Abstract
The persistence of West Nile virus (WNV) infections throughout the USA since its inception in 1999 and its continuous spread throughout the globe calls for an urgent need of effective treatments and prevention measures. Although the licensing of several WNV vaccines for veterinary use provides a proof of concept, similar efforts on the development of an effective vaccine for humans remain still unsuccessful. Increased understanding of biology and pathogenesis of WNV together with recent technological advancements have raised hope that an effective WNV vaccine may be available in the near future. In addition, rapid progress in the structural and functional characterization of WNV and other flaviviral proteins have provided a solid base for the design and development of several classes of inhibitors as potential WNV therapeutics. Moreover, the therapeutic monoclonal antibodies demonstrate an excellent efficacy against WNV in animal models and represent a promising class of WNV therapeutics. However, there are some challenges as to the design and development of a safe and efficient WNV vaccine or therapeutic. In this chapter, we discuss the current approaches, progress, and challenges toward the development of WNV vaccines, therapeutic antibodies, and antiviral drugs.
Key words: West Nile virus, Vaccine, Antiviral drug, Therapeutic antibody
Human WNV Diseases and the Need for Antiviral Drug or Vaccine
West Nile virus (WNV), a neurotropic RNA virus belonging to the flaviviridae family, is generally transmitted to human by infected mosquito bites, primarily by species [1, 2]. However, WNV can also be transmitted through other less frequent routes, including a transfusion of blood and blood components [3, 4], organ transplantation [5], breastfeeding [6], and congenital infections [7]. After an infected mosquito bite, WNV replicates in keratinocytes and skin-residential dendritic cells (Langerhans cells), and the latter cells carry the viruses to draining lymph nodes to cause viremia [8, 9]. Subsequently, WNV disseminates to the peripheral organs, such as spleen and liver, and possibly to spinal cord and brain. Human WNV infection may cause injury and death of neurons with various clinical manifestations, such as encephalitis, meningitis, flaccid paralysis, persistent neurologic sequelae, and possibly death, particularly in the elderly and immunocompromised individuals [1, 10, 11]. WNV strongly activates host immune responses, which play important roles in controlling viremia, viral dissemination to the central nervous system (CNS), and recovery from the disease [11]. However, the mechanism of WNV pathogenesis, including its tropism to neurons, CNS invasion, and viral or host factors that contribute to imbalance between viral pathology and host immunity still remain poorly understood.
Although WNV was first discovered in Uganda in 1937, it had been considered as a minor public health concern until its first appearance in the USA in 1999 [12]. Since then, it has dramatically spread to all the continental states of the USA and became an endemic disease throughout North America within a few years [13–16]. In the USA alone, there have been over 40,000 reported cases of WNV between 1999 and 2014, of which ~45 % were classified as neuroinvasive and claimed lives of nearly two thousand people [17]. However, the actual WNV burden is likely much higher than previously thought because only about 20 % of infected individual develop a clinical WNV disease [13]. It has been estimated that over three million individuals have been infected with WNV in the USA, of which about 780,000 had a symptomatic disease [18]. WNV also has potential to develop unusual clinical manifestations [19–21] and may involve in renal diseases [22, 23], myasthenia gravis [24], and myocarditis [25], suggesting that the range and severity of WNV disease may be even worse than previously believed. Importantly, increasing numbers of WNV outbreaks during the last 15 years have been associated with greater number of neuroinvasive cases and a higher rate of fatalities [16, 17]. However, no vaccine or antiviral therapeutic is currently available, which limits current treatments to only supportive care measures, such as intravenous fluids, antipyretics, respiratory support, and prevention of secondary infections. Considering the worldwide distribution of this virus and evidence of its potential to change in pathogenicity and transmission [26–30], there is an urgent need to develop safe and effective antiviral drugs or vaccines against WNV infection [31]. Intensive research during past decades has made significant progress in the design and development of several treatment and prevention methods for WNV infection (reviewed by [32–34]). Here, we discuss the current approaches and recent progress toward the development of vaccines, therapeutic antibodies, and antiviral drugs against WNV infection in humans.
WNV Structure and Therapeutic Targets
WNV is a spherical virus with 50 nm in diameter, which comprises an icosahedral nucleocapsid surrounded by a lipid envelope [35]. The virus contains a single-stranded, capped, and plus-sensed RNA genome of approximately 11 kb in size. The viral genome encodes a polyprotein precursor, which undergoes posttranslational processing by cellular and viral proteases to generate three structural proteins (capsid [C], premembrane [PrM], and envelope [E]), and seven non-structural proteins (NS1, NS2A, NS2B, NS3, NS4A, NS4B, and NS5). The structural proteins form virion structure, whereas the non-structural proteins play essential roles in the replication of viral genome, assembly of virion, and viral pathogenesis [36–38]. Thus, the structural and non-structural proteins of WNV may be potential targets for developing vaccines and antiviral therapeutics (Table 1).
Table 1.
Therapeutic targets of WNV structural and non-structural proteins
| Targets | Structural features | Functions | Targeting approaches |
|---|---|---|---|
| Envelope (E) | Contains a central domain I, a extended finger-like domain II, a immunoglobin-like domain III and a hydrophobic pocket between domain I and II; domain II contains a fusion loop; contains the major epitopes for B and T cells | Mediates virus binding to host cellular receptors and membrane fusion | Drug targeting to block viral entry, disrupt membrane fusion, and produce neutralizing monoclonal antibodies |
| Capsid (C) | Alpha-helical structural protein | Encapsidates viral RNA, induces host cell apoptosis [326], and to disrupt nucleosome formation | Internal deletion in capsid gene results in deficient replication and reduced pathogenicity [113] |
| Membrane (PrM) | Forms heterodimer with E protein | Virion assembly and fusion modulation [38] | Antigen for production of antibodies |
| NS1 | Secreted glycoprotein, contains epitopes for antibody production | Immune evasion activities [37] | Potent antigen for antibody production; ablation of NS1 glycosylation attenuates WNV |
| NS2A | Transmembrane protein, associates with endoplasmic reticulum membrane; component of replication complex | Virion assembly/maturation; antagonizes host immune responses | Alanine to proline substitution at position 30 of NS2A attenuates viral virulence [36] |
| NS2B | Consists of a 40-amino acid hydrophobic region, transmembrane protein, component of replication complex | The 40-amino acid hydrophobic region serves as cofactor for NS3 | NS2B cofactor activity can be targeted by inhibitors |
| NS3 | Multifunctional protein containing two functional domains; contains a shallow ATP binding pocket and an additional domain that is not present in human helicases | Serine protease (N-terminal); Helicase (C-terminal) | Substrate-based inhibitors can target NS3 protease; small-molecule inhibitors may target helicase domain |
| NS4B | Transmembrane protein, component of replication complex | Inhibits NS3 ATPase activity | NS4B forms ATP-binding site that may be targeted by a drug |
| NS4B | Transmembrane protein, component of replication complex | Participates in viral replication and immune evasion | Selected mutations [365] in NS4B attenuate WNV |
| NS5 | Multifunctional protein containing two functional domains | Methyltransferase and guanyltransferase (N-terminal); RNA-dependent RNA polymerase (C-terminal) | NS5 functions can be targeted by various inhibitors |
In a WNV virion structure, C protein encapsulates viral genomic RNA to form a nucleocapsid that is enveloped by a lipid bilayer into which trimmers of prM-E heterodimers form the spike-like projections. Among these structural proteins, E protein mediates crucial roles in binding to cellular receptors, membrane fusion, and entry of WNV into host cells, making it a key target for the development of vaccines, neutralizing antibodies, and entry inhibitors. Crystal structure analysis has confirmed that E protein folds into three structurally distinct ectodomains (EDs) termed EDI, EDII and EDIII [39–43]. Among these, the EDIII consists of the major neutralizing epitopes and is an antigen of choice to elicit production of neutralizing antibodies [43–46]. Based on the structural characterization of antiflaviviral monoclonal antibodies from both human and nonhuman primate, it appears that the epitopes of flaviviral E protein are more complex and diverse than previously thought [47–50]. In addition, mapping of B-cell and T-cell epitopes has led to the identification of many immunodominant epitopes in both structural and nonstructural proteins of WNV [51, 52].
Among the non-structural proteins, NS3 and NS5 are best-characterized, multifunctional proteins, both of which contain enzymatic activities that are essential for viral replication [53–55]. Such enzymatic functions of NS3 and NS5 have received considerable attention as potential targets for antiviral drug development [34, 54]. The NS3 protein contains two distinct functional domains. The N-terminal domain of NS3 (184 amino acid residues) has serine protease activity that requires a polypeptide cofactor NS2B for activation [54, 56, 57]. Recent X-ray crystallographic studies have shown that the conformation of β-loop of NS2B controls the substrate binding by NS2B/NS3 protease [58, 59]. In contrast, the C-terminal domain of NS3 functions as an RNA helicase, nucleoside triphosphatase, and RNA triphosphatase [60, 61]. Although the ATPase and helicase activities of NS3 function independently, NS4A protein has been suggested to regulate both of these activities [62]. Besides its role in cleaving the viral protein, the protease activity of NS3/NS2B may also contribute to host cell apoptosis and neuropathogenesis by cleaving host proteins [63]. Similarly, NS5 is another multifunctional protein containing N-terminal methyltransferase/guanyltransferase, and C-terminal RNA-dependent RNA polymerase (RdRp) activity [64, 65]. The N-terminal methyltransferase and guanylyltransferase activities of NS5 are essential for the formation of a cap structure in viral mRNA [66]. Thus, the functions of NS5 are crucial for both protection of viral genome and efficient translation of viral polyprotein. The N-terminal domain of NS5 contains multiple residues that can be phosphorylated by host protein kinases [67]. Besides its function in viral replication, NS5 also plays a role in viral pathogenesis by antagonizing host’s interferon response [28]. Other nonstructural proteins NS2A, NS2B, NS4A, and NS4B form the scaffold for the viral replication complex and also have roles in the replication of viral genome and host immune evasion [68–70]. Mutations in NS4B protein may attenuate WNV and other flaviviruses [68, 69]. In addition, a recent successful clinical trial of a hepatitis C virus NS5A inhibitor suggests that targeting non-structural proteins may be an ideal strategy to develop therapeutics against other flaviviruses, including WNV [71].
Current Approaches and Progress in WNV Vaccine Development
WNV infection induces potent activationof host immune responses that is critical for controlling viremia, viral dissemination into the CNS, and recovery from WNV diseases [1, 11]. Studies of WNV pathogenesis in animal models have demonstrated that humoral responses (antiviral antibodies) are essential in limiting viremia and neuroinvasive diseases [72, 73]. Thus, development of a vaccine that produces high titer of neutralizing antibodies would offer efficient protection against WNV infection [44]. Several epitopes for both B and T cells have been characterized in WNV proteins [52, 74–78]. In particular, the C-terminal EDIII of E protein that contains critical neutralizing epitopes is the major target for neutralizing antibodies against WNV infection [40, 44, 78, 79]. In addition to humoral immune response, cell mediated immunity by CD4+ and CD8+ T cells play critical roles in recovery from WNV infections [80–83]. Thus, the efficient generation of vaccine-induced immunity against WNV may also require activating and shaping of multiple effectors of adaptive immune response by early innate signaling pathways [84–86].
Vaccine Developments and Testing
Development of aneffective vaccine requires multiple steps from design and development to rigorous evaluation of both safety and efficacy (Fig. 1). Since the biology of vaccine-induced immunity and principles in vaccine development and testing have been extensively discussed before, we will not discuss these topics here. Immunization of the laboratory animals and subsequent challenge of those animals with a pathogen under controlled conditions is the common method for early evaluation of vaccine effectiveness. Various factors such as route of administration, immunogen dose, and type of adjuvant greatly influence the effectiveness of a vaccine candidate. Mouse models of WNV infection partially mimic the clinical course of WNV disease in humans, which not only help with the understanding of WNV pathogenesis but also facilitate the development and testing of WNV vaccines. In animal studies of vaccine efficacy, infected animals are observed for mortality and monitored for survival, pathology, seroconversion, immune responses, and vaccine safety. Plaque reduction neutralization test (PNRT) is a gold standard method to assess whether a candidate WNV vaccine induces neutralizing antibodies in both animals and humans, for which standard guidelines and methods are available [87]. Neutralizing antibody titer is correlated with protection against disease for other licensed flavivirus vaccines and is considered a key marker to assess vaccine efficacy [88]. For example, the protective threshold for a Japanese encephalitis virus vaccine is correlated with the titers of neutralizing antibodies, with a serum PRNT50 ≥ 1:10 considered protective [88]. Although WNV lineage 1 strains are commonly involved in human disease and were used in vaccine efficacy testing, the recent emergence of pathogenic lineage 2 strains in Europe has raised additional concerns in WNV vaccine efficacy studies, because WNV vaccine candidates based on lineage 1 strains may not protect against the lineage 2 strains. After promising results from animal studies, human WNV vaccines are further evaluated in terms of protection against natural challenge, as well as their safety and immunogenicity during series of clinical trials (phases I–III).
Fig. 1.

Overview of vaccine development process
Vaccine Development Approaches
Both traditional and modern approaches have been used for the development of WNV vaccines, and the most common approaches are listed below.
-based vaccine: By using the power of modern genetic tools, viral protein(s) can be expressed in a suitable vector to develop DNA vaccines against WNV infection [89, 90]. However, this strategy is sometimes hampered by poor immunogenicity and potential safety concerns, such as integration of foreign DNA into the host genome.
Chimeric/recombinant vaccine: This approach relies on the replacement of gene(s) of the established viral vaccine strain by equivalent WNV genes. Several live attenuated vaccines and viral vectors may be used as a backbone for developing recombinant WNV vaccine candidates, such as yellow fever virus (YFV) vaccine (YFV-17D) [91, 92], attenuated DENV serotype 4 (DENV4) [93, 94], HIV-based lentivirus vector vaccine [95], Schwarz strain of attenuated measles virus [96], vesicular stomatitis virus (VSV) vaccine vector [97, 98], and adenovirus A [98].
Live-attenuated vaccine: Attenuation of WNV can be achieved by classic cell culture passage or animal passage andtargeted genetic mutations. However, several potential challenges including residual pathogenicity, reversion to virulent strain, relative short self-life, and the demand of a safe biological production system need to be overcome.
Inactivated (killed) : Chemical inactivation of live viruses may be used to develop inactivated or killed virus particles. The limitations are the possibility of incomplete inactivation, short-lived immunity, and the requirement of multiple doses for efficient immunization.
Subunit or recombinant protein vaccine: This approach uses soluble recombinant protein(s) or protein expressed in virus-like particle (VLP) platform as a vaccine candidate. Success of this approach relies on optimum immunogenicity of a vaccine, as the protein vaccines generally require multiple boosts with strong adjuvants to provide acceptable efficacy.
Anti-WNV Vaccine Candidates Currently in Development and Clinical Trial
Although no vaccine is currently available against WNV infection in human, several WNV equine vaccines are available (Table 2). Several human vaccines are under development, and some are in clinical trials (Table 3). Their current status and approaches used for development are discussed below.
Table 2.
Licensed WNV vaccines in veterinary use
| Vaccine name | Company | Vaccine approach | Design/features | Status | References |
|---|---|---|---|---|---|
| West Nile-Innovator® DNA | Fort Dodge Animal Health/Pfizer | DNA | Plasmid DNA encoding WNV prM-E | Licensed (discontinued) | Davis et al. [89] |
| Vetera™ West Nile vaccine | Boehringer Ingelheim | Killed | Whole virus | Commercialized | |
| West Nile-Innovator® | Pfizer | Formalin inactivated | Whole virus | Commercialized | Ng et al. [127] |
| RecombiTek® | Merial | Recombinant vaccine | WNV prM-E in canarypox virus | Commercialized | Karaca et al. (2005) |
| PreveNile® | Intervet | Recombinant vaccine | WNV prM-E in yellow fever vaccine (17D) backbone | Licensed in 2006 (recalled in 2010 after severe reaction) |
Table 3.
Human WNV vaccine candidates in clinical trial
| Vaccine name (company) | Vaccine approach | Design/features | Status of development | Reference(s) |
|---|---|---|---|---|
| Chimeri-Vax-WN (Acambis, Sanofi-Pasteur) | Recombinant | WNV prM-E and E replacing capsid and non-structural protein of yellow fever vaccine strain (17D) | Phase I clinical trial completed, Phase II trial ongoing | Biedenbender et al. [366] |
| WN-DEN4 | Recombinant | WNV prM gene in a backbone of attenuated DEN-4 | Phase I clinical trial completed, Phase II trial ongoing | Pletnev et al. [94] |
| WN-80E | Subunit protein | Recombinant E protein lacking transmembrane domain | Phase I clinical trial completed | Lieberman et al. [136, 147] |
| WNVDNA017-00-VP (VRC in collaboration with Vical) | Plasmid based DNA vaccine | Plasmid DNA vector that express WNV-NY99 prM-Env under a cytomegalovirus promoter | Phase I clinical trial completed | Martin et al. [90]; Ledgerwood et al. [100] |
| HydroVax-001 (OHSU, NIH funded) | Inactivated | Chemical inactivation by H2O2 | Phase I clinical trial ongoing | http://www.nih.gov/news/health/jul2015/niaid-06.htm |
DNA-Based Vaccine
West Nile Innovator® DNA is the first licensed DNA vaccine for veterinary use following a successful demonstration of vaccine induced B and T cell-based immunity after immunization of mice and horses with DNA vaccines expressing prM and E genes of WNV [89] or the domain III (DIII) region of E gene [99]. This approachhas been widely used do develop various DNA vaccines against WNV. One of the first DNA vaccines introduced in phase I clinical trial was based on a circular plasmid DNA vector incorporating a cytomegalovirus (CMV) promoter to express the WNV-NY99 prM and E coding sequences in downstream of a modified JEV signal sequence (VRC-WNVDNA017-00-VP) [90]. Although no serious adverse effects were reported, its low immunogenicity hampered further development [90]. In an effort to improve immunogenicity of this vaccine, an additional regulatory element from human T-cell leukemia virus type 1 (HTLV-1) was incorporated in conjunction with the previously used CMV promoter [100] but without significant success when tested in the clinical trial [100]. Several studies have also tested carrier-conjugation and different inoculation routes [101, 102] to improve immunogenicity and efficient delivery of DNA vaccines. For example, a DNA vaccine expressing full length of truncated WNV E gene derivatives conjugated to the P28 region of C3d (a complement protein) induced strong IgG titers and efficient protection of mice when vaccinated by gene gun method [103]. In another study, a plasmid DNA vector expressing the ectodomain of WNV E protein into linear polyethyleneimine (lPEI) nanoparticles covalently bound to mannose was developed. However, this conjugation failed to generate sufficient E-protein specific humoral responses, despite the boosting of the vaccinated mice with recombinant E protein induced a significant increase in neutralizing antibodies [104].
Large deletions of capsid gene in the flaviviral RNAs result in a failure to produce infectious virions but retain the ability to replicate viral RNA genome and express prM and E proteins [105, 106]. This novel property has been used to develop several plasmids DNA (pDNA) vectors that after transfection produce single-round infectious particles (SRIPs), which in turn produce virus-like particles (VLP) containing viral surface proteins without viral genome. This strategy has been used to develop several candidate DNA vaccines against flavivirus by expressing E and prM proteins in a plasmid vector and forming VLPs. Using this approach, several plasmid-DNA constructs were developed as candidate DNA vaccines against WNV. This type of DNA vaccines encode for single-round infectious particles expressing E/prM [107–109], a full-length cDNA copy of attenuated WNV Kunjin strain [110], or ectodomain of E protein [111].
Live Attenuated Vaccine
Cell culture or animalpassage used to be conventional methods to develop live-attenuated vaccines. The advents of genetic manipulation techniques make it feasible to introduce targeted mutations into the viral genome and attenuate viruses. Using this approach, a WNV vaccine (RepliVAX WN) developed by an internal deletion of a region in capsid gene [112, 113] has been shown to induce neutralizing antibodies and protective immune responses in mice [114], hamsters [108], and nonhuman primates [109]. RepliVAX WN strongly activates B cell population secreting anti-NS1 IgG antibody and induces prolonged activation of memory CD8+, CD4+, and NS1 specific plasma cells [115]. Innate immune signaling pathways, such as TLR3 and MyD88-dependent signaling pathway are involved in strong activation of B cell response, development of germinal center, generation of long-lived plasma cells, and production of antibodies following immunization with RelpiVax WN vaccine [116]. In addition, another live attenuated WNV vaccine developed by generating mutations in glycosylation sites of E and NS1 proteins induces neutralizing antibodies and protective immune responses in mice [117]. Similarly, approaches of introduction of mutations in NS4B [69], NS2A [36], or E gene that were previously characterized to attenuate JEV-SA-14142 [118] have also been used to develop attenuated WNV vaccine candidates.
Chimeric/Recombinant Vaccine
Using this approach, a recombinant live attenuated WNV vaccine for veterinary use was developed and licensed in 2004 by Merial (RecombiTEK). This vaccine expresses WNV prM and E proteins in a canarypox virus backbone [119, 120]. Using a similar approach, a chimeric vaccine (ChimeriVax-WN02) has been developed by replacing prM and E genes in YFV vaccine strain (YFV-17D) with WNV-NY99 prM and E genes [91, 121]. ChimeriVax-WN02 was the first recombinant WNV vaccine candidate tested in clinical trial. Introduction of three mutations responsible for attenuation of JEV (SA14-14-2) in equivalent positions of WNV E gene further attenuated ChimeriVax-WN02. Similarly, DENV4 vaccine candidate (rDEN4Δ30), attenuated through a 30-nucleotide deletion in the 3′ untranslated region (UTR) of the viral genome, was further engineered to express WNV-NY99 prM and E [122, 123]. After preclinical evaluation in mice, geese, and monkeys, rDEN4Δ30 showed strong immunogenicity in the clinical trial [94]. In addition, a chimeric DENV2 vaccine candidate expressing the WNV NY99 prM and E proteins has been shown to protect mice from infection with WNV NY99 strain [124]. Another recombinant WNV vaccine based on influenza vaccine (FLU-NA-DIII) was developed by cloning DIII of WNV E into the N-terminal region of neuraminidase of influenza virus. This vaccine candidate induced WNV-specific neutralizing IgG and protected mice against lethal WNV infection [125]. Similarly, a recombinant adenoviral vaccine vector (CAdVax-WNVII) expressing all three structural proteins (C, prM, and E) along with NS1 of WNV induced neutralizing antibodies in mice [98]. Several other recombinant WNV vaccines have also been developed by expressing WNV protein in the backbones of attenuated measles virus [96], vesicular stomatitis virus [97], and herpes virus-1 [126].
Inactivated (Killed) Vaccine
The most commonapproach to develop non-replicating inactivated viral vaccines is to inactivate entire virus particles by using chemicals. A formalin-inactivated WNV vaccine based on WNV-NY99 strain was the first successful veterinary vaccine (marketed by Pfizer as West Nile Innovator®) licensed in 2003 [127]. Another veterinary WNV vaccine using killed virus was also licensed by USDA (marketed by Boehringer Ingelheim as Vetera™ WNV). Recently, an inactivated WNV vaccine (WN-VAX) based on WNV NY99 protects mice against lethal WNV infection and exhibits immunogenicity in monkeys [128]. In addition to WNV NY99, formalin inactivation of WNV IRS98 strain induces neutralizing antibody and protects immunized geese [129]. As an alternative to traditional formalin-based vaccines, a novel hydrogen peroxide (H2O2) inactivation approach has been recently used to produce a whole-virus vaccine against WNV [130, 131]. Mice immunized with H2O2-inactivated WNV vaccine candidate developed high serum neutralizing titers, and offered complete protection of vaccinated mice against lethal WNV challenge [130]. One of such H2O2 inactivated vaccine (HydroVax-001) has been recently introduced into phase I clinical trial. Although inactivation of virulent WNV virus strain has been successfully achieved by chemical-inactivation method [127, 129], use of a naturally attenuated Kunjin strain of WNV [132, 133], or chemically synthesized virus by cDNA system [134] as starting material has also been proposed.
Subunit, VLP, or Recombinant Protein Vaccine
Several studies demonstrated that soluble recombinant protein or VLP based approach could serve to develop WNV vaccines [135–139]. VLP are specialized subviral particles that lack of viral genome and solely contain viral structural proteins [140, 141] or express viral proteins on envelope membranes [142, 143]. Different vectors and production system were evaluated for development of various subunit vaccines against WNV. For example, a recombinant truncated form of WNV E protein produced in Escherichia coli induced neutralizing antibodies and protected mice from lethal WNV challenge [46, 144]. In addition, a recombinant truncated WNV E protein produced in the SF+ insect cells via baculovirus infection induced neutralizing antibodies and protected mice and hamsters from WNV infection [145]. Recombinant baculovirus was also used to express WNV prM and E proteins in mammalian cells under the CMV promoter, with or without vesicular stomatitis virus glycoprotein (Bac-G-prM/E). Such vaccines induced robust immune responses when inoculated in mice and produced both neutralizing antibodies and inflammatory cytokines [146]. In a recently proposed novel approach, known as pseudotyping, a retrovirus Gag polyprotein forms a VLP scaffold to display the ectodomain of human membrane glycoprotein (CD16) that was fused to the high affinity IgE receptor gamma chain (RIgE). Using this retrovirus based VLPs platform, a WNV vaccine was generated by replacement of the CD16 ectodomain in CD16-RIgE glycoprotein with EDIII of WNV, which induced neutralizing antibodies in mice [139].
A recombinant E protein of WNV-NY99 produced in Drosophila S2 cells (WNV-80E, developed by Hawaii Biotech) is the only WNV subunit vaccine candidate that has been tested in phase I clinical trial. Although preclinical studies revealed WNV-specific neutralizing antibody responses in vaccinated animals [136, 147, 148], the immunogenicity of this vaccine in humans was low. To increase immunogenicity, conjugation of recombinant proteins with nanoparticles or pathogen associated molecular patterns (PAMPs) as carrier/adjuvant have been tested. For instance, a recombinant WNV E protein administered with unmethylated CpG oligonucleotide adjuvant or loaded onto CpG-modified nanoparticles strongly activated dendritic cells and lymphocytes and elicited Th1-dominant immune responses by producing high titers of IgG2a and IgG2b in immunized mice [149, 150]. Similarly, mice injected with DIII of WNV E conjugated with bacterial flagellin (STF2∆.EIII) [137] or VLP derived from bacteriophage AP205 engineered to express DIII of WNV E (DIII-C-AP205) [138] also significantly increased neutralizing antibody production and protected the immunized mice.
Potential Novel Approaches for WNV Vaccine Development
Despite the intensive efforts in development of WNV vaccines, only a few reached the clinical trial stages. Of those in clinical trials, most candidate vaccines fail to demonstrate efficient immunity and safety. Development of new tools for antigen screening, expanded understanding of immunological correlates of vaccine induced-immunity, and discovery of novel adjuvants for vaccine delivery may facilitate the design and the development of WNV vaccines. For example, knowledge of genomic information and bioinformatics has been used for in silico identification of candidate antigens and development of vaccines by a novel method called “reverse vaccinology” [151, 152]. This comprehensive tool can quickly identify all potential antigens coded in the genome and may be used to develop a novel viral vaccine [153]. Development of vaccine against group B streptococci proved the potential of this approach [151]. Similarly, a “structural approach” that improves antigenicity of vaccines by rational designing has been developed by utilizing the knowledge of immunology, structural biology, and bioinformatics [154]. In addition, increased understanding of immunogenetics and role of environmental and host factors that determine the variation of vaccine immunity may offer new approaches to design a more effective vaccine against WNV infection in humans.
Antibody-Based Therapy: A Promising WNV Therapeutic
Therapeutic monoclonal antibodies (mAbs) or hyperimmune sera have been successfully used for prophylaxis of a number of infectious and noninfectious diseases, including WNV infection. In recent years, the number of mAbs in preclinical development and clinical trials has been increased significantly [155]. So far, nearly 50 mAbs have been approved for therapeutics by US FDA, including a humanized mAb Synagis (palivizumab) for preventive use against respiratory syncytial virus (RSV) infection in neonates and immunocompromised individuals [156–158]. Many mAbs have been developed against viruses, such as SARS-CoV, influenza, HIV-1, and other (re)emerging viruses including WNV [155, 159–163]. Some of them showed excellent therapeutic potential for clinical use in humans.
WNV infection induces a potent humoral immune response, which is essential in controlling viremia and limiting WNV dissemination to the CNS [72, 73]. Hyperimmune sera, γ-globulin, or affinity-purified antibodies harvested from WNV-infected humans and animals protect both wild-type and immunocompromised micefrom WNV challenge in laboratory conditions [164–166]. In addition, WNV patients who received antibodies from the WNV seropositive donors recovered from WNV infection [167–171]. These studies not only encouraged the efforts toward the development of human or humanized monoclonal antibodies against WNV, but also led to the discovery of several potent monoclonal antibodies that showed efficient protection of mice and hamsters from WNV infection [172–175]. Among these, a humanized anti-WNV mAb (Hu-E16) that binds to a highly conserved epitope in WNV E protein blocks viral fusion and provide post-exposure therapeutic potential [172]. This antibody is currently being assessed for its potential use as a WNV therapeutic antibody [176]. The phase I clinical trial showed that another humanized recombinant antibody targeting E protein of WNV (known as MGAWN1) has a good safety and tolerance profile in healthy humans [177], however, the phase II trial to assess its efficacy in WNV infection failed due to poor enrollment of participants. Besides the development of whole antibody, recombinant fusion proteins are also generated from single-chain antibody fragment of the variable region. Such antibody fragments that target E protein may be potential candidates for immunoprophylaxis and therapy of WNV infections. A recombinant human single-chain variable region antibody fragments (Fv-Fc) fusion protein has a protective role against WNV infection in mice [178].
One of the potential limitations of this approach is antibody-dependent enhancement (ADE), a phenomenon by which infection of some viruses is enhanced by virus-reactive antibodies resulting in more efficient virus entry through Fc receptor-mediated pathways. This phenomenon plays a role in the pathology of severe dengue infection and has also been observed in WNV in vitro [179]. Although the role of ADE in WNV disease in unclear, the development of a therapeutic antibody against WNV should address this potential issue. Another limitation of antibody-based therapeutics is high production cost, which limits mAb scalability. Producing therapeutic proteins, including antibodies in plants, may be a promising solution. Feasibility of this approach has been affirmed by the successful production of anti-WNV monoclonal antibody Hu-E16 in plants (Nicotiana benthamiana) (MAb-pE16) [180, 181]. The plant-derived MAb-pE16 confers a potent neutralizing activity in vitro without ADE, efficiently binds to complement and Fc receptors, and protects mice against lethal WNV- challenge with similar potency as their mammalian-cell counterparts [180, 182, 183].
Antibodies employseveral mechanisms to control WNV and other viral infections, including blockage of viral entry, Fc-dependent viral clearance, complement-mediated viral lysis, and antibody-dependent cytotoxicity of infected cells. Most of the current researches in the development of therapeutic antibodies against WNV are designed and tested for efficient neutralization potential [184, 185]. Increased understandings of the biology of antibody Fc regions, in particular, the roles of glycan in Fc mediated functions may facilitate the design and development of high-quality antibody through glycoengineering [186, 187]. Such engineering of antibody Fc region may be used to overcome ADE, modulate pharmacokinetics, and enhance Fc mediated effector functions, such as enhancement of antibody-dependent cell mediated cytotoxicity (ADCC), complement binding, and phagocytosis [188].
Recent technological advancement not only in development, production, and purification but also in ease of achieving desirable quality, efficacy, and safety required for the FDA approval makes monoclonal antibodies a promising therapeutic option. Thus, monoclonal antibodies may prove useful for WNV prophylaxis and therapy particularly in the elderly and immunocompromised individuals with limited ability to respond to a vaccine. To meet its therapeutic goal, a controlledclinical trial of therapeutic antibody should ensure its prophylactic and therapeutic efficacy along with optimal dose and timing of administration across the range of patient groups.
Moving Towards Anti-WNV Drug Discovery: Recent Approaches and Future Directions
Development of effective therapeutics have been successful in treating many viral diseases including influenza, HIV-1, hepatitis C virus (HCV), and hepatitis B virus (HBV). WNV causes transient viremia in human and animal models that is associated with its dissemination to brain and development of more severe disease [73, 189–192], suggesting that reducing viral loads by an antiviral drug during the early phase of infection may offer efficient control of WNV or lessen the chances of progression to neuroinvasive diseases. In addition, antiviral drugs are particularly useful for the elderly and the immunocompromised patients who may fail to develop efficient vaccine-induced immunity. Recent progress in the structural characterization of WNV and other flaviviruses broadens the understanding of WNV biology and provides a foundation for the development of small molecule inhibitors for WNV therapeutics [34, 193]. In addition, better understanding of the pathogenesis of WNV and other flaviviruses has offered new opportunities for designing many different classes of promising antiviral therapeutics by targeting both viral replication and the host cell metabolism.
Approaches for Drug Discovery
The development of an antiviral drug is a multistep process that takes years before it reaches the market. A general overview of a drug development process is outlined in Fig. 2. Hit-to-lead is an initial stage in a drug discovery, where small molecule hits are screened and further evaluated to identify promising lead compounds with a therapeutic potential. Recent progress in the development of multiple approaches for designing, screening, identification, and validation of hit compound (reviewed by [194–199]) have witnessed growing interests in the field of drug development. Significant progress in structural and functional characterization of both structural and nonstructural proteins of WNV and other flaviviruses has facilitated identification of therapeutic targets and hit-to-lead screening. For example, characterization of pseudo-atomic structure of mature and immature WNV [35], atomic resolution structure of WNV and other flaviviral protein by X-ray crystallography and nuclear magnetic resonance (NMR) spectroscopy [39, 41, 48, 200–203], and structural characterization of binding of a neutralizing monoclonal antibody to E protein of WNV [48] have greatly increased our understanding of both structural and functional aspects of potential therapeutic targets. Two approaches have been commonly used for small molecule inhibitor screening include target-based approach and cell-based approach. For target-based screening, several methods can be applied, including enzyme activity-based screening, fragment-based screening, affinity-based screening, structure-based rational designing, and in silico docking [197, 198, 204–207]. In contrast, cell-based approaches use viral infection and replication-dependent assays to identify inhibitors [208]. Each of these approaches presents their unique sets of merits and challenges. For instance, it is generally difficult to identify a target and also achieve specificity by cell-based assay because such identified inhibitors may potentially affect multiple steps of viral infection cycles and may target both viral and host proteins. Although inhibitors identified by cell-based assay may prove useful as antiviral drug candidates, these compounds could also act nonspecifically; thus further elucidation of their mechanism of action is required. The target-based approaches are highly efficient in screening process, however, an inhibitor screened by such method may require further modification for effective cellular permeability and validation for its antiviral activity, selectivity and toxicity by using cell-based assays. In the final stages, lead compounds are selected for in vitro and in vivo pharmacokinetic profiling, including efficacy, plasma stability (half-life), exposure, bioavailability, and preclinical toxicity before planning a clinical trial.
Fig. 2.
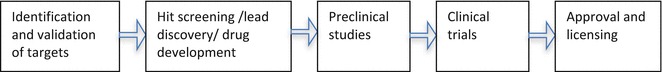
Steps in antiviral drug development
Current Status of Anti-WNV Drug Development
The most common strategies for antiviral drug development include blocking virus attachment or entry into host cells and inhibiting viral replication, either by targeting on viral components or host cells metabolism. There has been a significant progress in development and testing of potential antivirals against flaviviruses including WNV (reviewed by [34, 209, 210]). Several natural and synthetic compounds, antiviral peptides and siRNAs have been identified to target both structural and nonstructural proteins of WNV and evaluated for their potential therapeutic roles. Other approaches include targeting host cell metabolism and physiology and modulating host immune system by using antiviral cytokines as potential therapeutics against WNV. Current status and strategy used to develop antiviral drugs targeting WNV and other flaviviruses are described below.
Natural and Synthetic Compounds as Small Molecule Inhibitors of WNV
Several natural and synthetic compounds have been identified to target both structural and non-structural proteins of flaviviruses. Most of these compounds are designed to target DENV and some of them also show antiviral activities against WNV and other flaviviruses. As these compounds are diverse in their chemistry, they are discussed below based on their modes of action.
Viral Entry/Fusion Inhibitors
The E protein of WNV and other flaviviruses play key roles in viral entry into host cells by mediating viral attachment to host cell receptors and subsequent membrane fusion [35, 41, 211, 212]. After binding to host cell receptors, WNV enters into cells through a clathrin-independent endocytosis process followed by a low-pH-dependent viral uncoating in the endosome to release viral genome into the cytoplasm for replication [213]. Inhibitors that disrupt the interaction of E protein with cell receptors or inhibit membrane fusion would be a potential antiviral against WNV. Two successful HIV drug Maraviroc (CCR5 antagonist) and Enfuvirtide (a peptide inhibitor) that respectively block viral entry [214] and membrane fusion [215] attest to this antiviral strategy. The flaviviral E glycoprotein contains several functional sites such as a hydrophobic pocket, the receptor-binding domain and stem domain that may be targeted by inhibitors. Among these, the hydrophobic ligand-binding pocket in a hinge region between domain I and II of E protein plays an important role in low-pH-mediated membrane fusion process and is a unique target for developing small-molecule inhibitors against flaviviruses [216, 217]. Various screening approaches were used to identify inhibitors against DENV and other flaviviruses that bind into this hydrophobic pocket and interfere with the conformational changes of E protein [207, 218–221], Most of these inhibitors were designed and tested against DENV, some of which were reported to exhibit antiviral activities against WNV [207, 220]. However, these compounds failed in the further drug development due to their undesirable properties, such as low solubility and cytotoxicity.
The domain III of E glycoprotein that mediates receptor-binding can be potentially targeted by developing inhibitors that can disrupt the viral attachment to host cell receptors. Neutralizing antibodies against E protein have proven the potential of this strategy. Several compounds have also been shown to interfere with the binding of flaviviruses to host cell receptors [222–226]. However, lack of understanding of cell receptor for WNV has hampered the success of this approach. Identification of cellular receptors for WNV and understanding of virus–receptor interaction may provide new opportunities to identify small molecule inhibitors that interrupt the binding of WNV to host cell receptors.
NS3 Protease Inhibitors
Viral proteases are essential for WNV life cycle for they cleave the viral polyprotein precursors into functional proteins. Successful development and licensing of protease inhibitor against HIV-1 [227, 228] and HCV [229, 230] provides the proof of concept and feasibility for similar targeting of proteases of other viruses. The N-terminal domain of flaviviral NS3 (amino acids 1–169) has serine protease activity whereas a hydrophobic region of NS2B protein serves as cofactor to activate the enzymatic activity of NS3 [53, 231, 232]. The NS3 protease of WNV processes the viral polyprotein precursor into structural and non-structural proteins and disruption of this activity is lethal for WNV [54, 57]. In addition, WNV NS2B/NS3 protease can also cleave host proteins and may contribute to neuropathogenesis [63]. Recent progress in the expression of stable NS2B/NS3 and identification of the high-affinity substrate for this viral enzyme has promoted large-scale screening of protease inhibitors for WNV and other flaviviruses. A wide range of assays, such as conventional enzyme-substrate based detection, HPLC, ELISA, and high-throughput fluorescence-based detection methods have been developed for screening of viral protease inhibitors [233–236]. Thus, NS3 protease is an attractive target for the development of antiviral against WNV and other flaviviruses (reviewed by [237]).
Except for aprotinin, apancreatic trypsin inhibitor, most of the classical inhibitors of serine protease do not inhibit flaviviral NS2B/NS3 protease activity [238, 239]. Although aprotinin is a potent inhibitor of flaviviral protease, this compound was withdrawn from the market in 2008 due to safety issues [240]. To screen and identify small molecule inhibitors of flavivirus protease, both high throughput screening and structure-based drug designing have been used. These strategies are based on the identification of allosteric inhibitors that target the interface of NS2B-NS3 protease, or the active site of NS3 protease. The former strategy may overcome nonspecificity of the latter due to the largely conserved active sites of the human and viral serine proteinases. A number of inhibitors for WNV NS2B/NS3 protease have been identified by in silico docking or high-throughput screen using in vitro enzyme activity-based assays [34, 59, 206, 241–249]. However, most of these compounds failed to demonstrate potent antiviral activity in cell culture. Although a few compounds identified by these approaches show anti-WNV activity in cell-based assays [62, 206, 242], none of the inhibitors has progressed beyond the hit optimization stage. Discovery of NS2B/NS3 protease inhibitors has been hampered largely due to the difficulties in obtaining co-crystal structures of inhibitor-protease complexes. Moreover, because of the weak binding affinity of NS2B/NS3 active site due to its flat and charged nature, the design of potent small molecule inhibitors by structure-based method becomes difficult [53, 242, 250, 251].
NS3 Helicase/Nuclease Inhibitors
The helicases are enzymes that unwind nucleic acidby using energy derived from hydrolysis of NTP. The C-terminal domain of NS3 of WNV contains helicase/nuclease activities and plays important roles in virulence and pathogenesis [252–254]. High throughput assays that measure helicase activity by monitoring helicase-catalyzed strand separation in real-time by using radioactive or fluorescent-labeled oligonucleotides have been developed to screen helicase inhibitors [255–258].
Several small molecule inhibitors targeting helicase of HCV and HIV-1 have been developed [259]. By using the substrate-based assay, a few compounds have been identified and evaluated in vitro against NTPases/helicases of WNV and other flaviviruses [260–262]. However, inhibitory effects of WNV helicase by these compounds are specific to either DNA or RNA substrate. For example, a compound named 4,5,6,7-tetrabromobenzotriazole (TBBT), a halogenated benzotriazole, inhibits NS3 helicase, but not NTPase activity [260]. A series of ring-expanded nucleoside/nucleotide analogs (RENs) also inhibit NTPases/helicases activities of flaviviruses, including WNV, HCV, and JEV [263, 264], however, these compounds did not show any promising anti-WNV activity in cell culture. A nucleoside analog imidazo[4,5-d]pyridazine nucleosides [265], and a broad- spectrum antiparasitic drug named ivermectin [266] inhibit NS3 helicase and also show anti-WNV activity in cell culture.
RNA-Dependent RNA Polymerase Inhibitors
The RNA-dependent RNA polymerase (RdRp) activity of C-terminal NS5 protein of WNV and other flaviviruses is an attractive target for developing antiviral agents [55, 267–269]. Two approaches used to target WNV RdRp include nucleoside inhibitors (NIs) or non-nucleosides inhibitors (NNIs). NIs (also known as type 1 inhibitors) are nucleoside/nucleotide analogs that target the active sites of the polymerase and generally compete with natural NTP substrates of RdRp to block their incorporation into viral genome during replication and lead to incomplete replication or mutations of viral genome. The success of NIs against several viruses including HIV-1, herpesviruses, HBV, and HCV has already proved the therapeutic potential of this class of compounds [270–272]. In addition, NI generally displays broad-spectrum antiviral activities across related RNA viruses suggesting its potential as pan-flaviviral therapeutics. Various cell-based and cell-free assays have been developed for high-throughput screening of flaviviral RdRp inhibitors [273–276].
So far, several NIs that inhibit WNV, DENV, and other RNA viruses have been identified [34]. For example, favipiravir (T-705; 6-fluoro-3-hydroxy-2-pyrazinecarboxamide) and related compounds selectively inhibit viral RNA-dependent RNA polymerase and have potent anti-influenza activity [277, 278]. This antiviral drug is currently being evaluated in clinical trials against influenza virus. In addition, favipiravir also blocks replication of many other RNA viruses, including WNV and are promising drug candidate against a broad range of RNA viral diseases [279]. Two other nucleoside analogs called 7-deaza-2′-C-methyl-adenosine and 5-aza-7-deazaguanosine (ZX-2401), which are the derivatives of triphosphates of 2′-C-methyl- adenosine and 2′-C-methyl-guanosine, respectively, are also broad-spectrum antiviral compounds targeting viral RdRp that inhibit DENV, HCV and WNV [280–282]. Similarly, two other NI inhibitors, NITD-008 (beta-D-2′-ethynyl-7-deaza-adenosine triphosphate) and NITD203 (3′,5′-O-diisobutyryl-2′-C-acetylene-7-deaza-7-carbamoyladenosine) inhibit all four of DENV serotypes and WNV.
In contrast to NI inhibitors, antiviral NNI inhibitors (also known as type 2 inhibitors) interfere with the function of viral polymerase by occupying its allosteric sites, thus preventing viral RNA synthesis. Analysis of RdRp crystal structure of WNV and DENV3 revealed a cavity that plays a critical role in viral replication, suggesting a potential target for screening of structure-based allosteric inhibitors [55, 283]. N-sulfonylanthranilic acids derivatives identified by high-throughput screening are examples of allosteric inhibitors of RdRp activity of DENV [284]. However, these compound were specific to DENV and did not show any activity against WNV RdRp. A recent study demonstrated that a conformational change occurred in DENV-3 polymerase after binding with an inhibitor [285]. However, a similar antiviral activity of NNI inhibitors targeting polymerase of WNV has not been reported yet.
Methyltransferase Inhibitor
Messenger RNA (mRNA) of WNV possesses a 5′ cap that plays important roles in stability of mRNA and its translation. The methyltransferase (MTase) activity of the N-terminal domain of NS5 is responsible for N-7 and 2′ O- methylation of the viral RNA cap [64, 286] [287]. In addition, MTaseactivity is also responsible for evading host’s antiviral interferon response and plays an important role in WNV pathogenesis [288]. Several structural and functional studies along with identification of several potential inhibitors suggest that targeting MTase represents a novel approach for the development of novel therapeutics against WNV and other flaviviruses [289–294]. Flaviviruses MTase catalyzes sequential methylations of the viral RNA cap using S-adenosyl-l-methionine (SAM) as the methyl donor and contains a single binding site for SAM in its crystal structure [289, 294]. In addition to MTase activity, binding of GTP has been shown in MTase domain of several members of flavivirus [295]. Several assays have been developed for high-throughput screening of methyltransferase inhibitors by structural-based and ligand-based methods [296, 297]. Rational design of SAM analogs has identified several inhibitors targeting MTase activity of DENV and WNV [34, 298].
Nonspecific inhibition of host MTase is one of the potential drawbacks of SAM analogs. A specific inhibition of flaviviral, but not host, MTase can be achieved by targeting a pocket near the SAM-binding site [290, 298]. Two nucleoside analogs were identified that potently inhibited the MTase of WNV without inhibiting human MTase. One of these compounds (GRL-003) showed antiviral activity against WNV in cell culture [299]. In addition, several screening studies against YFV and DENV NS5 have identified hits targeting MTase activity, some of which showed antiviral activity against WNV in cell culture [204, 300, 301]. However, an extensive multistage molecular docking approach to screen a library of about 5 millions of commercial compounds against two active sites of DENV MTase/GTase failed to identify any specific hits [302]. Recently, 5′-silylated nucleoside scaffold derived from 3′-azidothymidine (AZT) demonstrated antiviral activity against WNV and DENV, which binds MTase [303].
Antiviral Peptides
Several potential tools, including rational design and phase display library, have been developed for high-throughput screening of specific antiviral peptides [304, 305]. Enfuvirtide, a 36-amino-acid peptide based on the stem region of the HIV gp41, exemplifies an efficient antiviral peptide currently in clinical use [306]. Thus, antiviral peptides may serve as a novel therapeutic measure against WNV. Several antiviral peptides targeting both structural and non-structural protein of WNV and other flavivirus have been identified.
Targeting WNV E protein by antiviral peptides is a potential strategy that blocks virus attachment and entry into the host cells. Several short antiviral peptides (13–16 amino acid residuals) that bind to WNV E protein have been identified by screening of a murine brain cDNA phage display library [307]. One of those peptides (P9) reduces viremia and fatality after WNV infection in mice. P9 can efficiently penetrate the murine blood–brain barrier, implying that it may have antiviral activity in the CNS [307]. Similarly, a peptide inhibitor (WN83) targeting domain II of WNV E protein designed by using a physicochemical algorithm approach potently inhibits WNV infectivity [308]. Another peptide designed to target domain II of DENV E shows antiviral activity against both DENV and WNV [308]. In addition, a rational drug design approach has been used to identify a peptidomimetic that mimic NS2B/NS3 protease substrate and inhibits its activity. The mechanism proposed for the peptidomimetic is that NS2B/NS3 cleaves between P1 and P1′ in a peptide substrate consisting of P2P1P1′, where P1 and P2 are basic amino acids (Arg or Lys) and P1′ is a side-chain amino acid (Gly, Ser, or Ala) [56, 231]. Thus, a preferred peptide substrate contains several positively charged amino acids. A common method for screening peptide inhibitors of NS2B/NS3 protease employs a fluorophore conjugated peptide substrate containing basic amino acids at the P1 and P2 positions. Cleavage of peptide substrate by NS2B/NS3 protease results in a release of fluorophore and increase of fluorescence [242, 243]. Several peptide inhibitors of NS2B/NS3 protease have been identified against WNV [62, 309–311]. A novel agmatine dipeptide inhibitor with improved inhibitory activity against WNV NS2B/NS3 has been recently identified [309]. In addition, a recombinant peptide called retrocyclin-1 (RC-1) has been shown to inhibit NS2B/NS3 protease [312]. However, most of these peptides showed poor activity in the cell-based assay and has not been tested for their in vivo efficacy. Thus, all of the peptide inhibitor of NS2B/NS3 protease that has been identified so far failed at the early development stages. Potential limitations of this approach include poor pharmacokinetic properties due to charged nature of peptide, lack of specificity, requirement of intravenous delivery, rapid degradation in plasma, and costly production.
Small Interfering RNAs (siRNA)
RNA interference (RNAi) is a cellular process first described in the nematode Caenorhabditis elegans [313, 314]. This process specifically degrades RNA in a sequence-specific manner and is conserved in mammalian cells [315, 316]. RNAi is a natural defense of eukaryotic cells against viral infections, and may be a promising strategy for developing a potential antiviral therapeutic. Numerous siRNA targets were identified in the genomic region of WNV encoding both structural and non-structural proteins, and siRNA targeting these proteins effectively inhibits WNV replication [191, 317–321]. Besides the siRNA targeting coding regions, siRNA that targets noncoding regions have also been identified to inhibit WNV replication in a sequence-specific manner [191]. Although anti-WNV siRNAs efficiently block viral replication in cell cultures, similar successes are difficult to achieve in animal models [191]. Quick degradation by serum nucleases, failure to reach target cells, and rapid renal excretion due to their small size and anionic character are hindering the clinical application of antiviral siRNAs. Several delivery systems, including cell-penetrating peptide [322, 323], nanoparticles [324, 325], and viral vectors [326], may improve siRNA stability and enhance delivery efficiency. Despite many challenges, use of antiviral siRNA as anti-WNV therapeutics remains promising.
Targeting Cytokines/Chemokine Signaling as Therapeutics
Cytokines signaling controls diverse immune functions during infection, autoimmune disease, and cancer. Various immunomodulatory or immunostimulatory cytokines and chemokines have been identified to play a protective or pathological role in WNV infection. For examples, type-I interferons (IFNs) [327, 328], interleukin (IL)-23 [192], interferon-γ (IFN-γ) [83], IL-1β [329], macrophage migration inhibitory factor (MIF) [330], CXCL10 [331], and CCL5 [332, 333] protect against WNV infection, whereas IL-10 [190] and IL-22 [334] favor WNV pathogenicity. Pharmacological blockade of IL-10 by neutralizing antibody has been shown to protect mice against WNV challenge [190]. Type I IFNs (IFNα/β) inhibit many flaviviruses including WNV and have been used as therapeutics against hepatitis C virus [335]. Although the therapeutic effect of type I IFNs in WNV has yet to be evaluated, its application may be limited due to the antagonistic role of WNV NS5 protein in IFN signaling [336, 337]. Interestingly, treatment with pegylated IFN-λ, also known as a type III interferon, has been recently shown to protect mice against lethal WNV infectionby decreasing blood–brain barrier permeability [338]. Thus, strategies targeting the expression of cytokine and chemokine, blocking their signaling, or direct use of recombinant cytokines may be novel approaches for treating WNV infection or controlling its pathology.
Inhibitors Targeting the Host
Viruses utilize host cellular system for entry, genome replication, transcription, synthesis of viral proteins, and production of viral progenies. In addition, interactions of viral proteins with cellular proteins may evade host immune defense and favor viral replication and pathogenesis. Several host pathways and enzymes including clathrin-mediated endocytosis cyclophilins [339], ubiquitin-proteasome system [340], unfolded protein response [341], nucleotide biosynthesis [342, 343], post-translational protein modification [344–346], and lipid metabolism [347–349] have been suggested in flavivirus replication and pathogenesis. Targeting host factors may be used as a strategy for developing antiviral therapeutic against flaviviruses, including WNV infection [350–352].
So far, many inhibitors targeting host proteins have been developed and tested against WNV and other flaviviruses, such as HCV. Host cyclophilin, a family of cellular peptidyl-prolyl isomerases, may serve as a component of flavivirus replication complex and play a role in flaviviral replication. Targeting this enzyme by cyclosporine inhibits replication of WNV [339]. Targeting lipid signaling and metabolism by a bioactive lipid signaling modulator 4-hydroxyphenyl retinamide (4-HPR, fenretinide) also inhibits replication of WNV and other flaviviruses. Similarly, ribavirin and mycophenolic acid target inosine monophosphate dehydrogenase (IMPDH), an enzyme in purine biosynthesis, and thereby inhibit replication of flaviviruses [342, 343]. In addition, NITD-982 and brequinar that block pyrimidine biosynthesis also inhibit replication of broad range of RNA viruses, including WNV and other flaviviruses [353, 354]. Besides blocking viral genome replication, antiviral targeting ofother steps, such asvirus maturation, assembly, and viral dissemination into brain has also been suggested and tested against WNV. For instance, inhibitors of alpha-glucosidase I and II, enzymes that play a role in processing of N-linked oligosaccharides of the viral glycoproteins, also inhibit WNV and other flaviviruses [205, 355]. Although the mechanism by which WNV enters the brain is still poorly understood, the two potential routes include axonal retrograde transport (ART) from the peripheral nervous system and direct hematogenous diffusion via a breakdown in the blood–choroid plexus barrier [356]. Nocodazole, a microtubule inhibitor that blocks ART, delays WNV entry into brain [357]. The 3′ or the 5′ terminal stem-loop in flaviviral RNA contains essential cis-acting elements and plays important roles in viral replication [358, 359]. Interestingly, a range of cellular proteins have been identified to interact with 3′ stem-loop of flaviviral RNAs [352, 359–362], suggesting a potential strategy to design inhibitors targeting this virus– host protein interaction.
Targeting host factors may raise a higher barrier to viral resistance emergence and provide broad-spectrum antiviral effects. However, current understanding of virus–host cell interaction and research on targeting of host factors to block viral infections are still limited. In addition, there are some potential drawbacks of this approach, including undesirable drug-induced side-effects and difficulties for drug delivery into brain to control WNV encephalitis. Further understanding of virus–host interaction will facilitate identification of novel antiviral agents.
Conclusions and Perspectives
Development and testing of various methods for treatment and prevention of WNV infection, such as protective vaccines, therapeutic antibodies, antiviral compounds, peptides, and siRNA have been proposed and intensively studied. Although a number of WNV veterinary vaccines have already been licensed and are in use for years, human vaccine candidates are still in various stages of development and testing. Some therapeutic antibodies that show excellent efficacy in small animal models and are currently being tested in clinical trials represent a promising class of WNV therapeutic. Recent technological advancement and increased understanding of the biology of WNV and other flaviviruses along with structural/functional characterization of viral proteins have provided a solid foundation for the development of small molecule inhibitors as future WNV therapeutics. However, efforts for development of an effective drug for prevention or control of WNV infection in human still remain unsuccessful. Some of the reasons include a low incidence of diseases, low commercial interest by pharmaceutical companies, high cost of mass vaccination, and difficulties with running clinical trials due to unpredictable and sporadic nature of WNV outbreaks [363, 364]. Another challenge for developing successful WNV therapeutics is to ensure safety and efficacy in target populations that mostly include children, elderly, and immune-compromised individuals. Despite all these difficulties, the quest for development of effective treatment and prevention methods against WNV infection are likely to be facilitated by recent technological advancement and should continue to meet the public health needs.
Acknowledgements
The authors are very thankful to Dr. Dobrivoje S. Stokic for his critical reading. This work is supported by funding from Wilson Research Foundation, Jackson, MS, NIH R15AI113706, and the University of Southern Mississippi.
Contributor Information
Tonya M. Colpitts, Phone: +11803-216-3421, Email: tonya.colpitts@uscmed.sc.edu
Fengwei Bai, Email: fengwei.bai@usm.edu
References
- 1.Colpitts TM, Conway MJ, Montgomery RR, Fikrig E. West Nile virus: biology, transmission, and human infection. Clin Microbiol Rev. 2012;25:635–648. doi: 10.1128/CMR.00045-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kuno G, Chang GJ. Biological transmission of arboviruses: reexamination of and new insights into components, mechanisms, and unique traits as well as their evolutionary trends. Clin Microbiol Rev. 2005;18:608–637. doi: 10.1128/CMR.18.4.608-637.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pealer LN, Marfin AA, Petersen LR, Lanciotti RS, Page PL, Stramer SL, Stobierski MG, Signs K, Newman B, Kapoor H, Goodman JL, Chamberland ME, West Nile Virus Transmission Investigation Team Transmission of West Nile virus through blood transfusion in the United States in 2002. N Engl J Med. 2003;349:1236–1245. doi: 10.1056/NEJMoa030969. [DOI] [PubMed] [Google Scholar]
- 4.Stramer SL, Fang CT, Foster GA, Wagner AG, Brodsky JP, Dodd RY. West Nile virus among blood donors in the United States, 2003 and 2004. N Engl J Med. 2005;353:451–459. doi: 10.1056/NEJMoa044333. [DOI] [PubMed] [Google Scholar]
- 5.Centers for Disease, Control, and Prevention West Nile virus transmission via organ transplantation and blood transfusion – Louisiana, 2008. MMWR Morb Mortal Wkly Rep. 2009;58:1263–1267. [PubMed] [Google Scholar]
- 6.Blazquez AB, Saiz JC. West Nile virus (WNV) transmission routes in the murine model: intrauterine, by breastfeeding and after cannibal ingestion. Virus Res. 2010;151:240–243. doi: 10.1016/j.virusres.2010.04.009. [DOI] [PubMed] [Google Scholar]
- 7.Alpert SG, Fergerson J, Noel LP. Intrauterine West Nile virus: ocular and systemic findings. Am J Ophthalmol. 2003;136:733–735. doi: 10.1016/S0002-9394(03)00452-5. [DOI] [PubMed] [Google Scholar]
- 8.Lim PY, Behr MJ, Chadwick CM, Shi PY, Bernard KA. Keratinocytes are cell targets of West Nile virus in vivo. J Virol. 2011;85:5197–5201. doi: 10.1128/JVI.02692-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ye C, Abraham S, Wu H, Shankar P, Manjunath N. Silencing early viral replication in macrophages and dendritic cells effectively suppresses flavivirus encephalitis. PLoS One. 2011;6:e17889. doi: 10.1371/journal.pone.0017889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hayes EB, Sejvar JJ, Zaki SR, Lanciotti RS, Bode AV, Campbell GL. Virology, pathology, and clinical manifestations of West Nile virus disease. Emerg Infect Dis. 2005;11:1174–1179. doi: 10.3201/eid1108.050289b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Samuel MA, Diamond MS. Pathogenesis of West Nile Virus infection: a balance between virulence, innate and adaptive immunity, and viral evasion. J Virol. 2006;80:9349–9360. doi: 10.1128/JVI.01122-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nash D, Mostashari F, Fine A, Miller J, O'Leary D, Murray K, Huang A, Rosenberg A, Greenberg A, Sherman M, Wong S, Layton M, West Nile Outbreak Response Working Group The outbreak of West Nile virus infection in the New York City area in 1999. N Engl J Med. 2001;344:1807–1814. doi: 10.1056/NEJM200106143442401. [DOI] [PubMed] [Google Scholar]
- 13.Mostashari F, Bunning ML, Kitsutani PT, Singer DA, Nash D, Cooper MJ, Katz N, Liljebjelke KA, Biggerstaff BJ, Fine AD, Layton MC, Mullin SM, Johnson AJ, Martin DA, Hayes EB, Campbell GL. Epidemic West Nile encephalitis, New York, 1999: results of a household-based seroepidemiological survey. Lancet. 2001;358:261–264. doi: 10.1016/S0140-6736(01)05480-0. [DOI] [PubMed] [Google Scholar]
- 14.Hayes CG. West Nile virus: Uganda, 1937, to New York City, 1999. Ann N Y Acad Sci. 2001;951:25–37. doi: 10.1111/j.1749-6632.2001.tb02682.x. [DOI] [PubMed] [Google Scholar]
- 15.Hayes EB, Komar N, Nasci RS, Montgomery SP, O'Leary DR, Campbell GL. Epidemiology and transmission dynamics of West Nile virus disease. Emerg Infect Dis. 2005;11:1167–1173. doi: 10.3201/eid1108.050289a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gubler DJ. The continuing spread of West Nile virus in the western hemisphere. Clin Infect Dis. 2007;45:1039–1046. doi: 10.1086/521911. [DOI] [PubMed] [Google Scholar]
- 17.CDC (2015) West Nile virus. http://www.cdc.gov/westnile/statsmaps/index.html
- 18.Petersen LR, Carson PJ, Biggerstaff BJ, Custer B, Borchardt SM, Busch MP. Estimated cumulative incidence of West Nile virus infection in US adults, 1999–2010. Epidemiol Infect. 2013;141:591–595. doi: 10.1017/S0950268812001070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Szatmary G, Leis AA. Concurrent West Nile virus infection in pneumococcal meningitis: clinical and MRI features. J Neuroimaging. 2015;25:312–315. doi: 10.1111/jon.12125. [DOI] [PubMed] [Google Scholar]
- 20.Leis AA, Stokic DS, Polk JL, Dostrow V, Winkelmann M. A poliomyelitis-like syndrome from West Nile virus infection. N Engl J Med. 2002;347:1279–1280. doi: 10.1056/NEJM2002c021587. [DOI] [PubMed] [Google Scholar]
- 21.Leis AA, Stokic DS. Neuromuscular manifestations of West Nile virus infection. Front Neurol. 2012;3:37. doi: 10.3389/fneur.2012.00037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Saxena V, Xie G, Li B, Farris T, Welte T, Gong B, Boor P, Wu P, Tang SJ, Tesh R, Wang T. A hamster-derived West Nile virus isolate induces persistent renal infection in mice. PLoS Negl Trop Dis. 2013;7:e2275. doi: 10.1371/journal.pntd.0002275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Barzon L, Pacenti M, Palu G. West Nile virus and kidney disease. Expert Rev Anti Infect Ther. 2013;11:479–487. doi: 10.1586/eri.13.34. [DOI] [PubMed] [Google Scholar]
- 24.Leis AA, Szatmary G, Ross MA, Stokic DS. West Nile virus infection and myasthenia gravis. Muscle Nerve. 2014;49:26–29. doi: 10.1002/mus.23869. [DOI] [PubMed] [Google Scholar]
- 25.Kushawaha A, Jadonath S, Mobarakai N. West Nile virus myocarditis causing a fatal arrhythmia: a case report. Cases J. 2009;2:7147. doi: 10.1186/1757-1626-2-7147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Davis CT, Ebel GD, Lanciotti RS, Brault AC, Guzman H, Siirin M, Lambert A, Parsons RE, Beasley DW, Novak RJ, Elizondo-Quiroga D, Green EN, Young DS, Stark LM, Drebot MA, Artsob H, Tesh RB, Kramer LD, Barrett AD. Phylogenetic analysis of North American West Nile virus isolates, 2001–2004: evidence for the emergence of a dominant genotype. Virology. 2005;342:252–265. doi: 10.1016/j.virol.2005.07.022. [DOI] [PubMed] [Google Scholar]
- 27.Ebel GD, Carricaburu J, Young D, Bernard KA, Kramer LD. Genetic and phenotypic variation of West Nile virus in New York, 2000–2003. Am J Trop Med Hyg. 2004;71:493–500. [PubMed] [Google Scholar]
- 28.Moudy RM, Meola MA, Morin LL, Ebel GD, Kramer LD. A newly emergent genotype of West Nile virus is transmitted earlier and more efficiently by Culex mosquitoes. Am J Trop Med Hyg. 2007;77:365–370. [PubMed] [Google Scholar]
- 29.Prow NA, Setoh YX, Biron RM, Sester DP, Kim KS, Hobson-Peters J, Hall RA, Bielefeldt-Ohmann H. The West Nile virus-like flavivirus Koutango is highly virulent in mice due to delayed viral clearance and the induction of a poor neutralizing antibody response. J Virol. 2014;88:9947–9962. doi: 10.1128/JVI.01304-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.van den Hurk AF, Hall-Mendelin S, Webb CE, Tan CS, Frentiu FD, Prow NA, Hall RA. Role of enhanced vector transmission of a new West Nile virus strain in an outbreak of equine disease in Australia in 2011. Parasit Vectors. 2014;7:586. doi: 10.1186/s13071-014-0586-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Martina BE, Koraka P, Osterhaus AD. West Nile virus: is a vaccine needed? Curr Opin Investig Drugs. 2010;11:139–146. [PubMed] [Google Scholar]
- 32.Brandler S, Tangy F. Vaccines in development against West Nile virus. Viruses. 2013;5:2384–2409. doi: 10.3390/v5102384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Beasley DW. Vaccines and immunotherapeutics for the prevention and treatment of infections with West Nile virus. Immunotherapy. 2011;3:269–285. doi: 10.2217/imt.10.93. [DOI] [PubMed] [Google Scholar]
- 34.Lim SP, Shi PY. West Nile virus drug discovery. Viruses. 2013;5:2977–3006. doi: 10.3390/v5122977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mukhopadhyay S, Kim BS, Chipman PR, Rossmann MG, Kuhn RJ. Structure of West Nile virus. Science. 2003;302:248. doi: 10.1126/science.1089316. [DOI] [PubMed] [Google Scholar]
- 36.Liu WJ, Wang XJ, Clark DC, Lobigs M, Hall RA, Khromykh AA. A single amino acid substitution in the West Nile virus nonstructural protein NS2A disables its ability to inhibit alpha/beta interferon induction and attenuates virus virulence in mice. J Virol. 2006;80:2396–2404. doi: 10.1128/JVI.80.5.2396-2404.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chung KM, Liszewski MK, Nybakken G, Davis AE, Townsend RR, Fremont DH, Atkinson JP, Diamond MS. West Nile virus nonstructural protein NS1 inhibits complement activation by binding the regulatory protein factor H. Proc Natl Acad Sci U S A. 2006;103:19111–19116. doi: 10.1073/pnas.0605668103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mukherjee S, Lin TY, Dowd KA, Manhart CJ, Pierson TC. The infectivity of prM-containing partially mature West Nile virus does not require the activity of cellular furin-like proteases. J Virol. 2011;85:12067–12072. doi: 10.1128/JVI.05559-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nybakken GE, Nelson CA, Chen BR, Diamond MS, Fremont DH. Crystal structure of the West Nile virus envelope glycoprotein. J Virol. 2006;80:11467–11474. doi: 10.1128/JVI.01125-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Oliphant T, Nybakken GE, Engle M, Xu Q, Nelson CA, Sukupolvi-Petty S, Marri A, Lachmi BE, Olshevsky U, Fremont DH, Pierson TC, Diamond MS. Antibody recognition and neutralization determinants on domains I and II of West Nile Virus envelope protein. J Virol. 2006;80:12149–12159. doi: 10.1128/JVI.01732-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kanai R, Kar K, Anthony K, Gould LH, Ledizet M, Fikrig E, Marasco WA, Koski RA, Modis Y. Crystal structure of West Nile virus envelope glycoprotein reveals viral surface epitopes. J Virol. 2006;80:11000–11008. doi: 10.1128/JVI.01735-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yuan F, Lou Z, Li X, Chen YW, Bell JI, Rao Z, Gao GF. Refolding, crystallization and preliminary X-ray structural studies of the West Nile virus envelope (E) protein domain III. Acta Crystallogr Sect F Struct Biol Cryst Commun. 2005;61:421–423. doi: 10.1107/S1744309105008195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li L, Barrett AD, Beasley DW. Differential expression of domain III neutralizing epitopes on the envelope proteins of West Nile virus strains. Virology. 2005;335:99–105. doi: 10.1016/j.virol.2005.02.011. [DOI] [PubMed] [Google Scholar]
- 44.Heinz FX, Stiasny K. Flaviviruses and flavivirus vaccines. Vaccine. 2012;30:4301–4306. doi: 10.1016/j.vaccine.2011.09.114. [DOI] [PubMed] [Google Scholar]
- 45.Wang T, Anderson JF, Magnarelli LA, Bushmich S, Wong S, Koski RA, Fikrig E. West Nile virus envelope protein: role in diagnosis and immunity. Ann N Y Acad Sci. 2001;951:325–327. doi: 10.1111/j.1749-6632.2001.tb02708.x. [DOI] [PubMed] [Google Scholar]
- 46.Wang T, Anderson JF, Magnarelli LA, Wong SJ, Koski RA, Fikrig E. Immunization of mice against West Nile virus with recombinant envelope protein. J Immunol. 2001;167:5273–5277. doi: 10.4049/jimmunol.167.9.5273. [DOI] [PubMed] [Google Scholar]
- 47.de Alwis R, Smith SA, Olivarez NP, Messer WB, Huynh JP, Wahala WM, White LJ, Diamond MS, Baric RS, Crowe JE, Jr, de Silva AM. Identification of human neutralizing antibodies that bind to complex epitopes on dengue virions. Proc Natl Acad Sci U S A. 2012;109:7439–7444. doi: 10.1073/pnas.1200566109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kaufmann B, Nybakken GE, Chipman PR, Zhang W, Diamond MS, Fremont DH, Kuhn RJ, Rossmann MG. West Nile virus in complex with the Fab fragment of a neutralizing monoclonal antibody. Proc Natl Acad Sci U S A. 2006;103:12400–12404. doi: 10.1073/pnas.0603488103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kaufmann B, Vogt MR, Goudsmit J, Holdaway HA, Aksyuk AA, Chipman PR, Kuhn RJ, Diamond MS, Rossmann MG. Neutralization of West Nile virus by cross-linking of its surface proteins with Fab fragments of the human monoclonal antibody CR4354. Proc Natl Acad Sci U S A. 2010;107:18950–18955. doi: 10.1073/pnas.1011036107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vogt MR, Moesker B, Goudsmit J, Jongeneelen M, Austin SK, Oliphant T, Nelson S, Pierson TC, Wilschut J, Throsby M, Diamond MS. Human monoclonal antibodies against West Nile virus induced by natural infection neutralize at a postattachment step. J Virol. 2009;83:6494–6507. doi: 10.1128/JVI.00286-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.De Filette M, Chabierski S, Andries O, Ulbert S, Sanders NN. T cell epitope mapping of the e-protein of West Nile virus in BALB/c mice. PLoS One. 2014;9:e115343. doi: 10.1371/journal.pone.0115343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lanteri MC, Heitman JW, Owen RE, Busch T, Gefter N, Kiely N, Kamel HT, Tobler LH, Busch MP, Norris PJ. Comprehensive analysis of West Nile virus-specific T cell responses in humans. J Infect Dis. 2008;197:1296–1306. doi: 10.1086/586898. [DOI] [PubMed] [Google Scholar]
- 53.Erbel P, Schiering N, D'Arcy A, Renatus M, Kroemer M, Lim SP, Yin Z, Keller TH, Vasudevan SG, Hommel U. Structural basis for the activation of flaviviral NS3 proteases from dengue and West Nile virus. Nat Struct Mol Biol. 2006;13:372–373. doi: 10.1038/nsmb1073. [DOI] [PubMed] [Google Scholar]
- 54.Chappell KJ, Stoermer MJ, Fairlie DP, Young PR. West Nile Virus NS2B/NS3 protease as an antiviral target. Curr Med Chem. 2008;15:2771–2784. doi: 10.2174/092986708786242804. [DOI] [PubMed] [Google Scholar]
- 55.Malet H, Egloff MP, Selisko B, Butcher RE, Wright PJ, Roberts M, Gruez A, Sulzenbacher G, Vonrhein C, Bricogne G, Mackenzie JM, Khromykh AA, Davidson AD, Canard B. Crystal structure of the RNA polymerase domain of the West Nile virus non-structural protein 5. J Biol Chem. 2007;282:10678–10689. doi: 10.1074/jbc.M607273200. [DOI] [PubMed] [Google Scholar]
- 56.Chambers TJ, Weir RC, Grakoui A, McCourt DW, Bazan JF, Fletterick RJ, Rice CM. Evidence that the N-terminal domain of nonstructural protein NS3 from yellow fever virus is a serine protease responsible for site-specific cleavages in the viral polyprotein. Proc Natl Acad Sci U S A. 1990;87:8898–8902. doi: 10.1073/pnas.87.22.8898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chambers TJ, Droll DA, Tang Y, Liang Y, Ganesh VK, Murthy KH, Nickells M. Yellow fever virus NS2B-NS3 protease: characterization of charged-to-alanine mutant and revertant viruses and analysis of polyprotein-cleavage activities. J Gen Virol. 2005;86:1403–1413. doi: 10.1099/vir.0.80427-0. [DOI] [PubMed] [Google Scholar]
- 58.Ekonomiuk D, Caflisch A. Activation of the West Nile virus NS3 protease: molecular dynamics evidence for a conformational selection mechanism. Protein Sci. 2009;18:1003–1011. doi: 10.1002/pro.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ekonomiuk D, Su XC, Ozawa K, Bodenreider C, Lim SP, Otting G, Huang D, Caflisch A. Flaviviral protease inhibitors identified by fragment-based library docking into a structure generated by molecular dynamics. J Med Chem. 2009;52:4860–4868. doi: 10.1021/jm900448m. [DOI] [PubMed] [Google Scholar]
- 60.Wengler G, Wengler G. The carboxy-terminal part of the NS 3 protein of the West Nile flavivirus can be isolated as a soluble protein after proteolytic cleavage and represents an RNA-stimulated NTPase. Virology. 1991;184:707–715. doi: 10.1016/0042-6822(91)90440-M. [DOI] [PubMed] [Google Scholar]
- 61.Wengler G, Wengler G. The NS 3 nonstructural protein of flaviviruses contains an RNA triphosphatase activity. Virology. 1993;197:265–273. doi: 10.1006/viro.1993.1587. [DOI] [PubMed] [Google Scholar]
- 62.Shiryaev SA, Ratnikov BI, Chekanov AV, Sikora S, Rozanov DV, Godzik A, Wang J, Smith JW, Huang Z, Lindberg I, Samuel MA, Diamond MS, Strongin AY. Cleavage targets and the D-arginine-based inhibitors of the West Nile virus NS3 processing proteinase. Biochem J. 2006;393:503–511. doi: 10.1042/BJ20051374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ramanathan MP, Chambers JA, Pankhong P, Chattergoon M, Attatippaholkun W, Dang K, Shah N, Weiner DB. Host cell killing by the West Nile Virus NS2B-NS3 proteolytic complex: NS3 alone is sufficient to recruit caspase-8-based apoptotic pathway. Virology. 2006;345:56–72. doi: 10.1016/j.virol.2005.08.043. [DOI] [PubMed] [Google Scholar]
- 64.Ray D, Shah A, Tilgner M, Guo Y, Zhao Y, Dong H, Deas TS, Zhou Y, Li H, Shi PY. West Nile virus 5′-cap structure is formed by sequential guanine N-7 and ribose 2′-O methylations by nonstructural protein 5. J Virol. 2006;80:8362–8370. doi: 10.1128/JVI.00814-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tan BH, Fu J, Sugrue RJ, Yap EH, Chan YC, Tan YH. Recombinant dengue type 1 virus NS5 protein expressed in Escherichia coli exhibits RNA-dependent RNA polymerase activity. Virology. 1996;216:317–325. doi: 10.1006/viro.1996.0067. [DOI] [PubMed] [Google Scholar]
- 66.Henderson Brittney R., Saeedi Bejan J., Campagnola Grace, Geiss Brian J. Analysis of RNA Binding by the Dengue Virus NS5 RNA Capping Enzyme. PLoS ONE. 2011;6(10):e25795. doi: 10.1371/journal.pone.0025795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Keating JA, Bhattacharya D, Lim PY, Falk S, Weisblum B, Bernard KA, Sharma M, Kuhn RJ, Striker R. West Nile virus methyltransferase domain interacts with protein kinase G. Virol J. 2013;10:242. doi: 10.1186/1743-422X-10-242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Grant D, Tan GK, Qing M, Ng JK, Yip A, Zou G, Xie X, Yuan Z, Schreiber MJ, Schul W, Shi PY, Alonso S. A single amino acid in nonstructural protein NS4B confers virulence to dengue virus in AG129 mice through enhancement of viral RNA synthesis. J Virol. 2011;85:7775–7787. doi: 10.1128/JVI.00665-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wicker JA, Whiteman MC, Beasley DW, Davis CT, Zhang S, Schneider BS, Higgs S, Kinney RM, Barrett AD. A single amino acid substitution in the central portion of the West Nile virus NS4B protein confers a highly attenuated phenotype in mice. Virology. 2006;349:245–253. doi: 10.1016/j.virol.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 70.Xie X, Wang QY, Xu HY, Qing M, Kramer L, Yuan Z, Shi PY. Inhibition of dengue virus by targeting viral NS4B protein. J Virol. 2011;85:11183–11195. doi: 10.1128/JVI.05468-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gao M, Nettles RE, Belema M, Snyder LB, Nguyen VN, Fridell RA, Serrano-Wu MH, Langley DR, Sun JH, O'Boyle DR, 2nd, Lemm JA, Wang C, Knipe JO, Chien C, Colonno RJ, Grasela DM, Meanwell NA, Hamann LG. Chemical genetics strategy identifies an HCV NS5A inhibitor with a potent clinical effect. Nature. 2010;465:96–100. doi: 10.1038/nature08960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Diamond MS, Shrestha B, Marri A, Mahan D, Engle M. B cells and antibody play critical roles in the immediate defense of disseminated infection by West Nile encephalitis virus. J Virol. 2003;77:2578–2586. doi: 10.1128/JVI.77.4.2578-2586.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Diamond MS, Sitati EM, Friend LD, Higgs S, Shrestha B, Engle M. A critical role for induced IgM in the protection against West Nile virus infection. J Exp Med. 2003;198:1853–1862. doi: 10.1084/jem.20031223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Parsons R, Lelic A, Hayes L, Carter A, Marshall L, Evelegh C, Drebot M, Andonova M, McMurtrey C, Hildebrand W, Loeb MB, Bramson JL. The memory T cell response to West Nile virus in symptomatic humans following natural infection is not influenced by age and is dominated by a restricted set of CD8+ T cell epitopes. J Immunol. 2008;181:1563–1572. doi: 10.4049/jimmunol.181.2.1563. [DOI] [PubMed] [Google Scholar]
- 75.Larsen MV, Lelic A, Parsons R, Nielsen M, Hoof I, Lamberth K, Loeb MB, Buus S, Bramson J, Lund O. Identification of CD8+ T cell epitopes in the West Nile virus polyprotein by reverse-immunology using NetCTL. PLoS One. 2010;5:e12697. doi: 10.1371/journal.pone.0012697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sun EC, Zhao J, Liu NH, Yang T, Ma JN, Geng HW, Wang LF, Qin YL, Bu ZG, Yang YH, Lunt RA, Wang LF, Wu DL. Comprehensive mapping of West Nile virus (WNV)- and Japanese encephalitis virus serocomplex-specific linear B-cell epitopes from WNV non-structural protein 1. J Gen Virol. 2012;93:50–60. doi: 10.1099/vir.0.034900-0. [DOI] [PubMed] [Google Scholar]
- 77.Sun EC, Ma JN, Liu NH, Yang T, Zhao J, Geng HW, Wang LF, Qin YL, Bu ZG, Yang YH, Lunt RA, Wang LF, Wu DL. Identification of two linear B-cell epitopes from West Nile virus NS1 by screening a phage-displayed random peptide library. BMC Microbiol. 2011;11:160. doi: 10.1186/1471-2180-11-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Throsby M, Ter Meulen J, Geuijen C, Goudsmit J, de Kruif J. Mapping and analysis of West Nile virus-specific monoclonal antibodies: prospects for vaccine development. Expert Rev Vaccines. 2007;6:183–191. doi: 10.1586/14760584.6.2.183. [DOI] [PubMed] [Google Scholar]
- 79.Sultana H, Foellmer HG, Neelakanta G, Oliphant T, Engle M, Ledizet M, Krishnan MN, Bonafe N, Anthony KG, Marasco WA, Kaplan P, Montgomery RR, Diamond MS, Koski RA, Fikrig E. Fusion loop peptide of the West Nile virus envelope protein is essential for pathogenesis and is recognized by a therapeutic cross-reactive human monoclonal antibody. J Immunol. 2009;183:650–660. doi: 10.4049/jimmunol.0900093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Getts DR, Matsumoto I, Muller M, Getts MT, Radford J, Shrestha B, Campbell IL, King NJ. Role of IFN-gamma in an experimental murine model of West Nile virus-induced seizures. J Neurochem. 2007;103:1019–1030. doi: 10.1111/j.1471-4159.2007.04798.x. [DOI] [PubMed] [Google Scholar]
- 81.Shrestha B, Diamond MS. Role of CD8+ T cells in control of West Nile virus infection. J Virol. 2004;78:8312–8321. doi: 10.1128/JVI.78.15.8312-8321.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Shrestha B, Ng T, Chu HJ, Noll M, Diamond MS. The relative contribution of antibody and CD8+ T cells to vaccine immunity against West Nile encephalitis virus. Vaccine. 2008;26:2020–2033. doi: 10.1016/j.vaccine.2008.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Shrestha B, Wang T, Samuel MA, Whitby K, Craft J, Fikrig E, Diamond MS. Gamma interferon plays a crucial early antiviral role in protection against West Nile virus infection. J Virol. 2006;80:5338–5348. doi: 10.1128/JVI.00274-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lazear HM, Pinto AK, Ramos HJ, Vick SC, Shrestha B, Suthar MS, Gale M, Jr, Diamond MS. Pattern recognition receptor MDA5 modulates CD8+ T cell-dependent clearance of West Nile virus from the central nervous system. J Virol. 2013;87:11401–11415. doi: 10.1128/JVI.01403-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Miller JD, van der Most RG, Akondy RS, Glidewell JT, Albott S, Masopust D, Murali-Krishna K, Mahar PL, Edupuganti S, Lalor S, Germon S, Del Rio C, Mulligan MJ, Staprans SI, Altman JD, Feinberg MB, Ahmed R. Human effector and memory CD8+ T cell responses to smallpox and yellow fever vaccines. Immunity. 2008;28:710–722. doi: 10.1016/j.immuni.2008.02.020. [DOI] [PubMed] [Google Scholar]
- 86.Koup RA, Douek DC. Vaccine design for CD8 T lymphocyte responses. Cold Spring Harb Perspect Med. 2011;1:a007252. doi: 10.1101/cshperspect.a007252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Roehrig JT, Hombach J, Barrett AD. Guidelines for plaque-reduction neutralization testing of human antibodies to dengue viruses. Viral Immunol. 2008;21:123–132. doi: 10.1089/vim.2008.0007. [DOI] [PubMed] [Google Scholar]
- 88.Hombach J, Solomon T, Kurane I, Jacobson J, Wood D. Report on a WHO consultation on immunological endpoints for evaluation of new Japanese encephalitis vaccines, WHO, Geneva, 2–3 September, 2004. Vaccine. 2005;23:5205–5211. doi: 10.1016/j.vaccine.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 89.Davis BS, Chang GJ, Cropp B, Roehrig JT, Martin DA, Mitchell CJ, Bowen R, Bunning ML. West Nile virus recombinant DNA vaccine protects mouse and horse from virus challenge and expresses in vitro a noninfectious recombinant antigen that can be used in enzyme-linked immunosorbent assays. J Virol. 2001;75:4040–4047. doi: 10.1128/JVI.75.9.4040-4047.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Martin JE, Pierson TC, Hubka S, Rucker S, Gordon IJ, Enama ME, Andrews CA, Xu Q, Davis BS, Nason M, Fay M, Koup RA, Roederer M, Bailer RT, Gomez PL, Mascola JR, Chang GJ, Nabel GJ, Graham BS. A West Nile virus DNA vaccine induces neutralizing antibody in healthy adults during a phase 1 clinical trial. J Infect Dis. 2007;196:1732–1740. doi: 10.1086/523650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Arroyo J, Miller CA, Catalan J, Monath TP. Yellow fever vector live-virus vaccines: West Nile virus vaccine development. Trends Mol Med. 2001;7:350–354. doi: 10.1016/S1471-4914(01)02048-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Dayan GH, Pugachev K, Bevilacqua J, Lang J, Monath TP. Preclinical and clinical development of a YFV 17 D-based chimeric vaccine against West Nile virus. Viruses. 2013;5:3048–3070. doi: 10.3390/v5123048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Pletnev AG, Claire MS, Elkins R, Speicher J, Murphy BR, Chanock RM. Molecularly engineered live-attenuated chimeric West Nile/dengue virus vaccines protect rhesus monkeys from West Nile virus. Virology. 2003;314:190–195. doi: 10.1016/S0042-6822(03)00450-1. [DOI] [PubMed] [Google Scholar]
- 94.Pletnev AG, Swayne DE, Speicher J, Rumyantsev AA, Murphy BR. Chimeric West Nile/dengue virus vaccine candidate: preclinical evaluation in mice, geese and monkeys for safety and immunogenicity. Vaccine. 2006;24:6392–6404. doi: 10.1016/j.vaccine.2006.06.008. [DOI] [PubMed] [Google Scholar]
- 95.Iglesias MC, Frenkiel MP, Mollier K, Souque P, Despres P, Charneau P. A single immunization with a minute dose of a lentiviral vector-based vaccine is highly effective at eliciting protective humoral immunity against West Nile virus. J Gene Med. 2006;8:265–274. doi: 10.1002/jgm.837. [DOI] [PubMed] [Google Scholar]
- 96.Despres P, Combredet C, Frenkiel MP, Lorin C, Brahic M, Tangy F. Live measles vaccine expressing the secreted form of the West Nile virus envelope glycoprotein protects against West Nile virus encephalitis. J Infect Dis. 2005;191:207–214. doi: 10.1086/426824. [DOI] [PubMed] [Google Scholar]
- 97.Iyer AV, Pahar B, Boudreaux MJ, Wakamatsu N, Roy AF, Chouljenko VN, Baghian A, Apetrei C, Marx PA, Kousoulas KG. Recombinant vesicular stomatitis virus-based West Nile vaccine elicits strong humoral and cellular immune responses and protects mice against lethal challenge with the virulent West Nile virus strain LSU-AR01. Vaccine. 2009;27:893–903. doi: 10.1016/j.vaccine.2008.11.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Schepp-Berglind J, Luo M, Wang D, Wicker JA, Raja NU, Hoel BD, Holman DH, Barrett AD, Dong JY. Complex adenovirus-mediated expression of West Nile virus C, PreM, E, and NS1 proteins induces both humoral and cellular immune responses. Clin Vaccine Immunol. 2007;14:1117–1126. doi: 10.1128/CVI.00070-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ramanathan MP, Kutzler MA, Kuo YC, Yan J, Liu H, Shah V, Bawa A, Selling B, Sardesai NY, Kim JJ, Weiner DB. Coimmunization with an optimized IL15 plasmid adjuvant enhances humoral immunity via stimulating B cells induced by genetically engineered DNA vaccines expressing consensus JEV and WNV E DIII. Vaccine. 2009;27:4370–4380. doi: 10.1016/j.vaccine.2009.01.137. [DOI] [PubMed] [Google Scholar]
- 100.Ledgerwood JE, Pierson TC, Hubka SA, Desai N, Rucker S, Gordon IJ, Enama ME, Nelson S, Nason M, Gu W, Bundrant N, Koup RA, Bailer RT, Mascola JR, Nabel GJ, Graham BS, Team VRCS. A West Nile virus DNA vaccine utilizing a modified promoter induces neutralizing antibody in younger and older healthy adults in a phase I clinical trial. J Infect Dis. 2011;203:1396–1404. doi: 10.1093/infdis/jir054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zhao Z, Wakita T, Yasui K. Inoculation of plasmids encoding Japanese encephalitis virus PrM-E proteins with colloidal gold elicits a protective immune response in BALB/c mice. J Virol. 2003;77:4248–4260. doi: 10.1128/JVI.77.7.4248-4260.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Prow TW, Chen X, Prow NA, Fernando GJ, Tan CS, Raphael AP, Chang D, Ruutu MP, Jenkins DW, Pyke A, Crichton ML, Raphaelli K, Goh LY, Frazer IH, Roberts MS, Gardner J, Khromykh AA, Suhrbier A, Hall RA, Kendall MA. Nanopatch-targeted skin vaccination against West Nile virus and Chikungunya virus in mice. Small. 2010;6:1776–1784. doi: 10.1002/smll.201000331. [DOI] [PubMed] [Google Scholar]
- 103.Dunn MD, Rossi SL, Carter DM, Vogt MR, Mehlhop E, Diamond MS, Ross TM. Enhancement of anti-DIII antibodies by the C3d derivative P28 results in lower viral titers and augments protection in mice. Virol J. 2010;7:95. doi: 10.1186/1743-422X-7-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.De Filette M, Soehle S, Ulbert S, Richner J, Diamond MS, Sinigaglia A, Barzon L, Roels S, Lisziewicz J, Lorincz O, Sanders NN. Vaccination of mice using the West Nile virus E-protein in a DNA prime-protein boost strategy stimulates cell-mediated immunity and protects mice against a lethal challenge. PLoS One. 2014;9:e87837. doi: 10.1371/journal.pone.0087837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kofler RM, Heinz FX, Mandl CW (2004) A novel principle of attenuation for the development of new generation live flavivirus vaccines. Arch Virol Suppl 191–200 [DOI] [PubMed]
- 106.Kofler RM, Aberle JH, Aberle SW, Allison SL, Heinz FX, Mandl CW. Mimicking live flavivirus immunization with a noninfectious RNA vaccine. Proc Natl Acad Sci U S A. 2004;101:1951–1956. doi: 10.1073/pnas.0307145101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Chang DC, Liu WJ, Anraku I, Clark DC, Pollitt CC, Suhrbier A, Hall RA, Khromykh AA. Single-round infectious particles enhance immunogenicity of a DNA vaccine against West Nile virus. Nat Biotechnol. 2008;26:571–577. doi: 10.1038/nbt1400. [DOI] [PubMed] [Google Scholar]
- 108.Widman DG, Ishikawa T, Winkelmann ER, Infante E, Bourne N, Mason PW. RepliVAX WN, a single-cycle flavivirus vaccine to prevent West Nile disease, elicits durable protective immunity in hamsters. Vaccine. 2009;27:5550–5553. doi: 10.1016/j.vaccine.2009.07.016. [DOI] [PubMed] [Google Scholar]
- 109.Widman DG, Ishikawa T, Giavedoni LD, Hodara VL, Garza Mde L, Montalbo JA, Travassos Da Rosa AP, Tesh RB, Patterson JL, Carrion R, Jr, Bourne N, Mason PW. Evaluation of RepliVAX WN, a single-cycle flavivirus vaccine, in a non-human primate model of West Nile virus infection. Am J Trop Med Hyg. 2010;82:1160–1167. doi: 10.4269/ajtmh.2010.09-0310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Hall RA, Nisbet DJ, Pham KB, Pyke AT, Smith GA, Khromykh AA. DNA vaccine coding for the full-length infectious Kunjin virus RNA protects mice against the New York strain of West Nile virus. Proc Natl Acad Sci U S A. 2003;100:10460–10464. doi: 10.1073/pnas.1834270100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Schneeweiss A, Chabierski S, Salomo M, Delaroque N, Al-Robaiy S, Grunwald T, Burki K, Liebert UG, Ulbert S. A DNA vaccine encoding the E protein of West Nile virus is protective and can be boosted by recombinant domain DIII. Vaccine. 2011;29:6352–6357. doi: 10.1016/j.vaccine.2011.04.116. [DOI] [PubMed] [Google Scholar]
- 112.Suzuki R, Winkelmann ER, Mason PW. Construction and characterization of a single-cycle chimeric flavivirus vaccine candidate that protects mice against lethal challenge with dengue virus type 2. J Virol. 2009;83:1870–1880. doi: 10.1128/JVI.01891-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Mason PW, Shustov AV, Frolov I. Production and characterization of vaccines based on flaviviruses defective in replication. Virology. 2006;351:432–443. doi: 10.1016/j.virol.2006.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Widman DG, Ishikawa T, Fayzulin R, Bourne N, Mason PW. Construction and characterization of a second-generation pseudoinfectious West Nile virus vaccine propagated using a new cultivation system. Vaccine. 2008;26:2762–2771. doi: 10.1016/j.vaccine.2008.03.009. [DOI] [PubMed] [Google Scholar]
- 115.Nelson MH, Winkelmann E, Ma Y, Xia J, Mason PW, Bourne N, Milligan GN. Immunogenicity of RepliVAX WN, a novel single-cycle West Nile virus vaccine. Vaccine. 2010;29:174–182. doi: 10.1016/j.vaccine.2010.10.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Xia J, Winkelmann ER, Gorder SR, Mason PW, Milligan GN. TLR3- and MyD88-dependent signaling differentially influences the development of West Nile virus-specific B cell responses in mice following immunization with RepliVAX WN, a single-cycle flavivirus vaccine candidate. J Virol. 2013;87:12090–12101. doi: 10.1128/JVI.01469-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Whiteman MC, Li L, Wicker JA, Kinney RM, Huang C, Beasley DW, Chung KM, Diamond MS, Solomon T, Barrett AD. Development and characterization of non-glycosylated E and NS1 mutant viruses as a potential candidate vaccine for West Nile virus. Vaccine. 2010;28:1075–1083. doi: 10.1016/j.vaccine.2009.10.112. [DOI] [PubMed] [Google Scholar]
- 118.Yu L, Robert Putnak J, Pletnev AG, Markoff L. Attenuated West Nile viruses bearing 3′SL and envelope gene substitution mutations. Vaccine. 2008;26:5981–5988. doi: 10.1016/j.vaccine.2008.08.064. [DOI] [PubMed] [Google Scholar]
- 119.Siger L, Bowen RA, Karaca K, Murray MJ, Gordy PW, Loosmore SM, Audonnet JC, Nordgren RM, Minke JM. Assessment of the efficacy of a single dose of a recombinant vaccine against West Nile virus in response to natural challenge with West Nile virus-infected mosquitoes in horses. Am J Vet Res. 2004;65:1459–1462. doi: 10.2460/ajvr.2004.65.1459. [DOI] [PubMed] [Google Scholar]
- 120.Minke JM, Siger L, Karaca K, Austgen L, Gordy P, Bowen R, Renshaw RW, Loosmore S, Audonnet JC, Nordgren B (2004) Recombinant canarypoxvirus vaccine carrying the prM/E genes of West Nile virus protects horses against a West Nile virus-mosquito challenge. Arch Virol Suppl 221–230 [DOI] [PubMed]
- 121.Monath TP, Liu J, Kanesa-Thasan N, Myers GA, Nichols R, Deary A, McCarthy K, Johnson C, Ermak T, Shin S, Arroyo J, Guirakhoo F, Kennedy JS, Ennis FA, Green S, Bedford P. A live, attenuated recombinant West Nile virus vaccine. Proc Natl Acad Sci U S A. 2006;103:6694–6699. doi: 10.1073/pnas.0601932103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Durbin AP, Karron RA, Sun W, Vaughn DW, Reynolds MJ, Perreault JR, Thumar B, Men R, Lai CJ, Elkins WR, Chanock RM, Murphy BR, Whitehead SS. Attenuation and immunogenicity in humans of a live dengue virus type-4 vaccine candidate with a 30 nucleotide deletion in its 3′-untranslated region. Am J Trop Med Hyg. 2001;65:405–413. doi: 10.4269/ajtmh.2001.65.405. [DOI] [PubMed] [Google Scholar]
- 123.Durbin AP, Wright PF, Cox A, Kagucia W, Elwood D, Henderson S, Wanionek K, Speicher J, Whitehead SS, Pletnev AG. The live attenuated chimeric vaccine rWN/DEN4Delta30 is well-tolerated and immunogenic in healthy flavivirus-naive adult volunteers. Vaccine. 2013;31:5772–5777. doi: 10.1016/j.vaccine.2013.07.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Huang CY, Silengo SJ, Whiteman MC, Kinney RM. Chimeric dengue 2 PDK-53/West Nile NY99 viruses retain the phenotypic attenuation markers of the candidate PDK-53 vaccine virus and protect mice against lethal challenge with West Nile virus. J Virol. 2005;79:7300–7310. doi: 10.1128/JVI.79.12.7300-7310.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Yamshchikov G, Borisevich V, Seregin A, Chaporgina E, Mishina M, Mishin V, Kwok CW, Yamshchikov V. An attenuated West Nile prototype virus is highly immunogenic and protects against the deadly NY99 strain: a candidate for live WN vaccine development. Virology. 2004;330:304–312. doi: 10.1016/j.virol.2004.09.014. [DOI] [PubMed] [Google Scholar]
- 126.Rosas CT, Tischer BK, Perkins GA, Wagner B, Goodman LB, Osterrieder N. Live-attenuated recombinant equine herpesvirus type 1 (EHV-1) induces a neutralizing antibody response against West Nile virus (WNV) Virus Res. 2007;125:69–78. doi: 10.1016/j.virusres.2006.12.009. [DOI] [PubMed] [Google Scholar]
- 127.Ng T, Hathaway D, Jennings N, Champ D, Chiang YW, Chu HJ. Equine vaccine for West Nile virus. Dev Biol (Basel) 2003;114:221–227. [PubMed] [Google Scholar]
- 128.Muraki Y, Fujita T, Matsuura M, Fuke I, Manabe S, Ishikawa T, Okuno Y, Morita K. The efficacy of inactivated West Nile vaccine (WN-VAX) in mice and monkeys. Virol J. 2015;12:54. doi: 10.1186/s12985-015-0282-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Samina I, Havenga M, Koudstaal W, Khinich Y, Koldijk M, Malkinson M, Simanov M, Perl S, Gijsbers L, Weverling GJ, Uytdehaag F, Goudsmit J. Safety and efficacy in geese of a PER.C6-based inactivated West Nile virus vaccine. Vaccine. 2007;25:8338–8345. doi: 10.1016/j.vaccine.2007.09.055. [DOI] [PubMed] [Google Scholar]
- 130.Pinto AK, Richner JM, Poore EA, Patil PP, Amanna IJ, Slifka MK, Diamond MS. A hydrogen peroxide-inactivated virus vaccine elicits humoral and cellular immunity and protects against lethal West Nile virus infection in aged mice. J Virol. 2013;87:1926–1936. doi: 10.1128/JVI.02903-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Amanna IJ, Raue HP, Slifka MK. Development of a new hydrogen peroxide-based vaccine platform. Nat Med. 2012;18:974–979. doi: 10.1038/nm.2763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Scherret JH, Mackenzie JS, Hall RA, Deubel V, Gould EA. Phylogeny and molecular epidemiology of West Nile and Kunjin viruses. Curr Top Microbiol Immunol. 2002;267:373–390. doi: 10.1007/978-3-642-59403-8_18. [DOI] [PubMed] [Google Scholar]
- 133.Gray TJ, Burrow JN, Markey PG, Whelan PI, Jackson J, Smith DW, Currie BJ. West Nile virus (Kunjin subtype) disease in the northern territory of Australia—a case of encephalitis and review of all reported cases. Am J Trop Med Hyg. 2011;85:952–956. doi: 10.4269/ajtmh.2011.11-0165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Orlinger KK, Holzer GW, Schwaiger J, Mayrhofer J, Schmid K, Kistner O, Noel Barrett P, Falkner FG. An inactivated West Nile virus vaccine derived from a chemically synthesized cDNA system. Vaccine. 2010;28:3318–3324. doi: 10.1016/j.vaccine.2010.02.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Martina BE, Koraka P, van den Doel P, van Amerongen G, Rimmelzwaan GF, Osterhaus AD. Immunization with West Nile virus envelope domain III protects mice against lethal infection with homologous and heterologous virus. Vaccine. 2008;26:153–157. doi: 10.1016/j.vaccine.2007.10.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Lieberman MM, Clements DE, Ogata S, Wang G, Corpuz G, Wong T, Martyak T, Gilson L, Coller BA, Leung J, Watts DM, Tesh RB, Siirin M, Travassos da Rosa A, Humphreys T, Weeks-Levy C. Preparation and immunogenic properties of a recombinant West Nile subunit vaccine. Vaccine. 2007;25:414–423. doi: 10.1016/j.vaccine.2006.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.McDonald WF, Huleatt JW, Foellmer HG, Hewitt D, Tang J, Desai P, Price A, Jacobs A, Takahashi VN, Huang Y, Nakaar V, Alexopoulou L, Fikrig E, Powell TJ. A West Nile virus recombinant protein vaccine that coactivates innate and adaptive immunity. J Infect Dis. 2007;195:1607–1617. doi: 10.1086/517613. [DOI] [PubMed] [Google Scholar]
- 138.Spohn G, Jennings GT, Martina BE, Keller I, Beck M, Pumpens P, Osterhaus AD, Bachmann MF. A VLP-based vaccine targeting domain III of the West Nile virus E protein protects from lethal infection in mice. Virol J. 2010;7:146. doi: 10.1186/1743-422X-7-146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Chua AJ, Vituret C, Tan ML, Gonzalez G, Boulanger P, Ng ML, Hong SS. A novel platform for virus-like particle-display of flaviviral envelope domain III: induction of Dengue and West Nile virus neutralizing antibodies. Virol J. 2013;10:129. doi: 10.1186/1743-422X-10-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Wang CY, Miyazaki N, Yamashita T, Higashiura A, Nakagawa A, Li TC, Takeda N, Xing L, Hjalmarsson E, Friberg C, Liou DM, Sung YJ, Tsukihara T, Matsuura Y, Miyamura T, Cheng RH. Crystallization and preliminary X-ray diffraction analysis of recombinant hepatitis E virus-like particle. Acta Crystallogr Sect F Struct Biol Cryst Commun. 2008;64:318–322. doi: 10.1107/S1744309108007197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Krammer F, Schinko T, Messner P, Palmberger D, Ferko B, Grabherr R. Influenza virus-like particles as an antigen-carrier platform for the ESAT-6 epitope of Mycobacterium tuberculosis. J Virol Methods. 2010;167:17–22. doi: 10.1016/j.jviromet.2010.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Carriere C, Gay B, Chazal N, Morin N, Boulanger P. Sequence requirements for encapsidation of deletion mutants and chimeras of human immunodeficiency virus type 1 Gag precursor into retrovirus-like particles. J Virol. 1995;69:2366–2377. doi: 10.1128/jvi.69.4.2366-2377.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Hsu M, Zhang J, Flint M, Logvinoff C, Cheng-Mayer C, Rice CM, McKeating JA. Hepatitis C virus glycoproteins mediate pH-dependent cell entry of pseudotyped retroviral particles. Proc Natl Acad Sci U S A. 2003;100:7271–7276. doi: 10.1073/pnas.0832180100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Ledizet M, Kar K, Foellmer HG, Wang T, Bushmich SL, Anderson JF, Fikrig E, Koski RA. A recombinant envelope protein vaccine against West Nile virus. Vaccine. 2005;23:3915–3924. doi: 10.1016/j.vaccine.2005.03.006. [DOI] [PubMed] [Google Scholar]
- 145.Bonafe N, Rininger JA, Chubet RG, Foellmer HG, Fader S, Anderson JF, Bushmich SL, Anthony K, Ledizet M, Fikrig E, Koski RA, Kaplan P. A recombinant West Nile virus envelope protein vaccine candidate produced in Spodoptera frugiperda expresSF+ cells. Vaccine. 2009;27:213–222. doi: 10.1016/j.vaccine.2008.10.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Zhu B, Ye J, Lu P, Jiang R, Yang X, Fu ZF, Chen H, Cao S. Induction of antigen-specific immune responses in mice by recombinant baculovirus expressing premembrane and envelope proteins of West Nile virus. Virol J. 2012;9:132. doi: 10.1186/1743-422X-9-132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Lieberman MM, Nerurkar VR, Luo H, Cropp B, Carrion R, Jr, de la Garza M, Coller BA, Clements D, Ogata S, Wong T, Martyak T, Weeks-Levy C. Immunogenicity and protective efficacy of a recombinant subunit West Nile virus vaccine in rhesus monkeys. Clin Vaccine Immunol. 2009;16:1332–1337. doi: 10.1128/CVI.00119-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Watts DM, Tesh RB, Siirin M, Rosa AT, Newman PC, Clements DE, Ogata S, Coller BA, Weeks-Levy C, Lieberman MM. Efficacy and durability of a recombinant subunit West Nile vaccine candidate in protecting hamsters from West Nile encephalitis. Vaccine. 2007;25:2913–2918. doi: 10.1016/j.vaccine.2006.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Demento SL, Bonafe N, Cui W, Kaech SM, Caplan MJ, Fikrig E, Ledizet M, Fahmy TM. TLR9-targeted biodegradable nanoparticles as immunization vectors protect against West Nile encephalitis. J Immunol. 2010;185:2989–2997. doi: 10.4049/jimmunol.1000768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Chu JH, Chiang CC, Ng ML. Immunization of flavivirus West Nile recombinant envelope domain III protein induced specific immune response and protection against West Nile virus infection. J Immunol. 2007;178:2699–2705. doi: 10.4049/jimmunol.178.5.2699. [DOI] [PubMed] [Google Scholar]
- 151.Rappuoli R. Reverse vaccinology, a genome-based approach to vaccine development. Vaccine. 2001;19:2688–2691. doi: 10.1016/S0264-410X(00)00554-5. [DOI] [PubMed] [Google Scholar]
- 152.Moriel DG, Scarselli M, Serino L, Mora M, Rappuoli R, Masignani V. Genome-based vaccine development: a short cut for the future. Hum Vaccin. 2008;4:184–188. doi: 10.4161/hv.4.3.6313. [DOI] [PubMed] [Google Scholar]
- 153.Bruno L, Cortese M, Rappuoli R, Merola M. Lessons from reverse vaccinology for viral vaccine design. Curr Opin Virol. 2015;11:89–97. doi: 10.1016/j.coviro.2015.03.001. [DOI] [PubMed] [Google Scholar]
- 154.Cozzi R, Scarselli M, Ferlenghi I. Structural vaccinology: a three-dimensional view for vaccine development. Curr Top Med Chem. 2013;13:2629–2637. doi: 10.2174/15680266113136660187. [DOI] [PubMed] [Google Scholar]
- 155.Marasco WA, Sui J. The growth and potential of human antiviral monoclonal antibody therapeutics. Nat Biotechnol. 2007;25:1421–1434. doi: 10.1038/nbt1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Faverio LA, Piazza FM, Johnson SA, Darnell ME, Ottolini MG, Hemming VG, Prince GA. Immunoprophylaxis of group B respiratory syncytial virus infection in cotton rats. J Infect Dis. 1997;175:932–934. doi: 10.1086/513993. [DOI] [PubMed] [Google Scholar]
- 157.Johnson S, Oliver C, Prince GA, Hemming VG, Pfarr DS, Wang SC, Dormitzer M, O'Grady J, Koenig S, Tamura JK, Woods R, Bansal G, Couchenour D, Tsao E, Hall WC, Young JF. Development of a humanized monoclonal antibody (MEDI-493) with potent in vitro and in vivo activity against respiratory syncytial virus. J Infect Dis. 1997;176:1215–1224. doi: 10.1086/514115. [DOI] [PubMed] [Google Scholar]
- 158.Scott LJ, Lamb HM. Palivizumab. Drugs. 1999;58:305–311. doi: 10.2165/00003495-199958020-00009. [DOI] [PubMed] [Google Scholar]
- 159.Prabakaran P, Zhu Z, Xiao X, Biragyn A, Dimitrov AS, Broder CC, Dimitrov DS. Potent human monoclonal antibodies against SARS CoV, Nipah and Hendra viruses. Expert Opin Biol Ther. 2009;9:355–368. doi: 10.1517/14712590902763755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Ye J, Shao H, Perez DR. Passive immune neutralization strategies for prevention and control of influenza A infections. Immunotherapy. 2012;4:175–186. doi: 10.2217/imt.11.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Bonsignori M, Alam SM, Liao HX, Verkoczy L, Tomaras GD, Haynes BF, Moody MA. HIV-1 antibodies from infection and vaccination: insights for guiding vaccine design. Trends Microbiol. 2012;20:532–539. doi: 10.1016/j.tim.2012.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Gong R, Chen W, Dimitrov DS. Candidate antibody-based therapeutics against HIV-1. BioDrugs. 2012;26:143–162. doi: 10.2165/11631400-000000000-00000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Kwong PD, Mascola JR. Human antibodies that neutralize HIV-1: identification, structures, and B cell ontogenies. Immunity. 2012;37:412–425. doi: 10.1016/j.immuni.2012.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Engle MJ, Diamond MS. Antibody prophylaxis and therapy against West Nile virus infection in wild-type and immunodeficient mice. J Virol. 2003;77:12941–12949. doi: 10.1128/JVI.77.24.12941-12949.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Ben-Nathan D, Lustig S, Tam G, Robinzon S, Segal S, Rager-Zisman B. Prophylactic and therapeutic efficacy of human intravenous immunoglobulin in treating West Nile virus infection in mice. J Infect Dis. 2003;188:5–12. doi: 10.1086/376870. [DOI] [PubMed] [Google Scholar]
- 166.Kreil TR, Eibl MM. Pre- and postexposure protection by passive immunoglobulin but no enhancement of infection with a flavivirus in a mouse model. J Virol. 1997;71:2921–2927. doi: 10.1128/jvi.71.4.2921-2927.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Haley M, Retter AS, Fowler D, Gea-Banacloche J, O'Grady NP. The role for intravenous immunoglobulin in the treatment of West Nile virus encephalitis. Clin Infect Dis. 2003;37:e88–e90. doi: 10.1086/377172. [DOI] [PubMed] [Google Scholar]
- 168.Hamdan A, Green P, Mendelson E, Kramer MR, Pitlik S, Weinberger M. Possible benefit of intravenous immunoglobulin therapy in a lung transplant recipient with West Nile virus encephalitis. Transpl Infect Dis. 2002;4:160–162. doi: 10.1034/j.1399-3062.2002.01014.x. [DOI] [PubMed] [Google Scholar]
- 169.Saquib R, Randall H, Chandrakantan A, Spak CW, Barri YM. West Nile virus encephalitis in a renal transplant recipient: the role of intravenous immunoglobulin. Am J Kidney Dis. 2008;52:e19–e21. doi: 10.1053/j.ajkd.2008.03.042. [DOI] [PubMed] [Google Scholar]
- 170.Shimoni Z, Niven MJ, Pitlick S, Bulvik S. Treatment of West Nile virus encephalitis with intravenous immunoglobulin. Emerg Infect Dis. 2001;7:759. doi: 10.3201/eid0704.017432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Rager-Zisman B, Ben Nathan D. Efficacy of prophylactic and therapeutic human immunoglobulin on West Nile virus infection. Isr Med Assoc J. 2003;5:691. [PubMed] [Google Scholar]
- 172.Oliphant T, Engle M, Nybakken GE, Doane C, Johnson S, Huang L, Gorlatov S, Mehlhop E, Marri A, Chung KM, Ebel GD, Kramer LD, Fremont DH, Diamond MS. Development of a humanized monoclonal antibody with therapeutic potential against West Nile virus. Nat Med. 2005;11:522–530. doi: 10.1038/nm1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Morrey JD, Siddharthan V, Olsen AL, Roper GY, Wang H, Baldwin TJ, Koenig S, Johnson S, Nordstrom JL, Diamond MS. Humanized monoclonal antibody against West Nile virus envelope protein administered after neuronal infection protects against lethal encephalitis in hamsters. J Infect Dis. 2006;194:1300–1308. doi: 10.1086/508293. [DOI] [PubMed] [Google Scholar]
- 174.Ledizet M, Kar K, Foellmer HG, Bonafe N, Anthony KG, Gould LH, Bushmich SL, Fikrig E, Koski RA. Antibodies targeting linear determinants of the envelope protein protect mice against West Nile virus. J Infect Dis. 2007;196:1741–1748. doi: 10.1086/523654. [DOI] [PubMed] [Google Scholar]
- 175.Morrey JD, Siddharthan V, Olsen AL, Wang H, Julander JG, Hall JO, Li H, Nordstrom JL, Koenig S, Johnson S, Diamond MS. Defining limits of treatment with humanized neutralizing monoclonal antibody for West Nile virus neurological infection in a hamster model. Antimicrob Agents Chemother. 2007;51:2396–2402. doi: 10.1128/AAC.00147-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Zhang S, Vogt MR, Oliphant T, Engle M, Bovshik EI, Diamond MS, Beasley DW. Development of resistance to passive therapy with a potently neutralizing humanized monoclonal antibody against West Nile virus. J Infect Dis. 2009;200:202–205. doi: 10.1086/599794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Beigel JH, Nordstrom JL, Pillemer SR, Roncal C, Goldwater DR, Li H, Holland PC, Johnson S, Stein K, Koenig S. Safety and pharmacokinetics of single intravenous dose of MGAWN1, a novel monoclonal antibody to West Nile virus. Antimicrob Agents Chemother. 2010;54:2431–2436. doi: 10.1128/AAC.01178-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Gould LH, Sui J, Foellmer H, Oliphant T, Wang T, Ledizet M, Murakami A, Noonan K, Lambeth C, Kar K, Anderson JF, de Silva AM, Diamond MS, Koski RA, Marasco WA, Fikrig E. Protective and therapeutic capacity of human single-chain Fv-Fc fusion proteins against West Nile virus. J Virol. 2005;79:14606–14613. doi: 10.1128/JVI.79.23.14606-14613.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Mehlhop E, Ansarah-Sobrinho C, Johnson S, Engle M, Fremont DH, Pierson TC, Diamond MS. Complement protein C1q inhibits antibody-dependent enhancement of flavivirus infection in an IgG subclass-specific manner. Cell Host Microbe. 2007;2:417–426. doi: 10.1016/j.chom.2007.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.He J, Lai H, Engle M, Gorlatov S, Gruber C, Steinkellner H, Diamond MS, Chen Q. Generation and analysis of novel plant-derived antibody-based therapeutic molecules against West Nile virus. PLoS One. 2014;9:e93541. doi: 10.1371/journal.pone.0093541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.He J, Lai H, Brock C, Chen Q. A novel system for rapid and cost-effective production of detection and diagnostic reagents of West Nile virus in plants. J Biomed Biotechnol. 2012;2012:106783. doi: 10.1155/2012/106783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Chen Q. Plant-made vaccines against West Nile virus are potent, safe, and economically feasible. Biotechnol J. 2015;10(5):671–680. doi: 10.1002/biot.201400428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Lai H, Engle M, Fuchs A, Keller T, Johnson S, Gorlatov S, Diamond MS, Chen Q. Monoclonal antibody produced in plants efficiently treats West Nile virus infection in mice. Proc Natl Acad Sci U S A. 2010;107:2419–2424. doi: 10.1073/pnas.0914503107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Diamond MS, Pierson TC, Fremont DH. The structural immunology of antibody protection against West Nile virus. Immunol Rev. 2008;225:212–225. doi: 10.1111/j.1600-065X.2008.00676.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Oliphant T, Diamond MS. The molecular basis of antibody-mediated neutralization of West Nile virus. Expert Opin Biol Ther. 2007;7:885–892. doi: 10.1517/14712598.7.6.885. [DOI] [PubMed] [Google Scholar]
- 186.Maynard J, Georgiou G. Antibody engineering. Annu Rev Biomed Eng. 2000;2:339–376. doi: 10.1146/annurev.bioeng.2.1.339. [DOI] [PubMed] [Google Scholar]
- 187.Filpula D. Antibody engineering and modification technologies. Biomol Eng. 2007;24:201–215. doi: 10.1016/j.bioeng.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 188.Herter S, Birk MC, Klein C, Gerdes C, Umana P, Bacac M. Glycoengineering of therapeutic antibodies enhances monocyte/macrophage-mediated phagocytosis and cytotoxicity. J Immunol. 2014;192:2252–2260. doi: 10.4049/jimmunol.1301249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Bai F, Kong KF, Dai J, Qian F, Zhang L, Brown CR, Fikrig E, Montgomery RR. A paradoxical role for neutrophils in the pathogenesis of West Nile virus. J Infect Dis. 2010;202:1804–1812. doi: 10.1086/657416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Bai F, Town T, Qian F, Wang P, Kamanaka M, Connolly TM, Gate D, Montgomery RR, Flavell RA, Fikrig E. IL-10 signaling blockade controls murine West Nile virus infection. PLoS Pathog. 2009;5:e1000610. doi: 10.1371/journal.ppat.1000610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Bai F, Wang T, Pal U, Bao F, Gould LH, Fikrig E. Use of RNA interference to prevent lethal murine West Nile virus infection. J Infect Dis. 2005;191:1148–1154. doi: 10.1086/428507. [DOI] [PubMed] [Google Scholar]
- 192.Town T, Bai F, Wang T, Kaplan AT, Qian F, Montgomery RR, Anderson JF, Flavell RA, Fikrig E. Toll-like receptor 7 mitigates lethal West Nile encephalitis via interleukin 23-dependent immune cell infiltration and homing. Immunity. 2009;30:242–253. doi: 10.1016/j.immuni.2008.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Lim SP, Wang QY, Noble CG, Chen YL, Dong H, Zou B, Yokokawa F, Nilar S, Smith P, Beer D, Lescar J, Shi PY. Ten years of dengue drug discovery: progress and prospects. Antiviral Res. 2013;100:500–519. doi: 10.1016/j.antiviral.2013.09.013. [DOI] [PubMed] [Google Scholar]
- 194.Walters WP, Namchuk M. Designing screens: how to make your hits a hit. Nat Rev Drug Discov. 2003;2:259–266. doi: 10.1038/nrd1063. [DOI] [PubMed] [Google Scholar]
- 195.Hughes JP, Rees S, Kalindjian SB, Philpott KL. Principles of early drug discovery. Br J Pharmacol. 2011;162:1239–1249. doi: 10.1111/j.1476-5381.2010.01127.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Mayr LM, Bojanic D. Novel trends in high-throughput screening. Curr Opin Pharmacol. 2009;9:580–588. doi: 10.1016/j.coph.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 197.Zhu Z, Cuozzo J. Review article: high-throughput affinity-based technologies for small-molecule drug discovery. J Biomol Screen. 2009;14:1157–1164. doi: 10.1177/1087057109350114. [DOI] [PubMed] [Google Scholar]
- 198.Davies TG, Tickle IJ. Fragment screening using X-ray crystallography. Top Curr Chem. 2012;317:33–59. doi: 10.1007/128_2011_179. [DOI] [PubMed] [Google Scholar]
- 199.Bergsdorf C, Ottl J. Affinity-based screening techniques: their impact and benefit to increase the number of high quality leads. Expert Opin Drug Discov. 2010;5:1095–1107. doi: 10.1517/17460441.2010.524641. [DOI] [PubMed] [Google Scholar]
- 200.Ma L, Jones CT, Groesch TD, Kuhn RJ, Post CB. Solution structure of dengue virus capsid protein reveals another fold. Proc Natl Acad Sci U S A. 2004;101:3414–3419. doi: 10.1073/pnas.0305892101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Li L, Lok SM, Yu IM, Zhang Y, Kuhn RJ, Chen J, Rossmann MG. The flavivirus precursor membrane-envelope protein complex: structure and maturation. Science. 2008;319:1830–1834. doi: 10.1126/science.1153263. [DOI] [PubMed] [Google Scholar]
- 202.Zhang Y, Zhang W, Ogata S, Clements D, Strauss JH, Baker TS, Kuhn RJ, Rossmann MG. Conformational changes of the flavivirus E glycoprotein. Structure. 2004;12:1607–1618. doi: 10.1016/j.str.2004.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Zhang Y, Kaufmann B, Chipman PR, Kuhn RJ, Rossmann MG. Structure of immature West Nile virus. J Virol. 2007;81:6141–6145. doi: 10.1128/JVI.00037-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Geiss BJ, Stahla-Beek HJ, Hannah AM, Gari HH, Henderson BR, Saeedi BJ, Keenan SM. A high-throughput screening assay for the identification of flavivirus NS5 capping enzyme GTP-binding inhibitors: implications for antiviral drug development. J Biomol Screen. 2011;16:852–861. doi: 10.1177/1087057111412183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Gu B, Ouzunov S, Wang L, Mason P, Bourne N, Cuconati A, Block TM. Discovery of small molecule inhibitors of West Nile virus using a high-throughput sub-genomic replicon screen. Antiviral Res. 2006;70:39–50. doi: 10.1016/j.antiviral.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 206.Johnston PA, Phillips J, Shun TY, Shinde S, Lazo JS, Huryn DM, Myers MC, Ratnikov BI, Smith JW, Su Y, Dahl R, Cosford ND, Shiryaev SA, Strongin AY. HTS identifies novel and specific uncompetitive inhibitors of the two-component NS2B-NS3 proteinase of West Nile virus. Assay Drug Dev Technol. 2007;5:737–750. doi: 10.1089/adt.2007.101. [DOI] [PubMed] [Google Scholar]
- 207.Kampmann T, Yennamalli R, Campbell P, Stoermer MJ, Fairlie DP, Kobe B, Young PR. In silico screening of small molecule libraries using the dengue virus envelope E protein has identified compounds with antiviral activity against multiple flaviviruses. Antiviral Res. 2009;84:234–241. doi: 10.1016/j.antiviral.2009.09.007. [DOI] [PubMed] [Google Scholar]
- 208.Green N, Ott RD, Isaacs RJ, Fang H. Cell-based assays to identify inhibitors of viral disease. Expert Opin Drug Discov. 2008;3:671–676. doi: 10.1517/17460441.3.6.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Baharuddin A, Hassan AA, Sheng GC, Nasir SB, Othman S, Yusof R, Othman R, Rahman NA. Current approaches in antiviral drug discovery against the Flaviviridae family. Curr Pharm Des. 2014;20:3428–3444. doi: 10.2174/13816128113199990635. [DOI] [PubMed] [Google Scholar]
- 210.Botting C, Kuhn RJ. Novel approaches to flavivirus drug discovery. Expert Opin Drug Discov. 2012;7:417–428. doi: 10.1517/17460441.2012.673579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Vlaycheva L, Nickells M, Droll DA, Chambers TJ. Neuroblastoma cell-adapted yellow fever virus: mutagenesis of the E protein locus involved in persistent infection and its effects on virus penetration and spread. J Gen Virol. 2005;86:413–421. doi: 10.1099/vir.0.80314-0. [DOI] [PubMed] [Google Scholar]
- 212.Heinz FX, Auer G, Stiasny K, Holzmann H, Mandl C, Guirakhoo F, Kunz C. The interactions of the flavivirus envelope proteins: implications for virus entry and release. Arch Virol Suppl. 1994;9:339–348. doi: 10.1007/978-3-7091-9326-6_34. [DOI] [PubMed] [Google Scholar]
- 213.Chu JJ, Ng ML. Infectious entry of West Nile virus occurs through a clathrin-mediated endocytic pathway. J Virol. 2004;78:10543–10555. doi: 10.1128/JVI.78.19.10543-10555.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Kuritzkes DR. HIV-1 entry inhibitors: an overview. Curr Opin HIV AIDS. 2009;4:82–87. doi: 10.1097/COH.0b013e328322402e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Garg H, Viard M, Jacobs A, Blumenthal R. Targeting HIV-1 gp41-induced fusion and pathogenesis for anti-viral therapy. Curr Top Med Chem. 2011;11:2947–2958. doi: 10.2174/156802611798808479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Modis Y, Ogata S, Clements D, Harrison SC. A ligand-binding pocket in the dengue virus envelope glycoprotein. Proc Natl Acad Sci U S A. 2003;100:6986–6991. doi: 10.1073/pnas.0832193100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Bressanelli S, Stiasny K, Allison SL, Stura EA, Duquerroy S, Lescar J, Heinz FX, Rey FA. Structure of a flavivirus envelope glycoprotein in its low-pH-induced membrane fusion conformation. EMBO J. 2004;23:728–738. doi: 10.1038/sj.emboj.7600064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Li Z, Khaliq M, Zhou Z, Post CB, Kuhn RJ, Cushman M. Design, synthesis, and biological evaluation of antiviral agents targeting flavivirus envelope proteins. J Med Chem. 2008;51:4660–4671. doi: 10.1021/jm800412d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Poh MK, Yip A, Zhang S, Priestle JP, Ma NL, Smit JM, Wilschut J, Shi PY, Wenk MR, Schul W. A small molecule fusion inhibitor of dengue virus. Antiviral Res. 2009;84:260–266. doi: 10.1016/j.antiviral.2009.09.011. [DOI] [PubMed] [Google Scholar]
- 220.Wang QY, Patel SJ, Vangrevelinghe E, Xu HY, Rao R, Jaber D, Schul W, Gu F, Heudi O, Ma NL, Poh MK, Phong WY, Keller TH, Jacoby E, Vasudevan SG. A small-molecule dengue virus entry inhibitor. Antimicrob Agents Chemother. 2009;53:1823–1831. doi: 10.1128/AAC.01148-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Zhou Z, Khaliq M, Suk JE, Patkar C, Li L, Kuhn RJ, Post CB. Antiviral compounds discovered by virtual screening of small-molecule libraries against dengue virus E protein. ACS Chem Biol. 2008;3:765–775. doi: 10.1021/cb800176t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Vervaeke P, Alen M, Noppen S, Schols D, Oreste P, Liekens S. Sulfated Escherichia coli K5 polysaccharide derivatives inhibit dengue virus infection of human microvascular endothelial cells by interacting with the viral envelope protein E domain III. PLoS One. 2013;8:e74035. doi: 10.1371/journal.pone.0074035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Lin LT, Chen TY, Lin SC, Chung CY, Lin TC, Wang GH, Anderson R, Lin CC, Richardson CD. Broad-spectrum antiviral activity of chebulagic acid and punicalagin against viruses that use glycosaminoglycans for entry. BMC Microbiol. 2013;13:187. doi: 10.1186/1471-2180-13-187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Lee E, Pavy M, Young N, Freeman C, Lobigs M. Antiviral effect of the heparan sulfate mimetic, PI-88, against dengue and encephalitic flaviviruses. Antiviral Res. 2006;69:31–38. doi: 10.1016/j.antiviral.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 225.Pujol CA, Ray S, Ray B, Damonte EB. Antiviral activity against dengue virus of diverse classes of algal sulfated polysaccharides. Int J Biol Macromol. 2012;51:412–416. doi: 10.1016/j.ijbiomac.2012.05.028. [DOI] [PubMed] [Google Scholar]
- 226.Balzarini J. Carbohydrate-binding agents: a potential future cornerstone for the chemotherapy of enveloped viruses? Antivir Chem Chemother. 2007;18:1–11. doi: 10.1177/095632020701800101. [DOI] [PubMed] [Google Scholar]
- 227.Gunthard HF, Aberg JA, Eron JJ, Hoy JF, Telenti A, Benson CA, Burger DM, Cahn P, Gallant JE, Glesby MJ, Reiss P, Saag MS, Thomas DL, Jacobsen DM, Volberding PA, International Antiviral Society USAP Antiretroviral treatment of adult HIV infection: 2014 recommendations of the International Antiviral Society-USA Panel. JAMA. 2014;312:410–425. doi: 10.1001/jama.2014.8722. [DOI] [PubMed] [Google Scholar]
- 228.Patick AK, Potts KE. Protease inhibitors as antiviral agents. Clin Microbiol Rev. 1998;11:614–627. doi: 10.1128/cmr.11.4.614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Lin C, Kwong AD, Perni RB. Discovery and development of VX-950, a novel, covalent, and reversible inhibitor of hepatitis C virus NS3.4A serine protease. Infect Disord Drug Targets. 2006;6:3–16. doi: 10.2174/187152606776056706. [DOI] [PubMed] [Google Scholar]
- 230.Perni RB, Almquist SJ, Byrn RA, Chandorkar G, Chaturvedi PR, Courtney LF, Decker CJ, Dinehart K, Gates CA, Harbeson SL, Heiser A, Kalkeri G, Kolaczkowski E, Lin K, Luong YP, Rao BG, Taylor WP, Thomson JA, Tung RD, Wei Y, Kwong AD, Lin C. Preclinical profile of VX-950, a potent, selective, and orally bioavailable inhibitor of hepatitis C virus NS3-4A serine protease. Antimicrob Agents Chemother. 2006;50:899–909. doi: 10.1128/AAC.50.3.899-909.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Falgout B, Pethel M, Zhang YM, Lai CJ. Both nonstructural proteins NS2B and NS3 are required for the proteolytic processing of dengue virus nonstructural proteins. J Virol. 1991;65:2467–2475. doi: 10.1128/jvi.65.5.2467-2475.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Kim YM, Gayen S, Kang C, Joy J, Huang Q, Chen AS, Wee JL, Ang MJ, Lim HA, Hung AW, Li R, Noble CG, le Lee T, Yip A, Wang QY, Chia CS, Hill J, Shi PY, Keller TH. NMR analysis of a novel enzymatically active unlinked dengue NS2B-NS3 protease complex. J Biol Chem. 2013;288:12891–12900. doi: 10.1074/jbc.M112.442723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Sudo K, Yamaji K, Kawamura K, Nishijima T, Kojima N, Aibe K, Shimotohno K, Shimizu Y. High-throughput screening of low molecular weight NS3-NS4A protease inhibitors using a fluorescence resonance energy transfer substrate. Antivir Chem Chemother. 2005;16:385–392. doi: 10.1177/095632020501600605. [DOI] [PubMed] [Google Scholar]
- 234.Sudo K, Inoue H, Shimizu Y, Yamaji K, Konno K, Shigeta S, Kaneko T, Yokota T, Shimotohno K. Establishment of an in vitro assay system for screening hepatitis C virus protease inhibitors using high performance liquid chromatography. Antiviral Res. 1996;32:9–18. doi: 10.1016/0166-3542(95)00969-8. [DOI] [PubMed] [Google Scholar]
- 235.Berdichevsky Y, Zemel R, Bachmatov L, Abramovich A, Koren R, Sathiyamoorthy P, Golan-Goldhirsh A, Tur-Kaspa R, Benhar I. A novel high throughput screening assay for HCV NS3 serine protease inhibitors. J Virol Methods. 2003;107:245–255. doi: 10.1016/S0166-0934(02)00255-0. [DOI] [PubMed] [Google Scholar]
- 236.Kakiuchi N, Komoda Y, Komoda K, Takeshita N, Okada S, Tani T, Shimotohno K. Non-peptide inhibitors of HCV serine proteinase. FEBS Lett. 1998;421:217–220. doi: 10.1016/S0014-5793(97)01566-4. [DOI] [PubMed] [Google Scholar]
- 237.Brecher M, Zhang J, Li H. The flavivirus protease as a target for drug discovery. Virol Sin. 2013;28:326–336. doi: 10.1007/s12250-013-3390-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Aleshin AE, Shiryaev SA, Strongin AY, Liddington RC. Structural evidence for regulation and specificity of flaviviral proteases and evolution of the Flaviviridae fold. Protein Sci. 2007;16:795–806. doi: 10.1110/ps.072753207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Leung D, Schroder K, White H, Fang NX, Stoermer MJ, Abbenante G, Martin JL, Young PR, Fairlie DP. Activity of recombinant dengue 2 virus NS3 protease in the presence of a truncated NS2B co-factor, small peptide substrates, and inhibitors. J Biol Chem. 2001;276:45762–45771. doi: 10.1074/jbc.M107360200. [DOI] [PubMed] [Google Scholar]
- 240.Mangano DT, Miao Y, Vuylsteke A, Tudor IC, Juneja R, Filipescu D, Hoeft A, Fontes ML, Hillel Z, Ott E, Titov T, Dietzel C, Levin J, Investigators of The Multicenter Study of Perioperative Ischemia Research Group, Ischemia Research, and Education Foundation Mortality associated with aprotinin during 5 years following coronary artery bypass graft surgery. JAMA. 2007;297:471–479. doi: 10.1001/jama.297.5.471. [DOI] [PubMed] [Google Scholar]
- 241.Ekonomiuk D, Su XC, Ozawa K, Bodenreider C, Lim SP, Yin Z, Keller TH, Beer D, Patel V, Otting G, Caflisch A, Huang D. Discovery of a non-peptidic inhibitor of West Nile virus NS3 protease by high-throughput docking. PLoS Negl Trop Dis. 2009;3:e356. doi: 10.1371/journal.pntd.0000356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Mueller NH, Pattabiraman N, Ansarah-Sobrinho C, Viswanathan P, Pierson TC, Padmanabhan R. Identification and biochemical characterization of small-molecule inhibitors of West Nile virus serine protease by a high-throughput screen. Antimicrob Agents Chemother. 2008;52:3385–3393. doi: 10.1128/AAC.01508-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Mueller NH, Yon C, Ganesh VK, Padmanabhan R. Characterization of the West Nile virus protease substrate specificity and inhibitors. Int J Biochem Cell Biol. 2007;39:606–614. doi: 10.1016/j.biocel.2006.10.025. [DOI] [PubMed] [Google Scholar]
- 244.Samanta S, Lim TL, Lam Y. Synthesis and in vitro evaluation of West Nile virus protease inhibitors based on the 2-{6-[2-(5-phenyl-4H-{1,2,4]triazol-3-ylsulfanyl)acetylamino]benzothiazol-2-ylsul fanyl}acetamide scaffold. ChemMedChem. 2013;8:994–1001. doi: 10.1002/cmdc.201300114. [DOI] [PubMed] [Google Scholar]
- 245.Sidique S, Shiryaev SA, Ratnikov BI, Herath A, Su Y, Strongin AY, Cosford ND. Structure-activity relationship and improved hydrolytic stability of pyrazole derivatives that are allosteric inhibitors of West Nile Virus NS2B-NS3 proteinase. Bioorg Med Chem Lett. 2009;19:5773–5777. doi: 10.1016/j.bmcl.2009.07.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Aravapalli S, Lai H, Teramoto T, Alliston KR, Lushington GH, Ferguson EL, Padmanabhan R, Groutas WC. Inhibitors of Dengue virus and West Nile virus proteases based on the aminobenzamide scaffold. Bioorg Med Chem. 2012;20:4140–4148. doi: 10.1016/j.bmc.2012.04.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Gao Y, Samanta S, Cui T, Lam Y. Synthesis and in vitro evaluation of West Nile virus protease inhibitors based on the 1,3,4,5-tetrasubstituted 1H-pyrrol-2(5H)-one scaffold. ChemMedChem. 2013;8:1554–1560. doi: 10.1002/cmdc.201300244. [DOI] [PubMed] [Google Scholar]
- 248.Lai H, Dou D, Aravapalli S, Teramoto T, Lushington GH, Mwania TM, Alliston KR, Eichhorn DM, Padmanabhan R, Groutas WC. Design, synthesis and characterization of novel 1,2-benzisothiazol-3(2H)-one and 1,3,4-oxadiazole hybrid derivatives: potent inhibitors of Dengue and West Nile virus NS2B/NS3 proteases. Bioorg Med Chem. 2013;21:102–113. doi: 10.1016/j.bmc.2012.10.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Cregar-Hernandez L, Jiao GS, Johnson AT, Lehrer AT, Wong TA, Margosiak SA. Small molecule pan-dengue and West Nile virus NS3 protease inhibitors. Antivir Chem Chemother. 2011;21:209–217. doi: 10.3851/IMP1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Luo D, Wei N, Doan DN, Paradkar PN, Chong Y, Davidson AD, Kotaka M, Lescar J, Vasudevan SG. Flexibility between the protease and helicase domains of the dengue virus NS3 protein conferred by the linker region and its functional implications. J Biol Chem. 2010;285:18817–18827. doi: 10.1074/jbc.M109.090936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Robin G, Chappell K, Stoermer MJ, Hu SH, Young PR, Fairlie DP, Martin JL. Structure of West Nile virus NS3 protease: ligand stabilization of the catalytic conformation. J Mol Biol. 2009;385:1568–1577. doi: 10.1016/j.jmb.2008.11.026. [DOI] [PubMed] [Google Scholar]
- 252.Brault AC, Huang CY, Langevin SA, Kinney RM, Bowen RA, Ramey WN, Panella NA, Holmes EC, Powers AM, Miller BR. A single positively selected West Nile viral mutation confers increased virogenesis in American crows. Nat Genet. 2007;39:1162–1166. doi: 10.1038/ng2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Mertens E, Kajaste-Rudnitski A, Torres S, Funk A, Frenkiel MP, Iteman I, Khromykh AA, Despres P. Viral determinants in the NS3 helicase and 2 K peptide that promote West Nile virus resistance to antiviral action of 2′,5′-oligoadenylate synthetase 1b. Virology. 2010;399:176–185. doi: 10.1016/j.virol.2009.12.036. [DOI] [PubMed] [Google Scholar]
- 254.Borowski P, Niebuhr A, Mueller O, Bretner M, Felczak K, Kulikowski T, Schmitz H. Purification and characterization of West Nile virus nucleoside triphosphatase (NTPase)/helicase: evidence for dissociation of the NTPase and helicase activities of the enzyme. J Virol. 2001;75:3220–3229. doi: 10.1128/JVI.75.7.3220-3229.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Boguszewska-Chachulska AM, Krawczyk M, Stankiewicz A, Gozdek A, Haenni AL, Strokovskaya L. Direct fluorometric measurement of hepatitis C virus helicase activity. FEBS Lett. 2004;567:253–258. doi: 10.1016/j.febslet.2004.04.072. [DOI] [PubMed] [Google Scholar]
- 256.Hanson AM, Hernandez JJ, Shadrick WR, Frick DN. Identification and analysis of inhibitors targeting the hepatitis C virus NS3 helicase. Methods Enzymol. 2012;511:463–483. doi: 10.1016/B978-0-12-396546-2.00021-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Mukherjee S, Hanson AM, Shadrick WR, Ndjomou J, Sweeney NL, Hernandez JJ, Bartczak D, Li K, Frankowski KJ, Heck JA, Arnold LA, Schoenen FJ, Frick DN. Identification and analysis of hepatitis C virus NS3 helicase inhibitors using nucleic acid binding assays. Nucleic Acids Res. 2012;40:8607–8621. doi: 10.1093/nar/gks623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Tani H, Akimitsu N, Fujita O, Matsuda Y, Miyata R, Tsuneda S, Igarashi M, Sekiguchi Y, Noda N. High-throughput screening assay of hepatitis C virus helicase inhibitors using fluorescence-quenching phenomenon. Biochem Biophys Res Commun. 2009;379:1054–1059. doi: 10.1016/j.bbrc.2009.01.020. [DOI] [PubMed] [Google Scholar]
- 259.Kwong AD, Rao BG, Jeang KT. Viral and cellular RNA helicases as antiviral targets. Nat Rev Drug Discov. 2005;4:845–853. doi: 10.1038/nrd1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Borowski P, Deinert J, Schalinski S, Bretner M, Ginalski K, Kulikowski T, Shugar D. Halogenated benzimidazoles and benzotriazoles as inhibitors of the NTPase/helicase activities of hepatitis C and related viruses. Eur J Biochem. 2003;270:1645–1653. doi: 10.1046/j.1432-1033.2003.03540.x. [DOI] [PubMed] [Google Scholar]
- 261.Bretner M, Schalinski S, Haag A, Lang M, Schmitz H, Baier A, Behrens SE, Kulikowski T, Borowski P. Synthesis and evaluation of ATP-binding site directed potential inhibitors of nucleoside triphosphatases/helicases and polymerases of hepatitis C and other selected Flaviviridae viruses. Antivir Chem Chemother. 2004;15:35–42. doi: 10.1177/095632020401500104. [DOI] [PubMed] [Google Scholar]
- 262.Ujjinamatada RK, Baier A, Borowski P, Hosmane RS. An analogue of AICAR with dual inhibitory activity against WNV and HCV NTPase/helicase: synthesis and in vitro screening of 4-carbamoyl-5-(4,6-diamino-2,5-dihydro-1,3,5-triazin-2-yl)imidazole-1-beta-D-ribo furanoside. Bioorg Med Chem Lett. 2007;17:2285–2288. doi: 10.1016/j.bmcl.2007.01.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Zhang N, Chen HM, Koch V, Schmitz H, Liao CL, Bretner M, Bhadti VS, Fattom AI, Naso RB, Hosmane RS, Borowski P. Ring-expanded ("fat") nucleoside and nucleotide analogues exhibit potent in vitro activity against flaviviridae NTPases/helicases, including those of the West Nile virus, hepatitis C virus, and Japanese encephalitis virus. J Med Chem. 2003;46:4149–4164. doi: 10.1021/jm030842j. [DOI] [PubMed] [Google Scholar]
- 264.Zhang N, Chen HM, Koch V, Schmitz H, Minczuk M, Stepien P, Fattom AI, Naso RB, Kalicharran K, Borowski P, Hosmane RS. Potent inhibition of NTPase/helicase of the West Nile Virus by ring-expanded ("fat") nucleoside analogues. J Med Chem. 2003;46:4776–4789. doi: 10.1021/jm030277k. [DOI] [PubMed] [Google Scholar]
- 265.Borowski P, Lang M, Haag A, Schmitz H, Choe J, Chen HM, Hosmane RS. Characterization of imidazo[4,5-d]pyridazine nucleosides as modulators of unwinding reaction mediated by West Nile virus nucleoside triphosphatase/helicase: evidence for activity on the level of substrate and/or enzyme. Antimicrob Agents Chemother. 2002;46:1231–1239. doi: 10.1128/AAC.46.5.1231-1239.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Mastrangelo E, Pezzullo M, De Burghgraeve T, Kaptein S, Pastorino B, Dallmeier K, de Lamballerie X, Neyts J, Hanson AM, Frick DN, Bolognesi M, Milani M. Ivermectin is a potent inhibitor of flavivirus replication specifically targeting NS3 helicase activity: new prospects for an old drug. J Antimicrob Chemother. 2012;67:1884–1894. doi: 10.1093/jac/dks147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Davidson AD. Chapter 2. New insights into flavivirus nonstructural protein 5. Adv Virus Res. 2009;74:41–101. doi: 10.1016/S0065-3527(09)74002-3. [DOI] [PubMed] [Google Scholar]
- 268.Selisko B, Dutartre H, Guillemot JC, Debarnot C, Benarroch D, Khromykh A, Despres P, Egloff MP, Canard B. Comparative mechanistic studies of de novo RNA synthesis by flavivirus RNA-dependent RNA polymerases. Virology. 2006;351:145–158. doi: 10.1016/j.virol.2006.03.026. [DOI] [PubMed] [Google Scholar]
- 269.Malet H, Masse N, Selisko B, Romette JL, Alvarez K, Guillemot JC, Tolou H, Yap TL, Vasudevan S, Lescar J, Canard B. The flavivirus polymerase as a target for drug discovery. Antiviral Res. 2008;80:23–35. doi: 10.1016/j.antiviral.2008.06.007. [DOI] [PubMed] [Google Scholar]
- 270.De Francesco R, Carfi A. Advances in the development of new therapeutic agents targeting the NS3-4A serine protease or the NS5B RNA-dependent RNA polymerase of the hepatitis C virus. Adv Drug Deliv Rev. 2007;59:1242–1262. doi: 10.1016/j.addr.2007.04.016. [DOI] [PubMed] [Google Scholar]
- 271.De Clercq E. Recent highlights in the development of new antiviral drugs. Curr Opin Microbiol. 2005;8:552–560. doi: 10.1016/j.mib.2005.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Pilger BD, Cui C, Coen DM. Identification of a small molecule that inhibits herpes simplex virus DNA polymerase subunit interactions and viral replication. Chem Biol. 2004;11:647–654. doi: 10.1016/j.chembiol.2004.01.018. [DOI] [PubMed] [Google Scholar]
- 273.Wang YK, Rigat KL, Roberts SB, Gao M. A homogeneous, solid-phase assay for hepatitis C virus RNA-dependent RNA polymerase. Anal Biochem. 2006;359:106–111. doi: 10.1016/j.ab.2006.09.013. [DOI] [PubMed] [Google Scholar]
- 274.Lee JC, Tseng CK, Chen KJ, Huang KJ, Lin CK, Lin YT. A cell-based reporter assay for inhibitor screening of hepatitis C virus RNA-dependent RNA polymerase. Anal Biochem. 2010;403:52–62. doi: 10.1016/j.ab.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 275.Lahser FC, Malcolm BA. A continuous nonradioactive assay for RNA-dependent RNA polymerase activity. Anal Biochem. 2004;325:247–254. doi: 10.1016/j.ab.2003.10.034. [DOI] [PubMed] [Google Scholar]
- 276.Ranjith-Kumar CT, Wen Y, Baxter N, Bhardwaj K, Cheng Kao C. A cell-based assay for RNA synthesis by the HCV polymerase reveals new insights on mechanism of polymerase inhibitors and modulation by NS5A. PLoS One. 2011;6:e22575. doi: 10.1371/journal.pone.0022575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Smee DF, Tarbet EB, Furuta Y, Morrey JD, Barnard DL. Synergistic combinations of favipiravir and oseltamivir against wild-type pandemic and oseltamivir-resistant influenza A virus infections in mice. Future Virol. 2013;8:1085–1094. doi: 10.2217/fvl.13.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Tarbet EB, Vollmer AH, Hurst BL, Barnard DL, Furuta Y, Smee DF. In vitro activity of favipiravir and neuraminidase inhibitor combinations against oseltamivir-sensitive and oseltamivir-resistant pandemic influenza A (H1N1) virus. Arch Virol. 2014;159:1279–1291. doi: 10.1007/s00705-013-1922-1. [DOI] [PubMed] [Google Scholar]
- 279.Furuta Y, Gowen BB, Takahashi K, Shiraki K, Smee DF, Barnard DL. Favipiravir (T-705), a novel viral RNA polymerase inhibitor. Antiviral Res. 2013;100:446–454. doi: 10.1016/j.antiviral.2013.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Ojwang JO, Ali S, Smee DF, Morrey JD, Shimasaki CD, Sidwell RW. Broad-spectrum inhibitor of viruses in the Flaviviridae family. Antiviral Res. 2005;68:49–55. doi: 10.1016/j.antiviral.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 281.Olsen DB, Eldrup AB, Bartholomew L, Bhat B, Bosserman MR, Ceccacci A, Colwell LF, Fay JF, Flores OA, Getty KL, Grobler JA, LaFemina RL, Markel EJ, Migliaccio G, Prhavc M, Stahlhut MW, Tomassini JE, MacCoss M, Hazuda DJ, Carroll SS. A 7-deaza-adenosine analog is a potent and selective inhibitor of hepatitis C virus replication with excellent pharmacokinetic properties. Antimicrob Agents Chemother. 2004;48:3944–3953. doi: 10.1128/AAC.48.10.3944-3953.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Schul W, Liu W, Xu HY, Flamand M, Vasudevan SG. A dengue fever viremia model in mice shows reduction in viral replication and suppression of the inflammatory response after treatment with antiviral drugs. J Infect Dis. 2007;195:665–674. doi: 10.1086/511310. [DOI] [PubMed] [Google Scholar]
- 283.Yap TL, Xu T, Chen YL, Malet H, Egloff MP, Canard B, Vasudevan SG, Lescar J. Crystal structure of the dengue virus RNA-dependent RNA polymerase catalytic domain at 1.85-angstrom resolution. J Virol. 2007;81:4753–4765. doi: 10.1128/JVI.02283-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Niyomrattanakit P, Chen YL, Dong H, Yin Z, Qing M, Glickman JF, Lin K, Mueller D, Voshol H, Lim JY, Nilar S, Keller TH, Shi PY. Inhibition of dengue virus polymerase by blocking of the RNA tunnel. J Virol. 2010;84:5678–5686. doi: 10.1128/JVI.02451-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Noble CG, Lim SP, Chen YL, Liew CW, Yap L, Lescar J, Shi PY. Conformational flexibility of the dengue virus RNA-dependent RNA polymerase revealed by a complex with an inhibitor. J Virol. 2013;87:5291–5295. doi: 10.1128/JVI.00045-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Egloff MP, Benarroch D, Selisko B, Romette JL, Canard B. An RNA cap (nucleoside-2′-O-)-methyltransferase in the flavivirus RNA polymerase NS5: crystal structure and functional characterization. EMBO J. 2002;21:2757–2768. doi: 10.1093/emboj/21.11.2757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Zhou Y, Ray D, Zhao Y, Dong H, Ren S, Li Z, Guo Y, Bernard KA, Shi PY, Li H. Structure and function of flavivirus NS5 methyltransferase. J Virol. 2007;81:3891–3903. doi: 10.1128/JVI.02704-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Daffis S, Szretter KJ, Schriewer J, Li J, Youn S, Errett J, Lin TY, Schneller S, Zust R, Dong H, Thiel V, Sen GC, Fensterl V, Klimstra WB, Pierson TC, Buller RM, Gale M, Jr, Shi PY, Diamond MS. 2′-O methylation of the viral mRNA cap evades host restriction by IFIT family members. Nature. 2010;468:452–456. doi: 10.1038/nature09489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Dong H, Chang DC, Hua MH, Lim SP, Chionh YH, Hia F, Lee YH, Kukkaro P, Lok SM, Dedon PC, Shi PY. 2′-O methylation of internal adenosine by flavivirus NS5 methyltransferase. PLoS Pathog. 2012;8:e1002642. doi: 10.1371/journal.ppat.1002642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Dong H, Liu L, Zou G, Zhao Y, Li Z, Lim SP, Shi PY, Li H. Structural and functional analyses of a conserved hydrophobic pocket of flavivirus methyltransferase. J Biol Chem. 2010;285:32586–32595. doi: 10.1074/jbc.M110.129197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Benarroch D, Egloff MP, Mulard L, Guerreiro C, Romette JL, Canard B. A structural basis for the inhibition of the NS5 dengue virus mRNA 2′-O-methyltransferase domain by ribavirin 5′-triphosphate. J Biol Chem. 2004;279:35638–35643. doi: 10.1074/jbc.M400460200. [DOI] [PubMed] [Google Scholar]
- 292.Milani M, Mastrangelo E, Bollati M, Selisko B, Decroly E, Bouvet M, Canard B, Bolognesi M. Flaviviral methyltransferase/RNA interaction: structural basis for enzyme inhibition. Antiviral Res. 2009;83:28–34. doi: 10.1016/j.antiviral.2009.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Selisko B, Peyrane FF, Canard B, Alvarez K, Decroly E. Biochemical characterization of the (nucleoside-2′O)-methyltransferase activity of dengue virus protein NS5 using purified capped RNA oligonucleotides (7Me)GpppAC(n) and GpppAC(n) J Gen Virol. 2010;91:112–121. doi: 10.1099/vir.0.015511-0. [DOI] [PubMed] [Google Scholar]
- 294.Dong H, Zhang B, Shi PY. Flavivirus methyltransferase: a novel antiviral target. Antiviral Res. 2008;80:1–10. doi: 10.1016/j.antiviral.2008.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Issur M, Geiss BJ, Bougie I, Picard-Jean F, Despins S, Mayette J, Hobdey SE, Bisaillon M. The flavivirus NS5 protein is a true RNA guanylyltransferase that catalyzes a two-step reaction to form the RNA cap structure. RNA. 2009;15:2340–2350. doi: 10.1261/rna.1609709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Barral K, Sallamand C, Petzold C, Coutard B, Collet A, Thillier Y, Zimmermann J, Vasseur JJ, Canard B, Rohayem J, Debart F, Decroly E. Development of specific dengue virus 2′-O- and N7-methyltransferase assays for antiviral drug screening. Antiviral Res. 2013;99:292–300. doi: 10.1016/j.antiviral.2013.06.001. [DOI] [PubMed] [Google Scholar]
- 297.Lim SV, Rahman MB, Tejo BA. Structure-based and ligand-based virtual screening of novel methyltransferase inhibitors of the dengue virus. BMC Bioinformatics. 2011;12(Suppl 13):24. doi: 10.1186/1471-2105-12-S13-S24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Lim SP, Sonntag LS, Noble C, Nilar SH, Ng RH, Zou G, Monaghan P, Chung KY, Dong H, Liu B, Bodenreider C, Lee G, Ding M, Chan WL, Wang G, Jian YL, Chao AT, Lescar J, Yin Z, Vedananda TR, Keller TH, Shi PY. Small molecule inhibitors that selectively block dengue virus methyltransferase. J Biol Chem. 2011;286:6233–6240. doi: 10.1074/jbc.M110.179184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Chen H, Liu L, Jones SA, Banavali N, Kass J, Li Z, Zhang J, Kramer LD, Ghosh AK, Li H. Selective inhibition of the West Nile virus methyltransferase by nucleoside analogs. Antiviral Res. 2013;97:232–239. doi: 10.1016/j.antiviral.2012.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Stahla-Beek HJ, April DG, Saeedi BJ, Hannah AM, Keenan SM, Geiss BJ. Identification of a novel antiviral inhibitor of the flavivirus guanylyltransferase enzyme. J Virol. 2012;86:8730–8739. doi: 10.1128/JVI.00384-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Luzhkov VB, Selisko B, Nordqvist A, Peyrane F, Decroly E, Alvarez K, Karlen A, Canard B, Qvist J. Virtual screening and bioassay study of novel inhibitors for dengue virus mRNA cap (nucleoside-2′O)-methyltransferase. Bioorg Med Chem. 2007;15:7795–7802. doi: 10.1016/j.bmc.2007.08.049. [DOI] [PubMed] [Google Scholar]
- 302.Podvinec M, Lim SP, Schmidt T, Scarsi M, Wen D, Sonntag LS, Sanschagrin P, Shenkin PS, Schwede T. Novel inhibitors of dengue virus methyltransferase: discovery by in vitro-driven virtual screening on a desktop computer grid. J Med Chem. 2010;53:1483–1495. doi: 10.1021/jm900776m. [DOI] [PubMed] [Google Scholar]
- 303.Vernekar SK, Qiu L, Zhang J, Kankanala J, Li H, Geraghty RJ, Wang Z. 5′-Silylated 3′-1,2,3-triazolyl thymidine analogues as inhibitors of West Nile virus and dengue virus. J Med Chem. 2015;58:4016–4028. doi: 10.1021/acs.jmedchem.5b00327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Castel G, Chteoui M, Heyd B, Tordo N. Phage display of combinatorial peptide libraries: application to antiviral research. Molecules. 2011;16:3499–3518. doi: 10.3390/molecules16053499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Sillerud LO, Larson RS. Design and structure of peptide and peptidomimetic antagonists of protein-protein interaction. Curr Protein Pept Sci. 2005;6:151–169. doi: 10.2174/1389203053545462. [DOI] [PubMed] [Google Scholar]
- 306.Root MJ, Steger HK. HIV-1 gp41 as a target for viral entry inhibition. Curr Pharm Des. 2004;10:1805–1825. doi: 10.2174/1381612043384448. [DOI] [PubMed] [Google Scholar]
- 307.Bai F, Town T, Pradhan D, Cox J, Ashish Ledizet M, Anderson JF, Flavell RA, Krueger JK, Koski RA, Fikrig E. Antiviral peptides targeting the West Nile virus envelope protein. J Virol. 2007;81:2047–2055. doi: 10.1128/JVI.01840-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Hrobowski YM, Garry RF, Michael SF. Peptide inhibitors of dengue virus and West Nile virus infectivity. Virol J. 2005;2:49. doi: 10.1186/1743-422X-2-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Lim HA, Ang MJ, Joy J, Poulsen A, Wu W, Ching SC, Hill J, Chia CS. Novel agmatine dipeptide inhibitors against the West Nile virus NS2B/NS3 protease: a P3 and N-cap optimization study. Eur J Med Chem. 2013;62:199–205. doi: 10.1016/j.ejmech.2012.12.043. [DOI] [PubMed] [Google Scholar]
- 310.Schuller A, Yin Z, Brian Chia CS, Doan DN, Kim HK, Shang L, Loh TP, Hill J, Vasudevan SG. Tripeptide inhibitors of dengue and West Nile virus NS2B-NS3 protease. Antiviral Res. 2011;92:96–101. doi: 10.1016/j.antiviral.2011.07.002. [DOI] [PubMed] [Google Scholar]
- 311.Knox JE, Ma NL, Yin Z, Patel SJ, Wang WL, Chan WL, Ranga Rao KR, Wang G, Ngew X, Patel V, Beer D, Lim SP, Vasudevan SG, Keller TH. Peptide inhibitors of West Nile NS3 protease: SAR study of tetrapeptide aldehyde inhibitors. J Med Chem. 2006;49:6585–6590. doi: 10.1021/jm0607606. [DOI] [PubMed] [Google Scholar]
- 312.Rothan HA, Han HC, Ramasamy TS, Othman S, Rahman NA, Yusof R. Inhibition of dengue NS2B-NS3 protease and viral replication in Vero cells by recombinant retrocyclin-1. BMC Infect Dis. 2012;12:314. doi: 10.1186/1471-2334-12-314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Fire A, Xu S, Montgomery MK, Kostas SA, Driver SE, Mello CC. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature. 1998;391:806–811. doi: 10.1038/35888. [DOI] [PubMed] [Google Scholar]
- 314.Montgomery MK, Xu S, Fire A. RNA as a target of double-stranded RNA-mediated genetic interference in Caenorhabditis elegans. Proc Natl Acad Sci U S A. 1998;95:15502–15507. doi: 10.1073/pnas.95.26.15502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Meister G, Landthaler M, Dorsett Y, Tuschl T. Sequence-specific inhibition of microRNA- and siRNA-induced RNA silencing. RNA. 2004;10:544–550. doi: 10.1261/rna.5235104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Meister G, Tuschl T. Mechanisms of gene silencing by double-stranded RNA. Nature. 2004;431:343–349. doi: 10.1038/nature02873. [DOI] [PubMed] [Google Scholar]
- 317.Geiss BJ, Pierson TC, Diamond MS. Actively replicating West Nile virus is resistant to cytoplasmic delivery of siRNA. Virol J. 2005;2:53. doi: 10.1186/1743-422X-2-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Kumar P, Lee SK, Shankar P, Manjunath N. A single siRNA suppresses fatal encephalitis induced by two different flaviviruses. PLoS Med. 2006;3:e96. doi: 10.1371/journal.pmed.0030096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.McCown M, Diamond MS, Pekosz A. The utility of siRNA transcripts produced by RNA polymerase i in down regulating viral gene expression and replication of negative- and positive-strand RNA viruses. Virology. 2003;313:514–524. doi: 10.1016/S0042-6822(03)00341-6. [DOI] [PubMed] [Google Scholar]
- 320.Ong SP, Choo BG, Chu JJ, Ng ML. Expression of vector-based small interfering RNA against West Nile virus effectively inhibits virus replication. Antiviral Res. 2006;72:216–223. doi: 10.1016/j.antiviral.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 321.Ong SP, Chu JJ, Ng ML. Inhibition of West Nile virus replication in cells stably transfected with vector-based shRNA expression system. Virus Res. 2008;135:292–297. doi: 10.1016/j.virusres.2008.04.014. [DOI] [PubMed] [Google Scholar]
- 322.Kumar P, Wu H, McBride JL, Jung KE, Kim MH, Davidson BL, Lee SK, Shankar P, Manjunath N. Transvascular delivery of small interfering RNA to the central nervous system. Nature. 2007;448:39–43. doi: 10.1038/nature05901. [DOI] [PubMed] [Google Scholar]
- 323.Gao Y, Wang ZY, Zhang J, Zhang Y, Huo H, Wang T, Jiang T, Wang S. RVG-peptide-linked trimethylated chitosan for delivery of siRNA to the brain. Biomacromolecules. 2014;15:1010–1018. doi: 10.1021/bm401906p. [DOI] [PubMed] [Google Scholar]
- 324.Paul AM, Shi Y, Acharya D, Douglas JR, Cooley A, Anderson JF, Huang F, Bai F. Delivery of antiviral small interfering RNA with gold nanoparticles inhibits dengue virus infection in vitro. J Gen Virol. 2014;95:1712–1722. doi: 10.1099/vir.0.066084-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Hasan W, Chu K, Gullapalli A, Dunn SS, Enlow EM, Luft JC, Tian S, Napier ME, Pohlhaus PD, Rolland JP, DeSimone JM. Delivery of multiple siRNAs using lipid-coated PLGA nanoparticles for treatment of prostate cancer. Nano Lett. 2012;12:287–292. doi: 10.1021/nl2035354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Yang Y, Wu C, Wu J, Nerurkar VR, Yanagihara R, Lu Y. Inhibition of West Nile virus replication by retrovirus-delivered small interfering RNA in human neuroblastoma cells. J Med Virol. 2008;80:930–936. doi: 10.1002/jmv.21164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Samuel MA, Diamond MS. Alpha/beta interferon protects against lethal West Nile virus infection by restricting cellular tropism and enhancing neuronal survival. J Virol. 2005;79:13350–13361. doi: 10.1128/JVI.79.21.13350-13361.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Lazear HM, Pinto AK, Vogt MR, Gale M, Jr, Diamond MS. Beta interferon controls West Nile virus infection and pathogenesis in mice. J Virol. 2011;85:7186–7194. doi: 10.1128/JVI.00396-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Ramos HJ, Lanteri MC, Blahnik G, Negash A, Suthar MS, Brassil MM, Sodhi K, Treuting PM, Busch MP, Norris PJ, Gale M., Jr IL-1beta signaling promotes CNS-intrinsic immune control of West Nile virus infection. PLoS Pathog. 2012;8:e1003039. doi: 10.1371/journal.ppat.1003039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Arjona A, Foellmer HG, Town T, Leng L, McDonald C, Wang T, Wong SJ, Montgomery RR, Fikrig E, Bucala R. Abrogation of macrophage migration inhibitory factor decreases West Nile virus lethality by limiting viral neuroinvasion. J Clin Invest. 2007;117:3059–3066. doi: 10.1172/JCI32218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Klein RS, Lin E, Zhang B, Luster AD, Tollett J, Samuel MA, Engle M, Diamond MS. Neuronal CXCL10 directs CD8+ T-cell recruitment and control of West Nile virus encephalitis. J Virol. 2005;79:11457–11466. doi: 10.1128/JVI.79.17.11457-11466.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Crawford A, Angelosanto JM, Nadwodny KL, Blackburn SD, Wherry EJ. A role for the chemokine RANTES in regulating CD8 T cell responses during chronic viral infection. PLoS Pathog. 2011;7:e1002098. doi: 10.1371/journal.ppat.1002098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Glass WG, Lim JK, Cholera R, Pletnev AG, Gao JL, Murphy PM. Chemokine receptor CCR5 promotes leukocyte trafficking to the brain and survival in West Nile virus infection. J Exp Med. 2005;202:1087–1098. doi: 10.1084/jem.20042530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Wang P, Bai F, Zenewicz LA, Dai J, Gate D, Cheng G, Yang L, Qian F, Yuan X, Montgomery RR, Flavell RA, Town T, Fikrig E. IL-22 signaling contributes to West Nile encephalitis pathogenesis. PLoS One. 2012;7:e44153. doi: 10.1371/journal.pone.0044153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.Shepherd J, Brodin HF, Cave CB, Waugh NR, Price A, Gabbay J. Clinical- and cost-effectiveness of pegylated interferon alfa in the treatment of chronic hepatitis C: a systematic review and economic evaluation. Int J Technol Assess Health Care. 2005;21:47–54. doi: 10.1017/S0266462305050063. [DOI] [PubMed] [Google Scholar]
- 336.Laurent-Rolle M, Boer EF, Lubick KJ, Wolfinbarger JB, Carmody AB, Rockx B, Liu W, Ashour J, Shupert WL, Holbrook MR, Barrett AD, Mason PW, Bloom ME, Garcia-Sastre A, Khromykh AA, Best SM. The NS5 protein of the virulent West Nile virus NY99 strain is a potent antagonist of type I interferon-mediated JAK-STAT signaling. J Virol. 2010;84:3503–3515. doi: 10.1128/JVI.01161-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Lubick KJ, Robertson SJ, McNally KL, Freedman BA, Rasmussen AL, Taylor RT, Walts AD, Tsuruda S, Sakai M, Ishizuka M, Boer EF, Foster EC, Chiramel AI, Addison CB, Green R, Kastner DL, Katze MG, Holland SM, Forlino A, Freeman AF, Boehm M, Yoshii K, Best SM. Flavivirus antagonism of type I interferon signaling reveals prolidase as a regulator of IFNAR1 surface expression. Cell Host Microbe. 2015;18:61–74. doi: 10.1016/j.chom.2015.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.Lazear HM, Daniels BP, Pinto AK, Huang AC, Vick SC, Doyle SE, Gale M, Jr, Klein RS, Diamond MS. Interferon-lambda restricts West Nile virus neuroinvasion by tightening the blood-brain barrier. Sci Transl Med. 2015;7:284ra259. doi: 10.1126/scitranslmed.aaa4304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Qing M, Yang F, Zhang B, Zou G, Robida JM, Yuan Z, Tang H, Shi PY. Cyclosporine inhibits flavivirus replication through blocking the interaction between host cyclophilins and viral NS5 protein. Antimicrob Agents Chemother. 2009;53:3226–3235. doi: 10.1128/AAC.00189-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Kanlaya R, Pattanakitsakul SN, Sinchaikul S, Chen ST, Thongboonkerd V. The ubiquitin-proteasome pathway is important for dengue virus infection in primary human endothelial cells. J Proteome Res. 2010;9:4960–4971. doi: 10.1021/pr100219y. [DOI] [PubMed] [Google Scholar]
- 341.Umareddy I, Pluquet O, Wang QY, Vasudevan SG, Chevet E, Gu F. Dengue virus serotype infection specifies the activation of the unfolded protein response. Virol J. 2007;4:91. doi: 10.1186/1743-422X-4-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.Diamond MS, Zachariah M, Harris E. Mycophenolic acid inhibits dengue virus infection by preventing replication of viral RNA. Virology. 2002;304:211–221. doi: 10.1006/viro.2002.1685. [DOI] [PubMed] [Google Scholar]
- 343.Leyssen P, Balzarini J, De Clercq E, Neyts J. The predominant mechanism by which ribavirin exerts its antiviral activity in vitro against flaviviruses and paramyxoviruses is mediated by inhibition of IMP dehydrogenase. J Virol. 2005;79:1943–1947. doi: 10.1128/JVI.79.3.1943-1947.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.Kariwa H, Murata R, Totani M, Yoshii K, Takashima I. Increased pathogenicity of West Nile virus (WNV) by glycosylation of envelope protein and seroprevalence of WNV in wild birds in Far Eastern Russia. Int J Environ Res Public Health. 2013;10:7144–7164. doi: 10.3390/ijerph10127144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345.Murata R, Eshita Y, Maeda A, Maeda J, Akita S, Tanaka T, Yoshii K, Kariwa H, Umemura T, Takashima I. Glycosylation of the West Nile virus envelope protein increases in vivo and in vitro viral multiplication in birds. Am J Trop Med Hyg. 2010;82:696–704. doi: 10.4269/ajtmh.2010.09-0262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346.Whiteman MC, Wicker JA, Kinney RM, Huang CY, Solomon T, Barrett AD. Multiple amino acid changes at the first glycosylation motif in NS1 protein of West Nile virus are necessary for complete attenuation for mouse neuroinvasiveness. Vaccine. 2011;29:9702–9710. doi: 10.1016/j.vaccine.2011.09.036. [DOI] [PubMed] [Google Scholar]
- 347.Poh MK, Shui G, Xie X, Shi PY, Wenk MR, Gu F. U18666A, an intra-cellular cholesterol transport inhibitor, inhibits dengue virus entry and replication. Antiviral Res. 2012;93:191–198. doi: 10.1016/j.antiviral.2011.11.014. [DOI] [PubMed] [Google Scholar]
- 348.Martin-Acebes MA, Blazquez AB, Jimenez de Oya N, Escribano-Romero E, Saiz JC. West Nile virus replication requires fatty acid synthesis but is independent on phosphatidylinositol-4-phosphate lipids. PLoS One. 2011;6:e24970. doi: 10.1371/journal.pone.0024970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349.Medigeshi GR, Hirsch AJ, Streblow DN, Nikolich-Zugich J, Nelson JA. West Nile virus entry requires cholesterol-rich membrane microdomains and is independent of alphavbeta3 integrin. J Virol. 2008;82:5212–5219. doi: 10.1128/JVI.00008-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350.Sessions OM, Barrows NJ, Souza-Neto JA, Robinson TJ, Hershey CL, Rodgers MA, Ramirez JL, Dimopoulos G, Yang PL, Pearson JL, Garcia-Blanco MA. Discovery of insect and human dengue virus host factors. Nature. 2009;458:1047–1050. doi: 10.1038/nature07967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351.Fischl W, Bartenschlager R. Exploitation of cellular pathways by dengue virus. Curr Opin Microbiol. 2011;14:470–475. doi: 10.1016/j.mib.2011.07.012. [DOI] [PubMed] [Google Scholar]
- 352.Brinton MA. Host factors involved in West Nile virus replication. Ann N Y Acad Sci. 2001;951:207–219. doi: 10.1111/j.1749-6632.2001.tb02698.x. [DOI] [PubMed] [Google Scholar]
- 353.Qing M, Zou G, Wang QY, Xu HY, Dong H, Yuan Z, Shi PY. Characterization of dengue virus resistance to brequinar in cell culture. Antimicrob Agents Chemother. 2010;54:3686–3695. doi: 10.1128/AAC.00561-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 354.Wang QY, Bushell S, Qing M, Xu HY, Bonavia A, Nunes S, Zhou J, Poh MK, Florez de Sessions P, Niyomrattanakit P, Dong H, Hoffmaster K, Goh A, Nilar S, Schul W, Jones S, Kramer L, Compton T, Shi PY. Inhibition of dengue virus through suppression of host pyrimidine biosynthesis. J Virol. 2011;85:6548–6556. doi: 10.1128/JVI.02510-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355.Chang J, Wang L, Ma D, Qu X, Guo H, Xu X, Mason PM, Bourne N, Moriarty R, Gu B, Guo JT, Block TM. Novel imino sugar derivatives demonstrate potent antiviral activity against flaviviruses. Antimicrob Agents Chemother. 2009;53:1501–1508. doi: 10.1128/AAC.01457-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 356.Suen WW, Prow NA, Hall RA, Bielefeldt-Ohmann H. Mechanism of West Nile virus neuroinvasion: a critical appraisal. Viruses. 2014;6:2796–2825. doi: 10.3390/v6072796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357.Hunsperger EA, Roehrig JT. Nocodazole delays viral entry into the brain following footpad inoculation with West Nile virus in mice. J Neurovirol. 2009;15:211–218. doi: 10.1080/13550280902913255. [DOI] [PubMed] [Google Scholar]
- 358.Davis WG, Basu M, Elrod EJ, Germann MW, Brinton MA. Identification of cis-acting nucleotides and a structural feature in West Nile virus 3′-terminus RNA that facilitate viral minus strand RNA synthesis. J Virol. 2013;87:7622–7636. doi: 10.1128/JVI.00212-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 359.Li W, Li Y, Kedersha N, Anderson P, Emara M, Swiderek KM, Moreno GT, Brinton MA. Cell proteins TIA-1 and TIAR interact with the 3′ stem-loop of the West Nile virus complementary minus-strand RNA and facilitate virus replication. J Virol. 2002;76:11989–12000. doi: 10.1128/JVI.76.23.11989-12000.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360.Blackwell JL, Brinton MA. BHK cell proteins that bind to the 3′ stem-loop structure of the West Nile virus genome RNA. J Virol. 1995;69:5650–5658. doi: 10.1128/jvi.69.9.5650-5658.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 361.Davis WG, Blackwell JL, Shi PY, Brinton MA. Interaction between the cellular protein eEF1A and the 3′-terminal stem-loop of West Nile virus genomic RNA facilitates viral minus-strand RNA synthesis. J Virol. 2007;81:10172–10187. doi: 10.1128/JVI.00531-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362.Shi PY, Li W, Brinton MA. Cell proteins bind specifically to West Nile virus minus-strand 3′ stem-loop RNA. J Virol. 1996;70:6278–6287. doi: 10.1128/jvi.70.9.6278-6287.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 363.Zohrabian A, Hayes EB, Petersen LR. Cost-effectiveness of West Nile virus vaccination. Emerg Infect Dis. 2006;12:375–380. doi: 10.3201/eid1203.050782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 364.Monath TP. Prospects for development of a vaccine against the West Nile virus. Ann N Y Acad Sci. 2001;951:1–12. doi: 10.1111/j.1749-6632.2001.tb02680.x. [DOI] [PubMed] [Google Scholar]
- 365.Welte Thomas, Xie Guorui, Wicker Jason A., Whiteman Melissa C., Li Li, Rachamallu Aparna, Barrett Alan, Wang Tian. Immune responses to an attenuated West Nile virus NS4B-P38G mutant strain. Vaccine. 2011;29(29-30):4853–4861. doi: 10.1016/j.vaccine.2011.04.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 366.Biedenbender Rex, Bevilacqua Joan, Gregg Anne M., Watson Mike, Dayan Gustavo. Phase II, Randomized, Double-Blind, Placebo-Controlled, Multicenter Study to Investigate the Immunogenicity and Safety of a West Nile Virus Vaccine in Healthy Adults. The Journal of Infectious Diseases. 2011;203(1):75–84. doi: 10.1093/infdis/jiq003. [DOI] [PMC free article] [PubMed] [Google Scholar]


