Publisher Summary
This chapter illustrates the recommendations and guidelines of the World Health Organization (WHO) concerning water, sanitation, and health. The recommendations and guidelines are evaluated in the light of disease caused by human pathogenic viruses. The guidelines outline a preventive management framework for safe drinking water. The framework includes health-based targets to assist national authorities who are normally responsible to set the targets for the protection of public health from risks by exposure to drinking water. Assessing the adequacy of systems, defining and monitoring control measures, and establishing management plans are the three components of the so-called water safety plans. Achievement of health-based targets may be verified by independent surveillance to assess the safety of the drinking water through additional verification or audit-based approaches. This framework for safe drinking water can be adapted according to environmental, social, economic, and cultural circumstances of drinking water provision on the national, regional, and local level. The chapter concludes that viruses could be considered as biocolloids with specific properties such as size, shape, structure, charge, composition, and genome. These viral characteristics determine their behavior in the environment, resistance to natural inactivation and treatment, and disinfection processes. For each (re-)emerging virus these properties may be known or could be assessed predicting the effectiveness of possible intervention measures for prevention of waterborne disease.
In this chapter the recommendations and guidelines of the World Health Organization (WHO) concerning water, sanitation and health will be evaluated in the light of disease caused by human pathogenic viruses. The focus will be on drinking water safety.
WHO guidelines for drinking water quality
From end product monitoring to prevention
In 1983–1984 and 1993–1997, respectively, the WHO published the first and second editions of the Guidelines for Drinking Water Quality (GDWQ). The development of these Guidelines in the 1980s was a significant departure from previous ‘international standards’, emphasising their advisory nature to national governments and the importance of their adaptation to take account of national and local socio-cultural, environmental and economic circumstances. This philosophy was maintained in the second edition, which provided guidance on many more individual chemicals. In 1995, it was decided to revise the guidelines to account for advancing scientific knowledge with respect to drinking water quality. This especially related to microbial hazards and infectious disease risks. Since the early 1990s, a process of ‘rolling revision’ of the GDWQ has led to more frequent updating including additional publications regarding the chemical and microbiological aspects of drinking water quality and toxic cyanobacteria in water and addenda to the Guidelines themselves. All these publications and the Guidelines are freely available on the Internet (http://www.who.int/water_sanitation_health/dwq).
The third edition of the WHO GDWQ was launched in September 2004 at the ‘World Water Congress’ in Marrakech, Morocco. According to these guidelines, access to safe drinking water is a component of effective policy for health protection. Requirements to ensure drinking water safety include both minimum procedures and specific guideline values as described in the WHO GDWQ, which also describes how to use these requirements. The multiple barrier approach, including source protection, appropriate levels of treatment and protection of water safety during distribution, is a basic principle for the reduction of health consequences by consumption of drinking water.
The guidelines outline a preventive management ‘framework for safe drinking water’ (Fig. 1 ). The framework includes ‘health-based targets’ to assist national authorities who are normally responsible to set the targets for the protection of public health from risks by exposure to drinking water. Water suppliers are responsible to meet these targets by the most appropriate means under local circumstances by specific control measures in the drinking water supply and also by defining management actions in case of regular situations or incidents. Assessing the adequacy of systems, defining and monitoring control measures and establishing management plans are the three components of the so-called ‘water safety plans’ (WSPs). Achievement of health-based targets may be verified by independent surveillance to assess the safety of the drinking water through additional verification or audit-based approaches. This framework for safe drinking water can be adapted according to environmental, social, economic and cultural circumstances of drinking water provision on the national, regional or local level.
Fig. 1.
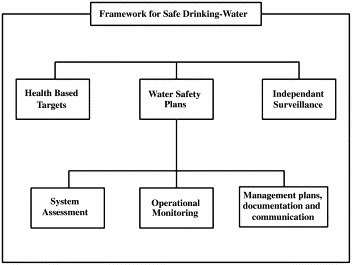
Framework for safe drinking water (WHO, 2004).
The fourth edition of the WHO GDWQ is planned for 2009 that would include viewpoints on topics such as safe drinking water for vulnerable groups. Addenda for this edition have been published in 2006 and 2007 (http://www.who.int/water_sanitation_health/dwq).
Water-related viruses
The WHO GDWQ concerns prevention of waterborne diseases by exposure to microbial, chemical and radiological hazards in the drinking water. Microbial hazards are still considered to be of major concern for providing safe drinking water in both developed and developing countries. Infectious diseases caused by pathogenic bacteria, viruses and parasites (e.g. protozoa and helminths) are the most common and widespread health risk associated with drinking water.
The true waterborne diseases are part of a wider range of water-related disease. Water-related disease may be water-based, water-washed, waterborne or be transmitted by water-related insect vectors (Bradley, 1977). Insect vectors breeding in water or insect vectors only biting near water cause water-related infectious disease. Some viruses are water-related such as dengue virus and the flavivirus causing yellow fever. These viruses are transmitted via the bite of specific mosquitoes. Water-based diseases are typically caused by parasitic worms not virus infections in which waterborne hosts support an essential part of the life cycle of the infecting agent. Waterborne diseases are caused by the ingestion of water contaminated by human and/or animal excreta and secreta containing pathogenic bacteria, viruses or parasites, which are passively carried in the water supplies. Most water-related viruses are waterborne such as poliomyelitis virus, rotavirus, norovirus (NoV) and hepatitis A and E viruses (HAV and HEV, respectively). Water-washed diseases are the result of lack of water. This leads to poor personal hygiene and allows skin or eye infections to develop and spread easily through personal contact or contact with contaminated water. An example of a water-washed virus is adenovirus though some adenoviruses are typically waterborne. Most waterborne diseases can also be water-washed because inadequate personal hygiene facilitates faecal–oral disease transmission.
Besides the well-known water-transmitted viruses (see Chapters 2–4), infection and disease may be associated with (re-)emerging viruses such as the water-related chikungunya virus and emerging viruses such as severe acute respiratory syndrome (SARS) coronavirus and avian influenza virus (see Text box 1 ). Also, mutation and recombination of existing virus strains may lead to (re-)emergence such as described for vaccine-derived poliovirus (see Chapter 4). As a group, water-transmitted viruses can cause a wide variety of infections and symptoms involving different routes of transmission, routes and sites of infection and routes of excretion. Most water-transmitted virus infections occur asymptomatically or cause diarrhoea and self-limiting gastroenteritis in humans. Some waterborne viruses may cause respiratory infections, conjunctivitis, hepatitis and diseases that have high mortality rates; such as aseptic meningitis, encephalitis and paralysis. In addition, some of these viruses such as specific human enteroviruses have been linked to chronic diseases such as myocarditis and type 1 diabetes mellitus (Witso et al., 2006). As yet other long-term health effects, such as cancer, are not readily associated epidemiologically with water-transmitted viral disease (Ashbolt, 2004).
Text box 1.
The WHO publication Emerging Issues in Water and Infectious Disease provides knowledge and guidance to information sources on the evolution of infectious disease, the emerging waterborne pathogens, new environments and technologies that may influence emergence. It describes scientific advances in water microbiology and changes in human behaviour and vulnerability to broaden awareness and enhance preparedness about the changing world and its associated hazards (http://www.who.int/water_sanitation_health/emerging/emerging.pdf).
Faecal–oral transmission is the most well-known route of infection with waterborne viruses though other excreta (e.g. urine) and secreta (e.g. sweat) may also play a role in virus transmission via water which is less well studied. Transmission through aerosols and droplets derived from contaminated water may also lead to virus infection. A recent example of such environmental transmission of predominantly person-to-person transmitted flu-like viruses was the spread of SARS coronavirus in the large, private apartment complex Amoy Gardens in Hong Kong in 2003 (McKinney et al., 2006).
Since water-transmitted viruses do not grow in water, their numbers are determined by the extent of excretion/secretion into the environment followed by subsequent disintegration (see Chapter 5). The number of water-transmitted viruses in the water is determined by shedding of viruses by symptomatic patients as well as asymptomatically infected humans. Moreover, initially zoonotic viruses may be excreted by both the animal and the human host (see Text box 2 ).
Text box 2.
The WHO book on Waterborne Zoonoses: Identification, Causes and Control provides a critical and balanced assessment of current knowledge about waterborne zoonoses and identifies strategies and research needs for anticipating and controlling future emerging water-related diseases (http://www.who.int/water_sanitation_health/diseases/zoonoses.pdf).
Subsequent virus disintegration in the environment depends on the specific sensitivity of the virus to inactivation, e.g. by radiation, temperature and its tendency to attach or aggregate with particles (reviewed by John and Rose, 2005). Viruses that are efficiently transmitted via blood or respiratory droplets generally have a limited resistance in the water, such as human immunodeficiency virus (HIV), whereas other, mainly faecal–oral transmission of viruses such as poliovirus, are much more resilient (Moore, 1993). This range of viral resistance in the water is defined by the virus properties such as size, shape, charge, etc. The possible public health threat of (re-)emerging viruses should be assessed based on these particle characteristics. For example, SARS lead to the investigation of previously described respiratory agents such as Legionella. The newly identified SARS coronavirus was initially characterised as a respiratory virus without estimating its potential for transmission via water (Ksiazek et al., 2003). Subsequent research showed the common high level of excretion in faeces from infected individuals (Ding et al., 2004). Experimental studies showed stability of infectious virus for several days in different liquid environments (Duan et al., 2003). This outlines the need for a proper assessment of the potential role of drinking water in SARS coronavirus transmission and in general in the transmission of emerging viruses. Another public health concern arises from the high infectivity as determined for most viruses emphasising the significance of the presence of each infectious virion in water for human use. The probability of infection from exposure to one or a few virus particles in water has been described for several waterborne viruses (Haas et al., 1993; Lindesmith et al., 2003).
Health-based targets
Faecally derived pathogens are currently the principal concerns in setting health-based targets for microbial safety of drinking water. Health-based targets are set by national authorities to protect and improve drinking-water quality such as with respect to the quantity and type of viruses present and, consequently, human health from viral disease. Health-based targets to reduce the waterborne disease burden may take different forms and are best derived using quantitative risk assessment, taking into account local conditions and hazards including epidemiological evidence of waterborne disease. International guidance on good practice is available to assist this (WHO, 2004). Typically performance and health-outcome targets are suitable as types of health-based targets for microbial hazards such as human pathogenic viruses in drinking water (Table 1 ).
Table 1.
Health-based targets as applicable for microbial, e.g. viral, hazards
| Type of target | Nature of target | Typical applications | Assessment |
|---|---|---|---|
| Health outcome | |||
|
|
|
|
|
|
|
|
| Performance | |||
|
|
|
|
|
|
|
|
Health outcome target
The health outcome target is the target type for which health effects and drinking-water management are most closely linked, but is not often applicable in practice and other types are more frequently used. Health-outcome targets are most useful in case of a measurable burden of water-transmitted disease, e.g. that associated with the presence of human pathogens in small water supplies both in developing and developed countries. It requires effortless and reliable monitoring of changes in exposure. Several aspects may hamper establishing the attributable risk of waterborne viral disease. First of all, it may be difficult to distinguish viral disease from disease with similar symptoms but caused by other pathogens such as bacteria and parasites. Gastroenteritis may manifest itself with very general complaints such as abdominal cramps, fever, nausea, vomiting, diarrhoea, etc. On the other hand, projectile vomiting is characteristic for specific viruses, i.e. NoV, and bloody diarrhoea is quite uncommon with viral gastroenteritis. The same holds true for hepatitis that may be associated with either one of two specific waterborne viruses (HAV and HEV) or one other virus out of five different virus families but also with alcohol abuse or, though uncommonly, microsporidia infection. Second, it may not be possible to discern water-transmitted disease from disease contracted via other routes such as person to person, utensils or food. And in case of food, the transmission route may involve water such as preparation of lemonade or ice cubes and irrigation or washing of crops (see Text box 3 ) with virally contaminated water. The virus infections and symptoms resulting from either described route of transmission will be the same. Moreover, in the presence of other obvious high-risk exposures the drinking water may not be considered as the source of infection and disease, for instance in case of bottled water versus fruits or vegetables consumed raw. Nevertheless, if locally food-borne disease encompasses a higher burden as compared with waterborne disease, then it should be given correspondingly greater attention taking also into account costs and impacts of available interventions.
Text box 3.
The WHO Guidelines for the safe use of wastewater, excreta and grey water describe health-based targets and good management practices to safeguard the health benefits such as better nutrition and food security for households and at the same time control the possible negative health impacts from exposure to hazardous substances such as viruses (WHO, 2006a; http://www.who.int/water_sanitation_health/wastewater/gsuww/en).
A third aspect hindering the estimation of waterborne viral disease burden is the incubation period; it may take several weeks for some viral diseases such as hepatitis to manifest themselves. The time of peak contamination in the source, failure in treatment or contamination in the distribution may be long gone. Also the drinking water may comply with regulations involving bacterial counts but these may be insufficient with respect to reduction of human pathogenic viruses. And fourth, the primary source may be drinking water but in case of high-level secondary transmission from person to person this may go unnoticed. However, in recent years methodology and awareness have improved greatly resulting in more and more publications on drinking-water disease outbreaks (Poullis et al., 2005). Remarkably few outbreaks were described associated with exposure to enteroviruses in drinking or recreational waters. Recently, large outbreaks with hundreds of cases of meningitis were reported for both types of exposure (Hauri et al., 2005; Amvrosieva et al., 2006). Also, the causative agent of the first reported waterborne outbreak of hepatitis E in Delhi, India in 1955 and 1956 was not identified until the 1990s with the advances in molecular and immunodiagnostics (Worm et al., 2002).
Health outcome-based targets may also be based on the results of quantitative microbial risk assessment (QMRA; see Chapter 8). In these cases, health outcomes are estimated based on information concerning exposure and dose–response relationships. However, with regard to QMRA also there are limitations in the available data and models (see Section Risk management). In addition, uncertainties such as short-term fluctuations in water quality may have a major impact on overall health risks—including those associated with background rates of disease and outbreaks—and are a particular focus of concern in expanding application of QMRA. Nevertheless, QMRA may be very useful in directly assessing data gaps if not in actual assessment of risks for consumption of drinking water. The results of a QMRA may be employed as a basis to define water quality targets or may provide the basis for development of performance targets (see Section Performance target).
Performance target
In contrast with health outcome targets, performance targets are frequently applied to the control of microbial hazards in piped supplies of any size though there is less accrued experience in their application to source protection or distribution than there is to treatment processes and systems (http://www.who.int/water_sanitation_health/dwq/en/safepipedwater.pdf). Performance targets are also readily applied to wastewater re-use systems (WHO, 2006a). Performance targets aid the selection and use of control measures to achieve acceptable source water quality and treatment efficiency. These control measures must ensure the prevention of pathogens breaching the barriers of source protection, treatment and distribution systems or preventing growth within the distribution system.
Targets for the removal of pathogens preferably are derived from location-specific data on source water quality. The source water may be groundwater or surface water or a mixture of both. Groundwater may be initially free of pathogenic viruses because of age. If water seeps through soil before reaching the groundwater, it may be effectively purified with respect to viruses depending on soil and virus type (Zhuang and Jin, 2003). However, shallow and/or unconfined groundwater may be contaminated through leaking of viruses originating from host ex-/secretions near the source or through flaws in the extraction process (see Text box 4 ).
Text box 4.
The WHO book on Protecting Groundwater for Health: Managing the Quality of Drinking Water Sources is a tool for intersectoral development of strategies to protect groundwater for health providing different points of entry (http://www.who.int/water_sanitation_health/gdwqrevision/groundwater/en/).
In case of surface water, the number and type of human pathogenic viruses depend on the proximity of the viral reservoirs and dilution and inactivation of the viruses between point or diffuse source and the intake location for drinking (water production). Rainwater may also be used as drinking water. This type of source water may be more likely to be microbially contaminated by animal faeces if the rainwater is collected on rooftops with birds and rodents in the vicinity. Hantavirus infection is widely spread among rodents such as different types of mice and rats. On the other hand, poor hygienic and sanitation conditions associated with the design of the reservoir or tap may lead to viruses originating from human faeces. To date no study has been conducted for the presence of human pathogenic viruses in rainwater. Alternatively, seawater may be used as a source for drinking water by use of desalination. However, both bacteriophages and human pathogenic viruses were found to be very prevalent in seawaters (Shuval, 2003). Viruses seem to be readily inactivated in natural seawaters (Wetz et al., 2004). So far nothing is known about the reduction of human pathogenic viruses by desalination but membrane systems such as reverse osmosis and electrodialysis, which are the newest approach to desalination, are known to be very efficient in virus removal. Though desalination remains one of the most expensive ways to produce drinking water, these new advances and the increasing water scarcity may push the use of seawater (Reuther, 2000; http://www.who.int/water_sanitation_health/gdwqrevision/desalination/en/).
The quality of source water with respect to human pathogenic viruses thus highly depends on the circulation of particular viruses in the human population and with respect to zoonotic viruses in the animal population. Specific clinical data or source water quality data may not be available for human pathogenic viruses or for the location. In both developing and developed regions, viral disease is often classified as non-bacterial, probably viral, but never confirmed. In absence of specific data, national or regional data and/or data on viruses such as bacterial viruses (bacteriophages) may serve as input for setting performance targets. In this respect, free data sharing among researchers and risk managers is a key to successful reduction of the environmental spread of pathogenic viruses and reduced burden of water-transmitted viral disease.
Depending on the specific source water quality, the required performance target as in reduction of viruses by treatment can be set. These requirements concern control measures that must ensure both sufficient and robust virus reduction. The treatment efficiency that is needed to produce drinking water of acceptable quality with respect to viruses can be estimated by means of a system assessment (see Section Risk management). Alternatively in case of a high disease burden the efficiency of point-of-use treatment may be assessed with a surveillance programme. A multiple barrier approach including several treatment steps will facilitate a constant drinking water quality. However, besides knowledge of source water quality this requires trained operators and straightforward risk management actions (see Section System assessment). Moreover, the viral water quality often varies rapidly and over a wide range (Westrell et al., 2006). Treatment processes should be evaluated with respect to handling of short-term fluctuations as well as their potential to be the source of short-term fluctuations.
The role of the distribution system in the fate of human pathogenic viruses in drinking water so far has not been the focus of many studies (Skraber et al., 2005). The question is whether human pathogenic viruses benefit from their transport in the distribution system, for instance by the presence of biomass or biofilms preventing their inactivation. Or that growth in the distribution system may be considered as an additional treatment step aiding virus reduction in drinking water before consumption. Performance requirements are also important in certification of devices for drinking water treatment and for pipe installation that prevents ingress. Leaks or human error leading to unintended incorrect connections may be an important attributor to virus-associated disease outbreaks (Jardine et al., 2003).
The choice of an index virus to be able to target the group of human pathogenic viruses is very useful not only to indicate source water quality but also to estimate the reduction of viruses in the treatment of the source water and virus dynamics in the distribution system. Including different viruses reflecting the diverse characteristics with respect to their replication rates, natural reduction and reduction by treatment will improve the eventual drinking water quality by optimising the drinking water supply from source to tap. For viruses transmitted by the faecal–oral route, drinking water is only one vehicle of transmission. Contamination of food, hands, utensils and clothing can also play a role, particularly when domestic sanitation and hygiene are poor. Since water is the most important transmission route for some viruses whereas others are mainly transmitted via food or person to person, improvements in the quality and availability of water, in excreta disposal and in general hygiene are all important in reducing faecal–oral transmission of viruses. In combination with target microbes representing other pathogen groups, i.e. bacteria, parasites and helminths, performance targets can be developed that encompass both control challenges and health significance for a broad range of pathogens. In addition to source water quality, treatment efficiency and distribution, disease burden and dose–response relationships for specific pathogens serve as the basic parameters for the derivation of performance targets. Performance targets may be derived in relation to exposure to specific pathogens; however, account should be taken of the impact of short-term peaks in virus concentration on overall exposure and therefore the risk of drinking water disease.
Reference level of risk
To be able to establish the possible public health priority of reduction of water-transmitted viral disease as compared with other burdens of disease, a reference level of risk for the comparison of diseases is useful. A common denominator for disease burden enables a consistent approach for dealing with each hazard. Water-transmitted disease may be compared with disease contracted via other pathways. Similarly, viral diseases may be compared with disease associated with other pathogen groups or with each other. The hazard identification may be hampered, though, by the equivalence of disease symptoms of viral and non-viral diseases as explained above for gastroenteritis and hepatitis that may have alternative causes. A common metric taking into account differing probabilities, severities and duration of effects is the disability adjusted life year (DALY) widely used by WHO in the GDWQ. The range of differing severities, including acute, delayed and chronic effects and both morbidity and mortality associated with the specific viral pathogen have to be known. In addition, infection with a water-transmitted virus may have more severe disease outcome in vulnerable individuals as compared with the general population. This, for instance, holds true for HEV infection with a relatively high mortality rate among pregnant women (Rab et al., 1997).
In two ways viruses transmitted via water may be specifically significant as compared with other routes of transmission or other pathogens. One, major changes in viruses leading to a possibly more virulent, infectious or pathogenic virus type may result from recombination; this in contrast with other pathogens. In particular recombination may occur if the host is exposed to multiple virus variants from the same species. These double infections are more likely to occur as a result of consumption of drinking water or water-contaminated foods but not of person-to-person transmission. Two, consumption of virus-contaminated drinking water from a reservoir or served by a piped distribution reaches a relatively large part of the population rapidly leading to many exposures of humans and perhaps animals. And more generally, by means of the described approach employing a reference level of risk it may become evident that waterborne viral disease is a far more important public health challenge than frequently appreciated.
Water safety plans
Hazard analysis and critical control point (HACCP) has been a breakthrough in the control of food safety initially applied for the safety of foods for the manned space programme in the 1960s. The principles of HACCP are based on developing an understanding of the system, prioritising risks and ensuring that appropriate control measures are in place to reduce risks to an acceptable level. These same principles have been long applied in drinking water safety management and more recently have been refined and tailored to the context of drinking water (Havelaar, 1994) following the application of HACCP by several water utilities in the world (see example for Australia in http://www.who.int/wsportal/wsp/en/). Such a systematic management approach to water safety by the water suppliers was translated by WHO into a so-called WSP that incorporates system protection and process control. The WSP objectives are to ensure safe drinking water through good water supply practice. This involves preventing contamination of source waters, treating the water to the extent necessary to meet health-based targets and preventing contamination during storage, distribution and handling of drinking water. A WSP comprises system assessment and design, operational monitoring and management plans, including documentation and communication, concerning all aspects of the drinking water supply from catchment to consumer. The WHO GDWQ asks for the inclusion of management plans as an integral part of the WSPs. These plans should describe actions to be taken during normal operation or incident conditions. The system assessment should be documented (including upgrade and improvement). Furthermore, monitoring and communication plans and supporting programmes should be described. In this way the management and control of drinking water supply are safeguarded and continued through systematic and detailed assessment and prioritisation of hazards and the operational monitoring of barriers or control measures. Moreover, in case of emergencies plans of action are in place with consent of the people involved, which can be readily initiated, and these plans have been rehearsed in emergency drills. The control measures as described in the WSP will be effective to protect public health from water-related viral disease. WSP issues, specifically with respect to human pathogenic viruses in drinking water, are discussed below.
System assessment
Risk assessment has been used for decades, initially for decision making in psychotherapy and vaccination strategies, and today for management of pandemics such as influenza and AIDS. In 1993, the application of this tool was first described for exposure to pathogenic viruses in treated drinking water, since it is difficult to estimate risks posed from exposure to low levels using epidemiology (Haas et al., 1993). According to the US National Academy of Sciences, the successive steps of the risk assessment include the hazard identification, exposure assessment, dose–response modelling and risk characterisation to estimate the probability and consequences of infection—here specifically for individuals consuming various levels of viruses in the drinking water. Risk assessment should be considered as an ongoing process to allow advances in research and technology to be fed back into the risk characterisation. As stated in the WHO GDWQ, a system assessment establishes the capacity of a drinking-water supply to deliver water of a quality that meets the health-based targets. This also includes the assessment of design criteria for new systems and upgrading of existing systems.
Hazard identification
The word virus translated from Latin means slimy liquid, slime or poison, especially of snakes’ venom; any harsh taste or smell. Clearly, a virus as intended in this book is unwanted in drinking water and should be considered a hazard: a biological agent that has the potential to cause harm. A number of issues have to be taken into account to determine those viruses that may be water-related and need to be considered in the specific system assessment.
Human and animal sources
The viruses that may cause disease in humans may be derived from humans or animals. More specifically, numerous human pathogenic viruses are shed in human and animal excreta (i.e. faeces, urine, vomitus), secreta (i.e. saliva, tears, semen, mucus, sweat) and blood of infected individuals. In addition, viruses may target specific host organs such as the liver or the heart and therefore these organs will harbour viruses. Porcine HEVs phylogenetically related to human HEV strains were shown to be shed in different bodily fluids and tissues of pigs such as faeces and liver (De Deus et al., 2007), but it is yet unknown if HEV is shed in human fluids other than serum and faeces. Viruses account for the highest infection risks through accidental exposure of health care workers (Tarantola et al., 2006), but often a broad investigation of viruses in bodily fluids of humans has not been done leaving broad knowledge gaps (Table 2 ).
Table 2.
Incomplete but informative list of virus types present in human excreta and secreta
| Faeces | Urine | Saliva | Tears | Sweat | Reference | |
|---|---|---|---|---|---|---|
| Adenovirus | + | + | + | + | ? | Ramsay et al. (2002), Hatakeyama et al. (2006), Kaye et al. (2005) |
| Aichivirus | + | ? | ? | ? | ? | Yamashita et al. (2000) |
| Astrovirus | + | ? | ? | ? | ? | Guix et al. (2005) |
| Enterovirus | + | + | + | ? | ? | Ramsay et al. (2002), Muir et al. (1993) |
| Hepatitis A virus | + | ? | + | ? | ? | Mackiewicz et al. (2004) |
| Hepatitis C virus | + | ? | + | ? | + | Beld et al. (2000), Ortiz-Movilla et al. (2002) |
| Hepatitis E virus | + | ? | ? | ? | ? | Singh et al. (1998) |
| Norovirus | + | ? | ? | ? | ? | Herrmann et al. (1985) |
| Parvovirus | ? | ? | + | ? | ? | Ramsay et al. (2002) |
| Picobirnavirus | + | ? | ? | ? | ? | Cascio et al. 1996, Banyai et al. (2003) |
| Polyomavirus | + | + | ? | ? | ? | Hatakeyama et al. (2006), Berger et al. (2006) |
| Reovirus | + | ? | ? | ? | ? | Giordano et al. (2002) |
| Rotavirus | + | ? | ? | ? | ? | Bowdre (1983) |
| Sapovirus | + | ? | ? | ? | ? | Phan et al. (2006) |
| SARS coronavirus | + | + | + | ? | + | Wang et al. (2004), Ding et al. (2004) |
| TT virus | + | – | + | + | – | Matsubara et al. (2000) |
Note: +, studied and confirmed; –, studied but not detected; ?, no studies included in publications shown by search engine NCBI PubMed.
Typically numerous viruses are shed in human faeces (reviewed by Carter, 2005). Most of these viruses replicate in the gastrointestinal tract and are referred to as enteric viruses (see Chapters 2 and 3). Some viruses are renowned for their excretion in urine such as polyomavirus JC but also human papillomavirus (HPV), cytomegalovirus, hepatitis B viruses (HBV) and more recently SARS coronaviruses. The most common viruses to be secreted in saliva are HBV, HPV and cytomegalovirus, but recently HAV, a well-known food- and waterborne virus, was also associated with human saliva (Mackiewicz et al., 2004). In addition, saliva and throat wash of SARS patients were positive for the presence of the specific coronavirus (Wang et al., 2004). Hepatitis C viruses replicate in sweat glands leading to virus release in sweat of patients (Ortiz-Movilla et al., 2002). Torque teno virus (TTV) DNA was detected in tears as well as in saliva, breast milk, semen, blood, faeces and vaginal fluid whereas no evidence was found for the presence of the virus in urine and sweat.
For viruses shed in bodily fluids and organs the probability of being transmitted via water is largely determined by their circulation in the population, their ability to reach water resources and their inactivation rate in water. These issues may be unknown besides the existing knowledge gaps on virus shedding in the absence of specific research efforts or lack of access to the results by means of international, peer-reviewed journals or other accessible publications. Also, epidemiological evidence for viruses to be water-related may be lacking but this may be a research bias as explained in Section Health outcome target.
Drinking water outbreaks
If information on specific virus types causing waterborne outbreaks exists (see Chapters 2 and 3) then outbreak data should be collected including the number of patients, their exposure and the number and type of viruses in the contaminated water to characterise the hazard and determine a dose–response relation (the latter instead of or in addition to human volunteer or animal studies). Numerous waterborne outbreaks of mainly NoV-associated gastroenteritis have been described originating from contaminated drinking water but often the descriptions are anecdotical or at best an epidemiological association was determined between exposure of the patients and the common water source. With the improvement of detection methods with respect to sensitivity and specificity the causative agent can be detected more often in the water and typed with similar sequences in the patients and the common water source. Especially screening of patient samples for the virus followed by specific design of the detection method for monitoring the environment enhances the chance of success in confirming the link (Duizer and Kooomans, 2006; Hoebe et al., 2004). In some instances, the cause of the drinking water contamination is resolved and the drinking water was found to be contaminated by sewage through pump failure or blockage of a sewage system. In other cases, inadequate or failing treatment processes lead to insufficient removal of viruses from source waters for drinking water production. Only recently enteric cytopathic human orphan (ECHO) virus type 30 has been identified as an important waterborne agent of meningitis in different parts of the world. The possibility for enteroviruses to be transmitted via drinking water (Amvrosieva et al., 2006) or recreational water (Hauri et al., 2005) was not recognised previously though numerous enterovirus-associated meningitis outbreaks have been described for decades. These data confirm the value of outbreak investigations.
Selection and prioritisation of viral hazards
To be able to select and prioritise human pathogenic viruses to be included in the system assessment, criteria concerning the extent of the health risk, the waterborne transmission and the quantitative risk assessment need to be set. First of all, location-specific information on the prevalence of the virus infection and disease in the population is essential though it may be substituted by, for instance, knowledge on human pathogenic viruses in sewage or surface waters. Furthermore, viral disease outcomes, ranging from asymptomatic through severe defects to death (Table 3 ), largely determine the health-based targets, since prevention of a high disease burden will be a more effective intervention as compared with prevention of disease with an overall low burden (see Section Health-based targets). The burden of viral disease involves the severity of disease as well as prevalence. For instance, if overall more individuals experience episodes of mild, limited viral gastroenteritis in a specific region then patients with a more severe health outcome, viral hepatitis, should also be considered in setting the health-based targets. Besides being dependent on the pathogenicity of the virus, the severity of viral disease also depends on the susceptibility of the host. The disease burden of rotavirus in high-income countries is much lower than in low-income countries with a much higher susceptible fraction of the population and more severe outcome of the infection (Havelaar and Melse, 2003; http://www.who.int/water_sanitation_health/bathing/en/).
Table 3.
Disease outcomes of human pathogenic viruses
| Disease outcome | Causative viral agent | Reference |
|---|---|---|
| Conjunctivitis | Adenovirus, enterovirus | Tavares et al. (2006) |
| Gastroenteritis | Adenovirus, aichivirus, astrovirus, enterovirus, norovirus, picobirnavirus, rotavirus, sapovirus | Glass et al. (2001) |
| Hepatitis | Hepatitis A–G virus, TT virus | Luo et al. (1999) |
| Leukoencephalopathy | Polyomavirus | Khalili et al. (2006) |
| Meningitis | Enterovirus, reovirus | Johansson et al. (1996) |
| Myocarditis | Adenovirus, enterovirus, parvovirus | |
| Poliomyelitis | Enterovirus | |
| SARS | SARS coronavirus |
The extent of the health risk associated with a specific virus is also determined by the ability to treat the virus infection and the availability of the treatment. Though the area of antiviral treatment is rapidly advancing, there is no cure for many viruses and in many countries there are no means to administer drugs to people in need. Vaccination strategies to prevent viral disease have been very successful. Smallpox was eradicated in 1978 and the eradication of poliomyelitis is in sight. (Re-)emergence of vaccine-preventable viral diseases should be monitored such as disease caused by vaccine-derived polioviruses, which can be efficiently transmitted via water (see Chapter 4). The possibility of a virus to spread epidemically and/or endemically is important in waterborne transmission because of the level of viruses circulating in the asymptomatic and symptomatic population and the total burden of viruses shed into the environment, and if relevant, also those viruses derived from animals. In case of HEV it was found that the consumption of liver and blood of domestic pigs, wild boar and/or deer leads to infections with HEV variants causing hepatitis E in the exposed individuals (Li et al., 2005). The human HEV strains were phylogenetically related to the animal variants (Lu et al., 2006). Moreover, HEV transmission is primarily water- and food-borne but may also be transmitted via blood transfusion, contact with sewage or animals or vertical transmission (Singh et al., 2003; Vaidya et al., 2003; Li et al., 2005). These routes of HEV transmission appear to be more efficient as compared with person-to-person transmission (Somani et al., 2003). Other possible zoonotic viruses such as some porcine NoV and sapoviruses (SaV) are genetically or antigenically related to human strains (Wang et al., 2006). Recombinants within NoV and SaV occur for human and pig strains, and the high prevalence and sub-clinical infection rate of these viruses in pigs raise questions of whether pigs may be reservoirs for human strains or for the emergence of new human and porcine recombinants. The occurrence of zoonotic virus strains stresses the need to take animal reservoirs into consideration regarding source protection and when assessing the health risk for drinking water.
Environmental surveillance
In addition to disease surveillance in the population to whom the (produced) drinking water is delivered, environmental surveillance can aid the identification of the viral hazards by typing and quantification. Data resulting from studies on human pathogenic viruses in sewage and surface waters are accumulating. Raw sewage waters were shown to contain human enteric viruses such as enteroviruses, reoviruses, rotaviruses, NoV and HEV (Clemente-Casares, 2003; Villena et al., 2003; Lodder and de Roda Husman, 2005). In most studies presence/absence data were shown but the virus concentrations were not determined. Though some viruses such as specific entero- and adenoviruses can be cultured, there is no susceptible cell line for many other human pathogenic viruses. In this case, the virus concentration may be estimated from molecular data collected by (semi-)quantitative and/or real-time polymerase chain reaction (PCR). Typically 106 viral genomes can be detected in raw sewage as compared with 103 infectious viruses (Schvoerer et al., 2001; Laverick et al., 2004; Lodder and de Roda Husman, 2005; van den Berg et al., 2005). In these studies the applied sewage-treatment processes approximately resulted in a limited 1–2 log10-units reduction as assessed by culture or molecular techniques. In general, primary and secondary sewage-treatment processes do not efficiently reduce the virus concentration in contrast with tertiary processes (Fleischer et al., 2000; Schvoerer et al., 2001; Gehr et al., 2003; van den Berg et al., 2005; Myrmel et al., 2006). Bacteriophages may be useful in assessing reduction of human viruses by wastewater treatment processes (Lucena et al., 2004). Wastewater treatment is warranted to reduce viral load in wastewaters discharging onto receiving waters (Godfree and Farrell, 2005). However, since human viruses enter wastewater at high numbers wastewater will still contain viruses even after treatment. Therefore, in addition to wastewater overflows treated effluents will have to be taken into account as a significant source of viral pollution for wastewater used in agriculture and for surface waters used for aquaculture and fishery, recreational purposes or as a source for drinking water production. Moreover, both raw and treated sewage may contaminate groundwater through leakage (Abbaszadegan et al., 1999; Borchardt, 2003; Borchardt et al., 2004).
Common sources of faecal pollution, directly or indirectly contaminating surface waters with human pathogenic viruses, include raw and treated sewage, wash-off of animal manure, faeces from wildlife such as waterfowl or deer, grazing animals and vermin in and around reservoirs, backflow from unprotected connections and sewer cross connections. In river water samples enteroviruses, reoviruses, rotaviruses, HAV, astroviruses, TTV and NoV were detected in different countries around the world but these results are biased since most researches were done in Europe (Matsuura et al., 1993; Gilgen et al., 1997; Schvoerer et al., 2000; Hot et al., 2003; Denis-Mize et al., 2004; Hörman et al., 2004; Haramoto et al., 2005, Villar et al., 2006). Viruses originating from discharge of treated-sewage water onto the receiving surface waters used for drinking water production (or recreational purposes) pose a health risk (Schernewski and Julich, 2001). Most papers on viruses in surface waters document presence/absence data only but to be able to estimate the infectious risk by consumption of drinking water quantitative data are necessary. In some studies, the virus concentrations were determined in river water varying from 0.001 to 10 infectious entero- and reoviruses per litre to 5 to 5000 genomes for NoV and rotaviruses per litre of river water (Haramoto et al., 2005; Lodder and de Roda Husman, 2005). Location-specific data on concentration and types of viruses in source waters and inactivation rates under the specific conditions in the specified waters are preferred for the hazard identification. In the absence of such data, virus data from other locations that may be considered comparable may also be employed. For instance, if treated and untreated urban sewage are discharged then it could be assumed that virus types such as enteroviruses and rotaviruses may be present and virus concentrations could be contracted from available studies. If there is sewage discharge from slaughterhouses, faecal pollution from wildlife such as wild boar or deer or wash-off of animal manure HEV and SaV could be present and may be carried to receiving waters. In case of rodent vermin hantaviruses could be expected.
Exposure assessment
In some regions in the world source waters that are used directly as drinking water receive human and animal excreta and secreta originating from bathers, animals, boats or indirectly from, for instance, wastewater discharges. In this case no reduction of viruses will take place before consumption. In other regions, the source waters are treated before use as drinking water. To be able to assess the ability of the system of concern to provide safe drinking water the viral hazards in the drinking water should be studied quantitatively.
Pathogenic viruses in drinking water
A few studies have shown the presence of human pathogenic viruses in untreated or treated drinking water used in different regions around the world (Leclerc et al., 2000). As described surface waters may be contaminated with viruses originating from a diversity of sources. Similar human and animal sources may contaminate groundwater that is insufficiently protected from external influences. This may be the case with semi- or unconfined groundwater from sandy saturated zones or from limestone or marl or with pre-treated, artificially infiltrated surface water or with bank filtered water. Groundwater was shown to be positive for viruses in 8 or 16% of the samples analysed (Borchardt, 2003; Fout et al., 2003). Reovirus, rotavirus, enterovirus, HAV and NoV were detected by molecular methods (Fout et al., 2003). None of the samples was positive in case cell culture was applied to detect infectious enterovirus (Fout et al., 2003). Another study revealed virus positivity in well waters by molecular (50%) and cell culture methods (5%; Borchardt et al., 2004). The presence of NoV genomes could not be confirmed in mineral waters whether spring water or finished product (Lamothe et al., 2003). Other studies confirmed virus-contaminated groundwater to be the source of outbreaks of acute gastroenteritis among hundreds of people (Parshionikar, 2003; Kim et al., 2005). Interestingly in one of the studies the contamination appeared to be transient since none of the sequential well water samples were virus positive (Borchardt, 2003). This may be due to short-term fluctuations in pathogen load caused by epidemics in the human or animal population, seasonal variations and/or incidents. The latter may encompass upstream (raw or treated) wastewater discharges, building of upstream sewage treatment plant or discharge, digging, drilling or maintenance near production site, injecting manure near unconfined source or discharge of polder waters near intake. Also heavy rainfall events, high river-/stream discharge and flow, high groundwater level, flooding of catchment/production site, thawing of (faecally contaminated) ice on reservoirs, frost leading to high numbers of birds on reservoirs and high numbers of birds (or other game) may be associated with peaks in faecal contamination of groundwater and also surface water.
Evidence has been published on the presence of human pathogenic viruses in treated water since drinking water treatment processes may have a limited capacity for virus reduction. Infectious rotavirus and enterovirus were detected in 83% of treated drinking water samples associated with rainfall but not with the presence of bacterial indicators (Keswick et al., 1984). In the African region, using cell culture, Ali et al. (2004) reported the presence of cultivable enteroviruses in 7 out of 30 finished drinking water samples at concentrations ranging from 5 to 33 plaque-forming units per litre. In Asian and African countries, integrated cell culture PCR enabled the detection of enteroviruses (Vivier et al., 2004), adenoviruses (Van Heerden et al., 2003) or both (Lee and Kim, 2002; Lee and Jeong, 2004) in drinking water. With the use of only molecular methods the presence of several pathogenic viruses such as rotavirus (Divizia et al., 2004; van Zyl et al., 2004), astrovirus (Gofti-Laroche et al., 2003) and NoV (Kukkula et al., 1999; Haramoto et al., 2004) were detected in drinking water in European, African and Asian countries. In another study in Thailand, no HAV was detected in tap waters, but in Brazil, 20% of river and tap waters were HAV positive (Kittigul, 2006; Villar et al., 2006). In Canada, outbreaks were identified associated with the presence of HAV and NoV in drinking water (Schuster et al., 2005). In case of direct evidence for the presence of pathogenic viruses in treated or untreated drinking water by either molecular or cell culture or other techniques, the quantity and host specificity should really be determined to be able to perform QMRA.
Pathogenic viruses in source water and virus reduction by treatment
The absence of pathogenic viruses in finished drinking waters does not exclude their presence but may merely indicate a lack of sensitivity of the detection techniques and/or the representativeness of the sampling. As shown with the recent advances in virus concentration and detection techniques more and more often human viruses are detected in tap water. However, at low dose viruses are still significant with respect to infectious and disease risk by exposure to drinking water because of their infectivity and pathogenicity and because of the large exposed population. An alternative approach in case of low virus concentrations in the finished waters is their detection in source waters combined with the assessment of the efficiency of drinking water treatment processes with respect to virus reduction. This approach ideally involves location-specific, quantitative information on the virus concentration in the source waters and virus reduction by treatment to estimate the virus concentration in the drinking water. In addition, information on the virus analysis, such as virus recovery rate of the method, infectivity and host specificity of the virus, are needed. Moreover, the local habits of drinking water consumption of the consumers should be known.
The recovery of the applied concentration–detection procedure should be determined to be able to accurately estimate the actual virus concentration. Loss of virus particles during the analysis will lead to underestimation of the infectious risk. Virus recovery rates depend on the employed concentration and detection methods, the volume of water to be concentrated, the turbidity of the water and the characteristics of the target virus (Senouci et al., 1996; Hill et al., 2005; Olszewski et al., 2005; Polaczyk et al., 2006). In order to determine the recovery rate of the sample analysis the procedure that is followed includes analysis of the viruses in the natural water sample and analysis of the same sample seeded with a specific dose of a known virus. This virus commonly involves the use of cultivable viruses such as specific enterovirus types or bacteriophages. These viruses are assumed to behave similarly to each other but differences in recovery between human and bacterial viruses have been reported (Hill et al., 2005). Moreover, these model viruses often have undergone several passages through the susceptible cell line and are therefore considered cell line adapted strains, which will behave differently as compared with naturally occurring human pathogenic viruses in the environment. However, in the absence of alternative approaches experiments with model viruses need to be done preferably coinciding with each sample analysis since virus recovery rates have been found to be highly variable even when the virus and water type are kept constant (Denis-Mize et al., 2004). In addition, in seed experiments often high virus levels are used to easily recover the model virus. But studies have shown that at the natural low dose virus recovery is lower and more variable (Fuhrman et al., 2005). Though many studies were undertaken in the past years, further optimisation of methods with respect to virus recovery is still warranted.
Since virus genomes may be present in the source water long after the virus particle has lost its ability to infect a host cell knowledge on the infectivity of the target virus is necessary to limit overestimation of the virus concentration and therefore the infectious risk. On the one hand, it may be argued that the detection of genomes of human pathogenic viruses in the drinking water is a necessary condition for the presence of infectious viruses. The employment of culture and molecular techniques in the detection of human pathogenic viruses in drinking water both encompass advantages and disadvantages. Susceptible cell lines are known for only some waterborne viruses and not for NoV (Duizer et al., 2004) and such cell lines may not be suitable for implementation as routine and robust diagnostic tools. Also, cell culture assays are laborious and expensive. On the other hand, plaque assays give quantitative results assuming that one plaque originates from one initial infectious virus particle. The latter may be another point of discussion (Teunis et al., 2005).
Often virus strains and types belonging to one virus family have a long history of developing their own host specificity (Fields et al., 2002). These genetically related viruses may be detected by the use of the same molecular or culture technique but not each virus strain will be capable to infect the human host. In case of the detection of human and animal, but non-zoonotic virus strains the infectious risk would be overestimated. Examples of such virus families are enteroviruses and adenoviruses (Fong and Lipp, 2005).
A few studies have been done on human pathogenic viruses at the intake point of drinking water suppliers. During an extensive study period of 9 years, 9% of source waters for drinking water production were positive for the presence of reovirus at relatively low titres but enterovirus or adenovirus were not detected (Sedmak et al., 2005). Rotaviruses were detected in source waters by use of molecular techniques (Van Zyl et al., 2004). The use of indicators for the presence of human pathogenic viruses in source waters has been reported with highly variable success with respect to correlation between index and indicator organisms (Bosch, 1998). The QMRA ideally involves location-specific, quantitative information on the virus concentration in the source waters that is representative for the time period including potentially wide quality fluctuations within it. Alternatively, literature data may be used but as shown such data are scarce.
Also location-specific, quantitative information is needed on virus reduction by treatment. Treatment may include a multiple barrier approach and/or point-of-use treatment in the home. Indicators such as somatic phages or F-specific phages with similar characteristics (size, surface charge, shape etc.) may be used in experiments to determine the efficiency of virus reduction (Table 4 ). These viruses that prey on specific bacterial hosts occur at higher numbers, which facilitates their detection after treatment (Payment, 1991). The ease and relative cost of phage detection is another advantage over human pathogenic viruses. Comparative analyses of the index and indicator virus reduction by the specific treatment step should show first that these viruses behave similarly as was performed by Payment and Franco (1993).
Table 4.
Characteristics of human pathogenic viruses and their possible indicators (Ferguson et al., 2003)
| Virus type | Size (nm) | pI | Phage type | Size (nm) | pI |
|---|---|---|---|---|---|
| Poliovirus 1 | 29 | 7.2 | PM2 | 60 | 7.3 |
| Poliovirus 2 | 29 | 4.5–6.5 | PM2 | 60 | 7.3 |
| Coxsackie virus A/B | 29 | 6.6-8.2 | ΦX174, PM2 | 26–32, 60 | 6.6, 7.3 |
| ECHO virus | 29 | 5.3–6.4 | ΦX174, Qβ | 26–32, 24 | 6.6, 5.3 |
| Hepatitis A virus | 27 | 2.8 | MS2 | 26 | 3.9 |
| Hepatitis E virus | 30 | – | – | – | – |
| Norovirus | 25 | 4.9♯ | Qβ, PRD1 | 24, 63 | 5.3, 4.2 |
| Sapovirus | 34 | – | – | – | – |
| Reovirus | 75 | 3.9 | MS2 | 26 | 3.9 |
| Rotavirus | 70 | – | – | – | – |
| Astrovirus | 29 | – | – | – | – |
| Adenovirus | 60–80 | – | – | – | – |
Note: ECHO, enteric cytopathogenic human orphan; Reo, respiratory enteric orphan; –, unknown; nm nanometer; pI, isoelectric point; ♯, determined for Norwalk-like virus particles.
Field data on pathogen removal or inactivation derived from the applied treatment plant is the most significant input for the QMRA but may be difficult because of low-level contamination or lack of methodology. Some field studies have been described. Analysis of viruses in drinking water produced by coagulation, sedimentation, sand filtration and chlorination showed 83% of the samples positive for infectious rotavirus and/or enterovirus (Keswick et al., 1984). The overall reduction of enteroviruses for similar processes in a 1-year study at 7 treatment plants was 99.97% with different coxsackie virus types B, polioviruses and echovirus identified in the finished drinking waters at an average concentration of 0.0006 infectious viruses per litre (Payment et al., 1985). Though in another study including 6 waterworks 11 out of 55 samples of partially treated waters were positive for different types of enteroviruses, no viruses were detected in 100 samples of finished drinking water (van Olphen et al., 1984). The evaluation of enteric virus and bacteriophages removal at three treatment plants did not show any positive results for viral presence in finished waters (Payment and Franco, 1993). Conventional treatment was insufficient for the reduction of enteroviruses in case of highly polluted intake water (Ali et al., 2004). Pilot plants on site are usually very similar to the operational plant and less troublesome for the retrieval of data because of the smaller scale. The removal of feline calicivirus and different phages was evaluated during conventional drinking water treatment ranging from 1.85 to 3.21 log10 units (Gerba et al., 2003). Laboratory experiments, for instance, with the specific source water and sand from an applied rapid sand filter will also generate useful data. Comparison of the removal of viruses and phages for a point-of-use treatment unit showed (more than) 5 log10-units reduction for somatic phages and MS2 coliphages and poliovirus, HAV, adenoviruses, rotaviruses and astrovirus (Grabow et al., 1999). If facilities for collection of field, pilot plant or laboratory data are missing, literature data on similar systems as determined by specific control parameters may be employed but though not complete, the overview above shows again that limited data are available on human pathogenic viruses.
Fate and behaviour of viruses during transport and storage
Human pathogenic viruses may be present in tap water depending on the concentration in the source water and in case of treatment on the efficiency of the treatment processes with respect to virus reduction. The concentration of pathogenic viruses in the drinking water can be used to assess the health risk associated with the consumption of tap water, as has been done for different viruses (see Section Risk characterisation). However, the previous studies have not taken into account the dynamics of human pathogenic viruses meaning the variations in the prevalence and infectivity of viruses during transport and storage. First of all, though human and animal viruses do not grow during transport and storage, sanitation and hygiene problems may cause (re-)introduction of pathogens in the drinking water that may survive for the duration of storage as was shown for bacteriophages. In case of a piped distribution system, the virus concentration in tap water is determined by the viral burden of the source water on one hand and reduction by applied treatment processes (if this is the case) and during transport on the other hand. Reduction of viruses in drinking water may be caused if temperatures are high, i.e., most viruses are readily inactivated at over 20°C (John and Rose, 2005). Exposure of the viruses to, for instance, sunlight as well as other environmental factors will decrease the numbers of viruses rapidly (Lytle and Sagrapanti, 2005). Again (re-)introduction of human pathogenic viruses may occur during maintenance and repair practices (Havelaar, 1994). Second, biofilms may play an important role with respect to virus concentrations in the finished waters (Fig. 2 ; Skraber et al., 2005). Since it has been shown that viruses can attach to biofilms, there is a possibility that drinking water biofilms accumulate pathogenic viruses present in the water entering the distribution system acting as an additional removal process. On exposure to single viruses or sloughs containing viruses released from biofilms the consumer could be infected. Data on fate and behaviour of human pathogenic viruses during transport and storage are extremely limited but this information is of importance with respect to intervention measures.
Fig. 2.
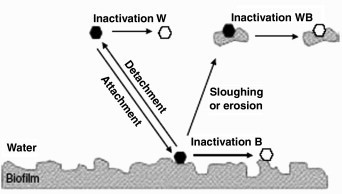
Parameters that control virus behaviour in drinking water distribution systems. Viral inactivation was considered as follows. ‘Inactivation W’ corresponds to the inactivation of virus in the water phase; ‘Inactivation B’ corresponds to the inactivation of virus in biofilm; ‘Inactivation BW’ corresponds to the inactivation of virus entrapped or attached to biofilm and released into the water phase by sloughing or erosion. Solid hexagon, free or particle-associated virus that is able to infect target cells; open hexagon, free or particle-associated virus that is not able to multiply anymore. From Skraber et al. (2005).
Consumption of drinking water
Here consumption of drinking water is discussed as route of transmission for human viruses disregarding exposure of the skin, eyes, ears and other body parts. Besides estimation of the concentration of virus in drinking water the assessment of the health risk posed by consumption of drinking water requires estimation of the volume of drinking water ingested and the response of the host to the ingested dose of viruses. Since waterborne viruses are readily inactivated at high temperatures the risk relates to the use of unboiled drinking water, e.g. excluding the consumption of tea but including the water used for washing salad and for making ice cubes.
Global data on the consumption of drinking water are limited especially for the developing regions. In studies carried out in Canada, The Netherlands, the United Kingdom and the USA, the average daily per capita consumption was usually found to be less than 2 l, but there was considerable variation between individuals in different countries and within the same country. The daily median intake of cold tap water in The Netherlands was 0.052 l, in Germany 0.15 l, in the United Kingdom 0.475 l, in Sweden 0.8 l and in Australia 0.5–1 l (Mons et al., 2005; Westrell et al., 2006). Another study in The Netherlands showed a daily median intake of cold tap water of 0.153 l, threefold higher than the study mentioned above (Teunis et al., 1996). As water intake will vary with climate, physical activity and culture, the above studies, which were conducted in temperate zones, can give only a limited view of consumption patterns throughout the world. At temperatures above 25°C, e.g. there is a sharp rise in fluid intake, largely to meet the demands of an increased sweat rate (Howard and Bartram, 2005). Other factors such as changes in cultural behaviour because of public awareness through information to the public or changes in the drinking-water demand or supply may also play a role in the habits of drinking water consumption. In the WHO GDWQ with respect to microbial hazards, per capita daily consumption of 1 l of unboiled water was assumed.
Dose–response modelling
The number of virus particles necessary to initiate an infection or even disease in the susceptible host after ingestion may be as low as a few particles or even a single particle (Ward et al., 1986; Lindesmith et al., 2003). The host may not experience symptoms though often acute symptoms will occur. These symptoms may be mild, severe and/or life-threatening (see Chapters 2, 3 and 4). A virus infection may become chronic and/or health effects may be delayed, so-called sequelae. Each of these health outcomes may result from infection with one and the same virus variant. For instance, infection with coxsackie virus variant B4 may pass unnoticed, cause gastroenteritis, cause myocarditis or diabetes or lead to infant death. The dose–response relation describes the quantitative relation between the intensity of exposure, i.e. the dose (here pathogenic viruses in drinking water), and the frequency of the occurrence of the adverse health effect within the exposed population of hosts, i.e. the response (here number of cases with specific symptoms in the human population). Available dose–response data have been obtained mainly from clinical studies including healthy adult volunteers but (premature) infants were included in some other studies (Teunis et al., 1996). The dose–response relations have been determined for oral ingestion of some specific variants of waterborne rotavirus and enteroviruses during volunteer studies (Table 5 ).
Table 5.
Infectivity of specific waterborne virus variants
| Organisms | Symptom scored | ID50 | |
|---|---|---|---|
| Rotavirusa | Excretion | 2.65×10−1 | 6.11 |
| Echovirus 12b | Excretion | 1.76×10−3 | 1.05×103 |
| Poliovirus | |||
| 1 sm | Excretion | 3.88×10−1 | 1.411 |
| 1 LSc2ab | Excretion | 7.14×10−4 | 6.93×104 |
| 1 | Excretion | 9.10×10−3 | 76.2 |
| 3 Fox (infants) | Excretion | 1.90×10−1 | 5.513 |
| 3 Fox (premature) | Excretion | 2.66×10−1 | 5.05 |
Source: From Teunis et al. (1996).
Note:, conditional probability of infection; ID50, number of microorganisms required to cause infection in 50% of experimentally infected animals, a measure of infectivity.
Administered in pH-buffered solution.
Rejected at the 95% level, within 99% confidence range for the deviance from maximum possible likelihood.
As shown in Table 5 the probability of infection after consumption of one infectious virus may range from 0.000714 for poliovirus type 1 LSc2ab to 0.388 for poliovirus type 1 sm. The number of viruses that cause infection in 50% of the human volunteers may vary from 1.411 poliovirus type 1 sm to 69,300 poliovirus type 1 LSc2ab. These findings demonstrate the variability in infectivity not just between virus families but also between virus types and even between virus variants affecting the QMRA. On the other hand, it may reflect differences in exposed populations (from neonates to adults) and ways of exposure (ingestion to bathing). In case of a specific human pathogenic virus variant detected in a known fraction of the drinking water than specific dose–response relation should be used. However, often exposure is determined for a broad class of pathogens, like enterovirus concentrations as determined by use of a specific susceptible cell line (most commonly Buffalo green monkey, BGM). For such a large family of viruses a dose–response relation can be defined, but this should include an additional level of variation: differences in infectivity (and/or pathogenicity) between related virus strains. A hierarchic dose–response model could be applied, accounting for variability both within and between pathogenic virus strains (Teunis, personal communication).
Besides viral characteristics, such as high infectivity and recombination, the host characteristics largely determine the adverse health effects. Genetic predisposition may render an individual susceptible or insusceptible to a specific virus infection as described for HIV (Paxton et al., 1996) and NoV (Lindesmith et al., 2003). Other host susceptibility factors include nutrition, age, pregnancy and immune status. Some of these factors are preventable such as nutrition whereas genetic background cannot be altered.
Risk characterisation
The concentration of pathogenic viruses in the drinking water (either directly determined or derived from location-specific data on human pathogenic viruses in source water and virus reduction by treatment) in combination with the dose–response relation can be used to assess the health risk associated with the consumption of drinking water. Several QMRA studies were performed to estimate the risk for exposure to drinking water contaminated with adenovirus, rotavirus and coxsackie virus (Regli et al., 1991; Haas et al., 1993; Gerba et al., 1996; Crabtree et al., 1997; Mena et al., 2003; Van Heerden et al., 2005). These studies involve static QMRA models ignoring the dynamics of a viral disease process in the exposed population with respect to the different transmission routes for the same pathogen, immunity, secondary transmission and genetic predisposition. These factors are included in a dynamic QMRA model (Eisenberg et al., 1998) but these have not been applied to date for estimation of the health risk associated with the consumption of virally contaminated drinking water. Anyway, the degree of confidence in the final risk estimate will largely depend on the assumptions, the uncertainties and variability of the data on which the QMRA is built and these need to be determined. Moreover, a level of tolerable risk needs to be set by the risk managers. For human pathogenic viruses to date the most common tolerable risk level was established at less than one infection in a population of 10,000 persons per year but this is to be discussed and decided by all parties involved in drinking water safety. More recently the WHO GDWQ use a reference level of risk of 10−6 DALY per person per year.
Risk management
Given the assessment of the risk of viral disease from consumption of drinking water, the risk managers have to select and implement appropriate control measures. These may involve cost-effectiveness reasoning. After implementation of a selected intervention, the effectiveness needs to be evaluated by surveillance of viral disease. In addition, environmental surveillance analysing the numbers of viral pathogens in the drinking water or alternatively in the source waters may be helpful in evaluating source protection interventions. Interventions may vary from additional research in case the risk estimate is unacceptably uncertain. In case of an unacceptable health risk the intervention may concern an additional treatment step in the process specifically designed to reduce human pathogenic viruses from the source water. Alternatively, the intervention may involve enhanced source water protection by barring virus-positive animal hosts or reducing contamination in distribution.
Besides a system assessment, the WSPs of each drinking water supply should include operational monitoring. Control measures need to be identified that control identified risks and ensure health-based targets are met. For each control measure identified, an appropriate means of operational monitoring should be defined that will ensure that any deviation from required performance is detected in time. Operational monitoring normally focuses on simple, cheap and rapid tests and as such microbial testing is rarely applicable.
For purposes of verification as opposed to operational monitoring, E. coli bacteria are measured. In case of the presence of this faecal indicator, the drinking water could be contaminated with human pathogenic viruses. The appropriate management action could, for instance, be a boiling water advisory. It would be inappropriate to perform retesting of the same sample or resampling because the basis for E. coli testing as a verification measure is the good performance of the test as laid down in standard operating procedures and its use as indicator. Retesting and resampling would take even more time and this should be spent on finding the fault or source of contamination to be able to protect public health. Again end product monitoring is useful in this way but system assessment and operational monitoring can elucidate the actual ability of the drinking water system to provide safe drinking water also with respect to human pathogenic viruses.
Risk communication
Communication of the viral risk estimate and the proposed risk management measures contributes to the improvement of the drinking water safety with respect to human pathogenic viruses. Dissemination involves not only risk managers (policy makers and inspectors) and risk assessors but consumers and suppliers of drinking water alike. For this purpose tools such as brochures and websites should be attuned and transparent. In The Netherlands, a webtool for the assessment of risk by non-experts was launched simultaneously with the Inspectorate guideline for analysis of microbial safety of drinking water (De Roda Husman and Medema, 2006) to be used by Dutch policy makers and inspectors and will be released for use by other interested parties.
Concluding remarks and recommendations
The global supply of safe drinking water should be considered with an open mind to the possible viral threats. The potential transmission of (re-)emerging human pathogenic viruses should be assessed based on research. For instance, foot-and-mouth disease viruses were considered to be solely respiratory pathogens and therefore their potential as possible waterborne pathogens was disregarded. Not that these viruses display a major threat to the drinking water supply or human health (Schijven et al., 2005) but animal health is endangered and at least the risk should be assessed instead of hypothesised. Recently this has received a great deal of attention for the emerging disease SARS: the associated coronavirus was evaluated with respect to possible shedding and transmission routes including water. The same is true for avian influenza viruses which occasionally infect humans from their animal reservoirs mostly by close contact; the potential risk of waterborne transmission was estimated (WHO, 2006b). Theoretically, viruses could be considered as biocolloids with specific properties such as size, shape, structure, charge, composition, genome, etc. These viral characteristics determine their behaviour in the environment, resistance to natural inactivation and treatment and disinfection processes. For each (re-)emerging virus these properties may be known or could be assessed predicting the effectiveness of possible intervention measures for prevention of waterborne disease. In this respect, free data sharing among researchers and risk managers in times of emergencies and disasters as encouraged by WHO is one of the keys to successful reduction of the environmental spread of pathogenic viruses and reduced burden of water-transmitted viral disease.
References
- Abbaszadegan M., Stewart P., LeChevallier M. A strategy for detection of viruses in groundwater by PCR. Appl Environ Microbiol. 1999;65:444–449. doi: 10.1128/aem.65.2.444-449.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali M.A., Al-Herrawy A.Z., El-Hawaary S.E. Detection of enteric viruses, Giardia and Cryptosporidium in two different types of drinking water treatment facilities. Water Res. 2004;38:3931–3939. doi: 10.1016/j.watres.2004.06.014. [DOI] [PubMed] [Google Scholar]
- Amvrosieva T.V., Paklonskaya N.V., Biazruchka A.A., Kazinetz O.N., Bohush Z.F., Fisenko E.G. Enteroviral infection outbreak in the Republic of Belarus: principal characteristics and phylogenetic analysis of etiological agents. Cent Eur J Publ Health. 2006;14(2):67–73. doi: 10.21101/cejph.a3369. [DOI] [PubMed] [Google Scholar]
- Ashbolt N.J. Microbial contamination of drinking water and disease outcomes in developing regions. Toxicology. 2004;198(1–3):229–238. doi: 10.1016/j.tox.2004.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banyai K., Jakab F., Reuter G., Bene J., Uj M., Melegh B., Szucs G. Sequence heterogeneity among human picobirnaviruses detected in gastroenteritis outbreak. Arch Virol. 2003;148:2281–2291. doi: 10.1007/s00705-003-0200-z. [DOI] [PubMed] [Google Scholar]
- Beld M., Sentjens R., Rebers S., Weel J., Wertheim-van Dillen P., Sol C., Boom R. Detection and quantitation of hepatitis C virus RNA in feces of chronically infected individuals. J Clin Microbiol. 2000;38(9):3442–3444. doi: 10.1128/jcm.38.9.3442-3444.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger J.R., Miller C.S., Mootoor Y., Avdiushko S.A., Kryscio R.J., Zhu H. JC virus detection in bodily fluids: clues to transmission. Clin Infect Dis. 2006;43:e9–e12. doi: 10.1086/504947. [DOI] [PubMed] [Google Scholar]
- Borchardt M.A. Incidence of enteric viruses in groundwater from household wells in Wisconsin. Appl Environ Microbiol. 2003;69(2):1172–1180. doi: 10.1128/AEM.69.2.1172-1180.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borchardt M.A., Haas N.L., Hunt R.J. Vulnerability of drinking-water wells in la Crosse, Wisconsin, to enteric-virus contamination from surface water contributions. Appl Environ Microbiol. 2004;70:5937–5946. doi: 10.1128/AEM.70.10.5937-5946.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosch A. Human enteric viruses in the water environment: a minireview. Int Microbiol. 1998;1:191–196. [PubMed] [Google Scholar]
- Bowdre J.H. Viral gastroenteritis and laboratory detection of rotavirus. Am J Med Technol. 1983;49(9):665–668. [PubMed] [Google Scholar]
- Bradley D. Health aspects of water supplies in tropical countries. In: Water, Wastes and Health in Hot Climates (Feachem R, McGarry M, Mara D, editors). Chichester: Wiley; 1977; pp. 3–17.
- Carter M.J. Enterically infecting viruses: pathogenicity, transmission and significance for food and waterborne infection. J Appl Microbiol. 2005;98(6):1354–1380. doi: 10.1111/j.1365-2672.2005.02635.x. [DOI] [PubMed] [Google Scholar]
- Cascio A., Bosco M., Vizzi E., Giammanco A., Ferraro D., Arista S. Identification of picobirnavirus from faeces of Italian children suffering from acute diarrhea. Eur J Epidemiol. 1996;12(5):545–547. doi: 10.1007/BF00144011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemente-Casares P. Hepatitis E virus epidemiology in industrialized countries. Emerg Infect Dis. 2003;9:448–454. doi: 10.3201/eid0904.020351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crabtree K.D., Gerba C.P., Rose J.B., Haas C.N. Waterborne adenovirus: a risk assessment. Water Sci Technol. 1997;35:1–6. [Google Scholar]
- De Deus N., Seminati C., Pina S., Mateu E., Martin M., Segales J. Detection of hepatitis E virus in liver, mesenteric lymph node, serum, bile, and faeces of naturally infected pigs affected by different pathological conditions. Vet Microbiol. 2007;119(2–4):105–114. doi: 10.1016/j.vetmic.2006.08.027. [DOI] [PubMed] [Google Scholar]
- Denis-Mize K., Fout G.S., Dahling D.R., Francy D.S. Detection of human enteric viruses in stream water with RT-PCR and cell culture. J Water Health. 2004;2(1):37–47. [PubMed] [Google Scholar]
- De Roda Husman A.M., Medema G.J. Dutch Environmental Inspectorate guideline ‘Assessment of the microbial safety of drinking water’. Artikelcode. 2006;5318 [Google Scholar]
- Ding Y., He L., Zhang Q., Huang Z., Che X., Hou J., Wang H., Shen H., Qiu L., Li Z., Geng J., Cai J., Han H., Li X., Kang W., Weng D., Liang P., Jiang S. Organ distribution of severe acute respiratory syndrome (SARS) associated coronavirus (SARS-CoV) in SARS patients: implications for pathogenesis and virus transmission pathways. J Pathol. 2004;203(2):622–630. doi: 10.1002/path.1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Divizia M., Gabrieli R., Donia D., Macaluso A., Bosch A., Guix S., Sanchez G., Villena C., Pinto R.M., Palombi L., Buonuomo E., Cenko F., Leno L., Bebeci D., Bino S. Waterborne gastroenteritis outbreak in Albania. Water Sci Technol. 2004;50:57–61. [PubMed] [Google Scholar]
- Duan S.M., Zhao X.S., Wen R.F., Huang J.J., Pi G.H., Zhang S.X., Han J., Bi S.L., Ruan L., Dong X.P., SARS Research Team Stability of SARS coronavirus in human specimens and environment and its sensitivity to heating and UV irradiation. Biomed Environ Sci. 2003;16(3):246–255. [PubMed] [Google Scholar]
- Duizer E., Schwab K.J., Neill F.H., Atmar R.L., Koopmans M.P., Estes M.K. Laboratory efforts to cultivate noroviruses. J Gen Virol. 2004;85:79–87. doi: 10.1099/vir.0.19478-0. [DOI] [PubMed] [Google Scholar]
- Duizer E., Koopmans M. Tracking foodborne viruses: Lessons from noroviruses. In: Motarjemi A., editor. Emerging foodborne pathogens. CRC Press; Boca Raton, USA: 2006. pp. 77–110. [Google Scholar]
- Eisenberg J.N., Seto E.Y., Colford J.M., Jr, Olivieri A., Spear R.C. An analysis of the Milwaukee Cryptosporidiosis outbreak based on a dynamic model of the infection process. Epidemiology. 1998;9(3):255–263. [PubMed] [Google Scholar]
- Ferguson C., de Roda Husman A.M., Altavilla N., Deere D., Ashbolt N. Fate and transport of surface water pathogens in watersheds. Crit Rev Environ Sci Technol. 2003;33(3):299–361. [Google Scholar]
- Fields B.N., Knipe D.M., Howley P.M., editors. Fields Virology. 4th ed. Lippincott Williams and Wilkins; Philadelphia, PA: 2002. [Google Scholar]
- Fleischer J., Schlafmann K., Otchwemah R., Botzenhart K. Elimination of enteroviruses, other enteric viruses, F-specific coliphages, somatic coliphages and E. coli in four sewage treatment plants of Southern Germany. J Water Supply Res Technol. 2000;49:127–137. [Google Scholar]
- Fong T.T., Lipp E.K. Enteric viruses of humans and animals in aquatic environments: health risks, detection, and potential water quality assessment tools. Microbiol Mol Biol Rev. 2005;69(2):357–371. doi: 10.1128/MMBR.69.2.357-371.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fout G.S., Martinson B.C., Moyer M.W., Dahling D.R. A multiplex reverse-transcription-PCR method for detection of human enteric viruses in groundwater. Appl Environ Microbiol. 2003;69(6):3158–3164. doi: 10.1128/AEM.69.6.3158-3164.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuhrman J.A., Liang X., Noble R.T. Rapid detection of enteroviruses in small volumes of natural waters by real-time quantitative reverse transcriptase PCR. Appl Environ Microbiol. 2005;71(8):4523–4530. doi: 10.1128/AEM.71.8.4523-4530.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehr R., Wagner M., Veerasubramanian P., Payment P. Disinfection efficiency of peracetic acid, UV and ozone after enhanced primary treatment of municipal wastewater. Water Res. 2003;37:4573–4586. doi: 10.1016/S0043-1354(03)00394-4. [DOI] [PubMed] [Google Scholar]
- Gerba C.P., Rose J.B., Haas C.N., Crabtree K.D. Waterborne rotavirus: a risk assessment. Water Res. 1996;30:2929–2940. [Google Scholar]
- Gerba C.P., Riley K.R., Nwachuku N., Ryu H., Abbaszadegan M. Removal of Encephalitozoon intestinalis, calicivirus, and coliphages by conventional drinking water treatment. J Environ Sci Health A Tox Hazard Subst Environ Eng. 2003;38(7):1259–1268. doi: 10.1081/ese-120021124. [DOI] [PubMed] [Google Scholar]
- Gilgen M., Germann D., Luthy J., Hubner P. Three-step isolation method for sensitive detection of enterovirus, rotavirus, hepatitis A virus, and small round structured viruses in water samples. Int J Food Microbiol. 1997;37:189–199. doi: 10.1016/s0168-1605(97)00075-5. [DOI] [PubMed] [Google Scholar]
- Giordano M.O., Martinez L.C., Isa M.B., Ferreyra L.J., Canna F., Pavan J.V., Paez M., Notario R., Nates S.V. Twenty year study of the occurrence of reovirus infection in hospitalized children with acute gastroenteritis in Argentina. Pediatr Infect Dis J. 2002;21(9):880–882. doi: 10.1097/00006454-200209000-00021. [DOI] [PubMed] [Google Scholar]
- Glass RI, Bresee J, Jiang B, Gentsch J, Ando T, Fankhauser R, Noel J, Parashar U, Rosen B, Monroe SS. Gastroenteritis viruses: an overview. Novartis Foundation Symposium; 2001; 238; pp. 5–19. Discussion; pp. 19–25. [DOI] [PubMed]
- Godfree A., Farrell J. Processes for managing pathogens. J Environ Qual. 2005;34(1):105–113. doi: 10.2134/jeq2005.0105. [DOI] [PubMed] [Google Scholar]
- Gofti-Laroche L., Gratacap-Cavallier B., Demanse D., Genoulaz O., Seigneurin J.M., Zmirou D. Are waterborne astrovirus implicated in acute digestive morbidity (E.M.I.R.A. study)? J Clin Virol. 2003;27:74–82. doi: 10.1016/s1386-6532(02)00130-0. [DOI] [PubMed] [Google Scholar]
- Grabow W.O., Clay C.G., Dhaliwal W., Vrey M.A., Muller E.E. Elimination of viruses, phages, bacteria and cryptosporidium by a new generation Aquaguard point-of-use water treatment unit. Zentralbl Hyg Umweltmed. 1999;202(5):399–410. [PubMed] [Google Scholar]
- Guix S., Bosch A., Pinto R.M. Human astrovirus diagnosis and typing: current and future prospects. Lett Appl Microbiol. 2005;41(2):103–105. doi: 10.1111/j.1472-765X.2005.01759.x. [DOI] [PubMed] [Google Scholar]
- Haas C.N., Rose J.B., Gerba C., Regli S. Risk assessment of virus in drinking water. Risk Anal. 1993;13(5):545–552. doi: 10.1111/j.1539-6924.1993.tb00013.x. [DOI] [PubMed] [Google Scholar]
- Haramoto E., Katayama H., Ohgaki S. Detection of noroviruses in tap water in Japan by means of a new method for concentrating enteric viruses in large volumes of freshwater. Appl Environ Microbiol. 2004;70(4):2154–2160. doi: 10.1128/AEM.70.4.2154-2160.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haramoto E., Katayama H., Oguma K., Ohgaki S. Application of cation-coated filter method to detection of noroviruses, enteroviruses, adenoviruses and torque tenoviruses in the Tamagawa river in Japan. Appl Environ Microbiol. 2005;71(5):2403–2411. doi: 10.1128/AEM.71.5.2403-2411.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatakeyama N., Suzuki N., Yamamoto M., Kuroiwa Y., Hori T., Mizue N., Tsutsumi H. Detection of BK virus and adenovirus in the urine from children after allogeneic stem cell transplantation. Pediatr Infect Dis J. 2006;25(1):84–85. doi: 10.1097/01.inf.0000195613.00368.df. [DOI] [PubMed] [Google Scholar]
- Hauri A.M., Schimmelpfennig M., Walter-Domes M., Letz A., Diedrich S., Lopez-Pila J., Schreier E. An outbreak of viral meningitis associated with a public swimming pond. Epidemiol Infect. 2005;133(2):291–298. doi: 10.1017/s0950268804003437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havelaar A.H. Application of HACCP to drinking-water supply. Food Control. 1994;5:145–152. [Google Scholar]
- Havelaar AH, Melse JM. Quantifying public health risks in the WHO Guidelines for Drinking-Water Quality: a burden of disease approach. Report 734301022/2003; Bilthoven, the Netherlands: RIVM; 2003.
- Herrmann J.E., Nowak N.A., Blacklow N.R. Detection of Norwalk virus in stools by enzyme immunoassay. J Med Virol. 1985;17(2):127–133. doi: 10.1002/jmv.1890170205. [DOI] [PubMed] [Google Scholar]
- Hill V.R., Polaczyk A.L., Hahn D., Narayanan J., Cromeans T.L., Roberts J.M., Amburgey J.E. Development of a rapid method for simultaneous recovery of diverse microbes in drinking water by ultrafiltration with sodium polyphosphate and surfactants. Appl Environ Microbiol. 2005;71(11):6878–6884. doi: 10.1128/AEM.71.11.6878-6884.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoebe C.J., Vennema H., de Roda Husman A.M., van Duynhoven Y.T. Norovirus outbreak among primary schoolchildren who had played in a recreational water fountain. J Infect Dis. 2004;189(4):699–705. doi: 10.1086/381534. [DOI] [PubMed] [Google Scholar]
- Hörman A., Rimhanen-Finne R., Maunula L., von Bonsdorff C.H., Torvela N., Heikinheimo A., Hanninen M.L. Campylobacter spp., Giardia spp., Cryptosporidium spp., noroviruses, and indicator organisms in surface water in southwestern Finland, 2000–2001. Appl Environ Microbiol. 2004;70:87–95. doi: 10.1128/AEM.70.1.87-95.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hot D., Legeay O., Jacques J., Gantzer C., Caudrelier Y., Guyard K., Lange M., Andreoletti L. Detection of somatic phages, infectious enteroviruses and enterovirus genomes as indicators of human enteric viral pollution in surface water. Water Res. 2003;37:4703–4710. doi: 10.1016/S0043-1354(03)00439-1. [DOI] [PubMed] [Google Scholar]
- Howard G., Bartram J. Effective water supply surveillance in urban areas of developing countries. J Water Health. 2005;3(1):31–43. [PubMed] [Google Scholar]
- Johansson P.J., Sveger T., Ahlfors K., Ekstrand J., Svensson L. Reovirus type 1 associated with meningitis. Scand J Infect Dis. 1996;28(2):117–120. doi: 10.3109/00365549609049060. [DOI] [PubMed] [Google Scholar]
- Jardine C., Hrudey S., Shortreed J., Craig L., Krewski D., Furgal C., McColl S. Risk management frameworks for human health and environmental risks. J Toxicol Environ Health B Crit Rev. 2003;6(6):569–720. doi: 10.1080/10937400390208608. [DOI] [PubMed] [Google Scholar]
- John D.E., Rose J.B. Review of factors affecting microbial survival in groundwater. Environ Sci Technol. 2005;39(19):7345–7356. doi: 10.1021/es047995w. [DOI] [PubMed] [Google Scholar]
- Kaye S.B., Lloyd M., Williams H., Yuen C., Scott J.A., O’Donnell N., Batterbury M., Hiscott P., Hart C.A. Evidence for persistence of adenovirus in the tear film a decade following conjunctivitis. J Med Virol. 2005;77(2):227–231. doi: 10.1002/jmv.20440. [DOI] [PubMed] [Google Scholar]
- Keswick B.H., Gerba C.P., DuPont H.L., Rose J.B. Detection of enteric viruses in treated drinking water. Appl Environ Microbiol. 1984;47(6):1290–1294. doi: 10.1128/aem.47.6.1290-1294.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalili K., Gordon J., White M.K. The polyomavirus, JCV and its involvement in human disease. Adv Exp Med Biol. 2006;577:274–287. doi: 10.1007/0-387-32957-9_20. [DOI] [PubMed] [Google Scholar]
- Kim S.H., Cheon D.S., Kim J.H., Lee D.H., Jheong W.H., Heo Y.J., Chung H.M., Jee Y., Lee J.S. Outbreaks of gastroenteritis that occurred during school excursions in Korea were associated with several waterborne strains of norovirus. J Clin Microbiol. 2005;43(9):4836–4839. doi: 10.1128/JCM.43.9.4836-4839.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kittigul L. Detection and characterization of hepatitis A virus in water samples in Thailand. J Appl Microbiol. 2006;100(6):1318–1323. doi: 10.1111/j.1365-2672.2006.02876.x. [DOI] [PubMed] [Google Scholar]
- Ksiazek T.G., Erdman D., Goldsmith C.S., Zaki S.R., Peret T., Emery S., Tong S., Urbani C., Comer J.A., Lim W., Rollin P.E., Dowell S.F., Ling A.E., Humphrey C.D., Shieh W.J., Guarner J., Paddock C.D., Rota P., Fields B., DeRisi J., Yang J.Y., Cox N., Hughes J.M., LeDuc J.W., Bellini W.J., Anderson L.J., SARS Working Group A novel coronavirus associated with Severe acute respiratory syndrome. New Engl J Med. 2003;348(20):1953–1966. doi: 10.1056/NEJMoa030781. [DOI] [PubMed] [Google Scholar]
- Kukkula M., Maunula L., Silvennoinen E., von Bonsdorff C.H. Outbreak of viral gastroenteritis due to drinking water contaminated by Norwalk-like viruses. J Infect Dis. 1999;180:1771–1776. doi: 10.1086/315145. [DOI] [PubMed] [Google Scholar]
- Lamothe G.T., Putallaz T., Joosten H., Marugg J.D. Reverse transcription-PCR analysis of bottled and natural mineral waters for the presence of noroviruses. Appl Environ Microbiol. 2003;69(11):6541–6549. doi: 10.1128/AEM.69.11.6541-6549.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laverick M.A., Wyn-Jones A.P., Carter M.J. Quantitative RT-PCR for the enumeration of noroviruses (Norwalk-like viruses) in water and sewage. Lett Appl Microbiol. 2004;39:127–136. doi: 10.1111/j.1472-765X.2004.01534.x. [DOI] [PubMed] [Google Scholar]
- Leclerc H., Edberg S., Pierzo V., Delattre J.M. Bacteriophages as indicators of enteric viruses and public health risk in groundwaters. J Appl Microbiol. 2000;88(1):5–21. doi: 10.1046/j.1365-2672.2000.00949.x. [DOI] [PubMed] [Google Scholar]
- Lee H.K., Jeong Y.S. Comparison of total culturable virus assay and multiplex integrated cell culture-PCR for reliability of waterborne virus detection. Appl Environ Microbiol. 2004;70:3626–3632. doi: 10.1128/AEM.70.6.3632-3636.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S.H., Kim S.J. Detection of infectious enteroviruses and adenoviruses in tap water in urban areas in Korea. Water Res. 2002;36:248–256. doi: 10.1016/s0043-1354(01)00199-3. [DOI] [PubMed] [Google Scholar]
- Li T.C., Chijiwa K., Sera N., Ishibashi T., Etoh Y., Shinohara Y., Kurata Y., Ishida M., Sakamoto S., Takeda N., Miyamura T. Hepatitis E virus transmission from wild boar meat. Emerg Infect Dis. 2005;11(12):1958–1960. doi: 10.3201/eid1112.051041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindesmith L., Moe C., Marionneau S., Ruvoen N., Jiang X., Lindblad L., Stewart P., LePendu J., Baric R. Human susceptibility and resistance to Norwalk virus infection. Nat Med. 2003;9:548–553. doi: 10.1038/nm860. [DOI] [PubMed] [Google Scholar]
- Lodder W.J., de Roda Husman A.M. Presence of noroviruses and other enteric viruses in sewage and surface waters in the Netherlands. Appl Environ Microbiol. 2005;71(3):1453–1461. doi: 10.1128/AEM.71.3.1453-1461.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu L., Li C., Hagedorn C.H. Phylogenetic analysis of global hepatitis E virus sequences: genetic diversity, subtypes and zoonosis. Rev Med Virol. 2006;16(1):5–36. doi: 10.1002/rmv.482. [DOI] [PubMed] [Google Scholar]
- Lucena F., Duran A.E., Moron A., Calderon E., Campos C., Gantzer C., Skraber S., Jofre J. Reduction of bacterial indicators and bacteriophages infecting faecal bacteria in primary and secondary wastewater treatments. JAppl Microbiol. 2004;97:1069–1076. doi: 10.1111/j.1365-2672.2004.02397.x. [DOI] [PubMed] [Google Scholar]
- Luo K.X., Zhang L., Wang S.S., Nie J., Yang S.C., Liu D.X., Liang W.F., He H.T., Lu Q. An outbreak of enterically transmitted non-A, non-E viral hepatitis. J Viral Hepat. 1999;6(1):59–64. doi: 10.1046/j.1365-2893.1999.6120132.x. [DOI] [PubMed] [Google Scholar]
- Lytle C.D., Sagrapanti J.L. Predicted inactivation of viruses of relevance to biodefense by solar radiation. J Virol. 2005;79(22):14244–14252. doi: 10.1128/JVI.79.22.14244-14252.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackiewicz V., Dussaix E., Le Petitcorps M.F., Roque-Afonso A.M. Detection of hepatitis A virus RNA in saliva. J Clin Microbiol. 2004;42(9):4329–4331. doi: 10.1128/JCM.42.9.4329-4331.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsubara H., Michitaka K., Horiike N., Yano M., Akbar S.M., Torisu M., Onji M. Existence of TT virus DNA in extracellular body fluids from normal healthy Japanese subjects. Intervirology. 2000;43(1):16–19. doi: 10.1159/000025018. [DOI] [PubMed] [Google Scholar]
- Matsuura K., Ishikura M., Nakayama T., Hasegawa S., Morita O., Katori K., Uetake H. Ecological studies on reovirus pollution of rivers in Toyama Prefecture. II. Molecular epidemiological study of reoviruses isolated from river water. Microbiol Immunol. 1993;37:305–310. doi: 10.1111/j.1348-0421.1993.tb03214.x. [DOI] [PubMed] [Google Scholar]
- McKinney K.R., Gong Y.Y., Lewis T.G. Environmental transmission of SARS at Amoy gardens. J Environ Health. 2006;68(9):26–30. [PubMed] [Google Scholar]
- Mena K.D., Gerba C.P., Haas C.N., Rose J.B. Risk assessment of waterborne coxsackie virus. J Am Water Works Assoc. 2003;95:122–131. [Google Scholar]
- Mons M, Blokker M, van der Wielen J, Medema G, Sinclair M, Hulshof K, Dangendorff F, Hunter P. Estimation of the consumption of cold tap water for microbiological risk assessment. EU project MICRORISK (contract EVK1-CT-2002-00123); 2005. http://217.77.141.80/clueadeau/microrisk/uploads/finalreportwp5.pdf, retrieved in November, 2006. [DOI] [PubMed]
- Moore B.E. Survival of Human Immunodeficiency Virus (HIV), HIV-Infected lymphocytes, and poliovirus in water. Appl Environ Microbiol. 1993;59(5):1437–1443. doi: 10.1128/aem.59.5.1437-1443.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muir P., Nicholson F., Jhetam M., Neogi S., Banatvala J.E. Rapid diagnosis of enterovirus infection by magnetic bead extraction and polymerase chain reaction detection of enterovirus RNA in clinical specimens. J Clin Microbiol. 1993;31(1):31–38. doi: 10.1128/jcm.31.1.31-38.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myrmel M., Berg E.M., Grinde B., Rimstad E. Enteric viruses in inlet and outlet samples from sewage treatment plants. J Water Health. 2006;4(2):197–209. [PubMed] [Google Scholar]
- Olszewski J., Winona L., Oshima K.H. Comparison of 2 ultrafiltration systems for the concentration of seeded viruses from environmental waters. Can J Microbiol. 2005;51(4):295–303. doi: 10.1139/w05-011. [DOI] [PubMed] [Google Scholar]
- Ortiz-Movilla N., Lazaro P., Rodriguez-Inigo E., Bartolome J., Longo I., Lecona M., Pardo M., Carreno V. Hepatitis C virus replicates in sweat glands and is released into sweat in patients with chronic hepatitis C. J Med Virol. 2002;68(4):529–536. doi: 10.1002/jmv.10238. [DOI] [PubMed] [Google Scholar]
- Parshionikar S.U. Waterborne outbreak of gastroenteritis associated with a norovirus. Appl Environ Microbiol. 2003;69(9):5263–5268. doi: 10.1128/AEM.69.9.5263-5268.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxton W.A., Martin S.R., Tse D., O’Brien T.R., Skurnick J., VanDevanter N.L., Padian N., Braun J.F., Kotler D.P., Wolinsky S.M., Koup R.A. Relative resistance to HIV-1 infection of CD4 lymphocytes from persons who remain uninfected despite multiple high-risk sexual exposure. Nat Med. 1996;2(4):412–417. doi: 10.1038/nm0496-412. [DOI] [PubMed] [Google Scholar]
- Payment P. Fate of human enteric viruses, coliphages, and Clostridium perfringens during drinking-water treatment. Can J Microbiol. 1991;37(2):154–157. doi: 10.1139/m91-023. [DOI] [PubMed] [Google Scholar]
- Payment P., Franco E. Clostridium perfringens and somatic coliphages as indicators of the efficiency of drinking water treatment for viruses and protozoan cysts. Appl Environ Microbiol. 1993;59(8):2418–2424. doi: 10.1128/aem.59.8.2418-2424.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payment P., Trudel M., Plante R. Elimination of viruses and indicator bacteria at each step of treatment during preparation of drinking water at seven water treatment plants. Appl Environ Microbiol. 1985;49(6):1418–1428. doi: 10.1128/aem.49.6.1418-1428.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phan T.G., Trinh Q.D., Yagyu F., Sugita K., Okitsu S., Muller W.E., Ushijima H. Outbreak of sapovirus infection among infants and children with acute gastroenteritis in Osaka City, Japan during 2004–2005. J Med Virol. 2006;78(6):839–846. doi: 10.1002/jmv.20632. [DOI] [PubMed] [Google Scholar]
- Polaczyk AL, Roberts JM, Hill VR. Evaluation of 1MDS electropositive microfilters for simultaneous recovery of multiple microbe classes from tap water. J Microbiol Meth 2006 doi:10.1016/j.mimet.2006.08.007. [DOI] [PubMed]
- Poullis D.A., Attwell R.W., Powell S.C. The characterization of waterborne-disease outbreaks. Rev Environ Health. 2005;20(2):141–149. [PubMed] [Google Scholar]
- Rab M.A., Bile M.K., Mubarik M.M., Asghar H., Sami Z., Siddiqi S., Dil A.S., Barzgar M.A., Chaudhry M.A., Burney M.I. Water-borne hepatitis E virus epidemic in Islamabad, Pakistan: a common source outbreak traced to the malfunction of a modern water treatment plant. Am J Trop Med Hyg. 1997;57(2):151–157. doi: 10.4269/ajtmh.1997.57.151. [DOI] [PubMed] [Google Scholar]
- Ramsay M., Reacher M., O’Flynn C., Buttery R., Hadden F., Cohen B., Knowles W., Wreghitt T., Brown D. Causes of morbiliform rash in a highly immunised English population. Arch Dis Child. 2002;87:202–206. doi: 10.1136/adc.87.3.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regli S., Rose J.B., Haas C.N., Gerba C.P. Modelling the risk from Giardia and viruses in drinking water. J Am Water Works Assoc. 1991;83:76–84. [Google Scholar]
- Reuther C.G. Saline solutions: the quest for fresh water. Environ Health Perspect. 2000;108(2):A78–A80. doi: 10.1289/ehp.108-a78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schernewski G., Julich W.D. Risk assessment of virus infections in the Oder estuary (southern Baltic) on the basis of spatial transport and virus decay simulations. Int J Hyg Environ Health. 2001;203:317–325. doi: 10.1078/1438-4639-00046. [DOI] [PubMed] [Google Scholar]
- Schijven J.F., Rijs G.B., de Roda Husman A.M. Quantitative risk assessment of FMD virus transmission via water. Risk Anal. 2005;25(1):13–21. doi: 10.1111/j.0272-4332.2005.00563.x. [DOI] [PubMed] [Google Scholar]
- Schuster C.J., Ellis A.G., Robertson W.J., Charron D.F., Aramini J.J., Marshall B.J., Medeiros D.T. Infectious disease outbreaks related to drinking water in Canada, 1974–2001. Can J Pub Health. 2005;96(4):254–258. doi: 10.1007/BF03405157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schvoerer E., Bonnet F., Dubois V., Cazaux G., Serceau R., Fleury H.J., Lafon M.E. PCR detection of human enteric viruses in bathing areas, waste waters and human stools in Southwestern France. Res Microbiol. 2000;151:693–701. doi: 10.1016/s0923-2508(00)90132-3. [DOI] [PubMed] [Google Scholar]
- Schvoerer E., Ventura M., Dubos O., Cazaux G., Serceau R., Gournier N., Dubois V., Caminade P., Fleury H.J., Lafon M.E. Qualitative and quantitative molecular detection of enteroviruses in water from bathing areas and from a sewage treatment plant. Res Microbiol. 2001;152:179–186. doi: 10.1016/s0923-2508(01)01190-1. [DOI] [PubMed] [Google Scholar]
- Sedmak G., Bina D., Macdonald J., Couillard L. Nine-year study of the occurrence of culturable viruses in source water for two drinking water treatment plants and the influent and effluent of a Wastewater Treatment Plant in Milwaukee, Wisconsin (August 1994 through July 2003) Appl Environ Microbiol. 2005;71(2):1042–1050. doi: 10.1128/AEM.71.2.1042-1050.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senouci S., Maul A., Schwartzbrod L. Comparison study on three protocols used to concentrate poliovirus type 1 from drinking water. Zentralbl Hyg Umweltmed. 1996;198(4):307–317. [PubMed] [Google Scholar]
- Shuval H. Estimating the global burden of thalassogenic diseases: human infectious diseases caused by wastewater pollution of the marine environment. J Water Health. 2003;1(2):53–64. [PubMed] [Google Scholar]
- Singh S, Mohanty A, Joshi YK, Deka D, Mohanty S, Panda SK. Mother-to-child transmission of hepatitis E virus infection. Indian J Pediatr 2003; 70: 37–39 [DOI] [PubMed]
- Singh V., Singh V., Raje M., Nain C.K., Singh K. Routes of transmission in the hepatitis E epidemic of Saharanpur. Trop Gastroenterol. 1998;19(3):107–109. [PubMed] [Google Scholar]
- Skraber S., Schijven J.F., Gantzer C., de Roda Husman A.M. Pathogenic viruses in drinking water biofilms: a public health risk? Biofilms. 2005;2:1–13. [Google Scholar]
- Somani S.K., Aggarwal R., Naik S.R., Srivastava S., Naik S. A serological study of intrafamilial spread from patients with sporadic hepatitis E virus infection. J Viral Hepat. 2003;10(6):446–449. doi: 10.1046/j.1365-2893.2003.00458.x. [DOI] [PubMed] [Google Scholar]
- Tarantola A., Abiteboul D., Rachline A. Infection risks following accidental exposure to blood or body fluids in health care workers: a review of pathogens transmitted in published cases. Am J Infect Control. 2006;34(6):367–375. doi: 10.1016/j.ajic.2004.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavares F.N., Costa E.V., Oliveira S.S., Nicolai C.C., Baran M., da Silva E.E. Acute hemorrhagic conjunctivitis and coxsackie virus A24v, Rio de Janeiro, Brazil, 2004. 1. Emerg Infect Dis. 2006;12(3):495–497. doi: 10.3201/eid1203.051173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teunis PFM, Heijden OG van der, Giessen JWB van der, Havelaar AH. The dose-response relation in human volunteers for gastro-intestinal pathogens. RIVM Report 284550002 1996. Freely available from www.rivm.nl.
- Teunis P.F.M., Lodder W.J., Heisterkamp S.H., de Roda Husman A.M. Mixed plaques: statistical evidence how plaque assays may underestimate virus concentrations. Water Res. 2005;39:4240–4250. doi: 10.1016/j.watres.2005.08.012. [DOI] [PubMed] [Google Scholar]
- Vaidya S.R., Tilekar B.N., Walimbe A.M., Arankalle V.A. Increased risk of hepatitis E in sewage workers from India. J Occup Environ Med. 2003;45(11):1167–1170. doi: 10.1097/01.jom.0000088874.43855.2f. [DOI] [PubMed] [Google Scholar]
- Van den Berg H., Lodder W., van der Poel W., Vennema H., de Roda Husman A.M. Genetic diversity of noroviruses in raw and treated sewage water. Res Microbiol. 2005;156:532–540. doi: 10.1016/j.resmic.2005.01.008. [DOI] [PubMed] [Google Scholar]
- Van Heerden J., Ehlers M.M., Van Zyl W.B., Grabow W.O. Incidence of adenoviruses in raw and treated water. Water Res. 2003;37:3704–3708. doi: 10.1016/S0043-1354(03)00245-8. [DOI] [PubMed] [Google Scholar]
- Van Heerden J., Ehlers M.M., Vivier J.C., Grabow W.O. Risk assessment of adenoviruses detected in treated drinking water and recreational water. J Appl Microbiol. 2005;99(4):926–933. doi: 10.1111/j.1365-2672.2005.02650.x. [DOI] [PubMed] [Google Scholar]
- Van Olphen M., Kapsenberg J.G., van de Baan E., Kroon W.A. Removal of enteric viruses from surface water at eight waterworks in the Netherlands. Appl Environ Microbiol. 1984;47:927–932. doi: 10.1128/aem.47.5.927-932.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Zyl W.B., Williams P.J., Grabow W.O., Taylor M.B. Application of a molecular method for the detection of group A rotaviruses in raw and treated water. Water Sci Technol. 2004;50(1):223–228. [PubMed] [Google Scholar]
- Villar L.M., de Paula V.S., Diniz-Mendes L., Lampe E., Gaspar A.M. Evaluation of methods used to concentrate and detect hepatitis A virus in water samples. J Virol Meth. 2006;137(2):169–176. doi: 10.1016/j.jviromet.2006.06.008. [DOI] [PubMed] [Google Scholar]
- Villena C., El-Senousy W.M., Abad F.X., Pinto R.M., Bosch A. Group A rotavirus in sewage samples from Barcelona and Cairo: emergence of unusual genotypes. Appl Environ Microbiol. 2003;69:3919–3923. doi: 10.1128/AEM.69.7.3919-3923.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vivier J.C., Ehlers M.M., Grabow W.O. Detection of enteroviruses in treated drinking water. Water Res. 2004;38:2699–2705. doi: 10.1016/S0043-1354(01)00433-X. [DOI] [PubMed] [Google Scholar]
- Wang Q.H., Souza M., Funk J.A., Zhang W., Saif L.J. Prevalence of noroviruses and sapoviruses in swine of various ages determined by reverse transcription-PCR and microwell hybridization assays. J Clin Microbiol. 2006;44(6):2057–2062. doi: 10.1128/JCM.02634-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W.K., Chen S.Y., Liu I.J., Chen Y.C., Chen H.L., Yang C.F., Chen P.J., Yeh S.H., Kao C.L., Huang L.M., Hsueh P.R., Wang J.T., Sheng W.H., Fang C.T., Hung C.C., Hsieh S.M., Su C.P., Chiang W.C., Yang J.Y., Lin J.H., Hsieh S.C., Hu H.P., Chiang Y.P., Wang J.T., Yang P.C., Chang S.C., SARS Research Group of the National Taiwan University/National Taiwan University Hospital Detection of SARS-associated coronavirus in throat wash and saliva in early diagnosis. Emerg Infect Dis. 2004;10(7):1213–1219. doi: 10.3201/eid1007.031113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward R.L., Bernstein D.I., Young E.C., Sherwood J.R., Knowlton D.R., Schiff G.M. Human rotavirus studies in volunteers: determination of infectious dose and serological response to infection. J Infect Dis. 1986;154(5):871–880. doi: 10.1093/infdis/154.5.871. [DOI] [PubMed] [Google Scholar]
- Westrell T., Andersson Y., Stenström T.A. Drinking water consumption patterns in Sweden. J Water Health. 2006;04:511–522. [PubMed] [Google Scholar]
- Wetz J.J., Lipp E.K., Griffin D.W., Lukasik J., Wait D., Sobsey M.D., Scott T.M., Rose J.B. Presence, infectivity, and stability of enteric viruses in seawater: relationship to marine water quality in the Florida Keys. Mar Pollut Bull. 2004;48(7–8):698–704. doi: 10.1016/j.marpolbul.2003.09.008. [DOI] [PubMed] [Google Scholar]
- WHO (World Health Organization). Guidelines for drinking water quality. Geneva: World Health Organization; 2004. http://www.who.int/water_sanitation_health/dwq
- WHO (World Health Organization). Guidelines for the safe use of wastewater, excreta and greywater. Geneva: World Health Organization; 2006a. ISBN, 92 4 154686 7.
- WHO (World Health Organization). Review of latest available evidence on risks to human health through potential transmission of avian influenza (H5N1) through water and sewage. 2006b. Freely available from http://www.who.int/water_sanitation_health/emerging/h5n1background.pdf.
- Witso E., Palacios G., Cinek O., Stene L.C., Grinde B., Janowitz D., Lipkin W.I., Ronningen K.S. Natural circulation of human enteroviruses: high prevalence of human enterovirus A infections. J Clin Microbiol. 2006;44(11):4095–4100. doi: 10.1128/JCM.00653-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worm H.C., van der Poel W.H., Brandstatter G. Hepatitis E: an overview. Microb Infect. 2002;4(6):657–666. doi: 10.1016/s1286-4579(02)01584-8. [DOI] [PubMed] [Google Scholar]
- Yamashita T., Sugiyama M., Tsuzuki H., Sakae K., Suzuki Y., Miyazaki Y. Application of a reverse-transcription-PCR for identification and differentiation of Aichi virus, a new member of the picornavirus family associated with gastroenteritis in humans. J Clin Microbiol. 2000;38(8):2955–2961. doi: 10.1128/jcm.38.8.2955-2961.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuang J., Jin Y. Virus retention and transport through Al-oxide coated sand columns: Effects of ionic strength and composition. J Contam Hydrol. 2003;60(3–4):193–209. doi: 10.1016/s0169-7722(02)00087-6. [DOI] [PubMed] [Google Scholar]


