Abstract
Background
Patients with Parkinson's disease commonly suffer from speech and voice difficulties such as impaired articulation and reduced loudness. Speech and language therapy (SLT) aims to improve the intelligibility of speech with behavioural treatment techniques or instrumental aids.
Objectives
To compare the efficacy and effectiveness of novel SLT techniques versus a standard SLT approach to treat Parkinsonian speech problems.
Search methods
We identified relevant, published prior to 11th April 2011, by electronic searches of numerous literature databases including CENTRAL, MEDLINE and CINAHL, as well as handsearching relevant conference abstracts and examining reference lists in identified studies and other reviews.
Selection criteria
Only randomised controlled trials (RCT) of one type of speech and language therapy versus another were included.
Data collection and analysis
Two review authors independently extracted data and resolved differences by discussion.
Main results
Six trials involving 159 patients satisfied the inclusion criteria. Data could not be analysed from one trial due to changes in patient numbers and from a second because the data provided were not in a usable format. All trials reported intelligibility measures but a statistically significant result was only reported for the diagnostic rhyme test used in the study of Lee Silverman Voice Treatment ‐LOUD (LSVT‐LOUD) versus a modified version of this therapy (LSVT‐ARTIC). In this case a difference of 12.5 points (95% confidence interval (CI) ‐22.2 to ‐2.8; P = 0.01) between the mean changes in favour of the LSVT‐LOUD group was reported for a speech sample overlaid with Babble noise; this difference was not reproduced for the two additional noise conditions under which the speech samples were assessed. LSVT‐LOUD also outperformed LSVT‐ARTIC and Respiration therapy (RT) in improving loudness, with a difference in reading a sample text of 5.0 dB (95%CI ‐8.3 to ‐1.7; P = 0.003) and 5.5 dB (95% CI 3.4 to 7.7; P < 0.00001) respectively, and a difference in monologue speech of 2.9 dB (95% CI 0.6 to 5.2; P = 0.01) versus RT.
Authors' conclusions
Considering the small patient numbers in these trials, there is insufficient evidence to support or refute the efficacy of any form of SLT over another to treat speech problems in patients with Parkinson's disease.
Keywords: Humans, Bias, Dysarthria, Dysarthria/etiology, Dysarthria/therapy, Language Therapy, Language Therapy/methods, Parkinson Disease, Parkinson Disease/complications, Randomized Controlled Trials as Topic, Speech Intelligibility, Speech Therapy, Speech Therapy/methods
Plain language summary
Speech and language therapy for speech problems in Parkinson’s disease
Many people with Parkinson's disease suffer from disorders of speech. The most frequently reported speech problems are weak, hoarse, nasal or monotonous voice, imprecise articulation, slow or fast speech, difficulty starting speech, impaired stress or rhythm, stuttering and tremor. People with the condition also tend to give fewer non‐verbal cues such as using facial expression to convey information. These disabilities tend to increase as the disease progresses and can lead to serious problems with communication.
This review compares the benefits of one form of speech and language therapy (SLT) versus another for individuals with Parkinson's disease. Relevant trials were identified by electronic searches of 16 biomedical literature databases, various registers of clinical trials and examination of the reference lists of identified studies and other reviews.
Only randomised controlled trials were included in this review. These are studies in which two groups of patients were compared, each group receiving a different form of SLT, with patients assigned to the groups in a random fashion to reduce potential for bias.
Six trials were found with a total of 159 patients. Methods varied so much that meta‐analysis of the results was not possible. Considering the small number of patients and the methodological flaws in these studies, there is insufficient evidence to support the use of one form of SLT over another for the treatment of speech problems in individuals with Parkinson's disease.
Background
For definition of terms see Table 1: Glossary. Speech problems are common in Parkinson's disease (PD) and increase in frequency and intensity with progression of the disease (Streifler 1984; Sapir 2001). Dysarthria is a collective name for a group of speech disorders resulting from disturbances in muscular control of the speech mechanism due to damage of the central nervous system. It designates problems in oral communication due to paralysis, weakness or incoordination of the speech musculature (Darley 1969). Common characteristics of Parkinsonian dysarthria are monotony of pitch and volume (dysprosody), reduced stress, imprecise articulation, variations in speed resulting in both inappropriate silences and rushes of speech, and a breathy hoarseness to the speech (hypophonia) reflecting the difficulty the patient has in synchronising talking and breathing (Logemann 1978; Stewart 1995). Many of these features are attributed to hypokinesia (paucity of movement) and rigidity which are considered to be cardinal features of PD (Mawdsley 1971). Patients with PD also suffer from cognitive impairment which leads to difficulties in language selection, language understanding, coordination and dual tasks (talking and walking) as well as emotional intent and understanding. These issues do not come under the umbrella of dysarthric speech but impact on the ability of individuals to participate in spoken communication. As a result, the title of this review has been changed from ‘dysarthria’ to include the full complexity of ‘speech problems’ in PD.
1. Glossary.
| TERM | DEFINITION |
| Amplitude | The maximum absolute value of a periodically varying quantity. For a sound wave, the maximum variation in pressure relative to static conditions (e.g. atmospheric pressure). Small variations produce weak (or quiet) sounds whilst large variations produce strong (or loud) sounds. (See loudness below). |
| Articulation | The production of vowels and consonants using both the moving parts of the mouth (e.g. tongue and lips) and the fixed structure of the mouth (e.g. hard and soft palate). It does not involve the voice box. |
| Concealment of Allocation | The process used to conceal foreknowledge of group assignment, which should be seen as distinct from blinding. The allocation process should be impervious to any influence by the person making the allocation. Adequate methods of allocation concealment include: centralised randomisation schemes (telephone randomisation) or sequentially numbered opaque sealed envelopes. |
| Decibel (dB) | A unit used to express relative difference in power or intensity, usually between two acoustic or electric signals, equal to ten times the common logarithm (i.e. base 10) of the ratio of the two levels. i.e. 10 log10 (W2/W1) where W1 is the reference power level and W2 is the quantity being specified in dB relative to W1. It is commonplace to want to express in decibels, quantities that are related not to power, but power2. Examples include sound pressure and voltage. In such cases the expression for the decibel level becomes 20 log10 (p2/p1).So that individual quantities can be specified, default reference values are defined for sound pressure (20´10‐6 Pascals), sound power (10‐6 watts) and sound intensity (10‐12 watts per square meter). For other quantities (e.g. voltage) a value of unity is often used implicitly. The reference level for sound pressure (corresponding to 0 dB) was originally set as an approximation to the threshold of human hearing. A whisper has an intensity of ˜30 dB, normal speech ˜60 dB, a shout ˜90 dB and a jet aircraft ˜120 dB. |
| Dysarthria | Dysarthria is a collective name for a group of speech disorders resulting from disturbances in muscular control of the speech mechanism due to damage of the central nervous system. It designates problems in oral communication due to paralysis, weakness or incoordination of the speech musculature. |
| Dysprosody | Abnormal prosody (see prosody). Loss of the 'melody' of speech. |
| Frequency | The number of complete cycles of a periodic process occurring per unit time. For sound waves this is the number of times the pressure variation cycle occurs in one second. The unit used to measure frequency is the hertz (Hz) (see below). |
| Fundamental Frequency (F0) | The fundamental frequency is the inverse of the period (T0); i.e. F0 = 1/T0. For complex sounds such as speech, F0 will normally correspond to the frequency of the lowest harmonic. It is measured in hertz (see below). The aim of S< is to increase the F0 of Parkinsonian speech as this leads to improved intelligibility. See also Pitch (see below). |
| Fundamental Frequency Variability | The variation in fundamental frequency (see above) of speech. Measured as the standard deviation of F0 in hertz or semitones (STSD). The aim of S< is to increase F0 variation and thus decrease the monotonicity of the patient's speech. See also Pitch. |
| Hertz (Hz) | Hertz is the unit of frequency expressed in cycles (sound waves) per second. |
| Hypophonia | A breathy hoarseness to the speech. |
| Intelligibility | Degree of clarity with which utterances are understood by average listeners. It is influenced by articulation, rate, fluency, vocal quality and intensity (see below). |
| Intensity (of Sound) | The sound power propagating through a unit area of the sound field in a given direction. For example the sound intensity of a point source radiating spherical waves and of a given sound power, will diminish as the distance from the source is increased, in proportion to the inverse of the square of this distance (1/distance2). It is a vector quantity since it specifies both a magnitude and direction, therefore direct measurement is not straightforward. Sound intensity has units of watts per square metre, but can also be expressed in decibels (see above). Sound intensity is related to the square of the sound pressure, but the exact relationship depends on the characteristics of the sound field. |
| Intention‐To‐Treat Data Analysis | Data are analysed according to the randomisation allocation, irrespective of protocol violations and withdrawals. Withdrawals, and therefore missing data points, are usually compensated for by using the last observation carried forward (LOCF). Intention‐to‐treat analyses are favoured in assessments of effectiveness as they mirror the non‐compliance and treatment changes that are likely to occur when the intervention is used in practice and because of the risk of attrition bias when participants are excluded from the analysis. |
| Loudness | Loudness can be measured either subjectively or objectively. Subjectively loudness is the perceptual correlate of amplitude (see above). Equal steps in subjective loudness are roughly equal to logarithmic steps in amplitude. It is also logarithmically correlated to intensity, an increase of 6‐10 dB is equivalent to a doubling in perceptual loudness. Objective measurements of loudness measure the sound's intensity (see below), usually using the decibel scale (see above) and are described as Volume or Sound Pressure Level (see below). |
| Monotonicity | A lack in variation of both loudness (see above) and pitch (see below). |
| Palilalia | Speaker reiterates many times a word, phrase or sentence which they have just spoken, sometimes with increasing rate and decreasing audibility. |
| Period (T0) | The length of each sound wave (cycle) in time is called the period of a waveform. It is equal to 1/frequency. |
| Per Protocol Data Analysis | Data are analysed according to what therapy the patients received, rather than according to their randomised allocation. Withdrawals are removed from the analysis. This form of data analysis risks attrition bias. |
| Phonation | The mechanism of producing sounds with the vocal folds (also known as vocal cords). |
| Pitch | The perceptual correlate of frequency (see above). Normally, the pitch of a complex sound is a function of its fundamental frequency (see above). Equal steps in pitch are roughly equal to logarithmic steps in amplitude. |
| Prosody | Prosody is defined as that aspect of spoken language which consists in correct placing of pitch and stress on syllables and words. It is responsible for conveying subtle changes of meaning independently of words or grammatical order. In addition to this semantic role, it makes a major contribution to the emotional content of speech. |
| Rainbow Passage | A reading passage that is phonetically balanced and has all the vowel and consonant sounds present in the English language. |
| Reference values for sound pressure, sound power and sound intensity (P0) | So that individual quantities can be specified in terms of decibels, default reference values are defined for sound pressure (20´10‐6 Pascals), sound power (10‐6 watts) and sound intensity (10‐12 watts per square meter). For other quantities (e.g. voltage) a value of unity is often used implicitly. The reference level for sound pressure (corresponding to 0 dB) was originally set as an approximation to the threshold of human hearing. However this equivalence has since been questioned. |
| Respiration | Breathing |
| Sound Pressure and Sound Pressure Level (SPL) | Sound pressure is the root mean square (r.m.s) variation in pressure from the static value (e.g. the atmospheric pressure) and is measured in Pascals. The r.m.s variation in pressure from the static value (e.g. the atmospheric pressure). Sound pressure is measured in Pascals, but can be expressed in decibels (see above), 20 log10(sound pressure/20´10‐6) whereupon it is referred to as sound pressure level. Sound pressure is a scalar quantity and is therefore relatively easy to measure, for example a microphone responds to sound pressure. The reference level for sound pressure (corresponding to 0 dB) was originally set as an approximation to the threshold of human hearing. However this equivalence has since been questioned. |
| Volume | Equivalent to loudness (see above). |
Four approaches to speech therapy are available: behavioural treatment techniques (drill, exercise), instrumental aids including prosthetic and augmentative devices, medication, and surgical procedures. Pharmacotherapy and surgery have a limited role in the management of specific motor impairments such as speech disorders, particularly those that emerge during the later stages of the disease. It has been suggested that the behavioural treatment techniques of speech and language therapy (SLT) may be more effective in improving the intelligibility of speech in Parkinson's disease. Even then, "compensated intelligibility" rather than "normal speech" may be considered the more limited goal of SLT (Rosenbek 1985).
A 2009 patient survey by Parkinson’s UK showed that only 34% of patients with PD in England reported receiving SLT (Parkinson's disease society 2008). This low referral rate does not accord with the advice in most published guidelines which suggests that SLT should always be made available for the management of PD (NCC‐CC 2006).
This review compares the efficacy of one type of speech and language therapy with another for speech problems in patients with PD. Another review examines trials that compare speech and language therapy with placebo or no intervention (Herd In Press).
Objectives
To compare the efficacy and effectiveness of novel SLT techniques versus an alternative SLT approach in patients with PD.
Methods
Criteria for considering studies for this review
Types of studies
We considered all randomised controlled trials comparing two types of speech and language therapy for inclusion in the study. We accepted both random and quasi‐random methods of allocation.
Types of participants
Patients with a diagnosis of PD (as defined by the authors of the studies)
Any duration of PD
All ages
Any drug therapy
Any duration of treatment
Types of interventions
One speech and language therapy technique versus a second.
Types of outcome measures
1. Speech and voice production parameters (i.e. measures of impairment). (a) Total impairments (e.g. Dysarthria rating scales, Intelligibility rating scales). (b) Objective and subjective acoustic measures of speech samples (e.g. pitch, loudness, sentence length etc.). (c) Measures of laryngeal activity (e.g. fibre optic laryngoscopy, stroboscopy). (d) Level of communication participation. 2. Activities of daily living (e.g. Sickness Impact Profile (SIP) communication subsection (Bergner 1981)). 3. Handicap and quality of life measures, both disease‐specific (e.g. Parkinson's Disease Questionnaire ‐ 39, PDQ39) and generic (e.g. Short Form ‐ 36, SF36). 4. Depression rating scales (e.g. Beck Depression Index (BDI) (Beck 1961)). 5. Adverse effects. 6. Carer outcomes (e.g. Carer strain index). 7. Economic analysis.
We examined both the short‐term and the long‐term effects of the interventions.
Search methods for identification of studies
1. The review is based on the search strategy of the Movement Disorders Group and also the following more general search strategy:
a. Dysarthria OR speech OR speak OR intelligibility OR dysprosody OR hypophonia OR monotonicity OR phonate b. ((Speech OR speak OR language OR voice OR vocal OR articulate OR sing) near (task OR therapy OR treatment OR train OR councel OR intervention OR exercise OR drill OR rehabilitation)) OR silverman OR LSVT c. Parkinson OR Parkinson's disease OR Parkinsonism d. (#a AND #b AND #c) OR (#a and #c)
See Appendix 1 for sample search (MEDLINE). This strategy was adapted for each electronic database.
We identified relevant trials by electronic searches of general biomedical and science databases: The Cochrane Controlled Trials Register (CENTRAL) searched without date limiters, CENTRAL (MEDLINE database searched through Ovid (1966‐2011) and PubMed (2010‐2011 for data not yet released onto Ovid Medline), EMBASE (1974‐2011), CINAHL (1982‐2011), ISI‐SCI ((1981‐2011); rehabilitation databases: AMED (1985‐2011), MANTIS (1880‐2000), REHABDATA (1956‐2011), REHADAT, GEROLIT (1979‐2011); English language databases of foreign language research and third world publications: Pascal (1984‐2000), LILACS (1982‐2011), MedCarib (17th Century‐2000), Journal@rchive (19th century‐2011), AIM (1993‐2000), IMEMR (1984‐2011) and handsearching of appropriate conference proceedings Relevant trials were included on the Group's specialised register of randomised controlled trials.
2. We also searched The CenterWatch Clinical Trials listing service, controlled_trials.com, ClinicalTrials.gov, CRISP, PEDro, NIDRR and NRR for relevant trials.
3. The reference lists of located trials and review articles.
4. Grey literature (e.g. conference abstracts, theses and internal reports) were searched. This included The International Congress on Parkinson's disease (1999, 2001), The International Congress of Parkinson's Disease and Movement Disorders (1990, 1992, 1994, 1996, 1997, 1998, 2002, 2004, 2005, 2006, 2007, 2008, 2009, 2010, 2011), The American Academy of Neurology 51st annual meeting (1999) and the Congress of the European Federation of Neurological Societies (2003, 2004, 2005, 2006, 2007, 2008, 2009, 2010). We searched the following grey literature databases: OpenSIGLE (1980‐2011), ISI‐ISTP (1982‐2000), Proquest(1999‐2011), Conference Papers Index (1982‐2011) Ethos (1970 ‐2011) and Index to Theses (1716 ‐ 2011).
Further details on this search strategy are available in the Group's module within The Cochrane Library (www.cochrane.org). This includes explanations of the acronyms, sources and web sites.
Data collection and analysis
The review authors independently assessed the studies identified by the search strategy. We resolved disagreements about inclusions by discussion.
We contacted the authors of trials in the cases where further trial information was required for full analysis. We assessed full papers for methodological quality by recording the method of randomisation, concealment of allocation and blinding of assessors to treatment group, whether an intention‐to‐treat analysis was used and the number of patients lost to follow‐up.
Two review authors (CPH and CLT) independently abstracted eligible data onto standardised forms, checked for accuracy and amalgamated the information. We resolved disagreements about inclusions by discussion.
We combined the results of each trial using standard meta‐analytic methods to estimate an overall effect for one type of speech and language therapy intervention versus another.
For all identified continuous variables, we calculated the mean difference between treatment arms using weighted mean difference methods (Fleiss 1993). In summary, this involved for each trial, calculating the mean change (and standard deviation) from baseline to the post‐intervention time point for both intervention groups. The mean difference and its variance between arms for each trial could then be calculated. In some studies the standard deviation for the mean change was not reported, in these cases, we imputed this standard deviation using the standard deviations for the baseline and final scores. To do this we used the following formula to estimate the variance of the change in score:
var diff = var pre + var post – 2r√(var pre var post)
where var diff is the variance of the change score; var pre is the variance of the baseline score; var post is the variance of the final score and r is the correlation between the pre‐ and post‐ treatment scores. We assumed a correlation co‐efficient of 0.5, which is a conservative estimate, to reduce the chance of false positive results (Higgins 2011).
We then combined these values using weighted mean difference methods to give the overall pooled estimate of the mean difference, with 95% confidence interval, for one speech and language therapy intervention versus another. A result with a value of P < 0.05 is considered to be statistically significant.
Results
Description of studies
See Table 2: Characteristics of included studies and Figure 1: PRISMA flow diagram.
2. Key Characteristics of Included Studies.
| Study | Number of patients analysed | Mean age | Mean Hoehn & Yahr score | Duration of therapy | Therapy A | Therapy B |
| Constantinescu 2011 | 34 | 70 | 1.6 | 16 hours/1 month | LSVT ‐ online delivery | LSVT ‐ face‐to‐face delivery |
| Halpern 2007 | 18 | 68 | not reported | 13 hours 20 mins/1 month | LSVT ARTIC ‐ enhanced articulation | LSVT LOUD ‐ increased loudness |
| Healy 2002 | 26 | 66 | not reported | 1 hour session or 6 hour long sessions over 6 weeks | Alphabet board | Pacing board |
| Lowit 2010 | 10 | 63 | 2.9 | 6 hours/6 weeks | Altered auditory feedback ‐ in ear device | Traditional rate reduction therapy |
| Ramig 1995 | 45 | 65 | 2.5 | 16 hours/1 month | LSVT ‐ increased loudness | Respiration therapy |
| Scott 1983 | 26 | 66 | n/a | 10 hours/2 weeks | Prosodic exercises with visual feedback | Prosodic exercises alone |
| Total | 159 |
LSVT: Lee Silverman Voice Treatment
1.
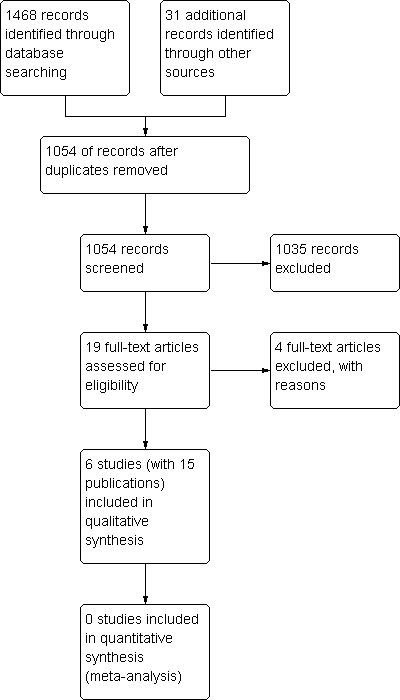
PRISMA diagram.
We found five randomised controlled trials comparing two methods of speech and language therapy for speech disorders in a total of 125 patients with PD (Scott 1983; Ramig 1995; Healy 2002; Halpern 2007; Lowit 2010). In addition to this, we included a trial of 34 patients that compared two different delivery methods for the same speech and language therapy technique (Constantinescu 2011).
Trial design
Five trials (Scott 1983; Ramig 1995; Healy 2002; Halpern 2007; Constantinescu 2011) were parallel group trials; four were single‐centre studies and one was a multi‐centre study; and one trial (Lowit 2010) had a cross‐over design, from which we included the pre‐cross‐over data in this review.
Participants
Scott 1983, the mean age of the patients was 66 years in both groups, but the visual stimulation group had only two females (of 13), whereas the prosodic exercises group had seven (of 13). The stage of PD was not assessed. In Ramig 1995, the 45 patients were stratified by a large number of criteria including age, sex and stage of disease before randomisation. This lead to the ages of the participants in the two groups being similar (64 and 66 years) as was the disease stage (Hoehn and Yahr 2.7 and 2.3). However, the split of the sexes was uneven with the Lee Silverman Voice Treatment (LSVT) group having 19% females whilst the respiration therapy group had 37%. The two groups in Lowit 2010 were age‐matched at an average of 63 years. There was a large difference in Hoehn and Yahr scores and male/female ratio for this trial with the Altered Auditory Feedback group scoring an average of 1.8 at baseline and having only one in five females and the Traditional Therapy group score averaging 3.3 with three in five patients being female. Stratification parameters used in Halpern 2007 ensured an even distribution of female participants across the two groups (two in LSVT‐ARTIC and three in LSVT‐LOUD) and matched the groups for age with an average of 66 and 71 for LSVT‐ARTIC and LSVT LOUD respectively and Hoehn and Yahr score (on medication) for which both groups averaged 2.2. The groups taking part in Constantinescu 2011 were matched in age 70.5 (LSVT Artic), 66.1 (LSVT LOUD), male/female ratio 6/2 (LSVT Artic), 7/3 (LSVT LOUD) and Hoehn and Yahr stage on medication score 2.2 (LSVT Artic) and 2.2 (LSVT LOUD). Duration of condition was not reported for this study. Baseline characteristics for the four groups in Healy 2002 were not reported, the mean age of all participants was 65.6. Of the 26 participants in this trial, nine were female.
Interventions
See Table 3: Summary of Interventions
3. Summary of Interventions.
| Intervention | Used in Trials | Description of intervention | Target Symptom | Duration/Dose | Treatment Setting | Designed specifically for treatment of PD | Delivered by trained therapist Yes/No |
| Lee Silverman Voice Therapy (LSVT) | Ramig 95 Halpern 2007 Constantinescu 2011 | A high effort intensive treatment using tasks such as pushing hands together, pushing down or lifting on the arms of a chair while phonating to stimulate increased vocal fold adduction. Patients were cued to think LOUD and encouraged to use the same loud, good quality voice generated by sustained phonation during speech tasks. | Quiet voice | 4 sessions per week for 4 weeks with each session lasting 1 hour (16 hours) | Ramig 95: Outpatients Halpern 2007: Not reported Constantinescu 2011: Outpatients | YES | YES |
| Articulation focussed LSVT (LSVT‐ARTIC) | Halpern 2007 | Following same general principles as LSVT but sustained phonation tasks are replaced with articulation drills such as repitition of sounds 'pa', 'ta' and 'ka', cue used for speech tasks is ENUNCIATE. | Poor articulation | 4 sessions per week for 4 weeks with each session lasting 1 hour (16 hours) | Not reported | YES | YES |
| Respiratory Therapy | Ramig 1995 | High effort intensive treatment aimed at increased respiratory muscle activity. Tasks included maximising inhalation and exhalation, maximum duration of voiceless continuents /s/ and /f/ and sustained intraoral air pressure. Visual feedback of excursion of ribcage and abdomen during vocal tasks was provided. No attention was directed toward increasing vocal loudness. | Reduced respiratory effort | 4 sessions per week for 4 weeks with each session lasting 1 hour (16 hours) | Outpatients | NO | YES |
| Alphabet Board | Healy 2002 | Board with grid containing letters of the alphabet in order is used. Speaker points to initial letter of each word they speak on the board. | Palilalia | 6 sessions over 6 weeks OR a single session, each session lasting 1 hour (6 hours OR 1 hour) | Home | NO | YES |
| Pacing Board | Healy 2002 | Wooden ruler with series of raised divisions at 30 mm intervals. Speaker is required to place their finger in each section consecutively as they speak each word. Tactile feedback helps to control speech rate. | Palilalia | 6 sessions over 6 weeks OR a single session, each session lasting 1 hour (6 hours OR 1 hour) | Home | YES | YES |
| Altered Auditory Feedback (AAF) | Lowit 2010 | In‐ear device is worn which disrupts the normal auditory feedback loop causing the speaker to hear their speech in an altered fashion reducing rate of speech. Patients trialled both delayed auditory feedback (DAF) which delivers speech signal to speaker after a short delay and frequency shifted feedback (FSF) which delivers signal in real time but with an altered pitch. | Palilalia | 6 sessions over 6 weeks with each session lasting approx. 1 hour (6 hours) | Home | NO | UNCLEAR |
| Traditional Rate Reduction Therapy (TT) | Lowit 2010 | Focussed on identifying most suitable strategy for reducing speech rate e.g. increasing pauses or stretching out articulation. Tasks increased in complexity heirarchically from reading short phrases to conversational speech. Self appraisal through feedback and listening to recordings was developed. Other speech aspects such as volume or intonational variation were also addressed where necessary. | Palilalia | 6 sessions over 6 weeks with each session lasting approx. 1 hour (6 hours) | Home | YES | YES |
| Prosodic Exercises (PE) with Cueing | Scott 83 | Exercises aimed to increase patients awareness of speech problem, emphasising the importance of volume and intonation and using a visual feedback system (vocalite ‐ a voice‐operated light source). | Prosodic abnormality | 5 sessions per week for two weeks with each session lasting 1 hour (10 hours) | Home | UNCLEAR | YES |
| Prosodic Exercises (PE) without Cueing | Scott 83 | Same exercises used in PE with cueing but without use of vocalite. | Prosodic abnormality | 5 sessions per week for two weeks with each session lasting 1 hour (10 hours) | Home | UNCLEAR | YES |
Scott 1983 treated their patients for 10 hours over two weeks. Ramig 1995 and Constantinescu 2011 treated their patients for 16 hours over one month. Halpern 2007 delivered a similar treatment schedule of 16 sessions lasting 50 minutes each over one month. Lowit 2010 provided weekly hour‐long sessions for six weeks. Scott 1983 and Lowit 2010 treated their patients at home and Ramig 1995, Halpern 2007 and Constantinescu 2011 conducted their therapy in an outpatient setting. Healy 2002 split their participants between four groups and for each of the two interventions there was a group who received a single hour‐long session and a group who received six sessions, each an hour‐long over six weeks.
A variety of intervention methods were used in the studies: Scott et al considered the Parkinsonian speech abnormality to be a dysprosody (see Glossary Table 1). It is responsible for conveying subtle changes of meaning independently of word or grammatical order. In addition to this semantic role, it makes a major contribution to the emotional content of speech. Scott 1983 used prosodic exercises (PE) aimed at improving the patients’ prosodic abnormality by increasing their awareness of the problem and emphasising the importance of volume and intonation. The other arm of the study used the same prosodic exercises but also gave the patients visual feedback using a 'Vocalite'. The Vocalite is a voice‐operated light source designed to enable the patient to monitor some of the prosodic features of their own speech. The trial therefore examined the influence of the Vocalite only.
Ramig 1995 used respiration therapy (RT) aimed at increasing respiratory muscle activity, thus increasing respiratory volumes and subglottal air pressure. Patients were given visual feedback of their rib cage and abdominal movements via a 'Respigraph' in some of their treatment sessions. The other arm of the study used the LSVT method. LSVT is a high effort intensive treatment that aims to increase vocal loudness through increasing vocal adduction, 'thinking loud' and increasing respiratory effort; it is sometimes referred to as LSVT‐LOUD. These techniques were also used by Halpern 2007 where the comparison was LSVT‐LOUD versus LSVT‐ARTIC, which uses broadly the same techniques as LSVT‐LOUD but with the cue ‘Enunciate’ to focus on articulation. LSVT‐LOUD was also studied by Constantinescu 2011 in a non‐inferiority trial investigating the efficacy of online delivery of the techniques compared with traditional face‐to‐face delivery.
Speech rate reduction was the focus of the Lowit 2010 trial and an in‐ear device was used which altered the way a speaker hears their own voice by changing the pitch or delaying the sound. The control arm in this trial was traditional rate reduction therapy using behavioural techniques of pause insertion or stretching out articulation. Healy 2002 also studied two rate reduction techniques. Two of the groups were trained with an alphabet chart, in an attempt to control their rate of speech they were instructed to point to the initial letter of each word they said on the chart. The remaining two groups used a pacing board which is divided into sections, the speaker places their finger into each section consecutively for each word spoken.
Outcome measures
Scott 1983 measured prosodic abnormality and intelligibility. Ramig 1995 measured a very wide variety of outcomes. Objective and subjective measures of intelligibility, volume, monotonicity and an objective measure of pitch were included, as well as measures of depression (Beck Depression Index (BDI) (Beck 1961)); and activities of daily living that were affected by poor speech as measured with the communication and social interaction subsections of the Sickness Impact Profile (SIP) (Bergner 1981). Constantinescu 2011 measured a variety of outcomes including acoustic measures of loudness during a variety of speech formats, acoustic frequency and perceptual measures of loudness and intelligibility. Halpern 2007 measured loudness and intelligibility using the diagnostic rhyme test (DRT) and Lowit 2010 measured speech rate and intelligibility from long connected speech tasks. Healy 2002 reported measures of speaking rate and intelligibility for a variety of speaking modes as well as results of an oro‐motor assessment for which movement of lips, tongue and soft palate and voice quality are rated. This study group also included reported level of voluntary use of devices outside of therapy sessions as an outcome measure.
Risk of bias in included studies
See Table 2 and Figure 2 for summary of the methodological quality of the trials. It is impossible to blind patients and treating therapists in trials comparing the efficacy of two types of speech and language therapy. This leaves such trials open to performance and attrition bias. Blinded assessors were used for all included trials, so detection bias is unlikely in these studies.
2.
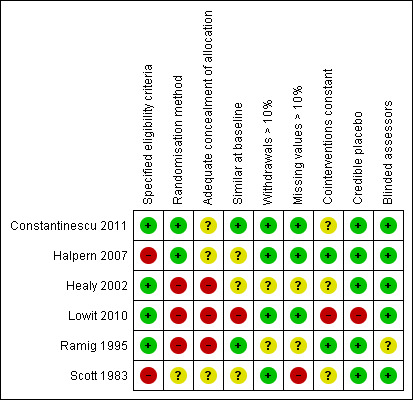
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Randomisation method and concealment of allocation
Scott 1983 used random number tables to randomise the patients. Although the therapist was blinded to the treatment group, the allocation was carried out by one of the authors of the paper. Personal communication with the authors revealed that this author saw only a few patients, therefore, the chance of selection bias is small. Ramig 1995 pulled numbers out of a hat to randomise patients into their trial and Healy 2002 used sealed envelopes. Though these are a truly random methods, they are open to manipulation as concealment of allocation can easily be compromised. Halpern 2007 and Constantinescu 2011 both used computer‐generated random number allocation. Concealment of allocation was not reported for either of these trials, therefore, cannot be confirmed and selection bias cannot therefore, be ruled out, despite the use of a sophisticated method to generate the allocation. Lowit 2010 used alternate allocation to randomise.This method is poor as it is not truly random and concealment of allocation cannot be achieved thus, there is a high risk of selection bias.
Eligiblity criteria
The eligibility criteria for the trials were broad. Scott 1983 treated patients with PD who had a speech disorder. They excluded those they considered to have a 'subjectively slight' communication disorder, intellectual impairment, history of stroke or other disorder likely to affect speech, significant hearing impairment, or those with varying drug therapy. Ramig 1995 excluded patients without idiopathic PD or with laryngeal pathology. The authors rated their patient's speech deficits on a five‐point scale along with their primary speech and/or voice characteristics at baseline. Healy 2002 included patients with a diagnosis of PD who were able to cope with individual therapy sessions. They also required participants to have a faster than normal speech rate or evidence of ‘palilalia’ (see Glossary Table 1), with or without other dysarthric features. Exclusion criteria for this trial were a score of nine or below on the Middlesex Elderly Assessment of Mental State ((MEAMS); Golding 1989); unclear diagnosis of PD; recent or anticipated change in Parkinsonian‐related medication; previous exposure to the pacing techniques used in this study. Halpern 2007 stated only that patients had PD. Inclusion criteria for Lowit 2010 were a diagnosis of idiopathic PD and a speech impairment severe enough to warrant treatment. Patients were excluded if there was a presence of significant dementia, history of deep brain stimulation or speech and language therapy for dysarthria symptoms during the previous 12 months or presence of speech and language problems other than those caused by PD. Constantinescu 2011 included patients with a diagnosis of hypokinetic dysarthria (mild to severe) with PD impacting on communication, a videolaryngoscopic evaluation of the vocal fold structure and movement consistent with PD, with the ability to produce speech of an increased loudness when guided to do so and on a consistent drug regimen for PD. Exclusion criteria for this group consisted of speech or language difficulties unrelated to PD, an additional co‐existing neurological disorder, respiratory difficulties inconsistent with PD, cognitive difficulties resulting in the inability to provide informed consent, a severe uncorrected visual and/or auditory disturbance, a history of alcohol abuse or participation in LSVT within 12 months of this study.
Patient numbers
We found only six randomised controlled trials comparing two methods of speech and language therapy for speech disorders in a total of 159 Parkinson's patients (see Table 2 for patient numbers in each trial). With such a small number of patients examined, it is possible that there was bias in the selection of patients, therefore, the applicability of the data to the general population with PD is questionable. The lack of power due to the small number of patients also increases the risk of a false negative result.
Similarity at baseline
In all trials, females were under‐represented (only 28%) while in the general Parkinson's population, the incidence is around 50%. Scott 1983 gave no indication of the severity of PD for its patients. It is accepted that the Hoehn and Yahr score assesses physical disability and does not have a speech component and so would be a very crude measure of speech impairment. However, it has been shown that impairment in speaking ability increases in frequency and intensity with the progress of the disease (Streifler 1984; Sapir 2001). Scott 1983 did not give a measure of overall speech impairment, whilst Ramig 1995 used a five‐point rating scale to summarise the speech impairments of the patients. Matching of baseline characteristics between the two arms is difficult to achieve for the small groups studied in the trials included in this review. This is highlighted in Lowit 2010 as there is a difference of 1.5 points between the average Hoehn and Yahr scores for the two groups. Healy 2002 could not be analysed for similarity as baseline characteristics for the groups were not provided.
Description of speech and language therapy methods
It is important that other speech and language therapists have enough details of the methods used in the studies to be able to reproduce them accurately. The methods of speech and language therapy were all described in broad terms in the papers. Ramig 1995 and Scott 1983 also referred readers to books that detailed the prosodic exercises (Halliday 1970) and LSVT (Ramig 1995b) in greater depth. The respiration therapy used in Ramig 1995 was also well referenced. A website reference was provided for further details of the in‐ear devices used in Lowit 2010 (www.casafuturatech.com). .
One speech and language therapist administered treatment to both groups in Scott 1983. Clinicians were randomly assigned to patients in Ramig 1995 and Constantinescu 2011 to deliver either high treatment; daily homework was given out to patients in these trials. Two therapists were involved in the delivery of treatment in Ramig 1995 and four therapists in Constantinescu 2011. Therapists involved in Healy 2002 instructed patients on the use of two rate‐control devices in either a single session or six sessions and then patients were encouraged to practice with the devices during their daily activities. Halpern 2007 and Lowit 2010 did not discuss the number or the type of health professionals involved in delivery of their interventions.
Drug therapy
The drug therapy of the patients was constant in Scott 1983 and Halpern 2007, but in Ramig 1995 the drug therapy was only kept constant for the duration of the speech and language therapy. During the two‐year follow‐up period the patients were 'optimally medicated'. Although various drug therapies can affect speech quality (Biary 1988; Dann 1994; Stewart 1995), this is ethically unavoidable in a trial of this length. Lowit 2010 stated that two of the 10 participants in their trial had changed their drug regimen during the treatment phase; the group assignments for these two patients were not reported. Although Constantinescu 2011 stated in their inclusion criteria that a stable drug regimen was required they failed to report whether or not the participants maintained this throughout the trial period. Healy 2002 did not report details of whether drug therapies were constant throughout the trial period for their patients.
Data analysis
This review discusses differences between the effectiveness of one type of SLT and an alternative, as such, there is no distinction in our analysis between two therapies which have both improved an outcome and two therapies which have both had no effect or made things worse, all are reported as ‘no difference’. For a discussion of the effectiveness of these techniques see Cochrane review ‘Speech and language therapy for speech problems in Parkinson’s disease’(Herd In Press).
Scott 1983, Ramig 1995 and Lowit 2010 analysed data on a per protocol basis rather than on an intention‐to‐treat basis. This compromises the randomisation and could lead to attrition and detection bias. In Lowit 2010, one patient withdrew from the TT group after baseline assessment. In Ramig 1995 after 24 months, seven of the 19 patients who started in the RT group and five of the 26 patients in the LSVT group, had withdrawn from the study. Interpretation of the results from this trial was further complicated by subsets of the patients being examined in greater detail for certain parameters (e.g. laryngoscopic analysis, aerodynamic analysis, perceptual voice quality analysis). It is not made clear which of the original 45 patients were examined in each of these subsets. In Scott 1983, two of the final 12 patients were missing from the baseline assessment in their PE‐only group. Halpern 2007 and Constantinescu 2011 had no withdrawals and so it is assumed they used an intention‐to‐treat design in their protocol, although this was not stated. It was unclear from the information provided whether any patients withdrew from Healy 2002.
Only Constantinescu 2011 statistically compared the change in a given outcome measure (i.e. score after therapy ‐ score at baseline) between the two therapy groups (i.e. change due to therapy A versus change due to therapy B). All other trials statistically compared the change in an outcome for each therapy group individually over time. This means that these trials do not examine whether one form of SLT is better than another, only that improvements occurred after a given therapy. The results from four trials were analysed for statistical significance. Outcomes from Scott 1983 and Healy 2002 could not be included in this due to missing baseline data and data provided in a non‐usable format respectively.
Effects of interventions
See Summary of Results Tables: Table 4; Table 5; Table 6; Table 7; Table 8. Significant results are statistically significant at the P < 0.05 level. The trial designs of the identified studies were so different that meta‐analysis of the results was inappropriate.
4. Summary of Results ‐ Scott 83.
| Subsection | Outcome | Mean Difference pre‐/post‐treatment |
| General | Prosodic abnormality score | ‐0.25 |
| Intelligibility | Rating score (0‐3) | 0.25 |
| Visual analogue scale (0‐100) | 21.4 | |
| Mean Difference = (Mean change due to PE + cues) ‐ (Mean change due to PE alone) | No statistical analysis available for mean change. |
5. Summary of Results ‐ Ramig 95.
| Subsection | Outcome | Mean Difference pre/post treatment | 95% CI pre‐/post‐ treatment | P value pre‐/post‐ treatment | Mean Difference pre‐/12 month follow‐up | 95% CI pre‐/12 month follow‐up | P value pre‐/12 month follow‐up | Mean Difference pre‐/24 month follow‐up | 95% CI pre/24 month follow‐up | P value pre‐/24 month follow‐up |
| Intelligibility | Patient assessed | 3.6 | ‐7.0, 14.2 | 0.50 | ||||||
| Carer assessed | 10.7 | ‐7.2, 28.6 | 0.24 | |||||||
| Loudness | Patien assessed | 3.3 | ‐10.7, 17.3 | 0.64 | ||||||
| Carer assessed | 1.6 | ‐19.9, 23.1 | 0.88 | |||||||
| Monologue | 2.9 | 0.6, 5.7 | 0.01 | 3.8 | 1.2, 6.4 | 0.004 | 1.3 | ‐2.0, 4.6 | 0.44 | |
| Reading | 5.5 | 3.4, 7.7 | < 0.00001 | 2.8 | 0.2, 5.4 | 0.03 | 2.9 | ‐0.3, 6.1 | 0.08 | |
| Sustained phonation | 14.3 | 11.5, 17.1 | < 0.00001 | 9.5 | 6.0, 13.0 | < 0.00001 | 7.3 | 3.3, 11.3 | 0.0004 | |
| Monotonicity | Patient assessed | 8.7 | ‐4.6, 22.0 | 0.2 | ||||||
| Care assessed | 3.1 | ‐19.0, 25.2 | 0.78 | |||||||
| Monologue | 0.5 | 0.01, 0.9 | 0.04 | 0.8 | 0.1, 1.4 | 0.02 | 0.8 | 0.1, 1.5 | 0.03 | |
| Reading | 0.3 | 0.0, 0.6 | 0.07 | 0.3 | ‐0.1, 0.7 | 0.12 | 0.2 | ‐0.1, 0.6 | 0.2 | |
| Pitch | Monologue | 8.2 | ‐5.4, 21.8 | 0.24 | ‐3.2 | ‐17.4, 11.0 | 0.6 | |||
| Reading | 4.9 | ‐7.4, 17.2 | 0.43 | 2.9 | ‐11.2, 17.0 | 0.7 | ||||
| ADL | SIP ‐ communication | ‐14.5 | ‐29.4, 0.4 | 0.06 | ‐12.8 | ‐32.7, 7.2 | 0.21 | |||
| SIP ‐ social interaction | ‐0.4 | ‐6.8, 6.0 | 0.9 | ‐4.6 | ‐16.4, 7.2 | 0.45 | ||||
| Depression | BDI | ‐1.1 | ‐4.7, 2.5 | 0.55 | ‐0.1 | ‐5.6, 5.5 | 0.98 | |||
| Hoarseness | Therapist assessed | ‐17.2 | ‐34.3, ‐0.1 | 0.05 | ||||||
| Breathiness | Therapist assessed | ‐23.8 | ‐45.5, ‐2.1 | 0.03 | ||||||
| Mean Change = (Mean change due to LSVT) ‐ (Mean change due to Respiration) |
ADL: activities of daily living BDI: Beck Depression Index LSVT: Lee Silverman Voice Treatment SIP: Sickness Impact Profile
6. Summary of Results ‐ Halpern 2007.
| Subsection | Outcome | Mean Difference pre‐/post‐treatment | 95% CI pre‐/post‐treatment | P value pre‐/post‐treatment |
| Loudness | Reading | ‐5.0 | ‐8.3, ‐1.7 | 0.003 |
| Intelligibility | DRT babble noise | ‐12.5 | ‐22.2, ‐2.8 | 0.01 |
| DRT shopping mall noise | ‐11.6 | ‐24.7, 1.5 | 0.08 | |
| DRT no noise | ‐2.1 | ‐5.9, 1.8 | 0.29 |
DRT: Diagnostic Rhyme Test
7. Summary of Results ‐ Lowit 2010.
| Subsection | Outcome | Mean Difference pre‐/post‐treatment | 95% CI pre‐/post‐treatment | P value pre‐/post‐treatment | Mean Difference pre‐/12 month follow‐up | 95% CI pre‐/12 month follow‐up | P value pre‐/12 month follow‐up |
| Articulation Rate | Reading | ‐0.7 | ‐1.3, ‐0.2 | 0.01 | ‐0.8 | ‐1.4, ‐0.2 | 0.007 |
| Intelligibility | Reading | ‐23.9 | ‐44.5, ‐3.3 | 0.02 | ‐5.5 | ‐28.5, 17.5 | 0.64 |
| Monologue | 0.1 | ‐2.5, 2.7 | 0.94 | 0.0 | ‐2.1, 2.1 | 0.97 |
CI: confidence interval
8. Summary of Results ‐ Constantinescu 2011.
| Subsection | Outcome | Mean Difference pre‐/post‐treatment | 95% CI pre‐/post‐treatment | P value pre‐/post‐treatment |
| Intelligibility | Overall articulatory precision | 9.1 | ‐0.7, 18.9 | 0.07 |
| Overall speech intelligibility in conversation | ‐9.1 | ‐20.7, 2.6 | 0.13 | |
| Percentage word intelligibility | ‐2.1 | ‐7.8, 3.6 | 0.46 | |
| Percentage sentence intelligibility | ‐1.4 | ‐4.0, 1.2 | 0.30 | |
| Communication efficiency ratio | 0.0 | ‐0.2, 0.2 | 1.00 | |
| Loudness | Reading | 0.3 | ‐1.7, 2.2 | 0.81 |
| Monologue | 0.0 | ‐1.9, 1.9 | 0.99 | |
| Sustained phonation | ‐10.0 | ‐12.9, ‐7.2 | < 0.00001 | |
| Monotonicity | Vocal glides | 0.4 | ‐4.1, 4.9 | 0.87 |
| Therapist assessed | 6.5 | ‐2.7, 15.7 | 0.17 | |
| Duration of phonation | Sustained phonation | 0.4 | ‐2.7, 3.4 | 0.81 |
| Loudness level | Therapist assessed | ‐7.7 | ‐20.2, 4.8 | 0.22 |
| Loudness variability | Therapist assessed | 5.4 | ‐2.9, 13.6 | 0.20 |
| Roughness | Therapist assessed | 9.2 | 1.5, 16.9 | 0.02 |
| Breathiness | Therapist assessed | 5.5 | ‐3.4, 14.5 | 0.23 |
Scott 1983 Therapists involved in Scott 1983 assessed tapes of the 23 patients' speech. They used a seven‐point prosodic abnormality score, which assessed volume, pitch, tone, intonation, vocal quality (hoarseness, tremor etc), rate and rhythm. One point was scored for an abnormality of any item, zero if normal. Scott 1983 measured intelligibility in two ways, firstly using an ad hoc rating scale (zero to three) and secondly with a visual analogue scale (zero to 100). Scott 1983 concentrated solely on prosody and intelligibility as outcome measures in this study. No other outcomes were measured. The statistical significance of all Scott 1983 outcome measures could not be assessed as an additional patient was included in the exercises‐alone group post‐treatment data sets.
Ramig 1995 Ramig 1995 was unique in following their patient groups for two years. Unfortunately, the data were analysed in a per‐protocol rather than intention‐to‐treat manner. From the 45 patients that started the trial, 12 patients withdrew at later time points and were removed from the baseline data in subsequent publications. Potentially the baseline data could have shifted over time due to these withdrawals.
Intelligibility was assessed with a visual analogue scale (zero to 100) as used in Scott 1983, but in this study, ratings were carried out by the patients themselves and their carers prior to treatment and immediately post‐treatment. The differences in the change of the mean intelligibility scores between the RT group and the LSVT group were not statistically significant (P = 0.5 as assessed by the patients and P = 0.24 as assessed by the carers).
For objective outcomes, loudness was described as sound pressure level (SPL) or intensity (see Glossary: Table 1). Patients and carers assessed loudness on a 100‐point visual analogue scale. There was no difference in the change of the mean scores between the treatment groups (P = 0.64 as assessed by the patients or P = 0.88 as assessed by the carers). The mean objective loudness of the patient's monologue improved by 2.9 dB (95% confidence interval (CI) 0.6 to 5.2; P = 0.01) more with LSVT than with RT immediately after treatment. This significant result was maintained 12 months later with a reported 3.8 dB (95% CI 1.2 to 6.4; P = 0.004) difference between the two groups. After 24 months, the difference was no longer significant (P = 0.44). A similar pattern was observed in the loudness of the patients' voices reading a standard passage (the Rainbow Passage). Immediately after LSVT, the patient's reading loudness improved by 5.5 dB (95% CI 3.4 to 7.7; P < 0.00001) when compared with RT and at 12 months the difference was 2.8 dB (95% CI 0.2 to 5.4; P = 0.03). The difference between therapy groups was no longer significant at 24 months (P = 0.08). For sustained phonation, the difference between the two groups was significant at 14.3 dB (95% CI 11.5 to 17.0; P < 0.00001) immediately after treatment and was maintained at 12 months (9.5 dB; 95% CI 6.0 to 13.0; P < 0.00001) and 24 months (7.3 dB; 95% CI 3.3 to 11.3; P = 0.0004) after treatment.
Monotonicity was assessed by patients and their carers on a visual analogue scale in Ramig 1995. The difference in the change of the mean monotonicity scores between the groups was not significant in both cases (P = 0.2 as assessed by the patients and P = 0.78 as assessed by the carers). The objective measure of monotonicity was fundamental frequency (pitch) variability (see Glossary: Table 1). This is a measure of the standard deviation of the lowest pitch of a patient's voice over a period of time. In this case it was measured in semitones (ST) for monologue speech and reading a passage. During a monologue the groups’ speech varied by 0.47 semitones (95% CI 0.01 to 0.93; P = 0.04) more with LSVT than with RT immediately after treatment. This difference was maintained 24 months later, when it was 0.77 semitones (95% CI 0.06 to 1.48; P = 0.03). No difference was observed in the pitch variability of the patients' voice when reading a standard passage (the Rainbow Passage) immediately after treatment. Follow‐up assessments of this outcome up to 24 months later also failed to distinguish between the groups.
Perceptual ratings of voice quality assessed by speech pathologists, who were blinded to the type of therapy received, were also reported. Breathiness (23.8 points; 95% CI ‐45.5 to ‐2.1; P = 0.03) and hoarseness (17.2 points; 95% CI ‐34.3 to ‐0.1; P = 0.05) showed greater improvements in the LSVT group than the RT group.
The pitch (fundamental frequency) of the patient's voice was measured objectively from monologue speech and reading a standard passage; both methods failed to distinguish between the therapy groups. Neither the initial post–treatment result nor the follow‐up results gave differences between the groups that were statistically significant. Similarly, for the increase in the pitch of the patients' voice reading a standard passage (the Rainbow Passage), the differences were not statistically significant at either time point.
Communication aspects of QOL were assessed in Ramig 1995 using the SIP communication and social interaction subsections. SIP is a highly validated scale that uses a number of rated statements to assess the severity of the impact of a disease on QOL. There was no difference between the LSVT group and the RT group for these outcome measures. Depression was assessed using the BDI. The difference between the two groups was not statistically significant immediately after therapy or at 12‐month follow‐up.
No assessment was made in Ramig 1995 of activities of daily living and no economic analysis was performed.
Healy 2002 The number of syllables per minute for a sample of 22 sentences (reading), a story telling and conversational speech was assessed by a therapist as well as percentage of intelligible words in the sample for the sentence reading and intelligibility rating (scale of one to eight where eight is normal speech) for spontaneous conversational speech. An Oro‐motor assessment was carried out by the therapist, for which movement of lips, tongue and soft palate and voice quality are rated on a scale of one to eight where eight is normal. This study group also included reported level of voluntary use of devices outside of therapy sessions as an outcome measure, this was self‐rated by patients (scale of one to 10, where zero = never used). Baseline data were not reported for this trial and the results for the two different treatment schedule groups were amalgamated for each intervention, precluding statistical analysis for this review.
Halpern 2007 The Diagnostic Rhyme Test (DRT), which uses rhyming pairs differing only in their initial consonant, was used to measure intelligibility before and after LSVT‐ARTIC and LSVT‐LOUD delivered in Halpern 2007. Analysis of the recorded voice samples played under three different noise conditions was carried out. LSVT‐LOUD gave a greater improvement than LSVT‐ARTIC for babble noise 12.5 points (95% CI ‐22.2 to ‐2.8; P = 0.01). No statistically significant difference was recorded for the other two conditions (P = 0.08 for shopping mall noise and P = 0.29 for no noise).
Those receiving LSVT‐LOUD showed a greater improvement in the loudness of reading the DRT word list difference of 5.0 dB (95%CI ‐8.3 to ‐1.7; P = 0.003).
Halpern 2007 only reported measures of intelligibility and loudness.
Lowit 2010 Lowit 2010 measured the intelligibility of two different speaking modes using different methods. For monologue speech a nine‐point Likert scale showed no difference between the Altered Auditory Feedback (AAF) and the Traditional Therapy (TT) groups either immediately after therapy or six weeks later. The TT group showed the greatest improvement for reading a passage as measured by the direct magnitude estimation (DME): the difference for ratings immediately after therapy of 23.9 points (95% CI ‐44.5 to ‐3.3; P = 0.02) was significant, however after six weeks the difference was no longer statistically significant (P = 0.64).
Articulation rate was improved by 0.73 syllables/sec (95% CI ‐1.33 to ‐0.13; P = 0.02) more in the AAF group than in the TT group immediately after therapy. After six weeks the difference between the two groups had increased slightly to 0.83 (95% CI ‐1.43 to ‐0.23; P = 0.007).
Constantinescu 2011 Non‐inferiority of online LSVT delivery compared with face‐to‐face delivery was supported by non‐ significant differences in: objective measures of loudness of monologue speech (P = 1.0 for the primary outcome measure), reading loudness (P = 0.8), duration of sustained phonation (P = 0.8) and maximum fundamental frequency range (P = 0.9) taken from vocal glides. The only objective measure which showed a statistically significant difference between the two delivery methods was sustained phonation, for which the result was 10.0 dB (95% CI ‐12.8 to ‐7.2; P < 0.00001) in favour of face‐to‐face LSVT.
Perceptual ratings of intelligibility were also reported for Constantinescu 2011. The Assessment of Intelligibility of Dysarthric Speech (ASSIDS) (Yorkston 1981), during which patients read 50 words and 22 sentences of increasing length which were randomised prior to the assessment, was used to analyse percentage sentence intelligibility, percentage word intelligibility, and communication efficiency( CER). All parameters showed no significant difference between the two methods of delivery for LSVT. Monologue speech was assessed for perceptual voice ratings of breathiness, roughness and overall articulatory precision (OAP). No significant differences were reported for OAP and breathiness. One of the few significant differences recorded was for roughness which showed a difference between the mean changes from baseline of 9.2 points (95% CI 1.5 to 16.9; P = 0.02) in favour of face‐to‐face delivery. Patients were also asked to read a passage before and after therapy, and their speech during this task was used to obtain perceptual ratings of loudness level (P = 0.22), loudness variability (P = 0.2) and pitch variability (P = 0.2). All outcome measures for this task supported the non‐inferiority of online delivery of LSVT as the differences between the mean changes of the two groups were not statistically significant.
Discussion
Summary of main results
Six randomised controlled trials were found comparing two forms of speech and language therapy (159 patients). These trials varied considerably in their methodology so the results could not be combined by meta‐analysis. One study examined the impact of visual feedback on prosodic exercise techniques. Another compared the Lee Silverman Voice Therapy (LSVT) technique with respiration therapy. LSVT, in its original form (LSVT‐LOUD), was also compared with a modified version of itself with cues and emphasis centred around articulation (LSVT‐ARTIC). A third trial also studied LSVT, comparing two methods of delivering the treatment, to provide evidence for the non‐inferiority of online sessions compared with face‐to‐face delivery. Two trials studying rate reduction techniques were found. One of these trials compared an in‐ear device giving altered auditory feedback (AAF) with traditional rate reduction behavioural therapy methods and the other compared an alphabet board and a pacing board.
LSVT improved loudness and monotonicity more than RT in a variety of speaking modes in Ramig 1995. The result was maintained for 12 months in the objective measures of monologue and reading loudness and for 24 months in the objective measures of sustained phonation loudness and monotonicity. Patient and carer ratings of these outcomes failed to distinguish between SLT methods but therapist ratings of voice quality did show a significantly greater improvement in the LSVT group.
Halpern 2007 assessed patients for intelligibility and loudness of speech pre‐ and immediately post‐treatment but they did not follow up the patients. Loudness improved more in the LSVT‐LOUD group than in the LSVT‐ARTIC group. Intelligibility of recorded speech was assessed three times with different types of background noise overlaid onto it, only the babble noise condition distinguished between the two groups with LSVT‐LOUD showing a greater improvement.
The non‐inferiority of online delivery of LSVT versus face‐to‐face delivery of the therapy was supported by non‐significant differences in loudness, intelligibility and monotonicity in Constantinescu 2011. Only pre‐ and immediately post‐treatment assessments were carried out in this trial.
Despite improving the articulation rate of speakers by a greater amount than traditional therapy (TT), AAF in‐ear devices were less successful than TT at improving the intelligibility of speech.
Limited evidence is presented in this review in favour of LSVT (or LSVT‐LOUD) over RT and LSVT‐ARTIC and supporting the non‐inferiority of online delivery of LSVT compared with traditional face‐to‐face delivery of the same techniques. Larger trials are required to confirm these findings.
Overall completeness and applicability of evidence
Outcome measures
It can be argued that intelligibility is the most critical outcome to be measured in speech and language therapy trials. If this global measure does not improve then it is irrelevant to the patient how many other objective speech quality measures improve; they still cannot be understood. All included trials assessed intelligibility and almost all results were not statistically significant. The exception to this was the perceptual ratings of speech recordings overlaid with babble noise (Halpern 2007) for which LSVT‐LOUD gave a greater improvement than LSVT‐ARTIC (‐12.5 points; 95% CI ‐22.2 to ‐2.8; P = 0.01). The non‐inferiority of online delivery of LSVT was supported by the intelligibility results of Constantinescu 2011, who reported no statistically significant difference between the improvements in both acoustic and perceptual measures for this group and the face‐to‐face delivery group.
Parkinsonian speech is often characterised by a quiet voice. This can exacerbate problems with intelligibility as listeners strain to hear what is being said by the patient. All three trials for LSVT included loudness as an outcome measure. Patients showed a greater improvement from LSVT than alternative therapies with a difference for reading of 5.0 dB (95%CI ‐8.3 to ‐1.7; P = 0.003) (Halpern 2007) and 5.5 dB (95% CI 3.4 to 7.7; P < 0.00001) (Ramig 1995) and for monologue of 2.9 dB (95% CI 0.6 to 5.2; P = 0.01) (Ramig 1995). Significant differences were recorded 12 months after therapy. Objective measures of monotonicity of a monologue also favoured the LSVT method (0.47 semitones; 95% CI 0.01 to 0.93; P = 0.04). Constantinescu 2011 reported no significant difference between online LSVT and face‐to‐face delivery in reading and monologue loudness and monotonicity. Sustained phonation loudness was one of the few outcome measures which did not support the equivalence of the two methods of delivery and this result was significant in favour of face‐to‐face delivery.
The prosodic abnormality score, which assesses volume, pitch, tone, intonation, vocal quality (hoarse, tremor etc), rate and rhythm, does not appear to have been independently assessed for validity.
Adverse events were not reported by any of the trials included in this review. Although the risk associated with speech and language therapy is low, patients could be affected by vocal strain or abuse during high effort exercises.
Quality of life (QOL) is recognised as a vital assessment in modern clinical trials so that the impact of a given therapy can be assessed in the light of its perceived value to the patient. Only Ramig 1995 attempted to measure this using the communication and social interaction subsections of SIP which failed to distinguish between the two therapies studied.
Depression in trial participants could affect compliance and effectiveness of therapy. In turn this outcome may be improved due to time and attention or specific therapeutic techniques. It is important to measure depression as a number of surveys (Karlsen 1999; GPDS 2000; Zach 2004; Visser 2008) have found depression to be the main contributor to a reduction in quality of life due to PD. Ramig 1995 used the BDI to assess depression in their patients. It did not detect any improvement in either of the two therapies examined. On average, the patients assessed were not depressed as defined by this scale.
Approximately 75% of patients with PD live with a partner, who is usually of a similar age and may have disabilities of their own (Lloyd 1999). The impact of caring for a person with PD can be severe (O'Reilly 1996) and it would be hoped that an intervention such as speech and language therapy could have a positive effect on the carer's life as well as the patient's. Although Ramig 1995 did ask the carers for their assessment of the patient's speech, they did not ask what impact poor communication with the patient had on their stress and strain levels and their QOL. All other trials failed to assess carer outcomes.
No health economics analysis of speech and language therapy has been performed which precludes an understanding of the economic value of this therapy. The results of non‐inferiority trials such as Constantinescu 2011 contribute useful data to evaluate the most cost effective modes of delivery in future health economics analyses.
Speech and language therapy methodology
There is no consensus amongst therapists on which SLT method to use or whether it should be a combination of methods. A recent survey of speech and language therapists in the UK (Miller 2011), showed a high proportion of patients with PD referred for SLT receive only an assessment, advice and review service. When treatment methods were employed, voice quality was most commonly addressed with LSVT or other vocal loudness exercises and intelligibility was treated with pacing/rate control exercises supported by work on loudness. Psychosocial and language strategies were rarely employed by the therapists surveyed despite these being flagged as important reasons for referral. Over 75% of all therapists surveyed wanted further training and over half of these specifically desired training in LSVT techniques. As part of the same study, a survey of SLT provision was carried out with patients with PD and their carer’s (Miller 2011b). Of the 83 patients who had received any treatment from a speech and language therapist, 56% had their sessions in a local clinic or hospital outpatients setting and 37% were visited in their own home. Median duration of therapy for those treated was four weeks with 68% attending a single weekly session, a further 22%, who were predominantly receiving LSVT, had four or more therapy sessions per week. Most sessions (80%) lasted between 30 to 60 minutes.
Speech and language therapy terminology
The terminology in this review has been aimed at a general clinical audience unlike some of the trial reports. It is hoped that this will improve understanding by non‐SLT specialists. In an attempt to make reading the original reports easier, we have included a glossary in this review (Table 1: Glossary).
The same outcome measure was often labelled differently in different trials (e.g. volume and sound pressure level) which adds further to the confusion. It was also difficult for a non‐specialist to determine the value of any given change in the vocal characteristics measured in these trials. Care should be taken when writing reports of speech therapy that an association is made with the direction and size of change in a given outcome measure and its impact on the communication ability of the patient.
Quality of the evidence
The methodological quality of the trials and the standard of the reporting was mixed, but Scott 1983 and Ramig 1995 were published before the CONSORT guidelines were published (CONSORT 1996). The trials used insufficient numbers of patients to avoid making false positive or false negative conclusions and to reduce the possibility of selection bias. It can be argued that the primary outcome of interest in SLT trials should be improved intelligibility which was an outcome in all included trials.
The trials included in this review used a variety of randomisation methods, but all omitted to report whether concealment of allocation had been achieved, leaving the risk of selection bias unclear or high in all cases. It is vital that eligibility criteria, including type and severity of PD as well as co‐existing conditions, are stated so that the population treated during the trial is well defined. This enables treating physicians to assess the results of the trials for relevance to their patients.
There is no difference between the prevalence of PD in men and that in women (Tanner 1996). Only 28% of the patients enrolled into the trials were female and so the trial groups were not truly representative of the general PD population.
Authors' conclusions
Implications for practice.
Considering the small number of patients examined and the methodological flaws in the studies, it is unsafe to draw any conclusions regarding the efficacy of one form of speech and language therapy in preference over another for the treatment of speech problems in PD.
Implications for research.
To obtain proof of the efficacy of speech and language therapy for speech disorders in patients with PD, large randomised placebo‐controlled trials are required. After this, large RCTs are needed to demonstrate the most effective form of SLT to treat speech disorders in PD. All of these trials should use a rigorous method of randomisation and adequate concealment of allocation. Data should be analysed on an intention‐to‐treat basis. Trials should be reported according to CONSORT guidelines (CONSORT 1996).The methodological shortcomings highlighted in this review have a significant bearing on the conduct of future speech and language therapy trials in Parkinson's disease.
Firm diagnostic criteria should be used (e.g. UK Parkinson's Disease Brain Bank Criteria) (Gibb 1988).
Inclusion and exclusion criteria should be clear and trials should aim to enrol uniform cohorts of patients with PD.
Investigators should clarify at what stage of the disease speech and language therapy is being evaluated.
Trials must have sufficient numbers of patients to avoid false negative or false positive conclusions.
Trials must include a clear description of the two therapeutic interventions.
The patients should be followed for at least six months after treatment to assess the duration of any benefit from the SLT intervention.
Regardless of the scale used, trials should report whether scores on impairment and disability refer to the 'on' or 'off' phase.
Suitable clinimetrically sound outcome measures should be chosen so that the efficacy and effectiveness of SLT can be assessed and an economic analysis performed. Outcomes which have meaning to patients should be used wherever possible since they need to know the value of SLT in practical terms.
The data must be analysed on an intention‐to‐treat basis and the change in an outcome measure must be compared statistically across the two therapy groups.
What's new
| Date | Event | Description |
|---|---|---|
| 9 July 2012 | New citation required but conclusions have not changed | New citation: conclusions not changed |
| 9 July 2012 | New search has been performed | Searches had been rerun and new studies were incorporated |
History
Protocol first published: Issue 4, 2000 Review first published: Issue 2, 2001
| Date | Event | Description |
|---|---|---|
| 14 October 2010 | Amended | This review is currently being updated. In the meantime, readers should note that the data reviewed in it goes back up to 2000. New evidence might have been published subsequent to the current version. This new evidence will be evaluated during the updating process. |
| 13 November 2008 | Amended | Converted to new review format. |
| 28 February 2001 | New citation required and conclusions have changed | Substantive amendment |
Acknowledgements
We would like to thank the authors of the included studies who assisted in providing unpublished data and clarification of their methods. Thanks also to the people contacted whilst locating unpublished trials. Thanks to Dr Richard Barham at the National Physics Laboratory for his assistance with the glossary, to Ashwini Sreekanta at the University of Birmingham for her work on the search strategy and to Maxwell Barnish at the University of East Anglia for his contribution to the search for studies.
Appendices
Appendix 1. MEDLINE search strategy
1. randomized controlled trial.pt.
2. controlled clinical trial.pt.
3. randomized.ab.
4. placebo.ab.
5. drug therapy.fs.
6. randomly.ab.
7. trial.ab.
8. groups.ab.
9. 1 or 2 or 3 or 3 or 4 or 5 or 6 or 7 or 8
10. exp animals/ not humans.sh.
11. 9 not 10
12. exp Parkinson disease/
13. Parkinson$.tw.
14. 12 or 13
15. exp speech disorders/
16. exp articulation disorders/
17. dysarthr*.tw.
18. (speech or speak*).tw.
19. intelligib*.tw.
20. dysprod*.tw.
21. hypophoni*.tw.
22. monoton*.tw.
23. phon*.tw.
24. 15 or 16 or 17 or 18 or 19 or 20 or 21 or 22 or 23
25. exp "rehabilitation of speech and language disorders"/ or exp language therapy/ or exp myofunctional therapy/ or exp speech, alaryngeal/ or exp speech, esophageal/ or exp speech therapy/ or exp voice training/
26. ((speech or speak* or language or voice or vocal* or articulate* or sing*) adj3 (task* or therap* or treat* or train* or counsel* or intervention* or exercise* or drill)).tw.
27. (Silverman* or LSVT).tw.
28. 25 or 26 or 27
29. 11 and 14 and 24 and 28
30. 11 and 14 and 28
31. 29 or 30
Data and analyses
Comparison 1. LSVT versus high respiratory effort treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Patient assessed loudness | 1 | 45 | Mean Difference (IV, Fixed, 95% CI) | 3.30 [‐10.71, 17.31] |
| 2 Carer assessed loudness | 1 | 20 | Mean Difference (IV, Fixed, 95% CI) | 1.60 [‐19.89, 23.09] |
| 3 Patient assessed monotonicity | 1 | 45 | Mean Difference (IV, Fixed, 95% CI) | 8.7 [‐4.56, 21.96] |
| 4 Carer assessed monotonicity | 1 | 20 | Mean Difference (IV, Fixed, 95% CI) | 3.10 [‐19.03, 25.23] |
| 5 Patient assessed intelligibility | 1 | 52 | Mean Difference (IV, Fixed, 95% CI) | 3.60 [‐6.98, 14.18] |
| 6 Carer assessed intelligibility | 1 | 20 | Mean Difference (IV, Fixed, 95% CI) | 10.70 [‐7.21, 28.61] |
| 7 SPL Reading | 1 | 147 | Mean Difference (IV, Fixed, 95% CI) | 3.62 [2.36, 4.89] |
| 7.1 Pre/Post | 1 | 45 | Mean Difference (IV, Fixed, 95% CI) | 5.54 [3.35, 7.73] |
| 7.2 Pre/6 months | 1 | 35 | Mean Difference (IV, Fixed, 95% CI) | 2.4 [‐0.05, 4.85] |
| 7.3 Pre/12 months | 1 | 35 | Mean Difference (IV, Fixed, 95% CI) | 2.8 [0.24, 5.36] |
| 7.4 Pre/24 months | 1 | 32 | Mean Difference (IV, Fixed, 95% CI) | 2.90 [‐0.31, 6.11] |
| 8 SPL Monologue | 1 | 89 | Mean Difference (IV, Fixed, 95% CI) | 2.49 [1.22, 3.76] |
| 8.1 Pre/Post | 1 | 29 | Mean Difference (IV, Fixed, 95% CI) | 2.9 [0.63, 5.17] |
| 8.2 Pre/6 months | 1 | 21 | Mean Difference (IV, Fixed, 95% CI) | 1.6 [‐0.71, 3.91] |
| 8.3 Pre/12 months | 1 | 21 | Mean Difference (IV, Fixed, 95% CI) | 3.8 [1.21, 6.39] |
| 8.4 Pre/24 months | 1 | 18 | Mean Difference (IV, Fixed, 95% CI) | 1.30 [‐2.01, 4.61] |
| 9 Fundamental frequency reading | 1 | 109 | Mean Difference (IV, Fixed, 95% CI) | 3.96 [‐3.66, 11.58] |
| 9.1 Pre/Post | 1 | 43 | Mean Difference (IV, Fixed, 95% CI) | 4.90 [‐7.37, 17.17] |
| 9.2 Pre/6 months | 1 | 33 | Mean Difference (IV, Fixed, 95% CI) | 3.80 [‐9.63, 17.23] |
| 9.3 Pre/12 months | 1 | 33 | Mean Difference (IV, Fixed, 95% CI) | 2.90 [‐11.20, 17.00] |
| 10 Frequency variability monologue | 1 | 93 | Mean Difference (IV, Fixed, 95% CI) | 0.57 [0.28, 0.85] |
| 10.1 Pre/post | 1 | 29 | Mean Difference (IV, Fixed, 95% CI) | 0.47 [0.01, 0.93] |
| 10.2 Pre/6 months | 1 | 22 | Mean Difference (IV, Fixed, 95% CI) | 0.43 [‐0.14, 1.00] |
| 10.3 Pre/12 months | 1 | 22 | Mean Difference (IV, Fixed, 95% CI) | 0.75 [0.13, 1.37] |
| 10.4 Pre/24 months | 1 | 20 | Mean Difference (IV, Fixed, 95% CI) | 0.77 [0.06, 1.48] |
| 11 Frequency variability reading | 1 | 141 | Mean Difference (IV, Fixed, 95% CI) | 0.24 [0.08, 0.41] |
| 11.1 Pre/post | 1 | 43 | Mean Difference (IV, Fixed, 95% CI) | 0.27 [‐0.03, 0.57] |
| 11.2 Pre/6 months | 1 | 33 | Mean Difference (IV, Fixed, 95% CI) | 0.18 [‐0.16, 0.52] |
| 11.3 Pre/12 months | 1 | 33 | Mean Difference (IV, Fixed, 95% CI) | 0.29 [‐0.08, 0.66] |
| 11.4 Pre/24 months | 1 | 32 | Mean Difference (IV, Fixed, 95% CI) | 0.23 [‐0.12, 0.58] |
| 12 Fundamental frequency monologue | 1 | 73 | Mean Difference (IV, Fixed, 95% CI) | 3.28 [‐4.73, 11.30] |
| 12.1 Pre/post | 1 | 29 | Mean Difference (IV, Fixed, 95% CI) | 8.2 [‐5.35, 21.75] |
| 12.2 Pre/6 months | 1 | 22 | Mean Difference (IV, Fixed, 95% CI) | 4.30 [‐9.60, 18.20] |
| 12.3 Pre/12 months | 1 | 22 | Mean Difference (IV, Fixed, 95% CI) | ‐3.2 [‐17.43, 11.03] |
| 13 BDI self rating of depression | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐0.79 [‐3.82, 2.23] |
| 13.1 Pre/post | 1 | 35 | Mean Difference (IV, Fixed, 95% CI) | ‐1.1 [‐4.72, 2.52] |
| 13.2 Pre/12 months | 1 | 25 | Mean Difference (IV, Fixed, 95% CI) | ‐0.07 [‐5.60, 5.46] |
| 14 SIP Communication | 1 | 59 | Mean Difference (IV, Fixed, 95% CI) | ‐13.88 [‐25.80, ‐1.96] |
| 14.1 Pre/post | 1 | 35 | Mean Difference (IV, Fixed, 95% CI) | ‐14.5 [‐29.37, 0.37] |
| 14.2 Pre/12 months | 1 | 24 | Mean Difference (IV, Fixed, 95% CI) | ‐12.76 [‐32.71, 7.19] |
| 15 SIP social interaction | 1 | 59 | Mean Difference (IV, Fixed, 95% CI) | ‐1.35 [‐6.98, 4.28] |
| 15.1 Pre/post | 1 | 35 | Mean Difference (IV, Fixed, 95% CI) | ‐0.40 [‐6.80, 6.00] |
| 15.2 Pre/12 months | 1 | 24 | Mean Difference (IV, Fixed, 95% CI) | ‐4.6 [‐16.42, 7.22] |
| 16 Hoarseness | 1 | 20 | Mean Difference (IV, Fixed, 95% CI) | ‐17.20 [‐34.29, ‐0.11] |
| 17 Breathiness | 1 | 20 | Mean Difference (IV, Fixed, 95% CI) | ‐23.8 [‐45.50, ‐2.10] |
| 18 SPL sustained phonation | 1 | 148 | Mean Difference (IV, Fixed, 95% CI) | 11.12 [9.43, 12.81] |
| 18.1 Pre/post | 1 | 45 | Mean Difference (IV, Fixed, 95% CI) | 14.3 [11.52, 17.08] |
| 18.2 Pre/6 months | 1 | 35 | Mean Difference (IV, Fixed, 95% CI) | 10.6 [6.98, 14.22] |
| 18.3 Pre/12 months | 1 | 35 | Mean Difference (IV, Fixed, 95% CI) | 9.5 [6.04, 12.96] |
| 18.4 Pre/24 months | 1 | 33 | Mean Difference (IV, Fixed, 95% CI) | 7.3 [3.29, 11.31] |
1.1. Analysis.
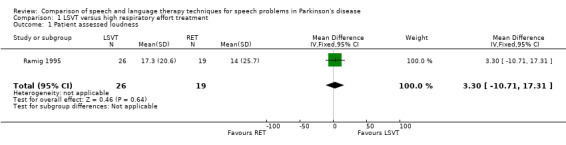
Comparison 1 LSVT versus high respiratory effort treatment, Outcome 1 Patient assessed loudness.
1.2. Analysis.
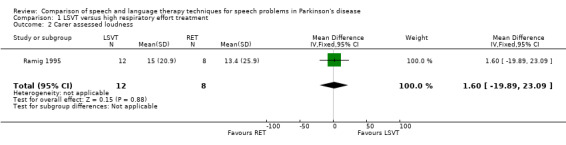
Comparison 1 LSVT versus high respiratory effort treatment, Outcome 2 Carer assessed loudness.
1.3. Analysis.
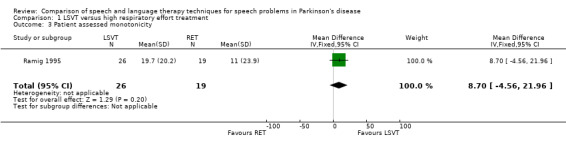
Comparison 1 LSVT versus high respiratory effort treatment, Outcome 3 Patient assessed monotonicity.
1.4. Analysis.
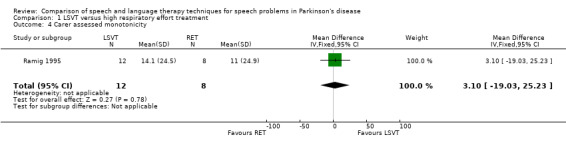
Comparison 1 LSVT versus high respiratory effort treatment, Outcome 4 Carer assessed monotonicity.
1.5. Analysis.
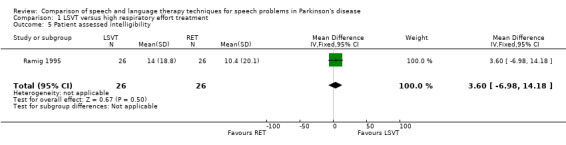
Comparison 1 LSVT versus high respiratory effort treatment, Outcome 5 Patient assessed intelligibility.
1.6. Analysis.
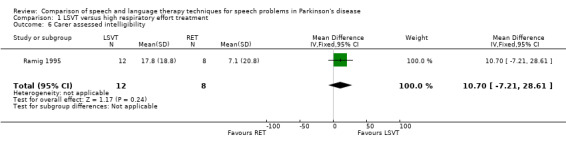
Comparison 1 LSVT versus high respiratory effort treatment, Outcome 6 Carer assessed intelligibility.
1.7. Analysis.
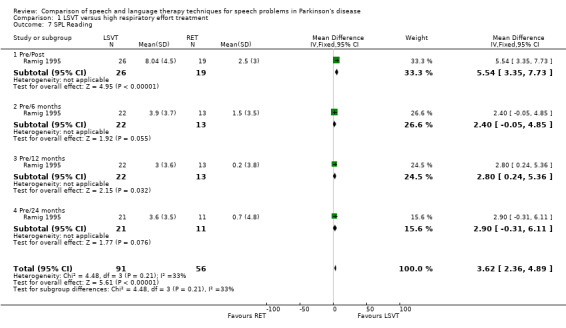
Comparison 1 LSVT versus high respiratory effort treatment, Outcome 7 SPL Reading.
1.8. Analysis.
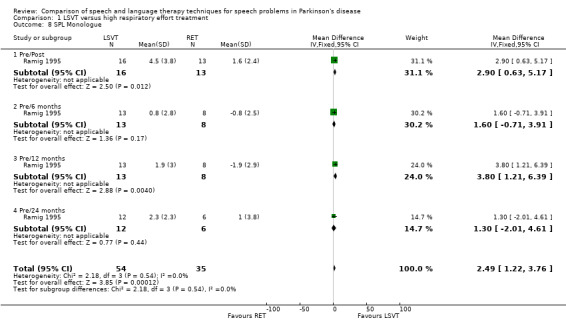
Comparison 1 LSVT versus high respiratory effort treatment, Outcome 8 SPL Monologue.
1.9. Analysis.
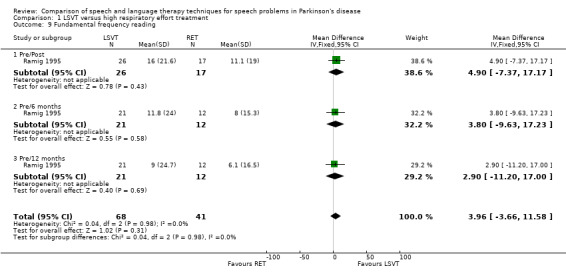
Comparison 1 LSVT versus high respiratory effort treatment, Outcome 9 Fundamental frequency reading.
1.10. Analysis.
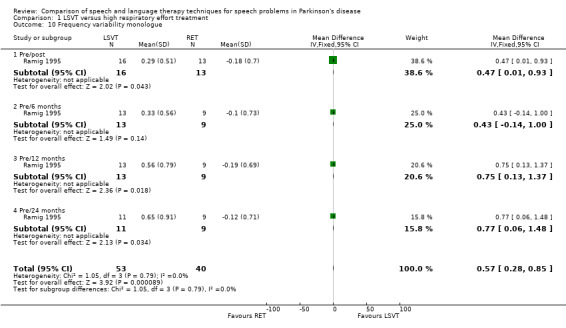
Comparison 1 LSVT versus high respiratory effort treatment, Outcome 10 Frequency variability monologue.
1.11. Analysis.
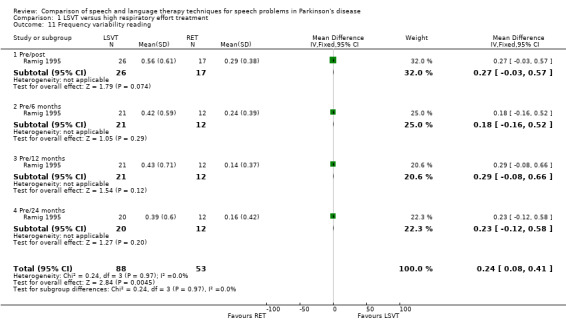
Comparison 1 LSVT versus high respiratory effort treatment, Outcome 11 Frequency variability reading.
1.12. Analysis.
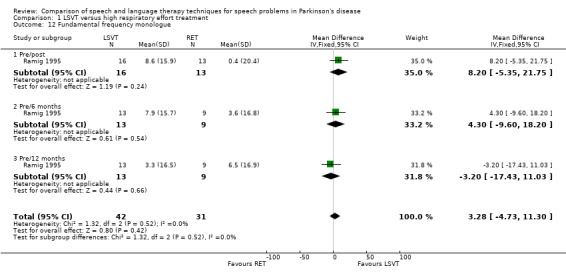
Comparison 1 LSVT versus high respiratory effort treatment, Outcome 12 Fundamental frequency monologue.
1.13. Analysis.
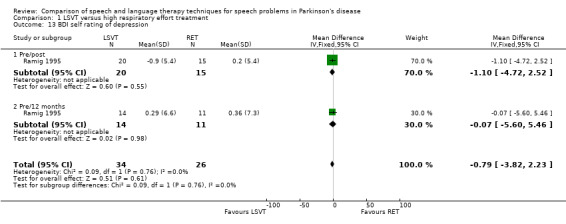
Comparison 1 LSVT versus high respiratory effort treatment, Outcome 13 BDI self rating of depression.
1.14. Analysis.
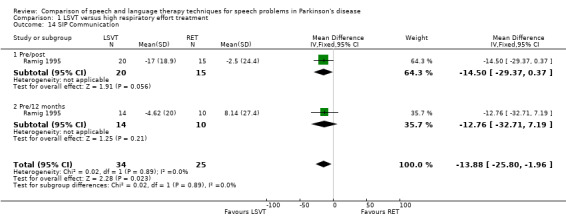
Comparison 1 LSVT versus high respiratory effort treatment, Outcome 14 SIP Communication.
1.15. Analysis.
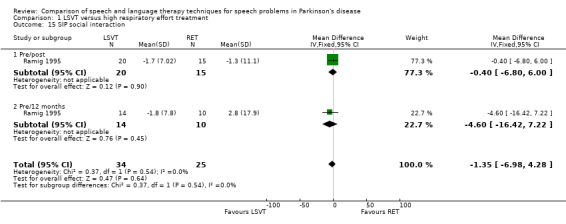
Comparison 1 LSVT versus high respiratory effort treatment, Outcome 15 SIP social interaction.
1.16. Analysis.
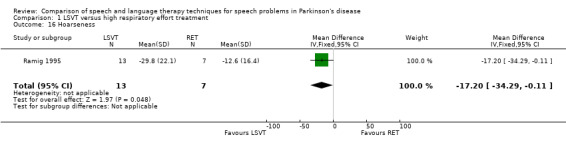
Comparison 1 LSVT versus high respiratory effort treatment, Outcome 16 Hoarseness.
1.17. Analysis.
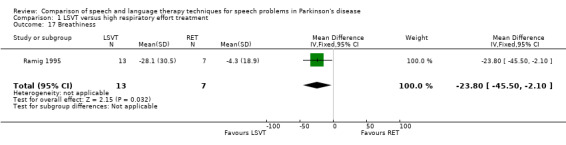
Comparison 1 LSVT versus high respiratory effort treatment, Outcome 17 Breathiness.
1.18. Analysis.
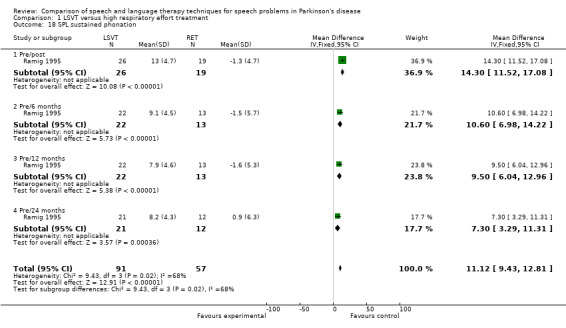
Comparison 1 LSVT versus high respiratory effort treatment, Outcome 18 SPL sustained phonation.
Comparison 2. LSVT Artic versus LSVT Loud.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 SPL Reading Pre/Post | 1 | 18 | Mean Difference (IV, Fixed, 95% CI) | ‐5.03 [‐8.32, ‐1.74] |
| 2 DRT Score Babble Noise Pre/Post | 1 | 18 | Mean Difference (IV, Fixed, 95% CI) | ‐12.46 [‐22.15, ‐2.77] |
| 3 DRT Score Shopping Mall Noise Pre/Post | 1 | 18 | Mean Difference (IV, Fixed, 95% CI) | ‐11.64 [‐24.73, 1.45] |
| 4 DRT Score No Noise Pre/Post | 1 | 18 | Mean Difference (IV, Fixed, 95% CI) | ‐2.06 [‐5.90, 1.78] |
2.1. Analysis.
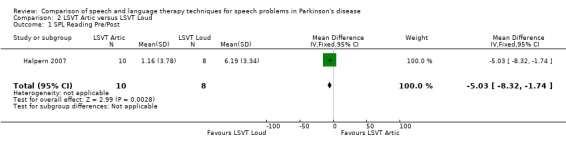
Comparison 2 LSVT Artic versus LSVT Loud, Outcome 1 SPL Reading Pre/Post.
2.2. Analysis.
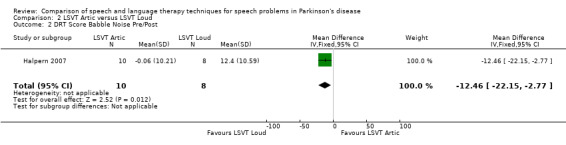
Comparison 2 LSVT Artic versus LSVT Loud, Outcome 2 DRT Score Babble Noise Pre/Post.
2.3. Analysis.
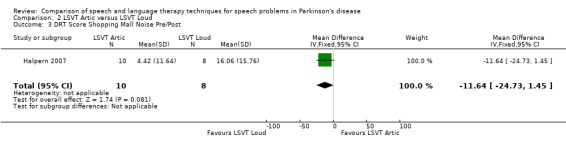
Comparison 2 LSVT Artic versus LSVT Loud, Outcome 3 DRT Score Shopping Mall Noise Pre/Post.
2.4. Analysis.
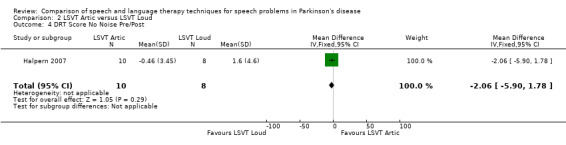
Comparison 2 LSVT Artic versus LSVT Loud, Outcome 4 DRT Score No Noise Pre/Post.
Comparison 3. Altered Auditory Feedback versus Traditional Rate Reduction Therapy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Speech Rate Reading Pre/Post | 1 | 9 | Mean Difference (IV, Fixed, 95% CI) | ‐0.73 [‐1.33, ‐0.13] |
| 2 Speech Rate Reading Pre/6 Week Follow‐up | 1 | 9 | Mean Difference (IV, Fixed, 95% CI) | ‐0.83 [‐1.43, ‐0.23] |
| 3 Intelligibility Reading Pre/Post | 1 | 10 | Mean Difference (IV, Fixed, 95% CI) | ‐23.89 [‐44.46, ‐3.32] |
| 4 Intelligibility Reading Pre/6 Week Follow‐up | 1 | 10 | Mean Difference (IV, Fixed, 95% CI) | ‐5.48 [‐28.49, 17.53] |
| 5 Intelligibility Monologue Pre/Post | 1 | 10 | Mean Difference (IV, Fixed, 95% CI) | 0.10 [‐2.48, 2.68] |
| 6 Intelligibility Monologue Pre/6 Week Follow‐up | 1 | 10 | Mean Difference (IV, Fixed, 95% CI) | 0.04 [‐2.06, 2.14] |
3.1. Analysis.
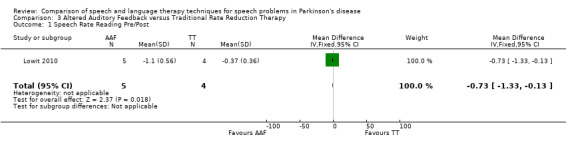
Comparison 3 Altered Auditory Feedback versus Traditional Rate Reduction Therapy, Outcome 1 Speech Rate Reading Pre/Post.
3.2. Analysis.
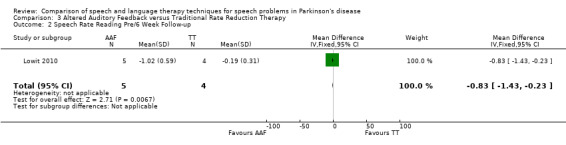
Comparison 3 Altered Auditory Feedback versus Traditional Rate Reduction Therapy, Outcome 2 Speech Rate Reading Pre/6 Week Follow‐up.
3.3. Analysis.
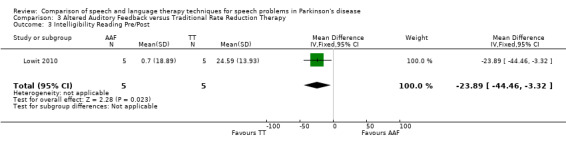
Comparison 3 Altered Auditory Feedback versus Traditional Rate Reduction Therapy, Outcome 3 Intelligibility Reading Pre/Post.
3.4. Analysis.
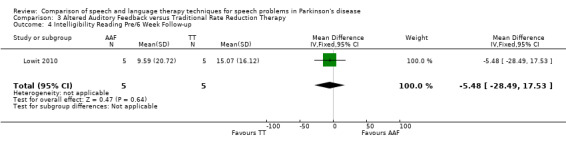
Comparison 3 Altered Auditory Feedback versus Traditional Rate Reduction Therapy, Outcome 4 Intelligibility Reading Pre/6 Week Follow‐up.
3.5. Analysis.
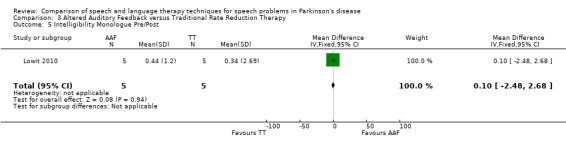
Comparison 3 Altered Auditory Feedback versus Traditional Rate Reduction Therapy, Outcome 5 Intelligibility Monologue Pre/Post.
3.6. Analysis.
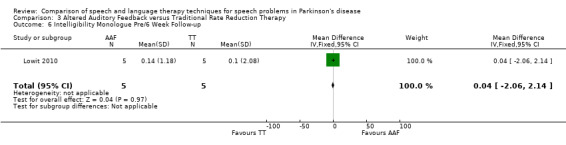
Comparison 3 Altered Auditory Feedback versus Traditional Rate Reduction Therapy, Outcome 6 Intelligibility Monologue Pre/6 Week Follow‐up.
Comparison 4. Online LSVT versus face‐to‐face LSVT.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 SPL Monologue | 1 | 34 | Mean Difference (IV, Fixed, 95% CI) | ‐0.01 [‐1.89, 1.87] |
| 2 SPL sustained vowel phonation | 1 | 34 | Mean Difference (IV, Fixed, 95% CI) | ‐10.01 [‐12.85, ‐7.17] |
| 3 SPL reading | 1 | 34 | Mean Difference (IV, Fixed, 95% CI) | 0.25 [‐1.74, 2.24] |
| 4 Duration of phonation | 1 | 34 | Mean Difference (IV, Fixed, 95% CI) | 0.37 [‐2.64, 3.38] |
| 5 Max fundamental frequency range | 1 | 34 | Mean Difference (IV, Fixed, 95% CI) | 0.39 [‐4.12, 4.90] |
| 6 Breathiness | 1 | 34 | Mean Difference (IV, Fixed, 95% CI) | 5.53 [‐3.44, 14.50] |
| 7 Roughness | 1 | 34 | Mean Difference (IV, Fixed, 95% CI) | 9.2 [1.49, 16.91] |
| 8 Loudness level | 1 | 34 | Mean Difference (IV, Fixed, 95% CI) | ‐7.74 [‐20.23, 4.75] |
| 9 Loudness variability | 1 | 34 | Mean Difference (IV, Fixed, 95% CI) | 5.38 [‐2.85, 13.61] |
| 10 Pitch variability | 1 | 34 | Mean Difference (IV, Fixed, 95% CI) | 6.49 [‐2.67, 15.65] |
| 11 Overall articulatory precision | 1 | 34 | Mean Difference (IV, Fixed, 95% CI) | 9.14 [‐0.66, 18.94] |
| 12 Overall speech intelligibility in conversation | 1 | 34 | Mean Difference (IV, Fixed, 95% CI) | ‐9.08 [‐20.74, 2.58] |
| 13 Percentage word intelligibility | 1 | 34 | Mean Difference (IV, Fixed, 95% CI) | ‐2.13 [‐7.84, 3.58] |
| 14 Percentage sentence intelligibility | 1 | 34 | Mean Difference (IV, Fixed, 95% CI) | ‐1.37 [‐3.98, 1.24] |
| 15 Communication efficiency ratio | 1 | 34 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐0.15, 0.15] |
4.1. Analysis.
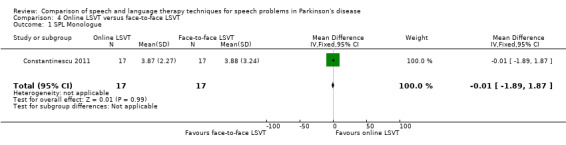
Comparison 4 Online LSVT versus face‐to‐face LSVT, Outcome 1 SPL Monologue.
4.2. Analysis.
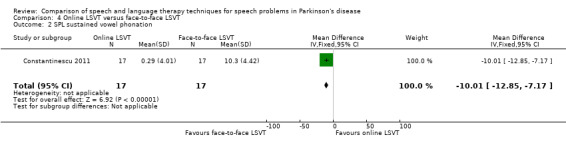
Comparison 4 Online LSVT versus face‐to‐face LSVT, Outcome 2 SPL sustained vowel phonation.
4.3. Analysis.
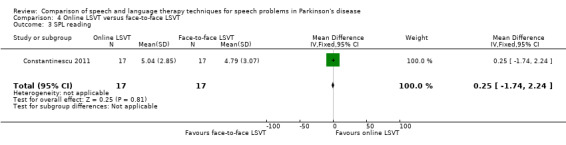
Comparison 4 Online LSVT versus face‐to‐face LSVT, Outcome 3 SPL reading.
4.4. Analysis.
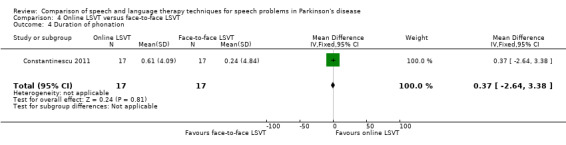
Comparison 4 Online LSVT versus face‐to‐face LSVT, Outcome 4 Duration of phonation.
4.5. Analysis.
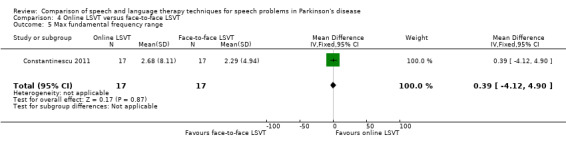
Comparison 4 Online LSVT versus face‐to‐face LSVT, Outcome 5 Max fundamental frequency range.
4.6. Analysis.
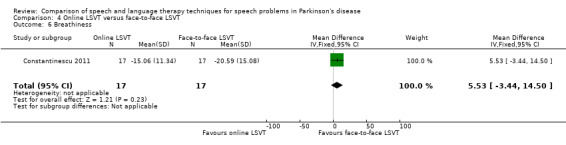
Comparison 4 Online LSVT versus face‐to‐face LSVT, Outcome 6 Breathiness.
4.7. Analysis.
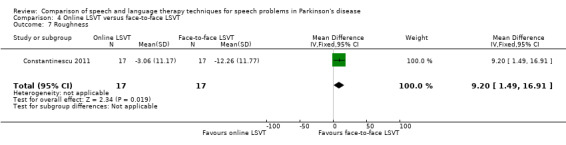
Comparison 4 Online LSVT versus face‐to‐face LSVT, Outcome 7 Roughness.
4.8. Analysis.
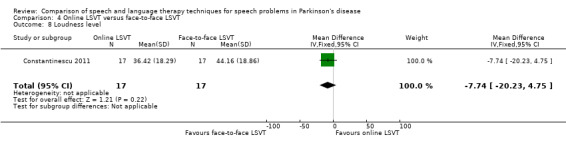
Comparison 4 Online LSVT versus face‐to‐face LSVT, Outcome 8 Loudness level.
4.9. Analysis.
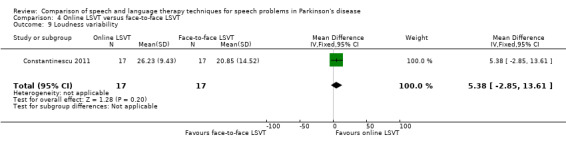
Comparison 4 Online LSVT versus face‐to‐face LSVT, Outcome 9 Loudness variability.
4.10. Analysis.
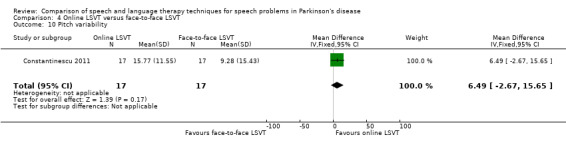
Comparison 4 Online LSVT versus face‐to‐face LSVT, Outcome 10 Pitch variability.
4.11. Analysis.
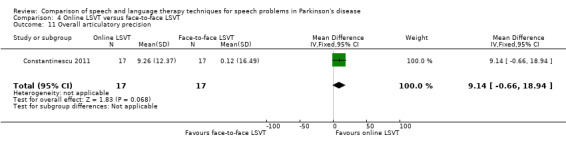
Comparison 4 Online LSVT versus face‐to‐face LSVT, Outcome 11 Overall articulatory precision.
4.12. Analysis.
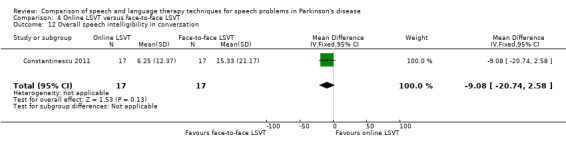
Comparison 4 Online LSVT versus face‐to‐face LSVT, Outcome 12 Overall speech intelligibility in conversation.
4.13. Analysis.
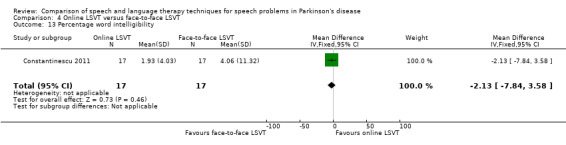
Comparison 4 Online LSVT versus face‐to‐face LSVT, Outcome 13 Percentage word intelligibility.
4.14. Analysis.
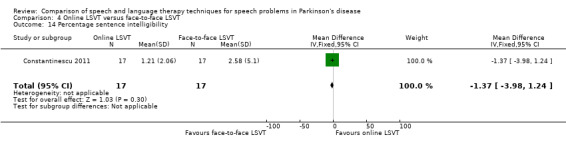
Comparison 4 Online LSVT versus face‐to‐face LSVT, Outcome 14 Percentage sentence intelligibility.
4.15. Analysis.
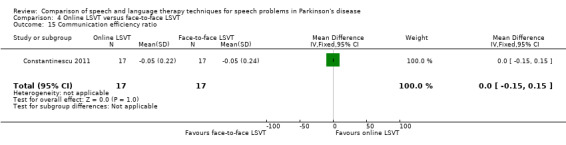
Comparison 4 Online LSVT versus face‐to‐face LSVT, Outcome 15 Communication efficiency ratio.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Constantinescu 2011.
| Methods | Parallel group non‐inferiority trial design. Based upon their dysarthria severity classification the participants were stratified and then assigned to a treatment group using a computerised random number generator. The primary investigator generated the random number and assigned the participants in order of recruitment to the study, concealment of allocation was not confirmed in the published information. Data from the first 8 participants recruited to the study were used to carry out a power calculation and the resulting target, plus an additional 10% to allow for some drop‐out from the study, was met. It is assumed that data would have been analysed on an intention‐to‐treat basis if any patients had withdrawn from the study, although this was not stated. Treatment was administered in an outpatient setting, in 4 sessions per week for 4 weeks with each session lasting 1 hour. Assessments were carried out pre‐ and post‐treatment and assessors were blinded to treatment group. | |
| Participants | 17 participants in novel (online delivery of LSVT) and 17 in standard (face‐to‐face delivery of LSVT). No drop‐outs were mentioned in the published material. Patients mean age 70.65 (online), 69.59 (face‐to‐face); male/female 14/3 (online), 13/4 (face‐to‐face); Hoehn and Yahr 1.59 (online), 1.53 (face‐to‐face); duration of condition/years 5.39 (online), 6.88 (face‐to‐face). Inclusion criteria: presence of hypokinetic dysarthria (mild to severe) with PD impacting on communication; a videolaryngoscopic evaluation of the vocal fold structure and movement consistent with PD; ability when guided to increase loudness; and a consistent drug regimen for PD. Exclusion criteria: speech and/or language difficulties unrelated to PD; an additional co‐existing neurological disorder; respiratory difficulties inconsistent with PD; cognitive difficulties resulting in the inability to provide informed consent; a severe uncorrected visual and/or auditory disturbance; a history of alcohol abuse; and participation in the LSVT within 12 months of this study. | |
| Interventions | Novel treatment: LSVT, a high effort intensive treatment increasing vocal loudness through increasing vocal fold adduction, thinking loud and increased respiratory effort, delivered via PC‐based video conferencing between patient and speech‐language pathologist in a separate room. Standard treatment: Same LSVT methods delivered face‐to‐face by speech‐language pathologist. | |
| Outcomes | Primary: Sound pressure levels Secondary (acoustic): Phonation time, maximum fundamental frequency range Secondary (perceptual): Breathiness, roughness, overall articulatory precision, loudness level, pitch loudness and variation, participant satisfaction |
|
| Notes | Participant satisfaction questionnaire only completed by novel arm group so data cannot be used in this review. Subgroup analysis by dysarthria severity was carried out. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Specified eligibility criteria | Low risk | Inclusion criteria stated |
| Randomisation method | Low risk | Randomised by computer random number generator |
| Adequate concealment of allocation | Unclear risk | Concealment of allocation not stated |
| Similar at baseline | Low risk | Groups similar in age, duration of condition, severity of condition and male/female ratio |
| Withdrawals > 10% | Low risk | No withdrawals |
| Missing values > 10% | Low risk | No missing data |
| Cointerventions constant | Unclear risk | Consistent drug regimen stated as inclusion criteria but no report on whether this was maintained throughout trial period |
| Credible placebo | Low risk | Equal time spent training each group |
| Blinded assessors | Low risk | Patients instructed not to divulge their treatment allocation to assessors |
Halpern 2007.
| Methods | Parallel group trial design. The participants were assigned to a treatment group using a computerised randomisation method. It is assumed that data would have been analysed on an intention‐to‐treat basis if any patients had withdrawn from the study, although this was not stated. Treatment was administered in 4 sessions per week for 4 weeks with each session lasting 50 minutes. Assessments were carried out pre‐ and post‐treatment. | |
| Participants | 8 participants in novel (Articulation‐focused LSVT) and 10 in standard (Loudness‐focused LSVT). No drop‐outs were mentioned in the published material. Patients mean age 70.5 (LSVT Artic), 66.1 (LSVT LOUD); male/female 6/2 (LSVT Artic), 7/3 (LSVT LOUD); Hoehn and Yahr (stage on meds) score 2.2 (LSVT Artic) and 2.2 (LSVT LOUD); duration of condition was not assessed. No inclusion or exclusion criteria stated. | |
| Interventions | Novel Therapy: LSVT Artic, following same general principles as LSVT as described for Ramig 1995 but with focus on articulation and cue used is ENUNCIATE. Standard therapy, LSVT Loud, as described for Ramig 1995 with original LOUD cue used. | |
| Outcomes | Speech intelligibility measured using DRT for no noise, shopping mall noise and babble noise conditions. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Specified eligibility criteria | High risk | Criteria not stated |
| Randomisation method | Low risk | Randomised by computer random number generator |
| Adequate concealment of allocation | Unclear risk | Concealment of allocation not stated |
| Similar at baseline | Unclear risk | Groups similar in age, severity of condition and male/female ratio |
| Withdrawals > 10% | Low risk | No withdrawals |
| Missing values > 10% | Low risk | No missing data |
| Cointerventions constant | Low risk | Stable drug regimen confirmed through correspondence with author |
| Credible placebo | Low risk | Same time and attention for both groups |
| Blinded assessors | Low risk | Blinding of assessors confirmed through correspondence with author |
Healy 2002.
| Methods | Parallel group trial design. The participants were assigned to a treatment group using a sealed envelope randomisation method. It is unreported whether data were analysed on an intention‐to‐treat basis or if any patients had withdrawn from the study. Treatment was administered in a single hour long session or in 6 sessions each lasting an hour over 6 weeks. Assessments were carried out pre‐ and post‐treatment and at 1 week, 6 weeks and 6 months after treatment. | |
| Participants | Baseline characteristics were not stated for the different groups. Inclusion criteria: diagnosis of PD, faster than normal speech rate/evidence of palilalia with or without other dysarthric features, able to cope with individual therapy sessions and consent to take part on this basis. Exclusion criteria: score of 9 or below on Middlesex Elderly Assessment of Mental State (MEAMS) (Golding 1989) indicating cognitive impairment, unclear diagnosis of PD, recent or anticipated change in Parkinsonian‐related medication, previous exposure to pacing therapy techniques used in study, Parkinson speakers with normal/slow speech rates, inability to cope with individual therapy sessions lasting up to two hours e.g. due to poor concentration or excessive fatigue. | |
| Interventions | Therapy 1, alphabet board. Speaker points to the initial letter of each word spoken on the board this task is intended to control rate of speech. Therapy 2, Pacing board. Wooden ruler with series of raised divisions at 30 mm intervals. Speaker is required to place their finger in each section as they speak each word. Tactile feedback is intended to help control speech rate. | |
| Outcomes | Sentence rate and intelligibility (assessment of intelligibility of dysarthric speech using 22 sentences) Narrative rate Spontaneous speech rate and intelligibility from conversation Oro‐motor assessment, Frenchay dysarthria assessment Reported use of devices |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Specified eligibility criteria | Low risk | Inclusion and exclusion criteria stated |
| Randomisation method | High risk | Sealed envelope randomisation method |
| Adequate concealment of allocation | High risk | Sealed envelope randomisation method |
| Similar at baseline | Unclear risk | Baseline characteristics of groups not stated |
| Withdrawals > 10% | Unclear risk | N numbers for time points not stated |
| Missing values > 10% | Unclear risk | Means given with N numbers for time points not stated |
| Cointerventions constant | Unclear risk | No report of whether drug regimens were constant throughout trial |
| Credible placebo | Low risk | Matching treatment schedules for each technique |
| Blinded assessors | Low risk | Audiotapes analysed by a qualified speech and language therapist blinded to participants group |
Lowit 2010.
| Methods | Cross‐over trial, data from first treatment session obtained through correspondence with author. The participants were randomly assigned to a treatment group using alternate allocation by order of entry into study. Data have been analysed on as per protocol basis. Treatment was administered in the patients homes, in 1 session per week for 4 weeks with each session lasting 50 ‐ 60 minutes. Assessments were carried out pre‐ and post‐treatment and assessors were blinded to treatment group, time of assessment and feedback preference for in‐ear device. | |
| Participants | 5 participants in novel (altered auditory feedback (AAF) in‐ear device) and 5 in standard (traditional rate reduction therapy (TT)). 1 patient was unable to participate in post treatment analysis of articulation rate. Patients mean age 62.6 (AAF), 63.0 (TT); male/female 4/1 (AAF), 2/3 (TT); Hoehn and Yahr 1.8 (AAF), 3.3 (TT); duration of condition was not assessed. Inclusion criteria: Idiopathic PD and a speech impairment severe enough to warrant treatment. Exclusion criteria: Presence of significant dementia, history of deep brain stimulation or speech and language therapy for dysarthria symptoms during the last 12 months, presence of speech and language problems other than those caused by PD. | |
| Interventions | Novel therapy, AAF is an in‐ear device worn by patients which disrupts the normal auditory feedback loop causing a slowing in the rate of speech. Participants used delayed auditory feedback (DAF) and frequency shifted feedback (FSF) and were encouraged to choose their preferred setting as part of this trial. Standard therapy, TTfocused on identifying the most suitable strategy for reducing speech rate e.g. increasing pauses or stretching out articulation. | |
| Outcomes | Articulation rate from reading a passage Intelligibility from reading a passage and a monologue task |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Specified eligibility criteria | Low risk | Inclusion criteria stated |
| Randomisation method | High risk | Quasi‐random method, alternate allocation |
| Adequate concealment of allocation | High risk | Quasi‐random method, alternate allocation |
| Similar at baseline | High risk | Groups differ in mean stage of disease (Hoehn and Yahr) |
| Withdrawals > 10% | Low risk | 1 drop‐out from 10 recruited to trial |
| Missing values > 10% | Low risk | 1 drop‐out from 10 recruited to trial |
| Cointerventions constant | High risk | Two patients had to change their medication during treatment |
| Credible placebo | High risk | In‐ear device compared with behavioural exercises |
| Blinded assessors | Low risk | Experimenter who processed rate data and judges who rated intelligibility were blinded to treatment group |
Ramig 1995.
| Methods | Parallel group design. Randomised by number picked out of a hat, so no concealment of allocation. Analysed on a per protocol basis. Treated as outpatients for 16 hours over 1 month. Assessed at baseline, immediately after treatment, 6, 12, 24 and 48 months later. Assessors were blinded. | |
| Participants | 26 patients in novel LSVT) group and 19 in standard (respiration therapy) group. After two years, 5 drop‐outs in LSVT group and 7 in respiration group. Patients mean age 63.5 (LSVT), 65.6 (respiration); male/female 21/5 (LSVT), 12/7 (respiration); Hoehn and Yahr 2.7 (LSVT), 2.3 (respiration); duration of condition/years 8.3 (LSVT), 5.9 (respiration). No inclusion criteria stated. Exclusion criteria: not idiopathic PD, having laryngeal pathology. | |
| Interventions | Novel treatment: LSVT, a high effort intensive treatment increasing vocal loudness through increasing vocal fold adduction, thinking loud and increased respiratory effort. Standard treatment: Respiration therapy, aimed at increased respiratory muscle activity. Visual feedback was provided. Drugs stable during therapy period, and patients were 'optimally medicated' throughout follow‐up period. | |
| Outcomes | Fundamental frequency Sound pressure levels Intelligibility Beck Depression Index Sickness Impact Profile ‐ (communication and social interaction) UPDRS Hoehn & Yahr etc All measured in 'on' phase. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Specified eligibility criteria | Low risk | Exclusion criteria stated |
| Randomisation method | High risk | Numbers pulled out of a hat |
| Adequate concealment of allocation | High risk | Numbers pulled out of a hat randomisation method |
| Similar at baseline | Low risk | Groups similar in age, duration of condition, severity of condition and male/female ratio |
| Withdrawals > 10% | Unclear risk | Patient numbers decreased in subsequent publications but it is unclear whether these are subgroup studies, withdrawals or missing values. |
| Missing values > 10% | Unclear risk | Patient numbers decreased in subsequent publications but it is unclear whether these are subgroup studies, withdrawals or missing values. |
| Cointerventions constant | Low risk | Stable drug regimen throughout treatment period, optimally medicated for follow‐up |
| Credible placebo | Low risk | Same time and attention for both groups |
| Blinded assessors | Unclear risk | Perceptual measures were rated blindly |
Scott 1983.
| Methods | Parallel group design. Randomised using random number tables. Allocation was concealed from therapist. Data analysed on a per protocol basis. Treated at home for 10 hours over 2 weeks. Assessed at baseline, immediately after therapy and 3 months later. The prosodic abnormality score was scored by an unblinded and a blinded assessor and the mean taken. The remainder of the outcomes were assessed blinded. | |
| Participants | 13 per arm of study. After 3 months, 3 drop‐outs in novel (visual stimulation) group, 1 drop‐out in standard (prosodic exercises) group. Patients mean age 66 in both arms; male/female 7/6 (visual), 11/2 (prosodic); duration of condition/years 13 (visual), 10 (prosodic); Hoehn and Yahr score was not assessed. Inclusion criteria: patients with PD with a speech disorder. Exclusion criteria: Subjectively slight communication difficulty, evidence of intellectual impairment, history of stroke or other disorder likely to affect speech, significant impairment of hearing, drug therapy likely to vary, unlikely to co‐operate in trial. | |
| Interventions | Novel treatment: Prosodic exercises with visual stimulation (Vocalite). The exercises aimed to improve prosodic abnormality by increasing the patients awareness of the problem and emphasising the importance of volume and intonation. Standard treatment: Prosodic exercises alone. Drug therapy was constant. | |
| Outcomes | Prosodic abnormality score Intelligibility rating Visual analogue score of intelligibility | |
| Notes | After 2 weeks of standard therapy this group had a further week of prosodic exercises with the visual stimulation of a Vocalite. Therefore, data after the first 2 week period were not used in this review. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Specified eligibility criteria | High risk | Inclusion criteria stated |
| Randomisation method | Unclear risk | Random number tables |
| Adequate concealment of allocation | Unclear risk | Concealment of allocation not stated |
| Similar at baseline | Unclear risk | Groups similar in age and duration of condition, male/female ratio differs between groups, stage of disease not reported |
| Withdrawals > 10% | Low risk | Withdrawals reach high risk level for 3‐month follow‐up data not included in this review |
| Missing values > 10% | High risk | Missing data reached high risk level for 3‐month follow‐up data not included in this review |
| Cointerventions constant | Unclear risk | Stable drug regimen |
| Credible placebo | Low risk | Same time and attention for both groups |
| Blinded assessors | Low risk | Prosodic abnormality score was scored by an unblinded and a blinded assessor and the mean taken. Intelligibility outcomes were assessed blinded. |
AAF: altered auditory feedback DAF: delayed auditory feedback DRT: Diagnostic Rhyme Test FSF: frequency shifted feedback LSVT: Lee Silverman Voice Treatment MEAMS: Middlesex Elderly Assessment of Mental State PD: Parkinson's disease TT: traditional rate reduction therapy
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Adams 2002 | This RCT examined the motor learning principles involved in the acquisition and retention of novel speech skills. Intervention did not aim to improve everyday speech of participants, but studied the ability of the patients with PD to learn to speak at a target rate. Outcomes were not relevant to this review question. |
| de Swart 2003 | This trial investigated the effects of Pitch Limiting Voice Treatment (PVLT), "speak loud and low" compared with LSVT, "think loud, think shout". Both treatments were aimed to increase loudness but the novel PLVT therapy aimed to do this without raising the vocal pitch and laryngeal muscle tension. The patients withPD in this trial were not randomised, all took part in both types of therapy. |
| Pacchetti 2000 | This RCT studied the effects of music therapy versus physical therapy on the motor and emotional health of people with PD, by combining movement and stimulation of different sensory pathways. No speech outcomes were investigated. |
| Tindall 2009 | This trial studies voice treatment delivered via a videophone and compares to historical data for traditional delivery. The study is therefore not randomised or adequately controlled. |
LSVT: Lee Silverman Voice Treatment PD: Parkinson's disease PVLT: Pitch Limiting Voice Treatment RCT: randomised controlled trial
Contributions of authors
C Herd carried out the majority of the searching for eligible studies. C P Herd and C L Tomlinson extracted the data from the included studies. All reviewers were involved in the writing of the review. C P Herd was the primary updating author and K H O Deane was the original author.
Sources of support
Internal sources
City Hospital NHS Trust, UK.
External sources
Cochrane Centre, UK.
NHS Research and Development Programme for People with Physical and Complex Disabilities; Project PCD2/A1/250, UK.
Conference grant from The Royal Society, UK.
Conference grant from Pharmacia Upjohn, UK.
-
CSO, Chief Scientist Office, UK.
Author funding
Declarations of interest
Authors C P Herd, C H Smith, M C Brady, C Sackley and C E Clarke are part of a team performing a pilot trial comparing LSVT versus NHS SLT versus attention control (PD COMM). The views expressed here are those of the authors and not necessarily those of the Chief Scientist Office.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Constantinescu 2011 {published data only}
- Constantinescu G, Theodoros D, Russell T, Ward E, Wilson S, Wootton R. Treating disordered speech and voice in Parkinson's disease online: a randomized controlled non‐inferiority trial. International Journal of Language & Communication Disorders 2011;46(1):1‐16. [DOI] [PubMed] [Google Scholar]
Halpern 2007 {published data only}
- Halpern A, Spielman J, Ramig L, Cable J, Panzer I, Sharpley A. The effects of loudness and noise on speech intelligibility in Parkinson's disease. Movement Disorders 2007;22(16):S105. [Google Scholar]
Healy 2002 {published data only}
- Healy V. A comparison of the efficacy of two methods of rate control in the speech of people with Parkinson's disease. Internal report, Manchester Royal Infirmary 2002.
Lowit 2010 {published data only}
- Lowit A, Dobinson C, Timmins C, Howell P, Kroger B. The effectiveness of traditional methods and altered auditory feedback in improving speech rate and intelligibility in speakers with Parkinson's disease. International Journal of Speech‐Language Pathology 2010;12(5):426‐36. [DOI] [PubMed] [Google Scholar]
Ramig 1995 {published and unpublished data}
- Baumgartner CA, Sapir A, Ramig LO. Perceptual voice quality changes following phonatory‐respiratory effort treatment (LSVT) vs. respiratory effort treatment for individuals with Parkinson's disease. Submitted to Journal of Voice. [DOI] [PubMed]
- Ramig LO, Countryman S. O'Brien C, Hoehn M, Thompson L. Intensive speech treatment for patients with Parkinson's disease: Short‐ and long‐term comparison of two techniques. Neurology 1996;47:1496‐1504. [DOI] [PubMed] [Google Scholar]
- Ramig LO, Dromey C. Aerodynamic mechanisms underlying treatment‐related changes in vocal intensity in patients with Parkinson's disease. Journal of Speech and Hearing Research 1996;39:798‐807. [DOI] [PubMed] [Google Scholar]
- Ramig LO, Hoyt P, Seeley E, Sapir S. Voice treatment (LSVT) for IPD: Perceptual findings. Parkinsonism and related disorders 1999;5(Supplement):S42. [Google Scholar]
- Ramig LO, Sapir S, Countryman S, Pawlas AA, O'Brien C, Hoehn M, et al. Intensive voice treatment (LSVT (R)) for patients with Parkinson's disease: a 2 year follow up. Journal of Neurology Neurosurgery and Psychiatry 2001;71(4):493‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramig LOS, Countryman S, Thompson LL, Hori Y. Comparison of two forms of intensive speech treatment for Parkinson's disease. Journal of Speech and Hearing Research 1995;38:1232‐51. [DOI] [PubMed] [Google Scholar]
- Sapir S, Ramig LO, Hoyt P, Countryman S, O'Brien C, Hoehn M. Speech loudness and quality 12 months after intensive voice treatment (LSVT (R)) for Parkinson's disease: A comparison with an alternative speech treatment. Folia Phoniatrica et Logopaedica 2002;54(6):296‐303. [DOI] [PubMed] [Google Scholar]
- Smith ME, Ramig LO, Dromey C, Perez KS, Samandari R. Intensive voice treatment in Parkinson's disease: Laryngostroboscopic findings. Journal of Voice 1995;9(4):453‐9. [DOI] [PubMed] [Google Scholar]
Scott 1983 {published and unpublished data}
- Scott S, Caird FI. Speech therapy for Parkinson's disease. Journal of Neurology, Neurosurgery and Psychiatry 1983;46:140‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott S, Caird FI, Williams BO. The effect of speech therapy on communication. In: S. Scott, F.I. Caird, B.O. Williams editor(s). Communication in Parkinson's disease. Beckenham, Kent: Croom Helm Ltd, 1985:47‐60. [Google Scholar]
References to studies excluded from this review
Adams 2002 {published data only}
- Adams SG, Page AD, Jog M. Summary feedback schedules and speech motor learning in Parkinson's disease. Journal of Medical Speech‐Language Pathology 2002;10(4):215‐20. [Google Scholar]
de Swart 2003 {published data only}
- Swart BJM, Willemse SC, Maassen BAM, Horstink MWIM. Improvement of voicing in patients with Parkinson's disease by speech therapy. Neurology 2003;60(3):498‐500. [DOI] [PubMed] [Google Scholar]
Pacchetti 2000 {published data only}
- Pacchetti C, Mancini F, Aglieri R, Fundaro C, Martignoni E, Nappi G. Active music therapy in Parkinson's disease: An integrative method for motor and emotional rehabilitation. Psychosomatic Medicine 2000;62(3):386‐93. [DOI] [PubMed] [Google Scholar]
Tindall 2009 {published data only}
- Tindall LR, Huebner RA. The impact of an application of telerehabilitation technology on caregiver burden. International Journal of Telerehabilitation 2009;1(1):3‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Additional references
Beck 1961
- Beck AT, Ward CH, Mendelsohn M, Mock J, Erbaugh J. An inventory for measuring depression. Archives of General Psychiatry 1961;4(6):561‐71. [DOI] [PubMed] [Google Scholar]
Bergner 1981
- Bergner M, Bobbitt RA, Carter WB, Gilson BS. The Sickness Impact Profile: development and final revision of a health status measure. Medical Care 1981;19(8):787‐805. [DOI] [PubMed] [Google Scholar]
Biary 1988
- Biary, N, P.A. Pimental, P.W. Langenberg. A double‐blind trial of clonazepam in the treatment of parkinsonian dysarthria.. Neurology 1988;38:255‐258. [DOI] [PubMed] [Google Scholar]
CONSORT 1996
- Begg C, Cho M, Eastwood S, Horton R, Moher D, Olkin I, et al. Improving the quality of reporting of randomized controlled trials. The CONSORT statement. Journal of the American Medical Association 1996;276(8):637‐9. [DOI] [PubMed] [Google Scholar]
Dann 1994
- Dann N. Saunders H, Hunter PC, Hughes AJ. The response of parkinsonian dysarthria to dopaminergic stimulation. Movement Disorders 1994;9(Supplement 1):83. [Google Scholar]
Darley 1969
- Darley, Aronson, Brown. Motor Speech Disorders. Philadelphia: Saunders, 1969. [Google Scholar]
Fleiss 1993
- Fleiss JL. The statistical basis of meta‐analysis. Statistical Methods in Medical Research 1993;2(2):121‐45. [DOI] [PubMed] [Google Scholar]
Gibb 1988
- Gibb WRG, Lees AJ. The relevance of the Lewy body to the pathogenesis of idiopathic Parkinson's disease. Journal of Neurology, Neurosurgery and Psychiatry 1988;51:745‐52. [DOI] [PMC free article] [PubMed] [Google Scholar]
Golding 1989
- Golding E. The Middlesex Elderly Assessment of Mental State (MEAMS). Suffolk, UK: Thames ValleyTest Company. Suffolk, England: Thames Valley Test Company, 1989.
GPDS 2000
- The Global Parkinson's Disease Survey. An insight into quality of life with Parkinson's disease. The Parkinson's Disease Society of the United Kingdom 2000.
Halliday 1970
- Halliday MAK. A course in spoken English: Intonation. London: Oxford University Press, 1970. [Google Scholar]
Herd In Press
- Herd C, Tomlinson CL, Deane KH O, Brady MC, Smith C, Sackley C, Clarke CE. Speech and language therapy versus placebo or no intervention for speech problems in Parkinson's disease.. Cochrane Database of Systematic Reviews In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S. Cochrane handbook for systematic reviews of interventions. Version 5.1.0. The Cochrane Collaboration 2011, 2011. [Google Scholar]
Karlsen 1999
- Karlsen KH, Larsen JP, Tandeburg E, Maeland JG. Influence of clinical and demographic variables on quality of life in patients with Parkinson's disease. Journal of Neurology, Neurosurgery and Psychiatry 1999;66(4):431‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lloyd 1999
- Lloyd M. The new community care for people for people with Parkinson's disease and their carers. In: Percival R, P. Hobson editor(s). Parkinson's Disease. Studies in Psycological and Social Care. London: BPS Books, 1999:13‐59. [Google Scholar]
Logemann 1978
- Logemann JA, Fisher HB, Boshes B, Blonsky ER. Frequency and cooccurance of vocal tract dysfunctions in the speech of a large sample of Parkinson patients. Journal of Speech and Hearing Disorders 1978;43:47‐57. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Mawdsley 1971
- Mawdsley C, Gamsu CV. Periodicity of speech in Parkinson's disease. Nature 1971;231(5301):315‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Miller 2011
- Miller N, Noble E, Jones D, Deane KHO, Gibb C. Survey of speech and language therapy provision for people with Parkinson's disease in the United Kingdom: patients' and carers' perspectives. International Journal of Language & Communication Disorders 2011;46(2):179‐88. [DOI] [PubMed] [Google Scholar]
Miller 2011b
- Miller N, Deane KHO, Jones D, Noble E, Gibb C. National survey of speech and language therapy provision for people with Parkinson's disease in the United Kingdom: therapists' practices. International Journal of Language & Communication Disorders 2011;46(2):189‐201. [DOI] [PubMed] [Google Scholar]
NCC‐CC 2006
- The national collaborating centre for chronic conditions. Parkinson's disease: National clinical guideline for diagnosis and management in primary and secondary care. Royal College of Physicians, London. London: Royal College of Physicians, 2006.
O'Reilly 1996
- O'Reilly F, Finnan F, Allwright S, Davey Smith G, Ben‐Shlomo Y. The effects of caring for a spouse with Parkinson's disease on social, psychological and physical well‐being. British Journal of General Practice 1996;46:507‐12. [PMC free article] [PubMed] [Google Scholar]
Parkinson's disease society 2008
- Parkinson's disease society. Life with Parkinson's today ‐room for improvement. Parkinson's disease society, London 2008.
Ramig 1995b
- Ramig LO, Pawlas A, Countryman S. The Lee Silverman Voice Treatment (LSVT): A practical guide to treating the voice and speech disorders in Parkinson's disease. Iowa City: University of Iowa, 1995. [Google Scholar]
Rosenbek 1985
- Rosenbek, LaPointe. The dysarthrias: Description, diagnosis and treatment. In: Johns DF editor(s). Clinical management of neurogenic communicative disorders. 2nd Edition. Boston: Little Brown and Co, 1985. [Google Scholar]
Sapir 2001
- Sapir S, Pawlas AA, Ramig LO, Countryman S, O'Brien C, Hoehn MM, et al. Voice and speech abnormalities in Parkinson disease: Relation to severity of motor impairment, duration of disease, medication, depression, gender, and age. Journal of Medical Speech‐Language Pathology 2001;9(4):213‐26. [Google Scholar]
Stewart 1995
- Stewart C, Winfield L, Hunt A, Bressman SB, Fahn S, Blitzer A, et al. Speech dysfunction in early Parkinson's disease. Movement Disorders 1995;10(5):562‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Streifler 1984
- Streifler M, Hofman S. Disorders of verbal expression in Parkinsonism. Advances in Neurology 1984;40:385‐93. [MEDLINE: ] [PubMed] [Google Scholar]
Tanner 1996
- Tanner CMJ, Hubble P, Chan P. Epidemiology and genetics of Parkinson's disease. In: Watts R. L, W. C. Koller editor(s). Movement Disorders. Neurologic Principles and Practise. New York: McGraw Hill, 1996:137‐60. [ISBN: 0‐07‐035203‐8] [Google Scholar]
Visser 2008
- Visser M, Rooden SM, Verbaan D, Marinus J, Stiggelbout AM, Hilten JJ. A comprehensive model of health‐related quality of life in Parkinson's disease. Journal of Neurology 2008;255(10):1580‐7. [DOI] [PubMed] [Google Scholar]
Yorkston 1981
- Yorkston KM, Beukelman DR. Assessment of Intelligibility of Dysarthric Speech. Austin, TX: PRO‐ED, 1981. [Google Scholar]
Zach 2004
- Zach M, Friedman A, Slawek J, Derejko M. Quality of life in polish patients with long‐lasting Parkinson's disease. Movement Disorders 2004;19(6):667‐72. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Deane 2001b
- Deane K, Whurr R, Playford ED, Ben‐Shlomo Y, Clarke CE. Speech and language therapy for dysarthria in Parkinson's disease: a comparison of techniques. Cochrane Database of Systematic Reviews 2001, Issue 2. [DOI: 10.1002/14651858.CD002814] [DOI] [PubMed] [Google Scholar]


