Abstract
Interleukin-35 is a potent suppressive cytokine of the IL-12 family. Although other members of the IL-12 family are produced mainly by antigen-presenting cells (APCs), IL-35 is produced by regulatory T (Treg) cells and suppresses cell proliferation. It has been shown to play an important role in many disease models and has been recently shown to have additional functions aside from inhibition of proliferation, including inducing its own expression in non-Treg cells. In this chapter, we discuss the history and current status of IL-35 biology, as well as suggest where the field might move in the future.
Keywords: IL-35, STAT heterodimer, Suppression, Treg
The IL-12 Family Members of Heterodimeric Cytokines
Cytokines comprise a critical mediator of cell-to-cell communication. Although T-cell–APC contacts in the form of pMHC:TCR and costimulation are important for initiating the immune response, cytokines are essential for shaping it, inducing differentiation into particular programmed effector lineages and aiding in the generation of memory. In addition, cytokines can have direct effector function by inducing cell activation or apoptosis, modulation of proliferation, and alteration of metabolism.
Although some cytokine families are predominantly pro-inflammatory or anti-inflammatory, the IL-12 cytokines family is unusual in having particularly broad immunomodulatory activities. The IL-12 family belongs to the IL-6 superfamily of type 1 cytokines, which is characterized by cytokines with multisubunit receptors (Jones and Vignali 2011; Vignali and Kuchroo 2012).These cytokines have a wide variety of functions in the hematopoietic lineages.
The IL-12 family was formed with the discovery of IL-12, a heterodimer of p35 (encoded by Il12a) and p40 (encoded by Il12b), linked by disulfide bonds (Fig. 15.1). This family is characterized by heterodimers of alpha-subunits consistent with the structure of type 1 cytokines and beta-subunits that are structurally similar to soluble cytokine receptors (Jones and Vignali 2011; Vignali and Kuchroo 2012). This structure thought to have evolved from the IL-6 superfamily of cytokines. Many IL-6 superfamily members utilize gp130 as a signal-transducing subunit (IL-6, IL-11, CNTF, LIF, OSM, CLF, etc.). Beta-subunits structurally resemble signal-transducing subunits as well as cytokine-binding proteins (Jones and Vignali 2011). IL-12 was shown to be critical for induction of T helper 1 (Th1) cell differentiation and interferon (IFN)-γ production. The generation of knockout mice for Il12a and Il12b confirms the physiological importance of IL-12 in a variety of in vivo models, including experimental autoimmune encephalomyelitis (EAE) (Bettelli et al. 2007). As the Th1/Th2 paradigm suggested that Th1 cells would be important for EAE disease pathogenesis, it was anticipated that Il12b −/− mice would be resistant to EAE. However, the observation that Il12a −/− were susceptible to EAE was surprising and suggested a more complex biology. This idea prompted researchers to suggest that perhaps p40 was playing a role as a subunit for another cytokine, leading to the discovery of the p19 alpha-chain and the demonstration that it pairs with the p40 beta-chain via a disulfide linkage to form IL-23. This new cytokine, while originally thought to activate memory cells, was shown to induce the differentiation of Th17 cells, a predominantly inflammatory T cell subset. In the coming years, new cytokines have be added to the IL-12 family based on structural homology. IL-27, a heterodimer of p28 (encoded by Il27) and Epstein–Barr virus-induced gene 3 (Ebi3, encoded by Ebi3), which lacks disulfide linkage, was shown to be produced by recently activated antigen-presenting cells (APCs) (Fig. 15.1). It was first thought to be a proinflammatory cytokine, because stimulation of naïve T cells with IL-27 induces Il12rb2 expression, greatly enhancing T-cell sensitivity to IL-12 (Devergne et al. 1996; Hall et al. 2012). However, recent data have highlighted a more immunomodulatory role for IL-27. For instance, IL-27 can induce the generation of IL-10-producing Tr1 cells while inhibiting Th17 and peripherally induced Treg (pTreg) induction. It is also notable that several IL-12 family member subunits have been suggested to have functional roles as homodimers. IL12p40 homodimers, p28 homodimers (IL-30), and Ebi3 homodimers have been reported with various functions resulting from their antagonistic activity via receptor blockade (Hall et al. 2012; Kempe et al. 2009; Vignali and Kuchroo 2012). Recently, p28 has been shown to have an agonistic function in T cells, although it remains to be determined whether these homodimers have physiological roles in vivo (Garbers et al. 2013).
Fig. 15.1.
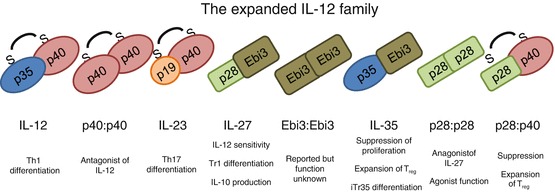
The growing interleukin (IL)-12 family. The IL-12 family of cytokines is characterized by subunit sharing and pairing. Some, such as IL-12 and IL-23, are linked via disulfide linkage, whereas others (those utilizing Ebi3) are held together by other structural forces. The functions of the IL-12 family are many and varied, often related to differentiation. The presence of homodimers of subunits has been reported, and these often have been implicated in acting as natural antagonists of bioactive cytokine signaling
It is clear that the IL-12 cytokine family mediates a broad and varied portfolio of immunological events (Vignali and Kuchroo 2012). Thus, it seems likely that new insights are likely to emerge and new members of this family may also remain to be discovered. However, in this chapter we focus exclusively on interleukin-35, the newest and perhaps most elusive member of this family.
Discovery of IL-35 and Identification of Its Cellular Source
When a second IL-12 family cytokine beta-chain, Ebi3, was discovered in 1996 (Devergne et al. 1996), there was considerable interest in identifying its “natural” binding partner. This quest continued for some time as p28 was identified 6 years after the discovery of Ebi3 (Pflanz et al. 2002). In 1997, it was shown that Ebi3 could pair with p35 in transfection experiments (Devergne et al. 1997). Although it was suggested that placental trophoblasts might coexpress Ebi3 and p35 (Devergne et al. 1997, 2001), it was not clear whether this was a bona fide heterodimeric cytokine, what its predominant cellular source might be, and its function in vivo.
A decade later, detailed analysis of the Treg cell gene expression “signature” revealed Ebi3 as an upregulated gene and potential Foxp3 target (Collison et al. 2007; Hill et al. 2007; Knoechel et al. 2006; Zheng et al. 2007). Given that Ebi3 is a p40-related member of the IL-12 family, a cytokine family that was thought to be exclusively produced by APCs, increased expression in Treg cells was surprising. In 2007, the Vignali lab identified IL-35 as a p35:Ebi3 heterodimer important for Treg cell function (Collison et al. 2007). Importantly, Ebi3- and Il12a-deficient Treg cells, stimulated in isolation or co-cultured with wild-type conventional cells, displayed limited suppressive capabilities compared with their wild-type controls. Furthermore, recombinant 2A-linked “native” or a single-chain “hyperkine” IL-35 suppressed T-cell proliferation. Around the same time, another group used a p35:Ebi3 single-chain hyperkine-Fc fusion protein to abrogate collagen-induced arthritis in mice (Niedbala et al. 2007). However, this study seemed to indicate a role for IL-35 in the expansion of Tregs rather than the suppression of proliferation. These contrasting data in vitro confirmed some earlier observations, but a physiological role and source for IL-35 in vivo seemed to be lacking (Devergne et al. 1997; Niedbala et al. 2007). In all, these early observations suggested that IL-35 may be an important member of the suppressive cytokine “community,” joining transforming growth factor (TGF)-β and IL-10 as soluble mediators of suppression.
The Function(s) of IL-35
The discovery of IL-35 as a Treg-derived suppressive cytokine has sparked considerable interest. Although there is clearly much to learn about this intriguing cytokine, several recent studies have begun to provide important insight into its function (Fig. 15.2). The primary function of IL-35 appears to be the induction of cell-cycle arrest in many cell types (Bettini et al. 2012; Collison et al. 2007; Wirtz et al. 2011). The ability of IL-35 to suppress cellular proliferation may be a critical component of the Treg cell armament, especially in inflammatory environments (Bettini et al. 2012; Collison et al. 2007, 2009; Wirtz et al. 2011). It has been suggested that IL-35 can expand Treg cells, although the mechanism of this expansion is unknown and the observation has yet to be confirmed in vivo (Niedbala et al. 2007). It is possible that that Treg cells are merely resistant to IL-35 and thus are selected for in this context, or it may be that IL-35 blocks factors that would hamper Treg cell function.
Fig. 15.2.
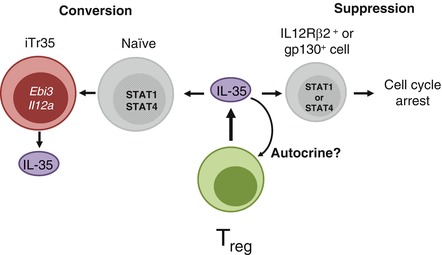
Multiple functions of IL-35. IL-35-treated T cells expressing gp130 or IL12Rβ2 signal via STAT1 or STAT4, respectively, and induce cell-cycle arrest (suppression). Treg cell-derived IL-35 can also suppress activated T cells via a STAT1:STAT4 heterodimer, and induce Ebi3 and Il12a transcription and IL-35 production (iTr35 conversion). Finally, IL-35 may be able to act on Treg cells in an autocrine fashion to program stability, but these findings have yet to be fully elucidated
Many studies have implicated IL-35 in various disease models. Initial studies suggested that IL-35 could ameliorate inflammatory environments, such as in models of inflammatory bowel disease (IBD) (Collison et al. 2007). Early in vivo functions of IL-35 were attributed to the expansion of regulatory cells or the suppression of Th17 cells, specifically in the progression of experimental arthritis (Kochetkova et al. 2010; Niedbala et al. 2007; Yang et al. 2008). IL-35 was also shown to be important in inhibiting the clearance of Listeria infection (Yang et al. 2008). Ebi3 −/− animals have exacerbated central nervous system (CNS) pathology in response to coronavirus infection (Tirotta et al. 2013). Use of an IL-35 expression construct in mice led to attenuated autoimmune-induced CNS demyelination (Zandian et al. 2011), decreased experimental colitis (Wirtz et al. 2011), and reduced severity of airway inflammation and antibody production in a model of dust mite allergy (Huang et al. 2011). Transgenic NOD mice expressing IL-35 in pancreatic beta cells in the islets of Langerhans have substantially decreased diabetes and immune pathology (Bettini et al. 2012). Further analysis of IL-35 function in vivo is clearly required, but the general consensus is that IL-35 exhibits an inhibitory phenotype in a broad range of disease models.
The suppressive cytokines TGF-β and IL-10 have the capacity to convert CD4+ T cells in to in vitro-generated induced Treg (iTreg) that suppress via TGF-β or IL-10, respectively (Vignali et al. 2008). It seemed logical that IL-35 might have a similar function as a mediator of “infectious tolerance,” the notion that cells which are suppressed can themselves become suppressive, passing the tolerant state from one cell to another (Gravano and Vignali 2012). Indeed, naive CD4+ T cells stimulated in the presence of IL-35 are not only suppressed but are converted to an IL-35-producing iTreg population termed iTr35 (Collison et al. 2010). Interestingly, these cells do not express Foxp3 or suppress via TGF-β or IL-10 and are implicated in several disease models, especially cancer, as tumors are enriched for Foxp3– IL-35-producing cells.
Although there is mounting evidence supporting an inhibitory role for murine IL-35, evidence supporting a role for human IL35 remains limited. It has been suggested that human IL-35 may be suppressive (Collison et al. 2010; Jones et al. 2012), but initial studies could not detect IL-35 production by human Treg cells ex vivo or following short-term stimulation (Bardel et al. 2008). Furthermore, retroviral-mediated overexpression of FOXP3 in human T cells could not induce EBI3 or IL12A expression (Allan et al. 2008). However, most Treg cells isolated from human samples are not particularly suppressive ex vivo and do not always express high levels of FOXP3. Indeed, IL-35 has been detectable in human samples from a variety of disease states (Langhans et al. 2010; Liu et al. 2011; Seyerl et al. 2010). Furthermore, hepatitis C virus-specific human Tregs have been shown to suppress via IL-35 in response to HCV epitopes (Langhans et al. 2010). IL-35 has also been shown to be produced by human Tregs in response to rhinovirus infection, implicating PD-L1 and CD169 in its induction (Seyerl et al. 2010). As in the mouse, it also appears that human CD4+ T cells exposed to IL-35 can express and mediate suppression via IL-35 (Collison et al. 2010). Although most of the focus has been on production of IL-35 by CD4+ regulatory T-cell populations, an intriguing recent study has suggested that a human CD8+ T-cell subpopulation can regulate antitumor immunity in prostate cancer via IL-35 production and CTLA-4 (Olson et al. 2012). This finding is particularly intriguing because CD8+ cells express a functional receptor for IL-35 that could induce IL-35 production (Collison et al. 2012). Also recently, a study has suggested that IL-35 may be produced by tumor cells, inducing cell-cycle arrest and promoting apoptosis among themselves as well as tumor-infiltrating cells (Long et al. 2013). Some cancer lines were also shown to express functional receptors for IL-35 as well as IL-35 itself. Furthermore, IL-35-negative tumor lines in which IL-35 was overexpressed gained the ability to mediate cell-cycle arrest and apoptosis (Long et al. 2013). This ability may serve as a mechanism for tumors to evade the immune system and establish long-term tolerance, promoting disease progression. Such observations are encouraging, but it will be critical to fully evaluate the functional capacity and scope of human IL-35, moving forward to determine the potential of its therapeutic utilization or blockade.
Cellular Targets of IL-35: Lessons from Its Receptor
Based on structural homology between the subunits of IL-35 and other members of the IL-12 family, it was likely that IL-35 would utilize one or more of the receptor subunits associated with this family. Utilizing cells deficient in these receptor subunits, we demonstrated a role for gp130 and IL-12Rβ2 in IL-35-mediated suppression (Collison et al. 2012). However, although gp130- and IL-12Rβ2 double-deficient cells were completely resistant to IL-35-mediated suppression, those lacking only a single chain of the receptor could still signal and suppress, especially in vivo. This observation was surprising as other members of the IL-12 family lose their signaling capacity in the absence of a single receptor chain (Garbers et al. 2012; Vignali and Kuchroo 2012). Intriguingly, signaling via gp130 or IL-12Rβ2 alone was not sufficient to mediate IL-35 production and iTr35 conversion. Subsequent biochemical analysis and microscopy approaches revealed that IL-35 can utilize three receptors: gp130 homodimers and IL-12Rβ2 homodimers, which were sufficient to mediate suppression, and gp130:IL12Rβ2 heterodimers, which mediate suppression and iTr35 conversion (Fig. 15.3). This conversion occurs through STAT1:STAT4 heterodimers, which can bind to regions within the Ebi3 and Il12a promoters and initiate transcription. What remains unclear is how gp130 or IL-12Rβ2 homodimers can stimulate STAT1 or STAT4 activation and mediate suppression in the absence of the other chain.
Fig. 15.3.
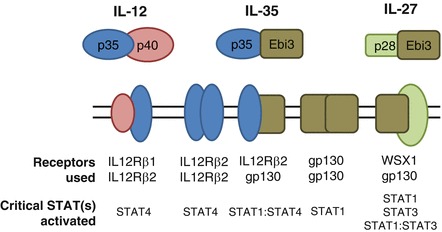
IL-35 utilizes three receptors. Although IL-12 and IL-27 utilize a single receptor consisting of two chains, IL-35 is unique in that it can also signal, at least in part, through homodimers of its receptor chains. However, in the absence of one of the receptor chains, its function is limited merely to suppression, whereas the full receptor of gp130:IL-12Rβ2 can generate STAT1:STAT4 heterodimers, which program IL-35 production and iTr35 conversion
IL-12Rβ2 is primarily restricted to the hematopoietic lineage and is really only expressed on a small proportion of cells [activated conventional CD4+ and CD8+ T cells, natural killer (NK) cells, dendritic cells (DCs), and some B cells). Interestingly, CD8+ regulatory cells generated in prostate tumors have been shown to suppress via IL-35 production, suggesting that perhaps the presence of IL-12Rβ2 and gp130 on activated CD8+ T cells may sensitize them for conversion to IL-35 production (Olson et al. 2012). However, gp130 is ubiquitously expressed throughout the body. As IL-35 can mediate suppression through gp130 homodimers, this implies that most cells could be susceptible to IL-35-mediated suppression. This is an exciting thought but it highlights questions regarding the potency of IL-35 in vivo and implications for clinical use. Does IL-35-mediated Treg suppression require closer contact and direct delivery to its target cells? Is IL-35 removed from the microenvironment by the myriad of receptor-positive cells in vivo? Is there a method for therapeutic intervention by which one could specifically deliver IL-35 to its intended targets, sparing gp130+ bystander cells?
Conundrums Associated with IL-35
IL-35 has proven to be an extraordinarily hard cytokine to study. A key issue is that purified biologically active recombinant IL-35 is still not commercially available and has not been reported. This restriction is in part because IL-35 is poorly secreted and may be somewhat unstable (Jones et al. 2012). Generally Fc fusion proteins or hyperkines are used to study heterodimeric cytokines (such as IL-27), but attempts to generate functional IL-35 in this manner have had mixed results. Although 293T cells coexpressing p35 and Ebi3 secrete bioactive IL-35, the amount is low and it has not been successful purified (Collison et al. 2007, 2009; Jones et al. 2012). Important studies are being undertaken currently to determine the fundamental biology of IL-35, which may help shed light on problems with its production and purification as a research reagent. Interestingly, p35 utilizes two potential start codons, the first encoding a nonclassical, extraordinarily long signal peptide (Kollet et al. 2001; Vaidyanathan et al. 2001, 2003), raising the possibility that IL-35 may be processed differently than are other cytokines. These hypotheses parallel the initial observations that Ebi3 may also be membrane associated and have alternative processing (Devergne et al. 1996). Solving these inherent problems in IL-35 expression, production, and purification may help elucidate the biology of this mysterious cytokine. Such studies may pave the way for future use of IL-35 as a research reagent and a tool for therapeutic intervention.
Given that IL-35 is a multisubunit cytokine, genetic models of simple gene deletion complicate any interpretation of published data (Collison and Vignali 2008). For instance, Il12a −/− mice lack both IL-35 and IL-12, thus clouding whether the phenotype observed is caused by one cytokine or the other. An attractive idea would be to find sites in Ebi3 and p35 that would mediate binding with IL-12, IL-27, or IL-35, respectively, and make mutant mice that would thus be deficient in only one cytokine, rather than both (Vignali and Kuchroo 2012). Given that IL-35 is a heterodimeric cytokine, one might anticipate that mutations could be made that would abrogate binding to one binding partner versus the other. However, extensive mutagenesis of Ebi3 and p35 has suggested that this may not be the case. Although IL-12 and IL-27 have conserved regions of homology crucial to subunit pairing, p35 and Ebi3 appear to pair in an unconventional manner (Jones et al. 2012). However, using point mutants discovered by these mutagenesis studies could pave the way for generation of mice that are specifically deficient in IL-12 or IL-27, leaving IL-35 pairing and function intact.
Future Directions in IL-35 Biology
Many important questions still remain regarding IL-35 beyond the recombinant production issues detailed here.
First, a more detailed, temporally- and cell type-restricted assessment of IL-35 function in a variety of disease settings is essential, using a combination of IL-35-specific neutralizing monoclonal antibodies (mAbs) and floxed Ebi3 and Il12a mice.
Second, a detailed analysis of which cell types produce IL-35, and when, is needed, using flow cytometric cytokine-staining approaches and reporter mice. Similar studies also need to be performed for IL-35 receptor expression and function.
Third, understanding the mechanism by which IL-35 promotes cell-cycle arrest is of critical importance. It is especially important to delineate whether other functions of IL-35 (such as iTr35 conversion) are dependent on the capacity to be “suppressed” or are independent of these signaling mechanisms.
Fourth, although IL-35 has been shown to signal via a STAT1:STAT4 heterodimer, it is still unclear how a heterodimer is formed (as opposed to STAT homodimers). Evaluating potential mechanisms of heterodimer formation in the context of IL-35 can, hopefully, shed light on the general role of STAT heterodimers in immunity. Furthermore, elucidating how STAT heterodimers function distinctly from homodimers has implications for all of cytokine biology. Given that many cytokines with distinct functions utilize the same STATs, determining the mechanism by which STAT heterodimers promote alternative transcription and cellular function will provide fundamental insight into how these critical pathways are modulated.
Fifth, if IL-35 is shown to expand Treg populations (Niedbala et al. 2007), it is extremely important to interrogate any “autocrine” activity of IL-35 on Tregs. Tregs do express gp130, but they do not express high levels of IL-12Rβ2, suggesting that they would be insensitive to the “converting” functions of IL-35. It may stand to reason that IL-35 acts to block gp130-utilizing cytokines that would otherwise hamper suppressive function, such as IL-6.
Finally, clearly it is extremely important to determine to the fullest extent the role that IL-35 plays in human Treg function, given the opposing findings in this matter. Only then might the full therapeutic utility and potential of IL-35 be realized.
Acknowledgments
This work was supported by the National Institutes of Health (R01 AI091977 and AI039480 to D.A.A.V.; F32 AI098383 to G.M.D.), NCI Comprehensive Cancer Center Support CORE grant (CA21765, to D.A.A.V.), and the American Lebanese Syrian Associated Charities (ALSAC, to D.A.A.V.).
Contributor Information
Takayuki Yoshimoto, Phone: 333516141, Email: yoshimot@tokyo-med.ac.jp.
Tomohiro Yoshimoto, Phone: +81-798456789, Email: tomo@hyo-med.ac.jp.
Dario A. A. Vignali, Email: vignali.lab@stjude.org
References
- Allan SE, Song-Zhao GX, Abraham T, McMurchy AN, Levings MK. Inducible reprogramming of human T cells into Treg cells by a conditionally active form of FOXP3. Eur J Immunol. 2008;38:3282–3289. doi: 10.1002/eji.200838373. [DOI] [PubMed] [Google Scholar]
- Bardel E, Larousserie F, Charlot-Rabiega P, Coulomb-L’Hermine A, Devergne O. Human CD4+ CD25+ Foxp3+ regulatory T cells do not constitutively express IL-35. J Immunol. 2008;181:6898–6905. doi: 10.4049/jimmunol.181.10.6898. [DOI] [PubMed] [Google Scholar]
- Bettelli E, Korn T, Kuchroo VK. Th17: the third member of the effector T cell trilogy. Curr Opin Immunol. 2007;19:652–657. doi: 10.1016/j.coi.2007.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bettini M, Castellaw AH, Lennon GP, Burton AR, Vignali DA. Prevention of autoimmune diabetes by ectopic pancreatic beta-cell expression of interleukin-35. Diabetes. 2012;61:1519–1526. doi: 10.2337/db11-0784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collison LW, Vignali DA. Interleukin-35: odd one out or part of the family? Immunol Rev. 2008;226:248–262. doi: 10.1111/j.1600-065X.2008.00704.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collison LW, Workman CJ, Kuo TT, Boyd K, Wang Y, Vignali KM, Cross R, Sehy D, Blumberg RS, Vignali DA. The inhibitory cytokine IL-35 contributes to regulatory T-cell function. Nature (Lond) 2007;450:566–569. doi: 10.1038/nature06306. [DOI] [PubMed] [Google Scholar]
- Collison LW, Pillai MR, Chaturvedi V, Vignali DA. Regulatory T cell suppression is potentiated by target T cells in a cell contact, IL-35- and IL-10-dependent manner. J Immunol. 2009;182:6121–6128. doi: 10.4049/jimmunol.0803646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collison LW, Chaturvedi V, Henderson AL, Giacomin PR, Guy C, Bankoti J, Finkelstein D, Forbes K, Workman CJ, Brown SA, et al. IL-35-mediated induction of a potent regulatory T cell population. Nat Immunol. 2010;11:1093–1101. doi: 10.1038/ni.1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collison LW, Delgoffe GM, Guy CS, Vignali KM, Chaturvedi V, Fairweather D, Satoskar AR, Garcia KC, Hunter CA, Drake CG, et al. The composition and signaling of the IL-35 receptor are unconventional. Nat Immunol. 2012;13:290–299. doi: 10.1038/ni.2227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devergne O, Hummel M, Koeppen H, Le Beau MM, Nathanson EC, Kieff E, Birkenbach M. A novel interleukin-12 p40-related protein induced by latent Epstein–Barr virus infection in B lymphocytes. J Virol. 1996;70:1143–1153. doi: 10.1128/jvi.70.2.1143-1153.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devergne O, Birkenbach M, Kieff E. Epstein-Barr virus-induced gene 3 and the p35 subunit of interleukin 12 form a novel heterodimeric hematopoietin. Proc Natl Acad Sci USA. 1997;94:12041–12046. doi: 10.1073/pnas.94.22.12041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devergne O, Coulomb-L’Hermine A, Capel F, Moussa M, Capron F. Expression of Epstein–Barr virus-induced gene 3, an interleukin-12 p40-related molecule, throughout human pregnancy: involvement of syncytiotrophoblasts and extravillous trophoblasts. Am J Pathol. 2001;159:1763–1776. doi: 10.1016/S0002-9440(10)63023-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garbers C, Hermanns HM, Schaper F, Muller-Newen G, Grotzinger J, Rose-John S, Scheller J. Plasticity and cross-talk of interleukin 6-type cytokines. Cytokine Growth Factor Rev. 2012;23:85–97. doi: 10.1016/j.cytogfr.2012.04.001. [DOI] [PubMed] [Google Scholar]
- Garbers C, Spudy B, Aparicio-Siegmund S, Waetzig GH, Sommer J, Holscher C, Rose-John S, Grotzinger J, Lorenzen I, Scheller J. An interleukin-6 receptor-dependent molecular switch mediates signal transduction of the IL-27 cytokine subunit p28 (IL-30) via a gp130 receptor homodimer. J Biol Chem. 2013;288:4346–4354. doi: 10.1074/jbc.M112.432955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gravano DM, Vignali DA. The battle against immunopathology: infectious tolerance mediated by regulatory T cells. Cell Mol Life Sci. 2012;69:1997–2008. doi: 10.1007/s00018-011-0907-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall AO, Silver JS, Hunter CA. The immunobiology of IL-27. Adv Immunol. 2012;115:1–44. doi: 10.1016/B978-0-12-394299-9.00001-1. [DOI] [PubMed] [Google Scholar]
- Hill JA, Feuerer M, Tash K, Haxhinasto S, Perez J, Melamed R, Mathis D, Benoist C. Foxp3 transcription-factor-dependent and -independent regulation of the regulatory T cell transcriptional signature. Immunity. 2007;27:786–800. doi: 10.1016/j.immuni.2007.09.010. [DOI] [PubMed] [Google Scholar]
- Huang CH, Loo EX, Kuo IC, Soh GH, Goh DL, Lee BW, Chua KY. Airway inflammation and IgE production induced by dust mite allergen-specific memory/effector Th2 cell line can be effectively attenuated by IL-35. J Immunol. 2011;187:462–471. doi: 10.4049/jimmunol.1100259. [DOI] [PubMed] [Google Scholar]
- Jones LL, Vignali DA. Molecular interactions within the IL-6/IL-12 cytokine/receptor superfamily. Immunol Res. 2011;51:5–14. doi: 10.1007/s12026-011-8209-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones LL, Chaturvedi V, Uyttenhove C, Van Snick J, Vignali DA. Distinct subunit pairing criteria within the heterodimeric IL-12 cytokine family. Mol Immunol. 2012;51:234–244. doi: 10.1016/j.molimm.2012.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempe S, Heinz P, Kokai E, Devergne O, Marx N, Wirth T. Epstein–Barr virus-induced gene-3 is expressed in human atheroma plaques. Am J Pathol. 2009;175:440–447. doi: 10.2353/ajpath.2009.080752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoechel B, Lohr J, Zhu S, Wong L, Hu D, Ausubel L, Abbas AK. Functional and molecular comparison of anergic and regulatory T lymphocytes. J Immunol. 2006;176:6473–6483. doi: 10.4049/jimmunol.176.11.6473. [DOI] [PubMed] [Google Scholar]
- Kochetkova I, Golden S, Holderness K, Callis G, Pascual DW. IL-35 stimulation of CD39+ regulatory T cells confers protection against collagen II-induced arthritis via the production of IL-10. J Immunol. 2010;184:7144–7153. doi: 10.4049/jimmunol.0902739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kollet J, Witek C, Gentry JD, Liu X, Schwartzbach SD, Petro TM. Deletional analysis of the murine IL-12 p35 promoter comparing IFN-gamma and lipopolysaccharide stimulation. J Immunol. 2001;167:5653–5663. doi: 10.4049/jimmunol.167.10.5653. [DOI] [PubMed] [Google Scholar]
- Langhans B, Braunschweiger I, Arndt S, Schulte W, Satoguina J, Layland LE, Vidovic N, Hoerauf A, Oldenburg J, Sauerbruch T, Spengler U. Core-specific adaptive regulatory T-cells in different outcomes of hepatitis C. Clin Sci (Lond) 2010;119:97–109. doi: 10.1042/CS20090661. [DOI] [PubMed] [Google Scholar]
- Liu F, Tong F, He Y, Liu H. Detectable expression of IL-35 in CD4+ T cells from peripheral blood of chronic hepatitis B patients. Clin Immunol. 2011;139:1–5. doi: 10.1016/j.clim.2010.12.012. [DOI] [PubMed] [Google Scholar]
- Long J, Zhang X, Wen M, Kong Q, Lv Z, An Y, Wei XQ. IL-35 over-expression increases apoptosis sensitivity and suppresses cell growth in human cancer cells. Biochem Biophys Res Commun. 2013;430(1):364–369. doi: 10.1016/j.bbrc.2012.11.004. [DOI] [PubMed] [Google Scholar]
- Niedbala W, Wei XQ, Cai B, Hueber AJ, Leung BP, McInnes IB, Liew FY. IL-35 is a novel cytokine with therapeutic effects against collagen-induced arthritis through the expansion of regulatory T cells and suppression of Th17 cells. Eur J Immunol. 2007;37:3021–3029. doi: 10.1002/eji.200737810. [DOI] [PubMed] [Google Scholar]
- Olson BM, Jankowska-Gan E, Becker JT, Vignali DA, Burlingham WJ, McNeel DG. Human prostate tumor antigen-specific CD8+ regulatory T cells are inhibited by CTLA-4 or IL-35 blockade. J Immunol. 2012;189:5590–5601. doi: 10.4049/jimmunol.1201744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pflanz S, Timans JC, Cheung J, Rosales R, Kanzler H, Gilbert J, Hibbert L, Churakova T, Travis M, Vaisberg E, et al. IL-27, a heterodimeric cytokine composed of EBI3 and p28 protein, induces proliferation of naive CD4(+) T cells. Immunity. 2002;16:779–790. doi: 10.1016/S1074-7613(02)00324-2. [DOI] [PubMed] [Google Scholar]
- Seyerl M, Kirchberger S, Majdic O, Seipelt J, Jindra C, Schrauf C, Stockl J. Human rhinoviruses induce IL-35-producing Treg via induction of B7-H1 (CD274) and sialoadhesin (CD169) on DC. Eur J Immunol. 2010;40:321–329. doi: 10.1002/eji.200939527. [DOI] [PubMed] [Google Scholar]
- Tirotta E, Duncker P, Oak J, Klaus S, Tsukamoto MR, Gov L, Lane TE. Epstein–Barr virus-induced gene 3 negatively regulates neuroinflammation and T cell activation following coronavirus-induced encephalomyelitis. J Neuroimmunol. 2013;254(1-2):110–116. doi: 10.1016/j.jneuroim.2012.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaidyanathan H, Gentry JD, Weatherman A, Schwartzbach SD, Petro TM. Differential response of the murine IL-12 p35 gene to lipopolysaccharide compared with interferon-gamma and CD40 ligation. Cytokine. 2001;16:1–9. doi: 10.1006/cyto.2001.0938. [DOI] [PubMed] [Google Scholar]
- Vaidyanathan H, Zhou Y, Petro TM, Schwartzbach SD. Intracellular localization of the p35 subunit of murine IL-12. Cytokine. 2003;21:120–128. doi: 10.1016/S1043-4666(03)00016-4. [DOI] [PubMed] [Google Scholar]
- Vignali DA, Kuchroo VK. IL-12 family cytokines: immunological playmakers. Nat Immunol. 2012;13:722–728. doi: 10.1038/ni.2366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vignali DA, Collison LW, Workman CJ. How regulatory T cells work. Nat Rev Immunol. 2008;8:523–532. doi: 10.1038/nri2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirtz S, Billmeier U, McHedlidze T, Blumberg RS, Neurath MF. Interleukin-35 mediates mucosal immune responses that protect against T-cell-dependent colitis. Gastroenterology. 2011;141:1875–1886. doi: 10.1053/j.gastro.2011.07.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Yang M, Htut TM, Ouyang X, Hanidu A, Li X, Sellati R, Jiang H, Zhang S, Li H, et al. Epstein–Barr virus-induced gene 3 negatively regulates IL-17, IL-22 and ROR gamma t. Eur J Immunol. 2008;38:1204–1214. doi: 10.1002/eji.200838145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zandian M, Mott KR, Allen SJ, Dumitrascu O, Kuo JZ, Ghiasi H. Use of cytokine immunotherapy to block CNS demyelination induced by a recombinant HSV-1 expressing IL-2. Gene Ther. 2011;18:734–742. doi: 10.1038/gt.2011.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Y, Josefowicz SZ, Kas A, Chu TT, Gavin MA, Rudensky AY. Genome-wide analysis of Foxp3 target genes in developing and mature regulatory T cells. Nature (Lond) 2007;445:936–940. doi: 10.1038/nature05563. [DOI] [PubMed] [Google Scholar]


