Abstract
The immune and neuroendocrine systems are closely involved in the regulation of metabolism at peripheral and central hypothalamic levels. In both physiological (meals) and pathological (infections, traumas and tumors) conditions immune cells are activated responding with the release of cytokines and other immune mediators (afferent signals). In the hypothalamus (central integration), cytokines influence metabolism by acting on nucleus involved in feeding and homeostasis regulation leading to the acute phase response (efferent signals) aimed to maintain the body integrity.
Peripheral administration of cytokines, inoculation of tumor and induction of infection alter, by means of cytokine action, the normal pattern of food intake affecting meal size and meal number suggesting that cytokines acted differentially on specific hypothalamic neurons. The effect of cytokines-related cancer anorexia is also exerted peripherally. Increase plasma concentrations of insulin and free tryptophan and decrease gastric emptying and d-xylose absorption. In addition, in obesity an increase in interleukin (IL)-1 and IL-6 occurs in mesenteric fat tissue, which together with an increase in corticosterone, is associated with hyperglycemia, dyslipidemias and insulin resistance of obesity-related metabolic syndrome. These changes in circulating nutrients and hormones are sensed by hypothalamic neurons that influence food intake and metabolism.
In anorectic tumor-bearing rats, we detected upregulation of IL-1β and IL-1 receptor mRNA levels in the hypothalamus, a negative correlation between IL-1 concentration in cerebro-spinal fluid and food intake and high levels of hypothalamic serotonin, and these differences disappeared after tumor removal. Moreover, there is an interaction between serotonin and IL-1 in the development of cancer anorexia as well as an increase in hypothalamic dopamine and serotonin production. Immunohistochemical studies have shown a decrease in neuropeptide Y (NPY) and dopamine (DA) and an increase in serotonin concentration in tumor-bearing rats, in first- and second-order hypothalamic nuclei, while tumor resection reverted these changes and normalized food intake, suggesting negative regulation of NPY and DA systems by cytokines during anorexia, probably mediated by serotonin that appears to play a pivotal role in the regulation of food intake in cancer.
Among the different forms of therapy, nutritional manipulation of diet in tumor-bearing state has been investigated. Supplementation of tumor bearing rats with ω-3 fatty acid vs. control diet delayed the appearance of tumor, reduced tumor-growth rate and volume, negated onset of anorexia, increased body weight, decreased cytokines production and increased expression of NPY and decreased α-melanocyte-stimulating hormone (α-MSH) in hypothalamic nuclei. These data suggest that ω-3 fatty acid suppressed pro-inflammatory cytokines production and improved food intake by normalizing hypothalamic food intake-related peptides and point to the possibility of a therapeutic use of these fatty acids.
The sum of these data support the concept that immune cell-derived cytokines are closely related with the regulation of metabolism and have both central and peripheral actions, inducing anorexia via hypothalamic anorectic factors, including serotonin and dopamine, and inhibiting NPY leading to a reduction in food intake and body weight, emphasizing the interconnection of the immune and neuroendocrine systems in regulating metabolism during infectious process, cachexia and obesity.
Introduction
The quote an “An army marches on its stomach”, often attributed to Napoleon, links the rallying of bodily defenses with the appropriate marshalling of nutrient responses. In this analogy General Baron De Jomini (Fig. 1 ), Napoleon's Quartermaster, represents the “hypothalamus” which plays an important role in defining the magnitude and temporal profile of hypothalamic nuclei responses by integrating these with the responses from the rest of the brain (central events) and initiating hormonal release by the hypothalamic–pituitary–adrenal axis (HPA). He assessed the magnitude and type of the danger encountered by Napoleon's troops in a battle (afferent signals) and ensured the army had appropriate supplies to react in a timely fashion and to behave by a measured response (efferent signals) to overcome the immediate threat. Like any good army, communication pathways exist between the immune system and the brain allowing bi-directional regulation of the immune- and the brain-initiated behavioral responses, thereby maintaining homeostatic regulation of the body and stability of the army.
Fig. 1.

General Baron De Jomini.
Napoleon and De Jomini were less successful when it came to long-term campaigns under adverse conditions as reflected by the retreat from Moscow. So too the hypothalamus reacts to a foreign stimulus by mounting an acute phase response via modulating the neuroendocrine system and through the HPA axis to altered peripheral metabolism of carbohydrates, proteins and fats to ensure an immediate energy-rich milieu to sustain immune function, while optimally conserving long-term body energy status. However, like Napoleon's long-term campaign, the acute phase response is ill suited for protracted immune challenges, such as morbid obesity or Crohn's disease, which result in chronic and life-threatening conditions.
The term acute phase response refers to the inflammatory response of the host occurring shortly after any tissue injury. The purpose of the acute phase response is to prevent further injury of an organ, to limit the growth of the infective organism, to remove harmful molecules and to activate the repair processes to return the organ to normal function. The acute phase response is characterized by the systemic inflammatory signs of fever, anorexia, somnolence and depression, which are a reflection of the integration of multiple neuro-endocrine, immunological, metabolic and neurological changes in response to the afferent stimulus. The intensity of the acute phase response varies with the acute stimulus and when it becomes overwhelming conditions, such as ileitis or obesity produce profound morphologic and metabolic changes that induce chronic illness and impair survival.
The transmission of the peripheral immune information to the brain is carried out by two different pathways: (i) blood-borne mechanisms: cytokines reach the brain by crossing blood–brain barrier via active transport (Banks et al., 1991), by means of circumventricular organs, such as vascular organ of the lamina terminalis, median eminence and area postrema (which lack a blood–brain barrier so that fenestrated capillaries allow plasma passage; Blatteis, 1992) or by binding to cerebral blood vessel endothelium leading to the release of other second messengers such as prostaglandins (Ericsson et al., 1997). (ii) By means of the vagus nerve (Goehler et al., 1997, Goehler et al., 1999, Goehler et al., 2000; Ek et al., 1998; Mascarucci et al., 1998; Hosoi et al., 2000). Stimulation of vagus nerve by pathogens or immune cells-derived mediators is followed by the activation of neurons in the nucleus tractus solitarius (Konsman et al., 2000), which send projections to hypothalamic and limbic areas involved in regulation of feeding behavior (Ricardo and Koh, 1978). In the vagally mediated immunosignal to the brain, dendritic cells play an important role given its prominent localization within the vagus nerve and associated paraganglia (Goehler et al., 1999). Apart from the direct stimulation of vagal afferents by immune cell-derived mediators, these signals can activate chemoreceptive cells located in the vagal paraganglia, which are penetrated by blood and lymph vessels, allowing vagal paraganglia to sense compounds circulating in blood or lymph (Goehler et al., 2000).
Afferent signals and central events-behavioral and physiological components
A number of examples are sighted below to demonstrate our understanding of the common features of the acute response and function of the afferent signal mechanism(s) that impinge on central event, initiating the acute phase response as manifested by changes in behavior using food intake, and its components (meal size and meal number) as a biological index. To illustrate these points we will use data based on our work and augment it with data from the literature.
Responses to oral food
Ingested nutrients are strong macromolecular antigens that actively challenge the gut's immune defense mechanism. These consist of intestinal lymphoid cells that secrete intraluminal immunoglobulins and local cytokines that (i) further recruit immune cells from the circulation to the gut lymphoid tissue, and (ii) simultaneously mount a systemic acute phase immune response, mediated via afferent blood-borne and neuroendocrine pathways to the liver and ultimately to the hypothalamus. Hansen et al. examined the effect of a single high-protein meal on peripheral immune response by measuring blood mononuclear cells, plasma concentrations of tumor necrosis factor-α (TNF-α), IL-6 and cortisol and growth hormone (Hansen et al., 1997). After the 30 min meal (Fig. 2A ) a significant rise of peripheral neutrophils within 15 min of completing the meal occurred and which remained elevated for 3.5 h. At the same time, a significant decrease in circulating lymphocytes occurred accompanied by a sharp rise in cortisol that started to increase during the meal and peaked shortly thereafter (Fig. 2B). An increase in plasma cytokines levels was not detected. In a follow up “cafeteria-diet” paradigm rat study, an increase in IL-1β mRNA expression occurred in liver and hypothalamus, while an associated decrease in IL-1 receptor accessory proteins (IL-1RAP) mRNA (reflecting IL-1's binding and signaling capacity) occurred in liver and brain stem (Hansen et al., 1998).
Fig. 2.
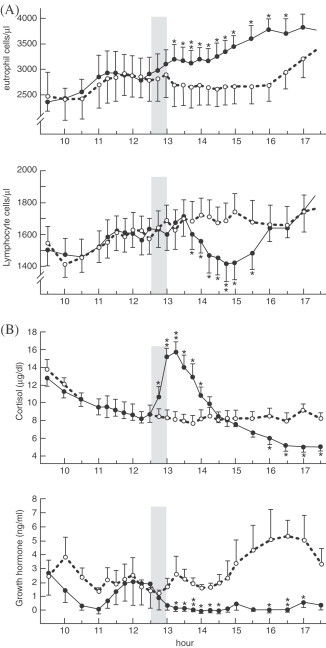
Effect of a single high-protein meal on peripheral immune response. (A) After the 30 min meal a significant rise of neutrophils within 15 min of completing the meal occurred and which remained elevated for 3.5 h. (B) At the same time, a significant decrease in circulating lymphocytes occurred accompanied by a sharp rise in cortisol that started to increase during the meal and peaked shortly thereafter. (From Hansen et al., 1997).
Both studies present interesting results. The intrameal rise in cortisol indicates that afferent signals, such as nutrients or hormones, rapidly reached the hypothalamus, particularly the arcuate nucleus (ARC), probably via blood-borne afferent stimuli through the median eminence and via gastrointestinal vagal afferents. The ARC projects to the paraventricular nucleus (PVN) which releases corticotropin-releasing factor (CRF) into the portal plexus at the median eminence eliciting the synthesis and release of adrenocorticotropin hormone (ACTH) from the anterior pituitary that stimulated the adrenal gland to secrete cortisol, which influenced the migration of immune cell from the circulation into extra-vascular gut tissue to support local immune function (Ottaway and Husband, 1994). The increase in IL-1β mRNA in the hypothalamus and the decrease in IL-1RAP mRNA in brain stem confirms the role of the vagal visceral chemosensory pathway as one of the routes for afferent signals from the gastrointestinal tract to hypothalamic nuclei via the dorsomotor vagal complex of the medulla. Despite Hansen et al.'s inability to detect cytokines in the circulation in response to the physiological event of a single meal, in a pathological model of shock-induced intestinal injury, Deitch et al. demonstrated that the gut liberates cytokines (Deitch et al., 1994). Concentrations of IL-6 and TNF were significantly higher in portal blood than cardiac blood.
In response to the increased intraportal IL-1β, Niijima (1996) demonstrated an increased vagal afferent electrical activity in a dose-dependent response with a simultaneous decreased efferent sympathetic splanchnic activity and a simultaneous increase in vagal thymic nerve activity (see section on Efferent signals). Similar findings were reported by Niijima and Meguid in response to intraportal arginine, an amino acid known to enhance immunity (Niijima and Meguid, 1998) by stimulating increased T-cell release from the thymus and inhibiting the spleen from taking up circulating lymphocytes. The net result is to enhance the circulating numbers and phagocytical active T and B cells (Fig. 3 ).
Fig. 3.
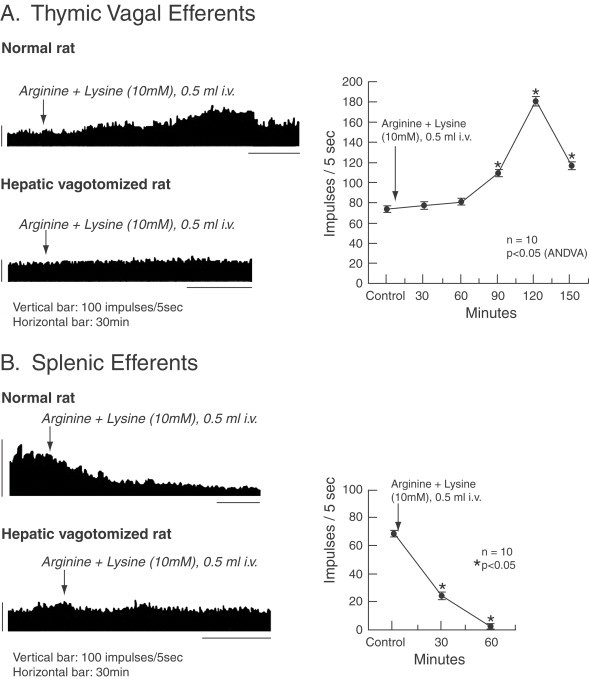
Effect of intravenous administration of a mixture solution of Arg and Lys (10 mM, 0.5 ml) on the efferent activity of the (A) thymic branch of the vagus nerve and (B) the efferent activity of the splenic nerve in normal and hepatic vagotomized rats. *p<0.05. (From Niijima and Meguid, 1998).
Responses to continuous intravenous nutrients
In a parallel series of studies we continuously infused graded caloric amounts of intravenous nutrients for 3 or 9 days (total parenteral nutrition) into the rat, while measuring the response of oral food intake, meal patterns, peripheral hormones, cytokines, hepatic vagal afferent activity and intrahypothalamic monoamines (Opara et al., 1996). Figure 4 shows that graded amounts of total parenteral nutrition lead to a graded compensatory decrease in oral intake. The graded compensatory decrease in oral intake persisted for the duration of the infusion. Plasma glucose and insulin significantly increase and whereas hepatic glycogen concentrations decreased, hepatic triglyceride concentrations significantly increased (Meguid et al., 1991). Simultaneously, plasma TNF-α and peripheral blood monocyte IL-1 also significantly increased (Opara et al., 1995b), while intraportal nutrients decreased hepatic vagal firing rates (Niijima and Meguid, 1994). Interestingly, the response of the hepatic vagal afferent firing rate varied according to the type of amino acid infused intraportally. Thus, of 15 different amino acids infused intraportally (10 mmol in 0.1 ml), eight were excitatory and increased the vagal afferent firing rate, while the others were inhibitory and decreased the firing rate. The change in afferent activity to the hypothalamus may affect reflex regulation of the visceral functions and thereby influence appetite (Niijima and Meguid, 1995). Intralateral hypothalamic area (LHA) neuron dopaminergic activity in response to total parenteral nutrition or its constituent's nutrients was measured by microdialysis (Meguid et al., 1993). LHA-DA levels rose and remained elevated during a continuous 3-h peripheral total parenteral nutrition infusion. Similar increases in LHA-DA occurred during peripheral glucose, fat and amino acid infusion. However, after cessation of these peripherally infused solutions, glucose was the only solution where the percent of DA decrease below baseline for 3 h. A similar relationship was determined between LHA-DA to oral intake in normal rats. The ingestion of a single meal induced a rise in LHA-DA, as measured via microdialysis, that was double in magnitude to that induced by to a meal one-half the size (Meguid et al., 1995). A reciprocal relationship exists between the LHA and ventromedial hypothalamus (VMH) in food intake regulation. Thus, the relationship of DA to the VMH was also explored. DA-VMH concentrations decreased during eating, and the degree and duration of decrease after the meal corresponded to the size of the meal. When the decreased postmeal VMH-DA level had returned to baseline, rats ate once more. We infer from the data that in normal rats eating was associated with decreased DA levels in the VMH, that was followed by a lag time during which no additional eating occurred suggesting that VMH-DA levels contributed to determining the duration of the intermeal interval and hence by influence meal frequency (Meguid et al., 1997).
Fig. 4.
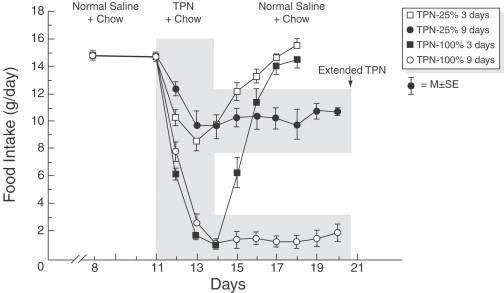
Spontaneous food intake in rats receiving total parenteral nutrition (TPN-25 or TPN-100) for 3 or 9 days. TPN-25 indicates the amount of total parenteral nutrition that provides 25% of a rat's daily caloric intake; TPN-100, the amount of total parenteral nutrition that provides 100% of the rat's daily caloric intake. Values are the mean±SEM. Total parenteral nutrition decreased spontaneous food intake in proportion to the amount infused. (From Campos et al., 1990).
These studies present two striking results. First, the increased circulating nutrients and insulin led to a form of anorexia by compensating for the increased caloric intake. Second, the stress of the continuous hypertonic infusion increased TNF-α and IL-1 contributing to the cytokine mechanism that decreases oral intake in the rat. The ARC, which is the nodal point in hypothalamic regulation of energy balance, promptly senses these nutrients. The leptin- and ghrelin-responsive ARC neurons affect the activity of neurons in PVN, VMH and LHA and other key effector central sites (Elias et al., 1998, Elias et al., 1999; Cowley et al., 1999; Saper et al., 2002), which contain orexigenic and anorexigenic neuropeptides, including orexin-A and -B, cholecystokinine and melanin concentrating hormone (MCH). Many of these central sites are linked to the hypophysiothropic, behavioral and autonomic adaptive responses to changes in energy status (Zigman and Elmquist, 2003). The ARC project neurons to the LHA that plays a key role in ingestive behavior and energy balance, because of the neurochemical phenotypes of the cells express melanocortin hormones and orexin (see section on Central integration). These neurons also synthesize and release DA that “stimulate” or “inhibit” regulatory control over the HPA (Meguid et al., 1995). Thus, although the rise in LHA-DA in our study could have been anticipated, the fall in LHA-DA after the cessation of only the intravenous glucose, supports the concept of the glucose-sensing capacity of the neurons in the ARC, PVN and the LHA (Elmquist and Marcus, 2003). No such response occurred with cessation of the complex solutions that constitute total parenteral nutrition, fat emulsion or amino acid solution, all of which are compound solutions. These data indicate that blood-borne factors are sensed by the hypothalamus but particularly by the ARC and that this sensing mechanism constitutes part of the acute phase response system. At the same time, the increase in TNF-α and IL-1 was likely sensed by the brain via the afferent hepatic vagus because when a sub-diaphragmatic vagotomy was performed, vagotomized rats consumed more food during total parenteral nutrition. They did this by increasing the frequency, size and duration of a meal, suggesting that the influence of blood-borne nutrient and insulin on the ARC was greater than the effect that TNF-α had in decreasing food intake vial abdominal vagus (Yang et al., 1992; Opara et al., 1995b). Based on these results it appears that the cytokine response to the continuous infusion of hypertonic nutrients has a quantitatively greater inhibitory effect on food intake than the continuous nutrient supply.
Afferent signals from a peripheral tumor
Another model that we have used extensively in our laboratory to gain insight into the integrative acute phase response to peripheral signals is the methylcholanthrene sarcoma without inducing metastases in the rat model that induces anorexia as the tumor grows. When 106 methylcholanthrene-sarcoma tumor cells are injected into the flank of a Fischer rat a palpable tumor is detected after 10 days . The tumor's exponential growth results in a 1 cm3 mass between 16 and 20 days (Meguid et al., 1987). During tumor growth, meal number gradually declines with time, but food intake is maintained by a compensatory increase in meal size between 18 and 20 days. When this compensation fails, food intake dramatically decreases and anorexia is behaviorally manifested. Interferon-γ (INF-γ) was detected in the tumor tissue, while we measured a significant increase in IL-1β and in IL-1 receptor 1 mRNA expression in the hypothalamus, cerebellum and hippocampus. Simultaneously, we detected an increase in IL-1RAP 1 and 2 in the liver confirming activity of IL-1 in the periphery (Fig. 5 ; Turrin et al., 2004).
Fig. 5.
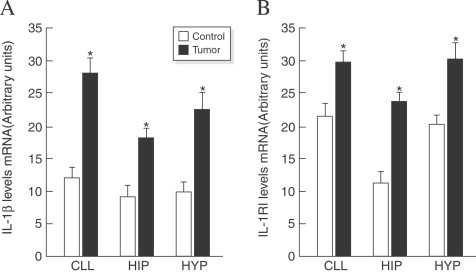
(A) Increased expression of IL-1 β mRNA levels in cerebellum (CLL), hippocampus (Hip) and hypothalamus (Hyp) with peripheral tumor growth and (B) IL-1 RI mRNA levels in controls or tumor-bearing rats. Values (means±SE; n=8 for each group) were standardized to arbitrary units. *p<0.05 vs. controls. (From Turrin et al., 2004).
These studies clearly indicate that the peripherally growing tumor is challenged by immune cells to elaborate both TNF-α and IL-1β. Yang et al. (1994) demonstrated that a 3-day infusion of subclinical concentrations of TNF-α and IL-1 induced anorexia via a synergistic effect, each having no biological effect when infused separately. These act not only on both the parvocellular and the magnocellular neuronal population of the PVN to modulate the acute phase response to induce anorexia thereby decreasing meal size and meal number, which are the components of food intake as measured by our Automated Computerized Rat Eater Meter (Meguid et al., 1990; Meguid et al., 1998); but also on other brain regions involved with locomotion to conserve energy and on memory to respond appropriately to the acute phase response event. Understanding the component of food intake i.e., whether it is meal number or/and meal size, decrease or change, is a useful behavioral index. It reflects where in the hypothalamus and on which neuron population afferent signals, such as cytokines, act. Using an injection of fetal serotonergic or dopaminergic cell suspensions into the LHA, VMN or supraoptic nucleus (SON) we demonstrated that either meal size or meal number or both could be manipulated, suggesting that these neurotransmitters had co-receptors on the primary neuropeptide food-regulating neurons (Meguid et al., 1999).
Responses to a viral and bacterial infection
During an outbreak of a sialodacryoadenitis viral infection in our rat colony we detected that food intake markedly decreased when the rats become clinically symptomatic (Sato et al., 2001c). This reduction in food intake occurred via a decrease in meal size not adequately compensated by an increase in meal number that occurred during both the light and dark phase. This pattern is similar to that which we described in anorexia of indomethacin-induced ulcerative ileitis that is accompanied by an increase in plasma concentrations of TNF-α (Veerabagu et al., 1996), but differed from that observed in anorexia of bacterial lipopolysaccharide-induced infections (Langhans et al., 1991a; Porter et al., 1998) and in cancer anorexia (Meguid et al., 2000). Table 1 compares the different responses of both components of food intake to different stimuli.
Table 1.
Different responses of both components of food intake, meal number (MN) and meal size (MZ), to different stimuli
| Stimuli | MN | MZ | Reference |
|---|---|---|---|
| TPN | ↓ | ↓ | Meguid et al. (1991) |
| MDP | ↓ | ↔ | Langhans et al. (1991a) |
| IL1-α | ↓ | ↓ | Debonis et al. (1995) |
| Ulcerative ileitis | ↔ | ↓ | Veerabagu et al. (1996) |
| LPS | ↓ | ↔ | Porter et al. (1998) |
| Tumor | ↓ | ↓ | Meguid et al. (2000) |
| SDA | ↔ | ↓ | Sato et al. (2001c) |
Note: ↔ No change; ↓ decrease. LPS, lipopolysaccharide; MDP, muramyl dipeptide; TPN, total parenteral nutrition; SDA, sialodacryoadenitis.
The reduced food intake during sialodacryoadenitis infection, which is a corona virus, is probably due to TNF-α because corona viruses induce an increase in this cytokine (Itoh et al., 1991) and using anti-TNF-α agent inhibits TNF-α induced anorexia (Porter et al., 2000), while the participation of IL-1β seems to be limited given that even in IL-1β-deficient mice the anorexia induced by influenza virus is severe (Kozak et al., 1995). The effect of TNF-α on food intake may be partly mediated by changes in hypothalamic DA levels, because in a cecal ligation and puncture septic rat model studied in our laboratory, a progressive decrease in VMH-DA concentrations associated with anorexia was demonstrated (Torelli et al., 2000). Besides the role of TNF-α in sialodacryoadenitis-induced anorexia the participation of other factors, including IL-2, IL-6, IL-8 and/or INT-γ has been suggested (Conn et al., 1995; Plata-Salaman, 1996; Arsenijevic and Richard, 1999).
During bacterial infection IL1-β seems to play and important role in anorexia induction (Von Meyenburg et al., 2003). MohanKumar et al. (1999) found a marked increase in DA, norepinephrine (NE), serotonin (5-HT) and 5-hydroxyindoleacetic acid (5-HIAA) in PVN, as well as an increase in DA concentrations in ARC, two nuclei involved in the control of food intake, after lipopolysaccharide intraperitoneal administration. These changes are completely blocked by treatment with IL-1ra, suggesting the participation of IL-1β in these monoamine metabolism alterations, which is able to induce changes in neurotransmitters concentrations in specific hypothalamic areas (MohanKumar et al., 1998).
Gonadal hormones and sexual dimorphic-based acute phase response
Another critical model studied in our laboratory that has profound influence on hypothalamic integration of immune function and metabolism is the sex of the study model given that gender differences exist in the acute phase response (Coe and Ross, 1983; Hirai and Limaos, 1990; Spitzer and Zhang, 1996a, Spitzer and Zhang, 1996b).
Although daily food intake based on 100 g-body weight is similar in males and females rats, during a 44-day observation period of weight-gain rate in young growing adults was sevenfold greater in males than in females, and the pattern of food intake differed between both sexes. Thus, while in males the constancy of food intake was achieved by an increase on meal size compensated by a decrease in meal number the striking observation in comparably aged female rats is that their food intake is relatively stable, because there are cyclical and reciprocally recurring changes in both meal size and meal number (Fig. 6 ), which are synchronized with the 3–4 days estrous cycle, such that meal number is greatest during estrous phase and meal size is small, while during the met-estrous cycle meal size is largest and meal number is the lowest (Laviano et al., 1996).
Fig. 6.
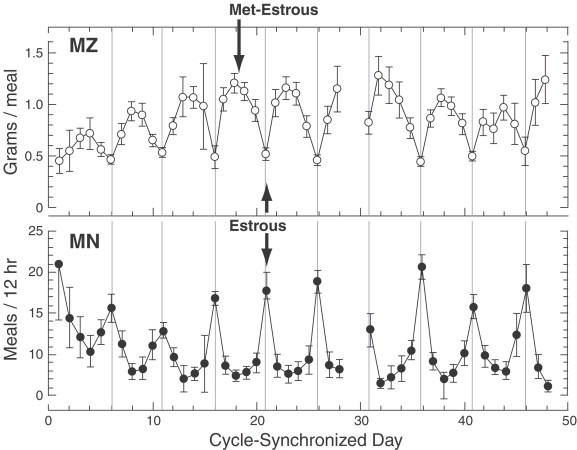
Food intake in female rats is relatively stable by means of cyclical and reciprocally recurring changes in both meal size (MZ) and meal number (MN), which are synchronized with the 3–4 day estrous cycle), such that meal number is greatest during estrous phase and meal size is small, while during the met-estrous cycle meal size is largest and meal number is the lowest. (From Laviano et al., 1996).
Estrogen modulates the neurons in suprachiasmatic nucleus (SCN, Hansen et al., 1978, Hansen et al., 1979), the nucleus tractus solitarius (Eckel and Geary, 2001; Eckel et al., 2002), preoptic area (Dagnault and Richard, 1997), VMH (Beatty et al., 1974) and parvocellular division of PVN (Butera and Beikirch, 1989; Eckel and Geary, 2001; Eckel et al., 2002) to reduce food intake, which is not mediated by CRF (Dagnault and Richard, 1997) or by cholecystokinine (Flanagan-Cato et al., 1998; Eckel et al., 2002). In our studies, the role played by the gonadal hormones in this different behavior of food intake pattern was tested by ovariectomy, which resulted in a loss of cyclic feeding pattern, and an increase in daily food intake caused primarily by an increase in light phase meal size. This pattern of feeding behavior was reversed and normalized after exogenous estrogen restoration, resulting in body weight preservation (Varma et al., 1999). In contrast, orchiectomy reduced food intake by reduction of meal number. This pattern was normalized after exogenous testosterone was given, reversing weight loss (Chai et al., 1999). Our interpretation of the differences in the feeding pattern in females may be explained teleologically by the need to find a mate. Foraging for food during estrous exposes the female to maximum number of potential males, necessitating an increase in meal number but lower meal size, while during met-estrous, when the chances of mating are lowest the meal size are greatest. No such similar evolutionary need is necessary in male rats.
However, in the context of the acute phase response to a noxious stimulus, these data become important because the distribution of meals during the day and night are regulated by the circadian control of energy homeostasis. Thus the sex of the subject, which is receiving the insult, is critical in determining the response. In the female rat, energy regulation occurs primarily by changing meal size, not meal number. Estradiol acts directly on the SCN (Hansen et al., 1978, Hansen et al., 1979) the main circadian oscillator, to influence the daily rhythm of food intake by changes in both its own receptors and their activity, and by changes in diurnal rhythms of other critical neurotransmitters such as dopamine, norepinephrine, and serotonin, and receptors such as alpha 1, beta 1 and 2 in specific hypothalamic nuclei including the VMH and SCN which influence the response to the acute phase response via the HPA and cortisol response. Thus, Watanobe and Yoneda observed a greater release of ACTH in female than in male rats in response to the intravenous administration of lipoprotein polysaccharide (Watanobe and Yoneda, 2003). However there were no changes in plasma IL-1β, IL-6 or TNF-α. There were also no changes in tissue concentrations of CRF and arginine–vasopressin in the medial basal hypothalamus and in the anterior pituitary (AP). There were no changes in the binding characteristic of IL-6 in the medial basal hypothalamus or AP but the number of the IL-1β and TNF-α binding sites, but not in the binding affinities in the medial basal hypothalamus altered significantly after gonadectomy in response to a lipopolysaccharide challenge. These sexual differences were restored after hormonal restoration in response to lipopolysaccharide. The results suggest that the hypothalamic sensitivity to peripheral IL-1 β and TNF-α is an important mechanism underlying the sexual dimorphic ACTH response to lipopolysaccharide in rats.
In a recent study designed to gain insight into the sex differences of basic nonspecific and specific immune responses intracellular type I and II cytokine production by stimulated male and female lymphocytes and monocytes in a whole-blood preparation was measured by flow cytometry. An increased percentage of IL-12, IL-1β and TNF-α was found in men compared to women suggesting that gender differences in the balance between specific and nonspecific immune response existed in men compared to women (Bouman et al., 2004; Posma et al., 2004). Thus, it is apparent that the acute phase response is different in males and in females, explaining in part, the greater survival of the female.
Overwhelming the acute phase response by massive accumulation of subcutaneous fat
The next example that we cite is based on our observations of obesity-induced inflammatory changes in adipose tissue (Hotamisligil et al., 1993; Wellen and Hotamisligil, 2003; Xu et al., 2003; Fantuzzi, 2005). In a series of studies Sprague–Dawley pups were made obese using a high-energy diet (Ramos et al., 2003). We found that the ratio of mesenteric fat to subcutaneous fat for IL-6, TNF-α, corticosterone and their gene profiles as measured by GeneChip Rat UG34A Gene Chip (Affymetrix, Santa Clara, CA) was significantly elevated relative to nonobese mesenteric fat and contributed to the hyperglycemic and hyperdyslipidemia of obesity. In response to weight loss induced by gastric bypass these inflammatory mediators normalized. As summarized schematically in Fig. 7 , as obesity develops the size of the adipocyte increases, which stimulates it to increase the synthesis and release of TNF-α. This stimulates both pre-adipocyte and endothelial cells within the surrounding fat to produce monocyte chemo-attractant protein increasing further intraadipose migration of monocytes. The stimulated mature adipocyte also synthesizes leptin and vascular endothelial growth factor contributing to angiogenesis, while the accumulating free fatty acids induce oxidative stress to the vascular endothelium, in a similar process to arteriosclerosis. The infiltrating macrophages secrete IL-6, IL-1β, TNF-α and corticosterone. The net effect is to increase insulin resistance and induce the biochemical picture of type 2 diabetes mellitus.
Fig. 7.
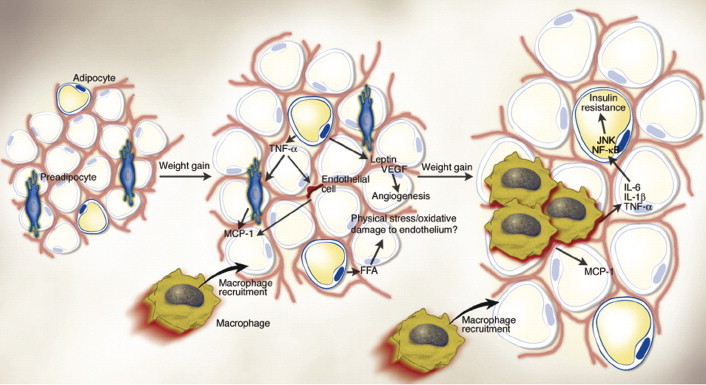
Obese adipose tissue is characterized by inflammation and progressive infiltration by macrophages as obesity develops. Changes in adipocyte and fat pad size led to physical changes in the surrounding area and modifications of the paracrine function of the adipocyte. For example, in obesity, adipocytes begin to secrete low levels of TNF-α, which can stimulate preadipocytes to produce monocyte chemoattractant protein-1 (MCP-1). Similarly, endothelial cells also secrete MCP-1 in response to cytokines. Thus, either preadipocytes or endothelial cells could be responsible for attracting macrophages to adipose tissue. The early timing of MCP-1 expression prior to that of other macrophage markers during the development of obesity also supports the idea that it is produced initially by cells other than macrophages. Increased secretion of leptin (and/or decreased production of adiponectin) by adipocytes may also contribute to macrophage accumulation by stimulating transport of macrophages to adipose tissue and promoting adhesion of macrophages to endothelial cells, respectively. It is conceivable, also, that physical damage to the endothelium, caused either by sheer size changes and crowding or oxidative damage resulting from an increasingly lipolytic environment, could also play a role in macrophage recruitment, similar to that seen in atherosclerosis. Whatever the initial stimulus to recruit macrophages into adipose tissue is, once these cells are present and active, they, along with adipocytes and other cell types, could perpetuate a vicious cycle of macrophage recruitment, production of inflammatory cytokines, and impairment of adipocyte function. (From Wellen et al., 2003).
Brain modulation of systemic inflammation: The nicotinic anti-inflammatory pathway
The last example that we would like to use is a recently completed experiment in our rat tumor model in which we have demonstrated cytokine production. This stimulus has been modified by the external application of nicotine.
As previously described, a large bulk of data exists showing that brain's activity including behavioral responses like feeding is heavily influenced by cytokines or in broader terms by inflammation. These interactions occurring at the cellular and molecular levels between inflammatory mediators and aminergic as well as peptidergic neurons explain the occurrence of fever, anorexia and cachexia, and may provide an important step based on which we can develop pathogenesis-based therapeutic strategies.
However, the hypothalamus not only integrates immune inputs to adjust metabolism and behavior, but it appears to influence the immune response as well. In other words, and probably via a feedback system already demonstrated in many physiologic responses, inflammation influences the brain which in turn influence inflammation. There is a good evidence that the brain, via the vagus nerve, can control systemic inflammation in animal models (Borovikova et al., 2000; Wang et al., 2003). In particular, it appears that acetylcholine, the main neurotransmitter of the vagus nerve, can inhibit the production of pro-inflammatory cytokines by signaling through nicotinic receptors of macrophages (Fig. 8 ; Ulloa, 2005). Consequently, this mechanism has been called “the nicotinic anti-inflammatory pathway”, since acetylcholine exerts its anti-inflammatory effects via the a7-nicotinic–acetylcholine receptor (a7 nAChR; Wang et al., 2004). Interestingly, nicotine is more efficient than acetylcholine at inhibiting cytokine production since nicotine is a more selective cholinergic agonist. This evidence may explain the well-established clinical knowledge that Crohn's disease, which can be described as a chronic inflammatory status of the intestine mediated and sustained by cytokines, is less prevalent and severe among smokers than among nonsmokers.
Fig. 8.
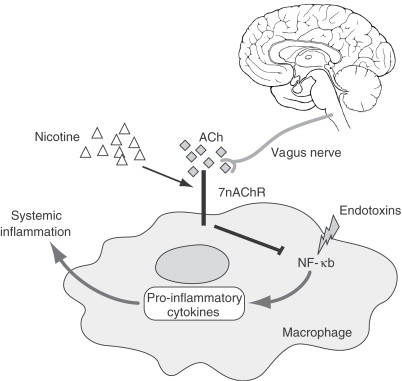
After injury, injection or trauma, endotoxins activate a number of macrophage intracellular pathways, including the NF-κB pathway, which is critical for the production of proinflammatory cytokines. Cytokines then promote and sustain systemic inflammation. To compensate for the increasing inflammatory status, the vagus nerve releases acetylcholine (ACh) which signals through the a7-nicotinic-acetylcholine receptor (a7 nAChR) to inhibit NF-κB induced macrophage activation and cytokine production. Compared to ACh, nicotine is more selective at activating a7 nAChR and efficient at inhibiting proinflammatory cytokines. This pathway has been named as “the nicotinic anti-inflammatory pathway” (Adapted from Ulloa, 2005).
The molecular mechanisms responsible for the anti-inflammatory effects of nicotine are currently being detailed. It appears that nicotine prevents the endotoxin-induced activation of the nuclear factor-κB (NF-κB) pathway, which is critical for the production of pro-inflammatory cytokines (Li and Verma, 2002), via a7 nAChR signaling (Wang et al., 2004).
The potential therapeutic exploitations of the nicotinic anti-inflammatory pathway are many, and are currently being tested. We have been previously shown that nicotine-induced reduction of food intake is mediated via derangement of brain neurochemistry (Miyata et al., 1999; Ramos et al., 2004a) in normal rats. More recently, we hypothesized that nicotine administration may improve food intake in anorectic tumor-bearing rats via its signaling through the nicotinic anti-inflammatory pathway. To test this hypothesis we used the Fischer rat-methylcholanthrene-sarcoma model, since this animal model of cancer-induced anorexia serves very well to the hypothesis since anorexia is mediated by systemic and central hyperproduction of TNF-α and IL-1 (Smith and Kluger, 1993; Opara et al., 1995a; Chance et al., 2003), and pharmacological inhibition of these two cytokines has been demonstrated effective in ameliorating anorexia (Torelli et al., 1999; Laviano et al., 2000). First, we tested whether repeated nicotine administration had any permanent effects on food intake of normal rats, and we observed that nicotine (three injections/day for three consecutive days each week) reduces food intake in a dose-dependent manner, as expected, but this effect progressively fades away with time, and disappears after 3 weeks. In tumor-bearing rats, we decided to test different injection schedules of the minimal effective dose, and preliminary results shows that repeated nicotine administration improves food intake in anorectic tumor-bearing rats and prolongs survival. Analyses are being carried out to test whether these important effects are associated with reduced production of pro-inflammatory cytokines.
Summary of afferent signals
The sums of these divergent and dissimilar studies reveal common immune responses that modify behavior and initiate an immune response by (i) monocytes elaborating cytokines in response to either a physiological or a pathological event; (ii) peripheral immune sensors detect these: the efferent vagal fibers in the gut/liver or the somatic preganglionic fibers in the tissue; and (iii) transduced via an increase in vagal efferent activity is transmitted to the dorsomotor vagal complex of the medulla, which projects onto the hypothalamus including the preoptic area with activation of the PVN (Elmquist and Saper, 1996); (iv) an afferent neural response leads to an increased release of T cells from the thymus and a delayed uptake and destruction of B cells by the spleen and (v) a neuroendocrine event occurs between the hypothalamic nuclei to modulate the metabolic event via the HPA axis.
Central Integration
Figure 9 shows the relationship between the first- and second-order neurons in the ARC, and their susceptibility to hormones and cytokines. Much data contributing to our understanding of central regulatory function has emanated from our studies on continuous intravenous infusion of nutrients and cancer anorexia.
Fig. 9.
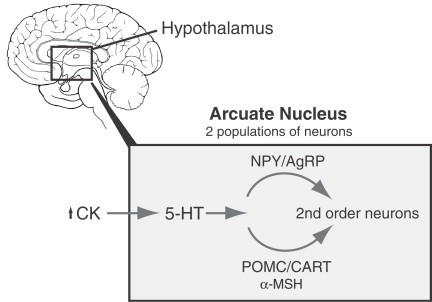
Peripheral signals such as cytokines (CK) reach the hypothalamus, specifically the arcuate nucleus (ARC), where they interact with two neuronal populations, which project to second-order neuronal signalling pathways. Neuropeptide Y/Agouti-related peptide (AgRP) neurons stimulate food intake. Pro-opiomelanocortin (POMC)/cocaine and amphetamine-regulated transcript (CART) neurons inhibit food intake. The effects of cytokines on hypothalamus seem to be mainly mediated by 5-HT. (From Laviano, et al., 2003).
As tumor progresses and grows, interaction between tumor and immune system are established affecting body metabolism from cellular to behavioral level both peripherally and centrally. Tumor growth leads to anorexia (food intake reduction) (Meguid et al., 1987; Kurzer et al., 1988; Makarenko et al., 2003; Meguid et al., 2004; Ramos et al., 2004c). Cytokines acting in endocrine, paracrine and autocrine fashion, play a key role in this relationship establishing a link between tumor and metabolism as well as behavior. During tumor development there is an imbalance between pro-inflammatory, IL-1, IL-6, TNF-α and anti-inflammatory cytokines, such as IL-10, that causes changes in monoaminergic and peptidergic systems, most of them identified in feeding and energy homeostasis control, in both whole brain and hypothalamus (Noguchi et al., 1996; Cravo, 2000; Plata-Salaman, 2000; Makarenko et al., 2002, Makarenko et al., 2003).
Although there is much evidence showing the involvement of peripheral IL-1 in cancer anorexia pathogenesis, it has been demonstrated that IL-1 is also synthesizing at CNS which together with that synthesized peripherally acts directly in the CNS to inducing anorexia (Gelin et al., 1991; Plata-Salaman, 1991; Plata-Salaman and Ffrench-Mullen, 1992; Yang et al., 1994; Plata-Salaman et al., 1998; Turrin et al., 2004). Cytokine receptors have been found in the CNS including hypothalamus (Cunningham and De Souza, 1993) with highest abundance in VMH (Yabuuchi et al., 1994). Furthermore, small pathophysiological dose of IL-1α are required to induce anorexia when these are injected centrally, while pharmacological dose are needed to obtain the same effect when injected peripherally (Plata-Salaman, 1996; Plata-Salaman et al., 1996). We measured the content of IL-1α in cerebrospinal fluid obtained from tumor-bearing rats, achieved by inoculation of methylcholanthrene-induced sarcoma cells, finding reduction of food intake in tumor-bearing rats during anorectic phase compared to pre-anorectic phase as well as to controls. Furthermore, cerebrospinal fluid IL-1α correlated negatively with food intake and positively with tumor weight (Opara et al., 1995a) indicating that central IL-1α plays a role in the pathogenesis of cancer anorexia to conserve energy and to induce mobilization of nutrients for the defense of the host. The data also suggest that other anorectic cytokines (IL-6, IL-8 and TNF-α) may contribute to this phenomenon. These data agree with previous reports showing that IL-1α, IL-1β, IL-8 and TNF-α exert a general anorectic effect at the central level (Chance and Fischer, 1991; Plata-Salaman and Ffrench-Mullen, 1992; Fantino and Wieteska, 1993; Plata-Salaman and Borkoski, 1993; Yang et al., 1999).
Arcuate nucleus
Several experimental findings point the ARC as one of the hypothalamic structures playing a role in the effects of cytokines, particularly IL-1. The medial part of the ARC contains cells that express IL-1R1 (Ericsson et al., 1995) and is activated by systemic IL-1 administration (Herkenham et al., 1998; Reyes and Sawchenko, 2002). Besides, the medial part of the ARC can bind peripheral peptides or proteins because of its localization close to median eminence.
MohanKumar et al. (1999) showed in adult male rats that after intraperitoneal administration of lipopolysaccharide (10 μg/kg body weight) there was an increase (more than twofold) in DA concentrations on ARC compared to control rats. However, lipopolysaccharide treatment did not alter the content of other neurotransmitters, such as 5-HT, NE or its metabolites, in ARC. IL-1β may mediate these changes in DA given that the pretreatment with IL-1ra avoid the increase in DA content (MohanKumar et al., 1999; Fig. 10 ). In addition, systemic lipopolysaccharide administration increases noradrenergic and serotonergic metabolism (Delrue et al., 1994) and activates tryptophan hydroxylase (the first enzyme in the route of synthesis of monoamines) and tyrosine hydroxylase (the rate-limiting enzyme in monoamine synthesis) in several brain areas including ARC, PVN and posterior pituitary gland (MohanKumar et al., 1999; Nolan et al., 2000; De Laurentiis et al., 2002). In a more recent study, Gonzalez et al. (2004) found that intracerebroventricular injection of lipopolysaccharide in male rats caused a transitory and strong immunoreaction to IL-1β in ARC microglial cells parallel with a decline in the number of tyrosine hydroxylase-and tyrosine hydroxylase mRNA-positive cells and in tyrosine hydroxylase activity (the rate-limiting enzyme in monoamine synthesis) in median eminence and, at 12 h, an elevation in prolactin concentrations in serum. These results suggest that hypothalamic catecholaminergic system are involved in the control of autonomic and neuroendocrine responses to peripheral and central inflammation.
Fig. 10.
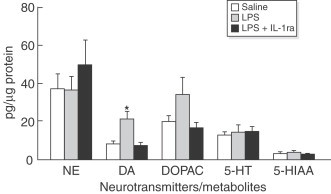
Effects of lipopolysaccharide (LPS) on neurotransmitter concentrations in the arcuate nucleus (ARC). Lipopolysaccharide treatment increased the concentration of DA in the ARC p<0.05. Treatment with IL-1ra completely blocked this effect. Other neurotransmitters were unaffected. (From MohanKumar et al., 1999).
Other hypothalamic systems involved in the regulation of energy homeostasis affected by cytokines are neuropeptide Y (NPY) and α-MSH and pro-opiomelanocortin (POMC). Thus, in tumor-bearing rats at the onset of anorexia NPY immunoreactivity in ARC was lower than in nontumor-bearing rats while α-MSH was greater (Ramos et al., 2005) and these changes were reverted when the rats were fed with ω-3FA as well as the inhibition of food intake. In tumor-bearing rats fed with ω-3FA diet NPY immunoreactivity in ARC was greater than in tumor-bearing chow-fed rats (Ramos et al., 2005). After peripheral administration of IL-1β in rats Reyes and Sawchenko, (2002) detected greater percentage of activated neurons expressing NPY, which co-express Agouti-related peptide (AgRP) (Broberger et al., 1998) and POMC, which co-express Cocaine-amphetamine regulated transcript (CART) (Elias et al., 1998), than after vehicle administration by measuring of Fos induction and these changes were accompanied by a decline in food intake.
In a recent study carried out in C57BL/6J mice, Rossi-George et al. (2005) have described a significant increase in cFos expression in ARC, as well as in PVN, after intraperitoneal injection of 10 μg of staphylococcal enterotoxin A, a superantigen that activates T lymphocytes and induces production of different cytokines such as TNF-α, INT-γ and IL-2 (Bette et al., 1993; Rosendahl et al., 1997) and have neurobiological actions including the activation of HPA axis, sympathetic nervous system and anorexia (Shurin et al., 1997; Kusnecov et al., 1999; Del Rey et al., 2002; Pacheco-Lopez et al., 2004), or 5 μg of lipopolysaccharide (Fig. 11A ) along with a reduction in food intake associated to anxiety/fear-like process and to cFos activation in limbic brain regions. The cFos induction after staphylococcal enterotoxin A seems to be mediated by TNF-α, which plasma levels were increased after staphylococcal enterotoxin A injection, given that in TNF−/− mutant mice cFos induction was not observed (Rossi-George et al., 2005; Fig. 11B).
Fig. 11.
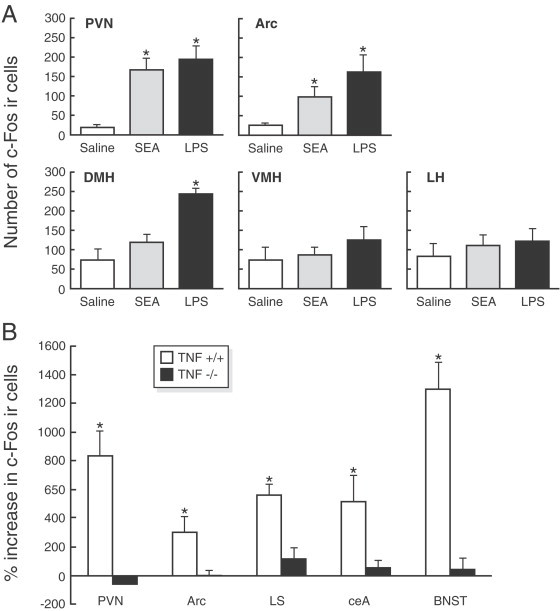
(A) Mean number of c-Fos-immunoreactive cells in hypothalamic nuclei 2 h after intraperitoneal injection with saline, 10 μg of SEA, or 5 μg of lipopolysaccharide (LPS). Each bar represents the mean±SEM. *p<0.05 relative to saline. (B) Percentage increase in the number of c-Fos-immunoreactive cells in the brains of SEA-challenged wild-type (TNF+/+) and TNF-α knockout (TNF-/-) mice. For each individual animal that was given an injection of SEA, the quantitation for each brain region was expressed as a percentage above the group mean of the corresponding saline-injected control of the same strain. The mean number of c-Fos-positive cells in wild-type saline-injected and saline-injected TNF-α knockout mice did not differ. (From Rossi-George et al., 2005).
Paraventricular nucleus
Elmquist and Saper (1996) demonstrated, using cFos as an immunohistochemical marker of neuronal activity, that neurons in both the autonomic and endocrine components of the PVN were activated by lipopolysaccharide. Several of the activated cell groups directly projected to the PVN including the visceral motor complex, median preoptic nucleus, ventromedial preoptic area, nucleus of the stria terminalis, parabrachial nucleus, ventrolateral medulla and nucleus tractus solitarius. These findings indicate that the stimulation of the immune system activates cell groups from medial nervous systems that project on to the PVN and are consistent with the postulate that the PVN plays a key role in integrating diverse physiological cues into the varied manifestations that constitute the cerebral component of the acute phase response (Elmquist and Saper, 1996).
Cytokines induce their anorectic effects partly via the effects on the neurons of the PVN. In support of this, anorexia induced by experimental colitis in rats can be prevented by intracerebroventricular administration of IL-1ra that leads to a 18-fold reduction in PVN 5-HT associated with a significant increase in food intake (El-Haj et al., 2002).
In normal rats, the inhibition of 5-HT synthesis or the blockage of their receptors in PVN causes an increase of NPY concentration suggesting a link between both regulators (Currie and Coscina, 1997). This corresponds to the data that reports an inhibition in NPY system in different types of anorexia (Pich et al., 1992; Broberger et al., 1997, Broberger et al., 1999), particularly those achieved after ventricular administration of ciliary neurotrophic factor, known to cause a decrease in NPY gene expression (Pu et al., 2000), or those associated with cancer (Chance et al., 1994a, Chance et al., 1994b, Chance et al., 1998). Under these experimental conditions the injection of NPY into the PVN in tumor-bearing rats results in a decrease in food intake while in their pair-fed controls a significant increase in food intake occurs, suggesting a refractory feeding response to NPY in the tumor-bearing rats mediated by unknown mechanisms (Chance et al., 1994a; Inui, 1999). Furthermore, the infusion of NPY into the III ventricle inhibits and reverses the anorexia induced by both pathophysiological and pharmacological concentrations of IL1-β (Sonti et al., 1996). As shown in Fig. 12 , our results are in keeping with these data, revealing a significant reduction in the NPY in PVN of tumor-bearing rats (Ramos et al., 2004c) accompanied by an increase in PVN 5-HT. Figure 13 of immunohistochemical sections of the hypothalamus, support the finding by showing increased staining of 5-HT1B receptor proteins, indicating, that there is substantial serotoninergic innervations in PVN (Card and Moore, 1988; Makarenko et al., 2005b). NPY, one of the most potent orexigenic agents (Dryden et al., 1994) can affect many different neuronal systems given its wide distribution and its ubiquitous receptors in the hypothalamus. Of these the serotoninergic system has co-receptors (Fig. 13) since, as we have mentioned above, there is a close association between NPY-ir fibers and hypothalamic neurons expressing 5-HT1B receptor in normal and in tumor-bearing rats (Makarenko et al., 2002). This interaction is bi-directional, since, in normal rats, an increase in NPY release with its subsequent enhanced of intake in food, has been reported after PVN injection of 5-HT receptor antagonist (Dryden et al., 1995). Furthermore, intraperitoneal or intracerebroventricular injection of serotonin agonist decrease the NPY concentration and inhibit stimulatory effect on food intake (Rogers et al., 1997; Currie et al., 2002). In recent studies, we have reported in tumor-bearing rats abnormal levels of hypothalamic 5-HT, NPY and DA at the onset of anorexia (Fig. 14 ). There is an increase in PVN 5-HT concentrations along with a decrease in NPY and DA concentrations. To confirm these findings, the tumor was resected with the expectation that the observed changes normalized. After tumor resection, food intake subsequently normalized and the concentration of both the monoamines and the NPY also return to normal values (Meguid et al., 2004; Ramos et al., 2004c). Using a pair fed vs. control group we verified that the reduction of food intake was induced by the changes observed in NPY and 5-HT in the tumor-bearing rats, while DA reduction seems to be due to the decrease in food intake because the decline in food intake also occurred in the pair-fed group. These data suggest the existence of a dynamic interaction between brain amines and NPY in tumor-bearing rats.
Fig. 12.
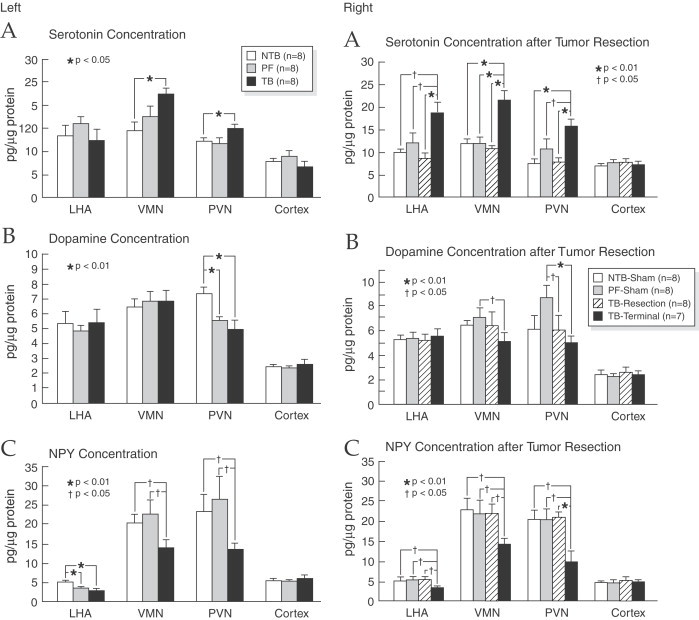
Hypothalamic serotonin, and DA and NPY concentrations in LHA, VMN, and PVN at the onset of anorexia (left) and after tumor resection and terminal state (right). In tumor-bearing rats there was a significant reduction in the NPY in PVN of tumor-bearing rats accompanied by an increase in PVN 5-HT and an increase in bilateral VMH 5-HT content with a concomitant decrease of DA concentrations content while NPY decreases. LHA 5-HT content was increased in rats who were allowed to live until terminal state, while LHA NPY concentration was lower in tumor-bearing rats than in non-tumor-bearing rats at both the onset of anorexia (mean day 19) and at terminal state. (From Ramos et al., 2004c).
Fig. 13.
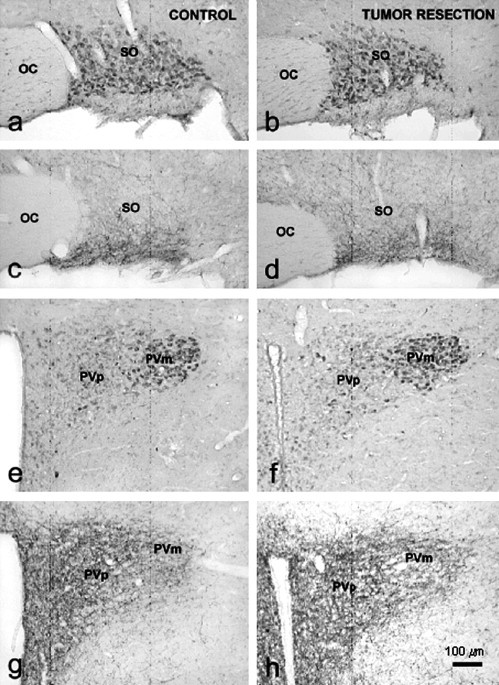
Immunocytochemical visualization of 5-HT1B-receptors (a, b, e and f) and NPY immunoreactive fibers (c, d, g and h) in the hypothalamus of Control and tumor resected (TB-R) rats. SO, supraoptic; PVm, magnocellular part of paraventricular nucleus; PVp, parvocellular part of paraventricular nucleus; OC, optic chiasm. In tumor-bearing rats there was an increased staining of 5-HT1B receptor proteins indicating a substantial serotoninergic innervations in PVN. (From Makarenko et al., 2005b).
Fig. 14.
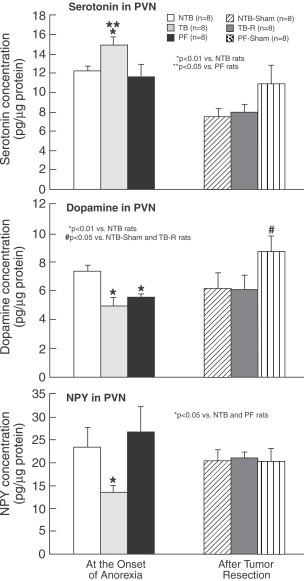
Hypothalamic PVN 5-HT, DA and NPY concentrations. In tumor-bearing rats, there was an increase in PVN 5-HT concentrations along with a decrease in NPY and DA concentrations. These changes normalized after tumor resection. Values are mean±SEM. *p<0.01 vs. NTB rats; **p<0.05 vs. PF (pair fed) rats. *# p<0.05 vs. NTB (Sham) and tumor-bearing rats. (From Meguid et al., 2004).
The changes in serotoninergic system at the onset of anorexia not only affect the levels of this neurotransmitter, but also the expression of 5-HT1B receptors, one of the most important serotonin receptors mediating the anorectic effect of 5-HT receptors (Barnes and Sharp, 1999; Makarenko et al., 2002), as we have reported very recently (Makarenco et al., 2005a) using a peroxidase–antiperoxidase immunocytochemical methods and semiquantitative image analysis of 5-HT1B receptors immunostaining. This study shows the same hypothalamic distribution of this receptor in both tumor-bearing rats and nontumor-bearing rats, but higher immunostaining intensity in most neurons of the magnocellular PVN (but not in parvocellular division). It is generally accepted that most of the magnocellular neurons of the PVN, and also of the SON, produce oxytocin and vasopressin (Sawchenko and Swanson, 1983), which besides their involvement in water balance control, participate in feeding regulation exerting an anorectic action (Arletti et al., 1990; Olson et al., 1991). The activation of 5-HT1B receptors on the magnocellular neurons modulates the release of both hormones and in turn food intake. Furthermore, it has been described that more than 95% of the oxytocin and vasopressin neurons in SON and PVN also express NPY-Y5 receptors (Campbell et al., 2001). In a subsequent study using the same methods as we used in our reported studies we showed that the changes reported at the onset of anorexia in hypothalamic distribution of NPY and 5-HT1B receptors are reverted after tumor resection (Makarenko et al., 2005a). These data show that tumor resection, and therefore the removal of the effect of cytokines, results not only in an enhancement of food intake, reaching the normal levels, but also in a reversible changes of hypothalamic orexigenic and anorectic modulators.
Ventromedial hypothalamus
As indicated by the data of Elmquist and Saper (1996) another primary hypothalamic site where cytokines regulates metabolism is VMH, a known satiety center, which send potent excitatory signals to ARC POMC neurons (Sternson et al., 2005). We injected a pathophysiological quantity of IL-1α into the VMH of rats and caused a significant reduction of food intake (Yang et al., 1999). The mechanism of action may involve the modulation of the serotoninergic and dopaminergic systems, because when we were measuring the release of 5-HT and DA's metabolites we found an increase in the concentrations of 5-HT, 5-HIAA (the main metabolite of 5-HT) and DA just after the injection of IL-1α in VMH. This remained above basal levels during the next 40–60 min, respectively (Yang et al., 1999), clearly linking the anorectic effect of IL-1α and the early development of satiety to neurotransmitters, particularly enhance serotoninergic activity. Other authors have also demonstrated the relationship between cytokines and hypothalamic neurotransmitters (Plata-Salaman, 1997). These interactions also extend to neuropeptides and hormones, because Smagin et al. (1996) reported an enhancement in NE hypothalamic content concurrent to an increase in plasma ACTH and corticosterone concentrations after both intravenous and intraperitoneal injection of IL-1β, indicating that the anorectic effect of this cytokine can be mediated by an increase in hypothalamic NE given that this neurotransmitter regulate HPA axis (Plotsky et al., 1989).
In tumor-bearing rats, we have found an upregulation of D1- and D2- receptor mRNA expression in VMH during anorectic period (Sato et al., 2001b, Fig. 15 ). Our data suggest that tumor-released cytokines regulates food intake through modulation of dopaminergic activity that may take place in VMH as well as in SON (Sato et al., 2001b, Sato et al., 2001a). It has been demonstrated that VMH serotoninergic activity enhanced during cancer anorexia in methylcholanthrene tumor-bearing rats and returned to control levels along with a normalization of food intake once tumor was removed supporting the involvement of serotoninergic system in IL-1α-induced anorexia (Blaha et al., 1996). Furthermore, VMH microinjection of mianserin, 5-HT1c/2 antagonist receptor, or IL-1ra, an endogenous inhibitor of IL-1α in anorectic methylcholanthrene tumor-bearing rats improve food intake by an increase in meal number without effect on meal size, as measured using the Automated Computerized Rat Eater Meter (Meguid et al., 1990), while in normal rats this effect does not occur (Laviano et al., 2000). These findings suggest that under normal conditions VMH 5-HT has only a relative importance in the control of meal number and meal size. However, during tumor growth VMH 5-HT participation in the control of food intake homeostasis becomes relatively important. By integrating these data we suggest that during the progressive tumor growth IL-1α may cause anorexia by a central mechanism that involves VMH 5-HT (Kuriyama et al., 1990).
Fig. 15.
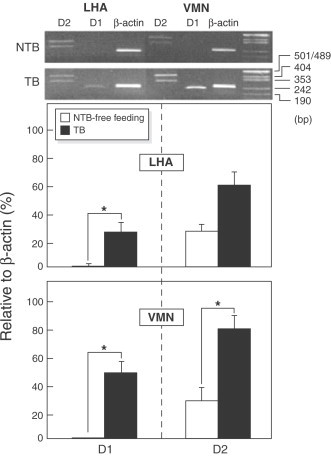
D1- and D2-receptor mRNA expression in the LHA and VMN in anorectic tumor-bearing and non-tumor-bearing free-feeding control rats. Data are expressed as percent change relative to β-actin (β-Act). *p<0.05. In tumor-bearing rats, an upregulation of D1- and D2- receptor mRNA expression in VMH and LHA during anorectic period was found. (From Sato et al., 2001b).
As shown in Fig. 12 Ramos et al. (2004c) found an increase in bilateral VMH 5-HT content with a concomitant decrease of DA concentrations content in tumor-bearing rats at the onset of anorexia, while NPY decreases. These changes, that were accompanied by a reduction in total body fat in tumor-bearing rats as well as a significant decrease of food intake, are normalized 9 days after tumor resection, while these indices continued to be abnormal in tumor-bearing control rats until death.
Lateral hypothalamic area
In our studies, as shown in Fig. 12, 5-HT and DA concentration in LHA in tumor-bearing rats was not significantly different from nontumor-bearing rats at the onset of anorexia but 5-HT content was increased in rats which were allowed to live until the terminal state, while NPY concentration was lower in tumor-bearing rats than in nontumor-bearing rats at both onset of anorexia (mean day 19) and at the terminal state. And these changes were accompanied by a significant reduction in food intake during the study and thus were associated with a reduction in total body fat (Ramos et al., 2004c). As described above, after tumor resection, performed to validate the tumor as the etiology of the acute phase response cytokine changes, the changes reported had reverted 9 days after tumor resection, while these indices continued to be abnormal in their tumor-bearing control cohorts until their death.
In tumor-bearing rats there is an increase of mRNA of both D1- and D2- receptor in LHA (Sato et al., 2001b; Fig. 15). Furthermore, LHA DA release is increased during cancer anorexia (Chance et al., 1991). However, using microdialysis, we found that continuous peripheral IL-1α infusion in normal rats did not cause modifications in LHA DA content (Yang and Meguid, 1995), suggesting that other cytokines were also a contributory factor involved in the biochemical changes observed in LHA in cancer anorexia.
Supraoptic nucleus
The SON, besides its participation in the control of water balance, are also involved in the regulation of food intake by mean of the secretion of vasopressin and oxytocin (anorectic neurohypophyseal hormones) into hypothalamic–pituitary portal circulation (Arletti et al., 1989: Langhans et al., 1991b) after stimulation of D2 receptors by DA released by dopaminergic neurons from the ventrotegmental area in the brain stem. Sato et al. (2001a) have reported that an injection of D2 receptor antagonist (sulpiride, 4 μg/0.5 μl) into bilateral SON of anorectic tumor-bearing rats results in an increase in both meal size and meal number leading to an improvement of food intake (Fig. 16 ) and therefore in body weight as we have also observed when sulpiride was injected into LHA and VMH (Sato et al., 2001b), although in these nuclei the increase in food intake is achieved by an increase in meal number only.
Fig. 16.
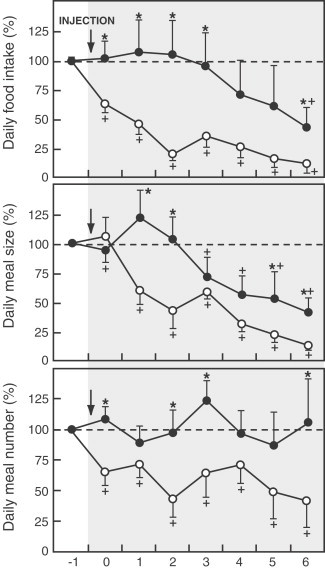
Changes in food intake (top panel), meal size (middle panel) and meal number (lower panel) after an injection of sulpiride/saline into the supraoptic nucleus in tumor-bearing rats. Food intake, meal size and meal number on day −1 before injections was defined as 100%. *p<0.05 vs. control group. +p<0.05 vs. data on day 0 in each group. D2 receptor antagonist injection caused an increase in both meal size and meal number leading to an improvement of food intake. (From Sato et al., 2001a).
Efferent response of acute phase response
During infection, tissue injury, inflammatory or malignant process immune-derived cytokines exert strong neuroendocrine effects resulting in marked changes in host homeostasis. One of the most affected metabolic pathways is carbohydrate metabolism. IL-1, one of the main inflammation mediators, has the capacity to elevate glucocorticoid levels by stimulating hypothalamic CRF-producing neurons (Berkenbosch et al., 1987; Sapolsky et al., 1987; Del Rey et al., 1998). It has been observed that IL-1β intraperitoneal administration increases glucocorticoid and glucagon production, that stimulate the mobilization of glucose stores, and decreases hepatic glycogen content in mice, but these changes are accompanied by hypoglycemia (Del Rey and Besedovsky, 1987; Del Rey et al., 1998) which is even more marked under fasting conditions and is sustained even after glucose load (Fig. 17 ), suggesting that the glucose fast mobilized from the liver is rapidly incorporated into other tissues such as fat or muscle by an increase in glucose transport elicited by IL-1 (Garcia-Welsh et al., 1990; Bird et al., 1990; Shikhman et al., 2001; Fischereder et al., 2003). Further, it has been demonstrated that 2-deoxy-glucose uptake by peripheral tissues (heart, spleen, lung, liver and tumor) was enhanced in mice bearing IL-1β-secreting tumor (Metzger et al., 2004); this increase in glucose uptake may be mediated by a nondependent insulin enhancement of hepatic mRNA expression of the glucose transporter 3 (GLUT-3) by IL-1β. IL-1β, as well as IL-6, inhibit the enhancement of glycogen deposition induced by insulin in primary rat hepatocyte cultures increasing [14C]-glycogen degradation, decreasing [14C]-glucose incorporation into glycogen, stimulating glycogen phosphorylase activity and inhibiting glycogen synthase activity (Kanemaki et al., 1998) which are the rate-limiting enzymes in glycogen metabolism. It was also reported that the inhibition of glycogen synthesis by pro-inflammatory cytokines in both in vitro and in vivo models by Kitano et al. (2002) and Metzger et al. (2004) respectively, as well as the inhibition of gluconeogenesis in vitro by Yerkovich et al. (2004). It has been observed that cytokines inhibit gluconeogenesis induced by glucagon (Stadler et al., 1995; Christ and Nath, 1996). The inhibition of hepatic glucose synthesis was elicited by a downregulation of phosphoenolpyruvate carboxykinase and glucose-6-phosphatase activities induced by cytokines (Metzger et al., 2004; Yerkovich et al., 2004) as well as by the action of cytokines on other enzymes of gluconeogenesis or glycolysis (Ceppi et al., 1992; Metzger et al., 1997; Maitra et al., 2000).
Fig. 17.
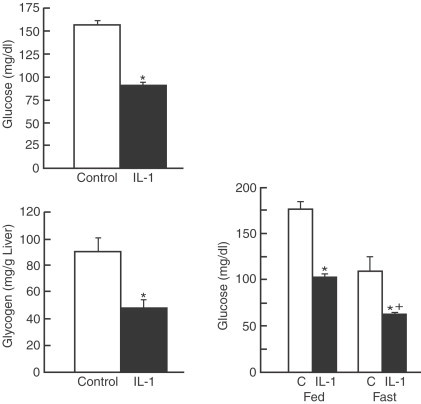
Intraperitoneal injection of IL-1β induced hypoglycemia accompanied by a decrease in glycogen content in mouse liver (left). Each bar represent mean±SEM. *p<0.05 vs. control mice,+p<0.05 vs. fed ad libitum mice. The hypoglycemia is even more marked under fasting conditions (24 h fasting, right). (From Del Rey et al., 1998).
The hypoglycemic effect of cytokines may be due to, at least in part, an increase in insulin levels (Del Rey and Besedovsky, 1987) but is more probable that it can be mediated by activation of brain IL-1 receptors (Del Rey et al., 1998) given that the hypoglycemic effect was also found in insulin-resistant diabetic mice and in adrenalectomized mice, where there is no hyperinsulinemia (Del Rey and Besedovsky, 1989), and this central action may involve effects on central mechanisms controlling glucose homeostasis leading to a downregulation of glucose set point (Del Rey and Besedovsky, 1992; Del Rey et al., 1998). In this central effect of IL-1, hypothalamic catecholamines seen to play a role counteracting the effect of cytokines on glucose concentrations given that its central depletion accentuated hypoglycemia. (Del Rey et al., 1998).
One of the interfaces between cytokines and glucose metabolism may be 5-HT, as was proposed by MohanKumar et al. (1999). These authors suggest that the increase in PVN 5-HT activity observed in rats after lipopolysaccharide intraperitoneal injection, which is mediated by IL-1β, could play a role in HPA axis activation given that 5-HT fibers innervate CRF perikarya (Sawchenko et al., 1983) and changes in PVN 5-HT concentrations markedly altered CRF release (Feldman et al., 1987).
During inflammation and infection process there are many changes in host lipid and lipoprotein metabolism including an increase on adipose tissue lipolysis, hepatic reesterification of fatty acid and hepatic lipogenesis as well as a decline in fatty acid oxidation in several tissues such as liver, heart and skeletal muscle (Lanza-Jacoby and Tabares, 1990; Takeyama et al., 1990; Feingold et al., 1992; Hardardottir et al., 1994; Khovidhunkit et al., 2004). These changes can be achieved by administration of lipopolysaccharide and pro-inflammatory cytokines suggesting that these proteins are involved in the mediation of many of the host metabolic responses that take place during inflammation and infectious diseases (Hardardottir et al., 1994). Many of these effects of cytokines on lipid metabolism are mediated by the modulation of synthesis and activity of some enzymes involved in the metabolism of lipids. For example, lipopolysaccharide and cytokines reduce mRNA expression of fatty acid translocase and fatty acid transport protein in muscle, heart and adipose tissue of Syrian hamster (Memon et al., 1998a). Further, lipopolysaccharide, TNF-α and IL-1 decrease mRNA levels and activity of acyl-CoA synthetase in several tissues including liver and adipose tissue of Syrian hamster (Memon et al., 1998b; Fig. 18 ) enhancing fatty acid reesterification, suppressing fatty acid oxidation and stimulating lipogenesis and therefore leading to elevated plasma triglycerides and very low-density lipoprotein (Feingold et al., 1991; Memon et al., 1993; Nachiappan et al., 1994).
Fig. 18.
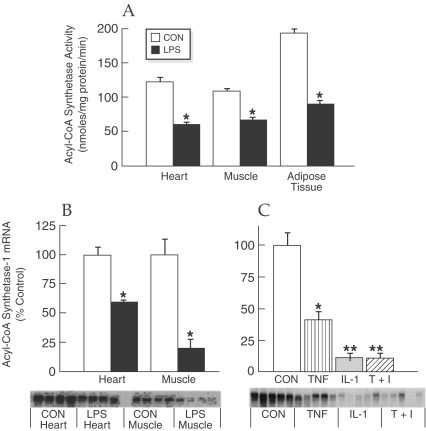
(A) Effect of intraperitoneal lipopolysaccharide (LPS) on acylCoA synthetase 1 (ACS1) activity in adipose tissue, heart and muscle of Syrian hamsters. *p<0.001; (B) Effect of intraperitoneal lipopolysaccharide on ACS1 mRNA levels in heart and muscle. Values are means±SEM. *p<0.001; (C) Effect of tumor necrosis factor (TNF), interleukin-1 (IL-1) and the combination of TNF and IL-1 (T 1 I) on ACS1 mRNA levels in liver of Syrian hamsters. *p<0.002, **p<0.001. (From Memon et al., 1998b).
Besides these effects on carbohydrate metabolism, IL-1 acts on protein and lipid metabolism (Del Rey and Besedovsky, 1987; Klasing, 1988; Argiles et al, 1989; Kanemaki et al., 1998; Kitano et al., 2002; Matsuki et al., 2003; Metzger et al., 2004; Khovidhunkit et al., 2004; Yerkovich et al., 2004) probably due to the triggering of neuroendocrine responses given that IL-1 induces the release of CRF (Sapolsky et al., 1987), melanocortins and other neuropeptides (Tocci and Schmidt, 1997) as well as a direct effect on metabolic activity of different tissues such as skeletal muscle, liver and adipose tissue. IL-1 acts directly on lipid metabolism by inhibiting lipoprotein lipase activity, which control the availability of lipid fuel in the body (Beutler and Cerami, 1985; Doerrler et al., 1994; Matsuki et al., 2003) and decreasing intestinal lipid absorption and lipid accumulation (Argiles et al., 1989). Furthermore, this cytokines can modulate adipocyte function by suppressing the synthesis of fatty acid transport proteins in adipose tissue and the adipocyte maturation in vitro (Gregoire et al., 1992; Memon et al., 1998a). Using an IL-1ra-deficient (IL-1ra−/−) mice Matsuki et al. (2003) showed that excess IL-1 signaling suppresses weight gain and decreases fat mass without changes in food intake, but the morphology and cell volume of adipocytes is not altered compared with those of the wild-type (Fig. 19 ), as well as the hypothalamic expression of adiponectin, leptin and resistin or the expression levels of different anorectic and orexigenic hypothalamic feeding regulators but have impaired lipid storage and lipid uptake into adipose tissue, these defects being more accentuated in males than in females. Furthermore, the high IL-1 signaling causes a decrease in serum leptin, insulin and triacylglycerol, and but an enhancement of insulin sensitivity (Fig. 20 ).
Fig. 19.
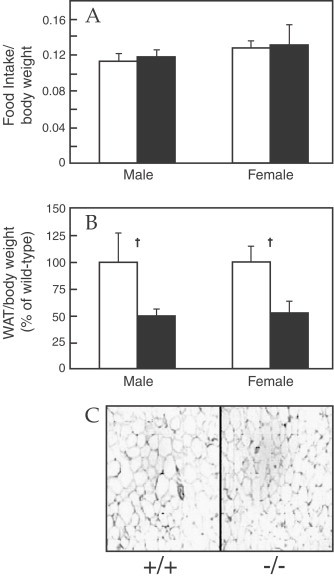
Using a IL-1ra-deficient (IL-1ra−/−) mice it has been shown that excess IL-1 signaling suppress weight gain and decrease fat mass without changes in food intake, but the morphology and cell volume of adipocytes is not altered compared with those of wild type. (A) Food intake per body weight, (B) white adipose tissue (WAT) weight per body weight and (C) Paraffin sections of WAT from epididymal fat pads in IL-1Ra−/− mice. IL-1Ra−/− (shaded bars, −/−) and wild-type (white bars, +/+) mice and IL-1Ra−/−. Data are expressed as the mean±SEM. *p<0.05, † and p<0.01 vs. wild-type mice. (From Matsuki et al., 2003).
Fig. 20.
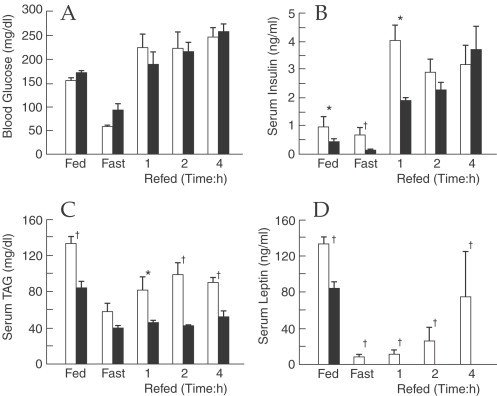
Decreased serum levels of insulin, triacylglycerol (TAG), and leptin in IL-1Ra−/− mice. (A) Blood glucose, (B) serum insulin, (C) TAG and (D) leptin levels in body weight-matched wild-type (white bars) and IL-1Ra−/− (shaded bars) mice. Data are expressed as the mean±SEM. *p<0.05, †, p<0.01 vs. wild-type mice. (From Matsuki et al., 2003).
There are some evidences that suggest the participation of IL-6 in the regulation of lipid metabolism. Thus, IL-6-deficient mice develop obesity along with obesity-related metabolic disorders and these alterations are partially abolished by exogenous IL-6 administration (Wallenius et al., 2002a). Furthermore, it has been observed that intracerebroventricular administration of this cytokine acutely stimulate energy expenditure (Rothwell et al., 1991; Wallenius et al., 2002a) and decreases the weight of mesenteric and retroperitoneal fat pads and circulating leptin levels (Wallenius et al., 2002b; Fig. 21 ). Also in mice bearing an IL-6-secreting tumor for 18 days a reduction in body fat is observed (Metzger et al., 2001). In healthy humans, a negative correlation between IL-6 cerebrospinal fluid levels and total body weight, subcutaneous and total body fat and serum leptin (Stenlof et al., 2003). Ciliary neurotrophic factor, which is structurally related to IL-6, has been shown to reduce body fat in mice fed with diet-induced obesity (Gloaguen et al., 1997; Lambert et al., 2001) and also affect protein metabolism by inducing protein degradation (Espat et al., 1996).
Fig. 21.
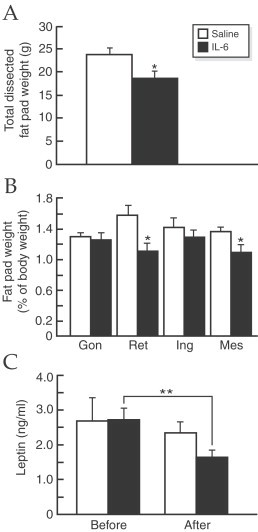
Dissected fat pads and serum leptin. Three intraabdominal fat pads (gonadal (Gon), retroperitoneal (Ret) and mesenteric (Mes)) and the inguinal (Ing) fat pad (a subcutaneous fat pad in the groin) were dissected. (A) The total weight of the dissected fat pads after two weeks of intracerebroventricular treatment with saline or IL-6 (0.4 l g/day). (B) Comparison between the relative weights of the different dissected fat pads (% of body weight) after saline and IL-6 treatment. (C) Leptin levels before and after 2 weeks of intracerebroventricular treatment with saline or IL-6 treatment. (A,B) *p<0:05, vs. control, (C) **p<0:01 vs. before IL-6 treatment. (From Wallenius et al., 2002b).
The efferent signals of acute phase response and the interaction between immune system and hypothalamus–pituitary–thyroid axis play an important role because of the effects of the thyroid hormones on metabolism. Immune-derived cytokines, mainly pro-inflammatory cytokines such as TNF-α, IL-1β and IL-6, stimulate the growth and the function of thyroid cells (Armstrong and Klein, 2001). It has been observed that serum thyroid-stimulating hormone concentrations decreases during 5 h following a single injection of IL-1β (Dubuis et al., 1988) followed by a decline in total tetra-iodothyronine and an increase in free tetra-iodothyronine in rats (Wang et al., 1998). Similar results have been reported after continuous infusion of TNF-α, IL-1β and IL-6 in rats (Hermus et al., 1992; Sweep et al., 1992).
Other peripheral mechanisms of cytokines may be the enhancement of the availability of tryptophan (the 5-HT precursor) to maintain an elevated 5-HT turnover (Dunn, 1992) given that brain 5-HT synthesis depends on the brain availability of this amino acid (Schaechter and Wurtman, 1990) which is positively correlated to plasma-free tryptophan concentration (Fernstrom and Wurtman, 1972). In different anorexia animal models (Kurzer et al., 1988; Meguid et al., 1992; Muscaritoli et al., 1996; Laviano et al., 1999) and anorectic patients with different diseases (Cangiano et al., 1994; Laviano et al., 1997; Aguilera et al., 2000) an increase in plasma and brain free tryptophan concentrations and brain serotoninergic activity has been reported suggesting a connection between anorexia disease, circulating tryptophan, brain serotoninergic activity and cytokines. Moreover, in anorectic tumor-bearing rats, enhanced free tryptophan circulating levels decrease to normal levels after tumor removal thereby improving food intake (Cangiano et al., 1994). The subcutaneous administration of IL-1α to normal rats during 2 days caused a rise in plasma-free tryptophan associated to a decrease of food intake and subsequently to a reduction in carcass adiposity and body weight (Sato et al., 2003; Fig. 22 ). Although the injection of tryptophan increases plasma free and total tryptophan there was not a clear effect on food intake. This lack of effect may be due to the newly synthesized 5-HT in the hypothalamus that is not released (Schaechter and Wurtman, 1990). Moreover, the neuronal activity is a decisive factor to hypothalamic 5-HT release, and IL-1α is able to modulate neuronal activity (Bartholomew and Hoffman, 1993). Insulin may contribute to the anorectic effect of IL-1α taking into account that circulating insulin increases after cytokine injection and that there is a negative correlation between food intake and plasma insulin (Sato et al., 2003). It is known that peripheral insulin increase plasma tryptophan and decreases other neutral amino acids (Fernstrom and Wurtman, 1972) that compete with free tryptophan for brain entry (Fernstrom and wurtman, 1972; Landel et al., 1987) leading to an enhanced availability of hypothalamic tryptophan and subsequently to a rise in 5-HT production that may be released after the increase in neuronal activity induced by IL-1α.
Fig. 22.
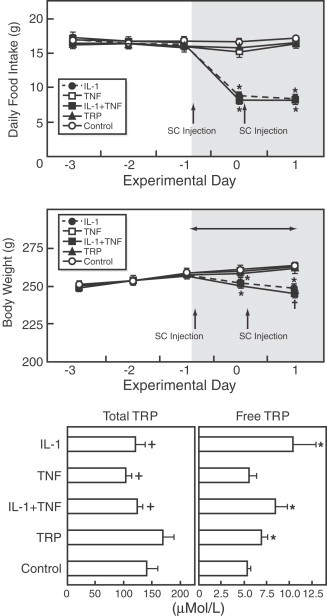
(A) Daily food intake before and after subcutaneous injections of cytokines or tryptophan. The shaded area indicates the period influenced by the injections. *p<0.005 vs. the TNF, TRP and control groups. (B) Body weight before and after subcutaneous injections of cytokines or tryptophan. Before injections, there was no significant difference in body weight among the five groups. The asterisks indicate that the changes in body weight in the IL-1 and IL-1+TNF groups were significantly different vs the other three groups, and, furthermore, that the change in the IL-1+TNF group was greater than that of the IL-1 group. * p<0.05 vs. the TNF, TRP and control groups. (C) Plasma-total and -free tryptophan among the five groups. *p<0.05 vs. the control group.+p<0.05 vs. the TRP group. # p<0.05 vs. the TNF, TRP and control groups. The data were mean±SEM. (From Sato et al., 2003).
Purpose
As we have mentioned above, during infectious disease, trauma or cancer process, host respond with a generalized defense reaction called acute phase response which is characterized by alterations in immune, metabolic, endocrine and neural functions as well as behavior (Baumann and Gauldie, 1994) aimed to inhibit the proliferation and spread of the pathogens. These behavioral alterations, such as fever, somnolence, lethargy or anorexia, called “sickness behavior” (Hart, 1990) are mediated by cytokines and are adaptive and beneficial for the host at least during the first phases of infectious or trauma process (Hart, 1988, Hart, 1990). For instance, anorexia reduces the energy expenditure due to search for food and decrease the growth of the pathogenic agents by reducing the availability of nutrients coming from food such as free iron, which is indispensable for bacterial proliferation (Weinberg, 1984). However, although these modifications are an essential part in response to different challenges, excessive or long-term production of cytokines or synthesis of cytokines in incorrect biological context compromises host survival and are associated with pathology and mortality in many different diseases. For example, prolonged changes in structure and function of lipoproteins may contribute to atherogenesis (Khovidhunkit et al., 2004). Therefore, potentially beneficial immunotherapies based on long-term cytokines administration cannot be applied because of these harmful side effects, particularly in the CNS (Smith et al., 1990).
Therapy to reverse acute effects of cytokines on acute phase response
Cytokine metabolism and actions can be modulated not only by pharmacological procedures but also by nutrients. There are some evidences that strongly suggest a role of dietary factors in the regulation of cytokines production (Sasaki et al., 1999; Das et al., 2003; Sato et al., 2003). In particular, it has been observed that diets rich in long-chain ω-3 polyunsaturated fatty acids, such as eicosapentaenoic acid or docosahexaenoic acid inhibits the production of IL-1 and TNF-α (Endres et al., 1989; Sasaki et al., 1999) as well as reduces its biological activity, and more specifically those related to food intake (Sato et al., 2003). Further, experimental and clinical studies with cancer patients have shown a reduction of weight loss when the diet was enriched with ω-3 fatty acids from fish oil (Dagnelie et al., 1994; Barber et al., 1999). Moreover in tumor-bearing rats, diets rich in eicosapentaenoic acid diminish tumor growth and ameliorate cachexia (Jho et al., 2002). A marked improvement in food intake, and its two components, body weight and tumor progression in methylcholanthrene tumor-bearing rats fed with ω-3 fatty acids-supplemented diet compared with tumor-bearing rats fed with chow diet are reported (Ramos et al., 2004b, Ramos et al., 2005; Fig. 23 ). This diet retarded the appearance of the tumor and reduced its size and weight as it has been described previously (Bartoli et al., 1993; Dagnelie et al., 1994; Chen and Istfan, 2000) and avoided the decrease in both meal size and meal number at the onset of anorexia and in body weight resulting in no difference between tumor-bearing rats fed with ω-3 fatty acids-enriched diet and control groups. The effects of ω-3 fatty acids on the progression of the tumor may be mediated by the inhibition of smooth cells proliferation and the subsequent reduction of vascularization of the tumor (Kremer and Robinson, 1991; Rose et al., 1991). The ω-3 fatty acids sustain food intake during tumor growth by means of maintaining both meal number and meal size, delaying the onset of anorexia and thus preventing body weight loss. It is likely that the inhibition of TNF-α and IL-1 and leukotriene B4, which enhance IL-1 production, exerted by ω-3 fatty acids (Kunkel and Chensue, 1985; Rola-Pleszczynski and Lemaire, 1985) as well as the inhibition of mononuclear cell proliferation (Meguid and Pichard, 2003) be responsible for the beneficial effects of these fatty acids on food intake. This assumption is supported by the fact that the ARC expression of TNF-α and IL-1 in tumor-bearing rats fed with diet rich in ω-3 fatty acids was lower than in tumor-bearing rats fed with chow diet (Ramos et al., 2004b). Furthermore, we found an increase in ARC and PVN NPY expression in tumor-bearing rats fed with ω-3 fatty acids supplemented diet, measured by mean of microarray analysis. We also reported increased levels of NPY immunoreactivity in ARC and magnocellular division of PVH, but not in parvocellular PVH or SON, and decreased levels of α-MSH in magnocellular PVN and ARC, but not in parvocellular section of PVN, as well as a decrease in immunoreactivity of 5-HT1B receptor in SON (Ramos et al., 2005; Fig. 24 ). The ω-3 fatty acids mechanism(s) of action is/are not completely known. It has been proposed that ω-3 fatty acids may affect the neuronal membrane by inhibiting the production of eicosanoids, which are lipid-derived modulators and among them arachidonic acid is the most important of their precursors. This action alters the phospholipids composition of cellular membrane leading to the alteration of different membrane functions, such as those related to neurotransmitter receptors, second messenger and transport proteins (Meterissian et al., 1995). On the other hand, this inhibition not only affects numerous biological responses but also modulates neurotransmission via inhibition of metabolism and actions of arachidonic acid and its derivatives (Bazan et al., 1997). These data suggest that the effects of ω-3 fatty acids on food intake may be mediated by the modulation of the balance of hypothalamic orexigenic and anorectic factors via inhibition of cytokines production. Further, these data point out the possibility of the therapeutic use of the ω-3 fatty acids at earlier stages of tumor progression for preventing body weight loss and ameliorating anorexia as well as for inhibiting tumor growth.
Fig. 23.
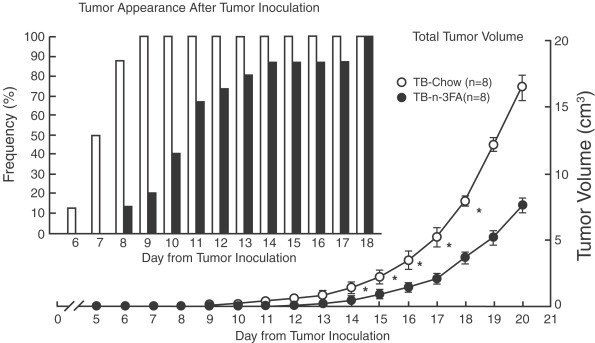
Tumor appearance and changes of volume in tumor-bearing (TB) rats. Tumor appearance occurred in 100% (8 of 8) within 9 days after tumor inoculation in tumor-bearing Chow rats; in tumor-bearing ω-3 fatty acids rats, tumor appearance occurred in 20% (2 of 8) within 9 days and in 100% (8 of 8) on day 18 after tumor inoculation. *p〉λτ∼0.05 vs. tumor-bearing ω-3 fatty acids rats. FA, fatty acid. (From Ramos et al., 2004b).
Fig. 24.
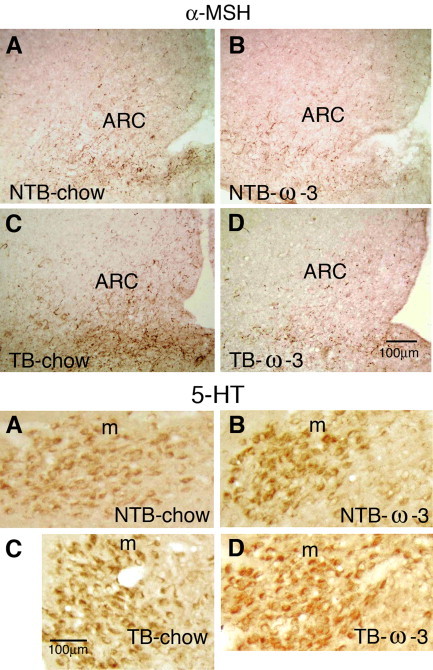
α-MSH immunoreactivity in ARC (top). In non tumor-bearing rats (panels A and B), ω-3 fatty acids-supplemented diet did not influence α-MSH immunoreactivity. Following tumor injection, an increased expression of α-MSH occurred in tumor-bearing Chow (panel C). The use of a ω-3 fatty acids supplemented diet prevented the increase of α-MSH immunoreactivity in tumor-bearing ω-3 fatty acids rats (panel D). This was demonstrated by the less intense immunoreactivity (64% lower) in tumor-bearing ω-3 fatty acids vs. tumor-bearing Chow. 5-HT1B-receptor immunoreactivity in magnocellular PVN (mPVN, bottom). Serotonin is anorexigenic and therefore, 5-HT1B-receptors should be upregulated after tumor inoculation. This figure shows that, after tumor inoculation, the increase in 5-HT1B-receptors was less pronounced in rats fed a ω- 3 fatty acids supplemented diet vs. rats fed chow (panels D vs. C). In tumor-bearing Chow vs. non-tumor-bearing Chow (panel C vs. A), there was an increase of 40% in 5-HT1B-receptor immunoreactivity (p<0.05), while in tumor-bearing ω-3 fatty acids vs. non tumor-bearing ω-3 fatty acids (panels D vs. B), a nonsignificant increase of 14% occurred, demonstrating that the ω-3 fatty acids supplemented diet prevented the upregulation of 5-HT1B-receptors. Scale bar=100 μm. V, ventricle. (From Ramos et al., 2005.)
To this emerging therapy may now be added the idea of the use of nicotine in a sociably acceptable manner, and preliminary studies in our laboratory in tumor-bearing rats show promising results (Personal observations, Laviano and Meguid, 2005).
Abbreviations
- ACTH
adrenocorticotropin hormone
- AP
anterior pituitary
- ARC
arcuate nucleus
- DA
dopamine
- CRF
Corticotropin-releasing factor
- HPA
hypothalamus–pituitary–adrenal
- 5-HT
serotonin
- IL
interleukin
- IL-1ra
interleukin-1α receptor antagonist
- IL-1RI
IL-1 receptor type I
- IL-1RAP
interleukin-1 receptor accessory protein
- INT-γ
interferon gamma
- LHA
lateral hypothalamic area
- NPY
neuropeptide Y
- POMC
pro-opiomelanocortin
- PVN
paraventricular nucleus
- SCN
suprachiasmatic nucleus
- SON
supraoptic nucleus
- TNF-α
tumor necrosis factor-α
- VMH
ventromedial hypothalamus
Acknowledgment
This work was supported in part by NIH 3568-CA 70239, The American Institute of Cancer 22861, American Diabetes Association 35243, and by funding from NCI International Exchange Fellowship and the Hendrix Fund of SUNY Upstate Medical University.
References
- Aguilera A., Selgas R., Codoceo R., Bajo A. Uremic anorexia: a consequence of persistently high brain serotonin levels? The tryptophan/serotonin disorder hypothesis. Perit. Dial. Int. 2000;20:810–816. [PubMed] [Google Scholar]
- Argiles J.M., Lopez-Soriano F.J., Evans R.D., Williamson D.H. Interleukin-1 and lipid metabolism in the rat. Biochem. J. 1989;259:673–678. doi: 10.1042/bj2590673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arletti R., Benelli A., Bertolini A. Influence of oxytocin on feeding behavior in the rat. Peptides. 1989;10:89–93. doi: 10.1016/0196-9781(89)90082-x. [DOI] [PubMed] [Google Scholar]
- Arletti R., Benelli A., Bertolini A. Oxytocin inhibits food and fluid intake in rats. Physiol. Behav. 1990;48:825–830. doi: 10.1016/0031-9384(90)90234-u. [DOI] [PubMed] [Google Scholar]
- Armstrong M.D., Klein J.R. Immune-endocrine interactions of the hypothalamus–pituitary–thyroid axis: integration, communication and homeostasis. Arch. Immunol. Ther. Exp. (Warsz), 2001;49:231–237. [PubMed] [Google Scholar]
- Arsenijevic D., Richard D. A predominant role for INF-γ in infection induced cachexia in comparison to TNF-α (abstract) Appetite. 1999;33:232. [Google Scholar]
- Banks W.A., Ortiz L., Plotkin S.R., Kastin A.J. Human interleukin (IL) 1 alpha, murine IL-1 alpha and murine IL-1 beta are transported from blood to brain in the mouse by a shared saturable mechanism. J. Pharmacol. Exp. Ther. 1991;259:988–996. [PubMed] [Google Scholar]
- Barber M.D., Ross J.A., Voss A.C., Tisdale M.J., Fearon K.C. The effect of an oral nutritional supplement enriched with fish oil on weight-loss in patients with pancreatic cancer. Br. J. Cancer. 1999;81:80–86. doi: 10.1038/sj.bjc.6690654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes N.M., Sharp T. A review of central 5-HT receptors and their function. Neuropharmacology. 1999;38:1083–1152. doi: 10.1016/s0028-3908(99)00010-6. [DOI] [PubMed] [Google Scholar]
- Bartholomew S.A., Hoffman S.A. Effects of peripheral cytokine injections on multiple unit activity in the anterior hypothalamic area of the mouse. Brain Behav. Immun. 1993;7:301–316. doi: 10.1006/brbi.1993.1030. [DOI] [PubMed] [Google Scholar]
- Bartoli G.M., Palozza P., Marra G., Armelao F., Franceschelli P., Luberto C., Sgarlata E., Piccioni E., Anti M. ω-3 PUFA and alpha-tocopherol control of tumor cell proliferation. Mol. Aspects. Med. 1993;14:247–252. doi: 10.1016/0098-2997(93)90011-2. [DOI] [PubMed] [Google Scholar]
- Baumann H., Gauldie J. The acute phase response. Immunol. Today. 1994;15:74–80. doi: 10.1016/0167-5699(94)90137-6. [DOI] [PubMed] [Google Scholar]
- Bazan N.G., Packard M.G., Teather L., Allan G. Bioactive lipids in excitatory neurotransmission and neuronal plasticity. Neurochem. Int. 1997;30:225–231. doi: 10.1016/s0197-0186(96)00020-4. [DOI] [PubMed] [Google Scholar]
- Beatty W.W., O’Brien D.A., Vilberg T.R. Suppression of feeding by intrahypothalamic implants of estradiol in male and female rats. Bull. Psychonom. Soc. 1974;3:273–274. [Google Scholar]
- Berkenbosch F., van Oers J., del Rey A., Tilders F., Besedovsky H. Corticotropin-releasing factor-producing neurons in the rat activated by interleukin-1. Science. 1987;238:524–526. doi: 10.1126/science.2443979. [DOI] [PubMed] [Google Scholar]
- Bette M., Schafer M.K., van Rooijen N., Weihe E., Fleischer B. Distribution and kinetics of superantigen-induced cytokine gene expression in mouse spleen. J. Exp. Med. 1993;178:1531–1539. doi: 10.1084/jem.178.5.1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beutler B.A., Cerami A. Recombinant interleukin 1 suppresses lipoprotein lipase activity in 3T3-L1 cells. J. Immunol. 1985;135:3969–3971. [PubMed] [Google Scholar]
- Bird T.A., Davies A., Baldwin S.A., Saklatvala J. Interleukin 1 stimulates hexose transport in fibroblasts by increasing the expression of glucose transporters. J. Biol. Chem. 1990;265:13578–13583. [PubMed] [Google Scholar]
- Blaha V., Yang Z.-J., Meguid M.M., Laviano A., Zadack Z., Rossi-Fanelli F. Cancer anorexia is modulated by interaction of hypothalamic-VMN dopamine (DA) and serotonin (5-HT) and not solely 5-HT as currently thought. Surg. Forum. 1996;47:517–520. [Google Scholar]
- Blatteis C.M. Role of the OVLT in the febrile response to circulating pyrogens. Prog. Brain Res. 1992;91:409–412. doi: 10.1016/s0079-6123(08)62360-2. [DOI] [PubMed] [Google Scholar]
- Borovikova L.V., Ivanova S., Zhang M., Yang H., Botchkina G.I., Watkins L.R., Wang H., Abumrad N., Eaton J.W., Tracey K.J. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature. 2000;405:458–462. doi: 10.1038/35013070. [DOI] [PubMed] [Google Scholar]
- Bouman A., Schipper M., Heineman M.J., Faas M.M. Gender difference in the non-specific and specific immune response in humans. Am. J. Reprod. Immunol. 2004;52:19–26. doi: 10.1111/j.1600-0897.2004.00177.x. [DOI] [PubMed] [Google Scholar]
- Broberger C., De Lecea L., Sutcliffe J.G., Hokfelt T. Hypocretin/orexin- and melanin-concentrating hormone-expressing cells form distinct populations in the rodent lateral hypothalamus: relationship to the neuropeptide Y and agouti gene-related protein systems. J. Comp. Neurol. 1998;402:460–474. [PubMed] [Google Scholar]
- Broberger C., Johansen J., Brismar H., Johansson C., Schalling M., Hokfelt T. Changes in neuropeptide Y receptors and pro-opiomelanocortin in the anorexia (anx/anx) mouse hypothalamus. J. Neurosci. 1999;19:7130–7139. doi: 10.1523/JNEUROSCI.19-16-07130.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broberger C., Johansen J., Schalling M., Hokfelt T. Hypothalamic neurohistochemistry of the murine anorexia (anx/anx) mutation: altered processing of neuropeptide Y in the arcuate nucleus. J. Comp. Neurol. 1997;387:124–135. doi: 10.1002/(sici)1096-9861(19971013)387:1<124::aid-cne10>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Butera P.C., Beikirch R.J. Central implants of diluted estradiol: independent effects on ingestive and reproductive behaviors of ovariectomized rats. Brain Res. 1989;491:266–273. doi: 10.1016/0006-8993(89)90062-0. [DOI] [PubMed] [Google Scholar]
- Campbel R.E., French-Mullen J.M., Cowley M.A., Smith M.S., Grove K.L. Hypothalamic circuitry of neuropeptide Y regulation of neuroendocrine function and food intake via the Y5 receptor subtype. Neuroendocrinology. 2001;74:106–119. doi: 10.1159/000054676. [DOI] [PubMed] [Google Scholar]
- Campos A.C., Oler A., Meguid M.M., Chen T.Y. Liver biochemical and histological changes with graded amounts of total parenteral nutrition. Arch. Surg. 1990;125:447–450. doi: 10.1001/archsurg.1990.01410160033006. [DOI] [PubMed] [Google Scholar]
- Cangiano C., Testa U., Muscaritoli M., Meguid M.M., Mulieri M., Laviano A., Cascino A., Preziosa I., Conversano L., Rossi-Fanelli F. Cytokines, tryptophan and anorexia in cancer patients before and after surgical tumor ablation. Anticancer Res. 1994;14:1451–1455. [PubMed] [Google Scholar]
- Card J.P., Moore R.Y. Neuropeptide Y localization in the rat suprachiasmatic nucleus and periventricular hypothalamus. Neurosci. Lett. 1988;88:241–246. doi: 10.1016/0304-3940(88)90217-0. [DOI] [PubMed] [Google Scholar]
- Ceppi E.D., Knowles R.G., Carpenter K.M., Titheradge M.A. Effect of treatment in vivo of rats with bacterial endotoxin on fructose 2,6-bisphosphate metabolism and L-pyruvate kinase activity and flux in isolated liver cells. Biochem. J. 1992;284:761–766. doi: 10.1042/bj2840761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chai J.-K., Blaha V., Meguid M.M., Yang Z.-J., Varma M., Laviano A. Use of orchiectomy and testosterone replacement to explore meal number to meal size relationship in male rats. Am. J. Physiol. 1999;45:R1366–R1373. doi: 10.1152/ajpregu.1999.276.5.R1366. [DOI] [PubMed] [Google Scholar]
- Chance W.T., Balasubramaniam A., Dayal R., Brown J., Fischer J.E. Hypothalamic concentration and release of neuropeptideY into microdialysates is reduced in anorectic tumor-bearing rats. Life Sci. 1994;54:1869–1874. doi: 10.1016/0024-3205(94)90144-9. [DOI] [PubMed] [Google Scholar]
- Chance W.T., Balasubramaniam A., Sheriff S., Fischer J.E. Possible role of neuropeptide Y in experimental cancer anorexia. Adv. Exp. Med. Biol. 1994;354:185–201. doi: 10.1007/978-1-4899-0939-8_14. [DOI] [PubMed] [Google Scholar]
- Chance W.T., Fischer J.E. Aphagic and adipsic effects of interleukin-1. Brain Res. 1991;568:261–264. doi: 10.1016/0006-8993(91)91406-q. [DOI] [PubMed] [Google Scholar]
- Chance W.T., Sheriff S., Dayal R., Balasubramaniam A. Refractory hypothalamic alpha-MSH satiety and AGRP feeding systems in rats bearing MCA sarcomas. Peptides. 2003;24:1909–1919. doi: 10.1016/j.peptides.2003.09.019. [DOI] [PubMed] [Google Scholar]
- Chance W.T., Sheriff S., Kasckow J.W., Regmi A., Balasubramaniam A. NPY messenger RNA is increased in medial hypothalamus of anorectic tumor-bearing rats. Regul. Pept. 1998;75–76:347–353. doi: 10.1016/s0167-0115(98)00087-1. [DOI] [PubMed] [Google Scholar]
- Chen Z.Y., Istfan N.W. Docosahexaenoic acid is a potent inducer of apoptosis in HT-29 colon cancer cells. Prostaglandins Leukot. Essent. Fatty Acids. 2000;63:301–308. doi: 10.1054/plef.2000.0218. [DOI] [PubMed] [Google Scholar]
- Christ B., Nath A. Impairment by interleukin 1 beta and tumour necrosis factor alpha of the glucagon-induced increase in phosphoenolpyruvate carboxykinase gene expression and gluconeogenesis in cultured rat hepatocytes. Biochem. J. 1996;320:161–166. doi: 10.1042/bj3200161. Erratum in: Biochem. J., 1997 321: 903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coe J.E., Ross M.J. Hamster female protein. A divergent acute phase protein in male and female Syrian hamsters. J. Exp. Med. 1983;157:1421–1433. doi: 10.1084/jem.157.5.1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conn C.A., McClellan J.L., Maassab H.F., Smitka C.W., Majde J.A., Kluger M.J. Cytokines and the acute phase response to influenza virus in mice. Am. J. Physiol. 1995;268:R78–R84. doi: 10.1152/ajpregu.1995.268.1.R78. [DOI] [PubMed] [Google Scholar]
- Cowley M.A., Pronchuk N., Fan W., Dinulescu D.M., Colmers W.F., Cone R.D. Integration of NPY, AGRP, and melanocortin signals in the hypothalamic paraventricular nucleus: evidence of a cellular basis for the adipostat. Neuron. 1999;24:155–163. doi: 10.1016/s0896-6273(00)80829-6. [DOI] [PubMed] [Google Scholar]
- Cravo M.L., Gloria L.M., Claro I. Metabolic responses to tumor disease and progression: tumor–host interaction. Clin. Nutr. 2000;19:459–465. doi: 10.1054/clnu.2000.0140. [DOI] [PubMed] [Google Scholar]
- Cunningham E.T., Jr., De Souza E.B. Interleukin 1 receptors in the brain and endocrine tissues. Immunol. Today. 1993;14:171–176. doi: 10.1016/0167-5699(93)90281-o. [DOI] [PubMed] [Google Scholar]
- Currie P.J., Coiro C.D., Niyomchai T., Lira A., Farahnmmand F. Hypothalamic paraventricular 5-hydroxytryptamine: receptor specific inhibition of NPY-stimulated eating and energy metabolism. Pharmacol. Biochem. Behav. 2002;71:709–716. doi: 10.1016/s0091-3057(01)00671-2. [DOI] [PubMed] [Google Scholar]
- Currie P.J., Coscina D.V. Stimulation of 5-HT(2A/2C) receptors within specific hypothalamic nuclei differentially antagonizes NPY-induced feeding. NeuroReport. 1997;8:3759–3762. doi: 10.1097/00001756-199712010-00020. [DOI] [PubMed] [Google Scholar]
- Dagnault A., Richard D. Involvement of the medial preoptic area in the anorectic action of estrogens. Am. J. Physiol. 1997;272:R311–R317. doi: 10.1152/ajpregu.1997.272.1.R311. [DOI] [PubMed] [Google Scholar]
- Dagnelie P.C., Bell J.D., Williams S.C., Bates T.E., Abel P.D., Foster C.S. Effect of fish oil on cancer cachexia and host liver metabolism in rats with prostate tumors. Lipids. 1994;29:195–203. doi: 10.1007/BF02536729. [DOI] [PubMed] [Google Scholar]
- Das U.N., Ramos E.J., Meguid M.M. Metabolic alterations during inflammation and its modulation by central actions of omega-3 fatty acids. Curr. Opin. Clin. Nutr. Metab. Care. 2003;6:413–419. doi: 10.1097/01.mco.0000078981.18774.5e. [DOI] [PubMed] [Google Scholar]
- Debonis D., Meguid M.M., Laviano A., Yang Z.J., Gleason J.R. Temporal changes in meal number and meal size relationship in response to rHu IL-1α. NeuroReport. 1995;6:1752–1756. doi: 10.1097/00001756-199509000-00011. [DOI] [PubMed] [Google Scholar]
- Deitch E.A., Xu D., Franko L., Ayala A., Chaudry I.H. Evidence favoring the role of the gut as a cytokine-generating organ in rats subjected to hemorrhagic shock. Shock. 1994;1:141–144. doi: 10.1097/00024382-199402000-00010. [DOI] [PubMed] [Google Scholar]
- De Laurentiis A., Pisera D., Caruso C., Candolfi M., Mohn C., Rettori V., Seilicovich A. Lipopolysaccharide- and tumor necrosis factor-alpha-induced changes in prolactin secretion and dopaminergic activity in the hypothalamic–pituitary axis. Neuroimmunomodulation. 2002;10:30–39. doi: 10.1159/000064412. [DOI] [PubMed] [Google Scholar]
- Del Rey A., Besedovsky H. Interleukin 1 affects glucose homeostasis. Am. J. Physiol. 1987;253:R794–R798. doi: 10.1152/ajpregu.1987.253.5.R794. [DOI] [PubMed] [Google Scholar]
- Del Rey A., Besedovsky H. Antidiabetic effects of interleukin 1. Proc. Natl. Acad. Sci. USA. 1989;86:5943–5947. doi: 10.1073/pnas.86.15.5943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Rey A., Besedovsky H.O. Metabolic and neuroendocrine effects of pro-inflammatory cytokines. Eur. J. Clin. Invest. 1992;22:10–15. [PubMed] [Google Scholar]
- Del Rey A., Kabiersch A., Petzoldt S., Besedovsky H.O. Involvement of noradrenergic nerves in the activation and clonal deletion of T cells stimulated by superantigen in vivo. J. Neuroimmunol. 2002;127:44–53. doi: 10.1016/s0165-5728(02)00096-6. [DOI] [PubMed] [Google Scholar]
- Del Rey A., Monge-Arditi G., Besedovsky H.O. Central and peripheral mechanisms contribute to the hypoglycemia induced by interleukin-1. Ann. NY. Acad. Sci. 1998;840:153–161. doi: 10.1111/j.1749-6632.1998.tb09559.x. [DOI] [PubMed] [Google Scholar]
- Delrue C., Deleplanque B., Rouge-Pont F., Vitiello S., Neveu P.J. Brain monoaminergic, neuroendocrine, and immune responses to an immune challenge in relation to brain and behavioral lateralization. Brain Behav Immun. 1994;8:137–152. doi: 10.1006/brbi.1994.1013. [DOI] [PubMed] [Google Scholar]
- Doerrler W., Feingold K.R., Grunfeld C. Cytokines induce catabolic effects in cultured adipocytes by multiple mechanisms. Cytokine. 1994;6:478–484. doi: 10.1016/1043-4666(94)90074-4. [DOI] [PubMed] [Google Scholar]
- Dryden S., Frankish H.M., Wang Q., Williams G. Neuropeptide Y and energy balance: one-way ahead from the treatment of obesity. J. Clin. Invest. 1994;24:293–308. doi: 10.1111/j.1365-2362.1994.tb01089.x. [DOI] [PubMed] [Google Scholar]
- Dryden S., Wang Q., Frankish H.M., Pickavance L., Williams G. The serotonin (5-HT) antagonist metysergide increases neuropeptide Y (NPY) synthesis and secretion in the hypothalamus of the rat. Brain Res. 1995;699:12–18. doi: 10.1016/0006-8993(95)00841-d. [DOI] [PubMed] [Google Scholar]
- Dubuis J.M., Dayer J.M., Siegrist-Kaiser C.A., Burger A.G. Human recombinant interleukin-1 beta decreases plasma thyroid hormone and thyroid stimulating hormone levels in rats. Endocrinology. 1988;123:2175–2181. doi: 10.1210/endo-123-5-2175. [DOI] [PubMed] [Google Scholar]
- Dunn A.J. Endotoxin-induced activation of cerebral catecholamine and serotonin metabolism: comparison with interleukin-1. J. Pharmacol. Exp. Ther. 1992;261:964–969. [PubMed] [Google Scholar]
- Eckel L.A., Geary N. Estradiol treatment increases feeding induced c-Fos expression in the brains of ovariectomized rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2001;281:R738–R746. doi: 10.1152/ajpregu.2001.281.3.R738. [DOI] [PubMed] [Google Scholar]
- Eckel L.A., Houpt T.A., Geary N. Estradiol treatment increases CCK-induced c-Fos expression in the brains of ovariectomized rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2002;283:R1378–R1385. doi: 10.1152/ajpregu.00300.2002. [DOI] [PubMed] [Google Scholar]
- Ek M., Kurosawa M., Lundeberg T., Ericsson A. Activation of vagal afferents after intravenous injection of interleukin-1beta: role of endogenous prostaglandins. J. Neurosci. 1998;18:9471–9479. doi: 10.1523/JNEUROSCI.18-22-09471.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Haj T., Poole S., Farthing M.J., Ballinger A.B. Anorexia in a rat model of colitis: interaction of interleukin-1 and hypothalamic serotonin. Brain Res. 2002;927:1–7. doi: 10.1016/s0006-8993(01)03305-4. [DOI] [PubMed] [Google Scholar]
- Elias C.F., Aschkenasi C., Lee C., Kelly J., Ahima R.S., Bjorbaek C., Flier J.S., Saper C.B., Elmquist J.K. Leptin differentially regulates NPY and POMC neurons projecting to the lateral hypothalamic area. Neuron. 1999;23:775–786. doi: 10.1016/s0896-6273(01)80035-0. [DOI] [PubMed] [Google Scholar]
- Elias C.F., Lee C., Kelly J., Aschkenasi C., Ahima R.S., Couceyro P.R., Kuhar M.J., Saper C.B., Elmquist J.K. Leptin activates hypothalamic CART neurons projecting to the spinal cord. Neuron. 1998;21:1375–1385. doi: 10.1016/s0896-6273(00)80656-x. [DOI] [PubMed] [Google Scholar]
- Elmquist J.K., Marcus J.N. Rethinking the central causes of diabetes. Nat. Med. 2003;9:645–647. doi: 10.1038/nm0603-645. [DOI] [PubMed] [Google Scholar]
- Elmquist J.K., Saper C.B. Activation of neurons projecting to the paraventricular hypothalamic nucleus by intravenous lipopolysaccharide. J. Comp. Neurol. 1996;374:315–331. doi: 10.1002/(SICI)1096-9861(19961021)374:3<315::AID-CNE1>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Endres S., Ghorbani R., Kelley V.E., Georgilis K., Lonnemann G., van der Meer J.W., Cannon J.G., Rogers T.S., Klempner M.S., Weber P.C. The effect of dietary supplementation with n-3 polyunsaturated fatty acids on the synthesis of interleukin-1 and tumor necrosis factor by mononuclear cells. N. Engl. J. Med. 1989;320:265–271. doi: 10.1056/NEJM198902023200501. [DOI] [PubMed] [Google Scholar]
- Ericsson A., Arias C., Sawchenko P.E. Evidence for an intramedullary prostaglandin-dependent mechanism in the activation of stress-related neuroendocrine circuitry by intravenous interleukin-1. J. Neurosci. 1997;17:7166–7179. doi: 10.1523/JNEUROSCI.17-18-07166.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ericsson A., Liu C., Hart R.P., Sawchenko P.E. Type 1 interleukin-1 receptor in the rat brain: distribution, regulation, and relationship to sites of IL-1-induced cellular activation. J. Comp. Neurol. 1995;361:681–698. doi: 10.1002/cne.903610410. [DOI] [PubMed] [Google Scholar]
- Espat N.J., Auffenberg T., Rosenberg J.J., Rogy M., Martin D., Fang C.H., Hasselgren P.O., Copeland E.M., Moldawer L.L. Ciliary neurotrophic factor is catabolic and shares with IL-6 the capacity to induce an acute phase response. Am. J. Physiol. 1996;271:R185–R190. doi: 10.1152/ajpregu.1996.271.1.R185. [DOI] [PubMed] [Google Scholar]
- Fantino M., Wieteska L. Evidence for a direct central anorectic effect of tumor-necrosis-factor-alpha in the rat. Physiol. Behav. 1993;53:477–483. doi: 10.1016/0031-9384(93)90141-2. [DOI] [PubMed] [Google Scholar]
- Fantuzzi G. Adipose tissue, adipokines, and inflammation. J. Allergy Clin. Immunol. 2005;115:911–919. doi: 10.1016/j.jaci.2005.02.023. [DOI] [PubMed] [Google Scholar]
- Feingold K.R., Soued M., Adi S., Staprans I., Neese R., Shigenaga J., Doerrler W., Moser A., Dinarello C.A., Grunfeld C. Effect of interleukin-1 on lipid metabolism in the rat. Similarities to and differences from tumor necrosis factor. Arterioscler. Thromb. 1991;11:495–500. doi: 10.1161/01.atv.11.3.495. [DOI] [PubMed] [Google Scholar]
- Feingold K.R., Staprans I., Memon R.A., Moser A.H., Shigenaga J.K., Doerrler W., Dinarello C.A., Grunfeld C. Endotoxin rapidly induces changes in lipid metabolism that produce hypertriglyceridemia: low doses stimulate hepatic triglyceride production while high doses inhibit clearance. J. Lipid. Res. 1992;33:1765–1776. [PubMed] [Google Scholar]
- Feldman S., Conforti N., Melamed E. Paraventricular nucleus serotonin mediates neurally stimulated adrenocortical secretion. Brain Res. Bull. 1987;18:165–168. doi: 10.1016/0361-9230(87)90186-9. [DOI] [PubMed] [Google Scholar]
- Fernstrom J.D., Wurtman R.J. Brain serotonin content: physiological regulation by plasma neutral amino acids. Science. 1972;178:414–416. doi: 10.1126/science.178.4059.414. [DOI] [PubMed] [Google Scholar]
- Fischereder M., Schroppel B., Wiese P., Fink M., Banas B., Schmidbauer S., Schlondorff D. Regulation of glucose transporters in human peritoneal mesothelial cells. J. Nephrol. 2003;16:103–109. [PubMed] [Google Scholar]
- Flanagan-Cato L.M., King J.F., Blechman J.G., O’Brien M.P. Estrogen reduces cholecystokinin-induced c-Fos expression in the rat brain. Neuroendocrinology. 1998;67:384–391. doi: 10.1159/000054337. [DOI] [PubMed] [Google Scholar]
- Garcia-Welsh A., Schneiderman J.S., Baly D.L. Interleukin-1 stimulates glucose transport in rat adipose cells. Evidence for receptor discrimination between IL-1 beta and IL-1 alpha. FEBS Lett. 1990;269:421–424. doi: 10.1016/0014-5793(90)81207-5. [DOI] [PubMed] [Google Scholar]
- Gelin J., Moldawer L.L., Lonnroth C., Sherry B., Chizzonite R., Lundholm K. Role of endogenous tumor necrosis factor alpha and interleukin 1 for experimental tumor growth and the development of cancer cachexia. Cancer Res. 1991;51:415–421. [PubMed] [Google Scholar]
- Gloaguen I., Costa P., Demartis A., Lazzaro D., Di Marco A., Graziani R., Paonessa G., Chen F., Rosenblum C.I., Van der Ploeg L.H., Cortese R., Ciliberto G., Laufer R. Ciliary neurotrophic factor corrects obesity and diabetes associated with leptin deficiency and resistance. Proc. Natl. Acad. Sci. USA. 1997;94:6456–6461. doi: 10.1073/pnas.94.12.6456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goehler L.E., Gaykema R.P., Hansen M.K., Anderson K., Maier S.F., Watkins L.R. Vagal immune-to-brain communication: a visceral chemosensory pathway. Auton. Neurosci. 2000;85:49–59. doi: 10.1016/S1566-0702(00)00219-8. [DOI] [PubMed] [Google Scholar]
- Goehler L.E., Gaykema R.P., Nguyen K.T., Lee J.E., Tilders F.J., Maier S.F., Watkins L.R. Interleukin-1β in immune cells of the abdominal vagus nerve: a link between the immune and nervous systems? J. Neurosci. 1999;19:2799–2806. doi: 10.1523/JNEUROSCI.19-07-02799.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goehler L.E., Relton J.K., Dripps D., Kiechle R., Tartaglia N., Maier S.F., Watkins L.R. Vagal paraganglia bind biotinylated interleukin-1 receptor antagonist: a possible mechanism for immune-to-brain communication. Brain Res. Bull. 1997;43:357–364. doi: 10.1016/s0361-9230(97)00020-8. [DOI] [PubMed] [Google Scholar]
- Gonzalez M.C., Abreu P., Barroso-Chinea P., Cruz-Muros I., Gonzalez-Hernandez T. Effect of intracerebroventricular injection of lipopolysaccharide on the tuberoinfundibular dopaminergic system of the rat. Neuroscience. 2004;127:251–259. doi: 10.1016/j.neuroscience.2004.04.057. [DOI] [PubMed] [Google Scholar]
- Gregoire F., De Broux N., Hauser N., Heremans H., Van Damme J., Remacle C. Interferon-gamma and interleukin-1 beta inhibit adipoconversion in cultured rodent preadipocytes. J. Cell. Physiol. 1992;151:300–309. doi: 10.1002/jcp.1041510211. [DOI] [PubMed] [Google Scholar]
- Hansen K., Sickelmann F., Pietrowsky R., Fehm H.L., Born J. Systemic immune changes following meal intake in humans. Am. J. Physiol. 1997;273:R548–R553. doi: 10.1152/ajpregu.1997.273.2.R548. [DOI] [PubMed] [Google Scholar]
- Hansen M.K., Taishi P., Chen Z., Krueger J.M. Cafeteria feeding induces interleukin-1beta mRNA expression in rat liver and brain. Am. J. Physiol. 1998;274:R1734–R1739. doi: 10.1152/ajpregu.1998.274.6.R1734. [DOI] [PubMed] [Google Scholar]
- Hansen S., Sodersten P., Eneroth P., Srebro B., Hole K. A sexually dimorphic rhythm in oestradiol-activated lordosis behaviour in the rat. J. Endocrinol. 1979;82:267–274. doi: 10.1677/joe.0.0830267. [DOI] [PubMed] [Google Scholar]
- Hansen S., Sodersten P., Srebo B. A daily rhythm in the behavioral sensitivity of the female rat to oestradiol. J. Endocrinol. 1978;77:381–388. doi: 10.1677/joe.0.0770381. [DOI] [PubMed] [Google Scholar]
- Hardardottir I., Grunfeld C., Feingold K.R. Effects of endotoxin and cytokines on lipid metabolism. Curr. Opin. Lipidol. 1994;5:207–215. doi: 10.1097/00041433-199405030-00008. [DOI] [PubMed] [Google Scholar]
- Hart B.L. Biological basis of the behavior of sick animals. Neurosci. Biobehav. Rev. 1988;12:123–137. doi: 10.1016/s0149-7634(88)80004-6. [DOI] [PubMed] [Google Scholar]
- Hart B.L. Behavioral adaptations to pathogens and parasites: five strategies. Neurosci. Biobehav. Rev. 1990;14:273–294. doi: 10.1016/s0149-7634(05)80038-7. [DOI] [PubMed] [Google Scholar]
- Herkenham M., Lee H.Y., Baker R.A. Temporal and spatial patterns of c-fos mRNA induced by intravenous interleukin-1: a cascade of non-neuronal cellular activation at the blood–brain barrier. J. Comp. Neurol. 1998;400:175–196. doi: 10.1002/(sici)1096-9861(19981019)400:2<175::aid-cne2>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Hermus R.M., Sweep C.G., van der Meer M.J., Ross H.A., Smals A.G., Benraad T.J., Kloppenborg P.W. Continuous infusion of interleukin-1 beta induces a nonthyroidal illness syndrome in the rat. Endocrinology. 1992;131:2139–2146. doi: 10.1210/endo.131.5.1425414. [DOI] [PubMed] [Google Scholar]
- Hirai C.Y., Limaos E.A. Effect of sex steroids on the circulating levels of alpha 2-macroglobulin in injured rats. Braz. J. Med. Biol. Res. 1990;23:1021–1024. [PubMed] [Google Scholar]
- Hosoi T., Okuma Y., Nomura Y. Electrical stimulation of afferent vagus nerve induces IL-1beta expression in the brain and activates HPA axis. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2000;279:R141–R147. doi: 10.1152/ajpregu.2000.279.1.R141. [DOI] [PubMed] [Google Scholar]
- Hotamisligil G.S., Shargill N.S., Spiegelman B.M. Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science. 1993;259:87–91. doi: 10.1126/science.7678183. [DOI] [PubMed] [Google Scholar]
- Inui A. Cancer anorexia–cachexia syndrome: are neuropeptides the key? Cancer Res. 1999;59:4493–4501. [PubMed] [Google Scholar]
- Itoh T., Iwai H., Ueda K. Comparative lung pathology of inbred strain of mice resistant and susceptible to Sendai virus infection. J. Vet. Med. Sci. 1991;53:275–279. doi: 10.1292/jvms.53.275. [DOI] [PubMed] [Google Scholar]
- Jho D.H., Babcock T.A., Tevar R., Helton W.S., Espat N.J. Eicosapentaenoic acid supplementation reduces tumor volume and attenuates cachexia in a rat model of progressive non-metastasizing malignancy. JPEN J. Parenter. Enteral Nutr. 2002;26:291–297. doi: 10.1177/0148607102026005291. [DOI] [PubMed] [Google Scholar]
- Kanemaki T., Kitade H., Kaibori M., Sakitani K., Hiramatsu Y., Kamiyama Y., Ito S., Okumura T. Interleukin 1β and interleukin 6, but not tumor necrosis factor α, inhibit insulin-stimulated glycogen synthesis in rat hepatocytes. Hepatology I. 1998;27:1296–1303. doi: 10.1002/hep.510270515. [DOI] [PubMed] [Google Scholar]
- Khovidhunkit W., Kim M.S., Memon R.A., Shigenaga J.K., Moser A.H., Feingold K.R., Grunfeld C. Effects of infection and inflammation on lipid and lipoprotein metabolism: mechanisms and consequences to the host. J. Lipid. Res. 2004;45:1169–1196. doi: 10.1194/jlr.R300019-JLR200. [DOI] [PubMed] [Google Scholar]
- Kitano T., Okumura T., Nishizawa M., Liew F.Y., Seki T., Inoue K., Ito S. Altered response to inflammatory cytokines in hepatic energy metabolism in inducible nitric oxide synthase knockout mice. J. Hepatol. 2002;36:759–765. doi: 10.1016/s0168-8278(02)00061-2. [DOI] [PubMed] [Google Scholar]
- Klasing K.C. Nutritional aspects of leukocytic cytokines. J. Nutr. 1988;118:1436–1446. doi: 10.1093/jn/118.12.1436. [DOI] [PubMed] [Google Scholar]
- Konsman J.P., Luheshi G.N., Bluthe R.M., Dantzer R. The vagus nerve mediates behavioural depression, but not fever, in response to peripheral immune signals; a functional anatomical analysis. Eur. J. Neurosci. 2000;12:4434–4446. doi: 10.1046/j.0953-816x.2000.01319.x. [DOI] [PubMed] [Google Scholar]
- Kozak W., Zheng H., Conn C.A., Soszynski D., van der Ploeg L.H., Kluger M.J. Thermal and behavioral effects of lipopolysaccharide and influenza in interleukin-1 beta-deficient mice. Am. J. Physiol. 1995;269:R969–R977. doi: 10.1152/ajpregu.1995.269.5.R969. [DOI] [PubMed] [Google Scholar]
- Kremer J.M., Robinson D.R. Studies of dietary supplementation with omega 3 fatty acids in patients with rheumatoid arthritis. World Rev. Nutr. Diet. 1991;66:367–382. doi: 10.1159/000419305. [DOI] [PubMed] [Google Scholar]
- Kunkel S.L., Chensue S.W. Arachidonic acid metabolites regulate interleukin-1 production. Biochem. Biophys. Res. Commun. 1985;128:892–897. doi: 10.1016/0006-291x(85)90130-5. [DOI] [PubMed] [Google Scholar]
- Kuriyama K., Hori T., Mori T., Nakashima T. Actions of interferon alpha and interleukin- 1 beta on the glucose-responsive neurons in the ventromedial hypothalamus. Brain. Res. Bull. 1990;24:803–810. doi: 10.1016/0361-9230(90)90143-n. [DOI] [PubMed] [Google Scholar]
- Kurzer M.J., Janiszewsky J., Meguid M.M. Aminoacid profiles in tumor bearing and non-tumor bearing malnourished rats. Cancer. 1988;62:1492–1496. doi: 10.1002/1097-0142(19881015)62:8<1492::aid-cncr2820620808>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Kusnecov A.W., Liang R., Shurin G. T-lymphocyte activation increases hypothalamic and amygdaloid expression of CRH mRNA and emotional reactivity to novelty. J. Neurosci. 1999;19:4533–4543. doi: 10.1523/JNEUROSCI.19-11-04533.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert P.D., Anderson K.D., Sleeman M.W., Wong V., Tan J., Hijarunguru A., Corcoran T.L., Murray J.D., Thabet K.E., Yancopoulos G.D., Wiegand S.J. Ciliary neurotrophic factor activates leptin-like pathways and reduces body fat, without cachexia or rebound weight gain, even in leptin-resistant obesity. Proc. Natl. Acad. Sci. USA. 2001;98:4652–4657. doi: 10.1073/pnas.061034298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landel A.M., Lo C.C., Meguid M.M. Observations on predicted brain influx rates of neurotransmitter precursors. Effects of tumor, operative stress with tumor removal, and postoperative TPN of varying amino acid compositions. Cancer. 1987;59:1192–1200. doi: 10.1002/1097-0142(19870315)59:6<1192::aid-cncr2820590627>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- Langhans W., Balkowski G., Savoldelli D. Differential feeding responses to bacterial lipopolysaccharide and muramyl dipeptide. Am. J. Physiol. 1991;261:R659–R664. doi: 10.1152/ajpregu.1991.261.3.R659. [DOI] [PubMed] [Google Scholar]
- Langhans W., Delprete E., Scharrer E. Mechanisms of vasopressin's anorectic effect. Physiol. Behav. 1991;49:169–176. doi: 10.1016/0031-9384(91)90251-i. [DOI] [PubMed] [Google Scholar]
- Lanza-Jacoby S., Tabares A. Triglyceride kinetics, tissue lipoprotein lipase, and liver lipogenesis in septic rats. Am. J. Physiol. 1990;258:E678–E685. doi: 10.1152/ajpendo.1990.258.4.E678. [DOI] [PubMed] [Google Scholar]
- Laviano A., Cangiano C., Fava A., Muscaritoli M., Mulieri G., Rossi-Fanelli F. Peripherally injected IL-1 induces anorexia and increases brain tryptophan concentrations. Adv. Exp. Med. Biol. 1999;467:105–108. doi: 10.1007/978-1-4615-4709-9_15. [DOI] [PubMed] [Google Scholar]
- Laviano A., Cangiano C., Preziosa I., Riggio O., Conversano L., Cascino A., Ariemma S., Rossi-Fanelli F. Plasma tryptophan levels and anorexia in liver cirrhosis. Int. J. Eat. Disord. 1997;21:181–186. doi: 10.1002/(sici)1098-108x(199703)21:2<181::aid-eat9>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- Laviano A., Gleason J.R., Meguid M.M., Yang Z.-J., Cangiano C., Rossi-Fanelli F. Effects of intra-VMN mianserin and IL-1ra on meal number in anorectic tumor-bearing rats. J. Investig. Med. 2000;48:40–48. [PubMed] [Google Scholar]
- Laviano A., Meguid M.M., Gleason J.R., Yang Z.-J., Renvyle T. Comparison of long-term feeding pattern between male and female Fischer 344 rats: influence of estrous cycle. Am. J. Physiol. 1996;270:R413–R419. doi: 10.1152/ajpregu.1996.270.2.R413. [DOI] [PubMed] [Google Scholar]
- Laviano A., Meguid M.M., Rossi-Fanelli F. Cancer anorexia: clinical implications, pathogenesis, and therapeutic strategies. Lancet Oncol. 2003;4:686–994. doi: 10.1016/s1470-2045(03)01247-6. [DOI] [PubMed] [Google Scholar]
- Li Q., Verma I.M. NF-kappaB regulation in the immune system. Nat. Rev. Immunol. 2002;2:725–734. doi: 10.1038/nri910. [DOI] [PubMed] [Google Scholar]
- Maitra S.R., Wang S., Brathwaite C.E., El-Maghrabi M.R. Alterations in glucose-6-phosphatase gene expression in sepsis. J. Trauma. 2000;49:38–42. doi: 10.1097/00005373-200007000-00005. [DOI] [PubMed] [Google Scholar]
- Makarenko I.G., Meguid M.M., Ugrumov M.V. Distribution of serotonin 5-HT1B receptors in the normal rat hypothalamus. Neurosci. Lett. 2002;328:155–159. doi: 10.1016/s0304-3940(02)00345-2. [DOI] [PubMed] [Google Scholar]
- Makarenko I.G., Meguid M.M., Gatto l., Chen C., Ramos E.J.B., Goncalves C.G., Ugrumov M.V. Normalization of hypothalamic serotonin (5-HT1B) receptor and NPY in cancer anorexia after tumor resection: an immunocytochemical study. Neurosci. Lett. 2005;383:322–327. doi: 10.1016/j.neulet.2005.04.031. [DOI] [PubMed] [Google Scholar]
- Makarenko I.G., Meguid M.M., Gatto I., Goncalves C.G., Ramos E.J.B., Chen C., Ugrumov M.V. Hypothalamic 5-HT1B- receptor changes in the anorectic tumor bearing rats. Neurosci. Lett. 2005;376:71–75. doi: 10.1016/j.neulet.2004.11.026. [DOI] [PubMed] [Google Scholar]
- Makarenko I.G., Meguid M.M., Gatto L., Chen C., Ugrumov M.V. Decreased NPY innervation of the hypothalamic nuclei in rats with cancer anorexia. Brain Res. 2003;961:100–108. doi: 10.1016/s0006-8993(02)03850-7. [DOI] [PubMed] [Google Scholar]
- Mascarucci P., Perego C., Terrazzino S., De Simoni M.G. Glutamate release in the nucleus tractus solitarius induced by peripheral lipopolysaccharide and interleukin-1 beta. Neuroscience. 1998;86:1285–1290. doi: 10.1016/s0306-4522(98)00105-5. [DOI] [PubMed] [Google Scholar]
- Matsuki T., Horai R., Sudo K., Iwakura Y. IL-1 plays an important role in lipid metabolism by regulating insulin levels under physiological conditions. J. Exp. Med. 2003;198:877–888. doi: 10.1084/jem.20030299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meguid M.M., Chen T.-Y., Yang Z.-J., Campos A.C., Hitch D.C., Gleason J.R. Effects of continuous graded TPN on feeding indexes and metabolic concomitants in rats. Am. J. Physiol. 1991;260:E126–E140. doi: 10.1152/ajpendo.1991.260.1.E126. [DOI] [PubMed] [Google Scholar]
- Meguid M.M., Fetissov S.O., Miyata G., Torelli G.F. Feeding pattern in obese Zucker rats after dopaminergic and serotonergic LHA grafts. Neuroreport. 1999;10:1049–1053. doi: 10.1097/00001756-199904060-00028. [DOI] [PubMed] [Google Scholar]
- Meguid M.M., Kawashima Y., Campos A.C.L., Gelling P., Hill T.W., Chen T.-Y., Hitch D.C., Mueller W.J., Hammond W.G. Automated computerized rat eater meter: description and application. Physiol. Behav. 1990;48:759–763. doi: 10.1016/0031-9384(90)90222-p. [DOI] [PubMed] [Google Scholar]
- Meguid M.M., Landel A.M., Lo C.-C., Rivera D. Effect of tumor and tumor removal on DNA, RNA, protein tissue content and survival of methylcholanthrene sarcoma-bearing rat. Surg. Res. Commun. 1987;1:261–271. [Google Scholar]
- Meguid M.M., Laviano A., Rossi-Fanelli F. Food intake equals meal size times mean number. Appetite. 1998;31:404. doi: 10.1006/appe.1998.0209. [DOI] [PubMed] [Google Scholar]
- Meguid M.M., Muscaritoli M., Beverly J.L., Yang Z.J., Cangiano C., Rossi-Fanelli F. The early cancer anorexia paradigm: changes in plasma-free tryptophan and feeding indexes. J. Parenter. Enteral. Nutr. 1992;16:56S–59S. doi: 10.1177/014860719201600604. [DOI] [PubMed] [Google Scholar]
- Meguid M.M., Pichard C. Cytokines: the mother of catabolic mediators. Curr. Opin. Clin. Nutr. Metab. Care. 2003;6:383–386. doi: 10.1097/01.mco.0000078982.18774.85. [DOI] [PubMed] [Google Scholar]
- Meguid M.M., Ramos E.J.B., Laviano A., Varma M., Sato T., Cheng C., Qi Y., Das U.N. Tumor anorexia: effects on neuropeptide Y and monoamines in paraventricular nucleus. Peptides. 2004;25:261–266. doi: 10.1016/j.peptides.2004.01.012. [DOI] [PubMed] [Google Scholar]
- Meguid M.M., Sato T., Torelli G.F., Laviano A., Rossi-Fanelli F. An analysis of temporal changes in meal number and meal size at onset of anorexia in male tumor-bearing rats. Nutrition. 2000;16:305–306. doi: 10.1016/s0899-9007(00)00259-8. [DOI] [PubMed] [Google Scholar]
- Meguid M.M., Yang Z.J., Koseki M. Eating induced rise in LHA-dopamine correlates with meal size in normal and bulbectomized rats. Brain Res. Bull. 1995;36:487–490. doi: 10.1016/0361-9230(95)92128-3. [DOI] [PubMed] [Google Scholar]
- Meguid M.M., Yang Z.J., Laviano A. Meal size and number: relationship to dopamine levels in the ventromedial hypothalamic nucleus. Am. J. Physiol. 1997;272:R1925–R1930. doi: 10.1152/ajpregu.1997.272.6.R1925. [DOI] [PubMed] [Google Scholar]
- Meguid M.M., Yang Z.-J., Montante A. Lateral hypothalamic dopaminergic neural activity in response to TPN. Surgery. 1993;114:400–406. [PubMed] [Google Scholar]
- Memon R.A., Feingold K.R., Moser A.H., Fuller J., Grunfeld C. Regulation of fatty acid transport protein and fatty acid translocase mRNA levels by endotoxin and cytokines. Am. J. Physiol. 1998;274:E210–E217. doi: 10.1152/ajpendo.1998.274.2.E210. [DOI] [PubMed] [Google Scholar]
- Memon R.A., Fuller J., Moser A.H., Smith P.J., Feingold K.R., Grunfeld C. In vivo regulation of acyl-CoA synthetase mRNA and activity by endotoxin and cytokines. Am. J. Physiol. 1998;275:E64–E72. doi: 10.1152/ajpendo.1998.275.1.E64. [DOI] [PubMed] [Google Scholar]
- Memon R.A., Grunfeld C., Moser A.H., Feingold K.R. Tumor necrosis factor mediates the effects of endotoxin on cholesterol and triglyceride metabolism in mice. Endocrinology. 1993;132:2246–2253. doi: 10.1210/endo.132.5.8477669. [DOI] [PubMed] [Google Scholar]
- Meterissian S.H., Forse R.A., Steele G.D., Thomas P. Effect of membrane free fatty acid alterations on the adhesion of human colorectal carcinoma cells to liver macrophages and extracellular matrix proteins. Cancer Lett. 1995;89:145–152. doi: 10.1016/0304-3835(94)03659-7. [DOI] [PubMed] [Google Scholar]
- Metzger S., Begleibter N., Barash V., Drize O., Peretz T., Shiloni E., Chajek-Shaul T. Tumor necrosis factor inhibits the transcriptional rate of glucose-6-phosphatase in vivo and in vitro. Metabolism. 1997;46:579–583. doi: 10.1016/s0026-0495(97)90197-9. [DOI] [PubMed] [Google Scholar]
- Metzger S., Hassin T., Barash V., Pappo O., Chajek-Shaul T. Reduced body fat and increased hepatic lipid synthesis in mice bearing interleukin-6-secreting tumor. Am. J. Physiol. Endocrinol. Metab. 2001;281:E957–E965. doi: 10.1152/ajpendo.2001.281.5.E957. [DOI] [PubMed] [Google Scholar]
- Metzger S., Nusair S., Planer D., Barash V., Pappo O., Shilyansky J., Chajek-Shaul T. Inhibition of hepatic gluconeogenesis and enhanced glucose uptake contribute to the development of hypoglycemia in mice bearing interleukin-1beta-secreting tumor. Endocrinology. 2004;145:5150–5156. doi: 10.1210/en.2004-0323. [DOI] [PubMed] [Google Scholar]
- Miyata G., Meguid M.M., Fetissov S.O., Torelli G.F., Kim H.J. Nicotine's effect on hypothalamic neurotransmitters and appetite regulation. Surgery. 1999;126:255–263. [PubMed] [Google Scholar]
- MohanKumar S.M., MohanKumar P.S., Quadri S.K. Specificity of interleukin-1beta-induced changes in monoamine concentrations in hypothalamic nuclei: blockade by interleukin-1 receptor antagonist. Brain Res. Bull. 1998;47:29–34. doi: 10.1016/s0361-9230(98)00037-9. [DOI] [PubMed] [Google Scholar]
- MohanKumar S.M., MohanKumar P.S., Quadri S.K. Lipopolyssacharide-induced changes in monoamines in specific areas of the brain: blockade by interleukin-1 receptor antagonist. Brain Res. 1999;10:232–237. doi: 10.1016/s0006-8993(99)01206-8. [DOI] [PubMed] [Google Scholar]
- Muscaritoli M., Meguid M.M., Beverly J.L., Yang Z.J., Cangiano C., Rossi-Fanelli F. Mechanism of early tumor anorexia. J. Surg. Res. 1996;60:389–397. doi: 10.1006/jsre.1996.0064. [DOI] [PubMed] [Google Scholar]
- Nachiappan V., Curtiss D., Corkey B.E., Kilpatrick L. Cytokines inhibit fatty acid oxidation in isolated rat hepatocytes: synergy among TNF, IL-6, and IL-1. Shock. 1994;1:123–129. doi: 10.1097/00024382-199402000-00007. [DOI] [PubMed] [Google Scholar]
- Niijima A. The afferent discharges from sensors for interleukin 1 beta in the hepatoportal system in the anesthetized rat. J. Auton. Nerv. Syst. 1996;61:287–291. doi: 10.1016/s0165-1838(96)00098-7. [DOI] [PubMed] [Google Scholar]
- Niijima A., Meguid M.M. Parenteral nutrients in rat suppress hepatic vagal afferent signals from portal vein to hypothalamus. Surgery. 1994;116:294–301. [PubMed] [Google Scholar]
- Niijima A., Meguid M.M. An electrophysiological study on amino acid sensors in the hepato-portal system in the rat. Obes. Res. 1995;5:741S–745S. doi: 10.1002/j.1550-8528.1995.tb00494.x. [DOI] [PubMed] [Google Scholar]
- Niijima A., Meguid M.M. Influence of systemic arginine–lysine on immune organ function: an electrophysiology study. Brain Res. Bull. 1998;45:437–441. doi: 10.1016/s0361-9230(97)00349-3. [DOI] [PubMed] [Google Scholar]
- Noguchi Y., Yoshikawa T., Matsumoto A., Svaninger G., Gelin J. Are cytokines possible mediators of cancer cachexia? Surg. Today. 1996;26:467–475. doi: 10.1007/BF00311551. [DOI] [PubMed] [Google Scholar]
- Nolan Y., Connor T.J., Kelly J.P., Leonard B.E. Lipopolysaccharide administration produces time-dependent and region-specific alterations in tryptophan and tyrosine hydroxylase activities in rat brain. J. Neural. Transm. 2000;107:1393–1401. doi: 10.1007/s007020070003. [DOI] [PubMed] [Google Scholar]
- Olson B.R., Drutarowsky M.D., Chow M.S., Hruby V.J., Stricker E.M., Verbalis J.G. Oxytocin and an oxytocin agonist administered centrally decrease food intake in rats. Peptides. 1991;12:113–118. doi: 10.1016/0196-9781(91)90176-p. [DOI] [PubMed] [Google Scholar]
- Opara E.I., Laviano A., Meguid M.M., Yang Z.-J. Correlation between food intake and CSF IL-l in anorectic tumor-bearing rats. NeuroReport. 1995;6:750–752. doi: 10.1097/00001756-199503270-00011. [DOI] [PubMed] [Google Scholar]
- Opara E.I., Meguid M.M., Yang Z.-J., Chai J.-K., Veerabagu M. Tumor necrosis factor-α and TPN-induced anorexia. Surgery. 1995;18:756–762. doi: 10.1016/s0039-6060(05)80046-7. [DOI] [PubMed] [Google Scholar]
- Opara E.I., Meguid M.M., Yang Z.J., Hammond W.G. Studies on the regulation of food intake using rat total parenteral nutrition as a model. Neurosci. Biobehav. Rev. 1996;20:413–443. doi: 10.1016/0149-7634(95)00027-5. [DOI] [PubMed] [Google Scholar]
- Ottaway C.A., Husband A.J. The influence of neuroendocrine pathways on lymphocyte migration. Immunol. Today. 1994;15:511–517. doi: 10.1016/0167-5699(94)90206-2. [DOI] [PubMed] [Google Scholar]
- Pacheco-Lopez G., Niemi M.B., Kou W., Harting M., Del Rey A., Besedovsky H.O., Schedlowski M. Behavioural endocrine immune-conditioned response is induced by taste and superantigen pairing. Neuroscience. 2004;129:555–562. doi: 10.1016/j.neuroscience.2004.08.033. [DOI] [PubMed] [Google Scholar]
- Pich E.M., Messori B., Zoli M., Ferraguti F., Marrama P., Biagini G., Fuxe K., Agnati L.F. Feeding and drinking responses to neuropeptide Y injections in the paraventricular hypothalamic nucleus of aged rats. Brain Res. 1992;575:265–271. doi: 10.1016/0006-8993(92)90089-r. [DOI] [PubMed] [Google Scholar]
- Plata-Salaman C.R. Immunoregulators in the nervous system. Neurosci. Biobehav. Rev. 1991;15:185–215. doi: 10.1016/s0149-7634(05)80001-6. [DOI] [PubMed] [Google Scholar]
- Plata-Salaman C.R. Cytokine action in the nervous system at pathophysiological versus pharmacological concentrations. Adv. Exp. Med. Biol. 1996;402:191–197. doi: 10.1007/978-1-4613-0407-4_25. [DOI] [PubMed] [Google Scholar]
- Plata-Salaman C.R. Anorexia during acute and chronic disease: relevance of neurotransmitter-peptide-cytokine interactions. Nutrition. 1997;13:159–160. doi: 10.1016/s0899-9007(96)00295-x. [DOI] [PubMed] [Google Scholar]
- Plata-Salaman C.R. Central nervous system mechanisms contributing to the cachexia–anorexia syndrome. Nutrition. 2000;16:1009–1012. doi: 10.1016/s0899-9007(00)00413-5. [DOI] [PubMed] [Google Scholar]
- Plata-Salaman C.R., Borkoski J.P. Interleukin-8 modulates feeding by direct action in the central nervous system. Am. J. Physiol. 1993;265:R877–R882. doi: 10.1152/ajpregu.1993.265.4.R877. [DOI] [PubMed] [Google Scholar]
- Plata-Salaman C.R., Ffrench-Mullen J.M. Intracerebroventricular administration of a specific IL-1 receptor antagonist blocks food and water intake suppression induced by interleukin-1 beta. Physiol. Behav. 1992;51:1277–1279. doi: 10.1016/0031-9384(92)90321-r. [DOI] [PubMed] [Google Scholar]
- Plata-Salaman C.R., Oomura Y., Kai Y. Tumor necrosis factor and interleukin-1 beta: suppression of food intake by direct action in the central nervous system. Brain Res. 1998;448:106–114. doi: 10.1016/0006-8993(88)91106-7. [DOI] [PubMed] [Google Scholar]
- Plata-Salaman C.R., Sonti G., Borkoski J.P., Wilson C.D., French-Mullen J.M. Anorexia induced by chronic central administration of cytokines at estimated pathophysiological concentrations. Physiol. Behav. 1996;60:867–875. [PubMed] [Google Scholar]
- Plotsky P.M., Cunningham E.T., Jr., Widmaier E.P. Catecholaminergic modulation of corticotropin-releasing factor and adrenocorticotropin secretion. Endocr. Rev. 1989;10:437–458. doi: 10.1210/edrv-10-4-437. [DOI] [PubMed] [Google Scholar]
- Porter M.H., Arnold M., Langhans W. Lipopolysaccharide-induced anorexia following hepatic portal vein and vena cava administration. Physiol. Behav. 1998;64:581–584. doi: 10.1016/s0031-9384(98)00082-1. [DOI] [PubMed] [Google Scholar]
- Porter M.H., Hrupka B.J., Altreuther G., Arnold M., Langhans W. Inhibition of TNF-alpha production contributes to the attenuation of LPS-induced hypophagia by pentoxifylline. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2000;279:R2113–R2120. doi: 10.1152/ajpregu.2000.279.6.R2113. [DOI] [PubMed] [Google Scholar]
- Posma E., Moes H., Heineman M.J., Faas M.M. The effect of testosterone on cytokine production in the specific and non-specific immune response. Am. J. Reprod. Immunol. 2004;52:237–243. doi: 10.1111/j.1600-0897.2004.00216.x. [DOI] [PubMed] [Google Scholar]
- Pu S., Dhillon H., Moldawer L.L., Kalra P.S., Kalra S.P. Neuropeptide Y counteracts the anorectic and weight reduction effects of ciliary neurotropic factor. J. Neuroendocrinol. 2000;12:827–832. doi: 10.1046/j.1365-2826.2000.00526.x. [DOI] [PubMed] [Google Scholar]
- Ramos E.J., Meguid M.M., Zhang L., Miyata G., Fetissov S.O., Chen C., Suzuki S., Laviano A. Nicotine infusion into rat ventromedial nuclei and effects on monoaminergic system. Neuroreport. 2004;15:2293–2297. doi: 10.1097/00001756-200410050-00030. [DOI] [PubMed] [Google Scholar]
- Ramos E.J.B., Middelton F.A., Laviano A., Sato T., Romanova I., Das U., Cheng C., Qi Y., Meguid M.M. Effects of omega -3 fatty acid supplementation on tumor-bearing rats. J. Am. Coll. Surg. 2004;199:716–723. doi: 10.1016/j.jamcollsurg.2004.07.014. [DOI] [PubMed] [Google Scholar]
- Ramos E.J.B., Romanova I., Suzuki S., Cheng C., Ugrumov M.V., Sato T., Goncalves C.G., Meguid M.M. Effects of omega -3 fatty acid on orexigenic and anorexigenic modulators at the onset of anorexia. Brain Res. 2005;1046:157–164. doi: 10.1016/j.brainres.2005.03.052. [DOI] [PubMed] [Google Scholar]
- Ramos E.J.B., Suzuki S., Meguid M.M., Laviano A., Sato T., Cheng C., Das U. Changes in hypothalamic neuropeptide Y and monoaminergic system in tumor-bearing rats: Pre- and post-tumor resection and death. Surgery. 2004;136:270–276. doi: 10.1016/j.surg.2004.03.016. [DOI] [PubMed] [Google Scholar]
- Ramos E.J., Xu Y., Romanova I., Middleton F., Chen C., Quinn R., Inui A., Das U., Meguid M.M. Is obesity an inflammatory disease? Surgery. 2003;134:329–335. doi: 10.1067/msy.2003.267. [DOI] [PubMed] [Google Scholar]
- Reyes T.M., Sawchenko P.E. Involvement of the arcuate nucleus of the hypothalamus in interleukin-1-induced anorexia. J. Neurosci. 2002;22:5091–5099. doi: 10.1523/JNEUROSCI.22-12-05091.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricardo J.A., Koh E.T. Anatomical evidence of direct projections from the nucleus of the solitary tract to the hypothalamus, amygdala, and other forebrain structures in the rat. Brain Res. 1978;153:1–26. doi: 10.1016/0006-8993(78)91125-3. [DOI] [PubMed] [Google Scholar]
- Rogers P., McKibbin P.E., Williams G. Acute fenfluramine administration reduces neuropeptide Y concentration in specific hypothalamic regions of the rat: possible implications for the anorectic effect of fenfluramine. Peptides. 1997;12:251–255. doi: 10.1016/0196-9781(91)90007-c. [DOI] [PubMed] [Google Scholar]
- Rola-Pleszczynski M., Lemaire I. Leukotrienes augment interleukin 1 production by human monocytes. J. Immunol. 1985;135:3958–3961. [PubMed] [Google Scholar]
- Rose D.P., Connolly J.M., Meschter C.L. Effect of dietary fat on human breast cancer growth and lung metastasis in nude mice. J. Natl. Cancer. Inst. 1991;83:1491–1495. doi: 10.1093/jnci/83.20.1491. [DOI] [PubMed] [Google Scholar]
- Rosendahl A., Hansson J., Antonsson P., Sekaly R.P., Kalland T., Dohlsten M. A mutation of F47 to A in staphylococcus enterotoxin A activates the T-cell receptor Vbeta repertoire in vivo. Infect. Immun. 1997;65:5118–5124. doi: 10.1128/iai.65.12.5118-5124.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi-George A., Urbach D., Colas D., Goldfarb Y., Kusnecov A.W. Neuronal, endocrine, and anorexic responses to the T-cell superantigen staphylococcal enterotoxin A: dependence on tumor necrosis factor-alpha. J. Neurosci. 2005;25:5314–5322. doi: 10.1523/JNEUROSCI.0687-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothwell N.J., Busbridge N.J., Lefeuvre R.A., Hardwick A.J., Gauldie J., Hopkins S.J. Interleukin-6 is a centrally acting endogenous pyrogen in the rat. Can. J. Physiol. Pharmacol. 1991;69:1465–1469. doi: 10.1139/y91-219. [DOI] [PubMed] [Google Scholar]
- Saper C.B., Chou T.C., Elmquist J.K. The need to feed: homeostatic and hedonic control of eating. Neuron. 2002;36:199–211. doi: 10.1016/s0896-6273(02)00969-8. [DOI] [PubMed] [Google Scholar]
- Sapolsky R., Rivier C., Yamamoto G., Plotsky P., Vale W. Interleukin-1 stimulates the secretion of hypothalamic corticotropin-releasing factor. Science. 1987;238:522–524. doi: 10.1126/science.2821621. [DOI] [PubMed] [Google Scholar]
- Sasaki T., Kudoh K., Uda Y., Ozawa Y., Shimizu J., Kanke Y., Takita T. Effects of isothiocyanates on growth and metastaticity of B16-F10 melanoma cells. Nutr. Cancer. 1999;33:76–81. doi: 10.1080/01635589909514751. [DOI] [PubMed] [Google Scholar]
- Sato T., Fetissov S.O., Meguid M.M., Miyata G., Chen C. Intra-supraoptic nucleus sulpiride improves anorexia in tumor-bearing rats. Neuroreport. 2001;12:2429–2432. doi: 10.1097/00001756-200108080-00028. [DOI] [PubMed] [Google Scholar]
- Sato T., Laviano A., Meguid M.M., Chen C., Rossi-Fanelli F., Hatakeyama K. Involvement of plasma leptin, insulin and free tryptophan in cytokine-induced anorexia. Clin. Nutr. 2003;22:139–146. doi: 10.1054/clnu.2002.0609. [DOI] [PubMed] [Google Scholar]
- Sato T., Meguid M.M., Fetissov S.O., Chen C., Zhang L. Hypothalamic dopaminergic receptor expressions in anorexia of tumor-bearing rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2001;281:R1907–R1916. doi: 10.1152/ajpregu.2001.281.6.R1907. [DOI] [PubMed] [Google Scholar]
- Sato T., Meguid M.M., Quinn R.H., Zhang L., Chen C. Feeding behavior during sialodacryoadenitis viral infection in rats. Physiol. Behav. 2001;72:721–726. doi: 10.1016/S0031-9384(01)00420-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawchenko P.E., Swanson L.W. Hypothalamic integration: organization of the paraventricular and supraoptic nuclei. Annu. Rev. Neurosci. 1983;6:269–324. doi: 10.1146/annurev.ne.06.030183.001413. [DOI] [PubMed] [Google Scholar]
- Sawchenko P.E., Swanson L.W., Steinbusch H.W., Verhofstad A.A. The distribution and cells of origin of serotonergic inputs to the paraventricular and supraoptic nuclei of the rat. Brain Res. 1983;277:355–360. doi: 10.1016/0006-8993(83)90945-9. [DOI] [PubMed] [Google Scholar]
- Schaechter J.D., Wurtman R.J. Serotonin release varies with brain tryptophan levels. Brain Res. 1990;532:203–210. doi: 10.1016/0006-8993(90)91761-5. [DOI] [PubMed] [Google Scholar]
- Shikhman A.R., Brinson D.C., Valbracht J., Lotz M.K. Cytokine regulation of facilitated glucose transport in human articular chondrocytes. J. Immunol. 2001;167:7001–7008. doi: 10.4049/jimmunol.167.12.7001. [DOI] [PubMed] [Google Scholar]
- Shurin G., Shanks N., Nelson L., Hoffman G., Huang L., Kusnecov A.W. Hypothalamic-pituitary-adrenal activation by the bacterial superantigen staphylococcal enterotoxin B: role of macrophages and T cells. Neuroendocrinology. 1997;65:18–28. doi: 10.1159/000127161. [DOI] [PubMed] [Google Scholar]
- Smagin G.N., Swiergiel A.H., Dunn A.J. Peripheral administration of interleukin-1 increases extracellular concentrations of norepinephrine in rat hypothalamus: comparison with plasma corticosterone. Psychoneuroendocrinology. 1996;21:83–93. doi: 10.1016/0306-4530(95)00019-4. [DOI] [PubMed] [Google Scholar]
- Smith B.K., Kluger M.J. Anti-TNF-alpha antibodies normalized body temperature and enhanced food intake in tumor-bearing rats. Am. J. Physiol. 1993;265:R615–R619. doi: 10.1152/ajpregu.1993.265.3.R615. [DOI] [PubMed] [Google Scholar]
- Smith J.W., Urba W.J., Steis R.G., Janik J.E., Fenton R.G., Sharfman W.H., Conlon K.C., Sznol M., Creekmore S.P., Wells N., Elwood L., Keller J., Hestdal K., Ewel C., Rossio J., Kopp W.C., Shimuzi M., Oppenheim J.J., Longo D.L. Phase I trial of interleukin 1 alpha (IL-1 alpha) alone and in combination with indomethacin. Lymphokine Res. 1990;9:568. [Google Scholar]
- Sonti G., Ilyin S.E., Plata-Salaman C.R. Neuropeptide Y blocks and reverses interleukin-1β-induced anorexia in rats. Peptides. 1996;17:517–520. doi: 10.1016/0196-9781(96)00016-2. [DOI] [PubMed] [Google Scholar]
- Spitzer J.A., Zhang P. Protein tyrosine kinase activity and the influence of gender in phagocytosis and tumor necrosis factor secretion in alveolar macrophages and lung-recruited neutrophils. Shock. 1996;6:426–433. doi: 10.1097/00024382-199612000-00007. [DOI] [PubMed] [Google Scholar]
- Spitzer J.A., Zhang P. Gender differences in neutrophil function and cytokine-induced neutrophil chemoattractant generation in endotoxic rats. Inflammation. 1996;20:485–498. doi: 10.1007/BF01487041. [DOI] [PubMed] [Google Scholar]
- Stadler J., Barton D., Beil-Moeller H., Diekmann S., Hierholzer C., Erhard W., Heidecke C.D. Hepatocyte nitric oxide biosynthesis inhibits glucose output and competes with urea synthesis for L-arginine. Am. J. Physiol. 1995;268:G183–G188. doi: 10.1152/ajpgi.1995.268.1.G183. [DOI] [PubMed] [Google Scholar]
- Stenlof K., Wernstedt I., Fjallman T., Wallenius V., Wallenius K., Jansson J.O. Interleukin-6 levels in the central nervous system are negatively correlated with fat mass in overweight/obese subjects. J. Clin. Endocrinol. Metab. 2003;88:4379–4383. doi: 10.1210/jc.2002-021733. [DOI] [PubMed] [Google Scholar]
- Sternson S.M., Shepherd G.M., Friedman J.M. Topographic mapping of VMH → arcuate nucleus microcircuits and their reorganization by fasting. Nat. Neurosci. 2005;8:1356–1363. doi: 10.1038/nn1550. [DOI] [PubMed] [Google Scholar]
- Sweep C.G., van der Meer M.J., Ross H.A., Vranckx R., Visser T.J., Hermus A.R. Chronic infusion of TNF-alpha reduces plasma T4 binding without affecting pituitary-thyroid activity in rats. Am. J. Physiol. 1992;263:E1099–E1105. doi: 10.1152/ajpendo.2006.263.6.E1099. [DOI] [PubMed] [Google Scholar]
- Takeyama N., Itoh Y., Kitazawa Y., Tanaka T. Altered hepatic mitochondrial fatty acid oxidation and ketogenesis in endotoxic rats. Am. J. Physiol. 1990;259:E498–E505. doi: 10.1152/ajpendo.1990.259.4.E498. [DOI] [PubMed] [Google Scholar]
- Tocci M.J., Schmidt J.A. Interleukin-1: structure and function. In: Remick D.G., Friedland J.S., editors. Cytokines in Health and Disease. second edition. Marcel Dekker, Inc.; New York: 1997. pp. 1–27. [Google Scholar]
- Torelli G.F., Meguid M.M., Miyata G., Fetissov S.O., Carter J.L., Kim H.J., Muscaritoli M., Rossi-Fanelli F. VMN hypothalamic dopamine and serotonin in anorectic septic rats. Shock. 2000;13:204–208. doi: 10.1097/00024382-200003000-00006. [DOI] [PubMed] [Google Scholar]
- Torelli G.F., Meguid M.M., Moldawer L.L., Edwards 3rd C.K., Kim H.J., Carter J.L., Laviano A., Rossi-Fanelli F. Use of recombinant human soluble TNF receptor in anorectic tumor-bearing rats. Am. J. Physiol. 1999;277:R850–R855. doi: 10.1152/ajpregu.1999.277.3.R850. [DOI] [PubMed] [Google Scholar]
- Turrin N.P., Ilyin S.E., Gayle D.A., Plata-Salaman C.R., Ramos E.J., Laviano A., Das U.N., Inui A., Meguid M.M. Interleukin, system activation in anorectic catabolic tumor-bearing rats. Curr. Opin. Clin. Nutr. Metab. Care. 2004;7:419–426. doi: 10.1097/01.mco.0000134373.16557.92. [DOI] [PubMed] [Google Scholar]
- Ulloa L. The vagus nerve and the nicotinic anti-inflammatory pathway. Nat. Rev. Drug. Discov. 2005;4:673–684. doi: 10.1038/nrd1797. [DOI] [PubMed] [Google Scholar]
- Varma M., Chai J.-K., Meguid M.M., Laviano A., Gleason J.R., Yang Z.-J., Blaha V. Effect of estradiol and progesterone on daily rhythm in food intake and feeding patterns in Fischer rats. Physiol. Behav. 1999;68:99–107. doi: 10.1016/s0031-9384(99)00152-3. [DOI] [PubMed] [Google Scholar]
- Veerabagu M.P., Opara E.I., Meguid M.M., Nandi J., Oler A., Holtzapple P.G., Levine R.A. Mode of food intake reduction in Lewis rats with indomethacin-induced ulcerative ileitis. Physiol. Behav. 1996;60:381–387. [PubMed] [Google Scholar]
- von Meyenbrg C., Langhans W., Hrupka B.J. Evidence for a role of the 5-HT2C receptor in central lipopolysaccharide-, interleukin-1β-, and leptin-induced anorexia. Pharmacol. Biochem. Behav. 2003;74:1025–1031. doi: 10.1016/s0091-3057(03)00030-3. [DOI] [PubMed] [Google Scholar]
- Wallenius K., Wallenius V., Sunter D., Dickson S.L., Jansson J.O. Intracerebroventricular interleukin-6 treatment decreases body fat in rats. Biochem. Biophys. Res. Commun. 2002;293:560–565. doi: 10.1016/S0006-291X(02)00230-9. [DOI] [PubMed] [Google Scholar]
- Wallenius V., Wallenius K., Ahren B., Rudling M., Carlsten H., Dickson S.L., Ohlsson C., Jansson J.O. Interleukin-6-deficient mice develop mature-onset obesity. Nat. Med. 2002;8:75–79. doi: 10.1038/nm0102-75. [DOI] [PubMed] [Google Scholar]
- Wang J., Griggs N.D., Tung K.S., Klein J.R. Dynamic regulation of gastric autoimmunity by thyroid hormone. Int. Immunol. 1998;10:231–236. doi: 10.1093/intimm/10.2.231. [DOI] [PubMed] [Google Scholar]
- Wang H., Liao H., Ochani M., Justiniani M., Lin X., Yang L., Al-Abed Y., Wang H., Metz C., Miller E.J., Tracey K.J., Ulloa L. Cholinergic agonists inhibit HMGB1 release and improve survival in experimental sepsis. Nat. Med. 2004;10:1216–1221. doi: 10.1038/nm1124. [DOI] [PubMed] [Google Scholar]
- Wang H., Yu M., Ochani M., Amella C.A., Tanovic M., Susarla S., Li J.H., Wang H., Yang H., Ulloa L., Al-Abed Y., Czura C.J., Tracey K.J. Nicotinic acetylcholine receptor alpha7 subunit is an essential regulator of inflammation. Nature. 2003;421:384–388. doi: 10.1038/nature01339. [DOI] [PubMed] [Google Scholar]
- Watanobe H., Yoneda M. A mechanism underlying the sexually dimorphic ACTH response to lipopolysaccharide in rats: sex steroid modulation of cytokine-binding sites in the hypothalamus. J. Physiol. 2003;547:221–232. doi: 10.1113/jphysiol.2002.032169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg E.D. Iron withholding: a defense against infection and neoplasia. Physiol. Rev. 1984;64:65–102. doi: 10.1152/physrev.1984.64.1.65. [DOI] [PubMed] [Google Scholar]
- Wellen K.E., Hotamisligil G.S. Obesity-induced inflammatory changes in adipose tissue. J. Clin. Invest. 2003;112:1785–1788. doi: 10.1172/JCI20514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H., Barnes G.T., Yang Q., Tan G., Yang D., Chou C.J., Sole J., Nichols A., Ross J.S., Tartaglia L.A., Chen H. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J. Clin. Invest. 2003;112:1821–1830. doi: 10.1172/JCI19451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yabuuchi K., Minami M., Katsumata S., Satoh M. Localization of type I interleukin-1 receptor mRNA in the rat brain. Brain Res. Mol. Brain Res. 1994;27:27–36. doi: 10.1016/0169-328x(94)90180-5. [DOI] [PubMed] [Google Scholar]
- Yang Z.J., Blaha V., meguid m.m., Laviano A., Oler A., Zadak Z. Interleukin-1α injection into ventromedial hypothalamic nucleus of normal rats depress food intake and increases release of dopamine and serotonin. Pharmacol. Biochem. Behav. 1999;1:61–65. doi: 10.1016/s0091-3057(98)00136-1. [DOI] [PubMed] [Google Scholar]
- Yang Z.J., Koseki M., Meguid M.M., Gleason J.R., Debonis D. Synergistic effect of rhTNF-alpha and rhIL-1 alpha in inducing anorexia in rats. Am. J. Physiol. 1994;267:R1056–R1064. doi: 10.1152/ajpregu.1994.267.4.R1056. [DOI] [PubMed] [Google Scholar]
- Yang Z.J., Meguid M.M. Continuous systemic interleukin-1 alpha infusion suppresses food intake without increasing lateral hypothalamic dopamine activity. Brain Res. Bull. 1995;36:417–420. doi: 10.1016/0361-9230(94)00212-j. [DOI] [PubMed] [Google Scholar]
- Yang Z.-J., Ratto C., Gleason J.R., Bellantone R., Crucitti F., Meguid M.M. Influence of anterior subdiaphragmatic vagotomy and TPN on rat feeding behavior. Physiol. Behav. 1992;51:919–926. doi: 10.1016/0031-9384(92)90071-9. [DOI] [PubMed] [Google Scholar]
- Yerkovich S.T., Rigby P.J., Fournier P.A., Olynyk J.K., Yeoh G.C. Kupffer cell cytokines interleukin-1beta and interleukin-10 combine to inhibit phosphoenolpyruvate carboxykinase and gluconeogenesis in cultured hepatocytes. Int. J. Biochem. Cell. Biol. 2004;36:1462–1472. doi: 10.1016/j.biocel.2003.10.022. [DOI] [PubMed] [Google Scholar]
- Zigman J.M., Elmquist J.K. Minireview: from anorexia to obesity — the yin and yang of body weight control. Endocrinology. 2003;144:3749–3756. doi: 10.1210/en.2003-0241. [DOI] [PubMed] [Google Scholar]


