Abstract
The lymphoid, renal, pulmonary, and hepatic lesions of naturally occurring postweaning multisystemic wasting syndrome (PMWS) affected pigs have been studied by means of immunohistology. Ten conventionally reared pigs showing acute clinical signs of PMWS were selected from a farm on which animal were seronegative to porcine reproductive and respiratory virus and to Aujeszky’s disease virus. All pigs were positive in tests for porcine circovirus type 2 by ISH and IHC. Monoclonal and polyclonal antibodies to CD3, CD79α, CD45RA (3C3/9), lysozyme, SLA-II-DQ (BL2H5), and MAC387 were used to characterise cells in PMWS lesions. The most relevant changes were reduction or loss of B and T lymphocytes, increased numbers of macrophages, and partial loss and redistribution of antigen presenting cells throughout lymphoid tissues compared to uninfected controls. The characteristics of lymphoid lesions in the present study strongly suggest an immunosuppressive effect of PMWS in affected pigs.
Keywords: Swine, Lymphoid lesions, Postweaning multisystemic wasting syndrome, PMWS, PCV2
1. Introduction
Postweaning multisystemic wasting syndrome (PMWS) is a disease characterised mainly by growth retardation in postweaning pigs; since the first description (Clark, 1997) it has now been observed and described in several countries all around the world (Allan and Ellis, 2000). Although porcine circovirus type 2 (PCV2) was consistently associated with PMWS in early studies (Segalés et al., 1997), only recently it has been experimentally demonstrated to be the etiological agent of PMWS (Harms et al., 2001, Bolin et al., 2001).
It has been suggested that PMWS may be related with immunosuppression in pigs (Segalés et al., 2001). Typical microscopic lymphoid damage in PMWS affected tissues (Segales et al., 1997, Rosell et al., 1999), and the association of the disease with opportunistic pathogens commonly associated with immunosuppression (Clark, 1997, Carrasco et al., 2000), help to support this hypothesis. Furthermore, some studies demonstrated that the over stimulation of infected pig’s immune system, or routine vaccination could potentiate PCV2 infection and subsequent development of wasting disease (Allan et al., 2000, Krakowka et al., 2001). Flow cytometry and immunohistochemical (IHC) studies have been performed to determine the changes on leukocyte populations of conventional pigs affected with PMWS (Shibahara et al., 2000, Segales et al., 2001, Darwich et al., 2002, Sarli et al., 2001). The most relevant findings reported in these studies were decrease of circulating B cells (Darwich et al., 2002) and loss of lymphocytes in B-cell areas (Shibahara et al., 2000). Moreover, a decrease in CD4+ and/or CD8+ T lymphocytes has also been described (Sarli et al., 2001, Segales et al., 2001, Darwich et al., 2002).
Although some studies have been focused on the immunopathological study of lymphoid lesions of PMWS affected pigs (Kiupel et al., 1999, Sarli et al., 2001), little data have been gathered regarding the alterations of the cell populations in the most representative lesions associated with PMWS. Therefore, the aim of this study was to characterise the lymphoid, renal, pulmonary, and hepatic lesions of natural PMWS affected pigs by means of IHC methods.
2. Materials and methods
2.1. Pigs
Ten 2.5-month-old conventional pigs suffering from acute PMWS-like clinical signs were selected from a 1300 sow, farrow-to-finish operation. The herd had previously been diagnosed with PMWS, and was seronegative to porcine reproductive and respiratory syndrome virus (PRRSV), Aujeszky’s disease virus (ADV), and porcine parvovirus (PPV). In this farm, sows were regularly vaccinated against ADV (aqueous live vaccine), PPV, and erysipelas (combined killed vaccine). Fattening pigs were vaccinated against ADV at 2 and 3 months of age. PRRSV vaccination had never been used on this farm.
Pigs were killed by an intravenous sodium pentobarbital injection and a complete necropsy then performed on all animals. Samples of the following tissues were collected: lungs, thymus, tonsils, lymph nodes (mesenteric, superficial inguinal and submandibular), spleen, liver, kidney and ileum. Tissues were fixed in 10% neutral buffered formalin, embedded in paraffin wax, sectioned at 3 μm, and stained with haematoxylin and eosin (HE) for microscopic study. For IHC and in situ hybridisation (ISH), serial 4 μm thick sections of all tissues were cut and placed on silane (3-(triethoxysilyl)-propylamine) coated slides.
Five 1.5- to 2.5-month-old, conventional pigs seronegative to PCV2, PRRSV, ADV, transmissible gastroenteritis virus, porcine respiratory coronavirus, swine influenza virus, PPV, Actinobacillus pleuropneumoniae and Erysipelothrix rhusiopathiae, were used as controls for normal tissues (Chianini et al., 2001).
2.2. PCV2 detection
ISH and IHC to detect PCV2 were performed in formalin-fixed, paraffin-embedded tissue, following previously published procedures (Rosell et al., 1999).
For ISH a PCV2 specific oligonucleotide was used (Rosell et al., 2000a). Briefly, sections were placed on Probe-on-Plus glass microscope slides (Fisher Scientific, Pittsburgh, USA). A workstation was used to control the temperature of the hybridisation reactions and various incubations, and to minimise reagent consumption. Tissue sections were deparaffinised, and rehydrated through graded alcohols to Automotion Buffer (Biømeda Corp., CA, USA). Proteolytic digestion was achieved with 0.3% pepsin, and incubation with 100% formamide for 5 min at 105 °C was done. Hybridisation was performed for 5 min at 105 °C and 30 min at 37 °C. After stringent washes with saline sodium citrate buffers, an anti-digoxigenin antibody conjugated with alkaline phosphatase was applied. Colour was developed with nitroblue tetrazolium dye. The sections were counterstained with fast green stain, dehydrated and mounted.
IHC was performed with a polyclonal anti-PCV2 antibody using a previously described protocol (Sorden et al., 1999). Briefly, tissue sections were deparaffinised with xylene and rehydrated through graded alcohols. Endogenous peroxidase activity was blocked by incubation with hydrogen peroxide 3% in distilled water for 30 min. Tissue sections were rinsed in 0.1 M Tris-buffered saline (pH 7.6) and incubated with 20% normal goat serum solution in 0.1 M Tris-buffered saline for 1 h at room temperature. PCV2 antibody was used at 1/200 dilution in 0.1 M Tris-buffered saline, and incubated overnight at 4 °C. Biotinylated goat anti-rabbit (1/400) was used as a secondary antibody, for 1 h at room temperature. An avidin–biotin peroxidase (ABC) method (Pierce, IL, USA) diluted 1/100 in 0.1 Tris-buffered saline was applied for 1 h at room temperature. Sections were finally incubated in diaminobenzidine (DAB)–hydrogen peroxide solution for 10 min, counterstained with Harris’ haematoxylin, dehydrated, coverslipped and examined microscopically.
Sections from a previous case of PMWS diagnosed in Spain (Segalés et al., 1997) were used as positive control for both IHC and ISH techniques. Negative controls consisted of archives samples of lung and lymphoid tissues of pigs from an experimental farm with no history of PWMS or PCV2 infection.
2.3. Immune system cell characterisation
Cell markers used to classify the different cell phenotypes present in the lymphoid and no lymphoid tissues are shown in Table 1 . T cells were identified using an anti-human CD3 antibody. B cells were labelled with an anti-human CD79α (HM57) and anti-swine CD45RA (3C3/9) antibodies. Macrophages were detected with anti-human lysozyme antibody. Antigen presenting cells (APCs) were identified with an anti-SLA-II-DQ (BL2H5) antibody. Polymorphonuclear granulocytes (PMNG) were labelled with a MAC387 antibody.
Table 1.
Details of the primary antibodies used in the immunohistochemical study
| Specificity | pAb/mAb (clone) | Host of origin | Treatment | Dilution | Source |
| CD3 | pAb | Human | Pronasea | 1/150 | Dako (Denmark) |
| CD79α | mAb (HM57) | Human | Microwaveb | 1/25 | Dako (Denmark) |
| L1 | mAb (MAC387) | Human | Pronase | 1/200 | Dako (Denmark) |
| Lysozyme | pAb (A099) | Human | Trypsinc | 1/100 | Dako (Denmark) |
| CD45RA | mAb (3C3/9) | Swine | No treatment | Undilutedd | INIA Lab |
| SLA-DQ | mAb (BL2H5) | Swine | Pronase | Undilutedd | INIA Lab |
Incubation with 0.1% pronase for 10min at 37°C.
Incubation with citrate buffer (pH 6) for 20min at 100°C.
Incubation with 0.1% trypsin for 2h at 37°C.
Culture surnatant.
All the antibodies were used with a standard ABC method following previously described procedures (Chianini et al., 2001). Briefly, tissue sections were deparaffinised in xylene and rehydrated in graded alcohols to distilled water. Endogenous peroxidase activity was blocked by incubation with hydrogen peroxide 3% in distilled water for 30 min. After pronase, trypsin or citrate buffer treatment, depending on the antibody used, tissue sections were rinsed in 0.1 M Tris-buffered saline (pH 7.6) and incubated with 20% normal goat serum solution in 0.1 M Tris-buffered saline for 1 h at room temperature. The slides were then incubated overnight at 4 °C with one of the primary antibodies. Biotinylated goat anti-mouse (1/200) and biotinylated goat anti-rabbit (1/400) were used as secondary antibodies, for 1 h at room temperature. An ABC complex (Pierce, IL, USA) diluted 1/100 in 0.1 Tris-buffered saline was applied for 1 h at room temperature. Sections were finally incubated in diaminobenzidine (DAB)–hydrogen peroxide solution for 10 min, counterstained with Harris’ haematoxylin, dehydrated, coverslipped and examined microscopically. As negative controls, irrelevant primary antibodies at the same dilution were used in substitution of the specific antibodies.
3. Results
3.1. Macroscopic lesions
At necropsy, cranioventral pulmonary consolidation (9 out of 10 pigs), and enlargement of inguinal and mesenteric (jejunal) lymph nodes (8/10) were the most obvious finding. Other lesions observed in the PMWS affected pigs were non-collapsed lungs (3/10), parakeratosis of the oesophageal zone of the stomach (3/10), slight atrophy of nasal turbinates (3/10), and multifocal size white spots in the outer renal cortex (2/10). Gross lesions were not observed in the control pigs.
3.2. Microscopic lesions
The main lesions observed in the lymphoid organs were depletion of lymphocytes in B- and T-cell areas and infiltration of lymphoid tissues by histiocytes. Based on the intensity of these abnormalities, three stages of the infection were determined: stage I mild lesions (2 animals out of 10); stage II moderate lesions (3/10); stage III severe lesions (5/10). A detailed description of each stage follows with a summary in Table 2 .
Table 2.
Histological and immunohistochemical results
| Stages | Microscopic lesions |
PCV2 detectiona |
Cell markersa |
||||||||||
| Lymphocyte depletionb |
Histiocytic cellsc | MGCc | ICIBc | IHC | ISH | CD79α | 3C3/9 | CD3 | Lysozyme | BL2H5 | MAC387 | ||
| B zones | T zones | ||||||||||||
| Controls | − | − | + | − | − | − | − | +++ | +++ | +++ | + | +++ | + |
| I | + | − | + | + | − | + | + | ++ | ++ | +++ | + | +++d | + |
| II | ++ | + | ++ | +++ | + | ++ | ++ | − | + | ++ | ++ | +++d | ++ |
| III | +++ | ++ | +++ | + | ++ | +++ | +++ | − | − | + | +++ | ++ | ++ |
Staining has been graded as (−) no stained cells, (+) low number of stained cells, (++) moderate number of stained cells, and (+++) high number of stained cells.
Lymphocyte depletion degree: (−) absence; (+) slight; (++) moderate; (+++) severe.
Presence of histiocytic cells, MGC and intracytoplasmic inclusion bodies (ICIB) has been graded as (−) absence, (+) low numbers, (++) moderate numbers, and (+++) large numbers.
Different distribution compared to control cases.
3.2.1. Stage I
Mild depletion of B-cell areas and mild infiltration of histiocytes and few multinucleate giant cells (MGCs), mainly in the germinal centres of follicular areas of lymph nodes, tonsil, spleen and Peyer’s patches were consistently observed at this stage. Furthermore, in lymph nodes, mild infiltration of the medulla-like tissue by histiocytes was also noted. Scattered circulating granulocytes, mainly eosinophils, and occasional mitotic and apoptotic figures were found in all lymphoid tissues.
Mild, multifocal thickening of the alveolar septae due to the presence of mononuclear cells mainly lymphocytes and scattered histiocytes was observed in the lung of both pigs. In one of them, randomly distributed, multifocal aggregates of lymphocytes and plasma cells were noted in the hepatic and renal parenchyma.
3.2.2. Stage II
The main histopathological findings in lymph nodes, tonsil, spleen and Peyer’s patches of animals at this stage were moderate lymphocyte depletion of B-cell areas, mild lymphocyte depletion in T-cell areas, and a variable number of infiltrating histiocytes in follicular and interfollicular areas. MGCs were observed in lymphoid organs, especially in the lymph nodes of two animals. In one animal, small, round nucleated cells and degenerated granulocytes were found in the cytoplasm of MGCs. Large single or smaller multiple spherical basophilic inclusions were present in the cytoplasm of scattered histiocytes in tonsil and mesenteric lymph node of one animal. In the tonsil of the same animal, a large number of eosinophils infiltrated the tissues and a tonsillar crypt. In the ileal Peyer’s patches and submandibular lymph node of two animals many mitotic figures were observed. Apoptotic figures were occasionally observed in all lymphoid tissues.
In lungs, mild neutrophilic exudation in bronchi, bronchioles and alveoli, and mild, multifocal thickening of the alveolar septa due to the presence of lymphocytes and scattered macrophages were observed. Increased numbers of alveolar macrophages were detected in one animal.
Mild randomly distributed multifocal or periportal lymphoplasmacytic infiltrations were sporadically observed in the liver; one pig also had multifocal lymphoplasmacytic infiltrations in renal medulla and cortex.
3.2.3. Stage III
Lymph nodes follicles were drastically reduced or absent. Subcapsular, interfollicular, and medulla-like tissues were invaded by a large numbers of histiocytes. Large single or smaller multiple cytoplasmic spherical, basophilic inclusions were occasionally observed in the histiocytes. MGCs were also found scattered in the cortical areas of lymph nodes. Activated high endothelial venules were observed in the lymph nodes of one animal. Occasional mitotic and apoptotic figures were sometimes located in follicular areas of all animals. Furthermore, a large number of circulating polymorphonuclear granulocytes, mainly eosinophils, were constantly seen, except in submandibular lymph nodes. Changes in tonsil were dominated by marked loss of lymphocytes in B- and T-cell areas and macrophage infiltration. Numerous eosinophils were constantly found in follicular and interfollicular tissue, and inside the crypt lamina. Numerous of mitotic figures were observed, whereas apoptotic cells and MGCs were only sporadically present. Splenic changes were characterised by diminution of lymphocytes and infiltration of histiocytic cells in periarteriolar lymphoid sheaths (PALS) and follicles. Occasionally, scattered MGCs and eosinophils in the red pulp were seen.
Changes in Peyer’s patches consisted in lymphocytic depletion in B- and T-cell areas associated to macrophage infiltration. Small multiple basophilic cytoplasmic inclusion bodies were observed in macrophages infiltrating follicular areas. Scattered mitotic and apoptotic figures were also seen. Thymic changes were characterised by diminution of thymocytes, especially in the medulla, and histiocytic infiltration in the cortex and medulla.
In all pigs at stage III, macrophage infiltration and diminution or loss of bronchus-associated lymphoid tissue (BALT) were prominent features in lung. Furthermore, neutrophilic exudation in bronchi, bronchioli and alveoli was a constant finding, associated with moderate to severe thickening of the alveolar septae due to the presence of mononuclear cells, mainly lymphocytes and fewer macrophages.
Lymphoplasmacytic multifocal or perilobular infiltration, and diffuse hydropic degeneration of hepatocytes were the most frequent changes observed in the liver. Mild to moderate, multifocal lymphoplasmacytic infiltration was observed in the renal cortex of one animal.
3.3. PCV2 detection
PCV2 nucleic acid and antigen were consistently detected in the lymphoid tissues of all animals, whereas detection of PCV2 was variable in non-lymphoid tissues. In follicular lymphoid tissues, PCV2 nucleic acid and antigen were labelled in the cytoplasm of macrophages, follicular dendritic cells, interdigitating cells and MGCs (however, the nuclei of scattered macrophages, follicular dendritic cells and lymphocytes were also occasionally stained). In lymphoid tissues, the intensity of PCV2 detection correlated with the severity of the histological lesions. Results are summarised in Table 2.
3.3.1. Stage I
PCV2 nucleic acid and antigen in follicular lymphoid tissues were mainly confined in infiltrating macrophages and dendritic cells of follicular areas, and in scattered interdigitating cells and lymphocytes in T-cell areas. Furthermore, in Peyer’s patches, PCV2 was labelled in histiocytic cells in the lamina propria and in intraepithelial lymphocytes. In thymus, PCV2 was observed in thymic corpuscles and in scattered macrophages of the medulla and cortex. PCV2 nucleic acid or antigen was not detected in lung, liver, and kidney.
3.3.2. Stage II
PCV2 nucleic acid and antigen was observed in infiltrating macrophages, MGCs (Fig. 1 ) and interdigitating or dendritic cells in the cortex of lymph nodes. In tonsil, PCV2 was mainly located in macrophages and dendritic cells of follicular areas, around the crypt and in a few scattered lymphocytes in interfollicular areas. In spleen, PCV2 was observed in histiocytic cells of PALS and in circulating cells in the red pulp. In lungs, a variable amount of PCV2-positive histiocytic cells were found in BALT. PCV2-positive Kupffer cells were occasionally observed in liver. In one case, PCV2 was detected in the cytoplasm of macrophages and MGCs in a nephritic focus.
Fig. 1.
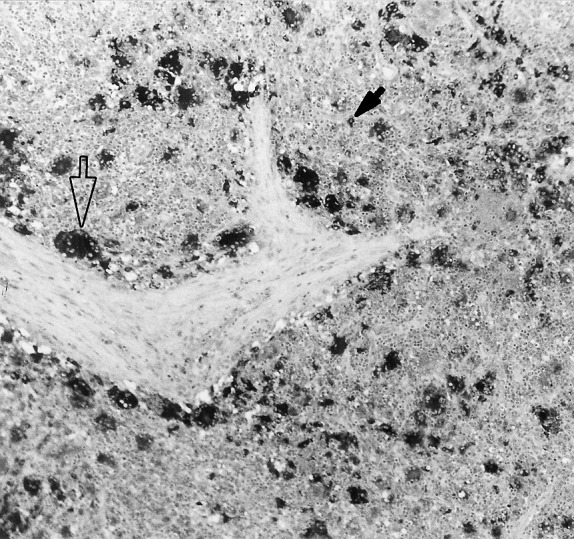
Lymph node from a pig in stage II. Immunolabelling of PCV2 antigen within the cytoplasm of MGCs (open arrow) and macrophages (filled arrow) in formalin-fixed, paraffin-embedded tissue, using the ABC method and Mayer’s haematoxylin counterstain (original magnification 10×).
3.3.3. Stage III
PCV2 distribution in lymphoid organs was similar to that of stage II; but viral nucleic acid and antigen were also detected in the cytoplasm of macrophages infiltrating the medulla-like area of lymph nodes. In lung, a discrete PCV2 staining was found in BALT macrophages, alveolar septae, peribronchiolar and peribronchial macrophages, and in the macrophages of intrabronchiolar exudates. In liver, PCV2 antigen and, in a smaller number of cases, nucleic acid were found in Kupffer cells and in perilobular and periportal macrophages. In one animal, PCV2 was demonstrated in a small focus of macrophages located in the kidney.
3.4. Immune system cells characterisation
The most consistent findings were reduction or loss of follicular CD79α- and 3C3/9-positive cells, diminution in T-cell areas of CD3-positive lymphocytes, increase of subcapsular and peritrabecular lysozyme-positive macrophages, and partial loss and redistribution of BL2H5-positive APCs throughout lymphoid tissues. A varying number of circulating MAC387-stained PMNs were constantly observed in lymphoid and non-lymphoid tissues of all animals. These results are summarised in Table 2. Normal distribution of the cell markers used in this study is described elsewhere (Chianini et al., 2001).
3.4.1. Stage I
In follicular organs, a decreased number of CD79α-stained lymphocytes were observed in germinal centres and mantle zones of mildly affected cases. 3C3/9 antibody-stained germinal centres of all follicular immune system organs, similar to control cases. In spleen, a moderate number of 3C3/9 antibody-stained cells were observed in the marginal zones, as in control animals. The majority of T-cell areas were visibly delimited resembling control animals, and CD3-stained (Fig. 2 A) lymphocytes were only slightly diminished. In Peyer’s patches, the distribution of CD3-positive cells was comparable to control animals, being more numerous in the lamina propria and in an intraepithelial localisation than in domes, and germinal centres.
Fig. 2.
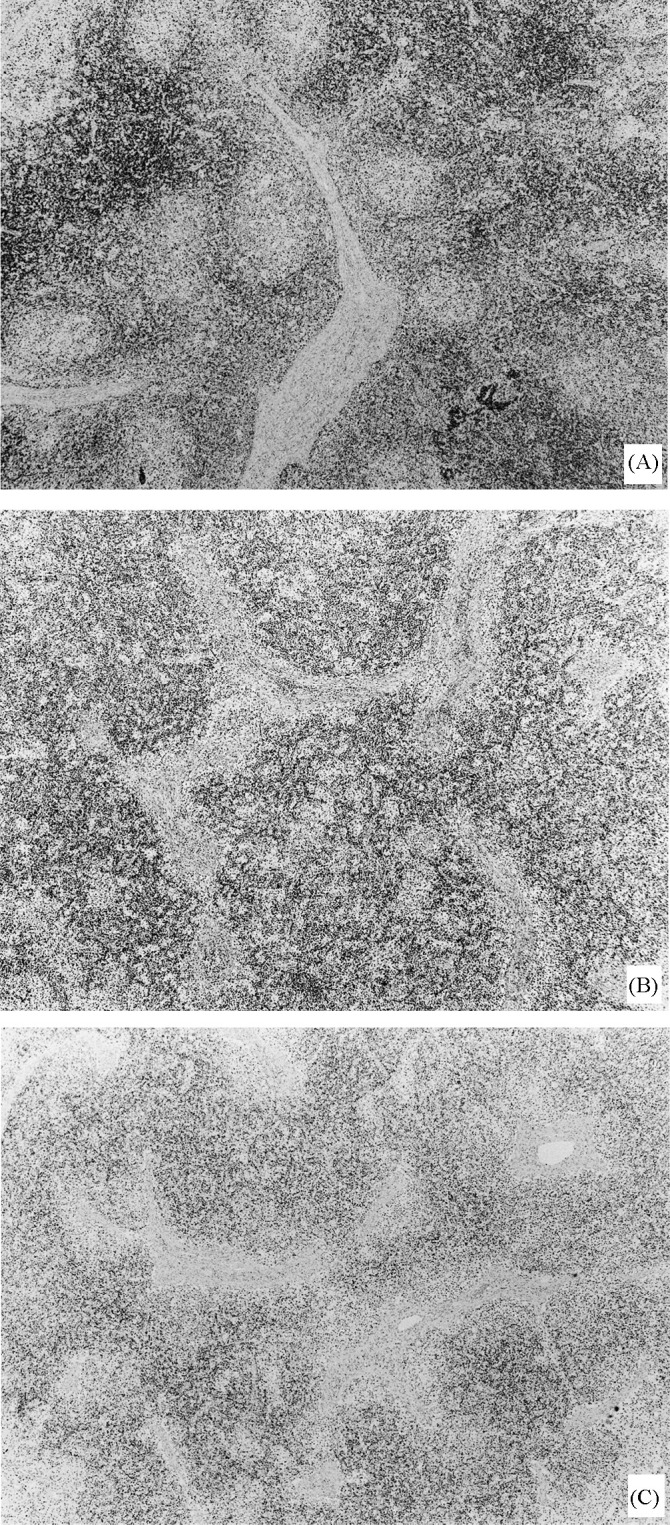
Lymph node from pigs in stages I (A), II (B), and III (C). Note slight (A), moderate (B) and severe (C) diminished number of CD3 lymphocytes, and disorganisation of T-cell areas (B, C). Immunolabelling with anti-CD3 in formalin-fixed, paraffin-embedded tissue, using the ABC method and Mayer’s haematoxylin counterstain (original magnification 4×).
In follicular lymphoid organs, lysozyme-positive cells were depicted in the germinal centre of the follicles. In lymph nodes, a slightly increased number of lysozyme-positive histiocytic cells were found in medulla-like tissues and peritrabecular zones. In tonsil, the majority of lysozyme-positive cells were detected in follicular zones and around the crypts, whose epithelium was also occasionally stained. In spleen, lysozyme-stained cells were detected in follicular and marginal zones. In the Peyer’s patches, lysozyme-stained histiocytes were found in the lamina propria.
BL2H5 antibody diffusely stained the surface and occasionally the cytoplasm of histiocytic cells and many endothelial cells in all lymphoid organs. In follicular lymphoid organs, BL2H5 antibody-stained follicular dendritic cells in germinal centres, similar to control animals, whereas lymphocytes in B-cell areas were unstained. In thymus, a larger number of BL2H5-positive cells were found in the medulla compared to control animals.
MAC387 antibody stained a moderate to high number of circulating PMNs in the red pulp of the spleen and in smallest numbers in the peritrabecular sinuses of lymph nodes. In tonsil, a variable number of scattered circulating PMNs were depicted with MAC387. In the Peyer’s patches positive PMNs were found in the lamina propria of intestinal villi. Very few circulating PMNs were seen in the medulla and cortex of the thymus; in some cases thymic corpuscles were faintly stained with this antibody.
In lung, circulating CD3-positive lymphocytes were constantly observed, in BALT and peribronchiolar and peribronchial areas. Lysozyme stained a variable number of histiocytic cells in BALT and in the alveolar septae. BL2H5-positive cells were mainly located in BALT and in the alveolar septae, but frequently found also in peribronchiolar and peribronchial areas.
In one animal, CD3-stained lymphocytes, lysozyme- and BL2H5-stained macrophages, and MAC387-stained PMNs were observed in randomly distributed multifocal inflammatory infiltrates in hepatic parenchyma and renal pelvis.
BL2H5 antibody stained a larger number of endothelial cells of glomerular and interstitial vessels, compared to control animals.
3.4.2. Stage II
In some lymph nodes, small numbers of CD79α-stained cells were observed in the mantle zone of follicles, but other lymph nodes showed an absence of staining. In follicular lymphoid organs, 3C3/9 antibody only stained lymphocytes in marginal zone and follicles of spleen. In lymph nodes, tonsil, and spleen, T-cell areas were sometimes disorganised and showed a slight to moderate diminution of CD3-stained cells (Fig. 2B). In T-cell areas of Peyer’s patches and spleen, CD3-positive cells were mildly reduced in number.
An increase of lysozyme-positive histiocytes in lymph nodes was observed in subcapsular areas and medulla-like tissues. In tonsil, many lysozyme-stained cells were seen diffusely distributed throughout. In two animals, the cytoplasm of numerous MGCs strongly stained with lysozyme. Several lysozyme-positive histiocytic cells showed perinuclear granular staining in lymphoid organs. A large number of scattered tingible body macrophages were abundantly depicted with lysozyme in thymic cortex, tonsil, and in smaller numbers in spleen and lymph nodes.
BL2H5 antibody stained the surface and sometimes the cytoplasm of histiocytes, MGCs (Fig. 3 ), and endothelial cells in all lymphoid tissues studied. In lymph nodes, the majority of BL2H5-positive cells were observed in the medulla-like and peritrabecular areas, whereas scattered positive-stained cells were observed in the cortical area. In thymus, BL2H5 antibody-stained cells in the medulla, similarly to animals in stage I.
Fig. 3.
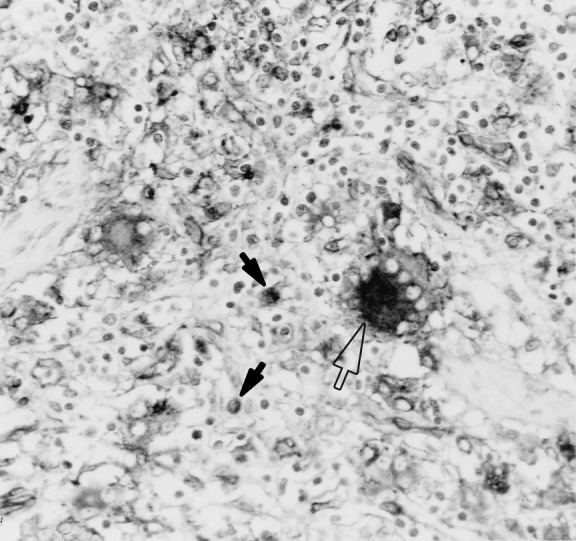
Lymph node from a pig in stage II. Labelling of cytoplasm and surface of MGCs (open arrow) and macrophages (filled arrows) in the interfollicular area. Immunolabelling with anti-SLA-II-DQ (BL2H5) in formalin-fixed, paraffin-embedded tissue, using the ABC method and Mayer’s haematoxylin counterstain (original magnification 40×).
MAC387-positive cells had a similar pattern of distribution to animals from stage I, being more numerous especially in the red pulp of the spleen and in the sinus of lymph nodes. Occasionally, positive PMNs were located within the cytoplasm of MGCs.
In lung, CD3, BL2H5 and lysozyme antibodies showed a similar staining pattern to stage I. MAC387 antibody-stained PMNs strongly and macrophages faintly inside bronchi, bronchioles and alveoli, as well as in alveolar septae.
In liver, CD3, lysozyme and BL2H5-positive cells were observed in mild inflammatory infiltrates localised in periportal areas or randomly distributed foci.
In kidney, one animal had a large number of CD3- and BL2H5-stained cells in the renal pelvis, within interstitial inflammatory foci. Circulating MAC387-stained PMNs were consistently observed, sometimes associated with small foci of inflammation.
3.4.3. Stage III
In follicular lymphoid tissues, no CD79α- or 3C3/9-stained cells were found in B-cell areas, and only scattered 3C3/9-positive cells were observed in the marginal zone of the spleen. Reduction of CD3-positive cells was especially noticeable in lymph nodes (Fig. 2C) and spleen. The majority of lymph nodes and spleens, showed undefined T-cell areas, and, in lymph nodes, CD3-positive lymphocytes showed a diffuse disorganised pattern of distribution, invading subcapsular and peritrabecular areas. In spleen, a moderate depletion of CD3-stained cells was observed in PALS. T-cell dependent interfollicular zones were maintained in tonsil, showing a moderate to severe degree of CD3 lymphocytes depletion. In Peyer’s patches, only a slight decrease of CD3-stained cells was observed. In thymus, a slight reduction of medullary mature CD3-positive thymocytes was occasionally observed.
An increased number of lysozyme-positive macrophages was apparent, and mimicked the distribution of infiltrating histiocytes observed in the histological study. In lymph nodes, lysozyme-positive cells were constantly observed in medullary and peritrabecular zones (Fig. 4 ), and infiltrating the cortex. In tonsil, lysozyme-stained macrophages were uniformly distributed, but especially numerous around the crypts (Fig. 5 ) in which positive staining epithelium was also occasionally present. Positive lysozyme inclusion bodies and MGCs were also observed. In spleen, the lysozyme antibody-stained infiltrating macrophages in PALS and scattered macrophages in the marginal zones and red pulp. In Peyer’s patches, lysozyme-positive macrophages were mainly observed in follicular areas and domes. A variable number of tingible body macrophages were stained with the lysozyme antibody in thymic cortex, and in smaller numbers in Peyer’s patches, lymph nodes and spleen.
Fig. 4.
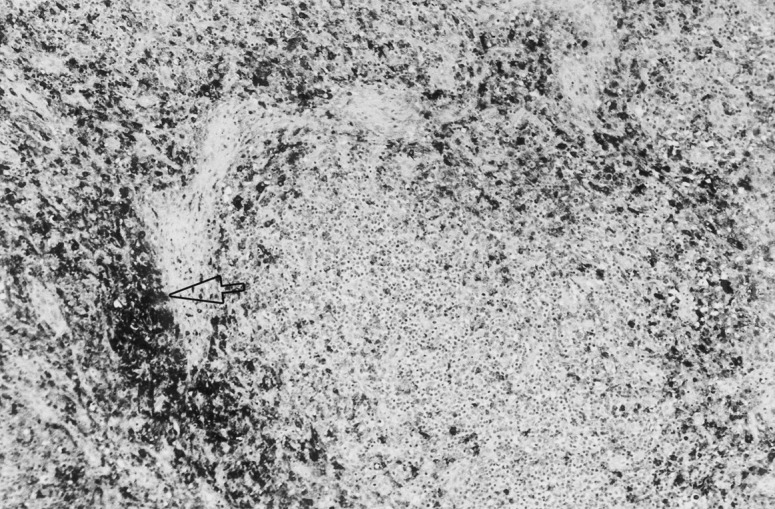
Lymph node from a pig in stage III. There are a large number of macrophages infiltrating a peritrabecular area (arrow). Immunolabelling with anti-lysozyme in formalin-fixed, paraffin-embedded tissue, using the ABC method and Mayer’s haematoxylin counterstain (original magnification 10×).
Fig. 5.
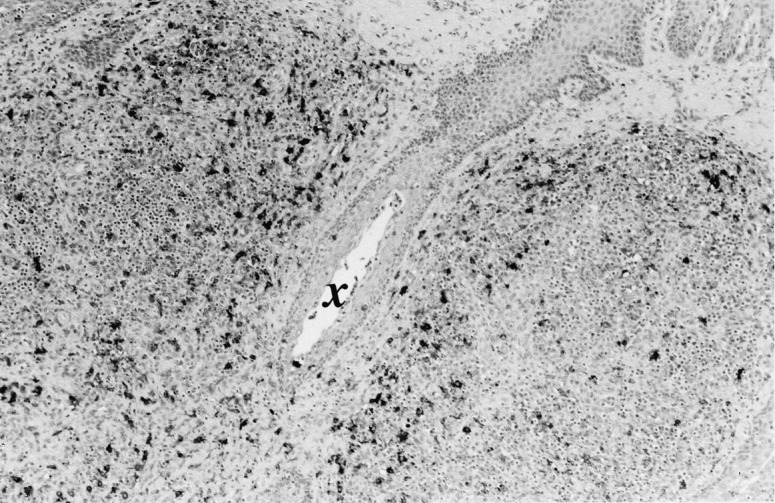
Tonsil from a pig in stage III. Note infiltrating macrophages around a crypt (×). Immunolabelling with anti-lysozyme in formalin-fixed, paraffin-embedded, using the ABC method and Mayer’s haematoxylin counterstain (original magnification 10×).
The cell surface and occasionally the cytoplasm of histiocytic and MGCs and many endothelial cells stained with BL2H5 antibody. The positive cells were diffusely distributed in all lymphoid tissues. The largest number of stained cells was observed in the peritrabecular areas of lymph nodes and in PALS of the spleen. In Peyer’s patches, stained cells were mainly observed in the follicular zones, and in more variable numbers in the lamina propria of intestinal villi. In thymus, similar to moderately affected cases, BL2H5 antibody stained the surface of interdigitating cells and macrophages in the medulla, cortico-medullary junction and cytoplasmic processes in the cortex.
In this group of animals, MAC387-stained PMNs with a similar distribution to stage II animals. In lung, circulating CD3 lymphocytes were observed in these cases. The lysozyme antibody stained a variable number of histiocytic cells in the alveolar septae and less frequently in the bronchi and bronchioles. Positive BL2H5 cells were mainly located in the alveolar septae, but also found in peribronchiolar and peribronchial locations. In a very few cases, histiocytic BL2H5-stained cells were observed inside bronchi or bronchioles. Large numbers of MAC387-positive PMNs and few macrophages were seen inside bronchi, bronchioles and alveoli, as well as in alveolar septae.
In liver and kidney a similar pattern of staining was seen with all antibodies to stage I animals.
4. Discussion
The present work describes the changes of immune system cell distribution in different organs of pigs with naturally occurring PMWS using previously described IHC methods (Chianini et al., 2001). The more significant changes are reduction or loss of B cells, diminution of T lymphocytes, increase of subcapsular and peritrabecular macrophages, and partial loss and redistribution of APCs throughout lymphoid tissues. The extent of the mentioned changes was strongly correlated with the severity of PMWS histological lesions and the amount of PCV2 antigen and/or nucleic acid detected by IHC and ISH tests.
Macroscopic lesions observed in lymph nodes, lungs, and kidneys in the present study had been previously reported by other authors (Clark, 1997, Rosell et al., 1999). The low incidence of renal macroscopic lesion found in this work may be due to the small number of pigs studied. However, much larger study, also reported a low number of pigs with gross renal lesions (Quintana et al., 2001).
Microscopic lymphoid lesions consisting of lymphocyte depletion, histiocytic infiltration, MGCs, and cytoplasmic inclusions, are considered characteristic of PMWS affected pigs (Clark, 1997, Rosell et al., 1999). Varying degrees of lymphocytic depletion and histiocytic infiltration were observed in all pigs studied; however, significant numbers of MGCs were only observed in stage II affected cases. Activation of the immune system is considered crucial for the formation of this cell type in tonsil and adenoid tissues of human immunodeficiency virus affected patients (Orenstein and Wahl, 1999). Our results suggest that a similar phenomenon with formation of MGCs only occur in more immunologically responsive PCV2 infected animals. Further studies on PCV2 pathogenetic mechanisms and a larger number of pigs are necessary to clarify this point.
Among non-lymphoid tissues, interstitial pneumonia was the main finding, whereas suppurative bronchopneumonia observed in stages II and III was associated with intercurrent bacterial infections (data not shown). Hepatic and renal lesions associated with PCV2, as described in other reports (Rosell et al., 2000a, Rosell et al., 2000b), were only sporadically observed in our study; the selection methods and the larger number of pigs used by these authors might explain this discrepancy.
Concurrent ISH for PCV nucleic acid (without differentiation between PCV1 and PCV2) and IHC for several cell markers confirmed macrophages to be the predominant virus containing cells in PMWS affected pigs (Kiupel et al., 1999). In our study, the application of a larger panel of cell markers, and the use of a PCV2-specific probe for ISH (Rosell et al., 2000a) demonstrated that APCs in general, and not only macrophages, are target cells for PCV2 infection. On the other hand, we found that lymphocytes are only sporadically infected, similarly to other authors’ results (Kiupel et al., 1999). The relation between severity of lesions in secondary lymphoid tissues and presence of PCV2 nucleic acid has been already documented (Darwich et al., 2002). In the present study, thymus, a primary lymphoid organ, was also investigated. In this organ, PCV2 nucleic acid or antigen was detected only in a few histiocytic cells of the medulla, suggesting that thymocytes and T cells might be more resistant to PCV2 infection.
The decrease of CD79α- and CD45RA-positive lymphocytes observed in our study agreed with the results of other IHC studies in tissue (Kiupel et al., 1999, Shibahara et al., 2000, Sarli et al., 2001) and peripheral blood leukocytes using flow cytometry (Segales et al., 2001, Darwich et al., 2002) of PMWS affected pigs. These studies described CD4+, CD8+, and CD4+/CD8+ lymphocyte changes in frozen tissues or in peripheral blood of PMWS affected pigs (Sarli et al., 2001, Segales et al., 2001, Darwich et al., 2002). In our study, T-cell depletion was evaluated with CD3 antibody, a pan-T lymphocyte marker, which makes comparison impossible with previous authors. T naïve lymphocytes, which are positive for both CD45RA and CD3 antibodies (Chianini et al., 2001), were still observed in the marginal zone of the spleen of all studied pigs, suggesting that this cell subpopulation is not adversely affected in PMWS when compared with healthy animals.
PCV2 have been described as inducing apoptosis of B lymphocytes, which would explain B-cell areas depletion in PMWS affected pigs (Shibahara et al., 2000). Recent studies, including the present one, have now demonstrated that depletion also occurs, to a lesser degree, in T-cell areas (Sarli et al., 2001). Death from apoptosis is a fundamental process in regulating T- and B-cell populations and also in regulating cell viral infection (Alcami and Koszinowski, 2000, Kramer, 2000). Therefore, apoptosis might be a significant mechanism of lymphocyte depletion observed in PMWS. If and how PCV2 might play a role in apoptosis up-regulation is still unknown.
Histiocytic like cells infiltrating immune system organs in PMWS have been identified as macrophages (Kiupel et al., 1999, Shibahara et al., 2000, Sarli et al., 2001). In our study heavy infiltrations of lysozyme-positive macrophages were observed in stages II and III, with a similar pattern of distribution to PCV2 infected cells. Recent studies on viral immune evasion mechanisms explain how DNA viruses are able to acquire immune response genes of the host, which blocks or subvert the host anti-virus immune and inflammatory responses (Alcami and Koszinowski, 2000, Haig, 2001). PCV2 may inhibit one of several host anti-virus responses, such as apoptosis, chemokine production, or interferon production allowing macrophages to survive longer. Further in vivo and in vitro studies are still needed to better explain the pathogenesis of lymphocyte depletion, and macrophage infiltration characterising PMWS.
Lysozyme and SLA-II-DQ (BL2H5) staining of MGCs confirmed the macrophage origin of these cells. BL2H5 antibody cytoplasm labelling the of these cells is not a surprising finding since MHC II is firstly assembled to the antigen in the endoplasmic reticulum before transportation to the cell surface (Calafat et al., 1994). BL2H5 antibody demonstrated that APCs and PCV2 infected cells have a very similar pattern of distribution, reinforcing the observation that all types of APCs could be PCV2 infection target cells (Rosell et al., 1999), not just macrophages as previously suggested (Kiupel et al., 1999). In stages I and II, APCs number, but not their distribution, was very similar to control cases, whereas in stage III a smaller number of these cells were observed. This finding can be explained by the replacement of B cells by infiltrating macrophages, since B-lymphocytes are also APCs. In our study, only the most severely affected pigs showed a decreased number of APCs; however, because these animals were killed it is impossible to predict what changes would have occurred if they had survived longer. It may well be that the most significant APCs loss occurs in the terminal stage of disease.
Increase of MAC387-stained PMNs observed in almost all tissues in stages II and III might be a consequence of secondary infections occurring in the pneumonic lungs (data not shown). In this work, MAC387 antibody faintly stained some macrophages in suppurative foci in lung, unlike control animals in which staining was never observed in macrophages (Chianini et al., 2001). These macrophages could have phagocyted granulocyte material, resulting in positive staining; however, stronger macrophage stimulation could also be the reason for L1 protein expression recognised by the MAC387 antibody.
Unlike other viral diseases such as hog cholera or PRRS that provoke transitory suppression of the immune system (Drew, 2000, Summerfield et al., 2000), PMWS shows severe unique lymphoid lesions (Rosell et al., 1999). PCV2 have been confirmed as the causal agent of these lesions (Kennedy et al., 2000) and the disease can be reproduced by this virus alone (Bolin et al., 2001, Harms et al., 2001). Further characterisations of lymphoid lesions of this study support the hypothesis that PMWS affected pig are immunosuppressed (Sarli et al., 2001, Segales et al., 2001, Darwich et al., 2002). Pathological stages I, II, and III defined in this work might be comparable to three distinct clinical stages of the disease (initial, intermediate and final), but some of these differences can be related to variation in immunological responses of individual pigs to PCV2 infection. Further experimental studies should be performed to better understand PMWS pathogenesis.
Acknowledgements
The authors wish to thank Dr. Robert Higgins (VLA, Lasswade, UK) for critically reviewing the manuscript and Ms. Silvia Usero, Ms. Blanca Perez, and Mr. Pere Losada for technical assistance. Francesca Chianini was a doctoral Marie Curie fellow, Grant no. FMBICT983417. This work was partly funded by the QLRT-PL-199900307 Project from the European Commission’s Fifth Framework Programme 1998–2002, and the 2-FEDER-1997-1341 project, I+D National Plan (Spain).
References
- Alcami A, Koszinowski U.H. Viral mechanisms of immune evasion. Immunol. Today. 2000;21:447–455. doi: 10.1016/S0167-5699(00)01699-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allan G.M, Ellis J.A. Porcine circoviruses: a review. J. Vet. Diagn. Invest. 2000;12:3–14. doi: 10.1177/104063870001200102. [DOI] [PubMed] [Google Scholar]
- Allan G.M, McNeilly F, Kennedy S, Meehan B, Ellis J, Krakowka S. Immunostimulation, PCV-2 and PMWS. Vet. Rec. 2000;147:170–171. [PubMed] [Google Scholar]
- Bolin S.R, Stoffregen W.C, Nayar G.P, Hamel A.L. Postweaning multisystemic wasting syndrome induced after experimental inoculation of cesarean-derived, colostrum-deprived piglets with type 2 porcine circovirus. J. Vet. Diagn. Invest. 2001;13:185–194. doi: 10.1177/104063870101300301. [DOI] [PubMed] [Google Scholar]
- Calafat J, Nijenhuis M, Janssen H, Tulp A, Dusseljee S, Wubbolts R, Neefjes J. Major histocompatibility complex class II molecules induce the formation of endocytic MIIC-like structures. J. Cell. Biol. 1994;126:967–977. doi: 10.1083/jcb.126.4.967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrasco L, Segalés J, Bautista M.J, Gómez-Villamandos J.C, Rosell C, Ruiz-Villamor E, Sierra M.A. Intestinal chlamydial infection concurrent with postweaning multisystemic wasting syndrome in pigs. Vet. Rec. 2000;146:21–23. doi: 10.1136/vr.146.1.21. [DOI] [PubMed] [Google Scholar]
- Chianini F, Majó N, Segalés J, Domínguez J, Domingo M. Immunological study of the immune system cells in paraffin-embedded tissues of conventional pigs. Vet. Immunol. Immunopathol. 2001;82:245–255. doi: 10.1016/S0165-2427(01)00364-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark E.G. Post-weaning multisystemic wasting syndrome. Proc. Am. Assoc. Swine Pract. 1997;28:499–501. [Google Scholar]
- Darwich L, Segalés J, Domingo M, Mateu E. Changes in CD4(+), CD8(+), CD4(+) CD8(+), and immunoglobulin M-positive peripheral blood mononuclear cells of postweaning multisystemic wasting syndrome-affected pigs and age-matched uninfected wasted and healthy pigs correlate with lesions and porcine circovirus type 2 load in lymphoid tissues. Clin. Diagn. Lab. Immunol. 2002;9:236–242. doi: 10.1128/CDLI.9.2.236-242.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drew T. A review of evidence for immunosuppression due to porcine reproductive and respiratory syndrome virus. Vet. Res. 2000;31:27–39. doi: 10.1051/vetres:2000106. [DOI] [PubMed] [Google Scholar]
- Haig D.M. Subversion and piracy: DNA viruses and immune evasion. Res. Vet. Sci. 2001;70:205–219. doi: 10.1053/rvsc.2001.0462. [DOI] [PubMed] [Google Scholar]
- Harms P.A, Sorden S.D, Halbur P.G, Bolin S.R, Lager K.M, Morozov I, Paul P.S. Experimental reproduction of severe disease in CD/CD pigs concurrently infected with type 2 porcine circovirus and porcine reproductive and respiratory syndrome virus. Vet. Pathol. 2001;38:528–539. doi: 10.1354/vp.38-5-528. [DOI] [PubMed] [Google Scholar]
- Kennedy S, Moffett D, McNeilly F, Meehan B, Ellis J, Krakowka S, Allan G.M. Reproduction of lesions of postweaning multisystemic wasting syndrome by infection of conventional pigs with porcine circovirus type 2 alone or in combination with porcine parvovirus. J. Comp. Pathol. 2000;122:9–24. doi: 10.1053/jcpa.1999.0337. [DOI] [PubMed] [Google Scholar]
- Kiupel M, Stevenson G.W, Kanitz C.L, Anothayanontha L, Latimer K.S, Mittal S.K. Cellular localization of porcine circovirus in postweaning pigs with chronic wasting disease. Eur. J. Vet. Pathol. 1999;5:77–82. [Google Scholar]
- Krakowka S, Ellis J.A, McNeilly F, Ringler S, Rings D.M, Allan G. Activation of the immune system is the pivotal event in the production of wasting disease in pigs infected with porcine circovirus-2 (PCV-2) Vet. Pathol. 2001;38:31–42. doi: 10.1354/vp.38-1-31. [DOI] [PubMed] [Google Scholar]
- Kramer P.H. CD95’s deadly mission in the immune system. Nature. 2000;407:789–794. doi: 10.1038/35037728. [DOI] [PubMed] [Google Scholar]
- Orenstein J.M, Wahl S. The macrophage origin of the HIV-expressing multinucleated giant cells in hyperplastic tonsils and adenoids. Ultrastruc. Pathol. 1999;23:79–91. doi: 10.1080/019131299281734. [DOI] [PubMed] [Google Scholar]
- Quintana J, Segales J, Rosell C, Calsamiglia M, Rodriguez-Arrioja G.M, Chianini F, Folch J.M, Maldonado J, Canal M, Plana-Duran J, Domingo M. Clinical and pathological observations on pigs with postweaning multisystemic wasting syndrome. Vet. Rec. 2001;149:357–361. doi: 10.1136/vr.149.12.357. [DOI] [PubMed] [Google Scholar]
- Rosell C, Segales J, Plana-Duran J, Balasch M, Rodriguez-Arrioja G.M, Kennedy S, Allan G.M, McNeilly F, Latimer K.S, Domingo M. Pathological, immunohistochemical, and in-situ hybridisation studies of natural cases of postweaning multisystemic wasting syndrome (PMWS) in pigs. J. Comp. Pathol. 1999;120:59–78. doi: 10.1053/jcpa.1998.0258. [DOI] [PubMed] [Google Scholar]
- Rosell C, Segales J, Domingo M. Hepatitis and staging of hepatic damage in pigs naturally infected with porcine circovirus type 2. Vet. Pathol. 2000;37:687–692. doi: 10.1354/vp.37-6-687. [DOI] [PubMed] [Google Scholar]
- Rosell C, Segales J, Ramos-Vara J.A, Folch J.M, Rodriguez-Arrioja G.M, Duran C.O, Balasch M, Plana-Duran J, Domingo M. Identification of porcine circovirus in tissues of pigs with porcine dermatitis and nephropathy syndrome. Vet. Rec. 2000;146:40–43. doi: 10.1136/vr.146.2.40. [DOI] [PubMed] [Google Scholar]
- Sarli G, Mandrioli L, Laurenti M, Sidoli L, Cerati C, Rolla G, Marcato P.S. Immunohistochemical characterisation of the lymph node reaction in pig post-weaning multisystemic wasting syndrome (PMWS) Vet. Immunol. Immunopathol. 2001;83:53–67. doi: 10.1016/s0165-2427(01)00363-4. [DOI] [PubMed] [Google Scholar]
- Segalés J, Sitjar M, Domingo M, Dee S, Del Pozo M, Noval R, Sacristán C, De Las Heras A, Ferro A, Latimer K.S. First report of postweaning multisystemic wasting syndrome in Spain. Vet. Rec. 1997;141:600–601. [PubMed] [Google Scholar]
- Segalés J, Alonso F, Rosell C, Pastor J, Chianini F, Campos E, Lopez-Fuertes L, Quintana J, Rodriguez-Arrioja G, Calsamiglia M, Pujols J, Dominguez J, Domingo M. Changes in peripheral blood leukocyte populations in pigs with natural postweaning multisystemic wasting syndrome (PMWS) Vet. Immunol. Immunopathol. 2001;81:37–44. doi: 10.1016/s0165-2427(01)00326-9. [DOI] [PubMed] [Google Scholar]
- Shibahara T, Sato K, Ishikawa Y, Kadota K. Porcine circovirus induces B lymphocyte depletion in pigs with wasting disease syndrome. J. Vet. Med. Sci. 2000;62:1125–1131. doi: 10.1292/jvms.62.1125. [DOI] [PubMed] [Google Scholar]
- Sorden S.D, Harms P.A, Nawagitgul P, Cavanaugh D, Prem P.S. Development of a polyclonal-antibody-based immunohistochemical method for the detection of type 2 porcine circovirus in formalin-fixed, paraffin-embedded tissue. J. Vet. Diagn. Invest. 1999;11:528–530. doi: 10.1177/104063879901100607. [DOI] [PubMed] [Google Scholar]
- Summerfield A, Knoetig S.M, Tschudin R, McCullough K.C. Pathogenesis of granulocytopenia and bone marrow atrophy during classical swine fever involves apoptosis and necrosis of uninfected cells. Virology. 2000;272:50–60. doi: 10.1006/viro.2000.0361. [DOI] [PubMed] [Google Scholar]


