51.1 Introduction
Now that the strategies needed to build a sustainable research career have been discussed, it is important to be cognizant of the mission of the funding agency, the National Institutes of Health (NIH), to select the most appropriate funding mechanism. This chapter provides details of the most important NIH grant funding mechanisms and subsequent sections explain in much more detail the preparation and submission of the application, and cover the NIH peer review process.
With the goal of improving public health, NIH funds the most meritorious scientific research projects within available funding limits. Peer review is the process that evaluates each submitted application for its scientific and technical merit. In general, the scientific merit of the proposed research project is the most important factor that determines whether it is funded. However, it is important to focus not only on elegant science, but also on the institute's mission and its programatic needs. The impact of the proposed study on public health, the feasibility of the proposed study, and selection of the appropriate funding mechanism also are very important for building a successful and sustainable personal research portfolio.
Therefore, it is important to write a successful NIH grant application with a clear understanding of NIH administrative grant award policies and procedures, and this section provides the potential applicant with some help navigating the system. Because the NIH supports different types of grants (and contracts), it may be difficult to select the appropriate funding mechanism when writing an application. Therefore, this chapter helps with that selection and provides the investigator with an overview of some common funding mechanisms.
In determining the most appropriate funding mechanism, investigators first should be aware of the requirements of each mechanism, and then assess their ability to compete successfully for funds. Some award mechanisms are specific for PhDs and others for MDs. Figure 51.1 illustrates a career path for PhDs and Figure 51.2 for MDs. Note that there also are award mechanisms common to both career paths. Potential applicants should choose the award type that matches their area(s) of interest. To select the most appropriate award options, refer to the career-stage graphics (Figure 51.1 and Figure 51.2).
Figure 51.1.
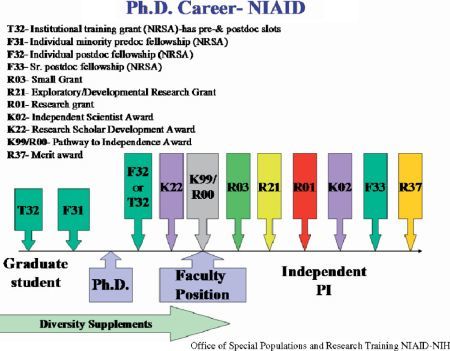
Potential career path for an investigator with a PhD (See Color Plates).
Figure 51.2.
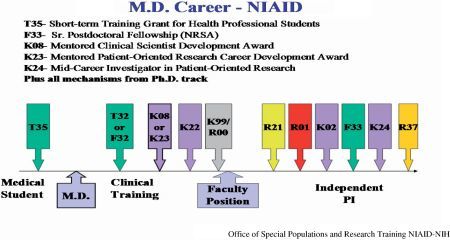
Potential career path for investigator with an MD (See Color Plates).
As mentioned, NIH uses variety of award mechanisms to meet its mission and programs. NIH institutes and centers (ICs) may vary in the way they use grant mechanisms. However, it should be noted that not all ICs accept applications for all mechanisms. Thus, potential applicants should periodically check the National Institute of Allergy and Infectious Disease grants and contracts website at http://www.niaid.nih.gov/ncn/grants/default_grants.htm for more details.
All grant applications submitted to the NIH must be submitted in response to a funding opportunity announcement (FOA), which is a notice in Grants.gov for a federal grant funding opportunity. FOAs are released by NIH ICs and are published simultaneously in the NIH Guide for Grants and Contracts (http://grants2.nih.gov/grants/guide/index.html) and at Grants. gov (http://www.grants.gov). Potential applicants can obtain an application package as well as general instructions for completing an electronic grant applications package for a particular FOA (http://era.nih.gov/ElectronicReceipt/find_app). Investigators may submit their research as an investigator-initiated grant application, known as “unsolicited” research, or submit in response to FOAs known as a program announcement (PA), a request for application (RFA), or a request for proposals (RFP). As mentioned earlier, institutes publish PAs and RFAs in the NIH Guide to Grants and Contracts and in Grants.gov as FOAs. Contract RFPs are published in FedBizOps (http://www.fedbizops.gov).
It is important to determine and choose the award mechanism that fits the investigator's research goal and also addresses the mission and programmatic needs of the participating ICs. Therefore, it is wise to contact the program officer at the IC to discuss the appropriateness of the funding mechanism as it relates to the proposed area(s) of science and the investigator's stage along the career path.
51.2 Investigator-Initiated Research (Unsolicited Applications)
The principal investigator (PI) of an investigator-initiated application has the responsibility of selecting the subject area, which should be exciting, significant, and important. PIs should capitalize on their strengths and find creative, novel and/or innovative ideas within their areas of expertise that would make an impact on public health. Once a topic has been selected, the PI should assess the literature to ensure the topic fills a research gap, search databases to see what has already been done, and assess other resources available (see Part V, Identifying Research Resources and Funding Opportunities) to leverage one's research prowess and expertise. Then, the researcher should decide the type of funding mechanism that fits, based on his or her career level and the needs of the research. Generally, PIs submit a basic research project grant (R01), which gives a substantial level and duration of funding support.
Potential applicants for their first independent research grant should apply through a NIH-wide parent PA that covers investigator-initiated research. Applicants also should check institute-specific PAs, because some institutes do not partici-pate—or may not accept—applications in all research areas in response to a parent announcement, or they may have institute-specific requirements.
A comprehensive list of all funding mechanisms used by NIH or NIAID can be found at http://www.niaid.nih.gov/ncn/grants/mechan.htm or http://grants.nih.gov/grants/funding/ac.pdf. Listed below are some of the main NIH and NIAID investigator-initiated grant funding mechanisms with brief descriptions.
51.2.1 Research Grants (R Series)
NIH has developed the parent announcement for investigator-initiated or unsolicited applications. Several parent announcements are available and are listed below. This list is by no means exhaustive. For a full list, see the website: http://grants1.nih.gov/grants/guide/parent_announcements.htm. Note that in general, all NIH grants that are funded successfully are awarded to the investigator's institution, which administers them on behalf of a principal investigator.
NIH Research Project Grant Program (R01): This mechanism provides support for a specified and focused investigator-initiated research project for 1 to 5 years. Applicants should provide preliminary data to support their research and there can be more than one principal investigator on an R01 grant; see http://www.niaid.nih.gov/ncn/newsletters/2007/0329.htm. This is a very competitive grant mechanism and the budget varies based on the proposed research. For more information see the PA: http://grants.nih.gov/grants/guide/pa-files/PA-07-070.html.
NIH Small Grant Program (R03): This mechanism provides limited funding for a short period of time to support a variety of projects including pilot or feasibility studies, secondary analysis of existing data, small, self-contained research projects, development of new research technology, etc. Funding is limited to up to 2 years in direct costs generally up to $50,000 per year. For more information see the PA: http://grants.nih.gov/grants/guide/pa-files/PA-06-180.html.
NIH Academic Research Enhancement Award (AREA; R15): AREA grants support small research projects, new or expanded, in the biomedical and behavioral sciences conducted by students and faculty in health professional schools and other academic components that have not been major recipients of NIH research grant funds. Direct costs are limited to $150,000 over the entire project period—up to 3 years— with a limited eligibility. For more information see the PA: http://grants.nih.gov/grants/guide/pa-files/PA-06-042.html.
NIH Exploratory/Developmental Research Grant Award (R21): This mechanism is for new, innovative, exploratory and developmental research projects. Preliminary data are generally not required for this mechanism; however, supportive data are helpful. Funding is limited to up to 2 years and the combined budget for direct costs for the 2-year project period usually may not exceed $275,000. For more information, see the PA: http://grants.nih.gov/grants/guide/pa-files/PA-06-181.html.
NIH Clinical Trial Planning Grant Program (R34): This is a planning grant mechanism designed to support the development of clinical protocol and the preparation of essential documents for clinical trial implementation grants. A R34 planning grant is awarded for 1 year up to $150,000 in direct costs. For detailed information about NIAID investigator-initiated clinical trial planning and implementation grants, see the website: http://www.niaid.nih.gov/ncn/clinical/R34.htm.
Small Business Technology Transfer (STTR; R41/R42): This mechanism is designed to stimulate a partnership of ideas and technologies between innovative small businesses and research institutions through federally funded research or research and development (R&D). This mechanism assists the small business and research communities in commercializing innovative technologies. A phase I grant (R41) is to establish the technical and scientific merit and feasibility of the proposed research (R) or R&D efforts. A phase II (R42) is to continue the research or R&D efforts initiated in phase I. Eligibility is limited to U.S. investigators. For more information, see the PA: http://grants.nih.gov/grants/guide/pa-files/PA-07-281.html.
Small Business Innovative Research (SBIR; R43/44): This mechanism stimulates technological innovation in the private sector by supporting research or R&D for for-profit institutions for ideas that have potential for commercialization. Phase I (R43) is to establish the technical and scientific merit and feasibility of the proposed R/R&D efforts. Phase II (R44) is to continue the research or R&D effort initiated in phase I. Eligibility is limited to U.S. investigators. For more information see the PA: http://grants.nih.gov/grants/guide/pa-files/PA-07-280.html.
- Resource Grants: The following represent some of the more frequently used types of grant programs that provide research-related support or access to resources. A comprehensive list of all funding mechanisms used by NIH and NIAID can be found at: http://www.nlm.nih.gov/ep/Grants.html and http://www.niaid.nih.gov/ncn/grants/mechan.htm.
- NIH Support for Conferences and Scientific Meetings (R13 and U13): These mechanisms support high quality conferences and scientific meetings that are relevant to NIH and NIAID scientific mission and to the public health. Potential applicants should request advanced permission from the funding IC to submit an application. NIAID funds scientific meetings through conference grants (R13) or cooperative agreements (U13) for up to 5 years. Non-U.S. institutions are not eligible to apply. Follow the guidelines provided in the PA: http://grants.nih.gov/grants/guide/pa-files/PA-06-041.html.
- Resource-Related Research Projects (R24): This mechanism supports research projects to enhance the capacity of resources that serve biomedical research. It is generally used to provide resources where multiple fields of expertise are needed to focus on a single complex question in biomedical research. Currently, there is no PA for this mechanism. Potential applicants should visit the IC website and contact the program officer for that science area.
51.2.2 NIH Research Training and Research Career Development Opportunities (F, K, and T series)
In general, NIH provides research-training opportunities and career development awards to research doctorates or health professional doctorates to gain education and experience in the biomedical field. NIH and NIAID have a variety of training programs for early to mid-career stage investigators. Focused training is a fundamental part of the critical path toward developing a sustainable research effort and portfolio. NIAID awards grants for different career stages and types of research, e.g., basic, clinical, and patient-oriented research and also offers training and career development in biodefense and emerging infectious diseases.
Extramural Research Training & Research Career Opportunities: NIH and NIAID support a variety of pred-octoral and postdoctoral training opportunities to non-federal scientists at universities, hospitals, and at other research institutions throughout the U.S. (extramural; outside of NIH) and in numerous countries. The main goals of the training program are to have highly trained scientists meet the nation's future needs and to develop tomorrow's scientists in the biomedical field. During the past several decades, NIAID has faced and continues to address many global challenges, e.g., HIV/AIDS, pandemic influenza, SARS, drug-resistant tuberculosis, other emerging and reemerging infectious diseases, allergic diseases, potential biowarfare threats as part of Project Bioshield, and the development of radiation countermeasures. Below are some of the examples of training and career grants that are funded by NIH or NIAID. For further information go to the NIAID or the Office of Extramural Research websites: http://www.niaid.nih.gov/ncn/training/advice/training_dev_grants.htm or http://grants1.nih.gov/training.
All NIAID training and career development awards (except for the pathway to independence award mechanism, K99/R00) require either U.S. citizenship or legal residence (“green card”). Potential candidates should check the eligibility and visa requirements for each mechanism in the PA. Potential applicants also should see frequently asked questions at: http://grants.nih.gov/training/q&a.htm and the new investigators program web page at: http://grants.nih.gov/grants/new_investigators/index.htm.
- Fellowships (Fs): The National Research Service Award (NRSA) provides support to pre- and postdoctoral trainees for independent research projects in basic or clinical scientific areas within the NIAID mission. These are individual awards under a mentor; most awardees are PhDs. If the candidates are predoctoral students and are members of an underrepresented minority group, have a disability, or come from a disadvantaged background, the academic institution can nominate them for a fellowship that provides up to 5 years of support for biomedical, behavioral sciences, or health services research. Training can be provided at domestic or non-U.S. institutions. For more information see: http://www.niaid.nih.gov/ncn/training/advice/fellowships.htm.
- NRSA Pre-doctoral Fellowships (F31): This is an NIH-wide program that provides funding under the National Research Service Award to predoctoral students with disabilities, from disadvantaged backgrounds, or from underrepresented racial and ethnic groups. It provides a stipend and a limited amount of tuition funds. Potential applicants must be enrolled or accepted into a PhD or MD, PhD program in biomedical and behavioral sciences or health services research. For further information and eligibility check the PA: http://grants1.nih.gov/grants/guide/pa-files/PA-07-002.html.
- NRSA Postdoctoral Fellowships (F32): This program is for postdoctoral candidates only and provides fellowships to individuals who have a doctoral degree from an accredited institution. The award supports promising applicants who have the potential to become productive, independent investigators, but may not be used to support studies leading to the MD, DO, or other similar health-professional degrees, or residency training. Research clinicians must devote full time to their proposed research training and confine clinical duties to those activities that are part of the research-training program. For further information and eligibility check the PA: http://grants1.nih.gov/grants/guide/pa-files/PA-07-107.html.
- NRSA Senior Fellowships (F33): This program generally supports faculty, MDs or PhDs on sabbaticals. Potential applicants must have at least 7 years of research experience after the doctoral degree and the proposed research must expand experience and offer career benefits. This program is not designed for postdoctoral level scientists seeking to enhance their research experience prior to independence. For further information and eligibility check the announcement: http://grants1.nih.gov/grants/guide/pa-files/PA-07-172.html.
- Career Development Awards (Ks): This program awards individuals with MD and PhD degrees who wish to develop careers in biomedical research. These are more senior awards than the fellowship awards mentioned above. Some awards are mentored and others are not. Below are some specific career award mechanisms and detailed information on the Ks Kiosk can be found at: http://www.niaid.nih.gov/ncn/training/advice/career_dev.htm.
- For Individuals with a PhD Degree (Figure 51.1): For the PhD, there are several award mechanisms from which to choose. Most awards support individuals who have been accepted or are ready for a faculty position. Some award mechanisms listed below may be eligible for candidates with an MD degree. Potential candidates should check each announcement for eligibility.
-
i.Mentored Research Scientist Development Award (K01): This mechanism is for new faculty members who need additional supervised research experience. Both MDs and PhDs in the fields of epidemiology, modeling, and outcomes research are eligible to apply. For detailed information see the PA: http://grants1.nih.gov/grants/guide/pa-files//PA-06-001.html.
-
ii.Independent Scientist Award (K02): This program provides support to more established “senior” investigators who have a doctoral degree and have an independent research project grant (R01 or equivalent). This award protects at least 75% of the effort for the awardees to focus on research program development. Most successful candidates are assistant professors or just-promoted associate professors. For detailed information see the PA: http://grants1.nih.gov/grants/guide/pa-files/PA-06-527.html.
-
iii.Senior Scientist Award (K05): This mechanism provides time and salary support to senior and established investigators. Not all NIH institutes participate in this program. For detailed information check the PA: http://grants.nih.gov/grants/guide/pa-files/PA-00-021.html.
-
iv.Research Scholar Development Award (K22): This program provides research support to both MD and PhD candidates during the transition from the postdoctoral position to an assistant professor at an academic institution. Candidates with more than 5 years of research experience in a postdoctoral position are not eligible to apply. This mechanism is funded for 2 years with no renewal option. The application has two phases. In phase 1 the application is submitted for peer review of scientific merit. To qualify for phase 2, the applicant must be offered a position as an assistant professor with an independent laboratory, available start-up funds, and minimal teaching or other responsibilities. This award is used differently by the ICs and centers that participate. Potential applicants should review the PA at http://grants.nih.gov/grants/guide/pa-files/PAR-07-347.html and the institutes' website for preferences.
-
v.Mentored Quantitative Research Development Award (K25): This program is for junior faculty members with an advanced degree in engineering or quantitative science such as a PhD or MSEE who would like to apply their experience to biomedical science. There are limited eligibility requirements; for detailed information, check the PA: http://grants1.nih.gov/grants/guide/pa-files/PA-06-087.html.
-
vi.Other K Awards: There are other K awards for individuals interested in stem cell research or mouse pathobiology (K18). Detailed information may be found at http://grants.nih.gov/grants/guide/pa-files/PAR-02-069.html and http://grants.nih.gov/grants/guide/pa-files/PAR-99-065.html websites respectively. The academic award (K07) is used to recruit research faculty into areas where there is a growing need for the research and instructional capabilities. For further information see the PA: http://grants.nih.gov/grants/guide/pa-files/PA-00-070.html.
-
i.
- For Individuals with an MD Degree or Equivalent Clinical Degree (Figure 51.2): Individuals with a health professional doctorate degree, the MD have an opportunity to choose from different career development award mechanisms. Most of these awards support individuals who have completed their clinical training and have accepted a faculty position.
-
i.Mentored Clinical Scientist Development Award (K08): This program provides awards to candidates interested in basic research project and have a health professional doctoral degree such as the MD, DO, DVM or equivalent degree, and a professional license to practice in the United States. For further information see the PA: http://grants1.nih.gov/grants/guide/pa-files/PA-06-512.html.
-
ii.Mentored Clinical Scientist Development Program (K12): This program provides awards to specific institutions to establish career development programs for clinicians to become independent patient-oriented researchers. Some, but not all, NIH institutes participate in this program. For further information check the institute's website and their PA.
-
iii.Mentored Patient-Oriented Research Career Development Award (K23): This program provides awards to candidates interested in patient-oriented research projects. Candidates must have a health professional doctorate degree or its equivalent. Candidates with PhD degrees are eligible if the degree is in a clinical field and they usually perform clinical duties. For further information check the PA: http://grants1.nih.gov/grants/guide/pa-files/PA-05-143.html.
-
iv.Mid-Career Investigator Award in Patient-Oriented Research (K24): This program is for established mid-career clinicians or PhDs who are committed to patient oriented research and are willing to serve as a mentor primarily for junior scientists engaged in clinical research. The principal investigator should have concurrent research support, such as R01, or pharmaceutical company funding, or the equivalent. For further information check the PA: http://grants1.nih.gov/grants/guide/pa-files/PA-04-107.html.
-
v.NIH Pathway to Independence (PI) Award (K99/R00): This is a transition award for postdoctoral researchers moving to assistant professor positions. The program is for both clinical or research doctorate degree candidates with no more than 5 years of postdoctoral research training at the time of initial application or subsequent resubmis-sions. The award provides funds for up to 5 years and it consists of two phases. The first phase provides 1 to 2 years of mentored support for postdoctoral research scientists (K99) and the second phase provides up to 3 years of independent support (R00) contingent on securing an independent research position. U.S. citizenship and green card are not required for this mechanism. For eligibility check the NIH PA: http://grants.nih.gov/grants/guide/pa-files/PA-06-133.html.
-
i.
- Training Grants (Ts): The national research service award (NRSA) institutional research training grant (T32) and NRSA short-term institutional research training grant (T35) awards fund training programs for pre- and postdoctoral candidates in basic or clinical scientific areas for MDs, PhDs, and DVMs. These are multi-slot awards administered by U.S. institutions only. Senior investigators at academic institutions generally apply for T32 grants and trainees work in a mentor's laboratory. For further detail check the website: http://www.niaid.nih.gov/ncn/training/advice/t32_qualifying.htm.
- Ruth L. Kirschstein National Research Service Award (NRSA) Institutional Research Training Grants (T32): This funding mechanism provides awards to support institutional training grants at eligible institutions for graduate and postdoctoral researchers in biomedical and behavioral science. The goal of the program is to prepare a highly diverse group of individuals for careers that have a significant impact on the health-related research needs of the nation. Trainees must be citizens or non-citizen nationals of the United States, or have been lawfully admitted in the United States for permanent residence and have a valid “green card” or other legal document of such status in the United States. Individuals on temporary or student visas are not eligible for support by the NRSA. For further information check the PA: http://grants1.nih.gov/grants/guide/pa-files/PA-07-172.html.
- Ruth L. Kirschstein National Research Service Award Short-Term Institutional Research Training Grants (T35): This grant mechanism exclusively provides support for intensive, short-term research training for students in health professional schools during the summer. In addition, the short-term institutional research training grant may be used to support other types of predoctoral and postdoctoral training in focused, often emerging scientific areas relevant to the mission of the funding NIH institute or center. For further information see the PA: http://grants1.nih.gov/grants/guide/pa-files/PA-05-117.html.
Women in Biomedical Careers: NIH has established an Office of Research on Women's Health (ORWH), under the Office of the NIH Director. This office develops opportunities and programs to support the recruitment and retention of women to advance their careers in the biomedical field. NIH has a working group on women in biomedical careers to work with extramural community and the public on the issue of the advancement of women in research careers. Several initiatives have been issued by NIH ICs for advancing novel science in women's health. For further information see the website: http://womenin-science.nih.gov.
Research Supplements: This program supports underrep-resented minority high school, undergraduate, graduate, and medical students, as well as postdoctoral scientists and faculty members. Awards are supplements to funded research grants and provide salary, supplies and travel. Funds are added to a grant for training researchers from underrepre-sented groups. Note: diversity supplements replace research supplements for underrepresented minorities and research supplements for individuals with disabilities. For further information check the website: http://www.niaid.nih.gov/ncn/training/advice/research_supp.htm and the PA: http://grants2.nih.gov/grants/guide/pa-files/PA-05-015.html.
NIH Loan Repayment Program (LRPs): The NIH loan repayment program is designed to help recruit and retain highly qualified clinicians, dentists, and other health professionals with doctoral-level degrees in biomedical and behavioral research careers. There are two kinds of LRPs: one for health professionals pursuing careers in clinical, pediatric, health disparities, or contraception and infertility research (Extramural LRP), and another for health professionals in research positions and fellowships (clinical and basic science) in the NIH intramural laboratories (intramural LRP). For more information about the NIH LRPs visit the website: http://www.lrp.nih.gov.
- Intramural Research Training & Research Career Opportunities: This program is open to scientists at all career levels and provides opportunities for non-US scientists to conduct collaborative research at the NIH. There are several research and training opportunities at the NIH intramural (within NIH) laboratories. These opportunities range from summer programs for high school students through employment for postdoctoral scientists. Below are examples of some of the training opportunities. For more information check the website: http://www.training.nih.gov.
- Training in NIAID Intramural Laboratories: NIAID offers both basic and clinical research training programs for students and postdoctoral fellows, e.g., postdoctoral intramural research training award (IRTA) and National Research Council (NRC) research associateship program. There are two programs for non-U.S. scientists through NIH visiting programs: the visiting fellow program, which is open to applicants with a doctoral degree or equivalent and 5 years or less of research experience, and the visiting scientist program is open to applicants with a doctoral degree or equivalent and at least 6 years of research experience. For detailed information see the website: http://www.training.nih.gov/postdoctoral. NIAID postdoctoral fellowship positions application can be submitted online. See: http://www.training.nih.gov/webforms/postdoctoral/application/adIndex.aspx?strSearch=NIAID.
- Office of Training and Special Emphasis Programs (OTSEP): This NIH Office actively recruits students interested in exploring careers in biomedical research. The OTSEP program is designed to facilitate training programs that promote diversity in NIAID laboratories. The intramural NIAID research opportunities program recruits underrepresented minority students interested in exploring career opportunities in allergy, immunology, and infectious diseases. The summer internship program in biomedical research provides an opportunity to high school and undergraduate students to join one of the NIH or NIAID laboratories to work with world-leading scientists in the field of biomedical research. Students 16 years of age or older who are U.S. citizens or hold a green card and are currently enrolled at least half-time in high school or an accredited U.S. college or university are eligible to apply. Students who have been accepted into a college or university may also apply. For more information check following websites: http://www3.niaid.nih.gov/about/organization/dir/otsep.htm and http://www.training.nih.gov/student.
51.3 Program Project/Center Grants (P series; Solicited or Unsolicited Applications)
The P series grants are large, multidisciplinary, multi-project, long-term research programs with a common theme. NIH ICs issue FOAs to indicate their interest in funding these types of program grants. Multi-project grants may be investigator-initiated or submitted in response to an FOA. Potential applicants should not only follow the instructions provided in the PHS 398, but also should check the IC's websites for additional instructions. Furthermore, before considering applying for a multi-project grant, potential applicants should speak with NIAID program staff. Pre-application approval is required for the acceptance of an investigator-initiated unsolicited program grant application. Some multi-project application mechanisms are not accepted as investigator-initiated applications, but are submitted in response to FOAs (RFAs or PAs). NIAID has special instructions for the preparation of the multi-project applications. For detailed information see the NIAID website at: http://www.niaid.nih.gov/ncn/grants/multi/index.htm.
Research Program Project Grant (P01): This mechanism supports integrated, multidisciplinary, multi-project research projects, often long-term research programs with an objective or theme involving a number of independent investigators who share knowledge, experience, expertise, and common resources. Each project contributes or is directly related to the common theme of program, thus forming synergistic research activities and projects directed toward a well-defined research program goal. NIAID usually accepts unsolicited program project applications with at least two interrelated research projects having a common theme and shared resources, cores or facilities. In general, there is no specific budgetary limit for these applications unless specified in FOA. Note: If the application is not in response to an RFA, potential applicants should discuss ideas with the program officer and should obtain pre-approval for the submission of the application. Applications for more than $500,000 in direct costs in any given year require a pre-approval (prior to the submission) from the funding institute.
Center Core Grants (P30): This multi-project mechanism supports shared resources and facilities for a multidisci-plinary research team or a group of investigators focusing on a common research topic to extend and enhance the effectiveness of the research. The core center grants provide accessible resources to extramural community (see Part V, Identifying Research Resources and Funding Opportunities), fostering interaction and collaborations between investigators at multiple institutions to promote a multifac-eted approach to a common theme. Generally, this mechanism is available only through a RFA or a PA. To be eligible for a core center grant, the potential applicant's institution already must have a substantial program base and investigators that will benefit from shared resources. For eligibility, visit the IC websites and and FOAs (RFA or PA).
Specialized Center Grants (P50): This award mechanism supports multi-project grants in all aspects of R&D, from basic to clinical, and may involve ancillary support activities such as patient care. Like P30 grants, this mechanism is available only through an RFA. Investigator-initiated applications are not accepted for this mechanism. There is substantial program staff involvement after the award. Centers also may serve as regional or national resources for special research. For further information and instructions for preparation of a multi-project center grant see the NIAID website: http://www.niaid.nih.gov/ncn/grants/multi/index.htm.
Exploratory Grants (P20): This mechanism often is used to support planning activities associated with large multi-project program project grants, e.g., P50 center grants, which are usually by RFA only.
51.4 Responding to an Institute-Specific FOAs (Solicited Applications)
Institute-specific FOAs, formerly known as RFAs, RFPs, and PAs, state the areas in which institutes or centers have interest and would like applications to be submitted. Applying in response to an initiative could be advantageous to potential applicants studying similar scientific areas, because there is often set-aside money allocated to fund these applications. However, competition for set-aside funds is vigorous, and such a strategy may or may not enhance one's chances of getting funds. NIAID promotes basic and applied research in scientific areas that pose an emerging opportunity. NIAID also must respond to new, emerging diseases, such as West Nile virus, pandemic influenza, re-emerging diseases, such as malaria and multi-drug resistant tuberculosis, as well as Project BioShield mandates, such as radiation countermea-sures. NIAID program divisions fulfill their scientific requirements by collectively issuing RFAs, RFPs, and PAs in the NIH Guide at: http://grants.nih.gov/grants/guide/index.html and in Grants.gov http://www.grants.gov as FOAs and by supporting a broad array of investigator-initiated studies.
RFA: RFAs are initiatives sponsored by one or more NIH institutes or centers to stimulate research in well-defined scientific areas identified by the funding institute. RFAs have a single receipt date, set aside funds, and indicate the number of awards likely to be made. In responding to an RFA, applicants must respond to the specifics of the announcement. Investigators should check the NIAID concepts page at http://www.niaid.nih.gov/ncn/budget/in-main.htm for highest priority areas. Applications are peer-reviewed based on the five NIH standard review criteria and RFA-specific review criteria, if any (see Part V, Preparing and Submitting a Competitive Grant Application). Competitive applications are funded based on scientific and technical merit, programmatic relevance, and availability of funds. Applicants should capitalize on their strengths when responding to an initiative and seek collaborations to build their sustainable personal research portfolio. Before writing the application, applicants should contact the program officer or the review staff listed in the announcement to discuss the science and the Institute's priorities. NIAID RFAs are published as FOAs in Grants. gov: http://www.grants.gov. To find active NIAID initiatives, go to: http://www.niaid.nih.gov/ncn/budget/opps.htm. If an RFA uses more than one funding mechanism, Grants. gov will list a separate FOA for each mechanism.
RFP: An RFP is an initiative sponsored by an NIH institute that requests proposals for a contract to meet a specific need of that institute. Proposals consist of two parts: technical and business. The technical part includes a description of the project, personnel, and facilities to carry out the proposed work. The business part includes cost information. RFPs, like RFAs, have a set proposal receipt date but are published in FedBizOps, the Federal Business Opportunities website at http://www.fbo.gov; sometimes they are listed in the NIH Guide to Grants and Contracts. Contract procurement legally binds the federal agency (NIH IC) and the offeror—the recipient of the funds—obligating the offeror to provide a product or service as defined by NIAID and obligates the institute to pay for that product or service. To find published initiatives, go to NIAID's R&D contracts page: http://www.niaid.nih.gov/contract/default.htm. Potential offerors should check http://www.fedbi-zopps.gov routinely for RFPs and amendments. NIAID does not notify offerors directly of changes or amendments to the RFPs. RFPs are indexed on the NIAID website Fed-BizOpps, but amendments appear only on FedBizOpps.
Institute-Specific PAs: PAs generally request grant applications, which are investigator—initiated grants in specific scientific areas. PAs are often broader in scientific scope than RFAs. Most PAs do not have set-aside funds, although some do. NIAID publishes PAs in the NIH Guide and in Grants.gov as FOAs. These initiatives have standard receipt dates, and usually open for 3 years. PAs also have special requirements for applicants. For further information check the NIAID funding opportunity website: http://www.niaid.nih.gov/ncn/budget/opps.htm.
51.5 Support for International Research
The NIAID research mission of conducting and supporting research in infectious, allergic and immunologic diseases is of global importance. NIAID has two international offices to coordinate domestic and international efforts for emerging and re-emerging infectious diseases. The Office of Global Research coordinates and supports collaborative studies in developing countries. This office responds to new disease outbreaks, and facilitates partnership with the Fogarty International Center, the World Health Organization, the State Department and the Center for Disease Control. The other office of the NIAID, the Office of International Extramural Activities, manages international research awards. NIAID has established various international research programs: NIAID intramural research training and collaborative research; non-U.S. investigator-initiated awards; U.S. investigator awards with international components; domestic training programs; bilateral scientific agreements with non-U.S. governments or organizations; multilateral programs with the World Health Organization, UNAIDS, and European Union; and most importantly, through interagency agreements with other federal agencies. NIAID has very active multidisciplinary research programs to build and maintain research capacity in developing countries. To see the complete list of NIAID international grants and contracts, visit the NIAID funding website, http://www.niaid.nih.gov/ncn/grants/int/default.htm; resources available to seek collaborations are described in Part V, Identifying Research Resources and Funding Opportunities.
Most NIH grants are awarded to domestic institutions. However, non-U.S. investigators or grantee institutions do not need U.S. affiliation or citizenship to apply for research project grants (R01s), small grants (R03s), or exploratory/ developmental grants (R21s) unless specified in the FOA. However, some grant types do have a citizenship requirement, including small business and training grants. Non-U.S. investigators should read the guidelines and eligibility requirements at http://www.niaid.nih.gov/ncn/grants/basics/basics_a2.htm. In general, NIH does not award program projects, centers, resources, institutional national research services awards, business grants, or construction grants to non-U.S. institutions. It is very important for non-U.S. investigators to focus on their strengths, such as unique expertise, resources not available in the U.S. or regionally important diseases and patient populations. Applications are assessed on the relevance of the proposed research to NIH or NIAID missions, whether similar work is being done or can be done in the U.S., and whether there is a need for the research in a global health perspective.
The NIH Fogarty International Center, the international component of the NIH, addresses global health challenges through innovative and collaborative research and training programs and supports and advances the NIH mission through international partnerships. To address these needs, the Fogarty International Center (FIC) and NIAID support collaborations with domestic and international partners in international scientific research and training to reduce disparities in global health. Examine the brochure “International Opportunities in Biomedical Research and Training” at http://www.fic.nih.gov/news/publications/interop_03-2004.htm. The website describes programs and other international opportunities supported by the NIH. The NIH Fogarty International Center provides a variety of training programs in biomedical fields. Below are some of the Fogarty research training opportunities at http://www.fic.nih.gov/programs/training_grants/index.htm.
AIDS International Training and Research Program (AITRP): This program provides grant awards to U.S. institutions to support HIV-AIDS related research training with institutions in low- and middle-income countries. The grantees at U.S. institutions collaborate with non-U.S. institutions to develop joint research training programs. Details are at: http://www.fic.nih.gov/programs/training_grants/aitrp/index.htm#introduction.
FIC/Ellison Clinical Research for US Graduate Students: The Fogarty Center, in collaboration with the Ellison Medical Foundation and NIAID, provides 1-year clinical research training experiences for U.S. graduate students in developing countries. Details can be seen at: http://www.fic.nih.gov/programs/training_grants/fic_ellison.htm.
Global Infectious Disease Research Training Program (GID): The Fogarty Center and the CDC have collabora-tively developed this program to address research training related to infectious diseases that are predominantly endemic in developing countries. Details can be seen at: http://www.fic.nih.gov/programs/training_grants/gid.htm.
Informatics Training for Global Health (ITGH): This program supports informatics training in low- and middle-income countries in partnership with U.S. institutions and investigators. Details can be seen at: http://www.fic.nih.gov/programs/training_grants/itgh/index.htm.
International Research Ethics Education and Career Development Award: The Fogarty Center in partnership with several NIH institutions including NIAID support domestic and international educational and training opportunities in international bioethics related to research conducted in low- and middle-income countries. Details can be seen at: http://www.fic.nih.gov/programs/training_grants/bioethics/index.htm.
International Clinical, Operational and Health Services Research Training Award for AIDS and TB (ICOHRTA AIDS/TB): This program awards grants to U.S. investigators' institutions to support clinical, collaborative, multidis-ciplinary, international, operational, and health services at U.S. and non-US collaborating sites and mentored research and training in low- and middle-income countries. Details can be seen at: http://www.fic.nih.gov/programs/training_grants/icohrta/index.htm.
International Clinical, Operational and Health Services Research Training Award for AIDS and TB (ICOHRTA AIDS/TB): This program awards grants to institutions located in low- and middle-income countries where AIDS, TB or both are widespread to strengthen their capacity to conduct clinical, operational and health services research. Details can be seen at: http://www.fic.nih.gov/programs/training_grants/icohrta/aids_tb.htm.
International Collaborative Genetics Research Training Program: This program promotes international collaborative activities between U.S. and non-U.S. investigators to build institutional infrastructure for the advancement of the genetic science and develop scientists and health specialist with expertise in human genetics. Details can be seen at: http://www.fic.nih.gov/programs/training_grants/genetics.htm.
International Research Scientist Development Award (IRSDA): This program provides opportunities to junior U.S. scientists and postdoctoral candidates to develop careers in international health research and establish collaborations in developing countries. The program is similar to NIH career development award (K01) but the main focus is the developing world. It supports basic, behavioral and clinical scientists. Details can be seen at: http://www.fic.nih.gov/programs/training_grants/irsda.htm.


