Abstract
A new assay was developed for rapid and antemortem diagnosis of canine distemper (CD). This immunochromatography (IC)-based assay, which employs two monoclonal anti-CDV antibodies, was compared with nested PCR. When serial dilutions of purified CDV were tested, the CDV detection limits of the nested PCR and IC assays were 2 × 102 TCID50/ml and 5 × 102 TCID50/ml, respectively. Nasal irrigation fluid, conjunctival swabs, and blood lymphocytes from 66 dogs suspected to have CD were tested. Preliminary IC experiments revealed that the optimal sample volume and reaction time were 100 μl and 5 min, respectively. Relative to nested PCR, the sensitivity and specificity of the IC assay was maximal (100% and 100%, respectively) when conjunctival swabs were tested. This is significant because conjunctival swab specimens are easy to obtain in the early phase of CD infection. However, with blood lymphocytes and nasal samples, the IC assay was slightly less sensitive (89.7% and 85.7%, respectively) and specific (94.6% and 100%, respectively) than nested PCR. Since this novel IC assay does not require special instruments, it is a simple enough for dog owners to use. Since early detection of CD would allow appropriate treatment and quarantine to be instituted quickly, such a test would help reduce the morbidity and mortality associated with CD help to prevent its spread to other animals.
Keywords: CDV, Immunochromatography, PCR
1. Introduction
Canine distemper virus (CDV) is a member of the genus Morbillivirus of the Paramyxoviridae and is related closely to the viruses responsible for measles, rinderpest, peste des petits ruminants, phocine distemper, dolphin distemper, porpoise distemper, and equine morbillivirus (Fenner, 1976, Haas and Barrett, 1996, Osterhaus et al., 1995). Canine distemper (CD) is a highly contagious disease that affects dogs of all ages. It is associated with high morbidity and mortality and occurs worldwide. Dogs that have been infected naturally with CD show commonly systemic signs of the disease such as vomiting, diarrhoea and/or respiratory signs; sometimes they also exhibit generalized or localized myoclonus and the characteristic clinical symptoms of CDV encephalomyelitis (Frisk et al., 1999, Moritz et al., 2000, Okita et al., 1997). In the recent outbreaks of CD in Korea, the affected dogs could be classified clinically into two groups, namely, young dogs with CNS signs associated with respiratory and/or gastrointestinal symptoms, and old dogs with CNS signs alone. Thus, the clinical signs of CD vary depending on the age and immune status of the host as well as the virulence of the virus strain and the environmental conditions (Greene and Appel, 1990). Acutely infected dogs shed the virus in all bodily secretions regardless of whether they are symptomatic or not. Although the virus cannot be isolated from the bodily secretions of dogs with subacute distemper encephalomyelitis and persistent viral infection of the CNS, they may still transmit the virus (Appel, 1987).
To provide timely medical treatment that preserves the life of the infected dog (especially if neurological signs are present), it is important to have available an easy assay that can detect rapidly and accurately CDV. Such an assay would also help to exclude other diagnoses and prevent the further transmission of the disease. At present, CD can be diagnosed by virus isolation, immunofluorescence assays (IFA), RT-PCR, and real-time PCR (Frisk et al., 1999, Hoyland et al., 2003, Jozwik and Frymus, 2005, Shin et al., 2004), all of which are relatively time-consuming and laborious techniques. The ImmunoComb antibody test kit (Biogal Galed Labs, Israel) for CD is also used frequently in animal hospitals to decide when to vaccinate a pet, as it reveals the anti-CDV IgM and IgG antibody levels. However, to use this test to determine whether a dog is infected with CDV, the animal has to be tested twice over several days. This delay in diagnosis is a serious limitation of this test.
A new immunochromatography (IC)-based assay was developed for the antemortem diagnosis of CD. The sensitivity and specificity of this assay were compared with a nested PCR assay by testing conjunctival swabs, nasal irrigation fluid, and blood lymphocyte samples from dogs suspected to have CD.
2. Materials and methods
2.1. Field specimens and purified virus
In total, 158 specimens (53 nasal irrigation fluids, 39 conjunctival swab and 66 blood lymphocyte samples) were obtained from 66 dogs suspected of infection with CD. These samples were provided by nine private animal hospitals in Seoul from May 2005 to April 2006. All three samples (nasal, conjunctival and blood) were obtained from each of 38 dogs at the time they presented at the animal hospital. Of the remaining dogs, 16 provided blood and either nasal irrigation fluids or conjunctival swab samples, and 12 provided only blood. The conjunctival swabs and nasal irrigation fluids were each mixed with 1 ml PBS (phosphate-buffered saline, pH 7.4) while blood lymphocytes were separated by using a LeucoSep™ tube (Greiner, Cat. No. 163290, Austria). All of the samples were stored at −20 °C until use. The dogs suspected to have CD were Maltese dogs (n = 20), Yorkshire Terriers (n = 13), Shih Tzu (n = 13), Korea Jindo dogs (n = 6), Poodles (n = 5), American Cocker Spaniels (n = 4), Pugs (n = 3), and Miniature Schnauzers (n = 2). Most were young dogs who demonstrated mainly acute and systemic clinical signs. However, six dogs which were over 2 years of age showed nervous symptoms. The Choongang Vaccine Laboratory (Daedeok valley, Daejeon, Korea) kindly provided us with the Rockborn strain of CDV, canine coronavirus (CCV), canine parainfluenza viurs type 2 (CPIV-2) and rabies virus.
2.2. RT-PCR and nested PCR
Total RNA was extracted from the nasal irrigation fluid, conjunctival swab and blood lymphocyte samples by using the microcolumn technique-based QIAamp viral RNA Mini kit (Qiagen, Cat. No. 52906, USA). The RNA was then used in a reverse transcription reaction at 42 °C for 30 min and 94 °C for 15 min using a Perkin-Elmer Gene Amp PCR system 9600. The cDNA was amplified by using a one-step RT-PCR kit (Qiagen, Cat. No. 210212, USA) employing primers based on the conserved NP (nucleoprotein) regions of the CDV genome sequence that has been deposited in GeneBank (accession no. AJ009656) (Table 1 ). The PCR consisted of 35 cycles at 94 °C for 30 s, 63 °C for 30 s, and 72 °C for 30 s. Nested PCR was carried out in 20 μl volumes containing 1 μl of the RT-PCR product, 1× PCR buffer, 0.2 mM dNTPs, 2.5 U of Taq polymerase (TaKaRa EX Taq, Takara Bio, Inc.), and 0.2 μM of each nested sense and anti-sense primer (Table 1). Amplification consisted of 20 cycles of the same temperature profile used with the RT-PCR. The RT-PCR and nested PCR products were checked by electrophoresis on a 1.2% agarose gel and were of the expected sizes (433 and 223 bp, respectively).
Table 1.
Sequence and position of oligonucleotide primers used in RT-PCR and nested PCR
| Primer | Sequence 5′–3′ | Sense | Positiona |
|---|---|---|---|
| CDFb | GGATTCTGAGGCAGATGAGTTCTTC | + | 369–394 |
| CDRb | AGCTAATCCAGCTTCTACAATGTAGT | − | 776–801 |
| CDNFc | GTGACTGCTCCTGATACTGCAG | + | 544–565 |
| CDNRc | CACAAATCATTTCAGCAATTCTAGGCT | − | 740–766 |
The oligonucleotide position is based on the sequence of the accession no. AJ009656 CDV strain.
RT-PCR.
Nested PCR.
2.3. Monoclonal antibody preparation
Hybridomas that produce mouse anti-CDV monoclonal antibodies (Mabs) were generated as described by Coyle et al. (1992). Briefly, the popliteal lymph node cells of BALB/c mice (female, 6–8 weeks) that had been immunized in the footpad with purified CDV were fused with SP 2/0 myeloma cells. Hybridomas that produced CDV-specific Mabs in the screening test were selected and subcloned three times from single cells by limiting dilution. Murine ascites fluid was produced in BALB/c mice and IgG was isolated by affinity chromatography using HiTrap Protein G HP column (GE Healthcare, Cat. No. 17-0404-1, USA). Western blotting was performed as described previously to confirm the specificity of the Mabs (Vihinen-Ranta et al., 1996). The cloned Mabs were subtyped by using a Mouse MonoBb ID kit (Zymed Laboratory, Cat. No. 90-6550, USA). The two selected Mabs were designated as CDV-Mab 9D3 and 7B2.
2.4. Conjugation with colloidal gold
Anti-CDV-Mab 9D3 was dialyzed against 10 mM carbonate buffer (pH 9.0) for 1 h at 4 °C. The pH of the 0.01% (w/v) colloidal gold solution was adjusted to 9.0 with 0.1 M NaOH and 0.08 mg/ml of anti-CDV-Mab 9D3 was added. The mixture was gently mixed for 30 min, blocked with 1% bovine serum albumin for 15 min, and centrifuged at 30,000 × g for 20 min. The gold colloid conjugate pellets were suspended in 10 mM phosphate buffer (pH 7.2) containing 0.5% casein and the optical density was adjusted to 5 at 520 nm with 10 mM phosphate buffer (pH 7.2). The colloidal gold conjugate was stored at 4 °C until use.
2.5. Principle of the IC assay
The test strip was assembled with sample pad, conjugate pad, nitrocellulose membrane, and adsorption pad (cellulose paper). If a specimen containing CDV antigen is added to the sample pad, the gold conjugate migrates from the pad onto the cellulose membrane by capillary action. As the specimen and gold conjugate flow along the cellulose membrane, the CDV antigen in the specimen becomes sandwiched between the two antibodies, namely, the gold-conjugated anti-CDV-Mab 9D3 and the anti-CDV-Mab 7B2. A signal is then detected on the nitrocellulose membrane (the test line). The control line is covered with 1 mg/ml goat anti-mouse IgG while the test line is covered with 1 mg/ml CDV-Mab 7B2.
2.6. Calculation of diagnostic sensitivity and specificity
The specimens were classified as true positive or true negative by the gold standard method, namely, the nested PCR, and the IC assay results were classified as false positive or false negative if they disagreed with the nested PCR results. The diagnostic sensitivity was calculated as true positive/(true positive + false negative) while diagnostic specificity was calculated as true negative/(true negative + false positive). The results of both calculations were usually expressed as percentages.
3. Results
3.1. Monoclonal antibody production
Several hybridoma cell lines that were found during screening to express an anti-CDV-Mab were cloned by limiting dilution. Two clones were then selected, namely, CDV-Mab 9D3 and 7B2. Both produced IgG1 isotype antibodies that were confirmed to be different clones by using the antigen and antibody complex test that the CDV antigen becomes sandwiched between the gold-conjugated anti-CDV-Mab 9D3 and the anti-CDV-Mab 7B2 on the capture line (data not shown). Western blotting revealed that both purified monoclonal antibodies bound a 40 kDa CDV F1 (fusion) protein (Fig. 1 ).
Fig. 1.
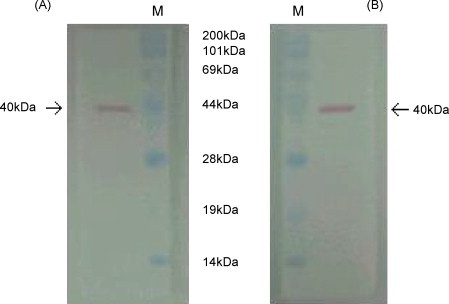
Western blotting analysis with CDV-Mab 9D3 (A) and 7B2 (B). The purified Rockborn strain of CDV was separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, transferred to nitrocellulose, and probed with the CDV-Mabs. Both Mabs bound to the 40 kDa F1 protein of CPV (M: molecular markers).
3.2. Measurement of the CDV detection limit of the IC assay by using purified CDV
A nested PCR assay served as the “gold standard” against which the IC assay was compared. The specificity of the nested PCR assay was first tested by using purified CCV, CPIV-2, or rabies virus as the RNA substrate. The nested PCR assay discriminated accurately CDV from all of these viruses (data not shown). The IC assay showed the same results (data not shown).
The CDV detection limits of the IC and nested PCR assays were tested by using the Rockborn CDV strain and diluting it serially 10-fold. The CDV detection limits of the nested PCR and IC assays were 2 × 102 TCID50/ml and 5 × 102 TCID50/ml, respectively.
3.3. Measurement of the sensitivity and specificity of the IC assay by using nasal irrigation fluid, conjunctival swab, and blood lymphocyte samples from dogs suspected to have CD
In total, 158 nasal, conjunctival swab and serum samples obtained from 66 dogs suspected to have CD were tested. Preliminary assays using 50 μl, 100 μl, 150 μl, and 200 μl volumes of 27 of the dog samples revealed that the best results were observed when 100 μl was used. When 100 μl was used, the optimal reaction time was on average 5 min (Fig. 2 ). These optimal conditions did not vary depending on whether the sample was a nasal, tear or blood sample. These parameters were then used when testing the remaining dog samples. Of the 158 samples, the IC assay yielded incorrect results with eight: six false negatives (three nasal irrigation and three blood samples) and two false positives (two blood samples) (Table 2 ).
Fig. 2.
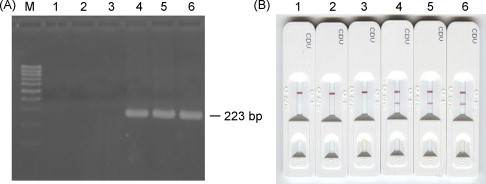
Detection of the CDV NP gene in nasal irrigation, conjunctival swab, and blood lymphocyte samples by nested PCR (A) and the IC assay (B). Lane M: 100 bp DNA ladder; lanes 1 and 4: nasal irrigation fluid samples; lanes 2 and 5: conjunctival swab samples; lanes 3 and 6: blood lymphocyte samples. Lanes 1–3: negative samples; lanes 4–6: positive samples.
Table 2.
Clinical signs, age and breed of CD-positive specimens
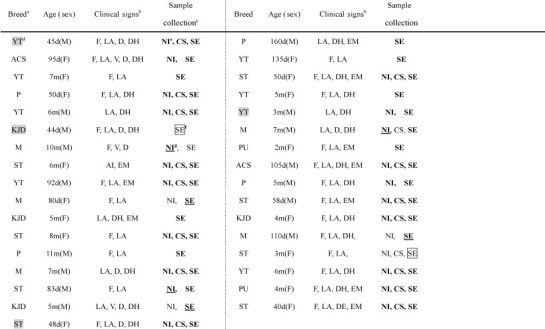
aP: Poodle; ST: Shih Tzu; ACS: American Cocker Spaniel; M: Maltese; KJD: Korea Jindo Dog; PU: Pug; YT: Yorkshire Terrier; MS: Miniature Schnauzer. bF: fever; LA: lack of appetite; V: vomiting; D: diarrhea; DH: dehydration; EM: emaciation. cBL: blood lymphocytes; NI: nasal irrigation fluid; CS: conjunctival swab. dPositives within 5 days post-vaccination (5 DPV) are shaded. ePositives according to nested PCR are bolded. fFalse positives according to IC are boxed. gFalse negatives according to IC are underlined.
Compared with the nested PCR assay, the overall sensitivity and specificity of the IC assay for all 158 specimens was 90.8% (59/65) and 97.8% (91/93), respectively. The sensitivity and specificity of the test was highest when conjunctival swab samples were tested (both 100%). High sensitivity and specificity was also observed when nasal irrigation samples (85.7% and 100%, respectively) and blood lymphocyte samples (89.7% and 94.6%, respectively) were tested (Table 3 ).
Table 3.
Analysis of the sensitivity and specificity of the IC assay relative to nested PCR results in detecting CDV in specimens from dogs suspected to have CDa
| IC assay result | No. of samples that are positive or negative upon nested PCR |
Total | |||||
|---|---|---|---|---|---|---|---|
| Nasal irrigation fluid (n = 53) |
Conjunctival swab (n = 39) |
Blood lymphocytes (n = 66) |
|||||
| + | − | + | − | + | − | ||
| + | 18 | 0 | 15 | 0 | 26 | 2 | 61 |
| − | 3 | 32 | 0 | 24 | 3 | 35 | 97 |
| Total | 21 | 32 | 15 | 24 | 29 | 37 | 158 |
The sensitivity and specificity of the IC assay relative to nested PCR results were, respectively, 85.7% (18/21) and 100% (32/32) for nasal irrigation fluid samples, 100% (15/15) and 100% (24/24) for conjunctival swab samples, and 89.7% (26/29) and 94.6% (35/37) for blood lymphocyte samples.
4. Discussion
The antemortem diagnosis of canine distemper is based on the demonstration of viral antigens in scrapings and body fluids such as conjunctival and vaginal smears, tracheal washings and urine sediment (Tipold et al., 1992). Various techniques have been used for this, including virus isolation, IFA, RT-PCR, and real-time PCR (Frisk et al., 1999, Hoyland et al., 2003, Jozwik and Frymus, 2005, Shin et al., 2004). However, the direct IFA gives false negative results in the subacute or chronic form of the disease (Jozwik and Frymus, 2005), and the other methods tend to be labor- and time-consuming. The Biogal's ImmunoComb antibody test kit, which measures the anti-CDV IgM or IgG levels in the dog and is usually used to determine when to vaccinate, can be used as a subsidiary measure to deduce CD infection. However, with this method, the same dog has to be re-tested after several days to determine whether the antibody levels are increasing, which reflects an ongoing immune response and probable infection with CDV. This delay in diagnosis is a serious limitation of this method.
A number of studies have examined which sample types are the most appropriate for use with PCR-based methods to detect CDV. One study using nested PCR analysis revealed that of 22 blood, 20 urine, 25 saliva, and 27 nasal swab samples from dogs suspected to have CD, 81.8%, 75%, 56%, and 70.3% tested positive, respectively (Shin et al., 2004). A similar study using nested PCR that detected CDV NP RNA revealed that of 29 serum, 16 whole blood, and 16 cerebrospinal fluid (CSF) samples, 86%, 88%, and 88% were positive, respectively (Frisk et al., 1999, Moritz et al., 2000). In contrast, hospitalized dogs with neurological disturbances but lacking the typical findings of distemper were tested, RT-PCR analysis failed to detect CDV RNA in any of the serum samples while only 1/5 (20%) whole blood and 2/5 (40%) CSF samples were positive. However, 4/5 (80%) urine and all 5 (100%) CSF samples were positive (Amude et al., 2006). Another study used RT-PCR to compare the various bodily secretions for the early detection of CDV in experimentally infected dogs (Kim et al., 2006). The conjunctival swab was found to be the most suitable specimen for early diagnosis of CD, as CDV amplicons were detected in the conjunctival swabs from all seven dogs at days 3–14 post-infection (Kim et al., 2006).
Compared to nested PCR in this study, the IC assay showed the highest sensitivity and specificity when conjunctival swabs were tested (both 100%). Therefore, conjunctival swab specimens are the most suitable specimens for early antemortem diagnosis of CD, probably because there is persistent shedding of CDV in the eye, unlike in other bodily compartments. Significantly, conjunctival swabs are easier to obtain than the other specimens. However, conjunctival swabs have to be collected in the early phase of infection (Kim et al., 2006).
The eight samples that yielded disparate results with nested PCR and the IC test (two false positives and six false negatives) were inoculated into 7-day-old eggs to determine whether they were truly positive or negative. The six false negative samples, but neither of the false positive samples, induced pocks on the chorioallantoic membrane (CAM) 7 days after inoculation. These pocks were found by RT-PCR and nucleotide sequence analysis to contain CDV genes. Thus, the overall sensitivity and specificity of the IC assay were 9.2% and 2.2% lower than those of the nested PCR, respectively. It is possible that vaccination may lead to a false positive if testing occurs within a few days after vaccination. Indeed, one of the false positives results identified by the IC assay (which was then found to be a true negative) was a Korea Jindo dog that had been tested 4 days post-vaccination. Notably, of the other dogs that were tested shortly after vaccination (two Yorkshire Terriers and one Shih Tzu were tested 3, 5, and 3 days post-vaccination, respectively), all were true positives as determined by both the IC and nested RT-PCR assays. Hence, it was not clear whether these animals were really infected or whether both assays have detected the vaccine strain (Table 2).
The IC assay has the advantage over many other field diagnostic techniques used in clinical practice in that the test procedure is simple, rapid, and can be performed by owners as well as veterinarians. This is particularly relevant since animals suspected to have an infectious disease are generally not hospitalized immediately. Due to these advantages, the IC assays detecting specific antibodies are available for other veterinary diseases, including CPV (Oh et al., 2006). An IC test for simultaneous detection of specific antibodies against Babesia caballi and Babesia equi in the horse has also been developed by using recombinant B. caballi 48 kDa rhoptry protein and recombinant truncated B. equi merozoite antigen 2 (Huang et al., 2006). However, the IC assays can be limited in terms of the amount of antigen they can detect, as large amounts of viral antigen are required to produce a clearly visible band. As a result, the interpretation of results may be affected by the subjectivity of the test operator. However, the IC assay developed for CDV is as good as nested RT-PCR when conjunctival swab samples are tested.
In conclusion, even though the IC assay is slightly less sensitive and specific than nested PCR, it could be a useful aid for early diagnosis of CDV in infected dogs, especially when conjunctival swabs are used. This assay has the advantage over other techniques in that it can be completed within 5 min without the need for special instruments. The ready availability of this assay to dog owners as well as veterinarians could help to reduce the morbidity and mortality of the disease as it would allow appropriate treatment to be instituted before full symptoms become evident.
Acknowledgement
This research was supported in part by the Joint HBI Corporation, Anyang, Kyunggi-do.
References
- Amude A.M., Alfieri A.A., Alfieri A.F. Antemortem diagnosis of CDV infection by RT-PCR in distemper dogs with neurological deficits without the typical clinical presentation. Vet. Rec. Commun. 2006;30:679–687. doi: 10.1007/s11259-006-3308-2. [DOI] [PubMed] [Google Scholar]
- Appel M.J. Canine distemper virus. In: Appel M.J., editor. Virus Infections of Carnivores. Elsevier Science Publishers B.V.; Amsterdam, The Netherlands: 1987. pp. 133–159. [Google Scholar]
- Coyle P.V., Wyatt D., McCaughey C., O’Neill H.J. A sample standardised protocol for the production of monoclonal antibodies against viral and bacterial antigens. J. Immunol. Methods. 1992;153:81–84. doi: 10.1016/0022-1759(92)90308-g. [DOI] [PubMed] [Google Scholar]
- Fenner F. Paramyxoviridae. Intervirology. 1976;7:59–60. [Google Scholar]
- Frisk A.L., König M., Moritz A., Baumgärtner W. Detection of canine distemper virus nucleoprotein RNA by reverse transcription-PCR using serum, whole blood, and cerebrospinal fluid from dogs with distemper. J. Clin. Microbiol. 1999;37:3634–3643. doi: 10.1128/jcm.37.11.3634-3643.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene C.E., Appel M.J. Infectious diseases of the dog and cat. In: Greene C.E., editor. Canine Distemper. 2nd ed. WB Saunders; Philadelphia, PA: 1990. [Google Scholar]
- Haas L., Barrett T. Rinderpest and other animal morbillivirus infections: comparative aspects and recent developments. J. Vet. Med. 1996;43:411–420. doi: 10.1111/j.1439-0450.1996.tb00333.x. [DOI] [PubMed] [Google Scholar]
- Hoyland J.A., Dixon J.A., Berry J.L., Davies M., Selby P.L., Mee A.P. A comparison of in situ hybridisation, reverse transcriptase-polymerase chain reaction (RT-PCR) and in situ-RT-PCR for the detection of canine distemper virus RNA in Paget's disease. J. Virol. Methods. 2003;109:253–259. doi: 10.1016/s0166-0934(03)00079-x. [DOI] [PubMed] [Google Scholar]
- Huang X., Xuan X., Verdida R.A., Zhang S., Yokoyama N., Xu L., Igarashi I. Immunochromatographic test for simultaneous serodiagnosis of Babesia caballi and B. equi infections in horses. Clin. Vaccine Immunol. 2006;13:553–555. doi: 10.1128/CVI.13.5.553-555.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jozwik A., Frymus T. Comparison of the immunofluorescence assay with RT-PCR and nested PCR in the diagnosis of canine distemper. Vet. Res. Commun. 2005;29:347–359. doi: 10.1023/b:verc.0000048528.76429.8b. [DOI] [PubMed] [Google Scholar]
- Kim D., Jeoung S.Y., Ahn S.J., Lee J.H., Pak S.I., Kwon H.M. Comparison of tissue and fluid samples for the early detection of canine distemper virus in experimentally infected dogs. J. Vet. Med. Sci. 2006;68:877–879. doi: 10.1292/jvms.68.877. [DOI] [PubMed] [Google Scholar]
- Moritz A., Frisk A.L., Baumgartner W. The evaluation of diagnostic procedures for the detection of canine distemper virus infection. Eur. J. Companion Anim. Pract. 2000;10:37–47. [Google Scholar]
- Oh J.S., Ha G.W., Cho Y.S., Kim M.J., An D.J., Hwang K.K., Lim Y.K., Park B.K., Kang B., Song D.S. One-step immunochromatography assay kit for detecting antibodies to canine parvovirus. Clin. Vaccine Immunol. 2006;13:520–524. doi: 10.1128/CVI.13.4.520-524.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okita M., Yanai T., Ochikubo F., Gemma T., Mori T., Maseki T., Yamanouchi K., Mikami T., Kai C. Histopathological features of canine distemper recently observed in Japan. J. Comp. Pathol. 1997;116:403–408. doi: 10.1016/s0021-9975(97)80057-6. [DOI] [PubMed] [Google Scholar]
- Osterhaus A.D.M.E., de Swart R.L., Vos H.W., Ross P.S., Kenter M.J.H., Barrett T. Morbillivirus infections of aquatic mammals: newly identified members of the genus. Vet. Microbiol. 1995;44:219–227. doi: 10.1016/0378-1135(95)00015-3. [DOI] [PubMed] [Google Scholar]
- Shin Y.J., Cho K.O., Cho H.S., Kang S.K., Kim H.J., Kim Y.H., Park H.S., Park N.Y. Comparison of one-step RT-PCR and a nested PCR for the detection of canine distemper virus in clinical samples. Aust. Vet. J. 2004;82:83–86. doi: 10.1111/j.1751-0813.2004.tb14651.x. [DOI] [PubMed] [Google Scholar]
- Tipold A., Vandevelde M., Jaggy A. Neurological manifestation of canine distemper virus infection. J. Small Anim. Pract. 1992;33:466–470. [Google Scholar]
- Vihinen-Ranta M., Lindfors E., Heiska L., Veijalainen P., Vuento M. Detection of canine parvovirus antigens with antibodies to synthetic peptides. Arch. Virol. 1996;141:1741–1748. doi: 10.1007/BF01718296. [DOI] [PubMed] [Google Scholar]


