Abstract
A focus formation assay (FFA) for detection and titration of porcine epidemic diarrhea virus (PEDV) in a micro-culture system using Vero cells and PAP staining technique was evaluated. A linear correlation between the virus dilution and virus titer determined by FFA was observed between the range of 10 and 30 foci per well. Comparative analysis between FFA and plaque assay showed no significant difference in estimating the titer of cell adapted PEDV. However, the culture time required for detecting the virus was considerably shorter for FFA. In addition, FFA had higher sensitivity for detecting field isolates of PEDV as well as positive identification of the virus with the antibody specific reaction. A broader range of dilutions and number of replicates may be used for titration. A FFA may be applied as an alternative method for detection and titration of PEDV.
Keywords: Porcine epidemic diarrhea virus, Focus formation assay, Plaque assay, Peroxidase–antiperoxidase staining
1. Introduction
Porcine epidemic diarrhea virus (PEDV) is a member of the family Coronaviridae and is closely related with Human coronavirus 229E, Transmissible gastroenteritis virus and Feline infectious peritonitis virus (Bridgen et al., 1993, Yaling et al., 1988). This virus causes porcine epidemic diarrhea, an enteric disease characterized by acute watery diarrhea and dehydration (DeBouck and Pensaert, 1980). The clinical and pathological symptoms are similar to transmissible gastroenteritis, making it difficult to differentiate between the two infections (Pospischil et al., 1981, Pritchard et al., 1999).
Current diagnostic procedures for confirming PEDV infection is based mostly on viral detection by immunohistochemistry, in situ hybridization, reverse transcription-polymerase chain reaction (RT-PCR) and dot-blot hybridization (Jung et al., 2003, Jung and Chae, 2005, Kim and Chae, 1999, Kim and Chae, 2000, Kim and Chae, 2002). Isolation and in vitro culture of PEDV using stable cell lines is difficult, and only a few strains have been cultured successfully in cell lines (Hofmann and Wyler, 1988, Kadoi et al., 2002, Kweon et al., 1999). Among the diagnostic methods used, the RT-PCR method is an easy, rapid, specific, and sensitive test for the detection of PEDV in Vero cells and from fecal samples and homogenized intestinal tissues (Kim and Chae, 2002, Song et al., 2006). However, RT-PCR only confirms the presence of the viral genome, and not necessarily the presence of infectious virions. Hence, it may not provide sufficient information for determining the presence of infectious virions. At present, plaque assay and immunofluorescence antibody tests have been used for measuring virus infectivity of PEDV in vitro (Hofmann and Wyler, 1988).
An alternative method that has not been considered yet for PEDV is the focus formation assay (FFA) using the peroxidase–antiperoxidase staining method. Estimating virus titer by FFA has been reported for several types of viruses like Mumps, Japanese encephalitis, Dengue, Chikungunya, Renal syndrome and Influenza and (Okuno et al., 1977, Okuno et al., 1985a, Okuno et al., 1985b, Okuno et al., 1990, Raharjo et al., 1986, Tanishita et al., 1984).
In this study, the use of the focus formation assay for determining the titer of KPEDV-9 and for detection of PEDV in specimens collected in the field was evaluated. The results were compared with an infectivity assay using the plaque assay.
2. Materials and methods
2.1. Cells and virus
KPEDV-9, a cell-adapted strain of PEDV from the Korean National Veterinary Research and Quarantine Services was cultured in African green monkey kidney cells (Vero, CCL-81) as described previously (Hofmann and Wyler, 1988). Briefly, KPEDV-9 was inoculated onto Vero cells grown to confluence with minimum essential medium (MEM, Gibco BRL) containing 5% fetal bovine serum (FBS, Hyclone) in a 15 cm diameter TC dish (SPL Life Science) then maintained in virus medium (MEM with 10 μg/ml trypsin) at 37 °C for 24 h. Culture medium was collected and centrifuged at 10,000 × g for 10 min to remove cell debris while infected cells were harvested by scraping and placed in 1 ml 100 mM NaCl, 10 mM Tris–Cl, 1 mM EDTA (STE buffer, pH 7.4). Progeny virions trapped in intracellular vesicles were released by repeated freezing and thawing and recovered into the supernatant after centrifugation at 10,000 × g for 10 min. All virus suspensions were kept at −80 °C prior to use.
2.2. Focus formation assay (FFA)
KPEDV-9 stock virus was examined by focus formation assay (FFA) following the peroxidase–antiperoxidase (PAP) method described by Okuno et al., 1985a, Okuno et al., 1985b, with some modifications. Vero cells were grown to confluence with MEM containing 5% FBS in a 96-well TC plate at 37 °C, 5% CO2 for 24 h. After washing twice with 10 mM phosphate buffered saline (PBS, pH 7.4), twofold serially diluted virus suspension was inoculated in quadruplicate wells and kept at 37 °C, 5% CO2 for 2 h. Inoculum was removed carefully and the cell monolayer was overlaid with virus medium containing 0.5% methyl cellulose (Sigma–Aldrich) then placed at 37 °C, 5% CO2 between 8 and 48 h. The overlay medium was removed carefully and cell monolayer was fixed with 5% formaldehyde in PBS for 20 min then washed three times with PBS. The cells were permeated with 1% Nonidet-P 40 (NP-40) in PBS for 20 min, washed three times with PBS then blocked with 4% skim milk (Difco) in PBS. Infected cells were detected by probing with mouse anti-PEDV polyclonal antisera (1:4000) at room temperature for 1 h, followed by biotinylated rabbit anti-mouse IgG (1:1,000, Vector Lab) at room temperature for 1 h, and strepavidin-biotinylated horseradish peroxidase (Vector Lab) for 30 min. Each step was preceded by washing the wells three times with PBS. Finally, the cell monolayer was incubated with 0.5 mg/ml 3,3′-diaminobenzidine tetrahydrochloride dihydrate containing NiCl and H2O2 in PBS (DAB solution, Vector Lab) for 10 min at room temperature. The reaction was stopped by removing the DAB solution and rinsing with deionized water. Clusters of infected cells (focus) stained dark-gray were counted under an inverted microscope (Zeiss) and reported as focus forming units (ffu).
2.3. Plaque assay
KPEDV-9 stock virus was titrated by plaque assay as described elsewhere (Vautherot, 1981). Tenfold dilution of the stock virus was inoculated in 35 mm TC dishes (SPL Life Science) containing confluent monolayer of Vero cells. After 2 h, the inoculum was removed and the cell monolayer was overlaid with virus medium containing 1% agarose (SeaKem) and kept at 37 °C, 5% CO2 for 24 h. The culture was then overlaid with virus medium containing 0.01% neutral red and 1% agarose, and kept at 37 °C, 5% CO2 for another 24–48 h while monitoring the appearance of plaques. The cell monolayer was fixed with 5% formaldehyde in PBS and stained permanently with 0.01% crystal violet solution (0.01% crystal violet, 2.5% ethanol in PBS).
2.4. FFA and plaque assay of field-collected specimens
Small intestines from five piglets (5–6-weeks-old) exhibiting clinical signs and symptoms of PEDV were collected from local farms in South Korea. Portions of the jejunum and ileum were homogenized in sterile PBS (1.5 ml PBS per 1 g tissue). The homogenized tissues were centrifuged at 6000 × g and the supernatant was aliquoted and stored at −80 °C prior to use. FFA and plaque assay were carried out as stated above.
2.5. Statistical analysis
Pearson's correlation coefficient was used to determine the range of ffu count that provided the most accurate estimate virus titer. The paired t-test with an α ≤ 0.05 was used to compare virus titer obtained by FFA and plaque assay.
3. Results
3.1. Immunostaining of cells infected with PEDV
KPEDV-9 infection of Vero cells was stopped at 8, 10 12 or 24 h post inoculation (p.i.) by fixing with 5% formaldehyde then processed for FFA. Clusters of cells, or foci, infected with KPEDV-9 were colored dark gray in contrast with non-infected cells after reaction of the bound immune complex with the DAB solution. At 8 h p.i., the infected cells did not exhibit discernable CPE (Fig. 1A). However, by 10 h p.i., several adjacent cells were infected with PEDV as shown by immunostaining and early signs of cell-to-cell fusion began to manifest (Fig. 1B). By 12 h p.i., syncytium formation was observed among several foci (Fig. 1C). Within 24 h p.i., several syncytia have fused making it difficult to identify individual syncytium (Fig. 1D).
Fig. 1.
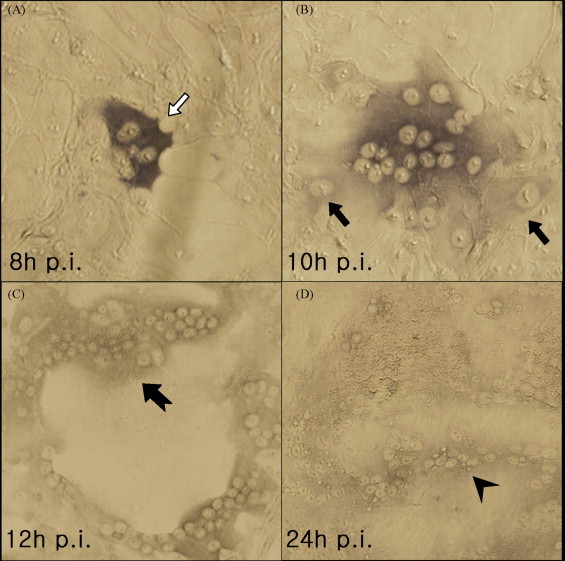
Localized areas of infection (focus) visualized by PAP staining technique under a light microscope. At 8 h p.i., individual cells infected with KPEDV-9 are shown having dark-gray stained cytoplasm ( ) due to reactivity of viral proteins deposited in the cytoplasm with anti-PEDV immune complex and DAB (A). At 10 h p.i., early signs of synyctium formation (multi-nucleated cells) and infection of adjacent cells (↑) are observed (B). At 12 h p.i., more than 50 nuclei (
) due to reactivity of viral proteins deposited in the cytoplasm with anti-PEDV immune complex and DAB (A). At 10 h p.i., early signs of synyctium formation (multi-nucleated cells) and infection of adjacent cells (↑) are observed (B). At 12 h p.i., more than 50 nuclei ( ) line up at the periphery of the syncytium (C). At 24 h p.i., there is nearly 100% cell-to-cell fusion through out the cell monolayer with the nuclei lined up in the middle of the syncytium (
) line up at the periphery of the syncytium (C). At 24 h p.i., there is nearly 100% cell-to-cell fusion through out the cell monolayer with the nuclei lined up in the middle of the syncytium (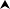 ) (D) (magnification: 40×).
) (D) (magnification: 40×).
3.2. Correlation of dilution and virus titer in FFA
Various dilutions of KPEDV-9 were used to determine the approximate virus titer by FFA. Based on the previous experiment, virus infection was arrested at 10 h p.i. and immunostained immediately using anti-PEDV polyclonal antisera and DAB. The number of stained clusters of cells, or foci, was used to calculate the titer of in focus forming units per milliliter of suspension (ffu/ml). As shown in Fig. 2 , KPEDV-9 suspension diluted 1:400 yielded 51.3 ± 6.2 ffu per well (or 100 μl virus suspension) while virus suspension diluted 1:12,800 yielded 2.4 ± 1.6 ffu per well. Using Pearson's correlation, virus dilutions which resulted with a foci count between 8.5 ± 1.9 and 28.0 ± 3.0 ffu showed the highest linear correlation (r = −0.902) and provided the most accurate representation of the virus titer.
Fig. 2.
Correlation between virus titer and virus dilution. Vero cells grown in 96-well tissue culture plates were infected with serially diluted KPEDV-9 and stained by PAP method 12 h p.i. (correlation coefficient, r = −0.902).
3.3. Titration of KPEDV-9 by FFA and plaque assay
After determining the range which rendered the highest correlation coefficient between dilution and titer of KPEDV-9 by FFA, a comparison with plaque assay was conducted. Four different stocks of KPEDV-9 were retrieved from −80 °C storage and tested by FFA and plaque assay in parallel infections. Table 1 shows the summary of virus titration using both methods. All four stocks had an approximate virus titer between 104 and 105 infectious units. Using the paired t-test, no significant difference between FFA and plaque assay was observed (α < 0.05).
Table 1.
Comparison of virus titer determined by plaque assay technique and focus forming assay (α ≤ 0.05)
| KPEDV-9a | pfu ml−1 | ffu ml−1 |
|---|---|---|
| Stock 1 | 1.68 × 105 | 1.71 × 105 |
| Stock 2 | 5.02 × 104 | 6.23 × 104 |
| Stock 3 | 4.13 × 104 | 8.50 × 104 |
| Stock 4 | 1.95 × 105 | 1.89 × 105 |
Different stocks of KPEDV-9. P-value = 1.220.
3.4. FFA versus plaque assay of cell adapted and PEDV specimens collected in the field
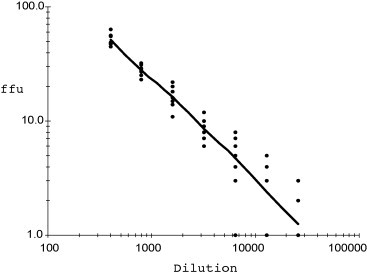
Positive infection of Vero cells by KPEDV-9, a cell adapted strain of PEDV, was confirmed by FFA at 8 h p.i. Within 48 h p.i., multiple overlapping syncytia were observed and made titration impossible (Fig. 3A). On the other hand, the appearance of virus plaques in the plaque assay setup was observed initially at 48 h p.i. Within 72 h p.i., several overlapping plaques were observed which made it difficult to determine accurately plaque count (Fig. 3B). Unlike the cell-adapted strain of KPEDV-9, primary isolates of PEDV do not cause cell-to-cell fusion of Vero cells (Hofmann and Wyler, 1988). This was also observed among the primary isolates of PEDV collected from local farms in South Korea. Fig. 3A shows the infectivity of five different primary isolates of PEDV. All 5 isolates (HS-01, HS-02, HS-05, PT-01 and IC-01) did not show typical CPE after infection of Vero cells even after 48 h p.i. Furthermore, adjacent cells were not stained, suggesting that the virus did not spread among surrounding cells. Similarly, plaque assay of the five primary isolates did not result in the formation of plaques even after 72 h p.i. (Fig. 3B).
Fig. 3.
Comparison of FFA and plaque assay with field-collected PEDV specimens. (A) PEDV infection by field-collected specimens is confirmed within 8 h p.i. and no increase in focus size is observed even after 48 h p.i. (magnification: 40×). (B) Plaque assay of KPEDV-9 showed clear plaques 48 h p.i. that increased in size by 72 h p.i. In contrast, field collected samples (HS-01, HS-02, HS-05, PT-01 and IC-01) did not show evident plaque after 72 h p.i. (NC: mock infected Vero cells at 72 h p.i.).
4. Discussion
A useful technique for evaluating virus infectivity is by measuring virus titer in cell cultures. One of the earliest techniques developed for determining the titer of infectious virions is the plaque assay method (Bachrach et al., 1957). A susceptible cell line is grown to confluence in a tissue culture dish and is inoculated with various dilutions of the virus. After considerable CPE is observed, staining with crystal violet allows clear visualization of areas of localized infection or plaques. This method has been employed for detection and titration of several arboviruses, herpesviruses, and coronaviruses, and has been the “gold standard” in characterizing virus infectivity (Russel, 1962, Takayama and Kim, 1976, Thomas and Dulac, 1976, Vautherot, 1981, Westaway, 1966).
In 1977, Okuno et al. introduced an alternative method for rapid titration of dengue type 4 using FFA. FFA uses a peroxidase–antiperoxidase (PAP) staining technique which allows visualization of infected cells under a dissecting or inverted microscope by probing the cells with specific antibodies and staining with a peroxidase-conjugated antibody and DAB substrate. This method was applied for detection of several other arboviruses and for evaluation of neutralizing antibodies (Jirakanjanakit et al., 1997, Okuno et al., 1978, Okuno et al., 1979, Okuno et al., 1990, Okuno et al., 1980, Raharjo et al., 1986). This method has also been applied to other types of viruses (Okuno et al., 1990, Tanishita et al., 1984).
FFA offers some advantages for PEDV over the plaque assay technique. One clear advantage is the ability to observe virus infected cells without discernable CPE. Since this method detects viral proteins in infected cells using antibodies that are virus-specific, it is possible to detect the presence of the virus as long as sufficient amount of viral proteins are expressed by the infected cells. In addition, the use of virus-specific antibodies also allows antigenic confirmation of PEDV. Previously, it was reported that the parental strain of PEDV CV777 infects only a small number of cells and fail to cause syncytium formation in Vero cells. CPE was observed only after several passages (Hofmann and Wyler, 1988). However, using the FFA, virus infection can be confirmed 8 h after initial infection of the cells. This allows detection of PEDV as well as measuring the titer of the stock on every passage of the virus. Furthermore, adaptation of the virus to Vero cells may be monitored by observing the size of the foci and comparing the rate of infection and spread of the virus to adjacent cells. Another advantage of FFA is the short incubation period required to detect cells infected with the virus. Unlike plaque assay, the presence of the virus may be confirmed within a few replication cycle of the virus. In contrast, the plaque assay requires several replication cycles of the virus before discernable CPE is observed. Finally, the 96-well microtiter plate scale for cell culture used in FFA provides the advantage of using more replicates and a large number of dilutions for detection and titration of the virus (Ishimine et al., 1987).
Based on the results of the FFA and plaque assay, there was no significant difference in sensitivity with respect to determining virus titer of cell-adapted PEDV. However, the time it took to determine the titer of PEDV by FFA was shorter compared with the plaque assay. Also, the FFA was able to detect infectious virions in field-collected specimens in which the plaque assay failed to demonstrate. These findings suggest that the FFA may be used for detection and titration of PEDV in a given sample among primary isolates. This method may also be used to measure the progression of PEDV infectivity and cell adaptation among different passages of the various PEDV isolates.
Acknowledgement
This research work was supported by the Korea Research Foundation Grant funded by the Korean Government (MOEHRD No. R05-2003-000-11395-0).
References
- Bachrach H.L., Callis J.J., Hess W.R., Patty R.E. A plaque assay for foot-and-mouth disease virus and kinetics of virus reproduction. Virology. 1957;4:224–236. doi: 10.1016/0042-6822(57)90060-0. [DOI] [PubMed] [Google Scholar]
- Bridgen A., Duarte M., Tobler K., Laude H., Ackermann M. Sequence determination of the nucleocapsid protein gene of the porcine epidemic diarrhea virus confirms that this virus is a coronavirus related to human coronavirus 229E and porcine transmissible gastroenteritis virus. J. Gen. Virol. 1993;74:1795–1804. doi: 10.1099/0022-1317-74-9-1795. [DOI] [PubMed] [Google Scholar]
- DeBouck P., Pensaert M.B. Experimental infection of pigs with a new porcine enteric coronavirus CV777. Am. J. Vet. Res. 1980;41:219223. [PubMed] [Google Scholar]
- Hofmann M., Wyler R. Propagation of the virus of porcine epidemic diarrhea in cell culture. J. Clin. Microbiol. 1988;26:22352239. doi: 10.1128/jcm.26.11.2235-2239.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishimine T., Tadano M., Fukunaga T., Okuno Y. An improved micromethod for infectivity assays and neutralization tests of dengue viruses. Biken J. 1987;30:39–44. [PubMed] [Google Scholar]
- Jirakanjanakit N., Sanohsomneing T., Yoksan S., Bhamarapravati N. The micro-focus reduction neutralization test for determining dengue and Japanese encephalitis neutralizing antibodies in volunteers vaccinated against dengue. Trans. R. Soc. Trop. Med. Hyg. 1997;91:614–617. doi: 10.1016/s0035-9203(97)90050-x. [DOI] [PubMed] [Google Scholar]
- Jung K., Kim J., Kim O., Kim B., Chae C. Differentiation between porcine epidemic diarrhea virus and transmissible gastroenteritis virus in formalin-fixed paraffin-embedded tissues by multiplex RT-nested PCR and comparison with in situ hybridization. J. Virol. Meth. 2003;108:41–47. doi: 10.1016/s0166-0934(02)00253-7. [DOI] [PubMed] [Google Scholar]
- Jung K., Chae C. RT-PCR-based dot blot hybridization for the detection and differentiation between porcine epidemic diarrhea virus and transmissible gastroenteritis virus in fecal samples using a non-radioactive digoxigenin cDNA probe. J. Virol. Meth. 2005;123:141–146. doi: 10.1016/j.jviromet.2004.09.012. [DOI] [PubMed] [Google Scholar]
- Kadoi K., Sugioka H., Satoh T., Kadoi B.K. The propagation of a porcine epidemic diarrhea virus in swine cell lines. Microbiologica. 2002;25:285–290. [PubMed] [Google Scholar]
- Kim O., Chae C. Application of reverse transcription polymerase chain reaction to detect porcine epidemic diarrhea virus in Vero cell culture. J. Vet. Diagn. Invest. 1999;11:537–538. doi: 10.1177/104063879901100610. [DOI] [PubMed] [Google Scholar]
- Kim O., Chae C. In situ hybridization for the detection and localization of porcine epidemic diarrhea virus in the intestinal tissues from naturally infected piglets. Vet. Pathol. 2000;37:62–67. doi: 10.1354/vp.37-1-62. [DOI] [PubMed] [Google Scholar]
- Kim O., Chae C. Comparison of reverse transcription polymerase chain reaction, immunohistochemistry, and in situ hybridization for the detection of porcine epidemic diarrhea virus. Can. J. Vet. Res. 2002;66:112–116. [PMC free article] [PubMed] [Google Scholar]
- Kweon C.H., Kwon B.J., Lee J.G., Kwon G.O., Kang Y.B. Derivation of attenuated porcine epidemic diarrhea virus (PEDV) as vaccine candidate. Vaccine. 1999;17:2546–2553. doi: 10.1016/S0264-410X(99)00059-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okuno Y., Igarashi A., Fukai K. Neutralization tests for dengue and Japanese encephalitis viruses by the focus reduction method using peroxidase–anti-peroxidase staining. Biken J. 1978;21:137–147. [PubMed] [Google Scholar]
- Okuno Y., Sasao F., Fukunaga T., Fukai K. An application of PAP (peroxidase–antiperoxidase) staining technique for the rapid titration of dengue virus type 4 infectivity. Biken J. 1977;20:29–33. [PubMed] [Google Scholar]
- Okuno Y., Tanaka K., Baba K., Maeda A., Kunita N., Ueda S. Rapid focus reduction neutralization test of influenza A and B viruses in microtiter system. J. Clin. Microbiol. 1990;28:1308–1313. doi: 10.1128/jcm.28.6.1308-1313.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okuno Y., Yamanishi K., Lwin S., Takahashi M. Micro-neutralization test for mumps virus using the 96-well tissue culture plate and PAP (peroxidase–antiperoxidase) staining technique. Microbiol. Immunol. 1985;29:327–335. doi: 10.1111/j.1348-0421.1985.tb00831.x. [DOI] [PubMed] [Google Scholar]
- Okuno Y., Fukunaga T., Tadano M., Okamoto Y., Ohnishi T., Takagi M. Rapid focus reduction neutralization test of Japanese encephalitis virus in microtiter system. Arch. Virol. 1985;86:129–135. doi: 10.1007/BF01314119. [DOI] [PubMed] [Google Scholar]
- Okuno Y., Fukunaga T., Srisupaluck S., Fukai K. A modified PAP (peroxidase–anti-peroxidase) staining technique using sera from patients with dengue hemorrhagic fever (DHF): 4 step PAP staining technique. Biken J. 1979;22:131–135. [PubMed] [Google Scholar]
- Okuno Y., Fukunaga T., Srisupaluck S., Kasemsam P., Dharakul C., Sangkawibha N. Serological and virological studies on patients with dengue hemorrhagic fever (DHF) in Chanthaburi province, Thailand. I. Serological studies on paired sera from DHF patients by neutralization (N), hemagglutination inhibition (HI) and staining tests. Biken J. 1980;23:113–121. [PubMed] [Google Scholar]
- Pospischil A., Hess R.G., Bachmann P.A. Light microscopy and ultrahistology of intestinal changes in pigs infected with epizootic diarrhoea virus (EDV): comparison with transmissible gastroenteritis (TGE) virus and porcine rotavirus infections. J. Vet. Med. B. 1981;28:564577. doi: 10.1111/j.1439-0450.1981.tb01774.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pritchard G.C., Paton D.J., Wibberley G., Ibata G. Transmissible gastroenteritis and porcine epidemic diarrhoea in Britain. Vet. Rec. 1999;144:616618. doi: 10.1136/vr.144.22.616. [DOI] [PubMed] [Google Scholar]
- Raharjo E., Tadano M., Okamoto Y., Okuno Y. Development of a micro-neutralization test for chikungunya virus. Biken J. 1986;29:27–30. [PubMed] [Google Scholar]
- Russel W.C. A sensitive and precise plaque assay for herpes virus. Nature. 1962;195:1028–1029. doi: 10.1038/1951028a0. [DOI] [PubMed] [Google Scholar]
- Song D.B., Kang B.K., Lee S.S., Yang J.S., Moon H.J., Oh J.S., Ha G.W., Jang Y.S., Park B.K. An internal control in the quantitative RT-PCR assay for quantitation of porcine epidemic diarrhea virus shedding in pigs. J. Virol. Meth. 2006;133:27–33. doi: 10.1016/j.jviromet.2005.10.021. [DOI] [PubMed] [Google Scholar]
- Takayama N., Kim A. An improved method for titration of mouse hepatitis virus type 3 in a mouse cell culture. Arch. Virol. 1976;52:347–349. doi: 10.1007/BF01315624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanishita O., Takahashi Y., Okuno Y., Yamanishi K., Takahashi M. Evaluation of focus reduction neutralization test with peroxidase–antiperoxidase staining technique for hemorrhagic fever with renal syndrome virus. J. Clin. Microbiol. 1984;20:1213–1215. doi: 10.1128/jcm.20.6.1213-1215.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas F.C., Dulac G.C. Transmissible gastroenteritis virus: plaques and plaque neutralization test. Can. J. Com. Med. 1976;40:171–174. [PMC free article] [PubMed] [Google Scholar]
- Vautherot J.F. Plaque assay for titration of bovine enteric coronavirus. J. Gen. Virol. 1981;56:451–455. doi: 10.1099/0022-1317-56-2-451. [DOI] [PubMed] [Google Scholar]
- Yaling Z., Ederveem K., Egberink H., Pensaert M.B., Horzinek M.C. Porcine epidemic diarrhea virus (CV777) and feline infectious peritonitis virus (FIPV) are antigenically related. Arch. Virol. 1988;102:63–71. doi: 10.1007/BF01315563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westaway E.G. Assessment and application of a cell line from pig kidney for plaque assay and neutralization tests with twelve group B arboviruses. Am. J. Epidemiol. 1966;84:439–456. doi: 10.1093/oxfordjournals.aje.a120657. [DOI] [PubMed] [Google Scholar]


