Abstract
The objective of this study was to elucidate the kinetics and magnitudes of specific IgA antibody responses in intestines of turkey poults infected with turkey coronavirus (TCV). Turkey poults were orally inoculated with TCV at 10 days of age. Intestinal segment cultures were administered for duodenum, jejunum, and ileum and the IgA antibody responses were analyzed at 1, 2, 3, 4, 6, or 9 weeks post-infection (PI) in two different experiments. The kinetics of virus-specific IgA antibody responses in duodenum, jejunum, and ileum were similar: gradually increased from 1 week PI, reached the peak at 3 or 4 weeks PI, and declined afterward. The virus-specific IgA antibody responses in duodenum, jejunum, and ileum showed negative correlation with duration of TCV antigen in the corresponding locations of intestine with Spearman’s correlation coefficient of −0.85 (p=0.034), −0.74 (p=0.096), and −0.75 (p=0.084), respectively. Moreover, the virus-specific IgA antibody responses in serum were positively correlated with that of duodenum (coefficient=0.829, p=0.042), jejunum (coefficient=0.829, p=0.042), and ileum (coefficient=0.771, p=0.072) segment cultures, suggesting that the induction of specific IgA response in serum was predictive of an IgA response in intestine. The results indicate that intestinal mucosal IgA antibodies to TCV are elicited in turkeys following infection with TCV. The local mucosal antibodies may provide protective immunity for infected turkeys to recover from TCV infection.
Keywords: Humoral, Immune responses, Immunoglobulin A, Mucosal immunity, Turkey coronavirus, Turkey poult enteritis
1. Introduction
Turkey coronaviral enteritis is an acute and highly contagious disease (Nagaraja and Pomeroy, 1997). Turkey poult enteritis associated with turkey coronaviral infection has contributed to significant economic losses for turkey producers in Indiana, North Carolina, and other states for the last several years and remained as a major threat to the turkey industry. Once the coronaviral enteritis is introduced into the areas with high concentrations of turkeys on a year-round basis, it is not easily eliminated and is encountered frequently in turkey poults (Nagaraja and Pomeroy, 1997). Treatments of the disease are often unsuccessful and there are currently no effective vaccines or medications to prevent the disease.
Protection against enteric infections is largely provided by the gastrointestinal immune system. Considerable efforts of developing vaccines to these infections have been made on strategies that may stimulate this compartment of the mucosal immune system. Animal studies have demonstrated that IgA antibody responses induced in intestinal mucosal lymphoid tissues are of fundamental importance in protection against intestinal infection by transmissible gastroenteritis coronavirus (VanCott et al., 1993) or human rotavirus (Yuan et al., 1996). Development of successful vaccines to these virus-induced diseases depends on understanding of the most efficient means of inducing virus-specific effector cells within gut-associated lymphoid tissue.
The assessment of intestinal immune responses has frequently relied on indirect measurements such as determination of specific antibodies in serum. However, measurement of antibodies of the systemic immune system may not be a reliable indicator for local mucosal immunity. Due to the ease of sample collection, measurement of antibody in the saline extract of feces has been used to assess intestinal immune responses. However, the complicated components in the gastrointestinal tract may affect the measurement of antibodies in feces such as local antibody degradation by proteases and sialidases. Nevertheless, the above-mentioned methods cannot provide information about where in the intestinal tract the specific antibody response occurs. Such information has specific relevance for the development of control and prevention strategies against enteric pathogens.
Intestinal segment culture has been successfully used for the measurement of mucosal antibody production in mouse (Khoury et al., 1994) and human (Losonsky et al., 1999). Since the whole tissue sample is used, the mucosal microenvironment remains intact. The humoral (such as cytokines) and cellular (such as accessory cells) factors required to produce antibody responses in the local intestinal tract are present. The antibody responses detected by intestinal segment cultures were positively correlated with the numbers of antibody secreting cells enumerated by enzyme-linked immunospot assay (ELISPOT) (Losonsky et al., 1999). The intestinal segment culture had been used to evaluate candidate vaccines consisting of rotavirus primate strain RRV, bovine strain WC3, or reassortant viruses between these animal viruses and human rotaviruses (Khoury et al., 1994).
Because turkey coronavirus (TCV) replicate only in mature villus epithelial cells in small intestine (Deshmukh et al., 1975), protection against infection of TCV is likely mediated by immune responses active at the intestinal mucosal surface. However, due to shortage of immunological reagents and methods specific for evaluation of turkey immunity, reports of intestinal mucosal immune responses in turkeys following exposure to TCV are limited. The antibodies to TCV in the intestine and bile were only qualitatively detected by immunodiffusion test (Nagaraja and Pomeroy, 1978) or a sandwich immunofluorescent antibody assay (IFA) (Nagaraja and Pomeroy, 1980). The interactions between the kinetics and magnitudes of mucosal IgA antibody responses in turkeys and acute turkey coronaviral infection and subsequent recovery of affected turkeys from the infection are not completely understood. This study was undertaken to elucidate the kinetic and magnitudes of intestinal IgA antibody responses in turkey poults infected with TCV by intestinal segment cultures.
2. Materials and methods
2.1. Turkey eggs and poults
Turkey eggs and 1-day-old turkey poults (British United Turkey of America, BUTA) of both sexes were obtained (Perdue Farm, Washington, IN). They were free of recognized pathogens for turkeys, including TCV. Turkey poults were housed in Horsfall-Bauer isolation units for 4 weeks and then transferred to isolated floor pens. Feed and water were provided ad libitum.
2.2. Virus isolation and propagation
TCV was isolated from intestines of 28-day-old turkey poults with outbreak of acute enteritis. Affected intestines were homogenized with 5-fold volume of phosphate-buffered saline (PBS) buffer, clarified by centrifugation at 3000×g for 10 min, and filtered through 0.45 and 0.22 μm membrane filters (Millipore Products Division, Bedford, MA). Filtrates containing only TCV, but not other viruses, were examined by electron microscopy (EM) and immunoelectron microscopy (IEM) according to procedures previously described (Dea and Garzon, 1991). For IEM, the turkey antisera specific for TCV was provided by Dr. Y.M. Saif (The Ohio State University, Wooster, OH) (Yu et al., 2000, Loa et al., 2001). Twenty-two days old embryonated turkey eggs were inoculated with 200 μl of the filtrate via amniotic sac route. Embryo intestines were harvested in 3 days. Harvested embryo intestines were processed and propagated serially as described above for five passages. Intestines of TCV-infected turkey embryos in the fifth passage were prepared as 20% suspension in PBS. The suspension was homogenized, clarified, filtered as described above, and used as inoculum. The inoculum was also examined with EM and IEM to confirm the presence of only TCV. The titer of virus was determined by inoculation of the suspension at 10-fold dilutions into groups of five 22-day-old embryonated turkey eggs. The 50% embryo infectious dose (EID50) was calculated by the method of Reed and Muench (1938). An inoculum containing 4×103 EID50/200 μl (Experiment 1) or 2×104 EID50/1 ml (Experiment 2) of TCV was prepared and used to experimentally infect turkey poults.
2.3. Experimental design
Two experiments were conducted with two separate hatches of turkey poults. Infection with TCV was confirmed at 3 days post-infection (PI) by examination of intestines of five turkey poults with IFA, histopathology, and EM in each experiment. For histopathology, intestines were fixed in 10% phosphate-buffered formalin for overnight, processed, embedded, sectioned, and stained with hematoxylin and eosin. For EM, intestinal contents were clarified by centrifugation at 5000×g for 30 min at 4 °C. The clarified samples were ultracentrifuged at 100 000×g for 2 h at 4 °C. Pellets were resuspended in distilled water, placed on 200-mesh formvar carbon-coated grid, and negatively stained with 2% of phosphotungstic acid, pH 6.5.
2.3.1. Experiment 1
Twenty 10-day-old turkey poults were orally inoculated with TCV-containing inoculum prepared as described previously. A control group of 20 age-matched birds was sham-inoculated with sterile PBS buffer. Five birds were randomly selected from each group and necropsied at 1, 2, 3, and 4 weeks PI. Duodenum, jejunum, and ileum were collected from each bird. Intestinal segment cultures were performed on duodenum, jejunum, and ileum, respectively. TCV-specific IgA antibody in the supernatants collected from duodenum, jejunum, or ileum cultures was determined by antibody-capture enzyme-linked immunosorbent assay (ELISA).
2.3.2. Experiment 2
Thirty 10-day-old turkey poults were orally inoculated with TCV-containing inoculum prepared as described previously. A control group of 30 age-matched birds was sham-inoculated with sterile PBS buffer. Five birds were randomly selected from each group and necropsied at 1, 2, 3, 4, 6, and 9 weeks PI. Blood was drawn from each bird and the serum collected was stored at −20 °C. Feces, duodenum, jejunum, and ileum were collected from each bird. The presence of TCV in duodenum, jejunum, and ileum was examined by IFA. Intestinal segment cultures were performed on duodenum, jejunum, and ileum, respectively. TCV-specific IgA antibody in serum, feces, and supernatants collected from duodenum, jejunum, or ileum cultures was determined by antibody-capture ELISA.
2.4. Immunofluorescent antibody assay for TCV antigen in the intestines
The IFA method for detection of TCV was described previously (Patel et al., 1975, Loa et al., 2000). Duodenum, jejunum, and ileum were frozen immediately, sectioned, and incubated with turkey antisera specific for TCV (Dr. Y.M. Saif) at a dilution of 1:40 at room temperature (25 °C) for 30 min. Intestinal sections were incubated with fluorescein isothiocyanate (FITC) conjugated goat anti-turkey IgG (H+L) antibodies (Kirkegaard and Perry Laboratories, Gaithersburg, MD) at a dilution of 1:40 at room temperature for 30 min. The intensity of positive IFA staining for TCV in the enterocytes was scored on a scale of 1 to 4 with 1 being no staining and 4 being the most intense staining. The distribution of positive IFA staining for TCV in the intestinal section was categorized into diffuse, multifocal, and focal and scored as 3, 2, and 1, respectively. The scores of intensity and distribution of positive IFA staining for TCV were added. The final value indicated the overall positive IFA staining in a section, with higher value indicating more positive staining.
2.5. Intestinal segment culture
The culture system for the measurement of intestinal antibody was modified from previously published methods for mice (Khoury et al., 1994) and human (Losonsky et al., 1999). A 0.5 cm long segment of duodenum was collected from the loop of duodenum adjacent to pancreas. A 0.5 cm long segment of jejunum adjacent to the Merckel’s diverticulum was collected. A 0.5 cm long segment of ileum in between the ceca was collected. The intestinal segments from the different poults were collected from the same locations. Segments of duodenum, jejunum, and ileum were collected under sterile conditions, opened longitudinally, and washed twice with calcium- and magnesium-free Hanks balanced salt solution (HBSS) (Gibco, Grand Island, NY) supplemented with 10 mM N-2-hydroxy-ethylpiperazine-N′-2-ethanesulfonic acid (HEPES) and 50 μg/ml of gentamicin (Sigma, St. Louis, MO). Each intestinal segment was placed in individual wells of a sterile 6-well culture plate containing 4 ml of RPMI-1640 culture medium supplemented with 10 mM HEPES, 10% sterile turkey serum, 0.25 μg/ml of amphotericin B, 100 units/ml of penicillin, 100 μg/ml of streptomycin, and 50 μg/ml of gentamicin. All intestinal segments were cultured for 5 days at 37 °C in an atmosphere of 95% O2 to 5% CO2. The culture supernatants were collected from each well and stored at −20 °C until used.
2.6. Preparation of anti-turkey IgA antiserum
Preparation of rabbit anti-turkey IgA antibodies was modified from previously described methods (Dohms et al., 1978, Beetham et al., 1993). Turkey IgA was purified from turkey bile by Sephacryl S-200 size-exclusion chromatography (Pharmacia, Peapack, NJ). Whole molecule IgA was reduced with 2-mercaptoethanol (Sigma) and alkylated with iodoacetamide (Sigma). Heavy and light chains were separated by Sephacryl S-100 size-exclusion chromatography. Antiserum to turkey IgA was generated in rabbits using the purified heavy chains as inoculums.
2.7. ELISA for antibodies to TCV
The ELISA method for detection of antibody to TCV was previously described (Loa et al., 2000). Supernatants of intestinal segment cultures, feces, and serum samples at a dilution of 1:3, 1:15, and 1:40, respectively, were added at 100 μl per well. Rabbit anti-turkey IgA antibodies were used at a dilution of 1:1600 for supernatants of intestinal segment cultures and serum samples and 1:800 for fecal samples. Horseradish peroxidase (HRPO)-conjugated goat anti-rabbit IgG (H+L) antibodies (Sigma) were used at a dilution of 1:400.
2.8. Statistical analysis
Calculated means from each group were compared by t-test with significance determined at p<0.05. The correlations among antibody responses in duodenum, jejunum, or ileum segment cultures and durations of TCV in corresponding intestinal segments or antibody responses in serum were determined by Spearman’s rank correlation test (McPherson, 2001).
3. Results and discussion
3.1. Duration of TCV in duodenum, jejunum, and ileum
The infection of turkey poults with TCV was confirmed at 3 days PI. The intestines of turkey poults were positive for TCV antigen by IFA. The turkey poults had acute atrophic enteritis by histopathology (data not shown). Turkey coronaviral particles in the intestinal contents were observed by EM (data not shown).
Duration of viral antigen in different locations of small intestine is shown in Fig. 1 . The presence of TCV in duodenum was apparent at 1 week PI as shown by high IFA score at 5.6. The IFA score for TCV antigen was markedly decreased to 1.4 at 2 weeks PI. Viral antigen was no longer detectable after 3 weeks PI. In jejunum and ileum, the IFA response to TCV was observed until 4 weeks PI. The scores of IFA response to TCV gradually declined from 7.0 at 1 week PI to 4.0 at 4 weeks PI in jejunum and from 6.2 at 1 week PI to 2.2 at 4 weeks PI in ileum. As in duodenum, the viral antigen was not detectable in jejunum or ileum by IFA at 6 and 9 weeks PI. Duodenum, jejunum, and ileum of non-infected control turkeys from each interval were negative for TCV. It was previously reported that TCV antigen in duodenum was consistently detectable from 1 to 6 days PI and only occasionally found at 14 and 24 days PI by IFA method while the viral antigen in jejunum and ileum was evident until 28 days PI (Patel et al., 1975). Similar findings that shorter duration of TCV in duodenum than in jejunum or ileum were also obtained in this study. The viral load in jejunum was higher than that in ileum in both studies.
Fig. 1.
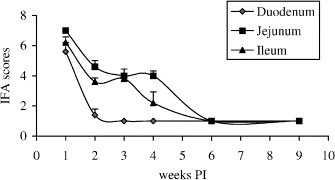
Duration of TCV in duodenum, jejunum, and ileum as determined by IFA. Turkey poults were orally infected with TCV at 10 days old. Intestinal segments of duodenum, jejunum, and ileum were separately collected from turkeys of infected or uninfected control groups at 1, 2, 3, 4, 6, and 9 weeks PI, and the IFA scores for TCV were determined as described in Section 2. The error bars represent the standard error of five samples.
3.2. Virus-specific IgA antibodies in intestinal segment cultures
Virus-specific IgA antibodies in supernatant fluids from intestinal segment cultures is shown in Fig. 2 (Experiment 1) and Fig. 3 (Experiment 2). The IgA response in duodenum cultures gradually increased from 1 to 4 weeks PI and reached the peak at ELISA reading of 0.67 in Experiment 1 and 1.24 in Experiment 2. The response declined at 6 and 9 weeks PI with ELISA readings at 1.14 and 0.93, respectively, in Experiment 2. In jejunum cultures, the IgA response gradually increased from 1 to 3 weeks PI and reached the peak at ELISA reading of 0.56 and slightly decreased at 4 weeks PI with ELISA reading of 0.42 in Experiment 1. The IgA response gradually increased from 1 to 4 weeks PI and reached the peak at ELISA reading of 1.04 in Experiment 2. The response declined at 6 and 9 weeks PI with ELISA readings at 0.51 and 0.45, respectively, in Experiment 2. In ileum cultures, the IgA response gradually increased from 1 to 4 weeks PI and reached the peak at ELISA reading of 0.41 in Experiment 1 and 0.88 in Experiment 2. The response declined at 6 and 9 weeks PI with ELISA readings at 0.72 and 0.82, respectively, in Experiment 2. Virus-specific IgA antibodies in supernatant fluids from duodenum, jejunum, or ileum cultures of non-infected control groups were undetectable throughout the entire experiments. The virus-specific IgA antibody responses in duodenum, jejunum, and ileum negatively correlated with the duration of TCV in the corresponding intestinal segments as determined by Spearman’s rank correlation test in Experiment 2. The correlation coefficients were −0.85 (p=0.034), −0.74 (p=0.096), and −0.75 (p=0.084) between IgA antibody responses and duration of TCV in duodenum, jejunum, and ileum, respectively.
Fig. 2.
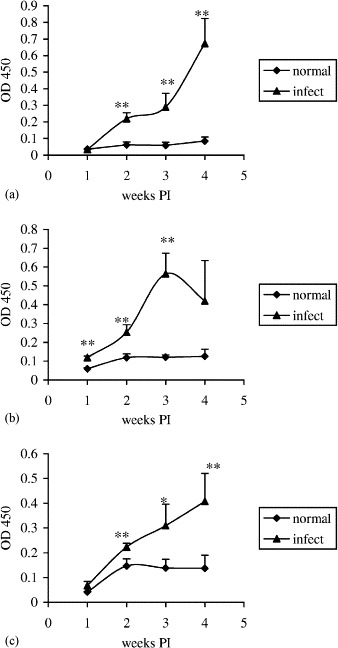
Kinetics of virus-specific IgA antibody responses in duodenum (a), jejunum (b), and ileum (c) segment cultures as determined by an antibody-capture ELISA in Experiment 1. Turkey poults were orally infected with TCV at 10 days old. Intestinal segments of duodenum, jejunum, and ileum were separately collected from turkeys of infected or uninfected control groups at 1, 2, 3, and 4 weeks PI and cultured. The virus-specific IgA antibodies in the culture supernatants were assessed by ELISA. The error bars represent the standard error of five samples. OD 450=optical density read at 450 nm. The significance of difference between infected and uninfected control groups is indicated at each time interval by asterisks (∗p<0.1; ∗∗p<0.05).
Fig. 3.
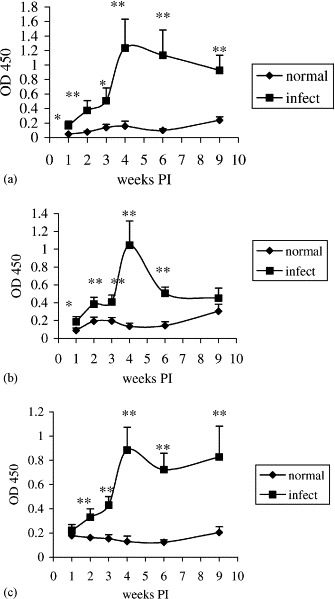
Kinetics of virus-specific IgA antibody responses in duodenum (a), jejunum (b), and ileum (c) fragment cultures as determined by an antibody-capture ELISA in Experiment 2. Turkey poults were orally infected with TCV at 10 days old. Intestinal segments of duodenum, jejunum, and ileum were separately collected from turkeys of infected or uninfected control groups at 1, 2, 3, 4, 6, and 9 weeks PI and cultured. The virus-specific IgA antibodies in the culture supernatants were assessed by ELISA. The error bars represent the standard error of five samples. OD 450=optical density read at 450 nm. The significance of difference between infected and uninfected control groups is indicated at each time interval by asterisks (∗p<0.1; ∗∗p<0.05).
These results revealed that local intestinal mucosal IgA antibodies was stimulated and reached the plateau in a similar fashion in different sites of intestine after infection with TCV. It was demonstrated that induction of virus-specific IgA antibody responses in local mucosal tissue was related to replication of porcine coronavirus (VanCott et al., 1993) and rotavirus (Khoury et al., 1994). The virus-specific IgA antibody response seemed to decline faster in jejunum than in duodenum or ileum when the viral antigen is no longer detectable at 6 and 9 weeks PI. Given the much longer stretch of jejunum in comparison with duodenum or ileum in turkey, the faster decline of IgA response in jejunum may be due to random dispersion of virus-specific antibody secreting cells in a much wider jejunum.
3.3. Virus-specific IgA antibodies in feces and serum
The IgA response in feces reached the peak at 3 weeks PI with ELISA reading of 0.69 in Experiment 2 (Fig. 4a ). The responses dramatically declined at 4 weeks PI with ELISA reading of 0.26 and no longer detectable at 6 and 9 weeks PI. Virus-specific IgA antibodies in feces of non-infected control groups remained undetectable throughout the entire experiments. The virus-specific IgA antibody responses in feces did not correlate with those of duodenum, jejunum, or ileum segment cultures. The lower level of virus-specific IgA antibodies in feces than that in intestinal segment cultures suggested that intestinal segment culture is a more sensitive method for detection of virus-specific IgA antibodies in intestinal mucosa. The appearance of accumulated IgA antibodies in feces was only detectable within a relatively small window of time at 3 and 4 weeks PI and markedly declined thereafter. In addition, it is difficult to determine where the accumulated virus-specific IgA antibodies in feces were produced from which segment of the intestine.
Fig. 4.
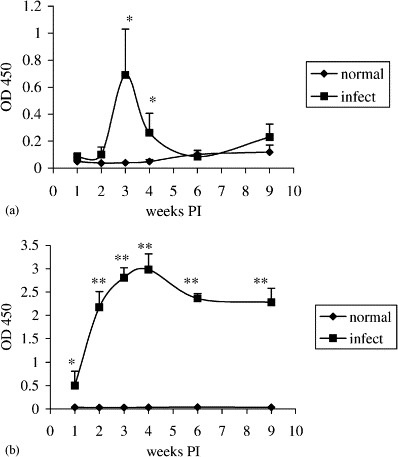
Kinetics of virus-specific IgA antibody responses in feces (a) or serum (b) as determined by an antibody-capture ELISA in Experiment 2. Turkey poults were orally infected with TCV at 10 days old. Feces or sera were collected from turkeys of infected or uninfected control groups at 1, 2, 3, 4, 6, and 9 weeks PI. Feces were diluted at 1:15 and sera were diluted at 1:40. The virus-specific IgA antibodies in the fecal and serum samples were assessed by ELISA. The error bars represent the standard error of five samples. OD 450=optical density read at 450 nm. The significance of difference between infected and uninfected control groups is indicated at each time interval by asterisks (∗p<0.1; ∗∗p<0.05).
The virus-specific IgA antibodies in serum were initially detected at 1 week PI, gradually increased from 1 to 4 weeks PI and reached the peak at ELISA reading of 2.98 in Experiment 2 (Fig. 4b). The response declined at 6 and 9 weeks PI with ELISA readings at 2.36 and 2.28, respectively. Virus-specific IgA antibodies in serum of non-infected control groups remained undetectable throughout the entire experiments. The virus-specific IgA antibody responses in serum positively correlated with those of duodenum (coefficient=0.829, p=0.042), jejunum (coefficient=0.829, p=0.042), and ileum (coefficient=0.771, p=0.072) segment cultures. Intestinal mucosal immune responses are initiated by uptake of antigens from mucosa surfaces into lymphoid tissues located in the mucosa where specific B cells are activated. The activated B cells may enter the circulation and migrate to local and distant mucosal tissues as well as systemic lymphoid tissues. In the target environments, terminal differentiation of the activated B cells occurs and the virus-specific antibodies are produced. The result in this study suggested that IgA antibody response in sera of turkeys might serve as an indicator for IgA antibody response in the intestine after TCV infection.
In conclusion, the results in this study showed that intestinal segment culture is a useful method for studies of intestinal mucosal IgA immunity. A reliable method for the measurement of local mucosal immune responses is crucial for the evaluation of local immune response to infections and the development of mucosa-based vaccines. Virus-specific IgA antibody responses to TCV were induced in intestinal mucosa of turkey poults infected with TCV. These IgA antibody responses were negatively correlated with the presence of TCV in the infected intestines. The elicited local mucosal antibodies may have provided protective immunity for infected turkeys to recover from TCV infection.
Acknowledgements
The authors thank the support provided by the Commission of Agriculture, State of Indiana, Pfizer Animal Health, North Carolina Turkey Spiking Mortality Task Force, and United States Department of Agriculture.
References
- Beetham P.K., Glick B., Dick J.W. A comparison of three isolation methods for obtaining immunoglobulin A from turkey bile. Avian Dis. 1993;37:1026–1031. [PubMed] [Google Scholar]
- Dea S., Garzon S. Identification of coronaviruses by the use of indirect protein A-gold immunoelectron microscopy. J. Vet. Diagn. Invest. 1991;3:297–305. doi: 10.1177/104063879100300405. [DOI] [PubMed] [Google Scholar]
- Deshmukh D.R., Sautter J.H., Patel B.L., Pomeroy B.S. Histopathology of fasting and bluecomb disease in turkey poults and embryos experimentally infected with bluecomb disease coronavirus. Avian Dis. 1975;20(4):631–640. [PubMed] [Google Scholar]
- Dohms J.E., Saif Y.M., Pitts J.E. Isolation of turkey immunoglobulin-A. Avian Dis. 1978;22(1):151–156. [PubMed] [Google Scholar]
- Khoury C.A., Brown K.A., Kim J.E., Offit P.A. Rotavirus-specific intestinal immune response in mice assessed by enzyme-linked immunospot assay and intestinal fragment culture. Clin. Diagn. Lab. Immunol. 1994;1(6):722–728. doi: 10.1128/cdli.1.6.722-728.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loa C.C., Lin T.L., Wu C.C., Bryan T.A., Thacker H.L., Hooper T., Schrader D. Detection of antibody to turkey coronavirus by antibody-capture enzyme-linked immunosorbent assay utilizing infectious bronchitis virus antigen. Avian Dis. 2000;44:498–506. [PubMed] [Google Scholar]
- Loa C.C., Lin T.L., Wu C.C., Bryan T., Thacker H.L., Hooper T., Schrader D. Humoral and cellular immune responses in turkey poults infected with turkey coronavirus. Poultry Sci. 2001;80:1416–1424. doi: 10.1093/ps/80.10.1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Losonsky G.A., Fantry G.T., Reymann M., Lim Y. Validation of a gastrointestinal explant system for measurement of mucosal antibody production. Clin. Diagn. Lab. Immunol. 1999;6(6):803–807. doi: 10.1128/cdli.6.6.803-807.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McPherson, G., 2001. Applying and Interpreting Statistics: A Comprehensive Guide, 2nd Edition. Springer, New York, pp. 457–458.
- Nagaraja K.V., Pomeroy B.S. Secretory antibodies against turkey coronaviral enteritis. Am. J. Vet. Res. 1978;39:1463–1465. [PubMed] [Google Scholar]
- Nagaraja K.V., Pomeroy B.S. Immunofluorescent studies on localization of secretory immunoglobulins in the intestines of turkeys recovered from turkey coronaviral enteritis. Am. J. Vet. Res. 1980;41:1283–1284. [PubMed] [Google Scholar]
- Nagaraja, K.V., Pomeroy, B.S., 1997. Coronaviral enteritis of turkeys (bluecomb disease). In: Calnek, B.W., Barnes, H.J., Beard, C.W., McDougald, L.R., Saif, Y.M. (Eds.), Diseases of Poultry, 10th Edition. Iowa State University Press, Ames, IA, pp. 686–692.
- Patel B.L., Deshmukh D.R., Pomeroy B.S. Fluorescent antibody test for rapid diagnosis of coronaviral enteritis of turkeys (bluecomb) Am. J. Vet. Res. 1975;36:1265–1267. [PubMed] [Google Scholar]
- Reed L.J., Muench H. A simple method for estimating fifty percent endpoints. Am. J. Hyg. 1938;27:493–497. [Google Scholar]
- VanCott J.L., Brim T.A., Simkins R.A., Saif L.J. Isotype-specific antibody-secreting cells to transmissible gastroenteritis virus and porcine respiratory coronavirus in gut- and bronchus-associated lymphoid tissues of suckling pigs. J. Immunol. 1993;150:3990–4000. [PubMed] [Google Scholar]
- Yu M., Ismail M.M., Qureshi M.A., Dearth R.N., Barnes H.J., Saif Y.M. Viral agents associated with poult enteritis and mortality syndrome: the role of a small round virus and a turkey coronavirus. Avian Dis. 2000;44:297–304. [PubMed] [Google Scholar]
- Yuan L., Ward L.A., Rosen B.I., To T.L., Saif L.J. Systemic and intestinal antibody-secreting cell responses and correlates of protective immunity to human rotavirus in a gnotobiotic pig model of disease. J. Virol. 1996;70(5):3075–3083. doi: 10.1128/jvi.70.5.3075-3083.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]


