Abstract
Porcine monomyeloid cell lines were established following transfection of primary porcine alveolar macrophage cultures with plasmid pSV3neo, carrying genes for neomycin resistance and SV40 large T antigen. The parental clone 3D4 exhibited a relatively rapid doubling time (25.5 h), high plating efficiency and mixed phenotype with respect to growth on a solid support. Single cell cloning of the 3D4 parent resulted in establishment of several cell lines; three of them designated 3D4/2, 3D4/21 and 3D4/31 were selected for further characterization. All three clones supported the replication of vesicular stomatitis virus (VSV), pseudorabies virus (PRV), classical swine fever virus (CSFV), swine vesicular disease virus (SVDV), swine poxvirus, African swine fever virus (ASFV), herpes simplex virus (HSV), parainfluenza virus, bovine adenovirus (BAV), vaccinia virus (VV), and porcine adenovirus (PAV). Under the conditions tested the cells did not support replication of porcine reproductive and respiratory syndrome virus (PRRSV). The swine myeloid character was confirmed for all three clones by fluorescence activated cell scanning (FACS) analysis using monoclonal antibodies 74-22-15 and DH59B, which recognize the pan-myeloid antigen cluster SWC3a. A subpopulation of each cell line was positive for nonspecific esterase activity and phagocytic activity to varying degrees depending on the media formulation. Cells from all three lines exhibited anchorage dependent growth when maintained in RPMI 1640 medium supplemented with 5–15% fetal bovine serum (FBS) and nonessential amino acids. Propagation in commercially formulated serum free media resulted in colony formation and growth in suspension. The addition of dimethyl sulfoxide (DMSO) or phorbol 12-myristate 13-acetate (PMA) to serum free media restored cell attachment. DMSO was also able to induce expression of CD14 monocyte marker in the 3D4/31 cell line maintained in FBS containing medium, as determined by FACS with monoclonal antibody CAM36A. Supplementation of RPMI medium with 10% porcine serum upregulated the expression of CD14 and induced expression of porcine macrophage markers recognized by antibodies 2B10 and 2G6 (Vet. Immunol. Immunopathol. 74 (2000) 163) in all three cell lines. The porcine myelomonocytic cell lines obtained may have a wide variety of applications in porcine virology and immunology.
Keywords: Pig, Myelomonocytic cells, Macrophages, Differentiation, Virus susceptibility
1. Introduction
Cells of the monocyte-macrophage lineage are important effectors and regulators of the immune response and also serve as target cells for replication of a number of viruses. Their infection can thus play an important role in the pathogenesis of a number of viral diseases. For example, macrophages are the only target cells for porcine respiratory and reproductive syndrome virus (PRRSV) (Rossow, 1998) and are the primary targets for African swine fever virus (ASFV) (Malmquist and Hay, 1960, Minguez et al., 1988). A number of other viruses that infect pigs, such as classical swine fever virus (CSFV), porcine circovirus 1 (PCV-1) (Mc Neilly et al., 1996), and porcine circovirus 2 (PCV-2) (Morozov et al., 1998, Kiupel et al., 1998) also replicate in cells of monocyte/macrophage lineage. Work was initiated to establish an immortalized porcine monocyte/macrophage cell line that could be used for propagation and study of selected porcine viruses. The availability of such a cell line would facilitate the isolation and study of these viruses by eliminating reliance on primary cell cultures, difficult to establish and standardize. For example, field strains of PRRSV and ASFV are currently isolated using porcine primary macrophages which are not only difficult to establish but cannot be frozen reliably for long term storage and use. More importantly, it has been shown that the phenotype of porcine monocytic cells at the time of isolation can be heterogeneous (McCullough et al., 1993) which, in turn, may effect the efficiency of ASFV replication. In order to overcome these difficulties, a combination of porcine alveolar macrophages and MA-104 cells has been recommended for the isolation of PRRSV (Benfield et al., 1999). A continuous porcine monocytic cell line with the potential to differentiate into macrophage type cells, would also be a valuable tool for viral pathogenesis and immune function studies. For these reasons, we sought to develop a continuous porcine monocyte/macrophage cell line and to determine its susceptibility to selected viruses.
2. Materials and methods
2.1. Cells
Alveolar monocyte cells were obtained from the lungs of euthanized 12-week-old (SPF) pigs from swine unit, Glenlea research station, University of Manitoba, which were part of nutritional study.
The cells were obtained by infusing 500 ml of cold, sterile phosphate buffered saline (PBS–0.01 M PO4) containing 2 mM ethylenediamine tetraacetic acid (EDTA), pH 7.4 and 20 U/ml nystatin (Life Technologies), and gently massaging to distribute the fluid through the lung parenchyma. The cells from each animal were kept separate throughout the process of purification, transfection and clone selection. Cells were concentrated and freed of debris as follows: lavage samples were first filtered through one layer of sterile gauze and then washed twice in Hanks balanced salt solution (HBBS) (Life Technologies) without calcium chloride and magnesium chloride by centrifugation for 10 min at 800×g at 25 °C. Cellular debris and mucus were removed from the packed cell pellet by pipette, the supernatant fluid was decanted, and the cell pellet was resuspended in RPMI 1640 (Sigma) cell culture medium supplemented with penicillin G sodium (1000 U/ml)/streptomycin sulfate (1 mg/ml) (Wisent), 10% (v/v) porcine serum (PorS) (Life Technologies) and 10% (v/v) gamma-irradiated fetal bovine serum (FBS) (Wisent). Resuspended cells were seeded into 24-well plates and incubated at 37 °C, 5% CO2. After 24 h the adherent cells were washed to remove residual mucus and cell debris and overlayed with the above described supplemented cell culture medium. Approximately 30% confluent cell monolayers were used for the transfection.
The following cell lines were obtained from the American Type Culture Collection (ATCC): porcine kidney (PK-15), swine testicle (ST), Madin–Darby bovine kidney (MDBK), African green monkey (Vero), Georgia bovine kidney (GBK), human diploid fibroblast (MARC-145) and rhesus monkey kidney (MA-104). The medium used for cell growth varied with the cell line as described in Hay et al. (1992).
2.2. Viruses and antisera
The following viruses were maintained routinely at the National Centre for Foreign Animal disease (NCFAD) (Winnipeg, Canada): ASFV/Vero cell adapted strain Lisbon 61, ASFV/Lillie SI 85, CSFV/Alfort 187, swine vesicular disease virus (SVDV)/UK 27/72, pseudorabies virus (PRV)/Shope; swinepox (SwPV)/VR 363, vesicular stomatitis virus (VSV)/Indiana. PRRSV was kindly provided by Dr R. Magar (AGR-LHVA) (St. Hyacinthe, Canada). The Veterinary Infectious Disease Organization (VIDO) (Saskatoon, Canada) routinely maintained: parainfluenzavirus (PIV3), bovine herpervirus-1 (BHV-1), VSV/New Jersey, bovine adenovirus-3 (BAV-3), herpes simplex virus-1 (HSV-1), porcine adenovirus-3 (PAV-3), bovine coronavirus (BCV), vaccinia virus (VV), bovine rotavirus (BRV)/C486, bovine respiratory syncytial virus (BRSV), and porcine parvovirus (PPV).
Swine antisera with specificity for ASFV/Spain, PAV and CSFV were produced at the NCFAD. Swine anti-PRRSV serum was kindly provided by Dr. Magar (AGR-LHVA, St. Hyacinthe, Canada).
2.3. Transfection and selection
Primary porcine alveolar macrophage cultures were transfected with pSV3-neo (ATCC) using Lipofectamine™ (Life Technologies). The procedure was as follows: primary porcine alveolar monocytes/macrophages were seeded in a 24-well plate (Nunc) and incubated for 24 h, 37 °C, 5% CO2 in RPMI supplemented with 20% FBS, nonessential amino acids (1:100) (Cellgro) and gentamycin (5 μg/ml) (Life Technologies). The transfection was carried out according to the protocol supplied by Life Technologies. Cells in each well were incubated in 1 ml of the transfection mixture (5 μg of plasmid DNA, 40 μl of Lipofectamine, 10% FBS, non-essential amino acids and gentamycin supplemented RPMI) for 28 h, at 37 °C in 5% CO2. The transfection medium was then replaced for 48 h with 20% FBS/RPMI supplemented with nonessential amino acids and gentamycin to allow the cells to recover.
The cells were placed under positive selective pressure using resistance to Geneticin (G-418 sulfate) (Life Technologies) on day 3, for a total period of 14 weeks. For the first 2 weeks Geneticin was maintained at a concentration of 600 μg/ml, after which the amount of Geneticin was decreased to 300 μg/ml. At this time the cells were subcloned into parental clones and the selective pressure on the parental clones continued for 12 weeks with a change of selective pressure medium every 4–5 days. The cells were then routinely maintained at 37 °C, 5% CO2 in RPMI supplemented with 10% FBS, nonessential amino acids (1:100), and gentamycin (5 μg/ml).
A limiting dilution method was used to generate single cell clones from the parental cell population, designated 3D4. Throughout the cloning process, cell cultures were maintained at 37 °C, 5% CO2 in RPMI supplemented with 20% FBS, nonessential amino acids (1:100) and gentamycin (5 μg/ml).
2.4. Growth characterization
2.4.1. Mean generation time
Cells were seeded in six-well plates at approximately 2×104 cells/cm2 under the conditions used for routine maintenance. Three wells were trypsinized after 24, 48 and 72 h incubation, and the cells from each well were counted. Doubling time (mean generation time=mgt) was calculated according the formula: N=N 02T/mgt.
2.4.2. Plating efficiency
Cells were seeded in duplicate 25 cm2 flasks at 8 cells/cm2 in the medium used for routine maintenance. After 5 days, the cell colonies in each flask were stained with crystal violet, counted and the average number of cell colonies was calculated.
2.4.3. Viability after freezing
Suspensions containing 107 cells/ml were frozen in freezing medium containing 10% dimethyl sulfoxide (DMSO) (Sigma) and 10% RPMI in FBS for 4 weeks in liquid nitrogen. Cell viability was determined by trypan blue exclusion (0.4% in PBS) (Life Technologies) immediately after thawing and at 18 h post seeding. The dead cell count at 18 h post seeding was deducted from the viable count immediately after thawing and used to estimate cell attachment/survival 18 h after seeding.
2.4.4. Growth curve
Growth curve was determined for the clones 3D4/2, 3D4/21 and 3D4/31. Cells were seeded in six-well plates at approximately 2×105 cells/well in 15% FBS/RPMI, nonessential amino acids (1:100) and gentamycin (5 μg/ml). Three wells per clone were trypsinized at 22, 28, 48, 72, 96, 116 and 142 h post seeding, and the cells from each well were counted. The obtained average cell count for each clone at each time point was plotted against the time.
2.4.5. Growth in serum-free and supplemented media
3D4/2, 3D4/21 and 3D4/31 cells were seeded in 24-well plates at either 2.5×104, 5×104 or 105 cells/cm2 in macrophage serum-free medium (Macrophage-SFM Medium) (Life Technologies), in serum-free lymphocyte medium (AIM-V*) (Life Technologies) or the medium used routinely to maintain these cells. Cells seeded at each concentration in each of the three media formulations were also subjected to various concentrations of phorbol 12-myristate 13-acetate (PMA) (Sigma) and/or DMSO. Cells were maintained for 120 h at 37 °C, 5% CO2 prior to examination by light microscopy and staining with crystal violet in order to assess visually the degree of monolayer formation.
2.5. Phagocytic activity
Each of the three clones was seeded into 8-chamber Nunc LabTek™ Chamber Slides at approximately 1.3×104 cells/ml and incubated at 37 °C, 5% CO2 for 24 h in one of the following media formulations: (1) RPMI containing 15% FBS, nonessential amino acids (1:100), gentamycin (5 μg/ml); (2) RPMI containing 15% FBS, nonessential amino acids (1:100), gentamycin (5 μg/ml), 150 nM PMA, 0.015% DMSO; (3) RPMI containing 15% FBS, nonessential amino acids (1:100), gentamycin (5 μg/ml), 1 μM PMA, 0.1% DMSO; (4) macrophage-SFM containing 1% DMSO, gentamycin (5 μg/ml); (5) macrophage-SFM, gentamycin (5 μg/ml). The medium was then replaced with fresh medium containing 1 μm red or green fluorescent, sulfate-modified, polystyrene, latex beads (0.025% v/v) (Sigma) and the cells were incubated for 1, 3, 5 or 18 h at either 37 or 39 °C, 5% CO2. Following the incubation, cells were gently washed five times with HBSS (containing 0.3 mM Ca2+ and 1 mM Mg2+), overlayed with HBSS and observed by fluorescent microscopy using an FITC or GFP Aqua filter for the green beads and an M2 filter for the red beads. The proportion of phagocyting cells was estimated using photographic images.
2.6. Fluorescence activated cell scanning (FACS)
1×106 cells were resuspended in 100 μl of PBS/sodium azide (0.1%) (Sigma) then pelleted at 800×g, 4 °C for 5 min and resuspended in 25 μl of a mAb solution specific to different cell surface markers or PBS (negative controls). Cells were incubated with the mAb for 60 min, on ice, washed twice with PBS/azide and then resuspended in the appropriate FITC conjugated rabbit anti-mouse F(ab′)2 secondary antibody solution. Incubation with the conjugate continued for 60 min on ice after which time the cells were washed and fixed with PBS/paraformaldehyde (0.5%). The samples were analyzed using a FACScan (Bencton Dickinson). The Lysis II program was used for all flow cytometric analyses.
The following mAb were kindly provided for this study: 2G6 and 2B10 by Dr A. Berndt (F. Schiller Univ., Jena, Germany), FY1H2 by Dr Yang through Dr Haverson (Univ. of Bristol, UK), K139.3E1A9c9g9 by Dr Haverson, (IAH-)CC51 by Dr Howard (IAH, Compton, UK).
Monoclonal antibodies 74-12-4, 76-2-11, and 76-7-4 we obtained from ATCC, 74-22-15, (BB23-)8E6, PT90A, PT36B, CAM36A and DH59B were obtained from VMRD Inc.
2.7. Non-specific esterase staining
Cell monolayers grown on coverslips were fixed for 5 min in 4% paraformaldehyde, incubated 1 h at 37 °C in pararosaniline-naphthyl acetate (Sigma) according to Yam et al. (1971) and counterstained with 0.033% light green.
2.8. Large T antigen expression
2.8.1. Western blot analysis for SV40 large T antigen
Cell lysate from clones 3D4/2, 3D4/21 and 3D4/31, along with the COS-7 lysate (PharMigen) as a positive control were probed with purified mouse anti-SV40 large T antigen monoclonal antibody (PharMigen). Binding of the antibody was detected using alkaline-phosphatase conjugated anti-mouse IgG (H+L) (Kirkegaard and Perry Laboratories) developed with 5-bromo-4-chloro-3-indolyl-phosphate/nitroblue tetrazolium (BCIP/NBT) (Kirkegaard and Perry Laboratories).
2.8.2. Detection of SV40 large T antigen by PCR
Genomic DNA from the continuous porcine clones 3D4/2, 3D4/21, and 3D4/31, and Eco RI restricted pSV3neo plasmid as a positive control template DNA were used in the PCR reactions. SV40 large T antigen sequences were amplified by PCR as described previously (Dhaene et al., 1999). The primers used amplify the retinoblastoma pocket-binding domain of the SV40 large T antigen producing a 105 bp amplicon.
2.8.3. Detection of SV40 large T antigen by immunofluorescence
Cell lines 3D4/2, 3D4/21, and 3D4/31 were grown on glass coverslips and fixed in 4% paraformaldehyde, 0.01 M PBS. The cells, permeabilized in ice-cold acid alcohol, were incubated with primary antibody Pab 101 (PharMingen), overlayed with FITC-conjugated goat anti-mouse IgG (H+L) (Life Technologies), and examined by fluorescence microscopy.
2.9. Virus susceptibility
Cells were seeded in 96-well plates at approximately 3.3×103 cells/cm2 and incubated overnight in one of the following maintenance media formulations: (1) RPMI containing 15% FBS, nonessential amino acids (1:100), gentamycin (5 μg/ml); (2) RPMI containing 15% FBS, nonessential amino acids (1:100), gentamycin (5 μg/ml), 1 μM PMA, 0.1% DMSO; (3) RPMI containing 15% FBS, nonessential amino acids (1:100), gentamycin (5 μg/ml), 1% DMSO; (4) macrophage-SFM, gentamycin (5 μg/ml); (5) RPMI containing 10% porcine serum (PorS), nonessential amino acids (1:100), gentamycin (5 μg/ml). The maintenance medium was then replaced with 10-fold dilutions of various virus stocks made in RPMI and incubated with the cells for 3 h at 37 °C, 5% CO2. After the adsorption period, the virus inoculum was removed and cells were washed once with RPMI and overlayed with their respective maintenance medium containing one-half the amount of specific serum. Cells were incubated for 5 days at 37 °C, 5% CO2 and then either fixed for immunostaining (ASFV, CSFV, PRRSV) or evaluated for the degree of cytopathic effect (CPE) (SVDV, VSV, PRV). In the case of cells infected with SwPV, the infected cell supernatant was titrated using a susceptible cell line (ST). ASFV was titrated on cells maintained in all five media formulations listed above whereas PRRSV was titrated on cells maintained in the first three media formulations. The following viruses: CSFV, SwPV, SVDV, PRV and VSV-Indiana were titrated on cells maintained in the first two media formulations.
Alternatively, some viruses were passed successively twice in each of the three cell lines and the supernatants from the second passage were then titrated (10-fold dilutions) on previously determined, susceptible cell lines along with the original virus inoculum. This method of determining virus susceptibility was carried out for: HSV-1 and PIV3, propagated routinely in Vero cells; BHV-1, VSV/NJ, PAV-3 and BAV-3, routinely propagated in MDBK cells; BCV and BRSV, routinely propagated in GBK cells; VV and BRV, propagated routinely in MA-104 cells; and for PRRSV, routinely propagated in Marc cells. Infection of the IPAM cell clones was carried out by incubating an 0.5 ml cell suspension (2×105 cells/ml), made in RPMI containing 7.5% FBS, nonessential amino acids (1:100) and gentamycin (5 μg/ml), with 0.5 ml of virus inoculum in a 12-well plate for 5 days at 37 °C, 5% CO2. Prior to preparing the cell suspension, the IPAM cell clones were maintained in the above medium containing 15% FBS.
2.10. Immunostaining
Cells were heat fixed and treated with PBS/acetone (20%) at room temperature for 10 min. Rehydration of cells was accomplished by overlaying the cells with PBS-T at room temperature for 30 min. They were then incubated with predetermined dilutions of swine antisera specific for ASFV, PRRSV or CSFV diluted in PBS-T, followed by incubation with horseradish peroxidase conjugated, affinity purified, goat anti-swine IgG-HRP (Kirkegaard & Perry Labs, Gaithersburg), each for 30 min at room temperature with PBS-T washes between incubation steps. Substrate (3-amino-9-ethyl-carbazole in 0.05 M acetate buffer containing hydrogen peroxide at a final concentration of 0.001%) was added as a last step, and incubated at room temperature for 15 min. Following PBS-T washes, the cells were washed with distilled water, heat dried, and the degree of immunostaining was determined by light microscopy.
3. Results
3.1. Parental and single cell clones
Twenty parental clones from two pigs were obtained following the transfection of cells derived from eight pigs. These clones grew in positive selection pressure medium for 14 weeks, at which time they all demonstrated the ability to support the replication of ASFV/Lisbon 61. Three basic phenotypes of cell growth were observed when the parental clones were maintained in RPMI supplemented with FBS: smooth monolayer, monolayer with ridges and vertical ridges with no discrete monolayer.
A time course analysis of phagocytic activity for selected parental clones, as determined by the proportion of cells in a monolayer containing latex beads, revealed that there was minimal activity 1 h after the addition of beads. After a 5 h incubation period, individual clones demonstrated differing levels of phagocytic activity which never exceeded the involvement of 50% of the cell monolayer, even at the 18 h time point.
Doubling times and plating efficiencies during cultivation were determined for ten selected parental clones (Table 1 ). Parental clone 3D4 was selected for further work based on: (1) the shortest doubling time; (2) a viability of 97.5–98% immediately after freezing and thawing; and (3) an estimated viability of 56% 18 h after seeding from a thawed state. Thin section electron microscopy did not reveal Papovaviridae or Retroviridae-like particles.
Table 1.
Phenotype, doubling time and plating efficiency of selected continuous porcine monocyte/macrophage parental clones propagated in RPMI supplemented with 15% FBS, nonessential amino acids (1:100) and gentamycin (5 μg/ml)
| Clone | Doubling time (h) | Plating efficiency (%) | Phenotype |
|---|---|---|---|
| 3D8 | 60 | 99 | Ridges |
| 3C10 | 68 | 79 | Ridges |
| 3E8 | 70 | 90 | Monolayer |
| 3G8 | 31.5 | 82.5 | Monolayer/ridge |
| 3D4 | 25.5 | 93.5 | Monolayer/ridge |
| 3G11 | 132.5 | 80 | Ridges |
| 4C5 | 71 | 82 | Monolayer/ridge |
| 4D8 | 120.5 | 90 | Monolayer/ridge |
| 3C8 | 88 | 85.5 | Monolayer |
| 3G5 | 55 | 79 | Monolayer/ridge |
Sixteen single cell clones, derived from the parental clone 3D4, maintained the phagocytic activity and the ability to support the replication of ASFV/Lisbon 61. Their mean generation times ranged from 12 to 52 h. Three single cell clones were selected for more detailed characterization based on their cell growth phenotype: 3D4/2 which grew as a monolayer with ridges, 3D4/21 which grew in vertical ridges with no discrete monolayer and 3D4/31 which grew in a smooth monolayer. All three clones tested negative for presence of mycoplasma by PCR and electron microscopy.
3.2. Detection of SV40 large T antigen
Analysis of 3D4/2, 3D4/21 and 3D4/31 cells by Western blotting and immunofluorescence, in either the exponential or the stationary growth phase, did not reveal the presence of the large T antigen. This was supported by the absence of a DNA sequence coding for this antigen, as determined by PCR analysis (data not shown).
3.3. Growth characterization of the IPAM clones 3D4/2, 3D4/21 and 3D4/31
The growth curves of clones 3D4/2, 3D4/21 and 3D4/31 are illustrated in Fig. 1 . After an initial lag phase, the cells started to divide until they reached the stationary phase at about 80 h post seeding. Propagation of the cell clones in serum-free media resulted in colony formation and growth in suspension, while in FBS supplemented medium, cells attached to polystyrene surfaces and formed a monolayer. The three clones grew in distinct ridges when propagated in RPMI supplemented with 10% PorS, losing the differential morphology observed when they were propagated in FBS supplemented medium.
Fig. 1.
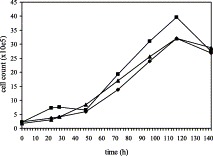
Growth curve of the continuous porcine myelomonocytic single cell clones 3D4/2 (♦), 3D4/21 (■) and 3D4/31 (▴) in 15% FBS/RPMI. Cells were seeded in six-well plates at approximately 2×105 cells/well in 15% FBS/RPMI, nonessential amino acids (1:100) and gentamycin (5 μg/ml). Three wells per clone were trypsinized at 22, 28, 48, 72, 96, 116 and 142 h post seeding, and the cells from each well were counted The obtained average cell count for each clone at each time point was plotted against the time.
When the three cell clones were propagated in two different, commercially available, serum free media (AIV-V and macrophage-SFM), supplementation of either medium with 1 μM PMA promoted cell attachment and growth in monolayers for all three cell clones seeded at 2.5×104, 5×104 or 1×105 cells/cm2. Using 1% DMSO as a supplement, only cells seeded at 1×105 cells/cm2 formed monolayers. Regardless of the cell seeding density, higher concentrations of both DMSO (2%) and PMA (10 μM) were toxic to all three cell clones.
3.4. Phagocytic activity of 3D4/2, 3D4/21 and 3D4/31 clones
Single cell clones 3D4/2, 3D4/21, and 3D4/31 were evaluated for their ability to phagocytose latex beads under various conditions. Very few cells in each clone were able to exhibit this ability when they were maintained in SFM. A noticeable increase in internalized beads was detected with the addition of 1% DMSO or 1 μM PMA to the medium (data not shown).
A time course analysis of phagocytosis for cells in the maintenance medium either in the presence or absence of PMA (1 μM or 150 nM) indicated that only a few cells had adsorbed beads after a 1 h incubation period. After 3 h, the proportion of cells with adsorbed beads increased with a few cells starting to internalize the beads. The proportion of cells with internalized beads further increased after both a 5-h and an overnight incubation period. There were no observable differences in the pattern of bead internalization among the clones and the time course of bead adsorption and internalization was similar at 37 and 39 °C.
The internalization of the latex beads by cells of all three clones was confirmed by electron microscopy.
3.5. Non-specific esterase staining
Very few cells in clones 3D4/2, 3D4/21 and 3D4/31 stained for non-specific esterase when maintained in SFM. The proportion of stained cells increased in media with FBS alone or supplemented with 1 μM PMA. Under these conditions, staining was restricted to specific areas of the monolayer, where the cells grew in ridge formations. The highest proportion of cells exhibiting non-specific esterase activity were detected, when the cells were maintained in RPMI containing 1% DMSO/FBS or 10% porcine serum. In this case, the stained cells were distributed evenly throughout the entire monolayer.
3.6. Fluorescence activated cell scanning for immune cell markers
First set of FACScan analyses for the presence of immune cell markers was performed on cells propagated in RPMI supplemented with 15% FBS. Monoclonal antibodies DH59B and 74-22-15 bound to cells from all three clones, although to varying degrees. The following mAbs did not exhibit binding to cells from any of the three cell clones: FY1H2, CC51, K139.3E1 A9c9g9, 74-12-4, 76-2-11, 76-7-4, 8E6, PT90A, PT36B. Second set of analyses was performed on cells cultivated overnight in different media prior to the FACScan. Fig. 2 illustrates the reactivity of cell clone 3D4/31 with mAbs 2G6 and 2B10. The cells displayed broad range of nonspecific fluorescence (likely due to their granular appearance), and the arbitrarily established cut of value for positive staining may have not captured all the stained cells. The FACS analysis is summarized in Fig. 3 . Regardless of the media used for propagation mAb 74-22-15 recognized majority of the cells from all three clones. DH59B antibody showed lower reactivity, but still recognized the cells in all types of medium. Monoclonal antibodies CAM36A, 2B10 and 2G6 labeled over 50% of cells from each clone grown in RPMI supplemented with 10% PorS. An increase in staining with mAb CAM36A was observed in cell clone 3D4/31 propagated in maintenance medium with 1% DMSO added. See Table 2 for specificity of individual antibodies.
Fig. 2.
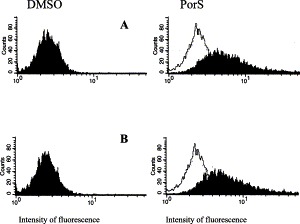
Immunofluorescent staining detected by flow cytometry of the clone 3D4/31 incubated overnight in either DMSO/FCS/RPMI (DMSO) or 10% porcine serum/RPMI (PorS), and labeled with antibody 2B10 (A) or antibody 2G6 (B). Cell control fluorescence intensity was in the range illustrated in panel PorS (no fill peak).
Fig. 3.
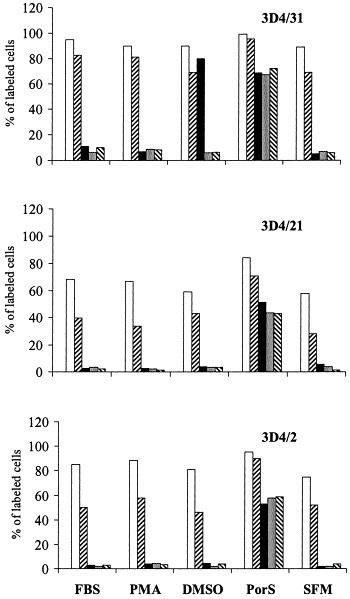
FACScan analysis of clones 3D4/2, 3D4/21 and 3D4/31 for immune cell surface markers. Cells were incubated in 15% FBS/RPMI (FBS), 1 μM PMA/FBS/RPMI (PMA), 1% DMSO/FCS/RPMI (DMSO), 10% porcine serum/RPMI (PorS), and macrophage-SFM medium (SFM), prior to labeling with the following antibodies: 74-22-15 (white column), DH59B (right leaning dashed column), CAM36A (black), 2B10 (dotted), 2G6 (left leaning dashed column). Primary antibodies were incubated with FITC conjugated rabbit anti-mouse F(ab′)2 secondary antibody, and analyzed using a Becton Dickinson FACScan.
Table 2.
List of monoclonal antibodies used in phenotyping against their markers, with their specificity
| Monoclonal antibody (isotype) | Marker | Cell type | Reference |
|---|---|---|---|
| 2G6 (IgG1) | 140–150 kDa | Mature resident tissue macrophages, e.g. alveolar macrophages, peripheral blood monocytes | Berndt et al., 2000 |
| 2B10 (IgG1) | 140–145 kDa | Subpopulation of macrophages from bronchoalveolar lavage, tissues macrophages | Berndt et al., 2000 |
| FY1H2 (IgG1) | CD3c | T-cell and activation marker group | Saalmuller et al., 1998 |
| K139.3E1A9c9g9 (IgG2a) | Ig light chain | B-cell subset | Saalmuller et al., 1998 |
| (IAH)-CC51 (IgG2b) | CD21 | B-cell subset | Saalmuller et al., 1998 |
| 74-12-4 (IgG2b) | Swine CD4a | T-cell subset | Lunney, 1994, Saalmuller et al., 1998 |
| 76-2-11 (IgG2ak) | Swine CD8a | T-cell subset | Lunney, 1994, Saalmuller et al., 1998 |
| 76-7-4 (IgG2ak) | Swine CD1 | B-cell subset | Lunney, 1994, Saalmuller et al., 1998 |
| 74-22-15 (IgG2a) | SWC3a | (swine) panmyeloid marker (macrophages) | Lunney, 1994, Alvarez et al., 2000, Saalmuller et al., 1998 |
| (BB23)-8E6 (IgG1) | CD3a | T-cell subset | Saalmuller et al., 1998 |
| PT90A (IgG2a) | Swine CD4a | T-cell subset | Lunney, 1994, Saalmuller et al., 1998 |
| PT36B (IgG1) | Swine CD8a | T-cell subset | Lunney, 1994, Saalmuller et al., 1998 |
| CAM36A IgG1 | CD14 | Monocyte cell subset | Brodersen et al., 1998 |
| DH59B IgG1 | SWC3a | (swine) panmyeloid marker (macrophages) | Lunney, 1994, Alvarez et al., 2000, Saalmuller et al., 1998 |
3.7. Virus susceptibility
All of the parental and single cell clones propagated in routine maintenance medium supported replication of ASFV, Lisbon-61. The single cell clones 3D4/2, 3D4/21 and 3D4/31 also supported the replication of VSV/Indiana, VSV/NJ, PRV, CSFV, SVDV, SwPV, HSV-1, PI3, BHV-1, BAV-3, VV, BRV and PAV-3, although they displayed some differential sensitivity to several viruses. Clone 3D4/21 did not support the replication of BHV-1 in contrast to the clones 3D4/2 and 3D4/31, and it replicated VV to a significantly lower titer than the other two clones. In contrast clone 3D4/21 could replicate BAV-3 to markedly higher titers (difference of 5 logs in TCID50) than clones 3D4/2 and 3D4/31. (Fig. 4 ) There was no significant difference (more than 1 log) in the titer of VSV-Indiana, PRV, SVDV and SPV grown in the three cell clones maintained in RPMI supplemented with FBS with or without the addition of 1 μM PMA. However, the titer of CSFV grown in 3D4/21 cells in maintenance medium, was significantly lower than that in the other two cell clones maintained in the same medium. The cell clones did not support the replication of BCV, BRSV and PRRSV, regardless of the media formulation.
Fig. 4.
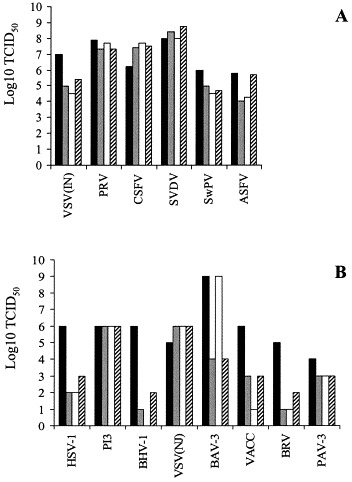
Virus susceptibility of the continuous porcine myelomonocytic cell lines 3D4/2, 3D4/21 and 3D4/31 in 7.5% FBS/RPMI, compare to the susceptibility of standard assay cells: 3D4/2 (dotted column), 3D4/21 (white), 3D4/31 (dashed), standard assay cells (black column). Panel A illustrates results when cells were seeded in 96-well plates and incubated overnight in RPMI containing 15% FBS. The maintenance medium was then replaced with 10-fold dilutions of various virus stocks made in RPMI and incubated with the cells for 3 h at 37 °C, 5% CO2. After the adsorption period, the virus inoculum was removed and cells were washed once with RPMI and overlayed with their respective maintenance medium containing 7.5% of FBS. Cells were incubated for 5 days at 37 °C, 5% CO2 and then either fixed for immunostaining (ASFV, CSFV) or evaluated for the degree of cytopathic effect (CPE) (SVDV, VSV, PRV). In the case of cells infected with SwPV, the infected cell supernatant was titrated using a susceptible cell line (ST). Panel B illustrates results when viruses were successively passed twice in each of the three cell lines and the supernatants from the second passage were then titrated (10-fold dilutions) on previously determined, susceptible cell lines along with the original virus inoculum. Infection of the IPAM cell clones was carried out by incubating a 0.5 ml cell suspension (2×105 cells/ml) made in RPMI containing 7.5% FBS with 0.5 ml of virus inoculum in a 12-well plate for 5 days at 37 °C, 5% CO2. Prior to preparing the cell suspension, the IPAM cell clones were maintained in the above medium containing 15% FBS.
The ability of the cells to replicate the cell culture adapted ASFV-Lisbon 61, and the field isolate of ASFV (Lillie SI/85) was tested with cells grown in different media (Table 3 ).
Table 3.
Effect of culture media formulation on ASFV replication in continuous porcine monocyte/macrophage clones 3D4/2, 3D4/21 and 3D4/31 in comparison to standard cell culture systems
| Medium/supplement | ASFV-Lisbon 61 (titer log 10) |
ASFV-Lillie (titer log 10) |
||||||
|---|---|---|---|---|---|---|---|---|
| Vero | 3D4/2 | 3D4/21 | 3D4/31 | PMBC | 3D4/2 | 3D4/21 | 3D4/31 | |
| (1) RPMI/10% FBS | 6.00 | 5.50 | 6.25 | 6.70 | 6.50 | 3.00 | 3.75 | 3.75 |
| (2) RPMI/10% FBS 1:M PMA | 5.80 | 5.80 | 6.50 | 3.00 | 4.00 | 2.75 | ||
| (3) RPMI/10% FBS 1% DMSO | 5.00 | 6.25 | 6.50 | 3.50 | 5.00 | 2.50 | ||
| (4) SFM | 4.75 | 3.75 | 6.00 | 3.50 | 3.50 | 2.75 | ||
| (5) *RPMI/10%PorS | 6.10* | 4.50 | 4.60 | 4.65 | 5.10* | ** | ** | ** |
New virus stocks.
Cells in the 10−1 virus dilution washed off. No IHC staining was detected in 10−2 virus dilution.
No major difference among the media formulation (1), (2), and (3) was observed for replication of ASFV-Lisbon 61 in any of the three clones. Clones 3D4/21 and 3D4/2 yielded significantly lower virus titers (more than 1 log TCID50) in SFM (4) when compared to the other media formulations, and all three clones yielded significantly lower virus titers in 10% porcine serum medium (5).
ASFV-Lillie replicated in the continuous monocyte/macrophage clones to titers about 3 logs lower than the titer obtained in primary peripheral blood leukocytes. No significant differences among the clones in the various media formulations were observed, except for 10% porcine serum medium (5), where the cells would repeatedly wash off in the 10−1 virus dilution wells and no IHC staining was detected in 10−2 virus dilutions (Table 3).
4. Discussion
Three myeloid (monocyte/macrophage) cell lines, designated 3D4/2, 3D4/21 and 3D4/31 were successfully passaged forty times without significant changes in growth characteristics. Their apparent loss of contact inhibition as demonstrated by formation of multilayer ridges and their viability after storage in liquid nitrogen at intermittent passage levels also support the argument that they are immortalized. Although the cells were resistant to high doses of the selective antibiotic G418 for a prolonged period of time when compared to immortalized porcine fibroblasts (Choi et al., 1990), the expression of the SV40 large T-antigen gene was not confirmed, suggesting that an alternative mechanism may account for the immortalization. Preliminary screening of the cells by electron microscopy for the presence of adventitious viruses, especially those with oncogenic potential, did not reveal virus like particles. Further work is needed to elucidate the mechanism of immortalization.
Monoclonal antibodies DH59B and 74-22-15 which recognize the porcine pan-myeloid cluster of differentiation SWC3a (Lunney, 1994, Alvarez et al., 2000) indicated that all three clones, 3D4/2, 3D4/21 and 3D4/31 are of the monomyeloid lineage. When grown in serum free medium the cells could be considered non-differentiated myeloid-type cells based on their: (1) anchorage independent behavior; (2) diminished phagocytic and non-specific esterase activity; and (3) lack of expression of CD14 and macrophage markers (identified by mAbs 2B10 and 2G6).
The cells grown in the maintenance medium supported replication of selected porcine viruses, except PRRSV. We hypothesized that by cultivation in the maintenance medium partial differentiation of the cells was achieved, based on phenotypic changes such as attachment to polystyrene surfaces, increase in the non-specific esterase and phagocytic activity. This is consistent with the work of others (Kreutz et al., 1992), who have shown that serum factors can induce partial maturation of primary monocytes in vitro. However, the degree of differentiation was not sufficient to ensure replication of PRRSV and satisfactory replication of field isolates of ASFV. We have therefore attempted to further differentiate/mature the cells by adding PMA or DMSO to medium containing FBS (Denholm and Stankus, 1995), or by using PorS supplemented medium (McCullough et al., 1999).
As the PMA stock solution must be prepared in DMSO, all working solutions contained traces of DMSO. These however are in quantities at least 10-fold lower than those reported to induce differentiation (Lackey and Cabot, 1983, Cellier et al., 1997). The argument that the PMA was the primary differentiating agent in the PMA formulations (Tsuchiya et al., 1982), was supported by our experiments. Addition of PMA increased the proportion of cells displaying non-specific esterase and phagocytic activities in all three clones, however, it did not change cell culture morphology, cell surface marker expression or sensitivity to viruses.
The addition of porcine serum to the culture media appeared to have the most pronounced effect on the cells based on growth characteristics and marker expression. However their sensitivity, based on GFP-reporter system (Pasick et al., 2002), to the field strain of ASFV-Lillie did not change, indicating that the differentiation of the continuous porcine alveolar monocyte cell lines did not reach a level of maturation necessary for satisfactory replication of field isolates of ASFV (Natale and McCullough, 1998). Two other findings suggest that the 3D4/2, 3D4/21 and 3D4/31 cells reached only intermediate state of differentiation in porcine serum: (1) high level of CD14 expression on their surface which is an indicator of monocyte-type cells (McCullough et al., 1997); and (2) relatively high proportion of the 3D4/2, 3D4/21 and 3D4/31 cells displaying non-specific esterase activity (Basta et al., 1999). Markers SWC1 and SWC9 will be used along with the CD14 and non-specific esterase activity to characterize cell differentiation in future work.
Addition of DMSO increased the proportion of cells displaying non-specific esterase activity in all three clones, upregulated the expression of CD14 in clone 3D4/31, and interestingly improved the capability of clone 3D4/21 to replicate the field isolate of ASFV/Lillie compare to the other clones. The best, but not optimal, conditions for replication of field isolate of ASFV in the immortalized macrophages were thus in clone 3D4/21 in the RPMI medium containing 10% FBS/1% DMSO (titer 105 compare to 106.5 in PBMC).
The reason for PRRSV not being able to replicate in the continuous porcine alveolar monocyte cell lines is not known. PRRSV has been reported to have strongly restricted tropism for only some sub-populations of porcine monocytes/macrophages that may depend on their differentiation and/or activation (Duan et al., 1997).
In summary the three porcine cell lines 3D4/2, 3D4/21 and 3D4/31 are monomyeloid cells with potential to differentiate into mature cells. It was demonstrated that under specific conditions they differentiated into cells having the characteristics of those at an intermediate stage of maturation. The cells supported replication of wide range of viruses, and may have the potential to support replication of monocytotropic viruses, which require mature-type cells for replication. Although the three cell lines have similar characteristics, they differ in the degree they support replication of some viruses, and in the degree they respond to differentiating agents. The ease of manipulation of these cells and the above mentioned biological characteristics make them potentially suitable for viral diagnostic and immunopathology applications.
Acknowledgements
We would like to thank Dr Michael Coulthart, Chief of Genetics and Prion Diseases, Health Canada, CSCHAH, Winnipeg for the use of their flow cytometry equipment, Dean Airey (Genetics and Prion Diseases) and Terry Beskorwayne (VIDO) for performing the FACScan analysis, Paul Chipman for the electron microscopy analysis of the cells, and Halina Kobylecka (NCFAD) for preparation of the primary alveolar macrophages. We would like to thank Dr Berndt (F. Schiller Univ., Jena) for kindly providing the macrophage marker antibodies 2B10 and 2G6, and to Dr. Hope Weiler, Human Nutritional Studies, University of Manitoba for providing porcine lungs used for preparation of primary cells. The work was supported by Canadian Food Inspection Agency from their technical development funds.
References
- Alvarez B., Sanchez C., Bullido R., Marina A., Lunney J., Alonso F., Ezquerra A., Dominguez J. A porcine cell surface receptor identified by monoclonal antibodies to SWC3 is a member of the signal regulatory protein family and associates with protein-tyrosine phosphatase SHP-1. Tissue Antigens. 2000;55:342–351. doi: 10.1034/j.1399-0039.2000.550408.x. [DOI] [PubMed] [Google Scholar]
- Basta S., Knoetig S.M., Spagnuolo-Weaver M., Allan G., McCullough K.C. Modulation of monocytic cell activity and virus susceptibility during differentiation into macrophages. J. Immunol. 1999;162:3961–3969. [PubMed] [Google Scholar]
- Benfield, D.A., Collins, J.E., Dee, S.A., Halbur, P.G., Joo, H.S., Lager, K.M., Mengeling, W.L., Murtaugh, M.P., Rossow, K.D., Stevenson, G.W., Zimmerman, J.J., 1999. Porcine reproductive and respiratory syndrome. In: Straw, B.E., D'Allaire, S., Mengeling, W.L., Taylor, D.J. (Eds.) Diseases of Swine, 8th edition, Iowa State University Press, Ames, Iowa, pp. 201–232.
- Berndt A., Heller M., Methner U., Kosmel H., Muller G. Monoclonal antibodies against porcine macrophages. Vet. Immunol. Immunopathol. 2000;74:163–177. doi: 10.1016/s0165-2427(00)00166-5. [DOI] [PubMed] [Google Scholar]
- Brodersen R., Bijlsma F., Gori K., Jensen K.T., Chen W., Dominguez J., Haverson K., Moore P.F., Saalmuller A., Sachs D., Slierendrecht W.J., Stokes C., Vainio O., Zuckermann F., Aasted B. Analysis of the immunological cross reactivities of 213 well characterized monoclonal antibodies with specificities against various leukocyte surface antigens of human and 11 animal species. Vet. Immunol. Immunopathol. 1998;64:1–13. doi: 10.1016/s0165-2427(98)00117-2. [DOI] [PubMed] [Google Scholar]
- Cellier M., Shustik C., Dalton W., Rich E., Hu J., Malo D., Schurr E., Gros P. Expression of the human NRAMP1 gene in professional primary phagocytes: studies in blood cells and in HL-60 promyelocytic leukemia. J. Leuk. Biol. 1997;61:96–105. doi: 10.1002/jlb.61.1.96. [DOI] [PubMed] [Google Scholar]
- Choi C.S., Murtaugh M.P., Molitor T.W. Establishment of transformed swine fibroblast cell lines using SV40 large T antigen. Arch. Virol. 1990;115:227–237. doi: 10.1007/BF01310532. [DOI] [PubMed] [Google Scholar]
- Denholm E.M., Stankus G.P. Changes in the expression of MCP-1 receptors on monocytic THP-1 cells following differentiation to macrophages with phorbol myristate acetate. Cytokine. 1995;7:436–440. doi: 10.1006/cyto.1995.0059. [DOI] [PubMed] [Google Scholar]
- Dhaene K., Verhulst A., Van Marck E. SV40 large T-antigen and human pleural mesothelioma. Screening by polymerase chain reaction and tyramine-amplified immunohistochemistry. Virchows Arch. 1999;435:1–7. doi: 10.1007/s004280050387. [DOI] [PubMed] [Google Scholar]
- Duan X., Nauwynck H.J., Pensaert M.B. Effects of origin and state of differentiation and activation of monocytes/macrophages on their susceptibility to porcine reproductive and respiratory syndrome virus (PRRSV) Arch. Virol. 1997;142:2483–2497. doi: 10.1007/s007050050256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay, R.J., Caputo, J., Chen, T.R., 1992. American Type Culture Collection, catalog of cell lines and hybridomas, 7th Edition, ATCC, Rockville.
- Kiupel M., Stevenson G.W., Mittal S.K., Clark E.G., Haines D.M. Circovirus-like viral associated disease in weaned pigs in Indiana. Vet. Pathol. 1998;35:303–307. doi: 10.1177/030098589803500411. [DOI] [PubMed] [Google Scholar]
- Kreutz M., Krause S.W., Hennemann A., Rehm A., Andreeses R. Macrophage heterogeneity and differentiation: defined serum-free culture conditions induce different types of macrophages in vitro. Res. Immunol. 1992;143:107–115. doi: 10.1016/0923-2494(92)80087-2. [DOI] [PubMed] [Google Scholar]
- Lackey R.J., Cabot M.C. Acetate utilization and fatty acid metabolism in phorbol ester and dimethyl sulfoxide-differentiated human leukemia cells. Cancer Lett. 1983;20:291–297. doi: 10.1016/0304-3835(83)90027-7. [DOI] [PubMed] [Google Scholar]
- Lunney J.K. Overview of the first international workshop to define swine leukocyte cluster of differentiation (CD) antigens. Vet. Immunol. Immunopathol. 1994;43:193–206. doi: 10.1016/0165-2427(94)90136-8. [DOI] [PubMed] [Google Scholar]
- Malmquist W.A., Hay D. Hemadsorption and cytopathic effect produced by African swine fever virus in swine bone marrow and buffy coat cultures. Am. J. Vet. Res. 1960;21:104–108. [PubMed] [Google Scholar]
- McCullough K.C., Schaffner R., Fraefel W., Kihm U. The relative density of CD-44 positive porcine monocytic cell populations varies between isolations and upon culture and influences susceptibility to infection by African swine fever virus. Immunol. Lett. 1993;37:83–90. doi: 10.1016/0165-2478(93)90136-p. [DOI] [PubMed] [Google Scholar]
- McCullough K.C., Schffner R., Natale V., Kim Y.B., Summerfield A. Phenotype of porcine monocytic cells: modulation of surface molecule expression upon monocyte differentiation into macrophages. Vet. Immunol. Immunopathol. 1997;58:265–275. doi: 10.1016/s0165-2427(97)00045-7. [DOI] [PubMed] [Google Scholar]
- McCullough K.C., Basta S., Knotig S., Gerber H., Schaffner R., Kim Y.B., Saalmuller A., Summerfield A. Intermediate stages in monocyte-macrophage differentiation modulate phenotype and susceptibility to virus infection. Immunology. 1999;98:203–212. doi: 10.1046/j.1365-2567.1999.00867.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mc Neilly F., Allan G.M., Foster J.C., Adair B.M., McNulty M.S., Pollock J. Effect of porcine circovirus infection on porcine alveolar macrophage function. Vet. Immunol. Immunopathol. 1996;49:295–306. doi: 10.1016/0165-2427(95)05476-6. [DOI] [PubMed] [Google Scholar]
- Minguez I., Rueda A., Dominguez J., Sanchez-Vizcaino J.M. Double labeling immunohictological study of African swine fever virus infected spleen and lymph nodes. Vet. Pathol. 1988;25:193–198. doi: 10.1177/030098588802500302. [DOI] [PubMed] [Google Scholar]
- Morozov I., Sirinarumitr T., Sorden S.D., Halbur P.G., Morgan M.K., Yoon K.J., Paul P.S. Detection of a novel strain of porcine circovirus in pigs with postweaning multisystemic wasting syndrome. J. Clin. Microbiol. 1998;36:2535–2541. doi: 10.1128/jcm.36.9.2535-2541.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natale V.I., McCullough K.C. Macrophage cytoplasmic vesicle pH gradients and vacuolar H+-ATPase: activities relative to virus infection. J. Leuk. Biol. 1998;64:302–310. doi: 10.1002/jlb.64.3.302. [DOI] [PubMed] [Google Scholar]
- Pasick, J., Weingartl, H., Hills, K., submitted for publication. Development of a green fluorescent protein reporter system for the detection of African swine fever virus infections (submitted for publication).
- Rossow K.D. Porcine reproductive and respiratory syndrome, review article. Vet. Pathol. 1998;35:1–20. doi: 10.1177/030098589803500101. [DOI] [PubMed] [Google Scholar]
- Saalmuller A., Pauly T., Lunney J.K., Boyd P., Aasted B., Sachs D.H. Overview of the second international workshop to define swine cluster of differentiation (CD) antigens. Vet. Immunol. Immunopathol. 1998;60:207–228. doi: 10.1016/s0165-2427(97)00098-6. [DOI] [PubMed] [Google Scholar]
- Tsuchiya S., Kobayashi Y., Goto Y., Okumura H., Nakae S., Konno T., Tada K. Induction of maturation in cultured human monocytic leukemia cells by a phorbol diester. Cancer Res. 1982;42:1530–1536. [PubMed] [Google Scholar]
- Yam L.T., Li C.Y., Crosby W.H. Cytochemical identification of monocytes and granulocytes. Am. J. Clin. Pathol. 1971;55:283–290. doi: 10.1093/ajcp/55.3.283. [DOI] [PubMed] [Google Scholar]


