Abstract
Background
There is a need for more research on all forms of rhinosinusitis. Progress in this area has been hampered by a lack of consensus definitions and the limited number of published clinical trials.
Objectives
To develop consensus definitions for rhinosinusitis and outline strategies useful in clinical trials.
Study design
Five national societies, The American Academy of Allergy, Asthma and Immunology; The American Academy of Otolaryngic Allergy; The American Academy of Otolaryngology Head and Neck Surgery; The American College of Allergy, Asthma and Immunology; and the American Rhinologic Society formed an expert panel from multiple disciplines. Over two days, the panel developed definitions for rhinosinusitis and outlined strategies for design of clinical trials.
Results
Committee members agreed to adopt the term “rhinosinusitis” and reached consensus on definitions and strategies for clinical research on acute presumed bacterial rhinosinusitis, chronic rhinosinusitis without polyposis, chronic rhinosinusitis with polyposis, and classic allergic fungal rhinosinusitis. Symptom and objective criteria, measures for monitoring research progress, and use of symptom scoring tools, quality-of-life instruments, radiologic studies, and rhinoscopic assessment were outlined for each condition.
Conclusions
The recommendations from this conference should improve accuracy of clinical diagnosis and serve as a starting point for design of rhinosinusitis clinical trials.
I. Preface
Recognizing a need for evidence-based rhinosinusitis guidelines, 5 national societies, The American Academy of Allergy, Asthma and Immunology (AAAAI); The American Academy of Otolaryngic Allergy (AAOA); The American Academy of Otolaryngology-Head and Neck Surgery (AAO-HNS); The American College of Allergy, Asthma and Immunology (ACAAI); and the American Rhinologic Society (ARS), convened a group of 30 physicians from a wide range of disciplines: allergy-immunology, otolaryngology, infectious disease, and radiology. Over 2 days, this panel worked together to develop definitions of rhinosinusitis for clinical research and to suggest clinical trial designs for studies that would allow for more appropriate use of pharmacologic, immunologic, and surgical interventions. Using an anonymous electronic audience response system, the committee was able to reach consensus (≥80% of committee members) on definitions and clinical research strategies for acute (bacterial) rhinosinusitis, chronic rhinosinusitis (CRS) without polyps, CRS with polyps, and allergic fungal rhinosinusitis (AFRS). Diversity of opinion was expressed on whether rhinosinusitis would best be characterized as an infection or an inflammatory condition. Current understanding of the terms infection and inflammation were therefore included in this discussion.
At this consensus conference, multiple viewpoints were discussed, and there was general agreement that no one causative factor fully explains or adequately accounts for the pathologic manifestations and clinical heterogeneity of rhinosinusitis. Histopathologically speaking, the inflammatory component of these disorders manifests as a mixed mononuclear inflammatory cell infiltrate, with neutrophils predominating in acute disease and eosinophils predominating in most chronic disease. Additionally, there has been an evolution of thought moving away from the notion that all of CRS can be explained on the basis of sinus ostial obstruction and persistent bacterial infection to an appreciation that CRS has a significant inflammatory component that might be caused simultaneously or independently by various factors. Evidence for the varying potential sources of this condition is discussed. These include but are not restricted to the possible roles of:
-
1
persistent infection as a factor in CRS, including biofilms and osteitis1, 2, 3, 4;
-
2
allergy and other disorders of immunity;
-
3
intrinsic factors of the upper airway;
-
4
superantigens from Staphylococcus aureus in CRS with nasal polyps5, 6;
-
5
colonizing fungi that induce and sustain eosinophilic inflammation7, 8, 9; and
-
6
metabolic perturbations, such as aspirin sensitivity.
It was emphasized that several mechanisms might be acting simultaneously or independently in a given patient. Thus, this document reviews various causative factors in rhinosinusitis and highlights areas in which their roles in rhinosinusitis are controversial and in which new information is emerging. Various physicians authored individual sections to serve as background information on the controversies and definitions presented later in this article. The document also presents a classification scheme for CRS on the basis of current knowledge and consensus opinion, and, furthermore, discusses the subjective and objective measures used in the diagnosis and evaluation of rhinosinusitis. Important factors in the design of clinical trials are discussed. Ultimately, consensus definitions for rhinosinusitis are put forth for:
-
1
acute presumed bacterial rhinosinusitis;
-
2
CRS without polyps;
-
3
CRS with polyps; and
-
4
classic AFRS.
Initial proposals are made for clinical trial designs, including an outline of suggested subjective and objective assessments applicable to these studies.
This group concluded that (1) promoting more research on both acute rhinosinusitis and CRS is essential, (2) a better understanding of the pathophysiology of these diseases is needed, and (3) study designs for the evaluation of potential therapeutic modalities for rhinosinusitis, as well as appropriate outcome studies, must be carefully considered.
These consensus recommendations are based on the clinical expertise of the participants, which is, in turn, based on a review and understanding of the clinical literature. They do not represent the position of any regulatory agency or pharmaceutical company. Much work needs to be done before definitive study designs for rhinosinusitis can be recommended, although this document represents an essential beginning to that process. The development of recommendations for study designs in the study of therapeutic modalities for the treatment of rhinosinusitis will be the responsibility of this collaborative group in the future.
The group decided by consensus to use the term rhinosinusitis instead of sinusitis throughout this document. This decision was based on the fact that sinusitis is almost always accompanied by concurrent nasal airway inflammation, and, in many cases, sinusitis is preceded by rhinitis symptoms. Therefore, it was believed that the use of the term rhinosinusitis more accurately describes the spectrum of infectious and inflammatory conditions previously grouped under the term sinusitis. The group endorsed and adopted the previously developed definition of the Sinus and Allergy Health Partnership Task Force for Rhinosinusitis: “Rhinosinusitis is a group of disorders characterized by inflammation of the mucosa of the nose and the paranasal sinuses.”
For acute rhinosinusitis, CRS without nasal polyposis, CRS with nasal polyposis, and classic AFRS, diagnostic criteria are outlined, including the pattern of symptoms that defines each one, the typical symptoms necessary to diagnose the disease, and the objective criteria required. Measurements for monitoring progress to determine clinical efficacy are also suggested. It is hoped that the establishment of a consensus of these definitions and recommendations by recognized experts in the diagnosis and assessment of rhinosinusitis will provide clinicians and researchers with the tools necessary for developing and implementing appropriate clinical studies and serve as a catalyst for further research of rhinosinusitis.
II. Executive summary
Rhinosinusitis is increasing in prevalence and incidence and has been estimated to affect approximately 31 million patients in the United States each year.10 It causes significant physical symptoms, negatively affects quality of life (QOL), and can substantially impair daily functioning. Advancing existing definitions that describe all manifestations of rhinosinusitis, discussed elsewhere as sinusitis, has proved to be difficult. This is due, in part, to the numerous causes of the condition, including viral, bacterial, fungal, and allergic causes; in addition, many patients have seemingly idiopathic disease. Rhinosinusitis is commonly divided into acute and chronic forms because these are 2 major categories that are listed in the International Classification of Diseases-Ninth Revision, Sixth Edition,11 although other classes (ie, subacute, recurrent acute, acute exacerbation of chronic, community acquired bacterial, and nosocomial) are described elsewhere in the medical literature.12
Acute rhinosinusitis is usually infectious in nature, whereas chronic disease might result from a wide range of processes. Related to the complexities of this health care problem and because of practical constraints, the primary focus of this article is to establish clear definitions of acute rhinosinusitis and CRS for research and to advance existing definitions for clinical care. These goals are achieved on the basis of evidence in the literature and consensus of opinions (>80% of committee members) for these proposed definitions.
There is a clear need for more research on all forms of rhinosinusitis. Not enough is understood about the pathophysiology of these conditions, and without better understanding, safer and more effective treatment options cannot be developed. To date, most clinical research, including drug trials, have focused on acute rhinosinusitis. Reasons for the limited number of therapeutic trials for CRS have included the lack of widespread acceptance of existing definitions for the disorder and the acknowledged difficulty in establishing the causes for this condition. As a result, clinicians have been left to use empiric guidelines or their best judgment in choosing interventions for the treatment of CRS. Likewise, there is a lack of evidence-based guidelines to aid in developing successful rhinosinusitis clinical trials. Notwithstanding the need for additional research, there is widely held agreement that careful consideration of parameters for trial designs and outcomes studies is required as a starting point.
Various causative factors play a role in rhinosinusitis, including microorganisms, allergic and nonallergic immunologic inflammation, and noninfectious, nonimmunologic causes. Infection is defined as the invasion and multiplication of microorganisms within sterile host tissues. Inflammation is a series of cellular and molecular responses designed to eliminate foreign agents and promote repair of damaged tissues. Histologic patterns of inflammation are a function of at least 3 factors: nature of the inciting agent, time of the observation, and immune status of the host.
The common cold involves both the nasal passages and the paranasal sinuses. During a cold, nasal fluid containing viruses, bacteria, and inflammatory mediators are blown into the sinuses where they produce inflammation, infection, or both. This results in mucosal edema, cellular infiltration, and mucus thickened by means of exocytosis of mucin from the numerous goblet cells in the sinus epithelium.
A sinus infection can be caused by one or more bacteria in high density (at least 1000 colony forming units [cfu]/mL); commonly isolated bacteria in patients with rhinosinusitis include Streptococcus pneumoniae, Haemophilus influenzae, and Moraxella catarrhalis. Bacterial rhinosinusitis can be classified as acute, subacute, or chronic, depending on the duration of the symptoms. The role of bacterial infection in children and adults with CRS is controversial. Bacterial superantigens, biofilms, and osteitis might play an important role in CRS and warrant further study.
AFRS is a distinct clinical subset of CRS in which patients will have positive evidence of allergy to the fungus colonizing their “allergic mucin” in the majority of cases. Patients with AFRS typically demonstrate 5 characteristics: gross production of eosinophilic mucin containing noninvasive fungal hyphae, nasal polyposis, specific radiographic findings, immunocompetence, and allergy to cultured fungi. The presentation of AFRS might be dramatic, giving rise to acute visual loss, gross facial dysmorphia, or complete nasal obstruction, but more often, the presentation is subtle. Recent studies suggest that fungi might play an alternate role in the development of CRS, whereby patients mount innate immune responses to colonizing fungi through non-IgE-mediated mechanisms. These responses are hypothesized to lead to local eosinophilic infiltration, inflammation, and tissue injury. This concept of “eosinophilic fungal rhinosinusitis” encompasses most patients with CRS.
There are documented allergic and immunologic factors associated with the development of rhinosinusitis. Clinically, perennial allergic rhinitis is a predisposing condition for acute bacterial rhinosinusitis. Histologically, CRS without nasal polyps (CRSsNP) is characterized by a predominantly neutrophilic inflammation with a lesser contribution of eosinophils, whereas CRS with nasal polyps (CRSwNP) is characterized by eosinophilic inflammation. Interleukin (IL) 5 and eotaxin could play a role in the latter process. Neither total IgE concentrations nor eosinophilic cationic protein (ECP), IL-4, or IL-5 concentrations in nasal polyps are different in atopic versus nonatopic subjects, suggesting a discordance between systemic allergic phenotype and local inflammatory mechanisms leading to eosinophilic inflammation in nasal polyps (NPs). A role has been proposed for IgE specific for staphylococcal-derived superantigens in the pathogenesis of CRS associated with nasal polyps.
Not all rhinosinusitis is inflammatory. Overactivity or underactivity of autonomic nerve pathways, abnormalities in leukotriene production or responsiveness, nociceptive dysfunction, or local irritation caused by gastroesophageal reflux are demonstrable in select subsets of patients with rhinosinusitis and could predispose to the pathogenesis of CRS. Defects in mucociliary clearance and antibody deficiency syndromes predispose to rhinosinusitis. Aspirin-associated respiratory disease also predisposes to rhinosinusitis.
Examining the histology of middle turbinate tissues from subjects with polypoid versus nonpolypoid disease might allow for distinction between these 2 entities. Samples from patients with CRSsNP versus CRSwNP generally show different patterns in cellular content and gross histologic changes within the tissue, especially with regard to fibrosis and edema. The mucosal lining in CRSsNP is characterized by basement membrane thickening, goblet cell hyperplasia, limited subepithelial edema, prominent fibrosis and mononuclear cell infiltration. Histomorphologic characterization of CRSwNP reveals frequent epithelial damage, a thickened basement membrane, and mostly edematous to sometimes fibrotic stromal tissue, with a reduced number of vessels and glands but virtually no neuronal structures.
Characteristic symptoms and signs of CRSwNP include nasal congestion, facial pain-pressure-fullness, postnasal drainage, hyposmia-anosmia, and the presence of bilateral NPs. Histologically, NPs typically show an inflammatory infiltrate with increased numbers of eosinophils. At least 4 processes might contribute to variable degrees to the inflammatory process of CRSwNP: (1) late-phase allergic inflammation in response to airborne allergens; (2) T-cell activation with production of IL-5, IL-13, and IFN-γ in response to fungal antigens (hyphae) in sinus mucus; (3) T-cell activation, cytokine production, and local IgE production in response to bacterial superantigens; and (4) dysregulation of sinus epithelium with overproduction of chemokines, such as regulated on activation, normal T cell expressed and secreted (RANTES).
Numerous subjective and objective assessment measures can be used in the diagnosis and evaluation of rhinosinusitis, including symptoms, QOL scores, rhinoscopic examination, imaging, and nasal-sinus challenges.
All relevant rhinosinusitis symptoms, their severity, and their time course should be documented. Characteristic symptoms and signs of rhinosinusitis include nasal congestion, facial pain-pressure-fullness, anterior and postnasal drainage, and hyposmia-anosmia. The symptom list is not necessarily different between patients with acute versus chronic disease, and some symptoms are present in patients with rhinitis who do not have evidence of sinusitis. A 7-point analog scale can be used to report individual symptom severity scores, a total rhinosinusitis severity score, a global severity score, an overall QOL score, and the effect of current and past treatments.
For a complete and thorough assessment of the morbidity associated with rhinosinusitis and the evaluation of treatment, it is imperative that the physical, social, emotional, and functional problems associated with this condition be measured in a valid way. Investigators should strive to report quality-of-life (QOL) data in a fashion that is most clinically meaningful. There are several validated rhinosinusitis outcome measures, and the instrument that seems best suited for the particular research question should be selected.
Anterior rhinoscopy is the basic tool of the physical examination that relates to determining the existence of pathology in the sinonasal passages. It is best to evaluate the patient after decongestion with topical decongestants. However, even with this method, examination of the nasal passages beyond the anterior portion can be limited. Nasal endoscopy helps identify erythema, edema, polyps or polypoid swelling, crusting, eosinophilic mucin, and mucopus or frank pus deep in the nasal cavity. Cultures obtained endoscopically are less invasive and associated with less morbidity; however, this technique was not found to be equivalent to antral puncture in children with sinus infections.
Although rhinosinusitis can be diagnosed in the majority of patients by using only the history and physical examination (including endoscopy), patients with persistent sinus disease often require imaging studies. These studies are an absolute requirement in patients undergoing functional endoscopic sinus surgery. Computed tomography (CT) has 2 major roles in the management of rhinosinusitis: (1) to define the anatomy of the sinuses before surgery and (2) to aid in the diagnosis and management of recurrent rhinosinusitis or CRS. Although magnetic resonance imaging (MRI) does not display the bony anatomy as does CT, it does provide an excellent display of the mucosa, and it is better than CT in distinguishing between bacterial-viral inflammatory disease and fungal concretions.
Nasal-sinus challenge is useful in defining the pathophysiology of rhinosinusitis and the interactions between the nose and the sinuses, as well as the lower airway. Nasal challenge has also been used to confirm the presence of allergy, to assess nasal threshold responses, and to study the mediators, inflammatory cells, and cytokines associated with rhinosinusitis.
The integrated airway syndrome, also called chronic inflammatory respiratory syndrome, has a wide spectrum of severity: at the low end, its manifestations are clinically evident in the form of rhinitis, and at the high end, manifestations can include asthma and possibly rhinosinusitis. There is a very strong link between the upper and lower airways: both allergic rhinitis and nonallergic rhinitis are risk factors for asthma; allergic rhinitis is almost ubiquitous in asthma; even in the absence of nasal symptoms, the nasal mucosa of patients with asthma shows evidence of inflammation; and the rhinitis of asthmatic patients tends to be more severe than the rhinitis of nonasthmatic patients. Moreover, allergic reactions and their inflammatory consequences appear to propagate systemically; therefore, the link between the nose, the sinuses, and the lower airways might be considered a systemic process.
Agreement on definitions, histopathology, and diagnostic criteria is an important prelude to the selection of an appropriate design for clinical studies of rhinosinusitis. The efficacy of a treatment modality for rhinosinusitis must be demonstrated through adequate and well-controlled studies showing that the intervention will have the effect that is claimed. Factors to consider in developing a protocol for such a study include (1) primary and secondary study objectives, (2) overall study design, (3) the basis for dose selection and route of administration, (4) the study population, (5) inclusion-exclusion criteria, (6) control subjects, (7) safety and efficacy outcome variables, and (8) statistical considerations, such as powering the study. For example, the prospective choice of end points is an important decision in designing clinical studies. Efficacy end points for studies that will form the basis of approval for such a treatment modality should be clinically relevant and validated.
III. Introduction
Rhinosinusitis is increasing in prevalence and incidence, and has been estimated to affect approximately 31 million patients in the United States each year.10 It causes significant physical symptoms, negatively affects QOL, and can substantially impair daily functioning. Advancing existing definitions that describe all manifestations of rhinosinusitis, discussed elsewhere as sinusitis, has proved to be difficult. This is due, in part, to the numerous causes of the condition, including viral, bacterial, fungal, and allergic causes; in addition, many patients have seemingly idiopathic disease. Rhinosinusitis is commonly divided into acute and chronic forms because these are 2 major categories that are listed in the International Classification of Diseases-Ninth Revision, Sixth Edition,11 although other classes (ie, subacute, recurrent acute, acute exacerbation of chronic, community acquired bacterial, and nosocomial) are described elsewhere in the medical literature.12
Acute rhinosinusitis is usually infectious in nature, whereas chronic disease might result from a wide range of processes. Related to the complexities of this health care problem and for practical constraints, the primary focus of this article is to establish clear definitions of acute rhinosinusitis and CRS for research and to advance existing definitions for clinical care. These goals are achieved on the basis of evidence in the literature and consensus of opinions of international leaders for these proposed definitions.
There is a clear need for more research on all forms of rhinosinusitis. Not enough is understood about the pathophysiology of these conditions, and without better understanding, safer and more effective treatment options cannot be developed. To date, most clinical research, including drug trials, have focused on acute rhinosinusitis. Reasons for the limited number of therapeutic trials for CRS have included the lack of widespread acceptance of existing definitions for the disorder and the acknowledged difficulty in establishing the causes for this condition. As a result, clinicians have been left to use empiric guidelines or their best judgment in choosing interventions for the treatment of CRS. Likewise, there is a lack of evidence-based guidelines to aid in developing successful rhinosinusitis clinical trials. Notwithstanding the need for additional research, there is widely held agreement that careful consideration of parameters for trial designs and outcomes studies is required as a starting point.
IV. Causative factors in rhinosinusitis
As a preface to this section, the terms infection and inflammation are discussed and defined. Infection typically induces an inflammatory response and has been defined in various ways. Although it is important to note that some choose to define infection as a microbial phenomenon characterized by an inflammatory response to the presence of microorganisms,14 others believe that true infection is defined as the invasion and multiplication of microorganisms in tissue. Additionally, they hold that infection is distinct from colonization by the immune response and development of disease in the host (J. Gwaltney, personal communication, 2004).
Inflammation is a series of cellular and molecular responses that are designed to eliminate foreign agents and promote repair of damaged tissues.15 It begins with a reaction of blood vessels, leading to the accumulation of fluid and leukocytes in extravascular tissues.16 There is increasing evidence that in addition to infection, immunologic inflammatory responses play major roles in the cause and pathophysiology of CRS.
In this article infection is distinguished from inflammation along the more traditional concepts of tissue invasion. It is acknowledged, however, that the histopathologic evidence of this distinction in all forms of rhinosinusitis is not carefully studied. Additionally, the 2 most hotly debated hypotheses to explain CRS relate to colonization of the sinonasal mucosa with microorganisms and the host response to their presence (eg, superantigens-producing S aureus and colonizing fungi). A substantial concern is that identifying rhinosinusitis as an infection alone might promote continued widespread use of antimicrobial agents. Current evidence to support their use, particularly in chronic disease, is limited, and there is an obvious concern that this will contribute to the increasing rates of antimicrobial resistance.
Histologic patterns of inflammation are a function of at least 3 factors: nature of the inciting agent, time of the observation, and immune status of the host. Timing is traditionally defined on the basis of clinical onset and duration of the response. Specifically, inflammation has been referred to as acute when signs or symptoms appear over minutes to hours, subacute when it spans days to weeks, and chronic when it occurs over weeks to months.15 The main pathologic characteristic of acute inflammation is the exudation of fluid and plasma proteins (edema) and the emigration of leukocytes, predominantly neutrophils. Chronic inflammation is histologically associated with the presence of lymphocytes, macrophages, and occasionally eosinophils and basophils and the proliferation of blood vessels, fibrosis, and tissue necrosis.16 A clear distinction between acute and chronic inflammation is somewhat artificial because of numerous overlapping patterns of inflammation.17 Despite the evolutionary benefits to inflammation and repair, alterations in the balance between proinflammatory and anti-inflammatory mediators can lead to harmful effects.
A. Microorganisms and rhinosinusitis
1. Viral infection
Summary Statements:
-
•
In the nonimmune individual, the nasal passages are unable to clear or inactivate an infecting virus.
-
•
The common cold involves both the nasal passages and the paranasal sinuses.
-
•
Evidence supports the concept that during a cold, nasal fluid containing viruses, bacteria, and inflammatory mediators might be blown into the sinuses, where they produce inflammation, infection, or both. Mucosal edema, cellular infiltration, and mucus thickened by exocytosis of mucin from the numerous goblet cells in the sinus epithelium are the result.
Although symptoms of the common cold have been recognized since antiquity, the first cold virus, rhinovirus, was not discovered until 1956.18 Within 30 years of its discovery, the replication strategy and atomic structure of the virus was determined.19 The rhinovirus enters the body through the nose by means of either contaminated fingers or large airborne particles.20 The virus is then transported in the mucus stream to the adenoid region of the nasopharynx, reaching an area where there are specialized lymphoepithelial cells (M cells) overlying lymphoid follicles.21, 22 These lymphoepithelial cells are rich in the rhinovirus receptor intercellular adhesion molecule 1 (ICAM-1).23
This series of events is very efficient. One of the central features in the pathogenesis of infections caused by rhinovirus is that in the nonimmune individual the nasal passages are unable to clear or inactivate the virus. For example, when 343 nonimmune healthy young adults were challenged by dropping rhinovirus in their nose, 321 (95%) of these individuals became infected.24 However, only three quarters of those who became infected had symptoms of illness, reflecting an inapparent infection rate similar to that observed under natural conditions. Initiation of rhinovirus infection is not only an efficient mechanism, but also occurs quite rapidly. After intranasal rhinovirus challenge of susceptible volunteers, newly produced virus was recovered in nasal secretions within 8 to 10 hours.25 This is the same amount of time required for rhinovirus replication in cell culture. Also, in this study symptoms were observed to appear after a relatively short time. Sore throat, nasal obstruction, and rhinorrhea were reported within 8 to 12 hours after virus challenge.
It is now recognized that the common cold not only involves the nasal passages but also the paranasal sinuses (Fig 1). Sinus CT scans obtained in 31 young adults with early common colds revealed frequent abnormalities in the sinus cavity.26 These abnormalities were observed in the maxillary sinus in 87% of the patients, the ethmoid sinus in 65%, the frontal in 32%, and the sphenoid in 39%. A subset of these patients underwent repeat CT scans 2 weeks later; most of the original changes resolved spontaneously after resolution of the corresponding upper respiratory tract infection. The findings of sinus abnormalities during colds have been confirmed in adults and children.27, 28 The nature of these findings has been debated, but one explanation is the development of thick exudates adhering to the sinus wall with such tenacity that the material is not moved by ciliary action. The epithelium of the sinus cavity contains a high concentration of goblet cells,29, 30 and exocytosis of large amounts of mucin might occur when these cells are stimulated. It is important to determine the nature of the abnormality because this has implications for understanding the pathogenesis of the process and the appropriate approach to its treatment. Whatever its nature, this self-limited process represents a viral rhinosinusitis that is occurring as part of the common cold.
Fig 1.
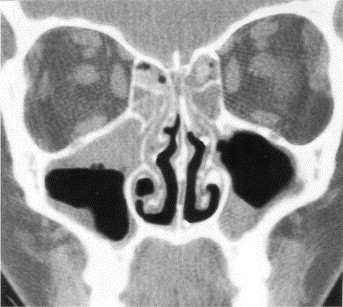
Sinus CT scan of a patient with viral rhinosinusitis showing abnormalities of the maxillary and ethmoid sinuses. Reprinted with permission from Arch Otolaryngol Head Neck Surg 1994;120:144. Copyrighted © 1994, American Medical Association. All Rights reserved.
An unusual finding on CT scanning of the sinus of a patient with a fresh common cold was closely evaluated to explore possible causes of sinus abnormalities during a common cold. The scan showed the maxillary sinus to be filled with what were unquestionable air bubbles, giving a frothy appearance to the material (Fig 2). 31 A sinus CT scan taken 3 days later showed typical findings associated with viral sinusitis “exudates” containing a few “air bubbles.” This led to the hypothesis that in this patient nasal fluid had been blown into the infundibulum and into the sinus, producing multiple air bubbles as the fluid exited the narrow lumen of the infundibulum under pressure and entered the relatively large sinus cavity. Later, the material was believed to have been thickened by means of exocytosis of mucin and coalesced to form an exudate.
Fig 2.
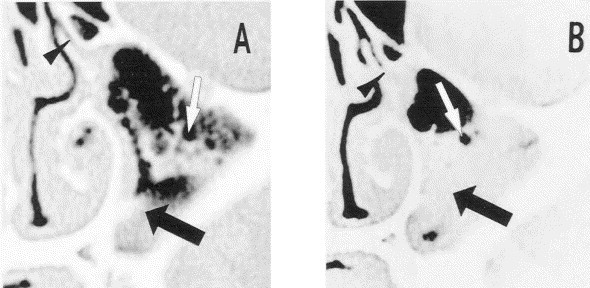
Coronal CT scan of the maxillary sinus of an adult with a common cold. A, Fourth day of illness showing multiple bubbles in the sinus cavity (white arrows), occlusion of the infundibulum (black arrowhead), and homogeneous abnormality along the medial wall and floor of the sinus cavity (black arrow). B, Seventh day of the illness showing occlusion of the infundibulum (black arrowhead) and homogenous abnormaility of the lower two thirds of the sinus cavity (black arrow). Few bubbles are still present in this material, but most of those present earlier have burst (white arrow). Reprinted with permission from Gwaltney JM, Jr, Hendley JO, Phillips CD, et al. Nose blowing propels nasal fluid into the paranasal sinuses. Clin Infect Dis 2000;30(2):387–92. Published by The University of Chicago Press. © 2000 by the Infectious Diseases Society of America.
Intranasal pressures were measured in volunteers during quiet respiration, nose blowing, sneezing, and coughing to determine how nasal fluid might be propelled into the sinus cavity.31 The mean ± SD maximal intranasal pressure was 66 ± 14 mm Hg during 35 nose blows, 4.6 ± 3.8 mm Hg during 13 sneezes, and 6.6 ± 3.8 mm Hg during 18 coughing bouts (Fig 3). Sneezing and coughing did not increase intranasal pressures to greater than those observed during quiet respirations. Contrast medium was placed in the pharynx of volunteers who then blew their nose, sneezed, or coughed to further investigate the pressure effects in the nasal passages of nose blowing, after which CT scans of the sinuses were obtained. Contrast medium appeared in one or more sinuses in 4 of the 4 subjects after a nose blow but not after sneezing or coughing (Fig 4). Calculations revealed that when the middle meatus is filled with viscous fluid, a single nose blow can propel up to 1 mL of this material into the maxillary sinus. These findings might explain the origin of the sinus cavity abnormalities in colds and also might explain why abnormalities are usually irregular in occurrence among various sinuses. One sinus might have considerable involvement, and another might be perfectly normal.
Fig 3.
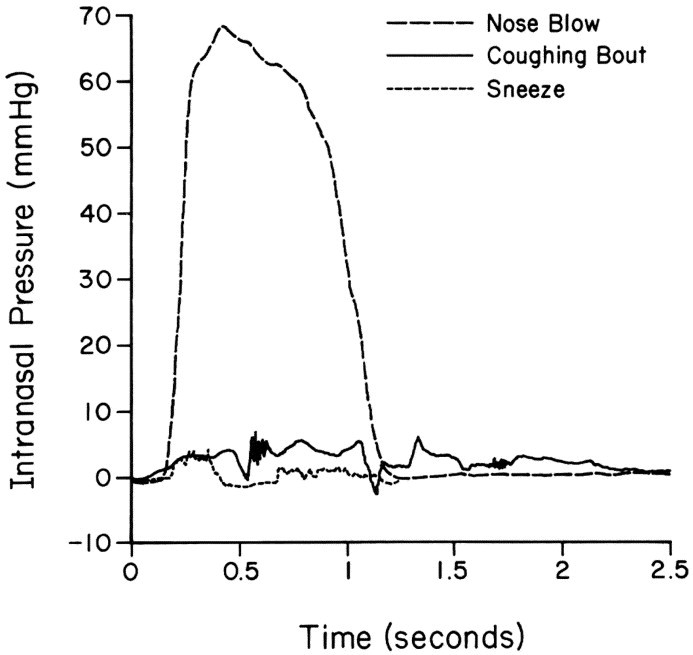
Intranasal pressure time histories for a representative nose blow, coughing bout, and sneeze shown on the same scale for comparison (dashed line, nose blow; solid line, coughing bout; dotted line, sneeze). Reprinted with permission from Gwaltney JM, Jr, Hendley JO, Phillips CD, et al. Nose blowing propels nasal fluid into the paranasal sinuses. Clin Infect Dis 2000;30(2):387–92. Published by The University of Chicago Press. © 2000 by the Infectious Diseases Society of America.
Fig 4.
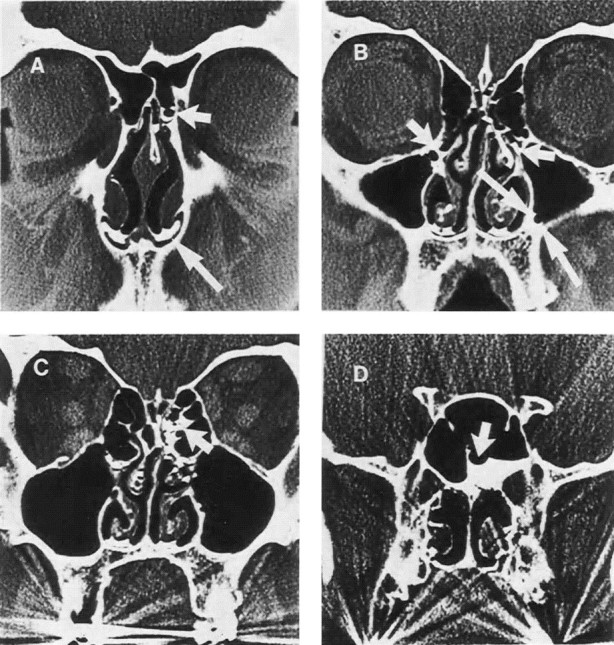
Sinus CT scan of an adult after instillation of contrast medium into the nasopharynx, followed by nose blowing. A, Contrast in an anterior ethmoid sinus cell (short arrow) and in the floor of the nasal cavities (long arrow). B, Contrast in the infundibulum bilaterally (short arrows) and in the maxillary sinus outlining a bubble (long arrows). C, Contrast in the posterior ethmoid sinus (arrow). D, Contrast in the sphenoid sinus outlining a bubble (arrow). Reprinted with permission from Gwaltney JM, Jr, Hendley JO, Phillips CD, et al. Nose blowing propels nasal fluid into the paranasal sinuses. Clin Infect Dis 2000;30(2):387–92. Published by The University of Chicago Press. © 2000 by the Infectious Diseases Society of America.
In summary, these findings support the hypothesis that during a cold, nasal fluid containing viruses, bacteria, and inflammatory mediators might be blown into the sinuses, where it produces inflammation, infection, or both and is thickened by means of exocytosis of mucin from the numerous goblet cells in the sinus epithelium. Thus the CT abnormalities observed in viral rhinosinusitis could be the result of inflammation alone or of viral infection of the cells in the sinus epithelium. In sinus puncture studies in patients with acute community-acquired rhinosinusitis, 15% of the sinus aspirates have yielded rhinovirus, 5% have yielded influenza virus, 3% have yielded parainfluenza virus, and 2% have yielded adenovirus.32 It is not known whether this actually represents viral replication in the sinus cavity. Some sinus aspirates have yielded both viruses and bacteria.
Criteria to define a case of viral rhinosinusitis are lacking. However, attention has been given to trying to define situations in which viral agents are not the sole cause; that is, the 0.5% to 2% of cases of viral rhinosinusitis that are estimated to be complicated by secondary bacterial infections.32, 33 However, it should be recognized that no studies have ever been conducted in which the sensitivity and specificity of various clinical findings have been evaluated and the comparison standard is a positive viral or bacterial sinus aspirate culture.33 The current clinical diagnostic criteria for a large proportion of the cases of acute community-acquired bacterial rhinosinusitis and for the use of antimicrobial treatment that is the most widely accepted today include a cold that is no better or worse after 10 to 14 days. Conversely, the current clinical diagnostic criteria for viral rhinosinusitis include a cold that is beginning to resolve after a few days and is better by a week to 10 days after onset. For purposes of research, the criteria standards for diagnosis of viral rhinosinusitis are a positive virus culture or detection of viral nucleic acid in cells of the sinus epithelium, indicating active viral replication.34
2. Bacterial infection
Summary Statements:
-
•
The most common cause of rhinosinusitis is a community-acquired viral infection that leads to a self-limited period of upper respiratory symptoms (nasal symptoms [ie, discharge], congestion, and cough). On occasion, there might be a secondary bacterial infection of the paranasal sinuses that requires specific antimicrobial therapy. These infections are characterized by the presence of one or more bacteria in high density (at least 1000 colony forming units per milliliter). Commonly isolated bacteria in patients with rhinosinusitis include S pneumonia, H influenzae, and M catarrhalis. Rhinosinusitis syndromes can be classified as acute, subacute, or chronic according to the duration of symptoms.
-
•
The role of bacterial infection in children and adults with CRS is controversial. Bacterial superantigens, biofilms, and osteitis might play a role in CRS and warrant further study.
Although the paranasal sinuses are believed to be sterile under normal circumstances, the upper respiratory tract, specifically the nose and nasopharynx, are heavily colonized with normal flora.32 Normal nasal flora in adults and children include coagulase-negative staphylococci (CNS), Corynebacterium species, and S aureus. In children the organisms frequently cultured from the nasal cavity include S pneumoniae, M catarrhalis, and H influenzae. Normal nasal-sinus flora in patients with CRS and the role of bacterial pathogens in CRS are poorly defined. In CRS the mucosal response to bacterial colonization or bacterial infection in an otherwise normal host is likely to be different than that in acute rhinosinusitis. Given this possibility, different criteria to define colonization and infection are probably needed but have not been established.
a. Microbiology of acute rhinosinusitis in children
The microbiology of paranasal sinus infection can be anticipated according to the age of the patient, clinical presentation, and immunocompetency of the host. Despite the substantial prevalence and clinical importance of rhinosinusitis in childhood, studies of the microbiology of acute and subacute rhinosinusitis in pediatric patients have been relatively limited. By using a study design similar to one described by investigators at the University of Virginia,35 an investigation of the microbiology of acute sinusitis in pediatric patients was conducted at the Children’s Hospital of Pittsburgh in 1979.36 Patients were eligible for this study if they were between 2 and 16 years of age and presented with one of 2 clinical pictures: onset with either persistent or severe respiratory symptoms.
Sinus radiographs were performed on eligible children with either of these 2 presentations. When a maxillary sinus aspirate (by using a transnasal approach) was performed on children presenting with either persistent or severe symptoms and significantly abnormal sinus radiographs, bacteria in high density were recovered from 70%.37 The bacterial isolates in their relative order of prevalence are shown in Table 1. S pneumoniae was most common, followed closely by M catarrhalis and H influenzae. Both H influenzae and M catarrhalis might be β-lactamase producing and thereby amoxicillin resistant. Approximately a third of S pneumoniae also exhibit intermediate or high resistance to penicillin. The H influenzae found in sinus aspirates, like those found in infected middle ear cavities, are almost always the nontypeable organisms, reflecting their frequent colonization of the nasopharynx, in contrast to H influenzae type b. Only a single anaerobic bacterial species, a peptostreptococcus, was isolated. No staphylococci were recovered. Mixed infection with heavy growth of 2 bacterial species was occasionally found. In 25% of patients with bilateral maxillary sinusitis, there were discordant bacterial culture results. In some patients one sinus aspirate was positive, whereas the other was negative. In the remaining patients different bacterial species were recovered from each.
Table 1.
Bacterial species cultured from 79 sinus aspirates in 50 children with acute rhinosinusitis
| Species | Single isolates | Multiple isolates | Total |
|---|---|---|---|
| Streptococcus pneumoniae | 14 | 8 | 22 |
| Moraxella catarrhalis | 13 | 2 | 15 |
| Haemophilus influenzae | 10 | 5 | 15 |
| Eikenella corrodens | 1 | 0 | 1 |
| Group A streptococcus | 1 | 0 | 1 |
| Group C streptococcus | 0 | 1 | 1 |
| α-Streptococcus | 1 | 1 | 2 |
| Peptostreptococcus | 0 | 1 | 1 |
| Moraxella species | 1 | 0 | 1 |
b. Microbiology of subacute rhinosinusitis in children
The signs and symptoms of children with subacute rhinosinusitis were described in 1989.38 These youngsters were evaluated in the context of several different comparative trials of antimicrobial therapy. All children had persistent respiratory symptoms (ie, nasal discharge, cough, or both lasting between 30 and 120 days). These children were generally in good health, with minimal constitutional complaints, except for their respiratory symptoms. Intermittent fever was a complaint in 25% of patients but was rarely documented at the time of presentation. Some of these children had previously received one or more courses of antimicrobial agents. In each case they either failed to respond to the antimicrobial agent or improved only slightly and experienced recurrence of symptoms after cessation of antibiotics. Table 2 shows the bacterial species cultured from 40 children. Again, the 3 predominant organisms were S pneumoniae, H influenzae, and M catarrhalis.38
Table 2.
Bacterial species cultured from 52 sinus aspirates in children with subacute rhinosinusitis
| Species | Single isolates | Multiple isolates | Total |
|---|---|---|---|
| Streptococcus pneumoniae | 9 | 3 | 12 |
| Haemophilus influenzae | 9 | 2 | 11 |
| Moraxella catarrhalis | 6 | 2 | 8 |
| Streptococcus pyogenes | 2 | 0 | 2 |
| Streptococcus viridans | 0 | 1 | 1 |
| Moraxella species | 0 | 1 | 1 |
c. Microbiology of CRS in children
There have been 9 studies of the microbiology of CRS in children between 1981 and 2001 (Table 3). 39, 40, 41, 42, 43, 44, 45, 46, 47 Three of these studies were prospective40, 42, 43 and 6 were retrospective. In all but one study, the maxillary sinus was sampled by means of transnasal aspiration. The most common criterion for evaluation was symptoms for at least 90 days. An attempt was made to sterilize the nose in only 5 of 9 investigations. Quantitation of bacteria was rarely performed. In part, this was a result of the frequent need for irrigation of the maxillary sinus to obtain sufficient material for culture. In 6 studies patients were receiving antibiotics up to the time that cultures were obtained. In 2 of the studies, normal nasal flora were the usual organisms recovered (ie, CNS and viridians streptococci). It is difficult to know what pathologic significance to ascribe to CNS. In the remaining studies the usual sinus pathogens were recovered in about 60% of cases (ie, H influenzae, S pneumoniae, and M catarrhalis, with H influenzae being most common). This was especially true when the criteria for entry included purulent secretions. In the remaining 30% to 40% of children, other organisms were recovered. Except for 2 studies, both performed by Brook and associates, anaerobes were rarely recovered from children with CRS.39, 40
Table 3.
Chronic rhinosinusitis in children
| Author | Criteria | No. | Age (y) | Sterilization | Quantitation | Off antibiotics | Microbiology |
|---|---|---|---|---|---|---|---|
| Brook, 198139 | ≥21 d | 40 | 6–16 | Yes | No | Yes | • 37/40 = +cx |
| • Anaerobes in all (GPC, GPR, GNR) | |||||||
| • Aerobes in 38% (GPC) | |||||||
| Otten and Grote, 198841 | ≥90 (purulent d/c) | 141 | 3–10 | NA | NA | NA | • 70% +cx: Usual acute flora |
| Tinkleman and Silk, 198946 | ≥30 d | 35 | 0.9–16 | Yes | Yes | No | • 63% +cx: Usual acute flora |
| Muntz and Lusk, 199145 | NA | 105 | 0.7–17 | NA | No (mucosa) | No | • Contaminants: majority |
| • Acute agents ∼ minority | |||||||
| • Bx of ethmoid cultured | |||||||
| Orobello et al, 1991*43 | ≥42 d (or recurrent) | 39 | 1.2–19 | Yes | Semi (irrigation) | No | • Contaminants: majority |
| • Usual acute flora: very light density | |||||||
| Otten, 1994*42 | ≥90 d (purulent d/c) | 79 | 2–12 | NA | No | Yes | • Usual pathogens |
| Brook et al, 2000*40 | ≥90 d (purulent) | 32 | 4–11 | Yes | No (irrigation) | Yes | • Usual pathogens and anaerobes |
| Slack et al, 200047 | ≥56 d | 119 | 0.8–14.5 | No | No (irrigation) | No | • Usual pathogens |
| • Occasional contaminants | |||||||
| Don et al, 200144 | ≥90 d | 70 | 0.9–15 | Yes | No (irrigation) | No | • Usual pathogens (60%) |
| • Contaminants |
Contaminants: CNS, α-strep, and coagulase-positive staphylococci.
+cx, Positive culture; GPC, gram-positive cocci; GPR, gram-positive rods; GNR, gram-negative rods; d/c, discharge; NA, not available; Bx, biopsy.
Prospective.
In patients with acute exacerbations of CRS characterized by persistent or intermittent episodes of purulent nasal discharge, the usual microorganisms associated with acute sinusitis are causative. However, in patients with chronic persistent rhinosinusitis (nasal congestion or nonspecific rhinorrhea or cough, alone or in combination), the role of bacterial agents is less clear. Most organisms have been recovered in low density, and frequently, these were recovered at a time when the patient was receiving antibiotics to which the organisms were susceptible. The lack of quantitation of organisms also complicates interpretation because the middle meatus in children is known to be colonized with the usual sinus pathogens. The persistence of symptoms despite multiple courses of appropriate antimicrobial agents in many children is counter to the notion that bacterial infection is a significant component of CRS. All of these observations support the hypothesis that bacterial infection has a minor role in many children with CRS.
d. Microbiology of acute community-acquired rhinosinusitis in adults
In adults bacteriologic information is derived mainly from cultures of mucus obtained by means of aspiration from the maxillary sinus, the most accessible of the paranasal sinuses. Although there is no certainty that cultures from the maxillary sinus can be extrapolated to all the other paranasal sinuses, the findings of sinus puncture studies performed in the United States and abroad have provided fairly similar results. In general, a sinus infection is caused by a single bacterial isolate in high density.35 In 25% of cases, 2 bacterial species, each in high density, were recovered.
The 2 most important causes of acute community-acquired rhinosinusitis in adults are S pneumoniae and nontypeable H influenzae (Table 4). These 2 species account for more than 75% of the bacterial isolates. One remarkable change observed by Gwaltney and colleagues between 1975 and 1991 was the increase in the prevalence of β-lactamase-producing H influenzae. In the first decade, β-lactamase-mediated resistance was rare; however, from 1986 through 1991, more than half of 29 strains of H influenzae were β-lactamase producing.32 There has been no increase in β-lactamase-positive H influenzae over the last 10 years, and this mechanism of resistance appears to have stabilized at less than 40% of isolates.48, 49 Next in frequency were streptococci other than pneumococci, such as streptococcal α and β strains, and anaerobic bacterial species. The role of anaerobes in acute community-acquired disease is variable. Although anaerobic bacteria have a more remarkable role in chronic sinus disease, they are not as established in acute sinus disease and account for only 2% to 6% of acute cases, some of which arise from primary dental pathology.
Table 4.
Community-acquired acute rhinosinusitis in adults
| Streptococcus pneumoniae | 41% |
| Haemophilus influenzae | 35% |
| Anaerobes | 7% |
| Streptococcus species | 7% |
| Moraxella catarrhalis | 4% |
| Staphylococcus aureus | 3% |
| Other | 4% |
S aureus and Streptococcus pyogenes are uncommon causes of acute rhinosinusitis in children and adults. The actual role of S aureus might occasionally be exaggerated when surrogate nasal cultures are substituted for sinus aspirates. Although uncommon, S aureus and S pyogenes may cause serious intracranial suppuration or, rarely, subperiosteal or orbital abscess as complications of acute rhinosinusitis.
e. Microbiology of nosocomial rhinosinusitis
Patients with nosocomial rhinosinusitis are usually those who require extended periods of intensive care (postoperative patients, burn victims, and patients with severe trauma) involving prolonged endotracheal or nasogastric intubation.50 Nasotracheal intubation provides a substantially higher risk for nosocomial sinusitis than orotracheal intubation.51 Nosocomial rhinosinusitis develops in approximately 25% of patients requiring nasotracheal intubation for more than 5 days.52 In contrast to community-acquired rhinosinusitis, samples taken from hospitalized patients usually contain pathogens that are gram-negative enterics (eg, Pseudomonas aeruginosa, Klebsiella pneumoniae, Enterobacter species, Proteus mirabilis, Serratia marcescens) and gram-positive cocci (occasionally streptococci and staphylococci).52, 53, 54, 55, 56 Whether these organisms actually cause the original sinus disease is unclear; however, they might represent postsurgical colonization of an environment with impaired mucociliary transport caused by the presence of a foreign body in the nasal cavity.
f. Microbiology of CRS in adults
In contrast to the agreement among investigators with regard to the microbiology of acute rhinosinusitis, there is disagreement with regard to the microbiology of CRS. The many factors that contribute to the difficulty in summarizing the literature include various methods used to sample the sinus cavity (ie, aspiration, irrigation, Calginate swab or biopsy), failure to sterilize the area through which the trocar or endoscope is passed, different sinuses or areas that are sampled (ie, ethmoid bulla or maxillary antrum or middle meatus), lack of assessment of the inflammatory response, lack of quantitation of bacteria, previous or current use of antibiotics, and variable patient selection (ie, age, duration, extent of disease, surgical or non surgical subjects, presence of nasal polyps, time of culture transport and method of culture).
Seven studies of patients with CRS performed since 1991 are shown in Table 5. 57, 58, 59, 60, 61, 62, 63 Three studies were prospective. The importance of a prospective investigation is that there is more assurance that patients are identified and cultures are processed in a standard fashion. CNS was the most common aerobic isolate in 5 of the 7 studies, often accompanied by S aureus and viridians streptococci. The absence of quantitation or performance of Gram stains in almost all studies prohibits an assessment of both the density of organisms and the accompaniment of an inflammatory response. Although CNS is traditionally discounted as a pathogen in both acute rhinosinusitis and CRS, its role as a pathogen in other body sites has been well documented and reviewed by Hsu et al59: neutropenic sepsis, infections of indwelling catheters, and in burn patients. Frequent bacterial recovery from swabs obtained from the middle meatus of healthy subjects suggests that these bacteria are commensals and likely contaminants.64 Nadel et al65 suggested that the difference might be of a quantitative nature. In the unusual situation in which a large number of white blood cells and organisms were present on Gram stains and there was heavy growth of CNS, the possibility of a true infection should be entertained.
Table 5.
CRS in adults 17 to 79 years of age
| Author | Year | No. of patients | Sterilization | Quantitation | Aspiration | Biopsy | Endoscopic | Antibiotic | WBC | Microorganism |
|---|---|---|---|---|---|---|---|---|---|---|
| Doyle and Woodham57 (6 wk) | 1991 | 59 | Yes | + (semi) | − | + | − | + | + | CNS; SA; GNR |
| Hoyt58 | 1992 | 197 | NA | NA | + | + | − | NA | − | CNS; SA; GNR |
| Hsu et al59 | 1998 | 34 | No | − | − | − | + | NA | NA | CNS; VS; GNR; SA |
| Biel et al60 (3 mo)* | 1998 | 174 | No | − | − | − | + | + | NA | CNS; SA; VS; anaerobes |
| Brook and Frazier61 (3 mo) | 2001 | 108 | Yes | − | + | − | − | NA | NA | SA; VS; PA; anaerobes; ++ |
| Jiang et al62 (3 mo)* | 2002 | 186 | Yes | − | − | − | + | NA | NA | CNS; GNR; SA |
| Finegold et al63* | 2002 | 150 | NA | − | + | − | NA | NA | GNR; ACS; anaerobes; ++; |
WBC, White blood cell; CNS, coagulase-negative staphylococcus; SA, Staphylococcus aureus; GNR, gram-negative enteric rods; NA, not available; VS, viridans streptococci; ACS, acute community-acquired pathogens; ++;, peptostreptococcus, prevotella, fusobacterium.
Prospective.
The surprising isolates in 5 of the 7 studies were gram-negative enteric rods, including P aeruginosa, K pneumoniae, P mirabilis, Enterobacter species, and Escherichia coli. Because these are rarely found in cultures of the middle meatus obtained from healthy individuals, their isolation from these symptomatic patients suggests 2 possibilities: (1) these organisms are causative, or (2) gram-negative organisms might colonize or secondarily infect because of underlying defects in host defense, such as impaired mucociliary clearance, nasal polyps in patients with CRS, or cystic fibrosis with the corresponding transport defect. Furthermore, the frequent use of antibiotics in these patients might promote the emergence of gram-negative bacterial colonization or infection.
An excellent illustration of the complexities of dealing with the microbiology of CRS is assessing the role of anaerobes in this condition. The isolation of anaerobes is critically dependent on culture techniques, and most studies have not used optimal techniques to isolate them. The frequency with which these organisms are recovered from patients who have been studied varies between zero and 100% and every number in between. In reviewing a series of studies, anaerobes were found primarily in the investigations of Finegold et al63 and Brook and Frazier.61 The reconciliation of these studies with all others and the significance of the recovery of these anaerobes is unclear.
In support of a role for anaerobic bacteria in chronic maxillary sinusitis, Finegold et al63 found recurrence of signs and symptoms twice as frequent when cultures showed anaerobic bacterial counts of greater than 103 cfu/mL. A role was further supported by the detection of antibodies (IgG) to 2 anaerobic organisms commonly recovered from sinus aspirates (Fusobacterium nucleatum and Prevotella intermedia). Antibody levels to these organisms decreased in the patients who responded to therapy and were cured but did not decrease in those in whom therapy failed.
Anaerobes have been identified in chronic sinusitis primarily when special techniques for their cultivation were used. The predominant isolates identified were pigmented Prevotella, Fusobacterium, and Peptostreptococcus species; the predominant aerobic bacteria were S aureus, M catarrhalis, and Haemophilus species. In several studies aerobic and anaerobic β-lactamase-producing bacteria were isolated from more than one third of patients studied.39, 66, 67, 68, 69 The β-lactamase-producing bacteria identified were S aureus, Haemophilus species, Prevotella species, and Fusobacterium species. Since 1974, a total of 1758 patients with CRS were evaluated in 18 studies using methods adequate for the recovery of anaerobic bacteria.63, 70, 71 Anaerobes were recovered in 12% to 93% of patients. The variability in recovery might result from differences in the methodologies used for transportation and cultivation, patient population, geography, and previous antimicrobial therapy.
Some investigators have argued that CRS represents a repeatedly damaged mucosal lining that has lost its normal state of sterility.43, 72, 73 These authors do not ascribe a major role for bacteria in the pathology of CRS unless there is an acute exacerbation characterized by purulent nasal discharge. Obviously, more work is needed to resolve these discrepant data. A suggested strategy would be to conduct a prospective investigation in which (1) patients are carefully identified and characterized, (2) cultures and Gram stains are obtained by using aseptic techniques with rigorous and standardized handling of specimens, (3) at least semiquantitative culture methods are used so that the density of bacteria can be assessed, and (4) the inflammatory infiltrate is characterized as neutrophilic or eosinophilic (which mark different pathogenic mechanisms).
g. New insights into the role of bacteria in CRS
1). Bacterial superantigens
A number of bacteria, viruses, and fungi can produce exotoxins (sometimes referred to as enterotoxins) that are able to activate T lymphocytes by cross-linking the MHC II molecule on antigen-presenting cells with the variable beta (Vβ) region of the T-cell receptor. These exotoxins are termed superantigens because they activate subpopulations representing up to 30% of T lymphocytes in contrast to classical antigens, which activate less than 0.01% of T lymphocytes. In addition, superantigens can also act as classical antigens, leading to concomitant generation of anti-superantigen antibodies. These includes antibodies of the IgE isotypes.5, 6
A potential role for superantigens from S aureus in the pathogenesis of nasal polyposis has been suggested and is discussed in the section “Factors involved in nasal polyposis.”
2). Biofilms
A biofilm is a communicating organization of microorganisms surrounded by a glycocalyx that frequently forms on an artificial or damaged biologic surface. Organisms living in a biofilm are relatively impervious to host defenses and antimicrobial agents. Bacterial biofilms have been elegantly demonstrated in an animal model of otitis media by using scanning electron microscopy and confocal microscopy.1 The possibility that a bacterial biofilm could be contributing to CRS has not been formally studied. This possibility is theoretically attractive and might help to explain the clinical situation in which patients frequently have negative cultures, improve symptomatically while receiving antibiotics, and relapse when antibiotics are withdrawn. In a biofilm, planktonic bacteria leave the biofilm, cause symptoms, and are susceptible to host defenses and antibiotics. However, the biofilm itself is relatively impervious to antimicrobial agents and is never eradicated. Mechanical debridement appears to be the only mechanism that resolves a biofilm. In some refractory patients this might explain improvement with surgery and irrigation.
3). Osteitis: the role of bone
To date, bacterial organisms have not been identified in the bone in either human subjects or animal models of CRS. However, in chronic osteomyelitis it is known that organisms can be scarce and difficult to identify. Whether bacteria induce bony remodeling because of associated inflammation or whether they truly infect bone is unknown.2 Areas of increased bone density and bony thickening are frequently seen on CT scans in areas of chronic inflammation and might be a marker of the chronic inflammatory process. However, during the initial phases of severe CRS, the effect frequently appears as rarefaction of the bony ethmoid partitions.
In one study bone specimens from 34 patients with CRS and 9 healthy control subjects were labeled with tetracycline by means of oral ingestion and then 2 weeks later with demeclocycline.3 The bone then underwent biopsy 3 to 7 days after completion of the second antibiotic course. In the patients with CRS, there was a significantly greater remodeling activity than in the control group, as demonstrated by significant separation of the 2 lines of fluorescence resulting from the tetracyclines. The bone was also evaluated for bone turnover semiquantitatively and qualitatively by applying techniques of histomorphometry. Indices evaluated included bone volume, osteoid surface, eroded surface, fibrosis, osteoblastic surface, and tetracycline labeling. Statistically significant differences were again obtained, and the bone turnover seen in the CRS group was similar to that seen in patients with osteomyelitis and trauma.
In rabbit studies of experimentally induced Pseudomonas maxillary sinusitis, Perloff et al4 demonstrated that not only does the bone become involved adjacent to the involved maxillary sinus but also that the inflammation typically spreads through the Haversian canals and might result in bone changes consistent with some degree of chronic osteomyelitis at a distance from the primary infection. A study by Khalid et al2 using both Pseudomonas species and S aureus in a rabbit study demonstrated similar results. Bone involvement was noted in 92% of the animals on the ipsilateral side to the infection, and in some specimens clear osteonecrosis was identified. Inflammatory bone changes were also noted on the contralateral side in 52% of the animals. The inflammation caused well-defined changes in the bone in rabbits, both adjacent to the infection and at a distance from the primary site of inflammation, which were compatible with a histologic diagnosis of chronic osteomyelitis. The inflammatory spread within the bone appears to occur as a result of well-defined changes in the Haversian canals, leading first to widening of the canals and increased vascularity, then to an inflammatory cellular collection within the canals, and later to fibrosis in the involved area. It is certainly possible that these changes, if further confirmed in patients, might, at least in part, explain why CRS is relatively resistant to medical therapy.
3. Fungal colonization-sensitization
Summary Statements:
-
•
AFRS is a distinct clinical subset of CRS in which patients will have positive evidence of fungal allergy to the fungus colonizing their allergic mucin in the majority of cases.
-
•
Those patients with AFRS typically demonstrate 5 characteristics: gross production of eosinophilic mucin containing noninvasive fungal hyphae, nasal polyposis, characteristic radiographic findings, immunocompetence, and allergy to cultured fungi.
-
•
The presentation of AFRS might be dramatic, giving rise to acute visual loss, gross facial dysmorphia, or complete nasal obstruction, but more often, the presentation is subtle.
-
•
Recent studies suggest that fungi can play an alternate role in the development of CRS, whereby patients become sensitized by colonizing fungi through a non-IgE-mediated mechanism. This sensitization is hypothesized to lead to local eosinophilic chemotaxis, inflammation, and tissue injury. This concept of eosinophilic fungal rhinosinusitis encompasses most patients with CRS.
The spectrum of fungal involvement in CRS runs from benign colonization to potentially life-threatening invasive disease. Fungal colonization of the nose and paranasal sinuses appears to be a common finding in both normal and diseased states, although there is considerable debate over the prevalence of colonization.7, 8, 9 Fungal colonization is presumed to be due to the ubiquitous nature of fungal spores in ambient air and the propensity of these spores to germinate in nasal and sinus mucus. In rare circumstances this leads to macroscopic fungal proliferation in the form of fungus balls (formerly referred to as mycetomas) or saprophytic growth of fungus. In these cases fungal mycelia accumulate and occupy available spaces within the nose and paranasal sinuses in the absence of significant mucosal inflammation. Treatment is simply directed toward extirpation of the offending fungal growth.74 Occurring more commonly than in the case of fungus balls, microscopic quantities of fungal hyphae in sinus mucus elicit an intense local immune response. In AFRS this gives rise to the pathognomonic feature of the disease, namely the presence of allergic mucin (described below). It is important to realize that AFRS and fungal balls represent noninvasive forms of fungal rhinosinusitis, which must be distinguished from invasive forms.
Invasive fungal rhinosinusitis is often an acute fulminant disease that carries a high mortality rate. Acute fulminant invasive fungal rhinosinusitis is usually caused by fungi such as Absidia species, Aspergillus species, Basidiobolus species, Mucor species, and Rhizopus species.75 However, in patients whose immunologic deficiency is mild or unapparent, invasive fungal rhinosinusitis might run a more indolent chronic course. The diagnosis is made on the basis of histologic evidence of invasive fungi in the nose and paranasal sinuses that is present for more than 12 weeks. Management requires repeated surgical debridements, correction of any immunologic deficiency, and long-term systemic and topical antifungal therapy. Despite close medical attention, all invasive cases of fungal rhinosinusitis can progress to a fatal outcome or become a recurrent problem. Chronic invasive fungal rhinosinusitis has been divided into granulomatous and nongranulomatous subtypes on the basis of histopathology; however, the clinical distinction in terms of prognosis and management between these 2 subtypes is not clear. Chronic invasive fungal rhinosinusitis has been specifically associated with Aspergillus species, Mucor species, Alternaria species, Curvularia species, Bipolaris species, Candida species, Sporothrix schenckii, and Pseudallescheria boydii.74, 76
Traditional classification of fungal rhinosinusitis emphasizes differentiating these diseases on the basis of the presence or absence of tissue invasion. Little emphasis has been placed on differentiation of fungal inflammation induced by colonization versus infection. There is little question that the invasive forms of fungal rhinosinusitis constitute infection, but the issue of whether the noninvasive forms represent infection versus inflammation in response to colonizing fungi offers more confusion. At present, current data suggest that the mucosal inflammatory process with noninvasive fungal colonization represents a noninfectious process.8, 77
a. Allergy to fungi
Unlike invasive forms of fungal rhinosinusitis, it is the potential for colonizing fungi to elicit allergic mucosal inflammation in the absence of invasion that characterizes AFRS. The ability of fungi or, more specifically, protein components of fungi to elicit IgE-mediated allergic mucosal inflammation is well documented.78 Moreover, when those sensitized individuals are placed in environments of high fungal exposure, symptoms of airway hyperresponsiveness increase significantly over those of nonsensitized individuals in similar situations.79 Virtually all studies of the pathophysiology of AFRS have been based on the premise that IgE-mediated allergy to one or more fungi underlie the disease, with the predominant finding of eosinophil-predominant tissue infiltration akin to late-phase allergic inflammation. In this way AFRS has features quite similar to those of allergic bronchopulmonary aspergillosis.80
b. Classic AFRS
Over the course of the past 25 years, AFRS has emerged as a clinically distinct subset of CRS. AFRS possesses characteristic clinical, radiographic, pathologic, and immunologic features.
1). History and physical
Occasionally, the presentation of AFRS might be dramatic, giving rise to acute visual loss, gross facial dysmorphia (described below), or complete nasal obstruction,80, 81, 82 but more often, the presentation of AFRS is subtle. Patients typically complain of gradual nasal airway obstruction and production of semisolid nasal crusts that, on inquiry, match the gross description of allergic fungal mucin. The development of nasal airway obstruction might have been so gradual that the patient is unaware of its presence. Pain is uncommon among patients with AFRS and suggests the concomitant presence of a bacterial rhinosinusitis.83, 84 In contrast to the often subtle symptoms of AFRS, physical findings are often more remarkable. The range of physical findings on examination is typically broad, ranging from nasal airway obstruction resulting from intranasal inflammation and polyposis to gross facial disfigurement and orbital or ocular abnormalities.81
2). Radiologic findings
The slow accumulation of allergic fungal mucin provides unique and rather predictable characteristics to the disease. Allergic fungal mucin is sequestered within involved paranasal sinus cavities, and its accumulation eventually leads to the increasingly well-recognized radiographic findings characteristic of AFRS (Table 6). A recent study of sinus CT scans from 45 patients with AFRS objectively supports several previous clinical observations.85 AFRS, although bilateral in 51% of the cases reviewed, caused asymmetric involvement of the paranasal sinuses in 78% of the cases. Bone erosion and extension of disease into adjacent anatomic areas was encountered in 20% of the patients and was more likely to occur in the presence of bilateral advanced disease. Expansion, remodeling, or thinning of involved sinus walls was common (and was thought to be due to the expansile nature of the accumulating mucin). These finding were corroborated by Nussenbaum, et al,86 who reviewed CT scans of 142 patients treated for AFRS at a single institution and also found demineralization of bone in approximately 20% of the subjects.
Table 6.
Characteristic radiographic findings for AFRS
| CT findings | MRI findings* | |
|---|---|---|
| Diagnosis requires | 1. At least one opacified paranasal sinus | |
| Other strongly supportive radiographic findings |
|
General
|
T1:
|
||
T2:
|
Optional but should not be used in the absence of CT.
Heterogeneous areas of signal intensity within paranasal sinuses filled with allergic fungal mucin are frequently identified on CT scans (Fig 5). Although these findings are not specific for AFRS, they remain relatively characteristic of the disease and might provide preoperative information supportive of a diagnosis of AFRS.85 Current evidence points to the presence of accumulations of heavy metals (eg, iron and manganese) and calcium salt precipitation within inspissated allergic fungal mucin as the most likely cause of these radiographic findings.85, 87 Desiccation of sinus contents might also contribute to the hyperdense areas seen on CT scans.
Fig 5.
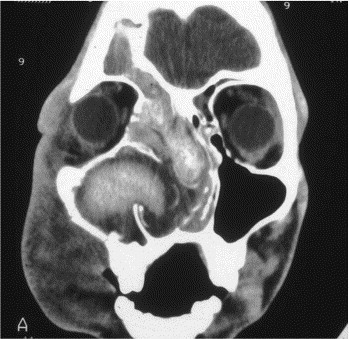
Soft tissue algorithm CT scan showing findings typical of AFRS. Note the heterogenous appearance within involved paranasal sinuses.
MRI can also provide information useful in the preoperative identification of allergic fungal mucin. This effect is more pronounced on T2-weighted images as a result of prolonged magnetic field relaxation times. The high protein and low water concentration of allergic fungal mucin, coupled with the high water content within surrounding edematous paranasal sinus mucosa, gives rise to rather specific magnetic resonance characteristics. The combined CT and MRI findings provide a radiographic appearance that is highly suggestive of AFRS.88, 89
3). Immunologic testing
A study by Manning and Holman84 prospectively compared 8 patients with culture-positive Bipolaris species AFRS with 10 control subjects with CRS. Both groups were evaluated with (1) RAST and ELISA inhibition to Bipolaris species-specific IgE and IgG antibodies and (2) skin testing with Bipolaris species antigen. All 8 patients with AFRS had positive skin test reactions to Bipolaris species antigen, as well as positive RAST and ELISA inhibition results to Bipolaris species-specific IgE and IgG. In comparison, 8 of the 10 control subjects had negative results on both skin and serologic testing.
Several other studies have also demonstrated a positive correlation between skin test and in vitro (RAST) responses for both to fungal and nonfungal antigens in patients with AFRS.84, 89 Moreover, patients with AFRS appear to demonstrate a broad sensitivity to a number of fungal and nonfungal antigens.90 On the basis of these and other studies, it is generally agreed that patients with AFRS will have positive evidence of fungal allergy to the fungus colonizing their allergic mucin in the majority of cases. In those cases not showing such a correlation, it might be that technical problems in fungal culture or a lack of skin testing reagents might explain the discrepancy. Sensitivity to numerous fungi has been indicated by means of both in vitro (RAST) and in vivo (skin testing) methods, although generally only a single fungus is isolated by means of culture of corresponding allergic fungal mucin. This has been previously thought to be the result of either a common fungal epitope or a genetic predisposition toward fungal allergy in AFRS. Recent work by Chrzanowski et al91 identified the presence of an 18-kd protein in allergic mucin obtained from patients with AFRS, which might represent a fungal panantigen.
Total IgE values are also generally increased in patients with AFRS, often to more than 1000 IU/mL, and have been proposed as a clinically useful indicator of AFRS disease activity.90, 92 In some cases fungus-specific IgG precipitins have also been detected analogous to those described in allergic bronchopulmonary aspergillosis.
4). Histologic characteristics of allergic mucin
It is the production of allergic mucin that is considered pathognomonic of AFRS. Grossly, allergic mucin is thick, tenacious, and highly viscous in consistency; its color can vary from light tan to brown or dark green.93, 94 It is the mucin, rather than paranasal sinus mucosa, that provides the histologic information necessary to make the diagnosis of AFRS.95, 96 Examination of mucosa and polyps obtained from involved paranasal sinuses reveal findings of chronic inflammation, usually with an abundance of eosinophils. Pathologic examination of these tissues should be done to establish that fungal invasion is not present.96
The histologic appearance of allergic mucin reveals the characteristic findings of branching noninvasive fungal hyphae within sheets of eosinophils and Charcot-Leyden crystals.97, 98, 99 Hematoxylin and eosin staining accentuates the mucin and cellular components of allergic fungal mucin but fails to stain the fungal hyphae. Fungi are recognized for a unique ability to absorb silver. This is the basis for various silver stains, such as Grocott’s or Gomori’s methamine silver stain, which stain fungi black or dark brown. Unfortunately, silver-based stains have high specificity but low sensitivity. A more sensitive method for identification of fungi has been recently developed that makes use of a fluorescein-labeled chitin-specific binding protein. In a study that compared mucus retrieved from 54 patients with CRS, use of this technique allowed for identification of fungal elements in 100% of specimens, whereas fungi were only detected in 41 (76%) of the 54 specimens by using a Grocott stain.100 Using this technique, Taylor et al100 identified fungal hyphae in the vast majority of sinus mucus samples obtained from patients with CRS, even though most of these patients lacked the other classic features of AFRS. This has become one of the major tenets of the hypothesis associated with the concept of eosinophilic fungal rhinosinusitis (see below).
5). Culture of fungi
Fungal cultures of allergic fungal mucin might provide supportive evidence for the diagnosis and subsequent treatment of AFRS but must be interpreted with caution. It is important to realize that the diagnosis of AFRS is neither established nor eliminated on the basis of the results of these cultures. The variable yield of fungal cultures (64% to 100%) renders AFRS in the presence of a negative fungal culture quite possible.84 Conversely, a positive fungal culture fails to confirm the diagnosis of AFRS because it might merely represent the presence of saprophytic fungal growth. For this reason, the histologic appearance of allergic mucin remains the most reliable indicator of AFRS.
6). Diagnostic criteria
The constellation of clinical, radiographic, and immunologic features help to define the disease and have been the focus of a number of diagnostic criteria.101, 102 Those patients with AFRS uniformly demonstrated 5 characteristics: gross production of eosinophilic mucin containing noninvasive fungal hyphae, nasal polyposis, characteristic radiographic findings, immunocompetence, and allergy to fungi.102 Taking into account the current literature, the diagnosis of AFRS is minimally dependent on identifying the combination of histologic evidence of fungal hyphae within eosinophilic mucin and host allergy to that fungus. The diagnosis might be suspected on the basis of physical examination or radiographic findings; however, in most cases the diagnosis is not established until sinus tissue and mucus obtained during sinus surgery have been reviewed. At the time of surgery, the patient might have a persistently opacified sinus cavity, and eosinophilic mucus plus polypoid tissue might be found to account for this opacification. Patients nearly always have type I allergic sensitivity to fungal antigens. Because of these distinctive features plus the distinctive complications of this disease, including bony erosion and facial dysmorphia, AFRS represents a distinct subset from the much broader group of patients with CRS.
c. Non-IgE-mediated eosinophilic fungal inflammation (eosinophilic fungal rhinosinusitis)
In 1999, a hypothesis of CRS was proposed by Ponikau et al103 that suggested colonizing fungi in sinus mucus play a much broader role in the pathogenesis of CRS. By using an ultrasensitive culture technique, 93% of 101 consecutive patients with CRS demonstrated positive fungal cultures from nasal lavage. Examination of surgically obtained specimens from these patients also revealed eosinophils and fungal hyphae in the sinus mucus of nearly all patients. It was also observed that 100% of a group of healthy control subjects had positive fungal cultures from nasal lavage. Conventional IgE-mediated allergy to fungi was not consistently observed in the patients with CRS. It was proposed that virtually all cases of CRS were associated with sensitization to colonizing fungi. It was further suggested that the term allergic fungal rhinosinusitis be replaced with eosinophilic fungal rhinosinusitis.103
An intriguing issue raised by this study is the possibility that certain fungi could elicit eosinophilic inflammation in the absence of conventional IgE in subjects with CRS. This concept was supported by in vitro studies in which peripheral blood mononuclear cells from patients with CRS were found to produce large quantities of IL-5 and IL-13 after exposure to certain fungal antigens.104 In contrast, peripheral blood mononuclear cells obtained from healthy control subjects failed to produce the same response. Thus patients with CRS show evidence of sensitization and immune activation in response to colonizing fungi in the nasal and sinus mucus, and this process might be responsible for the production of cytokines that recruit and activate eosinophils in CRS. For further discussion of this issue, see the section “Controversy 3: Should CRS be classified on the basis of the proposed definition of eosinophilic fungal rhinosinusitis.”
B. Allergic and immunologic factors of rhinosinusitis
Summary Statements:
-
•
Perennial allergic rhinitis appears to be a predisposing factor for acute bacterial rhinosinusitis.
-
•
CRS without nasal polyps is characterized by a predominantly neutrophilic inflammation with a lesser contribution of eosinophils; in contrast, nasal polyps are characterized by eosinophilic inflammation, and IL-5 and eotaxin have been shown to play a role in this process.
-
•
Neither total IgE concentrations nor ECP, IL-4, or IL-5 concentrations in nasal polyps are different in atopic versus nonatopic subjects, indicating a discordance between systemic allergic phenotype and local inflammatory mechanisms leading to eosinophilic inflammation in NP.
-
•
A role has been proposed for IgE specific staphylococcal-derived superantigens in the pathogenesis of CRS associated with nasal polyps.
1. Allergic inflammation
The contribution of allergic responses in CRS has long been controversial. Nonetheless, there is now evidence that at least perennial allergic rhinitis could be a facilitating factor for acute bacterial rhinosinusitis, as demonstrated in a prospective sinus CT scan study.105 Although seasonal allergic rhinitis has been shown to be a risk factor for orbital complications of acute rhinosinusitis in children,106 similar evidence is not available for acute rhinosinusitis in adults. In a mouse model allergic inflammation induced by means of sensitization to ovalbumin has also been demonstrated to augment the inflammatory response to acute bacterial infection.107 Furthermore, allergic reactivity is a poor prognostic factor after surgery in some, but not all, studies.108
Most studies of allergic factors in CRS involved studies of NPs. The results of these studies are unclear. Slightly less than half of the patients with CRS and NPs have associated allergies.109 Furthermore, seasonal allergen exposure does not increase symptoms or mediators in the nasal lavage of patients with NPs and ragweed sensitivity.110 However, there is a substantial discordance between skin prick tests and evidence of local IgE antibody levels in polyp homogenates.13 As early as 1982, Drake-Lee and McLaughlin111 reported their finding of IgE antibody in NPs and no difference in local IgE levels in allergic and nonallergic subjects. Recent studies have found IgE in NPs specific for enterotoxins from S aureus, which act as superantigens resulting in a multiclonal stimulation of T and B lymphocytes.13 Another study reported skewing of the Vβ phenotype of T lymphocytes in NPs toward those responsive to staphylococcal exotoxins detected in the tissues.112 It has been repeatedly demonstrated, at least qualitatively speaking, that neither IgE levels nor ECP, IL-4, or IL-5 concentrations in NPs differentiate atopic versus nonatopic subjects, indicating that the phenotype of systemic allergy defined as skin prick test positivity does not correlate with the local features of allergic inflammation in NPs.113 The full explanation for these findings remains elusive, but the implication is that local inflammatory mechanisms might be important in NP pathogenesis. Furthermore, a positive skin test response in a patient with CRS should not be interpreted as an allergic case of CRS. One exception to this rule is AFRS, in which a systemic allergic response to fungi colonizing the sinus mucus is demonstrated in the vast majority of cases (see section “Controversy 3: Should CRS be classified on the basis of the proposed definition of eosinophilic fungal rhinosinusitis”).
A significant body of work has been done to characterize the T-cell cytokine profile in NPs (reviewed below). Some of these studies subclassified NPs into allergic and nonallergic subtypes on the basis of results of allergy skin testing and the profile of T-cell cytokines found in NP tissue, but it is not clear that this distinction is important in the underlying disease pathogenesis because both allergic and nonallergic patients with NPs manifest prominent eosinophilic tissue infiltration (see section “Factors involved in nasal polyposis”).
Studies of patients with CRS without polyposis are limited but have also shown differences between subjects with and without allergic sensitivities.114, 115 The principal differences involve a greater degree of neutrophilic inflammation and a lesser degree of eosinophilic inflammation in nonallergic patients114; however, eosinophil infiltration is seen to some degree in both allergic and nonallergic patients analogous to the findings in NPs. Also similar to results in patients with NPs, the T-cell cytokine profiles of allergic and nonallergic subjects with CRS show differences precisely as described in subjects with NPs, namely that the full cadre of TH2 cytokines is found in allergic subjects, and a mixed TH1/TH2 profile is found in nonallergic subjects.115 However, once again, the degree of tissue infiltration with eosinophils is not substantially different in allergic and nonallergic subjects, raising the question of the relevance of systemic allergic phenotype to the underlying pathogenesis.
2. Other inflammatory features
In CRS (without NPs) a range of mediators and cytokines has been shown to be increased in comparison with levels seen in control tissue, mostly inferior turbinates. These include IL-1, IL-6, IL-8, tumor necrosis factor (TNF) α, IL-3, granulocyte-macrophage colony-stimulating factor (GM-CSF), ICAM-1, myeloperoxidase, and ECP.114, 116, 117, 118, 119 CRS is characterized by a predominantly neutrophilic inflammation, with a lesser contribution of eosinophils. Interestingly, vascular cell adhesion molecule 1 (VCAM-1), an adhesion molecule involved in selective eosinophil recruitment, and IL-5, a key cytokine for eosinophil survival and activity, have been shown not to be increased.117, 118 This cytokine and mediator profile resembles very much the profile found in acute viral rhinosinusitis, with the exception of a small, although significant, increase of ECP. These findings therefore suggest that the underlying pathologic process might involve unresolved inflammation after infection or a response to chronic infection. This profile is distinct from the pattern in NP.13, 118, 120
By comparison, many more studies have been done to describe the inflammation in NPs (see section “Factors involved in nasal polyposis”). A hallmark inflammatory feature is the presence of abundant eosinophils. A variety of mechanisms have been proposed to account for the presence of eosinophils in NPs, as discussed below.
As previously mentioned, a characteristic feature of NPs is the local production of IgE, with a more than 10-fold increase of IgE-producing plasma cells compared with that seen in control subjects. Analysis of specific IgE revealed a multiclonal IgE response in NP tissue and IgE antibodies to S aureus enterotoxins (SAEs) in about 60% of the patients and in about 80% of subjects with NPs and asthma.13 Total and specific IgE levels in polyp homogenates are only partially reflected in the serum of these patients.
The classical SAEs, especially toxic shock syndrome toxin 1 and staphylococcus protein A, are excellent candidates to induce multiclonal IgE synthesis by increasing the release of IL-4, as well as the expression of CD40 ligand on T cells and B7.2 on B cells.5, 6 Staphylococcus protein A furthermore interacts with the VH3 family of immunoglobulin heavy chain variable gene products and thus preferentiates plasma cells presenting such immunoglobulins on their surface, which leads to a VH3 bias.121 In fact, follicle-like aggregates can be found in nasal polyps expressing CD20+ B cells, CD3+ T cells, and IgE plasma cells but largely lacking CD1a+ dendritic antigen-presenting cells, supporting the concept of superantigen stimulation. SAEs furthermore stimulate T cells by binding to the variable β chain of the T-cell receptor, which induce cytokine production of IL-4 and IL-5, directly activate eosinophils and prolong their survival, and also might directly activate epithelial cells to release chemokines.122 SAEs furthermore activate antigen-presenting cells to increase antigen uptake. In fact, when comparing SAE-IgE+ NPs with SAE-IgE− NPs, the number of IgE+ cells and eosinophils is significantly increased. The more severe inflammation is also reflected by significantly increased levels of IL-5, ECP, and total IgE in the NPs. In conclusion, SAEs are able to induce a more severe eosinophilic inflammation, as well as the synthesis of a multiclonal IgE response with high total IgE concentrations in the tissue, which would suggest that SAEs are at least modifiers of disease in NP.122 Interestingly, similar findings have recently been reported in asthma, which is known to occur concurrently with nasal polyposiss.123 IgE antibody formation to SAE is rarely seen in CRS in the absence of NPs.
C. Noninfectious and nonimmunologic factors of rhinosinusitis
Summary Statements:
-
•
Overactivity or underactivity of autonomic nerve pathways, abnormalities in leukotriene production or responsiveness, nociceptive dysfunction, or local irritation caused by gastroesophageal reflux are demonstrable in select subsets of patients with rhinosinusitis and likely predispose to the pathogenesis of CRS.
-
•
Defects in mucociliary clearance and antibody deficiency syndromes might predispose to rhinosinusitis.
-
•
Aspirin-associated respiratory disease predisposes to rhinosinusitis.
Rhinosinusitis can be classified as resulting from either inflammatory or noninflammatory causes (Table 7). 124, 125 Within these classifications, each cause can be further divided. The following sections will review the various causal factors for rhinosinusitis, with emphasis on noninfectious and nonimmunologic causes.
Table 7.
Mechanistic classification of the differential diagnosis for rhinosinusitis
| Inflammatory leukocytes |
No inflammatory leukocytes |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Eosinophilia | Neutrophilia | Mixed or poorly defined | Epithelial dysplasia | Neural involvement |
Hormonal | Anatomic and structural changes | |||
| Trigeminal nociceptive dysfunction of “visceral sensations” | Parasympathetic dysfunction | Sympathetic: Vascoconstrictor dysfunction | Olfactory dysfunction | ||||||
|
|
|
|
|
|
|
|
|
|
NSAID, Nonsteroidal anti-inflammatory drug; VRL, vanilloid receptor-like; VR1, vanilloid receptor subtype 1, recently renamed transient receptor potential vanilloid receptor 1 (TRPV1); ACE, angiotensin-converting enzyme.
1. Presence of inflammatory changes
a. Eosinophilic rhinosinusitis
Conditions associated with eosinophilic tissue infiltration are summarized in controversy 2.
b. Neutrophilic rhinitis
The neutrophilic group includes acute bacterial sinusitis, cystic fibrosis, and chronic bacterial infections complicating immunodeficiencies and foreign bodies. Nasal polyps with neutrophilia in children are highly suggestive of cystic fibrosis.
c. Mixed inflammatory patterns
Complex mixed or as yet poorly defined nasal mucosal cell populations are present in viral infection, autoimmune diseases, and idiopathic diseases. The leukocytes attracted to the nasal and sinus mucosa vary with the chronology and specific virus causing acute common cold syndromes (rhinovirus, parainfluenza virus, adenovirus, coronavirus, and others), influenza (might cause epithelial destruction), potentially sterile (nonbacterial) rhinosinusitis, or adenoiditis. Because the time course of leukocyte invasion is different for specific leukocyte populations, it is necessary to synchronize the day after initiation of infection to follow this time course. This can be achieved only in longitudinal studies of groups inoculated with virus and not by cross-sectional studies in which the date of onset of the infection is not documented precisely. Autoimmune, vasculitic, and other complex syndromes are included in this group by virtue of these poorly defined, mixed cellular populations. Lymphocytic infiltrates of T cells with CD4 or CD8 derivations might be present but might also change with the stage or duration of illness. An example would be sarcoidosis, with its predominance of TH1 lymphocytes and macrophages in noncaseating granuloma.
d. Epithelial dysplasia
Epithelial changes occur as CRS progresses from mild to severe. The epithelium shows an inexorable trend from normal ciliated to goblet cells predominant, microvillous cell predominant, and ultimately squamous epithelium with breaches in the basement membrane and surface erythrocytes, indicating bleeding. This epithelial progression roughly parallels CT scan severity.126 Exposures to organic toxins, fine particulate material, oxidizing minerals (eg, iron), and other materials can lead to epithelial differentiation from ciliated to squamous metaplasia without leukocytosis. The olfactory mucosa might be particularly susceptible. An example of this type of response is the exposure to complex particulates. Some toxins cause an early and transient neutrophilic inflammation that clears rapidly once the exposure has ended. These types of changes have been examined in rodent and screening toxicologic studies, but there are much fewer data in human exposure situations.127
2. Noninflammatory changes
The second large group is the set of noninflammatory syndromes that do not show any changes in normal leukocyte infiltration.
a. Trigeminal dysfunction
Many of these disorders involve afferent trigeminal and efferent autonomic nerves. They are often dismissed as a functional disorder of nasal complaints without physical findings and lumped together as vasomotor rhinitis (idiopathic rhinitis). This is a misnomer that does no justice to the patient or his or her complaints. Perennial noninfectious, nonallergic rhinitis is an alternative term. There are no clear vascular, motor, or inflammatory cellular patterns. Inquiries about key historical issues can classify and direct therapy to these symptomatic and frustrated patients. These individuals appear to have increased afferent trigeminal nerve sensitivity to inhaled irritants, disordered axon response mechanisms, and potentially altered dorsal horn processing of nociceptive input that contribute to increased perception of these mucosal or visceral stimuli and hence greater complaints of symptoms. An alternative term, irritant rhinitis, describes the syndrome more accurately.128 Patients with irritant rhinitis typically complain of nasal congestion and rhinorrhea in response to weather, temperature and humidity changes, and irritants, such as tobacco smoke,129 gasoline fumes, perfumes and cleaning solutions, beer, and wine. An important function of type C nociceptive neurons is their role in immediate neurogenic responses to noxious stimuli.130 New information about the nature of nociceptive sensors, such as the capsaicin-sensitive ion channel receptor (VR1, recently renamed transient receptor potential vanilloid subfamily protein or TRPV1) offers a new understanding of the molecular mechanisms underlying responses to irritant gases, fine particulate material, cigarette smoke components, osmolarity, and temperature changes.131
b. Cholinergic rhinitis
The afferent stimuli can recruit overactive parasympathetic cholinergic reflexes that mediate cholinergic (muscarinic receptor M3-mediated) glandular secretion. This mucus hypersecretion might confound observations of mucopurulent discharge and therefore suggest that rhinosinusitis is present. The effectiveness of anticholinergic agents suggests that parasympathetic cholinergic outflow is the major factor contributing to chronic or long-lasting irritant-induced (eg, cold dry air in skiers) nasal discharge in nonallergic rhinitis. Acute stimulation of nociceptive nerves (eg, by eating capsaicin-laden foods that stimulate vanilloid receptor 1 bearing type C trigeminal neurons) also recruits overwhelming lacrimal, nasal, and salivary glandular discharge. When excessive, this is termed cholinergic rhinitis. Again, anticholinergic agents are effective at blocking this CNS trigeminal-facial (Vidian parasympathetic) nerve-mediated reflex.
c. Sympathetic dysfunction
Impaired sympathetic outflow can lead to default dilatation of venous sinusoids. This thickens the mucosa and reduces the cross-sectional area for airflow and therefore leads to obstructed nasal airflow. Horner syndrome is an example.
d. Other
Hormonal, structural, and neoplastic disorders also lead to symptoms, including referred pain suggestive of rhinosinusitis. The hormones of pregnancy are notorious for causing nasal obstruction symptoms that can be very problematic to patients. This congestion clears with delivery of the placenta. Hypothyroidism leads to sympathetic dysfunction with ineffective noradrenergic effects and the absence of venous sinusoid vasoconstriction that results in default blood pooling and thickening of the nasal mucosa. Drugs that block this vasoconstrictor function (central and peripheral acting antihypertensive agents) will also lead to mucosal thickening and nasal obstruction to airflow.
3. Nociceptive dysfunction in rhinosinusitis
The importance of nociceptive neural mechanisms and hyperalgesia in rhinosinusitis is demonstrated by studying the tenderness of the sinus regions.132 Although pain is the patient’s subjective complaint, tenderness to palpation is a function of spinal cord pain processing (hyperalgesia). Subjects with acute rhinosinusitis and CRS have significantly lower pain thresholds over their sinus regions compared with healthy control subjects (Fig 6). These studies validate the sign of sinus tenderness in the diagnosis of rhinosinusitis. Differences in sensitivity to usually nonpainful stimuli (allodynia) have not been studied in rhinosinusitis.
Fig 6.
Sinus pressure thresholds (mean ± 95% CI) decreased from the healthy control (non-chronic fatigue syndrome/no rhinosinusitis) to chronic fatigue syndrome/CRS group. Significant differences were found from non-chronic fatigue syndrome/no rhinosinusitis (*P < 0.05, **P < 0.001, ***P < 0.00001, and ****P < 0.0000001), chronic fatigue syndrome/CRS (#P < 0.01, ##P < 0.0001), and chronic fatigue syndrome/no rhinosinusitis (@.07 < P < 0.05, @@P < 0.02). Sinus thresholds were significantly reduced in both subjects with chronic fatigue syndrome and subjects without chronic fatigue syndrome with acute rhinosinusitis and CRS compared with the non-chronic fatigue syndrome/no rhinosinusitis control group. CFS, Chronic fatigue syndrome; sinusitis, rhinosinusitis.
Patients with allergic rhinitis had intermediate tenderness thresholds that were not significantly different from those of control subjects. Nerve growth factor133 or potentially other neurotrophins released by mast cells, lymphocytes, or other activated cells in allergic rhinitis might induce these hyperalgesic sensory changes. However, it has been a challenge to demonstrate these alterations in allergic rhinitis because severely symptomatic, untreated patients with allergic rhinitis must be challenged with highly painful doses of capsaicin to identify these responses. CRS might represent a better model to investigate neurogenic changes because of the larger magnitude of the hyperalgesia. Studies in patients with rhinosinusitis are just beginning.
An important finding of this study was that subjects with chronic fatigue syndrome, particularly those who complain of CRS, had significantly lower sinus pain thresholds than the control group, as well as the acute rhinosinusitis and CRS groups.132 This is of importance because many of these subjects have normal sinus CT scans and carry the diagnosis of nonallergic irritant rhinitis. Their inclusion in rhinosinusitis studies might confound study outcomes because they might not respond to any rhinosinusitis therapies. They can be identified by means of questionnaires and their systemic tenderness.
4. Aspirin-exacerbated respiratory disease
Aspirin-exacerbated respiratory disease is an adult-onset disorder defined as a triad of asthma, NPs, and rhinosinusitis.134 The disease progresses irrespective of whether the individual ingests cyclooxygenase 1 inhibitors (aspirin or nonsteroidal anti-inflammatory drugs), but ingestion triggers a severe upper and lower respiratory tract reaction. The disorder can be diagnosed by means of oral aspirin challenge134 or (outside the United States) by means of inhalation of lysine-aspirin (but this would not be useful in identifying pure nasal reactors).135, 136, 137, 138 Nasal inhalation of lysine-aspirin has also been studied, and although highly sensitive, it can be difficult for some patients to endure (20% withdraw), and it is not as specific as the oral challenge (86%).139, 140, 141
Antihistamines and high doses of oral steroids should be avoided before the challenge because these can prevent nasocular reactions to aspirin.142, 143 However, nasal steroids and leukotriene modifiers (zileuton and montelukast) can be continued because they do not appear capable of inhibiting nasocular reactions.144, 145 Leukotriene modifiers do not block the upper airway response in aspirin-sensitive patients because zileuton only inhibits the 5-lipoxygenase enzyme by about 40%, and the dose of montelukast appears to be a significant factor.
5. Gastroesophageal reflux disease
Gastroesophageal reflux disease (GERD) is typically produced by the reflux of stomach acid into the lower esophagus, especially when supine.146, 147 Acid can also reflux into the oropharynx, nose, and sinuses, producing upper airway symptoms, including rhinosinusitis.148 Symptoms include hoarseness, cough, postnasal drip, nasal congestion, and drainage; this condition is also referred to as supraesophageal reflux disease (SERD). Although rhinosinusitis symptoms can and do occur in patients with typical GERD,149 studies have shown that 57% to 94% of patients with ear, nose, and throat symptoms do not have typical GERD.150
The pathophysiology of this condition is believed to be direct contact of the upper airway with gastric contents, including acid and pepsin; duodenal contents, including bile acids and pancreatic enzymes like trypsin; or both. Vagal-mediated reflexes have also been implicated. Additional possible mechanisms include defective upper esophageal sphincter pressure, esophageal dysmotility, and poor acid clearance.151
The diagnosis of supraesophageal reflux is somewhat difficult. The tests commonly used to diagnose GERD are less effective in SERD.152 These include upper gastrointestinal examination, endoscopy, the Bernstein acid perfusion test, manometry, and reflux scintiscanning. More effective tests for making the diagnosis of SERD include 24-hour pH monitoring, the gold standard, or an empiric therapeutic trial.152 An empiric therapeutic trial would not be useful for a research study unless one first did a placebo-controlled empiric treatment trial to determine eligibility for the proposed research. The probe should be placed in the proximal esophagus (2 cm above the upper esophageal sphincter) or in the pharynx.153 When this is accomplished, there is high specificity (90% to 100%); however, sensitivity varies from 55% to 95%.152, 154
There are 2 studies that address the role of acid suppression in treating CRS.155, 156 Both studies were open treatment protocols in children with difficult-to-manage CRS. Dual pH probe monitoring was performed, but not all enrolled subjects in one of the trials had SERD.155 In any case patients were noted to improve in both studies.
6. Other contributive factors to rhinosinusitis (defects in mucociliary clearance and antibody deficiency syndromes)
A great deal has been written regarding the role of defects in mucociliary clearance and humoral immune deficiency as contributive factors to rhinosinusitis. These have been extensively reviewed in recent articles12, 157, 158, 159, 160, 161, 162, 163 and were therefore not discussed at length at the conference.
D. Histologic factors of CRS
Summary Statements:
-
•
Examining the histology of middle turbinate tissues from subjects with CRS suggests a distinction between cases of CRSsNP and cases of CRS with NPs (CRSwNP; ie, different patterns in cellular content and gross histologic changes within the tissue, especially with regard to fibrosis and edema).
-
•
The mucosal lining in CRSsNP is characterized by basement membrane thickening, goblet cell hyperplasia, limited subepithelial edema, prominent fibrosis, and mononuclear cell infiltration.
-
•
In contrast, CRSwNP reveals frequent epithelial damage, a thickened basement membrane, and mostly edematous to sometimes fibrotic stromal tissue, with a reduced number of vessels and glands but virtually no neuronal structures.
1. Polypoid versus nonpolypoid CRS
CRS is known to manifest as polypoid and nonpolypoid forms. Recent studies examining the histology of middle turbinate tissues from subjects with polypoid versus nonpolypoid disease support the distinction between them. In the study by Malekzadeh et al,164 preoperative sinus CT scans and histologic specimens of middle turbinates obtained during sinus surgery were examined retrospectively in 34 patients and compared with those of 7 control patients who underwent cosmetic and spenopalatine surgery. CT scan severity was classified according to the May classification.165 Tissue sections were stained for mucus cells in glands with Alcian Blue. Goblet cells were often not present because of epithelial metaplasia. The area of tissue sections below the epithelial basement membrane were assessed by means of digitized image analysis, and the percentage area stained blue was determined (Fig 7). Normal (May class 0) and mild (May class I) sinusitis showed approximately 6% mucous cells in nasal airway mucosa. A similar percentage was found in class II. However, these subjects could be divided into those with relatively normal histology and those who showed cobblestoned mucosa or small polyps during surgery. This was accompanied by suggestions of increased mucosal edema. As shown by Biedlingmaier and Trifillis,126 these subjects have goblet cell metaplasia with a decrease in ciliated cells. A major difference in mucosal histology was seen in class III. One population of subjects showed thickened mucosa on visual inspection and had glandular mucous cell hyperplasia, with 22% of the mucosa stained with Alcian Blue. This indicates that some mechanism was active to cause glandular hyperplasia. Potential mechanisms could be similar to those suggested for chronic bronchitis and murine models showing IL-13-induced goblet cell hyperplasia. The other population of patients had visual evidence of polyposis. The histology from the patients with polyps showed a decrease in percentage Alcian Blue area as disease severity worsened from class II to class IV (pansinusitis). Ultimately, the polypoid degeneration totally obliterated normal mucosal histology in the region of the polyp root. Patients with polypoid disease were also more likely to manifest changes of pansinusitis on sinus CT. These results strongly suggest that distinct molecular mechanisms underly the polypoid and glandular hypertrophy subsets of CRS.
Fig 7.
Two distinct histologic subsets of CRS. Glandular hypertrophy-hyperplasia is noted in May class III, with an increase in the percentage of the mucosal area occupied by mucous glands. In contrast, visually observed and histologic polypoid degeneration occurs in an exclusive and nonoverlapping group. Massive polyposis is found in pansinusitis (May class IV).
These distinct histologic patterns have been independently supported by several studies.166, 167 A similar dichotomy was seen in clinical, radiologic, and treatment responses by Eichel.169 Patients with polyposis detected by means of observation or CT scanning were more resistant to medical therapy and often needed a combination of surgical and long-term medical interventions. The polypoid disease was generally recurrent, despite the medical follow-up treatment. The nonpolypoid sinusitis group generally responded more favorably to medical therapy and in some cases had total resolution of symptoms.
As previously stated, the histology of the epithelium has also been noted to change with radiographic disease severity, as assessed with the May classification.126 Normal nasal epithelium is ciliated and pseudostratified. In class I and II these cells are replaced by goblet cells. In class III microvillous cells are the predominant population. Squamous metaplasia is present in class IV. Erythrocytes and patches of denuded epithelium are also seen. This suggests a mechanism for invasion of microbes through the usually protective lining. In essence, the nasal and sinus mucosa differentiates into a skin-like squamous epithelium. These epithelial changes offer novel ecologic niches for microbial colonization and invasion. This might explain the differences in bacterial organisms cultured from acute rhinosinusitis (presumably ciliated epithelium) versus CRS (microvillous to squamous epithelium) groups. These epithelial changes are amenable to treatment and might improve with intensive therapy after surgery.169
Polypoid and nonpolypoid CRS (CRSwNP vs CRSsNP) also generally show different patterns in cellular content and gross histologic changes within the tissue, especially edema and fibrosis formation. In the sinus fluid of patients with CRSsNP undergoing surgery, inflammatory cells are predominantly neutrophils, as is observed in acute sinusitis, but a low percentage of eosinophils, mast cells and basophils might also be found.170, 171 In a recent study evaluating the percentage of eosinophils (of 1000 inflammatory cells counted per vision field), 31 patients with untreated chronic sinusitis without NPs all had less than 10% eosinophils (overall mean, 2%), whereas in specimens from 123 untreated patients with NPs, 108 samples showed more than 10% eosinophils (overall mean 50%).172 Among the inflammatory cells, EG2+ (activated) eosinophils are a prominent and characteristic feature in about 80% of patients with CRSwNP.173 Eosinophils are localized around the vessels and glands and directly beneath the mucosal epithelium.174
The mucosal lining in patients with CRSsNP is characterized by basement membrane thickening, goblet cell hyperplasia, limited subepithelial edema, prominent fibrosis, and mononuclear cell infiltration. Histomorphologic characterization of NP tissue (CRSwNP) reveals frequent epithelial damage, a thickened basement membrane, and mostly edematous to sometimes fibrotic stromal tissue, with a reduced number of vessels and glands but virtually no neuronal structures.174, 175, 176 The stroma of mature polyps is mainly characterized by its edematous nature and consists of supporting fibroblasts and infiltrating inflammatory cells localized around empty pseudocyst formations. In small polyps, not larger than 5 mm, growing on normal-looking mucosa of the middle turbinate in patients with bilateral polyposis, early processes of polyp growth have been studied.177 Numerous subepithelial EG2+ eosinophils were present in the luminal compartment of the early-stage polyp, forming a cap over the central pseudocyst area. Fibronectin deposition was noticed around the eosinophils in the luminal compartment of the early-stage polyp and formed a network-like structure in the polyp center and within the pseudocysts. The presence of myofibroblasts was limited to the central pseudocyst area. Interestingly, albumin and probably other plasma proteins were deposited within the pseudocysts adjacent to the eosinophil infiltration. These observations suggest a central deposition of plasma proteins, regulated by the subepithelial eosinophilic inflammation, as a pathogenic principle of polyp formation and growth. The extravasated plasma, for reasons of distance, binding force, or extracellular matrix damage or abnormality, might not find its way to the airway surface.178
For additional discussion of the significance of polypoid versus nonpolypoid CRS, see the section below on controversy 1 (p. 29).
2. Infectious versus noninfectious-inflammatory CRS
Can we distinguish infectious and noninfectious-inflammatory subtypes of CRS on histologic grounds? Unfortunately, although other evidence presented in this conference would suggest that there might be infectious and noninfectious-inflammatory subtypes of CRS, there is a general lack of information to support or refute this on histologic grounds. This is an important area in need of further study.
E. Factors involved in nasal polyposis
Summary Statements:
-
•
Characteristic symptoms and signs of CRSwNP include nasal congestion, facial pain-pressure-fullness, postnasal drainage, hyposmia-anosmia, and the presence of bilateral NPs.
-
•
Histologically, NPs typically show a chronic inflammatory infiltrate with increased numbers of eosinophils.
-
•
At least 4 processes might contribute to variable degrees to the inflammatory process of CRSwNP: (1) late-phase allergic inflammation in response to airborne allergens; (2) T-cell activation with production of IL-5, IL-13, and IFN-γ in response to fungal antigens (hyphae) in sinus mucus; (3) T-cell activation, cytokine production, and local IgE production in response to bacterial superantigens; and (4) dysregulation of sinus epithelium with overproduction of chemokines, such as RANTES.
Most of what we know about the pathology of NPs comes from studies of inflammatory NPs; that is, those that would best fit the description of edematous, eosinophilic type NPs but might include some NPs with neutrophilic or mixed inflammation cells. Initial studies found heterogeneity in the appearance of NPs, despite the fact that all subjects had symptoms of CRS for a minimum of 12 weeks in association with a history of bilateral NPs and mucosal thickening on sinus CT scans.179 Likewise, other studies have reported heterogeneity in the histologic appearance of NPs.174, 175 The pathologic significance of this heterogeneity is unclear but should be kept in mind when interpreting data from NP studies.
The characteristic symptoms of CRSwNP include nasal congestion, facial pain-pressure-fullness, postnasal drainage, and hyposmia-anosmia. Facial pain and fever are uncommon. The most characteristic clinical appearance is that of bilateral NPs. In fact, the presence of unilateral NPs should prompt consideration of other conditions, such as AFRS, inverting papilloma, an antral choanal nasal polyp, other unusual polypoid lesions, or nasal tumors. On radiographic or sinus CT scanning, sinus mucosal thickening is usually present in multiple sinus areas bilaterally, along with bilateral NPs. When assessed by means of prick and intradermal skin testing, approximately 50% of patients are nonallergic. Overall, about 50% of patients have asthma, and 40% of patients have aspirin intolerance.180
1. Noninfectious CRS
In the majority of cases of CRS in which prominent polypoid tissue is present, the results of bacterial culture are negative. Even more sensitive polymerase chain reaction techniques have failed to demonstrate bacterial infection in most cases.181 This is consistent with a study in which antral punctures of the maxillary sinus were performed in 12 subjects with CRSwNP. A positive culture was found in only 3 patients.179 Because of the lack of evidence for bacterial infection, the lack of sinus pain-pressure and fever experienced by patients, and the typical appearance of NP tissue showing a pattern of chronic inflammation with a predominance of eosinophils and a relative paucity of neutrophils, CRSwNP has been referred to as noninfectious CRS.118, 182
2. What are early features of NPs?
Only one study has attempted to describe the early features of edematous, eosinophilic-type NPs. In this study the subjects had evidence for a developing polypoid lesion on the middle turbinate before ever having had polyps.177 An early feature in these lesions was the presence of eosinophils forming a subepithelial cap over a pseudocyst area filled with albumin. A later feature was a large pseudocyst area containing albumin surrounded by subepithelial eosinophils.
3. Hallmark inflammatory features of CRSwNP
Histologically, NPs show a chronic inflammatory infiltrate with increased numbers of eosinophils. There is an influx of CD34+ eosinophil-basophil bone marrow progenitor cells. Special stains typically reveal a mild increase in the number of mast cells and evidence of mast cell degranulation. Plasma cells are increased in comparison with the normal nasal mucosa.183 By means of immunohistochemical staining, the numbers of macrophages, neutrophils, and CD8+ T lymphocytes are normal. However, in one study of cells isolated from digested nasal polyp tissue, CD8+ T lymphocytes predominated over CD4+ T lymphocytes.184 Most studies have reported normal or mildly increased numbers of CD4+ T lymphocytes. However, there is an increase in activated T cells (CD45RO+),184 and dual immunostaining reveals an increase in the number of IL-5-producing T lymphocytes in both allergic and nonallergic patients.185 There is also increased expression of ICAM-1, VCAM-1, E-selectin, and P-selectin on NP endothelium186, 187 and increased local production of chemokines (eg, RANTES, and eotaxin), especially in the epithelium,185, 188 but also in the submucosal fibroblasts.189
Numerous cytokines and chemokines are overexpressed in NPs. With respect to T lymphocytes, the profile is a mixed TH1/TH2 cytokine profile. An increase in GM-CSF, IL-3, and IL-13 levels is also present, and their levels are relatively similar in allergic and nonallergic subjects. There is also increased expression of proinflammatory cytokines.186
In addition to cytokines and chemokines, other mediators, such as histamine, are also markedly increased in nasal polyps, exceeding levels of 4000 ng/mL.111 Increased levels of tryptase, histamine, and ECP have been reported in polyp tissue and in nasal lavage fluid from patients with NP compared with that seen in those without NP.111, 190 In addition, increased levels of IgA, IgE, IgG, and IgM in polyp fluid and tissue have been reported.
There is also evidence for remodeling in NPs, including an increase in glandular proliferation, increased numbers of blood vessels, an increase in α-SMA+ myofibroblasts, and deposition of collagen types I, III, and V.191, 192 Several profibrotic cytokines have been found to be increased in NP, including GM-CSF, transforming growth factor β (TGF-β), platelet-derived growth factor, fibroblast growth factor and vascular endothelial growth factor, epidermal growth factor, insulin-like growth factor, and IL-11.186, 193, 194, 195, 196 A significant amount of constitutive matrix metalloproteinase (MMP) 1 mRNA has been reported in NP fibroblasts, and this expression was found to be upregulated by cytokines.197
4. Role of mast cells in NPs
Mast cells are known to play a key role in IgE-mediated diseases but are also involved in non-IgE-mediated inflammatory diseases. Mast cells can be detected in both the epithelium and the stroma of NPs, as also seen in the nasal mucosa of patients with allergic rhinitis. By contrast to that in the allergic nasal mucosa, the majority of degranulated mast cells are localized to the deep stroma, suggesting that mast cells in NPs are not likely to be activated by inhalant allergens. These mast cells express a variety of cytokines, such as IL-4, IL-5, IL-6, IL-13, GM-CSF, TNF-β, and IL-8, and mast cell mediators, such as histamine and tryptase. IL-4 and IL-13 are capable of upregulating the release of RANTES, GM-CSF, stem cell factor, and thymus and activation-regulated chemokine from NP epithelial cells, fibroblasts, or both, indicating a vicious cycle perpetuating the eosinophilic inflammation. In fact, it was recently observed that there are increased levels of tryptase and ECP in recurrent NPs compared with levels found in fresh untreated NPs.190 Also, a good correlation was detected between the levels of ECP and tryptase. These findings are further supported by the observations of Di Lorenzo et al198 that the levels of tryptase and ECP in nasal lavage samples of patients with NPs correlated with symptom scores. Furthermore, histamine from mast cells can upregulate the production of fibronectin and chymase, and tryptase can upregulate the production of MMP-9.190 Because mast cells can be stimulated in a variety of ways other than conventional allergy, (eg, bacteria, virus, fungi, complement, or autoantibodies), mast cells might contribute to the induction of eosinophilic inflammation through the release of various inflammatory mediators and indirectly through the activation of structural cells, thus contributing to the formation and progression of NPs.
5. Mechanisms of eosinophil accumulation in CRSwNP
Several pathologic processes act in concert to promote the accumulation of eosinophils in NPs. These include infiltration of the NP by CD34+ eosinophil-basophil progenitor cells199; increase in the local survival of eosinophils in NP tissue (which is dependent on local IL-5)200; evidence for local production of GM-CSF and IL-3179; upregulation of endothelial VCAM-1 and P-selectin186, 187, 201; production of C-C chemokines in epithelium and NP fibroblasts185, 188, 189, 202; and local production of IL-13, which might contribute to adhesion molecule expression and enhance the action of IL-5 and eotaxin in airway tissue.120, 186
There is an increase in the local production of IL-5 in both allergic and nonallergic subjects with CRSwNP.185, 203 The majority of IL-5-producing cells in NPs are T lymphocytes (68%), with the remainder being primarily eosinophils (18%) and mast cells (14%).185 This at least suggests that an immune specific activation process might be involved in the disease process. IL-5 is the principal survival-promoting cytokine in NPs.200 Locally produced IL-5 might also serve as a systemic stimulus for bone marrow eosinophilipoiesis in these patients.
GM-CSF and IL-3 are abundantly produced in CRSwNP and correlate with the numbers of eosinophils present. These contribute to the sustained activation and survival of eosinophils in the NPs. Much of the local production of GM-CSF in NPs probably results from the autoactivation of eosinophils.
Proinflammatory cytokines, such as TNF-α and IL-1β, are also highly overexpressed. They promote NP inflammation through induction of endothelial adhesion molecules, including ICAM-1, VCAM-1, and P-selectin. Several investigators have found increased expression of these molecules in NPs.186, 187
Several chemokines are overproduced in NPs, including the C-X-C chemokine IL-8 and the C-C chemokines RANTES and eotaxin. It might be imprecise to state that IL-8 is dysregulated in CRS. Rather, production of IL-8 by epithelial cells might well be a part of the innate immune response to sinus infection. However, there is evidence for dysregulation of epithelial C-C chemokine production, including RANTES and eotaxin, in NP epithelium, and these chemokines might be important in promoting the local chemotaxis of eosinophils.177, 185, 188, 189, 202, 204
IL-13 is increased in NPs from both allergic and nonallergic subjects.186 The functions of IL-13 are mediated through the IL-4 receptor chain but are distinct from those produced by IL-4. Given the lack of evidence for overexpression of IL-4 in nonallergic patients, IL-13 might play an important role in disease pathogenesis.
According to the current understanding, IL-5 and eotaxin are the major factors in this eosinophilic inflammation, and IL-5 correlates significantly with ECP.13, 120 Very recently, the regulation of the IL-5 receptor, which exists in the soluble and transmembrane isoform, has been investigated.205 In NPs the probably antagonistic soluble isoform is upregulated, and the signal-transducing transmembrane isoform is downregulated, especially if associated with asthma.
6. T-cell phenotype in CRSwNP
Several groups have investigated the cytokine profile of T lymphocytes in NPs. Most have found a mixed phenotype of TH2 and TH1 cytokines, with evidence for local production of IL-5, IL-13, and IFN-γ.179, 184, 203, 206, 207 The cytokine profile is somewhat different in allergic and nonallergic subjects. A more characteristic TH2 cytokine profile is seen in subjects with CRSwNP and associated allergies, whereas the mixed TH1/TH2 cytokine profile is characteristic of the nonallergic subjects. However, both allergic and nonallergic subjects have increased IL-5 and IL-13 production,186, 208 and the extent of tissue eosinophil infiltration is indistinguishable in these 2 groups. Similar differences in the pattern of cytokines expressed has been found in allergic versus nonallergic subjects with CRSsNP.115 The local production of IL-5 by T lymphocytes is likely to be of great importance in promoting the survival of tissue eosinophils in NPs.120, 186, 200
7. Potential mechanisms of inflammation in NPs
On the basis of the pathologic features of CRSwNP and a limited number of investigations, it is possible to consider 4 processes that might contribute to the inflammatory process: (A) late-phase allergic inflammation in response to airborne allergens (in allergic subjects with CRSwNP); (B) T-cell activation with production of IL-5, IL-13, and IFN-γ in response to fungal antigens (hyphae) in sinus mucus; (C) T-cell activation, cytokine production, and local IgE production in response to bacterial superantigens; and (D) dysregulation of sinus epithelium with overproduction of chemokines, such as RANTES.
A. In allergic subjects with CRSwNP, the presence of the complete TH2 profile of cytokines suggests that late-phase allergic inflammation might contribute to the disease. However, on the basis of studies of Adkins et al,209 it is doubtful that airborne allergens penetrate into sinus cavities. This leaves open the question as to how the late-phase inflammatory process is driven in the sinuses. The answer might lie in systemic cross-talk of allergic inflammation (ie, the ability of allergen-induced inflammation at one site to induce a similar response at a remote site). This type of interaction has been demonstrated between the nose and the lungs,210, 211, 212 and preliminary studies suggest that a similar interaction might occur between the nose and the sinuses.213 Increased levels of IgE receptor (FcϵRI) expression were detected in NPs from atopic subjects, and the functional relevance of this could be to cause an increase in IgE-dependent histamine release from NP mast cells.183, 190 In these patients the levels of specific IgE have been found to be higher in the NP tissue compared with that found in the serum of the same patients, indicating local IgE synthesis.190 Because IgE itself can upregulate FcϵRI expression in mast cells, this can lead to a chronic activation of mast cells and the recurrence of NPs with eosinophilic inflammation.
B. Studies of antigen-specific immune responses in CRSwNP are very preliminary, but evidence suggests that peripheral blood T lymphocytes from patients with CRS proliferate and produce IL-5, IL-13, and IFN-γ in response to fungal antigens, particularly those from the dematacious fungi Alternaria and Candida species (see section “Non-IgE-mediated eosinophilic fungal inflammation”).104 This cytokine profile matches that found in NP tissues or T lymphocytes isolated from NP, therefore supporting the concept that this might be an important immune response pattern in CRSwNP. However, this response profile is not specific for fungi and can also be seen in response to superantigens.214
C. Bacterial infection might also be associated with IgE sensitization and increase in bacteria-specific IgE and a shift to a TH2-type cytokine profile.215 In fact, Bachert et al13 detected specific IgE to staphylococcal enterotoxins A and B in NPs and found that the levels of IgE correlated with the eosinophilic infiltration. They demonstrated multiclonal IgE, including specific IgE to staphylococcal enterotoxin A and staphylococcal enterotoxin B in 50% of bilateral eosinophilic NPs. Similar levels of superantigen-specific IgE were found in atopic and nonatopic subjects, suggesting a potential common inflammatory response in these 2 groups. Most of these subjects also had asthma. Because IgE can upregulate mast cell FcϵRI expression and mast cell activation, these observations further suggest a role for mast cells in regulating the chronic eosinophilic inflammation.
D. Holtzman et al216 described a mechanism of T-cell transmigration through the epithelium that involves ICAM-1 and the C-C chemokine RANTES. They proposed that the epithelium in asthma is “constitutively dysregulated” (ie, expressing ICAM-1 and producing RANTES independent of exogenous stimuli). This dysregulation does not appear to be associated with increased nuclear factor κB activation in the airway epithelium. The functional consequence of these actions is to facilitate T-lymphocyte migration through the epithelial compartment. They further showed that this dysregulation involves overactivity of the transcription factor signal transducer and activator of transcription 1 and does not require the presence of IFN-γ.217 Given the many similar inflammatory features of asthma and CRSwNP, it is possible that a similar type of dysregulation is present in CRSwNP.
V. How should we subclassify CRS?
A. Should CRS be subclassified?
An important issue discussed at the conference was whether current evidence was sufficient to subclassify CRS into distinct subcategories. This lead to considerable discussion and debate. The most controversial issues are summarized below, after which the consensus opinions expressed at the conference are summarized.
B. Controversy 1: should CRS be subclassified as without NPs versus with NPs?
Many published studies of CRS have made a distinction between CRS and CRS with concomitant NPs. Most of these studies have regarded patients with bilateral NPs as forming a distinct subset of the patients with CRS. At the conference, a discussion centered on whether CRS should be formally subclassified as CRSsNP and CRSwNP for the purposes of advancing our knowledge of the underlying pathologic processes involved in each and as a means of sharpening the focus of therapeutic trials. The consensus opinion was in favor of such a subclassification.
Evidence discussed previously in the sections “Histologic factors of CRS” and “Allergic and immunologic factors of rhinosinusitis” support the concept that different pathogenic processes are involved in CRSsNP and CRSwNP. In addition to differences in the inflammatory cellular infiltrate, cytokine and mediator profiles, and the immune response to SAEs, differences have also been described in remodeling processes in CRSsNP versus CRSwNP.177 The expression of TGF-β1 at the protein and RNA level is significantly higher in CRSsNP versus CRSwNP and linked to a fibrotic cross-anatomy.177 In contrast, edema and pseudocyst formation characterize CRSwNP, with only few areas of fibrosis. Furthermore, an imbalance of MMPs with an upregulation of MMP-7 and MMP-9 in CRSwNP has been found, whereas in CRSsNP MMP-9 and TIMP-1, a natural antagonist, are increased.218 This results in the enhancement of MMP-9 in CRSwNP, whereas in CRSsNP MMP-9 activity is inhibited.219 Differences in TGF-β1 and metalloproteinase levels might account for edema formation with albumin retention in CRSwNP versus fibrosis in CRSsNP.
Most published studies have required that patients have bilateral NPs visible in the middle meatus to satisfy the criteria for NPs. On the basis of histologic assessment, the presence of eosinophils and the general nature of the inflammatory response are similar in NPs and maxillary polypoid mucosa.179 In patients with previous surgeries, all evidence of polyposis might have been removed, but it is reasonable to classify such a patient postoperatively in the CRSwNP category, at least for a period of time. This might be the case, for instance, in a drug study in which the putative action of the drug might be to prevent the recurrence of NPs. In the earliest stage of polyposis, it is likely that CRSwNP could not be distinguished from CRSsNP. Focal polypoid mucosal changes on the middle turbinate have been suggested as an early feature of CRSwNP177; however, prospective studies testing this hypothesis have not been done. Other manifestations of polypoid tissue on the nasal turbinates or in the sinus cavities have unclear significance and do not satisfy the criteria for NPs. Thus for the purposes of classification of a patient with CRS, the presence of NPs in the middle meatus, either in the past or at present, defines the subset of patients with CRSwNP.
Although there are a paucity of studies directly comparing the clinical features of patients with CRSsNP and CRSwNP, the clinical differences between these subgroups can only be described in terms of general tendencies. The symptom of facial pain-pressure-fullness is generally less common and reduced sense of smell (hyposmia or anosmia) is generally more common in CRSwNP. However, there is a large overlap in the symptoms of each form of CRS, and for this reason, the same general symptom criteria were proposed to define CRS in each case (see section “Rhinosinusitis consensus definitions and clinical trial guidelines”). Patients with NPs are more likely to manifest blood eosinophilia, asthma, and aspirin sensitivity.176 Patients with CRSsNP appear more likely to manifest signs of bacterial infection and have been reported to have a better response to medical treatment.168 The development of CRSsNP has long been viewed as a result of abnormal ventilation and drainage of the sinuses to the nasal cavity. However, there is an increasing appreciation of the complexity of this disorder and acknowledgement that persistent inflammation is usually present in CRSsNP, frequently including some degree of tissue eosinophil infiltration.220 Glandular dysfunction might also play an important role in the pathogenesis of CRSsNP, as suggested by Malekzadeh et al.164
The phenotype of CRS also appears to affect prognosis after surgical or medical intervention. Using a rhinoscopic grading system, Kennedy et al108 found that patients with advanced mucosal polypoid changes preoperatively had a much higher rate of recurrence of disease and relapse after endoscopic surgery. Similarly, Subramanian et al221 found that patients with a past history or current evidence of NPs had a higher rate of relapse after intensive medical treatment. There is a high rate of recurrence of NPs despite surgical or medical treatment.
The extremely strong association between the development of classic AFRS and the presence of underlying NPs also deserves mention as a distinguishing feature between CRSsNP and CRSwNP. The reason for this close association is unknown, but the marked skewing of AFRS cases into the CRSwNP category was considered another argument for classifying CRSwNP as distinct from CRSsNP. Also, the clinical features of classic AFRS were considered distinctive enough to further subclassify the CRSwNP subgroup into 2 groups, with one represented by patients with classic AFRS and the other group represented by all other patients.
Another question highlights the diversity of opinion on this subject: Does polypoid swelling always evolve into a polyp? As such, are patients with polypoid swelling considered to be different from those with polyps? We acknowledge that there is an earlier intermediate stage of NP formation that is not addressed with the current classification scheme; however, this stage remains undefined and in need of further study.
C. Controversy 2: should CRS be classified as eosinophilic versus noneosinophilic?
A suggestion was made to classify CRS in terms of the presence or absence of mucosal infiltration with eosinophils or conversely on the basis of the presence of degranulating eosinophils in sinus mucus (eosinophilic mucin rhinosinusitis). The term chronic eosinophilic sinusitis syndromes or chronic eosinophilic sinusitis syndrome has also been suggested to emphasize the role of eosinophilic diseases in rhinosinusitis. Within the category of eosinophilic CRS would fall classic AFRS; eosinophilic inflammation without fungal hyphae (also described as eosinophilic mucin rhinosinusitis by Ferguson222); aspirin-exacerbated respiratory disease consisting of NPs-CRS, asthma, and aspirin sensitivity; and eosinophilic granuloma. In terms of the classification scheme in Fig 8, nearly all cases of CRSwNP and a subset of cases of CRSsNP would fall into the category of eosinophilic inflammation. Eosinophilic diseases limited predominantly to the nasal cavity are worth mentioning because of their strong association and potential overlap with rhinosinusitis. These include allergic rhinitis, nonallergic rhinitis with eosinophilia syndrome, and blood eosinophilia with nonallergic rhinitis with eosinophilia syndrome. Obviously, the finding of nasal and sinus eosinophilia is the common element that groups these very diverse and distinct pathogenic syndromes into a single classification that can then be further delineated by other findings, such as the presence of atopy, mucosal edema (polyposis), and fungal hyphae.
Fig 8.
Proposed subclassification of CRS.
The noneosinophilic category would include all other cases and could be broken down further into distinct subsets. For instance, one subset would include those with a predominance of neutrophilic inflammation, as well as most of those associated with vasomotor rhinitis, GERD, and sarcoidosis.
The rationale for this subclassification is that eosinophilic inflammation is an important feature of the pathogenesis of CRS, even though multiple causative factors, both allergic and nonallergic, might contribute to it. Another important observation is the strong clinical and pathologic association of the eosinophilic category with asthma. In contrast, the pathologic processes believed to be most likely in CRS without eosinophilic inflammation are those that impair local or systemic immunity (innate or acquired), mucociliary clearance, or sinus ventilation.
This proposed subclassification incorporates many of the concepts discussed at the consensus conference; however, it was not formally adopted. The level of tissue eosinophils needed to define CRS with eosinophilic inflammation has not been established. Furthermore, because histologic findings (including a quantification of eosinophils) are not readily available in patients who have not undergone sinus surgery, this classification scheme could not be applied clinically without obtaining sinus tissue.
D. Controversy 3: should CRS be classified on the basis of the proposed definition of eosinophilic fungal rhinosinusitis?
The recent hypothesis of Ponikau et al8 to describe CRS as eosinophilic fungal rhinosinusitis or eosinophilic fungal rhinosinusitis was discussed. This hypothesis was based on the finding of fungal hyphae in association with degranulating eosinophils in the sinus mucus of 93% of patients with CRS, regardless of the presence or absence of allergy, NPs, or other classic features of AFRS. The authors proposed that eosinophilic fungal rhinosinusitis accounts for the vast majority of cases of CRS. Their proposal has stimulated a great deal of controversy. One contentious issue pertains to the prevalence of finding fungal hyphae in sinus mucus and what level of hyphae would be considered abnormal. Using greatly refined mucus collection and staining methods, Ponikau et al8 found fungal hyphae in the mucus of nearly all patients with CRS. Confirmatory results were recently published from Graz, Austria, by using similar methods of mucus handling and histologic staining for fungal hyphae.223
The apparent differences between the findings of Ponikau et al8 and those of earlier reports might be due to the different techniques used for mucus handling and fungal staining, but because this has been a controversial issue, it will be helpful to have additional studies to confirm Ponikau et al’s observations. Part of the controversy seems to stem from the fact that an inflammatory process stimulated by fungal hyphae had previously been implicated in only a small subset of cases defined as AFRS. The Ponikau et al proposal to redefine CRS as AFRS seems to dismiss the importance of defining a small subset of cases as AFRS. However, the consensus opinion expressed at the conference was that the term classic AFRS should be retained as the name for the condition classically described as having distinct immunologic, allergic, clinical, and histologic features (see previous discussion of these features). If the Ponikau hypothesis is ultimately widely accepted, the subgroup of patients with classic AFRS are still likely to represent a distinct clinical subset based on these distinctive features.224
Given the emerging data from the Ponikau group, it is clear that the role of fungi in CRS pathogenesis could assume much greater importance than was previously ascribed to it on the basis of studies of classic AFRS.
E. Consensus classification scheme for CRS
A classification scheme for CRS, intended for both clinical use and clinical research, is presented in Fig 8. The distinction between factors that are directly evident in the disease versus factors that underlie the disease is somewhat arbitrary but was viewed as the most practical means for classifying CRS. Important distinguishing features in the scheme are (1) the presence or absence of NPs; (2) the presence or absence of eosinophilic or other inflammatory features; and (3) the presence or absence of fungal hyphae in sinus mucus. The role of bacterial infection as a causative factor in CRS remains controversial, but bacterial infection is regarded as a potentially important factor in both CRSsNP and CRSwNP. Similarly, other underlying or predisposing factors to the disease, such as mucus recirculation, humoral immune deficiency, abnormal mucociliary function, and allergic rhinitis, are extremely important and are listed. Anatomic abnormalities have anecdotally been listed as a predisposing factor for rhinosinusitis, but existing studies do not support this role. An important question arising from the classification scheme is how strongly are certain factors, such as bacterial infection, associated with either CRSsNP or CRSwNP. At present, it is not possible to provide definitive answers to this question. Precise classification of a patient in terms of inflammatory features, namely as having eosinophilic or other inflammatory features, requires evaluation of sinus tissue and sinus mucus. In cases in which this information is unavailable, the minimal clinical classification will be either CRSsNP or CRSwNP. However, for research purposes, the committee believes this information is essential to classify patients.
VI. Diagnosis and assessment of rhinosinusitis
A. Symptoms assessments
Summary Statements:
-
•
All relevant rhinosinusitis symptoms, their severity, and time course should be documented.
-
•
The symptom list is not necessarily different in patients with acute and chronic disease, and some symptoms are present in patients with only rhinitis.
-
•
A 7-point analog scale could be used to report individual symptom severity scores, a total rhinosinusitis severity score, a global severity score, an overall QOL score, and the effect of current and past treatments.
The history of patients who present with a possible diagnosis of rhinosinusitis should document all relevant symptoms, their severity, and their time course. Sinusitis is often preceded by and rarely occurs without rhinitis.225 Therefore for the purposes of accuracy and definition, the term rhinosinusitis is preferred, and all appropriate symptoms should be noted. Several classifications of relevant symptoms have been proposed.12, 226 The first, developed at a meeting sponsored by the AAAAI, lists symptoms associated with “acute bacterial rhinosinusitis” (Table 8). Another classification was developed through the Task Force on Rhinosinusitis, sponsored by the AAO-HNS. This one is less specific for the etiology of the rhinosinusitis (Table 9). Like the earlier list, symptoms are divided into major and minor groups. However, it is not clear whether these categories were based on the prevalence rates, the severity degree, or the specificity of the symptoms. Additional lists have been generated. These do not necessarily divide symptoms into major and minor categories.227 Some suggest symptoms such as facial erythema and maxillary toothache have high specificity but low sensitivity in the diagnosis of acute community-acquired bacterial rhinosinusitis.228, 229 Others state the same symptoms are seen with both acute rhinosinusitis and CRS, although they might be more vague in patients whose symptoms have persisted for a longer time.157 Although a single symptom or sign might have only fair sensitivity-specificity, the combination of symptoms has very good predictive value.
Table 8.
Acute bacterial rhinosinusitis*
Major symptoms
|
Minor symptoms
|
Acute bacterial rhinosinusitis probable if 2 or more major symptoms or 1 major symptom and 2 or more minor symptoms are present.
Table 9.
Symptoms associated with the diagnosis of rhinosinusitis*
Major symptoms
|
Minor symptoms
|
A diagnosis of rhinosinusitis is probable if 2 or more major symptoms or 1 major symptom and 2 or more minor symptoms are present. Facial pain-pressure-fullness alone does not constitute a suggestive history in the absence of another major nasal symptom or sign. Fever alone in acute sinusitis does not constitute a strongly suggestive history in the absence of another major nasal symptom or sign.
1. Relevant symptoms
The most recent relevant symptom survey was a modification of the clinical diagnostic criteria suggested by the AAO-HNS for CRS.230 In it, anterior and posterior purulent drainage were compressed into the single symptom of nasal discharge, and fever was omitted as a major symptom because this was not a study of acute rhinosinusitis but rather a study of patients with a disease duration of 12 weeks or longer. This survey was given to 322 patients (mean age, 42 years), and the percentage of patients with each symptom was tabulated (Table 10). Symptoms could be aggregated further into nasal symptoms (nasal obstruction, nasal discharge, and sense of smell), facial symptoms (facial congestion, facial pain-pressure-fullness, and headache), oropharyngeal symptoms (halitosis, dental pain, cough, and ear pain-pressure), and systemic symptoms (fever and fatigue).
Table 10.
Presenting symptoms of chronic rhinosinusitis: percent of patients with symptom
| Major symptoms | % of patients | Minor symptoms | % of patients |
|---|---|---|---|
| Nasal discharge | 82 | Headache | 83 |
| Nasal obstruction | 94 | Ear pain-pressure | 68 |
| Facial congestion | 85 | Halitosis | 53 |
| Facial pain-pressure-fullness | 83 | Dental pain | 50 |
| Loss of smell | 68 | Cough | 65 |
| Fever | 33 | ||
| Fatigue | 84 |
In CRS symptoms are generally the same as those seen in acute rhinosinusitis. However, in some patients, the symptoms might be mild or consist of only a single symptom, such as postnasal drip, or the patient might not be aware of sinus involvement at all (eg, in subjects with concurrent rhinosinusitis and asthma). In CRS the most common symptoms of importance for differential diagnosis are headache, facial pain, nasal obstruction, and discharge.
Headache might even be the only symptom in some patients (eg, those with chronic sphenoiditis). The location of the headache might vary depending on which sinuses are affected.158 However, headache or facial pain does not generally suggest rhinosinusitis in the absence of other signs and symptoms. Many causes of headaches are manifest in the anterior face. These include tension, migraine, cluster, and rebound headaches and tempromandibular joint dysfunction. Eye diseases and problems with accommodation can also cause periorbital pain. Tension headache is the most common type of headache. It can be described as tightness over the head and neck. It is not aggravated by physical activity, and the typical presenting symptoms of migraine are absent. Patients with tension headaches often believe they have rhinosinusitis because their pain is localized in the forehead and relief is obtained from over-the-counter sinus medications. Migraine headache is an idiopathic recurring disorder with attacks that last approximately 4 to 72 hours. Characteristic of migraine are unilateral location, pulsating quality, moderate-to-severe intensity, associated nausea, and phonophobia or photophobia.231 Some of the distinctive features of migraine with aura are the complex neurologic symptoms that develop before the onset of the acute headache. Migraine is also aggravated by routine physical activity. Migraine and rhinosinusitis can be present at the same time, and the migraine headaches might be stimulated or worsen because of the rhinosinusitis.
Nasal obstruction might be related to structural variations of the septum, abnormalities of the nasal pyramid, or hypertrophy and edema of the turbinates. Moderate-to-severe anatomic deviations of the septum might cause a constant unilateral obstruction. A tumor might also present with the symptom of nasal blockage. Unilateral nasal obstruction that increases with time, possibly with pain or bloody discharge, suggests a possible sinister pathology in the nasal, paranasal, or nasopharyngeal cavities. A foreign body or NP might also cause unilateral obstruction. Patients with rhinosinusitis and other mucosal diseases most often experience alternating nasal obstruction, usually combined with anterior discharge, postnasal discharge, or both. The mucus might vary in quantity, quality, and color somewhat, depending on the cause of the disorder. Rhinosinusitis symptoms and signs include those seen with allergic or nonallergic rhinitis.158 Nasal obstruction, nasal discharge, and hyposmia are all symptoms consistent with the diagnosis of rhinosinusitis.12
2. Symptom severity scoring
Once the relevant rhinosinusitis symptoms have been itemized, they need to be individually quantified. This will help define the magnitude of a patient’s disease and allow for more refined assessments of interventions. The scoring can be as simple as a dichotomy indicating the presence or absence of a given symptom. The most common symptom scoring range in clinical trials of upper respiratory diseases has 4 options: 0, none; 1, mild; 2, moderate; and 3, severe.227 A 6-point Likert scale would range as follows: 0, none-absent; 1, very mild; 2, mild; 3, moderate; 4, severe; and 5, very severe. This scale has been used to identify which symptoms are typically the most problematic for patients with CRS (Table 11). 230 Another scoring system option is a visual analogue scale that ranges from 0 (no symptoms) to 100 (maximum severity). The Joint Task Force on Practice Parameters (representing the AAAAI; the American College of Allergy, Asthma and Immunology; and the Joint Council of Allergy, Asthma and Immunology) has developed a method for assessing severity of symptoms of allergic rhinitis. It includes an assessment of nasal symptom severity, an assessment of nonnasal symptom severity, a global assessment of nasal and nonnasal symptom severity, an assessment of QOL issues related to allergic rhinitis, and the effectiveness and adverse profile of current and past rhinitis medications.232 This method for severity assessment of allergic rhinitis symptoms still requires internal and external validation. Nonetheless, it appears to have potential for both assessing patient management and facilitating clinical research. In addition, by using this method as a guideline, a variation of it can be suggested and could be adapted for evaluating rhinosinusitis. Although patients might characterize the severity of rhinosinusitis as mild, moderate, or severe on the basis of one dominating symptom, there is often a mixed degree of severity of the individual symptoms that comprise the full clinical picture.
Table 11.
Scores of presenting CRS symptoms (range, 0–5)
| Symptom | Mean score | 95% CI |
|---|---|---|
| Major symptoms | ||
| Nasal discharge | 2.6 | 2.4–2.7 |
| Nasal obstruction | 3.2 | 3.1–3.3 |
| Facial congestion | 2.7 | 2.5–2.9 |
| Facial pain-pressure-fullness | 2.5 | 2.4–2.7 |
| Loss of smell | 2.0 | 1.8–2.2 |
| Minor Symptoms | ||
| Headache | 2.6 | 2.4–2.8 |
| Ear pain-pressure | 1.9 | 1.7–2.1 |
| Halitosis | 1.2 | 1.1–1.4 |
| Dental pain | 1.3 | 1.1–1.4 |
| Cough | 1.7 | 1.5–1.9 |
| Fever | 0.7 | 0.6–0.8 |
| Fatigue | 2.6 | 2.4–2.8 |
CI, Confidence interval.
The recommendation of the Joint Task Force on Practice Parameters is to assess individual rhinitis symptom severity using a 7-point visual analog scale (Table 12). It is reported that with this range and intervals, data can be generated with a lower measurement error and a correspondingly higher precision compared with a 5-point equal interval scale.233, 234 A Likert scale was used by Juniper et al235 to validate that QOL instrument. A total rhinosinusitis symptom score can also be obtained by adding the scores of the individual symptoms. Because the duration of rhinosinusitis symptoms will be different for each patient, the assessment should specify the time frame over which symptom severity is being evaluated (eg, at a point in time-instantaneous, reflective over the past 24 hours, or reflective over the past 2 weeks). A global rhinosinusitis symptom severity score provides additional information about the status of the patient beyond what is learned by assessing individual symptoms and totaling their scores. It is generated by the patient rating his or her perception of the combination of the symptoms on the 7-point scale (Table 13).
Table 12.
Individual rhinosinusitis symptoms: Severity scoring assessment

Table 13.
Global assessment of rhinosinusitis symptom severity

QOL is a very important consideration in the evaluation of the severity of rhinosinusitis. Measuring it recognizes the effects of the disease, which might not otherwise be reported by patients or considered by clinicians. A scale similar to that used for symptom severity assessment can be used (Table 14). A visual analog scale can also be used to assess the effect of current and past therapy (including over-the-counter and prescription medications, complementary and alternative treatments, and surgical procedures) for a patient’s rhinosinusitis (Table 15). Failure of a medication used consistently should be contrasted with failure of one that was compromised by poor adherence to a regimen. The duration of treatment and both the benefits and adverse effects should be quantified.
Table 14.
Quality-of-life assessment for rhinosinusitis severity

Table 15.
Effect of current and past treatment assessments

B. QOL assessments
Summary Statements:
-
•
For a complete and through assessment of rhinosinusitis morbidity and the evaluation of treatment, it is imperative that the physical, social, and emotional problems associated with this condition be measured in a valid way.
-
•
Investigators should strive to report QOL data in a fashion that is most clinically meaningful.
-
•
There are several validated rhinosinusitis outcome measures, and the instrument that seems best suited for the particular research question should be selected.
QOL is a very important consideration in the evaluation of the severity of rhinosinusitis. QOL measurements reflect the effect symptoms have on the patient’s daily life. Outcomes research studies the effects of diverse therapies on patient outcome and is increasingly recognized by physicians, third-party payers, and the federal government as crucial for the demonstration of treatment effectiveness and the establishment of patient care guidelines.236, 237 One of the key features of outcomes research is the expanded definition of outcome. The new outcomes measures used in outcomes research include patient-based measures of symptoms, functional status, social and emotional consequences of disease and treatment, and satisfaction with care. Outcomes research refers to the degree of change of the physical, mental, emotional, or social states of being.237, 238Generally, outcomes refers to the outcomes of an intervention and the change in these states associated with a treatment or intervention.239 Outcomes can also change without intervention. Outcome measures can focus on the traditional hard biologic measures, such as blood pressure and laboratory values, or soft measures, such as pain and functional limitations.240
Health-related QOL assessment refers to the description of health and disability from the individual’s perspective.241, 242, 243 QOL instruments generally include measures of physical and emotional impairment, functional disability, and handicap.244, 245 Over the last 20 years, there has been a dramatic increase in the use of QOL instruments and the reporting of QOL outcomes. Unfortunately, not all results with QOL instruments are easy to understand or can be integrated into the clinical care of patients.
1. Problems in QOL reporting
There are multiple problems in the published literature regarding the reporting of QOL studies.246 These problems include (1) the use of unfamiliar scales; (2) failure to explain the clinical importance of the instrument, including the failure to use anchors; (3) failure to describe the minimally clinically important differences; (4) failure to differentiate between inferences for individuals and inferences for individuals versus groups; (5) documenting the responsiveness to change; (6) identifying sample size requirements and statistical power; and (7) multiple QOL end points, longitudinal time frame, and whether the data were analyzed according to an original plan.
2. Health status and health-related QOL in rhinosinusitis
Health status and health-related QOL instruments can be general or disease specific.247 General measures allow comparison across different disorders, severities of disease, and interventions, whereas disease-specific scales contain items most relevant to the condition under study and that are most likely to change with effective therapy. An example of a general instrument is the Medical Outcomes Study Short Form-36 (SF-36).248 Examples of disease-specific instruments are the Symptom Score,249 the Rhinosinusitis Outcome Measure-31,250 the Sino-Nasal Outcome Test-20,251 the Chronic Sinusitis Survey,239 and the Rhinosinusitis Disability Index (RSDI).252
a. Medical outcomes study SF-36
The Medical Outcomes Study SF-36 was originally developed for study of the use of health insurance.253 It contains 36 items and measures health status in 8 domains: Physical Functioning, Role Physical, Body Pain, General Health, Vitality, Social Functioning, Role Emotional, and Mental Health. Scores range from 0 to 100, with the higher score representing better functioning. Glicklich and Metson254 showed that patients with sinusitis had significantly lower scores when compared with the general population in the domains of Social Functioning, Body Pain, Vitality, and General Health. Some of these scores were similar to the disability experienced by patients who have back pain, chronic obstructive pulmonary disease, and angina.
b. Symptom score
The Symptom Score measures the severity of 6 sinusitis-related symptoms: nasal obstruction, problems with sense of smell, anterior rhinorrhea, postnasal discharge, headache, and facial pain. The severity of symptoms is assessed with a visual analogue scale (0–10). In the 24 patients who underwent functional endoscopic sinus surgery, there was a statistically significant difference (improvement) in all 6 symptoms.
c. Rhinosinusitis outcome measure-31.250
This is a 31-item instrument. The items are classified into 7 domains. For each item, there are 2 response scales: Magnitude and Importance. The Magnitude Scale has a 6-category response score, and the Importance Scale has a 4-category response score. The product of the Magnitude and Importance score creates the Symptom-Impact Score, a unique patient-specific score. The Rhinosinusitis Outcome Measure-31 (RSOM-31) requires approximately 20 minutes to complete and has documented response to clinical change.
The domains most affected (in order of severity) in a cohort of 142 patients with rhinosinusitis were Sleep, General Problems, Nasal, and Emotional. The RSOM score correlated with the Vitality, General Health, Social Functioning, and Role-Physical subscales of the SF-36.
d. Sino-Nasal Outcome Test-20.251
The Sino-Nasal Outcome Test-20 (SNOT-20) was derived from the RSOM through the elimination of 11 items. The Importance scale was revised to make scoring easier. The patient is requested to identify which of the 20 items are most important to them and that they hope will get better with therapy (to a maximum of 5). Two scores are derived: (1) Total Score, which is the mean score for all 20 items, and (2) Importance Score, which is the mean score for the items identified as important. The SNOT-20 was validated and demonstrated to be sensitive to change. Items identified as important had higher scores, on average, and showed greater change scores after treatment than items not identified as important.
The SNOT-20 has been used in an outcomes study sponsored by the AAAAI, numerous pharmaceutical-sponsored studies, and is currently being used by Royal College of Surgeons’ (United Kingdom) National Comparative Audit of Sino-Nasal Surgery. The Royal College of Surgeons’ audit is an outcomes study of 3200 patients undergoing sinonasal surgery with a 3-year follow-up (http://www.rcseng.ac.uk/surgical/research/ceu/projects_ongoing/proj_sinonasal_html).
e. Chronic Sinusitis Survey.239
The Chronic Sinusitis Survey is a 6-item, duration-based monitor of sinusitis-specific outcomes. The symptom-based section contains the following 3 items: pain or pressure, congestion or difficulty breathing through the nose, and nasal discharge or post nasal drip. The medication-based section contains these items: antibiotics, prescription nasal sprays, and sinus medications in pill form. The severity of symptoms are scaled 0 (none) to 4 (severe), and a total score is calculated by using a scoring algorithm that normalized scores from 0 (worst) to 100 (best).
f. RSDI.252
The RSDI is a broad-based, disease-specific instrument that is comprised of 30 items that are used to evaluate the physical, emotional, and social disabilities of CRS with or without polyps, aspirin sensitivity triad, allergic rhinitis, nonallergic rhinitis, acute rhinosinusitis, recurrent acute rhinosinusitis, and septal deviation with obstruction. The 30 items in the RSDI have been validated through test-retest, Cronbach α coefficient, and Spearman correlation. The RSDI has been used to evaluate patients with a variety of nasal disorders, sinus disorders, or both and to compare the effect of these disorders on the physical, functional, and emotional domains.
3. Criteria for choosing a particular QOL outcomes measure
When deciding which QOL outcome measure to use in a particular study, we recommend that the following criteria be used:
-
1
demonstrated test-retest reliability;
-
2
validity (measures what it purports to measure);
-
3
responsiveness to change;
-
4
ease of interpretability of the results;
-
5
degree of respondent burden; and
-
6
intended purpose of the outcome measure, (ie, diagnostic, assess response to therapy, or prognostic).
In summary, for a complete and through assessment of rhinosinusitis and evaluation of treatment, it is imperative that the physical, functional, and emotional problems associated with this condition be measured in a valid way. Without the incorporation of a good QOL instrument, there is no good rhinosinusitis outcomes research. There are many pitfalls in the reporting of QOL information. The investigators should strive to report QOL data in a fashion that is most clinically meaningful. There are several validated rhinosinusitis outcome measures, and the instrument that seems best suited for the particular research question should be selected.
C. Rhinoscopic assessments
Summary Statements:
-
•
Anterior rhinoscopy is the basic tool of the physical examination that relates to determining the existence of pathology in the sinonasal passages. It is best to evaluate the patient after decongestion with topical decongestants. However, even with this method, examination of the nasal passages beyond the anterior portion can be limited.
-
•
Nasal endoscopy helps identify erythema, edema, polyps or polypoid swelling, crusting, eosinophilic mucin, and mucopus or frank pus deep in the nasal cavity. It is most useful in the assessment and treatment of patients with refractory or chronic symptoms and in patients who have impending or existing complications of rhinosinusitis.
-
•
Cultures obtained endoscopically are less invasive and associated with less morbidity; however, this technique is not proved to be equivalent to antral puncture in children with sinus infections.
Rhinosinusitis has been traditionally diagnosed through careful history and physical examination. These techniques reveal important information necessary for diagnosis, treatment, and monitoring. However, symptoms provide information different from and not well correlated with endoscopy or imaging. Patients might be given an improper diagnosis and might be managed improperly on the basis of history alone.255 Therefore objective measures are increasingly perceived as necessary to accurately determine the presence or absence of rhinosinusitis. The 2 leading methods of objective assessment are nasal endoscopy and sinus imaging with CT. Endoscopy alone cannot be used to determine normalcy because rhinosinusitis can occur in sinus areas that endoscopy cannot detect. Similarly, abnormalities seen in imaging can be present without associated symptoms. Therefore both subjective and objective assessments have value.
1. Anterior rhinoscopy
Anterior rhinoscopy is the basic tool of the physical examination that most specifically relates to determining the existence of pathology in the sinonasal passages. It is best to evaluate the patient before and after decongestion with topical decongestants, such as oxymetazoline or neosynephrine. Before decongestion, the clinician evaluates the appearance of the anterior nasal passageways. Typically, it is only after decongestion that the middle turbinates can be directly visualized on anterior rhinoscopy. However, examination of the nasal passages beyond this is very limited when using this method. Septal deviations, seen in up to 79% of the normal population, can obstruct a more complete examination when assessing with anterior rhinoscopy.256
2. Nasal endoscopy
Nasal endoscopy not only plays an important role in the diagnosis of rhinosinusitis but also can assist with its treatment. Most clinicians currently using nasal endoscopy hold 6 tenets to be true: (1) patient symptoms can be an unreliable gauge of disease257; (2) endoscopy facilitates proper diagnosis and can detect disease missed on routine history and physical examination or even that missed on imaging studies; (3) discolored drainage (yellow to green) represents a pathologic process draining through the nasal passageways; (4) properly obtained endoscopic cultures are useful in identifying organisms that might be responsible for certain forms of rhinosinusitis; (5) the most important role of endoscopy is in the assessment and treatment of patients with refractory or chronic symptoms and in patients who have impending or existing complications of rhinosinusitis; and (6) endoscopy is well tolerated but is not without risk.258
In contrast to anterior rhinoscopy, endoscopy introduces brilliant illumination into the dark cavities and permits magnified direct visualization of the mucosa, the turbinates, and, in postsurgical patients, the sinuses. Nasal endoscopy helps identify erythema, edema, polyps or polypoid swelling, crusting, eosinophilic mucin, and mucopus or frank pus deep in the nasal cavity. The examiner can also identify pus emanating from the middle meatus or sphenoethmoidal recess and in the nasopharynx.
There are 2 types of endoscopes available for evaluating the sinonasal passages: flexible fiberoptic endoscopes and rigid telescopes. They differ mainly in terms of patient tolerance and safety. With regard to patient comfort and direct access to sinus cavities, flexible endoscopy is generally superior to rigid endoscopy. However, image clarity, the facility to obtain cultures and sample tissues, the ability to control epistaxis, and the ability to perform surgery is superior with rigid endoscopy. Photo documentation of an endoscopic evaluation (photoendoscopy) has been used by some as a research tool. Despite a difference in patient comfort, even rigid nasal endoscopy can be well tolerated. This is evidenced by unpublished data collected during an evaluation of the microbiology of the nasal cavities in 20 healthy medical students.259 The subjects underwent topical decongestion and anesthesia followed by rigid nasal endoscopy and were asked to rate their overall experience with rigid nasal endoscopy before culture sampling on a 1- to 5-point scale. On average, the subjects rated the experience between tolerable and mildly uncomfortable (2.5).259
Although it is generally a very safe and well-tolerated procedure, the most common adverse effects of endoscopy are patient discomfort-pain, epistaxis, and vasovagal events. With regard to patient comfort during endoscopy, it is worth noting that there appears to be decreased sensitivity in the nasal passageways of patients with nasal polyps.260, 261, 262 This, in part, might be explained by data that suggest that substance P levels are depleted in polyp tissues. This information also helps support the fact that patients can be an unreliable gauge of their own disease. Severe and very rare complications have been reported, albeit rarely, with office endoscopy, including orbital hematoma and death (associated with suctioning near the carotid artery).
Indications for nasal endoscopy during an office evaluation include assessments of symptomatic patients who are refractory to appropriate empiric therapy, who have unilateral disease without septal deviation, or who have severe and disabling symptoms. Endoscopy is also indicated if complications are suspected, if the patient is immunocompromised, or after sinus surgery, trauma, or both.
Although controversy exists over the value of endoscopically obtained cultures, many leaders who study nasal and sinus diseases collect them to guide therapy. Cultures should be obtained by skilled experienced endoscopists. Otherwise, the results from the specimen could be misleading.263, 264, 265 Endoscopic sampling can be performed with either a sterile swab or by aspiration into a sterile trap.266
3. Techniques for obtaining bacterial cultures
The sample of sinus secretions must be obtained from one of the paranasal sinuses without contamination by normal respiratory or oral flora to determine the microbiology of rhinosinusitis.267 These specimens can be collected by means of sinus puncture or endoscopically.
a. Sinus aspirates
Traditionally, bacterial specimens of the sinuses have been collected from sinus aspirates. The maxillary sinus is the most accessible. There are 2 nonendoscopic approaches to the maxillary sinus, either through the canine fossa or the inferior meatus. The nasal vestibule is heavily colonized with pathogenic bacteria, especially S aureus. Accordingly, sterilization of the nasal vestibule and the area beneath the inferior nasal turbinate is recommended. Contaminating nasal flora isolated in the sinus aspirate might be misconstrued as pathogenic. A topical anesthetic is used at the puncture site.
Acute infection is defined as the recovery of a bacterial species in high density (ie, a colony count of at least 103–104 cfu/mL) to avoid misinterpretation of culture results. This quantitative definition increases the probability that organisms recovered from the maxillary sinus aspirate truly represent in situ infection and not contamination. In fact, most sinus aspirates from infected sinuses are associated with colony counts in excess of 104 cfu/mL. If quantitative cultures cannot be performed, Gram staining of aspirated specimens affords semiquantitative data. If bacteria are readily apparent on a Gram stain, the approximate bacterial density is 105 cfu/mL. Of 12 cases in which an antral puncture showed at least 105 cfu/mL pathogens, the Gram stain demonstrated either organisms or white blood cells in all 12 and organisms and white blood cells in 9 of 12.264 The Gram stain is especially helpful if bacteria are seen on smear and the specimen fails to grow with standard aerobic culture techniques, in which case anaerobic organisms or other fastidious bacteria, such as a bacterial biofilm or an antibiotic-suppressed infection, should be suspected. Performance of a Gram stain will also permit an assessment of the local inflammatory response. The presence of many white blood cells in association with a positive bacterial culture in high density makes it likely that a bacterial infection is present. A Gram stain does not easily differentiate neutrophils from eosinophils, and therefore an eosinophil-rich smear with bacteria would be interpreted as showing many white blood cells by many laboratories. Alternatively, a paucity or absence of white blood cells in association with the presence of a positive culture in low density suggests that the bacteria are contaminating the culture rather than causing infection.
b. Endoscopic specimens
Recently, there has been interest in obtaining cultures of the middle meatus endoscopically as a surrogate for cultures from a sinus aspirate. The endoscopically obtained culture is less invasive and associated with less morbidity.264 Unfortunately, in healthy children the middle meatus has been shown to be colonized with the same bacterial species, S pneumoniae, H influenzae, and M catarrhalis, as are commonly recovered from children with sinus infection.268 Accordingly, this technique is controversial because of the potential for misinterpretation in children.
In 3 recent studies the bacterial species recovered from middle meatal samples of healthy adults were CNS in 35% to 50%, Corynebacterium species from 16% to 23%, and S aureus from 8% to 20%.64, 65, 269 The only organism serving both as a commensal and potential pathogen was S aureus. Several studies in adults have shown a good correlation between cultures of the middle meatus and the sinus aspirate in patients with acute sinusitis, especially when purulence is seen in the middle meatus164, 264, 270; however, other studies have not.271, 272 CNS is usually interpreted as a nonpathogen in acute sinusitis. Talbot et al264 correlated the results of endoscopically obtained cultures and cultures obtained from maxillary sinus aspirates. They reported no situations in which the puncture demonstrated CNS of greater than 105 cfu/mL; however, a swab of the middle meatus grew CNS in 6 of 53 patients. Interpretation of the pathogenicity of S aureus is more difficult. Two of 53 patients had greater than 105 cfu/mL, which correlated with the endoscopic swab. However, in an additional 6 patients, there was no agreement between sites.264
In rare instances neither a sinus aspirate nor a specimen obtained endoscopically is sufficient for the diagnosis of a sinus infection. In these instances, biopsy of the sinus mucosa and broth culture and appropriate stains might be required to ascertain the microbiology.
D. Imaging assessments
Summary Statements:
-
•
Although rhinosinusitis can be diagnosed in the majority of patients by using only clinical judgment, patients with recurrent or complicated sinus disease might require imaging studies. These studies are an absolute requirement in patients undergoing functional endoscopic sinus surgery.
-
•
CT has 2 major roles in rhinosinusitis: to define the anatomy of the sinuses before surgery and to aid in the diagnosis and management of recurrent rhinosinusitis or CSR.
-
•
Although MRI does not display the bony anatomy as does CT, it does provide an excellent display of the mucosa, and it is superior in distinguishing between bacterial-viral inflammatory disease and fungal concretions.
A critical assessment of the relative value of various imaging modalities for rhinosinusitis must consider not only the technical merits of each type of study but also the proposed application of the study for disease diagnosis, risk stratification, quantification of disease, response to medical or surgical intervention and disease prognostication.
1. Standard plain films of the nasal cavity and paranasal sinuses
Although plain film technology might be less costly compared with other diagnostic measures, it falls short of providing adequate diagnostic information. Plain films fail to provide information required on a patient’s anatomy, the paranasal sinus perimeter, and the extent of inflammatory disease. Also, plain films are inadequate to guide surgery.
Although plain films have limited utility as a screening tool or in children, an appropriate current view would be that the modality is useless in the demonstration of the regional morphology and precludes an accurate representation of the extent of disease.273, 274 In general, the marginal benefits of sinus plain films are insufficient to justify the exposure to radiation (regardless of how low it might be) afforded by this technology.
2. CT scanning
CT has 2 major roles in rhinosinusitis: to define the anatomy of the sinuses before surgery and to aid in the diagnosis and management of recurrent rhinosinusitis or CRS. Given its resolution of the regional bony anatomy and mucosa, it has proved to be the optimal modality in providing the anatomic roadmap for the surgeon performing functional endoscopic sinus surgery. Information afforded by the coronal plane has proven to correlate with the endoscopic information and has been the favored plane to study the patient’s anatomy and plan a surgical procedure.275, 276 The development of image-guided surgical equipment has been based primarily on CT information.
More recently, several authors have attempted to use the CT information, specifically the volume of inflammatory disease within the paranasal sinuses, in an attempt to stage patients with rhinosinusitis. The various staging systems are primarily focused on the presence of and the quantity of the inflammatory disease within the paranasal sinus.277, 278 The most accepted staging system is that by Lund-Mackay (Table 16). 279 Unfortunately, no system currently available allows clinicians to show or judge the evolution of this disease or to indicate prognosis.280, 281, 282, 283 Similarly, to date, a meaningful correlation has not been determined between symptoms and the presence of inflammatory disease within the various sinuses.277, 280, 282, 284, 285, 286
Table 16.
The Lund-Mackey Staging System
| R | L | |
|---|---|---|
| Sinus systems (0–2) | ||
| Maxillary | ||
| Anterior ethmoids | ||
| Posterior ethmoids | ||
| Sphenoid | ||
| Frontal | ||
| Ostiomeatal complex | ||
| Total | ||
| Anatomic variants (0–1) | ||
| Absent frontal sinus | ||
| Concha bullosa | ||
| Paradoxic middle turbinate | ||
| Everted uncinate process | ||
| Haller cells | ||
| Agger nasi cells |
3. Challenges in CT staging
Although results from the Lund-Mackay system279 appear to be the most reproducible, there are still clinical challenges not addressed by this method of classification. This and other current classification systems lack sufficient levels of gradation for tracking progression or reduction of the disease volume with adequate precision. Another problem is that the classifications currently used do not correlate well with symptoms.255 It is possible that considering the ostiomeatal channels and quantifying the volume of disease will add to the clinical value of future classification systems.
A staging system should:
-
•
provide an objective means of quantifying the volume of inflammatory mucosa and opacification;
-
•
be easy to use and require no formal training;
-
•
have high reproducibility, demonstrated by interobserver and intraobserver studies;
-
•
be able to quantify the patency of the ostiomeatal passageways (ie, specific ostiomeatal tight spots, such as the frontal recess, infundibulum, middle meatus, and sphenoethmoid recess).
Quantification of the patency of these structures would offer important additional information in staging disease and assessing progression and regression. Ostiomeatal patency might be an important indicator of response to medical or surgical treatment; however, this has not been formally shown. A more precise quantification might also provide a better measure of regression of disease in tight spots in association with a reduction of the volume of inflammatory disease.
4. MRI
Even though this imaging modality does not display the bony anatomy as does CT, it does provide an excellent display of the mucosa. It is superior in displaying extension of disease beyond the paranasal sinuses into the orbits and intracranial compartment. Bacterial and viral inflammations are indistinguishable; however, MRI is superior in differentiating between infectious inflammatory disease (bacterial or viral) and fungal concretions, and it is the most effective technology in isolating the presence of neoplasia in the morphologic area.275, 276, 287 Given its sensitivity in documenting the presence of fluid, MRI does not distinguish between inflammatory disease and the edematous mucosa seen during the nasal cycle. Additionally, MRI technology is less readily available, more expensive, and lengthier procedure than CT.
5. Proposals for improving currently available staging systems
Two cross-sectional imaging modalities are available, CT and MRI, and each is able to demonstrate mucosal inflammatory disease and therefore potentially useful in disease staging.
The Lund-Mackay system279 is the most objective and most reproducible. A major drawback is its inability to subgrade the volume of inflammatory disease in grade I, which can represent any degree of sinus involvement from greater than 0% to less than 100%. When evaluating a specific medical therapeutic agent, if grade 1 disease with 10% sinus involvement is cured, it is reduced to grade 0. However, if grade I disease with 90% involvement is reduced to 30%, a substantial improvement, the classification is still grade 1, suggesting there has been no change. Furthermore, this staging system does not take into account the patency of the ostiomeatal channels. These issues can be easily addressed by further stratifying grade 1 into 1A (1% to 33%), 1B (34% to 66%), and 1C (67% to 99%) and by noting the patency of the tight spots (ie, the frontal recess, middle meatus, infundibulum, and sphenoethmoid recess). These modifications should, of course, be evaluated for reproducibility and prognostic value (Table 17).
Table 17.
Proposal for CT rhinosinusitis staging system
| Sinus inflammation staging (0% inflammation) |
||||||||
|---|---|---|---|---|---|---|---|---|
| Right sinus |
Left sinus |
|||||||
| 0 (0%) | 1A (1%–33%) | 1B (34%–66%) | 1C (67%–99%) | 0 (0%) | 1A (1%–33%%) | 1B (34%–66%) | 1C (67%–99%) | |
| Sinus | ||||||||
| Maxillary | ||||||||
| Anterior ethmoids | ||||||||
| Posterior ethmoids | ||||||||
| Sphenoid | ||||||||
| Frontal | ||||||||
| Ostiomeatal complex | ||||||||
MRI, although more expensive than CT, could be used to assess the volume of inflammatory mucosa. The bright signal intensity of the T2-weighted images can be isolated on a computer workstation and 3-dimensionally reconstructed by the computer to provide a quantitative estimate of volume. A potential confounder is the edematous mucosa of the nasal cycle, which has the same signal as inflammatory disease and cycles from side to side. The edematous mucosa cannot be separated from infected mucosa and must be included in the 3-dimensional reconstruction. However, one would assume (hopefully correctly) that the volume of mucosa that cycles in the nasal cavity is constant in each individual.
E. Nasal-sinus challenge assessments
Summary Statements:
-
•
Nasal and sinus challenges provide a means to study the pathophysiology of disease and the interactions among the nose, sinuses, and lower airway.
-
•
Nasal challenges have also been used to confirm allergy, to assess nasal threshold responses, and to study mediators, inflammatory cells, and cytokines.
Nasal and sinus challenge studies have contributed to understanding the pathophysiology of nasal and sinus disease, as well as understanding the connection between the upper and lower airways. Baroody et al288 have been interested in the interaction between the nose and the paranasal sinuses. They first performed a double-blind, placebo-controlled, cross-over trial in 20 healthy nonallergic subjects to assess nasal versus sinus responsiveness to histamine. Subjects were treated with loratadine or placebo for 7 days and then underwent a nasal challenge with histamine. Twenty-four hours later, while receiving the medication, a catheter was placed in the sinus cavity, and the subjects underwent a sinus challenge with histamine. Not surprisingly, in patients receiving placebo, nasal challenge with histamine led to increasing vascular leak indicated by increasing levels of albumin in nasal lavage specimens. When treated with loratadine, an H1 antihistamine, the effect of histamine challenge was blocked. Although a similar effect was noted in the sinus challenge, the sinus mucosa was 10 times less sensitive to histamine compared with the nasal mucosa. A contralateral response, indicative of a nasonasal reflex, was also evaluated. Although the nasal challenge produced a significant reflex, no effect was noted with sinus challenges. This study showed the feasibility of challenging a sinus directly and suggested differences between the response of the sinus and nasal mucosa to the same stimulus.
Researchers have also investigated whether allergen challenge of the nose has the ability to induce inflammatory changes in the sinuses. Pelikan and Pelikan-Filipek289 conducted 73 nasal challenges with antigen in 37 patients with chronic maxillary sinusitis. This resulted in 41 positive nasal responses (in 29 patients), as measured by using rhinometry. Interestingly, 32 of the 41 challenges showed an increase in mucosal edema or opacification of the maxillary sinuses on plain radiographs. They concluded that there was a role of nasal allergy in some patients with chronic maxillary sinusitis. In another study, Baroody et al213 evaluated the effect of antigen challenge in the nose on inflammation within the sinus. Using a Sinojet (Atos Medical, distributed by Bivona Medical Technologies, Gary, Ind), an instrument used to obtain sinus lavage fluid from the maxillary sinus, they found that nasal allergen challenge induced an eosinophilic sinus mucosal response that was not seen with control challenge. In another study subjects were challenged to assess the effects of nasal allergen challenge on the ipsilateral versus the contralateral sinus. Although eosinophils were present on both sides, the number of eosinophils was significantly less on the contralateral side.290 A significant increase in maxillary sinus eosinophils was also found during the allergy season compared with that seen in patients out of season, confirming the findings of the nasal challenge studies.291 Overall, these results suggest that sinus inflammation occurs after nasal allergen challenge.
Adkins et al209 studied the ability of inhaled antigen to enter the sinuses. Radiolabeled ragweed pollen was sprayed intranasally in 5 nonallergic subjects. Using a CT scan, radiolabeled ragweed was only detectable in the nose, suggesting in this study that pollen itself was not inhaled into the maxillary sinus. However, as discussed earlier, Gwaltney et al31 performed a different study in which sinus CT scans were obtained after instillation of radiopaque contrast material into the nasopharynx. The contrast material entered the sinus in 4 of 4 patients after nose blowing, although not after sneezing or coughing. This suggests a mechanism by which allergen or virus could be propelled through secretions into the sinuses.
In addition to advancing the understanding of sinus pathophysiology, nasal-sinus challenges have other important applications in rhinosinusitis research. For instance, nasal challenge with lysine-aspirin has been used to confirm a history of aspirin sensitivity.292 Nasal challenges have also been used to confirm allergy, to assess nasal threshold responses, and to study the inflammatory cells and cytokines involved in allergic inflammation. For example, Keith and colleagues110 performed allergen challenges on patients with NPs and positive skin test responses and found them to be insensitive to challenge, implying that allergy was not important in the pathophysiology of this group of patients with NPs.
In summary, nasal and sinus challenges provide a means to study the pathophysiology of disease and the interactions among the nose, sinuses, and lower airway. Although not currently indicated, they might, in the future, have value in defining patients to be entered into clinical trials for the study of sinusitis.
F. Upper-lower airway assessment
Summary Statements:
-
•
The integrated airway syndrome, also called chronic inflammatory respiratory syndrome, has a wide spectrum of severity: at the low end, its manifestations are clinically evident in the form of rhinitis, and at the high end, manifestations include asthma and possibly rhinosinusitis.
-
•
The links between the upper and lower respiratory tract are strongly supported: both allergic rhinitis and non-allergic rhinitis are risk factors for asthma; allergic rhinitis is almost ubiquitous in asthma, even in the absence of nasal symptoms; the nasal mucosa of patients with asthma shows evidence of inflammation; and the rhinitis of asthmatic patients tends to be more severe than the rhinitis of nonasthmatic patients.
-
•
Allergic reactions and their inflammatory consequences appear to propagate systemically, and therefore the interactions between nasal, sinus, and lower airways might represent the manifestations of such a systemic process.
The nasal airways, the sinus cavities, the pharynx, the larynx, the trachea, and the intrathoracic airways are parts of one conduit with a common embryologic origin. Each of these parts appears to have specialized functions, but all parts are highly integrated. Although some illnesses might affect only selected parts, several others manifest themselves over the entire respiratory tract. The chronic allergic respiratory syndrome (and perhaps its nonallergic counterpart) is an example of the panairway affliction. Chronic rhinitis, rhinosinusitis, and asthma should be considered components of this syndrome and not independent nosologic entities.
A model has been proposed to integrate many epidemiologic, pathophysiologic, and clinical observations on rhinitis and asthma.293 A similar model could be proposed to integrate rhinitis and rhinosinusitis, as well as rhinosinusitis and asthma. The premise of this model is that the chronic allergic respiratory syndrome has a spectrum of severity. At the low end, its manifestations are clinically evident in the form of rhinitis, and at the high end, manifestations include asthma and possibly rhinosinusitis. The reason why the nose is in the center of the syndrome is because it constitutes the primary deposition site for aeroallergens. In the presence of rhinitis alone, the lack of clinical manifestations of the syndrome in the lower airways and the paranasal sinuses should not be interpreted as a lack of involvement. The lower airways of individuals who only have allergic rhinitis have been repeatedly found to be inflamed or even remodeled (increased thickness of the reticular basement membrane) compared with those of healthy control subjects.294 Also, lower airway hyperresponsiveness can be detected in a significant number of individuals with allergic rhinitis but without lower airway symptoms.295
Several observations support the aforementioned model. First, both allergic rhinitis and nonallergic rhinitis are risk factors for asthma in cross-sectional and longitudinal studies.296, 297, 298, 299 Second, rhinitis is almost ubiquitous in asthma.298, 300 Furthermore, the nasal mucosa of patients with asthma shows evidence of inflammation, even in the absence of nasal symptoms.301 Third, the rhinitis of asthmatic patients tends to be more severe than the rhinitis of nonasthmatic patients. Although this concept has not been adequately investigated and the available data are still in preliminary form,30, 302 recent epidemiologic evidence provides some support for this theory.299 Finally, data from pathologic and clinicoepidemiologic studies suggests that in asthmatic patients the severity of asthma and rhinitis tracks in parallel.303, 304, 305, 306, 307, 308, 309
An additional aspect of the relationship between nasal and lower airway disease in the context of the chronic allergic respiratory syndrome is that events that take place in the nasal cavities might affect the lower airways. A nasal allergic reaction induced by localized provocation, for example, can result in increased responsiveness in the lower airways310, 311 or even in late reductions in lung function.312 Inversely, treatment of allergic rhinitis with topical glucosteroids has been shown, in several studies, to improve various asthma outcomes.313, 314, 315, 316, 317, 318, 319, 320, 321 However, the mechanisms of this apparent interaction between the nasal and the lower airways are not clear. Obviously, many functions of the nose are known to benefit the lower airways, and it would not be surprising if deterioration or improvement of these functions accounted for the interactions. On the other hand, allergic reactions and their inflammatory consequences appear to propagate systemically, and the interactions between nasal and lower airways might represent the manifestations of such a systemic component.210, 211, 212
The data relating sinus disease to asthma are far less extensive; however, they indicate similar relationships between asthma and rhinosinusitis as between asthma and rhinitis. For example, almost ubiquitous presence of paranasal sinus abnormalities in patients with moderate-to-severe asthma has been reported in a study using computed tomography.322 Because they commonly coexist, testing pulmonary functions in patients with rhinosinusitis should always be considered. Evidence of eosinophilia in the sinus mucosa is stronger in patients with rhinosinusitis and asthma, as opposed to rhinosinusitis alone.323 Medical and surgical treatment of sinus disease appears to have beneficial effects on asthma outcomes, but the studies reporting such findings are not randomized, and the outcomes are frequently subjective.324, 325, 326 Thus because the links between the upper and lower airway are not fully understood, additional, careful, mechanistic, and therapeutic studies need to be conducted to further clarify the relationships of rhinosinusitis in these integrated respiratory syndromes.
VII. Clinical trial designs of rhinosinusitis
A. Issues compromising advances in rhinosinusitis research
Summary Statements:
-
•
Rhinosinusitis definitions for clinical trials or epidemiologic surveys are largely proposed on an ad hoc basis.
-
•
The use of certain markers might be inappropriate as outcome variables in clinical trials because they correlate poorly with clinical end points, such as symptoms.
-
•
Rhinosinusitis trials need to be concerned with timing issues, such as seasonal patterns and the duration of acute versus chronic studies.
1. Definitions
Several consensus documents have been published in recent years that have attempted to define rhinosinusitis or sinusitis.12, 157, 159, 327 Individual articles have also attempted to develop definitions.257 Despite this, disease definitions for clinical trials or epidemiologic surveys are largely proposed on an ad hoc basis. For example, studies designed to demonstrate the efficacy of novel antibiotics frequently study subjects with acute symptoms combined with the presence of fluid in the maxillary sinuses as demonstrated on the basis of air-fluid levels on plain radiography or CT scanning. Given regulatory guidelines by which this class of drug is approved, this definition is understandable. However, many patients present with a similar spectrum of acute symptoms and are treated on an empiric basis without confirmatory imaging tests.160, 161, 162, 328, 329 On review of published clinical trials, it is clear that even for the acute maxillary paradigm listed above, inclusion and exclusion criteria are inconsistent in regard to the demographics of the populations studied, the medications prohibited during any study, and the range of concomitant medications permitted.
Without a consensus on definitions, it is not surprising that the basic epidemiology is unclear. In large epidemiologic surveys it might be sufficient for a patient to report a diagnosis of CRS to be included in that category. In others, a CT scan or other objective confirmation might be required. Similarly, a variety of outcome scales and instruments have been used.330, 331, 332, 333, 334, 335 These issues have been discussed in general reviews.108, 336, 337, 338
2. Placebos
There are 2 main issues surrounding placebos: ethics and technical feasibility. There are certain clear situations in which a placebo group would be unethical (eg, for an acute, severe bacterial infection for which antibiotic treatment is indicated). In this case a standard antibiotic is generally appropriately used as a positive control. In other cases of suspected acute bacterial sinusitis, a placebo-controlled trial might be reasonable if appropriate rescue measures are included to protect the patients in the trial. Discontinuation of patients from such a trial could be a valid efficacy outcome variable. In addition, some clinical trials have demonstrated that antibiotics are not effective for rhinosinusitis. In such cases, a placebo control group might be ethical. Also, when adjunctive or prophylactic agents are being evaluated, it is often possible to design an appropriate placebo-controlled study.
Technical difficulties can occur when evaluating different formulations, such as topical versus systemic agents or oral versus parenteral agents. When it is clear that there is no infection, the selection of a placebo group might depend on whether that group is adversely affected by allowing the underlying disease processes (eg, inflammation) to continue untreated. If the baseline variability of different study groups is poorly defined or the duration between discrete episodes is long, a placebo run-in period might not be feasible.
3. Topical agents
The effects of topical agents on the nose and sinuses should be considered during the conduct of a clinical trial. In several European studies evaluating treatment modalities for rhinosinusitis, the use of topical α-adrenergic agonists was permitted as concomitant medication (reference). This will confound the trial data because this class of drug has been shown to increase sinus ostial diameter. Even if the incidence of use of such medications in study groups is similar, their additive effect might not be separable from the effect of the study drug.
There is a large body of literature discussing the effects of preservatives on the nasal mucosa. The overall conclusions, derived from a combination of in vitro and animal studies, are somewhat controversial as applied to clinical practice. Because the ciliary activity might be reduced by infection per se, any added effect caused by preservative-containing topical compounds might be inconsequential when studying infectious rhinosinusitis.
Topical agents introduced into the nose do not pass retrogradely into the paranasal sinuses through an intact osteomeatal unit. Several imaging studies have been conducted that failed to demonstrate retrograde transport. This fact must be considered when interpreting the results of a study of a topical agent in rhinosinusitis. The outcomes might be different for those who have had sinus surgery compared with those who have not.
Studies administering saline by means of nasal instillation demonstrated reduction in nasal blood flow, as measured by means of laser-Doppler velocimetry.339 Because intranasal saline has been shown to have a mild decongestant action, the use of even seemingly benign sprays should be controlled in studies of rhinosinusitis.339 In addition, vehicles used in drugs such as polyethylene glycol act as wetting agents and can produce a beneficial effect on nasal symptoms. Testing therapeutic agents that use such vehicles require appropriate controls. Ultimately, the potential beneficial and adverse effects of all components of a drug product other than the drug itself should be taken into consideration when analyzing the value of compounds for the treatment of rhinosinusitis.
4. Systemic agents
The clinical effect of repeated doses of various medications might be different from the effect seen after administration of a single dose because of such events as receptor downregulation or induction of pharmacologic tolerance. Other issues compromising the use of systemic agents for rhinosinusitis include their adverse effects on other organs and problems with drug-drug interactions.
5. Outcome measures
The use of certain markers (eg, imaging studies) might be inappropriate as end points in clinical trials because they do not correlate well with clinical end points (ie, symptoms). In studies of therapeutic agents for the treatment of rhinitis, both individual and composite symptom scores are well accepted as end points. In studies of nonantibiotic drugs for the management of rhinosinusitis, a condition for which there is poor correlation between CT findings and symptoms, CT findings alone can not be used as a surrogate in assessing efficacy.340, 341
Physicians routinely treat the entities of acute and chronic rhinitis and rhinosinusitis empirically on the basis of symptomatic presentation.161, 162, 163, 329, 342, 343 In established patients with rhinosinusitis who experience exacerbations, physicians often diagnose and prescribe without physical examination because there is little evidence that physical examination is helpful in establishing the diagnosis in patients who present with typical symptoms of rhinosinusitis. If patients are refractory to therapy or if their symptoms are atypical, a detailed physical examination is generally indicated. In some cases referral to a specialist, with performance of radiographic imaging, flexible or rigid nasal endoscopy, or both, might be appropriate.344 As experience has evolved with the use of coronal CT imaging of the sinuses, it is now accepted that appropriate timing of a CT scan is crucial. Gwaltney et al26 showed that positive scans could be demonstrated in acute upper respiratory tract infection. For CRS, a scan taken to demonstrate the extent of residual disease after maximal pharmacotherapy, to define anatomy before surgery, or both is appropriate.162 There have been several attempts to devise staging systems to define the extent of disease.345 Most of these have been developed as a guide to surgical staging and have not been validated in the context of assessing the natural history of the disease or in assessing the effect of nonsurgical intervention.
6. Time course
Clinical trials need to be conducted when there is an increased incidence of upper respiratory tract infections to obtain an adequate number of clinical trial subjects with acute episodes of rhinosinusitis. The seasonal epidemiology of patients with CRS has not been well defined. Patients with an underlying allergic diathesis might experience exacerbations at the time that the allergens to which they are sensitive are present in the environment. There might be acute infectious episodes that present at the time of increased airway reactivity. This could be due to the predisposition induced by allergic inflammation or independent of these effects. For studies conducted in different parts of the world, the duration of seasonal allergies and the specific pollens will vary, leading to disparate clinical effects.
Studies up to 1 year have been reported to study the effects of interventions on the incidence of exacerbations. The environmental and other variables that might change during this prolonged study period could be difficult to assess and control. Similarly, when seeking a past history of acute rhinosinusitis episodes, it is frequently difficult to pinpoint discrete episodes and even more difficult to document whether they were acute infectious episodes. Such episodes might have been treated empirically or through telephone consultation, and there is rarely objective confirmation of an active pathogen. To be pragmatic, it is often reasonable to define a set of symptoms that are consistent with sinus infection and to assume that the patient can recognize these retrospectively to categorize the sinus infection. Furthermore, because current medical practice includes diagnosing and treating empirically, one option would be to design clinical trial protocols on the basis of this real-life scenario. Because there is limited understanding of the natural history of the various types of rhinosinusitis, the decisions about a specific end point and follow-up time frame after an intervention might be arbitrary.
7. Allergic reactivity
The effects of allergic inflammation on the paranasal sinuses are of great interest, but their full effect remains poorly understood However, there is no entity that would be currently characterized as allergic rhinosinusitis.346 Pelikan and Pelikan-Filipek289 described a series of cases that demonstrated acute reversible opacification of the maxillary sinuses after topical antigen challenge, but this study did not examine sinus cavities pathologically. Several studies reported a higher incidence of acute sinusitis episodes in patients with allergic rhinitis compared with those without allergic rhinitis.163 If there was a clear association between allergic rhinitis exacerbations and sinus infections, the effect of allergen immunotherapy would be important to study. Similarly, the effect of other immunomodulating interventions should be studied. Given these facts, there is reason to believe that immunotherapy could confound the assessment of other treatments under evaluation, especially if the allergen dose is not stable. The seasonal effects of immunotherapy on patients who enter long-term clinical trials have not been determined.
8. Surgical therapy
Assessing the effects of surgery on CRS poses a special challenge. There is a chance that a published case might represent the best results of an individual surgeon or group highly experienced in a given technique. Case series of bilateral intranasal sphenoethmoidectomy have been published.340, 342, 347, 348, 338 The outcomes of endoscopic surgery have also been well described in several studies.108, 285, 349, 350 It might be difficult to compare techniques and outcomes from different surgeons. One key point is that the surgical outcome is dependent on the degree of mucosal disease present before the operation.108 The indications for performing sinus surgery might also vary somewhat by case series, with some patients undergoing surgery despite relatively normal sinus appearance on the preoperative sinus CT scan. It is not clear how these results compare with each other, and it is still difficult for a medical practitioner to decide the basis for referring a given patient to a surgeon other than anecdotal satisfaction. Ethical considerations must be evaluated before doing a parallel-group clinical trial in human subjects, with one group getting sham surgery. One interesting study in nasal polyposis involved operating on an unaffected side.351 Although it is difficult to blind or sham control surgical treatment, it is possible to and important to consider randomization to surgery versus no surgery with available rescue medication over a period of time.
B. Developing effective drug trial schemes for rhinosinusitis
Summary Statements:
-
•
Most trials for acute rhinosinusitis will likely be carried out in a primary care office setting, where sophisticated diagnostic techniques, such as CT and MRI, might not be readily available, and therefore the medical history in particular, and sometimes the physical examination, should be primarily used to diagnose the condition.
-
•
Therapeutic efficacy must be demonstrated through adequate and well-controlled studies showing that the intervention will have the effect it purports.
-
•
The prospective choice of end points is a critical part of drug development; efficacy end points for trials that will form the basis of approval should be clinically relevant, validated, and direct.
The majority of rhinosinusitis trials for new forms of therapy or new indications for already existing treatments are done in patients with acute rhinosinusitis. CRS is a serious and often debilitating disease; however, the poor pharmacologic response rates seen in this patient population, as well as the lack of understanding about disease classification, have made accurate efficacy assessment difficult. Therefore clearer definitions and categories of CRS are urgently needed.
1. Targeting the appropriate patient population
Targeting the correct patient population is essential when designing a study for ensuring real-world assessment of an intervention. Researchers developing a clinical trial design need to set diagnostic parameters so that the patients who can provide the most meaningful clinical results can be included. In patients with rhinosinusitis, this might mean distinguishing between viral and bacterial disease, as well as acute rhinosinusitis and CRS. Most trials for acute rhinosinusitis will likely be carried out in a primary care office setting, where sophisticated diagnostic techniques, such as CT and MRI, might not be readily available. For the purposes of research, patients with symptoms lasting less than 10 days should not be included in trials on presumed bacterial infections because symptoms that resolve before 10 days are usually indicative of viral rhinosinusitis. Symptoms that should be evaluated include purulent drainage, nasal congestion, facial pain, and headache. Even if these criteria are applied, consideration must be given to the high rate of spontaneous resolution (approximately 50%) in the population with acute (presumed bacterial) rhinosinusitis.343 Therefore, unless a sample size is large enough to effectively power the study, the results might fail to demonstrate drug efficacy.
2. Study outcome variables
Pharmacologic studies for rhinosinusitis either involve symptom-relief drugs or curative drugs. For symptom relievers, such as corticosteroids, antihistamines, decongestants, or mucolytics, the primary outcome variables used to evaluate efficacy should be improvement in symptoms and signs. For curative drugs, such as antibiotics, outcome variables could be cure rate or failure or recurrence rate. Other important outcome measures for both types of drugs are time to improvement and number of symptom-free days. Disease burden and QOL can also be important assessments. Depending on the type of drug, a study could also be designed to evaluate prophylaxis, safety, or both.
3. General study design
Studies are most often designed to focus on efficacy. Such studies need to be randomized, double-blind, and controlled. Because a key measure of efficacy in this type of study is symptom resolution, a control group is essential to quantify the placebo effect. The duration of treatment depends on the disease under study. In an acute rhinosinusitis study the screening phase would be very short because of the acute nature of the disease, and the treatment period should also be brief (eg, 2 weeks). Studies in patients with CRS will require treatment for much longer, typically a month or more, and should differentiate between CRSsNP and CRSwNP, focusing on different symptom patterns. Studies assessing prophylaxis need to run at least 12 months. Once the treatment period has ended, a follow-up period is necessary to ensure symptoms are truly resolved.
4. Inclusion and exclusion criteria
Inclusion criteria include predefined age limits (for either an adult or pediatric study) and clearly defined symptoms of acute rhinosinusitis for 10 to 28 days. Common exclusion criteria include immunocompromised patients, patients with ciliary disorders or any sort of permanent local obstruction, and, if it is an acute study, patients with CRS. Other confounding factors that are usually excluded are seasonal pollen allergy, large nasal polyps, and atrophic rhinitis.
5. Efficacy and safety outcomes
Outcome measures will vary depending on the drug class being studied. Most existing sinusitis studies have focused on antibiotic therapy. As such, a large body of information is available as guidance for appropriate outcome measures for these drugs. However, recent studies have also been done on nonantibiotic regimens, such as intranasal steroids. Controlled trials need to show drug safety for use under labeling conditions and provide substantial evidence of efficacy for recommended use. When assessing the safety of a drug, much depends on the agent and the drug class. Trials evaluating an already approved medication for a new indication will need much less focus on safety than trials for new molecular entities or a drug that is first in that class.
The prospective choice of end points is a critical part of drug development. Efficacy end points for trials that will form the basis of approval should be clinically relevant, validated, and direct. Also, the methods used to make these measurements should be accurate, precise, reproducible, and responsive. Choice of efficacy end points earlier in development might differ and might even be a surrogate end point, depending on the phase of development, goals of the study, and rationale for decision making. Because there is limited experience with CRS studies, statistically significant differences from placebo will be important to demonstrate initially. Clinical relevance might be more difficult to quantify. A clinically meaningful effect could be the time to reduce or recover from symptoms when receiving a study drug versus placebo rather than the outcome at a certain time point.
A number of potential problems exist when designing a rhinosinusitis study. Until recently, there has been a lack of consensus regarding classification and definition of various types of rhinosinusitis, inability to diagnose rhinosinusitis with high specificity, and varying standards of care; all of these factors make design of an effective clinical study difficult. In many respects acute rhinosinusitis and recurrent rhinosinusitis are easier to study then CRS. The stage was set for further refinement of the definitions of CRS, as proposed in this document through the development of a consensus definition for CRS by the Chronic Rhinosinusitis Task Force of the Sinus and Allergy Health Partnership.352
During an acute rhinosinusitis study, evidence of efficacy depends on the drug itself. For an adjunct therapy, the study needs to show that the combination is better than the regimen to which the experimental agent is added. For a stand-alone nonantibiotic drug, the study needs to show that the experimental medication is better than placebo. For a stand-alone antibiotic drug, the study needs to show that the experimental antimicrobial agent is similar or superior to an approved antibiotic, as well as showing some evidence of bacteriological cure (www.fda.cder/guidance: Acute Bacterial Sinusitis). For acute bacterial rhinosinusitis studies, clinical outcomes are measured by clinical cure or clinical failure, for example, a 20% difference between an antibiotic and placebo as an outcome at the end of 10 days of therapy. Microbiologic outcomes are described as documented eradication, presumed eradication, documented persistence, or presumed persistence. During a recurrent rhinosinusitis study, efficacy variables can include time to recurrence (possibly primary variable), severity of recurrence (secondary variable), and frequency of recurrence (secondary variable). Finally, it is important to determine that end points used to assess efficacy should be carefully selected to avoid confounding factors. Investigators need to define the clinically significant difference for a particular study. The ways to define clinically significant difference include a distribution-based approach with a standardized response mean and effect size and the preferred anchor-based approach, using global ratings of change.
VIII. Rhinosinusitis consensus definitions and clinical trial guidelines
What follows are the consensus definitions and disease classifications for acute rhinosinusitis, CRS without nasal polyposis, CRS with nasal polyposis, and classic AFRS, as well as suggested study schemes. Separate definitions are outlined for research and patient care. It is important to keep in mind that the consensus recommendations are based on the experience of the authors, and they should not be assumed to represent the position of any regulatory body or to be complete or final. Much work needs to be done before definitive rhinosinusitis trial schemes are established; therefore future panels should be planned to further define and refine appropriate clinical trials.
A. Definition
The committee decided by consensus to accept the term rhinosinusitis instead of sinusitis throughout the document. Sinusitis is almost always accompanied by concurrent nasal airway inflammation, and in many cases, sinusitis is preceded by rhinitis symptoms. Therefore the use of the term rhinosinusitis more accurately describes the spectrum of infectious and inflammatory conditions previously grouped under the term sinusitis. The group agreed to endorse and adopt the previously developed definition of the Sinus and Allergy Health Partnership Task Force with the following definition for rhinosinusitis: “Rhinosinusitis is a group of disorders characterized by inflammation of the mucosa of the nose and the paranasal sinuses.”352
Although the participants recognize the advantages in using the term rhinosinusitis rather than sinusitis, the committee also wants to emphasize that the term rhinosinusitis is not intended to be confused with or replace the term rhinitis, which refers to the various diseases primarily, but not exclusively, confined to the nose. It is important to maintain the distinction between rhinosinusitis and rhinitis both diagnostically and therapeutically, even though the conditions might have overlapping symptoms and signs. For instance, although the use of an antibiotic might be very appropriate for a case of acute rhinosinusitis, it would be unusual for a rhinopathy. Health care professionals are keenly aware of the problem of overuse of antibiotics for upper respiratory tract infections and are committed to adding greater precision to the diagnosis of both acute rhinosinusitis and CRS. In promulgating the definitions proposed in this meeting, experts in the field will need to continually stress the distinguishing features of rhinosinusitis and the importance of applying targeted diagnostic criteria when making therapeutic decisions.
In defining rhinosinusitis, as well as determining the criteria required to secure each of the aforementioned diagnoses, many factors have been considered, including the temporal nature of these disorders, clinical presentation, imaging data, histopathologic findings, causative factors (eg, microorganisms, aspirin sensitivity, and allergy), and differences in therapy.
B. Rhinosinusitis consensus research definitions and clinical trial guidelines
The following sections will discuss the research definitions and clinical trial guidelines as agreed upon by group consensus (≥80% of committee members). For each condition, entrance diagnostic criteria are outlined, including the pattern of symptoms that defines each particular classification, the typical symptoms necessary to diagnose disease, and measures of objective criteria required. These conditions are defined as they typically appear in the community and might not encompass all clinical scenarios encountered (eg, immunocompromised host). Measures for monitoring progress to determine clinical efficacy are also provided. These evaluations include monitoring individual symptoms, rating global symptom severity, assessing QOL, documenting objective clinical trial findings, and rating global response to treatment. A summary of the clinical trial guidelines can be found in Table 18.
Table 18.
Rhinosinusitis consensus research definitions and clinical trial guidelines
| Type of rhinosinusitis |
||||
|---|---|---|---|---|
| Acute (presumed bacterial) rhinosinusitis | CRS without nasal polyposis | CRS with nasal polyposis | AFRS | |
| Criteria for diagnosis | ||||
| Pattern of symptoms |
|
Symptoms present for ≥12 wk | ||
| Symptoms for diagnosis | Requires:
|
Requires ≥2 of the following symptoms:
|
Requires ≥2 of the following symptoms:
|
Requires ≥1 of the following symptoms:
|
| Objective documentation | Requires either
|
Requires both
|
Requires both
|
Requires
|
Other possible, but not required, documentation measures:
|
||||
Patients who have intracranial extension, have orbital cellulitis, or require hospitalization are considered to have severe disease but should be excluded from clinical trials of uncomplicated acute (presumed bacterial) rhinosinusitis.
1. Acute (presumed bacterial) rhinosinusitis
Acute rhinosinusitis is an inflammatory condition involving the paranasal sinuses, as well as the lining of the nasal passages, and it lasts up to 4 weeks (28 days). In the immunocompetent person living in the general community, acute rhinosinusitis is typically believed to be induced by viruses and does not require antibiotics for the first 10 to 14 days unless complicating features are present, at which point bacteria are presumed to be involved and antibiotics are often employed. These complicating features include severe headache or facial pain, high fever, and impending or actual complications to the eye, lung, or brain. Without any complicating feature present, after 10 to 14 days of symptoms consistent with rhinosinusitis and objective findings, bacteria are presumed to predominate, and the patient might benefit from initiating appropriate antibiotic therapy.353 Patients with acute rhinosinusitis typically present with varying degrees of the following symptoms: anterior purulent drainage, posterior purulent drainage, or both plus nasal obstruction, facial pain-pressure-fullness, or both. Relative to nasal inflammation, hyposmia can be present. Purulence arising from the sinonasal passages must be present to ensure this diagnosis. The nature of predominating organisms (viruses, bacteria, or fungi) in the immunocompromised host and intensive care unit patient are considered to be more variable, and these patients are not the target population of these definitions and clinical trial recommendations.
a. Research criteria for diagnosis
Patients with acute (presumed bacterial) rhinosinusitis must have symptoms present for a minimum of 10 days up to a maximum of 28 days. Additional individuals who have patterns that might qualify for inclusion are patients with severe disease who have the presence of nasal or postnasal purulent secretions for 3 to 4 days with high fever and patients whose symptoms initially regress but then worsen within the first 10 days. Symptoms required for diagnosis include anterior purulent drainage, posterior purulent drainage, or both plus nasal obstruction or facial pain-pressure-fullness. Patients who experience orbital cellulites or intracranial extension of the infection or who require hospitalization are considered to have severe disease and should be excluded automatically from clinical trials of uncomplicated acute (presumed bacterial) rhinosinusitis. Objective documentation for the diagnosis is required by either nasal airway examination for purulent drainage or radiographic evidence of acute rhinosinusitis. Purulent drainage should be noted beyond the nasal vestibule by means of either anterior rhinoscopy or endoscopy or as posterior pharyngeal drainage. Regarding imaging, plain sinus films, although certainly less costly, do have limitations and are generally less reliable than CT or MRI but might be adequate for an acute rhinosinusitis study.
b. Measures for monitoring progress in research setting
Individual symptoms that should be included in outcomes monitoring include drainage (anterior, posterior, or both), nasal obstruction, facial pain-pressure-fullness, diminished sense of smell, headache, ear pain-pressure, halitosis, dental pain-pressure, cough, fatigue, fever, and sleep disturbance. Individual symptoms should be rated on a set categoric scale. Symptom severity should also be rated on a global scale. For example, patients can be asked, “Overall, how bothered are you by your symptoms?” Optional responses would be as follows: 1, not bothered; 2, bothered a little; 3, bothered more than a little but not a lot; 4, bothered a lot; or 5, extremely bothered. Although standardized subjective QOL measurements play an important role in assessing a drug’s effectiveness in clinical trials, there was no consensus agreement as to whether to mandate QOL assessments for all trials of acute (presumed bacterial) rhinosinusitis.
Several objective evaluations should be used for monitoring efficacy. A physical examination is essential. Objective documentation should also be provided on the basis of either (1) a nasal airway examination for purulent drainage beyond the nasal vestibule by means of either anterior rhinoscopy or endoscopy or posterior pharyngeal drainage or (2) imaging by means of plain radiography or CT. Another objective measure that might be useful is obtaining and assessing bacterial cultures. Bacterial cultures were strongly recommended for studies of antibiotic treatment and provide valuable information for any therapeutic trial of acute bacterial rhinosinusitis. Finally, researchers can use the patient’s subjective global rating of response to treatment. For example, in response to the question, “Overall, how would you rate your response to treatment?,” patients could answer using a categorical scale as follows: −4, as bad as can be; −3, a lot worse; −2, more than a little worse; −1, a little worse; 0, same; +1, a little better; +2, more than a little better; +3, a lot better; or +4, as good as can be.
2. CRS with and without nasal polyposis
These are inflammatory conditions involving the paranasal sinuses, as well as the lining of the nasal passages that persist beyond 12 weeks. The diagnosis of CRS with or without nasal polyposis requires that symptoms must be present for 12 weeks or more. When 2 or more of the following symptoms are present, CRS might be strongly suspected: anterior mucopurulent drainage, posterior mucopurulent drainage, or both; nasal obstruction; facial pain-pressure-fullness; and decreased sense of smell. Objective documentation is required by means of direct visualization of the middle meatus through anterior rhinoscopy (after decongestion) or nasal endoscopy to assert the accurate diagnosis of CRS. Bilateral NPs are recorded as absent or present in the middle meatus to distinguish between CRSwNP and CRSsNP. Although physical examination could reveal unilateral polyposis, and this could represent CRSwNP, this unilateral appearance should always herald the suspicion of inverted papilloma or other sinonasal tumor. Thus in this clinical setting, an imaging study should be strongly considered (see below). In the absence of polyps, signs of inflammation, such as discolored mucus (not blood) or edema of the middle meatus or ethmoid area, must be seen to assert the diagnosis of CRS. A positive sinus CT scan is required for the research definition of both CRSsNP and CRSwNP. Rarely, incidental imaging findings can be used to make the diagnosis of CRS independent of symptoms and physical examination, but imaging studies alone might not be able to determine the presence or absence of polyps.
a. Research criteria for diagnosis of CRSsNP
Again, symptoms must be present for 12 weeks or more. Two or more of the following symptoms are required for diagnosis: anterior mucopurulent drainage, posterior mucopurulent drainage, or both; nasal obstruction; and facial pain-pressure-fullness. Required objective documentation requires endoscopy to exclude the presence of NPs and to document signs of inflammation, such as discolored mucus or edema of the middle meatus or ethmoid area. A positive imaging study by means of sinus CT is also required (see imaging section for criteria set forth).
b. Measures for monitoring progress for CRSsNP
Individual symptoms that should be included in end point monitoring are drainage (anterior or posterior), nasal obstruction, facial pain-pressure-fullness, diminished sense of smell, headache, ear pain-pressure, halitosis, dental pain-pressure, cough, fatigue, fever, and sleep disturbance. Individual symptoms should be rated on a set categoric scale. Global symptom severity should also be rated as outlined in the section “Acute (presumed bacterial) rhinosinusitis.” A validated QOL measurement should be performed to monitor progress. Optional recommended instruments include SF-36, SNOT-20, and RSDI.
Several objective measures can be used for determination of efficacy. A physical examination is essential. Objective documentation requires repeating endoscopy and sinus CT scan. The group did not reach consensus on any particular endoscopic or radiographic scoring system. Other potentially useful objective measures that might be useful include nasal patency measurements (which must be interpreted in light of lung function), such as the peak nasal inspiratory flow (PNIF); acoustic rhinometry; rhinomanometry; a smell identification test, quantification test, or both; a measure of mucociliary function; assessments of the cytologic pattern; and measurements of inflammatory factors in nasal mucus or epithelial samples. Finally, researchers can use the patient’s subjective global rating of response to treatment as outlined in the section “Acute (presumed bacterial) rhinosinusitis.”
c. Research criteria for diagnosis of CRSwNP
By definition, patients with the diagnosis of CRS with nasal polyposis require the presence of symptoms for 12 weeks or more. Two or more of the following symptoms are required for diagnosis: anterior mucopurulent drainage, posterior mucopurulent drainage, or both; nasal obstruction; and decreased sense of smell. Objective documentation requires both endoscopy to confirm the presence of bilateral polyps and imaging by means of CT with confirmation of bilateral mucosal disease.
d. Measures for monitoring progress of CRSwNP
Individual symptoms that should be included in end point monitoring are drainage (anterior or posterior), nasal obstruction, facial pain-pressure-fullness, diminished sense of smell, headache, ear pain-pressure, halitosis, dental pain-pressure, cough, fatigue, fever, and sleep disturbance. Individual symptoms should be rated on a categoric scale. Global symptom severity should also be rated as outlined previously. A validated QOL measurement should be performed. The group did not reach a consensus on which questionnaire should be used to monitor progress. Optional recommended instruments include SF-36, SNOT-20, and RSDI.
Several objective measures should be used for monitoring efficacy. A physical examination is essential. Required objective measures include endoscopy to assess the magnitude of NPs. Imaging should also be performed by means of CT to measure any changes in extent of disease. Nasal patency measurements (which must be interpreted in light of lung function), such as PNIF, acoustic rhinometry, and rhinomanometry, might also be useful. Other objective measures that might be useful include a smell identification test, a smell quantification test, or both; a measure of mucociliary function; assessments of the cytologic pattern; and measurements of inflammatory factors in nasal mucus or epithelial samples. Finally, the patient’s subjective global rating of response to treatment should be included as outlined previously.
3. Classic AFRS
AFRS is clinically diagnosed by meeting the criteria for CRS (with or without polyps) while demonstrating the presence of allergic mucin and evidence of fungal hypersensitivity by means of skin testing or in vitro blood testing. Positive fungal cultures, characteristic CT studies, and absence of tissue invasion in the immunocompetent host are not required to secure the diagnosis. However, these tests (cultures, CT, and pathology) are recommended for complete evaluation of these patients. Anecdotally, it is reported that should a patient become immunocompromised, AFRS condition could predispose the patient to acute invasive fungal rhinosinusitis.
a. Research criteria for diagnosis of classic AFRS
The diagnosis of AFRS requires that symptoms must be present for 12 weeks or more. One or more of the following symptoms are required for diagnosis: anterior nasal drainage, posterior nasal drainage, or both; nasal obstruction; decreased sense of smell; and facial pain-pressure-fullness. Required objective documentation includes endoscopy to document the presence of inflammation, such as edema of the middle meatus or ethmoid area, or NPs. Critical to the establishment of the diagnosis of AFRS is the identification of allergic mucin (histologically containing fungal hyphae and degranulating eosinophils). Imaging studies, by means of either CT or MRI, are required. These occasionally show pathognomonic features of AFRS but do so in less than 50% of cases. For consistency, the group agreed that sinus CT or MRI findings of sinus mucosal disease or sinus opacification must be present at some stage, such as preoperatively, but these features do not need to be present postoperatively. This might apply for instance, in a drug treatment trial, in cases in which the disease was clearly present before surgery and the other criteria for disease are met. Other required criteria include evidence of fungal-specific IgE (by means of skin testing or in vitro blood testing) and absence of histologic evidence of invasive fungal disease in sinus tissue. Other potentially useful but not required diagnostic criteria include a positive fungal culture result from sinus mucus, an increased total serum IgE level, and imaging by more than one technique (CT or MRI).
b. Measures for monitoring progress of classic AFRS
Individual symptoms that should be included in outcomes monitoring are drainage (anterior or posterior), nasal obstruction, facial pain-pressure-fullness, diminished sense of smell, headache, ear pain-pressure, halitosis, dental pain-pressure, cough, fatigue, fever, and sleep disturbance. Individual symptoms should be rated on a set scale. Global symptom severity should also be rated as outlined previously. A validated QOL measurement should be performed to monitor progress. Optional recommended instruments include SF-36, SNOT-20, and RSDI. Although a standardized QOL assessment should be included, the group did not reach a consensus on any one specific instrument.
Several objective measures of assessment should be used for determination of efficacy. A physical examination is essential. Objective assessments should include endoscopy, reviewing the initially described characteristics and grading the appearance of NPs. The group did not reach consensus on any particular endoscopic scoring systems. Imaging by means of CT or MRI should also be repeated. The group did not reach consensus for an image scoring system. Other potentially useful objective measures include the use of nasal patency measurements (which must be interpreted in light of lung function), such as PNIF; acoustic rhinometry; rhinomanometry; smell identification testing, smell quantification testing, or both; mucociliary function; assessments of the cytologic pattern; and measurements of inflammatory factors in nasal mucus or epithelial samples. A response to treatment global rating score should be included as outlined previously.
C. Rhinosinusitis patient care definitions
These definitions are summarized in Table 19. They differ from the research definitions only in terms of the objective documentation required for diagnosis as explained below.
Table 19.
Rhinosinusitis consensus definitions for patient care
| Type of rhinosinusitis |
||||
|---|---|---|---|---|
| Acute (presumed bacterial) rhinosinusitis | CRS without nasal polyposis | CRS with nasal polyposis | AFRS | |
| Criteria for diagnosis | ||||
| Pattern of symptoms |
|
• Symptoms present for ≥12 wk | ||
| Symptoms for diagnosis | Requires:
|
Requires ≥2 of the following symptoms:
|
Requires ≥2 of the following symptoms:
|
Requires ≥1 of the following symptoms:
|
| Objective documentation | Requires either
|
|
|
Requires
|
| Sinus CT imaging is not essential but is highly recommended because of tendency for bony erosions and extension of disease into adjacent anatomic areas. | ||||
Other possible, but not required, documentation measures:
|
||||
Patients who have intracranial extension, have orbital cellulitis, or require hospitalization are considered to have severe disease.
However, imaging studies alone might not be able to determine the presence or absence of polyps.
1. Acute (presumed bacterial) rhinosinusitis
The objective criteria are the same as those for the research definition.
2. CRS with and without nasal polyposis
For clinical diagnosis, sinus CT imaging is not essential but should be strongly considered. If symptoms or findings are equivocal, a sinus CT scan can confirm the diagnosis. Rarely, incidental imaging findings can be used to make the diagnosis of CRS independent of symptoms and physical examination, but imaging studies alone might not be able to determine the presence or absence of polyps.
3. Classic AFRS
Sinus CT imaging is not essential but is highly recommended because of the tendency for bony erosions and extension of disease into adjacent anatomic areas.
IX. Future directions
This conference focused on the development of definitions and clinical trial designs for 4 classifications of rhinosinusitis that encompass a large number of patients. However, guidelines still need to be refined and developed for other populations, including patients with acute presumed viral rhinosinusitis, unresolved or subacute rhinosinusitis, recurrent acute rhinosinusitis, acute exacerbations of CRS, and eosinophilic fungal rhinosinusitis. Furthermore, the benefits and risks of various interventions were not a focus of these proceedings.
Rhinosinusitis is complex. The understanding of it is still limited. Developing sound clinical trials that target its various causes will help clinicians gain a better understanding of how to effectively prevent and treat the detrimental health consequences associated with rhinosinusitis.
Acknowledgments
The leadership of the participating organizations would like to especially thank Drs Meltzer and Hamilos for the amount of personal time and effort devoted to the development and completion of this project. The editors and participating organizations are also grateful to the AAAAI for organizing this conference and to the national and international experts who participated. We are also very appreciative of Schering’s unrestricted grant to fund the meeting and the publication of the conference’s proceedings. We would like to thank Jerome Schultz for his administrative contributions for the meeting and publications, and Maria Bavishi for assistance in developing this consensus document, as well as Dr Jens Ponikau for providing an additional review of this manuscript.
Footnotes
This document was edited by Dr Nicklas in his private capacity and not in his capacity as a Medical Officer with the United States Food and Drug Administration (FDA). No official support or endorsement by the FDA is intended or should be inferred.
Disclosure of potential conflict of interest: E. O. Meltzer has consulting arrangements with Alcon, Altana, AstraZeneca, Aventis, Dey, Genentech, GlaxoSmithKline, Merck, Medpointe, Novartis, Pfizer, Schering-Plough, and Wyeth; receives grants-research support from Alcon, Altana, AstraZeneca, Aventis, Dey, Genentech, GlaxoSmithKline, Merck, Novartis, Pfizer, and Schering-Plough; and is on the Speaker’s Bureau for Aventis, AstraZeneca, Genentech, GlaxoSmithKline, Merck, Pfizer, and Schering-Plough, D. L. Hamilos—none disclosed. J. Hadley has consultant arrangements with Abbott, Aventis, Bayer, GlaxoSmithKline, Merck, GE Medical Systems, Pfizer, and Schering-Plough and is on the Speaker’s Bureau for Abbott, Aventis, Bayer, GlaxoSmithKline, Merck, Pfizer, and Schering-Plough. D. Lanza is on the Speaker’s Bureau for AstraZeneca. B. F. Marple has consulting arrangements with Alcon, Aventis, and Bayer and receives grants-research support from Aventis, GlaxoSmithKline, Abbott, Merck, and Bayer. R. Nicklas—none disclosed. C. Bachert—none disclosed. J. Baraniuk has consultant arrangements with Roche, Centecor, GlaxoSmithKline, Astra-Zeneca, and Aventis; has patent-licensing arrangements with Georgetown University; receives grants-research support from the National Institutes of Health; and is employed by Georgetown University. F. M. Baroody is on the Speaker’s Bureau for Merck & Co, Inc, and GlaxoSmithKline. M. S. Benninger has consultant arrangements with Aventis, GlaxoSmithKline, Ortho-McNeil, Abbott, and Bayer; receives grants-research support from Novartis and Bayer; is on the Speaker’s Bureau for Aventis and GlaxoSmithKline. I. Brook is on the Speaker’s Bureau for Abbott. B. A. Chowdhury—none disclosed. H. M. Druce is employed by Phys Inc. S. Durham has consultant arrangements with ALK Abello, UCB, and GlaxoSmithKline; receives grants-research support from ALK Abello and GlaxoSmithKline; and is on the Speaker’s Bureau for ALK Abello, UCB, Aventis, and WAO. B. Ferguson has consulting arrangements with Aventis, Abbott, and GlaxoSmithKline; is on the Speaker’s Bureau for Aventis, Astra Zeneca, Abbott, Merck, GlaxoSmithKline, and Pfizer; and is an advisor for Pfizer. J. M. Gwaltney, Jr—none disclosed. M. Kaliner has consultant arrangements with Aventis, Novartis, GlaxoSmithKline, Genentech, and Abbott; receives grants-research support from Astra Zeneca, Aventis, Boehringer-Ingelheim, GlaxoSmithKline, and Schering-Plough; is on the Speaker’s Bureau for Aventis, Abbott, GlaxoSmithKline, Medpointe, Novartis, and Genentech. D. Kennedy has consulting arrangements with Aventis, Novartis, Medtronic-Xomed, and Schering-Plough; received grants-research support from Novartis; and has patent-licensing arrangements with Medtronic-Xomed. V. Lund has consulting arrangements with Schering-Plough and Bayer. R. Naclerio has consulting arrangements with Merck and Schering-Plough; receives grants-research support from Corixa and Merck; and is on the Speaker’s Bureau for Merck and GlaxoSmithKline. R. Pawankar—none disclosed. J. F. Piccirillo—none disclosed. P. Rohane is employed by Celgene Corp. R. Simon—none disclosed. R. Slavin has consultant arrangements with Aventis; receives grants-research support from GlaxoSmithKline, Genentech, and AstraZeneca; and is on the Speaker’s Bureau of Aventis, Schering-Plough, Genentech, and AstraZeneca. A. Togias has consultant arrangement with AstraZeneca, Genentech, GlaxoSmithKline, Novartis, and Merck; receives grants-research support from Merck and Genentech; and is on the Speaker’s Bureau for Genentech, Merck, Novartis, Pfizer, and UCB. E. R. Wald receives grants-research support from Aventis, GlaxoSmithKline, Wyeth, Abbott, and Medimmune. J. Zinreich—none disclosed.
References
- 1.Post J.C. Direct evidence of bacterial biofilms in otitis media. Laryngoscope. 2001;111:2083–2094. doi: 10.1097/00005537-200112000-00001. [DOI] [PubMed] [Google Scholar]
- 2.Khalid A.N., Hunt J., Perloff J.R., Kennedy D.W. The role of bone in chronic rhinosinusitis. Laryngoscope. 2002;112:1951–1957. doi: 10.1097/00005537-200211000-00008. [DOI] [PubMed] [Google Scholar]
- 3.Kennedy D.W., Senior B.A., Gannon F.H., Montone K.T., Hwang P., Lanza D.C. Histology and histomorphometry of ethmoid bone in chronic rhinosinusitis. Laryngoscope. 1998;108:502–507. doi: 10.1097/00005537-199804000-00008. [DOI] [PubMed] [Google Scholar]
- 4.Perloff J.R., Gannon F.H., Bolger W.E., Montone K.T., Orlandi R., Kennedy D.W. Bone involvement in sinusitis: an apparent pathway for the spread of disease. Laryngoscope. 2000;110:2095–2099. doi: 10.1097/00005537-200012000-00023. [DOI] [PubMed] [Google Scholar]
- 5.Hofer M.F., Harbeck R.J., Schlievert P.M., Leung D.Y. Staphylococcal toxins augment specific IgE responses by atopic patients exposed to allergen. J Invest Dermatol. 1999;112:171–176. doi: 10.1046/j.1523-1747.1999.00492.x. [DOI] [PubMed] [Google Scholar]
- 6.Jabara H.H., Geha R.S. The superantigen toxic shock syndrome toxin-1 induces CD40 ligand expression and modulates IgE isotype switching. Int Immunol. 1996;8:1503–1510. doi: 10.1093/intimm/8.10.1503. [DOI] [PubMed] [Google Scholar]
- 7.Vennewald I., Henker M., Klemm E., Seebacher Fungal colonization of the paranasal sinuses. Mycosis. 1999;42:33–36. [PubMed] [Google Scholar]
- 8.Ponikau J.U., Sherris D.A., Kern E.B. The diagnosis and incidence of allergic fungal sinusitis. Mayo Clin Proc. 1999;74:877–884. doi: 10.4065/74.9.877. [DOI] [PubMed] [Google Scholar]
- 9.Catten M., Murr A., Goldstein J., Mhatre A., Lalwani A. Detection of fungi in the nasal mucosal using polymerase chain reaction. Laryngoscope. 2001;111:399–403. doi: 10.1097/00005537-200103000-00006. [DOI] [PubMed] [Google Scholar]
- 10.International Rhinosinusitis Advisory Board Infectious rhinosinusitis in adults: classification, etiology and management. Ear Nose Throat J. 1997;76:5–22. [PubMed] [Google Scholar]
- 11.Medicode. ICD-9-CM Expert for Hospitals. 6th ed. Ingenix-St Anthony’s Press; 2003. p. 137–l9.
- 12.Lanza D.C., Kennedy D.W. Adult rhinosinusitis defined. Otolaryngol Head Neck Surg. 1997;117(suppl):S1–S7. doi: 10.1016/S0194-59989770001-9. [DOI] [PubMed] [Google Scholar]
- 13.Bachert C., Gevaert P., Holtappels G., Johansson S.G., van Cauwenberge P. Total and specific IgE in nasal polyps is related to local eosinophilic inflammation. J Allergy Clin Immunol. 2001;107:607–614. doi: 10.1067/mai.2001.112374. [DOI] [PubMed] [Google Scholar]
- 14.Bone R.C., Balk R.A., Cerra F.B., The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Chest. 1992;101:1644–1655. doi: 10.1378/chest.101.6.1644. [DOI] [PubMed] [Google Scholar]
- 15.Chensue S., Ward P. Inflammation. In: Damjanov I., Linder J., editors. Anderson’s pathology. 10th ed. Mosby; St Louis: 1996. [Google Scholar]
- 16.Collins T. Acute and chronic inflammation. In: Cotran R., Kumar V., Collins T., editors. Robbins’ pathologic basis of disease. W. B. Saunders; Philadelphia: 1999. [Google Scholar]
- 17.Szekanecz Z., Koch A. Inflammation: cells, cytokines, and other mediators. In: Humes H., editor. Kelley’s textbook of internal medicine. 4th edition. Lippincott Williams & Wilkins; Philadelphia: 2000. [Google Scholar]
- 18.Gwaltney J. Historical eras of the common cold. In: Sande M., Root T., editors. Contemporary issues in infectious disease. Churchill Livingstone; New York: 1993. pp. 1–13. [Google Scholar]
- 19.Gwaltney J., Heinz B. Rhinovirus. In: Richman D., Whitley R., Hayden F., editors. Clinical virology. 2nd ed. ASM Press; Portland (OR): 2002. pp. 995–1018. [Google Scholar]
- 20.Hendley J.O., Gwaltney J.M., Jr. Mechanisms of transmission of rhinovirus infections. Epidemiol Rev. 1988;10:243–258. [PubMed] [Google Scholar]
- 21.Winther B., Gwaltney J.M., Jr, Mygind N., Turner R.B., Hendley J.O. Sites of rhinovirus recovery after point inoculation of the upper airway. JAMA. 1986;256:1763–1767. [PubMed] [Google Scholar]
- 22.Winther B., Innes D.J. The human adenoid. A morphologic study. Arch Otolaryngol Head Neck Surg. 1994;120:144–149. doi: 10.1001/archotol.1994.01880260016004. [DOI] [PubMed] [Google Scholar]
- 23.Winther B., Greve J.M., Gwaltney J.M., Jr Surface expression of intercellular adhesion molecule 1 on epithelial cells in the human adenoid. J Infect Dis. 1997;176:523–525. doi: 10.1086/517280. [DOI] [PubMed] [Google Scholar]
- 24.Gwaltney J.M., Jr, Hayden F.G. Psychological stress and the common cold. N Engl J Med. 1992;326:644–646. doi: 10.1056/NEJM199202273260915. [DOI] [PubMed] [Google Scholar]
- 25.Harris J.M., 2nd, Gwaltney J.M., Jr. Incubation periods of experimental rhinovirus infection and illness. Clin Infect Dis. 1996;23:1287–1290. doi: 10.1093/clinids/23.6.1287. [DOI] [PubMed] [Google Scholar]
- 26.Gwaltney J.M., Jr, Phillips C.D., Miller R.D., Riker D.K. Computed tomographic study of the common cold. N Engl J Med. 1994;330:25–30. doi: 10.1056/NEJM199401063300105. [DOI] [PubMed] [Google Scholar]
- 27.Puhakka T., Makela M.J., Alanen A. Sinusitis in the common cold. J Allergy Clin Immunol. 1998;102:403–408. doi: 10.1016/S0091-6749(98)70127-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kristo A., Uhari M., Luotonen J. Paranasal sinus findings in children during respiratory infection evaluated with magnetic resonance imaging. Pediatrics. 2003;111:e586–e589. doi: 10.1542/peds.111.5.e586. [DOI] [PubMed] [Google Scholar]
- 29.Mogensen C., Tos M. Quantitative histology of the maxillary sinus. Rhinology. 1977;15:129–140. [PubMed] [Google Scholar]
- 30.Mogensen C., Tos M. Quantitative histology of the normal sphenoidal sinus. Rhinology. 1978;16:203–213. [PubMed] [Google Scholar]
- 31.Gwaltney J.M., Jr, Hendley J.O., Phillips C.D., Bass C.R., Mygind N., Winther B. Nose blowing propels nasal fluid into the paranasal sinuses. Clin Infect Dis. 2000;30:387–391. doi: 10.1086/313661. [DOI] [PubMed] [Google Scholar]
- 32.Gwaltney J.M., Jr. Acute community-acquired sinusitis. Clin Infect Dis. 1996;23:1209–1225. doi: 10.1093/clinids/23.6.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gwaltney J.M., Jr, Wiesinger B.A., Patrie J.T. Acute community-acquired bacterial sinusitis: the value of antimicrobial treatment and the natural history. Clin Infect Dis. 2004;38:227–233. doi: 10.1086/380641. [DOI] [PubMed] [Google Scholar]
- 34.Arruda E., Boyle T.R., Winther B., Pevear D.C., Gwaltney J.M., Jr, Hayden F.G. Localization of human rhinovirus replication in the upper respiratory tract by in situ hybridization. J Infect Dis. 1995;171:1329–1333. doi: 10.1093/infdis/171.5.1329. [DOI] [PubMed] [Google Scholar]
- 35.Evans F.O., Jr, Sydnor J.B., Moore W.E. Sinusitis of the maxillary antrum. N Engl J Med. 1975;293:735–739. doi: 10.1056/NEJM197510092931502. [DOI] [PubMed] [Google Scholar]
- 36.Wald E.R., Milmoe G.J., Bowen A., Ledesma-Medina J., Salamon N., Bluestone C.D. Acute maxillary sinusitis in children. N Engl J Med. 1981;304:749–754. doi: 10.1056/NEJM198103263041302. [DOI] [PubMed] [Google Scholar]
- 37.Wald E.R., Reilly J.S., Casselbrant M. Treatment of acute maxillary sinusitis in childhood: a comparative study of amoxicillin and cefaclor. J Pediatr. 1984;104:297–302. doi: 10.1016/s0022-3476(84)81018-5. [DOI] [PubMed] [Google Scholar]
- 38.Wald E.R., Byers C., Guerra N., Casselbrant M., Beste D. Subacute sinusitis in children. J Pediatr. 1989;115:28–32. doi: 10.1016/s0022-3476(89)80324-5. [DOI] [PubMed] [Google Scholar]
- 39.Brook I. Bacteriologic features of chronic sinusitis in children. JAMA. 1981;246:967–969. [PubMed] [Google Scholar]
- 40.Brook I., Yocum P., Shah K. Aerobic and anaerobic bacteriology of concurrent chronic otitis media with effusion and chronic sinusitis in children. Arch Otolaryngol Head Neck Surg. 2000;126:174–176. doi: 10.1001/archotol.126.2.174. [DOI] [PubMed] [Google Scholar]
- 41.Otten F.W., Grote J.J. Treatment of chronic maxillary sinusitis in children. Int J Pediatr Otorhinolaryngol. 1988;15:269–278. doi: 10.1016/0165-5876(88)90082-1. [DOI] [PubMed] [Google Scholar]
- 42.Otten F.W. Conservative treatment of chronic maxillary sinusitis in children. Long-term follow-up. Acta Otorhinolaryngol Belg. 1997;51:173–175. [PubMed] [Google Scholar]
- 43.Orobello P.W., Jr, Park R.I., Belcher L.J. Microbiology of chronic sinusitis in children. Arch Otolaryngol Head Neck Surg. 1991;117:980–983. doi: 10.1001/archotol.1991.01870210052007. [DOI] [PubMed] [Google Scholar]
- 44.Don D.M., Yellon R.F., Casselbrant M.L., Bluestone C.D. Efficacy of a stepwise protocol that includes intravenous antibiotic therapy for the management of chronic sinusitis in children and adolescents. Arch Otolaryngol Head Neck Surg. 2001;127:1093–1098. doi: 10.1001/archotol.127.9.1093. [DOI] [PubMed] [Google Scholar]
- 45.Muntz H.R., Lusk R.P. Bacteriology of the ethmoid bullae in children with chronic sinusitis. Arch Otolaryngol Head Neck Surg. 1991;117:179–181. doi: 10.1001/archotol.1991.01870140067008. [DOI] [PubMed] [Google Scholar]
- 46.Tinkelman D.G., Silk H.J. Clinical and bacteriologic features of chronic sinusitis in children. Am J Dis Child. 1989;143:938–941. doi: 10.1001/archpedi.1989.02150200098025. [DOI] [PubMed] [Google Scholar]
- 47.Slack C.L., Dahn K.A., Abzug M.J., Chan K.H. Antibiotic-resistant bacteria in pediatric chronic sinusitis. Pediatr Infect Dis J. 2001;20:247–250. doi: 10.1097/00006454-200103000-00006. [DOI] [PubMed] [Google Scholar]
- 48.Felmingham D., Washington J. Trends in the antimicrobial susceptibility of bacterial respiratory tract pathogens—findings of the Alexander Project 1992–1996. J Chemother. 1999;11(suppl 1):5–21. doi: 10.1179/joc.1999.11.Supplement-2.5. [DOI] [PubMed] [Google Scholar]
- 49.Pfaller M.A., Jones R.N., Doern G.V., Kugler K. Bacterial pathogens isolated from patients with bloodstream infection: frequencies of occurrence and antimicrobial susceptibility patterns from the SENTRY antimicrobial surveillance program (United States and Canada, 1997) Antimicrob Agents Chemother. 1998;42:1762–1770. doi: 10.1128/aac.42.7.1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bach A., Boehrer H., Schmidt H., Geiss H.K. Nosocomial sinusitis in ventilated patients. Nasotracheal versus orotracheal intubation. Anaesthesia. 1992;47:335–339. doi: 10.1111/j.1365-2044.1992.tb02177.x. [DOI] [PubMed] [Google Scholar]
- 51.Mevio E., Benazzo M., Quaglieri S., Mencherini S. Sinus infection in intensive care patients. Rhinology. 1996;34:232–236. [PubMed] [Google Scholar]
- 52.O’Reilly M.J., Reddick E.J., Black W. Sepsis from sinusitis in nasotracheally intubated patients. A diagnostic dilemma. Am J Surg. 1984;147:601–604. doi: 10.1016/0002-9610(84)90122-3. [DOI] [PubMed] [Google Scholar]
- 53.Arens J.F., LeJeune F.E., Jr, Webre D.R. Maxillary sinusitis, a complication of nasotracheal intubation. Anesthesiology. 1974;40:415–416. doi: 10.1097/00000542-197404000-00024. [DOI] [PubMed] [Google Scholar]
- 54.Caplan E.S., Hoyt N.J. Nosocomial sinusitis. JAMA. 1982;247:639–641. [PubMed] [Google Scholar]
- 55.Kronberg F.G., Goodwin W.J., Jr. Sinusitis in intensive care unit patients. Laryngoscope. 1985;95:936–938. doi: 10.1288/00005537-198508000-00010. [DOI] [PubMed] [Google Scholar]
- 56.Miner J.D., Elliott C.L., Johnson C.W., McSoley T., Spahn J.G., Spahn T. Nosocomial sinusitis. Indiana Med. 1988;81:684–686. [PubMed] [Google Scholar]
- 57.Doyle P.W., Woodham J.D. Evaluation of the microbiology of chronic ethmoid sinusitis. J Clin Microbiol. 1991;29:2396–2400. doi: 10.1128/jcm.29.11.2396-2400.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hoyt W.H., 3rd Bacterial patterns found in surgery patients with chronic sinusitis. J Am Osteopath Assoc. 1992;92:209–212. [PubMed] [Google Scholar]
- 59.Hsu J., Lanza D.C., Kennedy D.W. Antimicrobial resistance in bacterial chronic sinusitis. Am J Rhinol. 1998;12:243–248. doi: 10.2500/105065898781390055. [DOI] [PubMed] [Google Scholar]
- 60.Biel M.A., Brown C.A., Levinson R.M. Evaluation of the microbiology of chronic maxillary sinusitis. Ann Otol Rhinol Laryngol. 1998;107:942–945. doi: 10.1177/000348949810701107. [DOI] [PubMed] [Google Scholar]
- 61.Brook I., Frazier E.H. Correlation between microbiology and previous sinus surgery in patients with chronic maxillary sinusitis. Ann Otol Rhinol Laryngol. 2001;110:148–151. doi: 10.1177/000348940111000210. [DOI] [PubMed] [Google Scholar]
- 62.Jiang R.S., Lin J.F., Hsu C.Y. Correlation between bacteriology of the middle meatus and ethmoid sinus in chronic sinusitis. J Laryngol Otol. 2002;116:443–446. doi: 10.1258/0022215021911248. [DOI] [PubMed] [Google Scholar]
- 63.Finegold S.M., Flynn M.J., Rose F.V. Bacteriologic findings associated with chronic bacterial maxillary sinusitis in adults. Clin Infect Dis. 2002;35:428–433. doi: 10.1086/341899. [DOI] [PubMed] [Google Scholar]
- 64.Gordts F., Halewyck S., Pierard D., Kaufman L., Clement P.A. Microbiology of the middle meatus: a comparison between normal adults and children. J Laryngol Otol. 2000;114:184–188. doi: 10.1258/0022215001905292. [DOI] [PubMed] [Google Scholar]
- 65.Nadel D.M., Lanza D.C., Kennedy D.W. Endoscopically guided cultures in chronic sinusitis. Am J Rhinol. 1998;12:233–241. doi: 10.2500/105065898781390000. [DOI] [PubMed] [Google Scholar]
- 66.Frederick J., Braude A. Anaerobic infections of the paranasal sinuses. N Engl J Med. 1974;290:135–137. doi: 10.1056/NEJM197401172900304. [DOI] [PubMed] [Google Scholar]
- 67.Carenfelt C., Lundberg C., Nord C.E., Wretlind B. Bacteriology of maxillary sinusitis in relation to quality of the retained secretion. Acta Otolaryngol. 1978;86:298–302. doi: 10.3109/00016487809124750. [DOI] [PubMed] [Google Scholar]
- 68.Brook I. Bacteriology of chronic maxillary sinusitis in adults. Ann Otol Rhinol Laryngol. 1989;98:426–428. doi: 10.1177/000348948909800605. [DOI] [PubMed] [Google Scholar]
- 69.Mustafa E., Tahsin A., Mustafa Ö., Nedret K. Bacteriology of antrum in adults with chronic maxillary sinusitis. Laryngoscope. 1994;104:321–324. doi: 10.1288/00005537-199403000-00013. [DOI] [PubMed] [Google Scholar]
- 70.Nord C.E. The role of anaerobic bacteria in recurrent episodes of sinusitis and tonsillitis. Clin Infect Dis. 1995;20:1512–1524. doi: 10.1093/clinids/20.6.1512. [DOI] [PubMed] [Google Scholar]
- 71.Klossek J.M., Dubreuil L., Richet H., Richet B., Beutter P. Bacteriology of chronic purulent secretions in chronic rhinosinusitis. J Laryngol Otol. 1998;112:1162–1166. doi: 10.1017/s0022215100142732. [DOI] [PubMed] [Google Scholar]
- 72.Van Cauwenberge P.B., Ingels K.J., Bachert C., Wang D.Y. Microbiology of chronic sinusitis. Acta Otorhinolaryngol Belg. 1997;51:239–246. [PubMed] [Google Scholar]
- 73.Wald E.R. Chronic sinusitis in children. J Pediatr. 1995;127:339–347. doi: 10.1016/s0022-3476(95)70061-7. [DOI] [PubMed] [Google Scholar]
- 74.Stringer S., Ryan M.W. Chronic invasive fungal rhinosinusitis. Otolaryngol Clin North Am. 2000;33:375–387. doi: 10.1016/s0030-6665(00)80012-2. [DOI] [PubMed] [Google Scholar]
- 75.Schell W.A. Unusual fungal pathogens in fungal rhinosinusitis. Otolaryngol Clin North Am. 2000;33:367–373. doi: 10.1016/s0030-6665(00)80011-0. [DOI] [PubMed] [Google Scholar]
- 76.Miloshev B., Davidson C.M., Gentles J.C., Sandison A.T. Aspergilloma of paranasal sinuses and orbit in Northern Sudanese. Lancet. 1966;1:746–747. doi: 10.1016/s0140-6736(66)90898-1. [DOI] [PubMed] [Google Scholar]
- 77.Marple B.F., Mabry R.L. Allergic fungal sinusitis: learning from our failures. Am J Rhinol. 2000;14:223–226. doi: 10.2500/105065800779954482. [DOI] [PubMed] [Google Scholar]
- 78.Horst M., Hejjaoui A., Horst V., Michel F.B., Bousquet J. Double-blind, placebo-controlled rush immunotherapy with a standardized Alternaria extract. J Allergy Clin Immunol. 1990;85:460–472. doi: 10.1016/0091-6749(90)90156-x. [DOI] [PubMed] [Google Scholar]
- 79.Downs S., Mitkakis T., Marks G. Clinical importance of Alternaria exposure in children. Am J Respir Crit Care Med. 2001;164:455–459. doi: 10.1164/ajrccm.164.3.2008042. [DOI] [PubMed] [Google Scholar]
- 80.Manning S.C., Vuitch F., Weinberg A.G., Brown O.E. Allergic aspergillosis: a newly recognized form of sinusitis in the pediatric population. Laryngoscope. 1989;99:681–685. doi: 10.1288/00005537-198907000-00003. [DOI] [PubMed] [Google Scholar]
- 81.Marple B.F., Gibbs S.R., Newcomer M.T., Mabry R.L. Allergic fungal sinusitis-induced visual loss. Am J Rhinol. 1999;13:191–195. doi: 10.2500/105065899781389740. [DOI] [PubMed] [Google Scholar]
- 82.Manning S.C., Schaefer S.D., Close L.G., Vuitch F. Culture-positive allergic fungal sinusitis. Arch Otolaryngol Head Neck Surg. 1991;117:174–178. doi: 10.1001/archotol.1991.01870140062007. [DOI] [PubMed] [Google Scholar]
- 83.Marple B. Allergic fungal sinusitis. Curr Opin Otolaryngol. 1999;7:383–387. [Google Scholar]
- 84.Manning S.C., Holman M. Further evidence for allergic pathophysiology in allergic fungal sinusitis. Laryngoscope. 1998;108:1485–1496. doi: 10.1097/00005537-199810000-00012. [DOI] [PubMed] [Google Scholar]
- 85.Mukherji S.K., Figueroa R.E., Ginsberg L.E. Allergic fungal sinusitis: CT findings. Radiology. 1998;207:417–422. doi: 10.1148/radiology.207.2.9577490. [DOI] [PubMed] [Google Scholar]
- 86.Nussenbaum B., Marple B.F., Schwade N.D. Characteristics of bony erosion in allergic fungal rhinosinusitis. Otolaryngol Head Neck Surg. 2001;124:150–154. doi: 10.1067/mhn.2001.112573. [DOI] [PubMed] [Google Scholar]
- 87.Zinreich S.J., Kennedy D.W., Malat J. Fungal sinusitis: diagnosis with CT and MR imaging. Radiology. 1988;169:439–444. doi: 10.1148/radiology.169.2.3174990. [DOI] [PubMed] [Google Scholar]
- 88.Manning S.C., Merkel M., Kriesel K., Vuitch F., Marple B. Computed tomography and magnetic resonance diagnosis of allergic fungal sinusitis. Laryngoscope. 1997;107:170–176. doi: 10.1097/00005537-199702000-00007. [DOI] [PubMed] [Google Scholar]
- 89.Mabry R.L., Manning S. Radioallergosorbent microscreen and total immunoglobulin E in allergic fungal sinusitis. Otolaryngol Head Neck Surg. 1995;113:721–723. doi: 10.1016/S0194-59989570011-0. [DOI] [PubMed] [Google Scholar]
- 90.Manning S.C., Mabry R.L., Schaefer S.D., Close L.G. Evidence of IgE-mediated hypersensitivity in allergic fungal sinusitis. Laryngoscope. 1993;103:717–721. doi: 10.1288/00005537-199307000-00002. [DOI] [PubMed] [Google Scholar]
- 91.Chrzanowski R.R., Rupp N.T., Kuhn F.A., Phillips A.E., Dolen W.K. Allergenic fungi in allergic fungal sinusitis. Ann Allergy Asthma Immunol. 1997;79:431–435. doi: 10.1016/S1081-1206(10)63039-6. [DOI] [PubMed] [Google Scholar]
- 92.Schubert M.S., Goetz D.W. Evaluation and treatment of allergic fungal sinusitis. I. Demographics and diagnosis. J Allergy Clin Immunol. 1998;102:387–394. doi: 10.1016/s0091-6749(98)70125-3. [DOI] [PubMed] [Google Scholar]
- 93.Marple B.F., Mabry R.L. Comprehensive management of allergic fungal sinusitis. Am J Rhinol. 1998;12:263–268. doi: 10.2500/105065898781390037. [DOI] [PubMed] [Google Scholar]
- 94.Corey J.P. Allergic fungal sinusitis. Otolaryngol Clin North Am. 1992;25:225–230. [PubMed] [Google Scholar]
- 95.Schnadig V.J., Rassekh C.H., Gourley W.K. Allergic fungal sinusitis. A report of two cases with diagnosis by intraoperative aspiration cytology. Acta Cytol. 1999;43:268–272. doi: 10.1159/000330991. [DOI] [PubMed] [Google Scholar]
- 96.Torres C., Ro J.Y., el-Naggar A.K., Sim S.J., Weber R.S., Ayala A.G. Allergic fungal sinusitis: a clinicopathologic study of 16 cases. Hum Pathol. 1996;27:793–799. doi: 10.1016/s0046-8177(96)90451-7. [DOI] [PubMed] [Google Scholar]
- 97.Miller J., Johnston A., Lamb D. Allergic aspergillosis of the maxillary sinuses. Proc Scott Thorac Soc. 1981;36:710–715. [Google Scholar]
- 98.Lamb D., Miller J., Johnston A. Allergic aspergillosis of the paranasal sinuses. J Pathol. 1982;137:56. [Google Scholar]
- 99.Katzenstein A.L., Sale S.R., Greenberger P.A. Allergic Aspergillus sinusitis: a newly recognized form of sinusitis. J Allergy Clin Immunol. 1983;72:89–93. doi: 10.1016/0091-6749(83)90057-x. [DOI] [PubMed] [Google Scholar]
- 100.Taylor M.J., Ponikau J.U., Sherris D.A. Detection of fungal organisms in eosinophilic mucin using a fluorescein-labeled chitin-specific binding protein. Otolaryngol Head Neck Surg. 2002;127:377–383. doi: 10.1067/mhn.2002.128896. [DOI] [PubMed] [Google Scholar]
- 101.Loury M.C., Leopold D.A., Schaefer S.D. Allergic Aspergillus sinusitis. Arch Otolaryngol Head Neck Surg. 1993;119:1042–1043. doi: 10.1001/archotol.1993.01880210136019. [DOI] [PubMed] [Google Scholar]
- 102.Bent J.P., 3rd, Kuhn F.A. Diagnosis of allergic fungal sinusitis. Otolaryngol Head Neck Surg. 1994;111:580–588. doi: 10.1177/019459989411100508. [DOI] [PubMed] [Google Scholar]
- 103.Ponikau J.U., Sherris D.A., Kita H., Kern E.B. Intranasal antifungal treatment in 51 patients with chronic rhinosinusitis. J Allergy Clin Immunol. 2002;110:862–866. doi: 10.1067/mai.2002.130051. [DOI] [PubMed] [Google Scholar]
- 104.Kita H. Presented at: 57th AAAAI Annual Meeting; March 2001; New Orleans (LA).
- 105.Berrettini S., Carabelli A., Sellari-Franceschini S. Perennial allergic rhinitis and chronic sinusitis: correlation with rhinologic risk factors. Allergy. 1999;54:242–248. doi: 10.1034/j.1398-9995.1999.00813.x. [DOI] [PubMed] [Google Scholar]
- 106.Holzmann D., Willi U., Nadal D. Allergic rhinitis as a risk factor for orbital complication of acute rhinosinusitis in children. Am J Rhinol. 2001;15:387–390. [PubMed] [Google Scholar]
- 107.Blair C., Nelson M., Thompson K. Allergic inflammation enhances bacterial sinusitis in mice. J Allergy Clin Immunol. 2001;108:424–429. doi: 10.1067/mai.2001.117793. [DOI] [PubMed] [Google Scholar]
- 108.Kennedy D.W., Wright E.D., Goldberg A.N. Objective and subjective outcomes in surgery for chronic sinusitis. Laryngoscope. 2000;110:29–31. doi: 10.1097/00005537-200003002-00008. [DOI] [PubMed] [Google Scholar]
- 109.Settipane G.A., Chafee F.H. Nasal polyps in asthma and rhinitis. A review of 6,037 patients. J Allergy Clin Immunol. 1977;59:17–21. doi: 10.1016/0091-6749(77)90171-3. [DOI] [PubMed] [Google Scholar]
- 110.Keith P.K., Conway M., Evans S. Nasal polyps: effects of seasonal allergen exposure. J Allergy Clin Immunol. 1994;93:567–574. doi: 10.1016/s0091-6749(94)70068-0. [DOI] [PubMed] [Google Scholar]
- 111.Drake-Lee A.B., McLaughlan P. Clinical symptoms, free histamine and IgE in patients with nasal polyposis. Int Arch Allergy Appl Immunol. 1982;69:268–271. doi: 10.1159/000233182. [DOI] [PubMed] [Google Scholar]
- 112.Bernstein J.M., Ballow M., Schlievert P.M., Rich G., Allen C., Dryja D. A superantigen hypothesis for the pathogenesis of chronic hyperplastic sinusitis with massive nasal polyposis. Am J Rhinol. 2003;17:321–326. [PubMed] [Google Scholar]
- 113.Bachert C., van Cauwenberge P. Nasal polyposis and sinusitis. In: Adkinson N., Yunginger J., Busse W., Bochner B., Holgate S., Simons E., editors. Allergy: principles and practice. 6th ed. Mosby; St Louis: 2003. [Google Scholar]
- 114.Demoly P., Crampette L., Mondain M., Enander I., Jones I., Bousquet J. Myeloperoxidase and interleukin-8 levels in chronic sinusitis. Clin Exp Allergy. 1997;27:672–675. [PubMed] [Google Scholar]
- 115.Ghaffar O., Durham S., Al-Ghamdi K. Expression of IgE heavy chain transcripts in the sinus mucosa of atopic and nonatopic patients with chronic sinusitis. Am J Respir Cell Mol Biol. 1998;18:706–711. doi: 10.1165/ajrcmb.18.5.3030. [DOI] [PubMed] [Google Scholar]
- 116.Rhyoo C., Sanders S.P., Leopold D.A., Proud D. Sinus mucosal IL-8 gene expression in chronic rhinosinusitis. J Allergy Clin Immunol. 1999;103:395–400. doi: 10.1016/s0091-6749(99)70462-8. [DOI] [PubMed] [Google Scholar]
- 117.Nonoyama T., Harada T., Shinogi J., Yoshimura E., Sakakura Y. Immunohistochemical localization of cytokines and cell adhesion molecules in maxillary sinus mucosa in chronic sinusitis. Auris Nasus Larynx. 2000;27:51–58. doi: 10.1016/s0385-8146(99)00042-5. [DOI] [PubMed] [Google Scholar]
- 118.Bachert C., Wagenmann M., Rudack C. The role of cytokines in infectious sinusitis and nasal polyposis. Allergy. 1998;53:2–13. doi: 10.1111/j.1398-9995.1998.tb03767.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Rudack C., Stoll W., Bachert C. Cytokines in nasal polyposis, acute and chronic sinusitis. Am J Rhinol. 1998;12:383–388. doi: 10.2500/105065898780708008. [DOI] [PubMed] [Google Scholar]
- 120.Bachert C., Wagenmann M., Hauser U., Rudack C. IL-5 synthesis is upregulated in human nasal polyp tissue. J Allergy Clin Immunol. 1997;99:837–842. doi: 10.1016/s0091-6749(97)80019-x. [DOI] [PubMed] [Google Scholar]
- 121.Roben P.W., Salem A.N., Silverman G.J. VH3 family antibodies bind domain D of staphylococcal protein A. J Immunol. 1995;154:6437–6445. [PubMed] [Google Scholar]
- 122.Bachert C., Gevaert P., van Cauwenberge P. Staphylococcus aureus enterotoxins: a key in airway disease? Allergy. 2002;57:480–487. doi: 10.1034/j.1398-9995.2002.02156.x. [DOI] [PubMed] [Google Scholar]
- 123.Bachert C., Gevaert P., Howarth P., Holtappels G., van Cauwenberge P., Johansson S.G. IgE to Staphylococcus aureus enterotoxins in serum is related to severity of asthma. J Allergy Clin Immunol. 2003;111:1131–1132. [PubMed] [Google Scholar]
- 124.Baraniuk J.N. Neurogenic mechanisms in rhinosinusitis. Curr Allergy Asthma Rep. 2001;1:252–261. doi: 10.1007/s11882-001-0016-4. [DOI] [PubMed] [Google Scholar]
- 125.Baraniuk J., Staevska M. Perennial nonallergic rhinitis. In: Lichtenstein L., Busse W., Geha R., editors. Current therapy in allergy immunology and rheumatology. 6th ed. Mosby; St Louis: 2003. [Google Scholar]
- 126.Biedlingmaier J.F., Trifillis A. Comparison of CT scan and electron microscopic findings on endoscopically harvested middle turbinates. Otolaryngol Head Neck Surg. 1998;118:165–173. doi: 10.1016/S0194-5998(98)80005-3. [DOI] [PubMed] [Google Scholar]
- 127.Barrow C. Hemisphere Publishing; Washington (DC): 1986. Toxicology of the nasal passages. [Google Scholar]
- 128.Baraniuk J., Naranch K., Maibach H., Clauw D. Irritant rhinitis in allergic, nonallergic, control and chronic fatigue syndrome populations. J Chronic Fatigue Syndr. 2000;7:3–31. [Google Scholar]
- 129.Baraniuk J., Naranch K., Maibach H., Clauw D. Tobacco sensitivity in chronic fatigue syndrome. J Chronic Fatigue Syndr. 2000;7:33–52. [Google Scholar]
- 130.Baraniuk J.N., Ali M., Yuta A., Fang S.Y., Naranch K. Hypertonic saline nasal provocation stimulates nociceptive nerves, substance P release, and glandular mucous exocytosis in normal humans. Am J Respir Crit Care Med. 1999;160:655–662. doi: 10.1164/ajrccm.160.2.9805081. [DOI] [PubMed] [Google Scholar]
- 131.Gunthorpe M.J., Benham C.D., Randall A., Davis J.B. The diversity in the vanilloid (TRPV) receptor family of ion channels. Trends Pharmacol Sci. 2002;23:183–191. doi: 10.1016/s0165-6147(02)01999-5. [DOI] [PubMed] [Google Scholar]
- 132.Naranch K., Park Y.J., Repka-Ramirez M.S., Velarde A., Clauw D., Baraniuk J.N. A tender sinus does not always mean rhinosinusitis. Otolaryngol Head Neck Surg. 2002;127:387–397. doi: 10.1067/mhn.2002.129038. [DOI] [PubMed] [Google Scholar]
- 133.Sanico A.M., Koliatsos V.E., Stanisz A.M., Bienenstock J., Togias A. Neural hyperresponsiveness and nerve growth factor in allergic rhinitis. Int Arch Allergy Immunol. 1999;118:154–158. doi: 10.1159/000024054. [DOI] [PubMed] [Google Scholar]
- 134.Stevenson D., Simon R. Sensitivity to aspirin and non-steroidal anti-inflammatory drugs. In: Middleton E., Reed C., Ellis E., Adkinson N., Yunginger J., Busse W., editors. Allergy: principles and practice. 6th ed. Mosby; St Louis: 2003. [Google Scholar]
- 135.Bianco S., Petrini G. Aspirin induced tolerance in aspirin-asthma detected by a new challenge test. J Med Sci. 1977;5:129–136. [Google Scholar]
- 136.Schmitz-Schumann V., Juhl E., Costabel U. Analgesic asthma-provocation challenge with acetylsalicylic acid. Atemw Lungenkrkh Jahrgang. 1985;10:479–485. [Google Scholar]
- 137.Phillips G.D., Foord R., Holgate S.T. Inhaled lysine-aspirin as a bronchoprovocation procedure in aspirin-sensitive asthma: its repeatability, absence of a late-phase reaction, and the role of histamine. J Allergy Clin Immunol. 1989;84:232–241. doi: 10.1016/0091-6749(89)90330-8. [DOI] [PubMed] [Google Scholar]
- 138.Melillo G., Padovano A., Cocco G., Masi C. Dosimeter inhalation test with lysine acetylsalicylate for the detection of aspirin-induced asthma. Ann Allergy. 1993;71:61–65. [PubMed] [Google Scholar]
- 139.Milewski M., Mastalerz L., Nizankowska E., Szczeklik A. Nasal provocation test with lysine-aspirin for diagnosis of aspirin-sensitive asthma. J Allergy Clin Immunol. 1998;101:581–586. doi: 10.1016/S0091-6749(98)70163-0. [DOI] [PubMed] [Google Scholar]
- 140.Patriarca G., Nucera E., DiRienzo V., Schiavino D., Pellegrino S., Fais G. Nasal provocation test with lysine acetylsalicylate in aspirin-sensitive patients. Ann Allergy. 1991;67:60–62. [PubMed] [Google Scholar]
- 141.Pawlowicz A., Williams W.R., Davies B.H. Inhalation and nasal challenge in the diagnosis of aspirin-induced asthma. Allergy. 1991;46:405–409. doi: 10.1111/j.1398-9995.1991.tb04218.x. [DOI] [PubMed] [Google Scholar]
- 142.Szczeklik A., Serwonska M. Inhibition of idiosyncratic reactions to aspirin in asthmatic patients by clemastine. Thorax. 1979;34:654–657. doi: 10.1136/thx.34.5.654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Nizankowska E., Szczeklik A. Glucocorticosteroids attenuate aspirin-precipitated adverse reactions in aspirin-intolerant patients with asthma. Ann Allergy. 1989;63:159–162. [PubMed] [Google Scholar]
- 144.Pauls J.D., Simon R.A., Daffern P.J., Stevenson D.D. Lack of effect of the 5-lipoxygenase inhibitor zileuton in blocking oral aspirin challenges in aspirin-sensitive asthmatics. Ann Allergy Asthma Immunol. 2000;85:40–45. doi: 10.1016/S1081-1206(10)62432-5. [DOI] [PubMed] [Google Scholar]
- 145.Stevenson D.D., Simon R.A., Mathison D.A., Christiansen S.C. Montelukast is only partially effective in inhibiting aspirin responses in aspirin-sensitive asthmatics. Ann Allergy Asthma Immunol. 2000;85:477–482. doi: 10.1016/S1081-1206(10)62575-6. [DOI] [PubMed] [Google Scholar]
- 146.Koufman J.A. Laryngopharyngeal reflux is different from classic gastroesophageal reflux disease. Ear Nose Throat J. 2002;81:7–9. [PubMed] [Google Scholar]
- 147.Koufman J.A., Belafsky P.C., Bach K.K., Daniel E., Postma G.N. Prevalence of esophagitis in patients with pH-documented laryngopharyngeal reflux. Laryngoscope. 2002;112:1606–1609. doi: 10.1097/00005537-200209000-00014. [DOI] [PubMed] [Google Scholar]
- 148.Richter J.E. Extraesophageal presentations of gastroesophageal reflux disease: an overview. Am J Gastroenterol. 2000;95(suppl):S1–S3. doi: 10.1016/s0002-9270(00)01071-6. [DOI] [PubMed] [Google Scholar]
- 149.Theodoropoulos D.S., Ledford D.K., Lockey R.F. Prevalence of upper respiratory symptoms in patients with symptomatic gastroesophageal reflux disease. Am J Respir Crit Care Med. 2001;164:72–76. doi: 10.1164/ajrccm.164.1.2006002. [DOI] [PubMed] [Google Scholar]
- 150.Richter J.E. Extraesophageal presentations of gastroesophageal reflux disease. Semin Gastrointest Dis. 1997;8:75–89. [PubMed] [Google Scholar]
- 151.Orlando R.C. The pathogenesis of gastroesophageal reflux disease: the relationship between epithelial defense, dysmotility, and acid exposure. Am J Gastroenterol. 1997;92(Suppl 4):3S–5S. [PubMed] [Google Scholar]
- 152.Richter J.E., Bradley L.A., DeMeester T.R., Wu W.C. Normal 24-hr ambulatory esophageal pH values. Influence of study center, pH electrode, age, and gender. Dig Dis Sci. 1992;37:849–856. doi: 10.1007/BF01300382. [DOI] [PubMed] [Google Scholar]
- 153.Belafsky P.C., Postma G.N., Amin M.R. Symptoms and findings of laryngopharyngeal reflux. Ear Nose Throat J. 2002;81(9 Suppl 2):10–13. [PubMed] [Google Scholar]
- 154.Vaezi M.F., Schroeder P.L., Richter J.E. Reproducibility of proximal probe pH parameters in 24-hour ambulatory esophageal pH monitoring. Am J Gastroenterol. 1997;92:825–829. [PubMed] [Google Scholar]
- 155.Phipps C.D., Wood W.E., Gibson W.S., Cochran W.J. Gastroesophageal reflux contributing to chronic sinus disease in children: a prospective analysis. Arch Otolaryngol Head Neck Surg. 2000;126:831–836. doi: 10.1001/archotol.126.7.831. [DOI] [PubMed] [Google Scholar]
- 156.DiBaise J.K., Olusola B.F., Huerter J.V., Quigley E.M. Role of GERD in chronic resistant sinusitis: a prospective, open label, pilot trial. Am J Gastroenterol. 2002;97:843–850. doi: 10.1111/j.1572-0241.2002.05598.x. [DOI] [PubMed] [Google Scholar]
- 157.Spector S.L., Bernstein I.L., Li J.T. Parameters for the diagnosis and management of sinusitis. J Allergy Clin Immunol. 1998;102(suppl):S107–S144. doi: 10.1016/s0091-6749(98)70045-4. [DOI] [PubMed] [Google Scholar]
- 158.Bachert C., Verhaeghe B. Differential diagnosis of rhinosinusitis. Fam Prac Recert. 2002;24(suppl):S8–S13. [Google Scholar]
- 159.Kaliner M.A., Osguthorpe J.D., Fireman P. Sinusitis: bench to bedside. Current findings, future directions. J Allergy Clin Immunol. 1997;99(suppl):S829–S848. [PubMed] [Google Scholar]
- 160.Jones N.S. Current concepts in the management of paediatric rhinosinusitis. J Laryngol Otol. 1999;113:1–9. doi: 10.1017/s0022215100143038. [DOI] [PubMed] [Google Scholar]
- 161.Desrosiers M., Frenkiel S., Hamid Q.A. Acute bacterial sinusitis in adults: management in the primary care setting. J Otolaryngol. 2002;31(suppl 2):2S–14S. [PubMed] [Google Scholar]
- 162.Mucha S.M., Baroody F.M. Sinusitis update. Curr Opin Allergy Clin Immunol. 2003;3:33–38. doi: 10.1097/00130832-200302000-00006. [DOI] [PubMed] [Google Scholar]
- 163.Dykewicz M.S. 7. Rhinitis and sinusitis. J Allergy Clin Immunol. 2003;111(suppl):S520–S529. doi: 10.1067/mai.2003.82. [DOI] [PubMed] [Google Scholar]
- 164.Malekzadeh S., Hamburger M.D., Whelan P.J., Biedlingmaier J.F., Baraniuk J.N. Density of middle turbinate subepithelial mucous glands in patients with chronic rhinosinusitis. Otolaryngol Head Neck Surg. 2002;127:190–195. doi: 10.1067/mhn.2002.126800. [DOI] [PubMed] [Google Scholar]
- 165.May M., Levine H., Schaitkin B. Results of surgery. In: Levine H., May M., editors. Rhinology and sinusology. Thieme Medical Publishing; New York: 1993. pp. 105–125. [Google Scholar]
- 166.Berger G., Kattan A., Bernheim J., Ophir D. Polypoid mucosa with eosinophilia and glandular hyperplasia in chronic sinusitis: a histopathological and immunohistochemical study. Laryngoscope. 2002;112:738–745. doi: 10.1097/00005537-200204000-00026. [DOI] [PubMed] [Google Scholar]
- 167.Kramer M.F., Ostertag P., Pfrogner E., Rasp G. Nasal interleukin-5, immunoglobulin E, eosinophilic cationic protein, and soluble intercellular adhesion molecule-1 in chronic sinusitis, allergic rhinitis, and nasal polyposis. Laryngoscope. 2000;110:1056–1062. doi: 10.1097/00005537-200006000-00031. [DOI] [PubMed] [Google Scholar]
- 168.Eichel B.S. A proposal for a staging system for hyperplastic rhinosinusitis based on the presence or absence of intranasal polyposis. Ear Nose Throat J. 1999;78 268–52. [PubMed] [Google Scholar]
- 169.Fang S.Y., Jin Y.T. Application of endoscopic sinus surgery to primary atrophic rhinitis? A clinical trial. Rhinology. 1998;36:122–127. [PubMed] [Google Scholar]
- 170.Georgitis J.W., Matthews B.L., Stone B. Chronic sinusitis: characterization of cellular influx and inflammatory mediators in sinus lavage fluid. Int Arch Allergy Immunol. 1995;106:416–421. doi: 10.1159/000236875. [DOI] [PubMed] [Google Scholar]
- 171.Stierna P., Carlsoo B. Histopathological observations in chronic maxillary sinusitis. Acta Otolaryngol. 1990;110:450–458. doi: 10.3109/00016489009107468. [DOI] [PubMed] [Google Scholar]
- 172.Jankowski R., Bouchoua F., Coffinet L., Vignaud J.M. Clinical factors influencing the eosinophil infiltration of nasal polyps. Rhinology. 2002;40:173–178. [PubMed] [Google Scholar]
- 173.Stoop A.E., van der Heijden H.A., Biewenga J., van der Baan S. Eosinophils in nasal polyps and nasal mucosa: an immunohistochemical study. J Allergy Clin Immunol. 1993;91:616–622. doi: 10.1016/0091-6749(93)90267-j. [DOI] [PubMed] [Google Scholar]
- 174.Kakoi H., Hiraide F. A histological study of formation and growth of nasal polyps. Acta Otolaryngol. 1987;103:137–144. doi: 10.3109/00016488709134709. [DOI] [PubMed] [Google Scholar]
- 175.Taylor M. Histochemical studies on nasal polypi. J Laryngol Otol. 1963;77:326–341. doi: 10.1017/s0022215100060692. [DOI] [PubMed] [Google Scholar]
- 176.Mygind N., Dahl R., Bachert C. Nasal polyposis, eosinophil dominated inflammation, and allergy. Thorax. 2000;55(suppl 2):S79–S83. doi: 10.1136/thorax.55.suppl_2.S79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Bachert C., Gevaert P., Holtappels G., Cuvelier C., van Cauwenberge P. Nasal polyposis: from cytokines to growth. Am J Rhinol. 2000;14:279–290. doi: 10.2500/105065800781329573. [DOI] [PubMed] [Google Scholar]
- 178.Persson C. Mucosal exudation mechanisms. Allergy Clin Immunol News. 1991;5:142. [Google Scholar]
- 179.Hamilos D.L., Leung D.Y., Wood R. Chronic hyperplastic sinusitis: association of tissue eosinophilia with mRNA expression of granulocyte-macrophage colony-stimulating factor and interleukin-3. J Allergy Clin Immunol. 1993;92:39–48. doi: 10.1016/0091-6749(93)90035-e. [DOI] [PubMed] [Google Scholar]
- 180.Hamilos D.L. Nasal polyps as immunoreactive tissue. Allergy Asthma Proc. 1996;17:293–296. doi: 10.2500/108854196778662200. [DOI] [PubMed] [Google Scholar]
- 181.Bucholtz G.A., Salzman S.A., Bersalona F.B. PCR analysis of nasal polyps, chronic sinusitis, and hypertrophied turbinates for DNA encoding bacterial 16S rRNA. Am J Rhinol. 2002;16:169–173. [PubMed] [Google Scholar]
- 182.Hamilos D.L., Leung D.Y., Muro S. GRbeta expression in nasal polyp inflammatory cells and its relationship to the anti-inflammatory effects of intranasal fluticasone. J Allergy Clin Immunol. 2001;108:59–68. doi: 10.1067/mai.2001.116428. [DOI] [PubMed] [Google Scholar]
- 183.Seki H., Otsuka H., Pawankar R. Nippon Jibiinkoka Gakkai Kaiho. 1992;95:1012–1021. doi: 10.3950/jibiinkoka.95.1012. [Studies on the function of mast cells infiltrating in nasal polyps] [DOI] [PubMed] [Google Scholar]
- 184.Sanchez-Segura A., Brieva J.A., Rodriguez C. T lymphocytes that infiltrate nasal polyps have a specialized phenotype and produce a mixed TH1/TH2 pattern of cytokines. J Allergy Clin Immunol. 1998;102:953–960. doi: 10.1016/s0091-6749(98)70333-1. [DOI] [PubMed] [Google Scholar]
- 185.Hamilos D.L., Leung D.Y., Huston D.P., Kamil A., Wood R., Hamid Q. GM-CSF, IL-5 and RANTES immunoreactivity and mRNA expression in chronic hyperplastic sinusitis with nasal polyposis (NP) Clin Exp Allergy. 1998;28:1145–1152. doi: 10.1046/j.1365-2222.1998.00380.x. [DOI] [PubMed] [Google Scholar]
- 186.Hamilos D.L., Leung D.Y., Wood R. Eosinophil infiltration in nonallergic chronic hyperplastic sinusitis with nasal polyposis (CHS/NP) is associated with endothelial VCAM-1 upregulation and expression of TNF-alpha. Am J Respir Cell Mol Biol. 1996;15:443–450. doi: 10.1165/ajrcmb.15.4.8879177. [DOI] [PubMed] [Google Scholar]
- 187.Jahnsen F.L., Haraldsen G., Aanesen J.P., Haye R., Brandtzaeg P. Eosinophil infiltration is related to increased expression of vascular cell adhesion molecule-1 in nasal polyps. Am J Respir Cell Mol Biol. 1995;12:624–632. doi: 10.1165/ajrcmb.12.6.7539273. [DOI] [PubMed] [Google Scholar]
- 188.Beck L.A., Stellato C., Beall L.D. Detection of the chemokine RANTES and endothelial adhesion molecules in nasal polyps. J Allergy Clin Immunol. 1996;98:766–780. doi: 10.1016/s0091-6749(96)70126-4. [DOI] [PubMed] [Google Scholar]
- 189.Nonaka M., Pawankar R., Saji F., Yagi T. Eotaxin synthesis by nasal polyp fibroblasts. Acta Otolaryngol. 1999;119:816–820. doi: 10.1080/00016489950180478. [DOI] [PubMed] [Google Scholar]
- 190.Pawankar R. Nasal polyposis: an update. Curr Opin Allergy Clin Immunol. 2003;3:1–6. doi: 10.1097/00130832-200302000-00001. editorial review. [DOI] [PubMed] [Google Scholar]
- 191.Wang Q.P., Escudier E., Roudot-Thoraval F., Abd-Al Samad I., Peynegre R., Coste A. Myofibroblast accumulation induced by transforming growth factor-beta is involved in the pathogenesis of nasal polyps. Laryngoscope. 1997;107:926–931. doi: 10.1097/00005537-199707000-00018. [DOI] [PubMed] [Google Scholar]
- 192.Molet S.M., Hamid Q.A., Hamilos D.L. IL-11 and IL-17 expression in nasal polyps: relationship to collagen deposition and suppression by intranasal fluticasone propionate. Laryngoscope. 2003;113:1803–1812. doi: 10.1097/00005537-200310000-00027. [DOI] [PubMed] [Google Scholar]
- 193.Coste A., Brugel L., Maitre B. Inflammatory cells as well as epithelial cells in nasal polyps express vascular endothelial growth factor. Eur Respir J. 2000;15:367–372. doi: 10.1034/j.1399-3003.2000.15b24.x. [DOI] [PubMed] [Google Scholar]
- 194.Xing Z., Jordana M., Braciak T., Ohtoshi T., Gauldie J. Lipopolysaccharide induces expression of granulocyte/macrophage colony-stimulating factor, interleukin-8, and interleukin-6 in human nasal, but not lung, fibroblasts: evidence for heterogeneity within the respiratory tract. Am J Respir Cell Mol Biol. 1993;9:255–263. doi: 10.1165/ajrcmb/9.3.255. [DOI] [PubMed] [Google Scholar]
- 195.Elovic A., Wong D.T., Weller P.F., Matossian K., Galli S.J. Expression of transforming growth factors-alpha and beta 1 messenger RNA and product by eosinophils in nasal polyps. J Allergy Clin Immunol. 1994;93:864–869. doi: 10.1016/0091-6749(94)90379-4. [DOI] [PubMed] [Google Scholar]
- 196.Chang C.H., Chai C.Y., Ho K.Y. Expression of transforming growth factor-beta 1 and alpha-smooth muscle actin of myofibroblast in the pathogenesis of nasal polyps. Kaohsiung J Med Sci. 2001;17:133–138. [PubMed] [Google Scholar]
- 197.Pawankar R. Mast cells in rhinitis. In: Watanabe T., Timmerman H., Yanai K., editors. Histamine research in the new millennium. Elsevier Science; Amsterdam: 2001. pp. 369–374. [Google Scholar]
- 198.Di Lorenzo G., Drago A., Esposito Pellitteri M. Measurement of inflammatory mediators of mast cells and eosinophils in native nasal lavage fluid in nasal polyposis. Int Arch Allergy Immunol. 2001;125:164–175. doi: 10.1159/000053811. [DOI] [PubMed] [Google Scholar]
- 199.Kim Y.K., Uno M., Hamilos D.L. Immunolocalization of CD34 in nasal polyposis. Effect of topical corticosteroids. Am J Respir Cell Mol Biol. 1999;20:388–397. doi: 10.1165/ajrcmb.20.3.3060. [DOI] [PubMed] [Google Scholar]
- 200.Simon H.U., Yousefi S., Schranz C., Schapowal A., Bachert C., Blaser K. Direct demonstration of delayed eosinophil apoptosis as a mechanism causing tissue eosinophilia. J Immunol. 1997;158:3902–3908. [PubMed] [Google Scholar]
- 201.Hamilos D.L., Thawley S.E., Kramper M.A., Kamil A., Hamid Q.A. Effect of intranasal fluticasone on cellular infiltration, endothelial adhesion molecule expression, and proinflammatory cytokine mRNA in nasal polyp disease. J Allergy Clin Immunol. 1999;103:79–87. doi: 10.1016/s0091-6749(99)70529-4. [DOI] [PubMed] [Google Scholar]
- 202.Minshall E.M., Cameron L., Lavigne F. Eotaxin mRNA and protein expression in chronic sinusitis and allergen-induced nasal responses in seasonal allergic rhinitis. Am J Respir Cell Mol Biol. 1997;17:683–690. doi: 10.1165/ajrcmb.17.6.2865. [DOI] [PubMed] [Google Scholar]
- 203.Hamilos D.L., Leung D.Y., Wood R. Evidence for distinct cytokine expression in allergic versus nonallergic chronic sinusitis. J Allergy Clin Immunol. 1995;96:537–544. doi: 10.1016/s0091-6749(95)70298-9. [DOI] [PubMed] [Google Scholar]
- 204.Nonaka M., Pawankar R., Saji F., Yagi T. Distinct expression of RANTES and GM-CSF by lipopolysaccharide in human nasal fibroblasts but not in other airway fibroblasts. Int Arch Allergy Immunol. 1999;119:314–321. doi: 10.1159/000024209. [DOI] [PubMed] [Google Scholar]
- 205.Gevaert P., Bachert C., Holtappels G. Enhanced soluble interleukin-5 receptor alpha expression in nasal polyposis. Allergy. 2003;58:371–379. doi: 10.1034/j.1398-9995.2003.00110.x. [DOI] [PubMed] [Google Scholar]
- 206.Miller C.H., Pudiak D.R., Hatem F., Looney R.J. Accumulation of interferon gamma-producing TH1 helper T cells in nasal polyps. Otolaryngol Head Neck Surg. 1994;111:51–58. doi: 10.1177/019459989411100111. [DOI] [PubMed] [Google Scholar]
- 207.Wagenmann M., Gärtner-Ackerboom M., Helmig P. Increased production of typ-2 and type-1 cytokines in nasal polyps. J Allergy Clin Immunol. 2000;105(suppl):S210. [Google Scholar]
- 208.Davidsson A., Danielsen A., Viale G. Positive identification in situ of mRNA expression of IL-6, and IL-12, and the chemotactic cytokine RANTES in patients with chronic sinusitis and polypoid disease. Clinical relevance and relation to allergy. Acta Otolaryngol. 1996;116:604–610. doi: 10.3109/00016489609137897. [DOI] [PubMed] [Google Scholar]
- 209.Adkins T.N., Goodgold H.M., Hendershott L., Slavin R.G. Does inhaled pollen enter the sinus cavities? Ann Allergy Asthma Immunol. 1998;81:181–184. doi: 10.1016/S1081-1206(10)62807-4. [DOI] [PubMed] [Google Scholar]
- 210.Braunstahl G.J., Kleinjan A., Overbeek S.E., Prins J.B., Hoogsteden H.C., Fokkens W.J. Segmental bronchial provocation induces nasal inflammation in allergic rhinitis patients. Am J Respir Crit Care Med. 2000;161:2051–2057. doi: 10.1164/ajrccm.161.6.9906121. [DOI] [PubMed] [Google Scholar]
- 211.Braunstahl G.J., Overbeek S.E., Kleinjan A., Prins J.B., Hoogsteden H.C., Fokkens W.J. Nasal allergen provocation induces adhesion molecule expression and tissue eosinophilia in upper and lower airways. J Allergy Clin Immunol. 2001;107:469–476. doi: 10.1067/mai.2001.113046. [DOI] [PubMed] [Google Scholar]
- 212.Braunstahl G.J., Overbeek S.E., Fokkens W.J. Segmental bronchoprovocation in allergic rhinitis patients affects mast cell and basophil numbers in nasal and bronchial mucosa. Am J Respir Crit Care Med. 2001;164:858–865. doi: 10.1164/ajrccm.164.5.2006082. [DOI] [PubMed] [Google Scholar]
- 213.Baroody F., deTineo M., Haney L., Clark K., Blair C., Naclerio R.A. Influx of eosinophils into the maxillary sinus after nasal challenge with allergen. J Allergy Clin Immunol. 2000;105(suppl):S70. [abstract] [Google Scholar]
- 214.Bachert C., van Zele T., Gevaert P., De Schrijver L., Van Cauwenberge P. Superantigens and nasal polyps. Curr Allergy Asthma Rep. 2003;3:523–531. doi: 10.1007/s11882-003-0065-y. [DOI] [PubMed] [Google Scholar]
- 215.Calenoff E., McMahan J.T., Herzon G.D., Kern R.C., Ghadge G.D., Hanson D.G. Bacterial allergy in nasal polyposis. A new method for quantifying specific IgE. Arch Otolaryngol Head Neck Surg. 1993;119:830–836. doi: 10.1001/archotol.1993.01880200030004. [DOI] [PubMed] [Google Scholar]
- 216.Holtzman M.J., Morton J.D., Shornick L.P. Immunity, inflammation, and remodeling in the airway epithelial barrier: epithelial-viral-allergic paradigm. Physiol Rev. 2002;82:19–46. doi: 10.1152/physrev.00020.2001. [DOI] [PubMed] [Google Scholar]
- 217.Sampath D., Castro M., Look D.C., Holtzman M.J. Constitutive activation of an epithelial signal transducer and activator of transcription (STAT) pathway in asthma. J Clin Invest. 1999;103:1353–1361. doi: 10.1172/JCI6130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Watelet J.B., Bachert C., Claeys C., Van Cauwenberge P. Matrix metalloproteinases MMP-7, MMP-9 and their tissue inhibitor TIMP-1: expression in chronic sinusitis vs nasal polyposis. Allergy. 2004;59:54–60. doi: 10.1046/j.1398-9995.2003.00364.x. [DOI] [PubMed] [Google Scholar]
- 219.Pawankar R., Watanabe S., Nonaka M. Differential expression of MMP-2 and 9 in the allergic nasal mucosa and nasal polyps. J Allergy Clin Immunol. 2004;113(2):S332. [abstract] [Google Scholar]
- 220.Bhattacharyya N., Vyas D.K., Fechner F.P., Gliklich R.E., Metson R. Tissue eosinophilia in chronic sinusitis: quantification techniques. Arch Otolaryngol Head Neck Surg. 2001;127:1102–1105. doi: 10.1001/archotol.127.9.1102. [DOI] [PubMed] [Google Scholar]
- 221.Subramanian H.N., Schechtman K.B., Hamilos D.L. A retrospective analysis of treatment outcomes and time to relapse after intensive medical treatment for chronic sinusitis. Am J Rhinol. 2002;16:303–312. [PubMed] [Google Scholar]
- 222.Ferguson B. Eosinophilic mucin rhinosinusitis: a distinct clinicopathological entity. Laryngoscope. 2000;110:799–813. doi: 10.1097/00005537-200005000-00010. [DOI] [PubMed] [Google Scholar]
- 223.Braun H., Stammberger H., Buzina W., Freudenschuss K., Lackner A., Beham A. Incidence and detection of fungi and eosinophilic granulocytes in chronic rhinosinusitis. Laryngorhinootologie. 2003;82:330–340. doi: 10.1055/s-2003-39777. [DOI] [PubMed] [Google Scholar]
- 224.Ferguson B. Definitions of fungal rhinosinusitis. Otolaryngol Clin North Am. 2000;33:227–235. doi: 10.1016/s0030-6665(00)80002-x. [DOI] [PubMed] [Google Scholar]
- 225.Lund V.J., Kennedy D.W. Quantification for staging sinusitis. The Staging and Therapy Group. Ann Otol Rhinol Laryngol Suppl. 1995;167:17–21. [PubMed] [Google Scholar]
- 226.Shapiro G.G., Rachelefsky G.S. Introduction and definition of sinusitis. J Allergy Clin Immunol. 1992;90:417–418. doi: 10.1016/0091-6749(92)90160-4. [DOI] [PubMed] [Google Scholar]
- 227.Meltzer E.O., Orgel H.A., Backhaus J.W. Intranasal flunisolide spray as an adjunct to oral antibiotic therapy for sinusitis. J Allergy Clin Immunol. 1993;92:812–823. doi: 10.1016/0091-6749(93)90058-n. [DOI] [PubMed] [Google Scholar]
- 228.Lund V., Gwaltney J., Baquero F. Infectious rhinosinusitis in adults: classification, etiology and management. Ear Nose Throat J. 1997;76(suppl):S5–S22. [PubMed] [Google Scholar]
- 229.Williams J.W., Jr, Simel D.L. Does this patient have sinusitis? Diagnosing acute sinusitis by history and physical examination. JAMA. 1993;270:1242–1246. [PubMed] [Google Scholar]
- 230.Bhattacharyya N. The economic burden and symptom manifestations of chronic rhinosinusitis. Am J Rhinol. 2003;17:27–32. [PubMed] [Google Scholar]
- 231.Levine H.L. Patients with headache and visual disturbance: a differentiation between migraine and sinonasal headache. Arch Otolaryngol Head Neck Surg. 2000;126:234–235. doi: 10.1001/archotol.126.2.234. [DOI] [PubMed] [Google Scholar]
- 232.Spector S.L., Nicklas R.A., Chapman J.A. Symptom severity assessment of allergic rhinitis: part 1. Ann Allergy Asthma Immunol. 2003;91:105–114. doi: 10.1016/s1081-1206(10)62160-6. [DOI] [PubMed] [Google Scholar]
- 233.Pemberton E. Technique for measuring the optimum rating scale for opinion measures. Sociol Soc Res. 1933;17:470–472. [Google Scholar]
- 234.Miller G.A. The magical number seven plus or minus two: some limits on our capacity for processing information. Psychol Rev. 1956;63:81–97. [PubMed] [Google Scholar]
- 235.Juniper E.F., Guyatt G.H., Andersson B., Ferrie P.J. Comparison of powder and aerosolized budesonide in perennial rhinitis: validation of rhinitis quality of life questionnaire. Ann Allergy. 1993;70:225–230. [PubMed] [Google Scholar]
- 236.Roper W.L., Winkenwerder W., Hackbarth G.M., Krakauer H. Effectiveness in health care. An initiative to evaluate and improve medical practice. N Engl J Med. 1988;319:1197–1202. doi: 10.1056/NEJM198811033191805. [DOI] [PubMed] [Google Scholar]
- 237.Piccirillo J.F. Outcomes research and otolaryngology. Otolaryngol Head Neck Surg. 1994;111:764–769. doi: 10.1177/019459989411100611. [DOI] [PubMed] [Google Scholar]
- 238.Clancy C.M., Eisenberg J.M. Outcomes research: measuring the end results of health care. Science. 1998;282:245–246. doi: 10.1126/science.282.5387.245. [DOI] [PubMed] [Google Scholar]
- 239.Gliklich R.E., Metson R. Techniques for outcomes research in chronic sinusitis. Laryngoscope. 1995;105:387–390. doi: 10.1288/00005537-199504000-00010. [DOI] [PubMed] [Google Scholar]
- 240.Feinstein A.R. Clinical biostatistics. XLI. Hard science, soft data, and the challenges of choosing clinical variables in research. Clin Pharmacol Ther. 1977;22:485–498. doi: 10.1002/cpt1977224485. [DOI] [PubMed] [Google Scholar]
- 241.Cella D.F. Quality of life: concepts and definition. J Pain Symptom Manage. 1994;9:186–192. doi: 10.1016/0885-3924(94)90129-5. [DOI] [PubMed] [Google Scholar]
- 242.Covinsky K.E., Wu A.W., Landefeld C.S. Health status versus quality of life in older patients: does the distinction matter? Am J Med. 1999;106:435–440. doi: 10.1016/s0002-9343(99)00052-2. [DOI] [PubMed] [Google Scholar]
- 243.Guyatt G.H. Making sense of quality-of-life data. Med Care. 2000;38:II175–II179. doi: 10.1097/00005650-200009002-00027. [DOI] [PubMed] [Google Scholar]
- 244.Gill T.M., Feinstein A.R. A critical appraisal of the quality of quality-of-life measurements. JAMA. 1994;272:619–626. [PubMed] [Google Scholar]
- 245.Lara-Munoz C., Feinstein A.R. How should quality of life be measured? J Investig Med. 1999;47:17–24. [PubMed] [Google Scholar]
- 246.Yueh B., Feinstein A.R. Abstruse comparisons: the problems of numerical contrasts of two groups. J Clin Epidemiol. 1999;52:13–18. doi: 10.1016/s0895-4356(98)00133-4. [DOI] [PubMed] [Google Scholar]
- 247.Streiner D., Norman G. Oxford University Press; New York: 1995. Health Measurement Scales. [Google Scholar]
- 248.Ware J., Snow K., Kosinski M., Gandek B. The Health Institute; Boston, MA: 1999. SF-36 Health Survey. Manual and Interpretation Guide. [Google Scholar]
- 249.Lund V.J., Holmstrom M., Scadding G.K. Functional endoscopic sinus surgery in the management of chronic rhinosinusitis. An objective assessment. J Laryngol Otol. 1991;105:832–835. doi: 10.1017/s0022215100117463. [DOI] [PubMed] [Google Scholar]
- 250.Piccirillo J., Edwards D., Haiduk A., Yonan C., Thawley S. Psychometric and clinimetric validity of the 31-item rhinosinusitis outcome measure (RSOM-31) Am J Rhinol. 1995;9:297–306. [Google Scholar]
- 251.Piccirillo J.F., Merritt M.G., Jr, Richards M.L. Psychometric and clinimetric validity of the 20-Item Sino-Nasal Outcome Test (SNOT-20) Otolaryngol Head Neck Surg. 2002;126:41–47. doi: 10.1067/mhn.2002.121022. [DOI] [PubMed] [Google Scholar]
- 252.Benninger M.S., Senior B.A. The development of the Rhinosinusitis Disability Index. Arch Otolaryngol Head Neck Surg. 1997;123:1175–1179. doi: 10.1001/archotol.1997.01900110025004. [DOI] [PubMed] [Google Scholar]
- 253.Ware J.E., Brook R., Davies-Avery A. The RAND Corporation; Santa Monica (CA): 1980. Conceptualization and measurement of health for adults in the Health Insurance Study. Volume I: Model of health and methodology. [Google Scholar]
- 254.Gliklich R.E., Metson R. The health impact of chronic sinusitis in patients seeking otolaryngologic care. Otolaryngol Head Neck Surg. 1995;113:104–109. doi: 10.1016/S0194-59989570152-4. [DOI] [PubMed] [Google Scholar]
- 255.Hwang P.H., Irwin S.B., Griest S.E., Caro J.E., Nesbit G.M. Radiologic correlates of symptom-based diagnostic criteria for chronic rhinosinusitis. Otolaryngol Head Neck Surg. 2003;128:489–496. doi: 10.1016/S0194-59980223295-7. [DOI] [PubMed] [Google Scholar]
- 256.Gray L.P. Deviated nasal septum. Incidence and etiology. Ann Otol Rhinol Laryngol Suppl. 1978;87:3–20. doi: 10.1177/00034894780873s201. [DOI] [PubMed] [Google Scholar]
- 257.Stankiewicz J.A., Chow J.M. Nasal endoscopy and the definition and diagnosis of chronic rhinosinusitis. Otolaryngol Head Neck Surg. 2002;126:623–627. doi: 10.1067/mhn.2002.125602. [DOI] [PubMed] [Google Scholar]
- 258.Lanza D. Diagnosis of chronic rhinosinusitis. Ann Otol Rhinol Laryngol. 2004;113:10–14. doi: 10.1177/00034894041130s504. [DOI] [PubMed] [Google Scholar]
- 259.Nadel D.M., Lanza D.C., Kennedy D.W. Endoscopically guided sinus cultures in normal subjects. Am J Rhinol. 1999;13:87–90. doi: 10.2500/105065899782106742. [DOI] [PubMed] [Google Scholar]
- 260.Stammberger H., Wolf G. Headaches and sinus disease: the endoscopic approach. Ann Otol Rhinol Laryngol Suppl. 1988;134:3–23. doi: 10.1177/00034894880970s501. [DOI] [PubMed] [Google Scholar]
- 261.Gungor A., Baroody F.M., Naclerio R.M., White S.R., Corey J.P. Decreased neuropeptide release may play a role in the pathogenesis of nasal polyps. Otolaryngol Head Neck Surg. 1999;121:585–590. doi: 10.1016/S0194-5998(99)70061-6. [DOI] [PubMed] [Google Scholar]
- 262.Fang S.Y., Shen C.L., Ohyama M. Presence of neuropeptides in human nasal polyps. Acta Otolaryngol. 1994;114:324–328. doi: 10.3109/00016489409126064. [DOI] [PubMed] [Google Scholar]
- 263.Vogan J.C., Bolger W.E., Keyes A.S. Endoscopically guided sinonasal cultures: a direct comparison with maxillary sinus aspirate cultures. Otolaryngol Head Neck Surg. 2000;122:370–373. doi: 10.1016/S0194-5998(00)70051-9. [DOI] [PubMed] [Google Scholar]
- 264.Talbot G.H., Kennedy D.W., Scheld W.M., Granito K. Rigid nasal endoscopy versus sinus puncture and aspiration for microbiologic documentation of acute bacterial maxillary sinusitis. Clin Infect Dis. 2001;33:1668–1675. doi: 10.1086/323813. [DOI] [PubMed] [Google Scholar]
- 265.Araujo E., Palombini B.C., Cantarelli V., Pereira A., Mariante A. Microbiology of middle meatus in chronic rhinosinusitis. Am J Rhinol. 2003;17:9–15. [PubMed] [Google Scholar]
- 266.Tantilipikorn P., Fritz M., Tanabodee J., Lanza D.C., Kennedy D.W. A comparison of endoscopic culture techniques for chronic rhinosinusitis. Am J Rhinol. 2002;16:255–260. [PubMed] [Google Scholar]
- 267.American Academy of Pediatrics, Subcommittee on Management of Sinusitis and Committee on Quality Improvement Clinical practice guideline: management of sinusitis. Pediatrics. 2001;108:798–808. doi: 10.1542/peds.108.3.798. [DOI] [PubMed] [Google Scholar]
- 268.Gordts F., Abu Nasser I., Clement P.A., Pierard D., Kaufman L. Bacteriology of the middle meatus in children. Int J Pediatr Otorhinolaryngol. 1999;48:163–167. doi: 10.1016/s0165-5876(99)00032-4. [DOI] [PubMed] [Google Scholar]
- 269.Klossek J.M., Dubreuil L., Richet H., Richet B., Sedallian A., Beutter P. Bacteriology of the adult middle meatus. J Laryngol Otol. 1996;110:847–849. doi: 10.1017/s0022215100135133. [DOI] [PubMed] [Google Scholar]
- 270.Gold S.M., Tami T.A. Role of middle meatus aspiration culture in the diagnosis of chronic sinusitis. Laryngoscope. 1997;107:1586–1589. doi: 10.1097/00005537-199712000-00002. [DOI] [PubMed] [Google Scholar]
- 271.Winther B., Vicery C., Gross C., Hendley O. Microbiology of the maxillary sinus in adults with chronic sinus disease. Am J Rhinol. 1996;10:347–350. [Google Scholar]
- 272.Kountakis S.E., Skoulas I.G. Middle meatal vs antral lavage cultures in intensive care unit patients. Otolaryngol Head Neck Surg. 2002;126:377–381. doi: 10.1067/mhn.2002.123444. [DOI] [PubMed] [Google Scholar]
- 273.Belden C.J., Zinreich S.J. Orbital imaging techniques. Semin Ultrasound CT MR. 1997;18:413–422. doi: 10.1016/s0887-2171(97)90003-2. [DOI] [PubMed] [Google Scholar]
- 274.Klose K.C., Elies W., Sondermann U. Rofo Fortschr Geb Rontgenstr Neuen Bildgeb Verfahr. 1991;155:199–206. [The accuracy of plain-film radiology in demonstrating the shadows of the pneumatic spaces of the skull—a comparison with computed tomography] [Google Scholar]
- 275.Melhem E.R., Oliverio P.J., Benson M.L., Leopold D.A., Zinreich S.J. Optimal CT evaluation for functional endoscopic sinus surgery. AJNR Am J Neuroradiol. 1996;17:181–188. [PMC free article] [PubMed] [Google Scholar]
- 276.Zinreich S., Kennedy D., Kumar A., Rosenbaum A., Arrington J., Johns M. MR imaging of the normal nasal cycle: comparison with sinus pathology. J Comput Assist Tomogr. 1988;12:1014–1019. doi: 10.1097/00004728-198811000-00019. [DOI] [PubMed] [Google Scholar]
- 277.Kennedy D.W. Prognostic factors, outcomes and staging in ethmoid sinus surgery. Laryngoscope. 1992;102:1–18. [PubMed] [Google Scholar]
- 278.Metson R., Gliklich R.E., Stankiewicz J.A. Comparison of sinus computed tomography staging systems. Otolaryngol Head Neck Surg. 1997;117:372–379. doi: 10.1016/S0194-5998(97)70129-3. [DOI] [PubMed] [Google Scholar]
- 279.Lund V.J., Mackay I.S. Staging in rhinosinusitis. Rhinology. 1993;31:183–184. [PubMed] [Google Scholar]
- 280.Lloyd G.A., Lund V.J., Scadding G.K. CT of the paranasal sinuses and functional endoscopic surgery: a critical analysis of 100 symptomatic patients. J Laryngol Otol. 1991;105:181–185. doi: 10.1017/s0022215100115300. [DOI] [PubMed] [Google Scholar]
- 281.Friedman W.H., Katsantonis G.P., Bumpous J.M. Staging of chronic hyperplastic rhinosinusitis: treatment strategies. Otolaryngol Head Neck Surg. 1995;112:210–214. doi: 10.1016/S0194-59989570238-5. [DOI] [PubMed] [Google Scholar]
- 282.Bhattacharyya T., Piccirillo J., Wippold F.J., 2nd Relationship between patient-based descriptions of sinusitis and paranasal sinus computed tomographic findings. Arch Otolaryngol Head Neck Surg. 1997;123:1189–1192. doi: 10.1001/archotol.1997.01900110039006. [DOI] [PubMed] [Google Scholar]
- 283.Levine H.L. Functional endoscopic sinus surgery: evaluation, surgery, and follow-up of 250 patients. Laryngoscope. 1990;100:79–84. doi: 10.1288/00005537-199001000-00016. [DOI] [PubMed] [Google Scholar]
- 284.Bhattacharyya N. Test-retest reliability of computed tomography in the assessment of chronic rhinosinusitis. Laryngoscope. 1999;109:1055–1058. doi: 10.1097/00005537-199907000-00008. [DOI] [PubMed] [Google Scholar]
- 285.Senior B.A., Kennedy D.W., Tanabodee J., Kroger H., Hassab M., Lanza D. Long-term results of functional endoscopic sinus surgery. Laryngoscope. 1998;108:151–157. doi: 10.1097/00005537-199802000-00001. [DOI] [PubMed] [Google Scholar]
- 286.Shields G., Seikaly H., LeBoeuf M. Correlation between facial pain or headache and computed tomography in rhinosinusitis in Canadian and U.S. subjects. Laryngoscope. 2003;113:943–945. doi: 10.1097/00005537-200306000-00006. [DOI] [PubMed] [Google Scholar]
- 287.Som P.M., Dillon W.P., Curtin H.D., Fullerton G.D., Lidov M. Hypointense paranasal sinus foci: differential diagnosis with MR imaging and relation to CT findings. Radiology. 1990;176:777–781. doi: 10.1148/radiology.176.3.2389036. [DOI] [PubMed] [Google Scholar]
- 288.Baroody F.M., Gungor A., deTineo M., Haney L., Blair C., Naclerio R.M. Comparison of the response to histamine challenge of the nose and the maxillary sinus: effect of loratadine. J Appl Physiol. 1999;87:1038–1047. doi: 10.1152/jappl.1999.87.3.1038. [DOI] [PubMed] [Google Scholar]
- 289.Pelikan Z., Pelikan-Filipek M. Role of nasal allergy in chronic maxillary sinusitis—diagnostic value of nasal challenge with allergen. J Allergy Clin Immunol. 1990;86:484–491. doi: 10.1016/s0091-6749(05)80203-9. [DOI] [PubMed] [Google Scholar]
- 290.Baroody F., Saengpanich S., deTineo M., Haney L., Votypka V., Naclerio R. Nasal allergen challenge leads to bilateral maxillary sinus eosinophil influx. J Allergy Clin Immunol. 2002;109(suppl):S84. [abstract] [Google Scholar]
- 291.Baroody F., Mucha S., de Tineo M., Malecker B., Naclerio R. Evidence of maxillary sinus inflammation in seasonal allergic rhinitis (SAR) [abstract] J Allergy Clin Immunol. 2003;111(suppl):S124. [Google Scholar]
- 292.Casadevall J., Ventura P.J., Mullol J., Picado C. Intranasal challenge with aspirin in the diagnosis of aspirin intolerant asthma: evaluation of nasal response by acoustic rhinometry. Thorax. 2000;55:921–924. doi: 10.1136/thorax.55.11.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Togias A. Rhinitis and asthma: evidence for respiratory system integration. J Allergy Clin Immunol. 2003;111:1171–1183. doi: 10.1067/mai.2003.1592. [DOI] [PubMed] [Google Scholar]
- 294.Djukanovic R., Lai C.K., Wilson J.W. Bronchial mucosal manifestations of atopy: a comparison of markers of inflammation between atopic asthmatics, atopic nonasthmatics and healthy controls. Eur Respir J. 1992;5:538–544. [PubMed] [Google Scholar]
- 295.Townley R.G., Ryo U.Y., Kolotkin B.M., Kang B. Bronchial sensitivity to methacholine in current and former asthmatic and allergic rhinitis patients and control subjects. J Allergy Clin Immunol. 1975;56:429–442. doi: 10.1016/0091-6749(75)90061-5. [DOI] [PubMed] [Google Scholar]
- 296.Leynaert B., Bousquet J., Neukirch C., Liard R., Neukirch F. Perennial rhinitis: an independent risk factor for asthma in nonatopic subjects: results from the European Community Respiratory Health Survey. J Allergy Clin Immunol. 1999;104:301–304. doi: 10.1016/s0091-6749(99)70370-2. [DOI] [PubMed] [Google Scholar]
- 297.Settipane R.J., Hagy G.W., Settipane G.A. Long-term risk factors for developing asthma and allergic rhinitis: a 23-year follow-up study of college students. Allergy Proc. 1994;15:21–25. doi: 10.2500/108854194778816634. [DOI] [PubMed] [Google Scholar]
- 298.Linneberg A., Henrik Nielsen N., Frolund L., Madsen F., Dirksen A., Jorgensen T. The link between allergic rhinitis and allergic asthma: a prospective population-based study. The Copenhagen Allergy Study. Allergy. 2002;57:1048–1052. doi: 10.1034/j.1398-9995.2002.23664.x. [DOI] [PubMed] [Google Scholar]
- 299.Guerra S., Sherrill D.L., Martinez F.D., Barbee R.A. Rhinitis as an independent risk factor for adult-onset asthma. J Allergy Clin Immunol. 2002;109:419–425. doi: 10.1067/mai.2002.121701. [DOI] [PubMed] [Google Scholar]
- 300.Kapsali T., Horowitz E., Togias A. Rhinitis is ubiquitous in allergic asthmatics. J Allergy Clin Immunol. 1997;99(suppl):S138. [abstract] [Google Scholar]
- 301.Simon R.A. The allergy-asthma connection. Allergy Asthma Proc. 2002;23(4):219–222. [PubMed] [Google Scholar]
- 302.Stephens L., Proud D., Togias A. Nasal cold, dry air (CDA) challenge results in stronger nasal and pulmonary responses in asthmatics compared to patients with rhinitis. J Allergy Clin Immunol. 1996;97:A315. [abstract] [Google Scholar]
- 303.Gaga M., Lambrou P., Papageorgiou N. Eosinophils are a feature of upper and lower airway pathology in non-atopic asthma, irrespective of the presence of rhinitis. Clin Exp Allergy. 2000;30:663–669. doi: 10.1046/j.1365-2222.2000.00804.x. [DOI] [PubMed] [Google Scholar]
- 304.Huse D., Hartz S., Russell M., Piercey G., Weiss S. Allergic rhinitis may worsen asthma symptoms in children: the international asthma outcomes registry. Am J Respir Crit Care Med. 1996;153:A860. [abstract] [Google Scholar]
- 305.Huse D., Hartz S., Klaus D., Piercey G., Richner R., Weiss S. Does allergic rhinitis exacerbate asthma symptoms in adults? The asthma outcomes registry. Eur Respir J. 1996;9(suppl):351S. [abstract] [Google Scholar]
- 306.Huse D., Russell M., Miller J., Weiss S., Hartz S. Asthma treatment costs are increased in children with allergic rhinitis. Eur Respir J. 1998;12(suppl):50S. [Google Scholar]
- 307.Huse D., Hartz S., Klaus D., Kuriyama N., Piercey G., Weiss S. Do symptoms of allergic rhinitis exacerbate asthma symptoms in adults? The asthma outcomes registry. Eur Respir J. 1998;12(suppl):50S. [Google Scholar]
- 308.Halpern M., Richner R., Guo C., de Lissovoy G., Togias A. Allergic rhinitis: a potential cause of increased asthma medication use, costs, and morbidity. J Asthma. 2004;41:117–126. doi: 10.1081/jas-120026069. [DOI] [PubMed] [Google Scholar]
- 309.Chanez P., Vignola A.M., Vic P. Comparison between nasal and bronchial inflammation in asthmatic and control subjects. Am J Respir Crit Care Med. 1999;159:588–595. doi: 10.1164/ajrccm.159.2.9801022. [DOI] [PubMed] [Google Scholar]
- 310.Corren J., Adinoff A.D., Irvin C.G. Changes in bronchial responsiveness following nasal provocation with allergen. J Allergy Clin Immunol. 1992;89:611–618. doi: 10.1016/0091-6749(92)90329-z. [DOI] [PubMed] [Google Scholar]
- 311.Tran N., Proud D., Scichilone N., Kosmas E., Togias A. The effect of multiple nasal antigen challenges on the asthmatic airways. J Allergy Clin Immunol. 2000;105(suppl):S282–S283. [Google Scholar]
- 312.Noureddine G., Thompson M., Brennan F. Nasal antigen challenge in asthmatics: effects of cetirizine on nasal and pulmonary responses. J Allergy Clin Immunol. 1994;93:177. [abstract] [Google Scholar]
- 313.Henriksen J.M., Wenzel A. Effect of an intranasally administered corticosteroid (budesonide) on nasal obstruction, mouth breathing, and asthma. Am Rev Respir Dis. 1984;130:1014–1018. doi: 10.1164/arrd.1984.130.6.1014. [DOI] [PubMed] [Google Scholar]
- 314.Welsh P.W., Stricker W.E., Chu C.P. Efficacy of beclomethasone nasal solution, flunisolide, and cromolyn in relieving symptoms of ragweed allergy. Mayo Clin Proc. 1987;62:125–134. doi: 10.1016/s0025-6196(12)61882-5. [DOI] [PubMed] [Google Scholar]
- 315.Corren J., Adinoff A.D., Buchmeier A.D., Irvin C.G. Nasal beclomethasone prevents the seasonal increase in bronchial responsiveness in patients with allergic rhinitis and asthma. J Allergy Clin Immunol. 1992;90:250–256. doi: 10.1016/0091-6749(92)90079-h. [DOI] [PubMed] [Google Scholar]
- 316.Watson W.T., Becker A.B., Simons F.E. Treatment of allergic rhinitis with intranasal corticosteroids in patients with mild asthma: effect on lower airway responsiveness. J Allergy Clin Immunol. 1993;91:97–101. doi: 10.1016/0091-6749(93)90301-u. [DOI] [PubMed] [Google Scholar]
- 317.Wood R.A., Eggleston P.A. The effects of intranasal steroids on nasal and pulmonary responses to cat exposure. Am J Respir Crit Care Med. 1995;151:315–320. doi: 10.1164/ajrccm.151.2.7842184. [DOI] [PubMed] [Google Scholar]
- 318.Foresi A., Pelucchi A., Gherson G., Mastropasqua B., Chiapparino A., Testi R. Once daily intranasal fluticasone propionate (200 micrograms) reduces nasal symptoms and inflammation but also attenuates the increase in bronchial responsiveness during the pollen season in allergic rhinitis. J Allergy Clin Immunol. 1996;98:274–282. doi: 10.1016/s0091-6749(96)70150-1. [DOI] [PubMed] [Google Scholar]
- 319.Pelucchi A., Chiapparino A., Mastropasqua B., Marazzini L., Hernandez A., Foresi A. Effect of intranasal azelastine and beclomethasone dipropionate on nasal symptoms, nasal cytology, and bronchial responsiveness to methacholine in allergic rhinitis in response to grass pollens. J Allergy Clin Immunol. 1995;95:515–523. doi: 10.1016/s0091-6749(95)70313-6. [DOI] [PubMed] [Google Scholar]
- 320.Dahl R., Baker R., Pauwels R. Seasonal rhinitis and asthma. Effects of topical nasal and/or orally inhaled fluticasone proponiate. The SPIRA study (FNM40001) J Allergy Clin Immunol. 2001;107(suppl):S154. [abstract] [Google Scholar]
- 321.Crystal-Peters J., Neslusan C., Crown W.H., Torres A. Treating allergic rhinitis in patients with comorbid asthma: the risk of asthma-related hospitalizations and emergency department visits. J Allergy Clin Immunol. 2002;109:57–62. doi: 10.1067/mai.2002.120554. [DOI] [PubMed] [Google Scholar]
- 322.Bresciani M., Paradis L., Des Roches A. Rhinosinusitis in severe asthma. J Allergy Clin Immunol. 2001;107:73–80. doi: 10.1067/mai.2001.111593. [DOI] [PubMed] [Google Scholar]
- 323.Harlin S.L., Ansel D.G., Lane S.R., Myers J., Kephart G.M., Gleich G.J. A clinical and pathologic study of chronic sinusitis: the role of the eosinophil. J Allergy Clin Immunol. 1988;81:867–875. doi: 10.1016/0091-6749(88)90944-x. [DOI] [PubMed] [Google Scholar]
- 324.Jankowski R., Moneret-Vautrin D.A., Goetz R., Wayoff M. Incidence of medico-surgical treatment for nasal polyps on the development of associated asthma. Rhinology. 1992;30:249–258. [PubMed] [Google Scholar]
- 325.Jankowski R., Pigret D., Decroocq F. Comparison of functional results after ethmoidectomy and nasalization for diffuse and severe nasal polyposis. Acta Otolaryngol. 1997;117:601–608. doi: 10.3109/00016489709113445. [DOI] [PubMed] [Google Scholar]
- 326.Rachelefsky G.S., Katz R.M., Siegel S.C. Chronic sinus disease with associated reactive airway disease in children. Pediatrics. 1984;73:526–529. [PubMed] [Google Scholar]
- 327.Johansson S.G., Hourihane J.O., Bousquet J. A revised nomenclature for allergy. An EAACI position statement from the EAACI nomenclature task force. Allergy. 2001;56:813–824. doi: 10.1034/j.1398-9995.2001.t01-1-00001.x. [DOI] [PubMed] [Google Scholar]
- 328.Garbutt J.M., Goldstein M., Gellman E., Shannon W., Littenberg B. A randomized, placebo-controlled trial of antimicrobial treatment for children with clinically diagnosed acute sinusitis. Pediatrics. 2001;107:619–625. doi: 10.1542/peds.107.4.619. [DOI] [PubMed] [Google Scholar]
- 329.Werning J.W., Preston T.W., Khuder S. Physician specialty is associated with differences in the evaluation and management of acute bacterial rhinosinusitis. Arch Otolaryngol Head Neck Surg. 2002;128:123–130. doi: 10.1001/archotol.128.2.123. [DOI] [PubMed] [Google Scholar]
- 330.Wasserfallen J.B., Gold K., Schulman K.A., Baraniuk J.N. Development and validation of a rhinoconjunctivitis and asthma symptom score for use as an outcome measure in clinical trials. J Allergy Clin Immunol. 1997;100:16–22. doi: 10.1016/s0091-6749(97)70189-1. [DOI] [PubMed] [Google Scholar]
- 331.Rosenfeld R.M. Pilot study of outcomes in pediatric rhinosinusitis. Arch Otolaryngol Head Neck Surg. 1995;121:729–736. doi: 10.1001/archotol.1995.01890070015005. [DOI] [PubMed] [Google Scholar]
- 332.Bucknall C.E. Definitions of severity and outcome measures. Respir Med. 1996;90:447–452. doi: 10.1016/s0954-6111(96)90169-9. [DOI] [PubMed] [Google Scholar]
- 333.Harvey R.P., Comer C., Sanders B. Model for outcomes assessment of antihistamine use for seasonal allergic rhinitis. J Allergy Clin Immunol. 1996;97:1233–1241. doi: 10.1016/s0091-6749(96)70190-2. [DOI] [PubMed] [Google Scholar]
- 334.Fahmy F.F., McCombe A., McKiernan D.C. Sino nasal assessment questionnaire, a patient focused, rhinosinusitis specific outcome measure. Rhinology. 2002;40:195–197. [PubMed] [Google Scholar]
- 335.Senior B.A., Glaze C., Benninger M.S. Use of the Rhinosinusitis Disability Index (RSDI) in rhinologic disease. Am J Rhinol. 2001;15:15–20. doi: 10.2500/105065801781329428. [DOI] [PubMed] [Google Scholar]
- 336.Druce H.M. Diagnosis of sinusitis in adults: history, physical examination, nasal cytology, echo, and rhinoscope. J Allergy Clin Immunol. 1992;90:436–441. doi: 10.1016/0091-6749(92)90165-x. [DOI] [PubMed] [Google Scholar]
- 337.Fokkens W.J. Thoughts on the pathophysiology of nonallergic rhinitis. Curr Allergy Asthma Rep. 2002;2:203–209. doi: 10.1007/s11882-002-0020-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.Hamilos D.L. Chronic sinusitis. J Allergy Clin Immunol. 2000;106:213–227. doi: 10.1067/mai.2000.109269. [DOI] [PubMed] [Google Scholar]
- 339.Druce H.M., Bonner R.F., Patow C., Choo P., Summers R.J., Kaliner M.A. Response of nasal blood flow to neurohormones as measured by laser-Doppler velocimetry. J Appl Physiol. 1984;57:1276–1283. doi: 10.1152/jappl.1984.57.4.1276. [DOI] [PubMed] [Google Scholar]
- 340.Havas T.E., Motbey J.A., Gullane P.J. Prevalence of incidental abnormalities on computed tomographic scans of the paranasal sinuses. Arch Otolaryngol Head Neck Surg. 1988;114:856–859. doi: 10.1001/archotol.1988.01860200040012. [DOI] [PubMed] [Google Scholar]
- 341.Mudgil S.P., Wise S.W., Hopper K.D., Kasales C.J., Mauger D., Fornadley J.A. Correlation between presumed sinusitis-induced pain and paranasal sinus computed tomographic findings. Ann Allergy Asthma Immunol. 2002;88:223–226. doi: 10.1016/S1081-1206(10)62000-5. [DOI] [PubMed] [Google Scholar]
- 342.Sinus and Allergy Health Partnership Antimicrobial treatment guidelines for acute bacterial sinusitis. Otolaryngol Head Neck Surg. 2000;123(suppl):S4–S31. [PubMed] [Google Scholar]
- 343.Hickner J.M., Bartlett J.G., Besser R.E., Gonzales R., Hoffman J.R., Sande M.A. Principles of appropriate antibiotic use for acute rhinosinusitis in adults: background. Ann Intern Med. 2001;134:498–505. doi: 10.7326/0003-4819-134-6-200103200-00017. [DOI] [PubMed] [Google Scholar]
- 344.Georgitis J.W., Druce H.M., Goldstein S., Upper Airway Allergy Committee Rhinopharyngolaryngoscopy. J Allergy Clin Immunol. 1993;91:961–962. doi: 10.1016/0091-6749(93)90355-j. [DOI] [PubMed] [Google Scholar]
- 345.Lund V.J., Kennedy D.W. Staging for rhinosinusitis. Otolaryngol Head Neck Surg. 1997;117(suppl):S35–S40. doi: 10.1016/S0194-59989770005-6. [DOI] [PubMed] [Google Scholar]
- 346.Slavin R.G., Leipzig J.R., Goodgold H.M. “Allergic sinusitis” revisited. Ann Allergy Asthma Immunol. 2000;85:273–276. doi: 10.1016/S1081-1206(10)62529-X. [DOI] [PubMed] [Google Scholar]
- 347.Mings R., Friedman W., Linford P. Five-year follow-up of the effects of bilateral intranasal sphenoethmoidectomy in patients with sinusitis and asthma. Am J Rhinol. 1988;2:13–16. [Google Scholar]
- 348.Friedman W.H. Surgery for chronic hyperplastic rhinosinusitis. Laryngoscope. 1975;85:1999–2011. doi: 10.1288/00005537-197512000-00005. [DOI] [PubMed] [Google Scholar]
- 349.Chambers D.W., Davis W.E., Cook P.R., Nishioka G.J., Rudman D.T. Long-term outcome analysis of functional endoscopic sinus surgery: correlation of symptoms with endoscopic examination findings and potential prognostic variables. Laryngoscope. 1997;107:504–510. doi: 10.1097/00005537-199704000-00014. [DOI] [PubMed] [Google Scholar]
- 350.Jakobsen J., Svendstrup F. Functional endoscopic sinus surgery in chronic sinusitis—a series of 237 consecutively operated patients. Acta Otolaryngol Suppl. 2000;543:158–161. doi: 10.1080/000164800454279. [DOI] [PubMed] [Google Scholar]
- 351.Blomqvist E.H., Lundblad L., Anggard A., Haraldsson P.O., Stjarne P. A randomized controlled study evaluating medical treatment versus surgical treatment in addition to medical treatment of nasal polyposis. J Allergy Clin Immunol. 2001;107:224–228. doi: 10.1067/mai.2001.112124. [DOI] [PubMed] [Google Scholar]
- 352.Benninger M., Ferguson B., Hadley J. Adult chronic rhinosinusitis: definitions, diagnosis, epidemiology, and pathophysiology. Otolaryngol Head Neck Surg. 2003;129(suppl):S1–S32. doi: 10.1016/s0194-5998(03)01397-4. [DOI] [PubMed] [Google Scholar]
- 353.Anon J., Jacobs M., Poole M. Antimicrobial treatment guidelines for acute bacterial rhinosinusitis. Otolaryngol Head Neck Surg. 2004;130:1–45. doi: 10.1016/j.otohns.2003.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]


