Abstract
Circadian clocks are evolutionary molecular machinery that dictate temporal regulation of physiology to maintain homeostasis. Disruption of circadian rhythm plays a key role in tumorigenesis and facilitates the establishment of cancer hallmarks. Conversely, oncogenic processes directly weaken circadian rhythms. Pharmacological modulation of core clock genes is a new approach in cancer therapy. The integration of circadian biology into cancer research offers new options for making cancer treatment more effective and would encompass the prevention, diagnosis, and treatment of this devastating disease. This review highlights the role of circadian clock in tumorigenesis, cancer hallmarks and will discuss how pharmacological modulation of circadian clock genes can lead to new therapeutic options.
Keywords: Circadian rhythms, circadian clock, cancer hallmarks, cancer therapy, tumorigenesis
The circadian clock is an emerging factor in cancer research
All forms of life exposed to day-night cycles and seasonal changes in daylength developed a prodigious molecular mechanism to keep track of time and optimize daily life during these periodic fluctuations. In complex multicellular organisms, the circadian clock coordinates through the establishment of circadian rhythms several physiological processes. Disruption in circadian rhythms thus predisposes the onset of numerous chronic diseases, (such as cancer and metabolic disorders) (1). A large body of work strongly supports the existence of a crosstalk between cancer and the circadian clock. Indeed, the escape from circadian physiologic discipline is relevant in tumorigenesis, where the homeostasis of cells and tissues is hijacked to fuel rapid and uncontrolled proliferation, cope with increased metabolic demands, trigger immune evasion, resistance to apoptosis, and allow for an inflammatory tumor-supportive environment (hallmarks of cancer) (2). Oncogenic processes may suppress the homeostatic balance imposed by the circadian clock in order to facilitate the instauration of cancer hallmarks. Conversely, other pieces of evidence show that circadian rhythms dysfunctions affect tumorigenesis and clock genes regulate several cancer hallmarks. Pharmacological manipulation of circadian clock components may unveil novel opportunities for cancer treatment. In this review article, we discuss the role of circadian rhythms disruption in tumorigenesis, clock genes dependent regulation of cancer hallmarks and new anticancer therapeutic strategies targeting the circadian clock.
Circadian clock – Cancer connection
The cellular and molecular organization of circadian rhythm offers a new framework to understand how circadian clocks or clock components reciprocally interact with cancer. The circadian clock is cell autonomous and is composed of 13 transcription regulators that participate in interlocked transcriptional feedback loops to generate their own oscillation (Fig. 1, Box 1). Cellular circadian clocks in mammals are organized in a hierarchical manner. The circadian clocks in ~20,000 neurons of the suprachiasmatic nuclei (SCN) region of the hypothalamus receive input from retinal ganglion cells (mRGC) expressing photopigment melanopsin. Light signals carried from the retina to the SCN ensures that the SCN neurons are in sync with the gradual changes in day length in different seasons (3). The SCN clocks directly or indirectly produce daily rhythms in body temperature and rhythms in several hormones which synchronize circadian clocks present in other parts of the brain and in peripheral organs (4). SCN also imposes a daily rhythm in sleep and activity. As animals and humans are likely to eat during the active-period of their sleep-wake cycle, the SCN indirectly drives a fasting-feeding rhythm. Additionally, feeding induces changes in body temperature and in hormones (including insulin), which further synchronize circadian clocks in many peripheral organs. At the cellular level, the circadian clock responds to extra-cellular cues or the cellular environment and regulates the expression or function of numerous genes through transcriptional and post-translational mechanisms.
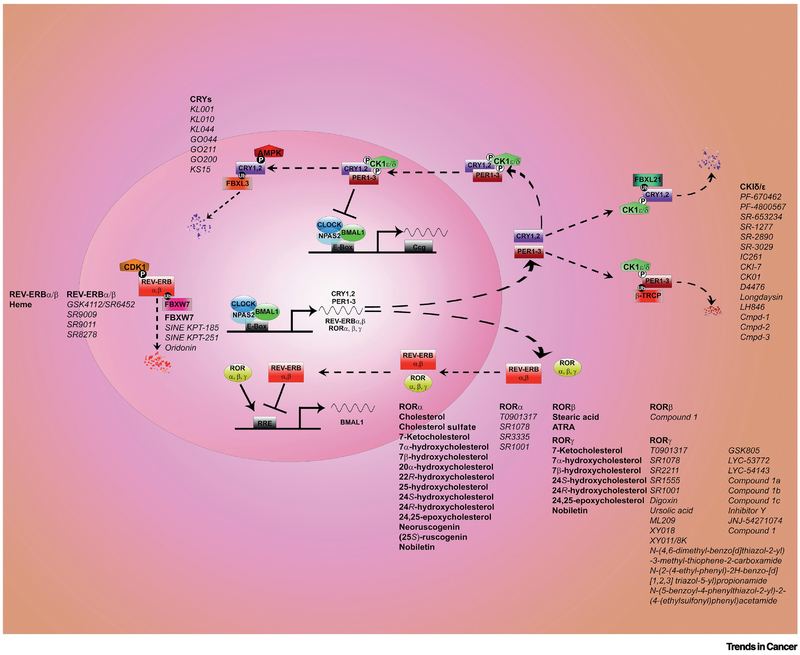
FIGURE 1. Circadian clock: molecular mechanisms, natural ligands and, chemical tools.
The circadian clock machinery generates everyday circadian rhythms. The core clock mechanism presents activators and repressors and consists of two main loops. Several regulatory layers (transcriptional, post-translational, cytoplasm-nuclear translocation) are in play to ensure rhythmicity. Transcriptional activators BMAL1 and CLOCK/NPAS2 (NPAS2 substitutes for CLOCK in the forebrain) bind to DNA element E-box motif and trigger the expression of the repressors Period (PER1, PER2, PER3), Cryptochrome (CRY1 and CRY2). Upon accumulation PERs and CRYs interact, they are phosphorylated by Casein Kinase 1δ/ε and translocate to the nucleus where they block the transcriptional activity of BMAL1 and CLOCK/NPAS2 thus inhibiting their own transcription. Post-translational mechanisms driven by AMPK and FBXL3 will lead CRYs to degradation in the nucleus; cytoplasmic degradation of CRYs is instead regulated by CK1δ/ε and FBXL21. Several chemical tools have been developed to modulate CRYs and CK1δ/ε as displayed. PERs are degraded in the cytoplasm upon phosphorylation by CK1δ/ε and β-TRCP. In an additional loop BMAL1 and CLOCK/NPAS2 activate the transcription of REV-ERBα/β and RORα/β/γ proteins. These are respectively transcriptional repressors and activators that further ensure rhythmicity of BMAL expression. Both REV-ERBα/β and RORα/β/γ are nuclear hormone receptors that are modulated by natural ligands (in bold in the figure). REV-ERBα/β and RORα/β/γ are rapidly becoming a target of interest for pharmacology and several small molecules (in italic in the figure) have been developed in the recent years to modulate their activity.
Text Box 1-. Molecular Regulation of the Circadian Clock.
The circadian oscillator in mammals is based on interlocked transcription-translation feedback-loops (Fig. 1). Transcription activators BMAL1 and CLOCK/NPAS2 bind to DNA element containing the E-box motif and induce the expression of the transcription repressors Cryptochrome (CRY1 and CRY2), and Period (PER1, PER2, PER3) genes. CRY, and PER protein complexes inhibit CLOCK-BMAL1 activity, thus inhibiting their own transcription. CRY, and PER proteins are degraded, thus restoring BMAL1-CLOCK activity. In an interlocked loop, CLOCK/BMAL activate the transcriptional factors REV-ERBα/β (5) and RORα/β/γ proteins (6). The REV-ERB and ROR proteins act as repressors and activators of Bmal1 transcription, respectively. A growing list of genes currently numbering a few dozen participate in finetuning transcription, translation, post-translational modification, sub-cellular localization, and degradation of core clock proteins.
There are four broad mechanisms by which circadian clock components are known to be involved in cancer initiation or progression: (A) Circadian clock components directly or indirectly regulate the expression of hundreds or thousands of genes in different cell types, which leads to daily rhythms in many cellular processes including nutrient metabolism, redox regulation, autophagy, DNA damage repair, protein folding, cellular secretion, etc. Daily rhythm in these cellular processes is an integral part of their homeostasis. Disruption of circadian rhythms also disrupt these cellular processes and creates a cellular environment conducive for tumorigenesis (i.e. metabolic reprogramming, redox imbalance, chronic inflammation, etc.). (B) Many circadian clock proteins also physically interact with proteins that participate in pathways relevant to cancer. Therefore, modulation of circadian clock function or expression of clock proteins can protect or promote cancer. (C) Circadian clock proteins and their interacting proteins “sense” the cellular environment. For example, change in the redox state of the cell can affect CLOCK/BMAL1 affinity to DNA. In addition, levels of co-factors (such as Heme binding to REV-ERB, Fig. 1), and activity of factors mediating post-translational modification (e.g., acetylation, phosphorylation) of circadian clock proteins are modulated by the nutrient status of the cell or in response to signaling events. Consequently, change in redox state, co-factors, and post-translational modifications brought about by oncogenic programs can change the stability, localization, or function of clock proteins. (D) The circadian clock regulates the expression of several secreted factors which can have paracrine or endocrine function. Some of these secreted factors, including cytokines, hormones, neurotransmitters, can signal through their cognate receptors and the downstream signaling pathways to affect clock function in order to entrain or synchronize clocks in different tissues. These endocrine factors can be biomarkers of circadian function in different tissues. However, some tumors can produce excessive amounts of such circadian clock relevant hormones or cytokines, which can also disrupt circadian clocks in distant organs.
Factors that disrupt normal circadian rhythms
The normal functioning of circadian clocks can be disrupted by genetic, environmental, and internal factors. Genetic mutations affecting the function of circadian clock components can disrupt normal circadian rhythms. Homologs of the clock core clock components, such as Per-1,-2,-3, Cry-1,-2, Rev-erb-α, -β, Ror-α,-β,-γ are not uniformly expressed in all tissues (7). Rather each homolog is preferentially expressed in certain tissues. Therefore, mutations in any one of these clock components are more likely to disrupt normal circadian rhythm in specific tissues, which may explain tissue-type specific tumor incidences in circadian mutant mice (discussed later).
Environmental factors can also affect the normal function of the circadian clock. In nature, the time of sunrise and food availability are highly predictable and do not change abruptly from one day to another. Therefore, the circadian clocks change only gradually with seasonal changes and are relatively slow in adjusting to rapid change in light onset or food availability. In response to an abrupt change in ambient light or food availability, normal circadian rhythm may be affected in several ways: (a) Light stimulus at certain times at night can completely abolish circadian rhythm for one to several days (8). (b) The circadian clock may take several days to adjust to a large change in the light:dark cycle. During this resetting period, the circadian rhythms are not in optimum synchrony with the ambient light:dark cycle and dependent rhythm in fasting-feeding. (c) When feeding occurs erratically over long periods of the 24 h day, or when animals or humans are exposed to continuous light, the absence of a clear feeding-fasting or light-dark cycle can disrupt circadian rhythms. (d) When the timing of feeding-fasting and light-dark cycles are not in a usual relationship that occurs in nature (e.g., feeding nocturnal rodents during the day, or eating late at night for humans), the circadian clocks are also disrupted. Circadian clocks in the brain regions that respond to light cues remain out of sync with peripheral organs that primarily respond to food time.
Finally, studies on disease states and circadian rhythms are shedding light on disease-clock interaction. Tumors in one organ can disrupt the normal homeostatic levels of systemic factors, such as cytokines, which subsequently disrupt circadian clock function in distant organs. Similarly, chronic activation or repression of certain signaling pathways within a cell can also affect normal circadian clock function within the same cell. In the following sections, we will discuss the link between these circadian rhythm disruptions and tumor initiation or progression.
Chronic circadian rhythm disruption facilitates tumor initiation and progression.
Emerging data in clinical and basic research are providing evidence linking chronic circadian disruption and cancer.
Human epidemiological studies.
The connection between chronic circadian rhythm disruption and cancer among free-living humans comes from epidemiological studies on cancer incidence and sleep disruption among shift workers. When shift workers work in the early morning, evening, or overnight shifts and try to maintain a day-active social life on off-days, such frequent and repetitive disruption of daily schedules results in misalignment of internal and external circadian cues. Several epidemiological studies have assessed cancer risk in shift workers, and the majority of these studies shows that a long period (more than 20 years) of shift work correlates with higher risk to develop breast, and prostate cancers (9). Similarly, another recent study confirmed that the risk of rectal cancer (hazard ratio) increased with long term exposure to shift work (10). In lieu of the elevated risk of cancer among shift workers, the International Agency for Research on Cancer (IARC), part of the World Health Organization (WHO) has enlisted circadian disruption as a probable human carcinogenic (Group 2A) in 2007 (11).
A note of caution is warranted in interpreting studies that examine the connection between shift work and cancer risk. Shift work is a loosely defined work schedule that can vary wildly in frequency, duration, and of nature of shiftwork. Shiftwork is often associated with other confounding factors such as alcohol consumption, smoking habits, food choices, activity levels, exposure to radiation, or other carcinogens. So, it is not surprising that some studies have found no clear link between shiftwork and increased cancer risk (9,10,12–16). While more studies are needed, at the moment, it is recommended to follow the IARC guidelines (11).
Outside of canonical shiftwork, many people in modern society also adopt a lifestyle that mimics aspects of shiftwork including eating a significant portion of calories late at night or spreading daily caloric intake over long periods of the 24 h day. Epidemiological studies on the length of overnight fasting or time of dinner have found relations between daily eating pattern and cancer risk. Women who eat all their calories within 11 hours, leaving 13 h of overnight fast have a significantly lower risk for breast cancer (17). A recent study from Spain has found eating dinner before 9 pm correlates with significantly reduced risk for prostate and breast cancer (18).
Conversely, enforcement of biological rhythms may impair cancer progression and recurrence, and increase the quality of life. For example, the presence of cortisol rhythms is a survival predictor in lung and breast cancer (19,20). Additional studies show that patients with metastatic colorectal cancer who have a robust rest/activity rhythm are associated with better survival and quality of life than patients with erratic periods of rest/activity and poor sleep (21,22).
Evidences in experimental animal models.
While human epidemiological studies offer a correlation between shiftwork induced circadian disruption and elevated cancer risks, controlled animal studies corroborate this link. Non-genetic approaches to circadian rhythm disruption in rodents involve SCN ablation and chronic jetlag. Surgical ablation of the SCN in rodents abolishes circadian activity-rest rhythms. Subjecting mice to a light-dark cycle that is advanced or delayed by several hours every week mimics chronic jet lag or rotating shiftwork in humans. Chronic jetlag or SCN ablation in normal mice, mice bearing tumors, or mice that are tumor-prone dramatically accelerates tumor initiation and progression (23–26).
Genetic disruption of several core clock genes in mice renders them tumor-prone without or in combination with carcinogenic agents or oncogenic stimuli. Mice harboring genetic alteration in Per2, or in Per1 and Per2 genes develop salivary gland hyperplasia, teratomas, lymphoma, liver, and ovarian cancers (23,27); and a single dose of irradiation accelerates tumor development in these mutants. Per2 mutant mice treated with the carcinogen diethylnitrosamine (DEN) are more susceptible to liver cancer than the wild type control mice (28).
Genetic disruption of Per2 also accelerates tumor formation in standard models of cancer. Per2 mutant in a ApcMin/+ genetic background further aggravates tumor formation compared to control mice (29). Similarly, Per2 mutation accelerates initiation and progression of lung tumors in KrasG12D and in KrasG12D; p53−/− mutants (24). Mice harboring the p53R172H and the PER2S662G mutations (present in individuals affected by familial advanced sleep phase syndrome) when tested for survival rate display a decrease in life span (30); similarly tumor development is more aggressive in mice lacking both Per2 and p53 when compared to p53 null mice (23).
Genetic disruption of other clock components also increases tumor formation. The clock mutation ClockΔ19/+ also cooperates with p53 mutations (30). Bmal1−/+ mice show lymphoma, liver and ovarian tumors, and the cancer incidences further increase when these mutants are irradiated (23). Similar observations are made in mice lacking Cry1 and Cry2 (23). Bmal1 deletion promotes lung tumorigenesis in cooperation with KrasG12D, while Cry2 mutation drives aggressive lymphoma development in EμMyc models. Mice with deleted RORγ also develop T-cell lymphomas (31). Interestingly alterations in clock genes that do not cause severe circadian dysfunctions have still a dramatic impact on tumorigenesis.
Paradoxically, other studies showed that Clock mutant mice, Per1−/− or Per2−/− and Cry1−/−;Cry2−/− mice are not tumor prone (32–34) and that clock genes may fuel tumorigenesis. In a cutaneous squamous tumor model driven by a dominant form of the RAS activator Son of Sevenless, BMAL1 deletion protects from tumor initiation and progression (35). Similarly, another study showed that CLOCK and BMAL1 are essential regulators of proliferation and stemness of acute myeloid leukemia (AML) and that lack of BMAL1 impairs leukemia growth in vivo (36). Furthermore, Cry1 and Cry2 deletions delay tumor development in the p53 null background possibly due to increased sensitivity of these triple mutants to genotoxic stress (37). It is, therefore, possible that clock genes may function either as tumor suppressors or as oncogenes. This dual function could be due to tissue-specific mechanisms, or it may reflect the existence of more complex clock gene dependent-mechanisms involved in the maintenance of homeostasis between stem cells, progenitors, and differentiated cells (35,36,38,39).
Finally, animal studies are also revealing the impact of tumors on circadian rhythms. Tumors can affect circadian rhythms in a tumor-free distal organ (40–42). In a mouse model, breast cancer causes alterations in the liver of the circadian expression of several genes including the core clock genes Rev-erbα, Per2, RORy, Clock; the authors suggest that this misalignment from physiological oscillations may result in oxidative stress, polyploidy, and inflammation (40). Similarly, Per1 and BMAL1 oscillations are attenuated in melanoma and melanoma-adjacent normal skin (41). These alterations extend to two organs that are sites for melanoma metastasis: lung and liver. Interestingly, this report also showed that in the SCN of tumor-bearing mice, BMAL1 rhythmic expression is lost. Finally, an additional study (42) shows that lung cancer, although not affecting the core clock machinery, induces a deep reprogramming of circadian rhythms in the liver, both at the level of transcripts and metabolites.
Genetic evidences in cancer patients.
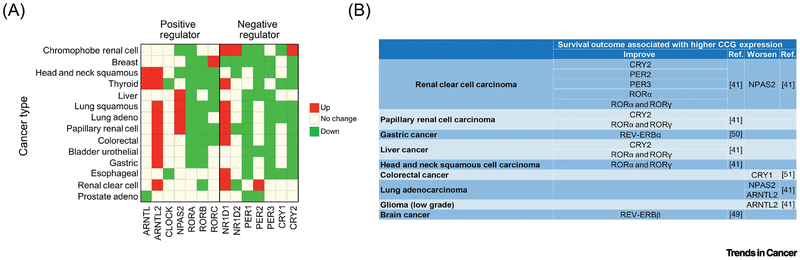
Traditionally, the increased mutation rate of a given gene in tumors offer strong support for the role of that gene in tumor initiation or progression. However, data available in the TCGA database suggest that the mutation frequency of clock genes in cancer is low (43). Despite sparsity of mutations in clock genes in tumors, analyses of survival trends in breast cancer showed that patients harboring a mutation in all the core clock genes or in all the PERs have a lower survival rate than patients carrying no mutations (43). Additionally, analyses of gene expression datasets (The Cancer Genome Atlas (TCGA) and the NCBI Gene Expression Omnibus (GEO)) show that the relative expression pattern of clock genes is significantly different from the pattern observed in non-tumor samples (43) (44) (Fig. 2).
FIGURE 2. Circadian clock genes expression in cancer patients and their association with survival outcome.
a) Altered circadian clock genes expression in various cancer types compared to the normal tissues. Green represents cancers with downregulated circadian clock genes; red, upregulated. b) Table indicating the survival outcome associated with core clock gene (CCG) expression.
The change in gene expression in the TCGA or GEO datasets is based on only one time-point. Hence it does not clearly reflect the diurnal expression pattern in tumor samples. However, improved algorithm (CYCLOPS) that can temporally order gene expression datasets have found important differences in circadian gene expression pattern in hepatocellular carcinoma (HCC) and in normal liver tissues. Such analyses show that a consistent fraction of rhythmic transcripts (nearly 15%, including PER1 and CRY1) do not display circadian oscillations in cancer (45). Similarly, gene expression analyses in metastatic melanoma suggest that circadian oscillations are impaired when compared to normal skin (46).
Altogether, these observations indicate circadian gene expression alterations are shared features of different tumor types. Such changes in clock gene expression in tumors may have functional relevance. Few studies have found changes in the expression of Rev-erb and Cry in tumor samples that influence the survival of glioblastoma, colorectal, and gastric cancer patients (Fig. 2) (43,47–49). Disruption of circadian gene expression can be caused not only by genetic events. For example, acidification of tumor microenvironment leads to circadian clock alterations (as discussed later (50) and autophagy, which is highly upregulated in several tumor types trigger CRY1 degradation (51). In summary, genetic and genomic analyses of tumors have indicated that changes in the expression of clock genes are more widespread than mutations in clock genes. This lead to the hypothesis that oncogenic programs may disrupt circadian regulation, which in turn further fuels tumor growth.
Circadian clock control of cancer hallmarks
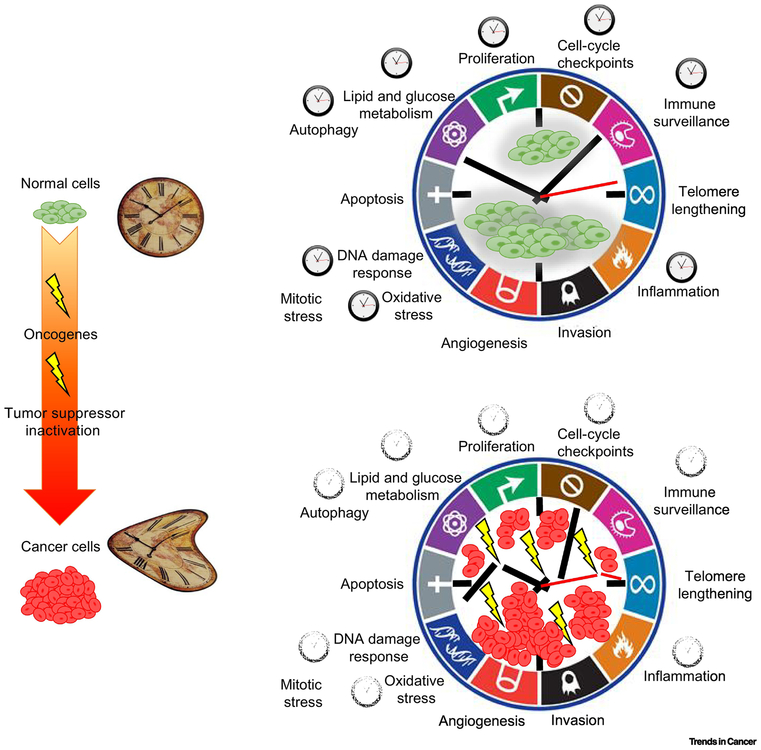
Several processes driving tumorigenesis have been extensively investigated and are known as “cancer hallmarks” (2). Unbiased circadian transcriptome studies and focused mechanistic studies are converging on the idea that in normal cells the circadian clock exerts tight control over several cancer hallmarks. Escape from circadian regulation may enable these cancer hallmarks to facilitate the transformation of normal cells to malignant cells. The following section highlights the molecular connections between the circadian clock and cancer hallmarks (Fig. 3, Key Figure).
FIGURE 3, Key Figure. Disruption of circadian rhythms during tumorigenesis and impact on cancer hallmarks.
Normal cells and tissues display circadian oscillations. A large body of evidence has connected circadian clock genes to several important regulatory nodes for cellular transformation. Given the pleiotropic role of the circadian clock in physiology it is not surprising that several cancer hallmarks are under circadian clock control. During cancer initiation circadian rhythms are disrupted by oncogenes; this suggests that loosening of the circadian clock function may facilitate the instauration of cancer hallmarks and play a fundamental role in tumorigenesis. Adapted from “Hallmarks of Cancer: The Next Generation,” 2011 Cell.
Sustaining proliferative signal.
In normal cells, DNA replication and cell cycle are tightly regulated by regulatory mechanisms known as checkpoints. Bypassing of these checkpoints leads to uncontrolled cell proliferation. In many organisms, DNA replication more likely occurs at night (52–55). This temporal regulation encourages cells to divide within a time-window, thereby restricting uncontrolled proliferation. Such night-time DNA replication supports the notion that circadian rhythm might have evolved as a flight-from-(UV) light mechanism to safeguard DNA.
We are beginning to understand how circadian clock machinery may exert temporal regulation on DNA replication and cell cycle. Many cell cycle regulators show daily rhythms in their expression: CDK2, CDK4, CDK6 (G1/S); Cdc25a, Cdc25b (G1/S, G2/M); the CDK inhibitors p21, p57, p27 and several cyclins including CycD1, CycD2, CycA1, CycA2, CycB1, and CycE1 (56,57). Some of these genes are directly controlled by the CLOCK/BMAL or the ROR/REV-ERB arms of the circadian oscillator (27,53,58)
One of the examples of circadian gating of the cell cycle is the temporal regulation of G1-S transition. P16-Ink4A is an important factor mediating the G1/S transition. It inhibits CDK4 and CDK6- mediated phosphorylation of pRB, thus sequestering E2F transcription factors in inactive pRb/E2F complexes leading to cell cycle arrest. Interaction between clock component PER2 and RNA binding protein NONO on p16 promoter drives a daily rhythm in p16-Ink4A expression (59). This correlates with the circadian rhythm in pRB phosphorylation (Ser807/811) (60). Circadian rhythm disruption releases this temporal regulation of G1-S transition and results in proliferation increase accompanied by pRB hyperphosphorylation and parallel upregulation of Myc, CycD1/3, and Cdt1 (60).
In addition to cell cycle regulators, tumor suppressors and oncogenes also show circadian regulation. One of the most important tumor suppressive proteins, p53, has circadian oscillations and its transcription, stability, and activity is modulated by BMAL1 and PER2 (61–63). Lack of PER2 impairs activation of p53 upon DNA damage possibly due to a role of PER2 in the stabilization and nuclear translocation of p53 (27,62,64,65). Similarly, downregulation of BMAL expression impairs p53-dependent induction of p21 (66). An additional mechanism is involved in the stabilization of the p53 protein. ATF4 (Activating transcription factor (ATF)/cAMP response element (CRE)-binding (CREB) 4) drives circadian expression of p14ARF (Cdkn2a) which is a known inhibitor of MDM2, the main ubiquitin ligase responsible for p53 protein stability (67). In addition, association with PER2 protects p53 from MDM2 mediated degradation (62). Clock genes, therefore, exert their control of p53 on multiple levels. Conversely, tumor suppressive mechanisms ensure the maintenance of circadian rhythmicity. Indeed, the p53 pathway (specifically p53 and MDM2) regulates PER2 levels (68,69) thus establishing a feedback loop potentially relevant for tumor suppression and anticancer therapies.
Another reciprocal interplay occurs between c-MYC and the circadian clock, c-MYC display circadian oscillations and is directly regulated by clock genes both at transcriptional (by BMAL1 and NPAS2) and post-translational levels (by CRY2) (27,70). This suggests that alteration of the circadian clock may modulate c-MYC activity. c-MYC can also affect circadian clock function through at least two different mechanisms (E-box dependent and independent). In a complex with MIZ1, c-MYC directly repress BMAL1, CLOCK, and NPAS2 (E-box independent) (71). In addition, c-MYC can upregulate the expression of the circadian repressors REV-ERBα (NR1D1) and REV-ERBβ by binding to the E-boxes present in their promoters (E-box dependent). Increased REV-ERBs consequently repress BMAL1 expression (72). Interestingly, MYC can also form a complex with BMAL and interfere with CLOCK/BMAL transcriptional activity at the Per1 promoter (73).
The RAS/MAPK pathway is highly active in different tumor types. Several pieces of evidence derived in non-cancerous tissue suggest that RAS activity can modulate circadian rhythms (reviewed in (74)). An important component of the RAS signaling pathway is PI3 kinase (PI3K), with pleiotropic effects in cancer and glucose metabolism. In non-malignant cells, PI3K seems to be required for BMAL1/CLOCK heterodimerization (75). This may explain a recent observation that aberrant RAS activation leads to alteration of circadian rhythms possibly via impairment of BMAL1/CLOCK activity and upregulation of Ink4a/Arf (76,77).
In summary, there is increasing evidence to support the notion that intricate reciprocal regulation among circadian clock components, oncogenes, tumor suppressors, and cell cycle regulators ensures homeostatic regulation of proliferation in normal cells. Chronic circadian rhythm disruption impairs this homeostasis and facilitates tumor initiation while oncogenic agents further disrupt circadian clock function to sustain proliferation.
Enabling replicative immortality.
Normal cells have limited replicative potential, while cancer cells have the hallmark of unlimited replicative potential (immortalization).
Telomerase, the essential enzyme that ensures correct maintenance of chromosomes ends (telomeres), plays a critical role in maintaining telomere length in cancer cells thus avoiding replicative senescence triggered by excessive telomere shortening. Remarkably this fundamental process is controlled by the clock machinery. Telomerase expression, is directly regulated by BMAL/CLOCK (78) possibly through the E-boxes present in the TERT gene promoter. Both the mRNA and protein level of TERT display circadian rhythms (in mice and humans). Thus, alterations in clock genes may directly affect TERT expression and facilitate cellular immortalization. Additionally, CLOCK-deficient mice have a shorter life span, and cells lacking CLOCK display short telomere length (78). Conversely restoring telomerase levels can reactivate circadian rhythms in senescent cells (79,80), thus suggesting the existence of a convoluted balance between the circadian clock, proliferation, and tumor suppression.
Genome instability & mutation.
A tight crosstalk exists between the DNA damage response pathway (DDR), DNA repair, and the circadian machinery. Many DNA repair genes show circadian rhythms in their mRNA expression and protein accumulation (7,56). Furthermore, several clock components physically interact with components of the DDR pathway. Upon DNA damage, PER1 forms complexes with ATM and Chk2 and modulates ATM activity. Reduced expression of PER1 impair ATM-dependent phosphorylation of Chk2 (81) and thereby impairs appropriate cellular response upon DNA damage. CRYs are structurally similar to photolyases, a class of enzymes involved in DNA repair of single strand breaks. Although mammalian CRYs lack DNA repair activity, CRY1 facilitates ATR interaction with TIM (Timeless) resulting in circadian rhythm in ATR activity (82). Indeed, in vivo upon treatment with cisplatin, a chemotherapeutic drug that induce DNA damage, the phosphorylation of the ATR target MCM2 is higher when CRY1 expression peaks. Similarly, CRY2 may facilitate interactions between ATR, TIM and Chk1 (83). Altogether circadian rhythms in CRYs and PERs along with the previously described interactions with key DDR pathway members may constitute a daily rhythm in normal cell’s ability to sense and repair damaged DNA.
DNA repair.
Not only DDR is regulated by the circadian clock but also DNA repair mechanisms and in particular the Nucleotide Excision Repair pathway. XPA is a key mediator of this pathway. Interestingly the promoter of Xpa gene contain E-boxes which mediates transcriptional activation by CLOCK/BMAL1 and repression by CRYs and PERs, which results in rhythmic expression of Xpa (84). DNA damage can also directly influence circadian rhythms. The potential mechanism involves poly-ADP ribose polymerase, which is activated by DNA damage to quickly produce polyADP ribose polymers from NAD, which may impact circadian clock function through NAD-dependent deacetylase Sirtuins. In fact, DNA damage is known to affect the circadian clock leading to changes in the phase of circadian rhythm (85,86) and deficiency of PARP1 also changes the pace of resetting of the circadian clock (87).
Deregulating cellular metabolism.
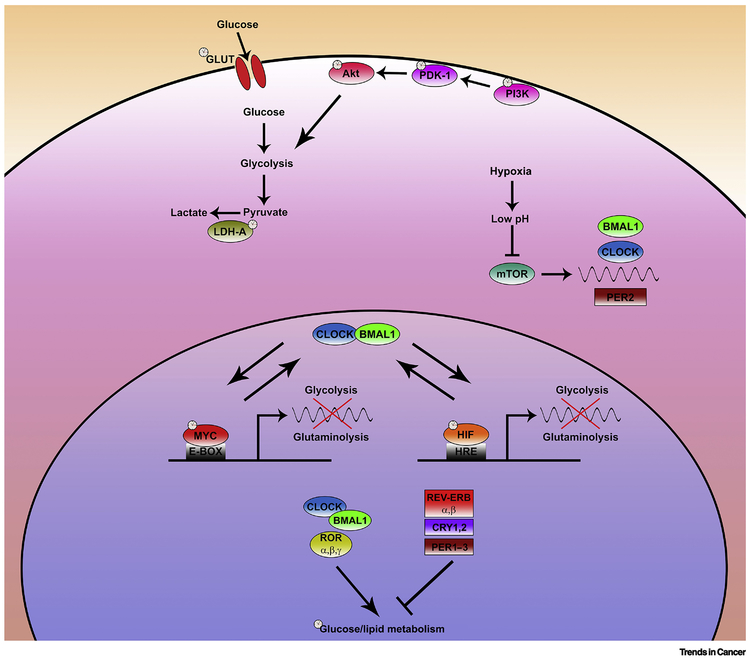
One of the conserved roles of the circadian clock in different organs is the regulation of catabolic and anabolic metabolism and regulation of cellular defense to oxidative stress that can arise from nutrient metabolism (88). As cancer cells have metabolic requirements that are distinct from normal cells, future studies will need to assess how circadian disruption facilitates cancer metabolism (Fig. 4).
FIGURE 4. Circadian clock interplay with cancer metabolic nodes.
Aerobic glycolytic switch is observed in many tumor types. Intriguingly circadian clock may control glycolysis in cancer cells through multiple steps. Several players sustaining aerobic glycolysis in cancer such as the PI3K/AKT cascade, GLUT transporters, LDHA, Myc and HIF are modulated by circadian rhythms. The extent of the interplay occurring between CLOCK, BMAL, MYC and HIF is not currently characterized and may unveil important connections affecting both glycolysis and glutaminolysis. Similarly, the tight interactions existing between glucose/lipid metabolism and clock genes have been only barely investigated for their relevance in cancer. Environmental changes associated with the cancerous state also affect the circadian clock. Hypoxia results in an acid microenvironment which block mTOR activity. mTOR has been recently suggested to play an important role in the translation of several core clock genes.
An interesting link was provided by a recent study showing that chronic jet lag drives hepatocellular carcinoma (HCC) by triggering the development of non-alcoholic fatty liver disease (25). Jet lag induces global changes in gene expression and alters the levels of several metabolites. These metabolic alterations lead to insulin resistance, non-alcoholic fatty liver disease, and liver steatohepatitis (25). This profound metabolic syndrome ultimately culminates with the development of HCC (25).
Elevated glycolytic metabolism (Warburg effect) feeds cancer cell energy requirements and at least two pathways are known to increase glycolysis in cancer cells. Constitutive activation of the PI3K/Pdk1/AKT pathway promotes the glycolytic program in cancer (89). Furthermore, the low oxygen level in the tumor also activates HIF1a, which in turn contributes to increased glycolysis. Circadian regulation of glucose metabolism in the context of cancer involves at least three interrelated mechanisms. The circadian clock controls the expression of several genes (e.g. glucose transporters and lactate dehydrogenase-A) involved in transport and metabolism of glucose in glycolytic and oxidative phosphorylation (90,91).
Circadian clock components also reciprocally interact with AKT and HIF1a pathways. Several components of the P13K/Pdk1/AKT pathway exhibit circadian rhythm in their protein accumulation (92). Conversely, activation of the AKT pathway affects the pace of the circadian clock (93). Similarly, circadian clock imposes circadian rhythm in HIF1α through both transcriptional and post-translational mechanisms (94–96). Conversely, hypoxia and HIF1α affect circadian rhythms through regulation of the circadian clock genes CRYs, Rorα, Per2, and Cry1 (95,97). In cancer cells hypoxia may impair circadian clock machinery through acidification of the microenvironment. Cellular acidification affects mTORC1 and disable mTORC1 dependent control of translation resulting in low protein levels of clock regulators (BMAL, CLOCK, and PER2) (50) (Fig. 4). Additionally, ChIP-seq analyses have shown significant overlap between BMAL and HIF1α targets (95). Such shared targets may explain the altered or absence of rhythms in several antioxidant enzymes in tumors which fosters oxidative stress (98).
Tumor-promoting inflammation - Avoiding immune destruction.
Aberrant inflammation, immune suppression, and evasion of immune surveillance by fostering cell proliferation, cell survival, angiogenesis, and tissue invasiveness play a critical role in tumorigenesis (99). The circadian clock and clock components regulate several aspects of immune system and inflammatory processes (100) (Fig. 5).
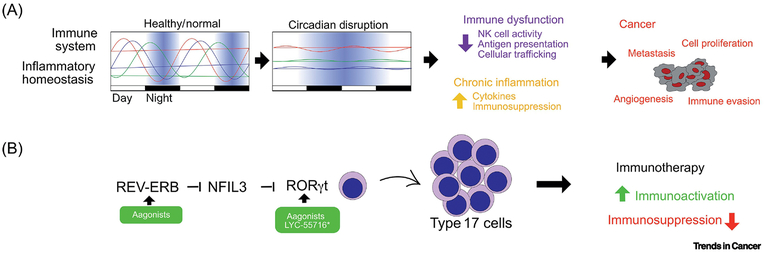
FIGURE 5. Role of the circadian clock in the tumor-immune interactions and immunotherapy.
The circadian clock imposes rhythms in key components of the immune system and maintain homeostasis in inflammatory processes. Disruption of circadian rhythms affects multiple immune system functions and leads to chronic inflammation. These alterations foster tumor initiation, progression and metastasis. Intriguingly, the crosstalk occurring between the circadian clock and the immune system can be exploited for the generation of innovative immunotherapy approaches. Indeed, the RORγ agonist Lyc-55716 is currently tested in clinical trials as monotherapy and in combination with anti PD-1 in patients with advanced solid tumors. Interestingly, pharmacological activation of RORγ has a dual effect: lead to the activation of Th17 cells and attenuate immunosuppression. A similar effect could be achieved by activation of REV-ERBs
Circadian disruption in rodents subjected to a jet-lag protocol resulted in alteration in the rhythms of cytolytic factors and cytokines. Circadian rhythm disruption therefore leads to attenuation of the natural killer (NK) cells activity and accelerates lung tumor growth (101).
Impairment of several core clock genes in mice models results in a massive dysregulation of proinflammatory cytokines that resemble chronic inflammation. Genetic perturbation of the circadian clock components – Per2 (102), Bmal (100), Cry (103), Rev-Erbs (104–106) – alter the level of several cytokines and chemokines (including GM-CSF, CCL2, CXCL6, CCR2, CX3CR1, IL-2, IL-10, IL-6, IL19, IL-1β, TNF-α, MCP-1/JE, MMP9, and IFN-γ) or blunt the dynamic difference of circadian time-dependent gene activation (100,107). Myeloid-specific knockout of BmaI1 results in elevated serum proinflammatory cytokines including IL6, IL1b, CCL2, and TNF leading to a proinflammatory state (100).. While some cytokines are direct transcriptional targets of clock components, some are indirectly regulated. For example, Bmal1 impose rhythmic oscillation in NRF2 which controls IL-1β expression, and Bmal1 deletion results in IL-1 β increased production (108).
The circadian clock components BMAL1, CLOCK, and CRYs modulate the function of NF-κB one of the central regulators of inflammation. Physiological downregulation of BmaI1 during an inflammatory response is associated with activation of NF-κB through phosphorylation of the p65 subunit (109). CLOCK associates with p65 and stimulates the transcription of NF-kB driven inflammatory genes (107). CRYs regulates NF-κB activation through modulation of adenylyl cyclase; an enzyme that controls cAMP levels and activity of the protein kinase A (100,102). PKA, promotes activation of NF-kB through phosphorylation of p65. Consequently, lack of CRYs in fibroblasts and macrophages results in higher expression of NF-κB targets including TNF, IL-6, and CXCL1 (103).
Impairment of the physiological oscillations of cytokines triggered by oncogenic transformation may not only impact the tumor microenvironment and neighboring tissues but also have an effect on distal organs (42). Indeed, mice bearing lung adenocarcinoma are characterized by an increase of IL-6 in the serum, which in turn affects circadian rhythms in gene expression and metabolism in multiple distant organs (liver, white adipose tissue, WAT, muscle). Such reprogramming of circadian gene expression could also play a role in cachexia and metastasis (41,42,110). Indeed hyperactivation of the IL-6 pathway in the WAT, observed in a well-established colon cancer model for the study of cachexia, lead to impairments in the rhythmicity of several key lipogenesis genes such as PPARγ, C/EBPα, FAS, Scd1, and Dgat2 (110). The authors also observed an abrogation of the rhythmicity of REV-ERBα and Per2 and altered expression of BMAL1 and CRY1. These perturbations may contribute to WAT depletion (110).
Evasion of immune surveillance, through immunosuppression, is critical to tumor progression (111). Aberrant release of proinflammatory cytokines such as CXCL12, IL-18, IL-1 β, IL-10 and others contribute to immunosuppression in the tumor microenvironment. Therefore, alterations affecting the circadian clock could also play a fundamental role in immunosuppression.
Among the immune suppressive cytokines, Tissue Growth Factor beta (TGFβ) is a chief mediator. TGFβ inhibits DC maturation (112), shifts the balance from Th1 to Th2 (113), suppresses cytotoxic T lymphocytes activation (114), and induces regulatory T cells (115). Components of the circadian clock have been implicated in TGFβ signaling under different physiological contexts (116–118). Interestingly, a recent study shows that high level of BMAL1 is associated with increased T-cell infiltration and activation in metastatic melanoma (46). Furthermore, patients with high BMAL1 expression respond better to anti-PD1 treatment that patients with low BMAL1 levels (46).
The circadian clock genes also play roles in immune cell differentiation, trafficking and function. In non-cancerous conditions BMAL1 orchestrates rhythmic oscillations of leukocyte numbers and together with Cry is known to play a role in B cell development (103). REV-ERBs are involved in the migration and cell adhesion of macrophages (106,118). Lack of CLOCK in mice leads to low levels of Th1 cells (119).BmaI, Clock, and Rev-Erb control differentiation of the Interleukin-17–producing CD4+ T helper (Th17) cells through RORγt and NFIL3 (119). How the circadian clock integrates these signals and modulates immune-tumor cell interaction in the tumor environment will deserve future investigations (Fig. 5).
Circadian Medicine – anticancer approaches based on circadian clocks
The science of circadian rhythm has led to three broad approaches for the prevention and management of diseases including cancer: sleep, diet, and medication timing. Sustaining a robust circadian rhythm through consistent daily behavioral patterns in sleep and eating may significantly reduce the risk for cancer. This topic is covered in recent articles (120,121). Timing of drugs to the appropriate phase of the patient’s circadian rhythm can potentially reduce adverse side effects and accelerate prognosis (122). This approach of timing therapy for improved prognosis is summarized in Box 2. There is an emerging approach to use drugs that directly or indirectly target circadian clock components to treat cancer. For simplicity, we will refer to this approach as “drugging the clock” and the compounds targeting clock components as “clock-drugs.”
Text Box 2: Chronotherapy, where do we stand?
Chronotherapy is an intervention performed at a specific time in order to maximize therapeutic response and minimize side effects. Most cancer chemotherapies affect highly proliferating cellular populations. Given the relationship between circadian clock, cell proliferation, DNA damage response and repair, it was hypothesized that timing the delivery of chemotherapeutic agents to follow circadian rhythms may maximize therapeutic efficacy but more importantly, minimize toxicities to non-cancerous cells. Indeed, several randomized control trials for breast (143), colorectal (144,145), and endometrial cancer (146) evaluating the timing of treatment showed reduced side effects including mucositis, stomatitis, and leukopenia. Two randomized control trials for colorectal cancer showed improved response rate and reduced toxicity in the chronotherapy group (144,145). While these results are promising larger studies lead to mixed results with small benefits in survival observed only in men (147). Although the principle is simple and intuitive chronotherapy remain confined to few trials due to the heterogeneity of treatments, tumor types and the variability of the results obtained. Indeed, in 2016 only 0.16% of clinical trials were taking the timing of the drug administration into account (148). Immunotherapy also comes with significant adverse effects, causing inflammatory response in a cohort of organs associated with the use of immune checkpoint inhibitors (149), or a septic-shock like syndrome due to a rapid release of cytokine associated with CAR-T cell therapy (Cytokine Release Syndrome, CRS). Thus, minimizing immune related toxicity is clinically important. Utilizing the knowledge of circadian physiology may improve his approach. Cytokine levels, immune cells trafficking, and acute inflammatory response are tightly regulated by the circadian clock. Mortality rates to septic shock can vary up to 8–10 fold in mice depending on when the endotoxin is introduced, and experimental disruption of the circadian clock abolished this diurnal difference (150–153). Whether CRS can be minimized by infusion of CAR T-Cell at a specific time of the day should be investigated. The cornerstone of chronotherapy is identifying and exploiting the presence of an oscillatory mechanism that upon selection of the right time provide optimized therapeutic windows. While this may pose technical and practical challenges (148), the recent advances in “-omics” technology, machine learning algorithms, programmable pumps for drug delivery, wearable devices and blood tests to easily assess in real-time circadian rhythms, may provide new opportunities for the integration of chronotherapy into precision medicine.
The rationale for drugging the clock to treat cancer are as follows: (A) Clock-drugs acting through one clock component can have pleiotropic benefits on multiple cancer hallmarks. Therefore, clock-drugs can have the same benefit as a combination of two or more drugs targeting different pathways. (B) Clock-drugs can sustain rhythms in normal tissues in which circadian function may be disrupted by paracrine factors from tumors. Therefore, clock-drugs can reduce secondary health burdens of cancer. (C) Due to the interlocked feedback loop nature of the circadian mechanism, a normal circadian clock is resilient to wide fluctuation in the cellular biochemical environment. This molecular resilience of a normal circadian clock may attenuate any adverse effect or toxicity of clock-drugs in normal cells. These rationales also support the idea of using clock-drugs alone or in combination with other cancer therapy.
Clock-drugs for cancer therapy.
Two types of clock-drugs are currently available: drugs that can directly modulate the activity of circadian core genes and drugs targeting regulators of circadian core clock genes. Drugs in the second category target proteins that phosphorylate or degrade clock components among other target proteins and hence are not specific for clock components. These include compounds targeting Casein Kinase 1δ, Casein Kinase 1ε, and the F-box protein Fbxw7. Two members of the Casein Kinase 1 family (CK1δ and CK1ε) are known to play an essential role on the regulation of the circadian clock machinery (Fig. 1) (123) and their pharmacological inhibition alters circadian rhythms in vivo (124). Both CK1δ/ε are highly expressed in several tumor types including leukemia, breast, pancreas, and ovarian cancer and they are targets of interest for anticancer therapy (125). Interestingly, new potent CK2 inhibitors recently developed affect the growth of human renal cell carcinoma (RCC) lines (126).
Fbxw7 promotes ubiquitination and degradation of REV-ERBα (127). Forcing nuclear retention of Fbxw7 by using specific inhibitors of nuclear export (SINE) impair tumor growth both in vitro and in xenograft models (128). A compound from the herbal extract Oridonin has some anticancer effects, which require Fbxw7 (129). However, it is unclear if the anticancer properties of compounds targeting CKs and Fbxw7 are mainly mediated through their circadian clock targets.
Clock-drugs have been recently developed as tools to directly target circadian clock components including RORγ, REV-ERBs, and CRYs. We will discuss a few examples of their potential use as cancer therapeutics. The observation that metastatic castrate-resistant prostate cancer (CRPC) tumors express high levels of RORγ and that RORγ activates the expression of the androgen receptor (AR), prompted researchers to test whether RORγ inhibition can affect prostate cancer (44). Importantly in vivo administration of RORγ antagonists blocks the growth of AR-expressing tumors and restores sensitivity toward enzalutamide (ENZ, an androgen signaling inhibitor currently used for prostate cancer treatment) in resistant tumors (44). By combining computational and functional genomic data RORγ was identified as a master regulator of pancreatic cancer stem cells (130). Pharmacological inhibition of RORγ impair tumor growth and improve survival in multiple pancreatic cancer in vivo models (130). Nobiletin, a small-molecule natural product, present in the citrus peel, with known anticancer properties was recently identified as an activator of RORs (131).
Multiple REV-ERBs agonists as reported in different studies show anticancer activity towards several tumor types and can affect glioblastoma growth in vivo (36,47,48,132,133). REV-ERBs potently repress expression of autophagy genes and de novo lipogenesis rate-limiting enzymes FASN and SCD1 (47,132). Both autophagy and de novo lipogenesis are crucial metabolic pathways that allow cancer cells to cope with their elevated metabolic demands. Pharmacological activation of REV-ERBs leads to the simultaneous suppression of both pathways, which are necessary for antitumor properties of REV-ERB agonists. Importantly, REV-ERB agonists anticancer activity is abolished upon Rev-erb downregulation, thus suggesting that REV-ERBs are potential targets for cancer treatment. These observations highlight how targeting of circadian core clock genes could result in combination therapy. Another well-characterized class of compounds are potent modulators of CRY proteins. In vitro observations are showing that pharmacological inhibition of CRY reduces cancer cell proliferation while having no effect on normal epithelial breast lines (134).
Clock-drugs in immunotherapy.
While the above-mentioned use of clock drugs is in vivo, one promising strategy involves ex-vivo use of clock-drugs on IL17A expressing T-cells to augment CAR T cell therapy. RORγt is a master transcription factor for Type 17 T cells. Supplementation of a RORγt agonist during ex-vivo expansion increases the antitumor activity of the Th17 cells engineered with CAR and mice with RORγt-primed T cells are protected from cancer when tumor cells are reintroduced in the animal (135,136).
RORγ agonists can also act as monotherapy in vivo (Fig. 5). Importantly administration of RORγ agonists displays antitumor properties due to the engagement of multiple tumor suppressive mechanisms, including boosting the activity of Th17 cells and blocking immunosuppression driven by Treg (135). Treatment with RORy results in increased production of IL-17A, GM-CSF and of the co-stimulatory receptor CD137 and CD226 thus promoting T-cell cell growth and survival. In addition, RORy agonists affect immunosuppression by attenuating the expression of co-inhibitory receptors PD-1 and TIGIT, and by blunting the activity of Treg through downregulation of CD39 and CD73 expression (135).
An optimized version of RORy agonist as a single agent (clinical trial NCT0292862) or in combination with pembrolizumab (anti PD-1, clinical trial NCT03396497) is currently in clinical trials for the treatment of patients with advanced, relapsed, or refractory solid tumors and no major adverse events were observed in the Phase I.
Concluding remarks
The emerging connection between circadian clock and cancer is raising new hope for cancer prevention and opening new opportunities for innovations in lifestyle intervention, genetic testing, diagnostic markers, chronotherapy (text Box 2), and finally novel therapeutics targeting clock components. Yet, future investigations are needed to address several key issues (see Outstanding Questions).
Outstanding questions.
Are core clock genes alterations driving tumorigenesis because of their effect on circadian rhythms?
What are the diagnostic criteria to assess the potential use and efficacy of clock-drugs in cancer?
Are there tumor types or stages where circadian rhythm disruption is no longer a limiting factor?
Can circadian clock alteration fuel metastasis and through which mechanisms?
Can restoration of circadian rhythms through behavioral and pharmacological intervention minimize the risk to develop cancer, impair tumor progression, and generate new therapeutic avenues?
Can we develop standard clinical practices incorporating circadian behavioral modification, non-prescription supplementation and chronotherapy of existing cancer drugs to improve efficacy and reduce side effects of contemporary cancer treatment?
Can we differentiate tumor-specific circadian clock alterations from the normal tissues and develop new diagnostic tools?
Can we exploit the interconnections occurring between circadian clock genes and immune system to derive novel and more safe immunotherapy approaches?
What are the off-target effects of existing tool compounds targeting specific circadian clock components? How to assess the contribution of their clock- and non-clock targets on therapeutic effects?
While long-term exposure to shiftwork is known to increase the risk of cancer, shiftwork is essential for modern society. Circadian science can reduce the risk of cancer among shift workers. Controlled clinical studies on novel lifestyle interventions combining optimal timing of food and light exposure among shift workers are needed to reduce cancer risk. Interestingly genetic variations in clock genes (such as Neuronal PAS domain protein 2, NPAS2 (rs23051560) (137), RORα (rs1482057 and rs12914272), CLOCK (rs11932595) (138), BMAL1 (rs2290035, rs2278749, rs969485), and ROR-b (rs3750420)(139) are associated with increased risk of breast cancer while other polymorphisms BMAL1 (rs2278749) CLOCK (rs3749474) ROR-β (rs3903529, rs3750420) NPAS2 (rs17024926) lead to a reduced risk (139). While the mechanistic relevance of these polymorphisms needs further characterization, they raise the possibility of genetic tests for clock gene polymorphisms as an additional screening tool to reduce cancer risk in shiftworkers. Stratification of different types of shiftwork and cancer risk can further improve employment policies for shiftworkers.
Diagnostic markers of circadian rhythm disruption and their predictive value for cancer risk is another area for innovation. Chronic circadian rhythm disruption in animal models may leave stable epigenetic changes. This has raised hope for diagnostic markers for circadian rhythm disruption.
Although cancer chronotherapy has been studied for decades, it is yet to be a standard practice in cancer treatment. There is limited data on the efficacy of chronotherapy beyond a handful of cancer drugs. Lack of a standardized circadian phase markers among cancer patients further complicates its adoption. Timing of chemotherapy is also constrained by hospital scheduling. Research in these specific areas along with the adoption of improved wearable devices and drug pumps for automatic delivery of cancer drugs at a specific time may leverage the value of chronotherapy.
Despite the excitement around potential of clock-drugs, it is not clear if some of the clock-drugs offer therapeutic benefits due to improving circadian rhythm or due to non-circadian rhythm function of the target in cellular function. At least some studies have shown that forcing circadian rhythmicity by acute administration of dexamethasone, forskolin, heat shock, and melatonin reinstate the circadian clock control of cell cycle genes thus reducing the proliferation of different cancer cell lines (140,141). However, we cannot rule out the possibility that some of the effects of clock-drugs, such as the effect of RORγ modulators on AR and T-cells, may be due to a function of the target protein in cellular mechanisms that are less dependent on a functional circadian rhythm. This question about the role of clock genes vs. circadian clock function in tumor needs additional investigation as it relates to which type of tumor would best benefit from clock drugs. In order to move this nascent strategy towards clinical applications a massive effort encompassing chemistry and pharmacology will be necessary to generate agents targeting circadian clock genes with optimized potency and specificity. As well it will be important in the future to assess the consequences of long-term treatment with clock drugs.
Several aspects of cancer-circadian clock axis are sparsely studied. It is not clear if tumor types that retain or have lost circadian rhythms differ in their response to existing cancer drugs. While several reports assessed the role of clock genes in cancer initiation more investigations are needed to characterize the involvement of clock genes in metastasis. Finally, many cancer drugs are known to leave a lasting impact on endocrine organs, which leads to additional complications among cancer survivors (142). As endocrine tissues are critical hubs in circadian regulation of whole-body physiology, diagnosis, and intervention in circadian endocrine perturbation can improve the quality of life of cancer survivors. In summary, the novel intersection between circadian rhythms and medicine has untapped potential to make a significant contribution to the prevention and treatment of cancer.
Highlights.
The circadian clock establishes daily rhythms in multiple physiological processes, to maintain homeostasis.
Circadian rhythm disruption and circadian core clock alterations promote cancer initiation.
The circadian clock machinery and circadian rhythms control several cancer hallmarks.
Pharmacological modulation of circadian clock provides a novel anticancer strategy.
GLOSSARY
- Diurnal rhythm
Any oscillating pattern that cycles every 24 hours
- Circadian rhythm
Any self-sustained, oscillating physiological processes that cycle with a near 24-hour period. It can adapt to the rhythmicity of external cues (such as light/dark cycle)
- Chronic jet lag
An experimental model mimicking frequent transmeridian flights or shiftwork, in which external circadian cues such as light or food are repeatedly shifted from the standard 12hr:12hr light:dark cycles or active/resting phase. Chronic repetitive deviation from a 24-hour period resulted in disrupted circadian rhythmicity in behavior, physiology, or gene expression
Footnotes
RESOURCES
iii https://www.cancer.gov/about-nci/organization/ccg/research/structural-genomics/tcga
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Sulli G, Manoogian ENC, Taub PR, Panda S. Training the Circadian Clock, Clocking the Drugs, and Drugging the Clock to Prevent, Manage, and Treat Chronic Diseases. Trends Pharmacol Sci. 2018;39(9):812–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011. March 4;144(5):646–74. [DOI] [PubMed] [Google Scholar]
- 3.Hatori M, Panda S. The emerging roles of melanopsin in behavioral adaptation to light. Trends Mol Med. 2010. October;16(10):435–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hastings MH, Maywood ES, Brancaccio M. Generation of circadian rhythms in the suprachiasmatic nucleus. Nat Rev Neurosci. 2018. August; 19(8):453–69. [DOI] [PubMed] [Google Scholar]
- 5.Cho H, Zhao X, Hatori M, Yu RT, Barish GD, Lam MT, et al. Regulation of circadian behaviour and metabolism by REV-ERB-alpha and REV-ERB-beta. Nature. 2012. May 3;485(7396): 123–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sato TK, Panda S, Miraglia LJ, Reyes TM, Rudic RD, McNamara P, et al. A functional genomics strategy reveals Rora as a component of the mammalian circadian clock. Neuron. 2004. August 19;43(4):527–37. [DOI] [PubMed] [Google Scholar]
- 7.Mure LS, Le HD, Benegiamo G, Chang MW, Rios L, Jillani N, et al. Diurnal transcriptome atlas of a primate across major neural and peripheral tissues. Science. 2018. 16;359(6381). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jewett ME, Kronauer RE, Czeisler CA. Light-induced suppression of endogenous circadian amplitude in humans. Nature. 1991. March 7;350(6313):59–62. [DOI] [PubMed] [Google Scholar]
- 9.Salamanca-Fernández E, Rodríguez-Barranco M, Guevara M, Ardanaz E, Olry de Labry Lima A, Sánchez MJ. Night-shift work and breast and prostate cancer risk: updating the evidence from epidemiological studies. An Sist Sanit Navar. 2018. August 29;41 (2):211–26. [DOI] [PubMed] [Google Scholar]
- 10.Papantoniou K, Devore EE, Massa J, Strohmaier S, Vetter C, Yang L, et al. Rotating night shift work and colorectal cancer risk in the nurses’ health studies. Int J Cancer. 2018. 01;143(11):2709–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Straif K, Baan R, Grosse Y, Secretan B, El Ghissassi F, Bouvard V, et al. Carcinogenicity of shift-work, painting, and fire-fighting. The lancet oncology. 2007. December;8(12): 1065–6. [DOI] [PubMed] [Google Scholar]
- 12.Leung L, Grundy A, Siemiatycki J, Arseneau J, Gilbert L, Gotlieb WH, et al. Shift Work Patterns, Chronotype, and Epithelial Ovarian Cancer Risk. Cancer Epidemiol Biomarkers Prev. 2019. March 6;28(5):987–995 [DOI] [PubMed] [Google Scholar]
- 13.Bhatti P, Cushing-Haugen KL, Wicklund KG, Doherty JA, Rossing MA. Nightshift work and risk of ovarian cancer. Occup Environ Med. 2013. April;70(4):231–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carter BD, Diver WR, Hildebrand JS, Patel AV, Gapstur SM. Circadian disruption and fatal ovarian cancer. Am J Prev Med. 2014. March;46(3 Suppl 1):S34–41. [DOI] [PubMed] [Google Scholar]
- 15.Wendeu-Foyet MG, Bayon V, Cénée S, Trétarre B, Rébillard X, Cancel-Tassin G, et al. Night work and prostate cancer risk: results from the EPICAP Study. Occup Environ Med. 2018. August;75(8):573–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cordina-Duverger E, Menegaux F, Popa A, Rabstein S, Harth V, Pesch B, et al. Night shift work and breast cancer: a pooled analysis of population-based case-control studies with complete work history. Eur J Epidemiol. 2018;33(4):369–79. [DOI] [PubMed] [Google Scholar]
- 17.Marinac CR, Natarajan L, Sears DD, Gallo LC, Hartman SJ, Arredondo E, et al. Prolonged Nightly Fasting and Breast Cancer Risk: Findings from NHANES (2009-2010). Cancer Epidemiol Biomarkers Prev. 2015. May;24(5):783–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kogevinas M, Espinosa A, Castelló A, Gómez-Acebo I, Guevara M, Martin V, et al. Effect of mistimed eating patterns on breast and prostate cancer risk (MCC-Spain Study). Int J Cancer. 2018. 15;143(10):2380–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sephton SE, Sapolsky RM, Kraemer HC, Spiegel D. Diurnal cortisol rhythm as a predictor of breast cancer survival. J Natl Cancer Inst. 2000. June 21;92(12):994–1000. [DOI] [PubMed] [Google Scholar]
- 20.Sephton SE, Lush E, Dedert EA, Floyd AR, Rebholz WN, Dhabhar FS, et al. Diurnal cortisol rhythm as a predictor of lung cancer survival. Brain Behav Immun. 2013. March;30 Suppl:S163–170. [DOI] [PubMed] [Google Scholar]
- 21.Mormont MC, Waterhouse J, Bleuzen P, Giacchetti S, Jami A, Bogdan A, et al. Marked 24-h rest/activity rhythms are associated with better quality of life, better response, and longer survival in patients with metastatic colorectal cancer and good performance status. Clin Cancer Res. 2000. August;6(8):3038–45. [PubMed] [Google Scholar]
- 22.Innominato PF, Komarzynski S, Palesh OG, Dallmann R, Bjarnason GA, Giacchetti S, et al. Circadian rest-activity rhythm as an objective biomarker of patient-reported outcomes in patients with advanced cancer. Cancer Med. 2018. September;7(9):4396–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee S, Donehower LA, Herron AJ, Moore DD, Fu L. Disrupting circadian homeostasis of sympathetic signaling promotes tumor development in mice. PLoS ONE. 2010. June 7;5(6):e10995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Papagiannakopoulos T, Bauer MR, Davidson SM, Heimann M, Subbaraj L, Bhutkar A, et al. Circadian Rhythm Disruption Promotes Lung Tumorigenesis. Cell metabolism. 2016. August 9;24(2):324–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kettner NM, Voicu H, Finegold MJ, Coarfa C, Sreekumar A, Putluri N, et al. Circadian Homeostasis of Liver Metabolism Suppresses Hepatocarcinogenesis. Cancer Cell. 2016. December 12;30(6):909–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Filipski E, King VM, Li X, Granda TG, Mormont M-C, Liu X, et al. Host circadian clock as a control point in tumor progression. J Natl Cancer Inst. 2002. May 1;94(9):690–7. [DOI] [PubMed] [Google Scholar]
- 27.Fu L, Pelicano H, Liu J, Huang P, Lee C. The circadian gene Period2 plays an important role in tumor suppression and DNA damage response in vivo. Cell. 2002. October 4;111(1):41–50. [DOI] [PubMed] [Google Scholar]
- 28.Mteyrek A, Filipski E, Guettier C, Okyar A, Levi F. Clock gene Per2 as a controller of liver carcinogenesis. Oncotarget. 2016. December 27;7(52):85832–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wood PA, Yang X, Taber A, Oh EY, Ansell C, Ayers SE, et al. Period 2 mutation accelerates ApcMin/+ tumorigenesis. Molecular cancer research: MCR. 2008. November;6(11): 1786–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gu X, Xing L, Shi G, Liu Z, Wang X, Qu Z, et al. The circadian mutation PER2(S662G) is linked to cell cycle progression and tumorigenesis. Cell Death Differ. 2012. March;19(3):397–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ueda E, Kurebayashi S, Sakaue M, Backlund M, Koller B, Jetten AM. High Incidence of T-Cell Lymphomas in Mice Deficient in the Retinoid-Related Orphan Receptor RORgamma. Cancer Res. 2002. February 1;62(3):901–9. [PubMed] [Google Scholar]
- 32.Antoch MP, Gorbacheva VY, Vykhovanets O, Toshkov IA, Kondratov RV, Kondratova AA, et al. Disruption of the circadian clock due to the Clock mutation has discrete effects on aging and carcinogenesis. Cell cycle (Georgetown, Tex. 2008. May;7(9): 1197–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gauger MA, Sancar A. Cryptochrome, circadian cycle, cell cycle checkpoints, and cancer. Cancer Res. 2005. August 1;65(15):6828–34. [DOI] [PubMed] [Google Scholar]
- 34.Antoch MP, Toshkov I, Kuropatwinski KK, Jackson M. Deficiency in PER proteins has no effect on the rate of spontaneous and radiation-induced carcinogenesis. Cell Cycle. 2013. December 1;12(23):3673–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Janich P, Pascual G, Merlos-Suárez A, Batlle E, Ripperger J, Albrecht U, et al. The circadian molecular clock creates epidermal stem cell heterogeneity. Nature. 2011. November 9;480(7376):209–14. [DOI] [PubMed] [Google Scholar]
- 36.Puram RV, Kowalczyk MS, de Boer CG, Schneider RK, Miller PG, McConkey M, et al. Core Circadian Clock Genes Regulate Leukemia Stem Cells in AML. Cell. 2016. April 7;165(2):303–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ozturk N, Lee JH, Gaddameedhi S, Sancar A. Loss of cryptochrome reduces cancer risk in p53 mutant mice. Proc Natl Acad Sci USA. 2009. February 24;106(8):2841–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Matsu-Ura T, Dovzhenok A, Aihara E, Rood J, Le H, Ren Y, et al. Intercellular Coupling of the Cell Cycle and Circadian Clock in Adult Stem Cell Culture. Mol Cell. 2016. 01;64(5):900–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Janich P, Toufighi K, Solanas G, Luis NM, Minkwitz S, Serrano L, et al. Human epidermal stem cell function is regulated by circadian oscillations. Cell Stem Cell. 2013. December 5; 13(6):745–53. [DOI] [PubMed] [Google Scholar]
- 40.Hojo H, Enya S, Arai M, Suzuki Y, Nojiri T, Kangawa K, et al. Remote reprogramming of hepatic circadian transcriptome by breast cancer. Oncotarget. 2017. May 23;8(21):34128–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.de Assis LVM, Moraes MN, Magalhães-Marques KK, Kinker GS, da Silveira Cruz-Machado S, Castrucci AM de L. Non-Metastatic Cutaneous Melanoma Induces Chronodisruption in Central and Peripheral Circadian Clocks. Int J Mol Sci. 2018. 03; 19(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Masri S, Papagiannakopoulos T, Kinouchi K, Liu Y, Cervantes M, Baldi P, et al. Lung Adenocarcinoma Distally Rewires Hepatic Circadian Homeostasis. Cell. 2016. May 5;165(4):896–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ye Y, Xiang Y, Ozguc FM, Kim Y, Liu C-J, Park PK, et al. The Genomic Landscape and Pharmacogenomic Interactions of Clock Genes in Cancer Chronotherapy. Cell Syst. 2018. March 28;6(3):314–328.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang J, Zou JX, Xue X, Cai D, Zhang Y, Duan Z, et al. ROR-γ drives androgen receptor expression and represents a therapeutic target in castration-resistant prostate cancer. Nat Med. 2016. May;22(5):488–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Anafi RC, Francey LJ, Hogenesch JB, Kim J. CYCLOPS reveals human transcriptional rhythms in health and disease. Proc Natl Acad Sci USA. 2017. 16; 114(20):5312–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.de Assis LVM, Kinker GS, Moraes MN, Markus RP, Fernandes PA, de Castrucci AML. Expression of the Circadian Clock Gene BMAL1 Positively Correlates With Antitumor Immunity and Patient Survival in Metastatic Melanoma. Front Oncol. 2018;8:185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sulli G, Rommel A, Wang X, Kolar MJ, Puca F, Saghatelian A, et al. Pharmacological activation of REV-ERBs is lethal in cancer and oncogene-induced senescence. Nature. 2018. January;553(7688):351–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang X, Wang N, Wei X, Yu H, Wang Z. REV-ERB± reduction is associated with clinicopathological features and prognosis in human gastric cancer. Oncol Lett. 2018. August; 16(2): 1499–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yu H, Meng X, Wu J, Pan C, Ying X, Zhou Y, et al. Cryptochrome 1 overexpression correlates with tumor progression and poor prognosis in patients with colorectal cancer. PLoS ONE. 2013;8(4):e61679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Walton ZE, Patel CH, Brooks RC, Yu Y, Ibrahim-Hashim A, Riddle M, et al. Acid Suspends the Circadian Clock in Hypoxia through Inhibition of mTOR. Cell. 2018. 28;174(1):72–87.e32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Toledo M, Batista-Gonzalez A, Merheb E, Aoun ML, Tarabra E, Feng D, et al. Autophagy Regulates the Liver Clock and Glucose Metabolism by Degrading CRY1. Cell Metab. 2018. August 7;28(2):268–281.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Plikus MV, Vollmers C, de la Cruz D, Chaix A, Ramos R, Panda S, et al. Local circadian clock gates cell cycle progression of transient amplifying cells during regenerative hair cycling. Proceedings of the National Academy of Sciences of the United States of America. 2013. June 4;110(23):E2106–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Matsuo T, Yamaguchi S, Mitsui S, Emi A, Shimoda F, Okamura H. Control mechanism of the circadian clock for timing of cell division in vivo. Science (New York, NY. 2003. October 10;302(5643):255–9. [DOI] [PubMed] [Google Scholar]
- 54.Bjarnason GA, Jordan R. Circadian variation of cell proliferation and cell cycle protein expression in man: clinical implications. Prog Cell Cycle Res. 2000;4:193–206. [DOI] [PubMed] [Google Scholar]
- 55.Smaaland R Circadian rhythm of cell division. Prog Cell Cycle Res. 1996;2:241–66. [DOI] [PubMed] [Google Scholar]
- 56.Wang J, Mauvoisin D, Martin E, Atger F, Galindo AN, Dayon L, et al. Nuclear Proteomics Uncovers Diurnal Regulatory Landscapes in Mouse Liver. Cell Metab. 2017. 10;25(1):102–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Soták M, Sumová A, Pacha J. Cross-talk between the circadian clock and the cell cycle in cancer. Ann Med. 2014. June;46(4):221–32. [DOI] [PubMed] [Google Scholar]
- 58.Grechez-Cassiau A, Rayet B, Guillaumond F, Teboul M, Delaunay F. The circadian clock component BMAL1 is a critical regulator of p21WAF1/CIP1 expression and hepatocyte proliferation. The Journal of biological chemistry. 2008. February 22;283(8):4535–42. [DOI] [PubMed] [Google Scholar]
- 59.Kowalska E, Ripperger JA, Hoegger DC, Bruegger P, Buch T, Birchler T, et al. NONO couples the circadian clock to the cell cycle. Proc Natl Acad Sci USA. 2013. January 29; 110(5): 1592–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lee Y, Lahens NF, Zhang S, Bedont J, Field JM, Sehgal A. G1/S cell cycle regulators mediate effects of circadian dysregulation on tumor growth and provide targets for timed anticancer treatment. PLoS Biol. 2019. April; 17(4):e3000228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jiang W, Zhao S, Jiang X, Zhang E, Hu G, Hu B, et al. The circadian clock gene Bmal1 acts as a potential anti-oncogene in pancreatic cancer by activating the p53 tumor suppressor pathway. Cancer Lett. 2016. February 28;371(2):314–25. [DOI] [PubMed] [Google Scholar]
- 62.Gotoh T, Vila-Caballer M, Santos CS, Liu J, Yang J, Finkielstein CV. The circadian factor Period 2 modulates p53 stability and transcriptional activity in unstressed cells. Mol Biol Cell. 2014. October 1;25(19):3081–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gotoh T, Kim JK, Liu J, Vila-Caballer M, Stauffer PE, Tyson J J, et al. Model-driven experimental approach reveals the complex regulatory distribution of p53 by the circadian factor Period 2. Proc Natl Acad Sci USA. 2016; 113(47): 13516–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gotoh T, Vila-Caballer M, Liu J, Schiffhauer S, Finkielstein CV. Association of the circadian factor Period 2 to p53 influences p53’s function in DNA-damage signaling. Mol Biol Cell. 2015. January 15;26(2):359–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhanfeng N, Chengquan W, Hechun X, Jun W, Lijian Z, Dede M, et al. Period2 downregulation inhibits glioma cell apoptosis by activating the MDM2-TP53 pathway. Oncotarget. 2016. May 10;7(19):27350–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mullenders J, Fabius AW, Madiredjo M, Bernards R, Beijersbergen RL. A large scale shRNA barcode screen identifies the circadian clock component ARNTL as putative regulator of the p53 tumor suppressor pathway. PloS one. 2009;4(3):e4798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Horiguchi M, Koyanagi S, Hamdan AM, Kakimoto K, Matsunaga N, Yamashita C, et al. Rhythmic control of the ARF-MDM2 pathway by ATF4 underlies circadian accumulation of p53 in malignant cells. Cancer Res. 2013. April 15;73(8):2639–49. [DOI] [PubMed] [Google Scholar]
- 68.Miki T, Matsumoto T, Zhao Z, Lee CC. p53 regulates Period2 expression and the circadian clock. Nat Commun. 2013;4:2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Liu J, Zou X, Gotoh T, Brown AM, Jiang L, Wisdom EL, et al. Distinct control of PERIOD2 degradation and circadian rhythms by the oncoprotein and ubiquitin ligase MDM2. Sci Signal. 2018. Nov 13;11(556). [DOI] [PubMed] [Google Scholar]
- 70.Huber AL, Papp SJ, Chan AB, Henriksson E, Jordan SD, Kriebs A, et al. CRY2 and FBXL3 Cooperatively Degrade c-MYC. Molecular cell. 2016. November 17;64(4):774–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Shostak A, Ruppert B, Ha N, Bruns P, Toprak UH, ICGC MMML-Seq Project, et al. MYC/MIZ1-dependent gene repression inversely coordinates the circadian clock with cell cycle and proliferation. Nat Commun. 2016. 24;7:11807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Altman BJ, Hsieh AL, Sengupta A, Krishnanaiah SY, Stine ZE, Walton ZE, et al. MYC Disrupts the Circadian Clock and Metabolism in Cancer Cells. Cell Metab. 2015. December 1;22(6): 1009–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Repouskou A, Prombona A. c-MYC targets the central oscillator gene Perl and is regulated by the circadian clock at the post-transcriptional level. Biochim Biophys Acta. 2016. April; 1859(4):541–52. [DOI] [PubMed] [Google Scholar]
- 74.Serchov T, Heumann R. Ras Activity Tunes the Period and Modulates the Entrainment of the Suprachiasmatic Clock. Front Neurol. 2017;8:264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Morishita Y, Miura D, Kida S. PI3K regulates BMAL1/CLOCK-mediated circadian transcription from the Dbp promoter. Biosci Biotechnol Biochem. 2016. June;80(6):1131–40. [DOI] [PubMed] [Google Scholar]
- 76.Relógio A, Thomas P, Medina-Pérez P, Reischl S, Bervoets S, Gloc E, et al. Ras-mediated deregulation of the circadian clock in cancer. PLoS Genet. 2014; 10(5):e1004338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.El-Athman R, Genov NN, Mazuch J, Zhang K, Yu Y, Fuhr L, et al. The Ink4a/Arf locus operates as a regulator of the circadian clock modulating RAS activity. PLoS Biol. 2017. December; 15(12):e2002940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chen W-D, Wen M-S, Shie S-S, Lo Y-L, Wo H-T, Wang C-C, et al. The circadian rhythm controls telomeres and telomerase activity. Biochem Biophys Res Commun. 2014. August 29;451 (3):408–14. [DOI] [PubMed] [Google Scholar]
- 79.Kunieda T, Minamino T, Katsuno T, Tateno K, Nishi J, Miyauchi FI, et al. Cellular senescence impairs circadian expression of clock genes in vitro and in vivo. Circulation research. 2006. March 3;98(4):532–9. [DOI] [PubMed] [Google Scholar]
- 80.Qu Y, Mao M, Li X, Liu Y, Ding J, Jiang Z, et al. Telomerase reconstitution contributes to resetting of circadian rhythm in fibroblasts. Mol Cell Biochem. 2008. June;313(1 –2): 11–8. [DOI] [PubMed] [Google Scholar]
- 81.Gery S, Komatsu N, Baldjyan L, Yu A, Koo D, Koeffler HP. The circadian gene perl plays an important role in cell growth and DNA damage control in human cancer cells. Mol Cell. 2006. May 5;22(3):375–82. [DOI] [PubMed] [Google Scholar]
- 82.Kang TH, Leem SH. Modulation of ATR-mediated DNA damage checkpoint response by cryptochrome 1. Nucleic acids research. 2014. April;42(7):4427–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Unsal-Kacmaz K, Mullen TE, Kaufmann WK, Sancar A. Coupling of human circadian and cell cycles by the timeless protein. Molecular and cellular biology. 2005. April;25(8):3109–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kang T-H, Reardon JT, Kemp M, Sancar A. Circadian oscillation of nucleotide excision repair in mammalian brain. Proc Natl Acad Sci USA. 2009. February 24;106(8):2864–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Oklejewicz M, Destici E, Tamanini F, Hut RA, Janssens R, van der Florst GTJ. Phase resetting of the mammalian circadian clock by DNA damage. Curr Biol. 2008. February 26; 18(4):286–91. [DOI] [PubMed] [Google Scholar]
- 86.Papp SJ, Fluber A-L, Jordan SD, Kriebs A, Nguyen M, Moresco JJ, et al. DNA damage shifts circadian clock time via Hausp-dependent Cry1 stabilization. Elife. 2015. March 10;4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Asher G, Reinke H, Altmeyer M, Gutierrez-Arcelus M, Hottiger MO, Schibler U. Poly(ADP-ribose) polymerase 1 participates in the phase entrainment of circadian clocks to feeding. Cell. 2010. September 17;142(6):943–53. [DOI] [PubMed] [Google Scholar]
- 88.Reinke H, Asher G. Crosstalk between metabolism and circadian clocks. Nat Rev Mol Cell Biol. 2019. April;20(4):227–41. [DOI] [PubMed] [Google Scholar]
- 89.Pavlova NN, Thompson CB. The Emerging Hallmarks of Cancer Metabolism. Cell metabolism. 2016. January 12;23(1):27–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Vollmers C, Gill S, DiTacchio L, Pulivarthy SR, Le HD, Panda S. Time of feeding and the intrinsic circadian clock drive rhythms in hepatic gene expression. Proc Natl Acad Sci USA. 2009. December 15;106(50):21453–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Dyar KA, Lutter D, Artati A, Ceglia NJ, Liu Y, Armenia D, et al. Atlas of Circadian Metabolism Reveals System-wide Coordination and Communication between Clocks. Cell. 2018. September 6; 174(6): 1571–1585.e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wang Y, Song L, Liu M, Ge R, Zhou Q, Liu W, et al. A proteomics landscape of circadian clock in mouse liver. Nat Commun. 2018. 19;9(1): 1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zheng X, Sehgal A. AKT and TOR signaling set the pace of the circadian pacemaker. Curr Biol. 2010. July 13;20(13): 1203–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Eckle T, Hartmann K, Bonney S, Reithel S, Mittelbronn M, Walker LA, et al. Adora2b-elicited Per2 stabilization promotes a HIF-dependent metabolic switch crucial for myocardial adaptation to ischemia. Nat Med. 2012. April 15;18(5):774–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wu Y, Tang D, Liu N, Xiong W, Huang H, Li Y, et al. Reciprocal Regulation between the Circadian Clock and Hypoxia Signaling at the Genome Level in Mammals. Cell Metab. 2017. 10;25(1):73–85. [DOI] [PubMed] [Google Scholar]
- 96.Peek CB, Levine DC, Cedernaes J, Taguchi A, Kobayashi Y, Tsai SJ, et al. Circadian Clock Interaction with HIF1α Mediates Oxygenic Metabolism and Anaerobic Glycolysis in Skeletal Muscle. Cell Metab. 2017. 10;25(1):86–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Adamovich Y, Ladeuix B, Golik M, Koeners MP, Asher G. Rhythmic Oxygen Levels Reset Circadian Clocks through HIF1α. Cell Metab. 2017. 10;25(1):93–101. [DOI] [PubMed] [Google Scholar]
- 98.Wilking M, Ndiaye M, Mukhtar H, Ahmad N. Circadian rhythm connections to oxidative stress: implications for human health. Antioxid Redox Signal. 2013. July 10; 19(2): 192–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Shalapour S, Karin M. Immunity, inflammation, and cancer: an eternal fight between good and evil. J Clin Invest. 2015. September;125(9):3347–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Scheiermann C, Gibbs J, Ince L, Loudon A. Clocking in to immunity. Nat Rev Immunol. 2018. July; 18(7):423–37. [DOI] [PubMed] [Google Scholar]
- 101.Logan RW, Zhang C, Murugan S, O’Connell S, Levitt D, Rosenwasser AM, et al. Chronic shift-lag alters the circadian clock of NK cells and promotes lung cancer growth in rats. J Immunol. 2012. March 15;188(6):2583–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Scheiermann C, Kunisaki Y, Frenette PS. Circadian control of the immune system. Nature reviews Immunology. 2013. March; 13(3): 190–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Cao Q, Zhao X, Bai J, Gery S, Sun H, Lin D-C, et al. Circadian clock cryptochrome proteins regulate autoimmunity. Proc Natl Acad Sci USA. 2017. 21; 114(47): 12548–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Lam MT, Cho H, Lesch HP, Gosselin D, Heinz S, Tanaka-Oishi Y, et al. Rev-Erbs repress macrophage gene expression by inhibiting enhancer-directed transcription. Nature. 2013. June 27;498(7455):511–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Gibbs JE, Blaikley J, Beesley S, Matthews L, Simpson KD, Boyce SH, et al. The nuclear receptor REV-ERBalpha mediates circadian regulation of innate immunity through selective regulation of inflammatory cytokines. Proceedings of the National Academy of Sciences of the United States of America. 2012. January 10;109(2):582–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Sato S, Sakurai T, Ogasawara J, Takahashi M, Izawa T, Imaizumi K, et al. A circadian clock gene, Rev-erbalpha, modulates the inflammatory function of macrophages through the negative regulation of Ccl2 expression. Journal of immunology. 2014. January 1; 192(1):407–17. [DOI] [PubMed] [Google Scholar]
- 107.Man K, Loudon A, Chawla A. Immunity around the clock. Science. 2016. 25;354(6315):999–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Early JO, Menon D, Wyse CA, Cervantes-Silva MP, Zaslona Z, Carroll RG, et al. Circadian clock protein BMAL1 regulates IL-1 β in macrophages via NRF2. Proc Natl Acad Sci USA. 2018. 04; 115(36):E8460–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Curtis AM, Fagundes CT, Yang G, Palsson-McDermott EM, Wochal P, McGettrick AF, et al. Circadian control of innate immunity in macrophages by miR-155 targeting Bmal1. Proc Natl Acad Sci USA. 2015. June 9;112(23):7231–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Tsoli M, Schweiger M, Vanniasinghe AS, Painter A, Zechner R, Clarke S, et al. Depletion of white adipose tissue in cancer cachexia syndrome is associated with inflammatory signaling and disrupted circadian regulation. PLoS ONE. 2014;9(3):e92966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Vinay DS, Ryan EP, Pawelec G, Talib WH, Stagg J, Elkord E, et al. Immune evasion in cancer: Mechanistic basis and therapeutic strategies. Semin Cancer Biol. 2015. December;35 Suppl:S185–98. [DOI] [PubMed] [Google Scholar]
- 112.Flavell RA, Sanjabi S, Wrzesinski SH, Licona-Limon P. The polarization of immune cells in the tumour environment by TGFbeta. Nat Rev Immunol. 2010. August;10(8):554–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Maeda FI, Shiraishi A. TGF-beta contributes to the shift toward Th2-type responses through direct and IL-10-mediated pathways in tumor-bearing mice. J Immunol. 1996. January 1;156(1):73–8. [PubMed] [Google Scholar]
- 114.Shalapour S, Font-Burgada J, Di Caro G, Zhong Z, Sanchez-Lopez E, Dhar D, et al. Immunosuppressive plasma cells impede T-cell-dependent immunogenic chemotherapy. Nature. 2015. May 7;521(7550):94–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Lv K, Zhang Y, Zhang M, Zhong M, Suo Q. Galectin-9 promotes TGF-β1-dependent induction of regulatory T cells via the TGF-β/Smad signaling pathway. Mol Med Rep. 2013. January;7(1):205–10. [DOI] [PubMed] [Google Scholar]
- 116.Nam D, Guo B, Chatterjee S, Chen M-H, Nelson D, Yechoor VK, et al. The adipocyte clock controls brown adipogenesis through the TGF-β and BMP signaling pathways. J Cell Sci. 2015. May 1;128(9):1835–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Chen W-D, Yeh J-K, Peng M-T, Shie S-S, Lin S-L, Yang C-H, et al. Circadian CLOCK Mediates Activation of Transforming Growth Factor-β Signaling and Renal Fibrosis through Cyclooxygenase 2. Am J Pathol. 2015. December; 185(12):3152–63. [DOI] [PubMed] [Google Scholar]
- 118.Eichenfield DZ, Troutman TD, Link VM, Lam MT, Cho H, Gosselin D, et al. Tissue damage drives co-localization of NF-κB, Smad3, and Nrf2 to direct Rev-erb sensitive wound repair in mouse macrophages. Elife. 2016. 27;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Yu X, Rollins D, Ruhn KA, Stubblefield JJ, Green CB, Kashiwada M, et al. TH17 cell differentiation is regulated by the circadian clock. Science. 2013. November 8;342(6159):727–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Erren TC, Morfeld P, Foster RG, Reiter RJ, Groß JV, Westermann IK. Sleep and cancer: Synthesis of experimental data and meta-analyses of cancer incidence among some 1,500,000 study individuals in 13 countries. Chronobiol Int. 2016;33(4):325–50. [DOI] [PubMed] [Google Scholar]
- 121.Sulli G, Manoogian ENC, Taub PR, Panda S. Training the Circadian Clock, Clocking the Drugs, and Drugging the Clock to Prevent, Manage, and Treat Chronic Diseases. Trends Pharmacol Sci. 2018;39(9):812–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Ballesta A, Innominato PF, Dallmann R, Rand DA, Lévi FA. Systems Chronotherapeutics. Pharmacol Rev. 2017;69(2):161–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Yang Y, Xu T, Zhang Y, Qin X. Molecular basis for the regulation of the circadian clock kinases CK1δ and CK1ε. Cell Signal. 2017;31:58–65. [DOI] [PubMed] [Google Scholar]
- 124.Kennaway DJ, Varcoe TJ, Voultsios A, Salkeld MD, Rattanatray L, Boden MJ. Acute inhibition of casein kinase 1δ/ε rapidly delays peripheral clock gene rhythms. Mol Cell Biochem. 2015. January;398(1 –2): 195–206. [DOI] [PubMed] [Google Scholar]
- 125.Rosenberg LH, Lafitte M, Quereda V, Grant W, Chen W, Bibian M, et al. Therapeutic targeting of casein kinase 1δ in breast cancer. Sci Transl Med. 2015. December 16;7(318):318ra202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Oshima T, Niwa Y, Kuwata K, Srivastava A, Hyoda T, Tsuchiya Y, et al. Cell-based screen identifies a new potent and highly selective CK2 inhibitor for modulation of circadian rhythms and cancer cell growth. Sci Adv. 2019. January;5(1):eaau9060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Zhao X, Hirota T, Han X, Cho H, Chong L-W, Lamia K, et al. Circadian Amplitude Regulation via FBXW7-Targeted REV-ERBα Degradation. Cell. 2016. June 16; 165(7): 1644–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Gao J, Azmi AS, Aboukameel A, Kauffman M, Shacham S, Abou-Samra A-B, et al. Nuclear retention of Fbw7 by specific inhibitors of nuclear export leads to Notch1 degradation in pancreatic cancer. Oncotarget. 2014. June 15;5(11):3444–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Huang H-L, Weng H-Y, Wang L-Q, Yu C-H, Huang Q-J, Zhao P-P, et al. Triggering Fbw7-mediated proteasomal degradation of c-Myc by oridonin induces cell growth inhibition and apoptosis. Mol Cancer Ther. 2012. May; 11(5): 1155–65. [DOI] [PubMed] [Google Scholar]
- 130.Lytle NK, Ferguson LP, Rajbhandari N, Gilroy K, Fox RG, Deshpande A, et al. A Multiscale Map of the Stem Cell State in Pancreatic Adenocarcinoma. Cell. 2019. April 18; 177(3):572–586.e22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.He B, Nohara K, Park N, Park Y-S, Guillory B, Zhao Z, et al. The Small Molecule Nobiletin Targets the Molecular Oscillator to Enhance Circadian Rhythms and Protect against Metabolic Syndrome. Cell Metab. 2016. April 12;23(4):610–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Solt LA, Wang Y, Banerjee S, Hughes T, Kojetin DJ, Lundasen T, et al. Regulation of circadian behaviour and metabolism by synthetic REV-ERB agonists. Nature. 2012. May 3;485(7396):62–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Grant D, Yin L, Collins JL, Parks DJ, Orband-Miller LA, Wisely GB, et al. GSK4112, a small molecule chemical probe for the cell biology of the nuclear heme receptor Rev-erbα. ACS Chem Biol. 2010. October 15;5(10):925–32. [DOI] [PubMed] [Google Scholar]
- 134.Chun SK, Chung S, Kim H-D, Lee JH, Jang J, Kim J, et al. A synthetic cryptochrome inhibitor induces anti-proliferative effects and increases chemosensitivity in human breast cancer cells. Biochem Biophys Res Commun. 2015. November 13;467(2):441–6. [DOI] [PubMed] [Google Scholar]
- 135.Hu X, Liu X, Moisan J, Wang Y, Lesch CA, Spooner C, et al. Synthetic RORγ agonists regulate multiple pathways to enhance antitumor immunity. Oncoimmunology. 2016;5(12):e1254854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Hu X, Majchrzak K, Liu X, Wyatt MM, Spooner CJ, Moisan J, et al. In Vitro Priming of Adoptively Transferred T Cells with a RORγ Agonist Confers Durable Memory and Stemnness In Vivo. Cancer Res. 2018. July 15;78(14):3888–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Monsees GM, Kraft P, Hankinson SE, Hunter DJ, Schernhammer ES. Circadian genes and breast cancer susceptibility in rotating shift workers. Int J Cancer. 2012. December 1; 131 (11):2547–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Truong T, Liquet B, Menegaux F, Plancoulaine S, Laurent-Puig P, Mulot C, et al. Breast cancer risk, nightwork, and circadian clock gene polymorphisms. Endocr Relat Cancer. 2014. August;21(4):629–38. [DOI] [PubMed] [Google Scholar]
- 139.Zienolddiny S, Haugen A, Lie J-AS, Kjuus H, Anmarkrud KH, Kjærheim K. Analysis of polymorphisms in the circadian-related genes and breast cancer risk in Norwegian nurses working night shifts. Breast Cancer Res. 2013;15(4):R53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Kiessling S, Beaulieu-Laroche L, Blum ID, Landgraf D, Welsh DK, Storch K-F, et al. Enhancing circadian clock function in cancer cells inhibits tumor growth. BMC Biol. 2017. 14; 15(1): 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Jung-Hynes B, Huang W, Reiter RJ, Ahmad N. Melatonin resynchronizes dysregulated circadian rhythm circuitry in human prostate cancer cells. J Pineal Res. 2010. August;49(1):60–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Gebauer J, Higham C, Langer T, Denzer C, Brabant G. Long-Term Endocrine and Metabolic Consequences of Cancer Treatment: A Systematic Review. Endocr Rev. 2019. June 1;40(3):711–67. [DOI] [PubMed] [Google Scholar]
- 143.Coudert B, Focan C, Genet D, Giacchetti S, Cvickovic F, Zambelli A, et al. A randomized multicenter study of optimal circadian time of vinorelbine combined with chronomodulated 5-fluorouracil in pretreated metastatic breast cancer patients: EORTC trial 05971. Chronobiol Int. 2008. September;25(5):680–96. [DOI] [PubMed] [Google Scholar]
- 144.Lévi FA, Zidani R, Vannetzel JM, Perpoint B, Focan C, Faggiuolo R, et al. Chronomodulated versus fixed-infusion-rate delivery of ambulatory chemotherapy with oxaliplatin, fluorouracil, and folinic acid (leucovorin) in patients with colorectal cancer metastases: a randomized multi-institutional trial. J Natl Cancer Inst. 1994. November 2;86(21): 1608–17. [DOI] [PubMed] [Google Scholar]
- 145.Lévi F, Zidani R, Misset JL. Randomised multicentre trial of chronotherapy with oxaliplatin, fluorouracil, and folinic acid in metastatic colorectal cancer. International Organization for Cancer Chronotherapy. Lancet. 1997. September 6;350(9079):681–6. [DOI] [PubMed] [Google Scholar]
- 146.Gallion HH, Brunetto VL, Cibull M, Lentz SS, Reid G, Soper JT, et al. Randomized phase III trial of standard timed doxorubicin plus cisplatin versus circadian timed doxorubicin plus cisplatin in stage III and IV or recurrent endometrial carcinoma: a Gynecologic Oncology Group Study. J Clin Oncol. 2003. October 15;21(20):3808–13. [DOI] [PubMed] [Google Scholar]
- 147.Giacchetti S, Bjarnason G, Garufi C, Genet D, lacobelli S, Tampellini M, et al. Phase III trial comparing 4-day chronomodulated therapy versus 2-day conventional delivery of fluorouracil, leucovorin, and oxaliplatin as first-line chemotherapy of metastatic colorectal cancer: the European Organisation for Research and Treatment of Cancer Chronotherapy Group. J Clin Oncol. 2006. August 1;24(22):3562–9. [DOI] [PubMed] [Google Scholar]
- 148.Selfridge JM, Gotoh T, Schiffhauer S, Liu J, Stauffer PE, Li A, et al. Chronotherapy: Intuitive, Sound, Founded...But Not Broadly Applied. Drugs. 2016. October;76(16): 1507–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Postow MA, Sidlow R, Hellmann MD. Immune-Related Adverse Events Associated with Immune Checkpoint Blockade. N Engl J Med. 2018. 11;378(2):158–68. [DOI] [PubMed] [Google Scholar]
- 150.Nguyen KD, Fentress SJ, Qiu Y, Yun K, Cox JS, Chawla A. Circadian gene Bmal1 regulates diurnal oscillations of Ly6C(hi) inflammatory monocytes. Science. 2013. September 27;341 (6153): 1483–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Guerrero-Vargas NN, Salgado-Delgado R, Basualdo MDC, García J, Guzmán-Ruiz M, Carrero JC, et al. Reciprocal interaction between the suprachiasmatic nucleus and the immune system tunes down the inflammatory response to lipopolysaccharide. J Neuroimmunol. 2014. August 15;273(1–2):22–30. [DOI] [PubMed] [Google Scholar]
- 152.Liu J, Malkani G, Mankani G, Shi X, Meyer M, Cunningham-Runddles S, et al. The circadian clock Period 2 gene regulates gamma interferon production of NK cells in host response to lipopolysaccharide-induced endotoxic shock. Infect Immun. 2006. August;74(8):4750–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Deng W, Zhu S, Zeng L, Liu J, Kang R, Yang M, et al. The Circadian Clock Controls Immune Checkpoint Pathway in Sepsis. Cell Rep. 2018. July 10;24(2):366–78. [DOI] [PMC free article] [PubMed] [Google Scholar]