Abstract
Acute leukemia represents the most common pediatric malignancy comprising diverse subtypes with varying prognosis and treatment outcomes. New and targeted treatment options are warranted for this disease. Patient-derived xenograft (PDX) models are increasingly being used for preclinical testing of novel treatment modalities. A novel approach involving targeted error-corrected RNA sequencing using ArcherDX HemeV2 kit was employed to compare 25 primary pediatric acute leukemia samples and their corresponding PDX samples. A comparison of the primary samples and PDX samples revealed a high concordance between single nucleotide variants and gene fusions whereas other complex structural variants were not as consistent. The presence of gene fusions representing the major driver mutations at similar allelic frequencies in PDX samples compared to primary samples and over multiple passages confirms the utility of PDX models for preclinical drug testing. Characterization and tracking of these novel cryptic fusions and exonal variants in PDX models is critical in assessing response to potential new therapies.
Keywords: error-corrected sequencing, genomics, patient derived xenograft models, pediatric cancers, structural variants
Introduction
Genomic characterization of the somatic landscape is essential for the robust clinical evaluation and classification of pediatric leukemias [1]. Somatic variants can inform both diagnosis and prognostication, as well as guide therapy decisions [2]. The development and validation of new targeted therapies for pediatric leukemias is dependent on the availability of pre-clinical models capable of recapitulation of the disease. Patient-derived orthotopic xenograft models (PDX) are routinely used in disease modeling for preclinical drug evaluation [3]. Although several studies have been conducted to understand the stability and suitability of PDX models, the majority of these efforts have focused on adult-derived leukemias and the characterization of single nucleotide variants (SNVs) [4].
Chromosomal rearrangements generating gene fusions and other structural variants (StVs) are more common in pediatric malignancies compared to adults [5]. These StVs and SNVs have demonstrated a different landscape for diagnostic, prognostic, and therapeutic value. Of note, pediatric leukemias are genomically heterogenous and require a broad spectrum of molecular biology techniques to fully characterize. Additionally, StVs are difficult to identify via short read DNA-seq approaches, and recent research has demonstrated the power and utility of identifying SNVs in RNA molecules [6].
Acute lymphoblastic leukemia (ALL) is the most common type of cancer in children and adolescents. ALL represents 20% of all cancers diagnosed in individuals with less than 20 years of age [7]. In general, survival in ALL has improved significantly over the past 40 years with more than 90% of patients now surviving. Acute myeloid leukemia (AML) is the second most common type of leukemia diagnosed in children. AML has an overall survival rate that is less than 65%. In all children with AML, and many with ALL, survival comes at the expense of intensive chemotherapy. New strategies are needed, as are preclinical models that reflect the clinical disease.
The goal of this study was to characterize complex genomic variants in pediatric leukemias and describe and monitor these variants in preclinical PDX models in comparison with the primary samples. The ability to track complex genomic lesions in primary samples and across passage in PDX lines is essential in ensuring that that the model can be used for biologic and therapeutic modeling. RNA next generation sequencing (NGS) techniques enable a sensitive and broad approach for analyzing complex genomic lesions and identifying clinically relevant novel somatic mutations associated with pediatric leukemias.
Methods
Patient samples and consent
All samples used in this study were procured by the Nemours Biobank following written informed consent. For majority of samples, leukemic cells were isolated from human bone marrow aspirates with the exception of NTPL-59 and NTPL-109, which were isolated from apheresis products by Ficoll density gradient centrifugation and provided to us under an Institutional Review Board approved protocol (Nemours Office of Human Subjects Protection IRB# 267207). Summary of the subject’s characteristics are presented in Table 1.
Table 1.
Summary of leukemic samples utilized
| Patient characteristic | AML | ALL |
|---|---|---|
| No. | 5 | 20 |
| Age (yr), median (range) | 10 (1.5-14) | 5.5 (1-16) |
| Sex | ||
| Male | 40 | 55 |
| Female | 60 | 45 |
| Race | ||
| Caucasian | 60 | 35 |
| African American | 0 | 25 |
| Hispanic | 20 | 20 |
| Samples collected at diagnosis | 80 | 95 |
| Cytogenetically normal (by karyotype analysis) | 0 | 55 |
| Bone marrow origin | 100 | 90 |
| Peripheral blood origin | 0 | 10 |
| Average leukemic blast percentage | 76 | 78 |
Values are presented as percentage unless otherwise indicated.
AML, acute myeloid leukemia; ALL, acute lymphoblastic leukemia.
Generation of PDX models
PDX models were generated as described previously [8] using a protocol approved by the Nemours Institutional Animal Care and Use Committee. Leukemic cells from patient samples were injected into immune-deficient NSG-B2m mice (stock no. 010636, Jackson Laboratories, Bar Harbor, ME, USA) via the tail vein. Disease progression was examined by determination of the percentage of human leukemic cells in mouse peripheral blood by flow cytometry. Mice were closely monitored for experimental endpoints such as increased leukemic burden, weight loss greater than 20% body weight, hunched back, and impaired mobility. Mice meeting end point criteria were euthanized using a method consistent with the guidelines of the American Veterinary Medical Association. Leukemic cells were isolated from the femurs and spleen post euthanasia and used for serial transplantation in a new cohort of mice. Bioauthentication and validation of PDX sample with matching primary sample was performed by subjecting the DNA samples to AmpFISTR Identifiler PCR Amplification Kit (Applied Biosystems, Foster City, CA, USA).
Error-corrected sequencing library preparation and sequencing
To optimize detection of structural and copy number variants in RNA we prepared RNA–error-corrected sequencing libraries using the ArcherDX (Boulder, CO, USA) FusionPlex HemeV2 Kit (catalog no. AB0012) per manufacturer’s protocols. Total RNA was extracted using RNeasy Mini Kit (Qiagen, Hilden, Germany). Nucleic acid quantity and quality was then assessed using the Agilent (Santa Clara, CA, USA) TapeStation 4200 following the manufacturer’s protocol and using the High Sensitivity RNA Screen Tape (catalog no. 5067-5579). cDNA was made from 50 ng of RNA using the QIAseq kit. Each library was sequenced on the Illumina NextSeq platform (San Diego, CA, USA). The gene fusion data produced by the Archer panel was initially correlated with diagnostic fluorescence in situ hybridization data available for each primary sample.
Bioinformatics
The data was processed via ArcherDX Analysis platform (v5.1.3), hosted in the cloud by Amazon Web Services, including fastq trimming, read deduplication, genome alignment, and variant detection and annotation. The analysis pipeline contains the following applications: ABRA [9], bamaddrg, bcftools, bedtools, blast [10], bowtie2 [11], bwa, EMBOSS, fastqc, freeBayes [12], Lofreq [13], MiXCR [14], Muscle, samtools, VEP [15], Velvet [16], HTSeq [17], complete-striped-smith-waterman-library, JBrowse [18], JQuery DataTables, Django Solo, and plot.js.
Fastq files were analyzed via fastqc for library quality, and error corrected reads (hamming distance of 2) were aligned to the genome build hg19 using BWA and bowtie2, and alignment files were processed via GATK best practices [19]. SNVs and short InDels (≤ 20 bp) were detected from the genomic alignments (forced reference mapping) by freeBayes and Lofreq, whereas large structural variants and cryptic fusions were detected via de novo assembly approaches. A minimum of three reads per unique molecular barcode (UMI) was required for the downstream process of de-duplication and error-correction [20]. Variants were filtered based on depth of error-corrected sequencing bins, minimum of 3, that supported the call. All regions in which variants called required a total read depth >100×, and a minimal base quality score (phred) of 20 was applied. The ExAC database was used to annotate common variants.
Variant allele frequencies (VAF) were calculated for SNVs based on number reads mapped to that location supporting the alternative allele versus the total number of reads mapped to that genome location. VAFs for StVs are calculated by analyzing the number of reads supporting the wild type sequence/junction, compared to the number of reads supporting the novel junction. R statistics was used for making scatter plots, specifically ggplot2 [21]. Alignment files (bam) were visualized via integrative genome browser (IGV). The fastq data is publicly available via short read archives under the following accession number (will add upon acceptance of manuscript).
Results
Comparison of RNA StVs and SNVs between primary and PDX AML samples
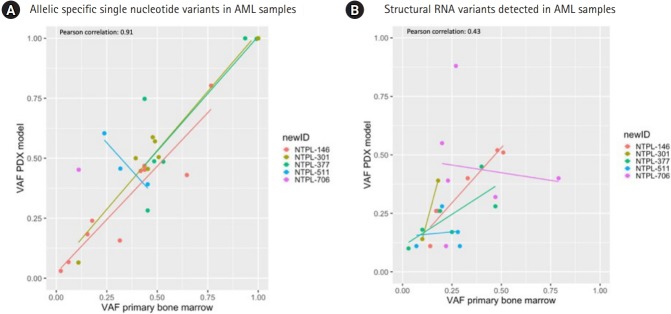
To determine the concordance of RNA variants between primary and PDX samples for pediatric AML, a targeted RNA sequencing panel approach (HemeV2; ArcherDx) was utilized. In this report, we analyzed 5 AML primary-PDX sample pairs, and in total 31 allelic specific SNVs were identified with the following distribution: 1 frameshift, 11 missense, 2 splice region and 17 untranslated region (UTR) variants (Supplementary Table 1). Five UTR variants were present at a VAF of 1 in both primary and PDX AML samples. The absolute change in VAFs between primary and PDX samples was less than 0.2 for 27 SNVs. A few variants increased in VAF in the PDX (MYC, CDKN2A, and NOTCH1), other variants reduced in VAF in PDX samples (CCND3 and ABL2) (Fig. 1A, Supplementary Table 1).
Fig. 1.
Summary of primary and xenograft RNA variants in acute myeloid leukemia (AML). (A) Allelic specific single nucleotide variants. Variant allele frequency (VAF) at time of diagnosis, x-axis is plotted versus the VAF in the xenograft model, y-axis. (B) Structural RNA variants. VAF at time of diagnosis, x-axis is plotted versus the VAF in the xenograft model, y-axis. PDX, patient-derived xenograft.
VAFs for all RNA StVs including gene fusions and alternative exon usage variants were graphed between the primary and PDX AML samples and results are displayed (Fig. 1B). Four unique gene fusions (KMT2A-MLLT1, KMT2A-MLLT3, NUP-98-NSD1, and the reciprocal NSD1-NUP98) were identified in the primary AML samples and PDX samples. Additionally, 5 exon duplications/deletions were identified in CEBPA and IRF4 (Supplementary Table 2).
Multiple retained introns (n=14) were identified in the 5 primary and PDX AML samples in the following genes: ZCCHC7, ABL1, JAK2, IRF8, TAL1, CEBPG, ETV6, KMT2A, MLLT10, KLF2, and PRDM16 (Supplementary Table 2). The SNVs were more concordant between primary and PDX samples compared to StVs (Pearson correlation coefficient, 0.91; p = 5.12e-13 and 0.43; p = 0.036 respectively). Among the StVs, fusions were identified at similar VAFs in primary and PDX samples, whereas the alternative exon usage variants showed greater variability. Interestingly, the 2 AML samples with KMT2A gene rearrangements (NTPL-146 and NTPL-377) showed higher level of concordance between VAFs for StVs as well as SNVs.
Comparison of RNA StVs and SNVs between primary and PDX T-ALL and B-ALL samples
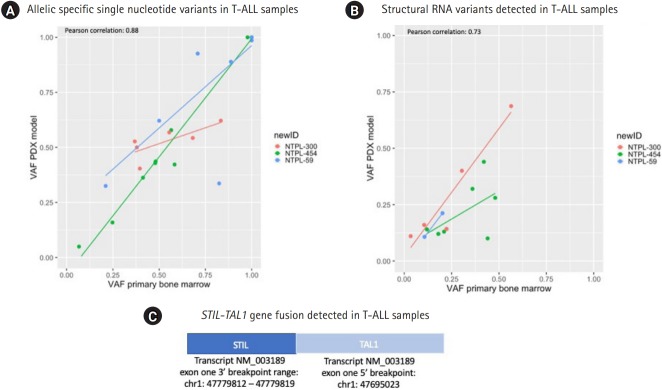
To determine the concordance of RNA variants between primary and PDX samples for pediatric ALL, samples target RNA sequencing approach was utilized. The correlation coefficients of VAF between primary and PDX T-cell ALL (T-ALL) samples identified across 3 primary and PDX T-ALL samples were similar between SNVs and StVs (Pearson correlation coefficient, 0.88; p = 6.12e-10 and 0.73; p = 0.003 respectively) (Fig. 2). In total, 25 allelic specific RNA SNVs were identified in the primary and PDX T-ALL samples: 3 frameshift, 8 missense, and 14 UTR variants. Six UTR variants had VAF = 1 in primary and PDX T-ALL samples. Three variants had absolute VAFs greater than 0.25; 1 of these SNVs (NOTCH1 frameshift variant) reduced in VAF in PDX samples, while 2 (CEBPA missense variants) showed gains in PDX samples. NTPL-454 had a strong correlation between SNV VAFs in the primary and PDX models (Fig. 2A; green line), whereas NTPL-59 and NTPL-300 were not as consistent with VAFs for SNVs.
Fig. 2.
Summary of primary and xenograft RNA variants in T-cell acute lymphoblastic leukemia (T-ALL). (A) Allelic specific single nucleotide variants. Variant allele frequency (VAF) at time of diagnosis, x-axis is plotted versus the variant allele frequency in the xenograft model, y-axis. (B) Structural RNA variants. VAF at time of diagnosis, x-axis is plotted versus the VAF in the xenograft model, y-axis. (C) STIL-TAL1 gene fusion identified in 2 of the T-ALL samples. PDX, patient-derived xenograft.
In total 14 StVs were identified in the primary and PDX models for T-ALL samples; 4 unique fusions (STIL-TAL1, SPTAN1-ABL1), 7 retained introns (EIF4A, IRF8, KMT2A, NF1, SETD2), and 3 molecules with exon duplications (BCL11B and ZCCH7). NTPL-300 was the most concordant for VAF of StVs in primary and PDX T-ALL samples (Fig. 2B). Of interest, 2 of the T-ALL samples had a STIL-TAL gene fusion, which was recently published as a potential driver / founder event [22].
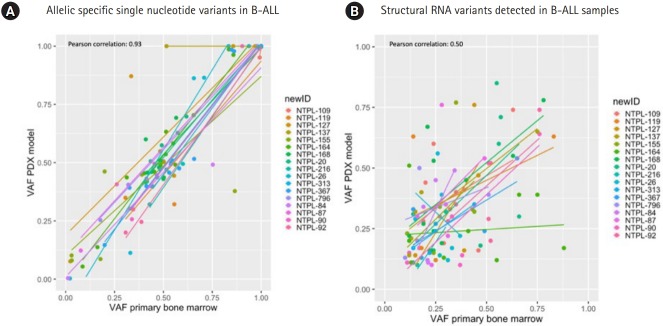
The correlation between VAF from primary to PDX samples was analyzed for RNA StVs and SNVs in 17 B-cell ALL (B-ALL) samples. In total 114 RNA SNVs were identified in the primary and PDX B-ALL samples, and of those variants 4 were frameshift, 25 missense, 5 splice region, 2 stop gained and the rest were UTR variants (Supplementary Table 1). Twenty-two UTR variants (RUNX1, IKZF3, CHIC2, CCND2, BCL2) were detected at identical VAF of 1 in primary and PDX B-ALL samples. Five variants (4.4%) had VAFs greater than 0.25; CHID1, ABL2 UTR variants showed decreased VAF, and BCR, CCND2, NOTCH1 SNVs showed increased VAF in PDX samples.
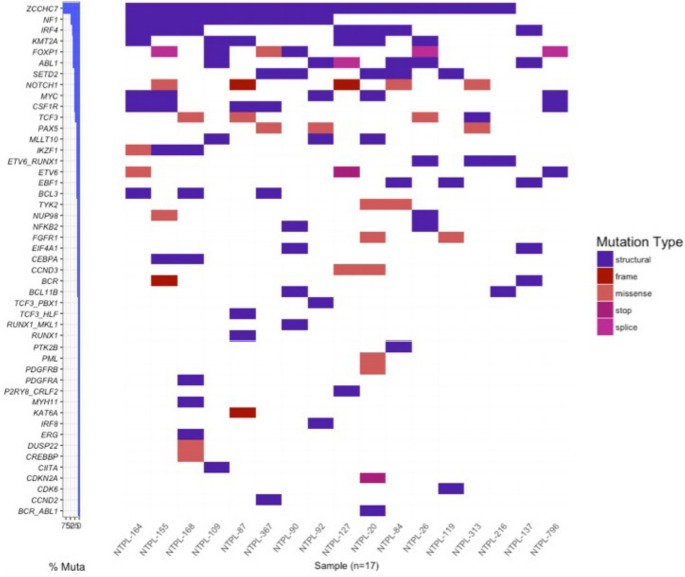
The correlation between SNV VAFs from primary to PDX B-ALL samples was higher than the correlation between StV VAFs (Pearson correlation coefficient, 0.93; p = 2.2e-16 and 0.5; p = 9.5e-8, respectively) (Fig. 3A, B). Eight of 17 samples possessed a gene fusion (BCR-ABL1, ETV6-RUNX1, P2RY8-CRLF2, RUNX1-MKL1, TCF3-HLF, TCF3-PBX1). The VAFs for StVs, especially the alternate exon usage variants, were more variable in these samples, similar to AML samples. Interestingly, 15 out of 18 of the B-ALL samples had a retained intron in ZCCHC7 involving intron 2, which was persistent in PDX samples (Fig. 3B). Two AML and 1 T-ALL sample also showed a similar retained intron variant (Supplementary Table 2). ZCCHC7 intron 2 has been mapped to hotspot for breakpoints in B-ALL [23]. ZCCHC7 topped the waterfall graph used to analyze and visualize the most commonly altered genes in B-ALL samples (Fig. 4).
Fig. 3.
Summary of primary and xenograft RNA variants in B-cell acute lymphoblastic leukemia (B-ALL). (A) Allelic specific single nucleotide variants. Variant allele frequency (VAF) at time of diagnosis, x-axis is plotted versus the VAF in the xenograft model, y-axis. (B) Structural RNA variants. VAF at time of diagnosis, x-axis is plotted versus the VAF in the xenograft model, y-axis. PDX, patient-derived xenograft.
Fig. 4.
Waterfall graph for single nucleotide variants (SNVs) and structural variants (StVs) detected in B-cell acute lymphoblastic leukemia samples. Genes with either a coding SNV or StV were plotted (y-axis) per sample (x-axis). Mutations are colored based on type.
Discussion
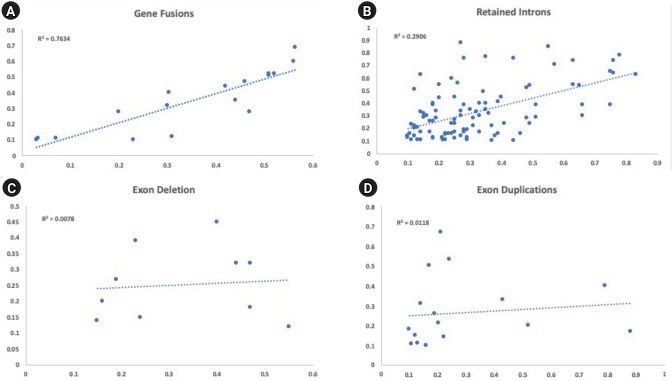
Sequencing of primary acute leukemia patient samples and matching PDX samples showed concordance between the detected variants and their allelic frequencies for the majority of variants tested. The percentage of all variants with absolute delta VAFs <0.2 was 86.7%. This percentage was higher in SNVs (93.6%) compared to StVs (79.6%) across all primary and PDX samples analyzed. Among the different categories of StVs, the allelic frequencies of fusion genes, which are considered to be driver mutations, matched most consistently between the primary and PDX samples (Fig. 5). Our data validate this novel sequencing approach for detection and tracking of diverse variants in primary leukemic samples and corresponding PDX lines.
Fig. 5.
Comparison of variant allele frequencies of structural variants between primary bone marrow samples (x-axis) and matched xenograft sample (y-axis). (A) The variant allele frequencies for all gene fusions were plotted between the primary and xenograft model (R2 = 0.7634). (B) The variant allele frequencies for all retained introns were plotted between the primary and xenograft model (R2 = 0.2906). (C) The variant allele frequencies for all exon deletion were plotted between the primary and xenograft model (R2 = 0.0078). (D) The variant allele frequencies for all exon duplications were plotted between the primary and xenograft model (R2 = 0.0118).
We identified several SNVs, but no StVs, with sustained VAF = 1 in primary and PDX samples across all leukemia subtypes. These SNVs in genes ABL1, BCL2, CCND2, CHIC2, IKZF3, RUNX1, and MECOM, likely represent the germline mutations. Several germline variants, including UTR variants have been shown to be associated with disposition to hematological malignancies [24]. Future characterization of these variants will determine the relevance of these germline UTR variants.
Retained intron variants were detected in all samples except NTPL-59. Retention of introns serves as another mode of regulation of gene expression [25]. Alternative splicing of multi-exon genes in patients with AML compared to normal CD34+ cells has been observed [26]. Such alternative exon usage variants were associated with oncogene expression and drug resistance [27]. Further work is required to understand the biological and clinical significance of alternative exon usage variants.
As we have shown previously, error-correction via the introduction of a nucleic acid-specific UMI allows the removal of NGS errors, retaining only true mutations and significantly improving the sensitivity of NGS [28-30]. In this study, we paired the error-correction strategy with anchored-multiplexed PCR (AMP) chemistry for the quantitative detection of complex structural RNA variants. Recently Benayed et al. [31] published an RNA sequencing approach similar to the one outlined in this manuscript (MSK targeted RNA panel using ArcherDx), and demonstrated that their MSK-IMPACT DNA panel missed cancer-related and targetable mutations in greater than 15% of lung cancer patients. They leveraged ArcherDx FusionPlex technology (identical to our approach) to identify these cases. Additionally, several recent studies have demonstrated the use of AMP technology (ArcherDx) for identifying rare and complex structural variants in pediatric cancers [32,33].
Taken together, advanced sequencing techniques are required to accurately detect and annotate complex StVs that are commonly associated with pediatric leukemias. Such complex variants, including StVs, are not detectable using DNA and short read sequencing technology such as Illumina sequencing platform. Additionally, the RNA molecules that are generated from these complex genomic rearrangements can be difficult to capture. Using an RNA sequencing approach with AMP technology and short read sequencing platform described in this study, pediatric PDX models could be appropriately characterized and validated for concordance of somatic mutations with respect to primary samples. Such analysis is not feasible using standard DNA sequencing techniques. This is one of the first reports to describe pediatric PDX samples using an RNA sequencing approach.
Acknowledgments
The authors would like to thank the Nemours Center for Cancer and Blood Disorders, Nemours Biobank, and the Nemours Biomedical Research Department for supporting this work. This work was supported by the NIH NCI CA211711-01 (PI Druley), Leukemia Research Foundation of Delaware (PI Kolb), and B+ Foundation (PI Barwe).
Footnotes
Authors’ Contribution
Conceptualization: SPB, AG, EAK, ELC. Data curation: SPB, AG, NM, TED. Formal analysis: ELC. Writing - original draft: SPB, AG, NM, TED, EA, ELC. Writing - review & editing: SPB, AG, NM, TED, EA, ELC.
Conflicts of Interest
No potential conflict of interest relevant to this article was reported.
Supplementary Materials
Supplementary data including two tables can be found with this article online at https://doi.org/10.5808/2020.18.1.e6.
Single nucleotide variants detected per subject.
Structural variants detected per subject.
References
- 1.Taylor J, Xiao W, Abdel-Wahab O. Diagnosis and classification of hematologic malignancies on the basis of genetics. Blood. 2017;130:410–423. doi: 10.1182/blood-2017-02-734541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang M, Lindberg J, Klevebring D, Nilsson C, Mer AS, Rantalainen M, et al. Validation of risk stratification models in acute myeloid leukemia using sequencing-based molecular profiling. Leukemia. 2017;31:2029–2036. doi: 10.1038/leu.2017.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hidalgo M, Amant F, Biankin AV, Budinska E, Byrne AT, Caldas C, et al. Patient-derived xenograft models: an emerging platform for translational cancer research. Cancer Discov. 2014;4:998–1013. doi: 10.1158/2159-8290.CD-14-0001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang K, Sanchez-Martin M, Wang X, Knapp KM, Koche R, Vu L, et al. Patient-derived xenotransplants can recapitulate the genetic driver landscape of acute leukemias. Leukemia. 2017;31:151–158. doi: 10.1038/leu.2016.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moorman AV, Chilton L, Wilkinson J, Ensor HM, Bown N, Proctor SJ. A population-based cytogenetic study of adults with acute lymphoblastic leukemia. Blood. 2010;115:206–214. doi: 10.1182/blood-2009-07-232124. [DOI] [PubMed] [Google Scholar]
- 6.Poirion O, Zhu X, Ching T, Garmire LX. Using single nucleotide variations in single-cell RNA-seq to identify subpopulations and genotype-phenotype linkage. Nat Commun. 2018;9:4892. doi: 10.1038/s41467-018-07170-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ward E, DeSantis C, Robbins A, Kohler B, Jemal A. Childhood and adolescent cancer statistics, 2014. CA Cancer J Clin. 2014;64:83–103. doi: 10.3322/caac.21219. [DOI] [PubMed] [Google Scholar]
- 8.Gopalakrishnapillai A, Kolb EA, Dhanan P, Bojja AS, Mason RW, Corao D, et al. Generation of pediatric leukemia xenograft models in NSG-B2m mice: comparison with NOD/SCID mice. Front Oncol. 2016;6:162. doi: 10.3389/fonc.2016.00162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mose LE, Wilkerson MD, Hayes DN, Perou CM, Parker JS. ABRA: improved coding indel detection via assembly-based realignment. Bioinformatics. 2014;30:2813–2815. doi: 10.1093/bioinformatics/btu376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 11.Langmead B, Salzberg SL. Fast gapped-read alignment with Bowtie 2. Nat Methods. 2012;9:357–359. doi: 10.1038/nmeth.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Clevenger J, Chavarro C, Pearl SA, Ozias-Akins P, Jackson SA. Single nucleotide polymorphism identification in polyploids: a review, example, and recommendations. Mol Plant. 2015;8:831–846. doi: 10.1016/j.molp.2015.02.002. [DOI] [PubMed] [Google Scholar]
- 13.Wilm A, Aw PP, Bertrand D, Yeo GH, Ong SH, Wong CH, et al. LoFreq: a sequence-quality aware, ultra-sensitive variant caller for uncovering cell-population heterogeneity from high-throughput sequencing datasets. Nucleic Acids Res. 2012;40:11189–11201. doi: 10.1093/nar/gks918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bolotin DA, Poslavsky S, Mitrophanov I, Shugay M, Mamedov IZ, Putintseva EV, et al. MiXCR: software for comprehensive adaptive immunity profiling. Nat Methods. 2015;12:380–381. doi: 10.1038/nmeth.3364. [DOI] [PubMed] [Google Scholar]
- 15.McLaren W, Gil L, Hunt SE, Riat HS, Ritchie GR, Thormann A, et al. The Ensembl variant effect predictor. Genome Biol. 2016;17:122. doi: 10.1186/s13059-016-0974-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zerbino DR, Birney E. Velvet: algorithms for de novo short read assembly using de Bruijn graphs. Genome Res. 2008;18:821–829. doi: 10.1101/gr.074492.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Anders S, Pyl PT, Huber W. HTSeq: a Python framework to work with high-throughput sequencing data. Bioinformatics. 2015;31:166–169. doi: 10.1093/bioinformatics/btu638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Skinner ME, Uzilov AV, Stein LD, Mungall CJ, Holmes IH. JBrowse: a next-generation genome browser. Genome Res. 2009;19:1630–1638. doi: 10.1101/gr.094607.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.DePristo MA, Banks E, Poplin R, Garimella KV, Maguire JR, Hartl C, et al. A framework for variation discovery and genotyping using next-generation DNA sequencing data. Nat Genet. 2011;43:491–498. doi: 10.1038/ng.806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Young AL, Challen GA, Birmann BM, Druley TE. Clonal haematopoiesis harbouring AML-associated mutations is ubiquitous in healthy adults. Nat Commun. 2016;7:12484. doi: 10.1038/ncomms12484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wickham H. ggplot2: Elegant Graphics for Data Analysis. New York: Springer-Verlag; 2016. [Google Scholar]
- 22.Furness CL, Mansur MB, Weston VJ, Ermini L, van Delft FW, Jenkinson S, et al. The subclonal complexity of STIL-TAL1+ T-cell acute lymphoblastic leukaemia. Leukemia. 2018;32:1984–1993. doi: 10.1038/s41375-018-0046-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Heinaniemi M, Vuorenmaa T, Teppo S, Kaikkonen MU, Bouvy-Liivrand M, Mehtonen J, et al. Transcription-coupled genetic instability marks acute lymphoblastic leukemia structural variation hotspots. Elife. 2016;5:e13087. doi: 10.7554/eLife.13087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kelly JL, Novak AJ, Fredericksen ZS, Liebow M, Ansell SM, Dogan A, et al. Germline variation in apoptosis pathway genes and risk of non-Hodgkin's lymphoma. Cancer Epidemiol Biomarkers Prev. 2010;19:2847–2858. doi: 10.1158/1055-9965.EPI-10-0581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jacob AG, Smith CW. Intron retention as a component of regulated gene expression programs. Hum Genet. 2017;136:1043–1057. doi: 10.1007/s00439-017-1791-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Adamia S, Haibe-Kains B, Pilarski PM, Bar-Natan M, Pevzner S, Avet-Loiseau H, et al. A genome-wide aberrant RNA splicing in patients with acute myeloid leukemia identifies novel potential disease markers and therapeutic targets. Clin Cancer Res. 2014;20:1135–1145. doi: 10.1158/1078-0432.CCR-13-0956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dvinge H, Bradley RK. Widespread intron retention diversifies most cancer transcriptomes. Genome Med. 2015;7:45. doi: 10.1186/s13073-015-0168-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Crowgey EL, Mahajan N, Wong WH, Gopalakrishnapillai A, Barwe SP, Kolb EA, et al. Error-corrected sequencing strategies enable comprehensive detection of leukemic mutations relevant for diagnosis and minimal residual disease monitoring. BMC Med Genomics. 2020;13:32. doi: 10.1186/s12920-020-0671-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wong WH, Tong RS, Young AL, Druley TE. Rare event detection using error-corrected DNA and RNA sequencing. J Vis Exp. 2018;(138):57509. doi: 10.3791/57509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Young AL, Wong TN, Hughes AE, Heath SE, Ley TJ, Link DC, et al. Quantifying ultra-rare pre-leukemic clones via targeted error-corrected sequencing. Leukemia. 2015;29:1608–1611. doi: 10.1038/leu.2015.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Benayed R, Offin M, Mullaney K, Sukhadia P, Rios K, Desmeules P, et al. High yield of RNA sequencing for targetable kinase fusions in lung adenocarcinomas with no mitogenic driver alteration detected by DNA sequencing and low tumor mutation burden. Clin Cancer Res. 2019;25:4712–4722. doi: 10.1158/1078-0432.CCR-19-0225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lebedeva S, Zerkalenkova E, Soldatkina O, Maschan M, Maschan AA, Novichkova G, et al. Novel KMT2A partner gene NUTM2A revealed by anchored multiplex PCR in ALL. Blood. 2019;134(Suppl 1):5203. [Google Scholar]
- 33.Surrey LF, MacFarland SP, Chang F, Cao K, Rathi KS, Akgumus GT, et al. Clinical utility of custom-designed NGS panel testing in pediatric tumors. Genome Med. 2019;11:32. doi: 10.1186/s13073-019-0644-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Single nucleotide variants detected per subject.
Structural variants detected per subject.