Abstract
It is well known that piglets congenitally infected with porcine reproductive and respiratory syndrome virus (PRRSV) can be viremic at birth, and that preweaning mortality due to secondary infections often increases during acute outbreaks of PRRS. Therefore, an immunosuppressive effect of in utero infection has been suggested. The aim of the present study was to characterise the changes of leukocyte populations in piglets surviving in utero infection with PRRSV. A total of 27 liveborn uninfected control piglets and 22 piglets infected transplacentally with a Danish strain of PRRSV were included. At 2 and 4 weeks of age, 21 of 22 (96%) and 7 of 14 (50%) examined infected piglets were still viremic, whereas PRRSV could not be detected in the six infected piglets examined at 6 weeks of age. Flow cytometry analysis was used to determine the phenotypic composition of leukocytes in peripheral blood and bronchoalveolar lavage fluid (BALF) of 2-, 4- and 6-week-old infected piglets and age-matched uninfected controls. The key observation in the present study is that high levels of CD8+ cells constitute a dominant feature in peripheral blood and BALF of piglets surviving in utero infection with PRRSV. In BALF, the average high level of CD8+ cells in 2-week-old infected piglets (33.4±12.6%) was followed by a decline to 7.3±3.0 and 11.1±3.0% at 4 and 6 weeks of age. BALF of control piglets contained 1.6±0.9, 2.3±1.8 and 1.9±0.5% CD8+ cells, only. In peripheral blood, however, the average number of CD8+ cells remained at high levels in the infected piglets throughout the post-natal experimental period (2.8±1.9, 2.9±1.8 and 3.2±1.7×106 CD8+ cells/ml at 2, 4 and 6 weeks, respectively). In the controls, the average levels of CD8+ cells were 0.9±0.2, 1.9±1.7 and 1.6±0.5×106/ml, respectively. Furthermore, the numbers of CD2+, CD4+CD8+ and SLA-classII+ cells, respectively, in peripheral blood, together with the levels of CD2+ and CD3+ cells in BALF were increased in the infected piglets infected in utero compared to the uninfected controls.
The kinetic analyses carried out in the present study reflect that in utero infection with PRRSV modulates immune cell populations in peripheral blood and BALF of surviving piglets. The observed changes are characterised by high levels of CD8+ cells supporting an important role of these cells in PRRSV infection. The present results, however, do not support the existence of post-natal immunosuppression following in utero infection with PRRSV.
Keywords: Pigs, PRRSV, In utero, Flow cytometry, Leukocyte subpopulations
Abbreviations: SLA, swine leukocyte antigen; FSC, forward light scattering; SSC, side light scattering; BALF, bronchoalveolar lavage fluid; PPAM, porcine pulmonary alveolar macrophages; WBC, white blood cells; CD, cluster of differentiation
1. Introduction
Porcine reproductive and respiratory syndrome (PRRS) is a severe disease in pigs characterised by reproductive failure in sows and gilts, increase in preweaning mortality and pneumonia in young growing pigs. The causal agents of the disease is a positive-stranded enveloped RNA virus (Wensvoort et al., 1991) designated PRRS virus (PRRSV) which primarily replicates in macrophages.
The clinical signs of PRRS vary widely, possibly due to a variety of modifying factors, e.g. differences in genetic susceptibility, environmental factors, management, immune status, virus strain differences or a combination of more factors (Robertson, 1992, Halbur et al., 1995, Polson, 1995; Goldberg et al., 2000a, Goldberg et al., 2000b).
Field reports describe an increased incidence and severity of secondary infections, e.g. Streptococcus suis, Haemophilus parasuis, Mycoplasma hyopneumonia, Actinobaccillus pleuropneumonia, Salmonella spp. and swine influenza virus, in swine herds following PRRSV infection (Keffaber et al., 1992, Collins and Rossow, 1993, Zeman et al., 1993, Done and Paton, 1995). Although the current experimental evidence for any interaction of PRRSV with other pathogens is ambiguous (for review, see Drew, 2000), a number of reports support the assumption that PRRSV may exacerbate secondary infections (Carvalho et al., 1997, Feng et al., 2001, Galina et al., 1994, Van Reeth et al., 1996, Wills et al., 2000). If indeed there is an increased susceptibility to other pathogens following PRRSV infection, an underlying mechanism could be suppression of the immune system in infected pigs, as also often suggested. Based on clinical and experimental observations, it appears likely that PRRSV interacts with the immune system of the pig paving the way for increased susceptibility to secondary infections. Drew (2000) summarised the present knowledge concerning immunomodulation by PRRSV and concluded that (i) immunomodulation is likely to be present, at local level, in the lung, (ii) general immunosuppression is possible, but not proven, (iii) the degree of such immunomodulation and its significance for piglet health is unclear, (iv) certain experimental evidence for enhancement of the immune system exists.
The majority of the studies dealing with the influence of PRRSV on immune functions of the pig have been carried out either in vitro (Choi et al., 1994, Oleksiewicz and Nielsen, 1999, Thanawongnuwech et al., 1997, Bautista and Molitor, 1999, Lopez-Fuertes et al., 2000, Thanawongnuwech et al., 2001) or in vivo using young pigs infected at various ages (Shimizu et al., 1996, Vezina et al., 1996, Bautista and Molitor, 1997, Nielsen and Bøtner, 1997, Albina et al., 1998, Kawashima et al., 1999, Lopez-Fuertes et al., 1999, Labarque et al., 2000, Samsom et al., 2000). However, no previous studies have addressed the hypothesis of immune changes in piglets following in utero infection with PRRSV. It is well known that piglets can be viremic at birth (Bøtner et al., 1994, Kranker et al., 1998, Mengeling et al., 1998), and that preweaning mortality due to secondary infections often increases during acute outbreaks of PRRS (Stevenson et al., 1993, Done and Paton, 1995, Feng et al., 2001, Nielsen et al., 2002). Feng et al. (2001) and Nielsen et al. (2002) suggested an immunosuppressive effect of in utero infection with PRRSV, and therefore we decided to study the interaction of PRRSV with various facets of the immune system of surviving piglets infected in utero.
In the present paper, we describe the studies on the phenotypic composition of leukocytes in peripheral blood and bronchoalveolar fluid of 2-, 4- and 6-week-old piglets infected in utero with PRRSV.
2. Materials and methods
2.1. Animals
Four healthy, pregnant sows were procured from a PRRSV-seronegative swine herd. The sows had been routinely vaccinated against porcine parvovirus (PPV) prior to service. Approximately 2 months before estimated farrowing, the sows were transferred to the animal isolation units at the Danish Veterinary Institute.
2.2. Experimental design
In preliminary experiments, we developed a PRRSV pig infection model which provides only few dead foetuses but high numbers of in utero infected piglets surviving transplacental infection with PRRSV. This model was applied in the present study: on day 90 of gestation, two pregnant sows (infected sows 97 and 208) were challenged with a sixth porcine pulmonary alveolar macrophages (PPAM) cell culture passage of a Danish PRRSV isolate (Bøtner et al., 1994) with a titre of 107 TCID50/ml. One ml of challenge virus (107 TCID50) was diluted to a total volume of 4 ml in Eagle’s minimum essential medium (EMEM) and given intranasally to each sow, 2 ml per nostril. At the same stage of gestation, two sows (control sows 199 and 303) were sham-inoculated with EMEM. General health, appetite, and rectal body temperature of the sows were monitored daily.
For virological and serological examination, serum blood samples were collected from sows on days 0 and 14 post-inoculation (PI), at farrowing, and at weaning of the piglets at 4 weeks of age. All live piglets were blood sampled before ingestion of colostrum, and 2, 4 and 6 weeks after birth. Serum samples were obtained for virological and serological examination, and EDTA-stabilised samples for total white blood cell (WBC) counts and flow cytometry analysis. From stillborn pigs and from piglets dying during the observation period, lung and spleen tissue, and thoracic fluids were collected.
Two, 4 and 6 weeks after birth, numbers of 8, 8 and 6 infected piglets, and 7, 11 and 9 control piglets, respectively, were euthanised for examination. At necropsy, tissue samples collected from the left lung lobes and the spleen were pooled at weight ratio of 4:1 for the individual pigs for virological examination. Furthermore, lung tissues were routinely processed for histopathological examination.
Bronchoalveolar lavage fluid (BALF) was collected as follows. After euthanasia, the lungs, heart and trachea were removed. Before removal of the left lung for examination (see above), the left main bronchial branch was cross-clamped in order to prevent escape of fluid in connection with subsequent lavage. The trachea was cannulated, and the lobes of the right lung were repeatedly washed with lung lavage fluid consisting of PBS supplemented with antibiotics (streptomycin, 0.1 g/l; neomycin, 0.05 g/l; penicillin, 106 IU/l; amphotericin, 8 mg/l). The amount of fluid varied from 200 to 500 ml, depending on the size of piglet. Routinely, the recovered BALF cells were washed once. However, when reddening of the pellet indicated the presence of erythrocytes, an initial wash with 0.83% NH4Cl was performed to lyse these cells. The centrifugations were carried out at 800×g at 20 °C. The cell pellet was resuspended in EMEM supplemented with 20% FCS to a final concentration, typically between 5 and 10×107 cells/ml. Ten percent DMSO was added, and ampoules containing 1.5 ml cell suspension were stored on liquid nitrogen until use.
All experimental procedures and animal management protocols were undertaken in accordance with the requirements of the Danish Animal Experiments Inspectorate.
2.3. Virus isolation
Examination of serum, thoracic fluids, and lung/spleen tissue homogenates for PRRSV was carried out using PPAM as previously described (Bøtner et al., 1994). Furthermore, the tissue homogenates were examined for other porcine viruses known to be present among Danish pigs, i.e. porcine adenovirus, swine influenza virus, porcine enterovirus, porcine parvovirus (PPV), porcine respiratory coronavirus, haemagglutinating encephalomyelitis virus, and porcine circovirus types 1 (PCV1) and 2 (PCV2) according to the standard procedures used at DVI.
2.4. Serological examination
Sera and thoracic fluids were examined for antibodies to PRRSV in an immunoperoxidase monolayer assay (IPMA), carried out as a double test using a European PRRSV strain and an American PRRSV strain in parallel (Sørensen et al., 1998).
2.5. Immunological examination
2.5.1. Total WBC counts
The WBC were counted using a semi-automated animal blood cell counter (Vet abc™, ABX, Montpellier, France).
2.5.2. Flow cytometry analyses
For the phenotyping of leucocytes in peripheral blood and BALF by flow cytometry, both single-labelling and double-labelling methods for defining the different subpopulations were employed. To this end, the following murine monoclonal antibodies (mAb) directed against or cross-reactive with porcine leukocyte cluster of differentiation (CD) antigens were used: anti-SLA-classI (anti-SLAI) (mAb PT85A, Davis et al., 1987; VMRD Inc., Pullman, WA, USA) reacting with all nucleated cells; anti-SLA-classII (anti-SLAII) (mAb MSA3, Hammerberg and Schurig, 1986; the hybridoma of this mAb was kindly placed at our disposal by Dr. A. Saalmüller, Federal Research Centre for Virus Diseases of Animals, Tübingen, Germany) reacting with B cells, some T cells, monocytes/macrophages; anti-CD3 (mAb PPT3, Yang et al., 1996; mAb 3E8, VMRD Inc., Pullman, WA, USA) reacting with T cells; anti-wCD21 (mAb CC51, Naessens et al., 1990; HB271, ATCC, Manassas, VA, USA) reacting with bovine and cross-reacting with porcine B cells; anti-SWC3 (mAb 74-22-15, Pescovitz et al., 1984) reacting with monocytes, macrophages and granulocytes; anti-CD2 (mAb MSA4, Hammerberg and Schurig, 1986) reacting with T cells, natural killer (NK) cells and thymocytes; anti-CD4 (mAb 74-12-4, Pescovitz et al., 1984) reacting with T helper (Th) cells; anti-CD8 (mAb 76-2-11, Pescovitz et al., 1984) reacting with cytotoxic T (Tc) cells and NK cells. The hybridomas of the latter 4 mAb were kindly placed at our disposal by Dr. J.K. Lunney, USDA, Beltsville, MD, USA. Hybridoma supernatants were produced at DVI, Lindholm, for all but two mAbs (anti-SLAI and anti-CD3). Extrathymic cells reacting with both mAb 74-12-4 and mAb 76-2-11 are characterised as CD4+CD8+ memory cells (Zuckermann, 1999). An overview of characterisation of swine leukocytes using differentiation antigens is given by Samsom et al. (2000).
A FACScan flow cytometer (Becton Dickinson, San José, CA, USA) was used for the analyses. For phenotyping, four parameters were stored: forward light scattering (FSC), sideward light scattering (SSC), green (FL1) and orange (FL2) fluorescence. For viability test using propidium iodide, FL3 was stored. In each test, 10,000 cells were counted, and data were analysed using CellQuest software (Becton Dickinson).
2.5.3. Single-colour flow cytometry
2.5.3.1. Blood samples
An indirect immunofluorescence staining of whole blood using a lyse/wash procedure was used. A volume of 100 μl EDTA-stabilised blood was incubated with 25 or 50 μl of mAb (undiluted hybridoma culture supernatant, except for mAb PT85A which was diluted mouse ascites fluid) for 30 min. Two millilitres of 10% FACS™ lysing solution (Becton Dickinson) were added and the samples were then incubated for 10 min.
After washing with 2 ml PBS supplemented with 1% pig serum and 0.1% sodium azide (washing buffer), the cells were incubated with 15 μl fluorescein isothiocyanate (FITC)-conjugated rabbit anti-mouse IgG F(ab′)2 fragment (F0313, DAKO, Denmark) diluted in washing buffer for 30 min. After an additional wash, the cells were finally resuspended in FACSFlow™ sheat-fluid (Becton Dickinson). All incubations were performed at room temperature in the dark and centrifugations were carried out at 490×g at 20 °C. Controls without any reagents, or with secondary antibody, only, were included for each animal. The samples were stored at 4 °C until flow cytometric examination within 3 h.
For B cell detection a three-step method was used. The cells were labelled with anti-wCD21 followed by lysing and washing as above. The cells were then incubated for 30 min at room temperature with 25 μl biotin-conjugated goat–anti-mouse (BIOGAM) IgG (Jackson Immuno Research Laboratories (JIRL) Inc., West Grove, PA, USA) diluted in washing buffer. After another washing, the cells were incubated with R-phycoerythrin (RPE)-conjugated streptavidin (R0438, DAKO, Denmark). Otherwise, the procedure was as above.
Generally, the single-colour analysis was carried out on cells within the mononuclear cell (MNC) region, corresponding to lymphocytes and monocytes, individually gated for each animal using FSC/SSC dot plot (Fig. 1 a). For B cell analysis, however, the lymphocyte region was used in order to avoid interpretation errors due to non-specific staining of monocytes. Differentiation of leukocyte populations was done using the anti-SWC3 mAb 74-22-15 labelled samples in a FL1-SWC3/SSC dot plot (Fig. 1b).
Fig. 1.
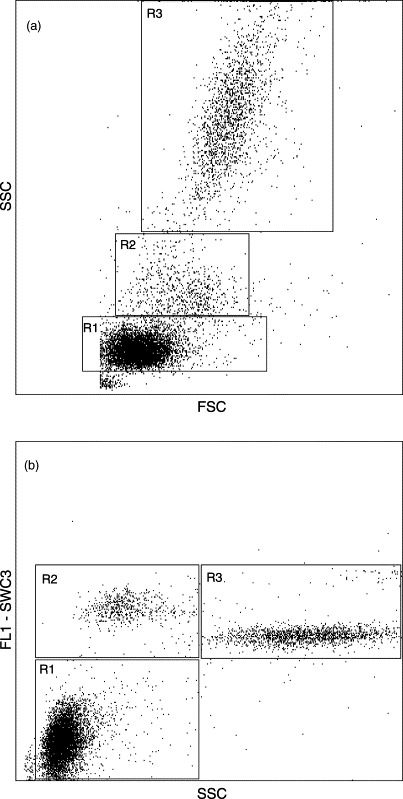
FSC/SSC (a) and FL1-SWC3/SSC (b) dot plots of peripheral blood leukocytes from a representative 4-week-old uninfected control piglet. Analysis gates for lymphocytes (R1) and mononuclear cells (lymphocytes (R1) and monocytes (R2)) were defined on the basis of cell size and granularity (a) combined with differentiation of leukocyte populations using the anti-SWC3 mAb 74-22-15 reactive with monocytes/macrophages and granulocytes (b). SWC3− cells with low granularity were defined as lymphocytes (R1), SWC3+ cells with low granularity as monocytes (R2), and SWC3+ cells with high granularity as granulocytes (R3). Mononuclear cell analysis was performed using R1+R2.
2.5.3.2. BALF cells
An indirect three-step immunofluorescence staining with wash/but without lysing of BALF cells was used. Initially, BALF cells were thawed at 37 °C and immediately transferred to 10 ml 37 °C warm EMEM. After washing, approximately 106 cells were incubated for 30 min with the various mAbs also used for the blood samples. Hereafter, the procedure used was similar to that for single-colour labelling of B cells (described above).
For cell viability analysis, BALF cells were labelled with propidium iodide, 1 μg/ml (Sigma).
2.5.4. Double-colour flow cytometry
Monoclonal antibodies mAb 74-12-11 (anti-CD4) and mAb 76-2-11 (anti-CD8) were purified from hybridoma cell culture supernatants by protein A chromatography and labelled with FITC or biotin, respectively, using standard protocols. A direct immunofluorescence staining of whole blood using a lyse/wash procedure was used. CD4/CD8 double-stained samples were prepared by using 100 μl EDTA-stabilised blood incubated with 25 μl FITC-conjugated anti-CD4 and 25 μl biotin-conjugated anti-CD8 for 30 min. After one wash, RPE-conjugated streptavidin was added, and the samples were incubated for 30 min.
After another wash, the cells were finally resuspended in sheat-fluid, stored at 4 °C and examined by flow cytometry within 3 h.
Cells labelled with one of the conjugated mAbs, only, and cells labelled with FITC- or biotin-conjugated irrelevant isotype controls served as controls. Analysis was done on cells within the lymphocyte region (Fig. 1, R1).
2.6. BALF cytospin samples
For detection of cell-associated PRRSV, cytospin slides were prepared from BALF cells thawed at 37 °C and immediately transferred to 10 ml 37 °C EMEM. Approximately 18×104 cells were immersion-fixed in 4% neutral buffered paraformaldehyde for 2 min followed by a incubation in 0.05% Triton X-100 for 10 min. The prepared cytospin samples were incubated overnight with the mAb SDOW 17 raised against the nucleocapsid protein of PRRSV (Nelson et al., 1993). After a brief rinse in TBS the cells were incubated with BIOGAM IgG (JIRL) for 30 min followed by a incubation with Vectastain®ABC Elite reagent (Vector Laboratories Inc., Burlingame, CA, USA) for 30 min. The cytospin samples were then developed in diaminobenzidine, mounted and examined by light microscopy. The entire slide was examined for positive cells. To elucidate the composition of BALF cell populations, slides were stained with Hemacolor (Merck KGaA, Darmstadt, Germany).
2.7. Statistical analysis
Student’s t-test was used for statistical evaluation of differences between the control groups and the infected groups. Three levels of significance were considered: ** P<0.05, ** P<0.01 and *** P<0.001.
3. Results
None of the sows showed any signs of clinical disease throughout the experimental period. The two control sows delivered a total of 29 liveborn and 1 stillborn piglet. The two infected sows delivered 23 liveborn and 3 stillborn piglets. One infected piglet died on day 11 PI.
At necropsy, the control piglets neither showed signs of macro- nor microscopical pathological lesions. In the infected group, gross lung lesions characterised by multi-focal tan-mottled areas were observed in six of eight piglets, four of eight piglets and four of six piglets necropsied at 2, 4 and 6 weeks of age, respectively. Furthermore, histopathological examination showed interstitial pneumonia of varying intensity in all infected piglets. The observed lung lesions were typical for PRRSV-induced pneumonia (Halbur et al., 1995, Lager and Halbur, 1996, Kranker et al., 1998). Peritonitis was observed in one 4- and one 6-week-old piglet as well as pleuritis in a 6-week-old piglet, all three from infected sow 208.
3.1. Virus isolation and serology
3.1.1. Sows
The detection of PRRSV in serum from infected sow 97 on days 14 and 21 PI, and in infected sow 208 on day 14 PI showed that both virus-inoculated sows had been infected. Furthermore, both sows had seroconverted on day 14 PI. Control sows remained negative throughout the experiment.
3.1.2. Piglets
For all liveborn piglets from the infected sows, PRRSV was isolated from precolostral serum samples at birth (Table 1 ) implying that virus had crossed the placental barrier and subsequently infected foetuses. PRRSV was detected in one of the stillborn pigs. At 2, 4 and 6 weeks of age, respectively, PRRSV was detected in serum from 21/22 (96%), 7/14 (50%) and 0/6 (0%) infected piglets, respectively, at these stages. Examination of tissue samples collected at necropsy at 2, 4 and 6 weeks of age revealed that PRRSV was present in 8/8, 4/8 and 0/6 infected piglets, respectively. In addition, PRRSV-specific antibodies were detected in 41% of the infected piglets at birth, before colostral intake, indicating that these foetuses had been infected at least some days earlier. At 2 weeks of age and later, all infected piglets were antibody-positive.
Table 1.
Examination for PRRSV in liveborn piglets delivered by sows infected with PRRSV on day 90 of gestation
| Experimental group | Sow ID | Age of piglets |
|||
| At birtha | 2 weeksb | 4 weeksb | 6 weeksb | ||
| Infected | 97 | 9/9c | 8/8 | ||
| 208 | 14/14 | 13/14 | 7/14 | 0/6 | |
| Total | 23/23 | 21/22 | 7/14 | 0/6 | |
| Controls | 199 | 0/16 | 0/14 | 0/14 | 0/9 |
| 303 | 0/13 | 0/13 | 0/6 | ||
| Total | 0/29 | 0/27 | 0/20 | 0/9 | |
Precolostral samples.
Serum samples.
Number of positive piglets/number of piglets examined.
All piglets from the control sows remained negative for PRRSV and antibodies to PRRSV throughout the experiment. Finally, no other viruses than PRRSV were isolated from any of the sows or piglets.
3.2. Total and differential WBC counts
Total WBC counts were significantly higher for infected piglets than control piglets at 2 and 4 weeks of age (P<0.001), but not at 6 weeks of age (Fig. 2 a). The number of lymphocytes increased over time in both groups. Although the mean percentage of lymphocytes was around 50% for both groups (data not shown), the absolute numbers were significantly higher in the infected piglets at 2 (P<0.001) and 4 (P<0.05) weeks of age (Fig. 2b). Also the numbers of granulocytes were significantly increased in the infected piglets at 2 and 4 weeks of age (P<0.001) (Fig. 2c). The percentage of monocytes was lower in the infected piglets than in controls at 2 and 4 weeks of age (P<0.001), but higher at 6 weeks of age (P<0.01) (data not shown). The absolute monocyte counts, however, were significantly higher for the infected piglets at 6 weeks of age (P<0.01) (Fig. 2d).
Fig. 2.
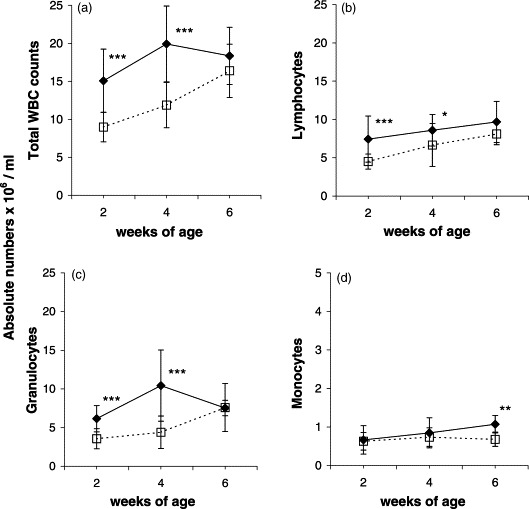
Variations over time in absolute numbers of total WBC (a), lymphocytes (b), granulocytes (c) and monocytes (d) in peripheral blood of uninfected control (□) and in utero PRRSV-infected (♦) piglets. Each dot represents the average±S.D. of examined piglets at the various age. At 2, 4 and 6 weeks of age, respectively, 27, 20 and 9 control piglets, and 22, 14 and 6 infected piglets were examined. Significant difference between age-matched controls and infected piglets: *P<0.05; **P<0.01; ***P<0.001.
3.3. Characterisation of blood leukocyte subpopulations
The distribution of various leukocyte subpopulations in peripheral blood was determined by flow cytometry analysis.
The SLAI mAb PT85A was used to define nucleated cells, in order to exclude red blood cells, sporadically present in the cell preparations, from the analyses.
The absolute number of CD2+ cells was markedly higher for the infected piglets than for the controls (Fig. 3 a). However, due to great variation within the infected groups, statistical significant difference between groups was seen at 2 weeks of age (P<0.001), only. The increase of total CD2+ cells was not simply due to the overall increase of WBC counts, since the mean percentage of CD2+ cells in the MNC fraction (Fig. 1, R1+R2) was also significantly higher for the infected piglets (63±8, 58±8 and 52±5%) than the controls (52±9, 51±8 and 44±5%) at 2 (P<0.001), and 4 and 6 (P<0.05) weeks after birth.
Fig. 3.
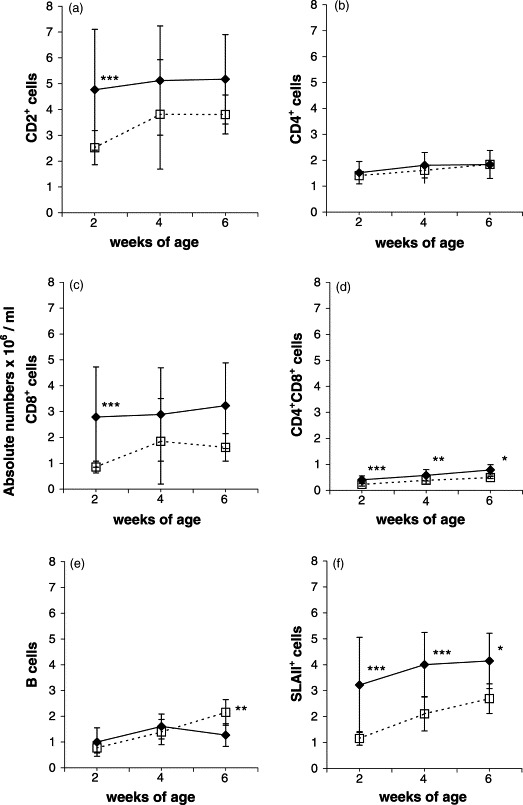
Variations over time in absolute numbers of various leukocyte subpopulations in peripheral blood of uninfected control (□) and in utero PRRSV-infected (♦) piglets. Each dot represents the average±S.D. of examined piglets at the various age. At 2, 4 and 6 weeks of age, respectively, 27, 20 and 9 control piglets, and 22, 14 and 6 infected piglets were examined. Significant difference between age-matched controls and infected piglets: *P<0.05; **P<0.01; ***P<0.001.
The mean percentage of CD4+ cells (in MNC fraction) was markedly lower in the infected piglets (21±5 and 18±2%) than controls (28±4 and 22±3%) at 2 (P<0.001) and 6 (P<0.05) weeks of age, but at almost the same level at 4 weeks of age (infected piglets: 20±4%, controls: 23±4%). The absolute numbers of CD4+ cells, however, showed no significant difference between the groups at any stage (Fig. 3b).
The mean percentage of CD8+ cells (in MNC fraction) was significantly higher in the infected piglets (33±11, 31±10 and 31±8%) than in the controls (17±3, 23±10 and 18±4%) at 2 (P<0.001), 4 (P<0.05) and 6 (P<0.01) weeks of age. Also, the absolute number of CD8+ cells was markedly higher for the infected piglets than for the controls. Due to great variation within the infected groups, however, statistically significant difference between groups was observed at 2 weeks (P<0.001) only (Fig. 3c).
The level of CD4/CD8 double-positive cells increased over time in both groups. The mean percentage of CD4+CD8+ cells (in lymphocyte region, Fig. 1, R1) was significantly higher in the infected piglets (8±1, 9±1.5 and 11±2.5%) compared to controls (6±1, 6±1.5 and 6±1%) at 2 and 4 (P<0.001) and 6 (P<0.01) weeks of age. This difference was paralleled by significantly higher absolute numbers of double-positive cells for the infected piglets at 2 (P<0.001), 4 (P<0.01) and 6 (P<0.05) weeks of age (Fig. 3d).
Compared to the controls, the mean percentages of B cells (in lymphocyte region, Fig. 1, R1) for the infected piglets (12±6 and 14±2%) were significantly lower than for the controls (15±3 and 23±3%) at 2 and 6 weeks (P<0.01), but at the same level (20%) at 4 weeks. The level of B cells increased over time, however, a sudden drop was observed in 6-week-old infected piglets. At this stage, the absolute number of B cells was significantly enhanced in the controls compared to the infected piglets (P<0.01) (Fig. 3e).
At 2, 4 and 6 weeks of age, the mean percentage of SLAII+ cells in the MNC fraction was significantly higher (P<0.001) in the infected piglets (41±11, 46±7 and 42±3%) than in the controls (23±3, 30±4 and 31±4%). In parallel, the absolute number of SLAII+ cells was significantly higher in infected piglets than in controls at weeks 2 and 4 (P<0.001) and week 6 (P<0.05) (Fig. 3f).
3.4. Examination of BALF cells
BALF cell subpopulations were characterised by flow cytometry analysis. Berndt and Müller (1997) described distinction of BALF subpopulations on the basis of size and granularity. In the present study, two BALF cell populations were characterised on the basis of size and granularity (Fig. 4 a), and reactivity with anti-classI mAb PT85A and granularity (Fig. 4b). One population of small SLAI+ cells with low granularity was defined as lymphocytic and monocytic cells (Fig. 4a and b, R1). The second population consisted of SLAI+ cells with high granularity (Fig. 4a and b, R2). These cells were also found to be SLAII+, and were defined as macrophages.
Fig. 4.
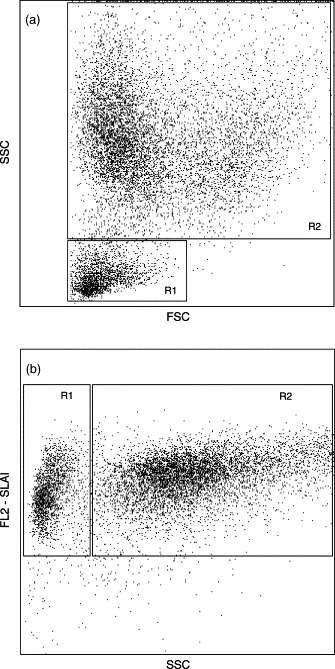
FSC/SSC (a) and FL2-SLAI/SSC (b) dot plots of BALF cells from a representative 4-week-old piglet infected in utero with PRRSV. Two cell populations were characterised on the basis of size and granularity (a) and reactivity with anti-SLAI mAb PT85A and granularity (b). One population of SLAI+, small cells with low granularity was defined as lymphocytic and monocytic cells (R1). The second population consisted of SLAI+ cells with high granularity (R2). These cells were also found to be SLAII+, and were defined as macrophages. The dot plot setup shown in (b) was used for further analyses.
On average, the BALF cell populations consisted of 96–98% SLAI+ cells, indicating that contamination with non-nucleated cells, i.e. erythrocytes, was low. There was no significant difference within or between groups.
The viability (measured as exclusion of propidium iodide) of BALF cells from 2-, 4- and 6-week-old piglets was 84±7, 91±2 and 91±3%, respectively, for the controls, and 77±6, 89±4 and 95±1%, respectively, for the infected piglets. The proportion of macrophages (Fig. 4, R2) in BALF was significantly lower in the infected piglets than controls at 2, 4 (P<0.001) and 6 (P<0.01) weeks of age (Fig. 5 a). In the control piglets, the proportion of macrophages in BALF averaged 93±3% at 2 and 4 weeks, and 93±1% at 6 weeks, whereas the infected had only 40±14, 77±7 and 76±10% macrophages at 2, 4 and 6 weeks of age, respectively. Cells other than macrophages falling in region R1 (Fig. 4) were characterised by their reactivity with mAb against various CD-antigens.
Fig. 5.
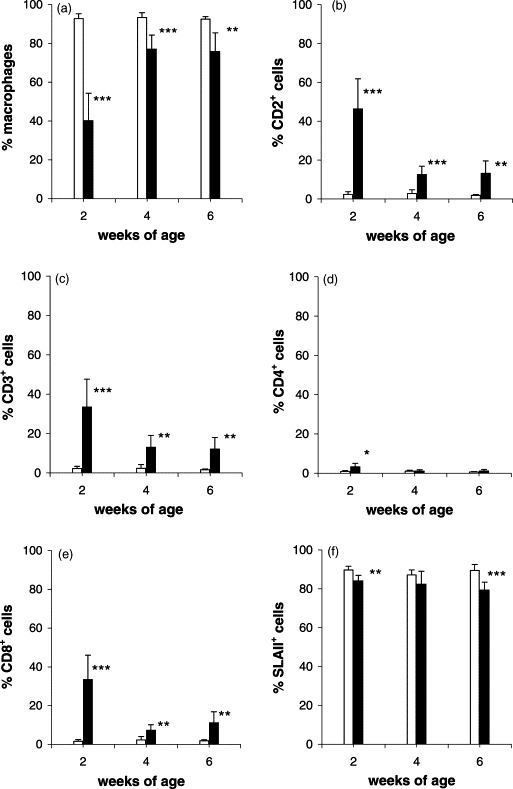
Flow cytometric characterisation of BALF cells recovered from lungs of uninfected control (□) and in utero PRRSV-infected (■) piglets at various stages after birth. Each bar represents the average percentage±S.D. (upper lines) of the number of examined piglets. At 2, 4 and 6 weeks of age, respectively, 7, 8 and 9 control piglets, and 7, 8 and 6 infected piglets were examined. Significant difference between age-matched controls and infected piglets: *P<0.05; **P<0.01; ***P<0.001.
In the controls, only low numbers of cells expressed the CD2, CD3, CD4 and CD8, antigens, respectively (Fig. 5b–e). In the infected piglets, however, the percentages of CD2+ and CD3+ cells were dramatically increased, and levels were significantly higher than in controls at 2 (P<0.001), 4 and 6 (P<0.01) weeks of age, the difference being most pronounced in 2-week-old piglets (Fig. 5b and c).
The proportion of CD4+ cells was low in all piglets (range 0.6±0.2 to 3.3±1.82%), but significantly higher (P<0.05) in infected 2-week-old piglets than in controls (Fig. 5d).
At all three stages, the percentage of CD8+ cells was significantly higher in the infected piglets than in the controls (P<0.001, P<0.01 and P<0.01), most pronounced at 2 weeks of age, where infected piglets had 33.4±12.6% CD8+ cells in contrast to only 1.6±0.9% in controls (Fig. 5e).
At all ages, the proportion of SLAI+ cells was lower in the infected piglets than in the controls (Fig. 5f). In infected 2-week-old piglets, the percentage of SLAII+ macrophages was low (Fig. 5a) but increased numbers of SLAII+ cells belonging to the monocytic/lymphocytic region (Fig. 4, R1) were detected in these animals (data not shown).
The percentages of B cells remained below 1% (range 0.1–0.9%) in all piglets with no significant difference between groups (data not shown).
Examination of cytospin slides revealed that PRRSV virus could not be detected in BALF cells of any of the controls, but in all infected piglets. However, the level of PRRSV-positive cells was low and estimated to average approximately 0.2% (range 0.05–0.3) in 2-week-old piglets, and 0.02% (range 0.05–0.1) in 4- and 6-week-old piglets, indicating a decrease of positive cells over time. The virus-positive cells were defined as macrophages, and cells with strong as well as weaker staining were observed. Furthermore, morphological evaluation of the slides revealed that various cell types could be detected. In all the control piglets, macrophages were the most prominent cell type with only few lymphocytes present. Granulocytes were either not detected or sporadically seen, only. The macrophages appeared round, uniform with a homogeneous nucleus as previously described for germ-free piglets (Gehrke and Pabst, 1990). In the infected piglets, the cell composition appeared much more inhomogeneous. Macrophages with differing morphology (varying size, more irregularly shaped, and increased numbers of cytoplasmic processes), monocytes, lymphocytes, plasma cells and epithelial cells could be detected. Compared to the controls, the greatest variation was seen in the 2-week-old infected piglets. Granulocytes were only sporadically seen in a few infected piglets, and never exceeding more than 1–2% of the BALF cell population.
4. Discussion
The present experiment provides evidence that in utero infection with PRRSV modulates leukocyte subpopulations in peripheral blood and BALF of surviving piglets, especially characterised by the occurrence of high levels of CD8+ cells.
In other experiments, PRRSV has been shown to induce a transient reduction in the number of blood lymphocytes in the early phase of infection (Christianson et al., 1993, Shimizu et al., 1996, Nielsen and Bøtner, 1997). The fact that a such reduction was not observed in our study is likely to reflect that the piglets were not any more in the acute phase of infection, since they had been infected at least 2 weeks prior to the initial haematological examination at 2 weeks of age. In the present experiment, the infection did not significantly change the numbers of CD4+ cells in peripheral blood. This result corresponds to previous observations in pigs infected with PRRSV at 8 weeks of age (Albina et al., 1998), but not to the transient decrease in the number of CD4+ cells in peripheral blood after PRRSV-infection of 54-day-old and 4.5-month-old pigs, respectively, as demonstrated by Shimizu et al. (1996) and Nielsen and Bøtner (1997). Most likely, this discrepancy is related to variations in experimental design. However, it cannot be excluded that a number of other factors may influence and explain conflicting observations on the kinetics of subpopulations in PRRSV-infected pigs, e.g. variations in pathogenicity among PRRSV strains or post-natal versus congenital infection.
PRRSV-specific proliferation of peripheral blood MNC from pigs infected with PRRSV has been demonstrated for a period of more than 3 months after first detection by 4 weeks post-inoculation (Bautista and Molitor, 1997, Lopez-Fuertes et al., 1999). In addition, Bautista and Molitor (1997) showed that PRRSV-immunised pigs developed memory T cells that not only responded faster on a second virus exposure and with a higher magnitude to in vitro stimulation with PRRSV antigens, but that they were able to mount an antigen-specific in vivo delayed type hypersensitivity reaction in response to intradermal challenge with PRRSV. CD4+CD8+ extrathymic lymphocytes represent a portion of porcine memory T cells, and the CD4/CD8 double-positive cells have been suggested to play a major role in protective immunity (Zuckermann, 1999). The present demonstration of significantly increased numbers of CD4+CD8+ cells in peripheral blood of the infected piglets compared to the controls most likely reflects the development of PRRSV-specific memory cells. However, the importance of these memory cells in the immune response against PRRSV still remains to be defined.
The present study does not explain the role of the significantly higher numbers of SLAII+ cells found in the infected piglets throughout the experiment. However, since SLAII molecules are involved in the presentation of foreign antigens to the immune system, it may appear likely that the increased numbers of SLAII+ cells reflect a persistent and enhanced level of immunological activity in these animals compared to the controls.
Previous reports have described enhancements of CD8+ cells in peripheral blood (Shimizu et al., 1996, Albina et al., 1998) and BALF (Samsom et al., 2000) after infection of growing pigs with PRRSV. In our study, we demonstrated that high levels of CD8+ cells also constitute a dominant feature of piglets surviving in utero infection with PRRSV. Abnormal high, and persistent, numbers of CD2+ and CD8+ cells were observed in peripheral blood of 2-, 4- and 6-week-old infected piglets. Most likely, the increase of CD2+ cells is mainly due to an augmentation in the number of CD8+ cells, since the latter express both antigens. CD8+ cells may belong to the cytotoxic T cell population as well as the natural killer (NK) cell population, but the labelling method used in the present experiment does not discriminate between these two different types of cytolytic cells. Characteristically, the high level of CD8+ cells in peripheral blood of the infected piglets was paralleled by an augmented percentage of CD8+ cells in BALF, indicating an influx of these cells into the bronchoalveolar space. However, the decrease in the proportion of CD8+ cells observed over time in BALF was not seen in peripheral blood, possibly indicating that the effect of the infection may decrease earlier in the lungs than in peripheral blood.
The biological significance of the increased levels of CD8+ cells in PRRSV infected pigs has not yet been defined. CD8+ cells are precursors for cytolytic lymphocytes (i.e. cytotoxic T cells and NK cells) which are important cells for the elimination of virus-infected cells. Albina et al. (1998) suggested that CD8+ cells could play a role in the control of virus replication, since viremia started to decline shortly after the proliferation of CD8+ cells. In our experiment, all infected piglets were viremic at 2 weeks of age, but none at 6 weeks. At this stage, however, PRRSV-positive BALF cells were still present, although few in number. This persistence of virus may explain why the level of CD8+ cells in peripheral blood and BALF remained high in the infected piglets also 6 weeks after birth. Our results support the hypothesis of CD8+ cells as an important factor in the control of PRRSV replication. However, studies by Shimizu et al. (1996) indicated that a certain as yet unidentified physiological stimulus induced as a consequence of PRRSV infection may be the cause of the proliferation of CD8+ cells, possibly effectors being helper cells or immuno-stimulating cytokines induced by PRRSV, as suggested by Albina et al. (1998). In contrast to the study by Albina et al. (1998) showing no changes in the level of peripheral blood granulocytes as a result of infection with PRRSV, we found significantly higher numbers of blood granulocytes in the infected piglets at 2 and 4 weeks of age. Since there were no indications of concurrent bacterial infections to explain the increased level of granulocytes, the possible importance of this event remains to be elucidated.
In the control piglets, the composition of the BALF cells, i.e. predominantly alveolar macrophages with only few lymphocytes present, corresponded to the reference values for healthy specific pathogen-free (SPF) pigs established by Ganter and Hensel (1997). The cellular shift in BALF of the in utero infected piglets, represented by increased levels of mononuclear cells in parallel with decreased proportions of macrophages (Fig. 5a–e) correlate well with previous observations after infection of 4- and 8–10-week-old SPF piglets with PRRSV (Shibata et al., 1997, Samsom et al., 2000). The latter authors, however, demonstrated that the number of cells of the macrophage/monocyte lineage in BALF did not decrease, but increased in accordance with an increase of the total number of BALF cells after infection with PRRSV. In the present study, we did only semi-quantitatively determine the total number of cells in BALF. However, the markedly higher amounts of cells recovered from 2- and 4-week-old infected compared to control piglets indicated that the in utero infected piglets had increased numbers of BALF cells. Granulocytes are rarely found in normal porcine BALF (Gehrke and Pabst, 1990). The few granulocytes found in the BALF in our experiment (together with the virological results) indicate that no other infection than PRRSV was present in the piglets. The remarkably lower levels of CD3+ cells compared to CD2+ cells observed for 2-week-old infected piglets (compare Fig. 5b and c), but not later, most likely reflect that these CD2+ populations—in addition to cytotoxic T lymphocytes which stain with anti-CD3 mAbs—comprised a proportion of NK cells which do not stain with anti-CD3 mAbs. This is in accordance with the demonstration of a strong influx of both NK cells and cytotoxic T lymphocytes into the bronchoalveolar space during PRRSV-infection in pigs as shown by Samsom et al. (2000). In contrast to the latter authors who did not find an influence of the infection on the number of CD4+ cells, we demonstrated significantly higher numbers of CD4+ cells in BALF from 2-week-old infected piglets, possibly indicating a role of these cells in the respiratory immune response. The low level of B cells in BALF of both infected piglets and controls indicate that these cells do not play any role for the elimination of the respiratory infection, in accordance with the observation that PRRSV persisted in the infected pigs in the presence of high levels of specific antibodies (data not shown).
The present experiment showed that PRRSV could be detected in BALF cells of all infected piglets 2, 4 and 6 weeks after birth. In accordance with the observation by Shibata et al. (1997) who isolated PRRSV in BALF samples as late as 49 days post-inoculation, we detected the virus in BALF cells at least 6 weeks after infection, i.e. in 6-week-old piglets. At that stage, virus could not be isolated from serum, demonstrating that PRRSV may persist in BALF cells long after serum viremia.
The decreased viability of BALF cells, in particular macrophages, observed in 2-week-old infected piglets may be due to a direct cytocidal effect of the virus (Chiou et al., 2000), although the cell death may also be by apoptosis (Sirinarumitr et al., 1998, Oleksiewicz and Nielsen, 1999) which could serve as a host defence to quench viral replication.
To conclude, in utero infection with PRRSV profoundly affects the immune cell populations in surviving piglets. On the contrary, the present results do not support the existence of any immunosuppression following in utero PRRSV-infection. The significantly increased post-natal levels of cells with the CD2, CD3 and CD8 phenotypes in peripheral blood and BALF indicate a long-lasting activation of the immune system. However, further studies will be needed to determine the precise role of these T cell subpopulations in the infection. In order to elucidate the importance of the CD8+ cells, current experiments at the Danish Veterinary Institute aim to determine the effects of mAb-induced in vivo CD8+ cell-depletion of piglets on the course of PRRSV-infection.
In this paper, we do not describe putative consequences of the PRRSV-infection on the function of the various immune cells. During the present animal experiment, however, a number of functional tests were also carried out. Thus, studies on cytokine profiles in PBMC and lymph node cells (Aasted et al., 2002) and in BALF cells (Johnsen et al., 2002) demonstrated that in utero infection with PRRSV may alter the expression of cytokines in surviving piglets. In addition, infected piglets showed inhibition of alveolar lung macrophage phagocytosis (U. Riber, personal communication), indicating a state of immunosuppression.
Acknowledgements
The assistance of the technical staff in the Cell Culture Laboratory and the Swine Virology Laboratory at DVI, Lindholm is highly appreciated. Likewise, we thank Bent Eriksen and co-workers for taking care of the animals. The preparation of the FITC- and biotin-conjugated purified mAbs was kindly carried out by Søren Kamstrup, DVI, Lindholm. The present study was financially supported by The Danish Veterinary and Agricultural Research Council.
References
- Aasted B, Bach P, Nielsen J, Lind P. Cytokine profiles in peripheral blood mononuclear cells and lymph node cells from piglets infected in utero with porcine reproductive and respiratory syndrome virus. Clin. Diagn. Lab. Immunol. 2002;9:1229–1234. doi: 10.1128/CDLI.9.6.1229-1234.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albina E, Piriou L, Cariolet R, Hospitalier R.L. Immune responses in pigs infected with porcine reproductive and respiratory syndrome virus (PRRSV) Vet. Immunol. Immunopathol. 1998;61:49–66. doi: 10.1016/S0165-2427(97)00134-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bautista E.M, Molitor T.W. Cell-mediated immunity to porcine reproductive and respiratory syndrome virus in swine. Viral Immunol. 1997;10:83–94. doi: 10.1089/vim.1997.10.83. [DOI] [PubMed] [Google Scholar]
- Bautista E.M, Molitor T.W. IFN gamma inhibits porcine reproductive and respiratory syndrome virus replication in macrophages. Arch. Virol. 1999;144:1191–1200. doi: 10.1007/s007050050578. [DOI] [PubMed] [Google Scholar]
- Berndt A, Müller G. Heterogeneity of porcine alveolar macrophages in experimental pneumonia. Vet. Immunol. Immunopathol. 1997;56:279–287. doi: 10.1016/s0165-2427(97)00009-3. [DOI] [PubMed] [Google Scholar]
- Bøtner A, Nielsen J, Bille-Hansen V. Isolation of porcine reproductive and respiratory syndrome (PRRS) virus in a Danish swine herd and experimental infection of pregnant gilts with the virus. Vet. Microbiol. 1994;40:351–360. doi: 10.1016/0378-1135(94)90122-8. [DOI] [PubMed] [Google Scholar]
- Carvalho L.F.O.S, Segales J, Pijoan C. Effect of porcine reproductive and respiratory syndrome virus on subsequent Pasteurella multocida challenge in pigs. Vet. Microbiol. 1997;55:241–246. doi: 10.1016/S0378-1135(96)01324-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiou M.-T, Jeng C.-R, Chueh C.-H, Cheng C.-H, Pang V.F. Effects of porcine reproductive and respiratory syndrome virus (isolate tw91) on porcine alveolar macrophages in vitro. Vet. Microbiol. 2000;71:9–25. doi: 10.1016/s0378-1135(99)00159-5. [DOI] [PubMed] [Google Scholar]
- Choi, C., Gustafson, K., Chinsakchai, S., Hill, H., Molitor, T., 1994. Heterogeneity of porcine alveolar macrophage subpopulations: immune functions and susceptibility to PEARS virus. In: Proceedings of the 13th IPVS Congress, Bangkok, Thailand, June 1994, pp. 26–30.
- Christianson W.T, Choi C.S, Collins J.E, Morrison R.B, Joo H.S. Pathogenesis of porcine reproductive and respiratory syndrome virus infection in mid-gestation sows and fetuses. Can. J. Vet. Res. 1993;57:262–268. [PMC free article] [PubMed] [Google Scholar]
- Collins, J.E., Rossow, K.D., 1993. Pathogenesis of PRRS. In: Proceedings of the Allen D. Leman Swine Conference, University of Minnesota, USA, pp. 47–48.
- Davis W.C, Marusic S, Lewin H.A, Splitter G.A, Perryman L.E, McGuire T.C, Gorman J.R. The development and analysis of species specific and cross reactive monoclonal antibodies to leukocyte differentiation antigens and antigens of the major histocompatibility complex for use in the study of the immune system in cattle and other species. Vet. Immunol. Immunopathol. 1987;15:337–376. doi: 10.1016/0165-2427(87)90005-5. [DOI] [PubMed] [Google Scholar]
- Done S.H, Paton D.J. Porcine reproductive and respiratory syndrome: clinical disease, pathology and immunosuppression. Vet. Rec. 1995;136:32–35. doi: 10.1136/vr.136.2.32. [DOI] [PubMed] [Google Scholar]
- Drew T.W. A review of evidence for immunosuppression due to porcine reproductive and respiratory syndrome virus. Vet. Res. 2000;31:27–39. doi: 10.1051/vetres:2000106. [DOI] [PubMed] [Google Scholar]
- Feng W, Laster S.M, Tompkins M, Brown T, Xu J.S, Altier C, Gomez W, Benfield D, McCaw M.B. In utero infection by porcine reproductive and respiratory syndrome virus is sufficient to increase susceptibility to challenge by Streptococcus suis type II. J. Virol. 2001;75:4889–4895. doi: 10.1128/JVI.75.10.4889-4895.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galina L, Pijoan C, Sitjar M, Christianson W.T, Rossow K, Collins J.E. Interaction between Streptococcus suis serotype-2 and porcine reproductive and respiratory syndrome virus in specific pathogen free piglets. Vet. Rec. 1994;134:60–64. doi: 10.1136/vr.134.3.60. [DOI] [PubMed] [Google Scholar]
- Ganter M, Hensel A. Cellular variables in bronchiolar lavage fluids (BALF) in selected healthy pigs. Res. Vet. Sci. 1997;63:215–217. doi: 10.1016/s0034-5288(97)90023-0. [DOI] [PubMed] [Google Scholar]
- Gehrke I, Pabst R. Cell composition and lymphocyte subsets in the bronchoalveolar lavage of normal pigs of different ages in comparison with germfree and pneumonic pigs. Lung. 1990;168:79–92. doi: 10.1007/BF02719678. [DOI] [PubMed] [Google Scholar]
- Goldberg T.L, Hahn E.C, Weigel R.M, Scherba G. Genetic, geographical and temporal variation of porcine reproductive and respiratory syndrome virus in Illinois. J. Gen. Virol. 2000;81:171–179. doi: 10.1099/0022-1317-81-1-171. [DOI] [PubMed] [Google Scholar]
- Goldberg T.L, Hahn E.C, Weigel R.M, Scherba G. Associations between genetics, farm characteristics and clinical disease in field outbreaks of porcine reproductive and respiratory syndrome virus. Prev. Vet. Med. 2000;43(4):293–302. doi: 10.1016/S0167-5877(99)00104-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halbur P.G, Paul P.S, Frey M.L, Landgraf J, Eernisse K, Meng X.J, Lum M.A, Andrews J.J, Rathje J.A. Comparison of the pathogenecity of two US porcine reproductive and respiratory syndrome virus isolates with that of the Lelystad virus. Vet. Pathol. 1995;32:648–660. doi: 10.1177/030098589503200606. [DOI] [PubMed] [Google Scholar]
- Hammerberg C, Schurig G.G. Characterization of monoclonal antibodies directed against swine leukocytes. Vet. Immunol. Immunopathol. 1986;11:107–121. doi: 10.1016/0165-2427(86)90092-9. [DOI] [PubMed] [Google Scholar]
- Johnsen C.K, Bøtner A, Kamstrup S, Lind P, Nielsen J. Cytokine mRNA profiles in bronchoalveolar cells of piglets experimentally infected in utero with porcine reproductive and respiratory syndrome virus: association of sustained expression of IFN-gamma and IL-10 after viral clearance. Viral Immunol. 2002;15:549–556. doi: 10.1089/088282402320914494. [DOI] [PubMed] [Google Scholar]
- Kawashima K, Narita M, Yamada S. Changes in macrophage and lymphocyte subpopulations of lymphoid tissues from pigs infected with the porcine reproductive and respiratory syndrome virus (PRRSV) Vet. Immunol. Immunopathol. 1999;71(3–4):257–262. doi: 10.1016/s0165-2427(99)00102-6. [DOI] [PubMed] [Google Scholar]
- Keffaber K, Stevenson G, Van Alstine W, Kanitz C, Harris L, Gorcyca D, Schlesinger K, Schultz R, Chladek D, Morrison R. SIRS virus infection in nursery/grower pigs. Am. Assoc. Swine Pract. Newslett. 1992;4:38–40. [Google Scholar]
- Kranker S, Nielsen J, Bille-Hansen V, Bøtner A. Experimental inoculation of swine at various stages of gestation with a Danish isolate of porcine reproductive and respiratory syndrome virus (PRRSV) Vet. Microbiol. 1998;61:21–31. doi: 10.1016/s0378-1135(98)00176-x. [DOI] [PubMed] [Google Scholar]
- Labarque G.G, Nauwynck H.J, Van Reeth K, Pensaert M.B. Effect of cellular changes and onset of humoral immunity on the replication of porcine reproductive and respiratory syndrome virus in the lungs of pigs. J. Gen. Virol. 2000;81:1327–1334. doi: 10.1099/0022-1317-81-5-1327. [DOI] [PubMed] [Google Scholar]
- Lager K.M, Halbur P.G. Gross and microscopic lesions in porcine fetuses infected with porcine reproductive and respiratory syndrome virus. J. Vet. Diagn. Invest. 1996;8:275–282. doi: 10.1177/104063879600800301. [DOI] [PubMed] [Google Scholar]
- López-Fuertes L, Doménech N, Alvarez B, Ezquerra A, Dominguez J.M, Alonso F. Analysis of cellular immune response in pigs recovered from respiratory and reproductive syndrome infection. Virus Res. 1999;64:33–42. doi: 10.1016/s0168-1702(99)00073-8. [DOI] [PubMed] [Google Scholar]
- López-Fuertes L, Campos E, Doménech N, Ezquerra A, Castro J.M, Dominingues J, Alonso F. Porcine reproductive and respiratory syndrome (PRRS) virus down-modulates TNF-α production in infected macrophages. Virus Res. 2000;69:41–46. doi: 10.1016/s0168-1702(00)00172-6. [DOI] [PubMed] [Google Scholar]
- Mengeling W.L, Lager K.M, Vorwald A.C. Clinical effects of porcine reproductive and respiratory syndrome virus on pigs during the early postnatal interval. Am. J. Vet. Res. 1998;59:52–55. [PubMed] [Google Scholar]
- Naessens J, Newson J, Howard C.J, Parsons K, Jones B. Characterization of a bovine leukocyte differentiation antigen 145,000 MW restricted to B lymphocytes. Immunology. 1990;69:525–530. [PMC free article] [PubMed] [Google Scholar]
- Nelson E.A, Christopher-Hennings J, Drew T, Wensvoort G, Collins J.E, Benfield D.A. Differentiation of US and European isolates of porcine reproductive and respiratory syndrome virus by monoclonal antibodies. J. Clin. Microbiol. 1993;31:3184–3189. doi: 10.1128/jcm.31.12.3184-3189.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen J, Bøtner A. Hematological and immunological parameters of 4 1/2-month-old pigs infected with PRRS virus. Vet. Microbiol. 1997;55:289–294. doi: 10.1016/s0378-1135(96)01334-x. [DOI] [PubMed] [Google Scholar]
- Nielsen J, Bøtner A, Bille-Hansen V, Oleksiewicz M.B, Storgaard T. Experimental inoculation of late term pregnant sows with a field isolate of porcine reproductive and respiratory syndrome vaccine-derived virus. Vet. Microbiol. 2002;84:1–13. doi: 10.1016/S0378-1135(01)00450-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oleksiewicz M.B, Nielsen J. Effect of porcine reproductive and respiratory syndrome virus (PRRSV) on alveolar lung macrophage survival and function. Vet. Microbiol. 1999;66:15–27. doi: 10.1016/s0378-1135(98)00309-5. [DOI] [PubMed] [Google Scholar]
- Pescovitz M.D, Lunney J.K, Sachs D.H. Preparation and characterization of monoclonal antibodies reactive with porcine PBL. J. Immunol. 1984;133:368–375. [PubMed] [Google Scholar]
- Polson, D.D., 1995. Assessing the cost of endemic reproductive and sucklet pig disease in the swine breeding herd. In: Proceedings of the Second International Symposium on Porcine Reproductive and Respiratory Syndrome (PRRS), Copenhagen, Denmark, 9–10 August, pp. 47–48.
- Robertson, I.B., 1992. Porcine reproductive and respiratory syndrome (blue-eared pig disease): some aspects of its epidemiology. In: Proceedings of the Society for Veterinary Epidemiology and Preventive Medicine, Edinburgh, UK, 1–3 April, pp. 24–38.
- Samsom J.N, de Bruin T.G.M, Voermans J.J.M, Meulenberg J.J.M, Pol J.M.A, Bianchi A.T.J. Changes of leukocyte phenotype and function in the broncho-alveolar lavage fluid of pigs infected with porcine reproductive and respiratory syndrome virus: a role for CD8+ cells. J. Gen. Virol. 2000;81:497–505. doi: 10.1099/0022-1317-81-2-497. [DOI] [PubMed] [Google Scholar]
- Shibata I, Mori M, Uruno K, Samegai Y, Okada M. In vivo replication of porcine reproductive and respiratory syndrome virus in swine alveolar macrophages and change in the cell population in bronchoalveolar lavage fluid after infection. Vet. Med. Sci. 1997;59:539–543. doi: 10.1292/jvms.59.539. [DOI] [PubMed] [Google Scholar]
- Shimizu M, Yamada S, Kawashima K, Ohashi S, Shimizu S, Ogawa T. Changes of lymphocyte subpopulations in pigs infected with porcine reproductive and respiratory syndrome (PRRS) virus. Vet. Immunol. Immunopathol. 1996;50:19–27. doi: 10.1016/0165-2427(95)05494-4. [DOI] [PubMed] [Google Scholar]
- Sirinarumitr T, Zhang Y, Kluge J.P, Halbur P.G, Paul P.S. A pneumo-virulent United States isolate of porcine reproductive and respiratory syndrome virus induces apoptosis in bystander cells both in vitro and in vivo. J. Gen. Virol. 1998;79:2989–2995. doi: 10.1099/0022-1317-79-12-2989. [DOI] [PubMed] [Google Scholar]
- Sørensen K.J, Strandbygaard B, Bøtner A, Madsen E.S, Nielsen J, Have P. Blocking ELISA’s for the distinction between antibodies against European and American strains of porcine reproductive and respiratory syndrome virus. Vet. Microbiol. 1998;60:169–177. doi: 10.1016/s0378-1135(98)00159-x. [DOI] [PubMed] [Google Scholar]
- Stevenson G.W, Van Alstine W.G, Kanitz C.L. Endemic porcine reproductive and respiratory syndrome virus infection of nursery pigs in two swine herds without current reproductive failure. J. Vet. Diagn. Invest. 1993;5:432–434. doi: 10.1177/104063879300500322. [DOI] [PubMed] [Google Scholar]
- Thanawongnuwech R, Thacker E.L, Halbur P.G. Effect of porcine reproductive and respiratory syndrome virus (PRRSV) (isolate ATCC VR-2385) infection on bactericidal activity of porcine pulmonary intravascular macrophages (PIMs): in vitro comparisons with pulmonary alveolar macrophages (PAMs) Vet. Immunol. Immunopathol. 1997;59:323–335. doi: 10.1016/s0165-2427(97)00078-0. [DOI] [PubMed] [Google Scholar]
- Thanawongnuwech R, Young T.F, Thacker B.J, Thacker E.L. Differential production of proinflammatory cytokines: in vitro PRRSV and Mycoplasma hyopneumoniae co-infection model. Vet. Immunol. Immunopathol. 2001;79:115–127. doi: 10.1016/s0165-2427(01)00243-4. [DOI] [PubMed] [Google Scholar]
- Van Reeth K, Nauwynck H, Pensaert M. Dual infections of feeder pigs with porcine reproductive and respiratory syndrome virus followed by porcine respiratory coronavirus or swine influenza virus: a clinical and virological study. Vet. Microbiol. 1996;48:325–335. doi: 10.1016/0378-1135(95)00145-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vezina S.A, Loemba H, Fournier M, Dea S, Archambault D. Antibody production and blastogenic response in pigs experimentally infected with porcine reproductive and respiratory syndrome virus. Can. J. Vet. Res. 1996;60:94–99. [PMC free article] [PubMed] [Google Scholar]
- Wensvoort G, Terpstra C, Pol J.M.A, ter Laak E.A, Bloemraad M, de Kluyver E.P, Kragten C, van Buiten L, den Besten A, Wagenaar F, Broekhuijsen J.M, Moonen P.L.J.M, Zetstra T, de Boer E.A, Tibben H.J, de Jong M.F, van’t Veld P, Groenland G.J.R, van Gennep J.A, Voets M.T, Verheijden J.H.M, Braamskamp J. Mystery swine disease in The Netherlands: the isolation of Lelystad virus. Vet. Q. 1991;13:121–130. doi: 10.1080/01652176.1991.9694296. [DOI] [PubMed] [Google Scholar]
- Wills R.W, Gray J.T, Fedorka-Gray P.J, Yoon K.-J, Ladely S, Zimmerman J.J. Synergism between porcine reproductive and respiratory syndrome virus (PRRSV) and Salmonella choleraesuis in swine. Vet. Microbiol. 2000;71:177–192. doi: 10.1016/S0378-1135(99)00175-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H, Oura C.A.L, Kirkham P.A, Parkhouse M.E. Preparation of monoclonal anti-porcine CD3 antibodies and preliminary characterization of porcine T lymphocytes. Immunology. 1996;88:577–585. doi: 10.1046/j.1365-2567.1996.d01-682.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeman D, Neiger M, Yaeger M, Nelson E, Benfield D, Leslie-Steen P, Thomson J, Miskimins D, Daly R, Minchart M. Laboratory investigation of PRRS virus infection in three swine herds. J. Vet. Diagn. Invest. 1993;5:522–528. doi: 10.1177/104063879300500404. [DOI] [PubMed] [Google Scholar]
- Zuckermann F.A. Extrathymic CD4/CD8 double positive cells. Vet. Immunol. Immunopathol. 1999;72:55–66. doi: 10.1016/s0165-2427(99)00118-x. [DOI] [PubMed] [Google Scholar]


