Abstract
Background
Babesia spp. are apicomplexan parasites which infect a wide range of mammalian hosts. Historically, most Babesia species were described based on the assumed host specificity and morphological features of the intraerythrocytic stages. New DNA-based approaches challenge the traditional species concept and host specificity in Babesia. Using such tools, the presence of Babesia DNA was reported in non-specific mammalian hosts, including B. canis in feces and tissues of insectivorous bats, opening questions on alternative transmission routes. The aim of the present study was to evaluate if B. canis DNA can be detected in tissues of laboratory rodents following oral inoculation with infected ticks.
Methods
Seventy-five questing adult Dermacentor reticulatus ticks were longitudinally cut in two halves and pooled. Each pool consisted of halves of 5 ticks, resulting in two analogous sets. One pool set (n = 15) served for DNA extraction, while the other set (n = 15) was used for oral inoculation of experimental animals (Mus musculus, line CD-1 and Meriones unguiculatus). Blood was collected three times during the experiment (before the inoculation, at 14 days post-inoculation and at 30 days post-inoculation). All animals were euthanized 30 days post-inoculation. At necropsy, half of the heart, lung, liver, spleen and kidneys were collected from each animal. The presence of Babesia DNA targeting the 18S rRNA gene was evaluated from blood and tissues samples. For histopathology, the other halves of the tissues were used. Stained blood smears were used for the light microscopy detection of Babesia.
Results
From the 15 pools of D. reticulatus used for the oral inoculation, six were PCR-positive for B. canis. DNA of B. canis was detected in blood and tissues of 33.3% of the animals (4 out of 12) inoculated with a B. canis-positive pool. No Babesia DNA was detected in the other 18 animals which received B. canis-negative tick pools. No Babesia was detected during the histological examination and all blood smears were microscopically negative.
Conclusions
Our findings demonstrate that B. canis DNA can be detected in tissues of mammalian hosts following ingestion of infected ticks and opens the question of alternative transmission routes for piroplasms.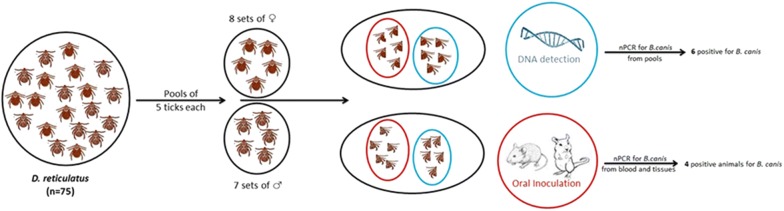
Keywords: Babesia canis, Dermacentor reticulatus, Mouse, Gerbil, Oral inoculation
Background
Babesia spp. are apicomplexan parasites which infect a wide range of mammalian hosts and often cause important clinical disease in domestic animals, and occasionally in wildlife and humans [1–4]. All Babesia spp. for which the life-cycle is known are transmitted by ticks during blood-feeding [5, 6].
Historically, Babesia species were described based on the assumed host specificity and morphology of the intraerythrocytic stages [7, 8]. Nevertheless, new molecular approaches do challenge the traditional species concept and host specificity of Babesia spp. [9]. Using such tools, the presence of Babesia DNA was reported in mammalian hosts that were not previously known to be susceptible to the infection [8]. Piroplasms ‘specific’ to horses (B. caballi and Theileria equi) were found in the blood of dogs from Croatia and Romania [10, 11]. Babesia canis and Babesia vulpes (reported as T. annae and Babesia microti-like piroplasm), considered to be canid-specific piroplasms (reported in wolves, red foxes, golden jackals) were identified in cats from Portugal [12, 13].
Dermacentor reticulatus, the only known vector of B. canis [14–16] is a relatively generalist tick. Although the immature stages of D. reticulatus feed principally on rodents, B. canis has never been reported in this group of hosts. Recent studies reported B. canis DNA in the feces, heart tissues, and engorged ticks (Ixodes simplex, I. vespertilionis) of European bats [17–19]. These findings might suggest alternative routes of transmission, such as oral ingestion of infected D. reticulatus ticks [17]. Such routes are well known for other tick-borne haemoprotozoans, such as Hepatozoon canis or H. americanum which are transmitted to dogs following ingestion of infected Rhipicephalus [20], Haemaphysalis [21] and Amblyomma [22–24] ticks. Apicomplexans of the genus Hemolivia are also known to be transmitted via tick ingestion to their vertebrate hosts [25–28]. These routes of transmission seem to represent evolutionary adaptations related to the feeding habits of the vertebrate hosts [29]. Carnivores are likely ingesting ticks when they feed on their prey [30, 31], while tortoises ingest ticks when feeding on vegetation [25]. Hence, such a route could be also the case for the repeated findings of B. canis DNA in bats. In this context, the aim of our study was to evaluate if oral ingestion of ticks infected with B. canis results into presence of detectable DNA in tissues of otherwise unexpected hosts.
Methods
Ticks
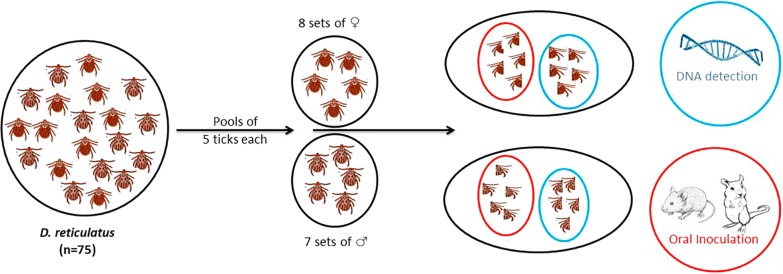
In May 2018, a total of 109 ticks were collected from Lazuri (47° 53′ 15″ N, 22° 53′ 30″ E), Satu-Mare County, Romania, by flagging. The ticks were kept in an aerated large plastic tube, in which a small piece of wet cotton was placed to maintain humidity until further processing. Morphological identification was performed individually for each tick using morphological keys [32]. Only the ticks identified as adult Dermacentor reticulatus (n = 75; 40 females and 35 males) were further used for the experimental trials. The 75 D. reticulatus ticks were divided in 15 pools, each containing 5 ticks of the same sex (8 pools of females and 7 pools of males). Each tick was longitudinally cut in half, while still alive. One pool set (15 pools) was used for DNA extraction, while the other set of pools (15 pools) was used for oral inoculation of experimental animals (Fig. 1).
Fig. 1.
Schematic representation of experimental protocol
Experimental animals and study design
The total number of animals used in this study was 32 (16 adult mice, Mus musculus line CD-1 and 16 adult Meriones unguiculatus). They were housed individually in commercial plastic cages. The animals were maintained in standard conditions. Each tick pool was triturated together with a drop of physiological saline. From the obtained suspension, half the volume was inoculated orally to a mouse (n = 15) and the other half to a gerbil (n = 15), using a plastic single-use pipette. Additionally, one mouse and one gerbil were used as uninfected negative controls and inoculated only with physiological saline.
From both mice and gerbils blood was collected at three time points (30 μl was collected from each animal at each collection time): (i) before the inoculation of tick triturate in order to exclude the presence of piroplasms (molecular assay described below); (ii) at 14 days post-inoculation (pi); and (iii) at 30 days pi. At the first two collections, the blood was collected from both, mice and gerbils, by lateral tail vein puncture after light sedation with isoflurane. At the third collection blood was collected from the retro-orbital sinus, also under light isoflurane sedation. Each blood sample was mixed with PBS buffer (170 μl) and kept at 4 °C until DNA extraction. A small drop of blood from each sample at each sampling time was used for smears.
After the oral inoculation, each animal was clinically evaluated daily for a period of 30 days. At 30 days pi, the animals were euthanized by prolonged narcosis with isoflurane. The necropsy was performed, and from each animal half of the heart and spleen, a lobe of lung and liver, and one kidney were collected for DNA isolation; the other parts of these organs were fixed in 10% formalin for histopathological examination.
Molecular assays
Genomic DNA from ticks and tissues was isolated using a commercial kit (Isolate II Genomic DNA Kit; Bioline, London, UK) according to the manufacturer’s instructions. Nested PCR amplifications targeting the 18S rDNA gene (561 bp) were performed using two sets of primer pairs [33, 34]. The amplification profile used was described previously [35]. DNA isolated from the blood of a dog from Romania which was naturally infected with B. canis was used as a positive control. The sample was confirmed to be positive for Babesia spp. using the same protocols [33, 34] followed by sequencing. A DNA-free water was used as a negative control. PCR products were visualized by electrophoresis in a 1.5% agarose gel stained with RedSafe™ 20,000× nucleic acid staining solution (Chembio, St Albans, UK). Their molecular weight was assessed by comparison with a molecular marker (Hyperladder IV; Bioline). PCR-positive amplicons were purified using Isolate II PCR and Gel Kit (Bioline) and sequenced (Macrogen Europe, Amsterdam, Netherlands). The sequences were compared to those available in GenBank using the Basic Local Alignment Search Tool (BLAST) analysis (BLASTn algorithm).
Haematological and histological examination
Two smears made from blood collected at 14 and 30 days pi from each experimental animal were Giemsa-stained and examined. At least 100 fields were examined under immersion oil objective (100× magnification) before the sample was considered free of piroplasms. All B. canis PCR-positive tissues were used for histopathological examination. The samples were routinely processed, embedded in paraffin wax, cut into 3–4 µm sections, and stained with haematoxylin and eosin (H&E). For each positive sample, two slides were examined using an Olympus BX51 microscope. The photomicrographs were taken using an Olympus SP 350 digital camera and Olympus stream image-analysis software (Olympus Corporation, Tokyo, Japan).
Results
The health condition of the animals did not change for the entire period of the study.
Molecular analyses
From the total number of 15 pools of D. reticulatus used for the oral inoculation of mice and gerbils, six were found positive for B. canis DNA (Table 1). In experimental animals, B. canis DNA was identified in blood and tissues of four animals (Table 1). Babesia canis DNA was not detected in tissues of animals which received non-infected tick pool suspension. Control animals were also PCR-negative. All the smears collected from the experimental animals were microscopically negative for the presence of Babesia spp.
Table 1.
Presence of Babesia sp. DNA in tick pools, blood and tissues
| Host | Sample | Pool | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| P1 | P2 | P3 | P4 | P5 | P6 | P7 | P8 | P9 | P10 | P11 | P12 | P13 | P14 | P15 | ||
| PCR tick | + | − | + | − | + | − | + | − | − | + | − | − | + | − | − | |
| Mouse | B1 | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − |
| B2 | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | |
| H | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | |
| L | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | |
| S | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | |
| K | − | − | − | − | − | − | − | − | − | + | − | − | − | − | − | |
| Gerbil | B1 | − | − | − | − | + | − | − | − | − | + | − | − | − | − | − |
| B2 | − | − | − | − | + | − | − | − | − | − | − | − | + | − | − | |
| H | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | |
| L | − | − | − | − | − | − | − | − | − | + | − | − | − | − | − | |
| S | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | |
| K | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | |
Abbreviations: B1, blood collected at 14 days pi; B2, blood collected at 30 days pi; H, heart; L, liver; S, spleen; K, kidney
Babesia canis DNA was present in the blood collected at 14 days pi in two gerbils (P5 and P10) but at the end of the experiment (30 days pi) only the blood of one of these gerbils (P5) remained positive. In one gerbil (P13), the blood tested positive only at 30 days pi. At the tissue level, only the liver of one gerbil (P10) and the kidney of one mouse (P10) were PCR-positive for B. canis. All the smears collected from the experimental animals were microscopically negative for the presence of Babesia spp.
The sequences analysis revealed the presence of one genetic variant in all the 6 infected tick pools (GenBank: MK836022). The BLAST analysis of the positive sequences showed a similarity of 100% with several B. canis isolates from dog blood (e.g. GenBank: MK571831.1 and MK571830.1), ticks (e.g. GenBank: MK070118.1) and a golden jackal (e.g. GenBank: KX712122.1). The sequences of B. canis found in the blood and tissues of infected animals were all identical with the sequences found in the respective positive tick pool, but not identical with the positive control used.
Histopathological examination
The hepatic parenchyma of the gerbil P5 showed diffuse congestion and randomly distributed foci of coagulative necrosis associated with small numbers of neutrophils and macrophages (Fig. 2). The portal areas were multifocally infiltrated with mononuclear cells dominated by lymphocytes, macrophages and few neutrophils. Both portal tracts and sinusoids showed individual and small groups of macrophages containing a finely granular yellow-brown pigment (hemosiderin) (Fig. 3).
Fig. 2.
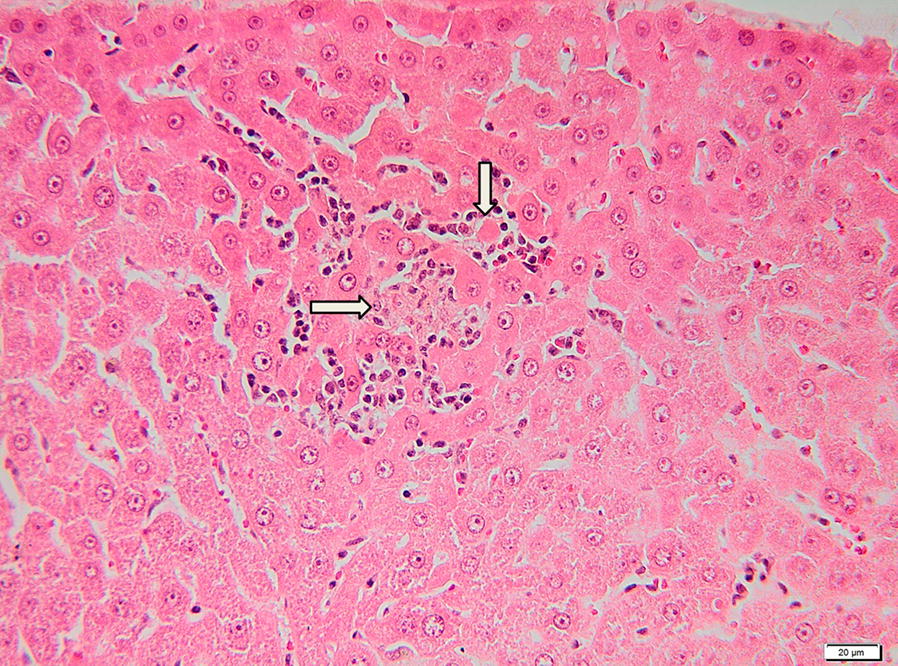
Histological section of the liver of a gerbil positive for B. canis DNA. The microphotograph is showing focal hepatic necrosis with neutrophils and macrophage infiltration (arrows). H&E staining. Scale-bar: 20 µm
Fig. 3.
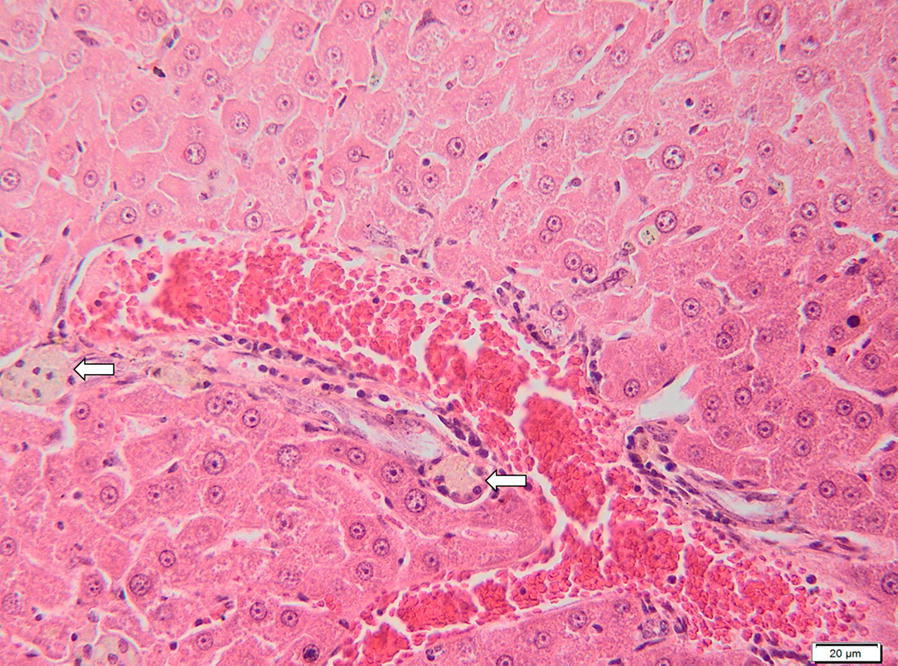
Liver of a gerbil positive for B. canis DNA. The image represents the portal tracts and sinusoids which presents individual and small groups of macrophages containing a fine granular yellow-brown pigment (hemosiderin) (arrows). H&E staining. Scale-bar: 20 µm
The renal parenchyma of the mouse P10 presented moderate renal congestion, particularly in the cortex; the interstitium was multifocally infiltrated by small numbers of mature lymphocytes, neutrophils and macrophages (Fig. 4). The renal proximal convoluted tubes were affected by vacuolar (hydropic) degeneration and coagulative necrosis: haemoglobin casts were occasionally found within the renal tubules (Fig. 5a, b). Mild glomerular hypercellularity was also observed in the positive cases.
Fig. 4.
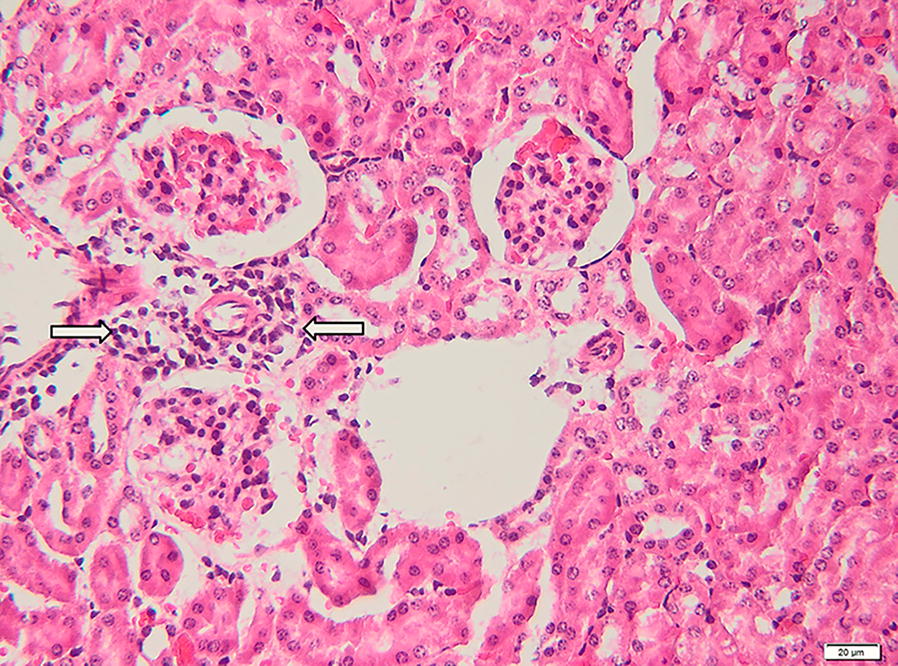
Kidney of a mouse positive for B. canis DNA. Microscopically, the perivascular areas and the tubule interstitium are mildly infiltrated with lymphocytes, neutrophils and macrophages (arrows). H&E staining. Scale-bar: 20 µm
Fig. 5.
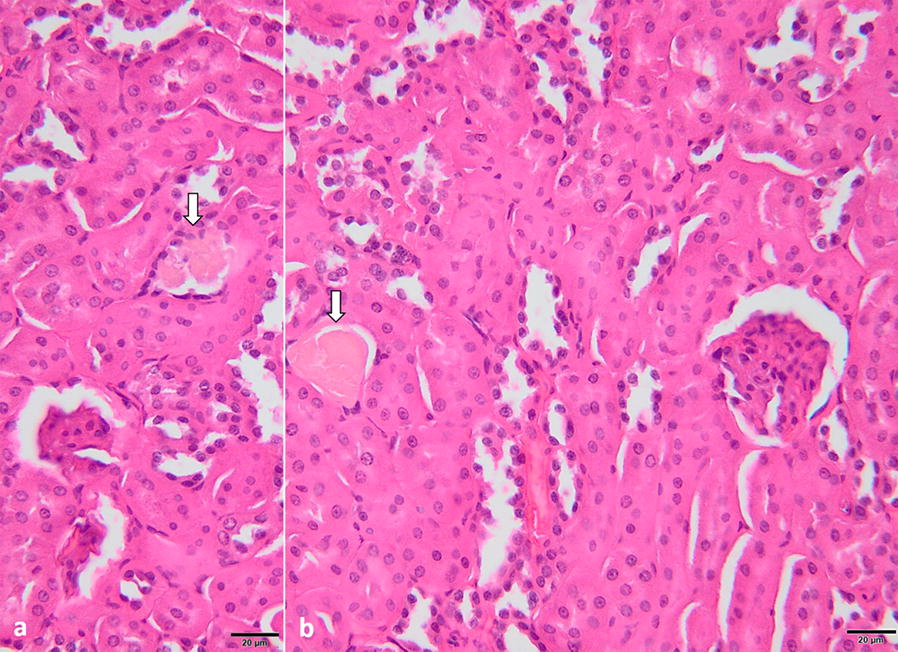
a, b Histological section of the kidney of a mouse positive for B. canis DNA. The microphotograph is showing area of hydropic degeneration and coagulative necrosis; the presence of haemoglobin casts can be also observed (arrows). H&E staining. Scale-bar: 20 µm
Discussion
This study presents experimental evidence for the presence of B. canis DNA in tissues of animals following the oral inoculation of B. canis-positive ticks. Although our study demonstrated that Babesia DNA can be found in tissues of animals after ingestion of infected ticks, this is not a proof of infection. The presence of the DNA does not imply the survival of the babesiae. To demonstrate this and the infection following oral inoculation of infected ticks, more detailed studies are probably needed, including the use of experimentally infected ticks. However, our study offers a possible explanation for the presence of B. canis DNA in the tissues of non-canid hosts, including the multiple reports in bats. As the use of bats for experimental studies is virtually impossible due to strict regulations, ethical issues, logistics and costs, we have designed our experiment using rodent models.
With more than 1200 species worldwide, bats’ diet is very diverse and depends on the geographical distribution of the bat species. All bats in Europe are insectivorous (except the Egyptian fruit bat) and belong to both orders, Yangochiroptera and Yinpterochiroptera [36]. Their diet may span many species, including flies, mosquitos, beetles, moths, crickets, grasshoppers, bees [37–40], but there are no reports of feeding on ticks. Interestingly, there are few recent reports of the presence of DNA of various piroplasmids and other hemoparasites of non-chiropteran hosts in samples collected from bats. Hornok et al. [17] reported the presence of B. canis DNA in feces of Nyctalus noctula, Myotis alcathoe, Myotis daubentonii, and Pipistrellus pygmaeus collected in Hungary. Corduneanu et al. [18] found DNA of B. canis, B. gibsoni and Hepatozoon canis in tissues of N. noctula and P. pipistrellus collected in the Czech Republic, Hungary and Romania. Additionally, Hornok et al. [19] reported the presence of several piroplasmid species in engorged bat-associated ticks (larvae of I. vespertilionis, and larvae, nymphs and females of I. simplex) collected from M. daubentonii, M. dasycneme, Eptesicus serotinus, Miniopterus schreibersii and Rhinolophus hipposideros in Hungary and Romania. All these reports are based on the detection of partial 18S rDNA, which was considered as inconclusive by Uilenberg et al. [8], who recommended experimental transmission studies as more conclusive. As the known vectors of all these haemoparasites are ticks which do not feed on bats, the origin of this DNA remains uncertain, and raised the idea of the possibility of oral transmission.
Ingestion of ticks can occur either during grooming or predation of infested hosts in carnivores [31, 41] or accidentally from the vegetation in herbivores [25]. For some tick-borne apicomplexan parasites, the oral transmission with infected ticks is the main route of infection, as is the case of Hepatozoon spp. in carnivores [30] or Hemolivia spp. in tortoises [25]. However, in these cases, it is unclear how the infective stages disseminate from the intestine to the target tissues in the body (i.e. direct penetration of the gut or invasion of various hosts’ cells followed by blood migration) [24]. However, as yet, no tick DNA has been detected in bat feces [17], but no extensive studies have been completed.
Oral transmission of Babesia microti was demonstrated by Malagon & Tapia [42]. They infected mice by ingestion of blood and by cannibalism. The infection rate of mice which were orally inoculated with blood was 3.7% (5/135) and by cannibalism was 15.1% (12/79). The presence of parasites was detected on blood smears at the beginning of the experiment, at 7 days pi, followed by collection of blood at 7-day intervals until 1 month [42]. In our study, the rate of DNA presence after oral ingestion with triturated ticks was 16.66% (1/6) in mice, and 50% (3/6) in gerbils.
Gerbils are good experimental models for babesiosis caused by B. divergens, as they develop an acute and fatal form of the disease after intraperitoneal inoculation. Most of the clinical signs appear after 3 days pi with the animals dying after 5 days pi [43, 44]. The hepatic tissue of gerbils infected with B. divergens presented dilated sinusoids with macrophages, inflammation of the stroma and hyperplasia of the Kupffer cells and the spleen presented disorganization of the architecture [43–45]. Experimental and spontaneous infections of dogs with B. canis and B. gibsoni showed diffuse periportal and centrilobular hepatitis, with the presence of hemosiderin in Kupffer cells [46–48]. In dogs infected with B. canis histopathological changes included hepatocyte vacuolation, dilatation of hepatic sinusoids and degenerative changes, particularly in the proximal tubes of kidneys [46]. In dogs naturally infected with B. canis vacuolar-hydropic degeneration, especially at the level of proximal convolute tubes and also necrosis of renal tubular epithelial cells was noticed [47]. In dogs infected with B. gibsoni, the kidneys presented an increased number of cells in the glomeruli area [48]. All these findings are consistent with our findings, but no other evidence of infection (i.e. no Babesia stages) was recorded.
Conclusions
Although the presence of DNA of B. canis in the tissues of rodents experimentally inoculated via oral ingestion with infected ticks is not a conclusive proof of the infection or the viability of the piroplasms, it still demonstrates the persistence of parasite DNA and raises further questions regarding alternative routes of transmission or controversial PCR diagnostic results.
Acknowledgements
We are grateful for the help of our collaborators during field work and sample collection: Paul Aurel Bercheşan, Marian Bocan and Svetlin Borisov.
Abbreviation
- pi
post-infection
Authors’ contributions
AC and ADM wrote the manuscript. AC, TDU collected the material for the study, AC identified the tick species performed the laboratory work and analysis of the data. TDU, BS, MT helped with the animal handling, blood collection, animal necropsy and histopathological examination of the samples. ADM and DM participated in manuscript correction. All authors read and approved the final manuscript.
Funding
This study was financially supported by UEFISCDI project PCCDI 57/2018.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Ethics approval and consent to participate
The project was approved by Institutional Research Ethics Committee USAMV CN (Approval no. 50/30.03.2017) and authorized by Regional State Veterinary Authority (authorization no. 50/30.03.2017).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Alexandra Corduneanu, Email: alexandra.corduneanu@usamvcluj.ro.
Teodor Dan Ursache, Email: teodor.ursache@usamvcluj.ro.
Marian Taulescu, Email: marian.taulescu@usamvcluj.ro.
Bogdan Sevastre, Email: bogdan.sevastre@usamvcluj.ro.
David Modrý, Email: modryd@vfu.cz.
Andrei Daniel Mihalca, Email: amihalca@usamvcluj.ro.
References
- 1.Solano-Gallego L, Baneth G. Babesiosis in dogs and cats: expanding parasitological and clinical spectra. Vet Parasitol. 2011;181:48–60. doi: 10.1016/j.vetpar.2011.04.023. [DOI] [PubMed] [Google Scholar]
- 2.Schnittger L, Rodriguez AE, Florin-Christensen M, Morrison DA. Babesia: a world emerging. Infect Genet Evol. 2012;12:1788–1809. doi: 10.1016/j.meegid.2012.07.004. [DOI] [PubMed] [Google Scholar]
- 3.Vannier EG, Diuk-Wasser MA, Mamoun CB, Krause PJ. Babesiosis. Infect Dis Clin N Am. 2015;29:357–370. doi: 10.1016/j.idc.2015.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alvarado-Rybak M, Solano-Gallego L, Millán J. A review of piroplasmid infections in wild carnivores worldwide: importance for domestic animal health and wildlife conservation. Parasit Vectors. 2016;9:538. doi: 10.1186/s13071-016-1808-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hunfeld K, Hildebrandt A, Gray JS. Babesiosis: recent insights into an ancient disease. Int J Parasitol. 2008;38:1219–1237. doi: 10.1016/j.ijpara.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 6.Irwin P. Canine babesiosis: from molecular taxonomy to control. Parasit Vectors. 2009;2(Suppl. 1):S4. doi: 10.1186/1756-3305-2-S1-S4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Homer MJ, Aguilar-Delfin I, Telford SR, 3rd, Krause PJ, Persing DH. Babesiosis. Clin Microbiol Rev. 2000;13:451–469. doi: 10.1128/CMR.13.3.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Uilenberg G, Gray J, Kahl O. Research on Piroplasmorida and other tick-borne agents: are we going the right way? Ticks Tick Borne Dis. 2018;9:860–863. doi: 10.1016/j.ttbdis.2018.03.005. [DOI] [PubMed] [Google Scholar]
- 9.Schreeg ME, Marr HS, Tarigo JL, Cohn LA, Bird DM, Scholl EH, et al. Mitochondrial genome sequences and structures aid in the resolution of Piroplasmida phylogeny. PLoS One. 2016;11:e0165702. doi: 10.1371/journal.pone.0165702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Beck A, Tatjana Z, Beck R, Vojta L, Mrljak V, Marinculic A, et al. Diversity of Babesia and Theileria species in symptomatic and asymptomatic dogs in Croatia. Int J Parasitol. 2009;39:843–848. doi: 10.1016/j.ijpara.2008.12.005. [DOI] [PubMed] [Google Scholar]
- 11.Gallusová M, Qablan MA, D’Amico G, Obomik M, Petrželková KJ, Modrý D. Piroplasms in feral and domestic equines in rural areas of the Danube Delta, Romania, with survey of dogs as a possible reservoir. Vet Parasitol. 2014;206:287–292. doi: 10.1016/j.vetpar.2014.10.018. [DOI] [PubMed] [Google Scholar]
- 12.Criado-Fornelio A, Martinez-Marcos A. Presence of Mycoplasma haemofelis, Mycoplasma haemominutum and piroplasmids in cats from southern Europe: a molecular study. Vet Microbiol. 2003;93:307–317. doi: 10.1016/S0378-1135(03)00044-0. [DOI] [PubMed] [Google Scholar]
- 13.Vilhena H, Martinez-Diaz VL, Cardoso L, Vieira L, Altet L, Francino O, et al. Feline vector-borne pathogens in the north and centre of Portugal. Parasit Vectors. 2013;6:99. doi: 10.1186/1756-3305-6-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Földvári G, Pave Š, Szekeres S, Majoros G, Sprong H. Dermacentor reticulatus: a vector on the rise. Parasit Vectors. 2016;9:314. doi: 10.1186/s13071-016-1599-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Olivieri E, Zanzani SA, Latrofa MS, Lia RP, Dantas-Torres F, Otranto D, et al. The southernmost foci of Dermacentor reticulatus in Italy and associated Babesia canis infection in dogs. Parasit Vectors. 2016;9:213. doi: 10.1186/s13071-016-1502-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Solano-Gallego L, Sainz Á, Roura X, Estrada-Peña A, Miró G. A review of canine babesiosis: the European perspective. Parasit Vectors. 2016;9:336. doi: 10.1186/s13071-016-1596-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hornok S, Estók P, Kováts D, Flaisz B, Takács N, Szőke K, et al. Screening of bat faeces for arthropod-borne apicomplexan protozoa: Babesia canis and Besnoitia besnoiti-like sequences from Chiroptera. Parasit Vectors. 2015;8:441. doi: 10.1186/s13071-015-1052-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Corduneanu A, Sándor AD, Mihalca AD, Hrazdilová K, Modrý D, Hornok S. Molecular evidence of canine pathogens in tissues of European bats. In: Proc. 17th International bat research conference Durban, South Africa. 2016; p. 50–1. https://www.researchgate.net/publication/332833641.
- 19.Hornok S, Szöke K, Kováts D, Estók P, Görföl T, Boldogh SA, et al. DNA of piroplasms of ruminants and dogs in ixodid bat ticks. PLoS One. 2016;11:e0167735. doi: 10.1371/journal.pone.0167735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Baneth G, Samish M, Shkap V. Life cycle of Hepatozoon canis (Apicomplexa: Adeleorina: Hepatozoidae) in the tick Rhipicephalus sanguineus and domestic dog (Canis familiaris) J Parasitol. 2007;93:283–299. doi: 10.1645/GE-494R.1. [DOI] [PubMed] [Google Scholar]
- 21.Murata T, Inoue M, Tateyama S, Taura Y, Nakama S. Vertical transmission of Hepatozoon canis in dogs. J Vet Med Sci. 1993;55:867–868. doi: 10.1292/jvms.55.867. [DOI] [PubMed] [Google Scholar]
- 22.Mathew JS, Ewing SA, Panciera RJ, Woods JP. Experimental transmission of Hepatozoon americanum to dogs by the Gulf Cost tick, Amblyomma maculatum. Vet Parasitol. 1998;80:1–14. doi: 10.1016/S0304-4017(98)00189-7. [DOI] [PubMed] [Google Scholar]
- 23.Forlano M, Scofield A, Elisei C, Fernandes KR, Ewing SA, Massard CL. Diagnosis of Hepatozoon spp. in Amblyomma ovale and its experimental transmission in domestic dogs in Brazil. Vet Parasitol. 2005;134:1–7. doi: 10.1016/j.vetpar.2005.05.066. [DOI] [PubMed] [Google Scholar]
- 24.Rubini AS, dos Santos Paduan K, Cavalcante GG, Ribolla PE, OʼDwyer LH. Molecular identification and characterization of canine Hepatozoon species from Brazil. Parasitol Res. 2005;97:91–93. doi: 10.1007/s00436-005-1383-x. [DOI] [PubMed] [Google Scholar]
- 25.Široký P, Kamler M, Modrý D. Prevalence of Hemolivia mauritanica (Apicomplexa: Adeleina: Haemogregarinidae) in natural populations of tortoises of the genus Testudo in the East Mediterranean region. Folia Parasitol. 2005;52:359–361. doi: 10.14411/fp.2005.049. [DOI] [PubMed] [Google Scholar]
- 26.Paperna I. Hemolivia mauritanica (Haemogregarinidae: Apicomplexa) infection in the tortoise Testudo graeca in the Near East with data on sporgonous development in the tick vector Hyalomna aegypticum. Parasite. 2006;13:267–273. doi: 10.1051/parasite/2006134267. [DOI] [PubMed] [Google Scholar]
- 27.Široký P, Kamler M, Frye FL, Fictum P, Modrý D. Endogenous development of Hemolivia mauritanica (Apicomplexa: Adeleina: Haemogregarinidae) in the marginated tortoise Testudo marginata (Reptilia: Testudinidae): evidence from experimental infection. Folia Parasitol. 2007;54:13–18. doi: 10.14411/fp.2007.002. [DOI] [PubMed] [Google Scholar]
- 28.Kvičerová J, Hypša V, Dvořáková N, Mikulíček P, Jandzik D, Gardner MG, et al. Hemolivia and Hepatozoon: haemogregarines with tangled evolutionary relationships. Protist. 2014;165:688–700. doi: 10.1016/j.protis.2014.06.001. [DOI] [PubMed] [Google Scholar]
- 29.de Sousa KC, Fernandes MP, Herrera HM, Benevenute JL, Santos FM, Rocha FL, et al. Molecular detection of Hepatozoon spp. in domestic dogs and wild mammals in southern Pantanal, Brazil with implications in the transmission route. Vet Parasitol. 2017;237:37–46. doi: 10.1016/j.vetpar.2017.02.023. [DOI] [PubMed] [Google Scholar]
- 30.Giannelli A, Lia RP, Annoscia G, Buonavoglia C, Lorusso E, Dantas-Torres F, et al. Rhipicephalus turanicus, a new vector of Hepatozoon canis. Parasitology. 2017;144:730–737. doi: 10.1017/S003118201600250X. [DOI] [PubMed] [Google Scholar]
- 31.Demoner LC, Magro NM, da Silva MRL, de Paula Antunes JMA, Calabuig CIP, O’Dwyer LH. Hepatozoon spp. infections in wild rodents in an area of endemic canine hepatozoonosis in south-eastern Brazil. Ticks Tick Borne Dis. 2013;78:59–64. doi: 10.1016/j.ttbdis.2016.04.002. [DOI] [PubMed] [Google Scholar]
- 32.Estrada-Peña A, Mihalca AD, Petney TN. Ticks of Europe and North Africa. A guide to species identification. Cham: Springer; 2017. [Google Scholar]
- 33.Zintl A, Finnerty EJ, Murphy TM, De Waal T, Gray JS. Babesias of red deer (Cervus elaphus) in Ireland. Vet Res. 2011;42:7. doi: 10.1186/1297-9716-42-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hodžić A, Alić A, Fuehrer HP, Harl J, Wille-Piazzai W, Duscher GG. A molecular survey of vector-borne pathogens in red foxes (Vulpes vulpes) from Bosnia and Herzegovina. Parasit Vectors. 2015;8:88. doi: 10.1186/s13071-015-0692-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Corduneanu A, Hrazdilová K, Sándor AD, Matei IA, Ionică AM, Barti L, et al. Babesia vesperuginis, a neglected piroplasmid: new hosts and geographical records, and phylogenetic relations. Parasit Vectors. 2017;10:598. doi: 10.1186/s13071-017-2536-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Teeling EC, Springer MS, Madsen O, Bates P, O’Brien SJ, Murphy WJ. A molecular phylogeny for bats illuminates biogeography and the fossil record. Science. 2005;307:580–584. doi: 10.1126/science.1105113. [DOI] [PubMed] [Google Scholar]
- 37.Jones G. Bats. In: MacDonald D, editor. The encyclopedia of mammals (2nd ed.). Oxford: Oxford University Press; 2001. p. 754–75.
- 38.Dietz C, Nill D, von Helversen O. Bats of Britain, Europe and Northwest Africa. London: A&C Black; 2009. [Google Scholar]
- 39.Krüger F, Clare EL, Symondson WO, Keišs O, Pētersons G. Diet of the insectivorous bat Pipistrellus nathusii during autumn migration and summer residence. Mol Ecol. 2014;23:3672–3683. doi: 10.1111/mec.12547. [DOI] [PubMed] [Google Scholar]
- 40.Wray AK, Jusino MA, Banik MT, Palmer JM, Kaarakka H, White JP, et al. Incidence and taxonomic richness of mosquitoes in the diets of little brown and big brown bats. J Mammal. 2018;99:668–674. doi: 10.1093/jmammal/gyy044. [DOI] [Google Scholar]
- 41.Johnson EM, Panciera RJ, Allen KE, Sheets ME, Beal JD, Ewing SA, et al. Alternate patway of infection with Hepatozoon americanum and the epidemiologic importance of predation. J Vet Intern Med. 2009;23:1315–1318. doi: 10.1111/j.1939-1676.2009.0375.x. [DOI] [PubMed] [Google Scholar]
- 42.Malagon F, Tapia JL. Experimental transmission of Babesia microti infection by the oral route. Parasitol Res. 1994;80:645–648. doi: 10.1007/BF00932947. [DOI] [PubMed] [Google Scholar]
- 43.Dkhil MA. Hepatic tissue damage induced in Meriones ungliculatus due to infection with Babesia divergens - infected erythrocytes. Saudi J Biol Sci. 2010;17:129–132. doi: 10.1016/j.sjbs.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dkhil MA, Abdel-Baki AS, Al-Quraishy S, Abdel-Moneim AE. Hepatic oxidative stress in Mongolian gerbils experimentally infected with Babesia divergens. Ticks Tick Borne Dis. 2013;4:346–351. doi: 10.1016/j.ttbdis.2013.01.002. [DOI] [PubMed] [Google Scholar]
- 45.Dkhil MA, Al-Quraishy S, Al-Khalifa MS. The effect of Babesia divergens infection on the spleen of Mongolian gerbils. BioMed Res Int. 2014;2014:483854. doi: 10.1155/2014/483854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Irwin PJ, Hutchinson GW. Clinical and pathological findings of Babesia infection in dogs. Aust Vet J. 1991;68:204–209. doi: 10.1111/j.1751-0813.1991.tb03194.x. [DOI] [PubMed] [Google Scholar]
- 47.Máthé A, Dobos-Kovács M, Vӧrӧs K. Histological and ultrastructural studies of renal lesions in Babesia canis infected dogs treated with imidocarb. Acta Vet Hung. 2007;55:511–523. doi: 10.1556/AVet.55.2007.4.10. [DOI] [PubMed] [Google Scholar]
- 48.Wozniak EJ, Barr BC, Thomford JW, Yamane I, Donough SP, Moore PF, et al. Clinical, anatomic, and immunopathologic characterization of Babesia gibsoni infection in the domestic dog (Canis familiaris) J Parasitol. 1197;83:692–699. doi: 10.2307/3284248. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.