Abstract
Background
Lauric acid (LA), a common constituent of coconut oil, is used as food additives and supplements in various formulations. Despite various potential pharmacological properties, no scientific evidence on its dose-related toxicity and safety is available till date.
Objective
The current study was conducted to evaluate acute oral toxicity of LA on normal rats.
Methods
The study was conducted in accordance with the Organization for Economic Co-operation and Development guidelines (OECD 423) with slight modifications. LA was administered orally to female Sprague Dawley (SD) rats (n = 6/group) at a single dose of 300 and 2,000 mg/kg body weight, respectively, while normal control received vehicle only. Animals from all the three groups were monitored for any behavioural and toxicological changes and mortality for two weeks. Food and fluid consumption, body weight was monitored on daily basis. At the end (on day 15th) of the experimental period, blood was collected for haematological and biochemical analysis. Further, all the animals were euthanized, and internal organs were harvested for histopathological investigation using four different stainings; haematoxylin and eosin, Masson trichrome, Periodic Acid Schiff and Picro Sirius Red for gross pathology through microscopical observation.
Results
The study results showed no LA treatment-related mortality and morbidity at two different dosages. Daily food and water consumption, body weight, relative organ weight, haematological, and biochemical analysis were observed to be normal with no severe alterations to the internal tissues.
Conclusion
The current finding suggests that single oral administration of LA, even up to 2,000 mg/kg body weight, did not exhibit any signs of toxicity in SD rats; thus, it was safe to be used on disease models in animals.
Keywords: Lauric acid, Acute oral toxicity, Haematological parameters, Biochemical parameters, Histopathology, Relative organ weight, Food and water intake, H&E staining, Masson trichrome staining
Introduction
Plant-based products are considered as the primary source for food additives and ingredients in various natural products. Natural substances are added in the food to enhance its flavour, taste and appearance. Various plant additives are being used for centuries to preserve various health care products and supplements (Amen et al., 2019; Arya et al., 2012a). According to the World Health Organization (WHO) recommendation, food additives must pass through the safety screen prior to use in any products. Additives in the form of crude plant or extracts are needed to ensure processed food remains safe, and in good condition, a variety of plants extracts contain specific phytochemicals whose biological activities is not known. It is possible that some unwanted phytocompounds might lead to toxic effects or effects products preserving capacity, stability and even the nutritional value of the products (World Health Organization, 2015; Yakob, Uyub & Sulaiman, 2012). However, chemical characterization, proper dosing regimen, and insufficient knowledge of the toxicity data constraint limit the usage of additives and herbal medicine (Deng et al., 2013). Thus, it is the current need to evaluate the possible toxicity of the plant extract and phytocompounds before its therapeutic use.
Lauric acid (LA) is the primary (>50%) composition of coconut oil (Sheela et al., 2016). It is a medium-chain fatty acid (MCFA) found abundantly in the coconut oil, palm kernel oil and laurel oil, which constitutes about half of the fatty acid (Beare-Rogers, Dieffenbacher & Holm, 2001; Ubgogu, Onyeagba & Chigbu, 2006). It is also found in various kinds of foods, such as fruits, seeds, and breast milk (Alves et al., 2017; Silberstein et al., 2013; Uday Kumar et al., 2014). It possesses pleiotropic biological activities, including potent anti-microbial effects (Dayrit, 2015), beneficial effect on the cardiovascular system (CVS) by increasing high-density lipoproteins (HDL) and reducing blood pressure and heart rate in hypertensive rats (Ahola et al., 2017). Moreover, it also prevents testosterone-induced prostatic hyperplasia (Babu et al., 2010), and promotes apoptosis in breast and cancerous endometrial cells (Lappano et al., 2017).
Despite various pharmacological properties, scientific evidence on the toxicological and safety profile of LA is not available. This is the first study aimed at investigating the acute toxicity of LA following the Organization of Economic Cooperation and Development (OECD 423) guideline in female Sprague Dawley (SD) rats with slight modifications. In this study, we evaluated numerous parameters, like body weight, food and water intake, haematology, biochemistry, and the histopathological changes in various tissues as shown in Fig. 1.
Figure 1. Study design.
Materials & Methods
Ethical consideration
The acute toxicity study of LA was accomplished in accordance with the guidelines stipulated by the Institutional Animal Care and Use Committee (IACUC), Faculty of Medicine, University of Malaya, Kuala Lumpur, Malaysia with animal ethics approval no: 2018-210605/TAY/R/AA (2018295).
Reagents
Lauric acid (MKCF8837, purity more than 98%) Sigma-Aldrich (St Louis, MO 63103 USA 314-77-5765), Tween-20 (C01-TW121-00, Systerm, UK), normal saline 0.9% (B Braun),10% neutral buffered formalin solution pH 7.4 (R&M, UK), Haematoxylin (Merck, USA) and Eosin (Systerm, UK), Masson trichrome (Abcam150686), Periodic Acid Schiff (Abcam150680) and Picro Sirius Red stain kit (Abcam 150681).
All other chemicals and solvents were of analytical grade.
Experimental animals
In this study, eighteen (18) healthy specific pathogens-free (SPF) female SD rats, weighing 240–250 gram (g), age 8–10 weeks old were procured from the Animal Experimental Unit (AEU), Faculty of Medicine, University of Malaya. The AEU facility at the University of Malaya is certified by the Association for Assessment and Accreditation of Laboratory Animal Care International (AAALAC International). All the animals were randomly segregated in three different groups (six animals in each group) and housed in polypropylene cages for seven days to acclimatise laboratory environment prior to the experiments. A consistent environment of 12-hour light/dark cycle, temperature 22 ± 2 °C, and humidity of 50 ± 10% were maintained. The animals were allowed a standard rodent pellet diet and reverse osmosis (RO) water ad libitum throughout the experimental period.
Acute oral toxicity test
Acute toxicity study was conducted to assess whether LA produce any mortality or other adverse reactions upon oral administration of dose (300 and 2,000 mg/kg body weight), according to guideline 423 provided by the OECD for the acute toxicity class method procedure (OECD, 2001) with minor amendments (OECD, 2002). Before the experiment, all the animals were carefully weighed, marked, and grouped accordingly:
Group I (n = 6): Vehicle (Tween 20/normal saline)-treated
Group II (n = 6): Lauric acid-treated, dose 300 mg/kg body weight
Group III (n = 6): Lauric acid-treated, dose 2,000 mg/kg body weight.
The LA was dissolved in Tween 20 and diluted with normal saline 0.9% to get the desired dose (Alves et al., 2017). Animals fasted overnight before dosing, and treatment groups received freshly prepared single doses of LA suspension at 300 and 2,000 mg/kg body weight, while animals in the control group received Tween 20 or normal saline 0.9% suspension equally based on individual body weight. Each gavage volume was 10 mL/kg body weight. All efforts were made to lessen experimental animals’ suffering, especially in handling and oral gavage.
Daily observations
After oral gavage, diet was withheld except water for 2–3 h while animals were monitored individually with special attention in the first half-hour till completion of first 24 h followed by daily cage-side examination for fourteen days for any signs of toxicity including general behavioural in skin, eyes, touch, activeness, and movement changes. All the changes observed were well documented before and after the gavage.
Body weight, food, and water consumption
The body weight of the individual animal was carefully noted before and after oral administration of the LA or vehicle, then weekly and lastly on day fifteen (euthanized day). Daily food (g/day), and water (ml/ day) intake was noted on daily basis in all the three groups for fourteen days.
Haematology and biochemical analysis
On day fourteen, all the animals fasted overnight for 12 h, only water was allowed. All the animals were euthanized in the morning by an intraperitoneal (IP) injection of ketamine (80 mg/kg) & xylazine (7 mg/kg), and blood was collected via micro-capillary tubes from the retro-orbital plexus region into two different sets of test tubes, one containing anticoagulant, ethylene diaminotetraacetic acid (EDTA) for haematological tests including red blood cells (RBC), white blood cells (WBC), platelets count, haemoglobin (Hb), packed cell volumes (PCV), mean corpuscular volume (MCV), mean corpuscular haemoglobin (MCH), mean corpuscular haemoglobin concentration (MCHC), neutrophil, lymphocytes, monocytes, and eosinophils and the other without additives for clinical biochemistry tests like urea, creatinine, calcium, inorganic phosphate, uric acid, sodium, potassium, chloride, total cholesterol (TC), high-density lipoproteins (HDL), low-density lipoproteins (LDL), triglycerides (TG), total (HDL) ratio, albumin, globulin, albumin-globulin (A/G) ratio, ALP, AST and ALT levels. The collected blood samples were then sent to an ISO-certified blood-testing laboratory.
Measurement of relative organ weight
Internal organs such as adrenal glands, brain, eyes, heart, kidneys, liver, lungs, ovaries, spleen, stomach, urinary bladder, uterus, and cervix/vagina were isolated through a fine incision in the abdomen at the midline of the euthanized animals. All the harvested tissues were cleaned with phosphate buffer saline (PBS), wiped with clean blotting paper and weighed on a calibrated weighing balance. Relative organ weight (ROW) of all the animals was noted in proportion to body weight according to the following equation:
ROW =
Histopathological analysis
Histopathological examination was conducted on the harvested liver, kidney, heart, lungs, brain, spleen, and pancreas in all the animals. Tissues were preserved in 10% neutral, buffered formalin solution for 48 h at 4 °C for histopathological study. Paraffin-embedded specimens of selected tissues were cut into 4–5 µm thick samples and stained with haematoxylin and eosin (H&E), Masson trichrome, Periodic Acid Schiff (PAS) and Picro Sirius Red staining as per the manufacturer protocol detailed in the kits. Later, all the stained tissues were sectioned and observed under the fluorescence compound microscope (40X) (Eclipse Ni-U, Nikon Corporation, Japan).
Statistical analysis
All statistical results were expressed as the mean ± standard deviation (SD). The comparison between the treated and control group was performed by a one-way analysis of variance (ANOVA) using statistical software, SPSS package version 22 (IBM, SPSS Statistics, Inc., Chicago, IL, USA) to evaluate the significant differences. The (p < 0.05) compared to the control group was considered statistically significant.
Results
Acute toxicological evaluation and daily observation
Animals in LA (300 and 2,000 mg/kg body weight) treated group were normal and healthy during fourteen days study period. No signs of mortality or sudden death were observed compared to the untreated group animals. No significant changes in the temperature, skin, eye colour, food and water intake were observed. Furthermore, no behavioural changes or signs of diarrhoea, coma, sedation and tremors were noted as compared to the control group Table 1. Experimental results suggest that LD50 of LA should be higher than 2,000 mg/kg body weight.
Table 1. The behavioural and general response of animals after oral administration of LA.
| Observation | Control | LA 300 mg/kg | LA 2,000 mg/kg |
|---|---|---|---|
| Temperature | n | n | n |
| Skin changes | no | no | no |
| Change eyes colour | no | no | no |
| General physique | n | n | n |
| Diarrhoea | no | no | no |
| Coma | no | no | no |
| Drowsiness | no | no | no |
| Breathing difficulty | no | no | no |
| Sedation | no | no | no |
| Tremor | no | no | no |
| Death | no | no | no |
Notes.
- n
- normal
- no
- not observed
Effect of LA on body weight, food and water consumption
Body weight, food & water intake in the LA treated groups (300 and 2,000 mg/kg body weight) were noted to be non-significant compared to the control group. However, LA treated groups showed slight changes in the body weight, food and water consumption on day fourteen, compared to the untreated group, as shown in Tables 2 and 3. The mean body weight of the control group was noted as 244.71 ± 3.13 g, whereas treatment groups showed 247.53 ± 2.96, and 240.63 ± 4.76 g, respectively. On day fourteen, the mean body weight gained by the control group was 14.81 ± 0.064 g, while LA treated animals (300 and 2,000 mg/kg body weight) displayed non-significant gain in the body weight (14.93 ± 0.68 and 15.36 ± 0.76 g), respectively. The percentage mean in the body weight of all the treated animals was non-significant in comparison to the control group (p > 0.05), as shown in Table 2.
Table 2. Effect of LA on body weight (g) assessment.
| Week | Control | LA 300 mg/kg | LA 2,000 mg/kg | F-value | P-value |
|---|---|---|---|---|---|
| Day 0 | 244.71 ± 3.13 | 247.53 ± 2.96 | 240.63 ± 4.76 | 1.464 | 0.263 |
| Week 1% | 7.64 ± 0.46 | 7.52 ± 0.22 | 7.87 ± 0.35 | 0.149 | 0.863 |
| Week 2% | 14.81 ± 0.064 | 14.93 ± 0.68 | 15.36 ± 0.76 | 0.113 | 0.894 |
Notes.
Values are expressed as the mean standard deviation (n = 6 for each group, female). One-way ANOVA (*p < 0.05) significant value.
Table 3. Effect of LA on food and water consumption.
| Week | Control | LA 300 mg/kg | LA 2,000 mg/kg | F-value | P-value |
|---|---|---|---|---|---|
| Food (g) | |||||
| Week 1st | 136.35 ± 5.55 | 150.38 ± 19.65 | 133.84 ± 9.18 | 0.265 | 0.570 |
| Week 2nd | 139.50 ± 13.13 | 123.07 ± 54.79 | 142.61 ± 12.82 | ||
| Water (ml) | |||||
| Week 1st | 204.28 ± 23.17 | 211.42 ± 31.58 | 200.71 ± 67.23 | 2.609 | 0.071 |
| Week 2nd | 184.28 ± 13.36 | 172.85 ± 7.55 | 187.14 ± 14.67 | ||
Notes.
Values are expressed as the mean ± standard deviation (n = 6 for each group, female). One-way ANOVA (*p < 0.05) significant value.
Moreover, LA administration (300 and 2,000 mg/kg) showed non-significant changes (p > 0.05) in the food and water consumption, compared to the control group, as shown in Table 3.
Effect of LA on relative organ weight
The relative organ weight of the harvested tissues—adrenal glands, brain, eyes, heart, kidney, liver, lungs, ovaries, spleen, stomach, urinary bladder, uterus and cervix/vagina, revealed non-significant changes post-LA treatment (300 and 2,000 mg/kg body weight) compared with the control group animals (p > 0.05), as shown in Table 4.
Table 4. Effect of LA on relative organ weight (g).
| Organ | Control | LA 300 mg/kg | LA 2,000 mg/kg | F-value | P-value |
|---|---|---|---|---|---|
| Adrenal gland (left) | 0.018 ± 0.001 | 0.019 ± 0.002 | 0.019 ± 0.002 | 0.638 | 0.698 |
| Adrenal gland (right) | 0.020 ± 0.004 | 0.018 ± 0.003 | 0.019 ± 0.001 | 0.537 | 0.595 |
| Brain | 0.643 ± 0.032 | 0.626 ± 0.069 | 0.632 ± 0.051 | 0.162 | 0.852 |
| Eye (left) | 0.041 ± 0.002 | 0.042 ± 0.003 | 0.044 ± 0.003 | 1.632 | 0.228 |
| Eye (right) | 0.043 ± 0.001 | 0.043 ± 0.002 | 0.045 ± 0.004 | 1.218 | 0.323 |
| Heart | 0.277 ± 0.016 | 0.290 ± 0.019 | 0.286 ± 0.012 | 0.960 | 0.405 |
| Kidney (left) | 0.285 ± 0.012 | 0.300 ± 0.024 | 0.280 ± 0.025 | 1.480 | 0.259 |
| Kidney (right) | 0.288 ± 0.014 | 0.297 ± 0.020 | 0.275 ± 0.013 | 2.640 | 0.104 |
| Liver | 2.981 ± 0.254 | 2.918 ± 0.367 | 2.931 ± 0.344 | 0.062 | 0.940 |
| Lungs | 0.476 ± 0.038 | 0.469 ± 0.028 | 0.502 ± 0.029 | 1.693 | 0.217 |
| Ovary (left) | 0.032 ± 0.008 | 0.031 ± 0.005 | 0.033 ± 0.010 | 0.062 | 0.940 |
| Ovary (right) | 0.033 ± 0.005 | 0.033 ± 0.009 | 0.033 ± 0.006 | 0.003 | 0.997 |
| Pancreas | 0.292 ± 0.043 | 0.359 ± 0.074 | 0.306 ± 0.056 | 2.117 | 0.115 |
| Spleen | 0.220 ± 0.018 | 0.209 ± 0.027 | 0.195 ± 0.013 | 2.284 | 0.136 |
| Stomach | 0.754 ± 0.111 | 0.762 ± 0.184 | 0.939 ± 0.238 | 1.890 | 0.185 |
| Urinary bladder | 0.037 ± 0.003 | 0.041 ± 0.007 | 0.037 ± 0.004 | 0.922 | 0.419 |
| Uterus | 0.398 ± 0.054 | 0.445 ± 0.211 | 0.258 ± 0.315 | 2.579 | 0.109 |
| Cervix/Vagina | 0.081 ± 0.017 | 0.075 ± 0.011 | 0.074 ± 0.008 | 0.445 | 0.445 |
Notes.
Values are expressed as the mean ± standard deviation (n = 6 for each group, female). One-way ANOVA (*p < 0.05) significant value.
Effect of LA on haematology and clinical biochemistry
The effect on haematological analysis is summarised in Table 5. Parameters including RBC, WBC, platelets count, Hb, PCV, MCV, MCH, MCHC, neutrophil, lymphocytes, monocytes, and eosinophils shows non-significant changes (p > 0.05) after oral administration of LA (300 and 2,000 mg/kg body weight) when compared with the control animals. Importantly, all the values were within the reference range, compared to the control group.
Table 5. Effect of LA on haematology.
| Parameters | Control | LA 300 mg/kg | LA 2,000 mg/kg | F-value | P-value |
|---|---|---|---|---|---|
| RBCs (1012/L) | 7.350 ± 0.187 | 7.616 ± 0.584 | 7.666 ± 0.216 | 1.232 | 0.320 |
| WBCs (109/L) | 3.883 ± 0.491 | 3.866 ± 1.237 | 3.533 ± 0.960 | 0.163 | 0.851 |
| Platelets (109/L) | 953.333 ± 68.677 | 865.000 ± 356.083 | 846.666 ± 154.749 | 1.091 | 0.361 |
| Hb (g/dl) | 13.716 ± 0.183 | 13.783 ± 0.523 | 13.833 ± 0.265 | 0.553 | 0.587 |
| PCV (HCT%) | 44.666 ± 1.210 | 36.833 ± 18.082 | 44.500 ± 1.378 | 0.393 | 0.682 |
| MCV fL | 52.333 ± 16.366 | 57.500 ± 2.345 | 57.333 ± 2.732 | 0.259 | 0.775 |
| MCH pg | 18.000 ± 0.894 | 17.666 ± 1.211 | 17.500 ± 0.836 | 0.377 | 0.692 |
| MCHC g/dL | 31.333 ± 0.816 | 31.500 ± 0.836 | 31.166 ± 0.752 | 0.260 | 0.775 |
| Neutrophil (%) | 24.000 ± 6.131 | 25.500 ± 7.582 | 32.500 ± 5.319 | 3.002 | 0.080 |
| Lymphocyte (%) | 64.000 ± 5.966 | 67.666 ± 7.004 | 61.833 ± 4.215 | 1.528 | 0.249 |
| Monocytes (%) | 4.333 ± 1.632 | 3.500 ± 1.760 | 3.500 ± 1.643 | 0.492 | 0.621 |
| Eosinophils (%) | 2.500 ± 0.547 | 2.333 ± 0.516 | 2.33 ± 0.516 | 0.200 | 0.821 |
Notes.
Values are expressed as the mean standard deviation (n = 6 for each group, female). One-way ANOVA (*p < 0.05) significant value.
Similarly, the biochemical analysis of liver and renal function tests, including urea, creatinine, calcium, inorganic phosphate, uric acid, sodium, potassium, chloride, TC, HDL, LDL, TG, total HDL ratio, albumin, globulin, A/G ratio, ALP, AST and ALT levels for LA treatment groups were not affected and observed to be normal (non-significant (p > 0.05)), when compared with the control group as presented in Table 6.
Table 6. Effect of LA on clinical biochemistry.
| Clinical biochemistry | Control | 300 mg/kg | 2,000 mg/kg | F-value | P-value |
|---|---|---|---|---|---|
| Urea (mmol/L) | 6.633 ± 1.040 | 6.966 ± 0.977 | 8.200 ± 1.240 | 3.427 | 0.059 |
| Creatinine (Umol/L) | 36.000 ± 4.898 | 28.833 ± 13.044 | 37.000 ± 5.656 | 1.579 | 0.239 |
| Calcium (mmol/L | 2.530 ± 0.062 | 2.500 ± 0.109 | 2.403 ± 0.095 | 3.126 | 0.073 |
| Inorganic Phosphate (mmol/L) | 3.195 ± 0.350 | 3.401 ± 0.642 | 3.900 ± 0.536 | 2.871 | 0.088 |
| Uric Acid (mmol/L) | 0.211 ± 0.290 | 0.106 ± 0.048 | 0.098 ± 0.041 | 0.813 | 0.462 |
| Sodium (mmol/L) | 145.00 ± 2.097 | 145.833 ± 1.471 | 145.333 ± 2.338 | 0.263 | 0.772 |
| Potassium (mmol/L) | 5.566 ± 0.163 | 5.900 ± 0.433 | 5.616 ± 0.248 | 2.105 | 0.156 |
| Chloride(mmol/L) | 98.500 ± 1.048 | 99.000 ± 2.449 | 100.500 ± 2.073 | 1.711 | 0.214 |
| TC (mmol/L) | 2.033 ± 0.436 | 2.300 ± 0.517 | 1.966 ± 0.307 | 1.012 | 0.387 |
| HDL(mmol/L) | 0.623 ± 0.082 | 0.640 ± 0.089 | 0.590 ± 0.098 | 0.474 | 0.632 |
| LDL (mmol/L) | 0.868 ± 0.198 | 0.933 ± 0.377 | 0.733 ± 0.398 | 0.550 | 0.588 |
| TG (Umol/L) | 1.783 ± 0.231 | 1.483 ± 0.487 | 1.116 ± 0.636 | 2.879 | 0.087 |
| Total HDL Ratio | 3.383 ± 0.231 | 3.516 ± 0.444 | 3.400 ± 0.236 | 0.309 | 0.739 |
| Total protein (g/dL) | 70.833 ± 2.926 | 71.500 ± 3.507 | 66.833 ± 3.816 | 3.236 | 0.068 |
| Albumin (g/dL) | 42.166 ± 4.445 | 44.500 ± 4.888 | 43.333 ± 3.076 | 0.461 | 0.639 |
| Globulin(g/dL) | 24.333 ± 1.211 | 25.333 ± 1.211 | 23.666 ± 0.816 | 3.519 | 0.056 |
| A/G ratio | 1.800 ± 0.894 | 1.800 ± 0.109 | 1.833 ± 0.121 | 0.192 | 0.827 |
| AP (IU/L) | 53.666 ± 5.750 | 57.166 ± 11.669 | 68.33 ± 25.453 | 1.293 | 0.303 |
| SGOT /AST (IU/L) | 165.166 ± 10.703 | 200.166 ± 35.204 | 174.33 ± 18.250 | 3.315 | 0.056 |
| SGPT /ALT(IU/L) | 36.666 ± 1.966 | 36.000 ± 3.741 | 40.500 ± 19.065 | 0.278 | 0.761 |
Notes.
Values are expressed as the mean ± standard deviation (n = 6 for each group, female). One-way ANOVA (*p < 0.05) significant value.
Effects of LA on Histopathology of internal organs
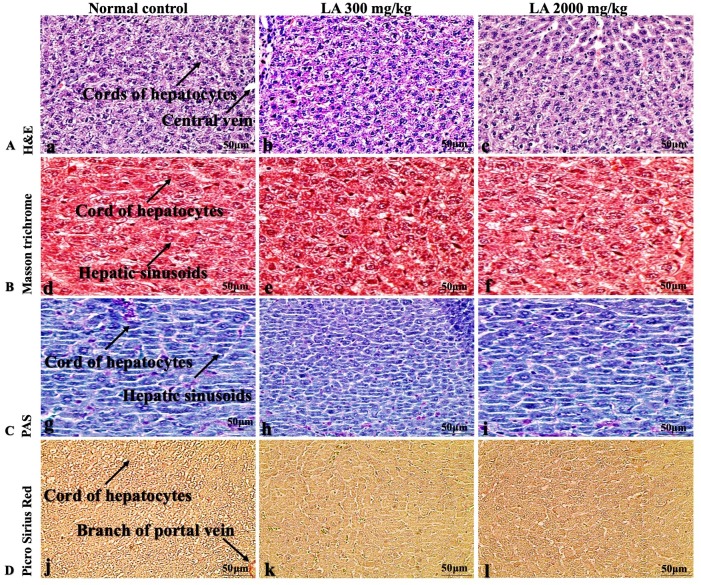
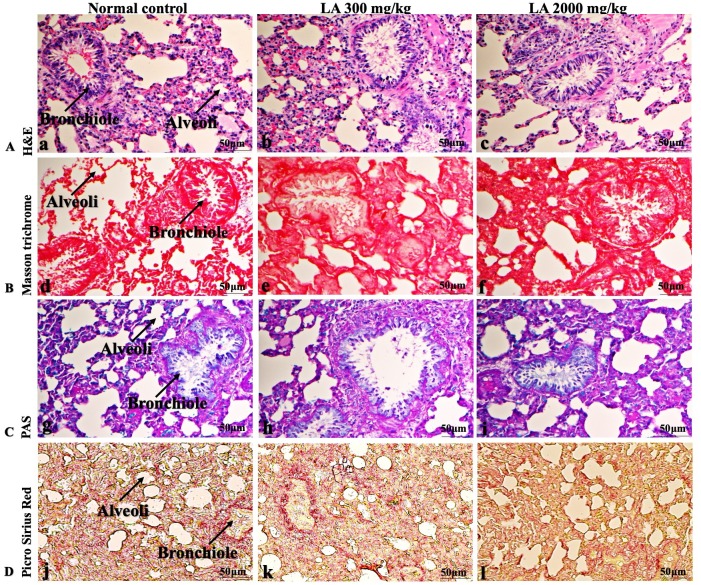
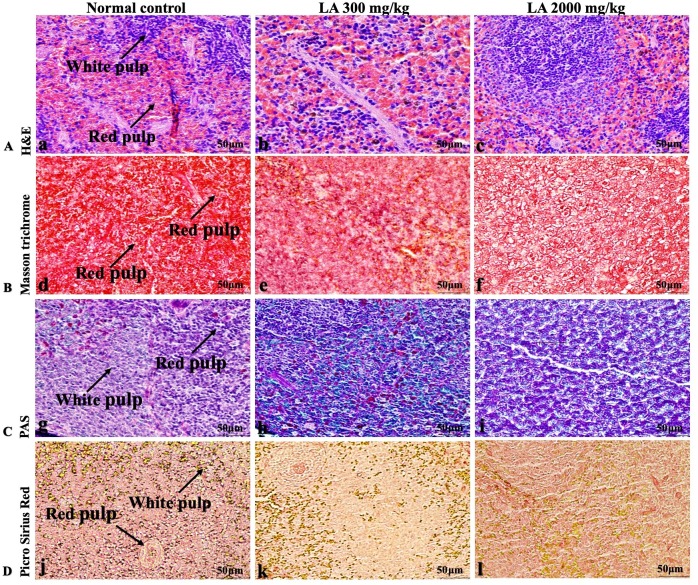
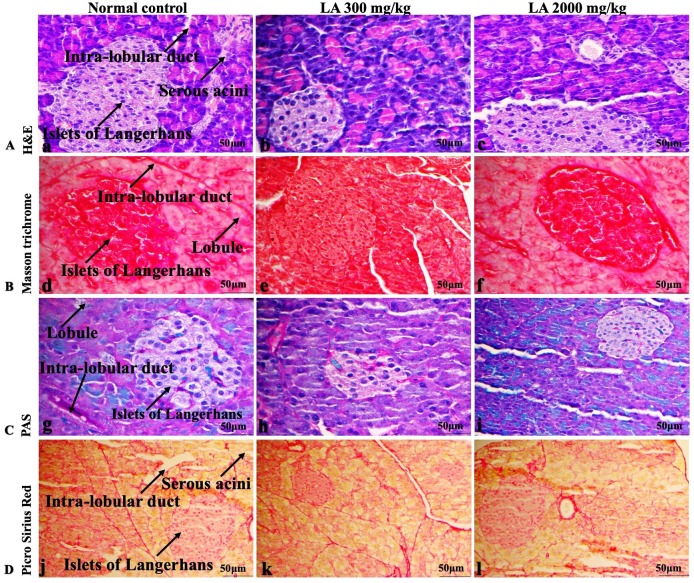
The histopathological analysis involves macroscopic and microscopic examination of processed and fixed tissues to visualise various components and structural alterations in the tissues (Greaves, 2011; Khoo et al., 2018). H&E staining is the most common and widely used technique to essentially identify morphological changes during diagnosis and screening of any malignancies in the tissues (Fischer et al., 2008). However, Masson trichrome staining remains reliable for the cytoplasm, which gives in-depth and selective detail about the connective tissue (Tsounapi et al., 2017). PAS staining assesses the changes in glycogen content within muscle fibre sections (Arya et al., 2015; Fairchild & Fournier, 2004). Moreover, the collagen content is analysed with the help of Picro Sirius Red staining, which provides a clear understanding of collagen accumulation in the tissues (Lattouf et al., 2014).
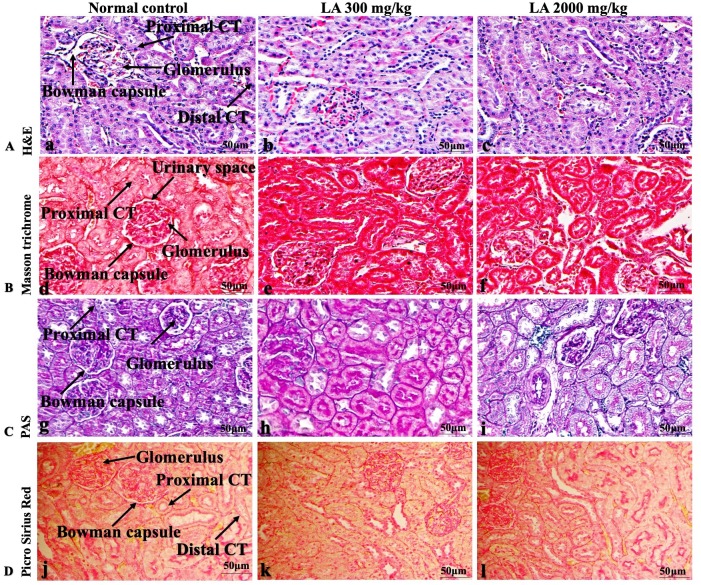
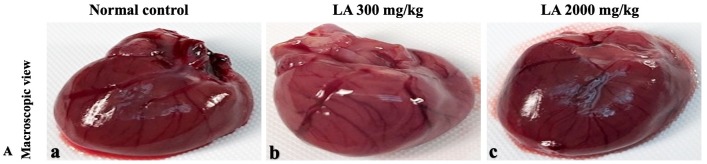
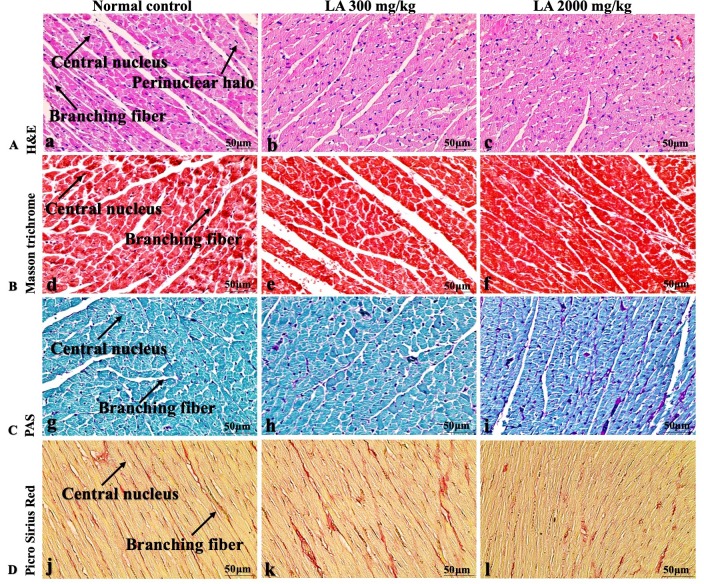
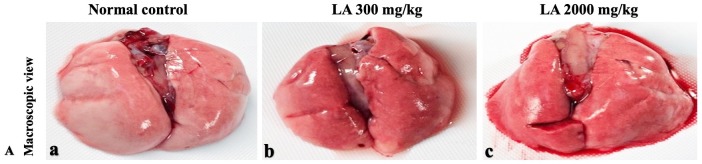
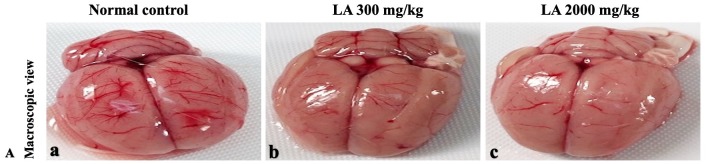
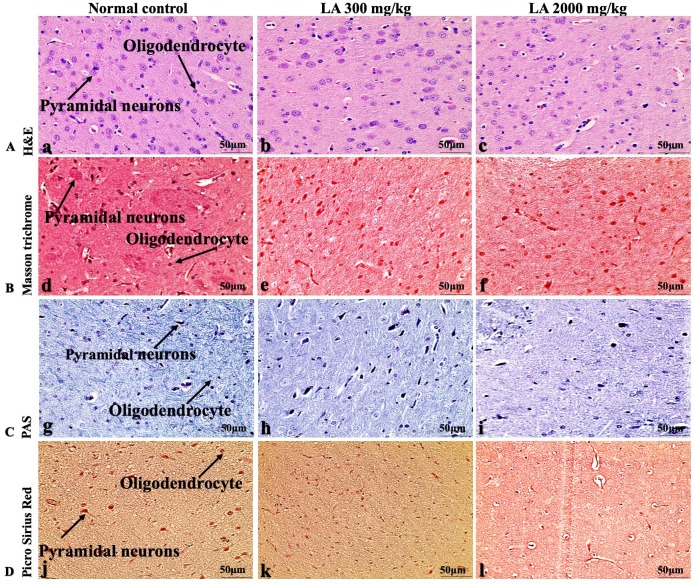
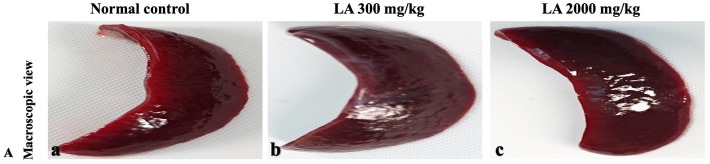
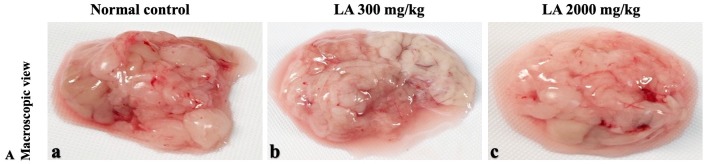
In brief, macroscopic analysis of liver sections (Fig. 2) in LA-treated rats (300 and 2,000 mg/kg body weight) displays no alterations in the colour or texture, importantly no changes to hepatocytes, hepatic sinusoids, central vein, and portal triad were observed when compared with the control group (Fig. 3). Kidney showed no structural degeneration, and colour changes in the renal cortex and tubules, without any tubular necrosis and inflammation in LA-treated animals compared to untreated animals, as displayed (Figs. 4 and 5). The photomicrographs of the heart (Figs. 6 and 7) tissue showed a normal size of myocardial fibre and filaments when compared with the control group. Lung tissues (Figs. 8 and 9) appeared to be normal, with no abnormal changes noted in colour. Moreover, microscopic observation displayed thin inter-alveolar septa, clear alveoli and alveolar sacs with the absence of inflammatory cells, including neutrophils, or thickening of alveolar septa, when compared with the untreated group. Furthermore, brain (Figs. 10 and 11) tissues showed normal morphology with the regular shape of the pyramidal neuron and uniform distribution of glial cells in LA-treated and control animals. Macroscopic texture (Figs. 12 and 13) exhibited normal and regular appearance with white and red pulp without any sign of apoptotic, necrotic lesions or megakaryocytosis compared with the control group. Microscopic appearance of the pancreas (Figs. 14 and 15) appeared with healthy acinar, clear round or oval-shaped Islets cells in cluster form, with lightly stained cytoplasm in the LA-treatment and control animals. Thus, the study results suggest that treatment with minimum and maximum doses of LA (300 & 2,000 mg/kg body weight) did not cause any tissue damage or morphological alteration at the cellular level.
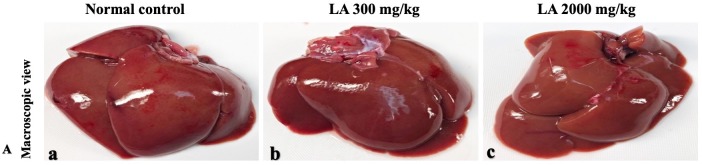
Figure 2. Macroscopic view of liver tissues of female SD rats in acute oral toxicity study (A) Normal control, (B) LA 300 mg/kg, (C) LA 2,000 mg/kg.
LA treated animals presenting normal reddish colour closed to the normal control group, after single oral doses of LA (300 mg/kg and 2,000 mg/kg).
Figure 3. Microscopic analysis of liver tissues of female SD rats in acute oral toxicity study, presenting normal morphology after single oral doses of LA (300 mg/kg and 2,000 mg/kg).
H&E (A), Masson trichrome (B), PAS (C), and Picro Sirius Red (D). 40× original magnification.
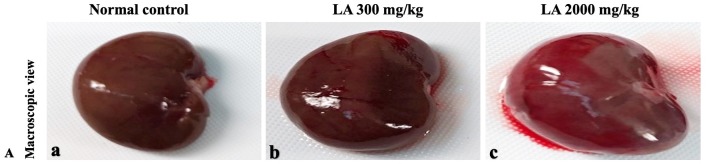
Figure 4. Macroscopic view of kidneys of female SD rats in acute oral toxicity study, presenting normal shape and appearance after single oral doses of LA (300 mg/kg and 2,000 mg/kg) (A) Normal control, (B) LA 300 mg/kg, (C) LA 2,000 mg/kg.
Figure 5. Microscopic analysis of kidney tissues of female SD rats in acute oral toxicity study, presenting normal morphology after single oral doses of LA (300 mg/kg and 2,000 mg/kg).
H&E (A), Masson trichrome (B), PAS (C), and Picro Sirius Red (D). 40× original magnification.
Figure 6. Macroscopic view of heart of female SD rats in acute oral toxicity study, presenting normal shape and appearance after single oral doses of LA (300 mg/kg and 2,000 mg/kg) (A) Normal control, (B) LA 300 mg/kg, (C) LA 2,000 mg/kg.
Figure 7. Microscopic analysis of heart tissues of female SD rats in acute oral toxicity study, presenting normal morphology after single oral doses of LA (300 mg/kg and 2,000 mg/kg).
H&E (A), Masson trichrome (B), PAS (C), and Picro Sirius Red (D). 40× original magnification.
Figure 8. Macroscopic view of lungs of female SD rats in acute oral toxicity study, presenting normal shape and appearance after single oral doses of LA (300 mg/kg and 2,000 mg/kg) (A) Normal control, (B) LA 300 mg/kg, (C) LA 2,000 mg/kg.
Figure 9. Microscopic analysis of lungs tissues of female SD rats in acute oral toxicity study, presenting normal morphology after single oral doses of LA (300 mg/kg and 2,000 mg/kg).
H&E (A), Masson trichrome (B), PAS (C), and Picro Sirius Red (D). 40× original magnification.
Figure 10. Macroscopic view of brain of female SD rats in acute oral toxicity study, presenting normal shape and appearance after single oral doses of LA (300 mg/kg and 2,000 mg/kg) (A) Normal control, (B) LA 300 mg/kg, (C) LA 2,000 mg/kg.
Figure 11. Microscopic analysis of brain tissues of female SD rats in acute oral toxicity study, presenting normal morphology after single oral doses of LA (300 mg/kg and 2,000 mg/kg).
H&E (A), Masson trichrome (B), PAS (C), and Picro Sirius Red (D). 40× original magnification.
Figure 12. Macroscopic view of spleen of female SD rats in acute oral toxicity study, presenting normal shape and appearance after single oral doses of LA (300 mg/kg and 2,000 mg/kg) (A) Normal control, (B) LA 300 mg/kg, (C) LA 2,000 mg/kg.
Figure 13. Microscopic analysis of spleen tissues of female SD rats in acute oral toxicity study, presenting normal morphology after single oral doses of LA (300 mg/kg and 2,000 mg/kg).
H&E (A), Masson trichrome (B), PAS (C), and Picro Sirius Red (D). 40× original magnification.
Figure 14. Macroscopic view of pancreas of female SD rats in acute oral toxicity study, presenting normal shape and appearance after single oral doses of LA (300 mg/kg and 2,000 mg/kg) (A) Normal control, (B) LA 300 mg/kg, (C) LA 2,000 mg/kg.
Figure 15. Microscopic analysis of pancreas tissues of female SD rats in acute oral toxicity study, presenting normal morphology after single oral doses of LA (300 mg/kg and 2,000 mg/kg).
H&E (A), Masson trichrome (B), PAS (C), and Picro Sirius Red (D). 40× original magnification.
Discussion
Toxicological studies of plant-based ingredients or food additives are important to ensure processed food remains safe and in good condition throughout its preservation to avoid any disease conditions. Herbs or plants additives used in dietary supplements, nutraceuticals, should be investigated for toxicity, despite their traditional claims (Figueredo et al., 2018; Lulekal et al., 2013).
Lauric acid revealed various pharmacological activities, but its safety profile still remains unevaluated. The current study is undertaken to screen the safety, and toxicity of LA at two different dose levels in normal rats, following OECD 423 guidelines.
No morbidity or mortality was noticed in LA-treated animals throughout fourteen days of the experimental period. Moreover, in line with previous study of Arya et al. (2012b), no changes were observed in general behaviour of treated animals when compared with the control group, suggesting that oral ingestion of LA neither cause any alterations nor produced any internal changes in female SD rats with doses up to 2,000 mg/kg body weight.
Body weight changes are the basic parameter which indicates the health status of animals. However, abrupt variations in the body weight indicate the toxic nature of the chemicals or drugs (Dongmo et al., 2019). In this study, LA treatment and control group recorded a normal gain in body weight, indicating, LA does not cause any physiological alterations that affects the normal growth in the animals, which is in agreement with the previous study published by Clemente et al. (2019). Similarly, organ weight variation is the fundamental and perceptive factor correlating organ to body weight index, which is important and critical in determining drug toxicity linked to treatment-related effects (Sellers et al., 2007). It is believed that medicinal plants in high dose could cause toxic or even lethal effects when administered in vivo (Silveira, Bandeira & Arrais, 2008; Siwe et al., 2015). Results from this study showed no difference in ROW in the treatment group when compared to control group, which indicates that LA is safer to the internal organs, supported by an earlier study published by Porwal, Khan & Maheshwari (2017). Studies have proven that ingesting toxic substances could suppress appetite, thus leading to less caloric intake (Madingou et al., 2016). During fourteen days observation period, no significant changes were noted in water and food consumption (Table 5), which shows that LA has no effect on the appetite of the animals, which is in line with the previous findings published by De Oliveira et al. (2020); Tonholo et al. (2020).
The haematopoietic system is crucial in the production of blood cells that accelerate metabolic functions and enhance normal proliferation in the living organism which act and delineate as a vital tool. This system is profound and directly or indirectly susceptible to chemical-induced injury, affecting physiological and pathological status in both humans and animals (Da Silva et al., 2018; Traesel et al., 2014). In the current findings, LA administration indicate non-significant changes in the essential blood cells and related parameters RBC, WBC, platelets count, Hb PCV, Mean MCV, MCH, MCHC, Neutrophil, Lymphocyte, Monocytes and Eosinophils, suggesting non-toxic nature of LA on haematological parameters and substantiating an understanding of normal haematopoiesis, which is in line with the previous findings published by Deyno et al. (2020) and Thai et al. (2020).
The liver is the central metabolic organ playing a key role in body metabolic functions where drug and chemical metabolism, biotransformation and detoxification occurs (Doherty, 2016). When hepatic cellular damage occurs due to hepatotoxic substances, the sensitive markers including transaminase serum enzymes ALT & AST start releasing from the cytosol and translocate into the bloodstream, leading to mild or severe liver injury (Figueredo et al., 2018; Hazarika et al., 2019; Ramaiah, 2011). In our study, LA did not elevate these transaminase enzymes, considering non-toxic nature of LA at different dose levels. Similar findings were noted by Moorthy, Khoo & Palanisamy (2019), where oral administration of the geraniin-enriched extract at 2,000 mg/kg body weight did not affect the biochemical parameters. Similarly, LA treatment did not change renal functions including serum creatinine and urea levels, which are used as an essential index to estimate renal damage (Arya et al., 2014; Mariappan et al., 2011; Meguellati et al., 2019). The elevated level of creatinine and urea in the blood displays malfunctioning of the kidney (Brondani et al., 2017; Feriani et al., 2017; Kifayatullah et al., 2015). In addition, serum electrolyte levels of LA treated animals observed to be in normal ranges. Overall, biochemical parameters are in line with previous reports of Bedi & Krishan (2019) and Thinkratok, Suwannaprapha & Srisawat (2014).
Histopathological investigation plays a significant role in determining tissue morphology through microscopical evaluations. The analysis of internal organs are identical and consider as the prime safety tool to determine any histological perturbations or deformities in the tissues (Chitra, Ramaswamy & Suba, 2015; Prabu, Panchapakesan & Raj, 2013). Therefore, the histopathological judgements concrete the haematological and biochemical findings and further substantiate the biological response factors (Jaganathan et al., 2012). Histopathological examination (macroscopic and microscopic) of internal organs including liver, kidney, heart, lungs, brain, spleen, and pancreas revealed a regular architecture, no morphological alterations were observed in LA treatment groups compared to the control group. Tissue degeneration or anomalies leading to necrosis, apoptosis or fibrosis are the basic parameters to determine toxicity in internal organs (De Lima et al., 2017).
The liver is the metabolic connective tissue which controls biliary and vascular traffic. Administration of LA at the minimum and maximum dose did not cause structural degeneration or accumulation in the liver tissues, displaying normal clusters of hepatocytes and parenchymal cells housed in cord-like arrangements. In this study, microscopical features of liver tissue is in agreement with the published data by Khoo et al. (2018) who postulated that changes to these cellular events might potentially be associated with the accumulation of exogenous chemicals and their metabolites may cause dose-dependent toxicity or damage to the liver (Debelo et al., 2016).
Similarly, LA administration did not alter kidney tissue, which showed organised pyramidal structure without any tubular necrosis and inflammation in the cortex and inner portion of the medulla region. However, the toxic effect of any compound on the kidney is demonstrated by changes in the serum creatinine or urea level (Gopi, Jacob & Mathur, 2016). Finding from this study reveals the harmless nature of LA on renal function by establishing the outcomes from histopathological analysis, which did not notice lesion or necrotic marks upon LA administration. Likewise, LA treatment on heart tissues displayed normal architecture of myocardial fibre and filaments which is in line with Greaves (2011) findings, who reported no morphological changes in heart tissues upon treatment with the phytocompounds. In a similar way, lungs of LA-treated animals showed no treatment-related morphological alterations, and our findings are in agreement with Aamir et al. (2019) who reported that no toxicological alteration was noted upon Arjunolic acid treatment in normal female SD rats. Furthermore, brain tissues showed normal morphology upon LA treatment, with no alterations to the cell types approaching through normal characteristics based on tissue pattern recognition, including neurons, glia, and microglia cells without any modulation to perivascular region. Thus, our study is supported by previous findings by Pravallika et al. (2019) who reported no structural and morphological changes in brain tissues following treatment with ethanolic extract of Centella asiatica plant. Moreover, LA treatment did not alter pancreas and spleen tissue which shows normal architecture, as we know rodent pancreas and spleen are diffused and soft compare to human tissues, which is in line with the previously published study by Prabu, Panchapakesan & Raj (2013) that reveals no morphological variation in tissues of control and hydroalcoholic extract of polyherbal treatment groups.
The present study expressed that oral administration of LA did not cause any morbidity or mortality at the selected doses, and LD50 values were greater than 2,000 mg/kg body weight. Based on the current findings, LA should remain in toxicity class 5, which exhibited the lowest toxicity (LD50 > 2,000 mg/kg body weight) as per the OECD 423 guideline, under chemical labelling and classification (OECD, 2001).
Conclusions
The findings of the current study showed that LA did not cause any significant changes upon oral administration up to 2,000 mg/kg body weight in female SD rats; no treatment-related signs of toxicity or mortality were noted. Moreover, histopathological examination exhibits no remarkable changes in the harvested tissues. Thus, it provides basic information on the toxicity and safety profile of LA, indicating low toxicity in the analysed parameters. This study could be helpful in the selection of dosages for future subacute or chronic disease model. However, in future, well-designed treatment strategies on pharmacological aspects should be carried out to set a clear understanding of safety and efficacy. Therefore, further validation on LA would enable to decide effective therapeutic dose prior to its long-term treatment as well as before the development of any health care products.
Supplemental Information
Values are expressed as the mean ±standard deviation (n = 6 for each group, female)
Acknowledgments
We are thankful to the University of Malaya for providing animal facilities.
Funding Statement
The current study was funded by the Taylors University Flagship Research Grant (TUFR/2017/002/01) under the umbrella of Ageing and Quality of Life. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Additional Information and Declarations
Competing Interests
The authors declare there are no competing interests.
Author Contributions
Hidayat Ullah Khan conceived and designed the experiments, performed the experiments, analyzed the data, prepared figures and/or tables, authored or reviewed drafts of the paper, and approved the final draft.
Khurram Aamir performed the experiments, analyzed the data, prepared figures and/or tables, authored or reviewed drafts of the paper, and approved the final draft.
Sreenivas Patro Sisinthy and Narendra Babu Shivanagere Nagojappa analyzed the data, authored or reviewed drafts of the paper, and approved the final draft.
Aditya Arya conceived and designed the experiments, authored or reviewed drafts of the paper, provided instrument and chemicals for smooth operations in laboratory work, and approved the final draft.
Field Study Permissions
The following information was supplied relating to field study approvals (i.e., approving body and any reference numbers):
The study was approved by Institutional Animal Care and Use Committee IACUC, Faculty of Medicine University of Malaya, ethics number: 2018-210605/TAY/R/AA(2018925)
Data Deposition
The following information was supplied regarding data availability:
Raw data is available in the Supplemental Files.
References
- Aamir et al. (2019).Aamir K, Khan HU, Hossain CF, Afrin MR, Shaik I, Salleh N, Giribabu N, Arya A. Oral toxicity of arjunolic acid on hematological, biochemical and histopathological investigations in female Sprague Dawley rats. PeerJ. 2019;7:e8045. doi: 10.7717/peerj.8045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahola et al. (2017).Ahola AJ, Lassenius MI, Forsblom C, Harjutsalo V, Lehto M, Groop P-H. Dietary patterns reflecting healthy food choices are associated with lower serum LPS activity. Scientific Reports. 2017;7:6511. doi: 10.1038/s41598-017-06885-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alves et al. (2017).Alves NFB, De Queiroz TM, De Almeida Travassos R, Magnani M, De Andrade Braga V. Acute treatment with lauric acid reduces blood pressure and oxidative stress in spontaneously hypertensive rats. Basic & Clinical Pharmacology & Toxicology. 2017;120:348–353. doi: 10.1111/bcpt.12700. [DOI] [PubMed] [Google Scholar]
- Amen et al. (2019).Amen OM, Sarker SD, Ghildyal R, Arya A. Endoplasmic reticulum stress activates unfolded protein response signaling and mediates inflammation, obesity, and cardiac dysfunction: therapeutic and molecular approach. Frontiers in Pharmacology. 2019;10:1–22. doi: 10.3389/fphar.2019.00977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arya et al. (2012a).Arya A, Achoui M, Cheah S-C, Abdelwahab SI, Narrima P, Mohan S, Mustafa MR, Mohd MA. Chloroform fraction of Centratherum anthelminticum (L.) seed inhibits tumor necrosis factor alpha and exhibits pleotropic bioactivities: inhibitory role in human tumor cells. Evidence-Based Complementary and Alternative Medicine. 2012a;2012:1–11. doi: 10.1155/2012/627256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arya et al. (2015).Arya A, Al-Obaidi MMJ, Karim RB, Taha H, Khan AK, Shahid N, Sayem AS, Looi CY, Mustafa MR, Mohd MA. Extract of Woodfordia fruticosa flowers ameliorates hyperglycemia, oxidative stress and improves β-cell function in streptozotocin–nicotinamide induced diabetic rats. Journal of Ethnopharmacology. 2015;175:229–240. doi: 10.1016/j.jep.2015.08.057. [DOI] [PubMed] [Google Scholar]
- Arya et al. (2014).Arya A, Al-Obaidi MMJ, Shahid N, Noordin MIB, Looi CY, Wong WF, Khaing SL, Mustafa MR. Synergistic effect of quercetin and quinic acid by alleviating structural degeneration in the liver, kidney and pancreas tissues of STZ-induced diabetic rats: a mechanistic study. Food and Chemical Toxicology. 2014;71:183–196. doi: 10.1016/j.fct.2014.06.010. [DOI] [PubMed] [Google Scholar]
- Arya et al. (2012b).Arya A, Cheah SC, Looi CY, Taha H, Mustafa MR, Mohd MA. The methanolic fraction of Centratherum anthelminticum seed downregulates pro-inflammatory cytokines, oxidative stress, and hyperglycemia in STZ-nicotinamide-induced type 2 diabetic rats. Food and Chemical Toxicology. 2012b;50:4209–4220. doi: 10.1016/j.fct.2012.08.012. [DOI] [PubMed] [Google Scholar]
- Babu et al. (2010).Babu SV, Veeresh B, Patil AA, Warke Y. Lauric acid and myristic acid prevent testosterone induced prostatic hyperplasia in rats. European Journal of Pharmacology. 2010;626:262–265. doi: 10.1016/j.ejphar.2009.09.037. [DOI] [PubMed] [Google Scholar]
- Beare-Rogers, Dieffenbacher & Holm (2001).Beare-Rogers J, Dieffenbacher A, Holm J. Lexicon of lipid nutrition (IUPAC Technical Report) Pure and Applied Chemistry. 2001;73:685–744. doi: 10.1351/pac200173040685. [DOI] [Google Scholar]
- Bedi & Krishan (2019).Bedi O, Krishan P. Investigations on acute oral toxicity studies of purpurin by application of OECD guideline 423 in rodents. Naunyn-Schmiedeberg’s Archives of Pharmacology. 2019;393(4):565–571. doi: 10.1007/s00210-019-01742-y. [DOI] [PubMed] [Google Scholar]
- Brondani et al. (2017).Brondani JC, Reginato FZ, Da Silva Brum E, De Souza Vencato M, Lhamas CL, Viana C, Da Rocha MIUM, De Freitas Bauermann L, Manfron MP. Evaluation of acute and subacute toxicity of hydroethanolic extract of Dolichandra unguis-cati L. leaves in rats. Journal of Ethnopharmacology. 2017;202:147–153. doi: 10.1016/j.jep.2017.03.011. [DOI] [PubMed] [Google Scholar]
- Chitra, Ramaswamy & Suba (2015).Chitra B, Ramaswamy R, Suba V. Toxicity evaluation of Pũrṅa Cantirotaya Centũram, a Siddha medicine in Wistar rats. International Scholarly Research Notices. 2015;2015:1–10. doi: 10.1155/2015/473296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemente et al. (2019).Clemente M, Miguel M, Felipe K, Gribner C, Moura P, Rigoni A, Fernandes L, Carvalho J, Hartmann I, Piltz M. Acute and sub-acute oral toxicity studies of standardized extract of Nasturtium officinale in Wistar rats. Regulatory Toxicology and Pharmacology. 2019;108:104443. doi: 10.1016/j.yrtph.2019.104443. [DOI] [PubMed] [Google Scholar]
- Da Silva et al. (2018).Da Silva ARH, Lopes LQS, Cassanego GB, De Jesus PR, Figueredo KC, Santos RCV, Lopes GHH, De Freitas Bauermann L. Acute toxicity and antimicrobial activity of leaf tincture Baccharis trimera (Less) Biomedical Journal. 2018;41:194–201. doi: 10.1016/j.bj.2018.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dayrit (2015).Dayrit FM. The properties of lauric acid and their significance in coconut oil. Journal of the American Oil Chemists’ Society. 2015;92:1–15. doi: 10.1007/s11746-014-2562-7. [DOI] [Google Scholar]
- De Lima et al. (2017).De Lima FF, Traesel GK, Menegati SELT, Dos Santos AC, Souza RIC, De Oliveira VS, Sanjinez-Argandoña EJ, Cardoso CAL, Oesterreich SA, Do Carmo Vieira M. Acute and subacute oral toxicity assessment of the oil extracted from Attalea phalerata Mart ex Spreng. pulp fruit in rats. Food Research International. 2017;91:11–17. doi: 10.1016/j.foodres.2016.11.019. [DOI] [PubMed] [Google Scholar]
- De Oliveira et al. (2020).De Oliveira NA, Sandini TM, Cornelio-Santiago HP, Martinelli ECL, Raspantini LER, Raspantini PC, Momo C, De Oliveira AL, Fukumasu H. Acute and subacute (28 days) toxicity of green coffee oil enriched with diterpenes cafestol and kahweol in rats. Regulatory Toxicology and Pharmacology. 2020;110:104517. doi: 10.1016/j.yrtph.2019.104517. [DOI] [PubMed] [Google Scholar]
- Debelo et al. (2016).Debelo N, Afework M, Debella A, Makonnen E, Ergete W, Geleta B. Assessment of hematological, biochemical and histopathological effects of acute and sub-chronic administration of the aqueous leaves extract of thymus schimperi in rats. Journal of Clinical Toxicology. 2016;6:2161–0495. [Google Scholar]
- Deng et al. (2013).Deng Y-X, Cao M, Shi D-X, Yin Z-Q, Jia R-Y, Xu J, Wang C, Lv C, Liang X-X, He C-L. Toxicological evaluation of neem (Azadirachta indica) oil: acute and subacute toxicity. Environmental Toxicology and Pharmacology. 2013;35:240–246. doi: 10.1016/j.etap.2012.12.015. [DOI] [PubMed] [Google Scholar]
- Deyno et al. (2020).Deyno S, Abebe A, Tola MA, Hymete A, Bazira J, Makonnen E, Alele PE. Acute and sub-acute toxicity of Echinops kebericho decoction in rats. BMC Complementary Medicine and Therapies. 2020;20:2. doi: 10.1186/s12906-019-2794-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doherty (2016).Doherty DG. Immunity, tolerance and autoimmunity in the liver: a comprehensive review. Journal of Autoimmunity. 2016;66:60–75. doi: 10.1016/j.jaut.2015.08.020. [DOI] [PubMed] [Google Scholar]
- Dongmo et al. (2019).Dongmo OLM, Epoh NJ, Tadjoua HT, Yousuf S, Telefo PB, Tapondjou LA, Choudhary MI. Acute and sub-acute toxicity of the aqueous extract from the stem bark of Tetrapleura tetrapteura Taub.(Fabaceae) in mice and rats. Journal of Ethnopharmacology. 2019;236:42–49. doi: 10.1016/j.jep.2019.02.026. [DOI] [PubMed] [Google Scholar]
- Fairchild & Fournier (2004).Fairchild TJ, Fournier PA. Glycogen determination using periodic acid-schiff: artifact of muscle preparation. Medicine and Science in Sports and Exercise. 2004;36:2053–2058. doi: 10.1249/01.mss.0000147586.85615.c4. [DOI] [PubMed] [Google Scholar]
- Feriani et al. (2017).Feriani A, Del Mar Contreras M, Talhaoui N, Gómez-Caravaca AM, Taamalli A, Segura-Carretero A, Ghazouani L, El Feki A, Allagui MS. Protective effect of Globularia alypum leaves against deltamethrin-induced nephrotoxicity in rats and determination of its bioactive compounds using high-performance liquid chromatography coupled with electrospray ionization tandem quadrupole–time-of-flight mass spectrometry. Journal of Functional Foods. 2017;32:139–148. doi: 10.1016/j.jff.2017.02.015. [DOI] [Google Scholar]
- Figueredo et al. (2018).Figueredo KC, Guex CG, Reginato FZ, Da Silva ARH, Cassanego GB, Lhamas CL, Boligon AA, Lopes GHH, De Freitas Bauermann L. Safety assessment of Morus nigra L. leaves: acute and subacute oral toxicity studies in Wistar rats. Journal of Ethnopharmacology. 2018;224:290–296. doi: 10.1016/j.jep.2018.05.013. [DOI] [PubMed] [Google Scholar]
- Fischer et al. (2008).Fischer AH, Jacobson KA, Rose J, Zeller R. Hematoxylin and eosin staining of tissue and cell sections. Cold Spring Harbor Protocols. 2008;2008 doi: 10.1101/pdb.prot4986. pdb.prot4986. [DOI] [PubMed] [Google Scholar]
- Gopi, Jacob & Mathur (2016).Gopi S, Jacob J, Mathur KY. Acute and subchronic oral toxicity studies of hydrogenated curcuminoid formulation ‘CuroWhite’ in rats. Toxicology Reports. 2016;3:817–825. doi: 10.1016/j.toxrep.2016.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greaves (2011).Greaves P. Histopathology of preclinical toxicity studies: interpretation and relevance in drug safety evaluation. Academic Press; 2011. [Google Scholar]
- Hazarika et al. (2019).Hazarika I, Geetha K, Sundari PS, Madhu D. Acute oral toxicity evaluation of extracts of Hydrocotyle sibthorpioides in wister albino rats as per OECD 425 TG. Toxicology Reports. 2019;6:321–328. doi: 10.1016/j.toxrep.2019.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaganathan et al. (2012).Jaganathan R, Ravinayagam V, Panchanadham S, Palanivelu S. Troxicological, biochemical and histopathological evaluation of Tridham, a siddha medicine in Wistar albino rats. Journal of Biochemical Technology. 2012;4:541–548. [Google Scholar]
- Khoo et al. (2018).Khoo L, Foong Kow A, Maulidiani M, Lee M, Tan C, Shaari K, Tham C, Abas F. Hematological, biochemical, histopathological and 1H-NMR metabolomics application in acute toxicity evaluation of clinacanthus nutans water leaf extract. Molecules. 2018;23:2172. doi: 10.3390/molecules23092172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kifayatullah et al. (2015).Kifayatullah M, Mustafa MS, Sengupta P, Sarker MMR, Das A, Das SK. Evaluation of the acute and sub-acute toxicity of the ethanolic extract of Pericampylus glaucus (Lam.) Merr. in BALB/c mice. Journal of Acute Disease. 2015;4:309–315. doi: 10.1016/j.joad.2015.06.010. [DOI] [Google Scholar]
- Lappano et al. (2017).Lappano R, Sebastiani A, Cirillo F, Rigiracciolo DC, Galli GR, Curcio R, Malaguarnera R, Belfiore A, Cappello AR, Maggiolini M. The lauric acid-activated signaling prompts apoptosis in cancer cells. Cell Death Discovery. 2017;3:17063. doi: 10.1038/cddiscovery.2017.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lattouf et al. (2014).Lattouf R, Younes R, Lutomski D, Naaman N, Godeau G, Senni K, Changotade S. Picrosirius red staining: a useful tool to appraise collagen networks in normal and pathological tissues. Journal of Histochemistry & Cytochemistry. 2014;62:751–758. doi: 10.1369/0022155414545787. [DOI] [PubMed] [Google Scholar]
- Lulekal et al. (2013).Lulekal E, Asfaw Z, Kelbessa E, Van Damme P. Ethnomedicinal study of plants used for human ailments in Ankober District, North Shewa Zone, Amhara region, Ethiopia. Journal of Ethnobiology and Ethnomedicine. 2013;9:63. doi: 10.1186/1746-4269-9-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madingou et al. (2016).Madingou NOK, Traore A, Souza A, Mounanga MMB, Samseny RRA, Ouedraogo S, Traore AS. Preliminary studies of acute and sub-chronic toxicity of the aqueous extract of Guibourtia tessmannii (Harms) J. Leonard stem barks (Caesalpiniaceae) in mice and rats. Asian Pacific Journal of Tropical Biomedicine. 2016;6:506–510. doi: 10.1016/j.apjtb.2016.04.001. [DOI] [Google Scholar]
- Mariappan et al. (2011).Mariappan G, Saha B, Sutharson L, Singh A, Garg S, Pandey L, Kumar D. Analgesic, anti-inflammatory, antipyretic and toxicological evaluation of some newer 3-methyl pyrazolone derivatives. Saudi Pharmaceutical Journal. 2011;19:115–122. doi: 10.1016/j.jsps.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meguellati et al. (2019).Meguellati H, Ouafi S, Saad S, Djemouai N. Evaluation of acute, subacute oral toxicity and wound healing activity of mother plant and callus of Teucrium polium L. subsp. geyrii Maire from Algeria. South African Journal of Botany. 2019;127:25–34. doi: 10.1016/j.sajb.2019.08.023. [DOI] [Google Scholar]
- Moorthy, Khoo & Palanisamy (2019).Moorthy M, Khoo JJ, Palanisamy UD. Acute oral toxicity of the ellagitannin geraniin and a geraniin-enriched extract from Nephelium lappaceum L rind in Sprague Dawley rats. Heliyon. 2019;5:e02333. doi: 10.1016/j.heliyon.2019.e02333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OECD (2001).Organisation for Economic Cooperation and Development (OECD) 423: Acute oral toxicity—acute toxic class method. OECD; Paris: 2001. [Google Scholar]
- OECD (2002).Organisation for Economic Cooperation and Development (OECD) Guidelines for the testing of chemicals/section 4: health effects test no. 423, acute oral toxicity-acute toxic class method. OECD; Paris: 2002. [Google Scholar]
- Porwal, Khan & Maheshwari (2017).Porwal M, Khan N, Maheshwari K. Evaluation of acute and subacute oral toxicity induced by ethanolic extract of Marsdenia tenacissima leaves in experimental rats. Scientia Pharmaceutica. 2017;85:29. doi: 10.3390/scipharm85030029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prabu, Panchapakesan & Raj (2013).Prabu P, Panchapakesan S, Raj CD. Acute and sub-acute oral toxicity assessment of the hydroalcoholic extract of Withania somnifera roots in Wistar rats. Phytotherapy Research. 2013;27:1169–1178. doi: 10.1002/ptr.4854. [DOI] [PubMed] [Google Scholar]
- Pravallika et al. (2019).Pravallika PL, Mohan GK, Rao KV, Shanker K. Biosynthesis, characterization and acute oral toxicity studies of synthesized iron oxide nanoparticles using ethanolic extract of Centella asiatica plant. Materials Letters. 2019;236:256–259. doi: 10.1016/j.matlet.2018.10.037. [DOI] [Google Scholar]
- Ramaiah (2011).Ramaiah SK. Preclinical safety assessment: current gaps, challenges, and approaches in identifying translatable biomarkers of drug-induced liver injury. Clinics in Laboratory Medicine. 2011;31:161–172. doi: 10.1016/j.cll.2010.10.004. [DOI] [PubMed] [Google Scholar]
- Sellers et al. (2007).Sellers RS, Mortan D, Michael B, Roome N, Johnson JK, Yano BL, Perry R, Schafer K. Society of toxicologic pathology position paper: organ weight recommendations for toxicology studies. Toxicologic Pathology. 2007;35:751–755. doi: 10.1080/01926230701595300. [DOI] [PubMed] [Google Scholar]
- Sheela et al. (2016).Sheela DL, Nazeem PA, Narayanankutty A, Manalil JJ, Raghavamenon AC. In silico and wet lab studies reveal the cholesterol lowering efficacy of lauric acid, a medium chain fat of coconut oil. Plant Foods for Human Nutrition. 2016;71:410–415. doi: 10.1007/s11130-016-0577-y. [DOI] [PubMed] [Google Scholar]
- Silberstein et al. (2013).Silberstein T, Burg A, Blumenfeld J, Sheizaf B, Tzur T, Saphier O. Saturated fatty acid composition of human milk in Israel: a comparison between Jewish and Bedouin women. The Israel Medical Association Journal. 2013;15:156–159. [PubMed] [Google Scholar]
- Silveira, Bandeira & Arrais (2008).Silveira PFD, Bandeira MAM, Arrais PSD. Pharmacovigilance and adverse reactions to the medicinal plants and herbal drugs: a reality. Revista Brasileira de Farmacognosia. 2008;18:618–626. doi: 10.1590/S0102-695X2008000400021. [DOI] [Google Scholar]
- Siwe et al. (2015).Siwe GT, Enow-Orock GE, Amang AP, Mezui C, Dongmo AB, Tan PV. Acute and subacute toxicological assessment of the leaf aqueous extract of Eremomastax speciosa (Acanthaceae) in wistar rats. Journal of Advances in Medical and Pharmaceutical Sciences. 2015;4:1–13. [Google Scholar]
- Thai et al. (2020).Thai H, Nguyen CT, Thach LT, Tran MT, Mai HD, Nguyen TTT, Le GD, Van Can M, Dai Tran L, Bach GL. Characterization of chitosan/alginate/lovastatin nanoparticles and investigation of their toxic effects in vitro and in vivo. Scientific Reports. 2020;10:1–15. doi: 10.1038/s41598-019-56847-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thinkratok, Suwannaprapha & Srisawat (2014).Thinkratok A, Suwannaprapha P, Srisawat R. Safety assessment of hydroethanolic rambutan rind extract: acute and sub-chronic toxicity studies. Indian Journal of Experimental Biology. 2014;52(10):989–995. [PubMed] [Google Scholar]
- Tonholo et al. (2020).Tonholo D, Maltarollo V, Kronenberger T, Silva I, Azevedo P, Oliveira R, Souza L, Tagliati C. Preclinical toxicity of innovative molecules: In vitro, in vivo and metabolism prediction. Chemico-Biological Interactions. 2020;315:108896. doi: 10.1016/j.cbi.2019.108896. [DOI] [PubMed] [Google Scholar]
- Traesel et al. (2014).Traesel GK, De Souza JC, De Barros AL, Souza MA, Schmitz WO, Muzzi RM, Oesterreich SA, Arena AC. Acute and subacute (28 days) oral toxicity assessment of the oil extracted from Acrocomia aculeata pulp in rats. Food and Chemical Toxicology. 2014;74:320–325. doi: 10.1016/j.fct.2014.10.026. [DOI] [PubMed] [Google Scholar]
- Tsounapi et al. (2017).Tsounapi P, Honda M, Dimitriadis F, Kawamoto B, Hikita K, Muraoka K, Saito M, Sofikitis N, Takenaka A. Impact of antioxidants on seminal vesicles function and fertilizing potential in diabetic rats. Asian Journal of Andrology. 2017;19:639–646. doi: 10.4103/1008-682X.186871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ubgogu, Onyeagba & Chigbu (2006).Ubgogu O, Onyeagba R, Chigbu O. Lauric acid content and inhibitory effect of palm kernel oil on two bacterial isolates and Candida albicans. African Journal of Biotechnology. 2006;5:1045–1047. [Google Scholar]
- Uday Kumar et al. (2014).Uday Kumar D, Christopher V, Sobarani D, Nagendra Sastry Y. Lauric acid as potential natural product in the treatment of cardiovascular disease: a review. Journal of Bioanalysis and Biomedicine. 2014;6(2):037–039. doi: 10.4172/1948-593X.1000107. [DOI] [Google Scholar]
- World Health Organization (2015).World Health Organization . WHO guidelines on safety monitoring of herbal medicines in pharmacovigilance systems. 2004. World Health Organization; Geneva: 2015. [Google Scholar]
- Yakob, Uyub & Sulaiman (2012).Yakob HK, Uyub AM, Sulaiman SF. Toxicological evaluation of 80% methanol extract of Ludwigia octovalvis (Jacq.) PH Raven leaves (Onagraceae) in BALB/c mice. Journal of Ethnopharmacology. 2012;142:663–668. doi: 10.1016/j.jep.2012.05.035. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Values are expressed as the mean ±standard deviation (n = 6 for each group, female)