Abstract
Background
Approximately 15.5 million deaths from cardiovascular diseases occur every year. About half are due to acute myocardial infarction (AMI), and 80% occur in low- and middle-income countries. Therefore, low-cost therapies would be invaluable. Although glucose-insulin-potassium (GIK) infusion and low-molecular-weight heparin (LMWH) appear to be promising in AMI, the available trials are inconclusive and these treatments require rigorous evaluation.
Methods
The Clinical Trial of Reviparin and Metabolic Modulation in Acute Myocardial Infarction Treatment and Evaluation-Estudios Clínicos Latino America (CREATE-ECLA) study is a randomized controlled trial in ST-elevation AMI patients evaluating a 24-hour infusion of Glucose-Insulin-Potassium (GIK) intravenous vs usual care (control) on 30-day mortality in 20 000 patients from 21 countries. Patients from India and China (n = 15 000) are also randomized using a factorial design to receive low-molecular-weight heparin (Reviparin) or placebo injection twice daily for 7 days to assess the impact on the composite outcomes of death, reinfarction or stroke (first co-primary outcome) or the composite + refractory ischemia (second co-primary outcome).
Results
Twenty thousand two hundred and one (20,201) GIK/control patients and 15,570 Reviparin/placebo patients have been included, with results expected in November 2004.
Conclusions
The CREATE-ECLA trial will provide definitive answers to the role of 2 practical, promising and low-cost therapies, LMWH and GIK, in AMI patients. If effective, these therapies could be used in small medical centers in low- and middle- income countries. The experiences in this trial indicate that large trials of important questions can be successfully conducted in resource-poor settings, by academic groups without industry involvement.
See related article on page 924.
Approximately 15.5 million deaths from cardiovascular diseases (CVD) occur every year.1 Of these, about half are due to acute myocardial infarction (AMI), and over 80% of these occur in low- and middle-income countries (LIC and MIC), especially in the Indian subcontinent and China. Aspirin, thrombolytic therapy, β-blockers and angiotensin converting enzyme (ACE)-inhibitors improve prognosis in AMI,2 and have been adopted widely. Recent trials suggest that primary percutaneous coronary angioplasty (PCI) offers some benefit over thrombolytic therapy,3 but rapid access to primary PCI is limited in most parts of the world. Combinations of newer anti-platelet regimens4 or direct thrombin inhibitors5 have not yielded clear incremental benefit, but cause increased bleeding. Although heparin is commonly used after AMI, especially in patients receiving a fibrin-specific thrombolytic agent, and some trials of low-molecular-weight heparin (LMWH) appear promising, there is no convincing evidence that these agents reduce mortality in patients receiving thrombolytic therapy and aspirin (Table I, Table IIA, Table IIB, A and II, B).6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18 Although LMWH reduces reinfarction, there appears to be no impact on mortality. Furthermore, there are significant increases in major bleeds and a trend towards more hemorrhagic strokes with both agents (Table IIA, Table IIB, A and II, B). Therefore, the net benefit-risk ratio is not clear. Further, some of the larger trials were not blinded, so one cannot exclude the potential for biases in the ascertainment of outcomes that involve judgement (eg, early reinfarction). The uncertainty about the net benefits and risks of LMWH is reflected in no clear recommendation for the use of thrombin inhibitors in AMI, especially in those receiving a thrombolytic agent that is not fibrin-specific. Although UFH is widely used after a fibrin-specific thrombolytic agent, this is based on improved coronary artery patency in 2 small trials, which did not have the power to reliably assess the impact on clinical outcomes such as death, reinfarction, bleeding, or strokes. Therefore, a large definitive trial of LMWH vs placebo, is urgently needed.
Table I.
Trials of subcutaneous or intravenous unfractionated heparin in patients with acute myocardial infarction treated with thrombolytic therapy
| Trial | Treatment | N | Any death, n (%) | Reinfarction, n (%) | Any stroke, n (%) |
|---|---|---|---|---|---|
| SC heparin versus control | |||||
| ISIS-37 | SC Heparin + ASA | 20656 | 2132 (10.3) | 378 (1.9) | 261 (1.3) |
| ASA alone | 20643 | 2189 (10.6) | 414 (2.0) | 240 (1.2) | |
| GISSI-28 | SC Heparin + ASA | 10361 | 968 (9.3) | 218 (2.1) | 115 (1.1) |
| ASA alone | 10407 | 983 (9.4) | 239 (2.3) | 119 (1.1) | |
| IV heparin versus SC heparin | |||||
| GUSTO-16 | SK + IV Heparin | 10410 | 763 (7.4) | 438 (4.0) | 144 (1.4) |
| SK + SC Heparin | 9841 | 712 (7.2) | 343 (3.4) | 117 (1.2) |
SC, Subcutaneous, IV, intravenous; ASA, aspirin.
Table IIA.
Trials of low molecular weight heparin versus placebo in patients with acute myocardial infarction
| Trial | Treatment | N | Setting | Duration | Death | Re-MI | Major Bleeds | Hemorrhagic Stroke |
|---|---|---|---|---|---|---|---|---|
| FRAMI 19979 | Dalteparin | 338 | Started 8 hrs after TT | Hospital period | 23 | 6 | 11 | 3 |
| Placebo | 338 | 23 | 8 | 1 | 0 | |||
| Glick 199610 | Clexane | 43 | Started 5 days after TT | 25 days | 0 | 2 | 0 | 0 |
| Placebo | 60 | 1 | 13 | 0 | 0 | |||
| BIOMACS II 199911 | Dalteparin | 54 | Adjunct to SK | 1 day | 4 | 8 | 2 | 0 |
| Placebo | 47 | 6 | 2 | 0 | 0 | |||
| AMI-SK12 | Enoxaparin | 253 | Adjunct to SK | 3–8 days | 17 | 6 | 12 | 0 |
| Placebo | 243 | 3–8 days | 17 | 18 | 6 | 1 | ||
| Total | LMWH vs. | 688 | – | – | 44 (6.4%) | 22 (3.2%) | 25 (3.6%) | 3 (0.44%) |
| Placebo | 688 | – | – | 47 (6.8%) | 41 (6.0%) | 7 (1.0%) | 1 (0.15%) | |
| OR (95% CI)* | – | – | – | 0.75(0.36–1.55) | 0.54(0.33–0.91) | 3.00(1.50–6.00) | 2.01(0.40–9.99) |
Re-MI, Reinfarction; TT, thrombolytic therapy; SK, streptokinase.
The odds ratio (OR) and 95% confidence intervals (CI) are calculated using the Yusuf-Peto modification of the Mantel-Haenszel method.35
Table IIB.
Trials of low molecular weight heparin versus unfractionated heparin in acute myocardial infarction
| Trial | Treatment | N | Setting | Duration | Death | Re-MI | Major bleeds | Hemorrhagic stroke |
|---|---|---|---|---|---|---|---|---|
| Baird 199813 | Enoxaparin | 149 | Adjunct to TT | 4 days | 9 | 22 | NR | NR |
| UFH | 151 | 16 | 30 | |||||
| HART II 200014 | Enoxaparin | 200 | Adjunct to tPA | 3 days | 9 | NR | 7 | 2 |
| UFH | 200 | 10 | NR | 6 | 2 | |||
| TETAMI15 | Enoxaparin | 604 | AMI patients ineligible for reperfusion | 2–8 days | 42 | 15 | 9 | 4 |
| UFH | 620 | 41 | 18 | 8 | 4 | |||
| ENTIRE TIMI 2316 | Enoxaparin | 324 | Adjunct to TNK and Abx | Max. 8 days | 10 | 6 | 17 | 4 |
| UFH | 159 | Min. 36 hours | 5 | 13 | 6 | 1 | ||
| ASSENT 317 | Enoxaparin | 2040 | Adjunct to TNK | Max. 7 days | 109 | 54 | 62 | 18 |
| UFH | 2038 | 48 hours | 122 | 86 | 44 | 19 | ||
| ASSENT 3 | Enoxaparin | 818 | Prehospital adjunct to TNK | Max 7 days | 61 | 29 | 33 | 18 |
| PLUS18 | UFH | 821 | 48 hours | 49 | 48 | 23 | 8 | |
| TOTAL | LMWH vs | 4135 | – | – | 240/3971(5.8%) | 126/3935(3.2%) | 128/3986(3.2%) | 46/3986(1.2%) |
| UFH | 3989 | – | – | 243/3989(6.1%) | 195/3789(5.1%) | 87/3838(2.3%) | 34/3838(0.89%) | |
| OR (95% CI)* | – | – | – | 0.97(0.81–1.17) | 0.61(0.48–0.76) | 1.38(1.05–1.81) | 1.30(0.84–2.03) |
Re-MI, Reinfarction; NR, not recorded; TT, thrombolytic therapy; tPA, tissue plasminogen Activator; Abx, abciximab; TNK, tenecteplase.
The odds ratio (OR) and 95% confidence intervals (CI) are calculated using the Yusuf-Peto modification of the Mantel-Haenszel method.35
Metabolic modulation in AMI with glucose-insulin-potassium (GIK) infusions was proposed in the 1960s. GIK suppresses myocardial uptake of free fatty acids, thereby reducing myocardial oxygen requirements and improving ventricular contractility. It also increases intramyocellular potassium. These effects may reduce life-threatening arrhythmias, and improve ventricular function, which could reduce mortality. A meta-analysis of 15 small trials19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34 involving almost 5 000 patients indicates an 18% relative-risk reduction in mortality (P = .03), but with wide CIs (Table III and Figure 1)35 However, trials which evaluated high doses of glucose and insulin reported a 30% relative-risk reduction (RRR) in mortality (P = .03) compared to a non-significant 11% RRR with low-dose GIK. Most of these trials antedate thrombolytic agents or aspirin, and some had incomplete follow-up or lack of information on the integrity of randomization. Although encouraging, a large definitive trial of GIK in the context of modern management of AMI is required. Despite the low costs of GIK and potential global applicability, a large trial of this question is yet to be done, perhaps because of a lack of commercial interest, and complex rules which hamper the conduct of large trials of academically-driven questions with little external funding.
Table III.
Design of the Randomized trials of GIK vs. control in acute myocardial infarction, and GIK regimen used
| STUDY | Year | 2N | Duration of infusion | GIK regimen |
|---|---|---|---|---|
| Mittra19 | 1965 | 170 | 14 days | Low dose: Oral or I.V.: Oral: 240 g oral glucose O.D. + 10 U SC soluble insulin B.I.D. +52–78 mEq effervescent potassium O.D., or I.V.: 21/170 patients received 10% dextrose 500 cc + 10 U soluble insulin + 20 mEq KCl infused at 40–60 drops/minutes (1.5–2 L/day) for 3–4 days, then oral dose upto 14 days, versus usual care control |
| Pilcher20 | 1967 | 102 | 14 days | Low dose: Oral: using Mittra regimen above, versus oral placebo tablets |
| Pentecost21 | 1968 | 200 | 48 h | Low dose: I.V. 10% dextrose 1500 cc + 30 U soluble insulin + 30 mEq potassium for first 24 hours; 10% dextrose 1000 cc + 30 U soluble insulin + 20 mEq potassium for second 24 hours, versus usual care control |
| M.R.C.22 | 1968 | 968 | 14 days | Low dose: Oral or I.V.: Oral: 160 g glucose in water 1500 mL per day + 20 U SC soluble insulin per day + 52 mEq potassium per day, or I.V.: 40 mL of 50% glucose, then 10% glucose in water 1500 mL per 24 hours + 30 U soluble insulin per 24 hours + 45 mEq KCl per day, versus oral placebo (starch and lactose) control |
| Hjermann23 | 1971 | 204 | 10 days | Low dose: Oral: 200 g glucose per day + 16 U long acting insulin per day + 55 mEq potassium per day, versus placebo juice containing sodium cyclamate and placebo SC insulin control |
| Heng24 | 1977 | 27 | 6–12 h | High dose: I.V.: 50% glucose (5.5 mmol/kg) infused for 10 minutes at 2.0 mL/kg + 0.4 U/kg soluble insulin, then 50% glucose (4.2 mmol/kg/hr) + 0.3 U/kg soluble insulin + 0.15 mmol/kg KCl infused at 1.5 mL/kg/hr; or 50% glucose (5.55 mmol/kg) infused at 2.0 mL/kg for 10 minutes (4.2 mmol/kg/hr), then 50% glucose infused at 1.5 mL/kg/hr (4.2 mmol/kg/hr), versus control of normal saline solution infused for 10 minutes at 2 mL/kg, then at 1.5 mL/kg/hr (4.2 mmol/kg/hr) |
| Stanley25 | 1978 | 110 | 48 h | High dose: I.V.: 300 g glucose + 50 U regular insulin + 80 mEq KCl/L infused at 1.5 mL/kg/hr, versus control solution of half-normal saline |
| Rogers26 | 1979 | 134 | 48 h | High dose: I.V.: 300 g glucose + 50 U insulin + 80 mEq potassium/L infused at 1.5 mL/kg/hr, versus control solution of half-normal saline at a rate to keep catheter patent |
| Mantle27 | 1981 | 85 | 48 h | High dose: I.V.: 300 g glucose + 50 U insulin + 80 mEq KCl/L infused at 1.5 mL/kg/hr for 48 hrs, then 0.45% NaCl + 5000 U/L heparin at “keep open” rate of 20 mL/hr through CV and PA lumen for one day, versus control of same heparinized 0.45% NaCl solution through both lumens of catheter at “keep open” rate for whole study period (3 days) |
| Whitlow28 | 1982 | 28 | 48 h | High dose: I.V.: 300 g glucose + 50 U regular insulin + 80 mEqKCL/L infused at 1.5 mL/kg/hr for 48 hrs, then 0.45% NaCl for 2 days at 20 mL/hr, versus control of 0.45% NaCl at 20 mL/hr for 4 days |
| Salter29 | 1987 | 17 | 48 h | High dose: I.V.: 300 g glucose + 50 U regular insulin + 80 mEq potassium/L infused at 1.5 mL/kg/hr versus control of 5% dextrose in water at 1.5 mL/kg/hr |
| Malmberg30 | 1995 | 620 | 24 h | High dose: I.V.: 5% glucose 500 cc + 80 U soluble insulin (no potassium) infused at 30 mL/hr and rate adjusted to blood glucose nomogram, then s.c. soluble insulin 3 times daily and medium-long acting insulin once daily for 3 months with dosage for stable normoglycemia, versus usual care only (insulin if clinically indicated) |
| Diaz31 | 1998 | 407 | 24 h | Low or High dose: I.V.: Low dose: 10% glucose + 20 U insulin + 40 mmol KCL infused at 1.0 mL/kg/hr/hr High dose: 25% glucose + 50 U soluble insulin + 80 mmol KCL infused at 1.5 mL/kg/hr versus usual care control |
| Ceremuzynski32 | 1999 | 962 | 24 h | Low dose I.V.: 10% dextrose 1000 mL + 32 U rapid insulin + 20 U Humulin R insulin (mixed insulin for only 369/954 patients; due to hypoglycemia, remaining patients received only 20 U short-acting Humulin R in infusion) + 6.0 g potassium chlorate infused at 42 mL/hr, versus control of 0.9% NaCl 1000 mL at 42 mL/h for 24 hrs |
| Diaz-Araya33 | 2002 | 20 | 24 h | High dose: I.V.: 30% glucose + 50 U insulin + 40 mM KCl/L infused at 1.5 mL/kg/hr versus control of normal saline solution at 1.5 mL/kg/hr |
| van der Horst34 | 2003 | 940 | 8–12 h | High dose: I.V.: 20% glucose in 500 mL water + 80 mmol KCl infused at 3.0 mL/kg/hr, with infusion of 50 U short-acting insulin in 50 mL 0.9% NaCl with infusion rate adjusted to blood glucose nomogram versus control of no infusion |
Figure 1.

Overall results of trials of GIK vs control on all-cause in-hospital mortality.36 The trial by Ceremuzynski measured outcomes at 35 days. Data from individual trials are combined utilizing a modified Mantel-Haenszel method.35, 36
Over the last 6 years, a group of us from several countries decided to perform 2 similar trials, the CREATE trial and the ECLA trial (Estudios Clínicos Latin America Study Group) aimed at reliably evaluating the role of high-dose GIK in 20 000 patients. In addition, in India and China (CREATE), we simultaneously evaluated a LMWH, Reviparin, in about 15 000 subjects. Although the 2 trials were designed and initiated separately, given the substantial similarity of the GIK components, an early decision was made to merge and standardize the 2 trials to more reliably evaluate GIK. Below, we describe the design of this study, the baseline characteristics, and discuss the challenges faced in establishing this trial.
The CREATE trial
The CREATE trial was initiated in 2000 by investigators from Canada, China, and India without external funding. It utilized “opportunistic” meetings and internal funds from the Population Health Research Institute (PHRI) at McMaster University and Hamilton Health Sciences. The study design was simple, data collection parsimonious and focused on major clinical outcomes. We emphasized data quality and adherence to the study regimen. This was facilitated by training investigators, data entry through the internet, central data review, central event adjudication of key outcomes, and judicious on-site monitoring.
Objectives of the trial
-
•
For GIK, the primary outcome was a reduction in 30-day mortality compared to usual care.
-
•
For Reviparin compared to placebo there were 2 co-primary outcomes: The first co-primary outcome was the composite of death, MI, or stroke at 7 days; the second co-primary outcome included the above + severe ischemia with ECG changes at 7 days.
Design and drug administration
The CREATE trial utilizes a partial 2 × 2 factorial design comparing Reviparin (a low-molecular-weight heparin) to placebo given for 7 days or until discharge (if discharge is earlier than 7 days [double blind]), and GIK vs control (open) given for 24 hours. All patients in India and China were randomized to both parts of the trial (n = 15,570 patients), whereas those from Pakistan were only included in the GIK component (total of 20,201 patients). All patients presenting with suspected AMI and ST-segment elevation or new left bundle branch block within 12 hours of symptom onset, and who were without contraindications to heparin or GIK and who provided written consent or witnessed oral consent were randomized (Figure 2 -Study design flow chart). It was recommended that study drugs be initiated within 15 minutes of thrombolytic therapy. The dose of Reviparin or placebo was weight-based. Patients < 50 kg received 3436 IU antiXa Ph Eur units* of Reviparin every 12 hours subcutaneously; those between 50–75 kg received 5153 IU antiXa Ph Eur units* every 12 hours; and those > 75 kg received 6871 IU antiXa Ph Eur units* every 12 hours. Drugs and placebo were provided by Abbott Laboratories. In patients undergoing primary PTCA, open-label unfractionated heparin could be used for up to 24 hours, with study medication being initiated thereafter, 1 hour after removal of the sheath. All other non-study thrombin inhibitors were not allowed, unless there was a clinical need, in which case blinded-study medication was discontinued.
Figure 2.
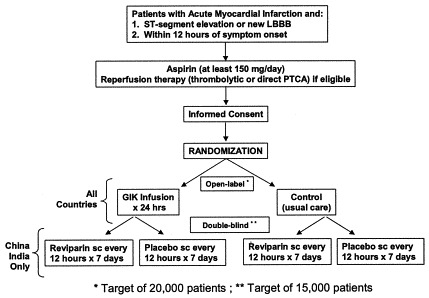
CREATE-ECLA partial factorial study design.
Glucose-insulin-potassium was prepared by adding 25 IU of insulin (regular or human) and 40 mmol of KCl to a 500 mL bag of 25% glucose, and infused through a dedicated 14-gauge peripheral intravenous (IV) site at a rate of 1.5 mL/kg/h for 24 hours (regimen identical to ECLA pilot).31 Patients randomized to the control group received usual care. Glucose, potassium and sodium levels were checked at baseline, 6 hours, and 24 hours. Adjustments to the rate of infusion of GIK were allowed based on blood glucose and potassium levels and on the Killip Class status of the patient. Fluid intake and output for the first 24 hours was recorded in all subjects.
All other aspects of patient management were at the discretion of the local treating physician. All centers obtained local Ethics Committee approval, and in addition, the Project Office obtained approval from the Institutional Review Board of the Hamilton Health Sciences and McMaster University, Hamilton, Canada.
The ECLA study
The ECLA group had independently completed a pilot trial in 1996, with promising results with high-dose GIK.31 Utilizing the high-dose GIK regimen, the main ECLA trial was initiated in mid-1998 in Latin America, with later expansion to Kuwait, Europe, the USA, and Australasia; this component recruited 3804 patients. Given the identical nature of the GIK regimen in CREATE and ECLA, and primary outcomes (30-day mortality) and similarity in data-collection between the 2 trials, the 2 trials were merged in November 2002 for evaluation of GIK. This ensured that 20 000 patients would be randomized by mid-2004. Harmonization of key aspects (definitions, data collection, etc.) of the 2 studies occurred.
Study organization
The study involves 274 centers in China, 67 in India, 4 in Pakistan, 46 in Argentina, 22 in Brazil, 1 in Kuwait, 16 in Venezuela, 9 in North America, and 14 in Europe. Data from centers in China were sent by mail to the National Center (NC) in Beijing, from Indian Centers to the NC in Bangalore, from Pakistani centers to the NC in Karachi, and from all other centers to the ECLA office in Rosario, Argentina. These regional coordinating centers entered the data into a web-based Oracle database (other than in ECLA) that was connected online to the Population Health Research Institute (PHRI) in Hamilton, Canada. Extensive consistency and edit checks, and central-event adjudication ensured high data quality. Data from the ECLA trial were transferred to the PHRI at regular intervals, where additional data checks were conducted prior to statistical analyses and reports.
An independent Data and Safety Monitoring Board (DSMB) was formed by joining the Boards that had been set up for each of the 2 trials. One member of the ECLA DSMB (S. Yusuf) stepped down from the joint Board as soon as the studies were combined and remained blinded to the subsequent data. At that time, < 1000 subjects had been reviewed by the ECLA DSMB. The newly constituted Board periodically reviewed the accumulating data on efficacy and safety. Three formal interim analyses occurred when 25%, 50% and 75% of the data were available. For the first 2 looks, the boundary for benefit was 4 SDs (χ2 of 16; P < .0001) for 30-day mortality (GIK) or the first co-primary outcome for Reviparin. For the third look, the boundary was 3.5 (χ2 of 12.25; P < .00047) deviations. The boundary had to remain crossed on 2 successive examinations of the data about 3 months apart, to ensure robustness and consistency of results.
Power
Anticipating a 12% rate at 7 days in the placebo group for the first co-primary outcome of death, MI, or stroke with 15 000 patients, there would be 93% power to detect a 15% relative-risk reduction with Reviparin. For the GIK comparison, with a 10% death rate at 30 days in the control group, with 20 000 subjects, there would be 95% power to detect a RRR of 15%, and 80% power to detect a RRR of 11.7%.
Patient recruitment
Recruitment commenced in August 1998 in South America, July 2001 in China, January 2002 in India, and October 2003 in Pakistan (Figure 3). By July 9, 2004, the overall trial had recruited 20,201 patients, with 15,507 into the component evaluating Reviparin.
Figure 3.
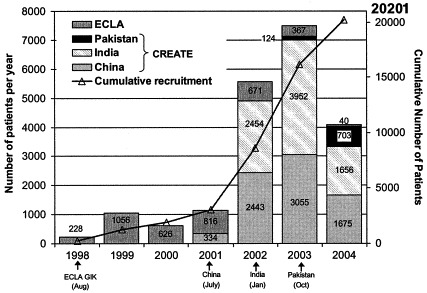
CREATE-ECLA Number of patients recruited per year between August 20, 1998 – July 9, 2004.
Baseline characteristics
Table IV summarizes key baseline characteristics. About two thirds of the patients were randomized in < 6 hours of symptom onset. 84.6% of the patients were in Killip Class I, with 15.4% having some evidence of heart failure. The baseline glucose level is 9.0 mmol/L and potassium is 4.0 mEq/L. Thrombolytic therapy was used in 74.1%, and primary PCI in 9.1%. Rates of reperfusion therapy were higher in India (94.0%) compared to China (62.1%). Of the patients in India and China, pre-randomization heparin was given in 9.4% of patients, and non-study heparin after randomization was used in only 9.9% of patients. Reviparin or matching placebo was given in 98.7% of individuals and GIK was given in 98.0% of patients randomized to GIK. Aspirin was used in 97.3%, clopidogrel in 48.6%, β-blockers in 70.0%, ACE inhibitors in 72.4%, lipid-lowering medications in 67.7% (other than in ECLA, where this information was not collected), and calcium channel blockers in 8.8%. Thus, a high proportion of patients received proven pharmacologic therapies and there was excellent adherence to the allocated study treatments.
Table IV.
Key characteristics of CREATE-ECLA trial patients
| Baseline characteristic | CREATE |
ECLA | Overall | ||
|---|---|---|---|---|---|
| India | China | Pakistan | |||
| Number of patients | 8060 | 7510 | 827 | 3804 | 20,201 |
| Number of case report forms | 8060 | 7510 | 827 | 3798 | 20,195 |
| Age (mean & SD) | 55.5 (11.8) | 62.7 (11.9) | 55.4 (11.3) | 57.9 (12.4) | 58.6 (12.4) |
| % Males | 82.2 | 70.7 | 80.9 | 80.9 | 77.6 |
| Onset of symptoms to randomization (%) | |||||
| <6 Hours | 65.1 | 57.6 | 53.1 | 78.1 | 64.3 |
| 6–12 Hours | 34.9 | 42.1 | 46.9 | 20.9 | 35.4 |
| Median time (hours) | 4.5 | 5.2 | 5.7 | 3.7 | 4.7 |
| Previous MI (%) | 7.0 | 7.9 | 7.4 | 11.3 | 8.1 |
| Diabetes (%) | 22.8 | 11.2 | 23.9 | 18.6 | 17.7 |
| Hypertension (%) | 28.0 | 40.6 | 48.6 | 47.0 | 37.1 |
| Weight (kg) | 64.0 (10.6) | 66.5 (11.8) | 68.3 (9.8) | 77.4 (14.4) | 67.7 (12.8) |
| Blood pressure (mmHg) | 129.21/84.1 | 126.0/78.9 | 127.1/80.5 | 134.6/81.5 | 129.0 81.5 |
| Heart rate (beats/min) | 84.2 | 77.5 | 80.6 | 78.0 | 79.0 |
| Killip class >1 (%) | 14.4 | 18.1 | 10.9 | 12.2 | 15.4 |
| Mean glucose (mmol/L) | 9.1 (4.9) | 8.6 (4.3) | 9.2 (5.3) | 9.7 (4.7) | 9.0 (4.7) |
| Medications in hospital (%) | |||||
| Thrombolytic therapy | 91.9 | 52.5 | 89.7 | 75.3 | 74.1 |
| Direct PCI | 2.5 | 10.0 | 3.7 | 22.4 | 9.1 |
| Aspirin | 97.9 | 95.8 | 99.6 | 98.4 | 97.3 |
| Ticlopidine/clopidogrel | 80.2* | 27.8 | 78.6* | 16.0 | 48.6 |
| IV Nitrates | 59.4 | 91.8 | 60.6 | 70.2 | 73.5 |
| β-Blocker | 70.0 | 61.5 | 91.7 | 82.1 | 70.0 |
| ACE inhibitor | 73.6 | 71.7 | 85.5 | 68.2 | 72.4 |
| Lipid lowering | 62.3 | 71.3 | 88.0 | NR | NR |
| Calcium antagonist | 6.1 | 12.8 | 3.4 | 7.8 | 8.8 |
NR, Not recorded.
The high rates of use of a thienopyride is due to the availability of a combination tablet of aspirin + clopidogrel.
Challenges faced during the conduct of the trial
This study has been a formidable undertaking and has been successful in recruiting the overall target of 20 000 patients. The lack of external funding required that investigator meetings were generally attached to other meetings, although annual regional meetings were held in India and China. The centers in ECLA were not provided with any compensation and the centers in CREATE (India, China, and Pakistan) received modest compensation per patient to cover their visible costs. Despite this, over the last 2 years of the study, recruitment rates averaged 7000 patients per year. The National Coordinating Centers obtained national regulatory approvals and licences for drug imports. These processes were extremely bureaucratic and slow (9–18 months in each country), and were chiefly designed to be done by pharmaceutical companies or independent contract research organizations (CRO) at considerable expense. A quote from a CRO in India for obtaining approval and monitoring the trial exceeded the entire cost of running the study in India (note that, in most countries, there is no streamlined and efficient mechanism to obtain regulatory approvals for studies conducted independent of industry). Several of the participating centers did not have institutional review boards (IRBs) or ethics committees, so national and independent IRBs had to be used. There were considerable initial delays in receiving the packaged drugs (Reviparin/placebo) from the company, which was in the midst of a takeover, and the study was not considered to be of sufficiently high priority within the new organization. For most centers, the CREATE study was the first trial in which they had participated, so considerable training (at small study meetings and by phone) was needed. Despite their enthusiasm and the relative simplicity of the study, some centers initially had problems with accurate completion of forms, complying with certain aspects of the study protocol, and maintaining careful documents. Therefore, we instituted a system of on-site monitoring and training which trained staff at sites in CREATE, and provided support. This improved protocol adherence and data quality considerably. For randomization in India, the study initially used sealed envelopes (due to lack of low-cost, reliable and accessible telephone lines 24 hours-a-day at all hospitals). Despite multiple safeguards, allocation errors occurred in a few patients. Therefore, despite higher costs, randomization was switched to a 24-hour central telephone system in Bangalore, India, which substantially reduced the allocation error rate. In China, 24-hour central randomization was used throughout, and so few errors occurred.
Given that the centers were compensated very modestly, the study was repeatedly threatened by the withdrawal of centers who wished to participate in “competing” trials, sponsored by pharmaceutical companies with higher rates of compensation. This substantially slowed recruitment into ECLA so that, instead of the original target of 10 000 patients over 3 years, only 4000 patients were randomized over 6 years. This was less of a problem in India and China, where only a few centers stopped randomizing by the end of 2003 to participate in other trials. Nevertheless, most centers had made important contributions to the trial. During 2003, the epidemic of Severe Acute Respiratory Syndrome (SARS) in China adversely affected recruitment and several participating hospitals were closed down, or stopped admitting AMI patients.
What lessons have we learned from the CREATE-ECLA experience and collaboration? First, if important generic questions are incorporated into a very simple protocol, sufficient numbers of physicians are still willing and able to collaborate at little or no reimbursement. Second, the “merger” of CREATE and ECLA was possible because of long-standing previous collaboration between the investigators from the 2 groups, and a mutual commitment to sharing credit. This led to ensuring a statistically robust, reliable result regarding GIK, rather than the potential for an inconclusive result from the ECLA study alone. Third, the bureaucratic hurdles for conducting this trial in India and China were formidable and took between 9–18 months to overcome, chiefly through the tenacity, determination and dedication of the National Coordinators in Beijing and Bangalore. Fourth, even in a very simple trial, some streamlined and sensible (“helpful and supportive” as opposed to “policing”) monitoring could help in improving study quality, especially when centers had not previously participated in trials. Fifth, much of the increasing regulatory bureaucracy imposed by guidelines such as the “Good Clinical Practice” guidelines may not enhance the quality of the study or improve patient safety more than careful attention to a few key aspects of study design, conduct (such as proper randomization and complete and unbiased outcome ascertainment), and periodic review of the data by an independent DSMB. In fact, there is a danger that these well-intentioned guidelines could prevent the conduct of important low-cost academic trials of generic (non-pharmaceutical) questions by imposing burdensome and expensive processes which may be of little scientific, medical, or ethical value. Sixth, peer review and governmental organizations should develop mechanisms to support international trials and epidemiologic studies. Current funding mechanisms do not generally support such studies in multiple countries, especially if they are low- or middle-income countries.
In conclusion, the CREATE-ECLA trial will provide definitive answers regarding the value of 2 practical and promising therapies (LMWH and GIK) in AMI. These therapies, if proven to be effective, could be used even in small medical centers in low- and middle-income countries. The practical experiences in conducting this trial indicate how large trials of important questions can be successfully conducted in resource-poor settings, by academic groups without industry involvement. However, such studies are uncommon and, therefore, a large number of important questions remain unaddressed. This emphasizes the need to develop funding structures and regulations that will facilitate global low-cost trials of important public health questions.
Footnotes
Salim Yusuf is supported by an Endowed Chair of the Heart and Stroke Foundation of Ontario and a Senior Scientist Award from the Canadian Institutes of Health Research (CIHR). Shamir R. Mehta is supported by a CIHR New Investigator award.
Appendix.
The following persons participated in the CREATE-ECLA International GIK Study: International Steering Committee: S. Yusuf (Principal Investigator and Chairman), S.R. Mehta (Co-Principal Investigator and Project Director), R. Diaz (Joint Principal Investigator—ECLA), E. Paolasso (Joint Principal Investigator—ECLA), P. Pais (Principal Investigator—India), S. Reddy (Co-Principal Investigator India), L. Liu (Principal Investigator—China), K. Kazmi (Principal Investigator—Pakistan), R.J. Ahmed, L. Cronin (Global Study Coordinators), D. Xavier (Coordinator—India), J. Zhu (Coordinator—China), J. Tai (Coordinator—Pakistan), C. Xie (Project Statistician); Indian Steering Committee: R. Gupta, K.K. Haridas, T.M. Jaison, P.P. Joshi, P.G. Kerkar, A.K. Maity, S.C. Manchanda, S. Naik, P. Pais, D. Prabhakaran, S. Reddy, B. Singh, S. Thanikachalam, D. Xavier; Chinese Steering Committee: X.J. Bai, T. Chen, J.J. Cui, T.X. Cui, S.Y. Fu, H. Ge, Q.L. Li, S.M. Li, W. Li, Y.Q. Li, L. Liu, Y.H. Liu, Z.R. Lu, S.P. Ma, D. Qiao, Y.C. Song, N.L. Sun, L.H. Wang, S.W. Wang, W. Wang, N. Wu, Y.S. Wu, C.B. Xu, S.C. Xu, Z.M. Xu, G.J. Yang, H.S. Yang, C.Z. Zhang, S.T. Zhang, W.J. Zhang, J.C. Zhou, J. Zhu; ECLA-GIK 2 Steering Committee: W. Almahmeed, A. Avezum, P. Castro, R. Corbalán, R. Díaz, R. Pitarch Flors, B.M. Lombana, L. Marano, D. Mcguire, A. Orlandini, E. Paolasso, J. E. Isea Perez, L.Soares Piegas, F. Van De Werf, H. White, M. Zubaid; Pakistani Steering Committee: A.M. Faruqi, K. Kazmi, I. Rasool, K. Soomro, J. Tai, H. ul Banna; Population Health Research Institute, Hamilton, Canada: S. Yusuf, S.R. Mehta, R.J. Ahmed, L. Cronin, S. Pavlov (Database Programmer), C. Xie, J. Pogue, F. Zhao, I. Tsuluca, M. Molec, I. Holadyk-Gris, K. Ahmed; India National Coordinating Office (NCO), Institute of Population Health and Clinical Research, St. John's National Academy of Health Sciences, Bangalore, India: P. Pais, D. Xavier, D. Freeda, S. Lidwin; Indian Adjudication Committee: M. Chenniappan, B. Isaac, S.S. Iyengar, T.M. Jaison, P. Joshi, S.P. Kalantri, S.K. Kaushik, P.G. Kerkar, U.K. Mahorkar, J. Narendra, S.K. Paul, M.J. Santhosh, B.K.S. Sastry, B. Singh, S.B. Siwach, K. Varghese; China NCO, Beijing Hypertensive League Institute, Beijing, China: L. Liu, J. Zhu, H. Yang, Y. Yang, X. Zhang, H. Tan, J. Tang, X. Li, L. Yan, Y. Zhang, J. Li; Chinese Adjudication Committee: M.Y. Bai, Y.Q. Jiang, S.Y. Lang, X.Y. Shi, Y.C. Song, Z.R. Tian, K. Wang, D.H. Yan, S.Y. Yu; Estudios Clínicos Latino América, Rosario, Santa Fé, Argentina: A. Pascual, H. Ozcoidi, C. Cuesta, D. Wojdyla, G.M. Font, M.I. Genisans; Pakistan NCO, Cardiology Section, Department of Medicine, Aga Khan University Hospital, Karachi, Pakistan: K. Kazmi, J. Tai, I. Chandna, M. Rafiq, N. Dadani, S.S. Fatima, S. Rehman; Pakistani Adjudication Committee: S. Adil, K. Bhojomal, A. Hameed, S.A. Khan; Data and Safety Monitoring Board: P. Sleight (Chair), C. Baigent, J. Hirsh, W. Taylor, G. Tognoni; Abbott GmbH & Co. KG (Reviparin Sponsor): P. Bacher, N. Bender, U. Legler, U. Magin, U. Raschke; Investigators—Argentina: Berazategui: M. San Mauro; Capital Federal: M.A.Brito, A. Alves De Lima, R.A. Ahuad Guerrero, R. Nordaby; Cordoba: R.J. Barcudi, J. Bono, R.E. Ledesma, H.R. Ramos; Coronel Suarez: A. Caccavo; Corrientes: S.M. Macin; Haedo: S.N. Ferreyra Cantante; La Plata: G.D. Caime, L. R. Cartasegna, O.A. Perrino; Mendoza: A.J. Gambarte, E. Marzetti; Merlo: M. Garrido; Olavarria: R.J. Balado; Posadas: C.B. Martinez; Quilmes: A.A. Fernandez; Resistencia: E. Ferro Queirel; Rio Grande: J.O. Balbi; Rio IV: G. Amuchastegui; Rosario: G. Covelli, A. Gentile, C.E. Girino, J. López, R. Monti, A.D. Orlandini, O. Pellizon, J.L. Ramos, G. Zapata; Salta: C.A. Cuneo, J.A. Sanchez; San Luis: J.P. Albisu; Santa Fe: M.A. Berli, M.A. Hominal; Santa Teresita: R.A. Peleteiro Mariño; Tandil: V. Mezzina; Tucuman: E.M. Avila, P. Baselga, C.R. Castellanos, H.L. Luciardi, L.L. Lobo Marquez, J. Muntaner; Belgium: Leuven: F. Van De Werf; Brazil: Belo Horizonte: E.M. Good God, G. Reis; Blumenau: D.M. Mello Soares; Porto Alegre: L.C. Bodanese, E. Manenti, L. Prestes; Rio De Janeiro: J.G. De Castro Amino; Sao Paulo: R. Uchoa Azevedo, A.C.C. Carvalho, R.F. Ramos; Chile: Las Condes: M. Alcaino; Santiago: P. Castro Galvez, R. Corbalán Herreros; China: An Yang: H. Liu; Angang: R. Wang; Anshan: Z.C. Liu, X. Tian, G. Wang, Y. Zhang; Baoding: Z. Nan, J. Zhang; Bazhou: C. Zai; Beijing: W. Chen, M. Gao, D. Hu, S. Jia, D.X. Li, Q. Li, W. Li, S.L. Liu, Y. Sun, B. Wang, G.F. Xie, Z. Xu, X. Yang, M. Zhao, X. Zhao; Beipiao: Z. Fang; Benxi: S.Y. Liu; Cangzhou: Z. Ma; Changchun: Y. Jiang, S.M. Li; Changsha: Z. Zhen; Chendu: F. Huo; Chengde: H.G. Yang; Chengwu: H. Liu; Chifeng: Y. Miao; Chongqing: C.M. Yang; Dalian: X. Chi, Z.X. Liu, S. Zhou; Dandong: Y. Sun; Dashiqiao: F.S. Zhou; Fenyang: R. Guo; Gaoping: K. Jing; Guan: Z. Xu; Haicheng: S. Ren, J. Zhao; Hebei: H. Bai, H. Bai, C. Cheng, J. Cheng, X. Hao, H. Li, W.G. Li, S. Wang, W. Zhang; Heilongjiang: L. Li, Y. Sun; Helongjiang: S. Fu, J. Shao, X. Tan; Henan: S. Chen, J. Fu; Hengshui: Q. Zheng; Huai Ren: J. Ma; Inner Mongolia: H. Chen, H. Ma; Jiamusi: L. Gong; Jiaozhou: Z. Zhang; Jiaxiang: F. Li; Jilin: B. Yang; Jinan: L.S. Zhou; Jinlin: Z. Wang; Jinning: X. Sun; Jinzhou: G. Tao; Jiujiang: Q. Wang; Jun: J. Li; Lankao: X. Guo; Lian Yungang: X. Wang; Liaocheng: K. Zai; Liaoning: Q. Cui, S. Fan, H. Li, W. Liu, Q. Meng, G. Qi, Y. Qin, G. Wang, N. Wang, G. Xu, X. Yin, Q. Zhang, S. Zhang, Y. Zhang, Z. Zhang; Liaoyang: R. Liu, F. Wang; Linfen: Y. Zhang; Lingbao: W.K. Li; Lingshou: H. Zhang; Linyi: X. Xu; Longkou: R. Ma; Luoyang: F. Guan, T. Yang; Mongolia: R. Zhqo; Nanjing: J. Huang; Nanle: A. Li; Neimeng: J. Zhou; Panjin: X. You; Qi: L. Hao; Qingdao: F. Zhangfang; Qinyuang: X. Ma; Ruyang: C. Shen; Sanhe: Q. Li; Shangdong: Z. Hou; Shanghai: N.F. Zho; Shangqiu: G. Huang; Shanxi: P. Guo, J. Lou, Q.P. Wang, Z. Wang; Shenyang: X. Jiang, Z. Li, D. Tian, S. Wang, Z.D. Wu, M.G. Yang; Shi Jiazhuang: Z.C. Li; Taian: S.G. Yang; Tangshan: Y. Tu; Taonan: C. He; Tianjin: Y. Cao, Y. Han; Wangdu: J.H. Yang; Wangrong: S. Dong; Wuxiang: D.F. Li; Xiang Cheng: Q.F. Zhang; Xianxian: Z. Fan; Xinjiang: D.Q. An; Xinxiang: J. Liu; Xiping: G. Yang; Xiuwu: X.C. Xu; Xuzhou: Y. Xia; Yantai: H. Xu; Yanzhou: T. Wang; Yichun: D. Li; Yingkou: J. Wei; Yongji: P. Yang; Yuci: C.Y. Liu; Zhengzhou: S. Shang; Zhumadian: Y.G. Zhang; Colombia: Bogota: E. Hernandez Leyva; Manizales: N.Cano Lopez; Dominican Republic: Santo Domingo: A.R. Gonzalez Medina; Greece: Athens: J. E. Kanakakis, J.N. Nanas, P. D. Papazoglou; India: Adoni: J. Srinivas, B. Srinivasulu; Ahmedabad: S. Dani, J. Prajapati; Alappuzha: G. Deepak, J.F. Shallam; Ambur: K.J. Nesaraj; Amritsar: A. Kumar, R.K. Sharma; Annamalainagar: S. Balasubramaniyan, N. Chidambaram, R. Umarani; Bangalore: S. Chandra, S. Dwivedi, B. Isaac, R. Kishore, B.J. Kumar, Y. Kumble, S. Mehrotra, P.R. Nayak, S.S. Ramesh, M.J. Santhosh, P.K. Shetty, K. Varghese; Bhopal: S.K. Trivedi; Bikaner: R. Beniwal, A. Kalla, R.B. Panwar; Calicut: K.G. Alexander, A.V. Bindu, A. Nambiar; Chennai: D. Barkavi, A. Kalanidhi, T. Pradeep, J. Rajesh, M. Ramesh, S. Shanmugasundaram, S. Thanikachalam; Cochin: K.K. Haridas, P. Kumar; Doraha: G. Sidhu, R. Singh; Ernakulam: K.N. Pradeep; Ghaziabad: A. Kumar, A. Mittal; Gulbarga: J.B. Bijapure, M.S. Rao; Guntur: N.G. Mohanarjun, M.B. Rao; Hyderabad: B.R. Babu, N. Dinesh, R.K. Jain, P.A. Jiwani, S.R. Naik, T.N.C. Padmanabhan, B.S. Raju, R. Rajaram, A.S.V.N. Rao, D. Rao, V.S.P. Rao, B.K.S. Sastry, S. Sinha; Indore: A. Bharani, G. Verma; Jaipur: R. Gupta, R. K. Tongia, S. Kalra, S. Sharma; Jodhpur: R. Mehrotra, S. Sanghvi, O.P. Soni; Kolkata: A.D. Biswas, A.K. Maity, S.K. Paul; Kottayam: J. Boben, G. Jacob, J. Joseph; Lucknow: A. Puri, V.K. Puri, H. Singh; Ludhiana: R. Calton, T.M. Jaison; Meerut: G. K. Aneja; Mumbai: P.G. Kerkar, P. Nyayadhish, P.J. Nathani, S.K. Rane; Nagpur: M. Fulwani, A.S. Jain, P.P. Joshi, A. Khan, U.K. Mahorkar, R.G. Salkar, A. Somani, R. Wadhwani, S.D. Zawar; Nanded: V.E. Shegokar, S.L. Tungikar; Nashik: V. Vijan; New Delhi: B. Singh, R. Trehan; Patiala: A. Garg, H. Singh, S. Verma; Pune: S. Borade, D. Duggal, J. Hiremath; Rohtak: Jagdish, V.K. Katyal, S.B. Siwach; Shimoga: H.R. Devendrappa, J. Narendra, Ratnakar; Thrissur: P.B. Latha, E.B. Manoj, P.P. Mohanan; Trichy: M. Chenniappan, P. Gandhimadhinathan, K. Jeremaiah, B.S.V. Raj, R.Udaysankar; Udaipur: J.K. Chhaparwal, S.K. Kaushik; Vellore: S.T. Chandy, S.N. Gupta, S. Raghavan; Vijayawada: P. Ramesh, V.S. Reddy, P. Srinivas; Vishakapatnam: K.D. Rao, B.R Malipeddi, G.S.R. Murthy; Wardha: R. Joshi, S.P. Kalantri, S. Patil; Italy: Busto Arsizio: S. Tredici; Erba: D. Agnelli; Milano: E. Assanelli, L. Marano; Monza: A. Bozzano, Scorrano (Le): L. Marsano; Torino: R. Crivello, A. Nejrotti; Kuwait: Safat: A. Abdulminem, H. Saad, M. Zubaid; Mexico: Mexico City: J. Martinez Sanchez; San Luis Potosi: J. Carrillo Calvillo; New Zealand: Auckland: H. White; Nelson: A. Hamer; Pakistan: Karachi: I. Chandna, A.M. Faruqi, M. Rafiq, I. Rasool, K. Soomro; Lahore: H.U. Banna; Panama: Bella Vista: B.M. Lombana; Spain: Manacor: J. Lopez Ferre, R. Pitarch Flors; U.S.A.: Alexandria: R.J. Freedman; Dallas: D. Mcguire; Myrtle Beach: N. Transk III; United Arab Emirates: Abu Dhabi: W. Almahmeed; Venezuela: Caracas: D. Almeida; Catia: P.J.C. Lujan; El Llanito: C. Mata, J.E. Isea Perez; La Guaira: C. Becerra, K. Gonzalez, F. Torres; Maturin: M.A. Alvarez; Valencia: E. Carrillo.
References
- 1.Yusuf S., Reddy S., Ounpuu S. Global burden of cardiovascular diseases: part I: general considerations, the epidemiologic transition, risk factors, and impact of urbanization. Circulation. 2001;104:2746–2753. doi: 10.1161/hc4601.099487. [DOI] [PubMed] [Google Scholar]
- 2.Yusuf S., Wittes J., Friedman L. Overview of results of randomized clinical trials in heart disease: I. Treatments following myocardial infarction. JAMA. 1988;260:2088–2093. [PubMed] [Google Scholar]
- 3.Keeley E.C., Boura J.A., Grines C.L. Primary angioplasty versus intravenous thrombolytic therapy for acute myocardial infarction: a quantitative review of 23 randomised trials. Lancet. 2003;361:13–20. doi: 10.1016/S0140-6736(03)12113-7. [DOI] [PubMed] [Google Scholar]
- 4.The GUSTO Investigators Reperfusion therapy for acute myocardial infarction with fibrinolytic therapy or combination reduced fibrinolytic therapy and platelet glycoprotein IIb/IIIa inhibition: the GUSTO V randomised trial. Lancet. 2001;357:1905–1914. doi: 10.1016/s0140-6736(00)05059-5. [DOI] [PubMed] [Google Scholar]
- 5.White H., Hirulog and Early Reperfusion or Occlusion (HERO)-2 Trial Investigators Thrombin-specific anticoagulation with bivalirudin versus heparin in patients receiving fibrinolytic therapy for acute myocardial infarction: the HERO-2 randomised trial. Lancet. 2001;358:1855–1863. doi: 10.1016/s0140-6736(01)06887-8. [DOI] [PubMed] [Google Scholar]
- 6.The GUSTO Investigators An international randomized trial comparing four thrombolytic strategies for acute myocardial infarction. N Engl J Med. 1993;329:673–682. doi: 10.1056/NEJM199309023291001. [DOI] [PubMed] [Google Scholar]
- 7.ISIS-3 (Third International Study of Infarct Survival) Collaborative group ISIS-3: a randomized comparison of streptokinase vs tissue plasminogen activator vs anistreplase and of aspirin plus heparin vs aspirin alone among 41 229 cases of suspected acute myocardial infarction. Lancet. 1992;339:754–769. [PubMed] [Google Scholar]
- 8.Gruppo Italiano Per Lo Studio Della Sopravvivenza Nell'Infarto Miocardico GISSI-2: a factorial randomized trial of alteplase versus streptokinase and heparin versus no heparin among 12 490 patients with acute myocardial infarction. Lancet. 1990;336:65–71. [PubMed] [Google Scholar]
- 9.Kontny F., Dale J., Abildgaard U., FRAMI study group Randomized trial of low molecular weight heparin (dalteparin) in prevention of left ventricular thrombus formation and arterial embolism after acute anterior myocardial infarction: the Fragmin in Acute Myocardial Infarction (FRAMI) Study. J Am Coll Cardiol. 1997;30:962–969. doi: 10.1016/s0735-1097(97)00258-1. [DOI] [PubMed] [Google Scholar]
- 10.Glick A., Kornowski R., Michowich Y. Reduction of reinfarction and angina with use of low-molecular-weight heparin therapy after streptokinase (and heparin) in acute myocardial infarction. Am J Cardiol. 1996;77:1145–1148. doi: 10.1016/s0002-9149(96)00152-x. [DOI] [PubMed] [Google Scholar]
- 11.Frostfeldt G., Ahlberg G., Gustafsson G. Low-molecular-weight heparin (dalteparin) as adjuvant treatment to thrombolysis in acute myocardial infarction—a pilot study: biochemical markers in acute coronary syndromes (BIOMACS II) J Am Coll Cardiol. 1999;33:627–633. doi: 10.1016/s0735-1097(98)00612-3. [DOI] [PubMed] [Google Scholar]
- 12.Simoons M., Krzeminska-Pakula M., Alonso A. Improved reperfusion and clinical outcome with enoxaparin as an adjunct to streptokinase thrombolysis in acute myocardial infarction: The AMI-SK study. Eur Heart J. 2002;23:1282–1290. doi: 10.1053/euhj.2001.3083. [DOI] [PubMed] [Google Scholar]
- 13.Baird S.H., McBride S.J., Trouton T.G. Low molecular weight heparin versus unfractionated heparin following thrombolysis in myocardial infarction. J Am Coll Cardiol. 1998;31:191A. [Google Scholar]
- 14.Ross A.M., Molhoek P., Lundergan C. Randomized comparison of enoxaparin, a low-molecular-weight heparin, with unfractionated heparin adjunctive to recombinant tissue plasminogen activator thrombolysis and aspirin: second trial of Heparin and Aspirin Reperfusion Therapy (HART II) Circulation. 2001;104:648–652. doi: 10.1161/hc3101.093866. [DOI] [PubMed] [Google Scholar]
- 15.Cohen M., Gensini G.F., Maritz F. The safety and efficacy of subcutaneous enoxaparin versus intravenous unfractionated heparin and tirofiban versus placebo in the treatment of acute ST-segment elevation myocardial infarction patients ineligible for reperfusion (TETAMI): a randomized trial. J Am Coll Cardiol. 2003;42:1348–1356. doi: 10.1016/s0735-1097(03)01040-4. [DOI] [PubMed] [Google Scholar]
- 16.Antman E.M., Louwerenburg H.W., Baars H.F. Enoxaparin as adjunctive antithrombin therapy for ST-elevation myocardial infarction: results of the ENTIRE-Thrombolysis in Myocardial Infarction (TIMI) 23 Trial. Circulation. 2002;105:1642–1649. doi: 10.1161/01.cir.0000013402.34759.46. Erratum in: Circulation 2002 Jun 11;105:2799. [DOI] [PubMed] [Google Scholar]
- 17.The Assessment of the Safety and Efficacy of a New Thrombolytic Regimen (ASSENT)-3 Investigators Efficacy and safety of tenecteplase in combination with enoxaparin, abciximab, or unfractionated heparin: the ASSENT-3 randomised trial in acute myocardial infarction. Lancet. 2001;358:605–613. doi: 10.1016/S0140-6736(01)05775-0. [DOI] [PubMed] [Google Scholar]
- 18.Wallentin L., Goldstein P., Armstrong P.W. Efficacy and safety of tenecteplase in combination with the low-molecular-weight heparin enoxaparin or unfractionated heparin in the prehospital setting: the Assessment of the Safety and Efficacy of a New Thrombolytic Regimen (ASSENT)-3 PLUS randomized trial in acute myocardial infarction. Circulation. 2003;108:135–142. doi: 10.1161/01.CIR.0000081659.72985.A8. [DOI] [PubMed] [Google Scholar]
- 19.Mittra B. Potassium, glucose, and insulin in treatment of myocardial infarction. Lancet. 1965;2:607–609. doi: 10.1016/s0140-6736(65)90516-7. [DOI] [PubMed] [Google Scholar]
- 20.Pilcher J., Etishamudin M., Exon P. Potassium, glucose and insulin in myocardial infarction. Lancet. 1967;1:1109. doi: 10.1016/s0140-6736(67)90470-9. [DOI] [PubMed] [Google Scholar]
- 21.Pentecost B.L., Mayne N.M., Lamb P. Controlled trial of intravenous glucose, potassium, and insulin in acute myocardial infarction. Lancet. 1968;1:946–948. doi: 10.1016/s0140-6736(68)90903-3. [DOI] [PubMed] [Google Scholar]
- 22.Medical Research Council Working Party on the Treatment of Myocardial Infarction Potassium, glucose, and insulin treatment for acute myocardial infarction. Lancet. 1968;2:1355–1360. [PubMed] [Google Scholar]
- 23.Hjermann I. A controlled study of peroral glucose, insulin and potassium treatment in myocardial infarction. Acta Med Scand. 1971;190:213–218. doi: 10.1111/j.0954-6820.1971.tb07419.x. [DOI] [PubMed] [Google Scholar]
- 24.Heng M.K., Norris R.M., Singh B.N. Effects of glucose and glucose-insulin-potassium on haemodynamics and enzyme release after acute myocardial infarction. Br Heart J. 1977;39:748–757. doi: 10.1136/hrt.39.7.748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stanley A.W.H., Prather J.W. Glucose-insulin-potassium, patient mortality and the acute myocardial infarction: results from a prospective randomized study. Circulation. 1978;57(suppl II):II, 62. Abstract. [Google Scholar]
- 26.Rogers W.J., McDaniel H.G., Mantle J.A. Prospective randomized trial of glucose-insulin-potassium in acute myocardial infarction: effects of hemodynamics, short and long-term survival. J Am Coll Cardiol. 1983;1:628. doi: 10.1016/0002-9149(79)90081-x. [DOI] [PubMed] [Google Scholar]
- 27.Mantle J.A., Rogers W.J., Smith R. Clinical effects of glucose-insulin-potassium on left ventricular function in acute myocardial infarction: results from a randomized clinical trial. Am Heart J. 1981;102:313–324. doi: 10.1016/0002-8703(81)90303-3. [DOI] [PubMed] [Google Scholar]
- 28.Whitlow P.L., Rogers W.J., Smith L.R. Enhancements of left ventricular function by glucose-insulin-potassium infusion in acute myocardial infarction. Am J Cardiol. 1982;49:811–820. doi: 10.1016/0002-9149(82)91963-4. [DOI] [PubMed] [Google Scholar]
- 29.Salter L.F., Green C.E., Kent K.M. Metabolic support during coronary reperfusion. Am Heart J. 1987;114:54–58. doi: 10.1016/0002-8703(87)90306-1. [DOI] [PubMed] [Google Scholar]
- 30.Malmberg K., Ryden L., Efendic S. Randomized trial of insulin-glucose infusion followed by subcutaneous insulin treatment in diabetic patients with acute myocardial infarction (DIGAMI Study): effect on mortality at 1 year. J Am Coll Cardiol. 1995;26:57–65. doi: 10.1016/0735-1097(95)00126-k. [DOI] [PubMed] [Google Scholar]
- 31.Díaz R., Paolasso E.A., Piegas L.S. Metabolic modulation of acute myocardial infarction: the ECLA glucose-insulin-potassium pilot trial. Circulation. 1998;98:2227–2234. doi: 10.1161/01.cir.98.21.2227. [DOI] [PubMed] [Google Scholar]
- 32.Ceremuzynski L., Budaj A., Czepiel A. Low-dose glucose-insulin-potassium is ineffective in acute myocardial infarction: results of a randomized multicenter pol-GIK trial. Cardiovascular Drugs and Therapy. 1999;13:191–200. doi: 10.1023/a:1007787924085. [DOI] [PubMed] [Google Scholar]
- 33.Diaz-Araya G., Nettle D., Castro P. Oxidative stress after reperfusion with primary coronary angioplasty: lack of effect of glucose-insulin-potassium infusion. Crit Care Med. 2002;30:417–421. doi: 10.1097/00003246-200202000-00025. [DOI] [PubMed] [Google Scholar]
- 34.van der Horst I.C., Zijlstra F., van't Hof A.W. Glucose-insulin-potassium infusion in patients treated with primary angioplasty for acute myocardial infarction: the glucose-insulin-potassium study: a randomized trial. J Am Coll Cardiol. 2003;42:784–791. doi: 10.1016/s0735-1097(03)00830-1. [DOI] [PubMed] [Google Scholar]
- 35.Yusuf S., Peto R., Lewis J. Beta blockade during and after myocardial infarction: an overview of the randomized trials. Prog Cardiovasc Dis. 1985;XXVII:335–371. doi: 10.1016/s0033-0620(85)80003-7. [DOI] [PubMed] [Google Scholar]
- 36.Mehta SR. The CREATE Study : rationale, design and methods. July 2002. MS Thesis, McMaster University, Hamilton, Canada


