Abstract
Mannose binding lectin (MBL) generally plays a protective role during viral infection, yet MBL-mediated complement activation promotes Ross River virus (RRV)-induced inflammatory tissue destruction, contributing to arthritis and myositis. As MBL binds to carbohydrates, we hypothesized that N-linked glycans on the RRV envelope glycoproteins act as ligands for MBL. Using a panel of RRV mutants lacking the envelope N-linked glycans, we found that MBL deposition onto infected cells was dependent on the E2 glycans. Moreover, the glycan-deficient viruses exhibited reduced disease and tissue damage in a mouse model of RRV-induced myositis compared to wild-type RRV, despite similar viral load and inflammatory infiltrates within the skeletal muscle. Instead, the reduced disease induced by glycan-deficient viruses was linked to decreased MBL deposition and complement activation within inflamed tissues. These results demonstrate that the viral N-linked glycans promote MBL deposition and complement activation onto RRV-infected cells, contributing to the development of RRV-induced myositis.
Keywords: Ross River virus, Alphavirus, Mannose-binding lectin, Complement, N-linked glycan
Highlights
-
•
Mannose-binding lectin promotes induction of complement-mediated arthritis and myositis during Ross River virus infection.
-
•
Mannose Binding Lectin deposition onto Ross River virus-infected cells is dependent on glycans on the viral E2 glycoprotein.
-
•
Viral mutants lacking E2 glycans exhibit reduced disease in a model of Ross River virus-induced arthritis and myositis.
-
•
Ross River virus E2 glycan mutants cause reduced Mannose Binding Lectin deposition and complement activation.
1. Importance
Mannose binding lectin (MBL), a lectin that can initiate the host complement cascade, generally has a protective role following viral infection. However, in the context of alphavirus-induced disease, MBL-mediated complement activation is pathologic, promoting Ross River virus (RRV)-induced inflammatory tissue destruction within the musculoskeletal system. MBL binds to glycosylated proteins, thus we hypothesized that the RRV envelope N-linked glycans promote MBL binding, complement activation, and disease. Using a panel of mutant viruses lacking one or more envelope glycans, we found that the RRV E2 glycans are required for MBL binding to infected cells and subsequent disease. Mice infected with a virus lacking both E2 glycans exhibited reduced disease and tissue damage, and decreased MBL binding and complement activation compared to mice infected with the wild type virus. These results suggest that interactions between MBL and the viral N-linked glycans play a major role in development of alphavirus-induced inflammatory disease.
2. Introduction
Arthritogenic alphaviruses such as Ross River virus (RRV) and chikungunya virus (CHIKV) are mosquito-borne viruses that cause outbreaks of infectious arthritis and myositis in many regions of the world. Both RRV and CHIKV share similar disease symptoms that are characterized by debilitating polyarthritis and myositis that frequently results in myalgia and arthralgia. Studies in both humans and mice have identified a critical role for the host inflammatory response in the development of disease and immunopathology following infection, with macrophages playing an essential role in damage to the musculoskeletal system (Lidbury et al., 2000, Morrison et al., 2006, Herrero et al., 2013, Herrero et al., 2011).
We have previously demonstrated that the host complement system initiated by mannose binding lectin (MBL) also has a critical role in development of disease (Gunn et al., 2012). Interestingly, rather than regulating the infiltration of macrophages and other inflammatory cells into the musculoskeletal system, MBL-mediated complement activation following RRV infection contributes to disease by regulating inflammatory cell activation within the inflamed tissue through complement receptor 3 (CR3) (Gunn et al., 2012, Morrison et al., 2008). The C3 cleavage product iC3b produced following complement activation can bind to CR3 on inflammatory cells including macrophages and neutrophils to induce phagocytosis of iC3b-opsonized pathogens, and can also initiate cytotoxic effector programs through Syk-mediated outside-in signaling that leads to tissue damage in autoimmune disorders (Ross, 2000, Abram and Lowell, 2009, Hirahashi et al., 2006). In the absence of MBL, C3, or CR3 expression of cytotoxic inflammatory mediators within muscle tissue are reduced in RRV-infected mice, correlating with reduced disease, but viral tropism and viral burden are unaffected (Gunn et al., 2012, Morrison et al., 2008). Importantly, production of the CR3 ligand iC3b is reduced in the absence of MBL following RRV infection (Gunn et al., 2012), suggesting that MBL-mediated complement activation regulates production of iC3b and subsequent activation phenotype of CR3-bearing inflammatory cells present within the tissues.
MBL-mediated complement activation is initiated by the recognition of terminal sugars on glycosylated proteins by the carbohydrate recognition domain (CRD) of MBL (reviewed in Takahashi et al., 2006). The alphavirus glycoproteins E1 and E2 contain three to four N-linked glycosylation sites that are glycosylated with N-linked glycans (Strauss and Strauss, 1994). During structural protein synthesis, the E2 and E1 glycoproteins are processed through the ER and Golgi to generate mature E2-E1 heterodimers, which self-assemble into trimeric spikes at the plasma membrane. Alphaviruses bud from the plasma membrane and each budding alphavirus virion incorporates 80 glycoprotein spikes to make up the viral envelope. The E2 glycoprotein is prominently displayed on the surface of the virus and on the surface of infected cells, and for most alphaviruses, two of the N-linked glycans are located on E2. The RRV E2 N200 glycan is located on one side of the tip of the protruding E2 “petal” on the glycoprotein spike (E2 N200), and the E2 N262 glycan appears to be located between the trimeric spikes (Pletnev et al., 2001). Thus, the RRV E2 N-linked glycans are surface exposed and are in a key position to interact with host proteins. The RRV E2 N-linked glycans are glycosylated with a combination of high mannose (E2 N200) and complex (E2 N262) glycans when produced in mammalian cells (Shabman et al., 2008). Therefore, while we have previously demonstrated that MBL does not directly bind to free RRV virions (Gunn et al., 2012), we hypothesized that MBL might interact with the N-linked glycans on the RRV envelope glycoproteins when they are displayed on the surface of infected cells.
In this study, we used RRV mutants that lack one or both of the two N-linked glycosylation sites on E2 to demonstrate that the E2 N-linked glycans are required for MBL binding to infected cells and subsequent induction of virus-induced disease. While RRV-infected cells are readily bound by MBL, this activity was lost when the cells were infected with RRV lacking both N-linked glycans on the viral E2 glycoprotein. Furthermore, viruses lacking either E2 glycosylation site caused reduced RRV disease in mice, and a virus lacking both sites was further attenuated. Our results support a model of RRV pathogenesis wherein the E2 N-linked glycans promote activation of the lectin complement pathway by MBL, resulting in activation of CR3-bearing inflammatory cells and subsequent damage within inflamed tissues.
3. Materials and methods
3.1. Ethics statement
All mouse studies were performed in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. All mouse studies were performed at the University of North Carolina (Animal Welfare Assurance #A3410-01) using protocols approved by the University of North Carolina Institutional Animal Care and Use Committee (IACUC; Protocol #10–204 and 11–224). All studies were performed in a manner designed to minimize pain and suffering in infected animals, and any animal that exhibited severe disease signs was euthanized immediately in accordance with IACUC approved endpoints.
3.2. Viruses and cells
WT RRV is derived from the infectious clone of RRV T48 (pRR64), and the E2 N-linked glycan mutants were generated previously by site directed mutagenesis of N-linked glycosylation sites in E2 in pRR64 (Shabman et al., 2008). The viral stocks used in this study were generated as described in (Morrison et al., 2006). Primary myoblasts were generated from skeletal muscle from one day-old C57BL/6J mice, and muscle was dissociated by type I collagenase (Worthington Biochemicals) and grown in DMEM supplemented with 6% FBS. To differentiate cells, the medium was replaced with DMEM containing 3% FBS. Baby hamster kidney (BHK-21) cells were cultured in MEM alpha supplemented with 10% FBS and L-glutamine. Human embryonic kidney (HEK) 293T cells were cultured in DMEM supplemented with 10% FBS.
3.3. MBL deposition onto myotubes
Differentiated myotubes (C2C12 or primary cells from C57BL/6J mice) were either mock-infected or infected with RRV WT or E2 DM at an MOI of 20. At 18 hpi, culture medium was removed and cells were incubated in medium containing either 10% serum from WT or MBL-DKO mice for an additional 30 min. Cells were washed with PBS containing 400 mM NaCl and harvested in lysis buffer. Cell lysates were analyzed by immunoblot analysis by standard techniques. Densitometry was performed using ImageJ (NIH), and values were normalized either to actin, RRV E2, or RRV capsid.
3.4. Immunofluorescence
BHK-21 cells were infected at MOI of 1 with either diluent alone (mock), RRV WT or E2 DM. At 12 hpi, medium was removed and cells were incubated in HBSS with 5 mM CaCl2 with or without 10 µg/ml rhMBL (R&D Systems) for 30 min. Cells were washed with PBS, fixed with PFA, and stained using standard techniques using the following antibodies: α-MBL-C (SCBT 1:50); α-RRV (ATCC 1:1000); Alexa Fluor 488-α mouse (Invitrogen 1:1000); and Alexa Fluor 594 α-rabbit (Invitrogen 1:1000). Slides were mounted with ProLong Gold with DAPI, (Invitrogen) and imaged by fluorescence microscopy. Images were processed using ImageJ (NIH).
3.5. RRV antigen detection by flow cytometry
BHK-21 or HEK 293T cells were infected at an MOI of 5 with either diluent (mock), RRV WT, or the indicated RRV E2 mutants. At 12 hpi, media was removed, cells were washed with PBS, and cells were harvested using cell dissociation buffer (Gibco). Cells were then spun down and stained for extracellular RRV antigen using α-RRV (ATCC) and FITC-conjugated α-mouse (eBioscience). Cells were then fixed in 4% PFA and analyzed by flow cytometry as previously described (Morrison et al., 2008).
3.6. Mice
All mice used in this study were maintained and bred in house at the University of North Carolina (UNC) in accordance with UNC Institutional Animal Care and Use Committee guidelines. C57BL/6J mice were purchased from The Jackson Laboratories (Bar Harbor, ME).
3.7. Infection of mice with RRV
24-day-old C57BL/6J mice were inoculated with 103 PFU of RRV in diluent in the left rear footpad. Mice were weighed daily and assigned a clinical score based on hind limb weakness and altered gait on the following scale: 0 = no disease; 1 = mild loss of hind limb grip; 2 = moderate loss of hind limb grip; 3 = severe loss of hind limb grip; 4 = no hind limb grip and mild inability to right; 5 = no hind limb grip and complete inability to right; 6 = moribund.
3.8. Viral burden analysis
At indicated times post infection mice were sacrificed, perfused with 1× PBS, and indicated tissues were dissected and removed, weighed, and homogenized with glass beads in diluent. Viral titers within infected tissues were determined by plaque assay on BHK-21 cells from tissue homogenates.
3.9. Quantification of RRV genomes
Quadriceps muscle from infected mice were removed, and homogenized with glass beads in Trizol (Invitrogen). Total RNA was extracted and cDNA was generated from 1 µg of RNA by Superscript III reverse transcriptase. RRV genomes were amplified using a tagged RRV specific primer by qRT-PCR, and absolute numbers of RRV genomes were determined using a standard curve of serial dilutions ranging from 108 to 10° copies of RRV genomes.
3.10. Histological analysis, immunohistochemistry, and Evans Blue dye (EBD) analysis
At indicated times post infection, mice were sacrificed and perfused with 4% PFA. Tissues were paraffin embedded and 5 µm sections were generated and stained with hematoxylin and eosin to examine tissue pathology and inflammation or probed with a goat a-mouse C3 antibody (1:500 Cappel) for immunohistochemistry using the Vectastain ABC-AP kit (Vector Labs). To visualize tissue damage by EBD, mice were injected with 1% EBD as previously described (Morrison et al., 2007), and frozen sections were generated. Sections were visualized by either bright field or fluorescence microscopy.
3.11. Analysis of infiltrating inflammatory cells by flow cytometry
At indicated times post infection mice were sacrificed, and both quadriceps muscles were removed, minced, and digested and cells were isolated and analyzed as previously described (Gunn et al., 2012).
3.12. Statistical analysis
Data was analyzed for statistically significant differences using either Mann-Whitney analysis, one-way ANOVA, or t-test (p < 0.05 is considered significant). Statistical analyses were performed using GraphPad Prism 5 and the statistical programming language R (v.3.0.2).
4. Results
4.1. RRV E2 N-linked glycans contribute to MBL deposition onto infected cells
We have previously shown that MBL deposition is enhanced on cells following RRV infection, suggesting that some aspect of viral infection induces MBL binding to infected cells (Gunn et al., 2012). MBL can recognize terminal sugars on glycans and thus we hypothesized that the RRV E2 N-linked glycans mediate MBL binding to infected cells resulting in complement activation. Although MBL did not directly bind to RRV virion particles (Gunn et al., 2012), we hypothesized that the viral N-linked glycans may be more accessible when the viral glycoproteins are displayed on the cell surface compared to the virion, allowing MBL to bind the N-linked glycans on the viral glycoproteins located on the surface of infected cells.
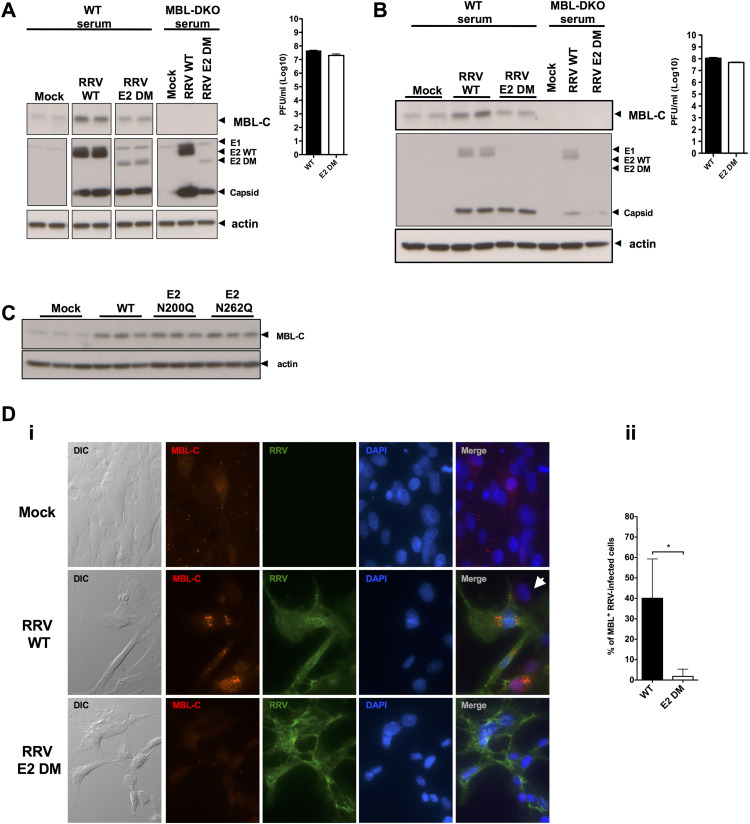
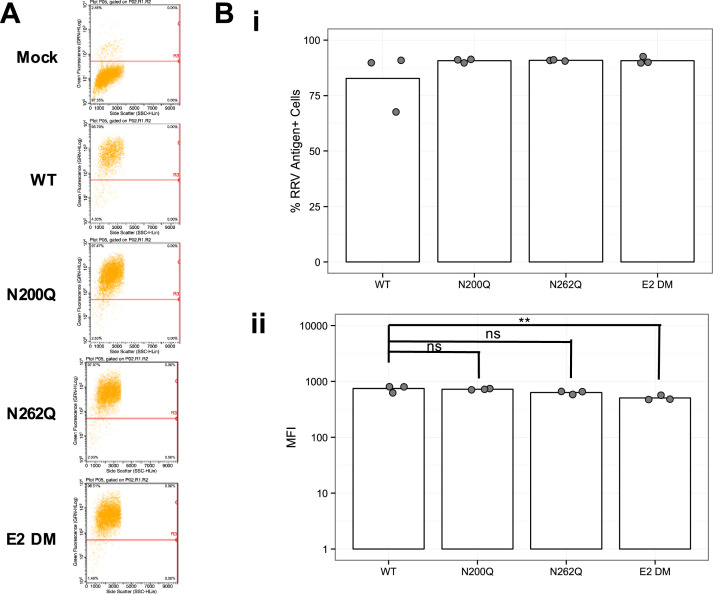
To test if the RRV E2 N-linked glycans were required for MBL binding to infected cells, we used a previously generated panel of mutant viruses lacking one or both E2 glycans: RRV E2 N200Q, E2 N262Q, and E2 N200;262Q (E2 DM) (Shabman et al., 2008). Mutant viruses lacking the E1 glycan demonstrated significant replication deficiencies in vitro (Shabman et al., 2008), and thus we did not evaluate this mutant in our study. Differentiated C2C12 myotubes or primary murine myotubes, which are derived from skeletal muscle, were infected with either wild-type RRV (RRV WT) or the glycan mutants, and infected cells were then incubated in medium containing serum from either naïve WT or MBL-DKO mice as a source of MBL. Cells were extensively washed to remove unbound MBL and free virus, and cell lysates were analyzed by western blot for levels of MBL. Consistent with our hypothesis, we observed a decrease in MBL deposition onto RRV E2 DM-infected C2C12 cells compared to RRV WT-infected cells, despite similar levels of viral replication ( Fig. 1A). Similar results were observed in primary myotubes (Fig. 1B). When MBL deposition was normalized against either the viral capsid protein or actin (Supplementary Figure 1), we observed a clear reduction in MBL deposition onto RRV E2 DM-infected cells as compared to wild-type. We did not observe MBL deposition in cells incubated with MBL-DKO serum, demonstrating both the specificity of the MBL western blots and indicating that we were detecting only exogenously added MBL in the assay, rather than modulation of MBL production by the different viruses within the infected cells. Interestingly, infection with the viruses lacking each glycan individually showed comparable MBL deposition levels to RRV WT (Fig. 1C), indicating that both N-linked glycans contribute to MBL deposition onto RRV-infected cells. To confirm the western blot analysis and to determine if MBL was binding to virally infected cells in a glycan-dependent manner, we evaluated MBL deposition by fluorescence microscopy. BHK-21 cells were infected with either diluent alone (mock-infected), RRV WT or RRV E2 DM, and subsequently incubated with recombinant human MBL. Cells were washed extensively, fixed, stained without permeabilization to only detect surface localization of MBL and RRV structural proteins, and imaged by fluorescence microscopy. As shown in Fig. 1Di, we observed enhanced deposition of MBL onto RRV-infected cells compared to mock-infected cells. Within the RRV WT-infected culture, we observed deposition primarily onto the infected cells rather than onto uninfected cells (indicated by white arrow), indicating that viral infection of the cell contributes to MBL deposition. Levels of MBL were reduced on cells infected with E2 DM compared to WT, despite comparable levels of RRV antigen between WT and E2 DM infection. Furthermore, the percentage of infected cells with MBL deposition was significantly reduced in cells infected with RRV E2 DM compared to WT (Fig. 1Dii). These results indicate that N-linked glycans on the E2 glycoprotein are required for MBL deposition on infected cells. However, while images in Fig. 1Di indicate equivalent levels of RRV antigen expression on the cell surface, western blotting with this antibody did not efficiently detect the E2 glycoprotein in cells infected with the E2 DM virus, which raised the possibility that the lack of N-linked glycans on the E2 protein may lead to reduced cell surface expression of E2 and decreased MBL binding. Therefore, we quantified RRV glycoprotein expression on the cell surface for each of the mutant viruses by flow cytometry. As shown in Fig. 2A and Bi, the E2 DM virus exhibited no defect in surface glycoprotein expression in BHK-21 cells as compared to either of the single mutant viruses, which still bind MBL (Fig. 1c). While we did observe a slight reduction in fluorescence intensity of the E2 mutants as compared to WT (Fig. 2Bii); this difference was less than two-fold. We observed a similar pattern in HEK 293T cells (data not shown). Therefore, it is unlikely that the reduced MBL deposition seen with the E2 DM virus is due to an overall reduction in cell surface glycoprotein expression. Taken together, these data support the hypothesis that MBL recognizes and binds to the E2 N-linked glycans on the surface of infected cells.
Fig. 1.
The RRV E2 N-linked glycans contribute to MBL deposition onto infected cells. (A) C2C12 myotubes were infected with either RRV WT or E2 DM, and incubated in WT or MBL-DKO mouse serum. Western blots of MBL-C deposition onto cells are shown (left) and viral titer (right) (B) Same as above, but primary C57BL/6 myotube cultures were infected with either RRV WT or E2 DM. Western blots of MBL-C deposition are shown (left) and viral titer (right). (C) C2C12 myotubes were infected with either RRV WT, E2 N200Q, or E2 N262Q and incubated with MBL-WT mouse serum and western blot of MBL-C deposition is shown. (D) Immunofluorescence of either mock (top row), RRV WT (middle row) or RRV E2 DM (bottom row)-infected BHK-21 cells incubated with rhMBL-C at 12 hpi. (i) Individual panels from left: DIC, MBL-C (Texas Red), RRV antigen (FITC), DAPI, and merge. No MBL-C binding was observed in cells incubated without MBL-C (data not shown). (ii) The percentage of infected cells with MBL deposition between RRV WT and E2 DM was determined by visual analysis of four independent fields. * p < 0.05 by Mann-Whitney analysis.
Fig. 2.
Envelope protein expression is not diminished by disruption of the N-linked glycans. (A) BHK-21 cells were infected with either RRV WT or a RRV glycan mutant at an MOI of 5 (n = 3 per virus). At 12 hpi, intact cells were dissociated from the dish and stained for RRV antigen (FITC). Cells were fixed overnight and RRV envelope expression was measured by flow cytometry. Representative dot plots are shown. (B) (i) The percentage of infected cells and (ii) mean fluorescence intensity (MFI) of RRV antigen staining in (A) were quantified. Data representative of two independent experiments. Error bars represent the standard deviation from the mean. One-way ANOVA performed for determination of statistical significance. * p < 0.05, ** p < 0.01.
4.2. The RRV E2 N-linked glycans contribute to severe disease
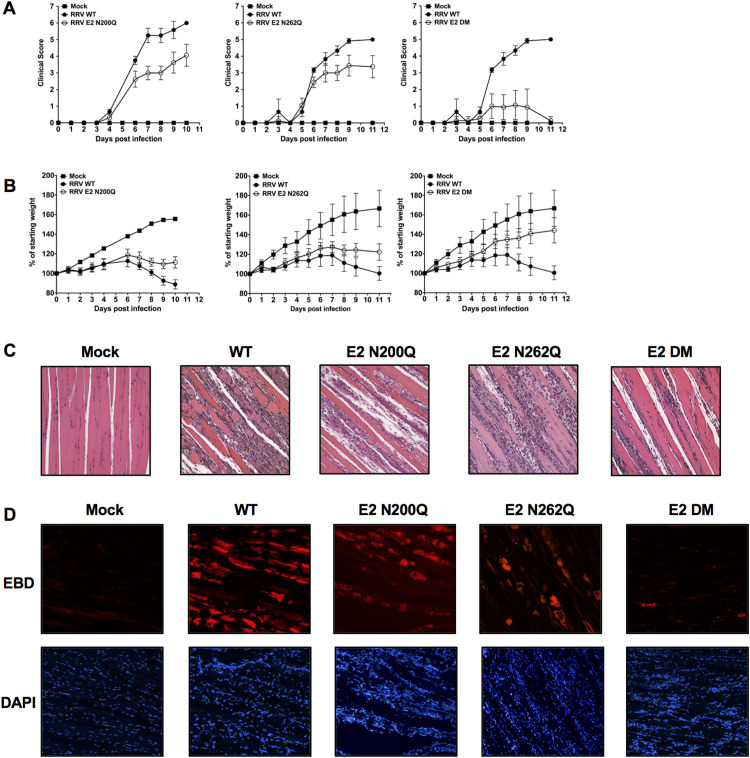
Given that the E2 N-linked glycans contributed to MBL binding to infected cells, and since MBL contributes to RRV-induced disease, we hypothesized that the E2 glycans also contributed to development of RRV-induced disease. Mice were infected with either RRV WT or the panel of glycan mutant viruses, and mice were weighed and assigned a clinical score as previously described (Morrison et al., 2006). Consistent with our previous studies, RRV WT-infected mice began to develop severe disease characterized by hind-limb dysfunction by 5 days post infection (dpi), with peak disease severity from 7 to 10 dpi ( Fig. 3A), and had reduced weight gain compared to mock-infected mice (Fig. 3B). Consistent with a previous report (Nelson et al., 2016), mice infected with viruses lacking either one of the E2 glycosylation sites (Fig. 3A E2 N200Q, left; E2 N262Q, middle) developed hind-limb dysfunction with approximately the same kinetics as WT-infected mice, but the peak disease severity was reduced in these mice. In addition, these mice had reduced weight loss compared to WT-infected mice (Fig. 3B E2 N200Q, left; E2 N262Q, middle), suggesting that each individual E2 glycan contributes to the severity of RRV-induced disease. Interestingly, mice infected with RRV E2 DM developed a very mild disease with little to no hind-limb dysfunction, and continued to gain weight throughout the course of infection (Fig. 3A-B, right). Notably, the disease in the E2 DM-infected mice was remarkably similar to the disease induced in MBL-DKO and complement deficient mice (Gunn et al., 2012, Morrison et al., 2007). Taken together, these data indicate while each of the E2 glycans contribute to disease severity individually, both of the E2 N-linked glycans are required for development of maximal disease, and suggests that the N-linked glycans have a key role in mediating RRV pathogenesis.
Fig. 3.
The RRV E2 N-linked glycans contribute to severe disease. (A-C) Twenty-four day old C57BL/6 were either mock-infected, or infected with either RRV WT or a RRV glycan mutant. Mice were assigned a clinical score (A) or weight loss (B) as described in the Experimental Procedures. Mice infected with RRV WT (solid circles) were compared directly to mice infected with E2 N200Q (open circle, left graph), E2 N262Q (open circle, middle graph) or E2 DM (open circle, right graph). Each data point represents the arithmetic mean ± SD and is representative of at least three independent experiments. (C) Tissue pathology and inflammation was examined at 10 dpi by H&E staining of quadriceps muscle sections. A representative section from mice infected with each virus strain is shown. (D) To assess damage within the muscle, mice infected with either RRV WT or the RRV glycan mutants were injected with EBD at 10 dpi, and frozen sections were generated. EBD positive muscle fibers were identified by fluorescence microscopy. Representative sections of each virus strain are shown.
4.3. RRV lacking one or both E2 N-linked glycans are attenuated for inflammatory pathology and tissue damage within quadriceps muscle
Infiltration of inflammatory cells into the skeletal muscle is a characteristic of RRV-induced disease, and much of the disease observed following RRV infection is due to the pathology mediated by inflammatory infiltrates (Lidbury et al., 2000, Hazelton et al., 1985, Clarris et al., 1975, Fraser et al., 1981). To assess the role of the E2 glycans in promoting inflammatory pathology following RRV infection, we analyzed H&E-stained quadriceps muscle sections from mice infected with either RRV WT or the glycan mutants at 10 dpi. As expected, we observed severe inflammatory pathology in the quadriceps of RRV WT-infected mice evidenced by the destruction of the fibrous architecture of the skeletal muscle and the presence of infiltrating cells (Fig. 3C). We observed the presence of infiltrating cells in the quadriceps muscle of mice infected with any of E2 glycan mutant viruses. However, consistent with reduced disease scores and previous reports (Nelson et al., 2016), the quadriceps muscles of mice infected with either E2 N200Q or E2 N262Q had moderate tissue damage that was reduced compared to WT-infected mice, as indicated by the presence of intact muscle fibers (Fig. 3C). In contrast, mice infected with E2 DM had intact skeletal muscle fibers with minimal tissue damage despite the presence of infiltrating cells (Fig. 3C).
To confirm that mice infected with the RRV glycan mutants had reduced tissue damage we administered Evans Blue dye (EBD) into either RRV WT or glycan mutant-infected mice at 10 dpi to visualize damaged muscle fibers within the quadriceps muscle. EBD is excluded from healthy cells, but taken up by damaged cells and can be visualized by fluorescent microscopy; thus, EBD+ tissues indicate tissue damage. As shown in Fig. 3D, we observed abundant EBD positive muscle fibers in quadriceps muscle from RRV WT-infected mice. There were fewer EBD positive fibers observed in mice infected with either of the single E2 glycan mutants compared to RRV WT-infected mice, consistent with the reduced clinical scores and pathology. Furthermore, EBD-positive muscle fibers were rare in mice infected with E2 DM, suggesting that both viral N-linked glycans are required for maximal induction of tissue damage.
4.4. The RRV E2 N-linked glycans contribute to complement activation in quadriceps muscle
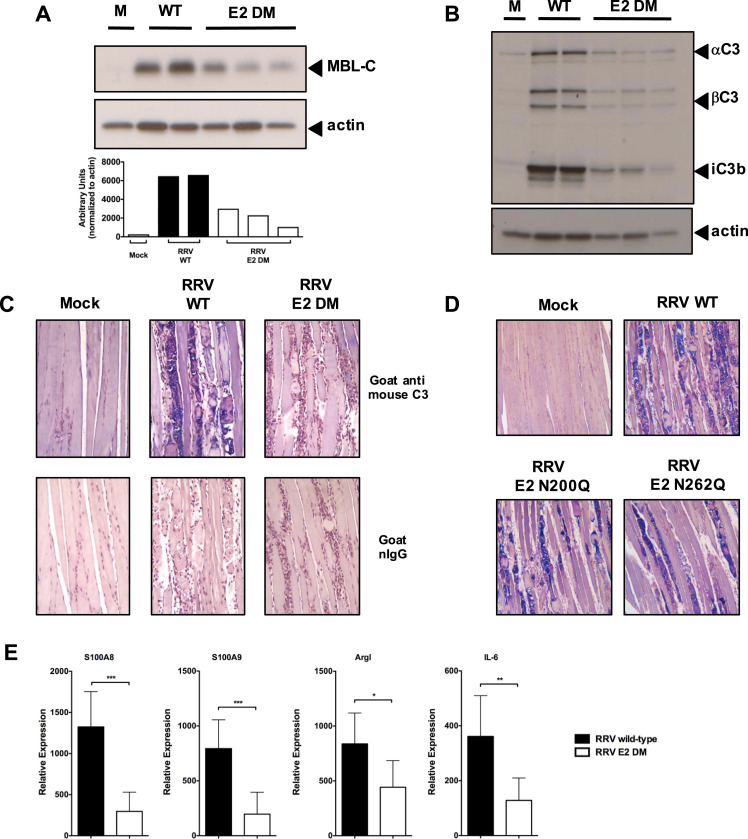
We have previously shown that complement activation following RRV infection is mediated primarily through MBL (Gunn et al., 2012). Given that we observed a decrease in MBL deposition within RRV E2 DM-infected cultures, and since the disease phenotype induced by E2 DM in WT mice was strikingly similar to the disease induced by WT RRV in MBL-DKO mice, we hypothesized that the presence of the E2 glycans contribute to MBL deposition and complement activation in the quadriceps muscle following infection. MBL-mediated complement activation is initiated by the recognition of terminal sugars on glycosylated proteins by the carbohydrate recognition domain of MBL, and binding of MBL to the ligand leads to formation of a C3 convertase by the MBL-associated serine proteases resulting in localized C3 deposition (Ricklin et al., 2010). We infected mice with either RRV WT or E2 DM, harvested quadriceps muscle at 7 dpi and analyzed the muscle homogenates by western blot for MBL-C levels. As shown in Fig. 4A, RRV WT-infected mice had elevated levels of MBL-C in the quadriceps at 7 dpi compared to mock-infected mice, and importantly, we observed reduced levels of MBL in RRV E2 DM-infected mice compared to RRV WT-infected mice, suggesting that the RRV E2 glycans contribute to MBL deposition within infected tissues.
Fig. 4.
The RRV E2 N-linked glycans contribute to complement activation in quadriceps muscle. (A) Top, Quadriceps muscle homogenates from mock-infected mice or mice infected with either RRV WT or E2 DM were analyzed by western blot analysis at 7 dpi to determine relative levels of MBL. Each lane represents an individual mouse. Bottom, Normalization of MBL deposition to actin by densitometry analysis. (B) To determine if complement activation differed between mice infected with RRV WT or E2 DM, we analyzed homogenized quadriceps muscles from either mock-infected or infected mice at 7 dpi by western blot using α-mouse C3 or α-mouse actin antibody. C3 cleavage products are indicated with solid arrowheads. Each lane represents an individual mouse. (C, D) C3 deposition was assessed by immunohistochemistry using an α-mouse C3 antibody on quadriceps muscle sections from mock-infected mice or mice infected with either RRV WT (C, D), E2 DM (C), E2 N200Q (D), or E2 N262Q (D) at 7 dpi. C3 positive areas are stained in blue. A representative section from each strain is shown. (E) Relative mRNA expression of C3-dependent inflammatory mediators and cytokines S100A9, S100A8, Arginase I, and IL-6 from quadriceps muscle from mice infected with either RRV WT (solid bar, n = 7) or E2 DM (open bar, n = 8) mice by quantitative real-time RT-PCR. Raw data values were normalized to 18S rRNA levels, and are graphically depicted as fold expression over mock-infected mice. Data from a single experiment is shown, but is representative of two independent experiments. *** p < 0.001; ** p < 0.005 * p < 0.05 by Student's t-test.
To determine if the reduced levels of MBL within the quadriceps muscle of RRV E2 DM-infected mice correlated with reduced complement activation, we analyzed quadriceps muscle homogenates from RRV WT or E2 DM-infected mice for levels of C3 cleavage products by western blot. Consistent with our hypothesis, we observed reduced levels of both the alpha and beta chain of C3 in E2 DM-infected mice compared to WT-infected mice (Fig. 4B), indicating that the E2 glycans are required for C3 deposition onto infected tissues. Importantly, the levels of the C3 cleavage product and CR3 ligand iC3b was significantly reduced in E2 DM-infected mice compared to WT-infected mice (Fig. 4B), indicating that the RRV E2 glycans are required for robust complement activation following infection.
As western blot analysis of C3 present in quadriceps muscle tissue does not distinguish between C3 produced by infiltrating inflammatory monocytes and C3 that is deposited onto infected tissues, we sought to further confirm that the RRV E2 glycans are required for C3 deposition onto skeletal muscle following infection by immunohistochemical staining of C3 on skeletal muscle sections. We infected mice with either RRV WT or E2 DM, and generated sections of the quadriceps muscle at 7 dpi. Consistent with results in Fig. 4B, we observed a reduction in C3 deposition onto the skeletal muscle from mice infected with RRV E2 DM compared to WT (Fig. 4C). Interestingly, we also observed reduced C3 deposition onto quadriceps muscle sections from mice infected with RRV E2 N200Q or E2 N262Q compared to RRV WT (Fig. 4D). The level of C3 deposition correlates with disease severity and is consistent with the role of complement in determining disease severity. As the E2 glycans were required for production of iC3b, we analyzed the expression of MBL, C3, and CR3-dependent pro-inflammatory mediators by real-time PCR to determine if their expression was also dependent on the E2 glycans. Indeed, mice infected with RRV E2 DM had reduced expression of MBL and C3-dependent pro-inflammatory genes compared to RRV WT (Fig. 4E), further supporting the hypothesis that the E2 N-linked glycans mediate complement activation following infection.
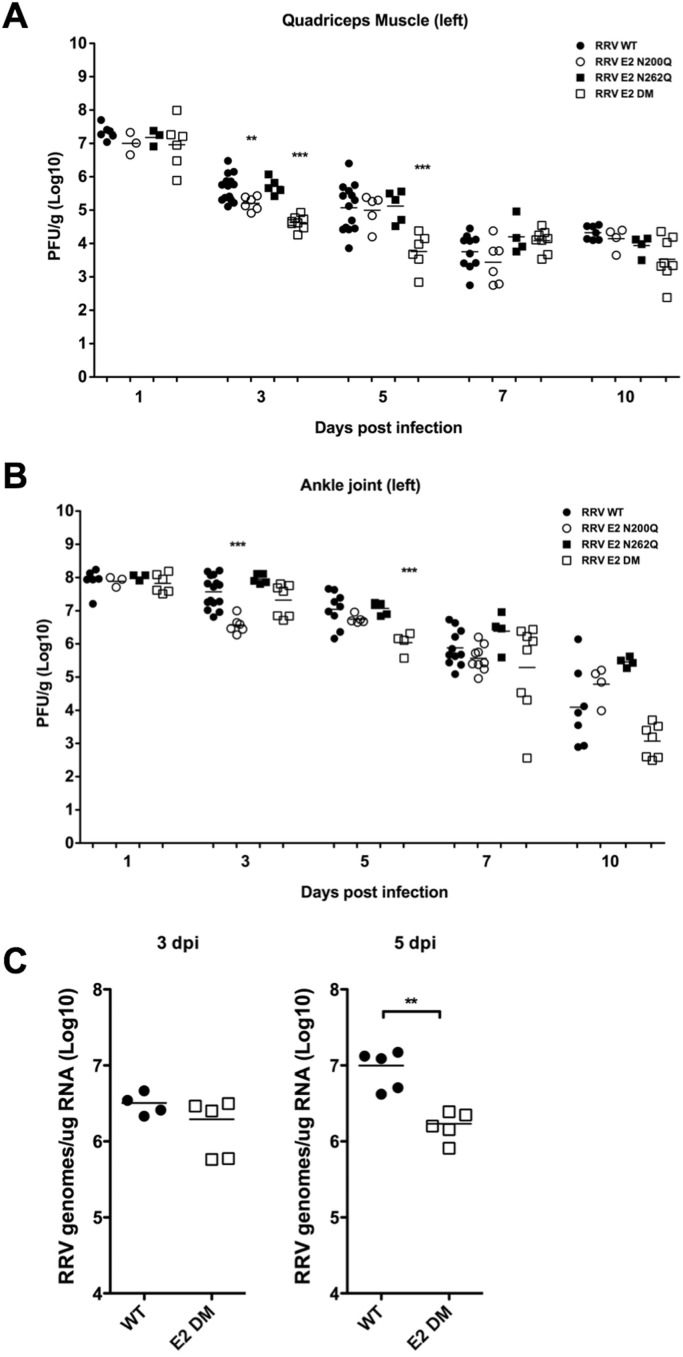
4.5. RRV glycan mutants retain the ability to replicate in vivo
Although the RRV E2 N-linked glycans are dispensable for replication in cell culture (Shabman et al., 2008), it is possible that the glycans might be required for efficient viral replication and dissemination in vivo. Therefore, we evaluated the viral titer within the quadriceps muscle and the ankle joints from either RRV WT, or glycan mutant-infected mice at multiple times post-infection by plaque assay. There was no difference in viral titer within the quadriceps and ankle joints at 1 dpi between any of the viruses, suggesting that the E2 glycans are not required for initial dissemination and replication within target tissues ( Fig. 5A-B). Overall, all of the glycan mutant viruses replicated at or near wild-type levels in target tissues, although there were some differences in titer observed at intermediate time points. At 3 dpi, we observed reduced viral titer within the quadriceps muscle in mice infected with E2 N200Q and E2 DM compared to WT (Fig. 5A). The E2 N262Q virus did not show any difference in titer compared to RRV WT at any time post-infection in either the ankle or the quadriceps muscle. At 5 dpi, only the RRV E2 DM titer is reduced in the quadriceps muscle compared to RRV WT, but importantly, at time points of severe disease, 7 and 10 dpi, no difference was observed in the quadriceps muscle between any of the viruses (Fig. 5A). Similar results were observed in the ankle joints, although there were significant differences in titer at day 5 in the ankle joint between RRV E2 DM and RRV WT, but these differences were not sustained at time points of peak disease severity (7 or 10 dpi) (Fig. 5B).
Fig. 5.
RRV lacking E2 N-linked glycans retain ability to replicatein vivo. Quadriceps muscle (A) and ankle joints (B) from mice infected with RRV WT (solid circles), E2 N200Q (open circles), E2 N262Q (solid squares) or E2 DM (open square) were assayed to determine viral titer at various times post infection. Viral titer was determined by plaque assay on BHK-21 cells. *** p < 0.005 ** p < 0.01 by one-way ANOVA. (C) Quantification of total numbers of RRV RNA genomes per µg of RNA from the quadriceps muscles of mice infected with either RRV WT (solid circles) or E2 DM (open squares). ** p < 0.01 by Student's t-test.
In order to confirm the plaque assay results, we also performed quantitative real time RT-PCR to determine the absolute numbers of RRV genomes per µg of RNA within the quadriceps muscle between WT and E2 DM virus at 3 and 5 dpi. We did not observe any differences in the number of RRV genomes between the two viruses at 3 dpi (Fig. 5C). This suggests that the overall levels of viral replication within the tissues are equivalent between the wild type and mutant virus at this time point. However, consistent with reduced viral titer at 5 dpi, we observed a similar decrease in viral RNA at 5 dpi in the quadriceps muscle between WT and E2 DM (Fig. 5C). Taken together, these data indicate that the loss of the glycans does not affect the overall ability of the virus disseminate to and replicate within target tissues, however the E2 DM does show reduced replication at intermediate time points.
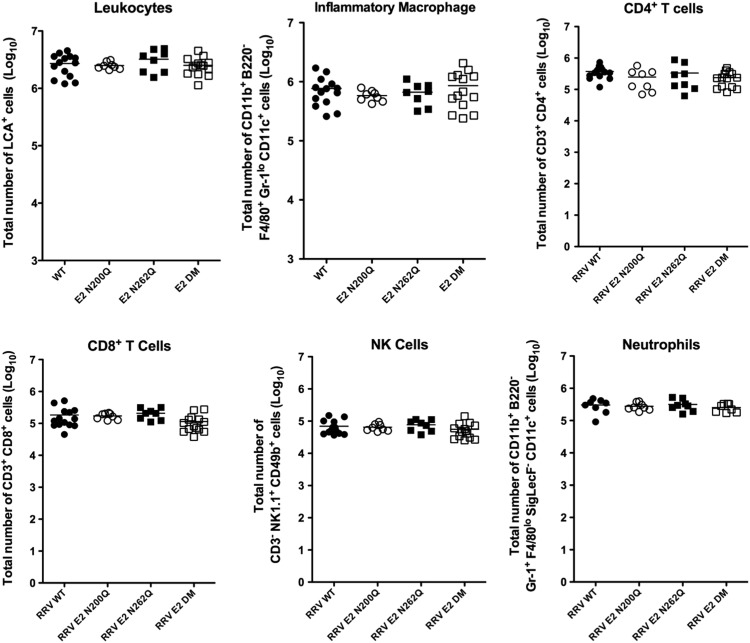
4.6. The RRV E2 glycan mutants and WT RRV induce equivalent inflammatory responses
RRV infection induces an inflammatory response that results in infiltration of inflammatory cells into target tissues such as the quadriceps muscle, subsequently leading to tissue damage (Morrison et al., 2006). Prior studies demonstrated that even though RRV-infected C3-/- and MBL-DKO mice were resistant to virus-induced disease, virus induced inflammatory cell recruitment was unaffected (Gunn et al., 2012, Morrison et al., 2007). Therefore, we evaluated the E2 glycan mutants to determine if virus induced inflammation was similar to that observed in C3 or MBL deficient animals by quantifying and characterizing inflammatory cell populations from whole quadriceps muscle of mice infected with the RRV WT or each of the mutant viruses by flow cytometry. At 10 dpi, which is a time point of peak inflammation within tissues, we did not observe any differences in the total magnitude or the composition of the inflammatory cell population between RRV WT and the glycan mutants ( Fig. 6). Total numbers of leukocytes in the quadriceps muscle were equivalent between mice in all groups (Fig. 6), indicating that the E2 N-linked glycans do not affect infiltration of inflammatory cells into skeletal muscle. Furthermore, the numbers of inflammatory macrophages, which are thought to drive much of the tissue damage during RRV infection (Lidbury et al., 2000, Lidbury et al., 2008), and other cell types including CD4+ and CD8+ T cells, NK cells, and neutrophils, were equivalent between RRV WT and the glycan mutants (Fig. 6). These data suggest that loss of the E2 glycans does not significantly affect the induction of the infiltration or composition of the inflammatory cells into the quadriceps muscle following RRV infection.
Fig. 6.
The RRV E2 glycan mutants and WT RRV induce equivalent inflammation within skeletal muscle. Leukocytes were isolated from the quadriceps muscle of mice infected with RRV WT (solid circles), E2 N200Q (open circles), E2 N262Q (solid squares) or E2 DM (open squares) mice at 10 dpi. Cells were characterized and quantified by flow cytometry using the markers indicated on Y-axis. Total numbers of leukocytes, inflammatory macrophages and other inflammatory cell types are shown. Each data point represents a single animal, and data presented in the figure are combined from three independent experiments.
5. Conclusions
We report herein that the RRV E2 N-linked glycans bind MBL and contribute to activation of complement, ultimately enhancing RRV-mediated tissue destruction. As MBL binds to carbohydrates, we hypothesized that the viral N-linked glycans displayed on RRV-infected cells act as ligands for MBL-mediated complement activation. Thus, to determine whether the viral N-linked glycans contribute to MBL-mediated complement activation during RRV infection, we generated a panel of RRV mutants that lack the E2 N-linked glycans: N200Q, N262Q, or N200;262Q (double mutant, or DM). Consistent with our hypothesis, reduced MBL deposition was observed onto skeletal muscle cells infected with RRV E2 DM compared to RRV wild-type, suggesting that the viral N-linked glycans drive MBL deposition onto infected cells. We next determined whether the E2 N-linked glycans are required for severe disease using a mouse model of RRV-induced myositis. RRV mutants lacking one or both glycans fail to induce severe disease in vivo, with mice infected with the glycan mutants exhibiting significantly reduced inflammatory pathology and tissue damage in quadriceps muscle compared to mice infected with wild-type RRV, despite similar viral loads within infected quadriceps muscle and ankle joints at times of peak disease. Importantly, MBL deposition and complement activation within the skeletal muscle of RRV E2 DM infected mice was reduced compared to wild-type virus, consistent with our hypothesis that the viral E2 glycans drive complement activation within inflamed tissues. Because RRV infection is associated with infiltration of inflammatory leukocytes into the quadriceps muscle, we next tested whether the E2 N-linked glycans modulate recruitment of inflammatory cells into the muscle. However, similar numbers of infiltrating inflammatory leukocytes within skeletal muscle were observed for all viruses, suggesting that complement activation, rather than inflammatory cell recruitment, is differentially activated by the E2 N-linked glycan mutants and contributes to disease. Thus, these data demonstrate that the RRV E2 N-linked glycans present on infected cells contribute to severe RRV-induced disease through MBL-mediated activation of complement.
6. Discussion
Mosquito-borne alphaviruses such as RRV and CHIKV are emerging viruses that are a significant cause of explosive outbreaks of infectious arthralgia and myalgia in humans. The host complement system through MBL plays a critical role in mediating development of disease and tissue damage through activation of inflammatory cells that infiltrate into the muscle and joints following infection (Gunn et al., 2012, Morrison et al., 2008, Morrison et al., 2007). In this study, we demonstrate that the RRV envelope N-linked glycans contribute to MBL deposition and complement activation leading to the development of severe virus-induced damage to the musculoskeletal system.
The results presented in this study demonstrate that viral N-linked glycans promote MBL binding to RRV infected cells and that MBL deposition and subsequent complement activation is reduced in tissues from animals infected with E2 DM (Fig. 1, Fig. 4). Given the central role that MBL, complement activation, and subsequent inflammatory cell activation through CR3 plays in the pathogenesis of RRV-induced disease, it is likely that the major mechanism of attenuation for the E2 DM virus is through reduced MBL binding, complement activation, and production of the CR3 ligand iC3b leading to reduced activation of macrophage within tissues. However, given that the E2 DM virus did show reduced levels of replication at intermediate times post infection (Fig. 5A), we cannot rule out the possibility that at least some of the impact on virus-induced disease with the E2 DM virus is due to transient reduction in viral load. However, it is important to point out that the decrease in viral load at 3 and 5 dpi did not affect the recruitment of inflammatory cells into the sites of viral replication (Fig. 6), and that viral loads were indistinguishable at 7 dpi, which is a time when severe disease is readily apparent with the wild type virus (Fig. 5A-B). Furthermore, we and others showed that disease is reduced in mice infected with either of the single glycan mutants that exhibit either no difference in viral loads compared to RRV WT (E2 N262Q mutant) or modest differences in viral loads (E2 N200Q mutant) (Nelson et al., 2016), and the reduction in disease in these mice correlates with reduced C3 deposition within infected tissues. Thus, these data support the hypothesis that the E2 N-linked glycans primarily affect RRV pathogenesis through complement activation.
Beyond activation of the complement system, the E2 N-linked glycans likely modulate other aspects of the disease (both protective and pathologic) through differential induction of innate immune responses via interactions with carbohydrate binding proteins such as C-type lectins, and toll-like receptors (Shabman et al., 2008). In addition, while we have focused on the role of the E2 glycans in mediating RRV-induced disease in this study, the E1 glycan was recently shown to modulate RRV pathogenesis, as a mutant virus lacking the E1 glycan (E1 N141Q) demonstrated enhanced clearance from tissues, resulting in reduced inflammation and pathology (Nelson et al., 2016). Interestingly, the E1 N141Q mutant enhanced circulating IFNγ levels, whereas viruses lacking the E2 glycans did not affect IFNγ production, suggesting that the E1 and E2 glycans differentially interact with the immune system (Nelson et al., 2016). Future studies aimed at identifying and dissecting the differential interactions of the E1 and E2 glycans with the innate immune system may reveal novel mechanisms through which viral N-linked glycans modulate viral-induced inflammatory disease.
The interaction between MBL and viral N-linked glycans has been demonstrated in a number of other virus systems including HIV, SARS-CoV, Ebola virus, and West Nile virus (Ezekowitz et al., 1989, Zhou et al., 2010, Ip et al., 2005, Hart et al., 2002, Fuchs et al., 2010, Ji et al., 2005). In these systems, MBL recognizes and binds to high mannose glycans on the envelope glycoproteins, and leads either to direct neutralization of virus, or aids in inhibiting virus infection by preventing or abrogating attachment and entry into host cells. In contrast to the protective role of MBL for other viruses, MBL clearly plays a pathologic role during RRV infection and these studies provide new insights into the role of viral N-linked glycans in driving this pathologic process. Furthermore, studies with other viruses have demonstrated that the interaction between MBL and the virus particle is important for MBL-mediated neutralization (Zhou et al., 2010, Fuchs et al., 2010, Ji et al., 2005). In contrast to those studies, we have several lines of evidence that demonstrate MBL interacts with RRV-infected cells through the E2 N-linked glycans on the surface of the infected cell rather than with the virion particle. First, MBL deposition onto infected cells in vitro and within infected tissues in vivo are elevated compared to mock infection (Gunn et al., 2012). Second, MBL deposition is reduced in cells and tissues infected with RRV E2 DM compared to WT independent of viral replication indicating that the E2 glycans promote MBL binding. Finally, we observed MBL deposition onto RRV-infected cells rather than uninfected cells, which was reduced in E2 DM-infected cells, despite surface expression of the RRV glycoproteins on E2 DM-infected cells. Thus, while the individual E2 glycans appear to have little impact on replication in either mammalian or mosquito cells (Shabman et al., 2008, Nelson et al., 2016), we cannot rule out the possibility that loss of the N-linked glycans leads to conformational changes in E2, which could diminish MBL binding. Our results suggest that the viral N-linked glycans are essential for promoting MBL deposition onto the surface of infected cells. In this way, the E2 N-linked glycans contribute to pathology and disease in the mammalian host.
In addition to providing new insights into the pathogenesis of alphavirus-induced disease, these findings suggest that targeting interactions between the viral N-linked glycans and MBL may have therapeutic potential for treating RRV-induced disease. Indeed, a recent study using a MASP competitive binding inhibitor which blocks activation of the lectin pathway resulted in abrogation of RRV-induced disease (Banda et al., 2014). Thus, blocking the ability of MBL to interact with the viral N-linked glycans through inhibition of downstream proteases, such as MASP proteins, may be a viable therapeutic strategy that can prevent the severe pathology associated with RRV induced disease, while maintaining the protective aspects of the host innate immune response. Furthermore, given that the viral N-linked glycan mutants are attenuated for disease, but not replication, it may be possible to incorporate N-linked glycan mutants into live attenuated alphavirus vaccines for enhanced safety.
In summary, we have demonstrated that the interaction between the host innate immune protein MBL and the RRV E2 N-linked glycans contribute to the pathogenesis of RRV-induced arthritis and myositis through activation of the host complement system. Our data supports a therapeutic strategy of specifically targeting the interaction between the RRV E2 N-linked glycans and MBL through the use of MBL inhibitors in the treatment of RRV and other virus-induced inflammatory diseases.
Acknowledgements
We thank the members of the Heise lab for helpful scientific discussions, and in particular would like to thank Martin Ferris for assistance with statistical analysis. We would also like to thank Janice Weaver and the LCCC/DLAM histopathology core facility and Bob Bagnell and the UNC microscopy facility.
Acknowledgments
Funding sources
This work was supported by the National Institutes of Health [grant numbers R01 AR47190, U19 AI 109761, U19 AI100625, and U54 AI 057157].
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.virol.2017.12.022.
Appendix A. Supplementary material
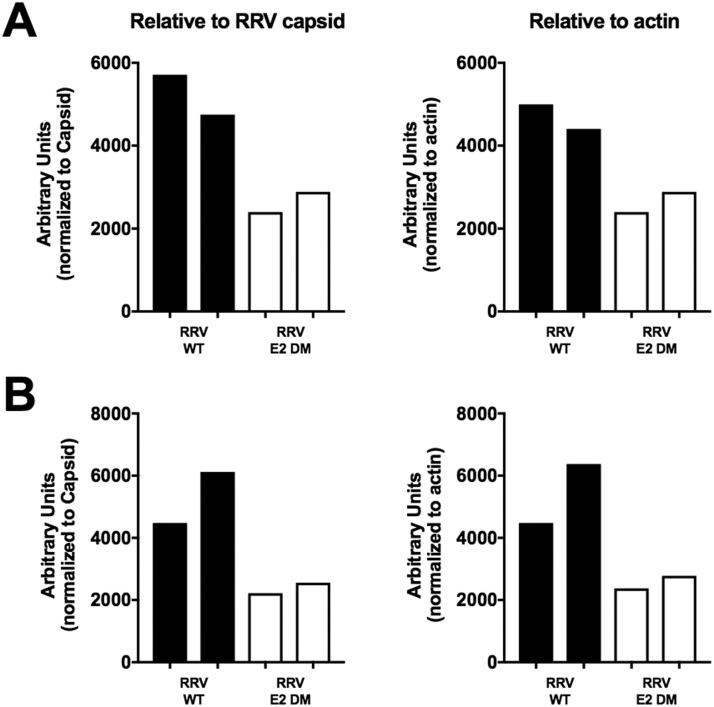
Fig. S1.
Quantification of MBL deposition. MBL-C deposition measured in Fig. 1A (A) or Fig. 1B (B) was normalized by densitometry analysis relative to viral RRV capsid protein (left graphs), or actin (right graphs).
References
- Abram C.L., Lowell C.A. The ins and outs of leukocyte integrin signaling. Annu. Rev. Immunol. 2009;27:339–362. doi: 10.1146/annurev.immunol.021908.132554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banda N.K., Mehta G., Kjaer T.R., Takahashi M., Schaack J., Morrison T.E., Thiel S., Arend W.P., Holers V.M. Essential role for the lectin pathway in collagen antibody-induced arthritis revealed through use of adenovirus programming complement inhibitor MAp44 expression. J. Immunol. 2014;193:2455–2468. doi: 10.4049/jimmunol.1400752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarris B.J., Doherty R.L., Fraser J.R., French E.L., Muirden K.D. Epidemic polyarthritis: a cytological, virological and immunochemical study. Aust. N. Z. J. Med. 1975;5:450–457. doi: 10.1111/j.1445-5994.1975.tb03056.x. [DOI] [PubMed] [Google Scholar]
- Ezekowitz R.A., Kuhlman M., Groopman J.E., Byrn R.A. A human serum mannose-binding protein inhibits in vitro infection by the human immunodeficiency virus. J. Exp. Med. 1989;169:185–196. doi: 10.1084/jem.169.1.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser J.R., Cunningham A.L., Clarris B.J., Aaskov J.G., Leach R. Cytology of synovial effusions in epidemic polyarthritis. Aust. N. Z. J. Med. 1981;11:168–173. doi: 10.1111/j.1445-5994.1981.tb04226.x. [DOI] [PubMed] [Google Scholar]
- Fuchs A., Lin T.Y., Beasley D.W., Stover C.M., Schwaeble W.J., Pierson T.C., Diamond M.S. Direct complement restriction of flavivirus infection requires glycan recognition by mannose-binding lectin. Cell Host Microbe. 2010;8:186–195. doi: 10.1016/j.chom.2010.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunn B.M., Morrison T.E., Whitmore A.C., Blevins L.K., Hueston L., Fraser R.J., Herrero L.J., Ramirez R., Smith P.N., Mahalingam S., Heise M.T. Mannose binding lectin is required for alphavirus-induced arthritis/myositis. PLoS Pathog. 2012;8:e1002586. doi: 10.1371/journal.ppat.1002586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart M.L., Saifuddin M., Uemura K., Bremer E.G., Hooker B., Kawasaki T., Spear G.T. High mannose glycans and sialic acid on gp120 regulate binding of mannose-binding lectin (MBL) to HIV type 1. AIDS Res. Hum. Retrovir. 2002;18:1311–1317. doi: 10.1089/088922202320886352. [DOI] [PubMed] [Google Scholar]
- Hazelton R.A., Hughes C., Aaskov J.G. The inflammatory response in the synovium of a patient with Ross River arbovirus infection. Aust. N. Z. J. Med. 1985;15:336–339. doi: 10.1111/j.1445-5994.1985.tb04048.x. [DOI] [PubMed] [Google Scholar]
- Herrero L.J., Nelson M., Srikiatkhachorn A., Gu R., Anantapreecha S., Fingerle-Rowson G., Bucala R., Morand E., Santos L.L., Mahalingam S. Critical role for macrophage migration inhibitory factor (MIF) in Ross River virus-induced arthritis and myositis. Proc. Natl. Acad. Sci. U.S.A. 2011;108:12048–12053. doi: 10.1073/pnas.1101089108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrero L.J., Sheng K.C., Jian P., Taylor A., Her Z., Herring B.L., Chow A., Leo Y.S., Hickey M.J., Morand E.F., Ng L.F., Bucala R., Mahalingam S. Macrophage migration inhibitory factor receptor CD74 mediates alphavirus-induced arthritis and myositis in murine models of alphavirus infection. Arthritis Rheum. 2013;65:2724–2736. doi: 10.1002/art.38090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirahashi J., Mekala D., Van Ziffle J., Xiao L., Saffaripour S., Wagner D.D., Shapiro S.D., Lowell C., Mayadas T.N. Mac-1 signaling via Src-family and Syk kinases results in elastase-dependent thrombohemorrhagic vasculopathy. Immunity. 2006;25:271–283. doi: 10.1016/j.immuni.2006.05.014. [DOI] [PubMed] [Google Scholar]
- Ip W.K., Chan K.H., Law H.K., Tso G.H., Kong E.K., Wong W.H., To Y.F., Yung R.W., Chow E.Y., Au K.L., Chan E.Y., Lim W., Jensenius J.C., Turner M.W., Peiris J.S., Lau Y.L. Mannose-binding lectin in severe acute respiratory syndrome coronavirus infection. J. Infect. Dis. 2005;191:1697–1704. doi: 10.1086/429631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji X., Olinger G.G., Aris S., Chen Y., Gewurz H., Spear G.T. Mannose-binding lectin binds to Ebola and Marburg envelope glycoproteins, resulting in blocking of virus interaction with DC-SIGN and complement-mediated virus neutralization. J. Gen. Virol. 2005;86:2535–2542. doi: 10.1099/vir.0.81199-0. [DOI] [PubMed] [Google Scholar]
- Lidbury B.A., Simeonovic C., Maxwell G.E., Marshall I.D., Hapel A.J. Macrophage-induced muscle pathology results in morbidity and mortality for Ross River virus-infected mice. J. Infect. Dis. 2000;181:27–34. doi: 10.1086/315164. [DOI] [PubMed] [Google Scholar]
- Lidbury B.A., Rulli N.E., Suhrbier A., Smith P.N., McColl S.R., Cunningham A.L., Tarkowski A., van Rooijen N., Fraser R.J., Mahalingam S. Macrophage-derived proinflammatory factors contribute to the development of arthritis and myositis after infection with an arthrogenic alphavirus. J. Infect. Dis. 2008;197:1585–1593. doi: 10.1086/587841. [DOI] [PubMed] [Google Scholar]
- Morrison T.E., Whitmore A.C., Shabman R.S., Lidbury B.A., Mahalingam S., Heise M.T. Characterization of Ross River virus tropism and virus-induced inflammation in a mouse model of viral arthritis and myositis. J. Virol. 2006;80:737–749. doi: 10.1128/JVI.80.2.737-749.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison T.E., Fraser R.J., Smith P.N., Mahalingam S., Heise M.T. Complement contributes to inflammatory tissue destruction in a mouse model of Ross River virus-induced disease. J. Virol. 2007;81:5132–5143. doi: 10.1128/JVI.02799-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison T.E., Simmons J.D., Heise M.T. Complement receptor 3 promotes severe ross river virus-induced disease. J. Virol. 2008;82:11263–11272. doi: 10.1128/JVI.01352-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson M.A., Herrero L.J., Jeffery J.A., Hoehn M., Rudd P.A., Supramaniam A., Kay B.H., Ryan P.A., Mahalingam S. Role of envelope N-linked glycosylation in Ross River virus virulence and transmission. J. Gen. Virol. 2016;97:1094–1106. doi: 10.1099/jgv.0.000412. [DOI] [PubMed] [Google Scholar]
- Pletnev S.V., Zhang W., Mukhopadhyay S., Fisher B.R., Hernandez R., Brown D.T., Baker T.S., Rossmann M.G., Kuhn R.J. Locations of carbohydrate sites on alphavirus glycoproteins show that E1 forms an icosahedral scaffold. Cell. 2001;105:127–136. doi: 10.1016/s0092-8674(01)00302-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricklin D., Hajishengallis G., Yang K., Lambris J.D. Complement: a key system for immune surveillance and homeostasis. Nat. Immunol. 2010;11:785–797. doi: 10.1038/ni.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross G.D. Regulation of the adhesion versus cytotoxic functions of the Mac-1/CR3/alphaMbeta2-integrin glycoprotein. Crit. Rev. Immunol. 2000;20:197–222. [PubMed] [Google Scholar]
- Shabman R.S., Rogers K.M., Heise M.T. Ross River virus envelope glycans contribute to type I interferon production in myeloid dendritic cells. J. Virol. 2008;82:12374–12383. doi: 10.1128/JVI.00985-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss J.H., Strauss E.G. The alphaviruses: gene expression, replication, and evolution. Microbiol. Rev. 1994;58:491–562. doi: 10.1128/mr.58.3.491-562.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K., Ip W.E., Michelow I.C., Ezekowitz R.A. The mannose-binding lectin: a prototypic pattern recognition molecule. Curr. Opin. Immunol. 2006;18:16–23. doi: 10.1016/j.coi.2005.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y., Lu K., Pfefferle S., Bertram S., Glowacka I., Drosten C., Pohlmann S., Simmons G. A single asparagine-linked glycosylation site of the severe acute respiratory syndrome coronavirus spike glycoprotein facilitates inhibition by mannose-binding lectin through multiple mechanisms. J. Virol. 2010;84:8753–8764. doi: 10.1128/JVI.00554-10. [DOI] [PMC free article] [PubMed] [Google Scholar]