Abstract
Alcohol-based products for skin antisepsis have a long history of safety and efficacy in the United States and abroad. However, alcohol alone lacks the required antimicrobial persistence to provide for the sustained periods of skin antisepsis desired in the clinical environment. Therefore, alcohol-based products must have a preservative agent such as iodine/iodophor compounds, chlorhexidine gluconate, or zinc pyrithione, to extend its antimicrobial effects. Iodine, iodophors, and chlorhexidine gluconate are well-characterized antimicrobials and preservatives. The thrust of our effort was to examine the characteristics of the lesser-known zinc pyrithione and to evaluate its utility as a preservative in the formulation of alcohol-based products for skin antisepsis. This work includes a literature review of current zinc pyrithione applications in drugs and cosmetics, a safety and toxicity evaluation, consideration of the proposed mechanisms of antimicrobial action, in vitro and in vivo efficacy data, and a discussion of the mechanisms that confer the desired antimicrobial persistence. In addition, alcohol-based, zinc pyrithione-preserved, commercially available products of skin antisepsis are compared with other commercially available antimicrobials used for skin antisepsis and with additional alcohol-based products with different preservatives. The authors' conclusion is that zinc pyrithione is not only a safe and effective antimicrobial but that its use in certain alcohol-based formulations results in antimicrobial efficacy exceeding that of iodine and chlorhexidine gluconate.
Alcohol-based products for skin antisepsis have enjoyed a long history of safety and efficacy in the United States, as well as in many countries abroad. Examples of use include surgical scrubs, health care personnel handwashes, patient preoperative skin preparations, injection/catheter site preparations, preoperative antiseptic shower solutions, hand rubs/sanitizers, and tinctures such as those of iodine.1, 2, 3, 4, 5 Of the antimicrobials routinely used for skin antisepsis (alcohols, chlorhexidine gluconate [CHG], iodine/iodophors, parachlorometaxylenol [PCMX], triclosan, and quaternary ammonium compounds), alcohols are by far the fastest acting and most efficacious.6, 7, 8, 9, 10, 11 Almost exclusively, the short-chain, aliphatic alcohols—ethanol, isopropanol, and, in Europe, n-propanol—are used for skin and hand antisepsis. They have excellent activity against bacteria, fungi, and enveloped (and some nonenveloped) viruses.10 Alcohols may be used either alone or in combination with other antimicrobials to increase the efficacy and confer substantivity (persistence).
The Centers for Disease Control (CDC) and Prevention in its “Guideline for Prevention of Surgical Site Infection, 1999” acknowledged the utility of alcohol-based products in preoperative hand/forearm antisepsis and preoperative skin preparation.12 In late 2002, the recommendations of the Healthcare Infection Control Practices Advisory Committee and the HICPAC/SHEA/APIC/IDSA Hand Hygiene Task Force were published.9 This report recognized the speed and broad antimicrobial spectrum of alcohol, codified uses for alcohol-based products in skin antisepsis practices, and noted the lack of antimicrobial persistence associated with alcohol when used alone. The FDA also noted alcohol's lack of antimicrobial persistence; however, it allows a preservative agent to be incorporated into the vehicle (defined as the product without the active ingredient) to provide for the persistent antimicrobial effect necessary to sustain a reduction in the number of bacteria for 6 hours postapplication.4 The preservative supplies the required persistent antimicrobial activity for these formulations to meet or exceed the established agency efficacy. Preservative systems in some of the currently marketed alcohol-based products for skin antisepsis include iodine/iodophors compounds, CHG, and zinc pyrithione (ZPT). Although much has been written about the antimicrobial effects and the preservative potential of iodine and CHG, little has been published regarding these characteristics as attributes of ZPT. Therefore, this article includes a brief review of the published literature related to the development, safety, efficacy, and clinical utility of ZPT and evaluating its merits as a preservative for alcohol-based products of skin antisepsis.
Characteristics of zinc pyrithione
Synthesis, structure, and solubility
The natural antibiotic aspergillic acid contains a cyclic hydroxamic acid functional group in a pyrazine nucleus. Attempts to develop synthetic methods for introducing heterocyclic rings into the hydroxamic acid group present in aspergillic acid led in 1950 to the preparation for N-hydroxy-2-pyridinethione (HPT). The synthesis was achieved by conversion of a 2-pyridyl ether to its N-oxide, followed by dealkylation.13 Reaction of 2-bromopyridine-N-oxide with thiourea forms 2-pyridyl-N-oxide-isothiourea hydrobromide and, followed by treatment with aqueous sodium carbonate, produces N-hydroxy-2-pyridinethione (HPT).14 This compound was shown to have potent antimicrobial properties. In vitro, 1 μg HPT would inhibit Staphylococcus aureus.15 Thus, this synthetic analog was 30 times more potent as an antimicrobial than the native aspergillic acid. Later, HPT was shown to have extremely potent activity against gram-positive and gram-negative bacterial species as well as strong activity against yeasts and fungi: eg, Aspergillus, Trichophyton species, Candida albicans, and Cryptococcus species.16 Cox, in the mid-1950s, reviewed the uses of pyridine-N-oxides and noted that the mercapto derivatives formed quaternary ammonium compounds or heavy metal salts, which were described as effective fungicides and antibacterial agents and suggested their uses in pharmaceutic preparations.17 As a zinc chelate of HPT, ZPT exists in the monomeric form as 2 pyridine rings bound to a central zinc atom by bonds between the zinc atom and the sulphur and oxygen molecules of the pyridine ring structures.18, 19 ZPT is practically insoluble in water, organic solvents, or surfactants—a property that can be problematical when formulating with the compound.20 The structure of ZPT is presented in Fig 1.
Fig 1.
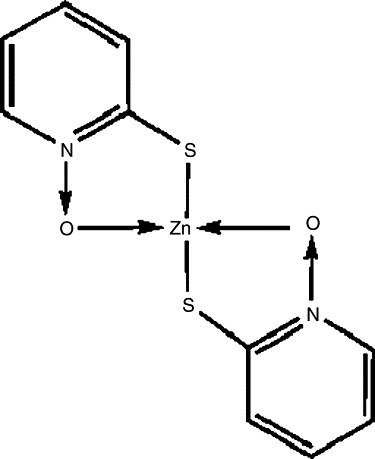
Structure of ZPT.
Applications
ZPT has a long history of numerous medical, scientific, and industrial applications. The compound has a master file registered with the FDA. Of some 1350 compounds screened, ZPT was one of the most active antifungal and antibacterial compounds examined.20, 21 For example, Pityrosporum ovale is thought to play a pathogenic role in skin conditions such as seborrheic dermatitis and dandruff.22 This led one well-known pharmaceutic company to conduct safety evaluations of ZPT in a shampoo formulation.21 The result was an antidandruff product, Head & Shoulders (Procter & Gamble, Cincinnati, Ohio) that is currently sold over-the-counter with 1.0% ZPT as the active ingredient. Presently, the FDA acknowledges the efficacy of ZPT in the treatment of dandruff and seborrheic dermatitis.23, 24 The ZPT-based shampoo has shown to be effective in treating tinea versicolor,25 and it is highly effective for psoriasis of the scalp.26
Cosmetic preservation has been a frequent use of ZPT for many years.18 ZPT has proven to be effective, even at low concentrations, against both gram-positive and gram-negative bacteria and fungi. It is compatible with most commonly used cosmetic ingredients; has a good toxicity profile for this type of application; and can be safely used to minimize discoloration, off-odors, and emulsion breaks because of microorganisms. ZPT helps to prevent spoilage of cosmetics because of microbial contamination during use, is found in GoJo Hand Cleaner products (GOJO Industries, Inc. Akron, Ohio), and in over-the-counter antimicrobials such as Lanacane (Combe, Inc.,White Plains, NY).27 Finally, ZPT has also been used in commercial laundries for inhibiting mold growth on fabrics.20
Safety and toxicity
In addition to a 50-year product history, the safety and toxicity of ZPT has been formally evaluated in animal models and by in vitro and human in vivo studies. When ZPT was applied to intact skin of monkeys with surfactants, the absorption was only 0.20% of the amount applied.28 Concentrations of ZPT in the blood stream following topical application are below the threshold of detection. The absorbed dose would likely be further reduced in humans by the fact that hand skin has a lower permeability than scalp skin.29, 30 ZPT does not induce primary skin irritation or sensitization in human skin.30 Finally, when a 2.0% suspension of ZPT was instilled into the conjunctival sacs of the eyes of 6 albino rabbits, ZPT produced only slight irritation of the conjunctival vessels, which lasted 3 days, but no corneal opacities were observed.31 Ocular toxicity is well known to be associated with other preservative systems such as CHG, and skin irritation and sensitization with iodine and CHG-based products has been documented.10, 32, 33, 34
Antimicrobial activity
The available data on the mode of antimicrobial action suggest that ZPT is membrane active, as indicated by the inhibition of uptake of several unrelated substrates in both bacteria and fungi35, 36, 37, 38 and the observed depolarization of the transmembrane potential in Neurospora crassa.39 The effects of an antimicrobial agent on substrate transport and related metabolism may be used as indicators of the membrane activity of the test agent.40 In turn, these effects may be reflected as a reduction in intracellular ATP levels.41 ZPT is a poor inhibitor of substrate catabolism. Subinhibitory concentrations of the biocide greatly reduce intracellular ATP levels in both Escherichia coli and Pseudomonas aeruginosa. This is thought to be due to the action of ZPT on the gram-negative bacterial membrane.42 Further investigation of the action at the membrane suggests that ZPT forms stable interactions with the bacterial membrane phospholipid phosphatidylethanolamine. This may result in the disaggregation of the phospholipid head structure at the outer membrane and may also indicate chelation of phosphorylethanolamine head groups from the core structure of the external lipopolysaccharide. This would further disrupt the membrane.43 In addition, current-voltage analysis demonstrates that the depolarization of the bacterial membrane is accompanied by a decrease in membrane electrical conductance in a manner consistent with inhibition of the primary proton pump and consistent with a mode of action of ZPT on plasma membrane ion channels. Therefore, ZPT inhibits membrane transport via a direct or indirect effect on the primary proton pump that energizes transport, and the site of action of ZPT is likely to be intracellular rather than extracellular.42 Other studies on the mode of action of pyridine-N-oxides has demonstrated their potent bactericidal activity to be linked to their ability to chelate: ie, to form cyclic complexes with the ions of heavy metals.44 Additional investigations reported by Hyde and Nelson have suggested that other mechanisms may be applicable.18 The authors propose that the pyrithione is an antimetabolite of the pyridine derivative pyridoxal and suggest that the activity of ZPT may be analogous to the inhibition of microbial folate production by sulfa drugs. Finally, dipole structure of the molecule creates a pseudoquaternary ammonium group, providing yet another potential mode of antimicrobial action for ZPT. Multiple mechanisms appear to be at work, suggesting that antimicrobial resistance is unlikely to develop. Reports of the development of antimicrobial resistance are not readily available, and further detailed investigation may be appropriate.
In vitro efficacy data
The in vitro composite data in Table 1 document the broad spectrum of antimicrobial activity of ZPT as well as quantify the inhibitory capacity of this compound when tested against numerous bacteria, yeast, and fungi (Personal communications from Ron Jones, M. D., Professor and Director, Division of Medical Microbiology, Director, Anti-Infectives Research Center and Special Microbiology Laboratories, Department of Pathology, University of Iowa College of Medicine, Iowa City, Ia, January 1993).20, 22, 27, 42 The antimicrobial range of ZPT is sufficient to include many pathogenic, opportunistic, and saprophytic organisms. Inhibitory concentrations are often achieved at a level of 40 μg/mL (40 ppm) or less for numerous pathogenic species.
Table 1.
Mean minimal inhibitory concentration of ZPT for selected bacteria, yeast, and fungi∗
| Organism tested | Number of strains/strain identification | MIC 50 in ppm | MIC 90 in ppm |
|---|---|---|---|
| Staphylococcus aureus | 10 strains | 10 | 10 |
| CNS† | 10 strains | 10 | 10 |
| Enterococcus species‡ | 10 strains | 20 | 20 |
| Streptococcus faecalis | ATCC 19433 | 10 | 20 |
| Bacillus cereus | ATCC 11778 | 10 | 10 |
| Sarcina lutea | ATCC 9341 | 10 | 20 |
| Escherichia coli | 10 strains | 10 | 10 |
| Proteus vulgaris | ATCC 9920 | 10 | 10 |
| Pseudomonas aeruginosa | NCIMB 10548 | ND | 13 |
| Citrobacter species§ | 10 strains | 20 | 40 |
| Acinetobacter species¶ | 10 strains | 40 | 150 |
| Serratia marcescens | 10 strains | 20 | 40 |
| Salmonella/Shigella# | 10 strains | 20 | 20 |
| Xanthomonas maltophilia | 10 strains | 150 | 300 |
| Morganella morganii | 5 strains | 40 | 150 |
| Enterobacter species∗∗ | 10 strains | 20 | 20 |
| Klebsiella species†† | 10 strains | 20 | 20 |
| Dermatophytes‡‡ | 5 strains | 10 | 10 |
| Aspergillus species§§ | 5 strains | 10 | 10 |
| Candida species¶¶ | 5 strains | 10 | 10 |
MIC, Mean minimal inhibitory concentration; ND, not done; ppm, parts per million.
Includes S epidermidis (4 strains), S hominis (1 strain), S saprophyticus (3 strains), and S simulans (2 strains).
Includes E faecalis (5 strains), E faecium (2 strains), E avium (1 strain), E durans (1 strain), and E raffinosus (1 strain).
Includes C diversus (6 strains) and C freundi (4 strains).
Includes A anitratus (8 strains and A lwoffi (2 strains).
Includes S enteritidis (6 strains) and S sonnei (4 strains).
Includes E cloacae (7 strains) and E aerogenes (3 strains).
Includes K oxytoca (2 strains) and K pneumoniae (8 strains).
Includes 1 strain each of M canis, M gypseum, T mentagrophytes, T rubrum, and Trichophyton spp.
Includes A flavus (2 strains), A fumigatus (2 strains), and A terreus (1 strain).
Includes C glabrata (1 strain), C krusei (1 strain), C lusitaniae (1 strain), C parapsilosis (1 strain), and C tropicalis (1 strain).
In vivo efficacy and persistence data
In 1979, Leyden et al45 developed a novel in vivo assay now known as the persistence (substantivity) test. This significant development was used to demonstrate the in vivo efficacy of ZPT in topical antisepsis applications. The assay determines the ability of the test agent to establish a reservoir in the stratum corneum. Substantive agents that diffuse into the stratum corneum or bind chemically to it will not be readily removed either by loss from the surface or by absorption. The antibacterial effect will therefore last several days. For this assay, 0.5 mL of the test agent is applied with a pipette twice daily for 4 consecutive days to the entire volar forearm. Twenty-four hours after the last application, occlusive dressings are applied. If an antimicrobial effect is demonstrable, the test is repeated, and the posttreatment interval is extended to 72 hours. The geometric means for bacterial counts per square centimeter are determined. For this study, 1.0% ZPT was compared with an equal concentration of CHG. The results of this 8-subject study demonstrated antimicrobial parity at 24 hours; however, at the 72-hour time point, ZPT provided a greater persistent effect than the comparator (ZPT = 120 vs CHG = 87,000 colonies/cm2 recovered). The suppression of the bacterial counts on the forearm 72 hours postapplication for ZPT is significantly greater than for chlorhexidine gluconate. These data support the superior efficacy and the persistence of ZPT for this indication.
In addition, Leyden et al45 devised an “expanded flora test” to evaluate further the in vivo antimicrobial efficacy and persistence of topical antiseptics. This test measures the ability of an agent to suppress a dense and flourishing population of organisms. For this study, the forearm is wrapped with several layers of impermeable plastic film and then sealed at the wrist and below the elbow for 24 hours to expand the resident flora. After expansion of the resident flora, 2, 2.5 cm2 areas on each arm are treated with 0.1 mL of the test agent administered by a plastic tuberculin syringe. All sites are immediately covered with a 5.0-cm2 section of impermeable plastic film. Encircling the limb with plastic tape as the final step occlusively seals the site. A strip of 1.25-cm (0.5 in.)-wide, white-backed adhesive tape is placed between each test site to prevent the possibility of translocation of test agents and organisms from 1 site to another. Sites are cultured at 6, 24, and 48 hours, depending on the stability of the test agent. The geometric mean of surviving organisms is determined by standard culture techniques. For the Lynden study reviewed here ( Table 2), 70% ethanol and 1.0% ZPT were compared in a 10-subject panel. As expected, ethanol induced a prompt, significant reduction in bacterial flora that was still measurable at the 6-hour sample when compared with the untreated control sample. The lack of an equally dramatic antibacterial effect 6 hours postapplication for ZPT suggests that a quick-acting antiseptic, such as alcohol, should be combined with it. In this fashion, the alcohol (which lacks persistence) will produce an immediate bactericidal action, and the ZPT (which lacks a dramatic quick kill) will produce a prolonged suppression of the skin bacterial flora. This concept is supported by the ZPT data obtained at the 24- and 48-hour sample points.
Table 2.
Expanded flora test: bacterial counts/cm2 forearm skin for 1.0% ZPT versus 70% ethanol45
| Sample time postmicrobial expansion |
|||
|---|---|---|---|
| Antimicrobial | 6 hours | 24 hours | 48 hours |
| ZPT 1.0% | 65,110 | 19 | 0 |
| Ethanol 70% | 14,000 | 4,694,000 | ND |
| Control untreated | 1,320,000 | 6,904,000 | 2,133,000 |
ND, Not done.
Persistence mechanism
Following topical application, ZPT is deposited in the epidermis; however, the largest proportion of ZPT is deposited on the outer layers of the stratum corneum.27, 45 ZPT is soluble in sebum, and, when applied to the skin, the ZPT in sebum is localized in the hair follicles. No transepidermal penetration occurs. The articles provided do not suggest whether the “dissolution” is of the intact ZPT or whether the zinc is first chelated by a separate mechanism, thus leaving the more soluble pyrithione available to penetrate via the follicle. Others46 used ZPT with radioactive sulfur to monitor tissue location so that the pyrithione moiety would be observed. Deposited particles of ZPT appear to adhere firmly to the hair and stratum corneum and to withstand copious rinsing.46 These adherent particles probably act as a reservoir and are responsible for the prolonged antimicrobial effect (persistence). Diminution of the amount of antibacterial effect with time is probably due to a great extent to normal turnover of the stratum corneum. Also, solubilized ZPT would eventually purge and/or break down further. It is pertinent to note that sebum and topical lipids can inactivate many antimicrobials47; examples include hexachlorophene and benzethonium chloride. ZPT is not only soluble in sebum but retains its antimicrobial activity.
Zinc pyrithione formulations with alcohol
Given the significant history, safety, and diversity of ZPT, it is only logical that other significant uses would be found for this antimicrobial. Despite the limited solubility and the formulation challenges that are characteristic of the compound, ZPT has been used successfully and most recently as a preservative system for several marketed alcohol-based products of skin antisepsis, at concentrations of 0.25% for waterless applications and up to 1.0% for water-aided products. These include surgical scrubs, health care personnel handwashes, patient preoperative skin preparations, and preoperative bath or shower solutions.1, 2, 3, 5, 48 (Products identified here include: Triseptin Water-aided Brush-free Surgical Scrub, Triseptin Waterless Brush-free Surgical Scrub, Triseptin Water-Optional Healthcare Personnel Handwash and Rub, Triseptin Hand and Body Antiseptic, Actiprep One-Step Preoperative Preparation Solution, all produced by Healthpoint, Ltd., Fort Worth, Tex.) In each case, these products are compliant with the FDA's OTC Drug Monograph and command in vitro and in vivo antimicrobial efficacy as required for immediacy of kill, persistence, and residual effect.
Hobson et al was the first to document in vivo the value of ZPT as a preservative system for an alcohol-based, water-aided surgical scrub formulation. The study compared the efficacy of 70% alcohol preserved with ZPT to a second scrub containing only 4.0% CHG and a third scrub containing only 7.5% iodine. Their data were obtained following 11 scrubs over a 5-day period.1, 4 The requirements for successful performance include log10 reductions from baseline of not less than 1.0 for day 1, 2.0 for day 2, and 3.0 for day 5. The immediacy of kill provided by the alcohol-based product on day 1 was readily demonstrated by the nearly 3.0-log10 reduction from baseline counts. The antimicrobial effect becomes enhanced on days 2 and 5 as a result of the contributions provided in part by the preservative system. At the end of the 5-day test period, the superiority of alcohol preserved with ZPT was readily apparent (4.78-log10 reduction from baseline) when compared with CHG or iodine-based products (3.43- and 1.27-log10 reduction from baseline, respectively) for the same day 5 time point. In a second in vivo study, a different formulation of an alcohol-based, ZPT-preserved, waterless surgical scrub was compared with another waterless, alcohol-based scrub containing 1.0% CHG as a preservative (Avagard, 3M Health Care, St Paul, Minn). As may be observed in Fig 2, the products performed similarly on days 1 and 2 (alcohol/ZPT log10 reductions from baseline = 2.32 and 3.07, respectively; alcohol/CHG log10 reductions from baseline = 2.08 and 2.87, respectively). However, by day 5, the antimicrobial efficacy of the alcohol/ZPT product was notably better (log10 reduction from baseline = 3.68) than that of the alcohol/CHG product (log10 reduction from baseline = 3.09) because the ZPT-preserved formulation more readily exceeded the 3.0-log10 reduction required for the surgical scrub indication.4 In a separate in vivo study, the cosmetic and skin-conditioning properties of the 2 waterless formulations were judged to be equal (personal communication, Dr. Ronald Rizer; manuscript submitted for publication to AORN Journal).
Fig 2.
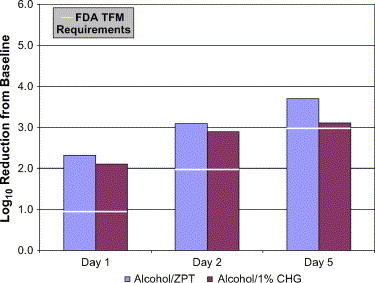
Comparison of alcohol-based surgical scrubs with different preservative systems.
Additional testing was performed with the waterless alcohol/ZPT formulation to assess its virucidal capacity against some common human pathogens. These included human coronavirus (HCoV, ATCC VR-740, both HCoV and SARS-HCoV belong to the virus family Coronaviridae), the human immunodeficiency virus type 1 (HIV-1, from Zepto Metrix Corp. of Buffalo, NY), hepatitis A virus (HAV, from CREM, University of Ottawa), herpes simplex type 1 (HSV-1, ATCC VR-260), and human rotavirus (strain Wa, ATCC VR-2018). These agents represent both RNA and DNA viruses, some with envelopes and others without. The HAV and the human rotavirus represent 2 nonenveloped RNA agents (one linear single-stranded, the other segmented double stranded) that are relatively resistant to known antimicrobials. Using standard virologic techniques, the viral agents were cultured in cell lines appropriate for propagation (see Table 3) to levels at or above the required titer of 106.00 infectious units/mL. The viruses were then harvested and exposed for 3 minutes (30 seconds for the human coronavirus) to the test formulation diluted by virus inoculum to a 90% concentration. Following exposure to the test product, the residual infectious viral population was determined for each virus, employing techniques appropriate for that virus. All testing was performed per American Society for Testing and Materials (ASTM) method E 1052-96, a Standard Test Method for Efficacy of Antimicrobial Agents Against Viruses in Suspension.50 The results of this effort are summarized in Table 3. The test criterion for classification as a virucide is not less than a 3.0-log10 reduction in the postexposure virus population, beyond any observed cytotoxicity. Exposure to the product produced a 3- to 4-log10 reduction in all virus populations tested, thereby meeting or exceeding the standard for classification as a virucide.
Table 3.
Virucidal effects of an alcohol-based, ZPT-preserved, surgical scrub
| Virus | Virus classification | Cell line | Initial CCID50/mL | Posttreatment log10 reduction | Detection method |
|---|---|---|---|---|---|
| Human coronavirus (SARS virus family) | SS RNAenveloped | MRC-5 | 105.77 | 4.27 | CPE∗ |
| Herpes simplex virus | DS DNAenveloped | VERO | ≥106.67 | >3.17 | Plaque assay |
| Human immunodeficiency virus | SS RNA enveloped | CEM | ≥107.50 | ≥4.0 | ELISA for p24 antigen |
| Hepatitis A virus | SS RNA nonenveloped | FRhK-4 | ≥106.50 | ≥3.0 | CPE∗ |
| Human rotavirus | DS RNA nonenveloped | MA-104 | ≥106.50 | ≥3.0 | Plaque assay |
Cytopathic effects.
Seal and Paul-Cheadle confirmed in vivo the antimicrobial efficacy and thus the value of alcohol products formulated with ZPT for added persistence.5 Their report demonstrated the superiority of an alcohol-based, ZPT-preserved, preoperative shower solution used in combination with a similarly formulated patient preoperative skin preparation. The combination of an alcohol-based, ZPT-preserved, preoperative shower solution and a similarly formulated patient preoperative skin preparation was compared with an iodine-based system (7.5% scrub and 10.0% PVPI paint) using identical application schedules in a well-controlled, human in vivo study. For this study, the subjects refrained from the use of any antimicrobial products for 2 weeks to allow for stabilization of the normal skin flora. Pretreatment cultures were obtained of the selected sites (groin) to obtain “baseline” of skin flora, to which posttreatment data could be compared and log10 reduction values calculated. The subjects then washed with the preoperative shower products at T = −12 and T = −6 hours. The preoperative surgical site preparations were applied at T = 0, and samples were collected at 10 minutes and from 6 hours out to 72 hours postapplication of the surgical site preparation. The alcohol-based products demonstrated superior efficacy at nearly every time point. At 72 hours (3 days) following the last product application, the alcohol-based, ZPT-preserved system continued to provide significant antimicrobial action as documented by a 2.0-log10 or a 99.0% reduction in the colony-forming units present at baseline. This compares with a <0.50-log10 reduction for the same time point with the iodine-based products. The extended period of antimicrobial persistence that is associated with the ZPT-preserved, alcohol-based system would allow for skin closure in a prolonged state of antisepsis and could result in a lower incidence of surgical site infections.
Discussion
Alcohol provides fast-acting, broad-spectrum antimicrobial activity. However, the requirement for persistence in topical antiseptics cannot be achieved with alcohol alone. Chlorhexidine gluconate, quaternary ammonium compounds, iodine, and triclosan have persistence features and have been added to alcohol solutions for antisepsis in Europe and/or the United States for many years. Antimicrobial persistence has been reported with chlorhexidine gluconate and triclosan, but it is slow to develop and is not always profound.32, 34, 46
As a preservative (persistence agent) for topical antisepsis, ZPT was found to have a safety profile and/or antimicrobial efficacy that exceeds iodine, chlorhexidine gluconate, and triclosan.1, 5, 32, 46, 49, 50 For these reasons, the antimicrobial persistence associated with ZPT has recently been incorporated successfully into commercially acceptable, FDA-compliant, patented products for surgical hand antisepsis and preoperative antiseptics. In conclusion, ZPT is a safe and effective antimicrobial suitable for use as a preservative system with alcohol formulations. The data confirm that ZPT contributes positively toward the overall antimicrobial efficacy of alcohol-based products in which it is used. Additional studies would prove useful in verifying the clinical relevance of this observation. Finally, it is likely that additional uses for this antimicrobial will be found as we continue the struggle with ever increasing resistance to current antibiotics and antimicrobials.
Fort Worth, Texas
References
- 1.Hobson D.W., Woller W., Anderson L., Guthery E. Development and evaluation of a new alcohol-based surgical hand scrub formulation with persistent antimicrobial characteristics and brushless application. Am J Infect Control. 1998;26:507–512. doi: 10.1016/s0196-6553(98)70024-0. [DOI] [PubMed] [Google Scholar]
- 2.Hobson D.W., Seal D.S. Antimicrobial body washes. In: Paulson D.S., editor. Handbook of topical antimicrobials: industrial applications in consumer products and pharmaceuticals. Marcel Dekker; New York: 2003. pp. 221–240. [Google Scholar]
- 3.Hobson D.W., Seal L.A. Brushless surgical scrubbing and handwashing. In: Paulson D.S., editor. Handbook of topical antimicrobials: industrial applications in consumer products and pharmaceuticals. Marcel Dekker; New York: 2003. pp. 167–180. [Google Scholar]
- 4.Food and Drug Administration. 21 CFR Parts 333 and 369. Tentative final monograph for health-care antiseptic drug products: proposed rule. Federal Regulation Part III. (June 17, 1994). p. 31402-52.
- 5.Seal L., Paul-Cheadle D. A systems approach to preoperative surgical patient skin preparation. Am J Infect Control. 2004;32:57–62. doi: 10.1016/j.ajic.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 6.Ayliffe G.A. Surgical scrub and skin disinfection. Am J Infect Control. 1984;5:23–27. doi: 10.1017/s0195941700058756. [DOI] [PubMed] [Google Scholar]
- 7.Larson E.L. APIC guidelines for infection control practice: guideline for use of topical antimicrobial agents. Am J Infect Control. 1988;16:253–266. doi: 10.1016/s0196-6553(88)80005-1. [DOI] [PubMed] [Google Scholar]
- 8.Rotter M.L. Alcohols for antisepsis of hands and skin. In: Ascenzi J.M., editor. Handbook of disinfectants and antiseptics. Marcel Dekker; New York: 1996. pp. 177–233. [Google Scholar]
- 9.Boyce J.M., Pittet D. Guideline for hand hygiene in the health care setting. Am J Infect Control. 2002;30:S1–S46. doi: 10.1067/mic.2002.130391. [DOI] [PubMed] [Google Scholar]
- 10.McDonnell G., Russell A.D. Antiseptics and disinfectants: activity, action, and resistance. Clin Microbiol Rev. 1999;12:147–179. doi: 10.1128/cmr.12.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Walker E.B. Quaternary ammonium compounds. In: Paulson D., editor. Handbook of topical antimicrobials: industrial applications in consumer products and pharmaceuticals. Marcel Dekker; New York: 2003. pp. 99–116. [Google Scholar]
- 12.Mangram A.J., Horan T.C., Pearson M.L., Silver L.C., Jarvis W.R. Guideline for the prevention of surgical site infection. Infect Control Hosp Epidemiol. 1999;20:247–278. doi: 10.1086/501620. [DOI] [PubMed] [Google Scholar]
- 13.Lott W.A., Shaw E. Analogs of aspergillic acid. II. Various antibacterial heterocyclic hydroxamic acids. J Am Chem Soc. January 1949;71:70–73. doi: 10.1021/ja01169a020. [DOI] [PubMed] [Google Scholar]
- 14.Shaw E., Bernstein J., Losee K., Lott W.A. Analogs of aspergillic acid. IV. Substituted 2-bromopyridine-N-oxides and their conversion to cyclic thiohydroxamic acids. J Am Chem Soc. 1950;72:4362–4364. [Google Scholar]
- 15.United States Patent 2,734,903.
- 16.Pansy F.E., Stander H., Koerber W.L., Donovick R. In vitro studies with 1-hydroxy-2 (1H) pyridinethione. Proc Soc Exp Biol Med. 1953;82:122–124. doi: 10.3181/00379727-82-20041. [DOI] [PubMed] [Google Scholar]
- 17.Cox AJ. Pyridine N-oxides and their uses. Manufacturing Chemist October 1957.
- 18.Nelson J.D., Hyde G.A. Sodium and zinc omadine as cosmetic preservatives. Cosmetics and Toiletries. 1981;96:87–90. [Google Scholar]
- 19.Barnett B.L., Kretschmar H.C., Hartman F.A. Structural characterization of Bis (n-oxopyridine-2-thionato) zinc (II) Inorg Chem. 1977;16:1834–1838. [Google Scholar]
- 20.Olin Chemicals product data: Zinc Omadine and Sodium Omadine antimicrobial agents; 1993.
- 21.Snyder F.H., Buehler E.V., Winek C.L. Safety evaluation of zinc 2-pyridinethiol 1-oxide in a shampoo formulation. Toxicol Appl Pharmacol. 1965;7:425–437. doi: 10.1016/0041-008x(65)90144-4. [DOI] [PubMed] [Google Scholar]
- 22.Van Cutsem J., Gerven F.V., Fransen J., Schrooten P., Janssen P.A.J. The in vitro antifungal activity of ketoconazole, zinc pyrithione, and selenium sulfide against Pityrosporum and their efficacy as a shampoo in the treatment of experimental pityrosporosis in guinea pigs. J Am Acad Dermatol. 1990;22:993–998. doi: 10.1016/0190-9622(90)70140-d. [DOI] [PubMed] [Google Scholar]
- 23.Food and Drug Administration. 21 CFR Parts 348 and 358. Dandruff, seborrheic dermatitis, and psoriasis drug products for over-the-counter human use: tentative final monograph. Federal Regulation (July 30, 1986).
- 24.Opdyke D.L., Burnett C.M., Brauer E.W. Anti-seborrhoeic qualities of zinc pyrithione in a cream vehicle. II. Safety evaluation. Food Cosmetic Toxicol. 1967;5:321–331. doi: 10.1016/s0015-6264(67)83057-8. [DOI] [PubMed] [Google Scholar]
- 25.Faergemann J., Fredriksson T. An open trial of the effect of a zinc pyrithione shampoo in tinea versicolor. Dermatology. 1980;25:667–668. [PubMed] [Google Scholar]
- 26.Crutchfield C.E., Lewis E.J., Zelickson B.D. The highly effective use of topical zinc pyrithione in the treatment of psoriasis. Dermatol Online J. 1997;3:3–5. [PubMed] [Google Scholar]
- 27.Olin Chemicals product data: Omadine antimicrobials for cosmetic preservation: combine broad spectrum antimicrobial activity and wide compatibility with cosmetic ingredients; 1993.
- 28.Gibson W.B., Calvin G. Percutaneous absorption of zinc pyridinethione in monkeys. Toxicol Appl Pharmacol. 1978;43:425–437. doi: 10.1016/s0041-008x(78)80002-7. [DOI] [PubMed] [Google Scholar]
- 29.Parran J.J. Deposition on the skin of particles of antimicrobial agents from detergent bases. J Invest Dermatol. 1965;45:86–88. doi: 10.1038/jid.1965.97. [DOI] [PubMed] [Google Scholar]
- 30.Wedig J.H., Maibach H.I. Percutaneous penetration of dipyrithione in man: effect of skin color (race) J Am Acad Dermatol. 1981;5:433–438. doi: 10.1016/s0190-9622(81)70105-1. [DOI] [PubMed] [Google Scholar]
- 31.Black J.G., Howes D. Toxicity of pyrithiones. Clin Toxicol. 1978;31:1–26. doi: 10.3109/15563657808988226. [DOI] [PubMed] [Google Scholar]
- 32.Paulson D.S. Current topical antimicrobials. In: Paulson D.S., editor. Topical antimicrobial testing and evaluation. Marcel Dekker; New York: 1999. pp. 53–59. [Google Scholar]
- 33.Paulson D.S. Chlorhexidine gluconate. In: Paulson D.S., editor. Handbook of topical antimicrobials: industrial applications in consumer products and pharmaceuticals. Marcel Dekker; New York: 2003. pp. 117–122. [Google Scholar]
- 34.Rosenberg A., Alatary S.D., Peterson A.F. Safety and efficacy of the antiseptic chlorhexidine gluconate. Surg Gynecol Obstet. 1976;143:789–792. [PubMed] [Google Scholar]
- 35.Chandler C.J., Segel I.H. Mechanism of the antibacterial action of pyrithione: effects on membrane transport, ATP levels and protein synthesis. Antimicrob Agents Chemother. 1978;14:60–68. doi: 10.1128/aac.14.1.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Friedman S.A. University of San Diego; San Diego (CA): 1981. Studies on the mode of antimicrobial action of metal complexing thiohydroxamic acids against Escherichia coli. [PhD thesis] [Google Scholar]
- 37.Khattar M.M., Salt W.G., Stretton J.R. The influence of pyrithione on the growth of microorganisms. J Appl Bacteriol. 1988;64:265–272. doi: 10.1111/j.1365-2672.1988.tb03384.x. [DOI] [PubMed] [Google Scholar]
- 38.Khattar M.M., Salt W.G., Stretton J.R. Growth and survival of Klebsiella pneumoniae in the presence of pyrithione. J Chromatogr. 1989;1:224–226. [PubMed] [Google Scholar]
- 39.Ermolayeva E., Sanders D. Mechanism of pyrithione-induced membrane depolarization in Neurospora crassa. Appl Environ Microbiol. 1995;61:33–90. doi: 10.1128/aem.61.9.3385-3390.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gilbert P., Barber J., Ford J. Interaction of biocides with model membranes and isolated membrane fragments. In: Denyer S.P., Hugo W.B., editors. Mechanism of action of chemical biocides. Blackwell Scientific Publications; Oxford: 1991. pp. 55–170. [Google Scholar]
- 41.Harold F.M. Conservation and transformation of energy by bacterial membranes. Bacteriol Rev. 1972;36:172–320. doi: 10.1128/br.36.2.172-230.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dinning A.J., Al-Adham I.S.I., Eastwood I.M., Austin P., Collier P.J. Pyrithione biocides as inhibitors of bacterial ATP synthesis. J Appl Microbiol. 1998;85:141–146. doi: 10.1046/j.1365-2672.1998.00478.x. [DOI] [PubMed] [Google Scholar]
- 43.Dinning A.J., Al-Adham I.S.I., Austin P., Charlton M., Collier P.J. Pyrithione biocide interactions with bacterial phospholipid head groups. J Appl Microbiol. 1998;85:132–140. doi: 10.1046/j.1365-2672.1998.00477.x. [DOI] [PubMed] [Google Scholar]
- 44.Albert A., Rees C.W., Tomlinson A.J.H. The influence of chemical constitution on anti-bacterial activity. Part VIII. 2-Mercaptopyridine-N-oxide, and some general observations on metal binding agents. Br J Exp Pathol. 1956;37:500–511. [PMC free article] [PubMed] [Google Scholar]
- 45.Leyden J.J., Stewart R., Kligman A.M. Updated in vivo methods for evaluating topical antimicrobial agents on human skin. J Invest Dermatol. 1979;72:165–170. doi: 10.1111/1523-1747.ep12676347. [DOI] [PubMed] [Google Scholar]
- 46.Rutherford T., Black J.G. The use of autoradiography to study the localization of germicides in skin. Br J Dermatol. 1969;81(Suppl 4):75–86. [Google Scholar]
- 47.Parran J.J., Brinkman R.E. The effect of human skin surface lipids upon the activity of antimicrobial agents. J Invest Dermatol. 1965;65:89–92. doi: 10.1038/jid.1965.98. [DOI] [PubMed] [Google Scholar]
- 48.Gruendemann B.J., Bjerke N.B. Is it time for brushless scrubbing with an alcohol-based agent? AORN J. 2001;74:859–873. doi: 10.1016/s0001-2092(06)61504-4. [DOI] [PubMed] [Google Scholar]
- 49.Paulson D.S. Efficacy evaluation of a 4% chlorhexidine gluconate solution as a full body shower wash. Am J Infect Control. 1993;21:205–209. doi: 10.1016/0196-6553(93)90033-z. [DOI] [PubMed] [Google Scholar]
- 50.American Society for Test Materials (ASTM) Method E 1052-96, a standard test method for efficacy of antimicrobial agents against viruses in suspension.


