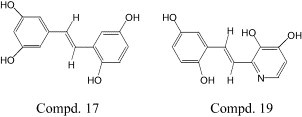
Abstract
Stilbene derivatives have wide range of activities. In an effort to find other potential activities of this kind of compounds, 17 derivatives, including resveratrol, were synthesized. Twelve of them were evaluated for their antiviral potential against severe acute respiratory syndrome (SARS)-CoV-induced cytopathicity in Vero E6 cell culture. The result showed that SARS virus was totally inhibited by compounds 17 and 19 ( ≤ 0.5 mg ml–1) and no significant cytotoxic effects were observed in vitro.
Keywords: Severe acute respiratory syndrome, Coronavirus, Stilbene derivatives
Abbreviations: CPE, cytopathic effect; SARS, severe acute respiratory syndrome
Graphical abstract
Seventeen stilbene derivatives were synthesized and 12 of them were evaluated for their antiviral potential against SARS-CoV-induced cytopathicity in Vero E6 cell culture. Compound 17 and 19 showed viral inhibition.
1. Introduction
Stilbene derivatives are widely distributed in nature, which are thought to be phytoalexins. There is a growing interest in stilbene derivatives because many activities have been observed in some of the naturally occurring as well as some of the synthetic stilbenes. Activities include antimicrobial [1], [2], [3], antioxidant [4], [5], antileukemic [6], anti-platelet aggregative [7], [8], protein tyrosine kinase inhibitory [9], anti-inflammatory [10], [11], anticarcinogenic activity [12], [13], anti-HIV [14], [15] and anti-herpes simplex virus [16]. In the course of our research for potential activities of stilbene derivatives, we designed different hydroxyl substituted sites against resveratrol and kept the trans structure to simulate this kind of phytoalexins.
In early 2003, severe acute respiratory syndrome (SARS) broke out in China and other countries. Many scholars and researchers were engaged in the search of anti-SARS agents and vaccines. At the same time, we also used this kind of compounds to the urgent antiviral filtration in vitro. The result was exciting that the change of hydroxyl group's site and the introduction of nitrogen atom were beneficial to the anti-SARS activity. Especially the substituted sites of hydroxyl groups in compounds 17 and 19 have been demonstrated to be a key structure element of anti-SARS virus.
No one knows the likelihood of evolution of SARS-CoV in human and animals. Moreover the complete understanding of pathogenesis of SARS remains tentative. In this study, we evaluated such stilbene derivatives for their potential to inhibit SARS-CoV replication for the first time and thought this discovery would be beneficial to the anti-SARS-CoV development.
2. Chemistry
In order to prepare a variety of stilbene derivatives with hydroxyl groups, we used methoxyl materials as starting materials to avoid the oxidation of hydroxyl compounds. Also we introduced one pyridine ring in place of one of the benzene rings for the purpose of evaluation the activity change. Preparation of aim compounds was accomplished as given in Scheme 1 . According to this scheme, 3,5-dimethoxybenzyl bromide, 2-chloromethyl-3, 4-dimethoxy pyridine or 2-chloromethyl-3,5-dimethyl-4-methoxypyridine were heated with triethyl phosphite in the presence of (n-Bu)4NI to give the phosphonates (2–4) (Michaelis–Arbuzov reaction [17]). In turn, the reaction of aryl aldehyde with the anion of phosphates formed in situ with sodium hydride gave (E)-stilbene derivatives (5–14) (with almost none of (Z)-isomer) together with water soluble diethyl phosphate (Wittig–Horner reaction [18], [19]). But the yield of pyridine containing derivatives is poor. Further methoxyl derivatives were demethylated with BBr3 in dichloromethane.
Scheme 1.
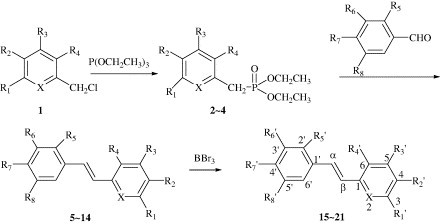
Synthesis of compounds 5–21. (a) P(OCH2CH3)3, (n-Bu)4NI, 100–120 °C; (b) methoxy benzaldehyde, NaH, THF, room temperature; (c) BBr3, CH2Cl2, 20–40 °C.
3. Biological results and discussion
In this study the antiviral potential of twelve compounds (7, 8, 10, 11, 14–21) was evaluated in vitro for their inhibitory effect of SARS virus. Cytotoxicity in Vero E6 cells was measured before the antiviral activity. The compounds that showed cytotoxity to Vero E6 were weeded out first. The remainder was studied their inhibitory activity. Only compounds 17 and 19 could inhibit the replication of SARS virus in Vero E6 cells in concentration ≤ 0.5 mg ml–1 (2.05 mM). There was no cytotoxicity to Vero E6 in concentration ≥ 2 mg ml–1 (8.20 mM) (Table 3 ). It showed that both of them could inhibit SARS virus in vitro. Compounds 17 and 19 clearly inhibited the cytopathic effect (CPE) induced by infection with SARS-CoV (Fig. 1 ). As compared with cinanserin which inhibits SARS virus at 66 μM (IC90) [20], the result in our research seems to be not so good. But the concentration 0.5 mg ml–1 is not the terminal of the inhibition, because our research could not undergo in present situation in our country.
Table 3.
Inhibitory effect of compounds 17 and 19 on SARS virus in vitro
| Compounds | Experiment number | Chemical control | Final concentration of compound (mg ml–1) |
Viral control (100TCID50) | ||
|---|---|---|---|---|---|---|
| 2 | 1 | 0.5 | ||||
| 17 | 1 | – | – | – | – | +++ |
| 2 | – | – | – | – | +++ | |
| 3 | – | – | – | – | +++ | |
| 19 | 1 | – | – | – | – | +++ |
| 2 | – | – | – | – | +++ | |
| 3 | – | – | – | – | +++ | |
– = no cytotoxicity, no CPE; +++ = CPE > 75% cells with CPE.
Fig. 1.
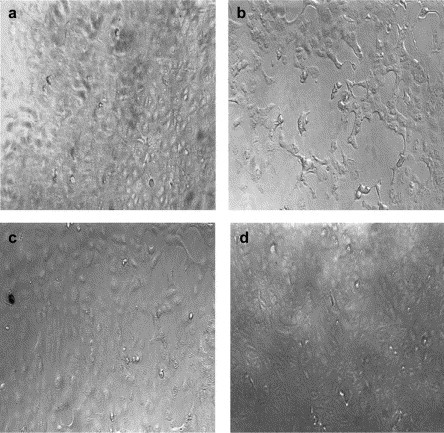
Cytopathic effect (CPE) of compounds 17 and 19 on replication of SARS-CoV in Vero E6 cells. (a) The normal Vero E6 cells were cultivated with Eagle’s medium containing 10% fetal calf serum. (b) The Vero E6 cells infected with 100TCID50 SARS virus. (c) The infected cells were treated with compound 17 for 72h. (d) The infected cells were treated with compound 19 for 72h.
The methoxy stilbene derivatives (7, 8, 10, 11, 14) showed no cytotoxity. Compounds 7 and 10, which were methyl ethers of compounds 17 and 19, respectively, could inhibit 50% replication of SARS virus in Vero E6 cells in concentration 1 mg ml–1. Compounds 8, 11 and 14 had no inhibition in the evaluation. Although compounds 17 and 19 had one same part, the compound 21 that had the same part showed no activity. It seemed that the whole molecular structure was necessary to the inhibitory activity and hydroxy group was prior to methoxy group. However, the structure–activity relationship was still unclear in our present study. Maybe the further research will reveal the relationship between the substituent and activity (Table 1, Table 2).
Table 1.
Substituent for compounds 2–14
| Compounds | X | R1 | R2 | R3 | R4 | R5 | R6 | R7 | R8 |
|---|---|---|---|---|---|---|---|---|---|
| 2 | C | OCH3 | H | OCH3 | H | ||||
| 3 | N | H | H | OCH3 | OCH3 | ||||
| 4 | N | H | CH3 | OCH3 | CH3 | ||||
| 5 | C | OCH3 | H | OCH3 | H | H | H | OCH3 | H |
| 6 | C | OCH3 | H | OCH3 | H | H | OCH3 | H | OCH3 |
| 7 | C | OCH3 | H | OCH3 | H | OCH3 | H | H | OCH3 |
| 8 | C | OCH3 | H | OCH3 | H | OCH3 | H | OCH3 | H |
| 9 | N | H | H | OCH3 | OCH3 | H | OCH3 | H | OCH3 |
| 10 | N | H | H | OCH3 | OCH3 | OCH3 | H | H | OCH3 |
| 11 | N | H | H | OCH3 | OCH3 | OCH3 | H | OCH3 | H |
| 12 | N | H | CH3 | OCH3 | CH3 | H | OCH3 | H | OCH3 |
| 13 | N | H | CH3 | OCH3 | CH3 | OCH3 | H | H | OCH3 |
| 14 | N | H | CH3 | OCH3 | CH3 | OCH3 | H | OCH3 | H |
Table 2.
Substituent for compounds 15–21
| Compounds | X | R1′ | R2′ | R3′ | R4′ | R5′ | R6′ | R7′ | R8′ |
|---|---|---|---|---|---|---|---|---|---|
| 15 | C | OH | H | OH | H | H | H | OH | H |
| 16 | C | OH | H | OH | H | H | OH | H | OH |
| 17 | C | OH | H | OH | H | OH | H | H | OH |
| 18 | N | H | H | OH | OH | H | OH | H | OH |
| 19 | N | H | H | OH | OH | OH | H | H | OH |
| 20 | N | H | CH3 | OH | CH3 | H | OH | H | OH |
| 21 | N | H | CH3 | OH | CH3 | OH | H | H | OH |
4. Conclusions
We have synthesized (E)-stilbene derivatives bearing hydroxyl groups and in some of them used pyridine ring in place of one benzene ring. Two of these compounds possessed antiviral activity against SARS in vitro and till now this has never been reported.
5. Experimental protocols
5.1. Synthesis
Melting points were determined in capillary tubes on a Buchi oil bath apparatus and uncorrected. Spectra were obtained as follows: LC/Q-Tof MS, 1H NMR spectra on Varian INOVA 400 MHz spectrometer with TMS as the internal standard, IR spectra on FT-IR Nicolet 20 spectrometer. Thin layer chromatography (TLC) was carried out on Si gel plates (60 F254, Merck).
All reagents were commercially available and used as received.
5.1.1. Diethyl [3,5-dimethoxybenzyl]phosphonate (2) [21]
Triethyl phosphate (2.7 ml, 16 mmol) was added to the 3,5-dimethoxybenzyl bromide (2.3 g, 10 mmol) containing a catalytic amount of tetrabutyl-ammonium iodine, and the mixture was heated at 110–130 °C for 5–6 h. Excess triethyl phosphite was removed by heating at 80–90 °C under vacuum (< 5 kPa) to yield 2 as a light yellow oil. 1H NMR (CDCl3) δ 1.27 (t, 3 J HH = 7.2 Hz, 6H, OCH2CH 3), 3.09 (d, 2 J HP = 21.6 Hz, 2H, PCH 2), 3.78 (s, 6H, OCH 3), 4.04 (quint, 3 J HH=3 J HP = 7.2 Hz, 4H, OCH 2CH3), 6.35 (s, 1H, Hpara of C6H4), 6.46 (s, 2H, Hortho of C6H4) ppm; IR (KBr) 2939, 2839, 1685, 1598, 1512, 1461, 1257 (vs, P=O), 1206, 1160 (w, P–O–C), 1029 (vs, P–O), 969, 835 cm−1.
5.1.2. Diethyl [3,4-dimethoxypyridine-2-methylene]phosphonate (3)
The compound was synthesized in the same manner as for 2 as a viscous orange liquid and used directly in the next step. 1H NMR (CDCl3) δ 1.28 (t, 3 J HH= 7.2 Hz, 6H, OCH2CH 3), 2.23 (d, J = 1.2 Hz, 3H, CH3), 2.32 (s, 3H, CH3), 3.42 (d, 2 J HP = 22.0 Hz, 2H, PCH 2), 3.75 (s, 3H, OCH 3), 4.09 (quint, 3 J HH = 3 J HP = 7.2 Hz, 4H, OCH 2CH3), 8.19 (s, 1H, H of C5NH) ppm; IR (KBr) 2982, 2944, 1583, 1489, 1447, 1425, 1302, 1253 (vs, P=O), 1163 (w, P–O–C), 1053 (vs, P–O), 1027 (vs, P–O), 965, 827, 781 cm−1.
5.1.3. Diethyl [3,5-dimethyl-4-methoxypyridine-2-methylene]phosphonate (4)
The compound was synthesized in the same manner as for 2 as a viscous liquid and used directly in the next step. 1H NMR (CDCl3) δ 1.30 (t, 3 J HH = 7.2 Hz, 6H, OCH2CH 3), 3.48 (d, 2 J HP = 22.4 Hz, 2H, PCH2), 3.90 (s, 3H, OCH3), 3.92 (s, 3H, OCH 3), 4.13 (quint, 3 J HH = 3 J HP= 7.2 Hz, 4H, OCH 2CH3), 6.76 (dd, 3 J HH=5.6 Hz, 6 J HP = 1.6 Hz, 1H, Hpara of C5NH2), 8.19(d, 3 J HH = 5.6 Hz, 1H, Hmeta of C5NH2) ppm; IR (KBr) 2983, 2933, 1590, 1566, 1475, 1396, 1253 (vs, P=O), 1164 (w, P–O–C), 1054 (vs, P–O), 1029 (vs, P–O), 965, 873, 850, 807, 777, 753 cm−1.
5.1.4. (E)-3,4′,5-Trimethoxystilbene (5)
The product of first step was dissolved in dry THF (20 ml) and stirred at 0–5 °C. Sodium hydride (0.6 g, 25 mmol) was added to the well-stirred phosphonate ester solution. After 30 min, the aldehyde (10 mmol) in THF (30 ml) was added dropwise, and the mixture was allowed to stir at room temperature for 8–16 h. The mixture was then cooled to 0 °C, and the excess sodium hydride was quenched with water (10 ml). The reaction mixture was then poured on ice, followed by addition of 1 M HCl to pH 6, and the product was extracted with ethyl acetate(4 × 50 ml). The organic layers were combined and washed with saturated solution of sodium chloride (2 × 30 ml). The ethyl acetate layer was dried over anhydrous sodium sulfate and evaporated. The residue was purified by recrystallization and gave 1.7 g (65.9%, total yield of the first two steps) of 5 as a slightly yellow crystal, m.p. 56–57 °C dec. 1H NMR (CDCl3) δ 3.83 (s, 9H, OCH3), 6.38 (s, 1H, H-4), 6.65 (s, 2H, H-2,6), 6.90 (d, J = 8.8 Hz, 2H, H-3′,5′), 6.91 (d, J = 16.4 Hz, 1H, H-α), 7.04 (d, J = 16.4 Hz, 1H, H-β), 7.44 (d, J = 8.8 Hz, 2H, H-2′,6′) ppm; IR (KBr) 2936, 2836, 1598, 1512, 1459, 1426, 1253, 1154, 962, 832 cm−1.
5.1.5. (E)-3,3′,5,5′-Tetramethoxystilbene (6)
The compound was synthesized in the same manner as for 5 in 83.0% yield as a white crystal, m.p. 135–136 °C dec. 1H NMR (CDCl3) δ 3.84 (s, 12H, OCH3), 6.40 (t, J = 2.0 Hz, 2H, H-4, 4′), 6.67(d, J = 2.0 Hz, 4H, H-2, 2′,6, 6′), 7.01(s, 2H, H-α, β) ppm; IR (KBr) 2999, 2939, 2839, 1594, 1462, 1428, 1207, 1194, 1151, 1065, 943, 865, 837, 825 cm−1.
5.1.6. (E)-2′,3,5,5′-Tetramethoxystilbene (7)
The compound was synthesized in the same manner as for 5 in 72.6% yield as a milk white amorphous solid, m.p. 54–55 °C dec. 1H NMR (CDCl3) δ 3.81(s, 3H, OCH3), 3.83 (s, 6H, OCH3), 3.84 (s, 3H, OCH3), 6.39 (t, J = 1.6 Hz, 1H, H-4), 6.69 (d, J = 1.6 Hz, 2H, H-2, 6), 6.79 (dd, 3 J = 8.4 Hz, 4 J = 2.8 Hz, 1H, H-4′), 6.83(d, J = 8.4 Hz, 1H, H-3′), 7.13 (d, J = 2.8 Hz, 1H, H-6′), 7.02, 7.42 each 1H (d, J = 16.4 Hz, H-α, β) ppm; IR (KBr) 3005, 2941, 2833, 1593, 1498, 1463, 1240, 1206, 1154, 1063, 1052, 966, 862, 804 cm−1.
5.1.7. (E)-2′,3,5,4′-Tetramethoxystilbene (8)
The compound was synthesized in the same manner as for 5 in 83.0% yield as a pale yellow crystal, m.p. 81–82 °C dec. 1H NMR (CDCl3) δ 3.82 (s, 9H, OCH3), 3.86 (s, 3H, OCH3), 6.36 (t, 4 J = 2.0 Hz, 1H, H-4), 6.46 (d, 4 J = 2.4 Hz, 1H, H-3′), 6.51 (dd, 4 J = 2.4 Hz, 3 J = 8.4 Hz, 1H, H-5′), 6.67 (d, 4 J = 2.0 Hz, 2H, H-2, 6), 7.49 (d, 3 J = 8.4 Hz, 1H, H- 6′), 7.36, 6.94 each 1H (d, 3 J = 16.4 Hz, 2H, H-α, β) ppm; IR (KBr) 3001, 2944, 2840, 1629, 1590, 1504, 1461, 1428, 1295, 1196, 1155, 1062, 1029, 968, 837, 818 cm−1.
5.1.8. (E)-3′,5,5′, 6-Tetramethoxystilbene-2-nitrogen (9)
The compound was synthesized in the same manner as for 5 in 23.2% yield as a pale yellow solid, m.p. 88–90 °C dec. 1H NMR (CDCl3) δ 3.83(s, 6H, OCH3), 3.88(s, 3H, OCH3), 3.93(s, 3H, OCH3), 6.43(t, J = 2.0 Hz, 1H, H-4′), 6.78(d, J = 2.0 Hz, 2H, H-2′, 6′), 6.75 (d, J = 5.2 Hz, 1H, H-4), 8.27 (d, J = 5.2 Hz, 1H, H-3), 7.47, 7.71 each 1H (d, J = 16.0 Hz, 2H, H-α, β) ppm; IR (KBr) 2927, 2836, 1597, 1476, 1458, 1423, 1280, 1270, 1203, 1158, 1148, 980, 818 cm−1.
5.1.9. (E)-2′,5,5′, 6-Tetramethoxystilbene-2-nitrogen (10)
The compound was synthesized in the same manner as for 5 in 20.7% yield as a pale yellow crystal, m.p. 100–101 °C dec. 1H NMR (CDCl3) δ 3.82(s, 3H, OCH3), 3.85(s, 3H, OCH3), 3.87(s, 3H, OCH3), 3.92 (s, 3H, OCH3), 6.73 (d, 3 J = 5.6 Hz, 1H, H-4), 7.23 (d, 4 J = 2.4 Hz, 1H, H-6′), 6.83 (dd, 3 J = 8.8 Hz, 4 J = 2.4 Hz, 1H, H-4′), 6.85 (d, 3 J = 8.8 Hz, 1H, H-3′), 8.28 (d, 3 J= 5.6 Hz, 1H, H-3), 8.08, 7.52 each 1H (d, 3 J = 16.4 Hz, 2H, H-α, β) ppm; IR (KBr) 2995, 2944, 2833, 1629, 1573, 1495, 1286, 1241, 1208, 1069, 994, 978, 854, 817, 802 cm−1.
5.1.10. (E)-2′,5,4′, 6-Tetramethoxystilbene-2-nitrogen (11)
The compound was synthesized in the same manner as for 5 in 27.5% yield as a white amorphous solid, m.p. 131–133 °C dec. 1H NMR (CDCl3) δ 3.84 (s, 3H, OCH3), 3.86 (s, 3H, OCH3), 3.87 (s, 3H, OCH3), 3.91 (s, 3H, OCH3), 6.47 (d, 4 J = 2.0 Hz, 1H, H-3′), 6.52 (dd, 3 J = 8.4, 4 J = 2.0 Hz, 1H, H-5′), 6.70 (d, 3 J = 5.2 Hz, 1H, H-4), 7.61 (d, 3 J = 8.4 Hz, 1H, H-6′), 8.27 (d, 3 J = 5.2 Hz, 1H, H-3), 7.43, 8.03 each 1H (d, 3 J = 16.4 Hz, 2H, H-α, β) ppm; IR (KBr) 2961, 2940, 2837, 1624, 1602, 1573, 1504, 1478, 1290, 1278, 1207, 1063, 1031, 998, 939, 821 cm−1.
5.1.11. (E)-4,6-Dimethyl-3′,5,5′-trimethoxystilbene-2-nitrogen (12)
The compound was synthesized in the same manner as for 5 in 34.7% yield as a white needle crystal, m.p. 94–95 °C dec. 1H NMR (CDCl3): δ 2.27 (s, 3H, CH3), 2.35 (s, 3H, CH3), 3.77 (s, 3H, OCH3), 3.83 (s, 6H, OCH3), 6.42 (t, 4 J = 2.0 Hz, 1H, H-4′), 6.75 (d, 4 J = 2.0 Hz, 2H, H-2′, 6′), 8.26 (s, 1H, H-3), 7.29, 7.63 each 1H (d, 3 J = 15.6 Hz, 2H, H-α, β) ppm; IR (KBr) 2959, 2936, 2838, 1632, 1592, 1465, 1266, 1207, 1159, 1071, 994, 964, 819 cm−1.
5.1.12. (E)-4,6-Dimethyl-2′,5,5′-trimethoxystilbene-2-nitrogen (13)
The compound was synthesized in the same manner as for 5 as a golden oil; 1H NMR (CDCl3) δ 2.24 (s, 3H, CH3), 2.33 (s, 3H, CH3), 3.74 (s, 3H, OCH3), 3.79 (s, 3H, OCH3), 3.83 (s, 3H, OCH3), 6.79 (dd, 3 J = 9.0 Hz, 4 J = 2.8 Hz, 1H, H-4′), 6.83 (d, 3 J = 9.0 Hz, 1H, H-3′), 7.16(d, 4 J = 2.8 Hz, 1H, H-6′), 8.25(s, 1H, H-3), 7.37, 7.95 each 1H (d, J = 16.0 Hz, 2H, H-α, β) ppm; IR (KBr) 2939, 2834, 1629, 1581, 1551, 1496, 1469, 1277, 1220, 1076, 1045, 1027, 978, 880, 853, 803 cm−1.
5.1.13. (E)-4,6-Dimethyl-2′,5,4′-trimethoxystilbene-2-nitrogen (14)
The compound was synthesized in the same manner as for 5 in 34.7% yield as a yellow needle crystal, m.p. 108–110.5 °C dec. 1H NMR (CDCl3): δ 2.25 (s, 3H, CH3), 2.33(s, 3H, CH3), 3.75 (s, 3H, OCH3), 3.84 (s, 3H, OCH3), 3.87 (s, 3H, OCH3), 6.51 (dd, 3 J = 8.4 Hz, 4 J = 2.0 Hz, 1H, H-5′), 6.47 (d, 4 J = 2.0 Hz, 1H, H-3′), 7.53 (d, 3 J = 8.4 Hz, 1H, H-6′), 8.25 (s, 1H, H-3), 7.30, 7.90 each 1H (d, 3 J = 15.6 Hz, 2H, H-α, β) ppm; IR (KBr) 2993, 2938, 2837, 1620, 1604, 1580, 1506, 1469, 1296, 1279, 1209, 1104, 1074, 1033, 1004, 986, 943, 818 cm−1.
5.1.14. (E)-3,4′,5-Trihydroxystilbene (15)
Boron tribromide (3.4 ml, 36 mmol) in CH2Cl2 (20 ml) was added to a stirred solution of 5 (4 mmol) in CH2Cl2 (50 ml) at –4 to 0 °C. The mixture was allowed to warm to 25–35 °C, stirred for 3 h, then poured into ice-water, and extracted with ethyl acetate (80 ml × 3). The organic layers were combined and washed with saturated solution of sodium chloride (1 × 30 ml). The ethyl acetate layer was dried over anhydrous sodium sulfate and evaporated. The residue was purified by recrystallization and gave 0.7–0.9 g (75.0–90.2%) of 15 as amorphous solid, m.p. 251–252 °C dec [2]. 1H NMR (DMSO-d6) δ 6.23 (s, 1H, H-4), 6.44 (s, 2H, H-2,6), 6.74 (d, 3 J = 16.4 Hz, 1H, H-α), 6.90 (d, 3 J = 16.4 Hz, 1H, H-β), 6.80 (d, 3 J = 8.2 Hz, 2H, H-3′,5′), 7.31(d, 3 J = 8.2 Hz, 2H, H-2′,6′) ppm; IR (KBr) 3298, 1606, 1589, 1514, 1445, 1327, 1249, 1154, 1010 ,988, 966, 832 cm−1.
5.1.15. (E)-3,3′,5,5′-Tetrahydroxystilbene (16)
The compound was synthesized in the same manner as for 15 in 96.0% yield as pale yellow crystal, m.p. > 300 °C dec [2]. 1H NMR (DMSO-d6) δ 6.11(s, 2H, H-4,4′), 6.36(d, 4 J = 1.6 Hz, 4H, H-2,2′,6, 6′), 6.79(s, 2H, H-α, β) ppm; IR (KBr) 3538, 3449, 3240, 1602, 1516, 1462, 1387, 1346, 1312, 1259, 1158, 1005, 966, 956, 836 cm−1.
5.1.16. (E)-2′,3,5′,5-Tetrthydroxystilbene (17)
The compound was synthesized in the same manner as for 15 in 84.5% yield as pale pink amorphous solid, m.p. 212–214 °C dec. 1H NMR (DMSO-d6) δ 6.29 (s, 1H, H-4), 6.53 (d, 4 J = 2.0 Hz, 2H, H-2, 6), 6.60 (m, 1H, H-4), 6.71 (d, 3 J = 8.4 Hz, 1H, H-3), 6.99 (d, 4 J = 2.8 Hz, 1H, H-6), 7.34, 6.87 each 1H (d, 3 J = 16.4 Hz, 2H, H-α, β) ppm; IR (KBr) 3589, 3359, 1621, 1599, 1507, 1493, 1476, 1351, 1340, 1202, 1163, 1148, 989, 966, 862, 831, 810 cm−1; API-ES: 243 (M – H+), 279 (M + Cl–), 487 (2M – H+), 523 (2M + Cl–). Q-TOFMS m/z [M + 1]+ 245.0811 (calculated for C14H13O4, 245.0814).
5.1.17. (E)-3′,5,5′,6-Tetrhydroxystilbene-2-nitrogen (18)
The compound was synthesized in the same manner as for 15 in 98.9% yield as pale yellow amorphous solid, m.p. > 300 °C; 1H NMR (DMSO-d6) δ 6.17(d, 4 J = 2.2 Hz, 2H, H-2′, 6′), 6.22 (t, 4 J = 2.2 Hz, 1H, H-4′), 7.51 (d, 3 J = 6.5 Hz, 1H, H-3), 6.18 (d, 3 J = 6.5 Hz, 1H, H-4), 6.99, 7.54 each 1H (d, 3 J = 16.5 Hz, 2H, H-α, β) ppm; IR (KBr) 3414, 3330, 1620, 1609, 1597, 1555, 1507, 1446, 1356, 1144, 1107, 1063, 1008, 991, 962, 825, 794, 734, 680 cm−1. API-ES: 244 (M – H+), 280 (M + Cl–), 489 (2M – H+), 525 (2M + Cl–). Q-TOFMS m/z [M + 1]+ 246.0767 (calculated for C13H12NO4, 246.0766).
5.1.18. (E)-2′,5,5′,6-Tetrahyroxystilbene-2-nitrogen (19)
The compound was synthesized in the same manner as for 15 in 99.7% yield as pale yellow amorphous solid, m.p. > 300 °C dec. 1H NMR (DMSO-d6)δ 6.90(d, 3 J = 8.8 Hz, 1H, H-3′), 6.73(d, 4 J = 3.0 Hz, 1H, H-6′), 6.58(dd, 4J = 3.0 Hz, 3 J = 8.8 Hz, 1H, H-4′), 7.47(d, 3 J = 6.7 Hz, 1H, H-3), 6.18(d, 3 J = 6.7 Hz, 1H, H-4), 7.16, 7.54 each 1H (d, 3 J = 16.9 Hz, 2H, H-α, β) ppm; IR (KBr) 3246, 3145, 2690, 1639, 1603, 1503, 1414, 1373, 1243, 1211, 1004, 980, 969, 857, 842, 816 cm−1; API-ES: 244 (M – H+), 280 (M + Cl–), 489 (2M – H+), 525 (2M + Cl–). Q-TOFMS m/z [M + 1]+ 246.0777 (calculated for C13H12NO4, 246.0766).
5.1.19. (E)-4,6-Dimethyl-3′,5,5′-trihydroxystilbene-2-nitrogen (20)
The compound was synthesized in the same manner as for 15 in 94.0% yield as pale yellow amorphous solid, m.p. 260 °C (oxy.) dec. 1H NMR (DMSO-d 6) δ 1.91 (s, 3H, CH3), 2.06 (s, 3H, CH3), 6.47 (d, 4 J = 2.2 Hz, 2H, H-2′, 6′), 6.23 (t, 4 J = 2.2 Hz, 1H, H-4′), 7.56 (s, 1H, H-3), 7.05, 7.11 each 1H (d, 3 J = 16.6 Hz, 2H, H-α, β) ppm; IR (KBr) 3239, 1624, 1594, 1478, 1442, 1377, 1343, 1310, 1281, 1157, 1091, 1013, 997, 961, 834 cm−1; API-ES: 292(M + Cl–), 513 (2M – H+), 549 (2M + Cl–). Q-TOFMS m/z [M + 1]+ 258.1136 (calculated for C15H16NO3, 258.1130).
5.1.20. (E)-4,6-Dimethyl-2′,5,5′-trihydroxystilbene-2-nitrogen (21)
The compound was synthesized in the same manner as for 15 in 62.8% yield as pale yellow amorphous solid, m.p. 240 °C (oxy.) dec. 1H NMR (DMSO-d6) δ 1.89(s, 3H, CH3), 2.03(s, 3H, CH3), 6.72 (d, 3 J = 8.5 Hz, 1H, H-3′), 6.95 (d, 4 J = 3.0 Hz, 1H, H-6′), 6.61 (dd, 4 J = 3.0 Hz, 3 J = 8.5 Hz, 1H, H-4′), 7.47 (s, 1H, H-3), 7.14, 7.39 each 1H (d, 3 J = 16.6 Hz, 2H, H-α, β) ppm; IR (KBr) 3313, 1641, 1606, 1589, 1537, 1500, 1446, 1379, 1279, 1238, 1173, 1092, 970, 823 cm−1; MS: 258(M + H); Q-TOFMS m/z [M + 1]+ 258.1141 (calculated for C15H16NO3, 258.1130).
5.2. Assay method
Vero E6 cells were cultured in our laboratory, College of Life Science and Bioengineering, Beijing University of Technology. BJ 9-2b SARS virus was provided by Institute of Microbiology and Epidemiology, Academy of Military Medical Sciences. Vero E6 cells (1.2 × 104 cells per well) were inoculated in triplicate in 200 μl of Eagle's medium containing 10% fetal calf serum in a 96 wells plate. After 2 days cultivation, the cells grew to a thin layer, then the cells were infected with 100 TCID50 SARS virus in 0.1 ml medium and incubated at 37 °C, 5% CO2, humidified incubator for 2 hours. After a 2 h adsorption, the supernatant was discarded to remove the free virus. Chemicals in a series of 2 × dilation in 0.2 ml medium were added into the cell wells, the chemical control without virus and the viral control without chemical in 0.2 ml medium were also added into the cell wells, they were then cultivated in the same incubator for 3 days and the CPE of cells were recorded.
References
- 1.Wieslaw P., Bogdan K. IL Farmaco. 1999;54:584–587. [Google Scholar]
- 2.Ali M.A., Kondo K., Tsuda Y. Chem. Pharm. Bull. (Tokyo) 1992;40:1130–1136. [Google Scholar]
- 3.Boonlaksiri C., Oonanant W., Kongsaeree P., Kittakoop P., Tanticharoen M., Thebtaranonth Y. Phytochem. 2000;54:415–417. doi: 10.1016/s0031-9422(00)00074-1. [DOI] [PubMed] [Google Scholar]
- 4.Cai Y.J., Fang J.G., Ma L.P., Yang L., Liu Z.L. Biochim. Biophys. Acta. 2003;1637:31–38. doi: 10.1016/s0925-4439(02)00174-6. [DOI] [PubMed] [Google Scholar]
- 5.Matsuda H., Morikawa T., Toguchida I., Park J.Y., Harima S., Yoshikawa M. Bioorg. Med. Chem. 2001;9:41–50. doi: 10.1016/s0968-0896(00)00215-7. [DOI] [PubMed] [Google Scholar]
- 6.Zheng J.B., Ramirez V.D. Biochem. Biophys. Res. Commun. 1999;261:499–503. doi: 10.1006/bbrc.1999.1063. [DOI] [PubMed] [Google Scholar]
- 7.Orsini F., Pelizzoni F., Verotta L., Aburjai T. J. Nat. Prod. 1997;60:1082–1087. doi: 10.1021/np970069t. [DOI] [PubMed] [Google Scholar]
- 8.Aburjai T.A. Phytochem. 2000;55:407–410. doi: 10.1016/s0031-9422(00)00341-1. [DOI] [PubMed] [Google Scholar]
- 9.Thakkar K., Geahlen R.L., Cushman M. J. Med. Chem. 1993;36:2950–2955. doi: 10.1021/jm00072a015. [DOI] [PubMed] [Google Scholar]
- 10.G.H. Chen, W. Liu, J.X. Li, W.M. John, P.C.T. Application, 2001 WO 0142231.
- 11.G.H. Chen, W. Liu, J.X. Li, W.M. John, P.C.T. Application, 2002 WO 02057219.
- 12.Jiang M.S., Cai L.N., Udeani G.O., Slowing K.V., Thomas C.F., Beecher C.W.W., Fong H.H.S., Farnsworth N.R., Kinghorn A.D., Mehta R.G., Moon R.C., Pezzuto J.M. Science, 1997;275:218–220. doi: 10.1126/science.275.5297.218. [DOI] [PubMed] [Google Scholar]
- 13.Yang L.M., Lin S.J., Hsu F.L., Yang T.H. Bioorg. Med. Chem. Lett. 2002;12:1013–1015. doi: 10.1016/s0960-894x(02)00092-6. [DOI] [PubMed] [Google Scholar]
- 14.Wang X., Heredia A., Song H., Zhang Z., Yu B., Davis C., Redfield R. J. Pharm. Sci. 2004;93:2448–2457. doi: 10.1002/jps.20156. [DOI] [PubMed] [Google Scholar]
- 15.Likhitwitayawuid K., Sritularak B., Benchanak K., Lipipun V., Mathew J., Schinazi R.F. Nat. Prod. Res. 2005;19:177–182. doi: 10.1080/14786410410001704813. [DOI] [PubMed] [Google Scholar]
- 16.Docherty J.J., Fu M.M.H., Stiffler B.S., Limperos R.J., Pokabla C.M., DeLucia A.L. Antivir. Res. 1999;43:135–145. doi: 10.1016/s0166-3542(99)00042-x. [DOI] [PubMed] [Google Scholar]
- 17.Bhatacharya A.K., Thyagarajan G. Chem. Rev. 1981;81:415–430. [Google Scholar]
- 18.Piechucki C. Synthesis 3. 1976:187–188. [Google Scholar]
- 19.Baker R., Sims R.J. Synthesis 2. 1981:117. [Google Scholar]
- 20.Chen L., Gui C., Luo X., Yang Q., Günther S., Scandella E., Drosten C., Bai D., He X., Ludewig B., Chen J., Luo H., Yang Y., Yang Y., Zou J., Thiel V., Chen K., Shen J., Shen X., Jiang H. J. Virol. 2005;11:7095–7103. doi: 10.1128/JVI.79.11.7095-7103.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Murias M., Handler N., Erker T., Pleban K., Ecker G., Saiko P., Szekeres T., Jäger W. Bioorg. Med. Chem. 2004;12:5571–5578. doi: 10.1016/j.bmc.2004.08.008. [DOI] [PubMed] [Google Scholar]


