Abstract
Study objective
Patients with communicable diseases may require respiratory isolation to reduce the chance of transmission to health care workers and the public. This project was conducted to determine whether negative-pressure isolation for multiple patients can be achieved quickly and effectively using general hospital space not previously dedicated to respiratory isolation.
Methods
The physical therapy gymnasium was the area designated to test the ability to create a negative-pressure isolation environment in a large space. The conversion was planned in advance of an unscheduled drill to convert the space. Four high-efficiency particulate air (HEPA) filtered forced air machines were used to generate negative pressure. The units were vented to the outside air by a 25-foot length of 10-inch-diameter reusable duct. We evaluated the time needed for equipment setup and room conversion and noted any subjective difficulty with either setup or operation of the equipment. We measured the ability of the equipment to generate a negative air pressure relative to adjacent areas and determined the noise levels created during the use of different combinations of machines at various power settings.
Results
After drill activation and the request for equipment setup, 1 hour was required to convert the physical therapy gymnasium into an operational negative-pressure environment. The room pressure readings “high” power ranged from −1.5 to −13 Pa (−0.006 to −0.052 inches of water), and noise levels ranged from 70 to 76 dB. Calculated air changes per hour using 1, 2, 3, or 4 units running simultaneously at “high” power were 4.1, 8.2, 12.3, and 16.4, respectively. Using 4 units at once running at “low” power setting yielded 8.2 air changes per hour and generated a room pressure reading of −8.0 Pa, or −0.032 inches of water.
Conclusion
Portable HEPA filtered forced air units are an effective means of creating large patient care areas with the negative-pressure environment required for respiratory isolation. This design results in a significantly lower-cost alternative compared with construction of individual rooms or units with similar capability and can be retrofitted to existing space. This type of unit would allow treatment of many more patients than current hospital capability would permit and would be an important asset in meeting the needs created by bioterrorism or a naturally occurring epidemic.
Introduction
Patients with communicable diseases, such as influenza and tuberculosis, often require respiratory isolation precautions to reduce the chance of transmission. Isolation has been effectively accomplished using a single patient room with negative air pressure and a ventilation system separated from the rest of the hospital.1
Airborne infection isolation refers to the isolation of patients infected with organisms spread by airborne droplet nuclei less than 5 μm in diameter. This isolation area receives 6 to 12 air changes per hour and is under negative pressure so that the direction of airflow is from the outside adjacent space (eg, corridor) into the room. The air in an airborne infection isolation room is preferably exhausted to the outside, but may be recirculated, provided that the return air is filtered through a high- efficiency particulate air (HEPA) filter. The use of personal respiratory protection is indicated for persons entering these rooms.2 Negative-pressure rooms are generally expensive to build, with new construction costs, according to a 1988 figure, ranging from $40,000 to $50,000 per room.3
Recent naturally occurring outbreaks of influenza or severe acute respiratory syndrome (SARS) have underscored the need to develop a flexible capacity for isolation to contain an outbreak.4 The risk of bioterrorism with a transmissible pathogen such as smallpox or plague has necessitated planning for the contingency of having to isolate large numbers of patients. Such planning calls for airborne infection isolation that is well beyond the scope of current isolation practices at most hospitals and requires either retrofitting existing facilities or constructing new ones.
Given the expense of either approach, we sought a means for mass isolation that would be inexpensive and feasible at most facilities. This article describes a project designed to transform existing space within a hospital into an airborne infection isolation unit with the capacity to isolate large numbers of patients in a negative-pressure environment. This exercise was designed to assess whether the negative-pressure environment needed to isolate multiple patients can be achieved quickly and effectively using existing hospital space.
Materials and methods
In designing the project's specification, we used the Centers for Disease Control and Prevention (CDC) Healthcare Infection Control Practices Advisory Committee Guidelines for Environmental Infection Control in Healthcare Facilities from 2003.4 We assembled a team of mechanical engineers, safety personnel, physicians, industrial hygienists, and construction personnel to design, fabricate, and assemble the material required for the conversion. The personnel costs were estimated to be less than $2,000. Planning and work began approximately 6 weeks before the unannounced exercise was to be conducted. Our clinical engineering department performed all work in-house.
The physical therapy gymnasium at our facility was used on April 28, 2003, to test the ability to create a negative air pressure environment in a large space not previously designed for such a use (Figure 1 ). This area required several minor modifications to allow use of the equipment and to effectively isolate the air in the gymnasium from that of the rest of the hospital. The gymnasium area is shown in Figure 1 as “large gymnasium” and encompasses 2,930 square feet, with a 10-foot ceiling, for a total air volume of 29,300 cubic feet.
Figure 1.
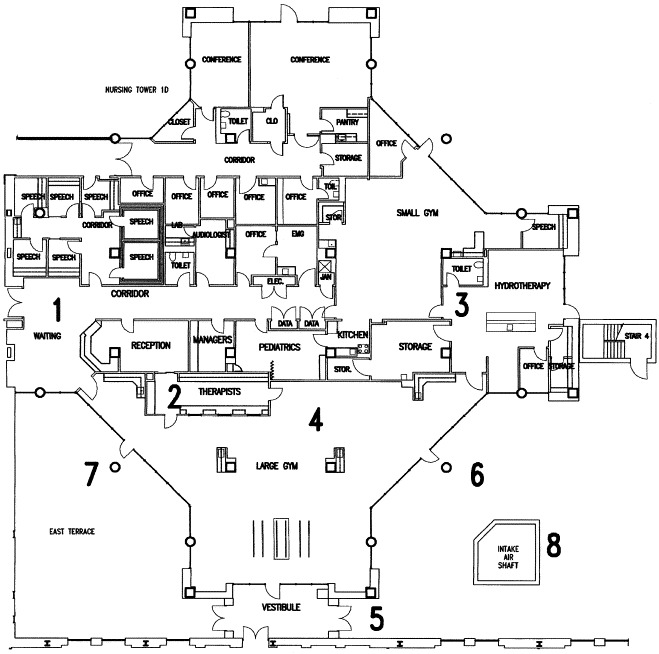
Architectural drawing of the physical therapy gymnasium illustrating its conversion to negative-pressure isolation room. The anteroom (1) is an enclosed area with access from the outside. Staff work area (2) and toilet (3) are isolated from the patient care area (4). Patient and EMS entrance is through the door adjacent to the vestibule (5). Air is exhausted after HEPA filtration through a fabricated steel insert placed in the doorway (6). The other doorway (7) may be used to avoid proximity to the air intake column (8), but room dimensions made this doorway less desirable.
The waiting area for the physical therapy gymnasium (labeled “waiting” on Figure 1) would be used as an anteroom for health care workers entering the negative-pressure area. This waiting area is separated from the main hospital by a fixed door, over which an airtight seal was applied using plastic barriers that can be zippered open and closed. The anteroom contains cabinets for supplies, a sink for hand washing, and ample space for donning and doffing personal protective garments and equipment. The anteroom serves as an added barrier to fomite transmission to the outside.
A work area within the physical therapy department was used because it could be closed off from the rest of the gymnasium by a door and a glass wall (labeled “therapists” in Figure 1), which allowed workers to be separated from patients while still being able to monitor activity within the physical therapy gymnasium. This workroom was equipped with previously installed computers, telephones, a fax machine, and other telecommunications equipment that would be essential for patient care.
Four Aramsco FA 2000 EC model HEPA-filtered forced air machines (Aramsco, Inc., Thorofare, NJ) were used for this test. Each unit is capable of moving 1,000 to 2,000 cubic feet of air per minute, depending on whether a “low” or “high” setting is used. Each unit weighs 160 pounds and is 26.5 inches high by 32.5 inches wide by 37.5 inches deep. The cost of each unit was US$731.25. Each air handler runs on 115 V, 60 Hz, and 13 A of electricity. Four dedicated 120-V, 20-A emergency power circuits were installed before the drill at a location convenient for placement of the airflow units and near the external door.
The units were vented to the outside air by a reusable duct 10 inches in diameter and 25 feet long (Aramsco model FA 600, Aramsco, Inc.). A prefabricated plywood insert, with 4 holes cut to a 12-inch diameter, was secured and sealed to enclose the gymnasium's exterior doorframe. The ducts were attached to the insert and individually secured with round collars to create an airtight seal (Figure 2 ).
Figure 2.

Photograph of HEPA filtered forced air machine installation with door insert already in place.
Sheet-metal blanks were fabricated to provide an airtight cover for existing air returns. These blanks facilitate rapid conversion to an isolation area by preventing contaminated air from flowing back into the main heating, ventilating, and air conditioning (HVAC) system once negative pressure has been created. Supply vents were left open to passively supply air into the sealed gymnasium.
The operational test evaluated the time needed to set up equipment. Any subjective difficulty with either setup or operation of the equipment was noted. The ability of the equipment to generate a negative air pressure and the noise levels created during the use of different combinations of machines and power settings were measured.
The pressures were evaluated using a TSI DP-CALC micromanometer (model 8705; TSI, Inc., Shoreview, MN). The DP-CALC measures static, total, and velocity pressures, as well as pressure decreases across filters, coils, fans, and diffusers. The microprocessor-based DP-CALC uses the latest electronic pressure transducer technology, eliminating sensitivity caused by the orientation of the meter. The micromanometer measures and displays differential pressure readings from −1,245 to 3,735 Pa (−9.3 to 28.0 mm Hg, or −5 to 15 inches of H2O). Zeroing function allows rapid and simple recalibration of the instrument.
Noise levels were measured with a Metrosonics db-3100 dosimeter sound level meter (Metrosonics, Inc., Oconomowoc, WI). We obtained separate readings using 1, 2, 3, or 4 units at once using the “high” power setting and using 4 units at once on “low” power setting.
Results
After the request for equipment setup, 1 hour was required to convert the physical therapy gymnasium into an operational negative-pressure environment, which included the time needed to move the equipment from a basement storage area, place the door insert, set up the airflow units, connect the ducts to the insert, block the air returns, and start the airflow machines. Six workers were able to accomplish this task with minimal assistance from 2 other staff members.
The pressure readings (in Pascal units), noise levels (in decibels), and calculated air changes per hour using 1, 2, 3, or 4 units at once at “high” power and using 4 units at once on “low” power setting are shown in the Table . The calculated air changes per hour changed proportionately according to whether 1, 2, 3, or 4 units were used. The negative pressure reading increased with additional units. The noise level did not appreciably change according to the number of units used or whether power was set at “low” or “high.”
Table.
The following pressure readings, noise level, and air changes per hour were obtained during the tests.∗
| No. of HEPA Units Operating | Power Level | Pressure Readings Pa (Inches of Water) | Noise Levels, dB | Air Changes per Hour (Calculated) |
|---|---|---|---|---|
| 1 | High | −1.5 (−0.006) | Not tested | 4.1 |
| 2 | High | −4.5 (−0.018) | 74 | 8.2 |
| 3 | High | −8.0 (−0.032) | Not tested | 12.3 |
| 4 | High | −13.0 (−0.052) | 76 | 16.4 |
| 4 | Low | −8.0 (−0.032) | 70 | 8.2 |
Calculations of air changes per hour are number of units × (2,000 cubic feet per minute [cfm] on high setting or 1,000 cfm on low setting) × 60 minutes divided by 29,300 (cubic feet of room). Example based on 4 units on high setting: (4 units × 2,000 cfm × 60 minutes) ÷ 29,300 = 16.4 air changes/hour.
Limitations
Limitations of the project include the test being conducted when patients were not scheduled to be present and that there was no measure of possible delays caused by patient movement or blockage of access to required areas. Time requirements could vary, depending on the number of personnel available to perform the conversion of the room and movement of equipment. Different sizes or shapes of rooms may present different requirements, and further testing would be required to confirm the effectiveness of this technique in other locations.
Our estimate of a 30 patient-bed capacity was based on rough measurements, and we did not place any simulated patients in this area, nor did we simulate any medical procedures during this evaluation. These tests were conducted with new machines and filters; older machines or clogged filters could limit the effective air exchange capacity. This, however, should be less of a problem, given that the environment will not have large particulate matter but only microscopic contaminants.
The CDC guidelines4 specify that the exhaust system “shall terminate above roof level either through an accessible HEPA filter or vertically through a self-draining stack discharging not less than 7 feet above the roof.” We did not add this requirement to the project because it would have required a vertical stack approximately 80 feet high. Ideally, air should exhaust above the level of the roof, but the HEPA filtration should prevent contamination of the rest of the building.
Another limitation is that we did not require testing, adjusting, and balancing by a qualified, independent, certified agency before acceptance as a certified negative-pressure isolation room. In actual practice, we would need to ascertain that the hallway outside the physical therapy room is free of infectious particles, which was not tested during our drill.
Discussion
In a 1993 study, Fraser et al5 surveyed 7 hospitals with a total of 3,574 hospital rooms and found that only 121 rooms (3.4%) were designed to have negative-pressure ventilation suitable for respiratory isolation. Although only a small fraction of rooms were designated for respiratory isolation, the performance of these existing negative-pressure rooms was not tested regularly. Many high-risk areas, such as ICUs and emergency departments, were not equipped to provide any degree of respiratory isolation.
Industrial hygienists from the New York State Department of Health evaluated 140 designated isolation rooms in 38 facilities within New York State during 1992 to 1998. The rooms were located in the following settings: hospitals (59%), correctional facilities (40%), and nursing homes (1%). Each room was tested with visible smoke for directional airflow into the patient room (ie, negative air pressure relative to adjacent areas). Inappropriate outward airflow was observed in 38% of the isolation rooms tested. These findings indicate that a balanced ventilation system does not guarantee inward airflow direction. Devices that continuously monitor and, in some cases, control pressurization were noted to have poor reliability.6
Infections among health care workers have been a common feature of SARS since its emergence. The majority of these infections have occurred in locations where infection-control precautions either had not been instituted or had been instituted but were not followed. Previously recommended infection-control precautions from 2002 included the use of negative-pressure isolation rooms; N95 or higher level of respiratory protection; gloves, gowns, and eye protection; and careful hand hygiene. A 2003 report from the CDC demonstrated that a cluster of SARS cases among health care workers in a hospital occurred despite apparent compliance with recommended infection-control precautions. In fact, hospitals, because of infection of health care workers, appeared to be sites of SARS epidemic amplification during the epidemic of 2002 to 20037; therefore, HEPA filtration and ultraviolet germicidal irradiation are recommended as additional infection control measures.4
Increasing hospital capacity to care for patients requiring respiratory isolation is a current focus of emergency preparedness efforts nationwide (Health Resources and Services Administration/CDC grant guidance 2003). The recognition that a mass casualty incident as a result of an intentionally released agent or an epidemic from a naturally occurring outbreak of illness could overwhelm current isolation capabilities has resulted in many suggested plans to handle such an event. Christiana Care Health System and the Delaware Health Care Preparedness Committee sought to examine these methods and design a system to meet our needs.
We began with a fundamental goal of maximizing the ability to provide care at current hospital sites before moving to an alternate location, which, by necessity, required us to search for surge capacity within the current physical plants of our hospitals. Plans to use facilities such as those described in the Modular Emergency Medical System plan, including use of Neighborhood Emergency Help Centers or acute care centers, or the use of a designated quarantine hospital were placed farther down our list of options. Hospitals were asked to identify areas that were not continuously used for patient care but could be opened to provide patient care on an emergency basis.
Ideal locations for a respiratory isolation unit were considered, and several characteristics were identified. The room should have immediate access from the outdoor environment and will almost certainly be on the ground floor, which limits the risk of contamination to other areas of the hospital and requires adequate door width, firm (concrete or paved) pathways, and accessibility to emergency medical services (EMS) units, with minimal stretcher movement requirements. The standards would match those for a hospital emergency exit.
The room should have doors or windows that can be replaced or fit with ventilation ducts to allow connection of the airflow units. These connections should be easily installed or made available for conservation of time. The room should be located at a peripheral location of the hospital to limit the number of air intake and return units affected. Blocking off an endpoint in the ventilation system presents far fewer difficulties than trying to isolate a more central area.
Before use, as many modifications as possible should be installed in the area chosen, which includes power modifications, ventilation duct installation or template fabrication, design of air-return barriers, and construction of shelving or any other structure or equipment that will be needed to operate the unit. Ideally, these modifications will not affect the current operation of the chosen unit.
The physical therapy gymnasium at Christiana Hospital met the specifications and was able to be fit with methods to seal the usual ventilation system. Removal of one exterior door and replacement with a plywood insert equipped with round collars for the flexible ductwork allowed the creation of negative air pressure within the gymnasium area and ventilation of filtered air to the outside of the hospital. In large health care facilities with central HVAC systems, sealed windows help to ensure the efficient operation of the system, especially with respect to creating and maintaining pressure differentials. Sealing the windows in isolation areas helps minimize the risk of airborne contamination to the outside.4
There was some concern about the proximity of the exhaust ports to the elevated air intake shaft. In addition, the air intake shaft and the patient arrival area appear to be in close proximity to the exhaust from this space. The American Institute of Architects standards stipulate that for new or renovated construction (1) exhaust outlets be placed more than 25 feet from air intake systems; (2) the bottom of outdoor air intakes for HVAC systems be 6 feet above ground or 3 feet above roof level; and (3) exhaust outlets from contaminated areas be situated above the roof level and arranged to minimize the recirculation of exhausted air back into the building.8 HEPA filtration of exhaust air from airborne isolation rooms is not required, providing that the exhaust is properly located to prevent reentry into the building. During planning, we determined that placement of exhaust outlets above the roof level was impractical for our ground floor conversion. Instead, we chose to use HEPA filtration of the exhaust air as a precaution, noting that the air intake shaft is over 30 feet above ground, which is not apparent in the drawing.
Air from toilet rooms or other soiled areas is usually exhausted directly to the atmosphere through a separate duct exhaust system. Air from rooms housing tuberculosis patients is exhausted to the outside if possible or passed through a HEPA filter before recirculation. Ultraviolet germicidal irradiation can be used as an adjunct air-cleaning measure, but it cannot replace HEPA filtration.9 We did not test an ultraviolet germicidal irradiation unit because the need for one in this setting is unclear. The use of ultraviolet lamps and HEPA filtration in a single unit offers only minimal infection-control benefits over those provided by the use of a HEPA filter alone.10
The portable airflow units were used to draw air through the care area, creating air changes in excess of the current CDC guidelines that specify 12 air changes per hour for particles smaller than 5 μm.4 The pressure gradient generated was acceptable and exceeded the minimum of 5 Pa (−0.02 inches of water) required for asbestos removal projects and on medical isolation facilities, which require a minimum of 2.5 Pa (−0.01 inches of water) for an isolation room.4., 8. This gradient indicated a pressure differential that would ensure isolation of air from this patient care area away from the rest of the hospital. The total cost of the project was less than $6,000, including the 4 portable airflow HEPA units, flexible ductwork, door and air return conversion, and installation of dedicated high-output power outlets. We estimated that 30 patients could be cared for comfortably within this environment, and although they would be in close proximity, there would still be adequate separation between them to allow sufficient room for staff to work.
Noise generation produced by the units was an area of concern. Total noise levels were well below the Occupational Safety and Health Administration (OSHA) standard for 8-hour or 16-hour work periods (90 and 85 dB, respectively; OSHA occupational noise exposure standard 29 CFR 1910.95) and would also fall below the 24-hour limit (approximately 83 dB). This 24-hour level is a potentially important consideration for patient safety. The level of 76 dB, although below maximal occupational levels, would be louder than operation of a household vacuum (70 dB per National Institute for Occupational Safety and Health 1973 source) and would still potentially be annoying. Methods to decrease the noise exposure could include using more units on the low setting, designing methods to deflect noise away from patient care areas, or maximizing the distance between patients and the airflow units.
In conclusion, this project describes the conversion of existing space and offers a significantly lower-cost alternative to construction of individual rooms or units with similar capabilities. The chosen rooms can be modified in advance to allow rapid conversion and generation of the negative-pressure environment. This type of unit would allow treatment of many more patients than current hospital capability would permit and would be an important asset to meeting the needs that would be created by a bioterrorist attack or a naturally occurring epidemic. We conclude that use of portable HEPA filtered forced air units is an effective means of creating large patient care areas with negative-pressure environments.
Acknowledgments
We thank Steven A. Gondek, David Parker, AS, and Joseph Hughes, BS, for their technical assistance in designing and fabricating materials for the project.
Footnotes
Presented in part at the National Bioterrorism Hospital Preparedness Program (sponsored by the Health Resources and Services Administration), Washington, DC, November 2003.
The equipment was purchased with funds from the Health Resources and Services Administration–sponsored National Bioterrorism Hospital Preparedness Program (grant 4U3RMC00021-01).
Reprints not available from the authors.
References
- 1.Garner J.S. The CDC Hospital Infection Control Practices Advisory Committee. Am J Infect Control. 1993;21:160–162. doi: 10.1016/0196-6553(93)90009-s. [DOI] [PubMed] [Google Scholar]
- 2.Sehulster L.M., Chinn R.Y.W., Arduino M.J. American Society for Healthcare Engineering/American Hospital Association; Chicago, IL: 2004. Guidelines for Environmental Infection Control in Health-Care Facilities: Recommendations From CDC and the Healthcare Infection Control Practices Advisory Committee (HICPAC) [Google Scholar]
- 3.McPherson D.C., Jackson M.M., Rogers J.C. Evaluating the cost of the body substance isolation system. J Healthcare Material Mgmt. 1988;6:20–28. [PubMed] [Google Scholar]
- 4.Chowell G., Fenimore P.W., Castillo-Garsow M.A. SARS outbreaks in Ontario, Hong Kong and Singapore: the role of diagnosis and isolation as a control mechanism. J Theor Biol. 2003;224:1–8. doi: 10.1016/S0022-5193(03)00228-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fraser V.J., Johnson K., Primack J. Evaluation of rooms with negative pressure ventilation used for respiratory isolation in seven midwestern hospitals. Infect Control Hosp Epidemiol. 1993;14:623–628. doi: 10.1086/646654. [DOI] [PubMed] [Google Scholar]
- 6.Pavelchak N., DePersis R.P., London M. Identification of factors that disrupt negative air pressurization of respiratory isolation rooms. Infect Control Hosp Epidemiol. 2000;21:191–195. doi: 10.1086/501742. [DOI] [PubMed] [Google Scholar]
- 7.Centers for Disease Control and Prevention. Cluster of severe acute respiratory syndrome cases among protected health-care workers: Toronto, Canada, April 2003. MMWR Morb Mortal Wkly Rep. 2003;52:433–436. [PubMed] [Google Scholar]
- 8.American Institute of Architects Press; Washington, DC: 2001. American Institute of Architects. Guidelines for Design and Construction of Hospital and Health Care Facilities, 2001. [Google Scholar]
- 9.Burroughs H.E.B. Sick building syndrome: fact, fiction, or facility? In: Hansen W., editor. A Guide to Managing Indoor Air Quality in Health Care Organizations. Joint Commission on Accreditation of Health Care Organizations; Oakbrook Terrace, IL: 1997. pp. 3–13. [Google Scholar]
- 10.ECRI Health devices evaluation of mobile high efficiency filter air cleaners (MHEFACs) ECRI. 1997;26:367–388. [PubMed] [Google Scholar]


