Abstract
MicroRNAs are known to be important in a variety of cancer types. The specific expression and roles of miR-338-3p in the context of gastric cancer, however, remains largely unknown. In this study, we found that miR-338-3p was expressed significantly lower in established/primary human gastric cancer cells than that in human gastric epithelial cells; miR-338-3p is also decreased in human gastric cancer tissues and was positively associated with the worse prognosis of patients with gastric cancer. Enforced expression of miR-338-3p could inhibit cell growth, survival, and proliferation, while inducing cell apoptosis. In addition, miR-338-3p negatively regulated SOX5 expression through directly binding to the 3′-untranslated region of SOX5, and an inverse correlation was found between miR-338-3p and SOX5 messenger RNA expression in gastric cancer tissues. Furthermore, miR-338-3p-induced inactivation of Wnt/β-catenin signaling was greatly abrogated by SOX5 upregulation. Finally, we found that hypoxic conditions were linked with reduced miR-338-3p expression in the context of gastric cancer. In conclusion, miR-338-3p acts as a tumor suppressor in gastric cancer, possibly by directly targeting SOX5 and blocking Wnt/β-catenin signaling. These findings might provide novel therapeutic targets for gastric cancer.
Keywords: miR-338-3p, proliferation, SOX5, ·gastric cancer, Wnt signaling
Introduction
Gastric cancer (GC) is the most common malignant tumor of the digestive system and remains the leading cause of cancer-related deaths in developing countries.1 Despite advances in surgical techniques and combined chemotherapy strategies, the 5-year overall survival (OS) rate of most patients with GC is still below 30% due to the lack of specific biomarkers for early GC diagnosis.2 There is thus a clear need further explore the molecular mechanisms of GC development and progression so as to design better therapeutic regimens.
MicroRNAs (miRNAs), which are noncoding RNAs that are very short in length,3 directly bind to target messenger RNA (mRNA) 3′-untranslated regions (3′UTRs), thereby suppressing translation and/or driving mRNA degradation.4,5 A wide range of miRNAs are able to modulate tumor cell proliferation, migration, and invasion, as well as other biological processes.6-8 Many miRNAs have also been shown to be expressed abnormally in specific cancer types in humans, wherein they can function in either an oncogenic or tumor suppressor role, controlling target gene expression in a tumor type–specific manner.9,10 As such, miRNAs may represent attractive therapeutic targets in human cancer. Herein, we examined the potential role of miR-338-3p in human GC.
Materials and Methods
Human Tissues
We obtained 96 fresh paired human GC and adjacent normal gastric tissue samples following surgical resection, with microscopy used to guide the separation of tumor and nontumor tissues from Lishui Municipal Central Hospital. Hematoxylin and eosin staining of these tissues was utilized as a means of confirming pathology. The Ethics Committee of Lishui Municipal Central Hospital approved all studies described herein, which were consistent with the Declaration of Helsinki. All patients provided written informed consent. The approval number is 2018-LLSC-12-02.
Cell Culture
We purchased HEK293T cells; the MKN-45, MGC803, and SGC-7901 GC lines; and the GES-1 normal gastric epithelial line from the Type Culture Collection of the Chinese Academy of Sciences (Shanghai, China). Dulbecco’s modified Eagle medium (Invitrogen, Carlsbad, California) supplemented using 10% fetal bovine serum (Invitrogen) was used for all cell culture in a 5% CO2 incubator at 37°C. We additionally established 2 sets of primary GC cells and primary gastric epithelium cells from 2 male stage III GC donors (aged 55 and 56), as previously described.11
Cell Transfection
MiR-338-3p mimics, controls (miR-NC), small interfering RNAs (siRNAs) targeting SOX5 (si-SOX5), or controls (si-NC) were produced by Gene Pharma (Shanghai, China) and transfected into cells using Lipofectamine 2000 (Invitrogen) for transfection based on provided protocols.
Quantitative Real-Time Polymerase Chain Reaction
Trizol (Invitrogen) was used for extracting total RNA, which was quantified using an ND-2000 spectrophotometer (Nano Drop Technologies, Wilmington, Delaware). Complementary DNA was generated using M-MLV (Promega, Wisconsin, Madison, WI, USA), and then a SYBR Premix Ex Taq Kits (TaKaRa, Tokyo, Japan) were used on an Applied Biosystems 7900HT platform (Thermo Fisher Scientific, Waltham, MA, USA) for measuring relative gene expression. Normalization of miRNA and mRNA expression was conducted using U6 and GAPDH, respectively, and the 2−ΔΔCt method was used to compare gene expression.
Primers were as follows:
MiR-338-3p
F 5′-GTTTCTGCCGCGTTCAAC-3′,
R 5′-GAACAGCCTTGTTGCTTTCG-3′;
SOX5
F 5′-AGGAAGCGGTCGAGACGG-3′,
R 5′-CGCCTTCGAAGATGGCGTTG-3′;
GAPDH
F 5′-TGACTTCAACAGCGACACCCA-3′,
R 5′-CACCCTGTTGCTGTAGCCAAA- 3′;
U6
F 5′-GACCGAGTGTAGCAAGG-3′,
R 5′-GTTCTTCCGAGAACATATAC-3′.
Cell Viability, EdU Proliferation, Colony Formation, and TUNEL Assays
These assays were conducted as in previous studies.12
Bioinformatic Predictions and Luciferase Reporter Assays
Target Scan (http://www.targetscan.org/) was utilized as a means of predicting miR-338-3p target mRNAs. These predictions were validated using Lipofectamine 2000 to cotransfect HEK293T cells with the wild-type or mutant (Mut) constructs and either miR-338-3p mimics or miR-NC. After 48 hours, luciferase activity was assessed based on the instructions provided in the Dual-Luciferase Reporter Assay kit (Promega).
Western Blotting
Radio immunoprecipitation assay (RIPA) buffer supplemented using protease/phosphatase inhibitors (Roche, Mannheim, Germany) was used to lyse cells, after which a bovine serum albumin (BSA) assay (Key Gen Biotech, Guangzhou, China) was used to quantify protein levels in lysates. Equal protein amounts were separated via 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis followed by transfer onto polyvinylidene difluoride membranes. These blots were probed using appropriate primary antibodies and then protein was detected via enhanced chemiluminescence. All antibodies were purchased from Santa Cruz Biotechnology (California, USA).
TOP/FOP Flash Reporter Assay
Plasmids encoding TOP or FOP flash along with appropriate TCF/LEF DNA binding sites came from Upstate Biotechnology (New York). Cells were first plated into 24-well plates followed by Lipofectamine 2000-mediated transfection with these TOP Flash or FOP Flash constructs and 10 ng of the control pRL-TK Renilla luciferase vector (Promega). After 24 hours, luciferase activity was measured as above and normalized to Renilla luciferase activity.
Statistical Analysis
Data are means ± standard deviation. SPSS version 15.0 was employed for statistical testing, with P < .05 as the significance threshold.
Results
Human GC Samples Exhibit Decreased MiR-338-3p Expression
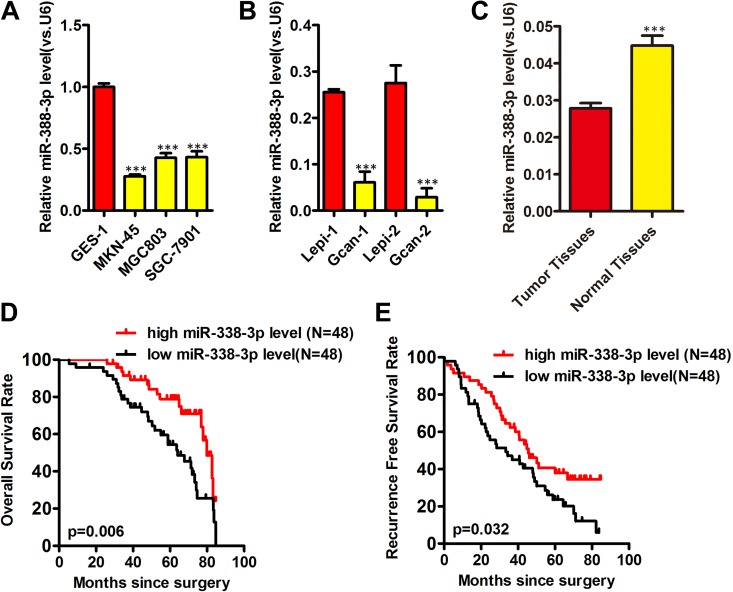
We first assessed the levels of miR-338-3p present within human GC cells via quantitative real-time polymerase chain reaction (qRT-PCR), revealing decreased levels of this miRNA in the MKN-45, MGC803, and SGC-7901 GC lines relative to control GES-1 cells, with A MKN-45 cells exhibiting the lowest expression (Figure 1A).We further found lower miR-338-3p expression in patient-derived primary gastric tumor cells as compared to primary gastric epithelial cells (Figure 1B). Consistent with this, miR-338-3p expression was reduced in GC tissues (Figure 1C) relative to surrounding normal gastric epithelial tissue (Figure 1C). This thus indicates that human GC cells exhibit decreased miR-338-3p expression.
Figure 1.
Human gastric cancer samples exhibit decreased miR-338-3p expression and its correlation with gastric cancer prognosis. Human gastric tumor (A, B) or normal epithelium (C), miR-338-3p expression was assessed via qRT-PCR, with U6 used for normalization (A-C). D, E, Kaplan-Meier curve of overall survival for and recurrence-free survival for miR-338-3p expression. *P < .05, **P < .01, ***P < .001. Gcan-1 and Gcan-2 cells are primary gastric cancer cells; Lepi-1 and Lepi-2 denote primary gastric epithelium cells.
Loss of MiR-338-3p Expression Is Correlated With Poor Prognosis of GC
To illustrate the correlation between the expression of miR-338-3p and the clinical characteristics of patients with GC, all the 96 patients with GC were divided into miR-338-3p-high or miR-338-3p-low group according to the median value of miR-338-3p expression level. The results showed that low expression of miR-338-3p was significantly correlated with the lymphatic metastasis (P = .005) and advanced tumor node metastasis (TNM) stage (P < .001; Table 1). To evaluate whether the aberrant expression of miR-338-3p was a potential biomarker for the prognosis of patients with GC, log-rank test was performed to analyze the correlation between the expression of miR-338-3p and the survival of patients with GC. The data showed that lower expression of miR-338-3p was significantly correlated with the worse prognosis of patients with GC (OS, P = .006, Figure 1D; recurrence-free survival [RFS], P = .032, Figure 1E). Univariate and multivariate analysis showed that low miR-338-3p expression correlated significantly with OS (hazard ratio [HR] = 2.217, P = 0.021) and RFS (HR = 2.501, P = 0.019; Table 2). These observations implied a tumor suppressor role of miR-338-3P in GC development.
Table 1.
Clinicopathological Characteristics and Expression of MiR-338-3p Level in Gastric Cancer.
| Variables | Cases | MiR-338-3p Level | P Value | |
|---|---|---|---|---|
| Low Expression, N = 48 | High Expression, N = 48 | |||
| Age (years) | ||||
| ≥60 | 68 | 28 | 40 | |
| <60 | 28 | 20 | 8 | |
| Gender | 1 | |||
| Female | 21 | 7 | 14 | |
| Male | 75 | 41 | 34 | |
| Tumor diameter (cm) | .194 | |||
| ≥5 | 53 | 12 | 41 | |
| <5 | 43 | 36 | 7 | |
| Differentiation | .113 | |||
| Low/middle | 70 | 28 | 42 | |
| Well | 26 | 20 | 6 | |
| Lymphatic metastasis | .005a | |||
| Yes | 65 | 24 | 41 | |
| No | 31 | 24 | 7 | |
| TNM stage | .000a | |||
| I-II | 44 | 25 | 19 | |
| III-IV | 52 | 23 | 29 | |
Abbreviation: TNM, tumor node metastasis.
a P < .05.
Table 2.
Univariate and Multivariate Cox Regression Analyses of Overall Survival and Disease-Free Survival in Patients With Gastric Cancer.
| Variables | Univariate Analyses | Multivariate Analyses | ||||
|---|---|---|---|---|---|---|
| Hazard Ratio | 95% CI | P | Hazard Ratio | 95% CI | P | |
| Overall survival | ||||||
| Age (years): ≥60/<60 | 0.635 | 0.389-2.928 | .426 | – | – | – |
| Gender: Male/female | 0.542 | 0.331-1.387 | .329 | – | – | – |
| Tumor diameter (cm): ≥5/<5 | 2.876 | 1.235-3.254 | .017 | 1.347 | 1.152-2.364 | .019 |
| Differentiation grade: Poor+ moderate/well | 3.562 | 1.206-4.159 | .022 | 2.611 | 1.145-3.152 | .025 |
| TNM stage: III/I + II | 2.184 | 1.635-3.212 | .031 | 2.826 | 1.256-2.807 | .045 |
| Lymph node metastasis: Yes/no | 3.375 | 2.317-4.059 | .032 | 3.104 | 1.879-3.624 | .043 |
| MiR-338-3p level: Low/high | 2.392 | 1.761-3.569 | .007 | 2.217 | 1.695-2.798 | .021 |
| Recurrence-free survival | ||||||
| Age (years): ≥ 60/<60 | 0.667 | 0.325-3.928 | 0.388 | – | – | – |
| Gender: Male/female | 0.947 | 0.439-1.307 | .429 | – | – | – |
| Tumor diameter (cm): ≥5/<5 | 2.643 | 1.985-3.568 | .032 | 2.162 | 1.297-3.714 | .042 |
| Differentiation grade: Poor + moderate/well | 3.246 | 1.761-3.527 | .032 | 2.754 | 1.451-3.245 | .041 |
| TNM stage: III/I + II | 3.134 | 1.756-3.975 | .022 | 2.788 | 1.984-3.884 | .035 |
| Lymph node metastasis: Yes/no | 3.563 | 1.716-4.112 | .031 | 2.109 | 1.325-3.754 | .043 |
| MiR-338-3p level: Low/high | 2.574 | 1.172-4.319 | .011 | 2.501 | 1.265-3.801 | .019 |
Abbreviations: HR, hazard ratio; 95% CI, 95% confidence interval.
Overexpression of MiR-338-3p Impairs the Growth of GC Cells
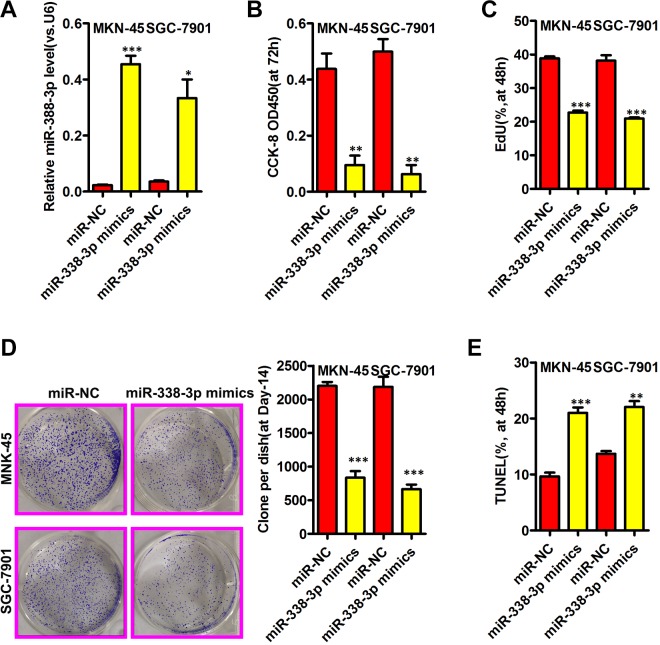
We next explored how overexpressing miR-338-3p influenced GC cell proliferation via transfecting MKN-45 and SGC-7901 cells with miR-338-3p mimics or control constructs. At 48 hours posttransfection, cells transfected with the mimic exhibited significantly elevated miR-338-3p expression relative to miR-NC-transfected cells (Figure 2A). A Cell Counting Kit-8 assay revealed miR-338-3p overexpression to be associated with a clear suppression of MKN-45 and SGC-7901 cell proliferation (Figure 2B), and EdU incorporation was similarly disrupted by this miR-338-3p overexpression (Figure 2C), as was colony formation (Figure 2D). TUNEL staining revealed a significant increase in the rates of apoptosis in MKN-45 and SGC-7901 cells with elevated miR-338-3p expression (Figure 2E).
Figure 2.
MiR-338-3p inhibits gastric cancer growth. A, The miR-338-3p expression in indicated gastric cancer was assessed via qRT-PCR. B, C, The cell viability and proliferation were assessed by CCK8 and EdU assays, respectively. D, Representative images of colony formation were shown on the left and quantification of colony numbers shown on the right. E, The cell apoptosis was measured by TUNEL assays. *P < .05, **P < .01, ***P < .001. CCK8 indicates Cell Counting Kit-8; qRT-PCR, quantitative real-time polymerase chain reaction.
MiR-338-3p Targets the SOX5 mRNA
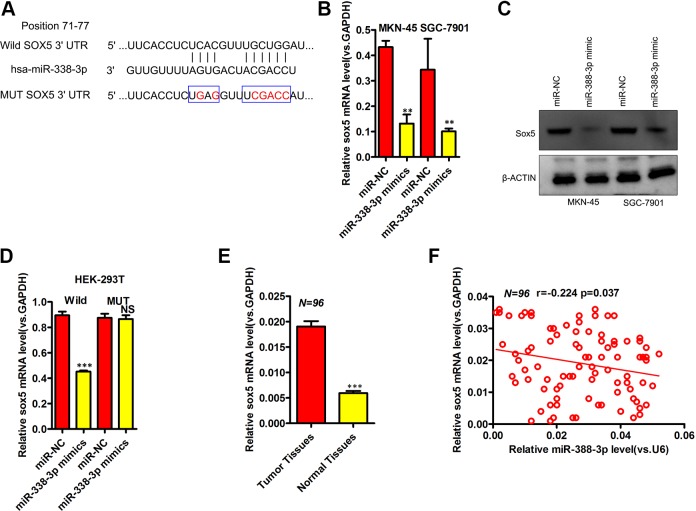
We next assessed the molecular mechanisms whereby miR-338-3p influences GC, using bioinformatics tools in order to predict target genes corresponding to this miRNA. Through this analysis, we were able to identify SOX5 as a putative miR-338-3p target (Figure 3A). Through qRT-PCR and Western blotting, we were able to confirm decreased expression of SOX5 in MKN-45 and SGC-7901 cells transfected with miR-338-3p mimics, consistent with this prediction (Figure 3B and C). To determine whether miR-338-3p was able to directly bind the SOX5 3′UTR, we further conducted luciferase reporter assays that confirmed miR-338-3p to be able to decrease luciferase activity from the pmirGLO-SOX5-3′UTR WT but not from the pmirGLO-SOX5-3′UTR Mut vectors (Figure 3D). We further used qRT-PCR to assess SOX5 expression in 96 paired GC and normal gastric epithelial tissue samples, revealing a significant increase in SOX5 levels in the tumor samples relative to matched normal controls (Figure 3E). In addition, SOX5 and miR-338-3p expression were significantly negatively correlated with one another in GC tissues (Figure 3F, Spearman r = −0.224). Together, these findings therefore indicated that SOX5 is a direct miR-338-3p target.
Figure 3.
MiR-338-3p directly targets SOX5 in gastric cancer. A, The alignment of the miR-338-3p sequence with that of predicted SOX5-binding sites. B, SOX5 expression was assessed via qRT-PCR in MKN-45 and SGC-7901 cells following miR-338-3p or miR-NC transfection. C, SOX5 levels were assessed via Western blotting in MKN-45 and SGC-7901 cells following miR-338-3p or miR-NC transfection. D, Dual-luciferase reporter assays were used to reveal a reduction in luminescence upon miR-338-3p mimic cotransfection with the SOX5-WT vector, without a comparable effect for the SOX5-Mut vector in HEK293T cells. E, Measurement of SOX5 expression via qRT-PCR in 96 paired gastric cancer and normal gastric epithelial tissue samples. F, Pearson correlation analysis of miR-338-3p and SOX5 expression in gastric cancer samples. *P < .05, **P < .01, ***P < .001. Mut indicates mutant; qRT-PCR, quantitative real-time polymerase chain reaction; WT, wild-type.
MiR-338-3p Targeting of SOX5 Inhibits GC Cell Wnt/β-Catenin Signaling
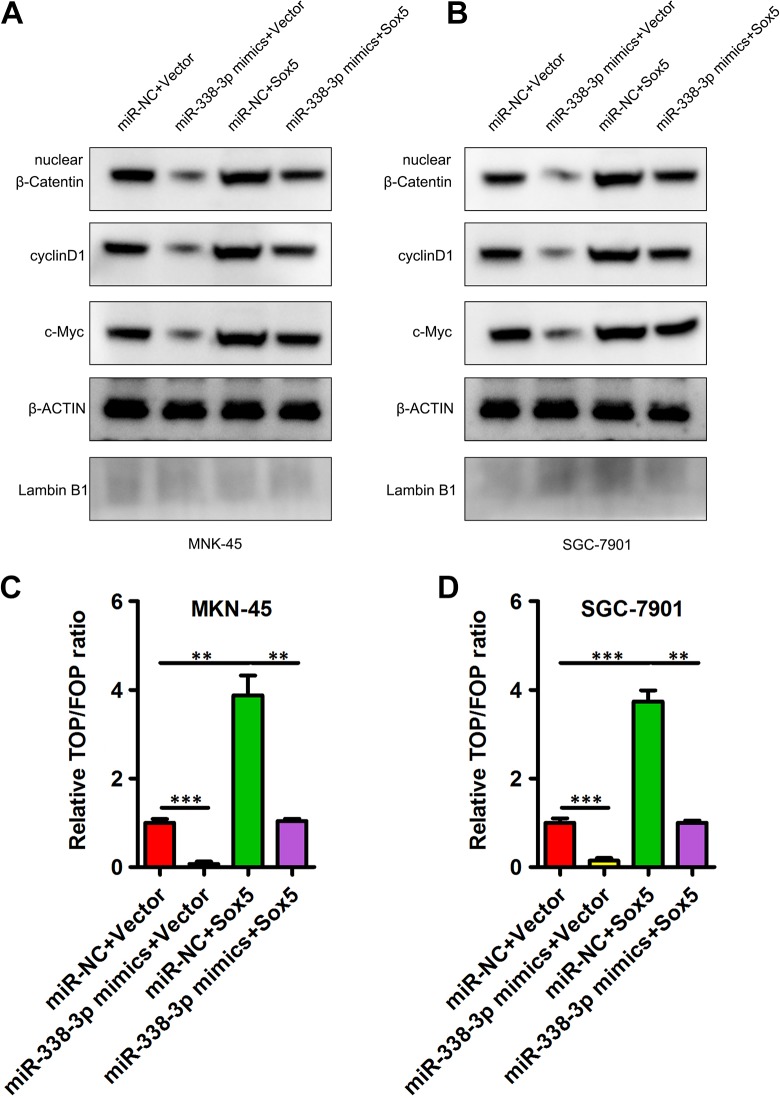
Wnt/β-catenin signaling serves as a key mediator of GC progression, with this signaling activity being closely linked to poorer patient prognosis and a higher grade of tumor malignancy.13 Previous work suggests that SOX5 is able to mediate gastric tumor progression through promotion of Wnt/β-catenin signaling.14 As such, we next explored the potential ability of miR-338-3p to modulate Wnt/β-catenin signaling through its influence on the expression of SOX5. We found that when overexpressed, miR-338-3p was able to markedly suppress β-catenin nuclear localization, and this coincided with decreased cyclin D1 and c-myc levels, whereas when SOX5 expression was increased, these effects were reversed (Figure 4A and B). In contrast, when SOX5 expression was upregulated; nuclear β-catenin, c-myc, and cyclin D1 levels rose; and miR-338-3p cotransfection reversed this upregulation to a significant degree (Figure 4A and B). Using a TOP/FOP luciferase activity assay, we were further able to provide clear evidence that miR-338-3p overexpression suppressed Wnt/β-catenin signaling, and elevated SOX5 expression reversed this, with the converse also being true with miR-338-3p suppressing to observed enhancement of Wnt/β-catenin signaling in cells upregulating SOX5 (Figure 4C and D). This thus demonstrated that miR-338-3p is able to suppress Wnt/β-catenin signaling via inhibiting SOX5. Together, these data thus allow us to conclude that miR-338-3p can inhibit the progression of GC via targeting SOX5 and thus interfering with Wnt/β-catenin signaling.
Figure 4.
SOX5 upregulation overcomes miR-338-3p-mediated disruptions to Wnt/β-catenin signaling. A, B, How miR-338-3p or SOX5 influenced nuclear β-catenin, c-myc, and cyclin D1 levels were assessed via Western blotting. C, D, The TOP/FOP luciferase assay was utilized as a means of assessing how miR-338-3p and SOX5 influenced Wnt/β-catenin signaling. *P < .05, **P < .01, ***P < .001.
Hypoxic Conditions Drive MiR-338-3p Decreased in GC
Through regulation of hypoxia-inducible factor 1α (HIF-1α), hypoxia can modulate gene expression,15 and as such we sought to determine whether miR-338-3p expression was influenced by hypoxia in the context of GC. When MKN-45 and SGC-7901 cells were grown in a hypoxic 1% oxygen environment, they exhibited both elevated HIF-1α expression and a gradual decline in expression of miR-338-3p (Figure 5A; P < .001 for 24, 48, and 72 hours). Hypoxia can regulate gene expression in a HIF-1α-dependent and HIF-1α-independent manner.15 To assess whether hypoxia-induced downregulation of miR-338-3p was HIF-1α dependent, we used siRNA to knock down HIF-1α in MKN-45 and SGC-7901 cells, which prevented this hypoxia-induced loss of miR-338-3p expression (Figure 5B, P < .05). We further assessed whether knocking down HIF-2α had a similar impact, but found that doing so did not alter downregulation of miR-338-3p in response to hypoxic conditions (Figure 5C). This suggests that hypoxia can reduce miR-338-3p expression in an HIF-1α-dependent manner.
Figure 5.
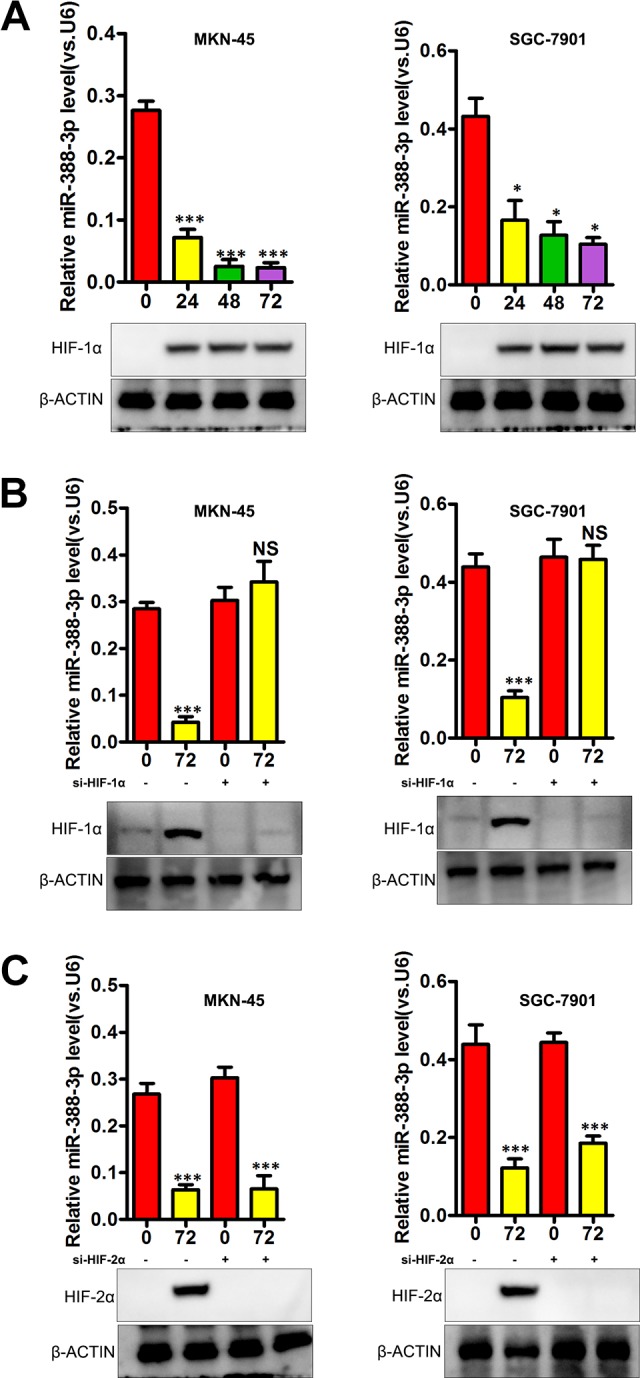
Hypoxia drives reduced miR-338-3p expression in gastric cancer. A, Levels of miR-338-3p expression under normoxic and hypoxic conditions. B, HIF-1α was knocked down using a specific siRNA in MKN-45 and SGC-7901 cells, reducing hypoxia-induced miR-338-3p downregulation at 48 hours. C, siRNA-mediated HIF-2α knockdown failed to alter hypoxia-induced miR-338-3p downregulation at 48 hours. *P < .05, **P < .01, ***P < .001. HIF-1α indicates hypoxia-inducible factor 1α; siRNA, small interfering RNA.
Discussion
Gastric cancer is a deadly disease, with China exhibiting the highest mortality rate for this form of cancer.16-18 The specific mechanisms governing GC oncogenesis, however, remain unclear, making it of great importance that novel targets be identified for the therapeutic treatment of individuals with this form of cancer. Many researchers have recently made progress in the use of miRNA-based cancer therapeutic strategies in order to specifically target genes known to drive tumor proliferation, survival, or metastasis, thereby allowing for the control of tumor effector functionality and growth.6-8 Herein, we identified miR-338-3p as a miRNA with markedly decreased expression in GC cells and tissues. We observed that low miR-338-3p levels correlated with several malignant pathological characteristics, including the lymphatic metastasis and advanced TNM stage, suggesting that miR-338-3p may be associated with GC progression. Survival analysis showed that miR-338-3p deficiency in tumor tissues was associated with a shorter survival and higher risk of recurrence in patients with GC. MiR-338-3p expression in tumor tissues was found to be an independent prognostic factor of survival and recurrence. These data revealed a previously unknown role of miR-338-3p in GC progression.
MicroRNAs are known to control gene expression via target mRNA 3′UTR binding. To assess how miR-338-3p affects GC, we therefore sought to identify its target genes in GC cell lines. Through a bioinformatics prediction, we identified SOX5 as a target of this miRNA, and we chose to validate this finding based on previous reports, indicating that SOX5 is known to be a key regulator of GC development and progression.19 Multiple miRNAs have been shown to target SOX5, such as miR-497-5p,20 miR-139-5p,21 and miR-146a-5p.22 We were able to use qRT-PCR, Western blotting, and luciferase reporter assays to confirm that miR-338-3p directly bound to and regulated SOX5 mRNA expression. As a member of the Sox family of transcription factors, SOX5 plays a major role in mediating embryonic development and the determination of cell fate.23 Aberrant expression of SOX5 was observed in various cancer types.24,25 We detected an elevation of SOX5 mRNA in tumor tissues from patients with GC compared with normal tissues. We further determined that SOX5 and miR-338-3p expression were negatively correlated with one another in gastric tumor tissues. Downregulation of SOX5 produced a similar phenotype to that yielded by overexpressing miR-338-3p with respect to GC cell proliferation. Together, our results thus provide clear evidence that miR-338-3p may influence GC progression at least in part via suppressing SOX5 expression. As such, this suggests the possibility that targeted therapies modulating the miR-338-3p/SOX5 pathway could offer novel opportunities to improve the survival of patient with GC.
Hypoxia is a common feature of all solid tumors and plays an essential role in tumor occurrence and development.26 The hypoxia signaling pathway is known to regulate multiple steps of tumorigenesis and keys oncogenes, such as FAM13A played a key role in lung cancer under hypoxia.27-29 Gastric cancer was also reported to display hypoxia, which could be a potential therapeutic target.30 Previous studies indicated that the cross-talk between Wnt/β-catenin and hypoxia signaling pathway, but the mechanism has not well documented due to the complexity of the network between Wnt/β-catenin and hypoxia signaling pathway. As mentioned above, SOX5 serves important roles in various cancer types24,25; however, the association of SOX5 with hypoxia in GC remains largely unclear. In this study, we for the first time provided the evidence of cross-talk between canonical Wnt/β-catenin and hypoxia signaling in that hypoxia led to suppress miR-338-3p expression in GC and enhanced SOX5 expression in a HIF-1α-dependent manner, suggesting that the hypoxia pathway might promote oncogenic Wnt/β-catenin signaling by recruiting HIF-α/miR-338-3p/SOX5 axis. In the current study, we found that miR-338-3p was suppressed by HIF-1α; however, the exactly mechanism that HIF-1α decrease miR-338-3p remains unclearly needed to be explored in the further. In general, these findings may enlarge our views on the interaction of Wnt/β-catenin and hypoxia signaling pathways in GC.
In summary, we found that miR-338-3p expression is reduced in the context of GC in human tissue samples and cells. Overexpression of miR-338-3p in MKN-45 and SGC-7901 cells markedly disrupted their ability to proliferate, likely at least in part via targeting SOX5. Our results thus highlight potential novel therapeutic strategies for treating GC. Our future research efforts will focus on further exploring differential expression of miR-338-3p and SOX5 in a variety of different GC subtypes, in addition to exploring their clinical and diagnostic significance to further understand the optimal clinical utility for these markers of GC.
Abbreviations
- GC
gastric cancer
- HIF-1α
hypoxia-inducible factor 1α
- HR
hazard ratio
- mRNA
messenger RNA
- miRNAs
microRNAs
- Mut
mutant
- OS
overall survival
- qRT-PCR
quantitative real-time polymerase chain reaction
- RFS
recurrence-free survival
- siRNAs
small interfering RNAs
- TNM
tumor node metastasis
- 3′UTRs
3′-untranslated regions
- WT
wild-type
Footnotes
Authors’ Note: Jing-Jing Zheng and Qiao-Yan Que contributed equally in this work. The Ethics Committee of Lishui Municipal Central Hospital approved all studies described herein, which were consistent with the Declaration of Helsinki. The approval number is 2018-LLSC-12-02. All patients provided written informed consent.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was funded by Zhejiang Administration of Traditional Chinese Medicine (2017ZB100).
ORCID iD: Jun-sheng Ni  https://orcid.org/0000-0002-3465-9116
https://orcid.org/0000-0002-3465-9116
References
- 1. Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65(2):87–108. [DOI] [PubMed] [Google Scholar]
- 2. Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136(5):E359–E386. [DOI] [PubMed] [Google Scholar]
- 3. Kim VN. MicroRNA biogenesis: coordinated cropping and dicing. Nat Rev Mol Cell Biol. 2005;6(5):376–385. [DOI] [PubMed] [Google Scholar]
- 4. Calin GA, Croce CM. MicroRNA signatures in human cancers. Nat Rev Cancer. 2006;6(11):857–866. [DOI] [PubMed] [Google Scholar]
- 5. Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell. 2009;136(2):215–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Aigner A. MicroRNAs (miRNAs) in cancer invasion and metastasis: therapeutic approaches based on metastasis-related miRNAs. J Mol Med (Berl). 2011;89(5):445–457. [DOI] [PubMed] [Google Scholar]
- 7. Rottiers V, Näär AM. MicroRNAs in metabolism and metabolic disorders. Nat Rev Mol Cell Biol. 2012;13(4):239–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cho WC. MicroRNAs: potential biomarkers for cancer diagnosis, prognosis and targets for therapy. Int J Biochem Cell Biol. 2010;42(8):1273–1281. [DOI] [PubMed] [Google Scholar]
- 9. Xia H, Li Y, Lv X. MicroRNA-107 inhibits tumor growth and metastasis by targeting the BDNF-mediated PI3K/AKT pathway in human non-small lung cancer. Int J Oncol. 2016;49(4):1325–1333. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 10. Lu C, Xie Z, Peng Q. MiRNA-107 enhances chemosensitivity to paclitaxel by targeting antiapoptotic factor Bcl-w in non small cell lung cancer. Am J Cancer Res. 2017;7(9):1863–1873. [PMC free article] [PubMed] [Google Scholar]
- 11. Kuwata T, Yanagihara K, Iino Y, et al. Establishment of novel gastric cancer patient-derived xenografts and cell lines: pathological comparison between primary tumor, patient-derived, and cell-line derived xenografts. Cells. 2019;8(6):pii585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zhang B, Lu HY, Xia YH, Jiang AG, Lv YX. Long non-coding RNA EPIC1 promotes human lung cancer cell growth. Biochem Biophys Res Commun. 2018;503(3):1342–1348. [DOI] [PubMed] [Google Scholar]
- 13. Denysenko T, Annovazzi L, Cassoni P, Melcarne A, Mellai M, Schiffer D. WNT/β-catenin signaling pathway and downstream modulators in low- and high-grade glioma. Cancer Genomics Proteomics. 2016;13(1):31–45. [PubMed] [Google Scholar]
- 14. Martinez-Morales PL, Quiroga AC, Barbas JA, Morales AV. SOX5 controls cell cycle progression in neural progenitors by interfering with the WNT-beta-catenin pathway. EMBO Rep. 2010;11(6):466–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Choudhry H, Harris AL, McIntyre A. The tumour hypoxia induced non-coding transcriptome. Mol Aspects Med. 2016;47-48:35–53. [DOI] [PubMed] [Google Scholar]
- 16. Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64(1):9–29. [DOI] [PubMed] [Google Scholar]
- 17. Siegel RL, Fedewa SA, Miller KD, et al. Cancer statistics for Hispanics/Latinos, 2015. CA Cancer J Clin. 2015;65(6):457–480. [DOI] [PubMed] [Google Scholar]
- 18. Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66(2):115–132. [DOI] [PubMed] [Google Scholar]
- 19. Wang X, Ju Y, Zhou MI, Liu X, Zhou C. Upregulation of SOX9 promotes cell proliferation, migration and invasion in lung adenocarcinoma. Oncol Lett. 2015;10(2):990–994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Li G, Wang K, Wang J, Qin S, Sun X, Ren H. . MiR-497-5p inhibits tumor cell growth and invasion by targeting SOX5 in non-small-cell lung cancer. J Cell Biochem. 2019;120(6):10587–10595. [DOI] [PubMed] [Google Scholar]
- 21. Yang B, Zhang W, Sun D, et al. Downregulation of miR-139-5p promotes prostate cancer progression through regulation of SOX5. Biomed Pharmacother. 2019;109:2128–2135. [DOI] [PubMed] [Google Scholar]
- 22. Si C, Yu Q, Yao Y. Effect of miR-146a-5p on proliferation and metastasis of triple-negative breast cancer via regulation of SOX5. Exp Ther Med. 2018;15(5):4515–4521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hersh CP, Silverman EK, Gascon J, et al. SOX5 is a candidate gene for chronic obstructive pulmonary disease susceptibility and is necessary for lung development. Am J Respir Crit Care Med. 2011;183(11):1482–1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Storlazzi CT, Albano F, Lo Cunsolo C, et al. Upregulation of the SOX5 by promoter swapping with the P2RY8 gene in primary splenic follicular lymphoma. Leukemia. 2007;21(10):2221–2225. [DOI] [PubMed] [Google Scholar]
- 25. Chen X, Fu Y, Xu H, et al. SOX5 predicts poor prognosis in lung adenocarcinoma and promotes tumor metastasis through epithelial–mesenchymal transition. Oncotarget. 2018;9(13):10891–10904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Xu W, Zhou W, Cheng M, et al. Hypoxia activates Wnt/beta-catenin signaling by regulating the expression of BCL9 in human hepatocellular carcinoma. Sci Rep. 2017;7:40446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ziółkowska-Suchanek I, Mosor M, Podralska M, et al. FAM13A as a novel hypoxia-induced gene in non-small cell lung cancer. J Cancer. 2017;8(19):3933–3938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Eisenhut F, Heim L, Trump S, et al. FAM13A is associated with non-small cell lung cancer (NSCLC) progression and controls tumor cell proliferation and survival. Oncoimmunology. 2016;6(1):e1256526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Yu Y, Mao L, Lu X, et al. Functional variant in 3′UTR of FAM13A is potentially associated with susceptibility and survival of lung squamous carcinoma. DNA Cell Biol. 2019;38(11):1269–1277. doi:10.1089/dna.2019.4892. [DOI] [PubMed] [Google Scholar]
- 30. Bishop T, Ratcliffe PJ. Signaling hypoxia by hypoxia-inducible factor protein hydroxylases: a historical overview and future perspectives. Hypoxia (Auckl). 2014;2:197–213. [DOI] [PMC free article] [PubMed] [Google Scholar]