Abstract
In three successive experiments, the immune functions of pigs persistently infected with the porcine reproductive and respiratory syndrome virus (PRRSV) have been evaluated. Non-specific immune responses were analyzed over a period of 12 weeks post-infection (PI). In addition, the capacity of PRRSV-infected pigs to develop an efficient immune response against pseudorabies virus (PRV) glycoproteins and to resist to a subsequent virulent challenge was investigated. Our results demonstrate that PRRSV produced minor effects on the immune system of pigs. The skin delayed type hypersensitivity (DTH) in response to phytohemagglutinine injection was slightly diminished one week after challenge, but was restored thereafter. However, three weeks after the infection, the total white blood cell count, and the number of CD2+, CD8+ and IgM+ cells were enhanced. The increase in numbers of CD8+ cells persisted for three consecutive weeks. Serum immunoglobulins in infected pigs also increased by week 3 PI and up to 8 weeks PI. These results show that PRRSV may have stimulating effects on the pig immune system during the phase of long-lasting infection. After immunization with PRV glycoproteins, the production of anti-PRV antibodies and skin DTH response against PRV glycoproteins were not affected. On the contrary, following a virulent PRV challenge, PRRSV-infected pigs developed a better secondary antibody response and their resistance to the infection was as effective as in control pigs. Taken together, our data do not support a systemic immunosuppressive effect of PRRSV, during the persistent phase of infection. Other mechanisms may therefore apply to explain the emergence of secondary infections in endemically infected herds.
Keywords: Porcine reproductive and respiratory syndrome, Antibody, Pig, Lymphocytes, Delayed type hypersensitivity
Abbreviations: DMEM, Dubelcco's modified Eagle's medium; DTH, delayed type hypersensitivity; EDTA, ethylene diamine tetra-acetic; ELISA, enzyme-linked immunosorbent assay; FCS, foetal calf serum; FITC, fluorescein isothiocyanate; IPMA, immunoperoxydase monolayer assay; LDH, lactate dehydrogenase; MAbs, monoclonal antibodies; PBMC, peripheral blood mononuclear cells; PBS, phosphate buffer saline; PHA, phytohemagglutinine; PI, post-infection; PRRSV, porcine reproductive and respiratory syndrome; PRV, pseudorabies (Aujesky's disease) virus; RNA, ribonucleotide acid; RPMI, Rosewell Park Memorial Institute; SPF, specific pathogen free; LDV, lactate dehydrogenase-elevating virus; CTLs, cytotoxic T lymphocytes; TCID, tissue culture infective doses; IU, international unit; WBC, white blood cells
1. Introduction
The porcine reproductive and respiratory syndrome (PRRS) is a new disease of swine caused by a small enveloped-RNA-virus recently characterized as an arterivirus (Conzelmann et al., 1993; Meulenberg et al., 1994). The syndrome mainly produces stillbirth, piglet mortality and respiratory disorders on all categories of pigs. After a severe pandemic phase, the disease has become endemic in many pig-producing countries, with a majority of herds being persistently infected for several years (Stevenson et al., 1993). The persistence of the virus within pigs and herds (Zimmerman et al., 1992; Albina et al., 1994) is often associated with the recurrence of secondary infections (Blaha, 1992; Stevenson et al., 1993). Therefore, PRRS virus has been suspected to have immunosuppressive effects on pigs.
Many researchers have investigated under experimental conditions the possible effects of PRRSV on the disease resistance of pigs. Their results were often contradictory. Some authors evidenced a negative effect of PRRSV when pigs were super-infected with Streptococcus suis (Galina et al., 1994), Mycoplasma hyorhinis (Shimizu et al., 1994; Kawashima et al., 1996), porcine respiratory coronavirus or influenza virus (Van Reeth et al., 1994, Van Reeth et al., 1996a). Others did not show any interaction between PRRSV and Pasteurella multocida (Cooper et al., 1995), Mycoplasma hyopneumoniae (Albina et al., 1995; Van Alstine et al., 1996), Haemophilus parasuis, S. suis or Salmonella cholerasuis (Cooper et al., 1995). Recently, Van Reeth et al. (1996a)reported that PRRSV effects on pig resistance to influenza virus challenge were not reproducible from one experiment to another. Several reasons, including differences in individual pig susceptibility, could explain such a variation. However, the time interval existing between the challenges seems to play a major role. Van Reeth et al. (1996b)recently confirmed that the clinical incidence of a dual infection with PRRSV and influenza virus were more pronounced when the challenges were distant from 7 days instead of 3. Furthermore, when challenges were 14 days apart, the clinical outcome was not worse than with single virus infection.
In summary, PRRSV may have detrimental effects on the disease resistance of pigs, during the acute phase of infection. Presumably, these effects are restricted to the lung, the initial site of virus replication. All the studies published so far demonstrate that local and systemic immunity of pigs is restored within 2 to 3 weeks after PRRSV infection. However, the virus persists for longer periods and to our knowledge, nothing has been yet documented on the possible effects of PRRSV on the immune system of pigs during this persistent infection.
The objective of this study was to investigate the consequences of a persistent infection on the immune responses of growing pigs. To answer the objective, we included in our protocol the measurement of non-specific immune functions as well as antigen-driven responses. The latter were carried out after an intramuscular injection of PRV glycoproteins. The efficacy of anti-PRV immune responses was secondarily tested by a virulent PRV challenge.
2. Materials and methods
2.1. Animals and experimental protocols
Three experiments were carried out with a total of 66 8-week old SPF or conventional pigs. The first experiment was designed to evaluate PRRSV effects on anti-PRV antibody production after immunization with PRV glycoproteins, and on subsequent disease resistance after a virulent PRV challenge. The second experiment was a reproduction of the first trial, but including pigs of different health status (Table 1 ). The last experiment was finally carried out to investigate the effects of PRRSV on lymphocyte subsets and on delayed type hypersensitivity. Pigs were allocated to 8 experimental groups and submitted to different treatments as described in Table 1. Each group was housed in separated isolation facility. PRRSV challenge consisted in the administration of 3 ml in each nostril of strain SDRPI5D titrating 100 tissue culture infective doses (TCID) 50/ml, followed one week later by intravenous injection of 5 ml of the same strain titrating 104 TCID 50/ml. This strain was an eighth passage of a Spanish isolate recovered from a sick piglet (generous gift from Dr. Plana Duran, Sobrino Laboratories, Cyanamid). It has proved before to be highly pathogenic for pregnant sows and young piglets (Plana Duran et al., 1992). Two weeks after initial PRRSV infection, the pigs were immunized intramuscularly with 2 ml of a commercial anti-PRV vaccine. This vaccine consisted of purified PRV glycoproteins in water/oil adjuvant. At 17 weeks old, the pigs were challenged intranasally with PRV strain AUJII3K titrating 106.1 TCID 50/ml (3 ml in each nostril). The inoculum was a clarified culture supernatant of primary pig kidney cells infected with a second passage of the strain 75V19 isolated by Andries et al. (1978). During the whole experimental period, the pigs were observed daily and any clinical manifestations were noted. Rectal temperatures and pig weights were respectively recorded daily and weekly. Blood samples were collected weekly. Nasal swabs were collected after PRV challenge as indicated in the results. At the end of the experiments, the pigs were anaesthetized intravenously with 20 mg/kg of thiopental sodium (Nesdonal, Rhône Mérieux, France), then bleeded. At necropsy, gross lesions were eventually recorded.
Table 1.
Constitution of the experimental groups and description of the protocols
| Experimental group (number of pigs) | Health status of pigs | Age at PRRSV challenge | Age at PRV immunization | Age at PRV challenge | Age at necropsy |
|---|---|---|---|---|---|
| Experiment 1 | |||||
| Group 1 (control for PRV, n=9) | SPF | − | − | 17 weeks | 20 weeks |
| Group 2 (control for PRRSV, n=8) | SPF | − | 10 weeks | 17 weeks | 20 weeks |
| Group 3 (PRRSV-infected, n=9) | SPF | 8 weeks | 10 weeks | 17 weeks | 20 weeks |
| Experiment 2 | |||||
| Group 4 (control for PRV, n=8) | Conventional | − | − | 17 weeks | 20 weeks |
| Group 5 (control for PRRSV, n=8) | Conventional | − | 10 weeks | 17 weeks | 20 weeks |
| Group 6 (PRRSV-infected, n=8) | Conventional | 8 weeks | 10 weeks | 17 weeks | 20 weeks |
| Experiment 3 | |||||
| Group 7 (control for PRRSV, n=8) | SPF | − | 10 weeks | − | 15 weeks |
| Group 8 (PRRSV-infected, n=8) | SPF | 8 weeks | 10 weeks | − | 15 weeks |
Three successive experiments with 66 pigs were carried out. In each experiment, one group of pigs was challenged with PRRSV at 8 weeks of age. Two weeks later, these groups and one additional group in each experiment (the `control for PRRSV' group) were immunized with PRV glycoproteins. At 17 weeks of age, all pigs were challenged with virulent PRV. Groups 1 and 4 (the `control for PRV' groups) consisted of non-PRRSV-infected and non-PRV-immunized pigs and were included to control the severity of PRV challenge.
2.2. Virus titrations
PRRSV in the sera of infected pigs was titrated according to the method of Kaerber (1931). Briefly, ten-fold serial dilutions of the sera were inoculated onto alveolar macrophages. Cytopathic effects were observed daily up to 7 days post-infection (PI). The 50% endpoint dilution was determined and titre was expressed as TCID 50/ml. PRV in nasal swabs was titrated as described by Vannier et al. (1991). Briefly, nasal mucus samples were weighed and re-suspended in 2 ml of culture medium. Suspensions were then titrated as described for PRRSV, but titres were finally adjusted to the initial mucus weight and expressed as TCID 50/100 mg mucus.
2.3. Antibody titrations
In order to estimate PRRSV effects on the ability of infected pigs to produce antibodies, serum immunoglobulins as well as anti-PRRSV and anti-PRV antibodies were titrated. An antibody-capture-ELISA was developed for the titration of serum immunoglobulins. Briefly, polystyrene 96-well flat-bottom microtitre ELISA plates (Nunc maxisorb) were coated overnight at 4°C with a rabbit anti-swine immunoglobulins G (heavy and light chains) (Biosys, France) appropriately diluted in carbonate buffer (pH=9.6). The plates were washed five times with phosphate buffer saline (PBS) containing 0.1% Tween 20 (Merck, France). Two-fold serial dilutions of test sera were applied for 1 h at 37°C. Serial dilutions from 6600 to 1.61 ng/ml of a swine IgG purified in the laboratory by salt precipitation, gel filtration and ion-exchange chromatography, were included as a standard in each microtitre plate. After incubation, the plates were washed five times with PBS–Tween, then a rabbit anti-swine immunoglobulins (light and gamma chains) (Dakopath, France) was added for 30 min at 37°C. After five washings with PBS–Tween, the substrate orthophenylene–diamine (Sigma, France) was added into each well and the coloration allowed to develop for 15 min at room temperature. Absorbance was read at 490 nm using an ELISA plate reader (MR 7000, Dynatech, France). The concentration of serum immunoglobulins was calculated from the alignment of sample and standard titration curves. Results from one assay to another were standardized by the application of a corrective coefficient deduced from the alignment of the purified IgG titres. Anti-PRRSV antibodies were titrated by the immunoperoxydase monolayer assay (IPMA) developed by Wensvoort et al. (1991), secondarily adapted in our laboratory (Baron et al., 1992). PRRSV-neutralizing antibodies were titrated with or without the addition of 20% fresh swine serum, according to the method described by Yoon et al. (1994). Anti-PRV antibodies were titrated using an immunoperoxydase technic derived from the IPMA. PRV-neutralizing antibodies were titrated according to the method described by Vannier et al. (1991).
2.4. Delayed type hypersensitivity (DTH)
Skin delayed type hypersensitivity tests were carried out to assess the ability of lymphocytes to proliferate in vivo. The DTH responses were measured against phytohemagglutinine 0.033 mg/ml (PHA, HA16 Welcome) and purified PRV glycoproteins 0.2 mg/ml (generous gift from Dr. Brun, Rhone Merieux Laboratories). One hundred microlitres of each stimulating solution were injected intradermally in two adjacent points of the ear skin. Buffer without mitogen nor antigen was included in duplicate tests to provide corresponding controls. Prior to the injections, the skin was thoroughly disinfected with ethanol 60%. Injections were performed at different times PI as indicated in the results. Twenty-four hours after the injections, skin indurations were measured using a thickness gauge calliper. The average thickness of two corresponding indurations was considered as the final result.
2.5. Total and differential white blood cell counts
Total and differential white blood cell counts were carried out to determine if PRRSV effects on DTH could originate from changes in cell sub-populations. Blood samples collected on EDTA were used for total white blood cell (WBC) counts. WBC were counted using an automated cell counter (ABX Minosvet, France). Polynuclear and mononuclear differential cell counts were performed on blood smears stained with May–Grunwald–Giemsa dyes. Heparinized blood samples were used for all subsequent studies on mononuclear cell sub-populations. Heparinized blood was diluted to 1/3 in sterile PBS (pH=7.2) and slowly layered onto a Ficoll–Hypaque cushion (Pharmacia Biotech, Sweden). After centrifugation at 1500g for 15 min at 18°C, PBMC were collected at the interface and washed twice in sterile PBS. Cells were then enumerated on Malassez cell. Viability of cells as estimated by trypan blue dye exclusion was always higher than 95%.
The distribution of lymphocyte sub-populations was determined by flow cytometry. PBMC were adjusted to a final concentration of 2×105 cells in 0.1 ml of sterile PBS (pH=7.2) with 2% of foetal calf serum (FCS). They were incubated at 4°C for 30 min with fifty microlitres of either mouse monoclonal antibodies against swine CD2 (MSA4, Hammerberg and Schurig, 1986), CD4 (74-12-4, Pescovitz et al., 1984), CD8 (11-295-33, provided by Saalmuller, Federal Research Centre for Virus Disease of Animals, Tubingen, Germany) or IgM (Van Zaane and Hulst, 1987). The cells were washed three times in PBS–2% FCS. Fifty microlitres of appropriate dilution in PBS–2% FCS of fluorescein isothiocyanate (FITC)-conjugated goat anti-mouse immunoglobulin G (Dakopath, France) and 20 mg/ml of propidium iodide (Sigma, France) were added for 30 min at 4°C. The cells were washed two times with PBS–2% FCS, one time with PBS–1% paraformaldehyde and finally re-suspended in PBS–1% paraformaldehyde. The cells were analyzed with a Facsort flow cytometer (Becton Dickinson). Isotypic unrelevant mouse monoclonal antibodies (IgG1, IgG2a and IgG2b MAbs) were included in the first run of cell analysis to check the specificity of cell labelling with anti-CD MAbs. Dead cells were excluded by red fluorescence resulting from propidium iodide incorporation.
2.6. Statistical analysis
Comparisons of results between experimental groups were performed using the non-parametric Kruskal–Wallis test from Systat Software (SPSS, USA).
3. Results
3.1. Persistence of PRRSV in infected pigs
As illustrated in Fig. 1 , a majority of infected pigs remained viraemic for 3 consecutive weeks after challenge. On the fourth and fifth weeks, the percentage of viraemic pigs progressively declined. The averaged virus titres in groups 6 and 8 were not statistically different (Fig. 1). Clinically, PRRSV challenge resulted in a recurrent hyperthermia of moderate intensity (≤41°C). In some pigs, hyperthermic waves lasting for 1 to 3 consecutive days, occurred over a period of 15 to 25 days PI. However, more than 50% of the infected pigs never exhibited an increase in rectal temperature. This result explained why in PRRSV-infected pigs, significant increases of rectal temperatures were irregular (i.e. on days 4, 5, 7, 9, 14, 15, 16, 17, 21, 22 and 24 PI). A significant growth retardation in PRRSV-infected pigs was also observed on weeks 1, 2 or 3 PI (data not shown).
Fig. 1.

Detection of PRRSV in the sera of infected pigs at different weeks post-infection. Histogram bars represent the percentage of viraemic pigs and vertical numbers give the corresponding mean PRRSV titres for groups 6 and 8. Titres are expressed in TCID 50 ml±standard deviations. Viraemia persisted up to 5 or 6 weeks post-infection. After PRV infection, PRRSV viraemia was not re-activated.
3.2. Effect of PRRSV on antibody production
After PRRSV challenge, the total amount of serum immunoglobulins in PRRSV-infected pigs was enhanced by 3 weeks PI and remained higher than in uninfected pigs for the 5 following weeks (Fig. 2 ). Nonetheless, the observed differences were only significant statistically on weeks 4, 5 and 8. Specific antibodies to PRRSV were detectable with the IPMA test by two weeks after infection and peaked at about 5 weeks PI (Fig. 3 ). Neutralizing antibodies were not detected before 4 weeks PI and maximum log titre (1.49) was only achieved on week 12 PI. However, the complement-associated neutralizing antibodies were detectable by 2 weeks PI and the titre reached 2.77 on week 10 (Fig. 3). Between PRRSV-infected pigs and uninfected pigs, the specific antibody responses after PRV immunization were indistinguishable (see illustration for PRV neutralizing antibodies in Fig. 4 ; similar results obtained with anti-PRV antibodies titrated by IPMA are not shown). However, after PRV infection, PRRSV-infected pigs showed a better secondary antibody response (Fig. 4).
Fig. 2.

Immunoglobulin concentration in the sera of pigs from experiment 2. Results are presented in average concentrations in mg/ml (histogram bars) and standard deviations (upper lines). Different letters indicate significative differences between groups (p<0.05). Serum immunoglobulins were titrated at different weeks after PRRSV challenge in group 6 (group 5=control group). Pigs from groups 5 and 6 were immunized with PRV glycoproteins at week 2. By 3 weeks post-infection, immunoglobulin concentration became higher in group 6 compared to group 5. All pigs included in this experiment were challenged with PRV at week 9 (group 4=control of PRV challenge). In all groups, an increase in serum immunoglobulin concentration was observed after PRV challenge. This increase was even higher in the pigs of group 4 which were not pre-immunized against PRV.
Fig. 3.

Titration of anti-PRRSV antibodies by the immunoperoxydase assay (IPMA) or the seroneutralization test in absence (SNT) or in presence (SNT+COMP) of serum complement. Results are presented in average log titres (histogram bars) and standard deviations (upper lines). All pigs were challenged at week 0, then, 2 weeks later, immunized with PRV glycoproteins. At week 9, they were challenged with PRV. At weeks 0 and 1 after PRRSV infection, antibodies were not detectable by IPMA (the detection threshold for this test was 3.22 log titre). Thereafter, the log titre increased to a maximum of 9. In contrast, neutralizing antibodies were detectable to low titres by 2 or 3 weeks post-infection.
Fig. 4.

Titration of PRV-neutralizing antibodies. Results are presented in average log titres (histogram bars) and standard deviations (upper lines). Different letters indicate significative differences between groups (p<0.05). Pigs from groups 3 and 6, were infected with PRRSV at week 0 (groups 2 and 5=control groups). All pigs were immunized with PRV glycoproteins at week 2, leading to a primary antibody response in conventional pigs (groups 5 and 6). In contrast, antibodies were not detectable in SPF pigs (groups 2 and 3). After PRV challenge (at week 9), a clear secondary antibody response arose in groups 5 and 6 whereas antibodies became detectable in the other groups. Differences in neutralizing antibody titres between PRRSV-infected and control pigs were observed at week 10 and 11.
3.3. Effect of PRRSV on cell sub-populations
Blood cell distributions over the whole experimental period are presented on Table 2 Table 3 . In experiment 2, PRRSV did not affect the total WBC count nor the number of polynuclear and mononuclear cells. In experiment 3, infected pigs showed a higher total WBC count and increased numbers of CD2+, CD8+ and IgM+ cells, three weeks after challenge. Significant differences extended to week 4 for CD2+ cells and to weeks 4 and 5 for CD8+ cells. Consequently, the CD4/CD8 ratio on weeks 3, 4 and 5 PI, was significantly enhanced in group 8 compared to group 7. The increase of total CD8+ and IgM+ cell counts was not simply due to the overall increase of WBC count, since relative percentages of these cell subsets among PBMC were also significantly increased (data not shown). Surprisingly, on weeks 0 and 1 PI, the numbers of polynuclear cells in group 7 surpassed those in group 8. At this time, differences in daily weight gains were also higher in group 7 compared to group 8 (data not shown). These differences were assumed to originate from the elimination of two pigs from group 8, shortly before PRRSV challenge. This modification in the group constitution could have been responsible for social stress and change in feeding behaviour leading to transient physiological modifications.
Table 2.
Distribution of white blood cell sub-populations over the 12 week-experimental period
| Weeks post-infection PRRSV | 0 | 1 | 2 | 3 | 4 | 5 | 8 | 10 | 11 | 12 |
|---|---|---|---|---|---|---|---|---|---|---|
| Total WBC count (×103 cells/mm3 blood) | ||||||||||
| Group 4 | − | − | − | − | − | − | 19.8±4.2 | 21.5±2.3 | 20.3±5.5 | |
| Group 5 | 19.0±3.9 | 18.6±5.2 | 20.6±4.7 | 18.6±1.7 | 18.0±2.3 | 18.5±3.5 | 19.0±2.4 | 20.4±4.0 | 22.7±5.4 | 18.2±2.4 |
| Group 6 | 20.0±2.6 | 17.0±3.1 | 19.8±4.0 | 18.8±1.8 | 19.5±1.8 | 18.0±1.8 | 17.6±2.4 | 22.6±3.9 | 23.4±4.9 | 18.1±3.7 |
| Group 7 | 11.0±1.6 | 11.4±3.0 | 15.1±3.3 | 13.2±2.3 a | 14.5±2.6 | 15.0±2.1 | − | − | − | − |
| Group 8 | 12.7±2.2 | 11.5±0.6 | 12.7±3.8 | 16.1±1.8 b | 16.6±2.3 | 14.8±2.8 | − | − | − | − |
| Polynuclear cells (×103 cells/mm3 blood) | ||||||||||
| Group 4 | − | − | − | − | − | − | 7.0±3.1 | 13.0±3.8 a | 11.7±2.4 a | 10.1±5.8 |
| Group 5 | 8.4±3.4 | 7.1±2.5 | 7.7±4.7 | 5.5±1.8 | 5.4±1.5 | 6.2±1.8 | 6.1±1.0 | 5.8±1.6 b | 9.3±2.6 b | 6.2±1.7 |
| Group 6 | 7.6±1.8 | 7.9±2.5 | 8.2±2.2 | 7.1±1.9 | 6.1±2.2 | 5.9±1.5 | 5.9±1.0 | 9.1±3.2 b | 12.0±5.8 a | 6.3±2.9 |
| Group 7 | 3.1±0.6 a | 3.5±1.1 a | 4.4±1.9 | 3.8±1.4 | 4.3±1.5 | 4.7±1.8 | − | − | − | − |
| Group 8 | 5.3±0.9 b | 4.6±0.9 b | 4.3±1.5 | 5.0±1.7 | 4.7±1.3 | 5.3±2.8 | − | − | − | − |
| Mononuclear cells (×103 cells/mm3 blood) | ||||||||||
| Group 4 | − | − | − | − | − | − | 12.8±2.5 | 8.5±2.7 a | 10.8±1.6 | 10.2±1.1 |
| Group 5 | 10.6±3.5 | 11.6±3.3 | 12.9±4.6 | 13.1±2.2 | 12.6±3.1 | 12.3±2.3 | 15.1±6.2 | 14.5±4.0 b | 13.4±4.6 | 12.1±2.7 |
| Group 6 | 12.4±2.8 | 9.1±1.9 | 11.5±4.1 | 11.6±1.8 | 13.4±2.1 | 12.1±2.1 | 11.7±1.8 | 13.6±2.7 b | 11.4±2.0 | 11.8±1.8 |
| Group 7 | 8.0±1.2 | 7.9±2.7 | 10.7±1.9 | 9.4±1.3 | 10.1±2.2 | 10.2±1.8 | − | − | − | |
| Group 8 | 7.3±2.2 | 6.9±0.6 | 8.4±2.9 | 11.1±1.9 | 11.9±2.6 | 9.5±1.6 | − | − | − | − |
Groups 6 and 8 consisted of pigs infected with PRRSV at week 0. Groups 5 and 7 consisted of control pigs for PRRSV infection. Pigs from groups 5 to 8 were immunized with PRV glycoproteins at week 2. Group 4 consisted of control pigs for PRV glycoprotein immunization. Pigs from groups 7 and 8 were sacrificed at week 7 whereas the three other groups were challenged with PRV at week 9, then sacrificed 3 weeks later. Results are expressed as average cell numbers±standard deviations. Different letters between groups indicate significant differences (p<0.05).
Table 3.
Distribution of lymphocyte sub-populations over the 5 week-experimental period for groups 7 and 8
| Weeks post-infection PRRSV | 0 | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|---|
| CD2+ cells (×103 cells/mm3 blood) | ||||||
| Group 7 (control) | 5.0±0.7 | 4.5±1.5 | 6.6±1.3 | 4.9±0.7 a | 5.3±1.2 a | 5.7±1.1 |
| Group 8 (PRRSV) | 4.8±1.6 | 4.1±0.7 | 5.3±2.4 | 7.1±1.5 b | 7.9±2.5 b | 6.4±1.5 |
| CD4+ cells (×103 cells/mm3 blood) | ||||||
| Group 7 (control) | 3.9±1.1 | 2.7±1.3 | 3.2±1.0 | 2.5±0.4 | 2.6±0.6 | 3.2±0.8 |
| Group 8 (PRRSV) | 3.5±1.2 | 2.4±0.8 | 2.6±1.2 | 2.7±1.0 | 2.8±0.8 | 2.8±0.7 |
| CD8+ cells (×103 cells/mm3 blood) | ||||||
| Group 7 (control) | 1.6±0.3 | 1.9±0.7 | 3.2±0.6 | 2.4±0.5 a | 2.7±0.5 a | 2.6±0.5 a |
| Group 8 (PRRSV) | 1.7±0.7 | 2.0±0.4 | 2.9±1.4 | 4.7±1.4 b | 5.1±2.3 b | 3.8±0.9 b |
| CD4/CD8 ratio | ||||||
| Group 7 (control) | 2.5±0.8 | 1.5±0.7 | 0.9±0.4 | 1.1±0.2 a | 0.9±0.2 a | 1.2±0.3 a |
| Group 8 (PRRSV) | 2.2±0.7 | 1.1±0.6 | 0.9±0.2 | 0.6±0.3 b | 0.6±0.2 b | 0.8±0.2 b |
| IgM+ cells (×103 cells/mm3 blood) | ||||||
| Group 7 (control) | − | 1.1±0.3 a | 0.9±0.1 a | 0.6±0.1 a | 0.7±0.1 | 0.8±0.2 |
| Group 8 (PRRSV) | − | 0.6±0.1 b | 0.7±0.5 b | 3.1±1.6 b | 1.5±1.4 | 0.9±0.3 |
Pigs from group 8 were challenged with PRRSV at week 0. All pigs from groups 7 and 8 were immunized with PRV glycoproteins at week 2. Results are expressed as average cell numbers±standard deviations. Different letters indicate significant differences (p<0.05).
Following PRV infection, striking modifications on cell distribution were observed (Table 2, Table 3). For all the infected pigs, total WBC counts increased by 14% and 22% on average, at one and two weeks post-challenge, respectively. This increase resulted from the increased number of polynuclear cells. In group 4, the polynuclear cell population was more important than in the other two groups on week 10. On the other hand, mononuclear cells in this group was significantly diminished on week 10 (Table 2). Excepted on week 11 and for polynuclear cells, no differences were seen in blood cell sub-populations between PRRSV-infected pigs and uninfected pigs. Three weeks after PRV challenge, all cell counts returned to pre-infection levels, excepted for polynuclear cells in group 4 which were still higher.
3.4. Effect of PRRSV on delayed type hypersensitivity
One week after PRRSV challenge, the DTH responses of infected pigs injected with PHA, were lower than in uninfected pigs (Table 4 ). However, the differences were not significant on weeks 3 and 5 PI. In addition, PRRSV-infected pigs and uninfected pigs showed the same DTH responses after intradermal injections of PRV glycoproteins. Interestingly, our results demonstrate that the anti-PRV DTH reactions developed shortly after priming (i.e. one week).
Table 4.
Results of skin delayed type hypersensitivity tests
| Weeks post-infection | PRRSV | Experiment 2 |
Experiment 3 |
||
|---|---|---|---|---|---|
| Group 5 (control) | Group 6 (PRRSV) | Group 7 (control) | Group 8 (PRRSV) | ||
| −1 | PHA | − | − | 2.03±0.77 | 2.13±0.46 |
| 1 | PHA | 2.28±0.49 a | 1.36±0.38 b | 2.25±0.61 a | 1.22±0.65 b |
| PRV | − | − | 0.11±0.63 | −0.14±0.83 | |
| 3 | PHA | 2.96±0.99 | 2.09±0.73 | 1.75±0.6 | 1.59±0.46 |
| PRV | − | − | 2.72±1.3 | 2.89±1.52 | |
| 5 | PHA | 1.88±0.44 | 2.44±0.74 | 1.56±0.42 | 1.38±0.52 |
| PRV | − | − | 1.88±0.94 | 1.53±0.81 | |
Pigs were injected intradermally with 100 μl of either phytohemagglutinine (PHA, 0.033 mg/ml) or pseudorabies virus glycoproteins (PRV, 0.2 mg/ml). Skin reactions were measured 24 h later. Results are expressed in mm. Different letters indicate significant differences (p<0.05).
3.5. Protective value of immunity induced in PRRSV-infected pigs after the injection of PRV glycoproteins
The capacity of the immune system of pigs to mount a protective response after a single injection of PRV glycoproteins was evaluated in PRRSV-infected pigs and in uninfected pigs by a virulent PRV challenge. During the first week after PRV challenge, PRRSV-infected pigs have lost between 0.5 and 0.7 kg/day. Their weight losses were however not significantly different from those of pigs uninfected with PRRSV. In contrast, pigs unprimed with PRV glycoproteins lost more than 1.1 kg/day. These differences between the experimental groups were also seen with rectal temperatures (data not shown). Serum antibody concentrations in groups 5 and 6 increased after PRV challenge but no differences were detected between the two groups (Fig. 2). However, serum immunoglobulins in group 4 dramatically increased 2 and 3 weeks after PRV challenge (Fig. 2). PRRSV effects on anti-PRV antibody response after PRV challenge have been described previously (see Fig. 4). In PRV-primed pigs, the degree and duration of PRV excretion after challenge were reduced (Fig. 5 ). PRV excretion in PRRSV-infected pigs was of same duration than in uninfected pigs. However, PRV titres tended to be higher, 3 and 4 days after PRV challenge.
Fig. 5.
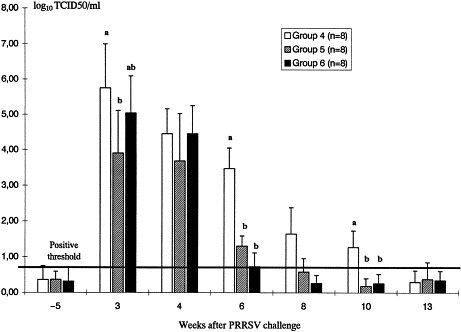
PRV excretion in nasal swabs. Results are presented in average log TCID 50/mg mucus (histogram bars) and standard deviations (upper lines). Different letters indicate significative differences between groups (p<0.05). In group 4, PRV excretion extended for 10 days after infection: the pigs were not immunized with PRV glycoproteins before the challenge. In contrast, PRV excretion lasted for 6 days in pigs from groups 5 and 6, which were immunized with PRV glycoproteins 7 weeks before the challenge. No differences in the level and duration of PRV excretion were seen between PRRSV-infected and control pigs.
4. Discussion
PRRSV infection of pigs under experimental conditions often leads to a long-lasting viraemia. We found in previous experiments that a double challenge one week apart with low doses of PRRSV generated a long-lasting and reproducible viraemia in infected pigs (data not shown). Our results effectively demonstrate that a long-lasting infection is consistently achieved, the infected pigs remaining viraemic for 5 to 6 consecutive weeks. As initially foreseen, all the PRRSV-infected pigs were viraemic when PRV immunizations were carried out, i.e. 14 days after the initial infection. At this time, it was assumed that the acute effects of PRRSV on the immune system of pigs had disappeared (see below). However, the virus was still present in the blood circulation, with possibly, additional incidence on the immune functions. The establishment of a persistent infection could result from the inability of the anti-viral defences to control the virus replication. In the present report, we confirmed that PRRSV is a poor and late inducer of neutralizing antibodies (see also Yoon et al., 1994). Recently, Yoon et al. (1996)established that virus neutralization in vivo requires a minimal titre of serum neutralizing antibodies. The log titre that efficiently neutralized the virus in vivo was estimated to be 2.8. Such a titre was achieved by a passive transfer of concentrated PRRSV-specific IgG (Yoon et al., 1996). Since the neutralizing antibody titre in the sera of convalescent pigs is generally lower (maximum log titre was 1.49 in our study), it can be assumed that neutralization in vivo is not an effective immune response for controlling the virus infection. In contrast, low amounts of neutralizing antibodies have been shown to significantly enhance the duration and the level of viraemia in pigs or the infection of alveolar macrophages in vitro (Yoon et al., 1996).
In the present study, we have observed an increase of CD2+ and CD8+ cells at weeks 3 and 4 after PRRSV infection. Levels of CD8+ cells were still higher in PRRSV-infected pigs than in uninfected pigs, at week 5 PI. The increase of CD2+ cells is likely to be due to the increase in CD8+ cells, since the latter express both markers. Our results are fully in agreement with those of Shimizu et al. (1996)who also demonstrated an increase of CD8+ cells on days 28 and 35 PI. The origin of this change is unclear. It is apparently not a direct effect of the virus on the CD8+ cells, since PBMC do not proliferate in vitro when they are inoculated with PRRSV (Shimizu et al., 1996). This result suggests that intermediate immune effectors are necessary to induce CD8 cell proliferation. These effectors which remain to be identified, could consist of helper cells or immuno-stimulating cytokines induced by the virus. The biological significance of this change in CD8+ cell numbers infected pigs is not well understood. Interestingly, viraemia starts to decline shortly after the proliferation of these CD8+ cells. The possible connection between these two events suggest that CD8+ cells could play a role in the control of virus replication. CD8+ cells are precursors of cytotoxic T lymphocytes (CTLs). CTLs are efficient immune effectors, with an important role in the elimination of virus-infected cells. Whether the proliferation of these CD8+ cells in PRRSV-infected pigs corresponds to the generation of a high number of PRRSV-specific CTLs or results from a non-specific polyclonal activation, remains to be elucidated. Recently, Bautista et al. (1996)reported that the cell immune responses against PRRSV were not detectable before 4 weeks PI and reached their maximum on week 7. These cell responses involved mainly CD4+ but also CD8+ cells. They also arose at a period of time PI which was consistent with the expected time of virus clearance. This observation would support our first hypothesis concerning the importance of these immune cells in the control of virus replication. The fact that efficient immune responses against the virus developed lately after the initial infection, also opens an interesting field of investigations on virus evasion strategies. In this area, a direct inhibitory effect of the virus on the normal anti-viral immune responses should be first considered.
PRRSV induced only minor negative effects on the non-specific immune responses of pigs. The in vivo lymphocyte response to PHA slightly decreased one week after infection. The origin of this change is not clear since the number of blood lymphocytes was not significantly decreased. This would suggest that PRRSV induced a low and transient suppression on lymphocyte functions in vivo. Since no definite proof of PRRSV replication within lymphocytes has been provided so far, we can also hypothesize that the infection may indirectly affect the lymphocyte functions by the generation of cytokines with potential suppressive effects. In other experiments, PRRSV has been shown to induce, 3 and 7 days after infection, a significant reduction of the number of blood lymphocytes and of their proliferative responses in vitro (Christianson et al., 1993; Vézina et al., 1996). Taken together, these results suggest that PRRSV may have a negative effect on the pig immune system, during the acute phase of infection. However, the real significance of this effect on the immune competence and disease resistance of pigs is yet to be determined.
Three weeks after PRRSV challenge, total WBC and IgM+ cell counts in infected pigs were significantly increased. If the increase of WBC can be merely explained by the increase of CD2+/CD8+ cells (see above), however, the reason for the change in IgM+ cells is not presently understood. Another surprising finding was the increase in serum immunoglobulin concentration from 3 to 8 weeks PI. Whether these modifications rely on a polyclonal activation of B cells followed by a differentiation in antibody-secreting cells and then an increased production of antibodies, has to be confirmed. In their study, Vézina et al. (1996) observed a spontaneous in vitro proliferation of blood lymphocytes collected from PRRSV-infected pigs. This polyclonal activation in the absence of mitogens occurred with blood samples collected at 7 and 14 days after initial infection, suggesting that a viral protein may act as a mitogen or a superantigen on some lymphocyte sub-populations. In this study, the lineage of the proliferating cells was not determined.
After PRV challenge, a secondary anti-PRV antibody response arose in all vaccinated pigs, the response being higher in pigs previously infected by PRRSV. Since PRRSV was not detected in the pig sera when the secondary anti-PRV response arose, we assume that PRRS acted, in some way, in up-regulating the degree of the primary response to PRV glycoproteins, leading subsequently to an enhanced secondary response. Although the anti-PRV antibody response and delayed type hypersensitivity were obviously not increased after PRV immunization, the concentration of serum immunoglobulins and the amount of WBC, CD2+/CD8+ and IgM+ cells were however enhanced in PRRSV-infected pigs. Therefore, it can be anticipated that PRRSV has stimulated at least one component of the immune response raised against PRV glycoproteins. This component which remains to be identified would have ultimately led to an improved secondary antibody response. The studies of Molitor et al. (1992)and Brun et al. (1994)also support a virus-dependent enhancement of the humoral immune responses of infected pigs. These investigators have reported that PRRSV could actually enhance the antibody production after immunization with inactivated PRV and Brucella abortus antigens, or after a virulent challenge with influenza virus.
Despite positive effects of PRRSV on the anti-PRV antibody response after PRV challenge, the disease resistance of PRRSV-infected pigs was not significantly improved compared to uninfected pigs. We assume that the effects of PRRSV on anti-PRV immune responses were not sufficient to provide supplementary protection against the PRV challenge. On the other hand, achievement of a better protection against this severe challenge would probably require the recruitment of other immune mechanisms such as CTLs.
In the present report, we have compared the incidence of two different virus infections in the same pigs. In contrast with PRRSV, PRV infection has led to pronounced effects on many immunological and clinical parameters. Our results with PRV are in agreement with those of Page et al. (1992). These authors demonstrated that PRV challenge resulted within 6 days in an increase of polynuclear cells and a pronounced lymphopenia. In addition, CD8+ cells and CD4/CD8 ratio decreased during the week after challenge. These alterations support a general immunosuppressive effect of PRV, as reported elsewhere (see review of Chinsakchai and Molitor, 1994). Thus, PRV appears to be more detrimental than PRRSV for the immune system of pig. Interestingly, the incidence of PRRSV infection was similar on SPF and conventional pigs. However, total and differential WBC counts and anti-PRV antibody production were higher in conventional pigs compared to SPF pigs, whereas daily weight gains were lower. These discrepancies may result from the improved health status of SPF pigs for which the immune system is less reactive and the growth is generally better.
In conclusion, our data do not support the hypothesis of a persistent suppressive effect of PRRSV on the humoral immune responses of pigs. The antibody production was not enhanced, immune cell distributions remained unchanged, immunization against PRV glycoproteins and subsequent disease resistance to a virulent PRV challenge was not adversely affected. Our study supports and extends previous findings which concluded that PRRSV had no effect on immune responses of pigs and subsequent disease resistance to various microorganisms (Cooper et al., 1995; Van Reeth et al., 1996b). The apparent increased disease susceptibility of PRRSV-infected pigs, as reported elsewhere (Galina et al., 1994; Van Reeth et al., 1996a), could be ascribed to the local disruption of the first lines of defence. This disruption could be due to the virus replication in alveolar macrophages and the consequent local inflammatory reaction. Recurrent secondary infections, observed in some herds, could be explained by successive phases of acute infection in new susceptible pigs, thus, facilitating the establishment, the proliferation and the dissemination of any opportunistic pathogens.
Acknowledgements
We thank Gaelle Bourbao and Catherine Houdayer (Centre National d'Etudes Vétérinaires et Alimentaires, CNEVA) for her technical assistance, Bernard Charley (Institut National de la Recherche Agronomique, INRA) for fruitful advice and Philippe Vannier (CNEVA) for reviewing the manuscript. Special acknowledgements to Trevor Drew (Central Veterinary Laboratory, Weybridge, UK) for his revision and fruitful comments on the manuscript, and also for interesting discussions in the field of PRRS immunology.
References
- Albina E, Madec F, Cariolet R, Torrison J. Immune response and persistence of the porcine reproductive and respiratory syndrome virus in infected pigs and farm units. Vet. Rec. 1994;134:567–573. doi: 10.1136/vr.134.22.567. [DOI] [PubMed] [Google Scholar]
- Albina E, Kobisch M, Cariolet R, Morvan P, Keranflec'h A, Beaurepaire B, Hutet E, Labbé A. Le syndrome dysgénésique et respiratoire du porc (SDRP): étude expérimentale des effets de l'infection sur la réponse immunitaire et la résistance aux infections Aujeszky et Mycoplasma hyopneumoniae chez le porc en croissance. Journées Rech Porcine en France. 1995;27:107–116. [Google Scholar]
- Andries K, Pensaert M.B, Vandeputte J. Effect of experimental infection with pseudorabies (Aujeszky's disease) virus on pigs with maternal immunity from vaccinated sows. Am. J. Vet. Res. 1978;39:1282–1285. [PubMed] [Google Scholar]
- Baron T, Albina E, Leforban Y, Madec F, Guilmoto H, Plana Duran J, Vannier P. Report on the first outbreak of porcine reproductive and respiratory syndrome (PRRS) in France: diagnosis and viral isolation. Ann. Rech. Vet. 1992;23:161–166. [PubMed] [Google Scholar]
- Bautista, E.M., Meulenberg, J.M.M., Choi, C.S., Pol, J., Molitor, T.W., 1996. PRRSV-specific responses in infected and vaccinated pigs. Proceedings of 14th International Pig Veterinary Society Congress, Bologna, Italy, 7–10 July, 63.
- Blaha, Th., 1992. Epidemiological investigations into PEARS in Germany: consequences in fattening pigs. Proceedings of 12th International Pig Veterinary Society Congress, The Hague, The Netherlands, 17–20 August, 126.
- Brun, A., Charreyre, C., Vaganay, A., Reynaud, G., 1994. Porcine reproductive respiratory syndrome: role of the togavirus and other infectious agents in the respiratory diseases of swine. Proceedings of 13th International Pig Veterinary Society Congress, Bangkok, Thailand, 26–30 June, 1, 52.
- Chinsakchai S, Molitor T.W. Immunobiology of pseudorabies virus infection in swine. Vet. Immunol. Immunopathol. 1994;43:107–116. doi: 10.1016/0165-2427(94)90126-0. [DOI] [PubMed] [Google Scholar]
- Christianson W.J, Choi C.S, Collins J.E, Molitor T.W, Morrison R.B, Joo H.S. Pathogenesis of porcine reproductive and respiratory syndrome virus infection in mid-gestation sows and fetuses. Can. J. Vet. Res. 1993;57:262–268. [PMC free article] [PubMed] [Google Scholar]
- Conzelmann K.K, Visser N, Van Woensel P, Thiel H.J. Molecular characterization of porcine reproductive and respiratory syndrome virus, a member of the arterivirus group. Virology. 1993;193:329–339. doi: 10.1006/viro.1993.1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper V.L, Doster A.R, Hesse R.A, Harris N.B. Porcine reproductive and respiratory syndrome: NEB-1 PRRSV infection did not potentiate bacterial pathogens. J. Vet. Diagn. Invest. 1995;7:313–320. doi: 10.1177/104063879500700303. [DOI] [PubMed] [Google Scholar]
- Galina L, Pijoan C, Sitjar M, Christianson W.T, Rossow K, Collins J.E. Interaction between Streptococcus suis serotype 2 and porcine reproductive and respiratory syndrome virus in specific pathogen free piglets. Vet. Rec. 1994;134:60–64. doi: 10.1136/vr.134.3.60. [DOI] [PubMed] [Google Scholar]
- Hammerberg C, Schurig G.G. Characterization of monoclonal antibodies directed against swine leukocytes. Vet. Immunol. Immunopathol. 1986;11:107–121. doi: 10.1016/0165-2427(86)90092-9. [DOI] [PubMed] [Google Scholar]
- Kaerber G. Beitrag zur kollektiven behandlung pharmakologischer. Reihenversuche. Arch. Exp. Pathol. Parmakol. 1931;142:480–483. [Google Scholar]
- Kawashima K, Yamada S, Kobayashi H, Narita M. Detection of porcine reproductive and respiratory syndrome virus and Mycoplasma hyorhinis antigens in pulmonary lesions of pigs suffering from respiratory distress. J. Comp. Path. 1996;114:315–323. doi: 10.1016/S0021-9975(96)80053-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meulenberg J.J.M, Hulst M.M, De Meijer E.J, Mooner P.L.J.M, Den Besten A, De Kluyer E.P, Wensvoort G, Moormann R.J.M. Lelystad virus belongs to a new virus family, comprising lactate dehydrogenase-elevating virus, equine arteritis virus, and simian hemorrhagic fever virus. Arch. Virol. 1994;9:441–448. doi: 10.1007/978-3-7091-9326-6_43. [DOI] [PubMed] [Google Scholar]
- Molitor, T., Leitner, G., Choi, C., Risdahl, J., Rossow, K., Collins, J., 1992. Modulation of host immune responses by SIRS virus. Proceedings of American Association of Swine Practitioners Annual Meeting, 4, 27–28.
- Page G.R, Wang F.-I, Hahn E.C. Interaction of pseudorabies virus with porcine peripheral blood lymphocytes. J. Leukocyte Biol. 1992;52:441–448. doi: 10.1002/jlb.52.4.441. [DOI] [PubMed] [Google Scholar]
- Pescovitz M.D, Lunney J.K, Sachs D.H. Preparation and characterization of monoclonal antibodies reactive with porcine PBL. J. Immunol. 1984;133:368–375. [PubMed] [Google Scholar]
- Plana Duran J, Vayreda M, Vilarassa J, Bastons M, Rosell R, Martinez M, San Gabriel A, Pujols J, Badiola J.L, Ramos J.A, Domingo M. Porcine epidemic abortion and respiratory syndrome (mystery swine disease). Isolation in spain of the causative agent and experimental reproduction of the disease. Vet. Microbiol. 1992;33:203–211. doi: 10.1016/0378-1135(92)90048-x. [DOI] [PubMed] [Google Scholar]
- Shimizu M, Yamada Y, Morozumi T, Kobayashi H, Mitani K, Ito N, Kubo M, Kimura K, Kobayashi M, Yamamoto K, Miura Y, Yamamoto T, Watanabe K. Isolation of porcine reproductive and respiratory (PRRS) virus from Heko-Heko disease of pigs. J. Vet. Med. Sci. 1994;56:389–391. doi: 10.1292/jvms.56.389. [DOI] [PubMed] [Google Scholar]
- Shimizu M, Yamada Y, Kawashima K, Ohashi S, Shimizu S, Ogawa T. Changes of lymphocyte subpopulations in pigs infected with porcine reproductive and respiratory syndrome (PRRS) virus. Vet. Immunol. Immunopathol. 1996;50:19–27. doi: 10.1016/0165-2427(95)05494-4. [DOI] [PubMed] [Google Scholar]
- Stevenson G.W, Van Alstine W.G, Kanitz C.L, Keffaber K.K. Endemic porcine reproductive and respiratory syndrome virus infection of nursery pigs in two swine herds without current reproductive failure. J. Vet. Diagn. Invest. 1993;5:432–434. doi: 10.1177/104063879300500322. [DOI] [PubMed] [Google Scholar]
- Van Alstine W.G, Stevenson G.W, Kanitz C.L. Porcine reproductive and respiratory syndrome virus does not exacerbate Mycoplasma hyopneumoniae infection in young pigs. Vet. Microbiol. 1996;49:297–303. doi: 10.1016/0378-1135(95)00191-3. [DOI] [PubMed] [Google Scholar]
- Van Reeth, K., Koyen, A., Pensaert, M., 1994. Clinical effects of dual infections with porcine epidemic abortion and respiratory syndrome virus, porcine respiratory coronavirus and swine influenza virus. Proceedings of 13th International Pig Veterinary Society Congress, Bangkok, Thailand, 26–30 June, 51.
- Van Reeth K, Nauwynck H, Pensaert M. Dual infections of feeder pigs with porcine reproductive and respiratory syndrome virus followed by porcine respiratory coronavirus or swine influenza virus: a clinical and virological study. Vet. Microbiol. 1996;48:325–335. doi: 10.1016/0378-1135(95)00145-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Reeth, K., Van Hoof, D., Willems, L., Pensaert, M., 1996b. A clinical study of dual infections with porcine reproductive—respiratory syndrome virus and swine influenza virus administered with different time intervals. Proceedings of 14th International Pig Veterinary Society Congress, Bologna, 7–10 July, 59.
- Van Zaane D, Hulst M.M. Monoclonal antibodies against porcine immunoglobulin isotypes. Vet. Immunol. Immunopathol. 1987;16:23–36. doi: 10.1016/0165-2427(87)90171-1. [DOI] [PubMed] [Google Scholar]
- Vannier P, Hutet E, Bourgueil E, Cariolet R. Level of virulent virus excreted by infected pigs previously vaccinated with different glycoprotein deleted Aujeszky's disease vaccines. Vet. Microbiol. 1991;29:213–223. doi: 10.1016/0378-1135(91)90129-4. [DOI] [PubMed] [Google Scholar]
- Vézina S.-A, Loemba H, Fournier M, Dea S, Archambault D. Antibody production and blastogenic response in pigs experimentally infected with porcine reproductive and respiratory syndrome virus. Can. J. Vet. Res. 1996;60:94–99. [PMC free article] [PubMed] [Google Scholar]
- Wensvoort G, Terpstra C, Pol J.M.A, Ter Laak E.A, Bloemrad M, Dekluyver E.P, Kragtan C, Van Buiten L, Den Bensten A, Wagenaar F, Boekhuijsen J.M, Moonen P.L, Zetstra T, De Boer E.A, Tibben H.J, De Jong M.F, Van't Veld P, Groenl G.J.R, Van Gennep J.A, Voets M.Th, Verheijden J.H.M, Braamskamp J. Mystery swine disease in the Netherlands: the isolation of Lelystad virus. Vet. Q. 1991;13:121–130. doi: 10.1080/01652176.1991.9694296. [DOI] [PubMed] [Google Scholar]
- Yoon I.J, Joo H.S, Goyal S.M, Molitor T.W. A modified serum neutralization test for the detection of antibody to porcine reproductive and respiratory syndrome virus in swine sera. J. Vet. Diagn. Invest. 1994;6:289–292. doi: 10.1177/104063879400600326. [DOI] [PubMed] [Google Scholar]
- Yoon K.-Y, Wu L.-L, Zimmerman J, Hill H.T, Platt K.B. Antibody-dependent enhancement (ADE) of porcine reproductive and respiratory syndrome virus (PRRSV) infection in pigs. Viral Immunol. 1996;9:51–63. doi: 10.1089/vim.1996.9.51. [DOI] [PubMed] [Google Scholar]
- Zimmerman, J., Sanderson, T., Ernisse, K., Hill, H., Frey, M., 1992. Transmission of SIRS virus in convalescent animals to commingled penmates under experimental conditions. Proceedings of American Association of Swine Practitioners Annual Meeting, 4, 25.


