Abstract
Background
Recombinant human growth hormone (rhGH) increases protein synthesis, therefore it is used in burns with a total body surface area (TBSA) greater than 40%, where there is frequently an increase in protein breakdown and a decrease in protein synthesis. This change in protein metabolism correlates with poor wound healing of the burn and donor sites.
Objectives
To determine the effects of rhGH on the healing rate of burn wounds and donor sites in people with burns.
Search methods
For this first update we searched the Cochrane Wounds Group Specialised Register (searched 04 September 2014); The Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library 2014, Issue 8); Database of Abstracts of Reviews of Effects (DARE) (The Cochrane Library 2014, Issue 3); Ovid MEDLINE (1950 to September Week 4 2014); Ovid MEDLINE (In‐Process & Other Non‐Indexed Citations September 8, 2014); Ovid EMBASE (1980 to 2014 Week 35); and EBSCO CINAHL (1982 to 8 September 2014).
Selection criteria
Randomised controlled trials (RCTs) comparing rhGH with any comparator intervention, e.g. oxandrolone or placebo, in adults or children with burns.
Data collection and analysis
Two review authors independently selected studies, assessed trial quality and extracted data. The primary outcomes were the healing of the burn wound and donor sites and the occurrence of wound infections. The secondary outcomes were mortality rate, length of hospital stay, scar assessment, and adverse events: hyperglycaemia and septicaemia.
Main results
We included 13 RCTs (701 people). Six of the RCTs included only children aged 1 to 18 years and seven involved only adults (from 18 to 65 years of age). The mean TBSA of the included participants was greater than 49%. Twelve studies compared rhGH with placebo and one study compared rhGH with oxandrolone. Two trials found that compared with placebo, burn wounds in adults treated with rhGH healed more quickly (by 9.07 days; 95% confidence interval (CI) 4.39 to 13.76, I² = 0%). The donor site healing time was significantly shorter in rhGH‐treated adults compared with placebo‐treated participants (by 3.15 days; 95% CI 1.54 to 4.75, I² = 0%). Two studies in children with the outcome of donor site healing time could be pooled and the donor site healing time was shorter in the rhGH‐treated children (by 1.70 days; 95% CI 0.87 to 2.53, I² = 0%). No studies reporting the outcome of wound infection were found. The incidence of hyperglycaemia was higher in adults during rhGH treatment compared with placebo (risk ratio (RR) 2.43; 95% CI 1.54 to 3.85), but not in children. Pooling the studies of adults and children yielded a significantly higher incidence of hyperglycaemia in the rhGH‐treated participants (RR 2.65; 95% CI 1.68 to 4.16).
Authors' conclusions
There is some evidence that using rhGH in people with large burns (more than 40% of the total body surface area) could result in more rapid healing of the burn wound and donor sites in adults and children, and in reduced length of hospital stay, without increased mortality or scarring, but with an increased risk of hyperglycaemia. This evidence is based on studies with small sample sizes and risk of bias and requires confirmation in higher quality, adequately powered trials.
Keywords: Adolescent; Adult; Aged; Child; Child, Preschool; Humans; Infant; Middle Aged; Young Adult; Transplant Donor Site; Anabolic Agents; Anabolic Agents/therapeutic use; Body Surface Area; Burns; Burns/drug therapy; Burns/pathology; Human Growth Hormone; Human Growth Hormone/therapeutic use; Oxandrolone; Oxandrolone/therapeutic use; Randomized Controlled Trials as Topic; Recombinant Proteins; Recombinant Proteins/therapeutic use; Wound Healing; Wound Healing/drug effects
Plain language summary
Human growth hormone for treating burns and skin graft donor sites
Growth hormone is produced by the pituitary gland. For decades, it could only be obtained by extraction from pituitary glands but more recently it has been produced through genetic engineering and made available for therapy as recombinant human growth hormone (rhGH). The aim of this review was to determine the effects of rhGH when used to treat burns and skin graft donor sites and to determine its safety compared with other treatments.
A burn that affects more than 40% of total body surface area affects the entire body. In people with such large burns, metabolism increases, as represented by a higher heart rate. This state of increased metabolism is called hypermetabolism. Hypermetabolism consumes high levels of energy Part of this energy is obtained through the breakdown of the patient’s own muscles, which leads to wasting. This breaking down of tissues into smaller molecules to release energy is called catabolism. However, such catabolism does not provide sufficient energy for the hypermetabolic state. This shortage of energy and building molecules leads to prolonged burn wound and donor site healing. In children, this shortage also leads to growth retardation. This catabolic state can be treated with anabolic agents that reverse the protein breakdown. One of the anabolic agents recommended for such a treatment approach is recombinant growth hormone.
We found 13 eligible randomised controlled trials (RCTs) involving 701 people for inclusion in this review. There is some evidence that recombinant growth hormone therapy in people with burns covering more than 40% of the total body surface area helps burn wounds and donor sites heal more rapidly and reduce the length of hospital stay, without increased mortality or increased scarring. We found it difficult to assess the quality of these studies due to poor reporting therefore we cannot be completely confident in their results.
Summary of findings
Summary of findings for the main comparison. Recombinant human growth hormone compared with placebo for treating burns and donor sites.
| Recombinant human growth hormone compared with placebo for treating burns and donor sites | ||||||
| Patient or population: Settings: burn centres Intervention: recombinant human growth hormone Comparison: | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Recombinant human growth hormone | ||||||
| Healing time of burn wounds in days for adults | The mean healing time of burn wounds in days for adults in the intervention groups was 9.07 lower (4.39 to 13.76 lower) |
36 (2 studies) | ⊕⊕⊝⊝ low1,2,3,4 | |||
| Donor site healing time in days for adults | The mean donor site healing time in days for adults in the intervention groups was 3.15 lower (1.54 to 4.75 lower) | 36 (2 studies) | ⊕⊕⊝⊝ low1,2,3,4 | |||
| Donor site healing time in days for children | The mean donor site healing time in days for children in the intervention groups was 1.70 lower (0.87 to 2.53 lower) | 73 (2 studies) |
⊕⊕⊝⊝ low13 | |||
| Mortality in adults and children | Study population5 | RR 0.53 (0.22 to 1.29) | 324 (5 studies) | ⊕⊕⊝⊝ low5,6,7 | ||
| 7 per 100 | 4 per 100 (2 to 9) | |||||
| Low5 | ||||||
| 5 per 100 | 3 per 100 (1 to 6) | |||||
| High5 | ||||||
| 13 per 100 | 7 per 100 (3 to 17) | |||||
| Septicaemia in adults | Study population8 | RR 0.61 (0.31 to 1.22) | 267 (4 studies) | ⊕⊕⊝⊝ low1,8,9 | ||
| 13 per 100 | 8 per 100 (4 to 16) | |||||
| Low8 | ||||||
| 4 per 100 | 2 per 100 (1 to 5) | |||||
| High8 | ||||||
| 13 per 100 | 8 per 100 (4 to 16) | |||||
| Hyperglycaemia in adults and children | Study population10 | RR 2.65 (1.68 to 4.16) | 340 (5 studies) | ⊕⊕⊝⊝ low2,10,11 | ||
| 11 per 100 | 30 per 100 (19 to 48) | |||||
| Low10 | ||||||
| 0 per 100 | 0 per 100 (0 to 0) | |||||
| High10 | ||||||
| 19 per 100 | 50 per 100 (32 to 79) | |||||
| Length of hospital stay in days for adults | The mean length of hospital stay in days for adults in the intervention groups was 12.55 lower (8 to 17.09 lower) | 99 (4 studies) | ⊕⊕⊝⊝ low1,12 | |||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Method of randomisation, allocation concealment and blinding not reported. 2 Small sample sizes of studies and wide confidence interval of estimate. 3 Patients in study of Sun 1998 had larger and deeper burns. 4 In the studies of Sun 1998 and Luo 2000 the way of assessment of the healing time was not reported. 5 The low and high risk values are the two extreme numbers of mortality in the control groups from two studies. 6 Method of randomisation and blinding in four studies and allocation concealment in five studies not reported. 7 Mortality is a rare outcome and the number of included patients in three studies was small. 8 The low and high risk values are the two extreme numbers of septicaemia in the control groups from two studies. 9 Septicaemia is a rare outcome and the number of included patients in three studies was small. 10 The low and high risk values are the two extreme numbers of hyperglycaemia in the control groups from two studies. Small sample sizes in four studies and wide confidence interval of estimate. 11 Method of randomisation and blinding in three studies and allocation concealment in five studies not reported. 12 Small sample sizes in four studies and wide confidence interval of estimate. Patients in control group of study of Lu 2004 received glutamine orally and no placebo injections. 13 Method of randomisation in one study and allocation concealment in two studies not reported.
Background
Description of the condition
Fire‐related burns (not including scalds or electric or chemical burns) were responsible for an estimated 322,000 deaths globally in 2002 (Mock 2008). Mortality rates due to these burns vary greatly among different regions of the world, ranging from 11.6 deaths per 100,000 people per year in Southeast Asia to 1.0 deaths per 100,000 people in high‐income countries (Mock 2008).
People with a major thermal injury experience a hypermetabolic response (an increase in metabolic activity). This hypermetabolic response is characterised by a hyperdynamic circulatory response, with increased body temperature; increased oxygen and glucose consumption; increased carbon dioxide production; hyperglycaemia (raised blood sugar levels); peripheral insulin resistance; glycogenolysis, proteolysis and lipolysis (i.e., the breakdown of glycogen, protein and lipids, respectively); the loss of lean body mass; and muscle and bone wasting. These symptoms can last for one to two years after the burn injury (Herndon 2004; Przkora 2005; Przkora 2006; Jeschke 2008; Branski 2009). The intensity of this response depends on the percentage of the total body surface area (TBSA) involved. The response is most frequently observed in burns with a TBSA greater than 40%. The hypermetabolic response is associated with sympathetic nervous system stimulation, increased levels of stress hormones (catecholamines, glucagon and glucocorticoids) and the release of inflammatory mediators (e.g., tumour necrosis factor, interleukin). In the severely burned patient, hypermetabolism correlates with protein catabolism (breakdown), which appears as muscle wasting and weakness, the loss of lean body mass, poor wound healing of the burn site and/or donor sites and immune system depression. Protein catabolism is an imbalance between protein synthesis and breakdown. The breakdown of muscles yields free amino acids for building proteins in the burn wound (Demling 2000; Pereira 2005).
Burn wounds can be treated with autologous skin transplantations using skin taken from another part of the burn victim's body. The donor sites of these transplants can be harvested more than once. Shortening the healing time of the donor sites can accelerate the person's recovery. The healing time of the donor sites is influenced by the person's nutritional state and is prolonged during severe hypermetabolism (Hart 2000).
Description of the intervention
Growth hormone is produced by the pituitary gland. For decades, growth hormone could only be obtained by extraction from the pituitary glands of corpses; however since 1985 it has been genetically engineered and is currently available for therapy as recombinant human growth hormone (rhGH). This hormone can be administered once or twice daily through subcutaneous or intramuscular injection. The usual dose for metabolic indications is 0.1 to 0.2 mg/kg/day, and it can be administered for a time period ranging from days to one year (Van Loon 1998). The price for 5 mg/mL is approximately USD 300; and treatment for a person with a body weight of 50 kg is USD 300 to 600 per day. New depot forms of rhGH, which enable the injections to be given once or twice a month, are becoming available. The drug is administered at bedtime to mimic the physiological pattern of growth hormone secretion. There are various rhGH formulations available on the market, including Genotropin®, Humatrope®, Nutropin®, Nutropin AQ®, Protropin®, Serostim® and Zorbitive®. The rhGH indications approved by the Food and Drug Administration (FDA) and the European Medicines Agency (EMEA) for giving rhGH are growth disturbance in children and growth hormone deficiency in adults.
How the intervention might work
Due to its potent anabolic (growth‐stimulating) properties, rhGH can be used to limit the catabolism of burned tissue (Demling 2000; Pereira 2005). Its anabolic effects increase the cellular uptake of amino acids, nitrogen retention and protein synthesis and the release of insulin‐like growth factor (IGF). It can have a direct effect on the skin due to the presence of growth hormone receptors on epidermal cells. The hypermetabolic response after burn injuries persists, despite improvements in surgical and nursing care. The modulation of hormonal imbalances after burn injuries was introduced in the 1990s, and investigated in animal studies and clinical trials (Herndon 1990; Klein 1998; Ramirez 1998; Singh 1998; Jeschke 1999a; Jeschke 1999b; Aili 2001; Hart 2001; Przkora 2006a; Branski 2009). Herndon 1990 showed reduced donor site healing times and a reduced length of hospital stay in children with burns who were treated with rhGH. Human growth hormone administration is associated with side effects, including hyperglycaemia and reactions at the injection site (e.g., nodules, erythema, pain and swelling). Long‐term treatment at doses of 0.1 mg/kg/day has been considered safe and well tolerated (Van Loon 1998).
Why it is important to do this review
The precise role of recombinant human growth hormone (rhGH) in the treatment of burns and skin donor sites is uncertain. Treatment with rhGH has been used with success to stimulate growth in children with growth retardation, including those with growth hormone deficiency, Turner and Nooan syndromes (Bryant 2007), small size for gestational age, Prader‐Willi syndrome, idiopathic short stature (Bryant 2007) and cystic fibrosis (Krysiak 2007). In adults, growth hormone therapy is indicated in people with growth hormone deficiency, and it has positive effects on the lipid profile, muscle strength, cardiac function and quality of life. In another study with thermally injured rats, rhGH attenuated the decrease in IGF‐1 after burns (Jeschke 1999b). Although randomised controlled trials (RCTs) are available there are no published systematic reviews or evidence‐based guidelines to direct clinical decision‐making in the costly use of rhGH to treat burn wounds.
Objectives
To determine the safety and effects of recombinant human growth hormone on the healing rate of burn wounds and donor sites in people with burns.
Methods
Criteria for considering studies for this review
Types of studies
We included only randomised controlled trials (RCTs).
Types of participants
We included trials that included people of any age who had a burn wound covering more than 20% TBSA with more than 10% full‐thickness (i.e., a third‐degree burn). Trials involving any type of burn injury were eligible (e.g., chemical, scald or flame burns). We included trials with donor sites only when the donor sites were used to treat burn wounds.
Types of interventions
We considered studies for inclusion if recombinant human growth hormone (rhGH, any regimen) was compared with any comparable intervention, for example, testosterone analogues (e.g., oxandrolone), β‐blockade (propranolol) or placebo.
Types of outcome measures
Primary outcomes
Burn wound healing. Objective measures of the healing of transplanted and non‐operated burn wounds included the time to complete wound healing or the proportion of the burn wound that healed completely (epithelialised) within a specific time period.
Donor site healing. Objective measures of donor site healing included the healing time (in days) for the first and successive donor sites or the number of wounds that healed completely during the trial period.
Wound infection (as defined by the trial authors).
Mortality rate.
Secondary outcomes
Length of hospital stay
Any objective measurement of scar formation. The most frequently used measure of scar evaluation is the Vancouver Scar Scale (Sullivan 1990; Baryza 1995). This is a clinical scale that has been tested for reliability and validity (Nedelec 2000). Another reliable and valid scar scale is the Patient and Observer Scar Assessment Scale (POSAS) (Draaijers 2004; Vercelli 2009). The Seattle Scar Scale (Yeong 1997) and Hamilton Scale (Crowe 1998) are used to assess scars using photographs of burn wounds.
Adverse events (e.g., hyperglycaemia, septicaemia).
Quality of life measures, such as the Burns Specific Health Scale (BSHS) questionnaire (Blades 1982).
Search methods for identification of studies
The search methods for the original review can be found in Appendix 1
Electronic searches
For this first update we searched the following electronic databases to find reports of relevant RCTs:
Cochrane Wounds Group Specialised Register (searched 4 September 2014);
The Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library 2014, Issue 8);
Database of Abstracts of Reviews of Effects (DARE) (The Cochrane Library 2014, Issue 3);
Ovid MEDLINE (1950 to September Week 4 2014);
Ovid MEDLINE (In‐Process & Other Non‐Indexed Citations September 8, 2014);
Ovid EMBASE (1980 to 2014 Week 35);
EBSCO CINAHL (1982 to 8 September 2014);
PEDro, the Physiotherapy Evidence database (1980 to 8 September 2014);
National Research Register (NRR) Archive (https://portal.nihr.ac.uk/Pages/NRRArchive.aspx);
OAIster (http://oaister.umdl.umich.edu/o/oaister/) international institutional digital repository search engine;
Web of Science (1975 to 8 September 2014);
Dissertation abstracts (www.dissonline.de; www.theses.com; www.proquest.co.uk/products.pq/descriptions/pqdt.shtml), searched 8 September 2014;
U.S. Food and Drug Administration (www.fda.gov), searched 8 September 2014;
European Medicines Agency (www.ema.europa.eu), searched 8 September 2014.
We used the following strategy to search The Cochrane Central Register of Controlled Trials (CENTRAL):
#1 MeSH descriptor: [Burns] explode all trees 1195 #2 (burn or burns or burned or scald*):ti,ab,kw 3823 #3 (thermal* NEXT injur*):ti,ab,kw 154 #4 #1 OR #2 OR #3 4024 #5 MeSH descriptor: [Growth Hormone] explode all trees 3004 #6 (growth NEXT hormone*):ti,ab,kw 4425 #7 rhGH:ti,ab,kw 382 #8 somatotropin:ti,ab,kw 113 #9 #5 OR #6 OR #7 OR #8 4455 #10 #4 AND #9 65
Additional search strategies for Ovid MEDLINE, Ovid EMBASE and EBSCO CINAHL can be found in Appendix 2.
We did not restrict studies with respect to language, date of publication or study setting. We considered and included abstracts and letters if we were able to obtain complete manuscripts from the study author(s).
Searching other resources
Manual searches
We searched the following journals manually:
Annals of Burns and Fire Disasters (1987 to September 2014);
(http://www.journalofburnsandwounds.com). Since 2008, this journal has been called ePlasty (http://www.eplasty.com) (2004 to 2014).
We searched scientific conference proceedings for the following associations (1995 to 2014) manually:
Asia‐Pacific Burns Association;
European Burns Association (EBA);
American Burn Association (ABA);
Australian and New Zealand Burns Association (ANZBA);
Mediterranean Council for Burns and Fire Disasters (MBC);
European Paediatric Burn Club (ECPB);
South African Burns Society;
International Society for Burn Injury (ISBI).
We performed manual searches of conference proceedings to identify papers, authors and grey literature related primarily to managing the hypermetabolic stress response. When only abstracts were available, we contacted the authors to request further information and full publications.
Grey literature and unpublished studies
We contacted authors and recognised experts in the field of acute burn management. We made formal requests for unpublished manuscripts by email to corresponding authors from the short list of identified publications.
We reviewed the following grey literature Internet web sites for appropriate studies and we contacted the authors as described above:
National Library of Medicine (http://www.nlm.nih.gov/nichsr/ehta/chapter10.html);
New York Academy of Medicine (http://www.nyam.org/library/grey.shtml);
GrayLIT Network (http://www.osti.gov/graylit/).
We reviewed the Trials Central and Current Controlled Trials databases for relevant studies registered since 2000. We searched the reference lists of retrieved articles and other systematic reviews and meta‐analyses identified using the above methods.
Data collection and analysis
Selection of studies
Two review authors independently screened the titles and abstracts of all records retrieved by the searches against the inclusion criteria to exclude obviously irrelevant studies. We retrieved as full text all citations that appeared to be relevant, and two review authors independently assessed these records against the inclusion criteria. In all instances, we resolved differences of opinion by discussion among the review authors.
Data extraction and management
Two review authors independently extracted data from the studies using paper data collection forms developed for this purpose. The standardised forms allowed the extraction of specific data, such as:
country of origin;
depth of the burn wound, size of the burn wound, burn type;
care setting;
number of participants randomised to each trial arm;
eligibility criteria and key baseline participant data;
details of the intervention (e.g., dosage, duration, type) that each group received;
details of any co‐interventions;
primary and secondary outcome(s) (with definitions);
outcomes data for primary and secondary outcomes (by group);
duration of follow‐up;
number of withdrawals (by group);
adverse events;
funding sources.
We resolved all differences by discussion among the review authors. Each review author checked the data independently for accuracy and one review author (WET) entered the data into Review Manager 5 (RevMan 2011). The second review author verified the data entered (RSB).
Assessment of risk of bias in included studies
Two review authors independently assessed each included study using the Cochrane Collaboration tool for assessing the risk of bias (Higgins 2011a). This tool addresses six specific domains, namely: sequence generation, allocation concealment, blinding, incomplete outcome data, selective outcome reporting and other issues (e.g., extreme baseline imbalance) (Appendix 3). We assessed blinding and the completeness of outcome data for each outcome separately. We completed a 'Risk of bias' table for each eligible study and discussed any disagreements amongst all review authors to achieve a consensus. We present the assessment of the risk of bias using a 'Risk of bias' summary figure, which presents all of the judgements in a study‐by‐entry cross‐tabulation (Figure 1). This display of internal validity indicates the weight the reader may give to each study's results.
1.

'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
Measures of treatment effect
For data analysis, we followed the guidelines outlined in Section 9 of the Cochrane Handbook for Systematic Reviews of Interventions 5.0.1 (Deeks 2011). We reported dichotomous outcomes (e.g., mortality) as risk ratios (RRs) with 95% confidence intervals (CIs). For continuous data, we presented results as the mean differences (MD) with 95% Cls. We used the standardised mean difference (SMD) when the studies assessed the same outcome, but used different scales (e.g., Vancouver Scar Scale and POSAS for scar assessment). The time to complete wound healing is time‐to‐event data and the most appropriate way of summarising this type of data is to use survival analysis methods and express the intervention effect as a hazard ratio. It is not appropriate to analyse time‐to‐event data using methods for continuous outcomes (e.g., using mean times‐to‐event) because the relevant times are only known for the subset of participants who had the event. When time is analysed as a continuous variable and not all participants experience the event (i.e., they are censored) they are excluded from the analysis which almost certainly introduces bias. When all of the participants in a study had completely healed wounds (the event), the mean wound‐healing time could be used.
We used Review Manager Version 5 software to generate the figures and statistical analyses (RevMan 2011).
Unit of analysis issues
The analysis of the included studies took into account the level at which randomisation occurred.
Dealing with missing data
We analysed data according to the intention‐to‐treat principle, i.e., all people included in the study at the point of randomisation were analysed according to their assigned treatment group, regardless of whether treatment was completed. Whenever possible, we contacted the original investigators to request missing data. We did not impute data if the missing data were not provided after several attempts to contact the study author. The potential impact of missing data on the review findings is addressed in the Discussion section.
Assessment of heterogeneity
The study synthesis methods depended on study quality, design and heterogeneity. We explored both clinical and statistical heterogeneity. In statistical heterogeneity the reported effect size is not consistent across studies. Clinical heterogeneity is characterised by a difference in study design; for instance, in the mixes of participants and in the implementation of interventions. We assessed heterogeneity between study results statistically using the I² statistic (Higgins 2003). This approach examines the percentage of the total variation across studies that is due to heterogeneity rather than chance. If the heterogeneity was more than 50% we used a random‐effects model instead of a fixed‐effect model when pooling was appropriate.
Assessment of reporting biases
We planned to assess reporting bias graphically using a funnel plot. We planned to report separately for each primary outcome; however, it must be recognised that if the number of eligible studies is small, the results are likely to be inconclusive (Egger 1997). The number of studies that could be pooled was very small (two to five studies), therefore this portion of the analysis was not undertaken.
Data synthesis
We examined clinical and methodological heterogeneity with reference to the study population (gender, age and TBSA percentage), intervention and outcome. In the absence of clinical and statistical heterogeneity, we used a fixed‐effect model to pool data. We investigated the cause of heterogeneity. In the presence of statistical heterogeneity (as estimated by an I² greater than 50%), we either applied a random‐effects model to pool the data or did not pool the data but undertook a narrative overview.
Subgroup analysis and investigation of heterogeneity
When we identified studies that included both adults and children, we presented the main effects for children and adults separately, data permitting.
Sensitivity analysis
We considered studies to have a high risk of bias if randomisation sequence generation, allocation concealment and outcome assessment blinding methods were inadequate or unclear. We performed a sensitivity analysis that omitted studies with a high risk of bias.
Results
Description of studies
Results of the search
The searches retrieved a total of 78 records (Figure 2). Independent scrutiny of the titles and abstracts identified 27 potentially relevant articles. We assessed these in full‐text and subsequently excluded 14 studies. The remaining 13 studies were eligible for inclusion in the review. Six studies included only children and seven included only adults. Five studies (Barret 1999; Demling 1999; Jeschke 2000; de Oliveira 2004; Przkora 2006) could not be included in a quantitative synthesis (meta‐analysis). New searches for the first update of the review in September 2014 did not yield any further studies.
2.
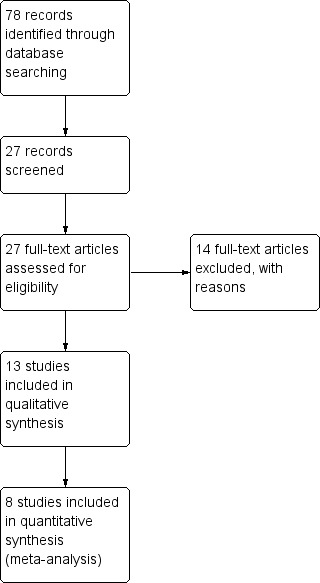
Study flow diagram.
Included studies
See: Characteristics of included studies.
Thirteen studies with 701 participants met the inclusion criteria. Six RCTs included 289 children (Herndon 1990; Gilpin 1994; Barret 1999; Jeschke 2000; de Oliveira 2004; Przkora 2006); seven RCTs included 412 adults (Sun 1998; Demling 1999; Luo 2000; Pelzer 2000; Losada 2002; Lu 2004; Chen 2005).
Design
Twelve RCTs were two‐armed trials with two independent groups, 11 trials compared rhGH injections with placebo injections (Herndon 1990; Gilpin 1994; Sun 1998; Barret 1999; Demling 1999; Jeschke 2000; Luo 2000; Pelzer 2000; Losada 2002; de Oliveira 2004; Chen 2005; Przkora 2006) one trial compared rhGH injections with oral oxandrolone (Demling 1999). One trial was a three‐arm trial comparing oral glycine, oral glutamine and oral glutamine with rhGH injections (Lu 2004); however only the last two arms were included in this review.
Sample size
The sample sizes ranged from 16 (Sun 1998) to 207 (Chen 2005) people. The child study sample sizes ranged from 27 (Herndon 1990) to 94 (Barret 1999) participants, with a mean of 47; the adult study sample sizes ranged from 16 (Sun 1998) to 207 (Chen 2005), with a mean of 48.
Setting
All 13 trials took place in hospitals (burn centres), but two trials also continued after patient discharge (de Oliveira 2004; Przkora 2006). Seven studies were performed in the United States (Herndon 1990; Gilpin 1994; Barret 1999; Demling 1999; Jeschke 2000; de Oliveira 2004; Przkora 2006), four in China (Sun 1998; Luo 2000; Lu 2004; Chen 2005), one in Germany (Pelzer 2000) and one in Spain (Losada 2002). Six trials took place in the same institution (Herndon 1990; Gilpin 1994; Barret 1999; Jeschke 2000; de Oliveira 2004; Przkora 2006). The following five studies described explicitly obtaining informed consent for the study from the patient or relatives: Pelzer 2000; Losada 2002; de Oliveira 2004; Chen 2005; Przkora 2006.
Participants
Six RCTs included 289 children (Herndon 1990; Gilpin 1994; Barret 1999; Jeschke 2000; de Oliveira 2004; Przkora 2006) and the other seven RCTs included 412 adults (Sun 1998; Demling 1999; Luo 2000; Pelzer 2000; Losada 2002; Lu 2004; Chen 2005). Ages ranged from one to 65 years. The mean TBSA of the included participants was greater than 40% and the mean full‐thickness (third‐degree) TBSA was greater than 20%. Nine studies reported the male‐to‐female ratio (Gilpin 1994; Barret 1999; Jeschke 2000; Luo 2000; Pelzer 2000; Losada 2002; de Oliveira 2004; Chen 2005; Przkora 2006). The percentage of males ranged from 60% (de Oliveira 2004) to 90% (Pelzer 2000), with a mean of 72%.
Interventions
Eleven trials compared rhGH with placebo (Herndon 1990; Gilpin 1994; Sun 1998; Barret 1999; Jeschke 2000; Luo 2000; Pelzer 2000; Losada 2002; de Oliveira 2004; Chen 2005; Przkora 2006). One trial compared rhGH with oxandrolone (Demling 1999) and one trial compared oral glutamine with oral glutamine combined with rhGH injections (Lu 2004). The doses of rhGH used were 0.05 mg/kg/day in two trials (de Oliveira 2004; Przkora 2006), 0.1 mg/kg/day in one trial (Demling 1999), 0.15 mg/kg/day in one trial (Losada 2002), 0.2 mg/kg/day in four trials (Herndon 1990; Gilpin 1994; Barret 1999; Jeschke 2000), 0.19 IU/kg/day in one trial (Chen 2005), 0.2 IU/kg/day in one trial (Lu 2004), 0.3 IU/kg/day in one trial (Sun 1998) and 0.5 IU/kg/day in two trials (Luo 2000; Pelzer 2000). The oxandrolone dose was 20 mg given orally (Demling 1999). In six of the 12 rhGH trials, the control injections contained saline (Herndon 1990; Sun 1998; Barret 1999; Jeschke 2000; Luo 2000; Chen 2005); in one study, the control injections contained water with cresol (a neutral carrier substance) (Pelzer 2000), and in four studies the control substance was not reported (Gilpin 1994; Losada 2002; de Oliveira 2004; Przkora 2006). In one study, 0.5 g/kg/day of glutamine was given orally to both the control group and to the rhGH group, but the control group received no placebo injections (Lu 2004).
Outcomes
Not all trials reported on all outcome measures. The following outcomes were reported: burn wound healing time in days (Sun 1998; Luo 2000), burn wound healing rate (on the 30th postburn day Sun 1998; Lu 2004), donor site healing time in adults (Sun 1998; Demling 1999; Luo 2000; Losada 2002), donor site healing time in children (Herndon 1990; Gilpin 1994), mortality (Sun 1998; Jeschke 2000; Pelzer 2000; Losada 2002; Chen 2005; of which one study described the mortality in children: Jeschke 2000), length of hospital stay in adults (Sun 1998; Demling 1999; Luo 2000; Losada 2002; Lu 2004) and in children (Herndon 1990; Jeschke 2000), percentage of children requiring reconstruction (Barret 1999), number of reconstructive procedures in children (Przkora 2006), scar scales in children (de Oliveira 2004), septicaemia (Sun 1998; Luo 2000; Losada 2002; Chen 2005) and hyperglycaemia (in adults: Demling 1999; Luo 2000; Pelzer 2000; Losada 2002; Chen 2005 and in children: Herndon 1990).
Excluded studies
See: Characteristics of excluded studies.
In total 14 studies were excluded from the review. Twelve of the excluded studies addressed none of the pre‐specified outcome measures, reporting metabolic outcome measures, growth, weight, cardiac and pulmonary function (Aarsland 1996; Klein 1998; Chrysopoulo 1999; Low 1999; Chai 2002a; Chai 2002b; Hart 2002; Suman 2003; Suman 2004; Mlcak 2005; Jeschke 2008; Branski 2009). In addition, one study was excluded because it was a controlled clinical trial and not a RCT (Herndon 1995) and one study was excluded because it had zero events of hyperglycaemia in the rhGH and placebo groups (Hart 2001).
Risk of bias in included studies
Details of the risk of bias assessment are provided in the table Characteristics of included studies. Additionally, a descriptive analysis of the studies is provided below. In general, study quality was assessed as having a low or unclear risk of bias. A summary of the risk of bias is provided in Figure 1.
Allocation
Adequate sequence generation
The method of randomisation was described in only two of the 13 studies (Herndon 1990; Pelzer 2000). In the other articles, no information was reported about the random component of the sequence generation process.
Allocation concealment
Allocation concealment was not clearly reported in any of the 13 studies.
Blinding
Four studies stated that the injection vials contents (saline or rhGH) were blinded for participants, nurses and physicians (Herndon 1990; Gilpin 1994; Barret 1999; Pelzer 2000). In one study, the drugs (oxandrolone) could not be blinded because one was provided parenterally and the other was given orally (Demling 1999). And in a second study blinding was not performed, because the control group received no injections but glutamine orally (Lu 2004). Only two studies reported using blinded outcome observers for scar assessment (Barret 1999; de Oliveira 2004).
Incomplete outcome data
All studies either had no incomplete data for the pre‐specified outcomes (Herndon 1990; Gilpin 1994; Sun 1998; Demling 1999; Jeschke 2000; Luo 2000; de Oliveira 2004) or the missing data were balanced in numbers across intervention groups (Barret 1999; Pelzer 2000; Losada 2002; Chen 2005 ; Przkora 2006). In Pelzer 2000, five of the 23 placebo group participants and seven of the 26 rhGH group participants dropped out, representing a 24% dropout rate for the entire group of participants. However, seven of the dropouts in this study were caused by mortality, which was an outcome measure. In Przkora 2006, which had a one‐year follow‐up, seven of the 19 placebo group participants and five of the 25 rhGH group participants dropped out, representing a 27% dropout rate for all participants.
Selective reporting
None of the studies provided a study protocol. The studies by Gilpin 1994; Jeschke 2000; Luo 2000; Pelzer 2000; Lu 2004 and Przkora 2006 were at low risk of bias for this domain as they reported the pre‐specified outcomes in the study. The studies by Barret 1999; Demling 1999; de Oliveira 2004 and Chen 2005 provided insufficient information about the criteria used to judge selective outcome reporting and are at unclear risk of bias. The outcome measures incorporated into this meta‐analysis from the studies by Herndon 1990; Sun 1998 and Losada 2002 were not pre‐specified in the methods of the articles and this represents a high risk of reporting bias for this domain.
Effects of interventions
See: Table 1
We analysed trials involving adults and children separately, except for the outcomes mortality and hyperglycaemia for which we analysed the trials in adults and children both separately and together.
Comparison of rhGH with placebo
Primary outcomes
Healing rate of burn wounds (adults)
For the primary outcome of the healing rate of burn wounds in adults, four studies compared recombinant human growth hormone (rhGH) and placebo and measured the outcome in three different ways (Sun 1998; Luo 2000; Pelzer 2000; Lu 2004).
Two studies reported mean wound healing time in days and we pooled them. The time to complete wound healing is time‐to‐event data but because all participants were followed until complete wound healing, the data can be analysed as if time is a continuous variable since no participants were censored. The mean wound healing time was statistically significantly shorter with rhGH treatment compared with placebo (Analysis 1.1: mean difference (MD) ‐9.07 days; 95% confidence interval (CI) ‐13.76 to ‐4.39; I² = 0%; Sun 1998; Luo 2000). We did not pool two studies that estimated the wound healing rate on the 30th post‐burn day (Lu 2004; Sun 1998) because of considerable heterogeneity (I² = 88%) and the compared treatments were different between the studies. Wound healing rate on the 30th post‐burn day could mean how many wounds have completely healed at day 30 or how much on average (percentage) is healed by day 30 and the definition of wound healing rate at day 30 was unclear in both Sun 1998 and Lu 2004. The wound healing rate on the 30th post‐burn day was significantly higher after rhGH treatment in both studies (Sun 1998 MD 20.10%; 95% CI 12.46 to 27.74; Lu 2004 MD 8.00%; 95% CI 4.58 to 11.42). The heterogeneity of these studies could be explained by a larger mean total % total body surface area (TBSA) and a larger mean % full‐thickness burn in the study by Sun 1998. In Lu 2004 the control group did not receive injections, as in the study by Sun 1998; instead controls received oral glutamine, which accelerated the wound healing rate in comparison with a third patient group that received oral glycine. Therefore, the wound healing rate difference in this study could be reduced by the glutamine effect. Pelzer 2000 did not report standard deviations of the wound closure index. The trial report states that the wound closure index was not significantly higher after 0.5 IU/kg/day of rhGH for 28 days, starting on second post‐burn day (P < 0.2); however, this could not be confirmed due to missing data.
1.1. Analysis.

Comparison 1 Comparison of rhGH with placebo, Outcome 1 Healing time of burn wounds in days for adults.
Donor site healing
Adults
Three studies involving adults reported this outcome (Sun 1998; Luo 2000; Losada 2002). Although time to complete wound healing is a type of time‐to‐event data and was not analysed in these studies using survival methods, all participants were followed until complete wound healing therefore the mean time to wound healing can be presented. We pooled two studies (Sun 1998; Luo 2000;) because pooling three studies caused considerable heterogeneity (I² = 81%). Donor site healing was statistically significantly shorter in the rhGH group compared with the other groups (Analysis 1.2: MD ‐3.15 days; 95% CI ‐4.75 to ‐1.54). The mean wound healing time was not significantly different between placebo and rhGH group in Losada 2002 (MD ‐0.28 days; 95% CI ‐0.94 to 0.38). The standard deviations of the donor site healing time were smaller in the Losada 2002 study than in the Sun 1998 and Luo 2000 studies. In Sun 1998 and Luo 2000 the method used to assess healing time was not reported; and in Losada 2002 the donor site was defined as being healed when it was adequate for harvesting as a new autograft donor site. This could have introduced a difference in the healing time variability in the studies.
1.2. Analysis.
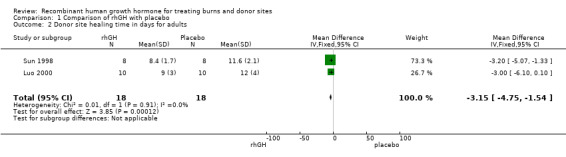
Comparison 1 Comparison of rhGH with placebo, Outcome 2 Donor site healing time in days for adults.
Children
We pooled two studies involving children (Herndon 1990; Gilpin 1994). The donor site healed significantly more quickly after rhGH treatment compared with placebo injections (Analysis 1.3: MD ‐1.70; 95% CI ‐2.53 to ‐0.87; I² = 0%)
1.3. Analysis.
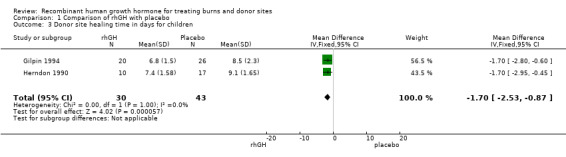
Comparison 1 Comparison of rhGH with placebo, Outcome 3 Donor site healing time in days for children.
Wound infections
No studies reported this outcome.
Mortality
We pooled data from the four studies involving adults (Sun 1998; Pelzer 2000; Losada 2002; Chen 2005). Overall there were 15 deaths; 5 in the rhGH groups and 10 in the placebo groups however this difference was not statistically significant, possibly due to lack of statistical power (Analysis 1.5.1: risk ratio (RR) 0.48; 95% CI 0.19 to 1.25; P = 0.13; I² = 0%). One study involving children (Jeschke 2000) found no statistically significant difference in mortality between rhGH and placebo (Analysis 1.5.2: RR 1.15; 95% CI 0.08 to 16.67). Combining the five studies in both adults and children resulted in no statistically significant difference in mortality between the rhGH and placebo groups (Analysis 1.5: RR = 0.53; 95% CI 0.22 to 1.29) however this comparison is underpowered with only 17 deaths in total therefore an effect on mortality cannot be ruled out.
1.5. Analysis.
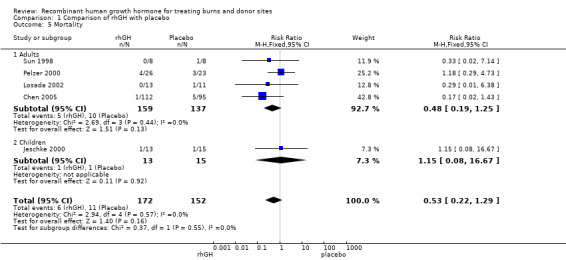
Comparison 1 Comparison of rhGH with placebo, Outcome 5 Mortality.
Secondary outcomes
Length of hospital stay
We pooled four studies involving adults (Sun 1998; Luo 2000; Losada 2002; Lu 2004). Hospital stay was significantly shorter in the rhGH group compared with the placebo group (Analysis 1.4.1: MD ‐12.55 days; 95% CI ‐17.09 to ‐8.00; I² = 0%).
1.4. Analysis.
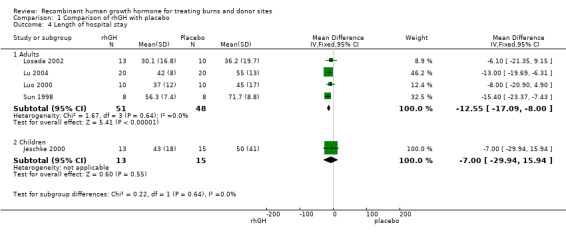
Comparison 1 Comparison of rhGH with placebo, Outcome 4 Length of hospital stay.
A study in children found no statistically significant difference in the length of hospital stay between the groups (Analysis 1.4.2: MD ‐7.00 days; 95% CI ‐29.94 to 15.94).
Burn scar formation
Three of the studies involving children reported this outcome (Barret 1999; de Oliveira 2004; Przkora 2006), but these studies could not be pooled because they used different methods to measure the outcome.
de Oliveira 2004 scored the Seattle, Hamilton and Vancouver scar scales at discharge and 6, 12 and 18 to 24 months after the burn. Photographs of the scars were evaluated using the Seattle and Hamilton Scar Scale. Clinically the most hypertrophic scars were assessed using the Vancouver Scar Scale. A higher score on these scar assessment scales indicates a worse outcome. The Seattle Scar Scale score was not significantly different in the rhGH group compared with the placebo group at discharge (MD ‐0.25; 95% CI ‐1.50 to 1.00), at six months (MD ‐0.05; 95% CI ‐1.55 to 01.45), nine months (MD ‐0.93; 95% CI ‐2.31 to 0.45), 12 months (MD ‐0.24; 95% CI ‐1.93 to 1.45) or 18 to 24 months after the burn (MD ‐0.78; 95% CI ‐2.91 to 1.35). The Hamilton Scar Scale was not significantly different in the rhGH group compared to the placebo group at discharge (MD ‐0.40; 95% CI ‐1.51 to 0.71) or at six months (MD ‐0.24; 95% CI ‐1.20 to 0.72), nine months (MD ‐0.50; 95% CI ‐2.05 to 1.05), 12 months (MD ‐0.50; 95% CI ‐2.09 to 1.09) or 18 to 24 months after burn (MD ‐0.83; 95% CI ‐2.51 to 0.85). The Vancouver Scar Scale was not significantly different in the rhGH group compared to the placebo group at discharge (MD ‐1.86; 95% CI ‐4.07 to 0.35), at six months (MD ‐1.51; 95% CI ‐4.04 to 1.02), at nine months (MD ‐1.60; 95% CI ‐5.56 to 2.36) or at 18 to 24 months after burn (MD ‐0.50; 95% CI ‐2.66 to 1.66); the Vancouver Scar Scale was significantly lower in the rhGH group compared with the placebo group at 12 months (MD ‐1.90; 95% CI ‐3.31 to ‐0.49).
One study, which provided no data, reported no significant difference in the Vancouver Scar Scale scores in the rhGH group compared with placebo after discharge or up to 24 months after the burn (Przkora 2006). Barret 1999 reported no difference between the burn scar rating scale scores of children who received a placebo and those who received rhGH during the acute phase of the hospital stay. These data could not be pooled because ranges should not be used to calculate standard deviations (Higgins 2011b).
Adverse events
Hyperglycaemia
Four adult studies (Luo 2000; Pelzer 2000; Losada 2002; Chen 2005; 300 participants, 72 events) and one child study (Herndon 1990; 40 participants, six events) reported the incidence of hyperglycaemia. In adults, the incidence was significantly higher in the rhGH group than in the placebo group (Analysis 1.6.1: RR 2.43; 95% CI 1.54 to 3.85; I² = 6%). In children whilst there was a difference in the incidence of hyperglycaemia (6 cases in the rHGH group and none in the placebo group) this difference was not statistically significant, probably due to lack of statistical power (Analysis 1.6.2: RR 10.74; 95% CI 0.65 to 178.65). Combining data for the adults and children yielded an incidence that was significantly higher in the rhGH group than in the placebo group (Analysis 1.6: RR 2.65; 95% CI 1.68 to 4.16; I² = 14%).
1.6. Analysis.
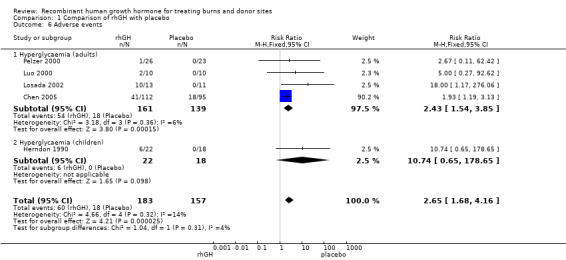
Comparison 1 Comparison of rhGH with placebo, Outcome 6 Adverse events.
Septicaemia
Four studies reported septicaemia data in adults and we pooled them (Sun 1998; Luo 2000; Losada 2002; Chen 2005). There was no statistically significant difference in the proportion of participants with septicaemia between rhGH and placebo (Analysis 1.7: RR 0.61; 95% CI 0.31 to 1.22; I² = 0%).
1.7. Analysis.
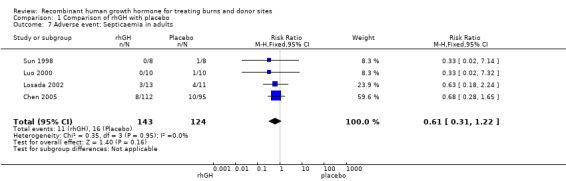
Comparison 1 Comparison of rhGH with placebo, Outcome 7 Adverse event: Septicaemia in adults.
Quality of life
No studies reported this outcome.
Comparison of rhGH with oxandrolone
One study compared rhGH with oxandrolone in adults (Demling 1999). There was no significant difference in the donor site healing time between the two treatment groups (Analysis 2.1: MD 0.00; 95% CI ‐1.64 to 1.64). All participants were followed until complete wound healing, therefore the time‐to‐event data are presented correctly. No participants were censored; therefore, the mean wound healing time is presented. The incidence of hyperglycaemia was significantly higher in the rhGH group than in the oxandrolone group (36 participants, 28 events; Analysis 2.2: RR 1.95; 95% CI 1.21 to 3.16).
2.1. Analysis.
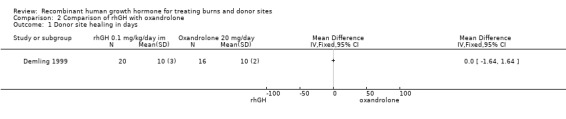
Comparison 2 Comparison of rhGH with oxandrolone, Outcome 1 Donor site healing in days.
2.2. Analysis.
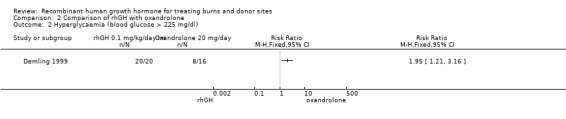
Comparison 2 Comparison of rhGH with oxandrolone, Outcome 2 Hyperglycaemia (blood glucose > 225 mg/dl).
Discussion
This systematic review summarises the best available evidence about the effects of recombinant human growth hormone (rhGH) for treating burns and donor sites. Thirteen randomised controlled trials met the inclusion criteria for this review. The mean total body surface area (TBSA) of the included participants was greater than 40%, and the mean full‐thickness (third‐degree) TBSA was greater than 20%. Therefore, all of the participants had serious burn injuries.
Our results indicate some preliminary evidence that burn wounds and donor sites in children and adults heal more rapidly when rhGH is administered and that length of hospital stay in adults is also shorter with rhGH compared to placebo. The statistically significant differences between rhGH and placebo administration are clinically meaningful and include a nine‐day reduction in the wound healing time in adults (Analysis 1.1) and a three‐day reduction in the donor site healing time in adults (Analysis 1.2) and a nearly two‐day reduction in the donor site healing time in children (Analysis 1.3). No evidence of a shorter hospital stay was reported when rhGH was administered to children. Whilst the differences in mortality between rhGH and placebo‐treated groups in adults and children were not statistically significant, mortality is a rare outcome and the number of included participants was small; therefore even after pooling there may not have been sufficient statistical power to detect a real difference in mortality as statistically significant. There was no evidence of a difference in septicaemia when rhGH and placebo groups in adults were compared. Three scar assessment scales showed no difference between children who received rhGH for a year after discharge and those who received placebo (de Oliveira 2004). Our results indicate that hyperglycaemia, as an adverse event, was more frequent after rhGH treatment than after placebo or oxandrolone therapy. Hyperglycaemia itself is associated with the hyper metabolic stress response and is related to increased mortality in severe burns (Gore 2001). The hyperglycaemia induced by rhGH is associated with insulin resistance (Gore 1991). However the effect of rhGH‐induced hyperglycaemia on hypermetabolism is unknown. There is evidence from one study (Demling 1999) that no difference exists in donor site healing, length of hospital stay or weight loss in groups of participants treated with rhGH or oxandrolone.
The summary of finding table, which was prepared with the software GRADEprofiler (GRADEpro), indicates clearly the low quality of the evidence for all the outcomes. No large effects were found in the studies. The risk of bias caused by imprecision was serious, because the studies included few participants and few events. This low quality of the evidence reduces the ability to make firm statements about what the evidence tells exactly about the treatment effects.
These results must therefore be interpreted with caution. The included studies often could not be pooled because they used different methods to measure outcomes. For instance, various studies measured wound healing time in adults in days, as a healing rate or with a wound closure index. Many of the studies included a small number of participants. No studies with wound healing time data in children could be found. We found no studies with wound infections as a primary outcome and no studies comparing rhGH and propranolol using the pre‐specified outcomes.
In general, the study quality was limited by failures to report randomisation techniques, allocation concealment and blinding. Eleven of the 13 studies included in this review did not mention the randomisation method, which may imply that several of these studies are not randomised controlled trials (RCTs), but controlled clinical trials. Blinding at outcome assessment is very important for outcome measures, such as the healing of burn wounds or donor sites, because it is difficult to determine when wound epithelisation is complete. Data, especially standard deviations, were sometimes missing, or outcomes were only presented in figures. The authors of the included studies could not (Barret 1999; Przkora 2006) or were not willing to (Pelzer 2000) provide missing data upon our request. The authors of this review cannot rule out selection bias and language bias when searching the Chinese literature. Due to the uncertainty about the amount of missing data, its impact on the findings of this review could not be estimated.
The costs of rhGH treatment are USD 300 to 600 per day, which could be counterbalanced by a reduction in hospital stay. However, no economic analyses to prove this were retrieved, and these costs are only a part of the overall expense of burn injury treatment. The high cost of this long‐term treatment could be a great disadvantage and hindrance to its wide application, especially in developing nations.
A review of metabolic support for burn injury that used some of the same literature included in this review came to the same conclusions about rhGH administration: it is associated with reduced wound healing time, reduced length of hospital stay and no increase in scarring, and increased hyperglycaemia as an adverse effect (Herndon 2004). A study by Ramirez 1998 analysed the records of 130 children who received rhGH in RCTs. These children were compared with 133 controls. No difference in mortality (2%) was found. Concern about the mortality related to rhGH administration arose after the publication of two RCTs that showed increased morbidity and mortality in non‐burned critically ill adults who were treated with rhGH (Takala 1999). The studies included in this review were not primarily designed to study the effect of rhGH on mortality and their sample sizes were small, therefore no definite conclusion can be drawn about the differences in mortality between rhGH‐ and placebo‐treated participants.
Authors' conclusions
Implications for practice.
There is some low quality evidence that the administration of recombinant human growth hormone (rhGH) results in quicker healing of burn wounds in adults and donor sites in adults and children and reduces hospital stay compared with placebo in people with large burns (>40% total body surface area). However use of rhGH appears to be associated with an increased risk of hyperglycaemia. The high costs of rhGH treatment during hospitalisation may be counterbalanced by the reduction in hospital stay, but no economic analyses have been published. The high cost of this long‐term treatment could be a great disadvantage and a hindrance to its wide application, especially in developing nations. There is some evidence that the effects of rhGH and oxandrolone are comparable, but with less hyperglycaemia, oxandrolone could be a promising alternative to rhGH.
Implications for research.
There is a need for large, well‐designed randomised controlled trials (RCTs) of the use of rhGH to treat large burns (TBSA of 40% or more) in both children and adults. Such RCTs should examine patient‐related outcomes and quality of life and include an economic analysis. To study mortality, the sample size should be large (> 500 participants). Such sample sizes can likely only be achieved with multicentre studies. The outcomes should be wound and donor site healing time (analysed using survival methods), length of hospital stay, mortality, hyperglycaemia, quality of life (for instance the Burns Specific Health Scale) and cost analysis. The intervention of rhGH injections should be compared with placebo and with oxandrolone as an intervention.
What's new
| Date | Event | Description |
|---|---|---|
| 9 September 2014 | New search has been performed | First update. |
| 9 September 2014 | New citation required but conclusions have not changed | New searches performed; no new trials identified; no change to conclusions. |
Acknowledgements
The authors would like to acknowledge the contribution of the peer referees who commented on this review: Wounds Group editors (Marian Brady, Dirk Ubbink, Ruth Foxlee), referees (Jane Burch, Richard Kirubakaran, Heather Cleland, Arturo Marti‐Carvajal, Amy Zelmer) and copy editor Jenny Bellorini.
Appendices
Appendix 1. Search methods used in the original version of this review 2012
For the original review we searched the following electronic databases to find reports of relevant RCTs:
Cochrane Wounds Group Specialised Register (searched 28 June 2012);
The Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library 2012, Issue 6);
Database of Abstracts of Reviews of Effects (DARE) (The Cochrane Library 2011, Issue 3);
Ovid MEDLINE (1950 to June Week 3 2012);
Ovid MEDLINE (In‐Process & Other Non‐Indexed Citations June 27, 2012);
Ovid EMBASE (1980 to 2012 Week 25);
EBSCO CINAHL (1982 to 21 June 2012)
PEDro, the Physiotherapy Evidence database (1980 to 7 February 2011);
National Research Register (NRR) Archive (https://portal.nihr.ac.uk/Pages/NRRArchive.aspx);
OAIster (http://oaister.umdl.umich.edu/o/oaister/) international institutional digital repository search engine;
Web of Science (1975 to 12 November 2010);
Dissertation abstracts (www.dissonline.de; www.theses.com; www.proquest.co.uk/products.pq/descriptions/pqdt.shtml), searched 14 January 2012;
U.S. Food and Drug Administration (www.fda.gov), searched 14 January 2012;
European Medicines Agency (www.ema.europa.eu), searched 14 January 2012.
We used the following strategy to search The Cochrane Central Register of Controlled Trials (CENTRAL):
#1 MeSH descriptor Burns explode all trees #2 ("burn" or "burns" or burned or scald*):ti,ab,kw #3 (thermal* NEXT injur*):ti,ab,kw #4 (#1 OR #2 OR #3) #5 MeSH descriptor Growth Hormone explode all trees #6 (growth NEXT hormone*):ti,ab,kw #7 rhGH:ti,ab,kw #8 somatotropin:ti,ab,kw #9 (#5 OR #6 OR #7 OR #8) #10 (#4 AND #9)
We combined the Ovid MEDLINE search with the Cochrane Highly Sensitive Search Strategy for identifying randomized trials in MEDLINE: sensitivity‐ and precision‐maximizing version (2008 revision) (Lefebvre 2011). The Ovid EMBASE and EBSCO CINAHL searches were combined with the trial filters developed by the Scottish Intercollegiate Guidelines Network (SIGN) (SIGN 2011). We did not restrict studies with respect to language, date of publication or study setting. We considered and included abstracts and letters if we were able to obtain complete manuscripts from the study author(s).
Appendix 2. Additional search strategies for the updated review
1 Ovid MEDLINE search strategy
1 exp Burns/ 2 (burn or burns or burned or scald*).tw. 3 (thermal adj injur*).tw. 4 or/1‐3 5 exp Growth Hormone/ 6 growth hormone*.tw. 7 rhGH.tw. 8 somatotropin.tw. 9 or/5‐8 10 4 and 9
2 Ovid EMBASE search strategy
1 exp burn/ 2 (burn or burns or burned or scald*).tw. 3 (thermal adj injur*).tw. 4 or/1‐3 5 exp growth hormone/ 6 growth hormone*.tw. 7 rhGH.tw. 8 somatotropin.tw. 9 or/5‐8 10 4 and 9
3 EBSCO CINAHL search strategy
S10S4 and S9 S9S5 or S6 or S7 or S8 S8TI rhGH or AB rhGH S7TI growth hormone* or AB growth hormone* S6TI somatotropin or AB somatotropin S5(MH "Somatotropin") S4S1 or S2 or S3 S3TI thermal* injur* or AB thermal* injur* S2TI ( burn or burns or burned or scald* ) or AB ( burn or burns or burned or scald* ) S1(MH "Burns")
Appendix 3. 'Risk of bias' criteria
1. Was the allocation sequence randomly generated?
Low risk of bias
The investigators describe a random component in the sequence generation process such as: referring to a random number table; using a computer random number generator; coin tossing; shuffling cards or envelopes; throwing dice; drawing of lots.
High risk of bias
The investigators describe a non‐random component in the sequence generation process. Usually, the description would involve some systematic, non‐random approach, for example: sequence generated by odd or even date of birth; sequence generated by some rule based on date (or day) of admission; sequence generated by some rule based on hospital or clinic record number.
Unclear
Insufficient information about the sequence generation process to permit judgement of Yes or No.
2. Was the treatment allocation adequately concealed?
Low risk of bias
Participants and investigators enrolling participants could not foresee assignment because one of the following, or an equivalent method, was used to conceal allocation: central allocation (including telephone, web‐based and pharmacy‐controlled randomisation); sequentially numbered drug containers of identical appearance; sequentially numbered, opaque, sealed envelopes.
High risk of bias
Participants or investigators enrolling participants could possibly foresee assignments and thus introduce selection bias, such as allocation based on: using an open random allocation schedule (e.g., a list of random numbers); assignment envelopes were used without appropriate safeguards (e.g., if envelopes were unsealed or nonopaque or not sequentially numbered); alternation or rotation; date of birth; case record number; any other explicitly unconcealed procedure.
Unclear
Insufficient information to permit judgement of Yes or No. This is usually the case if the method of concealment is not described or not described in sufficient detail to allow a definite judgement, for example if the use of assignment envelopes is described, but it remains unclear whether envelopes were sequentially numbered, opaque and sealed.
3. Blinding ‐ was knowledge of the allocated interventions adequately prevented during the study?
Low risk of bias
Any one of the following.
No blinding, but the review authors judge that the outcome and the outcome measurement are not likely to be influenced by lack of blinding.
Blinding of participants and key study personnel ensured, and unlikely that the blinding could have been broken.
Either participants or some key study personnel were not blinded, but outcome assessment was blinded and the non‐blinding of others unlikely to introduce bias.
High risk of bias
Any one of the following.
No blinding or incomplete blinding, and the outcome or outcome measurement is likely to be influenced by lack of blinding.
Blinding of key study participants and personnel attempted, but likely that the blinding could have been broken.
Either participants or some key study personnel were not blinded, and the non‐blinding of others likely to introduce bias.
Unclear
Any one of the following.
Insufficient information to permit judgement of Yes or No.
The study did not address this outcome.
4. Were incomplete outcome data adequately addressed?
Low risk of bias
Any one of the following.
No missing outcome data.
Reasons for missing outcome data unlikely to be related to true outcome (for survival data, censoring unlikely to be introducing bias).
Missing outcome data balanced in numbers across intervention groups, with similar reasons for missing data across groups.
For dichotomous outcome data, the proportion of missing outcomes compared with observed event risk not enough to have a clinically relevant impact on the intervention effect estimate.
For continuous outcome data, plausible effect size (difference in means or standardised difference in means) among missing outcomes not enough to have a clinically relevant impact on observed effect size.
Missing data have been imputed using appropriate methods.
High risk of bias
Any one of the following.
Reason for missing outcome data likely to be related to true outcome, with either imbalance in numbers or reasons for missing data across intervention groups.
For dichotomous outcome data, the proportion of missing outcomes compared with observed event risk enough to induce clinically relevant bias in intervention effect estimate.
For continuous outcome data, plausible effect size (difference in means or standardised difference in means) among missing outcomes enough to induce clinically relevant bias in observed effect size.
As‐treated analysis done with substantial departure of the intervention received from that assigned at randomisation.
Potentially inappropriate application of simple imputation.
Unclear
Any one of the following.
Insufficient reporting of attrition/exclusions to permit judgement of Yes or No (e.g., number randomised not stated, no reasons for missing data provided).
The study did not address this outcome.
5. Are reports of the study free of suggestion of selective outcome reporting?
Low risk of bias
Any of the following.
The study protocol is available and all of the studies pre‐specified (primary and secondary) outcomes that are of interest in the review have been reported in the pre‐specified way.
The study protocol is not available but it is clear that the published reports include all expected outcomes, including those that were pre‐specified (convincing text of this nature may be uncommon).
High risk of bias
Any one of the following.
Not all of the studies' pre‐specified primary outcomes have been reported.
One or more primary outcomes is reported using measurements, analysis methods or subsets of the data (e.g., subscales) that were not pre‐specified.
One or more reported primary outcomes were not pre‐specified (unless clear justification for their reporting is provided, such as an unexpected adverse effect).
One or more outcomes of interest in the review are reported incompletely so that they cannot be entered in a meta‐analysis.
The study report fails to include results for a key outcome that would be expected to have been reported for such a study.
Unclear
Insufficient information to permit judgement of Yes or No. It is likely that the majority of studies will fall into this category.
6. Other sources of potential bias
Low risk of bias
The study appears to be free of other sources of bias.
High risk of bias
There is at least one important risk of bias. For example, the study:
had a potential source of bias related to the specific study design used; or
stopped early due to some data‐dependent process (including a formal stopping rule); or
had extreme baseline imbalance; or
has been claimed to have been fraudulent; or
had some other problem.
Unclear
There may be a risk of bias, but there is either:
insufficient information to assess whether an important risk of bias exists; or
insufficient rationale or evidence that an identified problem will introduce bias.
Appendix 4. Glossary
a priori: in advance.
analogue (testosterone analogue): similar.
autologous: a transplantation in which the donor and recipient are the same person.
catabolism: the breakdown of substances in biochemical processes.
censored participants: people for whom the event of interest (for instance, complete wound healing or mortality) has not yet occurred.
clinical heterogeneity: differences in study designs; for instance, in the types of participants included or the implementation of interventions.
depot (forms of recombinant human growth hormone): a drug injected into the body for gradual release.
epithelialised: healed by becoming covered with epithelial cells (skin).
TBSA (total body surface area): an assessment of the burned area of the skin.
wasting (as used in this review rather than the more general meaning): to lose strength.
Data and analyses
Comparison 1. Comparison of rhGH with placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Healing time of burn wounds in days for adults | 2 | 36 | Mean Difference (IV, Fixed, 95% CI) | ‐9.07 [‐13.76, ‐4.39] |
| 2 Donor site healing time in days for adults | 2 | 36 | Mean Difference (IV, Fixed, 95% CI) | ‐3.15 [‐4.75, ‐1.54] |
| 3 Donor site healing time in days for children | 2 | 73 | Mean Difference (IV, Fixed, 95% CI) | ‐1.70 [‐2.53, ‐0.87] |
| 4 Length of hospital stay | 5 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 4.1 Adults | 4 | 99 | Mean Difference (IV, Fixed, 95% CI) | ‐12.55 [‐17.09, ‐8.00] |
| 4.2 Children | 1 | 28 | Mean Difference (IV, Fixed, 95% CI) | ‐7.0 [‐29.94, 15.94] |
| 5 Mortality | 5 | 324 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.53 [0.22, 1.29] |
| 5.1 Adults | 4 | 296 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.48 [0.19, 1.25] |
| 5.2 Children | 1 | 28 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.15 [0.08, 16.67] |
| 6 Adverse events | 5 | 340 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.65 [1.68, 4.16] |
| 6.1 Hyperglycaemia (adults) | 4 | 300 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.43 [1.54, 3.85] |
| 6.2 Hyperglycaemia (children) | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 10.74 [0.65, 178.65] |
| 7 Adverse event: Septicaemia in adults | 4 | 267 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.61 [0.31, 1.22] |
Comparison 2. Comparison of rhGH with oxandrolone.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Donor site healing in days | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2 Hyperglycaemia (blood glucose > 225 mg/dl) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Barret 1999.
| Methods | RCT, double‐blind | |
| Participants | 94 children (mean age 7.5 years) admitted to a US burn centre, ages between 1 and 18 years, n = 60 male. Burns > 40% TBSA + > 10% full‐thickness (third‐degree). Admitted within 3 days of injury. At least 1 donor site required. | |
| Interventions | rhGH 0.2 mg/kg/day (n = 45) or saline as a placebo (n = 49) administered by subcutaneous injection for the entire acute‐phase hospital stay (mean = 34.5 days, SD = 52.3) | |
| Outcomes | Burn Scar Rating Scale (Yeong 1997), % of people requiring reconstruction, number of plastic surgery operations in the first 2 years, time from injury to reconstructive operations in months. Burn scars were assessed by 3 experienced burn surgeons. | |
| Funding | Not reported | |
| Notes | Kappa interrater agreement was 0.78 for surface, 0.80 for border height, 0.72 for thickness, 0.81 for colour difference Only medians and ranges are given for Burn Scar Rating Scale categories, operations per patient and time from injury to reconstruction Ranges should not be used to estimate standard deviations (Higgins 2011b) The contacted authors could not provide additional data. Only data on percentage of people requiring reconstruction were included in this review. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Observers were blinded to treatment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Patient follow‐up was completed for 95% of the participants |
| Selective reporting (reporting bias) | Unclear risk | Unclear. Study protocol was not available. |
Chen 2005.
| Methods | RCT | |
| Participants | 219 adults enrolled in the study, from a burn centre in China. 12 people were lost; 207 people were analysed. Mean age: 36 years; 168 males, 39 females. Mean TBSA 61.5%; mean TBSA second‐degree burn 32%; mean TBSA third‐degree burn 19.6%. Included scalds, flame burns, chemical burns and electric burns. people with severe associated injuries were excluded. | |
| Interventions | rhGH 0.19 IU/kg/day (Gene Science®) (n = 112) or placebo saline (n = 95) were administered daily by subcutaneous injection morning or night beginning after a mean of 7.3 (SD = 2.8) days after injury and continuing for 10 to 16 days | |
| Outcomes | Mortality, hyperglycaemia (fasting blood‐glucose ≥ 10 mmol/L), septicaemia | |
| Funding | ||
| Notes | Article in Chinese. Informed consent for the study was obtained from participants or relatives. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Randomised blocks", no further details reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Method of blinding not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 219 adults were enrolled in the study, 12 participants were lost or rejected and 207 participants were analysed |
| Selective reporting (reporting bias) | Unclear risk | Unclear. Study protocol was not available. |
de Oliveira 2004.
| Methods | RCT, double‐blind. | |
| Participants | 62 children (mean age: 8.6 years) who were admitted to a US burn centre. Ages between 2 to 18 years, n = 37 male. The children had burns > 40% TBSA with second‐ or third‐degree facial burns. The participants were treated with autografts during the acute phase and pressure garments after discharge. | |
| Interventions | rhGH 0.05 mg/kg/day (n = 30) or placebo (n = 32) were administered by subcutaneous injection from the patient's discharge date until 1 year after the burn. 6 of the 30 participants received 0.1 mg/kg/day rhGH. | |
| Outcomes | Seattle Scar Scale (Yeong 1997), Hamilton Scar Scale (Crowe 1998) and Vancouver Scar Scale (Sullivan 1990; Baryza 1995) at 6, 9, 12 and 18 to 24 months post‐burn were administered by 3 observers. The Seattle and Hamilton Scar Scales were scored by evaluating photographs of faces and scars. The Vancouver Scar Scale was used for the clinical evaluation of participants. | |
| Funding | The rhGH was provided by Eli Lilly and Company | |
| Notes | Increased levels of IGF‐1 were found in the treatment group (for assessment of compliance) No mean scores and standard deviations were provided in the publication. Data were provided by the authors. The 3 scar assessment observers were blinded to the treatment. Informed consent for the study was obtained from the participants or relatives. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | Low risk | The 3 scar assessment observers were blinded to the treatment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing data for the Seattle, Hamilton and Vancouver Scar Scales |
| Selective reporting (reporting bias) | Unclear risk | Unclear. The study protocol was not available. |
Demling 1999.
| Methods | RCT, not blinded | |
| Participants | 36 adults admitted to a US burn centre in 1996 and 1997. Mean age: 48 years. Mean TBSA: 40%; mean full‐thickness: 29%; inhalation injury: 43%. | |
| Interventions | rhGH 0.1 mg/kg/day by intramuscular injection (n = 20) or 20 mg oral oxandrolone (n = 16) were administered once daily beginning between Days 7 and 10 post‐burn until the patient was ready for discharge to a rehabilitation centre | |
| Outcomes | Initial donor site healing time in days, as indicated by the atraumatic removal of the xeroform gauze. Net weight loss (kg) and nitrogen loss (g/day). | |
| Funding | ||
| Notes | The control group (n = 24) was not randomised (convenience sample), so the data of comparing rhGH with oxandrolone was included in the review, but the comparison with the control group was not. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | High risk | Not possible because the rhGH was administered parenterally and oxandrolone was administered orally |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing data for the healing time of donor sites and weight loss |
| Selective reporting (reporting bias) | Unclear risk | Unclear. The study protocol was not available. |
Gilpin 1994.
| Methods | RCT, double‐blind | |
| Participants | 46 children (mean age 7.8 years) admitted to a US burn centre. Ages were between 2 and 18 years; n = 33 male, n = 13 female. Flame or scald burns, > 40% TBSA + > 20% full‐thickness (third‐degree). Excision (except face and perineum) and grafting were completed within 48 hours of admission. Donor sites were harvested with electric dermatome and dressed with Scarlet Red®‐impregnated fine mesh gauze. | |
| Interventions | rhGH 0.2 mg/kg/day (n = 20) or placebo (n = 26) were administered by subcutaneous or intramuscular injection within 8 days of the burn on the morning of excision and until the burn wound was 95% closed or the initial donor site was healed | |
| Outcomes | The initial donor site's healing time in days, as indicated by the atraumatic removal of the Scarlet Red® gauze and assessed by 1 of 2 evaluators | |
| Funding | Supported by Genentech, Inc., San Francisco, California | |
| Notes | No standard deviations from the length of hospital stay in days. In this study, data from participants, who received rhGH for therapeutic reasons were also presented, but these data are not included in this review. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation method was not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Participants, physicians and nurses were blinded to the contents of the injection vials, which were provided by the manufacturer |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data for the healing time of donor sites |
| Selective reporting (reporting bias) | Low risk | The healing time of donor sites was reported for the 46 participants included in the RCT. Only one outcome measure was reported in this study. Study protocol was not available. |
Herndon 1990.
| Methods | RCT. Double‐blind, 2‐phase study. Randomisation was balanced for age, cause and extent of burn injury. | |
| Participants | 40 children (mean age: 8.6 years; range: 2 to 18 years) admitted to a US burn centre. Flame or scald burns, > 40% TBSA + > 20% full‐thickness (third‐degree). Excision (except face and perineum) and grafting occurred within 48 hours of admission. The donor sites were harvested with electric dermatome and dressed with Scarlet Red®‐impregnated fine mesh gauze. participants with severe associated injuries were excluded. | |
| Interventions | rhGH 0.1 mg/kg/day subcutaneous (n = 12, phase 1) or 0.2 mg/kg/day intramuscular (n = 10, phase 2) or placebo saline (total placebo n = 18; Phase 1: n = 12; Phase 2: n = 6) was administered by injection beginning at admission and continued throughout the hospitalisation period | |
| Outcomes | Healing time in days of the initial donor site as indicated by atraumatic removal of the Scarlet Red® gauze. Healing time for harvest 1, 2 and 3. Length of hospital stay per % TBSA burn. Hyperglycaemia, defined as elevated glucose levels necessitating exogenous insulin. Healing time of donor sites for harvest 1 reported for the 10 participants from phase 2 receiving 0.2 mg/kg/day rhGH and for the 17 participants of the combined placebo group used for this review. Length of hospital stay in days per % TBSA. | |
| Funding | Supported by Genentech, Inc., San Francisco, California | |
| Notes | This was a 2‐phase study. The placebo participants from the 2 phases were pooled and those data could not be split. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Random numbers chart" |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Participants, physicians and nurses were blinded to the contents of the injection vials, which were numbered by the drug company. Laboratory studies were conducted by independent laboratories to ensure that blinding was maintained. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 1 patient from the control group had missing outcome data for healing time of donor sites for study Phase 2. No participants were missing data for length of hospital stay. |
| Selective reporting (reporting bias) | High risk | Length of hospital stay was not a pre‐specified outcome. No study protocol was available. |
Jeschke 2000.
| Methods | RCT, double‐blind | |
| Participants | 28 children (mean age: 5.4 years; range: 1 to 16 years) admitted to a US burn centre; 17 males. > 40% TBSA + > 10% full‐thickness (third‐degree). Mean TBSA: 60%; mean third‐degree burn area: 50%. | |
| Interventions | rhGH 0.2 mg/kg/day (n = 13) or placebo (saline, n = 15) by subcutaneous injection within 3 days after injury and for at least 25 days afterward. Mean length of rhGH therapy: 34 days. | |
| Outcomes | Mortality. Acute phase proteins, constitutive hepatic proteins, cytokines and IGF‐1. | |
| Funding | ||
| Notes | Additional data were provided by the authors. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Method of blinding not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing data for mortality |
| Selective reporting (reporting bias) | Low risk | All outcome data were presented in a pre‐specified way. Only data for the outcome mortality were used for meta‐analysis. The study protocol was not available. |
Losada 2002.
| Methods | RCT, double‐blind | |
| Participants | 24 adults (mean age: 36.7 years; range: 18 to 65 years) admitted to a burn centre in Spain; 19 males. Flame or scald burns; > 40% TBSA and > 15% full‐thickness (third‐degree). Escharectomy and first grafting took place after a mean of 16.2 days. The donor sites were harvested with electric dermatome and dressed with hydrocolloid cellulose. participants with multiple traumas were excluded. | |
| Interventions | rhGH 0.15 mg/kg/day (Humatrope©) (n = 13) or placebo (n = 11) were administered by intramuscular injection in 2 equal doses beginning on the day of the first autograft and continuing until hospital discharge | |
| Outcomes | Donor site healing time. The donor site was classified as healed when it was adequate for re‐harvesting as a new autograft donor site. Mean number of skin grafts per patient Time admitted to the burn unit in absolute days or in relation to % TBSA or % full‐thickness |
|
| Funding | The study was supported by Lilly, S.A. | |
| Notes | The donor site area was evaluated daily by the same person Adverse effect: 1 patient had hyperglycaemia that required insulin therapy. Informed consent was obtained for the study from the patient or relatives. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Method of blinding not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data were missing for 1 control group patient |
| Selective reporting (reporting bias) | High risk | Mortality and septicaemia were not pre‐specified outcomes. The study protocol was not available. |
Lu 2004.
| Methods | RCT | |
| Participants | 60 adults, mean age 38.6 years, admitted in burn centre in China from March 2000 to June 2003. TBSA 30% to 80%; mean TBSA: 61.5%; > 20% third‐degree; mean third‐degree burn area: 33.1%. No participants had severe inhalation injury. Deep burns were treated with escharotomy and skin grafting. | |
| Interventions | 3 groups of n = 20 each. Control group: glycine orally as placebo (0.5 g/kg/day); glutamine + rhGH group: glutamine orally (0.5 g/kg/day) with 0.2 IU/kg/day rhGH by daily subcutaneous injection; glutamine group: only glutamine orally (0.5 g/kg/day). Treatment was administered from the 1st to 14th post‐burn day. | |
| Outcomes | Wound healing rate in % on 30th post‐burn day. Wound healing rate was not defined. Total hospital stay in days. | |
| Funding | ||
| Notes | Article in Chinese. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | The blinding method was not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No data were missing for wound healing rate and length of hospital stay |
| Selective reporting (reporting bias) | Low risk | All presented outcome data were pre‐specified, but the study protocol was not available |
Luo 2000.
| Methods | RCT | |
| Participants | 20 adults, (15 males, 5 females) admitted to a burn centre in China from July 1998 to July 1999. Mean age: 30.5 years; intervention group mean age: 32 years (SD = 4); control group mean age: 29 years (SD = 6). Mean 61% TBSA, mean 27% third‐degree TBSA. All participants underwent eschar excision < 4 days and skin autografting. | |
| Interventions | rhGH 0.5 IU/kg/day subcutaneously (n = 10) or normal saline subcutaneously from the 3rd to 17th post‐burn day | |
| Outcomes | Healing time of deep partial‐thickness burns and donor sites in days. Wound healing rate was not defined. Length of hospital stay in days. Hyperglycaemia (blood sugar > 12 mmol/L for 3 consecutive days). Mortality was zero in both groups, therefore these data with no events were not used in this meta‐analysis. | |
| Funding | ||
| Notes | Article in Chinese | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing data for the healing times of burn wounds and donor sites and duration of hospital stay |
| Selective reporting (reporting bias) | Low risk | All presented outcome data were pre‐specified, but the study protocol was not available |
Pelzer 2000.
| Methods | RCT, double‐blind | |
| Participants | 49 adults (mean age: 38.3 years; range: 18 to 60 years; 44 males) admitted to a burn centre in Germany, between 1995 and 1997. Abbreviated Burn Severity Index 7 to 11; > 20% TBSA. Early excision and autografting with mesh were performed. Donor sites were harvested with electric dermatome and dressed with Scarlet Red® ‐impregnated fine mesh gauze. participants with multiple injuries were excluded. | |
| Interventions | rhGH 0.5 IU/kg/day (Genotropin©; n = 26) or placebo, water with m‐cresol (n = 23), were administered by intramuscular injection in 2 equal doses beginning on the second day after trauma and continuing for 28 days | |
| Outcomes | The Wound Closure Index (WCI; Scott‐Conner 1986a) of the transplanted and un operated wounds. Wound healing was defined as complete epithelisation. Donor site healing time in days, as indicated by the atraumatic removal of the Scarlet Red® gauze. Wound healing was measured daily clinically and with photographs by the same person, who was blinded to treatment. | |
| Funding | Not reported | |
| Notes | 1 patient in the rhGH group was withdrawn because of hyperglycaemia; 4 in the rhGH group and 3 in the placebo group died (1 additional patient in the placebo group died 1 day after the study stopped). 4 participants withdrew their permission (2 in each group). 19 participants in the rhGH group and 18 in the placebo group were used for analysis. A WCI of 1 means a healing rate of 1% per day As‐treated or per‐protocol analyses were performed by the authors of the study No standard deviations of WCI and of healing time of donor sites in days were given, so this study was not used for the analysis of the healing time. Informed consent for the study was obtained from patient or relatives. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A randomisation list was provided by pharmaceutical firm |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | Low risk | The contents of the injection vials were masked. Wound healing was measured clinically and with photographs daily by the same person who was blinded to the treatment. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 7 participants were withdrawn in the treatment group and 5 were withdrawn in the placebo group. 1 patient in the rhGH group was withdrawn because of hyperglycaemia; 4 in the rhGH group and 3 in the placebo group died (1 additional patient in the placebo group died 1 day after the study stopped). 4 participants withdrew their permission (2 in each group). 19 participants in the rhGH group and 18 in the placebo group were used for analysis. |
| Selective reporting (reporting bias) | Low risk | All of the presented outcome data were pre‐specified, but the study protocol was not available |
Przkora 2006.
| Methods | RCT, double‐blind | |
| Participants | 44 children aged 19 or younger (mean age: 8 years; 30 males) were enrolled study between 1999 and 2004 for an additional year of examinations after 1 year of rhGH treatment post‐discharge from a US burn centre. TBSA > 40%; mean 56% TBSA; mean 47% third‐degree burns. The children were randomised upon discharge. | |
| Interventions | rhGH 0.05 mg/kg/day (n = 19) or placebo (n = 25) by subcutaneous injection from discharge date until 1 year after burn. The injections started on the day of discharge (equal to the time point at which wounds were 95% healed). After another year without rhGH treatment, 14 participants in the rhGH group and 18 in the placebo group completed the study. | |
| Outcomes | Outcomes were estimated one day before discharge and after 12 and 24 months post‐burn. Need for reconstructive operations. Hyperglycaemia. Lean body mass estimated with dual‐energy X‐ray absorptiometry (DEXA). Resting energy expenditure (REE). Echocardiography. Isokinetic strength measurement with Biodex® dynamometer for the dominant leg extensor at 150º/sec. Vancouver Scar Scale. The hyperglycaemia incidence was zero in both groups; therefore, these data with no events were not used in this meta‐analysis. | |
| Funding | rhGH was provided as a gift from Eli Lilly Corporation | |
| Notes | Compliance was measured with the Self‐Reported Compliance Questionnaire and with serum levels of insulin‐like growth factor‐1 (IGF‐1). Greater than 75% compliance with the daily study drug was necessary to remain in study. Scar assessment was performed by observers who were blinded to the treatment. Authors information: the data for left ventricular function are the not same as those from Mlcak 2005 Additional data were provided by the authors. No data from the scar assessment were available. Informed consent for the study was obtained from the patient or relatives. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 44 children were enrolled in the study; reconstructive procedure data were available for 32 participants. 5 participants in the treatment group and 7 participants in the control group did not complete the study. |
| Selective reporting (reporting bias) | Low risk | All presented outcome data were pre‐specified, but the study protocol was not available |
Sun 1998.
| Methods | Placebo‐controlled prospective study | |
| Participants | 16 adults (age range: 19 to 50 years) admitted to a burn centre in China between February 1996 and June 1997. Mean 81% TBSA; mean 61% third‐degree TBSA. All participants underwent eschar excision < 4 days and autografting with skin pulp. participants with associated injuries were excluded. | |
| Interventions | rhGH 0.3 IU/kg/day subcutaneously (n = 8) or 2 cc normal saline (n = 8) for 10 days, starting on the first postoperative day | |
| Outcomes | Grafted burn wound area and donor site healing time. Healing time was not defined. Wound healing rate at the 30th postoperative day. Duration of hospitalisation. Serum amino acid profile on Days 1 and 20 post‐burn. The hyperglycaemia incidence was zero in both groups; therefore, these data with no events were not used in this meta‐analysis. | |
| Funding | ||
| Notes | Article in Chinese | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing data for the healing time of burn wounds or donor sites and the duration of hospital stay |
| Selective reporting (reporting bias) | High risk | Mortality and septicaemia were not pre‐specified outcomes. No study protocol was available. |
IGF‐1: insulin‐like growth factor‐1; IU: international units; RCT: randomised controlled trial; rhGH: recombinant human growth hormone; SD: standard deviation; TBSA: total body surface area
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Aarsland 1996 | The excluded study addressed none of the pre‐specified outcome measures |
| Branski 2009 | The excluded study addressed none of the pre‐specified outcome measures |
| Chai 2002a | The excluded study addressed none of the pre‐specified outcome measures |
| Chai 2002b | The excluded study addressed none of the pre‐specified outcome measures |
| Chrysopoulo 1999 | The excluded study addressed none of the pre‐specified outcome measures |
| Hart 2001 | This study addressed none of the pre‐specified outcome measures except hyperglycaemia. The hyperglycaemia incidence was zero in the rhGH and placebo groups; therefore, these data with no events cannot be used in this meta‐analysis. |
| Hart 2002 | The excluded study addressed none of the pre‐specified outcome measures |
| Herndon 1995 | The study was not a RCT. Participants were not randomised. |
| Jeschke 2008 | The excluded study addressed none of the pre‐specified outcome measures |
| Klein 1998 | The excluded study addressed none of the pre‐specified outcome measures |
| Low 1999 | The excluded study did not address a pre‐specified outcome measure |
| Mlcak 2005 | The excluded study addressed none of the pre‐specified outcome measures |
| Suman 2003 | The excluded study addressed none of the pre‐specified outcome measures |
| Suman 2004 | The excluded study addressed none of the pre‐specified outcome measures |
RCT: randomised controlled trial
Contributions of authors
Roelf S. Breederveld: conceived the review question, developed the review, completed the first draft, made an intellectual contribution to the review and approved the final version prior to submission. Wim E. Tuinebreijer: developed the review, co‐ordinated the review development, completed the first draft of the review, made an intellectual contribution to the review and approved the final version prior to submission.
Contributions of the editorial base:
Nicky Cullum edited the review; advised on the methodology, interpretation and content; and approved the final review prior to submission. Sally Bell‐Syer co‐ordinated the editorial process; advised on the methodology, interpretation and content; and edited the review and the updated review. Amanda Briant ran some of the searches.
Sources of support
Internal sources
-
Red Cross Hospital, Beverwijk, Netherlands.
Use of library and internet facilities.
External sources
The National Institute for Health Research (NIHR) is the sole funder of the Cochrane Wounds Group, UK.
Declarations of interest
There are no known conflicts of interests.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Barret 1999 {published data only}
- Barret JP, Dziewulski P, Jeschke MG, Wolf SE, Herndon DN. Effects of recombinant human growth hormone on the development of burn scarring. Plastic and Reconstructive Surgery 1999;104(3):726‐9. [DOI] [PubMed] [Google Scholar]
Chen 2005 {published data only}
- Ghen GX, Han CM. Influence of recombinant human growth hormone on the prognosis of patients with severe burns a prospective multi‐center clinical trial. Zhonghua Shao Shang Za Zhi [Chinese Journal of Burns] 2005;21(5):347‐9. [PubMed] [Google Scholar]
Demling 1999 {published data only}
- Demling RH. Comparison of the anabolic effects and complications of human growth hormone and the testosterone analog, oxandrolone, after severe burn injury. Burns 199;25(3):215‐21. [DOI] [PubMed] [Google Scholar]
de Oliveira 2004 {published data only}
- Oliveira GV, Sanford AP, Murphy KD, Oliveira HM, Wilkins JP, Wu X, et al. Growth hormone effects on hypertrophic scar formation: a randomized controlled trial of 62 burned children. Wound Repair and Regeneration 2004;12(4):404‐11. [DOI] [PubMed] [Google Scholar]
Gilpin 1994 {published data only}
- Gilpin DA, Barrow RE, Rutan RL, Boemeling L, Herndon DN. Recombinant human growth hormone accelerates wound healing in children with large cutaneous burns. Annals of Surgery 1994;220(1):19‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Herndon 1990 {published data only}
- Herndon DN, Barrow RE, Kunkel KR, Broemeling L, Rutan RL. Effects of recombinant human growth hormone on donor‐site healing in severely burned children. Annals of Surgery 1990;212(4):424‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jeschke 2000 {published data only}
- Jeschke MG, Barrow RE, Herndon DN. Recombinant human growth hormone treatment in pediatric burn patients and its role during the hepatic acute phase response. Critical Care Medicine 2000;28(5):1578‐84. [DOI] [PubMed] [Google Scholar]
Losada 2002 {published data only}
- Losada F, García‐Luna PP, Gómez‐Cia T, Garrido M, Pereira JL, Marín F, et al. Effects of human recombinant growth hormone on donor‐site healing in burned adults. World Journal of Surgery 2002;26(1):2‐8. [DOI] [PubMed] [Google Scholar]
Lu 2004 {published data only}
- Lu CJ, Lin C, Xu JJ, Zhang P, Cao GZ, Hong BS. The influence of combined supplementation of glutamine and recombinant human growth hormone on the protein metabolism in severely burned patients. Chinese Journal of Burns 2004;20(4):220‐2. [PubMed] [Google Scholar]
Luo 2000 {published data only}
- Luo X, Cen Y, Yu R, Zhao J. Effectiveness of recombinant human growth hormone treatment for severe burn injury. Journal of West China University of Medical Sciences 2000;31(3):399‐401. [PubMed] [Google Scholar]
Pelzer 2000 {published data only}
- Pelzer M, Hartmann B, Blome‐Eberwein S, Raff T, Germann G. Effect of recombinant growth hormone on wound healing in severely burned adults. A placebo controlled, randomized double‐blind phase II study. Der Chirurg 2000;71(11):1352‐8. [DOI] [PubMed] [Google Scholar]
Przkora 2006 {published data only}
- Przkora R, Herndon DN, Suman OE, Jeschke MG, Meyer WJ, Chinkes DL, et al. Beneficial effects of extended growth hormone treatment after hospital discharge in pediatric burn patients. Annals of Surgery 2006;243(6):796‐801. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sun 1998 {published data only}
- Sun Y, Zhou Y, Jiang Z. The effect of growth hormone on wound healing rate in adult burns. Chinese Journal of Plastic Surgery and Burns 1998;14(4):277‐80. [PubMed] [Google Scholar]
References to studies excluded from this review
Aarsland 1996 {published data only}
- Aarsland A, Chinkes D, Wolfe RR, Barrow RE, Nelson SO, Pierrre E, et al. Beta‐blockade lowers peripheral lipolysis in burn patients receiving growth hormone. Rate of hepatic very low density lipoprotein triglyceride secretion remains unchanged. Annals of Surgery 1996;223(6):777‐89. [DOI] [PMC free article] [PubMed] [Google Scholar]
Branski 2009 {published data only}
- Branski LK, Herndon DN, Barrow RE, Kulp GA, Klein GL, Suman OE, et al. Randomized controlled trial to determine the efficacy of long‐term growth hormone treatment in severely burned children. Annals of Surgery 2009;250(4):514‐23. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chai 2002a {published data only}
- Chai J, Hao D, Wu Y, Shen C, Guo Z, Sheng Z. Severely burned patients after surgery: recombinant human growth hormone therapy its metabolic effects. Zhonghua Wai Ke Za Zhi [Chinese Journal of Burns] 2002;40(2):107‐11. [PubMed] [Google Scholar]
Chai 2002b {published data only}
- Chai J, Hao D, Wu Y, Shen C, Sheng Z. The effects of recombinant human growth hormone on the metabolism of branch chain amino acid in severely burned patients. Zhonghua Shao Shang Za Zhi [Chinese Journal of Burns] 2002;18(4):229‐31. [PubMed] [Google Scholar]
Chrysopoulo 1999 {published data only}
- Chrysopoulo MT, Jeschke MG, Ramirez RJ, Barrow RE, Herndon DN. Growth hormone attenuates tumor necrosis factor alpha in burned children. Archives of Surgery 1999;134(3):283‐6. [DOI] [PubMed] [Google Scholar]
Hart 2001 {published data only}
- Hart DW, Herndon DN, Klein G, Lee SB, Celis M, Mohan S, et al. Attenuation of posttraumatic muscle catabolism and osteopenia by long‐term growth hormone therapy. Annals of Surgery 2001;233(6):827‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hart 2002 {published data only}
- Hart DW, Wolf SE, Chinkes DL, Lai SO, Ramzy PI, Herndon DN. ß‐blockade and growth hormone after burn. Annals of Surgery 2002;236(4):450‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Herndon 1995 {published data only}
- Herndon DN, Hawkins HK, Nguyen TT, Pierre E, Cox R, Barrow RE. Characterization of growth hormone enhanced donor site healing in patients with large cutaneous burns. Annals of Surgery 1995;221(6):649‐59. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jeschke 2008 {published data only}
- Jeschke MG, Finnerty CC, Kulp GA, Przkora R, Mlcak R, Herndon DN. Combination of recombinant human growth hormone and propranolol decreases hypermetabolism and inflammation in severely burned children. Pediatric Critical Care Medicine 2008;9(2):209‐16. [DOI] [PubMed] [Google Scholar]
Klein 1998 {published data only}
- Klein GL, Wolf SE, Langman CB, Rosen CJ, Mohan S, Keenan BS, et al. Effects of therapy with recombinant human growth hormone on insulin‐like growth factor system components and serum levels of biochemical markers of bone formation in children after severe burn injury. Journal of Clinical Endocrinology and Metabolism 1998;83(1):21‐4. [DOI] [PubMed] [Google Scholar]
Low 1999 {published data only}
- Low JF, Herndon DN, Barrow RE. Effect of growth hormone on growth delay in burned children: a 3‐year follow‐up study. Lancet 1999;354(9192):1789. [DOI] [PubMed] [Google Scholar]
Mlcak 2005 {published data only}
- Mlcak RP, Suman OE, Murphy K, Herndon DN. Effects of growth hormone on anthropometric measurements and cardiac function in children with thermal injury. Burns 2005;31(1):60‐6. [DOI] [PubMed] [Google Scholar]
Suman 2003 {published data only}
- Suman OE, Thomas SJ, Wilkins JP, Mlcak RP, Herndon DN. Effect of exogenous growth hormone and exercise on lean mass and muscle function in children with burns. Journal of Applied Physiology 2003;94(6):2273‐81. [DOI] [PubMed] [Google Scholar]
Suman 2004 {published data only}
- Suman OE, Mlcak RP, Herndon DN. Effects of exogenous growth hormone on resting pulmonary function in children with thermal injury. Journal of Burn Care and Rehabilitation 2004;25(3):287‐93. [DOI] [PubMed] [Google Scholar]
Additional references
Aili 2001
- Aili Low JF, Barrow RE, Mittendorfer B, Jeschke MG, Chinkes DL, Herndon DN. The effect of short‐term growth hormone treatment on growth and energy expenditure in burned children. Burns 2001;27(5):447‐52. [DOI] [PubMed] [Google Scholar]
Baryza 1995
- Baryza MJ, Baryza GA. The Vancouver Scar Scale: an administration tool and its interrater reliability. Journal of Burn Care and Rehabilitation 1995;16(5):535‐8. [DOI] [PubMed] [Google Scholar]
Blades 1982
- Blades B, Mellis N, Munster A. A burn specific health scale. Journal of Trauma 1982;22(10):872‐5. [DOI] [PubMed] [Google Scholar]
Bryant 2007
- Bryant J, Baxter L, Cave CB, Milne R. Recombinant growth hormone for idiopathic short stature in children and adolescents. Cochrane Database of Systematic Reviews 2007, Issue 3. [DOI: 10.1002/14651858.CD004440.pub2] [DOI] [PubMed] [Google Scholar]
Crowe 1998
- Crowe JM, Simpson K, Johnson W, Allen J. Reliability of photographic analysis in determining change in scar appearance. Journal of Burn Care and Rehabilitation 1998;19(2):183‐6. [DOI] [PubMed] [Google Scholar]
Deeks 2011
- Deeks JJ, Higgins JPT, Altman DG (editors). Chapter 9: Analysing data and undertaking meta‐analyses. Higgins JPT, Green S, (editors). Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0.[updated March 2011] The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Demling 2000
- Demling RH, Seigne P. Metabolic management of patients with severe burns. World Journal of Surgery 2000;24(6):673‐80. [DOI] [PubMed] [Google Scholar]
Draaijers 2004
- Draaijers LJ, Tempelman FR, Botman YA, Tuinebreijer WE, Middelkoop E, Kreis RW, et al. The patient and observer scar assessment scale: a reliable and feasible tool for scar evaluation. Plastic and Reconstructive Surgery 2004;113(7):1960‐5. [DOI] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ 1997;315(7109):629‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gore 1991
- Gore DC, Honeycutt D, Jahoor F, Rutan T, Wolfe RR, Herndon DN. Effect of exogenous growth hormone on glucose utilization in burn patients. Journal of Surgical Research 1991;51(6):518‐23. [DOI] [PubMed] [Google Scholar]
Gore 2001
- Gore DC, Chinkes D, Heggers J, Herndon DN, Wolf SE, Desai M. Association of hyperglycaemia wit increased mortality after severe burn injury. Journal of Trauma 2001;51(3):540‐4. [DOI] [PubMed] [Google Scholar]
Hart 2000
- Hart DW, Wolf SE, Ghinkes DL, Gore DC, Mlcak RP, Beauford RB, et al. Determinants of skeletal muscle catabolism after severe burns. Annals of Surgery 2000;232(4):455‐65. [DOI] [PMC free article] [PubMed] [Google Scholar]
Herndon 2004
- Herndon DN, Tompkins RG. Support of the metabolic response to burn injury. Lancet 2004;363(9424):1895‐902. [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327:557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011a
- Higgins JPT, Altman DG, Sterne JAC (editors). Chapter 8: Assessing risk of bias in included studies. Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org. [Google Scholar]
Higgins 2011b
- Higgins JPT, Deeks JJ (editors). Chapter 7: Selecting studies and collecting data. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011] The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Jeschke 1999a
- Jeschke MG, Herndon DN, Wolf SE, DebRoy MA, Rai J, Lichtenbelt BJ, et al. Recombinant human growth hormone alters acute phase reactant proteins, cytokine expression, and liver morphology in burned rats. Journal of Surgical Research 1999;83(2):122‐9. [DOI] [PubMed] [Google Scholar]
Jeschke 1999b
- Jeschke MG, Chrysopoulo MT, Herndon DN, Wolf SE. Increased expression of insulin‐like growth factor‐I in serum and liver after recombinant human growth hormone administration in thermally injured rats. Journal of Surgical Research 1999;85(1):171‐7. [DOI] [PubMed] [Google Scholar]
Krysiak 2007
- Krysiak R, Gdula‐Dymek A, Bednarska‐Czerwinska A, Okopien B. Growth hormone therapy in children and adults. Pharmacological Reports 2007;59(5):500‐16. [PubMed] [Google Scholar]
Lefebvre 2011
- Lefebvre C, Manheimer E, Glanville J (editors). Chapter 6: Searching for studies.. In: Higgins JPT, Green S (editors), Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org. [Google Scholar]
Mock 2008
- Mock C, Peck M, Peden M, Krug E (editors). A WHO plan for burn prevention and care. Geneva: World Health Organization, 2008. [Google Scholar]
Nedelec 2000
- Nedelec B, Shankowsky HA, Tredget EE. Rating the resolving hypertrophic scar: comparison of the Vancouver Scar Scale and scar volume. Journal of Burn Care and Rehabilitation 2000;21(3):205‐12. [DOI] [PubMed] [Google Scholar]
Pereira 2005
- Pereira CT, Murphy KD, Herndon DN. Altering metabolism. Journal of Burn Care and Rehabilitation 2005;26(3):194‐9. [PubMed] [Google Scholar]
Przkora 2005
- Przkora R, Jeschke MG, Barrow RE, Suman OE, Meyer WJ, Finnerty CC, et al. Metabolic and hormonal changes of severely burned children receiving long‐term oxandrolone treatment. Annals of Surgery 2005;242(3):384‐9, discussion. [DOI] [PMC free article] [PubMed] [Google Scholar]
Przkora 2006a
- Przkora R, Barrow RE, Jeschke MG, Suman OE, Celis M, Sanford AP, et al. Body composition changes with time in pediatric burn patients. Journal of Trauma 2006;60(5):968‐71. [DOI] [PubMed] [Google Scholar]
Ramirez 1998
- Ramirez RJ, Wolf SE, Barrow RE, Herndon DN. Growth hormone treatment in pediatric burns: a safe therapeutic approach. Annals of Surgery 1998;228(4):439‐48. [DOI] [PMC free article] [PubMed] [Google Scholar]
RevMan 2011 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.1. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2011.
Scott‐Conner 1986a
- Scott‐Conner CE, Coil JA Jr, Conner HF, Mack ME. Wound Closure Index: a guide to prognosis in burned patients. Journal of Trauma 1986;26(2):123‐7. [DOI] [PubMed] [Google Scholar]
SIGN 2011
- Scottish Intercollegiate Guidelines Network (SIGN). Search filters. www.sign.ac.uk/methodology/filters.html#random 2011.
Singh 1998
- Singh KP, Prasad R, Chari PS, Dash RJ. Effect of growth hormone therapy in burn patients on conservative treatment. Burns 1998;24(8):733‐8. [DOI] [PubMed] [Google Scholar]
Sullivan 1990
- Sullivan T, Smith J, Kermode J, McIver E, Courtemanche DJ. Rating the burn scar. Journal of Burn Care and Rehabilitation 1990;11(3):256‐60. [DOI] [PubMed] [Google Scholar]
Takala 1999
- Takala J, Ruokonen E, Webster NR, Nielsen MS, Zandstra DF, Vundelinckx G, et al. Increased mortality associated with growth hormone treatment in critically ill adults. New England Journal of Medicine 1999;341(11):785‐92. [DOI] [PubMed] [Google Scholar]
Van Loon 1998
- Loon K. Safety of high doses of recombinant human growth hormone. Hormone Research 1998;49(Suppl 2):78‐81. [PubMed] [Google Scholar]
Vercelli 2009
- Vercelli S, Ferriero G, Sartorio F, Stissi V, Franchignoni F. How to assess postsurgical scars: a review of outcome measures. Disability and Rehabilitation 2009;31(25):2055‐63. [DOI] [PubMed] [Google Scholar]
Yeong 1997
- Yeong EK, Mann R, Engrav LH, Goldberg M, Cain V, Costa B, et al. Improved burn scar assessment with use of a new scar‐rating scale. Journal of Burn Care and Rehabilitation 1997;18(4):353‐5. [DOI] [PubMed] [Google Scholar]


