Abstract
Feline immunodeficiency virus (FIV) is a natural infection of domestic cats that results in acquired immunodeficiency syndrome resembling human immunodeficiency virus (HIV) infection in humans. The worldwide prevalence of FIV infection in domestic cats has been reported to range from 1 to 28%. Hence, an effective FIV vaccine will have an important impact on veterinary medicine in addition to being used as a small animal AIDS model for humans. Since the discovery of FIV reported in 1987, FIV vaccine research has pursued both molecular and conventional vaccine approaches toward the development of a commercial product. Published FIV vaccine trial results from 1998 to the present have been compiled to update the veterinary clinical and research communities on the immunologic and experimental efficacy status of these vaccines. A brief report is included on the outcome of the 10 years of collaborative work between industry and academia which led to recent USDA approval of the first animal lentivirus vaccine, the dual-subtype FIV vaccine. The immunogenicity and efficacy of the experimental prototype, dual-subtype FIV vaccine and the efficacy of the currently approved commercial, dual-subtype FIV vaccine (Fel-O-Vax FIV) are discussed. Potential cross-reactivity complications between commercial FIV diagnostic tests, Idexx Snap Combo Test® and Western blot assays, and sera from previously vaccinated cats are also discussed. Finally, recommendations are made for unbiased critical testing of new FIV vaccines, the currently USDA approved vaccine, and future vaccines in development.
Keywords: FIV, Vaccine, Review
1. Introduction
Feline immunodeficiency virus (FIV) is a lentivirus that causes chronic and progressive acquired immunodeficiency syndrome in domestic cats resembling human immunodeficiency virus (HIV) infection in humans (Pedersen et al., 1987, Siebelink et al., 1990). FIV has been reported worldwide with a prevalence rate ranging from 1 to 28% (Ishida et al., 1989, Yamamoto et al., 1989). Similar to HIV-1 with at least nine subtypes or clades (clades A–D, F–H, J, and K) in the predominant group M, FIV has been classified into five clades (clades A–E) with much smaller intraclade heterogeneity (Bachmann et al., 1997, Inoshima et al., 1998, Yamaguchi et al., 2002, Blackard et al., 2002). Although in the same Retrovirinae family as the oncovirus, feline leukemia virus (FeLV), FIV more closely resembles HIV in morphology, pathogenesis, and genetic sequence (Bendinelli et al., 1995, Yamamoto, 1999). As a result of the increase in worldwide prevalence and severity of the disease, the development of an effective vaccine is imperative for veterinary medicine (Fauci et al., 1991, Bendinelli et al., 1995). However, effective FIV vaccine development faces similar challenges and obstacles involving broad cross-subtype protection, individual mutations, and inter-subtype recombination, as does effective HIV vaccine development. This paper reviews the clinical disease manifestations produced by FIV infection, similar difficulties experienced with other controversial veterinary vaccines designed to protect both B-cells and T-cells, multiple approaches used to develop an effective FIV vaccine, and the process involved during the development of the first USDA approved commercial dual-subtype FIV vaccine.
2. Pathogenesis and infection
The hallmark of infection is depletion of CD4+ peripheral T-cells and reduction of CD4/CD8 ratios (Ackley et al., 1990, Taniguchi et al., 1991, Torten et al., 1991, Hoffmann-Fezer et al., 1992). Decreased CD4+ T-cell counts in infected cats have been reported in both natural and experimental infections (Ackley et al., 1990, Hoffmann-Fezer et al., 1992). However, select FIV vaccine strains consistently produce clinical illness characterized by recurrent high fever, increased incidence of secondary infection, leukopenia, CD4 loss, and CD4/CD8 inversion (Callanan et al., 1992, Diehl et al., 1995). Even with the loss of CD4+ T-cells, cytotoxic T-lymphocyte (CTL) activities develop early in FIV infections and persist during the asymptomatic stage, similar to HIV-1 infections (Song et al., 1992). These anti-FIV CTL activities are generally mediated by CD8+ T-cells and recognize whole virus and FIV Gag and Env proteins (Flynn et al., 1995, Li et al., 1995). In addition, CD8+ T-cells from FIV-infected cats produced soluble suppressor antiviral factors resembling the CD8-mediated antiviral factor (CAF) produced in HIV-positive individuals (Flynn et al., 1999, Choi et al., 2000). Infected cats possess strong T-helper activities (increased gamma interferon and IL-10 production), during early stages of FIV infection which gradually decrease with long-term infection (Barlough et al., 1991, Torten et al., 1991, Hoffmann-Fezer et al., 1992, Bendinelli et al., 1995, Liang et al., 2000). These T-cell dysfunctions and responses in FIV-infected cats closely mimic those of HIV-positive individuals (Rideout et al., 1992, Gebhard et al., 1999). B-cell dysfunctions involving suppressed primary antibody response (first time the antigen is presented to the B-cells) are less dramatic than T-cell dysfunctions (CD4 loss and CD4/CD8 inversion), and seem to be only in primary antibody responses to T-dependent antigens (Taniguchi et al., 1991, Torten et al., 1991). Virus neutralizing (VN) antibodies to FIV develop shortly after the production of anti-FIV CTL and provide additional immunity (Inoshima et al., 1996). Nevertheless, FIV infections persist in the infected animal and steadily destroy the immune system. Interestingly, elevated serum IgG levels are observed in infected cats, indicative of FIV-induced virus-specific B-cell hyperactivity similar to that observed in HIV (Hopper et al., 1989, Ackley et al., 1990). High-grade B-cell lymphomas at abnormal sites have also been reported in cats with natural and experimental FIV infections (Terry et al., 1995, Callanan et al., 1996). Thus, the FIV-induced immunological disease is paradoxical since the hyperimmunoactive state occurs shortly before or concurrently with the severe immunodeficiency syndrome in the infected animals. This presentation of the immune disorders closely resembles the presentation in human AIDS (Calabrese et al., 1991).
Symptoms most frequently observed in clinical cases of FIV infection are relatively mild and innocuous, including gingivitis, lymphadenopathy, and marginal leukopenia (Yamamoto et al., 1989, Client Information Series, 1990, Hartmann, 1998). The long asymptomatic phase generally persists for years during which time the immune system becomes progressively damaged until the infected animal develops persistent secondary diseases. These secondary infections frequently become resistant to antimicrobial therapy and eventually result in death (Yamamoto et al., 1989). Long-term survivors have been reported for FIV-infected cats similar to the long-term non-progressors reported for HIV-positive individuals (Bendinelli et al., 1995, Hofmann-Lehmann et al., 1997). The long-term survivors appear to make up a sizable population of naturally infected pet cats which can survive for many years with minimal clinical disease. In contrast, higher and earlier mortality rates have been reported in infected stray cats as a result of higher exposure to secondary infections that exacerbate the FIV disease (Yamamoto et al., 1989). The similarity between HIV and FIV in the disease manifestations and progression of viral infection promotes the use of FIV infection in cats as a model for identifying effective HIV vaccine strategies.
3. Potential of developing an effective AIDS lentivirus vaccine
In order to protect the household indoor/outdoor pet cat population from FIV infection and to control the increasing worldwide prevalence within reservoir stray cat populations, vaccine development is essential. However, efficacious vaccine immunity is dependent upon a healthy immune system. Both FIV and HIV-1 invade and destroy monocyte/macrophage and select T-cell populations within the immune system, mutate within the infected host cells, and create diverse strains with varying pathogenic characteristics. In addition, FIV infects B-cells (English et al., 1993). Consequently, vaccine development becomes increasingly difficult to achieve giving rise to major concerns regarding the development of a safe, efficacious product.
Similar questions arose with the earlier development of other commercial veterinary vaccines, such as the FeLV vaccine (Leukocell®, SmithKline Beecham/Pfizer Animal Health Group), the feline infectious peritonitis virus (FIPV) vaccine (Primucell®, SmithKline/Pfizer Animal Health), the Marek’s disease (MD) vaccine in chickens, and the experimental simian immunodeficiency virus (SIV) vaccine. Like FIV, FeLV attacks and destroys B- and T-lymphocytes and monocytes. Originally released in 1985, the initial vaccine exhibited marginal efficacy in experimental challenge studies. Recent studies with newer generation vaccines have demonstrated improved efficacy and safety in controlled experimental trials (Legendre et al., 1991, Hoover et al., 1996, Sparkes, 1997), although controversy still exists on the significance of these experimental trials in relation to natural infection (Norsworthy, 1993b, Sparkes, 1997). In addition, FeLV vaccines have been associated with vaccine-associated fibrosarcoma. Tumor formation has been attributed to a hyperproliferative state caused by excessive immunization with vaccines formulated in alum hydroxide based adjuvants (Sparkes, 1997, Hendrick and Brooks, 1994). This adjuvant has been considered the probable cause of vaccine-associated fibrosarcoma formation in cats (Hendrick and Brooks, 1994). Moreover, a new survey consisting of 31,671 cats from USA and Canada indicates that the incidence of vaccine-associated fibrosarcoma (0.63/10,000 cats) is lower than previously reported 1/10,000 cats and is not increasing (Gobar and Kass, 2002). Nevertheless, more studies will be needed to resolve this issue. Consequently, FeLV vaccination is generally not recommended for cats living indoors with a minimal risk of contracting the disease (Macy, 1994, Sparkes, 1997, Feline Medicine Advisory Panel, 2000).
Similar to FIV and HIV-1 in initial target cell infection, the corona virus, FIPV, initially invades monocytes and macrophages producing either a fatal effusive (wet) or non-effusive (dry) form of the disease (Norsworthy, 1993a). This controversial feline vaccine released in 1991 (Primucell®), consisted of attenuated temperature-sensitive live FIPV. Specific pathogen free (SPF) cats administered with intranasal vaccine exhibited significant efficacy in the manufacturer’s study (Gerber et al., 1990), whereas independent experimental studies demonstrated vaccine safety with minimal to no efficacy (Fehr et al., 1997, Scott, 1999). In additional studies, enhancement of FIP challenge infection was observed in vaccinated animals (Scott et al., 1995, McArdle et al., 1995).
Like FIV and FeLV, MD virus, an oncogenic herpes virus (serotype 1) specific to chickens, infects T- and B-lymphocytes producing neuropathy and neoplasia (Calnek, 1982, Calnek and Witter, 1997, Venugopal, 2000). This virus is highly cell-associated producing the disease only in chickens (Calnek and Witter, 1997). Contrary to popular scientific belief, vaccination against viruses which infect T- and B-lymphocytes is possible. Used as vaccine antigens, inactivated whole virus and inactivated infected-cell vaccines demonstrated efficacy in experimental and clinical trials (Calnek, 1982, Venugopal, 2000). Recent MD vaccines consisting of either live attenuated serotype 1 MD virus or non-pathogenic serotypes 2 or 3 avian herpes viruses, support earlier clinical trial results (Calnek, 1982). Consequently, the MD vaccine, the first successful vaccine to effectively control any neoplastic disease in man or animals, lends strong support for the idea that vaccine development is possible against viruses which infect both arms of the immune system (T- and B-lymphocytes) (Venugopal, 2000). Overall, the FeLV, FIP, and MD vaccines demonstrate that vaccines against viruses that infect and destroy immune cells are difficult to develop. However, the existence of USDA approved vaccines to these viruses in commercial market also provide optimism to the development of an effective FIV vaccine.
Promising results from recent SIV vaccine studies further support the view that vaccines against AIDS lentiviruses can be developed (Johnston and Flores, 2001, Nabel, 2001, Mooij and Heeney, 2002). A major reduction in SIV disease and virus load was observed in vaccinated macaques using either vector-based vaccines or DNA vaccines with and without cytokine adjuvantation or viral peptide boost (Johnston and Flores, 2001, Nabel, 2001, Mooij and Heeney, 2002). Partial protection correlated with strong CD8+ T-cell responses (Johnston and Flores, 2001, Nabel, 2001, Mooij and Heeney, 2002). Long-term follow-up of these animals will be required to determine whether the control of virus load and disease progression can be maintained with or without additional vaccination. Although sterilizing immunity was not observed, the virus virulence and viral challenge doses used may be considerably higher than those from a single natural exposure, and may thus represent excellent vaccine approaches for candidate HIV-1 vaccines. Protection against SIV infection has been observed with attenuated deletion mutant vaccines (Daniel et al., 1992, Desrosiers and Bolognesi, 1994, Wyand et al., 1996). However, this approach is considered to be impractical for clinical trials in humans. Results from these preclinical studies raise three important points: (1) vaccine protection against disease is easier to achieve than prevention of infection (i.e., sterilizing immunity); (2) the need to identify the duration of such partial protection; (3) the need to test the vector-based or DNA vaccine approaches against distinctly heterologous subtype clinical isolates.
4. FIV vaccine studies
Recent FIV vaccine trial results (1998 to present) are summarized in Table 1, Table 2, Table 3, Table 4 , whereas earlier vaccine trial results have been reported elsewhere (Elyar et al., 1997). A brief overview of these tables suggests that the majority of success achieved in experimental FIV vaccine trials has been made with inactivated whole virus or inactivated infected-cell conventional vaccines against more severe challenge systems. These severe challenge systems include in vivo-derived inocula, heterologous strains, and clinical isolates. In comparison, viral peptide vaccines have fared poorly (Table 3, Study 1–4), while DNA vaccines and vector vaccines have shown some promising results against weaker challenge systems.
Table 1.
Inactivated whole virus or whole viral protein vaccines
| Type of immunization | Immunization |
Type of adjuvanta | Challenge inoculum strain and dose (cell no. and CID50) and routeb, c, d | No. protected/no. challenged (% protected)* | Study no. (Ref.)e | ||
| Vaccine strainsb | Routec | Protocol (weeks) | |||||
| Whole virus | PET (A) + SHI (D) | s.c. | 0, 3, 9 | FD-1 | BANG, 10, i.v. | 4/5 (80)* | Study 1A (Pu et al., 2001) |
| Control (cells or PBS) | – | s.c. | 0, 3, 9 | FD-1 | BANG, 10, i.v. | 0/4 (0) | |
| Whole virus | PET (A) + SHI (D) | s.c. | 0, 3, 9, 12 | FD-1 | PET, 50, i.v. | 4/4 (100)* | Study 1B (Pu et al., 2001) |
| Whole virus | PET (A) | s.c. | 0, 3, 9, 12 | FD-1 | PET, 50, i.v. | 1/5 (20) | |
| Control (PBS) | – | s.c. | 0, 9, 12 | FD-1 | PET, 50, i.v. | 0/5 (0) | |
| Whole FIV | SHI (D) | s.c. | 0, 3, 9, 12 | FD-1 | SHI, 50, i.v. | 1/3 (33) | |
| Control (PBS) | – | s.c. | 0, 3, 9, 12 | FD-1 | SHI, 50, i.v. | 0/3 (0) | |
| Whole virus | GL8 (A) | rect (±i.p., in) | 0, 2, 4 | CT | GL8, 10m, rect | 0/12 (0) | Study 2 (Finerty et al., 2000) |
| Control (water) | – | rect | 0, 2, 4 | – | GL8, 10m, rect | 0/4 (0) | |
| Whole virus or proteins | M2 (B) | s.c. | 0, 3, 6, 9, 20 | IFA | M2, 10, i.v. | 11/16 (69) | Study 3 (Matteucci et al., 1999) |
| Control (IFA or none) | – | s.c. | 0, 3, 6, 9, 20 | IFA or none | M2, 10, i.v. | 7/12 (58) | |
| Whole virusf | M2 (B) | s.c. (±ivag, po)f | 0, 3, 6, 9, 20 | IFA ± CT | M2, 2×104 cells, ivag | 4/12 (33) | |
| Control (adjuvant)f | – | s.c. (±ivag, po)f | 0, 3, 6, 9, 20 | IFA ± CT | M2, 2×104 cells, ivag | 3/8 (37)g | |
| Whole virus | PET (A) | s.c. | 0, 3, 6 | MF59.0 | PET, 10, i.p. | 5/5 (100) | Study 4 (Hosie et al., 2000) |
| Control (adjuvant) | – | s.c. | 0, 3, 6 | MF59.0 | PET, 10, i.p. | 1/5 (20) | |
| Whole virus | PET (A) | s.c. | 0, 3, 6 | MF59.0 | GL8, 10, i.p. | 2/5 (40) | |
| Control (adjuvant) | – | s.c. | 0, 3, 6 | MF59.0 | GL8, 10, i.p. | 0/5 (0) | |
| Whole virus | PET (A) | s.c. | 0, 3, 6 | MF59.0 | AM6, 10, i.p. | 3/5 (60) | |
| Control (adjuvant) | – | s.c. | 0, 3, 6 | MF59.0 | AM6, 10, i.p. | 1/5 (20) | |
| Whole virus | M2 (B) | i.p. | 0, 4, 8, 12 | Homol-RBC | M2, 10, i.v. | 4/4 (100)* | Study 5 (Chiarantini et al., 1998) |
| Control (BSA) | – | i.p. | 0, 4, 8, 12 | Homol-RBC | M2, 10, i.v. | 0/4 (0) | |
| Whole virush | M2 (B) | i.p. | 0, 4, 8, 12, 16 | Homol-RBC | M2, 10, i.v. | 0/4 (0) | |
| Control (BSA)h | – | i.p. | 16 | Homol-RBC | M2, 10, i.v. | 0/4 (0) | |
Commercial Fort Dodge adjuvant (FD-1); cholera toxin (CT); incomplete Freund’s adjuvant (IFA); microfluidized oil/water emulsion (MF59.0); FIV proteins coated onto biotinylated homologous RBC (Homol-RBC).
FIV-Petaluma (PET); FIV-Shizuoka (SHI); FIV-Glasgow 8 (GL8); FIV-Milan 2 (M2).
Rectal (rect); intranasal (in); intravaginal (ivag); oral (po); rectal immunization followed by either i.p. or in (±i.p., in) immunization; s.c. immunization followed by either ivag or po immunization (±ivag, po) on week 20.
FIV-Amsterdam 6 (AM6).
Reference (Ref.).
20 week boost was given at 200mg of either IFA or cholera toxin (IFA/CT) as adjuvant using s.c. immunization followed by either intravaginal (s.c./ivag) or oral (s.c./po) immunization.
Four controls each were immunized with either IFA alone or IFA with CT boost on 20 weeks. Only combined result of the controls was available.
The four protected cats from above study were boosted with the same vaccine, while four new control cats were immunized with BSA biotinylated homologous RBC. Both groups were challenged with the same inoculum at the same dose as above.
Statistically significant difference from control when P<0.05.
Table 2.
Infected-cell vaccines (ICV) and recombinant vectored vaccines with and without ICV or SU protein boosts
| Type of immunizationa | Vaccine |
Immunization |
Type of adjuvantb | Challenge inoculum strain (clade) and dose (CID50) and routec, d, e | No. protected/no. challenged (% protect)f | Study no. (Ref.)g | ||
| Strain (clade)c | Dose (ug) | Routed | Protocol (weeks) | |||||
| Fixed FIV/FL-4 | PET (A) | 2.5×107 | TLN | 0, 2, 4 | Quil A | PET (A), 100, rect | 4/4 (100) | Study 1 (Finerty et al., 2002) |
| Fixed FeT-J | – | 2.5×107 | TLN | 0, 2, 4 | None | PET (A), 100, rect | 1/4 (25) | |
| Fixed FIV/FL-4 | PET (A) | 2.5×107 | TLN | 0, 2, 4 | Quil A | PET (A), 100, rect | 4/4 (100) | Study 2 (Stokes et al., 1999) |
| Fixed FeT-J | – | 2.5×107 | TLN | 0, 2, 4 | None | PET (A), 100, rect | 0/4 (0) | |
| Fixed FIV/MBM | M2 (B) | 3×107 | s.c. | 0, 3, 6, 16, 40, 64 | IFA | Field isol (na), na, contact expo | 12/12 (100) | Study 3 (Matteucci et al., 2000) |
| Control (none) | – | – | – | – | – | Field isol (na), na, contact expo | 9/14 (64) | |
| Fixed FIV-autoPBMC | Clone 19k1 (A) | 5×106 | i.v. + s.c. | 0, 2, 4, 6, 8, 16 | – | Clone19k1 (A), 10, i.m. | 0/3↑ (0) | Study 4 (Karlas et al., 1998, Karlas et al., 1999) |
| Fixed autoPBMC | – | 5×106 | i.v. + s.c. | 0, 2, 4, 6, 8, 16 | – | Clone19k1 (A), 10, i.m. | 0/2 (0) | |
| ALVAC-FIV | VFr (A) | 1×108 pfuh | i.m. | 0, 4, 8 | – | PET (A), 50, i.p. | 0/3 (0) | Study 5A (Tellier et al., 1998) |
| ALVAC-FIV + ICVh | VFr (A)/PET (A) | +2×108 cellsh | i.m. + s.c. | 0, 4, 8 | –/SAF-MDP | PET (A), 50, i.p. | 3/3 (100) | |
| ALVAC + ICVh | –/PET (A) | +2×108 cellsh | i.m. + s.c. | 0, 4, 8 | –/SAF-MDP | PET (A), 50, i.p. | 0/3 (0) | |
| ALVAC-FIV + ICVh, i | VFr (A)/PET (A) | +2×108 cellsh | i.m. + s.c. | 0, 4, 8 | –/SAF-MDP | BANG (A/B), 75, i.p.i | 0/3 (0) | |
| Control (none) | – | – | – | – | – | BANG (A/B), 75, i.p.i | 0/3 (0) | |
| pCI-NC vector/recSUj | GAS (A)/BANG (A/B) | 300/100 | in/s.c. | 0, 15, 30, 45 daysj | – | GAS (A), 1, i.p. | 1/4 (25) | Study 6 (Cuisinier et al., 1999) |
| pCI-NC vector/recSUj | GAS (A)/BANG (A/B) | 300/100 | i.m./s.c. | 0, 15, 30, 45 daysj | – | GAS (A), 1, i.p. | 0/4 (0) | |
| pCI vector/recSUj | –/BANG (A/B) | 300/100 | i.m./s.c. | 0, 15, 30, 45 daysj | – | GAS (A), 1, i.p. | 0/4 (0) | |
| pCI vector/PBSj | –/– | 300/– | i.m./s.c. | 0, 15, 30, 45 daysj | – | GAS (A), 1, i.p. | 0/4 (0) | |
Infected-cell vaccine and uninfected cells were inactivated by fixation with paraformaldehyde; FIV-PET infected feline T-cell line (FL-4) and uninfected feline T-cell line (FeT-J) developed by USA group; feline PBMC cell line (MBM) developed by Italian group; autologous peripheral blood mononuclear cells (autoPBMC); FIV envelope/Gag-protease (env/gag-pr) gene construct of canarypox virus vector (ALVAC-FIV) was grown in chicken embryo fibroblast (CEF); infected-cell vaccine (ICV) consisted of PET infected FL-4 cells; plasmid pCI vector construct of FIV nucleocapsid (pCI-NC vector); recombinant surface Env protein (recSU) expressed in E. coli system.
Quillaja saponin adjuvant (Quil A); incomplete Freund’s adjuvant (IFA); syntax adjuvant formulation muramylpeptide (SAF-MDP).
FIV-Petaluma (PET); FIV-Millan 2 (M2); infectious molecular clone (19k1) of FIV-AM19; FIV-Ville Franche (VFr); FIV-Gasser (GAS); FIV-Bangston (BANG) has Gag of clade A and Env of clade B (A/B).
Targeted lymph node (TLN); intranasal (in).
Rectal (rect); contact exposure (contact expo) with field cats infected with FIV; field isolates (field isol); information not available (na).
Percent protection (% protect); enhanced challenge virus load (↑) observed in this immunization group.
Reference (Ref.).
Immunized twice with either ALVAC-FIV or ALVAC vector (1×108pfu) followed by 1× immunization with ICV (+1×108 cell).
Three protected cats from above boosted with ICV and then challenged second time with distinctly heterologous FIV-BANG.
Immunized 2× with either pCI vector or pCI-NC vector on days 0 and 15 followed by 2× immunization with recSU on days 30 and 45.
Table 3.
Solubilized whole virus and viral peptide (synthetic and recombinant) vaccines
| Type of immunizationa | Vaccine |
Immunization |
Type of adjuvantb | Challenge inoculum strain (clade) and dose (CID50) and routec, d | No. protected/no. challengede | Study no. (Ref.)f | ||
| FIV strain (clade)c | Dose (ug) | Routeg | Protocol (weeks) | |||||
| ENV-C2 peptide | na | 200 | TLN | 0, 2, 4 | Quil A | GL8 (A), 10m, rectal | 0/4 | Study 1 (Finerty et al., 2002) |
| ENV-C2 peptide | na | 200 | Rectal | 0, 2, 4 | Quil A | GL8 (A), 10m, rectal | 0/4 | |
| Control (water) | – | – | Rectal | 0, 2, 4 | None | GL8 (A), 10m, rectal | 0/4 | |
| MAP V3 | GL8 (A) and PET (A) | 200 | Rectal (±i.p.) | 0, 2, 4 | CT | GL8 (A), 10m, rectal | 0/8 | Study 2A (Finerty et al., 2000) |
| MAP V3 | GL8 (A) and PET (A) | 200 | Rectal (±i.p.) | 0, 2, 4 | Quil A | GL8 (A), 10m, rectal | 0/8 | |
| MAP V3 | GL8 (A) and PET (A) | 200 | Rectal | 0, 2, 4 | – | GL8 (A), 10m, rectal | 0/4 | |
| Control (water) | – | – | Rectal | 0, 2, 4 | – | GL8 (A), 10m, rectal | 0/4 | |
| ptV3 | GL8 (A) and PET (A) | 200 | s.c. | 0, 2, 4 | IFA | GL8 (A), 10m, rectal | 0/4 | Study 2B (Finerty et al., 2000) |
| ptV3 | GL8 (A) and PET (A) | 200 | s.c. | 0, 2, 4 | ISCOMs | GL8 (A), 10m, rectal | 0/4 | |
| V3 | GL8 (A) and PET (A) | 200 | s.c. | 0, 2, 4 | CT | GL8 (A), 10m, rectal | 0/4 | |
| V3 | GL8 (A) and PET (A) | 200 | Rectal (±in) | 0, 2, 4 | CT | GL8 (A), 10m, rectal | 0/8 | |
| Control (water) | – | – | s.c. | 0, 2, 4 | – | GL8 (A), 10m, rectal | 0/4 | |
| swFIV | AM19 (A) | 10 | s.c. | 0, 4, 10 | ISCOMs | AM19 (A), 20, i.m. | 0/6 | Study 3 (Huisman et al., 1998) |
| swFIV + SU–TM | AM19 (A) | 10 | s.c. | 0, 4, 10 | ISCOMs (all) | AM19 (A), 20, i.m. | 0/6 | |
| swFIV + SU–TM + Gag | AM19/19k1 (A) | 10 | s.c. | 0, 4, 10 | ISCOMs (all) | AM19 (A), 20, i.m. | 0/5 | |
| swCrFK | – | 10 | s.c. | 0, 4, 10 | ISCOMs | AM19 (A), 20, i.m. | 0/6 | |
| SIV-Env | – | 10 | s.c. | 0, 4, 10 | ISCOMs | AM19 (A), 20, i.m. | 0/6 | |
| Control (PBS) | – | – | s.c. | 0, 4, 10 | – | AM19 (A), 20, i.m. | 0/6 | |
| recSU protein | Z2 (A) | 100 | s.c. | 0, 2, 4 | AlOH + QS-21 | Z2 (A), 20, i.p. | 0/5↓ | Study 4 (Leutenegger et al., 1998) |
| recSU glycoprotein | Z2 (A) | 100 | s.c. | 0, 2, 4 | AlOH + QS-21 | Z2 (A), 20, i.p. | 0/5↓ | |
| recSU glycoprotein | Z2 (A) | 100 | s.c. | 0, 2, 4 | FCA + rabNC | Z2 (A), 20, i.p. | 0/5↓ | |
| Control (PBS) | Z2 (A) | 100 | s.c. | 0, 2, 4 | – | Z2 (A), 20, i.p. | 0/7 | |
29 aa acid peptide to second conserved (C2) region of FIV surface Env; multiple antigenic peptide V3 region (MAP) of surface Env; palmitoyl thioester V3 peptide (ptV3); V3 region (V3) of surface Env; solubilized FIV-AM19 from infected CrFK cells (swFIV); recombinant vaccinia virus expressed Env glycoprotein of AM19 from which the cleavage site between SU and TM proteins (SU+TM); FIV Gag protein derived from FIV-AM19k1 (19k1) sequence; solubilized CrFK cell proteins (swCrFK); simian immunodeficiency virus Env (SIV-Env); phosphate buffered saline (PBS); recombinant surface Env (recSU) expressed either by E. coli or baculovirus expression system.
Quillaja saponin adjuvant (Quil A); cholera toxin (CT); incomplete Freund’s adjuvant (IFA); immune stimulating complexes (ISCOMs); all proteins formulated in ISCOMs (all); aluminum hydroxide (AlOH); stimulon saponin adjuvant (QS-21); Freund’s complete adjuvant (FCA); rabies nucleocapsid (rabNC).
FIV-Glasgow 8 (GL8); FIV-Petaluma (PET); FIV-Amsterdam 19 (AM19); infectious molecular clone (19k1) of AM19; FIV-Zurich 2 (Z2).
Mucosal (m) CID50.
Percent protection (% protect.); decreased FIV challenge load (↓) observed in this immunization group.
Reference (Ref.).
Targeted lymph node (TLN); all rectal immunization or rectal immunization followed by i.p. boosts (±i.p.); intranasal (in); all rectal immunization or rectal immunization followed by in boosts (±in).
Table 4.
DNA vaccines
| Type of immunizationa | Vaccine | Immunization |
Type of adjuvantb | Challenge inoculum strain and dose (CID50) and routec, d, e | No. protected/no. challengedf | Study no. (Ref.)g | |
| Strain (clade)c | Routeh, d | Protocol (weeks) | |||||
| Proviral DNA DIN | GL8 (A) | i.m. | 0, 4, 8 | – | PET, 25, i.p. | 1/6 | Study 1A (Dunham et al., 2002) |
| Proviral DNA DIN | GL8 | i.m. | 0, 4, 8 | IL-18 DNA | PET, 25, i.p. | 2/6 | |
| Proviral DNA DIN | GL8 | i.m. | 0, 4, 8 | IL12/IL18 DNA | PET, 25, i.p. | 2/6 | |
| Proviral DNA DRT | GL8 | i.m. | 0, 4, 8 | IL-18 DNA | PET, 25, i.p. | 2/6 | |
| Proviral DNA DRT | GL8 | i.m. | 0, 4, 8 | IL12/IL18 DNA | PET, 25, i.p. | 0/6 | |
| Control (pBR328) | – | i.m. | 0, 4, 8 | IL12/IL18 DNA | PET, 25, i.p. | 0/6 | |
| Proviral DNA DIN | GL8 (A) | i.m. | 0, 4, 8, 32 | – | PET, 25, i.p. (second)j | 1/1 | Study 1B (Dunham et al., 2002) |
| Proviral DNA DIN | GL8 | i.m. | 0, 4, 8, 32 | IL-18 DNA | PET, 25, i.p. (second)j | 2/2 | |
| Proviral DNA DIN | GL8 | i.m. | 0, 4, 8, 32 | IL12/IL18 DNA | PET, 25, i.p. (second)j | 1/1 | |
| Proviral DNA DRT | GL8 | i.m. | 0, 4, 8, 32 | IL-18 DNA | PET, 25, i.p. (second)j | 0/1 | |
| Control (pBR328)i | – | i.m. | (32)i | IL12/IL18 DNA | PET, 25, i.p. | 0/4 | |
| Proviral DNA DIN | GL8 (A) | i.m. | 32, 61 | – | GL8, 10, i.p. (third)j | 0/1↓ | Study 1C (Dunham et al., 2002) |
| Proviral DNA DIN | GL8 | i.m. | 32, 61 | IL-18 DNA | GL8, 10, i.p. (third)j | 0/2↓ | |
| Proviral DNA DIN | GL8 | i.m. | 32, 61 | IL12/IL18 DNA | GL8, 10, i.p. (third)j | 0/1↓ | |
| Control (PBS)i | – | i.m. | (61)i | – | GL8, 10, i.p. | 0/4 | |
| gp140 DNA MIDGE | Z2 (A) | i.e. | 0, 3, 6 | IL-12 DNA MIDGE | 25 TCID50, Z2 | 3/4↓ | Study 2 (Boretti et al., 2000, Leutenegger et al., 2000) |
| gp140 DNA MIDGE | Z2 | i.e. | 0, 3, 6 | IL-16 DNA MIDGE | 25 TCID50, Z2 | 0/4↓ | |
| gp140 DNA MIDGE | Z2 | i.e. | 0, 3, 6 | CpGs | 25 TCID50, Z2 | 0/4↓ | |
| gp140 DNA MIDGE | Z2 | i.e. | 0, 3, 6 | – | 25 TCID50, Z2 | 0/4 | |
| Control (gold particle) | Z2 | i.e. | 0, 3, 6 | – | 25 TCID50, Z2 | 0/4 | |
| Proviral DNA DVIF | PPR | i.m. | 0, 43 | – | PPR | 3/3 | Study 3 (Lockridge et al., 2000) |
| Control (media) | – | i.m. | 0, 43 | – | PPR | 0/2 | |
| Proviral DNA DRT | PET (A) | i.m. | 0, 4, 8 | IFN-g DNA | 10 PET | 1/6 | Study 4 (Hosie et al., 2000) |
| Proviral DNA DRT | GL8 (A) | i.m. | 0, 4, 8 | IFN-g DNA | 10 PET | 2/6 | |
| Control (adjuvant) | – | i.m. | 0, 4, 8 | IFN-g DNA | 10 PET | 0/6 | |
| Proviral DNA DRT | PET | i.m. | 0, 4, 8 | IFN-g DNA | 10 GL8 | 0/6 | |
| Proviral DNA DRT | GL8 | i.m. | 0, 4, 8 | IFN-g DNA | 10 GL8 | 0/6 | |
| Control (adjuvant) | – | i.m. | 0, 4, 8 | IFN-g DNA | 10 GL8 | 1/6 | |
| Proviral DNA DRT | PET (F-14) | i.m. | 0, 10, 23 | IFN-g DNA | 25, F-14 | 3/5 | Study 5 (Hosie et al., 1998) |
| Proviral DNA DRT | PET (F-14) | i.m. | 0, 10, 23 | – | 25, F-14 | 1/5 | |
| Control (adjuv/none) | PET (F-14) | i.m. | 0, 10, 23/none | IFN-g DNA or none | 25, F-14 | 0/10 | |
| Proviral DNA DRT | PET (F-14) | i.m. | 0, 4, 8 | IFN-g DNA | 25, F-14 | 2/5 | |
| Control (adjuvant) | PET (F-14) | i.m. | 0, 4, 8 | IFN-g DNA | 25, F-14 | 0/5 | |
| Proviral DNA DAP-1 | TM2 | ivag | 0 | – | 5×106 cells, TM2 | 2/2 | Study 6 (Kohmoto et al., 1998) |
| Control (none) | – | – | – | – | 5×106 cells, TM2 | 0/3 | |
FIV integrase gene deleted (DIN) proviral DNA; FIV reverse transcriptase gene deleted (DRT) proviral DNA; controls consist of either plasmid (pBR328), PBS, carrier (gold particle), culture media (media), adjuvant (adjuv), or no immunization (none); plasmid pBR328 used for proviral DNA construct; minimalistic, immunogenic defined gene expression (MIDGE) vector construct of FIV Env gene (gp140 DNA) coated onto gold particles; AP-1 binding site deleted infectious molecular clone (proviral DNA DAP-1).
Molecular adjuvantation with either interleukin 18 (IL-18 DNA), IL-12 plus IL-18 (IL12/IL18 DNA), and interferon-g (IFN-g DNA).
FIV-Glasgow 8 (GL8); FIV-Zurich 2 (Z2); FIV-San Diego PPR (PPR), FIV-Petaluma (PET); infectious molecular clone of PET (F-14); FIV-TM2 (TM2) from Japanese group.
Intraepidermal (i.e.); intravaginal (ivag).
All the challenge strains are wild type without deletions.
Decreased FIV challenge load (↓) observed in this immunization group as compared to control.
Reference (Ref.).
First immunization of vif gene deleted proviral DNA (DVIF) at 600mg followed by second immunization at 300mg; innoculated intravaginally with infectious molecular clone (proviral DNA AP-1) infected feline T-cell line (MYA-1).
Single immunization with either pBR328 plasmid or PBS at the time when other vaccine groups received the last boost.
Protected cats from above study received a second challenge (second) of PET and those protected from second challenge received a third challenge (third) of GL8.
Vaccine prophylaxis is influenced not only by the FIV strains used as vaccine antigens but also by the strain and source of the challenge inoculum. Multiple laboratories have demonstrated that single-strain FIV vaccine can protect cats against homologous (same strain) and slightly heterologous (different strain) FIV challenges of the same subtype (Yamamoto et al., 1993, Elyar et al., 1997, Hosie et al., 2000). In contrast, few studies show enhancement of FIV infection even against homologous challenge (Hosie et al., 1992, Elyar et al., 1997, Karlas et al., 1999). Technical differences in vaccine inactivation procedure, cell types used for growing vaccine virus, vaccination schedule, adjuvant used, vaccine doses, and variable virulence between FIV strains have produced conflicting results in vaccine efficacy studies (Hosie et al., 2000). In one study, inactivated whole virus vaccinated cats exhibited greater protection against in vitro-derived homologous challenge (FIVPET) compared to more virulent heterologous FIV challenge (FIVAM6, FIVGL8) (Hosie et al., 2000). Nevertheless, even with virulent challenge strains, significant reductions in virus load and early inverted CD4/CD8 ratios were observed in these whole virus vaccinated cats compared to unvaccinated control or DNA-based vaccinated cats. However, other investigators have reported partial protection against homologous challenge with FIV DNA vaccine in other FIV strains (Boretti et al., 2000, Lockridge et al., 2000).
Inactivated single-strain (whole virus) vaccine protection has been reported using in vivo-derived inoculum against challenges with homologous FIV strains but not against in vivo-derived challenges with heterologous FIV strains (Hesselink et al., 1999, Matteucci et al., 1996, Pu et al., 2001). Single-strain vaccines have only provided adequate protection against homologous and closely related strains but not against moderately to greatly heterologous FIV strains (Hosie et al., 1995, Elyar et al., 1997, Hesselink et al., 1999). To date, conventional inactivated single-subtype FIV vaccines and molecular-derived vaccines (plasmid DNA, recombinant vectored, recombinant peptide vaccines) have been untested or unsuccessful at protecting cats against heterologous subtype FIV challenges using in vivo-derived inoculum (Elyar et al., 1997). Recent studies suggest that homologous FIV vaccine protection using in vitro-derived inoculum challenges may not provide protection against challenges using in vivo-derived inoculum (Hesselink et al., 1999, Matteucci et al., 1996, Pu et al., 2001). In vivo-derived inoculum consists of plasma or infected-cells derived from cats infected with in vivo passaged laboratory isolates which contain quasi-species of FIV and closely simulate natural conditions. In a study using single-subtype FIV vaccine consisting of fixed infected-cell FIVM2 isolate, SPF cats were protected against homologous FIV challenge with in vivo-derived inoculum (Matteucci et al., 1996). When placed in a free-roaming shelter for 22 months with natural FIV-infected cats, this single-subtype vaccine protected the vaccinated group of cats (0/12 FIV infection rate) whereas 5/14 of the unvaccinated control group became infected (Matteucci et al., 2000). A major limitation of inactivated infected-cell vaccines is the short duration of vaccine immunity compared to inactivated whole virus vaccines (Matteucci et al., 1997, Tellier et al., 1998). Since the level of the FIV exposure during natural transmission remains unknown, the efficacy evaluation using a contact challenge system represents the ultimate test for any commercial FIV vaccine. Consequently, a modified approach to single-strain FIV vaccine designs may be required for developing a vaccine that can provide broad spectrum protection against common FIV subtypes (A and B) and in vivo-derived inoculum.
As demonstrated by the synergistic protective properties of the multi-serotype inactivated whole virus MD vaccines, the use of multi-serotype/multi-subtype vaccines is not a novel idea in veterinary medicine (Venugopal, 2000). The multi-subtype vaccine approach to broaden immunity and protection was first introduced to the AIDS vaccine community using an FIV vaccine model (Yamamoto et al., 1996). The first dual-subtype FIV vaccine, consisting of inactivated subtype A- and subtype D-infected-cells, provided protection against in vitro-derived homologous strains but was not tested against heterologous strains (Yamamoto et al., 1996, Hohdatsu et al., 1997). An improved dual-subtype FIV vaccine, consisting of inactivated whole viruses of subtypes A and D, elicited strong anti-FIV cellular immunity and broad spectrum VN antibody activities (Pu et al., 2001). In addition, it provided broadened protection for cats against homologous and heterologous challenges using in vivo-derived inoculum (Pu et al., 2001). However, this FIV vaccine has not yet been field tested against natural-exposed FIV-infected animals. The precedent for using a whole virus vaccine in cats has been established with FeLV vaccine. No known cases of accidental infection due to improper inactivation of the virus have been reported with FeLV vaccines. With improved inactivation methods of vaccine virus and better adjuvant formulations, inactivated or killed vaccine approach will continue to be useful in future veterinary vaccine development.
5. Immune correlate of vaccine protection
Until recently, most veterinary vaccines were developed to provide protection via induction of antiviral humoral immunity, specifically VN antibodies. This VN antibody approach was also used in early HIV/SIV vaccine development. However, a failure to experimentally demonstrate protection using surface envelope-based vaccines in non-human primates and a lack of correlation between VN antibody production and protection, dampened enthusiasm towards developing a vaccine that relies mainly on antibody immunity (Johnston and Flores, 2001, Nabel, 2001, Mooij and Heeney, 2002). Subsequently, the AIDS vaccine community modified their view by advocating the concept that an efficacious vaccine against AIDS viruses will require strong antiviral cellular immunity to confer protection (Johnston and Flores, 2001, Nabel, 2001, Mooij and Heeney, 2002). Consequently, developing vaccines which induce cellular immunity for protection such as recombinant vectored vaccines and DNA vaccines became the primary focus for AIDS vaccine research (Johnston and Flores, 2001, Nabel, 2001, Schnell, 2001, Mooij and Heeney, 2002).
DNA vaccines primarily elicit cellular immunity, whereas recombinant vectored vaccines elicit cellular immunity and additional antibodies aimed at the specific targeted proteins (Johnston and Flores, 2001, Nabel, 2001, Schnell, 2001, Mooij and Heeney, 2002). Antibody levels are dependent upon the physicochemical character and the expression level of the non-vector targeted proteins. Reduction of virus load and prevention of disease signs/symptoms were observed in animals immunized with DNA vaccines, although complete protection was not achieved in either the HIV- or SIV-infected non-human primate models or FIV-infected domestic cats (Hosie et al., 1998, Johnston and Flores, 2001, Nabel, 2001, Mooij and Heeney, 2002). Since a single identifiable immune correlate of vaccine protection has eluded AIDS researchers for almost two decades, a more comprehensive prime-boost approach incorporating both humoral and cellular immunity was developed against AIDS lentiviruses. This new vaccine approach generally involves priming with the DNA or the recombinant vectored vaccine followed by a viral-vectored or viral subunit protein boost which induces both cellular and antibody immunity (Johnston and Flores, 2001, Nabel, 2001, Schnell, 2001, Mooij and Heeney, 2002).
Current findings from AIDS vaccine research suggest that CD4+ T-helper, CD8+ T-cell, and CTL activity is important for vaccine immunity (Johnston and Flores, 2001, Nabel, 2001, Mooij and Heeney, 2002). CTL and strong T-helper activity against FIV has been observed with DNA, recombinant vectored, and inactivated whole virus vaccines (Flynn et al., 1995, Tellier et al., 1997, Lockridge et al., 2000, Pu et al., 2001, Dunham et al., 2002). Among these FIV vaccine designs, VN antibody induction was more consistent in cats immunized with conventional inactivated virus vaccine followed by recombinant vectored vaccine (Tellier et al., 1998, Giannecchini et al., 2001, Pu et al., 2001). Although, inactivated infected-cell vaccine may induce higher VN antibody levels compared to inactivated whole virus vaccine, the duration of protection may be shorter in the inactivated infected-cell vaccine group (Matteucci et al., 1997). Unlike human AIDS research which test HIV vaccine antibodies for their ability to neutralize both laboratory and clinical isolates, FIV vaccine researchers have not tested their vaccine antibodies against non-adapted clinical isolates (Matteucci et al., 1997, Pu et al., 2001). FIV laboratories now test the cross-neutralizing ability of sera from naturally and experimentally infected cats against clinical and laboratory isolates (Inoshima et al., 1998, Pu et al., 2001). This laboratory testing for neutralizing antibody against non-adapted clinical isolates may become a valuable mechanism for evaluating the cross-strain efficacy of future commercial FIV vaccines.
Like HIV, the detection and titer of VN antibodies to FIV are affected by the type of indicator cells (lymphoid cell lines vs. non-lymphoid CrFK cell lines) used in the VN assay (Giannecchini et al., 2001). For FIV VN assays, lymphoid cell lines have been reported to produce a more accurate titer during natural infection (Giannecchini et al., 2001). Cross-neutralizing antibody analysis against clinical isolates have not been performed on sera from cats immunized with candidate FIV vaccines (inactivated dual-subtype virus vaccine and inactivated FIVM2-infected-cell vaccine) (Matteucci et al., 1996, Matteucci et al., 2000, Pu et al., 2001). Both candidate vaccines reportedly protected cats against either in vivo-derived heterologous subtype FIV strains or contact challenge with natural isolates (Matteucci et al., 1996, Matteucci et al., 2000, Pu et al., 2001). However, VN antibody titer analysis has been conducted for homologous (FIVM2) virus using the inactivated FIVM2-infected-cell vaccine sera (Giannecchini et al., 2001). In this study, protection correlated with the presence of higher VN antibody titer (Giannecchini et al., 2001). Using sera from inactivated dual-subtype FIV vaccinated cats, a limited number of homologous and heterologous laboratory isolates were tested for cross-neutralizing antibodies (Pu et al., 2001). Increased types and levels of VN antibodies to heterologous laboratory isolates were reported with the dual-subtype FIV vaccine compared to single-subtype vaccines, further supporting the current concept of AIDS lentiviral vaccine protection requiring both arms of immunity (Pu et al., 2001).
6. Implication of challenge studies
Unlike earlier efficacy studies of commercial FeLV vaccines, newly developed FIV vaccines have come under increased scrutiny due to recent advances in human and non-human primate AIDS research, including technical improvements in virus detection methods and increased understanding of challenge conditions upon vaccine efficacy (Hesselink et al., 1999, Giannecchini et al., 2001, Pu et al., 2001). The demonstration of protection against both homologous and heterologous challenges has become a standard feature of AIDS lentivirus vaccine development for commercial veterinary use. USDA approved, veterinary vaccines are dependent upon demonstrating efficacy using laboratory grown challenge inoculum. In HIV/SIV studies with non-human primates and in vitro studies, molecular cloned and laboratory isolates did not accurately reflect the immunogenicity and virological activity reported for clinical isolates (D’Souza et al., 1997, Beaumont et al., 2000). Furthermore, inactivated whole virus SIV vaccine studies revealed the need to use challenge inoculum grown in different cells from the vaccine virus in order to prevent false protection resulting from non-specific anti-cellular activities (Stott, 1991). Consequently, recent experimental FIV vaccine studies incorporated four approaches: (1) early passaged inoculum grown in primary peripheral blood mononuclear cells (PBMC); (2) molecularly cloned inoculum; (3) in vivo-derived inoculum; (4) contact challenges to simulate natural conditions (Yamamoto et al., 1993, Matteucci et al., 1996, Huisman et al., 1998, Karlas et al., 1999; Matteucci et al., 2000; Pu et al., 2001). Of the four approaches utilized, laboratory grown primary PBMC inoculum has proven to be the most reproducible and inexpensive approach. High titer cell-free purified inoculum can be readily produced in large quantities as laboratory grown inoculum which allows for an increased titer retention for a longer duration of time under cryopreservation. Thus, screening potential vaccines can be conducted efficiently and affordable with laboratory grown inoculum followed by limited testing of in vivo-derived inoculum.
In contrast to the laboratory grown inoculum approach, the in vivo-derived approach produced multiple limitations with inoculum production, titer retention, and increased costs, while the contact challenge studies approach produced limitations with feasibility, virus transmission, and increased costs. Limitations of in vivo-derived inoculum included: (1) the amount of inoculum produced was limited by the number of donor animals required to obtain the plasma or the cells at optimal viremic stage; (2) the high titers of virus were difficult to consistently achieve in vivo; (3) the production of in vivo-derived inoculum was more costly and technically more difficult to time the blood collection with peak viremia; (4) the biological effects of the plasma or the cellular component of the inoculum were more variable among the recipient animals. Limitations with contact transmission of FIV included: (1) potentially prolonged exposure periods (possibly 2–3 years) before sufficient seroconversion; (2) natural transmission dose may be too low for rapid transmission of FIV; (3) the route of FIV transmission may limit the rate of FIV transmission (Yamamoto et al., 1989, Matteucci et al., 2000).
Another approach is to use molecularly cloned FIV, SIV, and SHIV (SIV backbone with HIV Env and regulatory genes) as challenge inocula (Johnson, 1996, Huisman et al., 1998, Karlas et al., 1999, Girard et al., 1999). The use of such inocula has provided valuable information on the virology and pathogenesis of AIDS lentiviruses (Zou et al., 1997, Lockridge et al., 1999). However, controversy has arisen on the use of such inocula as vaccine challenge, as was recently highlighted in experimental vaccine trial using SHIV challenge (Nicodemus et al., 2001, Garrett, 2001). Underlying concerns with such cloned challenge inocula are that they do not mimic natural transmission and thus protection may be potentially easier to achieve than against primary isolates or wild type inocula consisting of quasi-species. Although the use of pathogenic cloned virus can provide rapid readout of vaccine-mediated protection from clinical disease, there are additional concerns that such pathogenic clones do not recapitulate the more prolonged course of HIV infection in humans (Nicodemus et al., 2001, Garrett, 2001).
Many vaccines are designed to provide sterilizing immunity following administration. However, in the absence of sterilizing immunity, recent AIDS vaccine studies have advocated vaccine efficacy based on prevention of disease manifestations (Johnston and Flores, 2001, Nabel, 2001, Mooij and Heeney, 2002). Using high-dose challenge with virulent strains, a major reduction in both virus load and CD4 loss was observed in the disease-protected vaccinated animals compared to the unvaccinated animals (Boretti et al., 2000, Johnston and Flores, 2001, Nabel, 2001, Dunham et al., 2002, Mooij and Heeney, 2002). The duration of the reduced viral load is still unknown (Johnston and Flores, 2001, Mooij and Heeney, 2002). High-dose in vivo inoculum and/or early passages of select FIV strains have consistently caused clinical illness in addition to CD4 loss and CD4/CD8 inversion (Callanan et al., 1992, Diehl et al., 1995). The use of a high-dose challenge or a highly virulent challenge strain may require modifications in the standard of judging vaccine efficacy, since no vaccine may be able to provide complete protection against such a rigorous challenge. Vaccine efficacy evaluation should be based on the effect on virus load, CD4 counts, CD4/CD8 ratio, and specific FIV antibody titer for active infection.
A controversy currently exists over the frequency of vertical transmission in experimental conditions and of transmission in domestic cats in nature. Serosurveys of young kittens suggest that vertical transmission of FIV is uncommon (Yamamoto et al., 1989, Lawler and Evans, 1997). Direct exposure to FIV-positive saliva or blood via bite wounds is considered to be the major mode of natural transmission. Other transmission modes include exposure to contaminated body fluid (saliva or blood) during grooming and sharing of communal food (Lawler and Evans, 1997). This observation is supported by findings from experimental studies using oral inoculation with FIV-infected culture fluid or blood (Elyar et al., 1997, Obert and Hoover, 2000, Burkhard et al., 2002). The oral inoculation dose needed for infection was much higher compared to the dose required for parenteral (intravenous and intraperitoneal) inoculation (Elyar et al., 1997, Obert and Hoover, 2000, Burkhard et al., 2002). Intravenous (IV) inoculation required the smallest inoculum dose (Elyar et al., 1997, Hartmann et al., 2001). Thus, natural FIV transmission is limited by the dose present in saliva and peripheral blood combined with the route of exposure. As seen in experimental contact studies, salivary FIV levels may be too low for rapid transmission. FIV blood levels may be sufficiently higher than FIV saliva levels, but exposure to blood is limited by the presence of open lesion(s) or of appropriately aggressive behavior. Exposing intact SPF male cats to naturally infected cats with oral lesions or to naturally infected intact male cats may increase the rate of transmission. However, contact exposure studies with only intact male cats may raise ethical issues. Nevertheless, epidemiological surveys of FIV-positive cats suggest that FIV infections occur more frequently in male cats (3:1 ratio) compared to female cats and in stray cats compared to household cats, supporting the current view that male cat aggressive behavior is a predisposing factor to infection. These contact studies are essential to determine the value of FIV vaccines in protecting cats from natural transmission.
Compared to other feline viruses (FeLV, FIP, feline herpes virus), contact transmission of FIV requires long-term exposure (months to years) with natural infected cats. According to contact studies and epidemiological surveys, disease manifestation is not common during the early phases of natural infection, supporting the view that natural transmission occurs at low doses (Hartmann, 1998, Matteucci et al., 2000). In addition to a prerequisite prolonged exposure period, questions also arise concerning the dose requirements for natural transmission which remains elusive, the virulence of natural occurring populations, and the maintenance of high virus loads in body fluids of different FIV strains as opposed to laboratory strains. Consequently, a moderate approach using the appropriate FIV isolate and dose to produce CD4 decrease (12–24 weeks post-inoculation) without CD4/CD8 inversion, may be necessary to realistically approximate natural transmission and initially screen individual cats for infection. Although contact challenge studies (natural transmission) may not be feasible for commercial vaccine validation due to the prolonged exposure period, these obstacles should not preclude researchers from testing commercial vaccines against contact challenges since contact challenge systems closely mimic natural conditions. Reproducible, independent, scientific studies will become increasingly important in the future for improving the quality of new commercialized vaccines and established vaccines.
7. Conflict with current FIV diagnostic tests
Unlike the standard FeLV diagnostics which detect viral antigen in body fluids (blood and tears), current FIV diagnostics rely on the detection of FIV antibodies in the peripheral blood (Feline Medicine Advisory Panel, 2001, Hartmann et al., 2001). FIV infection does not release sufficient levels of virus in circulation for conventional enzyme immunoassays (EIA) to detect viral antigens consistently. Sensitive PCR-based assays have been used to detect FIV infection in experimentally infected cats (Vahlenkamp et al., 1996, Klein et al., 1999, Klein et al., 2001, Pedersen et al., 2001). The sensitivity and specificity of these assays should be compared to FIV antibody-based commercial assays prior to use due to the expense of these sensitive assays compared to conventional assays. The recent approval of a PCR-based assay for HIV-1 diagnostics sets the precedent for use of such assay systems as an additional test to the antibody-based assays (antibody-specific EIA assays and confirmatory Western blot analysis) in current use (Bootman and Kitchin, 1994). The impact to the current antibody-based FIV diagnostics will vary according to the type of FIV vaccine released for commercial use. The conventional inactivated FIV vaccine, for released this year, has a major conflict with current FIV diagnostics including the confirmatory Western blot analysis (Fig. 1 ). This vaccine, depending on the antigenic level of the commercial vaccine, induces broad spectrum antibody production to different FIV proteins with long-lasting titers. However, if appropriately inactivated, this vaccine will not result in viral antigen or genome production and will not conflict with PCR-based assays or conventional antigen-specific EIA assays. In contrast, live attenuated vaccines can cause significant conflict with current commercial FIV diagnostics. Also, potential conflicts may arise with future viral PCR- or protein-based diagnostics depending upon the level of attenuation. Since the current approach for testing attentuated FIV vaccines is based on viral regulatory gene deletion mutants, the attentuated virus will contain genes for viral structural proteins which are detectable by current FIV diagnostics. If improperly or insufficiently attenuated vaccines are used on immunocompromized hosts, the vaccine virus may escape the host compromized immune system and produce sufficient vaccine virus in circulation to interfere with current as well as future diagnostics.
Fig. 1.
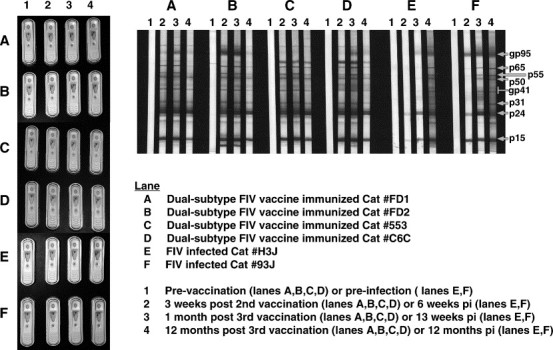
FIV immunoblot and commercial FIV test results of cats immunized with the dual-subtype FIV vaccine or experimentally infected with FIVPET (20 CID50). Sera tested for FIV antibodies were from cats (two each) experimentally immunized with two different sources of dual-subtype FIV vaccine (immunoblot/Snap Combo lanes A, B, C and D) or experimentally infected with FIV (immunoblot/Snap Combo lanes E and F). These sera were tested for the presence of FIV antibodies using immunoblot analysis and commercial Idexx SnapTM Combo (FeLV and FIV) Test Kit. The immunoblot analysis was performed at serum dilution of 1:100 using a published method (Pu et al., 2001) and the Snap test was performed as recommended by the Idexx Laboratories, Inc. Dual-subtype FIV vaccine produced by Fort Dodge Animal Health commercial company (USDA approved product) (lanes A and B) and those produced by our laboratory (lanes C and D) are shown for comparison. Immunogenicity and efficacy of our dual-subtype FIV vaccine has been previously reported (Pu et al., 2001). Both Snap test and immunoblot results demonstrate that vaccinated cats will develop antibodies reactive to current FIV diagnostics. Both vaccinations induced antibodies to the full spectrum of FIV antigens, including antibodies to the envelope (gp95). Interestingly, the cats immunized with the USDA approved dual-subtype FIV vaccine had more consistent and long-lasting antibodies to the envelope compared to cats immunized with our experimental dual-subtype FIV vaccine (2 of 2 cats vs. 1 of 2 cats positive after 1 year post-vaccination).
Most veterinary virus vaccines are either conventional inactivated whole virus or live attenuated virus vaccines (USDA, 2001). In the last 5 years, live recombinant vectored vaccines have been introduced to the veterinary market (Yamanouchi et al., 1998, USDA, 2001, van Kampen, 2001). Most notable and most widespread veterinary vectored vaccines are the rabies vaccine vectored by vaccinia, fowlpox, and canarypox viruses which are not virulent in unnatural hosts. These recombinant vectored vaccines incorporate gene sequence(s) of the targeted viral protein in the vector genome region that does not interfere with the targeted gene expression (Schnell, 2001). The incorporated gene(s) must include genes for protective FIV epitopes and must be expressed at a sufficient level for the vaccinated host to mount a specific immune response. Predominant CTL and VN antibody epitopes reside on FIV Gag proteins and envelope glycoproteins, respectively (Lombardi et al., 1993, Egberink et al., 1994, Flynn et al., 1995). These viral proteins also make up over 75% of the structural proteins and contain the major epitopes for immunodiagnostics. Although vectored vaccines are generally known for their ability to generate cellular immunity compared to humoral immunity, specific antibodies against targeted virus can be produced using this vaccine system. Thus, modifications in current FIV diagnostic tests must be made for detection of FIV antibodies to viral proteins of active infection instead of FIV antibodies present on the targeted gene products expressed by the vaccine vector. In contrast, recombinant vectored vaccines will have minimal to no conflict with viral PCR- and protein-based diagnostics if the vector virus used preferentially infects the above described non-leukocytes (avian pox viruses) and the targeted protein(s) are not released into circulation.
8. Summary: impact of FIV vaccine to AIDS vaccine development and feline medicine
Since the discovery of FIV in 1986, the goals of the FIV vaccine studies have been: (1) to identify effective vaccine designs which may serve as models for effective HIV vaccine designs in humans; (2) to develop an effective FIV vaccine for pet cats. Recent United States Department of Agriculture approval (USDA, 2002, Fort Dodge Animal Health, 2002a) of Fel-O-Vax FIV, a dual-subtype FIV vaccine, has led to the commercial release of this vaccine by Fort Dodge Animal Health (FDAH, Fort Dodge, IA) on 14 July, 2002. Without an additional boost, the protection rate of this dual-subtype FIV vaccine after 1 year is 67% (18/27 cats) against an intramuscular heterologous FIV challenge (Fort Dodge Animal Health, 2002a). The same challenge inoculum resulted in a 74% infection rate (25/34 cats) in unvaccinated control cats. In the second trial using a slightly modified vaccine and immunization schedule (i.e., 8 instead of 12 weeks old kittens), the protection rate for vaccinated kittens rose to 84% (21/25 cats) after the same heterologous challenge compared to 90% infection rate (17/19 cats) in the unvaccinated control cats after 1 year (Fort Dodge Animal Health, 2002b). Although the vaccine appears to provide immunity for at least 1 year, contact exposure trials that simulate natural transmission are needed which compliment the parenteral challenge inoculations performed by FDAH.
As a consequence of the FeLV vaccine-associated fibrosarcoma, release of a commercial veterinary FIV vaccine will raise similar concerns. The policies adopted by veterinary practitioners for FeLV vaccine administration will probably be applied to FIV vaccine administration. The impact on the current FIV diagnostic tests will be an important issue requiring careful monitoring by practitioners. FIV diagnostics, requiring molecular technology to develop advanced assays with increased sensitivity and specificity, must keep abreast with the development and release of new commercial FIV vaccines. Equally as important to developing and commercially producing a reliable, safe, and efficacious vaccine for the veterinary community, FIV researchers must continue to educate the AIDS community about and promote the value of an FIV vaccine model for HIV vaccine development.
Acknowledgements
This work was funded by NIH R01 AI30904 and JKY Miscellaneous Donors Fund. Dr. Yamamoto is the inventor of record on a University of Florida held patent and may be entitled to royalties from companies that are developing commercial products that are related to the research described in this paper.
References
- Ackley C.D., Yamamoto J.K., Levy N.B., Pedersen N.C., Cooper M.D. Immunologic abnormalities in pathogen-free cats experimentally infected with feline immunodeficiency virus. J. Virol. 1990;64:5652–5655. doi: 10.1128/jvi.64.11.5652-5655.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmann M.H., Mathiason-Dubard C., Learn G.H., Rodrigo A.G., Sodora D.L., Mazzetti P., Hoover E.A., Mullins J.I. Genetic diversity of feline immunodeficiency virus: dual infection, recombination, and distinct evolutionary rates among envelope sequence clades. J. Virol. 1997;71:4241–4253. doi: 10.1128/jvi.71.6.4241-4253.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlough J.E., Ackley C.D., George J.W., Levy N., Acevedo R., Moore P.F., Rideout B.A., Cooper M.D., Pedersen N.C. Acquired immune dysfunction in cats with experimentally induced feline immunodeficiency virus infection: comparison of short-term and long-term infection. J. Acquir. Immune Defic. Syndr. 1991;4:219–227. [PubMed] [Google Scholar]
- Beaumont T., Broersen S., van Nuenen A., Juisman J.G., de Roda Husman A.-M., Heeney J.L., Schuitemaker J. Increased neutralization sensitivity and reduced replicative capacity of human immunodeficiency virus type 1 after short-term in vivo or in vitro passage through chimpanzees. J. Virol. 2000;74:7699–7707. doi: 10.1128/jvi.74.17.7699-7707.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bendinelli M., Pistello M., Lombardi S., Poli A., Garzelli C., Matteucci D., Ceccherini-Nelli L., Malvaldi G., Tozzini F. Feline immunodeficiency virus: an interesting model for AIDS studies and an important cat pathogen. Clin. Microbiol. Rev. 1995;8:87–112. doi: 10.1128/cmr.8.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackard J.T., Cohen D.E., Mayer K.H. Human immunodeficiency virus superinfection and recombination: current state of knowledge and potential clinical consequences. Clin. Infect. Dis. 2002;34:1108–1114. doi: 10.1086/339547. [DOI] [PubMed] [Google Scholar]
- Bootman J.S., Kitchin P.A. Reference preparation in the standardisation of HIV-1 PCR—an international collaborative study. J. Virol. Meth. 1994;49:1–8. doi: 10.1016/0166-0934(94)90050-7. [DOI] [PubMed] [Google Scholar]
- Boretti F.S., Leutenegger C.M., Mislin C., Hofmann-Lehmann R., Konig S., Schroff M., Junghans C., Fehr D., Huettner S.W., Habel A., Flynn J.N., Aubert A., Pedersen N.C., Wittig B., Lutz H. Protection against FIV challenge infection by genetic vaccination using minimalistic DNA constructs for FIV Env gene and feline IL-12 expression. AIDS. 2000;14:1749–1757. doi: 10.1097/00002030-200008180-00009. [DOI] [PubMed] [Google Scholar]
- Burkhard M.J., Mathiason C.K., O’Halloran K., Hoover E.A. Kinetics of early FIV infection in cats exposed via the vaginal versus intravenous route. AIDS Res. Hum. Retrov. 2002;18:217–226. doi: 10.1089/08892220252781284. [DOI] [PubMed] [Google Scholar]
- Calabrese L.H., Kelley D.M., Myers A., O’Connell M., Easley K. Rheumatic symptoms and human immunodeficiency virus infection. The influence of clinical and laboratory variables in a longitudinal cohort study. Arthritis Rheum. 1991;34:257–263. doi: 10.1002/art.1780340302. [DOI] [PubMed] [Google Scholar]
- Callanan J.J., Thompson H., Toth S.R., O’Neil B., Lawrence C.E., Willett B., Jarrett O. Clinical and pathological findings in feline immunodeficiency virus experimental infection. Vet. Immunol. Immunopathol. 1992;35:3–13. doi: 10.1016/0165-2427(92)90116-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callanan J.J., Jones B.A., Irvine J., Willett B.J., McCandlish I.A.P., Jarrett O. Histologic classification and immunophenotype of lymphosarcomas in cats with naturally and experimentally acquired feline immunodeficiency virus infections. Vet. Pathol. 1996;33:264–272. doi: 10.1177/030098589603300302. [DOI] [PubMed] [Google Scholar]
- Calnek B.W. Marek’s disease vaccines. Dev. Biol. Stand. 1982;52:401–405. [PubMed] [Google Scholar]
- Calnek, B.W., Witter, R.L., 1997. Marek’s disease. In: Calnek, B.W., Barnes, H.J., Beard, C.W., McDougald, L.R., Saif, Y.M. (Eds.), Diseases of Poultry, 10th ed. Iowa State University Press, Ames, Iowa, pp. 369–413.
- Chiarantini L., Matteucci D., Pistello M., Mancini U., Mazzetti P., Massi C., Giannecchini S., Lonetti I., Magnani M., Bendinelli M. AIDS vaccination studies using an ex vivo feline immunodeficiency virus model: homologous erythrocytes as a delivery system for preferential immunization with putative protective antigens. Clin. Diag. Lab. Immunol. 1998;5:235–241. doi: 10.1128/cdli.5.2.235-241.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi I.S., Hokanson R., Collisson E.W. Anti-feline immunodeficiency virus (FIV) soluble factor(s) produced from antigen-stimulated feline CD8(+) T lymphocytes suppresses FIV replication. J. Virol. 2000;74:676–683. doi: 10.1128/jvi.74.2.676-683.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Client Information Series, 1990. Feline immunodeficiency virus. Feline Pract. 18 21–24.
- Cuisinier A.M., Meyer A., Chatrenet B., Verdier A.S., Aubert A. Attempt to modify the immune response developed against FIV gp120 protein by preliminary FIV DNA injection. Vaccine. 1999;17:415–425. doi: 10.1016/s0264-410x(98)00212-6. [DOI] [PubMed] [Google Scholar]
- D’Souza M.P., Livnat D., Bradac J.A., Bridges S.H. Evaluation of monoclonal antibodies to human immunodeficiency virus type 1 primary isolates by neutralization assays: performance criteria for selecting candidate antibodies for clinical trials. AIDS Clinical Trials Group Antibody Selection Working Group. J. Infect. Dis. 1997;175:1056–1062. doi: 10.1086/516443. [DOI] [PubMed] [Google Scholar]
- Daniel M.D., Kirchhoff F., Czajak S.C., Sehgal P.K., Desrosiers R.C. Protective effects of a live attenuated SIV vaccine with a deletion in the nef gene. Science. 1992;258:1938–1941. doi: 10.1126/science.1470917. [DOI] [PubMed] [Google Scholar]
- Desrosiers R.C., Bolognesi D.P. Controversies in science: a live virus AIDS vaccine? J. NIH Res. 1994;6:54–62. [Google Scholar]
- Diehl L.J., Mathiason-Dubard C.K., O’Neil L.L., Obert L.A., Hoover E.A. Induction of accelerated feline immunodeficiency virus disease by acute-phase virus passage. J. Virol. 1995;69:6149–6157. doi: 10.1128/jvi.69.10.6149-6157.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunham S.P., Flynn J.N., Rigby M.A., Macdonald J., Bruce J., Cannon C., Golder M.C., Hanlon L., Harbour D.A., Mackay N.A., Spibey N., Jarrett O., Neil J.C. Protection against feline immunodeficiency virus using replication defective proviral DNA vaccines with feline interleukin-12 and -18. Vaccine. 2002;20:1483–1496. doi: 10.1016/s0264-410x(01)00507-2. [DOI] [PubMed] [Google Scholar]
- Egberink H., Keldermans L., Schuurman N., Stam J., Hesselin W., van Vliet A., Verschoor E., Horzinek M., de Ronde A. Monoclonal antibodies to immunodominant and neutralizing domains of the envelope surface protein of feline immunodeficiency virus. J. Gen. Virol. 1994;75:889–893. doi: 10.1099/0022-1317-75-4-889. [DOI] [PubMed] [Google Scholar]
- Elyar J., Tellier M.C., Soos J.M., Yamamoto J.K. Perspectives on FIV vaccine development. Vaccine. 1997;15:1437–1444. doi: 10.1016/s0264-410x(97)00056-x. [DOI] [PubMed] [Google Scholar]
- English R.V., Johnson C.M., Gebhard D.H., Tompkins M.B. In vivo lymphocyte tropism of feline immunodeficiency virus. J. Virol. 1993;67:5175–5186. doi: 10.1128/jvi.67.9.5175-5186.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fauci A., Schnittman S., Poli G., Koenig S., Pantaleo G. Immunopathogenic mechanisms of human immunodeficiency virus (HIV) infection. Ann. Inter. Med. 1991;114:678–693. doi: 10.7326/0003-4819-114-8-678. [DOI] [PubMed] [Google Scholar]
- Fehr D., Holznagel E., Bolla S., Hauser B., Herrewegh A.A.P.M., Horzinek M.C., Lutz H. Placebo-controlled evaluation of a modified live virus vaccine against feline infectious peritonitis: safety and efficacy under field conditions. Vaccine. 1997;15:1101–1109. doi: 10.1016/S0264-410X(97)00006-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feline Medicine Advisory Panel, 2000. Report of the American Association of Feline Practitioners and Academy of Feline Medicine Advisory Panel on Feline Vaccines, pp. 1–27 (http://www.aafponline.org/about/guidelines.htm). [DOI] [PMC free article] [PubMed]
- Feline Medicine Advisory Panel, 2001. Report of the American Association of Feline Practitioners and Academy of Feline Medicine Advisory Panel on Feline Retrovirus Testing and Management (http://www.aafponline.org/about/guidlines.htm). [DOI] [PMC free article] [PubMed]
- Finerty S., Stokes C.R., Gruffydd-Jones T.J., Hillman T.J., Reeves N.A., Whiting C.V., Schaaper W.M., Dalsgaard K., Harbour D.A. Mucosal immunization with experimental feline immunodeficiency virus (FIV) vaccines induces both antibody and T cell responses but does not protect against rectal FIV challenge. Vaccine. 2000;18:3254–3265. doi: 10.1016/s0264-410x(00)00131-6. [DOI] [PubMed] [Google Scholar]
- Finerty S., Stokes C.R., Gruffydd-Jones T.J., Hillman T.J., Barr F.J., Harbour D.A. Targeted lymph node immunization can protect cats from a mucosal challenge with feline immunodeficiency virus. Vaccine. 2002;20:49–58. doi: 10.1016/s0264-410x(01)00323-1. [DOI] [PubMed] [Google Scholar]
- Flynn J.N., Beatty J.A., Cannon C.A., Stephens E.B., Hosie M.J., Neil J.C., Jarrett O. Involvement of Gag- and Env-specific cytotoxic T lymphocytes in protective immunity to feline immunodeficiency virus. AIDS Res. Hum. Retrov. 1995;11:1107–1113. doi: 10.1089/aid.1995.11.1107. [DOI] [PubMed] [Google Scholar]
- Flynn J.N., Cannon C.A., Sloan D., Neil J.C., Jarrett O. Suppression of feline immunodeficiency virus replication in vitro by a soluble factor secreted by CD8+ T lymphocytes. Immunology. 1999;96:220–229. doi: 10.1046/j.1365-2567.1999.00690.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fort Dodge Animal Health, 2002a. Fort Dodge Animal Health received USDA approval for a feline immunodeficiency virus vaccine for cats. Press Release March 25, 2002 (http://www.wyeth.com/news/pressed_and_released/pr03_25_2002.html).
- Fort Dodge Animal Health, 2002b. Fel-O-Vax FIV product insert released on July 14, 2002.
- Garrett, L., 2001. Skeptical about AIDS vaccine/testing method questioned. Newsday September 5, 2001 (http://www.aegis.com/news/newsday/2001/ND010901.html).
- Gebhard D.H., Dow J.L., Childers T.A., Alvelo J.I., Tompkins M.B., Tompkins W.A.F. Progressive expansion of an l-selectin-negative CD8 cell with anti-feline immunodeficiency virus (FIV) suppressor function in the circulation of FIV-infected cats. J. Infect. Dis. 1999;180:1503–1513. doi: 10.1086/315089. [DOI] [PubMed] [Google Scholar]
- Gerber J.D., Ingersoll J.D., Gast A.M., Christianson K.K., Selzer N.L., Landon R.M., Pfeiffer N.E., Sharpee R.L., Bechenhauer W.H. Protection against feline infectious peritonitis by intranasal inoculation of a temperature-sensitive FIPV vaccine. Vaccine. 1990;8:536–542. doi: 10.1016/0264-410X(90)90004-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giannecchini S., del Mauro D., Matteucci D., Bendinelli M. AIDS vaccination studies using an ex vivo feline immunodeficiency virus model: reevaluation of neutralizing antibody levels elicited by a protective and a nonprotective vaccine after removal of antisubstrate cell antibodies. J. Virol. 2001;75:4424–4429. doi: 10.1128/JVI.75.9.4424-4429.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girard M., Habel A., Chanel C. New prospects for the development of a vaccine against human immunodeficiency virus type 1: an overview. CR Acad. Sci. III. 1999;322:959–966. doi: 10.1016/s0764-4469(00)87193-0. [DOI] [PubMed] [Google Scholar]
- Gobar G.M., Kass P.H. World wide web-based survey of vaccination practices, postvaccinal reactions and vaccine site-associated sarcomas in cats. JAVMA. 2002;220:1477–1482. doi: 10.2460/javma.2002.220.1477. [DOI] [PubMed] [Google Scholar]
- Hartmann K. Feline immunodeficiency virus infection: an overview. Vet. J. 1998;155:123–137. doi: 10.1016/S1090-0233(98)80008-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartmann K., Werner R.M., Egberink H., Jarrett O. Comparison of six in-house tests for the rapid diagnosis of feline immunodeficiency and feline leukemia virus infection. Vet. Rec. 2001;149:317–320. doi: 10.1136/vr.149.11.317. [DOI] [PubMed] [Google Scholar]
- Hendrick M.J., Brooks J.J. Postvaccinal sarcomas in the cat: histology and immunochemistry. Vet. Pathol. 1994;31:126–129. doi: 10.1177/030098589403100121. [DOI] [PubMed] [Google Scholar]
- Hesselink W., Sondermeijer P., Pouwels H., Verblakt E., Dhore C. Vaccination of cats against feline immunodeficiency virus (FIV): a matter of challenge. Vet. Microbiol. 1999;69:109–110. doi: 10.1016/s0378-1135(99)00096-6. [DOI] [PubMed] [Google Scholar]
- Hoffmann-Fezer G., Thum J., Ackley C., Herbold M., Mysliwietz J., Thefeld S., Hartmann K., Kraft W. Decline in CD4+ cell numbers in cats with naturally acquired feline immunodeficiency virus infection. J. Virol. 1992;66:1484–1488. doi: 10.1128/jvi.66.3.1484-1488.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann-Lehmann R., Holznagel E., Ossent P., Lutz H. Parameters of disease progression in long-term experimental feline retrovirus (feline immunodeficiency virus and feline leukemia virus) infections: hematology, clinical chemistry, and lymphocyte subsets. Clin. Diag. Lab. Immunol. 1997;4:33–42. doi: 10.1128/cdli.4.1.33-42.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohdatsu T., Okada S., Motokawa K., Yamamoto J.K., Koyama H. Effect of dual-subtype vaccine against feline immunodeficiency virus infection. Vet. Microbiol. 1997;58:155–165. doi: 10.1016/S0378-1135(97)00164-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoover E.A., Mullins J.I., Chu H.-J., Wasmoen T.L. Efficacy of an inactivated feline leukemia virus vaccine. AIDS Res. Hum. Retrov. 1996;12:379–383. doi: 10.1089/aid.1996.12.379. [DOI] [PubMed] [Google Scholar]
- Hopper C.D., Sparkes A.H., Gruffydd-Jones T.J., Crispin S.M., Muir P., Harbour D.A., Stokes C.R. Clinical and laboratory findings in cats infected with feline immunodeficiency virus. Vet. Rec. 1989;125:341–346. doi: 10.1136/vr.125.13.341. [DOI] [PubMed] [Google Scholar]
- Hosie M.J., Osborne R., Reid G., Neil J.C., Jarrett O. Enhancement after feline immunodeficiency virus vaccination. Vet. Immunol. Immunopathol. 1992;35:191–197. doi: 10.1016/0165-2427(93)90149-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosie M.J., Osborne R., Thompson F.J., Yamamoto J.K., Neil J.C., Jarrett O. Protection against homologous but not heterologous challenge induced by inactivated feline immunodeficiency virus vaccines. J. Virol. 1995;69:1253–1255. doi: 10.1128/jvi.69.2.1253-1255.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosie M.J., Flynn J.N., Cannon C., Dunsford T., Mackay N.A., Argyle D., Willet B.J., Miyazawa T., Onions D.E., Jarrett O., Neil J.C. DNA vaccination affords significant protection against feline immunodeficiency virus infection without inducing detectable antiviral antibodies. J. Virol. 1998;72:7310–7319. doi: 10.1128/jvi.72.9.7310-7319.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosie M.J., Dunsford T., Klein D., Willett B.J., Cannon C., Osborne R., MacDonald J., Spibey N., Mackay N., Jarrett O., Neil J.C. Vaccination with inactivated virus but not viral DNA reduces virus load following challenge with a heterologous and virulent isolate of feline immunodeficiency virus. J. Virol. 2000;74:9403–9411. doi: 10.1128/jvi.74.20.9403-9411.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huisman W., Karlas J.A., Siebelink K.H.J., Huisman R.C., de Ronde A., Francis M.J., Rimmelzwaan G.F., Osterhaus A.D.M.E. Feline immunodeficiency virus subunit vaccines that induce neutralising antibodies but no protection against challenge infection. Vaccine. 1998;16:181–187. doi: 10.1016/s0264-410x(97)00184-9. [DOI] [PubMed] [Google Scholar]
- Inoshima Y., Ikeda Y., Kohmoto M., Pecoraro M.R., Shimojima M., Shimojima Y., Inada G., Kawaguchi Y., Tomonaga K., Miyazawa T., Mikami T. Persistence of high virus neutralizing antibody titers in cats experimentally infected with feline immunodeficiency virus. J. Vet. Med. Sci. 1996;58:925–927. doi: 10.1292/jvms.58.925. [DOI] [PubMed] [Google Scholar]
- Inoshima Y., Mihyzawa T., Kohmoto M., Ikeda Y., Sato E., Hohdatsu T., Mathiason-Dubard C., Hoover E.A., Mikami T. Cross virus neutralizing antibodies against feline immunodeficiency virus genotypes A, B, D and E. Archiv. Virol. 1998;143:157–162. doi: 10.1007/s007050050275. [DOI] [PubMed] [Google Scholar]
- Ishida T., Washizu T., Toriyabe K., Motoyoshi S., Tomoda I., Pedersen N. Feline immunodeficiency virus infection in cats of Japan. J. Am. Vet. Med. Assoc. 1989;194:221–225. [PubMed] [Google Scholar]
- Johnson R.P. Macaque models for AIDS vaccine development. Curr. Opin. Immunol. 1996;8:554–560. doi: 10.1016/s0952-7915(96)80046-x. [DOI] [PubMed] [Google Scholar]
- Johnston M.I., Flores J. Progress in HIV vaccine development. Curr. Opin. Pharmacol. 2001;1:504–510. doi: 10.1016/s1471-4892(01)00086-8. [DOI] [PubMed] [Google Scholar]
- Karlas J.A., Siebelink K.H.J., van Peer M.A., Huisman W., Rimmelzwaan G.F., Osterhaus A.D.M.E. Accelerated viraemia in cats vaccinated with fixed autologous FIV-infected cells. Vet. Immunol. Immunopathol. 1998;65:353–356. doi: 10.1016/s0165-2427(98)00166-4. [DOI] [PubMed] [Google Scholar]
- Karlas J.A., Siebelink K.H.J., van Peer M.A., Huisman W., Cuisinier A.M., Rimmelzwaan G.F., Osterhaus A.D.M.E. Vaccination with experimental feline immunodeficiency virus vaccines, based on autologous infected cells, elicits enhancement of homologous challenge infection. J. Gen. Virol. 1999;80:761–765. doi: 10.1099/0022-1317-80-3-761. [DOI] [PubMed] [Google Scholar]
- Klein D., Janda P., Steinborn R., Muller M., Salmons B., Gunzburg W.H. Proviral load determination of different feline immunodeficiency virus isolates using real-time polymerase chain reaction: influence of mismatches on quantification. Electrophoresis. 1999;20:291–299. doi: 10.1002/(SICI)1522-2683(19990201)20:2<291::AID-ELPS291>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- Klein D., Leutenegger C.M., Bahula C., Gold P., Hofmann-Lehmann R., Salmons B., Lutz H., Gunzburg W.H. Influence of preassay and sequence variations on viral load determination by a multiplex real-time reverse transcriptase-polymerase chain reaction for feline immunodeficiency virus. J. Acquir. Immune Defic. Syndr. 2001;26:8–20. doi: 10.1097/00126334-200101010-00002. [DOI] [PubMed] [Google Scholar]
- Kohmoto M., Miyazawa T., Sato E., Uetsuka K., Nishimura Y., Ikeda Y., Inada G., Doi K., Mikami T. Cats are protected against feline immunodeficiency virus following vaccination with a homologous AP-1 binding site-deleted mutant. Archiv. Virol. 1998;143:1839–1845. doi: 10.1007/s007050050422. [DOI] [PubMed] [Google Scholar]
- Lawler, D.F., Evans, R.H., 1997. Strategies for controlling viral infections in feline populations. In: August, J.R. (Ed.), Consultations in Feline Internal Medicine, vol. 3. Saunders, Philadelphia, PA, Chapter 76, pp. 603–610.
- Legendre A.M., Hawks D.M., Sebring R., Rohrbach B., Chavez L., Chu H.-J., Acree W.M. Comparison of the efficacy of three commercial feline leukemia virus vaccines in a natural challenge exposure. J. Am. Vet. Med. Assoc. 1991;199:1456–1462. [PubMed] [Google Scholar]
- Leutenegger C.M., Hofmann-Lehmann R., Holznagel E., Cuisinier A.M., Wolfensberger C., Duquesne V., Cronier J., Allenspach K., Aubert A., Ossent P., Lutz H. Partial protection by vaccination with recombinant feline immunodeficiency virus surface glycoproteins. AIDS Res. Hum. Retrov. 1998;14:275–283. doi: 10.1089/aid.1998.14.275. [DOI] [PubMed] [Google Scholar]
- Leutenegger C.M., Boretti F.S., Mislin C.N., Flynn J.N., Schroff M., Habel A., Junghans C., Koenig-Merediz S.A., Sigrist B., Aubert A., Pedersen N.C., Wittig B., Lutz H. Immunization of cats against feline immunodeficiency virus (FIV) infection by using minimalistic immunogenic defined gene expression vector vaccines expressing FIV gp140 alone or with feline interleukin-12 (IL-12), IL-16, or a CpG motif. J. Virol. 2000;74:10447–10457. doi: 10.1128/jvi.74.22.10447-10457.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J., Brown W.C., Song W., Carpino M.R., Wolf A.M., Grant C.K., Elder J.H., Collisson E.W. Retroviral vector-transduced cells expressing the core polyprotein induce feline immunodeficiency virus-specific cytotoxic T-lymphocytes from infected cats. Virus Res. 1995;38:93–109. doi: 10.1016/0168-1702(95)00050-z. [DOI] [PubMed] [Google Scholar]
- Liang Y., Hudson L.C., Levy J.K., Ritchey J.W., Tompkins W.A., Tompkins M.B. T cells overexpressing interferon-gamma and interleukin-10 are found in both the thymus and secondary lymphoid tissues of feline immunodeficiency virus-infected cats. J. Infect. Dis. 2000;181:564–575. doi: 10.1086/315226. [DOI] [PubMed] [Google Scholar]
- Lockridge, K.M., Himathongkham, S., Sawai, E.T., Chienand, M., Sparger, E.E., 1999. The feline immunodeficiency virus vif gene is required for productive infection of feline peripheral blood mononuclear cells and monocyte-derived macrophages. Virology 261, 25–30. [DOI] [PubMed]
- Lockridge K.M., Chien M., Dean G.A., Cole K.S., Montelaro R.C., Luciw P.A., Sparger E.E. Protective immunity against feline immunodeficiency virus induced by inoculation with vif-deleted proviral DNA. Virology. 2000;273:67–79. doi: 10.1006/viro.2000.0395. [DOI] [PubMed] [Google Scholar]
- Lombardi S., Garzelli C., la Rosa C., Zaccaro L., Spector S., Malvaldi G., Tozzini F., Esposito F., Bendinelli M. Identification of a linear neutralization site within the third variable region of the feline immunodeficiency virus envelope. J. Virol. 1993;67:4742–4749. doi: 10.1128/jvi.67.8.4742-4749.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macy, D.W., 1994. Vaccination against feline retroviruses. In: August, J.R. (Ed.), Consultations in Feline Internal Medicine, vol. 2. Saunders, Philadelphia, PA, Chapter 5, pp. 33–39.
- Matteucci D., Pistello M., Mazzetti P., Giannecchini S., del Mauro D., Zaccaro L., Bandecchi P., Tozzini F., Bendinelli M. Vaccination protects against in vivo-grown feline immunodeficiency virus even in the absence of detectable neutralizing antibodies. J. Virol. 1996;70:617–622. doi: 10.1128/jvi.70.1.617-622.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matteucci D., Pistello M., Mazzetti P., Giannecchini S., del Mauro D., Lonetti I., Zaccaro L., Pollera C., Specter S., Bendinelli M. Studies of AIDS vaccination using an ex vivo feline immunodeficiency virus model: protection conferred by a fixed-cell vaccine against cell-free and cell-associated challenge differs in duration and is not easily boosted. J. Virol. 1997;71:8368–8376. doi: 10.1128/jvi.71.11.8368-8376.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matteucci D., Pistello M., Mazzetti P., Giannecchini S., Isola P., Merico A., Zaccaro L., Rizzuti A., Bendinelli M. AIDS vaccination studies using feline immunodeficiency virus as a model: immunisation with inactivated whole virus suppresses viraemia levels following intravaginal challenge with infected cells but not following intravenous challenge with cell-free virus. Vaccine. 1999;18:119–130. doi: 10.1016/s0264-410x(99)00189-9. [DOI] [PubMed] [Google Scholar]
- Matteucci D., Poli A., Mazzetti P., Sozzi S., Bonci F., Isola P., Zaccaro L., Giannecchini S., Calandrella M., Pistello M., Specter S., Bendinelli M. Immunogenicity of an anti-clade B feline immunodeficiency fixed-cell virus vaccine in field cats. J. Virol. 2000;74:10911–10919. doi: 10.1128/jvi.74.23.10911-10919.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McArdle F., Tennant B., Kelly D.F., Gaskell C.J., Gaskell R.M. Independent evaluation of a modified live FIPV vaccine under experimental conditions (University of Liverpool experience) Feline Pract. 1995;23:67–71. [Google Scholar]
- Mooij P., Heeney J.L. Rational development of prophylactic HIV vaccines based on structural and regulatory proteins. Vaccine. 2002;20:304–321. doi: 10.1016/s0264-410x(01)00373-5. [DOI] [PubMed] [Google Scholar]
- Nabel G.J. Challenges and opportunities for development of an AIDS vaccine. Nature. 2001;410:1002–1007. doi: 10.1038/35073500. [DOI] [PubMed] [Google Scholar]
- Nicodemus, J., Sbai, H., De Groot, A.S., 2001. Urgency and Optimism at the AIDS Vaccine 2001 Conference, AIDScience vol. 1, October 4–11, 2001 (http://aidscience.org/Articles/aidscience009.pdf).
- Norsworthy, G.D. (Ed.), 1993a. Feline infectious peritonitis. In: Feline Practice. Lippincott, Philadelphia, PA, pp. 352–359.
- Norsworthy, G.D. (Ed.), 1993b. Feline leukemia virus diseases. In: Feline Practice. Lippincott, Philadelphia, PA, pp. 360–368.
- Obert L.A., Hoover E.A. Relationship of lymphoid lesions to disease course in mucosal feline immunodeficiency virus type C infection. Vet. Pathol. 2000;37:386–401. doi: 10.1354/vp.37-5-386. [DOI] [PubMed] [Google Scholar]
- Pedersen N.C., Ho E.W., Brown M.L., Yamamoto J.K. Isolation of a T-lymphotropic virus from domestic cats with an immunodeficiency-like syndrome. Science. 1987;235:790–793. doi: 10.1126/science.3643650. [DOI] [PubMed] [Google Scholar]
- Pedersen N.C., Leutenegger C.M., Woo J., Higgins J. Virulence differences between two field isolates of feline immunodeficiency virus (FIV-APetaluma and FIV-CGammar) in young adult specific pathogen free cats. Vet. Immunol. Immunopathol. 2001;79:53–67. doi: 10.1016/s0165-2427(01)00252-5. [DOI] [PubMed] [Google Scholar]
- Pu R., Coleman J., Omori M., Mison M., Huang C., Arai M., Tanabe T., Yamamoto J.K. Dual-subtype FIV vaccine protects cats against in vivo swarms of both homologous and heterologous subtype FIV isolates. AIDS. 2001;15:1–13. doi: 10.1097/00002030-200107060-00004. [DOI] [PubMed] [Google Scholar]
- Rideout B.A., Moore P.F., Pedersen N.C. Persistent up regulation of MHC class II antigen expression on T-lymphocytes from cats experimentally infected with feline immunodeficiency virus. Vet. Immunol. Immunopathol. 1992;35:71–81. doi: 10.1016/0165-2427(92)90122-7. [DOI] [PubMed] [Google Scholar]
- Schnell M.J. Viral vectors as potential HIV-1 vaccines. FEMS Microbiol. Lett. 2001;200:123–129. doi: 10.1111/j.1574-6968.2001.tb10703.x. [DOI] [PubMed] [Google Scholar]
- Scott, F.W., 1999. Evaluation of risks and benefits associated with vaccination against corona virus infections in cats. In: Schultz, R.D. (Ed.), Veterinary Vaccines and Diagnostics. Academic Press, San Diego, pp. 347–358. [DOI] [PMC free article] [PubMed]
- Scott F.W., Corapi W.V., Olsen C.W. Independent evaluation of a modified live FIPV vaccine under experimental conditions (Cornell experience) Feline Pract. 1995;23:74–76. [Google Scholar]
- Siebelink K.H.J., Chu I.-H., Rimmelzwaan G.F., Weijer K., Herwijnen R.V., Knell P., Egberink H.F., Bosch M.L., Osterhaus A.D.M.E. Feline immunodeficiency virus (FIV) infection in the cat as a model for HIV infection in man: FIV-induced impairment of immune function. AIDS Res. Hum. Retrov. 1990;6:1373–1378. doi: 10.1089/aid.1990.6.1373. [DOI] [PubMed] [Google Scholar]
- Song W., Collison E., Billingsley P., Brown W. Induction of feline immunodeficiency virus specific cytotoxic T-cell responses from experimentally infected cats. J. Virol. 1992;66:5409–5417. doi: 10.1128/jvi.66.9.5409-5417.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparkes A.H. Feline leukaemia virus: a review of immunity and vaccination. J. Small Anim. Pract. 1997;38:187–194. doi: 10.1111/j.1748-5827.1997.tb03339.x. [DOI] [PubMed] [Google Scholar]
- Stokes C.R., Finerty S., Gruffydd-Jones T.J., Sturgess C.P., Harbour D.A. Mucosal infection and vaccination against feline immunodeficiency virus. J. Biotechnol. 1999;73:213–221. doi: 10.1016/s0168-1656(99)00139-x. [DOI] [PubMed] [Google Scholar]
- Stott E.J. Anti-cell antibody in macaques. Nature. 1991;353:393. doi: 10.1038/353393a0. [DOI] [PubMed] [Google Scholar]
- Taniguchi A., Ishida T., Washizu T., Tomoda I. Humoral immune response to T-cell dependent and independent antigens in cats infected with feline immunodeficiency virus. J. Vet. Med. Sci. 1991;53:333–339. doi: 10.1292/jvms.53.333. [DOI] [PubMed] [Google Scholar]
- Tellier M.C., Soos J.M., Pu R., Pollock D., Yamamoto J.K. Development of FIV-specific cytolytic T-lymphocyte responses in cats upon immunization with FIV vaccines. Vet. Microbiol. 1997;57:1–11. doi: 10.1016/s0378-1135(97)00081-3. [DOI] [PubMed] [Google Scholar]
- Tellier M.C., Pu R., Pollock D., Vitsky A., Tartaglia J., Paoletti E., Yamamoto J.K. Efficacy evaluation of prime-boost protocol: canarypoxvirus-based feline immunodeficiency virus (FIV) vaccine and inactivated FIV-infected cell vaccine against heterologous FIV challenge in cats. AIDS. 1998;12:11–18. doi: 10.1097/00002030-199801000-00002. [DOI] [PubMed] [Google Scholar]
- Terry A., Callanan J.J., Fulton R., Jarrett O., Neil J.C. Molecular analysis of tumors from feline immunodeficiency virus (FIV)-infected cats: an indirect role for FIV? Int. J. Cancer. 1995;61:227–232. doi: 10.1002/ijc.2910610215. [DOI] [PubMed] [Google Scholar]
- Torten M., Franchini M., Barlough J.E., George J.W., Mozes E., Lutz J., Pedersen N.C. Progressive immune dysfunction in cats experimentally infected with feline immunodeficiency virus. J. Virol. 1991;65:2225–2230. doi: 10.1128/jvi.65.5.2225-2230.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- USDA, 2001. Center for Veterinary Biologics. In: UDSA Veterinary Biological Products: Licensee and Permittees, June 2001. USDA, IA (http://www.aphis.usda.gov/vs/cvb/lpd).
- USDA, 2002. USDA issues license for feline immunodeficiency virus vaccine for cats. Press release March 2002 (http://www.aphis.usda.gov/lpa/press/2002/03/catvac.txt).
- Vahlenkamp T.W., de Ronde A., Horzinek M.C., Egberink H.F. Quantification of feline immunodeficiency virus (FIV) RNA in the plasma of infected cats. Berlin Munch. Tierarztl. Wochenschr. 1996;109:265–269. [PubMed] [Google Scholar]
- van Kampen K.R. Recombinant vaccine technology in veterinary medicine. Vet. Clin. North Am. Small Anim. Pract. 2001;31:535–538. doi: 10.1016/s0195-5616(01)50607-5. [DOI] [PubMed] [Google Scholar]
- Venugopal K. Marek’s disease: an update on oncogenic mechanisms and control. Res. Vet. Sci. 2000;69:17–23. doi: 10.1053/rvsc.2000.0396. [DOI] [PubMed] [Google Scholar]
- Wyand M.S., Manson K.H., Garcia-Moll M., Montefiori D.C., Desrosiers R.C. Vaccine protection by a triple deletion mutant of simian immunodeficiency virus. J. Virol. 1996;70:3724–3733. doi: 10.1128/jvi.70.6.3724-3733.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi J., Vallari A.S., Swanson P., Bodelle P., Kaptue L., Ngansop C., Zekeng L., Gurtler L.G., Devare S.G., Brennan C.A. Evaluation of HIV type 1 group O isolates: identification of five phylogenetic clusters. AIDS Res. Hum. Retrov. 2002;18:269–282. doi: 10.1089/088922202753472847. [DOI] [PubMed] [Google Scholar]
- Yamamoto, J.K., 1999. Feline immunodeficiency virus (Retroviridae). In: Granoff, A., Webster, R.G. (Eds.), Encyclopedia of Virology, 2nd ed. Academic Press, San Diego, pp. 535–541.
- Yamamoto J.K., Hansen H., Ho E.W., Morishita T.Y., Okuda T., Sawa T.R., Nakamura R.M., Pedersen N.C. Epidemiologic and clinical aspects of feline immunodeficiency virus (FIV) infection in cats from the continental United States and Canada and possible mode of transmission. J. Am. Vet. Med. Assoc. 1989;194:213–220. [PubMed] [Google Scholar]
- Yamamoto J.K., Hohdatsu T., Olmsted R.A., Pu R., Louie H., Zochlinski H., Acevedo V., Johnson H.M., Soulds G.A., Gardner M.B. Experimental vaccine protection against homologous and heterologous strains of feline immunodeficiency virus. J. Virol. 1993;67:601–605. doi: 10.1128/jvi.67.1.601-605.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto, J.K., Mison, M., Elyar, J., Tellier, M., Pu, R., 1996. Efficacy evaluation of conventional dual-subtype FIV vaccine. In: Proceedings of the IX International Conference on AIDS, Vancouver, Canada, July 6–10, 1996 (oral presentation by M. Tellier). M. Tellier received Young Investigator Award for this abstract in the IX International Conference on AIDS.
- Yamanouchi K., Barrett T., Kai C. New approaches to the development of virus vaccines for veterinary use. Rev. Sci. Tech. 1998;17:641–653. doi: 10.20506/rst.17.3.1125. [DOI] [PubMed] [Google Scholar]
- Zou W., Lackner A.A., Simon M., Durand-Gasselin I., Galanaud P., Desrosiers R.C., Emilie D. Early cytokine and chemokine gene expression in lymph nodes of macaques infected with simian immunodeficiency virus is predictive of disease outcome and vaccine efficacy. J. Virol. 1997;71:1227–1236. doi: 10.1128/jvi.71.2.1227-1236.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]


