Abstract
The interplay between avian reovirus (ARV) replication and apoptosis and proteasome pathway was studied in cultured cells. It is shown that inhibition of the proteasome did not affect viral entry and host cell translation but had influence on ARV replication and ARV-induced apoptosis. Evidence is provided to demonstrate that ubiquitin-proteasome blocked ARV replication at an early step in viral life cycle. However, viral transcription and protein translation were also reduced markedly after addition of proteasome inhibitor MG132. Treatment of BHK-21 cells with the MG132 markedly decreased virus titer as well as prevented virus-induced apoptosis. The expression of ARV proteins σC, σA, and σNS was also reduced markedly, suggesting that suppression of virus replication is due to down-regulation of these ARV proteins by ubiquitin-proteasome system. MG132 was also shown to suppress ARV σC-induced phosphrylation of p53 on serine 46, caspase 3 activities, and DNA fragmentation leading to complete inhibition of ARV-induced apoptosis.
Keywords: Avian reovirus, Proteasome inhibitor MG132, Ubiquitin-proteasome pathway, Apoptosis
1. Introduction
Avian reovirus (ARV) causing arthritis, chronic respiratory diseases, and malabsorption syndrome leads to considerable economic losses (Kibenege and Wilcox, 1983). ARV-encoded proteins, including at least 10 structural proteins and 4 non-structural proteins have been demonstrated (Varela and Benavente, 1994, Bodelon et al., 2001). In the σ-class proteins of ARV, protein σC, is a minor outer-capsid protein of ARV that is encoded by the largest open reading frame of the S1 segment (Varela and Benavente, 1994, Bodelon et al., 2001). It is not only an attachment protein (Martinez-Costas et al., 1997) but also an apoptosis inducer (Shih et al., 2004). Some studies have suggested that σC is the target for type-specific neutralizing antibodies while σB is target for group-specific antibodies (Wickramasinghe et al., 1993). Protein P10 is a viroporin (Bodelon et al., 2002) responsible for ARV-induced cell fusion (Bodelon et al., 2001). A recent report suggests that P17 retards cell growth by activation of P53 pathway (Liu et al., 2005). ARV σA, encoded by the genome segment S2 (Yin et al., 2000), has been identified as a double-stranded RNA (dsRNA) binding protein (Yin et al., 2000) and is an inhibitor of the double-stranded RNA-dependent protein kinas (Gonzalez-Lopez et al., 2003). Another ARV protein, σNS, encoded by the genome segment S4 (Chiu and Lee, 1997), has been reported for its single-stranded RNA (ssRNA) binding activity (Yin and Lee, 1998).
The ubiquitin-proteasome pathway, a major intracellular protein degradation pathway in eukaryotic cells (Myung et al., 2001), plays an important role in a wide variety of cellular functions, including signal transduction, antigen processing, cell cycle regulation, transcription regulation, DNA repair, and apoptosis (Ciechanover, 1994, Glickman and Ciechanover, 2002). In the present study, attempts were made to explore the role of proteasome inhibition in ARV infectivity and the mechanisms involved in the proteasome inhibitor suppression of ARV replication and apoptosis induction in cultured cells.
It was shown previously that ARV σC is an apoptosis inducer that causes apoptosis by linking Src kinase to p53-mitochondrial pathway in cultured cells (Shih et al., 2004, Lin et al., 2006). Using the proteasome inhibitor MG132 to inhibit the cellular proteasome pathway, it was found that MG132 could reduce ARV-induced apoptosis, cytopathic effect (CPE), virus titer, and protein expression. Inhibition of the ubiquitin-proteasome pathway did not affect viral entry and host cell translation. To our knowledge, this is the first finding demonstrating that ARV replication and ARV-induced apoptosis could be suppressed by MG132.
2. Materials and methods
2.1. Antibodies
Two monoclonal antibodies against ARV σA and σNS were kindly provided by Dr. Long H. Lee (Hou et al., 2001, Pai et al., 2003, Huang et al., 2005). The monoclonal antibody against ARV σC was a laboratory stock (Hsu et al., 2006). The monoclonal antibody, which detects ser15-phosphorylated p53 was purchased from R&D systems Inc. (Minneapolis, MN, USA). The polyclonal antibodies against GFP and actin were from Invitrogen (Carlsbad, CA, USA) and Chemicon (Temecula, CA, USA), respectively.
2.2. Cell viability assay
A modified 3,4-(5-dimethylthiazol-2-yl)-5-(3-carboxyme-thoxy phenyl)-2-(4-sulfophenyl)-2H-tetrazolium salt (MTT) assay, which measures mitochondrial function, was used to determine cell viability according to the manufacturer's instruction (Promega, Madison, WI, USA). BHK-21 cells were pretreated with various concentrations of MG132 and then infected with ARV s1133 (MOI = 5) for 1 h. Cell viability was assessed by MTT assay at 18 h post-infection (h.p.i.).
2.3. Virus titer
ARV s1133 propagated in BHK-21 cells was described previously (Liu et al., 2003, Liu et al., 2004). To study the effect of MG132 on ARV replication and virus internalization, BHK-21 cells were infected with ARV at various MOIs or treated with various concentrations of MG132. Virus titers were determined at 18 h.p.i. by a plaque assay and Western blot for confirmation of expression of σA, σNS, and σC proteins. Representative morphological changes of BHK-21 cells treated with MG132 or DMSO were observed by a phase-contrast microscopy at 18 h.p.i.
To explore the effect of proteasome inactivation during the course of ARV infection, BHK-21 cells were treated with MG132 at different times. The cells were washed to remove the drug and further incubated until 18 h. Whole cell lysates were collected at the indicted time points and a plaque assay was done for each lysate to determine the virus yield during preceding 6-h period.
2.4. Plasmid construction and reverse transcription and polymerase chain reaction (RT-PCR)
The GFP gene was derived from the commercial vector pIκB-GFP (Invitrogen). Following respective treatments with Bam HI, Klenow, and Not I, the GFP fragment separated from pIκB-GFP was cloned into pcDNA3.1 (−) vector. The construct was named pcDNA-GFP. In addition, the construction of pcDNA-σC has been described previously (Shih et al., 2004).
To investigate the effect of MG132 on ARV production was through the reduction of ARV RNA levels, RNA extracted from BHK-21 cells 18 h.p.i. in the presence or absence of MG132 was amplified by RT-PCR. The primers for amplification of σA and GAPDH were as follows: σA forward primer, 5′-GGCTTCTACTTCTCCTCGAAG ACTC-3′ (identical to nucleotides 700–724) and σA reverse primer, 5′-AGAAGTCATTA GCCTCCTGCGT TA-3′ (complementary to nucleotides 1597–1625); GAPDH forward primer, 5′-CATTGACCTCAACTACATGG-3′, GAPDH reverse primer, 5′-TTGCCCA CAGCCTTGGCAGC-3′. Reverse transcription was carried out at 50 °C for 30 min. PCR reactions were subjected to 35 cycles consisting of denaturation for 1 min at 94 °C, annealing for 1 min at 55 °C and, extension for 90 s at 72 °C, and one final extension cycle at 72 °C for 7 min.
2.5. Western blot and immunofluorescent assay (IFA)
Cells in 60 mm dishes were infected with ARV at 5 MOI or transfected with 5 μg of plasmid DNA using superfect reagent (Qiagen, Valencia, USA). After incubation for 1 h, the medium was removed and cells were grown for an additional 18 h. Cells were harvested, washed, and lysed in 0.5 ml RIPA lysis buffer (50 mM Tris, pH 7.4, 150 mM NaCl, 1% Nonidet p-40, 1 mM PMSF). Both the supernatant and pellet were collected, mixed with Laemmli sample buffer and boiled for 5 min. After resolving the samples on a 12% SDS-PAGE, the proteins were transferred to PVDF membranes. The GFP and ARV σC, σA, and σNS proteins were detected using their respective antibodies described above. The signal was detected by ECL reagent (Amersham Pharmacia Biotech, Hong Kong) and visualized by autoradiography.
For IFA assay, ARV s1133-infected BHK-21 cells were fixed in a mixture of 50% acetone and 50% methanol for 10 min, and then incubated with an anti-σC monoclonal antibody (Hsu et al., 2006). The bound antibody was visualized by immunostaining with FITC-conjugated second antibody raised against mouse IgG.
2.6. Detection of the ser46-phosphorylated p53, active caspase 3, and DNA ladder in ARV-infected BHK-21 cells
To study whether ARV-induced apoptosis could be blocked in BHK-21 cells by MG132, Western blot detection of the level of ser46-phosphorylated p53, active caspase 3 staining, and DNA fragmentation analysis were carried out. BHK-21 cells were either pre-incubated 30 min before infection or 2 h.p.i. with MG132. Cell lysates were collected and assayed from infected cells 18 h after treatment. Active caspase 3 in living cells was examined as described previously (Chulu et al., 2007).
3. Results
3.1. Protease inhibitor MG132 reduces viral progeny release and ARV-induced CPE
It was found that different doses of MG132 caused only slight cytotoxicity to BHK-21 cells (data not shown). ARV replication in untreated cells resulted in the generation of a viral titer of 107.5 PFU/ml after 18 h.p.i., wherease proteasome inhibition reduced the viral titer in a dose-dependent manner (Fig. 1A). The results indicated that the proteasome inhibitor significantly blocked ARV production, suggesting that the proteasome may play an important role in ARV replication. In this study, viral titers in the whole cell lysates harvested at 18 h.p.i. were reduced dramatically by MG132 (Fig. 1A), suggesting that the effect is due to the reduction in virus growth rather than to a delay in virus release. Different amounts of input virus change the absolute amount of progeny virus but do not affect significantly the percentage of reduction in viral titers, suggesting that the antiviral properties of MG132 are independent of the multiplicity of infection (Fig. 1B). In addition, ARV-induced CPE is characterized by cell fusion, detachment of infected cells from culture flask, cell lysis and death. ARV-induced CPE and cell death were reduced dramatically after the addition of MG132 (Fig. 1C).
Fig. 1.
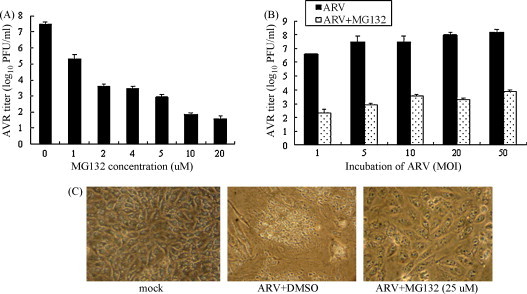
Proteasome inhibitor MG132 reduces ARV replication. (A) BHK-21 cells were infected with ARV (MOI = 5) and treated with various concentrations of MG132. Virus titers at 18 h.p.i. were determined by a plaque assay. (B) BHK-21 cells were infected with ARV at various MOIs and then treated with 25 μM of MG132 for 18 h. Virus titers at 18 h.p.i. were determined by a plaque assay. (C) Representative morphological changes of BHK-21 cells treated with MG132 or DMSO at 18 h.p.i. by a phase-contrast microscopy.
3.2. The ubiquitin-proteasome system is involved in an early step of virus replication but does not block virus internalization
Fig. 2A shows the experimental design for defining the major target of ubiquitin-proteasome system by following the MG132 treatment of ARV-infected cells at different time points in the viral life cycle. Fig. 2B shows that virus titer of the group treated for 0–6 h was 3.5 log units lower than the titer of the untreated sample. The virus titer of the groups treated for 6–12 h or 12–18 h was 2 and 0.5 log units lower than the titer of the non-treated sample. The kinetic study suggested that the ubiquitin-proteasome system is involved most likely at an early step in the virus life cycle, since the inhibitory effect of MG132 was seen primarily in the 0–6 h treatment group (Fig. 2B).
Fig. 2.
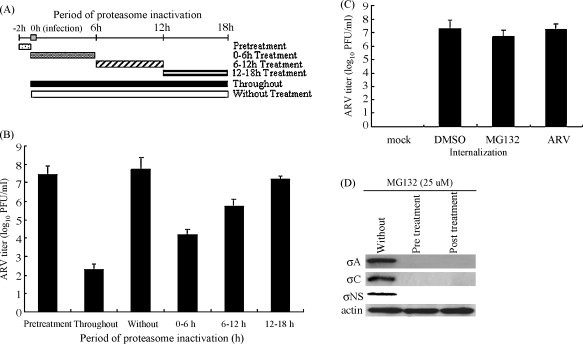
Effects of proteasome inactivation at increasing periods of time after infection and effect of MG132 on virus internalization. (A) Experimental design for pulse treatment with MG132 is shown. (B) BHK-21 cells were treated with MG132 during different time windows. The virus titer at each time point was examined by a plaque assay. (C–D) Effect of MG132 on virus internalization was evaluated. BHK-21 cells were either pre-incubated 30 min before infection or 2 h.p.i. with MG132. Whole cell extracts prepared from infected cells 18 h after treatment were assayed by examination of virus titer (C) and Western blot assay for the presence of σA, σNS, and σC expression (D) in ARV-infected BHK-21 cells. Values are means ± standard errors (SE) of three independent experiments.
As describe above, the MG132 has an inhibitory effect on ARV during early infection times. To dissect further each step in the presence of the MG132 to reveal the possible mechanism of inhibition, the virus internalization assay was carried out. There was no difference in virus titer in the presence or absence of MG132 (Fig. 2C). Viral proteins σA and σNS are important for ARV replication while σC is an apoptosis inducer. To determine whether the inhibitory effects of MG132 (25 uM) on ARV replication and apoptosis induction depend on their activities, we also examined their expression levels in ARV-infected BHK-21 cells. The results indicated that the expression of σA, σC, and σNS was reduced significantly in BHK-21cells either pre-incubated 30 min before infection or 2 h.p.i. with MG132 (Fig. 2D), suggesting that the ubiquitin-proteasome system is not involved in virus internalization.
3.3. ARV transcription and protein expression were suppressed by MG132
RT-PCR was carried out to investigate whether the effect of MG132 on ARV production was through the reduction in ARV RNA levels. The viral mRNA levels were dramatically decreased in ARV-infected BHK-21 cells treated with the MG132 (Fig. 3A). The ARV life-cycle consists of several steps. To determine which step(s) in the virus life cycle, the MG132 targeted, the reduction of viral titers was tested from whole cell lysates treated with proteasome inhibitor either 30 min before virus infection or 2 h.p.i. The results showed that exposure to MG132 inhibited significantly viral protein production 2 h.p.i. (Fig. 2D), suggesting that inhibition of viral replication by MG132 is likely not dependent on the blockage of viral entry into host cells.
Fig. 3.
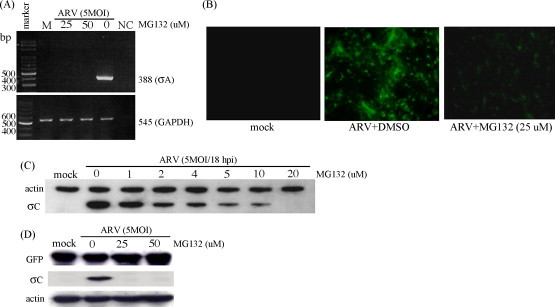
Proteasome inhibition decreases viral transcription and protein translation in ARV-infected cells. (A) The effects of proteasome inhibitor on ARV RNA level were examined. Total RNAs isolated from ARV-infected BHK-21 cells 18 h.p.i. after treatment with MG132 were subject to a RT-PCR analysis of ARV RNA. GAPDH was used as a control. M: mock infection; NC: water. (B) Representative IFA staining of ARV-infected BHK-21 cells used to detect σC expression. (C) BHK-21 cells were pre-incubated with various concentration of proteasome inhibitor MG132. Cell lysates were collected 18 h.p.i. and Western blot for σC was performed. This same blot was also stained with an antibody against actin to illustrate equal protein loading. Mock: without ARV. (D) To examine whether the protease inhibitor MG132 inhibits host cell translation, the pcDNA-GFP construct was transfected into ARV-infected BHK-21cells treated with various concentrations of MG132. Cell lysates were collected from infected cells 18 h.p.i. were assayed by Western blot for the presence of GFP and σC protein expression in ARV-infected BHK-21 cells.
Cells positive for σC expression were examined at 18 h.p.i. by IFA and Western blot assay in the presence of different concentrations of MG132. The MG132-treated cells showed significant reduction in amount of cells positive for ARV σC expression (Fig. 3B). In the present study, treatment with MG132 decreased σC expression in a dose-dependent manner (Fig. 3C).
The pcDNA-GFP construct was transfected into cells infected with different MOIs to understand whether MG132 can influence protein translation in BHK-21 cells. Fig. 3D shows that the expression of GFP protein was not affected but the expression of ARV σC was suppressed in ARV-infected cells in the presence of various concentration of MG132. Taken together, the results suggested that proteasome inhibition decreased viral replication via suppression of protein translation.
3.4. The expression of ser46-phosphorylated p53 and active caspase 3 in ARV-infected BHK-21 cells was suppressed by MG132
In this study, the level of ser46-phosphorylated p53 was reduced significantly in the presence of MG132 in either BHK-21 cells pre-incubated 30 min before infection or 2 h.p.i. with MG132 in ARV-infected BHK-21 cells (Fig. 4A). In comparison to without MG132 or DMSO-treated groups, caspase 3 activated in ARV-infected BHK-21 cells treated with MG132 was reduced (Fig. 4B). Addition of proteasome inhibitor in the medium also inhibited internucleosomal DNA cleavage, demonstrating that ARV-induced apoptosis could be suppressed by MG132 (Fig. 4C).
Fig. 4.
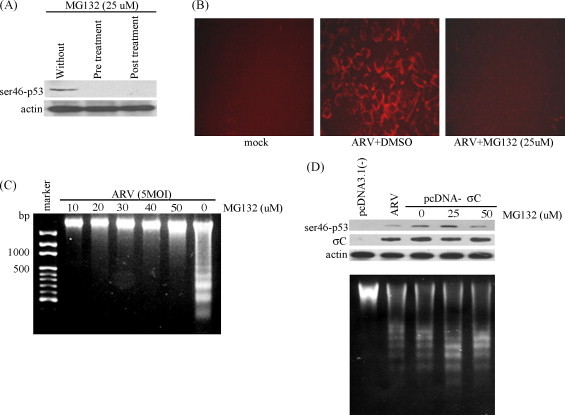
Inhibition of ARV σC-induced apoptosis by a protease inhibitor MG132. (A) BHK-21 cells were either pre-incubated 30 min before infection or 2 h.p.i. with MG132. Cell lysates collected from infected cells 18 h after treatment were assayed by Western blot assay for the presence of ser46-phosphorylated p53 protein expression in ARV-infected BHK-21 cells. (B) Activated caspase 3 staining of ARV-infected BHK-21 cells was carried out to examine inhibition of ARV-induced apoptosis by MG132. Mock and ARV-infected BHK-21 cells treated with or without MG132 were stained18 h.p.i. (C) BHK-21 cells were pre-incubated 30 min before infection with MG132. Cell lysates were collected from infected cells 18 h after treatment with various concentrations of MG132 and assayed by DNA fragmentation analysis. (D) BHK-21 cells were transfected with construct pcDNA-σC and then cells were treated with various concentrations of MG132. Cell lysates were collected 18 h after transfection and assayed by Western blot assay for the presence of ser46-phosphorylated p53 and σC protein expression (upper panel). DNA fragmentation assay was performed to check whether apoptosis was also induced in mock-treated, ARV-infected, and MG132-treated cells (lower panel). Actin, mock-transfection with pcDNA3.1 (−) and ARV-infected cells were used controls, respectively.
Fig. 4D shows that the transient expression of σC was not affected in ARV-infected BHK-21 cells in the presence of various concentrations of MG132. This confirmed further that the MG132 could not affect host cell translation. It was also seen that the elevated level of ser46-phosphorylated p53 (Fig. 4D; upper panel) and apoptosis induction in BHK-21 cells (Fig. 4D; lower panel) was due to ARV σC expression. With the exception of mock-transfection with the plasmid pcDNA3.1 (−), both ARV-infected cells without MG132 treatment and ARV σC-transfected cells with MG132 treatment showed internucleosomal DNA cleavage (Fig. 4D; lower panel).
4. Discussion
The 26S proteasome is a large multi-subunit complex that degrades selectively intracellular proteins. It is involved in a wide variety of mediated proteolytically intracellular process, such as apoptosis, cell cycle progression, transcriptional control, and metabolic regulation (Hilt and Wolf, 2000). Studies by many research groups have shown that various viruses have evolved sophisticated mechanisms to use or manipulate the host ubiquitin-proteasome pathway for their own needs. Retroviruses require the proteasome for budding from cells (Schuber et al., 2000), murine coronavirus for its transfer from endosome to cytoplasm during viral entry (Yu and Lai, 2005), minute virus of virus (MVM) and reovirus for nuclear translocation (Connolly et al., 2000, Ros and Kempf, 2004), and human cytomegalovirus for inducing cell cycle progression (Kalejta et al., 2003). Adenovirus requires active proteasomes to promote late gene expression (Galinier et al., 2002), Coxsackievirus for viral RNA transcription and protein synthesis (Luo et al., 2003), and tobacco mosaic virus for degradation movement protein (Reichel and Beachy, 2000). To date, whether the ubiquitin-proteasome pathway-mediated ARV replication and apoptosis induction is related to ubinquintin process or the proteasome degradation remains largely unknown. This study was therefore aimed at elucidating whether ARV also uses the ubiquitin-proteasome pathway for it's own benefit and other mechanisms for cell regulation. In this study, various aspects of the ARV-proteasome interplay were characterized. A proteasome inhibitor MG132 was used to explore the effect of proteasome inhibition on ARV replication and apoptosis induction by ARV. It was observed that MG132 was fully active against ARV when present at the beginning of the infection and a very limited effect was observed when present later after infection, suggesting that inhibition of ubiquitin-proteasome system did not affect virus entry and internalization and blocked ARV replication at an early step in viral life cycle.
Infection with avian reovirus causes severe CPE and apoptosis in both avian and mammalian cells. During the past several years, it was demonstrated that ARV σC could induce apoptosis in both culture cells and chicken tissues (Shih et al., 2004, Lin et al., 2007). Studies by our laboratory have demonstrated further that ARV-induced apoptosis by linking Src kinase to p53-mitochondrial pathway (Lin et al., 2006, Chulu et al., 2007). A recent report demonstrated that p53 regulates bax expression and translocation into the mitochondria leading to cytochrome c release into the cytoplasm, activating the caspase pathway that finally leads to cell apoptosis (Chulu et al., 2007). To date, the levels of ARV RNA transcription and protein synthesis in infected cells in the presence of MG132 were not clear. In the present study, the ARV RNA transcription and protein synthesis were shown to be inhibited dramatically in the ARV-infected cells after treatment with MG132. The expression of ser46-phosphorylated p53 and active caspase 3 in ARV-infected BHK-21 cells was reduced markedly following treatment with MG132. This suggested that MG132 decreased ARV infectivity and possessed the anti-apoptotic effect that may be due to reduced viral replication, especially down-regulation of σC expression (Fig. 3B–C).
How does the ubiquitin-proteasome pathway regulate ARV replication? Perhaps the ubiquitination of viral proteins is required for ubiquitin-proteasome mediated viral replication, such as ssRNA- and dsRNA-binding proteins σNS and σA which are essential for ARV replication. Elucidation of the ubiquitin-proteasome pathways involved in avian reovirus replication and induction of apoptosis will contribute important new information leading to better understanding of the mechanisms by which viruses in general cause cell death and diseases. Further study is undergoing to address these questions.
In conclusion, it was shown that proteasome inhibitor reduces ARV replication through inhibition of viral RNA transcription and protein synthesis, thus preventing ARV-induced apoptosis. The results suggest that ARV may have developed certain mechanisms, such as the ubiquitin-proteasome pathway, to inhibit apoptosis or viral replication by inactivating the p53 pathway.
Acknowledgement
This work was supported by the National Science Council (NSC-96-2313-B-020-001 & NSC-95-2313-B-020-009-MY3), Taiwan.
References
- Bodelon G., Labrada L., Martinez-Costas J., Benavente J. The avian reovirus genome segment S1 is a functionally tricistronic gene that express one structural and two nonstructural proteins in infected cells. Virology. 2001;290:181–191. doi: 10.1006/viro.2001.1159. [DOI] [PubMed] [Google Scholar]
- Bodelon G., Labrada L., Martinez-Costas J., Benavente J. Modification of late membrane permeability in avian reovirus-infected cells: viroporin activity of the S1-encoded nonstructural p10 protein. J. Biol. Chem. 2002;277:17789–17796. doi: 10.1074/jbc.M202018200. [DOI] [PubMed] [Google Scholar]
- Chiu C.J., Lee L.J. Cloning and nucleotide sequencing of the S4 genome segment of avian reovirus S1133. Arch. Virol. 1997;142:2515–2520. doi: 10.1007/s007050050258. [DOI] [PubMed] [Google Scholar]
- Chulu L.J., Lee L.H., Lee Y.C., Liao S.H., Shih W.L., Liu H.J. Apoptosis induction by avian reovirus through P53 and mitochondrial pathways. Biochem. Biophys. Res. Commun. 2007;356:529–535. doi: 10.1016/j.bbrc.2007.02.164. [DOI] [PubMed] [Google Scholar]
- Ciechanover A. The ubiquitin-proteasome proteolytic pathway. Cell. 1994;79:13–21. doi: 10.1016/0092-8674(94)90396-4. [DOI] [PubMed] [Google Scholar]
- Connolly D.L., Rodgers S.E., Clarke P., Ballard D.W., Kerr L.D., Tyler K.L., Dermody T.S. Reovirus-induced apoptosis requires activation of transcription factor NF-kappaB. J. Virol. 2000;74(7):2981–2989. doi: 10.1128/jvi.74.7.2981-2989.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galinier R., Gout E., Lortat-Jacob H., Wood J., Chroboczek J. Adenovirus protein involved in virus internalization recruits ubiquitin-protein ligase. Biochemistry. 2002;41:14299–14305. doi: 10.1021/bi020125b. [DOI] [PubMed] [Google Scholar]
- Glickman M.H., Ciechanover A. The ubiquitin-proteasome proteolytic pathway: destruction for the sake of construction. Physiol. Rev. 2002;82:373–428. doi: 10.1152/physrev.00027.2001. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Lopez C., Martinez-Costas J., Esteban M., Benavente J. Evidence that avian reovirus σA protein is an inhibitor of the double-stranded RNA-dependent protein kinase. J. Gen. Virol. 2003;84:1629–1639. doi: 10.1099/vir.0.19004-0. [DOI] [PubMed] [Google Scholar]
- Hilt W., Wolf D.H. In: Proteasome: The World Of Regulatory Proteolysis. Hilt W., Wolf D.H., editors. Eurekah. Co. Austin and Lanes Biosciences; Georgetown, TX: 2000. [Google Scholar]
- Hsu J., Wang C.Y., Lee L.H., Shih W.L., Chang C.I., Cheng H.L., Chulu J.L.C., Ji W.T., Liu H.J. Characterization of monoclonal antibodies against avian reovirus S1133 σC protein produced in insect cells and their application in detection of ARV isolates. Avian Pathol. 2006;35:320–326. doi: 10.1080/03079450600823386. [DOI] [PubMed] [Google Scholar]
- Hou H.S., Su Y.P., Shieh H.K., Lee L.H. Monoclonal antibodies against different epitopes of nonstructural protein σ NS of avian reovirus S1133. Virology. 2001;282:168–175. doi: 10.1006/viro.2001.0814. [DOI] [PubMed] [Google Scholar]
- Huang P.H., Li Y.J., Su Y.P., Lee L.H., Liu H.J. Epitope mapping and functional analysis of σA and σNS proteins of avian reovirus. Virology. 2005;332:584–595. doi: 10.1016/j.virol.2004.12.005. [DOI] [PubMed] [Google Scholar]
- Kalejta R.F., Bechtel J.T., Shenk T. Human cytomegalovirus pp71 stimulates cell cycle progression by inducing the proteasome-dependent degradation of the retinoblastoma family of tumor suppressors. Mol. Cell Biol. 2003;23:1885–1895. doi: 10.1128/MCB.23.6.1885-1895.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kibenege F.S.B., Wilcox G.E. Tenosynovitis in chickens. Vet. Bull. 1983;53:431–443. [Google Scholar]
- Lin P.Y., Liu H.J., Yu F.L., Hsu H.Y., Lee J.W., Shih W.L. Avian reovirus activates a novel proapoptotic signal by linking Src to p53. Apoptosis. 2006;11:2179–2193. doi: 10.1007/s10495-006-0291-6. [DOI] [PubMed] [Google Scholar]
- Lin H.Y., Chuang S.T., Chen Y.T., Shih W.L., Chang C.D., Liu H.J. Avian reovirus-induced apoptosis related to tissue injury. Avian Pathol. 2007;36:155–159. doi: 10.1080/03079450701261262. [DOI] [PubMed] [Google Scholar]
- Liu H.J., Lee L.H., Hsu H.W., Kuo L.C., Liao M.H. Molecular evolution of avian reovirus: evidence for genetic diversity and reassortment of the S-class genome segments and multiple cocirculating lineages. Virology. 2003;314:336–349. doi: 10.1016/s0042-6822(03)00415-x. [DOI] [PubMed] [Google Scholar]
- Liu H.J., Lee L.H., Shih W.L., Li Y.J., Su H.Y. Rapid characterization of avian reoviruses using polylogenetic analysis, reverse transcription-polymerase chain reaction and restriction enzyme fragment length polymorphism. Avian Pathol. 2004;33:171–180. doi: 10.1080/03079450310001652130. [DOI] [PubMed] [Google Scholar]
- Liu H.J., Lin P.Y., Lee J.W., Hsu H.Y., Shih W.L. Retardation of cell growth by avian reovirus p17 through the activation of p53 pathway. Biochem. Biophys. Res. Commun. 2005;336:709–715. doi: 10.1016/j.bbrc.2005.08.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo H., Zhang J., Cheung C., Suarez A., McManus M.M., Yang D. Proteasome inhibition reduces coxsackievirus B3 replication in murine cardiomyocytes. Am. J. Pathol. 2003;163:381–385. doi: 10.1016/S0002-9440(10)63667-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Costas J., Grande A., Varela R., Garcia-Martinez C., Benavente J. Protein architecture of avian reoviris S1133 and identification of the cell attachment protein. J. Virol. 1997;71:59–64. doi: 10.1128/jvi.71.1.59-64.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myung J., Kim K.B., Crews C.M. The ubiquitin-proteasome proteolytic pathway and proteasome inhibitors. Med. Res. Rev. 2001;21:245–273. doi: 10.1002/med.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pai W.C., Shien J.H., Liu H.J., Lee L.H. Characterization of monoclonal antibodies against avian reovirus S1133 protein σA synthesized in Escherichia coli. Vet. Microbiol. 2003;91:309–323. doi: 10.1016/s0378-1135(02)00308-5. [DOI] [PubMed] [Google Scholar]
- Reichel C., Beachy R.N. Degradation of tobacco mosaic virus movement protein by the 26S proteasome. J. Virol. 2000;74:3330–3337. doi: 10.1128/jvi.74.7.3330-3337.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ros C., Kempf C. The ubiquitin-proteasome machinery is essential for nuclear translocation of incoming minute virus of mice. Virology. 2004;324:350–360. doi: 10.1016/j.virol.2004.04.016. [DOI] [PubMed] [Google Scholar]
- Schuber U., Ott D.E., Chertova E.N., Welker R., Tessmer U., Princiotta M.F., Bennink J.R., Krausslich H.G., Yewdell J.W. Proteasome inhibition interferes with gag polyprotein processing, release, and maturation of HIV-1 and HIV-2. Proc. Natl. Acad. Sci. U.S.A. 2000;97:13057–13062. doi: 10.1073/pnas.97.24.13057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih W.L., Hsu W.H., Liao M.H., Lee L.H., Liu H.J. Avian reovirus σC protein induces apoptosis in cultured cells. Virology. 2004;321:65–74. doi: 10.1016/j.virol.2003.12.004. [DOI] [PubMed] [Google Scholar]
- Varela R., Benavente J. Protein coding assignment of avian reovirus S1133. J. Virol. 1994;68:6775–6777. doi: 10.1128/jvi.68.10.6775-6777.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickramasinghe R., Meanger J., Enriguez C.E., Wilcox G.E. Avian reoviruses proteins associated with neutralization of virus infectivity. Virology. 1993;194:688–696. doi: 10.1006/viro.1993.1309. [DOI] [PubMed] [Google Scholar]
- Yin H.S., Lee L.H. Identification and characterization of RNA-binding activities of avian reovirus non-structural protein σNS. J. Gen. Virol. 1998;79:1411–1413. doi: 10.1099/0022-1317-79-6-1411. [DOI] [PubMed] [Google Scholar]
- Yin Y.S., Shieh J.H., Lee L.H. Synthesis in Escherichia coli of ARV core protein σA and its dsRNA-binding activity. Virology. 2000;266:33–41. doi: 10.1006/viro.1999.0020. [DOI] [PubMed] [Google Scholar]
- Yu G.Y., Lai M.C. The ubiquitin-proteasome system facilitates the transfer of murine coronavirus from endosome to cytoplasm during virus entry. J. Virol. 2005;79:644–648. doi: 10.1128/JVI.79.1.644-648.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]


