Abstract
Transmissible gastroenteritis virus (TGEV) and porcine epidemic diarrhea virus (PEDV) are major etiological agents of diarrhea and death in piglets. Multiplex real-time reverse transcriptase (RT)-PCR was developed for simultaneous differential quantification of each virus in a single reaction tube, using Cy5- and FAM-labeled TaqMan-probes based on sequences from the TGEV and PEDV nucleocapsid genes. The copy numbers for transcripts of TGEV and PEDV were quantified using this assay over a range from 9 × 107 to 9 × 101 copies and 7 × 107 to 7 × 101 copies, respectively. The variability of the intra-assay and inter-assay were evaluated using standard solutions of each transcript, with coefficients of variation (CV) less than 3.43 and 3.33%, respectively. Piglets were experimentally infected with virulent TGEV and PEDV, and the amounts of virus from the onset of diarrhea were measured. Samples obtained from farms experiencing PED or TGE were quantified between 102 and 105 RNA copies. In conclusion, this assay provides an effective etiological diagnostic tool for detecting and quantifying viral loads. The assay may also prove useful for detecting infections, ultimately leading to better disease control on farms.
Keywords: Transmissible gastroenteritis virus (TGEV), Porcine epidemic diarrhea virus (PEDV), Multiplex real-time RT-PCR, Quantification
1. Introduction
Porcine transmissible gastroenteritis virus (TGEV) and porcine epidemic diarrhea virus (PEDV), members of the family Coronaviridae, are causes of viral enteritis, which destroys villus enterocytes in pigs of all ages (Debouck and Pensaert, 1980, Pensaert, 1999, Saif and Wesley, 1999). Both diseases are spread by the oral uptake of feces from infected pigs, resulting in vomiting, watery diarrhea, dehydration, and high mortality in nursery piglets (Pensaert, 1999, Saif and Wesley, 1999).
Conventional diagnostic assays use either antibodies or viral antigens to detect viral gastroenteritis. Several vaccines used for sows in Korea elicit immunoglobins (Igs) in maternal milk that help to protect offspring from infection. Therefore, serological examination cannot be used with these animals, since the vaccine does not distinguish between vaccinated animals and those infected with the wild-type virus. Several diagnostic assays are available for detecting viral antigens, including virus isolation, fluorescence assay, immune electron microscopy, in situ hybridization, and enzyme-linked immunosorbent assay (ELISA; Callebaut et al., 1982, Van Nieuwstadt et al., 1988, Sueyoshi et al., 1995, Pensaert, 1999, Shibata et al., 2000, Kim and Chae, 2001, Rodák et al., 2005). However, none of these techniques can be used for early detection of the viral antigen because they require clinical samples from the intestines of dead pigs and are cumbersome to use, resulting in rather long detection times. Reverse transcriptase polymerase chain reaction (RT-PCR), which includes single and multiplex RT-PCR, has proven to be a convenient and sensitive assay for detection of PEDV and TGEV (Paton et al., 1997, Kim et al., 2000, Kim et al., 2001). This technique also has its limitations; it has lower sensitivity (∼10–100 times) than real-time PCR, viral loads cannot be measured, and identifying the virus by agarose gel electrophoresis is time consuming (Keyaerts et al., 2006). RT-PCR also poses the potential risk of cross contamination during mass screening within the reverse transcription step. These drawbacks reduce the overall effectiveness of using RT-PCR to detect virus in new outbreaks and previous outbreak sites (Van Rijn et al., 2004, Ophuis et al., 2006).
Real-time PCR has the advantages that it is very accurate and sensitive, allowing for high-throughput screening and quantification of viral loads using small volumes. Therefore, it shows considerable promise as a potential alternative to molecular assays for the detection of viral RNA in clinical samples (Giulietti et al., 2001, Bustin, 2000). Recently, real-time RT-PCR using light-upon-extension (LUX) fluorogenic primers have been reported for the detection of TGEV (Chen et al., 2004). In this study, multiplex real-time RT-PCR was developed for differential detection and quantification of the viral loads of TGEV and PEDV in diarrhea from both experimentally and naturally infected piglets using two sets of primers and different colored probes in a single reaction tube.
2. Materials and methods
2.1. Viruses
The TGEV 175L and PEDV SM98 strains were isolated from the intestines and feces of infected piglets, respectively. The TGEV 175L strain was cultured in swine testicle (ST) cells using alpha-minimum essential medium (α-MEM) supplemented with 5% fetal calf serum and antibiotic/mycotic solution. The PEDV SM98 strain was cultured in African green monkey kidney cells (Vero cells) using the same medium. The TGEV 175L and PEDV SM98P strains were titrated to 1 × 105.0 and 1 × 104.0 TCID50/ml, respectively. The specificity of the assay was evaluated with the PEDV CV777 strain (Kocherhans et al., 2001), TGEV Purdue and Miller strain (Kapke and Brian, 1986, Wesley et al., 1990), reovirus Jones strain (Dermody et al., 1991), enterovirus Sukyung stain (isolated strain), rotavirus Gotffried, and OSU strain (Li and Gorziglia, 1993). Viral RNA was extracted from each sample using the RNeasy® Mini kit (Qiagen) according to the manufacturer's instructions.
2.2. Designing primers and probes
The primers and probes used for multiplex real-time RT-PCR were designed and synthesized in cooperation with TIB MOLBIOL (TIB MOLBIOL Syntheselabor GmbH, Germany). The TGEV and PEDV sequences were aligned using Clustal X (version 1.81; Thompson et al., 1997), and the highly conserved regions within each virus genome were identified. Primers and probes corresponding to the conserved regions were designed using sequence alignments of the nucleocapsid (N) genes from 10 strains of TGEV (Purdue 46-MAD, NC002306; TO14, AF302264; TS, DQ201447; SC-Y, DQ443743; Miller M6, DQ811785; TH-98, AY676604; HN2002, AY587884; 96-1933, AF104420; FS772/70, Y00542) and from five strains of PEDV (CV777, AF353511; Chinju99, AF237764; CH/S, DQ35524; S, DQ35223; JS2001, AY539715). The four primers were designed with a similar T m that was ∼10 °C lower than that of the probes to prevent any dimeric interactions from forming. For color multiplexing, the probe for TGEV was labeled with the 5′-reporter dye 6-carboxyfluorescein (FAM) and the 3′-quencher BHQ1, and the probe for PEDV was labeled with the 5′-reporter dye Cy-5 and the 3′-quencher BHQ2. The sequences and amplicon sizes of the primers and probes are listed in Table 1 . The specificity of the primers and probes was confirmed against random nucleotide sequences using a BLAST search in GenBank databases located in the National Center for Biotechnology Information (NCBI, http://www.ncbi.nlm.nih.gov/BLAST/).
Table 1.
Sequences of primers and probes used in this study
| Virus | Primer and probe name | Sequence (5′–3′) | Nucleotide position | Amplicon size (bp) |
|---|---|---|---|---|
| TGEV | TGENF TGENR | GCAGGTAAAGGTGATGTGACAA ACATTCAGCCAGTTGTGGGTAA | 27,637−27,756a | 120 |
| TGE-FAM | 6FAM-TGGCACTGCTGGGATTGGCAACGA-BHQ1 | 27,707–27,730 | ||
| PEDV | PEDNF PEDNR | CGCAAAGACTGAACCCACTAATTT TTGCCTCTGTTGTTACTTGGAGAT | 26,679−26,876b | 198 |
| PED-Cy5 | Cy5-TGTTGCCATTGCCACGACTCCTGC-BHQ3 | 26,819–26,842 | ||
The position of the primer is based on the Purdue strain of TGEV.
The position of the primer is based on the CV777 strain of PEDV.
2.3. Standard curves and variability of the assay
Run-off transcripts were generated for use as standards in the assay with the MEGAshortscript T7 kit (Ambion, USA) following the manufacturer's suggestions. Briefly, a reaction mixture with a final volume of 20 μl containing 150 ng of the PCR product, 2 μl of each nucleotide triphosphate, 2 μl of enzyme mix, and 2 μl of reaction buffer was incubated at 37 °C overnight for in vitro transcription. The cDNA was removed by digestion with 2 U of RNase-free DNase I for 15 min at 37 °C. After precipitating with sequential ethanol solutions, the transcripts were dissolved in 200 μl of RNase-free H2O and stored at −70 °C until used. The concentration of transcripts was determined using an ND-1000 NanoDrop spectrophotometer (NanoDrop Technologies, USA) at 260 nm, from which the molecular number was calculated (Fronhoffs et al., 2002). Tenfold serial dilutions of the transcripts were prepared at concentrations of 9 × 107 to 9 × 101 copies of TGEV and 7 × 107 to 7 × 101 copies of PEDV per 2 μl volume and stored at −80 °C. Multiplex real-time RT-PCR was performed using these standard solutions to obtain standard curves. Intra-assay variability was evaluated with three independent samples of each virus tested in triplicate, demonstrating copy numbers of 9 × 107, 9 × 104, and 9 × 102 for TGEV and 7 × 107, 7 × 104, and 7 × 102 for PEDV. Inter-assay variability was also determined by testing the same three samples in triplicate over 3 days.
2.4. Multiplex real-time RT-PCR
The quantitative one-step RT-PCR kit (Invitrogen Life Technologies™, USA) was used for multiplex real-time RT-PCR. Multiplex real-time RT-PCR was carried out in a 20 μl reaction containing 2 μl of RNA or transcripts, 0.5 μl of both TGEV forward and reverse primer, 0.5 μl of both PEDV forward and reverse primer, 0.5 μl of PEDV-Cy5 probe, 0.5 μl of TGEV-FAM probe, 0.8 μl of ThermoScript™ plus/ Platinum® Taq Enzyme Mix, 10 μl of 2× ThermoScript Reaction Mix (a final concentration of 3 mM MgCl2), and 4.2 μl of water. The reaction took place using an iCycler IQ™ multicolor real-time detection system (Bio-Rad, USA) under the following conditions: initial reverse transcription at 58 °C for 30 min, followed by initial denaturation at 95 °C for 5 min, 40 cycles of denaturation at 95 °C for 30 s, and annealing and extension at 60 °C for 1 min. The optical data were analyzed using iCycler iQ™ Optical System Software (version 3.1). For each determination, the threshold lines used were the automatic settings at 185.2 and 72.9 relative fluorescence units (RFU) for specific product analysis of TGEV and PEDV, respectively.
To confirm the differential detection of TGEV and porcine respiratory coronavirus (PRCV), multiplex one-step RT-PCR was performed using a one-step RT-PCR kit (Qiagen). Primers were designed to amplify the spike gene, including the deletion region of TGEV, leading to differentiation from PRCV (forward, 5′-GCCATTGATTTATGGAGACA-3′; reverse, 5′-GTATAAAACCTCCTGGCTGT-3′). Briefly, multiplex one-step RT-PCR was carried out in a final reaction volume of 25 μl containing 5 μl of RNA, 1 μl each of the forward and reverse primers (0.8 pmol), 1 μl of dNTP mix (0.2 mM each dNTP), 1 μl of enzyme mix (2.5 U), 5 μl of 5× buffer, and 11 μl of RNase-free water. PCR was performed under the following conditions: initial reverse transcription at 45 °C for 30 min, followed by initial denaturation at 94 °C for 15 min, and then 35 cycles of 94 °C for 45 s, 53 °C for 45 s, and 72 °C for 1 min. The amplicons were a 782-bp fragment of TGEV and 161-bp fragment of PRCV.
2.5. Clinical samples from experimentally infected piglets
Samples of diarrhea were taken from 3-day-old piglets inoculated with each virus. Two piglets were inoculated orally with 1 ml of virulent TGEV (Jeon3 strain, Korean isolated strain, 106.0 PID50/ml) and another two with PEDV using 1 ml of virulent PEDV (SM98 strain, 102.0 LD50/ml). The two groups of piglets were maintained in separate isolation units with their respective sows. Neither sow used in this study had antibodies for TGEV or PEDV, as confirmed by a serum neutralization test. Stool samples were collected with cotton swabs from piglets with diarrhea daily until death. The samples were diluted 1:10 in phosphate buffered saline (PBS, 0.1 M, pH 7.2), vortexed, and clarified by centrifugation for 10 min at 4000 × g to eliminate fecal debris. Viral RNA was extracted from 300 μl of the diluted sample and eluted in 30 μl RNase–DNase free water. RNA was extracted to quantify the viral load by multiplex real-time RT-PCR.
2.6. Clinical samples from naturally infected piglets
Stool and intestinal specimens were obtained from the Provincial Institute for Animal Health; these had been collected from infected swine on six Korean farms (farms KS, KB, KK, KD, IY for PED, and KL for TGE) between December 2005 and May 2006 (Table 2 ). The institute initially screened the samples as PEDV or TGEV positive with an indirect fluorescent assay using cryocut-sections and RT-PCR. RNA was extracted by vortexing 1 g of stool sample with 10 ml of PBS or by grinding 1 g of intestine with 5 ml of MEM. The specimens and extracted RNA were stored at −70 °C until used. The viral load of each sample was measured in duplicate using multiplex real-time RT-PCR.
Table 2.
Details of the outbreak farms, samples, and the quantification of viral loads
| Farm | No. of sows | Percentage mortality | Result of diagnosis | Viral loads (log10 copies.) | Sample |
|---|---|---|---|---|---|
| KS | 88 | 48.3 (290/600)a | PED | 2.66 | Feces |
| KB | 516 | 41.2 (140/340) | PED | 2.94 | Feces |
| KK | 100 | 50.0 (10/20) | PED | 4.63 | Feces |
| KD | 1000 | 66.6 (1000/1500) | PED | 2.97 | Feces |
| IY | 56 | 100.0 (100/100) | PED | 4.82 | Feces |
| KL | 100 | 20.0 (200/1000) | TGE | 2.87 | Intestine |
The number of dead piglets/number of infected piglets.
3. Results
3.1. Multiplex reaction parameters
The primer and probe sequences were designed from the conserved regions of the TGEV and PEDV nucleocapsid genes for universal detection of the strains used. The concentration of primers and probes was set at 0.5 μM to reach the threshold cycle (C T-value) rapidly and to ensure optimal fluorescence intensity while avoiding any mutual interference. Varying or increasing the probe/primer concentrations delayed the C T-value at entire high concentrations of transcript and lowered sensitivity toward both viruses. Standard conditions were used for the annealing temperature and cycle number (as stated in Section 2) for the multiplex real-time RT-PCR when evaluating the optimal working conditions for the primers and probes. The specificity of the assay was evaluated using other diarrhea-causing viruses such as enterovirus, rotavirus, and reovirus. No cross-reactivity was detected between the viruses in the multiplex real-time RT-PCR (data not shown).
3.2. Evaluation of multiplex real-time RT-PCR
Transcripts of TGEV (9 × 1012) and PEDV (7 × 1012) were used for quantifying the viral RNA load. Standard curves were plotted using 10-fold serial dilution stock solutions for the multiplex real-time RT-PCR (Fig. 1 ). As shown in Fig. 1, the standard curves of TGEV and PEDV were plotted with slopes of −3.214 and −3.219, respectively. The curves were used to determine the viral load of each sample, with detection limits at 9 × 101 TGEV and 7 × 101 PEDV copies.
Fig. 1.
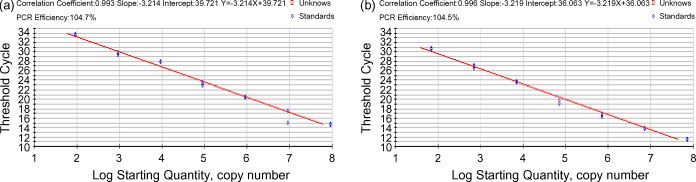
Standard curves for the 10-fold dilution stock solutions using 9 × 107 to 9 × 101 copies of TGEV transcripts (a) and 7 × 107 to 7 × 101 copies of PEDV transcripts (b) CT values (y-axis) are dependent on the log of the input amount of transcripts (x-axis).
The reproducibility and precision of the assay was confirmed by evaluating the variations in the intra-assay using transcript standards with 107, 104, and 102 range copies for each virus in three independent runs with three samples.
The intra-assay variability of TGEV was low with a coefficient of variation (CV) ranging between 0.78 and 1.85% at 9 × 102 copies, between 0.25 and 2.14% at 9 × 104 copies, and between 0.53 and 2.22% at 9 × 107 copies. The variability for PEDV was also low, in the range of 0.50–3.43% for each copy. Inter-assay variability was also evaluated using the same transcript standards in three assay runs performed over 3 days. The variability of CV was low, with values between 1.56 and 1.83% for TGEV and 2.21 and 3.33% for PEDV. The 10-fold serial dilutions of TCID50/ml (between 105 and 1.0) were assayed for both TGEV and PEDV. The 105.0 TCID50/ml TGEV had an RNA viral load of 107.72 copies, while the 104.0 TCID50/ml PEDV had 106.89 copies.
3.3. Application of multiplex real-time RT-PCR to clinical samples
Piglets inoculated with virulent TGEV shed the virus on either the 2nd or 3rd day, followed by profuse diarrhea that persisted until death 3–5 days after inoculation. One of the piglets died earlier than the other piglets, 3 days after inoculation. The load of TGEV shed in diarrhea was 104.80 copies on the 2nd day, peaking at 105.34 copies on the 4th day, and then decreasing until death (Fig. 2 ).
Fig. 2.
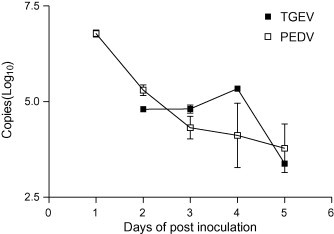
Detection and quantification of TGEV and PEDV shed in diarrhea from 3-day-old piglets inoculated with TGEV and PEDV using multiplex real-time RT-PCR.
At peak levels, more PEDV was shed than TGEV from the 1st day after inoculation until death. Approximately 106.78 copies of PEDV were shed in diarrhea on the 1st day, with the copy number decreasing over the course of the experiment (Fig. 2).
Once the assay conditions were established, the assay was applied to clinical samples from naturally infected piglets. The amount of PEDV RNA from clinical samples varied between 102 and 105 copies (Table 2). TGEV had 102.87 copies by real-time RT-PCR (Table 2). Non-specific reactions sometimes occurred with samples with late C T values (C T > 35), which were considered artifacts. The viral load for only one clinical sample of TGEV was evaluated, since there have been only a few reported TGE outbreaks in Korea over the past several years.
4. Discussion
PED is a major cause of viral diarrhea in piglets that results in heavy economic losses in Korea each year. Data on PED outbreaks have been compiled by the Animal Infectious Disease Data Management System (AIMS) of the National Veterinary Research & Quarantine Service (http://www.maf.go.kr/user.tdf?a=user.maf_portal.data.DataApp&c=1002&mc=03040200&fn=stat04_02.htm) every year since 2000, following the first report by Kweon et al. (1993). It shows that PED outbreaks have been recorded consistently. The majority of piglets infected with PEDV experienced symptoms of diarrhea that ultimately resulted in death. Interestingly, PEDV commonly infects finishing pigs in Europe (Pritchard et al., 1999). TGE outbreaks, unlike PED outbreaks, decreased until late 2005, according to the AIMS data. Chae et al. (2000) reported that PRCV, a mutant strain of TGEV, could be responsible for the recent reduction in the number of outbreaks of TGE in Korea.
Multiplex real-time RT-PCR provides rapid and sensitive laboratory detection and quantification of multiple specific targets in one test tube while reducing the number of experimental steps. This minimizes the risk of contamination, ensuring a better understanding of the epidemiology of an outbreak.
In developing multiplex real-time RT-PCR, the conditions for the assay were optimized by selecting primers and probes based on the N genes of the Purdue 46-MAD strain of TGEV and the CV777 strain of PEDV, which are highly conserved sequences with low homology (Sánchez et al., 1990, Kocherhans et al., 2001). The primers and probe for PEDV, the genome sequences of which had up to two mismatches from only a small group of reference strains, proved very effective and were capable of detecting PEDV in all strains, including the field strains used in this study. Probes labeled with different reporter dyes to minimize the overlap in emission wavelengths were prepared. The protocol then was optimized by using the same concentrations for both sets of primers and probes, developing an assay that could detect and differentiate TGEV and PEDV without cross-interference. This assay was specific for TGEV and PEDV, and no cross-reaction with other diarrhea-causing viruses was found.
To calculate the viral loads of unknown samples, standard curves were prepared with run-off transcripts by direct transcription using a T7 promoter (Stram et al., 2004). Similar dynamic ranges were obtained from 10-fold serial dilutions of both transcripts, and the detection limits were determined to be 90 and 70 copies of TGEV and PEDV, respectively. The variability of the assay using both transcripts was low, which was similar to or slightly higher than the amplification data from the intra- and inter-assay compared to reports for real-time PCR (Pugnale et al., 2006, Keyaerts et al., 2006). Using standard curves, the amount of viral RNA in infected ST and VERO cells was estimated to compare the sensitivity of this method with conventional PCR. The detection limit of the assay was 1 TCID50/ml for both viruses (data not shown), which is ∼10-fold higher than previously reported (Paton et al., 1997, Kim et al., 2000, Kim et al., 2001). RT-PCR-based dot blot hybridization reported by Jung and Chae (2005) shows more sensitive than multiplex real-time RT-PCR. However, real-time RT-PCR generates results within 3 h, which is significantly faster than the more than 1 day required to complete RT-PCR-based dot blot hybridization.
The efficiency of the multiplex real-time RT-PCR assay was determined by testing clinical samples from experimentally infected piglets. Both viral loads in diarrhea were measured starting 1 or 2 days after inoculation with each virus, continuing until death. The viral loads of TGEV were first calculated on day 2, peaked on day 4, and then decreased until death. For PEDV, the viral load reached its highest level on day 1 and then decreased until death. In this study, the virus shedding time, viral loads, and period of viral persistence differed from those previously reported due to differences in titers, the size of the viral inoculum, and the ages of the experimental pigs. De Arriba et al. (2002) reported that PEDV peaked after 5 days as determined by fecal swabs and decreased for 8 days after inoculation.
The viral loads of PEDV and TGEV obtained from samples in naturally infected piglets varied by farm. According to Song et al. (2006), viral levels and piglet mortality show a low degree of correlation; some of our data were consistent with that report. Nevertheless, a correlation was noted: the larger the viral load detected by multiplex real-time RT-PCR, the higher the rate of piglet mortality. The results show that this assay can be used to determine viral loads in pig herds, which makes it an additional factor for evaluating the status of the affected farms. Furthermore, farms experiencing a disease outbreak can use information on viral loads to determine the most appropriate treatment, such as selection of vaccine type to protect their herds against future epidemics as described for quantitative RT-PCR using a PEDV internal control by Song et al. (2006). To optimize calculation of viral load by this assay, virus loads must be evaluated on farms where outbreaks are just beginning or ending to elucidate the potential transmission of both viral infections on pig farms.
It is very important to differentiate the two viral diarrhea diseases rapidly, as they can be difficult to distinguish clinically in infected piglets, so that veterinarians can quickly control outbreaks and understand the entire epidemiology. This study showed that multiplex real-time RT-PCR with standard curves is a useful method for differential and quantitative diagnosis of piglets infected with diarrhea-causing TGEV and PEDV. Therefore, this assay may be useful as an alternative to the current diagnostic tools used for the detection of viruses, which tend to underestimate the infection status of outbreaks on farms.
Acknowledgment
This study was financed by a grant from the National Veterinary Research and Quarantine Service, Korean Ministry of Agriculture and Forestry.
References
- Bustin S.A. Absolute quantification of mRNA using real-time reverse transcription polymerase chain reaction assays. J. Mol. Endocrinol. 2000;25:169–193. doi: 10.1677/jme.0.0250169. [DOI] [PubMed] [Google Scholar]
- Callebaut P., Debouck P., Pensaert M. Enzyme-linked immunosorbent assay for the detection of the coronavirus-like agent and its antibodies in pigs with porcine epidemic diarrhea. Vet. Microbiol. 1982;7:295–306. doi: 10.1016/0378-1135(82)90009-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chae C., Kim O., Choi C., Min K., Cho W.-S., Kim J., Tai J.H. Prevalence of porcine epidemic diarrhoea virus and transmissible gastroenteritis virus infection in Korean pigs. Vet. Rec. 2000;147(21):606–608. doi: 10.1136/vr.147.21.606. [DOI] [PubMed] [Google Scholar]
- Chen R., Huang W., Lin Z., Zhou Z., Yu H., Zhu D. Development of a novel real-time RT-PCR assay with LUX primer for the detection of swine transmissible gastroenteritis virus. J. Virol. Method. 2004;122:57–61. doi: 10.1016/j.jviromet.2004.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Arriba M.L., Carvajal A., Pozo J., Rubio P. Isotype-specific antibody-secreting cells in systemic and mucosal associated lymphoid tissues and antibody response in serum of conventional pigs inoculated with PEDV. Vet. Immunol. Immunopathol. 2002;84:1–16. doi: 10.1016/S0165-2427(01)00386-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debouck P., Pensaert M. Experimental infection of pigs with a new porcine enteric coronavirus, CV 777. Am. J. Vet. Res. 1980;41:219–223. [PubMed] [Google Scholar]
- Dermody T.S., Schiff L.A., Nibert M.L., Coombs K.M., Fields B.N. The S2 gene nucleotide sequences of prototype strains of the three reovirus serotypes: characterization of reovirus core protein sigma 2. J. Virol. 1991;65:5721–5731. doi: 10.1128/jvi.65.11.5721-5731.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fronhoffs S., Totzke G., Stier S., Wernert N., Rothe M., Bruning T., Koch B., Sachinidis A., Vetter H., Ko Y. A method for the rapid construction of cRNA standard curves in quantitative real-time reverse transcription polymerase chain reaction. Mol. Cell. Probe. 2002;16:99–110. doi: 10.1006/mcpr.2002.0405. [DOI] [PubMed] [Google Scholar]
- Giulietti A., Overbergh L., Valckx D., Decallonne B., Bouillon R., Mathieu C. An overview of real-time quantitative PCR: applications to quantify cytokine gene expression. Methods. 2001;25:386–401. doi: 10.1006/meth.2001.1261. [DOI] [PubMed] [Google Scholar]
- Jung K., Chae C. RT-PCR-based dot blot hybridization for the detection and differentiation between porcine epidemic diarrhea virus and transmissible gastroenteritis virus in fecal samples using a non-radioactive digoxigenin cDNA probe. J. Virol. Method. 2005;123:141–146. doi: 10.1016/j.jviromet.2004.09.012. [DOI] [PubMed] [Google Scholar]
- Kapke P.A., Brian D.A. Sequence analysis of the porcine transmissible gastroenteritis coronavirus nucleocapsid protein gene. Virology. 1986;151:41–49. doi: 10.1016/0042-6822(86)90102-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keyaerts E., Vijgen L., Maes P., Duson G., Neyts J., Van Ranst M. Viral load quantitation of SARS-coronavirus RNA using a one-step real-time RT-PCR. Int. J. Infect. Dis. 2006;10:32–37. doi: 10.1016/j.ijid.2005.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim B., Chae C. In situ hybridization for the detection of transmissible gastroenteritis virus in pigs and comparison with other methods. Can. J. Vet. Res. 2001;65:33–37. [PMC free article] [PubMed] [Google Scholar]
- Kim O., Choi C., Kim B., Chae C. Detection and differentiation of porcine epidemic diarrhoea virus and transmissible gastroenteritis virus in clinical samples by multiplex RT-PCR. Vet. Rec. 2000;146:637–640. doi: 10.1136/vr.146.22.637. [DOI] [PubMed] [Google Scholar]
- Kim S.Y., Song D.S., Park B.K. Differential detection of transmissible gastroenteritis virus and porcine epidemic diarrhea virus by duplex RT-PCR. J. Vet. Diagn. Invest. 2001;13:516–520. doi: 10.1177/104063870101300611. [DOI] [PubMed] [Google Scholar]
- Kocherhans R., Bridgen A., Ackermann M., Tobler K. Completion of the porcine epidemic diarrhoea coronavirus (PEDV) genome sequence. Virus Genes. 2001;23:137–144. doi: 10.1023/A:1011831902219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kweon C.H., Kwon B.J., Jung T.S., Kee Y.J., Hur D.H., Hwang E.K., Rhee J.C., An S.H. Isolation of porcine epidemic diarrhea virus (PEDV) in Korea. Korean J. Vet. Res. 1993;33:249–254. [Google Scholar]
- Li B., Gorziglia M. VP4 serotype of the Gottfried strain of porcine rotavirus. J. Clin. Microbiol. 1993;31:3075–3077. doi: 10.1128/jcm.31.11.3075-3077.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ophuis R.J., Morrissy C.J., Boyle D.B. Detection and quantitative pathogenesis study of classical swine fever virus using a real-time RT-PCR assay. J. Virol. Method. 2006;131:78–85. doi: 10.1016/j.jviromet.2005.07.008. [DOI] [PubMed] [Google Scholar]
- Paton D., Ibata G., Sands J., McGoldrick A. Detection of transmissible gastroenteritis virus by RT-PCR and differentiation from porcine respiratory coronavirus. J. Virol. Method. 1997;66:303–309. doi: 10.1016/S0166-0934(97)00055-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pensaert M.B. Porcine epidemic diarrhea. In: Straw B.E., D’Allaire S., Mengeling W.L., Taylor D.I., editors. Diseases of Swine. 8th ed. Iowa State University Press; Ames: 1999. pp. 179–185. [Google Scholar]
- Pritchard G.C., Paton D.J., Wibberley G., Ibata G. Transmissible gastroenteritis and porcine epidemic diarrhoea in Britain. Vet. Rec. 1999;144:616–618. doi: 10.1136/vr.144.22.616. [DOI] [PubMed] [Google Scholar]
- Pugnale P., Latorre P., Rossi C., Crovatto K., Pazienza V., De Gottardi A., Negro F. Real-time multiplex PCR assay to quantify hepatitis C virus RNA in peripheral blood mononuclear cells. J. Virol. Method. 2006;133:195–204. doi: 10.1016/j.jviromet.2005.11.007. [DOI] [PubMed] [Google Scholar]
- Rodák L., Valíček L., Šmíd B., Nevoránková Z. An ELISA optimized for porcine epidemic diarrhea virus detection in faeces. Vet. Microbiol. 2005;105:9–17. doi: 10.1016/j.vetmic.2004.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saif L.J., Wesley R.D. Transmissible gastroenteritis and porcine respiratory coronavirus. In: Straw B.E., D’Allaire S., Mengeling W.L., Taylor D.I., editors. Diseases of Swine. 8th ed. Iowa State University Press; Ames: 1999. pp. 295–325. [Google Scholar]
- Sánchez C.M., Jiménez G., Laviada M.D., Correa I., Suné C., Bullido M., Gebauer F., Smerdou C., Callebaut P., Escribano J., Enjuanes L. Antigenic homology among coronaviruses related to transmissible gastroenteritis virus. Virology. 1990;174:410–417. doi: 10.1016/0042-6822(90)90094-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata I., Tsuda T., Mori M., Ono M., Sueyoshi M., Uruno K. Isolation of porcine epidemic diarrhea virus in porcine cell cultures and experimental infection of pigs of different ages. Vet. Microbiol. 2000;72:173–182. doi: 10.1016/S0378-1135(99)00199-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song D.S., Kang B.K., Lee S.S., Yang J.S., Moon H.J., Oh J.S., Ha G.W., Jang Y.S., Park B.K. Use of an internal control in a quantitative RT-PCR assay for quantitation of porcine epidemic diarrhea virus shedding in pigs. J. Virol. Method. 2006;133:27–33. doi: 10.1016/j.jviromet.2005.10.021. [DOI] [PubMed] [Google Scholar]
- Stram Y., Kuznetzova L., Guini M., Rogel A., Meirom R., Chai D., Yadin H., Brenner J. Detection and quantitation of akabane and aino viruses by multiplex real-time reverse-transcriptase PCR. J. Virol. Method. 2004;116:147–154. doi: 10.1016/j.jviromet.2003.11.010. [DOI] [PubMed] [Google Scholar]
- Sueyoshi M., Tsuda T., Yamazaki K., Yoshida K., Nakazawa M., Sato K., Minami T., Iwashita K., Watanabe M., Suzuki Y., Mori M. An immunohistochemical investigation of porcine epidemic diarrhoea. J. Comp. Pathol. 1995;113:59–67. doi: 10.1016/S0021-9975(05)80069-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson J.D., Gibson T.J., Plewniak F., Jeanmougin F., Higgins D.G. The ClustalX windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 1997;24:4876–4882. doi: 10.1093/nar/25.24.4876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Nieuwstadt A.P., Cornelissen J.B., Vreeswijk J. Solid phase immune electron microscopy for diagnosis of transmissible gastroenteritis in pigs. Res. Vet. Sci. 1988;44(3):286–294. doi: 10.1016/S0034-5288(18)30859-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Rijn P.A., Wellenberg G.J., Hakze-van der Honing R., Jacobs L., Moonen P.L., Feitsma H. Detection of economically important viruses in boar semen by quantitative real-time PCR technology. J. Virol. Method. 2004;120:151–160. doi: 10.1016/j.jviromet.2004.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wesley R.D., Woods R.D., Cheung A.K. Genetic basis for the pathogenesis of transmissible gastroenteritis virus. J. Virol. 1990;64:4761–4766. doi: 10.1128/jvi.64.10.4761-4766.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]


