Highlights
-
•
Multiple canine respiratory pathogens were differentiated simultaneously.
-
•
Wild type and vaccine strains of canine distemper virus were distinguished.
-
•
Results were read with naked eye and no reader equipment was needed.
-
•
High sensitivity, specificity and efficiency.
-
•
Low money and time costs.
Keywords: Bordetella bronchiseptica, Canine distemper virus, Canine herpesvirus, Influenza virus, Mycoplasma cynos, Oligonucleotide microarray
Abstract
Canine respiratory diseases are commonly seen in dogs along with co-infections with multiple respiratory pathogens, including viruses and bacteria. Virus infections in even vaccinated dogs were also reported. The clinical signs caused by different respiratory etiological agents are similar, which makes differential diagnosis imperative. An oligonucleotide microarray system was developed in this study. The wild type and vaccine strains of canine distemper virus (CDV), influenza virus, canine herpesvirus (CHV), Bordetella bronchiseptica and Mycoplasma cynos were detected and differentiated simultaneously on a microarray chip. The detection limit is 10, 10, 100, 50 and 50 copy numbers for CDV, influenza virus, CHV, B. bronchiseptica and M. cynos, respectively. The clinical test results of nasal swab samples showed that the microarray had remarkably better efficacy than the multiplex PCR-agarose gel method. The positive detection rate of microarray and agarose gel was 59.0% (n = 33) and 41.1% (n = 23) among the 56 samples, respectively. CDV vaccine strain and pathogen co-infections were further demonstrated by the microarray but not by the multiplex PCR-agarose gel. The oligonucleotide microarray provides a highly efficient diagnosis alternative that could be applied to clinical usage, greatly assisting in disease therapy and control.
1. Introduction
Respiratory infection is a common problem in dogs (Mochizuki et al., 2008). The etiological agents involved in canine infectious respiratory disease are complex. Viral infectious agents include canine distemper virus (CDV), influenza virus, canine herpesvirus (CHV), canine adenovirus 2 (CAV-2), canine parainfluenza virus (CPIV), canine respiratory coronavirus (CRCoV) (Jeoung et al., 2013). Bordetella bronchiseptica and Mycoplasma cynos are the most significant bacterial etiologic agents (Mochizuki et al., 2008, Priestnall et al., 2014). Canine distemper (CD) is a highly contagious and fatal disease in dogs caused by CDV. CDV belongs to the Paramyxoviridae family. Although live attenuated vaccines have been used for many years to control distemper, CDV continues to cause outbreaks, particularly in young dogs (Demeter et al., 2010). Previous studies reported that vaccinated dogs were infected with CDV in Mexico and Japan (Simon-Martinez et al., 2008, Uema et al., 2005). It is therefore necessary to discriminate between wild type and vaccine type strains because the attenuated CDV vaccines are used worldwide. The hemagglutinin (H) gene revealed pronounced genetic diversity and was employed to detect and differentiate between different CDV strains (An et al., 2008, Si et al., 2010, Uema et al., 2005). There is no specific therapy for animals with canine distemper besides supportive treatment (Deem et al., 2000). The influenza A virus belongs to the family Orthomyxoviridae. The matrix gene is highly conserved and was often used for virus detection (Fouchier et al., 2000). Influenza virus causes sustained transmission among dogs, leading to respiratory disease outbreaks, e.g. H3N8 (Crawford et al., 2005, Payungporn et al., 2008) and H3N2 (Song et al., 2009).Treatment with the antiviral medicine oseltamivir has been approved in humans, but its use in dogs still needs evaluation (Beeler, 2009). Canine herpesvirus (CHV) is a member of the Herpesviridae family. Infection in older dogs appears to be restricted to the upper respiratory tract (Buonavoglia and Martella, 2007). The CHV glycoprotein B gene is often used as the virus detection target because it is highly conserved (Decaro et al., 2010). Limited studies with antiviral agents such as vidarabine are inconclusive, but immediate recognition and treatment is essential to have any possibility of success (Creevy, June 2013). B. bronchiseptica is a major bacteria cause of kennel cough in dogs. An upstream flagellin gene sequence was employed for specific detection (Hozbor et al., 1999). Most B. bronchiseptica strains are sensitive to aminoglycosides, extended-spectrum third generation penicillin, tetracycline, quinolone and trimethoprim-sulfamethoxazole (Mattoo and Cherry, 2005). Mycoplasmas cynos was demonstrated as the only mollicute found associated with canine infectious respiratory disease (Chalker et al., 2004). PCR detection was developed based on its 16S/23S rRNA intergenic spacer (IGS) (Chalker, 2004). Treatment with none β-lactam antibiotics such as doxycycline or tetracycline should be effective against most canine mycoplasmas (Chalker, 2005).
Infections with mixed respiratory pathogens in dogs are commonly seen and the clinical signs are similar, which makes differential diagnosis necessary (Chalker et al., 2004, Chvala et al., 2007, Erles et al., 2004, Jeoung et al., 2013, Mochizuki et al., 2008). Simultaneous canine vurial respiratory disease detection using multiplex PCR has been reported (Jeoung et al., 2013), but the etiogenic bacteria were not involved and the wild and vaccine types of CDVs could not be distinguished. The microarray is a promising alternative that can detect hundreds or thousands of genes in parallel. This study attempts to develop an oligonucleotide microarray system for simultaneous detection and differentiation of dog respiratory pathogens, including wild strains and vaccine strains of CDVs, influenza virus, CHV, B. bronchiseptica and M.cynos. These pathogens were chosen based on the disease fatality and the particular medicine options. The oligonucleotide microarray could provide an excellent diagnosis approach with better efficacy and efficiency to comprehensively distinguish clinically significant dog respiratory pathogens, including viruses and bacteria at the same time. The proposed device might greatly assist in disease control and therapy.
2. Materials and methods
The flow chart of experimental design is shown on Supplement Fig. 1.
2.1. Samples
The wild type CDV (NTU311) and CIV (A/canine/Taiwan/E01/2014) were isolated from the School of Veterinary Medicine, National Taiwan University. The CDV vaccine strains were obtained commercially, including Onderstepoort strain (Nobivac® LDHPPi, Boxmeer, the Netherlands and Fort Dodge® Mas 5-CvK/4L, the Puppyshot® Booster, IA), Distemperoid strain (Nobivac® Canine 1-DAPPvL2 + Cv, NE), Lederle strain (Virbac® Canigen DHAPPiLR, Carros, France), BA strain (Merial® Eurican DHPPI2-L, Lyon, France) and N-CDV strain (Pfizer® Vanguard Plus 5 L4 CV, Lincoln, NE). CHV, CAV-2, CPIV and CRCoV were from the positive clinical samples proven by PCR and sequencing at the Laboratory of Molecular Biology, School of Veterinary Medicine, National Taiwan University. B.bronchiseptica was from commercial vaccine (Nobivac® KC, Boxmeer, the Netherlands). Mycoplasma cynos (ATCC®27544™) was shared from the Agricultural Technology Research Institute, Miao-Li, Taiwan.CAV-2, CPIV and CRCoV were used for specificity test of multiplex PCR and oligonucleotide microarrays as negative controls. Fifty-six nasal swab samples were from 56 dogs that were presented at National Taiwan University Veterinary Hospital from January to October of 2015 and showed clinical respiratory signs.
2.2. Nucleic acid extraction, reverse transcription and uniplex PCR
Nucleic acid of the reference pathogens and clinical samples, including both DNA and RNA, was extracted using PetNAD™ Nucleic Acid Co-prep kit (GeneReach Biotechnology Corp., Taiwan) in accordance with the manufacturer’s instructions. RNA was further reverse transcribed to DNA using Transcriptor High Fidelity cDNA Synthesis Kit (Roche, Mannheim, Germany) based on the manufacturer’s instructions. PCR primers were from original or modification of the published ones and the target genes of CDV, CIV, CHV, B. bronchiseptica and M. cynos were hemagglutinin gene, matrix gene, glycoprotein B gene, flagellin gene and 16S/23S rRNA IGS region, respectively (Table 1 ). The uniplex PCR was carried out in a reaction volume of 25 μl containing 2 μl of template, 0.5 μl of each primer (10 μM) and 5 μl of 5× taq Master Mix (Protech, Taipei, Taiwan). The amplification thermal profile for each pathogen is described below. (1) CDV: 94 °C for 3 min, 34× (94 °C for 30 s, 50 °C for 30 s, and 72 °C for 40 s), 72 °C for 5 min; (2) CIV: 94 °C for 2 min, 34× (94 °C for 1 min, 55 °C for 2 min, and 72 °C for 3 min), 72 °C for 10 min; (3) CHV: 94 °C for 3 min, 34× (94 °C for 30 s, 50 °C for 30 s, and 72 °C for 40 s), 72 °C for 5 min; (4) B. bronchiseptica: 94 °C for 4 min, 34× (94 °C for 1 min, 57 °C for 30 s, and 72 °C for 40 s), 72 °C for 5 min; (5) M. cynos: 94 °C for 5 min, 34× (94 °C for 1 min, 54 °C for 40 s, and 72 °C for 1 min), 72 °C for 5 min. Ten μl PCR products were separated in 2.0% agarose gel, run in 400 ml 0.5× TBE buffer with 20 μl (10 mg/ml) ethidium bromide at 100 V for 50 min and visualized under UV light.
Table 1.
PCR primers used in this study.
| Pathogen | Target gene | Name | Sequence (5′ → 3′) | Direction | Product | Reference |
|---|---|---|---|---|---|---|
| CDV | Hemagglutinin | CDHF1d | CATGGGAACCCTTYGGRGG | Forward | 531 bp | Modified from thesisa |
| CDHR1 | CATCCCAYACAAAACATTCAA | Reverse | ||||
| Influenza virus | Matrix | M52C | CTTCTAACCGAGGTCGAAACG | Forward | 245 bp | (Fouchier et al., 2000) |
| M253R | AGGGCATTTTGGACAAAKCGTCTA | Reverse | ||||
| CHV | Glycoprotein B | CHVF2 | TGGTCTGGAAGCACATATGC | Forward | 427 bp | Thesisb |
| CHVR2 | TCAGTATGAGCACCATCTCG | Reverse | ||||
| B. bronchiseptica | Flagellin | Fla3m | AGGCACCTGCCCCATCTC | Forward | 291 bp | (Hozbor et al., 1999) |
| Fla2m | AGGCTCCCAAGAGAGAAAGG | Reverse | ||||
| M. cynos | 16S/23S rRNA IGS | Myc1 | CACCGCCCGTCACACCA | Forward | 449 bp | Modified from reference (Chalker, 2004) |
| Myc2 | CAAGGCATCCACCAAAAACTCC | Reverse |
Chen, H.C.M. 2002. Combination of polymerase chain reaction and gene chip for the rapid detection of canine viral diseases (master’s thesis). National Taiwan University, Taipei, Taiwan.
Huang, C.Y. 2006. Viral Nucleic Acid Diagnostic Assays for Canine Infectious Respiratory Disease and Analysis of Clinical Cases (master’s thesis). National Taiwan University, Taipei, Taiwan.
2.3. Multiplex PCR development
DNA from the reference pathogens and clinical samples were subjected to multiplex PCR, whose reaction solution was prepared the same as the uniplex PCR except that the five pairs of primers were 5′ end-biotinylated and employed at the same time. The multiplex PCR thermal profile was 94 °C for 3 min, 34× (94 °C for 30 s, 50 °C for 30 s, and 72 °C for 40 s), 72 °C for 5 min. Ten μl PCR products were separated in 2.0% agarose gel, run in 400 ml 0.5× TBE buffer with 20 μl (10 mg/ml) ethidium bromide at 100 V for 60 min and visualized under UV light.
2.4. Probe design
Microarray generic probe used to detect each pathogen was designed based on the most conserved sequence within the amplicon amplified by each pair of primers. Probes used to differentiate different CDV types were designed based on the amplicon sequence difference among different types. All of the probes were derived from the alignment and analyses of the nucleotide sequences retrieved from the GenBank, and conducted using the MegAlign program (DNASTAR, Madison, WI). Each probe sequence and its target strains are listed in Table 2 .
Table 2.
Oligonucleotide microarray probes used in this study.
| Pathogen | Name | Sequence (5′ → 3′) | Target strains |
|---|---|---|---|
| CDV | CDVG | CCCATTTAGACTAACTACCAAGGGTA | Generic |
| CDVW1 | TTCACTGTKACCCCYCAT | Wild type strains in Taiwan, Japan, South Korea and China | |
| CDVW2 | CCAGGGAGTCGAGTGGAA | Wild type strains in Japan and South Korea that CDVW1 cannot bind | |
| CDVW3 | TCATCGAGTCCAATGTAG | Wild type strains in China that CDVW1 cannot bind | |
| CDVV1 | ACATATCACGAAGTGATCATGCGA | CDV traditional vaccine strains (Onderstepoort, Distemperoid, Lederle and BA strains) | |
| CDVV2 | GGAAGATTTCTTTTACGTAC | CDV contemporary vaccine strain (N-CDV strain) | |
| Influenza virus | IVG | CAGGCCCCCTCAAAGCCGAGAT | Generic |
| CHV | CHVG | GAAGTTGATGCCAGATCTGTTTATCC | Generic |
| B. bronchiseptica | Bb | ACGGACGCCTGTCCCCGCAGGAA | B. bronchiseptica |
| M. cynos | Mc | GAGAGAACTTTTTTTCTCTCATGTTC | M. cynos |
2.5. Oligonucleotide microarray preparation and hybridization reaction
A tail composed of 15 T bases was added onto each 5’end of the oligonucleotide probe, including the positive control probe (an oligonucleotide from capsid protein VP1 of human enterovirus 71 gene, 5′-ATGAAGCATGTCAGGGCTTGGATACCTCG-3′), to enhance the cohesion strength between the probes and microarray substrate. Twenty μM of each probe was then spotted to each specific position on the microarray polymer substrate using DR. Easy spotter (DR. Chip Biotech, Miao-Li, Taiwan) and immobilized using Stratagene UV Stratalinker 1800 (Stratagene, Santa Clara, CA) with 0.6 J. The hybridization reaction between each DNA template and probe was carried out with DR. Chip DIY™ Kit (Dr. Chip Biotech, Miao-Li, Taiwan). The multiplex PCR product was denatured at 95 °C for 5 min and cooled in an ice bath for 5 min. To the microarray chamber was added 200 μl of Hybridization Buffer (containing the 5′ end-biotinylated oligonucleotide complementary to the positive control probe sequence). Two μl of denatured multiplex PCR product was added and incubated at 56 °C with vibration for 1 h. The sample was then washed twice with 250 μl of Washing Buffer at room temperature. The blocking reaction was then performed by mixing 0.2 μl of streptavidin conjugate alkaline phosphatase and 200 μl of Blocking Reagent at room temperature for 30 min, followed with washing twice with Washing Buffer. The colorimetric reaction was then implemented by adding 4 μl of NBT/BCIP and 196 μl of Detection Buffer in the chamber, developing in the dark at room temperature for 5–10 min, and washing twice with distilled water. The hybridization result was read directly with the naked eye.
2.6. Cloning, copy number calculation and detection limit comparison
Each uniplex PCR positive signal band on the agarose gel was extracted and purified using GenepHlow™ Gel/PCR Kit (Geneaid, New Taipei City, Taiwan) and then cloned into pGEM-T EasyVector Systems (Promega, Madison, WI) following the manufacturer’s instructions. The plasmids were transformed into Max EfficiencyDH5α chemically competent E. Coli (Invitrogen, Carlsbad, CA) which were further cultured on LB agar (Becton Dickson and Company, Sparks, MD). The colonies with successful inserts were confirmed by PCR using plasmid T7 and SP6 primers and then amplified in LB broth (Becton Dickson and Company, Sparks, MD). Plasmid DNA was extracted using Mini Plus™ Plasmid DNA Extraction System (Viogen, Shijr, Taiwan). The inserts were further sequenced using commercial service (Mission Biotech, Taipei, Taiwan). The obtained sequence was further checked using BLAST (http://blast.ncbi.nlm.nih.gov/Blast.cgi) to confirm nucleotide correctness. The plasmid DNA of CHV, B. bronchiseptica and M. cynos was quantified with a spectrophotometer (GeneQuant II, Pharmacia, Uppsala, Sweden). The DNA copy numbers were calculated and the DNA was diluted serially in TE buffer for the multiplex PCR templates. The plasmid DNA of CDV and CIV was linearized with restriction enzyme (SacI, New England Biolabs, Ipswich, MA) and the 3’overhang was conversed with the DNA polymerase (Klenow, Promega, Madison, WI). In vitro transcription was performed using Riboprobe in vitro Transcription Systems (Promega, Madison, WI) with T7 RNA Polymerase according to the manufacturer’s recommendations. Remaining DNA was removed using RQ1 RNase-Free DNase (Promega, Madison, WI). The viral RNA was quantified using a spectrophotometer (GeneQuant II, Pharmacia, Uppsala, Sweden) and the copy number was calculated. The RNA was serially diluted 10-fold in DEPC treated water and used as templates for the following reverse transcription and multiplex PCR. The agarose gel and oligonucleotide microarray detection limits were then compared. Paired-samples t-test was used to evaluate the difference between agarose gel and microarray for clinical samples using SPSS 16.0 (IBM Corp, NY. USA).
3. Results
3.1. Uniplex and multiplex PCR
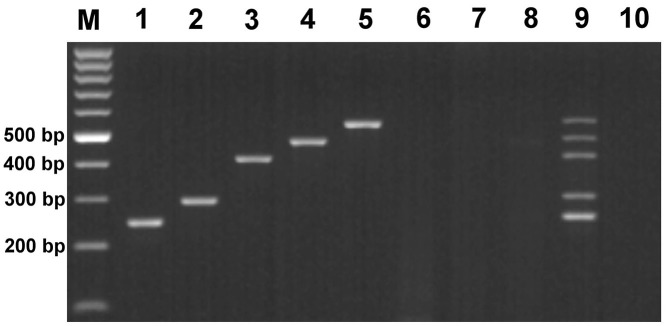
Each uniplex PCR could specifically amplify its target pathogen and show the corresponding band on gels (Supplement Fig. 2). The multiplex PCR was successfully developed with the annealing temperature at 50 °C. The product sizes of CIV, B. bronchiseptica, CHV, M. cynos and CDV were 245 bp, 291 bp, 427 bp, 449 bp and 531 bp, respectively. CAV-2, CPIV-2 and CRCoV were not amplified as expected (Fig. 1 ). Different strains of CDVs could not be distinguished on gels.
Fig. 1.
Multiplex PCR result of dog respiratory pathogens on an agarose gel. M: 100 bp ladder marker; 1: Influenza virus (245 bp); 2: B. bronchiseptica (291 bp); 3: CHV (427 bp); 4: M. cynos (449 bp); 5: CDV (531 bp); 6: CAV-2; 7: CPIV; 8: CRCoV; 9: Mixture of all pathogens containing 1–5. 10: Negative control.
3.2. Oligonucleotide microarray
Dog respiratory pathogens were tested using oligonucleotide microarrays following the multiplex PCR. All pathogens were unambiguously detected and differentiated with no cross-reactions found among the non-related probes (Fig. 2 ). The wild type and vaccine type CDVs were also well distinguished. CAV-2, CPIV-2, CRCoV and a nasal swab sample from a healthy dog were employed as negative controls. The results indicated good detection specificity among the selected pathogens and that the normal nasal flora would not bring about non-specific microarray signals.
Fig. 2.
Detection and differentiation of dog respiratory pathogens using oligonucleotide microarrays. (A) Microarray map. The meaning of each probe and its detecting strains are shown in Table 2. P: positive control. (B) The microarray detection results.
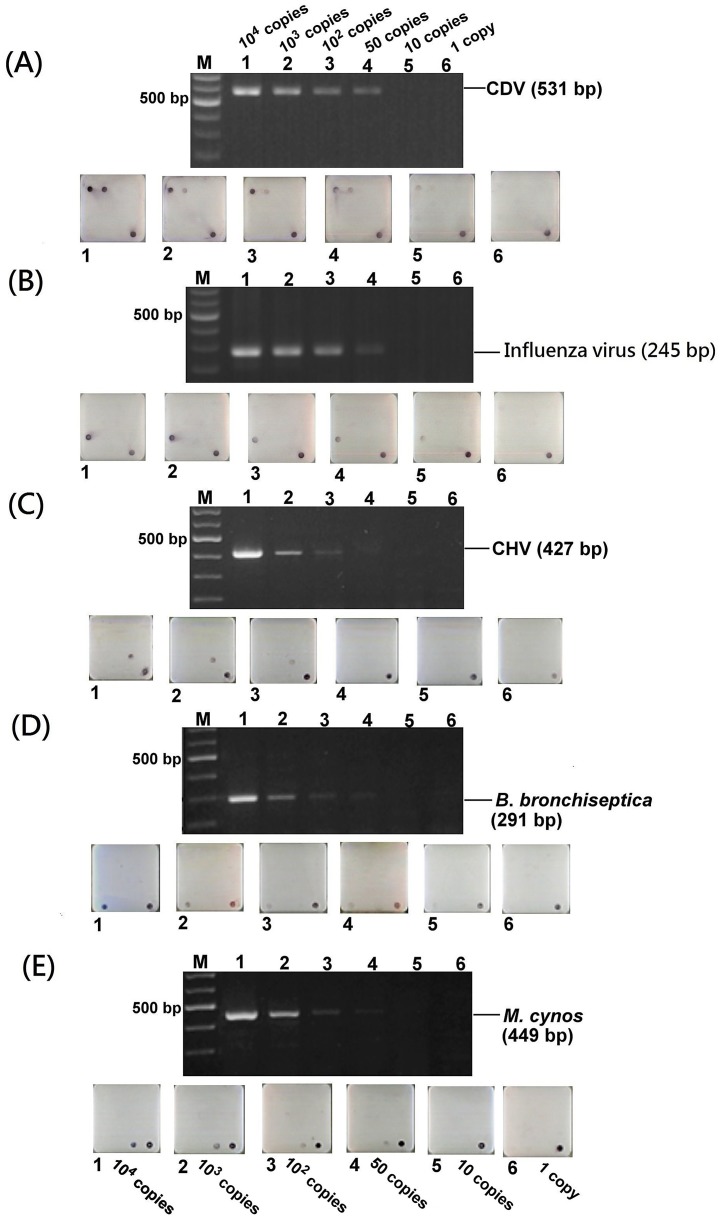
3.3. Detection limit test and comparison
The detection limit results of agarose gel and the microarray after multiplex PCR are shown in Fig. 3 . The gel detection limits of CDV (wild type NTU311 strain), CIV, CHV, B. bronchiseptica and M. cynos were 50, 50, 100, 100 and 100 copy numbers, respectively. The microarray detection limits of CDV (wild type NTU311 strain), CIV, CHV, B. bronchiseptica and M. cynos were 10, 10, 100, 50 and 50 copy numbers, respectively. The CDV vaccine strains, including probe CDVV1 targeted Onderstepoort, Distemperoid, Lederle and BA strains and probe CDVV2 targeted N-CDV strain, displayed the same detection limit results as the wild strain (data not shown).
Fig. 3.
Detection limit comparison on agarose gel and microarray. (A) CDV (wild type NTU311 strain). (B) Influenza virus. (C) CHV. (D) B. bronchiseptic. (E) M. cyno. M: 100 bp ladder marker; 1: 104 copies; 2: 103 copies; 3: 102 copies; 4: 50 copies; 5: 10 copies; 6: 1 copy.
3.4. Clinical sample test
Fifty-six nasal swab samples from 56 dogs showing respiratory signs were tested using the oligonucleotide microarray after multiplex PCR. Agarose gel results were compared concurrently (Table 3 ). The agarose gel and microarray detection results of certain representative samples are shown in supplement Fig. 3. The detection positive rates for the microarray and agarose gel were 59.0% (n = 33) and 41.1% (n = 23), respectively. The frequency of positive results was statistically different between these two methods (p value is 0.005 < 0.05). The oligonucleotide microarray showed exceeding differentiation effectiveness. One sample was differentiated as a CDV traditional vaccine strain among the total 25 single CDV infection positive samples. Co-infections of CDV and influenza virus, CDV and CHV, CDV and B. bronchiseptica were demonstrated by microarrays but none were revealed by agarose gels.
Table 3.
Pathogen detection and differentiation results of 56 clinical samples tested by agarose gel and oligonucleotide microarray after multiplex PCR.
| Detection method | No. of pathogen type |
|||||
|---|---|---|---|---|---|---|
| Negative | CDV | B. bronchiseptica | CDV+ influenza virus | CDV + CHV | CDV+ B. bronchiseptica | |
| Agarose gel | 33 | 23 | 0 | 0 | 0 | 0 |
| Microarray | 23 | 25a | 2 | 1b | 2c | 3d |
aTwenty-four were CDV wild type strains and one was CDV traditional vaccine strain among these 25 CDV positive samples tested on microarrays.
b,c,dAll of the CDV co-infection samples showed CDV wild type strains.
4. Discussion
The clinical signs caused by respiratory pathogens, including viruses and bacteria, are similar. Co-infections with multiple pathogens are also frequently seen (Chalker et al., 2004, Erles et al., 2004, Jeoung et al., 2013) which makes differential diagnosis imperative to enact correct treatment. The introduction of live attenuated CDV vaccines in the 1950s and their extensive use have drastically reduced the CD incidence in dogs. However, CD outbreaks involving virulent viruses introduced into a partly immune population have still been observed (Iwatsuki et al., 2000, Radtanakatikanon et al., 2013). Differentiation between wild type and vaccine type CDs is then necessary when performing CDV detection (Simon-Martinez et al., 2008, Wang et al., 2011). Based on the CDV H gene alignment and analysis of all CDV vaccines available in Taiwan, the CDV vaccine strains could be divided into two groups: traditional vaccine group (Onderstepoort strain, Distemperoid strain, Lederle strain and BA strain) and contemporary vaccine group (N-CDV strain, high homology to Vaccine X) (Chulakasian et al., 2010). Three canine respiratory viruses (CDV, CRCoV and influenza virus) have been reported distinguished using multiplex PCR (Jeoung et al., 2013). Four canine respiratory RNA viruses and two DNA viruses have been reported detected using multiplex RT-PCR and multiplex PCR, respectively (Piewbang et al., 2016). Wild type and traditional CDV vaccine strains could be discriminated using PCR-restriction fragment length polymorphism (Wang et al., 2011, Zhao et al., 2010), and cotemporary vaccine strain was able to be distinguished using refractory mutation system-PCR amplification (Chulakasian et al., 2010). However, no comprehensively integrated approach has ever been developed. Herein, an oligonucleotide microarray system was developed to simultaneously detect and differentiate clinical canine significant respiratory pathogens, including viruses, bacteria and traditional and contemporary CDV vaccine strains. The microarray signals were easily read with the naked eye requiring no additional reader equipment. The detection limit comparison and the clinical sample test findings demonstrated that the microarray had higher efficiency and sensitivity than the multiplex PCR-agarose gel method. The microarray CDV detection limit was 10 viral copies, which was even lower than the 23.2 copies using CDV hemi-nested RT-PCR (Di Francesco et al., 2012). Although real time RT-PCR can achieve similar low detection limit for these respiratory pathogens (Aeschbacher et al., 2015, Decaro et al., 2010, Nummi et al., 2015, Tatti et al., 2011, Wilkes et al., 2014), the machine cost is high. The microarray presented in this study compared to real time RT-PCR could be a feasibly alternative and especially preferable to the local under-equipped laboratories.
The microarray specificity also proved that no cross-reaction signals were observed among the unrelated probes. In the traditional approach, the agarose gel band needs to be cut and sequenced for confirmation of a doubtful result. However, the microarray accuracy could be ensured by omitting these steps since only complementary template-probe sequences hybrid and produce positive signals.
Although electrophoresis cost is 1.0 USD, it might increase to 9.0 USD if sequencing is needed for differentiation. Comparatively, the microarray cost is 3.0 USD but the array is reusable. No following gel imaging equipment and sequencing are demanded. The time cost from sample receipt to microarray readout is 4 h. However, it would take 3 days using traditional methods if sequencing was also included.
Probe CDVW1 was designed for the CDV wild type strains in Taiwan, Japan, South Korea and China. Probe CDVW2 and CDVW3 were for those in Japan, South Korea and China that CDVW1 cannot bind. However, CDVW2 and CDVW3 could not be tested in this study since these foreign strains were not available in Taiwan. Further experiments would be needed for CDVW2 and CDVW3 efficacy verification. In the microarray clinical sample tests, CDV, influenza virus, CHV and B. bronchiseptica were detected from the nasal swab samples but none of the M. cynos was captured. This might be due to the sampling method because M. cynos was only in the lower respiratory tract (Chalker et al., 2004). The detection rate of M. cynos might be higher if the samples were taken from the lower respiratory tract, e.g. bronchial-alveolar lavage. Because RNA viruses would be under constant mutation over time, the microarray efficacy might decrease if the mutations occur to the primer or probe sites. However, the microarray functionality could be extended by recruiting new probes, which would make this device more applicable for meeting future practical needs.
Acknowledgements
We thank the staff at National Taiwan University Veterinary Hospital for sample collection. This work was supported by the Ministry of Science and Technology, Taiwan (104-2622-B-002-009-CC2).
Footnotes
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.jviromet.2017.02.004.
Appendix A. Supplementary data
The following are Supplementary data to this article:
References
- Aeschbacher S., Santschi E., Gerber V., Stalder H.P., Zanoni R.G. Development of a real-time RT-PCR for detection of equine influenza virus. Schweiz. Arch. Tierheilkd. 2015;157:191–201. doi: 10.17236/sat00015. [DOI] [PubMed] [Google Scholar]
- An D.J., Yoon S.H., Park J.Y., No I.S., Park B.K. Phylogenetic characterization of canine distemper virus isolates from naturally infected dogs and a marten in Korea. Vet. Microbiol. 2008;132:389–395. doi: 10.1016/j.vetmic.2008.05.025. [DOI] [PubMed] [Google Scholar]
- Beeler E. Influenza in dogs and cats. Vet. Clin. North Am. Small Anim. Pract. 2009;39:251–264. doi: 10.1016/j.cvsm.2008.10.011. [DOI] [PubMed] [Google Scholar]
- Buonavoglia C., Martella V. Canine respiratory viruses. Vet. Res. 2007;38:355–373. doi: 10.1051/vetres:2006058. [DOI] [PubMed] [Google Scholar]
- Chalker V.J., Owen W.M., Paterson C., Barker E., Brooks H., Rycroft A.N., Brownlie J. Mycoplasmas associated with canine infectious respiratory disease. Microbiology. 2004;150:3491–3497. doi: 10.1099/mic.0.26848-0. [DOI] [PubMed] [Google Scholar]
- Chalker V.J. Taxonomy of the canine Mollicutes by 16S rRNA gene and 16S/23S rRNA intergenic spacer region sequence comparison. Int. J. Syst. Evol. Microbiol. 2004;54:537–542. doi: 10.1099/ijs.0.02869-0. [DOI] [PubMed] [Google Scholar]
- Chalker V.J. Canine mycoplasmas. Res. Vet. Sci. 2005;79:1–8. doi: 10.1016/j.rvsc.2004.10.002. [DOI] [PubMed] [Google Scholar]
- Chulakasian S., Lee M.S., Wang C.Y., Chiou S.S., Lin K.H., Lin F.Y., Hsu T.H., Wong M.L., Chang T.J., Hsu W.L. Multiplex amplification refractory mutation system polymerase chain reaction (ARMS-PCR) for diagnosis of natural infection with canine distemper virus. Virol. J. 2010;7:122. doi: 10.1186/1743-422X-7-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chvala S., Benetka V., Möstl K., Zeugswetter F., Spergser J., Weissenböck H. Simultaneous canine distemper virus, canine adenovirus type 2, and Mycoplasma cynos infection in a dog with pneumonia. Vet. Pathol. 2007;44:508–512. doi: 10.1354/vp.44-4-508. [DOI] [PubMed] [Google Scholar]
- Crawford P.C., Dubovi E.J., Castleman W.L., Stephenson I., Gibbs E.P., Chen L., Smith C., Hill R.C., Ferro P., Pompey J., Bright R.A., Medina M.J., Johnson C.M., Olsen C.W., Cox N.J., Klimov A.I., Katz J.M., Donis R.O. Transmission of equine influenza virus to dogs. Science. 2005;310:482–485. doi: 10.1126/science.1117950. [DOI] [PubMed] [Google Scholar]
- Creevy K.E. The Merck Veterinary Manual. 2013. Overview of canine herpesviral infection. http://www.merckvetmanual.com/mvm/generalized_conditions/canine_herpesviral_infection/overview_of_canine_herpesviral_infection.html (assessed 16.01.01) [Google Scholar]
- Decaro N., Amorisco F., Desario C., Lorusso E., Camero M., Bellacicco A.L., Sciarretta R., Lucente M.S., Martella V., Buonavoglia C. Development and validation of a real-time PCR assay for specific and sensitive detection of canid herpesvirus 1. J. Virol. Methods. 2010;169:176–180. doi: 10.1016/j.jviromet.2010.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deem S.L., Spelman L.H., Yates R.A., Montali R.J. Canine distemper in terrestrial carnivores: a review. J. Zoo Wildl. Med. 2000;31:441–451. doi: 10.1638/1042-7260(2000)031[0441:CDITCA]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Demeter Z., Palade E.A., Hornyák Á., Rusvai M. Controversial results of the genetic analysis of a canine distemper vaccine strain. Vet. Microbiol. 2010;142:420–426. doi: 10.1016/j.vetmic.2009.10.017. [DOI] [PubMed] [Google Scholar]
- Di Francesco C.E., Di Francesco D., Di Martino B., Speranza R., Santori D., Boari A., Marsilio F. Detection by hemi-nested reverse transcription polymerase chain reaction and genetic characterization of wild type strains of Canine distemper virus in suspected infected dogs. J. Vet. Diagn. Invest. 2012;24:107–115. doi: 10.1177/1040638711425700. [DOI] [PubMed] [Google Scholar]
- Erles K., Dubovi E.J., Brooks H.W., Brownlie J. Longitudinal study of viruses associated with canine infectious respiratory disease. J. Clin. Microbiol. 2004;42:4524–4529. doi: 10.1128/JCM.42.10.4524-4529.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fouchier R.A., Bestebroer T.M., Herfst S., Van Der Kemp L., Rimmelzwaan G.F., Osterhaus A.D. Detection of influenza A viruses from different species by PCR amplification of conserved sequences in the matrix gene. J. Clin. Microbiol. 2000;38:4096–4101. doi: 10.1128/jcm.38.11.4096-4101.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hozbor D., Fouque F., Guiso N. Detection of Bordetella bronchiseptica by the polymerase chain reaction. Res. Microbiol. 1999;150:333–341. doi: 10.1016/s0923-2508(99)80059-x. [DOI] [PubMed] [Google Scholar]
- Iwatsuki K., Tokiyoshi S., Hirayama N., Nakamura K., Ohashi K., Wakasa C., Mikami T., Kai C. Antigenic differences in the H proteins of canine distemper viruses. Vet. Microbiol. 2000;71:281–286. doi: 10.1016/s0378-1135(99)00172-8. [DOI] [PubMed] [Google Scholar]
- Jeoung H.Y., Song D.S., Jeong W.S., Lee W.H., Song J.Y., An D.J. Simultaneous detection of canine respiratory disease associated viruses by a multiplex reverse transcription-polymerase chain reaction assay. J. Vet. Med. Sci. 2013;75:103–106. doi: 10.1292/jvms.12-0287. [DOI] [PubMed] [Google Scholar]
- Mattoo S., Cherry J.D. Molecular pathogenesis, epidemiology, and clinical manifestations of respiratory infections due to Bordetella pertussis and other Bordetella subspecies. Clin. Microbiol. Rev. 2005;18:326–382. doi: 10.1128/CMR.18.2.326-382.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mochizuki M., Yachi A., Ohshima T., Ohuchi A., Ishida T. Etiologic study of upper respiratory infections of household dogs. J. Vet. Med. Sci. 2008;70:563–569. doi: 10.1292/jvms.70.563. [DOI] [PubMed] [Google Scholar]
- Nummi M., Mannonen L., Puolakkainen M. Development of a multiplex real-time PCR assay for detection of Mycoplasma pneumoniae, Chlamydia pneumoniae and mutations associated with macrolide resistance in Mycoplasma pneumoniae from respiratory clinical specimens. SpringerPlus. 2015;4:684. doi: 10.1186/s40064-015-1457-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payungporn S., Crawford P.C., Kouo T.S., Chen L.M., Pompey J., Castleman W.L., Dubovi E.J., Katz J.M., Donis R.O. Influenza A virus (H3N8) in dogs with respiratory disease, Florida. Emerg. Infect. Dis. 2008;14:902–908. doi: 10.3201/eid1406.071270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piewbang C., Rungsipipat A., Poovorawan Y., Techangamsuwan S. Development and application of multiplex PCR assays for detection of virus-induced respiratory disease complex in dogs. J. Vet. Med. Sci. 2016 doi: 10.1292/jvms.16-0342. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Priestnall S.L., Mitchell J.A., Walker C.A., Erles K., Brownlie J. New and emerging pathogens in canine infectious respiratory disease. Vet. Pathol. 2014;51:492–504. doi: 10.1177/0300985813511130. [DOI] [PubMed] [Google Scholar]
- Radtanakatikanon A., Keawcharoen J., Charoenvisal N.T., Poovorawan Y., Prompetchara E., Yamaguchi R., Techangamsuwan S. Genotypic lineages and restriction fragment length polymorphism of canine distemper virus isolates in Thailand. Vet. Microbiol. 2013;166:76–83. doi: 10.1016/j.vetmic.2013.05.015. [DOI] [PubMed] [Google Scholar]
- Si W., Zhou S., Wang Z., Cui S.J. A multiplex reverse transcription-nested polymerase chain reaction for detection and differentiation of wild-type and vaccine strains of canine distemper virus. Virol. J. 2010;7:86. doi: 10.1186/1743-422X-7-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon-Martinez J., Ulloa-Arvizu R., Soriano V.E., Fajardo R. Identification of a genetic variant of canine distemper virus from clinical cases in two vaccinated dogs in Mexico. Virol. J. 2008;175:423–426. doi: 10.1016/j.tvjl.2007.01.015. [DOI] [PubMed] [Google Scholar]
- Song D., Lee C., Kang B., Jung K., Oh T., Kim H., Park B., Oh J. Experimental infection of dogs with avian-origin canine influenza A virus (H3N2) Emerg. Infect. Dis. 2009;15:56–58. doi: 10.3201/eid1501.080755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatti K.M., Sparks K.N., Boney K.O., Tondella M.L. Novel multitarget real-time PCR assay for rapid detection of Bordetella species in clinical specimens. J. Clin. Microbiol. 2011;49:4059–4066. doi: 10.1128/JCM.00601-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uema M., Ohashi K., Wakasa C., Kai C. Phylogenetic and restriction fragment length polymorphism analyses of hemagglutinin (H) protein of canine distemper virus isolates from domestic dogs in Japan. Virus Res. 2005;109:59–63. doi: 10.1016/j.virusres.2004.10.008. [DOI] [PubMed] [Google Scholar]
- Wang F., Yan X., Chai X., Zhang H., Zhao J., Wen Y., Wu W. Differentiation of canine distemper virus isolates in fur animals from various vaccine strains by reverse transcription-polymerase chain reaction-restriction fragment length polymorphism according to phylogenetic relations in china. Virol. J. 2011;8:85. doi: 10.1186/1743-422X-8-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkes R.P., Sanchez E., Riley M.C., Kennedy M.A. Real-time reverse transcription polymerase chain reaction method for detection of canine distemper virus modified live vaccine shedding for differentiation from infection with wild-type strains. J. Vet. Diagn. Invest. 2014;26:27–34. doi: 10.1177/1040638713517232. [DOI] [PubMed] [Google Scholar]
- Zhao J.J., Yan X.J., Chai X.L., Martella V., Luo G.L., Zhang H.L., Gao H., Liu Y.X., Bai X., Zhang L., Chen T., Xu L., Zhao C.F., Wang F.X., Shao X.Q., Wu W., Cheng S.P. Phylogenetic analysis of the haemagglutinin gene of canine distemper virus strains detected from breeding foxes, raccoon dogs and minks in China. Vet. Microbiol. 2010;140:34–42. doi: 10.1016/j.vetmic.2009.07.010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.