Abstract
Background
It is unclear whether blood pressure (BP) should be altered actively during the acute phase of stroke.
Objectives
To assess the effect of lowering or elevating BP in people with acute stroke, and the effect of different vasoactive drugs on BP in acute stroke.
Search methods
We searched the Cochrane Stroke Group Trials Register (last searched June 2009), the Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library Issue 4, 2009), MEDLINE (1966 to October 2009), EMBASE (1980 to October 2009), and Science Citation Index (1981 to October 2009).
Selection criteria
Randomised trials of interventions that would be expected, on pharmacological grounds, to alter BP in patients within one week of the onset of acute stroke.
Data collection and analysis
Two review authors independently applied the trial inclusion criteria, assessed trial quality, and extracted data.
Main results
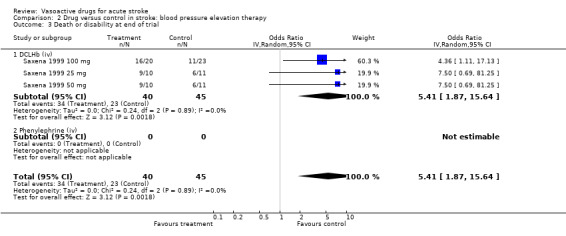
We identified 131 trials involving in excess of 18,000 patients; a further 13 trials are ongoing. We obtained data for 43 trials (7649 patients). Among BP‐lowering trials, beta receptor antagonists lowered BP (early systolic BP (SBP) mean difference (MD) ‐6.1 mmHg, 95% CI ‐11.4 to ‐0.9; late SBP MD ‐4.9 mmHg, 95% CI ‐10.2 to 0.4; late diastolic BP (DBP) MD ‐4.5 mmHg, 95% CI ‐7.8 to ‐1.2). Oral calcium channel blockers (CCB) lowered BP (late SBP MD ‐3.2 mmHg, 95% CI ‐5.4 to ‐1.1; early DBP MD ‐2.5, 95% CI ‐5.6 to 0.7; late DBP MD ‐2.1, 95% CI ‐3.5 to ‐0.7). Nitric oxide donors lowered BP (early SBP MD ‐10.3 mmHg, 95% CI ‐17.6 to ‐3.0). Prostacyclin lowered BP (late SBP MD, ‐7.7 mmHg, 95% CI ‐15.6 to 0.2; late DBP MD ‐3.9 mmHg, 95% CI ‐8.1 to 0.4). Among BP‐increasing trials, diaspirin cross‐linked haemoglobin (DCLHb) increased BP (early SBP MD 15.3 mmHg, 95% CI 4.0 to 26.6; late SBP MD 15.9 mmHg, 95% CI 1.8 to 30.0). None of the drug classes significantly altered outcome apart from DCLHb which increased combined death or dependency (odds ratio (OR) 5.41, 95% CI 1.87 to 15.64).
Authors' conclusions
There is not enough evidence to evaluate reliably the effect of altering BP on outcome after acute stroke. However, treatment with DCLHb was associated with poor clinical outcomes. Beta receptor antagonists, CCBs, nitric oxide, and prostacyclin each lowered BP during the acute phase of stroke. In contrast, DCLHb increased BP.
Plain language summary
Vasoactive drugs for acute stroke
In patients who have just had a stroke (a sudden catastrophe in the brain either because an artery to the brain blocks, or because an artery in or on the brain ruptures and bleeds) very high and very low blood pressure may be harmful. Drugs which raise low blood pressure or lower high blood pressure might benefit acute stroke patients. This review of 43 trials involving 7649 participants found that there was not enough evidence to decide if drugs which can alter blood pressure should or should not be used in patients with acute stroke. More research is needed.
Background
Description of the condition
Stroke is the third most common cause of death and the commonest cause of disability in the western world. Acute stroke, whether due to infarction or haemorrhage, is associated with high blood pressure in 75% of patients of whom 50% have a previous history of high blood pressure (International Society of Hypertension 2003). The mechanisms underlying hypertension in stroke are complex but pre‐existing hypertension (present in 50% to 60% of patients), hospitalisation stress, activation of the neuro‐endocrine pathways, and the Cushing reflex, each contribute (International Society of Hypertension 2003; Sprigg 2005). Low blood pressure is not common in acute stroke but it, like high blood pressure, is associated with a poor outcome (Castillo 2004; Leonardi‐Bee 2002; Vemmos 2004). Possible reasons for low blood pressure include potentially reversible conditions such as hypovolaemia, sepsis, impaired cardiac output secondary to cardiac failure, arrhythmias or cardiac ischaemia, and aortic dissection (Sprigg 2005).
Description of the intervention
Although debated more than 20 years ago, it still remains unclear whether hypertension should (Spence 1985) or should not (Yatsu 1985) be treated acutely following stroke. Recent guidelines recommend that acute lowering of blood pressure should be delayed for several days or even weeks unless blood pressure is higher than 220/120 mmHg, higher than 200/100 mmHg with end organ involvement (hypertensive encephalopathy, aortic dissection, cardiac ischaemia, pulmonary oedema, acute renal failure), or higher than 200/120 mmHg with primary intracerebral haemorrhage (PICH) (AHA‐HS 2007; AHA‐IS 2007; ESO 2008). Though the evidence is weak (class 1, level of evidence B) guidelines now recommend that patients who have elevated blood pressure and are otherwise eligible for treatment of recombinant tissue plasminogen activator (rtPA) may have their blood pressure lowered so that systolic blood pressure (SBP) is ≤ 185 mmHg and diastolic blood pressure (DBP) is ≤ 110 mmHg before thrombolysis using intravenous labetalol, nitropaste or nicardipine and it should be maintained below 180/105 mmHg for at least the first 24 hours after therapy (AHA‐IS 2007; ESO 2008). Similarly, guidelines recommend that causes of low blood pressure in the setting of acute stroke should be sought with a view to correcting reversible causes such as hypovolaemia and cardiac arrhythmias (AHA‐IS 2007; ESO 2008).
How the intervention might work
A number of small studies have assessed the relationship between blood pressure and outcome. A meta‐analysis of these and other studies found that elevated blood pressure was associated with a poor outcome (Willmot 2004). Data from 17,398 patients in the International Stroke Trial (IST) identified a U‐shaped relationship such that both low and high blood pressure was associated independently with increased early death and later death or dependency (Leonardi‐Bee 2002). A high blood pressure is also associated with increased early recurrence (Leonardi‐Bee 2002; Sprigg 2006). In ischaemic stroke, hypertension also appears to affect adversely through increasing the risk of cerebral oedema, but not haemorrhagic transformation (Leonardi‐Bee 2002) as shown in the IST analysis. Haematoma expansion is related to high blood pressure in patients with PICH although this relationship may be confounded by stroke severity and time to presentation (Bath 2003). Since cerebral autoregulation is lost following stroke (Burke 1986; Paulson 1990; Strandgaard 1973) such that cerebral blood flow becomes dependent on systemic blood pressure, some researchers have hypothesised that blood pressure should be increased (Sandercock 1992) after stroke to improve perfusion to the penumbral region, and several case series and small trials have been published. In a recent meta‐regression of blood pressure in acute stroke involving data from randomised controlled trials, large increases or reductions in blood pressure were associated with harm whereas moderate reductions were associated with a non‐significant reduction in death or dependency (Geeganage 2009).
Why it is important to do this review
This systematic review included randomised controlled trials (RCTs) of interventions that would be expected, on pharmacological grounds, to alter blood pressure in patients within one week of the onset of acute ischaemic or haemorrhagic stroke. A related review restricted inclusion to those trials which specifically studied the effect of changing blood pressure in acute stroke (BASC I). The aim of this review is to assess the effect of lowering or elevating blood pressure in people with acute stroke, and the effect of different vasoactive drugs on blood pressure in acute stroke.
Objectives
To determine whether lowering or elevating blood pressure in patients with acute stroke is safe and effective in reducing the risk of early and late death and functional dependency.
To determine the effect of vasoactive drugs on blood pressure patients with acute stroke.
Methods
Criteria for considering studies for this review
Types of studies
We included published and unpublished randomised or quasi‐randomised controlled trials (i.e. trials that used a non‐random method of treatment allocation, for example hospital number, date of birth or day of the week), of vasoactive drugs in acute ischaemic stroke or acute primary intracerebral haemorrhage where drug therapy was initiated within one week of stroke onset. We excluded uncontrolled studies, confounded controlled studies where the intervention was compared with another active therapy, and studies of patients with subarachnoid haemorrhage.
Types of participants
Adults (aged 18 years and over) of either sex with acute ischaemic or haemorrhagic stroke (within one week of onset) who were eligible for randomisation to either active treatment or placebo/open control.
Types of interventions
All randomised controlled acute stroke trials where vasoactive drugs were used in the acute treatment of stroke.
Types of outcome measures
Early (within one month) and end‐of‐trial mortality; early death or deterioration; end‐of‐trial mortality or dependency; blood pressure and heart rate at baseline, and during early (less than 24 hours) and late (24 to 72 hours) treatment; length of hospital stay and discharge destination. We defined disability or dependency as a Barthel Index 0 to 55 or Rankin score 3 to 5. We also noted the presence of 'hypotension' (however defined by trialists) where given.
Search methods for identification of studies
See the 'Specialized register' section in the Cochrane Stroke Group module.
We searched the Cochrane Stroke Group Trials Register, which was last searched by the Managing Editor in June 2009 using a search strategy designed to identify all relevant trials. In addition, we searched the Cochrane Database of Systematic Reviews (CDSR) and the Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library Issue 4, 2009), MEDLINE (1966 to October 2009) (Appendix 1), EMBASE (1980 to October 2009) (Appendix 2), and Science Citation Index (ISI Web of Science, 1981 to October 2009) (Appendix 3). We did not apply any language restrictions.
In an effort to identify further published, unpublished, and ongoing trials:
we searched reviews of hypertension in acute stroke from the CDSR and existing Cochrane and other stroke overviews relating to drugs which may alter blood pressure, including: calcium channel blockers (CCBs) (Horn 2001), nitric oxide (Bath 2002), pentoxifylline (Bath 2004/2), amphetamine (Martinsson 2007; Sprigg 2007), tirilazad (Tirilazad International Steering Committee 2001), naftidrofuryl (Leonardi‐Bee 2007), vinpocetine (Bereczki 2008) and prostacyclin (Bath 2004/1) as well as other generic reviews (Geeganage 2009);
we searched the Ongoing Trials section of Stroke and the Internet Stroke Center Stroke Trials Registry (Stroke Center) (October 2009);
we scanned the reference lists of relevant trials and existing review articles;
we contacted research workers in this field (see Acknowledgements);
we contacted the following pharmaceutical companies: Bayer (nimodipine), Napp (pentoxifylline), Novartis (isradipine), Lipha Sante (naftidrofuryl), Hoffmann la Roche (N Methyl D Aspartate), Hoechst (flunarizine) and UCB Pharma (piracetam) in 1999 for the previous version of the review.
Data collection and analysis
We identified and independently assessed published and unpublished trials and decided whether to include or exclude them. One review author (CG) identified data in published material and sought additional information from the principal investigators of the trials where necessary. We resolved disagreements by discussion. Where available, we re‐analysed individual patient data and used the resulting group data in preference to published data. We recorded information on the methods of randomisation, concealment of allocation, blinding, analysis (intention‐to‐treat or efficacy analysis), stroke type (ischaemia or haemorrhage), drug dose, route of administration (oral, transdermal or intravenous) and timing, blood pressure and heart rate (before and during treatment), numbers of deaths, functional disability, quality of life, length of stay, and adverse effects such as hypotension,
We assessed the methodological quality of trials, especially relating to concealment of allocation as detailed in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2008). We calculated the weighted estimate of the typical treatment effect across trials (odds ratio (OR) for binary data, mean difference (MD) for continuous data) using aggregated patient data in Review Manager 5.0 (RevMan 2008); this software also tests for heterogeneity between the trials.
Results
Description of studies
Where a trial used more than one dose of a particular drug then the reference is written as author followed by date followed by dose of drug. When referencing the whole trial the references for all the doses will be used (e.g. Saxena 1999 50 mg/Saxena 1999 100 mg). Blood pressure (BP) data were available in 43 trials including 7649 patients (Characteristics of included studies). We excluded more than 80 studies as the relevant data were unobtainable, either because they were not present in trial reports and could not be provided by trialists, or because they had been discarded or they could not be released until publication of the final trial reports (Characteristics of excluded studies).
The trials involved 16 combinations of drug classes and routes of administration: oral or sublingual angiotensin converting enzyme (ACE) inhibitors (perindopril, captopril and lisinopril); oral angiotensin receptor antagonists (ARA) (candesartan); oral beta receptor antagonists (βRA) (atenolol, propanolol); combined alpha and beta receptor antagonists (labetalol); oral thiazide diuretics (bendrofluazide), intravenous CCBs (flunarizine, isradipine, nimodipine); oral CCBs (nimodipine, nicardipine); intravenous DCLHb (a haemoglobin analogue); intravenous magnesium sulphate; intravenous naftidrofuryl; transdermal glyceryl trinitrate (a nitric oxide donor); intravenous piracetam; combined intravenous prostacyclin; intravenous glucose potassium insulin (GKI); intravenous insulin; intravenous phenylephrine; and intravenous and or oral mixed antihypertensive therapy (Characteristics of included studies).
Patients were recruited into trials within six to 168 hours from stroke onset; most were enrolled within 24 to 168 hours (Characteristics of included studies). Nine studies included patients who were hypertensive at the time of recruitment (Characteristics of included studies); the other studies involved patients with a range of BPs. Two trials studied phenylephrine and DCLHb which elevate BP (Saxena 1999 25 mg/Saxena 1999 50 mg/Saxena 1999 100 mg; Hillis 2003). Thirty‐eight trials were published and five trials unpublished (IMAGES Pilot; Lowe 1993; Pokrupa 1986; Strand 1984; Uzuner 1995/180 mg). Routes of administration included oral, intravenous (iv), transdermal, sublingual or combinations of these (Characteristics of included studies). The treatment duration varied from 24 hours to nine months (Characteristics of included studies). Some drugs were given in two phases, initially intravenously then orally (CCB, magnesium sulphate, naftidrofuryl, piracetam) (Characteristics of included studies). Combinations of intravenous and oral antihypertensive drugs were used to lower BP in the intensive as well as guideline group of INTERACT pilot trial (INTERACT pilot 2008). Three trials used transdermal glyceryl trinitrate (GTN) 5 mg daily for 12 days (Bath 2000); GTN 5 mg, 5/10 mg, 10 mg (Rashid 2003 5 mg/Rashid 2003 5/10 mg/Rashid 2003 10 mg) for 10 days; GTN 5 mg (Willmot 2006) for seven days. There was one dose escalation study of 8 mmol, 12 mmol, 16 mmol of magnesium sulphate over 24 hours (Muir 1995).
Risk of bias in included studies
The methods used in the 43 trials are summarised in the Characteristics of included studies table. All trials were double‐blind, with the exceptions of one single‐blinded (Saxena 1999 100 mg/Saxena 1999 25 mg/Saxena 1999 50 mg), four outcome‐blinded (Gray 2007; INTERACT pilot 2008; Rashid 2003 10 mg/Rashid 2003 5 mg/Rashid 2003 5/10 mg; Willmot 2006) and two open studies (Barer 1988 atenolol/Barer 1988 propanolol; Walters 2006). The method of randomisation was only given for 22 trials (Ahmed 2000 1 mg/Ahmed 2000 2 mg; Barer 1988 atenolol/Barer 1988 propanolol; Bath 2000; Bogousslavsky 1990; Dyker 1997; Eames 2005; Eveson 2007; Gray 2007; IMAGES Pilot; INTERACT pilot 2008; Kaste 1994/120 mg; Lees 1995; Limburg 1990; Lowe 1993; PASS 1995; Pokrupa 1986; Potter 2009 labetalol/Potter 2009 lisinopril; Rashid 2003 5 mg/Rashid 2003 5/10 mg/Rashid 2003 10 mg; Strand 1984; Walters 2006; Willmot 2006; Wimalarat 1994/120mg/Wimalarat 1994/240mg). All trials were analysed by the intention‐to‐treat analysis with the exception of two (Huczynski 1988; Martinez‐Vila 1990).
There were 23 single‐centred trials. All trials used computerised tomography (CT) to exclude patients with PICH with the exception of nine trials that included both types of stroke (Ahmed 2000 1 mg/Ahmed 2000 2 mg; Barer 1988 atenolol/Barer 1988 propanolol; Barer 1988/50 mg/Barer 1988/80 mg; Fagan 1988/120 mg/Fagan 1988/240 mg; Gray 2007; Potter 2009 labetalol/Potter 2009 lisinopril; Rashid 2003 5 mg/Rashid 2003 5/10 mg/Rashid 2003 10 mg; VENUS 1995; Willmot 2006). One trial only included patients with acute spontaneous intracerebral haemorrhage (ICH) diagnosed by CT (INTERACT pilot 2008). For the lisinopril study randomisation was done before neuroimaging and those with non‐ischaemic stoke were subsequently withdrawn from the study (Eveson 2007).
Effects of interventions
General
We identified a total of 131 trials involving in excess of 18,000 patients. However, data were only available for 43 trials involving 7649 patients. We excluded 86 trials as BP or outcome data were not available. The patients receiving placebo or control treatment in eight trials (Ahmed 2000 1 mg/Ahmed 2000 2 mg; Barer 1988 atenolol/Barer 1988 propanolol; Barer 1988/50 mg/Barer 1988/80 mg; Fagan 1988/120 mg/Fagan 1988/240 mg; Rashid 2003 5 mg/Rashid 2003 5/10 mg/Rashid 2003 10 mg; Saxena 1999 25 mg/Saxena 1999 50 mg/Saxena 1999 100 mg; Wimalarat 1994/120mg/Wimalarat 1994/240mg; Potter 2009 labetalol/Potter 2009 lisinopril) acted as controls for more than one group of actively treated patients; control participants in these studies were divided equally between each active treatment group to ensure that the total number of control participants was correct. This strategy is recommended by the Cochrane Stroke Group and avoids artificially inflating patient numbers and therefore narrowing confidence intervals.
Blood pressure
Baseline SBP was mismatched between the treatment and control groups across all treatments (MD ‐1.6 mmHg, 95% CI ‐2.8 to ‐0.4) and especially for intravenous CCBs (MD ‐6.6 mmHg, 95% CI ‐13.4 to 0.2) (Appendix 4). Several drug classes lowered BP, including: beta receptor antagonists (early SBP, MD ‐6.1 mmHg, 95% CI ‐11.4 to ‐0.9; late SBP MD ‐4.9 mmHg, 95% CI ‐10.2 to 0.4; late DBP MD ‐4.5 mmHg, 95% CI ‐7.8 to ‐1.2); oral CCBs (late SBP MD ‐3.2 mmHg, 95% CI ‐5.4 to ‐1.1; early DBP MD ‐2.5, 95% CI ‐5.6 to 0.7; late DBP MD ‐2.1, 95% CI ‐3.5 to ‐0.7); nitric oxide donors (early SBP MD ‐10.3 mmHg, 95% CI ‐17.6 to ‐3.0), and prostacyclin (late SBP MD ‐7.7 mmHg, 95% CI ‐15.6 to 0.2; late DBP MD ‐3.9 mmHg, 95% CI ‐8.1 to 0.4).
BP lowering is also seen for several other antihypertensive agents although the small number of participants studied meant that differences in BP were not always statistically significant. Drugs showing hypotensive properties included: angiotensin converting enzyme inhibitors, angiotensin receptor antagonists, bendrofluazide, intravenous CCBs and GKI. Neither magnesium, naftidrofuryl nor piracetam had appreciable effects on BP. INTERACT pilot 2008 lowered SBP (early MD ‐14.0 mmHg 95% CI ‐17.2 to ‐10.8, late MD ‐11.0 mmHg, 95% CI ‐14.0 to ‐8.0) in the intensive treatment versus guideline treatment arm.
In contrast, DCLHb increased BP (early SBP MD 15.3 mmHg, 95% CI 4.0 to 26.6; late SBP MD 15.9 mmHg, 95% CI 1.8 to 30.0). Intravenous phenylephrine also showed a trend towards an increase in SBP (MD 20.6, 95% CI ‐13.3 to 54.5) as compared to control.
Heart rate
Heart rate was lowered by beta blockers (early heart rate (HR) MD ‐6.8 beats/minute, 95% CI ‐9.6 to ‐4.0; late HR MD ‐9.3 beats/minute, 95% CI ‐12.0 to ‐6.6); and oral CCBs (late HR MD ‐2.8 beats/minute, 95% CI ‐3.9 to ‐1.7); and increased by nitric oxide donors (MD 6.3 beats/minute 95% CI 2.9 to 9.7). Intravenous CCBs, ACE inhibitors, naftidrofuryl, magnesium, and DCLHb did not alter heart rate.
Death or dependency
There was no evidence of an effect on death for any agent except DCLHb which significantly increased the odds of death or dependency (OR 5.41, 95% CI 1.87 to 15.64). A trend for an increase in combined end of trial death or disability was observed for oral CCBs (odds ratio (OR) 1.30, 95% CI 0.91 to1.86).
Hypotensive events
There was no significant difference for oral CCBs (total events four active, six control, OR 0.73, 95% CI 0.19 to 2.74) and mixed antihypertensive therapy (total events five active, six control, OR 1.24, 95% CI 0.33 to 4.7) in the number of hypotensive events. None of the other agents reported hypotensive events in trial publications.
Relationship between blood pressure and outcome
The numbers of trials and participants with data on BP and outcome were not identical and it was not possible to relate group differences in BP with group differences in outcome. This problem was compounded by biologically important differences in baseline BP between treated and control groups.
Discussion
Beta receptor antagonists, oral calcium channel blockers (CCBs), glyceryl trinitrate (GTN), prostacyclin and mixed antihypertensive therapy lowered blood pressure (BP) during the first three days of treatment. Angiotensin converting enzyme inhibitors, angiotensin receptor blockers, bendroflumethiazide, intravenous CCBs, insulin and GKI also appeared to lower BP as compared to the controls. In contrast, magnesium, naftidrofuryl, and piracetam had no effect on BP. Nevertheless, these observations may be partly confounded by mismatches in baseline BP (significantly so for intravenous CCBs). Baseline imbalances in BP would have profound effects on outcome and therefore change the BP‐outcome relationship. A definitive assessment as to whether these drugs change BP will be dependent on analysis of individual patient data from these trials. Unfortunately, individual patient data were not available for most of the included trials; the presence of these data would have addressed this issue. Further, the apparent BP‐reducing effect of GKI may be due to the confounding in the GIST trial, in which the controls were treated with intravenous saline, and the treatment group received intravenous dextrose, so the BP difference may not be attributable to the GKI lowering BP, but to the control group having less hypovolaemia/hypotension because of the saline (Gray 2007).
None of the drug classes altered outcome apart from diaspirin cross‐linked haemoglobin (DCLHb) which significantly increased combined death and dependency compared with control. There was no significant difference in outcome for CCBs, beta blockers, ACE‐I, magnesium, and nitric oxide. The relationship between BP and outcome could not be studied for methodological reasons in the present review. However, the relationship between BP and outcome based on many of the included trials has been assessed in a meta‐regression (Geeganage 2009). The results revealed a U‐shaped relationship between BP changes and outcome, with the lowest risk of death or combined death or dependency at the end of follow up in patients with BP reductions ranging from eight to 15 mmHg. Although large falls or increases in BP were associated with a higher risk of poor outcomes, a modest reduction may reduce death and combined death or dependency, although confidence intervals were wide and compatible with an overall benefit or hazard.
Authors' conclusions
Implications for practice.
Trials of vasoactive drugs in acute stroke reveal that beta receptor antagonists, oral calcium channel blockers (CCBs), glyceryl trinitrate (GTN), prostacyclin and mixed antihypertensive therapy each lower blood pressure (BP). In contrast, diaspirin cross‐linked haemoglobin (DCLHb) and phenylephrine increases BP. However, these data do not allow the effect of changing BP on outcome to be assessed. In the absence of definitive information, there is no clear indication for the deliberate alteration of BP during the first few days after stroke.
Implications for research.
The existing completed studies of vasoactive drugs in acute stroke are all small or medium sized (fewer than 1000 participants) and, hence, likely to be underpowered. One or more large trials (several thousand participants) are now required to determine whether altering (raising or lowering) BP can be safe and efficacious; such studies are ongoing (ENOS 2006; INTERACT 2 2007; SCAST 2005).
What's new
| Date | Event | Description |
|---|---|---|
| 1 October 2009 | New search has been performed | This review was updated in October 2009 and includes the following: (1) the addition of 11 completed trials involving 2281 patients; (2) the addition of 13 ongoing or planned trials. The previous version of the review included 32 trials involving 5368 patients. The conclusions of this review have not changed with the addition of the new data. |
| 1 October 2009 | New citation required but conclusions have not changed | Change of authors. |
History
Protocol first published: Issue 4, 2000 Review first published: Issue 4, 2000
| Date | Event | Description |
|---|---|---|
| 29 May 2008 | Amended | Converted to new review format. |
| 21 February 2007 | Amended | Substantive amendment. |
Acknowledgements
The Blood pressure in Acute Stroke Collaboration (BASC) comprises the following people.
Data collation and analysis, and review writing for this version of the review: Chamila Geeganage, Philip Bath (previous version: Fiona J Bath and R Iddenden)
Trialists: Ahmed N (Sweden), Asplund K (Sweden), Autret A (France), Barer D (UK), Bath PMW (UK), Bereczki D (Hungary), Bogousslavsky J (Switzerland), Chan YW (Hong Kong), Davis S (Australia), de Deyn PP (Belgium), Donnan G (Australia), Dyker AG (UK), Eveson D (UK), Fogelholm R (Finland), Gelmers HJ (Netherlands), Gray CS (UK), Grotta J (USA), Hachinski V (Canada), Hakim RP (Canada), Heiss WH (Germany), Herrschaft H (Germany), Hillis AB (USA), Horn J (Netherlands), Hsu CY (USA), Huczynski J (Poland), Kaste M (Finland), Koudstall PJ (Netherlands), Kramer G (Switzerland), Lees KR (UK), Limberg M (Netherlands), Lisk R (Cameroon), Lowe G (UK), Muir KW (UK), Mistri A (UK), Murphy JJ (UK), Orgogozo JM (France), Pokrupa RP (Canada), Rashid P (UK), Saxena R (Netherlands), Steiner T (UK), Strand T (Sweden), Uzuner N (Turkey), Wahlgren N (Sweden), Walters MR (UK), Willmot M (UK), Wimalaratna HSK (UK), Wong WJ (Taiwan).
Companies (for the previous version of this review): Bayer (Canada), Lipha Sante (France), UCB pharma (Belgium).
We are grateful to the Cochrane Stroke Group Editorial Board and external peer reviewers for their comments on this review. All the analyses and their interpretation reflect the opinions of BASC; no pharmaceutical company was involved in the analysis or interpretation of data, or in the writing of this review.
Appendices
Appendix 1. MEDLINE search strategy
01. stroke.mp. 02. infarction.mp. 03. exp brain Infarction/ 04. exp infarction, anterior cerebral artery/ 05. exp infarction, middle cerebral artery/ 06. exp infarction, posterior cerebral artery/ 07. exp brain ischemia/ 08. brain ischaemia.mp. 09. cerebral ischaemia.mp. 10. hemorrhage.mp. 11. exp cerebral hemorrhage/ 12. cerebral haemorrhage.mp. 13. 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 or 12 14. (nitrate or L‐arginine or thiazide or diuretics or beta blockers or calcium channel blockers or angiotensin‐converting enzyme inhibitors or ACE inhibitors or angiotensin receptor antagonists or rennin inhibitors or neuroprotective agents or alpha receptor antagonists or vasoconstrictors or adrenoceptor agonists or centrally acting antihyperten$ or vasodilators or hemodilution or haemodilution).mp. 15. (bendrofluazide or bendroflumethiazide or hydrochrlothiazide or atenolol or propanalol or bisoprolol or labetalol or nimodipine or nicardipine or amilodipine or felodipine or clinidipine or isradipine or nifedipine or nisolodipine or tirilazad or flunarazine or captopril or enalapril or lisinopril or perindopril or ramipril or candesartan or losartan or telmisartan or valsartan or clonidine or pentoxifylline or pentifylline or naftidrofuryl or prostacyclin or PGI2 or magnesium or papaverine or vinpocetin or piracetam or dopamine or dobutamine or adrenaline or noradrenaline or phenylephrine or amphetamine or caffeinol or caffeine or theophylline or diaspirin cross linked haemoglobin or DCLHb).mp. 16. 14 or 15 17. 13 and 16 18. (randomized controlled trial.pt. or controlled clinical trial.pt.or randomized.ab. or placebo.ab. or clinical trials as topic.sh. or randomly.ab. or trial.ti.) and humans.sh. 19. 17 and 18
Appendix 2. EMBASE search strategy
01. stroke.mp. 02. infarction.mp. 03. exp brain Infarction/ 04. exp brain infarction size/ 05. brain stem infarction 06. cerebellum infarction 07. brain ischemia.mp. 08. brain ischaemia.mp. 09. exp brain ischemia/ 10. cerebral ischaemia.mp. 11. hemorrhage.mp. 12. exp cerebral hemorrhage/ 13. cerebral haemorrhage.mp. 14. 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 or 12 or 13 or 14 15. (nitrate or L‐arginine or thiazide or diuretics or beta blockers or calcium channel blockers or angiotensin‐converting enzyme inhibitors or ACE inhibitors or angiotensin receptor antagonists or rennin inhibitors or neuroprotective agents or alpha receptor antagonists or vasoconstrictors or adrenoceptor agonists or centrally acting antihyperten$ or vasodilators or hemodilution or haemodilution).mp. 16. (bendrofluazide or bendroflumethiazide or hydrochrlothiazide or atenolol or propanalol or bisoprolol or labetalol or nimodipine or nicardipine or amilodipine or felodipine or clinidipine or isradipine or nifedipine or nisolodipine or tirilazad or flunarazine or captopril or enalapril or lisinopril or perindopril or ramipril or candesartan or losartan or telmisartan or valsartan or clonidine or pentoxifylline or pentifylline or naftidrofuryl or prostacyclin or PGI2 or magnesium or papaverine or vinpocetin or piracetam or dopamine or dobutamine or adrenaline or noradrenaline or phenylephrine or amphetamine or caffeinol or caffeine or theophylline or diaspirin cross linked haemoglobin or DCLHb).mp. 17. 15 or 16 18. 14 and 17 19. ((RANDOMIZED‐CONTROLLED‐TRIAL/ or RANDOMIZATION/ or CONTROLLED‐STUDY/ or MULTICENTER‐STUDY/ or PHASE‐3‐CLINICAL‐TRIAL/ or PHASE‐4‐CLINICAL‐TRIAL/ or DOUBLE‐BLIND‐PROCEDURE/ or SINGLE‐BLIND‐PROCEDURE/) or ((RANDOM* or CROSS?OVER* or FACTORIAL* or PLACEBO* or VOLUNTEER*) or ((SINGL* or DOUBL* or TREBL* or TRIPL*) adj3 (BLIND* or MASK*))).ti,ab) and human*.ec,hw,fs. 20. 18 and 19
Appendix 3. Science Citation Index search strategy
01. stroke.TS./TI 02. acute stroke.TS./TI. 03. cerebral infarction.TS./TI. 04. brain Infarction.TS./TI. 05. brain ischemia.TS./TI. 06. brain ischaemia.TS./TI. 07. brain ischemia.TS./TI. 08. cerebral ischaemia.TS./TI. 09. cerebral hemorrhage.TS./TI. 10. cerebral haemorrhage.TS./TI. 11. cerebral bleeding.TS./TI. 12. 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 or 12 or 13 or 14 13. (nitrate or L‐arginine or thiazide or diuretics or beta blockers or calcium channel blockers or angiotensin‐converting enzyme inhibitors or ACE inhibitors or angiotensin receptor antagonists or rennin inhibitors or neuroprotective agents or alpha receptor antagonists or vasoconstrictors or adrenoceptor agonists or centrally acting antihyperten$ or vasodilators or hemodilution or haemodilution.TS./TI. 14. (bendrofluazide or bendroflumethiazide or hydrochrlothiazide or atenolol or propanalol or bisoprolol or labetalol or nimodipine or nicardipine or amilodipine or felodipine or clinidipine or isradipine or nifedipine or nisolodipine or tirilazad or flunarazine or captopril or enalapril or lisinopril or perindopril or ramipril or candesartan or losartan or telmisartan or valsartan or clonidine or pentoxifylline or pentifylline or naftidrofuryl or prostacyclin or PGI2 or magnesium or papaverine or vinpocetin or piracetam or dopamine or dobutamine or adrenaline or noradrenaline or phenylephrine or amphetamine or caffeinol or caffeine or theophylline or diaspirin cross linked haemoglobin or DCLHb).TS./TI. 15. 13 or 14 16. 12 and 15 17. (randomized controlled trial.TI. or controlled clinical trial.TI.or randomized.TI. or placebo.TI. or clinical trials TI. or randomly.TI. or trial.TI.) and humans.TI. 18. 16 and 17
Appendix 4. Baseline haemodynamic measures for included studies
| Drug class | Baseline SBP | N | Baseline DBP | N | Baseline HR | N |
| MD (95% CI) | MD (95% CI) | MD (95% CI) | ||||
| BP lowering therapy | ||||||
| ACE inhibitors (po) | 1.79 (‐3.84 to 7.43) | 4 | ‐0.22 (‐4.34 to 3.89) | 4 | 0.22 (‐5.31 to 5.75) | 3 |
| ARA (po) | ‐2.00 (‐6.32 to 2.32) | 1 | 0.00 (‐2.97 to 2.97) | 1 | ||
| Beta blockers (po) | 0.34 (‐4.27 to 4.96) | 5 | 0.03 (‐3.75 to 3.80) | 5 | ‐0.36 (‐3.73 to 3.02) | 4 |
| Calcium channel blockers (iv) | ‐6.60 (‐13.37 to 0.16) | 6 | ‐1.72 (‐5.99 to 2.55) | 6 | ‐0.24 (‐3.18 to 2.71) | 4 |
| Calcium channel blockers (po) | 0.44 (‐2.82 to 1.94) | 14 | ‐0.11 (‐1.54 to 1.33) | 14 | ‐1.32 (‐2.77 to 0.13) | 9 |
| Glucose potassium insulin (iv) | ‐2.5 (‐6.29 to 1.29) | 1 | ||||
| Insulin (iv) | ‐7.00 (‐20.73 to 6.73) | 1 | 0.00 (‐9.08 to 9.08) | 1 | ||
| Magnesium (iv) | 1.42 (‐7.13 to 9.98) | 4 | 0.99 (‐4.37 to 6.35) | 4 | ‐1.79 (‐7.95 to 4.37) | 4 |
| Naftidrofuryl | ‐1.46 (‐7.95 to 5.02) | 2 | ‐0.18 (‐4.70 to 4.35) | 2 | 1.27(‐4.30 to 6.83) | 2 |
| Nitric oxide | 0.98 (‐6.13 to 8.08) | 5 | 3.9 (‐0.22 to 8.03) | 5 | 3.44 (‐2.29 to 9.17) | 5 |
| Other vasodilators (po) | ‐24.83 (‐48.89 to ‐0.77) | 1 | 8.33 (‐1.66 to 18.32) | 1 | ||
| Piracetam | ‐2.11 (‐6.39 to 2.16) | 2 | ‐0.39 (‐2.34 to 1.56) | 2 | ||
| Prostacyclin | ‐2.75 (‐10.87 to 5.36) | 3 | ‐4.84 (‐9.72 to 0.04) | 3 | ‐0.71 (‐5.52 to 4.11) | 3 |
| Thiazide diuretics (po) | ‐20.00(‐39.44 to ‐0.56) | 1 | ‐15.00 (‐29.51 to ‐0.49) | 1 | ||
| Unclassified or combined | ‐2.00 (‐5.61 to 1.61) | 1 | ‐4.00 (‐6.83 to ‐1.17) | 1 | ||
| Total | ‐1.59 (‐2.83 to ‐0.35) | 51 | ‐0.41 (‐1.37 to 0.55) | 50 | ||
| BP elevation therapy | ||||||
| DCLHb | 5.37 (‐3.59 to 14.34) | 3 | ‐2.56 (‐8.78 to 3.65) | 3 | 3.37 (‐2.43 to 9.17) | 3 |
| Phenylephrine | ‐27.5 (‐50.83 to ‐4.17) | 1 | ‐8.30 (‐19.13 to 2.53) | 1 | ||
| Total | ‐1.53 (‐15.15 to 12.09) | 4 | ‐3.73 (‐8.99 to 1.54) | 4 | 3.37 (‐2.43 to 9.17) | 3 |
Significant results are in bold type
CI: confidence interval DBP: diastolic blood pressure DCLHb: diaspirin cross‐linked haemoglobin HR: heart rate iv: intravenous MD: mean difference N: number of studies po: oral SBP: systolic blood pressure
Data and analyses
Comparison 1. Drug versus control in stroke: blood pressure lowering therapy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Early death (≤ 1 month) | 36 | 5134 | Odds Ratio (IV, Random, 95% CI) | 1.15 [0.98, 1.36] |
| 1.1 ACE inhibitors (po) | 3 | 164 | Odds Ratio (IV, Random, 95% CI) | 0.91 [0.26, 3.14] |
| 1.2 Beta blockers (po) | 5 | 434 | Odds Ratio (IV, Random, 95% CI) | 1.33 [0.58, 3.03] |
| 1.3 Calcium channel blockers (iv) | 6 | 738 | Odds Ratio (IV, Random, 95% CI) | 1.13 [0.76, 1.67] |
| 1.4 Calcium channel blockers (po) | 8 | 1802 | Odds Ratio (IV, Random, 95% CI) | 0.94 [0.63, 1.40] |
| 1.5 Insulin (iv) | 1 | 25 | Odds Ratio (IV, Random, 95% CI) | 3.00 [0.11, 80.95] |
| 1.6 Magnesium (iv) | 1 | 25 | Odds Ratio (IV, Random, 95% CI) | 1.05 [0.04, 29.24] |
| 1.7 Naftidrofuryl | 2 | 710 | Odds Ratio (IV, Random, 95% CI) | 1.16 [0.77, 1.75] |
| 1.8 Nitric oxide | 5 | 145 | Odds Ratio (IV, Random, 95% CI) | 1.00 [0.16, 6.11] |
| 1.9 Piracetam | 2 | 967 | Odds Ratio (IV, Random, 95% CI) | 1.38 [0.99, 1.92] |
| 1.10 Prostacyclin (iv) | 3 | 124 | Odds Ratio (IV, Random, 95% CI) | 0.51 [0.12, 2.23] |
| 2 Death at end of trial | 41 | 6648 | Odds Ratio (IV, Random, 95% CI) | 1.09 [0.96, 1.24] |
| 2.1 ACE inhibitors (po) | 3 | 155 | Odds Ratio (IV, Random, 95% CI) | 0.63 [0.21, 1.90] |
| 2.2 ARA (po) | 1 | 339 | Odds Ratio (IV, Random, 95% CI) | 0.38 [0.13, 1.11] |
| 2.3 Beta blockers (po) | 5 | 442 | Odds Ratio (IV, Random, 95% CI) | 1.10 [0.57, 2.14] |
| 2.4 Calcium channel blockers (iv) | 6 | 751 | Odds Ratio (IV, Random, 95% CI) | 1.17 [0.84, 1.63] |
| 2.5 Calcium channel blockers (po) | 10 | 1534 | Odds Ratio (IV, Random, 95% CI) | 0.97 [0.72, 1.29] |
| 2.6 GKI (iv) | 1 | 933 | Odds Ratio (IV, Random, 95% CI) | 1.14 [0.86, 1.51] |
| 2.7 Magnesium (iv) | 4 | 162 | Odds Ratio (IV, Random, 95% CI) | 0.64 [0.28, 1.48] |
| 2.8 Naftidrofuryl | 2 | 710 | Odds Ratio (IV, Random, 95% CI) | 1.19 [0.80, 1.77] |
| 2.9 Nitric oxide | 4 | 127 | Odds Ratio (IV, Random, 95% CI) | 1.01 [0.26, 4.00] |
| 2.10 Pentoxifylline | 0 | 0 | Odds Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.11 Piracetam | 2 | 967 | Odds Ratio (IV, Random, 95% CI) | 1.32 [0.96, 1.81] |
| 2.12 Prostacyclin (iv) | 3 | 124 | Odds Ratio (IV, Random, 95% CI) | 0.96 [0.17, 5.38] |
| 2.13 Unclassified or combined | 1 | 404 | Odds Ratio (IV, Random, 95% CI) | 0.81 [0.44, 1.50] |
| 3 Early death or deterioration (≤ 1month) | 15 | 2175 | Odds Ratio (IV, Random, 95% CI) | 1.07 [0.90, 1.28] |
| 3.1 ACE inhibitors (po) | 0 | 0 | Odds Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3.2 Beta blockers (po) | 4 | 357 | Odds Ratio (IV, Random, 95% CI) | 1.32 [0.84, 2.06] |
| 3.3 Calcium channel blockers (iv) | 3 | 254 | Odds Ratio (IV, Random, 95% CI) | 1.24 [0.75, 2.07] |
| 3.4 Calcium channel blockers (po) | 5 | 787 | Odds Ratio (IV, Random, 95% CI) | 1.04 [0.78, 1.39] |
| 3.5 Magnesium (iv) | 0 | 0 | Odds Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3.6 Naftidrofuryl | 2 | 710 | Odds Ratio (IV, Random, 95% CI) | 0.96 [0.71, 1.32] |
| 3.7 Nitric oxide | 0 | 0 | Odds Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3.8 Piracetam | 1 | 40 | Odds Ratio (IV, Random, 95% CI) | 0.21 [0.02, 2.25] |
| 3.9 Prostacyclin (iv) | 1 | 27 | Odds Ratio (IV, Random, 95% CI) | 1.96 [0.39, 9.93] |
| 4 Death or disability at end of trial | 29 | 3302 | Odds Ratio (IV, Random, 95% CI) | 1.11 [0.96, 1.29] |
| 4.1 ACE inhibitors (po) | 1 | 40 | Odds Ratio (IV, Random, 95% CI) | 1.11 [0.31, 4.03] |
| 4.2 Beta blockers (po) | 4 | 353 | Odds Ratio (IV, Random, 95% CI) | 1.19 [0.76, 1.85] |
| 4.3 Calcium channel blockers (iv) | 5 | 720 | Odds Ratio (IV, Random, 95% CI) | 1.13 [0.67, 1.91] |
| 4.4 Calcium channel blockers (po) | 6 | 1106 | Odds Ratio (IV, Random, 95% CI) | 1.30 [0.91, 1.86] |
| 4.5 Magnesium (iv) | 4 | 162 | Odds Ratio (IV, Random, 95% CI) | 0.73 [0.38, 1.43] |
| 4.6 Naftidrofuryl | 2 | 710 | Odds Ratio (IV, Random, 95% CI) | 0.97 [0.72, 1.32] |
| 4.7 Nitric oxide | 5 | 142 | Odds Ratio (IV, Random, 95% CI) | 1.31 [0.64, 2.65] |
| 4.8 Piracetam | 1 | 40 | Odds Ratio (IV, Random, 95% CI) | 0.37 [0.10, 1.36] |
| 4.9 Prostacyclin (iv) | 1 | 29 | Odds Ratio (IV, Random, 95% CI) | 2.60 [0.39, 17.16] |
| 5 Systolic blood pressure, early | 30 | 3473 | Mean Difference (IV, Random, 95% CI) | ‐6.95 [‐9.40, ‐4.51] |
| 5.1 ACE inhibitors (po) | 4 | 150 | Mean Difference (IV, Random, 95% CI) | ‐5.68 [‐18.32, 6.96] |
| 5.2 ARA (po) | 1 | 339 | Mean Difference (IV, Random, 95% CI) | ‐2.60 [‐6.92, 1.72] |
| 5.3 Beta blockers (po) | 5 | 397 | Mean Difference (IV, Random, 95% CI) | ‐6.14 [‐11.42, ‐0.87] |
| 5.4 Calcium channel blockers (iv) | 5 | 676 | Mean Difference (IV, Random, 95% CI) | ‐5.40 [‐12.86, 2.07] |
| 5.5 Calcium channel blockers (po) | 5 | 253 | Mean Difference (IV, Random, 95% CI) | ‐4.89 [‐11.01, 1.23] |
| 5.6 GKI (iv) | 1 | 933 | Mean Difference (IV, Random, 95% CI) | ‐11.10 [‐14.61, ‐7.59] |
| 5.7 Insulin (iv) | 1 | 25 | Mean Difference (IV, Random, 95% CI) | ‐2.20 [‐11.30, 6.90] |
| 5.8 Magnesium (iv) | 4 | 147 | Mean Difference (IV, Random, 95% CI) | ‐6.32 [‐14.64, 2.01] |
| 5.9 Nitric oxide | 5 | 145 | Mean Difference (IV, Random, 95% CI) | ‐10.32 [‐17.62, ‐3.02] |
| 5.10 Other vasodilators (po) | 1 | 4 | Mean Difference (IV, Random, 95% CI) | ‐7.16 [‐17.11, 2.79] |
| 5.11 Unclassified or combined | 1 | 404 | Mean Difference (IV, Random, 95% CI) | ‐14.0 [‐17.20, ‐10.80] |
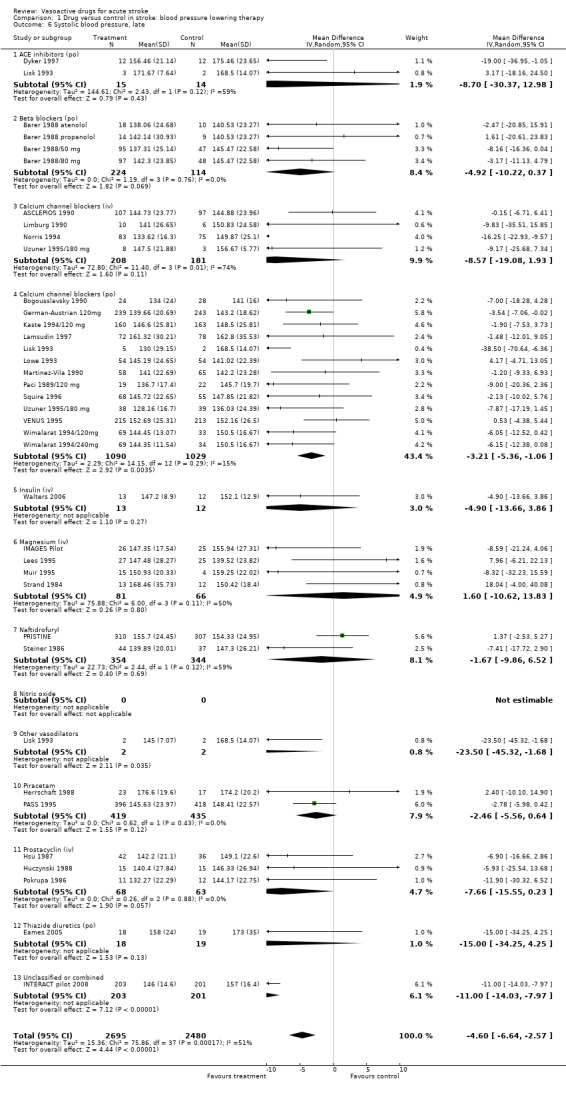
| 6 Systolic blood pressure, late | 35 | 5175 | Mean Difference (IV, Random, 95% CI) | ‐4.60 [‐6.64, ‐2.57] |
| 6.1 ACE inhibitors (po) | 2 | 29 | Mean Difference (IV, Random, 95% CI) | ‐8.70 [‐30.37, 12.98] |
| 6.2 Beta blockers (po) | 4 | 338 | Mean Difference (IV, Random, 95% CI) | ‐4.92 [‐10.22, 0.37] |
| 6.3 Calcium channel blockers (iv) | 4 | 389 | Mean Difference (IV, Random, 95% CI) | ‐8.57 [‐19.08, 1.93] |
| 6.4 Calcium channel blockers (po) | 13 | 2119 | Mean Difference (IV, Random, 95% CI) | ‐3.21 [‐5.36, ‐1.06] |
| 6.5 Insulin (iv) | 1 | 25 | Mean Difference (IV, Random, 95% CI) | ‐4.90 [‐13.66, 3.86] |
| 6.6 Magnesium (iv) | 4 | 147 | Mean Difference (IV, Random, 95% CI) | 1.60 [‐10.62, 13.83] |
| 6.7 Naftidrofuryl | 2 | 698 | Mean Difference (IV, Random, 95% CI) | ‐1.67 [‐9.86, 6.52] |
| 6.8 Nitric oxide | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6.9 Other vasodilators | 1 | 4 | Mean Difference (IV, Random, 95% CI) | ‐23.5 [‐45.32, ‐1.68] |
| 6.10 Piracetam | 2 | 854 | Mean Difference (IV, Random, 95% CI) | ‐2.46 [‐5.56, 0.64] |
| 6.11 Prostacyclin (iv) | 3 | 131 | Mean Difference (IV, Random, 95% CI) | ‐7.66 [‐15.55, 0.23] |
| 6.12 Thiazide diuretics (po) | 1 | 37 | Mean Difference (IV, Random, 95% CI) | ‐15.0 [‐34.25, 4.25] |
| 6.13 Unclassified or combined | 1 | 404 | Mean Difference (IV, Random, 95% CI) | ‐9.00 [‐14.03, ‐7.97] |
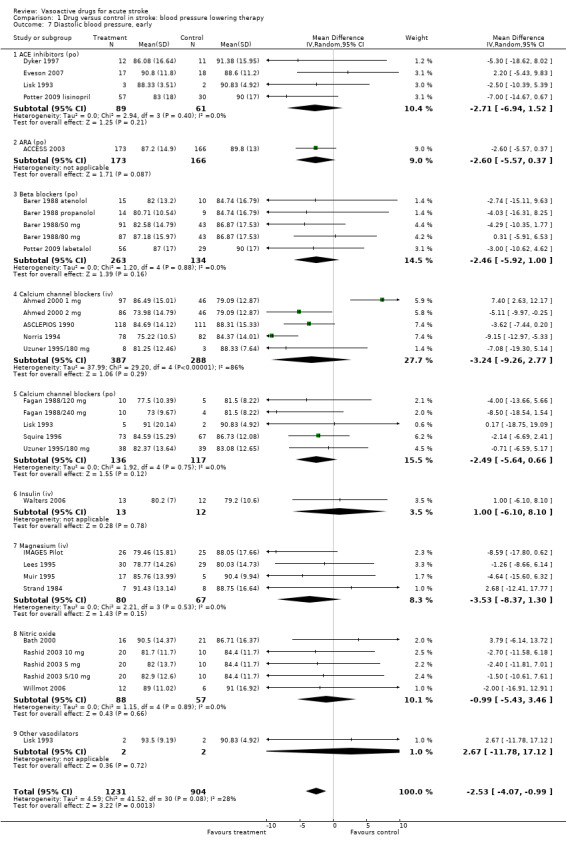
| 7 Diastolic blood pressure, early | 28 | 2135 | Mean Difference (IV, Random, 95% CI) | ‐2.53 [‐4.07, ‐0.99] |
| 7.1 ACE inhibitors (po) | 4 | 150 | Mean Difference (IV, Random, 95% CI) | ‐2.71 [‐6.94, 1.52] |
| 7.2 ARA (po) | 1 | 339 | Mean Difference (IV, Random, 95% CI) | ‐2.60 [‐5.57, 0.37] |
| 7.3 Beta blockers (po) | 5 | 397 | Mean Difference (IV, Random, 95% CI) | ‐2.46 [‐5.92, 1.00] |
| 7.4 Calcium channel blockers (iv) | 5 | 675 | Mean Difference (IV, Random, 95% CI) | ‐3.24 [‐9.26, 2.77] |
| 7.5 Calcium channel blockers (po) | 5 | 253 | Mean Difference (IV, Random, 95% CI) | ‐2.49 [‐5.64, 0.66] |
| 7.6 Insulin (iv) | 1 | 25 | Mean Difference (IV, Random, 95% CI) | 1.0 [‐6.10, 8.10] |
| 7.7 Magnesium (iv) | 4 | 147 | Mean Difference (IV, Random, 95% CI) | ‐3.53 [‐8.37, 1.30] |
| 7.8 Nitric oxide | 5 | 145 | Mean Difference (IV, Random, 95% CI) | ‐0.99 [‐5.43, 3.46] |
| 7.9 Other vasodilators | 1 | 4 | Mean Difference (IV, Random, 95% CI) | 2.67 [‐11.78, 17.12] |
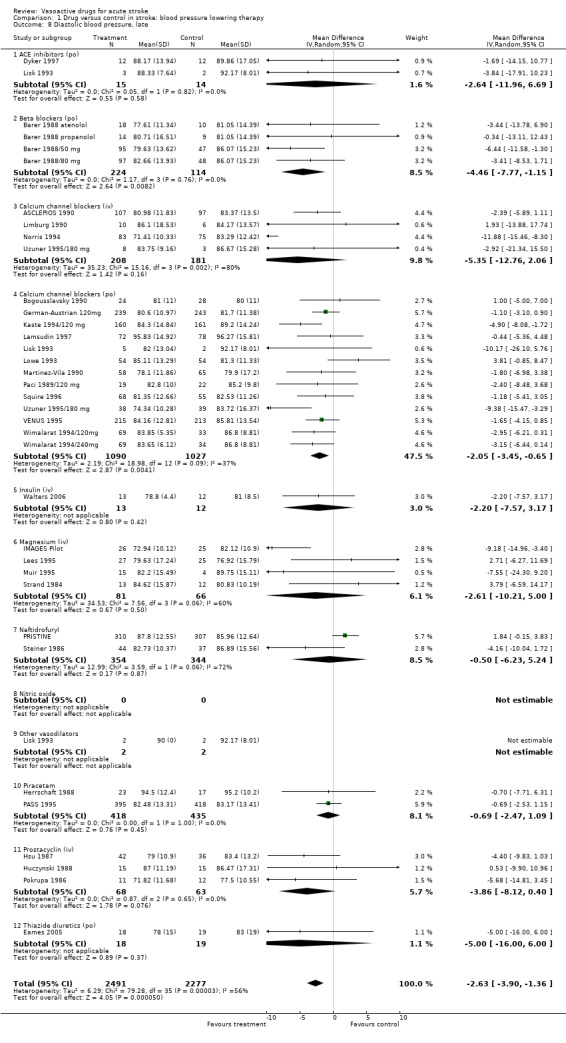
| 8 Diastolic blood pressure, late | 34 | 4768 | Mean Difference (IV, Random, 95% CI) | ‐2.63 [‐3.90, ‐1.36] |
| 8.1 ACE inhibitors (po) | 2 | 29 | Mean Difference (IV, Random, 95% CI) | ‐2.64 [‐11.96, 6.69] |
| 8.2 Beta blockers (po) | 4 | 338 | Mean Difference (IV, Random, 95% CI) | ‐4.46 [‐7.77, ‐1.15] |
| 8.3 Calcium channel blockers (iv) | 4 | 389 | Mean Difference (IV, Random, 95% CI) | ‐5.35 [‐12.76, 2.06] |
| 8.4 Calcium channel blockers (po) | 13 | 2117 | Mean Difference (IV, Random, 95% CI) | ‐2.05 [‐3.45, ‐0.65] |
| 8.5 Insulin (iv) | 1 | 25 | Mean Difference (IV, Random, 95% CI) | ‐2.20 [‐7.57, 3.17] |
| 8.6 Magnesium (iv) | 4 | 147 | Mean Difference (IV, Random, 95% CI) | ‐2.61 [‐10.21, 5.00] |
| 8.7 Naftidrofuryl | 2 | 698 | Mean Difference (IV, Random, 95% CI) | ‐0.50 [‐6.23, 5.24] |
| 8.8 Nitric oxide | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8.9 Other vasodilators | 1 | 4 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8.10 Piracetam | 2 | 853 | Mean Difference (IV, Random, 95% CI) | ‐0.69 [‐2.47, 1.09] |
| 8.11 Prostacyclin (iv) | 3 | 131 | Mean Difference (IV, Random, 95% CI) | ‐3.86 [‐8.12, 0.40] |
| 8.12 Thiazide diuretics (po) | 1 | 37 | Mean Difference (IV, Random, 95% CI) | ‐5.0 [‐16.00, 6.00] |
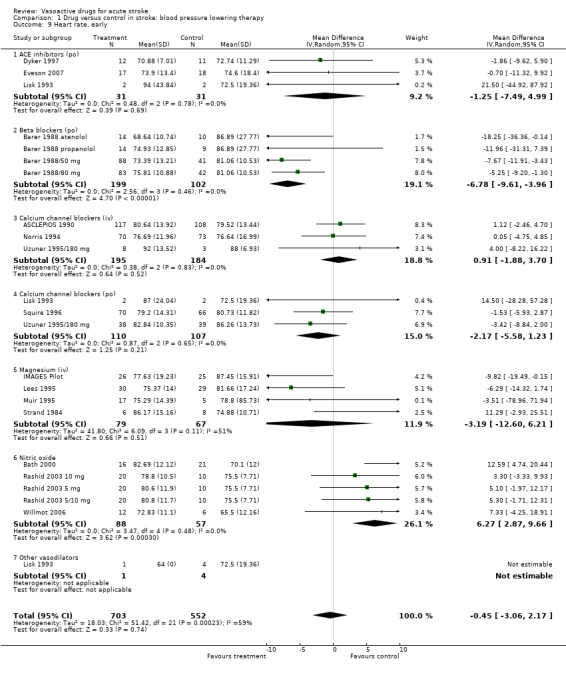
| 9 Heart rate, early | 20 | 1255 | Mean Difference (IV, Random, 95% CI) | ‐0.45 [‐3.06, 2.17] |
| 9.1 ACE inhibitors (po) | 3 | 62 | Mean Difference (IV, Random, 95% CI) | ‐1.25 [‐7.49, 4.99] |
| 9.2 Beta blockers (po) | 4 | 301 | Mean Difference (IV, Random, 95% CI) | ‐6.78 [‐9.61, ‐3.96] |
| 9.3 Calcium channel blockers (iv) | 3 | 379 | Mean Difference (IV, Random, 95% CI) | 0.91 [‐1.88, 3.70] |
| 9.4 Calcium channel blockers (po) | 3 | 217 | Mean Difference (IV, Random, 95% CI) | ‐2.17 [‐5.58, 1.23] |
| 9.5 Magnesium (iv) | 4 | 146 | Mean Difference (IV, Random, 95% CI) | ‐3.19 [‐12.60, 6.21] |
| 9.6 Nitric oxide | 5 | 145 | Mean Difference (IV, Random, 95% CI) | 6.27 [2.87, 9.66] |
| 9.7 Other vasodilators | 1 | 5 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
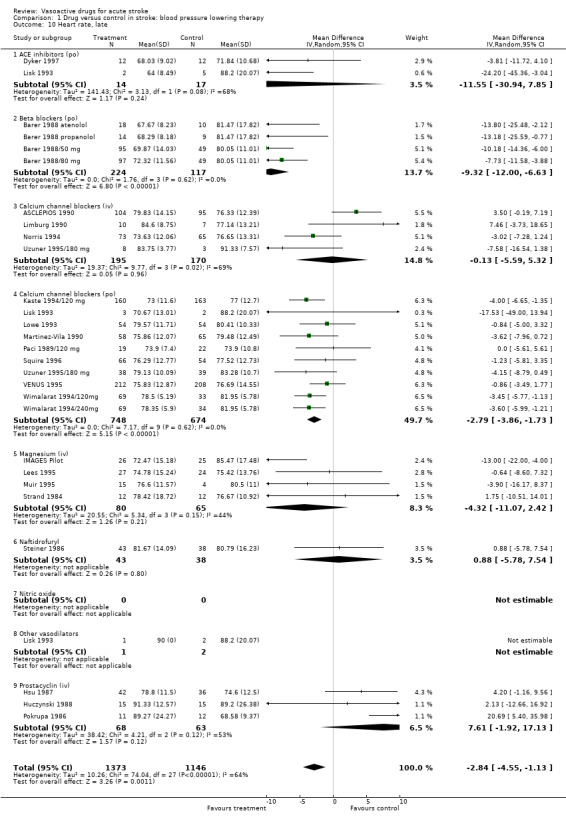
| 10 Heart rate, late | 26 | 2519 | Mean Difference (IV, Random, 95% CI) | ‐2.84 [‐4.55, ‐1.13] |
| 10.1 ACE inhibitors (po) | 2 | 31 | Mean Difference (IV, Random, 95% CI) | ‐11.55 [‐30.94, 7.85] |
| 10.2 Beta blockers (po) | 4 | 341 | Mean Difference (IV, Random, 95% CI) | ‐9.32 [‐12.00, ‐6.63] |
| 10.3 Calcium channel blockers (iv) | 4 | 365 | Mean Difference (IV, Random, 95% CI) | ‐0.13 [‐5.59, 5.32] |
| 10.4 Calcium channel blockers (po) | 10 | 1422 | Mean Difference (IV, Random, 95% CI) | ‐2.79 [‐3.86, ‐1.73] |
| 10.5 Magnesium (iv) | 4 | 145 | Mean Difference (IV, Random, 95% CI) | ‐4.32 [‐11.07, 2.42] |
| 10.6 Naftidrofuryl | 1 | 81 | Mean Difference (IV, Random, 95% CI) | 0.88 [‐5.78, 7.54] |
| 10.7 Nitric oxide | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10.8 Other vasodilators | 1 | 3 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10.9 Prostacyclin (iv) | 3 | 131 | Mean Difference (IV, Random, 95% CI) | 7.61 [‐1.92, 17.13] |
1.1. Analysis.
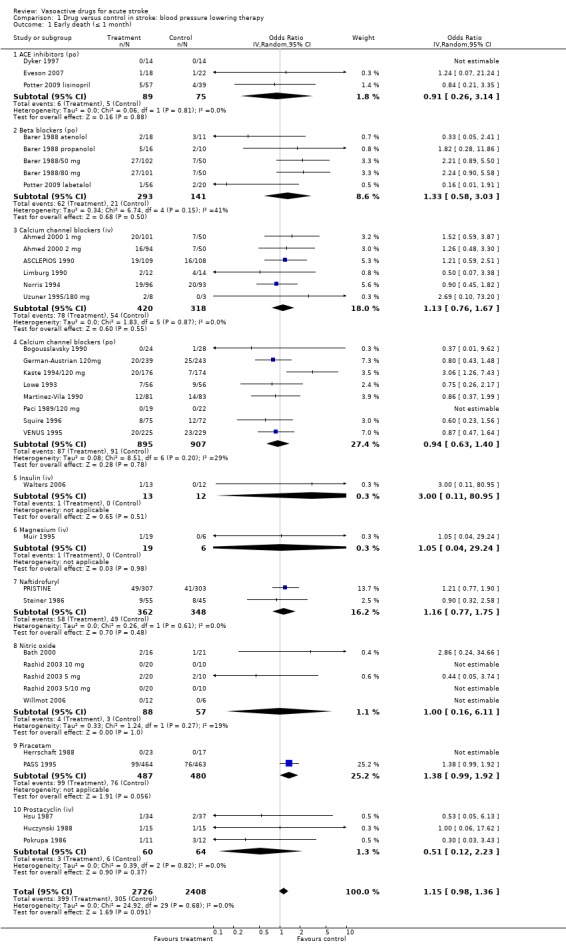
Comparison 1 Drug versus control in stroke: blood pressure lowering therapy, Outcome 1 Early death (≤ 1 month).
1.2. Analysis.
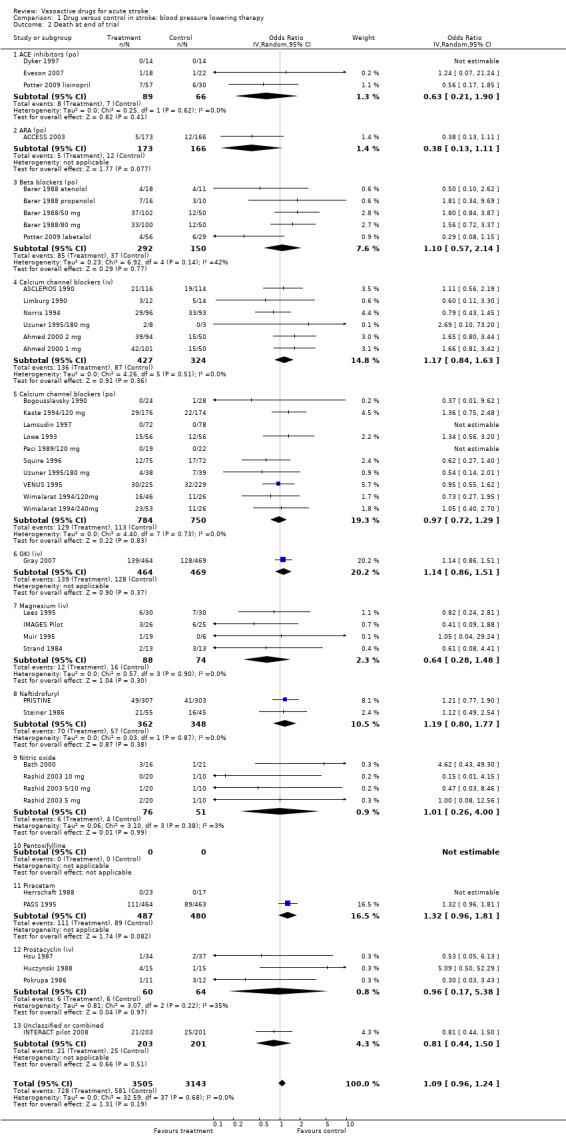
Comparison 1 Drug versus control in stroke: blood pressure lowering therapy, Outcome 2 Death at end of trial.
1.3. Analysis.
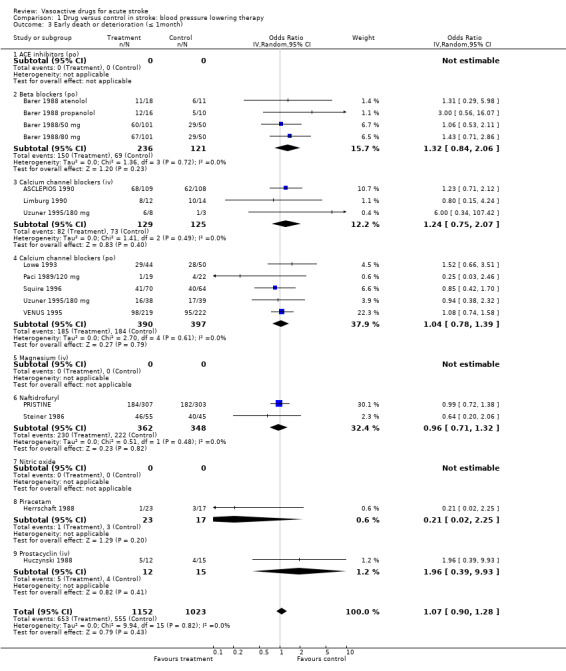
Comparison 1 Drug versus control in stroke: blood pressure lowering therapy, Outcome 3 Early death or deterioration (≤ 1month).
1.4. Analysis.
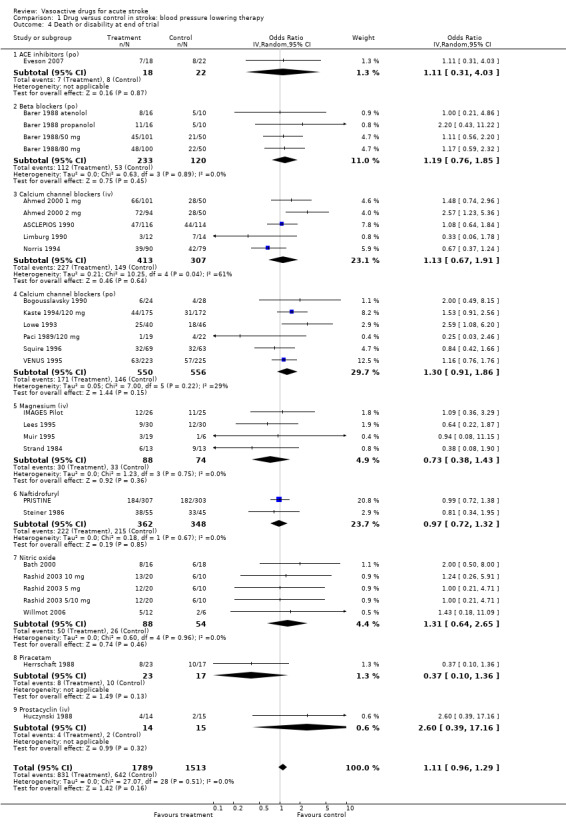
Comparison 1 Drug versus control in stroke: blood pressure lowering therapy, Outcome 4 Death or disability at end of trial.
1.5. Analysis.

Comparison 1 Drug versus control in stroke: blood pressure lowering therapy, Outcome 5 Systolic blood pressure, early.
1.6. Analysis.
Comparison 1 Drug versus control in stroke: blood pressure lowering therapy, Outcome 6 Systolic blood pressure, late.
1.7. Analysis.
Comparison 1 Drug versus control in stroke: blood pressure lowering therapy, Outcome 7 Diastolic blood pressure, early.
1.8. Analysis.
Comparison 1 Drug versus control in stroke: blood pressure lowering therapy, Outcome 8 Diastolic blood pressure, late.
1.9. Analysis.
Comparison 1 Drug versus control in stroke: blood pressure lowering therapy, Outcome 9 Heart rate, early.
1.10. Analysis.
Comparison 1 Drug versus control in stroke: blood pressure lowering therapy, Outcome 10 Heart rate, late.
Comparison 2. Drug versus control in stroke: blood pressure elevation therapy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Early death (≤ 1 month) | 1 | 15 | Odds Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.1 DCLHb (iv) | 0 | 0 | Odds Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.2 Phenylephrine (iv) | 1 | 15 | Odds Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
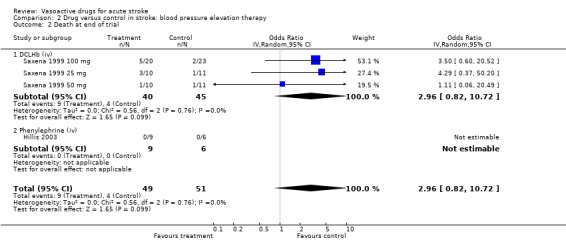
| 2 Death at end of trial | 4 | 100 | Odds Ratio (IV, Random, 95% CI) | 2.96 [0.82, 10.72] |
| 2.1 DCLHb (iv) | 3 | 85 | Odds Ratio (IV, Random, 95% CI) | 2.96 [0.82, 10.72] |
| 2.2 Phenylephrine (iv) | 1 | 15 | Odds Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Death or disability at end of trial | 3 | 85 | Odds Ratio (IV, Random, 95% CI) | 5.41 [1.87, 15.64] |
| 3.1 DCLHb (iv) | 3 | 85 | Odds Ratio (IV, Random, 95% CI) | 5.41 [1.87, 15.64] |
| 3.2 Phenylephrine (iv) | 0 | 0 | Odds Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Systolic blood pressure, early | 4 | 100 | Mean Difference (IV, Random, 95% CI) | 15.82 [5.10, 26.54] |
| 4.1 DCLHb (iv) | 3 | 85 | Mean Difference (IV, Random, 95% CI) | 15.29 [3.99, 26.58] |
| 4.2 Phenylephrine (iv) | 1 | 15 | Mean Difference (IV, Random, 95% CI) | 20.60 [‐13.31, 54.51] |
| 5 Systolic blood pressure, late | 3 | 85 | Mean Difference (IV, Random, 95% CI) | 15.90 [1.84, 29.96] |
| 5.1 DCLHb (iv) | 3 | 85 | Mean Difference (IV, Random, 95% CI) | 15.90 [1.84, 29.96] |
| 5.2 Phenylephrine (iv) | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6 Diastolic blood pressure, early | 4 | 100 | Mean Difference (IV, Random, 95% CI) | 5.11 [‐3.18, 13.39] |
| 6.1 DCLHb (iv) | 3 | 85 | Mean Difference (IV, Random, 95% CI) | 6.01 [‐4.35, 16.38] |
| 6.2 Phenylephrine (iv) | 1 | 15 | Mean Difference (IV, Random, 95% CI) | 0.5 [‐14.86, 15.86] |
| 7 Diastolic blood pressure, late | 3 | 85 | Mean Difference (IV, Random, 95% CI) | 1.94 [‐8.96, 12.83] |
| 7.1 DCLHb (iv) | 3 | 85 | Mean Difference (IV, Random, 95% CI) | 1.94 [‐8.96, 12.83] |
| 7.2 Phenylephrine (iv) | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8 Heart rate, early | 3 | 85 | Mean Difference (IV, Random, 95% CI) | 0.43 [‐6.36, 7.22] |
| 8.1 DCLHb (iv) | 3 | 85 | Mean Difference (IV, Random, 95% CI) | 0.43 [‐6.36, 7.22] |
| 8.2 Phenylephrine (iv) | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
2.1. Analysis.
Comparison 2 Drug versus control in stroke: blood pressure elevation therapy, Outcome 1 Early death (≤ 1 month).
2.2. Analysis.
Comparison 2 Drug versus control in stroke: blood pressure elevation therapy, Outcome 2 Death at end of trial.
2.3. Analysis.
Comparison 2 Drug versus control in stroke: blood pressure elevation therapy, Outcome 3 Death or disability at end of trial.
2.4. Analysis.
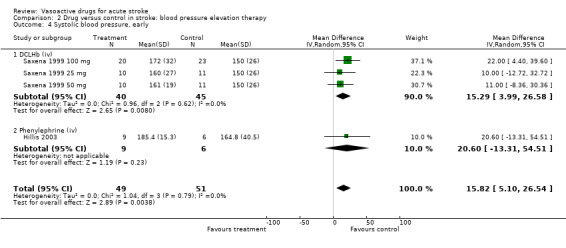
Comparison 2 Drug versus control in stroke: blood pressure elevation therapy, Outcome 4 Systolic blood pressure, early.
2.5. Analysis.
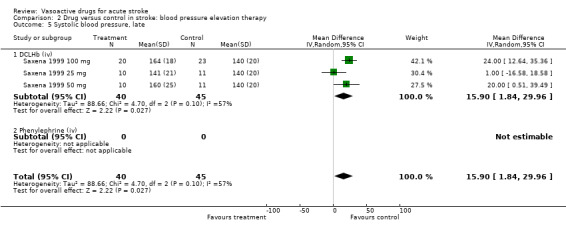
Comparison 2 Drug versus control in stroke: blood pressure elevation therapy, Outcome 5 Systolic blood pressure, late.
2.6. Analysis.
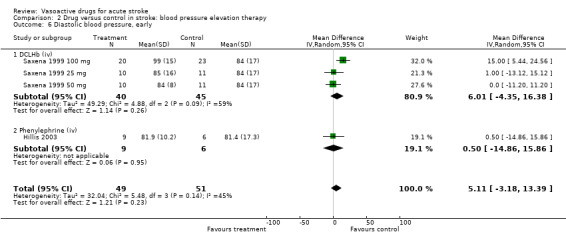
Comparison 2 Drug versus control in stroke: blood pressure elevation therapy, Outcome 6 Diastolic blood pressure, early.
2.7. Analysis.
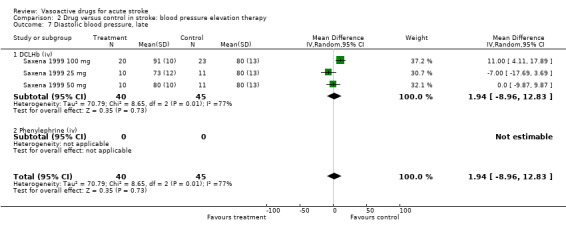
Comparison 2 Drug versus control in stroke: blood pressure elevation therapy, Outcome 7 Diastolic blood pressure, late.
2.8. Analysis.
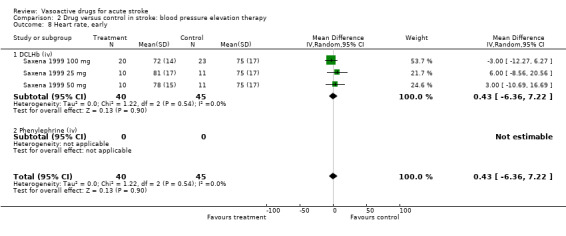
Comparison 2 Drug versus control in stroke: blood pressure elevation therapy, Outcome 8 Heart rate, early.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
ACCESS 2003.
| Methods | Multicentre, double‐blind, placebo‐controlled Method of randomisation not known | |
| Participants | Germany 339 patients ‐ T: 173, C:166 Age: T: 68.3 years; C: 67.8 years Male: T: 50%; C: 52% Inclusion: IS 100% CT Enrolment within 24 to 36 hours after admission | |
| Interventions | T: candesartan 4 mg po on day 1 and dose was increased to 8 or 16 mg if BP exceeded 160 mmHg systolic or 100 mmHg diastolic C: matching placebo Rx: 7 days | |
| Outcomes | BP was measured by a nurse or automatically Case fatality and disability using BI 3 months after the end of placebo‐controlled 7‐day period | |
| Notes | Exclusion: age > 85 years, > 70% stenosis of internal carotid artery, disorders in consciousness, cardiac failure, unstable angina, malignant hypertension, and high grade aortic or mitral stenosis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Probably done |
| Allocation concealment? | Unclear risk | Unclear from the publication |
| Blinding? | Low risk | Probably done |
| Completeness of follow‐up | Unclear risk | Unclear from the publication |
Ahmed 2000 1 mg.
| Methods | As for Ahmed 2000 2 mg | |
| Participants | — | |
| Interventions | — | |
| Outcomes | — | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Probably done |
| Allocation concealment? | Low risk | According to predetermined randomisation lists |
| Blinding? | Low risk | Probably done |
| Completeness of follow‐up | High risk | Probably not done 101 patients did not complete 21 days of treatment This includes 2 trial withdrawals |
Ahmed 2000 2 mg.
| Methods | Multicentre, double‐blind, placebo‐controlled Randomisation by predetermined randomisation list | |
| Participants | Sweden 295 patients: T1: 101, T2: 94, C: 100 Age: T1: 71.9 years , T2: 72.1 years, C: 71 years Male: T1: 45, T2: 45, C: 45 Inclusion: clinical diagnosis of ischaemic stroke in the carotid artery territory Enrolment: within 24 hours of ictus | |
| Interventions | T1: nimodipine iv 1 mg/hour for 5 days followed by oral nimodipine 30 mg qid for 16 days T2: nimodipine iv 2 mg/hour for 5 days followed by po nimodipine 30 mg qid for 16 days C: matching placebo | |
| Outcomes | Transformed Orgogozo score and transformed Barthel index score on the follow up at day 21 | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Probably done |
| Allocation concealment? | Low risk | According to predetermined randomisation lists |
| Blinding? | Low risk | Probably done |
| Completeness of follow‐up | High risk | Probably not done 101 patients did not complete 21 days of treatment This includes 2 trial withdrawals |
ASCLEPIOS 1990.
| Methods | Multicentre (40), double‐blind, placebo‐controlled Method of randomisation unknown ITT analysis | |
| Participants | European and Canadian 234 patients ‐ T:120, C:114 Age: 45 to 85 years Males: T: 76, C: 69 Patients with ischaemic MCA stroke presenting with hemiparesis or hemiplegia within 12 hours of onset 100% CT and/or MRI within 72 hours 1 patient > 12 hours (15 hours) and one patient < 45 years (44 years) | |
| Interventions | T: isradipine as continuous iv infusion (80 ug/hour) for 72 hours then po (2.5 mg bd) C: matching iv/po placebo Rx: for 28 days | |
| Outcomes | Assessments at baseline and days 1, 3, 7, 14, 28, 90 Neurological score (modified by Orgogozo et al (1993)); Barthel Index (extended to include death as worst possible outcome) Missing data: day 28: T: 11, C: 6; day 90: T: 4, C: 0 Blood pressure measured at baseline and days 1, 2, 3 (method of measurement unknown) | |
| Notes | Ex: Massive hemispheric damage; very mild stroke (neurological score > 65); any condition where previous neurological deficits might hinder ability to detect improvement from current stroke; other systemic diseases such as gastrointestinal system, liver, kidneys; acute or unstable cardiovascular disease, except AF; exposure to drugs that may interfere with safety or efficacy; pregnancy, lactation Data provided by J‐M Orgogozo (principal investigator) TIAs will be excluded and analysed separately | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Probably done |
| Allocation concealment? | Unclear risk | Unclear from the publication |
| Blinding? | Low risk | Probably done |
| Completeness of follow‐up | Unclear risk | Unclear from the publication |
Barer 1988 atenolol.
| Methods | Multicentre, open randomised controlled Separate randomisation schemes for each hospital ITT analysis | |
| Participants | UK 55 patients: T1: 18, T2:16, C:21 Mean age: T1: 73 years, T2: 72 years, C: 70 years Males: T1:12, T2:8, C:8 Inclusion: clinically diagnosed hemispheric strokes Patients should be conscious and able to swallow tablets Enrolment within 48 hours | |
| Interventions | T1: atenolol po 50 mg daily T2: propranolol 80 mg po daily Rx: 4 weeks | |
| Outcomes | Same time points used as Barer 1988 | |
| Notes | Same exclusions as Barer 1988 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Randomisation was done in block of 3 with separate schemes for each hospital |
| Allocation concealment? | Low risk | Probably done |
| Blinding? | High risk | Open randomised controlled trial |
| Completeness of follow‐up | Unclear risk | Unclear from the publication |
Barer 1988 propanolol.
| Methods | As for Barer 1988 atenolol | |
| Participants | — | |
| Interventions | ||
| Outcomes | — | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Probably done |
| Allocation concealment? | Low risk | Probably done |
| Blinding? | High risk | Open randomised controlled |
| Completeness of follow‐up | Unclear risk | 38 patients lost to follow up |
Barer 1988/50 mg.
| Methods | Single centre, double‐blind, placebo‐controlled Method of randomisation not known 38 patients lost to FU PP analysis | |
| Participants | UK 303 patients: T1:102, T2:101, C:100 Mean age: T1: 70.6 years, T2: 68.2 years, C: 69 years Males: T1: 53, T2: 57, C:49 Inclusion: clinically diagnosed hemispheric strokes Patients should be conscious and able to swallow tables CT not used Enrolment within 48 hours | |
| Interventions | T1: atenolol 50 mg po daily T2: slow release propranolol 80 mg po daily C: matching placebo Rx: 3 weeks | |
| Outcomes | Neurological assessments made at days 1 and 8 and months 1 and 6; full functional assessments made from day 8 onwards; death, functional outcome used ADL on an ordinal scale designed for patients with stroke; length of stay Method by which BP measured not given Early and late death and dependency data defined as ADL score of less than or equal to 4 No method given for BP measurements | |
| Notes | Ex: pre‐existing major physical or mental disability, taking beta blockers, contraindications to beta blockers i.e. heart rate ≤ 56 beats/minute, SBP < 100 mmHg, second or third degree heart block, heart failure or bronchospasm causing dyspnoea, history of asthma, insulin dependent diabetes, MI, other causes of seriously reduced cerebral perfusion | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Randomisation was done in block of 3 with separate schemes for each hospital |
| Allocation concealment? | Low risk | Probably done |
| Blinding? | High risk | Open randomised controlled trial |
| Completeness of follow‐up | High risk | Unclear from the publication |
Barer 1988/80 mg.
| Methods | As for Barer 1988/50 mg | |
| Participants | — | |
| Interventions | — | |
| Outcomes | — | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Probably done |
| Allocation concealment? | Low risk | Probably done |
| Blinding? | High risk | Open randomised controlled |
| Completeness of follow‐up | High risk | 38 patients lost to follow up |
Bath 2000.
| Methods | Single centre double‐blind, placebo‐controlled Randomisation by computer (with minimisation on age and mean arterial BP) ITT analysis | |
| Participants | UK 37 patients. T: 16, C: 21 Age: T: 76 years, C: 72 years Male T: 6, C: 12 Inclusion: ischaemic or haemorrhage stroke 100% CT Enrolment within 5 days: T: 4 patients enrolled > 5 days and C: 3 patients > 5 days Stroke type assessed clinically | |
| Interventions | T: transdermal GTN 5 mg C: matching placebo Rx: 12 days | |
| Outcomes | 24 hour ambulatory BP was measured before and during GTN treatment at days 0, 1 and 8 Ambulatory BP was monitored using a Spacelabs 90207 set to record thrice hourly during the day and hourly during the night Functional outcome Rankin scale and Barthel Index and case fatality at 3 months Late death and disability used Barthel, but if used Rankin there is 1 less missing value | |
| Notes | Ex: taking part in another trial | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Randomisation by computer (with minimisation on age and mean arterial BP) |
| Allocation concealment? | Low risk | Probably done |
| Blinding? | Low risk | Probably done |
| Completeness of follow‐up | Low risk | No loss of follow up |
Bogousslavsky 1990.
| Methods | Single centre, double‐blind, placebo‐controlled Randomisation by next random number on list 60 patients randomised but 8 excluded due to incorrect diagnosis Data from paper, PP analysis | |
| Participants | German 52 patients: T: 24, C: 28 Mean age T: 64, C: 65 (efficacy) Males 38 Inclusion: ischaemic stroke of mild to moderate severity (Mathew scale sum between 50 and 75), > 39 years and < 85 years Diagnosis: clinical and 100% CT scan Enrolment within 48 hours | |
| Interventions | T: nimodipine 30 mg po qid C: matching po placebo Rx: for 14 days Medical therapy allowed such as drugs against infection, hypertension, mild hypnotics, analgesics, volume substitution (including Dextran 40), low‐dose heparin (2 x 500 U/day) | |
| Outcomes | Impairment: Mathews score on day 1, 3, 5, 7, and 14, week 4 and month 4 BP and heart rate were checked twice daily and on week 4 and month 4 Number of hypotensives noted Method used for taking BP not given | |
| Notes | Ex: TIA, progressing stroke, coma, brain stem, ICH, SAH, recent MI, CCF, systemic infection, renal/hepatic failure, SBP < 100, DBP > 105, bradycardia (heart rate < 50 beats/minute), AV conduction disturbances, concomitant use of CCBs, piracetam, pentoxifylline, naftidrofuryl hydrogenoxalate, dihydroergotoxine, alpha methyl dopa Follow up 4 weeks and 4 months | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Randomisation by next random number on the list |
| Allocation concealment? | Low risk | Probably done |
| Blinding? | Low risk | Probably done |
| Completeness of follow‐up | Unclear risk | Unclear from the publication |
Dyker 1997.
| Methods | Double‐blind, placebo‐controlled Method of randomisation: computer‐generated random list prepared and held by Pharmacy Trials Department ITT analysis | |
| Participants | UK, single centre 28 patients: T: 14, C: 14 Mean age: 70 years Males: T: 9, C: 8 Inclusion: strokes with mild to moderate hypertension (170 to 250/95 to 120 mm Hg) 100% CT on entry Enrolment within 1 week Patients admitted on prescribed antihypertensive therapy had treatment discontinued for at least 48 hours before entry into the study | |
| Interventions | T: 4 mg perindopril po once daily C: matching placebo Rx: 2 weeks | |
| Outcomes | BP measured semi‐automatically pre‐treatment and hourly to 10 hours repeated at 24 hours and at 2 weeks Clinical and neurological assessment according to the NIH Stroke Scale made before study entry and repeated on day 15 | |
| Notes | Ex: severe carotid disease | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Computer‐generated random list prepared and held by pharmacy trials department |
| Allocation concealment? | Low risk | Probably done |
| Blinding? | Low risk | Probably done |
| Completeness of follow‐up | High risk | 28 recruited to the study with 24 completing the protocol |
Eames 2005.
| Methods | Double‐blind, placebo‐controlled, parallel group Block randomisation (4 per block) | |
| Participants | UK, single centre 37 patients: T: 18, C: 19 Age: 68 years Male: 86% Inclusion: neuroradiologically diagnosed ischaemic stroke with 24 hour BP > 135/85 mmHg Enrolment within 96 hours of stroke onset | |
| Interventions | T: bendrofluazide 2.5 mg po daily C: matching placebo Rx: 7 days | |
| Outcomes | Casual and non‐invasive beat‐to‐beat arterial BP level, cerebral blood flow velocity, ECG and transcutaneous carbon dioxide levels within 70+/‐20 hours of cerebral infarction and 7 days later were measured 24‐hour BP monitoring with Spacelabs 90207 and brachial artery BP with validated semi‐automatic BP monitor (Omron 711) | |
| Notes | Exclusion: history of previous stroke, dysphagia, symptoms lasting < 24 hours, or presented > 76 hours after symptom onset (to allow for 24 hour BP monitoring to be performed prior to randomisation) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Probably done |
| Allocation concealment? | Low risk | Probably done |
| Blinding? | Low risk | Probably done |
| Completeness of follow‐up | Low risk | 38 participants randomised, 19 to each group |
Eveson 2007.
| Methods | Double‐blind, placebo‐controlled, parallel group Randomisation by prepared and numbered identical study packs | |
| Participants | UK, single centre 40 patients. T: 18, C: 22 Age: T: 73 years, C: 75 years Male: 63% Inclusion: acute ischaemic stroke within the previous 24 hours with a mean casual SBP level ≥ 140 mm Hg or DBP level ≥ 90 mm Hg Randomisation done before neuroimaging and those with non‐ischaemic stroke were withdrawn from the study | |
| Interventions | T: 5 mg lisinopril po once daily C: matching placebo Rx: 14 days Dose was increased to 10 mg or 2 placebos on day 7 if SBP ≥ 140 mmHg or DBP ≥ 90 mmHg | |
| Outcomes | Casual brachial artery BP monitoring at 5‐minute intervals during a 30‐minute period with a validated monitor (A&D UA 767) NIHSS score at day 14, Barthel score and modified Rankin scale at day 14 and day 90 | |
| Notes | Ex: severe carotid stenosis, significant aortic stenosis, cardiac failure, MI within past 6 months, dysphagia, dehydration, adverse reactions to ACEI, and pre‐stroke modified Rankin score > 2 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Probably done |
| Allocation concealment? | Low risk | Probably done |
| Blinding? | Low risk | Probably done |
| Completeness of follow‐up | High risk | During 90‐day follow up 1 patient from lisinopril died, 2 placebo‐treated patients underwent rating before day 90 (1 moved to another hospital and 1 declined further study participation after the treatment period) |
Fagan 1988/120 mg.
| Methods | Multicentre, double‐blind, placebo‐controlled Randomisation technique not stated ITT analysis | |
| Participants | USA, 19 participants Age: > 45 years No genders given Inclusion: IS diagnosed on history and neurological examination Enrolment times not given | |
| Interventions | T: nimodipine (Miles Pharmaceuticals, USA) 120 mg/day po in 6 divided doses C: matching placebo Rx: for 21 days | |
| Outcomes | Brachial BP before and 30 and 60 minutes after each morning dose for 7 days BP methodology not stated DBP estimated from SBP and MAP given in paper | |
| Notes | Ex: concurrent calcium channel antagonists, antihypertensive agents (other than beta blockers) Admission times of concurrent medication always separated from study drug administration by at least 2 hours Part of a larger unpublished trial to evaluate the safety and efficacy of nimodipine | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Probably done |
| Allocation concealment? | Unclear risk | Unclear from the publication |
| Blinding? | Low risk | Probably done |
| Completeness of follow‐up | Unclear risk | Unclear from the publication |
Fagan 1988/240 mg.
| Methods | Multicentre, double‐blind, placebo‐controlled Randomisation technique not stated ITT analysis | |
| Participants | USA, 19 participants Age: > 45 years No genders given IS diagnosed on history and neurological examination Enrolment times not given | |
| Interventions | T: nimodipine (Miles Pharmaceuticals, USA) 240 mg/day po in 6 divided doses C: matching placebo Rx: for 21 days | |
| Outcomes | Brachial BP before and 30 and 60 minutes after each morning dose for 7 days BP methodology not stated DBP estimated from SBP and MAP given in paper | |
| Notes | Ex: concurrent calcium channel antagonists, antihypertensive agents (other than beta blockers) Admission times of concurrent medication always separated from study drug administration by at least 2 hours Part of a larger unpublished trial to evaluate the safety and efficacy of nimodipine | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Probably done |
| Allocation concealment? | Unclear risk | Unclear from the publication |
| Blinding? | Low risk | Probably done |
| Completeness of follow‐up | Unclear risk | Unclear from the publication |
German‐Austrian 120mg.
| Methods | Multicentre, double‐blind, placebo‐controlled Method of randomisation not known ITT analysis | |
| Participants | Germany and Austria, 16 centres 482 patients: T: 239, C: 243 Age: 40 to 80 years Inclusion: infarcts in anterior circulation 100% CT Enrolment within 48 hours | |
| Interventions | T: po nimodipine 30 mg qid C: matching placebo Optional concomitant drugs were haemodilution, low‐dose heparin, acetylsalicylic acid, digitalis, diuretics, antihypertensives, and sedatives Rx: 21 days | |
| Outcomes | Modified Mathew scale at baseline and days 1, 3, 5, 7, 14, 21 and 6 months Barthel Index at days 1 and 21. Method for measuring BP not given BP estimated from graphs in paper | |
| Notes | Ex: TIA, progressive stroke, vertebrobasilar ischaemia, coma, intracerebral bleeding or tumour, SAH, pregnancy, cardiac surgery within last 3 months, severe systemic illness, acute severe hepatic disease, bradycardia < 50 beats/minute, hypotension SBP < 100 mmHg, severe AV conduction block, renal insufficiency, severe systemic infections, severe cardiac insufficiency within last 3 months, other CCBs, PTX, naftidrofuryl, fetal bovine serum, piracetam, dihydroergotoxine, steroids and osmotic drugs Data taken from the paper | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Probably done |
| Allocation concealment? | Unclear risk | Unclear from the publication |
| Blinding? | Low risk | Probably done |
| Completeness of follow‐up | Unclear risk | Unclear from the publication |
Gray 2007.
| Methods | Multicentre, randomised controlled trial Blinded outcome assessments Randomisation: first 571 patients sealed envelopes, the rest by central randomisation service ITT analysis | |
| Participants | UK, 933 patients T: 464, C: 469 Mean age: 75 years Male: 45% Inclusion: acute ischaemic stroke or primary intracerebral haemorrhage with admission venous plasma glucose 6 to 17 mmol/L Enrolment within 24 hours of stroke onset | |
| Interventions | T: 500 ml GKI (of 10% dextrose, 20 mmol potassium chloride and 16U soluble recombinant human insulin) continuous iv infusion C: 0.9% normal saline Rx: 24 hours | |
| Outcomes | Death at 90 days, European stroke scale score, OCSP subtype, Glasgow Coma Scale at baseline Barthel index, mRS at 30 and 90 days | |
| Notes | Ex: SAH, isolated posterior circulation syndromes no physical disability, pure language disorders, renal failure, anaemia, coma, established history of insulin treated diabetes, previous disabling stroke, dementia or symptomatic cardiac failure | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Probably done |
| Allocation concealment? | Low risk | Treatment allocation was concealed |
| Blinding? | High risk | Probably not done |
| Completeness of follow‐up | High risk | Probably not done No loss of follow up for death at 90 days Day 90 mRS missing for 5 patients Day 90 Bartel Index missing for 30 patients |
Herrschaft 1988.
| Methods | Single centre, double‐blind, placebo‐controlled Randomisation technique not stated PP analysis FU: 4 lost | |
| Participants | German 44 participants: T: 24, C: 20 Mean age: T: 59 years, C: 54 years Males: T: 17, C:10 Inclusion: IS diagnosed on neurological examination and 100% CT, first stroke Enrolment within 5 days Proof of vascular stenoses or occlusions of the supplying or intracranial brain vessels by means of doppler sonography or cerebral angiography | |
| Interventions | T and C: continuous iv of 1000 ml Dextran 40 plus 2 x 150 ml Sorbit 40% daily during the first 3 days T and C: over 4 to 6 hours a daily infusion of 500 ml Dextran 40 from day 4 to day 14 T: 3 x 4 g/20 ml piracetam iv bolus day 1 to day 14; FU 28 days C: matching placebo T: 4.8 g piracetam po daily for following 14 days C: matching placebo po daily for following 14 days | |
| Outcomes | Neurological and psychiatric assessments using own scales at baseline and days 7, 14, 28 Organic brain psychosyndrome was determined using Lehrl and Erizgkeit short syndrome test Method of measuring BP not known | |
| Notes | Ex: patients with severe internal disease (heart and lung disease), liver or renal insufficiency, DM, fixed hypertonia, neoplasia, hematological and systemic diseases, patients who had earlier neurological diseases of a different nature, drug or alcohol abuse 4 patients were lost to follow up for following reasons: cardiac insufficiency, cardiac infarctus, pneumonia, gastrointestinal bleeding (T:1, C:3) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Probably done |
| Allocation concealment? | Unclear risk | Unclear from the publication |
| Blinding? | Low risk | Probably done |
| Completeness of follow‐up | High risk | 4 lost to follow up |
Hillis 2003.
| Methods | Pilot randomised controlled trial Method of randomisation not known FU: no losses | |
| Participants | USA, single centre 15 patients: T: 9, C: 6 Age: T: 59.1 years, C: 67.8 years Male: T: 2, C: 2 Inclusion: IS > 20% diffusion‐perfusion mismatch, quantifiable, stable or worsening aphasia, hemispatial neglect and/or hemiparesis Enrolment: up to 7 days from the onset of stroke symptoms Patients on any previous antihypertensive medication were discontinued prior to the initiation of the study 100% CT, MRI | |
| Interventions | T: iv phenylephrine was titrated to reach 10% to 20% increase MAP and continued for maximum of 72 hours After 24 hours the patients were started on midodrine (up to 10 mg ), fludrocortisone (up to 0.2 mg) and sodium chloride tablets while simultaneously weaning the iv phenylephrine By 4 weeks, midodrine, fludrocortisone, and sodium chloride were tapered as long as there was no concomitant clinical deterioration C: conventional management | |
| Outcomes | MAP measured BP measurement method not given NIHSS and cognitive tests on day 1, day 3 and 6 to 8 weeks | |
| Notes | Exclusion: CI or inability to tolerate MRI, cardiac ejection fraction < 25%, recent congestive heart failure, myocardial ischaemia, unstable angina, bradycardia, allergy to gadolinium, haemorrhage seen on initial CT, agitation requiring ongoing sedation, or MAP > 140 with no intervention | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Probably done |
| Allocation concealment? | Unclear risk | Unclear from the publication |
| Blinding? | Low risk | Probably done |
| Completeness of follow‐up | Low risk | 15 patients (T: 9, C: 6) no loss of follow up |
Hsu 1987.
| Methods | Multicentre, double‐blind, placebo‐controlled Randomisation technique not stated, stratified by thrombotic and embolic stroke ITT analysis | |
| Participants | USA, 5 centres T: 43, C: 37 Mean age: 65 years 49 male, 31 female Inclusion: IS 100% CT pre‐entry Enrolment within 24 hours | |
| Interventions | T: PGI2 (epoprostenol sodium, Upjohn Co, USA, and Wellcome, UK) iv infusion started at 1 ng/kg/min increased every 30 minutes until maximum rate of 10 ng/kg/min; infusion for 72 hours with gradual reduction of dose during last 12 hours C: solvent Rx: 3 days | |
| Outcomes | Death at 4 weeks (Neurological impairment assessed using Turnhill score at entry, day 3, weeks 1, 2 + 4) Method of BP measurement not known | |
| Notes | Ex: stupor, coma, psychiatric disorder, clinical intracranial hypertension, organ or systemic disease, bleeding risk, heparin Further information unavailable because original data discarded Data from unpublished manuscript | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Probably done |
| Allocation concealment? | Unclear risk | Unclear from the publication |
| Blinding? | Low risk | Probably done |
| Completeness of follow‐up | Unclear risk | Unclear from the publication |
Huczynski 1988.
| Methods | Single centre, double‐blind, placebo‐controlled Randomisation technique not stated PP analysis 5 lost to FU Withdrawals: T: 4, C: 1 | |
| Participants | Poland 30 patients: T: 15, C: 15 Mean age 61 years 16 male and 14 female Inclusion: IS in the territory of the internal carotid artery 100% EEG and CSF pre‐entry Enrolment 24 to 72 hours | |
| Interventions | T: PGI2 (Wellcome, UK, or Chinoin, WRL), daily 6 hour iv infusions at 2.5 to 5 ng/kg/min C: glycine solvent Rx: 2 weeks All patients given low‐molecular‐weight dextran | |
| Outcomes | Death at 4 weeks, neurological impairment assessed using modified Matthew score assessed at baseline, after each infusion, 3, 4 and 12 weeks Barthel and Rankin at 1, 2, 4, 12, 24 and 48 weeks | |
| Notes | Ex: heart failure, hyperglycaemia, uraemia, arrhythmia, hyperpyrexia, previous stroke, mild stroke | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Probably done |
| Allocation concealment? | Unclear risk | Unclear from the publication |
| Blinding? | Low risk | Probably done |
| Completeness of follow‐up | High risk | 5 lost to follow up |
IMAGES Pilot.
| Methods | Multicentre, double‐blind, placebo‐controlled Randomisation by telephone service provided by Clinphone ITT analysis | |
| Participants | UK 51 patients: T: 26, C: 25 Inclusion: clinically diagnosed acute stroke with limb weakness (NIHSS ≥ 1), symptoms present for at least an hour and treatment initiation possible within 12 hours of onset Age 18 or greater Previously independent in activities of daily living | |
| Interventions | T: iv magnesium sulphate given as 16 mmol over 15 minutes followed by 65 mmol over 24 hours C: matching placebo | |
| Outcomes | Death and death and disability at 3 months Disability < 60 on the Barthel Index | |
| Notes | Ex: co‐existing disease which is likely to prevent outcome assessment, renal impairment, intracerebral pathology other than IS, participation in another acute clinical trial, pregnancy, contraindication to magnesium | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Randomisation by telephone service provided by Clinphone |
| Allocation concealment? | Low risk | Probably done |
| Blinding? | Low risk | Probably done |
| Completeness of follow‐up | Low risk | No loss of follow up |
INTERACT pilot 2008.
| Methods | Open, blinded outcome, randomised trial Randomisation was done with minimisation through a password protected Internet‐based system ITT analysis | |
| Participants | International, multicentre 404 patients: T: 203, C: 201 Age: 63 years Male: 65% Inclusion: spontaneous ICH confirmed by CT and elevated SBP ( ≥ 2 measurements of 150 to 220 mmHg, recorded ≥ 2 minutes apart) 100% CT Enrolment: within 6 hours of ICH onset | |
| Interventions | T: early intensive lowering of BP (target SBP 140 mmHg) C: standard guideline based management of BP (target SBP 180 mmHg) Both groups have received oral as well as iv agents for lowering blood pressure Rx: for 7 days | |
| Outcomes | Proportional change in haematoma volume at 24 hours BP methodology not stated | |
| Notes | Exclusion: indication for intensive lowering of BP, contraindication to intensive lowering of BP, ICH secondary to structural cerebral abnormality or use of thrombolytic agent, IS within 30 days, deep coma (3 to 5 Glasgow Coma Scale), pre‐stroke disability or medical illness, and early planned decompressive neurosurgical intervention | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Randomisation was done with minimisation through a password‐protected Internet based system |
| Allocation concealment? | Low risk | Probably done |
| Blinding? | High risk | Open blinded outcome trial |
| Completeness of follow‐up | High risk | 1 patient from each group was lost to follow up at 90 days A further 9 patients were known to be alive, but dependency was not assessed at 90 days due to being unable to contact the patient or a relative |
Kaste 1994/120 mg.
| Methods | Multicentre, double‐blind, placebo‐controlled Randomisation used sealed envelopes, stratified by onset of therapy, age and stroke severity Tablets provided in identical numbered vials ITT analysis | |
| Participants | Finland, 3 centres 350 patients: T: 176, C: 174 Mean age: T: 57 years, C: 58 years Males: T: 122, C: 113 Inclusion: acute ischaemic hemispheric stroke 100% CT Enrolment within 48 hours | |
| Interventions | T: 30 mg nimodipine qid C: matching placebo Rx: 21 days | |
| Outcomes | Neurological evaluation (own score) at baseline, day 1, 7, 21 and months 3 and 12; mobility at 12 months Functional outcome, Rankin at 3 and 12 months ‐ grades 1 and 2 representing independence were considered good outcome Primary end points: Rankin at 12 months, neurological scale and death Used Rankin > 3 for dependence in this review Rankin scale missing 2 living patients in control and 1 living patient in treatment group Method of BP measurement unknown | |
| Notes | Ex: unconsciousness, dysphagia, TIA, dependence in ADL before stroke, brain stem infarction, complicated migraine, pregnancy, renal or hepatic or cardiac failure, severe systemic infection, serious psychiatric disturbance, terminal malignancy Data from published paper and author | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Randomisation used sealed envelopes, stratified by onset of therapy, age and stroke severity |
| Allocation concealment? | Low risk | Probably done |
| Blinding? | Low risk | Probably done |
| Completeness of follow‐up | Unclear risk | Unclear from the publication |
Lamsudin 1997.
| Methods | Multicentre, double‐blind, placebo‐controlled Method of randomisation not given ITT analysis | |
| Participants | Indonesia, 5 departments 150 patients Males: T: 46, C: 50 Inclusion: acute IS Enrolment within 24 hours | |
| Interventions | T1: 30 mg nimodipine tds and 500 mg aspirin tds T2: 500 mg aspirin tds Rx: 28 hours | |
| Outcomes | Canadian Neurological Scale at baseline, 7, 14, 21 and 28 days Barthel Index at baseline, 7 and 14 days | |
| Notes | Ex: coma, haemorrhage, tumour, infection, trauma, serious organic brain disease other than IS, need for ventilation, current use of CCBs, allergy to aspirin, pregnancy, hypotension (SBP < 100 mmHg), bradycardia (rate < 50), second or third degree heart block if patient did not have pacemaker, hepatic or renal dysfunction, congestive heart failure, pneumonia | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Probably done |
| Allocation concealment? | Low risk | Unclear from the publication |
| Blinding? | Low risk | Probably done |
| Completeness of follow‐up | Unclear risk | Unclear from the publication |
Lees 1995.
| Methods | Single centre, double‐blind, placebo‐controlled Randomisation performed in blocks of 10 according to a code devised and held by pharmacy ITT analysis | |
| Participants | UK 60 participants: T: 30, C: 30 Mean age: T: 69.2 years, C: 65.9 years 30 males and 30 females Inclusion: MCA strokes 100% CT scan Enrolment within 12 hours | |
| Interventions | T1: magnesium sulfate 8 mmol in 50 mL saline iv over 15 minutes, then 65 mmol in 100 mL saline continuous iv over 24 hours C: matching volumes of normal saline | |
| Outcomes | MCA Neurological Score (N score) and NIHSS at baseline days 5 and 90 Barthel Index and Rankin Scale at days 5 and 90 Assess of 10 metre walking time made Method of BP measurement not known | |
| Notes | Ex: pregnancy, renal failure, pre‐existing functional impairment such that post‐stroke assessment would be impaired (mRS ≤ 3) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Randomisation was performed in blocks of 10 according to a code devised and held by the pharmacy |
| Allocation concealment? | Low risk | Probably done |
| Blinding? | Low risk | Medical staff and patients were blind to treatment |
| Completeness of follow‐up | Unclear risk | Unclear from the publication |
Limburg 1990.
| Methods | Single centre, double‐blind, placebo‐controlled Randomisation using tables from manufacturer ITT analysis | |
| Participants | Netherlands 26 patients: T: 12, C: 14 Mean age: T: 67 years, C: 66 years Males: T: 3, C: 6 Inclusion: acute supratentorial brain infarction 100% CT Enrolment within 24 hours of ictus | |
| Interventions | T: flunarizine, iv bolus of 0.1 mg/kg body weight in 5% glucose solution, followed after 3 hours by continuous infusion of 0.3 mg/kg/24 hours during 72 hours, then po flunarizine for 11 days C: identical placebo Rx: 14 days | |
| Outcomes | Motricity Index, Rankin scale, Barthel Index, death Last follow up 6 months Barthel and Rankin used in review Method for measurement of BP not known | |
| Notes | Ex: lacunar syndromes, serious underlying diseases, previous disabling stroke, using CCBs | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Randomisation using tables from manufacturer |
| Allocation concealment? | Low risk | Probably done |
| Blinding? | Low risk | Probably done |
| Completeness of follow‐up | Unclear risk | Unclear from the publication |
Lisk 1993.
| Methods | Single centre, double‐blind, placebo‐controlled Randomisation technique not stated ITT analysis | |
| Participants | USA 16 patients: T1: 5, T2: 3, T3: 2, C: 6 Mean age: 66 years 4 male, 12 female Inclusion: all patients except 2 had MCA territory infarct SBP ≥ 170 mmHg or ≤ 220 mmHg, DBP ≥ 95 mmHg or ≤ 120 mmHg 100% CT pre‐entry Enrolment within 72 hours History or family with hypertension 6 had SBP < 170 mmHg and 3 had DBP < 95 mmHg (baseline measurements) | |
| Interventions | T1: po 20 mg nicardipine hydrochloride T2: 12.5 mg captopril T3: 0.1 mg clonidine hydrochloride C: placebo (dextrose and starch) every 8 hours for 3 days | |
| Outcomes | Neurological impairment assessed using NIHSS at baseline and daily BP taken in supine position with automatic monitors; every 10 minutes for the first hour after first dose of drug, then every hour for 6 hours Thereafter BP measured at 4‐hourly intervals during sleep and waking hours (standing where possible to check for postural hypotension) | |
| Notes | Ex: coma, significant neurological deficit from previous stroke, unstable cardiac disease including acute MI, severe heart failure or conduction defects, history of angioedema and collagen vascular disease, liver dysfunction with aspartate aminotransferase and or bilirubin levels greater than twice normal, brain stem strokes Data from published paper and individual patient data provided by author | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Probably done |
| Allocation concealment? | Unclear risk | Unclear from the publication |
| Blinding? | Low risk | Probably done |
| Completeness of follow‐up | Low risk | 16 patients were entered into the study: 6 received placebo, 10 had antihypertensive drugs All had follow‐up data |
Lowe 1993.
| Methods | Single centre, double‐blind, randomised, placebo‐controlled Stratification at entry into: A ‐ normal consciousness/face‐arm paresis B ‐ normal consciousness/hemiparesis or hemiplegia C ‐ altered consciousness/hemiparesis or hemiplegia Method of randomisation used, statistical table using 6 groups of 4 possible sequences of individual treatment ITT analysis |
|
| Participants | UK 112 patients: T: 56, C: 56 Age: 45 to 85 years Males: T: 37, C: 29 Inclusion: clinical diagnosis of acute cerebral hemispheric infarction, Barthel < 65 100% CT scan within 7 days Enrolment within 48 hours: T: 6 patients delay > 48 hours, C: 9 patients delay > 48 hours | |
| Interventions | If patients able to swallow po treatment may be initiated from the start of the trial T: 40 mg nimodipine tds C: identical placebo Rx: 16 weeks If concomitant therapy used like beta blockers or methyl dopa, iv treatment was to be initially titrated against BP | |
| Outcomes | Neurological outcome assessed by a 10‐item grading system and using the Medical Research Council (MRC) numeric grading for each item when applicable Functional outcome assessed by the Barthel Index Assessments at days 1, 4, 7, 10 and weeks 2, 4, 8, 12, 16, 20, 24 Barthel at 1 month missing 12 in treatment group and 6 in placebo group Barthel at 12 weeks missing 12 in treatment group and 6 in placebo group At 24 weeks 16 missing in treatment group and 10 in placebo group 1 patient died at day 215, i.e. later than 6 months BP measured at baseline, 24, 96 hours and 7, 10, 14 days and 1, 2, 3, 4, 5, 6 months Method used to measure BP not known | |
| Notes | Ex: disability due to other causes, MI in previous 4 weeks or decompensated heart failure, liver or renal failure, brainstem stroke, patient whose survival is not expected, causes of neurological deficits other than ischaemic hemispheric infarction | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Probably done |
| Allocation concealment? | Low risk | Probably done |
| Blinding? | Low risk | Probably done |
| Completeness of follow‐up | Unclear risk | Unclear from available data |
Martinez‐Vila 1990.
| Methods | Multicentre, placebo‐controlled Method of randomisation not given PP analysis 41 patients excluded blindly from efficacy analysis | |
| Participants | Spain, 4 centres 164 patients: T: 81, C: 83 Age range: 45 to 92 years Males: T:43, C:43 Inclusion: IS in internal carotid artery territory as by clinical examination 100% CT within 3 days Enrolment within 48 hours | |
| Interventions | T: 30 mg qid nimodipine po C: identical placebo Rx: 28 days Allowed drugs included heparin (5000 IU bid) and agents indicated for cerebral oedema and cardiovascular drugs (not CCBs) and antibiotic or anxiolytic drugs | |
| Outcomes | Slightly modified Mathews scale by Gelmers et al at baseline, 1, 3, 5, 7, 14, 21, 28 days BP data obtained from paper, using PP numbers Death data taken from paper using ITT data BP estimated from graphs in paper | |
| Notes | Ex: MI, renal failure, liver failure, severe systemic infections, poorly controlled DM, SBP < 100 mmHg, terminal malignancy, TIA, evolving strokes, coma | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Probably done |
| Allocation concealment? | Unclear risk | Unclear from the publication |
| Blinding? | Low risk | Probably done |
| Completeness of follow‐up | Unclear risk | Unclear from the publication |
Muir 1995.
| Methods | Single centre, double‐blind, randomised, placebo‐controlled Randomisation of 6 participants per group was planned ITT analysis | |
| Participants | UK 25 patients: T: 19, C: 6 Mean age: T1: 75 years, T2: 65 years, T3: 71 years, C: 68 years Males: T1: 4, T2: 5, T3: 4, C: 3 Inclusion: clinically diagnosed stroke CT within 72 hours of stroke Enrolment within 24 hours of ictus Stroke types classified according to OCSP criteria | |
| Interventions | T1: 8 mmol MgSO4 over 24 hours T2: 12 mmol MgSO4 over 24 hours T3: 16 mmol MgSO4 over 24 hours C: matching placebo | |
| Outcomes | Barthel ADL score and mRS on days 30 and 60 BP and heart rate were measured semi‐automatically by oscillometric recorders (Marquette) Method used for measuring BP not known | |
| Notes | Ex: pregnancy, coma, renal failure | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Probably done |
| Allocation concealment? | Unclear risk | Unclear from the publication |
| Blinding? | Low risk | Probably done |
| Completeness of follow‐up | Low risk | All completed the study protocol |
Norris 1994.
| Methods | Single centre, double‐blind, placebo‐controlled Randomisation method not given No method for concealment of allocation given 189 patients randomised, 164 analysed in paper ‐ due to 25 protocol violations Paper analysis PP, calculations here based on ITT data | |
| Participants | Canada 189 patients: T: 96, C: 93 Mean age: T: 71.1 years, C: 72.1 years Inclusion: IS, Toronto stroke scores of > 20 100% CT Enrolment within 48 hours 4 patients with delay > 48 hours: T: 3, C: 1 | |
| Interventions | T: iv nimodipine for first 10 days, 2 mg/hour, then po 180 mg/day for next 6 months C: identical placebo Rx: 6 months | |
| Outcomes | Neurological disability using Toronto scale at baseline 10, 15, and 30 days Functional disability at baseline, 6 months, and 1 year using 3 simple categories: minor or no disability, moderate disability and patients who were severely disabled or bedridden (used severely disabled for disability scoring) Toronto stroke scale missing 6 in treatment group and 14 in control group BP was measured at baseline then 2‐hourly for first day then 4‐hourly for day 2 and 8‐hourly day 3 to 10 Method by which BP was measured not given | |
| Notes | Ex: comatose patients, no motor weakness (e.g. aphasia only), brain stem strokes, previous strokes, CT scan not compatible with IS, on CCBs, terminal illness, renal or hepatic failure or heart block Data from published paper and from Bayer Canada | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Probably done |
| Allocation concealment? | Unclear risk | Unclear from the publication |
| Blinding? | Unclear risk | Unclear from the publication |
| Completeness of follow‐up | Unclear risk | Unclear from the publication |
Paci 1989/120 mg.
| Methods | Single centre, double‐blind, placebo‐controlled Method of randomisation unknown ITT analysis | |
| Participants | Italy 41 patients: T: 19, C: 22 Mean age: T: 62 years, C: 63 years Males: T: 11, C: 17 Inclusion: patients with sudden and persistent neurological deterioration due to a focal event in the carotid artery distribution 100% CT Enrolment within 12 hours | |
| Interventions | T: nimodipine 40 mg tds C: identical matching placebo Rx: 28 days Patients given supportive medication of 20% mannitol, antihypertensive agents and antibiotics | |
| Outcomes | Neurological deficit (Mathews slightly modified by Gelmers) baseline and at days 1, 2, 3, 5, 7, 14, 21, 28 Global assessment made at end of treatment ‐ good/fair/poor BP and heart rate recorded twice daily, method of recording not given | |
| Notes | Ex: TIA, progressing stroke, primary intracerebral haemorrhage (CT scan), systemic disorders, recent MI, CCF, abnormal hepatic, renal or pulmonary functions, previous history of complete stroke Data from published paper | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Probably done |
| Allocation concealment? | Unclear risk | Unclear from the publication |
| Blinding? | Low risk | Probably done |
| Completeness of follow‐up | High risk | Probably not complete 41 entered the study (T: 19, C: 22) and at the end of period of observation 18 nimodipine‐treated patients with 1 possible loss of follow up |
PASS 1995.
| Methods | Multicentre, double‐blind, placebo‐controlled Computer‐generated randomisation schedule stratified by study centre | |
| Participants | 55 centres in 10 European countries 927 patients: T: 464, C: 463 Mean age: T: 70 years, C: 71 years Males: T: 241, C: 238 Inclusion: clinical diagnosis of acute ischaemic supratentorial stroke with Orgogozo Scale of > 5 and < 70 100% CT within 24 hours of ictus Enrolment within 12 hours | |
| Interventions | T: 12 g piracetam (Nootropil) as initial iv bolus over 20 minutes, then 12 g daily for 4 weeks and 4.8 g daily for 8 weeks C: matching placebo RX: 12 weeks | |
| Outcomes | Assessment at 1 and 3 days and 1, 2, 4, 8 and 12 weeks Primary outcome: MCA neurological scale at week 4 Secondary outcome: modified Barthel at 12 weeks, first used after 3 days Method for BP measurement not given | |
| Notes | Ex: haemorrhage, coma (< 5 on Glasgow Coma Scale), previous stroke, confounding neurological or systemic illness, thrombolytic agents and haemodilution Dipyridamole and ticlopidine were prohibited during the first 4 weeks Non‐study medications allowed were CCBs, osmotic diuretics and heparin Concomitant aspirin not recommended for at least 24 hours | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Probably done |
| Allocation concealment? | Low risk | Probably done |
| Blinding? | Low risk | Probably done |
| Completeness of follow‐up | Unclear risk | Unclear from the publication |
Pokrupa 1986.
| Methods | Single centre, double‐blind, placebo‐controlled Randomisation by sealed numbered opaque envelope ITT analysis | |
| Participants | Canada 23 patients: T: 11, C: 12 Mean age: 63 years Males: T: 5, C: 6 Inclusion: completed cerebrovascular accidents 100% CT pre‐entry Enrolment within 48 hours of ictus 5 patients enrolled > 48 hours | |
| Interventions | T: PGI2 ("Cycloprostin", Upjohn Co, USA) 5 daily 8‐hour consecutive infusions weaned up from 2 to 10 mg/kg/min and tapered over last hour C: sterile diluent buffer (NaCl 0.147 w/v, glycine 0.188 w/v, NaOH, pH 10.5 +/‐ 0.3) Rx: 5 days | |
| Outcomes | Death at 5 days and 1, 2, and 4 weeks. (Neurological impairment rating at 5 days, and 1, 2, and 4 weeks; CT and PET at 5 to 9 days.) Method for measuring BP not known | |
| Notes | Ex: coma, complicating neurological conditions, heparin, malignant hypertension, uncontrolled DM, heart attack within 2 months, recent surgery Mixture of data used | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Randomisation by sealed numbered opaque envelope |
| Allocation concealment? | Unclear risk | Unclear from the publication |
| Blinding? | Low risk | Probably done |
| Completeness of follow‐up | Unclear risk | Unclear from the publication |
Potter 2009 labetalol.
| Methods | Multicentre, double‐blind, placebo‐controlled Randomisation by secure Internet central randomisation (with block size of 6) ITT analysis | |
| Participants | UK 172 patients: T: 56, C: 29 Mean age: 74 years Male 57% Inclusion: > 18 years of age, fixed neurological deficit > 60 minutes, clinical diagnosis of acute stroke Enrolment within 36 hours of ictus | |
| Interventions | Labetalol 50 mg po or matching placebo was initially given with the opportunity to repeat this at 4 hours and 8 hours after randomisation Thereafter patients were continued on 50 to 150 mg of labetalol twice daily for 2 weeks, including for dysphagic patients (after 72 hours intravenous labetalol was converted to oral or nasogastric labetalol depending on swallowing status in dysphagic patients) | |
| Outcomes | Death or dependency at 2 weeks Supine BP was measured with a validated A&D UA‐767 BP monitor with a cuff of a suitable size | |
| Notes | Ex: hypertensive encephalopathy, co‐existing cardiac or vascular emergency, SBP > 200 mmHg and/or DBP > 120 mmHg in association with primary intracerebral haemorrhage, pre‐existing antihypertensive therapy in patients without dysphagia, impaired level of consciousness, contraindication to trial therapy, premorbid mRS > 3, coexisting life threatening condition with life expectancy < 6 months, diagnosis of non‐stroke on subsequent neuroimaging | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Randomly assigned by secure Internet central randomisation (with a block size of 6) |
| Allocation concealment? | Low risk | Probably done |
| Blinding? | Low risk | Probably done |
| Completeness of follow‐up | High risk | 179 patients randomly assigned, 6 withdrawn due to a protocol violation or non‐stroke diagnosis and 1 withdrew consent |
Potter 2009 lisinopril.
| Methods | Multicentre, double‐blind, placebo‐controlled Randomisation by secure Internet central randomisation (with block size of 6) ITT analysis | |
| Participants | UK 172 patients: T: 56, C: 29 Mean age: 74 years Male 57% Inclusion: > 18 years of age, fixed neurological deficit > 60 minutes, clinical diagnosis of acute stroke Enrolment within 36 hours of ictus | |
| Interventions | Lisinopril or matching placebo was initially given at 5 mg po with an opportunity to repeat the dose at 4 hours and 8 hours after randomisation, with participants then continued on 5 to 15 mg of lisinopril once daily for up to 2 weeks | |
| Outcomes | Death or dependency at 2 weeks Supine BP was measured with a validated A&D UA‐767 BP monitor with a cuff of a suitable size | |
| Notes | Ex: hypertensive encephalopathy, co‐existing cardiac or vascular emergency, SBP > 200 mmHg and/or DBP > 120 mmHg in association with primary intracerebral haemorrhage, pre‐existing antihypertensive therapy in patients without dysphagia, impaired level of consciousness, contraindication to trial therapy, premorbid mRS > 3, coexisting life threatening condition with life expectancy < 6 months, diagnosis of non‐stroke on subsequent neuroimaging | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Randomly assigned by secure Internet central randomisation (with a block size of 6) |
| Allocation concealment? | Low risk | Probably done |
| Blinding? | Low risk | Probably done |
| Completeness of follow‐up | High risk | 179 patients randomly assigned, 6 withdrawn due to a protocol violation or non‐stroke diagnosis and 1 withdrew consent |
PRISTINE.
| Methods | Multicentre, double‐blind, placebo‐controlled Randomisation by minimisation Stratified by age and stroke severity ITT and PP analysis | |
| Participants | UK, Netherlands, Sweden: 9 centres 620 patients: T: 313, C: 307 Age: T: 72 years, C: 72 years Male: T: 161, C: 160 Inclusion: ACHI 100% CT Enrolment within 48 hours | |
| Interventions | T: naftidrofuryl fumarate 633 mg/day iv continuous for 7 days then orally for 6 months C: solvent and identical looking tablets Rx: 6 months | |
| Outcomes | Death Assessments were at entry and intervals to 1 year Method used for BP measurement not given | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Randomisation by minimisation |
| Allocation concealment? | Unclear risk | Unclear from the publication |
| Blinding? | Unclear risk | Probably done |
| Completeness of follow‐up | Unclear risk | Unclear from the publication |
Rashid 2003 10 mg.
| Methods | Open label blinded‐endpoint Dose comparison Controlled trial Randomisation by computer (with minimisation on age, gender, Scandinavian Neurological Stroke Scale and mean arterial pressure) FU: no losses ITT analysis | |
| Participants | UK, single centre 90 patients: T: 60, C: 30 Mean age: T: 70.8 years, C: 73.9 years Male: T: 28, C: 13 Inclusion: ischaemic or haemorrhagic stroke Enrolment within 72 hours of ictus Clinical stroke subtype at baseline and CT scanning within a week of stroke onset Any antihypertensive medication was stopped at the time of admission and recommenced after 10 days once the trial treatment phase was completed | |
| Interventions | Transdermal glyceryl trinitrate once daily: T1: 5 mg , T2: 5/10 mg, T3: 10 mg C: no patch Rx: 10 days | |
| Outcomes | 24 hour ambulatory BP monitoring was set to record 3 times per hour during the day and hourly during the night at days 0, 1, 4, 5 and 10 mRS, Barthel index and quality of life at 3 months | |
| Notes | Ex: SBP > 230 mmHg or < 100 mmHg, DBP > 130 mmHg or < 60 mmHg, heart rate > 130 beats/minute or < 50 beats/minute, mild stroke, coma, pre‐morbid dependence, or presence of illnesses that could confound neurological or functional evaluation (such as pre‐existing neurologic or psychiatric disorders) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Randomisation by computer (with minimisation on age, gender, Scandinavian Neurological Stroke Scale and mean arterial pressure) |
| Allocation concealment? | Low risk | Probably done |
| Blinding? | High risk | Probably not done |
| Completeness of follow‐up | Low risk | No loss of follow up |
Rashid 2003 5 mg.
| Methods | As for Rashid 2003 10 mg | |
| Participants | — | |
| Interventions | — | |
| Outcomes | — | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Randomisation by computer (with minimisation on age, gender, Scandinavian Neurological Stroke Scale and mean arterial pressure) |
| Allocation concealment? | Low risk | Probably done |
| Blinding? | High risk | Probably not done |
| Completeness of follow‐up | Low risk | No loss of follow up |
Rashid 2003 5/10 mg.
| Methods | As for Rashid 2003 10 mg | |
| Participants | — | |
| Interventions | — | |
| Outcomes | — | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Randomisation by computer (with minimisation on age, gender, Scandinavian Neurological Stroke Scale and mean arterial pressure) |
| Allocation concealment? | Low risk | Probably done |
| Blinding? | High risk | Probably not done |
| Completeness of follow‐up | Low risk | No loss of follow up |
Saxena 1999 100 mg.
| Methods | As for Saxena 1999 25 mg | |
| Participants | — | |
| Interventions | — | |
| Outcomes | — | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Probably done |
| Allocation concealment? | Unclear risk | Unclear from publications |
| Blinding? | High risk | Study was single‐blinded because of the prominent colour of the drug and the difficulty in manufacturing a proper placebo |
| Completeness of follow‐up | Unclear risk | Unclear from publications |
Saxena 1999 25 mg.
| Methods | Multicentre, single‐blind, placebo‐controlled Randomisation method not stated ITT analysis | |
| Participants | Europe 85 patients: T1: 10, T2: 10, T3: 20, C: 45 Mean age: T1, T2, T3: 68 years, C: 65 years 39 male and 46 female Inclusion: IS in the anterior circulation 100% CT scans Enrolment within 18 hours | |
| Interventions | T1: 25 mg/kg 10% DCLHb T2: 50 mg/kg 10% DCLHb T3: 100 mg/kg 10%DCLHb C: equal volume of 0.9% normal saline given every 6 hours for 73 hours Rx: 3 days | |
| Outcomes | Rankin at 3 months BP and heart rate measured every 15 minutes for approximately 72 hours | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Probably done |
| Allocation concealment? | Unclear risk | Unclear from publications |
| Blinding? | High risk | Study was single‐blinded because of the prominent colour of the drug and the difficulty in manufacturing a proper placebo |
| Completeness of follow‐up | Unclear risk | Unclear from publications |
Saxena 1999 50 mg.
| Methods | As for Saxena 1999 25 mg | |
| Participants | ||
| Interventions | ||
| Outcomes | — | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Probably done |
| Allocation concealment? | Unclear risk | Unclear from publications |
| Blinding? | High risk | Study was single‐blinded because of the prominent colour of the drug and the difficulty in manufacturing a proper placebo |
| Completeness of follow‐up | Unclear risk | Unclear from publications |
Squire 1996.
| Methods | Multicentre, double‐blind, placebo‐controlled Stratified for age and time of stroke onset ITT analysis | |
| Participants | UK, 16 centres 147 patients: T: 75, C: 72 Age: T: 69 years, C: 69 years Males: T: 50, C: 32 Inclusion: first ever IS, pre‐treatment motor arm or motor leg (NIHSS scale) of 2 or 3 100% CT scan within 72 hours Enrolment within 12 hours | |
| Interventions | T: lifarizine 250 ug/kg iv immediately plus lifarizine 60 mg bd C: matching placebo Rx: 6 days | |
| Outcomes | Death, NIH motor scores and Canadian Neurological scales days 26 to 30 and week 13; Rankin and Barthel scores at days 26 to 30 and week 13 T: 5 missing for Barthel at 4 weeks C: 8 missing for Barthel at 4 weeks T: 6 missing for Barthel at 3 months C: 9 missing for Barthel at 3 months Method for BP measurement not known | |
| Notes | Ex: NIH scale level of consciousness 2 or 3, previous stroke or neurological condition that may interfere with neurological or functional assessments, MI within last 4 months, left ventricular failure, SBP < 120 and DBP < 80 mmHg, history of ventricular arrhythmias or existing ECG abnormalities, AV block or IVCD, on CCBs or lipophilic beta blockers, premenopausal female, TIA, pre‐existing life‐threatening disease or systemic illness, endarterectomy or enrolled in other trial Data from paper and authors | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Probably done |
| Allocation concealment? | Unclear risk | Unclear from publications |
| Blinding? | Low risk | Probably done |
| Completeness of follow‐up | Unclear risk | Unclear from publication |
Steiner 1986.
| Methods | Single centre, double‐blind, placebo‐controlled Stratification based upon procedure similar to minimisation 18 patients secondarily excluded and 3 withdrawn 11 patients entered a short‐term active treatment group, these are included in the treatment arm ITT analysis | |
| Participants | UK, single centre 980 patients screened, 100 randomised: T: 55, C: 45 Mean age: 69.4 years (1 patient 81 years) 54 males Inclusion: ACHI, disabling hemiparesis, age 40 to 80 years 100% CT Enrolment within 1 week | |
| Interventions | T1: 600 mg naftidrofuryl iv daily for 10 days then 100 mg tds po for 9 months C: inactive vehicles to match T2: 1 in 3 patients starting treatment within 12 hours received active infusion and placebo capsules T1 and T2 treated as one here Rx: 9 months | |
| Outcomes | Neurological deficit measured at 24 and 48 to 72 hours, day 10 and disability and functional capacity (7‐point scale where 0 = normal, 5 = severely disabled and 6 = comatose, adapted from Rankins gradings) at weeks 3, 9, 10, 15, 24, 36, 52 Method for BP measurements not given | |
| Notes | Ex: coma, stroke but not ACHI, severe disability, severe intercurrent illness, incompatible medication on admission | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Assignment to active or placebo therapy followed stratification |
| Allocation concealment? | Unclear risk | Unclear from publication |
| Blinding? | Low risk | Probably done |
| Completeness of follow‐up | High risk | 18 patients secondarily excluded and 3 withdrawn |
Strand 1984.
| Methods | Single centre, double‐blind, placebo‐controlled Randomisation using serially numbered sealed envelopes; the code was held by pharmacy Stratified by delay of symptoms to randomisation, age and severity of symptoms ITT analysis | |
| Participants | Sweden 26 participants: T: 13, C: 13 Mean age: 74 years, T: 76.3 years, C: 71.5 years 14 males, 12 females 100% CT Enrolment within 36 hours | |
| Interventions | T: loading dose of 4 mmol magnesium sulfate iv over 10 minutes followed by continuous iv of 4 mmol magnesium sulfate during the following 8 hours, then after iv infusion one 250 mg magnesium hydroxide po, then 250 mg magnesium hydroxide po 8‐hourly for following 5 days C: equal volumes of isotonic saline and placebo pills Rx: 5 days | |
| Outcomes | Scandinavian Stroke Study Group (neurological score) at baseline, day 6 and 6 months Method of BP measurement not known | |
| Notes | Ex: SBP < 110 mmHg on admission, AV‐block II‐III, major renal impairment, respiratory insufficiency, pre‐existing functional impairment confusing proper evaluation of therapeutic effects, concomitant severe disorders, and ongoing anticoagulant treatment, plasma creatinine > 200 umol/l and EKG showing AV‐block II‐III | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Randomisation using serially numbered sealed envelopes |
| Allocation concealment? | Low risk | Randomisation code were held by pharmacy |
| Blinding? | Low risk | Probably done |
| Completeness of follow‐up | Unclear risk | Unclear from publication |
Uzuner 1995/180 mg.
| Methods | Single centre, randomised controlled trial ITT analysis | |
| Participants | Turkey 100 patients: T: 50, C: 50 IS: mean age: 63 years, 41 males and 36 females Primary intracerebral haemorrhage: mean age 65 years, 3 male, 8 female Inclusion: ischaemic and haemorrhagic strokes 100% CT pre‐entry Enrolment within 24 hours 16 patients enrolled after 24 hours (1 iv, 15 po) | |
| Interventions | T: IS ‐ nimodipine 180 mg/day (60 mg tds) po T: primary intracerebral haemorrhage: nimodipine 2 mg/hour iv for SAH or intracerebral haemorrhage C: matching po or iv placebo Rx: 2 days | |
| Outcomes | BP and pulse rate measured at basal and at 5, 15, 30 and 60 minutes in first hour and then every hour within the first 23 hours, then every 2 hours in the next 24 hours BP measured supine using automatic monitor (PETAS) Length of stay Glasgow Coma Scale | |
| Notes | Ex: 10 patients (T: 2, C: 8) treated with antihypertensive agents for malignant hypertension 2 patients with SAH (treated with iv nimodipine) excluded from our analysis We used unpublished paper and data supplied by author | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Probably done |
| Allocation concealment? | Unclear risk | Unclear from available data |
| Blinding? | Unclear risk | Unclear from available data |
| Completeness of follow‐up | Unclear risk | Unclear from available data |
VENUS 1995.
| Methods | Multicentre, double‐blind, randomised controlled trial Method of randomisation not known Patients randomised by general practitioners ITT analysis | |
| Participants | Netherlands, GP lead 454 patients: T: 225, C: 229 Males: T: 127, C: 142 Mean age: T: 70.5 years, C: 71.1 years Enrolment within 6 hours of ictus | |
| Interventions | T: nimodipine po 30 mg qid C: matching placebo Rx: 10 days | |
| Outcomes | Death, Barthel and Rankin done by telephone at 3 months Method for BP measurement not known | |
| Notes | Ex: SBP > 220 mmHg | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Randomised in equal blocks of 10, according to computer‐generated lists Numbered blocks contained 1 complete treatment or identical placebo course were sequentially distributed |
| Allocation concealment? | Low risk | Probably done |
| Blinding? | Low risk | Probably done |
| Completeness of follow‐up | Unclear risk | Unclear from publication |
Walters 2006.
| Methods | Single centre Randomisation done using standard algorithm by the local pharmacy production unit Allocation to insulin or control done in an open label design | |
| Participants | UK 25 patients: T: 13, C: 12 Mean age: 75 years Inclusion: acute ischaemic stroke Enrolment: within 24 hours of ictus 100% CT scan | |
| Interventions | T: iv insulin at a variable rate adjusted for target glucose concentration of 5 to 8 mmol/l C: iv crystalloid Rx: 2 days | |
| Outcomes | Mortality at 1 month | |
| Notes | Ex: known insulin requiring DM, patients with severe metabolic derangement, patients with clinical evidence of infection or CCF | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Randomisation performed using standard algorithm by the local pharmacy production unit |
| Allocation concealment? | Low risk | Probably done |
| Blinding? | High risk | Open label design |
| Completeness of follow‐up | Unclear risk | 25 patients recruited (T: 13, C: 12); no mention of loss of follow‐up |
Willmot 2006.
| Methods | Single centre Patient and measurement‐blinded Randomised controlled trial Randomisation by computer (with minimisation on age, sex, baseline SBP, baseline Scandinavian Stroke Score, hours from onset, presence of a visible stroke lesion on CT) FU: no losses ITT analysis | |
| Participants | UK 18 patients: T: 12, C: 6 Age: T: 69 years, C: 70.3 years Male: T: 2, C: 3 Inclusion: previously independent adult patients with a clinical stroke syndrome and limb weakness 100% CT Enrolment: within 5 days of ictus Prior antihypertensive medication was discontinued at the time of admission | |
| Interventions | T: transdermal glyceryl trinitrate 5 mg (Transiderm‐Nitro5, Novartis Pharmaceuticals) once daily C: no patch Rx: 7 days | |
| Outcomes | BP was measured immediately before the baseline xenon CT scan and immediately after the post‐treatment scan Peripheral SBP and DBP was measured in the non‐hemiparetic arm with a validated digital readout oscillometric device (Omron HEM‐705CP, Omron Corp, Tokyo, Japan) Central BP was assessed by applanation tonometry of the left radial artery and using the pulse wave analysis (PWA) system (Sphygmocor, Sydney, Australia) | |
| Notes | Ex: requirement for or contraindication to nitrate therapy, had a definite need for prior antihypertensive therapy or vasoactive drugs, co‐operate with scanning | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Randomly assigned using computerised minimisation |
| Allocation concealment? | Low risk | Probably done |
| Blinding? | High risk | Patient and measurement‐blinded |
| Completeness of follow‐up | Low risk | No loss of follow up |
Wimalarat 1994/120mg.
| Methods | Multicentre, double‐blind, randomised controlled trial Randomised by next random number on list Stratified according to the severity of stroke Treatment within 24 hours of stroke 34 patients secondarily excluded, leaving 181 with cerebral infarction PP analysis Comparing 2 different doses of nimodipine: 120 mg and 240 mg | |
| Participants | UK, 3 centres 215 patients: T: 58, C: 60; 181 patients analysed Mean age: T: 70 years, C: 71 years Males: T: 36, C: 33 Inclusion criteria: ischaemic cerebral hemisphere infarction, age range 45 to 85 years and with Barthel Index score of < 65 at entry 100% CT scan Enrolment within 24 hours | |
| Interventions | T1: 120 mg nimodipine daily for 16 weeks T2: 240 mg nimodipine daily for 16 weeks C: identical placebo | |
| Outcomes | Neurological assessment using Medical Research Council (MRC) score Functional assessment using Barthel Index All assessments at baseline, days 1, 4, 7 and weeks 2, 4, 8, 12, 16, 20 and 24 Method used for monitoring BP not known Deaths in this review are only out of those who completed and excluded withdrawals and secondary exclusions | |
| Notes | Ex: haemorrhage on CT scan, disability due to other causes inseparable from acute stroke, MI with last 6 months, renal or hepatic failure, patient in whom survival was not expected at the initial assessment, brain stem strokes Data used here not intention‐to‐treat 34 patients secondary excluded and 30 patients withdrawn BP measurements and outcome data obtained from author Data used was from the author and from the paper | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Probably done |
| Allocation concealment? | Unclear risk | Unclear from publication |
| Blinding? | Low risk | Probably done |
| Completeness of follow‐up | High risk | 30 patients withdrawn |
Wimalarat 1994/240mg.
| Methods | As for Wimalarat 1994/120mg but using 240 mg nimodipine T: 63 patients, C: 60 patients | |
| Participants | — | |
| Interventions | — | |
| Outcomes | — | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Probably done |
| Allocation concealment? | Unclear risk | Unclear from publication |
| Blinding? | Low risk | Probably done |
| Completeness of follow‐up | High risk | 30 patients withdrawn |
ACEI: angiotensin‐converting enzyme inhibitor ACHI: acute cerebral hemisphere infarction ADL: activities of daily living AF: atrial fibrillation AV: atrioventricular bd: twice a day BP: blood pressure C: control treatment CCB: calcium channel blocker CCF: congestive cardiac failure CI: cardiac index CSF: cerebrospinal fluid CT: computerised tomography DBP: diastolic blood pressure DHCLHb: diaspirin cross‐linked haemoglobin DM: diabetes mellitus ECG: echocardiogram EEG: electroencephalography Ex: exclusions FU: follow up GKI: glucose‐potassium‐insulin GTN: glyceryl trinitrate ICH: intracerebral haemorrhage IS: ischaemic stroke ITT: Intention to treat IU: international unit iv: intravenous MAP: mean arterial pressure MCA: middle cerebral artery MI: myocardial infarction MRI: magnetic resonance imaging mRS: modified Rankin Score NIHSS: National Institutes of Health Stroke Scale OCSP: Oxfordshire Community Stroke Project PET: positron emission tomography po: oral PP: per protocol PTX: pentoxifylline PGI2: prostacyclin PICH: primary intra cerebral haemorrhage qid: four times per day Rx: treatment SAH: subarachnoid haemorrhage SBP: systolic blood pressure sl: sublingual T: active treatment tds: three times per day TIA: transient ischaemic attack
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Albers 1995a | BP data not available |
| Albers 1995b | BP data not available |
| Ameriso 1992 | Haemorheological variables are studied This is part of the JP Mohr study |
| ANS 1992 | BP data in the immediate post‐stroke period not available |
| ATTACH 2006 | No control group |
| Autret 1992 | BP data not available |
| Bogousslavsky 2002 | Standard deviations for BP data not available |
| Britton 1980 | Unable to obtain data from the author |
| Brola 1998 | Unable to obtain data |
| Busse 1985 | Unable to obtain BP data |
| Cao 2003 | Confounded study (nimodipine + mannitol versus mannitol) |
| Capon 1983 | Unable to obtain data |
| CARING 2005 | Ongoing study Confounded (2 doses of nicardipine) |
| Chan 1993 | Unable to get BP data |
| Chandra 1995 | Oral versus intravenous nimodipine No control group |
| CHERISH 2006 | Confounded study (clinidipine versus losartan) |
| Davalos 1989 | Unable to obtain BP data |
| Dekoninck 1978 | Unable to obtain data |
| Domzal 1986 | Confounded study (vinpocetine versus aminophylline) |
| FIST 1996 | BP data not available |
| Galeas 1998 | Unable to obtain data |
| Gamez 1988 | Unable to obtain BP and outcome data |
| Geismar 1976 | Unable to obtain data from the author |
| Gelmers 1984/120 | BP data not available |
| Gelmers 1988/120 | BP data not available |
| Gladstone 2006 | BP data not available |
| Gray 1990 | On‐treatment BP data not available |
| Haley 1994/0.6 | Unable to obtain on‐treatment BP data |
| Haley 1994/2 | Unable to obtain on‐treatment BP data |
| Haley 1994/6 | Unable to obtain on‐treatment BP data |
| Hartmann 2005 | Confounded study (urapidil versus nifedipine) |
| Hoechst 1986 | After much correspondence the company Hoechst was unable to provide the data We continue to correspond |
| Holthoff 1990 | Unable to obtain BP data |
| Hsu 1988 | Author did not keep the raw BP data and referred us to Hoechst who seem unable to provide the data |
| Huber 1993 | Author did not have raw data for the trial but suggested Hoechst would We have corresponded over a period of 2 years with Hoechst and still unable to obtain the data |
| IMAGES 2004 | Unable to obtain BP data from authors |
| Infeld 1999 | BP data not available |
| Karoutas 1990 | Dr Karoutas died and the paper was never published No data could be retrieved from his personal archives |
| Kornhuber 1993 | Unable to obtain BP data |
| Lampl 2001 | BP data not available |
| Lipani 1984 | Cross‐over study Patients with TIA and other diseases (Parkinsonian syndrome) were also included |
| Martin 1985 | Raw data lost |
| Martinsson 2002 | BP data not available |
| MAST‐I | No BP data available in paper or from author |
| Meier 1991 | No BP data available for active and control groups |
| Miller 1984 | No control group |
| Ming 1990 | Unable to obtain BP data |
| Misra 2005 | BP data not available |
| Mohr 1992/120 | Unable to obtain BP data |
| Mohr 1992/240 | Unable to obtain BP data |
| Mohr 1992/60 | Unable to obtain BP data |
| Molnar 1979 | Confounded study |
| Mousavi 2004 | BP data not available |
| Nakamura 2007 | Confounded study (perindopril, candesartan or conventional therapy) |
| Nazir 2004 | On‐treatment BP data not available |
| NEST 1994/120 | On‐treatment BP data not available |
| NIMPAS 1997 | BP not taken |
| Oczkowski 1989 | BP data not available |
| Ohtomo 1986 | Confounded |
| Ohtomo 1987a | Unable to obtain any information for this study Author does not answer any correspondence |
| Ohtomo 1987b | Unable to obtain BP data |
| Orgogozo | Unable to obtain BP data |
| Piradov 1992 | Unable to obtain BP data |
| Piriyawat 2003 | No control group |
| Platt 1993 | Unable to obtain BP data |
| Popa 1995 | Not a trial of treatment but stopping |
| Rosenbaum 1991 | No control group |
| Saver 2004 | No control group |
| Sherman 1986/120 | Unable to obtain BP data |
| Sprigg 2007 | Study involves both acute and subacute stroke patients |
| Su 2004 | BP and outcome data not available |
| Suslina 1999 | Confounded study |
| Szakall 1998 | Sub‐acute trial and no placebo |
| Szczechowski 1994 | Unable to obtain data |
| TRUST 1990/120 | BP data not available |
| Vamosi 1976 | Study is confounded |
| Vamosi 1979 | Study is confounded |
| Wang 2004 | BP data not available |
| Wang 2006 | BP data not available |
| Wasilewski 1985 | Dr R Wasilewski now retired and all old documentation has been destroyed |
| Werner 1986 | No BP data available |
| Wong 1987 | Unable to get BP data |
| Woollard 1978 | Authors no longer have data; trying pharmaceutical company |
| Yu 2003 | Confounded study |
| Zhao 2003 | Unable to get BP data |
| Zorzon 1987 | An open non‐randomised clinical trial |
BP: blood pressure TIA: transient ischaemic attack
Characteristics of ongoing studies [ordered by study ID]
ACCOST 2006.
| Trial name or title | Acute Candesartan Cilexetil Outcomes Stroke Trial |
| Methods | Double‐blind, placebo‐controlled, phase IV randomised controlled trial |
| Participants | Patients presenting with a stroke within 72 hours having a mean BP >120/70 |
| Interventions | Candesartan cilexetil or matched placebo |
| Outcomes | Primary outcome: all‐cause mortality and mortality due to vascular causes Secondary outcomes: neurological recovery at 3 months (NIHSS), functional recovery at 3 months (mRS, Barthel) |
| Starting date | 2004 |
| Contact information | Christopher Gray, Sunderland Royal Hospital, Sunderland, Tyne and Wear, SR4 7TP, UK |
| Notes | Size: 50 participants Funding: City Hospitals Sunderland NHS Foundation Trust |
ASTART 2005.
| Trial name or title | Acute Stroke Treatment with Atorvastatin and Irbesartan (ASTART) |
| Methods | Randomised, placebo‐controlled |
| Participants | Clinical diagnosis of acute ischaemic stroke within 72 hours of onset |
| Interventions | Atorvastatin (80 mg) + irbesartan (150 mg) versus placebo |
| Outcomes | Effect on infarct size, cerebral perfusion and clinical outcome at 30 days |
| Starting date | — |
| Contact information | WA Centre for Health & Ageing (M573), University of Western Australia, 35 Stirling Highway, Crawley WA 6009, Australia |
| Notes | — |
ATACH‐2 2008.
| Trial name or title | Antihypertensive Treatment in Acute Cerebral Hemorrhage |
| Methods | 5‐year international, multicentre, open‐labelled, randomised, controlled, phase III clinical trial |
| Participants | Patients with co‐morbid hypertension and spontaneous ICH |
| Interventions | CCB, nicardipine iv |
| Outcomes | Efficacy of early, intensive antihypertensive treatment using iv nicardipine |
| Starting date | 2008 |
| Contact information | Adnan I Qureshi, University of Minnesota 12‐100 PWB 516 Delware St, SE Minneapolis, MN 55455 |
| Notes | Size: 60 participants Funding: NIH |
BLAST 2007.
| Trial name or title | Blood Pressure Lowering in Acute Stroke Trial |
| Methods | Randomised, double‐blind, placebo‐controlled |
| Participants | Acute ischaemic stroke patients with elevated BP within 48 hours of symptom onset |
| Interventions | Valsartan versus placebo orally daily for 7 days or until discharge |
| Outcomes | 30‐day Glasgow Outcome Scale, 30‐day modified Rankin scale and 30‐day Barthel Index |
| Starting date | 2007 |
| Contact information | Gregory W Albers, Stanford University School of Medicine |
| Notes | Funding: Stanford University, Novartis |
COSSACS 2005.
| Trial name or title | Continue Or Stop post‐Stroke Antihypertensives Collaborative Study |
| Methods | Multicentre, prospective, randomised, open, blinded endpoint study |
| Participants | Patients within 24 hours of acute ischaemic or hemorrhagic stroke and within 24 hours of last dose of antihypertensive therapy |
| Interventions | Continue or stop current antihypertensive therapy |
| Outcomes | Primary outcome: proportion of patients who are dead or dependent (defined by a mRS score > 2) at 14 days post‐stroke Secondary outcomes: BP changes, and neurological and functional status at 2 weeks and at 6 months post ictus |
| Starting date | 2002 |
| Contact information | T Robinson, Department of Cardiovascular Sciences, Aging and Stroke Research Group, University of Leicester, UK |
| Notes | Funding: The Health Foundation |
ENOS 2006.
| Trial name or title | Efficacy of Nitric Oxide in Stroke Trial |
| Methods | Prospective, international, multicentre, randomised, parallel group, double‐blind, placebo‐controlled, collaborative trial |
| Participants | Patients with hemorrhagic or ischaemic stroke who show motor weakness for at least 1 hour, who can be treated within 48 hours, and who have a pre‐stroke Rankin score > 3 |
| Interventions | Transdermal glyceryl trinitrate or placebo for 7 days |
| Outcomes | Primary outcome: mortality rate and Rankin score at 3 months Secondary outcomes: recurrent stroke, symptomatic deep vein thrombosis, symptomatic pulmonary embolism, or symptomatic intracranial haemorrhage at 7 days, major extracranial haemorrhage at 10 days, BP recorded during 7‐day treatment, length of hospital stay, discharge disposition, Barthel Index, quality of life as measured by EuroQol and abbreviated mental test score at 3 months |
| Starting date | 2001 |
| Contact information | Philip MW Bath, Division of Stroke Medicine, University of Nottingham, Clinical Sciences Building, City Hospital Campus, Hucknall Road, Nottingham NG5 1PB, UK |
| Notes | Size: 5000 participants (100 centres) Funding: BUPA Foundation, The Hypertension Trust, MRC, University of Nottingham |
FAST‐MAG 2005.
| Trial name or title | Field Administration of Stroke Therapy ‐ Magnesium Phase III Trial |
| Methods | Multicentre, randomised, double‐blind, placebo‐controlled trial |
| Participants | Patients (both cerebral infarction and intracerebral haemorrhage patients) as identified by the Los Angeles Prehospital Stroke Screen (LAPSS) whose neurological deficits have been present for at least 15 minutes, and who can be treated within 2 hours of symptom onset |
| Interventions | Magnesium sulphate iv or a matched placebo |
| Outcomes | Primary outcome: functional outcome at 90 days as measured by the mRS |
| Starting date | 2005 |
| Contact information | Jeffrey L Saver, Department of Neurology, UCLA Stroke Center, Los Angeles, California |
| Notes | Funding: National Institute for Neurological Disorders and Stroke, NIH, American Heart Association |
GRASP 2005.
| Trial name or title | Glucose Regulation in Acute Stroke Patients trial |
| Methods | Multicentre, randomised, controlled trial with 3 treatment arms |
| Participants | Adult acute ischaemic stroke patients with hyperglycaemia (glucose > 110 mg/dL) within 24 hours of stroke symptoms |
| Interventions | Tight glucose control, loose glucose control, or usual care |
| Outcomes | Primary outcome: rate of hypoglycaemic events (glucose < 55 mg/dL) The primary feasibility outcome is the frequency of participants in target range within 24 hours of treatment initiation |
| Starting date | 2005 |
| Contact information | Christiana E Hall, Karen C Johnston, University of Virginia, Department of Neurology #800394, Charlottesville, VA 22908 Telephone: +1 434 9245323 |
| Notes | Funding: NIH‐NINDS |
ICH ADAPT 2007.
| Trial name or title | IntraCerebral Hemorrahge Acutely Decreasing Arterial Pressure Trial |
| Methods | Multicentre, randomised, open label, blinded endpoint trial |
| Participants | Patients who have acute ICH, confirmed by CT diagnosis |
| Interventions | 10 mg iv bolus of labetalol or control |
| Outcomes | Primary outcome: imaging marker (peri‐hematomal rCBF, as measured with CT perfusion 2 hours after anti‐hypertensive therapy is initiated) |
| Starting date | 2007 |
| Contact information | Ken Butcher, Division of Neurology, 2E3.13 Walter C Mackenzie Health Sciences Centre, 8440 112 St, Edmonton, Alberta, T6G 2B7, Canada |
| Notes | Funding: University of Alberta University Hospital Foundation |
INTERACT 2 2007.
| Trial name or title | Intensive Blood Pressure Reduction in Acute Cerebral Haemorrhage |
| Methods | Randomised, open label, active control, parallel assignment, safety/efficacy study |
| Participants | Patients with acute stroke due to spontaneous ICH confirmed by clinical history and CT scan; at least 2 SBP measurements of ≥ 150 mmHg and ≤ 200 mmHg; recorded 2 or more minutes apart; within 6 hours of stroke onset |
| Interventions | Early intensive lowering of BP (target SBP 140 mmHg) or standard guideline based management of BP (target SBP 180 mmHg) |
| Outcomes | Primary outcome: mortality and dependency (according to a 3 to 5 score on the mRS at 3 months) |
| Starting date | 2007 |
| Contact information | Emma Heeley, The George Institute, Level 10, King George V Building, Royal Prince Alfred Hospital, Missenden Road, Camperdown NSW 2050, Australia |
| Notes | Funding: National Health and Medical Research Council of Australia (NHMRC) |
PASS II 1998.
| Trial name or title | Piracetam Acute Stroke Study II |
| Methods | Double‐blind, 2 parallel group, placebo‐controlled, multicentre trial |
| Participants | Acute stroke patients |
| Interventions | Piracetam iv or placebo |
| Outcomes | Primary outcome: aphasia at 4 and 12 weeks by the Frenchay Aphasia Screening Test |
| Starting date | 1999 |
| Contact information | Prof JM Orgogozo, Hospital Pellegrin, Place Amelie Raba‐Leon, France |
| Notes | Funding: UCB SA Belgium |
SCAST 2005.
| Trial name or title | Scandinavian Candesartan Acute Stroke Trial |
| Methods | Randomised, double‐blind, placebo‐controlled, multicentre phase III trial |
| Participants | Patients presenting with acute stroke within 30 hours and having a SBP ≥ 140 mmHg |
| Interventions | Candesarten cilexetil po (dose increasing from 4 to 16 mg daily) or placebo for 7 days |
| Outcomes | Primary outcome: death or disability at 6 months; combination of vascular death, MI or stroke during the first 6 months Secondary outcome: SSS at 7 days and 6 months; mRS at 1, 3 and 6 months; MMS score at 6 months; EuroQol score at 6 months |
| Starting date | 2005 |
| Contact information | Rune Aakvik, Department of Internal Medicine, University Hospital, N‐0407, Oslo, Norway |
| Notes | Size: 2500 participants Funding: Helse Øst RHF, AstraZeneca |
TAST 2007.
| Trial name or title | Effect of an angiotensin receptor antagonist on cerebral blood flow, cerebral perfusion pressure, and systemic and peripheral haemodynamics in patients with acute stroke |
| Methods | Single centre, interventional, randomised, double‐blind, placebo‐controlled trial |
| Participants | Ischaemic or haemorrhagic stroke within 5 days and systolic blood pressure > 140 mmHg |
| Interventions | Telmisartan 80 mg once a day or matched placebo |
| Outcomes | Quantitative cerebral blood flow (xenon CT figure) before and 1.5 hours after first treatment |
| Starting date | 2007 |
| Contact information | Division of Stroke Medicine, Clinical Sciences Building, City Hospital, Nottingham, NG5 1PB, United Kingdom |
| Notes | Funding: British Heart Foundation, University of Nottingham |
BP: blood pressure CCB: calcium channel blocker CT: computerised tomography ICH: intracerebral haemorrhage iv: intravenous MI: myocardial infarction MMS: Mini‐Mental State mRS: modified Rankin Score NIH: National Institutes of Health rCBF: regional cerebral blood flow SBP: systolic blood pressure SSS: Scandinavian Stroke Scale
Contributions of authors
Philip Bath was involved with the design, development of search strategies, analysis and writing. He is the study guarantor. Chamila Geeganage was involved with searches for studies, input of data into the latest version, analysis of the latest version, and writing.
Sources of support
Internal sources
No sources of support supplied
External sources
Trent NHS Executive (1998 to 2000), UK.
The Stroke Association (1998 ongoing), UK.
South Thames NHS Executive (1995 to 1997), UK.
Wolfson Foundation (1993 to 1998), UK.
Declarations of interest
PMW Bath was involved in three completed studies included in this review. He is the principal investigator of the ongoing Efficacy of Nitric Oxide in Stroke (ENOS) trial.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
ACCESS 2003 {published data only}
- Schrader J. The ACCESS study: evaluation of acute candesartan cilexetil therapy in stroke survivors. Stroke 2003;34:1699‐703. [DOI] [PubMed] [Google Scholar]
Ahmed 2000 1 mg {published and unpublished data}
- Ahmed N, Nasman P, Wahlgren NG. Effects of intravenous nimodipine on blood pressure and outcome after acute stroke. Stroke 2000;31:1250‐5. [DOI] [PubMed] [Google Scholar]
Ahmed 2000 2 mg {published and unpublished data}
- Ahmed N, Nasman P, Wahlgren NG. Effects of intravenous nimodipine on blood pressure and outcome after acute stroke. Stroke 2000;31:1250‐5. [DOI] [PubMed] [Google Scholar]
- Wahlgren NG, MacMahon DG, Keyser JD, Indredavik B, Ryman T. Intravenous nimodipine West European stroke trial (INWEST) of nimodipine in the treatment of acute ischaemic stroke. Cerebrovascular Diseases 1994;4:204‐10. [Google Scholar]
ASCLEPIOS 1990 {unpublished data only}
- Azcona A, Lataste X. Isradipine in patients with acute ischaemic cerebral infarction. Drugs 1990;40 Suppl 2:52‐7. [DOI] [PubMed] [Google Scholar]
Barer 1988 atenolol {unpublished data only}
- Barer DH, Cruickshank JM, Ebrahim SB, Mitchell JRA. Low dose beta blockade in acute stroke (BEST trial): an evaluation. BMJ 1988;296:737‐41. [DOI] [PMC free article] [PubMed] [Google Scholar]
Barer 1988 propanolol {unpublished data only}
- Barer DH, Cruickshank JM, Ebrahim SB, Mitchell JRA. Low dose beta blockade in acute stroke (BEST trial): an evaluation. BMJ 1988;296:737‐41. [DOI] [PMC free article] [PubMed] [Google Scholar]
Barer 1988/50 mg {published data only}
- Barer DH, Cruickshank JM, Ebrahim SB, Mitchell JRA. Low dose beta blockade in acute stroke (BEST trial): an evaluation. BMJ 1988;296:737‐41. [DOI] [PMC free article] [PubMed] [Google Scholar]
Barer 1988/80 mg {published data only}
- Barer DH, Cruickshank JM, Ebrahim SB, Mitchell JRA. Low dose beta blockade in acute stroke (BEST trial): an evaluation. BMJ 1988;296:737‐41. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bath 2000 {unpublished data only}
- Bath PMW, Pathansali R, Iddenden R, Bath FJ. The effect of transdermal glyceryl trinitrate, a nitric oxide donor, on blood pressure and platelet function in acute stroke. Cerebrovascular Diseases 2001;11:265‐72. [DOI] [PubMed] [Google Scholar]
Bogousslavsky 1990 {published data only}
- Bogousslavsky J, Regli F, Zumstein V, Kobberling W. Double‐blind study of nimodipine in non‐severe stroke. European Neurology 1990;30:23‐6. [DOI] [PubMed] [Google Scholar]
Dyker 1997 {published and unpublished data}
- Dyker AG, Grosset DG, Lees K. Perindopril reduces blood pressure but not cerebral blood flow in patients with recent cerebral ischemic stroke. Stroke 1997;28:580‐3. [DOI] [PubMed] [Google Scholar]
Eames 2005 {published and unpublished data}
- Eames PJ, Robinson TG, Panerai RB, Potter JF. Bendrofluazide fails to reduce elevated blood pressure levels in the immediate post‐stroke period. Cerebrovascular Diseases 2005;19:253‐9. [DOI] [PubMed] [Google Scholar]
Eveson 2007 {published and unpublished data}
- Eveson DJ, Robinson TG, Potter JF. Lisinopril for the treatment of hypertension within the first 24 hours of acute ischemic stroke and follow‐up. American Journal of Hypertension 2007;20:270‐7. [DOI] [PubMed] [Google Scholar]
Fagan 1988/120 mg {published data only}
- Fagan SC, Gengo FM, Bates V, Levine SR, Kinkel WR. Effect of nimodipine on blood pressure in acute ischemic stroke in humans. Stroke 1988;19:401‐2. [DOI] [PubMed] [Google Scholar]
Fagan 1988/240 mg {published data only}
- Fagan SC, Gengo FM, Bates V, Levine SR, Kinkel WR. Effect of nimodipine on blood pressure in acute ischemic stroke in humans. Stroke 1988;19:401‐2. [DOI] [PubMed] [Google Scholar]
German‐Austrian 120mg {published data only}
- Hacke W. German‐Austrian‐Multicenter Nimodipine Stroke Study. Neurology India 1989;3 Suppl:246. [Google Scholar]
- Hennerici MG, German‐Austrian Multicenter Nimodipine Stroke Study Group. Nimodipine in patients with acute ischemic stroke. Stroke 1990;21 Suppl:I‐127. [Google Scholar]
- Hornig CR, Kaps M, Kramer G, Busse O, Aichner F. Nimodipine in acute ischaemic stroke. Results of the Nimodipine German Austrian Stroke Trial. Stroke 1991;22:153. [Google Scholar]
- Kramer G, Tattenborn B, Rothacher G, Hacke W, Busse O, Hornig C, et al. Nimodipine German Austrian Stroke Trial. Neurology 1990;40 Suppl:415. [Google Scholar]
- Kramer G, Tettenborn B, Schmutzhard E, Aichner F, Schwartz A, Busse O, et al. Nimodipine in acute ischemic stroke. Cerebrovascular Diseases 1994;4:182‐8. [Google Scholar]
Gray 2007 {published data only}
- Gray CS, Hildreth AJ, Sandercock PA, O'Connell JE, Johnston DE, Cartlidge NEF, et al. Glucose‐potasium‐insulin infusions in the management of post‐stroke hyperglycaemia: the UK glucose insulin in stroke trial. Lancet Neurology 2007;6:397‐406. [DOI] [PubMed] [Google Scholar]
Herrschaft 1988 {published and unpublished data}
- Herrschaft H. The effectiveness of piracetam in acute cerebral ischemia in the human. A clinical controlled double‐blind study of piracetam/10% dextran 40 versus 10% dextran 40/placebo [Die wirksamkeit von piracetam bei der akuten zerebralen ischamie des menschen]. Medizinische Klinik 1988;83:667‐77. [PubMed] [Google Scholar]
Hillis 2003 {published and unpublished data}
- Hillis AE, Ulatowski JA, Barker PB, Torbey M, Ziai W, Beauchamp NJ, et al. A pilot randomised trial of induced blood pressure elevation: effects on function and focal perfusion in acute and subacute stroke. Cerebrovascular Diseases 2003;16:236‐46. [DOI] [PubMed] [Google Scholar]
Hsu 1987 {published data only}
- Hsu CY, Faught RE, Furlan AJ, Coull BM, Huang DC, Hogan EL, et al. Intravenous prostacyclin in acute nonhaemorrhagic stroke: a placebo‐controlled double‐blind trial. Stroke 1987;18:352‐8. [DOI] [PubMed] [Google Scholar]
Huczynski 1988 {published data only}
- Huczynski J, Gryglewski J, Trabka EK, Kiec AD, Mazur EP, Sotowska W, et al. Use of prostacyclin in patients with ischemic stroke. A double‐blind method II [Zastosowanie prostacykliny u chorych z udarem niedokrwiennym mozgu. Proba podwojnie slepa II]. Neurologia i Neurochirurgia Polska 1988;22(4):299‐304. [PubMed] [Google Scholar]
- Huczynski J, Kostka‐Trabka E, Sotowska W, Bieron K, Grodzinska L, Dembinska‐Kiec A, et al. Double‐blind controlled trial of the therapeutic effects of prostacyclin in patients with completed ischaemic stroke. Stroke 1985;16:810‐4. [DOI] [PubMed] [Google Scholar]
IMAGES Pilot {unpublished data only}
INTERACT pilot 2008 {published data only}
- Anderson CS, Huang Y, Wang JG, Arima H, Neal B, Peng B, et al. Intensive blood pressure reduction in acute cerebral haemorrhage trial (INTERACT): a randomised pilot trial. Lancet Neurology 2008;7(5):391‐9. [DOI] [PubMed] [Google Scholar]
Kaste 1994/120 mg {published and unpublished data}
- Kaste M, Fogelholm R, Erila T, Palomaki H, Murros K, Rissanen A, et al. A randomized, double‐blind, placebo‐controlled rial of nimodipine in acute ischemic hemispheric stroke. Stroke 1994;25:1348‐53. [DOI] [PubMed] [Google Scholar]
Lamsudin 1997 {published data only}
- Lamsudin R, Misbach J, Andradi, Petru Cahyadi A, Hadirioto S, Sumaryanto F. A controlled trial of nimodipine in acute ischaemic stroke. A multicenter Indonesian Neurological Association Study Groups. Indonesian Journal of Clinical Epidemiology and Biostatistics 1997;2:1. [Google Scholar]
Lees 1995 {published and unpublished data}
- Muir KW, Lees KR. A randomized, double‐blind, placebo‐controlled pilot trial of intravenous magnesium sulphate in acute stroke. Stroke 1995;26:1183‐8. [DOI] [PubMed] [Google Scholar]
Limburg 1990 {published data only}
- Limburg M, Hijdra A. Flunarizine in acute ischemic stroke: a pilot study. European Neurology 1990;30:121‐2. [DOI] [PubMed] [Google Scholar]
Lisk 1993 {published data only}
- Lisk DR, Grotta JC, Lamki LM, Tran HD, Taylor JW, Molony DA, et al. Should hypertension be treated after acute stroke? A randomized controlled trial using SPECT. Canadian Journal of Neurological Sciences 1993;Suppl 4:S246. [DOI] [PubMed] [Google Scholar]
- Lisk DR, Grotta JC, Lamki LM, Tran HD, Taylor JW, Molony DA, et al. Should hypertension be treated after acute stroke? A randomized controlled trial using single photon emission computed tomography. Archives of Neurology 1993;50:855‐62. [DOI] [PubMed] [Google Scholar]
Lowe 1993 {unpublished data only}
- Lowe DGO, Forbes CD. Nimodipine in acute cerebral hemispheric infarction. Unpublished report BAY e 9736/0449.
Martinez‐Vila 1990 {published data only}
- Martinez‐Vila E, Guillen F, Villanueva JA, Matias‐Guiu J, Bigorra J, Gil P, et al. Placebo‐controlled trial of nimodipine in the treatment of acute ischemic cerebral infarction. Stroke 1990;21:1023‐8. [DOI] [PubMed] [Google Scholar]
- Martinez‐Vila E, Martinez‐Lage J, Guillen F, Villanueva JA, Matias‐Guiu J, Biggorra J. Nimodipine in acute ischaemic stroke. New England Journal of Medicine 1988;319:249. [Google Scholar]
- Martinez‐Vila E, Matias‐Guiu J, Guillen F, Villanueva JA, Biggora J, Martinez‐Lage J. Nimodipine in acute ischaemic stroke. A controlled trial. Neurology India 1989;37 Suppl:148. [Google Scholar]
Muir 1995 {published and unpublished data}
- Muir KW, Lees KR. Dose optimization of intravenous magnesium sulphate after acute stroke. Stroke 1998;29:918‐23. [DOI] [PubMed] [Google Scholar]
- Muir KW, Lees KR. Dose‐ranging study of magnesium sulphate after acute stroke. European Journal of Neurology 1995;2 Suppl 2:7‐8. [Google Scholar]
Norris 1994 {published and unpublished data}
- Norris JW, Brun LH, Anderson BA. Intravenous nimodipine in acute ischaemic stroke. Cerebrovascular Diseases 1994;4:194‐6. [Google Scholar]
Paci 1989/120 mg {published data only}
- Paci A, Ottaviano P, Trenta A, Iannone G, Santis L, Lancia G, et al. Nimodipine in acute ischemic stroke: a double‐blind controlled study. Acta Neurologica Scandinavica 1989;80:282‐6. [DOI] [PubMed] [Google Scholar]
PASS 1995 {published and unpublished data}
- Deyn P‐P, Ruck J, Orgogozo J‐M, Deberdt W, for the Piracetam Study Group. The Piracetam in Acute Stroke Study (PASS). Stroke 1996;27(1):196. [Google Scholar]
- PASS study. Treatment of acute ischemic stroke with piracetam. Stroke 1997;28:2347‐52. [DOI] [PubMed] [Google Scholar]
Pokrupa 1986 {published and unpublished data}
- Pokrupa R, Hakim A, Villanueva J, Francis G, Wolfe L. Clinical study of prostacyclin infusion after acute ischemic stroke. Canadian Journal of Neurological Sciences 1986;13(2):165. [Google Scholar]
- Pokrupa RP, Hakim AM, Villanueva JA, Diksic M, Evans AE, Meyer E, et al. Prostacyclin infusion after acute cerebral infarction: clinical and PET studies. Unpublished manuscript.
Potter 2009 labetalol {published data only}
- Potter JF, Robinson TG, Ford GA, Mistri A, James M, Chernova J, et al. Controlling hypertension and hypotension immediately post‐stroke (CHHIPS): a randomised, placebo‐controlled, double‐blind pilot trial. Lancet Neurology 2008;8:48‐56. [DOI] [PubMed] [Google Scholar]
Potter 2009 lisinopril {published data only}
- Potter JF, Robinson TG, Ford GA, Mistri A, James M, Chernova J, et al. Controlling hypertension and hypotension immediately post‐stroke (CHHIPS): a randomised, placebo‐controlled, double‐blind pilot trial. Lancet Neurology 2008;8:48‐56. [DOI] [PubMed] [Google Scholar]
PRISTINE {unpublished data only}
- Steiner TJ. Naftidrofuryl in the treatment of acute cerebral hemisphere infarction (ACHI). Stroke 1996;27(1):195. [Google Scholar]
Rashid 2003 10 mg {published and unpublished data}
- Rashid P, Weaver C, Leonardi‐Bee J, Bath F, Fletcher S, Bath P. The effects of transdermal glyceryl trinitrate, a nitric oxide donor, on blood pressure, cerebral and cardiac haemodynamics, and plasma nitric oxide levels in acute stroke. Journal of Stroke and Cerebrovascular Diseases 2003;12(3):143‐51. [DOI] [PubMed] [Google Scholar]
Rashid 2003 5 mg {published and unpublished data}
- Rashid P, Weaver C, Leonardi‐Bee J, Bath F, Fletcher S, Bath P. The effects of transdermal glyceryl trinitrate, a nitric oxide donor, on blood pressure, cerebral and cardiac haemodynamics, and plasma nitric oxide levels in acute stroke. Journal of Stroke and Cerebrovascular Diseases 2003;12(3):143‐51. [DOI] [PubMed] [Google Scholar]
Rashid 2003 5/10 mg {published and unpublished data}
- Rashid P, Weaver C, Leonardi‐Bee J, Bath F, Fletcher S, Bath P. The effects of transdermal glyceryl trinitrate, a nitric oxide donor, on blood pressure, cerebral and cardiac haemodynamics, and plasma nitric oxide levels in acute stroke. Journal of Stroke and Cerebrovascular Diseases 2003;12(3):143‐51. [DOI] [PubMed] [Google Scholar]
Saxena 1999 100 mg {published data only}
- Saxena R, Wijnhoud AD, Carton H, Hacke W, Kaste M, Przybelski RJ, et al. Controlled safety study of a hemoglobin‐based oxygen carrier, DCLHb, in acute ischemic stroke. Stroke 1999;30:993‐6. [DOI] [PubMed] [Google Scholar]
Saxena 1999 25 mg {published data only}
- Saxena R, Wijnhoud AD, Carton H, Hacke W, Kaste M, Przybelski RJ, et al. Controlled safety study of a hemoglobin‐based oxygen carrier, DCLHb, in acute ischemic stroke. Stroke 1999;30:993‐6. [DOI] [PubMed] [Google Scholar]
Saxena 1999 50 mg {published data only}
- Saxena R, Wijnhoud AD, Carton H, Hacke W, Kaste M, Przybelski RJ, et al. Controlled safety study of a hemoglobin‐based oxygen carrier, DCLHb, in acute ischemic stroke. Stroke 1999;30:993‐6. [DOI] [PubMed] [Google Scholar]
Squire 1996 {published and unpublished data}
- Squire IB, Lees KR, Pryse‐Phillips W, Kertesz A, Bamford J. The effects of lifarizine in acute cerebral infarction: a pilot safety study. Cerebrovascular Diseases 1996;6:156‐60. [Google Scholar]
- Squire IB, Lees KR, Pryse‐Phillips W, Kertesz A, Bamford J, for the lifarizine Study Group. The effects of lifarizine in acute cerebral infarction: a pilot study. Annals of the New York Academy of Sciences 1995;765:317‐8. [DOI] [PubMed] [Google Scholar]
Steiner 1986 {published and unpublished data}
- Steiner TJ, Rose C. Randomized double‐blind placebo controlled clinical trial of naftidrofuryl in hemiparetic CT‐proven acute cerebral hemisphere infarction. Royal Society of Medicine Internal Congress Symposium (Series No 99, 85). 1986:85‐98.
- Steiner TJ, Rose C. Towards a model stroke trial. The single‐centre naftidrofuryl study. Neuroepidemiology 1986;5:121‐47. [DOI] [PubMed] [Google Scholar]
Strand 1984 {published and unpublished data}
- Strand T, Wester PO, CVD Group. A double blind randomized pilot trial of magnesium therapy in acute cerebral infarction. Proceedings of the 7th Scandinavian Meeting on Cerebrovascular Disease. Finland, Jyvaskyla, 14‐17 August 1993:37 (Abstract 19).
- Wester PO, Asplund K, Eriksson S, Hagg E, Lithner F, Strand T. Infusion of magnesium in patients with acute brain infarction. Acta Neurologica Scandinavica 1984;70(2):143. [DOI] [PubMed] [Google Scholar]
Uzuner 1995/180 mg {published data only}
- Uzuner N, Ozdemir G, Gucuyener D. The interaction between nimodipine and systemic blood pressure and pulse rate. Pan‐European Consensus Meeting on Stroke Management, Helsingborg, Sweden. 8‐10 November, 1985.
VENUS 1995 {unpublished data only}
- Limburg M, Horn J, Vermeulen M, for the VENUS Group. VENUS: Very Early Nimodipine Use in Stroke. Stroke 1995;26(2):353. [Google Scholar]
Walters 2006 {published and unpublished data}
- Walters MR, Weir CJ, Lees KR. A randomised controlled pilot study to investigate the potential benefit of intervention with insulin in hyperglycaemic acute ischaemic stroke patients. Cerebrovascular Diseases 2006;22:116‐22. [DOI] [PubMed] [Google Scholar]
Willmot 2006 {published and unpublished data}
- Willmot M, Ghadami A, Whysall B, Clarke W, Wardlaw J, Bath PMW. Transdermal glyceryl trinitrate lowers blood pressure and maintains cerebral blood flow in recent stroke. Hypertension 2006;47:1209‐15. [DOI] [PubMed] [Google Scholar]
Wimalarat 1994/120mg {published and unpublished data}
- Wimalaratna HSK, Capildeo R. Nimodipine in acute ischaemic cerebral hemisphere infarction. Cerebrovascular Diseases 1994;4:179‐81. [Google Scholar]
- Wimalaratna HSK, Capildeo R. Nimodipine in acute ischaemic stroke. Journal of Neurology 1990;237:146. [Google Scholar]
- Wimalaratna HSK, Capildeo R. What dosage of nimodipine for stroke? A randomised double‐blind controlled trial comparing two doses of nimodipine in acute ischaemic stroke. Neurology India 1998;37 Suppl:147. [Google Scholar]
Wimalarat 1994/240mg {published data only}
- Wimalaratna HSK, Capildeo R. Nimodipine in acute ischaemic stroke. Journal of Neurology 1990;237:146. [Google Scholar]
- Wimalaratna HSK, Capildeo R. Nimodopine in acute ischaemic cerebral hemisphere infarction. Cerebrovascular Diseases 1994;4:179‐81. [Google Scholar]
- Wimalaratna HSK, Capildeo R. What dosage of nimodipine for stroke? A randomised double‐blind controlled trial comparing two doses of nimodipine in acute ischaemic stroke. Neurology India 1998;37 Suppl:147. [Google Scholar]
References to studies excluded from this review
Albers 1995a {published data only}
- Albers GW, Atkinson RP, Kelley RE, Rosenbaum DM, the Dextrophan Study Group. Safety, tolerability, and pharmacokinetics of the N‐methyl‐D‐aspartate antagonist dextrorphan in patients with acute stroke. Stroke 1995;26:254‐8. [DOI] [PubMed] [Google Scholar]
Albers 1995b {published data only}
- Dextrorphan Study Group, Hoffmann‐La Roche. Safety tolerability and pharmacokinetics of the N‐methyl‐D‐aspartate antagonist R0‐01‐6794/706 in patients with acute ischemic stroke. Annals of the New York Academy of Science 1995;15(765):249‐61. [PubMed] [Google Scholar]
Ameriso 1992 {published data only}
- Ameriso SF, Wenby RB, Meiselman HJ, Fisher M. Nimodipine and the evolution of haemorrheological variables after acute ischemic stroke. Journal of Stroke and Cerebrovascular Diseases 1992;2:22‐5. [DOI] [PubMed] [Google Scholar]
ANS 1992 {published data only}
- American Nimodipine Study Group. Clinical trial of nimodipine in acute ischaemic stroke. Stroke 1992;23:3‐8. [DOI] [PubMed] [Google Scholar]
ATTACH 2006 {published data only}
- Qureshi AI. ATTACH ‐ Antihypertensive Treatment in Acute Cerebral Hemorrhage. Stroke Trials Directory, Internet Stroke Center: www.strokecenter.org/trials/. 2007.
- Qureshi AI. Antihypertensive Treatment of Acute Cerebral Hemorrhage (ATACH). Neurocritical Care 2007;6:56‐66. [DOI] [PubMed] [Google Scholar]
Autret 1992 {published data only}
- Autret A, Lehert P, Monsnier M. Study of naftidrofuryl in cerebral ischaemic accidents. Psychologie Medicale 1992;24(1):103‐14. [Google Scholar]
Bogousslavsky 2002 {published data only}
- Bogousslavsky J, Victor SJ, Salinas EO, Pallay A, Donnan GA, Fieschi C, et al. Fiblast (trafermin) in acute stroke: results of the European‐Australian phase II/III safety and efficacy trial. Cerebrovascular Diseases 2002;14:239‐51. [DOI] [PubMed] [Google Scholar]
Britton 1980 {published data only}
- Britton M, Faire U, Helmers C, Miah K, Rane A. Lack or effect of theophylline on the outcome of acute cerebral infarction. Acta Neurologica Scandinavica 1980;62:116‐23. [DOI] [PubMed] [Google Scholar]
Brola 1998 {published data only}
- Brola W, Czernicki J. The effect of pentoxifylline on treatment results and course of rehabilitation of patients after ischemic stroke. Polski Merkuriusz Lekarski 1998;4(21):116‐8. [PubMed] [Google Scholar]
Busse 1985 {published data only}
- Busse O, Hornig CR, Agnoli AL. Behandlung des ischamischen hirninfarktes mit naftidrofuryl eine randomisierte doppel blindstudie. Therapiewoche 1985;35:4147‐54. [Google Scholar]
Cao 2003 {published data only}
- Cao F, Sun S‐G, Tong E‐T, Luo F, Wang J. Clinical application of nimodipine in the treatment of acute intracerebral haemorrhage. Herald of Medicine (Yi Yao Dao Bao) 2003;22(2):82‐4. [Google Scholar]
Capon 1983 {unpublished data only}
- Bayer AG, Wuppertal, Germany. Unpublished work. Unpublished 1984.
CARING 2005 {published data only}
- Wang DZ, Honings D, Mathews M, Milbrandt J, Graumlich J, Avelino R. Open‐label prospective study to evaluate the efficacy and safety of double or triple concentrated intravenous nicardipine for treatment of hypertension in patients with ischemic stroke, intracerebral haemorrhage or subarachnoid hemorrhage ‐ the CARING trial. International Stroke Conference. 2005.
Chan 1993 {published data only}
- Chan YW, Kay CS. Pentoxifylline in the treatment of acute ischaemic stroke ‐ a reappraisal in Chinese stroke patients. Clinical and Experimental Neurology 1993;30:110‐6. [PubMed] [Google Scholar]
Chandra 1995 {published data only}
- Chandra B. A new form of management of stroke. Journal of Stroke and Cerebrovascular Diseases 1995;5:241‐3. [DOI] [PubMed] [Google Scholar]
- Chandra B. Nimodipine in the management of stroke. Journal of Neurology 1994;241 Suppl 1:S30. [Google Scholar]
CHERISH 2006 {published data only}
- Hong K‐S, Park J‐M, Kang D‐W, Lee Y‐S, Koo J‐S, Rha J‐H. Cilnidipine effect on high blood pressure and cerebral perfusion in ischemic stroke patients with hypertension. Proceedings of the International Stroke Conference. 2006.
- Park S‐H. CHERISH. Cilnidipine effect on high blood pressure and cerebral perfusion in ischemic stroke patients with hypertension. Stroke Trials Directory, Internet Stroke Center: www.strokecenter.org/trials/ 2006.
Davalos 1989 {published data only}
- Davalos A, Cendra E, Gonzalez B, Genis D, Teruel J, Ruibal A. Double‐blind randomized clinical trial of nicardipine vs placebo in acute ischemic stroke: clinical, radiological and biochemical evaluation of the ischaemic area. Preliminary results. Neurology India 1998;37 Suppl:283. [Google Scholar]
- Davalos EA, Cendra E, Genis D, Teruel J, Ruibal A, Musoles SD. Double blind controlled trial of nicardipine versus placebo in the treatment of the acute phase of cerebral infarction. Neurologia 1992;7(6):157. [Google Scholar]
Dekoninck 1978 {published data only}
- Dekoninck WJ, Jocquet P, Jacquy J, Henriet M. Comparative study of the clinical effect of vincamine + glycerol + placebo in the acute phase of stroke. Arzneimittel Forschung 1978;28:1654‐7. [PubMed] [Google Scholar]
Domzal 1986 {published data only}
- Domzal T, Kozlowski P, Zaleska B. Cavinton in the treatment of ischaemic cerebral stroke. Clinical and computerized‐tomographic evaluation. Neurologia i Neurochirurgia Polska 1986;20(3):234‐40. [PubMed] [Google Scholar]
FIST 1996 {published data only}
- Franke CL, Palm R, Dalby M, Schoonderwaldt HC, Hantson L, Eriksson B, et al. Flunarazine in stroke treatment (FIST): a double‐blind, placebo‐controlled trial in Scandinavia and the Netherlands. Acta Neurologica Scandinavica 1996;93:56‐60. [DOI] [PubMed] [Google Scholar]
Galeas 1998 {published data only}
- Galeas TH, Ziogas G, Valotasiou A, Galea B, Karapanos F, Lappas H. The role of magnesium (Mg) ‐ a national calcium (Ca) antagonist on the Ca channels of the patients in the treatment of acute ischaemic stroke. Proceedings of the Consensus Conference on Medical Management of Stroke, Royal College of Physicians of Edinburgh. May 1998.
Gamez 1988 {published data only}
- Marin Gamez N, Sota Mas JA, Aguilar Martinez JL, Bermudez Garcia JM, Salim A, Ramos Jimenez A, et al. A controlled double blind clinical trial of nicardipine versus placebo in acute focal cerebral ischaemia. Medicina Clinica 1988;90:690‐2. [PubMed] [Google Scholar]
Geismar 1976 {published data only}
- Geismar P, Marquardsen J, Sylvest J. Controlled trial of intravenous aminophylline in acute cerebral infarction. Acta Neurologica Scandinavica 1976;54:173‐80. [DOI] [PubMed] [Google Scholar]
Gelmers 1984/120 {published data only}
- Gelmers HJ. The effects of nimodipine on the clinical course of patients with acute ischemic stroke. Acta Neurologica Scandinavica 1984;69:232‐9. [DOI] [PubMed] [Google Scholar]
Gelmers 1988/120 {published data only}
- Gelmers HJ, Gorter K, Weerdt CJ, Weizer HJA. A controlled trial of nimodipine in acute ischemic stroke. New England Journal of Medicine 1988;318:203‐7. [DOI] [PubMed] [Google Scholar]
Gladstone 2006 {published data only}
- Gladstone DJ, Danells CJ, Armesto A, McIlroy WE, Staines R, Graham SJ, et al. Physiotherapy coupled with dextroamphetamine for rehabilitation after hemiparetic stroke. Stroke 2006;37:179‐85. [DOI] [PubMed] [Google Scholar]
Gray 1990 {published data only}
- Gray CS, French JM, Venables GS, Cartlidge NEF, James OFW, Bates D. A randomized double‐blind controlled trial of naftidrofuryl in acute stroke. Age and Ageing 1990;19:356‐63. [DOI] [PubMed] [Google Scholar]
Haley 1994/0.6 {published data only}
- The RANTTAS investigators. A randomized trial of tirilazad mesylate in patients with acute stroke (RANTTAS). Stroke 1996;27:1453‐8. [DOI] [PubMed] [Google Scholar]
Haley 1994/2 {published data only}
- The RANTTAS investigators. A randomized trial of tirilazad mesylate in patients with acute stroke (RANTTAS). Stroke 1996;27:1453‐8. [DOI] [PubMed] [Google Scholar]
Haley 1994/6 {published data only}
- The RANTTAS investigators. A randomized trial of tirilazad mesylate in patients with acute stroke (RANTTAS). Stroke 1996;27:1453‐8. [DOI] [PubMed] [Google Scholar]
Hartmann 2005 {published data only}
- Hartmann A, Pavlidis C, Dettmers C, Rommel T. The effect of antihypertensive drugs on intracranial pressure and cerebral blood flow in patients with acute stroke. Cerebrovascular Diseases 2005;19 Suppl 2:Abst 6. [Google Scholar]
Hoechst 1986 {published data only}
- Aschenbrenner KM. Addendum to the final report. Efficacy, safety and tolerance of Trental (pentoxifylline) in the treatment of acute non‐hemorrhagic focal cerebral infarction (12 ‐ 36 hours after onset). HRPI protocol 402 B ‐ modified, Hoechst AG 1987.
- Hentschel B, Forthofer R. Efficacy, safety and tolerance of Trental (pentoxifylline) in the treatment of acute non‐hemorrhagic focal cerebral infarction (12‐36 hours after onset). HRPI protocol 402 B ‐ modified. Final report. Hoechst 1986.
Holthoff 1990 {published data only}
- Heiss WD, Holthoff V, Pawlik G, Neveling M. Effect of nimodipine on regional cerebral glucose metabolism in patients with acute ischaemic stroke as measured by positron emission tomography. Journal of Cerebral Blood Flow and Metabolism 1990;10:127‐32. [DOI] [PubMed] [Google Scholar]
- Holthoff V, Beil C, Hartmann‐Klosterkotter U, Neveling M, Pawlik G, Herholz K, et al. Effect of nimodipine on glucose metabolism in the course of ischemic stroke. Stroke 1990;21 Suppl IV:IV 95‐7. [PubMed] [Google Scholar]
Hsu 1988 {published data only}
- Hoechst. Clinical/statistical report for pentoxifylline (Trental, BL 191). Protocol 402A. Hoechst report 1988.
- Hsu CY, Norris JW, Hogan EL, Bladin P, Dinsdale HB, Yatsu FM, et al. Pentoxifylline in acute nonhemorrhagic stroke. A randomised, placebo‐controlled double‐blind trial. Stroke 1988;19(6):716‐22. [DOI] [PubMed] [Google Scholar]
- Pentoxifylline Study Group. Pentoxifylline (PTX) in acute ischemic stroke. Stroke 1987;18(1):298. [Google Scholar]
Huber 1993 {published data only}
- Huber M, Hojer C, Fink G R, Neveling B, Kittner B, Heiss WD. Adenosine reuptake inhibition by propentofylline improves brain glucose metabolism as measured by FDG‐PET in human acute ischemic stroke. A randomized, placebo‐controlled, double‐blind study. 2nd International Conference on Stroke, Geneva 1993;124:2. [Google Scholar]
- Huber M, Kittner B, Hojer C, Fink GR, Neveling M, Heiss WD. Effect of propentofylline on regional cerebral glucose metabolism in acute ischemic stroke. Journal of Cerebral Blood Flow and Metabolism 1993;13:526‐30. [DOI] [PubMed] [Google Scholar]
IMAGES 2004 {published data only}
- IMAGES investigators. Magnesium for acute stroke (intravenous magnesium efficacy in stroke trial): randomised controlled trial. Lancet 2004;363:439‐45. [DOI] [PubMed] [Google Scholar]
Infeld 1999 {published data only}
- Infeld B, Davis SM, Donnan AD, Yasaka M, Lichtenstein M, Mitchell PJ, et al. Nimodipine and perfusion changes after stroke. Stroke 1999;30:1417‐23. [DOI] [PubMed] [Google Scholar]
Karoutas 1990 {published data only}
- Karoutas G. A randomized, double‐blind, placebo‐controlled study of piracetam in patients with acute ischaemic cerebral infarct in the carotid territory. Proceedings of Piracetam Symposium, Athens. 30 April 1990.
Kornhuber 1993 {published data only}
- Kornhuber HH, Hartung J, Herrlinger JD, Hertel G, Hulser PJ, Prange H, et al. Flunarazine in ischemic stroke: a randomised, multicentre, placebo‐controlled, double blind study. Neurological and Psychological Brain Research 1993;1:173‐80. [Google Scholar]
- Prange H, Hartung J, Hertel G, Herrlinger D, Hulser G, Kornhuber HH, et al. Treatment of acute stroke with flunarizine iv. Proceedings of the International Conference on Stroke, Geneva. 1991:39.
Lampl 2001 {published data only}
- Lampl Y, Gilad R, Geva D, Eshel Y, Sadeh M. Intravenous administration of magnesium sulphate in acute stroke: a randomised double blind study. Clinical Neuropharmacology 2001;24(1):11‐5. [DOI] [PubMed] [Google Scholar]
Lipani 1984 {unpublished data only}
- Lipani G. Clinical/experimental evaluations of the therapeutic efficacy and tolerability of the medical preparation "Cavinton" of Ayerst Italiana S.p.A. RGD document No 30709 1984.
Martin 1985 {published data only}
- Martin JF, Hamdy N, Nicholl J, Lewtas N, Bergvall U, Owen P, et al. Double‐blind controlled trial of prostacyclin in cerebral infarction. Stroke 1985;16:386‐90. [DOI] [PubMed] [Google Scholar]
Martinsson 2002 {published data only}
- Martinsson L, Wahlgren NG. Safety of dexamphetamine in acute ischaemic stroke: a randomised, double blind, controlled dose‐escalation trial. Stroke 2003;34:475‐81. [DOI] [PubMed] [Google Scholar]
MAST‐I {published data only}
- Multicentre Acute Stroke Trial‐Italy (MAST‐I) Group. Randomised controlled trial of streptokinase, aspirin and combination of both in treatment of acute ischaemic stroke. Lancet 1995;346:1509‐14. [PubMed] [Google Scholar]
Meier 1991 {published data only}
- Meier F, Wessel G, Thiele R, Gottschild D, Brandstatt H. Induced hypertension as an approach to treating acute cerebrovascular ischaemia: possibilities and limitations. Experimental Pathology 1991;42:257‐63. [DOI] [PubMed] [Google Scholar]
Miller 1984 {published data only}
- Miller VT, Coull BM, Yatsu FM, Shah AB, Beamer NB. Prostacyclin infusion in acute cerebral infarction. Neurology 1984;34:1431‐5. [DOI] [PubMed] [Google Scholar]
Ming 1990 {published data only}
- Ming A, Fritz VU, Winterton R, Esser J, Hinton S. Piracetam versus placebo in first, acute, non‐haemorrhagic, carotid territory stroke: a double‐blind pilot study. Proceedings of the Piracetam symposium, Athens, Greece. 1990:139‐51.
Misra 2005 {published data only}
- Misra UK, Kalita J, Ranjan P, Mandal SK. Mannitol in intracerebral haemorrhage: a randomized controlled study. Journal of the Neurological Sciences 2005;234:41‐5. [DOI] [PubMed] [Google Scholar]
Mohr 1992/120 {published data only}
- Mohr JP, The American Nimodipine study Group. Clinical trial of nimodipine in acute ischemic stroke. Stroke 1992;23:3‐8. [DOI] [PubMed] [Google Scholar]
Mohr 1992/240 {published data only}
- Mohr JP, The American Nimodipine study Group. Clinical trial of nimodipine in acute ischemic stroke. Stroke 1992;23:3‐8. [DOI] [PubMed] [Google Scholar]
Mohr 1992/60 {published data only}
- Mohr JP, The American Nimodipine study Group. Clinical trial of nimodipine in acute ischemic stroke. Stroke 1992;23:3‐8. [DOI] [PubMed] [Google Scholar]
Molnar 1979 {published data only}
- Molnar L, Vamosi B. The effect of cavinton and xavin on the redox changes in the brain of stroke patients. Proceedings of the Third Congress of the Hungarian Pharmacological Society, Budapest. 1979:369‐74.
Mousavi 2004 {published data only}
- Mousavi SA, Ziaei J, Saadatnia M. Magnesium sulphate in acute stroke: a randomised double blind clinical trial. Journal of Research in Medical Sciences 2004;4:7‐10. [Google Scholar]
Nakamura 2007 {published data only}
- Nakamura T, Uchiyama S, Tsutsumi Y, Shimizu Y, Iwata M. Renin‐angiotensin system blockade safely reduces blood pressure without affecting cerebral blood flow in patients with acute ischemic stroke. Stroke 2007;38(2):517. [DOI] [PubMed] [Google Scholar]
Nazir 2004 {published data only}
- Nazir FS, Overell JR, Bloster A, Hilditch TE, Reid JL, Lees KR. The effect of losartan on global and focal cerebral perfusion and on renal function in hypertensives in mild early ischaemic stroke. Journal of Hypertension 2004;22:989‐95. [DOI] [PubMed] [Google Scholar]
NEST 1994/120 {published and unpublished data}
- Hennerici M, Kramer G, North PM, Schmitz H, Tettenborn D. Nimodipine in the treatment of acute MCA ischaemic stroke. Cerebrovascular Diseases 1994;4:189‐93. [Google Scholar]
NIMPAS 1997 {published data only}
- Infeld B, Davis S, Donnan G. Nimodipine and perfusion after acute stroke (NIMPAS) progress report. Proceedings of the Stroke Society of Australian Annual Scientific Meeting, Sydney. 6‐7 October 1994.
Oczkowski 1989 {published data only}
- Oczkowski WJ, Hachinski VC, Bogousslavsky J, Barnett HJM, Curruthers SG. A double‐blind, randomized trial of PY108‐068 in acute ischemic cerebral infarction. Stroke 1989;20:604‐8. [DOI] [PubMed] [Google Scholar]
Ohtomo 1986 {published data only}
- Ohtomo E, Katsuzawa T, Araki S, Itoh E, Kuzuya F, Mijazaki M, et al. Clinical evaluation of LS‐121 in the treatment of cerebrovascular disorders. Clinical Evaluation 1986;14:279‐80. [Google Scholar]
Ohtomo 1987a {published data only}
- Mizushima Y, Toyota T, Okita K, Ohtomo E. Recent clinical studies on lipo‐PGE1 and lipo‐PGI2: PGE1 and PGI2 incorporated in lipid microspheres, for target delivery. Journal of Controlled Release 1994;28:243‐9. [Google Scholar]
Ohtomo 1987b {published data only}
- Ohtomo E, Nakajima K, Araki G, Itoh E, Mujazaki M, Sawach T, et al. Clinical evaluation of LS‐121 injection in the treatment of cerebral infarction and cerebral haemorrhage in acute and subacute stroke stages. Clinical Evaluation 1987;15:107‐42. [Google Scholar]
Orgogozo {unpublished data only}
- Orgogozo JM. A nicardipine stroke trial. Unpublished work 1995.
Piradov 1992 {published data only}
- Piradov MA. Piracetam in acute stroke. Cerebrovascular Diseases 1992;2:235. [Google Scholar]
Piriyawat 2003 {published data only}
- Piriyawat P, Labiche LA, Burgin WS, Aronowski JA, Grotta JC. Pilot dose‐escalation study of caffeine plus ethanol (caffeinol) in acute ischemic stroke. Stroke 2003;34:1242‐5. [DOI] [PubMed] [Google Scholar]
Platt 1993 {published data only}
- Platt D, Horn J, Summa JD, Schmitt‐Ruth R, Kauntz J, Kronert E. On the efficacy of piracetam in geriatric patients with acute cerebral ischaemia: a clinically controlled double‐blind study. Archives of Gerontology and Geriatrics 1993;16:149‐64. [DOI] [PubMed] [Google Scholar]
Popa 1995 {published data only}
- Popa G, Voiculescu V, Popa C, Stanescu A, Nistorescu A, Jepescu I. Stroke and hypertension. Antihypertensive therapy withdrawal. Romanian Journal of Neurology and Psychiatry 1995;33:29‐36. [PubMed] [Google Scholar]
Rosenbaum 1991 {published data only}
- Rosenbaum D, Zabramski J, Frey J, Yatsu F, Marler J, Spetzler R, et al. Early treatment of ischemic stroke with a calcium antagonist. Stroke 1991;22:437‐41. [DOI] [PubMed] [Google Scholar]
Saver 2004 {published data only}
- Saver JL, Kidwell C, Eckstein M, Starkman S. Prehospital neuroprotective therapy for acute stroke: results of the field administration of stroke therapy‐magnesium (FAST‐MAG) pilot trial. Stroke 2004;35:106‐8. [DOI] [PubMed] [Google Scholar]
Sherman 1986/120 {published data only}
- Sherman DG, Easton JD, Hart RG, Sherman CP, Battye R. Nimodipine in acute cerebral infarction: a double blind study of safety and efficacy. In: Battistini N, Fiorani P, Courbier R, Plum F, Fieschi C editor(s). Acute Brain Ischemia: Medical and Surgical Therapy. New York: Raven Press, 1986:257. [Google Scholar]
Sprigg 2007 {published data only}
- Sprigg N, Willmot MR, Gray LJ, Sunderland A, Pomeroy V, Walker M, et al. Amphetamine increases blood pressure and heart rate but has no effect on motor recovery or cerebral haemodynamics in ischaemic stroke: a randomised controlled trial (ISRCTN 36285333). Journal of Human Hypertension 2007;21:616‐24. [DOI] [PubMed] [Google Scholar]
Su 2004 {published data only}
- Su W, Luo S, Cai K, Gao P, Chu D‐F, Wang X‐D. Effect of flunarizine on ischemic penumbra in patients with acute ischemic cerebral infarction. Chinese Journal of Clinical Rehabilitation 2004;8(31):6948‐9. [Google Scholar]
Suslina 1999 {published data only}
- Suslina ZA, Fedorova TN, Kistenev BA, Khrapova EV, Maksimova MYU. Dynamics of lipid peroxidation in patients with acute ischemic stroke. Zhurnal Nevrologii I Psikhiatrii Imeni S.S. Korsakova 1999;99(7):33‐6. [PubMed] [Google Scholar]
Szakall 1998 {published data only}
- Szakall S, Boros I, Balkay L, Emri M, Fekete I, Kerenyi L, et al. Cerebral effects of a single dose of intravenous vinpocetine in chronic stroke patients: a PET Study. Journal of Neuroimaging 1998;8(4):197‐204. [DOI] [PubMed] [Google Scholar]
Szczechowski 1994 {published data only}
- Szczechowski L, Wajgt A. Ocena skutecznosci leczenia ostrych udarow niedokrwiennych dozylnym wlewem nimodypiny. Neurologia i Neurochirurgia Polska 1994;28:299‐306. [PubMed] [Google Scholar]
TRUST 1990/120 {published and unpublished data}
- Murphy J. UK Calcium antagonist trial (TRUST). Journal of Neurology 1990;237:130. [Google Scholar]
- Murphy J, Trust Study Group. Randomised, double‐blind, placebo‐controlled trial of nimodipine in acute stroke. Lancet 1990;336:1205‐9. [PubMed] [Google Scholar]
- Pandita‐Gunawardena ND, TRUST Investigators Group. A randomised, double‐blind, placebo controlled trial of nimodipine in acute stroke. Journal of the American Geriatric Society 1991;39:A11. [Google Scholar]
Vamosi 1976 {published data only}
- Vamosi B, Molnar L, Demeter J, Tury F. Comparative study of the effect of ethyl apovincaminate and xantinol nicotinate in cerebrovascular diseases. Arzneimittel Forschung 1976;26(10a):3‐7. [PubMed] [Google Scholar]
Vamosi 1979 {unpublished data only}
- Vamosi B, Molnar L, Gal J, Demeter J, Tury F. Cavinton (apovincaminate) and Xavin (xanthinolnicotinate) in acute cerebrovascular disorders (clinical effect, EEG, cerebral serial angiography). RDG document No 13977 1979.
Wang 2004 {published data only}
- Wang X, Xu J, Zhu B. Recent effect of magnesium sulphate on neurological function and the quality of life in patients with acute cerebral infarction. Chinese Journal of Clinical Rehabilitation 2004;8(34):7625‐7. [Google Scholar]
Wang 2006 {published data only}
- Wang L, Li J, Zhang Y. A clinical observation of efficacy of vinpocetine injection on acute cerebral haemorrhage. Progress in Pharmaceutical Sciences 2006;30(12):563‐5. [Google Scholar]
Wasilewski 1985 {unpublished data only}
- Wasilewski R. Effect of Cavinton treatment on the dynamics of the clinical syndromes of acute and chronic cerebral vascular disturbances. RGD document No 32140. 1985.
Werner 1986 {published data only}
- Werner J, Apecechea M, Schaltenbrand R, Fenz E. Clinical study to evaluate the efficacy and tolerance of vinpocetine iv added to standard therapy in patients suffering from an acute apoplectic insult. In: Bes A, et al. editor(s). Senile Dementias: Early Detection. John Libbey Eurotext, 1986:636‐41. [Google Scholar]
Wong 1987 {published data only}
- Wong WJ, Hu HH, Lo YK, Chu FL. A control trial of pentoxifylline plus glycerine in the treatment of acute ischemic stroke. Proceedings of the 7th Asian Oceanian Congress of Neurology, Bali. September 1987:45.
Woollard 1978 {published data only}
- Woollard ML, Pearson RM, Dorf G, Griffith D, James IM. Controlled trial of ornithine alpha ketoglutarate (OAKA) in patients with stroke. Stroke 1978;9:218‐22. [DOI] [PubMed] [Google Scholar]
Yu 2003 {published data only}
- Yu L, Zhao Y. Efficacy of aescine sodium, mannitol, and albumin on cerebral post‐infarction. China Pharmacist 2003;6(12):801‐2. [Google Scholar]
Zhao 2003 {published data only}
- Zhao Y, Dong Q, Jiang J. A clinical trial of the efficacy and safety of high‐dose naloxone at the early stage of cerebral haemorrhage secondary to hypertension. Journal of Apoplexy and Nervous Diseases 2003;20(3):261‐2. [Google Scholar]
Zorzon 1987 {published data only}
- Zorzon M, Monti F, Pio Luogo T, Cazzato G. La ticlopidina e la pentossifillina nell'infarto cerebrale acuto. Giornale di Clinica Medica 1987;11:569‐72. [PubMed] [Google Scholar]
References to ongoing studies
ACCOST 2006 {published data only}
- Gray C. ACCOST. Acute Candesartan Cilexetil Outcomes Stroke Trial. Stroke Trials Registry, Internet Stroke Center: www.strokecenter.org/trials. 2006.
ASTART 2005 {published data only}
- Beer C. Acute stroke treatment with atorvastatin and irbesartan. Australian New Zealand Clinical Trials Registry (ANZCTR) http://www.anzctr.org.au/ 2005.
ATACH‐2 2008 {published data only}
- Qureshi AI, Qureshi Z. Antihypertensive Treatment of Acute Cerebral Hemorrhage (ATACH) trial. Proceedings of the International Stroke Conference 2008. USA, New Orleans: American Stroke Association, 20‐22 February 2008.
BLAST 2007 {published data only}
- Albers GW. Blood Pressure Lowering in Acute Stroke Trial (BLAST). ClinicalTrials.gov 2007.
COSSACS 2005 {published data only}
- COSSACS Trial Group. COSSACS (Continue Or Stop post‐Stroke Antihypertensives Collaborative Study): rationale and design. Journal of Hypertension 2005;23(2):455‐8. [DOI] [PubMed] [Google Scholar]
ENOS 2006 {published data only}
- Bath PMW, and the ENOS Investigators. Efficacy of nitric oxide in stroke (ENOS) trial: prospective randomized controlled trial in acute stroke. European Journal of Neurology 2003;10 Suppl 1:59. [Google Scholar]
- Sprigg N, Gray LJ, Bath PMW. Continuing prior antihypertensive medication in acute stroke lowers blood pressure: data from the continue vs stop arm of the "Efficacy of Nitric Oxide in Stroke" (ENOS) trial. Cerebrovascular Diseases 2006;21 Suppl 4:144. [Google Scholar]
- The ENOS Trial Investigators. Glyceryl trinitrate vs. control, and continuing vs. stopping temporarily prior antihypertensive therapy, in acute stroke: rationale and design of the Efficacy of Nitric Oxide in Stroke (ENOS) trial (ISRCTN99414122). International Journal of Stroke 2006;1:245‐9. [DOI] [PubMed] [Google Scholar]
FAST‐MAG 2005 {published data only}
- Saver JL. FAST‐MAG, Field administration of stroke therapy ‐ magnesium phase III trial. International Stroke Conference. 2006.
GRASP 2005 {published data only}
- Johnston KC. Glucose regulation in acute stroke patients trial. International Stroke Conference. 2007.
ICH ADAPT 2007 {published data only}
- Butcher K. ICH ADAPT Intracerebral Hemorrhage Acutely Decreasing Arterial Pressure Trial. Stroke Trials Directory, Internet Stroke Center: www.strokecenter.org/trials 2007.
INTERACT 2 2007 {published data only}
- Anderson C. INTERACT Intensive Blood Pressure Reduction in Acute Cerebral Haemorrhage. Stroke Trials Directory, Internet Stroke Center: www.strokecenter.org/trials/ 2007.
PASS II 1998 {unpublished data only}
- Orgogozo JM. PASS II. Piracetam in acute ischaemic stroke II. Stroke Trials Directory, Internet Stroke Center: www.strokecenter.org/trials 1998.
SCAST 2005 {published data only}
- Aakvik R. SCAST. Scandinavian Candesartan Acute Stroke Trial. Stroke Trials Directory, Internet Stroke Center: www.strokecenter.org/trials 2006.
- Berge E, Aakvik R, Terent A, Boysen G. Scandinavian Candesartan Acute Stroke Trial (SCAST). Cerebrovascular Diseases 2006;21 Suppl 4:124‐5. [Google Scholar]
- Iuell RS, Aarhus D, Terent A, Boysen G, Thijs V, Berge E. SCAST ‐ Scandinavian Candesartan Acute Stroke Trial. Proceedings of the 16th European Stroke Conference. 2007.
TAST 2007 {published data only}
- Sare G. Effect of an angiotensin receptor antagonist on cerebral blood flow, cerebral perfusion pressure, and systemic and peripheral haemodynamics in patients with acute stroke. Current Controlled Trials (http://www.controlled‐trials.com/) 2007.
Additional references
AHA‐HS 2007
- Broderick J, Connolly S, Feldmann E, Hanley D, Kase C, Krieger D, et al. Guidelines for the management of spontaneous intracerebral hemorrhage in adults: 2007 update. Stroke 2007;38:2001‐23. [DOI] [PubMed] [Google Scholar]
AHA‐IS 2007
- Adams HP, Zoppo G, Alberts MJ, Bhatt DL, Brass L, Furlan A, et al. Guidelines for the early management of adults with ischemic stroke. Stroke 2007;38:1655‐711. [DOI] [PubMed] [Google Scholar]
BASC I
- Geeganage C, Bath PM. Interventions for deliberately altering blood pressure in acute stroke. Cochrane Database of Systematic Reviews 2008, Issue 4. [Art. No.: CD000039. DOI: 10.1002/14651858.CD000039.pub2.] [DOI] [PubMed] [Google Scholar]
Bath 2002
- Bath PMW, Willmot M, Leonardi‐Bee J, Bath‐Hextall FJ. Nitric oxide donors (nitrates), L‐arginine, or nitric oxide synthase inhibitors for acute stroke. Cochrane Database of Systematic Reviews 2002, Issue 4. [Art. No.: CD000398. DOI: 10.1002/14651858.CD000398] [DOI] [PubMed] [Google Scholar]
Bath 2003
- Bath P, Chalmers J, Powers W, Beilin L, Davis S, Lenfant C, et al. International Society of Hypertension (ISH): statement on the management of blood pressure in acute stroke. Journal of Hypertension 2003;21(4):665‐72. [DOI] [PubMed] [Google Scholar]
Bath 2004/1
- Bath PMW. Prostacyclin and analogues for acute ischaemic stroke. Cochrane Database of Systematic Reviews 2004, Issue 3. [Art. No.: CD000177. DOI: 10.1002/14651858.CD000177.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bath 2004/2
- Bath PMW, Bath‐Hextall FJ. Pentoxifylline, propentofylline and pentifylline for acute ischaemic stroke. Cochrane Database of Systematic Reviews 2004, Issue 3. [Art. No.: CD000162. DOI: 10.1002/14651858.CD000162.pub2.] [DOI] [PubMed] [Google Scholar]
Bereczki 2008
- Bereczki D, Fekete I. Vinpocetine for acute ischemic stroke. Stroke 2008;39:2404‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Burke 1986
- Burke AM, Younkin D, Gordon J, Goldberg H, Graham T, Kushner M, et al. Changes in cerebral blood flow and recovery from acute stroke. Stroke 1986;17:173‐8. [DOI] [PubMed] [Google Scholar]
Castillo 2004
- Castillo J, Leira R, Garcia MM, Serena J, Blanco M, Davalos A. Blood pressure decrease during the acute phase of ischemic stroke is associated with brain injury and poor stroke outcome. Stroke 2004;35:520‐7. [DOI] [PubMed] [Google Scholar]
ESO 2008
- The European Stroke Association (ESO) Executive Committee and the ESO Writing Committee. Guidelines for management of ischaemic stroke and transient ischaemic attack 2008. Cerebrovascular Diseases 2008;25:457‐507. [DOI] [PubMed] [Google Scholar]
Geeganage 2009
- Geeganage CM, Bath PMW. Relationship between therapeutic changes in blood pressure and outcomes in acute stroke: a meta‐regression. Hypertension 2009;54:775‐81. [DOI] [PubMed] [Google Scholar]
Higgins 2008
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.0 [updated February 2008]. The Cochrane Collaboration, 2008. Available from www.cochrane‐handbook.org.
Horn 2001
- Horn J, Limburg M. Calcium antagonists for acute ischemic stroke. Cochrane Database of Systematic Reviews 2000, Issue 1. [Art. No.: CD001928. DOI: 10.1002/14651858.CD001928] [DOI] [PubMed] [Google Scholar]
International Society of Hypertension 2003
- International Society of Hypertension Writing Group. International Society of Hypertension (ISH): Statement on the management of blood pressure in acute stroke. Journal of Hypertension 2003;21:665‐72. [DOI] [PubMed] [Google Scholar]
Leonardi‐Bee 2002
- Leonardi‐Bee J, Bath PMW, Phillips SJ, Sandercock PAG. Blood pressure and clinical outcomes in the International Stroke Trial. Stroke 2002;33:1315‐20. [DOI] [PubMed] [Google Scholar]
Leonardi‐Bee 2007
- Leonardi‐Bee J, Steiner T, Bath‐Hextall F. Naftidrofuryl for acute stroke. Cochrane Database of Systematic Reviews 2007, Issue 2. [Art. No.: CD005478. DOI: 10.1002/14651858.CD005478.pub2] [DOI] [PubMed] [Google Scholar]
Martinsson 2007
- Martinsson L, Hardemark H, Eksborg S. Amphetamines for improving recovery after stroke. Cochrane Database of Systematic Reviews 2007, Issue 1. [Art. No.: CD002090. DOI: 10.1002/14651858.CD002090.pub2.] [DOI] [PubMed] [Google Scholar]
Paulson 1990
- Paulson OB, Strandgaard S, Edvinsson L. Cerebral autoregulation. Cerebrovascular and Brain Metabolism Reviews 1990;2:161‐92. [PubMed] [Google Scholar]
RevMan 2008 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.0. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2008.
Sandercock 1992
- Sandercock P, Willems H. Medical treatment of acute ischaemic stroke. Lancet 1992;339:537‐9. [DOI] [PubMed] [Google Scholar]
Spence 1985
- Spence JD, Maestro RF. Hypertension in acute ischaemic strokes. Treat. Archives of Neurology 1985;42:1000‐2. [DOI] [PubMed] [Google Scholar]
Sprigg 2005
- Sprigg N, Bath PMW. Management of blood pressure in acute stroke. Practical Neurology 2005;5:218‐23. [Google Scholar]
Sprigg 2006
- Sprigg N, Gray LJ, Bath PMW, Boysen G, Deyn PP, Friss P, et al. Relationship between outcome and baseline blood pressure and other haemodynamic measures in acute ischaemic stroke: data from the TAIST trial. Journal of Hypertension 2006;24(7):1413‐8. [DOI] [PubMed] [Google Scholar]
Sprigg 2007
- Sprigg N, Willmot M, Gray LJ, Bath PMW. Effects of amphetamine on stroke recovery: a systematic review. Cerebrovascular Diseases 2007;23 Suppl 2:32. [Google Scholar]
Strandgaard 1973
- Strandgaard S, Olesen J, Skinhoj E, Lassen NA. Autoregulation of brain circulation in severe arterial hypertension. BMJ 1973;3:507‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Stroke Center
- Internet Stroke Center. Stroke Trials Registry. http://www.strokecenter.org/trials/.
Tirilazad International Steering Committee 2001
- Tirilazad International Steering Committee. Tirilazad for acute ischaemic stroke. Cochrane Database of Systematic Reviews 2001, Issue 4. [Art. No.: CD002087. DOI: 10.1002/14651858.CD002087] [DOI] [PubMed] [Google Scholar]
Vemmos 2004
- Vemmos KN, Tsivgoulis G, Spengos K, Zakopoulos N, Synetos A, Manios E, et al. U‐shaped relationship between mortality and admission blood pressure in patients with acute stroke. Journal of Internal Medicine 2004;255:257‐65. [DOI] [PubMed] [Google Scholar]
Willmot 2004
- Willmot M, Leonardi‐Bee J, Bath PMW. High blood pressure in acute stroke and subsequent outcome: a systematic review. Hypertension 2004;43:18‐24. [DOI] [PubMed] [Google Scholar]
Yatsu 1985
- Yatsu FM, Zivin J. Hypertension in acute ischaemic strokes. Not to treat. Archives of Neurology 1985;42:999‐1000. [DOI] [PubMed] [Google Scholar]


