Abstract
Blood-retinal barrier (BRB) includes inner BRB (iBRB) and outer BRB (oBRB), which are formed by retinal capillary endothelial (RCEC) cells and by retinal pigment epithelial (RPE) cells in collaboration with Bruch’s membrane and the choriocapillaris, respectively. Functions of the BRB are to regulate fluids and molecular movement between the ocular vascular beds and retinal tissues and to prevent leakage of macromolecules and other potentially harmful agents into the retina, keeping the microenvironment of the retina and retinal neurons. These functions are mainly attributed to absent fenestrations of RCECs, tight junctions, expression of a great diversity of transporters, and coverage of pericytes and glial cells. BRB existence also becomes a reason that systemic administration for some drugs is not suitable for the treatment of retinal diseases. Some diseases (such as diabetes and ischemia-reperfusion) impair BRB function via altering tight junctions, RCEC death, and transporter expression. This chapter will illustrate function of BRB, expressions and functions of these transporters, and their clinical significances.
Keywords: Blood-retinal barrier, Transporters, Retinal capillary endothelium, Retinal pigment epithelium, Diabetic retinopathy
General Introduction
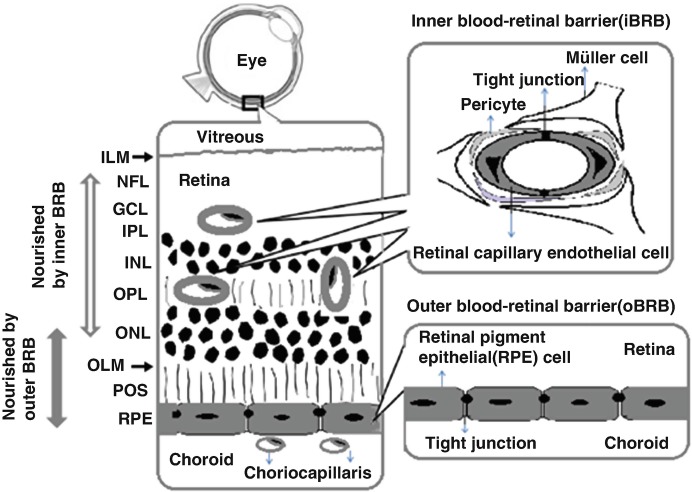
The retina has a unique position in that blood-retinal barrier (BRB) separates the retina from the circulating blood. The BRB regulates fluids and molecular movement between the ocular vascular beds and retinal tissues, prevents leakage of macromolecules and other potentially harmful agents into the retina, and keeps the microenvironment of the retina and retinal neurons. BBB existence is also a reason that systemic drug administration is not suitable for the treatment of retinal diseases. The BRB includes inner BRB (iBRB) and outer BRB (oBRB), which are formed by retinal capillary endothelial cells (RCECs) and by retinal pigment epithelial (RPE) cells in collaboration with Bruch’s membrane and the choriocapillaris, respectively (Fig. 10.1). It is well known that two third of the human retina is nourished by retinal capillaries via the iBRB and the remainder is attributed to choriocapillaris via the oBRB (Hosoya and Tomi 2005; Kur et al. 2012).
Fig. 10.1.
Schematic diagram of blood-retinal barrier (BRB). Symbol: ILM inner limiting “membrane,” NFL nerve fiber layer, GCL ganglion layer, IPL inner plexiform, INL inner nuclear layer, OPL outer plexiform, ONL outer nuclear layer, OLM outer limiting “membrane,” POS photoreceptor outer segments
The paracellular and transcellular transport across BRB are generally involved in the following five different mechanisms (Fig. 10.2) (Rizzolo et al. 2011):
Paracellular diffusion: Paracellular diffusion is mainly regulated by the tight junction. Tight junctions, boundaries between the apical and basolateral plasma membrane domains, are considered to be essential for the integrity of tissue barrier and the maintenance of cell polarity, which restrict paracellular movement of fluids and molecules between the blood and retina.
Facilitated diffusion: Transporters expressed in the plasma membrane allow the passage of preferred solutes across the monolayer along with a concentration gradient. An example is glucose transport via glucose transporter 1 (GLUT1).
Active transport: Transporters expressed in the plasma membrane consume ATP to move solutes against a concentration gradient or establish electrochemical gradients that drive vectorial transport through antiporters and cotransporters.
Transcytosis: Vesicles can invaginate and bud from the apical or basal membrane, traverse the cell, and fuse with the opposite membrane to release their contents on the opposite side of the cell. Normal BRB lacks transcytosis, which become a reason limiting transcellular passage (Chow and Gu 2017).
Solute modification: During transport, solutes can be degraded or transformed into something else. For example, in RPE, retinol enters the basal side of the RPE by receptor-mediated endocytosis and is delivered to microsomes, where retinol is transformed into cis-retinal. The cis-retinal transports across the monolayer and is endocytosed by photoreceptors and bound to opsin. Another example is CO2. CO2 is converted to HCO3− as it is transported from the apical to the basal side of the monolayer.
Fig. 10.2.
Mechanisms for the transepithelial transport of solutes in the BRB
The Inner Blood-Retinal Barrier (iBRB) and Outer Blood-Retinal Barrier (oBRB)
The iBRB is structurally similar to the blood-brain barrier (BBB). The RCECs connected by tight junctions are covered with pericytes and glial cells (Muller cells or astrocytes) (Cunha-Vaz et al. 2011). The iBRB is formed by the inner or outer capillary beds. The inner capillary bed lies in the ganglion nerve cell layer, and the iBRB function is induced by astrocytes. The outer capillary bed lies in the inner and outer plexiform layers, where function of BRB is regulated by Müller cells (Rizzolo et al. 2011).
The oBRB is established by RPE cells connected by tight junctions. RPE is a monolayer of pigmented cells situated between the neuroretina and the choroids. The apical membrane of RPE exhibiting long microvilli faces the light-sensitive outer segments of the photoreceptors cells, while its basolateral membrane faces the Bruch’s membrane, which separates the neural retina from the fenestrated endothelium of the choriocapillaris. It is different from the epithelium of the choroid plexus and other transporting epithelia that the apical membrane of RPE cells abuts a solid tissue rather than a lumen. Moreover, the transepithelial electrical resistance of RPE shows large species differences ranging from 135 to 600 Ω × cm2 (Rizzolo et al. 2011).
The main functions of the RPE (Kay et al. 2013; Simó et al. 2010; Willermain et al. 2014a) are to (1) transport nutrients, ions, and water or waste products; (2) absorb light and protect against photooxidation; (3) reisomerize all-trans-retinal into 11-cis-retinal, which is a key element of the visual cycle; (4) phagocyte shed photoreceptor membranes; (5) release K+ into the subretinal space to maintain constant excitability of the photoreceptors; (6) secrete growth factors such as pigmented epithelium-derived factor (PEDF) at the apical side and vascular endothelial growth factor (VEGF) at the basolateral side for the structural integrity of the retina; and (7) maintain the immune privilege of the eye due to its outer BRB function but also by interfering with signaling pathways coordinating the immune system.
Tight Junctions in the BRB
Tight junctions consist of specialized proteins such as occludins, claudins, and zonula occludens, which play an important role in maintaining the barrier function via regulating the transport of solutes and molecules across RCEC or RPE cell layers. Both the iBRB and the oBRB cells have tight junctions, but they differ in their organization and composition. Tight junctions of the oBRB are more concentrated at the apical side of the cell, whereas tight junctions of the iBRB are more dispersed between adherens junctions and gap junctions. The adherens junctions consist of vascular endothelial-cadherin and its associated proteins, such as catenins and plakoglobin, both of which are linked to the cytoskeleton (Dejana et al. 2008). The cell–cell contacts also contain additional adhesion molecules, such as platelet endothelial cell adhesion molecule-1 (PECAM-1), intercellular adhesion molecule 2 (ICAM2), endoglin, and other clusters (Dejana et al. 2008).
Astrocytes, Müller Cells, and Pericytes
Astrocytes, Müller cells, and pericytes, closely connected to blood vessels in the retina, are considered to influence activities of the BRB via secreting regulatory signal molecules such as glial cell line-derived neurotrophic factor (GDNF), transforming growth factor beta 1 (TGF-β1), and VEGF (Igarashi et al. 2000; Abukawa et al. 2009; Le 2017; Wisniewska-Kruk et al. 2012). In immortalized rat retinal capillary endothelial (TR-iBRB2), an in vitro model cell line of the iBRB, it was found that incubation with conditioned medium of Müller (TR-MUL5) cells increased alkaline phosphatase activity, induced expression of plasminogen activator inhibitor 1 (PAI-1) gene, and suppressed expression of an inhibitor of DNA binding 2 gene. TGF-β1, secreted from TR-MUL5 cells, showed similar effects, indicating that paracrine interactions occur between TR-iBRB2 and TR-MUL5 cells via secreting TGF-β1 (Abukawa et al. 2009). Müller cells are also involved in the regulation of retinal iron homeostasis. Loss of Müller cells was reported to break down the BRB and increase iron levels throughout the neurosensory retina of mice (Baumann et al. 2017). Müller cell loss or dysfunction often occurs in patients with macular telangiectasia type 2 (MacTel2) and diabetic retinopathy, which may explain the clinical findings that a patient with MacTel2 and a patient with diabetic retinopathy had the increased iron levels in RPE or neurosensory retina (Baumann et al. 2017). TGF-β was reported to impair function of BRB via simulating metalloproteinases (MMPs) from Müller cells, later degrading tight junction protein occludin (Behzadian et al. 2001). Moreover, interactions between endothelial cells and pericytes or astrocytes also increase the BRB properties through enhancing expression of tight junctions (Kim et al. 2009).
The pericytes play important roles in regulating vascular tone, secreting extracellular material, and being phagocytic. The frequency of pericyte coverage on human retinal capillaries was reported to be high up to 94.5%, substantially greater than that of human choriocapillaris (11%) (Chan-Ling et al. 2011), demonstrating that retinal microvasculature characterizes a uniquely high density of pericytes. The communication between pericytes and endothelial cells is mediated by diverse molecules such as angiopoietin, TGF-β1, platelet-derived growth factor-β (PDGF-β), and sphingosine-1-phosphate. In endothelial cells, genetic deletion of PDGF-β (Park et al. 2017) or blocking PDGF receptor beta using antibody (Ogura et al. 2017) could lead to severe vascular impairments such as vascular engorgement, leakage, severe hemorrhage, retinal detachment, and severely impaired pericyte coverage of the vessels. Moreover, pericyte loss also promotes pathologic angiogenesis. In consistence, pericyte dropout or loss in the retina is considered to be one of the earliest pathological changes in diabetic retinopathy. Moreover, hyperglycemia, advanced glycation end products, basement membrane thickening, and hypertension trigger pericyte apoptosis and dropout, all of which become reasons leading to diabetic retinopathy (Eshaq et al. 2017).
Major Drug Transporters in the Retina
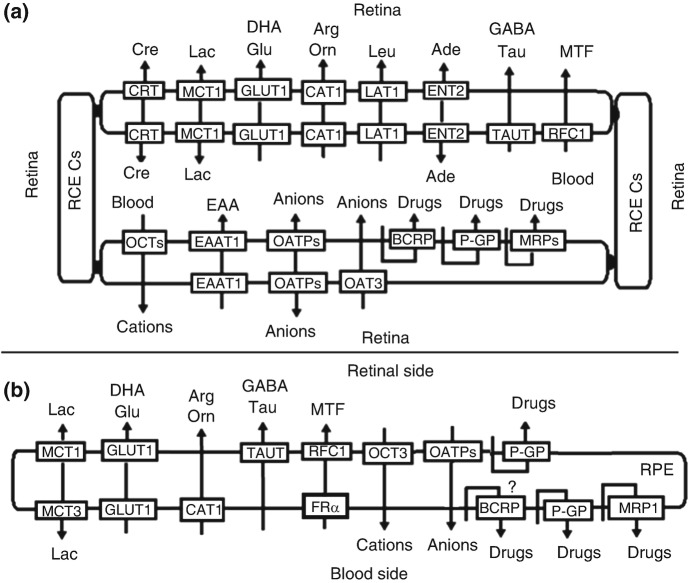
Several transporters, including the solute carrier (SLC) family and the ATP-binding cassette (ABC) family have been identified in the retina (Fig. 10.3). The SLC transporters utilize facilitated diffusion, or they couple an ion or electrochemical gradient to transfer their substrates across the cell membrane. ABC transporters, on the other hand, use ATP as the energy source to drive the transport.
Fig. 10.3.
Hypothetical localization and physiological function of several transporters in the iBRB (a) and the oBRB (b). Ade adenosine, Arg L-arginine, Cre creatine, DHA dehydroascorbic acid, EAA excitatory amino acid, GABA gamma-aminobutyric acid, Glu glucose, Lac lactate, Leu L-leucine, MTF methyltetrahydrofolate, Orn L-ornithine, RCECs retinal capillary endothelial cells, RPE retinal pigment epithelial (RPE) cells, Tau taurine
In the retina, neuronal cells, including photoreceptor cells, require a large amount of metabolic energy for phototransduction and neurotransduction metabolic substrates, such as D-glucose, amino acids, vitamins, and nucleosides. These compounds are hydrophilic, and their transport is often mediated by influx transporters, belonging to SLC family. The identified influx transporters in the retina include glucose transporter 1 (GLUT1), Na+-dependent multivitamin transporter (SMVT), taurine transporter (TAUT), cationic amino acid transporter 1 (CAT1), excitatory amino acid transporter 1 (EAAT1), L-type amino acid transporter 1 (LAT1), creatine transporter (CRT), nucleoside transporters, and monocarboxylate transporters (MCTs). A series of influx transporters for drugs such as organic cation transporters (OCTs), organic anion transporting polypeptides (OATPs), and organic anion transporters (OATs) have been also identified in the retina.
Influx Transporters
Glucose Transporter 1 (GLUT1/SLC2A1)
D-glucose is the main energy source for the retina, whose transport from the blood to the retina is mainly mediated by GLUT1 (Tomi and Hosoya 2004). GLUT1 is mainly localized at both luminal and abluminal membranes of the iBRB and the oBRB, although the expression of GLUT1 at abluminal membrane of the iBRB is approximately two- to threefold greater than that at its luminal membrane (Fernandes et al. 2003). The asymmetrical distribution of GLUT1 at the iBRB suggests that D-glucose transport is limited at the blood-to-luminal rather than the abluminal-to-interstitial interface. GLUT1 is also expressed in Müller cells (Hosoya et al. 2008a). In addition to hexoses, GLUT1 also transports dehydroascorbic acid from blood to the retina, where dehydroascorbic acid is converted to ascorbic acid. The uptake of dehydroascorbic acid by GLUT1 may be completely inhibited under diabetic conditions due to high glucose concentration (Minamizono et al. 2006).
Taurine Transporter (TAUT/SLC6A6)
Taurine, functioning as osmolyte and antioxidant, is the most abundant free amino acid in the retina, accounting for more than 50% of the free amino acid content in the rat retina, which is considered to be essential for maintenance of retinal structure. Taurine transport across BRB is mainly mediated by TAUT (Tomi et al. 2007).TAUT, an Na+- and Cl−-dependent transporter, is mainly expressed in apical membrane of RPE, ganglion cells, Müller cells (El-Sherbeny et al. 2004), and RCECs (Tomi et al. 2007, 2008). Some TAUT inhibitors such as β-alanine and hypotaurine may inhibit taurine transport across BRB. TAUT also mediates transport of β-alanine and gamma-aminobutyric acid (GABA) (Tomi et al. 2008; Usui et al. 2013). Hyperosmolar conditions could stimulate activity of TAUT, leading to increase in Vmax of taurine without affecting Km (El-Sherbeny et al. 2004; Yahara et al. 2010). Both in vivo and in vitro data demonstrated that taurine itself enhanced expression of retinal TAUT (Zeng et al. 2010). High glucose was reported to downregulate expression of retinal TAUT (Lee and Kang 2013; Stevens et al. 1999), which was attenuated by taurine treatment (Zeng et al. 2010). In consistence with this issue, 8-week and 12-week diabetic rats demonstrated significantly lower levels of retinal TAUT, and taurine treatment completely reversed the decreased expression of retinal TAUT by diabetes (Zeng et al. 2010). Similarly, TAUT deficiency was reported to lead to severe retinal degeneration in mice (Heller-Stilb et al. 2002). All these results demonstrated important roles of retinal TAUT in normal retinal development.
Cationic Amino Acid Transporter 1 (CAT1/SLC7A1)
L-arginine is the precursor molecule for the synthesis of nitric oxide (NO) by nitric oxide synthase (NOs). L-arginine transport from the blood to the retina across BRB is mediated by CATs. CAT1 is highly expressed at both luminal and abluminal membrane of RCECs, TR-iBRB2 cells, the basal membrane of PRE (Kubo et al. 2015; Tomi et al. 2009), and immortalized rat retinal pericyte cell line (TR-rPCT1 cells) (Zakoji et al. 2015). QT-PCR analysis showed that levels of CAT1 mRNA in TR-iBRB2 and the isolated rat RCECs were 25.9- and 796-fold greater than that of CAT3, respectively. CAT1-specific small interfering RNA, L-arginine, and L-lysine inhibited [3H]L-arginine uptake in TR-iBRB2 cells (Tomi et al. 2009). Moreover, high glucose exposure significantly inhibited L-arginine transport in TR-iBRB, and simvastatin might reverse high glucose-induced-alterations via increasing expression of endothelial NOS mRNA and NO production (Tun and Kang 2017).
L-ornithine is a cationic amino acid produced in the urea cycle and ingested from the diet. Clinical trials have suggested the beneficial effects of L-ornithine in the body, but long-term treatment or high concentrations of L-ornithine in the blood induce retinal toxicity, forming gyrate atrophy of the choroid and retina (Hayasaka et al. 2011), an autosomal recessive disease due to the genetic defect of ornithine aminotransferase. Both in vivo and in vitro data demonstrated that CAT1 mediates transport of [3H]L-ornithine across the BRB (Kubo et al. 2015). In RPE cells, it was reported that the basal-to-cell uptake of [3H]L-ornithine was greater than that of the apical-to-cell uptake, and the basal-to-cell transport was inhibited by L-ornithine, suggesting the involvement of CAT1 in the blood-to-cell transport of L-ornithine across the basal membrane of the oBRB (Kubo et al. 2015). Moreover, in human telomerase reverse transcriptase-RPE cells, CAT1 siRNA decreased both L-[14C]ornithine uptake and L-ornithine cytotoxicity (Kaneko et al. 2007). All these results indicate that reduction of the ornithine transport via inhibiting CAT1 may be a new target for treatment of gyrate atrophy.
L-Type Amino Acid Transporter 1 (LAT1/SLC7A5)
L-type amino acid transporters (LATs) prefer branched-chain and aromatic amino acids. LAT1 (SLC7A5) and LAT2 (SLC7A8) mRNAs were reported to be expressed in cultured human RPE cell line (ARPE-19 cells), but the level of LAT1 mRNA was 42-fold higher than that of LAT2 (Yamamoto et al. 2010). LAT1, an Na+-independent transporter, was also identified in TR-iBRB2, isolated rat RCECs, and primary cultured human RCECs (Tomi et al. 2005; Usui et al. 2013; Yamamoto et al. 2010), mediating blood-to-retina transport of large neutral amino acids including L-leucine (Tomi et al. 2005), L-histidine (Usui et al. 2013), and L-phenylalanine (Atluri et al. 2008). Some drugs such as L-dopa, melphalan, alpha-methyldopa, and gabapentin are substrates of LAT1 and LAT2 (del Amo et al. 2008), indicating the roles of LAT1 in retinal disposition of these drugs.
Creatine Transporter (CRT/SLC6As)
Creatine and phosphocreatine are required to maintain ATP needed for normal retinal function and development. Creatine is transported from the blood to the retina against the creatine concentration gradient via creatine transporter (CRT), an Na+- and Cl−-dependent transporter. The creatine transport from the blood to the retina is considered to be a major pathway for supplying creatine to the retina although local creatine is preferentially synthesized in the glial cells (Tachikawa et al. 2007; Nakashima et al. 2005a). In the inner retina, CRT is expressed in cells of intense metabolic activity, such as photoreceptors and selected cells, but not in glial cells (de Souza et al. 2012). CRT is also expressed at both the luminal and abluminal membranes of the iBRB (Nakashima et al. 2004). CRT expressed in the luminal membrane would mediate creatine supply to the retina, and CRT in the abluminal membrane may be involved in the metabolite uptake of creatine (Nakashima et al. 2004).
Monocarboxylate Transporters (MCTs/SLC16As)
The retina produces more L-lactic acid aerobically than any other tissues. In addition to D-glucose, L-lactic acid appears to be required as an energy source in photoreceptors. Transports of L-lactic acid and other monocarboxylates (such as pyruvate and the ketone bodies across cellular membranes) are facilitated by specific monocarboxylate transporters (MCTs). Four MCTs (including MCT1, MCT2, MCT3, and MCT4, encoded by SLC16A1, SLC16A7, SLC16A8, and SLC16A3, respectively) have been identified in the retina (Bergersen et al. 1999; Gerhart et al. 1999; Philp et al. 1998). MCT1 is detected on four retinal cell types: RPE, photoreceptor cells, Müller cells, and endothelial cells (Gerhart et al. 1999). MCT1 is mainly located in the apical membrane of RPE and in both the luminal and abluminal plasma membranes of RCECs (Bergersen et al. 1999; Gerhart et al. 1999). MCT2 is only found to be abundantly expressed on the inner (basal) plasma membrane of Müller cells and by glial cell processes surrounding retinal microvessels (Gerhart et al. 1999). High expression of MCT4 is only detected in the RPE of younger animals, but RPE of adult animals only show very weak expression (Bergersen et al. 1999). MCT3 is preferentially expressed in the basolateral membrane of the RPE (Philp et al. 1998, 2001), forming a heteromeric complex with the accessory protein CD147 (Philp et al. 2001). The absence of Mct3 was reported to impair expression of CD147 from the basolateral but not apical RPE of mice. Moreover, the amount of L-lactate in retinal of Mct3−/− mice was approximately four times higher than that from the wild-type retinas, accompanied by decreases in the magnitude of the light suppressible photoreceptor current (Daniele et al. 2008). These results demonstrate the pivotal roles of MCT3 in regulating the ionic composition of the outer retina.
MCTs also accept monocarboxylic acid drugs such as moxifloxacin (Barot et al. 2014) and nicotinate (Tachikawa et al. 2011) as their substrates, indicating that MCT-mediated transport at BRB is a possible route for delivery of monocarboxylic acid drugs to the retina (Hosoya et al. 2001).
Nucleoside Transporters
Adenosine is an important intercellular signaling molecule, showing a number of roles in retinal neurotransmission. Most of the adenosine in the retinal interstitial fluid originates from the catabolism of adenosine monophosphate, which is localized in the innermost process of Müller cells. Thus, almost all of the retinal adenosine is distributed in the neighborhood of the innermost process of Müller cells in the ganglion cell layer, inner plexiform layer, and inner nuclear layer. Adenosine in the blood may penetrate the iBRB via adenosine transport systems. Four transporters including two equilibrative nucleoside transporters (ENT1/(ENT2, SLC29A1/SLC29A2) and two concentrative nucleoside transporters (CNT1/CNT2, SLC28A1/SLC28A2) have been identified in the rat retina, although the expression of ENT2 mRNA was reported to be 5.5-fold greater than that of ENT1 mRNA. Among the four transporters, only CNT1 was not detected in TR-iBRB. Adenosine uptake in TR-iBRB2 cells is predominantly mediated by ENT2, which is strongly inhibited by adenosine, inosine, uridine, and thymidine, but neither nitrobenzylmercaptopurine riboside (NBMRP) nor dipyridamole, characterizing NBMPR- and dipyridamole-insensitive transport of adenosine. An in vivo study suggested that [3H]adenosine transport from the blood to the retina was also significantly inhibited by adenosine and thymidine, demonstrating that ENT2 most likely mediates adenosine transport at the iBRB (Nagase et al. 2006). ENT2 is also expressed in Müller cells, contributing to transport of adenosine and its metabolite hypoxanthine. ENT2 also mediates elimination of hypoxanthine from the retina (Akanuma et al. 2013a). In addition, ENT2 accepts some antiviral or anticancer nucleoside drugs, such as 3′-azido-3′-deoxythymidine, 2′3′-dideoxycytidine, 2′ 3′-dideoxyinosine, cladribine, cytarabine, fludarabine, gemcitabine, and capecitabine, as preferred substrates (Baldwin et al. 2004; Yao et al. 2001), indicating that ENT2 at the BRB could be a potential route for delivering nucleoside drugs from the circulating blood to the retina.
Folate Transport Proteins
Folates, water-soluble vitamins, play an essential role as cofactors for one-carbon metabolism in cells. Most of the folate in the plasma is in the reduced form, methyltetrahydrofolate. Folate transport from blood to the retina across BRB is mediated by some specific transport process. Three transport proteins, folate receptor-α (FRα), reduced folate carrier 1 (RFC1/SLC19A1), and proton-coupled folate transporter (PCFT/SLC46A1), have been described for folate uptake. FRα, a receptor for folate, is expressed in basolateral membrane of PRE and in retinal Müller cells, mediating the influx of its ligand into cells via receptor-mediated endocytosis (Bozard et al. 2010; Chancy et al. 2000). RFC1, a pH-sensitive transporter, is involved in folate−/OH−exchange. RFC1 is highly expressed in TR-iBRB2 cells, isolated rat RCECs, normal mouse RPE, and in cultured human RPE cells (Chancy et al. 2000; Hosoya et al. 2008b). Although both RFC1 and PCFT mRNA are expressed in TR-iBRB2 cells and isolated rat RCECs, the expression level of RFC1 mRNA was reported to be 83- and 49-fold greater than that of PCFT, respectively, indicating that RFC1 probably predominates at the iBRB. In TR-iBRB2 cells, transports of methotrexate, formyltetrahydrofolate and methyltetrahydrofolate are meidiated by RFC1 (Hosoya et al. 2008b). RFC1 is also expressed in RPE cells. Importantly, FRα and RFC1 are localized in the basolateral and apical membrane of the RPE, respectively, demonstrating that the two proteins work in a concerted manner to operate the vectorial transfer of folate across RPE from choroidal blood to the retina. Diabetes might downregulate expression and activity of RFC1 in mouse PRE. In ARPE-19 cells, it was reported that 6 h exposure of high glucose (45 mM) led to decreases in methyltetrahydrofolate uptake by 35%, which was consistent with decreases in levels of RFC1 mRNA and protein (Naggar et al. 2002). PCFT mRNA was detected in all tested retinal cells including primary cultures of ganglion, Müller, and RPE cells of the mouse retina and their cell lines (Umapathy et al. 2007), mediating H+-coupled transport of folate. In retinal Müller cells, PCFT and FRα are expressed and colocalized in the endosomal compartment, where the two proteins may work coordinately to mediate folate uptake (Bozard et al. 2010).
Organic Anion-Transporting Polypeptides (OATPs)
Several OATPs (OATPs for human and Oatps for animal), such as Oatp1a4, Oatp1c1, Oatp4a1, and Oatp1a5, have been detected in rat retina. Oatp1a4 and Oatp1c1 mRNA are predominantly expressed in isolated rat RCECs (Tomi and Hosoya 2004). Oatp1a4 proteins are detected on both the abluminal and luminal membrane of rat RCECs and RPE, but Oatp1a4 protein is preferentially localized on the abluminal membrane of the RCECs and the apical membrane of rat RPE (Akanuma et al. 2013b; Ito et al. 2002). Oatp1c1 protein is expressed in both the abluminal and luminal membrane of the RCECs and is preferentially expressed in the basolateral membrane of rat RPE (Akanuma et al. 2013b; Ito et al. 2002). Oatp1a5 protein is predominantly localized in optic nerve fibers, not in RPE (Gao et al. 2002; Ito et al. 2002). Expressions of Oatp1a4 and Oatp1c1 on two side membranes of BRB indicate their contributions to the transcellular transport of amphipathic organic anions including digoxin and [3H]estradiol 17-β glucuronide (E17βG) across the BRB in both the blood-to-retina and retina-to-blood directions. It was found that elimination rate constant of [3H]- E17βG from the vitreous humor of rats was 1.9-fold greater than that of [14C]D-mannitol. The efflux transport of E17βG from rat retina was significantly inhibited by organic anions probenecid, sulfobromophthalein, digoxin, and dehydroepiandrosterone sulfate (Katayama et al. 2006). Oatp4a1 is also expressed in the RPE, inner and outer nuclear layers, ganglion cell layer, and nerve fiber layer of rat eyes. In the cultured rat RPE cells, it was found that uptake of triiodothyronine, a known substrate of Oatp4a1, was significantly inhibited by OATP inhibitor sulfobromophthalein, indicating roles of OATPs in the transport of thyroid hormone in the retina (Ito et al. 2003).
Human OATP1A2 is expressed in photoreceptor bodies, somas of amacrine cells and RPE, and mediates the cellular uptake of all-trans-retinol (Chan et al. 2015; Gao et al. 2015), inferring roles of OATP1A2 in canonical visual cycle. The OATP1A2-mediated uptake of all-trans-retinol may be inhibited by chloroquine and hydroxychloroquine (Xu et al. 2016), which may provide novel insights into retinal dysfunction induced by certain drugs. For instance, digoxin and antimalarial drugs (chloroquine and hydroxychloroquine) have induced retinopathy (Kinoshita et al. 2014; Weleber and Shults 1981; Yaylali et al. 2013), which may be partly contributed to decrease in all-trans-retinol uptake into the retinal cells via inhibiting retinal OATP1A2 function, resulting in dysfunction of the canonical visual cycle and toxic accumulation of retinoids. Moreover, human OATP1A2 and OATP2B1 are abundantly expressed in retina amacrine neurons containing substance P and vasoactive intestinal peptide. The two peptides are also substrates of OATP1A2 and OATP2B1, demonstrating roles of OATP1A2 and OATP2B1 in reuptake of these neuropeptides released from retinal neurons and in the homeostasis of neuropeptides (Gao et al. 2015).
Organic Cation Transporters
Organic cation transporter 3 (Oct3) is detected in mouse RPE and in several cell types of the neural retina, including photoreceptor, ganglion, amacrine, and horizontal cells, where Oct3 participates in the clearance of dopamine, histamine, and neurotoxin 1-methyl-4-phenyl pyridinium (MPP+) from the subretinal space (Rajan et al. 2000). In cultured ARPE-19 cells, it was found that uptake of typical OCT3 substrate MPP+ was completely inhibited by several cationic drugs and monoamine neurotransmitters (dopamine and histamine) (Rajan et al. 2000), although an organic cation transporter, functionally similar to plasma membrane monoamine transporter (SLC29A4), was reported to mediate retina-to-blood transport of MPP+ at BRB (Kubo et al. 2017a).
l-carnitine is essential for the translocation of acylcarnitine esters into mitochondria for β-oxidation of long-chain fatty acids and ATP generation. l-carnitine transport in the retina is mainly mediated by organic cation/carnitine transporter 1/organic cation/carnitine transporter 2 (OCTN1/OCTN2 for human and Octn1/Octn2 for animal). QT-PCR analysis showed that the expressions of Octn2 mRNA in TR-iBRB2 and isolated rat RCECs were 27.3- and 45.9-fold greater than Octn1 mRNA, respectively, indicating that Octn2 predominantly accounts for the transport of acetyl-l-carnitine from the blood to the retina across the iBRB (Tachikawa et al. 2010). In consistence, acetyl-L-[3H]carnitine uptake in rat retina was significantly suppressed by L-carnitine and acetyl-L-carnitine. In TR-iBRB2 cells, OCTN substrates and inhibitors (as l-carnitine, acetyl-l-carnitine, tetraethylammonium, quinidine, and betaine) remarkably decreased uptake of l-[3H]carnitine and acetyl-l-[3H]carnitine (Tachikawa et al. 2010), further confirming roles of retina OCTNs in transport of L-carnitine.
Several novel organic cation transporters have been identified. Transport of the cationic drugs clonidine and diphenhydramine at the mouse BRB is mainly attributed to a carrier-mediated system. The transporter for transporting clonidine is an Na+-independent proton-antiporter and insensitive to the transmembrane potential. The transporter also transports other cationic drugs such as nicotine, tramadol, diphenhydramine, cocaine, verapamil, methadone, and oxycodone (Chapy et al. 2015). Transport of [3H]verapamil across the iBRB is also mediated by a novel organic cation transporter. In vivo data demonstrated that influx transport of verapamil across the BRB was about fivefold higher than that across the BBB. Verapamil (3 mM) and quinidine (10 mM), not pyrilamine (3 mM), slightly increased the retinal uptake of [3H]verapamil. However, these compounds markedly increased brain uptake of [3H]verapamil. Moreover, pyrilamine (40 mM) significantly reduced the retinal uptake index to 72.9% but not for the brain uptake index. These in vivo results clearly demonstrate that differently from the BBB, transport of verapamil across the BRB is mediated by both influx transporters and efflux transporters (Kubo et al. 2013a). Importantly, in P-GP-deficient rats, P-GP inhibitors (vinblastine and verapamil) inhibited verapamil uptake by the retina but not the brain, which conformed that [3H]verapamil is permeated across the BRB via influx transporters (Fujii et al. 2014). In human RPE cell lines (RPE/Hu and ARPE-19), it was found that verapamil uptake is active, pH-dependent, and independent of the membrane potential (Han et al. 2001), but in TR-iBRB2 cells, verapamil uptake was independent of pH (Kubo et al. 2013a), indicating that it has characteristics of influx transporter for verapamil in the iBRB was different from that in the oBRB. The verapamil uptake was inhibited by metabolic inhibitors, quinidine, pyrilamine, diphenhydramine, diltiazem, timolol, propranolol, and L-carnitine, but not by other known OCT/OCTN2 substrates nor inhibitors (such as tetraethylammonium, cimetidine, decynium-22, and MPP+) (Han et al. 2001; Kubo et al. 2013a). Similarly, [3H]pyrilamine uptake in TR-iBRB2 cells was also inhibited by verapamil and some cation compounds (such as desipramine, imipramine, propranolol, memantine, quinidine, and nipradilol), not tetraethylammonium, serotonin, choline, or choline. However, the transporter mediating pyrilamine uptake seemed not to be identical to verapamil transport system, because they showed different sensitivity to pH and L-carnitine. Moreover, kinetic analysis indicated that verapamil had no competitive effect on the pyrilamine uptake although verapamil uptake exhibited a competitive-like inhibition (Kubo et al. 2013a).
Transport of propranolol across iBRB is mediated by an influx carrier and was reduced by several organic cations (Kubo et al. 2013b). Propranolol uptake in TR-iBRB2 cells was also inhibited by some organic cations (such as pyrilamine, verapamil, imipramine) but not substrates nor inhibitors of OCTs (MPP+, tetraethylammonium, and cimetidine). The propranolol transport is pH dependent, Na+ independent, and L-carnitine insensitive, which is different from verapamil transport system (Kubo et al. 2013a, b). Blood-to-retina transport of nicotine across the iBRB is driven by an outwardly directed H+ gradient, which was stimulated by an outwardly directed H+ gradient and significantly inhibited by organic cations (pyrilamine and verapamil) but not OCT inhibitors (Kubo et al. 2014; Tega et al. 2015). These findings that substrate specificity of the cationic drug transport at the BRB is different from those of well-characterized organic cation transporters OCTs, OCTNs, and MATEs, suggesting the involvement of novel organic cation transporters in the influx transport of these cationic drugs across the BRB (Chapy et al. 2015; Kubo et al. 2013a, b, 2014).
Other Transporters
Organic anion transporter 3 (OAT3) is expressed in RCECs and TR-iBRB cells. Rat Oat3 is possibly located at the abluminal membrane of the RCECs, where it effluxes its substrates such as p-aminohippuric acid, benzylpenicillin, and 6-mercaptopurine from the vitreous humor/retina to the blood across the iBRB, limiting the retinal distribution of these substrates (Hosoya et al. 2009). Unlikely BBB, androgen receptor ligand dihydrotestosterone did not affect expression of Oat3 mRNA in TR-iBRB cells (Ohtsuki et al. 2005). Anion transporter inhibitor probenecid also inhibited transport of digoxin across BRB of rats, leading to significant increases in uptake retina index by 1.6-fold of control, indicating roles of OATs in efflux of digoxin across BRB (Toda et al. 2011).
Excitatory acid transporter 1 (EAAT1), an Na+-dependent high-affinity L-glutamate transporter, is localized on the abluminal membrane of the RCECs and mediates elimination of L-glutamate, a neuroexcitatory neurotransmitter, from the retina across the iBRB (Sakurai et al. 2015). Multivitamin transporter (SMVT/SLC5A6), an Na+-dependent transporter, was detected in TR-iBRB2 cells and isolated rat RCECs, mediating the uptake of vitamins and some essential cofactors such as biotin, pantothenic acid, and lipoic acid (Quick and Shi 2015). In vivo, [3H]biotin uptake by the rat retina was significantly inhibited by biotin and pantothenic acid. [3H]biotin uptake in TR-iBRB2 cells was also significantly inhibited by biotin, pantothenic acid, lipoic acid, and desthiobiotin (Ohkura et al. 2010). SMVT mRNA and protein were also detected in human RPE cells. Hypoxia induced expression and function of SMVT in human RPE cells, indicating that hypoxia may alter disposition of ophthalmic drugs (Vadlapatla et al. 2013). Riboflavin transport across blood-to-retina is mediated by riboflavin transporter (SLC52A/RFVT), an Na+- and Cl−-independent transporter. In TR-iBRB2 cells, two RFVTs RFVT2 (SLC52A2) and RFVT3 (SLC52A3) were detected (Kubo et al. 2017b).
Efflux Transporters
Efflux transporters mainly belong to ABC family transporters. Several ABC transporters, such as P-glycoprotein (P-GP/ABCB1), multidrug resistance proteins (MRPs/ABCCs for human and Mrps/ABccs for animal) (Asashima et al. 2006), and breast cancer resistance protein (BCRP/ABCG2), were identified in the BRB.
P-Glycoprotein (P-GP/ABCB1)
P-GP is mainly expressed at the iBRB. However, reports on P-GP in the oBRB are often contradictory, which may result from different tissues or cells from several species or different detection methods. In mice, it was found that the oBRB showed low or lack expression of P-GP (Chapy et al. 2016). In human fetal RPE and ARPE-19, no P-GP protein was detected by LC-MS/MS (Pelkonen et al. 2017). But, in the oBRB tissue of porcine, P-GP protein was measured to be 2.01 fmol/μg protein by LC-MS/MS, although it was less than that of the iBRB (8.70 fmol/μg protein) (Zhang et al. 2017a). Similarly, among three human RPE cell lines (ARPE-19, D407 and h1RPE), only ARPE-19 cells do not express P-GP. D407 and h1RPE cells express P-GP, but functional activity is demonstrable only in D407 cells (Constable et al. 2006). Expression and function of P-GP were also detected in both cultured human RPE and in D407 cells. Furthermore, P-GP immunoreactivity is predominantly associated with localization to both apical and basolateral cell membranes of human RPE (Kennedy and Mangini 2002). Importantly, expression and function of P-GP are also demonstrated in the mitochondria of D407 cells and upregulated by H2O2 (Zhang et al. 2017b). Expression and function of P-GP were also clearly demonstrated in the oBRB tissue of porcine. Permeabilities of verapamil and rhodamine in the retina-to-choroid direction were reported to be higher 5.5- and 2.6-fold than those in the opposite direction. Moreover, cellular calcein accumulation in the presence of verapamil was twice as strong as control (Steuer et al. 2005). PET data showed that P-GP inhibitor tariquidar significantly increased influx rate constant k1 across the BRB and total retinal distribution volume of (R)-[11C]-verapamil in human subjects, by 1.4-fold and 1.5-fold of baseline. In accordance with this, retinal efflux rate constant k2 was significantly decreased by 2.8 in the presences of P-GP inhibitor (Bauer et al. 2017).
It is worth noting that the impact of P-GP on BRB permeability to its substrates is greatly lower than that on BBB permeability (Chapy et al. 2016; Fujii et al. 2014). For example, uptake indexes of verapamil, quinidine, and digoxin in the retina of Abcb1a1−/− rats were 1.6-, 1.07-, and 3.7-fold of wild-type rats, respectively. But, the brain uptake parameters for verapamil, quinidine, and digoxin were high up to 8.3-, 12.3-, and 14.0-fold of wild-type rats, respectively. Quinidine, verapamil, and digoxin are substrates of P-GP, but P-GP inhibitors only inhibited transport of digoxin in the retina, which was different from the brain. These results indicate that P-GP may play a substantial role in the retinal distribution of digoxin, but not verapamil nor quinidine (Toda et al. 2011), which may partly be attributed to influx transporters for these drugs. Similarly, transport of [3H]-verapamil at BRB of mice was significantly increased by ~1.5-fold following P-GP inhibition using elacridar (5 μM) or valspodar (5 μM) and by 1.3-fold in triple knockout (Abcb1a/Abcb1b−/− and Abcg2−/−) mice compared with control wild-type mice, but extents of these alterations at the BRB were remarkably lower than those (5.6-fold for elacridar, 8.4-fold for valspodar, and 10.3-fold for triple knockout) at the BBB (Chapy et al. 2016).
Breast Cancer Resistance Protein (BCRP/ABCG2)
Likely to P-GP, BCRP is mainly located at the luminal membrane of RCECs (Asashima et al. 2006; Chapy et al. 2016), acting as the efflux transporter for photosensitive toxins and drugs in retinal tissue. In TR-iBRB2 cells, BCRP mediates cellular efflux of phototoxic compounds pheophorbide and protoporphyrin IX, which is inhibited by ABCG2 inhibitor Ko143 (Asashima et al. 2006). BCRP is often co-located with P-GP in the retina. In mice, P-GP and BCRP are uniformly expressed in the physiologically developing retinal vasculature of the neonatal mouse (Tagami et al. 2009). Expressions of P-GP and BCRP mRNA and proteins were also detected in the retinal vascular endothelial cells from the adult mouse retina (Tachikawa et al. 2008; Chapy et al. 2016).
Similarly to P-GP, the importance of BCRP efflux at the retina is less than that at the BBB. Triple knockout (Abcb1a/Abcb1b−/−:Abcg2−/−) and coadministration of BCRP inhibitor elacridar significantly increased the mitoxantrone entry rate to the mouse brain, with 3.3-fold increases of control mice, but these increases did not occur at the retina (Chapy et al. 2016).
Several reports have also demonstrated expression of BCRP in the oBRB, although contradictory may be often contradictory. LC-MS/MS analysis demonstrated no expression of BCRP in human fetal RPE and ARPE19 (Pelkonen et al. 2017), but high levels of BCRP protein were still detected in both the iBRR (22.8 fmol/μg protein) and the oBRB (2.76 fmol/μg protein) of pig (Zhang et al. 2017a). Among the tested three human RPE cell lines (ARPE-19, D407, and HRPEpiC) and bovine primary cells, BCRP was only detected in D407 cells (Mannermaa et al. 2009). However, BCRP mRNA was also detected in the neural retina, RPE eyecup, and primary mouse RPE cells. Immunoreactivity showed that the expression of BCRP is almost exclusively restricted to the RPE cell layer, mainly at the basolateral membrane of PRE (Gnana-Prakasam et al. 2011), indicating that the function of BCRP in the oBRB is to efflux of heme from the retina to choroidal blood, showing roles in retinal hemochromatosis. Iron overload downregulated BCRP expression in PRE, whose defective function in RPE may lead to increases in the cellular levels of phototoxin, thus contributing to oxidative stress and enhancing the progression of retinal diseases such as age-related macular degeneration (Gnana-Prakasam et al. 2011).
Multidrug Resistance-Associated Proteins (MRPs/ABCCs)
MRPs seem to be mainly expressed in the oBRB, but different tissues or cells from several species or detected methods often show different patterns of MRP expression. Mannermaa et al. (2009) investigated expressions of several MRPs in three human RPE cell lines (ARPE-19, D407 and HRPEpiC) and bovine primary RPE cells. The results showed that expressions of MRP1, MRP4, and MRP5 proteins were detected in the tested three human RPE cell lines. Unlike MRP1 and MRP4, Mrp5 protein was not detected in bovine primary RPE cells. MRP2 protein was only detected in the D407 cells (Mannermaa et al. 2009). Activities of MRP1 and MRP5 in ARPE-19 cells were also confirmed by the calcein-AM and CDCF efflux tests (Mannermaa et al. 2009), respectively. In PRE of porcine, permeability of known MRP substrate fluorescein in the retina-to-choroid direction across was reported to be higher 11.3-folds than that in opposite direction. The transport of fluorescein in the retina-to-choroid direction was blocked by probenecid, with the result that permeability was equalized in both directions, which was in line with expression of Mrps (Steuer et al. 2005).
LC-MS/LC-MS analysis demonstrated high expression of MRP1 in primary human PER cells, ARPE-19 cells, and oBRB tissue of porcine. High levels of MRP5 protein were only detected in primary human PER cells but not ARPE-19 cells nor oBRB tissue of porcine (Pelkonen et al. 2017; Zhang et al. 2017a). Mrp1 and Mrp4 were also detected in the basal membrane of the mouse RPE; in accordance, the efflux of [3H]-zidovudine from the retina might be inhibited by MRP inhibitor MK571 (Chapy et al. 2016). Moreover, Mrp4 was reported to be also uniformly expressed in the physiologically developing retinal vasculature of the neonatal mouse including the capillaries and large vessels (Tagami et al. 2009). RCECs of the adult mouse also showed expression of Mrp3, Mrp4, and Mrp6 mRNA (Tachikawa et al. 2008).
Generally, MRPs efflux a wide variety of endogenous compounds and therapeutic drugs. For example, MRP4 mediates cellular efflux of both cAMP and prostaglandin E2 (PGE2), indicating involvement of MRP4 in angiogenesis. In human RCECs, VEGF was reported to dose-dependently decrease expression of MRP4 mRNA and protein. Abcc4 knockdown using RNAi enhanced cell migration and attenuated serum starvation-induced cell apoptosis, assembled and aggregated into a massive tube-like structure (Tagami et al. 2010). Similarly, Abcc4 deficiency did not cause overt abnormalities in the development of the retinal vasculature of mice, but retinal vascular development was suppressed in response to forskolin administration. The forskolin-treated Abcc4−/− mice showed an increased number of Ki67-positive and cleaved caspase 3-positive RCECs and significant decreases in the amount of pericyte coverage and number of empty sleeves. Moreover, following exposure of hyperoxia, the Abcc4−/− mice showed a significant increase in the unvascularized retinal area. These results indicate that MRP4 may have its protective roles in the retinal vascular development by regulating the intracellular cAMP level (Matsumiya et al. 2012).
Many kinds of drug transporters, such as OATPs, OATs, P-GP, BCRP, and MRPs, are expressed at the BRB, mediating transport of therapeutic drugs across the BRB. These transporters often overlap substrate specificity. Thus net effect is attributed to their interplay. For example, the elimination of p-aminohippuric acid, benzylpenicillin, and 6-Mercaptopurine from vitreous humor is mediated by several transporters. P-aminohippuric acid, benzylpenicillin, and 6-Mercaptopurine are substrates of MRP4 (Uchida et al. 2007) and OAT3 (Hosoya et al. 2009), indicating that OAT3 and MRP4 in common contribute to the efflux transport of PAH, PCG, and 6-MP from the retina across the BRB. Another example is verapamil. Although P-GP is also highly expressed at the iBRB, P-GP has little involvement in the retinal uptake of verapamil, which is partly attributed to the existence of influx transporters (Chapy et al. 2015; Fujii et al. 2014). Transport of E17βG and dehydroepiandrosterone sulfate across BRB may be attributed to the combined effects of OATP1A4, OAT3, and MRP4 (Hosoya et al. 2011).
Alterations in BRB Function Under Disease Status and Clinic Significances
The BRB and Diabetic Retinopathy
Diabetic retinopathy, a complication of diabetes, is the leading cause of acquired blindness, which is involved in functional and structural changes of the BRB. Microvascular disorders are often diabetic retinopathy although other cells such as RPE cells are affected by diabetes. The vascular changes are clearly linked to the loss of visual acuity and clinical alterations in the retinal vasculature. Early vascular changes include leukostasis, aggregation of platelets, alteration in blood flow, degeneration of pericytes, and basement membrane thickening. The increased retinal vascular permeability associated with diabetic retinopathy may result from alterations in the tight junction and adherens junction complexes or from endothelial cell death. Macular edema is closely associated with the loss of visual acuity in diabetic retinopathy, which is attributed to the increased BRB permeability (Frey and Antonetti 2011; Arden and Sivaprasad 2011).
Inflammation and Diabetic Retinopathy
Retinal inflammation plays a major role in the pathogenesis of diabetic retinopathy. Hyperglycemia is considered as a pro-inflammatory environment via releasing some inflammatory cytokines [such as tumor necrosis factor-α (TNF-α), interleukin -1β (IL-1β), and interleukin-6 (IL-6)] and chemokine ligands (such as CCL2, CCL5, and CCL12)].These cytokines and chemokines induce the disorganization and redistribution of junctional proteins in microvasculature, leukocyte activation, release of intercellular adhesion molecule-1 (ICAM-1), and other cell adhesion molecules, in turn, increasing vascular permeability and further exacerbating the inflammatory milieu of the retina. For example, TNF-α downregulated expression of tight junction proteins (such as claudin-5 and ZO-1). Hyperglycemia upregulated levels of ICAM-1 and high mobility group box-1 (HMGB-1), which was in line with increases in leaky of Evans blue in rat retina (Ran et al. 2016). Streptozotocin (STZ)-induced diabetes significantly upregulated expression of retinal CCL2 and increased monocyte trafficking in rats. In accordance with this, intraocular injection of the CCL2 into nondiabetic rats also increased retinal monocyte trafficking, indicating the ability of the chemokine to attract monocytes/macrophages into retinal tissue. High glucose exposure was also reported to upregulate CCL2 expression in human RCECs. Contrarily, CCL2 deficiency prevented the increase in vascular permeability and monocyte trafficking in the retinas of diabetic rats (Rangasamy et al. 2014). Moreover, diabetes also significantly increased levels of retinal cathepsin D and CCL2, accompanied by increases in vascular permeability to albumin in the retinas of mice. Patients with diabetic macular edema also showed increases in levels of serum cathepsin D protein. In human RCECs, cathepsin D was reported to disrupt endothelial junctional barrier via increasing RhoA/ROCK cell contractility (Monickaraj et al. 2016). Significant increases in expression of CXC chemokine platelet factor-4 (PF-4/ CXCL4) were also found in both vitreous fluid from patients with proliferative diabetic retinopathy and the retinas of diabetic rats. In human RCECs, it was found that PF-4/CXCL4, an angiostatic chemokine, inhibited VEGF-induced signal transduction and inhibited cell migration (Nawaz et al. 2013). Platelet factor-4 variant (PF-4var/CXCL4L1) was also reported to inhibit VEGF-mediated hyperpermeability. In accordance, intravitreal PF-4var/CXCL4L1 or bevacizumab attenuated diabetes-induced BRB breakdown in rats, leading to decreases in vascular leakage by approximately 70% and 73%, respectively, compared with phosphate buffer saline injection. These effects were also associated with upregulation of occludin and vascular endothelial-cadherin and downregulation of hypoxia-inducible factor (HIF)-1α, VEGF, TNF-α, receptor for advanced glycation end products (RAGE), and caspase-3 (Abu El-Asrar et al. 2016). Nuclear factor-κB (NF-κB) pathway is also involved in the pathogenesis of diabetic retinopathy. It was reported that administration of NF-κB inhibitor dehydroxymethylepoxyquinomicin suppressed retinal adherent leukocytes, expression of inflammatory molecules (ICAM-1 and VEGF), and renin angiotensin system (RAS)-related molecules such as angiotensinogen and angiotensin-II receptor 1 (AT1-R) induced by diabetes (Nagai et al. 2007).
Vascular Endothelial Growth Factor (VEGF) and Diabetic Retinopathy
VEGF, a pro-angiogenic growth factor secreted preferentially from the basal surface of the RPE and Müller cells, modulates and maintains the extracellular space in and around the Bruch’s membrane and modulates the growth/density of endothelial cells in the choriocapillaris (Kay et al. 2013; Le 2017). Diabetic retinopathy is often associated with the increases in retinal VEGF levels (Kay et al. 2013; Lin et al. 2011; Nawaz et al. 2013). Animal and clinical trials have demonstrated that diabetes-induced increases in retinal VEGF levels are coincided with BRB breakdown (Le 2017).VEGF is involved in the pathogenesis of diabetic retinopathy via inducing retinal ICAM-1 expression, vascular permeability, leukostasis, BRB breakdown, or vascular lesions (Le 2017). In normal rats, it was found that VEGF164/VEGF-A was at least twice as potent as VEGF120 at inducing ICAM-1-mediated retinal leukostasis and BRB breakdown following intravitreous injections (Ishida et al. 2003). Moreover, blockade of endogenous VEGF-A with EYE001 significantly suppressed diabetes-induced retinal leukostasis and BRB breakdown, indicating that VEGF-A is an important isoform in the pathogenesis of diabetic retinopathy. Roles of VGEF in pathogenesis of diabetic retinopathy were confirmed by conditional VEGF knockout mice. It was found that conditional VEGF knockout significantly reduced leukostasis, expression of inflammatory biomarkers, depletion of tight junction proteins, numbers of acellular capillaries, and vascular leakage in the retina of diabetic mice (Wang et al. 2010). In general, VEGF shows its pathophysiologic effects in diabetic retinopathy in two ways (Deissler et al. 2014). One, VEGF increases vascular permeability and causes fluid extravasation and retinal edema via affecting endothelial tight junctions. Second, VEGF causes leukocyte aggregation in the retinal microvasculature, resulting in local cytokine production and inflammatory cell migration through the endothelium, both of which contribute to BRB breakdown. Anti-VEGF therapy with aflibercept, bevacizumab, or ranibizumab has been a hallmark strategy to prevent diabetic retinopathy in the clinic (Virgili et al. 2017), demonstrating efficiency of anti-VEGF drugs on the improvement of vision in people with diabetic macular edema. Some drugs such as brimonidine, memantine (Kusari et al. 2007, 2010), and corticosteroids (Edelman et al. 2005; Wang et al. 2008) also attenuate diabetic macular edema and retinopathy partly via affecting expression of retinal VEGF expression or downstream signal proteins of the VEGF receptor.
Hyperosmolar Stress and Diabetic Retinopathy
Diabetic retinopathy is associated with osmotic stress resulting from hyperglycemia and intracellular sorbitol accumulation. In vitro, it was reported that high glucose increased expression of the water channel aquaporin-1 (AQP1) and cyclooxygenase (COX)-2, increased activity of the osmolarity-sensitive transcription factor tonicity enhancer-binding protein (TonEBP), and enhanced endothelial migration and tubulization. These alterations by high glucose were reversed by AQP1 and TonEBP siRNA, indicating that high glucose-induced hyperosmolarity promotes angiogenesis and retinopathy via activating TonEBP (Madonna et al. 2016). It is generally accepted that glucose is reduced to sorbitol by aldose reductase, and sorbitol is eventually metabolized to fructose by sorbitol dehydrogenase. Intracellular sorbitol accumulation induces osmotic damage of the retinal vascular cells and RPE cells, loss of pericytes, basement membrane thickness, and oxidative stress, all of which contribute to iBRB rupture during diabetic retinopathy (Lorenzi 2007). RPE cells subjected to hyperosmolar stress also underwent osmoadaptative responses such as shrinkage of RPE cells, alterations in AQP expression, increases in VEGF expression, placental growth factor, monocyte chemoattractant protein-1, and basic fibroblast growth factor (Willermain et al. 2018), further impairing RPE function. Hyperosmolar conditions also increases activity and expression of TAUT in RPE cells, leading to increases in the uptake of taurine (El-Sherbeny et al. 2004). Moreover, hyperosmolar condition also increased expression of aldose reductase (Winges et al. 2016), in turn, further enhancing sorbitol accumulation.
Plasma Kallikrein-Kinin System (PKKs) and Diabetic Retinopathy
Accumulating evidences have demonstrated contribution of plasma kallikrein-kinin system (PKKs) to diabetic retinopathy. It was reported that levels of plasma prekallikrein and plasma kallikrein in vitreous from subjects with diabetic macular edema were increased to 2.0-fold and 11.0-fold, respectively, of those with a macular hole (Kita et al. 2015). In normal rats, intraocular injection of bradykinin dose-dependently might increase plasma extravasation, which was inhibited by bradykinin receptor 2 antagonist Hoe140 (Abdouh et al. 2008; Phipps et al. 2009). Similarly, the bradykinin receptor 2 agonist bradykinin might vasodilate retinal vessels in a concentration-dependent manner, which was completely blocked by Hoe140. But bradykinin receptor 1 agonist des-Arg9-bradykinin had no this effect. However, in diabetic rats, des-Arg9-bradykinin could also produce a concentration-dependent vasodilatation and was also inhibited by the bradykinin receptor 1 receptor antagonist des-Arg10-Hoe140 (Abdouh et al. 2003), which may be explained by these findings that the bradykinin receptor 1 in the retina is only minimally expressed under physiological conditions, but diabetes significantly upregulated expression of bradykinin receptor 1 without affecting bradykinin receptor 2 (Abdouh et al. 2008; Kita et al. 2015; Pouliot et al. 2012). Animal experiments have been demonstrated that diabetes-induced retinal vascular permeability is attenuated by plasma kallikrein inhibitor or bradykinin receptor antagonists (Abdouh et al. 2008; Catanzaro et al. 2012; Clermont et al. 2011; Pouliot et al. 2012) or plasma prekallikrein-gene deficiency (Kita et al. 2015). FOV-2304, a non-peptide selective bradykinin receptor 1 antagonist, also blocked retinal vascular permeability, inhibited leukocyte adhesion, and abolished the retinal mRNA expression of several inflammatory mediators in diabetic rats (Pruneau et al. 2010). These results indicate that the blockade of the PKKs is a promising therapeutic strategy for diabetic retinopathy.
Interestingly, the response of plasma kallikrein following injection into vitreous of diabetic rats was blocked by bradykinin receptor antagonist but not by bevacizumab. In mice, administration of VEGF receptor 2 antibody DC101 did not affect bradykinin-induced retinal thickening. Moreover, although increased VEGF levels were also observed in diabetic macular edema vitreous, no correlation of plasma kallikrein level and VEGF level was found. These results indicate that diabetic macular edema induced by PKKs is VEGF-independent (Kita et al. 2015). However, a report showed that plasma prekallikrein-gene deficiency partly decreased the VEGF-induced retinal vascular permeability and retinal thickening or TNFα-induced retinal thickening in mice. Systemic administration of plasma kallikrein inhibitor VA999272 also reduced VEGF-induced retinal thickening in both mice and rats, indicating that plasma kallikrein is required for the full effects of VEGF on retinal vascular permeability and retinal thickening (Clermont et al. 2016). Retinal hemorrhages also occur under diabetic condition. Intravitreal injection of autologous blood was reported to induce retinal vascular permeability and retinal leukostasis, which were ameliorated by plasma kallikrein inhibition. Intravitreal injections of exogenous plasma kallikrein also induced retinal vascular permeability, leukostasis, and retinal hemorrhage, indicating that retinal hemorrhage increases retinal vascular permeability and leukostasis partly via plasma kallikrein (Liu et al. 2013).
Real mechanisms that PKKs induce retinal vascular permeability and retinal thickening are not fully understood. Bradykinin was reported to evoke intracellular Ca2+ transients in primary human RPE, which was enhanced by pretreatment with TNF-α and/or IL-1β but inhibited by fasitibant chloride, a selective bradykinin receptor 2 antagonist. TNF-α and/or IL-1β enhanced bradykinin-induced Ca2+ response via increasing expression of both bradykinin receptor 2 and COX-2, as well as secretion of prostaglandin E1 and E2 into the extracellular medium (Catalioto et al. 2015). Intravitreal injections of either plasma kallikrein or collagenase, but not bradykinin, also induced retinal hemorrhage in rats. Proteomic analysis showed that plasma kallikrein increased collagen degradation in pericyte-conditioned medium and purified type IV collagen, indicating that plasma kallikrein leads to breakdown of BRB due to its collagenase-like activity (Liu et al. 2013). However, intravitreal injection of bradykinin also increased retinal vascular permeability, which might be prevented by both vasoinhibins and Hoe-140. In ARPE-19, bradykinin also increased permeability of the BRB and decreased endothelial monolayer resistance, which was attributed to redistribution of actin cytoskeleton, subsequently reorganization of tight and adherens junctions. These effects were reversed by NO synthase inhibitor L-NAME, vasoinhibins, and N-acetyl cysteine (Arredondo Zamarripa et al. 2014). Bradykinin could also inhibit TGF-β1-stimulated ARPE-19 cell proliferation, collagen I, fibronectin, and MMP-2 secretion as well as Akt phosphorylation via activating bradykinin receptor 2 (Cai et al. 2016). In normal rats, bradykinin-induced vasodilatation was involved in intracellular Ca2+ mobilization and products of the cyclooxygenase-2 (COX-2) pathway, but in STZ-diabetic rats, the vasodilatation in response to des-Arg9-bradykinin was involved in both calcium influx and intracellular calcium mobilization, which was blocked by GdCl3, 2,5-di-t-butylhydroquinone, and cADP ribose (Abdouh et al. 2003).
Renin Angiotensin System (RAS) and Diabetic Retinopathy
Clinical and experimental studies have demonstrated that abnormalities of the renin angiotensin system (RAS) may play a significant role in the progression of the diabetic retinopathy, presumably through local changes in the blood flow and the production of angiotensin II (Ang II) (Ola et al. 2017; Phipps et al. 2012). Besides circulating RAS, RAS locally exists in the retina. Animal experiments have demonstrated that diabetes increases components of RAS including prorenin, angiotensinogen, Ang II, angiotensin-converting enzyme (ACE), angiotensin-converting enzyme 2 (ACE2), and Ang II receptor 1 (AT1R) in the retina (Ola et al. 2017; Nagai et al. 2007; Verma et al. 2012). Several reports have shown that vitreous pool of proliferative diabetic retinopathy patients also exhibited an increased level of Ang II and other RAS components compared to nondiabetic subjects (Ola et al. 2017; Phipps et al. 2012). Rodent experiments have aslo revealed that ACE inhibitors and AT1R blockers could reduce diabetes-induced retinal microvascular damage with reductions in vascular leakage, decreased formation of acellular capillaries, and decreased expression of angiogenic factors such as VEGF (Mori et al. 2002; Wilkinson-Berka et al. 2007; Zhang et al. 2007). ACE inhibition or AT1R blockade also decreased diabetes-induced retinal leukostasis and upregulation of adhesion molecules (Mori et al. 2002; Chen et al. 2006; Silva et al. 2007), but AT2R blockade did not show this effect (Nagai et al. 2007).
AT1R antagonist candesartan could decrease diabetes-induced or Ang II-stimulated retinal vascular permeability without affecting retinal vascular permeability in normal rats although candesartan decreased hypertension both in diabetic and normal rats (Phipps et al. 2009). Ang II infusion also significantly increased expression of plasma kallikrein in rat retina. Bradykinin receptor 2 antagonist Hoe140 and plasma kallikrein inhibitor (ASP-440) might attenuate Ang II-induced retinal vascular permeability, indicating that activation of AT1R increases retinal vascular permeability partly via PKKs (Phipps et al. 2009). Clinical trial (Mauer et al. 2009) demonstrated effects of ACE inhibitor enalapril and the AT1R inhibitor losartan on progression of diabetic retinopathy to a similar extent, whose odds of retinopathy progression were reduced by 65% with enalapril treatment and 70% with treatment, respectively. Data from 5321 diabetic patients demonstrated that treatment with candesartan significantly reduced the incidence of development of diabetic retinopathy in type 1 diabetics without affecting its progression (Chaturvedi et al. 2008). But in type 2 diabetes, treatment with candesartan reduced the development of retinopathy and even resulted in a 34% regression of retinopathy compared with the control group (Sjølie et al. 2008). Taken together, these results suggest that RAS blockade may be useful for slowing progression and decreasing the severity of diabetic retinopathy.
ACE2 is expressed in the retina, which converts the vasoconstrictive and pro-inflammatory peptide Ang II into the vasodilatory and anti-inflammatory peptide angiotensin-(1-7) [Ang(1-7)], which exhibits its effects primarily through the receptor Mas. A report showed that STZ-induced diabetes significantly increased mRNA levels of the vasodeleterious axis of the RAS (angiotensinogen, renin, pro/renin receptor, ACE, and AT1R) in the retina of eNOS−/− mice, leading to increases in ratios of ACE/ACE2 and AT1R/Mas mRNA levels by approximately tenfolds and threefolds, respectively (Verma et al. 2012). The increases in vascular permeability, infiltrating CD45-positive macrophages, and activation of CD11b-positive microglial cells as well as formation of acellular capillaries in the retina of diabetic mice were attenuated by intraocular administration of adeno-associated virus-ACE2 or Ang-(1-7) vector (Verma et al. 2012). Intraocular administration of adeno-associated virus-ACE2 vector was also reported to attenuate the increase of acellular capillaries and leaky of macrophages/microglia in the retina of STZ-induced diabetic mice (Dominguez et al. 2016). Similarly, in STZ-induced diabetic rat retinas, the increased numbers of acellular capillaries were almost completely prevented by gene delivery of either ACE2 or Ang-(1-7) (Verma et al. 2012).
Diabetes is often associated with hypertension, and patients with hypertension are at a greater risk of developing diabetic complications including retinopathy, inferring that therapeutic effects of RAS blockade on diabetic retinopathy are also attributed to the reduction in blood pressure. Clinical trials have demonstrated no different effects on progression of retinopathy between captopril and atenolol treatments or between enalapril and nisoldipine in type 2 diabetes (Phipps et al. 2012). In consistence, diabetic spontaneously hypertensive rats (SHR) showed significantly higher number of ED1/microglial-positive cells and the expression of ICAM-1 in the retina than in control SHR. The SHR also possessed higher NF-κB p65 levels than Wistar Kyoto (WKY) rats. These abnormalities in diabetic SHR rats were completely prevented by losartan or complex of hydralazine+ reserpine +hydrochlorothiazide) (Silva et al. 2007). Similarly, SHR rats showed higher number of BrdU-positive retinal cells than WKY rats. A significant reduction in cell replication was found only in diabetic SHR, and this reduction was associated with enhanced p27Kip1, fibronectin, and VEGF retinal expressions and greater blood-retinal barrier breakdown (Lopes et al. 2008). These results indicate contribution of concomitant diabetes and hypertension to diabetic retinopathy. However, a rat experiment showed that ramipril, losartan, and nifedipine showed similar antihypertensive efficiencies, but ramipril and losartan showed stronger decreases in retinal leukostasis and expression of ICAM-1 induced by diabetes than nifedipine, indicating that their effects seem to be partly independent of blood pressure and to be associated with a decrease in ICAM-1 gene expression (Chen et al. 2006), which need further investigation.
It is worth noting that systemic Ang II reduces RPE renin production via stimulating AT1R and that systematic application of ACE inhibitors strongly activates local RAS in the retina, indicating that the systemic treatment with RAS blockade for retinal degeneration and systemic disease may cause side effects detrimental, or perhaps beneficial, to retinal disease (Strauß 2016) via affecting retinal RAS.
Alterations in Transport under Diabetic Status
Alterations in Glucose Transport
The retina and RPE are highly metabolically active tissues with substantial demands for glucose. In STZ-induced diabetic rats, Glut1 expression in retinal microvessels but not in RPE was decreased by approximately 50%, without altering the retina microvascular density, indicating that the fraction of the glucose entering the retina of diabetes is likely to be greater across the RPE than across the retinal vasculature (Badr et al. 2000). Decreases in expressions of Glut1 proteins not mRNA were also found in the retinas of diabetic GK rats and alloxan-treated rabbits (Fernandes et al. 2004). In TR-iBRB cells, high glucose exposure also decreased expression of Glut1 protein. Moreover, higher content of high molecular weight ubiquitin conjugates was found in both membrane fractions of diabetic retinas and endothelial cells treated with high glucose exposure, indicating that the decreased expression of Glut1 protein may be associated with its increased degradation by a ubiquitin-dependent mechanism (Fernandes et al. 2004). Importantly, although expressions of Glut1 on the luminal plasma membrane of the RCECs and in homogenates of the whole retina in diabetic rats were significantly decreased (about 55% and 36% of control rats, respectively) (Tang et al. 2000), retinal glucose levels were significantly elevated by fourfold to sixfold compared with the nondiabetic rats. It was also found that glucose influx increased with increasing plasma glucose in both diabetic and normal rats (Puchowicz et al. 2004). Dehydroascorbic acid transport across BRB is mediated by GLUT1; it was consistent with the decreases in Glut1 expression that [14C] dehydroascorbic acid transport across the BRB in STZ-induced diabetic rats was less than 35% of normal rats, inferring that hyperglycemia reduces the supply of vitamin C to the retina (Minamizono et al. 2006).
Alterations in H2O Transport
BRB maintains fluid homeostasis in the retina by removing fluid out of the retina via the retinal vasculature. Besides the ionic and osmotic gradients, transport H2O across BRB is mainly mediated by aquaporins (AQPs). Thirteen AQPs have been identified in mammals, most of which are expressed in the retina. Diabetes significantly altered the expression and distribution of retinal AQPs in the retina (Xia and Rizzolo 2017) in a species-dependent manner. For example, diabetes upregulated AQP5, 9, 11, and 12 but downregulated AQP0 in the RPE of rats (Hollborn et al. 2011). Diabetic retinopathy is often associated with cellular stressors (such as hypoxia and oxidative stress). In primary cultures of human RPE, it was found that exposure of stressors (chemical hypoxia, oxidative stress, VEGF, and high glucose) upregulated expression of AQP9 (Hollborn et al. 2012). AQP9, an aquaglyceroporin, is not only permeable to water but also to non-charged solutes, such as lactate. AQP9 expression is considered to be required for L-lactate to maintain retinal neuronal survival (Akashi et al. 2015), indicating that the upregulation of AQP9 in RPE cells may prevent lactic acidosis and subretinal edema under ischemic and oxidative stress conditions (Akashi et al. 2015; Hollborn et al. 2012). Hyperosmolarity could alter AQP expression. It was reported that 10 min exposure to an osmolar stress (400 mM sucrose or 200 mM NaCl) significantly decreased AQP4 expression in ARPE-19 cells (Willermain et al. 2014b). Under normal condition, expression of AQP1 in the rat retina is at minimal levels, diabetes increased expression of AQP1, and hypertension also enhanced expression of AQP4 under diabetic conditions, which were reversed by valsartan and metoprolol (Qin et al. 2012). Furthermore, intravitreal injection of VEGF also increased AQP4 expression in the retina of normal and diabetic rats. TGN-020, a selective AQP4 inhibitor, suppressed VEGF-induced enlargement of Müller cells and increases in intracellular levels of NO. Thus, the authors gave a conclusion that VEGF induced Müller cell swelling through the formation of NO and AQP4 channels (Kida et al. 2017). Importantly, although diabetes or administration of VEGF increased AQP4 expression in the retina of rats (Cui et al. 2012; Kida et al. 2017), AQP4 downregulation exacerbated diabetic retinopathy and aggravated inflammatory response (Cui et al. 2012). Thus, the cellular mechanisms mediating expression of AQPs by diabetes and their clinic significances need further investigation.
Alterations in Active Transport
Tight junctions maintain the ion gradients essential for transcellular transport mechanisms to function. Diabetes can directly decrease the ion gradients via decreasing Na+/K+-ATPase activity, contributing to retinal edema (Xia and Rizzolo 2017). Transport of L-lactic acid across the RPE is dependent on a pH gradient. Intracellular pH is regulated by Na+/HCO3− cotransporters. The Na+-dependent movement of HCO3− is actively driven by the physiologic Na+ gradient established by the Na+/K+-ATPase. Thus, inadequate activity of the Na+/K+-ATPase impaired transport of L-lactic acid. Taurine is the most abundant free amino acid in the retina, which functions as an antioxidant and may attenuate the spread of cell death in RPE cells. Diabetes and high glucose downregulate expression and activity of retinal TAUT (Zeng et al. 2010; Lee and Kang 2013) partly due to overexpression of aldose reductase (Nakashima et al. 2005b; Stevens et al. 1999). Taurine deficiency can lead to severe damage to photoreceptors (Ripps and Shen 2012). Thus, decreases in transport of retinal taurine by diabetes and subsequent taurine depletion from the retina could contribute to visual impairment.
Alterations in ABC Transporters
Some diseases may affect expression of retinal P-GP and BCRP. A report showed that 24-week STZ-induced diabetic mice demonstrated lower expression of retinal P-GP and BCRP, with the breakdown of the iBRB, which might be linked to the pathogenesis of early diabetic retinopathy (Li et al. 2017). In D407 cells, it was reported that high glucose exposure significantly decreased P-GP expression of both mRNA and protein levels, attenuated P-GP activity, and increased expressions of both mRNA and protein of inducible nitrate oxide synthase (iNOS). High glucose exposure also decreased expression of pregnane X receptor (PXR) mRNA. These alterations by high glucose were partially blocked by a selective iNOS inhibitor, whose effects were antagonized with the addition of L-arginine, a substrate for NO synthesis. These results demonstrate roles of iNOS induction in decreased P-GP expression and function at the human oBRB under hyperglycemic conditions (Zhang et al. 2012).
Diabetes is often associated with oxidative stress, which is a contributing factor to RPE cell dysfunction in diabetic retinopathy and age-related macular degeneration. In general, cellular antioxidants in RPE also play a critical role in combating oxidative stress; among the cellular antioxidant constituents, reduced glutathione (GSH) plays a significant role in cellular defense against pro-oxidants. MRP1, expressed in PRE, regulates levels of cellular GSH via efflux GSH. In ARPE-19 cells, it was found that MRP inhibitors (MK571 and sulfinpyrazone) significantly decreased GSH efflux by 50%. MRP1 silencing using MRP1-specific siRNA also caused a significant 60% reduction in GSH efflux. Moreover, the downregulation of MRP1 developed to resistance to H2O2-induced cell death. Contrarily, overexpression of MRP1 was susceptible to H2O2-induced cell death, which was consistent with lower cellular GSH levels in MRP1 overexpressed cells (Sreekumar et al. 2012). These results indicate that MRP1 shows its roles in oxidant-induced cell death via efflux GSH and that MRP1 may be a potential therapeutic target in pathological retinal degenerative disorders linked to oxidative stress.
Ischemia-Reperfusion/Hypoxia and BRB Breakdown
Retinal ischemia-reperfusion injury is associated with many ocular diseases such as acute retinal vein occlusion, diabetic retinopathy, and glaucoma. Oxidative injury is one of the complications after retinal ischemia-reperfusion injuries, accompanied by retinal swelling, neuronal cell death, and glial cell activation, due to oxidation stress and release of inflammatory cytokines and chemokines (Kaur et al. 2008). Ischemia-reperfusion was reported to significantly increase retinal mRNA expression of several pro-inflammatory cytokines such as IL-1β (3.2-fold), IL-6 (4.2-folds), TNF-α (5.6-fold), and CCL2 (116.9-folds) in the retinas, accompanied by significant increases in leaky of Evans Blue (3.8-fold) and number of CD45-positive cells (Gonçalves et al. 2016). The roles of TNF-α in cytokine-induced BRB breakdown were demonstrated in TNF-α-deficient mice. It was found that compared with wild-type mice, TNF-α deficiency significantly reduced leukocyte accumulation in retinal vessels by 80%, 100%, and 100% after intravitreous injection of VEGF, IL-1β, and platelet-activating factor (PAF), respectively. The absences of TNF-α significantly reduced retinal vascular permeability induced by injection of PAF, but not VEGF nor IL-1β. Moreover, TNF-α deficiency significantly reduced leukostasis and mild reduction in vascular leakage, but did not affect hypoxia-induced retinal neovascularization (Vinores et al. 2007). Ischemia-reperfusion was also reported to induce occludin Ser490 phosphorylation and ubiquitination, distribution of specific tight junction proteins, and activation and phosphorylation of VEGF receptor-2 (VEGFR-2) at tyrosine 1175, all of which contribute to the increased vascular permeability. Intravitreal injection of bevacizumab prevented VEGFR-2 activation, occludin phosphorylation, and vascular permeability induced by ischemia-reperfusion (Muthusamy et al. 2014). Furthermore, ischemia-reperfusion also altered distribution of glial fibrillary acidic protein (GFAP) and AQP4 in the retina. In normal rats, expression of GFAP was confined to astrocytes in ganglion layer; ischemia-reperfusion markedly increased expression of GFPA, whose expression was not just confined to the ganglion layer but also found in the Muller cell processes. AQP4 staining is discontinuous along the blood vessels and appeared weak in normal rats. AQP4 immunoreactivity surrounding retinal vessels became more intense in the ischemia-reperfusion retina (Li et al. 2011). The increased expression of AQP4 and alterations in its distribution led to increases in transport of water from blood vessels to the retinal tissues, contributing to edema formation in hypoxic conditions (Kaur et al. 2008; Li et al. 2011). Moreover, under normal conditions, Müller cells do not express GFAP, but many insults including hypoxia to the retina lead to a rapid upregulation of GFAP in these cells. Upregulation of GFAP in stressed Müller cells is often associated with an upregulation of heat shock proteins and alterations in cytoskeletal protein synthesis, swelling of Müller/astrocytes cells, and vascular leakage in hypoxic condition (Kaur et al. 2008). Caveolae-mediated vesicular transport is a system of transcellular transport of macromolecules in the BRB. Caveolin-1 is a principal structural component of caveolae. Under normal condition, expression of caveolin-1 in the RCECs contributes to the integrity of the BRB. Hypoxia increased expressions of caveolin-1 mRNA and protein and caveolin-1 siRNA significantly reduced hypoxia-induced albumin leakage in the retinas and neovascularization, which was in line with reduction of caveolin-1 mRNA and protein, indicating that overexpression of caveolin-1 in the retina is associated with BRB breakdown and neovascularization formation (Tian et al. 2012).
BRB breakdown by hypoxia is linked to induction of hypoxia-inducible factor (HIF)-1 in Müller cells. In vitro, it was found that unlikely to wild-type mice, hypoxia did not induce expression of abundance of VEGF in Müller cells from conditional Hif-1α knockout mice, which was consistent with decreases in expression of HIF-1, indicating that HIF-1α in Müller cells is a major mediator of ischemia-/hypoxia-induced VEGF overproduction in the retina. In vivo, hypoxia induces expression of HIF-1, VEGF, ICAM-1, vascular leakage, and retinal neovascularization in mice, which may be attenuated by conditional HIF-1α deficiency. Moreover, HIF-1α deficiency also reverses the diabetes-induced inflammation, vascular leakage, and leucostasis (Lin et al. 2011), indicating that Müller cell-derived HIF-1α is therefore a promising therapeutic target for diabetic retinopathy.
Acknowledgments
The project was in part supported by the National Natural Science Foundation of China (No. 81872930; 81573490) and “Double First-Class” University project (No. CPU2018GY22).
Contributor Information
Xiaodong Liu, Email: xdliu@cpu.edu.cn.
Guoyu Pan, Email: gypan@simm.ac.cn.
Li Liu, Email: liulee@cpu.edu.cn.
Xiaodong Liu, Email: xdliu@cpu.edu.cn.
References
- Abdouh M, Khanjari A, Abdelazziz N, Ongali B, Couture R, Hasséssian HM. Early upregulation of kinin B1 receptors in retinal microvessels of the streptozotocin-diabetic rat. Br J Pharmacol. 2003;140:33–40. doi: 10.1038/sj.bjp.0705210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdouh M, Talbot S, Couture R, Hasséssian HM. Retinal plasma extravasation in streptozotocin-diabetic rats mediated by kinin B(1) and B(2) receptors. Br J Pharmacol. 2008;154:136–143. doi: 10.1038/bjp.2008.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abu El-Asrar AM, Mohammad G, Nawaz MI, Abdelsaid M, Siddiquei MM, Alam K, et al. Variant (PF-4var)/CXCL4L1 inhibits diabetes-induced blood-retinal barrier breakdown. Invest Ophthalmol Vis Sci. 2016;56:1956–1964. doi: 10.1167/iovs.14-16144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abukawa H, Tomi M, Kiyokawa J, Hori S, Kondo T, Terasaki T, et al. Modulation of retinal capillary endothelial cells by Müller glial cell-derived factors. Mol Vis. 2009;15:451–457. [PMC free article] [PubMed] [Google Scholar]
- Akanuma SI, Hirose S, Tachikawa M, Hosoya KI. Localization of organic anion transporting polypeptide (Oatp) 1a4 and Oatp1c1 at the rat blood-retinal barrier. Fluids Barriers CNS. 2013;10:29. doi: 10.1186/2045-8118-10-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akanuma S, Soutome T, Hisada E, Tachikawa M, Kubo Y, Hosoya K. Na+-independent nucleoside transporters regulate adenosine and hypoxanthine levels in Müller cells and the inner blood-retinal barrier. Invest Ophthalmol Vis Sci. 2013;54:1469–1477. doi: 10.1167/iovs.12-10905. [DOI] [PubMed] [Google Scholar]
- Akashi A, Miki A, Kanamori A, Nakamura M. Aquaporin 9 expression is required for L-lactate to maintain retinal neuronal survival. Neurosci Lett. 2015;589:185–190. doi: 10.1016/j.neulet.2015.01.063. [DOI] [PubMed] [Google Scholar]
- Arden GB, Sivaprasad S. Hypoxia and oxidative stress in the causation of diabetic retinopathy. Curr Diabetes Rev. 2011;7:291–304. doi: 10.2174/157339911797415620. [DOI] [PubMed] [Google Scholar]
- Arredondo Zamarripa D, Díaz-Lezama N, Meléndez García R, Chávez Balderas J, Adán N, Ledesma-Colunga MG, et al. Vasoinhibins regulate the inner and outer blood-retinal barrier and limit retinal oxidative stress. Front Cell Neurosci. 2014;8:333. doi: 10.3389/fncel.2014.00333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asashima T, Hori S, Ohtsuki S, Tachikawa M, Watanabe M, Mukai C, et al. ATP-binding cassette transporter G2 mediates the efflux of phototoxins on the luminal membrane of retinal capillary endothelial cells. Pharm Res. 2006;23:1235–1242. doi: 10.1007/s11095-006-0067-2. [DOI] [PubMed] [Google Scholar]
- Atluri H, Talluri RS, Mitra AK. Functional activity of a large neutral amino acid transporter (LAT) in rabbit retina: a study involving the in vivo retinal uptake and vitreal pharmacokinetics of L-phenyl alanine. Int J Pharm. 2008;347:23–30. doi: 10.1016/j.ijpharm.2007.06.038. [DOI] [PubMed] [Google Scholar]
- Badr GA, Tang J, Ismail-Beigi F, Kern TS. Diabetes downregulates glut1 expression in the retina and its microvessels but not in the cerebral cortex or its microvessels. Diabetes. 2000;49:1016–1021. doi: 10.2337/diabetes.49.6.1016. [DOI] [PubMed] [Google Scholar]
- Baldwin SA, Beal PR, Yao SY, King AE, Cass CE, Young JD. The equilibrative nucleoside transporter family, SLC29. Pflugers Arch. 2004;447:735–743. doi: 10.1007/s00424-003-1103-2. [DOI] [PubMed] [Google Scholar]
- Barot M, Gokulgandhi MR, Agrahari V, Pal D, Mitra AK. Monocarboxylate transporter mediated uptake of moxifloxacin on human retinal pigmented epithelium cells. J Pharm Pharmacol. 2014;66:574–583. doi: 10.1111/jphp.12139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer M, Karch R, Tournier N, Cisternino S, Wadsak W, Hacker M, et al. Assessment of P-glycoprotein transport activity at the human blood-retina barrier with (R)-11C-verapamil PET. J Nucl Med. 2017;58:678–681. doi: 10.2967/jnumed.116.182147. [DOI] [PubMed] [Google Scholar]
- Baumann B, Sterling J, Song Y, Song D, Fruttiger M, Gillies M, et al. Conditional Müller cell ablation leads to retinal iron accumulation. Invest Ophthalmol Vis Sci. 2017;58:4223–4234. doi: 10.1167/iovs.17-21743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behzadian MA, Wang XL, Windsor LJ, Ghaly N, Caldwell RB. TGF-beta increases retinal endothelial cell permeability by increasing MMP-9: possible role of glial cells in endothelial barrier function. Invest Ophthalmol Vis Sci. 2001;42:853–859. [PubMed] [Google Scholar]
- Bergersen L, Jóhannsson E, Veruki ML, Nagelhus EA, Halestrap A, Sejersted OM, et al. Cellular and subcellular expression of monocarboxylate transporters in the pigment epithelium and retina of the rat. Neuroscience. 1999;90:319–331. doi: 10.1016/S0306-4522(98)00427-8. [DOI] [PubMed] [Google Scholar]
- Bozard BR, Ganapathy PS, Duplantier J, Mysona B, Ha Y, Roon P, et al. Molecular and biochemical characterization of folate transport proteins in retinal Müller cells. Invest Ophthalmol Vis Sci. 2010;51:3226–3235. doi: 10.1167/iovs.09-4833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai W, Wei Q, Liu Q, Ren C, Liu J, Zhang R, et al. Effect of bradykinin on TGF-β1-induced retinal pigment epithelial cell proliferation and extracellular matrix secretion. BMC Ophthalmol. 2016;16:199. doi: 10.1186/s12886-016-0373-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catalioto RM, Valenti C, Maggi CA, Giuliani S. Enhanced Ca(2+) response and stimulation of prostaglandin release by the bradykinin B2 receptor in human retinal pigment epithelial cells primed with proinflammatory cytokines. Biochem Pharmacol. 2015;97:189–202. doi: 10.1016/j.bcp.2015.07.034. [DOI] [PubMed] [Google Scholar]
- Catanzaro O, Labal E, Andornino A, Capponi JA, Di Martino I, Sirois P. Blockade of early and late retinal biochemical alterations associated with diabetes development by the selective bradykinin B1 receptor antagonist R-954. Peptides. 2012;34:349–352. doi: 10.1016/j.peptides.2012.02.008. [DOI] [PubMed] [Google Scholar]
- Chan T, Zhu L, Madigan MC, Wang K, Shen W, Gillies MC, et al. Human organic anion transporting polypeptide 1A2 (OATP1A2) mediates cellular uptake of all-trans-retinol in human retinal pigmented epithelial cells. Br J Pharmacol. 2015;172:2343–2353. doi: 10.1111/bph.13060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chancy CD, Kekuda R, Huang W, Prasad PD, Kuhnel JM, Sirotnak FM, et al. Expression and differential polarization of the reduced-folate transporter-1 and the folate receptor alpha in mammalian retinal pigment epithelium. J Biol Chem. 2000;275:20676–20684. doi: 10.1074/jbc.M002328200. [DOI] [PubMed] [Google Scholar]
- Chan-Ling T, Koina ME, McColm JR, Dahlstrom JE, Bean E, Adamson S, et al. Role of CD44+ stem cells in mural cell formation in the human choroid: evidence of vascular instability due to limited pericyte ensheathment. Invest Ophthalmol Vis Sci. 2011;52:399–410. doi: 10.1167/iovs.10-5403. [DOI] [PubMed] [Google Scholar]
- Chapy H, André P, Declèves X, Scherrmann JM, Cisternino S. A polyspecific drug/proton antiporter mediates diphenhydramine and clonidine transport at the mouse blood-retinal barrier. Br J Pharmacol. 2015;172:4714–47125. doi: 10.1111/bph.13246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapy H, Saubaméa B, Tournier N, Bourasset F, Behar-Cohen F, Declèves X, et al. Blood-brain and retinal barriers show dissimilar ABC transporter impacts and concealed effect of P-glycoprotein on a novel verapamil influx carrier. Br J Pharmacol. 2016;173:497–510. doi: 10.1111/bph.13376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaturvedi N, Porta M, Klein R, Orchard T, Fuller J, Parving HH, et al. Effect of candesartan on prevention (DIRECT-prevent 1) and progression (DIRECT-protect 1) of retinopathy in type 1 diabetes: randomised, placebo-controlled trials. Lancet. 2008;372:1394–1402. doi: 10.1016/S0140-6736(08)61412-9. [DOI] [PubMed] [Google Scholar]
- Chen P, Scicli GM, Guo M, Fenstermacher JD, Dahl D, Edwards PA, et al. Role of angiotensin II in retinal leukostasis in the diabetic rat. Exp Eye Res. 2006;83:1041–1051. doi: 10.1016/j.exer.2006.05.009. [DOI] [PubMed] [Google Scholar]
- Chow BW, Gu C. Gradual suppression of transcytosis governs functional blood-retinal barrier formation. Neuron. 2017;93:1325–1333. doi: 10.1016/j.neuron.2017.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clermont A, Chilcote TJ, Kita T, Liu J, Riva P, Sinha S, et al. Plasma kallikrein mediates retinal vascular dysfunction and induces retinal thickening in diabetic rats. Diabetes. 2011;60:1590–1598. doi: 10.2337/db10-1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clermont A, Murugesan N, Zhou Q, Kita T, Robson PA, Rushbrooke LJ, et al. Plasma kallikrein mediates vascular endothelial growth factor–induced retinal dysfunction and thickening. Invest Ophthalmol Vis Sci. 2016;57:2390–2399. doi: 10.1167/iovs.15-18272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constable PA, Lawrenson JG, Dolman DE, Arden GB, Abbott NJ. P-glycoprotein expression in human retinal pigment epithelium cell lines. Exp Eye Res. 2006;83:24–30. doi: 10.1016/j.exer.2005.10.029. [DOI] [PubMed] [Google Scholar]
- Cui B, Sun JH, Xiang FF, Liu L, Li WJ. Aquaporin 4 knockdown exacerbates streptozotocin-induced diabetic retinopathy through aggravating inflammatory response. Exp Eye Res. 2012;98:37–43. doi: 10.1016/j.exer.2012.02.013. [DOI] [PubMed] [Google Scholar]
- Cunha-Vaz J, Bernardes R, Lobo C. Blood-retinal barrier. Eur J Ophthalmol. 2011;21(Suppl 6):S3–S9. doi: 10.5301/EJO.2010.6049. [DOI] [PubMed] [Google Scholar]
- Daniele LL, Sauer B, Gallagher SM, Pugh EN, Jr, Philp NJ. Altered visual function in monocarboxylate transporter 3 (Slc16a8) knockout mice. Am J Physiol Cell Physiol. 2008;295:C451–C457. doi: 10.1152/ajpcell.00124.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Souza CF, Kalloniatis M, Christie DL, Polkinghorne PJ, McGhee CN, Acosta ML. Creatine transporter immunolocalization in aged human and detached retinas. Invest Ophthalmol Vis Sci. 2012;53:1936–1945. doi: 10.1167/iovs.11-8462. [DOI] [PubMed] [Google Scholar]
- Deissler HL, Lang GK, Lang GE. Capacity of aflibercept to counteract VEGF-stimulated abnormal behavior of retinal microvascular endothelial cells. Exp Eye Res. 2014;122:20–31. doi: 10.1016/j.exer.2014.02.024. [DOI] [PubMed] [Google Scholar]
- Dejana E, Orsenigo F, Lampugnani MG. The role of adherens junctions and VE-cadherin in the control of vascular permeability. J Cell Sci. 2008;121:2115–2122. doi: 10.1242/jcs.017897. [DOI] [PubMed] [Google Scholar]
- del Amo EM, Urtti A, Yliperttula M. Pharmacokinetic role of L-type amino acid transporters LAT1 and LAT2. Eur J Pharm Sci. 2008;35:161–174. doi: 10.1016/j.ejps.2008.06.015. [DOI] [PubMed] [Google Scholar]
- Dominguez JM, Hu P, Caballero S, Moldovan L, Verma A, Oudit GY, et al. Adeno-associated virus overexpression of angiotensin-converting enzyme-2 reverses diabetic retinopathy in type 1 diabetes in mice. Am J Pathol. 2016;186:1688–1700. doi: 10.1016/j.ajpath.2016.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelman JL, Lutz D, Castro MR. Corticosteroids inhibit GF-induced vascular leakage in a rabbit model of blood-retinal and blood-aqueous barrier breakdown. Exp Eye Res. 2005;80:249–258. doi: 10.1016/j.exer.2004.09.013. [DOI] [PubMed] [Google Scholar]
- El-Sherbeny A, Naggar H, Miyauchi S, Ola MS, Maddox DM, Martin PM, Ganapathy V, et al. Osmoregulation of taurine transporter function and expression in retinal pigment epithelial, ganglion, and Müller cells. Invest Ophthalmol Vis Sci. 2004;45:694–701. doi: 10.1167/iovs.03-0503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eshaq RS, Aldalati AMZ, Alexander JS, Harris NR. Diabetic retinopathy: breaking the barrier. Pathophysiology. 2017;24:229–241. doi: 10.1016/j.pathophys.2017.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandes R, Suzuki K, Kumagai AK. Inner blood-retinal barrier GLUT1 in long-term diabetic rats: an immunogold electron microscopic study. Invest Ophthalmol Vis Sci. 2003;44:3150–3154. doi: 10.1167/iovs.02-1284. [DOI] [PubMed] [Google Scholar]
- Fernandes A, Carvalho AL, Kumagai A, Seica R, Hosoya KI, Terasaki T, et al. Downregulation of retinal GLUT1 in diabetes by ubiquitinylation. Mol Vis. 2004;10:618–628. [PubMed] [Google Scholar]
- Frey T, Antonetti DA. Alterations to the blood-retinal barrier in diabetes: cytokines and reactive oxygen species. Antioxid Redox Signal. 2011;15:1271–1284. doi: 10.1089/ars.2011.3906. [DOI] [PubMed] [Google Scholar]
- Fujii S, Setoguchi C, Kawazu K, Hosoya K. Impact of P-glycoprotein on blood-retinal barrier permeability: comparison of blood-aqueous humor and blood-brain barrier using mdr1a knockout rats. Invest Ophthalmol Vis Sci. 2014;55:4650–4658. doi: 10.1167/iovs.13-13819. [DOI] [PubMed] [Google Scholar]
- Gao B, Wenzel A, Grimm C, Vavricka SR, Benke D, Meier PJ, et al. Localization of organic anion transport protein 2 in the apical region of rat retinal pigment epithelium. Invest Ophthalmol Vis Sci. 2002;43:510–514. [PubMed] [Google Scholar]
- Gao B, Vavricka SR, Meier PJ, Stieger B. Differential cellular expression of organic anion transporting peptides OATP1A2 and OATP2B1 in the human retina and brain: implications for carrier-mediated transport of neuropeptides and neurosteroids in the CNS. Pflugers Arch. 2015;467:1481–1493. doi: 10.1007/s00424-014-1596-x. [DOI] [PubMed] [Google Scholar]
- Gerhart DZ, Leino RL, Drewes LR. Distribution of monocarboxylate transporters MCT1 and MCT2 in rat retina. Neuroscience. 1999;92:367–375. doi: 10.1016/S0306-4522(98)00699-X. [DOI] [PubMed] [Google Scholar]
- Gnana-Prakasam JP, Reddy SK, Veeranan-Karmegam R, Smith SB, Martin PM, Ganapathy V. Polarized distribution of heme transporters in retinal pigment epithelium and their regulation in the iron-overload disease hemochromatosis. Invest Ophthalmol Vis Sci. 2011;52:9279–9286. doi: 10.1167/iovs.11-8264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonçalves A, Lin CM, Muthusamy A, Fontes-Ribeiro C, Ambróosio AF, Abcouwer SF, et al. Protective effect of a GLP-1 analog on ischemia/reperfusion induced blood–retinal barrier breakdown and inflammation. Invest Ophthalmol Vis Sci. 2016;57:2584–2592. doi: 10.1167/iovs.15-19006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han YH, Sweet DH, Hu DN, Pritchard JB. Characterization of a novel cationic drug transporter in human retinal pigment epithelial cells. J Pharmacol Exp Ther. 2001;296:450–457. [PubMed] [Google Scholar]
- Hayasaka S, Kodama T, Ohira A. Retinal risks of high-dose ornithine supplements: a review. Br J Nutr. 2011;106:801–811. doi: 10.1017/S0007114511003291. [DOI] [PubMed] [Google Scholar]
- Heller-Stilb B, van Roeyen C, Rascher K, Hartwig HG, Huth A, Seeliger MW, et al. Disruption of the taurine transporter gene (TauT) leads to retinal degeneration in mice. FASEB J. 2002;16:231–233. doi: 10.1096/fj.01-0691fje. [DOI] [PubMed] [Google Scholar]
- Hollborn M, Dukic-Stefanovic S, Pannicke T, Ulbricht E, Reichenbach A, Wiedemann P, et al. Expression of aquaporins in the retina of diabetic rats. Curr Eye Res. 2011;36:850–856. doi: 10.3109/02713683.2011.593108. [DOI] [PubMed] [Google Scholar]
- Hollborn M, Rehak M, Iandiev I, Pannicke T, Ulbricht E, Reichenbach A, et al. Transcriptional regulation of aquaporins in the ischemic rat retina: upregulation of aquaporin-9. Curr Eye Res. 2012;37:524–531. doi: 10.3109/02713683.2012.658133. [DOI] [PubMed] [Google Scholar]
- Hosoya KI, Tomi M. Advances in the cell biology of transport via the inner blood-retinal barrier: establishment of cell lines and transport functions. Biol Pharm Bull. 2005;28:1–8. doi: 10.1248/bpb.28.1. [DOI] [PubMed] [Google Scholar]
- Hosoya K, Kondo T, Tomi M, Takanaga H, Ohtsuki S, Terasaki T. MCT1-mediated transport of L-lactic acid at the inner blood-retinal barrier: a possible route for delivery of monocarboxylic acid drugs to the retina. Pharm Res. 2001;18:1669–1676. doi: 10.1023/A:1013310210710. [DOI] [PubMed] [Google Scholar]
- Hosoya K, Nakamura G, Akanuma S, Tomi M, Tachikawa M. Dehydroascorbic acid uptake and intracellular ascorbic acid accumulation in cultured Müller glial cells (TR-MUL) Neurochem Int. 2008;52:1351–1357. doi: 10.1016/j.neuint.2008.02.001. [DOI] [PubMed] [Google Scholar]
- Hosoya KI, Fujita K, Tachikawa M. Involvement of reduced folate carrier 1 in the inner blood-retinal barrier transport of methyltetrahydrofolate. Drug Metab Pharmacokinet. 2008;23:285–292. doi: 10.2133/dmpk.23.285. [DOI] [PubMed] [Google Scholar]
- Hosoya K, Makihara A, Tsujikawa Y, Yoneyama D, Mori S, Terasaki T, et al. Roles of inner blood-retinal barrier organic anion transporter 3 in the vitreous/retina-to-blood efflux transport of p-aminohippuric acid, benzylpenicillin, and 6-mercaptopurine. J Pharmacol Exp Ther. 2009;329:87–93. doi: 10.1124/jpet.108.146381. [DOI] [PubMed] [Google Scholar]
- Hosoya KI, Tomi M, Tachikawa M. Strategies for therapy of retinal diseases using systemic drug delivery: relevance of transporters at the blood–retinal barrier. Expert Opin Drug Deliv. 2011;8:1571–1587. doi: 10.1517/17425247.2011.628983. [DOI] [PubMed] [Google Scholar]
- Igarashi Y, Chiba H, Utsumi H, Miyajima H, Ishizaki T, Gotoh T, et al. Expression of receptors for glial cell line-derived neurotrophic factor (GDNF) and neurturin in the inner blood-retinal barrier of rats. Cell Struct Funct. 2000;25:237–241. doi: 10.1247/csf.25.237. [DOI] [PubMed] [Google Scholar]
- Ishida S, Usui T, Yamashiro K, Kaji Y, Ahmed E, Carrasquillo KG, et al. VEGF164 is proinflammatory in the diabetic retina. Invest Ophthalmol Vis Sci. 2003;44:2155–2162. doi: 10.1167/iovs.02-0807. [DOI] [PubMed] [Google Scholar]
- Ito A, Yamaguchi K, Onogawa T, Unno M, Suzuki T, Nishio T, et al. Distribution of organic anion-transporting polypeptide 2 (oatp2) and oatp3 in the rat retina. Invest Ophthalmol Vis Sci. 2002;43:858–863. [PubMed] [Google Scholar]
- Ito A, Yamaguchi K, Tomita H, Suzuki T, Onogawa T, Sato T, et al. Distribution of rat organic anion transporting polypeptide-E (oatp-E) in the rat eye. Invest Ophthalmol Vis Sci. 2003;44:4877–4884. doi: 10.1167/iovs.02-1108. [DOI] [PubMed] [Google Scholar]
- Kaneko S, Ando A, Okuda-Ashitaka E, Maeda M, Furuta K, Suzuki M, et al. Ornithine transport via cationic amino acid transporter-1 is involved in ornithine cytotoxicity in retinal pigment epithelial cells. Invest Ophthalmol Vis Sci. 2007;48:464–471. doi: 10.1167/iovs.06-0398. [DOI] [PubMed] [Google Scholar]
- Katayama K, Ohshima Y, Tomi M, Hosoya K. Application of microdialysis to evaluate the efflux transport of estradiol 17-β glucuronide across the rat blood-retinal barrier. J Neurosci Methods. 2006;156:249–256. doi: 10.1016/j.jneumeth.2006.03.012. [DOI] [PubMed] [Google Scholar]
- Kaur C, Foulds W, Ling EA. Blood–retinal barrier in hypoxic ischaemic conditions: basic concepts, clinical features and management. Prog Retin Eye Res. 2008;27:622–647. doi: 10.1016/j.preteyeres.2008.09.003. [DOI] [PubMed] [Google Scholar]
- Kay P, Yang YC, Paraoan L. Directional protein secretion by the retinal pigment epithelium: roles in retinal health and the development of age-related macular degeneration. J Cell Mol Med. 2013;17:833–843. doi: 10.1111/jcmm.12070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy BG, Mangini NJ. P-glycoprotein expression in human retinal pigment epithelium. Mol Vis. 2002;8:422–430. [PubMed] [Google Scholar]
- Kida T, Oku H, Horie T, Fukumoto M, Okuda Y, Morishita S, et al. Implication of VEGF and aquaporin 4 mediating Müller cell swelling to diabetic retinal edema. Graefes Arch Clin Exp Ophthalmol. 2017;255:1149–1157. doi: 10.1007/s00417-017-3631-z. [DOI] [PubMed] [Google Scholar]
- Kim JH, Kim JH, Yu YS, Kim DH, Kim KW. Recruitment of pericytes and astrocytes is closely related to the formation of tight junction in developing retinal vessels. J Neurosci Res. 2009;87:653–659. doi: 10.1002/jnr.21884. [DOI] [PubMed] [Google Scholar]
- Kinoshita J, Iwata N, Kimotsuki T, Yasuda M. Digoxin-induced reversible dysfunction of the cone photoreceptors in monkeys. Invest Ophthalmol Vis Sci. 2014;55:881–892. doi: 10.1167/iovs.13-13296. [DOI] [PubMed] [Google Scholar]
- Kita T, Clermont AC, Murugesan N, Zhou Q, Fujisawa K, Ishibashi T, et al. Plasma kallikrein-kinin system as a VEGF-independent mediator of diabetic macular edema. Diabetes. 2015;64:3588–3599. doi: 10.2337/db15-0317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubo Y, Kusagawa Y, Tachikawa M, Akanuma S, Hosoya K. Involvement of a novel organic cation transporter in verapamil transport across the inner blood-retinal barrier. Pharm Res. 2013;30:847–856. doi: 10.1007/s11095-012-0926-y. [DOI] [PubMed] [Google Scholar]
- Kubo Y, Shimizu Y, Kusagawa Y, Akanuma S, Hosoya K. Propranolol transport across the inner blood-retinal barrier: potential involvement of a novel organic cation transporter. J Pharm Sci. 2013;102:3332–3342. doi: 10.1002/jps.23535. [DOI] [PubMed] [Google Scholar]
- Kubo Y, Tsuchiyama A, Shimizu Y, Akanuma S, Hosoya K. Involvement of carrier-mediated transport in the retinal uptake of clonidine at the inner blood-retinal barrier. Mol Pharm. 2014;11:3747–3753. doi: 10.1021/mp500516j. [DOI] [PubMed] [Google Scholar]
- Kubo Y, Obata A, Akanuma S, Hosoya K. Impact of cationic amino acid transporter 1 on blood-retinal barrier transport of L-ornithine. Invest Ophthalmol Vis Sci. 2015;56:5925–5932. doi: 10.1167/iovs.15-17398. [DOI] [PubMed] [Google Scholar]
- Kubo Y, Yamamoto M, Matsunaga K, Usui T, Akanuma SI, Hosoya KI. Retina-to-blood transport of 1-methyl-4-phenylpyridinium involves carrier-mediated process at the blood-retinal barrier. J Pharm Sci. 2017;106:2583–2591. doi: 10.1016/j.xphs.2017.04.028. [DOI] [PubMed] [Google Scholar]
- Kubo Y, Yahata S, Miki S, Akanuma SI, Hosoya KI. Blood-to-retina transport of riboflavin via RFVTs at the inner blood-retinal barrier. Drug Metab Pharmacokinet. 2017;32:92–99. doi: 10.1016/j.dmpk.2016.09.006. [DOI] [PubMed] [Google Scholar]
- Kur J, Newman EA, Chan-Ling T. Cellular and physiological mechanisms underlying blood flow regulation in the retina and choroid in health and disease. Prog Retin Eye Res. 2012;31:377–406. doi: 10.1016/j.preteyeres.2012.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusari J, Zhou S, Padillo E, Clarke KG, Gil DW. Effect of memantine on neuroretinal function and retinal vascular changes of streptozotocin-induced diabetic rats. Invest Ophthalmol Vis Sci. 2007;48:5152–5159. doi: 10.1167/iovs.07-0427. [DOI] [PubMed] [Google Scholar]
- Kusari J, Zhou SX, Padillo E, Clarke KG, Gil DW. Inhibition of vitreoretinal VEGF elevation and blood-retinal barrier breakdown in streptozotocin-induced diabetic rats by brimonidine. Invest Ophthalmol Vis Sci. 2010;51:1044–1051. doi: 10.1167/iovs.08-3293. [DOI] [PubMed] [Google Scholar]
- Le YZ. VEGF production and signaling in Müller glia are critical to modulating vascular function and neuronal integrity in diabetic retinopathy and hypoxic retinal vascular diseases. Vis Res. 2017;139:108–114. doi: 10.1016/j.visres.2017.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee NY, Kang YS. The effects of bisphosphonates on taurine transport in retinal capillary endothelial cells under high glucose conditions. Adv Exp Med Biol. 2013;776:59–66. doi: 10.1007/978-1-4614-6093-0_7. [DOI] [PubMed] [Google Scholar]
- Li SY, Yang D, Yeung CM, Yu WY, Chang RCC, So KF, et al. Lycium barbarum polysaccharides reduce neuronal damage, blood-retinal barrier disruption and oxidative stress in retinal ischemia/reperfusion injury. PLoS One. 2011;6:e16380. doi: 10.1371/journal.pone.0016380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li MS, Xin M, Guo CL, Lin GM, Li J, Wu XG. Differential expression of breast cancer-resistance protein, lung resistance protein, and multidrug resistance protein 1 in retinas of streptozotocin-induced diabetic mice. Int J Ophthalmol. 2017;10:515–523. doi: 10.18240/ijo.2017.04.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin M, Chen Y, Jin J, Hu Y, Zhou KK, Zhu M, et al. Ischaemia-induced retinal neovascularisation and diabetic retinopathy in mice with conditional knockout of hypoxia-inducible factor-1 in retinal Müller cells. Diabetologia. 2011;54:1554–1566. doi: 10.1007/s00125-011-2081-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Clermont AC, Gao BB, Feener EP. Intraocular hemorrhage causes retinal vascular dysfunction via plasma kallikrein. Invest Ophthalmol Vis Sci. 2013;54:1086–1094. doi: 10.1167/iovs.12-10537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopes de Faria JM, Silva KC, Boer PA, Cavalcanti TC, Rosales MA, Ferrari AL, et al. A decrease in retinal progenitor cells is associated with early features of diabetic retinopathy in a model that combines diabetes and hypertension. Mol Vis. 2008;14:1680–1691. [PMC free article] [PubMed] [Google Scholar]
- Lorenzi M. The polyol pathway as a mechanism for diabetic retinopathy: attractive, elusive, and resilient. Exp Diabetes Res. 2007;2007:61038. doi: 10.1155/2007/61038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madonna R, Giovannelli G, Confalone P, Renna FV, Geng YJ, De Caterina R. High glucose-induced hyperosmolarity contributes to COX-2 expression and angiogenesis: implications for diabetic retinopathy. Cardiovasc Diabetol. 2016;15:18. doi: 10.1186/s12933-016-0342-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannermaa E, Vellonen KS, Ryhänen T, Kokkonen K, Ranta VP, Kaarniranta K, et al. Efflux protein expression in human retinal pigment epithelium cell lines. Pharm Res. 2009;26:1785–1791. doi: 10.1007/s11095-009-9890-6. [DOI] [PubMed] [Google Scholar]
- Matsumiya W, Kusuhara S, Hayashibe K, Maruyama K, Kusuhara H, Tagami M, et al. Forskolin modifies retinal vascular development in Mrp4-knockout mice. Invest Ophthalmol Vis Sci. 2012;53:8029–8035. doi: 10.1167/iovs.12-10781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauer M, Zinman B, Gardiner R, Suissa S, Sinaiko A, Strand T, et al. Renal and retinal effects of enalapril and losartan in type 1 diabetes. N Engl J Med. 2009;361:40–51. doi: 10.1056/NEJMoa0808400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minamizono A, Tomi M, Hosoya K. Inhibition of dehydroascorbic acid transport across the rat blood-retinal and -brain barriers in experimental diabetes. Biol Pharm Bull. 2006;29:2148–2150. doi: 10.1248/bpb.29.2148. [DOI] [PubMed] [Google Scholar]
- Monickaraj F, McGuire PG, Nitta CF, Ghosh K, Das A. Cathepsin D: an Mϕ-derived factor mediating increased endothelial cell permeability with implications for alteration of the blood-retinal barrier in diabetic retinopathy. FASEB J. 2016;30:1670–1682. doi: 10.1096/fj.15-279802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori F, Hikichi T, Nagaoka T, Takahashi J, Kitaya N, Yoshida A. Inhibitory effect of losartan, an AT1 angiotensin II receptor antagonist, on increased leucocyte entrapment in retinal microcirculation of diabetic rats. Bt J Ophthalmol. 2002;86:1172–1174. doi: 10.1136/bjo.86.10.1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthusamy A, Lin CM, Shanmugam S, Lindner HM, Abcouwer SF, Antonetti DA. Ischemia–reperfusion injury induces occludin phosphorylation/ubiquitination and retinal vascular permeability in a VEGFR-2-dependent manner. J Cereb Blood Flow Metab. 2014;34:522–531. doi: 10.1038/jcbfm.2013.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagai N, Izumi-Nagai K, Oike Y, Koto T, Satofuka S, Ozawa YK, et al. Suppression of diabetes-induced retinal inflammation by blocking the angiotensin ii type 1 receptor or its downstream nuclear factor-β pathway. Invest Ophthalmol Vis Sci. 2007;48:4342–4350. doi: 10.1167/iovs.06-1473. [DOI] [PubMed] [Google Scholar]
- Nagase K, Tomi M, Tachikawa M, Hosoya K. Functional and molecular characterization of adenosine transport at the rat inner blood-retinal barrier. Biochim Biophys Acta. 2006;1758:13–19. doi: 10.1016/j.bbamem.2006.01.011. [DOI] [PubMed] [Google Scholar]
- Naggar H, Ola MS, Moore P, Huang W, Bridges CC, Ganapathy V, et al. Downregulation of reduced-folate transporter by glucose in cultured RPE cells and in RPE of diabetic mice. Invest Ophthalmol Vis Sci. 2002;43:556–563. [PMC free article] [PubMed] [Google Scholar]
- Nakashima T, Tomi M, Katayama K, Tachikawa M, Watanabe M, Terasaki T, et al. Blood-to-retina transport of creatine via creatine transporter (CRT) at the rat inner blood–retinal barrier. J Neurochem. 2004;89:1454–1461. doi: 10.1111/j.1471-4159.2004.02437.x. [DOI] [PubMed] [Google Scholar]
- Nakashima T, Tomi M, Tachikawa M, Watanabe M, Terasaki T, Hosoya K. Evidence for creatine biosynthesis in Müller glia. Glia. 2005;52:47–52. doi: 10.1002/glia.20222. [DOI] [PubMed] [Google Scholar]
- Nakashima E, Pop-Busui R, Towns R, Thomas TP, Hosaka Y, Nakamura J, et al. Regulation of the human taurine transporter by oxidative stress in retinal pigment epithelial cells stably transformed to overexpress aldose reductase. Antioxid Redox Signal. 2005;7:1530–1542. doi: 10.1089/ars.2005.7.1530. [DOI] [PubMed] [Google Scholar]
- Nawaz MI, Van Raemdonck K, Mohammad G, Kangave D, Van Damme J, Abu El-Asrar AM, et al. Autocrine CCL2, CXCL4, CXCL9 and CXCL10 signal in retinal endothelial cells and are enhanced in diabetic retinopathy. Exp Eye Res. 2013;109:67–76. doi: 10.1016/j.exer.2013.01.008. [DOI] [PubMed] [Google Scholar]
- Ogura S, Kurata K, Hattori Y, Takase H, Ishiguro-Oonuma T, Hwang Y, et al. Sustained inflammation after pericyte depletion induces irreversible blood-retina barrier breakdown. JCI Insight. 2017;2:e90905. doi: 10.1172/jci.insight.90905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohkura Y, Akanuma S, Tachikawa M, Hosoya K. Blood-to-retina transport of biotin via Na(+)-dependent multivitamin transporter (SMVT) at the inner blood-retinal barrier. Exp Eye Res. 2010;91:387–392. doi: 10.1016/j.exer.2010.06.010. [DOI] [PubMed] [Google Scholar]
- Ohtsuki S, Tomi M, Hata T, Nagai Y, Hori S, Mori S, et al. Dominant expression of androgen receptors and their functional regulation of organic anion transporter 3 in rat brain capillary endothelial cells; comparison of gene expression between the blood-brain and -retinal barriers. J Cell Physiol. 2005;204:896–900. doi: 10.1002/jcp.20352. [DOI] [PubMed] [Google Scholar]
- Ola MS, Alhomida AS, Ferrario CM, Ahmad S. Role of tissue renin-angiotensin system and the chymase/angiotensin-(1-12) axis in the pathogenesis of diabetic retinopathy. Curr Med Chem. 2017;24:3104–3114. doi: 10.2174/0929867324666170407141955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park DY, Lee J, Kim J, Kim K, Hong S, Han S, et al. Plastic roles of pericytes in the blood-retinal barrier. Nat Commun. 2017;8:15296. doi: 10.1038/ncomms15296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelkonen L, Sato K, Reinisalo M, Kidron H, Tachikawa M, Watanabe M, et al. LC-MS/MS based quantitation of ABC and SLC transporter proteins in plasma membranes of cultured primary human retinal pigment epithelium cells and immortalized ARPE19 cell line. Mol Pharm. 2017;14:605–613. doi: 10.1021/acs.molpharmaceut.6b00782. [DOI] [PubMed] [Google Scholar]
- Philp NJ, Yoon H, Grollman EF. Monocarboxylate transporter MCT1 is located in the apical membrane and MCT3 in the basal membrane of rat RPE. Am J Phys. 1998;274:R1824–R1828. doi: 10.1152/ajpregu.1998.274.6.R1824. [DOI] [PubMed] [Google Scholar]
- Philp NJ, Yoon H, Lombardi L. Mouse MCT3 gene is expressed preferentially in retinal pigment and choroid plexus epithelia. Am J Physiol Cell Physiol. 2001;280:C1319–C1326. doi: 10.1152/ajpcell.2001.280.5.C1319. [DOI] [PubMed] [Google Scholar]
- Phipps JA, Clermont AC, Sinha S, Chilcote TJ, Bursell SE, Feener EP. Plasma kallikrein mediates angiotensin II type 1 receptor-stimulated retinal vascular permeability. Hypertension. 2009;53:175–181. doi: 10.1161/HYPERTENSIONAHA.108.117663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phipps JA, Jobling AI, Greferath U, Fletcher EL, Vessey KA. Alternative pathways in the development of diabetic retinopathy: the renin-angiotensin and kallikrein-kinin systems. Clin Exp Optom. 2012;95:282–289. doi: 10.1111/j.1444-0938.2012.00747.x. [DOI] [PubMed] [Google Scholar]
- Pouliot M, Talbot S, Sénécal J, Dotigny F, Vaucher E, Couture R. Ocular application of the kinin B1 receptor antagonist LF22-0542 inhibits retinal inflammation and oxidative stress in streptozotocin-diabetic rats. PLoS One. 2012;7:e33864. doi: 10.1371/journal.pone.0033864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruneau D, Bélichard P, Sahel JA, Combal JP. Targeting the kallikrein-kinin system as a new therapeutic approach to diabetic retinopathy. Curr Opin Investig Drugs. 2010;11:507–514. [PubMed] [Google Scholar]
- Puchowicz MA, Xu K, Magness D, Miller C, Lust WD, Kern TS, et al. Comparison of glucose influx and blood flow in retina and brain of diabetic rats. J Cereb Blood Flow Metab. 2004;24:449–457. doi: 10.1097/00004647-200404000-00010. [DOI] [PubMed] [Google Scholar]
- Qin Y, Ren H, Hoffman MR, Fan J, Zhang M, Xu G. Aquaporin changes during diabetic retinopathy in rats are accelerated by systemic hypertension and are linked to the renin-angiotensin system. Invest Ophthalmol Vis Sci. 2012;53:3047–3053. doi: 10.1167/iovs.11-9154. [DOI] [PubMed] [Google Scholar]
- Quick M, Shi L. The sodium/multivitamin transporter: a multipotent system with therapeutic implications. Vitam Horm. 2015;98:63–100. doi: 10.1016/bs.vh.2014.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajan PD, Kekuda R, Chancy CD, Huang W, Ganapathy V, Smith SB. Expression of the extraneuronal monoamine transporter in RPE and neural retina. Curr Eye Res. 2000;20:195–204. doi: 10.1076/0271-3683(200003)2031-9FT195. [DOI] [PubMed] [Google Scholar]
- Ran RJ, Zheng XY, Du LP, Zhang XD, Chen XL, Zhu SY. Upregulated inflammatory associated factors and blood-retinal barrier changes in the retina of type 2 diabetes mellitus model. Int J Ophthalmol. 2016;9:1591–1597. doi: 10.18240/ijo.2016.11.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rangasamy S, McGuire PG, Franco Nitta C, Monickaraj F, Oruganti SR, Das A. Chemokine mediated monocyte trafficking into the retina: role of inflammation in alteration of the blood-retinal barrier in diabetic retinopathy. PLoS One. 2014;9:e108508. doi: 10.1371/journal.pone.0108508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ripps H, Shen W. Review: taurine: a “very essential” amino acid. Mol Vis. 2012;18:2673–2686. [PMC free article] [PubMed] [Google Scholar]
- Rizzolo LJ, Peng S, Luo Y, Xiao W. Integration of tight junctions and claudins with the barrier functions of the retinal pigment epithelium. Prog Retin Eye Res. 2011;30:296–323. doi: 10.1016/j.preteyeres.2011.06.002. [DOI] [PubMed] [Google Scholar]
- Sakurai T, Akanuma S, Usui T, Kubo Y, Tachikawa M, Hosoya K. Excitatory amino acid transporter 1-mediated l-glutamate transport at the inner blood-retinal barrier: possible role in L-glutamate elimination from the retina. Biol Pharm Bull. 2015;38:1087–1091. doi: 10.1248/bpb.b15-00226. [DOI] [PubMed] [Google Scholar]
- Silva KC, Pinto CC, Biswas SK, Souza DS, de Faria JB, de Faria JM. Prevention of hypertension abrogates early inflammatory events in the retina of diabetic hypertensive rats. Exp Eye Res. 2007;85:123–129. doi: 10.1016/j.exer.2007.03.008. [DOI] [PubMed] [Google Scholar]
- Simó R, Villarroel M, Corraliza L, Hernández C, Garcia-Ramírez M. The retinal pigment epithelium: something more than a constituent of the blood-retinal barrier—implications for the pathogenesis of diabetic retinopathy. J Biomed Biotechnol. 2010;2010:190724. doi: 10.1155/2010/190724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjølie AK, Klein R, Porta M, Orchard T, Fuller J, Parving HH, et al. Effect of candesartan on progression and regression of retinopathy in type 2 diabetes (DIRECT-Protect 2): a randomised placebo-controlled trial. Lancet. 2008;372:1385–1393. doi: 10.1016/S0140-6736(08)61411-7. [DOI] [PubMed] [Google Scholar]
- Sreekumar PG, Spee C, Ryan SJ, Cole SP, Kannan R, Hinton DR. Mechanism of RPE cell death in α-crystallin deficient mice: a novel and critical role for MRP1-mediated GSH efflux. PLoS One. 2012;7:e33420. doi: 10.1371/journal.pone.0033420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steuer H, Jaworski A, Elger B, Kaussmann M, Keldenich J, Schneider H, et al. Functional characterization and comparison of the outer blood-retina barrier and the blood-brain barrier. Invest Ophthalmol Vis Sci. 2005;46:1047–1053. doi: 10.1167/iovs.04-0925. [DOI] [PubMed] [Google Scholar]
- Stevens MJ, Hosaka Y, Masterson JA, Jones SM, Thomas TP, Larkin DD. Downregulation of the human taurine transporter by glucose in cultured retinal pigment epithelial cells. Am J Phys. 1999;277(4 Pt 1):E760–E771. doi: 10.1152/ajpendo.1999.277.4.E760. [DOI] [PubMed] [Google Scholar]
- Strauß O. Pharmacology of the retinal pigment epithelium, the interface between retina and body system. Eur J Pharmacol. 2016;787:84–93. doi: 10.1016/j.ejphar.2016.03.066. [DOI] [PubMed] [Google Scholar]
- Tachikawa M, Hosoya K, Ohtsuki S, Terasaki T. A novel relationship between creatine transport at the blood-brain and blood-retinal barriers, creatine biosynthesis, and its use for brain and retinal energy homeostasis. Subcell Biochem. 2007;46:83–98. doi: 10.1007/978-1-4020-6486-9_5. [DOI] [PubMed] [Google Scholar]
- Tachikawa M, Toki H, Tomi M, Hosoya K. Gene expression profiles of ATP-binding cassette transporter A and C subfamilies in mouse retinal vascular endothelial cells. Microvasc Res. 2008;75:68–72. doi: 10.1016/j.mvr.2007.05.002. [DOI] [PubMed] [Google Scholar]
- Tachikawa M, Takeda Y, Tomi M, Hosoya K. Involvement of OCTN2 in the transport of acetyl-L-carnitine across the inner blood-retinal barrier. Invest Ophthalmol Vis Sci. 2010;51:430–436. doi: 10.1167/iovs.09-4080. [DOI] [PubMed] [Google Scholar]
- Tachikawa M, Murakami K, Martin PM, Hosoya K, Ganapathy V. Retinal transfer of nicotinate by H+-monocarboxylate transporter at the inner blood-retinal barrier. Microvasc Res. 2011;82:385–390. doi: 10.1016/j.mvr.2011.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tagami M, Kusuhara S, Honda S, Tsukahara Y, Negi A. Expression of ATP-binding cassette transporters at the inner blood-retinal barrier in a neonatal mouse model of oxygen-induced retinopathy. Brain Res. 2009;1283:186–193. doi: 10.1016/j.brainres.2009.05.095. [DOI] [PubMed] [Google Scholar]
- Tagami M, Kusuhara S, Imai H, Uemura A, Honda S, Tsukahara Y, et al. MRP4 knockdown enhances migration, suppresses apoptosis, and produces aggregated morphology in human retinal vascular endothelial cells. Biochem Biophys Res Commun. 2010;400:593–598. doi: 10.1016/j.bbrc.2010.08.109. [DOI] [PubMed] [Google Scholar]
- Tang J, Zhu XW, Lust WD, Kern TS. Retina accumulates more glucose than does the embryologically similar cerebral cortex in diabetic rats. Diabetologia. 2000;43:1417–1423. doi: 10.1007/s001250051548. [DOI] [PubMed] [Google Scholar]
- Tega Y, Kubo Y, Yuzurihara C, Akanuma S, Hosoya K. Carrier-mediated transport of nicotine across the inner blood-retinal barrier: involvement of a novel organic cation transporter driven by an outward H(+) gradient. J Pharm Sci. 2015;104:3069–3075. doi: 10.1002/jps.24453. [DOI] [PubMed] [Google Scholar]
- Tian XF, Xia XB, Xu HZ, Xiong SQ, Jiang J. Caveolin-1 expression regulates blood–retinal barrier permeability and retinal neovascularization in oxygen-induced retinopathy. Clin Exp Ophthalmol. 2012;40:e58–e66. doi: 10.1111/j.1442-9071.2011.02656.x. [DOI] [PubMed] [Google Scholar]
- Toda R, Kawazu K, Oyabu M, Miyazaki T, Kiuchi Y. Comparison of drug permeabilities across the blood–retinal barrier, blood–aqueous humor barrier, and blood–brain barrier. J Pharm Sci. 2011;100:3904–3911. doi: 10.1002/jps.22610. [DOI] [PubMed] [Google Scholar]
- Tomi M, Hosoya K. Application of magnetically isolated rat retinal vascular endothelial cells for the determination of transporter gene expression levels at the inner blood-retinal barrier. J Neurochem. 2004;91:1244–1248. doi: 10.1111/j.1471-4159.2004.02842.x. [DOI] [PubMed] [Google Scholar]
- Tomi M, Mori M, Tachikawa M, Katayama K, Terasaki T, Hosoya K. L-type amino acid transporter 1-mediated L-leucine transport at the inner blood-retinal barrier. Invest Ophthalmol Vis Sci. 2005;46:2522–2530. doi: 10.1167/iovs.04-1175. [DOI] [PubMed] [Google Scholar]
- Tomi M, Terayama T, Isobe T, Egami F, Morito A, Kurachi M, et al. Function and regulation of taurine transport at the inner blood-retinal barrier. Microvasc Res. 2007;73:100–106. doi: 10.1016/j.mvr.2006.10.003. [DOI] [PubMed] [Google Scholar]
- Tomi M, Tajima A, Tachikawa M, Hosoya K. Function of taurine transporter (Slc6a6/TauT) as a GABA transporting protein and its relevance to GABA transport in rat retinal capillary endothelial cells. Biochim Biophys Acta. 2008;1778:2138–2142. doi: 10.1016/j.bbamem.2008.04.012. [DOI] [PubMed] [Google Scholar]
- Tomi M, Kitade N, Hirose S, Yokota N, Akanuma S, Tachikawa M, et al. Cationic amino acid transporter 1-mediated L-arginine transport at the inner blood-retinal barrier. J Neurochem. 2009;111:716–725. doi: 10.1111/j.1471-4159.2009.06367.x. [DOI] [PubMed] [Google Scholar]
- Tun T, Kang YS. Effects of simvastatin on CAT-1-mediated arginine transport and NO level under high glucose conditions in conditionally immortalized rat inner blood-retinal barrier cell lines (TR-iBRB) Microvasc Res. 2017;111:60–66. doi: 10.1016/j.mvr.2017.01.002. [DOI] [PubMed] [Google Scholar]
- Uchida Y, Kamiie J, Ohtsuki S, Terasaki T. Multichannel liquid chromatography-tandem mass spectrometry cocktail method for comprehensive substrate characterization of multidrug resistance-associated protein 4 transporter. Pharm Res. 2007;24:2281–2296. doi: 10.1007/s11095-007-9453-7. [DOI] [PubMed] [Google Scholar]
- Umapathy NS, Gnana-Prakasam JP, Martin PM, Mysona B, Dun Y, Smith SB, et al. Cloning and functional characterization of the proton-coupled electrogenic folate transporter and analysis of its expression in retinal cell types. Invest Ophthalmol Vis Sci. 2007;48:5299–5305. doi: 10.1167/iovs.07-0288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usui T, Kubo Y, Akanuma S, Hosoya K. Β-alanine and l-histidine transport across the inner blood-retinal barrier: potential involvement in L-carnosine supply. Exp Eye Res. 2013;113:135–142. doi: 10.1016/j.exer.2013.06.002. [DOI] [PubMed] [Google Scholar]
- Vadlapatla RK, Vadlapudi AD, Ponnaluri VK, Pal D, Mukherji M, Mitra AK. Molecular expression and functional activity of efflux and influx transporters in hypoxia induced retinal pigment epithelial cells. Int J Pharm. 2013;454:444–452. doi: 10.1016/j.ijpharm.2013.06.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma A, Shan Z, Lei B, Yuan L, Liu X, Nakagawa T, et al. ACE2 and Ang-(1-7) confer protection against development of diabetic retinopathy. Mol Ther. 2012;20:28–36. doi: 10.1038/mt.2011.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinores SA, Xiao WH, Shen J, Campochiaro PA. TNF-α is critical for ischemia-induced leukostasis, but not retinal neovascularization nor VEGF-induced leakage. J Neuroimmunol. 2007;182:73–79. doi: 10.1016/j.jneuroim.2006.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virgili G, Parravano M, Evans JR, Gordon I, Lucenteforte E. Anti-vascular endothelial growth factor for diabetic macular oedema: a network meta-analysis. Cochrane Database Syst Rev. 2017;6:CD007419. doi: 10.1002/14651858.CD007419.pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang K, Wang Y, Gao L, Li X, Li M, Guo J. Dexamethasone inhibits leukocyte accumulation and vascular permeability in retina of streptozotocin-induced diabetic rats via reducing vascular endothelial growth factor and intercellular adhesion molecule-1 expression. Biol Pharm Bull. 2008;31:1541–1546. doi: 10.1248/bpb.31.1541. [DOI] [PubMed] [Google Scholar]
- Wang J, Xu X, Elliott MH, Zhu M, Le YZ. Müller cell-derived VEGF is essential for diabetes-induced retinal inflammation and vascular leakage. Diabetes. 2010;59:2297–2305. doi: 10.2337/db09-1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weleber RG, Shults WT. Digoxin retinal toxicity. Clinical and electrophysiological evaluation of a cone dysfunction syndrome. Arch Ophthalmol. 1981;99:1568–1572. doi: 10.1001/archopht.1981.03930020442007. [DOI] [PubMed] [Google Scholar]
- Wilkinson-Berka JL, Tan G, Jaworski K, Ninkovic S. Valsartan but not atenolol improves vascular pathology in diabetic Ren-2 rat retina. Am J Hypertens. 2007;20:423–430. doi: 10.1016/j.amjhyper.2006.09.018. [DOI] [PubMed] [Google Scholar]
- Willermain F, Libert S, Motulsky E, Salik D, Caspers L, Perret J, et al. Origins and consequences of hyperosmolar stress in retinal pigmented epithelial cells. Front Physiol. 2014;5:199. doi: 10.3389/fphys.2014.00199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willermain F, Janssens S, Arsenijevic T, Piens I, Bolaky N, Caspers L, et al. Osmotic stress decreases aquaporin-4 expression in the human retinal pigment epithelial cell line, ARPE-19. Int J Mol Med. 2014;34:533–538. doi: 10.3892/ijmm.2014.1791. [DOI] [PubMed] [Google Scholar]
- Willermain F, Scifo L, Weber C, Caspers L, Perret J, Delporte C. Potential interplay between hyperosmolarity and inflammation on retinal pigmented epithelium in pathogenesis of diabetic retinopathy. Int J Mol Sci. 2018;19:1056. doi: 10.3390/ijms19041056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winges A, Garcia TB, Prager P, Wiedemann P, Kohen L, Bringmann A, et al. Osmotic expression of aldose reductase in retinal pigment epithelial cells: involvement of NFAT5. Graefes Arch Clin Exp Ophthalmol. 2016;254:2387–2400. doi: 10.1007/s00417-016-3492-x. [DOI] [PubMed] [Google Scholar]
- Wisniewska-Kruk J, Hoeben KA, Vogels IM, Gaillard PJ, Van Noorden CJ, Schlingemann RO, et al. A novel co-culture model of the blood-retinal barrier based on primary retinal endothelial cells, pericytes and astrocytes. Exp Eye Res. 2012;96:181–190. doi: 10.1016/j.exer.2011.12.003. [DOI] [PubMed] [Google Scholar]
- Xia T, Rizzolo LJ. Effects of diabetic retinopathy on the barrier functions of the retinal pigment epithelium. Vis Res. 2017;139:72–81. doi: 10.1016/j.visres.2017.02.006. [DOI] [PubMed] [Google Scholar]
- Xu C, Zhu L, Chan T, Lu X, Shen W, Madigan MC, et al. Chloroquine and hydroxychloroquine are novel inhibitors of human organic anion transporting polypeptide 1A2. J Pharm Sci. 2016;105:884–890. doi: 10.1002/jps.24663. [DOI] [PubMed] [Google Scholar]
- Yahara T, Tachikawa M, Akanuma S, Hosoya K. Hypertonicity enhances GABA uptake by cultured rat retinal capillary endothelial cells. Drug Metab Pharmacokinet. 2010;25:611–615. doi: 10.2133/dmpk.DMPK-10-NT-057. [DOI] [PubMed] [Google Scholar]
- Yamamoto A, Akanuma S, Tachikawa M, Hosoya K. Involvement of LAT1 and LAT2 in the high- and low-affinity transport of L-leucine in human retinal pigment epithelial cells (ARPE-19 cells) J Pharm Sci. 2010;99:2475–2482. doi: 10.1002/jps.21991. [DOI] [PubMed] [Google Scholar]
- Yao SY, Ng AM, Sundaram M, Cass CE, Baldwin SA, Young JD. Transport of antiviral 3′-deoxy-nucleoside drugs by recombinant human and rat equilibrative, nitrobenzylthioinosine (NBMPR)-insensitive (ENT2) nucleoside transporter proteins produced in Xenopus oocytes. Mol Membr Biol. 2001;18:161–167. doi: 10.1080/09687680110048318. [DOI] [PubMed] [Google Scholar]
- Yaylali SA, Sadigov F, Erbil H, Ekinci A, Akcakaya AA. Chloroquine and hydroxychloroquine retinopathy-related risk factors in a Turkish cohort. Int Ophthalmol. 2013;33:627–634. doi: 10.1007/s10792-013-9748-0. [DOI] [PubMed] [Google Scholar]
- Zakoji N, Akanuma S, Tachikawa M, Hosoya K. Involvement of cationic amino acid transporter 1 in L-arginine transport in rat retinal pericytes. Biol Pharm Bull. 2015;38:257–262. doi: 10.1248/bpb.b14-00637. [DOI] [PubMed] [Google Scholar]
- Zeng K, Xu H, Mi M, Chen K, Zhu J, Yi L, et al. Effects of taurine on glial cells apoptosis and taurine transporter expression in retina under diabetic conditions. Neurochem Res. 2010;35:1566–1574. doi: 10.1007/s11064-010-0216-1. [DOI] [PubMed] [Google Scholar]
- Zhang JZ, Xi X, Gao L, Kern TS. Captopril inhibits capillary degeneration in the early stages of diabetic retinopathy. Curr Eye Res. 2007;32:883–889. doi: 10.1080/02713680701584123. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Li C, Sun X, Kuang X, Ruan X. High glucose decreases expression and activity of p-glycoprotein in cultured human retinal pigment epithelium possibly through iNOS induction. PLoS One. 2012;7:e31631. doi: 10.1371/journal.pone.0031631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Uchida Y, Hirano S, Ando D, Kubo Y, Auriola S, et al. Inner blood-retinal barrier dominantly expresses breast cancer resistance protein: comparative quantitative targeted absolute proteomics study of CNS barriers in pig. Mol Pharm. 2017;14:3729–3738. doi: 10.1021/acs.molpharmaceut.7b00493. [DOI] [PubMed] [Google Scholar]
- Zhang YH, Li J, Yang WZ, Xian ZH, Feng QT, Ruan XC. Mitochondrial expression and activity of P-glycoprotein under oxidative stress in outer blood-retinal barrier. Int J Ophthalmol. 2017;10:1055–1063. doi: 10.18240/ijo.2017.07.06. [DOI] [PMC free article] [PubMed] [Google Scholar]