1958;19(73):955–957. [PubMed] [Google Scholar]
- 6.Pfeiffer C.J. Gastric hypochlorhydria in ferret distemper. Can J Comp Med Vet Sci. 1967;31:135–138. [PMC free article] [PubMed] [Google Scholar]
- 7.Ryland L.M. A clinical guide to the pet ferret. Compend Cont Ed. 1983;8:25–33. [Google Scholar]
- 8.Ryland L.M., Bernard S.L., Gorham J.R. A clinical guide to the pet ferret. In: Rosenthal K.L., editor. Practical exotic animal medicine, the compendium collection. Veterinary Learning System; Trenton (NJ): 1997. pp. 122–129. [Google Scholar]
- 9.Davidson M. Canine distemper virus infection in the domestic ferret. Compend Cont Ed Pract Vet. 1986;8:448–453. [Google Scholar]
- 10.Stephenson C.B., Welter J., Thaker S.R. Canine distemper virus (CDV) infection of ferrets as a model for testing Morbillivirus vaccine strategies: NYVAC- and ALVAC-based CDV recombinants protect against symptomatic infection. J Virol. 1997;71(2):1506–1513. doi: 10.1128/jvi.71.2.1506-1513.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rzezutka A., Mizak B. Application of N-PCR for diagnosis of distemper in dogs and fur animals. Vet Mibrobiol. 2002;88:95–103. doi: 10.1016/s0378-1135(02)00097-4. [DOI] [PubMed] [Google Scholar]
- 12.Blair E.M., Chambers M.A., King H.A. Treating distemper in a young ferret. Vet Med. 1998;93(7):655–658. [Google Scholar]
- 13.Greenacre C.B. Incidence of adverse events in ferrets vaccinated with distemper or rabies vaccine:143 cases(1995–2001) J Am Vet Med Assoc. 2003;223(5):663–665. doi: 10.2460/javma.2003.223.663. [DOI] [PubMed] [Google Scholar]
- 14.Winsatt J., Jay M.T., Innes K.E. Serologic evaluation, efficacy, and safety of a commercial modified-live canine distemper vaccine in domestic ferrets. Am J Vet Res. 2001;62(5):736–740. doi: 10.2460/ajvr.2001.62.736. [DOI] [PubMed] [Google Scholar]
- 15.Carpenter J.W., Appel M.J., Erickson R.C. Fatal vaccine-induced canine distemper virus infection in black-footed ferrets. J Am Vet Med Assoc. 1976;169:961–964. [PubMed] [Google Scholar]
- 16.Kauffman C.A., Bergman A.G., O'Connor R.P. Distemper virus infection in ferrets: an animal model of measles-induced immunosuppression. Clin Exp Immunol. 1982;47:617–625. [PMC free article] [PubMed] [Google Scholar]
- 17.Rosenthal K.L. Respiratory diseases. In: Hillyer E.V., Quesenberry K.E., editors. Ferrets, rabbits and rodents clinical medicine and surgery. WB Saunders; Philadelphia: 1997. pp. 77–79. [Google Scholar]
- 18.Quesenberry K.E., Orcutt C. Basic approach to veterinary care. In: Quesenberry K.E., Carpenter J.W., editors. Ferrets, rabbits, and rodents clinical medicine and surgery. 2nd edition. WB Saunders; Philadelphia: 2004. pp. 13–24. [Google Scholar]
- 19.Kenyon A.J., Howard E., Buko L. Hyperglobulinemia in ferrets with lymphoproliferative lesions (Aleutian disease) Am J Vet Res. 1967;28:1167–1172. [PubMed] [Google Scholar]
- 20.Murakami M., Matsuba C., Une Y. Nucleotide sequence and polymerase chain reaction/restriction fragment length polymorphism analyses of Aleutian disease virus in ferrets in Japan. J Vet Diagn Invest. 2001;13:337–340. doi: 10.1177/104063870101300410. [DOI] [PubMed] [Google Scholar]
- 21.Porter H.C., Porter D.D., Larsen A.F. Aleutian disease in ferrets. Infect Immun. 1982;36:379–386. doi: 10.1128/iai.36.1.379-386.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Saifuddin M., Fox J.G. Identification of a DNA segment in ferret Aleutian disease virus similar to a hypervariable capsid region in mink Aleutian disease parvovirus. Arch Virol. 1996;141:1329–1339. doi: 10.1007/BF01718834. [DOI] [PubMed] [Google Scholar]
- 23.Ohshima K., Shen D.T., Henson J.B. Comparison of the lesions of Aleutian disease in mink and hypergammaglobulinemia in ferrets. Am J Vet Res. 1978;39(4):653–657. [PubMed] [Google Scholar]
- 24.Daoust P.Y., Hunter D.B. Spontaneous Aleutian disease in ferrets. Can Vet J. 1978;19:133–135. [PMC free article] [PubMed] [Google Scholar]
- 25.Palley L.S., Corning B.F., Fox J.C. Parvovirus-associated syndrome (Aleutian disease) in two ferrets. J Am Vet Med Assoc. 1992;201:100–106. [PubMed] [Google Scholar]
- 26.Morrisey J.K. Part II. Other diseases. In: Quesenberry K.E., Carpenter J.W., editors. Ferrets, rabbits, and rodents clinical medicine and surgery. 2nd edition. WB Saunders; Philadelphia: 2004. pp. 66–70. [Google Scholar]
- 27.Alexandersen S., Bloom M.E. Studies on the sequential development of acute interstitial pneumonia caused by Aleutian disease virus in mink kits. J Virol. 1987;61(1):81–86. doi: 10.1128/jvi.61.1.81-86.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Une Y., Wakimoto Y., Nakano Y. Spontaneous Aleutian disease in a ferret. J Vet Med Sci. 2000;62:553–555. doi: 10.1292/jvms.62.553. [DOI] [PubMed] [Google Scholar]
- 29.Oxenham M. Aleutian disease in the ferret. Vet Rec. 1990;126(23):585. [PubMed] [Google Scholar]
- 30.Stewart J.D., Rozengurt N. Aleutian disease in the ferret. Vet Rec. 1993;133:172. doi: 10.1136/vr.133.7.172-a. [DOI] [PubMed] [Google Scholar]
- 31.Welchman D de B., Oxenham M., Done S.H. Aleutian disease in domestic ferrets: diagnostic findings and survey results. Vet Rec. 1993;132:479–484. doi: 10.1136/vr.132.19.479. [DOI] [PubMed] [Google Scholar]
- 32.Wolfensohn H.H.L. Aleutian disease in laboratory ferrets. Vet Rec. 1994;134:100. doi: 10.1136/vr.134.4.100. [DOI] [PubMed] [Google Scholar]
- 33.Oxenham M. Aleutian disease in ferrets. Vet Rec. 1992;131(13):296. doi: 10.1136/vr.131.13.296-a. [DOI] [PubMed] [Google Scholar]
- 34.Kenyon A.J., Kenyon B.J., Hahn E.C. Protides of the Mustelidae immunoresponse of mustelids to Aleutian mink disease virus. Am J Vet Res. 1978;39:1011–1015. [PubMed] [Google Scholar]
- 35.Bloom M.E., Race R.E., Wolfinbarger J.B. Identification of a non-virion protein of Aleutian disease virus: mink with Aleutian disease have antibody to both virion and nonvirion proteins. J Virol. 1982;43:608–616. doi: 10.1128/jvi.43.2.608-616.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Haas L., Lochelt M., Kaaden O.R. Detection of Aleutian disease virus DNA in tissues of naturally infected mink. J Gen Virol. 1988;69:705–710. doi: 10.1099/0022-1317-69-3-705. [DOI] [PubMed] [Google Scholar]
- 37.Cheema A., Henson J.B., Gorham J.R. Aleutian disease of mink: prevention of lesions by immunosuppression. Am J Pathol. 1972;55:543–546. [PMC free article] [PubMed] [Google Scholar]
- 38.Aasted B., Alexandersen S., Hansen M. Treatment of neonatally Aleutian disease virus (ADV) infected mink kits with gamma-globulin containing antibodies to ADV reduces death rate of mink kits. Acta Vet Scand. 1988;29:323–330. doi: 10.1186/BF03548625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cho H.J., Greenfield J. Eradication of Aleutian disease of mink by eliminating positive counter immunoelectrophoresis reactors. J Clin Microbiol. 1978;7:18–22. doi: 10.1128/jcm.7.1.18-22.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shen D.T., Leendertsen L.W., Gorham J.R. Evaluation of chemical disinfectants for Aleutian disease virus of mink. Am J Vet Res. 1981;42(5):838–840. [PubMed] [Google Scholar]
- 41.Porter D.D., Larsen A.E., Porter H.G. The pathogenesis of Aleutian disease of mink. II. Response of mink to formalin treated diseased tissue and to subsequent challenge with virulent inoculum. Can J Comp Med. 1963;27:124–128. [PMC free article] [PubMed] [Google Scholar]
- 42.Gorham J.R., Evermann J.F., Ward A. Detection of coronavirus-like particles from mink. Can J Vet Res. 1990;54:383–384. [PMC free article] [PubMed] [Google Scholar]
- 43.Have P., Moving V., Svansson V. Coronavirus infection in mink (Mustela vison). Serological evidence of infection with a coronavirus related to transmissible gastroenteritis virus and porcine epidemic diarrhea virus. Vet Microbiol. 1992;31:1–10. doi: 10.1016/0378-1135(92)90135-G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Williams B.H., Kiupel M., West K.H. Coronavirus-associated epizootic catarrhal enteritis in ferrets. J Am Vet Med Assoc. 2000;217(4):526–530. doi: 10.2460/javma.2000.217.526. [DOI] [PubMed] [Google Scholar]
- 45.Hoefer H.L. Gastrointestinal diseases. In: Hiller E.V., Quesenberry K.E., editors. Ferrets, rabbits and rodents clinical medicine and surgery. 1st edition. WB Saunders; Philadelphia: 1997. pp. 26–36. [Google Scholar]
- 46.Doggart L. Viral disease of pet ferrets: part II. Aleutian disease, influenza, and rabies. Vet Technol. 1988;8:384–389. [Google Scholar]
- 47.Marois P., Boudreault A., DiFranco E. Response of ferrets and monkeys to intranasal infection with human, equine, and avian influenza viruses. Can J Comp Med. 1971;35:71–76. [PMC free article] [PubMed] [Google Scholar]
- 48.Shope R.E. The infection of ferrets with swine influenza virus. J Exp Med. 1934;60:49–61. doi: 10.1084/jem.60.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zitzow L.A., Rowe T., Morken T. Pathogenesis of avian influenza A (H5N1) viruses in ferrets. J Virol. 2002;76(9):4420–4429. doi: 10.1128/JVI.76.9.4420-4429.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Smith W., Andrews D.H., Laidlow P.O. The virus obtained from influenza patients. Lancet. 1933;2:66. [Google Scholar]
- 51.Smith W., Stuart-Harris C.H. Influenza infection of man from the ferret. Lancet. 1936;2:21. [Google Scholar]
- 52.Smith H., Sweet C. Lessons for human influenza from pathogenicity studies in ferrets. Rev Infec Dis. 1998;10:56–75. doi: 10.1093/clinids/10.1.56. [DOI] [PubMed] [Google Scholar]
- 53.Sweet C., Fenton R.J., Price G.E. The ferret as an animal model of influenza virus infection. In: Zak O., Sande M.A., editors. Handbook of animal models of infection. Academic Press; New York: 1999. pp. 989–998. [Google Scholar]
- 54.Basarab O., Smith H. Quantitative studies on the tissue localization of influenza virus in ferrets after intranasal and intravenous or extracordial inoculation. Br J Exp Pathol. 1969;50:612. [PMC free article] [PubMed] [Google Scholar]
- 55.Marini R.P., Adkins J.A., Fox J.G. Proven and potential zoonotic diseases of ferrets. J Am Vet Med Assoc. 1989;195:990–994. [PubMed] [Google Scholar]
- 56.Collie M.H., Rushton D.I., Sweet C. Studies of influenza infection in newborn ferrets. J Med Microbiol. 1980;13(4):561–571. doi: 10.1099/00222615-13-4-561. [DOI] [PubMed] [Google Scholar]
- 57.Buchman C.A., Swarts J.D., Seroky J.T. Otologic and systemic manifestations of experimental influenza A virus infection in the ferret. Otolaryngol Head Neck Surg. 1995;112(4):572–578. doi: 10.1177/019459989511200411. [DOI] [PubMed] [Google Scholar]
- 58.Rosenthal K.L. Respiratory disease. In: Quesenberry K.E., Carpenter J.W., editors. Ferrets, rabbits and rodents clinical medicine and surgery. 2nd edition. WB Saunders; Philadelphia: 2004. pp. 72–78. [Google Scholar]
- 59.Sweet C., Macartney J.C., Bird R.A. Differential distribution of virus and histological damage in the lower respiratory tract of ferrets infected with influenza viruses of differing virulence. J Gen Virol. 1981;54:103–114. doi: 10.1099/0022-1317-54-1-103. [DOI] [PubMed] [Google Scholar]
- 60.Coates D.M., Husseini R.H., Rushton D.I. The role of lung development in the age-related susceptibility of ferrets to influenza virus. Br J Exp Pathol. 1984;65:543. [PMC free article] [PubMed] [Google Scholar]
- 61.Sweet O., Jakeman K.J., Rushton I. Role of upper respiratory tract infection in the deaths occurring in neonatal ferrets infected with influenza virus. Microb Pathog. 1988;5:121–125. doi: 10.1016/0882-4010(88)90014-9. [DOI] [PubMed] [Google Scholar]
- 62.Glathe H., Lebhardt A., Hilgenfeld M. Intestinal influenza infection in ferrets (in German) Arch Exp Veterinarmed. 1984;38:771–777. [PubMed] [Google Scholar]
- 63.Kang E.S., Lee H.J., Boulet J. Potential for hepatic and renal dysfunction during influenza B infection, convalescence, and after induction of secondary viremia. J Exp Pathol. 1992;6:133–144. [PubMed] [Google Scholar]
- 64.Rowe T., Cho D.S., Bright R.A. Neurological manifestations of avian influenza viruses in mammals. Avian Dis. 2003;47:1122–1126. doi: 10.1637/0005-2086-47.s3.1122. [DOI] [PubMed] [Google Scholar]
- 65.De Boer G.F., Back W., Osterhaus A.D. An ELISA for detection of antibodies against influenza A nucleoprotein in humans and various animal species. Arch Virol. 1990;115:47–61. doi: 10.1007/BF01310622. [DOI] [PubMed] [Google Scholar]
- 66.Sweet C., Bird R.A., Cavanah D. The local origin of the febrile response induced in ferrets during respiratory infection with a virulent influenza virus. Br J Exp Pathol. 1979;60:300. [PMC free article] [PubMed] [Google Scholar]
- 67.Chen K.S., Bharaj S.S., King E.C. Induction and relief of nasal congestion in ferrets infected with influenza virus. Int J Exp Pathol. 1995;76:55–64. [PMC free article] [PubMed] [Google Scholar]
- 68.Husseini R.H., Collie M.H., Rushton D.I. The role of naturally-acquired bacterial infection in influenza-related death in neonatal ferrets. Br J Exp Pathol. 1983;64:559–569. [PMC free article] [PubMed] [Google Scholar]
- 69.Husseini R.H., Sweet C., Overton H. Role of maternal immunity in the protection of newborn ferrets against infection with a virulent influenza virus. Immunology. 1984;52(3):389–394. [PMC free article] [PubMed] [Google Scholar]
- 70.Fenton R.J., Bessell C., Spilling C.R. The effects of per oral or local aerosol administration of 1-aminoadamantane hydrochloride (amantadine hydrochloride) on influenza infections of the ferret. J Antimicrob Chemother. 1977;3(5):463. doi: 10.1093/jac/3.5.463. [DOI] [PubMed] [Google Scholar]
- 71.Fenton R.J., Morley P.J., Owens I.J. Chemoprophylaxis of influenza A virus infections, with single doses of zanamivir, demonstrates that zanamivir is cleared slowly from the respiratory tract. Antimicrob Agents Chemother. 1999;43(1):2642–2647. doi: 10.1128/aac.43.11.2642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Herlocher M.L., Truscon R., Fenton R. Assessment of development of resistance to antivirals in the ferret model of influenza virus infection. J Infec Dis. 2003;188(9):1355–1361. doi: 10.1086/379049. [DOI] [PubMed] [Google Scholar]
- 73.Potter C.W., Oxford J.S., Shore S.L. Immunity to influenza infection in ferrets. I. Response to live and killed virus. Br J Exp Pathol. 1972;53(2):153. [PMC free article] [PubMed] [Google Scholar]
- 74.Husseini R.H., Sweet C., Collie M.H. Elevation of nasal viral levels by suppression of fever in ferrets infected with influenza viruses of differing virulence. J Inf Dis. 1982;145(4):520–524. doi: 10.1093/infdis/145.4.520. [DOI] [PubMed] [Google Scholar]
- 75.Paisley J.W., Lauer B.A. Severe facial injuries to infants due to unprovoked attacks by pet ferrets. JAMA. 1988;259:2005. [PubMed] [Google Scholar]
- 76.Diesch S.L. Reported human injuries or health threats attributed to wild and exotic animals kept as pets (1971–1981) J Am Vet Med Assoc. 1982;18:382. [Google Scholar]
- 77.Applegate J.A., Walhout M.F. Childhood risks from the ferret. J Emerg Med. 1998;16(3):425–427. doi: 10.1016/s0736-4679(98)00008-0. [DOI] [PubMed] [Google Scholar]
- 78.National Association of State Public Health Veterinarians. Compendium of animal rabies control. J Am Vet Assoc. 1990;196:36–39. [Google Scholar]
- 79.Jenkins S.R., Osterholm M.T. Epidemiologists and public health veterinarians issue statement on ferrets. J Am Vet Med Assoc. 1994;205:534. [PubMed] [Google Scholar]
- 80.Centers for Disease Control . US Department of Health and Human Services; Washington, DC: 1986. Viral diseases: ferret rabies. Rabies surveillance, annual summary. [Google Scholar]
- 81.Niezgoda M., Briggs D.J., Shaddock J. Pathogenesis of experimentally induced rabies in domestic ferrets. Am J Vet Res. 1997;58(11):1327–1331. [PubMed] [Google Scholar]
- 82.Blancou J., Aubert J.F.A., Artois M. Rage expérimentale du furet [Mustela (putorius) furo] [Experimental rabies in the ferret (Mustela (putorius) furo)] Revue Méd Vét. 1982;133:553. (in French) [Google Scholar]
- 83.Niezgoda M., Briggs D.J., Shaddock J. Viral excretion in domestic ferrets (Mustela putorius furo) inoculated with a raccoon rabies isolate. Am J Vet Res. 1998;58(12):1629–1632. [PubMed] [Google Scholar]
- 84.Charlton K.M. The pathogenesis of rabies and other lyssaviral infections: recent studies. Curr Top Microbiol Immunol. 1994;187:95–119. doi: 10.1007/978-3-642-78490-3_6. [DOI] [PubMed] [Google Scholar]
- 85.Bell J.F., Moore G.J. Susceptibility of carnivore to rabies virus administered orally. Am J Epidemiol. 1971;93:176. doi: 10.1093/oxfordjournals.aje.a121244. [DOI] [PubMed] [Google Scholar]
- 86.Förster U. Zur frage der adaptationsfähigkeit von zwei in Mitteleuropa isolierten tollwutvirusstâmmen an eine domestizierte und zwei wildlebende spezies. Ein beitrag zur epidemiologic der tollwut 4. Mitteilung: übertragsversuche an frettchen mit einem nagerisolat. [The adaptability of two rabies virus strains isolated in central Europe to one domesticated and two wild-living species: a contribution to epidemiology of rabies. Part 4: transmission studies on ferret with rodent isolate] Zentralbl Veterinarmed. 1979;26:29–38. (in German) [PubMed] [Google Scholar]
- 87.Rupprecht C.E., Gilbert J., Pitts R. Evaluation of an inactivated rabies virus vaccine in domestic ferrets. J Am Vet Med Assoc. 1990;196:1614. [PubMed] [Google Scholar]
- 88.Hoover J.P., Baldwin C.A., Rupprecht C.E. Serologic response of domestic ferrets (Mustela putorius furo) to canine distemper and rabies virus vaccines. J Am Vet Med Assoc. 1989;194(2):234–238. [PubMed] [Google Scholar]
- 89.National Association of State Public Health Veterinarians Compendium of animal rabies prevention and control, 2003. Morb Mortal Wkly Rep. 2003;52(RR-5) [PubMed] [Google Scholar]
- 90.Rabies vaccines licensed in Canada. Canadian Food Inspection Agency, Animal Health and Production Division, Veterinary biologics section. Available at: http://www.inspection.gc.ca/english/anima/vetbio/prod/rabrage.shtml. Accessed October 2004.
- 91.Murray J. Vaccine injection-site sarcoma in a ferret. J Am Vet Med Assoc. 1998;213(7):955. [PubMed] [Google Scholar]
- 92.Hendrick M.J. Historical review and current knowledge of risk factors involved in feline vaccine-associated sarcomas. J Am Vet Med Assoc. 1998;213:1422–1423. [PubMed] [Google Scholar]
- 93.Hendrick M.J. Feline vaccine-associated sarcomas. Cancer Invest. 1999;17:273–277. doi: 10.3109/07357909909040597. [DOI] [PubMed] [Google Scholar]
- 94.Hendrick M.J., Dunagan C.A. Focal necrotizing granulomatous panniculitis associated with subcutaneous injection of rabies vaccine in cats and dogs: 10 cases (1988–1989) J Am Vet Med Assoc. 1991;198:304–305. [PubMed] [Google Scholar]
- 95.Carroll E.E., Dubielzig R.R., Schultz R.D. Cats differ from mink and ferrets in their response to commercial vaccines: A histologic comparison of early vaccine reactions. Vet Pathol. 2002;39:216–227. doi: 10.1354/vp.39-2-216. [DOI] [PubMed] [Google Scholar]
- 96.Lincoln J. Another interpretation of ferret's reaction to vaccination. J Am Vet Med Assoc. 2003;223(8):1112. [PubMed] [Google Scholar]
- 97.Fearneyhough M.G. Rabies postexposure prophylaxis. Vet Clin North Am. 2001;31(3):557–572. [PubMed] [Google Scholar]
- 98.Torres-Medina A. Isolation of an atypical rotavirus causing diarrhea in neonatal ferrets. Lab Animal Sc. 1987;37(2):167–171. [PubMed] [Google Scholar]
- 99.Suffin S.C., Prince G.A., Murk K.B. Ontogeny of the ferret humoral response. J Immunol. 1979;123:6–9. [PubMed] [Google Scholar]
- 100.Porter D.D., Larsen A.E., Cox N.A. Isolation of infectious bovine rhinotracheitis virus from Mustelidae. J Clin Microbiol. 1975;1:112–113. doi: 10.1128/jcm.1.1.112-113.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Smith P.C. Experimental infectious bovine rhinotracheitis virus infection of English ferrets (Mustela putorius furo L) Am J Vet Res. 1978;39(8):1369–1372. [PubMed] [Google Scholar]


