Abstract
Plant lectins are a unique group of proteins and glycoproteins with potent biological activity and have received widespread attention for many years. They can be found in wheat, corn, tomatoes, peanuts, kidney beans, bananas, peas, lentils, soybeans, mushrooms, tubers, seeds, mistletoe and potatoes among many others. Due to their ability to bind reversibly with specific carbohydrate structures and their abundant availability, plant lectins have commonly been used as a molecular tool in various disciplines of biology and medicine. Whilst once thought of being a dietary toxin, the focus on plant lectins has since shifted to understanding the useful properties of these lectins and utilizing them in medicinal applications to advance human health. This chapter reviews the current and potential applications of plant lectins in various areas of medically related research.
Keywords: Human Immunodeficiency Virus, Plant Lectin, Ribosome Inactivate Protein, Black Soybean, Mistletoe Lectin
Introduction
The word “lectin” originates from the Latin word “lego” which means, “to choose” or “pick out” [1]. Lectins are defined as sugar-binding proteins that are neither antibodies nor enzymes [2]. According to Rudiger and Gabius (2001), a glycoprotein must meet three distinct requirements to qualify as a lectin. Firstly, a lectin is a protein/glycoprotein that binds carbohydrate(s). Secondly, lectins are not immunoglobulins (antibodies). Thirdly, lectins do not biochemically modify the carbohydrates which they bind [3].
Lectins were first discovered more than 100 years ago in plants, when in 1888, Stillmark found that extracts of castor bean (Ricinus communis) seeds contained a protein that could agglutinate animal red blood cells [4]. Following the discovery of Stillmark, a number of other plant seed extracts demonstrated the same ability to agglutinate red blood cells, but escalating interest in lectins was sparked during the Second World War as a result of the awareness of using lectins for blood typing. Because some lectins were found to be specific for various blood types (A, B or O), and others were found to have specificities for different glycans, lectins were used for blood typing before blood transfusions were performed [5, 6]. Lectins have since been found in almost every plant species studied and are particularly abundant in the seeds of leguminous plants. They have also been found in various tissues and organs of many vertebrates and invertebrates [7]. Due to their biochemical properties, lectins have become a beneficial tool in several fields of biological research including immunology [8], study of membrane structure, cell recognition [9], cancer research [10], and clinical microbiology [11]. 10.1007/978-94-007-6214-5_2 in this book provides more interesting stories regarding the history of lectinology.
Despite a lack of complete understanding of their biological roles, lectins have been exploited for several years in many applications. The use of lectins has facilitated advancements in many areas of medical research. Lectins are promising candidates as useful therapeutic agents because they can recognize specific carbohydrate structures such as proteoglycans, glycoproteins, and glycolipids, resulting in the regulation of various cells via glycoconjugates and their physiological and pathological phenomena through host-pathogen interactions and cell–cell communications [12].
Types of Plant Lectins
Plant lectins can be categorized based on their overall mature structure into merolectins, hololectins, chimerolectins and superlectins (Fig. 5.1a). Firstly, merolectins are small monomeric lectins consisting exclusively of a single carbohydrate-binding domain. Due to their monovalent nature, they do not possess agglutinating activity, such as hevein isolated from the latex of Hevea brasiliensis [13]. Figure 5.1b shows the crystal structure of hevein with (4s)-2-methyl-2,4-pentanediol. Next are hololectins, also exclusively built up of carbohydrate-binding domains but containing at least two such domains that are either identical or very homologous and bind either the same or structurally similar sugar(s), and most plant lectins are included in this subgroup. One example is Concanavalin A which is a tetrameric protein and binds specifically α-d-mannosyl and α-d-glucosyl residues (two hexoses differing only by the alcohol on carbon 2). A refined structure of Concanavalin A with mannose at 2.0 angstroms resolution is shown in Fig. 5.1b [14]. Another example is peanut (Arachis hypogaea) agglutinin [15]. It is a 110-kDa, homotetrameric non-glycosylated protein (without RIP activity) and shows a specificity for the tumor-associated T-antigenic disaccharide Galβ1,3GalNAc. The third group is chimerolectins, which are fusion proteins consisting of one or more carbohydrate-binding domain(s) tandemly arrayed to an unrelated domain. An example of this is ricin, which can be classified as both lectin and type II ribosome inactivating protein (RIP) [16]. As shown in Fig. 5.1b, ricin consists of two parts; an A chain (with N-glycosidase activity/RIP activity) and a B chain (hemagglutinating/lectin activity) with the B chain capable of binding different carbohydrates, such as β-d-glucose and β-d-galactose as shown in the figure. Lastly, superlectins are a type of chimerolectins which consist of at least two structurally different domains which recognize structurally unrelated carbohydrates [17]. For instance, TxLC-I is a superlectin isolated from tulip bulbs, and it consists of a mannose-binding domain and an unrelated GalNAc-binding domain. Since there is no crystal data on plant superlectin, the crystal structure of a superlectin purified from Burkholderia cepacia (a Gram-negative bacterium), named BC2L-A, is used for demonstration (Fig. 5.1b) [18]. The N-terminal domain of BC2L-A is a novel TNF-α-like fucose-binding lectin while the C-terminal is a calcium-dependent bacterial lectin.
Fig. 5.1.
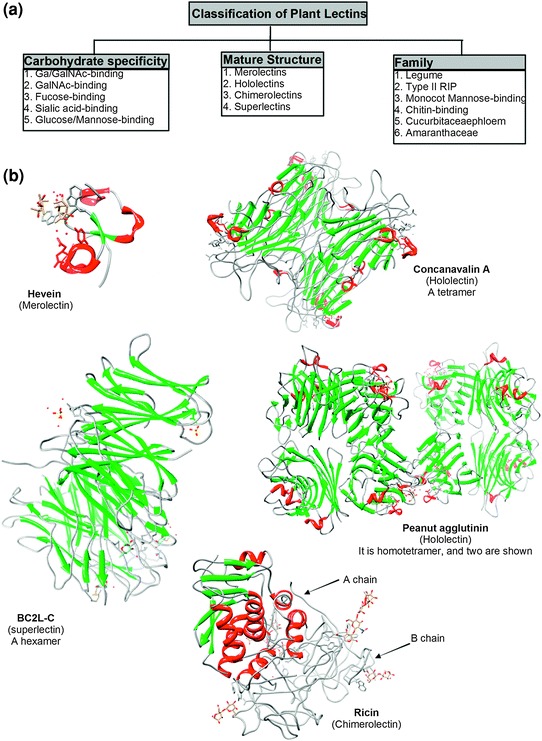
Classifications of plant lectins and the crystal structures of representative plant lectins under the classification standard of mature structure. Crystal data were from RCSB protein data bank and visualized by the UCSF chimera software as used previously [23]. Protein crystal structures were from PROTEIN DATA BANK: Hevein (PDB ID: 1Q9B); Concanavalin A (PDB ID: 5CNA) [14]; Ricin (PDB ID: 2AAI) [16]; Peanut agglutinin (PDB ID: 1CR7); BC2L-C (N-terminal domain: 2WQ4; C-terminal domain: 2XR4) [18]. Please note that BC2L-C is a bacterial lectin since currently no crystal structure of plant superlectin has been released. The coil, helix and strand are shown in dark grey, red and green, respectively
Plant lectins can also be grouped into different families according to some common features (legume lectins, type II ribosome-inactivating proteins, monocot mannose-binding lectins, and other lectins) [19]. Legume lectins are the best known lectin family. Lectin content in seeds is higher compared to the content in the bark, leaves, roots and stems of leguminous plants. Other smaller families of plants whose lectins have been characterized are Gramineae (cereals, such as wheat germ) and Solanaceae (potatoes and tomatoes). Monocot-binding lectins exhibit an exclusive specificity towards mannose and are built up of 1, 2, 3, or 4 subunits of about 12 kDa while chitin-binding lectins are composed of hevein domains [20]. For more information, readers are referred to an excellent review by Van Damme et al. (1998) describing in detail how plant lectins are structurally and evolutionarily related [21].
Because of the tremendous diversity of carbohydrate-binding specificities among the plant lectins, some researchers classify them according to the small carbohydrate haptens they recognize, e.g., galactose-binding lectins or GlcNAc-binding lectins (Fig. 5.1a). The specificity of a lectin is usually defined in terms of the monosaccharide(s) or glycosaccharides that inhibit lectin-induced agglutination [22]. This specificity is usually determined by comparing sugars on the basis of the minimum concentration needed to inhibit hemagglutination. If the lectin-binding carbohydrate is present freely dissolved and at a sufficiently high concentration, it will compete with the red cell glycoconjugates for the lectin and agglutination will not take place.
Medicinal Applications of Plant Lectins
Due to their versatility, lectins are frequently used in biological and medical research. The overwhelming success of plant lectins is based firstly on their highly specific carbohydrate-binding activity, and the biological effects they provoke in various organisms. Being able to procure reasonable amounts of pure lectin preparations also contributes to their success [20]. Consequently, screening of plant species in the search for lectins with new and useful biological properties has been taking place for several decades.
Plant Lectins in Serology
Because of their ability to distinguish carbohydrate determinants in human blood cells, lectins have historically been used for blood typing. Serology was the first discipline of medicine that relies on the specific biological activity of lectins [24]. Lectins agglutinate erythrocytes by binding to a carbohydrate-containing moiety on the surface of the cells and forming cross-bridges between them [4]. This useful property of lectins endows them the ability to discriminate between red blood cells that have different terminal, non-reducing sugars in the major glycoprotein that carries the blood group antigens [25]. For example, blood group A is determined by the presence of N-acetyl-galactosamine in the terminal, non-reducing end of the oligosaccharide portion of the receptor protein while group B is determined by galactose in this position. If both of these sugars are lacking, fucose is the terminal sugar and this determines blood group O. In this way, lectins with different specificities can be used in blood typing to differentiate between blood types. For readers seeking a comprehensive compilation of lectins that have been studied for blood group serology, the review by Judd (1980) is recommended [26].
Plant Lectins as Mitogens
A limited number of lectins from plants possess the unique ability to induce quiescent lymphocytes to grow and divide, a phenomenon known as mitogenic stimulation [27]. Mitogenic lectins mimic the action of antigens on lymphocytes, except that they activate a large proportion (as much as 70–80 %) of the cells, whereas antigens stimulate only specific clones, each of which compromise a tiny proportion, 0.1 % or less, of the total number of lymphocytes. Because of their ability to stimulate multiple lymphocyte clones, lectins are classified as polyclonal mitogens. One of the most valuable outcomes of this proliferative ability of lectins has been an increased understanding of the relationship between chromosomal abnormality and human diseases, which has tremendously helped in diagnosis [28]. Besides other cells, lymphocytes have been the usual target cells for mitogenic assays, and the study of lectin-lymphocyte interaction has made a significant contribution to elucidating the mechanism of lymphocyte activation and its control, further contributing to the understanding of cell growth and development.
The discovery of the first mitogenic lectin was made by Nowell in 1960, who found that the lectin of red kidney bean (Phaseolus vulgaris), known as phytohemagglutinin (PHA), possessed the ability to stimulate lymphocytes to undergo mitosis [29]. This discovery had a groundbreaking impact on immunology as it shattered the then prevailing opinion that lymphocytes were dead-end cells incapable of dividing or differentiating further [29]. Within a short time thereafter, several other lectins were proven to be mitogenic. Table 5.1 provides a few examples of plant lectins with mitogenic activity as such a lectin from the tuber of wild cobra lily [30], red kidney bean lectin [31], mushroom lectins [32, 33], Brazilian camaratu bean lectin [34], and jackfruit seed lectin [35] .
Table 5.1.
Mitogenic activity of selected plant lectins
| Lectin | MW (kDa) | Carbohydrate specificity | Cells | Activity | References |
|---|---|---|---|---|---|
| AFL | 13.5 | Asialofetuin | BALB/c splenocytes | increase in IL-2 | [30] |
| DRKBL | 67 | – | BALB/c splenocytes | Lower than Con A | [31] |
| GCL | 18 | Galactose | BALB/c splenocytes | More potent than Con A | [32] |
| VVL | 12.6 | Thyroglobulin | Mouse T cells | 10 fold more effective than Con A | [33] |
| CML | – | Mannose/Glucose | Human lymphocytes | Similar activity to Con A at 0.78 – 25 μg/ml dosage | [34] |
| Jacalin | 50 |
Galactose Mannose |
Human lymphocytes | 33.7 ± 15 × 103 ct/min | [35] |
AFL a lectin from tubers of Arisaema flavum (Schott.); DRKBL a lectin from Phaseolus vulgaris cv. Dark Red Kidney Bean; GCLGanoderma capense lectin; Con A Concanavalin A; VVLVolvariella volvacea lectin; CMLCratylia mollis seed lectin; Jacalin Jackfruit (Artocarpus heterophyllus) seed lectin
Concanavalin A, a lectin from the Jack bean, is an important mitogen because, in contrast to PHA, its activity can be inhibited by low concentrations of monosaccharides, for example, mannose. This finding provided proof that mitogenic stimulation is the result of binding of lectins to sugars on the surface of the lymphocytes and was among the earliest demonstrations for a biological role of cell surface sugars [36]. It has been suggested that mitogenic lectins interact with unique membrane components that may act as ‘stimulating receptors’, and that non-mitogenic lectins may not bind to these membrane components [28]. Mitogenic lectins are now tools for the study of signal transmission into cells and for the analysis of the biochemical events that occur during lymphocyte stimulation in vitro [36]. Such properties make them useful tools for the isolation and characterization of polysaccharides and glycoconjugates, in cancer research, as diagnostic tools for the investigation of early cell-membrane alterations and carbohydrate changes that accompany neoplastic processes, and in immunological studies.
Plant Lectins in Cancer Therapy
Cancer is one of the leading causes of death worldwide. Cancer is a deadly disease, where the abnormal behavior of a single cell type is difficult to treat by chemotherapy. It is important in cancer therapy that the treatment targets only the affected cells, leaving the normal cells undisturbed, which is quite difficult, especially in chemotherapy. Anti-cancer drugs available in the current market are not target-specific and elicit several side-effects and complications encountered in the clinical management of various forms of cancer, which highlights the urgent need for novel effective and less-toxic therapeutic approaches [37]. Recently, focus has shifted from using lectins to detect cancer to actually using lectins to combat cancer.
Evidence is now emerging that lectins are dynamic contributors to tumor cell recognition (surface markers), cell adhesion and localization, signal transduction across membranes, mitogenic stimulation, augmentation of host immune defense, cytotoxicity, and apoptosis [38]. A review by De Mejia and Prisecaru (2005) provides a comprehensive appraisal of the inhibitory effects of plant lectins on malignant cells in vitro and vivo [39]. Table 5.2 below adds some more recent selected plant lectins and lists their inhibitory effects on malignant cells.
Table 5.2.
Inhibitory effects of selected plant lectins on malignant cells
| Lectin | MW (kDa) | Carbohydrate specificity | Antiproliferative activity (IC50) | Mechanism of action | References |
|---|---|---|---|---|---|
| BTKL | 60 | Polygalacturonic acid | HepG2 (7.9 μM) | Apoptosis induction | [40] |
| Mitochondria damage | |||||
| FVML | 12 | – | L1210 (13 μM) | – | [41] |
| GCML | 18 | Galactose/galactosamine | L1210 (8 μM) | – | [32] |
| Hep G2 (16.5 μM) | |||||
| M1 (12.5 μM) | |||||
| PAL | 32 | Inulin | Hep G2 (2.1 μM) | – | [42] |
| MCF7 (3.2 μM) | |||||
| CNL | 15.9 | Asialofetuin and lactose | leukemic T (100 μg/ml) | binding to carbohydrate receptors on leukemic T cells | [43] |
| HCSL | 29 | D-galactose and N-acetyl-Dgalactosamine | HeLa (9 μg/ml) | Displayed a cytotoxic effect | [44] |
| FemX (11 μg/ml) | |||||
| ML-1 | 63 | Galactose | UISO-Mel6 (73.6 ng/ml) | Induction of apoptosis | [45] |
| MeWo (5084.0 ng/ml) | |||||
| FemX-1 (1.9 ng/ml) | |||||
| Lox (10.5 ng/ml) | |||||
| G361 (0.6 ng/ml) | |||||
| Con A | 104 | Mannose/Glucose | A375 (25 μg/ml) | Inducing apoptosis in both casp-8 and casp-9-dependent ways | [46] [47] |
| DRKBL | 67 | – | L1210 (1.6 μM) | – | [31] |
| GML | 50 | Melibiose | MCF7 (2.6 μM) | – | [48] |
| HepG2 (4.1 μM) | |||||
| DBL | 30 | Fructose | L1210 (3.6 μM) | Cytokine-inducing | [49] |
| HepG2 (25 μM) | |||||
| No production | |||||
| EAPL | 60 | Galactose | Hep G2 (34.8 μM) | Apoptosis-inducing | [50] |
| AFL | 13.5 | Asialofetuin | J774 | [30] | |
| P388D1 | |||||
| MCL | 130 | d-galactose and α-lactose | CNE 1 (6.9 μM) | Inducing apoptosis in both casp-8 and casp-9-dependent pathways; | [51] |
| CNE 2 (7.4 μM) | |||||
| Damage of mitochondria | |||||
| TDL | 48 | Mannose | Pro-01 (56.7 μM) | [52] | |
| Bre-04 (41.5 μM) | |||||
| Lu-04 (11.4 μM) | |||||
| SAL | 40 | Galactose | HeLa (6.25 μM) | – | [53] |
BTKLPhaseolus vulgariscv. Blue tiger king lectin; FVMLFlammulina velutipes mushroom lectin; GCMLGanoderma capense mushroom lectin; PALPholiota adipose mushroom lectin; CNL Ricin B-like lectin from mushroom Clitocybe nebularis; HCSLHaliclona crater sponge lectin; ML-1 European mistletoe lectin; Con A Concanavalin A; DRKBL Dark red kidney bean (Phaseolus vulgaris cv.) lectin; GML small glossy black soybean (Glycine max) lectin; DBL, Del Monte banana lectin; EAPLPhaseolus vulgaris cv. Extralong autumn purple bean lectin; AFL lectin from tubers of Wild Cobra Lily Arisaema flavum; MCLMomordica Charantia lectin; TDL lectin from fresh tubers of a medicinal herb Typhonium Divaricatum; SAL lectin from seeds of Sophora alopecuroide
Small glossy black soybean (Glycine max) lectin inhibited the proliferation of breast cancer and hepatoma cells [48]. An anti-tumor action mechanism of soybean lectins has been proposed involving the action of the lectins on tumor cell membranes, the reduction of tumor cell proliferation, the induction of tumor-specific cytotoxicity of macrophages, and apoptosis. Thus, tumor cells are more susceptible to attack by macrophages after treatment with lectins. Furthermore, lectins exert an immunomodulatory effect by altering interleukins production [54].
Mistletoe lectin (ML) is one of the most studied lectins in clinical trials and has demonstrated beneficial effects against cancer development. Additionally their mechanisms of action towards cancer treatment have been extensively studied. For example, in a study using European mistletoe lectin which demonstrated antiproliferative activity towards human melanoma cells, a significant number of melanoma cells started rounding up and exhibited cell shrinkage, chromatin condensation and nuclear fragmentation typical for apoptotic body formation indicating apoptotic cell death [45]. Three mistletoe lectins, I, II and III (ML-I, II, III) have been isolated [55]. Mistletoe lectin-I, which belongs to the type II ribosome-inactivating protein (RIP II) family and is composed of a catalytically active A-chain with rRNA N-glycosidase activity and a B-chain with carbohydrate binding properties, exerted potent cytotoxic effects on tumor cells. It also induced apoptosis through both caspase-8/FLICE independent of a death receptor pathway and via a p53-independent pathway following ionizing radiation. Meanwhile, mistletoe lectin-II induced apoptotic death in cancer cells involving the generation of intracellular hydrogen peroxide (H2O2) and activation of a caspase- 9-caspase-3 cascade [46].
The oral consumption of mistletoe lectins as an alternative therapy towards cancer therapy has been advocated by some parties as these lectins are resistant towards low pH in the stomach and are not affected by proteolytic enzymes in the stomach [56]. Pryme et al. (2007) provide a meticulous case report with more information on this topic [56]. However, the usefulness of mistletoe extracts in the treatment of malignant melanoma is still controversial for some. This may be in part due to the fact that the full molecular mechanisms underlying mistletoe treatment and how it works in vivo are still not completely clarified.
Lectins from several types of mushroom have demonstrated anti-proliferative activity including those from Flammulina velutipes [41], Ganoderma capense [32], Pholiota adipose [42] and Clitocybe nebularis [43]. A homodimeric 32.4-kDa lectin was isolated from fresh fruiting bodies of the mushroom Pleurotus citrinopileatus [57]. The lectin exerted strong anti-tumor activity in mice bearing sarcoma 180, and caused approximately 80 % inhibition of tumor growth when administered intraperitoneally at 5 mg/kg daily for a period of 20 days.
As many plant lectins have demonstrated anticancer properties in vitro, and in vivo, there clearly is a huge potential for their use as therapeutic agents in cancer treatment. Mechanisms of plant lectin action elucidated thus far include preferential binding to cancer cell membranes or their receptors, causing cytotoxicity, apoptosis, and inhibition of tumor growth. Plant lectins can be internalized into cells, causing cancer cell agglutination and/or aggregation [54]. Ingested lectins can also sequester the available body pool of polyamines, in this manner they prevent cancer cell growth. The immune system is also affected by alterations in the production of various interleukins, or by activation of certain protein kinases. Additionally, lectins can bind to ribosomes and inhibit protein synthesis. They also alter the cell cycle by inducing non-apoptotic G1-phase accumulation mechanisms, G2/M phase cell cycle arrest and apoptosis, and can trigger the caspase cascade. Lectins can also down-regulate telomerase activity and inhibit angiogenesis [54].
Future advances in cancer prevention, detection, and treatment could potentially be achieved by using plant lectins. These substances possess antitumor activity and anti-carcinogenic activity that could be beneficial in cancer treatment.
Plant Lectins as Antiviral Agents
Compounds with antiviral activity are generally of great medical interest and different modes of pharmaceutical actions have been described. The antiviral activity of plant lectins can be based on several mechanisms. The surfaces of retroviruses such as human immunodeficiency virus (HIV) and many other enveloped viruses are covered by virally-encoded glycoproteins. Glycoproteins gp120 and gp41 present on the HIV envelope are heavily glycosylated, with glycans estimated to contribute almost 50 % of the molecular weight of gp120. Agents that specifically and strongly interact with the glycans may disturb interactions between the proteins of the viral envelope and the cells of the host [58]. Sugar-binding proteins can crosslink glycans on the viral surface and prevent further interactions with the co-receptors.
HIV RT is a key enzyme of the HIV life cycle. Screening of HIV RT inhibitors is currently a strategy to search for anti-HIV drugs. Strikingly, the vast majority of plant lectins that are active against HIV possess carbohydrate specificity directed against mannose oligomers. Most HIV-inhibitory plant lectins are derived from the monocot families Amaryllidaceae, Orchidaceae and Alliaceae or the dicot families Fabaceae, Moraceae, Urticaceae and Cecropiaceae [58]. Table 5.3 lists several plant lectins such as Phaseolus vulgaris cv. Extralong autumn purple bean lectin [50], Del Monte banana lectin [49], black soybean lectin [48] and Pholiota adipose mushroom lectin [42] which all possessed anti-HIV RT activity.
Table 5.3.
Antiviral activity of selected plant lectins
| Name | MW (kDa) | Carbohydrate specificity | Anti-Viral activity (shown in MIC or as indicated) | Mechanism of action | References |
|---|---|---|---|---|---|
| EAPL | 60 | Galactose | HIV-1 (1.8 μM) | – | [50] |
| DBL | 30 | Fructose | HIV-1 (3 mM) | [49] | |
| APA | – | Mannose | Sars CoV corona virus (0.45 μg/ml) | Disruption of virus replication cycle | [59] |
| SGBSL | 50 | Melibiose | HIV-1 (2.82 μM) | [48] | |
| PAL | 32 | Inulin | HIV-1 (1.9 μM) | – | [42] |
| TDL | 48 | Mannose | HSV-II (3.1 μg/ml) | Bind on high-mannose | [52] |
| glycans on the surface of the virus particles | |||||
| CVN | 11 | – | HIV-1 laboratory strain RF (0.1 nM) | Binding on HIV surface envelope glycoprotein gp120 | [60, 61] |
| CVN | 11 | – | EboZV GP (50 nM) | – | [62] |
| Marburg GP (6-25 nM) | |||||
| CVN | 11 | – | Influenza A Virus Strains: | CV-N bound directly to and inactivated the | [63] |
| Sydney/05/07 (H3N3, 0.04 μg/ml) | viral particle | ||||
| Victoria/3/75 (H3N3, 0.005 μg/ml) | |||||
| Mem/8/99 (H3N3, 0.03 μg/ml) | |||||
| Mem/2/99 (H3N3, 0.15 μg/ml) | |||||
| Beijing/262/95 (H1N1, 0.11 μg/ml) | |||||
| Influenza B Virus Strains: | |||||
| Hong Kong/5/72 (0.5 μg/ml) | |||||
| Yamanashi/166/98 (0.04 μg/ml) | |||||
| Mem/3/99 (0.02 μg/ml) | |||||
| Lee/40 (0.026 μg/ml) | |||||
| CLL | 12 | Mannose | Poxvirus (around 30 μg/ml) | – | [64] |
EAPLPhaseolus vulgaris cv. Extralong autumn purple bean lectin; DBLDel Monte banana lectin; APA plant lectin isolated from leek; SGBSL small glossy black soybean (Glycine max) lectin; PALPholiota adiposa mushroom lectin; TDL lectin from fresh tubers of a medicinal herb Typhonium divaricatum (L.) Decne; CVN blue green algae Nostoc ellipsosporum lectin; CLL lectin from Crinum latifolium bulb; HIV-1 human immunodeficiency virus type 1; HSV-II herpes simplex virus type 2
Studies on plant lectins such as those from leek (Table 5.3) have shown that they can be potent inhibitors of coronaviruses by interfering with two targets in the viral replication cycle [59]. The first target was located early in the replication cycle, most probably viral attachment, and the second target was located at the end of the infectious virus cycle. The antiviral activity spectrum of plant lectins varies considerably, depending on the nature of their sugar specificity. In general, the mannose-specific plant lectins were found to be highly effective against coronaviruses [59].
A lectin from blue green algae (CV-N) exhibited a broad range of antiviral activities. It has been shown that CV-N binds with high affinity to HIV envelope protein gp120 [61] and also interacts with another surface glycoprotein, gp41 [63]. The specificity of CV-N, however, is not limited only to different strains of HIV and related retroviruses. CV-N inhibited the development of viral cytopathic effects of Ebola virus, binding to its surface envelope glycoprotein [62] and also blocked influenza A and B strains by binding to the hemagglutinin surface glycoprotein [63]. The results of viral pretreatment studies indicated that CV-N directly neutralized both influenza A and B viruses, including both H1N1 and H3N2 strains. However, the CV-N resistant influenza virus strain A/PR/8/34 was completely resistant to any direct neutralizing activity. Results suggested that CV-N bound directly to and inactivated the viral particle, preventing subsequent infection, and that both the likely molecular target for CV-N and the basis of CV-N resistance resided in the viral particle [63].
Unlike the majority of current antiviral therapeutics that act through inhibition of the viral life cycle, lectins can prevent penetration of the host cells by the viruses [60]. Antiviral lectins are best suited to topical applications and can exhibit lower toxicity than many currently used antiviral therapeutics. Additionally, these proteins are often resistant to high temperatures and low pH, as well as being odorless, which are favorable properties for potential microbicide drugs [60]. The mechanistic details on how lectins operate at a molecular level to inhibit virus growth need to be further explored to know the basis of their biological activity.
Plant Lectins as Antibacterial and Antifungal Agents
The growing resistance of microorganisms to convectional antimicrobial agents is a source of concern to clinical microbiologists all over the world. As a result, efforts are being made to develop antimicrobial agents from local sources for better chemotherapeutic effects. Lectins from plants could satisfy the demand for more natural antimicrobials as several studies have demonstrated the effectiveness of lectins as inhibitory compounds towards bacterial and fungal growth such as the examples listed in Table 5.4. Plant lectins have demonstrated antibacterial activity against many pathogens including Escherichia coli, Shigella dysenteriae, Staphylococcus aureus, Pseudomonas aeruginosa, Bacillus subtilis, and Klebsiella sp. Lectins have also demonstrated antifungal activity towards Aspergillus flavus, Trichoderma viride, Fusarium oxysporum, Fusarium moniliforme, Coprinus comatus, Rhizoctonia olani, Penicillium digitatum, Alternaria alternata, and Valsa mali. The use of lectins in clinical microbiology in a review by Slifkin and Doyle (1990) [11] as well as the antimicrobial activity from plant lectins article by Paiva et al. (2010) [65] is recommended for readers who seek further detailed information.
Table 5.4.
Antimicrobial activity of selected plant lectins
| Name | Antimicrobial activity | Lectin Specificity | Anti-microorganism activity (MIC or as indicated) | References |
|---|---|---|---|---|
| MOL | Antibacterial | – | E. coli | [66] |
| Shigella dysenteriae | ||||
| Staphylococcus aureus | ||||
| EUL | Antibacterial | – | S. aureus (1.5 μg/ml) | [67] |
| P. aeruginosa (1.5 μg/ml) | ||||
| Klebsiella sp. (1.5 μg/ml) | ||||
| Bacillus subtilis (16.5 μg/ml) | ||||
| Streptococcus sp. (16.5 μg/ml) | ||||
| Escherichia coli (16.5 μg/ml) | ||||
| AJL | Antibacterial | Bacillus subtilis (0.227 mg/ml) | [68] | |
| Antifungal | S. aureus (0.0567 mg/ml) | |||
| C. albicans (0.0567 mg/ml) | ||||
| PSL | Antifungal | Mannose | Aspergillus flavus (0.1 mg/ml) | [69] |
| Trichoderma viride(0.01 mg/ml) | ||||
| Fusarium oxysporum. (0.01 mg/ml) | ||||
| CFL | Antifungal | Mannose/glucose | Fusarium. moniliforme (-) | [70] |
| Aspergillus. flavus. (-) | ||||
| RKBL | Antifungal | Fusarium oxysporum (-) | [71] | |
| Coprinus comatus (-) | ||||
| Rhizoctonia solani (-) | ||||
| SAL | Antifungal | Galactose | Penicillium digitatum (IC50 = 3.125 μM) | [53] |
| Alternaria alternate (IC50 = 3.338 μM) | ||||
| CSL | Antifungal | Galactose, lactose, arabinose, etc. | Valsa mali (IC50 = 18 μM) | [72] |
MOL lectin purified from Drumstick (Moringa oleifera Lam.) leaves; EULEugenia uniflora seed lectin; AJL lectin from seeds of Archidendron jiringa; PSL lectin from Egyptian Pisum sativum seeds; CFL lectin from red cluster pepper (Capsicum frutescens); RKBL lectins from red kidney bean; SAL Lectin from seeds of Sophora alopecuroides; CSL lectin from caper (Capparis spinosa) seeds
The cell wall of bacteria not only precludes any interaction between the glycoconjugates on their membrane and carbohydrate-binding proteins but also prevents these proteins from penetrating the cytoplasm. Therefore, plant lectins cannot alter the structure and/or permeability of the membrane or disturb the normal intracellular processes of invading microbes. Therefore, if lectins play a role in the plant’s defense against bacteria, it must be through an indirect mechanism that is based on interactions with cell wall carbohydrates or extracellular glycans [73].
Similarly, plant lectins are not capable of binding to glycoconjugates on the fungal membranes or penetrating the cytoplasm due to the barrier formed by the cell wall. Thus, it is not likely that lectins directly inhibit fungal growth by changing the structure and/or permeability of the fungal membrane. However, there may be indirect effects produced by the binding of lectins to carbohydrates on the surface of the fungal cell wall [74].
This activity was concluded to be related to the lectin carbohydrate binding property, that might endow lectin molecules with binding activity towards certain carbohydrate components in the fungal cell wall affecting its activity and viability as most lectins recognize either N-acetylneuraminic acid, N-acetylglucosamine, N-acetylgalactosamine, galactose, mannose, or fucose [75]. Alternatively, it was stated that antifungal activity of some proteins or peptides is associated with chitin binding property and the active proteins should have a specific amino acid sequence and a cysteine/glycine rich chitin binding domain. This chitin binding property might simulate the carbohydrate binding property as chitin is composed of modified glucose subunits (N-acetyl glucose amine) which can be equally recognized by lectin as glucose [69]. Chitin binding might lead to the disruption of the fungal cell wall that increases toxicity, since chitin, which is a major component of the fungal wall, is a polymer of N-acetylglucosamine.
Plant Lectins in Drug Targeting
The idea to use lectins for drug delivery began when the use of tomato lectin (TL) to target the luminal surface of the small intestine was proposed [76]. The underlying principle behind lectin-mediated drug targeting is that most cell surface proteins and many lipids in cell membranes are glycosylated and these glycans can be the binding sites for lectins. The combination of a small number of sugars can produce a vast range of different chemical structures. Different cell types express different glycan arrays and diseased cells, such as transformed or cancerous cells, often express different glycans compared with their normal counterparts. Therefore, lectins could be used as carrier molecules to target drugs specifically to different cells and tissues [77]. By targeting cell types exclusively, the side effects of drugs could be minimized. Besides targeting specific cells, the lectin–sugar interaction can also been used to trigger vesicular transport into or across epithelial cells. The concept of bio-adhesion via lectins may be applied not only for the gastrointestinal tract [76] but also for other biological barriers like the nasal mucosa, the lungs, the buccal cavity, the eye [78] and the blood–brain barrier [77, 79]. Table 5.5 lists some examples of plant lectins from tomato [76], potato [78], wheat germ [79] and peanut [80] which have been studied to investigate their potential of use as drug targeting agents.
Table 5.5.
Potential drug targeting use of selected plant lectins
| Name | Source | Carbohydrate specificity | MW (kDa) | Potential use | References |
|---|---|---|---|---|---|
| AEL | Tomato | (GlcNAc)4 | 71 | Intestinal wall | [76] |
| Bioadhesive in the GI tract | |||||
| [81] | |||||
| STL | Potato | N-acetyl-d-glucosamine | – | Ocular (Corneal and conjunctival) | [78] |
| WGA | Wheat germ | N-acetyl-d-glucosamine | 38 | Blood brain barrier | [79] |
| Intestinal mucosal surface | |||||
| Sialic acid | |||||
| [82] | |||||
| PNA | Peanut | d-galactose | 110 | Intestinal mucosal surface | [80] |
AELLycopersicon esculenttun lectin; STLSolanum tuberosum lectin. WGA Wheat germ agglutinin; PNAArachis hypogaea lectin
Attempts to evaluate the binding of lectin candidates to non-histologically processed cell surfaces, must be done in order to systematically identify the appropriate receptors and lectin types for further studies. Further work will more precisely locate the lectin binding site on the tissue surface and will quantify the binding. Lectin toxicity and in vivo binding will then be considered prior to selecting the most promising candidates for formulation studies [78]. Lectins such as WGA could be useful as specific bioadhesive ligands for lipid nanoparticles intended for oral administration [82] once the bioadhesive properties and oral bioavailability efficiency are determined.
Results from preliminary studies performed so far can be taken as an indication that it may indeed be possible to exploit lectins of certain carbohydrate specificities for oral drug delivery and intestinal targeting. However, a great deal of research remains to be done before lectins can be used in practice. For a more thorough understanding, readers are directed to reviews by Lehr (2000) and Bies et al. (2004) which provides more information on lectin-mediated drug delivery and targeting, from their history to applications [77, 83].
Outlooks and Perspectives
Plant lectins have been and still are a subject of intense investigation. They have come a long way, since their first detection in plants as hemagglutinins, to their present status as recognition molecules with innumerable exciting functions and applications. As more plant lectins are isolated and further studies are conducted on the biological activities and mechanisms of action of lectins, the production of lectins can be improved and new applications of these lectins will be found or conceived. Lectins could be used as the next generation of medicines once research has contributed to their full understanding.
More research is still needed and a genomic and proteomic approach to elucidate and support the potential shown by lectins as anticancer, antimicrobial and drug delivery agents is warranted. The mechanistic details of how lectins operate at a molecular level need to be further explored to know the basis of their biological activity. Although there is still much to be learned about the effects of lectins, the area of research concerning plant lectins is constantly evolving and being updated. Thus the medicinal applications of plant lectins hold considerable potential and exciting discoveries lie ahead.
Biographies
Clara Bah
completed her Bachelor’s Degree in Chemical-Bioprocess Engineering at the University of Technology, Malaysia (UTM). After working as a research and development engineer, she completed a Postgraduate Diploma and Master’s of Science (Food Science) at the University of Otago, New Zealand. Her research topic explored the availability of bioactive compounds from fish eggs, with a particular focus on lectins. This work led to collaboration with Dr Evandro Fang and Prof. T.B. Ng of the School of Biomedical Sciences in the Chinese University of Hong Kong. Currently Clara is working towards completing her PhD at the University of Otago
Dr. Evandro Fei Fang
Dr. Evandro Fei Fang received his Ph.D. degree from The Chinese University of Hong Kong and currently works with Prof. Vilhelm A. Bohr on DNA repair and genome stability in human aging diseases. His research encompasses: (a) bioassay-guided isolation of antitumor constituents from medicinal plants and the molecular mechanisms involving apoptosis and autophagy pathways, and (b) the functions of mitochondria and mitophagy in sustaining human health and the molecular basis of their defects in the etiology of aging diseases. He has published over 30 papers in International Journals and 9 Book Chapters, and serves on the editorial board of Medicinal and Aromatic Plants. He was a finalist in the Young Investigator Award 2011 conducted by the Hong Kong Institute of Science.
Tzi Bun Ng
Tzi Bun Ng is Professor of Biochemistry at the Faculty of Medicine, The Chinese University of Hong Kong, HKSAR. After completing his B.Sc. studies at The University of Hong Kong, he obtained his Ph.D. degree from the Memorial University of Newfoundland in Canada. He pursued his postdoctoral training at University of California, San Francisco, USA, and sabbatical research at the Imperial College London, UK. His major research interests are the health-benefits of proteins and peptides including polysaccharopeptides, lectins, antimicrobial peptides, enzymes and protease inhibitors. In the past 30 years, his team made many contributions to the field of bioactive components, represented in more than 550 papers in International Peer-Reviewed Journals, over 60 invited Book Chapters, 2 Edited Books, and numerous other media. He currently sits on the editorial board of 9 International Journals and contributes actively in many scientific fields.
Contributor Information
Evandro Fei Fang, Phone: 4105588162, FAX: 4105588157, Email: fangfei1030@yahoo.com.cn.
Tzi Bun Ng, Phone: 39438031, FAX: 26035123, Email: b021770@mailserv.cuhk.edu.hk.
Evandro Fei Fang, Email: fange@mail.nih.gov.
Tzi Bun Ng, Email: b021770@mailserv.cuhk.edu.hk.
References
- 1.Boyd WC, Shapleigh E. Antigenic relations of blood group antigens as suggested by tests with lectins. J Immunol. 1954;73:226–231. [PubMed] [Google Scholar]
- 2.Barondes SH. Bifunctional properties of lectins: lectins redefined. Trends Biochem Sci. 1988;13:480–482. doi: 10.1016/0968-0004(88)90235-6. [DOI] [PubMed] [Google Scholar]
- 3.Rüdiger H, Gabius HJ. Plant lectins: occurrence, biochemistry, functions and applications. Glycoconj J. 2001;18:589–613. doi: 10.1023/A:1020687518999. [DOI] [PubMed] [Google Scholar]
- 4.Liener IE. Seed hemagglutinins. Econ Bot. 1964;18:27–33. doi: 10.1007/BF02903999. [DOI] [Google Scholar]
- 5.Renkonen KO. Studies on hemagglutinins present in seeds of some representatives of the family of Leguminoseae. Annales Medicinae Experimentalis et Biologiae Fenniae. 1948;26:66. [Google Scholar]
- 6.Boyd WC, Reguera RM. Hemagglutinating substances for human cells in various plants. J Immunol. 1949;62:333–339. [PubMed] [Google Scholar]
- 7.Goldstein IJ, Hughes RC, Monsigny M, Osawa T, Sharon N. What should be called a lectin. Nature. 1980;285:66. doi: 10.1038/285066b0. [DOI] [Google Scholar]
- 8.Fujita T. Evolution of the lectin-complement pathway and its role in innate immunity. Nat Rev Immunol. 2002;2:346–353. doi: 10.1038/nri800. [DOI] [PubMed] [Google Scholar]
- 9.Sharon N, Lis H. Lectins as cell recognition molecules. Science. 1989;246:227–234. doi: 10.1126/science.2552581. [DOI] [PubMed] [Google Scholar]
- 10.Mody R, Joshi SH, Chaney W. Use of lectins as diagnostic and therapeutic tools for cancer. J Pharmacol Toxicol Methods. 1995;33:1–10. doi: 10.1016/1056-8719(94)00052-6. [DOI] [PubMed] [Google Scholar]
- 11.Slifkin M, Doyle RJ. Lectins and their application to clinical microbiology. Clin Microbiol Rev. 1990;3:197–218. doi: 10.1128/cmr.3.3.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ogawa T., Watanabe M., Naganuma T., Muramoto K. (2011) Diversified carbohydrate-binding lectins from marine resources. J Amino Acids 2011 [DOI] [PMC free article] [PubMed]
- 13.Reyes-Lopez CA, Hernandez-Santoyo A, Pedraza-Escalona M, Mendoza G, Hernandez-Arana A, Rodriguez-Romero A. Insights into a conformational epitope of Hev b 6.02 (hevein) Biochem Biophys Res Commun. 2004;314:123–130. doi: 10.1016/j.bbrc.2003.12.068. [DOI] [PubMed] [Google Scholar]
- 14.Naismith J.H., Emmerich C., Habash J., Harrop S.J., Helliwell J.R., Hunter W.N., et al. Refined structure of concanavalin A complexed with methyl alpha-D-mannopyranoside at 2.0 A resolution and comparison with the saccharide-free structure. Acta crystallographica. Section D, Biol crystallogr. 1994;50:847–858. doi: 10.1107/S0907444994005287. [DOI] [PubMed] [Google Scholar]
- 15.Ravishankar R, Thomas CJ, Suguna K, Surolia A, Vijayan M. Crystal structures of the peanut lectin-lactose complex at acidic pH: retention of unusual quaternary structure, empty and carbohydrate bound combining sites, molecular mimicry and crystal packing directed by interactions at the combining site. Proteins. 2001;43:260–270. doi: 10.1002/prot.1037. [DOI] [PubMed] [Google Scholar]
- 16.Rutenber E, Katzin BJ, Ernst S, Collins EJ, Mlsna D, Ready MP, et al. Crystallographic refinement of ricin to 2.5 A. Proteins. 1991;10:240–250. doi: 10.1002/prot.340100308. [DOI] [PubMed] [Google Scholar]
- 17.Van Damme E.J.M., Peumans W.J., Pusztai A., Bardocz S. (1998) Handbook of plant lectins: prop biomed appl, Wiley
- 18.Sulak O, Cioci G, Lameignere E, Balloy V, Round A, Gutsche I, et al. Burkholderia cenocepacia BC2L-C is a super lectin with dual specificity and proinflammatory activity. PLoS Pathog. 2011;7:e1002238. doi: 10.1371/journal.ppat.1002238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lam SK, Ng TB. Lectins: production and practical applications. Appl Microbiol Biotechnol. 2011;89:45–55. doi: 10.1007/s00253-010-2892-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Peumans W, Van Damme EJ. Plant lectins: versatile proteins with important perspectives in biotechnology. Biotechnol Genet Eng Rev. 1988;15:199–228. [Google Scholar]
- 21.Van Damme EJM, Peumans WJ, Barre A, Rougé P. Plant lectins: A composite of several distinct families of structurally and evolutionary related proteins with diverse biological roles. Crit Rev Plant Sci. 1998;17:575–692. doi: 10.1080/07352689891304276. [DOI] [Google Scholar]
- 22.Goldstein I.J., Hughes R.C., Monsigny M., Osawa T., Sharon N. (1980) What should be called a lectin?, Published online: 08 May 1980; | doi:10.1038/285066b0 285: 66
- 23.Fang EF, Ng TB. Ribonucleases of different origins with a wide spectrum of medicinal applications. Biochim Biophys Acta. 1815;2011:65–74. doi: 10.1016/j.bbcan.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 24.Końska G, Wójtowicz U, Pituch-Noworolska A. Possible application of lectins in diagnostics and therapy. Part I. Diagnostic application. Przeglad lekarski. 2008;65:189–194. [PubMed] [Google Scholar]
- 25.Sequeira L. Lectins and their role in host-pathogen specificity. Annu Rev Phytopathol. 1978;16:453–481. doi: 10.1146/annurev.py.16.090178.002321. [DOI] [PubMed] [Google Scholar]
- 26.Judd WJ, Issitt PD. The role of lectins in blood group serology. Crit Rev Clin Lab Sci. 1980;12:171–214. doi: 10.3109/10408368009108729. [DOI] [PubMed] [Google Scholar]
- 27.Kilpatrick DC. Mechanisms and assessment of lectin-mediated mitogenesis. Mol Biotechnol. 1999;11:55–65. doi: 10.1007/BF02789176. [DOI] [PubMed] [Google Scholar]
- 28.Ashraf M.T., Khan R.H. Mitogenic lectins. Med Sci Monit. 2003;9:RA265–269. [PubMed] [Google Scholar]
- 29.Sharon N. Lectins: Carbohydrate-specific reagents and biological recognition molecules. J Biol Chem. 2007;282:2753–2764. doi: 10.1074/JBC.X600004200. [DOI] [PubMed] [Google Scholar]
- 30.Singh J, Singh J, Kamboj SS. A novel mitogenic and antiproliferative lectin from a wild cobra lily. Arisaema flavum. Biochem. Biophys. Res. Commun. 2004;318:1057–1065. doi: 10.1016/j.bbrc.2004.04.135. [DOI] [PubMed] [Google Scholar]
- 31.Xia L, Ng TB. A hemagglutinin with mitogenic activity from dark red kidney beans. J Chromatogr B. 2006;844:213–216. doi: 10.1016/j.jchromb.2006.07.042. [DOI] [PubMed] [Google Scholar]
- 32.Ngai PHK, Ng TB. A mushroom (Ganoderma capense) lectin with spectacular thermostability, potent mitogenic activity on splenocytes, and antiproliferative activity toward tumor cells. Biochem Biophys Res Commun. 2004;314:988–993. doi: 10.1016/j.bbrc.2003.12.196. [DOI] [PubMed] [Google Scholar]
- 33.Ho J.C.K., Sze S.C.W., Shen W.Z., Liu W.K. Mitogenic activity of edible mushroom lectins. Biochimica et Biophysica Acta (BBA) General Subjects. 2004;1671:9–17. doi: 10.1016/j.bbagen.2003.12.009. [DOI] [PubMed] [Google Scholar]
- 34.Maciel EVM, Araújo-Filho VS, Nakazawa M, Gomes YM, Coelho LCBB, Correia MTS. Mitogenic activity of Cratylia mollis lectin on human lymphocytes. Biologicals. 2004;32:57–60. doi: 10.1016/j.biologicals.2003.12.001. [DOI] [PubMed] [Google Scholar]
- 35.Pineau N, Aucouturier P, Brugier JC, Preud’homme JL. Jacalin: a lectin mitogenic for human CD4 T lymphocytes. Clin Exp Immunol. 1990;80:420–425. doi: 10.1111/j.1365-2249.1990.tb03304.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sharon N, Lis H. History of lectins: from hemagglutinins to biological recognition molecules. Glycobiology. 2004;14:53R–62R. doi: 10.1093/glycob/cwh122. [DOI] [PubMed] [Google Scholar]
- 37.Patel S, Goyal A. Recent developments in mushrooms as anti-cancer therapeutics: a review. 3. Biotech. 2012;2:1–15. doi: 10.1007/s13205-011-0036-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mody R, Joshi S, Chaney W. Use of lectins as diagnostic and therapeutic tools for cancer. J Pharmacol Toxicol Methods. 1995;33:1–10. doi: 10.1016/1056-8719(94)00052-6. [DOI] [PubMed] [Google Scholar]
- 39.De Mejía EG, Prisecaru VI. Lectins as bioactive plant proteins: a potential in cancer treatment. Crit Rev Food Sci Nutr. 2005;45:425–445. doi: 10.1080/10408390591034445. [DOI] [PubMed] [Google Scholar]
- 40.Fang EF, Pan WL, Wong JH, Chan YS, Ye XJ, Ng TB. A new Phaseolus vulgaris lectin induces selective toxicity on human liver carcinoma Hep G2 cells. Arch Toxicol. 2011;85:1551–1563. doi: 10.1007/s00204-011-0698-x. [DOI] [PubMed] [Google Scholar]
- 41.Ng TB, Ngai PHK, Xia L. An agglutinin with mitogenic and antiproliferative activities from the mushroom Flammulina velutipes. Mycologia. 2006;98:167–171. doi: 10.3852/mycologia.98.2.167. [DOI] [PubMed] [Google Scholar]
- 42.Zhang GQ, Sun J, Wang HX, Ng TB. A novel lectin with antiproliferative activity from the medicinal mushroom Pholiota adiposa. Acta Biochim Pol. 2009;56:415–421. [PubMed] [Google Scholar]
- 43.Pohleven J, Obermajer N, Sabotic J, Anzlovar S, Sepcić K, Kos J, et al. Purification, characterization and cloning of a ricin B-like lectin from mushroom Clitocybe nebularis with antiproliferative activity against human leukemic T cells. Biochim Biophys Acta. 2009;1790:173–181. doi: 10.1016/j.bbagen.2008.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pajic I, Kljajic Z, Dogovic N, Sladic D, Juranic Z, Gasic MJ. A novel lectin from the sponge Haliclona cratera: isolation, characterization and biological activity. Comp Biochem Physiol C: Toxicol Pharmacol. 2002;132:213–221. doi: 10.1016/S1532-0456(02)00068-6. [DOI] [PubMed] [Google Scholar]
- 45.Thies A, Nugel D, Pfüller U, Moll I, Schumacher U. Influence of mistletoe lectins and cytokines induced by them on cell proliferation of human melanoma cells in vitro. Toxicology. 2005;207:105–116. doi: 10.1016/j.tox.2004.09.009. [DOI] [PubMed] [Google Scholar]
- 46.Liu B, Min M-W, Bao J-K. Induction of apoptosis by Concanavalin A and its molecular mechanisms in cancer cells. Autophagy. 2009;5:432–433. doi: 10.4161/auto.5.3.7924. [DOI] [PubMed] [Google Scholar]
- 47.Liu B. Li C.-y., Bian H.-j., Min M.-w., Chen L.-f., Bao J.-k., Antiproliferative activity and apoptosis-inducing mechanism of Concanavalin A on human melanoma A375 cells. Arch Biochem Biophys. 2009;482:1–6. doi: 10.1016/j.abb.2008.12.003. [DOI] [PubMed] [Google Scholar]
- 48.Lin P, Ye X, Ng T. Purification of melibiose-binding lectins from two cultivars of Chinese black soybeans. Acta Biochim Biophys Sin. 2008;40:1029–1038. doi: 10.1111/j.1745-7270.2008.00488.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cheung AHK, Wong JH, Ng TB. Musa acuminata (Del Monte banana) lectin is a fructose-binding lectin with cytokine-inducing activity. Phytomedicine. 2009;16:594–600. doi: 10.1016/j.phymed.2008.12.016. [DOI] [PubMed] [Google Scholar]
- 50.Fang EF, Wong JH, Lin P, Ng TB. Biochemical and functional properties of a lectin purified from korean large black soybeans–a cultivar of glycine max. Protein Pept Lett. 2010;17:690–698. doi: 10.2174/092986610791190309. [DOI] [PubMed] [Google Scholar]
- 51.Fang EF, Zhang CZY, Ng TB, Wong JH, Pan WL, Ye XJ, et al. Momordica Charantia lectin, a type II ribosome inactivating protein, exhibits antitumor activity toward human nasopharyngeal carcinoma cells in vitro and in vivo. Cancer Prev Res (Phila) 2012;5:109–121. doi: 10.1158/1940-6207.CAPR-11-0203. [DOI] [PubMed] [Google Scholar]
- 52.Luo Y, Xu X, Liu J, Li J, Sun Y, Liu Z, et al. A novel mannose-binding tuber lectin from Typhonium divaricatum (L.) Decne (family Araceae) with antiviral activity against HSV-II and anti-proliferative effect on human cancer cell lines. J Biochem Mol Biol. 2007;40:358–367. doi: 10.5483/BMBRep.2007.40.3.358. [DOI] [PubMed] [Google Scholar]
- 53.Li T, Yin X, Liu D, Ma X, Lv H, Sun S. Isolation and characterization of a novel lectin with antifungal and antiproliferative activities from Sophora alopecuroides seeds. Acta Biochim Biophys Sin. 2012;44:606–613. doi: 10.1093/abbs/gms037. [DOI] [PubMed] [Google Scholar]
- 54.de Mejia EG, Bradford T, Hasler C. The anticarcinogenic potential of soybean lectin and lunasin. Nutr Rev. 2003;61:239–246. doi: 10.1301/nr.2003.jul.239-246. [DOI] [PubMed] [Google Scholar]
- 55.Schumacher U, Stamouli A, Adam E, Peddie M, Pfuller U. Biochemical, histochemical and cell biological investigations on the actions of mistletoe lectins I, II and III with human breast cancer cell lines. Glycoconj J. 1995;12:250–257. doi: 10.1007/BF00731327. [DOI] [PubMed] [Google Scholar]
- 56.Pryme IF, Tilrem P. Oral mistletoe lectins: A case for their use in cancer therapy. Cancer Ther. 2007;5:287–300. [Google Scholar]
- 57.Li YR, Liu QH, Wang HX, Ng TB. A novel lectin with potent antitumor, mitogenic and HIV-1 reverse transcriptase inhibitory activities from the edible mushroom Pleurotus citrinopileatus. Biochim Biophys Acta. 2008;1780:51–57. doi: 10.1016/j.bbagen.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 58.Balzarini J. Inhibition of HIV entry by carbohydrate-binding proteins. Antiviral Res. 2006;71:237–247. doi: 10.1016/j.antiviral.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 59.Keyaerts E, Vijgen L, Pannecouque C, Van Damme E, Peumans W, Egberink H, et al. Plant lectins are potent inhibitors of coronaviruses by interfering with two targets in the viral replication cycle. Antiviral Res. 2007;75:179–187. doi: 10.1016/j.antiviral.2007.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ziółkowska NE, Wlodawer A. others, Structural studies of algal lectins with anti-HIV activity. Acta Biochim Pol. 2006;53:617–626. [PubMed] [Google Scholar]
- 61.Boyd MR, Gustafson KR, McMahon JB, Shoemaker RH, O’Keefe BR, Mori T, et al. Discovery of cyanovirin-N, a novel human immunodeficiency virus-inactivating protein that binds viral surface envelope glycoprotein gp120: potential applications to microbicide development. Antimicrob Agents Chemother. 1997;41:1521–1530. doi: 10.1128/aac.41.7.1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Barrientos LG, Lasala F, Otero JR, Sanchez A, Delgado R. In vitro evaluation of cyanovirin-N antiviral activity, by use of lentiviral vectors pseudotyped with filovirus envelope glycoproteins. J Infect Dis. 2004;189:1440–1443. doi: 10.1086/382658. [DOI] [PubMed] [Google Scholar]
- 63.O’Keefe BR, Smee DF, Turpin JA, Saucedo CJ, Gustafson KR, Mori T, et al. Potent anti-influenza activity of cyanovirin-N and interactions with viral hemagglutinin. Antimicrob Agents Chemother. 2003;47:2518–2525. doi: 10.1128/AAC.47.8.2518-2525.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kaur A, Singh R. Singh Kamboj S., Singh J., J. Kotwal G., In vitro antiviral activity of Crinum latifolium lectin against poxvirus replication. Journal of Biological Sciences. 2008;8:1236–1240. doi: 10.3923/jbs.2008.1236.1240. [DOI] [Google Scholar]
- 65.Paiva P.M.G., Gomes F.S., Napole ∼ ao T.H., S\’a R.A., Correia M.T.S., Coelho L. (2010) Antimicrobial activity of secondary metabolites and lectins from plants. Curr Res Technol Educ Top Appl Microbiol Microb Biotechnol Badajoz: Formatex 396–406
- 66.Khatun S., Khan M.M.H., Ashraduzzaman M., Pervin F., Bari L., Absar N. Antibacterial Activity and Cytotoxicity of Three Lectins Purified from Drumstick (Moringa oleifera Lam.) Leaves. J Bio-Sci. 2009;17:89–94. [Google Scholar]
- 67.Oliveira M.d.l., Andrade C.a.s., Santos-Magalhães N.s., Teixeira J.a., Carneiro-da-Cunha M.g., et al. Purification of a lectin from Eugenia uniflora L. seeds and its potential antibacterial activity. Lett Appl Microbiol. 2008;46:371–376. doi: 10.1111/j.1472-765X.2007.02319.x. [DOI] [PubMed] [Google Scholar]
- 68.Charungchitrak S, Petsom A, Sangvanich P, Karnchanatat A. Antifungal and antibacterial activities of lectin from the seeds of Archidendron jiringa Nielsen. Food Chem. 2011;126:1025–1032. doi: 10.1016/j.foodchem.2010.11.114. [DOI] [Google Scholar]
- 69.Sitohy M, Doheim M, Badr H. Isolation and characterization of a lectin with antifungal activity from Egyptian Pisum sativum seeds. Food Chem. 2007;104:971–979. doi: 10.1016/j.foodchem.2007.01.026. [DOI] [Google Scholar]
- 70.Ngai PHK, Ng TB. A lectin with antifungal and mitogenic activities from red cluster pepper (Capsicum frutescens) seeds. Appl Microbiol Biotechnol. 2006;74:366–371. doi: 10.1007/s00253-006-0685-y. [DOI] [PubMed] [Google Scholar]
- 71.Ye XY, Ng TB, Tsang PW, Wang J. Isolation of a homodimeric lectin with antifungal and antiviral activities from red kidney bean (Phaseolus vulgaris) seeds. J Protein Chem. 2001;20:367–375. doi: 10.1023/A:1012276619686. [DOI] [PubMed] [Google Scholar]
- 72.Lam Sze K., Han Qi F., Ng Tzi B. Isolation and characterization of a lectin with potentially exploitable activities from caper (Capparis spinosa) seeds. Biosci Rep. 2009;29:293–299. doi: 10.1042/BSR20080110. [DOI] [PubMed] [Google Scholar]
- 73.Peumans W.J., Van Damme E.J. (1995) Lectins as plant defense proteins. Plant physiology 109 [DOI] [PMC free article] [PubMed]
- 74.Wong JH, Ng TB, Cheung RCF, Ye XJ, Wang HX, Lam SK, et al. Proteins with antifungal properties and other medicinal applications from plants and mushrooms. Appl Microbiol Biotechnol. 2010;87:1221–1235. doi: 10.1007/s00253-010-2690-4. [DOI] [PubMed] [Google Scholar]
- 75.Lis H, Sharon N. Lectins: Carbohydrate-Specific Proteins That Mediate Cellular Recognition. Chem Rev. 1998;98:637–674. doi: 10.1021/cr940413g. [DOI] [PubMed] [Google Scholar]
- 76.Naisbett B, Woodley J. The potential use of tomato lectin for oral drug delivery. 1. Lectin binding to rat small intestine in vitro. Int J Pharm. 1994;107:223–230. doi: 10.1016/0378-5173(94)90438-3. [DOI] [Google Scholar]
- 77.Bies C, Lehr C-M, Woodley JF. Lectin-mediated drug targeting: history and applications. Adv Drug Deliv Rev. 2004;56:425–435. doi: 10.1016/j.addr.2003.10.030. [DOI] [PubMed] [Google Scholar]
- 78.Nicholls TJ, Green KL, Rogers DJ, Cook JD, Wolowacz S, Smart JD. Lectins in ocular drug delivery: An investigation of lectin binding sites on the corneal and conjunctival surfaces. Int J Pharm. 1996;138:175–183. doi: 10.1016/0378-5173(96)04540-1. [DOI] [Google Scholar]
- 79.Fischer D, Kissel T. Histochemical characterization of primary capillary endothelial cells from porcine brains using monoclonal antibodies and fluorescein isothiocyanate-labelled lectins: implications for drug delivery. Eur J Pharm Biopharm. 2001;52:1–11. doi: 10.1016/S0939-6411(01)00159-X. [DOI] [PubMed] [Google Scholar]
- 80.Cai Q, Zhang Z-R. Lectin-mediated cytotoxicity and specificity of 5-fluorouracil conjugated with peanut agglutinin (5-Fu-PNA) in vitro. J Drug Target. 2005;13:251–257. doi: 10.1080/10611860500138505. [DOI] [PubMed] [Google Scholar]
- 81.Lehr CM, Bouwstra JA, Kok W, Noach AB, de Boer AG, Junginger HE. Bioadhesion by means of specific binding of tomato lectin. Pharm Res. 1992;9:547–553. doi: 10.1023/A:1015804816582. [DOI] [PubMed] [Google Scholar]
- 82.Liu Y, Wang P, Sun C, Zhao J, Du Y, Shi F, et al. Bioadhesion and enhanced bioavailability by wheat germ agglutinin-grafted lipid nanoparticles for oral delivery of poorly water-soluble drug bufalin. Int J Pharm. 2011;419:260–265. doi: 10.1016/j.ijpharm.2011.07.019. [DOI] [PubMed] [Google Scholar]
- 83.Lehr CM. Lectin-mediated drug delivery: the second generation of bioadhesives. Journal of controlled release: official journal of the Controlled Release Society. 2000;65:19–29. doi: 10.1016/S0168-3659(99)00228-X. [DOI] [PubMed] [Google Scholar]


