Abstract
Background
Acute pancreatitis creates a catabolic stress state promoting a systemic inflammatory response and nutritional deterioration. Adequate supply of nutrients plays an important role in recovery. Total parenteral nutrition (TPN) has been standard practice for providing exogenous nutrients to patients with severe acute pancreatitis. However, recent data suggest that enteral nutrition (EN) is not only feasible, but safer and more effective.Therefore, we sought to update our systematic review to re‐evaluate the level of evidence.
Objectives
To compare the effect of TPN versus EN on mortality, morbidity and length of hospital stay in patients with acute pancreatitis.
Search methods
Trials were identified by computerized searches of The Cochrane Controlled Trials Register, MEDLINE, and EMBASE. Additional studies were identified by searching Scisearch, bibliographies of review articles and identified trials. The search was undertaken in August 2000 and updated in September 2002, October 2003, November 2004 and November 2008. No language restrictions were applied.
Selection criteria
Randomized clinical trials comparing TPN to EN in patients with acute pancreatitis.
Data collection and analysis
Two reviewers independently abstracted data and assessed trial quality. A standardized form was used to extract relevant data.
Main results
Eight trials with a total of 348 participants were included. Comparing EN to TPN for acute pancreatitis, the relative risk (RR) for death was 0.50 (95% CI 0.28 to 0.91), for multiple organ failure (MOF) was 0.55 (95% CI 0.37 to 0.81), for systemic infection was 0.39 (95% CI 0.23 to 0.65), for operative interventions was 0.44 (95% CI 0.29 to 0.67), for local septic complications was 0.74 (95% CI 0.40 to 1.35), and for other local complications was 0.70 (95% CI 0.43 to 1.13). Mean length of hospital stay was reduced by 2.37 days in EN vs TPN groups (95% CI ‐7.18 to 2.44). Furthermore, a subgroup analysis for EN vs TPN in patients with severe acute pancreatitis showed a RR for death of 0.18 (95% CI 0.06 to 0.58) and a RR for MOF of 0.46 (95% CI 0.16 to 1.29).
Authors' conclusions
In patients with acute pancreatitis, enteral nutrition significantly reduced mortality, multiple organ failure, systemic infections, and the need for operative interventions compared to those who received TPN. In addition, there was a trend towards a reduction in length of hospital stay. These data suggest that EN should be considered the standard of care for patients with acute pancreatitis requiring nutritional support.
Keywords: Humans, Enteral Nutrition, Parenteral Nutrition, Acute Disease, Pancreatitis, Pancreatitis/mortality, Pancreatitis/therapy, Randomized Controlled Trials as Topic
Plain language summary
Nutritional support, through the intestine (enteral) versus by injection (parenteral) for people with acute pancreatitis
The pancreas is a gland that lies behind the stomach. It produces enzymes that help digestion. Acute pancreatitis is an inflammation in the pancreas which causes severe pains in the stomach. Extra nutrition is needed to recover. However the pancreas needs rest in order to repair. Nutrition must therefore be given either by a tube into the intestines (enteral) or by injection (parenteral). This review found that patients with acute pancreatitis receiving enteral nutrition have fewer episodes of death, systemic infections, multiple organ failure and operative interventions. This data suggests that EN should be considered the standard of care for patients with acute pancreatitis requiring nutritional support.
Summary of findings
Summary of findings for the main comparison. Enteral compared to parenteral nutrition for acute pancreatitis.
| Enteral compared to parenteral nutrition for acute pancreatitis | ||||||
| Patient or population: patients with acute pancreatitis Settings: Hospitals Intervention: Enteral Comparison: Parenteral nutrition | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Parenteral nutrition | Enteral | |||||
| Mortality | 158 per 1000 | 79 per 1000 (44 to 144) | RR 0.5 (0.28 to 0.91) | 348 (8 studies) | ⊕⊕⊝⊝ low1,2 | |
| Length of hospital stay | The mean Length of hospital stay in the intervention groups was 2.37 lower (7.18 lower to 2.44 higher) | 145 (4 studies) | ⊕⊕⊝⊝ low2,3 | |||
| Multiple Organ Failure(MOF) | 347 per 1000 | 191 per 1000 (128 to 281) | RR 0.55 (0.37 to 0.81) | 278 (6 studies) | ⊕⊕⊕⊝ moderate2 | |
| Operative Intervention | 335 per 1000 | 154 per 1000 (87 to 275) | RR 0.46 (0.26 to 0.82) | 316 (7 studies) | ⊕⊕⊕⊝ moderate4 | |
| Systemic infection | 311 per 1000 | 121 per 1000 (72 to 202) | RR 0.39 (0.23 to 0.65) | 259 (7 studies) | ⊕⊕⊕⊝ moderate4 | |
| Local septic complications | 183 per 1000 | 135 per 1000 (73 to 247) | RR 0.74 (0.4 to 1.35) | 246 (5 studies) | ⊕⊕⊕⊝ moderate4 | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 (McClave 1997) was underpowered and had reported no deaths in both groups this could be either because the population included patiens with mild acute pancreatitis who generally have good prognosis as described in (Abou‐Assi 2002) or death could have been masked because the study was terminated early due to difficulties in compliance and obtaining consent. 2 The majority of studies were of small sample size and with wide confidence intervals only one study (Petrov 2006) had significant results. 3 (McClave 1997) was terminated early and that probably affected the actual length of hospital stay. 4 The majority of studies were of small sample size and with wide confidence intervals only one study (Petrov 2006) had significant results.
Background
Description of the condition
Acute pancreatitis is a common hypermetabolic, hyperdynamic disease process of variable severity that has multiple etiologies and creates a catabolic stress state promoting a systemic inflammatory response and nutritional deterioration (Havala 1989; Steinberg 1994).
In severe acute pancreatitis, the inflammatory response following necrosis or inflammation of the pancreas and retroperitoneal tissues leads to increased caloric requirement and loss of protein mass (Havala 1989). This, combined with absence of oral intake, promotes progressive nutritional deterioration and persistent negative nitrogen balance that appears to be associated with a higher mortality rate as a result of loss of function and structural integrity of vital organs (Bradley 1993; Pisters 1992; Sitzmann 1989).
In critically ill patients, the gastrointestinal tract serves as a potential source of fuel for the immuno‐inflammatory response, since a metabolically deprived gut absorbs endotoxin and other bacterial products, which in turn stimulate endogenous cytokines (Fink 1991). Similar mechanisms have been shown in experimental acute pancreatitis to be a cause of the inflammatory response (Runkel 1991; Ryan 1993). It has also been shown that intestinal permeability increases in patients with severe acute pancreatitis (Ammori 1999). Failure to use the gut may lead to the development of nosocomial infections, sepsis, and organ failure (Hancke 1987; Helton 1990; Lange 1987; Russel 1983).
Description of the intervention
Nutritional status can deteriorate rapidly in patient with severe acute pancreatitis (Bradley 1993; Havala 1989; Pisters 1992; Sitzmann 1989), and subsequent recovery may be prolonged in the severely depleted patient (Keys 1950). Thus, early nutritional support plays an important role in the adjunctive management of these patients to ensure optimum recovery (Kalfarentzos 1991).
Total parenteral nutrition (TPN) has been the standard practice for providing exogenous nutrients to patients with severe acute pancreatitis, while avoiding pancreatic stimulation (Goodgame 1977; Grant 1984; Kalfarentzos 1991; VanGossum 1988). However, TPN is associated with certain disadvantages including cost (Twomey 1985), risk of catheter sepsis, and metabolic and electrolyte disturbances (Goodgame 1977; Grant 1984; Kalfarentzos 1991; VanGossum 1988).
How the intervention might work
Early enteral nutrition (EN) can maintain the integrity and function of intestinal mucosa (Buchman 1995; Maxton 1989). On the other hand, TPN may lead to malfunction of intestinal mucosal barrier, which may promote gut origin sepsis (Saadia 1990). This may be secondary to gut atrophy, or due to the absence of glutamine, which is not included in conventional solutions (Gardiner 1995).
Studies of other immuno‐inflammatory conditions associated with trauma (McClave 1986), thermal injury (Alexender 1980), and major surgery (Moore 1992) have shown that septic morbidity can be reduced by early introduction of EN.
One of the most important aspects of nutritional support in acute pancreatitis is to put the pancreas to rest, to avoid its stimulation (Povoski 1995). Pancreatic stimulation can be avoided if enteral nutrition is delivered to the small intestine distal to the ligament of Treitz, since nutrients infused into the jejunum result in minimal or negligible stimulation (Corcoy 1988).
Why it is important to do this review
EN has the advantages of being less expensive and having the potential for protecting the gut barrier (Scolapio 1999). However, EN has limitations that may reduce its applicability. These include the risk of proximal tube migration with subsequent pancreatic stimulation resulting in aggravation of pancreatitis, and the presence of ileus (McClave 1998; Scolapio 1999).
Recent data suggest that enteral nutrition is not only feasible, but safer and more effective than TPN in patients with acute pancreatitis. A number of national and international societies recommend EN, but these recommendations are mostly based on randomised controlled trials (Banks 2006; British Society of Gastroenterology 2005). Therefore, we sought to update our systematic review to re‐evaluate the level of evidence.
Objectives
The primary objective was to conduct a systematic review to compare the effect of total parenteral nutrition (TPN) versus enteral nutrition (EN) on mortality, morbidity and length of hospital stay in patients with acute pancreatitis.
Methods
Criteria for considering studies for this review
Types of studies
Randomized clinical trials, in which nutritional support with TPN was compared to EN in patients with acute pancreatitis.
Types of participants
Patients with a diagnosis of acute pancreatitis established by clinical presentation and elevated serum amylase. The study should include a recognized assessment of acute pancreatitis severity such as Ranson's criteria, Acute Physiology and Chronic Health Evaluation (APACHE) II score (Knaus 1985), Imrie classification (Blamey 1984), C‐reactive protein concentration, or Balthazar CT scan score (Balthazar 1990).
Types of interventions
Total parenteral nutrition (TPN) delivered through a central or peripheral venous line.
Enteral nutrition (EN) delivered through a nasoenteric feeding tube placed endoscopically or under fluoroscopy down into the jejunum at or below the level of ligament of Treitz, or confirmed radiologically after placement.
Types of outcome measures
We considered trials if any of the following clinical outcomes were reported:
Death
Length of hospital stay
Systemic inflammatory response syndrome (SIRS)
Multiple organ failure (MOF)
Operative intervention
Systemic infection (septicemia, urinary tract infection (UTI), pneumonia, line infection)
Local septic complications (pancreatic abscess formation, infected necrosis)
Other local complications (fluid collection, pseudocyst, sterile pancreatic necrosis, fistula)
Protection of gut mucosal barrier as estimated, indirectly, by changes in the serum level of IgM anti‐endotoxin core antibody (Endo CAb), total antioxidant capacity (TAC), Tumor Necrosis Factor (TNF), or Interlukin‐6 (IL‐6)
Search methods for identification of studies
Electronic searches
The following bibliographic databases were searched in order to identify relevant primary studies:
Cochrane Controlled Trials Register (CCTR)
MEDLINE
EMBASE
Trials were identified by searching MEDLINE 1966‐2008 November week 3, The Cochrane Library 4th quarter 2008, EMBASE 1980‐2008 week 49. We did not confine our search to English language publications. Searches in all databases were updated in September 2002, October 2003, November 2004 and December 2008. The Cochrane Highly Sensitive Search Strategy for identifying randomized trials in MEDLINE, sensitivity maximising version, Ovid format (Lefebvre 2008), was combined with the search terms to identify randomized controlled trials in MEDLINE. The MEDLINE search strategy was adapted for use in the other databases searched (Appendix 1; Appendix 2; Appendix 3).
Searching other resources
Additional studies were identified and included where relevant by searching Scisearch, the bibliographies of review articles and identified trials, and personal files. For ongoing trials, the following web site was searched: www.clinicaltrials.gov.
Data collection and analysis
Selection of studies
All retrieved studies' titles were scanned independently by two authors (MFT and ZHB), to assess whether it should be included or excluded. Disagreement was solved by consensus between them both, and full articles were retrieved for those found to be relevant. To avoid over‐representing duplicate studies in the review, duplicate publications were excluded.
Data extraction and management
Two reviewers (MFT and ZHB) extracted data using a standard form to record data independently. Recorded data were cross checked by the reviewers. If an agreement could not be reached by consensus, the third author (MA) was consulted. The data were entered into RevMan (version 5.0.20) for analysis. The following parameters were extracted: Number of deaths, SIRS, MOF, operative interventions, local septic complications (pancreatic abscess formation, infected necrosis), other local complications (fluid collection, pseudocyst, sterile pancreatic necrosis, fistula), systemic infection (septicemia, UTI, pneumonia, line infection), protection of gut mucosal barrier parameters, and length of hospital stay in days.
Assessment of risk of bias in included studies
Two authors (MFT and ZHB) independently assessed the selected trials for methodological quality using The Cochrane Collaboration method for assessing risk of bias (Higgins 2008). This set of criteria is based on evidence of associations between overestimates of effect and high risk of bias of the article such as sequence generation, allocation concealment, blinding, incomplete outcome data and selective reporting. The categories are defined below:
YES ‐ low risk of bias NO ‐ high risk of bias UNCLEAR ‐ uncertain risk of bias
We resolved disagreements by consensus, or, if this failed, by consulting a third author (MA).
Other validity criteria to assess studies included the following:
baseline comparability of treatment groups (Severity score)
presence of inclusion and exclusion criteria
intervention described in detail
definition of outcomes
stated time for outcome assessment
stated indications for further interventions
We planned on assessing for reporting and publication bias by funnel plots if ten or more studies were available for an outcome. This minimum number of studies was decided upon because if asymmetry were evident, tests for funnel plot asymmetry would have low power to distinguish chance from real asymmetry (Sterne 2008).
Measures of treatment effect
The extracted data from the trials was combined by calculating a pooled estimate of the relative risk and 95% confidence interval for dichotomous data using fixed‐effects model if the data was homogenous and a random‐effects model if the data was heterogenous indicated by a P value <0.1. Where continuous data outcomes were measured in a standard way across studies, the mean difference (and 95% confidence interval) was calculated; using a fixed‐effects model for homogenous data and a random‐effects model for heterogenous data. RevMan Software (version 5.0.20) was used for statistical analysis.
Results
Description of studies
Results of the search
The updated search generated 779 studies. No relevant ongoing trials were found upon searching www.clinicaltrials.gov. After screening, eleven were considered to be potentially eligible and from these, five were excluded. Six randomized clinical trials comparing EN and TPN fulfilled the criteria for consideration into the update of this review. In total, this review now includes eight trials comparing EN to TPN for acute pancreatitis.
Included studies
For details of included studies see Characteristics of included studies.
We included eight randomized controlled trials of published studies. Countries of publication varied with three from North America (Abou‐Assi 2002; McClave 1997; Louie 2005), one from Greece (Kalfarentzos 1997), Russia (Petrov 2006), Spain (Casas 2007), United Kingdom (Gupta 2003) and Hungary (Olah 2002); all were in English. We contacted authors of trials who did not report means and/or standard deviations of continuous outcomes such as length of hospital stay or measurements of gut mucosal barrier protection (Casas 2007; Gupta 2003; Kalfarentzos 1997). Two replied (Gupta 2003; Casas 2007), but only the latter was able to provide the missing data, and it was included. The eight included trials involved a total of 348 participants.
All these studies compared EN with TPN for acute pancreatitis. The number of participants included in each trial ranged between 17 and 89 and their ages ranged between 21 and 91 years.
Inclusion criteria
Inclusion criteria were fairly consistent among trials in diagnosis of acute pancreatitis that is, clinical signs with an elevated serum marker. Only one study (Louie 2005) did not mention how acute pancreatitis was diagnosed. All the trials included males and females except one trial that included only males (McClave 1997). Among these eight trials, five only included particpants with severe acute pancreatitis (SAP) (Casas 2007; Kalfarentzos 1997; Louie 2005; Petrov 2006; Gupta 2003), and one included a subgroup analysis of SAP patients (Abou‐Assi 2002). Criteria for diagnosing severity were variable, the most commonly used was the Acute Physiology and Chronic Health Evaluation (APACHE II) score followed by Ransons criteria.
Exclusion criteria
These varied, with three studies excluding pregnant women (Casas 2007; Gupta 2003; Petrov 2006), three others excluded young patients younger than 18 years old (Casas 2007; Louie 2005; Petrov 2006) and one less than 16 years of age (Gupta 2003). Another common feature to exclude participants was inability to initiate enteral feeding (Louie 2005; McClave 1997; Olah 2002).
Intervention
For the enteral group, the nasojejunal tube was inserted fluoroscopically or endoscopically in all the trials except two (Olah 2002; Gupta 2003), where the tube was inserted blindly and then confirmed by radiology. The TPN group had nutrition supplied through a central line. Two trials, in addition to using a central line in some patients, used a peripheral line for nutrition in others (Abou‐Assi 2002; McClave 1997).
Outcome measurements
Outcomes varied, with mortality being consistent among them all (see Characteristics of included studies).
Excluded studies
In total, eight studies were excluded (Hernandez‐Aranda1996; Windsor 1996; Windsor 1998; Powell 2000; Eckerwall 2006; Modena 2006; Ye 2007; Bao 2006 ). The first three were previously excluded from the search generated for the original review in 2000, while the latter four trials were excluded from the search that was done for the latest update of this review. One study was in Chinese and this was found to be initially relevant from its abstract, translation was undertaken and the study was then excluded because both groups were given TPN before initiating EN in the comparison group (Bao 2006). One compared TPN with TPN+EN (Ye 2007 ), the other compared TPN with nutrition delivered through a nasogastric rather than a nasojejunal tube (Eckerwall 2006), another compared EN to conventional treatment that did not include TPN (Powell 2000), and the final one was not randomized (Modena 2006).
Details of exclusion for the remaining studies are described in Characteristics of excluded studies.
Risk of bias in included studies
This is illustrated in (Figure 1 and Figure 2).
1.
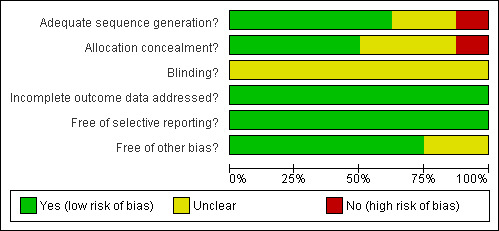
Methodological quality graph: review authors' judgements about each methodological quality item presented as percentages across all included studies.
2.
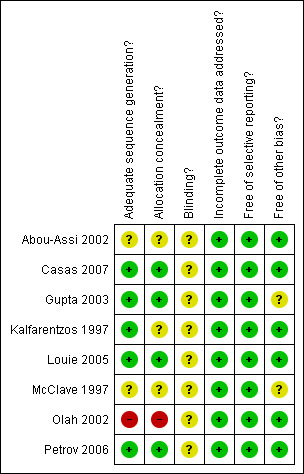
Methodological quality summary: review authors' judgements about each methodological quality item for each included study.
Allocation
Concealment of the allocation process was adequately done in half of the studies (Casas 2007; Louie 2005; Petrov 2006; Gupta 2003). For the other half, three did not report sufficient details to judge and were therefore categorized as being unclear (Kalfarentzos 1997; Abou‐Assi 2002; McClave 1997), and one reported an inadequate method by allocating according to the day of birth (Olah 2002).
Blinding
This was not addressed in any of the studies included. We acknowledge that blinding of the intervention is impossible, even attempts to blind radiologists, for example, would not work since the nasojejunal tube would be apparent on radiological images.
Incomplete outcome data
In all eight studies, all participants randomised had completed the study and there were no missing data for reported outcomes.
Selective reporting
All studies included all primary and secondary outcomes described in their introduction and/or methods in the results section in the pre‐specified way. One study did not provide details of the statistical methods used in some continuous outcomes, the authors were contacted and provided the missing data (Casas 2007).
Other potential sources of bias
Sources of funding that had involvement of one of their products in the intervention or comparison group were considered to be potential sources of bias if they did not provide further details on the extent of their involvement in the study. Consequently, a concern arose for two studies because of their source of funding (Gupta 2003; McClave 1997). Both trials did not provide any further detail of whether their funding agency had any role in the design or approval of publishing the study. In the other study (McClave 1997), the trial was stopped early for difficulty in recruitment, compliance and obtaining consent. Their study at that point showed significant difference of cost in favour of the intervention group and reported no deaths in either group. This was also considered to be an unclear contributor to bias because the absence of mortality may have been either due to the low risk population included in the study (mild pancreatitis), or the early termination may have masked it.
In reference to our pre‐specified criteria for considering funnel plots, we did not attempt to investigate for publication bias because fewer than ten studies were identified.
Effects of interventions
See: Table 1
See Data and analyses for relevant forest plots, and Table 1 for a summary of important outcomes and their level of evidence.
1. Mortality
This is the only outcome reported in all eight studies with a total number of 348 participants. The studies were homogenous and so a fixed‐effects model was used. The relative risk (RR) for death was 0.50 (95% CI 0.28 to 0.91; Analysis 1.1) (see Figure 3). Two studies had no deaths throughout the study period (Gupta 2003; McClave 1997). In one study (Abou‐Assi 2002), they analysed that death in two from the TPN group and four from the EN group occurred after resolution of acute pancreatitis and was attributed to other causes; cancer of the liver, cardiac surgery, squamous cell carcinoma of the pharynx and intracerebral hemorrhage. A meta‐analysis performed by excluding those patients resulted in a RR for death of 0.39 (95% CI 0.2 to 0.77; Analysis 1.2) (see Figure 4), being statistically significant in both cases.
1.1. Analysis.

Comparison 1 Enteral versus parenteral nutrition for acute pancreatitis, Outcome 1 Mortality.
3.
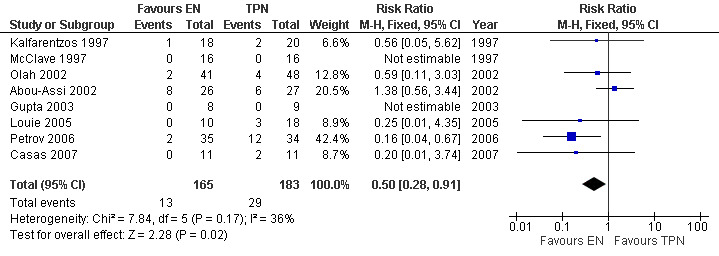
Forest plot of comparison: 1 Enteral versus parenteral nutrition for acute pancreatitis, outcome: 1.1 Mortality.
1.2. Analysis.

Comparison 1 Enteral versus parenteral nutrition for acute pancreatitis, Outcome 2 Mortality in patients with acute pancreatitis excluding those from (Abou‐Assi 2002) in which death resulted after resolution of acute pancreatitis and was attributed to other causes; cardiac surgery, cancer of the liver, squamous cell carcinoma of the pharynx and intracerebral hemorrhage..
4.
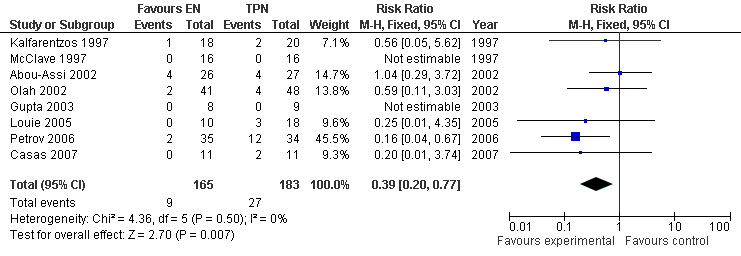
Forest plot of comparison: 1 Enteral versus parenteral nutrition for acute pancreatitis, outcome: 1.2 Mortality in patients with acute pancreatitis excluding those from (Kalfarentzos 1997) in which death resulted after resolution of acute pancreatitis and was attributed to other causes; cardiac surgery, cancer of the liver, squamous cell carcinoma of the pharynx and intracerebral hemorrhage..
Among those eight trials, five included only patients with severe acute pancreatitis (SAP); (Gupta 2003; Casas 2007; Louie 2005; Petrov 2006; Kalfarentzos 1997). Subgroup analysis was done by excluding one of them (Kalfarentzos 1997) because not all included participants were assessed for severity. The meta‐analysis showed a RR of 0.18 (95% CI 0.06 to 0.58; Analysis 1.3) (see Figure 5).
1.3. Analysis.

Comparison 1 Enteral versus parenteral nutrition for acute pancreatitis, Outcome 3 Mortality in patients with severe acute pancreatitis (SAP).
5.
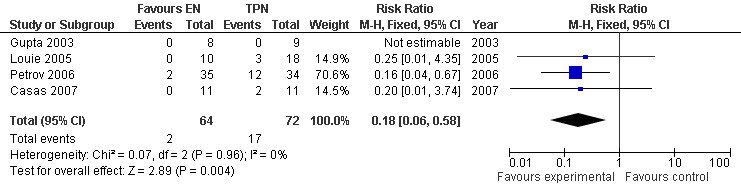
Forest plot of comparison: 1 Enteral versus parenteral nutrition for acute pancreatitis, outcome: 1.2 Mortality in patients with severe acute pancreatitis (SAP).
2. Length of hospital stay
Five studies reported this outcome (Casas 2007; Gupta 2003; Abou‐Assi 2002; McClave 1997; Kalfarentzos 1997), but only four were included in the meta‐analysis with a sample number of 145. Three studies provided sufficient data to be entered in the meta‐analysis (McClave 1997; Abou‐Assi 2002; Casas 2007), the latter by contacting the author for missing standard devation (SD) figures. One trial, (Kalfarentzos 1997) had missing SD, the author was contacted but no reply was received, so this was imputed by calculating the average of the SD of the other three studies in accordance with the recommendation stated in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2008a). The last remaining study (Gupta 2003) did not report any means or SD, the author was contacted but was unable to provide the missing data, and this study was therefore excluded from this meta‐analysis. A fixed‐effects model was applied since the studies were homogenous. The MD for length of hospital stay with EN was ‐2.37 (95% CI ‐7.18 to 2.44; Analysis 2.1) (see Figure 6).
2.1. Analysis.

Comparison 2 Enteral versus parenteral nutrition for acute pancreatitis, Outcome 1 Length of hospital stay.
6.
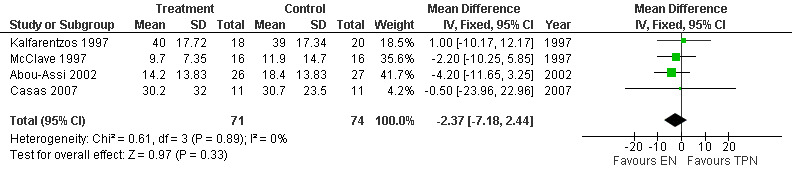
Forest plot of comparison: 2 Enteral versus parenteral nutrition for acute pancreatitis, outcome: 2.1 Length of hospital stay.
Among those four included studies (Casas 2007; Abou‐Assi 2002; McClave 1997; Kalfarentzos 1997), one included only patients with SAP (Casas 2007), and another included a subgroup analysis of patients with SAP for length of hospital stay only (Abou‐Assi 2002). We performed a subgroup analysis including both those trials ‐ including only the subgroup of the latter study ‐ and this resulted in a MD of ‐6.27 (95% CI ‐15.40 to 2.87; Analysis 2.2) (see Figure 7).
2.2. Analysis.

Comparison 2 Enteral versus parenteral nutrition for acute pancreatitis, Outcome 2 Subgroup analysis for length of hospital stay in patients with severe acute pancreatitis.
7.
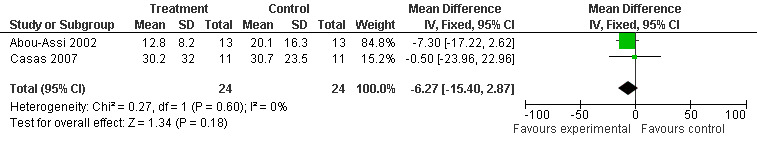
Forest plot of comparison: 2 Enteral versus parenteral nutrition for acute pancreatitis, outcome: 2.2 Subgroup analysis for length of hospital stay in patients with severe acute pancreatitis.
3. Systemic inflammatory response syndrome (SIRS)
This was reported in only one study (Casas 2007) where the RR for SIRS was 1.00 (95% CI 0.17 to 5.89; Analysis 3.1) (see Figure 8).
3.1. Analysis.

Comparison 3 Enteral versus parenteral nutrition for acute pancreatitis, Outcome 1 Systemic Inflammatory Response Response Syndrome(SIRS).
8.
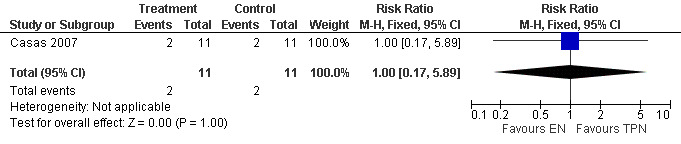
Forest plot of comparison: 3 Enteral versus parenteral nutrition for acute pancreatitis, outcome: 3.1 Systemic Inflammatory Response Response (SIRS).
4. Multiple organ failure (MOF)
Six studies collected data for this outcome comprising 278 participants (Abou‐Assi 2002; Olah 2002; Casas 2007; Gupta 2003; Louie 2005; Petrov 2006). The RR was 0.55 (95% CI 0.37 to 0.81; Analysis 4.1) (see Figure 9).
4.1. Analysis.

Comparison 4 Enteral versus parenteral nutrition for acute pancreatitis, Outcome 1 Multiple Organ Failure(MOF).
9.
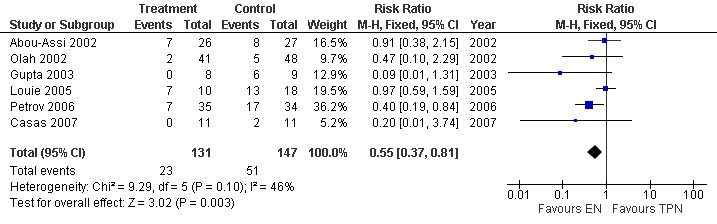
Forest plot of comparison: 4 Enteral versus parenteral nutrition for acute pancreatitis, outcome: 4.1 Multiple Organ Failure(MOF).
Four of those trials included only patients with SAP (Casas 2007; Gupta 2003; Louie 2005; Petrov 2006) and so a subgroup analysis was done that showed a RR of 0.46 (95% CI 0.16 to 1.29; Analysis 4.2) (see Figure 10).
4.2. Analysis.

Comparison 4 Enteral versus parenteral nutrition for acute pancreatitis, Outcome 2 MOF in Severe Acute Pancreatitis.
10.
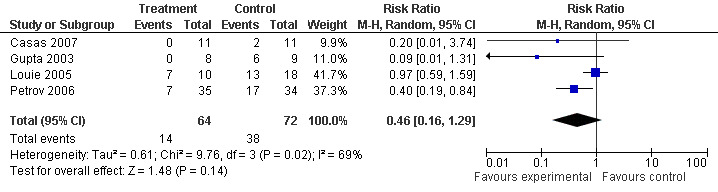
Forest plot of comparison: 4 Enteral versus parenteral nutrition for acute pancreatitis, outcome: 4.2 MOF in Severe Acute Pancreatitis.
5. Operative intervention
This was reported in seven studies with 316 participants (Casas 2007; Gupta 2003; Louie 2005; Petrov 2006; Abou‐Assi 2002; Kalfarentzos 1997; Olah 2002). Operative interventions showed a RR of 0.44 (95% CI 0.29 to 0.67; Analysis 5.1) (see Figure 11).
5.1. Analysis.

Comparison 5 Enteral versus parenteral nutrition for acute pancreatitis, Outcome 1 Operative Intervention.
11.
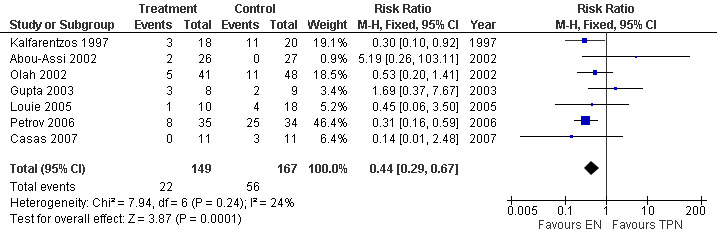
Forest plot of comparison: 5 Enteral versus parenteral nutrition for acute pancreatitis, outcome: 5.1 Operative Intervention.
In patients with SAP in four of those trials (Casas 2007; Gupta 2003; Louie 2005; Petrov 2006), the RR was 0.38 (95% CI 0.22 to 0.66; Analysis 5.2) (see Figure 12).
5.2. Analysis.

Comparison 5 Enteral versus parenteral nutrition for acute pancreatitis, Outcome 2 Operative intervention in SAP.
12.
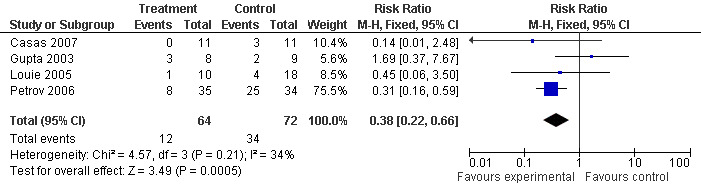
Forest plot of comparison: 5 Enteral versus parenteral nutrition for acute pancreatitis, outcome: 5.2 Operative intervention in SAP.
6. Systemic infection
This outcome was reported in seven trials with a total of 295 participant (Casas 2007; Gupta 2003; Louie 2005; Petrov 2006; Abou‐Assi 2002; Kalfarentzos 1997; McClave 1997 ), and showed a RR of 0.39 (95% CI 0.23 to 0.65; Analysis 6.1) (see Figure 13).
6.1. Analysis.

Comparison 6 Enteral versus parenteral nutrition for acute pancreatitis, Outcome 1 Systemic infection.
13.
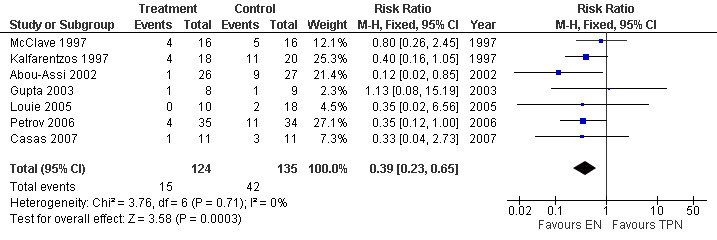
Forest plot of comparison: 6 Enteral versus parenteral nutrition for acute pancreatitis, outcome: 6.1 Systemic infection.
Among those, the four trials that included only patients with SAP (Casas 2007; Gupta 2003; Louie 2005; Petrov 2006), had a RR of 0.39 (95% CI 0.17 to 0.90; Analysis 6.2) (see Figure 14)
6.2. Analysis.

Comparison 6 Enteral versus parenteral nutrition for acute pancreatitis, Outcome 2 Systemic infection in SAP.
14.
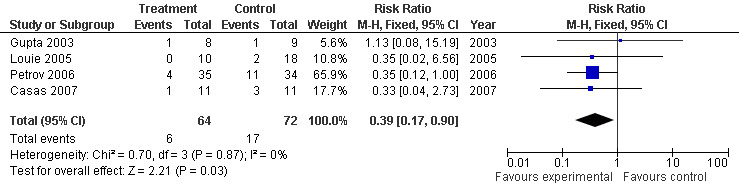
Forest plot of comparison: 6 Enteral versus parenteral nutrition for acute pancreatitis, outcome: 6.2 Systemic infection in SAP.
7. Local septic complications
Five studies assessed this outcome including a total of 246 participants. The RR for local septic complications with EN vs TPN was 0.74 (95% CI 0.40 to 1.35; Analysis 7.1) (Figure 15).
7.1. Analysis.

Comparison 7 Enteral versus parenteral nutrition for acute pancreatitis, Outcome 1 Local septic complications.
15.
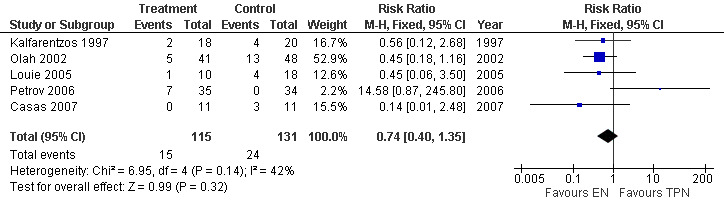
Forest plot of comparison: 7 Enteral versus parenteral nutrition for acute pancreatitis, outcome: 7.1 Local septic complications.
Subgroup analysis of the three trials with SAP (Casas 2007; Louie 2005; Casas 2007), had a RR of 0.93 (95% CI 0.07 to 12.59; Analysis 7.2) (see Figure 16).
7.2. Analysis.

Comparison 7 Enteral versus parenteral nutrition for acute pancreatitis, Outcome 2 Local septic complications in SAP.
16.
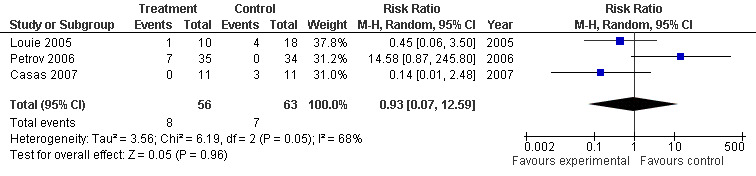
Forest plot of comparison: 7 Enteral versus parenteral nutrition for acute pancreatitis, outcome: 7.2 Local septic complications in SAP.
8. Other local complications
There were five studies that reported this outcome with a total sample size of 230 (Casas 2007; Louie 2005; Abou‐Assi 2002; Olah 2002; Kalfarentzos 1997). The RR for other local complications with EN vs TPN was 0.70 (95% CI 0.43 to 1.13; Analysis 8.1) (Figure 17).
8.1. Analysis.

Comparison 8 Enteral versus parenteral nutrition for acute pancreatitis, Outcome 1 Other local complications.
17.
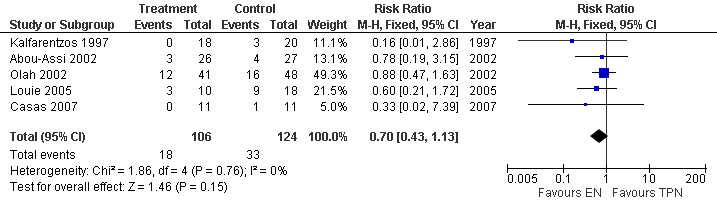
Forest plot of comparison: 8 Enteral versus parenteral nutrition for acute pancreatitis, outcome: 8.1 Other local complications.
Subgroup analysis of two of those trials with SAP (Casas 2007; Louie 2005), revealed a RR of 0.55 (95% CI 0.20 to 1.50; Analysis 8.2) (see Figure 18).
8.2. Analysis.

Comparison 8 Enteral versus parenteral nutrition for acute pancreatitis, Outcome 2 Other local complications in SAP.
18.
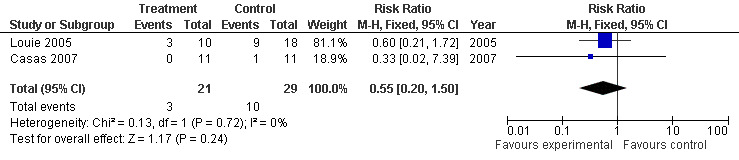
Forest plot of comparison: 8 Enteral versus parenteral nutrition for acute pancreatitis, outcome: 8.2 Other local complications in SAP.
9. Protection of gut mucosal barrier
This was reported in only two studies (Casas 2007; Gupta 2003). They each reported different parameters and there was insufficient data to include them in a meta‐analysis so a narrative description was used instead.
In the first study (Casas 2007), the authors measured serum levels of tumour necrosis factor alpha (TNF‐α) and Interleukine‐6 (IL‐6) at baseline, day 5 and day 10. This data was provided in graphs without absolute values of means or SD. Furthermore, no details were provided about which statistical measures were used. The author was contacted and provided the missing data, we used that to calculate the change in score from the baseline. Two decimals were taken and rounded to the closest whole number. For TNF‐α, the change in means from baseline was 59.3% for the EN group and ‐1.2% for the TPN group. On the other hand, IL‐6 showed 83.6% reduction from the baseline value compared to 58.7% for TPN. The study concluded that there were no significant differences observed between the two groups with a P value >0.05.
In (Gupta 2003), the study measured plasma glutamine levels, thiobarbituric acid‐reactive substances (TBARS), and the concentration of anti‐endotoxin antibodies (IgG and IgM) on admission, day 3 and day 7. These values were presented in medians and ranges. From the provided tables, we calculated the median change in score for each outcome. In the EN group, serum glutamine level showed a reduction of 6.3% compared to a 15.9% increase for TPN. For TBARS, there was a 56.7% and 95.6% increase from baseline for the EN and TPN group respectively. Moving on to the plasma anti‐endotoxin core antibodies, the EN group had a 44.8% and 21.2% reduction in the IgG and IgM levels respectively, while the TPN group revealed an increase of 28.4% and 38.8% of IgG and IgM levels respectively. The authors concluded that there was no difference in glutamine or TBARS concentrations between the groups at any time. Both IgG and IgM concentrations were raised in all patients on admission. IgG levels tended to fall over the study period in the EN group, while a significant rise was observed in the TPN group from day 3 to day 7 (P = 0.028). IgM antibodies fell significantly in the EN group, while in the TPN group, they had a non‐significant rise (P = 0.51).
Discussion
Summary of main results
One of the main concerns in severe acute pancreatitis is the high risk of mortality that is most commonly secondary to systemic infections and multiple organ failure. The findings of this review show significant benefits favouring EN over TPN by decreasing mortality, multiple organ failure, systemic infection, and operative interventions. In addition, there was a trend for a decrease in length of hospital stay, local septic complications and other local complications. All the trials resulted in outcomes that favour the EN group over the TPN group. The quality of the evidence supporting these findings is summarized in Table 1 showing low quality for mortality and length of hospital stay while all other outcomes were of moderate quality.
We undertook subgroup analysis of trials that included only patients with SAP for all outcomes to see if there would be a change in the magnitude of effect. This included four trials that included only patients with SAP (Casas 2007; Gupta 2003; Louie 2005; Petrov 2006), and one additional trial that included a subgroup analysis for SAP patients for length of hospital stay only (Abou‐Assi 2002). Interestingly, the main outcome that is most likely to affect decision makers; mortality, showed a dramatic reduction from a RR of 0.50 (95% CI 0.28 to 0.91) in all patients with acute pancreatitis to a RR of 0.18 (95% CI 0.06 to 0.58) in patients with SAP in favour of the EN group (see Figure 3; Figure 5). Furthermore, the need for operative interventions similarly showed a reduction in RR for patients with SAP receiving EN (see Figure 12), while systemic infections were similar in both groups (Figure 13; Figure 14). It was also observed that a trend in further reduction in length of hospital stay, the incidence of MOF, local septic complications and other local complications was evident in the EN group.(Figure 7; Figure 10; Figure 16; Figure 18). We find that these results are important and should be taken into consideration when encountering patients with SAP and deciding on the route of supplemental nutrition.
Overall completeness and applicability of evidence
All the studies we included involved patients with acute pancreatitis who received either TPN or EN. Endpoints of the studies were similar overall to the intended outcome measures of the review. Severity of pancreatitis varied among studies, with some including only patients with severe acute pancreatitis (SAP), others moderate, and some a combination of both. This should be kept in mind when taking decisions for different patients.
Quality of the evidence
Overall, our review included eight randomised controlled trials with a total of 348 participants. Key methodological limitations included inadequate method of allocation concealment in one study (Olah 2002); using day of birth for allocation and three others provided insufficient information to judge. However, although two studies were underpowered (McClave 1997 and Louie 2005) and the majority of studies included a rather small number of participants, they were consistent in their results in terms of favouring EN over TPN.
Potential biases in the review process
A number of limitations were encountered in the process of updating this review. The studies included were of small sample size, and two trials were underpowered to detect the desirable outcome (Louie 2005; McClave 1997).
A limitation we came across was the common finding of missing statistical measurements such as the standard deviation (SD) for length of hospital stay in Gupta 2003; Casas 2007; Kalfarentzos 1997. The authors were contacted, two replied, all missing data was provided by one (Casas 2007), one was unable to provide the missing data (Gupta 2003), and no response received from the remaining one. In Gupta 2003, the measurements of continuous outcomes were presented in medians and ranges. No other statistical measurement were provided to indicate if the data was skewed or not, furthermore, no other measurement of variance was provided to indirectly calculate the SD. Considering the rather small sample size (N=17) we assumed that it was skewed and did not include it in the meta‐analysis. Two studies did not directly provide the SD (Abou‐Assi 2002; McClave 1997 ) so it was calculated from the standard error (SE), and in one (Kalfarentzos 1997), it was imputed by calculating the average of the other three studies.
None of studies addressed any conflict of interest. In two studies (Gupta 2003; McClave 1997), the extent of involvement of their funding provider was not addressed. This might have been helpful considering that their product was used in the intervention group and their study revealed a positive result.
Agreements and disagreements with other studies or reviews
A couple of meta‐analyses have addressed the issue of route of nutrition in acute pancreatitis and its effect on a number of important outcomes. Marik and Zaloga (Marik 2004) included six trials with 263 participants and found significant reduction in infections, surgical interventions and length of hospital stay in the EN group compared to the TPN group. A reduction in mortality was also observed but was not statistically significant. Similarly, McClave and colleagues included seven trials with a total of 291 patients and found that the EN group had significant reduction in length of hospital stay and infectious morbidity compared to the TPN group (McClave 2006). The reduction in mortality was once again found to be insignificant. Both meta‐analyses concluded that EN should be the preferred route in patients with severe acute pancreatitis.
We disagree with McClave 2006 in the fact that the conclusions did not put into consideration the validity of the results described. On the other hand, Marik 2004 utilized a scale for assessment of risk of bias by Jadad and colleagues (Jadad 1996) that is an unreliable tool for assessment of validity suffering from the generic problems of scales (Jüni 1999).
Our review is in agreement with the above mentioned meta‐analyses in that the group receiving EN had less morbidity and mortality. Furthermore, it gives an updated and comprehensive overview of the topic at hand with results based on the risk of bias for each outcome by reliable tools that allow transparent and detailed report of factors affecting validity (Higgins 2008).
Authors' conclusions
Implications for practice.
The findings of this review support the use of EN in patients with acute pancreatitis requiring nutritional support over TPN. Patients receiving EN are less likely to suffer form MOF, systemic infections, operative interventions and, more importantly, death. The quality of evidence for these outcomes are of moderate quality (as shown in Table 1) except for death being of low quality. The best available evidence is in favour of EN.
Implications for research.
The current evidence suggests that enteral nutrition via a nasojejunal tube is superior to TPN in patients with acute pancreatitis. Nasojejunal tubes have their own difficulties in insertion, dislodgement and blockage. A number of trials have proposed yet an easier and cheaper method for nutrition; enteral nutrition to the stomach rather than to the jejunum and have shown its safety and effectiveness (Eatock 2005; Kumar 2006). Further well conducted randomized controlled trials comparing enteral nutrition via nasogastric tubes to nasojejunal tubes in patients with severe acute pancreatitis with adequately calculated sample sizes to test for equivalence are required before proposing this method of treatment in such patients. Outcomes that are necessary to address include mortality, multiple organ failure, systemic infections, length of hospital stay, the need for operative interventions, local septic complications, and SIRS.
What's new
| Date | Event | Description |
|---|---|---|
| 5 October 2010 | Amended | Contact details updated. |
History
Protocol first published: Issue 4, 2000 Review first published: Issue 2, 2001
| Date | Event | Description |
|---|---|---|
| 12 August 2009 | New citation required and conclusions have changed | Updated, new studies found, additions to the methods section, conclusions changed and additional authors contributed to the review. |
| 30 October 2008 | Amended | Converted to new review format. |
| 19 July 2004 | Amended | Minor update. |
| 2 July 2004 | New search has been performed | New studies sought but none found. |
| 30 October 2002 | New citation required and conclusions have changed | Substantive amendment. |
Acknowledgements
We would like to acknowledge the major contributions of Dr Ala Groof and Dr Derek Wilke for previous versions of this review, they would like to extend their thanks to Dr Arne Ohlsson for his efforts in teaching them how to conduct a systemic review during the Cochrane Reviews course at University of Toronto. We would like to thank those whom assisted us in hunting down the full articles for a number of studies; Racquel Simpson and Dr Ayman Abdo. For statistical support and guidance, we would like to thank Dr Shaffi from King Saud University.
Appendices
Appendix 1. MEDLINE SEARCH STRATEGY
Search strategy (MEDLINE 1966‐ to November Week 3 2008)
1 randomized controlled trial.pt. (269477) 2 controlled clinical trial.pt. (80776) 3 randomized.ab. (177355) 4 placebo.ab. (111337) 5 drug therapy.fs. (1318399) 6 randomly.ab. (128722) 7 trial.ab. (184716) 8 groups.ab. (890308) 9 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 (2386281) 10 humans.sh. (10826325) 11 9 and 10 (1953119) 12 exp pancreas/ (84903) 13 exp pancreatitis/ (37139) 14 panc$.tw. (173342) 15 or/12‐14 (200177) 16 exp enteral nutrition/ (12820) 17 exp parenteral nutrition/ (19417) 18 exp nutritional support/ (32587) 19 enteral$.tw. (11072) 20 parenteral$.tw. (36489) 21 TPN.tw. (3414) 22 PN.tw. (6162) 23 EN.tw. (20079) 24 or/16‐23 (85483) 25 15 and 24 (2919) 26 25 and 11 (571) 27 limit 26 to yr="2004 ‐ 2009" (175) 28 from 27 keep 1‐175 (175)
Appendix 2. EMBASE search strategy
Search strategy (EMBASE 1980‐ 2008 week 50) 1 exp randomized controlled trial/ (163768) 2 randomized controlled trial$.tw. (23434) 3 exp randomization/ (26367) 4 exp single blind procedure/ (7858) 5 exp double blind procedure/ (70768) 6 or/1‐5 (216794) 7 animal.hw. (2211384) 8 human.hw. (6476512) 9 7 not (7 and 8) (1981360) 10 6 not 9 (211800) 11 exp clinical trial/ (534057) 12 (clin$ adj3 stud$).ti,ab,tw. (113037) 13 (clin$ adj3 trial$).ti,ab,tw. (121139) 14 ((singl$ or doubl$ or treb$ or tripl$) adj3 (blind$ or mask$)).ti,ab,tw. (92614) 15 exp placebo/ (121040) 16 placebo$.ti,ab,tw. (108265) 17 random.ti,ab,tw. (84865) 18 (crossover$ or cross‐over$).ti,ab,tw. (38930) 19 or/11‐18 (834581) 20 19 not 9 (805886) 21 20 not 10 (608448) 22 exp comparative study/ (319229) 23 exp evaluation/ (53603) 24 exp prospective study/ (78778) 25 exp controlled study/ (2811967) 26 (control$ or prospective$ or volunteer$).ti,ab,tw. (1722211) 27 or/22‐26 (3848159) 28 27 not 9 (2744044) 29 10 or 21 or 28 (3077253) 30 exp pancreas/ (37058) 31 exp pancreatitis/ (29788) 32 panc$.tw. (125868) 33 or/30‐32 (140055) 34 exp Enteric Feeding/ (8044) 35 exp parenteral nutrition/ (17910) 36 enteral$.tw. (9682) 37 parenteral$.tw. (29889) 38 enteric.tw. (16233) 39 TPN.tw. (3038) 40 PN.tw. (5694) 41 EN.tw. (19185) 42 or/34‐41 (84474) 43 33 and 42 and 29 (1333) 44 limit 43 to yr="2004 ‐ 2009" (557) 45 from 44 keep 1‐557 (557)
Appendix 3. Cochrane central update
Database: All EBM Reviews ‐ Cochrane DSR, ACP Journal Club, DARE, CCTR, CMR, HTA, and NHSEED (2004‐2008) 1 exp pancreas/ (637) 2 exp pancreatitis/ (590) 3 panc$.ti,ab. (4089) 4 or/1‐3 (4362) 5 exp enteral nutrition/ (1046) 6 exp parenteral nutrition/ (1279) 7 exp nutritional support/ (2198) 8 enteral$.ti,ab. (2434) 9 parenteral$.ti,ab. (4576) 10 TPN.ti,ab. (560) 11 PN.ti,ab. (321) 12 EN.ti,ab. (1719) 13 or/5‐12 (8248) 14 4 and 13 (231) 15 limit 14 to yr="2004 ‐ 2008" [Limit not valid in DARE; records were retained] (59) 16 from 15 keep 4‐50 (47)
Data and analyses
Comparison 1. Enteral versus parenteral nutrition for acute pancreatitis.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mortality | 8 | 348 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.50 [0.28, 0.91] |
| 2 Mortality in patients with acute pancreatitis excluding those from (Abou‐Assi 2002) in which death resulted after resolution of acute pancreatitis and was attributed to other causes; cardiac surgery, cancer of the liver, squamous cell carcinoma of the pharynx and intracerebral hemorrhage. | 8 | 348 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.39 [0.20, 0.77] |
| 3 Mortality in patients with severe acute pancreatitis (SAP) | 4 | 136 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.18 [0.06, 0.58] |
Comparison 2. Enteral versus parenteral nutrition for acute pancreatitis.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Length of hospital stay | 4 | 145 | Mean Difference (IV, Fixed, 95% CI) | ‐2.37 [‐7.18, 2.44] |
| 2 Subgroup analysis for length of hospital stay in patients with severe acute pancreatitis | 2 | 48 | Mean Difference (IV, Fixed, 95% CI) | ‐6.27 [‐15.40, 2.87] |
Comparison 3. Enteral versus parenteral nutrition for acute pancreatitis.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Systemic Inflammatory Response Response Syndrome(SIRS) | 1 | 22 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.17, 5.89] |
Comparison 4. Enteral versus parenteral nutrition for acute pancreatitis.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Multiple Organ Failure(MOF) | 6 | 278 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.55 [0.37, 0.81] |
| 2 MOF in Severe Acute Pancreatitis | 4 | 136 | Risk Ratio (M‐H, Random, 95% CI) | 0.46 [0.16, 1.29] |
Comparison 5. Enteral versus parenteral nutrition for acute pancreatitis.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Operative Intervention | 7 | 316 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.44 [0.29, 0.67] |
| 2 Operative intervention in SAP | 4 | 136 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.38 [0.22, 0.66] |
Comparison 6. Enteral versus parenteral nutrition for acute pancreatitis.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Systemic infection | 7 | 259 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.39 [0.23, 0.65] |
| 2 Systemic infection in SAP | 4 | 136 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.39 [0.17, 0.90] |
Comparison 7. Enteral versus parenteral nutrition for acute pancreatitis.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Local septic complications | 5 | 246 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.40, 1.35] |
| 2 Local septic complications in SAP | 3 | 119 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.07, 12.59] |
Comparison 8. Enteral versus parenteral nutrition for acute pancreatitis.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Other local complications | 5 | 230 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.43, 1.13] |
| 2 Other local complications in SAP | 2 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.55 [0.20, 1.50] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Abou‐Assi 2002.
| Methods | Randomized Controlled Trial | |
| Participants | Number randomised: 53, intervention group 26, control group 27. Setting: Hospital (ICU and floor). Age: 46 (range 21‐91) years, mean intervention group 48(3), mean control group 50(3). Sex: Intervention; 16 males and 10 females, control group; 13 males and 14 females. Study period: January‐December 2000. Hospital Stay (days): Intervention group 12.2(1.9), control group 18.4(1.9). Diagnostic Criteria: For Acute Pancreatitis: Acute abdominal pain and 3‐fold elevation of serum pancreatic enzymes, amylase, and lipase, with a primary diagnosis of acute pancreatitis. For Severity: Patients who failed to show improvement were graded by Ransons criteria. Intervention group: Ranson's score: 3.1 (0.5), Control group: Ranson's score: 2.5(0.4). Inclusion criteria: All patients admitted with acute pancreatitis. Exclusion criteria: Not requiring nutritional support. Etiology: Not stated. |
|
| Interventions | Intervention group: Jejunal tubes ("Silk," Corpak, Wheeling, IL; "Stay‐Put," Novartis, St. Louis Park, MN; or "Dobhoff," Kendall HealthCare, Mansfield, MA) were placed by fluoroscopy or endoscopy. Tube feeding was commenced at 20 ml/h and increased progressively to goal rates over 48h. Control group: TPN was delivered via central vein catheters in patients in the ICUs and by peripheral catheter in floor patients, electrolytes were first corrected before full nutritional infusions were given. |
|
| Outcomes | Duration of hospitalizations Duration of interventional (EN or TPN) feeding. This also provided a measure of the rate of resolution of disease, the incidence of nutrition‐associated complications (i.e., hyperglycemia needing insulin for control and catheter‐related sepsis and septicemia) Tolerance to recommencement of oral feeding Cost effectiveness analysis |
|
| Notes | Funding: This work was supported in part by a Clinical Research Award from the American College of Gastroenterology and the Medical College of Virginia Hospitals Conflict of interest: Not stated Calculated sample size: Yes, it was calculated that they would have more than 80% power to reveal significant differences in nutritional outcome, complications, and costs if 50 patients were randomised. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Quote: "For the first 12 months, the study was applied to all patients admitted to our institution with the diagnosis of acute pancreatitis. Then for the 3 months afterward, the data collection was limited to only those patients with acute pancreatitis who were in need of nutritional support" Comment: Insufficient information to judge |
| Allocation concealment? | Unclear risk | Quote:"of those who did not improve, 19 (12.2%) were randomised to TPN (group TPN) and 20 (12.8%) to jejunal feeding (group EN) during the first 12 months of the study. Subsequently, 14 more patients were randomised in the next 3 months, to reach our goal number of 27 patients on TPN (group TPN) and 26 on jejunal feeding (group EN)" Comment: Insufficient information to judge. |
| Blinding? All outcomes | Unclear risk | Comment: Insufficient information to judge |
| Incomplete outcome data addressed? All outcomes | Low risk | Comment: No missing data. |
| Free of selective reporting? | Low risk | Comments: All outcomes stated in the methods were mentioned in the results section. |
| Free of other bias? | Low risk | |
Casas 2007.
| Methods | Randomized Controlled Trial | |
| Participants | Number randomised: 22; Intervention group 11, control group 11 Setting: Hospital ICU in Barcelona Age: >17 years, mean age intervention 61.2(+/‐ 16.6), mean age control 55.6 (+/‐15.6) Sex: Intervention group 8 males and 3 females, control group 8 males and 3 females Study period: Not stated Diagnostic criteria: Acute Pancreatitis: Abdominal pain and increased serum amylase and/or lipase levels at least 3 times the upper limit of the reference range. Severe Acute Pancreatitis: The diagnosis of SAP was made within 48 hours when two or more of the following criteria were evident: Acute Physiology and Chronic Health Evaluation (APACHE II) score ≥8, C‐reactive protein (CRP) level in excess of 150 mg/L, and/or a Balthazar D or E grade in the abdominal CT scan. Inclusion criteria: Patients of either sex who were 18 years or older with a SAP diagnosis of any etiology. Exclusion criteria: Under 18 years of age, pregnant women and relapsing chronic pancreatitis cases were excluded. Etiology: Intervention group; Biliary: 4, Alcohol: 1, Unkown: 3, Other: 3, Control group; Biliary: 7, Alcohol: 4 |
|
| Interventions | Intervention: TEN (PEPTISORB®, Nutricia S.R.L., Madrid) through a single‐lumen, 114‐cm long naso‐jejunal 10 F feeding tube whose tip was placed, under fluoroscopic screening, close to Treitz's ligament. The initial infusion rate was 25 ml/h with increases of 25 ml/4 h until requirements were reached. Control: TPN receive 24‐hour continuous infusion of TPN through a central venous catheter (subclavian/ jugular). Venous infusion was started at a rate of 40 ml/h and increased 20 ml/h every 4 hours until the required needs were met. |
|
| Outcomes | Evaluate inflammatory response (CRP, TNF‐α. IL‐6), visceral proteins (pre‐albumin, albumin). Rate of complications (SIRS, MOF, and infections). Number of surgical interventions. Length of hospital stay. Mortality rate. |
|
| Notes | Funding source: Study partially made with/a grant of the Instituto de Salud Carlos III. Conflict of interest: Not stated. Calculated sample size: Not stated. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Quote: "This prospective randomised trial included 22 consecutive patients who were distributed into two groups according to a computerized random number generation". Comment: Probably done. |
| Allocation concealment? | Low risk | Quote: "The option corresponding to each patient was placed in sealed envelopes that were opened immediately prior to inclusion". |
| Blinding? All outcomes | Unclear risk | Not addressed. |
| Incomplete outcome data addressed? All outcomes | Low risk | All 22 patients completed the study. No missing data. |
| Free of selective reporting? | Low risk | All outcomes mentioned in the introduction and methods were addressed in the results section. The authors were contacted and provided missing data and details of statistical methods used. |
| Free of other bias? | Low risk | |
Gupta 2003.
| Methods | Randomized Controlled Trial | |
| Participants | Number randomised: 21 (3 withdrew after recruitment, and 1 recovered rapidly and was discharged), intervention group 8, control group 9. Setting: Southampton General Hospital, UK. Age: >15, mean age of intervention group 65(56‐89) P=0.14, mean age of control group 57(38‐86). Sex: Intervention group 4 males and 4 females, control group 3 males and 6 females. Study period: November 1996 until April 1998. Diagnostic criteria: Acute pancreatitis was defined as abdominal pain and a serum amylase concentration of 1,000 U/l or more (normal range 70‐300 U/l). Severe Acute Pancreatitis was established by the presence of an acute physiology, age and chronic health evaluation score (APACHE II) of 6 or more. Inclusion criteria: Age > 15yrs male or female and all patients admitted with acute pancreatitis. Exclusion criteria: Pregnant. Etiology: Intervention group: Biliary stones: 3, Alcohol: 1, Unknown: 4, APACHE II: 8(6‐12) P=0.13, CRP: 54(15‐254), Control group: Biliary: 3, Alcohol: 5, Unknown: 1, APACHE II:10 (7‐14), CRP: 161(16‐290) |
|
| Interventions | Intervention: TEN delivered by nasojejunal dual lumen tubes (Medicina, Cookson, UK). The weighted nasojejunal tube was passed into the stomach, the patient was encouraged to sit up, or roll onto the right side, and subsequently a radiograph was taken to confirm the placement of the tube. Control: TPN delivered by a central intravenous line (Arrow triple lumen, Vygon, Cirencester) placed by a standard sterile technique. |
|
| Outcomes | The aim of the present paper was to report a prospective, randomised comparison of immediate TEN and TPN in patients with predicted severe acute pancreatitis in order to examine the effect of these different feeding regimes on fatigue, oxidative stress, plasma glutamine levels and endotoxaemia (IgG, IgM) | |
| Notes | Funding source: Nutricia Conflict of interest: Not stated Calculated sample size: Not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Quote: "Patients were randomised to TPN or TEN using sealed envelopes which had been prepared before the study commenced by a member of our department who had no other involvement with the study. These contained a card on which "Total parenteral nutrition" or "Total enteral nutrition" was written." Comment: Probably done. |
| Allocation concealment? | Low risk | Quote: "Patients were randomised to TPN or TEN using sealed envelopes which had been prepared before the study commenced by a member of our department who had no other involvement with the study. These contained a card on which "Total parenteral nutrition" or "Total enteral nutrition" was written." Comment: Probably done. |
| Blinding? All outcomes | Unclear risk | The study did not address this outcome. |
| Incomplete outcome data addressed? All outcomes | Low risk | No missing data. All 17 patients completed the study. |
| Free of selective reporting? | Low risk | Comments: All outcomes stated in the methods were mentioned in the results section. |
| Free of other bias? | Unclear risk | Comment: The study was supported by a grant from Nutricia, their product Nutrison was used for the TEN group. The study did not address whether Nutricia was or was not involved in the study design or approving the final paper. |
Kalfarentzos 1997.
| Methods | Randomized controlled trial. | |
| Participants | Number randomised: 38, intervention group 18, control group 20. Setting: Patras, Greece. Sex: Intervention group; 8 males and 10 females, control group; 7 males and 13 females. Age: Mean age of intervention group 63 +/‐10.7, mean age of control group 67.2 +/‐8.9 Study Period: July 1990 and December 1995. Diagnostic Criteria: Severity based on Imrie classification, or APACHE II score, C reactive protein concentration, Balthazar CT scan criteria. Inclusion criteria: The presence of three or more criteria according to the Imrie classification, or APACHEII score I4 of 8 or more, C‐reactive protein concentration greater than 120 mg/l within 48 h of admission, and grade D or E by computed tomography (CT) according to the Balthazar criteria. Exclusion criteria: Patients who were treated elsewhere for more than 2 days before admission to this hospital were not included. Etiology: Intervention group: Biliary 14, Alcohol 3, Others 1, APACHE II: 12.7(2.6) (8‐15) (APACHE II) scores were available for seven patients in the EF group and six who had TPN Imrie classification: 4.2(0.9) (3‐5) available for 16 patients in the enteral feeding (EF) group and 19 in the total parenteral nutrition (TPN) group. CRP conc.: 290 (157‐420) Based on 15 patients in the EF group and 17 in the TPN group. Balthazar CT Scan score: Grade D: 4 (4 out of 11), Grade E: 14 (14 out of 27). Control group: Biliary 16, Alcohol 2, Others 2, APACHE II: 11.8(1.9) (8‐15). Imrie classification: 4.6 (1.1) (3‐6), CRP conc.: 335 (140‐513), Balthazar CT Scan score: Grade D: 7, Grade E: 13. |
|
| Interventions | Intervention: Patients received enteral nutrition (Rebilan HN; Roussel Ulcef Nutrition, Montpellier, France) through a nasoenteric feeding tube, placed fluoroscopically (Flexifflo 10 fr, 115 cm; Ross Lab, Columbus, Ohio, USA). Control: Patients received parenteral nutrition containing; crystalline L‐amino acid (vamin 18 FE; Kabi Pharmacia, Stockholm, Sweden), carbohydrates in the form of dextrose, fat emulsion (Lipofudin long‐chain/medium chain triglycerides 20 per cent; Brain, Melsungen, Germany), vitamins, and minerals through a subclavian central venous line. |
|
| Outcomes | Complications during the course of the disease including death. Length of ICU stay. Length of hospital stay. Number of days on artificial ventilation. Number of days receiving tube feeding or TPN. Number of antibiotics used, Number of days on antibiotics. The mean cost of EN and TPN per day. | |
| Notes | Other outcomes addressed in the study:
Number of deaths.
Local septic complications.
Local other complications. Systemic sepsis. Funding source: Not stated. Conflict of interest: Not stated. Calculated sample size: not stated. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Qoute: "Patients were assigned on admission to one of two treatment groups by means of numbered envelopes" Comment: Probably done. |
| Allocation concealment? | Unclear risk | Not enough information to judge. |
| Blinding? All outcomes | Unclear risk | The study did not address this outcome. Comment: Probably not done. |
| Incomplete outcome data addressed? All outcomes | Low risk | No missing data. All 38 participants completed the study. |
| Free of selective reporting? | Low risk | All expected outcomes are addressed in detail in the methods section in terms of measurements and appear in the results section. |
| Free of other bias? | Low risk | |
Louie 2005.
| Methods | Randomized Controlled Trial | |
| Participants | Numer randomised: 28, intervention group 10, control group 18. Setting: An academic, multi‐institutional, tertiary care health system. Teaching hospitals associated with UofA Hospital. Age: >17, mean age of intervention group: 65.3 (SD 18.3), mean age of control group 59 (SD 15.3). Sex: Intervention group; 6 males and 4 females, control group; 9 males and 9 females. Study period: July/15/1999‐Dec/15/2001. Diagnostic Criteria: For acute pancreatitis: Quote: "Eligible patients were required to have acute pancreatitis" Comment: Not stated. For Severity: 1‐Ranson's criteria at admission or diagnosis. 2‐CT grading system for pancreatitis of Balthazar and associates. Inclusion criteria: Patients were required to have acute pancreatitis. A Ransons score (calculated by counting 1 point for each of the criteria met over the 48‐ hour period) of 3 or greater, and inability to tolerate oral fluids after a maximum time from admission of 96 hours. Exclusion criteria: Patients were excluded if they did not meet these criteria (Above), If they were younger than 18 years, unable to accept enteral nutrition via the gastrointestinal tract, or already receiving nutritional support. Etiology: Intervention group: Cancer: 0, TG: 1, ERCP: 0, Biliary stones: 5, Alcohol: 2, Unknown: 2, APACHE II: 11.8 (SD 8.3), Ranson's Score: 4.7 (1.3), Balthazar CT Scan score: 3.4 (SD 1.3). Control group: Cancer: 0, ERCP: 1, TG: 1, Biliary stones: 7, Alcohol: 4, Unknown: 5 APACHE II: 12.7 (SD 5.5), Ranson's Score: 5 (SD 1.8), Balthazar CT Scan score: 4.2 (SD 0.8). |
|
| Interventions | Intervention: Nasojejunal (NJ) feeding tubes were placed via gastroscopy and confirmed radiographically. Peptamen (Nestlé), a semielemental product with low fat content, was infused at 25 mL/h and increased by 10 mL/h every 6 hours, until the target rate was achieved. Control: In the PN group, long‐term vascular catheters were placed percutaneously and confirmed radiographically. PN was initially infused with a 10% dextrose solution and Intralipid (Baxter) at half of the calculated energy requirements; then increased over 2 days to achieve 100% of the target energy rate. |
|
| Outcomes | Attenuation of the inflammatory Response. The effectiveness of nutrition. The natural history and morbidity of pancreatitis. The morbidity from each nutritional modality. An economic evaluation of the nutrition technology |
|
| Notes | Funding source: Not stated. Conflicts of interest: Non declared. Ethical approval: Yes. Sample size calculation: Yes, they conservatively assumed a clinically important difference to be a mean of 2 days, with a standard deviation of 3 days, to achieve a 50% reduction in C‐reactive protein levels. To observe a difference between the groups, the total sample size was estimated at 58 participants to achieve an α= 0.05 and a β = 0.2. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Quote: "patients were blindly randomised to receive either PN or EN and stratified by hospital, by means of computer‐ generated assignment placed in sealed, opaque envelopes" Comment: Probably done. |
| Allocation concealment? | Low risk | Quote: "patients were blindly randomised to receive either PN or EN and stratified by hospital, by means of computer‐ generated assignment placed in sealed, opaque envelopes" Comment: Probably done. |
| Blinding? All outcomes | Unclear risk | Comment:The study did not address this. |
| Incomplete outcome data addressed? All outcomes | Low risk | Quote: "In all, 28 patients consented to participate and completed the study" Comment: No missing data. |
| Free of selective reporting? | Low risk | Comment: Probably yes, because all primary and secondary outcomes stated in the introduction were clearly presented in the result section. |
| Free of other bias? | Low risk | |
McClave 1997.
| Methods | Randomized controlled trial. | |
| Participants | Number randomised: 32, intervention group 16, control group 16. Age: Mean age of intervention group 47.6 +/‐4 years, mean age of control group 45.1+/‐ 4.2. Sex: Male. Study Period: Not stated. Diagnostic Criteria: Abdominal pain with elevated amylase and lipase, Severity based on Ranson criteria, APACHE III score (Knaus 1991), and MOF score. Inclusion criteria: Male, patients admitted to the hospital with acute pancreatitis or an acute flare of chronic pancreatitis or an acute flare of chronic pancreatitis, characterized by abdominal pain with elevated amylase and lipase. Exclusion criteria: Females & patients who had an evidence of short bowel syndrome, Crohns disease, major pancreatic resection, failure to start TEN or TPN within 48 hours of admission. After entry into the study patients were excluded if they failed to adhere to dietary restrictions or to the protocol terms for enteral tube placement. Etiology: Intervention group: EtOH%: 75.0+/‐11.2, control group: EtOH%: 62.5+/‐12.5. |
|
| Interventions | Intervention: They received enteral nutrition (Peptamen; Clintec Nutrition Company, Deerfeild, IL) through a nasoenteric tube placed endoscopically down into jejunum. Control: They received TPN through a central or peripheral venous line. | |
| Outcomes | Days to diet by mouth which was defined by the point at which the patient showed signs of clinical resolution, and was advanced to clear liquids by mouth.
Days to normal amylase.
Length of hospital stay.
Length of ICU stay.
Nosocomial infection.
Mortality.
Cost of nutritional support which was determined by assigning a dollar amount to endoscopic tube or IV line placement and volume of nutritional hyperalimentation infused. Other outcomes addressed in the study: Number of deaths. Systemic sepsis. Length of hospital stay. |
|
| Notes | Funding source: Supported by a research grant from Clintec nutrition company, Deerfield, Illinois. Conflict of interest: Not stated. Calculated sample size: Yes. "Sample size was calculated before the study based on achieving 80% power with a significance level of 0.05. A planned sample size of 20 per group afforded on 80% power to detect a difference of 1.8 days in length of hospitalisation assuming a standard deviation of 2.0 This sample size also afforded an 80% power to detect a $1000 difference in cost, assuming a standard deviation of 1100". Ethical approval: Yes. This study only compared males. No females. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Qoute: "Upon admission patients were prospectively randomised to receive either TEN or TPN". |
| Allocation concealment? | Unclear risk | Unclear. |
| Blinding? All outcomes | Unclear risk | The study did not address this outcome. |
| Incomplete outcome data addressed? All outcomes | Low risk | No missing data all; 32 participants completed the study. |
| Free of selective reporting? | Low risk | All expected outcomes are addressed in detail in the methods section in terms of measurements and appear in the results section. |
| Free of other bias? | Unclear risk | Their source of funding provided the nutritional product for the intervention group, and they did not address whether or not they were involved in the design, results or approval of the final paper. Secondly, the study was terminated early because of longer than anticipated duration of the study, difficulty in compliance and obtaining consent. Although a fairly reasonable explanation was provided, this may have masked reporting of some serious outcomes such as mortality. |
Olah 2002.
| Methods | Randomized Controlled Trial. | |
| Participants | Number randomised: 89, intervention group 41, control group 48. Setting: Surgical Ward of the Petz Alada'r Teaching Hospital Study Period: January 1, 1995 and May 31, 1996. Age: mean age of intervention group 47.2, mean age of control group 43.8 Sex: intervention group; 33 males and 8 females, control group; 42 males and 6 females. Diagnostic Critera: Clinical symptoms and laboratory signs of pancreatitis (plasma amylase>200 U/L, normal value <700 U/L) and brief histories of the disease. Severity Scale: 1‐ Imrie (Glascow) patients with an Imrie scores of at least 3. 2‐ C‐reactive protein value above 150 mg/L. 3‐ Severity was defined as a score above 6 on the Second Acute Physiology and Chronic Health Evaluation (APACHEII). Inclusion criteria: Patients with clinical symptoms and laboratory signs of acute pancreatitis. Exclusion criteria: Patients with good evidence of biliary tract disease, because those cases required other therapeutic interventions (endoscopic papillotomy, cholecystectomy and/or choledochotomy). Hospitalized patients with acute exacerbations of chronic pancreatitis and those in whom the placement of the feeding tube was not possible (patients unable to cooperate or patients who repeatedly removed their feeding tubes). Etiology: Intervention Group: Alcohol: 33, Other: 8. Control Group: Alcohol: 39, Other: 9. |
|
| Interventions | Intervention Group: A nasojejunal tube was placed within 24 h after admittance. The feeding tube (Angiomed duodenal set; 14‐F calibre; 4.67 mm; 150 cm long) was inserted into patients stomachs while they were in the surgical ward. The final placement of the tube through the pylorus, which is the most crucial step of the procedure, was performed by a radiologist. The correct position of the jejunal tube was confirmed by x ray. Enteral nutrition was started on the first day through the jejunal feeding tube placed into the second jejunal loop. Survimed OPD (Fresenius) elementary diet was administered (energy: 1 cal/mL protein 22.5 g/500 mL, osmolarity: 400 mOsmol/L). Jejunal feeding was governed by a gravity‐driven continuous drip. The dose was increased gradually and the maximum daily intake was reached within 2 to 3 days. The goal was to achieve a 30 kcal/kg of body weight energy intake in this group. Enteral feeding was continued in every patient for a minimum of 5 days and a maximum of 9 days. Control Group: The therapy protocol was placement of a nasogastric tube, gut rest (no oral feeding) and parenteral nutrition dextrose 10% (3 g/kg body weight), 10% amino acid solution (1 to 1.5 g/kg body weight) and 10% or 20% fat emulsion (0.8 to 1.3 g/kg body weight). The parenteral nutrition was an all‐in‐one venous admixture in most cases. The goal was to achieve a 30 kcal/kg of body weight energy intake in every patient. Parenteral nutrition was continued for a minimum of 5 days and a maximum of 16 days. |
|
| Outcomes | Primary outcome: Rates of septic complications, mortality, MOF. Other outcomes addressed in the study: Operative Intervention. Local septic complications. Other local complications |
|
| Notes | Funding source: Not stated. Conflict of interest: Not stated. Calculated sample size: No. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | High risk | Qoute: "89 patients were randomised into the two groups based on their birth dates. Patients who were born between the 1st and 15th of the month were assigned to group A, and patients born between the 16th and 31th were assigned to group B. Group A (n =48) received parenteral nutrition, and group B (n =41) received enteral feedings. Comment: Probably done. |
| Allocation concealment? | High risk | Qoute: "89 patients were randomised into the two groups based on their birth dates" Comment: Participants or investigators enrolling participants could possibly foresee assignments and thus introduce selection bias due to allocation based on date of birth. |
| Blinding? All outcomes | Unclear risk | The study did not address this outcome. |
| Incomplete outcome data addressed? All outcomes | Low risk | No missing data from outcomes. |
| Free of selective reporting? | Low risk | All expected outcomes are addressed in detail in the methods section in terms of measurements and appear in the results section |
| Free of other bias? | Low risk | |
Petrov 2006.
| Methods | Randomized Controlled Trial | |
| Participants | Number randomised 70, intervention group 35, control group 34. Setting: Intensive Care Unit. Age: >17 years old, median age (interquartile range) of intervention group 51(42‐67), median age (interquartile range) of control group 52(41‐70). Sex: Intervention group 27 females and 8 males, control group 24 females and 10 males. Diagnostic Criteria: Chemical and biochemical presentation (upper abdominal pain and serum amylase concentration at least 3 times the upper limit of the reference range). Severity Scale: (APACHEII) score of 8 or more, and/or a C‐reactive protein (CRP) level in excess of 150mg/l. Inclusion criteria: Patients with prognostically severe acute pancreatitis within 72 h of the onset of symptoms. Exclusion criteria: Age below 18 years and pregnancy. Etiology: Intervention Group: Biliary (stones): 11, Alcohol: 16, Others: 8, APACHEII: 12(10‐14), CRP Conc: 195(146‐216), Control Group: Biliary (stones): 13, Alcohol: 15, Others: 6, APACHEII: 12.5(11‐16), CRP Conc: 210(177‐246). |
|
| Interventions | For both groups: Nutritional support, supplying daily 30 kcal/kg and 1.5 g/kg of protein, based on ideal body weight, was commenced within 24 h of enrolment, patients received full supportive therapy as required; all patients received analgesia, antibiotic prophylaxis (ofloxacin plus metronidazole) and intravenous fluids. Intervention: Enteral feeding was through a radiologically placed nasojejunal feeding tube, distal to the ligament of Treitz. The position of a tube was confirmed by X‐ray, increased over 48 h to achieve 100% of the target energy rate. The standard enteral feed used was a semi‐elemental nutrition (Peptamen, Nestle), which is low in fat and higher in predigested protein than regular tube feeding formulas. Enteral feeding was commenced at a rate of 25 ml/h and increased by 10 ml/h every 6 h, until the desired caloric intake was reached. Control: TPN was delivered through a central venous catheter, it was initially infused with a 10% dextrose solution, 10% amino acid solution and 10% fat emulsion at half of the calculated energy requirements; then increased over 48 h to achieve 100% of the target energy rate. |
|
| Outcomes | Primary Outcome: Incidence of pancreatic infectious complications i.e. infected pancreatic necrosis and pancreatic abscess during hospital admission. Other reported outcomes: Incidence of noninfectious complications. Frequency of organ failure, (Marshall score was used). Need for operative intervention. CRP concentration. APACHE II score. Mortality. |
|
| Notes | Funding source: Not mentioned. Conflict of interest: Not mentioned. Calculated sample size: Yes. "A sample size of 68 patients was calculated in order to demonstrate a reduction of infectious complications in 45% of TPN patients to 12% in TEN patients (alpha 0.05, beta 0.80)." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Qoute: "Patients were randomised at admission by computerized random number generation to receive either enteral or parenteral feeding for a minimum of 7 days" Comment: Probably done. |
| Allocation concealment? | Low risk | Qoute: "Randomized at admission by computerized random number generation" Comment: Probably done. |
| Blinding? All outcomes | Unclear risk | The study did not address this outcome. |
| Incomplete outcome data addressed? All outcomes | Low risk | Qoute: "A total of 70 patients were randomised to receive either enteral or parenteral feeding. One patient who met the entry criteria was found at operation to have an acute mesenteric infarction and was thereby excluded from the study, leaving 35 patients in the TEN group, and 34 in the TPN group clinical characteristics of the two groups were similar". Comment: No missing data, except for one who was excluded for acute mesenteric infarction. |
| Free of selective reporting? | Low risk | All expected outcomes are addressed in detail in the methods section in terms of measurements and appear in the results section. |
| Free of other bias? | Low risk | |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Bao 2006 | TPN was given to both groups before initiating EN in the comparison group. |
| Eckerwall 2006 | Nasogastric (NG) rather than nasojejunal (NJ) tube was used. |
| Hernandez‐Aranda1996 | This is an RCT comparing the effect of TPN versus EN in post operative patients with severe acute pancreatitis. The study did not meet the inclusion criteria as the EN was delivered through a silicon catheter placed into the jejunum intra operatively. |
| Modena 2006 | Not randomized. |
| Powell 2000 | The study compared TEN to conventional therapy that included I.V antibiotics but no TPN. |
| Windsor 1996 | Abstract for the preliminary results for Windsor 1998. |
| Windsor 1998 | This is an RCT comparing the effect of TPN versus EN in patients with acute pancreatitis. This study did not meet the inclusion criteria as the enteral nutrition was delivered through feeding tube in severe acute pancreatitis patients only, while patients with mild to moderate pancreatitis received nutritional supplements by mouth. Furthermore, the final analysis for the outcomes did not distinguish between patients who received EN through feeding tube or per mouth. |
| Ye 2007 | Comparison was done between TPN group & PN+EN group. |
Differences between protocol and review
Methods
For the inclusion criteria we found it reasonable to include studies that inserted nasojejunal tubes blindly as long as they were confirmed by an X‐ray or other radiological methods since it complied with our objective of ensuring that the tube was positioned in the jejunum. Another addition was in the search strategy where the old RCT filters were replaced by the new ones described in Lefebvre 2008 because they have been found to be more sensitive. Another addition was the inclusion of searching for ongoing trials through www.clinicaltrials.gov. In addition, we replaced the old tool for assessment of risk of bias with the tool described in Higgins 2008 as it provides a more transparent and detailed assessment of validity of included studies. We planned on assessing for publication and reporting bias if ten or more studies were included in any outcome (Sterne 2008) as it is considered to be a threat for validity. In the outcomes section, we added line sepsis under systemic infections as it is recognized as an important source of infection that may affect morbidity and mortality. Instead of applying a random‐effects model for all outcomes, we made the decision based on the significance of heterogeneity: where studies were found to be significant for heterogeneity indicated by a P value < 0.1, a random‐effects model would be applied, while if they were homogenous, a fixed‐effects model would be applied. Finally, a 'Summary of findings' table was included in accordance with the recommendation of the Cochrane Handbook for Systematic Reviews of Interventions to provide a transparent and simple tabular format of the review’s main outcomes that are likely to be of importance to patients and decision makers (Schünemann 2008).
Authorship
Three new authors joined the group and were involved in the development and update of this review; (Al‐Ansary LA, Tashkandi MF and AlBalawi ZH). The first author remains unchanged; however, two previous authors whom contributed to development of the original review were unable to take part in the update or approval of this review and were therefore acknowledged for their efforts and previous contributions.
Contributions of authors
Mohammed Al‐Omran: Concept and design of the review, protocol development, and final approval of the manuscript to be submitted for publication.
Zaina AlBalawi: Selection of studies, quality assessment, data extraction, data analysis, update of the review.
Mariam Tashkandi: Selection of studies, quality assessment, data extraction, data analysis, update of the review.
Lubna Al‐Ansary: Critical revision for intellectual content, and final approval of the manuscript to be submitted for publication.
Ala Groof and Derek Wilke contributed to the development of the protocol and first published version of the review. They both took part in screening the studies for relevance, extracting data and assessment of quality of studies of the original review.
Sources of support
Internal sources
-
Cochrane Upper Gastrointestinal and Pancreatic Diseases Group, Canada.
Conducting the search, and assistance in retrieving full text for relevant articles.
External sources
-
J.W. Scott Health Sciences Library at University of Alberta, Canada.
Assistance in retrieving full text for relevant articles.
Declarations of interest
None known.
Edited (no change to conclusions)
References
References to studies included in this review
Abou‐Assi 2002 {published data only}
- Abou‐Assi S, Craig K, O’Keefe SJD. Hypocaloric jejunal feeding is better than total parenteral nutrition in acute pancreatitis: Results of a randomized comparative study. American Journal of Gastroenterology 2002;97:2255‐62. [DOI] [PubMed] [Google Scholar]
Casas 2007 {published data only}
- Casas M, Mora J, Fort E, Aracil C, Busquets D, Galter S, Jáuregui CE, Ayala E, Cardona D, Gich I, Farré A. Total enteral nutrition v.s total parenteral nutrition in patients with severe acute pancreatitis. Revista Española de Enfermedades Digestivas 2007;99(5):264‐269. [DOI] [PubMed] [Google Scholar]
Gupta 2003 {published data only}
- Gupta R, Patel K, Calder PC, Yaqoob P, Primrose JN, Johnson CD. A randomised clinical trial to assess the effect of total enteral and total parenteral nutritional support on metabolic, inflammatory and oxidative markers in patients with predicted severe acute pancreatitis (APACHE II ≥6). Pancreatology 2003;3:406‐13. [DOI] [PubMed] [Google Scholar]
Kalfarentzos 1997 {published data only}
- Kalfarentzos F, Kehagias J, Mead N, Kokkinis K, Gogos CA. Enteral nutrition is superior to parenteral nutrition in severe acute pancreatitis: results of a randomized prospective trial. British Journal of Surgery 1997;84:1665‐1669. [PubMed] [Google Scholar]
Louie 2005 {published data only}
- Louie BE, Noseworthy T, Hailey D, Gramlich LM, Jacobs P, Warnock GL. 2004 MacLean–Mueller Prize enteral or parenteral nutrition for severe pancreatitis: A randomized controlled trial and health technology assessment. Canadian Journal of Surgery 2005;48:298‐306. [PMC free article] [PubMed] [Google Scholar]
McClave 1997 {published data only}
- McClave SA, Greene LM, Snider HL, Mark LJK, Cheadle WG, Owens NA, Dukes LG, Goldsmith LJ. Comparison of the safety of early enteral versus parenteral nutrition in mild acute pancreatitis. Journal of Parenteral and Enteral Nutrition 1997;21:14‐20. [DOI] [PubMed] [Google Scholar]
Olah 2002 {published data only}
- Olah A, Pardavi G, Belagyi T, Nagy A, Issekutz A, Mohamed GE. Early nasojejunal feeding in acute pancreatitis is associated with a lower complication rate. Nutrition 2002;18:259‐262. [DOI] [PubMed] [Google Scholar]
Petrov 2006 {published data only}
- Petrov MS, Kukosh MV, Emelyanov NV. A Randomized controlled trial of enteral versus parenteral feeding in patients with predicted severe acute pancreatitis shows a significant reduction in mortality and in infected pancreatic complications with total enteral nutrition. Digestive Surgery 2006;23:336‐345. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Bao 2006 {published data only}
- Bao J, Jing D, Wang P, Wang X. Effects of early jejunum nutrition on the comprehensive therapy for severe acute pancreatitis. Chinese Journal of Gastroenterology 2006;11(5):259‐62. [Google Scholar]
Eckerwall 2006 {published data only}
- Eckerwall GE, Axelsson JB, Andersson RG. Early nasogastric feeding in predicted severe acute pancreatitis a clinical, randomized study. Annals of Surgery December 2006;244:959‐965. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hernandez‐Aranda1996 {published data only}
- Herandez‐Aranda JC, Gallo‐Chio B, Ramirez‐Barba EJ. Nutritional support in severe acute pancreatitis. Controlled clinical trial [Apoyo nuttricional en pancreatitis aguda grave. Ensayo clinico controlado]. Nutricion Hospitalaria 1996;11:160‐166. [PubMed] [Google Scholar]
Modena 2006 {published data only}
- Modena JT, Cevasco LB, Basot CA, Vicuna AO, Ramirez MP. Total enteral nutrition as prophylactic therapy for pancreatic necrosis infection in severe acute pancreatitis. Pancreatology 2006;6:58‐64. [DOI] [PubMed] [Google Scholar]
Powell 2000 {published data only}
- Powell JJ, Murchison JT, Fearon KCH, Ros JA, Siriwardena AK. Randomized controlled trial of the effect of early enteral nutrition on markers of the inflammatory response in predicted severe acute pancreatitis. British Journal of Surgery 2000;87:1375‐81. [DOI] [PubMed] [Google Scholar]
Windsor 1996 {published data only}
- Windsor ACJ, Li A, Guthrie A, Barnes E, Somers SS, Welsh F, Guillou PJ, Reynolds JV. Feeding the gut in acute pancreatitis: a randomized clinical trial of enteral versus parenteral nutrition. British Journal of Surgery 1996;83:689. [Google Scholar]
Windsor 1998 {published data only}
- Windsor ACJ, Kanwar S, Li AGK, Barnes E, Guthrie JA, Spark JI, Welsh F, Guillou PJ, Reynolds JV. Compared with parenteral nutrition, enteral feeding attenuates the acute phase response and improves disease severity in acute pancreatitis. Gut 1998;42:431‐435. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ye 2007 {published data only}
- Ye P, Wu W, Liu J, Xu K. Early enteral nutrition in the treatment of acute pancreatitis. Chinese Journal of Gastroenterology 2007;12(7):411‐3. [Google Scholar]
Additional references
Alexender 1980
- Alexender JW, MacMillan BG, Stinnert JD. Beneficial effects of aggressive protein feeding in severely burned children. Surgery 1980;192:505‐18. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ammori 1999
- Ammori BJ, Leeder PC, King RF, Barclay GR, Martin IG, Larvin M, McMahon MJ. Early increase in intestinal permeability in patients with severe acute pancreatitis: correlation with endotoxemia, organ failure, and mortality. Journal of Gastrointestinal Surgery 1999;3:252‐62. [DOI] [PubMed] [Google Scholar]
Balthazar 1990
- Balthazar EJ, Robinson DL, Megibow AJ, Ranson JHC. Acute pancreatitis: value of CT scan in establishing prognosis. Radiology 1990;174:331‐6. [DOI] [PubMed] [Google Scholar]
Banks 2006
- Banks PA, Freeman ML, and the Practice Parameters Committee of the American College of Gastroenterology. Practice Guidelines in Acute Pancreatitis. American Journal of Gastroenterology 2006;101:2379‐2400. [DOI] [PubMed] [Google Scholar]
Blamey 1984
- Blamey SL, Imrie CW, O'Neill J, Gilmour WH, Carter DC. Prognostic factors in acute pancreatitis. Gut 1984;25:1304‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bradley 1993
- Bradley EL III. A clinically based classification system for acute pancreatitis. Summary of the International Symposium of Acute Pancreatitis, Atlanta, Ga, September 11‐13. Arch Surg 1993;128:586‐90. [DOI] [PubMed] [Google Scholar]
British Society of Gastroenterology 2005
- British Society of Gastroenterology. United Kingdom guidelines for the management of acute pancreatitis. Gut 2005;54:1‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Buchman 1995
- Buchman AL, Moukarazel AA, Bhuta S, Belle M, Ament ME, Eckhert CD, Hollander D, Gornbein J, Kopple JD, Vijayaroghavan SR. Parenteral nutrition is associated with intestinal morphologic and functional changes in humans. Journal of Parenteral and Enteral Nutrition 1995;19:453‐60. [DOI] [PubMed] [Google Scholar]
Corcoy 1988
- Corcoy R, Ma Sanachez J, Domingo P, et al. Nutrition in patients with severe acute pancreatitis. Nutrition 1988;4:269‐75. [Google Scholar]
Eatock 2005
- Eatock FC, Chong P, Menezes N, Murray L, McKay CJ, Carter CR, Imrie CW. A randomized study of early nasogastric versus nasojejunal feeding in severe acute pancreatitis. American Journal of Gastroenterology 2005;100(2):432‐9. [DOI] [PubMed] [Google Scholar]
Fink 1991
- Fink MP. Gastrointestinal mucosal injury in experimental models of shock, trauma and sepsis. Critical Care Medicine 1991;19:627‐41. [DOI] [PubMed] [Google Scholar]
Gardiner 1995
- Gardiner KR, Kirk SJ, Rowlands BD. Novel substrates to maintain gut integrity. Nutrition Research Reviews 1995;8:43‐66. [DOI] [PubMed] [Google Scholar]
Goodgame 1977
- Goodgame JT, Fischer JE. Parenteral nutrition in the treatment of pancreatitis: effect on complications and mortality. Annals of Surgery 1977;186:651‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Grant 1984
- Grant JP, James S, Gratosowski V, Trexler KM. Total parenteral nutrition in pancreatic disease. Annals of Surgery 1984;200:627‐31. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hancke 1987
- Hancke E, Marlein G. Bacterial contamination of the pancreas with intestinal germs: A cause of acute suppurative pancreatitis. In: Berger HG editor(s). Acute Pancreatitis. Berlin: Spring‐Verlag, 1987:87‐9. [Google Scholar]
Havala 1989
- Havala T, Shronts E, Cerra F. Nutritinal support in acute pancreatitis. Gastroenterology Clinics of North America 1989;18:525‐42. [PubMed] [Google Scholar]
Helton 1990
- Helton WS. Intravenous nutrition in patients with acute pancreatitis. In: Rombeau JL editor(s). Clinical nutrition: Parenteral nutrition. Philadelphia: WB Saunders, 1990:442‐61. [Google Scholar]
Higgins 2008
- Higgins JPT, Altman DG (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Greens S (editors). Cochrane Handbook for Systematic Reviews of Interventions. Chichester (UK): John Wiley & sons, 2008. [Google Scholar]
Higgins 2008a
- Higgins JPT, Deeks JJ, Altman DG (editors). Chapter 16: Special topics in statistics. In: Higgins JPT, Green S (editors), Cochrane Handbook for Systematic Reviews of Interventions. Chichester (UK): John Wiley & Sons, 2008. [Google Scholar]
Jadad 1996
- Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJM, Gavaghan DJ, McQuay H. Assessing the quality of reports of randomized clinical trials: Is blinding necessary?. Controlled Clinical Trials 1996;17:1‐12. [DOI] [PubMed] [Google Scholar]
Jüni 1999
- Jüni P, Witschi A, Bloch R, Egger M. The hazards of scoring the quality of clinical trials for meta‐analysis. JAMA 1999;282:1054‐1060. [DOI] [PubMed] [Google Scholar]
Kalfarentzos 1991
- Kalfarentzos FE, Karavias DD, Karatzas TM Alevizatos BA, Androulakis JA. Total parenteral nutrition in severe acute pancreatitis. Journal of the American College of Nutrition 1991;10:156‐62. [DOI] [PubMed] [Google Scholar]
Keys 1950
- Keys A, Brozek J, Henschel A. The biology of human starvation. Minneapolis: University of Minnesota Press, 1950. [Google Scholar]
Knaus 1985
- Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE‐II: a severity of disease classification. Critical Care Medicine 1985;13:818‐29. [PubMed] [Google Scholar]
Knaus 1991
- Knaus WA, Wagner DP, Draper EA, et al. The APACHE III prognostic system. Risk prediction of hospital mortality for critically ill hospitalized patients. Chest 1991;100:1619‐1636. [DOI] [PubMed] [Google Scholar]
Kumar 2006
- Kumar A, Singh N, Prakash S, Saraya A, Joshi YK. Early enteral nutrition in severe acute pancreatitis: a prospective randomized controlled trial comparing nasojejunal and nasogastric routes. Journal of Clinical Gastroenterology 2006;40(5):431‐4. [DOI] [PubMed] [Google Scholar]
Lange 1987
- Lange JF, Gool J, Tytgat GN. The protective effect of a reduction in intestinal flora on mortality of acute haemorrhagic pancreatitis in the rat. Hepatogastroenterology 1987;34:28‐30. [PubMed] [Google Scholar]
Lefebvre 2008
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions. Wiley, 2008. [Google Scholar]
Marik 2004
- Marik PE, Zaloga GP. Meta‐analysis of parenteral nutrition versus enteral nutrition in patients with acute pancreatitis. BMJ 2004;328:1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
Maxton 1989
- Maxton DG, Menzies IS, Salvin B, Thompson RPH. Small intestinal function during enteral feeding and starvation in man. Clinical Science (Colch.) 1989;77:401‐6. [DOI] [PubMed] [Google Scholar]
McClave 1986
- McClave EE, Jones TN. Benefits of immediate jejunostomy feeding after major abdominal trauma ‐ a prospective randomized trial. Journal of Trauma 1986;26:874‐81. [DOI] [PubMed] [Google Scholar]
McClave 1998
- McClave SA, Snider H, Owens N, Sexton LK. Nutritional management in acute and chronic pancreatitis. Gastroenterology Clinics of North America 1998;27:421‐34. [DOI] [PubMed] [Google Scholar]
McClave 2006
- McClave SA, Chang WK, Dhaliwal R, Heyland DK. Nutrition support in acute pancreatitis: a systematic review of the literature. Journal of Parenteral and Enteral Nutrition 2006;30(2):143‐56. [DOI] [PubMed] [Google Scholar]
Moore 1992
- Moore FA, Feliciano DV, Andrassy RJ, McArdle AH, Booth FV, Morgenstein‐Wagner TB, Kellum JM Jr, Welling RE, Moore EE. Early enteral compared with parenteral nutrition reduces postoperative septic complications: the results of a meta‐analysis. Annals of Surgery 1992;216:172‐83. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pisters 1992
- Pisters PW, Ranson JHC. Nutritional support of acute pancreatitis. Surgery Gynecology & Obstetrics 1992;175:275‐84. [PubMed] [Google Scholar]
Povoski 1995
- Povoski SP, Nussbaum MS. Nutrition support in pancreatitis: fertile ground for prospective clinical investigation. Nutrition in Clinical Practice 1995;10:43‐44. [DOI] [PubMed] [Google Scholar]
Runkel 1991
- Runkel NS, Moody FG, Smith GS, Rodriguez MT, LaRocco MT, Miller TA. The role of the gut in the development of sepsis in acute pancreatitis. Journal of Surgical Research 1991;51:18‐23. [DOI] [PubMed] [Google Scholar]
Russel 1983
- Russel JC, Welch JP, Clark DG. Colonic complications of acute pancreatitis and pancreatic abscess. American Journal of Surgery 1983;146:558‐64. [DOI] [PubMed] [Google Scholar]
Ryan 1993
- Ryan CM, Schmidt J, Lawandrowski K, Compton CC, Rattner DW, Warshaw AL, Tompkins RG. Gut macromolecular permeability in pancreatitis correlates with severity of disease in rats. Gastroenterology 1993;104:890‐5. [DOI] [PubMed] [Google Scholar]
Saadia 1990
- Saadia R, Schein M, MacFarlance C, Boffard KD. Gut barrier function and the surgeon. British Journal of Surgery 1990;77:487‐92. [DOI] [PubMed] [Google Scholar]
Schünemann 2008
- Schünemann HJ, Oxman AD, Higgins JPT, Vist GE, Glasziou P, Guyatt GH. Chapter 11: Presenting results and Summary of findings tables. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions. Chichester (UK): John Wiley & Sons, 2008. [Google Scholar]
Scolapio 1999
- Scolapio JS, Malhi‐Chowla N, Ukleja A. Nutrition supplementation in patients with acute and chronic pancreatitis. Gastroenterology Clinics of North America 1999;28:695‐707. [DOI] [PubMed] [Google Scholar]
Sitzmann 1989
- Sitzmann JV, Steinborn PA, Zinner MJ, Cameron JL. Total parenteral nutrition and alternate energy substrates in treatment of severe acute pancreatitis. Surgery Gynaecology & Obstetrics 1989;168:311‐17. [PubMed] [Google Scholar]
Steinberg 1994
- Steinberg W, Tenner S. Acute pancreatitis. New England Journal of Medicine 1994;330:1198‐210. [DOI] [PubMed] [Google Scholar]
Sterne 2008
- Sterne JAC, Egger M, Moher D (editors). Chapter 10: Addressing reporting biases. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions. Chichester (UK): John Wiley & Sons, 2008. [Google Scholar]
Twomey 1985
- Twomey P, Patching S. Cost‐effectiveness of nutritional support. Journal of Parenteral and Enteral Nutrition 1985;9:3‐7. [DOI] [PubMed] [Google Scholar]
VanGossum 1988
- VanGossum A, Lemoyne M, Greig PD, Jeejeebhoy KN. Lipid associated total parenteral nutrition in patients with severe acute pancreatitis. Journal of Parenteral and Enteral Nutrition 1988;12:250‐5. [DOI] [PubMed] [Google Scholar]


