Abstract
After the outbreak of severe acute respiratory syndrome in Hong Kong, the importance of preventing nosocomial transmission of respiratory viruses has become a top priority in infection control. During the containment and early mitigation phases of the swine-origin influenza virus (S-OIV) A H1N1 pandemic, an infection control bundle consisting of multiple coherent measures was organised by our infection control team to minimise nosocomial transmission. This included repeated open staff forum achieving high attendance; early recognition of index cases among inpatients by liberal testing; early relief of sick staff from work; directly observed hand hygiene practice during outbreaks; and monitoring of compliance with infection control practice. During the first 100 days (from 1 May to 8 August 2009) when the first 100 laboratory-confirmed patients with S-OIV and 12 infected healthcare workers (HCWs) were identified, a total of 836 asymptomatic exposed persons (184 patients and 652 HCWs) were required to undergo a seven-day medical surveillance. The infection control nurses monitored them for the onset of symptoms. Four (0.48%) exposed persons (one house officer, two non-clinical staff, and one patient) were virologically confirmed with S-OIV. Not wearing a surgical mask either by the exposed persons during contact with the index cases (4/4 vs 264/832, P = 0.010) or vice versa (4/4 vs 300/832, P = 0.017, Fisher's exact test) were found to be significant risk factors for nosocomial acquisition of S-OIV.
Keywords: H1N1 virus, Infection control bundle, Influenza, Nosocomial infection
Introduction
Since the outbreak of severe acute respiratory syndrome (SARS) spreading from hospitals to the community in 2003, nosocomial infection by respiratory viruses has received the highest level of attention by the government and the public in Hong Kong.1 The hospital infection control team has been promoting infection control training for healthcare workers (HCWs), and implementing standard and transmission-based precautions with special reference to directly observed hand hygiene practice during outbreaks.2 When the outbreak of swine-origin influenza virus (S-OIV) A H1N1 infection occurred in Mexico and North America in early 2009, our infection control team immediately designed a series of strategic measures promulgated as an infection control bundle to enhance our colleagues' awareness and compliance to these measures. In Hong Kong, the first confirmed case of S-OIV was diagnosed in a traveller from Mexico on 1 May 2009.3 As of 20 August 2009, there were 8210 laboratory-confirmed cases with four deaths. Here, we report the effect of such measures on the occurrence of nosocomial S-OIV in our hospital.
Methods
Infection control preparedness for S-OIV
In collaboration with the stakeholders including hospital administration, clinicians, and nursing colleagues, a strategic infection control bundle was established (Box I ). Open staff forum and special education sessions were arranged by the hospital administration and infection control team. Nasopharygneal aspirates or nasopharyngeal flocked swabs were collected from cases, either patients or HCWs, who had upper respiratory tract infection (URTI) symptoms. Direct immunofluorescent antigen test for influenza A and other respiratory viruses (influenza B virus, respiratory syncytial virus, parainfluenza virus types 1, 2 and 3, and adenovirus) and reverse transcription–polymerase chain reaction (RT-PCR) for S-OIV H1 gene were performed, as previously described.3, 4 The infection control team checked with the microbiology laboratory on a daily basis for positive results. Cases were reviewed to determine the route of acquisition. For patients, they were considered as having community-acquired infection if they presented with URTI symptoms within 48 h of admission, without history of contact with any confirmed case in the hospital. For HCWs, they were considered as having community-acquired infection if they had confirmed cases in their household with no history of unprotected exposure to any confirmed case in the hospital. Otherwise, the case was classified as nosocomial infection. Unprotected exposure was defined as the contact within 1 m between a confirmed case and the exposed with both of them not having worn a surgical mask, whereas protected exposure meant that either one had worn a surgical mask during their contact.
Box I. Infection control strategic bundle in prevention of nosocomial transmission of swine-origin influenza virus (S-OIV) A H1N1.
-
1.‘Just-in-time’ education of infection control practice to healthcare workers
-
(i)Open infection control forum for all staff and special session for various clinical departments
-
(ii)Special session for staff who are attending isolation facilities
-
(iii)Special session for staff when inpatients and co-workers are confirmed with S-OIV
-
(i)
-
2.Enhanced infection control practice
-
(i)Enforcement of standard and transmission-based precaution in clinical area, especially with directly observed hand hygiene practice
-
(ii)Wearing surgical mask at all times in patient-care area and compliance on cough etiquette
-
(iii)Regular environmental cleaning with soap and water and ad-hoc environment cleaning with disinfectant (sodium hypochlorite 500 ppm) upon identification of confirmed case of S-OIV
-
(i)
-
3.Early recognition of index case in the hospitalised patients
-
(i)Triage of suspected patients in emergency room and admission to isolation facilities
-
(ii)Alertness of patients with nosocomial onset of upper respiratory tract infection and referral to isolation facilities
-
(iii)Implementation of rapid molecular diagnostic test with turnaround time within 24 h
-
(i)
-
4.Preventing introduction of index case to the hospitalised patients
-
(i)Promoting absenteeism for sick healthcare workers
-
(ii)7-day sick leave for infected healthcare workers
-
(iii)Wearing surgical mask for visitors in the hospital and promoting direct observed hand hygiene for visitors
-
(i)
-
5.Audit of infection control compliance
-
(i)Unobtrusive hand hygiene observation and monitoring the compliance of wearing surgical mask
-
(ii)Monitoring the consumption of alcohol-based hand rub in the hospital
-
(iii)Monitoring the incidence of nosocomial influenza A infection
-
(i)
-
6.Administrative support
-
(i)Provision of alcohol-based hand rub in every bed, all ward entrances and corridors
-
(ii)Provision of manpower and equipment for laboratory diagnostics and contact tracing
-
(iii)Co-ordination of infection control training sessions for staff
-
(i)
The incidence of nosocomial infection by influenza A virus and the other respiratory viruses expressed as number per 10 000 patient-days between 2007 and the first six months of 2009 were reviewed. The total patient-days of the hospital were obtained from the record office. Hand hygiene was promoted in 2006 and fully implemented in our hospital in 2008. Compliance of hand hygiene has been regularly audited by the infection control team according to a predetermined protocol.5 The consumption data of alcohol-based hand rub in terms of volume used per 100 admissions was retrieved from hospital administration.
Admission of patients for investigation for S-OIV
This study was performed in Queen Mary Hospital, a 1500-bed tertiary referral university-affiliated hospital with three adult isolation wards and one paediatric isolation ward in Hong Kong. The local hospital admission policies were modified during the various phases of S-OIV outbreak. In phase 1, the containment phase (from 1 May to 17 June 2009), all patients with influenza-like illness (ILI), defined as fever with a temperature of ≥38 °C with sore throat or cough, and returned from countries with confirmed cases of S-OIV in the preceding seven days, were admitted for single room isolation. Close contacts of the confirmed cases were quarantined. Close contacts were defined as individuals from the same household and flight passengers seated three rows in front or behind the confirmed cases. In phase 2, the early mitigation phase (from 18 June to 28 June 2009), all patients with laboratory-confirmed S-OIV were hospitalised for cohort nursing and consideration of antiviral therapy. During phase 3, the late mitigation phase (from 29 June 2009 onwards), only high risk patients (children aged ≤2 years or pregnant women) with S-OIV, and patients with clinical evidence of complicated influenza were admitted for further management.
Contact tracing of patients and HCWs with confirmed S-OIV
HCWs were monitored for URTI symptoms during and seven days after rotating out from the isolation facilities. For those patients diagnosed in the general wards, patients staying in the same cubicle and HCWs working in the same wards were subjected to contact tracing and underwent a seven-day medical surveillance which included daily monitoring of body temperature and URTI symptoms by the ward-in-charge and infection control team. If the patients or HCWs had unprotected exposure, oseltamivir (75 mg daily for 10 days) would be offered as post-exposure prophylaxis. For those with protected exposure, no oseltamivir was given. When S-OIV was confirmed in HCWs, a seven-day sick leave would be issued. The infection control team would follow up on the clinical progress of the staff, investigate the source of infection and classify it as community- or hospital-acquired infection. Contact tracing of the exposed inpatients and co-workers was followed by a seven-day medical surveillance. If the infected HCW had worn a surgical mask and practice hand hygiene during patient care, antiviral agent would not be prescribed to the exposed patients. However, oseltamivir (75 mg daily for 10 days) would be recommended to the co-workers as post-exposure prophylaxis if they had not worn a surgical mask during tea break, lunch, and dinner with the infected staff. The infected HCWs were interviewed by the infection control team for compliance with infection control practice according to a standard questionnaire. Risk factors for nosocomial infection by S-OIV in both patients and HCWs were investigated. Persons exposed to the same environment who were asymptomatic for URTI after seven days of medical surveillance were chosen as control.
Statistical analysis
Fisher's exact test was used to compare independent categorical variables between groups. All reported P-values were two-sided. P < 0.05 was considered statistically significant. Computation was performed using the SPSS Version 15.0 for Windows.
Results
Infection control preparedness for S-OIV
Between 1 May and 8 August 2009, 18 sessions of open staff forum with an overall attendance of 3698 (74.3%) out of 4976 hospital staff were held to update the medical knowledge and infection control practice to prevent acquisition of S-OIV. Along with the basic and special infection control training sessions previously held before the outbreak of S-OIV, 4618 (92.8%) of the hospital staff have attended infection control training in the past 18 months. Video demonstrations of gowning and degowning of personal protective equipment were provided to all isolation wards and uploaded to the hospital intranet for regular revision by frontline HCWs. Briefly, a surgical mask, protective eyewear and gown were recommended when the HCWs were within 1 m of contact with the suspected cases in isolation rooms. N95 respirator, cap and gloves were to be worn in addition to face shield and gown when aerosol generating procedures were performed.
The incidence of nosocomial infection of influenza A and other respiratory viruses gradually decreased from 0.25 and 0.86 per 10 000 patient-days in 2007, to 0.14 and 0.45 per 10 000 patient-days in 2008, and 0.05 and 0.15 per 10 000 patient-days, in the first six months of 2009. Previous audit of hand hygiene practice had shown a gradual increase in compliance in all clinical departments from 2006 (25%) to 2008 (59%). When a snapshot audit was performed in early August 2009, the overall hand hygiene compliance of hospital staff was consistently between 50 and 60%. The consumption of alcohol-based hand rub increased from 0.53 L per 100 admissions in 2006 to 7.78 L per 100 admissions in 2008. Since the gradual implementation of the infection control bundle to the full in May, 13.08 L per 100 admissions of alcohol-based hand rub have been consumed during the first six months of 2009.
Admission of patients for investigation for S-OIV
During the first 100 days (1 May to 8 August 2009) after the first case of S-OIV had occurred in Hong Kong, a total of 311 inpatients in Queen Mary Hospital were tested for S-OIV by RT-PCR. A total of 225 patients were admitted in phase 1, 71 in phase 2, and 15 in phase 3. There were 144 males and 167 females, including five pregnancies. The median age was 29 years (range: 53–87). They were admitted because of ILI after returning from countries with S-OIV (N = 163), asymptomatic close contact to confirmed cases (N = 65), local residents with confirmed S-OIV with or without risk factors for complications (N = 57), local residents with suspected S-OIV (N = 24), and symptomatic HCWs with history of contact with the confirmed patients (N = 2).
One hundred (32%) of 311 patients were positive for S-OIV by RT-PCR, with a male to female ratio of 47:53. The median age was 17 years (range: 6 months to 84 years). The confirmed cases were aged 13–19 years (45 cases, 45%), 20–39 years (20 cases, 20%), 6–12 years (13 cases, 13%), and ≤5 years (10 cases, 10%). Eight cases (8%) were aged 40–59 years, and four cases were aged ≥60 years. Fifty-four of 96 cases with ILI and four with mild pneumonia responded to oseltamivir. They were discharged at a median length of stay of four days (range: 1–32). Sixty-six patients were ethnic Chinese. Twenty-five patients were considered as imported cases and 75 patients as locally acquired, 27 of the latter due to local school outbreaks.
Contact tracing of patients and HCWs with confirmed S-OIV
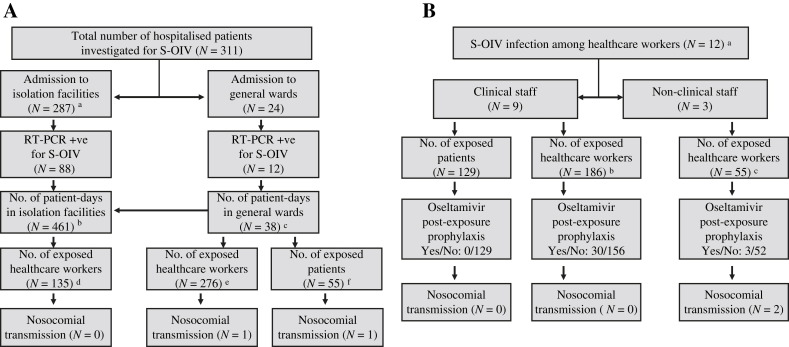
Twelve patients were diagnosed with S-OIV outside the isolation facilities in seven general wards from medical, paediatric, surgical, and orthopaedic specialties for a total of 38 patient-days, resulting in exposure of 55 inpatients in the same cubicle, and 276 HCWs serving in these wards (Figure 1 A). One infected house officer admitted lapses in infection control practice by not wearing a surgical mask and not using alcohol-based hand rub when setting up a peripheral venous catheter for a pre-symptomatic infected patient during the night call. The same index patient transmitted the infection to another patient staying in the same cubicle. Overall, nosocomial acquisition of S-OIV from hospitalised patients was two (0.43%) out of 466 exposed persons.
Figure 1.
(A) Contact tracing for hospitalised patients diagnosed to have swine-origin influenza virus (S-OIV) A H1N1. a Single room isolation until reverse transcription–polymerase chain reaction (RT-PCR) result available in phase 1 and cohort nursing of confirmed cases in phase 2. b Median length of stay: 4 days (range: 1–32). c Median length of stay: 2 days (range: 1–8), before confirmation of S-OIV. d Twenty-one doctors, 77 nurses, 37 ward assistants. e Sixty-one doctors, 133 nurses, 67 ward assistants, nine physiotherapists, two occupational therapists, three radiographers, one pharmacist. f Oseltamivir post-exposure prophylaxis was given to 32 patients with unprotected exposure. (B) Contact tracing for healthcare workers diagnosed to have S-OIV A H1N1. a Four doctors, four nurses, one ward assistant, one dispenser, one technician, and one clerk. b Forty-nine doctors, 99 nurses, 38 ward assistants. c Two nurses, 17 ward assistants, three technicians, and 33 pharmacists/dispensers.
In addition to the house officer who acquired S-OIV at work, another 11 HCWs had community-acquired S-OIV infection in eight different units, including surgery, paediatric, orthopaedic surgery, obstetric and gynaecology, oncology, psychiatry, endoscopy room, and general office (Figure 1B). Since all of these HCWs wore a surgical mask and practised hand hygiene with alcohol-based hand rub during patient care, none of the 129 exposed patients required post-exposure prophylactic oseltamivir or developed URTI symptoms in the seven-day medical surveillance period. Thirty-three (13.7%) out of 241 exposed co-workers received post-exposure prophylactic oseltamivir for unprotected exposure during tea break, lunch and dinner with the index cases. Two HCWs (one dispenser and one workman in pharmacy) acquired S-OIV from another dispenser during the pre-symptomatic shedding period because surgical mask was not mandatory in non-clinical areas. The nosocomial infection of S-OIV among hospital staff was two (0.83%) out of 241 exposed HCWs.
A total of four (0.48%) out of 836 exposed persons acquired S-OIV in the hospital environment during the study period. Not wearing surgical mask by the exposed persons during contact with the index cases (4/4 vs 264/832, P = 0.010) or vice versa (4/4 vs 300/832, P = 0.017, Fisher's exact test) was found to be a significant risk factor for nosocomial infection of S-OIV (Table I ).
Table I.
Risk factors for acquisition of swine-origin influenza virus (S-OIV) A H1N1 inside hospital during contact tracing of 836 exposed persons with contact to 100 infected patients and 12 infected healthcare workers
| Exposed persons infected with S-OIVa | Exposed persons not infected with S-OIVb | P-value | |
|---|---|---|---|
| (N = 4) | (N = 832) | ||
| Exposed persons residing or working in isolation facilities | 1.000 | ||
| Yes | 0 | 135 (16.2%) | |
| No | 4 (100%) | 697 (83.8%) | |
| Exposed persons wearing surgical mask during contact with the index case | 0.010 | ||
| Yes | 0 | 568 (68.3%) | |
| No or cannot recall | 4 (100%) | 264 (31.7%) | |
| Index persons wearing surgical mask during contact with the exposed | 0.017 | ||
| Yes | 0 | 532 (63.9%) | |
| No | 4 (100%) | 300 (36.1%) | |
| Exposed persons practising hand hygiene with alcohol-based hand rub after contact with the index case | 0.094 | ||
| Yes | 1 (25%) | 573 (68.9%) | |
| No or cannot recall | 3 (75%) | 259 (31.1%) | |
| Exposed persons receiving oseltamivir prophylaxis | 1.000 | ||
| Yes | 0 | 65 (7.8%) | |
| No | 4 (100%) | 767 (92.2%) |
Laboratory-confirmed with S-OIV in three healthcare workers and one patient.
Exposed persons who were either asymptomatic (N = 820) or tested negative for S-OIV (N = 12) in a seven-day medical surveillance.
Discussion
Infection control is the most important strategy in the control of S-OIV in the hospital before the availability of influenza vaccination. The implementation of infection control bundle in the first 100 days after the arrival of S-OIV in Hong Kong appeared to have minimised the nosocomial transmission of S-OIV among both patients and HCWs resulting in a secondary attack rate of 0.48%. It was much lower than a rate of 10–45% as reported in a recent review of published data on hospital-acquired influenza.6 Enhancement of staff awareness of infection control practice in both the hospital and community is essential for the programme's success and avoidance of misunderstanding. Our infection control teaching session has provided coverage for more than 90% of hospital staff. Whenever patients or HCWs were confirmed with S-OIV, the infection control team would visit the clinical and non-clinical units to explain the follow-up measures to the affected personnel. In particular, when the HCWs were undergoing medical surveillance, they were strongly advised not to have tea, lunch, and dinner with other co-workers because the shedding of influenza virus could occur one day before onset of symptoms. This measure is important for stopping the chain of transmission among HCWs. Therefore, HCWs were released from clinical duty and required to seek medical consultation for appropriate testing. Such measures radically changed the past practice of not collecting nasopharyngeal specimens in our staff clinic.
One HCW acquired S-OIV infection at the clinical area when he forgot to wear a surgical mask at night call. This shows that the routine use of surgical masks in all clinical areas is important in reducing the risk of nosocomial infection of S-OIV. According to our analysis, use of surgical masks by the exposed persons was associated with a lower risk of nosocomial acquisition of S-OIV, which was consistent with previous studies suggesting that wearing a surgical mask might decrease the risk of SARS infection.7 This measure discouraged the use of fingers to touch the mucous membranes of the nostrils and mouth since such spontaneous behaviour was not infrequently observed.8 Wearing surgical masks by the index persons as a form of source control also reduced the risk of transmission. As the use of surgical mask was mandatory for HCWs while in clinical areas, none of the 129 patients exposed to the clinical staff diagnosed with S-OIV during work acquired the infection despite not wearing masks themselves.
Self-reporting of compliance to hand hygiene by exposed persons was up to 70%, which was higher than the rate of 50–60% by unobtrusive hand hygiene observation during the study period. It may be related to recall bias by the exposed person. Unexpectedly the practice of hand hygiene after contact with the index cases did not reach statistical significance in reducing nosocomial infection of S-OIV in our study. This could be due to the small sample size in the group of infected persons. In fact, hand hygiene practice by using alcohol-based hand rub has been consistently promoted in our hospital since 2006 and directly observed hand hygiene (DOHH) has been strategically implemented during outbreaks. DOHH has been shown to be effective in reducing the frequency and the number of persons involved in the nosocomial outbreaks in our previous studies.2, 9
There are several limitations to this study. First, it was a quasi-experimental design to assess the nosocomial transmission of S-OIV, based on the epidemiological analysis of clinical symptoms of the exposed persons without serological confirmation. This might have underestimated the true incidence of nosocomial transmission. Second, the gradual decrease in the incidence of nosocomial influenza A and other respiratory virus infections may also be attributable to our ongoing promotion of infection control practice, which has been intensified at the time of the S-OIV pandemic. Third, the sustainability of this programme remains questionable since the workload of laboratory staff, infection control team, and the frontline HCWs has greatly increased during the current pandemic.
The recent finding of viral pneumonitis in animal models and mortality in immunocompetent young adults due to S-OIV have reminded us that this novel virus may be more pathogenic than seasonal influenza virus.10 As the first wave in the summer may be mild, this infection control bundle may play an even more impartant role during the second wave of this pandemic in the winter time.
Conflict of interest statement
None declared.
Funding source
This work was partly funded by Research Fund for the Control of Infectious Diseases.
References
- 1.Peiris J.S., Chu C.M., Cheng V.C. Clinical progression and viral load in a community outbreak of coronavirus-associated SARS pneumonia: a prospective study. Lancet. 2003;361:1767–1772. doi: 10.1016/S0140-6736(03)13412-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cheng V.C., Wu A.K., Cheung C.H. Outbreak of human metapneumovirus infection in psychiatric inpatients: implications for directly observed use of alcohol hand rub in prevention of nosocomial outbreaks. J Hosp Infect. 2007;67:336–343. doi: 10.1016/j.jhin.2007.09.010. [DOI] [PubMed] [Google Scholar]
- 3.Lau S.K., Chan K.H., Yip C.C. Confirmation of the first Hong Kong case of human infection by novel swine origin influenza A (H1N1) virus diagnosed using ultrarapid, real-time reverse transcriptase PCR. J Clin Microbiol. 2009;47:2344–2346. doi: 10.1128/JCM.00924-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chan K.H., Maldeis N., Pope W. Evaluation of the Directigen FluA + B test for rapid diagnosis of influenza virus type A and B infections. J Clin Microbiol. 2002;40:1675–1680. doi: 10.1128/JCM.40.5.1675-1680.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pittet D., Allegranzi B., Boyce J. The World Health Organization guidelines on hand hygiene in health care and their consensus recommendations. Infect Control Hosp Epidemiol. 2009;30:611–622. doi: 10.1086/600379. [DOI] [PubMed] [Google Scholar]
- 6.Voirin N., Barret B., Metzger M.H., Vanhems P. Hospital-acquired influenza: a synthesis using the Outbreak Reports and Intervention Studies of Nosocomial Infection (ORION) statement. J Hosp Infect. 2009;71:1–14. doi: 10.1016/j.jhin.2008.08.013. [DOI] [PubMed] [Google Scholar]
- 7.Wu J., Xu F., Zhou W. Risk factors for SARS among persons without known contact with SARS patients, Beijing, China. Emerg Infect Dis. 2004;10:210–216. doi: 10.3201/eid1002.030730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hendley J.O., Wenzel R.P., Gwaltney J.M., Jr. Transmission of rhinovirus colds by self-inoculation. N Engl J Med. 1973;288:1361–1364. doi: 10.1056/NEJM197306282882601. [DOI] [PubMed] [Google Scholar]
- 9.Cheng V.C., Tai J.W., Ho Y.Y., Chan J.F. Successful control of norovirus outbreak in an infirmary with the use of alcohol-based hand rub. J Hosp Infect. 2009;72:370–371. doi: 10.1016/j.jhin.2009.04.021. [DOI] [PubMed] [Google Scholar]
- 10.Itoh Y., Shinya K., Kiso M. In vitro and in vivo characterization of new swine-origin H1N1 influenza viruses. Nature. 2009;460:1021–1025. doi: 10.1038/nature08260. [DOI] [PMC free article] [PubMed] [Google Scholar]