Zoonoses are those diseases that are shared in nature by human beings and vertebrate animals [1], [2]. A more stringent definition identifies those diseases that are transmitted from vertebrate animals to human beings [1]. In the latter definition, there are relatively few diseases that are transmitted directly from horses to people. However, greater public attention has been focused on the broad group of emerging zoonoses, such as severe acute respiratory syndrome (SARS), influenza, bovine spongiform encephalopathy (BSE), and monkey pox. Likely emerging zoonotic agents like these will continue to increase in frequency with the encroachment of human populations on areas not commonly frequented by people and by rapid and widespread international movement of human beings and animals. Recently, the Institute of Medicine acknowledged that many microbes have apparent harmony with animals but are pathogenic to human beings [3]. The increasing recognition of emerging diseases is attributed to many factors, including worldwide trade, mass movement of people for leisure or work, increasing urbanization (ie, daycare facilities, prisons, homeless shelters), environmental changes, resource consumption, demographic changes like aging, and increasing numbers of immunocompromised patients. In addition, most emerging infectious diseases are considered zoonotic, and this report recommended the need for an interdisciplinary approach from a broad range of disciplines, including veterinary medicine, to address this phenomenon.
The focus of this article is on horses and the risk of zoonotic infections, especially in the hospital setting. There is a need to identify potential nosocomial and zoonotic disease events rapidly, with the purpose of preventing employee, owner, and animal illness. Also, in the context of emerging diseases, veterinary hospitals can provide a unique surveillance nidus to detect unusual disease events in animals and their owners. This is not an inclusive discussion of all diseases shared between horses and people but a synopsis of recent diseases of concern and the challenges they present. The following discussion includes (1) new and deadly agents, (2) old diseases that have resurfaced, (3) disease challenges that consume resources and energy, (4) diseases with complex webs of transmission, and (5) potential problems for immunocompromised human beings. A brief discussion of modes of disease transmission and infection control strategies is also highlighted from the human hospital perspective.
New and deadly agents
Horses are not immune to emerging diseases, many of which are zoonotic. Recently identified emerging diseases in horses include equine protozoal myeloencephalitis, clostridial enterocolitis, ehrlichiosis, Japanese encephalitis, vesicular stomatitis virus infection, Venezuelan equine encephalomyelitis (VEE), Hendra virus infections, and West Nile virus encephalitis. Viruses common to people and horses include rabies, influenza, vesicular stomatitis virus, Japanese B encephalitis virus, and a number of alpha viruses. Mosquitoes can carry Eastern equine encephalitis, Western equine encephalitis, VEE, and West Nile virus from birds to horses and people (Table 1 ). The likely bird source and mosquito vector may vary for each virus. Generally, neither horses nor people seem to be a significant source of transmission of these infections but are instead terminal hosts. The exception is VEE, in which horses can develop sufficient viremia to serve as amplifiers of the virus. Rare cases of VEE have been linked to inhalation of the virus in laboratory settings [2]. Direct transmission from horses to people in a veterinary setting is unlikely.
Table 1.
Characteristics of North American arboviral encephalitides in human beings
| Disease | Geographic distribution | Age group affected | Human mortality (%) | Neurologic sequelae (human) | Equine mortality (%) |
|---|---|---|---|---|---|
| Eastern equine encephalitis | West, Midwest | Children | 50–75 | 80% of survivors | 70–90 |
| La Crosse encephalitis | East, Gulf Coast, South | Children | <1 | low | — |
| St. Louis encephalitis | Central, West, South | Adults (>50 y) | 2–20 | 20% of survivors | — |
| Venezuelan equine encephalitis | South | Adults | 1 | Rare | 30–80 |
| Western equine encephalitis | Central, East | Infants and Adults (>50 y) | 5–15 | Moderate in infants, otherwise low | 20–50 |
| West Nile encephalitis | Across North America | Adults (>50 y) | 10 | Rare, acute flaccid paralysis syndrome | 30 |
Abbreviation: y, years.
The reality of emerging diseases and the potential impact on human and equine health was exemplified by the death of horses and people in Australia in 1994. This outbreak of severe respiratory disease affected 18 horses, their trainer, and a stablehand in Queensland, Australia [4]. Additionally, a 35-year-old man developed a brief episode of aseptic meningitis in August of 1994 after caring for two ill horses [5], [6]. He survived this initial infection but developed severe encephalitis resulting in death 13 months later. The 35-year-old patient assisted in necropsies of affected horses without gloves, a mask, or protective eyewear and likely had close contact with ill horses. Since 1994, there have been two other outbreaks recorded in horses. Three human cases have been attributed to these outbreaks. The level of contagiousness of Hendra virus is likely minimal, as evidenced by the identification of only small and infrequent outbreaks detected since 1994 and the lack of seroconversion in a large number of people and horses tested in Australia [7], [8]. These scenarios highlight new challenges that can affect horses and their human caretakers. The identified agent, the Hendra virus, is likely transmitted by direct contact with respiratory secretions of infected animals. Fruit bats (Pteropus sp) are the likely reservoirs.
New diseases are likely to be ongoing challenges in the future. These challenges require the clinician's awareness of new or unusual disease presentations and an awareness of measures to take when unusual cases are encountered. Also, this requires us to teach our veterinary students and technicians about basic infection control measures, including standard and transmission-based precautions. Standard precautions apply to all patients and stipulate that gloves should be worn to touch any of the following: blood, body fluids, secretions (except sweat), nonintact skin, and mucous membranes. Transmission-based precautions apply to disease-specific modes of transmission, such as contact precautions applied to a horse with dermatophytosis or droplet precautions applied to a horse with rabies. These human terms can be applied and modified to the emerging science of infection control in veterinary medicine.
Old threats become new
Some diseases have long been forgotten in developed countries because of improved management and the advent of antimicrobials; however, some of these diseases may still pose significant threats. This was highlighted by the diagnosis of Burkholderia mallei infection (glanders) in a military researcher in March of 2000 [9]. The last reported case of naturally acquired glanders in the United States was in 1945, and the most recent case demonstrated the difficulty of recognizing “nearly forgotten” diseases. The concern for these diseases is the potential they pose as engineered pathogens for terrorism. Some researchers suspect that strains of antimicrobial-resistant B mallei have already been produced. B mallei is currently endemic in Africa, Asia, the Middle East, and Central and South America and is known to cause infection in horses, mules, donkeys, sheep, goats, pigs, and human beings. It is traditionally transmitted by direct contact with infected animals. Other means of transmission include ingestion and inhalation, especially with the development of bioengineered strains, which were developed for warfare purposes. Clinical presentation varies by mode of transmission. Case fatality may be greater than 50% if untreated. Standard precautions are necessary to prevent transmission.
Horses, mules, and donkeys are most susceptible to illness; currently, there is no effective treatment [10]. There are four forms of infection: localized cutaneous infection of the skin or soft tissue; pneumonitis; sepsis; or a chronic form resulting in multiple abscesses of the liver, spleen, skin, or muscle. Disease control involves slaughter of ill and carrier animals. In human beings, the disease is manifested as pustular skin lesions or pulmonary disease after an incubation period that ranges from days to several weeks. The portal of entry is the skin or lungs. Regional adenopathy and systemic symptoms, such as fever and malaise, may be present. Dissemination of infection after 1 to 4 weeks results in metastatic abscesses, including lesions in visceral organs.
The interest in glanders as a biologic weapon stems from the fact that few organisms are required to cause disease; it is easily reproduced; high mortality is associated with inhalation; and there is a general lack of clinical recognition, which subsequently delays diagnosis and treatment. During World War I, horses scheduled for shipment to the Allies were deliberately exposed to B mallei. In a single year, the Soviet Union produced more than 2000 tons of dry glanders [11]. Glanders is an example of a disease that can have significant human health implications if used as a bioterrorism agent. Because this disease may not be easily recognized in people or animals, it is important for equine practitioners to be aware of this disease and to recognize that horses may serve as sentinels for a potential intentional biologic release.
Disease challenges that consume resources and energy
In the past two decades, the epidemiology of human rabies has changed. With the widespread use of rabies vaccine since the 1950s, human rabies cases have virtually disappeared. During the 1980s, most human rabies cases in the United States were attributed to exposure to wild dogs during foreign travel. These cases were infrequent. However, a disturbing trend appeared during the 1990s with an increase in human rabies cases attributed to bat exposures [12]. The reason for this re-emergence is not completely understood, except to highlight the adaptability of many of these emerging or re-emerging agents. Rabies is occasionally identified in horses; however, horses account for less than 1% of all rabid animals identified yearly. Since 1992, the number of reported equine cases ranges from 42 to 82. In the US literature, no documented human cases have been attributed to equine exposure, yet diligence is necessary because of the severity of human disease. Rabies in horses has a wide spectrum of clinical signs. Furthermore, there has been documented evidence of illness even in vaccinated horses [13]. Another interesting challenge, especially from rabid domestic animals, is the potential for large-scale human exposures. From 1990 through 1996, 22 large-scale episodes were reported in the United States [14]. Three of these episodes involved horses. Thirty-nine to 64 persons were potentially exposed to rabies in these three situations. This presents an economic and emotional challenge to identify potentially exposed cases and to ensure that they are receiving appropriate postexposure prophylaxis. Today, the current cost for postexposure prophylaxis for an unvaccinated person is approximately US $2000. Unfortunately, these situations do occur in veterinary hospital settings. The frequency of these situations is unknown, but they typify the challenges that can occur from potential zoonotic threats in a hospital setting. It is important that we minimize the impact of these risks through appropriate infection control procedures, pre-exposure vaccination of all personnel who are in contact with patient animals, rapid notification of appropriate staff, and limiting contact with suspect animals. In the veterinary hospital setting, our goal should be to avoid these mass exposure situations and to develop plans that are implemented before these situations occur. This should limit hospital liability and protect employee and public health.
Diseases with an insidious and complex web of transmission
Salmonella infections and outbreaks in veterinary teaching hospitals are an ongoing challenge. The economic losses and potential human health infections are singularly forcing administrators at veterinary teaching hospitals to review, modify, and improve their infection control procedures. The challenges for clinicians in hospital settings include the proportion of subclinical cases that come into the hospital, the hospital design and layout, the development of antimicrobial-resistant strains, and the ability of some strains of Salmonella to persist in the hospital environment. The limited resources that are available to develop an adequate surveillance programs complicate this issue.
This was highlighted in an outbreak that occurred at the University of Minnesota, Veterinary Teaching Hospital (VTH). In August of 1995, an increase in Salmonella cases was observed among horses at the VTH. In addition, Salmonella spp were isolated from two students who were in contact with infected horses and subsequently developed diarrhea. Serotyping revealed that the isolates were Salmonella Typhimurium [15]. Because of the human illness associated with the equine cases and prior collaborations with the State Health Department, isolates were subtyped by pulsed-field gel electrophoresis (PFGE) and tested for antimicrobial susceptibility. The isolates from people and horses had similar multidrug resistance profiles, and the PFGE patterns were identical or clonally related.
The disturbing element of this outbreak was how long it persisted. The identical PFGE pattern was identified in horses and the environment for several months ( Fig. 1). Often, Salmonella outbreaks are observed with a rapid succession of cases occurring over a short time. This was not the case in 1995 through 1996; sporadic cases were identified over a period of several months to a year. Salmonella persisted on environmental surfaces even after cleaning.
Fig. 1.
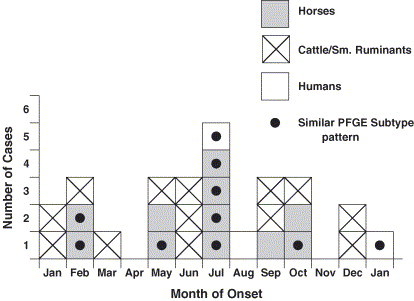
Salmonella cases in the Veterinary Teaching Hospital, St. Paul, Minnesota, 1995.
This pattern of disease transmission has been present in restaurant-associated outbreaks, with sporadic human cases detected over extended periods [16]. Furthermore, persistent environmental contamination was noted in some of these outbreaks. Another common feature among these restaurant-associated outbreaks was the associated employee illness. These types of outbreaks could be related to the low number of infectious organisms persisting in the environment, leading to low or moderate level transmission events over weeks to months. This may be the case in equine hospitals as well. Therefore, diligence is required to isolate and test diarrheic horses; limit personnel access; and train students, staff, and barn help to recognize and prevent continued transmission. Also, employee illness may serve as a point of recognition for potential outbreaks or nosocomial events. Employees with illness should promptly notify their hospital health officers or employers. Health officers should provide a mechanism by which employees can be evaluated by appropriate health care providers. These health care providers should be encouraged to submit appropriate diagnostic samples if a zoonotic illness is suspected. Furthermore, hospital administrators should work closely with occupational health providers to identify potentially infected employees and initiate a thorough surveillance program to identify suspect animal and human cases. Immunocompromised veterinary personnel, including those with malignancy, human immunodeficiency virus, or diabetes, and those receiving corticosteroid therapy or treatment with other immunotherapy agents are at particular risk. Mechanisms should be in place to reduce the risk of these employees being exposed to zoonotic pathogens. This may include temporary or permanent reassignment of duties, restriction of performing certain procedures or working with certain animals, and additional infection control education. These mechanisms should be dealt with prospectively so that employee (patient) confidentiality and occupation health and labor issues are appropriately addressed.
Immunocompromised owner or employee
There is an increasing percentage of the human population that is immunocompromised. This is likely a result of the increased survival time of persons with cancer and other serious diseases, the increasing number of people with drug- or disease-induced immunocompromising conditions (particularly HIV infection), and an aging population. Previously, it has been demonstrated that the risk of zoonotic infections to immunocompromised people is low [17], [18]. However, there is some concern about zoonotic disease acquisition when CD4 lymphocyte counts fall below 100 cells/mm3. The most common zoonotic diseases of concern in immunocompromised people include cryptosporidiosis, salmonellosis, and toxoplasmosis (Table 2 ). Transmission of these diseases to the immunocompromised patient can occur from direct contact with animals, but they are more often transmitted from inadequately cooked foods (ie, Toxoplasma gondii and Salmonella spp) or contaminated water sources (Cryptosporidium parvum). Estimates of these zoonotic infections in HIV patients are variable. Fourteen percent of HIV patients with diarrhea had cryptosporidiosis [19], whereas 20% to 47% of Toxoplasma-seropositive HIV patients developed toxoplasmic encephalitis [20], [21] and 10% of HIV patients developed salmonellosis [22].
Table 2.
Potential animal-associated infections among individuals with HIV infection
| Agent | Frequency in HIV patients | Sources | Likely animal sources | Likelihood of infection from contact with horse |
|---|---|---|---|---|
| Toxoplasma gondii | Common | Undercooked meats, unwashed produce, soil while gardening | Cats | None |
| Cryptosporidium | Moderate | Water, people, direct animal contact | Farm animals | Rare |
| Cryptococcus neoformans | Moderate | Soil, bird droppings | Birds | None |
| Salmonella | Moderate | Foods of animal origin, contaminated vegetables, direct animal contact | Reptiles, farm animals, cats | Moderate |
| Campylobacter | Low | Poultry, other meats of animal origin, raw milk, direct animal contact | Dogs, cats, farm animals | Rare |
| Bartonella henselae | Low | Cats | Cats | None |
| Giardia lamblia | Low | Person to person, water | Wild animals, dogs | None |
| Rhodococcus equi | Rare | Soil | Horses, pigs | Rare |
| Listeria monocytogenes | Rare | Soft cheeses, hot dogs, delicatessen meats, raw milk | Farm animals | Rare |
Data from Angulo FJ, Glaser CA, Juranek DD, Lappin MR, Regnery RL. Caring for pets of immunocompromised persons. J Am Vet Med Assoc 1994;205:1711–8; and Glaser CA, Angulo FJ, Rooney JA. Animal-associated opportunistic infections among persons infected with the human immunodeficiency virus. Clin Infect Dis 1994;18:14–24.
The risk of opportunistic zoonotic infections from horses is likely low. The two most likely agents are Salmonella and Rhodococcus equi. Also, documented animal-to-human and human-to-animal transmission of methicillin-resistant Staphylococcus aureus (MRSA) has occurred [23], [24]. MRSA is a frequent cause of nosocomial infection in the human hospital setting. Recently, MRSA has also been identified among healthy people who have not been hospitalized and likely acquired their infection from community contacts (ie, community-associated MRSA). Whether animals serve as a source of human MRSA infections is yet to be seen.
Until recently, R equi has been considered strictly an equine pathogen. The incidence of R equi infection in people has increased markedly with the emergence of HIV. The first reported human R equi infection was in 1967 in a 29-year-old man with an autoimmune disease who worked at a stockyard [25]. Since then, more than 100 cases have been described in the literature, with most occurring in immunocompromised patients, especially HIV-infected individuals. This facultative, intracellular, gram-positive coccobacillus is likely acquired from inhalation of contaminated soil, from inoculation of a wound or mucous membrane, or via ingestion [25]. The main route of human infection is unclear, but some have postulated that contact with farm animals and manure may account for one third of cases [25], [26]. Recently, virulence-associated antigens and plasmids have been identified. R equi virulence plasmid VapA commonly causes suppurative pneumonia in foals and is widespread on horse breeding farms [26]. However, only about 20% of R equi isolates from human infections express VapA. R equi virulence plasmid VapB has been found in the submaxillary lymph nodes of pigs, and some have postulated that pigs may serve as an important source for infections, especially in Southeast Asia [26].
Clinically, pulmonary involvement, including pneumonia, lung abscessation, and pulmonary nodules, is commonly described in human beings. Bacteremia is common in immunocompromised patients. A review of immunocompetent individuals with R equi infection identified 19 patients, 3 of whom had direct or indirect contact with horses or soil from horse farms [27]. R equi has been identified in other animals, including pigs, cattle, and goats. Instructions are given to immunocompromised patients, including transplant and HIV-infected patients, to avoid or limit contact with domestic animals. As greater proportions of our population are diagnosed with immunocompromising conditions, new challenges may present from organisms that are generally not considered zoonotic, such as R equi.
Infection control in the human hospital setting
There are five main transmission routes for infectious agents. These are contact, droplet, airborne, common vehicle, and vector borne. Contact transmission can be direct, involving body-to-body contact, or indirect, in which a contaminated intermediate inanimate carrier passes an infectious agent from one host to another. Enteric infections, such as salmonellosis or cryptosporidiosis, can be spread by direct contact or via contaminated objects. Droplets are large particles generated by sneezing, coughing, or talking. They are propelled for a short distance from the source individual and cannot remain suspended in the air. Airborne transmission involves small particles (<5 μm) that can remain in the air for prolonged periods and travel over long distances. Common vehicle transmission occurs by contamination of food, water, or equipment, whereas animals like flies, fleas, mosquitoes, and ticks transmit vector-borne infections.
In the human hospital setting, precautions can be taken to prevent transmission of infection by the contact, droplet, and airborne routes [28]. In all cases, standard precautions include hand hygiene; wearing gloves when touching blood, body fluids, secretions, excretions, and contaminated objects; and wearing masks, eye protection, and gowns when there is a risk of soiling or splashing with blood or other body fluids. Contact precautions require isolation of the patient to a private room (or cohorting patients with similar infections) and extend standard precautions to include the use of gowns and gloves on entering the patient's room. Droplet precautions require placement of the patient in a private room and wearing a mask when working within 3 ft of the patient. With airborne precautions, the patient is placed in a room with negative air pressure (6–12 air exchanges per hour), appropriate ventilation, and the use of an N95 mask (or equivalent) by persons entering the room. These precautions may not always be practical or applicable in the veterinary setting, but many are needed to prevent nosocomial and potential human infections. The common prevention measures include the use of protective clothing and gloves to prevent direct transmission of pathogens, personal protective equipment for other agents transmitted by droplet or airborne routes, adequate hand hygiene, hospital-based standard operating procedures, and appropriate use of vaccines.
Summary
Infectious agents are insidious, often changing to adapt to host defenses or treatment advances. Because these challenges will continue, the need to apply standard and transmission-based precautions is important not only in the human hospital setting but in the veterinary clinic setting. In addition, to prevent human infection and potential liability, clinics need to establish program algorithms to prevent disease spread for specific agents or planned procedures to respond to potential nosocomial and zoonotic disease events. These need to be done proactively. Furthermore, more money needs to be dedicated to establish infection control programs and to improve the science of infection control in the veterinary setting.
References
- 1.Swabe C. Williams & Wilkins; Baltimore: 1984. Veterinary medicine and human health. [Google Scholar]
- 2.Acha P., Szyfres B. 3rd edition. Pan American Health Organization; Washington, DC: 2003. Zoonoses and communicable diseases common to man and animals. [Google Scholar]
- 3.The National Academies Press; Washington, DC: 2003. Institute of Medicine of the National Academies. Microbial threats to health. [Google Scholar]
- 4.O'Sullivan J.D., Allworth A.M., Paterson D.L., Snow T.M., Boots R., Gleeson L.J. Fatal encephalitis due to novel paramyxovirus transmitted from horses. Lancet. 1997;349:93–95. doi: 10.1016/s0140-6736(96)06162-4. [DOI] [PubMed] [Google Scholar]
- 5.Barclay A.J., Paton D.J. Hendra (equine morbillivirus) Vet J. 2000;160:169–176. doi: 10.1053/tvjl.2000.0508. [DOI] [PubMed] [Google Scholar]
- 6.Anonymous. Another human case of equine morbillivirus disease in Australia. Emerg Infect Dis. 1996;2:71–72. doi: 10.3201/eid0201.960112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McCormack J.G., Allworth A.M., Selvey L.A., Selleck P.W. Transmissibility from horses to humans of a novel paramyxovirus, equine morbillivirus (EMV) J Infect. 1999;38:22–23. doi: 10.1016/s0163-4453(99)90023-3. [DOI] [PubMed] [Google Scholar]
- 8.Ward M.P., Black P.F., Childs A.J., Baldock F.C., Webster W.R., Rodwell B.J. Negative findings from serological studies of equine morbillivirus in the Queensland horse population. Aust Vet J. 1996;74:241–243. doi: 10.1111/j.1751-0813.1996.tb15412.x. [DOI] [PubMed] [Google Scholar]
- 9.Srinivasan A., Kraus C.N., DeShazer D., Becker P.M., Dick J.D., Spacek L. Glanders in a military research microbiologist. N Engl J Med. 2001;345:256–258. doi: 10.1056/NEJM200107263450404. [DOI] [PubMed] [Google Scholar]
- 10.Foreign animal diseases. United States Animal Health Association; Richmond (VA): 1998. United States Animal Health Association. Glanders; pp. 245–252. [Google Scholar]
- 11.Center for Food Security and Public Health. Glanders. Ames, IA: Iowa State University College of Veterinary Medicine; 2003. Available at: http://www.vetmed.iastate.edu/services/institutes/cfsph/FactSheets/glanders.pdf. Accessed June 7, 2004.
- 12.Messenger S.L., Smith J.S., Rupprecht C.E. Emerging epidemiology of bat-associated cryptic cases of rabies in humans in the United States. Clin Infect Dis. 2002;35:738–747. doi: 10.1086/342387. [DOI] [PubMed] [Google Scholar]
- 13.Green S.L., Smith L.L., Vernau W., Beacock S.M. Rabies in horses: 21 cases (1970–1990) J Am Vet Med Assoc. 1992;200:1133–1137. [PubMed] [Google Scholar]
- 14.Rotz L.D., Hensley J.A., Rupprecht C.E., Childs J.E. Large-scale human exposures to rabid or presumed rabid animals in the United States: 22 cases (1990–1996) J Am Vet Med Assoc. 1998;212:1198–1200. [PubMed] [Google Scholar]
- 15.Bender J.B., Hedberg C.W., Boxrud D.J., Besser J.M., Wicklund J.H., Smith K.E. Use of molecular subtyping in surveillance for Salmonella enterica serotype typhimurium. N Engl J Med. 2001;344:189–195. doi: 10.1056/NEJM200101183440305. [DOI] [PubMed] [Google Scholar]
- 16.Medus C.B.J., Smith K., Leano F.T., Besser J., Hedberg C.W. International Association of Food Protection; Minneapolis (MN): 2001. Foodworkers as a source for salmonellosis. [Google Scholar]
- 17.Angulo F.J., Glaser C.A., Juranek D.D., Lappin M.R., Regnery R.L. Caring for pets of immunocompromised persons. J Am Vet Med Assoc. 1994;205:1711–1718. [PubMed] [Google Scholar]
- 18.Glaser C.A., Angulo F.J., Rooney J.A. Animal-associated opportunistic infections among persons infected with the human immunodeficiency virus. Clin Infect Dis. 1994;18:14–24. doi: 10.1093/clinids/18.1.14. [DOI] [PubMed] [Google Scholar]
- 19.Guerrant R.L. Cryptosporidiosis: an emerging, highly infectious threat. Emerg Infect Dis. 1997;3:51–57. doi: 10.3201/eid0301.970106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Luft B.J., Remington J.S. Toxoplasmic encephalitis in AIDS. Clin Infect Dis. 1992;15:211–222. doi: 10.1093/clinids/15.2.211. [DOI] [PubMed] [Google Scholar]
- 21.Wong S.Y., Remington J.S. Biology of Toxoplasma gondii. AIDS. 1993;7:299–316. doi: 10.1097/00002030-199303000-00001. [DOI] [PubMed] [Google Scholar]
- 22.Hohmann E.L. Nontyphoidal salmonellosis. Clin Infect Dis. 2001;32:263–269. doi: 10.1086/318457. [DOI] [PubMed] [Google Scholar]
- 23.O'Rourke K. Methicillin-resistant Staphylococcus aureus: an emerging problem in horses? J Am Vet Med Assoc. 2003;223:1399–1400. [PubMed] [Google Scholar]
- 24.Seguin J.C., Walker R.D., Caron J.P., Kloos W.E., George C.G., Hollis R.J. Methicillin-resistant Staphylococcus aureus outbreak in a veterinary teaching hospital: potential human-to-animal transmission. J Clin Microbiol. 1999;37:1459–1463. doi: 10.1128/jcm.37.5.1459-1463.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Weinstock D.M., Brown A.E. Rhodococcus equi: an emerging pathogen. Clin Infect Dis. 2002;34:1379–1385. doi: 10.1086/340259. [DOI] [PubMed] [Google Scholar]
- 26.Takai S., Tharavichitkul P., Takarn P., Khantawa B., Tamura M., Tsukamoto A. Molecular epidemiology of Rhodococcus equi of intermediate virulence isolated from patients with and without acquired immune deficiency syndrome in Chiang Mai, Thailand. J Infect Dis. 2003;188:1717–1723. doi: 10.1086/379739. [DOI] [PubMed] [Google Scholar]
- 27.Kedlaya I., Ing M.B., Wong S.S. Rhodococcus equi infections in immunocompetent hosts: case report and review. Clin Infect Dis. 2001;32:E39–E46. doi: 10.1086/318520. [DOI] [PubMed] [Google Scholar]
- 28.Centers for Disease Control and Prevention. Issues in healthcare settings. Part II. Recommendations for isolation precautions in hospitals, 1994. Available at: www.cdc.gov/ncidod/hip/isolat/isopart2.htm. Accessed June 7, 2004.


