Abstract
Multiple sclerosis (MS) is an immune-mediated demyelinating disease that could be triggered by a viral infection. Coronaviruses induce an MS-like disease in rodents, are neuroinvasive in humans and can infect primary cultures of human astrocytes and microglia. Infection of the human astrocytic cell line U-373MG by the OC43 strain of human coronavirus caused an upregulation of IL-6, TNF-α, and MCP-1 mRNA expression. This virus also modulated the activity of matrix metalloproteinases-2 and -9 and augmented nitric oxide production in both U-373MG cells and the human microglial cell line CHME-5. Thus, a coronaviral infection of glial cells could lead to the production of inflammatory molecules that have been associated with central nervous system pathologies such as MS.
Keywords: Multiple sclerosis, Coronavirus, Cytokines, Chemokines, Matrix metalloproteinases, Nitric oxide
1. Introduction
Multiple sclerosis (MS) is an inflammatory demyelinating disease of the central nervous system (CNS) that is characterized by mononuclear cell infiltration into the CNS, myelin degradation and oligodendrocyte loss. It is the most common neurological disease affecting young adults (Ewing and Bernard, 1998). The etiology and pathogenesis of MS have yet to be elucidated but are probably multifactorial, involving both genetic and environmental factors (Ewing and Bernard, 1998). Several viruses have been associated with demyelinating processes (Sarchielli et al., 1993) and the human coronaviruses (HCoV) represent candidate environmental triggers of MS pathogenesis. The murine counterpart of HCoV, murine hepatitis virus (MHV), can induce an MS-like disease in genetically susceptible animals (Lane and Buchmeier, 1997) and HCoV are both neurotropic and neuroinvasive (Arbour et al., 1999a, Arbour et al., 1999b, Arbour et al., 2000). We have previously characterized acute and persistent infections of human neural cell lines by both known viral serogroups, OC43 and 229E (Lachance et al., 1998, Arbour et al., 1999a, Arbour et al., 1999b). Importantly, HCoV-OC43 RNA was detected in human brains (Murray et al., 1992, Stewart et al., 1992, Arbour et al., 2000). Interestingly, primary cultures of human astrocytes and microglia are susceptible to HCoV infection (Bonavia et al., 1997). Thus, this virus may be involved in demyelinating diseases by a mechanism of virus-induced myelin damage following infection of astrocytes and/or microglia.
It has been suggested that upon infection of glial cells, the upregulation of pro-inflammatory molecules might participate in the pathogenesis of several inflammatory CNS diseases (Chao et al., 1996, Sriram and Rodriguez, 1997). Given our in vitro observations of glial cell infection by HCoV, we hypothesize that HCoV may participate in MS pathogenesis through an indirect mechanism whereby glial cell infection in genetically susceptible individuals may lead to the upregulation of pro-inflammatory molecules that could participate in the destruction of the oligodendrocyte-myelin unit observed in MS.
Several inflammatory molecules including cytokines, chemokines, matrix metalloproteinases (MMPs), and nitric oxide (NO), have been associated with CNS pathologies. Moreover, a wide range of cytokines and chemokines have been detected in MS lesions. More specifically, IL-1α and β, IL-6, TNF-α, IFN-γ, MIP-1α, and MCP-1, have pro-inflammatory properties that could participate in CNS demyelination (Benveniste, 1997, Munoz-Fernandez and Fresno, 1998). Activated glial cells are known to secrete matrix metalloproteinases. Of these, MMPs-2, -3, -7 and -9 were shown to be able to cleave myelin basic protein (MBP) within the encephalitogenic portion of the protein; this would be expected to contribute to immune responses against MBP epitopes and demyelination (Chandler et al., 1995, Chandler et al., 1997, Cossins et al., 1997). Astrocytes and microglia can produce NO, a non-specific inflammatory mediator of oligodendrocyte death. Furthermore, active NO synthase was detected in astrocytes present at the level of MS plaques, at the lesion edge where demyelination occurs (Mitrovic et al., 1996).
In the present study, the capacity of human coronavirus infection to modulate the expression of pro-inflammatory molecules in astrocytes and microglia was assessed. The targeted molecules were those associated with MS pathology (Bilzer and Stitz, 1996, Benveniste, 1997, Giovannoni et al., 1997, Sarchielli et al., 1997, Link, 1998, Munoz-Fernandez and Fresno, 1998, Lee et al., 1999), those detected in animal models of MS such as experimental autoimmune encephalomyelitis (EAE; Cross et al., 1994, Renno et al., 1995, Benveniste, 1997, Clements et al., 1997, Kennedy et al., 1998), those modulated following infection by neurotropic viruses such as Theiler’s virus, MHV-JHM, human immunodeficiency virus (HIV), human T-lymphotropic virus-1 (Bilzer and Stitz, 1996), and finally those known to be expressed in glial cells (Reiling et al., 1994, Bilzer and Stitz, 1996).
We present in vitro data that is consistent with the hypothesis that the OC43 strain of HCoV, which we have shown to be neuroinvasive in humans, could be involved in CNS pathologies by an indirect mechanism. We show that HCoV-OC43 infection alters IL-6, TNF-α, and MCP-1 mRNA expression in infected astrocytes and upregulates NO production and MMP-2 and MMP-9 secretion in infected astrocytes and microglia. These results provide new insights into how infection by a neurotropic and neuroinvasive human virus might contribute to inflammatory immunopathologies of the CNS, such as MS.
2. Materials and methods
The HCoV-OC43 serogroup was chosen for these studies because we have previously shown that the glial cell lines tested are susceptible to infection by this coronavirus strain, which belongs to the same antigenic group as MHV, a murine coronavirus that can induce a pathology similar to MS in mice (Lai and Cavanagh, 1997). Since infiltrating T cells also contribute to MS pathology, and these cells are a major source of IFN-γ, we also evaluated the effect of an exogenous source of IFN-γ on the induction of inflammatory molecules in glial cells (Bilzer and Stitz, 1996).
2.1. Virus and cell lines
HCoV-OC43 was originally obtained from the American Type Culture Collection (ATCC, Manassas, VA), plaque purified twice, and grown on HRT-18 cells (ATCC) as previously described (Mounir and Talbot, 1992). The fifth passage of HCoV-OC43 stocks kept at −90°C, having a titer of 3.5×106 TCID50/ml, was used in all experiments.
The CHME-5 cell line consists of human fetal microglial cells immortalized by transfection with the SV40 large T antigen (Janabi et al., 1995). The U-373MG cell line was obtained from ATCC and was originally derived from a grade III human astrocytoma. Cells were grown in Dulbecco’s modified Eagle’s medium (DMEM), supplemented with 10% (v/v) heat-inactivated fetal bovine serum (FBS) and 50 μg/ml gentamicin (Gibco Laboratories, Grand Island, NY).
2.2. Expression of cytokines and chemokines in glial cell lines
For evaluation of mRNA expression, CHME-5 and U-373MG cell monolayers were grown to 80% confluence on plastic 140×20-mm Petri dishes. Positive controls for cytokine or chemokine expression were obtained by treating cells with 5 ng/ml phorbol myristate acetate (PMA; Sigma, Oakville, Ontario, Canada) or, for IL-12 induction, with 10 ng/ml of lipopolysaccharide (LPS; Sigma). To verify the influence of an exogenous source of IFN-γ, cells were treated with 100 U/ml of IFN-γ (Roche Diagnostics, Laval, Québec, Canada). Cells were infected with HCoV-OC43 at a multiplicity of infection (MOI) of 0.25 and treatments and infections were carried out for up to 3 days at 33°C in a humidified atmosphere containing 5% (v/v) CO2.
Total cellular RNA was extracted using TRIzol™ (Gibco). Five μg of RNA were reverse-transcribed according to manufacturer’s instructions (Roche Diagnostics) in the presence of 50 U of Expand™ Moloney murine leukemia virus reverse transcriptase (Mo-MuLV-RT), 60 U of RNAGuard™ RNase inhibitor (Roche Diagnostics) and 100 pmol of oligo(dT) (Roche Diagnostics) for 90 min at 42°C using 0.4 mM of deoxynucleotide triphosphates (dNTPs)-Na salt, 1× RT buffer (50 mM Tris–HCl, pH 8.3, 40 mM KCl, 5 mM MgCl2, 0.5%, v/v, Tween-20) and 10 mM dithiothreitol (DTT; Roche Diagnostics). Primers used for amplification of HCoV-OC43, glyceraldehyde 3-phosphate dehydrogenase (GAPDH), cytokines and chemokines are described in Table 1 . PCR conditions were optimized for salt concentration, amount of primer, number of cycles and annealing temperatures and are described in Table 2 . All primers were intron-spanning to allow potential DNA contamination to be assessed. A preliminary step involved amplification of GAPDH in all samples in order to compare amplification efficiencies. The concentration of cDNA was normalized using the integrated densitometric value (IS-1000 Digital Imaging System, Alpha Innotech Corporation, CA) obtained from GAPDH PCR amplification. The same concentrations were then used in all other amplification steps. PCR amplifications were performed using 2.5 U of Expand™ high-fidelity PCR system DNA polymerase (Roche Diagnostics) using the buffer supplied by the manufacturer (10 mM Tris–HCl, pH 8.3, 50 mM KCl) and 0.4 mM dNTPs-Li salt (Roche Diagnostics). A hot start was carried out at 94°C for 5 min followed by 5 min at 50–60°C (see Table 2). After addition of Expand™ polymerase, conditions used were: 1 min denaturation at 94°C, 2 min annealing, 2 min elongation at 72°C (annealing temperature and number of cycles are indicated in Table 2), followed by a final elongation step of 10 min at 72°C.
Table 1.
RT-PCR primers
| RNA | Sequence | Accession number |
|---|---|---|
| amplified | 5′→3′ | or reference |
| IL-1α | AGTGCTGCTGAAGGAGATGC | E04022 |
| AAGTGAGACTCCAGACCTACGC | ||
| IL-1β | GTGGCAATGAGGATGACTTG | M15330 |
| GCATCTTCCTCAGCTTGTCC | ||
| IL-6 | AGTCCTGATCCAGTTCCTGC | M14584 |
| TGACCAGAAGAAGGAATGCC | ||
| IL-10 | ACATGCTTCGAGATCTCCG | M57627 |
| GGTCTTGGTTCTCAGCTTGG | ||
| IL-12 | CAAGAACTTGCAGCTGAAGC | M86671 |
| GGATCAGAACCTAACTGCAGG | ||
| TGF-β | TTCAACACATCAGAGCTCCG | E00973 |
| ATAACCACTCTGGCGAGTCG | ||
| TNF-α | GGCAGTCAGATCATCTTCTCG | E00702 |
| ATGGCAGAGAGGAGGTTGAC | ||
| IFN-γ | AAATAATGCAGAGCCAAATTGTCTC | J00219 |
| TTGCAGGCAGGACAACCATTAC | ||
| MCP-1 | AACTGAAGCTCGCACTCTCG | X14768 |
| ATCTCCTTGGCCACAATGG | ||
| MIP-1α | TCACCTGCTCAGAATCATGC | AF043339 |
| GCTTGGTTAGGAAGATGACACC | ||
| GAPDH | GTGAAGGTCGGAGTCAACG | Arbour et al. (2000) |
| CACCTGGTGCTCAGTGTAGC | ||
| HCoV-OC43 | CCCAAGCAAACTGCTACCTCTCAG | Arbour et al. (1999a) |
| GTAGACTCCGTCAATATCGGTGCC |
Table 2.
PCR conditions
| RNA | Annealing | Quantity of | Number of | Concentration |
|---|---|---|---|---|
| amplified | Temperature | prime (pmol) | cycles | of MgCl2 |
| (°C) | (mM) | |||
| IL-1α | 60 | 20 | 30 | 2 |
| IL-1β | 60 | 50 | 25 | 2 |
| IL-6 | 60 | 20 | 30 | 2 |
| IL-10 | 60 | 50 | 30 | 2 |
| IL-12 | 55 | 50 | 32 | 2 |
| TGF-β | 60 | 20 | 27 | 1.5 |
| TNF-α | 60 | 20 | 30 | 1.5 |
| IFN-γ | 55 | 50 | 30 | 2 |
| MCP-1 | 61 | 50 | 27 | 2.5 |
| MIP-1α | 60 | 50 | 25 | 2 |
| GAPDH | 50 | 50 | 20 | 1.5 |
| HCoV-OC43 | 60 | 20 | 30 | 2 |
DNA amplicons were separated by electrophoresis on 1.5% (w/v) agarose gel and signals analyzed using the IS-1000 Digital Imaging System (Alpha Innotech Corporation). Volume integration of signals (AlphaImager™ software, Canberra Packard Canada) was normalized against the signals obtained from untreated cells, to yield the stimulation indices.
2.3. Secretion of matrix metalloproteinases by glial cells
The secretion and gelatinase activity of MMP-2 and MMP-9 were detected by gelatin-based zymography, using a modified version of the method of Heussen and Dowdle (1980). Supernatants of cells treated with PMA, infected with HCoV-OC43, or treated with conditioned media derived from infected cells (Giraudon et al., 1996) were harvested from cells cultured in medium without FBS. All samples were concentrated 10-fold by ultrafiltration using Centricon® YM-50 centrifugal filter devices (Amicon, Millipore Corporation, Bedford, MA). Twenty μl of samples were incubated with 5 μl non-reducing buffer (0.5 M Tris–HCl, pH 7.4, 10% (w/v) sodium dodecyl sulfate, 50% (v/v) glycerol) for 15 min at 37°C, and loaded onto 9% (w/v) polyacrylamide gels containing 0.4% (w/v) gelatin. After electrophoresis, gels were incubated twice in 2.5% (v/v) Triton X-100, rinsed with distilled water and then incubated in enzyme activation buffer (100 mM Tris–HCl, pH 7.4, 15 mM CaCl2) overnight at 37°C, with gentle rocking. After staining with Coomassie blue (0.1% (w/v) in acetic acid:methanol (1:3)), and destaining (acetic acid:methanol (1:3)), MMP-2 and MMP-9 activity was detected as clear bands of gelatin degradation. Incubation of the gel in activation buffer containing 10 mM EDTA, a specific inhibitor of Ca2+-dependent gelatinases/type IV collagenases, or 5 mM 1,10-phenanthroline, an inhibitor of Zn2+-dependent MMPs, served to confirm the specificity of the activities observed.
2.4. Production of nitric oxide by glial cells
Measurement of NO2 − in culture supernatants was performed using the Griess reagent according to the manufacturer’s instructions (Sigma), to evaluate NO production by cells (Green et al., 1982). Supernatants were distributed in 96-well microtiter plates and mixed with an equal volume of reagent solution. Absorbance was measured at 540 nm in an ELISA reader (BIO-TEK Instruments, Burlington, Vermont, USA). The NO2 − micromolar concentration was determined against a sodium nitrite standard, and background levels were subtracted using acellular medium.
3. Results
3.1. Cytokine and chemokine mRNA expression in infected glial cells
Expression of mRNA for the cytokines IL-1α, IL-1β, IL-6, IL-10, IL-12, TNF-α, TGF-β, IFN-γ, and the chemokines MIP-1α and MCP-1 was determined by semi-quantitative RT-PCR in the astrocytic and microglial cell lines. Upon HCoV-OC43 infection, we observed an upregulation in the expression of mRNA for IL-6 (Fig. 1A ), TNF-α (Fig. 1B) and MCP-1 (Fig. 1C) in the U-373MG astrocytic cell line. In contrast, the CHME-5 microglial cells expressed none of the molecules tested. The levels of IL-1α, IL-1β, and IL-12 were not altered following infection of U-373MG cells with HCoV-OC43 or upon treatment with IFN-γ and no expression of IL-10, TGF-β, IFN-γ or MIP-1α was detected in these cells (data not shown).
Fig. 1.
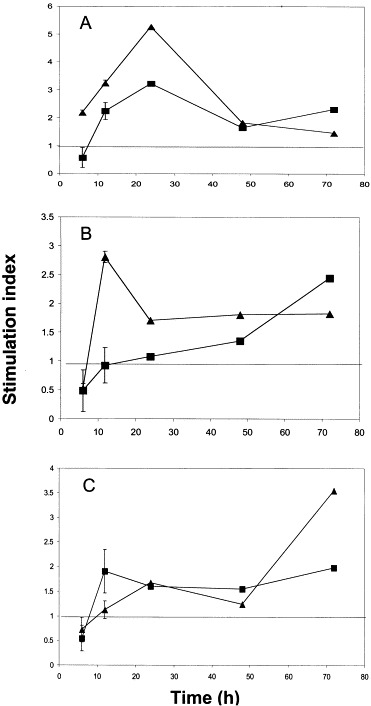
Upregulation of cytokine and chemokine mRNA expression in HCoV-OC43-infected and IFN-γ-treated U-373MG astrocytic cells. Stimulation indices were determined by semi-quantitative RT-PCR, using GAPDH as a housekeeping gene, and comparing to basal expression in non-stimulated cells. Data presented with error bar represent the mean±S.E. of three experiments. (A) IL-6; stimulation index for PMA-stimulated cells reached 15.7 at 6 h (data not shown). (B) TNF-α; stimulation index for PMA-stimulated cells reached 3.1 at 6 h (data not shown). (C) MCP-1; stimulation index for PMA-stimulated cells reached 2 at 6 h (data not shown). (■) HCoV-OC43; (▴) IFN-γ.
The kinetics of IL-6 induction after viral infection or IFN-γ treatment were similar, with a 3- and 5-fold maximal upregulation observed at 24 h, respectively (Fig. 1A). In both cases, IL-6 expression diminished after the 24-h time point, but remained above basal level (Fig. 1A). TNF-α mRNA expression was also upregulated in U-373MG cells after infection or IFN-γ treatment (Fig. 1B). IFN-γ treatment led to a maximal induction of 2.8-fold, reached at 24 h, which then decreased to a stable level of 1.8 for as long as 72 h. Viral infection caused a steady increase in TNF-α mRNA expression which peaked at 2.4-fold at 72 h, and further time points would be required to verify whether this upward trend is maintained (Fig. 1B). The expression of mRNA for the MCP-1 chemokine was also increased following either IFN-γ treatment or viral infection. While IFN-γ caused a steady increase in MCP-1 levels that reached 3.5-fold at 72 h, viral infection led to a 2-fold induction that peaked at 12 h and remained at this level (Fig. 1C).
3.2. Induction of matrix metalloproteinase secretion in glial cells
Since IL-6 and TNF-α have been reported to increase MMP activity (Giraudon et al., 1997), we evaluated the secretion of MMP-2 and MMP-9 (type IV collagenases) upon viral infection. Two major bands of gelatinolysis at 66 and 92 kDa were detected (Fig. 2 ) that were, respectively, identified as MMP-2 and pro-MMP-9 gelatinases, on the basis of their molecular mass and inhibition of activity by EDTA and 1,10-phenanthroline. In some instances, a third band of 82 kDa was observed which corresponds to active MMP-9 cleaved from its latent form, pro-MMP-9/92 kDa. While MMP-2 was constitutively expressed in both glial cell lines, its activity was increased at 24 h post-infection when compared to untreated cells. Both cell lines also expressed MMP-9. Untreated U-373MG astrocytic cells secreted very low levels of pro-MMP-9 which were significantly increased at 24 h post-infection, and a faint digestion site, corresponding to active MMP-9, was detected at 48 h post-infection (Fig. 2A). Similarly, the CHME-5 microglial cells showed MMP-9 secretion and an active form of the enzyme at 48 h (Fig. 2B). In order to determine whether increased MMP secretion was a direct consequence of viral infection or was due to soluble factors released upon viral infection, culture supernatants from infected cells were used to treat non-infected cells. While the supernatants were non-infectious, the kinetics of gelatinase activity was similar to that observed upon HCoV-OC43 infection (data not shown). These results strongly suggest that MMP secretion is mediated by soluble factors produced by infected cells.
Fig. 2.
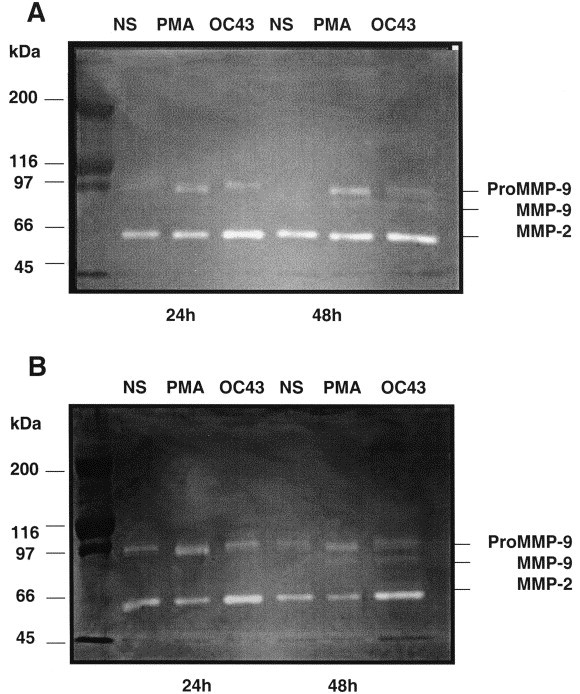
Gelatinase secretion in supernatants of: (A) U-373MG astrocytic cells and (B) CHME-5 microglial cells. Cells were stimulated or not (NS) for 24 and 48 h with PMA (100 ng/ml) or infected with HCoV-OC43 (MOI 0.25). Supernatants were collected, concentrated and assayed for their gelatinase content by gelatin-based zymography. Results are representative of three independent experiments.
3.3. Induction of nitric oxide production in glial cells
Since nitric oxide (NO) is a pro-inflammatory molecule that has been implicated in MS pathology (Bagasra et al., 1995) and astrocytes are the main producers of NO within the CNS (Lee and Brosnan, 1996), we evaluated NO production following HCoV infection. Untreated U-373MG astrocytic cells produced an average of 2.4 μM of NO at all time points tested (Fig. 3A ), presumably through the constitutive isoform of nitric oxide synthase (cNOS). Viral infection led to a 2-fold increase in NO production at 48 h, which was sustained up to 72 h (Fig. 3A). The CHME-5 microglial cells also produced NO but in lower quantities compared to that produced by U-373MG astrocytic cells (Fig. 3B).
Fig. 3.
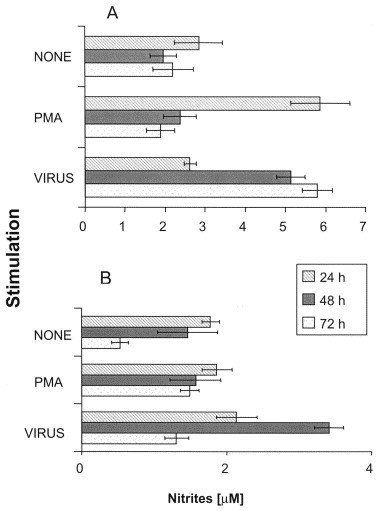
NO secretion by: (A) U-373MG astrocytic cells and (B) CHME-5 microglial cells cultured with different stimuli, for 24, 48, and 72 h. Data shown in each panel represent mean±S.E. of four or three independent experiments, respectively.
4. Discussion
We have shown by semi-quantitative RT-PCR the upregulation of mRNA expression for IL-6, TNF-α and MCP-1 following HCoV-OC43 infection or IFN-γ treatment of astrocytes. Moreover, detection of gelatinase activity by zymography suggested a modulation of MMP-2 and MMP-9 activity in infected astrocytes and microglia that was mediated by soluble molecules. Finally, nitrite quantification suggested that viral infection of both cell types led to the release of NO, presumably through iNOS activation. Thus, our data clearly show that the expression of pro-inflammatory molecules (cytokines, chemokines, MMPs, and NO) that have been associated with CNS pathologies is increased in glial cells following infection by the OC43 strain of human coronavirus.
Astrocytes are known to be the main producers of IL-6 within the CNS, and its expression can be induced by cytokines such as TNF-α, IL-1β, IFN-γ, and TGF-β (Bilzer and Stitz, 1996). The upregulation of IL-6 mRNA expression observed following HCoV infection was described after Theiler’s virus infection of astrocytes (Benveniste, 1998). The precise role of IL-6 in CNS pathologies remains unclear since it appears to have both pro- and anti-inflammatory properties through the promotion of leukocyte recruitment by induction of chemokine and adhesion molecules or the downregulation of TNF-α expression and the upregulation of soluble TNF-α receptor and IL-1R antagonist expression (Benveniste, 1998). Given that IL-6 induction following HCoV infection did not abrogate TNF-α induction in our study, this argues that IL-6 does not repress TNF-α induction in astrocytes. In addition, IL-6 was shown to modulate the expression of MMP and TIMP (tissue inhibitor of matrix metalloproteinase; Giraudon et al., 1997).
Infected U-373MG astrocytic cells showed a gradual and steady increase in TNF-α expression, which reached 2.8-fold at 72 h post-infection. This induction might be relevant to CNS pathologies, given that transgenic mice overexpressing murine TNF-α in the CNS spontaneously developed chronic CNS inflammation and treatment with anti-murine TNF-α antibody prevented demyelination (Probert et al., 1995). Moreover, it has been shown that TNF-α can cause oligodendrocyte death and myelin damage in vitro (Robbins et al., 1987). Finally, TNF-α can induce MMP and iNOS expression, which can be harmful to CNS cells (Lane et al., 1996, Giraudon et al., 1997). The contribution of TNF-α to demyelination remains controversial since it has been reported not to be required for the development of EAE (Frei et al., 1997).
Expression of the MCP-1 chemokine can be upregulated in CNS pathologies such as MHV-induced neurological disease in mice, EAE and MS (Glabinski and Ransohoff, 1999). Given that astrocytes are the main producers of MCP-1 within the CNS (Glabinski and Ransohoff, 1999), the increased expression of MCP-1 observed following HCoV-OC43 infection might contribute to MS pathology by promoting leukocyte infiltration into the CNS (Calvo et al., 1996). Lymphocyte infiltration in the CNS is well known to contribute to the pathology of MS (Bilzer and Stitz, 1996) and IFN-γ is an antiviral cytokine produced by activated Th1 and NK cells that has been detected within MS lesions (Munoz-Fernandez and Fresno, 1998).
Interestingly, systemic IFN-γ administration worsened the clinical state of MS patients (Munoz-Fernandez and Fresno, 1998). Since glial cells are not the main producers of IFN-γ (data not shown; Bilzer and Stitz, 1996) we found it pertinent to evaluate the effect of an exogenous source of IFN-γ, hypothetically produced by activated T cells, on expression of cytokines and chemokines. In this study, IFN-γ led to increased IL-6, TNF-α, and MCP-1 mRNA expression similar to that observed following HCoV infection. It is tempting to speculate that the combined effects of viral infection and lymphocyte-derived IFN-γ would have synergistic or additive effects that could contribute to inflammatory responses and MS pathology.
The CHME-5 microglial cells expressed none of the cytokines and chemokines tested. This could be a result of the transfection technique used to immortalize these cells, and serves to underline the need to pursue these studies with primary cultures. To our knowledge, this microglial cell line is the only one presently available worldwide. One current aim of our laboratory is to develop a new microglial cell line, to be used as an experimental tool for further studies of human coronavirus neurotropism and neuroinvasiveness.
There is growing evidence suggesting that MMP activity, in particular MMP-9, contributes to myelin degradation and promotes leukocyte trafficking across the blood–brain barrier (Goetzl et al., 1996, Chandler et al., 1997). It has been suggested that IFN-β used therapeutically in MS targets MMP-9 (Stuve et al., 1996). Also, MMPs can process pro-inflammatory cytokines, such as TNF-α and can degrade myelin basic protein to release immunogenic peptides (Cuzner and Opdenakker, 1999). We observed enhanced MMP-2 and MMP-9 secretion in infected cells. Since MMP-2 can cleave and activate MMP-9, the increased MMP-9 activity might be attributed in part to increased MMP-2 expression (Fridman et al., 1995). Increased activity of MMP-2 was reported in other viral infections, such as upon exposure to HIV-1 gp41 peptides (Chong et al., 1998). Interestingly, we also observed an upregulation of MMP-2 and MMP-9 expression in cells treated with supernatant derived from infected cells.
MMP synthesis and activity are regulated by gene transcription and translation, secretion, activation, or through inhibitors (Kleiner and Stetler-Stevenson, 1993). It would thus appear that HCoV-OC43 infection can influence the protease cascade indirectly through cellular factors induced following viral infection, such as IL-6 and TNF-α, as previously reported (Gottschall and Yu, 1995). It would therefore be of interest to evaluate the effect of these cytokines on MMP secretion and activity in glial cells. It is clear that cytokines and possibly viral proteins can regulate the synthesis, secretion and activity of MMPs. The combined effects of pro-inflammatory cytokines and MMP secretion on the health of the oligodendrocyte–myelin unit and their precise role in MS pathogenesis remain to be investigated. It is known, however, that MMPs can participate in autoimmunity by cleavage of myelin components and degradation of the extracellular matrix, thereby altering cell–cell connectivity. Since MMPs can sustain or direct the action of TNF-α by converting pro-TNF-α to its active form (Giraudon et al., 1996), it would appear that a self-amplification loop could exist in vivo.
We have shown increased NO production by infected astrocytes and microglia. Observations made in other laboratories suggest that astrocytes represent the main source of NO in the CNS (Lee and Brosnan, 1996), which is consistent with our in vitro results. It has been shown that human glial cells can be induced to express iNOS mRNA after exposure to pro-inflammatory cytokines such as IFN-γ and TNF-α (Ogura and Esumi, 1996). Thus, the increased NO production we observed might be attributed to TNF-α induction following infection. The observed pattern of NO production suggests that it is mediated by the transcription of the iNOS gene, since it is known to be tightly regulated and to generate greater amounts of NO (Liu et al., 1994). Given that in situ analysis of brain autopsies have shown iNOS immunoreactivity in the CNS of MS patients (Bagasra et al., 1995), viral infection might contribute to the pathology by promoting inflammatory cytokine release and iNOS induction.
In conclusion, astrocytes and microglia respond to infection by the OC43 strain of human coronavirus by producing various inflammatory molecules. This argues for an indirect inflammatory mechanism by which HCoV-OC43 could be implicated in an inflammatory pathology of the CNS, such as MS. We have shown that HCoV-OC43 can influence the expression of cytokines, chemokine and MMP and lead to an increased production of NO. These inflammatory mediators could act in concert to orchestrate an inflammatory pathology in the CNS.
Acknowledgements
This work was supported by grant MT-9203 from the Medical Research Council of Canada to P. J. Talbot. J. Edwards is grateful to the Institut Armand-Frappier for studentship support. We thank Francine Lambert for excellent technical assistance, and Nathalie Arbour for her important contributions to this study. We wish to thank Drs. Yves St-Pierre and Pierre Tremblay (INRS-Institut Armand-Frappier), and Drs. Pascale Giraudon, Arlette Bernard, and Marie-Françoise Bélin (Unité INSERM 433, Lyon, France) for their invaluable suggestions and advice.
References
- Arbour N., Côté G., Lachance C., Tardieu M., Cashman N.R., Talbot P.J. Acute and persistent infection of human neural cell lines bu human coronavirus OC43. J. Virol. 1999;73:3338–3350. doi: 10.1128/jvi.73.4.3338-3350.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arbour N., Ekandé S., Côté G., Lachance C., Chagnon F., Tardieu M., Cashman N.R., Talbot P.J. Persistent infection of human oligodendrocytic and neuroglial cell lines by human coronavirus 229E. J. Virol. 1999;73:3326–3337. doi: 10.1128/jvi.73.4.3326-3337.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arbour, N., Day, R., Newcombe, J., Talbot, P.J., 2000. Neuroinvasion by human respiratory coronaviruses. J. Virol. (in press). [DOI] [PMC free article] [PubMed]
- Bagasra O., Michaels F.H., Zheng Y.M. Activation of the inducible form of nitric oxide synthase in the brains of patients with multiple sclerosis. Proc. Natl. Acad. Sci. USA. 1995;92:12041–12045. doi: 10.1073/pnas.92.26.12041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benveniste E.N. Role of macrophages/microglia in multiple sclerosis and experimental allergic encephalomyelitis. J. Mol. Med. 1997;75:165–173. doi: 10.1007/s001090050101. [DOI] [PubMed] [Google Scholar]
- Benveniste E.N. Cytokine actions in the central nervous system. Cyt. Gr. Fact. Rev. 1998;9:259–275. doi: 10.1016/s1359-6101(98)00015-x. [DOI] [PubMed] [Google Scholar]
- Bilzer T., Stitz L. Immunopathogenesis of virus diseases affecting the central nervous system. Crit. Rev. Immunol. 1996;16:142–222. doi: 10.1615/critrevimmunol.v16.i2.20. [DOI] [PubMed] [Google Scholar]
- Bonavia A., Arbour N., Yong V.W., Talbot P.J. Infection of primary cultures of human neural cells by human coronaviruses 229E and OC43. J. Virol. 1997;71:800–806. doi: 10.1128/jvi.71.1.800-806.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvo C.F., Yoshimura T., Gelman M., Mallat M. Production of monocyte chemotactic protein-1 by rat brain macrophages. Eur. J. Neurosci. 1996;8:1725–1734. doi: 10.1111/j.1460-9568.1996.tb01316.x. [DOI] [PubMed] [Google Scholar]
- Chandler S., Coates R., Gearing A., Lury J., Wells G., Bone E. Matrix metalloproteinases degrade myelin basic protein. Neurosci. Lett. 1995;201:223–226. doi: 10.1016/0304-3940(95)12173-0. [DOI] [PubMed] [Google Scholar]
- Chandler S., Miller K.M., Clements J.M., Lury J., Corkill D., Anthony D.C.C., Adams S.E., Gearing A.J.H. Matrix metalloproteinases, tumor necrosis factor and multiple sclerosis: an overview. J. Neuroimmunol. 1997;72:155–161. doi: 10.1016/s0165-5728(96)00179-8. [DOI] [PubMed] [Google Scholar]
- Chao C.C., Hu S., Peterson P.K. Glia: the not so innocent bystanders. J. Neurovirol. 1996;4:234–239. doi: 10.3109/13550289609146886. [DOI] [PubMed] [Google Scholar]
- Chong Y.H., Seoh J.Y., Park H.K. Increased activity of matrix metalloproteinase-2 in human glial and neuronal cell lines treated with HIV-1 gp41 peptides. J. Mol. Neurosci. 1998;10:129–141. doi: 10.1007/BF02737124. [DOI] [PubMed] [Google Scholar]
- Clements J.M., Cossins J.A., Wells G.M.A., Corkill D.J., Helfrich K., Wood L.M., Pigott R., Stabler G., Ward G.A., Gearing A.J.H., Miller K.M. Matrix metalloproteinase expression during experimental autoimmune encephalomyelitis and effects of a combined matrix metalloproteinase and tumor necrosis factor-α inhibitor. J. Neuroimmunol. 1997;74:85–94. doi: 10.1016/s0165-5728(96)00210-x. [DOI] [PubMed] [Google Scholar]
- Cossins J.A., Clements J.M., Ford J., Miller K.M., Pigott R., Vos W., Van Der Valk P., De Groot C.J.A. Enhanced expression of MMP-7 and MMP-9 in demyelinating multiple sclerosis lesions. Acta Neuropathol. 1997;94:590–598. doi: 10.1007/s004010050754. [DOI] [PubMed] [Google Scholar]
- Cross A.H., Misko T.P., Lin R.F., Hickey W.F., Trotter J.L., Tilton R.G. Aminoguanidine, an inhibitor of inducible nitric oxide synthase, ameliorates experimental autoimmune encephalomyelitis in SJL mice. J. Clin. Invest. 1994;93:2684–2690. doi: 10.1172/JCI117282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuzner M.L., Opdenakker G. Plasminogen activators and matrix metalloproteinases, mediators of extracellular proteolysis in inflammatory demyelination of the central nervous system. J. Neuroimmunol. 1999;94:1–14. doi: 10.1016/s0165-5728(98)00241-0. [DOI] [PubMed] [Google Scholar]
- Ewing C., Bernard C.C. Insights into the aetiology and pathogenesis of multiple sclerosis. Immunol. Cell Biol. 1998;76:47–54. doi: 10.1046/j.1440-1711.1998.00718.x. [DOI] [PubMed] [Google Scholar]
- Frei K., Eugster H.P., Bopst M., Constantinescu C.S., Lavi E., Fontana A. Tumor necrosis factor alpha and lymphotoxin alpha are not required for induction of acute experimental autoimmune encephalomyelitis. J. Exp. Med. 1997;185:2177–2182. doi: 10.1084/jem.185.12.2177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fridman R., Toth M., Pena D., Mobashery S. Activation of progelatinase B (MMP-9) by gelatinase A (MMP-2) Cancer Res. 1995;55:2548–2555. [PubMed] [Google Scholar]
- Giovannoni G., Heales S.J.R., Silver N.C., O’Riordan J., Miller R.F., Land J.M., Clark J.B., Thompson E.J. Raised serum nitrate and nitrite levels in patients with multiple sclerosis. J. Neurol. Sci. 1997;145:77–81. doi: 10.1016/s0022-510x(96)00246-8. [DOI] [PubMed] [Google Scholar]
- Giraudon P., Buart S., Bernard A., Thomasset N., Bélin M.-F. Extracellular matrix-remodeling metalloproteinases and infection of the central nervous system with retrovirus human T-lymphotropic virus type 1 (HTLV-1) Prog. Neurobiol. 1996;49:169–184. doi: 10.1016/0301-0082(96)00017-2. [DOI] [PubMed] [Google Scholar]
- Giraudon P., Buart S., Bernard A., Bélin M.-F. Cytokines secreted by glial cells infected with HTLV-1 modulate the expression of matrix metalloproteinases (MMPs) and their natural inhibitor (TIMPs): possible involvement in neurodegenerative processes. Mol. Psy. 1997;2:107–110. doi: 10.1038/sj.mp.4000218. [DOI] [PubMed] [Google Scholar]
- Glabinski A.R., Ransohoff R.M. Chemokines and chemokine receptors in CNS pathology. J. Neurovirol. 1999;5:3–12. doi: 10.3109/13550289909029740. [DOI] [PubMed] [Google Scholar]
- Goetzl E.J., Banda M.J., Leppert D. Matrix metalloproteinases in immunity. J. Immunol. 1996;156:1–4. [PubMed] [Google Scholar]
- Gottschall P.E., Yu X. Cytokines regulate gelatinase A and B (matrix metalloproteinase 2 and 9) activity in cultured rat astrocytes. J. Neurochem. 1995;64:1520–1531. doi: 10.1046/j.1471-4159.1995.64041513.x. [DOI] [PubMed] [Google Scholar]
- Green L.C., Wagner D.A., Glogowski J., Skipper P.L., Wishnok J.S., Tannenbaum S.R. Analysis of nitrate, nitrite, and [15N] nitrate in biological fluids. Anal. Biochem. 1982;126:131–138. doi: 10.1016/0003-2697(82)90118-x. [DOI] [PubMed] [Google Scholar]
- Heussen C., Dowdle E.B. Electrophoretic analysis of plasminogen activators in polyacrylamide gels containing sodium dodecyl sulfate and copolymerized substrates. Anal. Biochem. 1980;102:196–202. doi: 10.1016/0003-2697(80)90338-3. [DOI] [PubMed] [Google Scholar]
- Janabi N., Peudenier S., Héron B., Ng K.H., Tardieu M. Establishment of human microglial cell lines after transfection of primary cultures of embryonic microglia cells with the SV40 large T antigen. Neurosci. Lett. 1995;195:105–108. doi: 10.1016/0304-3940(94)11792-h. [DOI] [PubMed] [Google Scholar]
- Kennedy K.J., Strieter R.M., Kunkel S.L., Lukacs N.W., Karpus W.J. Acute and relapsing experimental autoimmune encephalomyelitis are regulated by differential expression of the CC chemokines macrophage inflammatory protein-1α and monocyte chemotactic protein-1. J. Neuroimmunol. 1998;92:98–108. doi: 10.1016/s0165-5728(98)00187-8. [DOI] [PubMed] [Google Scholar]
- Kleiner D.E., Stetler-Stevenson W.J. Structural biochemistry and activation of matrix metalloproteases. Curr. Opin. Cell Biol. 1993;5:891–897. doi: 10.1016/0955-0674(93)90040-w. [DOI] [PubMed] [Google Scholar]
- Lachance C., Arbour N., Cashman N.R., Talbot P.J. Involvement of aminopeptidase N (CD13) in infection of human neural cells by human coronavirus 229E. J. Virol. 1998;72:6511–6519. doi: 10.1128/jvi.72.8.6511-6519.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai M.M., Cavanagh D. The molecular biology of coronaviruses. Adv. Virus Res. 1997;48:1–100. doi: 10.1016/S0065-3527(08)60286-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane T.E., Buchmeier M.J. Murine coronavirus infection: a paradigm for virus-induced demyelinating disease. Trends Microbiol. 1997;5:9–14. doi: 10.1016/S0966-842X(97)81768-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane T.E., Buchmeier M.J., Watry D.D., Fox H.S. Expression of inflammatory cytokines and inducible nitric oxide synthase in brains of SIV-infected rhesus monkeys: applications to HIV-induced central nervous system disease. Mol. Med. 1996;2:27–37. [PMC free article] [PubMed] [Google Scholar]
- Lee S.C., Brosnan S.C. Cytokine regulation of iNOS expression in human glial cells. Methods. 1996;10:31–37. doi: 10.1006/meth.1996.0075. [DOI] [PubMed] [Google Scholar]
- Lee M.A., Palace J., Stabler G., Ford J., Gearing A., Miller K. Serum gelatinase B, TIMP-1 and TIMP-2 levels in multiple sclerosis, a longitudinal clinical and MRI study. Brain. 1999;122:191–197. doi: 10.1093/brain/122.2.191. [DOI] [PubMed] [Google Scholar]
- Link H. The cytokine storm in multiple sclerosis. Mult. Sclerosis. 1998;4:12–15. doi: 10.1177/135245859800400104. [DOI] [PubMed] [Google Scholar]
- Liu W., Shafit-Zagardo B., Aquino D.A., Zhao M.L., Dickson D.W., Brosnan C.F., Lee S.C. Cytoskeletal alterations in human fetal astrocytes induced by interleukin-1 beta. J. Neurochem. 1994;63:1625–1634. doi: 10.1046/j.1471-4159.1994.63051625.x. [DOI] [PubMed] [Google Scholar]
- Mitrovic B., Parkinson J., Merrill J.E. An in vitro model of oligodendrocyte destruction by nitric oxide and its relevance to multiple sclerosis. Methods. 1996;10:501–513. doi: 10.1006/meth.1996.0127. [DOI] [PubMed] [Google Scholar]
- Mounir S., Talbot P.J. Sequence analysis of the membrane protein gene of human coronavirus OC43 and evidence for O-glycosylation. J. Gen. Virol. 1992;73:2731–2736. doi: 10.1099/0022-1317-73-10-2731. [DOI] [PubMed] [Google Scholar]
- Munoz-Fernandez M.A., Fresno M. The role of tumor necrosis factor, interleukin 6, interferon-γ and inducible nitric oxide synthase in the development and pathology of the nervous system. Prog. Neurobiol. 1998;56:307–340. doi: 10.1016/s0301-0082(98)00045-8. [DOI] [PubMed] [Google Scholar]
- Murray R.S., Brown B., Brian D., Cabirac G.F. Detection of coronavirus RNA and antigen in multiple sclerosis brain. Ann. Neurol. 1992;31:525–533. doi: 10.1002/ana.410310511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogura T., Esumi H. Nitric oxide synthase expression in human neuroblastoma cell line induced by cytokines. Neuroreport. 1996;7:853–856. doi: 10.1097/00001756-199603220-00003. [DOI] [PubMed] [Google Scholar]
- Probert L., Akassoglou K., Pasparakis M., Kontogeorgos G., Kollias G. Spontaneous inflammatory demyelinating disease in transgenic mice showing central nervous system-specific expression of tumor necrosis factor alpha. Proc. Natl. Acad. Sci. USA. 1995;92:11294–11298. doi: 10.1073/pnas.92.24.11294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiling N.J., Ulmer A.J., Duchrow M., Ernst M., Flad H.D., Hauschildt S. Nitric oxide synthase: mRNA expression of different isoforms in human monocytes/macrophages. Eur. J. Immunol. 1994;24:1941–1944. doi: 10.1002/eji.1830240836. [DOI] [PubMed] [Google Scholar]
- Renno T., Krakowski M., Piccirillo C., Lin J.Y., Owens T. TNF-alpha expression by resident microglia and infiltrating leukocytes in the central nervous system of mice with experimental allergic encephalomyelitis. Regulation by Th1 cytokines. J. Immunol. 1995;154:944–953. [PubMed] [Google Scholar]
- Robbins D.S., Shirazi Y., Drysdale B.E., Lieberman A., Shin H.S., Shin M.L. Production of cytotoxic factor for oligodendrocytes by stimulated astrocytes. J. Immunol. 1987;139:2593–2597. [PubMed] [Google Scholar]
- Sarchielli P., Trequattrini A., Usai F., Murasecco D., Gallai V. Role of viruses in the etiopathogenesis of multiple sclerosis. Acta Neurol. 1993;5:363–381. [PubMed] [Google Scholar]
- Sarchielli P., Orlacchio A., Vicinanza F., Pelliccioli G., Tognoloni M., Saccardi C., Gallai V. Cytokine secretion and nitric oxide production by mononuclear cells of patients with multiple sclerosis. J. Neuroimmunol. 1997;80:76–86. doi: 10.1016/s0165-5728(97)00136-7. [DOI] [PubMed] [Google Scholar]
- Sriram S., Rodriguez M. Indictment of the microglia as the villain in multiple sclerosis. Neurol. 1997;48:464–470. doi: 10.1212/wnl.48.2.464. [DOI] [PubMed] [Google Scholar]
- Stewart J.N., Mounir S., Talbot P.J. Human coronavirus gene expression in the brains of multiple sclerosis patients. Virology. 1992;191:502–505. doi: 10.1016/0042-6822(92)90220-J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuve O., Dooley N.P., Uhm J.H., Antel J.P., Francis G.S., Williams G., Yong V.W. Interferon beta-1b decreases the migration of T lymphocytes in vitro: effects on matrix metalloproteinase-9. Ann. Neurol. 1996;40:853–863. doi: 10.1002/ana.410400607. [DOI] [PubMed] [Google Scholar]


