Bronchiolitis is a lower respiratory tract infection of young children. Clinical features include expiratory wheezing, tachypnea, and hypoxia caused by obstruction of the small airways [1]. It has been suggested that the diagnosis of bronchiolitis would apply only to infants, but the use of the diagnosis varies greatly. In many clinical studies, all wheezing illnesses other than asthma in children younger than 3 years of age have been diagnosed as bronchiolitis. Asthma is a chronic inflammation of the airways, and clinically an acute asthma attack mimics bronchiolitis. The diagnosis of asthma should be used only after recurrent reversible wheezing episodes [2].
In the United States alone, an estimated 3% of all children are hospitalized for bronchiolitis in their first year of life, which is equivalent to more than 100,000 hospitalizations annually. Retrospective, hospital record–based studies have found that the prevalence of bronchiolitis increased in the 1980s and 1990s [3], [4]. The prevalence of asthma also has increased—from 3.6% to 6.2% from 1980 to 1996. From 1997 to 2000, the prevalence of asthma attacks has remained unchanged, however, suggesting that the burden from childhood asthma may have plateaued [5].
The relationship between bronchiolitis and the development of asthma and atopy (ie, immediate-type hypersensitivity) has been studied for many years. The development of atopy is particularly interesting because the persistent form of asthma is mainly atopic. It has been estimated that 50% of children with bronchiolitis have recurrent wheezing (assessed by the parents) or asthma (diagnosed by a physician) during the following 2 decades of life. The association between bronchiolitis and atopy defined as specific IgE antibodies or a positive skin prick test has been weak [6], [7].
Genetics and environmental influences, such as respiratory viral infections and allergen exposure, are associated closely with airway hyperreactivity. Respiratory viral infections predisposing to chronic asthma occur during infancy when immunologic maturation has not yet developed fully. It is crucial to understand how these different factors may contribute to the onset of asthma ( Fig. 1). It is well established that respiratory syncytial virus (RSV) bronchiolitis is associated strongly with recurrent wheezing and asthma, at least during the first decade of life [7], [8]. Preliminary findings suggest that rhinovirus-induced bronchiolitis is an even stronger risk factor and may be the first sign of asthma [9].
Fig. 1.
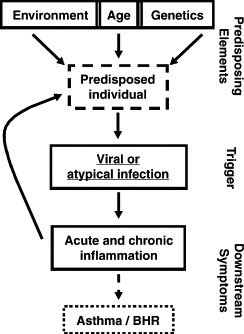
Multifactorial influences on the development of asthma. BHR, bronchial hyperreactivity. (From Openshaw PJ, Yamaguchi Y, Tregoning JS. Childhood infections, the developing immune system, and the origins of asthma. J Allergy Clin Immunol 2004;114:1276; with permission.)
Viral etiology of bronchiolitis and asthma
Many respiratory viruses can cause bronchiolitis ( Table 1). Many studies from the 1970s and 1980s have shown that RSV is the dominant causative agent, and it is virtually the only agent inducing epidemics. RSV infection has been detected in 50% to 70% of patients with bronchiolitis [10], [12], [14]. RSV is a rare pathogen in older hospitalized children [10], [14] because nearly all children have been infected with RSV within the first 2 years of life, and a child's initial RSV infection is typically the most severe. RSV epidemics usually begin yearly in the late fall and peak in November to March. In some countries, such as Finland, RSV infections occur in double-humped outbreaks in 2-year cycles [1], [14].
Table 1.
Studies on viral etiology of bronchiolitis
| Wheezing episodes/control subjects | Viral identification rates (%) |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Year of studya | Age (mo) | RSV | Rhinovirus | Enteroviruses | Parainfluenza virus | Influenza virus | Adenovirus | Coronavirus | hMPV | Total positive | |
| 1999 [10] | 22/17 | <24 | 68/0 | 41/41b | 1/0 | 0/1 | 0/1 | 82 | |||
| 2000 [11] | 84 | <12 | 54 | 19 | 12 | 0 | 0 | 13 | 0 | 74 | |
| 2002 [12] | 118 | <18 | 53 | 21 | 3 | 3 | 8 | 3 | 74 | ||
| 2003 [9] | 81 | <24 | 26 | 33 | 12 | 14 | 5 | 0 | 73 | ||
| 2004 [13], [14]c | 71 | 3–12 | 55 | 18 | 14 | 6 | 3 | 1 | 1 | 11 | 90 |
| 179/17 | 3–36 | 36 | 21/0d | 21/0d | 5 | 2 | 5 | 2 | 7 | 87 | |
Abbreviations: hMPV, human metapneumovirus; RSV, respiratory syncytial virus.
Including studies using polymerase chain reaction with a sampling period of >1 year.
With polymerase chain reaction; 23/25 using culture only.
New subgroup analysis.
Nontypable rhino-enterovirus: 15/18.
Studies using polymerase chain reaction (PCR) techniques have shown a prominent role for rhinovirus and enteroviruses in the etiology of acute bronchiolitis. Rhinovirus has been detected in 20% to 40% and enteroviruses in 10% to 20% of cases [10], [14]. Rhinovirus bronchiolitis often occurs during RSV epidemics. The clinical value of positive picornavirus PCR test has been questioned because RNA of these viruses has been detected in 20% to 40% of asymptomatic children [10], [13]. The authors have found that the degree of picornavirus PCR positivity markedly decreases over 2 to 3 weeks and disappears over 5 to 6 weeks after an acute wheezing episode, suggesting that positive picornavirus PCR is related to an acute symptomatic infection [13]. Rhinovirus outbreaks occur during the fall and spring, and enterovirus outbreaks usually occur only during the fall [14].
Viral respiratory infections commonly are associated with acute asthma ( Table 2). Rhinovirus is the main trigger of exacerbations, associated with 30% to 80% of cases. The community study of Johnston et al [15] reported picornaviruses by PCR in half of the cases with decreased peak expiratory flow. Of these, 57% were confirmed as rhinovirus by culture. Two studies have focused on viral etiologies in young children with acute asthma [14], [16]. Rhinovirus was found as an important viral agent in this patient group with a recovery rate of 27% to 44%.
Table 2.
Studies on viral etiology of acute asthma
| Wheezing episodes/control subjects | Viral identification rates (%) |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Year of studya | Age (y) | RSV | Rhinovirus | Enteroviruses | Parainfluenza virus | Influenza A/B virus | Adenovirus | Coronavirus | hMPV | Total positive | |
| 1995 [15] | 161 | 9–11 | 4 | 50b | 7 | 7 | 13 | 80c | |||
| 1999 [10]d | 48/42 | 2–16 | 6/0 | 71/36e | 2/0 | 2/0 | 2/0 | 6/0 | 83 | ||
| 1999 [16] | 71 | ≤2 | 24 | 44 | 10f | 4f | 10f | 5f | 5f | 86 | |
| 61 | >2 | 18 | 51 | 77 | |||||||
| 2003 [17] | 179 | 0.1–17 | 7 | 79 | 1 | 2 | 2 | 88 | |||
| 2004 [13], [14]g | 49/17 | 0.4–3 | 22 | 27/0 | 33/0 | 4 | 4 | 12 | 0 | 0 | 90 |
| 65/25 | 3–16 | 8 | 31/0h | 38/0h | 9 | 2 | 2 | 2 | 0 | 91 | |
Abbreviations: hMPV, human metapneumovirus; RSV, respiratory syncytial virus.
Including studies using polymerase chain reaction with a sampling period of >1 year.
Number is for picornaviruses, of which 57% were rhinoviruses, the remaining viruses could not be cultured and were classified as rhinoviruses because most enteroviruses culture easily.
In reported falls in peak expiratory flow.
Children with wheezing were included. Excluded were children with bronchopulmonary dysplasia or using corticosteroids within the previous week.
With polymerase chain reaction; 18/24% using culture only.
Information not available of different age groups.
New subgroup analyses.
Nontypable rhino-enterovirus: 12/18 in children age <3 y and 17/12 in children age ≥3 y.
In the authors' study, enteroviruses, which according to their name replicate most prolifically in the gastrointestinal tract, were related to acute asthma in 38% of the cases [14]. This finding is in agreement with the report of Rawlinson et al [17], who found enteroviruses by PCR in 29% of young children with well-documented asthma occurring in summer. Coronavirus has been found in 2% to 13% of children with acute exacerbations of asthma [10], [14], [15], [16]. Other viruses account for 10% or less of the cases. Influenza viruses, which yearly circulate in the community, are less important. RSV is a rare causative agent of acute asthma in children older than age 2 years.
Pathogenesis of bronchiolitis
The major risk factors for bronchiolitis include young age, passive smoke exposure, small lung size, a chronic underlying condition, and having older siblings [18], [19]. Genetic background also may influence the response to RSV infection because factors such as the polymorphisms of interleukin (IL)-8, IL-4, and its receptor and the surfactant protein D have been implicated in disease susceptibility and severity [20], [21], [22], [23].
The viral infection begins with viruses infecting airway epithelial cells, which are the primary site of replication. Numerous cytokines and chemokines are produced, which recruit and activate inflammatory cells. Innate and adaptive immune responses are triggered. The damaged airway together with an antiviral response causes epithelial edema and increased mucus production and vascular permeability. These events lead to narrowing of small bronchioles and consequent airway dysfunction and wheezing.
RSV infection activates signaling pathways in airway epithelium through a toll-like receptor 4 and by the generation of oxidative stress [24], [25]. Host cell recognition of viral RNA produced by viral replication initiates antiviral and proinflammatory responses within the cell. When newly synthesized viruses are released into the airway, the antiviral response is enhanced by mononuclear cells. Monocytes, macrophages, and probably dendritic cells secrete proinflammatory cytokines, such as IL-1, IL-8, tumor necrosis factor (TNF)-α, and interferon (IFN)-α, which further activate other cells and induce adhesion molecules. Because most recruited cells are neutrophils, it has been suggested that neutrophils and their activation products are important in the causation of airway obstruction. A few recruited cells are mononuclear cells and eosinophils. Lymphocytes also are recruited into the airways during the early stages of infection and are probably important in limiting the extent of infection and clearing virus-infected cells. Th1 and Th2 type responses have been implicated in infants with RSV bronchiolitis [26].
Most rhinoviruses are recognized by the intercellular adhesion molecule 1 (ICAM-1) [27], [28]. The rhinovirus-induced antiviral response, as in the case in RSV infection, is considered to be responsible for the clinical symptoms. The antiviral response to rhinovirus infection includes type 1 interferons and nitric oxide and the production of cytokines and chemokines, such as IL-1α/β, IL-8, IL-10, TNF-α, granulocyte-macrophage colony-stimulating factor, epithelial neutrophil-activating protein-78, RANTES (regulated on activation, normal T cell expressed and secreted), eotaxin 1/2, macrophage-inflammatory proteins, and leukotrienes, which influence the subsequent innate and specific immune response. Interferons are especially important in antiviral response because they are potent activators of antiviral effector cells as natural killer cells, CD8 T lymphocytes, and macrophages. The cellular response is mainly neutrophilic, but mast cells and eosinophils also infiltrate the infection site. Bronchial mucosal eosinophilic infiltrates have been found in biopsy specimens from healthy and asthmatic volunteers during an experimental rhinovirus infection [29]. The accumulation of eosinophils is influenced by IL-5, granulocyte-macrophage colony-stimulating factor, IL-8, RANTES, and eotaxin [27]. All of these except IL-5 have been produced by airway epithelial cells in vitro after a rhinovirus infection.
So far, only one report has been published comparing virus-specific inflammatory cell responses in bronchiolitis patients age younger than 2 years. According to Korppi et al [30], children with rhinovirus infection compared with children with RSV infection were older and presented more often with atopic dermatitis and blood eosinophilia. The groups did not differ in total serum IgE. Similarly, the authors have found a mean peripheral blood eosinophil count of 0.11 × 109/L and a neutrophil count of 3.48 × 109/L in children age younger than 2 years with the first wheezing attack induced by RSV, whereas in children with picornavirus bronchiolitis, the corresponding counts were 0.42 × 109/L and 5.87 × 109/L (unpublished findings). Although it is impossible to determine whether these differences are due to the virus, or whether they are related to a preexisting inflammation, there are great cellular differences in the courses of rhinovirus and RSV bronchiolitis. Eosinophilia and even neutrophilia in the peripheral blood are associated more clearly with rhinovirus bronchiolitis than RSV bronchiolitis.
Genetics of airway hyperreactivity
A parental history of childhood respiratory problems is an important risk factor for infantile lower respiratory tract illnesses. In the Tucson Children's Respiratory Study, the greatest risk was early onset of the parental illness [31]. A parental history of asthma or bronchiolitis with onset before age 3 years was associated with wheezing illnesses in offspring. The continuation of wheezing from early life until age 6 years also has a clear association with a maternal history of asthma and atopy [18]. Although these findings suggest a familial component in childhood wheezing, epidemiologic studies do not answer the question whether the risk is inherited genetically.
Since the 1990s, significant progress has been made in identifying the genes responsible for the development of asthma and atopy [32], [33], [34]. There are probably many susceptibility genes that act either alone or in combination with other genes increasing the risk of the disease [35]. Genetic studies are confounded by influences of genetic heterogeneity, heterogeneous phenotypes of asthma among the studied subjects, incomplete or low penetrance (despite a relatively high prevalence), and genotype-environment and gene-gene interactions [32], [36].
Reviews of genetic association studies have linked 60 genes to asthma [33], [34]. Of these, more than 30 have been replicated at least once, but less than 10 have been replicated in five or more studies. Many previous studies of the genetics of asthma have been criticized for being underpowered in view of the relatively modest effects of the individual genes. Nonreplicated studies may represent false-positive findings. Four interesting candidate genes have been highlighted in asthma, however. A role has been suggested for ADAM33 in airway remodeling and smooth muscle reactivity; for PHF11, in immunoregulation, especially that of B lymphocytes; for DPP10, in cytokine processing, especially in T cells; and for GPRA, in bronchial epithelial and smooth muscle surface receptor functions. Although it is difficult to separate atopy and bronchial hyperreactivity because of similarities in their regulatory networks, a definite genetic effect occurs in the two diseases.
Genetic predisposition to strong proinflammatory or weak anti-inflammatory capacity may increase the risk for atopic diseases. Regulatory T cells, which mediate their effects through anti-inflammatory cytokines, such as IL-10 and transforming growth factor-β, are able to suppress Th1 and Th2 cells [37]. Lower levels of IL-10 and transforming growth factor-β have been reported in asthmatic and atopic individuals [38], [39], whereas proinflammatory cytokines, such as the IL-1α genotype, have been more frequent in atopic subjects [40]. The matter is still controversial, however [41]. IL-10 responses have been highly influenced by viral infections in atopic asthmatics, and the responses in infants also seem to be different from responses in adults [42], [43].
Environmental influences on the immunopathogenesis of atopic asthma
Atopy and asthma may not be explained simply by the Th1/Th2 paradigm or abnormal proinflammatory or anti-inflammatory capacity, as suggested in a report by Heaton et al [36]. These investigators showed mixed Th1/Th2 immune responses in wheezing children. Although they confirmed that allergic diseases and asthma are associated with Th2 production (IL-5, eosinophilia, IgE production), they also showed that IFN-γ was associated with increased immediate skin test reactivity and with airway hyperreactivity [36]. These findings are in agreement with findings from a mouse model, suggesting that Th1 responses could increase the severity of allergic diseases and asthma [44]. IL-10 has been found to have a protective effect inhibiting immediate skin test reactions, as also stated earlier, but it also is associated with airway hyperreactivity in children without allergies and may increase the severity of airway disease in these subjects. The diversity of these responses may indicate that atopy is influenced not only by genetic heterogeneity, but also environmental effects, such as infections or exposure to allergens, which occur with varying intensities in different individuals [45].
Since 1989, the scope of the hygiene hypothesis has extended to environmental microbial burdens in general and the regulation of the pattern of immune responses in early life [46], [47]. Exposure to various environmental factors may influence inherited susceptibility to asthma and allergies by either increasing or decreasing the penetrance of predisposing genes. Interaction of viral infections in early life may be particularly important because RSV has been found to be an independent risk factor for the development of asthma [7], [8], [48], and a preliminary report suggests that this risk may be reduced by postponing the first RSV infection with RSV immunoglobulin [49]. IL-4 producing T cells responding to RSV and cat antigens have been reported to be more frequent in 7- and 8-year-old children with a history of RSV bronchiolitis [50], suggesting that early viral infection may affect Th2 polarization.
Repeated exposure to allergens may lead to the development of manifest atopy in early life, especially in immunologically susceptible individuals. Exposure to allergen during the first 2 years of life has predicted asthma better than exposure later in childhood [51]. A prophylactic reduction of exposure to house dust mite from birth has reduced the risk for wheeze and subsequent sensitization at school age [52]. It has been suggested that no other major factors independent of atopic status determine persistent childhood asthma, defined as bronchial hyperreactivity [18], [53]. In these children, symptoms can be produced with inhalation of allergen and reduced by moving the child to an allergen-free environment [54]. Respiratory viral infections are frequent triggers of acute bronchospasm also in allergic individuals, however [55]. Respiratory viral infections have been found to be more important seasonal triggers of exacerbations of asthma than pollen or spore counts [56].
Immunologic immaturity of infants
Human neonates exhibit decreased or aberrant innate, cellular, and humoral immune responses compared with adults. Many of the cells of the immune system are not intrinsically immature, but they lack the proper environmental influences to mount adult-type responses. The size of lymphocyte subpopulations shows marked changes during early life. The absolute number of B lymphocytes increases immediately after birth and remains unchanged until 2 years of age, then gradually decreases toward adult age. T lymphocytes increase after birth and decrease from 2 years to adulthood. Thymic involution starts at the age of 1 year and continues with a yearly loss of 3% [57]. The number of natural killer cells decreases over the first 2 months of life, then remains unchanged [58]. Cord blood has a decreased frequency of antigen-presenting dendritic cells, which present immunophenotypically with a higher degree of immaturity than adult dendritic cells [59]. Cord blood dendritic cells have a reduced ability to attain the mature adult phenotype and a reduced ability to activate naive CD4+ T cells to produce IFN-γ, suggesting that they are intrinsically preprogrammed against the generation of Th1 immune response [60].
Functionally, neonatal T cells proliferate poorly in response to antigenic and allogeneic stimulation. Human neonatal T cells produce lower levels of Th1 and Th2 cytokines than adult cells. It is well established that cord blood cells respond more with Th2 cytokines (IL-4, IL-5, IL-9, IL-13) and less with Th1 cytokines (IFN-γ, IL-2, IL-12, TNF-α). Poor production of IFN-γ may be attributed to decreased production of IL-12 [61]. Neonatal CD8 T cells produce high levels of IL-13, which may account for the type 2 bias [62]. It was reported that compared with adults, neonates have immature IL-10 and IFN-γ responses [63]. The neonatal Th1 response may not always be poorer than the adult Th1 response, but also can be dependent on the antigen [64].
During the first year of life, maternal IgG is replaced by neonatal IgM, IgG, and IgA. Many studies have shown that the primary T cell–dependent antibody response in the neonatal period is weak. Clinically, it is seen as a poor response to polysaccharide antigens. In a large study of 23-valent pneumococcal polysaccharide vaccine, poor antibody responses and no clinical protection were detected in children younger than 6 months of age [65]. A poor response to polysaccharide antigens coincides with the lack of marginal–zone CD21+ B cells and a high rate of cells coexpressing IgM and IgD [66].
Postnatal development of human immune responses is inadequately studied, but several single observations support the view that the human immune response mainly matures to the adult level during the first 2 years of life. B lymphocytes produce immunoglobulin levels close to adult levels, and the levels of lymphocyte subpopulations stabilize. The response to polysaccharide antigens can be detected in children older than age 2 years, and at the same age the histologic structure of the spleen is adult-type [66], [67]. By 1 year of age, almost all Vδ1 T cells in blood have activated and changed to memory T cells, reflecting immunologic maturation [68]. Mucosal immunity, immature at birth, usually develops fully in the first year of life [69]. It was reported that the recruitment of inflammatory cells to the nose is at full potential at age 2 years. The number of nasal Th2 driving (IL-4) and regulatory (IL-10) cytokine-positive cells has been found to decrease over the first 24 months of life [70].
All these observations support the view that the immune system is immature for the first 1 to 2 years of life and may be susceptible to delayed or permanent change induced by environmental factors, such as viral infections. It has been suggested that delayed maturation of immune responses may be a risk factor for allergies and asthma [71].
A neonatal mouse model has supported this hypothesis, showing that age at the first RSV infection determines the pattern of disease during reinfection in adulthood. The strongest Th2 responses were seen in mice primed at 1 day of age. Neonatal priming was followed by severe disease and an increased inflammatory cell response during reinfection at 12 weeks. Delayed priming led to less severe disease and enhanced IFN-γ production. RSV infection at a very young age has the potential to cause long-term alterations in the immune system [72]. It has been suggested further that chronicity or persistence of pathogens in the lung may perpetuate the inflammation, which is exacerbated by new infections [73]. This persistent inflammation drives the airway reactivity characteristic of asthma.
Development of recurrent wheezing and asthma after bronchiolitis
Long-term prospective studies have shown a link between bronchiolitis and asthma ( Table 3). In a study from the United Kingdom, Noble et al [82] monitored a cohort of 101 hospitalized infants with acute bronchiolitis (66% RSV positive) and 47 control infants. Abnormal pulmonary function still was found 9 to 10 years after hospitalization. Forced expiratory flow in 1 second (FEV1) and peak expiratory flow were 5% to 9% lower than in controls. Two to three times more episodes of wheeze and diagnosed asthma were reported in index children than in controls. The history of bronchiolitis was the only variable related to wheezing and diagnosis of asthma. Virus-specific (RSV positive or negative) analysis was not reported. No differences were found in histamine challenge and skin prick tests.
Table 3.
Important long-term studies of the link between bronchiolitis and reactive airway disease
| No. patients/controls | Viral etiology (%) | End points and results: patients versus controls (odds ratio, 95% CI P value) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| First authora | Year | Design | Age on entry (mo) | RSV | Other | Follow-up time (y) | Recurrent wheezing | Physician-diagnosed asthma | Abnormal pulmonary function test | Atopyb | |
| Sims [74] | 1978 | Ret | 35/35 | <12 | 100c | 8 | 51% vs 3% (P < .001)d | PEF 237 L/mm vs 265 L/min (P < .02) | NS | ||
| Pullan [75] | 1982 | Ret | 130/111 | <12 (mean 4) | 100c | 10 | 42% vs 19% (P < .001)d | NS | Exercise test or histamine challenge positive 25% vs 7% (P < .001) | NS | |
| Mok [76] | 1982 | Ret | 200/200 | <12 (mean 4) | 50c | 7 | 47% vs 17% (P < .01)d; 11% vs 1% (P < .01)e | 9% vs 2.5% (P < .05)d | FEV1 91% vs 95% predicted (P < .005), >10% fall after exercise 53% vs 37% (P < .05) | ||
| McConnochie [77] | 1984 | Ret | 59/177 | <24 | 8 | 44% vs 14% (P < 0.001)e | 25% vs 7% (P < 0.001)d, 19% vs 4% (P < 0.001)e | ||||
| McConnochie [78] | 1985 | Ret | 25/25 | <24 | 8–12 | FEF25–75 baseline 64% vs 75% predicted (P = .04), after cold air −9.0% vs −15.7% (P = .04) | |||||
| McConnochie [79] | 1989 | Ret | 51/102 | <24 | 13 | NS | NS | ||||
| Carlsen [80] | 1987 | Pro | 51/24 | <1 | 61 | 6f | 2 | No. episodes, median 3 vs 0 (P < .01) | NS | ||
| Osundwa [81] | 1993 | Ret | 70/70 | Mean 4 (range 3–8) | 100c | 2 | 44% vs 12.9% (P = .001)d | ||||
| Noble [82] | 1997 | Pro | 61/47 | Mean 4 (range 1–12) | 66 | 9–10 | 34% vs 13% (3.6, 1.3–9.8, P = .018)d | 39% vs 13% (4.4, 1.6–12, P = .004)e | Baseline PEF 93% vs 102% predicted (95% CI of differences 4.1–13.4, P < .001), FEV1 91% vs 96% predicted (0.5–9.6, P = .03) | NS | |
| Stein [7] | 1999 | Pro | 68/669 | <36 | 44 | 28g | 6 | Year 11: RSV 2.4, 1.3–4.6, P ≤ .01d; Year 13: other virus 3.1, 1.3–7.6, P ≤ .01d; negative test 2.1, 1–4.3, P ≤ .05d | Year 11: RSV FEV1 baseline 2.1, 2.1–2.2, P ≤.001; negative test 2.1, 2.1–2.2, P ≤.05 | NS | |
| 56/545 | 8 | ||||||||||
| 79/634 | 11 | ||||||||||
| 49/469 | 13 | ||||||||||
| Weber [83] | 1999 | Pro | 105/105 | Median 4 (quartiles 2–6) | 3 | 10% vs 1%e (IRR 7.4, 5.1–17.5)d | |||||
| Kneyber [84] | 2000 | Meta-analysish | 117/163 | <12 | 0–100 | <5 | <5 y: 36% vs 6% (5.5, 2.4–12.6)d; ≥5 y: 6% vs 3% (2.4, 0.7–8.4)d | NS | |||
| 230/321 | <12 | ≥5 | |||||||||
| Kotaniemi-Syrjänen [9] | 2003 | Pro | 44 | 1–24 | 23c | 45ci | 6 | 4.1, 1–16.8 (P = .047)j | NS | ||
| Sigurs [8], [48] | 2000 | Pro | 47/93 | <12 (mean 4) | 100c | 7 | Year 7: 68% vs 34% (P < .001)d Year 13: NS | Year 13: 37% vs 5.4% (P < .001)d, 28% vs 3.3% (P < .001)e | Year 13: baseline FEV1/FVC 85% vs 88% predicted (P = .001), after β2-agonist 88% vs 89% predicted (P = .043), fall in FEV1 after dry air hyperventilation 6.1% vs 4.6% (P = .047), reversibility NS | Year 7: 41% vs 22% (P = .039) Year 13: NSk | |
| 2004 | 13 | ||||||||||
| Piippo-Savolainen [85] | 2004 | Pro | 54/45 | Median 10 (range 1–24) | 19 | 30% vs 11% (3.4, 1.1–10.1)el | Abnormal pulmonary function 36% vs 11% (4.5, 1.5–13.2)l | NSk | |||
Abbreviations: FEF25–75, forced expiratory flow at 25–75% range; FEV1, forced expiratory flow in 1 second; IRR, incidence rate ratio; NS, nonsignificant; PEF, peak expiratory flow; Pro, prospective; Ret, retrospective; RSV, respiratory syncytial virus.
Only the last positive reports are included of studies with many interim analyses.
Confirmed by specific IgE antibodies or skin prick test.
Inclusion criteria.
Cumulative.
Currently or previous year.
Parainfluenza n = 2 (4%), rhinovirus n = 1 (2%).
Parainfluenza n = 68 (14%), other viruses n = 68 (14%) (including adenovirus, influenza virus, cytomegalovirus, rhinovirus, bacterial and mixed infections), negative test n = 129 (27%).
Rhinovirus n = 20 and virus negative n = 14.
Between rhinovirus-positive and rhinovirus-negative cases.
For any test positive.
Comparison included pneumonia group, which is not shown.
The Tucson Children's Respiratory Study followed a large cohort of children from birth to 13 years of age [7]. Although bronchiolitis was not confirmed by a physician in all cases, RSV lower respiratory tract illnesses were associated with an increased risk of frequent wheeze by age 6. The risk decreased markedly with age and was not significant by age 13. The index cases had a lower FEV1 than controls, but no difference was seen in the bronchodilator response. RSV lower respiratory tract illness was not related to atopic status on the basis of skin prick tests or serum IgE concentrations.
In a Swedish study, 47 hospitalized RSV-positive infants were followed for 13 years, and the last interim analyses were performed at age 7 years [8], [48]. The cumulative prevalence of asthma was 10 times higher, and the cumulative prevalence of any wheezing was almost twice more common in index cases than in controls at age 7 years. Allergic sensitization also was found almost twice more common in RSV children than in control subjects. At age 13 years, the occurrence of symptoms over the previous 12 months for asthma or recurrent wheezing was still more than five times higher in the RSV group than among the controls. RSV bronchiolitis had the highest independent risk ratio for current asthma. At this age, only borderline significance was found for sensitization to common inhaled allergens, however. Any positivity in skin prick tests or specific serum IgE concentrations did not show any difference, whereas dander-specific or pollen-specific tests showed a significant difference.
The longest prospective follow-up period of 19 years was reported in a Finnish study, which included 54 children hospitalized for bronchiolitis and 45 controls [85]. Two definitions for asthma were used: physician-diagnosed asthma and previously diagnosed asthma with recent asthmatic symptoms (physician-diagnosed asthma included). By these two definitions, asthma was present in 30% and 41% in the bronchiolitis group and in 11% in the control group. Lower baseline pulmonary function (ie, FEV1, FEV1/forced vital capacity, midexpiratory flow at 25% and 50% of forced vital capacity) was found in index cases, but no difference was seen on methacholine inhalation challenge. No significant difference was found in the prevalence of positive skin prick test reactions to common inhalant allergens. Sensitization to cat and dog dander was more than twice more common, however, in the bronchiolitis group than in the control group. The earlier studies reported a link between bronchiolitis and physician-defined asthma and abnormal pulmonary function occurring for 19 years [85]. For RSV bronchiolitis only, the link has been found to last for 13 years for physician-defined asthma [8]. No studies have reported a convincing link between bronchiolitis and current atopy after age 7 years.
Only two studies have evaluated the role of bronchiolitis induced by other viruses. Kotaniemi-Syrjanen et al [9] compared the development of asthma after RSV and rhinovirus bronchiolitis. An average of 6 years later, asthma was present in 10% of the RSV group subjects compared with 60% of the rhinovirus group subjects [9]. Although no significant difference was found, probably owing to small sample size, the difference was significant between rhinovirus-positive and rhinovirus-negative cases. The authors have confirmed the finding in a 1-year follow-up of hospitalized children after their first bout of bronchiolitis. Asthma (ie, three physician-verified wheezing attacks within 12 months) was diagnosed in 21% of children after an RSV infection and in 64% of children after rhinovirus infection [87]. These findings encourage a more critical approach to the earlier study of Stein et al [7]. They reported that RSV lower respiratory tract illness is an independent risk factor for the subsequent development of wheezing up to age 11 years, but not age 13. Stein et al [7] also reported, but did not adequately discuss, the finding that at age 13 years there still was a significant link between bronchiolitis and asthma in the groups of children with other virus or with negative microbiology. At age 11 years, the negative group also had lower FEV1 values. Rhinoviruses were not searched for by PCR techniques, and most of them probably were missed.
In addition to limited viral diagnostics, none of the aforementioned studies used or reported eosinophilia, which predicts the development of asthma, as an inclusion criterion or end point [88], [89]. Further limitations of earlier studies include the variable diagnostic criteria for bronchiolitis and asthma. Six studies have extended the age range of bronchiolitis over 12 months. The older the children are, the more likely they are to exhibit characteristics predisposing for persistent wheezing, such as eosinophilia, atopy, and rhinovirus infection [30]. The most common end points were recurrent wheezing reported by the parents and clinically diagnosed asthma, which are not reliable assessments and may overestimate lung impairment [2], [90]. Objective measurement of pulmonary function and physician-confirmed recurrent episodes of wheeze with a bronchodilator response are necessary for the diagnosis of asthma. Although baseline pulmonary function on follow-up is slightly decreased in the bronchiolitis groups of many studies, only two studies have shown evidence for bronchial hyperreactivity, characteristic of asthma [75], [78]. When considering the results of earlier studies and their limitations, a clear association can be seen between bronchiolitis and recurrent wheezing, but the association between bronchiolitis and atopic asthma is not yet convincing [91].
Rhinovirus-induced bronchiolitis may be a first sign of asthma, as suggested by Kotaniemi-Syrjanen et al [9]. In a cross-sectional emergency department study, rhinovirus-induced wheezing was associated most strongly with atopy and eosinophilia [10]. Children with rhinovirus bronchiolitis also have higher blood eosinophil counts than children with RSV, as discussed earlier [30], [89]. Immunologic events related to Th2 polarization seem to be important predisposing factors not only to the development of asthma, but also to rhinovirus-induced bronchiolitis. The link between these diseases is more likely to be attributable to this suggested immunologic anomaly that precedes or is induced by rhinovirus bronchiolitis rather than to structural damage to the airway as a result of bronchiolitis [19]. The hypothesis that airway inflammation before virus inoculation may be a risk factor for an adverse response to rhinovirus is supported by findings from an experimental rhinovirus infection in young adults with mild asthma. The study of Zambrano et al [92] has shown that subjects with high levels of IgE had greater lower respiratory tract symptom scores during the initial 4 days of the infection than the low IgE group. These subjects also had higher total blood eosinophil counts at baseline, increased eosinophil cationic protein in their nasal washes, and higher levels of expired nitric oxide at baseline and during peak cold symptoms. In agreement, another study has shown that on histamine challenge after experimental rhinovirus infection, allergic adult subjects show higher airway reactivity than healthy controls [93].
Why is rhinovirus infection such a common trigger of asthma exacerbations? First, the answer may be related to the expression of ICAM-1 by epithelial cells. ICAM-1 mediates viral binding, host infection, and antiviral response, and it is up-regulated by airway inflammation, including the rhinovirus infection itself [94]. Because Th2 cells predominate within the asthmatic airways, Bianco et al [95] addressed the question by studying the effects of Th2-associated and Th1-associated cytokines and experimental rhinovirus infections on ICAM-1 expression in epithelial cells in vitro. Th2-associated cytokines (IL-4, IL-5, IL-10, and IL-13) increased the ICAM-1 expression of uninfected and rhinovirus-infected cells, and these effects were dominant over the effects of IFN-γ. Second, a rhinovirus infection generates various inflammatory mediators, which probably enhance the ongoing inflammatory response in asthmatic airways at least in some circumstances [96]. Many studies have shown that rhinovirus infection can enhance lower airway histamine responses and eosinophil recruitment after allergen exposure [24]. Third, and probably most important, a defective IFN-γ response may not be able to limit the extent of the infection to upper airways. Peripheral blood mononuclear cells from atopic asthmatics have produced lower levels of IFN-γ and higher levels of IL-4 and IL-10 in response to rhinovirus infection than the cells from healthy subjects [42]. An experimental rhinovirus infection in atopic subjects has shown an inverse relationship between precold rhinovirus-induced IFN-γ secretion from peripheral blood mononuclear cells and peak virus shedding after inoculation [97]. Similarly, stronger Th1 responses in sputum cells (higher IFN-γ-to-IL-5 mRNA ratio) during induced colds have been found to be associated with milder cold symptoms and more rapid clearance of the virus [98]. Finally, the generation of IFN-γ has correlated directly with lung function [99]. These results together could explain why individuals with airway inflammation characterized by Th2 polarization and consequently by eosinophilia show increased susceptibility to and morbidity from rhinovirus infections and the associated exacerbation of asthma symptoms. The elevated secretion of IL-10 owing to rhinovirus infection may reduce partially the effectiveness of inflammatory mechanisms necessary for viral clearance and exacerbate airway inflammation and increase hyperreactivity.
Do viral infections contribute to sensitization to allergens? This is another important question, which cannot be answered exhaustively. Animal studies using experimental influenza virus, parainfluenza virus, and RSV infections have provided evidence that respiratory viral infections increase allergic sensitization to inhaled allergens and subsequently enhance airway inflammation, responsiveness, and obstruction [100], [101], [102], [103]. Respiratory viral infections have been shown to prevent tolerance induction and enhance IgE-mediated allergic sensitization to inhaled allergens when infection and sensitization have coincided. The possible mechanisms involve increased permeability of airway mucosa to allergens and the recruitment of dendritic cells to the respiratory epithelium. Consequently the sensitization could be facilitated by increased antigen uptake and more effective antigen presentation. It has been suggested that T cells, especially CD8+ T cells, IL-4, IL-5, and eosinophils are important regulators triggering airway hyperresponsiveness. Similarly, certain human respiratory viral infections may increase the risk of allergic sensitization by providing a local IL-4-high environment, as RSV studies show [50]. Recurrent respiratory viral infections with allergen exposure may contribute significantly to the onset of atopic asthma, but the full extent of these effects in humans remains to be evaluated.
Summary
Respiratory viral infections are closely related to bronchiolitis and acute asthma. RSV is the major causative agent of bronchiolitis in young infants, whereas rhinovirus is an important trigger of asthma exacerbations, but also is recognized increasingly in slightly older children with bronchiolitis. Bronchiolitis is followed by recurrent wheezing or asthma in 5% to 50% of children during the first 2 decades of life. Preliminary data indicate that rhinovirus bronchiolitis is a more potent risk factor for school-age asthma than RSV bronchiolitis. The authors hypothesize that an important link between bronchiolitis and asthma is a person's susceptibility to certain lower respiratory tract viral infections, especially to rhinovirus infection. This susceptibility probably is related to the strong genetic component of atopy and possibly to eosinophilic airway inflammation response, which are likely not only to increase susceptibility, but also to exacerbate rhinovirus infection. The immature immune system of children age 1 to 2 years may increase susceptibility to respiratory viral infections, and their immature immune system may be vulnerable to permanent change induced by environmental factors, such as viral infections. Recurrent respiratory viral infections, especially with allergen exposure, may contribute markedly to airway inflammation, airway hyperreactivity, and inception of atopic asthma. Lower respiratory tract rhinovirus infection in early life may be a first sign of asthma by identifying individuals with an underlying immunologic anomaly that predisposes to the development of asthma. Further studies are, needed to confirm this hypothesis.
Footnotes
The study was supported by the Turku University Foundation.
References
- 1.Ruuskanen O., Ogra P.L. Respiratory syncytial virus. Curr Probl Pediatr. 1993;23:50–79. doi: 10.1016/0045-9380(93)90003-U. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.National Asthma Education and Prevention Program Expert Panel Report Guidelines for the diagnosis and management of asthma: update on selected topics—2002. J Allergy Clin Immunol. 2002;110:S141–S219. [PubMed] [Google Scholar]
- 3.Shay D.K., Holman R.C., Newman R.D., Liu L.L., Stout J.W., Anderson L.J. Bronchiolitis-associated hospitalizations among US children, 1980–1996. JAMA. 1999;282:1440–1446. doi: 10.1001/jama.282.15.1440. [DOI] [PubMed] [Google Scholar]
- 4.Langley J.M., LeBlanc J.C., Smith B., Wang E.E. Increasing incidence of hospitalization for bronchiolitis among Canadian children, 1980–2000. J Infect Dis. 2003;188:1764–1767. doi: 10.1086/379740. [DOI] [PubMed] [Google Scholar]
- 5.Akinbami L.J., Schoendorf K.C. Trends in childhood asthma: prevalence, health care utilization, and mortality. Pediatrics. 2002;110:315–322. doi: 10.1542/peds.110.2.315. [DOI] [PubMed] [Google Scholar]
- 6.Forster J., Tacke U., Krebs H. Respiratory syncytial virus infection: its role in aeroallergen sensitization during the first two years of life. Pediatr Allergy Immunol. 1996;7:55–60. doi: 10.1111/j.1399-3038.1996.tb00107.x. [DOI] [PubMed] [Google Scholar]
- 7.Stein R.T., Sherrill D., Morgan W.J. Respiratory syncytial virus in early life and risk of wheeze and allergy by age 13 years. Lancet. 1999;354:541–545. doi: 10.1016/S0140-6736(98)10321-5. [DOI] [PubMed] [Google Scholar]
- 8.Sigurs N., Gustafsson P.M., Bjarnason R. Severe respiratory syncytial virus bronchiolitis in infancy and asthma and allergy at age 13. Am J Respir Crit Care Med. 2005;171:137–141. doi: 10.1164/rccm.200406-730OC. [DOI] [PubMed] [Google Scholar]
- 9.Kotaniemi-Syrjänen A., Vainionpää R., Reijonen T.M., Waris M., Korhonen K., Korppi M. Rhinovirus-induced wheezing in infancy—the first sign of childhood asthma? J Allergy Clin Immunol. 2003;111:66–71. doi: 10.1067/mai.2003.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rakes G.P., Arruda E., Ingram J.M. Rhinovirus and respiratory syncytial virus in wheezing children requiring emergency care: IgE and eosinophil analyses. Am J Respir Crit Care Med. 1999;159:785–790. doi: 10.1164/ajrccm.159.3.9801052. [DOI] [PubMed] [Google Scholar]
- 11.Andreoletti L., Lesay M., Deschildre A., Lambert V., Dewilde A., Wattre P. Differential detection of rhinoviruses and enteroviruses RNA sequences associated with classical immunofluorescence assay detection of respiratory virus antigens in nasopharyngeal swabs from infants with bronchiolitis. J Med Virol. 2000;61:341–346. doi: 10.1002/1096-9071(200007)61:3<341::AID-JMV10>3.0.CO;2-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Papadopoulos N.G., Moustaki M., Tsolia M. Association of rhinovirus infection with increased disease severity in acute bronchiolitis. Am J Respir Crit Care Med. 2002;165:1285–1289. doi: 10.1164/rccm.200112-118BC. [DOI] [PubMed] [Google Scholar]
- 13.Jartti T., Lehtinen P., Vuorinen T., Koskenvuo M., Ruuskanen O. Persistence of rhinovirus and enterovirus RNA after acute respiratory illness in children. J Med Virol. 2004;72:695–699. doi: 10.1002/jmv.20027. [DOI] [PubMed] [Google Scholar]
- 14.Jartti T., Lehtinen P., Vuorinen T. Respiratory picornaviruses and respiratory syncytial virus as causative agents of acute expiratory wheezing in children. Emerg Infect Dis. 2004;10:1095–1101. doi: 10.3201/eid1006.030629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Johnston S.L., Pattemore P.K., Sanderson G. Community study of role of viral infections in exacerbations of asthma in 9–11 year old children. BMJ. 1995;310:1225–1229. doi: 10.1136/bmj.310.6989.1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Freymuth F., Vabret A., Brouard J. Detection of viral, Chlamydia pneumoniae and Mycoplasma pneumoniae infections in exacerbations of asthma in children. J Clin Virol. 1999;13:131–139. doi: 10.1016/S1386-6532(99)00030-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rawlinson W.D., Waliuzzaman Z., Carter I.W., Belessis Y.C., Gilbert K.M., Morton J.R. Asthma exacerbations in children associated with rhinovirus but not human metapneumovirus infection. J Infect Dis. 2003;187:1314–1318. doi: 10.1086/368411. [DOI] [PubMed] [Google Scholar]
- 18.Martinez F.D., Wright A.L., Taussig L.M., Holberg C.J., Halonen M., Morgan W.J. Asthma and wheezing in the first six years of life. The Group Health Medical Associates. N Engl J Med. 1995;332:133–138. doi: 10.1056/NEJM199501193320301. [DOI] [PubMed] [Google Scholar]
- 19.Young S., O'Keeffe P.T., Arnott J., Landau L.I. Lung function, airway responsiveness, and respiratory symptoms before and after bronchiolitis. Arch Dis Child. 1995;72:16–24. doi: 10.1136/adc.72.1.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hull J., Thomson A., Kwiatkowski D. Association of respiratory syncytial virus bronchiolitis with the interleukin 8 gene region in UK families. Thorax. 2000;55:1023–1027. doi: 10.1136/thorax.55.12.1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lahti M., Löfgren J., Marttila R. Surfactant protein D gene polymorphism associated with severe respiratory syncytial virus infection. Pediatr Res. 2002;51:696–699. doi: 10.1203/00006450-200206000-00006. [DOI] [PubMed] [Google Scholar]
- 22.Hoebee B., Rietveld E., Bont L. Association of severe respiratory syncytial virus bronchiolitis with interleukin-4 and interleukin-4 receptor alpha polymorphisms. J Infect Dis. 2003;187:2–11. doi: 10.1086/345859. [DOI] [PubMed] [Google Scholar]
- 23.Heinzmann A., Ahlert I., Kurz T., Berner R., Deichmann K.A. Association study suggests opposite effects of polymorphisms within IL8 on bronchial asthma and respiratory syncytial virus bronchiolitis. J Allergy Clin Immunol. 2004;114:671–676. doi: 10.1016/j.jaci.2004.06.038. [DOI] [PubMed] [Google Scholar]
- 24.Gern J.E. Viral respiratory infection and the link to asthma. Pediatr Infect Dis J. 2004;23:S78–S86. doi: 10.1097/01.inf.0000108196.46134.a6. [DOI] [PubMed] [Google Scholar]
- 25.Heidema J., Kimpen J.L.L., van Bleek G.M. Pathogenesis of respiratory syncytial virus bronchiolitis: immunology and genetics. In: Kimpen J.L.L., Ramilo O., editors. Respiratory tract infections, pathogenesis and emerging strategies for control. Horizon Bioscience; Norfolk: 2004. pp. 233–252. [Google Scholar]
- 26.Tripp R.A., Moore D., Barskey A., 4th Peripheral blood mononuclear cells from infants hospitalized because of respiratory syncytial virus infection express T helper-1 and T helper-2 cytokines and CC chemokine messenger RNA. J Infect Dis. 2002;185:1388–1394. doi: 10.1086/340505. [DOI] [PubMed] [Google Scholar]
- 27.Message S.D., Johnston S.L. Host defense function of the airway epithelium in health and disease: clinical background. J Leukoc Biol. 2004;75:5–17. doi: 10.1189/jlb.0703315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ruuskanen O., Hyypiä T. Rhinovirus: is it really a relevant pathogen? In: Kimpen J.L.L., Ramilo O., editors. Respiratory tract infections, pathogenesis and emerging strategies for control. Horizon Bioscience; Norfolk: 2004. pp. 291–317. [Google Scholar]
- 29.Fraenkel D.J., Bardin P.G., Sanderson G., Lampe F., Johnston S.L., Holgate S.T. Lower airways inflammation during rhinovirus colds in normal and in asthmatic subjects. Am J Respir Crit Care Med. 1995;151:879–886. doi: 10.1164/ajrccm/151.3_Pt_1.879. [DOI] [PubMed] [Google Scholar]
- 30.Korppi M., Kotaniemi-Syrjänen A., Waris M., Vainionpää R., Reijonen T.M. Rhinovirus-associated wheezing in infancy: comparison with respiratory syncytial virus bronchiolitis. Pediatr Infect Dis J. 2004;23:995–999. doi: 10.1097/01.inf.0000143642.72480.53. [DOI] [PubMed] [Google Scholar]
- 31.Camilli A.E., Holberg C.J., Wright A.L., Taussig L.M. Parental childhood respiratory illness and respiratory illness in their infants. Group Health Medical Associates. Pediatr Pulmonol. 1993;16:275–280. doi: 10.1002/ppul.1950160502. [DOI] [PubMed] [Google Scholar]
- 32.Steinke J.W., Borish L., Rosenwasser L.J. Genetics of hypersensitivity. J Allergy Clin Immunol. 2003;111:S495–S501. doi: 10.1067/mai.2003.143. [DOI] [PubMed] [Google Scholar]
- 33.Weiss S.T., Raby B.A. Asthma genetics 2003. Hum Mol Genet. 2004;13:R83–R89. doi: 10.1093/hmg/ddh080. [DOI] [PubMed] [Google Scholar]
- 34.Kere J., Laitinen T. Positionally cloned susceptibility genes in allergy and asthma. Curr Opin Immunol. 2004;16:689–694. doi: 10.1016/j.coi.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 35.Xu J., Meyers D.A., Ober C., Collaborative Study on the Genetics of Asthma Genomewide screen and identification of gene-gene interactions for asthma-susceptibility loci in three US populations: collaborative study on the genetics of asthma. Am J Hum Genet. 2001;68:1437–1446. doi: 10.1086/320589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Heaton T., Rowe J., Turner S. An immunoepidemiological approach to asthma: identification of in-vitro T-cell response patterns associated with different wheezing phenotypes in children. Lancet. 2005;365:142–149. doi: 10.1016/S0140-6736(05)17704-6. [DOI] [PubMed] [Google Scholar]
- 37.Akdis C.A., Blesken T., Akdis M., Wuthrich B., Blaser K. Role of interleukin 10 in specific immunotherapy. J Clin Invest. 1998;102:98–106. doi: 10.1172/JCI2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Borish L., Aarons A., Rumbyrt J., Cvietusa P., Negri J., Wenzel S. Interleukin-10 regulation in normal subjects and patients with asthma. J Allergy Clin Immunol. 1996;97:1288–1296. doi: 10.1016/s0091-6749(96)70197-5. [DOI] [PubMed] [Google Scholar]
- 39.Arkwright P.D., Chase J.M., Babbage S., Pravica V., David T.J., Hutchinson I.V. Atopic dermatitis is associated with a low-producer transforming growth factor beta(1) cytokine genotype. J Allergy Clin Immunol. 2001;108:281–284. doi: 10.1067/mai.2001.117259. [DOI] [PubMed] [Google Scholar]
- 40.Karjalainen J., Hulkkonen J., Pessi T. The IL1A genotype associates with atopy in nonasthmatic adults. J Allergy Clin Immunol. 2002;110:429–434. doi: 10.1067/mai.2002.126784. [DOI] [PubMed] [Google Scholar]
- 41.Colavita A.M., Hastie A.T., Musani A.I. Kinetics of IL-10 production after segmental antigen challenge of atopic asthmatic subjects. J Allergy Clin Immunol. 2000;106:880–886. doi: 10.1067/mai.2000.110475. [DOI] [PubMed] [Google Scholar]
- 42.Papadopoulos N.G., Stanciu L.A., Papi A., Holgate S.T., Johnston S.L. A defective type 1 response to rhinovirus in atopic asthma. Thorax. 2002;57:328–332. doi: 10.1136/thorax.57.4.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.van Benten I.J., van Drunen C.M., Koevoet J.L. Reduced nasal IL-10 and enhanced TNFalpha responses during rhinovirus and RSV induced upper respiratory tract infection in atopic and non-atopic infants. J Med Virol. 2005;75:348–357. doi: 10.1002/jmv.20277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hansen G., Berry G., DeKruyff R.H., Umetsu D.T. Allergen-specific Th1 cells fail to counterbalance Th2 cell-induced airway hyperreactivity but cause severe airway inflammation. J Clin Invest. 1999;103:175–183. doi: 10.1172/JCI5155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Umetsu D.T. Revising the immunological theories of asthma and allergy. Lancet. 2005;365:98–100. doi: 10.1016/S0140-6736(05)17714-9. [DOI] [PubMed] [Google Scholar]
- 46.Strachan D.P. Hay fever, hygiene, and household size. BMJ. 1989;299:1259–1260. doi: 10.1136/bmj.299.6710.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rautava S., Ruuskanen O., Ouwehand A., Salminen S., Isolauri E. The hygiene hypothesis of atopic disease—an extended version. J Pediatr Gastroenterol Nutr. 2004;38:378–388. doi: 10.1097/00005176-200404000-00004. [DOI] [PubMed] [Google Scholar]
- 48.Sigurs N., Bjarnason R., Sigurbergsson F., Kjellman B. Respiratory syncytial virus bronchiolitis in infancy is an important risk factor for asthma and allergy at age 7. Am J Respir Crit Care Med. 2000;161:1501–1507. doi: 10.1164/ajrccm.161.5.9906076. [DOI] [PubMed] [Google Scholar]
- 49.Wenzel S.E., Gibbs R.L., Lehr M.V., Simoes E.A. Respiratory outcomes in high-risk children 7 to 10 years after prophylaxis with respiratory syncytial virus immune globulin. Am J Med. 2002;112:627–633. doi: 10.1016/s0002-9343(02)01095-1. [DOI] [PubMed] [Google Scholar]
- 50.Pala P., Bjarnason R., Sigurbergsson F., Metcalfe C., Sigurs N., Openshaw P.J. Enhanced IL-4 responses in children with a history of respiratory syncytial virus bronchiolitis in infancy. Eur Respir J. 2002;20:376–382. doi: 10.1183/09031936.02.00249902. [DOI] [PubMed] [Google Scholar]
- 51.Sporik R., Holgate S.T., Platts-Mills T.A., Cogswell J.J. Exposure to house-dust mite allergen (Der p I) and the development of asthma in childhood: a prospective study. N Engl J Med. 1990;323:502–507. doi: 10.1056/NEJM199008233230802. [DOI] [PubMed] [Google Scholar]
- 52.Arshad S.H., Bateman B., Matthews S.M. Primary prevention of asthma and atopy during childhood by allergen avoidance in infancy: a randomised controlled study. Thorax. 2003;58:489–493. doi: 10.1136/thorax.58.6.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sporik R., Ingram J.M., Price W., Sussman J.H., Honsinger R.W., Platts-Mills T.A. Association of asthma with serum IgE and skin test reactivity to allergens among children living at high altitude: tickling the dragon's breath. Am J Respir Crit Care Med. 1995;151:1388–1392. doi: 10.1164/ajrccm.151.5.7735590. [DOI] [PubMed] [Google Scholar]
- 54.Platts-Mills T.A., Rakes G., Heymann P.W. The relevance of allergen exposure to the development of asthma in childhood. J Allergy Clin Immunol. 2000;105:S503–S508. doi: 10.1016/S0091-6749(00)90051-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Green R.M., Custovic A., Sanderson G., Hunter J., Johnston S.L., Woodcock A. Synergism between allergens and viruses and risk of hospital admission with asthma: case-control study. BMJ. 2002;324:763. doi: 10.1136/bmj.324.7340.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Carlsen K.H., Orstavik I., Leegaard J., Hoeg H. Respiratory virus infections and aeroallergens in acute bronchial asthma. Arch Dis Child. 1984;59:310–315. doi: 10.1136/adc.59.4.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Steinmann G.G. Changes in the human thymus during aging. Curr Top Pathol. 1986;75:43–88. doi: 10.1007/978-3-642-82480-7_2. [DOI] [PubMed] [Google Scholar]
- 58.Comans-Bitter W.M., de Groot R., van den Beemd R. Immunophenotyping of blood lymphocytes in childhood. Reference values for lymphocyte subpopulations. J Pediatr. 1997;130:388–393. doi: 10.1016/s0022-3476(97)70200-2. [DOI] [PubMed] [Google Scholar]
- 59.Crespo I., Paiva A., Couceiro A., Pimentel P., Orfao A., Regateiro F. Immunophenotypic and functional characterization of cord blood dendritic cells. Stem Cells Dev. 2004;13:63–70. doi: 10.1089/154732804773099263. [DOI] [PubMed] [Google Scholar]
- 60.Langrish C.L., Buddle J.C., Thrasher A.J., Goldblatt D. Neonatal dendritic cells are intrinsically biased against Th-1 immune responses. Clin Exp Immunol. 2002;128:118–123. doi: 10.1046/j.1365-2249.2002.01817.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Marodi L. Down-regulation of Th1 responses in human neonates. Clin Exp Immunol. 2002;128:1–2. doi: 10.1046/j.1365-2249.2002.01873.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ribeiro-do-Couto L.M., Boeije L.C., Kroon J.S. High IL-13 production by human neonatal T cells: neonate immune system regulator? Eur J Immunol. 2001;31:3394–3402. doi: 10.1002/1521-4141(200111)31:11<3394::aid-immu3394>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 63.Kotiranta-Ainamo A., Rautonen J., Rautonen N. Imbalanced cytokine secretion in newborns. Biol Neonate. 2004;85:55–60. doi: 10.1159/000074959. [DOI] [PubMed] [Google Scholar]
- 64.Yu H.R., Chang J.C., Chen R.F. Different antigens trigger different Th1/Th2 reactions in neonatal mononuclear cells (MNCs) relating to T-bet/GATA-3 expression. J Leukoc Biol. 2003;74:952–958. doi: 10.1189/jlb.0902474. [DOI] [PubMed] [Google Scholar]
- 65.Mäkelä P.H., Sibakov M., Herva E. Pneumococcal vaccine and otitis media. Lancet. 1980;2:547–551. doi: 10.1016/s0140-6736(80)91989-3. [DOI] [PubMed] [Google Scholar]
- 66.Timens W., Boes A., Rozeboom-Uiterwijk T., Poppema S. Immaturity of the human splenic marginal zone in infancy: possible contribution to the deficient infant immune response. J Immunol. 1989;143:3200–3206. [PubMed] [Google Scholar]
- 67.Rijkers G.T., Sanders E.A., Breukels M.A., Zegers B.J. Infant B cell responses to polysaccharide determinants. Vaccine. 1998;16:1396–1400. doi: 10.1016/s0264-410x(98)00098-x. [DOI] [PubMed] [Google Scholar]
- 68.De Rosa S.C., Andrus J.P., Perfetto S.P. Ontogeny of gamma delta T cells in humans. J Immunol. 2004;172:1637–1645. doi: 10.4049/jimmunol.172.3.1637. [DOI] [PubMed] [Google Scholar]
- 69.Gleeson M., Cripps A.W. Development of mucosal immunity in the first year of life and relationship to sudden infant death syndrome. FEMS Immunol Med Microbiol. 2004;42:21–33. doi: 10.1016/j.femsim.2004.06.012. [DOI] [PubMed] [Google Scholar]
- 70.van Benten I.J., van Drunen C.M., Koopman L.P. Age- and infection-related maturation of the nasal immune response in 0–2-year-old children. Allergy. 2005;60:226–232. doi: 10.1111/j.1398-9995.2005.00684.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Martinez F.D., Holt P.G. Role of microbial burden in aetiology of allergy and asthma. Lancet. 1999;354:SII12–SII15. doi: 10.1016/s0140-6736(99)90437-3. [DOI] [PubMed] [Google Scholar]
- 72.Culley F.J., Pollott J., Openshaw P.J. Age at first viral infection determines the pattern of T cell-mediated disease during reinfection in adulthood. J Exp Med. 2002;196:1381–1386. doi: 10.1084/jem.20020943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Schwarze J., O'Donnell D.R., Rohwedder A., Openshaw P.J.M. Latency and persistence of respiratory syncytial virus despite T cell immunity. Am J Respir Crit Care Med. 2004;169:801–805. doi: 10.1164/rccm.200308-1203OC. [DOI] [PubMed] [Google Scholar]
- 74.Sims D.G., Downham M.A., Gardner P.S., Webb J.K., Weightman D. Study of 8-year-old children with a history of respiratory syncytial virus bronchiolitis in infancy. BMJ. 1978;1:11–14. doi: 10.1136/bmj.1.6104.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Pullan C.R., Hey E.N. Wheezing, asthma, and pulmonary dysfunction 10 years after infection with respiratory syncytial virus in infancy. BMJ. 1982;284:1665–1669. doi: 10.1136/bmj.284.6330.1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mok J.Y., Simpson H. Outcome of acute lower respiratory tract infection in infants: preliminary report of seven-year follow-up study. BMJ. 1982;285:333–337. doi: 10.1136/bmj.285.6338.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.McConnochie K.M., Roghmann K.J. Bronchiolitis as a possible cause of wheezing in childhood: new evidence. Pediatrics. 1984;74:1–10. [PubMed] [Google Scholar]
- 78.McConnochie K.M., Mark J.D., McBride J.T. Normal pulmonary function measurements and airway reactivity in childhood after mild bronchiolitis. J Pediatr. 1985;107:54–58. doi: 10.1016/s0022-3476(85)80614-4. [DOI] [PubMed] [Google Scholar]
- 79.McConnochie K.M., Roghmann K.J. Wheezing at 8 and 13 years: changing importance of bronchiolitis and passive smoking. Pediatr Pulmonol. 1989;6:138–146. doi: 10.1002/ppul.1950060303. [DOI] [PubMed] [Google Scholar]
- 80.Carlsen K.H., Larsen S., Bjerve O., Leegaard J. Acute bronchiolitis: predisposing factors and characterization of infants at risk. Pediatr Pulmonol. 1987;3:153–160. doi: 10.1002/ppul.1950030308. [DOI] [PubMed] [Google Scholar]
- 81.Osundwa V.M., Dawod S.T., Ehlayel M. Recurrent wheezing in children with respiratory syncytial virus (RSV) bronchiolitis in Qatar. Eur J Pediatr. 1993;152:1001–1003. doi: 10.1007/BF01957225. [DOI] [PubMed] [Google Scholar]
- 82.Noble V., Murray M., Webb M.S., Alexander J., Swarbrick A.S., Milner A.D. Respiratory status and allergy nine to 10 years after acute bronchiolitis. Arch Dis Child. 1997;76:315–319. doi: 10.1136/adc.76.4.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Weber M.W., Milligan P., Giadom B. Respiratory illness after severe respiratory syncytial virus disease in infancy in The Gambia. J Pediatr. 1999;135:683–688. doi: 10.1016/s0022-3476(99)70085-5. [DOI] [PubMed] [Google Scholar]
- 84.Kneyber M.C.J., Steyerberg E.W., de Groot R., Moll H.A. Long-term effects of respiratory syncytial virus (RSV) bronchiolitis in infants and young children: a quantitative review. Acta Pediatr. 2000;89:654–660. doi: 10.1080/080352500750043945. [DOI] [PubMed] [Google Scholar]
- 85.Piippo-Savolainen E., Remes S., Kannisto S., Korhonen K., Korppi M. Asthma and lung function 20 years after wheezing in infancy: results from a prospective follow-up study. Arch Pediatr Adolesc Med. 2004;158:1070–1076. doi: 10.1001/archpedi.158.11.1070. [DOI] [PubMed] [Google Scholar]
- 86.Sigurs N., Bjarnason R., Sigurbergsson F., Kjellman B., Bjorksten B. Asthma and immunoglobulin E antibodies after respiratory syncytial virus bronchiolitis: a prospective cohort study with matched controls. Pediatrics. 1995;95:500–505. [PubMed] [Google Scholar]
- 87.Lehtinen P, Ruohola A, Vuorinen T, Vanto T, Jartti T, Ruuskanen O. Development of asthma after first time viral bronchiolitis [Abstract]. Presented at Frontiers in Neonatal and Infant Immunity. Madrid, Spain, March 18–20, 2005.
- 88.Martinez F.D., Stern D.A., Wright A.L., Taussig L.M., Halonen M. Differential immune responses to acute lower respiratory illness in early life and subsequent development of persistent wheezing and asthma. J Allergy Clin Immunol. 1998;102:915–920. doi: 10.1016/s0091-6749(98)70328-8. [DOI] [PubMed] [Google Scholar]
- 89.Ehlenfield D.R., Cameron K., Welliver R.C. Eosinophilia at the time of respiratory syncytial virus bronchiolitis predicts childhood reactive airway disease. Pediatrics. 2000;105:79–83. doi: 10.1542/peds.105.1.79. [DOI] [PubMed] [Google Scholar]
- 90.Lowe L., Murray C.S., Martin L. Reported versus confirmed wheeze and lung function in early life. Arch Dis Child. 2004;89:540–543. doi: 10.1136/adc.2003.038539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Devulapalli C.S., Haaland G., Pettersen M., Carlsen K.H., Lodrup Carlsen K.C. Effect of inhaled steroids on lung function in young children: a cohort study. Eur Respir J. 2004;23:869–875. doi: 10.1183/09031936.04.00095304. [DOI] [PubMed] [Google Scholar]
- 92.Zambrano J.C., Carper H.T., Rakes G.P. Experimental rhinovirus challenges in adults with mild asthma: response to infection in relation to IgE. J Allergy Clin Immunol. 2003;111:1008–1016. doi: 10.1067/mai.2003.1396. [DOI] [PubMed] [Google Scholar]
- 93.Gern J.E., Calhoun W., Swenson C., Shen G., Busse W.W. Rhinovirus infection preferentially increases lower airway responsiveness in allergic subjects. Am J Respir Crit Care Med. 1997;155:1872–1876. doi: 10.1164/ajrccm.155.6.9196088. [DOI] [PubMed] [Google Scholar]
- 94.Papi A., Johnston S.L. Rhinovirus infection induces expression of its own receptor intercellular adhesion molecule 1 (ICAM-1) via increased NF-kappaB-mediated transcription. J Biol Chem. 1999;274:9707–9720. doi: 10.1074/jbc.274.14.9707. [DOI] [PubMed] [Google Scholar]
- 95.Bianco A., Sethi S.K., Allen J.T., Knight R.A., Spiteri M.A. Th2 cytokines exert a dominant influence on epithelial cell expression of the major group human rhinovirus receptor, ICAM-1. Eur Respir J. 1998;12:619–626. doi: 10.1183/09031936.98.12030619. [DOI] [PubMed] [Google Scholar]
- 96.Grunberg K., Timmers M.C., Smits H.H. Effect of experimental rhinovirus 16 colds on airway hyperresponsiveness to histamine and interleukin-8 in nasal lavage in asthmatic subjects in vivo. Clin Exp Allergy. 1997;27:36–45. doi: 10.1111/j.1365-2222.1997.tb00670.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Parry D.E., Busse W.W., Sukow K.A., Dick C.R., Swenson C., Gern J.E. Rhinovirus-induced PBMC responses and outcome of experimental infection in allergic subjects. J Allergy Clin Immunol. 2000;105:692–698. doi: 10.1067/mai.2000.104785. [DOI] [PubMed] [Google Scholar]
- 98.Gern J.E., Vrtis R., Grindle K.A., Swenson C., Busse W.W. Relationship of upper and lower airway cytokines to outcome of experimental rhinovirus infection. Am J Respir Crit Care Med. 2000;162:2226–2231. doi: 10.1164/ajrccm.162.6.2003019. [DOI] [PubMed] [Google Scholar]
- 99.Brooks G.D., Buchta K.A., Swenson C.A., Gern J.E., Busse W.W. Rhinovirus-induced interferon-gamma and airway responsiveness in asthma. Am J Respir Crit Care Med. 2003;168:1091–1094. doi: 10.1164/rccm.200306-737OC. [DOI] [PubMed] [Google Scholar]
- 100.Schwarze J., Mäkelä M., Cieslewicz G. Transfer of the enhancing effect of respiratory syncytial virus infection on subsequent allergic airway sensitization by T lymphocytes. J Immunol. 1999;163:5729–5734. [PubMed] [Google Scholar]
- 101.Schwarze J., Cieslewicz G., Joetham A. Critical roles for interleukin-4 and interleukin-5 during respiratory syncytial virus infection in the development of airway hyperresponsiveness after airway sensitization. Am J Respir Crit Care Med. 2000;162:380–386. doi: 10.1164/ajrccm.162.2.9903057. [DOI] [PubMed] [Google Scholar]
- 102.Mäkelä M.J., Kanehiro A., Dakhama A. The failure of interleukin-10-deficient mice to develop airway hyperresponsiveness is overcome by respiratory syncytial virus infection in allergen-sensitized/challenged mice. Am J Respir Crit Care Med. 2002;165:824–831. doi: 10.1164/ajrccm.165.6.2105062. [DOI] [PubMed] [Google Scholar]
- 103.Schwarze J., Gelfand E.W. Respiratory viral infections as promoters of allergic sensitization and asthma in animal models. Eur Respir J. 2002;19:341–349. doi: 10.1183/09031936.02.00254302. [DOI] [PubMed] [Google Scholar]


