Abstract
Bovine respiratory syncytial virus (BRSV) is an important cause of respiratory disease among calves in the Danish cattle industry. An experimental BRSV infection model was used to study the pathogenesis of the disease in calves. Broncho alveolar lung lavage (BAL) was performed on 28 Jersey calves, of which 23 were experimentally infected with BRSV and five were given a mock inoculum. The presence of the cytokine tumor necrosis factor α (TNF-α) in the BAL fluids was detected and quantified by a capture ELISA. TNF-α was detected in 21 of the infected animals. The amount of TNF-α in the BAL fluid of calves killed post inoculation day (PID) 2 and 4 was at the same very low level as in the uninfected control animals. Large amounts of TNF-α were detected on PID 6, maximum levels of TNF-α were reached on PID 7, and smaller amounts of TNF-α were seen on PID 8. The high levels of TNF-α appeared on the days where severe lung lesions and clinical signs were obvious and the amounts of BRSV-antigen were at their greatest. Although Pasteurellaceae were isolated from some of the BRSV-infected calves, calves treated with antibiotics before and through the whole period of the infection, as well as BRSV-infected calves free of bacteria reached the same level of TNF-α as animals from which bacteria were isolated from the lungs. It is concluded that significant quantities of TNF-α are produced in the lungs of the calves on PID 6–7 of BRSV infection. The involvement of TNF-α in the pathogenesis of, as well as the anti-viral immune response against, BRSV infection is discussed.
Abbreviations: PID, post inoculation day; BRSV, bovine respiratory syncytial virus; Mock, mock inoculum; BAL, broncho alveolar lung lavage; TNF-α, tumor necrosis factor-α
Keywords: Tumor necrosis factor-α, Bovine respiratory syncytial virus, Experimental calf pneumonia, Pathogenesis
1. Introduction
Enzootic pneumonia is a widespread disease in cattle populations throughout the world (Bryson, 1985). In Denmark, bovine respiratory syncytial virus (BRSV) plays a crucial role in the respiratory disease complex, leading to substantial losses within the calf rearing industry (Uttenthal et al., 1996). BRSV is a single-stranded RNA virus, which belongs to the genus Pneumovirus, classified in the subfamily Pneumovirinae in the family of Paramyxoviridae (Murphy et al., 1995). The virus is closely related to the human respiratory syncytial virus (HRSV) (Collins et al., 1996).
In cattle, BRSV induced disease ranges from fatal bronchointerstitial pneumonia to subclinical infections (Bryson, 1993). Calves which get seriously ill from BRSV infection develop severe coughing, tachypnea, dyspnea, mucopurulent nasal discharge, and high fever (Kimman et al., 1989, Belknap, 1993).
The gross lesions appear as large areas of consolidated tissue in the cranioventral parts of the lungs in which bronchi and bronchioli often are filled with mucopurulent exudate. Interstitial edema may be seen in all lobes, whereas emphysema typically is located in the diaphragmatic lobes (Kimman et al., 1989, Bryson, 1993). Microscopic findings consist of epithelial lesions in the bronchi, bronchioles and alveoli, which are filled or infiltrated with neutrophils and macrophages. Frequently, multinuclear syncytia as well as hyaline membranes can be found. Also, evidence of bronchiolar repair including epithelial hyperplasia and organisation of exudate are observed fairly early in the course of the infection (Kimman et al., 1989, Bryson, 1993). Immunostainings performed on lung tissue indicate that BRSV are located in the cranioventral lobes in most cases, and rarely in the caudal parts of the diaphragmatic lobes (Kimman et al., 1989, Ellis et al., 1996, Viuff et al., 1996). The similarity of the clinical signs and pathology appearing in BRSV-infected calves and HRSV-infected infants is noticeable (Baker, 1991). Hence, studies of BRSV-infections in calves also contribute valuable information to the disease in infants. Today, the available treatments and prophylactic precautions against the diseases are far from optimal. In fact, a vaccine against HRSV is still not available for children (Collins et al., 1996). Therefore, a great effort has been made to study the immune response and pathogenesis of the viral infection in ruminants (Kimman, 1993, Belknap et al., 1995).
Since lesions are present in areas of the lung where little or no virus is detected, one or several immunopathological mechanisms are assumed to be involved in the disease (Baker, 1991, Kimman, 1993). Moreover, the importance of different subpopulations of leukocytes in the lung during infection has been investigated (Kimman, 1993, Taylor et al., 1995). In particular, alveolar macrophages (AM), which phagocytise pathogens and secrete inflammatory and immunoregulatory cytokines, have attracted attention. Human or murine AM infected with HRSV in vitro produce large amounts of the cytokine, tumor necrosis factor-α (TNF-α) (Panuska et al., 1990, Becker et al., 1991). Today, accumulating data from in vivo investigations indicate that TNF-α is implicated in the pathogenesis of the disease. AM isolated from infants suffering from an acute RSV infection have been found to express TNF-α protein in a cell-associated manner (Midulla et al., 1993). Moreover, Matsuda et al. (1995) demonstrated the presence of TNF-α in the nasal discharge from HRSV-infected infants. In the murine HRSV-model, high levels of bioactive TNF-α were found in the lungs and sera of HRSV-infected BALBc mice, post inoculation day (PID) 2 (Hayes et al., 1994). Additionally, TNF-α has been shown to possess an antiviral effect against HRSV in vitro (Cirino et al., 1993, Merolla et al., 1995) as well as in mice in vivo (Neuzil et al., 1996). Therefore, the cytokine may also be involved in the immune defense directed against the RSV infection.
Hence, TNF-α seems to be an important cytokine to look for in the lungs of BRSV-infected calves.
The aims of this study were to investigate whether BRSV induces secretion of TNF-α in the lungs of infected calves, and if so, to match these findings with the clinical signs, the pathological changes and the appearance of the BRSV-antigen in the lungs of the animals.
To our knowledge, we submit the first evidence of TNF-α being present in the lungs of BRSV-infected calves.
2. Materials and methods
2.1. Viral strain/isolate and the preparation of inoculum
The third cell culture passage of a BRSV isolate designated 2022 was used as an inoculum for the experimental infections of calves. Origin and propagation of the virus are described in Larsen et al. (1998). Mock inoculum consisted of fetal calf lung cells (FBL cells) which were grown and frozen as the FBL cells the virus was propagated in. All inocula were tested free of bovine virus diarrhea virus (BVDV), bovine PI-3 virus (BPI-3V), bovine adeno virus (BAV), bovine corona virus (BCV), bovine entero virus (BEV), infectious bovine rhinotrachitis virus (IBRV), bovine reo virus, bacteria and bovine mycoplasmas.
2.2. The experimental BRSV model
An experimental model for BRSV infections in calves (Tjørnehøj, in preparation) was used to study the pathogenesis of the disease. Briefly, 7–14 day old male Jersey calves were purchased from closed herds and reared in isolation units following normal management procedures for calves. Eight to twenty-one week old calves were inoculated once by a 10 min aerosol exposure, followed by an intratracheal injection of viral inoculum with 104.6–5.2 tissue culture infectious dose50 (TCID50) of BRSV by each route. Mock inocula were diluted and administered in the same way as the viral inoculum. All calves in the experiments were tested free from infection with BVDV before inoculation.
After inoculation with virus, the general health status of the calves was monitored. The onset and degree of clinical signs were followed daily.
A total of 28 calves from five different experiments were included in the study. Twenty-three animals were given the BRSV inoculum and the rest of the calves received a mock inoculum. The calves were killed PID 2 (2), 4 (2), 6 (9), 7 (8) and 8 (2). Four of the calves killed PID 6 were treated systemically with a broad spectrum antibiotic, enrofloxacin (Baytril®) 2.5 mg/kg (Bayer), starting from 2 days before the inoculation with BRSV and throughout the experiment.
2.3. Necropsy
The lungs were immediately removed from the animals after exsanguination. Photographs were taken of the ventral and dorsal sides of the lungs. The extent of consolidated lung tissue was scored from 0 to 5, where the score 0 was given to lungs completely free of lung lesions; 1 to lungs with few spots (1–5%) of consolidated lung tissue, 2 to lungs with 5–15%, 3 to lungs with 15–30% and 4 to lungs with 30–50% of consolidated tissue. The score 5 was given to lungs where most of the tissue in the cranial, medial and accessory lobes and at least a third of the diaphragmatic lobes consisted of consolidated tissue (>50%).
2.4. Bronchoalveolar lavage (BAL)
BAL was performed on the left lung by flushing the bronchi of the lung with 100 ml of Eagles Minimum Essential Medium (MEM). After collection, the BAL fluids were supplemented with equal amounts of medium containing 1000 IU penicillin G/ml, 1 mg of streptomycin per ml and 0.005% amphotericin. BAL fluids for cytokine and viral examinations were immediately snapfrozen in liquid nitrogen, whereas the BAL fluids for cytospin preparations were kept at 4°C and processed within 8–10 h. Cells (3×104 to 5×104) were centrifuged (Shandon cytospin centrifuge, 3 min/1200 rpm) onto a coated slide (SuperFrost +), air dried for 5 min and fixed in 99% ethanol for 45 min at −20°C.
2.5. Demonstration of other infectious agents
To rule out the possibility of other agents causing disease or lesions in the lung, tissue from the right lung was tested for the presence of BPI-3V, BAV, and BCV by antigen enzyme-linked immunosorbent assay (ELISA) (Uttenthal et al., 1996). In addition, lung tissues were examined for viable BEV, IBRV, bovine reo virus, BPI-3V, BAV and BVDV. Briefly, supernatants of minced lung tissue were transferred to a monolayer of bovine kidney cells, and cytopathic effect (CPE) combined with immunofluorescence technique were used to confirm their presence. Also, tissue samples were taken from the lung, spleen and liver and cultured for bacteria. Finally, bronchial swabs were analyzed for the presence of Mycoplasma spp. The Danish Veterinary Laboratory and the Danish Veterinary Institute for Virus Research carried out these analyses.
2.6. Demonstration of BRSV in the lungs
Two grams of lung tissue were collected from each of nine different predetermined areas of the left lung representing the dorsal, medial and the ventral parts of all the lobes. The lung tissues were stored at −40°C until the examination for the presence of BRSV-antigen by an indirect antigen ELISA (Tjørnehøj et al., in preparation) was performed. The sum of the titers in each of the nine samples was used as a measurement of the level of BRSV-antigen in the lung tissue.
Lung lavage fluids and supernatants from lung tissue soaked in MEM were investigated for infectious BRSV (Tjørnehøj et al., in preparation). Samples were transferred to FBL cells and grown until CPE occurred. Two cell passages were made followed by indirect immunofluorescence test using hyperimmune guinea pig serum against BRSV and a FITC conjugated rabbit anti-guinea pig antibody (DAKO) to visualize BRSV in the cell culture.
2.7. Immunocytochemistry performed on BAL cells
Detection of BRSV-antigen in BAL cells was done by immunocytochemistry. Cytospin preparations were incubated for 10 min in a Tris-buffered saline (TBS: 0.05 M Tris, 0.15 M NaCl, pH 7.6) followed by a 15 min blocking with TBS, 5% swine serum. Slides were then incubated for 1 h with a bovine biotinylated hyperimmune serum against BRSV diluted 1:8000 in TBS containing 5% swine serum (Uttenthal et al., 1996). This was followed by a 1 h incubation with a streptavidin-alkaline phosphatase complex (DAKO). Three washes with TBS were performed between each incubation step. Finally, virus positive cells were visualized with Fast Red substrate (KemEnTec), and Harris Haematoxylen as a counterstain. Cytospin preparations of FBL cells infected with BRSV were used as positive control, while non-infected FBL cells and BAL cells from animals tested negative for BRSV served as negative controls. The immunostainings were evaluated by light microscopy. Each slide was given one of the following scores: 0 (no virus positive cells), 1(1–20% virus positive cells), 2 (20–40% virus positive cells), 3 (40–60% virus positive cells), 4 (60–80% virus positive cells) or 5 (80–100% virus positive cells).
Immunocytochemistry was also used to study the presence of TNF-α in the BAL cells. Rehydration and blocking were performed as described above. Cytospin preparations of BAL cells were incubated with a monoclonal antibody against TNF-α (Ellis et al., 1993), diluted 1:25 in TBS containing 5% swine serum for 1 h followed by 30 min incubation with EnVision (DAKO) and visualized with Fast Red substrate (KemEnTec). An isotype matched antibody (IgG1) (DAKO) diluted 1:25 was applied as a negative control for the immunocytochemistry procedure whereas cytospin preparations of freshly isolated BAL cells from uninfected calves were run as negative control cells. Cytospin preparations of BAM stimulated for 5 h with endotoxin 5 μg/ml (E. coli, O111: B4 (Sigma)) were used as positive controls.
2.8. ELISA to quantify TNF-α
The presence of the TNF-α in the lung lavage fluid was examined by a capture antigen ELISA. The monoclonal and polyclonal antibody used to detect TNF-α were described by Ellis et al. (1993). C96 maxisorb immunoplates (Nunc) were coated for 16–24 h with the monoclonal antibody 1D11-13 against TNF-α, diluted in coating buffer 1:1000 (4.53 mM NaHCO3, 1.82 mM Na2CO3, pH 9.6). The plates were washed six times with TBS containing 0.05% Tween (TBS-T) before triplicates of lung lavage diluted in TBS-T, 0.5% gelatin (TBS-T-g) were transferred to the wells and left overnight at 4°C. The following day the plates were washed with TBS-T, and incubated for 1 h with a polyclonal rabbit anti-TNF-α (pool 88) diluted 1:1500 in TBS-T-g. This was followed by a washing step, a 1 h incubation period with biotinylated goat anti-rabbit, H+L chain (Zymed) diluted 1:10.000, another washing step, and a 1 h incubation period with streptavidin-alkaline phosphatase (GibcoBRL) diluted 1:2000. Finally, p-nitrophenyl phosphate-substrate (GibcoBRL) was added to the wells and incubated for 20 min. The plates were read at 405 nm using 495 nm as a reference. A dilution of recombinant bovine TNF-α (Ciba-Geigy) was used as the standard. Plain medium was used as negative control. OD-values from the standard were plotted against ng/ml TNF-α. The dilution of the lavage fluid occurring in the lungs during the washing procedure was not taken into account when calculating the amounts of TNF-α.
2.9. Statistical analysis of the data
A two-tailed Student t-test was used to compare (1) the amount of TNF-α present in BRSV-infected calves and calves given the mock inoculum; (2) the amount of TNF-α present in BRSV-infected calves treated or not treated with antibiotics PID 6; and (3) the amount of TNF-α in BRSV-infected calves with or without bacteria in the lungs, PID 6–8. The data was log-transformed to achieve a normal distribution. P<0.05 was considered as significant.
3. Results
3.1. Clinical symptoms and gross pathology
Based on the temperatures and respiration rates recorded the day the animals were killed, the first clinical signs appeared in calves killed on days 2–4, some of which had mild coughing, but body temperature (39.3°C) and respiratory rate (40 min−1) within the normal range (Table 1 ). Severe clinical signs were seen in calves killed on PID 6–8, which exhibited depression, mucopurulent nasal discharge, severe coughing and dyspnea. Furthermore, respiratory rates ranging from 50 to 105 min were monitored in 16 of the 19 calves killed PID 6–8. Febrile reactions were present in the calves PID 6–8. In total, seven out of the nine calves killed on PID 6 and five out of the eight calves killed PID 7 reached temperatures between 39.3 and 40.9°C. One calf killed PID 8 had a normal temperature through the whole period. The other calf killed PID 8 dropped from 40.9°C on PID 7 to 39.7°C on PID 8 (results not shown).
Table 1.
Age, clinical signs, microbiology and the presence of infectious BRSV, BRSV-antigen and TNF-α in the lungs of individual calves experimentally infected with BRSVa
| Calf no. | PID | Age of calves (weeks) | Body temperature (°C) | Respiration (min) | Pasteurellacea bacteria in the lung tissue | Mycoplasma species in the bronchies | Infectious BRSV in the lung tissue | BRSV antigen score/BAL cells | TNF-α in BAL (ng/ml) |
| IX-1b | 2 | 14 | 38.4 | 35 | − | Ureaplasma | − | 0 | − |
| IX-2 | 15 | 38.4 | 40 | − | M. dispar | + | 2 | 0.8 | |
| Ureaplasma | |||||||||
| IX-3 | 13 | 38.5 | 30 | − | M. dispar | + | 1 | 0.5 | |
| Ureaplasma | |||||||||
| IX-4b | 4 | 11 | 38.8 | 38c | − | − | − | 0 | 1.1 |
| IX-5 | 14 | 38.9 | 40c | − | M. dispar | + | 3 | 0.5 | |
| Ureaplasma | |||||||||
| IX-6 | 10 | 39.2 | 34c | − | M. dispar | + | 3 | − | |
| Ureaplasma | |||||||||
| IX-7b | 6 | 13 | 39.3 | 38 | − | M. dispar | − | 0 | − |
| Ureaplasma | |||||||||
| IX-8 | 14 | 40.6 | 84 | H. somnus | M. dispar | + | 5 | 69.2 | |
| Ureaplasma | |||||||||
| IX-9 | 14 | 40.9 | 72 | H. somnus | M. dispar | + | 4 | 38.0 | |
| Ureaplasma | |||||||||
| XI-1d | 18 | 40.0 | 70 | − | − | + | 4 | 11.2 | |
| XI-2d | 16 | 39.5 | 75 | − | − | + | 4 | 94.6 | |
| XI-3d | 15 | 38.9 | 65 | − | Ureaplasma | + | 4 | 71.6 | |
| XI-4d | 8 | 38.8 | 35 | − | − | − | 2 | − | |
| XIV-1b | 21 | 38.6e | 28 | − | M. dispar | − | 0 | − | |
| XIV-2 | 17 | 37.8 | 44 | P. multocida | M. dispar | + | 4 | 13.5 | |
| XIV-3 | 21 | 40.3 | 104 | P. multocida | M. dispar | + | 3 | 29.8 | |
| M. bovirhinis | |||||||||
| XIV-4 | 21 | 40.3 | 76 | − | M. dispar | + | 4 | 19.3 | |
| M. bovirhinis | |||||||||
| X-9 | 7 | 14 | 39.0 | 90 | − | M. dispar | − | NDh | 40.6 |
| X-10 | 13 | 38.7 | 50 | − | − | + | ND | 20.8 | |
| X-11 | 18 | 39.3 | 50 | − | − | − | ND | 23.7 | |
| X-12 | 14 | 38.6 | 55 | − | − | − | ND | 40.9 | |
| XII-9 | 13 | 40.7 | 90 | P. multocida | M. dispar | + | ND | 25.2 | |
| XII-10 | 14 | 40.8 | 95 | P. multocida | M. dispar | − | ND | 256.9 | |
| XII-11 | 14 | 40.0 | 60 | − | M. dispar | − | ND | 200.1 | |
| XII-12 | 16 | 40.7 | 70 | − | M. dispar | − | ND | 53.4 | |
| IX-10b | 8 | 13 | 38.4f | 32 | − | M. dispar | − | 0 | − |
| M. bovirhinis | |||||||||
| IX-11 | 12 | 39.7 | 80 | P. multocida | M. dispar | +g | 3 | 3.7 | |
| M. bovirhinis | |||||||||
| Ureaplasma | |||||||||
| IX-12 | 15 | 39.1 | 38 | − | M. dispar | − | 3 | 10.5 | |
| Ureaplasma | |||||||||
The table presents data from the 28 calves described in this article. A roman figure followed by a number identifies a calf. The data are collected on the PID the calves were killed (if not otherwise stated). The score given to the BAL cells containing BRSV-antigen are defined as followed: 0 (no virus positive cells); 1 (1–20% positive virus cells); 2 (20–40% virus positive cells); 3 (40–60% virus positive cells); 4 (60–80% virus positive cells), 5 (all cells are virus positive).
Negative control calves (bold roman figures), which were given a mock inoculum.
The respiration rates were measured on PID 3.
The calves were treated with an antibiotic. Baytril® were administrated daily, from 2 days before inoculation with BRSV until the calves were killed on PID 6.
The temperature was taken PID 5.
The temperature was taken PID 7.
Infectious BRSV was found in the BAL fluid, but not the lung tissue.
ND: not done.
A few spots (1–5%) of dark red consolidated lung tissue were seen on PID 2–4 in the lungs of the calves infected with BRSV. These lesions could hardly be distinguished from the subacute to chronic lesions seen in some of the negative control calves. Small amounts of mucopurulent discharge were present in bronchi and trachea in both BRSV and mock-inoculated animals. Lungs from these calves were given a score from 0 to 1.
On PID 6–8, the infected calves had a moderate to severe exudative bronchopneumonia with 10–50% consolidated lung tissue (score 2–5). Average scores were 2.8 on PID 6, 3.3 on PID 7, and 3.5 on PID 8 (Fig. 1 A). Trachea and bronchi contained large amounts of mucopurulent to purulent discharge. In addition, there was widespread interstitial edema in all lobes and emphysema was found especially in the diaphragmatic lobe of the most affected calves.
Fig. 1.
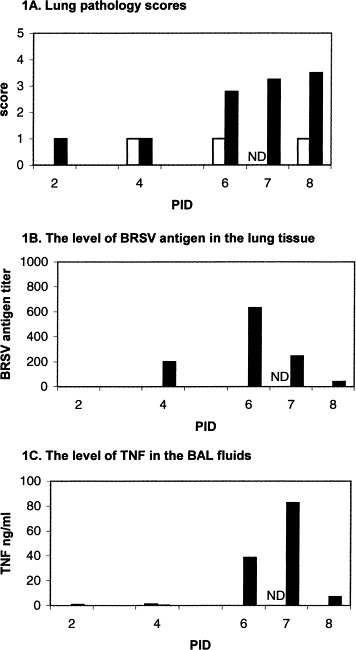
(A–C) Pathology scores and levels of BRSV antigen and TNF-α found in the lungs of calves experimentally infected with BRSV PID 2–8. The figures portray the mean values of calves infected with BRSV (■) where PID 2, 4 and 8 are each based on data from 2 calves, whereas PID 6 and 7 are based on 9 and 8 calves, respectively. Calves, which received the mock inoculum (□) are each represented by one animal PID 2, 4 and 8 and two animals on PID 6 (PID 7 is not done). (A) Pathology scores; based on the estimates of the amount of consolidated tissue present in the lungs of a calf, where the lungs without any lesions are given the score 0, and the lungs with extensive consolidated lung tissue in all lobes are given the score 5 (see Section 2); (B) the presence of BRSV antigen in the lung tissue described by antigen titers (see Section 2); (C) the levels of the cytokine TNF-α detected in BAL by ELISA; ND: not done.
3.2. Isolation of other infectious agents
No other bovine virus were isolated from the lower airways of the calves during the experiment. Mycoplasma dispar was present in almost every calf including the ones given the mock inoculum (Table 1). Moreover, it was possible to isolate Mycoplasma bovirhinis or Ureaplasma simultaneously in some individuals. Of the 23 BRSV-infected calves, six were found to be negative for mycoplasmas; three of these animals were treated with antibiotics (Table 1). No bacteria were isolated from BRSV-infected calves PID 2, PID 4, calves treated with antibiotics (PID 6), or any calf given the mock inoculum. Haemophilus somnus and Pasteurella multocida were isolated from two and five calves, respectively, of the 14 BRSV-infected calves killed on PID 6–8.
3.3. Detection of BRSV
The total BRSV titer of the left lung was calculated as the sum of the antigen titers measured in the nine different predetermined spots. BRSV antigens could not be detected on PID 2, while moderate values were seen on PID 4 (94–304) and high values appeared on PID 6 (400–1024) and PID 7 (96–768). One calf killed PID 6 and one killed PID 7 were negative for BRSV in the nine tested sites. The amount of detectable antigens decreased to very low levels (0–80) on PID 8 (Fig. 1B).
Infectious BRSV could be transferred from the lungs of the calves to fetal calf lung cells from PID 2 to 6. On PID 7, only two of eight calves contained infectious BRSV and on PID 8 all calves were negative for infectious BRSV in the lung tissue, however one calf had infectious virus in the BAL fluid (Table 1).
Immunocytochemistry was used to detect BRSV-antigen within the BAL-cells. BRSV-antigens were located in neutrophils, alveolar macrophages and epithelial cells. The amount of neutrophils in BAL increased dramatically from PID 4–8. The actual percentage of each cell population was difficult to establish, because many of the neutrophils and epithelial cells appeared in clumps. On PID 2 BRSV-antigen was only detected in few cells (score 1). On PID 4, the amount of cells containing BRSV increased to 40–50% (score 3) of the cells in the cytospin, whereas at least 75% (score 4) of all cells were positive on PID 6. The score 5 was given to a single animal, on PID 6. The number of BRSV positive cells declined to around 50% on PID 8 (score 3). No BRSV positive signals appeared in mock-inoculated calves (Table 1).
3.4. Detection of TNF-α
Significant amounts of TNF-α were found in the lungs of BRSV-infected calves compared to the uninfected calves (P<0.05). As demonstrated in Table 1 and Fig. 1C, detectable, but very low amounts (0.5–0.8 ng/ml) of TNF-α were found on PID 2 and 4. The cytokine level changed dramatically on PID 6 to values ranging from 11.2 to 94.6 ng/ml (a mean of 38.6 ng/ml), and increased even more on PID 7, where BAL contained from 20.8 to 256.9 ng/ml (a mean of 82.7 ng/ml). High TNF-α values were found in BAL from three of the four calves treated with antibiotics. One calf (no. XIV-4) was not affected by the BRSV-infection as the rest of the calves in the group, and neither BRSV-antigen nor TNF-α were detected in BAL from this animal. The TNF-α levels in BRSV-infected calves treated with antibiotic were not significantly different from the TNF-α levels in BRSV-infected calves not treated with antibiotic on PID 6 (P>0.05). Also, the TNF-α levels in calves from which P. multocida or H. somnus were isolated, were not different (P>0.05) from those calves where no bacteria were detected (PID 6–8). Lung lavages performed on two animals on PID 8 contained 3.7 and 10.5 ng/ml, respectively. In general, calves which had high levels of BRSV-antigens in the lung tissue had a fairly large amount of TNF-α in the lung wash. Extremely high TNF-α values were measured in two calves on PID 7. P. multocida was isolated in one of these cases. In contrast, TNF-α was only found in a small amount (1.0 ng/ml) in one mock-inoculated animal on PID 4 and could not be detected in the remaining uninfected control calves.
Furthermore, BAL cells from four BRSV-infected calves killed on PID 6 were examined for the presence of cell-associated TNF-α by immunocytochemistry. Many BAL cells were positive for TNF-α where some cells stained intensively or moderate for the cytokine and others only weakly. Therefore, no subpopulation of cells producing TNF-α could be identified by this method. BAL cells from calves, which stained intensively for BRSV-antigen, were strongly positive for TNF-α on parallel slides. The isotype matched control resulted in almost no background staining and no or few positive signals could be detected in a minority of the BAL-cells from non-infected calves.
4. Discussion
As several experiments have indicated that the proinflammatory and antiviral cytokine TNF-α is involved in the pathogenesis of RSV-infections in humans and mice (Panuska et al., 1990, Neuzil et al., 1996) we investigated if TNF-α is produced in the lungs of calves infected with BRSV. In agreement with what Hayes et al. (1994) found when they studied the experimental HRSV-infection in mice, we demonstrated that large amounts of TNF-α are produced in the lungs of calves experimentally infected with BRSV, but in contrast to their study, our RSV-studies were performed in the natural host.
Furthermore, the TNF-α measured in BAL of the BRSV infected calves is likely to be bioactive since results from the ELISA correlate well with the WEHI-164, clone 13, bioassay measurement of bioactive TNF-α (Ellis et al., 1993).
Being a proinflammatory cytokine, TNF-α is known to attract and activate neutrophil granulocytes and lymphocytes by itself or through the induction of other cytokines (Ohmann et al., 1990, Chiang et al., 1991, Persson et al., 1993). The cytokine has also been shown to be involved in the mechanisms leading to increased permeability of endothelium (Zeck-Kapp et al., 1990) and epithelium in lung inflammations (Li et al., 1995). Additionally, TNF-α has been shown to inhibit the alveolar type II epithelial cells production of surfactant phospholipid (Arias-Diaz et al., 1993) and surfactant proteins (Wispe et al., 1990, Pryhuber et al., 1996), a mechanism which has been suggested to contribute to the pathophysiology of adult respiratory distress syndrome (ARDS) (Arias-Diaz et al., 1993). Therefore, the biological effects induced by TNF-α could explain the presence of purulent exudate, edema and atelectasis in the BRSV-infected lung. Hence, we suggest that the high levels of TNF-α measured on PID 6–7 contribute to the severe lung lesions and clinical signs accompanying BRSV infection on PID 6–8.
Since both P. haemolytica and P. multocida are capable of inducing a harmful cytokine-response in lungs (Bienhoff et al., 1992, Yoo et al., 1995), the TNF-α measured in the BRSV-infected calves coinfected with Pasteurellaceae spp. could have been caused by the bacteria. However, the data suggest that BRSV was capable of inducing a significant secretion of TNF-α without any other contributing agents. Indeed there was no correlation between the presence of other infectious agents and the level of TNF-α.
In most cases, high levels of TNF-α were found in the calves which had large amounts of BRSV antigens in their lungs. Still, some calves which had very high levels of BRSV antigen, had only a moderate amount of TNF-α in their lung fluids (individual data not shown). This might be explained by the kinetics of TNF-α, which is known to be produced and degraded rather quickly. Thus, the level of the cytokine easily can change within hours (Adams et al., 1990, Horadagoda et al., 1994).
Previously, the alveolar macrophages have been shown to be the main source of TNF-α in the lung environment (Warren et al., 1989, Van Nhieu et al., 1993). However, like Yoo et al. (1995), we were unable to identify the cells responsible for the production of TNF-α in the lungs, as many BAL cells including some cell debris stained positive for the cytokine by immunocytochemistry in the BRSV-infected animals. This might be explained by the fact that TNF-α also appears in a cell-associated manner, partly because the cytokine binds to cells via its receptors (Ohmann et al., 1990), and also because TNF-α exists on the cell surface as a membrane-associated form (Nii et al., 1993). Compared to other bovine studies (Yoo et al., 1995), relatively high amounts of TNF-α were detected in the BAL fluids in this study, possibly because cell-associated TNF-α was included, since the BAL-cells were not separated from the lung wash suspensions before they were frozen.
Another potential contributor to the TNF-α present in the BAL is the airway epithelium. Although alveoli and bronchioli epithelial cell cultures have been shown to secrete IL1-α, IL6, IL8 and granulocyte macrophage stimulating factor (Arnold et al., 1994, Jiang et al., 1998, Patel et al., 1998), when infected in vitro with HRSV, controversy exists whether or not these cells also secrete TNF-α (Arnold et al., 1994, Patel et al., 1995).
In most viral infections the specific immune response consists of a humoral and a cell mediated immune response, which are responsible for the clearance of virus. In BRSV infection specific antibodies have been shown to appear in the BRSV-infected calves from PID 8–10, but the significance of both actively and maternally acquired antibodies in the clearance of BRSV is still unclear (Kimman, 1993). However, investigations performed by Taylor et al. (1995) indicate that cytotoxic T-lymphocytes could participate in the clearance of BRSV infections. In our study, the calves had few or no maternal antibodies (IgG1) against BRSV when they were inoculated with BRSV and IgM was not detected before PID 8, and only in one calf (data not shown). The presence of cytotoxic T-lymphocytes in the lungs of BRSV-infected calves is currently being investigated within the group.
In addition to specific immune responses, studies performed on HRSV-infected mice and cell cultures indicate that TNF-α also may play a role in the recovery of the infection (Merolla et al., 1995, Neuzil et al., 1996). In our study, no infectious BRSV could be isolated from the lungs of six out of eight BRSV-infected calves on the same day (PID 7) when TNF-α reached its maximum level in BAL. These findings imply that the cytokine could play an important role in the antiviral immune defense against BRSV infection in calves at a time where the specific immune response against BRSV has not been fully established.
The anti-viral effect of TNF-α is mainly established through the TNF-receptor p55, which induces cytotoxicity when it binds the cytokine (Wong et al., 1992, Tartaglia et al., 1993). This mechanism might play an important role in the RSV-infection since in vitro studies have shown that the TNF-receptor p55 is secreted in large amounts from lung epithelia cells infected with HRSV (Arnold et al., 1994). Interestingly, a homology was recently discovered between a conserved region in the G-protein of RSV and a domain within the TNF-receptor p55 (Langedijk et al., 1998). Therefore, one could speculate that RSV has evolved mechanisms by which it is able to interact with TNF-α, and thereby inhibit the antiviral effect of the cytokine. This interference may eventually result in the dramatic levels of TNF-α found in the lung.
Understanding of the pathogenesis of RSV infections is essential for finding a treatment to reduce the lung damage. Clearly, future experiments should involve inhibition of the TNF-α response early and late in the course of the viral infection. Intrapulmonary administration of recombinant bovine TNF-α or monoclonal antibodies against TNF-α or its receptors, is one way to continue these studies in calves. Moreover, drugs like dexamethasone and pentoxifylline which are known to inhibit the synthesis of TNF-α (Han et al., 1990, Balibrea et al., 1994) could be administered during the experimental BRSV infections.
Acknowledgements
The technical assistance of Anne Friis Petersen is gratefully acknowledged. The study was supported by Grant no. 9313833 from the Danish Agricultural and Veterinary Research Council and Grant no. 94-6 from the Danish Ministry of Food, Agriculture and Fisheries.
References
- Adams J.L, Semrad S.D, Czuprynski C.J. Administration of bacterial lipopolysaccharide elicits circulating tumor necrosis factor-alpha in neonatal calves. J. Clin. Microbiol. 1990;28:998–1001. doi: 10.1128/jcm.28.5.998-1001.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arias-Diaz J, Vara E, Garcia C, Gomez M, Balibrea J.L. Tumour necrosis factor-alpha inhibits synthesis of surfactant by isolated human type II pneumocytes. Eur. J. Surg. 1993;159:541–549. [PubMed] [Google Scholar]
- Arnold R, Humbert B, Werchau H, Gallati H, Konig W. Interleukin-8, interleukin-6, and soluble tumour necrosis factor receptor type I release from a human pulmonary epithelial cell line (A549) exposed to respiratory syncytial virus. Immunology. 1994;82:126–133. [PMC free article] [PubMed] [Google Scholar]
- Baker J.C. Human and bovine respiratory syncytial virus: immunopathologic mechanisms. Vet. Q. 1991;13:47–59. doi: 10.1080/01652176.1991.9694284. [DOI] [PubMed] [Google Scholar]
- Balibrea J.L, Arias-Diaz J, Garcia C, Vara E. Effect of pentoxifylline and somatostatin on tumour necrosis factor production by human pulmonary macrophages. Circ. Shock. 1994;43:51–56. [PubMed] [Google Scholar]
- Becker S, Quay J, Soukup J. Cytokine (tumor necrosis factor, IL-6, and IL-8) production by respiratory syncytial virus-infected human alveolar macrophages. J. Immunol. 1991;147:4307–4312. [PubMed] [Google Scholar]
- Belknap, E.B., 1993. Recognizing the clinical signs of BRSV infection. In: Baker, J.C. (Ed.), Proceedings of the Symposium on BRSV infection. Vet. Med. 88, 886–887.
- Belknap E.B, Ciszewski D.K, Baker J.C. Experimental respiratory syncytial virus infection in calves and lambs. J. Vet. Diagn. Invest. 1995;7:285–298. doi: 10.1177/104063879500700226. [DOI] [PubMed] [Google Scholar]
- Bienhoff S.E, Allen G.K, Berg J.N. Release of tumor necrosis factor-alpha from bovine alveolar macrophages stimulated with bovine respiratory viruses and bacterial endotoxins. Vet. Immunol. Immunopathol. 1992;30:341–357. doi: 10.1016/0165-2427(92)90104-x. [DOI] [PubMed] [Google Scholar]
- Bryson, D.G., 1985. Calf pneumonia. In: Proceedings of the Symposium on Bovine Respiratory Disease. Vet. Clin. North. Am. (Food Anim. Pract.) 1, 237–257. [DOI] [PMC free article] [PubMed]
- Bryson, D.G., 1993. Necropsy findings associated with BRSV pneumonia. In: Baker, J.C. (Ed.), Proceedings of the Symposium on BRSV infection. Vet. Med. 894–899.
- Chiang Y.W, Murata H, Roth J.A. Activation of bovine neutrophils by recombinant bovine tumor necrosis factor-alpha. Vet. Immunol. Immunopathol. 1991;29:329–338. doi: 10.1016/0165-2427(91)90023-6. [DOI] [PubMed] [Google Scholar]
- Cirino N.M, Panuska J.R, Villani A, Taraf H, Rebert N.A, Merolla R, Tsivitse P, Gilbert I.A. Restricted replication of respiratory syncytial virus in human alveolar macrophages. J. Gen. Virol. 1993;74:1527–1537. doi: 10.1099/0022-1317-74-8-1527. [DOI] [PubMed] [Google Scholar]
- Collins, P.L., McIntosh, K., Chanock, R.M., 1996. Respiratory Syncytial Virus. In: Field, B.N., Knipe, D.M. (Eds.), Fields Virology. Raven Press, New York, pp. 1313–1351.
- Ellis J.A, Godson D, Campos M, Sileghem M, Babiuk L.A. Capture immunoassay for ruminant tumor necrosis factor-alpha: comparison with bioassay. Vet. Immunol. Immunopathol. 1993;35:289–300. doi: 10.1016/0165-2427(93)90040-b. [DOI] [PubMed] [Google Scholar]
- Ellis J.A, Philibert H, West K, Clark E, Martin K, Haines D. Fatal pneumonia in adult dairy cattle associated with active infection with bovine respiratory syncytial virus. Can. Vet. J. 1996;37:103–105. [PMC free article] [PubMed] [Google Scholar]
- Han J, Thompson P, Beutler B. Dexamethasone and pentoxifylline inhibit endotoxin-induced cachectin/tumor necrosis factor synthesis at separate points in the signaling pathway. J. Exp. Med. 1990;172:391–394. doi: 10.1084/jem.172.1.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes P.J, Scott R, Wheeler J. In vivo production of tumour necrosis factor-alpha and interleukin-6 in BALB/c mice inoculated intranasally with a high dose of respiratory syncytial virus. J. Med. Virol. 1994;42:323–329. doi: 10.1002/jmv.1890420402. [DOI] [PubMed] [Google Scholar]
- Horadagoda A, Eckersall P.D, Hodgson J.C, Gibbs H.A, Moon G.M. Immediate responses in serum TNF alpha and acute phase protein concentrations to infection with Pasteurella haemolytica A1 in calves. Res. Vet. Sci. 1994;57:129–132. doi: 10.1016/0034-5288(94)90094-9. [DOI] [PubMed] [Google Scholar]
- Jiang Z, Kunimoto M, Patel J.A. Autocrine regulation and experimental modulation of interleukin-6 expression by human pulmonary epithelial cells infected with respiratory syncytial virus. J. Virol. 1998;72:2496–2499. doi: 10.1128/jvi.72.3.2496-2499.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimman, T.G., 1993. The immune response to and pathogenesis of BRSV infections. In: Baker, J.C. (Ed.), Proceedings of the Symposium on BRSV infection. Vet. Med. 1196–1204.
- Kimman T.G, Straver P.J, Zimmer G.M. Pathogenesis of naturally acquired bovine respiratory syncytial virus infection in calves: morphologic and serologic findings. Am. J. Vet. Res. 1989;50:684–693. [PubMed] [Google Scholar]
- Langedijk J.P, de G.B, Berendsen H.J, Van O.J. Structural homology of the central conserved region of the attachment protein G of respiratory syncytial virus with the fourth subdomain of 55-kDa tumor necrosis factor receptor. Virology. 1998;243:293–302. doi: 10.1006/viro.1998.9066. [DOI] [PubMed] [Google Scholar]
- Larsen L.E, Uttenthal A, Arctander P, Tjornehoj K, Viuff B, Rontved C, Ronsholt L, Alexandersen S, Blixenkrone-Moller M. Serological and genetic characterisation of bovine respiratory syncytial virus (BRSV) indicates that Danish isolates belong to the intermediate subgroup: no evidence of a selective effect on the variability of G protein nucleotide sequence by prior cell culture adaption and passages in cell culture or calves. Vet. Microbiol. 1998;62:265–279. doi: 10.1016/s0378-1135(98)00226-0. [DOI] [PubMed] [Google Scholar]
- Li X.Y, Donaldson K, Brown D, MacNee W. The role of tumor necrosis factor in increased airspace epithelial permeability in acute lung inflammation. Am. J. Respir. Cell Mol. Biol. 1995;13:185–195. doi: 10.1165/ajrcmb.13.2.7626286. [DOI] [PubMed] [Google Scholar]
- Matsuda K, Tsutsumi H, Okamoto Y, Chiba C. Development of interleukin 6 and tumor necrosis factor alpha activity in nasopharyngeal secretions of infants and children during infection with respiratory syncytial virus. Clin. Diagn. Lab. Immunol. 1995;2:322–324. doi: 10.1128/cdli.2.3.322-324.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merolla R, Rebert N.A, Tsiviste P.T, Hoffmann S.P, Panuska J.R. Respiratory syncytial virus replication in human lung epithelial cells: inhibition by tumor necrosis factor alpha and interferon beta. Am. J. Respir. Cell Mol. Biol. 1995;152:1358–1366. doi: 10.1164/ajrccm.152.4.7551395. [DOI] [PubMed] [Google Scholar]
- Midulla F, Villani A, Panuska J.R, Dab I, Kolls J.K, Merolla R, Ronchetti R. Respiratory syncytial virus lung infection in infants: immunoregulatory role of infected alveolar macrophages. J. Infect. Dis. 1993;168:1515–1519. doi: 10.1093/infdis/168.6.1515. [DOI] [PubMed] [Google Scholar]
- Murphy F.A, Famfuet C.M, Bishop D.H.L, Ghabrial S.A, Jarvis A.W, Martelli G.P, Mayo M.A, Summers M.D. Virus taxonomy: sixth report on taxonomy on the international comittee on taxonomy of viruses. Arch. Virol. Suppl. 1995;10:265–274. [Google Scholar]
- Neuzil K.M, Tang Y.W, Graham B.S. Protective Role of TNF-alpha in respiratory syncytial virus infection in vitro and in vivo. Am. J. Med. Sci. 1996;311:201–204. doi: 10.1097/00000441-199605000-00001. [DOI] [PubMed] [Google Scholar]
- Nii A, Sone S, Orino E, Ogura T. Induction of a 26-kDa membrane-form tumor necrosis factor (TNF)-alpha in human alveolar macrophages. J. Leukoc. Biol. 1993;53:29–36. doi: 10.1002/jlb.53.1.29. [DOI] [PubMed] [Google Scholar]
- Ohmann H.B, Campos M, McDougall L, Lawman M.J, Babiuk L.A. Expression of tumor necrosis factor-alpha receptors on bovine macrophages, lymphocytes and polymorphonuclear leukocytes, internalization of receptor-bound ligand, and some functional effects. Lymphokine Res. 1990;9:43–58. [PubMed] [Google Scholar]
- Panuska J.R, Midulla F, Cirino N.M, Villani A, Gilbert I.A, McFadden E.R, Jr., Huang Y.T. Virus-induced alterations in macrophage production of tumor necrosis factor and prostaglandin E2. Am. J. Physiol. 1990;259:L396–L402. doi: 10.1152/ajplung.1990.259.6.L396. [DOI] [PubMed] [Google Scholar]
- Patel J.A, Kunimoto M, Sim T.C, Garofalo R, Eliott T, Baron S, Ruuskanen O, Chonmaitree T, Ogra P.L, Schmalstieg F. Interleukin-1 alpha mediates the enhanced expression of intercellular adhesion molecule-1 in pulmonary epithelial cells infected with respiratory syncytial virus. Am. J. Respir. Cell Mol. Biol. 1995;13:602–609. doi: 10.1165/ajrcmb.13.5.7576697. [DOI] [PubMed] [Google Scholar]
- Patel J.A, Jiang Z, Nakajima N, Kunimoto M. Autocrine regulation of interleukin-8 by interleukin-1alpha in respiratory syncytial virus-infected pulmonary epithelial cells in vitro. Immunology. 1998;95:501–506. doi: 10.1046/j.1365-2567.1998.00640.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persson K, Larsson I, Hallen S.C. Effects of certain inflammatory mediators on bovine neutrophil migration in vivo and in vitro. Vet. Immunol. Immunopathol. 1993;37:99–112. doi: 10.1016/0165-2427(93)90058-c. [DOI] [PubMed] [Google Scholar]
- Pryhuber G.S, Bachurski C, Hirsch R, Bacon A, Whitsett J.A. Tumor necrosis factor-alpha decreases surfactant protein B mRNA in murine lung. Am. J. Physiol. 1996;270:L714–L721. doi: 10.1152/ajplung.1996.270.5.L714. [DOI] [PubMed] [Google Scholar]
- Tartaglia L.A, Rothe M, Hu Y.F, Goeddel D.V. Tumor necrosis factor’s cytotoxic activity is signaled by the p55 TNF receptor. Cell. 1993;73:213–216. doi: 10.1016/0092-8674(93)90222-c. [DOI] [PubMed] [Google Scholar]
- Taylor G, Thomas L.H, Wyld S.G, Furze J, Sopp P, Howard C.J. Role of T-lymphocyte subsets in recovery from respiratory syncytial virus infection in calves. J. Virol. 1995;69:6658–6664. doi: 10.1128/jvi.69.11.6658-6664.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uttenthal A, Jensen N.P, Blom J.Y. Viral aetiology of enzootic pneumonia in Danish dairy herds: diagnostic tools and epidemiology. Vet. Rec. 1996;139:114–117. doi: 10.1136/vr.139.5.114. [DOI] [PubMed] [Google Scholar]
- Van Nhieu T, Misset B, Lebargy F, Carlet J, Bernaudin J.F. Expression of tumor necrosis factor-alpha gene in alveolar macrophages from patients with the adult respiratory distress syndrome. Am. Rev. Respir. Dis. 1993;147:1585–1589. doi: 10.1164/ajrccm/147.6_Pt_1.1585. [DOI] [PubMed] [Google Scholar]
- Viuff B, Uttenthal A, Tegtmeier C, Alexandersen S. Sites of replication of bovine respiratory syncytial virus in naturally infected calves as determined by in situ hybridization. Vet. Pathol. 1996;33:383–390. doi: 10.1177/030098589603300403. [DOI] [PubMed] [Google Scholar]
- Warren J.S, Yabroff K.R, Remick D.G, Kunkel S.L, Chensue S.W, Kunkel R.G, Johnson K.J, Ward P.A. Tumor necrosis factor participates in the pathogenesis of acute immune complex alveolitis in the rat. J. Clin. Invest. 1989;84:1873–1882. doi: 10.1172/JCI114374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wispe J.R, Clark J.C, Warner B.B, Fajardo D, Hull W.E, Holtzman R.B, Whitsett J.A. Tumor necrosis factor-alpha inhibits expression of pulmonary surfactant protein. J. Clin. Invest. 1990;86:1954–1960. doi: 10.1172/JCI114929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong G.H, Tartaglia L.A, Lee M.S, Goeddel D.V. Antiviral activity of tumor necrosis factor is signaled through the 55-kDa type I TNF receptor. J. Immunol. 1992;149:3350–3353. [PubMed] [Google Scholar]
- Yoo H.S, Maheswaran S.K, Srinand S, Ames T.R, Suresh M. Increased tumor necrosis factor-alpha and interleukin-1 beta expression in the lungs of calves with experimental pneumonic pasteurellosis. Vet. Immunol. Immunopathol. 1995;49:15–28. doi: 10.1016/0165-2427(95)05453-d. [DOI] [PubMed] [Google Scholar]
- Zeck-Kapp G, Kapp A, Busse R, Riede U.N. Interaction of granulocytes and endothelial cells upon stimulation with tumor necrosis factor-alpha: an ultrastructural study. Immunobiology. 1990;181:267–275. doi: 10.1016/s0171-2985(11)80518-8. [DOI] [PubMed] [Google Scholar]


