Abstract
RT-PCR is the most sensitive assay for diagnosis of influenza, due to enhanced rapidity and sensitivity as compared to classical methods.
Hemi-nested RT-PCR was developed, targeting NP gene for influenza A and NS gene for influenza B, based on a previous single round RT-PCR method. The new method was compared with the previous technique for analytical sensitivity and specificity, and was applied to clinical samples from the lower and upper respiratory tract.
The analytical sensitivity of hemi-nested RT-PCR was 10 (influenza A) and 4 times (influenza B) higher than the previous method. A high specificity of the new hemi-nested RT-PCR assay was observed by using whole respiratory viruses.
When applied to lower respiratory tract specimens, the new method showed an increased rate of positivity as compared to the previous technique (9.3% versus 0.7% for influenza A, and 0.9% versus 0.2% for influenza B). Screening of upper respiratory tract samples collected during the seasonal 2005–2006 outbreak indicated 26.4% and 5.8% positivity for influenza A and B, respectively.
The results were confirmed by sequence analysis: apart from influenza B, both influenza A subtypes H3N2 and H1N1, associated with the seasonal outbreak, were detected.
Keywords: Influenza, Molecular diagnosis, RT-PCR, Respiratory infections
Infections due to A and B influenza virus cause substantial annual morbidity, hospitalization and mortality worldwide, especially in the case of infants, the elderly and immunocompromised patients (Lewis, 2006, Nicholson et al., 2003). However, influenza virus infections are often under-diagnosed. In addition, influenza viruses co-circulate with other respiratory pathogens that also cause influenza-like illness, hence it is important to differentiate influenza from other respiratory diseases (Nicholson et al., 2003). In addition to the opportunity to administer early antiviral treatment, when available, rapid diagnosis of viral respiratory infections is associated with more judicious antibiotic use, prevention of nosocomial spread, reduced length of hospital stay, and, ultimately, with reduced costs (Barenfanger et al., 2000, Cox and Subbarao, 1999, Woo et al., 1997). For these reasons, the availability of a sensitive tool for the diagnosis of influenza virus is of the utmost importance in order to optimize clinical management, reduce the risk of spread to contacts, and exclude influenza-like pathogens, as well as for the surveillance of influenza viruses in communities which, in turn, is important to provide information concerning the circulating subtypes, including potential avian influenza viruses.
Classical diagnosis of influenza virus infections is based on viral isolation in tissue culture and subsequent hemagglutination and neuraminidase subtyping by serological methods (McIntosh, 1996). However, these methods are cumbersome and time consuming, and require specialized personnel and tissue culture facilities. Rapid antigen detection tests have been developed and evaluated (Church et al., 2002, Sintchenko et al., 2002), showing good specificity and acceptable sensitivity, but RT-PCR (Wright et al., 1995), multiplex RT-PCR (Poddar, 2002) and real-time PCR (Hindiyeh et al., 2005, Smith et al., 2002) are at present considered to be the most sensitive assays for the detection of influenza viruses.
In this study a RT-PCR method, established originally by Wright et al. (1995), has been modified by designing an internal primer set to establish an hemi-nested RT-PCR.
The new hemi-nested RT-PCR was evaluated for analytical sensitivity and specificity, and for its ability to detect and concomitantly distinguish influenza viruses A and B in respiratory specimens.
The reference strains B/Singapore/222/79 and A/Pt.Chalmer/1/73(H3N2) were used as positive controls, and to set up plasmids containing, as the insert, the region targeted by each RT-PCR. The viruses were cultured on MDCK cells. All virus stocks were aliquoted and stored at −80 °C until use.
A total of 571 lower respiratory tract samples (365 sputum, 186 endotracheal aspirates and 20 bronchoalveolar lavages), were collected from 480 hospitalized patients, and consequently sent to the microbiology laboratories of three hospitals in Italy, during March 2004–May 2005.
In addition, 34 nasopharyngeal swabs from patients with influenza-like illness, were collected during a laboratory survey in the 2005–2006 seasonal outbreak (February–April 2006).
Lower respiratory tract samples were diluted 1:1 with Sputasol solution (Oxoid Ltd., Basingstoke, England) and underwent DNA/RNA extraction by using the Boom method (Boom et al., 1990) with Nuclisens reagents (bioMériux, Durham, NC, USA).
Unrelated DNA (β-globin DNA) and RNA (MS2 phage genome) controls were added to all samples before the extraction, and the target control sequences were amplified in parallel PCR and RT-PCR, to control the presence of inhibitors from the sample matrix.
Reverse transcription of RNA was carried out with random primers by using a Reverse Transcriptase Kit (Invitrogen, Milan, Italy) according to Manufacturer's instructions. The cDNA obtained was amplified by PCR, by using the GeneAmp DNA amplification reagent kit from Applied Biosystems (Roche, Branchburg, New Jersey USA).
For the detection of influenza A and B viruses, a single round RT-PCR targeting the NP and NS gene for influenza A and B, respectively and the new hemi-nested RT-PCR method were used. Positive and negative controls, containing viral RNA extracts and nuclease-free water, respectively, were included in each RT-PCR tube.
In the original single round RT-PCR described by Wright et al. (1995), the primers were ANP1/ANP2 for influenza A, and BNS1/BNS2 for influenza B. The amplicon size was 307 bp and 228 bp for influenza A and B, respectively. This method was used as reference to validate the new method. The new method was based on a modification of the previous technique, based on two round PCR with an hemi-nested format.
For the first round the same set of primers was used, with amplification conditions were modified slightly with respect to the original method. Specifically, 5 μl of cDNA was added to each PCR tube in the first round of amplification. PCR conditions included each specific primer at 0.2 μM in 50 μl final reaction volume, containing 1.5 U of Taq Gold DNA polymerase, 200 μM each dNTP, 10 mM Tris–HCl (pH 8.8), 75 mM potassium chloride and 1.5 mM magnesium chloride.
Amplification conditions were as follows: denaturation 15 min at 94 °C, then 30 cycles of 1 min at 94 °C, 2 min at 55 °C, 3 min at 72 °C. A final cycle with an elongation step of 7 min was included at the end. For the second round, two internal sense primers were designed: ANP3, 5′-AAAATCATGGCGTCCCAAGG-3′ (positions 40–59; GenBank accession number D00603), for influenza A virus and BNS3, 5′-AAATTGAGGTGGGTCCGGG-3′ (positions 53–71; GenBank accession number AY044170), for influenza B virus. 2.5 μl from the first amplification tube was used, and the reaction mixture was as for the first round. Amplification conditions were as follows: denaturation 15 min at 94 °C, then 35 cycles of 1 min at 94 °C, 1 min at 56 °C, 1 min at 72 °C. A final cycle with an elongation step of 7 min was included at the end. The amplified products (15 μl) were analyzed by agarose gel electrophoresis and visualized by UV fluorescence after staining with ethidium bromide. The amplicon size was slightly shorter than in the first round reaction (287 bp and 206 bp, respectively, Fig. 1 ).
Fig. 1.
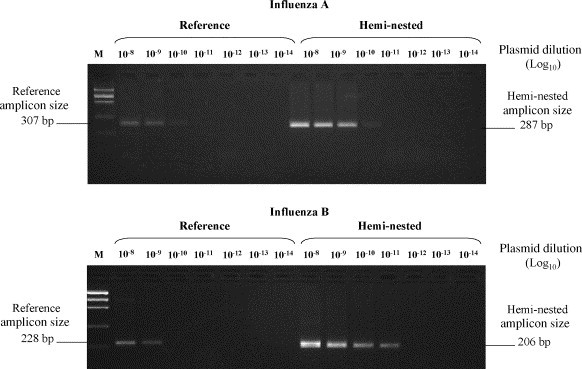
Detection of influenza A and B amplicons by hemi-nested and reference RT-PCR at limiting dilution of positive control plasmids containing the target sequences as the insert (influenza A plasmid concentration = 5 × 1010 copie/ml; influenza B plasmid concentration = 2.5 × 1011 copie/ml).
The new method was tested first on RNA extracted from virus stock. It was then applied to serial dilutions of 2 plasmids containing, as an insert, the target region from influenza A and B viruses, to assess the sensitivity of the modified amplification protocols.
PCR products (obtained by using ANP1/ANP2 and BNS1/BNS2) were cloned, to construct these plasmids. Specifically, amplified products were purified by the QIAquick® PCR Purification kit (Qiagen, GmbH, Germany), ligated into the pCR®4-TOPO® vector, contained in the TOPO TA Cloning® kit (Invitrogen, Life Technologies, Carlsbad, CA, USA), and transformed in competent Escherichia coli cells, by using the One Shot® TOP10 system (Invitrogen, Life Technologies, Carlsbad, CA, USA). Plasmid DNA was extracted by using QIAprep® Miniprep kit (Qiagen, GmbH, Germany). After transformation and plasmid extraction, a known copy number of the obtained recombinant plasmid was used to perform a limiting dilution PCR amplification, using 5 replicate tubes for each dilution. Sensitivity for the reaction was calculated by Epa Probit Analysis Program (version 1.5).
The specificity of the new hemi-nested RT-PCR assay was established by using a panel of plasmids containing target regions from the following respiratory viruses: human metapneumovirus (hMPV), adenovirus (AdV), parainfluenzavirus (PIV)-1 and PIV-3, respiratory syncytial virus (RSV), human rhinovirus (HRV), as well as human coronavirus (hCoV) OC43. In addition, preparations containing whole viruses (hMPV, PIV1 and 3, RSV, HRV) were tested.
The specificity of the new method in clinical samples was obtained by sequencing the amplicons from the hemi-nested RT-PCR, and by independent confirmation/subtyping based on sequencing of different target genes (HA, N, and M). Sequencing was performed on the automated ABI Prism 3100 instrument, by using BigDye Terminator cycle sequencing kit (Applied Biosystems, Warrington, UK).
Serial dilutions of two plasmids containing the target region for both viruses were tested in replicate assays, to establish analytical sensitivity. As shown in Fig. 1, the amplicons were detected at 1 and 2 higher 10-fold dilutions (for influenza A and B, respectively) by the new method as compared with the reference method. Sensitivity, established by probit analysis (i.e. the concentration of the target sequence resulting positive in 95% of cases) resulted in 10 and 70 copies for influenza A and B in reference RT-PCR, and 1 and 18 copies in hemi-nested RT-PCR, respectively.
As for specificity, none of the plasmids or whole virus preparations resulted positive or interfered with influenza A and B-specific RT-PCR assay. The results were reproduced on three PCR runs.
A total of 571 lower respiratory samples were tested by the two methods. The results, shown in Table 1 , show a significant increase of the rate of positivity to both influenza A and B by the new method as compared to the reference method. None of the samples resulting positive by the reference method tested negative by the hemi-nested method. The distribution of the cases detected with the hemi-nested RT-PCR was that expected on the basis of the seasonal influenza activity in the corresponding period, with a peak in January–February (Fig. 2 ). In addition, it is shown in Table 1 that about 50% of samples were also positive to other respiratory viruses.
Table 1.
Detection of influenza A and B in lower respiratory tract samples by single round RT-PCR and hemi-nested RT-PCR
| Assay | No. (%) of samples with the following results |
|
|---|---|---|
| Influenza A positive (co-infected with others respiratory viruses) | Influenza B positive (co-infected with others respiratory viruses) | |
| Single round | 4 (0.7%) | 1 (0.2%) |
| (2) | (0) | |
| Hemi-nested | 53 (9.3%) | 5 (0.9%) |
| (23) | (3) | |
Fig. 2.
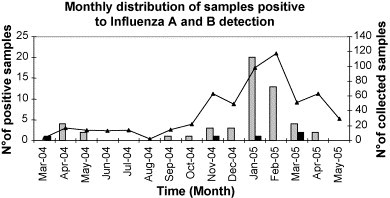
Monthly distribution of lower respiratory tract samples positive to influenza A and B by hemi-nested RT-PCR, during the period March 2004–May 2005. Continuous line: total number of samples collected. Grey bars: influenza A-positive sample. Black bars: influenza B-positive samples.
The new method was then applied to nasopharyngeal swabs from 34 patients with influenza-like illness collected in the 2005–2006 seasonal outbreak, of which 9 (26.4%) resulted positive to influenza A and 2 (5.8%) positive to influenza B. Also in this case, a relevant proportion of samples (n = 7; 20.6%) was positive to other respiratory viruses.
To confirm the specificity of the hemi-nested RT-PCR, 4 influenza A and 1 influenza B, were sequenced, indicating that in all cases the expected sequences were obtained.
Eighteen positive samples (2 influenza B and 16 influenza A) were subjected to amplification/sequencing for different target genes (i.e. HA, N and M for influenza A and HA for influenza B), for an independent confirmation/subtyping. This approach confirmed the presence of influenza A and B in all the 18 samples. In addition, HA and N sequence data from 9 of the samples indicated that both H3N2 (n = 5) and H1N1 (n = 4) subtypes were represented in the clinical series. In 7 samples, independent confirmation for influenza A was based only on the M gene sequence data. Influenza virus confirmation by sequencing/subtyping was also obtained in 4 samples containing other respiratory viruses (2 PIV1, 1 hCoV OC43 and 1 HRV), indicating that with the new method there is no interference for influenza virus detection, due to the concomitant presence of other viruses.
The analytical sensitivity of a new molecular method was establish for detecting influenza A and B, based on hemi-nested RT-PCR. The results indicate that the analytical sensitivity is improved approximately 10 times for influenza A and 5 times for influenza B in comparison to the reference method, and that the new method is specific.
As a result, the new method detected both viruses in a significantly higher proportion of clinical samples from lower (sputum, endotracheal aspirates and bronchoalveolar lavages) respiratory tract, as compared to single round RT-PCR. In addition, screening of nasopharyngeal swabs collected during the 2005–2006 winter seasonal outbreak of influenza by the new method indicated 26.4% and 5.8% positivity for influenza A and B, respectively.
A high specificity of the new method may be deduced from the fact that all positive samples undergoing sequence analysis were confirmed for the presence of influenza A or B viruses. Molecular subtyping indicated that the new method can detect both influenza A subtypes (H3N2 and H1N1) involved in the seasonal outbreak. Furthermore, the lack of interference can be deduced by the fact that a substantial proportion (26.1%) of specimens positive in the hemi-nested influenza RT-PCR were also positive to at least one additional respiratory virus, and sequence analysis confirmed the presence of influenza in multiply infected samples.
This study shows that the hemi-nested RT-PCR for influenza A and B viruses that has been developed is sensitive and specific, and suggests its improved diagnostic performance in the clinical setting.
However, the present method, based on multiple step PCR, may have some disadvantages, such as increased complexity, time, reagents use, costs and the possibility of contamination. Some of these disadvantages may be overcome by the use of carry over prevention systems, such as uracil-N-glycosylase (UNG), and one step RT-PCR kits. Both methods add further complexity to the operative steps, and cDNA preparation is more suitable for respiratory samples, often undergoing multiple amplification reactions for respiratory virus panels. The recent approach based on real time PCR may help solve most of these issues. However, it should be considered that the end point PCR is still used in many diagnostic laboratories, therefore the method described in this paper should be a valid option for detection of influenza viruses in both upper and lower respiratory tract clinical samples, substantially contributing to establish the etiology of lower respiratory tract diseases and of influenza-like illness, and to study the prevalence of respiratory viruses.
This study was supported in part by grants from the Italian Ministry of Health (“Fondi Ricerca Corrente”, “Ricerca Finalizzata”, and “Fondi per la creazione di un polo centralizzato per la crioconservazione”) to INMI L. Spallanzani. Neither funding source influenced the design, conduct or reporting of this study.
Carla Nisii and Anna Prygodzicz are gratefully acknowledged for assistance in writing the manuscript.
References
- Barenfanger J., Drake C., Leon N., Mueller T., Troutt T. Clinical and financial benefits of rapid detection of respiratory viruses: an outcomes study. J. Clin. Microbiol. 2000;38:2824–2828. doi: 10.1128/jcm.38.8.2824-2828.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boom R., Sol C.J., Salimans M.M., Jansen C.L., Wertheim-van Dillen P.M., van der Noordaa J. Rapid and simple method for purification of nucleic acids. J. Clin. Microbiol. 1990;28(3):495–503. doi: 10.1128/jcm.28.3.495-503.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Church D.L., Davies H.D., Mitton C., Semeniuk H., Logue M., Maxwell C., Donaldson C. Clinical and economic evaluation of rapid influenza a virus testing in nursing homes in calgary, Canada. Clin. Infect. Dis. 2002;34(6):790–795. doi: 10.1086/338960. [DOI] [PubMed] [Google Scholar]
- Cox N.J., Subbarao K. Influenza. Lancet. 1999;354(9186):1277–1282. doi: 10.1016/S0140-6736(99)01241-6. [DOI] [PubMed] [Google Scholar]
- Hindiyeh M., Levy V., Azar R., Varsano N., Regev L., Shalev Y., Grossman Z., Mendelson E. Evaluation of a multiplex real-time reverse transcriptase PCR assay for detection and differentiation of influenza viruses A and B during the 2001–2002 influenza season in Israel. J. Clin. Microbiol. 2005;43:589–595. doi: 10.1128/JCM.43.2.589-595.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis D.B. Avian flu to human influenza. Annu. Rev. Med. 2006;57:139–154. doi: 10.1146/annurev.med.57.121304.131333. Review. [DOI] [PubMed] [Google Scholar]
- McIntosh K. Diagnostic virology. In: Fields B.N., Knipe D.M., Howley P.M., editors. Fields Virology. 3rd ed. Lippincott-Raven; Philadelphia, PA: 1996. pp. 401–430. [Google Scholar]
- Nicholson K.G., Wood J.M., Zambon M. Influenza. Lancet. 2003;362(9397):1733–1745. doi: 10.1016/S0140-6736(03)14854-4. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poddar S.K. Influenza virus types and subtypes detection by single tube multiplex reverse transcription-polymerase chain reaction (RT-PCR) and agarose gel electrophoresis. J. Virol. Methods. 2002;99:63–70. doi: 10.1016/s0166-0934(01)00380-9. [DOI] [PubMed] [Google Scholar]
- Sintchenko V., Gilbert G.L., Coiera E., Dwyer D. Treat or test first? Decision analysis of empirical antiviral treatment of influenza virus infection versus treatment based on rapid test results. J. Clin. Virol. 2002;25(1):15–21. doi: 10.1016/s1386-6532(00)00182-7. [DOI] [PubMed] [Google Scholar]
- Smith A.B., Mock V., Melear R., Colarusso P., Willis D.E. Rapid detection of influenza A and B viruses in clinical specimens by Light Cycler real time PCR. J. Clin. Virol. 2002;28:51–58. doi: 10.1016/s1386-6532(02)00238-x. [DOI] [PubMed] [Google Scholar]
- Woo P.C., Chiu S.S., Seto W.H., Peiris M. Cost-effectiveness of rapid diagnosis of viral respiratory tract infections in pediatric patients. J. Clin. Microbiol. 1997;35:1579–1581. doi: 10.1128/jcm.35.6.1579-1581.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright K.E., Wilson G.A., Novosad D., Dimock C., Tan D., Weber J.M. Typing and subtyping of influenza viruses in clinical samples by PCR. J. Clin. Microbiol. 1995;33(5):1180–1184. doi: 10.1128/jcm.33.5.1180-1184.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]


