Abstract
Background
Despite medical therapies and surgical interventions for Parkinson's disease (PD), patients develop progressive disability. Physiotherapy aims to maximise functional ability and minimise secondary complications through movement rehabilitation within a context of education and support for the whole person. The overall aim is to optimise independence, safety, and well‐being, thereby enhancing quality of life.
Objectives
To assess the effectiveness of physiotherapy intervention compared with no intervention in patients with PD.
Search methods
We identified relevant trials by conducting electronic searches of numerous literature databases (e.g. MEDLINE, EMBASE) and trial registers, and by handsearching major journals, abstract books, conference proceedings, and reference lists of retrieved publications. The literature search included trials published up to the end of January 2012.
Selection criteria
Randomised controlled trials of physiotherapy intervention versus no physiotherapy intervention in patients with PD.
Data collection and analysis
Two review authors independently extracted data from each article. We used standard meta‐analysis methods to assess the effectiveness of physiotherapy intervention compared with no physiotherapy intervention. Trials were classified into the following intervention comparisons: general physiotherapy, exercise, treadmill training, cueing, dance, and martial arts. We used tests for heterogeneity to assess for differences in treatment effect across these different physiotherapy interventions.
Main results
We identified 39 trials with 1827 participants. We considered the trials to be at a mixed risk of bias as the result of unreported allocation concealment and probable detection bias. Compared with no intervention, physiotherapy significantly improved the gait outcomes of speed (mean difference 0.04 m/s, 95% confidence interval (CI) 0.02 to 0.06, P = 0.0002); two‐ or six‐minute walk test (13.37 m, 95% CI 0.55 to 26.20, P = 0.04) and Freezing of Gait questionnaire (‐1.41, 95% CI ‐2.63 to ‐0.19, P = 0.02); functional mobility and balance outcomes of Timed Up & Go test (‐0.63 s, 95% CI ‐1.05 to ‐0.21, P = 0.003), Functional Reach Test (2.16 cm, 95% CI 0.89 to 3.43, P = 0.0008), and Berg Balance Scale (3.71 points, 95% CI 2.30 to 5.11, P < 0.00001); and clinician‐rated disability using the Unified Parkinson’s Disease Rating Scale (UPDRS) (total ‐6.15 points, 95% CI‐8.57 to ‐3.73, P < 0.00001; activities of daily living: ‐1.36, 95% CI ‐2.41 to ‐0.30, P = 0.01; and motor: ‐5.01, 95% CI ‐6.30 to ‐3.72, P < 0.00001). No difference between arms was noted in falls (Falls Efficacy Scale: ‐1.91 points, 95% CI ‐4.76 to 0.94, P = 0.19) or patient‐rated quality of life (PDQ‐39 Summary Index: ‐0.38 points, 95% CI ‐2.58 to 1.81, P = 0.73). One study reported that adverse events were rare; no other studies reported data on this outcome. Indirect comparisons of the different physiotherapy interventions revealed no evidence that the treatment effect differed across physiotherapy interventions for any of the outcomes assessed.
Authors' conclusions
Benefit for physiotherapy was found in most outcomes over the short term (i.e. < 3 months) but was significant only for speed, two‐ or six‐minute walk test, Freezing of Gait questionnaire, Timed Up & Go, Functional Reach Test, Berg Balance Scale, and clinician‐rated UPDRS. Most of the observed differences between treatments were small. However, for some outcomes (e.g. speed, Berg Balance Scale, UPDRS), the differences observed were at, or approaching, what are considered minimal clinically important changes. These benefits should be interpreted with caution because the quality of most of the included trials was not high. Variation in measurements of outcome between studies meant that our analyses include a small proportion of the participants recruited.
This review illustrates that a wide range of approaches are employed by physiotherapists to treat patients with PD. However, no evidence of differences in treatment effect was noted between the different types of physiotherapy interventions being used, although this was based on indirect comparisons. A consensus menu of 'best practice' physiotherapy is needed, as are large, well‐designed randomised controlled trials undertaken to demonstrate the longer‐term efficacy and cost‐effectiveness of 'best practice' physiotherapy in PD.
Keywords: Humans, Physical Therapy Modalities, Activities of Daily Living, Gait, Parkinson Disease, Parkinson Disease/rehabilitation, Quality of Life, Randomized Controlled Trials as Topic, Walking, Watchful Waiting
Plain language summary
Physiotherapy for treatment of Parkinson's disease
In spite of various medical and surgical treatments for Parkinson's disease (PD), patients gradually develop significant physical problems. Physiotherapists aim to enable people with PD to maintain their maximum level of mobility, activity, and independence by monitoring their condition and targeting appropriate treatment. A range of approaches to movement rehabilitation are used, which aim to enhance quality of life by maximising physical ability and minimising problems related to Parkinson's over the whole course of the disease.
Only randomised controlled trials were included in this review. In these studies,a group of participants were given physiotherapy intervention and were compared with another group of participants, who did not receive physiotherapy. Participants were assigned to a group in random fashion so a fair test was established. Thirty‐nine randomised trials involving 1827 participants were identified as suitable for this review. The quality of the trials was not high because in many, methods were not reported adequately and blinding was not feasible. These trials assessed various physiotherapy interventions, so the trials were grouped according to the type of intervention being used (i.e. general physiotherapy, exercise, treadmill training, cueing, dance, or martial arts).
Improvement in all walking outcomes (except the 10‐ or 20‐metre walk test) was noted with physiotherapy intervention. However, these improvements were significant only for walking speed, walking endurance, and freezing of gait. Mobility and balance also improved with a physiotherapy intervention, with significant improvements reported in one test of mobility (the Timed Up & Go test, which times how long it takes a person to get up from a chair, walk a certain distance, then walk back to the chair and sit down) and in two tests of balance (one assessing how far a person can reach before he or she loses balance (Functional Reach Test) and another assessing multiple aspects of balance (Berg Balance Scale)). Clinician‐rated disability, using the Unified Parkinson’s Disease Rating Scale (UPDRS), was also improved with physiotherapy intervention. No difference was observed between the two groups in falls or patient‐rated quality of life. One study reported that adverse events were rare; no other studies reported data on this outcome. When the different physiotherapy interventions were compared, no evidence suggested that treatment effect differed across the physiotherapy interventions for any of the outcomes assessed.
This review provides evidence of the short‐term benefit of physiotherapy for the treatment of PD. Although most observed differences were small, improvements in walking speed, balance with the Berg Balance Scale, and clinician‐rated disability using the UPDRS were of a size that patients may consider them to be important. These benefits should be interpreted with caution because of the quality of the included trials, and the lack of common assessment of treatment effects. This affected the quantity of data that we could use for analysis.
Background
Parkinson’s disease (PD) is a complex neurodegenerative disorder (Rubenis 2007) with wide reaching implications for patients and their families. Although disability can occur at all stages of the disease (Deane 2001a), PD is progressive in nature, and so patients face increased difficulties with activities of daily living (ADL) (Kwakkel 2007) and various aspects of mobility such as gait, transfers, balance, and posture (Keus 2007b). Ultimately, this leads to decreased independence, inactivity, and social isolation (Keus 2007b), resulting in reduced quality of life (Schrag 2000).
The management of PD has traditionally centred on drug therapy, with levodopa viewed as the 'gold standard' treatment (Rascol 2002). However, even with optimal medical management, patients with PD experience deterioration in body function, daily activities, and participation (Nijkrake 2007). For this reason, support has been increasing for the inclusion of rehabilitation therapies as an adjuvant to pharmacological and neurosurgical treatment (Gage 2004; Nijkrake 2007), and a call for the move towards multidisciplinary management of this multidimensional condition (Robertson 2003; Rubenis 2007).
The physiotherapist is a member within this multidisciplinary team (Robertson 2008; Rubenis 2007), whose purpose is to maximise functional ability and minimise secondary complications through movement rehabilitation within a context of education and support for the whole person (Plant 2000; Deane 2001a). Physiotherapy for PD focuses on transfers, posture, upper limb function, balance (and falls), gait, and physical capacity and (in)activity by using cueing strategies, cognitive movement strategies, and exercise to optimise the patient’s independence, safety, and well‐being, thereby enhancing quality of life (Keus 2004; Keus 2007a).
Referral rates to physiotherapy for people with PD have historically been low (Mutch 1986; Yarrow 1999). However, in recent years, the number of referrals has increased, with a survey by Parkinson’s UK in 2008 reporting that 54% of the 13,000 members surveyed had seen a physiotherapist compared with 27% in a survey undertaken in 1998 (PDS 2008; Yarrow 1999). This rise in referrals may be attributed to two factors. First, guidelines published by the National Collaborating Centre for Chronic Conditions (Nat Collab Centre for Chronic Conditions 2006) recommended that physiotherapy be made available throughout all stages of the disease, raising the profile of the profession. This has been further supported by the publication of Dutch physiotherapy guidelines (Keus 2004), which provide specific information for physiotherapists involved in the management of PD. Second, a substantial increase has been noted in the number of trials completed over the past decade (particularly in the last five years), offering supportive evidence for the inclusion of physiotherapy in the management of PD (Keus 2009).
This Cochrane review assessing the effectiveness of physiotherapy intervention versus no physiotherapy intervention in patients with PD was first published in 2001, and included only 11 randomised controlled trials with a total of 280 participants (Deane 2001a). Most of the trials in the review reported a positive effect in favour of physiotherapy, but few outcome measures were statistically significant. This, combined with the presence of methodological flaws, small sample sizes, and the possibility of publication bias, led Deane et al. to conclude that evidence was insufficient to support or refute the efficacy of physiotherapy for PD (Deane 2001a). This review updates the previous Cochrane review. We appraised and synthesised relevant randomised controlled trials, and we conducted a meta‐analysis of outcomes where possible.
Objectives
To compare the effectiveness of physiotherapy intervention versus no physiotherapy intervention in participants with PD.
To indirectly compare the different physiotherapy interventions used within the various trials.
Methods
Criteria for considering studies for this review
Types of studies
For inclusion in the review, we considered all randomised controlled trials (including the first phase of cross‐over trials) comparing a physiotherapy intervention with no physiotherapy intervention (including placebo control). We included trials in which the no intervention arm used an active or credible placebo, as long as no physiotherapy was delivered to this group. We included only trials that implemented random methods of treatment allocation.
Types of participants
Participants with a diagnosis of PD (as defined by the authors of the studies):
Any duration of PD.
All ages.
Any drug therapy.
Any duration of physiotherapy treatment.
Types of interventions
Physiotherapy interventions aim to maximise functional ability and minimise secondary complications through movement rehabilitation within a context of education and support for the whole person. Physiotherapy encompasses a wide range of techniques, so we were inclusive in our definition of physiotherapy interventions (including those not delivered by a physiotherapist) with trials of general physiotherapy, exercise, treadmill training, cueing, dance, and martial arts included.
Types of outcome measures
Gait outcomes such as:
Two‐ or six‐minute walk test (m) - measures the number of metres a person can walk in two or six minutes, thereby providing a measurement of walking endurance (Kersten 2004).
Walking speed
10‐ or 20‐metre walk test (s) - measures the time in seconds that a person takes to walk 10 or 20 metres, thereby providing a measurement of gait speed (Kersten 2004).
Speed (m/s) - measures the rate of change of position, recorded in metres per second (Trew 2005).
Cadence (steps/min) - measures the number of steps taken in a given period, which is then converted into the number of steps taken per minute (Trew 2005).
Stride length (m) - measures the average distance (in metres) between two successive placements of the same foot (Whittle 1996).
Step length (m) - measures the average distance (in metres) between successive foot‐to‐floor contacts with opposite feet (Trew 2005).
Freezing of Gait Questionnaire - validated questionnaire for the assessment of freezing of gait. The questionnaire consists of six items, and scores range from 0 to 24, with higher scores corresponding to more severe freezing of gait (Giladi 2000).
Functional mobility and balance outcomes such as:
Timed Up & Go (s) - measures time taken in seconds for a person to get up from a chair, walk a certain distance (usually three metres), turn around, and walk back to the chair and sit down (Podsiadlo 1991).
Functional Reach Test (cm) - “the maximal distance one can reach forward beyond arm’s length, while maintaining a fixed base of support in the standing position” (Duncan 1990).
Berg Balance Scale - validated questionnaire designed to measure functional standing balance of the older adult. The measure consists of 14 items, and scores range from 0 to 56, with 0 to 20 = high fall risk; 21 to 40 = medium fall risk; and 41 to 56 = low fall risk (Berg 1992; Qutubuddin 2005).
Activity Specific Balance Confidence - 16‐item self‐report questionnaire that asks individuals to rate their confidence that they will maintain their balance in the course of daily activities. Each item is rated from 0% (no confidence) to 100% (complete confidence) (Powell 1995; Talley 2008).
Data on falls such as:
Number of patients falling - e.g. falls diary.
Falls Efficacy Scale - 10‐item patient‐reported questionnaire that measures how confident a person is at carrying out various activities of daily living (ADL). Items are rated from 1 to 10, with higher scores correlating with lower levels of confidence, and a total score of 70 or higher indicating that a person has a fear of falling (Tinetti 1990).
Falls Efficacy Scale International - 16‐item questionnaire that includes the 10 original items of the standard Falls Efficacy Scale, as well as six items regarding higher functioning and social activities. Each item is rated on a scale of 1 to 4, with 1 being ‘not concerned at all’ and 4 ‘very concerned’ (maximum score out of 64) (Yardley 2005).
Clinician‐rated impairment and disability measures such as:
Hoehn & Yahr - scale used to describe how symptoms of Parkinson's disease progress. Scale ranges from 0 to 5, with higher levels indicating greater disability (Hoehn 1967).
Unified Parkinson's Disease Rating Scale (UPDRS) - designed to assess motor impairment and disability in Parkinson’s disease. Higher scores correspond to greater disability (Fahn 1987).
Total - scores range from 0 to 176.
Mental - scores range from 0 to 16.
ADL - scores range from 0 to 52.
Motor - scores range from 0 to 108.
Webster Rating Scale - assessment of severity of disease and clinical impairment against 10 items using a scale of 0=normal to 3=maximum impairment: bradykinesia, rigidity, posture, upper extremity swing, gait, tremor at rest, facial expression, seborrhoea, speech, and self care. Scores range from 0 to 30, with higher scores indicating greater disease severity and disability (Webster 1968).
Columbia University Rating Scale - assessment of motor impairment and activities of daily living against 13 items, using a five‐point scale for each to yield a total score between 0=normal and 65=maximum disability (Yahr 1969).
Patient‐rated quality of life such as:
Parkinson's Disease Questionnaire39 (PDQ‐39) - PD‐specific health‐related quality of life questionnaire containing 39 items divided among eight domains. Scores range from 0 to 100, with higher scores corresponding to poorer quality of life (Jenkinson 1997; Peto 1995).
PDQUALIF - PD‐specific health‐related quality of life questionnaire containing 32 items in seven dimensions and one item of global health‐related quality of life. Total score ranges from 0 to 128, with higher scores indicating poorer quality of life (Welsh 2003).
PDQL - PD‐specific health‐related quality of life questionnaire containing 37 items grouped into four subscales. Item scores range from 1 to 5. The PDQL‐Summary Index ranges from 37 to 185, with higher scores reflecting better quality of life (Deboer 1996).
Short Form‐36 or ‐12 - generic short‐form health survey consisting of 36 or 12 questions. The SF‐36 consists of eight scaled scores assessing vitality, physical functioning, bodily pain, general health perceptions, physical role functioning, emotional role functioning, social role functioning, and mental health. Scores range from 0 to 100, with higher scores corresponding to better quality of life (Ware 1992).
Adverse events (e.g. fractures, pain).
Compliance (e.g. participant adherence, treatment fidelity).
Economic analysis.
Search methods for identification of studies
The review is based on the Movement Disorders Group search strategy and the following more general search strategy:
Physiotherapy OR physical therapy OR exercise OR rehabilitation.
Parkinson OR Parkinson's disease OR Parkinsonism.
#a AND #b.
Further details on this search strategy are available in the Group's module within The Cochrane Library (www.cochrane.org). This includes explanations of the acronyms, sources, and Websites.
We undertook a systematic search of the literature up to the end of January 2012 for publications or abstracts describing relevant trials. This included searching:
General biomedical and science electronic databases (without date limiters) including the Movement Disorders Review Group Specialized Register, The Cochrane Library, MEDLINE (1966‐2012), EMBASE (1974‐2012), CINAHL (1982‐2012), and ISI‐SCI (1981‐2012); rehabilitation databases: AMED (1985‐2012), REHABDATA (1995‐2012), REHADAT, and GEROLIT (1979‐2012); English language databases of foreign language research and third world publications: LILACS (1982‐2012), MedCarib (17th Century‐2012), and IMEMR (1984‐2012).
The Cochrane Controlled Trials Register, the CentreWatch Clinical Trials listing service, the metaRegister of Controlled Trials, ClinicalTrials.gov, RePORT, PEDro, NIDRR, and NRR.
Handsearching of general (Lancet, BMJ, JAMA) and specific journals (Movement Disorders, Neurology, Archives of Physical Medicine and Rehabilitation, Clinical Rehabilitation, Physiotherapy, Physical Therapy) from 2001 to the end of January 2012.
The reference lists of retrieved papers and review articles.
Abstract books and conference proceedings. This included The XIII International Congress on Parkinson's Disease (1999), The International Congress of Parkinson's Disease and Movement Disorders (1990, 92, 94, 96, 98, 2000, 02, 04, 05, 06, 07, 08, 09, 10, 11), World Congress on Parkinson's Disease and Related Disorders (2009, 2012), and The American Academy of Neurology 51st Annual Meeting (1999).
Grey literature databases (including theses): Conference Proceedings Citation Index (1982‐2010), DISSABS (1999‐2012), Conference Papers Index (1982‐2012), Index to Theses (1970‐2012), Electronic Theses Online Service (EThOS) (16th century‐2012), and ProQuest dissertations and theses databases (1861‐2012).
Data collection and analysis
Selection of studies
Abstracts of potentially relevant studies from search results were screened by two of the the four review authors involved in study selection (CT, SP, CH, LS). The full paper was obtained if the abstract did not provide sufficient information for investigators to determine eligibility for inclusion in the review. Disagreement was resolved by referral to an additional review author (RS). We contacted authors of potentially eligible studies for further information if details of the trial were unclear.
Data extraction and management
Four review authors (CT, SP, CM, and CH) independently assessed the identified papers and abstracts for trial details and outcome data, and each eligible study was considered by two of these four authors. This was validated by discussion, with any discrepancies resolved by consensus. We recorded trial details on a standard trial description form and included the following: trial name, trial group, authors, randomised comparison, treatment schedule (including duration, number of sessions, type of intervention), other therapy, eligibility criteria, method of randomisation, allocation concealment, blinding, accrual period, number of participants randomised, number of dropouts, duration of follow‐up, outcomes reported, use of intention‐to‐treat analysis, and publication date(s). Outcome data extracted included data on gait, functional mobility and balance, falls, clinician‐rated disability scale and patient‐rated quality of life, adverse events, compliance/withdrawals, and health economics where available.
We contacted the authors of any eligible unpublished studies to ask whether further details and data for their trial could be provided.
Assessment of risk of bias in included studies
We assessed the full papers for methodological quality by recording eligibility criteria, methods of randomisation and blinding, concealment of allocation, similarity of participants in treatment groups at baseline, cointervention(s) constant, use of active or credible placebo, whether an intention‐to‐treat analysis was performed, and the numbers of participants lost to follow‐up and missing values (see Risk of Bias tables under Characteristics of included studies).
Data synthesis
We combined the results of all trials using standard meta‐analytic methods to estimate an overall effect for physiotherapy intervention versus no physiotherapy intervention.
All outcomes with data available for meta‐analysis were continuous variables, so we calculated the mean difference between treatment arms using mean difference methods (Fleiss 1993). In summary, this involved calculating for each trial the mean change (and standard deviation) from baseline to the postintervention time point for the intervention and no intervention groups. From these, the mean difference and its variance between arms for each trial could be calculated. In some studies, the standard deviation for the mean change was not reported; in these cases, we imputed this standard deviation using the standard deviations for baseline and final scores. To do this, we used the following formula to estimate the variance of the change in score:
vardiff = varpre + varpost – 2r√(varpre varpost)
where vardiff is the variance of the change score; varpre is the variance of the baseline score; varpost is the variance of the final score; and r is the correlation between pretreatment and post‐treatment scores. We assumed a correlation co‐efficient of 0.5, which is a conservative estimate, to reduce the chance of false‐positive results (Higgins 2011).
These values were then combined using weighted mean difference methods to obtain the overall pooled estimate of the mean difference, with 95% confidence interval, for physiotherapy intervention versus no physiotherapy intervention (control).
If any trials with three or more intervention arms were identified, the following assumptions were made for the analysis:
If the trial was comparing two or more physiotherapy interventions within the same classification (see subgroup analysis later) versus no intervention, then we combined the data for these physiotherapy interventions to give one comparison of physiotherapy intervention versus no intervention.
If the trial was comparing two or more physiotherapy interventions in different classifications versus no intervention, then we included that trial in each relevant physiotherapy intervention classification. This meant that some trials were included multiple times in the analysis, and the control arms from these trials were counted more than once in the analysis.
The primary analysis was a comparison of physiotherapy intervention versus no physiotherapy intervention (control) based on change from baseline to the first assessment after the treatment period (which in most cases was immediately post intervention). This was chosen as the primary analysis for this review, as in most trials this was the main data analysis, and few trials reported data at longer‐term assessment points (i.e. after six months). Also, some trials allowed participants in the 'no intervention' arm to receive physiotherapy intervention after this point. So this allowed a clean comparison of physiotherapy intervention versus no physiotherapy intervention.
Subgroup analysis and investigation of heterogeneity
The different trials implemented various types of physiotherapy intervention. Therefore trials were divided according to the type of intervention administered:
General physiotherapy versus control.
Exercise versus control.
Treadmill versus control.
Cueing versus control.
Dance versus control.
Martial arts versus control.
To assess for differences between the different types of interventions involved, we performed indirect comparisons using tests of heterogeneity and I2 values to investigate whether the treatment effect differed across the different interventions (Deeks 2001; Higgins 2003). The I2 value describes the percentage of variability in effect estimates that is due to heterogeneity rather than to sampling error (chance) (Higgins 2003). These tests may suggest the possible superiority of one type of intervention over another, and may provide clinicians and patients with more reliable information upon which to base decisions about therapy. However, as with all subgroup comparisons, these analyses should be interpreted with caution and should be considered hypothesis generating (Assmann 2000; Clarke 2001).
Results
Description of studies
We identified 76 randomised trials of physiotherapy intervention in PD patients. We excluded 31 studies (see Characteristics of excluded studies). The reasons for excluding these trials were as follows: cross‐over study with data not presented for the first treatment period or cross‐over over a short period (e.g. 1 day) (n=6), not randomised or not properly randomised (n=7), no outcome measures relevant to our review (n=4), multidisciplinary therapy rehabilitation trial (n=4), study was confounded (n=2) and treatment given in trial was not usually used by physiotherapists (n=6), excessive number of withdrawals (n=1), and insufficient information (n=1). There were also six ongoing trials for which data were not yet available (see Characteristics of ongoing studies). Therefore, 39 trials were available for inclusion in the review compared with 11 in the 2001 review (Figure 1).
1.
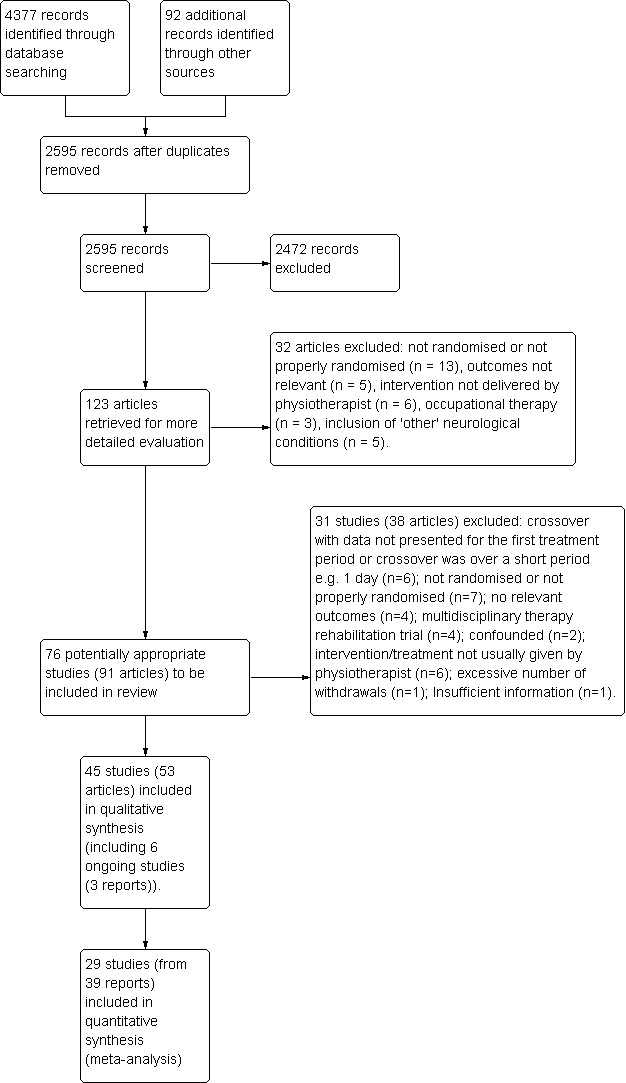
Study PRISMA flow diagram.
The number of participants randomly assigned into the 39 trials ranged from six to 153 participants, with 1827 participants randomly assigned in total (giving an average trial size of nearly 50 participants) (Characteristics of included studies). The assessment period ranged from three weeks to 12 months. The mean age of participants in the trials was 67 years, 64% were male, the mean Hoehn & Yahr stage was 2.4, and participants had had PD for approximately six years (Table 1).
1. Key Characteristics of Studies.
| Study | Number Randomised |
Mean Age (yrs) |
Mean Hoehn & Yahr Stage |
Duration of Disease (yrs) |
% Male | Duration of Treatment |
Design | Location | Type of Treatment |
| Allen 2010 | 48 | 67 | 8 | 54 | 48‐72 hrs/24 weeks | Parallel | Outpatient | Exercise | |
| Almeida 2012 | 42 | 68.4 | 5.3 | 74 | 9 hrs/6 weeks | Parallel | Outpatient | Cueing | |
| Ashburn 2007 | 142 | 72.15 | 3.13 | 8.35 | 61 | 42 hrs/6 weeks | Parallel | Home | Exercise |
| Boehm 2011 | 110 | 69.4 | 60 | 12 weeks | Cross‐over | Exercise | |||
| Cakit 2007 | 54 | 71.8 | 5.58 | 52 | 30‐min sessions/ 8 weeks | Parallel | Outpatient | Treadmill | |
| Canning 2008 | 20 | 61 | 9‐12 hrs/6 weeks | Parallel | Home | Treadmill | |||
| Cerri 1994 | 6 | 15 hrs/3 weeks | Parallel | Outpatient/Home | Exercise | ||||
| Chandler 1999 | 67 | 65.5 | 2.6 | 60 | 5 times/52 weeks | Parallel | Home | Physio | |
| de Bruin 2010a | 22 | 65.6 | 2.2 | 5.5 | 50 | 18 hrs/12 weeks | Parallel | Outpatient | Cueing |
| de Bruin 2010b | 13 | 3 per week/13 weeks | Parallel | Outpatient | Cueing | ||||
| Duncan 2012 | 62 | 70.3 | 2.5 | 56 | 24 hrs/2 weeks | Parallel | Dance | ||
| Ellis 2005 | 68 | 64 | 2.4 | 75 | 18 hrs/6 weeks | Cross‐over | Outpatient | Physio | |
| Fisher 2008 | 30 | 62.9 | 1.9 | 1.1 | 63 | 24 sessions/8 weeks | Parallel | Outpatient | Treadmill/Physio |
| Ganesan 2010 | 20 | 8 hrs/4 weeks | Parallel | Outpatient | Treadmill | ||||
| Goodwin 2009 | 130 | 71.1 | 2.5 | 8.7 | 57 | 10 weeks | Parallel | Outpatient | Exercise |
| Haase 2011 | 26 | 66 | 52 | 4 mins/single session | Parallel | Cueing | |||
| Hackney 2009 | 75 | 66.6 | 2.1 | 7.7 | 74 | 20 hrs/13 weeks | Parallel | Outpatient | Dance/Martial Arts |
| Homann 1998 | 15 | 14 units/5 weeks | Parallel | Outpatient | Physio | ||||
| Keus 2007 | 27 | 67.95 | 2.4 | 6.5 | 81 | 1 or 2 per week/10 weeks | Parallel | Outpatient | Physio |
| Klassen 2007 | 26 | 66.2 | 1.6 | 4.7 | 74 | 15‐30 hrs/12 weeks | Parallel | Outpatient | Exercise |
| Kurtais 2008 | 27 | 64.75 | 2.1 | 5 | 50 | 12 hrs/6 weeks | Parallel | Outpatient | Treadmill |
| Lehman 2005 | 11 | 75.8 | 6.5 | 73 | 5 per week/2 weeks | Parallel | Outpatient | Cueing | |
| Mak 2008 | 60 | 64 | 2.7 | 6 | 4‐6 hrs/4 weeks | Parallel | Outpatient | Cueing/Exercise | |
| Marjama‐Lyons 2002 | 30 | 24 hrs/12 weeks | Parallel | Outpatient | Martial Arts | ||||
| Meek 2010 | 39 | 64.2 | 4.9 | 79 | 12 weeks | Parallel | Outpatient | Exercise | |
| Nieuwboer 2007 | 153 | 67.1 | 2.8 | 7.5 | 58 | 4.5 hrs/3 weeks | Cross‐over | Home | Cueing |
| Protas 2005 | 18 | 72.5 | 2.9 | 7.6 | 100 | 24 hrs/8 weeks | Parallel | Outpatient | Treadmill |
| Purchas 2007 | 20 | 70 | 2.15 | 61 | 12 hrs/12 weeks | Cross‐over | Martial Arts | ||
| Sage 2009a | 53 | 66 | 3.5 | 54 | 18‐24 hrs/10‐12 weeks | Parallel | Outpatient | Exercise | |
| Schenkman 1998 | 51 | 70.9 | 2.7 | 74 | 22.5‐30 hrs/10 weeks | Parallel | Outpatient | Exercise | |
| Schilling 2008 | 18 | 59.2 | 2 | 61 | 2 per week/8 weeks | Parallel | Outpatient | Exercise | |
| Schmitz‐Hubsch 2006 | 56 | 63.5 | 5.8 | 77 | 16 hrs/24 weeks | Parallel | Outpatient | Martial Arts | |
| Shankar 2008 | 28 | 66 | 2.4 | 7.7 | 50 | 18 hrs/12 weeks | Parallel | Outpatient | Cueing |
| Shankar 2009 | 20 | 8 hrs/8 weeks | Parallel | Outpatient | Treadmill | ||||
| Stack 2012 | 47 | 12 hrs/4 weeks | Parallel | Home | Physio | ||||
| Stozek 2003 | 61 | 65.5 | 2.3 | 4.5 | 48 | 56 hrs/4 weeks | Parallel | Outpatient | Exercise |
| Taheri 2011 | 24 | 40 hrs/ 10 weeks | Parallel | Exercise | |||||
| Talakad 2011 | 60 | 8 hrs/4 weeks | Parallel | Outpatient | Physio/treadmill | ||||
| Thaut 1996 | 37 | 71.3 | 2.5 | 7.7 | 70 | 10.5 hrs/3 weeks | Parallel | Home | Exercise/Cueing |
One trial compared walking on a treadmill listening to music versus walking on a treadmill without music versus listening to music alone (Shankar 2009). We excluded the treadmill without music arm of this trial from the analysis as this was a confounded comparison.
Two three‐arm trials compared two exercise interventions with control. One compared exercise versus exercise and education versus control (Klassen 2007), and the other compared exercise versus PD SAFEx versus control (Sage 2009a). The exercise interventions being compared in these studies were considered suitably similar, so we combined the data from the two exercise arms within each trial to obtain one comparison of exercise versus control. Two three‐arm trials compared two cueing interventions with control. The overground and treadmill walking groups, each with equally spaced transverse lines as cues, were combined to obtain a single cueing versus control comparison (Almeida 2012). Finger tapping and arm swing interventions were similarly combined (Haase 2011). One four‐arm trial compared two types of dance (waltz/foxtrot and tango) and martial arts with control. We combined the two dance arms to obtain one comparison of dance versus control, as well as a martial arts versus control comparison (Hackney 2009).
Four other three‐arm trials contributed data to two of the different physiotherapy intervention comparisons. Two of these were trials of cueing versus exercise versus control, which contributed to both the cueing versus control and exercise versus control comparisons (Mak 2008; Thaut 1996). Another trial was of treadmill versus general physiotherapy versus control, which contributed to both the treadmill versus control and general physiotherapy versus control comparisons (Fisher 2008). The last trial, which provided no analysable data, contributed information to two comparisons: general physiotherapy versus control and treadmill versus control (Talakad 2011). The 39 trials therefore contributed data to 44 comparisons within the six different types of physiotherapy interventions - general physiotherapy versus control (n=7), exercise versus control (n=14), treadmill versus control (n=8), cueing versus control (n=9), dance versus control (n=2), and martial arts versus control (n=4).
Below is a summary of the characteristics of included studies. Details of individual studies are given in Characteristics of included studies.
General Physiotherapy versus Control
The seven trials of general physiotherapy versus control involved 244 participants (Chandler 1999; Ellis 2005; Fisher 2008; Homann 1998; Keus 2007b; Stack 2012; Talakad 2011)). Sixty participants split between physiotherapy and treadmill categories are not included in this total as the group splits were not given (Talakad 2011). The mean participant age was 65 years, 69% were male, the mean Hoehn & Yahr stage was 2.4, and mean duration of PD was four years. All trials were of parallel group design, except one, which used a cross‐over design (Ellis 2005). Treatment sessions took place over a period of four weeks to 12 months; duration of sessions was described by only two trials (Ellis 2005; Stack 2012). One trial used Bobath training for gait and posture (Homann 1998). The remaining trials provided multifaceted interventions encompassing movement strategies, exercise, hands‐on techniques, education, and advice, targeting a wide range of areas including gait, balance, transfers, posture, and physical fitness. Thus, general physiotherapy is a holistic intervention and on the whole uses a combination of techniques that do not routinely include complementary and/or alternative medicine such as acupuncture or hypnotherapy.
Exercise versus Control
The 14 trials of exercise versus control involved 769 participants (Allen 2010; Ashburn 2007; Boehm 2011; Cerri 1994; Goodwin 2009; Klassen 2007; Mak 2008; Meek 2010; Sage 2009a; Schenkman 1998; Schilling 2008; Stozek 2003; Taheri 2011; Thaut 1996). The mean participant age was 69 years, 60% were male, the mean Hoehn & Yahr stage was 2.6, and mean duration of PD was six years. Thirteen trials were of parallel group design, and one used a cross‐over design (Boehm 2011). Treatment sessions lasted from 30 minutes to two hours, and took place over a period of three to 24 weeks. Exercise involved a variety of different activities, including strengthening and balance training, walking, falls prevention, neuromuscular facilitation, resistance exercise and aerobic training, and education and relaxation techniques. Although sometimes multifaceted, the primary focus of these interventions was exercise delivery, and treatment was frequently categorised in this way by the trial authors.
Treadmill versus Control
The eight trials of treadmill versus control involved 179 participants (Cakit 2007; Canning 2008; Fisher 2008; Ganesan 2010; Kurtais 2008; Protas 2005; Shankar 2009; Talakad 2011). Sixty participants split between physiotherapy and treadmill categories are not included in this total, as the group splits were not given (Talakad 2011). The mean participant age was 68 years, 61% were male, the mean Hoehn & Yahr stage was 2.4, and mean duration of PD was five years. All trials used a parallel group design. Treatment sessions lasted from 30 to 60 minutes, and took place over a period of four to eight weeks. Treadmill training mainly involved participants walking on a treadmill with speed and/or incline adjustments. Three trials used body weight‐supported treadmill training (Fisher 2008; Ganesan 2010; Talakad 2011), and two trials provided gait and step training (Kurtais 2008; Protas 2005).
Cueing versus Control
The nine trials of cueing versus control involved 371 participants (Almeida 2012; de Bruin 2010a; de Bruin 2010b; Haase 2011; Lehman 2005; Mak 2008; Nieuwboer 2007; Shankar 2008; Thaut 1996). The mean participant age was 67 years, 59% were male, the mean Hoehn & Yahr stage was 2.6, and mean duration of PD was seven years. Eight of the trials were of parallel group design, and one used a cross‐over design (Nieuwboer 2007). Treatment sessions lasted from four to 30 minutes and took place over a period of a single session to 13 weeks. Three types of cueing were used in the trials: audio (music, spoken instructions), visual (computer images), and sensory (vibration). Six trials applied external cues during gait or gait‐related activity, and Mak (Mak 2008) used cues for the rehabilitation of sit‐to‐stand transfers.
Dance versus Control
The two trials of dance versus control involved 120 participants (Duncan 2012; Hackney 2009). The mean participant age was 69 years, 63% were male, the mean Hoehn & Yahr stage was 2.3, and mean duration of PD was seven years. Both trials used a parallel group design. Dance classes lasted one hour over 12 to 13 weeks, with a trained instructor teaching participants the tango, waltz, or foxtrot.
Martial Arts versus Control
The four trials of martial arts versus control involved 143 participants (Hackney 2009; Marjama‐Lyons 2002; Purchas 2007; Schmitz‐Hubsch 2006). The mean participant age was 65 years, 74% were male, the mean Hoehn & Yahr stage was 2.1, and mean duration of PD was six years. All the trials were of parallel group design, except one, which used a cross‐over design (Purchas 2007). Treatment lasted one hour and took place over a period of 12 to 24 weeks. Participants took classes on Tai Chi (three trials; Hackney 2009; Marjama‐Lyons 2002; Purchas 2007) or Qigong (one trial; Schmitz‐Hubsch 2006).
Risk of bias in included studies
See: Characteristics of included studies, risk of bias in included studies tables, risk of bias graph (Figure 2), and risk of bias summary (Figure 3).
2.
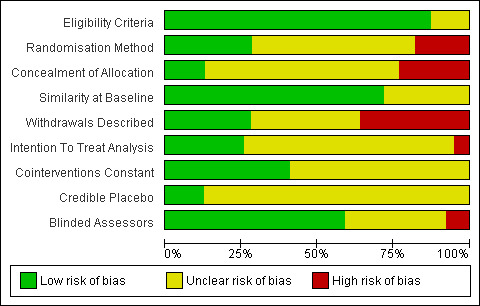
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
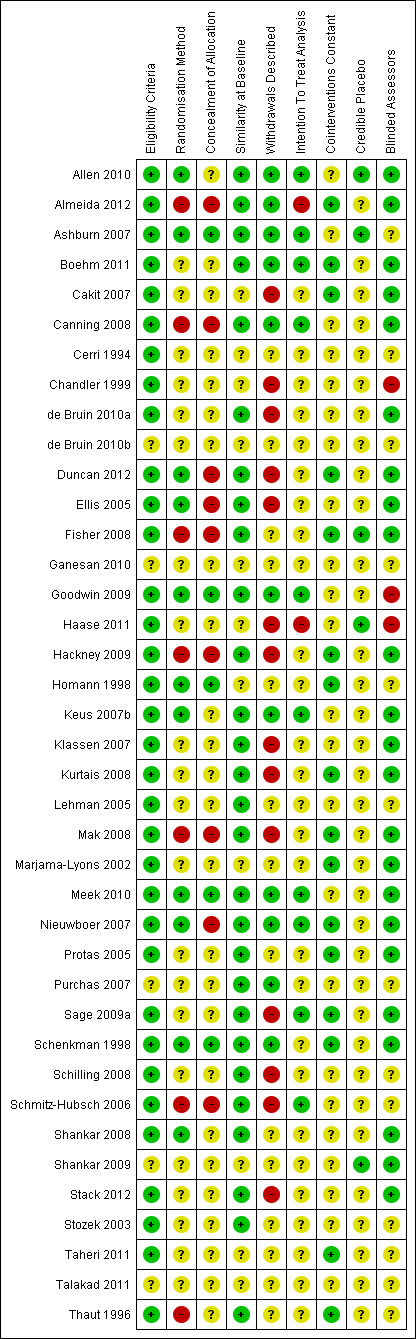
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Trial Design
Thirty‐five trials had a parallel design and four had a cross‐over design (Boehm 2011; Ellis 2005; Nieuwboer 2007; Purchas 2007). The cross‐over trials had no washout period, with participants assessed at baseline, after the first treatment period, and then after the second treatment period. Most trials looked at the short‐term effect of therapy by assessing participants at baseline and immediately or shortly after the physiotherapy intervention period (which ranged from two to 52 weeks). Ten of the parallel design trials (Almeida 2012; Ashburn 2007; Goodwin 2009; Klassen 2007; Lehman 2005; Mak 2008; Meek 2010; Schmitz‐Hubsch 2006; Stack 2012; Stozek 2003) reported additional data at assessment points after the treatment period had finished; this may have been at only one week or up to 12 months after the end of the treatment period.
Sample Size
Only six studies (15%; Allen 2010; Ashburn 2007; Duncan 2012; Ellis 2005; Goodwin 2009; Nieuwboer 2007) reported a sample size calculation in the trial report, three of which failed to achieve their target (Ashburn 2007; Duncan 2012; Goodwin 2009).
Eligibility Criteria
Eligibility criteria for the trials were broad and varied considerably across trials. The level of detail provided in the eligibility criteria was also variable, with some studies providing a detailed description of the entry criteria, and others just stating “patients with Parkinson’s disease.” Only eight trials (Cakit 2007; de Bruin 2010a; Homann 1998; Keus 2007b; Nieuwboer 2007; Schmitz‐Hubsch 2006; Shankar 2008; Stack 2012) stated that a diagnosis of PD by the United Kingdom Brain Bank Criteria (Gibb 1988) was required. It is vital that eligibility criteria are well defined, so that the trial participant population can be determined.
Randomisation Method and Concealment of Allocation
Only 18 trials (46%) described the randomisation method used, of which 11 trials used low‐risk methods (e.g. block randomisation, computer random number generators). No details on the randomisation method used were provided for the remaining 21 trials. Further, only 14 trials (36%) stated or gave adequate information that allowed the assessment of whether an adequate concealment of treatment allocation procedure had been used. Five trials were considered to be low risk by virtue of the fact that they used a central randomisation service, and the other nine were considered high risk (i.e. concealment of treatment allocation was potentially compromised - sealed envelopes, picking card or picking from a hat).
Blinding of Assessors
It would be impossible to blind participants and therapists to randomised treatment allocation in trials of physiotherapy. Therefore, such trials are open label by nature, and are consequently liable to the possibility of both performance and attrition bias. However, assessors could be blinded to try to reduce the possibility of bias. Twenty‐four (62%) of the thirty‐nine studies used blinded assessors (although in one study, the assessors correctly guessed the treatment allocation in nearly 30% of patients; unclear risk; Ashburn 2007), three used unblinded assessors so were classed as high risk, and in the other 12 studies, this information was not provided (classed as unclear risk).
Description of the No Intervention (Control) Group
In most trials (n=34), the control group did not receive any physiotherapy treatment or intervention; however, in five trials (Allen 2010; Ashburn 2007; Fisher 2008; Haase 2011; Shankar 2009), an active placebo was used that attempted to control for the time and attention involved in receiving physiotherapy intervention compared with no treatment. This included contact with a PD nurse, education classes, advice on falls prevention, and listening to music. The control groups were followed‐up and were assessed in the same manner as the intervention groups.
Cointerventions
Information on cointerventions was provided in 23 trials (59%), with participants continuing with their standard PD medication. In 16 trials, this drug therapy was kept stable (low risk) throughout the duration of the trial, whereas seven trials allowed variation (unclear risk). The remaining 16 trials did not describe drug therapy (unclear risk).
Similarity of Treatment Groups at Baseline
A description of the baseline characteristics of the trial participants is important for determination of whether the trial results are generalisable and for comparison of the characteristics of the two arms to ensure that the randomisation methods were successful.
Six trials (de Bruin 2010b; Ganesan 2010; Homann 1998; Marjama‐Lyons 2002; Taheri 2011; Talakad 2011) did not provide any information on the baseline characteristics of participants entered into the trial. Twenty‐eight (of the 33) trials that reported baseline data gave this information split by treatment group and showed participants to be similar at baseline. In ten trials; the baseline characteristics of the withdrawn participants were not given (Cakit 2007; de Bruin 2010a; Haase 2011; Hackney 2009; Klassen 2007; Kurtais 2008; Mak 2008; Purchas 2007; Sage 2009a; Schenkman 1998). This, along with the six studies that did not supply baseline data, meant that 261 (14%) of the 1827 randomly assigned participants were not characterised.
Data Analysis
Nine trials stated intention‐to‐treat as the primary method of analysis, although it was not always clear if patients who withdrew from the trial were included in the analysis. The number of patient withdrawals was classed as low risk (≤ 10% of trial participants withdrew) in seven of these nine trials. Three trials stated per protocol as the primary method of analysis. In the other 27 trials, the method of analysis was not described (unclear risk). Of these trials, 12 were considered high risk in terms of the proportion of patients that withdrew (i.e. > 10%), and in 14 trials, the number of participant withdrawals (if any) was not given (unclear risk).
Data Available for Analysis
Thirteen trials were reported in abstract form. We requested further information from authors; six (Boehm 2011; Haase 2011; Klassen 2007; Meek 2010; Purchas 2007; Shankar 2008;) provided additional information, and seven (Cerri 1994; de Bruin 2010b; Ganesan 2010; Homann 1998; Marjama‐Lyons 2002; Shankar 2009; Talakad 2011) requests were unsuccessful. Sufficient data were available for meta‐analysis for five of the 13 studies (Boehm 2011; Haase 2011; Klassen 2007; Meek 2010; Shankar 2009). Further, one trial had relevant data that could not be extracted as it was available only in graph form (Lehman 2005), and another trial published only median and interquartile range data, so their results could not be meta‐analysed in this format (Stack 2012). Therefore data were not available for meta‐analysis for ten trials, meaning that of the 39 trials, data available for analysis were provided by 29 trials.
Effects of interventions
Primary Analysis
Gait Outcomes
Two‐ or Six‐Minute Walk Test (m)
Data on the two‐ or six‐minute walk test were available from six trials for seven comparisons within four physiotherapy interventions (exercise, treadmill, dance, and martial arts). (Note: Hackney 2009 contributed data to both the dance and martial arts comparisons.) Two hundred forty‐two participants were included in this analysis. A benefit of borderline significance was identified, along with a greater increase in the distance walked in two or six minutes with physiotherapy intervention compared with no intervention (mean difference 13.37 m, 95% confidence interval (CI) 0.55 to 26.20; P = 0.04; see Figure 4). No evidence of heterogeneity was found between the individual trials (P = 0.44, I2 = 0%), nor did evidence suggest that the treatment effect differed across the four physiotherapy interventions (P = 0.19, I2 = 37%).
4.
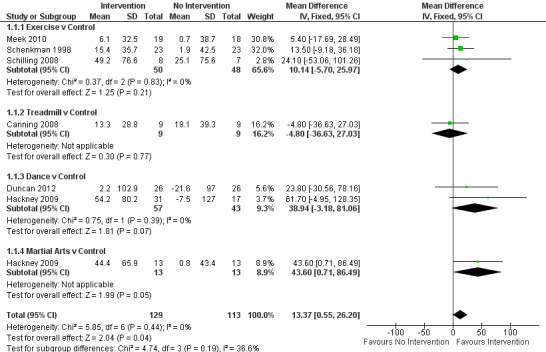
2‐ or 6‐Minute walk test (m).
Meek 2010 contributed to 2‐minute walk test. Hackney 2009, Schilling 2008, and Schenkman 1998 contributed to 6‐minute walk test.
10‐ or 20‐Metre Walk Test(s)
Data on the 10‐ or 20‐metre walk test were available from four trials for two physiotherapy interventions (exercise and treadmill). One hundred sixty‐nine participants were included in the analysis. Borderline significance was reported in favour of no intervention for the time taken to walk 10 or 20 metres (0.40 s, CI 0.00 to 0.80; P = 0.05; see Figure 5). No evidence of heterogeneity between individual trials was obtained (P = 0.19, I2 = 38%), nor did evidence indicate that the treatment effect differed across the two physiotherapy interventions (P = 0.51, I2 = 0%).
5.
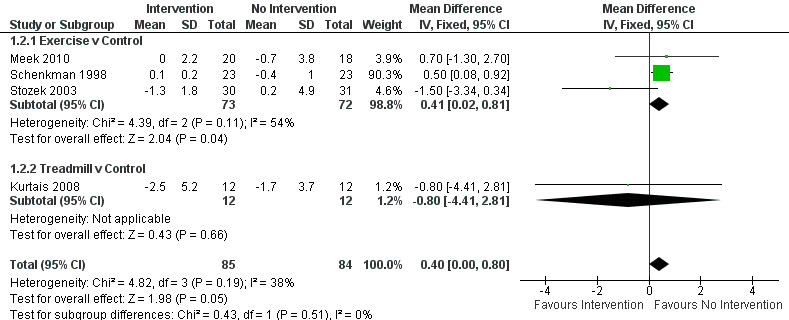
10‐ or 20‐m walk test (s).
Kurtais 2008 contributed to 20‐m walk test. Meek 2010, Schenkman 1998, and Stozek 2003 contributed to 10‐m walk test.
Speed (m/s)
Data on speed were available from 15 trials for 19 comparisons within all six physiotherapy interventions. (Note: Fisher 2008; Hackney 2009; Mak 2008; and Thaut 1996 all contributed data to two physiotherapy comparisons.) Eight hundred fourteen participants were included in this analysis. A significant benefit was reported for physiotherapy, with speed increased by 4 cm/s with a physiotherapy intervention compared with no intervention (0.04 m/s, CI 0.02 to 0.06; P = 0.0002; see Figure 6). No evidence of heterogeneity was obtained between the individual trials (P = 0.55, I2 = 0%), nor any evidence of heterogeneity found between the different types of physiotherapy intervention (P = 0.25, I2 = 25%).
6.
Speed (m/s).
Cadence (steps/min)
Data on cadence were available from seven trials for nine comparisons within four physiotherapy interventions (general physiotherapy, exercise, treadmill, and cueing). (Note: Fisher 2008 and Thaut 1996 contributed data to two physiotherapy comparisons). Three hundred fifty participants were included in this analysis. No significant difference in cadence was observed between the two treatment arms (‐1.57 steps/min, CI ‐3.81 to 0.67; P = 0.17).
Stride Length (m)
Data on stride length were available from six trials for nine comparisons within all six physiotherapy interventions. (Note: Fisher 2008, Hackney 2009, and Thaut 1996 contributed data to two physiotherapy comparisons.) Two hundred twenty‐five participants were included in this analysis. No difference in stride length was reported between the two treatment arms (0.03 m, 95% CI ‐0.02 to 0.08; P = 0.24).
Step Length (m)
Data on step length were available from five trials for six comparisons within four physiotherapy interventions (general physiotherapy, exercise, treadmill, and cueing). (Note: Fisher 2008 contributed data to both the general physiotherapy and treadmill comparisons.) Three hundred eighty‐three participants were included in this analysis. No difference in step length was noted between the two treatment arms (0.02 m, 95% CI 0.00 to 0.04; P = 0.06).
Freezing of Gait Questionnaire
Data from the Freezing of Gait Questionnaire were available from four trials for three physiotherapy interventions (exercise, cueing, and dance). Two hundred ninety‐eight participants were included in this analysis. A borderline significant benefit was noted, with freezing of gait questionnaire score improved by 1.4 points with a physiotherapy intervention compared with no intervention (‐1.41, 95% CI ‐2.63 to ‐0.19; P = 0.02, see Figure 7). No evidence of heterogeneity between the individual trials was found (P = 0.74, I2 = 0%), nor was there any evidence of heterogeneity between the different types of physiotherapy interventions (P = 0.55, I2 = 0%).
7.
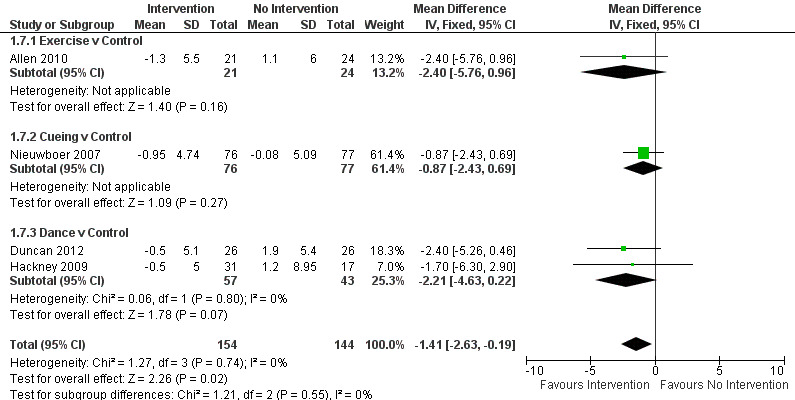
Forest plot of comparison: 1 Gait Outcomes, outcome: 1.7 Freezing of Gait Questionnaire.
Functional Mobility and Balance Outcomes
Timed Up & Go (s)
Data on the Timed Up & Go test were available from nine trials for ten comparisons within four physiotherapy interventions (exercise, cueing, dance, and martial arts). (Note: Hackney 2009 contributed data to both the dance and martial arts comparisons.) Six hundred thirty‐nine participants were included in this analysis. Overall, the time taken to complete the Timed Up & Go test was significantly improved (i.e. reduced) with physiotherapy intervention compared with no intervention (‐0.63 s, 95% CI ‐1.05 to ‐0.21; P = 0.003; see Figure 8). No heterogeneity was observed between the individual trials (P = 0.12, I2 = 36%), nor between the four physiotherapy interventions (P = 0.33, I2 = 12%).
8.
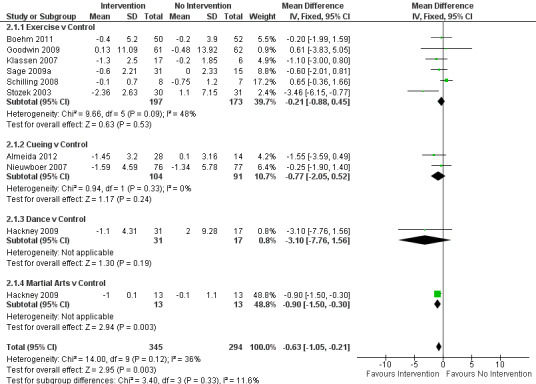
Timed Up & Go (s).
The results for the Hackney et al. martial arts comparison were heavily weighted in the analysis (48.8%) by very small standard deviations (Hackney 2009) compared with the other studies. It was also noted that in the trial publication, a nonsignificant (P = 0.093) effect of martial arts intervention was reported - a finding that contrasted with our data analysis, which reported a significant improvement (P = 0.003). The author was contacted to check whether the data reported in the paper were in fact standard errors, but they were confirmed as standard deviations. We therefore performed a sensitivity analysis to remove this study and found that the overall result became not significant (‐0.38 s, 95% CI ‐0.96 to 0.21; P = 0.21), so this result should be interpreted with caution.
Functional Reach Test (cm)
Data on the Functional Reach Test were available from four trials for two physiotherapy interventions (exercise and cueing). Three hundred ninety‐three participants were included in this analysis. Functional reach was significantly improved with physiotherapy intervention compared with no intervention (2.16 cm, 95% CI 0.89 to 3.43; P = 0.0008, see Figure 9). No evidence suggested heterogeneity between the individual trials (P = 0.15, I2 = 44%), nor did evidence indicate that the treatment effect differed across the two physiotherapy interventions (P = 0.48, I2 = 0%).
9.
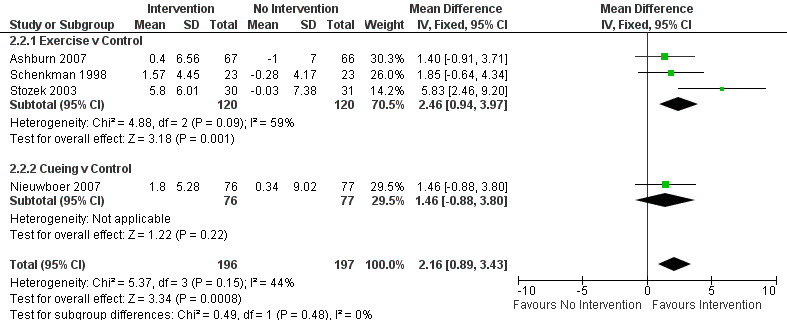
Functional Reach (cm).
Berg Balance Scale
Data on the Berg Balance Scale were available from five trials for six comparisons within four physiotherapy interventions (exercise, treadmill, dance, and martial arts). (Note: Hackney 2009 contributed data to both the dance and martial arts comparisons.) Three hundred eighty‐five participants were included in this analysis. The Berg Balance Scale was significantly better after physiotherapy intervention (3.71 points, 95% CI 2.30 to 5.11; P < 0.00001; see Figure 10). No evidence of heterogeneity between the individual trials was noted (P = 0.06, I2 = 53%), nor did evidence suggest that the treatment effect differed across the four physiotherapy interventions (P = 0.47, I2 = 0%).
10.
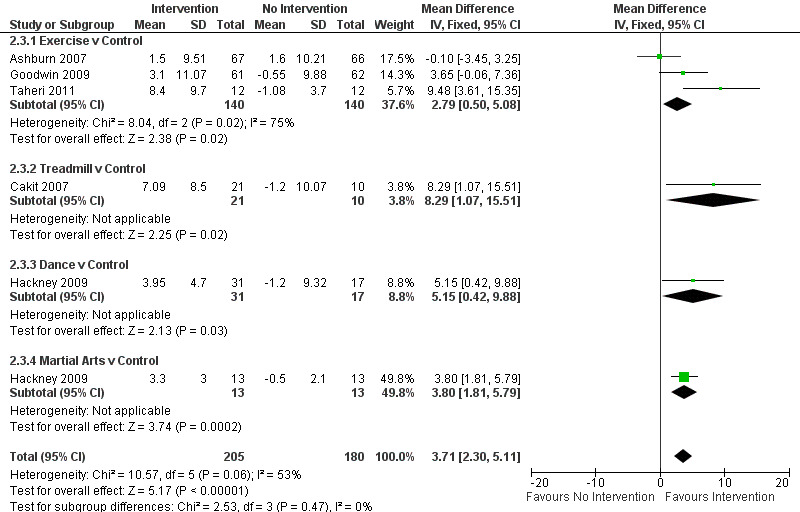
Berg Balance Scale.
Activity‐Specific Balance Confidence
Data on activity‐specific balance confidence were available from three trials for two physiotherapy interventions (exercise and cueing). Sixty‐six participants were included in this analysis. No difference between the two treatment arms was noted (2.40 points, 95% CI ‐2.78 to 7.57; P = 0.36).
Falls
Number of Falls
Seven trials (Ashburn 2007; Goodwin 2009; Marjama‐Lyons 2002; Meek 2010; Nieuwboer 2007; Protas 2005; Purchas 2007) attempted to record the number of falls during the trial period.This was usually done by means of a falls diary, which can be difficult to analyse and is subject to bias. Nevertheless, most of the individual trials reported a general trend for a reduction in the number of falls with intervention. However, when compared with the no intervention arm, this finding was not significant, except in one trial. Marjama‐Lyons 2002 reported a significant decrease in the chance of fall frequency with Tai Chi intervention when compared with no intervention.
Falls Efficacy Scale
Data on the Falls Efficacy Scale were available from four trials for four comparisons within two physiotherapy interventions (exercise and cueing). Three hundred fifty‐three participants were included in this analysis. No difference in the Falls Efficacy Scale was found between the two treatment arms (‐1.91 points, 95% CI ‐4.76 to 0.94; P = 0.19).
Clinician‐rated Disability
Only data on the Unified Parkinson’s Disease Rating Scale were available for meta‐analysis.
Unified Parkinson’s Disease Rating Scale (UPDRS)
Total
Data on the total UPDRS score were available from three trials for four comparisons within three physiotherapy interventions (general physiotherapy, exercise, and treadmill). (Note: Fisher 2008 contributed data to both the general physiotherapy and treadmill comparisons.) Two hundred seven participants were included in this analysis. Overall, the UPDRS total score was significantly improved with physiotherapy intervention compared with no intervention (‐6.15 points, 95% CI ‐8.57 to ‐3.73; P =< 0.00001; see Figure 11). Evidence of borderline heterogeneity was observed between the individual trials (P = 0.03, I2 = 67%), and between the different types of physiotherapy intervention (P = 0.01, I2 = 77%).
11.
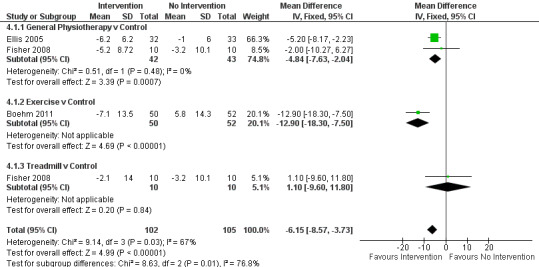
UPDRS - total.
Mental
Data on the mental sub‐scale of the UPDRS were available from two trials for three comparisons within two physiotherapy interventions (general physiotherapy and treadmill). (Note: Fisher 2008 contributed data to both the general physiotherapy and treadmill comparisons.) One hundred five participants were included in this analysis. No difference in UPDRS mental score was reported between the two treatment arms (‐0.44, 95% CI ‐0.98 to 0.09; P = 0.10).
Activities of Daily Living (ADL)
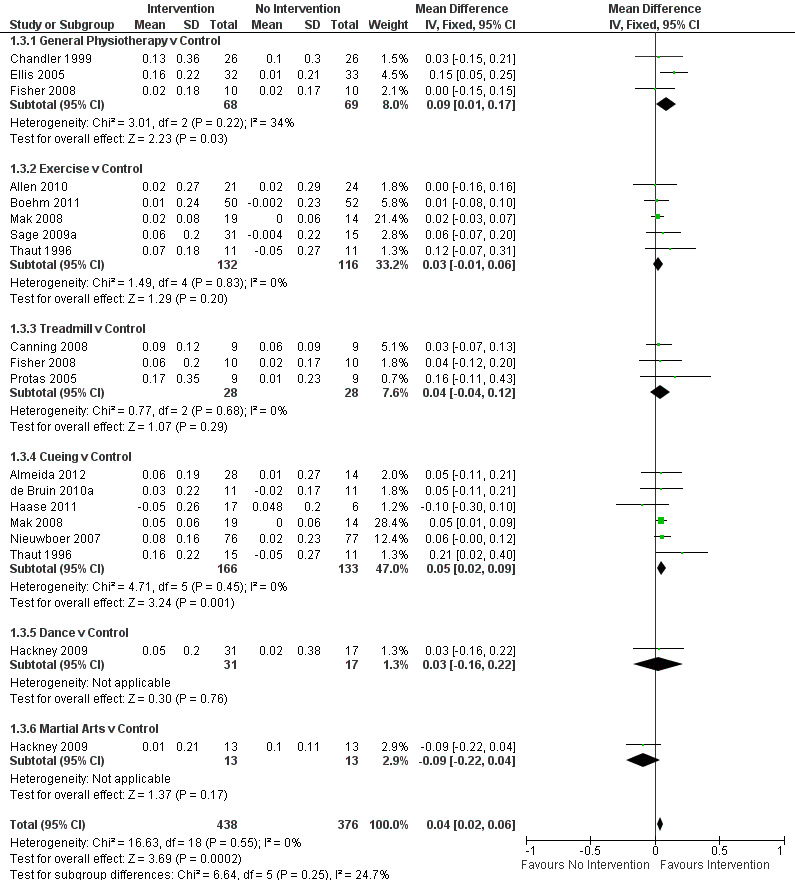
Data on the ADL sub‐scale of the UPDRS were available from three trials for four comparisons within three physiotherapy interventions (general physiotherapy, treadmill, and dance). (Note: Fisher 2008 contributed data to both the general physiotherapy and treadmill comparisons.) One hundred fifty‐seven participants were included in this analysis. Overall, the UPDRS ADL score was significantly improved with physiotherapy intervention compared with no intervention (‐1.36 points, 95% CI ‐2.41 to ‐0.30; P = 0.01; see Figure 12). No evidence of heterogeneity was observed between the individual trials (P = 0.28, I2 = 22%), nor was there any evidence of heterogeneity between the different types of physiotherapy intervention (P = 0.19, I2 = 40%).
12.
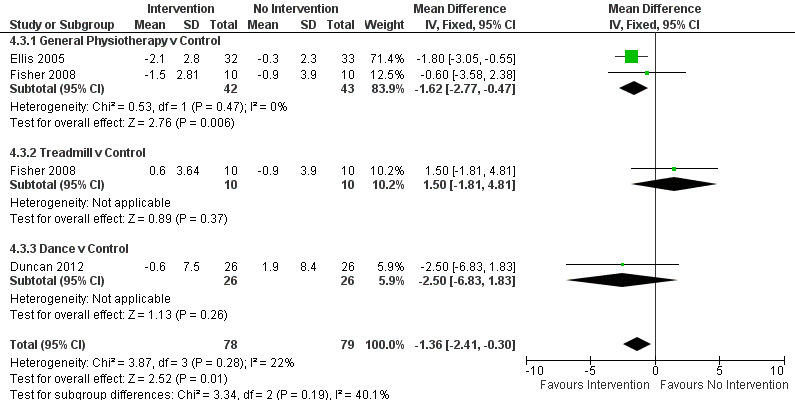
UPDRS - ADL.
Earhart 2010, MDS‐UPDRS.
Motor
Data on the motor sub‐scale of the UPDRS were available from 12 trials for 14 comparisons within all six physiotherapy interventions. (Note: Fisher 2008 and Hackney 2009 contributed data to two physiotherapy interventions.) Five hundred ninety‐three participants were included in this analysis. Overall, the UPDRS motor score was significantly improved with physiotherapy intervention compared with no intervention (‐5.01 points, CI ‐6.30 to ‐3.72; P < 0.00001; see Figure 13). Evidence indicated significant heterogeneity between the individual trials (P = 0.0009, I2 = 63%) and across the six physiotherapy interventions (P = 0.0001, I2 = 80%). A single outlying trial (Boehm 2011) was the source of this heterogeneity, as upon exclusion of this trial from the analysis, the result remained statistically significant (‐3.77 points, 95% CI ‐5.15 to ‐2.39; P < 0.00001), but the findings of tests for heterogeneity between trials (P = 0.44, I2 = 0%) and subgroups (P = 0.08, I2 = 50%) were no longer significant.
13.
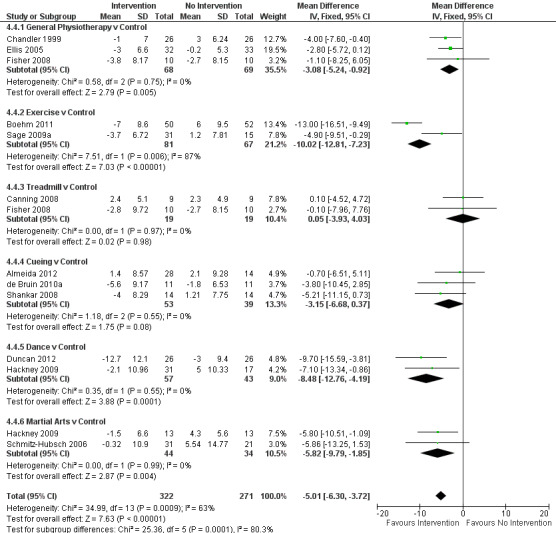
UPDRS - Motor.
Earhart 2010, MDS‐UPDRS.
Patient‐rated Quality of Life
Only data on the Parkinson’s Disease Questionnaire–39 (PDQ‐39) for the mobility domain and the summary index were available for meta‐analysis.
Parkinson’s Disease Questionnaire–39 (PDQ‐39)
Summary Index
Data on the Summary Index of the PDQ‐39 were available from seven trials for eight comparisons within all six physiotherapy interventions. (Note: Hackney 2009 contributed data to both the dance and martial arts comparisons.) Four hundred five participants were included in this analysis. No difference between treatment arms was observed in patient‐rated quality of life after physiotherapy intervention (‐0.38 points, 95% CI ‐2.58 to 1.81; P = 0.73).
Mobility
Data on the mobility domain of the PDQ‐39 were available from two trials for three comparisons within three physiotherapy interventions (general physiotherapy, dance, and martial arts). (Note: Hackney 2009 contributed data to both the dance and martial arts comparisons.) One hundred five participants were included in this analysis. No difference in the PDQ‐39 mobility score was observed between the two treatment arms (‐1.43, 95% CI ‐8.03 to 5.18; P = 0.67).
Adverse Events
No trials reported data on adverse events, and only one commented on adverse events, stating that none had occurred during treatment sessions (Goodwin 2009).
Compliance
Only fourteen of the thirty‐nine trials discussed patient compliance, with twelve (Allen 2010; Canning 2008, Duncan 2012, Ellis 2005; Goodwin 2009, Keus 2007b; Klassen 2007; Kurtais 2008; Meek 2010; Sage 2009a; Schenkman 1998; Schmitz‐Hubsch 2006) quantifying it in some form; however, this was difficult to analyse.
Health Economic
No trials reported data on health economic outcomes.
Subgroup Analysis
Only one outcome, the UPDRS motor sub‐scale, showed significant heterogeneity between the treatment effects of the different classes of interventions. In all other cases, no evidence of any differences was found. However, one outlying trial was the cause of this heterogeneity in the motor score (Boehm 2011); when this trial was excluded from the analysis, the result remained significant (−3.77 points, 95% CI ‐5.15 to ‐2.39; P < 0.001), but the test for between‐trial and between‐subgroup heterogeneity was no longer significant (P = 0.44 and P = 0.08, respectively).
Discussion
Summary of main results
This review updates the previous Cochrane review published in 2001 (Deane 2001a) comparing physiotherapy intervention versus no physiotherapy intervention for the treatment of PD. The review now includes 39 randomised trials and 1827 participants (compared with 11 trials and 280 participants in the 2001 review). It also compares the different types of physiotherapy interventions used in the treatment of PD, thus providing a comprehensive assessment of physiotherapy treatment. Many recent systematic reviews have focused on specific areas of physiotherapy such as exercise and cueing (Crizzle 2006; Goodwin 2008; Lim 2005; Nieuwboer 2008). Nowadays, physiotherapy for PD encompasses a wide range of methods and techniques ranging from standard NHS physiotherapy to exercise regimens and martial arts. Therefore, it is important that all forms of physiotherapy intervention are included, so that the true benefit (if any) of physiotherapy can be assessed. The review also includes a more comprehensive range of outcome measures compared with previous reviews (18 outcomes assessing gait, functional mobility and balance, falls, clinician‐rated Unified Parkinson’s Disease Rating Scale (UPDRS), and patient‐rated quality of life), thus providing the most reliable summary available of the current published evidence.
Physiotherapy Intervention versus No Physiotherapy Intervention
This review provides evidence of the short‐term (< three months) benefit of physiotherapy in the treatment of PD. All outcomes showed improvement with physiotherapy intervention compared with no intervention (except the 10‐ or 20‐metre walk test). However, significant benefits after physiotherapy intervention were observed only for the gait outcomes of speed, the two‐ or six‐minute walk test, and the Freezing of Gait questionnaire; the functional and mobility outcomes of the Timed Up & Go test, Functional Reach Test, and Berg Balance Scale; and the clinician‐rated UPDRS. It is of interest that the direction of the treatment effect favoured physiotherapy intervention in all outcome measures, except one. The absence of evidence in these outcomes is not necessarily evidence of the absence of benefit for physiotherapy. One possible reason for this may be the lack of data. More than 1800 participants were randomly assigned into the 39 trials included in this review, and 29 trials and 1577 participants (86% of total) provided data for analysis. However, the greatest quantity of data were provided for analysis of the outcome speed, and this included just 15 trials and 814 participants (52% of the total number of participants providing data). This general lack of extractable data means that results of this meta‐analysis should be interpreted with caution.
Gait
People with PD frequently have problems with gait, and treatment is usually targeted toward maximising exercise tolerance, improving the gait pattern, maintaining or increasing independence regarding mobility, and reducing the risk of falls. The most significant improvement among the outcomes assessing gait involved speed. In light of previous experimental evidence, it may be hypothesised that the improvement in speed is linked to an increase in step or stride length, or both, and that this in turn leads to a compensatory decrease in cadence (Morris 1994; Morris 1996). In this review, although a significant improvement in speed was observed, we found no difference in step length, stride length, or cadence. This could again be due to lack of data, as a smaller number of studies reported step and stride length and cadence (up to seven studies) compared with speed (15 studies). Thus, further data on the possible link between speed, cadence, step, and stride length are required.
Freezing of gait is a prevalent motor disturbance within PD, and it is known to have a detrimental impact on quality of life, as well as on gait and mobility (Moore 2007). We found a borderline significant difference in scores derived from the Freezing of Gait Questionnaire, but this was measured in only four trials (298 participants), again highlighting the need for further data in this important area.
Observed differences in the three significant gait outcomes (speed, the two‐ or six‐minute walk test, and freezing of gait) were relatively small. Therefore, their relevance and benefit to patients with PD must be put into context in terms of what is considered a minimally clinically important change (MCIC). Speed was significantly improved with physiotherapy intervention by 0.04 metres/s. Data on what is considered an MCIC are lacking for PD patients, but some data have been reported in stroke patients. In one study, it was reported that an increase in speed of just 0.03 and 0.13 metres/s could translate into a change from a limited household to an unlimited household walker, and from an unlimited household walker to a most‐limited community walker, respectively (Perry 1995). Our data are consistent with the findings reported by Perry (Perry 1995). For the two‐ or six‐minute walk test and freezing of gait, participants who received physiotherapy intervention were able to walk further over two or six minutes (by 13 m) and their Freezing of Gait score was improved by 1.4 points. Data on the MCIC are lacking for these outcomes, but although a 13‐m increase in distance walked would probably be considered clinically important, the importance of a 1.4‐point improvement in freezing of gait is less clear.
Functional Mobility and Balance
Changes in functional mobility and balance within PD have been well documented (Bloem 2001). Of the functional mobility and balance outcomes assessed within this review, significant improvements were observed in the Timed Up & Go test, Functional Reach Test, and Berg Balance Scale. The time taken to complete the Timed Up & Go test was significantly improved by 0.63 seconds with physiotherapy. Despite this significant change, the MCIC in PD patients is thought to be 11 seconds (Steffen 2008). Therefore, the small change observed within this review may not translate into a noticeable improvement in a person’s functional mobility.
A five‐point change is the MCIC on the Berg Balance Scale (Steffen 2008). In this review, a significant four‐point improvement in the Berg Balance Scale was noted after physiotherapy intervention. A greater evidence base is required to support or refute the clinical significance of this result. A significant improvement of 2 cm was also noted in the Functional Reach Test, but this is somewhat lower than the MCIC of 9 cm and 7 cm for the forward and backward Functional Reach Test (Steffen 2008).
Falls
Falls are a common and disabling problem within PD (Bloem 2001), with high clinical impact and serious cost implications to society. They are also a recurrent problem, with up to 51% of those falling reporting two or more falls per year (Wood 2002). Fear of falling has been recognised as a contributing factor to recurrent falls (Mak 2009). Within this review, fear of falling has been captured through the Falls Efficacy Scale (standard and international). No difference between treatment arms was observed for this outcome. This might be attributed to the small number of trials (and therefore participants) included within these analyses, but could also indicate that an improvement in balance does not automatically result in increased confidence in an individual’s ability not to fall. In turn, it could be hypothesised that improvement in balance does not directly equate to improved levels of mobility and independence. Although fear of falling was not reduced with physiotherapy within this review, it would be of interest to assess whether the number of falls was reduced, as this may be more relevant to patients. Unfortunately, data on this were poorly reported and were measured too variably within the trials; therefore, they could not be meta‐analysed. However, in the seven trials in which data on the number of falls were reported, a general trend toward a reduction in the number of falls with physiotherapy intervention was seen, but with no difference between the two treatment arms.
Clinician‐Rated Disability
Significant improvements after physiotherapy intervention were also observed for the clinician‐rated UPDRS (total, ADL, and motor scores). The UPDRS total score was improved by 6.2 points, the ADL score by 1.4 points, and motor score by 5.0 points. The MCIC for the UPDRS have been reported in two studies. One analysed data from two independent randomised controlled trials and concluded the MCIC to be eight points for the UPDRS total score, between two and three points for the ADL score, and five points for the motor score (Schrag 2006). The second study performed a cross‐sectional analysis on 653 PD participants, and reported MCIC of 2.3 to 2.7 points for motor and 4.1 to 4.5 points for total UPDRS (Shulman 2010). If the recommendations of both Schrag (Schrag 2006) and Shulman et al (Shulman 2010) are taken into account, it can be concluded that the significant improvements observed within this review are approaching or are MCICs (the MCICs for the UPDRS total, ADL, and motor scores lie within the confidence interval). This suggests that physiotherapy intervention is beneficial in improving motor symptoms and may positively impact ADL.
Patient‐Rated Quality of Life
No significant benefit of physiotherapy intervention for overall patient‐rated quality of life (measured using the Parkinson’s Disease Questionnaire (PDQ)‐39 Summary Index) or the mobility domain of the PDQ‐39 was noted, which is surprising in light of the significant improvements seen in UPDRS scores. Another study (Chandler 1999) assessed patient quality of life using the generic Short Form‐36 and also showed no effect of physiotherapy intervention.
Comparison of Different Physiotherapy Interventions
Although we found short‐term benefit for physiotherapy intervention in the treatment of PD, what is less clear is whether a certain type of physiotherapy intervention may provide greater benefit. This information would be of interest to both clinicians and patients, so that appropriate physiotherapy interventions that provide greater benefit can be delivered to patients with PD. To assess this, we categorised the various physiotherapy interventions used in the trials included in this review according to the type of treatment administered, and then compared them using tests for heterogeneity. We found no real evidence of any differences in the treatment effect between the different physiotherapy interventions used for any of the outcomes assessed. However, these were based on indirect comparisons (with limited data within each physiotherapy intervention) so should be interpreted with caution. They would be better assessed in trials directly comparing different types of physiotherapy interventions.
This lack of difference between the different types of physiotherapy intervention is perhaps not surprising. The content and delivery of the interventions used in the trials included within this review are diverse in nature and, although attempts were made to compare trials 'like for like' through the creation of different categories, the interventions delivered varied substantially within these categories. The variety in the therapies delivered is perhaps not surprising. By nature physiotherapists are autonomous professionals with differing sets of skills who work within their own scope of practice (Chartered Society of Physiotherapy), and so this variation in the interventions delivered within clinical trials may actually reflect clinical practice. Second, and perhaps more important, PD is recognised as a complex condition with an individualised presentation (van der Marck 2009). For this reason, Morris et al (Morris 2010) recognises the importance of the physiotherapist's understanding the specific experience of PD in each patient, and advocates that treatment is tailored to fit the individual’s complaints, lifestyle, and personal interests, as opposed to a 'one size fits all' approach. Over the past decade, steps have been taken to try to provide best practice consensus in the form of the Dutch KNGF guidelines for physical therapy in patients with Parkinson’s disease (Keus 2004). However, this publication provides a guidance framework rather than a 'recipe' for treatment. It is therefore important that physiotherapy interventions are compared against each other within rigorous trial designs to determine which are most effective. This will provide therapists with a menu of treatment strategies that are known to be effective, from which they can devise individualised interventions.
Quality of the evidence
Improvement in trial methodological quality and reporting has been noted since the last Cochrane review (Deane 2001a). The use of more robust randomisation methods, blinding, and intention‐to‐treat analyses had increased since the previous review but was still inadequate. Only 18 of the 39 trials provided information on the randomisation method (of which eleven were considered low risk), and only five used a central randomisation procedure to ensure concealment of treatment allocation. Twenty‐four used blinded assessors and nine reported using intention‐to‐treat analysis. The lack of information on this in many trial reports may not necessarily indicate lack of implementation within the trial, but without this information, the level of bias within the individual trials is difficult to assess. This does, therefore, reduce the amount of confidence that can be placed in the results of this meta‐analysis. The need for further improvement in the methodological quality of trials in physiotherapy for PD was noted in another recent systematic review (Kwakkel 2007). Future trials need to ensure that their designs fulfil the requirements of a methodologically sound, large, randomised controlled trial, and that the reporting follows the CONSORT guidelines (Schulz 2010).
The trials included in the review were relatively small, with most assessing the effects of physiotherapy intervention versus no physiotherapy intervention over a short period with limited follow‐up. The overall size of trials has increased (with an average of 46 participants per trial in this review compared with 25 in the previous review), but the number of small and underpowered trials remains a problem. Small trials may be subject to ‘random error’ (Doll 1980), and consequently may give rise to false‐negative or ‐positive results. To highlight this point, this review illustrates that any differences observed in the various outcome measures showing benefit for physiotherapy were quite small. So trials need to be large enough to detect these small but possibly clinically important differences.
Further, it must be noted that only 14 of the 39 trials discussed participant compliance. This is surprising in that compliance can be an important determinant of the outcomes measured in trials. Therefore, it would be beneficial if the level of compliance is measured in future trials.
Another limitation is that the follow‐up period in the trials included in this review was relatively short. Outcome measures were assessed by all trials at baseline and immediately or shortly after intervention had ceased (one or two weeks with one trial (Goodwin 2009) assessing at 10 weeks post intervention). Thus, this review is able to provide conclusions only on the short‐term benefits of physiotherapy. It is also important to consider results alongside the possibility of a so‐called honeymoon effect (Goetz 2008) in the period during or just after physiotherapy, which may inflate the treatment effect in favour of physiotherapy. Parkinson’s disease is a long‐term neurodegenerative disease, so it is important that the long‐term effect of treatment be assessed. Only 12 of the 39 trials followed‐up participants and reported further data during the post‐treatment period (but this could have been only one week or up to six months post the treatment period). The recommendations of the previous review were that participants should be followed‐up for at least six months, but only one trial (Schmitz‐Hubsch 2006) reported follow‐up data at six months post treatment completion. Long‐term data will provide valuable information about the duration of any improvement following therapy.
The outcome measures included in this review are standard physiotherapy and PD outcomes. However, PD is a multidimensional disease, and many important outcomes were poorly reported or were not reported, this includes data on the number of falls, depression and anxiety, adverse events, and the health of the caregiver supporting the person with PD. Further, no health economics analysis of physiotherapy intervention was reported; therefore little is known about the cost‐effectiveness and economic value of this therapy. Future trials should include these outcomes.
In summary, this review provides evidence of the short‐term (< three months) benefit of physiotherapy intervention for the treatment of PD. It is important to note that although most of the observed differences between the two treatments were small, the improvements observed for speed, Berg Balance Scale, and UPDRS scores were at levels considered to be of clinical importance. To clarify the long‐term (if any) benefit of physiotherapy, additional large, well‐designed randomised trials with a follow‐up of at least 12 months, alongside a health economics assessment, are needed to assess the impact of this treatment on all aspects of a patient's PD.
Authors' conclusions
Implications for practice.
Physiotherapy provides short‐term benefit in the treatment of PD. Significant benefits with physiotherapy intervention were observed for the following outcomes: two‐ or six‐minute walk test, speed, Freezing of Gait questionnaire, Timed Up & Go test, Functional Reach Test, Berg Balance Scale, and UPDRS total, ADL, and motor scores. Although most of the observed differences between the two treatment arms were small, the improvements seen for speed, Berg Balance Scale, and UPDRS scores occurred at levels that may be considered to be of clinical importance. These benefits should be interpreted with caution, however, because the quality of most of the included trials was not high.
The long‐term, if any, benefit of physiotherapy remains unidentified, as does which type of physiotherapy intervention should be delivered. Therefore, although this review has provided evidence that physiotherapy intervention may be of benefit to PD patients, it has also highlighted that further evidence is needed before firm conclusions can be made on the long‐term benefit and on which physiotherapy intervention should be used.
Implications for research.
Most of the studies in this review were small and had a short follow‐up period. It is clear that larger randomised controlled trials are required, particularly focusing on improving trial methodology and reporting. Rigorous methods of randomisation should be used and the allocation adequately concealed. Data should be analysed according to intention‐to‐treat principles, and trials should be reported according to the guidelines set out in the CONSORT statement (Schulz 2010).
A large variety of outcome measures were assessed in these trials, but data were sufficient only for meta‐analysis to be performed for eighteen outcomes. This variation in outcome selection and lack of extractable data resulted in a small proportion of included trials contributing to each outcome. This review illustrates the need for the universal employment of relevant, reliable, and sensitive outcome measures. Additionally, only one trial looked at the longer‐term benefit of physiotherapy intervention. To assess whether, or how long, any improvements due to physiotherapy intervention may last, it is important that long‐term follow‐up is performed.
No evidence indicates the best form of physiotherapy intervention. Comparisons of the different physiotherapy interventions described in this review were based on indirect comparisons between individual trials. A more reliable comparison would be obtained in large, randomised trials that directly compare different physiotherapy interventions.
This review highlights the variety of physiotherapy interventions being used in the treatment of PD. More specific trials with improved treatment strategies are needed to underpin the most appropriate choice of physiotherapy intervention and the outcomes measured.
What's new
| Date | Event | Description |
|---|---|---|
| 16 April 2013 | New citation required but conclusions have not changed | New studies added, conclusions unchanged. |
| 7 September 2012 | New search has been performed | Search updated to 31 January 2012. New studies added, conclusions unchanged. |
| 30 August 2011 | New search has been performed | Converted to new review format. Updated search till 31 December 2010. New studies, conclusions changed. |
History
Protocol first published: Issue 4, 2000 Review first published: Issue 3, 2001
| Date | Event | Description |
|---|---|---|
| 14 March 2001 | New citation required and conclusions have changed | Substantive amendment |
Acknowledgements
We recognise the contributions of all the original trialists and of the individuals who performed the trials that contributed to this meta‐analysis, and we thank the patients who agreed to help improve the assessment of PD treatment by taking part in these trials.
We acknowledge Parkinson’s UK for funding, as well as the Department of Health, whose core support for BCTU made this review possible.
We would also like to thank Alex Furmston, Kinga Malottki, Mohammad Tokhi, and Manijeh Ghods, who provided translations for foreign papers.
Data and analyses
Comparison 1. Gait Outcomes.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 2 or 6 Minute Walk Test (m) | 6 | 242 | Mean Difference (IV, Fixed, 95% CI) | 13.37 [0.55, 26.20] |
| 1.1 Exercise v Control | 3 | 98 | Mean Difference (IV, Fixed, 95% CI) | 10.14 [‐5.70, 25.97] |
| 1.2 Treadmill v Control | 1 | 18 | Mean Difference (IV, Fixed, 95% CI) | ‐4.80 [‐36.63, 27.03] |
| 1.3 Dance v Control | 2 | 100 | Mean Difference (IV, Fixed, 95% CI) | 38.94 [‐3.18, 81.06] |
| 1.4 Martial Arts v Control | 1 | 26 | Mean Difference (IV, Fixed, 95% CI) | 43.6 [0.71, 86.49] |
| 2 10 or 20m Walk Test (s) | 4 | 169 | Mean Difference (IV, Fixed, 95% CI) | 0.40 [0.00, 0.80] |
| 2.1 Exercise v Control | 3 | 145 | Mean Difference (IV, Fixed, 95% CI) | 0.41 [0.02, 0.81] |
| 2.2 Treadmill v Control | 1 | 24 | Mean Difference (IV, Fixed, 95% CI) | ‐0.8 [‐4.41, 2.81] |
| 3 Speed (m/s) | 15 | 814 | Mean Difference (IV, Fixed, 95% CI) | 0.04 [0.02, 0.06] |
| 3.1 General Physiotherapy v Control | 3 | 137 | Mean Difference (IV, Fixed, 95% CI) | 0.09 [0.01, 0.17] |
| 3.2 Exercise v Control | 5 | 248 | Mean Difference (IV, Fixed, 95% CI) | 0.03 [‐0.01, 0.06] |
| 3.3 Treadmill v Control | 3 | 56 | Mean Difference (IV, Fixed, 95% CI) | 0.04 [‐0.04, 0.12] |
| 3.4 Cueing v Control | 6 | 299 | Mean Difference (IV, Fixed, 95% CI) | 0.05 [0.02, 0.09] |
| 3.5 Dance v Control | 1 | 48 | Mean Difference (IV, Fixed, 95% CI) | 0.03 [‐0.16, 0.22] |
| 3.6 Martial Arts v Control | 1 | 26 | Mean Difference (IV, Fixed, 95% CI) | ‐0.09 [‐0.22, 0.04] |
| 4 Cadence (steps/min) | 7 | 350 | Mean Difference (IV, Fixed, 95% CI) | ‐1.57 [‐3.81, 0.67] |
| 4.1 General Physiotherapy v Control | 1 | 20 | Mean Difference (IV, Fixed, 95% CI) | ‐2.40 [‐11.12, 6.32] |
| 4.2 Exercise v Control | 2 | 68 | Mean Difference (IV, Fixed, 95% CI) | ‐1.70 [‐6.30, 2.90] |
| 4.3 Treadmill v Control | 2 | 38 | Mean Difference (IV, Fixed, 95% CI) | ‐0.04 [‐6.48, 6.39] |
| 4.4 Cueing v Control | 4 | 224 | Mean Difference (IV, Fixed, 95% CI) | ‐1.74 [‐4.70, 1.21] |
| 5 Stride Length (m) | 6 | 225 | Mean Difference (IV, Fixed, 95% CI) | 0.03 [‐0.02, 0.08] |
| 5.1 General Physiotherapy v Control | 1 | 20 | Mean Difference (IV, Fixed, 95% CI) | ‐0.02 [‐0.19, 0.15] |
| 5.2 Exercise v Control | 1 | 22 | Mean Difference (IV, Fixed, 95% CI) | 0.17 [‐0.03, 0.37] |
| 5.3 Treadmill v Control | 2 | 38 | Mean Difference (IV, Fixed, 95% CI) | 0.03 [‐0.07, 0.14] |
| 5.4 Cueing v Control | 3 | 71 | Mean Difference (IV, Fixed, 95% CI) | 0.07 [‐0.02, 0.17] |
| 5.5 Dance v Control | 1 | 48 | Mean Difference (IV, Fixed, 95% CI) | 0.07 [‐0.10, 0.24] |
| 5.6 Martial Arts v Control | 1 | 26 | Mean Difference (IV, Fixed, 95% CI) | ‐0.1 [‐0.23, 0.03] |
| 6 Step Length (m) | 5 | 383 | Mean Difference (IV, Fixed, 95% CI) | 0.02 [‐0.00, 0.04] |
| 6.1 General Physiotherapy v Control | 1 | 20 | Mean Difference (IV, Fixed, 95% CI) | ‐0.02 [‐0.10, 0.06] |
| 6.2 Exercise v Control | 2 | 148 | Mean Difference (IV, Fixed, 95% CI) | 0.01 [‐0.02, 0.04] |
| 6.3 Treadmill v Control | 1 | 20 | Mean Difference (IV, Fixed, 95% CI) | 0.01 [‐0.08, 0.10] |
| 6.4 Cueing v Control | 2 | 195 | Mean Difference (IV, Fixed, 95% CI) | 0.04 [0.01, 0.07] |
| 7 Freezing of Gait Questionnaire | 4 | 298 | Mean Difference (IV, Fixed, 95% CI) | ‐1.41 [‐2.63, ‐0.19] |
| 7.1 Exercise v Control | 1 | 45 | Mean Difference (IV, Fixed, 95% CI) | ‐2.40 [‐5.76, 0.96] |
| 7.2 Cueing v Control | 1 | 153 | Mean Difference (IV, Fixed, 95% CI) | ‐0.87 [‐2.43, 0.69] |
| 7.3 Dance v Control | 2 | 100 | Mean Difference (IV, Fixed, 95% CI) | ‐2.21 [‐4.63, 0.22] |
1.1. Analysis.
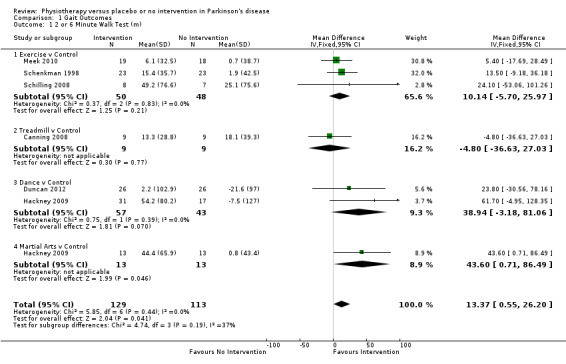
Comparison 1 Gait Outcomes, Outcome 1 2 or 6 Minute Walk Test (m).
1.2. Analysis.
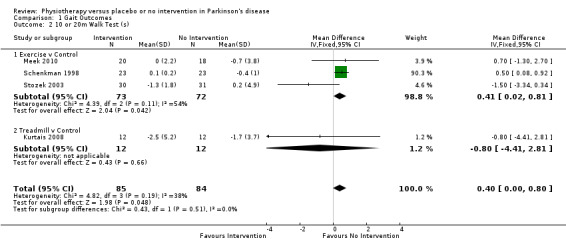
Comparison 1 Gait Outcomes, Outcome 2 10 or 20m Walk Test (s).
1.3. Analysis.
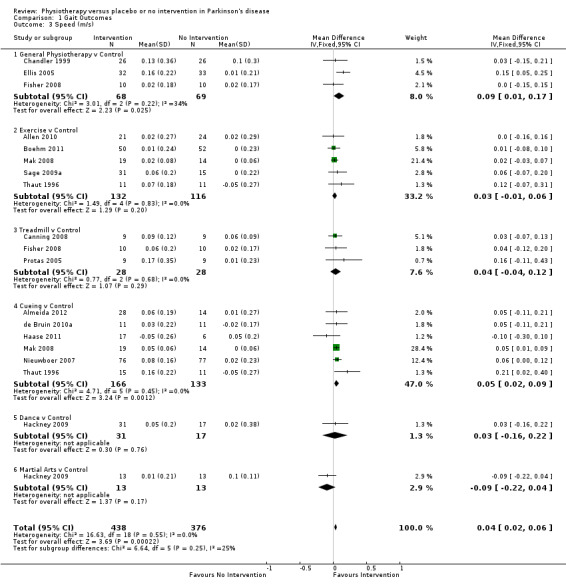
Comparison 1 Gait Outcomes, Outcome 3 Speed (m/s).
1.4. Analysis.
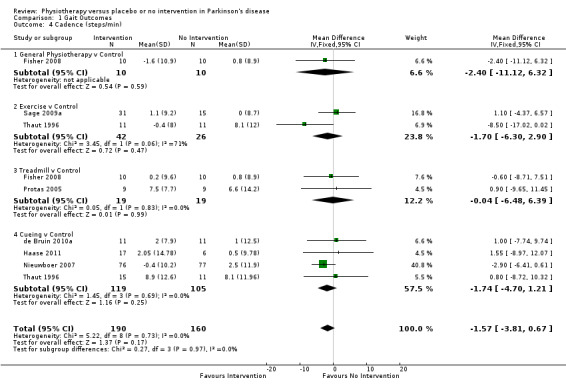
Comparison 1 Gait Outcomes, Outcome 4 Cadence (steps/min).
1.5. Analysis.
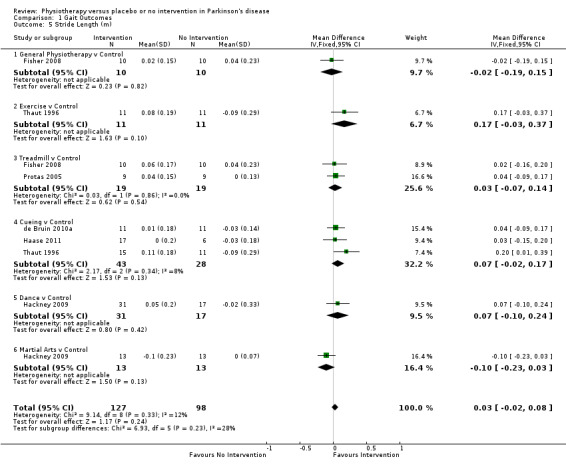
Comparison 1 Gait Outcomes, Outcome 5 Stride Length (m).
1.6. Analysis.
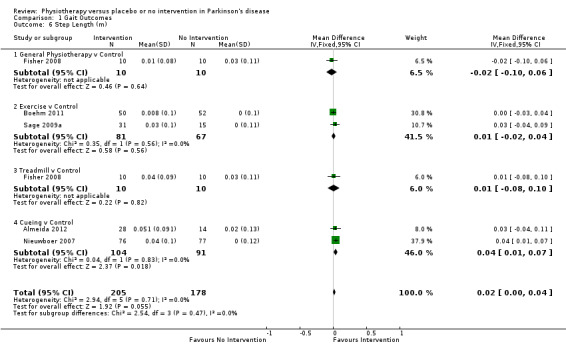
Comparison 1 Gait Outcomes, Outcome 6 Step Length (m).
1.7. Analysis.
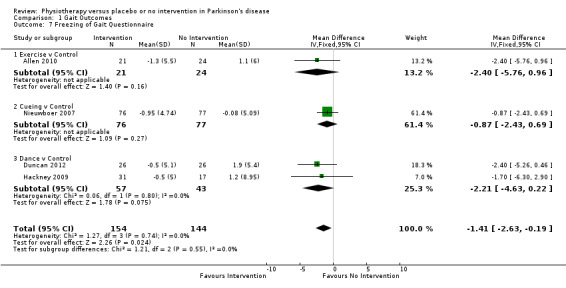
Comparison 1 Gait Outcomes, Outcome 7 Freezing of Gait Questionnaire.
Comparison 2. Functional Mobility and Balance Outcomes.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Timed Up & Go (s) | 9 | 639 | Mean Difference (IV, Fixed, 95% CI) | ‐0.63 [‐1.05, ‐0.21] |
| 1.1 Exercise v Control | 6 | 370 | Mean Difference (IV, Fixed, 95% CI) | ‐0.21 [‐0.88, 0.45] |
| 1.2 Cueing v Control | 2 | 195 | Mean Difference (IV, Fixed, 95% CI) | ‐0.77 [‐2.05, 0.52] |
| 1.3 Dance v Control | 1 | 48 | Mean Difference (IV, Fixed, 95% CI) | ‐3.10 [‐7.76, 1.56] |
| 1.4 Martial Arts v Control | 1 | 26 | Mean Difference (IV, Fixed, 95% CI) | ‐0.9 [‐1.50, ‐0.30] |
| 2 Functional Reach (cm) | 4 | 393 | Mean Difference (IV, Fixed, 95% CI) | 2.16 [0.89, 3.43] |
| 2.1 Exercise v Control | 3 | 240 | Mean Difference (IV, Fixed, 95% CI) | 2.46 [0.94, 3.97] |
| 2.2 Cueing v Control | 1 | 153 | Mean Difference (IV, Fixed, 95% CI) | 1.46 [‐0.88, 3.80] |
| 3 Berg Balance Scale | 5 | 385 | Mean Difference (IV, Fixed, 95% CI) | 3.71 [2.30, 5.11] |
| 3.1 Exercise v Control | 3 | 280 | Mean Difference (IV, Fixed, 95% CI) | 2.79 [0.50, 5.08] |
| 3.2 Treadmill v Control | 1 | 31 | Mean Difference (IV, Fixed, 95% CI) | 8.29 [1.07, 15.51] |
| 3.3 Dance v Control | 1 | 48 | Mean Difference (IV, Fixed, 95% CI) | 5.15 [0.42, 9.88] |
| 3.4 Martial Arts v Control | 1 | 26 | Mean Difference (IV, Fixed, 95% CI) | 3.80 [1.81, 5.79] |
| 4 Activity Specific Balance Confidence | 3 | 66 | Mean Difference (IV, Fixed, 95% CI) | 2.40 [‐2.78, 7.57] |
| 4.1 Exercise v Control | 2 | 38 | Mean Difference (IV, Fixed, 95% CI) | 3.63 [‐2.09, 9.36] |
| 4.2 Cueing v Control | 1 | 28 | Mean Difference (IV, Fixed, 95% CI) | ‐3.1 [‐15.18, 8.98] |
2.1. Analysis.
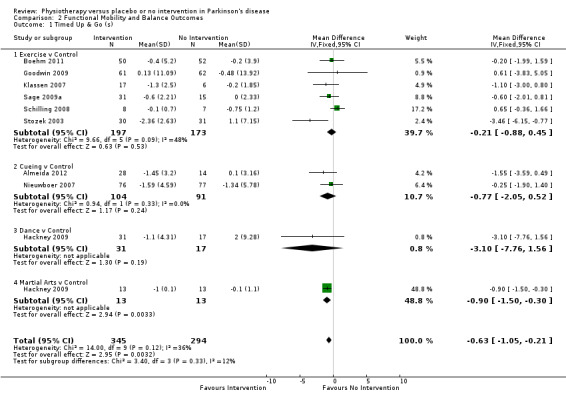
Comparison 2 Functional Mobility and Balance Outcomes, Outcome 1 Timed Up & Go (s).
2.2. Analysis.
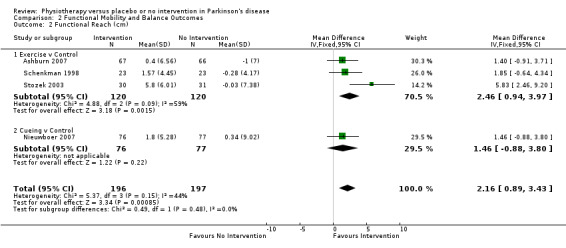
Comparison 2 Functional Mobility and Balance Outcomes, Outcome 2 Functional Reach (cm).
2.3. Analysis.
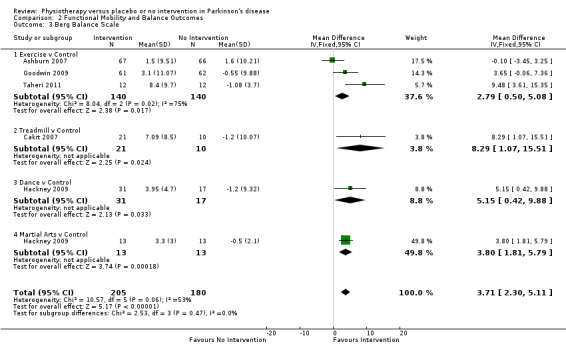
Comparison 2 Functional Mobility and Balance Outcomes, Outcome 3 Berg Balance Scale.
2.4. Analysis.
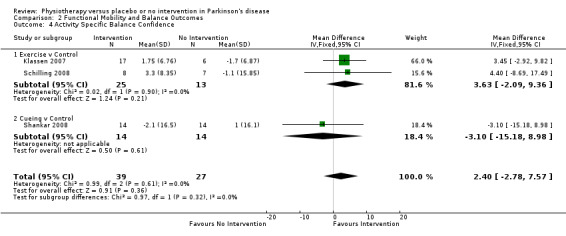
Comparison 2 Functional Mobility and Balance Outcomes, Outcome 4 Activity Specific Balance Confidence.
Comparison 3. Falls.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Falls Efficacy Scale | 4 | 353 | Mean Difference (IV, Fixed, 95% CI) | ‐1.91 [‐4.76, 0.94] |
| 1.1 Exercise v Control | 2 | 169 | Mean Difference (IV, Fixed, 95% CI) | ‐2.35 [‐5.38, 0.69] |
| 1.2 Treadmill v Control | 1 | 31 | Mean Difference (IV, Fixed, 95% CI) | ‐14.67 [‐39.11, 9.77] |
| 1.3 Cueing v Control | 1 | 153 | Mean Difference (IV, Fixed, 95% CI) | 3.32 [‐5.38, 12.02] |
3.1. Analysis.
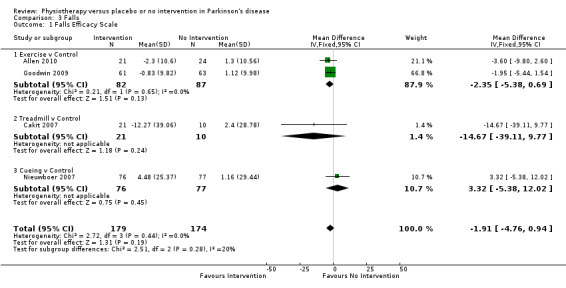
Comparison 3 Falls, Outcome 1 Falls Efficacy Scale.
Comparison 4. Clinician‐Rated Disability.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 UPDRS ‐ Total | 3 | 207 | Mean Difference (IV, Fixed, 95% CI) | ‐6.15 [‐8.57, ‐3.73] |
| 1.1 General Physiotherapy v Control | 2 | 85 | Mean Difference (IV, Fixed, 95% CI) | ‐4.84 [‐7.63, ‐2.04] |
| 1.2 Exercise v Control | 1 | 102 | Mean Difference (IV, Fixed, 95% CI) | ‐12.90 [‐18.30, ‐7.50] |
| 1.3 Treadmill v Control | 1 | 20 | Mean Difference (IV, Fixed, 95% CI) | 1.1 [‐9.60, 11.80] |
| 2 UPDRS ‐ Mental | 2 | 105 | Mean Difference (IV, Fixed, 95% CI) | ‐0.44 [‐0.98, 0.09] |
| 2.1 General Physiotherapy v Control | 2 | 85 | Mean Difference (IV, Fixed, 95% CI) | ‐0.47 [‐1.05, 0.11] |
| 2.2 Treadmill v Control | 1 | 20 | Mean Difference (IV, Fixed, 95% CI) | ‐0.3 [‐1.64, 1.04] |
| 3 UPDRS ‐ ADL | 3 | 157 | Mean Difference (IV, Fixed, 95% CI) | ‐1.36 [‐2.41, ‐0.30] |
| 3.1 General Physiotherapy v Control | 2 | 85 | Mean Difference (IV, Fixed, 95% CI) | ‐1.62 [‐2.77, ‐0.47] |
| 3.2 Treadmill v Control | 1 | 20 | Mean Difference (IV, Fixed, 95% CI) | 1.50 [‐1.81, 4.81] |
| 3.3 Dance v Control | 1 | 52 | Mean Difference (IV, Fixed, 95% CI) | ‐2.50 [‐6.83, 1.83] |
| 4 UPDRS ‐ Motor | 12 | 593 | Mean Difference (IV, Fixed, 95% CI) | ‐5.01 [‐6.30, ‐3.72] |
| 4.1 General Physiotherapy v Control | 3 | 137 | Mean Difference (IV, Fixed, 95% CI) | ‐3.08 [‐5.24, ‐0.92] |
| 4.2 Exercise v Control | 2 | 148 | Mean Difference (IV, Fixed, 95% CI) | ‐10.02 [‐12.81, ‐7.23] |
| 4.3 Treadmill v Control | 2 | 38 | Mean Difference (IV, Fixed, 95% CI) | 0.05 [‐3.93, 4.03] |
| 4.4 Cueing v Control | 3 | 92 | Mean Difference (IV, Fixed, 95% CI) | ‐3.15 [‐6.68, 0.37] |
| 4.5 Dance v Control | 2 | 100 | Mean Difference (IV, Fixed, 95% CI) | ‐8.48 [‐12.76, ‐4.19] |
| 4.6 Martial Arts v Control | 2 | 78 | Mean Difference (IV, Fixed, 95% CI) | ‐5.82 [‐9.79, ‐1.85] |
4.1. Analysis.
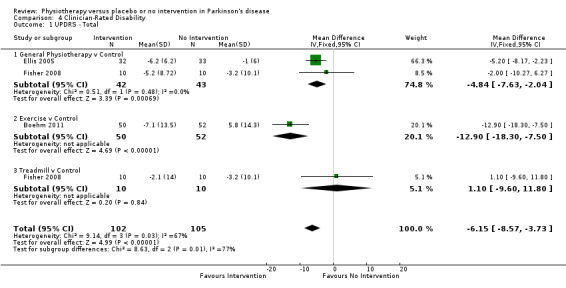
Comparison 4 Clinician‐Rated Disability, Outcome 1 UPDRS ‐ Total.
4.2. Analysis.
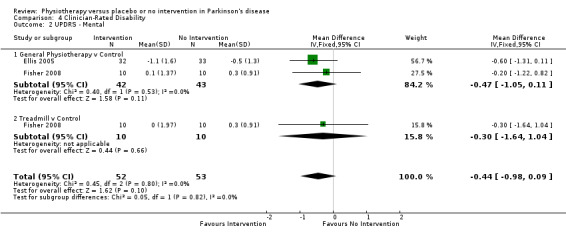
Comparison 4 Clinician‐Rated Disability, Outcome 2 UPDRS ‐ Mental.
4.3. Analysis.
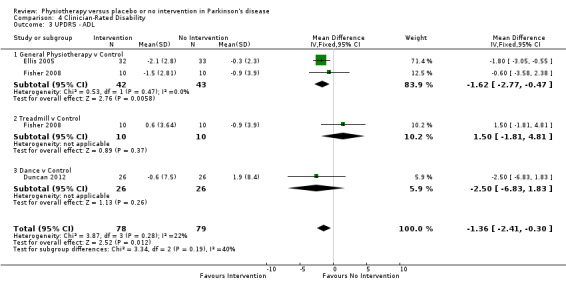
Comparison 4 Clinician‐Rated Disability, Outcome 3 UPDRS ‐ ADL.
4.4. Analysis.

Comparison 4 Clinician‐Rated Disability, Outcome 4 UPDRS ‐ Motor.
Comparison 5. Patient‐Rated Quality of Life.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 PDQ‐39 Summary Index | 7 | 405 | Mean Difference (IV, Fixed, 95% CI) | ‐0.38 [‐2.58, 1.81] |
| 1.1 General Physiotherapy v Control | 1 | 52 | Mean Difference (IV, Fixed, 95% CI) | 0.68 [‐6.84, 8.20] |
| 1.2 Exercise v Control | 3 | 104 | Mean Difference (IV, Fixed, 95% CI) | 0.32 [‐3.83, 4.48] |
| 1.3 Treadmill v Control | 1 | 18 | Mean Difference (IV, Fixed, 95% CI) | ‐0.7 [‐7.69, 6.29] |
| 1.4 Cueing v Control | 1 | 153 | Mean Difference (IV, Fixed, 95% CI) | ‐1.58 [‐5.45, 2.29] |
| 1.5 Dance v Control | 1 | 48 | Mean Difference (IV, Fixed, 95% CI) | ‐2.34 [‐8.83, 4.15] |
| 1.6 Martial Arts v Control | 1 | 30 | Mean Difference (IV, Fixed, 95% CI) | 3.05 [‐3.81, 9.91] |
| 2 PDQ‐39 Mobility | 2 | 105 | Mean Difference (IV, Fixed, 95% CI) | ‐1.43 [‐8.03, 5.18] |
| 2.1 General Physiotherapy v Control | 1 | 27 | Mean Difference (IV, Fixed, 95% CI) | 6.23 [‐3.85, 16.31] |
| 2.2 Dance v Control | 1 | 48 | Mean Difference (IV, Fixed, 95% CI) | ‐10.41 [‐22.50, 1.68] |
| 2.3 Martial Arts v Control | 1 | 30 | Mean Difference (IV, Fixed, 95% CI) | ‐3.65 [‐16.30, 9.00] |
5.1. Analysis.
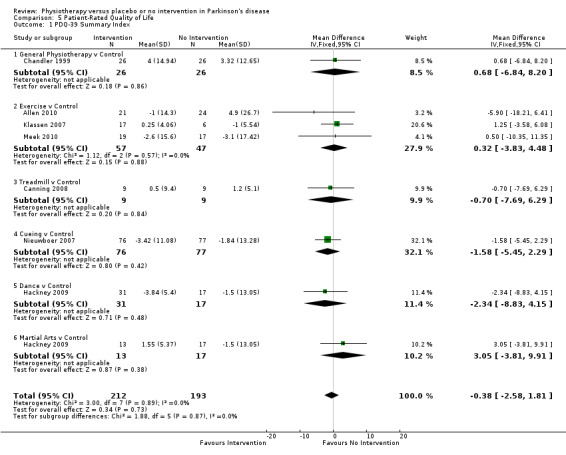
Comparison 5 Patient‐Rated Quality of Life, Outcome 1 PDQ‐39 Summary Index.
5.2. Analysis.
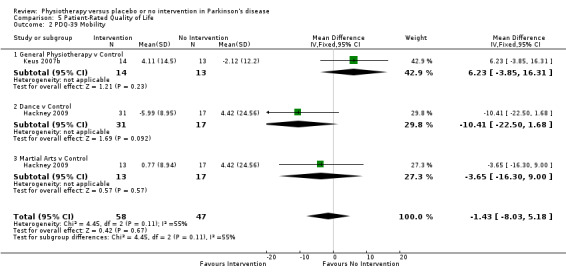
Comparison 5 Patient‐Rated Quality of Life, Outcome 2 PDQ‐39 Mobility.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Allen 2010.
| Methods | Parallel group design. Randomised using a randomisation schedule with randomly permuted block sizes, developed by an investigator not involved in subject recruitment or assessment. Data analysed on an intention‐to‐treat basis. Treated as outpatients and at home for 48‐72 hours over 6 months. Assessed at baseline and post intervention. Assessors were blinded. |
|
| Participants | 24 participants in the exercise group and 24 in the control group. 3 drop‐outs in the exercise group. Participants' mean age was 66 years (exercise) and 68 years (control); male/female 13/11 (exercise) and 13/11 (control); duration of PD 7 years (exercise) and 9 years (control). Hoehn and Yahr stage not reported. Inclusion criteria: diagnosis of idiopathic Parkinson's disease, able to walk independently (with or without an aid), fallen in the last year or deemed to be at risk of falling, 30‐80 years of age, and on the same PD medication for the past 2 weeks. Exclusion criteria: significant cognitive impairment (Mini Mental State Examination [MMSE] <24), had another neurological/musculoskeletal/cardiopulmonary/metabolic condition that would interfere with safe conduct of the training or testing protocol. |
|
| Interventions | Exercise: 40‐ to 60‐minute program of progressive lower limb strengthening and balance exercises (targeted leg muscle strength, balance, and freezing). Once‐monthly exercise classes, with the remaining exercise sessions at home. Control: usual care with advice on fall prevention and falls diary recording any fall. Drug therapy was allowed to vary. |
|
| Outcomes | PD falls risk score. Knee extensor strength. Coordinated stability. Sway. Maximum balance range in standing. Alternate step test. Freezing of Gait Questionnaire. Sit‐to‐stand time. Fast walking speed. Comfortable walking speed. Short physical performance battery. Falls Efficacy Scale - International. PDQ‐39. Participants were assessed in their home about 1 hour after taking their usual PD medication, and the order of measurements was standardised. |
|
| Notes | Participants in the exercise group who experienced freezing of gait were also instructed in cueing strategies to reduce freezing as part of their exercise program. Exercise group completed a mean of 70% of total prescribed exercise sessions. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Eligibility Criteria | Low risk | |
| Randomisation Method | Low risk | Randomly permuted block size. |
| Concealment of Allocation | Unclear risk | No information provided. |
| Similarity at Baseline | Low risk | |
| Withdrawals Described | Low risk | 6% overall, but all from exercise group. |
| Intention To Treat Analysis | Low risk | An intention‐to‐treat approach was used for all analyses. |
| Cointerventions Constant | Unclear risk | Allowed variation in levodopa therapy. |
| Credible Placebo | Low risk | Falls prevention advice given in both arms. |
| Blinded Assessors | Low risk | Assessors were blinded to group allocation. |
Almeida 2012.
| Methods | Parallel group design. Randomised by pulling allocation out of a hat. Analysed on a per protocol basis. Treated as outpatients for 9 hours over 6 weeks. Assessed at baseline, 6 weeks, and 12 weeks. Assessors were blinded for UPDRS III evaluation. |
|
| Participants | 14 participants in the Overground walking group (OG), 14 in the Treadmill walking group (TM), and 14 in the control group (CL). 2 dropouts in TM group, 1 dropout in CL group. Participants' mean age 73.9 years (OG), 63.9 years (TM), and 67.4 years (CL); male/female 12/2 (OG), 8/6 (TM), and 11/3 (CL); Hoehn and Yahr stage not stated; duration of PD not stated. Inclusion criteria: confirmed as having clinically typical Parkinson's disease by at least one movement disorders neurologist. Exclusion criteria: past history of neurological conditions other than Parkinson's disease, orthopaedic or visual disturbances that severely impaired walking ability, unable to independently walk down an 8‐meter GAITRite carpet for a total of 10 trials. |
|
| Interventions | OG: walk down equally spaced transverse lines presented on a 16‐m carpet. The cues were white lines of tape. Participants asked to walk across the lines, turn, and continue back. Spacings were set at 8% greater than the initial step length of any of the groups (70 cm). 30‐Minute session with mandatory 2‐minute break every 8 minutes, additional rest allowed if necessary, but a total of 24 minutes of walking was required for a gait session to be considered complete. TM: Walk on a treadmill presented with equally distributed standardised transverse white lines. Spacings were set at 8% greater than the initial step length of any of the groups (70 cm). 30‐minute session with mandatory 2‐minute break every 8 minutes, additional rest allowed if necessary, but a total of 24 minutes of walking was required for this gait session to be considered complete. CL: instructed to continue their usual activities. Participants were optimally medicated at time of all training and testing sessions and remained on stable regimen throughout trial period. |
|
| Outcomes | Step length. UPDRS III. Timed up and go. Gait speed. Cadence. Double support time. Step time. Step‐to‐step variability, Step time variability. 30‐Ssecond chair stand. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Eligibility Criteria | Low risk | Eligibility criteria stated. |
| Randomisation Method | High risk | Allocation pulled out of hat. |
| Concealment of Allocation | High risk | Allocation pulled out of hat. |
| Similarity at Baseline | Low risk | |
| Withdrawals Described | Low risk | Withdrawals at less than 10%. |
| Intention To Treat Analysis | High risk | Analysed on a per protocol basis. |
| Cointerventions Constant | Low risk | Participants maintained stable drug regiment throughout trial period. |
| Blinded Assessors | Low risk | Assessors blind for UPDRS III evaluation only. (This is the only subjective outcome.) |
Ashburn 2007.
| Methods | Parallel group design. Stratified by NHS using blocks of size four. Random allocation by telephoning the medical statistics group at University of Southampton. Participants were informed of their allocation by telephone. Data analysed on an intention‐to‐treat basis. Treated as outpatients 7 times a week for a 6‐week period, for a total period of 42 hours. Assessed at baseline, 8 weeks, and 6 months. |
|
| Participants | 70 participants in the exercise group and 72 in the control group. 6 dropouts in the exercise group and 8 in the control group. Participants' mean age 72.7 years (exercise), 71.6 years (control); male/female 38/32 (exercise), 48/24 (control); Hoehn and Yahr stage 3.14 (exercise), 3.11 (control); duration of PD 7.7 years (exercise), 9 years (control). Inclusion criteria: confirmed diagnosis of Parkinson's disease, independently mobile, living at home in the community, experienced more than one fall in the previous 12 months, passed a screening for gross cognitive impairment (Mini‐Mental State). Exclusion criteria: unable to participate in assessments because of pain, acute medical condition, in receipt of or soon to receive treatment. |
|
| Interventions | Exercise: personalised home‐based exercise and strategy programme. After assessment, treatment goals were established with participants, and exercises from the exercise menu were taught. Participants were visited weekly at home by a physiotherapist for approximately 1 hour. 6 levels of exercise progression comprised muscle strengthening, range of movement, balance training, and walking. Strategies of falls prevention and movement initiation and compensation taught by physiotherapist. Participants were asked to complete the exercises daily for max of 1 hour and to keep a record. Phoned monthly to encourage exercises. Control: usual care, contact with local Parkinson's disease nurse. Drug therapy was not described. |
|
| Outcomes | Self‐reported falls diary. Functional reach. Timed up and go test. Chair stand test. Berg balance test. Euroqol‐5d, QoL thermometer. Self‐assessment Parkinson's disease disability scale. Tests were carried out midway between drug doses. |
|
| Notes | At 6 months, 34% in the control group were participating in extra rehabilitation compared with 25% in the exercise group. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Eligibility Criteria | Low risk | |
| Randomisation Method | Low risk | Block randomisation (block size 4). |
| Concealment of Allocation | Low risk | Telephone call to central office. |
| Similarity at Baseline | Low risk | |
| Withdrawals Described | Low risk | 6% at 8 weeks and 8% at 6 months. |
| Intention To Treat Analysis | Low risk | Analysis was on an intention‐to‐treat basis. |
| Cointerventions Constant | Unclear risk | Drug therapy was not described. |
| Credible Placebo | Low risk | Controls had contact with Parkinson's disease nurse. |
| Blinded Assessors | Unclear risk | Assessor remained blind to group allocation but reported being aware of the allocation of 18 exercise and 11 control participants at 8 weeks, and 25 exercise and 14 control participants at 6 months. |
Boehm 2011.
| Methods | Cross‐over design. Random allocation generated and implemented by trial coordinator. Analysed on a per protocol basis. Treated for 12 weeks. Assessed at baseline, 12 weeks, and 24 weeks. Assessors were blinded for UPDRS III evaluation. |
|
| Participants | 55 participants in sensory attention focused exercise group (SAFE) and 55 in control group . 5 dropouts in SAFE group, 3 dropouts in control group. Participants' mean age 67.4 years (SAFE), 65.8 years (control); male/female 28/22 (SAFE), 30/22 (control); Hoehn and Yahr stage not stated; duration of PD 5.4 years (SAFE), 5.2 years (control). Inclusion criteria: idiopathic Parkinson's disease diagnosed by neurologist or movement disorders specialist according to international clinical diagnosis criteria, able to commit to study guidelines for 24 weeks. Exclusion criteria: score lower than 76 on 3MS (extended MMSE). |
|
| Interventions | Sensory attention focused exercise. Control: no intervention. Drug therapy was not described. |
|
| Outcomes | UPDRS I, II, & III. Timed up and go. Step length. Step length variability. Gait speed. Grooved peg‐board. 30 second chair stand. |
|
| Notes | Abstract and unpublished data. Baseline characteristics do not include dropouts. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Eligibility Criteria | Low risk | Eligibility criteria stated. |
| Randomisation Method | Unclear risk | Randomisation method not clear. |
| Concealment of Allocation | Unclear risk | Randomisation method not clear. |
| Similarity at Baseline | Low risk | |
| Withdrawals Described | Low risk | Withdrawals at 7%. |
| Intention To Treat Analysis | Low risk | Analysed on a per protocol basis. |
| Cointerventions Constant | Low risk | Drug therapy constant. |
| Blinded Assessors | Low risk | Assessors were blinded for UPDRS III evaluation. |
Cakit 2007.
| Methods | Parallel group design. Method of randomisation not stated. Method of analysis not described. Treated as outpatients for an unspecified time over 8 weeks (30‐minute sessions). Assessed at baseline and immediately after treatment. Assessors were blinded. |
|
| Participants | 27 participants in the treadmill group and 27 in the control group. 6 dropouts in the treadmill group, 17 dropouts in the control group. No baseline characteristics given for dropouts. Participants' mean age 71.8 years; male/female 16/15. The Hoehn and Yahr scores were not given. The mean duration of PD was 5.6 years. Inclusion criteria: Parkinson's disease patients who fulfilled the UK Parkinson's Disease Society Brain Bank Criteria, were medically stable, were able to walk 10‐metre distance at least 3 times with or without assistive device, able to provide informed consent. Exclusion criteria: participants who had neurological conditions other than idiopathic Parkinson's disease, scored greater than 3 in Hoehn and Yahr, scored less than 20 in MMSE, postural hypotension, cardiovascular disorders, class C or D exercise risk by the American College of Sports Medicine (ACSM) criteria, musculoskeletal disorders, visual disturbance or vestibular dysfunction limiting locomotion or balance. |
|
| Interventions | Treadmill: 8‐Week exercise program using incremental speed‐dependent treadmill training. Programme comprised stretching, range of motion exercise, and treadmill training. The treadmill session lasted for 30 minutes and participants were observed during treadmill training by a physiatrist, who gave no assistance in the actual performance of the movements. Maximum tolerated walking speed was determined before the training session began. This speed then was halved and was used for a 5‐minute warm‐up period. After the warm‐up period, the belt speed was increased by increments of 0.6 km/h every 5 minutes. When the belt speed was increased to the highest speed at which the participant could walk safely and without stumbling, this maximum‐achieved belt speed was maintained for 5 minutes and then was followed by 0.6‐km/h decrements. The participant maintained the rest of the treadmill session at this speed for 15 minutes. Control: no intervention. Drug therapy was constant during the trial. |
|
| Outcomes | Berg Balance Test. Dynamic Gait Index. Falls Efficacy Scale. Walking distance on treadmill. Tolerated maximum speed on treadmill (km/h). Examinations took place when participants were in the 'on' phase of medication. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Eligibility Criteria | Low risk | |
| Randomisation Method | Unclear risk | Method of randomisation not stated. |
| Concealment of Allocation | Unclear risk | Method of randomisation not stated. |
| Similarity at Baseline | Unclear risk | Baseline data given overall, not split by treatment group. |
| Withdrawals Described | High risk | 43% withdrawals. |
| Intention To Treat Analysis | Unclear risk | Method of analysis not described. |
| Cointerventions Constant | Low risk | Drug therapy was constant during the trial. |
| Blinded Assessors | Low risk | Assessors were blinded to group allocation. |
Canning 2008.
| Methods | Parallel group design. Randomised using opaque envelopes pre‐prepared by an investigator and randomly allocated by staff member not involved in the trial. Data analysed on an intention‐to‐treat basis.. Treated at home 4 times a week for 6 weeks, for a total of 12 to 16 hours. Assessed at baseline, 6 and 12 weeks. Assessors were blinded. |
|
| Participants | 10 participants in the treadmill group and 10 participants in the control group. Dropouts 2 (treadmill), 1 (control). Participants' mean age 60.7 years (treadmill), 62.9 years (control); male/female 5/5 (treadmill), 6/4 (control); Hoehn and Yahr stage not stated; duration of PD 6.1 years (treadmill), 5.2 years (control). Inclusion criteria: clinical diagnosis of idiopathic Parkinson's disease, aged 30 to 80 years, able to walk unaided but with subjective disturbance of gait and/or a Unified Parkinson's Disease Rating Scale (UPDRS) gait sub score of 1 or 2, sedentary, defined as performing less than 2 hours/week of leisure‐time physical activity over the prior 3 months, have adapted to current anti‐Parkinsonian medication for at least 2 weeks, is cognitively intact, has no freezing 'on' medication, Hoehn and Yahr stage 1 or 2. Exclusion criteria: motor fluctuations or dyskinesias that are disabling, requiring the use of a walking aid; more than one fall in the past 12 months, Mini‐Mental State Examination score < 24, exhibit other neurological or musculoskeletal conditions affecting walking, chest pain at rest or during exercise in the past 3 months, or heart attack, angioplasty, or heart surgery in the last 6 months. |
|
| Interventions | Treadmill: 30‐ to 40‐minutes sessions included 5‐minute warm up and cool down, sit‐to‐stand exercise and stretch exercises followed by treadmill walking. The intensity of training progressed over 6 weeks. Cognitive and manual tasks introduced during walking from week 4. Verbal and visual cues also provided for encouragement. 7 sessions were supervised in the home by a physiotherapist. Other sessions were completed independently. Control: advised to maintain current activity levels. 17 participants taking LD ranging from 100 to 1200 mg. 10 were also being treated with DA and 2 were also taking COMTI. Three (2 control and 1 experimental) were taking no PD medication. |
|
| Outcomes | 6‐Minute walk test. UPDRS - motor examination. PDQ‐39. Walking automaticity, speed of walking 10 m while performing a concurrent (cognitive or cognitive + physical) task, as expressed as a percentage of the walking speed of walking 10 m without performing the concurrent task. Walking consistency determined as the coefficient of variation for stride time and stride length recorded during the 6‐minute walk test. 7‐pt Likert scale to assess fatigue. Examinations took place during 'on' periods. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Eligibility Criteria | Low risk | |
| Randomisation Method | High risk | Randomised using opaque envelopes pre‐prepared by an investigator. |
| Concealment of Allocation | High risk | Randomly allocated by staff member not involved in the trial. |
| Similarity at Baseline | Low risk | |
| Withdrawals Described | Low risk | 10% dropout/withdrawal rate at 6 weeks (primary endpoint). Increases to 15% dropout/withdrawal rate by 12 weeks. |
| Intention To Treat Analysis | Low risk | Data analysed on an intention‐to‐treat basis. |
| Cointerventions Constant | Unclear risk | Not stated whether any changes to medications occurred during trial period. |
| Blinded Assessors | Low risk | Assessors were blinded to group allocation. |
Cerri 1994.
| Methods | Parallel group design. Randomisation method was not stated. Method of analysis not described. Treated as outpatients for 15 hours over 3 weeks followed by a home exercise program for 2 months, then the cycle was repeated. (Total of 30 hours therapy.) Assessed at baseline and immediately after treatment. Not stated whether assessors were blinded. |
|
| Participants | 3 participants in the exercise group and 3 in the control group. Dropouts not described. Participants' were all aged between 58 and 68 years at Hoehn and Yahr stage 3 and 4. No data given for the sex of the participants. Inclusion criteria: Parkinson's disease, stage 3 and 4 of Hoehn and Yahr scale, treated with L‐dopa for longer than 4 years with incomplete control of rigidity and tremor. No exclusion criteria stated. |
|
| Interventions | Exercise: Individual. Physical exercise program with neuromuscular facilitation techniques to improve posture, inhibit rigidity, and 'conscientize' movements. Control: Untreated. Drug therapy was allowed to vary during trial. |
|
| Outcomes | Webster Disability Scale. Activity of daily living. L‐dopa reduction. Not stated when examinations took place. |
|
| Notes | Abstract only. No means and SDs available. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Eligibility Criteria | Low risk | |
| Randomisation Method | Unclear risk | Randomisation method was not stated. |
| Concealment of Allocation | Unclear risk | Randomisation method was not stated. |
| Similarity at Baseline | Unclear risk | Only information given was that all participants were aged between 58 and 68 years and had Hoehn and Yahr stage 3 and 4. |
| Withdrawals Described | Unclear risk | Dropouts not described. |
| Cointerventions Constant | Unclear risk | 2 participants in the intervention group reduced dose of L‐dopa to avoid side effects. Allowed variation in medication. |
| Blinded Assessors | Unclear risk | Not stated whether assessors were blinded. |
Chandler 1999.
| Methods | Parallel group design. Randomisation method was not stated. Method of analysis not described. Treated at home, where they were assessed by a physiotherapist 5 times over a 12‐month period. The amount of physiotherapy was variable and depended on the participant's needs. Assessed at baseline and during the duration of the trial (at 3, 6, 9, and 12 months) (see Outcomes). Assessors were not blinded. |
|
| Participants | 32 participants in the physiotherapy group and 35 in the control group. Dropouts 6 (physiotherapy), 9 (control). Participants' mean age 65 years (physiotherapy), 66 years (control). 31 males and 21 females completed the study; Hoehn and Yahr for 47 of the participants, 2.6. Inclusion criteria: Idiopathic Parkinson's disease, not receiving physiotherapy, no access (including self‐referral) to a physiotherapy review system. No exclusion criteria stated. |
|
| Interventions | Physiotherapy: individualised, based on holistic approach in which empowerment of participants and caregivers was a strong element. Aimed to enhance the performance of activities. Gait and balance exercises using verbal, auditory, and visual cues. Exercises to reduce stiffness, improve muscle tone, and increase trunk rotation. Advice on transfers. Education in use of walking aids, reorganisation of environment to reduce hazards and facilitate movement. Leisure pursuits and social contacts encouraged after strategies were adopted to facilitate these. Relaxation techniques (audio tapes and aromatherapy) to improve sleep patterns. Aimed at reducing pain with education in postural awareness, exercise, TENS, and acupuncture. Referral to other health professionals and social services for aids and appliances.
Control: untreated. Drug therapy could vary. |
|
| Outcomes | Functional Independence Measure* Nottingham extended Activities Daily Living*. UPDRS - motor subsection*. Timed walk*. 9‐Hole peg test*. SF‐36 +. PDQ‐39 +. *Baseline, 3, 6, 9, 12 months. +Baseline, 6, 12 months. Not stated when during day examinations took place. |
|
| Notes | Participants referred to other health professionals and social services during trial. Occupational therapy component to the physiotherapy. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Eligibility Criteria | Low risk | |
| Randomisation Method | Unclear risk | Randomisation method was not stated. |
| Concealment of Allocation | Unclear risk | Randomisation method was not stated. |
| Similarity at Baseline | Unclear risk | Only gave information for age split by treatment group. |
| Withdrawals Described | High risk | 22% withdrawals. |
| Intention To Treat Analysis | Unclear risk | Method of analysis not described. |
| Cointerventions Constant | Unclear risk | Drug therapy could vary. |
| Blinded Assessors | High risk | Assessors were not blinded. |
de Bruin 2010a.
| Methods | Parallel group design. Method of randomisation not stated. Method of analysis not described. Treated as outpatients 3 times per week for a 13‐week period. Assessed at baseline and immediately after treatment. Assessors were blinded. |
|
| Participants | 16 participants in the cueing group and 17 participants in the control group. Dropouts 4 (cueing), 3 (control). Participants' mean age 64.1 years (cueing), 67.0 years (control); male/female 6/5 (cueing), 5/6 (control); Hoehn and Yahr 2.3 (cueing), 2.1 (control); mean duration of PD 6.4 years (cueing), 4.5 years (control). No baseline characteristics were given for the dropout. Inclusion criteria: diagnosis of Parkinson's disease (United Kingdom Brain Bank Criteria), Hoehn and Yahr stage 2 to 3, stable medication regimen, independently mobile without the use of a walking aid, and intact hearing. Exclusion criteria: diagnosis of less than 1 year, undergone deep brain stimulation surgery, experience regular freezing episodes, unable to ambulate independently in the community, presence of neurological disorders or comorbidities likely to affect gait, scoring 24 or less on the MMSE and/or already listening to music. |
|
| Interventions | Cueing: Walking at a self‐selected pace for 30 minutes, 3 times per week whilst listening to a preloaded music battery on an MP3 player. The music battery was individualised for each participant matching music preferences and the cadence of their preferred walking speed. Control: Continued with their regular activities. Drug therapy was not described. |
|
| Outcomes | Speed. Stride time. Stride length. Cadence. Stride time variability. UPDRS (III) score. Examined on medications at the same time of day. |
|
| Notes | Compliance in the intervention group was good. 2 subjects in the music group took a 1 week break. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Eligibility Criteria | Low risk | |
| Randomisation Method | Unclear risk | Randomisation method was not stated. |
| Concealment of Allocation | Unclear risk | Randomisation method was not stated. |
| Similarity at Baseline | Low risk | |
| Withdrawals Described | High risk | 21% withdrawals. |
| Intention To Treat Analysis | Unclear risk | Method of analysis not described. |
| Cointerventions Constant | Unclear risk | Drug therapy was not described. |
| Blinded Assessors | Low risk | UPDRS evaluator was blinded to subject group assignment. |
de Bruin 2010b.
| Methods | Parallel group design. Method of randomisation was not stated. Method of analysis not described. Treated as outpatient 3 times per week for 13 weeks. Assessed at baseline and post intervention. Not stated whether assessors were blinded. |
|
| Participants | 8 participants in the cueing group and 5 participants in the control group. No dropouts described. No baseline characteristics reported. Inclusion criteria: Parkinson's disease. No exclusion criteria. |
|
| Interventions | Cueing: Walking 3 times per week while listening to an individual music playlist. Playlists closely matched each individual's music preferences and preferred cadence. Control: Continued with their regular activities. Drug therapy was not described. |
|
| Outcomes | Spatiotemporal parameters approach, crossing and recovery steps of obstacle crossing were evaluated using a GAITRite mat. Step speed. Step length. Not stated when during the day examinations took place. |
|
| Notes | Abstract, only P values reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Randomisation Method | Unclear risk | Randomisation method was not stated. |
| Concealment of Allocation | Unclear risk | Randomisation method was not stated. |
| Similarity at Baseline | Unclear risk | No baseline characteristics reported. |
| Withdrawals Described | Unclear risk | No drop‐outs described. |
| Intention To Treat Analysis | Unclear risk | Method of analysis not described. |
| Cointerventions Constant | Unclear risk | Drug therapy was not described. |
| Blinded Assessors | Unclear risk | Not stated whether assessors were blinded. |
Duncan 2012.
| Methods | Parallel group design. Online random number generator used to perform group allocations. Data analysed on an intention‐to‐treat basis. Treated as outpatients for 104 hours over 12 months. Assessed at baseline, 3 months, 6 months, and 12 months. Assessor was blinded. |
|
| Participants | 32 participants in the dance group and 30 participants in the control group were analysed. 6 dropouts in dance group, 4 in control group. Participants mean age 70.6 years (dance), 69.2 (control). Mean Hoehn and Yahr stage 2.6 (dance), 2.5 (control). Male/female ratio 19/13 (dance), 16/14 (control). Duration of condition 5.4 years (dance), 6.9 (control). Inclusion criteria: Clinically defined 'definite PD.' Exclusion criteria: serious medical condition, evidence of abnormality other than PD‐related changes on brain imaging (previously done for clinical evaluations), history or evidence of musculoskeletal problem. |
|
| Interventions | Dance: tango class for 1 hour, twice weekly. Participants danced both leader and follower roles, changed partners frequently, and learned new steps and/or integrated previously learned steps in new ways at each class throughout the 12 months. Control: prescribed no exercise and told to go about living as usual. |
|
| Outcomes | MDS‐UPDRS‐III (primary). MDS‐UPDRS‐II & I. MiniBESTest balance test. Freezing of gait questionnaire. 6‐minute walk test Gait speed. Nine‐hole peg test. Participants were assessed while off medication (12‐hour withdrawal). |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Eligibility Criteria | Low risk | Eligibility criteria stated. |
| Randomisation Method | Low risk | Online random number generator. |
| Concealment of Allocation | High risk | Assigned by principal investigator using online random number generator. |
| Similarity at Baseline | Low risk | Baseline characteristics similar between groups. |
| Withdrawals Described | High risk | 16% withdrawals. |
| Intention To Treat Analysis | Unclear risk | Last observation carried forward. |
| Cointerventions Constant | Low risk | 12‐hour withdrawal before assessment. |
| Blinded Assessors | Low risk | Assessor was blind to group allocation. |
Ellis 2005.
| Methods | Cross‐over design. Block randomisation procedure was used in which each sealed envelope contained four Group A assignments and four Group B assignments. This process continued until a total of 68 subjects were randomly allocated. Method of analysis not described. Treated as outpatients 2 times a week for 6 weeks for a total of 18 hours (1.5‐hour sessions). Assessed at baseline, immediately after 1st treatment. Immediately before 2nd treatment and 3 months after 2nd treatment. Assessors were blinded. |
|
| Participants | 35 participants in the physiotherapy group and 33 in the control group. 11 dropouts. Participants' mean age 64 years (physiotherapy), 63 years (control); male/female ratio, 25/10 (physiotherapy), 26/7 (placebo); mean Hoehn & Yahr 2.5 (physiotherapy), 2.4 (control). Inclusion criteria: stable medication usage, Hoehn & Yahr stage 2 or 3, at least 1 score of 2 or more for at least 1 limb for the tremor, rigidity, or bradykinesia item of the UPDRS, ability to walk independently, age 35 to 75 years, no severe cognitive impairment (MMSE ≥24), no other severe neurologic, cardiopulmonary, or orthopaedic disorders, not having participated in a physical therapy or rehabilitation program in the previous 2 months. No exclusion criteria stated. |
|
| Interventions | Physiotherapy: 1.5‐hour‐long physical therapy session consisting of stretching, functional training, gait training, auditory cueing, balance, recreational, and relaxation. Control: medical therapy only. It was not stated whether drug therapy was kept constant during the trial. |
|
| Outcomes | Sickness Impact Profile (SIP‐68). UPDRS (Sections I, II, III). Comfortable walking speed. Assessments were performed at the same time of day and in the same order. Assessments were performed in the 'on' state for subjects who experience motor fluctuations. |
|
| Notes | Of the 68 subjects, 50 attended all treatment sessions. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Eligibility Criteria | Low risk | |
| Randomisation Method | Low risk | Blocked randomisation (block size 8) with sealed envelopes. |
| Concealment of Allocation | High risk | Sealed envelopes, which contained 8 group allocations (4 per group). |
| Similarity at Baseline | Low risk | |
| Withdrawals Described | High risk | 16% at the end of the trial. |
| Intention To Treat Analysis | Unclear risk | Method of analysis not described. |
| Cointerventions Constant | Unclear risk | It was not stated whether drug therapy was kept constant during the trial. |
| Blinded Assessors | Low risk | Assessors were blind to group allocation. |
Fisher 2008.
| Methods | Parallel group design. Randomisation was done by the subjects with their eyes closed; they selected a card corresponding to one of the three groups. Method of analysis not described. Treated as outpatients for 24 sessions over 8 weeks for both treatment arms, 6 sessions over 8 weeks for control group. Assessed at baseline and immediately post treatment. Assessors were blinded. |
|
| Participants | 10 participants in the treadmill group, 10 participants in the physiotherapy group and 10 participants in the control arm. No dropouts described. Participants' mean age, 64.1 years (treadmill), 61.5 years (physiotherapy), 63.1 years (control). Male/female ratio, 6/4 (treadmill), 5/5 (physiotherapy), 8/2 (control). Mean Hoehn and Yahr 1.9 in all 3 groups. Mean duration of PD 1.2 years (treadmill), 0.7 years (physiotherapy), 1.5 years (control). Inclusion criteria: early‐stage Parkinson's disease, diagnosis of Parkinson's disease within 3 years of study participation, Hoehn and Yahr stage 1 or 2, 18 years or older, medical clearance from primary care physician to participate in exercise programme, ability to walk. Exclusion criteria: medical or physical screening examination showed a score of less than 24 on the MMSE, revealed physician‐determined major medical problems such as cardiac dysfunction that would interfere with participation; subjects had musculoskeletal impairments or excessive pain in any joint that could limit participation in an exercise programme, had insufficient endurance and stamina to participate in exercise 3 times per week for a 1‐hour session. |
|
| Interventions | Treadmill: Level of intensity was defined by MET. High‐intensity exercise greater than 3 METs. Body weight supported (BWS) treadmill training. Goal of each session was to reach and maintain a MET > 3. Exercise progressed by decreasing BWS (initially 10% of subject's body weight) and physical assistance, increasing the treadmill speed and time on the treadmill, with the end goal for each subject to walk on the treadmill continuously for 45 minutes within the MET range. Physiotherapy: less than 3 METs. This group was representative of general or traditional physical therapy. Each 45‐minute session was individualised and consisted of activities from 6 categories: (1) passive range of motion and stretching, (2) active range of motion, (3) balance activities, (4) gait, (5) resistance training, (6) practice of functional activities and transitional movements. Control: zero intensity group. Six 1‐hour education classes taken over an 8‐week period. Drug therapy was constant during the trial. |
|
| Outcomes | UPDRS (Total, I, II, and III subscores). Hoehn and Yahr. Functional assessments. Walking test. Sit‐to‐stand test. Transcranial magnetic stimulation. All subjects took their customary medications at the same time relative to each assessment. |
|
| Notes | Subjects were allowed to continue their customary exercise routines and filled out a daily exercise diary. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Eligibility Criteria | Low risk | |
| Randomisation Method | High risk | Subjects self‐selected a card with eyes closed. |
| Concealment of Allocation | High risk | Subjects self‐selected a card with eyes closed. |
| Similarity at Baseline | Low risk | |
| Withdrawals Described | Unclear risk | No dropouts described. |
| Intention To Treat Analysis | Unclear risk | Method of analysis not described. |
| Cointerventions Constant | Low risk | All medication kept stable during course of study. |
| Credible Placebo | Low risk | Education classes attended by controls. |
| Blinded Assessors | Low risk | Assessors were blinded to group allocation. |
Ganesan 2010.
| Methods | Parallel group design. Method of randomisation not stated. Method of analysis not described. Treated as outpatients for 8 hours over 4 weeks. Assessed at baseline, and at 2 and 4 weeks. Not stated whether assessors were blinded. |
|
| Participants | Total of 20 participants. No baseline characteristics were reported. Inclusion criteria: idiopathic Parkinson's disease, stable doses of dopaminomimetic drugs. No exclusion criteria. |
|
| Interventions | Treadmill: partial weight supported treadmill gait training with 20% unweighing for 30 minutes per day, 4 times per week. Control: did not receive any specific intervention. Drugs were stable at time of randomisation. |
|
| Outcomes | UPDRS. Dynamic posturography. Berg Balance Scale. Tinetti performance orientated mobility assessment. Tinetti balance score. Gait score. Participants were assessed in best 'ON' state. |
|
| Notes | Abstract - only P values reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Randomisation Method | Unclear risk | Randomisation method was not stated. |
| Concealment of Allocation | Unclear risk | Randomisation method was not stated. |
| Similarity at Baseline | Unclear risk | No baseline characteristics were reported. |
| Withdrawals Described | Unclear risk | No information provided (abstract only). |
| Intention To Treat Analysis | Unclear risk | Method of analysis not described. |
| Cointerventions Constant | Unclear risk | Only information provided was that drugs were stable at time of randomisation. |
| Blinded Assessors | Unclear risk | Not stated whether assessors were blinded. |
Goodwin 2009.
| Methods | Parallel group design. Telephone randomisation external to the research team with 1:1 allocation in geographical cohorts. Data were analysed on an intention‐to‐treat basis. Treated as outpatients for 10 hours over 10 weeks, then home exercise for 10 weeks. Assessed at baseline and at 20 and 30 weeks. Assessors were not blinded. |
|
| Participants | 64 participants in the exercise group and 66 in the control group. 7 dropouts in total. Participants' mean age 72.0 years (exercise), 70.1 years (control). Male female ratio, 39/25 (exercise), 35/31 (control). Mean Hoehn and Yahr 2.6 (exercise), 2.4 (control). Mean duration of PD 9.1 years (exercise), 8.2 years (control). Inclusion criteria: diagnosis of idiopathic Parkinson's disease according to the UK Parkinson's Disease Society Brain Bank Criteria, self‐reported history of two or more falls in the past year, able to mobilise independently with/without a walking aid, resident in Devon, willingness to be randomly assigned and provide written informed consent. Exclusion criteria: needed supervision or assistance from another person to mobilise indoors, significant comorbidity that affects ability or safety to exercise (e.g. unstable angina, unstable diabetes, significant postural hypotension, severe pain, significant dyskinesia), unable to follow verbal or written instructions in English. |
|
| Interventions | Exercise: 10 weeks of supervised group strength and balance training plus 10 weeks of unsupervised home exercises. Control: usual care. Drug therapy could vary. |
|
| Outcomes | Falls Incidence. Number of fallers/recurrent faller. Fall‐related injuries. Berg Balance Scale. Timed Up and Go. Fall Efficacy Scale - International. EQ‐5D. Household and recreational physical activity (Phone‐FITT). Not stated when examinations took place. |
|
| Notes | Additional information and data obtained from author. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Eligibility Criteria | Low risk | Eligibility criteria stated. |
| Randomisation Method | Low risk | Telephone randomisation external to the research team with 1:1 allocation in geographical cohorts. |
| Concealment of Allocation | Low risk | Telephone randomisation external to the research team with 1:1 allocation in geographical cohorts. |
| Similarity at Baseline | Low risk | Characteristics of two groups similar. |
| Withdrawals Described | Low risk | 5% withdrawals. |
| Intention To Treat Analysis | Low risk | Data were analysed on an intention‐to‐treat basis. |
| Cointerventions Constant | Unclear risk | Participants changed their medication as appropriate as part of usual care. |
| Credible Placebo | Unclear risk | Control group received usual care. |
| Blinded Assessors | High risk | Assessors were not blinded. |
Haase 2011.
| Methods | Parallel group design. Block randomisation method used. Analysed on a per protocol basis Treated for a single session including 3 minutes of treatment. Assessment intervals not stated. Assessors not blinded. |
|
| Participants | 8 participants in the Finger tapping (FT) group, 12 in the Arm swinging group (AS) group, 6 in the control group. 1 dropout from the FT group and 2 from the AS group. Participants' mean age, 67 years (FT), 65 years (AS), 65 years (control); male/female 5/2 (FT), 5/5 (AS), 2/4 (control). Mean Hoehn and Yahr and mean duration of PD not stated. No baseline characteristics were given for dropouts. Inclusion criteria: able to walk independently without assistive devices for at least 14 m at a time no more than 4 times, Hoehn & Yahr stage 0 to 2. Exclusion criteria: severe perceptual deficits, medical complications. |
|
| Interventions | Rhythmic finger tapping exercise: participants instructed to tap on a metal plate (while seated) to the beat of an external auditory cue from a metronome set to 120% pretest walking cadence, for three, 1‐minute intervals with 30 seconds of rest between intervals. Rhythmic arm swing exercise: participants instructed to swing their arms (while seated) to the beat from a metronome set to 120% pretest walking cadence, for three, 1‐minute intervals with 30 seconds of rest between intervals. Control: participants were instructed to remain seated for 4 minutes. Drug therapy not described. |
|
| Outcomes | 9‐Hole peg test. UPDRS III & IV. Berg Balance Scale. Speed. Stride length. Cadence. |
|
| Notes | Abstract only. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Eligibility Criteria | Low risk | Eligibility criteria stated. |
| Randomisation Method | Unclear risk | Method of generating blocks not stated. |
| Concealment of Allocation | Unclear risk | Method of randomisation not clear. |
| Similarity at Baseline | Unclear risk | Full baseline characteristics not stated. |
| Withdrawals Described | High risk | Dropouts 12%. |
| Intention To Treat Analysis | High risk | Per protocol analysis. |
| Cointerventions Constant | Unclear risk | Drug therapy not described. |
| Credible Placebo | Low risk | Control group received same time and attention as intervention groups. |
| Blinded Assessors | High risk | Assessors were not blinded. |
Hackney 2009.
| Methods | Parallel group design. Randomisation was conducted by one author by selecting 1 of the 4 groups from a hat. Method of analysis not described. Treated as outpatients for 20 hours within 13 weeks (1‐hour sessions). Assessed at baseline and within one week of completing 20 sessions. Assessors were blinded. |
|
| Participants | 19 participants in the tango group, 19 in the waltz/foxtrot group, 17 in the Tai Chi group, and 20 in the control group. 5, 2, 4 and 3 dropouts from the tango, waltz/foxtrot, Tai Chi, and control group respectively. Participants' mean age, 68.2 years (tango), 66.8 years (waltz/foxtrot), 64.9 years (Tai Chi), 66.5 years (control); male/female 11/3 (tango), 11/6 (waltz/foxtrot), 11/2 (Tai Chi), 12/5 (control). Mean Hoehn and Yahr 2.1 (tango), 2.0 (waltz/foxtrot), 2.0 (Tai Chi), and 2.2 (control). Mean duration of PD 6.9 years (tango), 9.2 years (waltz/foxtrot), 8.7 years (Tai Chi), 5.9 years (control). No baseline characteristics were given for dropouts. Inclusion criteria: Hoehn and Yahr stages 1‐3, at least 40 years of age, could stand for at least 30 minutes, walk independently 3 or more metres with or without assistive device, diagnosis of idiopathic Parkinson's disease using diagnostic criteria for clinically defined 'definite PD' based on published standards, participants demonstrated clear benefit from levodopa, cognitively intact. Exclusion criteria: history of neurological deficit other than Parkinson's disease, dementia, another measure of cognitive function, and a separate part of the study not reported where all participants were required to perform a subtraction task while walking (all completed with 85% accuracy), considered cognitively intact. |
|
| Interventions | Dance: experienced professional ballroom dancer taught progressive tango or waltz/foxtrot lessons for 1 hour twice weekly. Instructor equally versed in both dances attempted to give all students equal attention. Both genders spent equal time leading and following dance roles. All steps done in closed practice position where participants maintain contact through upper extremities and face one another. Martial arts: Received progressive lessons for 1 hour twice weekly on Tai Chi's first and second circles including 37 postures of the Yang Short Style of Cheng Manching from an experienced instructor. Control: No intervention. Drug therapy was kept constant during the trial. |
|
| Outcomes | PDQ‐39. UPDRS III. Berg Balance Scale. Timed Up and Go. 6‐Minute walk test. Freezing of gait questionnaire. Forward and backward gait. Gait speed. Stride length. Single support time. Exit questionnaire. Tandem Stance Test (TS). One‐Leg Stance test (OLS). Assessments took place at a standardised time, when participants were in the 'on' state. |
|
| Notes | 1 participant was excluded from the study as the result of medication change. Participants were instructed not to change their habitual exercise routines. Data taken from all three publications. The tango and waltz/foxtrot arms assessed were suitably similar and were therefore combined to give one comparison of dance. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Eligibility Criteria | Low risk | |
| Randomisation Method | High risk | Conducted by one author by selecting 1 of the 4 groups from a hat. |
| Concealment of Allocation | High risk | Conducted by one author by selecting 1 of the 4 groups from a hat. |
| Similarity at Baseline | Low risk | |
| Withdrawals Described | High risk | 19% withdrawals. |
| Intention To Treat Analysis | Unclear risk | Method of analysis not described. |
| Cointerventions Constant | Low risk | Drug therapy was kept constant during the trial. |
| Blinded Assessors | Low risk | Assessors were blinded to group allocation. |
Homann 1998.
| Methods | Parallel group design. Participant's names were put into alphabetical order and then randomised using computer‐generated random number tables. Method of analysis not described. Treated as outpatients for 14 'units' over 5 weeks. Assessed at baseline and immediately after treatment. Not stated whether assessors were blinded. |
|
| Participants | 8 participants in physiotherapy group and 7 in placebo group. No dropouts were described. No baseline characteristics available from abstract. Inclusion criteria: idiopathic Parkinson's disease according to UK Brain Bank diagnostic criteria. No exclusion criteria. |
|
| Interventions | Physiotherapy: individual Bobath program focusing on proprioceptive skills to improve posture and gait. Control: untreated. Drugs were stable for duration of therapy. |
|
| Outcomes | UPDRS. Axial symptoms. Stride length. Walk speed. Stride cadence. |
|
| Notes | Abstract and poster only. No numerical data available. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Eligibility Criteria | Low risk | |
| Randomisation Method | Low risk | Participant's names were put into alphabetical order and then they were randomly assigned using computer‐generated random number tables. |
| Concealment of Allocation | Low risk | Based on information above, assumed treatment allocation performed once all patients recruited. |
| Similarity at Baseline | Unclear risk | No baseline characteristics available from abstract. |
| Withdrawals Described | Unclear risk | No dropouts described. |
| Intention To Treat Analysis | Unclear risk | Method of analysis not described. |
| Cointerventions Constant | Low risk | Drugs were stable for duration of therapy. |
| Blinded Assessors | Unclear risk | Not stated whether assessors were blinded. |
Keus 2007b.
| Methods | Parallel group design. Randomised in blocks of four in order of enrolment. Independently assigned with concealed allocation. The data was analysed on an intention‐to‐treat analysis. Treated as outpatients for an unspecified time, once or twice weekly for 10 weeks. Assessed at baseline and immediately after treatment. Assessor was blinded. |
|
| Participants | 14 participants in the physiotherapy group and 13 in the control group. 1 dropout from the control group. Participants' median age, 65 years (physiotherapy), 71 years (control). Male:female ratio, 11/3 (physiotherapy), 11/2 (control). Mean Hoehn and Yahr 2.4 in both groups. Mean duration of PD 7 years (physiotherapy), 6 years (control). Inclusion criteria: idiopathic Parkinson's disease according to the UK Parkinson's Disease Society Brain Bank Criteria, stable reaction to anti‐Parkinsonian medication, at least one mobility‐related activity limitation within core areas of physiotherapy practice in Parkinson's disease (gait, balance, posture, and transfers) experienced by the participant as important. Exclusion criteria: Hoehn and Yahr stage 5 during the 'on' period, physiotherapy within 4 months before randomisation, severe comorbidity influencing mobility or life threatening (e.g. cancer), not motivated to participate in physiotherapy, severe cognitive impairment defined by an MMSE score ≤ 24, presence of psychiatric impairments. |
|
| Interventions | Physiotherapy: Once‐ or twice‐weekly individual physiotherapy sessions. Delivered by a physiotherapist trained in the use of evidence‐based practice guidelines. Interventions included Parkinson's disease-specific techniques such as cueing, cognitive movement strategies, and general techniques such as training of balance, leg strength, and physical fitness. The intervention targeted balance, transfers, posture, and gait, dependent on the participant's main complaint. Control: no physiotherapy. It was not stated whether drug therapy was kept constant during the trial. |
|
| Outcomes | Patient preference outcome scale. The Parkinson Activity Scale. Mobility domain of the Dutch validated version of the Parkinson's disease questionnaire. Assessments took place during the participants' subjectively best 'on' phase. |
|
| Notes | Most participants received six to thirteen sessions of physiotherapy in the nine‐week period. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Eligibility Criteria | Low risk | |
| Randomisation Method | Low risk | Block size of 4. |
| Concealment of Allocation | Unclear risk | Independently assigned with concealed allocation. |
| Similarity at Baseline | Low risk | |
| Withdrawals Described | Low risk | 1 dropout from control group (4%). |
| Intention To Treat Analysis | Low risk | Data were analysed according to intention‐to‐treat principles. |
| Cointerventions Constant | Unclear risk | It was not stated whether drug therapy was kept constant during the trial. |
| Blinded Assessors | Low risk | Assessor was blind to group allocation. |
Klassen 2007.
| Methods | Parallel group design. Randomisation method was not stated. Method of analysis not described. Treated as outpatients for 45 hours (exercise and education), 30 hours (exercise only) over 12 weeks. Assessed at baseline, immediately, and 3 months after treatment. Assessors were blinded. |
|
| Participants | 9 participants in the exercise and education group, 9 in the exercise group, and 8 in the control group. 1 dropout (exercise and education), 1 (exercise), 2 (control). Median age 62 years (exercise and education), 70 years (exercise), 66.5 years (control). Male/female ratio, 7/2 (exercise and education), 5/3 (exercise), 5/1 (control). Hoehn and Yahr 1.9 (exercise and education), 1.4 (exercise), 1.5 (control). Years since diagnosis 4 years (exercise and education), 3 years (exercise), 7 years (control). No baseline characteristics given for dropouts. Inclusion criteria: clinical diagnosis of Parkinson's disease, 40 to 80 years of age, Hoehn and Yahr stages 1 to 3. Exclusion criteria: medical conditions that limit physical activity, dementia, or significant cognitive impairment. MMSE < 20, depression or other psychiatric disorder. Beck Depression Inventory II score > 20, other neurological conditions. |
|
| Interventions | Exercise and education: 1 hour and 15 minutes weekly of education delivered by physiotherapist, occupational therapist, speech language therapist, dietician, clinical psychologist, and social worker. Education consisted of active learning methods, action plan development, and discussion to complete each session. Report and discussion of action plan success/barriers to success at beginning of each session. An hour and 15 minutes twice weekly sessions of exercise, which consist of warm‐up, cool‐down, flexibility, and strengthening exercises, posture and balance training, progressive aerobic training, and functional task training (e.g. sit‐to‐stand). Exercise: as above, an hour 15 minutes twice weekly. Control: no intervention. It was not stated whether drug therapy was kept constant during the trial. |
|
| Outcomes | PDQ‐8. Stanford self‐efficacy for managing chronic disease scale. North Western University Disability Scale. Schwab and England ADL Scale. Activities Balance Confidence Scale. Timed Up & Go. Not stated when examinations took place. |
|
| Notes | Abstract and presentation slides only. The education and exercise and exercise only arms assessed were suitably similar and were therefore combined to give one comparison of exercise. Average attendance of the education and exercise classes ranged from 79.4% to 85.5%. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Eligibility Criteria | Low risk | |
| Randomisation Method | Unclear risk | Randomisation method was not stated. |
| Concealment of Allocation | Unclear risk | Randomisation method was not stated. |
| Similarity at Baseline | Low risk | |
| Withdrawals Described | High risk | 15% withdrawals |
| Intention To Treat Analysis | Unclear risk | Method of analysis not described. |
| Cointerventions Constant | Unclear risk | It was not stated whether drug therapy was kept constant during the trial. |
| Blinded Assessors | Low risk | Assessors were blinded to group allocation. |
Kurtais 2008.
| Methods | Parallel group design. Method of randomisation not stated. Method of analysis not described. Treated as outpatients 3 times a week for 6 weeks for a total period of 12 hours (40‐minute sessions). Assessed at baseline and 7 weeks after baseline assessments. Assessors were blinded. |
|
| Participants | 13 participants in the treadmill group and 14 in the control group. 1 dropout in the treadmill group and 2 in the control group. No baseline characteristics given for dropouts. Participants' mean age 63.8 years (treadmill), 65.7 years (control); male/female 5/7 (treadmill), 7/5 (control); Hoehn and Yahr 2.5 (treadmill), 2.2 (placebo). Duration of PD 5.3 years (treadmill), 5.4 years (control). Inclusion criteria: stable antiparkinsonian medication, ability to walk independently, not participated in a rehabilitation program in the previous 3 months. Exclusion criteria: severe cognitive impairments or severe musculoskeletal, cardiopulmonary, neurological, or other system disorders. |
|
| Interventions | Treadmill: gait training on a treadmill 3 times a week, attaining 70% to 80% of maximal heart rate. Either speed or incline was gradually increased over time. Control: untreated. Drug therapy was stable during the trial. |
|
| Outcomes | 20‐m walking time. Timed U‐turn task. Turning around a chair. Climbing up and down a flight of stairs at participants' preferred speed. Standing on one foot. Standing up from an armless chair. Rate global physical status. Cardiopulmonary fitness levels. Examinations were done during the participants 'on' phase. |
|
| Notes | Both groups were taught exercises to maintain flexibility and range of motion. One patient from the treadmill group was excluded as the result of noncompliance. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Eligibility Criteria | Low risk | |
| Randomisation Method | Unclear risk | Randomisation method was not stated. |
| Concealment of Allocation | Unclear risk | Randomisation method was not stated. |
| Similarity at Baseline | Low risk | |
| Withdrawals Described | High risk | 11% withdrawals. |
| Intention To Treat Analysis | Unclear risk | Method of analysis not described. |
| Cointerventions Constant | Low risk | Drug therapy was stable during the trial. |
| Blinded Assessors | Low risk | Assessors were blinded to group allocation. |
Lehman 2005.
| Methods | Parallel group design. Method of randomisation not stated. Method of analysis not described. Treated as outpatients for 10 days over 2 weeks. Assessed at baseline, immediately after, 1 week after and 1 month after intervention. |
|
| Participants | 5 participants in the cueing group and 6 participants in the control group. No dropouts described. Participants' mean age, 78 years (cueing), 74 years (control). Male/female ratio, 4/1 (cueing), 4/2 (control). Mean Hoehn and Yahr not stated. Duration of PD 7 years (cueing), 6.1 years (control). Inclusion criteria: participants with gait impairment due to Parkinson's disease, early‐stage Parkinson's disease. Exclusion criteria: persons with other neurological and/or orthopaedic impairments who could not walk the distances required of the training program were excluded. |
|
| Interventions | Cueing: 10‐day training programme of walking 1800 feet per day with instructions to 'take long steps.' One trip down the 30‐foot pathway is a length. Each training set consisted of 20 lengths. Participants completed three training sets each day. Controls: no change in lifestyle or medication. Drug therapy was not described. |
|
| Outcomes | Step length. Speed. Cadence. Examinations took place at the same time each day. |
|
| Notes | Data on graphs - limited data. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Eligibility Criteria | Low risk | |
| Randomisation Method | Unclear risk | Randomisation method was not stated. |
| Concealment of Allocation | Unclear risk | Randomisation method was not stated. |
| Similarity at Baseline | Low risk | |
| Withdrawals Described | Unclear risk | No dropouts described. |
| Intention To Treat Analysis | Unclear risk | Method of analysis not described. |
| Cointerventions Constant | Unclear risk | Drug therapy was not described. |
| Blinded Assessors | Unclear risk | Not stated whether assessors were blinded. |
Mak 2008.
| Methods | Parallel group design. Participants randomly allocated to groups by drawing lots. Method of analysis not described. Treated as outpatients for 4 hours (audio‐visual), 6 hours (exercise) over 4 weeks. Assessed at baseline, at 2 weeks, immediately after, and 2 weeks after treatment had ended. Assessor was blinded. |
|
| Participants | 21 participants in the cueing group, 21 participants in the exercise group, and 18 in the control group. 2 dropouts from the cueing group, 2 from the exercise group, and 4 from the control group. No baseline characteristics given for dropouts. Participants' mean age 63 (cueing), 66 (exercise), 63 (control). No data given for the sex of participants. Hoehn and Yahr stage 2.8 (cueing), 2.7 (exercise), and 2.7 (control). Duration of PD 5.9 years (cueing), 6.1 years (exercise), 5.9 years (control). Inclusion criteria: diagnosed with Parkinson's disease according to Quinn, stable on anti‐Parkinson's disease medications without dyskinesia, orthopaedic, arthritic, or heart problems, aged between 50 and 75 years, perform sit‐to‐stand independently, can follow instructions. No exclusion criteria stated. |
|
| Interventions | Cueing: audiovisual cued task‐specific training for 20 minutes three times per week. Received cued sit‐to‐stand training using Equitest‐Balance Master. Visual cue was given on a computer screen with verbal command as auditory cue. Each task lasted 2 minutes, repeated once with 30‐second rests in between. Exercise: 45 minutes of conventional exercise twice a week. Conventional mobility and strengthening exercises for flexors and extensors of trunk, hips, knees, and ankles, followed by sit‐to‐stand practice. Control: no treatment. Drugs stable during therapy. |
|
| Outcomes | Peak horizontal speed (used in meta‐analysis). Peak vertical speed. Movement time. 3D kinematics data of sit‐to‐stand. Not stated when during the day tests took place. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Eligibility Criteria | Low risk | |
| Randomisation Method | High risk | Drawing lots. |
| Concealment of Allocation | High risk | Drawing lots. |
| Similarity at Baseline | Low risk | |
| Withdrawals Described | High risk | 13% withdrawals. |
| Intention To Treat Analysis | Unclear risk | Method of analysis not described. |
| Cointerventions Constant | Low risk | Drugs stable during therapy. |
| Blinded Assessors | Low risk | Assessor was blinded to group allocation. |
Marjama‐Lyons 2002.
| Methods | Parallel group design. Method of randomisation not stated. Method of analysis not described. Treated as outpatients for 24 hours over 12 weeks. Assessed at baseline and immediately after treatment. Assessors were blinded. |
|
| Participants | 30 participants. No dropouts were described. No baseline characteristics available. Inclusion criteria: levodopa‐responsive Parkinson's disease, Hoehn and Yahr stage 1.5 to 3. No exclusion criteria. |
|
| Interventions | Martial arts: two one‐hour weekly Tai Chi classes. Control: continued baseline exercise program and added no new exercises. Drug therapy was stable during the study. |
|
| Outcomes | UPDRS motor score (part III). Fall frequency form. Balance master Limits of Stability. Global Assessment of Change. Examinations took place when participants were in the 'on' state. |
|
| Notes | All subjects did not practice Tai Chi before entry. Abstract. No means and SDs, just P values available. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Eligibility Criteria | Low risk | |
| Randomisation Method | Unclear risk | Randomisation method was not stated. |
| Concealment of Allocation | Unclear risk | Randomisation method was not stated. |
| Similarity at Baseline | Unclear risk | No baseline characteristics provided. |
| Withdrawals Described | Unclear risk | Dropouts not described. |
| Intention To Treat Analysis | Unclear risk | Method of analysis not described. |
| Cointerventions Constant | Low risk | Drug therapy was stable during study. |
| Blinded Assessors | Low risk | Assessors were blinded to group allocation. |
Meek 2010.
| Methods | Parallel group design. Participants were randomly assigned using computer‐generated random block sizes of four. The data were analysed on an intention‐to‐treat basis. Treated as outpatients for 12 sessions over 12 weeks. Assessed at baseline and immediately after treatment, and at 6 months. Assessor was blinded. |
|
| Participants | 20 participants in the exercise group and 19 in the control group. 1 dropout in the control group. Participants mean age, 63.4 years (exercise), 64.9 years (control); male/female ratio 15/5 (exercise), 16/3 (control); mean duration of PD 5.1 years (exercise), 4.7 (control). Mean Hoehn and Yahr was not reported. Inclusion criteria: diagnosis of idiopathic Parkinson's disease, aged 18 years or over, no cognitive, sensory, or psychological impairments that may prevent engagement in participation in the study or that put the participant at risk (judged by the referring clinician), able to participate in the study for its full duration, able to walk 10 m using any aid or assistance required. Exclusion criteria: participants unable to meet inclusion criteria, or those unwilling to participate, participants with additional impairments resulting in restriction of mobility, or any contraindications to exercise. |
|
| Interventions | Exercise: collaborated with fitness instructors to design a 3‐month individualised, progressive exercise program. Control: received usual care. Drug therapy was allowed to change during the study. |
|
| Outcomes | Physical Activity Scale for the Elderly. Accelerometer monitored physical activity. 10‐m walk test. 2‐minute walk test. Lower limb muscle strength and grip strength. Fatigue severity scale. PDQ‐39. Falls. No constraints on timing of assessments. |
|
| Notes | Abstract and further information provided by author. Gym attendance during the pooled intervention periods was high overall, with a mean of 14.5 visits and a median of 12 visits. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Eligibility Criteria | Low risk | |
| Randomisation Method | Low risk | Computer‐generated random block sizes of four. |
| Concealment of Allocation | Low risk | Randomisation done centrally. |
| Similarity at Baseline | Low risk | |
| Withdrawals Described | Low risk | 1 dropout in the control group (3%). |
| Intention To Treat Analysis | Low risk | Intention‐to‐treat analysis. |
| Cointerventions Constant | Unclear risk | Drug therapy was allowed to change during the study. |
| Blinded Assessors | Low risk | Assessors were blinded to group allocation. |
Nieuwboer 2007.
| Methods | Cross‐over design. Participants were randomly allocated in permuted blocks of six to an early or late intervention group by an independent investigator not involved in data analysis. Allocation was concealed by the use of opaque sealed envelopes. The data were analysed on an intention‐to‐treat basis. Treated at home for 4.5 hours over 3 weeks. Assessed at baseline, immediately after 1st and 2nd treatment, and at 12 weeks. Assessors were blinded. |
|
| Participants | 76 participants in the cueing group and 77 in the control group. 1 dropout in the cueing group. Participants' mean age, 66.9 years (cueing), 67.2 years (control). Male/female ratio 48/28 (cueing), 40/37 (control). Hoehn and Yahr 2.7 (cueing), 2.8 (control). Mean duration of PD 7 years (cueing), 8 years (control). Inclusion criteria: diagnosis of idiopathic Parkinson's disease (defined by the UK Brain Bank Criteria), Hoehn and Yahr stage 2 to 4, showing mild to severe gait disturbance with score > 1 on the UPDRS item 29, stable drug usage, age 18 to 80 years. Exclusion criteria: undergone deep brain stimulation or stereotactic neurosurgery, had cognitive impairment (MMSE < 24), had disorders interfering with participation in cueing training, including neurological (stroke, multiple sclerosis, tumour), cardiopulmonary (chronic obstructive disorders, angina pectoris), and orthopaedic (osteoarthritis, rheumatoid arthritis, and back pain) conditions, had predictable and long‐lasting off periods (score 1 on item 37 and score > 2 on item 39 on UPDRS). Had participated in a physiotherapy programme 2 months before starting the trial. |
|
| Interventions | Cueing: cueing programme delivered at home over 3 weeks by 1 therapist in 9 sessions lasting 30 minutes. A prototype cueing device specifically developed for the study provided 3 rhythmical cueing modalities: 1. auditory (a beep delivered through an ear piece), 2. visual (light flashes delivered through a light‐emitting diode attached to a pair of glasses), 3. somatosensory (pulsed vibrations delivered by a miniature cylinder worn under a wristband). Participants tried all cueing modalities in the first week, but trained with their preferred modality. Cued practice was applied during a variety of tasks and aimed to improve step length and walking speed, prevent freezing episodes, and improve balance. Control: no training. Drug therapy was kept constant throughout the trial. |
|
| Outcomes | Posture and gait score. Gait and balance measures (including 10‐m test of walking, gait speed, step length, step frequency, functional reach, timed single leg and tandem stance, Freezing of Gait Questionnaire, Timed Up and Go Test. Activity measures (including Nottingham Extended Activities of Daily Living Index, Falls Efficacy Scale). Participation measures (including Parkinson's Disease Questionnaire‐39, Carer Strain Index). Falls diary. Assessments were performed at the same time of day when participant was in the 'on' phase approximately 1 hour after drug intake. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Eligibility Criteria | Low risk | |
| Randomisation Method | Low risk | Permuted block size of 6. |
| Concealment of Allocation | High risk | Sealed envelopes. |
| Similarity at Baseline | Low risk | |
| Withdrawals Described | Low risk | 1 dropout in the cueing group (< 1%). |
| Intention To Treat Analysis | Low risk | The data were analysed on an intention‐to‐treat basis. |
| Cointerventions Constant | Low risk | All medication remained constant. |
| Blinded Assessors | Low risk | Assessors were blinded to group allocation. |
Protas 2005.
| Methods | Parallel group design. Method of randomisation not stated. Method of data analysis not described. Treated as outpatients for 24 hours over 8 weeks. Assessed at baseline and immediately after treatment. Assessors were blinded. |
|
| Participants | 9 participants in both groups. No dropouts described. Participants' mean age 71.3 years (treadmill), 73.7 years (control); male/female all male subjects for both groups. Mean Hoehn and Yahr 2.8 (treadmill), 2.9 (control). Duration of PD 7.1 years (treadmill), 8.1 years (control). Inclusion criteria: idiopathic Parkinson's disease, postural instability‐gait difficulty predominant Parkinson's disease, experiences with freezing episodes, and/or history of falls, stable regimen of antiparkinsonian medications, ability to stand and walk with or without assistance, stage 2 or 3 Hoehn and Yahr, scores of moderate or higher on all scales on the Neurobehavioural Cognitive Status Examination (Cognistat). No exclusion criteria. |
|
| Interventions | Treadmill: gait and step training 3 times per week. Using a harness for safety, the participant walks forward on a treadmill at fastest speed for 5 to 7 minutes, backwards at fastest self‐selected speed for 5 to 7 minutes. Then left and right sideways walking at fastest selected speed for 2 to 3 minutes each way. Participants then had 5 minutes of rest before starting step training, which consisted of turning on the treadmill suddenly to perturb the participant's standing balance (15‐20 forward and backward perturbations, 10‐15 left and right perturbations). Control: no intervention. Drug therapy was stable throughout the trial. |
|
| Outcomes | Gait speed. Cadence. Stride length. 5‐Step test. Reports of falls. Freezing of gait. Assessments took place when participants were at their best 'on' state. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Eligibility Criteria | Low risk | |
| Randomisation Method | Unclear risk | Randomisation method was not stated. |
| Concealment of Allocation | Unclear risk | Randomisation method was not stated. |
| Similarity at Baseline | Low risk | |
| Withdrawals Described | Unclear risk | No dropouts described. |
| Intention To Treat Analysis | Unclear risk | Method of data analysis not described. |
| Cointerventions Constant | Low risk | Drug therapy was stable throughout the trial. |
| Blinded Assessors | Low risk | Assessors were blinded to group allocation. |
Purchas 2007.
| Methods | Cross‐over design. Method of randomisation not stated. Method of data analysis not described. 1 session per week for a total of 12 weeks. Assessed at baseline and immediately after 1st and 2nd treatments. Not stated whether the assessors were blinded. |
|
| Participants | 10 participants in the martial arts group and 10 participants in the control group. One dropout from both groups. Mean age of participants 70 years in both groups. Male/female ratio, 7/2 (martial arts), 4/5 (control). Mean Hoehn and Yahr 2 (martial arts), 2.3 (control). No baseline characteristics given for dropouts. Inclusion criteria: maintenance phase of Parkinson's disease. No exclusion criteria. |
|
| Interventions | Martial arts: 1‐hour weekly Tai Chi training. Control: no treatment. Drug therapy not described. |
|
| Outcomes | Timed Up and Go Test. PDQ‐39. UPDRS. Hoehn and Yahr stage. Falls diary. Not stated when during the day examinations took place. |
|
| Notes | Abstract and poster only. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Eligibility Criteria | Unclear risk | Maintenance phase of Parkinson's disease. |
| Randomisation Method | Unclear risk | Randomisation method was not stated. |
| Concealment of Allocation | Unclear risk | Randomisation method was not stated. |
| Similarity at Baseline | Low risk | |
| Withdrawals Described | Low risk | 10% dropout |
| Intention To Treat Analysis | Unclear risk | Method of data analysis not described. |
| Cointerventions Constant | Unclear risk | Drug therapy was not described. |
| Blinded Assessors | Unclear risk | Not stated whether assessors were blinded. |
Sage 2009a.
| Methods | Parallel group design. Method of randomisation not stated. Data analysed on an intention‐to‐treat basis. Treated as outpatients for 18 hours (exercise), 20 to 24 hours (PDSAFEx) over 10 to 12 weeks. Assessed at baseline and after treatment. Assessors were blinded. |
|
| Participants | 17 participants in the exercise group, 21 participants in the PDSAFEx group, and 15 in control. 4 dropouts (exercise) and 3 dropouts (PDSAFEx). Participants' mean age 65.1 years (exercise), 64.2 years (PDSAFEx), 68.6 years (control). Male/female ratio, 6/7 (exercise), 12/6 (PDSAFEx), 7/8 (control). Hoehn and Yahr score not given. Duration of PD 3.2 years (exercise), 4.7 years (PDSAFEx), and 2.5 years (control). No baseline characteristics given for dropouts. Inclusion criteria: diagnosis of idiopathic Parkinson's disease with no other major medical, physiological, or neurological problem, a stable medication schedule, mild to moderate Parkinson's disease defined as a score of less than 35 on UPDRS motor section. |
|
| Interventions | Exercise: lower limb aerobic training, exercise for 30 minutes (5‐min warm‐up, 20‐min aerobic training, 5‐min cool‐down) three times a week in groups of 4 on Biostep semi‐recumbent elliptical's in the seated position. The machine was primarily leg driven with arms moving in a coordinated pattern. Intensity maintained by achieving a pace of 50 RPM, a heart rate of 60% to 75% of age‐related max, and a Borg rate of perceived exertion of below 5. PDSAFEx: sensory attention focused exercise for 40 to 60 minutes three times a week. 20 to 30 minutes of nonaerobic gait exercises focused on body coordination, followed by 20 to 30 minutes of sensory attention exercises using latex Thera‐bands attached to arm rests of office chairs. Exercises were completed with eyes closed and cued to the sensory feedback from specific portions of each exercise. Examples of exercises, tandem walking for balance and coordination, side stretches down side of chair for sensory feedback. Control: Nonexercise control group, maintained regular activity level. Drug therapy remained unchanged during the trial. |
|
| Outcomes | UPDRS III. Timed Up and Go. Spatiotemporal aspects of gait. Assessments took place when participants were at 'peak' dose (approximately 90 min after administration). |
|
| Notes | 3‐arm trial. The PD SAFEx and exercise arms assessed were suitably similar and were therefore combined to give one comparison of exercise. Both exercise groups attended an equivalent number of training sessions, overall 90%. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Eligibility Criteria | Low risk | |
| Randomisation Method | Unclear risk | Randomisation method was not stated. |
| Concealment of Allocation | Unclear risk | Randomisation method was not stated. |
| Similarity at Baseline | Low risk | |
| Withdrawals Described | High risk | 13% withdrawals. |
| Intention To Treat Analysis | Low risk | Statisicial analysis was done using intention‐to‐treat principles. |
| Cointerventions Constant | Low risk | Drug therapy remained unchanged during the trial. |
| Blinded Assessors | Low risk | Assessors were blinded to group allocation. |
Schenkman 1998.
| Methods | Parallel group design. Participants were stratified according to gender and then randomly assigned using computer‐generated assignment. Randomisation schedule kept in office of statistician until participants were assigned. Method of data analysis not described. Treated as outpatients for 30 hours over 10 to 13 weeks. Assessed at baseline and immediately after treatment. Assessors were blinded. |
|
| Participants | 27 participants in exercise group, 24 participants in control group. 4 dropouts from exercise group, 1 from control group. No baseline characteristics given for dropouts. Participants mean age 70.6 years (exercise), 71.2 years (control); male/female 18/5 (exercise), 16/7 (control); Hoehn and Yahr 2.6 (exercise), 2.7 (control). Inclusion criteria: Parkinson's disease as diagnosed by a neurologist, Hoehn and Yahr stage 2 or 3, functional axial rotation of 120 degrees or less to either side. Exclusion criteria: hospitalised within past 3 months, PD drugs changed in last month, other neurological disorders, Folstein MMSE < 23. |
|
| Interventions | Exercise: individual exercises to improve spinal flexibility and coordinated movement. Standardised programme included a series of exercises divided into 7 graduated stages, from supine to standing. Exercises learned at each stage are continued throughout with progressively higher level activities added. Exercises are incorporated into daily routine at end of formal training sessions. Control: no treatment. ('Wait listed' for exercise programme). Drug therapy constant during trial. |
|
| Outcomes | Functional axial rotation. Functional reach. Timed tests. Timed walk. Cervical and lumbar range of motion. Walking speed. Participants with fluctuations assessed during 'on' time. |
|
| Notes | Abstract, further information obtained from author. All 46 participants completed 30 treatment sessions within their allotted time. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Eligibility Criteria | Low risk | |
| Randomisation Method | Low risk | Computer‐generated assignment. |
| Concealment of Allocation | Low risk | Randomisation schedule kept in office of statistician until participants were assigned. |
| Similarity at Baseline | Low risk | |
| Withdrawals Described | Low risk | 10% withdrawals. |
| Intention To Treat Analysis | Unclear risk | Method of data analysis not described. |
| Cointerventions Constant | Low risk | Drug therapy was kept constant during the trial. |
| Blinded Assessors | Low risk | Assessors were blinded to group allocation. |
Schilling 2008.
| Methods | Parallel group design. Participants were gender matched, then randomly assigned. Method of randomisation not stated. Method of data analysis not described. Treated as outpatients for 16 sessions of an unspecified time over 8 weeks. Assessed at baseline and immediately after treatment. |
|
| Participants | 9 participants in the exercise group and 9 participants in the control group. 1 dropout form the exercise group, 2 dropouts from the control group. Participants' mean age 61.3 years (exercise), 57 years (control); male/female 5/4 (exercise), 6/3 (control); Hoehn and Yahr 2.1 (exercise), 1.9 (control). Inclusion criteria: mild to moderate Parkinson's disease, Hoehn and Yahr stage 1 to 2.5, ability to walk a 20‐foot path, turn, and return to the start without use of assistive device. Exclusion criteria: orthostatic hypotension, dementia (MMSE < 24), other significant comorbidities (i.e. stroke, severe degenerative osteoarthritis), other causes of Parkinsonism such as PSP, vascular PD, and multiple‐system atrophy as determined by board‐certified neurologist. |
|
| Interventions | Exercise: moderate volume, high‐load lower‐body resistance training twice weekly. After a warm‐up, participants performed three sets of 5 to 8 repetitions for the leg press, seated leg curl, and calf press under direct supervision of a Certified Strength and Conditioning Specialist. Subjects were instructed to lift the weight as fast as possible with good form and to slowly return the weight to the start position. Progression was planned so that when eight repetitions could be completed for all the sets, the weight was increased by 5% to 10%. Control: Continue standard care. Drug therapy was not described. |
|
| Outcomes | Maximum strength for the lower body. Activities‐specific balance confidence. Timed Up and Go. 6‐minute walk test. All testing done when participants were in their optimally medicated state, typically within 30 minutes to 2 hours of their first morning dose. |
|
| Notes | Control group given the opportunity to complete the training intervention after the 8‐week control period. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Eligibility Criteria | Low risk | |
| Randomisation Method | Unclear risk | Method of randomisation not stated. |
| Concealment of Allocation | Unclear risk | Method of randomisation not stated. |
| Similarity at Baseline | Low risk | |
| Withdrawals Described | High risk | 17% withdrawals. |
| Intention To Treat Analysis | Unclear risk | Method of data analysis not described. |
| Cointerventions Constant | Unclear risk | Drug therapy was not described. |
| Blinded Assessors | Unclear risk | Not stated whether assessors were blinded. |
Schmitz‐Hubsch 2006.
| Methods | Parallel group design. Participants were sorted randomly, matched for disease severity, presence or absence of dyskinesia, and type of clinical manifestation. Randomisation was carried out using a list of pseudonyms generated by one investigator and transferred by fax to a 2nd investigator. The data were analysed on an intention‐to‐treat basis. Treated as outpatients for 8 hours over 8 weeks, then for 0 hours for 8 weeks, then 8 hours for 8 weeks. Total of 16 hours over 24 weeks. Assessed at baseline, 3, 6, and 12 months. Not stated whether assessors were blinded. |
|
| Participants | 32 participants in the martial arts group and 24 in the control group. 2 dropouts in the martial arts group and 5 in the control group. Participants' mean age, 64 years (martial arts), 63 years (control); male/female 24/8 (martial arts), 19/5 (control). Hoehn and Yahr score not given. Duration of PD 6 years (martial arts) and 5.6 years (control). Inclusion criteria: participants diagnosed with Parkinson's disease according to the UK Brain Bank Criteria at any stage of the disease with or without motor complications, MMSE > 24. Exclusion criteria: previous practical experience with Qigong, recent (< 1 month), or planned change of medication, signs of central nervous system disease other than Parkinson's disease (e.g. aphasia, dementia) (defined by MMSE < 24). |
|
| Interventions | Martial arts: 1‐hour weekly group lesson of Qigong delivered by an experienced teacher. Exercises were carried out standing or in the sitting position adjusted to participants' physical abilities. Teacher repeatedly stressed importance of home self‐exercise. Control: no intervention. Drug therapy varied throughout the trial. |
|
| Outcomes | UPDRS III. PDQ‐39. Montogmery‐Asperg Depression Rating Scale. Nonmotor symptoms. Self‐reporting questionnaire. Assessments were carried out when participants were in the 'on' state (time of optimal medication effect as defined by the participant). Follow‐up assessments were done at similar times of the day. |
|
| Notes | Participants were asked not to change their medication during the study, but if their medical condition required adaptations, this would not lead to exclusion. Compliance at one year follow‐up was fair. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Eligibility Criteria | Low risk | |
| Randomisation Method | High risk | List of pseudonyms. |
| Concealment of Allocation | High risk | Randomisation was carried out using a list of pseudonyms generated by one investigator and transferred by fax to a 2nd investigator. |
| Similarity at Baseline | Low risk | |
| Withdrawals Described | High risk | 13% withdrawals. |
| Intention To Treat Analysis | Low risk | All analyses were carried out on an intention‐to‐treat‐basis. |
| Cointerventions Constant | Unclear risk | Drug therapy varied throughout the trial. |
| Blinded Assessors | Unclear risk | Not stated whether assessors were blinded. |
Shankar 2008.
| Methods | Parallel group design. Random allocation using computer‐generated random list. Method of analysis not described. Treated as an outpatient for 36 hours over 3 months. Assessed at baseline and immediately after treatment. Assessors were blinded. |
|
| Participants | 14 participants in the cueing group and 14 in the control group. No dropouts described. Participants' mean age, 70 years (cueing), 62 years (control); male/female 6/8 (cueing), 8/6 (control), mean Hoehn and Yahr score 2.4 (cueing), 2.3 (control). Duration of PD 7.5 years (cueing), 7.9 years (control). Inclusion criteria: diagnosis of idiopathic Parkinson's disease as per UK Brain Bank criteria, Hoehn & Yahr disease stages 2 and 3, stable Parkinson's disease medication for 1 month before baseline visit, ability to walk with headphones unaided for 30 minutes three times per week, absence of pre‐existing walking to music. Exclusion criteria: presence of dementia (MMSE < 26), presence of comorbidities that affect the ability to walk, hearing deficits. |
|
| Interventions | Cueing: walking for 30 minutes three times per week while listening to a battery of musical pieces. Music was self‐selected based on participant input and was cadence‐matched to the participant's ideal walking speed. Control: maintained their normal walking activity. Minor medication changes allowed, as deemed appropriate by team neurologist. |
|
| Outcomes | Gait and Balance Scale. UPDRS III. Adjusted PDQ‐39. Activities‐Specific Balance Confidence Scale. Not stated when examinations took place. |
|
| Notes | Abstract. Information on trial quality and data obtained from author. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Eligibility Criteria | Low risk | |
| Randomisation Method | Low risk | Computer‐generated random list. |
| Similarity at Baseline | Low risk | |
| Withdrawals Described | Unclear risk | No dropouts described. |
| Intention To Treat Analysis | Unclear risk | Method of analysis not described. |
| Cointerventions Constant | Unclear risk | Minor medication changes allowed, as deemed appropriate by team neurologist. |
| Blinded Assessors | Low risk | Assessors were blinded to group allocation. |
Shankar 2009.
| Methods | Parallel group design. Randomisation method not stated. Method of analysis not described. Treated as outpatients for 8 hours over 8 weeks. Assessed at baseline and immediately after treatment. Assessor was blinded. |
|
| Participants | 10 participants in the treadmill + cueing group, 10 participants in the cueing group. No dropouts described. Baseline characteristics given only for all three treatment groups combined, Mean age 64.4 years, 62% were male. Inclusion criteria: moderate Parkinson's disease. No exclusion criteria. |
|
| Interventions | Treadmill + cueing: walking on the treadmill with music for 30 min twice a week. Music was selected on the basis of participant input and was cadence‐matched to the participant's preferred walking speed. Treadmill: walking on the treadmill without music for 30 minutes twice a week. Cueing: listening to music for 30 minutes twice a week. Drug therapy was not described. |
|
| Outcomes | Gait and Balance Scale. UPDRS III. PDQ‐39. Not stated when examinations took place. |
|
| Notes | Abstract only. The 3rd arm, treadmill only, was excluded from our analysis |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Randomisation Method | Unclear risk | Randomisation method was not stated. |
| Concealment of Allocation | Unclear risk | Randomisation method was not stated. |
| Similarity at Baseline | Unclear risk | Baseline characteristics given only for all three treatments groups combined. |
| Withdrawals Described | Unclear risk | No dropouts described. |
| Intention To Treat Analysis | Unclear risk | Method of analysis not described. |
| Cointerventions Constant | Unclear risk | Drug therapy was not described. |
| Credible Placebo | Low risk | |
| Blinded Assessors | Low risk | Assessor was blinded to group allocation. |
Stack 2012.
| Methods | Parallel group design. Method of randomisation not stated. Method of analysis not stated. Treated at home for 12 hours over 4 weeks. Assessed at baseline, 4 weeks, 8 weeks and 12 weeks. Assessors blinded for all outcomes with the exception of forward reaches (FR). |
|
| Participants | 24 participants in physio group, 23 participants in control group. 8 drop‐outs in physio group, 4 in control group. Participants median age 75 years (physio), 74 years (control). Male/female ratio 17/7 (physio), 18/5 (control). Hoehn and Yahr 1.3 (physio), 1.7 (control). Duration of condition (median) 8 years (physio), 7 (control). Inclusion criteria: Parkinson's disease as per UK Brain Bank criteria, willing and able to take part in intervention, willing and able to complete outcome measures, score of at least 8/12 on the Middlesex elderly assessment of mental state, Hoehn and Yahr I‐IV, self report chair transfers as excessively slow or requiring much effort, assistance or repeated attempts or associated with a previous fall. |
|
| Interventions | Physio: Home‐based physiotherapy programme focused on chair transfers. Supervised exercises to enhance hip and knee extensor strength and trunk stability and flexibility. Teaching and learning movement strategies for safer and easier standing and sitting. Verbal cueing. Control: No physiotherapy. |
|
| Outcomes | PAS chair transfer. Sit‐to‐stand time. SAS score. SS‐180 turn time. Forward reach. UPDRS posture. HR‐QOL. Assessed in "ON" phase. |
|
| Notes | Data reported as median (IQR). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Eligibility Criteria | Low risk | Inclusion criteria stated. |
| Randomisation Method | Unclear risk | Method not stated. |
| Concealment of Allocation | Unclear risk | Method of randomisation not stated. |
| Similarity at Baseline | Low risk | Baseline characteristics similar in both arms. |
| Withdrawals Described | High risk | 26% withdrawals. |
| Intention To Treat Analysis | Unclear risk | Method of analysis not stated. |
| Cointerventions Constant | Unclear risk | Drug therapy not described. |
| Blinded Assessors | Low risk | Assessors blinded except for functional reach measurement. |
Stozek 2003.
| Methods | Parallel group design. Method of randomisation not stated. Method of analysis not described. Treated as outpatients for 56 hours over 4 weeks. Assessed at baseline, immediately and 1 month after treatment. Not stated whether assessors were blinded or not. |
|
| Participants | 30 participants in the exercise group and 31 participants in the control group. No dropouts described. Participants' mean age 64 years (exercise), 67 years (control); male/female 13/17 (exercise), 16/15 (control); Hoehn and Yahr 2.3 for both groups. Mean duration of PD 4.6 years (exercise), 4.3 years (control). Inclusion criteria: idiopathic Parkinson's disease diagnosed by a neurologist, disease stage based on the Hoehn and Yahr scale 1.5-beginning of 3, stable pharmacological treatment for at least the last 3 months, age 35 to 85, no other neurological disease or serious movement disorders, no contraindications for physical exercise, participants written consent to participate in the study. |
|
| Interventions | Exercise: complex rehabilitation for 2 hours twice daily for first 2 weeks, then once a day three times a week for 2 weeks, for a total of 28 sessions. Sensory reinforcements were used during all exercises: verbal, visual, auditory, extero‐ and proprioceptive stimulation. Complex rehabilitation consisted of relaxation and breathing exercises, exercises increasing the range of movement, functional exercises, exercises for posture, balance, gait, music‐dance exercises, mimic exercises of facial muscle and tongue, articulation and voice exercises, group therapy, and patient education. Control: without rehabilitation. It was not stated whether drug therapy was stable throughout the trial. |
|
| Outcomes | Functional reach test. Tinetti's Balance Performance Oriented Mobility Assessment. Static and dynamic balance. Timed Up and Go. 10‐m walk. Locomotion test. 360° turn. All assessments were carried out during one day in the morning when participants were in the 'on' phase. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Eligibility Criteria | Low risk | |
| Randomisation Method | Unclear risk | Randomisation method was not stated. |
| Concealment of Allocation | Unclear risk | Randomisation method was not stated. |
| Similarity at Baseline | Low risk | |
| Withdrawals Described | Unclear risk | No dropouts described. |
| Intention To Treat Analysis | Unclear risk | Method of analysis not described. |
| Cointerventions Constant | Unclear risk | It was not stated whether drug therapy was stable throughout trial. |
| Blinded Assessors | Unclear risk | Not stated whether assessors were blinded. |
Taheri 2011.
| Methods | Parallel group design. Method: randomisation method not stated. Method of analysis not stated. Treated for 40 hours over 10 weeks. Assessed before and after treatment period. Not stated whether assessors were blinded. |
|
| Participants | 12 participants in the physical therapy group and 12 in the control group. No dropouts described. Baseline characteristics not stated. Inclusion criteria: idiopathic Parkinson's disease, able to carry out activities of daily living independently, not part of any sports or physiotherapy treatment while participating in study, Hoehn & Yahr stage III. Exclusion criteria: secondary drawbacks (e.g. heart disease, arthritis, cognitive problems, not participating in experiments regularly). |
|
| Interventions | Physical therapy program: emphasis on tensional and supple exercises, chosen from Pito de Oto physical therapy and Donaron Rehabilitation centre, 5‐minute warm‐up of walking and exercises, 50 minutes of stretching and exercise, and 50minute cool‐down. Control group: no exercise. Both groups used the same doses of medications and were kept on the same medications throughout the trial. |
|
| Outcomes | Berg balance scale. Tinetti balance scale. |
|
| Notes | Translated from Farsi. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Eligibility Criteria | Low risk | Eligibility criteria described. |
| Randomisation Method | Unclear risk | Method not stated. |
| Concealment of Allocation | Unclear risk | Randomisation method not stated. |
| Similarity at Baseline | Unclear risk | Baseline characteristics not stated. |
| Withdrawals Described | Unclear risk | Withdrawals not described. |
| Intention To Treat Analysis | Unclear risk | Analysis method not stated. |
| Cointerventions Constant | Low risk | No medication changes occurred during trial period. |
| Blinded Assessors | Unclear risk | Not stated whether assessors were blinded. |
Talakad 2011.
| Methods | Parallel group design. Method of randomisation not stated. Method of analysis not described. Treated for 8 hours over 4 weeks. Assessed at baseline and after 4 weeks of intervention. Not stated whether assessors were blinded. |
|
| Participants | 60 participants were randomised into this trial. Dropouts were not described. Baseline characteristics of participants were not stated. Eligibility criteria not stated. |
|
| Interventions | Conventional gait training (CGT). Partial weight supported treadmill training: 20% unweighting. Control: no specific intervention. Drug therapy not described. |
|
| Outcomes | Dynamic posturography. UPDRS. Beat‐to‐beat finger blood pressure. |
|
| Notes | Abstract only. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Eligibility Criteria | Unclear risk | Eligibility criteria not stated. |
| Randomisation Method | Unclear risk | Method of randomisation not stated. |
| Concealment of Allocation | Unclear risk | Method of randomisation not stated. |
| Similarity at Baseline | Unclear risk | Baseline characteristics not stated. |
| Withdrawals Described | Unclear risk | Dropouts not described. |
| Intention To Treat Analysis | Unclear risk | Method of analysis not stated. |
| Cointerventions Constant | Unclear risk | Drug therapy not described. |
| Blinded Assessors | Unclear risk | Not stated whether assessors were blinded. |
Thaut 1996.
| Methods | Parallel group design. Randomised by a 'random draw,' but concealment of allocation unclear. Method of analysis not described. Treated at home or in the community for 10.5 hours over 3 weeks. Assessed in the laboratory at baseline and immediately after treatment. Not stated whether the assessors were blinded. |
|
| Participants | 15 participants in the cueing group, 11 participants in the exercise group, and 11 participants in the control group. No dropouts described. Participants' mean age, 69 (cueing), 74 (exercise), 71 (control); male/female 10/5 (cueing) 8/3 (exercise), 8/3 (control); Hoehn and Yahr 2.4 (cueing), 2.5 (exercise), 2.6 (control). Mean duration of PD 7.2 years (cueing), 5.4 years (exercise), 8.5 years (control). Inclusion criteria: idiopathic Parkinson's disease with significant gait deficits regarding speed, stride length, and cadence, but able to walk without physical assistance. No exclusion criteria. |
|
| Interventions | Cueing: Exercised for 30 minutes daily according to a prescribed program using rhythmic auditory stimulation (RAS). The RAS program consisted of walking on a flat surface, stair stepping, and stop‐and‐go exercises to rhythmically accentuated music at three different tempos. The tempos were labelled 'normal,' 'quick,' and 'fast.' Exercise (self‐paced therapy, SPT): performed their 300minute daily walking sessions without RAS, following the same training protocol and training exercises for the same length of time. Walking was divided equally into walking at normal pace, quick pace, and fast pace. Control: no treatment. Drugs stable during therapy. |
|
| Outcomes | Walk speed. Stride cadence. Stride length. EMG analysis on leg muscles. Footfall pattern. All testing done 90 to 120 minutes after first medication intake in morning. |
|
| Notes | 3 arms to the trial: RAS, SPT, and no treatment. SPT vs RAS are examined in 'A comparison of physiotherapy techniques for participants with Parkinson's disease.' Cochrane review. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Eligibility Criteria | Low risk | |
| Randomisation Method | High risk | Random draw. |
| Concealment of Allocation | Unclear risk | No information provided to allow assessment. |
| Similarity at Baseline | Low risk | |
| Withdrawals Described | Unclear risk | No dropouts described. |
| Intention To Treat Analysis | Unclear risk | Method of analysis not described. |
| Cointerventions Constant | Low risk | Medication remained stable throughout study. |
| Blinded Assessors | Unclear risk | Not stated whether assessors were blinded. |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Bergen 2002 | No outcome measures relevant to our review. |
| Blackinton 2002 | Initially identified as a suitable study for inclusion but was excluded because of the number of dropouts (47%; final number of participants analysed, n=8), which left the two groups unmatched by age and duration of Parkinson's disease. |
| Bridgewater 1997 | Although this trial was designed as an RCT, after discussion with the authors it was discovered that the method of randomisation was compromised. 'In order of response to advertising, subjects were allocated alternately to group A (period of exercise, then no exercise) and group B (control, then complimentary exercise classes).' Although alternate allocation is an acceptable method of randomisation, the authors went on to change participants from group A to B if their personal circumstances dictated that they would be unavailable for the physiotherapy (e.g. if they were leaving the state on holiday). We feel that this compromised the randomisation procedure, and therefore excluded the trial. |
| Byl 2009 | After email correspondence with the author, this trial was found out not to be randomised. |
| Chouza 2011 | Whole body vibration technique not usually used by Physiotherapists. |
| Christofoletti 2010 | Excluded as although the abstract for the study states 'randomised controlled trial,' after translation of the full paper, the study did not appear to be randomised; 'allocated to groups on convenience basis, following availability of participants at treatment site.' Attempted to contact author to clarify randomisation method but were unsuccessful. |
| Cianci 2010 | Excluded as confounded because of use of rolling walker. |
| Comella 1994 | The study did not report outcomes for the first assessment period and therefore has been excluded to prevent any bias of carryover or order effects. |
| Forkink 1996 | No outcome measures relevant to our review. |
| Formisano 1992 | Although this trial was controlled, the authors did not state that the allocation of participants into the two groups was random. |
| Gibberd 1981 | The study did not report outcomes for the first assessment period and therefore has been excluded to prevent any bias of carryover or order effects. |
| Guo 2009 | Multidisciplinary rehabilitation trail. Percentage component of physiotherapy was not specified, therefore unable to differentiate the contribution of physiotherapy to any change in the outcome measures. |
| Haas 2006 | Excluded as the study was a randomised cross‐over over a couple of hours. |
| Hurwitz 1989 | No outcome measures relevant to our review. |
| Kapur 2011 | Whole‐body vibration technique not usually used by physiotherapists. |
| Katsikitis 1996 | No outcome measures relevant to our review. |
| Kaut 2011 | Whole‐body vibration technique not usually used by physiotherapists. |
| King 2009 | Excluded as the study was a randomised cross‐over on the same day. |
| Knobl 2011 | Not properly randomised, placed in groups. |
| Koc 2012 | No patient numbers, methods, or data available; unable to make contact with authors. |
| Lee 2012 | Upon discussion with the authors, it was discovered that the method of randomisation was compromised as all patients in the control arm received delayed treatment and were added into the experimental group. |
| Patti 1996 | Multidisciplinary rehabilitation trail. Percentage component of physiotherapy was not specified, therefore unable to differentiate the contribution of physiotherapy to any change in the outcome measures. |
| Pohl 2003 | Randomised multiple intervention cross‐over, over 4 consecutive days. Randomisation was of the sequence of the interventions, therefore not RCT. |
| Rochester 2011 | Excluded, as the study was a randomised cross‐over over a couple of hours. |
| Sage 2009b | Upon contacting the author, it was found that the study was not properly randomised. |
| Stallibrass 2002 | The method of therapy used - Alexander Technique - is not used by physiotherapists. Therefore this trial was excluded. |
| Tickle‐Degnen 2010 | Multidisciplinary rehabilitation trail. Percentage component of physiotherapy was not specified, therefore unable to differentiate the contribution of physiotherapy to any change in the outcome measures. |
| Van Gerpen 2010 | Excluded as confounded because of the use of a four‐wheeled walker. |
| Wade 2003 | Multidisciplinary rehabilitation trail. Percentage component of physiotherapy was not specified, therefore unable to differentiate the contribution of physiotherapy to any change in the outcome measures. |
| Wells 1999 | Although not stated in the text, upon personal communication with the author, this trial was determined to be an RCT. However the method of therapy used - osteopathic manipulative treatment - is not used by physiotherapists. Therefore this trial was excluded. |
| Yen 2011 | No outcome measures relevant to our review. |
Characteristics of ongoing studies [ordered by study ID]
Canning 2009.
| Trial name or title | Exercise therapy for prevention of falls in people with Parkinson's disease: a randomised controlled trial. |
| Methods | Parallel group design. Randomisation was stratified by falls history (0‐10 falls in the previous 12 months/more than 10 falls in the previous 12 months) using a computer‐generated random number schedule with variable block sizes of 2 to 6. Randomisation was performed centrally by an investigator not involved in recruitment or assessments. Assessors were blinded. |
| Participants | 230 participants. Inclusion criteria: diagnosis of Idiopathic Parkinson's disease. Adapted to their current antiparkinsonian medication for at least 2 weeks. Aged 40 years or over. Able to walk independently (with or without walking aid). Have a history of falls (at least one fall in the previous 12 months) or are at risk of falls. Exclusion criteria: Mini‐Mental State Examination score of < 24. Suffer from unstable cardiovascular disease or other uncontrolled chronic conditions that would interfere with the safety and conduct of the training and testing protocol or interpretation of the results. |
| Interventions | Exercise: 40‐ to 60‐minute program of home‐based balance and leg strength exercises three times a week for 6 months. Participants can choose to participate in a once‐a‐month exercise class (for 6 months) conducted by a physiotherapist in association with the local Parkinson's NSW/ACT Support Group or hospital. Participants will be provided with a booklet containing safety precautions, instructions, and photographs of exercises for use in exercise sessions at home, as well as information sheets detailing strategies for managing freezing. In addition, they will be provided with a logbook for recording exercises completed and any adverse effects of exercise. Participants will also receive standardised falls prevention advice and will be provided with a falls diary for recording falls. Control: will have standardised falls prevention advice and will be provided with a falls diary for recording falls. |
| Outcomes | Falls diary*. Parkinson's Disease Falls Risk Score. Maximal muscle strength, knee extension (quadriceps). Step test component from the Berg Balance Scale. Short Physical Performance Battery. Freezing of Gait Questionnaire. SF12v2TM health survey. Falls Efficacy Scale International Questionnaire. Habitual Physical Activity Questionnaire. PDQ‐39. Positive and Negative Affect Schedule (PANAS). Total cost*. Tested at baseline and at the end of the 6‐month intervention period. *Data collected monthly. |
| Starting date | 01/05/2008. |
| Contact information | Dr Colleen Canning (c.canning@usyd.edu.au). Discipline of Physiotherapy, Faculty of Health Sciences, The University of Sydney. |
| Notes | Australian New Zealand Clinical Trials Registry Number: ACTRN12608000303347. |
Ledger 2008.
| Trial name or title | A randomised controlled trial (RCT) to evaluate use of auditory cueing device's (IACDs) on freezing and gait in people with Parkinson's disease (PD). |
| Methods | Randomly assigned using sealed, computer‐generated random numbers. |
| Participants | 47 participants. Inclusion criteria: Parkinson's disease, medically stable, willing to give informed consent, freeze at least once per week (minimum score of 2 on item 3 of the FOGQ) for at least 2 seconds (minimum score of 1 on item 4 of FOGQ), MMSE score greater than 24. Exclusion criteria: attending physiotherapy at time of recruitment, unwilling to give informed consent, not medically stable, cognitive impairment (MMSE score less than 24), acute comorbidity that prevents mobility. |
| Interventions | Cross‐over trial. Cueing: iPod containing an auditory cue in the form of a continuous metronome beat, individualised to participants' walking frequency (less than 10%). Participants instructed to listen to cueing when performing any mobility‐related tasks for 8 days. Control: iPod shuffle with no music or metronome beat for 8 days. |
| Outcomes | Freezing of Gait Questionnaire. Timed Up and Go Test. Modified Falls Efficacy Scale. 10‐Metre Walk Test. Tested on days 8, 15, and 23 and at 3 months. |
| Starting date | Study not yet open for recruitment. |
| Contact information | Dr Emma K Stokes (estokes@tcd.ie). |
| Notes | On days 1‐8 of the trial, both groups given an iPod with some music on it to allow all participants to become familiar with the device. They will be instructed to use the device only when sitting at home, and that the device should not be turned on when walking or performing any mobility‐related or daily tasks. NCT00727467. |
Martin 2009.
| Trial name or title | Home‐based rehabilitation to reduce falls in people with Parkinson's disease (PD): a randomised controlled trial. |
| Methods | Parallel group design. |
| Participants | 180 participants. Inclusion criteria: idiopathic Parkinson's disease, living in the community, Hoehn and Yahr stages 1 to 4. Exclusion criteria: suffer from cardiopulmonary, musculoskeletal, endocrine, or other medical condition that prevents safe participation in a home exercise program; participant or their carer/family are unwilling to have therapy and assessments in the home, are unable to communicate in English, have a dementia score MMSE score < 24, and unable to provide informed consent. |
| Interventions | Active intervention: 6‐week individualised home‐based rehabilitation program comprising a once‐weekly 1‐hour program delivered by a trained therapist, together with a once‐weekly 1‐hour self‐directed exercise program. The intervention is designed to provide participants with an integrated 'package' of evidence‐based therapy, including movement strategy training, strengthening, and falls education. Active control: 6‐week individualised home‐based 'life skills' program comprising a once‐weekly 1‐hour program delivered by a trained therapist, together with a once‐weekly self‐directed life skills home program. The active control is designed to provide education on taking medication, managing stress, driving, and other daily activities and will include content related to falls, physical exercise, and gait rehabilitation. |
| Outcomes | Fall frequency and injuries*. UPDRS total and motor. PDQ‐39. EuroQOL*. Tested at baseline, at 6 weeks, and at 12 months. *12 months only. |
| Starting date | 01/08/2008 |
| Contact information | Dr C Martin (cmartin@unimelb.edu.au). Centre for Health Exercise and Sports Medicine, School of Physiotherapy, The University of Melbourne. |
| Notes | ACTRN12608000390381. |
Schenkman 2009.
| Trial name or title | Exercise, physical function, and Parkinson's disease. |
| Methods | 3‐Arm parallel group design. |
| Participants | |
| Interventions | Exercise 1: general endurance training. Exercise 2: PD‐specific flexibility and functional training. Control: usual care based on a booklet based on the American Parkinson Foundation. |
| Outcomes | Continuous‐Scale Physical Functional Performance Test. Functional Reach. O2 consumption at a set walking speed. Assessed at baseline, after treatment, and at 10 and 16 months. |
| Starting date | 11/04/2003. |
| Contact information | Nancy Shinowara (shinowara@nih.gov). |
| Notes | Information obtained from CRISP/RePORT database. |
Schenkman 2012.
| Trial name or title | Endurance exercise. |
| Methods | Parallel group design. Method of randomisation not stated. Method of analysis not stated. Treatment schedule not stated. Assessment intervals not stated. Assessors were blinded. |
| Participants | Number of participants, group allocation, and dropouts were not described. Baseline characteristics were not stated. Eligibility criteria were not stated. |
| Interventions | Moderate exercise. Vigorous exercise. Drug therapy not described. |
| Outcomes | Adherence to exercise. UPDRS Motor. |
| Starting date | January 2012. |
| Contact information | Margaret Schenkman, University of Colarado, Denver. |
| Notes | Information obtained from ClinicalTrials.gov. |
Woo 2010.
| Trial name or title | The effectiveness of physiotherapy interventions for patients with Parkinson's disease. |
| Methods | 2‐Arm parallel group design. |
| Participants | Estimated enrolment: 112. Inclusion criteria: stable medication usage. Hoehn and Yahr stage II to IV. At least 1 score of 2 or more for at least 1 limb of the tremor, rigidity, or bradykinesia item of the Unified Parkinson's Disease Rating Scale (UPDRS). Able to walk independently. No severe cognitive impairments (Mini‐Mental State Examination - Chinese Cantonese version - score greater than 24). Exclusion criteria: other severe neurological, cardiopulmonary, or orthopedic disorders. Having participated in a physiotherapy or rehabilitation program in previous 2 months. |
| Interventions | Physiotherapy intervention: physiotherapy interventions including strengthening exercise, balance training, gait training with visual cue, gait training with treadmill. Education intervention: education classes. |
| Outcomes | Movement Disorder Society-Unified Parkinson's Disease Rating Scale. Levodopa equivalent daily dosage (LEDD). Timed Up and Go Test. Activities‐Specific Balance Confidence Scale (Chinese version). Parkinson's Disease Questionnaire (standard Chinese version). Number of injurious falls. |
| Starting date | 03/2010. |
| Contact information | CW Woo (woocx@ha.org.hk). |
| Notes | NCT01076712. |
Contributions of authors
Claire Tomlinson was involved in searching and selection of studies, data extraction, and analysis and interpretation of the review.
Smitaa Patel was involved in selection of studies, data extraction, and analysis and interpretation of the review.
Charmaine Meek was involved in data extraction and provided expert physiotherapy input into interpretation of the review.
Clare Herd was involved in searching and selection of studies, data extraction, and analysis and interpretation of the review for trials published from 2011 onwards.
Carl Clarke contributed to the design of the protocol and was involved in the interpretation of the review, providing clinical input.
Rebecca Stowe contributed to the design of the protocol and was involved in searching and selection of studies and analysis and interpretation of the review.
Laila Shah was involved in searching and selection of studies for the review.
Catherine Sackley contributed to the design of the protocol and provided expert physiotherapy input into the interpretation of the review.
Katherine Deane undertook the 2001 Cochrane Review and was involved in the interpretation of this review.
Keith Wheatley contributed to the design of the protocol and was involved in the interpretation of this review.
Natalie Ives contributed to the design of the protocol and was involved in the analysis and interpretation of this review.
Sources of support
Internal sources
No sources of support supplied
External sources
Parkinson's UK, UK.
Department of Health, UK.
Declarations of interest
Carl Clarke, Natalie Ives, Charmaine Meek, Smitaa Patel, Catherine Sackley, and Keith Wheatley are recruiting or are involved in the running of the UK PD REHAB trial.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Allen 2010 {published data only}
- Allen NE, Canning CG, Sherrington C, Lord SR, Latt MD, Close JCT, et al. The effects of an exercise program on fall risk factors in people with Parkinson's disease: a randomized controlled trial. Movement Disorders 2010;25(9):1217‐25. [DOI] [PubMed] [Google Scholar]
Almeida 2012 {published data only}
- Almeida QJ, Bhatt H. A manipulation of visual feedback during gait training in Parkinson's disease.. Parkinson's Disease 2012;Article no. 508720:1‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ashburn 2007 {published data only}
- Ashburn A, Fazakarley L, Ballinger C, Pickering R, McLellan LD, Fitton C. A randomised controlled trial of a home based exercise programme to reduce the risk of falling among people with Parkinson's disease. Journal of Neurology, Neurosurgery and Psychiatry 2007;78(7):678‐84. [DOI] [PMC free article] [PubMed] [Google Scholar]
Boehm 2011 {published data only}
- Boehm RL, Almeida QJ, Knobl P. Sensory attention focused exercise in Parkinson's disease: a randomized double‐crossover trial. Movement Disorders: 15th International Congress of Parkinson's Disease and Movement Disorders Toronto 2011;26:S331‐S332. [Google Scholar]
Cakit 2007 {published data only}
- Cakit BD, Saracoglu M, Genc H, Erdem HR, Inan L. The effects of incremental speed‐dependent treadmill training on postural instability and fear of falling in Parkinson's disease. Clinical Rehabilitation 2007;21(8):698‐705. [DOI] [PubMed] [Google Scholar]
Canning 2008 {published data only (unpublished sought but not used)}
- Canning CG, Allen NE, Dean CM, Goh L, Fung VS. Home‐based treadmill training for individuals with Parkinson's disease: a randomized controlled pilot trial.. Clinical Rehabilitation 2012;26(9):817‐826. [DOI] [PubMed] [Google Scholar]
- Canning CG, Allen NE, Fung VSC, Morris JGL, Dean CM. Home‐based treadmill walking for individuals with Parkinson's disease: a pilot randomized controlled trial. Movement Disorders 2008;23(Suppl 1):637. [Google Scholar]
Cerri 1994 {published data only}
- Cerri C, Arosio A, Biella AM, Premoselli S, Piccini L. Physical exercise therapy of Parkinson. Movement Disorders 1994;9(Suppl 1):68. [Google Scholar]
Chandler 1999 {published data only}
- Chandler C, Plant R. A targeted physiotherapy service for people with Parkinson's disease from diagnosis to end stage: a pilot study. In: R. Percival, P. Hobson editor(s). Parkinson's Disease: Studies in Psychological and Social Care. Leicester: BPS Books, 1999:256‐69. [Google Scholar]
- Chandler CS, Maher S, Harrison S, Plant R. A targeted physiotherapy service for people with Parkinson's disease from diagnosis to end stage - a pilot study. Parkinson's Disease Society Welfare Research Conference. London: Parkinson's Disease Society, 1997.
de Bruin 2010a {published data only}
- Bruin N, Bonfield S, Hu B, Suchowersky O, Doan J, Brown L. Walking while listening to music improves gait performance in Parkinson's disease. Movement Disorders 2008;23(Suppl 1):667. [Google Scholar]
- Bruin N, Doan JB, Turnbull G, Suchowersky O, Bonfield S, Hu B, et al. Walking with music is a safe and viable tool for gait training in Parkinson's disease: the effect of a 13‐week feasibility study on single and dual task walking. Parkinson's Disease 2010;Article ID 483530. [DOI] [PMC free article] [PubMed] [Google Scholar]
de Bruin 2010b {published data only}
- Bruin N, Doan J, Turnbull G, Bonfield S, Suchowersky O, Hu B, et al. The effects of a music accompanied walking program on obstacle crossing behaviours in people with Parkinson's disease. Movement Disorders 2010; Vol. 25, issue Suppl 3:S697.
Duncan 2012 {published and unpublished data}
- Duncan RP, Earhart GM. Randomized controlled trial of community‐based dancing to modify disease progression in Parkinson disease. Neurorehabilitation & Neural Repair 2012;26(2):132‐43. [DOI] [PubMed] [Google Scholar]
- Earhart GM, Rotello JMM, Duncan RP. Short‐term effects of a community‐based tango program on motor and non‐motor symptoms, activities of daily living, and motor complications in PD. Movement Disorders 2010; Vol. 25, issue Suppl 3:S697‐8.
Ellis 2005 {published data only}
- Ellis T, Goede CJ, Feldman RG, Wolters EC, Kwakkel G, Wagenaar RC. Efficacy of a physical therapy program in patients with Parkinson's disease: a randomized controlled trial. Archives of Physical Medicine and Rehabilitation 2005;86(4):626‐32. [DOI] [PubMed] [Google Scholar]
- Goede CJT, Ellis T, Wagenaar RC, Feldman RCH, Wolters E, Kwakkel G. Effects of group physiotherapy for patients with Parkinson's disease: a cross‐over trial. Nederlands Tijdschrift Fysiotherapie 2004;114(3):78‐82. [Google Scholar]
Fisher 2008 {published data only}
- Fisher BE, Wu AD, Salem GJ, Song J, Lin CH, Yip J, et al. The effect of exercise training in improving motor performance and corticomotor excitability in people with early Parkinson's disease. Archives of Physical Medicine and Rehabilitation 2008;89(7):1221‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ganesan 2010 {published data only}
- Ganesan M, Pal PK, Gupta A, Talakad S. Effect of partial weight supported treadmill gait training on balance in patients of Parkinson's disease. Parkinsonism & Related Disorders 2010; Vol. 16, issue Suppl 1:S66.
Goodwin 2009 {published and unpublished data}
- Goodwin V, Richards S, Ewings P, Taylor A, Campbell J. Preventing falls in Parkinson's disease: the GETuP trial. Parkinsonism & Related Disorders 2009;15(Suppl 2):S83. [Google Scholar]
- Goodwin VA, Richards SH, Ewings P, Taylor AH, Campbell JL. Preventing falls in Parkison's disease: the GETuP trial. Parkinsonism & Related Disorders 2009; Vol. 15, issue Suppl 2:S49.
- Goodwin VA, Richards SH, Henley W, Ewings P, Taylor AH, Campbell JL. An exercise intervention to prevent falls in people with Parkinson's disease: a pragmatic randomised controlled trial . Journal of Neurology, Neurosurgery & Psychiatry 2011;82(11):1232‐8. [DOI] [PubMed] [Google Scholar]
Haase 2011 {published data only}
- Haase M. The immediate effects of rhythmic arm swing and finger tapping exercises on gait of Parkinson's patients . ProQuest 2011.
Hackney 2009 {published data only}
- Hackney ME, Earhart GM. Effects of dance on movement control in Parkinson's disease: a comparison of Argentine tango and American ballroom. Journal of Rehabilitation Medicine 2009;41(6):475‐81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackney ME, Earhart GM. Health‐related quality of life and alternative forms of exercise in Parkinson disease. Parkinsonism & Related Disorders 2009;15:644‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackney ME, Earhart GM. Tai Chi improves balance and mobility in people with Parkinson disease. Gait & Posture 2008;28(3):456‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Homann 1998 {published and unpublished data}
- Homann CN, Crevenna R, Kojnig H, Kurzl B, Reinprecht S, Wenzel K, et al. Can physiotherapy improve axial symptoms in parkinsonian patients? A pilot study with the computerized movement analysis battery Zebris. Movement Disorders 1998;13(Suppl 2):234. [Google Scholar]
Keus 2007b {published data only}
- Keus SH, Bloem BR, Hilten JJ, Ashburn A, Munneke M. Effectiveness of physiotherapy in Parkinson's disease: the feasibility of a randomised controlled trial. Parkinsonism & Related Disorders 2007;13(2):115‐21. [DOI] [PubMed] [Google Scholar]
Klassen 2007 {published and unpublished data}
- Klassen L, Dal Bello‐Haas V, Sheppard M, Metcalfe A. Evaluating the benefits of group exercise and group exercise and education programs for individuals with Parkinson's disease. Physiotherapy 2007;93(Suppl 1):S91. [Google Scholar]
Kurtais 2008 {published data only}
- Kurtais Y, Kutlay S, Tur BS, Gok H, Akbostanci C. Does treadmill training improve lower‐extremity tasks in Parkinson disease? A randomized controlled trial. Clinical Journal of Sport Medicine 2008;18(3):289‐91. [DOI] [PubMed] [Google Scholar]
Lehman 2005 {published data only}
- Lehman DA, Toole T, Lofald D, Hirsch MA. Training with verbal instructional cues results in near‐term improvement of gait in people with Parkinson disease. Journal of Neurologic Physical Therapy 2005;29(1):2‐8. [DOI] [PubMed] [Google Scholar]
Mak 2008 {published data only}
- Mak MK, Hui‐Chan CW. Cued task‐specific training is better than exercise in improving sit‐to‐stand in patients with Parkinson's disease: a randomized controlled trial. Movement Disorders 2008;23(4):501‐9. [DOI] [PubMed] [Google Scholar]
- Mak MK, Hui‐Chan CW. Effect of 4‐week training with audio‐visual cues on sit‐to‐stand in Parkinsonian patients. Movement Disorders 2005;20(Suppl 10):388. [Google Scholar]
Marjama‐Lyons 2002 {published data only}
- Marjama‐Lyons J, Smith L, Mylar B, Nelson J, Holliday G, Seracino D. Tai Chi and reduced rate of falling in Parkinson's disease: a single‐blinded pilot study. Movement Disorders 2002;17(Suppl 5):190. [Google Scholar]
Meek 2010 {published and unpublished data}
- Meek C, Sackley CM, Clarke CE, Soundy AA, Winward C, Esser P, et al. Long‐term individual fitness enablement (LIFE) for Parkinson's disease: a feasibility study. Movement Disorders 2010; Vol. 25, issue Suppl 3:S713.
Nieuwboer 2007 {published data only}
- Nieuwboer A, Kwakkel G, Rochester L, Jones D, Wegen E, Willems AM. The effects of cueing therapy on gait and gait related mobility in people with Parkinson's disease: the RESCUE project. Movement Disorders 2006;21:S126. [Google Scholar]
- Nieuwboer A, Kwakkel G, Rochester L, Jones D, Wegen E, Willems AM, et al. Cueing training in the home improves gait‐related mobility in Parkinson's disease: the RESCUE trial. Journal of Neurology, Neurosurgery and Psychiatry 2007;78(2):134‐40. [DOI] [PMC free article] [PubMed] [Google Scholar]
Protas 2005 {published data only}
- Protas EJ, Mitchell K, Williams A, Qureshy H, Caroline K, Lai EC. Gait and step training to reduce falls in Parkinson's disease. NeuroRehabilitation 2005;20(3):183‐90. [PubMed] [Google Scholar]
Purchas 2007 {published and unpublished data}
- Purchas MA, MacMahon DG. The effects of Tai Chi training on general wellbeing and motor performance in patients with Parkinson's disease (PD): a pilot study. Movement Disorders 2007;22(Suppl 16):260. [Google Scholar]
Sage 2009a {published data only}
- Sage MD, Almeida QJ. Symptom and gait changes after sensory attention focused exercise vs aerobic training in Parkinson's disease. Movement Disorders 2009;24(8):1132‐8. [DOI] [PubMed] [Google Scholar]
Schenkman 1998 {published data only}
- Schenkman M, Cutson TM, Kuchibhatla M, Chandler J, Pieper CF, Ray L, et al. Exercise to improve spinal flexibility and function for people with Parkinson's disease: a randomised, controlled trial. Journal of the American Geriatrics Society 1998;46:1207‐16. [DOI] [PubMed] [Google Scholar]
Schilling 2008 {published data only}
- Schilling BK, Ledoux MS, Pfeiffer RF, Karlage RE, Weiss LW, Falvo MJ. Effects of lower‐body resistance training in persons with Parkinson's disease. Movement Disorders 2008;23(Suppl 1):639. [Google Scholar]
Schmitz‐Hubsch 2006 {published data only}
- Schmitz‐Hubsch T, Pyfer D, Kielwein K, Fimmers R, Klockgether T, Wullner U. Qigong exercise for the symptoms of Parkinson's disease: a randomized, controlled pilot study. Movement Disorders 2006;21(4):543‐8. [DOI] [PubMed] [Google Scholar]
Shankar 2008 {published and unpublished data}
- Shankar A, Bruin N, Bonfield S, Derwent L, Eliasziw M, Hu B, et al. Benefit of music therapy in patients with Parkinson's disease: a randomized controlled trial. Movement Disorders 2008;23(Suppl 1):608. [Google Scholar]
Shankar 2009 {published data only}
- Shankar A, Labelle N, Derwent L, Bonfield S, Eliasziw M, Hu B, et al. Treadmill‐walking with music shows a synergistic improvement in gait and balance in patients with Parkinson's disease: a randomized controlled trial. Movement Disorders 2009;24(Suppl 1):S281‐2. [Google Scholar]
Stack 2012 {published data only}
- Stack E, Roberts H, Ashburn A. The PIT: SToPP trial-A feasibility randomised controlled trial of home‐based physiotherapy for people with Parkinson's disease using video‐based measures to preserve assessor blinding. Parkinson's Disease 2012; Article Number: 360231:1‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Stozek 2003 {published data only}
- Stozek J, Rudzinska M, Longawa K, Szczudlik A. The effect of the complex rehabilitation on posture and gait in Parkinson disease. Neurologia i Neurochirurgia Polska 2003;37(Suppl 5):67‐81. [PubMed] [Google Scholar]
Taheri 2011 {published data only}
- Taheri H, Pejhan A, Taherzadeh J, Seyedahmadi M, Keavanloo F. Effect of a physical therapy program based on balance and gait in patients with Parkinson. Journal of Isfahan Medical School 2011;29(153):November. [Google Scholar]
Talakad 2011 {published data only}
- Gupta A, Ganesan M, Pal P, Talakkad S. Effect of partial weight‐supported treadmill gait training on balance in patients with Parkinson disease. 2011 Annual Assembly of the American Academy of Physical Medicine and Rehabilitation. 2011; Vol. 3, issue 10:S163‐4.
- Talakad S, Ganesan M, Gupta A, Pal PK, Trichur R. Effect of partial weight supported treadmill gait training on cardiovascular dynamics in patients with Parkinson's disease. Movement Disorders 2011;26(Suppl. 2):s173. [Google Scholar]
Thaut 1996 {published data only}
- Thaut MH, McIntosh GC, Rice RR, Miller RA, Rathbun J, Brault JM. Rhythmic auditory stimulation in gait training for Parkinson's disease patients. Movement Disorders 1996;11(2):193‐200. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Bergen 2002 {published data only}
- Bergen JL, Toole T, Elliott RG, Wallace B, Robinson K, Maitland CG. Aerobic exercise intervention improves aerobic capacity and movement initiation in Parkinson's disease patients. NeuroRehabilitation 2002;17(2):161‐8. [PubMed] [Google Scholar]
Blackinton 2002 {published data only}
- Blackinton MT, Summerall L, Waguespack K. Tertiary prevention in Parkinson disease: results from a preliminary study. Neurology Report 2002;26(3):160‐165. [Google Scholar]
Bridgewater 1997 {published data only}
- Bridgewater KJ, Sharpe MH. Aerobic exercise and early Parkinson's disease. Journal of Neurologic Rehabilitation 1996;10:233‐41. [Google Scholar]
- Bridgewater KJ, Sharpe MH. Trunk muscle training and early Parkinson's disease. Physiotherapy Theory and Practise 1997;13(2):139‐53. [Google Scholar]
Byl 2009 {published data only}
- Byl N. Enhancing safe mobility in patients with Parkinson's disease: effect of dual task training during aerobic and moderate exercise. Parkinsonism & Related Disorders 2009;15(Suppl 2):S122. [Google Scholar]
Chouza 2011 {published data only}
- Chouza M, Arias P, Vi¤as S, Cudeiro J. Acute effects of whole‐body vibration at 3, 6, and 9 hz on balance and gait in patients with Parkinson's disease. Movement Disorders 2011;26:920‐1. [DOI] [PubMed] [Google Scholar]
Christofoletti 2010 {published data only}
- Christofoletti G, Beinotti F, Borges G, Damasceno BP. Physical therapy improves the balance of patients with Parkinson's disease: a randomised controlled trial. Parkinsonism & Related Disorders 2010; Vol. 16, issue Suppl 1:S58.
- Christofoletti G, Freitas RT, Candido ER, Cardoso CS. Effectiveness of a physical therapy treatment on static and dynamic balance of subjects with Parkinson's disease. Fisioterapia e Pesquisa 2010;17(3):259‐63. [Google Scholar]
Cianci 2010 {published data only}
- Cianci H, Robinson K, Bunting‐Perry L, Sollenberger J, Nooregian J, Duda J. Are wheeled walkers with visual cues efficacious to treat freezing of gait in Parkinson's disease?. Parkinsonism & Related Disorders 2010; Vol. 16, issue Suppl 1:S64.
Comella 1994 {published data only}
- Comella CL, Stebbins GT, Brown‐Toms N, Goetz CG. Physical therapy and Parkinson's disease: a controlled clinical trial. Neurology 1994;44:376‐78. [DOI] [PubMed] [Google Scholar]
Forkink 1996 {published data only}
- Forkink A, Toole T, Hirsch MA, Lehman DA, Maitland CG. The effects of a balance and strengthening program on equilibrium in Parkinsonism. Working Paper Series: Pepper Institute on Ageing and Public Policy. Vol. PI‐96‐33, Tallahassee (FL): Florida State University, 1996. [Google Scholar]
- Toole T, Hirsch MA, Forkink A, Lehman DA, Maitland CG. The effects of a balance and strength training program on equilibrium in Parkinsonism: a preliminary study. NeuroRehabilitation 2000;14(3):165‐174. [PubMed] [Google Scholar]
Formisano 1992 {published data only}
- Formisano R, Pratesi L, Modarelli FT, Bonifati V, Meco G. Rehabilitation and Parkinson's disease. Scandinavian Journal of Rehabilitation Medicine 1992;24(3):157‐60. [PubMed] [Google Scholar]
Gibberd 1981 {published data only}
- Gibberd FB, Page NG, Spencer KM, Kinnear E, Hawksworth JB. Controlled trial of physiotherapy and occupational therapy for Parkinson's disease. British Medical Journal 1981;282(6271):1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibberd FB, Page NG, Spencer KM, Kinnear E, Williams JB. A controlled trial of physiotherapy for Parkinson's disease. In: Rose FC, Capildeo R editor(s). Recent Progress in Parkinson's Disease. Tunbridge Wells: Pitman Medical, 1981:401‐3. [Google Scholar]
Guo 2009 {published data only}
- Guo LP, Jiang YP, Yatsuya H, Yoshida Y, Sakamoto J. Group education with personal rehabilitation for idiopathic Parkinson's disease. Canadian Journal of Neurological Sciences 2009;36(1):51‐9. [DOI] [PubMed] [Google Scholar]
Haas 2006 {published data only}
- Haas CT, Turbanski S, Kessler K, Schmidtbleicher D. The effects of random whole‐body‐vibration on motor symptoms in Parkinson's disease. NeuroRehabilitation 2006;21(1):29‐36. [PubMed] [Google Scholar]
Hurwitz 1989 {published data only}
- Hurwitz A. The benefit of a home exercise regimen for ambulatory Parkinson's disease patients. Journal of Neuroscience Nursing 1989;21(3):180‐4. [DOI] [PubMed] [Google Scholar]
Kapur 2011 {published data only}
- Kapur SS, Stebbins GT, Goetz CG. Vibration therapy and Parkinson's disease. Movement Disorders 2011;26:s132. [Google Scholar]
Katsikitis 1996 {published data only}
- Katsikitis M, Pilowsky I. A controlled study of facial mobility treatment in Parkinson's disease. Journal of Psychosomatic Research 1996;40(4):387‐96. [DOI] [PubMed] [Google Scholar]
Kaut 2011 {published data only}
- Kaut O, Allert N, Coch C, Paus S, Grzeska A, Minnerop M, et al. Stochastic resonance therapy in Parkinson's disease. Neurorehabilitation 2011;28(4):353‐8. [DOI] [PubMed] [Google Scholar]
King 2009 {published data only}
- King LK, Almeida QJ, Ahonen H. Short‐term effects of vibration therapy on motor impairments in Parkinson's disease. Neurorehabilitation 2009;25(4):297‐306. [DOI] [PubMed] [Google Scholar]
Knobl 2011 {published data only}
- Knobl PE. An evaluation of motor imagery and exercise interventions in Parkinson's disease. ProQuest 2011.
- Knobl PE, Almeida QJ. Rehabilitation for Parkinson's disease: is it the sensory or attention focus that improves disease severity in sensory‐attention focused interventions?. Movement Disorders 2011;26(Suppl 2):s153. [Google Scholar]
Koc 2012 {published data only}
- Koc A. The effects of home exercise program on balance and activity of daily living in Parkinsonian patients. Parkinsonism and Related Disorders 2012;18:s153. [Google Scholar]
Lee 2012 {published data only}
- Lee HJ, Kim SY, Chae Y, Kim MY, Yin C, Jung WS, et al. QI dance in patient with Parkinson's disease. Parkinsonism and Related Disorders 2012;18(Suppl 2):S157. [Google Scholar]
Patti 1996 {published data only}
- Patti F, Reggio A, Nicoletti F, Sellaroli T, Deinite G, Nicoletti F. Effects of rehabilitation therapy on parkinsonians' disability and functional independence. Journal of Neurologic Rehabilitation 1996;10(4):223‐31. [Google Scholar]
Pohl 2003 {published data only}
- Pohl M, Rockstroh G, Ruckriem S, Mrass G, Mehrholz J. Immediate effects of speed‐dependent treadmill training on gait parameters in early Parkinson's disease. Archives of Physical Medicine and Rehabilitation 2003;84(12):1760‐6. [DOI] [PubMed] [Google Scholar]
Rochester 2011 {published data only}
- Rochester L, Baker K, Nieuwboer A, Burn D. Targeting dopa‐sensitive and dopa‐resistant gait dysfunction in Parkinson's disease: selective responses to internal and external cues. Movement Disorders 2011;26(3):430‐5. [DOI] [PubMed] [Google Scholar]
Sage 2009b {published and unpublished data}
- Sage MD, Almeida QJ. A Canadian approach to exercise rehabilitation: a systematic evaluation of strategies to reduce the symptoms of Parkinson's disease. Movement Disorders 2009;24(Suppl 1):S276‐7. [Google Scholar]
Stallibrass 2002 {published data only}
- Stallibrass C, Sissons P, Chalmers C. Randomized controlled trial of the Alexander Technique for idiopathic Parkinson's disease. Clinical Rehabilitation 2002;16(7):695‐708. [DOI] [PubMed] [Google Scholar]
Tickle‐Degnen 2010 {published data only}
- Tickle‐Degnen L, Ellis T, Saint‐Hilaire M, Thomas CA, Wagenaar RC. Efficacy of self‐management rehabilitation on quality of life outcomes in Parkinson's disease. Movement Disorders 2009;24(Suppl 1):S377‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tickle‐Degnen L, Ellis T, Saint‐Hilaire M, Thomas CA, Wagenaar RC. Self‐management rehabilitation and health‐related quality of life in Parkinson's disease: a randomized controlled trial. Movement Disorders 2010;25(2):194‐204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White DK, Wagenaar RC, Ellis TD, Tickle‐Degnen L. Changes in walking activity and endurance following rehabilitation for people with Parkinson disease. Archives of Physical Medicine and Rehabilitation 2009;90(1):43‐50. [DOI] [PubMed] [Google Scholar]
Van Gerpen 2010 {published data only}
- Gerpen J, Saucier M, Matthews M. Attentuating gain freezing and stride reduction in Pakinsonian patients with an attachable, adjustable laser (the Mobilaser): a pilot trial. Parkinsonism & Related Disorders 2010; Vol. 16, issue Suppl 1:S85.
Wade 2003 {published data only}
- Wade DT, Gage H, Owen C, Trend P, Grossmith C, Kaye J. Multidisciplinary rehabilitation for people with Parkinson's disease: a randomised controlled study. Journal of Neurology, Neurosurgery and Psychiatry 2003;74(2):158‐62. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wells 1999 {published data only}
- Wells MR, Giantinoto S, D'Agate D, Areman RD, Fazzini EA, Dowling D, Bosak A. Standard osteopathic manipulative treatment acutely improves gait performance in patients with Parkinson's disease. Journal of the American Osteopathic Association 1999;99(2):92‐8. [DOI] [PubMed] [Google Scholar]
Yen 2011 {published data only}
- Yi YC, Hwa LK, Hsia HM, Meei WR, Wu LT, Hwa LC, et al. Effects of virtual reality‐augmented balance training on sensory organization and attentional demand for postural control in people with Parkinson disease: a randomized controlled trial ... including Invited Commentary with Author Response. Physical Therapy 2011;91(6):862‐78. [DOI] [PubMed] [Google Scholar]
References to ongoing studies
Canning 2009 {published data only}
- Canning CG, Sherrington C, Lord SR, Fung VS, Close JC, Latt MD, Howard K, Allen NE, O'Rourke SD, Murray SM. Exercise therapy for prevention of falls in people with Parkinson's disease: a protocol for a randomised controlled trial and economic evaluation. BMC Neurology 2009;9:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ledger 2008 {published data only}
- Ledger S, Galvin R, Lynch D, Stokes EK. A randomised controlled trial evaluating the effect of an individual auditory cueing device on freezing and gait speed in people with Parkinson's disease. BMC Neurology 2008;8:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
Martin 2009 {published data only}
- Martin CL, Morris ME, Menz HB, Taylor NF, Watts JJ. Home‐based rehabilitation to reduce falls and disability in Parkinson's disease: protocol for a randomised controlled trial. Movement Disorders 2009;24(Suppl 1):S268. [Google Scholar]
Schenkman 2009 {published data only}
- Schenkman M, Shinowara N. Exercise, physical function and Parkinson's disease. Report Database 2009.
Schenkman 2012 {published data only}
- Schenkman M. Endurance exercise in Parkinson's disease. controlled_trials.com 2012.
Woo 2010 {published data only}
- Woo CW. The effectiveness of physiotherapy interventions in patients with Parkinson's disease, a randomized controlled trial. clinicaltrials.gov March 2010.
Additional references
Assmann 2000
- Assmann SF, Pocock SJ, Enos LE, Kasten LE. Subgroup analysis and other (mis)uses of baseline data in clinical trials. Lancet 2000;355(9209):1064‐9. [DOI] [PubMed] [Google Scholar]
Berg 1992
- Berg KO, Wooddauphinee SL, Williams JI. Measuring balance in the elderly - validation of an instrument. Canadian Journal of Public Health‐Revue Canadienne de Sante Publique 1992;83:S7‐S11. [PubMed] [Google Scholar]
Bloem 2001
- Bloem BR, Grimbergen YAM, Cramer M, Willemsen M, Zwinderman AH. Prospective assessment of falls in Parkinson's disease. Journal of Neurology 2001;248(11):950‐8. [DOI] [PubMed] [Google Scholar]
Chartered Society of Physiotherapy
- The Chartered Society of Physiotherapy. In: The Chartered Society of Physiotherapy, editor(s). Rules of Professional Conduct. 2nd Edition. London: The Chartered Society of Physiotherapy, 2002. [Google Scholar]
Clarke 2001
- Clarke M, Halsey J. DICE 2: a further investigation of the effect of chance in life, death and subgroup analyses. International Journal of Clinical Practice 2001;55(4):240‐2. [PubMed] [Google Scholar]
Crizzle 2006
- Crizzle AM, Newhouse IJ. Is physical exercise beneficial for persons with Parkinson's disease?. Clinical Journal of Sports Medicine 2006;16(5):422‐5. [DOI] [PubMed] [Google Scholar]
Deboer 1996
- Deboer AGEM, Wijker W, Speelman JD, Dehaes JCJM. Quality of life in patients with Parkinson's disease: development of a questionnaire. Journal of Neurology Neurosurgery and Psychiatry 1996;61(1):70‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Deeks 2001
- Deeks JJ, Altman DG, Bradburn MJ. Statistical methods for examining heterogeneity and combining results from several studies in meta‐analysis. In: Egger M, Davey Smith G, Altman DG editor(s). Systematic Reviews in Health Care: Meta‐analysis in Context. 2nd Edition. London: BMJ Publication Group, 2001. [Google Scholar]
Doll 1980
- Doll R, Peto R. Randomized controlled trials and retrospective controls. British Medical Journal 1980;280(6206):44. [DOI] [PMC free article] [PubMed] [Google Scholar]
Duncan 1990
- Duncan PW, Weiner DK, Chandler J, Studenski S. Functional reach: a new clinical measure of balance. Journals of Gerontology 1990;45(6):M192‐7. [DOI] [PubMed] [Google Scholar]
Fahn 1987
- Fahn S, Elton RL. UPDRS Development Committee. Unified Parkinson's Disease Rating Scale. In: Fahn S, Marsden CD, Calne DB, Goldestein M editor(s). Recent Developments in Parkinson's Disease. Florham Park, NJ: Macmillan Health Care Information, 1987:153‐63. [Google Scholar]
Fleiss 1993
- Fleiss JL. The statistical basis of meta‐analysis. Statistical Methods in Medical Research 1993;2(2):121‐45. [DOI] [PubMed] [Google Scholar]
Gage 2004
- Gage H, Storey L. Rehabilitation for Parkinson's disease: a systematic review of available evidence. Clinical Rehabilitation 2004;18(5):463‐82. [DOI] [PubMed] [Google Scholar]
Gibb 1988
- Gibb WRG, Lees AJ. The relevance of the Lewy body to the pathogenesis of idiopathic Parkinson's disease. Journal of Neurology Neurosurgery and Psychiatry 1988;51(6):745‐52. [DOI] [PMC free article] [PubMed] [Google Scholar]
Giladi 2000
- Giladi N, Shabtai H, Simon ES, Biran S, Tal J, Korczyn AD. Construction of freezing of gait questionnaire for patients with Parkinsonism. Parkinsonism & Related Disorders 2000;6(3):165‐70. [DOI] [PubMed] [Google Scholar]
Goetz 2008
- Goetz CG, Wuu J, McDermott MP, Adler CH, Fahn S, Freed CR, et al. Placebo response in Parkinson's disease: comparisons among 11 trials covering medical and surgical interventions. Movement Disorders 2008;23(5):690‐9. [DOI] [PubMed] [Google Scholar]
Goodwin 2008
- Goodwin VA, Richards SH, Taylor RS, Taylor AH, Campbell JL. The effectiveness of exercise interventions for people with Parkinson's disease: a systematic review and meta‐analysis. Movement Disorders 2008;23(5):631‐40. [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. British Medical Journal 2003;327(7414):557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0. The Cochrane Collaboration, 2011. [Google Scholar]
Hoehn 1967
- Hoehn MM, Yahr MD. Parkinsonism - onset, progression, and mortality. Neurology 1967;17(5):427. [DOI] [PubMed] [Google Scholar]
Jenkinson 1997
- Jenkinson C, Fitzpatrick R, Peto V, Greenhall R, Hyman N. The Parkinson's Disease Questionnaire (PDQ‐39): development and validation of a Parkinson's disease summary index score. Age and Ageing 1997;26(5):353‐7. [DOI] [PubMed] [Google Scholar]
Kersten 2004
- Kersten P. Principles of physiotherapy assessment and outcome measures. In: Stokes M editor(s). Physical Management in Neurological Rehabilitation. 2nd Edition. London: Elsevier Mosby, 2004:29‐46. [Google Scholar]
Keus 2004
- Keus S, Hendriks HJ, Bloem BR, Bredero‐Cohen AB, Goede CJ, Haaren M, et al. Clinical practice guidelines for physical therapy in patients with Parkinsons disease. Dutch Journal of Physiotherapy 2004;114(Suppl 3):1‐94. [Google Scholar]
Keus 2007a
- Keus SH, Bloem BR, Hendriks EJ, Bredero‐Cohen AB, Munneke M, Practice Recommendations Development Group. Evidence‐based analysis of physical therapy in Parkinson's disease with recommendations for practice and research. Movement Disorders 2007;22(4):451‐60. [DOI] [PubMed] [Google Scholar]
Keus 2009
- Keus SH, Munneke M, Nijkrake MJ, Kwakkel G, Bloem BR. Physical therapy in Parkinson's disease: evolution and future challenges. Movement Disorders 2009;24(1):1‐14. [DOI] [PubMed] [Google Scholar]
Kwakkel 2007
- Kwakkel G, Goede CJT, Wegen EEH. Impact of physical therapy for Parkinson's disease: a critical review of the literature. Parkinsonism & Related Disorders 2007;13(Suppl 3):S478‐87. [DOI] [PubMed] [Google Scholar]
Lim 2005
- Lim I, Wegen E, Goede C, Deutekom M, Nieuwboer A, Willems A, et al. Effects of external rhythmical cueing on gait in patients with Parkinson's disease: a systematic review. Clinical Rehabilitation 2005;19(7):695‐713. [DOI] [PubMed] [Google Scholar]
Mak 2009
- Mak MKY, Pang MYC. Fear of falling is independently associated with recurrent falls in patients with Parkinson's disease: a 1‐year prospective study. Journal of Neurology 2009;256(10):1689‐95. [DOI] [PubMed] [Google Scholar]
Moore 2007
- Moore O, Peretz C, Giladi N. Freezing of gait affects quality of life of peoples with Parkinson's disease beyond its relationships with mobility and gait. Movement Disorders 2007;22(15):2192‐5. [DOI] [PubMed] [Google Scholar]
Morris 1994
- Morris ME, Iansek R, Matyas TA, Summers JJ. The pathogenesis of gait hypokinesia in Parkinson's disease. Brain 1994;117:1169‐81. [DOI] [PubMed] [Google Scholar]
Morris 1996
- Morris ME, Iansek R, Matyas TA, Summers JJ. Stride length regulation in Parkinson's disease normalization strategies and underlying mechanisms. Brain 1996;119:551‐68. [DOI] [PubMed] [Google Scholar]
Morris 2010
- Morris ME, Martin CL, Schenkman ML. Striding out with Parkinson disease: evidence‐based physical therapy for gait disorders. Physical Therapy 2010;90(2):280‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mutch 1986
- Mutch WJ, Strudwick A, Roy SK, Downie AW. Parkinson's disease - disability, review, and management. British Medical Journal 1986;293(6548):675‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Nat Collab Centre for Chronic Conditions 2006
- National Collaborating Centre for Chronic Conditions. Parkinson's disease: national clinical guideline for diagnosis and management in primary and secondary care. London: Royal College of Physicians. 2006. [PubMed] [Google Scholar]
Nieuwboer 2008
- Nieuwboer A. Cueing for freezing of gait in patients with Parkinson's disease: a rehabilitation perspective. Movement Disorders 2008;23(Suppl 2):S475‐81. [DOI] [PubMed] [Google Scholar]
Nijkrake 2007
- Nijkrake MJ, Keus SH, Kalf JG, Sturkenboom IH, Munneke M, Kappelle AC, et al. Allied health care interventions and complementary therapies in Parkinson's disease. Parkinsonism & Related Disorders 2007;13(Suppl 3):S488‐94. [DOI] [PubMed] [Google Scholar]
PDS 2008
- Parkinson's Disease Society. Life WIth Parkinson's Today - Room for Improvement. London: Parkinson's Disease Society, 2008. [Google Scholar]
Perry 1995
- Perry J, Garrett M, Gronley JK, Mulroy SJ. Classification of walking handicap in the stroke population. Stroke 1995;26(6):982‐9. [DOI] [PubMed] [Google Scholar]
Peto 1995
- Peto V, Jenkinson C, Fitzpatrick R, Greenhall R. The development and validation of a short measure of functioning and well‐being for individuals with Parkinson's disease. Quality of Life Research 1995;4(3):241‐8. [DOI] [PubMed] [Google Scholar]
Plant 2000
- Plant R, Jones D, Walton G, Ashburn A, Lovgreen B, Handford F, et al. Physiotherapy for people with Parkinson's disease: UK best practice short report. Newcastle, UK: Institution of Rehabilitation, University of Northumbria, 2000.
Podsiadlo 1991
- Podsiadlo D, Richardson S. The Timed Up and Go - a test of basic functional mobility for frail elderly persons. Journal of the American Geriatrics Society 1991;39(2):142‐8. [DOI] [PubMed] [Google Scholar]
Powell 1995
- Powell LE, Myers AM. The Activities‐Specific Balance Confidence (ABC) Scale. Journals of Gerontology Series A‐Biological Sciences and Medical Sciences 1995;50(1):M28‐34. [DOI] [PubMed] [Google Scholar]
Qutubuddin 2005
- Qutubuddin AA, Pegg PO, Cifu DX, Brown R, McNamee S, Carne W. Validating the Berg Balance Scale for patients with Parkinson's disease: a key to rehabilitation evaluation. Archives of Physical Medicine and Rehabilitation 2005;86(4):789‐92. [DOI] [PubMed] [Google Scholar]
Rascol 2002
- Rascol O, Payoux P, Ferreira J, Brefel‐Courbon C. The management of patients with early Parkinson's disease. Parkinsonism & Related Disorders 2002;9(1):61‐7. [DOI] [PubMed] [Google Scholar]
Robertson 2003
- Robertson D. Developing and delivering services. In: Playford D, Thompson AJ editor(s). Neurological Rehabilitation of Parkinson's Disease. London: Martin Dunitz, 2003. [Google Scholar]
Robertson 2008
- Robertson D, Aragon A, Moore G, Whelan L. Rehabilitation and the interdisciplinary team. In: Playfer J, Hindle J editor(s). Parkinson's Disease in the Older Patient. 2nd Edition. Oxford: Radcliffe Publishing Ltd, 2008. [Google Scholar]
Rubenis 2007
- Rubenis J. A rehabilitational approach to the management of Parkinson's disease. Parkinsonism & Related Disorders 2007;13(Suppl 3):S495‐7. [DOI] [PubMed] [Google Scholar]
Schrag 2000
- Schrag A, Jahanshahi M, Quinn N. How does Parkinson's disease affect quality of life? A comparison with quality of life in the general population. Movement Disorders 2000;15(6):1112‐8. [DOI] [PubMed] [Google Scholar]
Schrag 2006
- Schrag A, Sampaio C, Counsell N, Poewe W. Minimal clinically important change on the Unified Parkinson's Disease Rating Scale. Movement Disorders 2006;21(8):1200‐7. [DOI] [PubMed] [Google Scholar]
Schulz 2010
- Schulz KF, Altman DG, Moher D. CONSORT 2010 Statement: updated guidelines for reporting parallel group randomised trials. British Medical Journal 2010;340:c332. [DOI] [PMC free article] [PubMed] [Google Scholar]
Shulman 2010
- Shulman LM, Gruber‐Baldini AL, Anderson KE, Fishman PS, Reich SG, Weiner WJ. The clinically important difference on the Unified Parkinson's Disease Rating Scale. Archives of Neurology 2010;67(1):64‐70. [DOI] [PubMed] [Google Scholar]
Steffen 2008
- Steffen T, Seney M. Test‐retest reliability and minimal detectable change on balance and ambulation tests, the 36‐Item Short‐Form Health Survey, and the Unified Parkinson Disease Rating Scale in people with parkinsonism. Physical Therapy 2008;88(6):733‐46. [DOI] [PubMed] [Google Scholar]
Talley 2008
- Talley KMC, Wyman JF, Gross CR. Psychometric properties of the Activities‐Specific Balance Confidence Scale and the Survey of Activities and Fear of Falling in older women. Journal of the American Geriatrics Society 2008;56(2):328‐33. [DOI] [PubMed] [Google Scholar]
Tinetti 1990
- Tinetti ME, Richman D, Powell L. Falls efficacy as a measure of fear of falling. Journals of Gerontology 1990;45(6):239‐43. [DOI] [PubMed] [Google Scholar]
Trew 2005
- Trew M, Everett T. Human Movement: An Introductory Text. 5th Edition. Edinburgh: Churchill Livingstone, 2005. [Google Scholar]
van der Marck 2009
- Marck MA, Kalf JG, Sturkenboom IH, Nijkrake MJ, Munneke M, Bloem BR. Multidisciplinary care for patients with Parkinson's disease. Parkinsonism & Related Disorders 2009;15(Suppl 3):S219‐23. [DOI] [PubMed] [Google Scholar]
Ware 1992
- Ware JE, Sherbourne CD. The Mos 36‐Item Short‐Form Health Survey (SF‐36) .1. Conceptual‐framework and item selection. Medical Care 1992;30(6):473‐83. [PubMed] [Google Scholar]
Webster 1968
- Webster DD. Critical analysis of disability in Parkinson's disease. Modern Treatment 1968;5(2):257. [PubMed] [Google Scholar]
Welsh 2003
- Welsh M, McDermott MP, Holloway RG, Plumb S, Pfeiffer R, Hubble J, et al. Development and testing of the Parkinson's disease quality of life scale. Movement Disorders 2003;18(6):637‐45. [DOI] [PubMed] [Google Scholar]
Whittle 1996
- Whittle M. Gait Analysis: An Introduction. 2nd Edition. Oxford: Butterworth‐Heinemann, 1996. [Google Scholar]
Wood 2002
- Wood BH, Bilclough JA, Bowron A, Walker RW. Incidence and prediction of falls in Parkinson's disease: a prospective multidisciplinary study. Journal of Neurology Neurosurgery and Psychiatry 2002;72(6):721‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Yahr 1969
- Yahr MD, Duvoisin RC, Schear MJ, Barrett RE, Hoehn MM. Treatment of parkinsonism with levodopa. Archives of Neurology 1969;21(4):343. [DOI] [PubMed] [Google Scholar]
Yardley 2005
- Yardley L, Beyer N, Hauer K, Kempen G, Piot‐Ziegler C, Todd C. Development and initial validation of the Falls Efficacy Scale‐International (FES‐I). Age and Ageing 2005;34(6):614‐9. [DOI] [PubMed] [Google Scholar]
Yarrow 1999
- Yarrow S. Members' 1998 survey of the Parkinson's Disease Scoiety of the United Kingdom. In: Percival R, Hobson P editor(s). Parkinson's Disease: Studies in Psychological and Social Care. Leicester: BPS Books, 1999:79‐92. [Google Scholar]
References to other published versions of this review
Deane 2001a
- Deane K, Jones DE, Playford ED, Ben‐Shlomo Y, Clarke CE. Physiotherapy versus placebo or no intervention in Parkinson's disease. Cochrane Database of Systematic Reviews 2001, Issue 3. [DOI: 10.1002/14651858.CD002817] [DOI] [Google Scholar]


