Abstract
Cases of Middle East respiratory syndrome coronavirus (MERS-CoV) continue to occur, making it one of the WHO´s targets for accelerated vaccine development. One vaccine candidate is based on live-attenuated measles virus (MV) vaccine encoding the MERS-CoV spike glycoprotein (MERS-S). MVvac2-MERS-S(H) induces robust humoral and cellular immunity against MERS-S mediating protection. Here, the induction and nature of immunity after vaccination with MVvac2-MERS-S(H) or novel MVvac2-MERS-N were further characterized. We focused on the necessity for vector replication and the nature of induced T cells, since functional CD8+ T cells contribute importantly to clearance of MERS-CoV. While no immunity against MERS-CoV or MV was detected in MV-susceptible mice after immunization with UV-inactivated virus, replication-competent MVvac2-MERS-S(H) triggered robust neutralizing antibody titers also in adult mice. Furthermore, a significant fraction of MERS CoV-specific CD8+ T cells and MV-specific CD4+ T cells simultaneously expressing IFN-γ and TNF-α were induced, revealing that MVvac2-MERS-S(H) induces multifunctional cellular immunity.
Keywords: Vaccine platform, Measles Virus, Multifunctional T cells, MERS Coronavirus, Antibody responses
1. Introduction
The Middle East respiratory syndrome coronavirus (MERS-CoV) is a member of the Coronaviridae family and emerged in 2012 in the Kingdom of Saudi Arabia (Zaki et al., 2012). Coronaviruses typically cause mild infections of the upper respiratory tract, but already in 2002, the severe acute respiratory syndrome CoV (SARS-CoV) with a mortality rate of about 10% among infected patients was introduced in the human population. SARS-CoV spread world-wide and caused more than 8000 diagnosed infections, but was contained within a year after its emergence (http://www.who.int/csr/sars/country/table2004_04_21/en/).
In contrast, infections with MERS-CoV are ongoing for more than 5 years, with 2103 laboratory-confirmed cases distributed over 27 countries with at least 733 deaths that were reported to the WHO by November 2017 (http://www.who.int/emergencies/mers-cov/en/). This apparent case-fatality rate of 35% is of grave concern, because epidemic spread as has been observed for SARS-CoV could result in a disastrous death toll. MERS-CoV has been introduced zoonotically by transmission from dromedary camels to human patients (Alagaili et al., 2014, Haagmans et al., 2014, Reusken et al., 2013a) and serological studies indicate wide-spread and early distribution among this animal host (Alagaili et al., 2014, Reusken et al., 2013b). Therefore, a continuous risk of transmission especially to persons in close contact to camels is evident. Fortunately, the human to human transmission rate has remained low. Aside of individuals with regular contact to camels, only health care workers or relatives of MERS-CoV patients have a considerable risk of infection (Alraddadi et al., 2016, Drosten et al., 2014), but still at a modest level. Nonetheless, the high case fatality rate, the recurrent outbreaks of MERS-CoV infections, and especially the risk of virus adaptation potentially resulting in epidemic or even pandemic spread make the development of an effective vaccine against MERS-CoV an international priority.
The efficacy of several vaccine candidates has been demonstrated in different animal models up to even dromedary camels (reviewed in (Okba et al., 2017)). One of these candidates, MVvac2-MERS-S(H) (Malczyk et al., 2015), is based on the measles virus (MV) vaccine platform technology (Mühlebach, 2017), and encodes the MERS-CoV spike protein (S) as an additional antigen in the backbone of recombinant MVvac2 (del Valle et al., 2007) resembling vaccine strain Moraten that is authorized and in use in the US since 1968. This candidate induces both robust humoral and functional cellular immune-responses against MERS-CoV. Moreover, MERS-CoV viral load and inflammation of the lung were significantly reduced in challenged mice that had been vaccinated with MVvac2-MERS-S(H), before (Malczyk et al., 2015).
While these experiments provided proof of concept for efficacy of this vaccine candidate, further mechanistic insights into the nature of the induced T cell responses remain to be elucidated. These are of special interest, since it has been shown that T cells are essential for clearance of the infection (Coleman et al., 2017, Zhao et al., 2014): Depletion of CD8+ T cells increased overall inflammation, bronchiolar inflammation, lymphocyte infiltration, and pleuritis at day 7 post-infection in mice (Coleman et al., 2017), while MERS CoV-susceptible mice depleted of all T cells were unable to clear the virus (Zhao et al., 2014). As an alternative to the spike glycoprotein, conserved (internal) structural proteins such as the nucleocapsid protein N are of special interest as putative target of anti-viral T cell responses to be triggered by future MERS vaccines (Agnihothram et al., 2014).
Therefore, we have also generated and characterized MERS-CoV N protein-encoding vaccine candidates based on the MVvac2 vaccine platform, in this study. To further characterize the induction of MERS CoV-specific immune responses, we first analyzed the necessity for viral replication for the induction of MERS CoV- and MV-specific immune responses using the highly immunogenic MVvac2-MERS-S(H) vaccine candidate. In addition, we characterized the functionality of CD8+ and CD4+ T cell responses in juvenile (6–12 week old) and adult (7 months of age) mice using flow cytometry and functional assays.
2. Material and methods
2.1. Cells
Vero (African green monkey kidney) (ATCC# CCL-81) and 293 T (ATCC CRL-3216) cell lines were purchased from ATCC (Manassas, VA, USA) and cultured in Dulbecco's modified Eagle's medium (DMEM, Biowest, Nuaillé, France) supplemented with 10% fetal bovine serum (FBS; Biochrom, Berlin, Germany) and 2 mM L-glutamine (L-Gln; Biochrom). JAWSII dendritic cells (ATCC CRL-11904) were purchased from ATCC and cultured in MEM-α with ribonucleosides and deoxyribonucleosides (GIBCO BRL, Eggenstein, Germany) supplemented with 20% FBS, 2 mM L-Gln, 1 mM sodium pyruvate (Biochrom), and 5 ng/mL murine GM-CSF (Peprotech, Hamburg, Germany). DC2.4 murine dendritic cells (Shen et al., 1997) were cultured in RPMI containing 10% FBS, 2 mM L-Gln, 1% non-essential aminoacids (Biochrom), 10 mM HEPES (pH 7,4), and 50 μM 2-Mercaptoethanol (Sigma-Aldrich, Steinheim, Germany). Cells were cultured at 37 °C in a humidified atmosphere containing 6% CO2 for a maximum of 6 months of culture after thawing of the original stock.
2.2. Plasmids
The codon-optimized gene encoding MERS-CoV-N (Genebank accession no. JX869059) flanked with AatII/MluI binding sites in plasmid pMA-RQ-MERS-N was obtained by gene synthesis (Invitrogen Life Technology, Regensburg, Germany). The antigen and the immediate early cytomegalovirus (CMV) promoter (Martin et al., 2006) were inserted into plasmids p(+)BR-MVvac2-ATU(P) (del Valle et al., 2007) or p(+)MVvac2-GFP(H) via MluI/AatII and SfiI/SacII, respectively, to generate p(+)PolII-MVvac2-MERS-N(P) or p(+)PolII-MVvac2-MERS-N(H). For construction of lentiviral transfervectors encoding MERS-CoV-N, the ORF of MERS-N was amplified by PCR with primers encompassing flanking restriction sites NheI/XhoI and template pMA-RQ-MERS-N. Details on primers and PCR are available upon request. PCR products were cloned into pCR2.1-TOPO (Invitrogen Life Technology) and fully sequenced. Intact antigen ORF was cloned into pCSCW2gluc-IRES-GFP (Hewett et al., 2007) using NheI/XhoI restriction sites to yield pCSCW2-MERS-N-IRES-GFP.
2.3. Production of lentiviral vectors and generation of antigen-expressing dendritic cell lines
Lentiviral vectors were produced and used for the generation of antigen-expressing dendritic cell lines as described, before (Malczyk et al., 2015). In short, HIV-1-derived vectors were generated using a standard 3 plasmid system and the transfer vector plasmid pCSCW2-MERS-N-IRES-GFP by PEI transfection. Subsequent purification after harvest of transfected 293 T cells yielded virus stocks used to transduce DC cell lines, which were single cell-sorted by FACS and selected for antigen expression.
2.4. Viruses
MERS-N encoding vaccine candidates MVvac2-MERS-N(P) and MVvac2-MERS-N(H) were rescued as described (Malczyk et al., 2015, Martin et al., 2006). Single syncytia were picked and overlaid onto 50% confluent Vero cells cultured in 6-well plates and harvested as “passage 0” (P0) by scraping and freeze-thaw cycle of cells at the time of maximal infection. Subsequent passages were generated as described for the following viruses. MERS-N encoding vaccine viruses in P3 were used for characterization, viruses in P4 for vaccination. MERS-S encoding vaccine virus MVvac2-MERS-S(H), and control virus MVvac2-ATU(P) (Malczyk et al., 2015) were also used in P4 for vaccination. Both as well as MVvac2-GFP(P) and MERS-CoV (isolate EMC/2012) (Zaki et al., 2012) used for neutralization assays were propagated and titrated on Vero cells by the method of Spearman and Kaerber (Hubert, 1984, Kärber, 1931). MVvac2-MERS-S(H) was inactivated by UV-irradiation using a CL-1000 UV crosslinker (UVP, Cambridge, UK). 100 μL of virus suspension in 48-well-plates on ice were exposed to UV light of 254 nm at 3 cm distance from the UV source of 1,85 × 105 μJ/cm2 for 30 min. Inactivation of virus was controlled by incubation of Vero cells with a control aliquot inactivated, in parallel. All virus stocks were stored in aliquots at −80 °C.
2.5. Western blot analysis
Cells were lysed and immunoblotted as previously described (Funke et al., 2008). A rabbit anti-MERS-CoV serum (1:1000) was used as primary antibody for MERS-CoV-N and a rabbit anti-MV-N polyclonal antibody (1:25,000) (Abcam) for MV-N detection. A donkey HRP-coupled anti-rabbit IgG (H&L) polyclonal antibody (1:10,000) (Rockland, Gilbertsville, PA) served as secondary antibody for both. Peroxidase activity was visualized with an enhanced chemiluminescence detection kit (Thermo Scientific, Bremen, Germany) on Amersham Hyperfilm ECL (GE Healthcare, Freiburg, Germany).
2.6. Animal experiments
All animal experiments were carried out in compliance with the regulations of German animal protection laws and as authorized by the RP Darmstadt. Six- to 12-week-old or 7 months old IFNAR-/--CD46Ge mice (Mrkic et al., 1998) deficient for type I IFN receptor and transgenically expressing human CD46 were inoculated intraperitoneally (i.p.) with 1 × 105 TCID50 of recombinant viruses or UV-inactivated vaccine preparations on days 0 and either on day 21 or 28. Mice were bled on days 0, 28, and 49 post initial infection (p.i.). Serum samples were stored at − 20 °C. Mice were euthanized on days 32, 42, or 49 p.i., and splenocytes were harvested for assessment of cellular immune responses.
2.7. Neutralization assay
Quantification of VNTs was done as described, before (Malczyk et al., 2015). In brief, mouse sera were serially diluted in 2-fold dilution steps in DMEM in duplicates. A total of 50 PFU of MVvac2-GFP(P) or 200 TCID50 of MERS-CoV (strain EMC/2012) were mixed with diluted sera and incubated at 37 °C for 1 h. Virus suspensions were added to 1 × 104 Vero cells seeded 4 h prior to assay in 96-well plates and incubated for 4 days at 37 °C. VNTs were calculated as the reciprocal of the highest mean dilution that abolished infection.
2.8. ELISpot analysis
Murine gamma interferon (IFN-γ) enzyme-linked immunosorbent spot (ELISpot) assays (eBioscience, Frankfurt, Germany) were performed according to the manufacturer's instructions using multiscreen immunoprecipitation (IP) ELISpot polyvinylidene difluoride (PVDF) 96-well plates (Merck Millipore, Darmstadt, Germany). 5 × 105 isolated splenocytes were co-cultured with different stimuli in 200 μL RPMI + 10% FBS, 2 mM L-Gln, and 1% penicillin-streptomycin for 36 h. For re-stimulation of MERS N-specific T cells, splenocytes were co-cultivated with 5 × 104 JAWSII, DC2.4 dendritic cells, or clones of either cell line encoding MERS-N. On the other hand, splenocytes were stimulated with 10 μg/mL MV bulk antigen (Serion Immunologics, Würzburg, Germany), 10 μg/mL MERS S-derived peptide S1165 (Biosynthesis Inc., Lewisville, TX, USA, (Channappanavar et al., 2014)), or 10 μg/mL SIINFEKL control peptide (SIN) of ovalbumin (aa 257–264) (InvivoGen, San Diego, CA, USA), as appropriate. For general T cell stimulation, 10 μg/mL Concanavalin A (ConA, Sigma-Aldrich, St. Louis, MO, USA) was used. As negative control, splenocytes were left untreated. After 36 h, cells were removed from the plates, and plates were incubated with biotin-conjugated anti-IFN-γ antibodies and avidin-HRP according to the manufacturer's instructions. 3-Amino-9-ethyl-carbazole (AEC; Sigma-Aldrich) substrate solution for development of spots was prepared according to the manufacturer's instructions using AEC dissolved in N,N-dimethylformamide (Merck Millipore) and used for peroxidase-dependent staining, afterwards. Spots were counted using an Eli.Scan ELISpot scanner (AE.L.VIS, Hamburg, Germany) and ELISpot analysis software Eli.Analyse V5.0 (AE.L.VIS).
2.9. Intracellular cytokine staining
For flow cytometry-based determination of cytokine expression by intracellular cytokine staining (ICS), splenocytes of vaccinated mice were isolated, and 2 × 106 splenocytes per mouse were cultivated in 200 μL RPMI1640 + 10% FBS, 2 mM L-Gln, 1 × non-essential amino acids (Biochrom), 10 mM HEPES, 1% penicillin-streptomycin, 50 μM β-mercaptoethanol, 10 μg/mL Brefeldin A (Sigma-Aldrich), and one of the stimuli also used for ELISpot analysis. For general T cell stimulation, 0.25 μg/mL tetradecanoylphorbol acetate (TPA, Sigma Aldrich) and 0.5 μg/mL Ionomycin (Iono, Sigma-Aldrich) were used as positive control, and only medium was used as negative control. Splenocytes were stimulated for 5 h at 37 °C. Subsequently, cells were stained with fixable viability dye eFluor450 (eBioscience), CD4-PE (1:2000) (BD, Franklin Lakes, NJ, USA), CD8-FITC (1:500) (BD), and, after permeabilization with Fixation/Permeabilization Solution (BD) and Perm/Wash Buffer (BD), stained with IFN-γ-APC (1:500) (BD) and TNF-α-Pe-Cy7 (1:500) (BD). Cells were fixed with ice-cold 1% paraformaldehyde (PFA) in PBS and analyzed via flow cytometry using an LSRII SORP flow cytometer (BD) and FCS Express software (De Novo Software, Glendale, CA, USA).
3. Results
3.1. Recombinant MVvac2 expressing MERS-CoV-N induces N-specific T cells in vaccinated animals
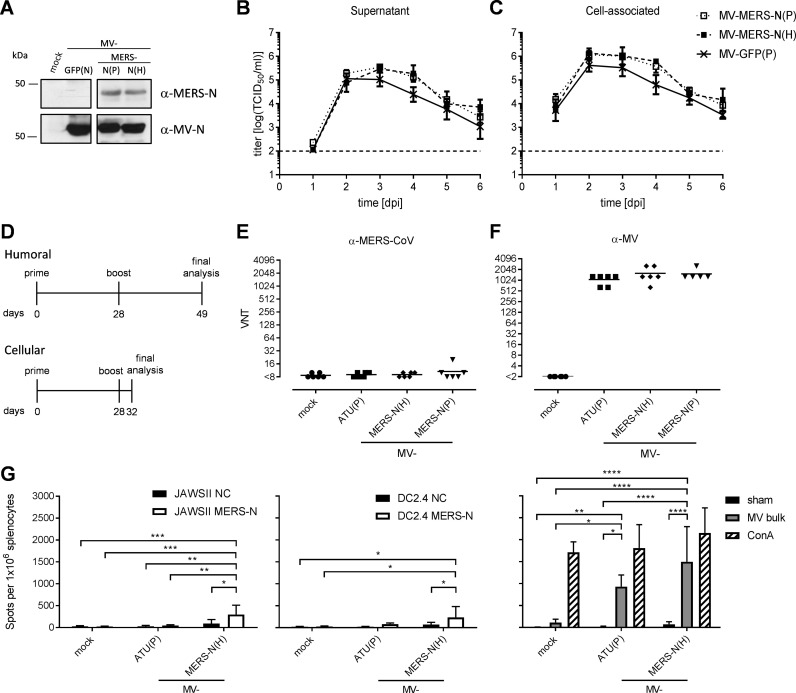
Since the nucleocapsid protein (N) of CoV is quite conserved, it is regarded as an appropriate target to induce anti-viral T cells. Therefore, MERS-CoV N was chosen as an alternative antigen to be expressed by the recombinant MV vaccine platform. Full-length MERS-N was cloned into two different additional transcription units (ATUs) either behind P (post P) or H (post H) cassettes of measles vaccine strain MVvac2 genome, and virus clones were successfully rescued and amplified in Vero cells with titers of up to 2 × 108 TCID50/mL. The essential verification of antigen expression by Western blot analysis of Vero cells infected with the MVvac2-MERS-N vaccines revealed expression of the N antigen with only little impact of the genomic position of the transgene cassette ( Fig. 1A), while growth kinetics showed no impairment of virus replication compared to the respective MVvac2-GFP(P) control virus (Fig. 1 B, C).
Fig. 1.
Generation, characterization, and immunogenicity of MVvac2-MERS-N (A) Immunoblot analysis of Vero cells infected at an MOI of 0.03 with MVvac2-MERS-N(P), MVvac2-MERS-N(H), or MVvac2-GFP(N) as depicted above the lanes. Uninfected cells served as mock control. Blots were probed with rabbit serum reactive against MERS-CoV (upper blot) or mAb directed against MV-N (lower blot). (B, C) Growth kinetics of recombinant MV on Vero cells infected at an MOI of 0.02 with MVvac2-MERS-N(P), MVvac2-MERS-N(H), or MVvac2-GFP(P). Virus from (B) supernatant or (C) cell-associated virus was harvested at indicated days post infection (dpi) and titrated on Vero cells. Means and standard deviation from three independent experiments. (D) Vaccination scheme for IFNAR-/--CD46Ge mice immunized with respective recombinant MV. (E, F) Analysis of virus neutralizing titers (VNT) in vaccinated animals on day 49 post prime immunization for complete neutralization of (E) 200 TCID50 of MERS-CoV or (F) 50 PFU of MV. Medium inoculated mice served as mock control. VNTs were calculated as the highest dilution abolishing infectivity. Dots represent single animals (n = 6); horizontal lines represent mean per group. The y-axis starts at the detection limit; all mice at the detection limit had no detectable VNT. (G) Secretion of IFN-γ after antigen-specific re-stimulation of splenocytes harvested 32 days post prime immunization and after co-culture with JAWSII (left) or DC2.4 (middle) dendritic cells transgenic for MERS-N (black) or untransduced controls (NC, white). (Right) To analyze cellular responses directed against MV, splenocytes were stimulated with 10 μg/mL MV bulk antigen (MV bulk) or left unstimulated (sham). The reactivity of splenocytes was confirmed by ConA treatment (10 μg/mL). TCID50, tissue culture infectious dose 50; One-way-ANOVA with Tukey multiple comparison. *: p < 0,05; **: p < 0,01; ***: p < 0001; ****: p < 0,0001.
To test the efficacy of the MVvac2-MERS-N candidate in vivo, genetically modified IFNAR-/--CD46Ge mice were chosen, since they are the prime small animal model for analysis of MV-derived vaccines (Mrkic et al., 1998). Thus, 6 mice per group were inoculated via the intraperitoneal (i.p.) route on days 0 and 28 with each time 1 × 105 TCID50 of MVvac2-MERS-N(P), MVvac2-MERS-N(H), or empty control virus MVvac2-ATU(P). Medium-inoculated mice served as negative controls. 21 days or four days after boost immunization, sera or splenocytes of immunized mice were sampled, respectively (Fig. 1D). As expected, all mice immunized with recombinant MV (including the control virus) developed high MV virus neutralizing titers (VNT) (512–2048 VNT, Fig. 1F). Little evidence for induction of neutralizing antibodies against MERS-CoV was found in all mice, as expected for the intra-particular antigen (Fig. 1E). No VNTs against MV or MERS-CoV were detected in control mice inoculated with medium alone.
To analyze splenocytes of animals vaccinated with MVvac2-MERS-N(H) or control animals inoculated with medium or MVvac2-ATU(P) by ELISpot assay for antigen-specific IFN-γ secretion, the antigen-specific T cells were re-stimulated in vitro by syngeneic murine DC cell lines (JAWSII and DC2.4), which had been genetically modified by lentiviral vector transduction to stably express MERS-N protein and thereby to present the respective T cell epitopes on MHC. Single cell clones were derived by flow cytometric sorting of single GFP-positive cells. Antigen expression by transduced DCs was verified by Western Blot analysis (data not shown). ELISpot assays using splenocytes of vaccinated animals in co-culture with JAWSII-MERS-N or DC2.4-MERS-N revealed about 200 IFN-γ secreting cells per 1 × 106 splenocytes after immunization with MVvac2-MERS-N(H) (Fig. 1G), which was significant over controls. Additionally, cellular immune responses targeting MV antigens were detected upon stimulation with MV bulk antigens in vaccinated mice that had received any recombinant virus, as expected. However, MV bulk antigens stimulated about 930–1500 IFN-γ secreting cells per 1 × 106 splenocytes of MV vaccinated animals, as described, before (Malczyk et al., 2015). Finally, splenocytes of all mice revealed a similar basic reactivity to unspecific T cell stimulation, as confirmed by similar numbers of IFN-γ secreting cells upon ConA treatment (Fig. 1G). Thus, the generated MV-based vaccine platform expressing MERS-N induces significant MERS N-specific cellular immune responses, as desired. In any case, humoral and cellular responses induced by vaccine candidate MVvac2-MERS-S had been considerably higher in previous analyses under similar conditions (Malczyk et al., 2015). Therefore, further characterization of anti-MERS-CoV immunity induced by MVvac2-derived vaccines proceeded with this MERS-S encoding vaccine candidate, which yielded approximately 5-fold higher numbers of reactive T cells after vaccination.
3.2. Live but not UV-inactivated MVvac2-MERS-S(H) induces humoral immune responses against MERS-CoV in IFNAR-/--CD46Ge mice
Since the MERS vaccine candidate MVvac2-MERS-S(H) induced robust protective humoral and cellular immune responses in IFNAR- /--CD46Ge mice (Malczyk et al., 2015), we were interested in the necessity of viral replication of this life-attenuated vaccine for the induction of MERS CoV-specific immunity. For these analyses IFNAR-/--CD46Ge mice were chosen as the animal model, again, since these mice are the standard animal model for analysis of MV-derived vaccines (Mühlebach, 2017), their genetic composition is compatible with an established MERS-CoV challenge model (Zhao et al., 2014), as shown, before (Malczyk et al., 2015), and their size allows housing under regularly available conditions opposed to dromedary camels, the only know natural host of MERS-CoV, to date.
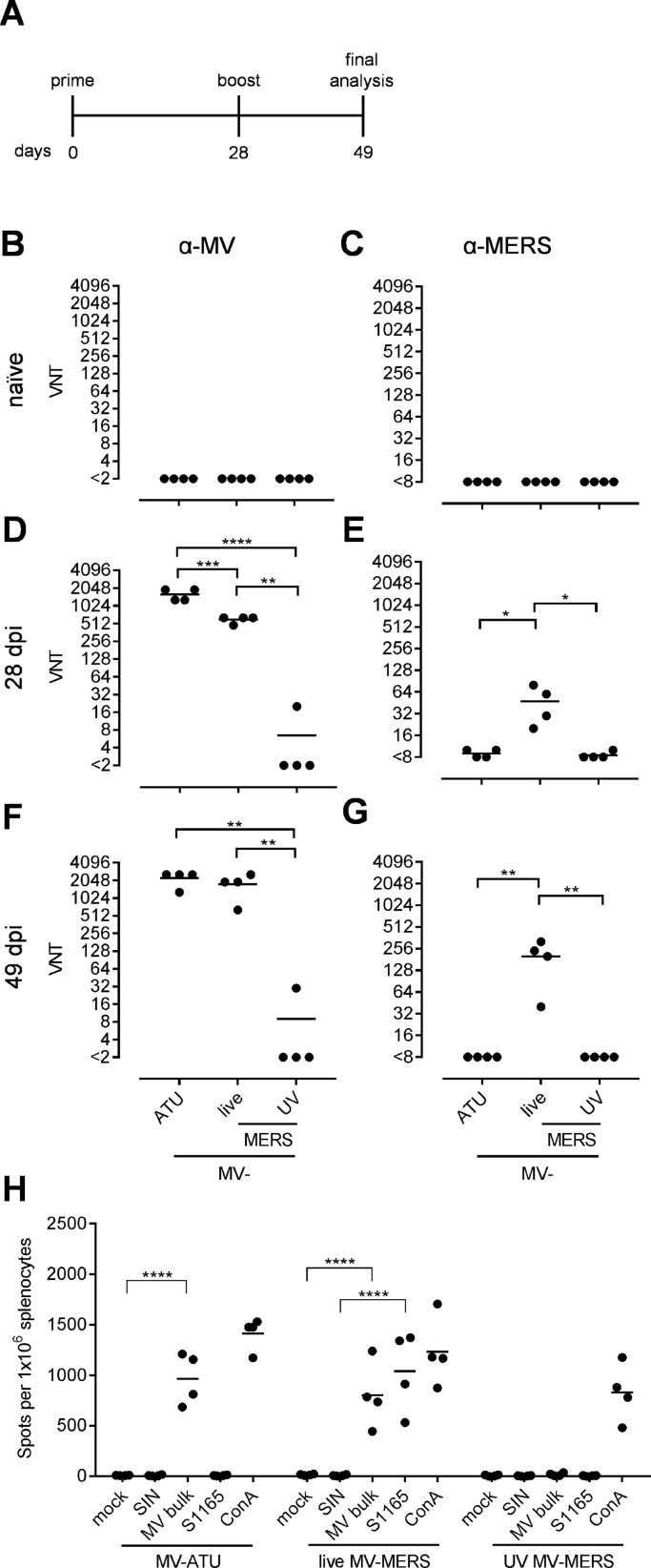
As all morbilliviruses, the MV-based vaccine virions are highly cell-associated, and transfer of antigenic protein within the vaccine preparation cannot be excluded. Therefore, we vaccinated these MV-susceptible mice with either 1 × 105 TCID50 of live or of the same formulation and quantity UV-inactivated MVvac2-MERS-S(H) in a prime-boost regimen ( Fig. 2A). MVvac2-ATU(P), which does not encode any additional antigen, was included as vector control. Blood was drawn from naïve mice on day 0 before vaccination, and on days 28 and 49 post-immunization. Serum samples were tested for their ability to neutralize MVvac2-GFP(P) (Fig. 2B, D, F) or MERS-CoV (Fig. 2C, E, G).
Fig. 2.
Humoral and cellular immune responses induced by live or UV-inactivated MV-MERS-S(H). (A) Schematic depiction of vaccination scheme for IFNAR-/--CD46Ge mice vaccinated with MVvac2-MERS-S(H), UV-inactivated MVvac2-MERS-S(H), or MVvac2-ATU(P) as vector control. (B-G) Virus neutralizing titers (VNT) in vaccinated mice. Titers of (B, D, F) MV or (C, E, G) MERS-CoV neutralizing antibodies in sera of (B, C) naїve mice, or in sera of mice after (D, E) prime- or (F, G) boost-immunization. One-way ANOVA with Tukey multiple comparison. * : p < 0,05; * *: p < 0,01; ***: p < 0001; ****: p < 0,0001. (H) Secretion of IFN-γ after antigen-specific re-stimulation of splenocytes. IFN-γ ELISpot analysis using splenocytes of mice vaccinated on days 0 and 28 with indicated vaccines isolated 21 days after boost immunization and after incubation with indicated stimuli (MERS-S peptide S1165, MV bulk antigen (MV bulk), immunodominant ovalbumin-derived SIINFEKL-peptide (SIN) as a peptide negative control) or untreated (mock). The reactivity of splenocytes was confirmed by Concanavalin A (ConA) treatment (10 μg/mL). The number of cells per 1 × 106 splenocytes represent the amount of cells expressing IFN-γ upon re-stimulation. Dots represent individual animals, horizontal bars mean. One-way ANOVA with Tukey multiple comparison. ****: p < 0,0001.
Sera of naïve mice had no neutralizing antibodies against either virus (Fig. 2B, C). After the first immunization, both live virus preparations induced neutralizing antibodies against MV, with MVvac2-ATU(P) triggering significantly higher titers (1280–1920 VNT) than MVvac2-MERS-S(H) (480–640 VNT). After the second immunization, anti-MV VNTs increased to titers of 640–2560 in both cohorts. In contrast, only one out of four animals in the UV-inactivated vaccine group had a borderline neutralizing antibody titer of 20 after the first immunization, and another animal had a titer of 30 after the boost. While MVvac2-ATU(P) and the UV-inactivated MVvac2-MERS-S(H) vaccine did not induce neutralizing antibodies against MERS-CoV above background levels over the course of the experiment, the group vaccinated with live MVvac2-MERS-S(H) developed titers around 50 after the first immunization and 40–320 (mean of 300) after the boost. Taken together, these data reveal that replication of the vaccine is necessary to induce functional antibody responses against MV and the additional antigen MERS-S.
3.3. Only vaccination with live MVvac2-MERS-S(H) elicits cellular immune responses
To assess the capacity of the different MVvac2-MERS-S(H) vaccine preparations to induce MERS-CoV S-specific cellular immune responses, splenocytes of mice, which had already been tested for humoral responses (Fig. 2A), were isolated and analyzed 49 days after immunization for antigen(Ag)-dependent IFN-γ secretion using ELISpot assay. The isolated splenocytes were re-stimulated with MERS-S immunodominant peptide S1165 (Channappanavar et al., 2014) or MV bulk antigen (MV bulk) to analyze MV-specific cellular immune responses. Ovalbumin-derived SIINFEKL-peptide (SIN) served as peptide negative control, or cells were left untreated (mock). Stimulation with concanavalin A (ConA) was used to confirm general T cell reactivity in splenocyte preparations (Fig. 2H). While splenocytes of all mice responded to ConA with 1000 to 1500 spots per 1 × 106 splenocytes, only those from animals vaccinated with live MVvac2-MERS-S(H) could be stimulated with MERS S-specific peptide S1165 reaching mean values of 1040 spots per 1 × 106 splenocytes. In contrast, splenocytes of the UV-inactivated group or control virus MVvac2-ATU(P) could not be re-stimulated to secrete IFN-γ. Furthermore, only replication-competent vaccine viruses induced MV-specific cellular immune responses in vaccinated mice. Re-stimulation with MV bulk Ag induced a mean of 800 and 970 spots per 1 × 106 splenocytes for MVvac2-MERS-S(H) or MVvac2-ATU(P) vaccinated mice, respectively. Consequently, replication of the vaccine candidate is essential to induce both arms of the immune system with responses against MV as well as the additional MERS-S antigen.
3.4. MVvac2-MERS-S(H) is immunogenic in adult mice
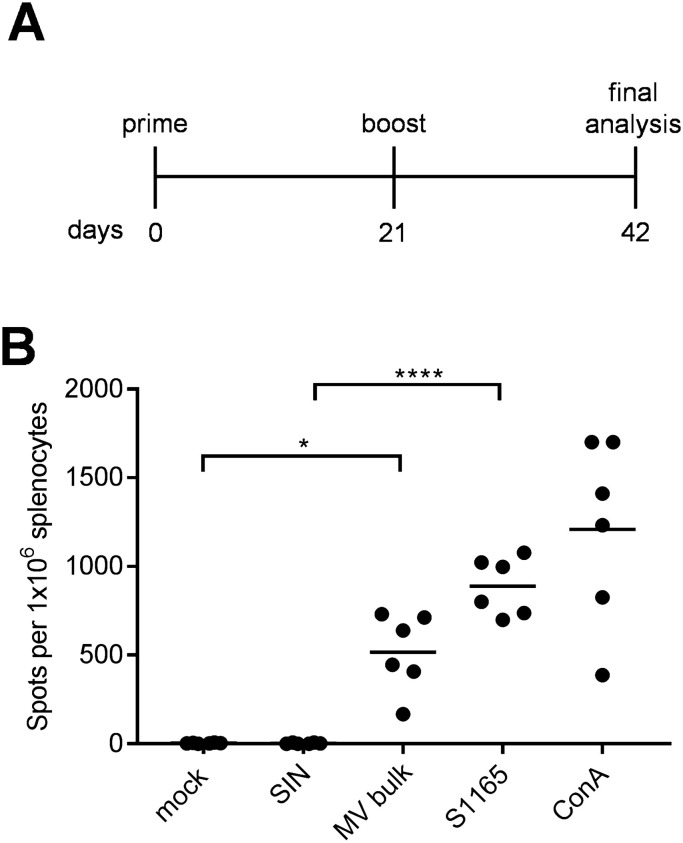
Usually, 6–12 weeks old juvenile mice are used for our immunization studies. To evaluate if there is an age-dependent change in vaccination efficacy, approximately 7 months-old mice were vaccinated with MVvac2-MERS-S(H) in a prime-boost vaccination scheme with 3 weeks between prime and boost vaccination ( Fig. 3A). Mice were sacrificed at day 42 post-immunization, and splenocytes were re-stimulated with MV-antigens or MERS-S peptide S1165. We found that reactive IFN-γ-secreting T cells were also specifically induced in mice of this age (Fig. 3B). A mean of 520 spots per 1 × 106 splenocytes was detected upon re-stimulation with MV bulk antigen, whereas 900 spots per 1 × 106 splenocytes were induced by re-stimulation with the MERS S-derived peptide S1165, illustrating that MV- and MERS-CoV-specific cellular immune responses are effectively induced in adult mice.
Fig. 3.
Characterization of T-cell responses against MERS-CoV in older mice. (A) Schematic depiction of vaccination scheme for at least 7 months old IFNAR-/--CD46Ge mice vaccinated with MVvac2-MERS-S(H) with splenocytes harvest 21 days after boost. (B) These splenocytes were re-stimulated with MERS-S peptide S1165, MV bulk Ag (MV bulk), ovalbumin-derived SIINFEKL-peptide (SIN) as peptide negative control, medium (mock), or Concanavalin A (ConA) as a general T-cell activator positive control. Dots represent individual animals, horizontal bars mean. One-way-Anova with Tukey multiple comparison. *: p < 0,05; ****: p < 0,0001.
3.5. Induced T cell responses are multifunctional
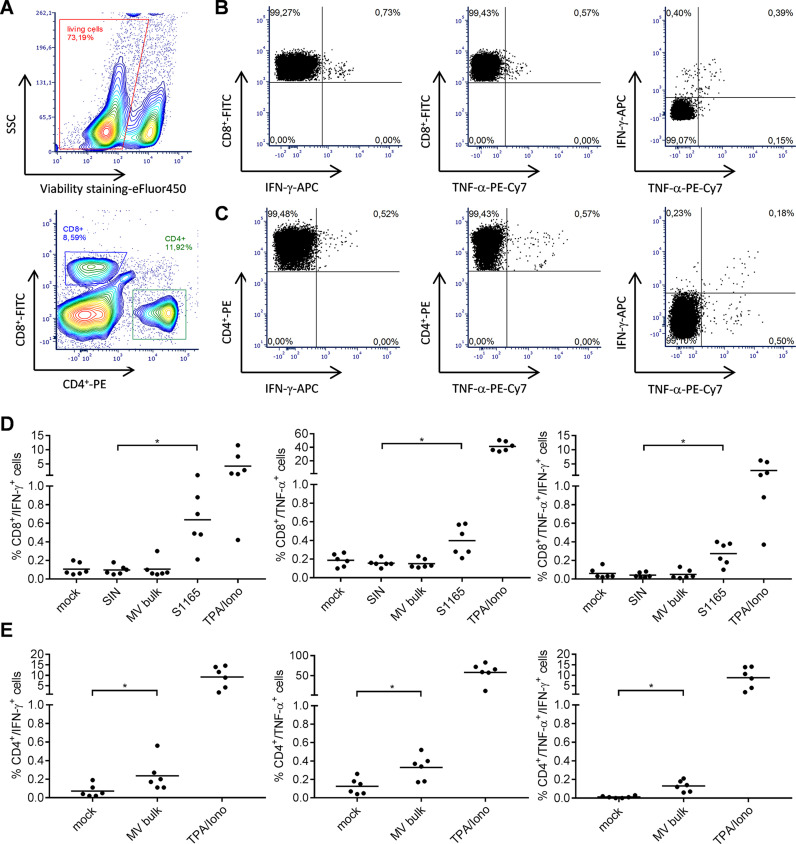
To gain more detailed insights in the quality of the observed T cell responses, we further characterized the responsive T cell populations by flow cytometry, determining the expression of CD8+ and CD4+ surface markers as well as IFN-γ and TNF-α upon re-stimulation with S1165 or MV bulk antigen. As a positive stimulus for T cell activation tetradecanoylphorbol-acetate and ionomycin (TPA/Iono) were used. Exocytosis of cytokines was blocked by addition of brefeldin A (10 μg/mL) during stimulation. Cells were permeabilized, labelled, and fixed for flow cytometry. The gating strategy excluded duplicates (not shown), selected for living cells ( Fig. 4A, upper panel), and separated CD8+ and CD4+ T cells (Fig. 4A, lower panel). Selected CD8+ T cells were then analyzed for their expression of IFN-γ (Fig. 4B left panel), TNF-α (Fig. 4B middle panel), or both (Fig. 4B right panel) as exemplarily shown for splenocytes re-stimulated with MERS-S peptide S1165. Likewise, CD4+ T cells expressing IFN-γ (Fig. 4C, left panel), TNF-α (Fig. 4C, middle panel), or both (Fig. 4C, right panel) are depicted after re-stimulation with MV bulk antigen.
Fig. 4.
Detection of multi-functional T-cell responses induced by vaccination with MVvac2-MERS-S(H). Harvested splenocytes of MVvac2-MERS-S(H) vaccinated mice (same as depicted in Fig. 3) were re-stimulated and subjected to intracellular staining (ICS) for IFN-γ and TNF-α and stained for extracellular T-cell markers CD4 and CD8 for flow cytometry analysis. (A - C) Gating strategy for analysis of CD8+ or CD4+ T-cells expressing IFN-γ or TNF-α within splenocytes stimulated with (B) S1165 peptide or (C) MV-bulk Ag. Duplicates (not shown) and dead cells (A) were excluded from analysis. (B, C) CD8+ and CD4+ cells were separately subjected to analysis for IFN-γ- (left panels), TNF-α- (middle panels) or double-positive cells (right panels). Quantification of flow cytometry data of (D) CD8+- and (E) CD4+-positive cells after incubation with indicated stimuli (MERS S-specific peptide S1165, MV bulk Ag (MV bulk), immunodominant Ovalbumin-derived SIINFEKL-peptide (SIN) as a peptide negative control, or untreated cells (mock); reactivity of splenocytes was confirmed by Tetradecanoylphorbol-acetate and Ionomycin (TPA/Iono) treatment (10 μg/mL). Dots represent individual animals, horizontal bars mean. Repeated-measures one-way ANOVA with Tukey multiple comparison. *: p < 0,05.
Vaccination with MVvac2-MERS-S(H) induced a significant amount of MERS S-specific CD8+ T cells expressing either IFN-γ (Fig. 4D, left panel) or TNF-α (Fig. 4D, middle panel), with means of 0.6% and 0.4% of total positive cells, respectively. Among those, a significant fraction of cells revealed to be multifunctional, with a mean of 0.3% of all CD8+ cells or 75% of the TNF-α − responsive cells being positive for both cytokines (Fig. 4D, right panel). Moreover, vaccination induced a significant fraction of vector-specific CD4+ T cells expressing IFN-γ (Fig. 4E, left panel), or TNF-α (Fig. 4E, middle panel) upon re-stimulation with MV bulk antigen. Among those, multifunctional CD4+ T cells expressing both cytokines were induced with a mean of about 0.1% (Fig. 4E, right panel). To conclude, vaccination with MVvac2-MERS-S(H) induces not only IFN-γ or TNF-α expressing T cells directed against MERS-CoV and MV, but also a significant fraction of multi-functional cytotoxic T cells specific for MERS-S and CD4+ T cells specific for MV antigens, illustrating that a broad and robust MERS-CoV-specific immune response is induced by vaccination with MVvac2-MERS-S(H).
4. Discussion
In this study, we aimed to understand the induction of immunity and the functionality of induced T cell responses after vaccination with MVvac2-MERS-S(H), a vaccine candidate that induces protective immunity against MERS-CoV in an appropriate animal model. In parallel, we generated and tested also alternative MV-based vaccine candidates expressing MERS-CoV N protein as conserved T cell antigen. MVvac2-MERS-N vaccine candidates indeed induced significant antigen-specific cellular immune responses in vaccinated transgenic mice, revealing that also MERS N-expression by recombinant MV may have a useful role to combat MERS-CoV. Since the immune responses induced by MERS-S expressing candidates had been nevertheless considerably higher, we proceeded with S-expressing vaccine virus to characterize the induction of anti-MERS-CoV immunity by MV-based vectors.
Using MVvac2-MERS-S(H), robust anti-MERS CoV immune responses were induced also in older mice, while replication of the vaccine vector was necessary to induce either arm of adaptive immunity against vector or pathogen. Furthermore, vaccination with MVvac2-MERS-S(H) triggered significant numbers of multifunctional MERS S-specific CD8+ T cells and MV-specific CD4+ T cells, simultaneously producing IFN-γ and TNF-α upon stimulation with respective antigens.
Since not only numbers, but also the quality of the induced MERS CoV-specific T cell responses might be relevant for protection against MERS-CoV, it is quite encouraging to see that approx. 50% of IFN-γ reactive CD8+ T cells also expressed TNF-α, whereas in reverse 75% of TNF-α-reactive CD8+ T cells co-expressed IFN-γ upon stimulation with the immune-dominant MERS-S peptide. This induction of multifunctional T cells is quite in accordance with previous studies, since the potential of MV during natural infection or the recombinant MV to induce multifunctional, antigen-specific T cells has already been demonstrated. Infection of macaques with wild-type MV induces polyfunctional T cells specific for MV proteins with increasing numbers of cells secreting IL-2, TNF-α, as well as IFN-γ over time (Nelson et al., 2017), and polyfunctional T cells directed against MV-H can be expanded from PBMC of human donors (Ndhlovu et al., 2010). Likewise, HIV-vaccine candidates MV1-F4, which encode Gag, RT, and Nef of an HIV-1 clade B or a clade C strain as foreign antigen, induce antigen-specific multifunctional T cells simultaneously expressing IFN-γ, TNF-α, and IL-2 in mice and also macaques (Stebbings et al., 2013, Stebbings et al., 2012). While the combination of IFN-γ and TNF-α indicates functional T cells with higher potency, in general, expression of IL-2 is a sign of the induction of CD8+ memory T cells (Williams et al., 2006). In our study, the strong correlation of IFN-γ and TNF-α expression thus indicates a high functionality of induced T cell responses. Extension of the antibody panel for IL-2 detection could yield further insight into the durability of these T cell responses induced by the MV vaccine platform in future studies.
Such multifunctional CD8+ T cells specific for MERS-CoV may become especially important, since mouse studies have shown that CD8+ T cells are crucial for clearance of MERS-CoV infection (Coleman et al., 2017, Zhao et al., 2014). Noteworthy, for other viral infections such as human immunodeficiency virus (HIV), modified vaccinia virus Ankara (MVA), or cytomegalovirus (CMV) the amount of just IFN-γ producing T cells does not correlate with CTL killing effectivity, but the multifunctionality of antigen-specific T cells inversely correlated with viral load (Betts et al., 2006, Lichterfeld et al., 2004, Precopio et al., 2007, Sandberg et al., 2001), further underlining the importance of multifunctionality.
Besides these cellular immune responses, also considerable humoral immunity was induced in vaccinated animals, here. The mean VNT was somewhat lower than expected (Malczyk et al., 2015), but still quite high. An alternative vaccine candidate derived from modified vaccinia virus Ankara, MVA-MERS-S, revealed protection in dromedary camels, the natural host for MERS-CoV (Haagmans et al., 2016). Passive immunotherapy with dromedary immune sera significantly reduced MERS-CoV titers in lung tissue of challenged mice, starting with a VNT of 160 (Zhao et al., 2015). Neutralizing antibody titers in reconvalescent plasma of human patients diagnosed with MERS were determined by microneutralization tests in two previous studies, and were on average at 175 (Arabi et al., 2016) or 58.3 (Zhao et al., 2017). Furthermore, a PRNT50 titer of at least just 50 was required to lower virus titers by more than 0.5 log in mice challenged after transfer of human reconvalescent plasma (Zhao et al., 2017). These titers were exceeded in this study. In addition, MVvac2-MERS-S(H) induced higher anti-MERS-S titers in C57/BL6 mice than MVA-MERS-S in Balb/c mice, when comparing studies that used similar virus titers for vaccination (Malczyk et al., 2015, Volz et al., 2015), thus indicating an at least comparable efficacy. Thus, also VNT determined here indicate efficacy and were anyway not statistically different from those published before for MVvac2-MERS-S(H) (Malczyk et al., 2015). Nevertheless, the exact correlates of protection for MERS-CoV remain to be determined in future studies, since it will be essential to evaluate the efficacy of the different vaccine candidates against this most important benchmark.
In contrast, UV-inactivated MVvac2-MERS-S(H) did not induce any antibodies able to neutralize MERS-CoV or MV. While neutralizing antibodies can in principle also be induced by inactivated vaccines or proteins, e.g. full-length or truncated MERS-S protein in combination with adjuvant (Wang et al., 2015). Obviously, the amount of MERS-S antigen within the MVvac2-MERS-S(H) vaccine formulation or the adjuvant effect of the inactivate were not sufficient during application. Therefore, replication of the MV-derived MERS vaccine candidate is necessary for the induction of immune responses both against vector and antigen of interest in vaccinated animals. Indeed, the induction of cellular immunity is usually more efficient by de novo expression of antigen after immunization. Consequently, the application of a replication competent vaccine platform is justified here to robustly induce potent responses of both arms of the adaptive immune system.
These powerful immune responses were not only induced in juvenile mice 6–12 weeks of age, but also in adult mice older than half a year of age. This is quite of interest, since adult vaccinees are also the target group for vaccination in response to the MERS-CoV outbreak, as defined in the target product profile by the WHO (http://www.who.int/blueprint/what/research-development/MERS_CoV_TPP_ 15052017.pdf). Remarkably, MV vaccine strain virus encoding Chikungunya virus (CHIKV) antigens was already tested in a phase I clinical trial in adult human vaccinees (18–45 years old) (Ramsauer et al., 2015). These adult test subjects all developed significant humoral immunity against CHIKV, despite their adult age and most interestingly also independent from measles pre-immunity.
Taken together, these study shows that MVvac2-MERS-S(H) induces surprisingly high numbers of multifunctional T cells specific for MERS-S also in adult test subjects, as a result from replication of the recombinant vector. Therefore, high quality cellular immune responses are induced in addition to the robust antibody responses by this vaccine candidate, further qualifying MVvac2-MERS-S(H) for evaluation as vaccine candidate against MERS-CoV. In parallel, MERS-N encoding MV can be a further option to generate protection against MERS in future studies and constructs.
Acknowledgements
This work was supported by the German Center for Infection Research (DZIF; TTU 01.802). The authors would like to thank Vivian Scheuplein, Jürgen Schnotz, and Daniela Müller for excellent technical assistance. The authors are indebted to Ron Fouchier for providing MERS-CoV strain EMC/2012, Kenneth Rock for DC2.4 cells, Roberto Cattaneo for providing the pB(+)MVvac2 construct, and Urs Schneider for providing the PolII Rescue System originally used to generate and to rescue recombinant MV vectors. The authors would further like to thank Bakhos Tannous for providing pCSCW2gluc-IRES-GFP. Moreover, the authors would like to thank Veronika von Messling for critically reading the manuscript.
References
- Agnihothram S., Gopal R., Yount B.L., Donaldson E.F., Menachery V.D., Graham R.L., Scobey T.D., Gralinski L.E., Denison M.R., Zambon M., Baric R.S. Evaluation of serologic and antigenic relationships between middle eastern respiratory syndrome coronavirus and other coronaviruses to develop vaccine platforms for the rapid response to emerging coronaviruses. J. Infect. Dis. 2014;209(7):995–1006. doi: 10.1093/infdis/jit609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alagaili A.N., Briese T., Mishra N., Kapoor V., Sameroff S.C., Burbelo P.D., Wit E. de, Munster V.J., Hensley L.E., Zalmout I.S., Kapoor A., Epstein J.H., Karesh W.B., Daszak P., Mohammed O.B., Lipkin W.I. Middle East respiratory syndrome coronavirus infection in dromedary camels in Saudi Arabia. mBio. 2014;5(2) doi: 10.1128/mBio.00884-14. (e00884-14) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alraddadi B.M., Al-Salmi H.S., Jacobs-Slifka K., Slayton R.B., Estivariz C.F., Geller A.I., Al-Turkistani H.H., Al-Rehily S.S., Alserehi H.A., Wali G.Y., Alshukairi A.N., Azhar E.I., Haynes L., Swerdlow D.L., Jernigan J.A., Madani T.A. Risk factors for Middle East respiratory syndrome coronavirus infection among healthcare personnel. Emerg. Infect. Dis. 2016;22(11):1915–1920. doi: 10.3201/eid2211.160920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arabi Y.M., Hajeer A.H., Luke T., Raviprakash K., Balkhy H., Johani S., Al-Dawood A., Al-Qahtani S., Al-Omari A., Al-Hameed F., Hayden F.G., Fowler R., Bouchama A., Shindo N., Al-Khairy K., Carson G., Taha Y., Sadat M., Alahmadi M. Feasibility of using convalescent plasma immunotherapy for MERS-CoV infection, Saudi Arabia. Emerg. Infect. Dis. 2016;22(9):1554–1561. doi: 10.3201/eid2209.151164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betts M.R., Nason M.C., West S.M., Rosa S.C., de, Migueles S.A., Abraham J., Lederman M.M., Benito J.M., Goepfert P.A., Connors M., Roederer M., Koup R.A. HIV nonprogressors preferentially maintain highly functional HIV-specific CD8+ T cells. Blood. 2006;107(12):4781–4789. doi: 10.1182/blood-2005-12-4818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Channappanavar R., Zhao J., Perlman S. T cell-mediated immune response to respiratory coronaviruses. Immunol. Res. 2014;59(1–3):118–128. doi: 10.1007/s12026-014-8534-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman C.M., Sisk J.M., Halasz G., Zhong J., Beck S.E., Matthews K.L., Venkataraman T., Rajagopalan S., Kyratsous C.A., Frieman M.B. CD8+ T cells and macrophages regulate pathogenesis in a mouse model of Middle East respiratory syndrome. J. Virol. 2017;91(1) doi: 10.1128/JVI.01825-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- del Valle J.R., Devaux P., Hodge G., Wegner N.J., McChesney M.B., Cattaneo R. A vectored measles virus induces hepatitis B surface antigen antibodies while protecting macaques against measles virus challenge. J. Virol. 2007;81(19):10597–10605. doi: 10.1128/JVI.00923-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drosten C., Meyer B., Müller M.A., Corman V.M., Al-Masri M., Hossain R., Madani H., Sieberg A., Bosch B.J., Lattwein E., Alhakeem R.F., Assiri A.M., Hajomar W., Albarrak A.M., Al-Tawfiq J.A., Zumla A.I., Memish Z.A. Transmission of MERS-coronavirus in household contacts. N. Engl. J. Med. 2014;371(9):828–835. doi: 10.1056/NEJMoa1405858. [DOI] [PubMed] [Google Scholar]
- Funke S., Maisner A., Mühlebach M.D., Koehl U., Grez M., Cattaneo R., Cichutek K., Buchholz C.J. Targeted cell entry of lentiviral vectors. Mol. Ther.: J. Am. Soc. Gene Ther. 2008;16(8):1427–1436. doi: 10.1038/mt.2008.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haagmans B.L., Al Dhahiry S.H.S., Reusken C.B.E.M., Raj V.S., Galiano M., Myers R., Godeke G.-J., Jonges M., Farag E., Diab A., Ghobashy H., Alhajri F., Al-Thani M., Al-Marri S.A., Al Romaihi H.E., Al Khal A., Bermingham A., Osterhaus A.D.M.E., AlHajri M.M., Koopmans M.P.G. Middle East respiratory syndrome coronavirus in dromedary camels: an outbreak investigation. Lancet Infect. Dis. 2014;14(2):140–145. doi: 10.1016/S1473-3099(13)70690-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haagmans B.L., van den Brand J.M.A., Raj V.S., Volz A., Wohlsein P., Smits S.L., Schipper D., Bestebroer T.M., Okba N., Fux R., Bensaid A., Solanes Foz D., Kuiken T., Baumgärtner W., Segalés J., Sutter G., Osterhaus A.D.M.E. An orthopoxvirus-based vaccine reduces virus excretion after MERS-CoV infection in dromedary camels. Science (N. Y., N. Y.) 2016;351(6268):77–81. doi: 10.1126/science.aad1283. [DOI] [PubMed] [Google Scholar]
- Hewett J.W., Tannous B., Niland B.P., Nery F.C., Zeng J., Li Y., Breakefield X.O. Mutant torsinA interferes with protein processing through the secretory pathway in DYT1 dystonia cells. Proc. Natl. Acad. Sci. USA. 2007;104(17):7271–7276. doi: 10.1073/pnas.0701185104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubert J. Bioassays: Spearman-Karber Method. 2nd ed. Hunt Publishing; Dubuque, IA: 1984. pp. 65–66. [Google Scholar]
- Kärber G. Beitrag zur kollektiven Behandlung pharmakologischer Reihenversuche. Archiv f. experiment. Pathol. U. Pharmakol. 1931;162(4):480–483. doi: 10.1007/BF01863914. [DOI] [Google Scholar]
- Lichterfeld M., Yu X.G., Waring M.T., Mui S.K., Johnston M.N., Cohen D., Addo M.M., Zaunders J., Alter G., Pae E., Strick D., Allen T.M., Rosenberg E.S., Walker B.D., Altfeld M. HIV-1-specific cytotoxicity is preferentially mediated by a subset of CD8(+) T cells producing both interferon-gamma and tumor necrosis factor-alpha. Blood. 2004;104(2):487–494. doi: 10.1182/blood-2003-12-4341. [DOI] [PubMed] [Google Scholar]
- Malczyk A.H., Kupke A., Prüfer S., Scheuplein V.A., Hutzler S., Kreuz D., Beissert T., Bauer S., Hubich-Rau S., Tondera C., Eldin H.S., Schmidt J., Vergara-Alert J., Süzer Y., Seifried J., Hanschmann K.-M., Kalinke U., Herold S., Sahin U., Cichutek K., Waibler Z., Eickmann M., Becker S., Mühlebach M.D. A highly immunogenic and protective middle east respiratory syndrome coronavirus vaccine based on a recombinant measles virus vaccine platform. J. Virol. 2015;89(22):11654–11667. doi: 10.1128/JVI.01815-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin A., Staeheli P., Schneider U. RNA polymerase II-controlled expression of antigenomic RNA enhances the rescue efficacies of two different members of the Mononegavirales independently of the site of viral genome replication. J. Virol. 2006;80(12):5708–5715. doi: 10.1128/JVI.02389-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mrkic B., Pavlovic J., Rülicke T., Volpe P., Buchholz C.J., Hourcade D., Atkinson J.P., Aguzzi A., Cattaneo R. Measles virus spread and pathogenesis in genetically modified mice. J. Virol. 1998;72(9):7420–7427. doi: 10.1128/jvi.72.9.7420-7427.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mühlebach M.D. Vaccine platform recombinant measles virus. Virus Genes. 2017;53(5):733–740. doi: 10.1007/s11262-017-1486-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ndhlovu Z.M., Oelke M., Schneck J.P., Griffin D.E. Dynamic regulation of functionally distinct virus-specific T cells. Proc. Natl. Acad. Sci. USA. 2010;107(8):3669–3674. doi: 10.1073/pnas.0915168107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson A.N., Putnam N., Hauer D., Baxter V.K., Adams R.J., Griffin D.E. Evolution of T cell responses during measles virus infection and RNA clearance. Sci. Rep. 2017;7(1):11474. doi: 10.1038/s41598-017-10965-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okba N.M., Raj V.S., Haagmans B.L. Middle East respiratory syndrome coronavirus vaccines: current status and novel approaches. Curr. Opin. Virol. 2017;23:49–58. doi: 10.1016/j.coviro.2017.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Precopio M.L., Betts M.R., Parrino J., Price D.A., Gostick E., Ambrozak D.R., Asher T.E., Douek D.C., Harari A., Pantaleo G., Bailer R., Graham B.S., Roederer M., Koup R.A. Immunization with vaccinia virus induces polyfunctional and phenotypically distinctive CD8(+) T cell responses. J. Exp. Med. 2007;204(6):1405–1416. doi: 10.1084/jem.20062363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsauer K., Schwameis M., Firbas C., Müllner M., Putnak R.J., Thomas S.J., Desprès P., Tauber E., Jilma B., Tangy F. Immunogenicity, safety, and tolerability of a recombinant measles-virus-based chikungunya vaccine: a randomised, double-blind, placebo-controlled, active-comparator, first-in-man trial. Lancet Infect. Dis. 2015;15(5):519–527. doi: 10.1016/S1473-3099(15)70043-5. [DOI] [PubMed] [Google Scholar]
- Reusken C.B., Ababneh M., Raj V.S., Meyer B., Eljarah A., Abutarbush S., Godeke G.J., Bestebroer T.M., Zutt I., Muller M.A., Bosch B.J., Rottier P.J., Osterhaus A.D., Drosten C., Haagmans B.L., Koopmans M.P. Middle East Respiratory Syndrome coronavirus (MERS-CoV) serology in major livestock species in an affected region in Jordan, June to September 2013. Eur. Surveill.: Bull. Eur. sur Les. Mal. Transm. = Eur. Commun. Dis. Bull. 2013;18(50):20662. doi: 10.2807/1560-7917.es2013.18.50.20662. [DOI] [PubMed] [Google Scholar]
- Reusken C.B.E.M., Haagmans B.L., Müller M.A., Gutierrez C., Godeke G.-J., Meyer B., Muth D., Raj V.S., Smits-De Vries L., Corman V.M., Drexler J.-F., Smits S.L., El Tahir Y.E., Sousa R., de, van Beek J., Nowotny N., van Maanen K., Hidalgo-Hermoso E., Bosch B.-J., Rottier P., Osterhaus A., Gortázar-Schmidt C., Drosten C., Koopmans M.P.G. Middle East respiratory syndrome coronavirus neutralising serum antibodies in dromedary camels: a comparative serological study. Lancet Infect. Dis. 2013;13(10):859–866. doi: 10.1016/S1473-3099(13)70164-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandberg J.K., Fast N.M., Nixon D.F. Functional heterogeneity of cytokines and cytolytic effector molecules in human CD8+ T lymphocytes. J. Immunol. (Baltim., Md.: 1950) 2001;167(1):181–187. doi: 10.4049/jimmunol.167.1.181. [DOI] [PubMed] [Google Scholar]
- Shen Z., Reznikoff G., Dranoff G., Rock K.L. Cloned dendritic cells can present exogenous antigens on both MHC class I and class II molecules. J. Immunol. (Baltim., Md.: 1950) 1997;158(6):2723–2730. [PubMed] [Google Scholar]
- Stebbings R., Février M., Li B., Lorin C., Koutsoukos M., Mee E., Rose N., Hall J., Page M., Almond N., Voss G., Tangy F. Immunogenicity of a recombinant measles-HIV-1 clade B candidate vaccine. PLoS One. 2012;7(11):e50397. doi: 10.1371/journal.pone.0050397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stebbings R., Li B., Lorin C., Koutsoukos M., Février M., Mee E.T., Page M., Almond N., Tangy F., Voss G. Immunogenicity of a recombinant measles HIV-1 subtype C vaccine. Vaccine. 2013;31(51):6079–6086. doi: 10.1016/j.vaccine.2013.09.072. [DOI] [PubMed] [Google Scholar]
- Volz A., Kupke A., Song F., Jany S., Fux R., Shams-Eldin H., Schmidt J., Becker C., Eickmann M., Becker S., Sutter G. Protective efficacy of recombinant modified vaccinia virus Ankara delivering Middle East respiratory syndrome coronavirus spike glycoprotein. J. Virol. 2015;89(16):8651–8656. doi: 10.1128/JVI.00614-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L., Shi W., Joyce M.G., Modjarrad K., Zhang Y., Leung K., Lees C.R., Zhou T., Yassine H.M., Kanekiyo M., Yang Z.-y., Chen X., Becker M.M., Freeman M., Vogel L., Johnson J.C., Olinger G., Todd J.P., Bagci U., Solomon J., Mollura D.J., Hensley L., Jahrling P., Denison M.R., Rao S.S., Subbarao K., Kwong P.D., Mascola J.R., Kong W.-P., Graham B.S. Evaluation of candidate vaccine approaches for MERS-CoV. Nat. Commun. 2015;6:7712. doi: 10.1038/ncomms8712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams M.A., Tyznik A.J., Bevan M.J. Interleukin-2 signals during priming are required for secondary expansion of CD8+ memory T cells. Nature. 2006;441(7095):890–893. doi: 10.1038/nature04790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaki A.M., van Boheemen S., Bestebroer T.M., Osterhaus A.D.M.E., Fouchier R.A.M. Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. N. Engl. J. Med. 2012;367(19):1814–1820. doi: 10.1056/NEJMoa1211721. [DOI] [PubMed] [Google Scholar]
- Zhao J., Alshukairi A.N., Baharoon S.A., Ahmed W.A., Bokhari A.A., Nehdi A.M., Layqah L.A., Alghamdi M.G., Al Gethamy M.M., Dada A.M., Khalid I., Boujelal M., Al Johani S.M., Vogel L., Subbarao K., Mangalam A., Wu C., Eyck P. ten, Perlman S., Zhao J. Recovery from the Middle East respiratory syndrome is associated with antibody and T-cell responses. Sci. Immunol. 2017;2(14) doi: 10.1126/sciimmunol.aan5393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J., Li K., Wohlford-Lenane C., Agnihothram S.S., Fett C., Zhao J., Gale M.J., Baric R.S., Enjuanes L., Gallagher T., McCray P.B., Perlman S. Rapid generation of a mouse model for Middle East respiratory syndrome. Proc. Natl. Acad. Sci. USA. 2014;111(13):4970–4975. doi: 10.1073/pnas.1323279111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J., Perera R.A.P.M., Kayali G., Meyerholz D., Perlman S., Peiris M. Passive immunotherapy with dromedary immune serum in an experimental animal model for Middle East respiratory syndrome coronavirus infection. J. Virol. 2015;89(11):6117–6120. doi: 10.1128/JVI.00446-15. [DOI] [PMC free article] [PubMed] [Google Scholar]