Abstract
Treatment guidelines developed by the Sinus and Allergy Health Partnership for acute bacterial rhinosinusitis (ABRS) were originally published in 2000. These guidelines were designed to: (1) educate clinicians and patients (or patients’ families) about the differences between viral and bacterial rhinosinusitis; (2) reduce the use of antibiotics for nonbacterial nasal/sinus disease; (3) provide recommendations for the diagnosis and optimal treatment of ABRS; (4) promote the use of appropriate antibiotic therapy when bacterial infection is likely; and (5) describe the current understanding of pharmacokinetic and pharmacodynamics and how they relate to the effectiveness of antimicrobial therapy. The original guidelines are updated here to include the most recent information on management principles, antimicrobial susceptibility patterns, and therapeutic options.
Burden of disease
An estimated 20 million cases of ABRS occur annually in the United States. According to National Ambulatory Medical Care Survey (NAMCS) data, sinusitis is the fifth most common diagnosis for which an antibiotic is prescribed. Sinusitis accounted for 9% and 21% of all pediatric and adult antibiotic prescriptions, respectively, written in 2002. The primary diagnosis of sinusitis results in expenditures of approximately $3.5 billion per year in the United States.
Definition and diagnosis of ABRS
ABRS is most often preceded by a viral upper respiratory tract infection (URI). Allergy, trauma, dental infection, or other factors that lead to inflammation of the nose and paranasal sinuses may also predispose individuals to developing ABRS.
Patients with a “common cold” (viral URI) usually report some combination of the following symptoms: sneezing, rhinorrhea, nasal congestion, hyposmia/anosmia, facial pressure, postnasal drip, sore throat, cough, ear fullness, fever, and myalgia. A change in the color or the characteristic of the nasal discharge is not a specific sign of a bacterial infection. Bacterial superinfection may occur at any time during the course of a viral URI. The risk that bacterial superinfection has occurred is greater if the illness is still present after 10 days. Because there may be cases that fall out of the “norm” of this typical progression, practicing clinicians need to rely on their clinical judgment when using these guidelines. In general, however, a diagnosis of ABRS may be made in adults or children with symptoms of a viral URI that have not improved after 10 days or worsen after 5 to 7 days. There may be some or all of the following signs and symptoms: nasal drainage, nasal congestion, facial pressure/pain (especially when unilateral and focused in the region of a particular sinus), postnasal drainage, hyposmia/anosmia, fever, cough, fatigue, maxillary dental pain, and ear pressure/fullness.
Physical examination provides limited information in the diagnosis of ABRS.
While sometimes helpful, plain film radiographs, computed tomography (CT), and magnetic resonance imaging scans are not necessary for cases of ABRS.
Microbiology of ABRS
The most common bacterial species isolated from the maxillary sinuses of patients with ABRS are Streptococcus pneumoniae, Haemophilus influenzae, and Moraxella catarrhalis, the latter being more common in children. Other streptococcal species, anaerobic bacteria and Staphylococcus aureus cause a small percentage of cases.
Bacterial resistance in ABRS
The increasing prevalence of penicillin nonsusceptibility and resistance to other drug classes among S pneumoniae has been a problem in the United States, with 15% being penicillin-intermediate and 25% being penicillin-resistant in recent studies. Resistance to macrolides and trimethoprim/sulfamethoxazole (TMP/SMX) is also common in S pneumoniae. The prevalence of β-lactamase-producing isolates of H influenzae is approximately 30%, while essentially all M catarrhalis isolates produce β-lactamases. Resistance of H influenzae to TMP/SMX is also common.
Antimicrobial treatment guidelines for ABRS
These guidelines apply to both adults and children. When selecting antibiotic therapy for ABRS, the clinician should consider the severity of the disease, the rate of progression of the disease, and recent antibiotic exposure. The guidelines now divide patients with ABRS into two general categories: (1) those with mild symptoms who have not received antibiotics within the past 4 to 6 weeks, and (2) those with mild disease who have received antibiotics within the past 4 to 6 weeks or those with moderate disease regardless of recent antibiotic exposure. The difference in severity of disease does not imply infection with a resistant pathogen. Rather, this terminology indicates the relative degree of acceptance of possible treatment failure and the likelihood of spontaneous resolution of symptoms—patients with more severe symptoms are less likely to resolve their disease spontaneously. The primary goal of antibiotic therapy is to eradicate bacteria from the site of infection, which, in turn, helps (1) return the sinuses back to health; (2) decrease the duration of symptoms to allow patients to resume daily activities more quickly; (3) prevent severe complications such as meningitis and brain abscess; and (4) decrease the development of chronic disease. Severe or life-threatening infections with or without complications are rare, and are not addressed in these guidelines.
Prior antibiotic use is a major risk factor associated with the development of infection with antimicrobial-resistant strains. Because recent antimicrobial exposure increases the risk of carriage of and infection due to resistant organisms, antimicrobial therapy should be based upon the patient’s history of recent antibiotic use. The panel’s guidelines, therefore, stratify patients according to antibiotic exposure in the previous 4 to 6 weeks.
Lack of response to therapy at ≥72 hours is an arbitrary time established to define treatment failures. Clinicians should monitor the response to antibiotic therapy, which may include instructing the patient to call the office or clinic if symptoms persist or worsen over the next few days.
The predicted bacteriologic and clinical efficacy of antibiotics in adults and children has been determined according to mathematical modeling of ABRS developed by Michael Poole, MD, PhD, based on pathogen distribution, resolution rates without treatment, and in vitro microbiologic activity.
Antibiotics can be placed into the following relative rank order of predicted clinical efficacy for adults: 90% to 92% = respiratory fluoroquinolones (gatifloxacin, levofloxacin, moxifloxacin), ceftriaxone, high-dose amoxicillin/clavulanate (4 g/250 mg/day), and amoxicillin/clavulanate (1.75 g/250 mg/day); 83% to 88% = high-dose amoxicillin (4 g/day), amoxicillin (1.5 g/day), cefpodoxime proxetil, cefixime (based on H influenzae and M catarrhalis coverage), cefuroxime axetil, cefdinir, and TMP/SMX; 77% to 81% = doxycycline, clindamycin (based on gram-positive coverage only), azithromycin, clarithromycin and erythromycin, and telithromycin; 65% to 66% = cefaclor and loracarbef. The predicted spontaneous resolution rate in patients with a clinical diagnosis of ABRS is 62%.
Antibiotics can be placed into the following relative rank order of predicted clinical efficacy in children with ABRS: 91% to 92% = ceftriaxone, high-dose amoxicillin/clavulanate (90 mg/6.4 mg per kg per day) and amoxicillin/clavulanate (45 mg/6.4 mg per kg per day); 82% to 87% = high-dose amoxicillin (90 mg/kg per day), amoxicillin (45 mg/kg per day), cefpodoxime proxetil, cefixime (based on H influenzae and M catarrhalis coverage only), cefuroxime axetil, cefdinir, and TMP/SMX; and 78% to 80% = clindamycin (based on gram-positive coverage only), cefprozil, azithromycin, clarithromycin, and erythromycin; 67% to 68% = cefaclor and loracarbef. The predicted spontaneous resolution rate in untreated children with a presumed diagnosis of ABRS is 63%.
Recommendations for initial therapy for adult patients with mild disease (who have not received antibiotics in the previous 4 to 6 weeks) include the following choices: amoxicillin/clavulanate (1.75 to 4 g/250 mg per day), amoxicillin (1.5 to 4 g/day), cefpodoxime proxetil, cefuroxime axetil, or cefdinir. While TMP/SMX, doxycycline, azithromycin, clarithromycin, erythromycin, or telithromycin may be considered for patients with β-lactam allergies, bacteriologic failure rates of 20% to 25% are possible. Failure to respond to antimicrobial therapy after 72 hours should prompt either a switch to alternate antimicrobial therapy or reevaluation of the patient (see Table 4).When a change in antibiotic therapy is made, the clinician should consider the limitations in coverage of the initial agent.
Recommendations for initial therapy for adults with mild disease who have received antibiotics in the previous 4 to 6 weeks or adults with moderate disease include the following choices: respiratory fluoroquinolone (eg, gatifloxacin, levofloxacin, moxifloxacin) or high-dose amoxicillin/clavulanate (4 g/250 mg per day). The widespread use of respiratory fluoroquinolones for patients with milder disease may promote resistance of a wide spectrum of organisms to this class of agents. Ceftriaxone (parenteral, 1 to 2 g/day for 5 days) or combination therapy with adequate gram-positive and negative coverage may also be considered. Examples of appropriate regimens of combination therapy include high-dose amoxicillin or clindamycin plus cefixime, or high-dose amoxicillin or clindamycin plus rifampin. While the clinical effectiveness of ceftriaxone and these combinations for ABRS is unproven; the panel considers these reasonable therapeutic options based on the spectrum of activity of these agents and on data extrapolated from acute otitis media studies. Rifampin should not be used as monotherapy, casually, or for longer than 10 to 14 days, as resistance quickly develops to this agent. Rifampin is also a well-known inducer of several cytochrome p450 isoenzymes and therefore has a high potential for drug interactions. Failure of a patient to respond to antimicrobial therapy after 72 hours of therapy should prompt either a switch to alternate antimicrobial therapy or reevaluation of the patient (see Table 4). When a change in antibiotic therapy is made, the clinician should consider the limitations in coverage of the initial agent. Patients who have received effective antibiotic therapy and continue to be symptomatic may need further evaluation. A CT scan, fiberoptic endoscopy or sinus aspiration and culture may be necessary.
Recommendations for initial therapy for children with mild disease and who have not received antibiotics in the previous 4 to 6 weeks include the following: high-dose amoxicillin/clavulanate (90 mg/6.4 mg per kg per day), amoxicillin (90 mg/kg per day), cefpodoxime proxetil, cefuroxime axetil, or cefdinir. TMP/SMX, azithromycin, clarithromycin, or erythromycin is recommended if the patient has a history of immediate Type I hypersensitivity reaction to β-lactams. These antibiotics have limited effectiveness against the major pathogens of ABRS and bacterial failure of 20% to 25% is possible. The clinician should differentiate an immediate hypersensitivity reaction from other less dangerous side effects. Children with immediate hypersensitivity reactions to β-lactams may need: desensitization, sinus cultures, or other ancillary procedures and studies. Children with other types of reactions and side effects may tolerate one specific β-lactam, but not another. Failure to respond to antimicrobial therapy after 72 hours should prompt either a switch to alternate antimicrobial therapy or reevaluation of the patient (see Table 5).When a change in antibiotic therapy is made, the clinician should consider the limitations in coverage of the initial agent.
The recommended initial therapy for children with mild disease who have received antibiotics in the previous 4 to 6 weeks or children with moderate disease is high-dose amoxicillin/clavulanate (90 mg/6.4 mg per kg per day). Cefpodoxime proxetil, cefuroxime axetil, or cefdinir may be used if there is a penicillin allergy (eg, penicillin rash); in such instances, cefdinir is preferred because of high patient acceptance. TMP/SMX, azithromycin, clarithromycin, or erythromycin is recommended if the patient is β-lactam allergic, but these do not provide optimal coverage. Clindamycin is appropriate if S pneumoniae is identified as a pathogen. Ceftriaxone (parenteral, 50 mg/kg per day for 5 days) or combination therapy with adequate gram-positive and -negative coverage may also be considered. Examples of appropriate regimens of combination therapy include high-dose amoxicillin or clindamycin plus cefixime, or high-dose amoxicillin or clindamycin plus rifampin. The clinical effectiveness of ceftriaxone and these combinations for ABRS is unproven; the panel considers these reasonable therapeutic options based on spectrum of activity and on data extrapolated from acute otitis media studies. Rifampin should not be used as monotherapy, casually, or for longer than 10 to 14 days as resistance quickly develops to this agent. Failure to respond to antimicrobial therapy after 72 hours of therapy should prompt either a switch to alternate antimicrobial therapy or reevaluation of the patient (see Table 5). When a change in antibiotic therapy is made, the clinician should consider the limitations in coverage of the initial agent. Patients who have received effective antibiotic therapy and continue to be symptomatic may need further evaluation. A CT scan, fiberoptic endoscopy or sinus aspiration and culture may be necessary.
Introduction
The Sinus and Allergy Health Partnership, a conjoint group initially sponsored by the American Academy of Otolaryngology Head and Neck Surgery, the American Academy of Otolaryngic Allergy and the American Rhinologic Society, in consultation with representatives of the Centers for Disease Control and Prevention (CDC), the Food and Drug Administration (FDA), and individuals from the fields of infectious disease, pediatric infectious disease, microbiology, and pharmacy have developed these guidelines as an educational tool for healthcare providers involved in managing patients with acute bacterial rhinosinusitis (ABRS). The guidelines, which were published in 2000, 1 were widely accepted; however, recent data (since the time of publication) and the approval of new antimicrobial agents/classes may impact the utility of those recommendations. As a result, the guidelines are updated here to include the most recent information on management principles, antimicrobial susceptibility patterns, and therapeutic options. Significant updates from the previous version of the guidelines include:
-
•
Diagnostic modalities, including serial sinus aspirate sampling;
-
•
Current antimicrobial susceptibility patterns in the United States;
-
•
Pharmacodynamic principles reflecting area under the concentration-time curve (AUC)/minimal inhibitory concentration (MIC) ratio as the parameter that correlates with efficacy for macrolides/azalides;
-
•
Antimicrobial treatment recommendations that reflect a better understanding of pharmacodynamic/pharmacokinetic (PK/PD) principles;
-
•
Consideration of new/other agents (eg, extended-release [adult] and extra strength [children] amoxicillin/clavulanate, cefdinir, telithromycin); and
-
•
Modification of the Poole model to include predicted bacteriologic outcomes in patients with bacteriologic disease and predicted clinical outcomes for a patient population with a clinical only diagnosis of ABRS
While this revised version includes many updates, much of this document is reprinted from the original recommendations because several key concepts have not changed since the time of their publication.
There are several issues we attempted to address during the process of writing this document: (1) the diagnosis of bacterial “sinusitis” is made too frequently—patients with viral illnesses of only a few days duration are inappropriately labeled as having bacterial disease and, therefore; (2) patients are prescribed an antibiotic that is not only ineffective against a viral pathogen but also has the risk of leading to; (3) increased resistance among respiratory tract pathogens, particularly Streptococcus pneumoniae.
In this document, the reader is taken through a stepwise approach to this complex disease. The burden, pathophysiology, and definition of ABRS are reviewed, along with the attributes and limitations of various diagnostic modalities. Also included in these guidelines is a critical review of antimicrobial treatment options for ABRS. Clinical trials conducted in this era of widespread antimicrobial resistance are just beginning to provide sufficient evidence to use as the basis for recommending treatment options but, in general, they are not sufficiently powered to be of objective use. In lieu of evidence, several factors may be useful to clinicians in selecting therapy for individual patients. These factors include pathogen distribution in ABRS, pharmacokinetic and pharmacodynamic principles, mechanisms of antimicrobial resistance, and data from in vitro surveillance studies. Many of these factors have been incorporated into a mathematical model that can be used to objectively compare various antimicrobial options for ABRS.
Our hope is that these guidelines will continue to be a well-accepted part of national and international efforts coordinated by the CDC and the FDA aimed at educating healthcare providers and patients about judicious antimicrobial use and avoidance of the abuse and overuse of these valuable agents The misuse of antibiotics should not be a replacement for spending time talking with and examining the patient and teaching that patient and/or family members the differences between viral and bacterial infections. We cannot rely on the pharmaceutical industry to continue to develop new drugs as organisms become resistant; rather, we must decrease unnecessary antimicrobial use as a means to reduce the spread of resistance.
We believe further research is necessary in order to (1) develop better methods to diagnose ABRS; (2) further explore the clinical application of the antibiotic recommendations presented in this document; (3) monitor antimicrobial resistance patterns among respiratory tract pathogens—especially for S pneumoniae and Haemophilus influenzae.
Viral versus bacterial rhinosinusitis
Each year in the United States, children and adults experience an average of 3 to 8 and 2 to 3 acute viral respiratory illnesses, respectively.2, 3 Up to 90% of these patients will actually have computed tomographic (CT) scan evidence of paranasal sinus involvement (ie, viral rhinosinusitis [VRS]).2, 4 Secondary bacterial infections, also referred to as ABRS, complicate a small number of viral infections and positive bacterial cultures can be obtained in roughly 0.5% to 2% of VRS episodes.2, 5 Approximately 20 million cases of ABRS would therefore be expected, based on the more than 1 billion viral respiratory illnesses that occur each year. Sinusitis accounted for 9% and 21% of all pediatric and adult antibiotic prescriptions, respectively, written in 2002.6 In addition to its public health implications, rhinosinusitis has a considerable economic impact. The most recent estimates suggest that expenditures attributable to ABRS total approximately $3.5 billion each year in the United States.7 In 2002, approximately $400 to $600 million was spent on antibiotic prescriptions for acute sinusitis.6, 8
Differentiating bacterial from viral rhinosinusitis often is a challenge because the clinical features of the two diseases are similar, and the common imaging modalities are not sufficiently sensitive or specific. As a result, clinicians often overtreat uncomplicated rhinosinusitis by readily prescribing antibiotics for the majority of patients with signs and symptoms of VRS (eg, headache, facial pain, nasal congestion, rhinorrhea, fever). Recent reports in the medical literature suggest that primary care physicians prescribe antibiotics for up to 85% to 98% of patients with clinically suspected rhinosinusitis.9, 10 The practice of treating uncomplicated VRS with antibiotics has two fundamental limitations: first, secondary bacterial infection complicates a relatively small proportion of cases and second, excessive antibiotic use is associated with consequences, both to individuals and to society as a whole.
As the total number of antibiotic prescriptions increased throughout the 1990s, antimicrobial resistance among respiratory tract pathogens emerged as a significant public health issue. Excessive antibiotic use is strongly associated with the development and spread of bacterial drug resistance.11, 12, 13, 14, 15, 16 Recent strategies promoting prudent and rational antimicrobial use have been implemented over the past several years. In 2000, there were 25 million fewer antibiotic prescriptions in the ambulatory care setting compared with 1992 (17% reduction).17 The most substantial reductions in antimicrobial prescribing have occurred for respiratory tract infections among children (<15 years of age). However, there was no significant change in the population-based antibiotic prescribing rate for sinusitis among children.18
In rhinosinusitis, two features of antibiotic prescribing are of particular concern. First is the frequent treatment of uncomplicated VRS with antimicrobials. Second is the selection of antimicrobial agents without documented efficacy or that are no longer effective due to the development of resistance. The continued goal of this panel is to develop guidelines for the judicious use of antibiotics in the treatment of ABRS.
Definition and diagnosis of ABRS
In 1997, the American Academy of Otolaryngology developed working definitions for sinusitis to clarify communications among healthcare providers and researchers.19 Sinusitis is generally preceded by rhinitis and rarely occurs without concurrent rhinitis; therefore, sinusitis is best described as rhinosinusitis. The terms acute, subacute, recurrent acute, and chronic rhinosinusitis were also reviewed and defined. This terminology was subsequently adopted by the Agency for Health Care and Policy Research in the development of their 1999 document on the Diagnosis and Treatment of Acute Bacterial Rhinosinusitis.20
Pathophysiology of ABRS
ABRS is most often preceded by a viral upper respiratory tract infection (URI). Allergy, trauma, or other environmental factors that lead to inflammation of the nose and paranasal sinuses may also predispose individuals to developing ABRS. Approximately 50% of common colds are caused by the human rhinovirus. Other viruses that cause rhinosinusitis include coronavirus, influenza A and B viruses, parainfluenza virus, respiratory syncytial virus, adenovirus, and enterovirus. Most of these viral infections occur in the early fall to early spring, and the incidence of sinusitis follows a similar pattern.
Human rhinovirus and coronavirus do not cause major epithelial damage, but influenza virus and adenovirus cause significant damage the nasal epithelium.21, 22 Human rhinovirus, for example, enters via the nose or lacrimal duct and attaches to ICAM-1 receptors on epithelial cells in the posterior nasopharynx.23 There is upregulation of the production of histamine, bradykinin, and various cytokines, including interleukin-1, interleukin-6, interleukin-8, tumor necrosis factor-α, and leukotriene C4. Viruses also have a substantial suppressive effect on neutrophil, macrophage and lymphocyte function. Effects on neutrophil function include diminished adherent, chemotactic, phagocytic, oxidative, secretory, and bactericidal functions. Viruses also suppress macrophage and lymphocyte function, resulting in patients with viral URIs being generally more vulnerable to secondary overgrowth and subsequent bacterial infection by pathogens residing in the nasopharynx, such as S pneumoniae and H influenzae. An animal model of nontypeable H influenzae adherence to respiratory epithelium was studied in the cotton rat with respiratory syncytial virus (RSV) infection.24 Colonization with nontypeable H influenzae increased to a maximum within 4 days of RSV infection compared to RSV negative controls and then declined over the subsequent 10 days. Systemic immunity to nontypeable H influenzae as measured by IgG-specific antibody to the outer membrane complex and bactericidal antibody did not influence colonization. These data suggest that colonization with nontypeable H influenzae is significantly affected by a concurrent infection with RSV24; however, the site of bacterial attachment is not known. The mechanism of attachment involves upregulation of expression of epithelial cell surface receptors including CEACAM1, ICAM-1, and PAF-r.25
Subsequent activation of inflammatory pathways and the parasympathetic nervous system generates the symptoms of rhinosinusitis. Fever, myalgia, and pharyngitis frequently associated with a viral URI tend to resolve after 5 days, whereas nasal congestion and cough may persist into the second and third week (Figure 1).26 Fever alone at day 10 is not suggestive of ABRS. The causes of secondary bacterial invasion of the sinuses are unknown, but a combination of factors such as nose blowing, 27 local/systemic immunity, the virulence of the virus, colonization of the nasopharynx with potential bacterial pathogens (eg, S pneumoniae) and various environmental factors may lead to conditions that are conducive for bacterial entry and growth in the sinuses.
Fig 1.
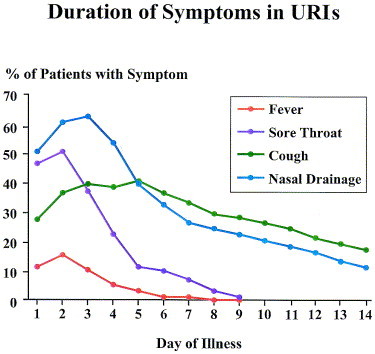
Duration of symptoms in rhinovirus URIs. There are three patterns of symptoms and resolution: (1) fever and myalgia; (2) sneezing and sore throat; and (3) cough and rhinorrhea, which are common and persistent in a significant proportion of patients. Persistence of these last two symptoms is entirely consistent with an uncomplicated rhinovirus infection.26
Because children experience an average of 3 to 8 viral URIs per year, the potential for inappropriate antibiotic use is high in this population.28 The mean duration of a viral URI ranges between 6.6 days (1- to 2-year-old children in home care) and 8.9 days (children <1-year-old in day care). Upper respiratory tract symptoms may, however, last more than 15 days in approximately 7% (1- to 3-year-old children in home care) to 13% (2- to 3-year-old children in day care) of cases. Children in day care are more likely to have protracted respiratory symptoms.29
Clinical diagnosis of ABRS
Patients with a common cold usually report some combination of the following symptoms: sneezing, rhinorrhea, nasal congestion, hyposmia/anosmia, facial pressure, postnasal drip, sore throat, cough, ear fullness, fever, and myalgia. Contrary to popular belief, a change in the color or the characteristic of the nasal discharge is not a specific sign of bacterial infection because after a few days of a viral infection, mucopurulent nasal secretions may occur due to an influx of neutrophils.30, 31, 32, 33, 34, 35 In a recent study, 35 the clinical signs and symptoms significantly associated in a multivariate model with the presence of bacteria included colored nasal discharge, facial pain, and radiologically determined maxillary sinusitis (complete opacity, air-fluid level, or mucosal thickening >10 mm). The model only had a sensitivity of 69% and a specificity of 64% and therefore could not be used either as a screening tool or as a diagnostic criterion for bacterial rhinosinusitis. The authors of this study concluded that the signs and symptoms of acute rhinosinusitis in patients with mild-to-moderate clinical presentations are poor predictors of the presence of bacteria.35
In a study by Gwaltney et al4 (n = 31), 87% of adults with acute onset of URI symptoms demonstrated inflammation within the nose and viscous secretions, sometimes with air bubbles, in the sinuses on CT scan. After 2 weeks without antibiotic therapy, repeat CT scans in 14 subjects revealed that 79% showed either disappearance or marked improvement in the previously identified abnormalities. The point at which a viral URI becomes superinfected with pathogenic bacteria may be determined with repeated sinus aspiration studies. Sinus aspiration studies in adults demonstrate significant bacterial growth in approximately 60% of patients with URI symptoms for 10 days or more.36 While duration of symptoms beyond 7 days is a moderately sensitive predictor of ABRS, it is relatively nonspecific because duration of symptoms does not reliably distinguish prolonged viral infection from ABRS.37 Individual cases may fall out of the “norm” of this typical progression and have specific findings suggesting bacterial infection (fever, facial erythema and swelling, and severe pain); therefore, clinicians need to rely on clinical judgment when using these guidelines. In general, a diagnosis of ABRS may be made in adults or children with a viral URI that has not resolved after 10 days or worsens after 5 to 7 days and is accompanied by some or all of the following signs or symptoms: nasal drainage, nasal congestion, facial pressure/pain (especially when unilateral and focused in the region of a particular sinus), postnasal drainage, hyposmia/anosmia, fever, cough, fatigue, maxillary dental pain, and ear pressure/fullness (Table 1).
Table 1.
| Nasal drainage |
| Nasal congestion |
| Facial pain/pressure (especially when unilateral and focused in the region of a particular sinus group) |
| Postnasal drip |
| Hyposmia/anosmia |
| Fever |
| Cough |
| Fatigue |
| Maxillary dental pain |
| Ear fullness/pressure |
A diagnosis of ABRS may be made in adults or children with a viral URI that is no better after 10 days (or worsens after 5–7 days) and is accompanied by some or all of these symptoms.
Modified from ref. 19.
Diagnostic modalities
Physical examination provides limited information in the diagnosis of ABRS. Several studies have evaluated whether certain signs or symptoms are specific to bacterial infection; however, these studies have methodologic limitations in that sinus aspiration was not used to document the presence or absence of bacterial infection.37 Unlike acute otitis media, in which the tympanic membrane and middle ear space are readily available for direct examination, the paranasal sinuses are hidden within the skull. Anterior rhinoscopy, with or without topical decongestant, allows examination of the mucosa of the inferior turbinate, secretions within the anterior nose, and the orientation of the nasal septum. Fiberoptic endoscopy allows visualization of the middle meatus, and direct culture of purulence in this region may correlate with cultures from maxillary sinus aspirates.38, 39 In a recent review of the literature, Benninger et al40 reported that there is 60% to 85% concordance between culture material obtained from endoscopically guided middle meatal swabs and maxillary sinus puncture. These studies, however, are limited by small sample sizes, and are therefore inadequate to make recommendations regarding the role of endoscopically guided middle meatal cultures as a formal method of identifying pathogens in ABRS at this time. A prospective study is currently underway to better answer this question. Other diagnostic modalities include transillumination, ultrasound, and radiological imaging. Transillumination has a 60% and 90% reproducibility rate for assessing disease within the maxillary sinuses and the frontal sinuses, respectively, but this does not differentiate bacterial from viral infection.41 B-mode ultrasound has replaced A-mode ultrasound for the diagnosis of diseases within the paranasal sinuses. However, because only the maxillary sinus can be adequately assessed, B-mode ultrasound has limited utility. A study correlating CT scan and B-mode ultrasound findings demonstrated a sensitivity of roughly 73% for the maxillary sinuses, 23% for the frontal sinuses and 11% for the ethmoids.42 Compared with clinical evaluation, the sensitivity of B-mode ultrasound was 36% and the specificity was 90%.43 Because ultrasound is technique-sensitive, there may be marked variations in the reliability of the information provided.44 Ultrasound cannot distinguish between viral and bacterial rhinosinusitis.
Plain film radiographs primarily reveal pathologic findings in the maxillary and frontal sinuses, whereas the ethmoids are poorly visualized using this imaging technique. Additionally, plain radiographs are imprecise at determining the extent of disease.45 A meta-analysis of six studies demonstrated that positive plain film radiographs have moderate sensitivity (76%) and specificity (79%) compared to maxillary sinus puncture, 20 and a negative radiograph has more diagnostic value than either a negative clinical examination or ultrasound. CT scans clearly detect abnormalities within the sinuses; however, as previously noted, abnormalities are frequently found on CT scans of patients with viral respiratory disease.4 Magnetic resonance imaging (MRI), without exposing patients to ionizing radiation, distinctly reveals mucosal thickening and fluid within the paranasal sinuses. In patients with maxillary sinusitis, serial MRI scans demonstrate mucosal thickening persisting for up to 8 weeks.46 Significant mucosal changes seen on CT or MRI may therefore persist significantly beyond microbiologic resolution of bacterial or viral disease. CT and MRI scans are not recommended for the routine management of ABRS, but they may be helpful in guiding the management for more complex cases.
Puncture of the maxillary sinus through the canine fossa or the inferior meatus provides material that may be cultured to identify bacterial isolates. Technical expertise is required to minimize complications, and the procedure is somewhat uncomfortable for the patient. Maxillary sinus puncture is not routinely used in cases of suspected ABRS. It is usually reserved for the research setting or for patients with more complicated infections. A novel technique—serial sinus aspirate sampling—devised by Anon, Ambrose, Jones et al, involves placing an indwelling catheter into the maxillary sinus. This technique has provided a means to determine actual time to eradication of various pathogens, compare change in symptoms as the bacterial population decreases, and evaluate antibiotic concentrations within the sinus fluid.47
Selection of antimicrobial therapy for ABRS
The primary reason for recommending antibiotic therapy for ABRS is because withholding the benefits of treatment unnecessarily exposes patients to unreasonable morbidity, particularly for those with more severe symptoms. However, the routine use of antimicrobial therapy for patients who experience mild sinus symptoms for a short duration (indicative of self-limiting viral rhinosinusitis) is generally not a reasonable option because of the risks associated with promoting resistance. Unfortunately, not all cases are straightforward, and the decision of whether—and when—to initiate antimicrobial therapy for an individual patient with signs and symptoms consistent with ABRS often requires consideration of potential risks and benefits of treatment. For example, there is a subset of patients who may experience prolonged, moderate to relatively severe symptoms that are more attributable to host factors (eg, immune response, anatomic abnormalities) rather than bacterial infection. For these patients, the benefits of initiating an earlier course of antimicrobial therapy might be appropriate.
The primary goal of antibiotic therapy for ABRS is to eradicate the bacterial pathogens from the site of infection, 48 which helps (1) decrease the duration of symptoms to allow patients to resume daily activities more quickly; (2) return the sinuses back to health; (3) prevent severe complications (eg, meningitis and brain abscess); and (4) decrease the likelihood of developing chronic disease. Severe or life-threatening infections with or without complications are rare, and are not addressed in these guidelines.
Clinical trials conducted in this era of widespread antimicrobial resistance are just beginning to provide some evidence to use as the basis for recommending specific antimicrobial treatment options but, in general, they are not sufficiently powered. In lieu of adequate evidence, several factors may be helpful to clinicians in selecting therapy for individual patients. These factors include pathogen distribution in ABRS, pharmacokinetic and pharmacodynamic principles of antimicrobial activity, mechanisms of antimicrobial resistance, and data from in vitro surveillance studies. Other factors, including symptom severity, the likelihood of infection with a resistant pathogen, and the likelihood of spontaneous resolution (based on the infecting pathogen) were included in the methodology used by the panel to objectively evaluate various antimicrobial options for ABRS. Each of these factors will be discussed in detail below.
Microbiology of ABRS
Bacteria are broadly classified into groups based on their cell-wall composition, morphologic characteristics, and metabolic requirements. The cell wall, an important determinant of inherent susceptibility or resistance for any bacterium to many antimicrobial agents, consists primarily of proteins, lipids, and a peptidoglycan layer. The peptidoglycan layer is composed of oligosaccharide chains cross-linked by short peptides that serve as the major structural component for maintaining cell-wall integrity. Although gram-positive and gram-negative bacteria share many common structural elements in their cell walls, the organization and content of these elements vary between these two bacterial classes (Figure 2). The cell wall of gram-positive bacteria consists almost entirely of a thick peptidoglycan layer fused to the outside of the cytoplasmic membrane. Gram-negative bacteria, however, have cell walls composed of a hydrophobic lipopolysaccharide capsule surrounding a lipoprotein-phospholipid membrane that contains small channels called porins. A thin peptidoglycan layer lies between the outer membrane and the inner cytoplasmic membrane. These two biological layers are separated by the periplasmic space. This space is an important site for degradation of antibiotics by drug-inactivating enzymes, such as β-lactamases, in gram-negative bacteria. Penicillin-binding proteins (PBPs), enzymes essential for cell-wall synthesis, are located in the cytoplasmic membrane. PBPs are found in gram-negative and -positive organisms. Altered PBPs, which have decreased affinity for β-lactams, have been identified in a variety of organisms.
Fig 2.
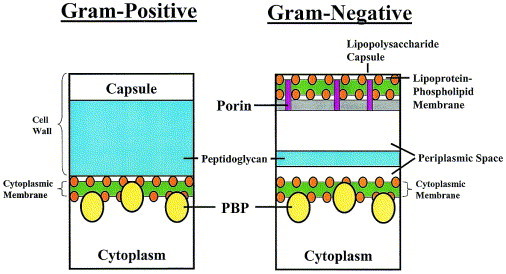
Gram-positive and gram-negative bacteria have different configurations of their cell walls, as noted in this illustration. Penicillin binding proteins (PBPs) play an important role in cell wall synthesis.
The most common bacterial isolates recovered from the maxillary sinuses of patients with ABRS are S pneumoniae, H influenzae, other streptococcal species, and Moraxella catarrhalis. A review of sinus aspiration studies performed in adults with ABRS suggests that S pneumoniae is isolated in approximately 20% to 43%, H influenzae in 22% to 35%, and M catarrhalis in 2% to 10% of aspirates (Figure 3). 2, 36, 49, 50, 51, 52 In children with ABRS, S pneumoniae is isolated in approximately 35% to 42%, while H influenzae and M catarrhalis each are recovered from about 21% to 28% of aspirates. Streptococcus pyogenes and anaerobes account for 3% to 7% (Figure 4). 36, 49, 50, 53, 54 Other bacterial isolates found in patients with ABRS include Staphylococcus aureus and anaerobes.36, 49, 50
Fig 3.
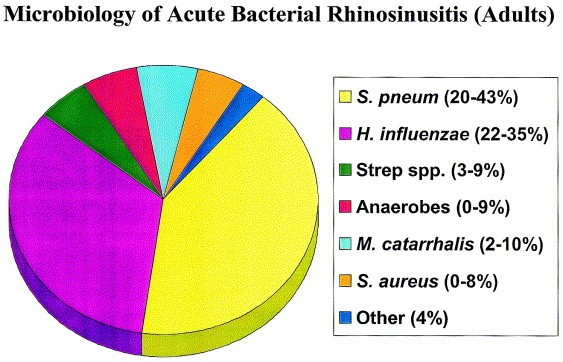
Prevalence of predominant pathogens associated with acute bacterial rhinosinusitis in adults.2, 36, 49, 50, 51, 52
Fig 4.
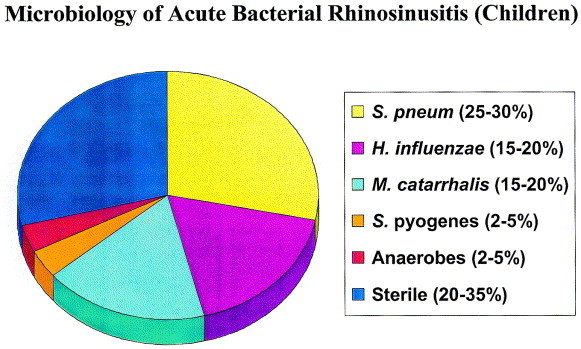
Prevalence of predominant pathogens associated with acute bacterial rhinosinusitis in children.36, 49, 50, 53, 54
Nasopharyngeal flora
Starting soon after birth, the nasopharynx is colonized with flora such as viridans streptococci, Corynebacterium species, Neisseria species and anaerobes. Colonization with “respiratory pathogens” occurs intermittently as discussed above, and by 12 months of age 70% of children are colonized by at least one of the three major respiratory pathogens: S pneumoniae, H influenzae, or M catarrhalis. Each pneumococcal strain persists in the nasopharynx for between 1 and 12 months, and point prevalence surveys have demonstrated that as many as two thirds of children have nasopharyngeal carriage of pneumococci.55 More than 90% of children are colonized with S pneumoniae by 3 years of age; the frequent serotypes/serogroups colonizing infants are 6, 9, 14, 19, and 23.56 Pneumococci also have a high frequency of genetic recombination, and strains carried in the nasopharynx may change serotype.57 Strains of nontypeable H influenzae also sequentially colonize the nasopharynx; this process starts in infancy. By 2 years of age, 44% of children have been colonized, with each strain being carried for 1 to 7 months (mean 2.2 months).58 Production of H influenzae-specific IgA results in eradication of carriage of a strain, which is followed by acquisition of a new strain with different surface proteins.
As is the case with S pneumoniae and H influenzae, M catarrhalis colonizes the nasopharynx, in early childhood; 78% of children are colonized by 2 years of age.59 Each child is sequentially colonized with different strains of M catarrhalis. Otitis-prone children are more frequently colonized than otherwise healthy children.
Colonization with “respiratory pathogens” increases considerably during winter and during periods of viral URI, which often results in these organisms causing bacterial otitis media and sinusitis.55 Pelton et al60 have recently reported that S pneumoniae was recovered from approximately 21% of nasopharyngeal cultures performed on healthy children versus 32% of cultures on the same children when presenting with acute otitis media (AOM). Further, a study Bernstein et al61 suggests there is a “to-and-fro” exchange of these organisms between the nasopharynx and the lateral nasal wall. Bacterial pathogens were isolated from 79% of adenoids and 46% of lateral walls of the nose in children undergoing adenoidectomy. Molecular typing of pairs of nontypeable H influenzae, S pneumoniae, and M catarrhalis revealed that in 16 of 18 pairs (89%) the identical strain was present in both sites simultaneously. In addition, administration of antimicrobials increases carriage of antimicrobial-resistant strains of these bacterial pathogens.62 Adults also have colonization of the nasopharynx, but duration of carriage is shorter than in children.63 A recent study64 demonstrated that one of the primary respiratory pathogens was recovered from the nasopharynx of approximately 75% of adults.
S pneumoniae
Pneumococci are gram-positive, catalase-negative, facultatively anaerobic spherical bacteria that are typically seen in pairs or chains. They are nutritionally fastidious, requiring complex media containing blood or serum for growth, and growth is often enhanced by a carbon dioxide-enriched atmosphere. S pneumoniae belongs to the α-hemolytic group of streptococci, and is distinguished from the viridans group by occurring in pairs, by the requirement for carbon dioxide for primary isolation, and for autolyzing in the presence of bile salts (bile solubility) and optochin (inhibition by optochin-containing disks). Pneumococci are usually encapsulated and the capsular polysaccharides are used for serological classification. There are 90 antigenically distinct capsular serotypes in 42 distinct serogroups. Some of the serotypes have common antigens and are grouped together in serogroups accounting for the designations of “6A” and “6B, ” for example, in serogroup 6.
The incidence of invasive pneumococcal disease varies with serotype, and the likelihood of infection with any given serotype is largely dependent on the virulence factors expressed by the bacteria. Pneumolysin and the polysaccharide capsule are two of the most widely known virulence factors for S pneumoniae. Infection caused by serotype 14 and serogroups 6, 9, 18, 19, and 23 is highest in children, while that caused by serotypes 3 and 8 is highest in adults. Serotypes 1, 5, and 7 and serogroup 4 tend to cause disease at similar frequency in all age groups. Further, it has been found that 12 serogroups account for about 80% of infections.65 Seven serotypes, 14, 6B, 19F, 18C, 23F, 4, and 9V (in order of decreasing frequency), accounted for 78% of isolates from blood, cerebrospinal fluid and middle ear sources of children in the United States.66 These are present in the 7-valent conjugated pneumococcal vaccine currently available in the United States.
Antimicrobial resistance is observed primarily in serotypes 6A, 6B, 9, 14, 19F, and 23F, which are the serotypes most frequently colonizing children. Because these are exposed to antimicrobial agents more commonly, they are the most likely to develop resistance.67 Serotypes 1, 3, 4, 5, 7, 11, 15, and 18 rarely acquire antibiotic-resistant genes.
The incidence of invasive pneumococcal infections is dependent on the time of year.68 Furthermore, the incidence of infection caused by resistant strains also may increase during winter months.69
Mechanisms of resistance among S pneumoniae
Resistance to β-lactams results following a stepwise alteration in PBPs, which leads to a decrease in the binding affinities of β-lactams.70 Varying degrees of resistance to penicillin and other β-lactams develop because changes can occur in multiple PBPs to alter the affinity for β-lactams.71 There are six known PBPs in S pneumoniae—1a, 1b, 2b, 2x, 2z, 3—and alterations in 1a, 2b, and 2x are most often associated with resistance to penicillin (penicillin minimum inhibitory concentrations [MICs] range from 0.25 μg/mL to >8 μg/mL compared to ≤0.06 μg/mL for susceptible strains).72
Macrolide resistance results primarily from alterations in ribosomal binding sites (due to a ribosomal methylase) or expression of an efflux mechanism.73, 74 There are two important genes responsible for macrolide-resistant strains that are most commonly encountered in the clinical setting: erm genes, which code for a ribosomal methylase and mef genes, which code for a macrolide-specific cell membrane-based efflux mechanism. The efflux mechanism confers a relatively moderate degree of resistance, compared to the high level of resistance seen in strains with altered ribosomal binding sites. The efflux mechanism is generally more common in the United States and is relatively uncommon in most other parts of the world. Recently, mechanisms of macrolide resistance were identified that could not be explained by any of the known resistance determinants.75 These novel mechanisms of macrolide resistance involve mutations in genes encoding ribosomal proteins (L4 or L22) or ribosomal RNA (23S rRNA). Mutations in genes for L22 and 23S rRNA result in increased macrolide MICs; however, the effect is variable (MIC range 0.25 μg/mL to >64 μg/mL). Mutations in genes for L4 generally confer high-level resistance (MICs >64 μg/mL).72, 76 Isolates of S pneumoniae expressing these mutant genes are rare but have been identified in several surveillance studies, 76, 77, 78, 79 and there have been several recent reports of macrolide treatment failures resulting from development of these mutations during therapy with macrolides.80, 81, 82, 83, 84 Ribosomal methylase also confers cross-resistance to clindamycin. Macrolide usage, particularly azithromycin, has been associated with the recent increase in S pneumoniae resistance to macrolides in the United States.15
Fluoroquinolone resistance results following mutations in targets binding sites of these agents, DNA gyrase and topoisomerase IV, rather than requiring the acquisition of foreign genes. Mutations in the parC gene that encodes for topoisomerase IV or in the gyrA gene encoding for the Gyr A subunit of DNA gyrase results in low-level quinolone resistance. Mutations in both genes results in the expression of high-level quinolone resistance. Although cross-resistance commonly occurs among the fluoroquinolones, the newest agents often remain active against some strains that have become resistant to older agents. A fluoroquinolone efflux mechanism (pmrA) also has been described for S pneumoniae.85
Resistance to trimethoprim and sulfonamides are also primarily a result of mutations in the target binding sites of these agents, dihydropteroate synthase and dihydrofolate reductase.
H influenzae
This organism belongs to the genus Haemophilus, which consists of small, pleomorphic, and facultatively anaerobic gram-negative bacilli. Most species have complex nutritional requirements, and growth is enhanced by a carbon dioxide-enriched atmosphere. H influenzae is characterized by its requirement for both hemin (X factor) and NAD (V factor). Strains of H influenzae may be either encapsulated or unencapsulated; encapsulated strains include six serotypes (serotypes a to f). However, nontypeable strains typically cause URIs such as otitis media, sinusitis, and acute exacerbations of chronic bronchitis; accordingly, the occurrence of these infections has not been affected by the use of type b vaccines.
Mechanisms of resistance among H influenzae
The primary mechanism of resistance to β-lactams is through the production of β-lactamases, 86 which hydrolyze the amide bond of the β-lactam ring, thus inactivating the antibiotic.
To overcome the effects of β-lactamase–mediated resistance, β-lactams that are less susceptible to hydrolysis, and specific β-lactamase inhibitors have been developed. Third-generation cephalosporins (eg, ceftriaxone and cefixime) are stable in the presence of β-lactamases, whereas clavulanic acid is a broad-spectrum irreversible inhibitor of β-lactamases. Because clavulanic acid is destroyed in the process of β-lactamase inhibition, it is often described as a “suicide inhibitor.” Combinations of β-lactams and β-lactamase inhibitors (eg, amoxicillin/clavulanic acid) often are useful for the treatment of many β-lactamase–producing bacteria including, H influenzae and M catarrhalis. Other β-lactamase inhibitors include tazobactam and sulbactam. It is important to note that β-lactamase inhibitors only serve to increase the amount of active β-lactam compound at the target site to exert its activity against otherwise susceptible bacteria. Therefore, if the bacteria are not inherently susceptible to β-lactam in the absence of β-lactamases, addition of a β-lactamase inhibitor will not make the organism susceptible. Alterations in PBPs also have been reported occasionally among strains of H influenzae, and these strains are referred to as β-lactamase–negative ampicillin-resistant (BLNAR). Resistance among BLNAR strains is attributable to alterations in PBPs 3a and 3b.87
Most gram-negative organisms have multiple efflux pumps to remove waste and foreign material; one efflux pump for H influenzae is chromosomally mediated via acrAB genes. Macrolides and azalides are substrates for these pumps and, as a result, these agents have intrinsically poor activity against H influenzae.88
M catarrhalis
This species consists of aerobic, oxidase-positive, gram-negative diplococci. It has much less fastidious growth requirements than either pneumococci or Haemophilus species, and will grow on simple media without blood or serum. The primary mechanism of β-lactam resistance expressed by M catarrhalis is β-lactamase production; however, the β-lactamases produced by M catarrhalis are different from those produced by H influenzae. As a result, some agents (eg, cefpodoxime proxetil, cefuroxime axetil) are less active against M catarrhalis than H influenzae (see Table 3).M catarrhalis is also intrinsically resistant to trimethoprim.86, 89
Table 3.
Susceptibility of respiratory tract isolates (1998 to 2000) to antimicrobial agents at PK/PD breakpoints89, 90
| Agent | Percentage of isolates susceptible at PK/PD breakpoint |
||||||
|---|---|---|---|---|---|---|---|
| Susceptibility breakpoint (μg/mL) (PK/PD) | S pneu-moniae (all) (n = 2901) | Penicillin-susceptible S pneu-moniae (n = 1845) | Penicillin-intermediate S pneumoniae (n = 382) | Penicillin-resistant S pneumoniae (n = 674) | H influ-enzae (n = 1919) | M catar-rhalis (n = 204) | |
| Amoxicillin | ≤2 | 91.6 | 100 | 100 | 63.6 | 70.2 | 7.3 |
| Amoxicillin HD∗ | ≤4 | 95.2 | 100 | 100 | 79.4 | 70.2 | 7.3 |
| Amox/Clav† | ≤2 | 92.1 | 100 | 99.7 | 66.3 | 98.3 | 100 |
| Amox/Clav HD/extended release∗, † | ≤4 | 95.2 | 100 | 100 | 79.4 | 99.8 | 100 |
| Cefaclor | ≤0.5 | 19.7 | 30.3 | 2.9 | 0.1 | 3.7 | 8.7 |
| Cefuroxime axetil | ≤1 | 72.6 | 99.9 | 68.8 | 0.0 | 82.8 | 50.5 |
| Cefixime | ≤1 | 66.3 | 96.7 | 35.3 | 0.4 | >99.9 | 100 |
| Ceftriaxone | ≤1 | 96.3 | 100 | 99.5 | 84.6 | >99.9 | 93.6 |
| Cefprozil | ≤1 | 71.8 | 99.7 | 63.1 | 0.4 | 23.2 | 9.2 |
| Cefpodoxime‡ | ≤0.5 | 75.4 | 99.7 | 67.4 | 0.7 | 100 | 85.0 |
| Cefdinir | ≤0.25 | 68.8 | 98.4 | 49.2 | 0.5 | 78.2 | 77.6 |
| Loracarbef | ≤0.5 | 7.6 | 10.3 | 6.5 | 0 | 9.6 | |
| Erythromycin | ≤0.25 | 72.0 | 92.6 | 49.7 | 28.0 | 0.0 | 100 |
| Clarithromycin | ≤0.25 | 72.3 | 92.8 | 51.0 | 28.2 | 0.0 | 100 |
| Azithromycin | ≤0.12 | 71.0 | 91.8 | 48.4 | 27.2 | 2.3 | 100 |
| Clindamycin | ≤0.25 | 90.6 | 97.9 | 81.4 | 75.8 | 0 | 0 |
| Ciprofloxacin | ≤1 | § | § | § | § | 100 | 100 |
| Levofloxacin | ≤2 | 99.1 | 99.0 | 99.7 | 99.1 | 100 | 100 |
| Gatifloxacin | ≤1 | 99.1 | 99.0 | 99.7 | 99.1 | 100 | 100 |
| Moxifloxacin | ≤1 | 99.2 | 99.0 | 100 | 99.3 | 100 | 100 |
| Doxycycline | ≤0.25 | 80.4 | 95.2 | 65.2 | 48.7 | 25.1 | 96.3 |
| TMP/SMX¶ | ≤0.5 | 63.7 | 86.4 | 46.1 | 11.3 | 78.1 | 19.3 |
The activity of telithromycin against S pneumoniae, H influenzae, and M catarrhalis depends on its PK/PD breakpoint, which is uncertain at this time. The activity of telithromycin is assumed to be similar to that of macrolides/azalides until further information becomes available.
Amox/clav, amoxicillin/clavulanate; NA, not applicable; PD, pharmacodynamic; PK, pharmacokinetic; TMP/SMX, trimethoprim/sulfamethoxazole. All values are based on PK/PD breakpoints, except for S pneumoniae, in which values are shown as PK/PD and new (Jan 2000) NCCLS breakpoints and for clindamycin and TMP/SMX, in which NCCLS breakpoints are used. Data are adapted from reference 88.
High-dose amoxicillin or amoxicillin-clavulanate as defined in text.
Shown as amoxicillin component.
Susceptibility data for cefpodoxime were obtained from the SENTRY database.90
The MICs of ciprofloxacin against some isolates of S pneumoniae are above the PK/PD breakpoint; therefore, ciprofloxacin does not reliably cover this organism.
Shown as TMP component.
Prevalence of antimicrobial resistance among isolates of S pneumoniae
Isolates of S pneumoniae with penicillin MICs ≤0.06 μg/mL are defined as penicillin-susceptible, whereas penicillin-intermediate strains have penicillin MICs of 0.12 to 1.0 μg/mL, and penicillin-resistant isolates of S pneumoniae have a penicillin MIC of ≥2 μg/mL. The latter two groups are often referred to as “penicillin-nonsusceptible, ” and the clinical significance of these varies with different β-lactams as will be discussed. Drug-resistant S pneumoniae (DRSP) connotes strains with penicillin MICs of ≥0.12 μg/mL and/or resistance to other classes of antibiotics. Multidrug-resistant S pneumoniae are defined as organisms resistant to three or more classes of antibiotics.
The increasing prevalence of isolates of S pneumoniae that are penicillin nonsusceptible has been a concern in the United States (Figure 5). 90 In the late 1980s and early 1990s penicillin-nonsusceptible S pneumoniae became a major concern in the United States.91, 92 The Alexander Project is a worldwide surveillance study that collects respiratory tract isolates from community-based physicians and utilizes PK/PD susceptibility breakpoints to evaluate the in vitro activity of various antimicrobial agents.89 Recent data from the US component of the Alexander project demonstrated that 12% of isolates were penicillin-intermediate and 25% were penicillin-resistant.
Fig 5.
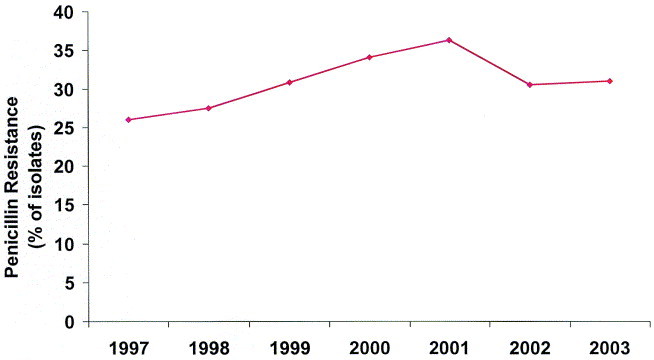
The prevalence of nonsusceptible (intermediate + resistant) S pneumoniae over the past several years in the United States.90
The prevalence of penicillin-nonsusceptibility appears to have peaked in 2001 at 36%, and has decreased to 31% in 2002.92 Resistance to other antimicrobial classes has also decreased. This trend may be attributable to several factors, including widespread use of the pneumococcal conjugate vaccine in children since 2000 as well as less overall antimicrobial use.
The overall US prevalence of resistance to trimethoprim/sulfamethoxazole (TMP/SMX), macrolides, doxycycline, and clindamycin was 37%, 29%, 21%, and 10%, respectively.89 Typically, resistance to these classes of antimicrobials is higher among penicillin-nonsusceptible isolates (Figure 6). 89 The respiratory fluoroquinolones (ie, gatifloxacin, levofloxacin, moxifloxacin) remain active against S pneumoniae, with fewer than 2% of all isolates being resistant.89 Data from the US component of the Alexander Project 1998–2000 demonstrate that 26% of S pneumoniae isolates were resistant to penicillin and two other classes of agents, and approximately 16% of isolates were resistant to any four classes of agents.89
Fig 6.
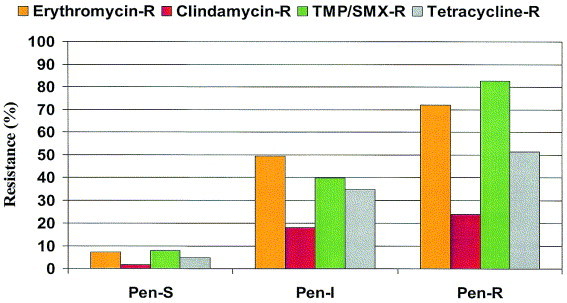
As resistance of S pneumoniae to penicillin rises, resistance to other antibiotics also increases. Drug-resistant S pneumoniae (DRSP) is an isolate with a penicillin MIC >0.06 μg/mL and/or resistance to other classes of antibiotics.89
Prevalence of antimicrobial resistance among isolates of H influenzae and M catarrhalis
The prevalence of β-lactamase–producing isolates of H influenzae varies slightly according to the particular study, ranging from 30% to 40% (Figure 7). 89, 90, 91 However, essentially all H influenzae isolates were susceptible to high-dose amoxicillin/clavulanate and cefixime.89 While BLNAR strains of H influenzae are rare in the United States, 89 they are more prevalent in other countries (eg, Japan).93
Fig 7.
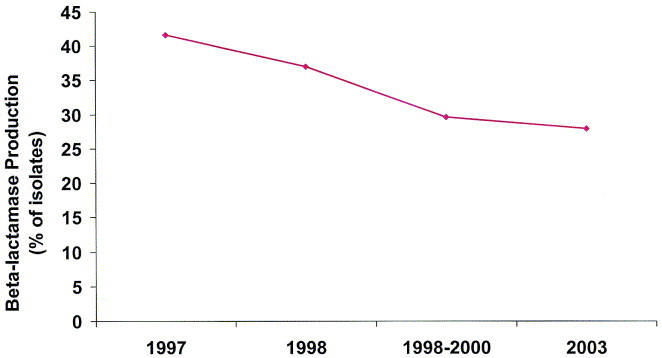
The prevalence of β-lactamase production by H influenzae over the past several years in the United States.89, 90, 91
Based on PK/PD susceptibility breakpoints, <1%, <1%, and approximately 3% of H influenzae isolates were susceptible to erythromycin, clarithromycin, and azithromycin, respectively.89 Approximately 22% of recent US H influenzae isolates were resistant to TMP/SMX. Data from the US component of the Alexander Project demonstrated that 92% of M catarrhalis isolates produced β-lactamases.89
Antimicrobial use and bacterial resistance
The extensive use of antibiotics may be associated with the development and spread of resistant microorganisms.11, 12, 13, 14, 15, 16 Nasopharyngeal carriage of resistant isolates of S pneumoniae is related to recent antimicrobial use as well as to living in a geographic region with a high volume of antibiotic use in children, 12, 94 and exposure to young children.95 The prevalence of β-lactamase–producing isolates of M catarrhalis was found to increase in proportion with cephalosporin use.11 In Finland, consumption of erythromycin was related to an increase in the prevalence of erythromycin-resistant group A streptococci.13 Furthermore, a steady and statistically significant decline in macrolide-resistant group A streptococci occurred after reducing the use of macrolide antibiotics for 2 years, which reinforces the rationale for judicious use of antibiotics.14
Assessment of antimicrobial activity
Numerous methods may be utilized to assess the in vitro activity of an antibiotic. Tests such as the minimal inhibitory concentration (MIC), minimal bactericidal concentration (MBC), and time-kill testing are valid methods for the assessment of antimicrobial activity. It is, however, important to understand the usefulness and limitations of each of these tests.
Antimicrobial activity is commonly evaluated by determining the MIC of a particular antibiotic against a specific bacterial strain (Figure 8). Therefore, if an MIC is reported as 2 μg/mL, the true inhibitory concentration is somewhere between 1 μg/mL and 2 μg/mL. Two other terms used are: MIC50, the lowest concentration that inhibits 50% of the isolates tested and MIC90, the lowest concentration that inhibits 90% of the isolates tested. It is extremely important to remember that the MIC is an in vitro characteristic of the antimicrobial and is determined under strictly adhered to conditions. Because environmental conditions at the site of infection rarely correspond to in vitro susceptibility test conditions, effects of elements such as oxygen tension, pH, and protein binding on the activity of the antimicrobial of interest need to be considered. Therefore, even if an organism appears susceptible in vitro, clinical failure may occur if in vivo conditions detract from the activity of the drug. Similarly, some host factors may actually improve the in vivo activity of an antimicrobial. Macrophages, opsonic factors, and complement may all act synergistically with an antibiotic and thus provide enhanced antibacterial activity over that which would be predicted in vitro. Additionally, many bacterial infections resolve spontaneously without the use of antimicrobial agents.
Fig 8.
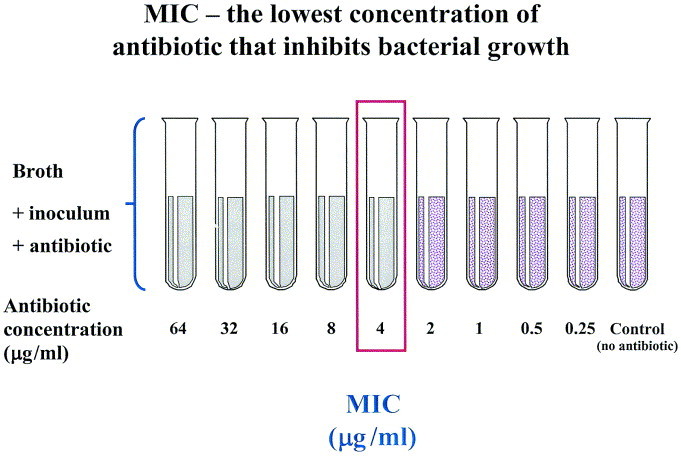
The MIC is the lowest concentration of the antimicrobial that results in the inhibition of growth of a microorganism. MICs are generally performed by placing a known inoculum of bacteria into media containing a range of doubling concentrations of the antimicrobial (ie, 0.5 μg/mL, 1 μg/mL, 2 μg/mL, 4 μg/mL, etc.). The MIC in this figure is 4 μg/mL.
While the MIC defines the amount of an antimicrobial necessary to inhibit the growth of a microbe, the MBC provides information regarding the concentration of drug required to kill the organism. The MBC, like the MIC, is an in vitro test that is subject to similar limitations in relation to clinical effectiveness. The MBC is calculated by determining concentrations of bacteria incubated in the presence of varying drug concentrations at time 0 and after 24 hours and is defined as the lowest concentration that results in a 99.9% reduction in viable count at 24 hours compared to the initial inoculum. The MBC values generally range from 0 to 2, doubling dilutions higher than MIC values. Because MICs are better standardized, less costly, and less labor intensive, they are used more often than are MBCs. However, if the MBC is much higher than the MIC (unless the drug is known to be bacteriostatic), the organism is said to display tolerance to the antimicrobial.
Pharmacokinetic/Pharmacodynamic principles
While MICs and MBCs are commonly utilized to describe the in vitro potency of antimicrobial agents, these measurements do not account for the pharmacokinetic properties of antimicrobial agents; therefore, their ability to predict therapeutic efficacy is limited.
The pharmacokinetics (ie, absorption, distribution, metabolism, and excretion) of many antimicrobials have been well established; however, the discipline of pharmacodynamics has only recently emerged. Pharmacodynamics describes the relationship between drug concentration and pharmacologic effect. For an antibiotic, it describes the relationship that exists between the drug concentration to which the bacteria is exposed at various sites of infection and bacterial killing. Pharmacodynamics attempts to integrate both microbiologic and pharmacokinetic data into more clinically relevant relationships. The evolution of this science has augmented the body of knowledge about how antimicrobials best treat infections. In addition, pharmacodynamics can be utilized to determine the impact of antimicrobial resistance. Consideration of pharmacodynamics can help define the MIC limit at which the pharmacokinetics of a specific antimicrobial drug would not be expected to result in treatment success. Pharmacodynamics has also established rational scientific principles that provide the basis for developing dosing strategies that optimize clinical outcomes.
Pharmacodynamically, in vivo bacterial killing may be described as a function of the duration of antimicrobial drug concentration over time relative to the MIC of that agent against a particular pathogen. The product of these pharmacokinetic parameters (drug concentration and time of drug exposure) in the bloodstream over the dosing interval is expressed as the AUC (Figure 9). Outcome of infection in animal models and human studies usually correlates with one of three pharmacodynamic parameters: (1) time of exposure of a bacteria to concentrations of the antibiotic exceeding the MIC of the agent against the pathogen (time above the MIC [T > MIC]); (2) ratio of peak serum concentration of the antimicrobial agent to the MIC of the agent against the pathogen (peak:MIC ratio), and (3) ratio of the AUC to the MIC of the agent against the pathogen (AUC:MIC ratio). Antimicrobial agents can thus be classified based on the pharmacodynamic parameter that best describes their in vivo pattern of bactericidal activity (Table 2).
Fig 9.
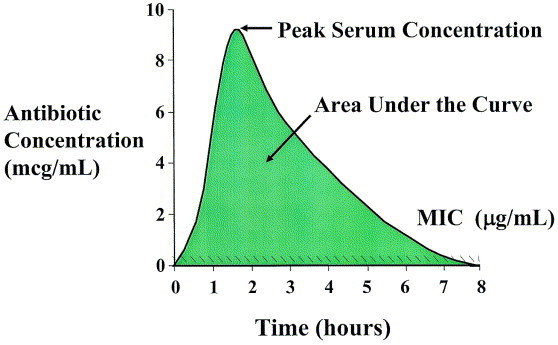
Pharmacodynamically, in vivo bacterial killing may be described as a function of the duration of an antimicrobial’s drug concentration over time relative to the MIC of that agent against a particular pathogen. The product of these pharmacokinetic parameters (drug concentration and time of drug exposure) over the dosing interval is expressed as the area under the concentration-time curve (AUC).
Table 2.
Antimicrobial agents classified by pattern of bactericidal activity
| Drug class | Pharmacodynamic class | Therapeutic goal (for S pneumoniae) |
|---|---|---|
| β-LactamsPenicillinsCephalosporins | Time-dependent | Time above MIC >40%-50% of the dosing interval |
| MacrolidesErythromycinClarithromycinAzithromycin | Time-dependent (with moderate to prolonged persistent effect) | AUC-to-MIC ratio of 25–35 |
| Ketolides | ||
| Telithromycin | Concentration-dependent | Unknown∗ |
| Fluoroquinolones | Concentration-dependent (with prolonged persistent effect) | AUC-to-MIC ratio of 25–35 |
| Gatifloxacin | ||
| Levofloxacin | ||
| Moxifloxacin | ||
Further research is needed.
Antimicrobials exhibiting time-dependent killing
β-Lactams are agents commonly used for respiratory tract infections that exhibit time-dependent killing. These agents do not kill more efficiently when the concentration exceeds a critical value. While a concentration that is two- to fourfold higher than the MIC is generally regarded as being optimal (ie, greatest likelihood of clinical success), further increasing the drug concentration beyond this magnitude does not improve the rate or extent of bacterial killing. These antibiotics exhibit time-dependent killing, and the best predictor of clinical outcome is the duration of time the concentration at the site of infection remains above the MIC (T > MIC) for the bacteria. In simplistic terms, the antibiotic needs to be at a high-enough concentration for a long-enough period of time at the site of infection. For β-lactams and extracellular pathogens, the free-drug concentration in serum is generally proportional to that in the interstitial fluid bathing the organism (protein-bound drug lacks antimicrobial activity). Therefore, the proportion of the dosing interval that the free-drug concentration in serum exceeds the antimicrobials MIC against a pathogen also reflects this parameter at most sites of infection. The amount of time that the free-drug concentration of a time-dependent antibiotic remains above the MIC (T > MIC) generally does not vary with the pathogen or the immunocompetence of the host. Data from in vitro pharmacokinetic simulations, animal models, and human clinical studies suggest that the T > MIC needed to achieve bacterial eradication should generally be >40% to 50% of the dosing interval for time-dependent antibiotics.96, 97 The optimal PK/PD parameter varies somewhat for β-lactams because of variability in the bacterial killing rate. For example, the T > MIC that correlates with optimal outcomes with carbapenems (15% to 25%) is slightly lower than with penicillins (30% to 40%) and cephalosporins (40% to 50%) because carbapenems have a more rapid bacterial killing effect.98
The relationship between the T > MIC and efficacy has been evaluated in patients with acute otitis media caused by S pneumoniae and H influenzae. Bacteriologic cure rates of 80% to 85% were observed when the T > MIC for various β-lactams were >40% to 50% of the dosing interval.99, 100 Moreover, in hospitalized patients with community-acquired pneumonia, no differences in clinical outcome were observed between patients receiving cefuroxime sodium as a 1500 mg per day continuous infusion (T > MIC = 100%) compared to 750 mg intermittently three times daily (estimated T > MIC = 50% to 60%).101 Thus, a serum concentration which is present for 40% to 50% of the dosing interval may be used to determine the susceptibility limit or “breakpoint” of an organism for a given dosing regimen. Additionally, the proportion of bacteria that are therefore susceptible can be based on the proportion of isolates with MICs at or below these susceptibility limits or breakpoints.
Antimicrobials exhibiting time-dependent killing with moderate to prolonged persistent effects
Macrolides/Azalides
Macrolides (eg, erythromycin and clarithromycin) and azalides (eg, azithromycin) exhibit time-dependent killing; however, because of the prolonged postantibiotic effect against gram-positive cocci and H influenzae 102, the pharmacodynamic parameter for these agents that correlates with efficacy is the AUC to MIC ratio rather than T > MIC. The AUC to MIC ratio that yields maximal efficacy with drugs from the macrolide and azalide class in animal models is approximately 25.103
Concern has been raised regarding the propensity of azithromycin to select for bacteria that are macrolide-resistant.104 The impact of community-based azithromycin use on the carriage and resistance of S pneumoniae has been prospectively studied.105 Single-dose azithromycin (20 mg/kg) was given to children with trachoma (a chronic disease caused by Chlamydia trachomatis) and to their household contacts who were children. Carriage rates of azithromycin-resistant S pneumoniae immediately before treatment and 2 to 3 weeks, 2 months, and 6 months after treatment were 2%, 55%, 35%, and 6%, respectively. The selective pressure of azithromycin may have allowed the growth and transmission of preexisting azithromycin-resistant strains.
One possible explanation for this observation relates to the long serum half-life of azithromycin and the long duration of subinhibitory concentrations of the drug.106 If the serum AUC for two antimicrobials, one with a short and the other with a long serum half-life, are compared with MIC values superimposed, a period or “window” for potential Darwinian selection can be plotted (Figure 10). For the antimicrobial with a short half-life, the duration of time between the drug concentration falling below the MIC and its total elimination from the body is relatively short compared to that of the antimicrobial with the longer half-life. For an antimicrobial with a 68-hour half-life (eg, azithromycin), total elimination from the body does not occur for 5 to 7 half-lives or 14 to 20 days. This period of subinhibitory concentrations of drug may be the pharmacodynamic explanation for the aforementioned observations. This concept is controversial and requires validation in future studies, but similar findings have recently been reported in a study from Israel.94
Fig 10.
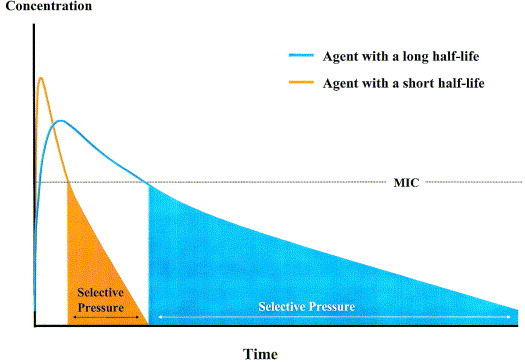
If the serum concentration-time curves (AUC) for two antimicrobials, one with a short and the other with a long serum half-life, are compared with MIC values superimposed, a period or “window” for potential Darwinian selection develops as illustrated in this plot.
Antimicrobials exhibiting concentration-dependent killing and prolonged persistent effects
Fluoroquinolones and ketolides exhibit a concentration-dependent mechanism of bacterial killing, in which they kill most efficiently when their concentrations are appreciably above the MIC of the pathogen.97, 107, 108 The goal of dosing regimen is to maximize drug concentration at the site of infection. The AUC:MIC ratio and the peak:MIC ratio are the major parameters correlating with efficacy. Fluoroquinolones eradicate organisms best at levels 10- to 12-fold higher than the MIC for the pathogen. Increases between 1 and 10 times the MIC of the S pneumoniae organism, the rate and extent of killing is increased, but the rate and extent of killing do not improve if the organism is initially susceptible to quinolones. If the organism has a range of susceptibilities, however, the rate and extent of killing favors the more potent in vitro agents.107, 108, 109, 110 If the optimal peak-to-MIC ratio is obtained, most bacteria die rapidly and consequently, the period of time over which the bacteria is exposed to the drug exposure is minimal.
Although peak-to-MIC ratios of >10:1 to 12:1 correlate with optimal bactericidal activity, 111 the AUC to MIC ratio is a better parameter for determining efficacy of fluoroquinolones for moderately susceptible bacteria, such as S pneumoniae.111 In fact, in most fluoroquinolone dose-fractionation studies, the AUC to MIC ratio has a better correlation with efficacy than peak to MIC ratio. Data obtained from several sources including animal models of sepsis, in vitro pharmacodynamic experiments, and clinical outcome studies indicate that the magnitude of the AUC to MIC ratio can be utilized to predict outcomes. Forrest et al112 demonstrated that an AUC to MIC ratio of ≥125 was associated with the highest bacterial eradication rates in the treatment of infections caused by gram-negative enteric pathogens. However, for gram-positive bacteria, it appears that effective AUC to MIC ratios can be appreciably lower. For instance, against S pneumoniae, an in vitro model of infection demonstrated that for levofloxacin and ciprofloxacin an AUC to MIC ratio of approximately 30 was associated with a 4-log reduction in bacterial titers; while ratios <30 were associated with significantly reduced rates of bacterial killing and in some instances bacterial regrowth.113 Similarly, Lister and Sanders114 reported that for levofloxacin and ciprofloxacin an AUC-to-MIC ratio of 32 to 44 was associated with maximal eradication of S pneumoniae in an in vitro model of infection. These observations are supported by data from non-neutropenic animal models of infection, in which survival was associated with an AUC-to-MIC ratio of 25 to 30 against the pneumococcus.115
Moreover, these observations from in vitro models of infection are further supported by clinical data. The relationship between microbiologic response and the AUC-to-MIC ratio for gatifloxacin and levofloxacin was recently evaluated in patients with pneumococcal respiratory tract infections.116 This analysis demonstrated that for gatifloxacin and levofloxacin, AUC-to-MIC ratios of at least 33.7 correlated with the eradication of S pneumoniae. AUC-to-MIC ratios >33.7 were associated with 100% of patients having a positive microbiologic response to therapy, while those patients with AUC-to-MIC ratios <33.7 had only a 64% response to therapy. The probability of attaining an AUC-to-MIC ratio exceeding 30 with currently approved doses varies among fluoroquinolones (moxifloxacin ≥ gatifloxacin ≥ levofloxacin).
Ketolides have not yet been approved for use in the United States, and their optimal PK/PD parameters have not yet been clearly established. Certain animal models of infection (eg, mouse thigh infection) suggest that the AUC-to-MIC ratio that correlates with efficacy against S pneumoniae for most ketolides is 25 to 50, 103, 117 whereas higher ratios (up to 100) improve survival.117 For one of the ketolides, telithromycin, the AUC-to-MIC ratio that correlates with efficacy for S pneumoniae may be much higher (between 50 and >200).118, 119, 120 Based on this uncertainty, we have considered telithromycin to be considered equivalent to currently available macrolides/azalides until subsequent data proves otherwise. Bacteriologic eradication rates in clinical trials to date with telithromycin suggest this agent may be valuable for the management of community-acquired respiratory tract infections, although the value of such studies is limited, as most bacteriologic outcomes are presumed outcomes based on clinical outcome.121
The PK/PD goals identified using animal models generally correlate with those in humans, and despite PK differences between animals and humans, the PD target is similar. This should not be surprising because the antimicrobial target is within the bacterial pathogen and not the mammalian host. However, the animal models often exclude host defenses (ie, neutrophils) to more clearly delineate the effects of antimicrobial therapy, and there are data to suggest that the PK/PD goal may be lower for certain agents (eg, ketolides) in the presence of adequate host defenses.122, 123 Furthermore, the PK/PD goals from animal models are calculated based on the assumption that serum concentrations approximate concentrations at the site of infection. However, certain agents (eg, macrolides) tend to accumulate at various sites of infection (eg, epithelial lining fluid), which may affect the PK/PD goal for these agents.124
Pharmacokinetic and pharmacodynamic principles play an important role in the evaluation and selection of antimicrobial therapy for ABRS and bacterial infections, in general. Once the PK/PD parameter that best predicts antimicrobial activity in vivo (ie, T > MIC, AUC:MIC ratio) is identified and the magnitude of the PK/PD parameter required for efficacy is determined (ie, PK/PD goal), resistance can be defined for situations in which the PK/PD goal cannot be achieved. The PK/PD goal generally does not change based on the site of infection, it is not affected by the dosing regimen or the infecting pathogen (including resistant strains), or the use of other agents in the same drug class (as long as free-drug concentrations are used).
Current NCCLS breakpoints125 for the same agent vary considerably, depending on the pathogen, whereas PK/PD breakpoints are the same for all pathogens. For S pneumoniae, PK/PD breakpoints are generally the same as, or within one-doubling–dilution of, NCCLS susceptibility breakpoints. As a result, both NCCLS and PK/PD breakpoints are similar at characterizing the activity of various agents against S pneumoniae.
However, there are significant differences between NCCLS and PK/PD susceptibility breakpoints for several antimicrobial agents against H influenzae, and the breakpoints used (NCCLS vs. PK/PD) can affect the interpretation of findings from surveillance studies.89, 126 As PK/PD breakpoints are based on the PK/PD relationships of the agents that result in successful clinical outcomes, rather than on MIC distributions of various species, the use of PK/PD breakpoints overcomes most of the limitations associated with use of NCCLS breakpoints.
Defining aantimicrobial susceptibility breakpoints
As previously discussed, pharmacokinetic/pharmacodynamic PK/PD parameters can be used to define susceptibility breakpoints for antibiotics. Defining susceptibility breakpoints for antimicrobial agents does not require special data sets or extensive in vitro or clinical studies. In fact, in most cases, PK/PD breakpoints can be determined from previously published data (ie, plasma concentration vs. time curves). For time-dependent agents (eg, β-lactams), the PK/PD breakpoint can be determined by identifying the timeframe (X-axis) that corresponds with 40% to 50% of the dosing interval and extrapolating that to the concentration (Y-axis) (Figure 11). A similar process can be used to determine the PK/PD breakpoint for concentration-dependent agents, in which the AUC (for the unbound serum fraction) is divided by 25 or 30.
Fig 11.
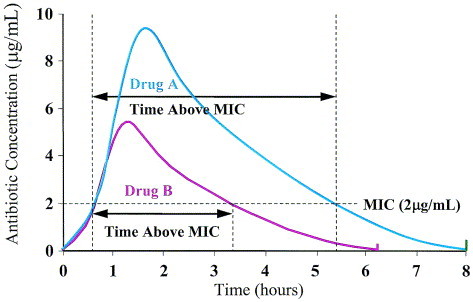
Determining PK/PD breakpoints: Time above the MIC. Schematic illustration of the serum pharmacokinetic profile of two time-dependent oral drug regimens over an 8-hour dosing interval. Drug A is present at 2 μg/mL for >50% of the dosing interval. Drug B is present at 2 μg/mL for approximately 35% of the dosing interval, but at 1 μg/mL for >50% of the dosing interval. Therefore, infections caused by a pathogens for which the MICs of both drugs are 2 μg/mL are more likely to be cured by Drug A rather than Drug B. Drug B would, however, be effective against strains where the MIC is ≤1 μg/mL, as Drug B is present at 1 μg/mL for >50% of the dosing interval. Drugs A and B can be two different time-dependent drugs, or two different dosing regimens of the same agent. A similar process can be done to determine the PK/PD breakpoint for concentration-dependent agents, in which the AUC (for the unbound serum fraction) is divided by 25 or 30.
Table 3 compares the susceptibility of isolates of S pneumoniae, H influenzae, and M catarrhalis to various antibiotics according to their PK/PD breakpoints.89 The panel used PK/PD breakpoints in preference to NCCLS125 or FDA breakpoints to allow unbiased comparisons of sinusitis pathogens using one breakpoint for each agent.
Monte Carlo simulations
As discussed in the previous section, two of the most important factors that influence the effectiveness of a particular antibiotic regimen are the drug exposure in the individual, which is reflected by the pharmacokinetics and pharmacodynamics of the drug, and the susceptibility of the infecting pathogen to the anti-infective agent selected for therapy, which is reflected by the MIC of the agent against the pathogen.
However, because of natural differences in biological systems, both MICs and human pharmacokinetic curves in serum distribute across a range of values.127, 128 Consequently, some bacterial strains are less susceptible to an antimicrobial agent than others and some are more susceptible than others. Similarly, some people absorb, metabolize, distribute, and excrete a drug more rapidly than others, and some more slowly, leading to considerable variations in pharmacokinetic parameters.129 As a result of these variations, antimicrobial-agent efficacy in vivo may differ from the in vitro prediction of drug susceptibility in some patients. To determine the true efficacy of an agent in every patient, the MIC of the causative organism against the agent used and the serum pharmacokinetics of the agent would need to be determined in each patient. This is, of course, physically impossible, and has only been performed on a small scale for both practical and ethical reasons.111 Pharmacokinetic/pharmacodynamic breakpoints are, therefore, currently determined from mean serum pharmacokinetic values, which do not reflect variations in pharmacokinetics from patient to patient, so that some patients will not achieve the target needed, while others will exceed the target.130 An example of this using susceptibility of gatifloxacin and levofloxacin against S pneumoniae is shown in Figure 12. 131 The susceptibility breakpoints for standard dosing regimens of these agents, based on unbound serum AUC divided by 30, is 1 μg/mL for gatifloxacin and 2 μg/mL for levofloxacin, and virtually all isolates are “susceptible” at these breakpoints (Figure 12, panels A and B). However, the modal MIC of gatifloxacin is 0.25 μg/mL, or one quarter of the breakpoint, whereas that of levofloxacin is 1 μg/mL or one half of the breakpoint.132 Variations in pharmacokinetics between patients are therefore more likely to result in levofloxacin not achieving its target more often than gatifloxacin, as gatifloxacin has a wider “safety margin” than does levofloxacin between MIC values and breakpoints. The variability in pharmacokinetics of these agents in patients enrolled in clinical trials, using dosing regimens of 400 mg once daily for gatifloxacin and 500 mg once daily for levofloxacin, is illustrated in panels C and D of Figure 12. AUC values for gatifloxacin varied from 8 to 500 (mean 64), while values for levofloxacin varied from 17 to 389 (mean 70). The “average” AUC and modal MIC values for these two agents would therefore result in “average” AUC:MIC ratios of 256 for gatifloxacin and 70 for levofloxacin. However, the variations in AUCs between patients and in MICs between isolates could result in AUC:MIC ratios varying from 1.5 to 6667 for gatifloxacin and from 3 to 2783 for levofloxacin.131
Fig 12.
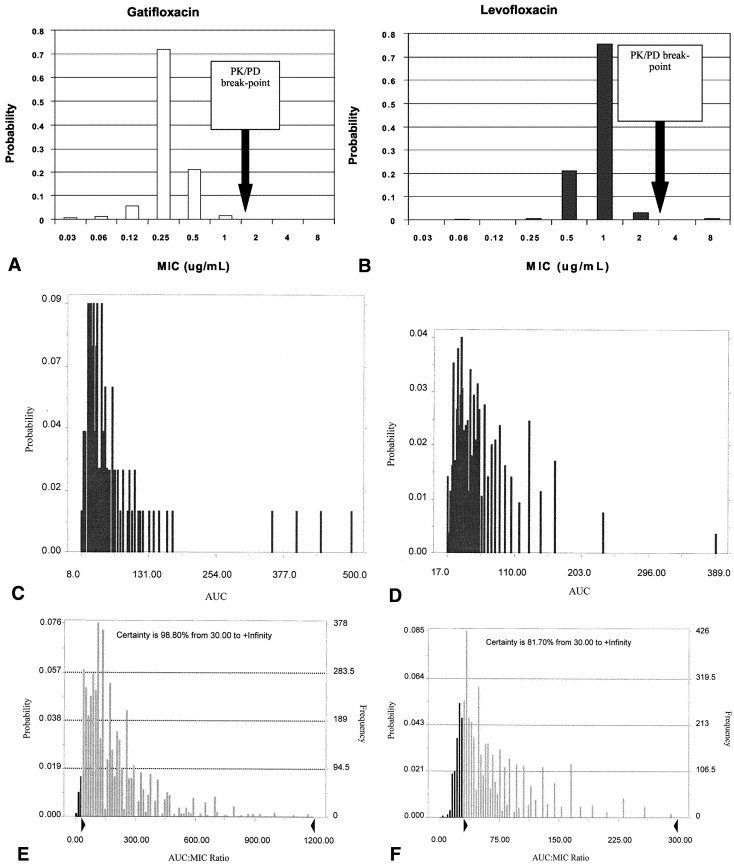
Monte Carlo simulation. (A, B) Distribution of gatifloxacin and levofloxacin MICs against S pneumoniae from the 1999–2000 Sentry Respiratory Surveillance Program study. (C) Distribution of gatifloxacin free-drug area under the concentration-time curve (AUC)0–24 (μg · h/mL) ratio in the patient population. (D) Distribution of levofloxacin free-drug area under the concentration-time curve (AUC)0–24 (μg · h/mL) ratio in the patient population. (E) Results of a 5000-patient Monte Carlo simulation for gatifloxacin based on MIC and AUC distributions presented in (A) and (C). The dark bars represent the number of simulated patients with AUC:MIC ratios <30, whereas the light bars represent patients with AUC:MIC ratios of ≥30. The probability of gatifloxacin attaining an AUC:MIC ratio of at least 30 is 98.80%. (F) Results of a 5000-patient Monte Carlo simulation for levofloxacin based on MIC and AUC distributions presented in (B) and (D). The dark bars represent the number of simulated patients with AUC:MIC ratios <30, whereas the light bars represent patients with AUC:MIC ratios of ≥30. The probability of levofloxacin attaining an AUC:MIC ratio of at least 30 is 81.7%. Reprinted with permission from reference.131
Monte Carlo simulation is a statistical method for estimating the probability of obtaining a desired target, which, in this case, is the pharmacokinetic/pharmacodynamic parameter needed to eradicate an infection, such as an AUC:MIC ratio of 30. Monte Carlo methods randomly select values from within a fixed range and selected to fit a probability distribution (eg, bell curve). Monte Carlo simulation can use individual values in two data sets, in this case MICs and AUCs, to generate random AUC:MIC ratios from randomly chosen MIC and AUC values. This can be done thousands of times, and the distribution of these results can be plotted.
The results of this simulation performed 5000 times on the data from panels A to D are shown in panels E and F of Figure 12. Based on these analyses, the AUC:MIC target value of ≥30 was achieved with 99% certainty for gatifloxacin and 82% for levofloxacin.131 Although the clinical implications of these findings have yet to be fully explored, such analyses provide further insight into optimal patient management. The FDA advisory committee on anti-infective drug products found Monte Carlo simulation, as presented by Drusano in October 1998, to be a reasonable approach to addressing these issues. Further study of these problems is needed, particularly to address variations in pharmacokinetics between each dose of an agent during a course of therapy in an individual patient as most data collected to date only reflected pharmacokinetics determined over one dosing interval in each patient.
Antimicrobial activity according to pharmacokinetic/pharmacodynamic breakpoints
Of oral agents, the respiratory fluoroquinolones have the greatest in vitro activity against the predominant pathogens. However, parenterally administered ceftriaxone may assure adequate concentration and provide better bacteriologic outcomes compared with oral antimicrobial therapy.
The relative antimicrobial activity against isolates of S pneumoniae based on PK/PD breakpoints, 89 can be listed as: gatifloxacin / levofloxacin / moxifloxacin (>99%); ceftriaxone / high-dose amoxicillin (± clavulanate [extended-release or extra strength]) (95% to 97%); amoxicillin (± clavulanate) / clindamycin (90% to 92%); cefpodoxime proxetil /cefuroxime axetil / cefdinir /erythromycin /clarithromycin / azithromycin / telithromycin / cefprozil / TMP/SMX / cefixime (63% to 75%); loracarbef / cefaclor (<20%).
The relative antimicrobial activity against H influenzae based on PK/PD breakpoints is: gatifloxacin / moxifloxacin / ceftriaxone / cefixime / cefpodoxime proxetil / extended-release and extra strength amoxicillin/clavulanate / amoxicillin/clavulanate (95% to 100%); cefuroxime axetil / cefdinir / TMP/SMX / amoxicillin (70% to 85%); cefprozil / cefaclor / loracarbef / doxycycline / erythromycin / clarithromycin / azithromycin / telithromycin (<25%).89, 90
The relative antimicrobial activity against M catarrhalis is: gatifloxacin / levofloxacin / moxifloxacin / cefixime / extended-release and extra strength amoxicillin/clavulanate / telithromycin / erythromycin / clarithromycin / azithromycin (100%); doxycycline/ceftriaxone / cefpodoxime proxetil / cefdinir (78% to 96%); cefuroxime axetil (50%); cefprozil / amoxicillin/ TMP/SMX / cefaclor/loracarbef (<20%).89, 90
Antimicrobial classes
Currently, the oral antimicrobial classes used to treat ABRS include: β-lactams, fluoroquinolones, macrolides/azalides, lincosamides, tetracyclines, and sulfonamides/trimethoprim, while one ketolide has undergone clinical study but has not yet been approved for clinical use by the FDA.
β-lactams
This class of antimicrobials—which are characterized by the presence of a β-lactam ring—includes numerous compounds, many with different spectra of activity. The β-lactams exert their antibacterial effect by inhibiting cell-wall synthesis and producing autolysis. This action is accomplished through the binding of the antimicrobial to the various PBPs in the cell wall.
Orally available agents include the penicillins (with and without β-lactamase–inhibitor compounds) and the cephalosporins. Cephalosporins have been modified to broaden the spectrum of antimicrobial activity, and increase stability in the presence of β-lactamases. The physicochemical properties of many oral cephalosporins make them less suitable than penicillin/amoxicillin when S pneumoniae is the infecting pathogen. Cephalosporins are inherently less active than penicillin/amoxicillin against S pneumoniae—many of these agents have baseline MICs that are fourfold higher than that of amoxicillin. Furthermore, cephalosporins are actively absorbed in the gastrointestinal tract, which limits the concentration that can be achieved, regardless of the magnitude of dose administered.
Amoxicillin and amoxicillin/clavulanate
A less potent but better-absorbed derivative of ampicillin, amoxicillin is relatively safe and well tolerated. Given its intrinsic activity and excellent bioavailability, amoxicillin is generally considered the most active of all oral β-lactams against streptococci, including pneumococci. While the addition of clavulanate to amoxicillin does not affect the intrinsic activity against S pneumoniae, clavulanate does preserve the activity of amoxicillin in the presence of β-lactamases.
Resistance to penicillin in isolates of S pneumoniae is relative and may be overcome by using higher doses of amoxicillin. While the “typical” adult amoxicillin dose is 1.5 to 1.75 g/day and the “typical” pediatric amoxicillin dose is 40 to 45 mg/kg per day, pharmacokinetic and pharmacodynamic research indicates that higher daily doses may be necessary to eradicate S pneumoniae with high MICs. Serum levels of amoxicillin increase linearly with the dose (ie, gastrointestinal absorption is not a limiting factor), and the difference in the incidence of adverse effects between lower and higher doses is negligible.
For the purposes of these guidelines, high-dose amoxicillin is defined as 4 g/day for adults and 90 mg/kg per day for children. High-dose amoxicillin/clavulanate is defined as 4 g of amoxicillin with 250 mg of clavulanate per day for adults, and 90 mg/kg per day of amoxicillin with 6.4 mg/kg per day of clavulanate (in two divided doses) for children. The only formulations of high-dose amoxicillin approved by the FDA are in combination with clavulanate, with separate formulations for adults and children. The adult formulation uses a modified-release mechanism to provide a pharmacokinetically enhanced version of amoxicillin/clavulanate. The pediatric formulation provides a 14:1 ratio of amoxicillin to clavulanate in an oral suspension.
High-dose amoxicillin (with or without clavulanate), appears to be safe and promising for respiratory tract pathogens, including penicillin-nonsusceptible S pneumoniae and β-lactamase–producing organisms (in combination with clavulanate).52, 133, 134, 135, 136
The intrinsic activity of amoxicillin against β-lactamase–negative strains of H influenzae is fair to good. Amoxicillin is 20 to 50 times less potent than third-generation cephalosporins (ie, cefixime, cefpodoxime), and occasional failures may be expected in infections caused by β-lactamase–negative strains of H influenzae treated with standard doses of amoxicillin. While the addition of clavulanate enhances the activity against β-lactamase–producing strains of H influenzae, drugs or formulations that optimize PK/PD performance help prevent treatment failures that occur when patients concentrate the drug at the site of infection (for varying reasons) to a less than average degree, especially when the average tissue concentration is close to the MIC of the pathogen (see discussion on Monte Carlo analyses). This is the reason why recent studies show that high-dose amoxicillin (± clavulanate) has significantly fewer bacteriologic failures against β-lactamase–negative H influenzae than lower doses, even though the in vitro susceptibility rate for regular doses of amoxicillin-clavulanate is 98%.
The addition of clavulanate does not appear to be a driving force in the development of resistance. When administered three times a day, amoxicillin/clavulanate has been associated with a high incidence of gastrointestinal side effects compared to most of its alternatives. The incidence is significantly less with twice-a-day dosing. In general, when the clavulanate dose exceeds approximately 10 mg/kg per day, diarrhea can become a problem.
Cefaclor
Cefaclor has poor activity against H influenzae, fair activity against penicillin-susceptible pneumococci, and no activity against DRSP. Therefore, cefaclor has poor overall efficacy against bacterial respiratory tract pathogens.
Cefdinir
Cefdinir is an extended-spectrum semisynthetic cephalosporin, for oral administration with activity against S pneumoniae that is comparable to second-generation agents (eg, cefuroxime axetil, cefpodoxime proxetil).137 Its activity against H influenzae is similar to cefuroxime axetil, but lower than that of cefpodoxime proxetil. Cefdinir is not appreciably metabolized and is eliminated principally via renal excretion. This agent is generally well tolerated, and the suspension formulation is very well accepted among children.138, 139
Cefixime
As the prototype oral third-generation oral cephalosporin, cefixime has potent activity against H influenzae but provides limited gram-positive coverage including S pneumoniae. Cefixime has no activity against staphylococci, may occasionally fail against even penicillin-susceptible pneumococci, and has no clinically significant activity against DRSP.
Cefpodoxime proxetil
Cefpodoxime proxetil, a third-generation oral cephalosporin, is a structural analog of ceftriaxone, and has similar activity to cefixime against respiratory pathogens. The activity of cefpodoxime proxetil is similar to that of cefuroxime axetil and cefdinir against S pneumoniae, but greater against H influenzae. Because of its spectrum of activity, cefpodoxime proxetil often is regarded as the preferred treatment for patients in whom treatment with high-dose amoxicillin or amoxicillin/clavulanate fails (or is intolerable). However, the clinical utility (ie, adherence) of the suspension formulation for children is often limited by its poor taste.
Cefprozil
Cefprozil is another good-tasting and well-tolerated broad-spectrum cephalosporin that has activity against S pneumoniae similar to cefdinir and cefuroxime axetil.137 However, cefprozil is markedly less active against H influenzae.
Ceftriaxone
Ceftriaxone is a semisynthetic, broad-spectrum cephalosporin antibiotic for intravenous (IV) or intramuscular (IM) administration. Ceftriaxone sodium is completely absorbed following IM administration with mean maximum plasma concentrations occurring between 2 and 3 hours post dosing. Multiple intravenous or IM doses ranging from 0.5 to 2 g at 12- to 24-hour intervals resulted in 15% to 36% accumulation of ceftriaxone above single-dose values.
Cefuroxime axetil
Parenteral cefuroxime sodium has a long-established history in the treatment of moderate-to-severe lower respiratory infections caused by H influenzae and S pneumoniae. An oral formulation, cefuroxime axetil, was introduced in the 1980s, it has demonstrated good potency, efficacy, and side effect profiles. The activity of cefuroxime axetil against S pneumoniae is similar to cefpodoxime and cefdinir. Cefuroxime axetil is less active than cefpodoxime against H influenzae.
Loracarbef
Loracarbef is comparable to cefaclor in its activity against pathogens in respiratory tract infections.
Fluoroquinolones
Gatifloxacin, levofloxacin, and moxifloxacin
Fluoroquinolones exert their bactericidal activity by binding to DNA gyrase and topoisomerase IV. This impedes the formation of supercoiled DNA, inhibits the relaxation of supercoiled DNA, and promotes double-strand DNA breakage. Ciprofloxacin has excellent activity against H influenzae and M catarrhalis, but the AUC-to-MIC ratio against S pneumoniae is only 10 to 20, whereas the target AUC-to-MIC ratio of fluoroquinolones for S pneumoniae is approximately 25 to 30.97, 140 Ciprofloxacin in combination with adequate gram-positive therapy (eg, clindamycin) could be used for patients with rhinosinusitis. The newer fluoroquinolones (gatifloxacin, levofloxacin, and moxifloxacin) have remarkable potency against H influenzae and M catarrhalis and, unlike ciprofloxacin, potency against gram-positive pathogens, including S pneumoniae.141 Gemifloxacin is another quinolone that is active against respiratory pathogens, but this agent is not currently approved for the management of sinusitis.
While the gastrointestinal absorption of these agents is inhibited by the coadministration of foods or supplements with certain multivalent cations (magnesium, aluminium, iron, ± calcium), they generally lack the safety concerns (ie, phototoxicity) observed with some other quinolones. Achilles tendon rupture (and other tendinopathies) is likely a class effect of the fluoroquinolones, and is a particular concern among patients with renal dysfunction/failure.142 The use of gemifloxacin in women for longer than 5 days is associated with an increased likelihood of rash. The fluoroquinolones are currently not approved for use in children in the United States.
The predominant concern surrounding fluoroquinolone use pertains to the selection of class resistance in organisms such as gram-negatives (especially Pseudomonas aeruginosa), staphylococci, and pneumococci. Recent data evaluating fluoroquinolones and the propensity to select resistant pathogens, especially S pneumoniae, suggest that the differences in pharmacodynamics are related to the frequency of resistance selection. While the specific pharmacodynamic criteria for resistance prevention are still to be established, it appears that the most potent agents are least likely to promote/select resistance.
Fluoroquinolones are increasingly being used as empiric therapy for the management of community-acquired respiratory tract infections, in part because of prevalent resistance to more traditional agents. As with most antimicrobial agents, development of resistance among S pneumoniae strains to one fluoroquinolone generally leads to cross-resistance to all members of the fluoroquinolone class, and there is evidence that inappropriate use of pharmacodynamically inferior fluoroquinolones is linked to the development of resistance and to clinical failures.143, 144, 145, 146 Because of this, fluoroquinolones should not be used indiscriminately, and the most pharmacodynamically potent fluoroquinolones should be used to treat the suspected pathogen. When the decision is made to use a fluoroquinolone, preference should be given to agents that are most likely to achieve optimal PK/PD parameters. The relative in vitro potency (based on PK/PD parameters) for several fluoroquinolones was moxifloxacin > gatifloxacin > levofloxacin. Higher doses of levofloxacin (750 mg/day) improve its PK/PD profile.
Macrolides/Azalides
Erythromycin, clarithromycin, and azithromycin
The macrolides include agents such as erythromycin and clarithromycin, whereas azithromycin, an azalide, is a closely related agent. These agents are active against gram-positive and some gram-negative bacteria. Bacterial ribosomes consist of a 50s subunit (comprised of a 23S and a 5S rRNA), a 30s subunit, and approximately 50 proteins. The mechanism of action of macrolides/azalides involves inhibition of RNA-dependent protein synthesis by binding to the 50S subunit of the bacterial ribosome—specifically at the polypeptide exit region. Although they are generally considered to be bacteriostatic, they are bactericidal against autolytic species such as pneumococci.
Macrolides exhibit better antibacterial activity in an environment with a neutral to basic pH. This physicochemical characteristic is due to the fact that at a low pH macrolides become positively charged and do not readily cross biological membranes. This effect is most pronounced for azithromycin because it carries a double-positive charge at a low pH.
All of the macrolides have good activity against macrolide susceptible pneumococci. However, the increasing prevalence of macrolide resistance to S pneumoniae is associated with a significant likelihood of clinical failure.147 Furthermore, resistance to macrolides has been correlated with increased macrolide use, 15 and of these agents, azithromycin use is more likely to select for resistant strains than clarithromycin use.148
While clarithromycin and azithromycin have slightly greater activity against H influenzae than erythromycin, most of the available eradication and efficacy studies suggest an activity that is similar to or marginally higher than that of placebo. There is some controversy surrounding the antimicrobial activity of metabolites (14-OH clarithromycin), the intracellular concentrations of the newer agents, and the effects of pH on MIC results, none of which impact the foregoing conclusions about the activity of these drugs for extracellular pathogens (ie, S pneumoniae and H influenzae). Macrolides/azalides are active against M catarrhalis.
Lincosamides
Clindamycin
Clindamycin, which is structurally different from the macrolides, also acts by binding the 50S ribosomal subunit of susceptible bacteria thereby suppressing protein synthesis. Clindamycin has a concentration-dependent mechanism of antimicrobial activity, 149 and this agent is used clinically for the treatment of susceptible gram-positive aerobes and anaerobes as well as many gram-negative anaerobes. It is not, however, active against H influenzae and M catarrhalis.
Tetracyclines
Doxycycline
These antibiotics inhibit bacterial growth via inhibition of RNA-dependent protein synthesis by reversibly binding to the 30S ribosomal subunit and prevent binding of t-RNA. A derivative of tetracycline, doxycycline has adequate activity against penicillin-susceptible pneumococci. Like other oral non-β–lactams, the likelihood of nonsusceptibility to doxycycline rises in pneumococcal strains exhibiting any degree of penicillin resistance.89 Doxycycline also has activity against M catarrhalis, but its activity against H influenzae is limited by its pharmacokinetics. Clinicians should be aware of the possibility of photosensitivity and infrequent esophageal caustic burns. Like the other tetracyclines, usage in children <8 years of age is contraindicated because of the possibility of tooth enamel discoloration.
Rifamycins
Rifampin
The prototype agent in this class is rifampin, which is a semisynthetic derivative of rifamycin B. Rifampin binds to the β subunit on RNA polymerase, which blocks RNA transcription (suppresses the initiation of chain formation), resulting in a bactericidal effect. Rifampin is active against a variety of intracellular and extracellular microorganisms, including gram-positive and -negative bacteria, fungi, and parasites. Recent surveillance studies150, 151 demonstrate that rifampin is active against approximately 99%, 96%, and 100% of S pneumoniae, H influenzae, and M catarrhalis isolates, respectively. While rifampin has been available for decades, the PK/PD profile of this agent is not known. Despite its activity against predominant respiratory pathogens, rifampin should not be used indiscriminately as monotherapy or for a prolonged duration because resistance to this agent develops rapidly.
At usual doses, the antimicrobial effect of rifampin is relatively specific to microorganisms (ie, mammalian RNA synthesis is not affected). Rifampin distributes widely throughout the body, and it is a well-known inducer of several cytochrome p450 isoenzymes and therefore has a high potential for drug interactions.
Folate inhibitors
Trimethoprim-sulfamethoxazole
Sulfonamides disrupt bacterial folic acid synthesis by inhibiting dihydropteroate synthase; this results in their bacteriostatic activity. TMP is a pyrimidine analog that inhibits dihydrofolate reductase. Because sulfonamides and trimethoprim block folic acid synthesis at different sites, they potentiate each other’s antimicrobial activity producing synergistic activity. High rates of resistance to these drugs are now present in pneumococci and H influenzae (∼25% to 30%).89 M catarrhalis is intrinsically resistant to trimethoprim. In addition, these agents can cause skin rash, erythema multiforme, and toxic epidermal necrolysis, which can be potentially fatal.
New antibiotics
Ketolides are a new class of semisynthetic antibiotics closely related to macrolides, designed, theoretically, to provide greater activity against respiratory tract pathogens, particularly against macrolide-resistant strains of S pneumoniae.121 The mechanism of action of ketolides is similar to that of macrolides; however, ketolides have a higher affinity for the target binding sites on 23S rRNA of the 50S ribosomal subunit, which is partly responsible for the greater in vitro activity against respiratory tract pathogens.152, 153 Ketolides have a concentration-dependent mechanism of antimicrobial killing.154 The structural modifications do not induce the ribosomal methylase-mediated resistance among pneumococcal strains with erm determinants that is common with macrolides and azalides.155 Furthermore, ketolides may retain activity against strains in which ribosomal methylase-mediated or efflux-mediated resistance mechanisms are present.156, 157
Andes et al122 evaluated the impact of neutrophils on the bacteriostatic activity of telithromycin against S pneumoniae in the murine thigh model and reported enhanced potency (1.8- to 24-fold increase) for the ketolides in the presence of neutrophils. Tessier et al158 studied 10 pneumococcal isolates in a murine thigh infection model and observed that telithromycin AUC/MIC ratios of approximately 200 correlated with bacteriostatic activity, whereas ratios of ≥1000 were needed to obtain bactericidal activity of the compound in this model.
Drusano and Preston159 have evaluated the pharmacodynamic profile of telithromycin 800 mg once daily in patients with community-acquired pneumonia. In this clinical study involving infected patients both microbiologic and clinical outcome were correlated with AUC/MIC ratios. The authors report that an AUC/MIC ratio of ≥3.375 resulted in 91% eradication or presumed eradication of the infecting pathogen. At the time of this writing, further research is needed to determine if these AUC/MIC ratios in patients with community-acquired pneumonia are relevant to sinusitis. As with the macrolides and azalides, ketolides have limited activity against H influenzae due to an efflux pump.
Oxazolidinones are a new class of antibiotics with a unique mechanism of action (protein synthesis inhibition) against gram-positive pathogens. Linezolid, the prototype agent from this class, is currently approved for more complicated infections but the emergence of linezolid-resistant gram-positive cocci has been reported. Glycylcyclines are advanced-generation tetracycline derivatives designed to overcome mechanisms of resistance to this class of antibiotics, and agents from this class are still in clinical development (at the time of this writing).
The Poole therapeutic outcome model
Evidence from controlled clinical trials represents the optimal basis for recommending antimicrobial therapy for ABRS. However, many of the current clinical trials evaluating antimicrobial therapy have methodologic limitations (eg, diagnostic criteria, comparators, endpoints, outcomes measures) that preclude them from being considered as evidence. Furthermore, the trends in antimicrobial resistance have changed dramatically over the past 10 years, which has affected the applicability of evidence from older clinical trials. While a large meta-analysis20 recently was conducted to evaluate the role of antimicrobial therapy (in general, and the role of specific agents/classes) in ABRS, many of the individual trials were conducted prior to the widespread resistance that is currently reported in S pneumoniae and H influenzae. As a result, the findings from this meta-analysis may not be applicable to the current treatment of ABRS.160 The methodology used in the present guidelines for evaluating antimicrobial therapy for ABRS does, however, take into account the current high levels of antibiotic-resistant organisms. In the absence of current evidence, it becomes more challenging to identify optimal therapy for ABRS. Rather than subjectively compiling a rank order of antimicrobial agents based on in vitro activity data and expert consensus, we utilized a more objective methodology to assessing treatment options. This approach involved the use of a mathematical model—the Poole therapeutic outcomes model—to predict treatment outcomes in ABRS.
The therapeutic outcomes model was extrapolated from work originally conducted by Marchant et al, 161 involving the correlation between in vivo potency of various antimicrobial agents with clinical outcomes using double-tympanocentesis methodology in otitis media. In these studies, Marchant et al observed that nonbacterial factors can result in discrepancies between bacteriologic and clinical outcomes. For example, an agent with a 100% bacteriologic cure rate will not necessarily have a 100% clinical cure rate because some patients will continue to experience symptoms resulting from nonbacterial factors (eg, viral infection, persistent middle ear effusion). Conversely, antimicrobial agents with relatively poor bacteriologic cure rates often appear to be more effective than expected because of the high rate of spontaneous resolution of symptoms. As a result, highly efficacious agents generally have only slightly better impact on clinical outcomes (ie, symptoms) compared with less-efficacious agents, despite more dramatic differences in bacteriologic outcomes.
The therapeutic outcomes model is a tool to help predict the likelihood of bacteriologic success with particular antimicrobial agents by accounting for various factors including: (1) the proportion of patients with a clinical diagnosis of acute bacterial rhinosinusitis and a positive sinus aspirate; (2) the clinical resolution of disease in the culture-negative patient group; (3) the distribution of pathogens frequently encountered in ABRS; (4) the spontaneous resolution rate associated each pathogen; and (5) the in vitro susceptibility of the predominant sinusitis pathogens to antimicrobial agents at PK/PD breakpoints (Figure 13). The therapeutic outcomes model also can predict overall clinical outcomes for the total patient group (ie, those with either bacterial or nonbacterial disease).
Fig 13.
Factors incorporated in the Poole therapeutic outcome model.
The first component of the mathematical model involves the process of accounting for nonbacterial disease. Among patients with nasal/sinus symptoms (who undergo sinus tap), approximately 35% will have negative bacterial cultures, with symptoms usually due to a primary viral process. The majority of these patients will achieve complete resolution of symptoms without antibiotic therapy. An additional 8% to 14% of patients will have persistent nasal/sinus symptoms, regardless of any antibiotic therapy prescribed. These persistent symptoms are likely due to nonbacterial causes of facial pain, nasal obstruction, or rhinorrhea (eg, allergy, headaches, and anatomic factors). The next component accounts for the distribution of pathogens in ABRS and the likelihood of spontaneous resolution associated with each of these pathogens (based on placebo-controlled trials in acute otitis media). Among bacterial infections—as mentioned above—the pathogen distribution in adults is S pneumoniae (33% to 41%), H influenzae (29% to 35%), and M catarrhalis (4% to 8%). Most studies suggest that 10% to 20% of the bacterial sinus infections are caused by pathogens other than S pneumoniae, H influenzae, or M catarrhalis. For this model, the percentages used for the pathogen distribution have been modified to yield a total of 100%. Also, “miscellaneous” pathogens are omitted from the model in this guideline revision because (1) the FDA does not typically consider them to be important pathogens, (2) adequate susceptibility data against these pathogens is lacking, and (3) the activity of a given antimicrobial agent against miscellaneous pathogens is likely to be close to the average of its activity against the three usual pathogens. The overall susceptibility pattern of a given agent is not substantially affected by the exclusion of these miscellaneous pathogens.
The pathogen distribution in children is S pneumoniae (25% to 30%), H influenzae (15% to 20%), and M catarrhalis (15% to 20%). Modifications of these values are made in the model to give a total of 100%. Spontaneous resolution rates used in this model were 30% for S pneumoniae, 60% for H influenzae, 80% for M catarrhalis and 50% for other pathogens. Spontaneous resolution rates will vary depending on duration of clinical observation, age, and status of mucosal health.
Based on these values, spontaneous resolution of symptoms would be expected in 47% of adults with documented bacterial infection (62% of the total, clinically diagnosed patient group). Among untreated children, spontaneous resolution of symptoms would be expected in 50% among those with bacterial infection (63% of the clinically defined pediatric sinusitis group).
The model also accounts for the effects of antimicrobial therapy on achieving clinical outcomes by using current susceptibility data for each organism at PK/PD breakpoints, because PK/PD susceptibility breakpoints for given dosing regimens have been shown to correlate with bacteriologic cure rates.97, 99 Resolution rates for the bacterially infected group and the total patient group are shown in a Marchant plot Fig 14, Fig 15. 89, 90 These data sets were used because complete MIC distributions were available and the proportion of isolates inhibited at PK/PD breakpoints could be determined. The outcomes of these calculations are shown as Marchant plots, showing predicted bacteriological outcomes in the bacterial infection group and the total patient group. The Marchant plot is only a relative rank order for the data used. Other surveillance data may, therefore, alter this relative rank order. The resolution rates are based on in vitro microbiologic efficacy and do not guarantee clinical outcome. However, in the absence of other evidence, the therapeutic outcomes model was used, as it was regarded as the best method available for objectively predicting clinical outcomes.
Fig 14.
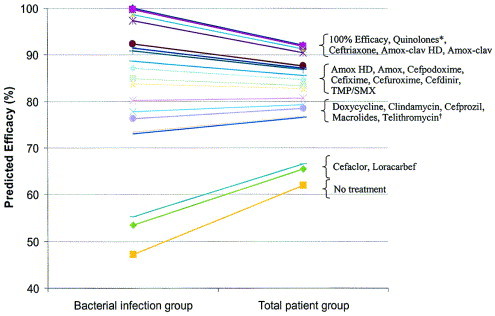
“Marchant” plot for antibiotics used to treat adult acute bacterial rhinosinusitis.89, 90 (∗Respiratory quinolone (ie, gatifloxacin, levofloxacin, moxifloxacin). †The activity of telithromycin against S pneumoniae, H influenzae, and M catarrhalis depends on its PK/PD breakpoints, which is uncertain at this time. The activity of telithromycin is assumed to be similar to that of macrolides/azalides until further information becomes available.)
Fig 15.
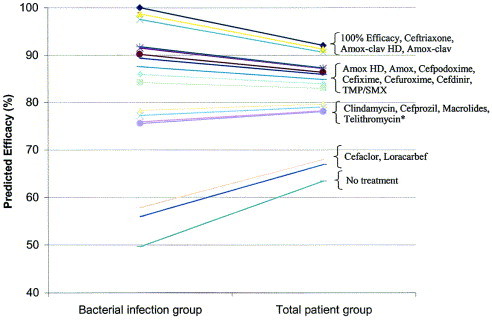
“Marchant” plot for antibiotics used to treat pediatric acute bacterial rhinosinusitis.89, 90 (∗The activity of telithromycin against S pneumoniae, H influenzae, and M catarrhalis depends on its PK/PD profile, which is uncertain at this time. The activity of telithromycin is assumed to be similar to that of macrolides/azalides until further information becomes available.)
A more detailed description of the therapeutic outcomes model is provided elsewhere in this supplement.162
Antimicrobial treatment guidelines
These recommendations are based on the therapeutic outcome model described below. Table 4, Table 5 summarize the panel’s antimicrobial treatment guidelines for adults and children, respectively. Multiple factors played a role in the antimicrobial selection process. Because serious intracranial and extrasinus complications associated with ABRS usually arise secondary to S pneumoniae infection, it is important for initial therapy to adequately cover S pneumoniae. Gram-negative coverage for H influenzae and M catarrhalis (in children) cannot be ignored, however. A rational approach to the treatment of ABRS should consider the aforementioned concerns along with the logical application of microbiology and the pharmacodynamic/pharmacokinetic principles.
Table 4.
Recommended antibiotic therapy for adults with ABRS
| Initial therapy | Calculated clinical efficacy(%)∗ | Calculated bacteriologic efficacy(%)∗ | Switch therapy options (no improvement or worsening after 72 hours)† |
|---|---|---|---|
| Mild disease‡ with no recent antimicrobial use (past 4–6 weeks)§ | |||
| Amoxicillin/clavulanate (1.75–4 g/250 mg/d)§, ∥ | 90–91 | 97–99 | |
| Amoxicillin (1.5–4 g/d)∥ | 87–88 | 91–92 | Gatifloxacin, levofloxacin, moxifloxacin |
| Cefpodoxime proxetil | 87 | 91 | Amoxicillin/clavulanate 4g/250 mg |
| Cefuroxime axetil | 85 | 87 | Ceftriaxone |
| Cefdinir | 83 | 85 | Combination Therapy¶ |
| β-Lactam allergic# | |||
| TMP/SMX | 83 | 84 | |
| Doxycycline | 81 | 80 | Gatifloxacin, levofloxacin, moxifloxacin |
| Azithromycin, clarithromycin, erythromycin | 77 | 73 | Rifampin plus clindamycin |
| Telithromycin∗∗ | 77 | 73 | |
| Mild disease‡ with recent antimicrobial use (past 4–6 weeks) or moderate disease‡ | |||
| Gatifloxacin/levofloxacin/moxifloxacin | 92 | 100 | |
| Amoxicillin/clavulanate (4 g/250 mg) | 91 | 99 | Reevaluate patient‡‡ |
| Ceftriaxone | 91 | 99 | |
| (Combination therapy)¶ | |||
| β-Lactam allergic# | |||
| Gatifloxacin, levofloxacin, moxifloxacin | 92 | 100 | Reevaluate patient†† |
| Clindamycin and rifampin†† | Reevaluate patient†† | ||
Clinical and bacterial efficacy (ie, clinical and microbiologic adequacy) is represented by the calculation from the Poole Therapeutic outcome model (see text) using the mean values of two surveillance data sets: the US component of the Alexander project (1998 to 2001) and SENTRY surveillance data. These values do not guarantee clinical success or failure.
When a change in antibiotic therapy is made, the clinician should consider the limitations in coverage of the initial antibiotic. The respiratory fluoroquinolones (gatifloxacin, levofloxacin, and moxifloxacin), ceftriaxone, and amoxicillin/clavulanate (4 g/250 mg) currently have the best coverage for both S pneumoniae and H influenzae. The terms mild and moderate are designed to aid in selecting antibiotic therapy.
The difference in severity of disease does not imply the presence or absence of antimicrobial resistance. Rather, this terminology indicates the relative degree of acceptance of possible therapeutic failure, and the likelihood of achieving spontaneous resolution of symptoms. The determination of disease severity lies with the clinician’s evaluation of the patient’s history and clinical presentation. Severe, life-threatening infection, with or without complications, is not addressed in these guidelines.
Prior antibiotic therapy within 4 to 6 weeks is a risk factor for infection with resistant organisms. Antibiotic choices should be based on this and other risk factors.
The total daily dose of amoxicillin and the amoxicillin component of amoxicillin/clavulanate can vary from 1.5 to 4 g/day. Lower daily doses (1.5 g/day) are more appropriate in mild disease in patients with no risk factors for infection with a resistant pathogen (including recent antibiotic use). Higher daily doses (4 g/day) may be advantageous in areas with a high prevalence of penicillin-resistant S pneumoniae or DRSP, for patients with moderate disease, for patients who may need better H influenzae coverage or for patients with risk factors for infection with a resistant pathogen. There is a greater potential for treatment failure or resistant pathogens in these patient groups.
Based on in vitro spectrum of activity; combination therapy using appropriate gram-positive and -negative coverage may be appropriate. Examples of combination therapy regimens include high-dose amoxicillin (4 g/day) or clindamycin plus cefixime, or high-dose amoxicillin (4 g/day) or clindamycin, plus rifampin. There is no clinical evidence at this time, however, of the safety or efficacy of these combinations.
Cephalosporins should be considered initially for patients with penicillin intolerance/non-Type I hypersensitivity reactions (eg, rash). TMP/SMX, doxycycline, macrolides, azalides, and ketolides are not recommended unless the patient is β-lactam allergic. Their effectiveness against the major pathogens of ABRS is limited, and bacterial failure of 20% to 25% is possible. A respiratory fluoroquinolone (eg, gatifloxacin, levofloxacin, moxifloxacin) is recommended for patients who have allergies to β-lactams or who have recently failed other regimens.
Telithromycin is not approved for use by the FDA (at the time of writing).
Reevaluation is necessary because the antibiotics recommended for initial therapy provide excellent activity against the predominant ABRS pathogens, including S pneumoniae and H influenzae. Additional history, physical examination, cultures, and/or CT scan may be indicated, and the possibility of other less common pathogens considered.
Rifampin is a well-known inducer of several cytochrome p450 isoenzymes and therefore has a high potential for drug interactions.
Table 5.
Recommended antibiotic therapy for children with ABRS
| Initial therapy | Calculated clinical efficacy (%)∗ | Calculated bacteriologic efficacy (%)∗ | Switch therapy options (no improvement or worsening after 72 hours)† |
|---|---|---|---|
| Mild disease‡ with no recent antimicrobial use (past 4 to 6 weeks)§ | |||
| Amoxicillin/clavulanate (90 mg/6.4 mg/kg per day)∥ | 91–92 | 97–99 | Amoxicillin clavulanate (90 mg/6.4 mg/kg per day) |
| Amoxicillin∥ | 86–87 | 90–92 | Ceftriaxone |
| Cefpodoxime proxetil | 87 | 92 | Combination therapy¶ |
| Cefuroxime axetil | 85 | 88 | |
| Cefdinir | 84 | 86 | |
| β-Lactam allergic# | |||
| TMP/SMX | 83 | 84 | Re-evaluate patient∗∗ |
| Azithromycin, clarithromycin, erythromycin | 78 | 76 | Combination therapy¶ |
| Mild disease‡ with recent antimicrobial use (past 4 to 6 weeks) or moderate disease‡ | |||
| Amoxicillin/clavulanate (90 mg/6.4 mg/kg per day)∥ | 92 | 99 | Reevaluate patient∗∗ |
| Ceftriaxone | 91 | 99 | |
| β-Lactam allergic# | |||
| TMP/SMX | 83 | 84 | Reevaluate patient∗∗ |
| Azithromycin, clarithromycin, erythromycinClindamycin†† | 7879 | 7678 | Combination therapy¶ (clindamycin or TMP/SMX plus rifampin) |
Clinical and bacterial efficacy (ie, clinical and microbiologic adequacy) is represented by the calculation from the Poole therapeutic outcome model (see text) using the mean values of two surveillance data sets: the US component of the Alexander project (1998 to 2001) and SENTRY surveillance data. These values do not guarantee clinical success or failure.
When a change in antibiotic therapy is made, the clinician should consider the limitations in coverage of the initial antibiotic. Ceftriaxone and high-dose amoxicillin/clavulanate currently have the best coverage for both S pneumoniae and H influenzae.
The terms mild and moderate are designed to aid in selecting antibiotic therapy. The difference in severity of disease does not imply the presence or absence of antimicrobial resistance. Rather, this terminology indicates the relative degree of acceptance of possible therapeutic failure, and the likelihood of achieving spontaneous resolution of symptoms. The determination of disease severity lies with the clinician’s evaluation of the patient’s history and clinical presentation. Severe, life-threatening infection, with or without complications, is not addressed in these guidelines.
Prior antibiotic therapy within 4 to 6 weeks is a risk factor for infection with resistant organisms. Antibiotic choices should be based on this and other risk factors.
The total daily dose of amoxicillin and the amoxicillin component of amoxicillin/clavulanate can vary from 45 to 90 mg/kg per day. Lower daily doses (45 mg/kg per day) are more appropriate in mild disease in patients with no risk factors for infection with a resistant pathogen (including recent antibiotic use). Higher daily doses (90 mg/kg per day) may be advantageous in areas with a high prevalence of penicillin-resistant S pneumoniae or DRSP, for patients with moderate disease, for patients who may need better H influenzae coverage or for patients with risk factors for infection with a resistant pathogen. There is a greater potential for treatment failure or resistant pathogens in these patient groups.
Based on in vitro spectrum of activity, combination therapy using appropriate gram-positive and -negative coverage may be appropriate. Examples of combination therapy regimens include high-dose amoxicillin (90 mg/kg per day) or clindamycin plus cefixime, or high-dose amoxicillin (90 mg/kg per day) or clindamycin, plus rifampin. Other combination therapy regimens may be appropriate for patients with β-lactam allergy. There is no clinical evidence at this time, however, of the safety or efficacy of these combinations.
Cephalosporins should be considered for patients with penicillin intolerance/non-Type I hypersensitivity reactions (eg, rash). TMP/SMX, macrolides, and azalides are not recommended unless the patient is β-lactam allergic. Their effectiveness against the major pathogens of ABRS is limited, and bacterial failure of 20% to 25% is possible.
Reevaluation is necessary because the antibiotics recommended for initial therapy provide excellent activity against the predominant ABRS pathogens, including S pneumoniae and H influenzae. Additional history, physical examination, cultures, and/or CT scan may be indicated, and the possibility of other less common pathogens considered.
Excluding β-lactams, clindamycin is the most active oral agent currently available with activity against approximately 90% of S pneumoniae isolates. It has no activity, however, against H influenzae or M catarrhalis.
The panel’s guidelines for adults and children with ABRS characterize two groups of patients: (1) those with mild disease who have not received antibiotics within the previous 4 to 6 weeks; and (2) those with mild disease who have received antibiotics within the prior 4 to 6 weeks and those with moderate disease (regardless of recent antibiotic exposure).
As mentioned previously, the primary reason why antimicrobial therapy is recommended for ABRS is to improve patient health. The terms mild and moderate have been integrated into the decision-making process to reflect the degree of patient discomfort (as evidenced by the symptom complex and the time course of the disease) and the likelihood of experiencing spontaneous resolution of those symptoms. In other words, patients with moderate disease are more likely to require therapeutic intervention to achieve resolution of their symptoms, and these patients are less likely to tolerate treatment failures. The differences in disease severity do not imply the presence or absence of antimicrobial resistance. Patients, however, may not always be neatly categorized based on this classification. An evaluation of disease severity requires clinical judgment gained only by the clinician familiar with the patient. Severe life-threatening infection with or without complications is not addressed in these guidelines.
Recent antibiotic use is a major risk factor associated with infection caused by resistant pathogens.163, 164 Other risk factors include age <5 years and attendance in day-care centers. Because recent antimicrobial exposure increases the risk of carriage and infection due to resistant organisms, antimicrobial therapy should be based on the patient’s history of recent antibiotic use. The panel’s guidelines stratify patients according to antibiotic exposure within the previous 4 to 6 weeks.
Lack of response to therapy at ≥72 hours is an arbitrary timeframe established to define treatment failures. Clinicians should monitor the patient’s response to antibiotic therapy, which may include instructing the patient to call the office or clinic if there is persistence or worsening of symptoms over the next few days.
The current recommendations for the duration of antimicrobial treatment for ABRS is 10 to 14 days, which is based on results of clinical trials that performed pre- and posttreatment sinus aspirates.36 The new technique of serial sinus sampling47 is designed to better define the optimal duration of treatment for ABRS.
Allergies to antibiotics (ie, β-lactams) or age limitations for certain antimicrobials (ie, fluoroquinolones) may preclude the use of optimal antimicrobials, and clinicians should be aware of the potential for treatment failure in these situations.
The panel used the therapeutic outcomes model as a tool in developing its antimicrobial recommendations. While the most recent and best data were used for this model, the panel realizes that resistance rates may change over time and may vary from community to community. The panel, therefore, will continue to revise the guidelines as resistance rates change and new antibiotics are introduced.
Antimicrobial choices
The following values calculated using the therapeutic outcomes model represent predicted clinical outcome in clinically diagnosed sinusitis. Clinical criteria were used in place of bacteriologic criteria (ie, bacteriologic outcome in patients with bacterial infection) because clinical outcomes are more consistent with what is encountered in everyday practice. While predicting bacteriologic outcomes in patients with bacterial infection may be ideal, the values obtained using the therapeutic outcomes model may be inconsistent with clinical experience. As mentioned previously, antibiotics should be reserved for patients with bacterial infection, with the primary goal of eradicating the pathogen from the site of infection. However, the limitations associated with differentiating bacterial from nonbacterial disease inevitably result in patients with nonbacterial disease receiving antibiotic therapy. Using antibiotics for patients with nonbacterial disease often dilutes or reduces the perceived bacteriologic efficacy of the antibiotic. This often is the case with bacteriologic findings from clinical trials that have methodologic limitations (eg, inclusion criteria, diagnostic criteria).
According to the therapeutic outcomes model, antibiotics can be placed into the following relative rank order of predicted clinical efficacy for adult patients: 90% to 92% = respiratory quinolones (gatifloxacin, levofloxacin, moxifloxacin), ceftriaxone, amoxicillin/clavulanate (4 g/250 mg per day), and amoxicillin/clavulanate (1.75 g/250 mg per day); 83% to 88% = high-dose amoxicillin (4 g/day), amoxicillin (1.5 g/day), cefpodoxime proxetil, cefixime (based on H influenzae and M catarrhalis coverage only), cefuroxime axetil, cefdinir, and TMP/SMX; 77% to 81% = doxycycline, clindamycin (based on gram-positive coverage only), cefprozil, azithromycin, clarithromycin, erythromycin, and telithromycin; 65% to 66% = cefaclor and loracarbef. The predicted spontaneous resolution rate for clinically diagnosed sinusitis in untreated adults with ABRS is 62%.
According to the Poole therapeutic outcomes model, antibiotics can be placed into the following relative rank order of predicted clinical efficacy in children: 91% to 92% = ceftriaxone, high-dose amoxicillin/clavulanate (90 mg/6.4 mg per kg per day), and amoxicillin/clavulanate (45 mg/6.4 mg per kg per day); 82% to 87% = high-dose amoxicillin (90 mg/kg per day), amoxicillin (45 mg/kg per day), cefpodoxime proxetil, cefixime (based on H influenzae and M catarrhalis coverage only), cefuroxime axetil, cefdinir, and TMP/SMX; 78% to 80% = clindamycin (based on gram-positive coverage only), cefprozil, azithromycin, clarithromycin, and erythromycin; 67% to 68% = cefaclor and loracarbef. The predicted spontaneous resolution rate in untreated children with ABRS is 63%.
The recommendations for patients who are not improving or are worsening at ≥72 hours of treatment are provided based on spectrum of activity of initial therapy against the major sinusitis pathogens. The estimated likelihood of a particular pathogen being encountered in patient failures with each type of initial therapy was utilized in lieu of obtaining a culture to guide “switch” therapy at 72 hours.
The recommendations for selecting antimicrobial therapy in the current guidelines are more focused compared with the previous guidelines. The decision to recommend fewer antimicrobial options, particularly for patients with moderate disease, was based on an evaluation of antimicrobial efficacy.
Recommendations for adult patients (see Table 4)
Recommendations for initial therapy for adult patients with mild disease and who have not received antibiotics in the previous 4 to 6 weeks include the following choices: amoxicillin/clavulanate (1.75 to 4 g/250 mg per day), amoxicillin (1.5 to 4 g/day), cefpodoxime proxetil, cefuroxime axetil, or cefdinir. While TMP/SMX, doxycycline, azithromycin, clarithromycin, erythromycin, or telithromycin may be considered for patients with β-lactam allergies, bacteriologic failure rates of 20% to 25% are possible. Failure to respond to antimicrobial therapy after 72 hours should prompt either a switch to alternate antimicrobial therapy or reevaluation of the patient (see Table 4). When a change in antibiotic therapy is made, the clinician should consider the limitations in coverage of the initial agent.
Recommendations for initial therapy for adults with mild disease who have received antibiotics in the previous 4 to 6 weeks or adults with moderate disease include the following choices: respiratory fluoroquinolone (gatifloxacin, levofloxacin, moxifloxacin) or high-dose amoxicillin/clavulanate (4 g/250 mg per day). The widespread use of respiratory fluoroquinolones for patients with milder disease may promote resistance (especially of gut organisms) to this class of agents. Ceftriaxone or combination therapy with adequate gram-positive and -negative coverage may also be considered. Examples of appropriate regimens of combination therapy include high-dose amoxicillin or clindamycin plus cefixime, or high-dose amoxicillin or clindamycin plus rifampin. When ceftriaxone is selected, a dose of 1 g/day IM or IV should be used for 5 days. This duration of therapy was arbitrarily extrapolated by the committee based on data from acute otitis media studies. Rifampin should not be used as monotherapy, casually, or for longer than 10 to 14 days, as resistance emerges rapidly to this agent.
Failure to respond to antimicrobial therapy after 72 hours should prompt either a switch to alternate antimicrobial therapy or reevaluation of the patient (see Table 4). When a change in antibiotic therapy is made, the clinician should consider the limitations in coverage of the initial agent. Patients who have received effective antibiotic therapy and continue to be symptomatic need further evaluation. A CT scan, fiberoptic endoscopy, or sinus aspiration for culture may be necessary.
When amoxicillin (± clavulanate) is selected for patients at risk for infection with penicillin-resistant S pneumoniae or DRSP (eg, recent antimicrobial use, immunodeficiency, frequent exposure to children attending day care, etc.), the high-dose regimen (ie, 4 g/250 mg) should be used.52
Recommendations for pediatric patients (see Table 5)
Recommendations for initial therapy for children with mild disease and who have not received antibiotics in the previous 4 to 6 weeks include the following: high-dose amoxicillin/clavulanate (90 mg/6.4 mg per kg per day), high-dose amoxicillin (90 mg/kg per day), cefpodoxime proxetil, cefuroxime axetil, or cefdinir. TMP/SMX, azithromycin, clarithromycin, or erythromycin is recommended if the patient has a history of immediate Type I hypersensitivity reaction to β-lactams. These antibiotics have limited effectiveness against the major pathogens of ABRS and bacterial failure is possible. The clinician should differentiate an immediate hypersensitivity reaction from other less dangerous side effects. Children with immediate hypersensitivity reactions to β-lactams may need: desensitization, sinus cultures, or other ancillary procedures and studies. Children with other types of reactions and side effects may tolerate one specific β-lactam, but not another.
The recommended initial therapy for children with mild disease who have received antibiotics in the previous 4 to 6 weeks or children with moderate disease is high-dose amoxicillin/clavulanate (90mg/6.4 mg per kg per day). Cefdinir, cefpodoxime proxetil, or cefuroxime axetil may be considered for patients with nonserious hypersensitivity reactions to penicillin. In such instances, cefdinir is the preferred agent based on patient acceptance.138, 139 TMP/SMX, azithromycin, clarithromycin, or erythromycin are recommended if the patient is β-lactam allergic (Type I hypersensitivity reaction). Ceftriaxone or combination therapy with adequate gram-positive and -negative coverage may also be considered. Examples of appropriate regimens of combination therapy include high-dose amoxicillin or clindamycin plus cefixime, or high-dose amoxicillin or clindamycin plus rifampin. When ceftriaxone is selected, a dose of 50 mg/kg per day IM or IV should be used for 5 days. This duration of therapy was arbitrarily extrapolated by the committee based on data from acute otitis media studies. Rifampin should not be used as monotherapy, casually, or for longer than 10 to 14 days as resistance emerges rapidly to this agent. Monotherapy with clindamycin for β-lactam–allergic patients is appropriate if S pneumoniae is identified as a pathogen.
Failure to respond to antimicrobial therapy after 72 hours should prompt either a switch to alternate antimicrobial therapy or reevaluation of the patient (see Table 4). When a change in antibiotic therapy is made, the clinician should consider the limitations in coverage of the initial agent.
When amoxicillin (± clavulanate) is selected for patients at risk for infection with penicillin-resistant S pneumoniae or DRSP (eg, recent antimicrobial use, day-care attendance, etc.), the high-dose regimen should be used. This recommendation is based on data from acute otitis media studies.165
Conclusions
These guidelines have been updated to provide the most recent information on management principles, antimicrobial susceptibility patterns, and therapeutic options. The treatment recommendations for ABRS in this document are based on a mathematical model using pathogen distribution and spontaneous resolution data and pharmacodynamically derived susceptibility values of the major ABRS pathogens, from which bacteriologic outcome can be predicted. The panel hopes these guidelines will continue to provide a rational approach to the need for antimicrobial therapy in bacterial rhinosinusitis, reduction in the use of antibiotics for nonbacterial infections, and the appropriate use antibiotics when bacterial disease is likely. These recommendations should help clinicians select antimicrobial therapy for patients with ABRS until more evidence from adequately designed clinical trials becomes available.
Acknowledgements
Acknowledgments: Antimicrobial Treatment Guidelines for Acute Bacterial Rhinosinusitis
The Sinus and Allergy Health Partnership acknowledges the following medical experts and specialists who wrote and edited the revision of these guidelines.
Note: The parenthetical listing indicates sponsorship of research grants, consultants, and speakers’ bureaus.
Jack B. Anon, MD, FACS (Chair), Clinical Professor, University of Pittsburgh School of Medicine (Abbott, Aventis, Bristol-Myers Squibb, GlaxoSmithKline, Ortho-McNeil)
Michael R. Jacobs, MD, PhD, Professor of Pathology and Medicine, Case Western Reserve University; Director of Clinical Microbiology, University Hospitals of Cleveland (Research grants: Abbott, Aventis, Bristol-Myers Squibb, Bayer, Daiichi, Dr Reddy’s Laboratory, Eli Lilly & Co., GlaxoSmithKline, Meiji, Ortho-McNeil, Pfizer, Inc., Rambaxy, Roche, TAP, Warner-Lambert, Wockhardt, Wyeth Ayerst/Lederle; Consultant: Abbott, Aventis, Bayer, GeneSoft, TAP, Wyeth Ayerst/Lederle; Speaker’s Bureau: Bayer, GlaxoSmithKline, Ortho-McNeil)
Michael D. Poole, MD, PhD, FACS, Professor and Chair, University of Texas at Houston Health Science Center-Medical School (Abbott, Alcon, Aventis, Bayer, Brisol-Myers Squibb, Daiichi, Merck, Novartis, Ortho-McNeill, Pharmacia-Upjohn, Roche) Dr. Poole also has received US patent approval for the Therapeutic Outcomes Model (patent pending)
Paul G. Ambrose, PharmD, Director, Anti-infective Clinical Research, Cognigen Corporation; Assistant Professor of Pharmacy, University of the Pacific
Michael S. Benninger, MD, Chair, Department of Otolaryngology–Head and Neck Surgery, Henry Ford Hospital (Abbott, Astra-Zeneca, Aventis, Bayer, GlaxoSmithKline, Novartis)
James A. Hadley, MD, FACS, Clinical Associate Professor, Otolaryngology, University of Rochester Medical Center (Astra-Zeneca, GlaxoSmithKline, Bristol-Myers Squibb, Aventis, Alcon)
William A. Craig, MD, Professor of Medicine, University of Wisconsin (GlaxoSmithKline, Abbott, Aventis, Roche, Schering-Plough, Eli Lilly & Co, Ortho-McNeil, Bristol-Myers Squibb)
The Sinus and Allergy Health Partnership and contributors acknowledge the efforts of Kevin Jarvis, PharmD, from BioCentric, Inc., for his efforts in revising, editing, and preparing this document for publication.
The Sinus and Allergy Health Partnership acknowledges the efforts of the following medical experts and specialists who contributed to the original drafting of these guidelines and/or its revision.
David R. Andes, MD, Assistant Professor of Medicine, University of Wisconsin
Joel M. Bernstein, MD, PhD, Clinical Professor of Otolaryngology and Pediatrics, State University of New York at Buffalo
Richard E. Besser, MD, Medical Epidemiologist, Respiratory Diseases Branch, National Center for Infectious Diseases/CDC
Scott F. Dowell, MD, MPH, Medical Epidemiologist, Respiratory Diseases Branch, National Center for Infectious Disease, Centers for Disease Control and Prevention
George L. Drusano, MD, Professor and Director, Division of Clinical Pharmacology, Departments of Medicine and Pharmacology, Albany Medical College
Michael E. Klepser, PharmD, Associate Professor, University of Iowa College of Pharmacy
Donald Leopold, MD, Professor and Chair, Department of Otolaryngology–Head and Neck Surgery, University of Nebraska
David Nicolau, PharmD, Center for Antiinfective Research and Development, Hartford Hospital
Alexander Radowsky, MD, Senior Medical Officer, National Institutes of Health (at the time of publication [2000], Dr Rakowsky was Medical Team Leader, Division of Anti-Infective Drug Products, Food and Drug Administration.∗
L. Barth Reller, MD, Professor of Medicine and Pathology/Director of Clinical Microbiology, Duke University Medical Center
Ellen R. Wald, MD, Professor of Pediatrics and Otolaryngology, University of Pittsburgh School of Medicine/Children’s Hospital of Pittsburgh
Deborah R. Zucker, MD, New England Medical Center/Tufts University School of Medicine
Footnotes
Some antibiotics discussed in this document currently are not approved by the US Food and Drug Administration for the treatment of maxillary sinusitis in adults or children, while the value of others approved when antimicrobial resistance rates were very low is now very limited.
The information contained in these guidelines does not necessarily reflect the views of the Food and Drug Administration.
References
- 1.Sinus and Allergy Health Partnership Antimicrobial treatment guidelines for acute bacterial Rhinosinusitis. Otolaryngol Head Neck Surg. 2000;123:S1–31. [PubMed] [Google Scholar]
- 2.Gwaltney J.M., Jr Acute community-acquired sinusitis. Clin Infect Dis. 1996;23:1209–1223. doi: 10.1093/clinids/23.6.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dowell S.F., Butler J.C., Giebink G.S. Acute otitis media: management and surveillance in an era of pneumococcal resistance—a report from the Drug-resistant Streptococcus pneumoniae Therapeutic Working Group. Pediatr Infect Dis J. 1999;18:1–9. [PubMed] [Google Scholar]
- 4.Gwaltney J.M., Jr, Phillips C.D., Miller R.D. Computed tomographic study of the common cold. N Engl J Med. 1994;330:25–30. doi: 10.1056/NEJM199401063300105. [DOI] [PubMed] [Google Scholar]
- 5.Berg O., Carenfelt C., Rystedt G. Occurrence of asymptomatic sinusitis in common cold and other acute ENT-infections. Rhinology. 1986;24:223–225. [PubMed] [Google Scholar]
- 6.Scott Levin™ Prescription Audit from Verispan, L.L.C., January-December 2002
- 7.Ray N.F., Baraniuk J.N., Thamer M. Healthcare expenditures for sinusitis in 1996: contributions of asthma, rhinitis, and other airway disorders. J Allergy Clin Immunol. 1999;103:408–414. doi: 10.1016/s0091-6749(99)70464-1. [DOI] [PubMed] [Google Scholar]
- 8.Physician Drug & Diagnosis Audit (PDDA) and SourceTM Prescription Audit (SPA) from Verispan, L.L.C., January-December 2002
- 9.Gonzales R., Steiner J.F., Lum A. Decreasing antibiotic use in ambulatory practice: impact of a multidimensional intervention on the treatment of uncomplicated acute bronchitis in adults. JAMA. 1999;281:1512–1519. doi: 10.1001/jama.281.16.1512. [DOI] [PubMed] [Google Scholar]
- 10.Dosh S.A., Hickner J.M., Mainous A.G., III Predictors of antibiotic prescribing for nonspecific upper respiratory infections, acute bronchitis, and sinusitis. J Fam Pract. 2000;49:407–414. [PubMed] [Google Scholar]
- 11.Nissinen A., Gronroos P., Huovinen P. Development of b-lactamase-mediated resistance to penicillin in middle-ear isolates of Moraxella catarrhalis in Finnish children, 1978–1993. Clin Infect Dis. 1995;21:1193–1196. doi: 10.1093/clinids/21.5.1193. [DOI] [PubMed] [Google Scholar]
- 12.Arason V., Kristinsson K., Sigurdsson J. Do antimicrobials increase the carriage rate of penicillin resistant pneumococci in children? Cross sectional prevalence study. BMJ. 1996;313:387–391. doi: 10.1136/bmj.313.7054.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Seppala H, Klaukka T, Lehtonen R, et al. Outpatient use of erythromycin: link to increased erythromycin resistance in Group A Streptococci. Clin Infect Dis 1995:1378–85 [DOI] [PubMed]
- 14.Seppala H., Klaukka T., Vuopio-Varkila J. The effect of changes in the consumption of macrolide antibiotics on erythromycin resistance in group A streptococci in Finland. N Engl J Med. 1997;337:441–446. doi: 10.1056/NEJM199708143370701. [DOI] [PubMed] [Google Scholar]
- 15.Hyde T.B., Gay K., Stephens D.S. Macrolide resistance among invasive Streptococcus pneumoniae isolates. JAMA. 2001;286:1857–1862. doi: 10.1001/jama.286.15.1857. [DOI] [PubMed] [Google Scholar]
- 16.Baquero F., Martinez-Beltran J., Loza E. A review of antibiotic resistance patterns of Streptococcus pneumoniae in Europe. J Antimicrob Chemother. 1991;28(Suppl C):31–38. doi: 10.1093/jac/28.suppl_c.31. [DOI] [PubMed] [Google Scholar]
- 17.McCaig L.F., Besser R.E., Hughes J.M. Antimicrobial drug prescriptions in ambulatory care settings, United States, 1992–2000. Emerg Infect Dis. 2003;9:432–437. doi: 10.3201/eid0904.020268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McCaig L.F., Besser R.E., Hughes J.M. Trends in antimicrobial prescribing rates for children and adolescents. JAMA. 2002;287:3096–3102. doi: 10.1001/jama.287.23.3096. [DOI] [PubMed] [Google Scholar]
- 19.Lanza D.C., Kennedy D.W. Adult rhinosinusitis defined. Otolaryngol Head Neck Surg. 1997;117:S1–7. doi: 10.1016/S0194-59989770001-9. [DOI] [PubMed] [Google Scholar]
- 20.AHCPR . Agency for Health Care Policy and Research; Rockville, MD: 1999. Diagnosis and treatment of acute bacterial rhinosinusitis. [Google Scholar]
- 21.Makela M.J., Puhakka T., Ruuskanen O. Viruses and bacteria in the etiology of the common cold. J Clin Microbiol. 1998;36:539–542. doi: 10.1128/jcm.36.2.539-542.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Winther B., Gwaltney J.M., Jr, Mygind N. Viral-induced rhinitis. Am J Rhinol. 1998;12:17–20. doi: 10.2500/105065898782102954. [DOI] [PubMed] [Google Scholar]
- 23.Greve J.M., Davis G., Meyer A.M. The major human rhinovirus receptor is ICAM-1. Cell. 1989;56:839–847. doi: 10.1016/0092-8674(89)90688-0. [DOI] [PubMed] [Google Scholar]
- 24.Patel J., Faden H., Sharma S. Effect of respiratory syncytial virus on adherence, colonization and immunity of non-typable Haemophilus influenzae: implications for otitis media. Int J Pediatr Otorhinolaryngol. 1992;23:15–23. doi: 10.1016/0165-5876(92)90075-z. [DOI] [PubMed] [Google Scholar]
- 25.Adderson EE, Ulett GC, Avadhanula V. Respiratory syncytial virus enhanced attachment of nontypeable Haemophilus influenzae to A549 epithelial cells [presentation B-797]. Presented at the 43rd Interscience Conference on Antimicrobial Agents and Chemotherapy; September 14–17, 2003; Chicago, Illinois
- 26.Gwaltney J.M., Jr, Hendley J.O., Simon G. Rhinovirus infections in an industrial population. II. Characteristics of illness and antibody response. JAMA. 1967;202:494–500. [PubMed] [Google Scholar]
- 27.Gwaltney J.M., Hendley J.O., Phillips C.D. Nose blowing propels nasal fluid into the paranasal sinuses. Clin Infect Dis. 2000;30:387–391. doi: 10.1086/313661. [DOI] [PubMed] [Google Scholar]
- 28.Monto A.S., Ullman B.M. Acute respiratory illness in an American community. The Tecumseh study. JAMA. 1974;227:164–169. [PubMed] [Google Scholar]
- 29.Wald E.R., Guerra N., Byers C. Upper respiratory tract infections in young children: duration of and frequency of complications. Pediatrics. 1991;87:129–133. [PubMed] [Google Scholar]
- 30.Hays G.C., Mullard J.E. Can nasal bacterial flora be predicted from clinical findings? Pediatrics. 1972;49:596–599. [PubMed] [Google Scholar]
- 31.Wald E.R. Purulent nasal discharge. Pediatr Infect Dis J. 1991;10:329–333. doi: 10.1097/00006454-199104000-00013. [DOI] [PubMed] [Google Scholar]
- 32.Wald E.R., Milmoe G.J., Bowen A. Acute maxillary sinusitis in children. N Engl J Med. 1981;304:749–754. doi: 10.1056/NEJM198103263041302. [DOI] [PubMed] [Google Scholar]
- 33.Winther B. Effects on the nasal mucosa of upper respiratory viruses (common cold) Dan Med Bull. 1994;41:193–204. [PubMed] [Google Scholar]
- 34.Winther B., Brofeldt S., Gronborg H. Study of bacteria in the nasal cavity and nasopharynx during naturally acquired common colds. Acta Otolaryngol (Stockh) 1984;98:315–320. doi: 10.3109/00016488409107569. [DOI] [PubMed] [Google Scholar]
- 35.LaCroix J.S., Ricchetti A., Lew D. Symptoms and clinical and radiological signs predicting the presence of pathogenic bacteria in acute rhinosinusitis. Acta Otolaryngol. 2002;122:192–196. doi: 10.1080/00016480252814216. [DOI] [PubMed] [Google Scholar]
- 36.Gwaltney J.M., Jr, Scheld W.M., Sande M.A. The microbial etiology and antimicrobial therapy of adults with acute community-acquired sinusitis: a fifteen-year experience at the University of Virginia and review of other selected studies. J Allergy Clin Immunol. 1992;90:457–461. doi: 10.1016/0091-6749(92)90169-3. [DOI] [PubMed] [Google Scholar]
- 37.Hickner J.M., Bartlett J.G., Besser R.E. Principles of appropriate antibiotic use for acute rhinosinusitis in adults: background. Ann Intern Med. 2001;134:498–505. doi: 10.7326/0003-4819-134-6-200103200-00017. [DOI] [PubMed] [Google Scholar]
- 38.Gold S.M., Tami T.A. Role of middle meatus aspiration culture in the diagnosis of chronic sinusitis. Laryngoscope. 1997;107:1586–1589. doi: 10.1097/00005537-199712000-00002. [DOI] [PubMed] [Google Scholar]
- 39.Brook I., Frazier E.H., Foote P.A. Microbiology of chronic maxillary sinusitis: comparison between specimens obtained by sinus endoscopy and by surgical drainage. J Med Microbiol. 1997;46:430–432. doi: 10.1099/00222615-46-5-430. [DOI] [PubMed] [Google Scholar]
- 40.Benninger M.S., Appelbaum P.C., Denneny J.C. Maxillary sinus puncture and culture in the diagnosis of acute rhinosinusitis: the case for pursuing alternative culture methods. Otolaryngol Head Neck Surg. 2002;127:7–12. doi: 10.1067/mhn.2002.124847. [DOI] [PubMed] [Google Scholar]
- 41.Williams J.W., Jr, Simel D.L. Does this patient have sinusitis? Diagnosing acute sinusitis by history and physical examination. JAMA. 1993;270:1242–1246. [PubMed] [Google Scholar]
- 42.Tiedjen K.U., Becker E., Heimann K.D. Value of B-image ultrasound in diagnosis of paranasal sinus diseases in comparison with computerized tomography. Laryngorhinootologie. 1998;77:541–546. doi: 10.1055/s-2007-997023. [DOI] [PubMed] [Google Scholar]
- 43.de Bock G.H., Houwing-Duistermaat J.J., Springer M.P. Sensitivity and specificity of diagnostic tests in acute maxillary sinusitis determined by maximum likelihood in the absence of an external standard. J Clin Epidemiol. 1994;47:1343–1352. doi: 10.1016/0895-4356(94)90078-7. [DOI] [PubMed] [Google Scholar]
- 44.Laine K., Maatta T., Varonen H. Diagnosing acute maxillary sinusitis in primary care: a comparison of ultrasound, clinical examination and radiography. Rhinology. 1998;36:2–6. [PubMed] [Google Scholar]
- 45.Zinreich S.J. Rhinosinusitis: radiologic diagnosis. Otolaryngol Head Neck Surg. 1997;117:S27–34. doi: 10.1016/S0194-59989770004-4. [DOI] [PubMed] [Google Scholar]
- 46.Leopold D.A., Sod E.W., Szeverenyi N.M. Clinical course of acute maxillary sinusitis documented by sequential MRI scanning. Am J Rhinol. 1994;8:19–28. [Google Scholar]
- 47.Ambrose P, Jones RN, Van Wart S, et al. Serial sinus aspirate sampling (SSAS): a novel technique for evaluating antimicrobial therapy of acute maxillary sinusitis (AMS) [presentation A-34]. Presented at the 43rd Interscience Conference on Antimicrobial Agents and Chemotherapy; September 14–17, 2003; Chicago, IL
- 48.Dagan R., Klugman K.P., Craig W.A. Evidence to support the rationale that bacterial eradication in respiratory tract infection is an important aim of antimicrobial therapy. J Antimicrob Chemother. 2001;47:129–140. doi: 10.1093/jac/47.2.129. [DOI] [PubMed] [Google Scholar]
- 49.Gwaltney J., Syndor A., Sande M. Etiology and antimicrobial treatment of acute sinusitis. Otol Rhinol Layrngol. 1981;90:68–71. doi: 10.1177/00034894810903s216. [DOI] [PubMed] [Google Scholar]
- 50.Berg O., Carenfelt C., Kronvall G. Bacteriology of maxillary sinusitis in relation to character of inflammation and prior treatment. Scand J Infect Dis. 1988;20:511–516. doi: 10.3109/00365548809032499. [DOI] [PubMed] [Google Scholar]
- 51.Brook I. Microbiology and management of sinusitis. J Otolaryngol. 1996;25:249–256. [PubMed] [Google Scholar]
- 52.Anon J, Ferguson B, Wynne B, et al. Pharmacokinetically enhanced amoxicillin/clavulanate 2000/125 mg twice daily in the treatment of acute bacterial sinusitis (ABS) in adults [Poster #300]. Presented at the 41st Annual Meeting of the Infectious Disease Society of America; October 9–12, 2003; San Diego, CA
- 53.Bluestone C.D., Stool S.E., Kenna M.A. 3rd ed. Saunders; Philadelphia: 1996. Pediatric otolaryngology. [Google Scholar]
- 54.Wald E.R., Reilly J.S., Casselbrant M. Treatment of acute maxillary sinusitis in childhood: a comparative study of amoxicillin and cefaclor. J Pediatr. 1984;104:297–302. doi: 10.1016/s0022-3476(84)81018-5. [DOI] [PubMed] [Google Scholar]
- 55.Faden H., Duffy L., Wasielewski R. Relationship between nasopharyngeal colonization and the development of otitis media in children. Tonawanda/Williamsville Pediatrics. J Infect Dis. 1997;175:1440–1445. doi: 10.1086/516477. [DOI] [PubMed] [Google Scholar]
- 56.Faden H., Waz M.J., Bernstein J.M. Nasopharyngeal flora in the first three years of life in normal and otitis-prone children. Ann Otol Rhinol Laryngol. 1991;100:612–615. doi: 10.1177/000348949110000802. [DOI] [PubMed] [Google Scholar]
- 57.Muller-Graf C.D., Whatmore A.M., King S.J. Population biology of Streptococcus pneumoniae isolated from oropharyngeal carriage and invasive disease. Microbiology. 1999;145:3283–3293. doi: 10.1099/00221287-145-11-3283. [DOI] [PubMed] [Google Scholar]
- 58.Faden H., Duffy L., Williams A. Epidemiology of nasopharyngeal colonization with nontypeable Haemophilus influenzae in the first 2 years of life. J Infect Dis. 1995;172:132–135. doi: 10.1093/infdis/172.1.132. [DOI] [PubMed] [Google Scholar]
- 59.Faden H., Harabuchi Y., Hong J.J. Epidemiology of Moraxella catarrhalis in children during the first 2 years of life: relationship to otitis media. J Infect Dis. 1994;169:1312–1317. doi: 10.1093/infdis/169.6.1312. [DOI] [PubMed] [Google Scholar]
- 60.Pelton S, Marchant CD, Christiansen D, Loughlin A. Temporal changes in Serotype Distribution and Antimicrobial Resistance among isolates of S. pneumoniae in Massachusetts [presentation G-890]. Presented at the 43rd Interscience Conference on Antimicrobial Agents and Chemotherapy; September 14–17, 2003; Chicago, IL
- 61.Bernstein J.M., Dryja D., Murphy T.F. Molecular typing of paired bacterial isolates from the adenoid and lateral wall of the nose in children undergoing adenoidectomy: implications in acute rhinosinusitis. Otolaryngol Head Neck Surg. 2001;125:593–597. doi: 10.1067/mhn.2001.120232. [DOI] [PubMed] [Google Scholar]
- 62.Dagan R., Leibovitz E., Greenberg D. Dynamics of pneumococcal nasopharyngeal colonization during the first days of antibiotic treatment in pediatric patients. Pediatr Infect Dis J. 1998;17:880–885. doi: 10.1097/00006454-199810000-00006. [DOI] [PubMed] [Google Scholar]
- 63.Ekdahl K., Ahlinder I., Hansson H.B. Duration of nasopharyngeal carriage of penicillin-resistant Streptococcus pneumoniae: experiences from the South Swedish Pneumococcal Intervention Project. Clin Infect Dis. 1997;25:1113–1117. doi: 10.1086/516103. [DOI] [PubMed] [Google Scholar]
- 64.Chi D.H., Hendley J.O., French P. Nasopharyngeal reservoir of bacterial otitis media and sinusitis pathogens in adults during wellness and viral respiratory illness. Am J Rhinol. 2003;17:209–214. [PubMed] [Google Scholar]
- 65.Scott J.A., Hall A.J., Dagan R. Serogroup-specific epidemiology of Streptococcus pneumoniae: associations with age, sex, and geography in 7, 000 episodes of invasive disease. Clin Infect Dis. 1996;22:973–981. doi: 10.1093/clinids/22.6.973. [DOI] [PubMed] [Google Scholar]
- 66.Butler J.C. Epidemiology of pneumococcal serotypes and conjugate vaccine formulations. Microb Drug Resist. 1997;3:125–129. doi: 10.1089/mdr.1997.3.125. [DOI] [PubMed] [Google Scholar]
- 67.Joloba M.L., Windau A., Bajaksouzian S. Pneumococcal conjugate vaccine serotypes of Streptococcus pneumoniae isolates and the antimicrobial susceptibility of such isolates in children with otitis media. Clin Infect Dis. 2001;33:1489–1494. doi: 10.1086/323027. [DOI] [PubMed] [Google Scholar]
- 68.Dowell S.F., Whitney C.G., Wright C. Seasonal patterns of invasive pneumococcal disease. Emerg Infect Dis. 2003;9:573–579. doi: 10.3201/eid0905.020556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Albanese B.A., Roche J.C., Pass M. Geographic, demographic, and seasonal differences in penicillin-resistant Streptococcus pneumoniae in Baltimore. Clin Infect Dis. 2002;34:15–21. doi: 10.1086/323674. [DOI] [PubMed] [Google Scholar]
- 70.Appelbaum P.C. Epidemiology and in vitro susceptibility of drug-resistant Streptococcus pneumoniae. Pediatr Infect Dis J. 1996;15:932–934. doi: 10.1097/00006454-199610000-00030. [DOI] [PubMed] [Google Scholar]
- 71.Jacobs M., Appelbaum P. Antibiotic resistant pneumococci. Rev Med Microbiol. 1995;6:77–93. [Google Scholar]
- 72.Nagai K., Davies T.A., Ednie L.M. Activities of a new fluoroketolide, HMR 3787, and its (des)-fluor derivative RU 64399 compared to those of telithromycin, erythromycin A, azithromycin, clarithromycin, and clindamycin against macrolide-susceptible or -resistant Streptococcus pneumoniae and S. pyogenes. Antimicrob Agents Chemother. 2001;45:3242–3245. doi: 10.1128/AAC.45.11.3242-3245.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Fasola E., Bajaksouzian S., Appelbaum P. Variation in erythromycin and clindamycin susceptibilities of Streptococcus pneumoniae in four test methods. Antimicrob Agents Chemother. 1997;41:129–134. doi: 10.1128/aac.41.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sutcliffe J., Grebe T., Tait-Kamradt A. Detection of erythromycin-resistant determinants by PCR. Antimicrob Agents Chemother. 1996;40:2562–2566. doi: 10.1128/aac.40.11.2562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tait-Kamradt A., Davies T., Cronan M. Mutations in 23S rRNA and ribosomal protein L4 account for resistance in pneumococcal strains selected in vitro by macrolide passage. Antimicrob Agents Chemother. 2000;44:2118–2125. doi: 10.1128/aac.44.8.2118-2125.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bozdogan B., Appelbaum P.C., Kelly L.M. Activity of telithromycin and seven other agents against 1034 pediatric Streptococcus pneumoniae isolates from ten central and eastern European centers. Clin Microbiol Infect. 2003;9:653–661. doi: 10.1046/j.1469-0691.2003.00597.x. [DOI] [PubMed] [Google Scholar]
- 77.Farrell D.J., Douthwaite S., Morrissey I. Macrolide Resistance by Ribosomal Mutation in Clinical Isolates of Streptococcus pneumoniae from the PROTEKT 1999–2000 Study. Antimicrob Agents Chemother. 2003;47:1777–1783. doi: 10.1128/AAC.47.6.1777-1783.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Reinert R.R., Wild A., Appelbaum P. Ribosomal mutations conferring resistance to macrolides in Streptococcus pneumoniae clinical strains isolated in Germany. Antimicrob Agents Chemother. 2003;47:2319–2322. doi: 10.1128/AAC.47.7.2319-2322.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Tait-Kamradt A., Davies T., Appelbaum P.C. Two new mechanisms of macrolide resistance in clinical strains of Streptococcus pneumoniae from Eastern Europe and North America. Antimicrob Agents Chemother. 2000;44:3395–3401. doi: 10.1128/aac.44.12.3395-3401.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kays M.B., Wack M.F., Smith D.W. Azithromycin treatment failure in community-acquired pneumonia caused by Streptococcus pneumoniae resistant to macrolides by a 23S rRNA mutation. Diagn Microbiol Infect Dis. 2002;43:163–165. doi: 10.1016/s0732-8893(02)00379-6. [DOI] [PubMed] [Google Scholar]
- 81.Musher D.M., Dowell M.E., Shortridge V.D. Emergence of macrolide resistance during treatment of pneumococcal pneumonia. N Engl J Med. 2002;346:630–631. doi: 10.1056/NEJM200202213460820. [DOI] [PubMed] [Google Scholar]
- 82.Butler J.C., Lennox J.L., McDougal L.K. Macrolide-resistant pneumococcal endocarditis and epidural abscess that develop during erythromycin therapy. Clin Infect Dis. 2003;36:e19–25. doi: 10.1086/344965. [DOI] [PubMed] [Google Scholar]
- 83.Pihlajamaki M., Kataja J., Seppala H. Ribosomal mutations in Streptococcus pneumoniae clinical isolates. Antimicrob Agents Chemother. 2002;46:654–658. doi: 10.1128/AAC.46.3.654-658.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Depardieu F., Courvalin P. Mutation in 23S rRNA responsible for resistance to 16-membered macrolides and streptogramins in Streptococcus pneumoniae. Antimicrob Agents Chemother. 2001;45:319–323. doi: 10.1128/AAC.45.1.319-323.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Gill M.J., Brenwald N.P., Wise R. Identification of an efflux pump gene, pmrA, associated with fluoroquinolone resistance in Streptococcus pneumoniae. Antimicrob Agents Chemother. 1999;43:187–189. doi: 10.1128/aac.43.1.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Felmingham D., Washington J. Trends in the antimicrobial susceptibility of bacterial respiratory tract pathogens—findings of the Alexander Project 1992–1996. J Chemother. 1999;11:5–21. doi: 10.1179/joc.1999.11.Supplement-2.5. [DOI] [PubMed] [Google Scholar]
- 87.Ubukata K., Shibasaki Y., Yamamoto K. Association of amino acid substitutions in penicillin-binding protein 3 with beta-lactam resistance in beta-lactamase-negative ampicillin-resistant Haemophilus influenzae. Antimicrob Agents Chemother. 2001;45:1693–1699. doi: 10.1128/AAC.45.6.1693-1699.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Peric M., Bozdogan B., Jacobs M.R. Effects of an efflux mechanism and ribosomal mutations on macrolide susceptibility of Haemophilus influenzae clinical isolates. Antimicrob Agents Chemother. 2003;47:1017–1022. doi: 10.1128/AAC.47.3.1017-1022.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Jacobs M.R., Felmingham D., Appelbaum P.C. The Alexander Project 1998–2000: susceptibility of pathogens isolated from community-acquired respiratory tract infection to commonly used antimicrobial agents. J Antimicrob Chemother. 2003;52:229–246. doi: 10.1093/jac/dkg321. [DOI] [PubMed] [Google Scholar]
- 90.Jones R. SENTRY Surveillance Program-US isolates; 2003
- 91.Jacobs M.R., Bajaksouzian S., Zilles A. Susceptibilities of Streptococcus pneumoniae and Haemophilus influenzae to 10 oral antimicrobial agents based on pharmacodynamic parameters: 1997 U.S. Surveillance study. Antimicrob Agents Chemother. 1999;43:1901–1908. doi: 10.1128/aac.43.8.1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Jones RN, Johnson DM, Sader HS, Fritsche TR. Recent declines in β-lactam and MLSB resistance among S. pneumoniae and age-related effects: report from the SENTRY Antimicrobial Surveillance Program (North America, 1997–2002) [presentation C2–926]. Presented at the 43rd Interscience Conference on Antimicrobial Agents and Chemotherapy; September 14–17, 2003; Chicago, IL
- 93.Hasegawa K., Yamamoto K., Chiba N. Diversity of ampicillin-resistance genes in Haemophilus influenzae in Japan and the United States. Microb Drug Resist. 2003;9:39–46. doi: 10.1089/107662903764736337. [DOI] [PubMed] [Google Scholar]
- 94.Dagan R, Greenberg D, Leiberman A, et al. The effect of azithromycin on carriage of antibiotic resistant S. pneumoniae in children depends on the prevalence of macrolide-resistance in the community [presentation G-898]. Presented at the 43rd Interscience Conference on Antimicrobial Agents and Chemotherapy; September 14–17, 2003; Chicago, IL
- 95.Gonzales R., Bartlett J.G., Besser R.E. Principles of appropriate antibiotic use for treatment of acute respiratory tract infections in adults: background, specific aims, and methods. Ann Intern Med. 2001;134:479–486. doi: 10.7326/0003-4819-134-6-200103200-00013. [DOI] [PubMed] [Google Scholar]
- 96.Vogelman B., Gudmundsson S., Leggett J. Correlation of antimicrobial pharmacokinetic parameters with therapeutic efficacy in an animal model. J Infect Dis. 1988;158:831–847. doi: 10.1093/infdis/158.4.831. [DOI] [PubMed] [Google Scholar]
- 97.Craig W. Pharmacokinetic/pharmacodynamic parameters: rationale for antibacterial dosing of mice and men. Clin Infect Dis. 1998;26:1–12. doi: 10.1086/516284. [DOI] [PubMed] [Google Scholar]
- 98.Craig WA, Andes DR. In vivo pharmacodynamic activity of faropenem against Streptococcus pneumoniae [abstract A-2094]. Presented at the 41st Interscience Conference on Antimicrobial Agents and Chemotherapy; December 16–19, 2001; Chicago, IL
- 99.Craig W.A., Andes D. Pharmacokinetics and pharmacodynamics of antibiotics in otitis media. Pediatr Infect Dis J. 1996;15:255–259. doi: 10.1097/00006454-199603000-00015. [DOI] [PubMed] [Google Scholar]
- 100.Craig W.A. Antimicrobial resistance issues of the future. Diagn Microbiol Infect Dis. 1996;25:213–217. doi: 10.1016/s0732-8893(96)00162-9. [DOI] [PubMed] [Google Scholar]
- 101.Ambrose PG, Quintiliani R, Nightingdale CH, et al. Continuous vs intermittent infusion of cefuroxime for the treatment of community-acquired pneumonia. Infect Dis Clin Pract 1997;7:463–70
- 102.Odenholt-Tornqvist I., Lowdin E., Cars O. Postantibiotic effects and postantibiotic sub-MIC effects of roxithromycin, clarithromycin, and azithromycin on respiratory tract pathogens. Antimicrob Agents Chemother. 1995;39:221–226. doi: 10.1128/aac.39.1.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Craig WA, Kiem S, Andes DR. Free Drug 24-Hr AUC/MIC is the PK/PD target that correlates with in vivo efficacy of macrolides, azalides, ketolides and clindamycin [abstract A-1264]. Presented at the 42nd Interscience Conference on Antimicrobial Agents and Chemotherapy; September 27–30, 2002; San Diego, CA
- 104.Jackson M., Burry V., Olson L. Breakthrough sepsis in macrolide resistant pneumococcal infection. Pediatr Infect Dis J. 1996;15:1049–1051. doi: 10.1097/00006454-199611000-00026. [DOI] [PubMed] [Google Scholar]
- 105.Leach A., Shelby-James T., Mayo M. A prospective study of the impact of community-based arithromycin treatment of trachoma on carriage and resistance of Streptococcus pneumoniae. Clin Infect Dis. 1997;24:356–362. doi: 10.1093/clinids/24.3.356. [DOI] [PubMed] [Google Scholar]
- 106.Guggenbichler J., Kastner U. In influence of antibiotics on the normal flora. Infect Med. 1998;15(Suppl A):15–22. [Google Scholar]
- 107.Moore R.D., Lietman P.S., Smith C.R. Clinical response to aminoglycoside therapy: importance of the ratio of peak concentration to minimal inhibitory concentration. J Infect Dis. 1987;155:93–99. doi: 10.1093/infdis/155.1.93. [DOI] [PubMed] [Google Scholar]
- 108.Preston S., Drusano G., Berman A. Pharmacodynamics of levofloxacin. A new paradigm for early clinical trials. JAMA. 1998;279:125–129. doi: 10.1001/jama.279.2.125. [DOI] [PubMed] [Google Scholar]
- 109.Drusano G.L., Johnson D.E., Rosen M. Pharmacodynamics of a fluoroquinolone antimicrobial agent in a neutropenic rat model of Pseudomonas sepsis. Antimicrob Agents Chemother. 1993;37:483–490. doi: 10.1128/aac.37.3.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Lister P.D., Sanders C.C. Pharmacodynamics of moxifloxacin, levofloxacin, and sparfloxacin against Streptococcus pneumoniae. J Antimicrob Chemother. 2001;47:811–818. doi: 10.1093/jac/47.6.811. [DOI] [PubMed] [Google Scholar]
- 111.Preston S.L., Drusano G.L., Berman A.L. Levofloxacin population pharmacokinetic and creation of a demographic model for prediction of individual drug clearance in patients with serious community-acquired infection. Antimicrob Agents Chemother. 1998;42:1098–1104. doi: 10.1128/aac.42.5.1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Forrest A., Nix D., Ballow C. Pharmacodynamics of intravenous ciprofloxacin in seriously ill patients. Anitmicrob Agents Chemother. 1993;37:1073–1081. doi: 10.1128/aac.37.5.1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Lacy M.K., Lu W., Xu X. Pharmacodynamic comparisons of levofloxacin, ciprofloxacin, and ampicillin against Streptococcus pneumoniae in an in vitro model of infection. Antimicrob Agents Chemother. 1999;43:672–677. doi: 10.1128/aac.43.3.672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Lister P.D., Sanders C.C. Pharmacodynamics of levofloxacin and ciprofloxacin against Streptococcus pneumoniae. J Antimicrob Chemother. 1999;43:79–86. doi: 10.1093/jac/43.1.79. [DOI] [PubMed] [Google Scholar]
- 115.Vesga O, Craig WA. Activity of levofloxacin against penicillin-resistant Streptococcus pneumoniae in normal and neutropenic mice. Presented at the 36th Interscience Conference on Antimicrobial Agents and Chemotherapy; September 15–18, 1996; New Orleans, LA
- 116.Ambrose P.G., Grasela D.M., Grasela T.H. Pharmacodynamics of fluoroquinolones against Streptococcus pneumoniae in patients with community-acquired respiratory tract infections. Antimicrob Agents Chemother. 2001;45:2793–2797. doi: 10.1128/AAC.45.10.2793-2797.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kim M.K., Zhou W., Tessier P.R. Bactericidal effect and pharmacodynamics of cethromycin (ABT-773) in a murine pneumococcal pneumonia model. Antimicrob Agents Chemother. 2002;46:3185–3192. doi: 10.1128/AAC.46.10.3185-3192.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Vesga O, Bonnat C, Craig WA. In vivo pharmacodynamic activity of HMR3647, a new ketolide [abstract F-255]. Presented at the 37th Interscience Conference on Antimicrobial Agents and Chemotherapy; September 28-October 1, 1997; Toronto, Ontario, Canada
- 119.Vesga O, Andes D, Craig WA. Comparative in vivo activity of HMR 3647, azithromycin, clarithromycin and roxithromycin against S. pneumoniae and Staphylococcus aureus [abstract F-258]. Presented at the 37th Interscience Conference on Antimicrobial Agents and Chemotherapy; September 28-October 1, 1997; Toronto, Ontario, Canada
- 120.Vesga O, Craig WA. Impact of macrolide resistance on the in vivo activity of a new ketolide, HMR 3647, against S. pneumoniae and S. aureus [abstract F-259]. Presented at the 37th Interscience Conference on Antimicrobial Agents and Chemotherapy; September 28-October 1, 1997; Toronto, Ontario, Canada
- 121.Zhanel G.G., Walters M., Noreddin A. The ketolides: a critical review. Drugs. 2002;62:1771–1804. doi: 10.2165/00003495-200262120-00006. [DOI] [PubMed] [Google Scholar]
- 122.Andes D, Vesga O, Craig W. Impact of neutrophils on the antimicrobial activity of ketolides in an animal infection model [abstract 1–19]. Presented at the Fourth International Conference on Macrolides, Azalides, Streptogramins, Ketolides and Oxazolidinones; January 21–23, 1998; Barcelona, Spain
- 123.Capitano B, Maglio D, Banevicius MA, et al. Bactericidal effect of cethromycin in an imunompetent murine pneumococcal pneumonia model [abstract A-1271]. Presented at the 42nd Interscience Conference on Antimicrobial Agents and Chemotherapy; September 27–30, 2002; San Diego, CA
- 124.Tessier P.R., Kim M.K., Zhou W. Pharmacodynamic assessment of clarithromycin in a murine model of pneumonia. Antimicrob Agents Chemother. 2002;46:1425–1434. doi: 10.1128/AAC.46.5.1425-1434.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.NCCLS. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; approved standard supplemental tables. NCCLS M7-A5/M100-S10 (M7) 2000;20:1–25
- 126.Jacobs MR, Bajaksouzian S, Windau A, et al. Effects of various test media on the activities of 21 antimicrobial agents against Haemophilus influenzae. J Clin Microbiol 2002;40:2369–3276 [DOI] [PMC free article] [PubMed]
- 127.Ambrose P.G., Garsela D.M. The use of Monte Carlo simulation to examine pharmacodynamic variance of drugs: fluoroquinolone pharmacodynamics against Streptococcus pneumoniae. Diagn Microbiol Infect Dis. 2000;38:151–157. doi: 10.1016/s0732-8893(00)00185-1. [DOI] [PubMed] [Google Scholar]
- 128.Dudley M.N., Ambrose P.G. Pharmacodynamics in the study of drug resistance and establishing in vitro susceptibility breakpoints: ready for prime time. Curr Opin Microbiol. 2000;3:515–521. doi: 10.1016/s1369-5274(00)00132-6. [DOI] [PubMed] [Google Scholar]
- 129.Ambrose P.G., Bhavnani S.M., Cirincione B.B. Gatifloxacin and the elderly: pharmacokinetic-pharmacodynamic rationale for a potential age-related dose reduction. J Antimicrob Chemother. 2003;52:435–440. doi: 10.1093/jac/dkg370. [DOI] [PubMed] [Google Scholar]
- 130.Ambrose P.G., Quintiliani R. Limitations of single point pharmacodynamic analysis. Pediatr Infect Dis J. 2000;19:769. doi: 10.1097/00006454-200008000-00025. [DOI] [PubMed] [Google Scholar]
- 131.Nicolau D.P., Ambrose P.G. Pharmacodynamic profiling of levofloxacin and gatifloxacin using Monte Carlo simulation for community-acquired isolates of Streptococcus pneumoniae. Am J Med. 2001;111(Suppl 9A):13S–18. doi: 10.1016/s0002-9343(01)01026-9. [DOI] [PubMed] [Google Scholar]
- 132.Jones R.N., Rubino C.M., Bhavnani S.M. Worldwide antimicrobial susceptibility patterns and pharmacodynamic comparisons of gatifloxacin and levofloxacin against Streptococcus pneumoniae: report from the Antimicrobial Resistance Rate Epidemiology Study Team. Antimicrob Agents Chemother. 2003;47:292–296. doi: 10.1128/AAC.47.1.292-296.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Schrag S.J., Pena C., Fernandez J. Effect of short-course, high-dose amoxicillin therapy on resistant pneumococcal carriage: a randomized trial. JAMA. 2001;286:49–56. doi: 10.1001/jama.286.1.49. [DOI] [PubMed] [Google Scholar]
- 134.Dagan R., Hoberman A., Johnson C. Bacteriologic and clinical efficacy of high dose amoxicillin/clavulanate in children with otitis media. Pediatr Infect Dis J. 2001;20:829–837. doi: 10.1097/00006454-200109000-00002. [DOI] [PubMed] [Google Scholar]
- 135.Piglansky L., Leibovitz E., Raiz S. Bacteriologic and clinical efficacy of high dose amoxicillin for therapy of acute otitis media in children. Pediatr Infect Dis J. 2003;22:405–413. doi: 10.1097/01.inf.0000065688.21336.fa. [DOI] [PubMed] [Google Scholar]
- 136.File T.M., Jr, Jacobs M.R., Poole M.D. Outcome of treatment of respiratory tract infections due to Streptococcus pneumoniae, including drug-resistant strains, with pharmacokinetically enhanced amoxicillin/clavulanate. Int J Antimicrob Agents. 2002;20:235–247. doi: 10.1016/s0924-8579(02)00130-9. [DOI] [PubMed] [Google Scholar]
- 137.Doern G.V. Activity of oral β-lactam antimicrobial agents versus respiratory tract isolates of Streptococcus pneumoniae, Haemophilus influenzae, and Moraxella catarrhalis in the era of antibiotic resistance. Otolaryngol Head Neck Surg. 2002;127:S17–23. doi: 10.1067/mhn.2002.130029. [DOI] [PubMed] [Google Scholar]
- 138.Powers J.L., Gooch W.M., III, Oddo L.P. Comparison of the palatability of the oral suspension of cefdinir vs. amoxicillin/clavulanate potassium, cefprozil, and azithromycin in pediatric patients. Pediatr Infect Dis J. 2000;19(12 Suppl):S174–180. doi: 10.1097/00006454-200012001-00008. [DOI] [PubMed] [Google Scholar]
- 139.Steele R.W., Thomas M.P., Begue R.E. Compliance issues related to the selection of antibiotic suspensions for children. Pediatr Infect Dis J. 2001;20:1–5. doi: 10.1097/00006454-200101000-00001. [DOI] [PubMed] [Google Scholar]
- 140.Craig W.A. Does the dose matter? Clin Infect Dis. 2001;33(Suppl 3):S233–237. doi: 10.1086/321854. [DOI] [PubMed] [Google Scholar]
- 141.Ambrose P., Owens R. New antibiotics in pulmonary critical care medicine; focus on advanced generation quinolones and cephalosporins. Semin Respir Crit Care Med. 2000;21:19–32. doi: 10.1055/s-2000-9927. [DOI] [PubMed] [Google Scholar]
- 142.Khaliq Y., Zhanel G.G. Fluoroquinolone-associated tendinopathy: a critical review of the literature. Clin Infect Dis. 2003;36:1404–1410. doi: 10.1086/375078. [DOI] [PubMed] [Google Scholar]
- 143.Kuehnert M.J., Nolte F.S., Perlino C.A. Fluoroquinolone resistance in Streptococcus pneumoniae. Ann Intern Med. 1999;131:312–313. doi: 10.7326/0003-4819-131-4-199908170-00023. [DOI] [PubMed] [Google Scholar]
- 144.Urban C., Rahman N., Zhao X. Fluoroquinolone-resistance Streptococcus pneumoniae associated with levofloxacin therapy. J Infect Dis. 2001;184:794–798. doi: 10.1086/323086. [DOI] [PubMed] [Google Scholar]
- 145.Kays M.B., Smith D.W., Wack M.F. Levofloxacin treatment failure in a patient with fluoroquinolone-resistant Streptococcus pneumoniae pneumonia. Pharmacotherapy. 2002;22:395–399. doi: 10.1592/phco.22.5.395.33185. [DOI] [PubMed] [Google Scholar]
- 146.Ross J.J., Worthington M.G., Gorbach S.L. Resistance to levofloxacin and failure of treatment of pneumococcal pneumonia. N Engl J Med. 2002;347:65–66. doi: 10.1056/NEJM200207043470115. [DOI] [PubMed] [Google Scholar]
- 147.Dagan R., Leibovitz E., Fliss D.M. Bacteriologic efficacies of oral azithromycin and oral cefaclor in treatment of acute otitis media in infants and young children. Antimicrob Agents Chemother. 2000;44:43–50. doi: 10.1128/aac.44.1.43-50.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Doern G.V. Antimicrobial use and emergence of antimicrobial resistance with Streptococcus pneumoniae in the United States. Clin Infect Dis. 2001;33(Suppl 3):S187–192. doi: 10.1086/321847. [DOI] [PubMed] [Google Scholar]
- 149.Christianson J, Andes DR, Craig WA. Pharmacodynamic characteristics of clindamycin against Streptococcus pneumoniae in a murine thigh-infection model [abstract A-1100]. Presented at the 41st Interscience Conference on Antimicrobial Agents and Chemotherapy; December 16–19, 2001; Chicago, IL
- 150.Doern G.V., Jones R.N., Pfaller M.A. Haemophilus influenzae and Moraxella catarrhalis from patients with community-acquired respiratory tract infections: antimicrobial susceptibility patterns from the SENTRY antimicrobial surveillance program (United States and Canada, 1997) Antimicrob Agents Chemother. 1999;43:385–389. doi: 10.1128/aac.43.2.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Doern G.V., Heilmann K.P., Huynh H.K. Antimicrobial resistance among clinical isolates of Streptococcus pneumoniae in the United States during 1999–2000, including a comparison of resistance rates since 1994–1995. Antimicrob Agents Chemother. 2001;45:1721–1729. doi: 10.1128/AAC.45.6.1721-1729.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Bertho G., Gharbi-Benarous J., Delaforge M. Conformational analysis of ketolide, conformations of RU 004 in solution and bound to bacterial ribosomes. J Med Chem. 1998;41:3373–3386. doi: 10.1021/jm970852i. [DOI] [PubMed] [Google Scholar]
- 153.Capobianco J.O., Cao Z., Shortridge V.D. Studies of the novel ketolide ABT-773: transport, binding to ribosomes, and inhibition of protein synthesis in Streptococcus pneumoniae. Antimicrob Agents Chemother. 2000;44:1562–1567. doi: 10.1128/aac.44.6.1562-1567.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Craig WA, Andes DR. Pattern of bactericidal activity with telithromycin against erythromycin-resistant Streptococcus pneumoniae in the murine thigh-infection model [abstract A-2098]. Presented at the 41st Interscience Conference on Antimicrobial Agents and Chemotherapy; December 16–19, 2001; Chicago, IL
- 155.Bonnefoy A., Girard A.M., Agouridas C. Ketolides lack inducibility properties of MLS(B) resistance phenotype. J Antimicrob Chemother. 1997;40:85–90. doi: 10.1093/jac/40.1.85. [DOI] [PubMed] [Google Scholar]
- 156.Agouridas C., Denis A., Auger J.M. Synthesis and antibacterial activity of ketolides (6-O-methyl-3-oxoerythromycin derivatives): a new class of antibacterials highly potent against macrolide-resistant and -susceptible respiratory pathogens. J Med Chem. 1998;41:4080–4100. doi: 10.1021/jm980240d. [DOI] [PubMed] [Google Scholar]
- 157.Rosato A., Vicarini H., Bonnefoy A. A new ketolide, HMR 3004, active against streptococci inducibly resistant to erythromycin. Antimicrob Agents Chemother. 1998;42:1392–1396. doi: 10.1128/aac.42.6.1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Tessier PR, Mattoes HM, Dandekar PK, et al. Pharmacodynamic profile of telithromycin against macrolide and fluoroquinolone resistant Streptococcus pneumoniae in a neutropenic thigh infection model. Antimicrob Agents Chemother. In Press [DOI] [PMC free article] [PubMed]
- 159.Drusano GL, Preston SL. Utility of an 800 mg dose of telithromycin for community-acquired pneumonia caused by extracellular pathogens: an assessment by pharmacodynamic modelling and monte carlo simulation [poster P1364]. Presented at: 12th European Congress of Clinical Microbiology and Infectious Disease; April 24–27, 2002; Milan, Italy
- 160.Ferguson B.J. Recommendations for the treatment of acute bacterial rhinosinusitis are NOT based on current microbial resistance patterns. Otolaryngol Head Neck Surg. 2000;123:665–667. doi: 10.1067/mhn.2000.110857. [DOI] [PubMed] [Google Scholar]
- 161.Marchant C., Carlin S., Johnson C. Measuring the comparative efficacy of antibacterial agents for acute otitis media: the “Pollyanna phenomenon”. J Pediatr. 1992;120:72–77. doi: 10.1016/s0022-3476(05)80601-8. [DOI] [PubMed] [Google Scholar]
- 162.Poole M.D. A mathematical therapeutic outcomes model for sinusitis. Otolaryngol Head Neck Surg. 2004;130:46S–50S. doi: 10.1016/j.otohns.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 163.Levine O.S., Farley M., Harrison L.H. Risk factors for invasive pneumococcal disease in children: a population-based case-control study in North America. Pediatrics. 1999;103:E28. doi: 10.1542/peds.103.3.e28. [DOI] [PubMed] [Google Scholar]
- 164.Pallares R., Gudiol F., Linares J. Risk factors and response to antibiotic therapy in adults with bacteremic pneumonia caused by penicillin-resistant pneumococci. N Engl J Med. 1987;317:18–22. doi: 10.1056/NEJM198707023170104. [DOI] [PubMed] [Google Scholar]
- 165.Dagan R., Hoberman A., Johnson C. Bacteriologic and clinical efficacy of high dose amoxicillin/clavulanate in children with acute otitis media. Pediatr Infect Dis J. 2001;20:829–837. doi: 10.1097/00006454-200109000-00002. [DOI] [PubMed] [Google Scholar]


