Abstract
Background and Objectives
Hyaluronan preserves the proliferation and differentiation potential of mesenchymal stem cells. Supplementation of low-concentration hyaluronan (SHA) in stem cells culture medium increases its proliferative rate, whereas coated-surface hyaluronan (CHA) maintains cells in a slow-proliferating mode. We have previously demonstrated that in CHA, the metabolic proliferative state of stem cells was influenced by upregulating mitochondrial biogenesis and function. However, the effect of SHA on stem cells’ energetic status remains unknown. In this study, we demonstrate the effect that low-concentration SHA at 0.001 mg/ml (SHA0.001) and high-concentration SHA at 5 mg/ml (SHA5) exert on stem cells’ mitochondrial function compared with CHA and noncoated tissue culture surface (control).
Methods and Results
Fast-proliferating human placenta-derived mesenchymal stem cells (PDMSCs) cultured on SHA0.001 exhibited reduced mitochondrial mass, lower mitochondrial DNA copy number, and lower oxygen consumption rate compared with slow-proliferating PDMSCs cultured on CHA at 5.0 (CHA5) or 30 µg/cm2 (CHA30). The reduced mitochondrial biogenesis observed in SHA0.001 was accompanied by a 2-fold increased ATP content and lactate production, suggesting that hyaluronan-induced fast-proliferating PDMSCs may rely less on mitochondrial function as an energy source and induce a mitochondrial functional switch to glycolysis.
Conclusions
PDMSCs cultured on both CHA and SHA exhibited a reduction in reactive oxygen species levels. The results from this study clarify our understandings on the effect of hyaluronan on stem cells and provide important insights into the effect of distinct supplementation methods used during cell therapies.
Keywords: Hyaluronan, Mesenchymal stem cells, Mitochondria, Cellular proliferation
Introduction
Stem cells exhibit an ability to undergo continuous proliferation and differentiate into diverse cell types that makes them a powerful tool in clinic applications, thus underscoring the importance of studying the biological mechanisms responsible for self-renewal. Unfortunately, during long-term cultivation, stem cells may spontaneously lose their proliferation and differentiation potential. Hyaluronan is a nonsulfated glycosaminoglycan component of the extracellular matrix that consists of repeating disaccharides of glucuronic acid and N-acetylglucosamine. Its viscous physical property enables it to function in tissue lubrication and homeostasis and coregulate cellular adhesion, migration, proliferation, and differentiation. Hyaluronan is a key component of the stem cell niche, as demonstrated by its abundance in stem cell surroundings. We have previously demonstrated that hyaluronan supplementation in vitro extends the proliferation and differentiation potential of mesenchymal stem cells (1). Murine-adipose derived mesenchymal stem cells cultured in coated hyaluronan (CHA) or medium supplemented hyaluronan (SHA) exhibit enhanced osteogenic potential and a reduced senescent population compared to the control (2). Similarly, culture of PDMSCs in CHA resulted in a delay in differentiation induction with an increased percentage of cells positive for MSC and pluripotent markers CD105, CD90, OCT-3/4, NANOG, and SSEA-4 (3). When PDMSCs were pretreated for a long period in CHA and then transferred onto normal tissue culture surface, enhanced osteogenic and chondrogenic potential was observed compared with the nontreatment group (1). These results suggest that hyaluronan-coated surfaces induce PMDSCs into a resting/quiescent state that preserved their stemness properties. Other recent reports have confirmed the ability of hyaluronan to promote adipogenic and chondrogenic differentiation and induce senescence delay (4). Previous studies have demonstrated a crucial function of hyaluronan on stem cell metabolism (5, 6). Mitochondria play a crucial role in the maintenance of self-renewal and differentiation of stem cells (7), and their function is improved by hyaluronan (8). We have recently reported for the first time that hyaluronan-coated surfaces reduced stem cell proliferation and upregulated mitochondrial biogenesis, which favored efficient mitochondrial function (3). The regulatory effect on stem cell proliferation depends on hyaluronan molecular weight, the concentration used, cell surface receptor signaling, and stem cell types (9, 10). Different hyaluronan supplementation methods (coated vs. medium supplemented) may cause distinct metabolic proliferative behaviors in stem cells. Hyaluronan supplementation in minute amounts on the culture medium induced an increase in proliferation in murine adipose-derived mesenchymal stem cells (mADSC) (2). Other reports have experimentally demonstrated the acceleration of stem cell proliferation by hyaluronan (10, 11). However, whether hyaluronan supplemented in the medium exerts changes in mitochondrial function to increase the cell proliferation rate remains unknown. In this study, we aimed to examine the effects that medium supplemented hyaluronan versus coated hyaluronan exert on mitochondrial function during hyaluronan-induced changes in stem cell proliferation. Understanding the influence of a biomaterial supplementation method in stem cells is pivotal during cell therapy, as it may affect its tissue regeneration mechanism.
Materials and Methods
PDMSC isolation, colonization, and maintenance
Full-term (38∼40 weeks of gestation) human placentas were obtained from Cesarean section births after pre-screening those negative for hepatitis (Hbs and Hbe), syphilis, and HIV. Informed consent forms were signed by the donors after introduction and explanation. All procedures were approved by the Institutional Review Board and were conducted in compliance with the Declaration of Helsinki. PDMSCs were isolated from the placenta’s chorionic villi as previously published (12) and cultured in low-glucose Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum and 100 U/mL gentamycin at 37℃ and 5% CO2. After cells achieved sub-confluency, PDMSCs were seeded on a polystyrene surface for further generation culture or on hyaluronan-supplemented plates for further experiments.
Preparation of different hyaluronan culture conditions
Hyaluronan solution was prepared by dissolving hyaluronan powder (Mw=1470 kDa; LifeCore, Chaska, MN, USA) in double-distilled water and diluting it to working concentrations before use, as previously described (12). Three kinds of culture system were applied: (1) “Control”, PDMSC cultured on regular tissue culture polystyrene surface; (2) “SHA”, PDMSC supplemented with hyaluronan in culture medium; and (3) “CHA”, PDMSC cultured on hyaluronan pre-coated surfaces. For preparation of hyaluronan-coated surfaces, hyaluronan solution was applied to a polystyrene surface, and the coated substratum was dried on a hot plate at 45℃ for 30 min. Different hyaluronan concentration in SHA (0.001, 0.1, 1, and 5 mg/ml) and CHA (0.5, 3, 5, and 30 μg/cm2) were screened. PDMSCs cultured on Control and with low concentrations of SHA were seeded at 1×104 cells/cm2, whereas PDMSCs cultured on SHA at 5mg/ml and CHA were seeded at 2.5×104 cells/cm2.
In vitro differentiation induction
Adipogenic, osteogenic and chondrogenic inductions of PDMSCs were performed according to StemProⓇ Adipogenesis, Osteogenesis, and Chondrogenesis Differentiation Kit manufacturer’s instructions (Gibco). PDMSCs at passage 2 were seeded on a polystyrene surface (control) and cultured in low-glucose DMEM–10% FBS for 3 days prior to differentiation induction. For adipogenic induction, PDMSCs were cultured on StemProⓇ Adipocyte Differentiation Basal Medium supplemented with StemProⓇ adipogenesis supplement and gentamycin (10 mg/ml, Gibco) for 34 days. Adipogenesis was assessed by oil red O staining (Sigma-Aldrich, Saint Louis, MO, USA). For osteogenic and chondrogenic differentiation induction, PDMSCs were cultured in StemProⓇ osteocyte/chondrocyte differentiation basal medium with StemProⓇ osteogenesis/chondrogenesis supplement and gentamycin reagent (10 mg/mL) for 34 days. Osteogenesis was assayed by alizarin red S stain (Sigma), and chondrogenesis was assayed by 1% alcian blue stain (Sigma) prepared in 0.1 N HCL. Cells were fixed prior to staining. Images were captured with an inverted light microscope (Leica Microsystems, Wetzlar, Germany).
Stem cell marker analysis
The expression of MSC markers in cells was analyzed by flow cytometry. PDMSCs were washed with Dulbecco’s phosphate buffered saline (DPBS, Gibco) and resuspended in flow cytometry staining (FACS) buffer (Thermo Fisher Scientific, Waltham, MA, USA) with anti-human CD90 FITC-conjugated, anti-human CD73 PE-conjugated, and anti-human CD105 APC-conjugated antibodies (1:20, BioLegend, San Diego, CA, USA) for 15 min at 25℃ in the dark. Cells were then washed with FACS buffer and resuspended in fixation buffer (Thermo Fisher Scientific). The fluorescence intensity of 10,000 cells was recorded on a flow cytometer (Guava, Merck, Darmstadt, Germany) and analyzed using FlowJo software with excitation/emission wavelengths of FITC at 494/520, PE at 496/578, and APC at 650/660.
Generation time and cell growth curve
The generation time of different generations of PDMSCs was determined by calculating accumulative population doubling. Accumulative population doubling was plotted against days by using the equation ΔPD=log(Nf/N0)/log2 (3). The curve represents the cumulative population doublings with time where each dot represents one passage approximately every 4 days. To construct the cell growth curve, we used trypan blue staining and a hemocytometer to count the cells every 24 h during an 8-day culture period.
Mitochondrial localization and measurement of mitochondrial mass
For mitochondrial localization and mitochondrial mass assays, PDMSCs harboring pAS3w-DsRed2-Mito clones were used. DsRed2-mito, which contains DNA sequences encoding the red fluorescent protein DsRed2 fused at the 3’ end of the mitochondrial targeting sequence from subunit VIII of cytochrome c oxidase, was sequentially ligated into the lentiviral vector pLKO_AS3w.puro as previously published (3). The success of the construction of the pAS3w-DsRed2-Mito clone was verified by PCR positive expression at 800 bp and >99% sequence alignment. For the mitochondrial localization experiments, monolayer PDMSCs expressing DsRed2-mito were washed with phosphate-buffered saline (PBS; pH 7.4) and fixed with 4% paraformaldehyde for 5 min at room temperature. Cells were rinsed several times in PBS and stained with 2 μg/mL 4’,6-diamidino-2-phenylindole (DAPI) for 5 min. Samples were analyzed with an immunofluorescence microscope (DM IRBE; Leica Mikrosysteme, Bensheim, Germany) with the excitation wavelength at 558 nm and emission wavelength at 583 nm by the Metamorph software or with an LSM-480 confocal microscope (Carl Zeiss, Oberkochen, Germany) and Zen 2010 software. Fluorescence intensity from the labeled mitochondria was quantified with the Metamorph software, as previously published (13). Briefly, transect lines were electronically drawn from the edge of the nucleus to the edge of the cell, pixel intensity was measured along the line and normalized. Microscopic examination of cells we calculated to obtain the percentage of cells with a perinuclear or homogenous mitochondrial distribution. For mitochondrial mass, cells expressing DsRed2-mito were harvested and resuspended in PBS. The fluorescence intensity of 10,000 cells was recorded by flow cytometry (FACScan; BD Biosciences, San Jose, CA, USA) using FL-2 with excitation/emission of 558 nm/583 nm and analyzed using WinMDI 2.9 software (J. Trotter, The Scripps Research Institute, La Jolla, CA, USA).
Determination of relative mtDNA copy number
For quantification of mtDNA copy number, total DNA was extracted by the phenol/chloroform/isoamyl (Amresco, Solon, OH, USA) method and subjected to QPCR using the LightCycler DNA Master SYBR Green I kit, as previously described (3). Briefly, the relative mtDNA copy number was measured by normalizing the crossing points in QPCR curves between mt-DNA encoded gene NADH dehydrogenase subunit 1 (mt-ND1) and 18S rRNA genes (internal control) using the Light Cycler RelQuant software. Results are expressed as fold changes relative to the control.
Measurement of intracellular ATP content
The intracellular ATP level was measured by the Bioluminescent Somatic Cell Assay Kit (Sigma-Aldrich) using an OrionL Microplate Luminometer (Berthold, Bad Wildbad, Germany), as previously described (3). Luminescence intensity was divided by total cell number.
Measurement of lactate production
Lactate production rate was measured using a Lactate Reagent kit (Fortress Diagnostics, Antrim, County Antrim, UK), as previously described (3). Absorbance results were measured using a SpectraMax M2e microplate reader (Molecular Devices, Sunnyvale, CA, USA) at 550 nm. Absorbance was divided by total cell number and time of incubation.
Measurement of intracellular reactive oxygen species
For measurement of H2O2 and O2 levels, cells were incubated in low-glucose DMEM containing 40 μM carboxy-H2DCFDA and 5 μg/ml hydroethidine (Invitrogen, Grand Island, NY, USA), respectively at 37℃ for 30 min. After staining, cells were re-suspended in PBS, and the fluorescence intensity of 10,000 cells was recorded on a flow cytometer with excitation wavelength at 488 and emission wavelengths at 535 and 580 nm for measurement of H2O2 and O2, respectively.
SILAC isotope labeling of PDMSC and LC-MS/MS-based quantitative proteomics
For proteomics studies, a stable isotope labeling by amino acid in cell culture (SILAC) approach was performed based on the incorporation of a ‘heavy’ (PDMSC cultured on polystyrene surface) or ‘light’ (PDMSC culture on CHA or SHA) form of the amino acid label into proteins for mass spectrometry (MS)-based quantitative proteomics following the established methods reported in our previous publication (14). The peptide samples obtained were subjected to protein identification and quantification by nanoscale liquid chromatography coupled to electrospray ionization mass spectrometry (nano-LC MS/MS, LTQ-Orbitrap, Thermo Fisher Scientific Incorporation, MA, USA). MaxQuant (1.3.0.5 version, Max Planck Institute of Biochemistry) and Perseus (1.3.0.4, Max Planck Institute of Biochemistry) programs were utilized to process proteomics data. Perseus was used to calculate the significance based on p-values, and p-values less than 0.05 were identified as significant.
Statistical analysis
Experimental results are expressed as means±SD of the samples performed at least in triplicate (n=3). Statistical analysis was performed using the paired t test by the Microsoft Office Excel 2010 statistical package. Results from at least n≥3 with p<0.05 were considered significant between the PDMSCs cultured in SHA0.001 and those in CHA or control. A difference is considered statistically significant when p value<.05.
Results
PDMSC increases the proliferation rate in low-concentration medium supplemented hyaluronan but reduces its proliferation in high-concentrated hyaluronan and hyaluronan-coated surfaces
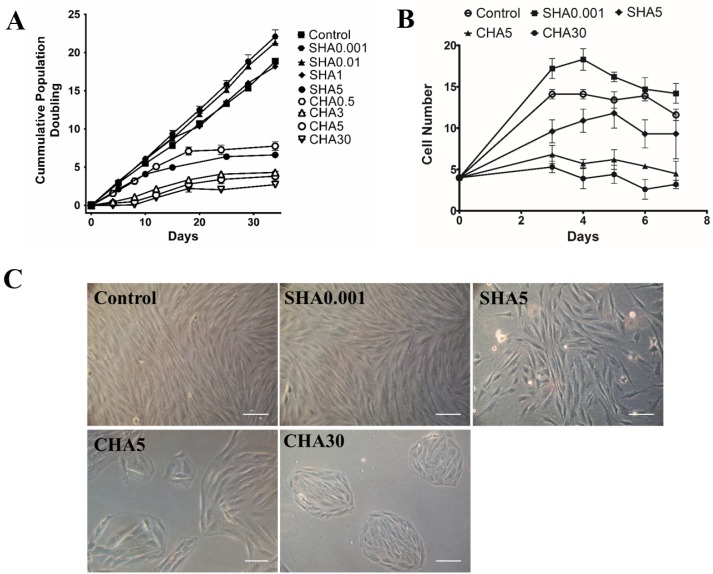
PDMSCs present the MSC characteristics of plastic adherence and fibroblastic morphology (Fig. 1A); adipogenic, chondrogenic, and osteogenic differentiation potential (Fig. 1B∼D); and positive expression of CD105, CD90, and CD73 markers (Fig. 1E). To confirm the effect of distinct supplementation methods of hyaluronan on the proliferation rate of PDMSCs, we passaged cells every 4 days for construction of a 34-day cumulative population doubling curve and investigated the effects of hyaluronan supplemented in the culture medium (medium supplemented hyaluronan, SHA) or supplemented as coated surfaces (coated hyaluronan, CHA). Medium supplemented hyaluronan at 0.001 and 0.01 mg/ml increased the proliferation rate of PDMSCs, with a 20% increase in cumulative population doubling of PDMSCs cultured in SHA0.001 compared with the control. As the concentration of hyaluronan increased in SHA groups, a gradual decrease in cell proliferation occurred in a hyaluronan concentration-dependent manner. Specifically, 1 mg/ml SHA reduced the proliferation of the control, reaching a 70% reduction in SHA5 (Fig. 2A). Supplementation of hyaluronan at increasing concentrations in CHA led to a concentration-dependent reduction in the proliferation rate of PDMSCs. Here, CHA3 exhibited greater reduction than SHA5, with a 90% decrease in CHA30 compared with the control (Fig. 2A). The cell growth curve results showed that effects of hyaluronan on PDMSC proliferation occurred immediately after hyaluronan treatment (Fig. 2B). PDMSCs cultured on control surfaces and SHA0.001 exhibited typical fibroblast-like morphology; however, at higher concentrations in SHA5, PDMSCs formed cell colonies (Fig. 2C) similar to CHA groups. Together, these results indicate that the different hyaluronan supplementation method exerts varying effects on cell proliferation. Low concentrations of SHA increase cell proliferation, whereas higher concentrations of SHA and CHA decrease cellular proliferation and promote cell colony formation.
Fig. 1.
PDMSCs possess plastic adherence characteristics, differentiate into MSC specific lineages, and express MSC CD Markers. PDMSCs cultured on control surface contained (A) fibroblastic morphology and plastic adherence characteristic; were assayed for differentiation ability by 32-day culture in (B) adipogenesis, (C) chondrogenesis, and (D) osteogenesis differentiation medium; and (E) positively express the MSC CD markers CD73, CD90, and CD105. Images obtained after oil red, alcian blue, and alizarin red, respectively. PE: Phycoerythrin, FITC: Fluorescein Isothiocyanate, APC: Allophycocyanin. Scale bar: 100 μm.
Fig. 2.
Different hyaluronan substratum exerted changes in PDMSCs cellular proliferation and morphology. (A) Cumulative population doubling of PDMSCs cultured for 34 days on control, medium supplemented hyaluronan (SHA 0.001, 0.01, 1, and 5 mg/ml), or coated hyaluronan (CHA 0.5, 3, 5, 30 μg/cm2). The drawing represents the cumulative population doublings with time where each dot represents one passage approximately every 4 days. Cumulative population doubling is expressed as mean±SD of at least triplicates; (B) cell growth curve and (C) morphological changes of PDMSCs cultured for 5 days in control, SHA 0.001 and 5 mg/ml; or CHA 5 and 30 μg/cm2. Scale bar: 100 μm. SHA: medium supplemented hyaluronan, CHA: coated hyaluronan. Three independent experiments were performed in control and experimental groups and data are represented in mean±SD.
Different hyaluronan supplementation methods altered mitochondrial mass and mitochondrial DNA copy number of PDMSCs
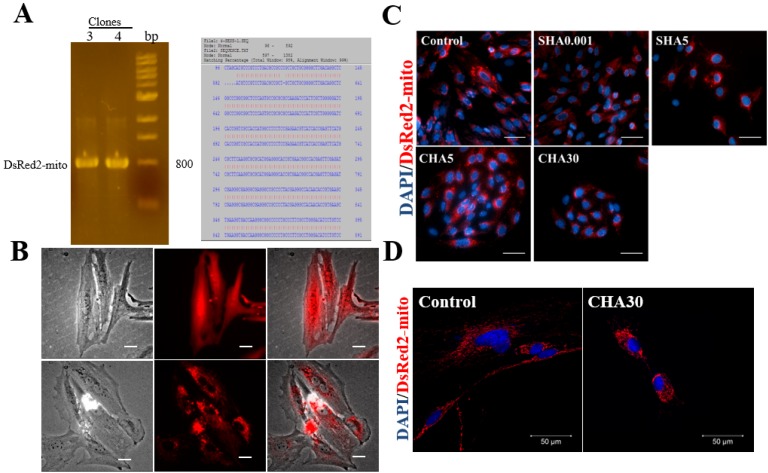
To measure the changes in mitochondrial localization of PDMSCs cultured in different hyaluronan supplementation methods, we constructed a lentiviral vector encoding mitochondrial-targeting DsRed2 (DsRed2-mito). The fidelity of our constructed vector was demonstrated by the positive expression of the clone at 800 bp and the 99% sequence similarity at the restriction enzyme cutting sites of NheI and PmeI (Fig. 3A). Successful mitochondrial localization in PDMSCs harboring DsRed2-mito was shown by the punctuated pattern characteristics of mitochondria compared with the diffused fluorescence pattern observed in PDMSCs with DsRed2-only (Fig. 3B). PDMSCs cultured on SHA5 contained a mitochondrial perinuclear distribution similar to CHA30 compared with the homogenous distribution observed in the control surface (Fig. 3C, 3D). Fluorescence intensity measurements indicated a great proportion of PDMSCs with mitochondrial perinuclear localization cultured in CHA30 and SHA5 compared with the control (Table 1).
Fig. 3.
Different hyaluronan substratum exerted changes in mitochondrial distribution of PDMSCs. Successful clones for lentivector pAS3W-DsRed2-mito after ligation of the vectors pLKOAS3w.puro and pDsRed2-mito. (A) PCR gels showed successful clone band at 800 bp; and sequence alignment of 99% from restriction enzyme cutting sites of NheI at 96 bp and PmeI at 892 bp. (B) Transfection of PDMSCs with pAS3W-DsRed2-mito enabled red fluorescence labelling of mitochondria specifically. Lentivector containing DsRed-mito showed fluorescence punctuated patterns when transfected into PDMSCs, whereas lentivector with only DsRed showed a diffused fluorescence pattern; (C) mitochondrial localization of PDMSCs cultured for 5 days on control surfaces, SHA 0.001 and SHA 5 mg/ml or CHA 5 and 30 μg/cm2. (D) Confocal images of mitochondrial distribution in hyaluronan-supplemented PDMSCs. Red fluorescence represents mitochondria through PDMSCs transfected with expression plasmid pAS3W-DsRed2-mito; blue fluorescence represents nucleus through DAPI staining. Scale bar: 100 μm. SHA: medium supplemented hyaluronan, CHA: coated hyaluronan, DAPI: 4’,6-diamidino-2-phenylindole.
Table 1.
Characteristics of mitochondrial distribution in PDMSCs
| Group | % Perinuclear | % Homogenous |
|---|---|---|
| Control | 20 | 80 |
| SHA 5 | 70 | 30 |
| CHA 30 | 80 | 20 |
SHA: medium supplemented hyaluronan, CHA: coated hyaluronan.
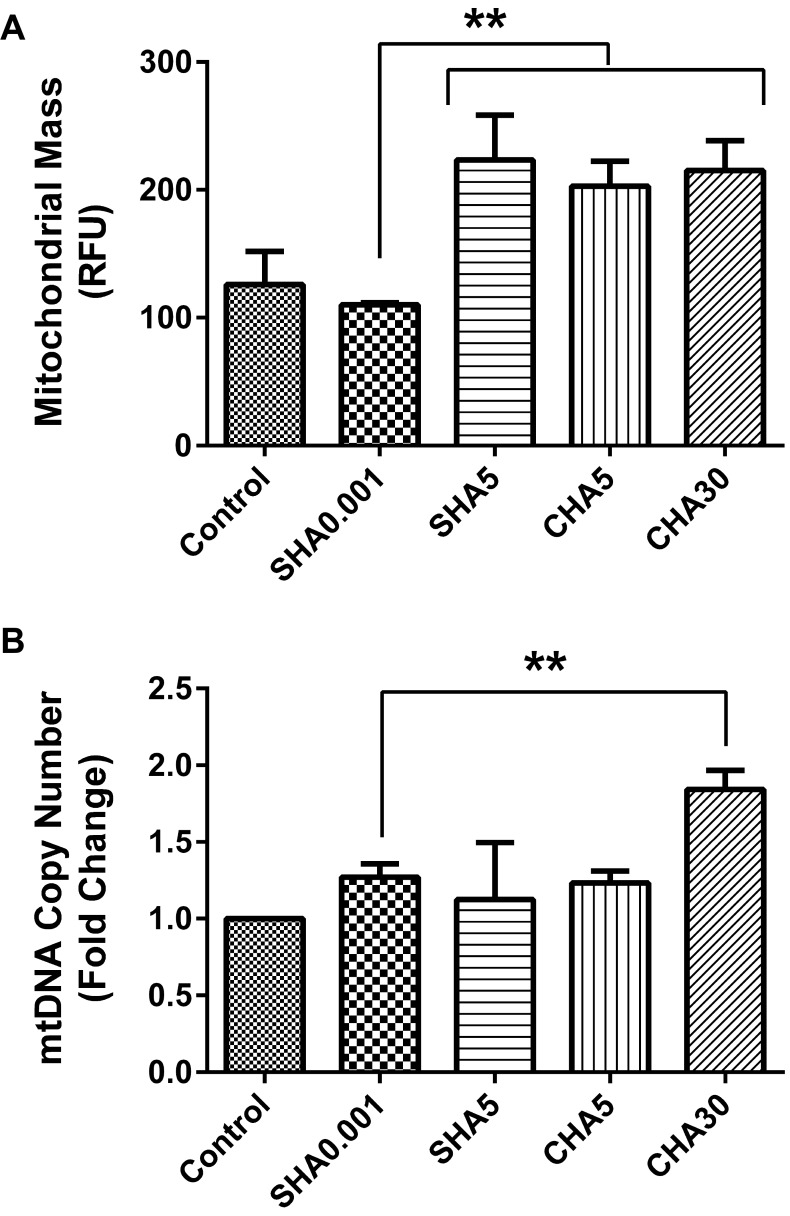
We previously demonstrated that the supplementation of hyaluronan on coated surfaces modulated mitochondrial biogenesis of PDSMCs. To elucidate whether SHA will differently alter mitochondrial biogenesis, we compared the mitochondrial mass and mtDNA copy number of medium supplemented hyaluronan versus hyaluronan-coated surfaces and control. Our data showed higher mitochondrial mass in slow-proliferating PDMSCs in SHA5, CHA5, and CHA30 compared with fast-proliferating PDMSCs at SHA0.001 and the control (Fig. 4A). mtDNA copy number increased in fast-proliferating PDMSCs in SHA0.001 compared to the control but remained lower than slow-proliferating PDMSC cultured in CHA30 (Fig. 4B). The reduced mitochondrial biogenesis observed in PDMSCs cultured in SHA0.001 suggest less mitochondrial utilization. This finding was the first indication that the effects of different hyaluronan supplementation methods (SHA or CHA) on cell proliferation triggered different levels of mitochondrial biogenesis.
Fig. 4.
Hyaluronan-induced fast-proliferative PDMSC had lower mitochondrial biogenesis. (A) Mitochondrial mass; (B) mtDNA copy number; All data is expressed as mean±SD of at least three replicates and analyzed by the paired t-test (*p<0.05, **p<0.01). Mitochondrial mass was analyzed by using PDMSCs transfected with expression plasmid pAS3w-DsRed2-mito. SHA: medium supplemented hyaluronan, CHA: coated hyaluronan, mtDNA: mitochondrial DNA, RFU: relative fluorescence unit.
Supplementation of hyaluronan in the medium and coated surfaces increases ATP content, modulates lactate production, and attenuates ROS production in PDMSCs
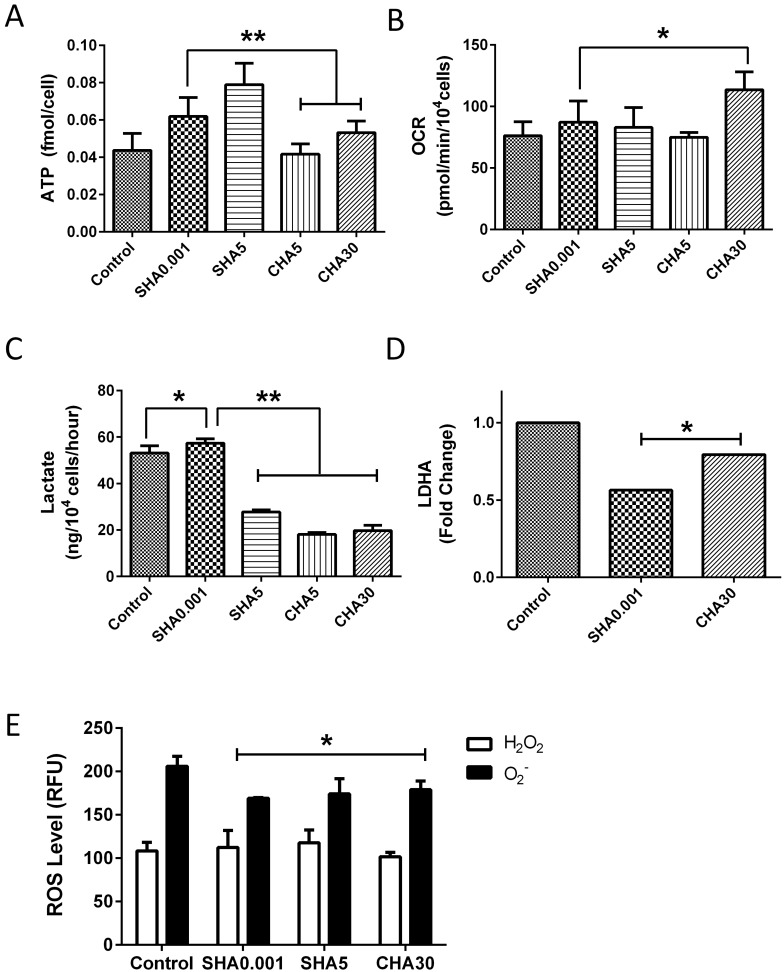
To ascertain whether changes in mitochondrial biogenesis in SHA and CHA were due to hyaluronan-induced changes in mitochondrial energy demand, we assayed oxygen consumption, ATP content, and levels of glycolysis, another nonmitochondrial energy pathway. ATP content was increased in a hyaluronan concentration-dependent manner in SHA and CHA compared with the control (Fig. 5A). PDMSCs cultured in SHA0.001 had a higher ATP content compared with CHA groups, with the highest levels observed in SHA5. However, oxygen consumption levels increased only in CHA30 compared with the control (Fig. 5B). Reactive oxygen species (ROS) are produced during mitochondrial respiration, and excess levels may be harmful to stem cells’ self-renewal and differentiation ability. To elucidate whether the observed hyaluronan-dependent increase in mitochondrial function causes an unwanted increase in ROS, which may be detrimental to stem cell fate, we assayed the levels of two types of ROS produced by the mitochondria: H2O2 and O2−. Interestingly, hyaluronan decreased the expression of both ROS radicals in PDMSCs (Fig. 5E). Furthermore, lactate levels, the end product of glycolysis, decreased in hyaluronan-induced slow-proliferating PDMSCs (Fig. 5C) but remained higher in fast-proliferating PDMSCs in SHA0.001. These results suggest glycolytic reliance for energy support in fast-proliferating PDMSCs and mitochondrial reliance in slow-proliferating PDMSCs. Our proteomics results confirmed reduced glycolysis in PDMSCs cultured on CHA as the expression of LDHA, a protein involved in lactate production, was decreased compared with the control (Fig. 5D). Taken together, our data suggest that different methods of mitochondrial-controlled proliferation and activity occur in PDMSCs cultured with SHA and CHA, possibly reflecting distinct mitochondria-supported proliferative mechanisms.
Fig. 5.
Higher ATP content and lactate production in hyaluronan-induced fast-proliferative PDMSCs. (A) ATP content; (B) OCR; (C) lactate production; (D) LDHA levels; and (E) ROS levels of PDMSCs cultured on control surface, SHA (0.001 mg/ml and 5 mg/ml), or CHA (5 and 30 μg/cm2). All data is expressed as mean±SD of at least three replicates and analyzed by paired t-test (*p<0.05, **p<0.01). SHA: medium supplemented hyaluronan, CHA: coated hyaluronan, ATP: adenosine triphosphate, OCR: oxygen consumption rate, ROS: reactive oxygen species, RFU: relative fluorescence unit, H2O2: hydrogen peroxide, O2−: superoxide anion.
Discussion
Our study demonstrated that the utilization of distinct hyaluronan culture substratum to modulate cellular proliferation induce different effects on the mitochondrial function of PDMSCs. The regulatory effect of hyaluronan on stem cell proliferation may vary depending on its molecular weight, the concentration used, supplementation method, and stem cell type. The supplementation of hyaluronan at low concentrations (0.001 mg/ml) in SHA increases PDMSC proliferation compared with control, which is consistent with our previous observations reported in mADSCs (2). Hyaluronan supplementation in the medium induces a dose-dependent increase in cell proliferation of amniotic-derived MSCs, and CD44 is highly expressed on the cell surface for regulation of cell proliferation (10). Interestingly, for the first time, we observed that increasing concentrations of hyaluronan in the culture medium (5 mg/ml) decreases the proliferation of PDMSCs in a manner similar to the decrease in proliferation observed with CHA5 and CHA30. A high percentage of primary rat calvarial osteoblasts cultured in the presence of sulfated hyaluronan derivatives resulted in reduced cell proliferation (15). Perhaps, the higher viscosity level provided by high concentrations of hyaluronan in the medium may be the main cause of the reduced proliferation observed. We previously demonstrated that the hyaluronan-induced slow-proliferating rate delayed differentiation induction and increased the percentage of cells positive for MSC and pluripotent markers CD105, CD90, OCT-3/4, NANOG, and SSEA-4. These results suggest that cells cultured on CHA acquired a slow-proliferating state that prolonged their undifferentiated state (3) with enhanced differentiation potential after transfer back into normal tissue culture surfaces (1). However, further studies are required to understand the mechanism of reduced cell proliferation utilized by hyaluronan when suspended at high concentrations in the culture medium.
PDMSCs cultured on CHA formed cell aggregates that decreased in size as hyaluronan concentration increased, and a similar phenomenon was observed in SHA5. Morphological observations of CHA forming cell aggregates that decreased in size with increasing hyaluronan concentrations were consistent with our previous observations (3). Stem cells form colonies as a means of maintaining their pluripotent characteristics. ESCs preserve colony forming characteristics for maintenance of differentiation ability, and long-term proliferative stem cells are present within these colonies (16). Hyaluronan plays an essential role in cell-cell contact, cell-substrate adhesion, and cell migration (17), suggesting a hyaluronan-related role in cell aggregation.
PDMSCs cultured at the highest hyaluronan concentrations (SHA5, CHA30) showed prominent mitochondrial perinuclear localization. Mitochondria are motile organelles (18), driven by their connections to microtubules by dynein guanosine triphosphatases (GTPases) (19). Mitochondria can localize in the plasma membrane, cytoplasm, or perinuclear areas. A perinuclear mitochondrial arrangement allows the mitochondria to have closer contact with the nucleus for more tightly controlled import and export of proteins from these two organelles for alteration of the bioenergetic status. Differential distribution of mitochondria in various cell types under different physiological conditions suggests a functional role for mitochondrial motility (19), serving as a signaling pathway within the cell (20). Perinuclear clustering of mitochondria is a useful marker of stem cell competence and multipotency (13). Different pluripotent stem cell models have been consistently observed with a prominent perinuclear localization of mitochondria (21, 22) and a wider perinuclear space than differentiated cells (23). Thus, the perinuclear mitochondrial localization induced after hyaluronan treatment suggests that hyaluronan preserves PDMSC stemness and contributes to a differential regulation of mitochondria.
Overall, our results showed that supplementation of hyaluronan in the culture medium or coated surfaces tended to differently modulate the mitochondrial mass, mtDNA copy number, ATP content, and mitochondrial oxygen consumption rate of PDMSCs. A decrease in mtDNA copy number and increase in lactate levels was observed in fast-proliferating PDMSCs (SHA0.001), whereas an increase in mtDNA copy number with decrease in lactate levels where observed in slow-proliferating PDMSCs (SHA5, CHA). Fast-proliferating MSCs exhibit an increase in glycolytic enzymes, increased lactate production and reduced mitochondrial respiration compared with their differentiated counterpart (24). Many reports have confirmed hyaluronan-induced regulation of mitochondrial function (25, 26). Furthermore, an increased reliance on glycolytic energy sources in stem cells has been suggested as a means of protection against detrimental ROS that may be produced if mitochondrial respiration is activated (27-31). Lactate production may be influenced by hyaluronan-induced change in the proliferative state of cells, thus explaining the increase in lactate production in fast-proliferative PDMSCs in SHA1 compared to SHA5. However, the expression of LDHA was not proportionally increased in SHA1. Specific metabolic pathways are differently activated depending on the type of stem cells and the lineage in which cells are differentiating into. ESCs has been shown to exhibit low MMP, higher levels of glutamine metabolism regulators and mitochondrial uncoupling protein 2 compared with differentiated cells (32). However, ESCs with longer generation time (slower proliferation) exhibit high mitochondrial activity with increased mitochondrial biogenesis and decreased overall ATP turnover (33). Consistent with the CHA-induced reduction in lactate production observed in PDMSCs, LDHA expression levels were also reduced. Although an increase in lactate production was observed in PDMSCs cultured in SHA0.001, our proteomics result revealed a nonproportional decrease in LDHA expression levels. Perhaps, other LDH isoforms could be involved in the maintenance of glycolysis. Further studies are required to understand the mechanism involved during HA-induced glycolysis during cell proliferation.
ROS levels were maintained at lower levels in SHA compared with the control, suggesting some antioxidant mechanism is provided by hyaluronan, as previously reported (3). It is possible that the physical characteristics of hyaluronan allows for the entrapment of iron ions that lead to the inhibition of the Fenton reaction that produces secondary oxidative species or that hyaluronan directly scavenges primary and secondary ROS as an antioxidant (34). Direct scavenging activity of hyaluronan against ROS/RNS through CD44 binding has been demonstrated (8), and diminished ROS levels has been reported after external supplementation of polymeric hyaluronan in fibroblasts (35). Oxidative DNA damage induced by H2O2, as reflected by induction of γH2AX, was distinctly reduced in cells that were exposed to H2O2 in the presence of hyaluronan, and the most pronounced reduction was observed with high-molecular weight hyaluronan (36, 37). The protective effect was still apparent but to a lesser degree when medium- and low-molecular weight hyaluronan were used (37). Stem cells seem to have several diverse mechanisms to protect the integrity of their DNA, such as allowing cells to maintain a low metabolic function that will diminish the possibility of oxidative stress, rapidly removing genotoxic agents from the cell through effective efflux pumps, and internalizing hyaluronan, which protects DNA from oxidants. Further studies are required to investigate the possible antioxidant properties of hyaluronan in our culture conditions. Taken together, our results clearly indicate that different hyaluronan-induced proliferative states in stem cells lead to the activation of distinct energetic status for the metabolic regulation of cell proliferation.
Hyaluronan directly controls PDMSC proliferation. The supply of hyaluronan in the medium (SHA, low concentration) of PDMSC culture plates can increase proliferation at early passages. In addition, culturing PDMSCs in high concentration SHA or on a hyaluronan-coated surface reduced the proliferation rate, resembling a dormant state. Our studies showed that hyaluronan modulates mitochondrial function and decreased ROS levels in PDMSCs, with an overall decrease in mitochondrial function, higher ATP content, and increased lactate production in hyaluronan-induced fast-proliferating PDMSCs. The results from this study increase our understanding on the influence of different hyaluronan supplementation methods on stem cells for future cell therapies.
Acknowledgments
We thank Davis Beltran for providing flow cytometry technical assistance for CD marker analysis. This work was supported by Research Grants NSC 99-3111-B-006-002; NSC 102-2325-B-006-012; and MOST-105-2622-8-006-010-TB1, from the Ministry of Science and Technology of Taiwan, and Secretaria Nacional de Ciencia, Tecnología, e Innovación research grant 09-2018-ITE17-R1-001 from Panama.
Footnotes
Potential Conflict of Interest
The authors have no conflicting financial interest.
Author Contributions
Mairim Alexandra Solis: Conception and design, collection and assembly of data, data analysis and interpretation, and manuscript writing.
Yau-Huei Wei: Contributed intellectually in the design of mitochondrial-related experiments and provided resources for pilot experiments.
Chiung-Hsin Chang, Chen-Hsiang Yu: Provided PDMSC resources.
Lynn L.H. Huang: All mitochondrial-related experiments were performed in her laboratory, financial and administrative support, provided resources and intellectual support, supervision of all experiments and study, contributed in edition, revision, and final approval of the manuscript.
References
- 1.Wong TY, Chang CH, Yu CH, Huang LLH. Hyaluronan keeps mesenchymal stem cells quiescent and maintains the differentiation potential over time. Aging Cell. 2017;16:451–460. doi: 10.1111/acel.12567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen PY, Huang LL, Hsieh HJ. Hyaluronan preserves the proliferation and differentiation potentials of long-term cultured murine adipose-derived stromal cells. Biochem Biophys Res Commun. 2007;360:1–6. doi: 10.1016/j.bbrc.2007.04.211. [DOI] [PubMed] [Google Scholar]
- 3.Solis MA, Wei YH, Chang CH, Yu CH, Kuo PL, Huang LL. Hyaluronan upregulates mitochondrial biogenesis and reduces adenoside triphosphate production for efficient mitochondrial function in slow-proliferating human mesenchymal stem cells. Stem Cells. 2016;34:2512–2524. doi: 10.1002/stem.2404. [DOI] [PubMed] [Google Scholar]
- 4.Alessio N, Stellavato A, Squillaro T, Del Gaudio S, Di Bernardo G, Peluso G, De Rosa M, Schiraldi C, Galderisi U. Hybrid complexes of high and low molecular weight hyaluronan delay in vitro replicative senescence of mesenchymal stromal cells: a pilot study for future therapeutic application. Aging (Albany NY) 2018;10:1575–1585. doi: 10.18632/aging.101493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chanmee T, Ontong P, Izumikawa T, Higashide M, Mochizuki N, Chokchaitaweesuk C, Khansai M, Nakajima K, Kakizaki I, Kongtawelert P, Taniguchi N, Itano N. Hyaluronan production regulates metabolic and cancer stem-like properties of breast cancer cells via hexosamine biosynthetic pathway-coupled HIF-1 signaling. J Biol Chem. 2016;291:24105–24120. doi: 10.1074/jbc.M116.751263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lambricht L, De Berdt P, Vanacker J, Leprince J, Diogenes A, Goldansaz H, Bouzin C, Préat V, Dupont-Gillain C, des Rieux A. The type and composition of alginate and hyaluronic-based hydrogels influence the viability of stem cells of the apical papilla. Dent Mater. 2014;30:e349–e361. doi: 10.1016/j.dental.2014.08.369. [DOI] [PubMed] [Google Scholar]
- 7.Xu X, Duan S, Yi F, Ocampo A, Liu GH, Izpisua Belmonte JC. Mitochondrial regulation in pluripotent stem cells. Cell Metab. 2013;18:325–332. doi: 10.1016/j.cmet.2013.06.005. [DOI] [PubMed] [Google Scholar]
- 8.Grishko V, Xu M, Ho R, Mates A, Watson S, Kim JT, Wilson GL, Pearsall AW 4th. Effects of hyaluronic acid on mitochondrial function and mitochondria-driven apoptosis following oxidative stress in human chondrocytes. J Biol Chem. 2009;284:9132–9139. doi: 10.1074/jbc.M804178200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cyphert JM, Trempus CS, Garantziotis S. Size matters: molecular weight specificity of hyaluronan effects in cell biology. Int J Cell Biol. 2015;2015:563818. doi: 10.1155/2015/563818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu RM, Sun RG, Zhang LT, Zhang QF, Chen DX, Zhong JJ, Xiao JH. Hyaluronic acid enhances proliferation of human amniotic mesenchymal stem cells through activation of Wnt/β-catenin signaling pathway. Exp Cell Res. 2016;345:218–229. doi: 10.1016/j.yexcr.2016.05.019. [DOI] [PubMed] [Google Scholar]
- 11.Joddar B, Kitajima T, Ito Y. The effects of covalently immobilized hyaluronic acid substrates on the adhesion, expansion, and differentiation of embryonic stem cells for in vitro tissue engineering. Biomaterials. 2011;32:8404–8415. doi: 10.1016/j.biomaterials.2011.07.083. [DOI] [PubMed] [Google Scholar]
- 12.Liu CM, Chang CH, Yu CH, Hsu CC, Huang LL. Hyaluronan substratum induces multidrug resistance in human mesenchymal stem cells via CD44 signaling. Cell Tissue Res. 2009;336:465–475. doi: 10.1007/s00441-009-0780-3. [DOI] [PubMed] [Google Scholar]
- 13.Lonergan T, Brenner C, Bavister B. Differentiation-related changes in mitochondrial properties as indicators of stem cell competence. J Cell Physiol. 2006;208:149–153. doi: 10.1002/jcp.20641. [DOI] [PubMed] [Google Scholar]
- 14.Wong TY, Chen YH, Liu SH, Solis MA, Yu CH, Chang CH, Huang LL. Differential proteomic analysis of human placenta-derived mesenchymal stem cells cultured on normal tissue culture surface and hyaluronan-coated surface. Stem Cells Int. 2016;2016:2809192. doi: 10.1155/2016/2809192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kunze R, Rösler M, Möller S, Schnabelrauch M, Riemer T, Hempel U, Dieter P. Sulfated hyaluronan derivatives reduce the proliferation rate of primary rat calvarial osteoblasts. Glycoconj J. 2010;27:151–158. doi: 10.1007/s10719-009-9270-9. [DOI] [PubMed] [Google Scholar]
- 16.Vazin T, Freed WJ. Human embryonic stem cells: derivation, culture, and differentiation: a review. Restor Neurol Neurosci. 2010;28:589–603. doi: 10.3233/RNN-2010-0543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen WY, Abatangelo G. Functions of hyaluronan in wound repair. Wound Repair Regen. 1999;7:79–89. doi: 10.1046/j.1524-475X.1999.00079.x. [DOI] [PubMed] [Google Scholar]
- 18.Forman DS, Lynch KJ, Smith RS. Organelle dynamics in lobster axons: anterograde, retrograde and stationary mitochondria. Brain Res. 1987;412:96–106. doi: 10.1016/0006-8993(87)91443-0. [DOI] [PubMed] [Google Scholar]
- 19.Frederick RL, Shaw JM. Moving mitochondria: establishing distribution of an essential organelle. Traffic. 2007;8:1668–1675. doi: 10.1111/j.1600-0854.2007.00644.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Murphy MP. Modulating mitochondrial intracellular location as a redox signal. Sci Signal. 2012;5:pe39. doi: 10.1126/scisignal.2003386. [DOI] [PubMed] [Google Scholar]
- 21.Mandal S, Lindgren AG, Srivastava AS, Clark AT, Banerjee U. Mitochondrial function controls proliferation and early differentiation potential of embryonic stem cells. Stem Cells. 2011;29:486–495. doi: 10.1002/stem.590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Prigione A, Fauler B, Lurz R, Lehrach H, Adjaye J. The senescence-related mitochondrial/oxidative stress pathway is repressed in human induced pluripotent stem cells. Stem Cells. 2010;28:721–733. doi: 10.1002/stem.404. [DOI] [PubMed] [Google Scholar]
- 23.Smith ER, Zhang XY, Capo-Chichi CD, Chen X, Xu XX. Increased expression of Syne1/nesprin-1 facilitates nuclear envelope structure changes in embryonic stem cell differentiation. Dev Dyn. 2011;240:2245–2255. doi: 10.1002/dvdy.22717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324:1029–1033. doi: 10.1126/science.1160809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lakshman M, Subramaniam V, Rubenthiran U, Jothy S. CD44 promotes resistance to apoptosis in human colon cancer cells. Exp Mol Pathol. 2004;77:18–25. doi: 10.1016/j.yexmp.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 26.Nolan MJ, Koga T, Walker L, McCarty R, Grybauskas A, Giovingo MC, Skuran K, Kuprys PV, Knepper PA. sCD44 internalization in human trabecular meshwork cells. Invest Ophthalmol Vis Sci. 2013;54:592–601. doi: 10.1167/iovs.12-10627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Folmes CD, Nelson TJ, Martinez-Fernandez A, Arrell DK, Lindor JZ, Dzeja PP, Ikeda Y, Perez-Terzic C, Terzic A. Somatic oxidative bioenergetics transitions into pluripotency-dependent glycolysis to facilitate nuclear reprogramming. Cell Metab. 2011;14:264–271. doi: 10.1016/j.cmet.2011.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guo HW, Yu JS, Hsu SH, Wei YH, Lee OK, Dong CY, Wang HW. Correlation of NADH fluorescence lifetime and oxidative phosphorylation metabolism in the osteogenic differentiation of human mesenchymal stem cell. J Biomed Opt. 2015;20:017004. doi: 10.1117/1.JBO.20.1.017004. [DOI] [PubMed] [Google Scholar]
- 29.Son MJ, Jeong BR, Kwon Y, Cho YS. Interference with the mitochondrial bioenergetics fuels reprogramming to pluripotency via facilitation of the glycolytic transition. Int J Biochem Cell Biol. 2013;45:2512–2518. doi: 10.1016/j.biocel.2013.07.023. [DOI] [PubMed] [Google Scholar]
- 30.Wu SB, Wei YH. AMPK-mediated increase of glycolysis as an adaptive response to oxidative stress in human cells: implication of the cell survival in mitochondrial diseases. Biochim Biophys Acta. 2012;1822:233–247. doi: 10.1016/j.bbadis.2011.09.014. [DOI] [PubMed] [Google Scholar]
- 31.Zhang J, Nuebel E, Daley GQ, Koehler CM, Teitell MA. Metabolic regulation in pluripotent stem cells during reprogramming and self-renewal. Cell Stem Cell. 2012;11:589–595. doi: 10.1016/j.stem.2012.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang L, Marsboom G, Glick D, Zhang Y, Toth PT, Jones N, Malik AB, Rehman J. Bioenergetic shifts during transitions between stem cell states (2013 Grover Conference series) Pulm Circ. 2014;4:387–394. doi: 10.1086/677353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Birket MJ, Orr AL, Gerencser AA, Madden DT, Vitelli C, Swistowski A, Brand MD, Zeng X. A reduction in ATP demand and mitochondrial activity with neural differentiation of human embryonic stem cells. J Cell Sci. 2011;124:348–358. doi: 10.1242/jcs.072272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Darzynkiewicz Z, Balazs EA. Genome integrity, stem cells and hyaluronan. Aging (Albany NY) 2012;4:78–88. doi: 10.18632/aging.100438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Saha P, Chowdhury AR, Dutta S, Chatterjee S, Ghosh I, Datta K. Autophagic vacuolation induced by excess ROS generation in HABP1/p32/gC1qR overexpressing fibroblasts and its reversal by polymeric hyaluronan. PLoS One. 2013;8:e78131. doi: 10.1371/journal.pone.0078131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.El-Safory NS, Lee CK. Cytotoxic and antioxidant effects of unsaturated hyaluronic acid oligomers. Carbohydr Polym. 2010;82:1116–1123. doi: 10.1016/j.carbpol.2010.06.042. [DOI] [Google Scholar]
- 37.Zhao H, Tanaka T, Mitlitski V, Heeter J, Balazs EA, Darzynkiewicz Z. Protective effect of hyaluronate on oxidative DNA damage in WI-38 and A549 cells. Int J Oncol. 2008;32:1159–1167. doi: 10.3892/ijo_32_6_1159. [DOI] [PMC free article] [PubMed] [Google Scholar]