Abstract
Feline odontoclastic resorptive lesions (FORL), previously known as `neck lesions,' are commonly known in domestic, but also in non-domestic cats. They are characterized by odontoclastic resorptive processes, which take place at the dental root and at the periodontium. Chronic inflammation of gingiva and periodontium is believed to be an important etiological factor in the development of FORL. In this context, various feline viruses have been discussed to play a relevant role in the pathogenesis of these lesions. The aim of this project was to determine in a blinded study the incidence of FORL in 10 cats which were infected for several years with feline immunodeficiency virus (FIV), but were otherwise free of feline viral infections (feline leukemia virus, feline calicivirus, feline herpesvirus, feline parvovirus, feline coronavirus, feline syncytium-forming virus). Nine age-matched controls were kept under identical conditions, but free of FIV. Subgingival resorptive lesions were found in six of 10 FIV-positive cats, but in three of nine controls only. FIV-positive cats had significantly more often gingivae with an increased tendency for bleeding upon probing than FIV-negative cats (p=0.0055), and they had slightly more often hyperplastic gingivae (p=0.0867). In conclusion, signs characteristic of FORL such as subgingival lesions, granulomatous or hyperplastic gingivae with a tendency for bleeding, were found significantly more often in FIV-positive cats than in the controls (p=0.0198). Therefore, it was concluded that FIV infection is an important factor for the occurrence of FORL, possibly through immune suppression or changes of the (sub)gingival micro-environment. However, non-infected control cats also showed some evidence of FORL in the absence of all tested viral infections. Therefore, factors other than viral infections must also play a role in the development of FORL in cats.
Keywords: Cats, Feline immunodeficiency virus (FIV), Feline odontoclastic resorptive lesions (FORL), Feline viruses, Neck lesions
1. Introduction
Feline odontoclastic resorptive lesions (FORL) that were previously known as `neck lesions', are common in domestic cats (Schneck and Osborn, 1976; Lyon, 1992; van Wessum et al., 1992; Harvey, 1993). They have also been reported in wild felids (Berger et al., 1995, Berger et al., 1996a). FORLs are characterized by a defect of enamel, dentine, and cementum (Schneck and Osborn, 1976; von Schlup and Stich, 1982; Okuda and Harvey, 1992a). The destructive lesions are the result of odontoclastic resorption (Schneck and Osborn, 1976). The process starts on the periodontal ligament. Lagoons, lacunae and resorptive canals are formed (Berger et al., 1996a). The missing coronal substance is filled with gingival granulation tissue (Harvey, 1995). In addition, reparative phases can be observed forming bone or cementum-like tissue, which can result in fusion of tooth an alveolar bone (ankylosis) (Okuda and Harvey, 1992a). In contrast to caries, healthy dental tissue borders directly onto the resorbed areas (von Schlup and Stich, 1982; Lyon, 1992). FORLs are progressive and severe loss of tooth structure may occur (Lyon, 1992). Lesions, which extend into the pulp chamber, are very painful upon probing and a reaction of the patient (`chattering' of the jaws) will be elicited even under general anesthesia (Lyon, 1992; Harvey, 1993). The premolars are the most likely to be affected, but lesions are also found in molars, canine and incisor teeth (von Schlup, 1982; van Wessum et al., 1992). Chronic inflammation of gingivae and periodontium is considered to be an important etiological factor in the development of FORL. Thereby, different feline viruses including immunodeficiency inducing agents (feline immunodeficiency virus, FIV; feline leukemia virus, FeLV) have been discussed to play a relevant role (Okuda and Harvey, 1992a). Beside FIV and FeLV, feline calicivirus (FCV), feline herpesvirus (FHV) and feline syncytium-forming virus (FeSFV) are shed in the saliva (Pedersen, 1992). Oral inflammation is found commonly in FIV-infected or FCV-infected cats, and most studies report increased incidence and severity of oral disease when compared with control cats (Knowles et al., 1989; Yamamoto et al., 1989; Tenorio et al., 1991; Williams and Aller, 1992; Diehl and Rosychuk, 1993). In contrast, most studies failed to demonstrate a strong relationship between FeLV infection and oral disease (Knowles et al., 1989; Tenorio et al., 1991; Williams and Aller, 1992; Diehl and Rosychuk, 1993), and feline coronavirus (FCoV) has rarely been associated with oral lesions (Pedersen, 1992). No data are available about the influence of feline viral infections on the occurrence of FORL. The aim of this study was to determine the incidence of FORL in cats that were infected for several years with FIV, but were otherwise free of feline viruses.
2. Animals, material and methods
Ten specified pathogen-free (SPF) cats from Ciba-Geigy AG, Werk Stein, Sisseln, Switzerland, were housed in a specialized facility at the University of Zurich (group 1). They were intraperitoneally infected with FIV Z2 (Morikawa et al., 1991) at the age of 17 weeks (Lehmann et al., 1991). By the time of the study, these cats had been FIV-positive for 6 years and 9 months, but were still clinically healthy (Hofmann-Lehmann et al., 1997). Nine cats were kept under identical conditions as age-matched FIV-negative controls (group 2). FIV was monitored by detection of antibodies (recombinant TM-ELISA, Western blot) and by virus isolation from blood lymphocytes (Lutz et al., 1988; Calzolari et al., 1995). In addition, the cats were checked for FeLV p27 antigen by sandwich ELISA (Lutz et al., 1983), and for antibodies by immunofluorescence assay to the following viral infections as described: FCoV (Osterhaus et al., 1977), FeSFV (Kölbl and Lutz, 1992), FCV and feline herpes virus (FHV) (Hofmann-Lehmann et al., 1996). Antibodies to feline parvovirus (FPV) were also determined by immunofluorescence assay. To this end, CrFK cell monolayers were infected with CPV-2 kindly provided by Dr. Daniela Fehr, Veterinaria AG, Zurich, Switzerland. They were incubated until CPE reached about 50% of the cells. Cells were trypsinized, washed in PBS twice and adjusted to 50 000 cells/ml in PBS. Slides were prepared as described for FCoV (Osterhaus et al., 1977). Sera were tested at serial four-fold diluations starting at 1:25. The assay was evaluated with samples of 29 SPF cats, vaccinated twice against FPV (Vetamun Parvo, Veterinaria AG, Zurich, Switzerland). Samples taken before vaccination yielded titers of less than 25, while samples collected 3 weeks after the second vaccination produced titers up to 1600. Lymphocyte subsets were determined by flow cytometry in platelet-free whole blood as described (Hofmann-Lehmann et al., 1997). The cats were fed canned and dry cat food (Whiskas and Brekkies, Effems AG, Zug, Switzerland). For the present study, the cats were clinically examined and a complete dental inspection was performed. Examination of the cats was accomplished by one of us (M.B.) not knowing the FIV-status of the animals. Cats never received any dental care before that moment. The dental examination was performed under general anesthesia of the cats. Each tooth was probed with a dental explorer, and a complete dental record was filled in for each cat. Special attention was focused to the occurrence of subgingival resorptive lesions and painfulness of these lesions, of hyperplastic gingival tissue, gingival granulation tissue and tartar, and to signs of inflammation or increased tendency of bleeding of the gingivae. Anatomical nomenclature of teeth was applied: I1–I3=incisor tooth 1–3, C=canine tooth, P2–P4=premolar tooth 2–4, M1=molar tooth 1, right side upper jaw=quadrant I, left side upper jaw=quadrant II, left side lower jaw=quadrant III, right side lower jaw=quadrant IV. In order to confirm the presence of FORL that were detected upon clinical examination, one cat underwent dental radiographs after euthanasia (which was necessary for other reasons). Frequencies of clinical observations and serological results were compared, using the two-sided Fisher's exact test. Numeric data were analyzed for significant differences with the non-parametric Mann–Whitney U-test. Differences were considered significant if p<0.05.
3. Results
All cats were examined for FeLV antigen, and antibodies to FHV, FCV, FPV, FCoV, FeSFV and FIV. They were all negative for FeLV p27 antigen by ELISA, and for antibodies to FHV (<1:20), FCV (<1:20), FPV (<1:25), FCoV (<1:25) and FeSFV (<1:25). Cats of group 1 from which FIV had been re-isolated numerous times (Hofmann-Lehmann et al., 1997), were positive for antibodies to TM measured by ELISA, while cats of group 2 were ELISA-negative (Table 1 ). Cats of group 1 had significantly lower absolute CD4+ lymphocyte counts (p=0.0025, Fig. 1 ), and significant lower CD4+:CD8+ ratio (p=0.0043, data not shown) than cats of group 2.
Table 1.
Results of examination for feline viral infections
| (a) Group 1 (FIV-positive) | |||||||
| Cat | FIV (ELISA + virus isolation) | Feline leukemia virus (ELISA) | Feline parvo- virus (IFA) | Feline calici- virus (IFA) | Feline herpes- virus (IFA) | Feline corona- virus (IFA) | Feline syncy-tium forming virus (IFA) |
| 257 | TM pos. | p27-neg. | <1:25 | <1:20 | <1:20 | <1:25 | <1:25 |
| 261 | TM pos. | p27-neg. | <1:25 | <1:20 | <1:20 | <1:25 | <1:25 |
| 262 | TM pos. | p27-neg. | <1:25 | <1:20 | <1:20 | <1:25 | <1:25 |
| 266 | TM pos. | p27-neg. | <1:25 | <1:20 | <1:20 | <1:25 | <1:25 |
| 275 | TM pos. | p27-neg. | <1:25 | <1:20 | <1:20 | <1:25 | <1:25 |
| 279 | TM pos. | p27-neg. | <1:25 | <1:20 | <1:20 | <1:25 | <1:25 |
| 280 | TM pos. | p27-neg. | <1:25 | <1:20 | <1:20 | <1:25 | <1:25 |
| 284 | TM pos. | p27-neg. | <1:25 | <1:20 | <1:20 | <1:25 | <1:25 |
| 285 | TM pos. | p27-neg. | <1:25 | <1:20 | <1:20 | <1:25 | <1:25 |
| 288 | TM pos. | p27-neg. | <1:25 | <1:20 | <1:20 | <1:25 | <1:25 |
| Total | 10/10 | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 |
| (b) Group 2 (FIV-negative controls) | |||||||
| 258 | TM neg. | p27-neg. | <1:25 | <1:20 | <1:20 | <1:25 | <1:25 |
| 259 | TM neg. | p27-neg. | <1:25 | <1:20 | <1:20 | <1:25 | <1:25 |
| 260 | TM neg. | p27-neg. | <1:25 | <1:20 | <1:20 | <1:25 | <1:25 |
| 263 | TM neg. | p27-neg. | <1:25 | <1:20 | <1:20 | <1:25 | <1:25 |
| 267 | TM neg. | p27-neg. | <1:25 | <1:20 | <1:20 | <1:25 | <1:25 |
| 269 | TM neg. | p27-neg. | <1:25 | <1:20 | <1:20 | <1:25 | <1:25 |
| 271 | TM neg. | p27-neg. | <1:25 | <1:20 | <1:20 | <1:25 | <1:25 |
| 281 | TM neg. | p27-neg. | <1:25 | <1:20 | <1:20 | <1:25 | <1:25 |
| 282 | TM neg. | p27-neg. | <1:25 | <1:20 | <1:20 | <1:25 | <1:25 |
| Total | 0/9 | 0/9 | 0/9 | 0/9 | 0/9 | 0/9 | 0/9 |
Fig. 1.
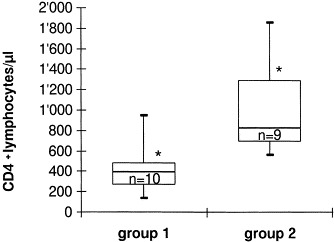
Box plots of absolute CD4+ lymphocyte counts of cats of group 1 or group 2 (*p=0.0025, Mann–Whitney U-test).
Gross visual dental inspection and probing with a dental explorer revealed subgingival resorption in six of 10 FIV-positive cats (group 1) and in only three of the FIV-negative controls of group 2 (Table 2 ). Resorptive lesions were confirmed by dental radiographs in one cat and were recognized as radiolucent areas as described (Harvey and Flax, 1992; Harvey, 1993; Berger et al., 1996b). In addition, ankylosis was demonstrated in some teeth in question. FIV-positive cats had significantly more often gingivae with increased tendency for bleeding upon probing than FIV-negative cats (p=0.0055), and they had slightly more often hyperplastic gingivae (p=0.0867). In sum, signs characteristic of FORL such as subgingival lesions, granulomatous or easily bleeding or hyperplastic gingiva were found significantly more often in FIV-positive cats (nine of 10) than in the controls (three of 9, p=0.0198). Signs of FORL were most frequently found in premolar teeth 3 and 4, to a lesser extent in molar and canine teeth, and not at all in incisor teeth. In addition, teeth were examined for the occurrence of tartar. The extent of tartar was scored for each tooth as strong positive (++=2), positive (+=1) or negative (0). Scores of all teeth of one cat were added up to a total tartar score. Surprisingly, FIV-positive cats had significantly lower mean tartar score than FIV-negative cats (p=0.0172, Table 2). Gingivitis was found in the majority of the FIV-positive cats (90%). This is significantly more often than for FIV-negative cats (p=0.0055, Table 2). Furthermore, FIV-positive animals suffered slightly more frequently from gingival tissue recession than the controls (p=0.867). Gingivae recession was found predominantly at the canine teeth.
Table 2.
Results of the dental examination
| (a) Group 1 (FIV-positive) | |||||||
|---|---|---|---|---|---|---|---|
| Cat | Subgingival resorptive lesion | Easily bleeding gingival tissue | Hyperplastic gingival tissue | Any sign of FORL | Tartar (total score) | Gingivitis | Gingival tissue recession |
| 257 | P4I | P4II | Yes | 8 | Yes | ||
| 261 | P2I, P4I, P4 II | P2I, P4I, P4II | Yes | 1 | Yes | ||
| 262 | P3IV, P4 IV | Yes | 2 | Yes | |||
| 266 | No | 2 | No | CII, CIII | |||
| 275 | P3III, P3IV, P4I P4I, P4II | P3I, P3II, P3III, P3IV, | P3I, P3III, P3IV | Yes | 12 | Yes | CI |
| 279 | P3I, P3II, P4II | P3II, P4II | Yes | 14 | Yes | ||
| 280 | P3II | P3IV, P4II, P4III | P3II | Yes | 0 | Yes | |
| 284 | P4I | P4I | Yes | 7 | Yes | CII | |
| 285 | CII, M1III | P3I, P4I, P4II | Yes | 17 | Yes | CIV | |
| 288 | P3I, P3II, P4II | Yes | 4 | Yes | |||
| Total | 6/10 | 8/10 | 4/10 | 9/10 | Mean 6.7 | 9/10 | 4/10 |
| (b) Group 2 (FIV-negative controls) | |||||||
| 258 | P3IV | – | Yes | 11 | No | – | |
| 259 | – | – | – | No | 8 | No | – |
| 260 | – | – | – | No | 14 | No | – |
| 263 | P3III, P3IV | P3III, P3IV | Yes | 10 | Yes | – | |
| 267 | P3III | – | – | Yes | 16 | No | – |
| 269 | – | – | – | No | 15 | No | – |
| 271 | – | – | – | No | 20 | No | – |
| 281 | – | – | – | No | 25 | Yes | – |
| 282 | – | – | – | No | 10 | No | – |
| Total | 3/9 | 1/9 | 0/9 | 3/9 | Mean 14.4 | 2/9 | 0/9 |
| p | ns | p=0.0055 | ns (p=0.0867) | p=0.0198 | p=0.0172 | p=0.0055 | ns (p=0.0867) |
4. Discussion
The aim of the study was to elucidate the influence of FIV infection on the occurrence of FORL. Ten cats (group 1) were infected with FIV for 6 years, but were otherwise free from feline viruses (FeLV, FHV, FPV, FCoV, FCV and FeSFV, Table 1). More often these cats showed clinical signs of FORL than the control animals or group 2 (Table 2). It is broadly accepted that the age of the cats does influence the extent of FORL (von Schlup, 1982; Coles, 1990; van Wessum et al., 1992; Harvey, 1993). In the present study, however, the animals had all the same age. They were offspring from the same litters. In addition, the two groups were controlled for sex of the animals – a factor that was found to be associated with the extent of FORL in one study only (van Wessum et al., 1992). The two groups were kept under identical conditions and food intake and the kind of food provided to the animals were identical. This is important as nutritional factors were suspected to influence the pathogenesis of FORL (von Schlup, 1982; Zetner, 1990, Zetner, 1992). Overall, the only obvious difference between groups 1 and 2 was the FIV status (Table 1). Therefore, it may be stated that FIV infection significantly increases the occurrence of FORL, at least after several years of infection. The exact pathogenesis that leads to increased incidence of resorptive lesions is unknown. However, it has to be recognized that FIV-infected cats of group 1 suffered more often from gingivitis than control animals (Table 2), an observation that is well-documented also under field conditions (Knowles et al., 1989; Sparger et al., 1989; Yamamoto et al., 1989). FIV is excreted through saliva of infected animals (Pedersen and Barlough, 1991). This might lead to a change of the local micro-environment, for example, to an alteration in local cytokine expression. Different cytokines such as interleukin-6 and interleukin-1β that are stimulators of osteoclastic cells, and transforming growth factor β that stimulates osteoblasts, have been postulated to influence the formation of FORL (Okuda and Harvey, 1992a, Okuda and Harvey, 1992b; Harvey, 1993). Furthermore, FIV infection leads to an impaired humoral and cellular immune response and finally to an AIDS-like syndrome in infected cats (Pedersen et al., 1987; Ishida and Tomoda, 1990; Pedersen and Barlough, 1991). CD4+ T lymphocytes progressively decline in FIV-infected cats (Barlough et al., 1991; Torten et al., 1991), but also elevated serum globulin fractions were demonstrated in such animals (Shelton et al., 1995; Hofmann-Lehmann et al., 1997). The FIV-infected cats of the present study had significantly lowered CD4+ blood lymphocyte counts (Fig. 1). Decreased, but also chronically activated immunity may have harmful effects in the oral cavity (Pedersen and Barlough, 1991). Interestingly, at the same time FIV-infected cats had significantly less tartar than the controls (Table 2). This might be an additional sign of a changed gingival micro-environmental climate in these cats.
It is also remarkable that the cats of group 2 showed evidence of some FORL (Table 2) even in the absence of all the viral infections tested (FIV, FeLV, FHV, FPV, FCoV, FCV and FeSFV, Table 1). Therefore, factors other than viral infections must play an important role in the development of FORL. The appearance of FORL is a rather recent phenomenon (van Wessum et al., 1992). Domestication and specifically the diets fed to domestic cats in the last 20 years have been hypothesized as a cause (von Schlup, 1982; Okuda and Harvey, 1992a; Zetner, 1992; Harvey, 1995). However, the influence of these factors must be minor as FORL were also found in free-ranging lions and a leopard (Berger et al., 1996a).
At the time of examination, the cats in the present study were 7 years and 3 months old. Under field conditions, 42% of cats at this age in Switzerland are reported to suffer from FORL (von Schlup, 1982). In the present study, only 33 percent of the non-infected control cats were affected (Table 2). As numbers are small, this might be a random difference. However, this fact may also be indicative for the influence of one of the viruses excluded in the present study (FeLV, FHV, FPV, FCoV, FCV and FeSFV) on the formation of FORL. Oral inflammation is also associated with infection by FCV (Knowles et al., 1989; Diehl and Rosychuk, 1993). Furthermore, FeSFV is known to lead to increased occurrence of gingivitis/stomatitis in infected cats (Kölbl and Lutz, 1992). Thus, these viruses could also influence the pathogenesis of FORL.
In conclusion, FIV was found to enhance the formation of FORLs. However, other factors must be as important, as virus-free cats also developed FORL, although to a lesser extent.
Acknowledgements
This study was supported by a grant from the Union Bank of Switzerland on behalf of a customer, and the European Concerted Action on Feline AIDS. The cat food was kindly donated by Effems AG, Zug, Switzerland. FHV, FCV and CPV were kindly provided by Dr. Daniela Fehr, Veterinaria AG, Zurich, Switzerland. We thank Dr. Margarete Akens, Felicitas Boretti, Peter Fidler and Celestine Wolfensberger for excellent technical assistance.
References
- Barlough J.E., Ackley C.D., George J.W., Levy N., Acevedo R., Moore P.F., Rideout B.A., Cooper M.D., Pedersen N.C. Acquired immune dysfunction in cats with experimentally induced feline immunodeficiency virus infection: Comparison of short-term and long-term infections. J. Acquir. Immune. Defic. Syndr. 1991;4:219–227. [PubMed] [Google Scholar]
- Berger M., Schawalder P., Stich H., Lussi A. Neck Lesion bei Grosskatzen; Untersuchungen beim Leoparden (Panthera pardus) Kleintierpraxis. 1995;40:537–549. [Google Scholar]
- Berger, M., Schawalder, P., Stich, H., Lussi, A., 1996a. Feline dental resorptive lesions in captive and wild leopards and lions. J. Vet. Dent. 13, 13–21
- Berger, M., Schawalder, P., Stich, H., Lussi, A., 1996b. Differentialdiagnose von resorptiven Zahnerkrankungen (FORL) und Karies. Schweiz. Arch. Tierheilkd. 138, 546–551 [PubMed]
- Calzolari M., Young E., Cox D., Davis D., Lutz H. Serological diagnosis of feline immundeficiency virus infection using recombinant transmembrane glycoprotein. Vet. Immunol. Immunopathol. 1995;46:83–92. doi: 10.1016/0165-2427(94)07008-u. [DOI] [PubMed] [Google Scholar]
- Coles S. The prevalence of buccal cervical root resorptions in Australian cats. J. Vet. Dent. 1990;7:14–16. [Google Scholar]
- Diehl K., Rosychuk R.A.W. Feline gingivitis–stomatitis–pharyngitis. Vet. Clin. North Am. Small Anim. Pract. 1993;23:139–153. doi: 10.1016/s0195-5616(93)50009-8. [DOI] [PubMed] [Google Scholar]
- Harvey C.E. Feline dental resorptive lesions. Semin. Vet. Med. Surg. (Small. Anim) 1993;8:187–196. [PubMed] [Google Scholar]
- Harvey, C.E., 1995. Feline oral pathology, diagnosis and management. Manual of Small Animal Dentistry. BSAVA, pp. 129–138
- Harvey C.E., Flax B.M. Feline oral–dental radiographic examination and interpretation. Vet. Clin. North Am. Small Anim. Pract. 1992;22:1279–1295. doi: 10.1016/s0195-5616(92)50127-9. [DOI] [PubMed] [Google Scholar]
- Hofmann-Lehmann R., Fehr D., Grob M., Elgizoli M., Packer C., Martenson J.S., O'Brien S.J., Lutz H. Prevalence of antibodies to feline parvovirus, calicivirus, herpesvirus, coronavirus and immunodeficiency virus and of feline leukemia virus antigen and the interrelationship of these viral infections in free-ranging lions in East Africa. Clin. Diagn. Lab. Immunol. 1996;3:554–562. doi: 10.1128/cdli.3.5.554-562.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann-Lehmann R., Holznagel E., Ossent P., Lutz H. Parameters of disease progression in long-term experimental feline retrovirus (feline immunodeficiency virus and feline leukemia virus) infections: Hematology, clinical chemistry and lymphocyte subsets. Clin. Diagn. Lab. Immunol. 1997;4:33–42. doi: 10.1128/cdli.4.1.33-42.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishida T., Tomoda I. Clinical staging of feline immunodeficiency virus infection. Jpn. J. Vet. Sci. 1990;52:645–648. doi: 10.1292/jvms1939.52.645. [DOI] [PubMed] [Google Scholar]
- Knowles J.O., Gaskell R.M., Gaskell C.J., Harvey C.E., Lutz H. Prevalence of feline calicivirus, feline leukaemia virus and antibodies to FIV in cats with chronic stomatitis. Vet. Rec. 1989;124:336–338. doi: 10.1136/vr.124.13.336. [DOI] [PubMed] [Google Scholar]
- Kölbl S., Lutz H. Die Infektion mit felinem Spumavirus (FeSFV): Häufigkeit bei Katzen in Oesterreich und Beziehung zur Infektion mit dem felinen Immunschwächevirus (FIV) Kleintierpraxis. 1992;37:307–318. [Google Scholar]
- Lehmann R., Franchini M., Aubert A., Wolfensberger C., Cronier J., Lutz H. Vaccination of cats experimentally infected with feline immunodeficiency virus, using a recombinant feline leukemia virus vaccine. J. Am. Vet. Med. Assoc. 1991;199:1446–1452. [PubMed] [Google Scholar]
- Lutz H., Pedersen N.C., Durbin R., Theilen G.H. Monoclonal antibodies to three epitopic regions of feline leukemia virus p27 and their use in enzyme-linked immunosorbent assay of p27. J. Immunol. Methods. 1983;56:209–220. doi: 10.1016/0022-1759(83)90413-1. [DOI] [PubMed] [Google Scholar]
- Lutz H., Arnold P., Hubscher U., Egberink H., Pedersen N., Horzinek M.C. Specificity assessment of feline T-lymphotropic lentivirus serology. Zentralbl. Veterinarmed. B. 1988;35:773–778. doi: 10.1111/j.1439-0450.1988.tb00559.x. [DOI] [PubMed] [Google Scholar]
- Lyon K.F. Subgingival odontoclastic resorptive lesions: Classification, treatment, and results in 58 cats. Vet. Clin. North Am. Small Anim. Pract. 1992;22:1415–1432. doi: 10.1016/s0195-5616(92)50135-8. [DOI] [PubMed] [Google Scholar]
- Morikawa S., Lutz H., Aubert A., Bishop D.H. Identification of conserved and variable regions in the envelope glycoprotein sequences of two feline immunodeficiency viruses isolated in Zurich. Switzerland. Virus Res. 1991;21:53–63. doi: 10.1016/0168-1702(91)90071-3. [DOI] [PubMed] [Google Scholar]
- Okuda, A., Harvey, C.E., 1992a. Etiopathogenesis of feline dental resorptive lesions. Vet. Clin. North Am. Small Anim. Pract. 22, 1385–1404 [DOI] [PubMed]
- Okuda, A., Harvey, C.E., 1992b. Immunohistochemical distributions of interleukins as possible stimulators of odontoclastic resorption activity in feline dental resorptive lesions. Proc. Vet. Dent. Forum. 6, 41–43
- Osterhaus A.D., Horzinek M.C., Reynolds D.J. Seroepidemiology of feline infectious peritonitis virus infections using transmissible gastroenteritis virus as antigen. Zentralbl. Veterinarmed. B. 1977;24:835–841. doi: 10.1111/j.1439-0450.1977.tb00976.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen N.C. Inflammatory oral cavity diseases of the cat. Vet. Clin. North Am. Small Anim. Pract. 1992;22:1323–1345. doi: 10.1016/S0195-5616(92)50130-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen N.C., Barlough J.E. Clinical overview of feline immunodeficiency virus. J. Am. Vet. Med. Assoc. 1991;199:1298–1305. [PubMed] [Google Scholar]
- Pedersen N.C., Ho E.W., Brown M.L., Yamamoto J.K. Isolation of a T-lymphotropic virus from domestic cats with an immunodeficiency-like syndrome. Science. 1987;235:790–793. doi: 10.1126/science.3643650. [DOI] [PubMed] [Google Scholar]
- Schneck G.W., Osborn J.W. Neck lesions in the teeth of cats. Vet. Rec. 1976;99:100. doi: 10.1136/vr.99.6.100. [DOI] [PubMed] [Google Scholar]
- Shelton G.H., Linenberger M.L., Persik M.T., Abkowitz J.L. Prospective hematologic and clinicopathological study of asymptomatic cats with naturally acquired feline immunodeficiency virus infection. J. Vet. Intern. Med. 1995;9:133–140. doi: 10.1111/j.1939-1676.1995.tb03286.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparger E.E., Luciw P.A., Elder J.H., Yamamoto J.K., Lowenstine L.J., Pedersen N.C. Feline immunodeficiency virus is a lentivirus associated with an AIDS-like disease in cats. AIDS. 1989;3:43–49. doi: 10.1097/00002030-198901001-00006. [DOI] [PubMed] [Google Scholar]
- Tenorio A.P., Franti C.E., Madewell B.R., Pedersen N.C. Chronic oral infections of cats and their relationship to persistent oral carriage of feline calici-, immunodeficiency, or leukemia viruses. Vet. Immunol. Immunopathol. 1991;29:1–14. doi: 10.1016/0165-2427(91)90048-h. [DOI] [PubMed] [Google Scholar]
- Torten M., Franchini M., Barlough J.E., George J.W., Mozes E., Lutz H., Pedersen N.C. Progressive immune dysfunction in cats experimentally infected with feline immunodeficiency virus. J. Virol. 1991;65:2225–2230. doi: 10.1128/jvi.65.5.2225-2230.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Wessum R., Harvey C.E., Hennet P. Feline dental resorptive lesions. Prevalence patterns. Vet. Clin. North Am. Small Anim. Pract. 1992;22:1405–1416. doi: 10.1016/s0195-5616(92)50134-6. [DOI] [PubMed] [Google Scholar]
- von Schlup D. Epidemiologische und morphologische Untersuchungen am Katzengebiss I. Mitteilung: Epidemiologische Untersuchungen. Kleintierpraxis. 1982;27:87–94. [Google Scholar]
- von Schlup D., Stich H. Epidemiologische und morphologische Untersuchungen am Katzengebiss. II Mitteilung: Morphologische Untersuchungen der neck lesions. Kleintierpraxis. 1982;27:179–188. [Google Scholar]
- Williams C.A., Aller M.S. Gingivitis/stomatitis in cats. Vet. Clin. North Am Small Anim. Pract. 1992;22:1361–1383. doi: 10.1016/s0195-5616(92)50132-2. [DOI] [PubMed] [Google Scholar]
- Yamamoto J.K., Hansen H., Ho E.W., Morishita T.Y., Okuda T., Sawa T.R., Nakamura R.M., Pedersen N.C. Epidemiologic and clinical aspects of feline immunodeficiency virus isolation in cats from the continental United States and Canada and possible mode of transmission. J. Am. Vet. Med. Assoc. 1989;194:213–220. [PubMed] [Google Scholar]
- Zetner K. Neck Lesions bei der Katze: Diagnostisch-aetiologische Untersuchungen über Zusammenhänge zwischen Röntgenbefund und Fütterung. Waltham Report. 1990;30:15–23. [Google Scholar]
- Zetner K. The influence of dry food and the development of feline neck lesions. J. Vet. Dent. 1992;9:4–6. [PubMed] [Google Scholar]


