Abstract
The distribution of different cells of the immune system has been studied in formalin-fixed paraffin-embedded tissues from conventionally reared healthy pigs, using immunohistological techniques. The samples collected were: lungs, tonsils, lymph nodes (mediastinal, mesenteric, inguinal and submandibular), pancreas, spleen, liver, kidney, adrenal gland, ileum and stomach. A total of six primary antibodies anti-CD3, anti-CD79α, Mac 387, anti-lysozyme, anti-CD45RA (3C3/9) and anti-SLA-II-DQ (BL2H5) were used with a standard avidin–biotin peroxidase (ABC) method. Anti-CD3 and anti-CD79α mAb-reacted, respectively with cells located in T cell areas and B cell areas. Mac 387 recognised circulating polymorphonuclear leukocytes, while anti-lysozyme-stained resident macrophages in all tissues. 3C3/9 and BL2H5, were assessed in formalin-fixed paraffin-embedded tissues for the first time. 3C3/9 identified B lymphocytes, in primary follicles and mantle zones, a subpopulation of T cells, especially located in the marginal zone of the spleen and a variable number of immunoblasts, in the germinal centres. BL2H5 reacted with B cells in the mantle zones of the follicles of lymphoid tissues, with dendritic and interdigitating cells in all studied lymphoid tissues and with a variable number of resting and activated T cells in the periarteriolar lymphoid sheath (PALs), marginal zone and red pulp of the spleen. Furthermore, it stained Kupffer and perivascular macrophages in the liver This study represents a detailed histological study of the distribution of the most important subpopulations of immune system cells in conventional, healthy pigs. In our view, these tools should be useful for future comparative studies in disease conditions.
Keywords: Pig, Immune system, Immunohistochemistry, Paraffin
1. Introduction
Immunohistochemistry is a useful technique for characterisation of immune system cells in tissues in pathogenic studies of diseases in humans and animals. Many antibodies have been developed that recognise cell populations in lymphoid organs, but the majority of them work only on frozen sections (Bianchi et al., 1992, Haverson et al., 1994, Denham et al., 1998). In such sections, the evaluation of morphological details is more difficult than in paraffin-embedded sections (Falk et al., 1994). For this reason, detailed studies of the distribution of the different cell populations in the swine immune system are hampered by the lack of antibodies that allow the detection of these cells in formalin-fixed, paraffin-embedded tissues. The cross-reactivity of some antibodies to human differentiation markers has allowed immunostaining of a few swine immune cell populations with protocols standardised in formalin-fixed, paraffin-embedded tissues. Cross-reacting polyclonal anti-human CD3 antibody is considered an excellent marker for swine T lymphocytes when used on paraffin sections (Mason et al., 1989). The identification of B lymphocytes is a more complex issue. Antibodies against several molecules, such as immunoglobulins (Ramos et al., 1992), CDw75, CD79α, CD79β and HLA-DR have been tested (Tanimoto and Ohtsuki, 1996). Among all these molecules, CD79α is one of the two polypeptide chains forming the CD79 molecule that is to say without the α, which has been considered the B cell equivalent of CD3 (Mason et al., 1995). In swine, mAb anti-CD79α has been described as a useful pan-B marker, in paraffin-embedded tissue, although it also stains cells of non-lymphoid origin (Tanimoto and Ohtsuki, 1996). More recently, a mAb to the largest isoform of porcine CD45 (clon 3C3/9, Bullido et al., 1997a) has been used for phenotypic and functional analyses of porcine lymphocyte subpopulations. The 3C3/9 antibody recognises all B lymphocytes and a subpopulation of T lymphocytes, and it has been applied only in frozen sections (Bullido et al., 1997a).
Antibodies exclusively detecting cells of the swine monocyte/macrophage series are rare, probably because of the high heterogeneity of this cell population. However, two antibodies have been described which stain these cells in paraffin-embedded tissues: polyclonal human anti-lysozyme (Falk et al., 1994) and monoclonal human anti-L1 (Mac 387) (Evensen, 1993). Furthermore, in swine and other species, it has been shown that Mac 387 also stains polymorphonuclear granulocytes (Evensen, 1993, Christgau et al., 1998).
Other important cells in lymphoid tissues are antigen presenting cells (APC). To our knowledge, immunohistological studies on the distribution of these cells in swine tissues have not been done. Recently, Bullido et al. (1997b) have developed a monoclonal antibody (BL2H5) recognising swine leukocyte antigens class II (SLA-DQ). This antibody has allowed the study of APC distribution in swine by flow cytometry (Bullido et al., 1997b), but it has not been used for immunohistochemistry in formalin-fixed paraffin-embedded tissues.
The aim of this work was to describe the normal distribution of different immune system cells populations using immunohistochemical techniques in formalin-fixed paraffin-embedded tissues of conventional, healthy pigs, to further compare to altered patterns in disease affecting the lymphoid tissues.
2. Materials and methods
2.1. Animals and sampling
Five 6–10-week-old conventional pigs were used in this study. Pigs were seronegative to circovirus type 2, porcine reproductive and respiratory syndrome virus, Aujeszky’s disease virus, transmissible gastroenteritis virus, porcine respiratory coronavirus, swine influenza virus, porcine parvovirus, Actinobacillus pleuropneumoniae and Erysipelothrix rhusiopathiae.
Pigs were killed by intravenous injection of sodium pentobarbital and a complete necropsy was performed on all animals. Samples of the following tissues were collected: lungs, tonsils, lymph nodes (mediastinal, mesenteric, inguinal and submandibular), pancreas, spleen, liver, kidney, adrenal gland, ileum and stomach. Tissues were fixed 48 h in neutral-buffered 10% formalin, embedded in paraffin wax, sectioned at 3 μm, and stained with haematoxylin and eosin (HE). All the investigated tissues showed a normal histological structure when stained with HE. For immunohistochemistry, serial 4 μm-thick sections of all tissues were cut and placed on silane (3-(trietoxysilyl)-propylamine) coated slides.
2.2. Immunohistochemistry
A total of six primary antibodies (anti-CD3, anti-CD79α, Mac 387, anti-lysozyme, anti-CD45RA (3C3/9) and anti-SLA-II-DQ mAb BL2H5 (see Table 1 ) were used with a standard avidin–biotin peroxidase (ABC) method. Previously described protocols were used for CD3, CD79α (Tanimoto and Ohtsuki, 1996), Mac 387 and lysozyme (Evensen, 1993) immunostaining, whereas standardisation of the protocols had to be carried out for 3C3/9 and BL2H5 antibodies. Briefly, tissue sections were deparaffinised with xylene and rehydrated through graded alcohols. Endogenous peroxidase activity was blocked by incubation with hydrogen peroxide 3% in distilled water for 30 min. After pronase, trypsin or microwave treatment, depending on the antibody used (see Table 1), tissue sections were rinsed in 0.1 M Tris-buffered saline (pH=7.6) and incubated with 20% normal goat serum solution in 0.1 M Tris-buffered saline for 1 h at room temperature. Titration experiments were carried out to obtain the optimal dilution for the different antibodies. All antibodies were incubated at 4°C overnight. Biotinylated goat anti-mouse (1/200) and biotinylated goat anti-rabbit (1/400) were used as secondary antibodies, for 1 h at room temperature. An ABC complex (Pierce, IL) diluted 1/100 in 0.1 Tris-buffered saline was applied for 1 h at room temperature. Sections were finally incubated in diaminobenzidine (DAB)–hydrogen peroxide solution for 10 min, counterstained with Harris’s haematoxylin, dehydrated, covered with a coverslip and examined microscopically. As negative controls, irrelevant primary antibodies at the same dilution were used in substitution of the specific antibodies.
Table 1.
Details of the primary antibodies used in the immunohistochemical study
| Specificity | pAb/mAb (clone) | Host of origin | Treatment | Dilution | Source |
| CD3 | pAb | Human | Pronasea | 1/150 | Dako (Denmark) |
| CD79α | mAb (HM57) | Human | Microwaveb | 1/25 | Dako (Denmark) |
| L1 | mAb (Mac 387) | Human | Pronase | 1/200 | Dako (Denmark) |
| Lysozyme | pAb (A099) | Human | Trypsinc | 1/100 | Dako (Denmark) |
| CD45RA | mAb (3C3/9) | Swine | No treatment | Undilutedd | INIA laboratory |
| SLA-DQ | mAb (BL2H5) | Swine | Pronase | Undilutedd | INIA laboratory |
Incubation with 0.1% pronase for 10min at 37°C.
Incubation with citrate buffer (pH=7.6) for 12 cycles of 1min.
Incubation with 0.1% trypsin for 2h at 37°C.
Culture supernatant.
3. Results
3.1. CD3
The anti-CD3 antibody stained the surface and cytoplasm of cells located in T cell areas of lymph nodes (Fig. 1 a), tonsil, spleen, Peyer’s patches and thymus. In lymph node, tonsil and Peyer’s patches these areas corresponded to interfollicular areas and, in the spleen, to periarteriolar lymphoid sheaths (PALs). In the thymus, medullary thymocytes stained more intensely than cortical thymocytes. Many positive cells were observed in the lamina propia and in an intraepithelial location in the ileum. A variable number of positive cells were also observed in the germinal centre of lymphoid follicles of lymph nodes, tonsil, spleen and Peyer’s patches and in the subcapsular sinus of the lymph nodes. In the dome region of the Peyer’s patches, in the marginal zone of the spleen and in the bronchus-associated lymphoid tissue (BALT) of the lung, scattered positive cells were observed. Furthermore, variable numbers of CD3-positive circulating cells were detected in blood vessels of all the studied tissues.
Fig. 1.
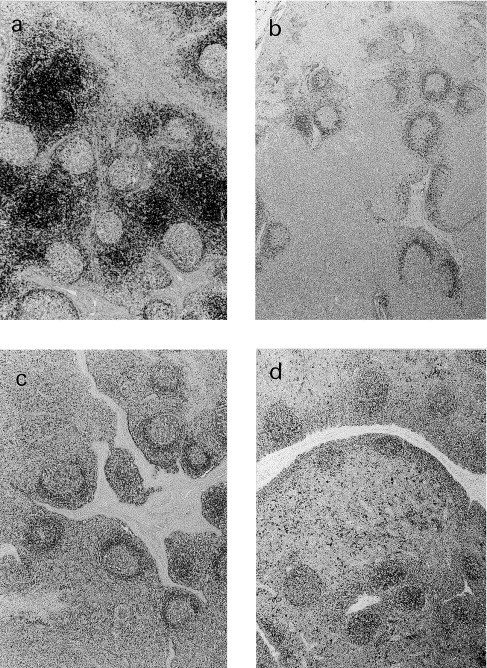
Immunohistochemical staining of formalin-fixed paraffin-embedded porcine lymph node sections with anti-CD3 (a), anti-CD79α (b), anti-CD45RA (3C3/9) (c), and anti-SLA-II-DQ (BL2H5) (d) antibody, using the ABC method. Mayer’s haematoxylin conterstain ×4. T cell areas stain strongly with anti-CD3 (a). Primary follicles and the mantle zone of secondary follicles are depicted with CD79α and 3C3/9 (b and c). The BL2H5 antibody detected lymphatic follicles, and scattered positive cells in the interfollicular area (d).
3.2. CD79α
The anti-CD79α antibody labelled the surface and cytoplasm of small, round cells with a centrally-located nuclei and a narrow rim of cytoplasm, localised in B cell areas. The positive reaction was mainly observed in primary follicles and in the mantle zone of secondary follicles of lymph nodes (Fig. 1, Fig. 2 ), tonsil, spleen, and Peyer’s patches. Most of the cells located in the dome region of Peyer’s patches and in the aggregated lymphoid follicles of the gastric tract mucosa were also positive. In the germinal centres of secondary follicles, cells with a larger amount of cytoplasm, stained strongly positive. Scattered positive cells were observed in the medulla of thymus. Among non-lymphoid tissues, smooth muscle cells and endothelial cells of small blood vessels gave occasionally a positive reaction.
Fig. 2.
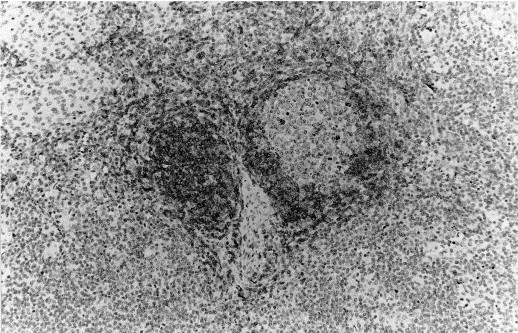
Immunohistochemical staining of formalin-fixed paraffin-embedded porcine lymph node section with anti-CD79α using the ABC method. Mayer’s haematoxylin conterstain ×20. A primary follicle and the mantle zone of a secondary follicle show immunoreactivity. Scattered cells in the germinal centre of the secondary follicle stain strongly positive.
3.3. Lysozyme
Incubation with anti-lysozyme antibody revealed a granular to diffuse cytoplasmic reaction of lobulated or kidney-shaped nuclei cells with an abundant cytoplasm. Scattered positive cells were observed in the germinal centres of lymphoid follicles of lymph nodes, tonsil, spleen, and Peyer’s patches. In the PALs and marginal zone of the spleen-isolated positive cells were detected. In the thymus, positivity was observed in scattered cells of the cortical and medullary area. Thymic corpuscles stained also positive. Cells located in the medulla-like subcapsular area of the lymph node were strongly positive (Fig. 3 ), while cells in the lumen of the pulmonary alveoli showed only a weak reaction. In the liver, cells lining the sinusoids stained strongly positive. Among non-lymphoid tissues, renal proximal tubular cells, epithelial lining cells of the tonsillar crypts, epithelial cells of gastric glands and endothelial cells of high endothelial venules in some lymph nodes stained.
Fig. 3.
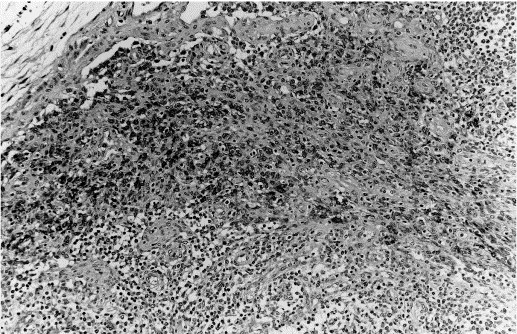
Immunohistochemical staining of formalin-fixed paraffin-embedded porcine lymph node section with anti-lysozyme using the ABC method. Mayer’s haematoxylin conterstain ×20. Strongly positive cells are observed in the medulla-like subcapsular area.
3.4. Mac 387
Mac 387 antibody strongly stained the cytoplasm of circulating polymorphonuclear granulocytes which had a variable distribution in the tissues studied, but were constantly observed in the red pulp of the spleen. Under our conditions, cells with morphology of monocyte–macrophage lineage were not stained with this antibody.
3.5. 3C3/9
The 3C3/9 antibody labelled the surface and cytoplasm of two cellular types localised mainly in B cell areas. One cellular type had small, round nuclei and a scant amount of cytoplasm, and the other showed more abundant cytoplasm. Positive cells were observed in primary and secondary follicles of the lymph nodes (Fig. 1c), tonsil, spleen, and Peyer’s patches. In the secondary follicles, the strongest stained area was the mantle zone. In the germinal centre, a small number of cells gave a weak positive reaction. In the Peyer’s patches, positivity was present in the corona of the follicles and, in the dome region, a large number of cells were also positive. In the spleen, a variable number of positive cells were observed in the marginal zone and in the PALs (Fig. 4 a). In the aggregated lymphoid follicles of the gastric tract mucosa, many positive cells were distributed in the periphery of the aggregates and only a few positive cells were observed in the centre of the follicles. In the liver, scattered circulating cells stained positive with 3C3/9 antibody.
Fig. 4.
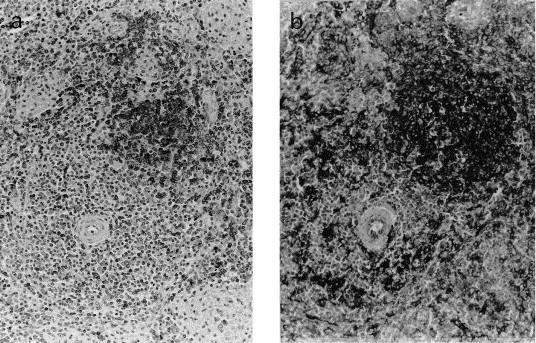
Immunohistochemical staining of formalin-fixed paraffin-embedded porcine spleen immunostained with CD45RA (3C3/9) (a), and anti-SLA-II-DQ (BL2H5) (b) using the ABC method. Mayer’s haematoxylin conterstain ×20. Primary follicle showing reaction with 3C3/9 (a), and BL2H5 (b). Scattered positive cells in the PALs and marginal zone with 3C3/9 (a). Numerous cells show strong positive reaction with BL2H5 (b), in the PALS, and in the marginal zone.
3.6. BL2H5
Incubation with BL2H5 antibody gave a strong reticular pattern of staining of the surface of two different cell types. One cell type was formed by small round cells with round, central-located nuclei and a narrow rim of cytoplasm, while the other was constituted of larger cells with central kidney-shaped nuclei, surrounded by an abundant cytoplasm with or without cytoplasmic processes. In the mantle zone of lymphatic follicles, all cells stained positive and, morphologically, these cells belonged to the small type group. Larger stained cells were observed in follicular germinal centres, and in interfollicular areas of lymph nodes (Fig. 1d), tonsils and Peyer’s patches. Furthermore, in the spleen, stained cells belonging to both types were found randomly distributed in the PALs, in the marginal zone and in the red pulp as isolated cells (Fig. 4b). In the thymus, large positive cells were mainly localised in the medulla, whereas in the cortex, only a few cells stained positive. In the dome region of the Peyer’s patches and in the aggregated lymphoid follicles of the gastric tract mucosa, a large number of positive cells of both types were observed. In the liver, few small cells lining the sinusoid and some perivascular cells reacted with mAb BL2H5. Staining was also observed in some endothelial cells of small blood vessels in some of the studied tissues.
4. Discussion
During the last decade, new emergent viral diseases causing important lesions in the lymphoid tissues have been described in pigs (Molitor et al., 1997, Rosell et al., 1999). The swine also has become an increasingly interesting experimental model to investigate transplant rejection, and a potential donor for xenografts (Sundt and Sachs, 1992, Bennet et al., 2000). Detailed knowledge of the normal distribution of cell populations of the immune system is required to understand pathogenesis of changes in these processes. The present work investigated this normal distribution by immunohistochemical techniques in paraffin-embedded tissues of conventional pigs, using four available antibodies and two mAbs which reactivity in paraffin-embedded tissues had not been characterised previously.
Polyclonal anti-human CD3 has been considered a specific pan T cells antibody in paraffin-embedded tissue (Mason et al., 1989). In our study, distribution of positive cells in normal lymphoid and non-lymphoid porcine tissues agreed with a previous description of other authors (Tanimoto and Ohtsuki, 1996), confirming the specificity of this antibody for T cells in swine tissues. T cell areas in lymphoid organs are clearly depicted with this antibody. In our study, in addition to T cell zones (Tanimoto and Ohtsuki, 1996), scattered cells with lymphoid morphology appeared stained in the marginal zone of the spleen.
Immunohistological detection of B cells has been done with anti-immunoglobulins antibodies (Ramos et al., 1992). Recently, anti-CD79α, the B cell equivalent to CD3, was found to be a useful marker for swine normal and neoplastic B cells in formalin-fixed, paraffin-embedded tissues (Tanimoto and Ohtsuki, 1996). As expected, the majority of CD79α-positive cells observed in our study were identified as B lymphocytes from its shape and location. In the germinal centres, stained cells with a larger amount of cytoplasm could correspond to immunoblasts. In addition, few CD79α-positive cells were also found in the thymic medulla, and in the marginal zone of the spleen.
Polyclonal anti-human lysozyme and monoclonal anti-human Mac 387 have been described as useful markers to stain cells of the monocyte/macrophage series in swine paraffin sections (Evensen, 1993). In the present study, lysozyme-identified resident macrophages in tissues (Kupffer cells, alveolar macrophages, intraglomerular mesangial cells, tingible-body macrophages in the cortex of the thymus, spleen sinus macrophages, etc.) and other non-lymphoid cells (renal proximal tubular cells, epithelial lining cells of the tonsillar crypts, epithelial cells of gastric glands and endothelial cells of high endothelial venules) which may also contain this enzyme. On the contrary, Mac 387 failed to detect macrophages, staining only polymorphonuclear granulocytes. A possible explanation to this fact might be that the L1 protein is not consistently expressed in swine macrophages. Other authors (Whyte et al., 1996, Smith et al., 1998) have also reported inconsistency of results of Mac 387 staining.
In a previous study, 3C3/9 has been shown to stain different subpopulations of lymphocytes, particularly, follicular lymphocytes and a small number of cells scattered in the interfollicular areas of swine frozen lymphoid sections (Bullido et al., 1997a). These results were confirmed in the present study, in paraffin-embedded tissues. Furthermore, in the present investigation, other organs were studied with this marker for the first time, as the spleen, the stomach and the liver. This antibody reacted with two different cellular types. One corresponded to lymphocytes for shape and location. A part of these cells could be considered B lymphocytes, on the basis of their location in primary follicles and mantle zones, while the other part could be B or T lymphocytes. In fact, it has been demonstrated that, in addition to B cells, naı̈ve T cells also express the high molecular weight isoform of CD45 (CD45RA) (Mackay, 1993, Bullido et al., 1997a). The other cellular type, with a more abundant cytoplasm and located in the germinal centres correspond to immunoblasts.
Histocompatibility class II antigens are present in a limited number of cell types. In the swine, they are expressed on all B cells, on APC and in a variable number of resting and activated T cells (Saalmuller et al., 1991, Bullido et al., 1997b). In the present investigation, it was possible to identify these cells, and to study their distribution by using an anti-SLA-II-DQ, the BL2H5 molecule. This antibody was used for the first time in an immunohistochemical study. Positive cells with round central nuclei and a small rim of cytoplasm were considered lymphocytes, while large cells were recognised to be macrophages, dendritic cells or interdigitating cells, depending on their distribution in the lymphoid organs. Lymphocytes observed in the mantle zone of the follicles in lymph node, tonsils, spleen and Peyer’s patches were considered B lymphocytes. Scattered small positive cells in the PALs, marginal zone and red pulp of the spleen could be considered T lymphocytes. In the dome region of the Peyer’s patches and in the aggregated lymphoid follicles of the gastric tract mucosa both B and T cells are found. Positive large cells in follicular germinal centres of lymph nodes, tonsils, spleen and Peyer’s patches were identify as follicular dendritic cells, while positive staining in the interfollicular areas was due to the presence of interdigitating cells. In the medulla of the thymus, positivity was attributed to the epithelial network and interdigitating cells. In the liver, staining was restricted to Kupffer cells and perivascular macrophages.
In conclusion, this work represents a detailed histological study of the distribution of the most important subpopulations of immune system cells in conventional, healthy pigs. In our view, these tools will allow studies on the histological alterations of the immune system cells in disease conditions.
Acknowledgements
The authors wish to thank Ms Silvia Usero, Ms Blanca Perez, and Mr Pere Losada for technical assistance. Francesca Chianini is a doctoral Marie Curie fellow (FMBICT983417). This work was partly funded by the QLRT-PL-199900307 Project from the European Commission’s Fifth Framework Programme 1998–2002, and the 2-FEDER-1997-1341 project, I+D National Plan (Spain).
References
- Bennet W., Sundberg B., Lundgren T., Tibell A., Groth C.G., Richards A., White D.J., Elgue G., Larsson R., Nilsson B., Korsgren O. Damage to porcine islets of Langherhans after exposure to human blood in vitro, or after intraportal transplantation to cynomolous monkeys: protective effects of sCR1 and heparin. Transplantation. 2000;69:711–719. doi: 10.1097/00007890-200003150-00007. [DOI] [PubMed] [Google Scholar]
- Bianchi A.T., Zwart R.J., Jeurissen S.H., Moonen-Leusen H.W. Development of the B- and T-cell compartments in porcine lymphoid organs from birth to adult life: an immunohistological approach. Vet. Immunol. Immunopathol. 1992;33:201–221. doi: 10.1016/0165-2427(92)90182-p. [DOI] [PubMed] [Google Scholar]
- Bullido R., Goméz del Moral M., Domenéch N., Alonso F., Ezquerra A., Domı́nguez J. Monoclonal antibodies to a high molecular weight isoform of porcine CD45: biochemical and tissue distribution analyses. Vet. Immunol. Immunopathol. 1997;56:151–162. doi: 10.1016/s0165-2427(96)05728-5. [DOI] [PubMed] [Google Scholar]
- Bullido R., Domenéch N., Alvarez B., Alonso F., Babı́n M., Ezquerra A., Ortuño E., Domı́nguez J. Characterisation of five monoclonal antibodies specific for swine class II major histocompatibility antigens and crossreactivity studies with leukocytes of domestic animals. Dev. Compar. Immunopathol. 1997;21:311–322. doi: 10.1016/s0145-305x(97)00008-6. [DOI] [PubMed] [Google Scholar]
- Christgau M., Caffesse R.G., Newland J.R., Schmalz G., D’Souza R.N. Characterisation of immunocompetent cells in the diseased canine periodontium. J. Histochem. Cytochem. 1998;46:1443–1454. doi: 10.1177/002215549804601213. [DOI] [PubMed] [Google Scholar]
- Denham S., Zwart R.J., Whittall J.T.D., Pampusch M., Corteyn A.H., Bianchi A.T.J., Murtaugh M.P., Parkhouse R.M.E., Tlaskalova H., Sinkora J., Sinkora M., Rehakova Z. Monoclonal antibodies putatively identifying porcine B cells. Vet. Immunol. Immunopathol. 1998;60:317–328. doi: 10.1016/s0165-2427(97)00108-6. [DOI] [PubMed] [Google Scholar]
- Evensen Ø. An immunohistochemical study on the cytogenetic origin of pulmonary multinucleate giant cells in porcine dermatosis vegetans. Vet. Pathol. 1993;30:162–170. doi: 10.1177/030098589303000209. [DOI] [PubMed] [Google Scholar]
- Falk E., Fallon J., Mailhac A., Fernadez-Ortiz A., Meyer B.J., Weng D., Shah P.K., Badimon J.J., Fuster V. Muramidase: a useful monocyte/macrophage immunocytochemical marker in swine of special interest in experimental cardiovascular disease. Cardiovasc. Pathol. 1994;3:183–189. doi: 10.1016/1054-8807(94)90028-0. [DOI] [PubMed] [Google Scholar]
- Haverson K., Bailey M., Higgins V.R., Bland P.W., Stokes C.R. Characterisation of monoclnal antibodies specific for monocytes, macrophages and granulocyte from porcine peripheral blood and mucosal tissues. J. Immunol. Meth. 1994;170:233–245. doi: 10.1016/0022-1759(94)90398-0. [DOI] [PubMed] [Google Scholar]
- Mackay C.R. Immunological memory. Adv. Immunol. 1993;53:217–265. doi: 10.1016/s0065-2776(08)60501-5. [DOI] [PubMed] [Google Scholar]
- Mason D.Y., Cordell J., Brown M., Pallesen G., Ralfkiaer E., Rothbard J., Crumpton M., Gatter K.C. Detection of T cells in paraffin wax-embedded tissue using antibodies against a peptide sequence from the CD3 antigen. J. Clin. Pathol. 1989;42:1194–1200. doi: 10.1136/jcp.42.11.1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason D.Y., Cordell J.L., Brown M.H., Borst J., Pulford K., Jaffe E., Ralfkiaer E., Dallenbach F., Stein H., Pileri S., Gatter J.K. CD79α: a novel marker for B-cell neoplasm in routinely processed tissue samples. Blood. 1995;86:1453–1459. [PubMed] [Google Scholar]
- Molitor T.W., Bautista E.M., Choi C.S. Immunity to PRRSV: double-edged sword. Vet. Microbiol. 1997;55:265–276. doi: 10.1016/S0378-1135(96)01327-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos J.A., Ramis A.J., Marco A., Domingo M., Rabanal R., Ferrer L. Histochemical and immunohistochemical study of the mucosal lymphoid system in swine. Am. J. Vet. Res. 1992;53:1418–1426. [PubMed] [Google Scholar]
- Rosell C., Segalés J., Plana-Durán J., Balasch M., Rodrı́guez-Arrioja G.M., Kennedy S., Allan G.M., McNeilly F., Latimer K.S., Domingo M. Pathological, immunohistochemical and in situ hybridisation studies of postweaning multysystemic wasting syndrome (PMWS) in pigs. J. Comp. Pathol. 1999;120:307–313. doi: 10.1053/jcpa.1998.0258. [DOI] [PubMed] [Google Scholar]
- Saalmuller A., Weiland F., Reddehase M. Resting porcine T lymphocytes expressing class II major histocompatibility antigen. Immunobiology. 1991;183:102–114. doi: 10.1016/S0171-2985(11)80190-7. [DOI] [PubMed] [Google Scholar]
- Smith K.J., Graham J.S., Skelton H.G., Hamilton T., O’Leary T., Okerberg C.V., Moeller R., Hurst C.G. Sensitivity of cross-reacting anti-human antibodies in formalin-fixed porcine skin: including antibodies to proliferation antigens and cytokeratins with specificity in the skin. J. Dermatol. Sci. 1998;18:19–29. doi: 10.1016/s0923-1811(98)00018-8. [DOI] [PubMed] [Google Scholar]
- Sundt Arn T.M., III., Sachs D.H. Patterns of T-cell-accessory cell interaction in the generation of primary alloresponses in the pig. Transplantation. 1992;54:911–916. doi: 10.1097/00007890-199211000-00027. [DOI] [PubMed] [Google Scholar]
- Tanimoto T., Ohtsuki Y. Evaluation of antibodies reactive with porcine lymphocytes and lymphoma cells in formalin-fixed paraffin-embedded, antigen-retrieved tissue sections. AJVR. 1996;57:853–859. [PubMed] [Google Scholar]
- Whyte A., Ockleford C.D., Byrne S., Hubbard A., Woolley S.T. Leukocyte and endothelial cell adhesion molecule expression in porcine histiocytic leiomyofibrosarcoma. J. Comp. Pathol. 1996;115:429–440. doi: 10.1016/s0021-9975(96)80076-4. [DOI] [PubMed] [Google Scholar]


