Abstract
The United States health care system and patient populations have changed substantially over the past several decades. The practice of infection control also has evolved since the landmark Study on the Efficacy of Nosocomial Infection Control project, and infection control professionals (ICPs) must continue to develop the knowledge and skills necessary to practice infection prevention and control. Practice analyses of infection control conducted between 1982 and 2001 were analyzed to determine changes in practice. These data reflect a 145% increase in infection control activities over a 20-year period. However, resources for infection control and prevention have not kept pace with this increased activity. In addition, the current trend toward mandatory reporting of health care-associated infections (HAIs) among several states will add more tasks for ICPs with limited resources, at the risk of spending less time on prevention and control activities. In keeping with its philosophy of quality health care and responsible public reporting, the Association of Professionals in Infection Control and Epidemiology, Inc, continues to explore the issue of mandatory reporting of HAIs.
The major infection control movement emerged in the United States in the 1970s. And by the mid-1970s, thousands of hospitals throughout the country had infection surveillance and control programs (ISCPs) in place. However, it was not until the landmark Study on the Efficacy of Nosocomial Infection Control (SENIC), which was conducted by the Centers for Disease Control (CDC) in the mid-1970s, that the link between ISCPs and the reduction of nosocomial infections (NIs) in acute care facilities was established.1 The SENIC demonstrated that effective ISCPs were associated with a 32% reduction in NIs.2 The SENIC project found that, prior to 1970, very few hospitals in the United States had an infection control professional (ICP), but, by 1977, 80% of the hospitals had at least 1 ICP.3 In the early 1970s, the CDC recommended that hospitals have at least 1 full-time equivalent (FTE) ICP for every 250 beds4; however, the SENIC found that only 28% of the ICPs surveyed worked full-time specifically on infection control activities, and the amount of time was related to hospital size, with an overall average of 24 hours per week.3 The extensive 1976 revisions to the Joint Commission on Accreditation of Hospitals (JCAH) guidelines for infection control, which formally recommended a position responsible for surveillance, added further impetus to include ICP positions to hospital infection control programs.5
Until the SENIC, there were no published studies that assessed the dimensions of infection control practice; therefore, the SENIC project included a study of the role of the ICP. Nearly all (94%) of ICPs surveyed in 1976-1977 were registered nurses (RNs), with two thirds of these having a diploma in nursing, one fourth a baccalaureate degree, and 8% an associate degree. The SENIC also identified 5 practice activities among ICPs: surveillance, policy development, training, epidemic investigations, and consulting3 ( Table 1). However, there were no certification standards for the ICP at the time of the SENIC project.
Table 1.
Evolution of infection control practice 1976-2002
| SENIC project |
CBIC practice analyses |
||||
|---|---|---|---|---|---|
| 1976∗ | 1982† | 1987‡ | 1992§ | 1996¶ | 2001# |
| Practice dimensions: n = 5 | Practice dimensions: n = 8 | Practice dimensions: n = 8 | Practice dimensions: n = 5 | Practice dimensions: n = 5 | Practice dimensions: n = 6 |
| Number of tasks: N/A | Number of tasks: 60 | Number of tasks: 67 | Number of tasks: 95 | Number of tasks: 127 | Number of tasks: 147 |
| Surveillance | Patient care practices | Patient care practices | Infectious process | Identification of infectious disease processes | Identification of infectious disease processes |
| Policy development | Infectious diseases | Infectious diseases | Surveillance/epidemiologic investigation | Surveillance/epidemiologic investigation | Surveillance/epidemiologic investigation |
| Training | Epidemiology and statistics | Epidemiology and statistics | Transmission of infection | Preventing/controlling transmission of infectious agents | Preventing/controlling transmission of infectious agents |
| Epidemic investigations | Microbiologic practices | Microbiologic practices | Management/communication | Program management/communication | Program management/communication |
| Consulting | Sterilization/disinfection | Sterilization/disinfection | Education | Education | Education and research |
| Education | Education | Infection control aspects of employee health | |||
| Employee health services | Employee health services | ||||
| Management/communication | Management/communication | ||||
SENIC, Study on the Efficacy of Nosocomial Infection Control; CBIC, Certification Board of Infection Control.
Emori et al, 1980.
Shannon et al, 1984.
Larson et al, 1988.
Bjerke et al, 1993.
Turner et al, 1999.
Goldrick et al, 2002.
The Association for Practitioners in Infection Control, Inc. (APIC), a multidisciplinary organization, was established in 1972 to meet the education and practice needs of ICPs in the United States. The name was changed to the Association for Professionals in Infection Control and Epidemiology, Inc, in 1994 to recognize the organization's maturation and evolution into the broader context of health care delivery in the United States.6 APIC's sister organization, Community and Hospital Infection Control Association-Canada (CHICA-Canada), was incorporated in 1976 for ICPs practicing in Canada.7
The APIC developed 8 educational standards for infection control practice in 1980, which included epidemiology; microbiology; infectious diseases; sterilization, disinfection, and sanitation; patient care practices; education; management and communication; and employee health.8 These standards were consolidated into The APIC Curriculum for Infection Control practice, which was published in 1983.9
Practice analyses for infection control practice: 1982-2001
The first infection control practice analysis (PA) was conducted in 1982 at the request of the Certification Board of Infection Control (CBIC), which was established in 1981.10 The first PA survey collected demographic data and data from a Task Inventory Rating Scale, which consisted of 99 task statements, categorized according to the 8 Educational Standards of APIC.8 A randomized stratified sample of 600 ICPs who would receive the PA questionnaire was deliberately skewed toward larger hospitals (>100 beds) because the PA committee felt that these hospitals would most likely employ ICPs who performed the full range of infection control activities. Based on inclusion/exclusion criteria, a total of 317 ICP respondents' data were used for analysis to determine key tasks for infection control practice.11 Most (88%) of respondents in the 1982 PA survey were RNs who worked in community hospitals (78%) with >200 beds (73%). All but 4% of the respondents' hospitals were accredited by the JCAH.12 Although the majority of the respondents were full-time employees, 17% spent less than 20 hours/week in infection control, and many held multiple positions within the hospital; however, 54% of the ICPs surveyed indicated that infection control was their primary responsibility. The majority (72%) of respondents had been in infection control practice between 2 and 10 years, and nearly all (96%) had attended educational programs in infection control. Sixty tasks (activities) related to the 8 areas/dimensions of infection control practice were identified as relevant in the 1982 PA11 (Table 1). The 1982 PA was the basis for the first infection control certification examination, which was offered in the United States in 1983.11 It also defined and described the scope of infection control practice for the first time and established a baseline for measuring progress and changes in practice.11 Recertification for ICPs is required every 5 years; therefore, the CBIC repeats the PA every 5 years.10
By the 1980s, infection control practice had expanded beyond the acute care setting. Infection control practice also had changed considerably. New infectious diseases such as acquired immunodeficiency syndrome had emerged, prospective payment systems for hospitals were in place, and the Joint Commission on Accreditation of Health Care Organizations (JCAHO; formerly JCAH) instituted outcome measures as part of the accreditation process. These changes required new or enhanced skills for the ICP. Therefore, in 1987, 5 years after the first infection control PA, a study was conducted to update and revalidate the original (1982) PA to ensure that the content of the certification examination was a valid representation of infection control practice. A modified Delphi technique was used between 2 panels of ICPs and further validated by a third panel of subject matter experts for a total of 29 ICPs. There was a high level of agreement between respondents on the 1982 PA, the 1987 ICPs, and the expert panel, providing content validity for the certification examination based on current infection control practice. Eight new tasks (activities) were identified for a total of 67 tasks in 8 areas (dimensions) of infection control practice (Table 1). These additional tasks “reflect[ed] an expanding role of the ICP as a planner and manager.”13
The CBIC conducted its second PA survey among ICPs in 1992, which included a random sample of Canadian ICPs for the first time. The original 1982 PA list was used with modifications to reflect current infection control practice and terminology. A total of 577 responses were available for analysis. The majority (87%) of respondents were RNs; 9% held an associate degree; 44% held a baccalaureate degree; and 24% held an advanced degree.14 The majority of respondents had 9 years or more in infection control practice (56%), worked in hospitals with greater than 200 beds (64%) less than 40 hours a week (52%), and were certified in infection control (72%). Ninety-five tasks identified in the 1992 PA were organized into 5 major practice dimensions describing the responsibilities of ICPs in the United States and Canada: infectious process, surveillance/epidemiologic investigation, transmission of infection, management and communication, and education14 (Table 1); however, new tasks were added, and outdated tasks were eliminated.14
Each PA builds on those conducted previously and is an important component of ensuring content validity that reflects current infection control practice. This process of content validation involves systematic collection of information that describes behaviors and activities (tasks) performed by occupants of the practice in question.15 Because of the numerous changes in health care delivery, which affected the scope and practice of infection control since the 1992 PA, a more contemporary analysis of infection control practice was needed in the 1996 PA survey conducted by the CBIC. The application of decision rules ensured that the resulting certification examination accurately reflected infection control practice in the United States and Canada, regardless of the region or size of facility. A representative sample across all health care settings was selected from the APIC and the CHICA-Canada ICPs to receive the 1996 PA questionnaire.16 A return of 1530 responses significantly exceeded the N = 1067 required by power analysis. As in past PA surveys, the majority (85%) of respondents in the 1996 survey were RNs. Thirteen percent of respondents held an associate degree; 41% held a baccalaureate degree; and 22% had an advanced degree.16 The majority of respondents in the 1996 PA survey worked in acute care settings (65%), worked in facilities with greater than 100 beds (68%), and worked less than 40 hours per week in infection control (62%). Nineteen percent of the respondents worked in long-term care (14%), mental health facilities (3%), and rehabilitation centers (2%). Thirty-five percent of the respondents had 10 or more years of experience in infection control practice, but less than half (48%) were certified in infection control.16 The completed inventory on the 1996 PA resulted in127 tasks organized into 5 major practice areas,16 as listed in Table 1.
The APIC/CHICA-Canada Infection Control and Epidemiology: Professional and Practice Standards were published in 1999.17 The document has the following 2 sections: (1) professional standards that the ICP is expected to meet or exceed and (2) practice standards that the ICP is capable of meeting, regardless of applicability to the specific practice setting. These professional and practice standards were synthesized into the most current PA survey, which was conducted by the CBIC in 2001, to ensure that the resulting content outline was consistent with current standards of infection control practice. The primary source for the 2001 PA survey questionnaire was the 1997 Infection Control Professional Task List.16 The target population for the survey consisted of ICPs in the United States and Canada who met the eligibility requirements for taking the certification examination.
A final sample of 1306 responses were available for analysis in the 2001 PA survey, which significantly exceeded the N = 1067 required by power analysis. Therefore, results from this sample could be generalized to ICPs in the United States and Canada. In the continuing process of serving the international infection control community, the survey instrument also was distributed to a selected sample of international ICPs.18 Table 1 outlines the 6 major areas of infection control practice identified in the 2001 PA. Two changes in the major areas of practice were made. These included a category that incorporates both education and research. Research had not been considered in previous PAs; however, because there were only 5 tasks associated with the “research” category, it was combined with the “education” category, and the category was renamed “Education and Research.” Several new tasks associated with employee health also were identified. As a result, all aspects of employee health were separated into a new category: “Infection Control Aspects of Employee Health,” which had previously been included in the category that addressed preventing and controlling the transmission of infectious agents.18 Table 1 indicates that 147 tasks related to the 6 areas of infection control practice were identified in the 2001 PA.
The majority of respondents in the 2001 PA survey was RNs (80%), held a baccalaureate degree or advanced degree (73%), and was certified in infection control (84%). More than half (55%) of the respondents had been in infection control practice more than 10 years. Most of the respondents (56%) worked in an acute care hospital with ≥200 beds (51%) with ≤1 full-time equivalent ICP (59%), and nearly half (49%) worked 40 or more hours a week. Eight percent of the respondents worked in long-term care (compared with 14% in 1996), 4% worked in public health, and 2% worked in mental health facilities.18
The 2001 PA reflected changes in the practice of infection prevention/control and applied epidemiology at that time and identified the responsibilities of the ICP both in the United States and in Canada.18 However, several new infection control activities have been identified since 2001. To remain current, the CBIC will conduct its next PA in 2006, which will include tasks related to changes in infection control practice since 2001.
Table 1 illustrates that the practice of infection control evolved significantly between 1976 and 2001. Figure 1 shows the increase in infection control tasks from 60 to 147 between 1982 and 2001, a 145% increase over a 20-year period, with little or no additional resources allotted to infection control programs during that time. Although the 2001 PA survey18 was undertaken early in 2001, long before the September 11, 2001, attacks on the United States and the Department of Homeland Security was formed, the CDC developed a plan to upgrade the nation's public health infrastructure to respond to acts of biological terrorism.19 In 1999, the APIC and the CDC developed a bioterrorism readiness plan “… to serve as a tool for [ICPs] and health care epidemiologists to guide the development of practical and realistic response plans for their institutions in preparation for a real or suspected bioterrorism attack.”20 As a result, many ICPs across the United States developed policies and procedures based on the APIC and the CDC recommendations to prepare their health care facilities to deal with bioterrorism, and, although bioterrorism was not a separate infection control practice category listed in the 2001 PA,18 the tasks related to attacks of biologic warfare are another layer in the practice of infection control, which deals with the prevention and control of the transmission of infectious agents (Table 1). Since the September 11 attacks, there has been a substantial increase in ICPs involvement in emergency preparedness and management for bioterrorist attacks and emerging infections. For example, the recent emergence of severe acute respiratory syndrome (SARS) and the threat of a new influenza pandemic are further examples of the need for due diligence in infection control. The main lesson learned from the SARS outbreak was that it was contained through the conscientious application of enhanced infection control measures at the national and local levels. These same measures will defeat SARS should it reemerge. Control of an emerging infection requires swift action by health care providers and an adequate public health infrastructure.21
Fig 1.
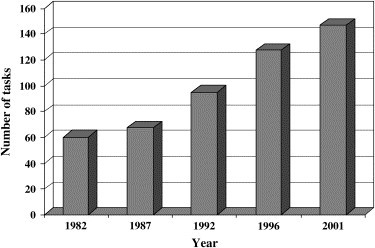
Increase in infection control tasks by year.
Changes and regulations affecting infection control practice
As change in the health care system continues, infection control practice also must evolve. ICPs are instrumental in ensuring that their health care facilities comply with new regulations and guidelines, which include education of health care workers on proper hand hygiene, prevention of needlestick injuries, isolation techniques, and appropriate use of personal protective equipment, and are prepared for emerging and reemerging infectious diseases. In addition, changes in the US health care infrastructure occurred during the past 20 years when many hospitals merged or became part of multihospital health systems that included health care facilities across the continum of care.
These changes in the organization and delivery of health care services prompted an expansion of the role of many ICPs to include not only the responsibility for infection control programs in acute care and/or long-term care facilities but the added responsibility for nonacute health services such as freestanding surgery centers, medical and dental clinics, rehabilitation units, child and adult day care centers, and home care. However, ICPs report that resources for the infection control staff have remained static despite the need to respond to emerging infections and implementation of new regulations and guidelines. Emergence of multidrug-resistant microorganisms in all health care facilities also has necessitated increased ICP activity.22 O'Boyle et al used a Delphi method to study infection control tasks in addition to those found in the 1996 PA, along with additional responsibilities.22 A panel of experts (n = 32) from 20 states, who represented ICPs in acute care, long-term care, and community settings, identified 14 new tasks added to the 127 listed in the 1996 PA. The Delphi panel also estimated the percentage of time used in the major infection control practice domains.22 The activity with the greatest average estimated time was surveillance (27%), followed by education (16%), prevention (14%), and communication (14%). The activity with the least average estimated time was control measures (8%). Lack of adequate resources was seen as influencing ICPs' ability to perform tasks across all infection control functions.22 The panelists in the study determined that a ratio of 0.8 to 1.0 ICP for every 100 occupied acute care beds was adequate infection control staffing.22
Despite the additional responsibilities involved in the practice of infection control, the ratio of 1 ICP for every 250 acute care beds has continued in many health care facilities in the United States since the 1976 SENIC project.2 However, the ratio of ICPs per 250 beds was a recommendation that was not evidence based. Two recent reports recognized that the complexity of the current practices of ICPs has changed considerably since the SENIC and that the old ratio of 1 ICP per 250 beds is no longer adequate. The authors also recommend that infection control programs be based on the scope of the infection control program rather than bed size.23, 24 In the past year alone, 5 new guidelines for infection control practice were published. ICPs must be aware of these guidelines and implement those recommendations that are strongly evidence based.25 The CDC recently released draft documents of revised isolation procedures and tuberculosis prevention guidelines. When the final documents are published, ICPs must implement the new recommendations. In addition, revised JCAHO infection control standards, which went into effect in January 2005, include an emergency management plan (Standard IC.6.10) that requires health care facilities to respond to epidemics and infections that may require expansion of patient care over extended periods of time.26
The first report, which addressed the ratio of ICPs per number of beds since the SENIC project, was published in The Canadian Journal of Infection Control in the summer of 2001.27 A Canadian Infection Control Alliance, consisting of infection control experts, was asked to reach consensus on the key components and resources needed to support effective infection prevention and control programs across the health care continuum: acute care settings, long-term care facilities, and community and home care settings. The Alliance recommended 3 full-time equivalent ICPs for every 500 beds in acute care settings and 1 full-time equivalent ICP for every 150 to 250 beds in long-term care facilities. The projected needs for ICPs in each of these settings was determined based on the expertise represented by members of the Alliance.27, 28 The Canadian study was an important step “towards the development of a ‘validated’ model for [infection prevention and control programs]” since the SENIC29; therefore, the study was reprinted in the American Journal of Infection Control in 2004 (Health Canada, 2004). It should be noted, however, that the 2001 PA,18 the O'Boyle et al study,22 and the Canadian Infection Control Alliance study27 were conducted before the September 11 attacks on the United States and therefore do not reflect current infection control practice, which now includes emerging infections and bioterrorism preparedness.
One state, New Jersey, recently recognized the importance of infection control programs and published revised hospital licensure regulations that mandate an ICP ratio of 1 full-time ICP per 200 adjusted-occupied beds as a minimum standard. The adjusted-occupied bed calculation takes into account patient days, outpatient factors, and case mix. Also mandated was certification by the Certification Board in Infection Control and Epidemiology for all ICPs within 5 years of beginning infection control practice.30
Reporting of health care-associated infections
The 1999 landmark Institute of Medicine (IOM) report on medical errors identified nosocomial infection surveillance as a model for voluntary patient safety reporting systems.31 The National Nosocomial Infection Surveillance (NNIS) System, created by the CDC in 1970 to establish a national nosocomial infections database, is the nation's largest and oldest performance measurement system devoted to hospital-acquired infections.32
The NNIS system started with 62 hospitals in 1970, and, by 2000, approximately 315 hospitals were participating in the NNIS system. Participation in the NNIS system is voluntary and involves only acute care facilities in the United States. Surveillance data are collected uniformly by trained ICPs using standardized protocols that target inpatients at high risk of infection and are reported routinely to the CDC at which they are aggregated into a national database. Participating hospitals are assured by law that the CDC will not provide any information that would identify any individual or institution and that the data received will be held in strict confidence.32
ICPs in the NNIS system collect data for selected “surveillance components”: adult and pediatric intensive care units, high-risk nursery, and surgical patients, using standard CDC definitions that include both clinical and laboratory criteria. NNIS data provide benchmarks to guide hospitals within and outside the NNIS system to improve efforts aimed at reducing infection rates. NNIS reports are published in the biomedical literature on a regular basis, with the latest providing data from January 1992 through June 2004.33 The infrastructure of the NNIS system offers a national resource on which to build improved voluntary patient safety monitoring efforts, as outlined in the 1999 IOM report.34
Other than the NNIS system, there currently is no national standardized method for collecting hospital infection data. In addition, because each hospital monitors those infections and procedures that are most risky for their specific patient populations, all hospitals do not monitor the same infections. Nonetheless, by early 2005, 5 states--Pennsylvania, Missouri, Illinois, Virginia, and Florida--had new regulations, which mandate that ICPs report HAIs to state agencies; however, each state's requirements differ. For example, the Missouri regulations call for the “methodologies and systems for data collection established by the federal Centers for Disease Control and Prevention National Nosocomial Infection Surveillance System, or its successor …”35 The Florida bill, on the other hand, would allow patients to request and obtain information about hospital infection rates.36 Mandatory reporting of HAIs is currently pending in at least 30 other states.6 Legislation for mandatory reporting of HAIs in California was vetoed by Governor Schwarzenegger, making the following case:37
“… Infection control programs have considerable merit and are currently in effect. The Department of Health Services and the Joint Commission on Accreditation of Health Care Organizations scrutinize hospital infection control programs and the National Quality Initiative is expected to more than double the number of quality indicators tracked by May 2005. This calls into question the need of a new program to address this issue .… The absence of data auditing and review by impartial clinical experts may call into question the quality and ultimate validity of the data on hospital-acquired infections ….”
The Healthcare Infection Control Practices Advisory Committee (HICPAC) (formerly the Hospital Infection Control Practices Advisory Committee), which is authorized under the Public Health Service Act, advises the Secretary, Department of Health and Human Services (DHHS) and the CDC regarding the practice of infection control and strategies for surveillance, prevention, and control of HAIs, antimicrobial resistance, and related events in settings in which health care is provided. The Committee also advises the CDC on periodic updating of existing guidelines, development of new guidelines, and other policy statements regarding the prevention of HAIs.38
In response to the recent states' legislation regarding mandatory public reporting of HAIs, HICPAC released a document, Guidance on Public Reporting of Healthcare-Associated Infections, which includes recommendations for use by policy makers and organizations that are tasked to design and implement public reporting systems for HAIs. However, based on an extensive review of the scientific literature, HICPAC found no conclusive evidence for or against public reporting of HAIs as a method to prevent or control their occurrence in health care settings.39
APIC'S commitment to quality health care
The APIC supports the right of consumers and purchasers of health care to expect quality health care and responsible public reporting of performance indicators.6 In its 1998 Position Paper entitled “Release of Nosocomial Infection Data,”40 the APIC outlined specific guidelines for interhospital comparison of HAI surveillance data. These included (1) trained ICPs who use standardized protocols for data collection, (2) maintenance of a continued level of surveillance over time, (3) consistent use of valid case definitions for identifying infections, (4) appropriate use of denominator data and time periods for rate-based data, and (4) risk stratification to control for different levels of illness among patients.40 In keeping with its philosophy, mandatory reporting of HAIs has become a high priority issue for the Association. On March 14, 2005, the APIC released its “Position on Mandatory Public Reporting of Healthcare-Associated Infections.”6 The APIC continues to explore this issue with other stakeholders and to identify and develop informational resources to assist its members at the local level.6
Summary
The United States health care system and patient populations have changed substantially over the past several decades. The practice of infection control also has evolved, and ICPs must continue to develop the knowledge and skills necessary to practice infection prevention and control as changes in health care, standards, guidelines, and regulations evolve. Practice analyses of infection control conducted between 1982 and 2001 reflect an increase in infection control activities from 60 to 147 tasks in 6 areas of infection control practice, a 145% increase over a 20-year period. However, resources have not kept pace with the increase in infection control activities. In addition, the recent trend toward public reporting of HAIs will add more tasks for ICPs with limited resources, at the risk of spending less time on prevention and control activities. In keeping with its philosophy of quality health care and responsible public reporting, the APIC continues to explore this issue.
Chatham, Massachusetts
Footnotes
Preparation of manuscript was supported by the Association for Professionals in Infection Control and Epidemiology (APIC) Research Foundation, Washington, DC.
References
- 1.Haley R.W., Shachtman R.H. The emergence of infection surveillance and control programs in US hospitals: an assessment, 1976. Am J Epidemiol. 1980;111:574–591. doi: 10.1093/oxfordjournals.aje.a112935. [DOI] [PubMed] [Google Scholar]
- 2.Haley R.W., Culver D.H., White J.W., Morgan W.M., Emori T.G., Munn V.P. The efficacy of infection surveillance and control programs in preventing nosocomial infections in US hospitals. Am J Epidemiol. 1985;121:182–205. doi: 10.1093/oxfordjournals.aje.a113990. [DOI] [PubMed] [Google Scholar]
- 3.Emori T.G., Haley R.W., Stanley R.C. The infection control nurse in US hospitals, 1976-1977: characteristics of the position and its occupant. Am J Epidemiol. 1980;111:592–607. doi: 10.1093/oxfordjournals.aje.a112936. [DOI] [PubMed] [Google Scholar]
- 4.Mallison G. A hospital program for control of nosocomial infections. Assoc Practitioners Infect Control Newsletter. 1974;2:1–6. [Google Scholar]
- 5.Joint Commission on Accreditation of Hospitals (JCAH) JCAH; Chicago, IL: 1976. Accreditation manual for hospitals. [Google Scholar]
- 6.Association for Professionals in Infection Control and Epidemiology (APIC). Available from: www.apic.org. Accessed December 3, 2004.
- 7.Community and Hospital Infection Control Association (CHICA)-Canada. Available from: http://www.chica.org/association.html. Accessed December 3, 2004.
- 8.Association for Practitioners in Infection Control (APIC) Educational standards of the Association for Practitioners in Infection Control. Am J Infect Control. 1981;9:42A. [Google Scholar]
- 9.Soule B.M., editor. Volumes I and II. Kendall/Hunt Publishing Company; Dubuque: 1983. (The APIC curriculum for infection control practice). [Google Scholar]
- 10.Certification Board of Infection Control and Epidemiology (CBIC). Available from: www.cbic.org. Accessed.
- 11.Shannon R., McArthur B.J., Weinstein S., Pugliese G., Jackson M.M., Lynch P. Part II: tasks, knowledge, and abilities for practice. Am J Infect Control. 1984;12:187–196. doi: 10.1016/0196-6553(84)90096-8. [DOI] [PubMed] [Google Scholar]
- 12.McArthur B.J., Pugliese G., Weinstein S., Shannon R., Lynch P., Jackson M.M. A task analysis of infection control practitioners, 1982. Part I: methodology and demography. Am J Infect Control. 1984;12:88–95. doi: 10.1016/0196-6553(84)90022-1. [DOI] [PubMed] [Google Scholar]
- 13.Larson E., Eisenberg R., Soule B.M. Validating the certification process for infection control practice. Am J Infect Control. 1988;16:198–205. doi: 10.1016/0196-6553(88)90060-0. [DOI] [PubMed] [Google Scholar]
- 14.Bjerke N.B., Fabrey L.J., Johnson C.B., Bennett G., Schollenberger D., Jacobsen D. Job analysis 1992: infection control practitioner. Am J Infect Control. 1993;21:51–57. doi: 10.1016/0196-6553(93)90224-r. [DOI] [PubMed] [Google Scholar]
- 15.Knapp J.E., Knapp L.G. Practice analysis: building the foundation for validity. In: Impara J.C., editor. Licensure testing: purposes, procedures and practices. Buros Institute of Mental Measurements; Lincoln, NE: 1995. pp. 93–116. [Google Scholar]
- 16.Turner J.G., Kolenc K., Docken L. Job analysis 1996: infection control practitioner. Am J Infect Control. 1999;27:145–157. doi: 10.1016/s0196-6553(99)70091-x. [DOI] [PubMed] [Google Scholar]
- 17.Horan-Murphy E., Barnard B., Chenoweth C., Friedman C., Hazuka B., Russell B. APIC/CHICA-Canada infection control and epidemiology: professional and practice standards. Am J Infect Control. 1999;27:47–51. doi: 10.1016/s0196-6553(99)70073-8. [DOI] [PubMed] [Google Scholar]
- 18.Goldrick B.A., Dingle D.A., Gilmore G.K., Curchoe R.M., Placker C.L., Fabrey L.J. Practice analysis for infection control and epidemiology in the new millennium. Am J Infect Control. 2002;30:437–448. doi: 10.1067/mic.2002.127706. [DOI] [PubMed] [Google Scholar]
- 19.Centers for Disease Control and Prevention (CDC) Biological and chemical terrorism: strategic plan for preparedness and response. MMWR. 2000;49(RR04):1–14. [PubMed] [Google Scholar]
- 20.Centers for Disease Control and Prevention (CDC). Bioterrorism readiness plan: a template for healthcare facilities. Available from: http://www.cdc.gov/ncidod/hip/Bio/13apr99APIC-CDCBioterrorism.PDF. Accessed December 10, 2004.
- 21.Public Health Grand Rounds, October 2003. Available from University of North Carolina Web site: http://www.publichealthgrandrounds.unc.edu/sars/webcast.htm. Accessed December 11, 2003.
- 22.O'Boyle C., Jackson M.M., Henly S.J. Staffing requirements for infection control programs in US health care facilities: Delphi project. Am J Infect Control. 2002;30:321–333. doi: 10.1067/mic.2002.127930. [DOI] [PubMed] [Google Scholar]
- 23.Friedman C., Barnette M., Buck A.S., Ham R., Harris J.A., Hoffman P. Requirements for infrastructure and essential activities of infection control and epidemiology in out-of-hospital settings: a consensus panel report. Am J Infect Control. 1999;27:418–430. doi: 10.1016/s0196-6553(99)70008-8. [DOI] [PubMed] [Google Scholar]
- 24.Scheckler W.E., Brimhall D., Buck A.S., Farr B.M., Friedman C., Garibaldi R.A. Requirements for infrastructure and essential activities of infection control and epidemiology in hospitals: a consensus panel report. Society for Healthcare Epidemiology of America. Am J Infect Control. 1998:47–60. doi: 10.1016/s0196-6553(98)70061-6. [DOI] [PubMed] [Google Scholar]
- 25.Jarvis W.R. The state of the science of health care epidemiology, infection control, and patient safety, 2004. Am J Infect Control. 2004;32:496–503. doi: 10.1016/j.ajic.2004.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Joint Commission on Accreditation of Healthcare Organizations (JCAHO) JCAHO; Chicago, IL: 2005. Joint Commission on Accreditation of Healthcare Organizations, 2005 Critical access hospitals surveillance, prevention and control of infection. [Google Scholar]
- 27.Health Canada, Division of Occupational and Nosocomial Infections Development of a resource model for infection prevention and control programs (IPCPs) in acute, long term, and home care settings: conference proceedings of the Infection Prevention and Control Alliance. Can J Infect Control. 2001;16:35–39. doi: 10.1016/j.ajic.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 28.Health Canada, Division of Occupational and Nosocomial Infections Development of a resource model for infection prevention and control programs (IPCPs) in acute, long term, and home care settings: conference proceedings of the Infection Prevention and Control Alliance. Am J Infect Control. 2004;32:2–6. doi: 10.1016/j.ajic.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 29.Goldrick B. Canadian report: ICP needs across the health care continuum. Am J Infect Control. 2004;32:1. doi: 10.1016/j.ajic.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 30.State of New Jersey. NJAC. 8:43G-14.3, 14.5, 2004. Available from: http://www.state.nj.us/health/hcsa/njac843g.pdf. Accessed December 15, 2004.
- 31.Institute of Medicine (IOM) National Academy Press; Washington, DC: 1999. To err is human. [Google Scholar]
- 32.Centers for Disease Control and Prevention (CDC). About NNIS. Available from: http://www.cdc.gov/ncidod/hip/NNIS/@nnis.htm. Accessed December 15, 2004.
- 33.NNIS System National nosocomial infections surveillance (NNIS) system report, data summary from January 1992 through June 2004, issued October 2004. Am J Infect Control. 2004;32:470–485. doi: 10.1016/S0196655304005425. [DOI] [PubMed] [Google Scholar]
- 34.Richards C., Emori T.G., Edwards J., Fridkin S., Tolson J., Gaynes R. Characteristics of hospitals and infection control professionals participating in the National Nosocomial Infections Surveillance System 1999. Am J Infect Control. 2001;29:400–403. doi: 10.1067/mic.2001.118408. [DOI] [PubMed] [Google Scholar]
- 35.State of Missouri. S1279, 2004.
- 36.State of Florida. HB1629, 2004, Section 11. Available from APIC Web site: http://www.apic.org/Content/NavigationMenu/GovernmentAdvocacy/MandatoryReporting/Abouttheissue/about_the_issue.htm. Accessed October 3, 2005.
- 37.Consumer Union. September 30, 2004. Available from: http://www.consumersunion.org. Accessed January 4, 2005.
- 38.Healthcare Infection Control Practice Advisory Committee (HICPAC). Available from: http://www.cdc.gov/ncidod/hip/HICPAC/Hicpac.htm. Accessed January 4, 2005.
- 39.Healthcare Infection Control Practice Advisory Committee (HICPAC). Available from: http://www.cdc.gov/ncidod/hip/PublicReportingGuide.pdf. Accessed January 4, 2005.
- 40.Association for Professionals in Infection Control and Epidemiology (APIC) APIC position paper: release of nosocomial infection data. APIC News. 1998;17:1–5. [Google Scholar]


