Abstract
A DNA vaccine against infectious bronchitis virus (IBV) can induce specific humoral and cell-mediated immunity. However, compared to conventional vaccines, DNA vaccines usually induce poor antibody responses. To develop a more potent IBV DNA vaccine formulations, a monocistronic vector encoding the nucleocapsid protein of IBV and a bicistronic vector separately encoding the nucleocapsid protein and immune-stimulatory interleukin-2 were constructed. When the DNA vaccines were administered to the quadriceps muscle of chickens, the induced humoral and cellular responses were evaluated. There was a significant difference in ELISA antibody levels elicited by either monocistronic or bicistronic DNA vaccines. The percentage of CD3+, CD3+CD8+ and CD3+CD4+ subgroups of peripheral blood T-lymphocytes in chickens immunized with bicistronic DNA vaccine were higher than those in chickens immunized with monocistronic DNA vaccine. When chickens were challenged with a virulent strain of IBV, the protective efficacy could be enhanced significantly after immunization with bicistronic DNA vaccine. These results demonstrated that bicistronic DNA vaccine is an effective approach to increase IBV DNA vaccine immunogenicity.
Keywords: Infectious bronchitis virus, DNA vaccine, Nucleocapsid protein, Bicistronic plasmid
1. Introduction
Infectious bronchitis (IB) is an acute and highly contagious respiratory disease of chickens. It is still a major health problem in the chicken industry in the world. Vaccination to control IB has been practiced for over half a century (Bijlenga et al., 2004, Cavanagh and Naqi, 2003, Cavanagh, 2003). Such conventional vaccines, although generally effective, do have some disadvantages. Attenuated vaccines, which generally induce long-lasting immunity, have a risk of insufficient attenuation and/or genetic instability (Cook et al., 1986). The limitations of inactivated vaccines include high cost for manufacturing and lack of long-term immunity. Therefore a new generation of IB vaccines is called for.
Infectious bronchitis virus (IBV) is an enveloped coronavirus that contains an unsegmented, single-stranded, positive-sense RNA genome. In addition to the internally localized nucleocapsid (N) protein, the virion is composed of spike protein, membrane protein and a small envelope protein. N protein carries epitopes inducing cross-reactive antibodies and is the most abundant virus-derived protein produced throughout infection (Seah et al., 2000, Boots et al., 1992, Seo et al., 1997, Yu et al., 2001). The carboxyl end of N protein has CTL epitopes for MHC compatible chickens (Seo and Collisson, 1997). Chickens inoculated with DNA plasmids expressing the carboxyl end of N were protected from IBV infection (Seo et al., 1997).
In recent years, the development of DNA vaccines as a potentially safe alternative has been explored. DNA immunization is an important vaccination strategy that has many characters desirable for an ideal vaccine, including induction of broad immune responses, long-lasting immunity and simple and cheap production. Experimental DNA vaccines against viral, bacterial, and parasitic disease have been described (Tacket et al., 1999, Strugnell et al., 1997, Kalinna, 1997) and DNA vaccines have been licensed for two nonhuman applications: one for West Nile virus for horses (Powell, 2004) and the other for infectious hematopoietic necrosis virus for salmon (Lorenzen and Lapatra, 2005). Hence DNA vaccines appear to be a useful technology.
It is believed that delivery of only a single DNA plasmid of antigen is not optimal to protect against infection. Adjuvants are used widely in various vaccine formulations for the enhancement of immune responses. Among the adjuvants, cytokines have been explored extensively to augment the potency of DNA vaccines (Gurunathan et al., 2000, Reyes-Sandoval and Ertl, 2001, Scheerlinck, 2001, Barouch et al., 2004, Calarota and Weiner, 2004, Stevenson, 2004, Barouch, 2006). Interleukin-2 (IL-2), initially known as T-cell growth factor, is a powerful immunoregulatory lymphokine which produced by lectin- or antigen-activated T-cells (Morgan et al., 1976). It is secreted by mature T-lymphocytes upon stimulation and certain T-cell lymphoma cell lines constitutively. Plasmid IL-2 has been investigated as a potential vaccine adjuvant in several studies and has been shown to increase DNA vaccine protective immunity against pathogens (Diane and Carlos, 2004, Rompato et al., 2006, Bu et al., 2003).
In the present study, a bicistronic plasmid encoding the nucleocapsid protein and immune-stimulatory interleukin-2 has been constructed and its immunogenicity and protective effect in chickens has been evaluated. It has been shown that the delivery of a bicistronic plasmid containing N gene and IL-2 can accelerate specific antibody induction with an increase T-cell responses. The use of bicistronic vector may enable more efficient delivery of both antigen and cytokine in DNA vaccination and promote synergistic responses.
2. Materials and methods
2.1. Virus, experimental animals, and cells
The specific-pathogen-free (SPF) chicken embryos were purchased from Shangdong Institute of Poultry Science, Shandong, PR China. Chickens were hatched and housed in a specific-pathogen-free environment at the Laboratory Animal and Resources Facility, Sichuan University. The nephropathogenic strain of IBV, SAIBk strain was propagated in the allantoic cavities of 10-day-old SPF embryonated chicken eggs, and then harvested allantoic fluid 36 h post-inoculation. The 50% chicken infection dose (EID50) was determined by inoculating serial 10-fold dilutions of virus into 10-day-old SPF embryonated chicken eggs. Vero cells were cultured in Dulbecco's modified Eagle's medium (Gibco) supplemented with 10% FBS, 100 U/ml penicillin and 100 μg/ml streptomycin at pH 7.2 and were kept at 37 °C with 5% carbon dioxide.
2.2. Construction of monocistronic and bicistronic plasmids
The pIRES-EGFP/DsRed bicistronic plasmid which was constructed and identified previously, was used to construct monocistronic and bicistronic DNA vaccines. This plasmid contained two fluorescent protein genes which were inserted into the multiple cloning sites (MCS) located on either side of the internal ribosome entry site from the encephalomyocarditis virus (ECMV). The IBV N gene was subcloned from recombinant plasmid pIBVN into EGFP of pIRES-EGFP/DsRed between NheI and XhoI restriction sites, and generated the plasmid pIRES-N/DsRed. The IL-2 gene was amplified by PCR from the plasmid pDNAIL-2 as a template. The forward primer (5′-CCAGGATCCACCATGATGTG AAG-3′) and reverse primer (5′-GAAGCGGCCGCAGATTAGTTAGC-3′) specific for the IL-2 gene were employed. The PCR product of IL-2 was digested with BamHI and NotI and ligated into similarly digested pIRES-N/DsRed, then the bicistronic plasmid pIRES-N/IL2 was created. The plasmid pIRES-N/DsRed was digested with BamHI and NotI, gel purified and blunt ended to create monocistronic pIRES-N. Identification of the recombinant plasmids was performed by double enzymes digestion and DNA sequencing.
2.3. In vitro expression of the plasmid DNA
Six-well tissue culture plates were seeded with Vero cells (106/well), and the cells were grown until they were about 70% confluent. The purified plasmids, pIRES-N, pIRES-N/IL-2 and pIRES were transfected respectively into the Vero cells with lipofectamine according to the manufacturer's instructions (Invitrogen, CA, USA). The expression products were identified after 36–48 h.
2.3.1. RT-PCR analysis
The transfected cells were harvested after 36 h and total cellular RNA was prepared from the transfected cells by TRIZOL reagent (Gibco BRL, USA). The transcription products were detected by reverse-transcription polymerase chain reaction (RT-PCR) with specific primer sets for the N gene and IL-2 as listed. N forward primer: 5′-CATCTCGAGTCTTTTATCATGGCAAGC-3′, reverse primer: 5′-GGCGAATTCATTAGAGTTCATTTTCAC-3′; IL-2 forward primer: 5′-CCAGGATCCACCATGATGTGCAAAG-3′, reverse primer: 5′-GAAGCGGCCGCAGATTAGTTAGC-3′.
2.3.2. Indirect immunofluorescence analysis
The medium was aspirated 48 h after transfection, and the cells were washed once with phosphate-buffered-saline (PBS), fixed with 100% acetone for 10 min at −20 °C, then washed three times for 5 min each with PBS. Thereafter, transfected cells were incubated at 37 °C for 1 h with the antibody, which was antiserum of rabbit to IBV. Then the cells were washed twice for 5 min each with PBS and incubated for a further 1 h at 37 °C with the secondary FITC-conjugated goat-anti-rabbit IgG antibody that included 0.05% azovan blue (purpose of using azovan blue was to identify the positive Vero cells and negative Vero cells). The positive Vero cells expressing N protein were stained green; the negative Vero cells were stained red. The cells were washed twice with PBS and analyzed the expression of the recombinant plasmid by fluorescence microscopy.
2.4. Immunization of chickens with plasmid DNA vaccines
The plasmids pIRES-N/IL2, pIRES-N, pIRES were amplified in Escherichia coli JM 109 and extracted using the alkaline lysis method as described previously (Sambrook et al., 1989). After purification by PEG8000 precipitation, the plasmids were resuspended in phosphate-buffered saline (PBS, pH 7.2) and kept at −20 °C until used for immunization. For vaccination, the chickens were randomly divided into four groups (n = 20 each). The 7-day-old chickens were injected intramuscularly into the quadriceps muscle with 150 μg of plasmid pIRES-N/IL2 (group 1). A second group was given 150 μg of plasmid pIRES-N (group 2). Other groups included chickens administered with 150 μg of empty vector pIRES (group 3), and chickens injected with 0.5 ml PBS only (group 4). All groups were boosted with an equivalent dose at 21 days after the initial inoculation.
2.5. Detection of anti-IBV specific antibodies
Pre-vaccination sera were collected from all vaccinated chickens. Blood was also collected before booster vaccination as well as before challenge. Sera were stored at −20 °C for serologic analysis. Total serum immunoglobulin G (IgG) specific for IBV was measured by indirect enzyme-linked immunosorbent assay (ELISA) as described previously, with brief modifications: ELISA plates were coated with IBV lysate at 5 μg/ml in carbonate buffer, PH 9.6, for overnight at 4 °C and blocked with 10% non-fat dried milk in PBS at 37 °C for 3 h. Serum samples were tested in 1:20 dilution in 10% dried milk in PBST. IgG against IBV was revealed with horseradish peroxidaselabeled goat-anti-chicken conjugate diluted 1:2000 in PBST. The substrate solution used was TMB microwell peroxidase. After 20 min of incubation in the dark, the reaction was stopped by the addition of 100 μl of 2 M H2SO4, and the optical density at 450 nm was measured in an ELISA microplate reader. Sera were run in duplicate. Negative and positive control sera were included in each assay. Total serum immunoglobulin G (IgG) specific for IBV are represented as the optical density.
2.6. Analysis of CD4+, CD8+ and CD3+ T-lymphocytes
Peripheral blood samples from immunized chicken were collected from the jugular vein in 2.5 ml syringes preloaded with 0.2 ml of sodium heparin to prevent clotting on day 7 after the boosting vaccination. Peripheral blood mononuclear cells were isolated from each blood sample by Ficoll-Hypaque density gradient centrifugation. PBMC were adjusted to 1 × 107 cells/ml. The 100 μl of samples (1 × 106 cells) were incubated for 1 h at room temperature with antibody as follows: mouse anti-chicken CD4-PE, mouse anti-chicken CD8-FITC, mouse anti-chicken CD3-SPRD (BD Biosciences Pharmingen). Leukocyte samples were triply labeled with CD3, CD4 and CD8 antibodies. The samples were processed on fluorescence activated cell sorter.
2.7. Virus challenge experiment
All of the chickens were challenged with 100EID50 of the IBV SAIBk strain in 0.1 ml by the nasal-ocular route at 15 days after the boosting immunization. The challenged chickens were examined daily for signs of clinical illness such as coughing, sneezing, ataxia, dyspnea or death for 2 weeks. Dead chickens were necropsied to confirm death by IBV infection. The challenged chickens generally began to show clinical signs from 4 to 10 days after challenge. Chickens in each group were euthanized at 14 days post-infection. Necropsies were performed immediately postmortem and kidney tissues were collected for further detection of virus.
2.8. Detecting of virus in kidney tissues by RT-PCR
The kidney tissues were incised individually from either the dead or euthanized chickens at 14 days post-challenged. The virus in the kidney tissues of the challenged chicken was detected by RT-PCR. Total RNA was extracted using Trizol LS reagent and subjected to RT-PCR using primers directed to the 3′ untranslated region (forward primer: 5′-GATGAGGAGAGGAACAATGC-3′; reverse primer: 5′-TGGGCGTCCTAGTGCTGT-3′). Total protection was defined as negative for the presence of virus in the kidney.
2.9. Statistical analysis
Data were analyzed using the one-sided Student's t test. Differences were considered statistically significant with P < 0.05.
3. Results
3.1. Construction of monocistronic and bicistronic DNA vaccine
The monocistronic and bicistronic DNA vaccine plasmids encoding IBV N protein and IL-2 were constructed using bicistronic plasmid pIRES-EGFP/DsRed as shown in Fig. 1 .
Fig. 1.
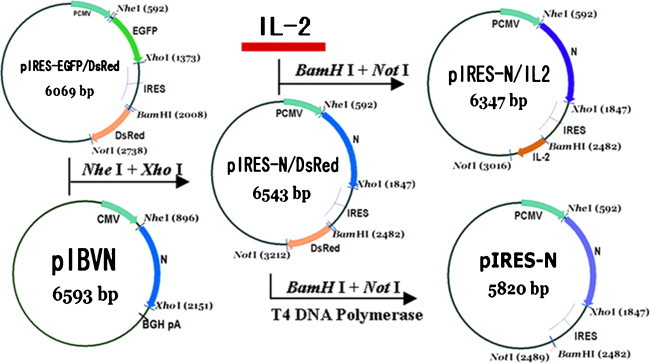
Strategy for construction of the monocistronic and bicistronic DNA vaccines.
3.2. Transcription of recombinant plasmids in Vero cells
To confirm the transcription of constructs pIRES-N/IL2, pIRES-N in a eukaryotic system, the plasmids were transfected respectively into Vero cells. Total RNA was extracted from transfected cells at 38 h and analyzed by RT-PCR for the presence of each corresponding mRNA. The predicted RT-PCR products were of 1.3 kb in size for N and 0.6 kb for IL2, all of which were confirmed by gel electrophoresis. No specific band of a similar size was seen in any of the mRNA samples in the absence of reverse transcription (Fig. 2 ). This result showed that constructs encoding N and IL-2 gene can be transcribed successfully in the eukaryotic system.
Fig. 2.
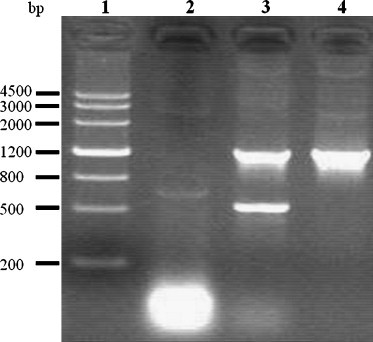
Detection the transcription of the plasmids by RT-PCR. Eukaryotic expression of the plasmids: pIRES-N/IL2, pIRES-N in Vero cells. Total RNA was extracted from Vero cells 36 h after transfection with pIRES-N/IL2, pIRES-N. The templates used for RT-PCR were as follows: lane 1, Marker III; lane 2, negative control; lane 3, plasmid pIRES-N/IL2; lane 4, plasmid pIRES-N.
3.3. Expression of recombinant plasmids in Vero cells
The expression of pIRES-N/IL2, pIRES-N was demonstrated by indirect immunofluorescence assay. After transfection with lipofectamine, the transfected cells displayed positive signals for the protein and located cytoplasm, where there was green fluorescence. Expression of N protein was not detected in transfected empty plasmid control cells (Fig. 3 ). This result shows that constructs encoding N protein can be successfully expressed in the eukaryotic system.
Fig. 3.
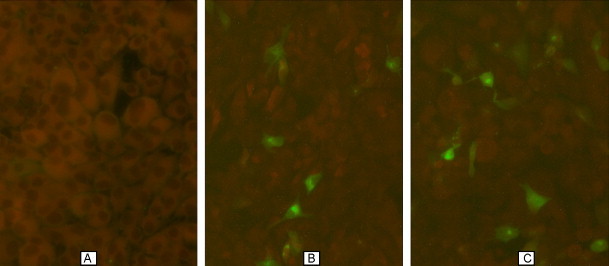
Indirect-immunofluorescence detection of the expressed N protein in Vero cell. (A) Cells transfected with the pIRES plasmid showed negative results, (B) cells transfected with the pIRES-N plasmid showed positive results and (C) cells transfected with the pIRES-N/IL2 plasmid showed positive results.
3.4. Humoral responses to IBV in chickens vaccinated with recombinant plasmids
The plasmids pIRES-N/IL2, pIRES-N induced detectable antibodies to IBV Ag in chickens one week after injection and the levels increased with subsequent vaccination. There was no specific antibody response in the group of chickens receiving PBS and pIRES plasmid. There was a significant difference in ELISA antibody levels (P < 0.05) elicited by either monocistronic or bicistronic DNA vaccines since the 14th-day after first inoculation. The result suggests that the bicistronic pIRES-N/IL2 can enhance humoral responses (Fig. 4 ).
Fig. 4.
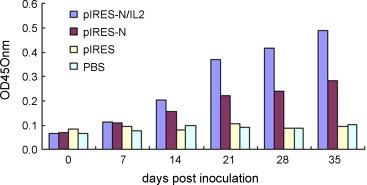
ELISA antibodies of different vaccines inoculated groups. Sera from all of animals were sampled weekly. The result was obtained from average of five sera in each group.
3.5. Cellular immune responses induced by recombinant plasmids vaccination
Peripheral blood lymphocytes were analyzed by flow cytometry on day 7 after the boosting immunization. The percentage of CD3+ and CD4+CD3+ T-lymphocytes significantly higher (P < 0.05) was observed from chickens immunized with pIRES-N/IL2 than the pIRES-N group. The percentage numbers of CD8+CD3+ T-lymphocytes subgroups of the pIRES-N/IL2-vaccinated group were higher than the pIRES-N-vaccinated group but no significant difference (P > 0.05) (Fig. 5 ).
Fig. 5.
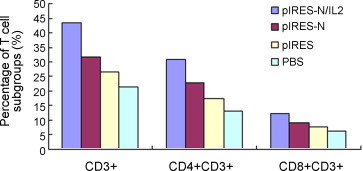
The percentage of CD3+, CD4+CD3+ and CD8+CD3+ T-lymphocytes of different vaccines inoculated groups. This test was performed at one week after boosting immunization.
3.6. Protection after challenge
Mortality, kidney infection and percent protection after challenge of chickens were summarized in Table 1 . Chickens that started to show clinical signs or died from viral infection did so beginning on day 5 after challenge. The chickens immunized with either control vector pIRES or PBS were not protected and developed coughing, nasal discharge, dyspnea. The death rate of the pIRES and PBS immunized chickens was 55 and 65% at 14 days after challenge, respectively. The death rate of the pIRES-N/IL2 DNA vaccine immunized chickens was only 5%, lower than that of the chickens injected with the pIRES-N DNA vaccine. To evaluate the level of protective response after challenge, the collected kidney samples were analyzed by RT-PCR. PCR results indicated that 20 and 50% of birds vaccinated with the pIRES-N/IL2 and pIRES-N plasmids were positive for the presence of virus in the kidney, respectively. All chickens immunized with either control vector pIRES or PBS were positive in RT-PCR test. The protection percent of group that vaccinated with the pIRES-N alone was higher than that of the empty vector or PBS. The group vaccinated with the pIRES-N/IL2 DNA vaccine (80%) had the highest protection rate in all vaccinated groups. This suggests that the plasmid expressing both N protein and IL-2 offers enhanced resistance against a virulent IBV challenge.
Table 1.
The mortality and protection rate of different groups challenged by virulent strain SIBK of IBV
| Groups | No. of death | No. of affecteda | Mortalityb (%) | Protection ratec (%) |
|---|---|---|---|---|
| pIRES-N/IL2 | 1/20 | 4/20 | 5 | 80 |
| pIRES-N | 3/20 | 10/20 | 15 | 50 |
| pIRES | 11/20 | 20/20 | 55 | 0 |
| PBS | 13/20 | 20/20 | 65 | 0 |
Affected was determined by RT-PCR positive bird from dead and euthanized chickens’ kidneys.
Mortality was recorded for each day after challenge and is presented as total number of dead chickens in each group.
Percent protection was determined by the number of unaffected chickens/total number of chickens.
4. Discussion
In order to increase the efficiency of this immunization procedure, cytokine was co-expressed with the viral protein. This strategy has already been used successfully to enhance the effect of DNA vaccination procedures (Li et al., 2006, Chow et al., 1997, Henke et al., 2004). The pIRES-EGFP/DsRed bicistronic plasmid which was constructed and identified previously was used to construct monocistronic and bicistronic DNA vaccines. The plasmid pIRES-EGFP/DsRed enabled the simultaneous translation of two genes of interest from the same RNA transcript. Each gene is cloned into one of the multiple cloning sites on either side of the internal ribosomal entry site of the encephalomyocarditis virus (EMCV). The entire construct is under control of the cytomegalovirus (CMV) immediate-early promoter allowing the expression of two individual proteins from one plasmid. In this study, an experimental immunization strategy was developed and tested against IBV. Two recombinant plasmids pIRES-N/IL2, pIRES-N were constructed. These recombinant plasmids were inoculated in chickens and tested in a protection-challenge experiment, demonstrating that vaccination with the co-expression plasmid pIRES-N/IL2 can induce stronger immune response than vaccination with pIRES-N. Thus, it seems that vaccination with a bicistronic DNA vaccine expressing both IBV N protein and IL-2 may elicit potent immune response.
In this study, the level of specific antibodies developed in pIRES-N/IL2 group was higher than that of pIRES-N group. However, the precise role of antibodies for the control of IBV infection remains controversial. Some reports have shown that circulating antibody titer did not correlate with protection from IBV infection (Gelb et al., 1998, Gough and Alexander, 1979, Raggi and Lee, 1965). Other studies demonstrated that humoral immunity plays an important role in disease recovery and virus clearance (Cook et al., 1991, Thompson et al., 1997, Toro and Fernandez, 1994). The antibody-dependence of the mechanism of protection against the disease remains unclear, suggesting an important role of the T-cell response in efficacy protection.
In order to evaluate recombinant plasmids induced T-cell response, peripheral blood lymphocytes were analyzed by flow cytometry. Results of T-lymphocytes subgroup detection indicated, that the percentage numbers of CD3+, CD4+CD3+ and CD8+CD3+ T-lymphocytes subgroups in pIRES-N/IL2 vaccinated chickens group were higher than those in the pIRES-N vaccinated chickens. This demonstrated that IL-2 has the ability to stimulate T-cell growth. It has been shown that cell-mediated immunity to IBV is also induced and is believed to be a protective mechanism in IBV infection. CD8+ CTL are critical in the control of infectious bronchitis in poultry (Collisson et al., 2000, Dhinakar and Jones, 1997, Seo et al., 2000). CD4+ T-cell responses may increase the proliferation, maturation and functional activity of CD8+ CTL, providing increased help for B-cells and directly producing antiviral cytokines. By increasing the number of T-cells at the same site being able to respond to N antigen, there might have been a limiting effect on viral replication leading to better protection.
To investigate the level of protection elicited by pIRES-N/IL2, vaccinated chickens were challenged with a nephropathogenic strain of IBV. Chickens that received the pIRES-N/IL2 plasmid DNA were better protected than that administered with the plasmid pIRES-N. Mortality of pIRES-N/IL2 group was lower than that of pIRES-N group. The protection rate of pIRES-N/IL2 group was the highest in all the vaccination groups, possibly indicating protective immunity overcome by virus aggressiveness. These results suggested that vaccination with the co-expression plasmid of N protein gene and IL-2 may have increased the protection rate against challenge.
Results of immune response and viral challenge showed that the group inoculated with pIRES-N/IL2 provided stronger immune response and better protection rate than pIRES-N vaccinated group. This indicated that IL-2 might effectively enhanced humoral-mediated immune and cell-mediated immune responses to some extent. This was consistent with the result of previous reports (Li et al., 2006, Chow et al., 1997, Henke et al., 2004). These results demonstrated that bicistronic DNA vaccine is an effective approach to increase IBV DNA vaccine immunogenicity.
Our results showing the induction of both antibody and T-cell responses against the IBV challenge in chickens demonstrate that the delivery of antigens and cytokines via bicistronic vectors is feasible in the chicken model.
Acknowledgement
This work was supported by the foundation of Chinese National Programs for High Technology Research and Development (project number 2006AA10A205).
References
- Barouch D.H. Rational design of gene-based vaccines. J. Pathol. 2006;208:283–289. doi: 10.1002/path.1874. [DOI] [PubMed] [Google Scholar]
- Barouch D.H., Letvin N.L., Seder R.A. The role of cytokine DNAs as vaccine adjuvants for optimizing cellular immune responses. Immunol. Rev. 2004;202:266–274. doi: 10.1111/j.0105-2896.2004.00200.x. [DOI] [PubMed] [Google Scholar]
- Bijlenga G., Cook J.K.A., Gelb J., de Wit J.J. Development and use of the H strain of avian infectious bronchitis virus from The Netherlands as a vaccine: a review. Avian Pathol. 2004;33:550–557. doi: 10.1080/03079450400013154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boots A.M., Benaissa-Trouw B.J., Hesselink W., Rijke E., Schrier C., Hensen E.J. Induction of anti-viral immune responses by immunization with recombinant-DNA encoded avian coronavirus nucleocapsid protein. Vaccine. 1992;10:119–124. doi: 10.1016/0264-410X(92)90028-I. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bu J., Song Y., Rompato G., Burgess D.J., Garmendia A.E. Co-delivery of IL-2 or liposomes augment the responses of mice to a DNA vaccine for pseudorabies virus IE80. Comp. Immunol. Microbiol. Infect. Dis. 2003;26:175–187. doi: 10.1016/s0147-9571(02)00050-4. [DOI] [PubMed] [Google Scholar]
- Calarota S.A., Weiner D.B. Enhancement of human immunodeficiency virus type 1-DNA vaccine potency through incorporation of T-helper 1 molecular adjuvants. Immunol. Rev. 2004;199:84–99. doi: 10.1111/j.0105-2896.2004.00150.x. [DOI] [PubMed] [Google Scholar]
- Cavanagh D. Severe acute respiratory syndrome vaccine development: experiences of vaccination against avian infectious bronchitis coronavirus. Avian Pathol. 2003;32:567–582. doi: 10.1080/03079450310001621198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanagh D., Naqi S.A. Infectious bronchitis. In: Saif Y.M., Barnes H.J., Glisson J.R., Fadly A.M., McDougald L.R., Swayne D.E., editors. Diseases of Poultry. 11th edition. Iowa State University Press; Ames, Iowa: 2003. pp. 101–119. [Google Scholar]
- Chow Y., Huang W., Chi W., Chu Y., Tao M. Improvement of hepatitis B virus DNA vaccines by plasmids coexpressing hepatitis B surface antigen and interleukin-2. J. Virol. 1997;71:169–178. doi: 10.1128/jvi.71.1.169-178.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collisson E.W., Pei J., Dzielawa J., Seo S.H. Cytotoxic T lymphocytes are critical in the control of infectious bronchitis in poultry. Dev. Comp. Immunol. 2000;24:187–200. doi: 10.1016/s0145-305x(99)00072-5. [DOI] [PubMed] [Google Scholar]
- Cook J.K., Smith H.W., Huggins M.B. Infectious bronchitis immunity: its study in chickens experimentally infected with mixtures of infectious bronchitis virus and Escherichia coli. J. Gen. Virol. 1986;67:1427–1434. doi: 10.1099/0022-1317-67-7-1427. [DOI] [PubMed] [Google Scholar]
- Cook J.K., Davison T.F., Huggins M.B., McLauthlan P. Effect of in vivo bursectomy on the course of an infectious bronchitis virus infection in line C White Leghoen chickens. Arch. Virol. 1991;118:225–234. doi: 10.1007/BF01314032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhinakar R.G., Jones R.C. Cross-reactive cellular immnue responses in chickens vaccinated with live infectious bronchitis virus vaccine. Avain Pathol. 1997;26:641–649. doi: 10.1080/03079459708419240. [DOI] [PubMed] [Google Scholar]
- Diane J.H., Carlos H.R. Partial protection against infectious bursal disease virus through DNA-mediated vaccination with the VP2 capsid protein and chicken IL-2 genes. Vaccine. 2004;22:1249–1259. doi: 10.1016/j.vaccine.2003.09.021. [DOI] [PubMed] [Google Scholar]
- Gelb J., Jr., Nix W.A., Gellman S.D. Infectious bronchitis virus antibodies in tears and their relationship to immunity. Avian Dis. 1998;42:364–374. [PubMed] [Google Scholar]
- Gough R.E., Alexander D.J. Comparison of duration of immunity in chickens infected with a live infectious bronchitis vaccine by three different routes. Res. Vet. Sci. 1979;26:329–332. [PubMed] [Google Scholar]
- Gurunathan S., Klinman D.M., Seder R.A. DNA vaccines: immunology, application, and optimization. Annu. Rev. Immunol. 2000;18:927–974. doi: 10.1146/annurev.immunol.18.1.927. [DOI] [PubMed] [Google Scholar]
- Henke A., Chiang C.S., Zell R., Stelzner A. Co-expression of interleukin-2 to increase the efficacy of DNA vaccine-mediated protection in coxsackievirus B3-infected mice. Antiviral Res. 2004;64:131–136. doi: 10.1016/j.antiviral.2004.06.009. [DOI] [PubMed] [Google Scholar]
- Kalinna B.H. DNA vaccines for parasitic infections. Immunol. Cell Biol. 1997;75:370–375. doi: 10.1038/icb.1997.58. [DOI] [PubMed] [Google Scholar]
- Li W.R., Niu B., Wang J.W., Feng Z.J., Wang D.X. Coexpression of interleukin-2 enhances the immunization effect of a DNA vaccine expressing herpes simplex 1 glycoprotein D. Acta Virol. 2006;50:251–256. [PubMed] [Google Scholar]
- Lorenzen N., Lapatra S.E. DNA vaccines for aquacultured fish. Rev. Sci. Tech. 2005;24:201–213. [PubMed] [Google Scholar]
- Morgan D.A., Ruscetti F., Giallo F.W. Selective in vitro growth of T lymphocytes from normal human bone marrows. Science. 1976;193:1007–1008. doi: 10.1126/science.181845. [DOI] [PubMed] [Google Scholar]
- Powell K. DNA vaccines: back in the saddle again? Nat. Biotechnol. 2004;22:799–801. doi: 10.1038/nbt0704-799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raggi L.G., Lee G.G. Lack of correlation between infectivity, serologic response and challenge results in immunization with an avian infectious bronchitis vaccine. J. Immunol. 1965;94:538–543. [PubMed] [Google Scholar]
- Reyes-Sandoval A., Ertl H.C. DNA vaccines. Curr. Mol. Med. 2001;1:217–243. doi: 10.2174/1566524013363898. [DOI] [PubMed] [Google Scholar]
- Rompato G., Ling E., Chen Z., Kruiningen H.V., Garmendia A.E. Positive inductive effect of IL-2 on virus-specific cellular responses elicited by a PRRSV-ORF7 DNA vaccine in swine. Vet. Immunol. Immunopathol. 2006;109:151–160. doi: 10.1016/j.vetimm.2005.08.015. [DOI] [PubMed] [Google Scholar]
- Sambrook J., Fritsch E.F., Maniatis T. 2nd ed. Cold Spring Harbor Laboratory Press; 1989. Molecular Cloning—A Laboratory Manual. pp. 24–28. [Google Scholar]
- Scheerlinck J.Y. Genetic adjuvants for DNA vaccines. Vaccine. 2001;19:2647–2656. doi: 10.1016/s0264-410x(00)00495-3. [DOI] [PubMed] [Google Scholar]
- Seah J.N., Yu L., Kwang J. Location of linear B-cell epitopes on infectious bronchitis virus nucleocapsid protein. Vet. Microbiol. 2000;75:11–16. doi: 10.1016/s0378-1135(00)00202-9. [DOI] [PubMed] [Google Scholar]
- Seo S.H., Collisson E.W. Specific cytotoxic T lymphocytes are involved in in vivo clearance of infectious bronchitis virus. J. Virol. 1997;71:5173–5177. doi: 10.1128/jvi.71.7.5173-5177.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo S.H., Wang L., Smith R., Collisson E.W. The carboxylterminal 120-residue polypeptide of infectious bronchitis virus nucleocapsid induces cytotoxic T lymphocytes and protects chickens from acute infection. J. Virol. 1997;71:7889–7894. doi: 10.1128/jvi.71.10.7889-7894.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo S.H., Pei J., Briles W.E., Dzielawa J., Collisson E.W. Adoptive transfer of infectious bronchitis virus primed alpha beta T cells bearing CD8 antigen protects chicks from acute infection. Virology. 2000;269:183–189. doi: 10.1006/viro.2000.0211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson F.K. DNA vaccines and adjuvants. Immunol. Rev. 2004;199:5–8. doi: 10.1111/j.0105-2896.2004.00146.x. [DOI] [PubMed] [Google Scholar]
- Strugnell R.A., Drew D., Merciece J., DiNatale S., Firez N., Dunstan S.J., Simmons C.P., Vadolas J. DNA vaccine for bacterial infections. Immunol. Cell Biol. 1997;75:364–369. doi: 10.1038/icb.1997.57. [DOI] [PubMed] [Google Scholar]
- Tacket C.O., Roy M.J., Widera G., Swain W.F., Broome S., Edelman R. Phase I safety and immune response studies of a DNA vaccine encoding hepatitis B surface antigen delivered by a gene delivery device. Vaccine. 1999;19:2826–2829. doi: 10.1016/s0264-410x(99)00094-8. [DOI] [PubMed] [Google Scholar]
- Thompson G., Mohammed H., Bauman B., Naqi S. Systemic and local antibody responses to infectious bronchitis virus in chickens inoculated with infectious bursal disease virus and control chickens. Avian Dis. 1997;41:519–527. [PubMed] [Google Scholar]
- Toro H., Fernandez I. Avian infectious bronchitis: specific lachrymal IgA level and resistance against challenge. Zentralbl. Veterinarmed. B. 1994;41:467–472. doi: 10.1111/j.1439-0450.1994.tb00252.x. [DOI] [PubMed] [Google Scholar]
- Yu L., Liu W., Schnitzlein W.M., Tripathy D.N., Kwang J. Study of protection by recombinant fowl poxvirus expressing C-terminal nucleocapsid protein of infectious bronchitis virus against challenge. Avian Dis. 2001;45:340–348. [PubMed] [Google Scholar]


