Abstract
An extensive use of pharmaceuticals and the widespread practices of their erroneous disposal measures have made these products contaminants of emerging concern (CEC). Especially, active pharmaceutical ingredients (APIs) are ubiquitously detected in surface water and soil, mainly in the aquatic compartment, where they do affect the living systems. Unfortunately, there is a huge gap in the availability of ecotoxicological data on pharmaceuticals’ environmental behavior and ecotoxicity which force EMEA (European Medicines Agency) to release guidelines for their risk assessment. In silico modeling approaches are vital tools to exploit the existing information to rapidly emphasize the potentially most hazardous and toxic pharmaceuticals and prioritize the most environmentally hazardous ones for focusing further on their experimental studies. The quantitative structure–activity relationship (QSAR) models are capable of predicting missing properties for toxic end-points required to prioritize existing, or newly synthesized chemicals for their potential hazard. This chapter reviews the information regarding occurrence and impact of pharmaceuticals and their metabolites in the environment along with their persistence, environmental fate, risk assessment, and risk management. A bird’s eye view about the necessity of in silico methods for fate prediction of pharmaceuticals in the environment as well as existing successful models regarding ecotoxicity of pharmaceuticals are discussed. Available toxicity endpoints, ecotoxicity databases, and expert systems frequently used for ecotoxicity predictions of pharmaceuticals are also reported. The overall discussion justifies the requirement to build up additional in silico models for quick prediction of ecotoxicity of pharmaceuticals economically, without or involving only limited animal testing.
Key words: APIs, Ecotoxicity, CEC, In silico, Pharmaceuticals, QSAR, Risk assessment, Risk management, Waste management
Introduction
Pharmaceuticals are one of the indispensable products with unquestionable benefits to human health and lifestyle. Regrettably, due to overuse of pharmaceuticals together with their improper disposal, unwanted residues of active pharmaceutical ingredients (APIs) have been found in different compartments of environment since 1970 [1, 2]. Pharmaceuticals are deliberately designed to have an explicit mechanism of action (MOA) and exercise an effect on specific organs, tissues, or cells in living systems and many of them are persistent in the body [3]. Thus, pharmaceuticals and their unaltered metabolites can affect humans as well as animals when entered into the environment by diverse sources and routes. Also the MOA designed for human could be different for another type of species and even potentially “safe” drugs could have serious effects on them from biological ladder. This feature makes pharmaceuticals unique from other chemicals and this is the one and only reason to assess the potential acute and chronic effects of pharmaceuticals in diverse environmental compartments. It is quite obvious that the toxicity of pharmaceuticals on organisms in aquatic and nonaquatic environment is due to their long persistent and bioaccumulative nature [4]. One of the notable effects is the increased resistance of infectious microorganisms to numerous antibiotics due to the overuse of pharmaceuticals in humans and pet animals [5].
The ridiculously excess use of pharmaceutical products was well reported [5, 6] showing significant negative consequences for environment and individual health system in the last decades [6, 7]. Thus, increasing levels of detected and measured medicine residues in the environment make pharmaceuticals as Contaminants of Emerging Concern (CEC) [8, 9]. Around 600 APIs or their metabolites and transformation products have been found in the environment, specifically in surface water and sewage effluent as well as in ground water and in the soil samples for more than 71 countries all over the world [7, 10]. More than 200 APIs from therapeutic classes of painkillers, vascular drugs, antibiotics, and antidepressants are identified in aquatic and terrestrial compartments in concentration ranging from few ng/L to 1000 μg/L [11]. Majority of APIs are partially degraded or treated in waste water treatment plants (WWTPs) and finally discharged in the aquatic environment [12], leading to a ubiquitous and uninterrupted contamination [13]. A good amount of contaminants are released through improper disposals and excretion through feces and urines into the sewage system [14]. Other significant sources of pharmaceuticals are hospitals and industries, whose effluents are loaded with very high concentration of APIs and their metabolites [15].
One of the frequently used pharmaceutical classes is antibiotics which are poorly metabolized after ingestion, providing a fraction from 25% to 75% which may leave the bodies in an unchanged form after consumption [16]. Under nationwide study of “emerging pollutants” in 139 rivers in 30 states of the USA, the US Geological Survey (USGS) detected biologically active compounds of diverse therapeutic classes [17]. The cardiovascular drug propranolol and antiepileptic drugs like carbamazepine and clofibrate have been reported in sewage treatment plants [18, 19]. Commonly used beta blockers (e.g., Metoprolol around 1.54 μg/L) and beta-sympathomimetics, estrogens (e.g., 17β-estradiol equal to 0.013 μg/L) [20], analgesic and anti-inflammatory drugs (e.g., Diclofenac up to 1.2 μg/L) [21], lipid lowering agents (e.g., clofibrinic acid up to 0.2 μg/L) [22], and antiepileptic drugs (e.g., Carbamazepine up to 2.1 μg/L) [21] were detected in major river water. Interestingly, there is strong evidence of nonprescription drugs like cotinine, caffeine, and acetaminophenone in samples of potable water in Atlanta, Georgia [23]. Gemfibrozil and carbamazepine were detected in drinking waters in ten cities in Canada by Tauber [24]. Under sex hormones, estrogens have been detected in plasticizers and preservatives, while 17α-ethinylestradiol (EE2), an active component of contraceptive pills, has been identified in groundwater and tap water samples [25].
The European Medicines Evaluation Agency (EMEA) and the Food and Drug Administration (FDA) of USA (US FDA) introduced risk assessment guidelines due to continuous detection of human and veterinary pharmaceuticals and their residues into the environment. The EMEA guidelines for the assessment of potential environmental risks were released in 2006 [26]. According to the US FDA guidelines, applicants have to present an environmental assessment report when the probable concentration of the API in the aquatic environment is ≥1 μg/L [27]. Previously, the FDA Center for Drug Evaluation and Research (CDER) issued a guidance document ‘Guidance for Industry for the Submission of an Environmental Assessment in Human drug Application and Supplements’ in 1995 [28]. Due to the emerging concern of their hazards, directive 2004/27/EC [29, 30] for human medicine and directive 2004/28/EC [31] for veterinary medicine required an Environmental Risk Assessment for marketing authorization of new pharmaceuticals products. The European Union Directive 2013/39/EU [32] included two pharmaceuticals (diclofenac and 17-alpha-ethynilestradiol (EE2)) and a natural hormone (17-beta-estradiol (E2)) in a first watch list of ten substances for the European Union monitoring of water. Furthermore, the watch list was amended in 2015 with directive 2015/495/EU [33], and three macrolide antibiotics (azithromycin, clarithromycin, and erythromycin), an additional natural hormone (estrone E1), a UV filter (octinoxate), and an antioxidant used as a food additive (butylated hydroxytoluene) were included [34].
The usage of pharmaceuticals is so extensive, and it is expected to intensify due to ageing of population in Europe and the USA, that the risk assessment requires a huge number of experimental data ensuing high costs, high time demand, and animal testing for in vivo testing. Regrettably, the number of available experimental data is very limited. The available data are also limited to specific species and assessed for particular environment compartment. In absence of sufficient experimental data, quantitative structure–activity/toxicity relationship (QSAR /QSTR) approach represents a convincing substitute to predict the possible hazards, from their chemical structure information [35]. Thus, to fill the data gaps, government and nongovernment regulatory authorities suggest the use of in silico methods for prediction of the physicochemical properties, toxicological activity, distribution, fate, etc. of pharmaceuticals along with their effects on environment and living systems much before they enter into market for usage. Therefore, usage of QSAR as one of the nonexperimental methods is noteworthy in order to lessen time, animal usage and cost involvement in toxicity prediction of pharmaceuticals [36, 37]. Persistent and Bioaccumulative (PB) behavior of pharmaceuticals was studied by Howard and Muir [38] employing QSAR models. More than 1200 pharmaceuticals were screened and prioritized for overall Persistence, Bioaccumulation and Toxicity (PBT) potential using the Insubria PBT Index and the US-EPA PBT Profiler by Sangion and Gramatica [39]. The European Union Commission’s Scientific Committee on Toxicity, Ecotoxicity and Environment (CSTEE) had recommended the use of QSAR models for screening purposes of pharmaceutical ingredients [40]. In recent times, a good number of software or expert systems are available for ecotoxicity prediction. A widely used online modeling tool to predict ecotoxicity of chemicals by QSAR is ECOSAR [41, 42].
Although few QSAR studies have been performed to fill the data gap in ecotoxicity of pharmaceuticals [43–46], there is a significant lack of knowledge about the environmental fate of a huge number of pharmaceuticals and their metabolites. Thus, generation of in silico models for pharmaceuticals’ ecotoxicity is the need of hour. This chapter intends to provide information regarding occurrence of pharmaceuticals and their residues in the environment, their persistence, environmental fate, and toxicity along with application of QSAR models and expert systems to predict risk and fate properties of pharmaceuticals. Concise ideas about commonly used endpoints or test batteries, available databases and expert systems employed for swift ecotoxicity predictions of pharmaceuticals are discussed.
Ecotoxicity of Pharmaceuticals: A General Overview
Source and Entry Routes
To understand the ecotoxicity of pharmaceuticals, the first step is to identify their sources and entry routes into the environment. Major sources and familiar pathways for environmental pollution of pharmaceuticals are illustrated below.
Household disposal: Due to lack or improper instructions about medication disposal, in many cases expired and unused medicines are dumped through the toilet or via waste bins, before being transferred to landfill sites as terrestrial ecosystem hazards. According to a study, unused medication were found due to modification of drugs by the doctor (48.9%), or self-discontinuation (25.8%), and the study identified that the common approach of disposal was throwing unused drugs in the trash (76.5%) or flushing them down the drain (11.2%) [47].
Industrial waste: One of the major sources of API , in-process and quality control failed final materials are generated from pharmaceutical industries. Though industries are following the Good Manufacturing Practices (GMP), still a large number of evidence is there for significant pharmaceutical emissions from industries. Concentrations up to several mg/L of pharmaceutical wastes have been identified in effluents for single compounds, specifically in Asian countries [48].
Hospital influent and effluent: Hospital influents and effluents are another noteworthy source according to several researches and it is proved that the eradication of the pharmaceuticals is partial. According to a study, 16 pharmaceuticals including antiepileptics and anti-inflammatories were detected in the hospital waste water [49].
Continuous introduction of diagnostic compounds: Iodinated X-ray contrast media like iomeprol, iohexol, and iopromide are generally employed as diagnostic tools for capturing detailed X-ray images of soft tissues and thereafter eliminated without appropriate treatment which helps in persistence of these wastes for a long period of time in the ecosystem [50].
Human excreta: APIs as well as their metabolites are excreted through the human excreta which is another imperative source of pharmaceutical waste. The complexity of risk assessment is increased manifold as the risk of metabolites is entirely dissimilar from the API.
Aquaculture: Sewage treatment plant (STP) sludge is routinely used as fertilizer in agriculture. Along with that, antibiotics have been also employed in aquaculture primarily for therapeutic purposes and as prophylactic agents (erythromycin, oxytetracycline, premix, sarafloxacin, sulfonamides) according to Food and Agriculture Organization (FAO) [51].
Manure, animal husbandry, and veterinary medicine: Urine and feces of animals in addition to direct application of veterinary medication in aqua farming leads to soil contamination besides contaminating both the surrounding surface and groundwater during rain [52].
Plant agriculture: Antibiotics like streptomycin with oxytetracycline are very commonly employed in plant agriculture in controlling bacterial diseases of flower and fruits. They are principally used for apple, pear, and related fruit trees for controlling fire blight caused by Erwinia amylovora. Studies have suggested that antibiotics applied to plants account for <0.5% of total antibiotic use in the USA [53].
We have demonstrated most common and significant sources, routes and fate of pharmaceuticals in Fig. 1.
Fig. 1.
Sources, routes and fate of pharmaceuticals in different compartments of environment
Occurrence and Effects
Pharmaceuticals are frequently employed in healthcare, farming, and aquaculture nowadays. The defined daily doses (DDDs) of consumed drugs can be found in the European Surveillance of Antimicrobial Consumption (ESAC) homepage [54]. A hefty number of research covering evidence of pharmaceuticals in water bodies, sewage and effluent treatment plant, manure, soil, and air dust have been performed. Pharmaceuticals are multicomponent mixtures rather than isolated pure substances, which will either be transformed by physical and chemical means and/or subsequently biotransformed by microorganisms. Therefore, multi-component mixtures are the primary concerning issue for the ecotoxicity assessment. Another point of concern is that majority of molecules can be neutral, cationic, anionic, or zwitterionic which makes the risk assessment study trickier. Thus, pharmaceuticals are evaluated for their acute toxicity by standard tests following the guidelines of Organisation for Economic Co-operation and Development (OECD), US EPA, International Organization for Standardization (ISO) employing standard laboratory endpoints like zooplankton, algae, and other invertebrates and fish. The most toxic and concerning classes were antibiotics, antibacterials, analgesics , cardiovascular drugs, antidepressants, and antipsychotics.
Antibiotics: Quinolones (mostly ciprofloxacin) have been identified in the hospital effluent up to a low μg/L range while β-lactams like carbapenems, monobactams, penicillins, cephalosporins, and β-lactamase inhibitors were detected in the lower μg/L range in hospital effluent as well as in the influent of a municipal STP [55]. Antibiotics like tetracycline, oxytetracycline, chlortetracycline, sarafloxacin, and cyclosporine A are largely found in the ecosystem and the most concerning issue is they have quite slow biodegradability in soil. Chlortetracycline and tetracycline were detected in ten out of 12 soil samples by Hamscher et al. [56]. As antibiotics have the ability to affect the microorganisms in sewage systems and WWTP, the inhibition of wastewater bacteria may critically influence degradation and nitrification process of organic matter [57]. Carucci [58] et al. reported noteworthy inhibition of the nitrification showed by lincomycin. Ciprofloxacin was identified to be active against Vibrio fisheri at a concentration of 5 mg/L [59]. Processes like transcription and translation are largely affected by macrolides, tetracyclines, lincosamides, P-aminoglycosides, and pleuromutilins for plants. Not only this, metabolic pathways like folate biosynthesis, fatty acid biosynthesis, and chloroplast replication are influenced by sulfonamides, triclosan, and fluoroquinolones, respectively [60]. Along with water bodies, microorganism and plants, antibiotics have the ability to affect the degradation of organic matter of soils and sediments to a large extent. A transitory effect on sulfate reduction was also identified when antibiotics were present in sediment [61]. Chloramphenicol was banned for food-producing animals within the USA and the EU in 1994 as it generated severe hazardous effects including myelosuppression to farmers [62]. In present times, the most significant effect is the surfacing of resistance due to improper and uncontrolled usage of antibiotics as medicine for human and animals as well as animal husbandry. The most well-known examples are methicillin-resistant Staphylococcus aureus (MRSA), vancomycin-resistant enterococci (VRE), and multiresistant pseudomonads [63].
Blood lipid lowering agents: Proliferation of peroxisomes in rodent liver is caused by fibrates and statins which have the ability to suppress synthesis of the juvenile hormone in insects. Additionally, they produce damaging effect to protozoan parasites, inhibiting growth and development. Zebrafish (Danio rerio) and amphibians can be highly affected by fibrates when present even at micromolar concentrations [3]. Quinn et al. [64] categorized bezafibrate as damaging for nontarget organisms with EC50 concentration between 10 and 100 mg/L and gemfibrozil as toxic with EC50 concentration between 1 and 10 mg/L. Fibrates have been evaluated by usual toxicity assays and the following no-observed-effect-concentration (NOEC) was detected for clofibric acid in C. dubia is NOEC (7 days) = 640 μg/L, for the rotifer B. Calyciflorus is NOEC (2 days) = 246 μg/L, and in early life stages of zebrafish is NOEC (10 days) = 70 mg/L [65]. Clofibrate is harmful to aquatic organisms with LC50 values of 7.7–39.7 mg/L. The most sensitive organism toward clofibrate is the fish Gambusia holbrooki [LC50 (96 h) = 7.7 mg/L]. Misra et al. [66] reported that clofibrate has no effect on in vitro growth of T. bruceii but reduces the incidence of P. berghei and the invasiveness as well as development of Acanthomoeba culbertsoni in exposed mammalian hosts.
Analgesics and nonsteroidal anti-inflammatory drugs (NSAIDs): NSAIDs have been detected in higher concentrations especially in surface water, ranging between 0.4 ng/L and 15 μg/L. Among NSAIDs, paracetamol, diclofenac, and ibuprofen are the most quantitatively found [67]. Cleuvers [68] checked that acute toxicities of NSAIDs were moderately low with concentration (EC50) attained in Daphnia in the range from 68 to 166 mg/L and from 72 to 626 mg/L in case of algae. Observed EC50 values for chronic toxicity are 23.8 mg/L, 23.6 mg/L, and 38.2 mg/L for diclofenac, ibuprofen, and naproxen, respectively in surface water. The NASIDs can reach in the environment with concentrations up to >1 μg/L due to their extensive usage and required pharmacokinetic and pharmacodynamic properties. Among NSAIDs, diclofenac has the highest acute toxicity with the effective concentrations below 100 mg/L and frequently detected in wastewater at a median concentration of 0.81 μg/L, whereas the maximal concentration in wastewater and surface water is up to 2 μg/L [69]. Acetylsalicylic acid affected reproduction in D. longispina and D. magna at concentrations of 1.8 mg/L [69]. Another frequently prescribed NSAID is paracetamol which is present in surface waters with concentration of 78.17 μg/L and within the range of 20 ng/L to 4.3 μg/L in STP effluents. The detected concentrations are higher than the stipulated no-effect concentration (PNEC) of 9.2 μg/L [3].
Beta-blockers: Propranolol showed the highest acute toxicity and log K ow supporting the fact that it is one of the strongest membrane stabilizers among the examined beta-blockers by Huggett et al. [70]. The NOEC and lowest-observed-effect-concentration (LOEC) of propranolol affecting reproduction in C. dubia were 125 and 250 μg/L. In case of H. azteca, reproduction affected after 27 days of exposure in at 100 μg/L [70]. In aquatic compartment, propranolol negatively affects survival, swimming behavior, and phototaxis of free-living aquatic stages of trematodes. Fathead minnows exposed to atenolol throughout embryo–larval growth showed LOEC and NOEC values for growth rate of 10 mg/L and 3.2 mg/L, respectively [3]. With 48-h exposure to propranolol, LC50 values of 1.6 mg/L, 29.8 mg/L, and 0.8 mg/L were obtained for D. magna, H. azteca, and C. dubia respectively, while acute exposure to nadolol did not affect the survival of the invertebrates [3]. Encystment of the protozoan Entamoeba invadens was inhibited in the presence of metoprolol [71].
Anticancer drugs: These are one of the most toxic therapeutic classes, designed to kill the cancer cells. They possess genotoxic, mutagenic, carcinogenic, teratogenic, and fetotoxic properties. One of the interesting points is that 14–53% of the drugs can be excreted in unaltered form through urine and feces, making them lethal for aquatic, soil organisms as well as for living systems and ecosystems [72]. Methotrexate has been reported to show acute effects in the ciliate Tetrahymena pyriformis with an EC50 of 45 mg/L and teratogenicity for fish embryos with an EC50 of 85 mg/L after 48 h of exposure in both cases [73]. Cyclophosphamide and methotrexate demonstrated immunosuppressant property to cause a proliferation in disease incidence and intensity in host–parasite systems [74]. Highly proliferative species like the ciliate Tetrahymena pyriformis showed acute toxicity to methotrexate with concentration of EC50 (48 h) = 45 mg/L [75]. Surprisingly, Methotrexate has no or little effect on definite protozoans like Babesia bovis, Toxoplasma gondii, and Leishmania tropica, as these organisms have dissimilar mechanisms of drug metabolism [76]. Again, cyclophosphamide emerges to have a minute effect on them. Development and growth of helminths in mammalian and bird hosts were destructively effected by cyclophosphamide and methotrexate. Abnormal teratogenicity was observed in fish embryos at higher concentrations of methotrexate [EC50 (48 h) = 85 mg/L] [76].
CNS affecting drugs: Fluoxetine, a serotonin reuptake inhibitor (SSRI), is one of the acute toxic pharmaceuticals with toxicity ranging from EC50 (48 h, alga) = 0.024 mg/L to LC50 (48 h) = 2 mg/L [18]. Sertraline demonstrated highly toxic response to rainbow trout (LC50 of 0.38 mg/L) at a 96-h exposure [77]. SSRIs showed growth inhibitions for algae and chronic toxicity tests confirmed they were sensitive with NOEC values below 1 mg/L [78]. On the contrary, organism like C. vulgaris was identified to be the least sensitive species for all SSRIs tested. Fluvoxamine showed a rise to the highest EC50 values for all algae species tested with concentration range between 3563 and 10,208 μg/L. Nitrazepam, benzodiazepines, and diazepam were recognized to amplify the number of microfilariae of Setavia cervi liberated from the lungs into the peripheral blood circulation in rats [79]. Caffeine was identified to stimulate the growth of P. falciparum and P. gallinaceum, while the mood stabilizer valproic acid and the antipsychotic haloperidol efficiently inhibited the in vitro growth of T. gondii [80]. Antiepileptics like carbamazepine and diazepam were categorized as potentially harmful to aquatic organisms as majority of the acute toxicity data was below 100 mg/L [65].
Sex hormones: These are one of the significant therapeutic classes emerged as the most serious aquatic environmental hazards due to their widespread use as human contraceptives. A synthetic estrogen named Ethinylestradiol (EE2) is generally found in oral contraceptive pills with evident estrogenic effects in fish. The EE2 concentrations below 1 ng/L creates striking effects in fertilization process, egg production and decreased expression of secondary male sex characteristics to fathead minnows. Lifelong contact of zebrafish to EE2 (with concentration of 5 ng/L) has led to reproductive failure due to the nonexistence of secondary male sex characteristics [81]. Exposure to 17β-estradiol caused an increased susceptibility to the protozoan T. gondii in mice, while increased pathology occurred in mammals infected with Leishmania mexicana amazonensis and exposed to either estradiol or testosterone [82]. Estradiol increased the vulnerability of cyprinids to hemoflagellates by the repression of lymphocyte proliferation [83]. Hydrocortisone can increase the intensity of ectoparasitic infections in fish at reasonably high concentrations. The detected concentrations of estrogenic products are typically below 50 ng/L in the effluent of STP and WWTP. On the contrary, high concentrations of 17α-estradiol and estriol (about 180 ng/L and 590 ng/L, respectively) were found in the USA [84].
Antiparasitic compounds: Antiparasitic compounds doramectin and ivermectin, with concentration of 0.112 mg/kg and 1.85 mg/kg, respectively were detected in dung of a farmhouse in the UK [85]. Interestingly, the concentrations of these drugs in soil were noticeably lower up to 0.046 mg/kg for the same farm [85]. Grønvold et al. [86] found that fenbendazole and ivermectin affect the endurance of the nematode Pristionchus maupasi at concentrations of 10–20 mg/kg and higher than 3 mg/kg, respectively. Svendsen et al. [87] reported that fenbendazole and ivermectin did not affect earthworms; however, the disappearance in dung was affected by the avermectin but not by the fenbendazole. Sun et al. [88] detected avermectin B1A in soil with the compost worm Eisenia fetida at a concentration of 17.1 mg/kg (LC50).
Antivirals: The emergence of Relenzas (zanamivir) and Tamiflu, neuraminidase inhibitors, in the USA began after the influenza pandemic (H1N1) in 2009 [89]. Tamiflu overpowered Relenza due to its relative ease of administration. Tamiflu, a prodrug form, is converted to the active molecule oseltamivir carboxylate (OC) in the liver. Generally, 80% of an oral dose of Tamiflu is excreted as OC through urine and the remaining fractions are excreted as oseltamivir ethylester-phosphate (OP) in the feces. Thus, both the API and its active metabolite ultimately are projected to attain a mean of 2–12 mg/L in WWTPs during moderate and severe pandemics, respectively [89]. The OC concentrations ranging from 293 to 480 ng/L have been reported in river waters charged with WWTP effluents during the 2009 pandemic [90].
Pharmaceutical Hazardous Wastes and Their Treatment
The definition of medical waste according to EPA is “all waste materials generated at health care facilities, such as hospitals, clinics, physicians’ offices, dental practices, blood banks, and veterinary hospitals/clinics, as well as medical research facilities and laboratories.” Among the medical waste, pharmaceutical waste is the most prominent and perilous ones. The USA has spent around $2.5 billion for the disposal of medical waste in 2012. Interestingly, with annual growth of 4.8%, by 2017, the increased cost is expected to $3.2 billion [91]. For instance, just hospitals in the USA produce more than 5.9 million tons of waste annually. Therefore, one can imagine the level of hazardous intensity generated by medical waste all over the world as all healthcare activities considered to humans generated medical wastes. The danger increased to manifold by mishandling or improper disposal of these medical wastes. Therefore, persons engaged to proper risk assessment and management must be aware with types of medical wastes especially pharmaceutical ones along with different approaches to treat them efficiently to minimize the hazards to environment and living systems. A typical list of pharmaceutical hazardous waste with few examples is illustrated in Fig. 2 and most commonly employed treatment for pharmaceutical as well as medical waste to avoid high risk of ecotoxicity is reported in Fig. 3.
Fig. 2.
Types of pharmaceutical hazardous wastes with few examples [Color of the boxes for the medical waste represents the color of the waste container]
Fig. 3.
Different ways of treatment for medical wastes to avoid high risk of ecotoxicity
Environmental Risk Assessment of Pharmaceuticals
Risk assessment is the process of assessing the concentration, occurrence, and level of environment and human exposure of a pharmaceutical product [92]. The key aim of environmental risk assessment (ERA) should be risk mitigation and risk management. The conclusion of the ERA report should be based on scientific reasoning supported by adequate ecotoxicity studies. The outline of the registration process and the ERA consist of European Commission and Council directives and regulations on registration, European policy, case law, and global (trade) agreements.
Risk Assessment Approaches
The most commonly employed risk assessment approaches of pharmaceuticals and their metabolites in various environment compartments are discussed below [93].
Hazard Identification
The first and foremost step for risk assessment is the identification of source and occurrence of hazards which supports the intensity of risk of a pharmaceutical . Majority of scientists highly relied on in vivo data, but due to huge deficiency of reasonable data for majority of pharmaceuticals related to specific species and definite environment compartment, greater effort should be offered on the efficient usage of in vitro assays and in silico analysis, as well as the use of computational techniques in systems biology [94].
Dose-Response Assessment
Detection of threshold dose of the toxic effect is imperative for scientific risk assessment of any hazards. Dose-response information over a wide range of test concentrations should be performed through quantitative high throughput screening (q-HTS). Additionally, sensitive assays should be able to detect toxicity at very low doses or below environmental levels experienced by living organisms. There should be sufficient scope available to extrapolate adversarial responses and to assess critical concentrations data employing statistical approaches [95].
Dose and Species Extrapolation
Major drawbacks of risk assessment are low-dose toxicity and lack of interspecies extrapolation data. In some cases, regulatory authorities and government organizations have implemented in silico models and expert systems as alternatives to deal with these problems. In vitro to in vivo extrapolation and physiologically based pharmacokinetic (PBPK) models are agreeable to sensitivity, variability, and uncertainty analysis using conventional tools [96].
Risk Characterization
The final phase of the ecological risk assessment is the risk characterization which integrates the analyses from the exposure and ecological effects characterization along with the doubts, hypothesis, strengths and limitations of the analyses. The risk characterization has two major components: risk estimation and risk description. Again, risk estimation compares integrated exposure and effects data in context of Levels of Concern (LOCs) and states the potential for risk [97].
Deterministic Approach and Calculation of Risk Quotients
The EPA uses a deterministic approach or the risk quotient (RQ) to evaluate toxicity to environmental exposure which is calculated by dividing a point estimate of exposure by a point estimate of effects. Calculation of RQ are based upon ecological effects data, hazards use data, fate and transport data, and estimates of exposure to the hazards. Thus, the estimated environmental concentration (EEC) is compared to an effect level, such as an LC50 (the concentration where 50% of the organisms die.)
RQ = Exposure/Toxicity
Environmental Risk Assessment Modeling of Pharmaceuticals
The risk assessment model considers the safety issues and RQ of individual pharmaceutical products. The most common approaches are offered in the guidance for environmental assessments for regulatory drug approvals by the US FDA [28] or by the European Medicines Agency (EMA) [26]. It is important to evaluate exposure of any pharmaceutical by the following ways previous to model a toxicological study [98]:
For modeling purpose, the exposure is assessed in the form of occurrence or the environmental concentration to which the biological system is exposed along with the duration and frequency being not on the concentrations to which individual living system is exposed. Exposure is also dependent on many miscellaneous factors such as sorption effects, metabolism, transformation processes, and fate.
The life cycle of any organism must be taken into account for understanding the effect of pharmaceuticals on them.
The MOA of pharmaceuticals needs to be determined to depict each step of molecular and functional effects.
Proper understanding of the pathways and target sites of pharmaceuticals in the biological system.
The bioavailability and toxicokinetic properties of the pharmaceutical need to be studied.
Complete pharmacokinetic and pharmacodynamic information are required to understand the absorption, distribution, metabolism, excretion, and toxicity pattern.
The hazard generated from inherent toxicity of the pharmaceuticals according to their chemical properties is needed to be studied.
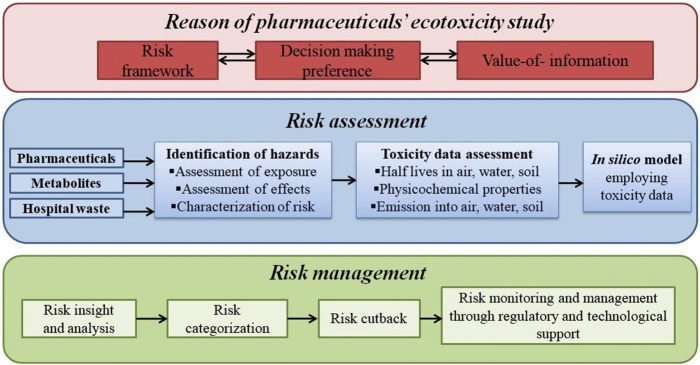
The most important steps for risk assessment and management process are reported in Fig. 4.
Fig. 4.
Reasons for ecotoxicity study along with steps for risk assessment and risk management due to pharmaceuticals hazards
Environmental Risk Management of Pharmaceuticals
The risk management can be defined as follows: “the process of identifying, evaluating, selecting, and implementing actions to reduce risk to human health and to ecosystems. The goal of risk management is scientifically sound, cost-effective, integrated actions that reduce or prevent risks while taking into account social, cultural, ethical, political, and legal considerations” [99]. The process of risk management caused by pharmaceuticals hazards must be balanced with cost benefit and practical to implement.
Accomplishment of Preventive Measures
Pharmaceuticals are one of the must have emergency products which cannot be stopped for use but the possible risks of them related to environmental can be managed by executing apposite preventative measure and safeguard. A set of guidelines has been set by the EMEA in 2006 as safety measures for risk management:
Initial assessment of risk for individual products,
Each pharmaceutical package should have appropriate product labeling and summary product characteristics (SPC),
Educated patients about the possible toxicity toward humans as well as environment through Package leaflet (PL),
Safe and appropriate storage as well as disposal of pharmaceutical products,
Minimizing the Input of Pharmaceutical Hazards into the Environment
To reduce the input of pharmaceutical products and their metabolites, the following steps can be employed effectively.
Awareness and Training
Awareness and training about occurrence and effect of individual pharmaceutical products along with their corresponding effects toward environment is the most crucial step. In addition, knowledge about disposal process of diverse types of pharmaceutical hazards is the first step to reduce the input of those hazards into the ecosystem. The awareness need to be spread among the shareholders, stakeholders and community using the pharmaceuticals, including patients, doctors and nurses, and pharmacists. The most important role need to be played by industries as they are the major source of pharmaceutical hazards and many of them are APIs when they are released into the environment without adequate waste treatment. In addition, each raw material, in process molecules and API should consist of material safety data sheets (MSDSs) intended to provide workers and emergency personnel with process for handling that product safely with information like: physical data, toxicity and health hazards, first aid, reactivity, storage, disposal, protective equipment, and spill-handling procedures. People related to risk management should possess information about the drug flows from the diverse sources of households, industries, hospitals and pharmacy [100].
High-End and Advanced Sewage Treatment
Most of the risk management procedures can be controlled with improvement of sewage treatment. Implication of sophisticated and enhanced waste water as well as sewage treatment can diminish the hormonal effects to living systems, ecotoxicity and pathogenic effects of the effluent to manifold. Recently, advanced effluent sewage treatment has been practiced comprehensively and performed employing photochemical oxidation, filtration, and adsorption processes [101].
Green and Viable Pharmacy
The final approach is the knowledge of green and sustainable pharmacy which supports environmentally benign compounds which after coming into the contact with environment will be degraded with minimum hazardous effects in no time [100]. In the present scenario, it is the least practiced methods, but in long term of sustainability, it is the need of hour.
Furthermore, possible measures, roles and action to reduce the ecotoxicity imposed through pharmaceutical hazards by diverse stakeholders are addressed in Table 1 for improved understanding .
Table 1.
Roles of different stakeholders to reduce the pharmaceutical induces ecotoxicity
| Stakeholders | Possible measures, roles and action |
|---|---|
| Doctors |
• Prescribed only required medicine. • Awareness about their hazardous properties. |
| Pharmacist |
• Awareness and information about usage and disposal to patients. • Take back system of unused medicine if possible. |
| Patients |
• Improvement of compliance and proper disposal. • Consumption of medicine only if prescribed by a doctor. |
| Hospitals |
• Amalgamation of the delivering pharmacy/wholesaler about the handling of expired medicaments. • Proper implementation of rules and regulation by hospital authorities for the disposal of pharmaceuticals and other medicaments. |
| Industries |
• Periodical report of environmental assessment related data of individual pharmaceuticals along with raw materials, in process ingredients. • Complete information about analytical methods and results. • Proper packaging and labeling with proper uses, storage and disposal. • Improved drug delivery systems so that smaller doses are needed. • Environment friendly packaging with extended shelf life of packing material. |
| Drinking water |
• Extended monitoring of water for hospital, industry and pathological centers. • Advanced treatment of water • Suitable approach to complete or up to acceptable limit removal of waste. |
| Waste water treatment |
• Tertiary treatment methods such as ozonation, activated carbon adsorption, or nanofiltration. • Separate and careful piping between waste water and rain water. |
| Authorities |
• Instigation and back up of communication between all stakeholders. • Implementation of threshold limits for each pharmaceuticals and other medicaments for different environmental compartments. |
| Policies and regulations |
• Annexation of every APIs and formulation product in environmental legislation. • Updated regulation for the management of out dated and new as well as existing medicaments. |
| Green pharmacy |
• Fast and trouble free degradability of pharmaceutical products and supplements. • Improvement of synthesis and renewable feedstock for preparation of environment friendly pharmaceuticals. |
Global Regulatory Bodies Concerning Pharmaceuticals Hazard and Risk Quantification
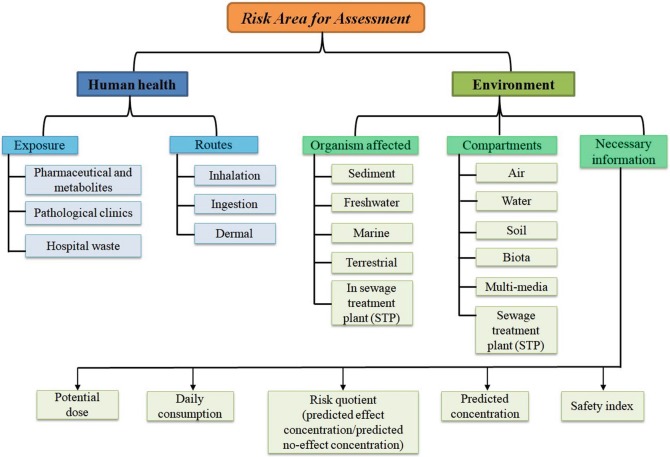
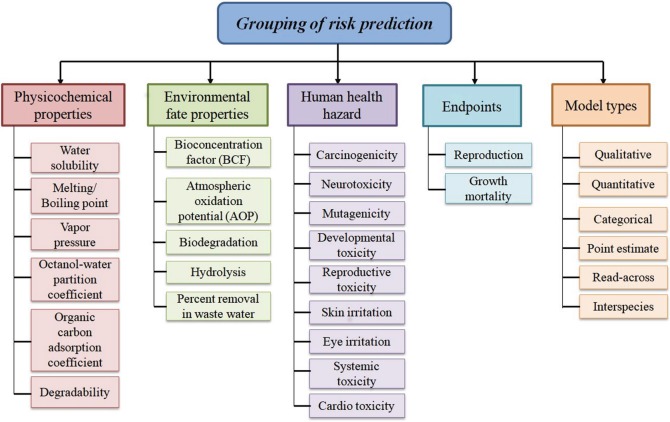
Increasing exposure of pharmaceutical wastes in the environment is an affair of anxiety and a hot topic worldwide. The risk of pharmaceutical hazards are directly related to the environment and indirectly related to human health to a great extent. There is a strong need to predict physicochemical properties, environmental fate, effects of pharmaceuticals and their metabolites, as concerned experimental data for different compartments of the environment are absent for huge number of pharmaceuticals till today. A good number of government regulatory authorities related to environment safety consider in silico approaches like structure–activity relationship (SAR) and QSAR to predict the hazardous effect and fate of untested and newly introduced pharmaceuticals as fast as possible with economical way using less animal testing [26, 28, 102–106]. To predict the human health or environmental hazards due to exposure of pharmaceuticals, in silico models are utilized by the OECD and the developed models are organized as searchable databases which are intended for providing risk assessment and management resources. The models are good sources as screening tools for pharmaceutical databases when there is missing of chemical-specific data for establishing priorities for risk assessment and for evaluating issues of potential concern [102]. Commonly used endpoints for ecotoxicity modeling and areas where QSAR models can be employed for risk prediction are reported in Table 2. Regulatory bodies responsible for the risk identification, assessment and management of pharmaceuticals ecotoxicity across the globe are listed in Table 3. Areas for risk assessment and grouping of information for risk prediction of human health and environment according to OECD’s guidelines are illustrated in Figs. 5 and 6, respectively.
Table 2.
A list of endpoints for modeling purpose under OECD and areas where QSAR models can be employed
| Endpoints for modeling under OECD | Areas where QSAR models can be used |
|---|---|
|
• Physical-chemical properties: Boiling point, melting point, vapor pressure, octanol–water partition coefficient, organic carbon–water partition coefficient and water solubility • Ecological effects on endpoints: long-term toxicity, acute Daphnia toxicity, Acute fish toxicity, terrestrial toxicity, algal-toxicity, marine organism toxicity, microorganism toxicity in sewage treatment plant • Environmental fate: Biodegradation, hydrolysis in water, atmospheric oxidation, and bioaccumulation ▪ effects: Acute oral, acute inhalation, eye irritation, acute dermal, skin sensitization, skin irritation, repeated dose toxicity, reproductive toxicity, genotoxicity, systemic toxicity, developmental toxicity, mutagenicity, carcinogenicity, etc. |
• Prioritization of existing pharmaceuticals for toxicity to environment • Classification and labeling of new pharmaceuticals • Risk assessment of new and existing pharmaceuticals • Guiding experimental design of regulatory tests or testing strategies • Providing mechanistic information • Filling up the large data gaps • Building a proper database of each pharmaceutical to different species regarding ecotoxicity • Development of expert systems for each therapeutic class for diverse compartments of the environment • Construction of efficient interspecies models to extrapolate data from one species to another species when data of a specific species is missing |
Table 3.
Global regulatory agencies which deal with the environmental risk assessment and risk management of pharmaceuticals
| Regulatory agencies | Objective | Responsibility and method of risk assessment |
|---|---|---|
| AEA (Australian Environment Agency) | Advises clients on the environmental hazards and potential risks associated with the production, use and disposal of chemicals. AEA is a member of the Society of Ecotoxicology and Chemistry (SETAC) | AEA has undertaken reports for the Department of Sustainability, Environment, Water, Population and Communities (DSEWPaC), particularly with respect to their environmental assessments performed on new and existing agricultural and veterinary chemicals for the Australian Pesticides and Veterinary Medicine Authority (APVMA), and industrial chemicals for the National Industrial Chemicals Notification and Assessment Scheme (NICNAS). |
| CDER (Center for drug evaluation and research) | CDER reviews New Drug Applications to ensure that the drugs are safe and effective. Its primary objective is to ensure that all prescription and over-the-counter (OTC) medications are safe and effective when used as directed. | An assessment of risk to the environment is required for manufacture, use and distribution of human drugs under the National Environment Policy Act of 1969 and an environmental assessment procedure was developed by the US Food and Drug Administration (US FDA) as a part of the registration procedure for new human pharmaceutical drugs. Additionally, in 1995, the FDA-CDER issued a new guidance for the Submission of an Environmental Assessment in Human drugs. In 1997 the FDA implemented a Note for Guidance paper in which all drugs entering the aquatic compartment at levels below 1 μg/L Predicted Environmental Concentration (PECEFFLUENT) were exempted from a detailed risk assessment. |
| EMEA (European agency for the evaluation of medicinal products) | EMEA exhibits the scope and legal basis for risk assessment of pharmaceuticals and outlines the general considerations and the recommended stepwise procedure for their risk assessment. The guideline considers the specific features of pharmaceuticals, e.g., the use of available pharmacological information. Previously environmental risk assessments performed mainly on acute ecotoxicity data, but in recent time EMEA draft has proposed to include pharmacokinetic and pharmacodynamic data for environmental risk assessment. |
Environmental risk assessment is divided into three phases: (a) Phase I: Pre-screening and estimation of based on the drug only, irrespective of its route of administration, pharmaceutical form, metabolism and excretion (b) Phase II Tier A: Screening and initial prediction of risk where all relevant data should be taken into account, e.g., data on physical-chemical properties, primary and secondary pharmacodynamics, toxicology, metabolism, excretion, degradability, and persistence of the drug substance and/or relevant metabolites (c) Phase II Tier B: Extended and substance and compartment-specific risk assessment. Information from the refined data set is available comprising information on route(s) of excretion; and qualitative and quantitative information on excreted compounds, and possibly additional long-term toxicity data |
| EU-CSTEE (European Union Commission’s scientific committee on toxicity, ecotoxicity and environment) | The CSTEE has identified the need for a proactive approach in obtaining data on the environmental effects of pharmaceuticals. Thus, it is recognized that a prioritization procedure needs to be developed for environmental risk assessment of pharmaceuticals, and that this should follow the general scheme for chemicals described in the White Paper for future EU chemicals policy i.e., REACH guideline. | QSAR is the first step in gaining more general knowledge on the risk assessment issue as an alternative to nonanimal method. In contrast to the amount of analytical data, information about the ecotoxicological effects of drug residues is scrubby. To create a broader basis for the evaluation of the ecotoxicological relevance of pharmaceutical compounds, proper documentation of their effects and the reason are identified and documented. |
| MHLW (Ministry of Health, Labour and Welfare of Japan) | The MHLW constructed a research group to build up a concept on the regulation of pharmaceuticals for environmental safety in 2007. The regulation system is similar to that of general chemicals in Japan and the Guideline by EMEA. The main function of this group is to establish a risk-benefit analysis committee for the pharmaceuticals which have a high risk for environmental organisms and to human health. | The risk assessment is judged by the PEC/PNEC (Predicted Environmental Concentration/Predicted No Effect Concentration) ratio or ΣPECi/PNECi. In addition, the Organization for Pharmaceutical Safety and Research (OPSR) conducted compliance reviews on application data. This was followed by the integration of the aforementioned Evaluation Center, OPSR, and part of the Medical Devices Center to form a new independent administrative organization, the Pharmaceutical and Medical Devices Agency (PMDA). The MHLW and PMDA handle a wide range of activities from clinical studies to approval reviews, reviews throughout post-marketing stage, and pharmaceutical safety measures. |
| NICNAS (National Industrial Chemicals Notification and Assessment Scheme) | NICNAS was established in July 1990 under the Industrial Chemicals (Notification and Assessment) Act 1989 by Australian Government Department of Health. A range of state, territory and Commonwealth government agencies share regulatory responsibility for chemical safety in Australia, with each chemical being regulated according to its use, whether as a pharmaceuticals, veterinary medicine, pesticide, food additive or industrial chemical. |
The major responsibility of NICNAS are: • Assessing new industrial chemicals for human health and/or environmental effects • Maintaining the Australian Inventory of Chemical Substances (AICS) • Circulation of information on the human health and environmental impacts of chemicals and recommending on their safe use • Registering new industrial chemicals |
| REACH (Registration, Evaluation, Authorisation and Restriction of Chemicals) | Aims to improve the protection of human health and the environment through the better and earlier identification of the intrinsic properties of chemical substances. | “No data no market”: the REACH Regulation places responsibility on industry to manage the risks from chemicals and to provide safety information on the substances. Manufacturers and importers of substances have a general obligation to submit a registration to the European Chemicals Agency for each substance manufactured or imported in quantities of 1 tonne or more per year per company |
| SECIS (Swedish Environmental Classification and Information System for pharmaceuticals) | An authorized regulatory body which was initiated in 2005 by the Swedish Association for the Pharmaceutical Industry. The rationale of the classification system is to offer the public and health care sectors with environmental information about all active pharmaceutical ingredients (API) on the Swedish market up to now. | To improve risk management decision making, sufficient knowledge about environmental exposures and effects in nontarget species for all relevant pharmaceutical substances is needed. Within SECIS, the pharmaceutical companies provide environmental data and classify their products according to predefined criteria and a guidance document. The guidance document is developed for the purposes of SECIS, but it is based on the European Medicines Agency (EMA) guideline for environmental risk assessment of pharmaceuticals and the European Commission Technical Guidance Document (TGD). |
| UBA (Federal Environment Agency) | The German Medicines Act provides that the UBA is responsible for the environmental risk assessment. The UBA started assessing the environmental impact of veterinary and human pharmaceuticals in an authorization routine in 1998 and 2003, respectively. | The UBA already assessed around 180 veterinary and around 240 human pharmaceutical formulations. Filtering concepts established between UBA and the authorization agency responsible for veterinary medicines focused the ERA on antibiotics, parasiticidal substances and analgesics. Cytostatic medicines, hormones and contrast agents dominated the human medicine dossiers assessed by UBA. |
| VICH (International Cooperation on Harmonization of Technical Requirements for Registration of Veterinary Medicinal Products) | VICH is a trilateral (EU–Japan–USA) program aimed at harmonizing technical requirements for veterinary product registration was officially launched in April 1996. The initiative to begin the harmonization process came about in 1983 when the first International Technical Consultation on Veterinary Drug Registration (ITCVDR) was held. | Veterinary medicinal products (VMPs) are regulated for environment safety as described in Environmental Impact Assessment for VMPs; Phase I in 2000 and Phase II in 2004. |
Fig. 5.
Areas of risk assessment and modeling for ecotoxicity prediction as stated to the OECD
Fig. 6.
Category of information included in predicting health and environmental effects according to the OECD guidelines
In Silico Modeling in Ecotoxicity of Pharmaceuticals
Computational methods intend to harmonize in vitro and in vivo toxicity tests to potentially curtail the necessity for animal testing, reduce the cost and time of experiments, and improve toxicity prediction and safety assessment. Additionally, computational approaches have an exclusive benefit of being competent to approximate chemicals for its toxicity even before they are synthesized [102]. With increasing concern about the ecotoxicity and human health, the storage, distribution and release of pharmaceuticals after their usage to the environment are controlled and regulated at various levels by governments and regulatory bodies. Applications of assortment of in silico tools are very much constructive option to provide sufficient information in a regulatory decision making context in the absence of experimental data [102]. Most commonly employed predictive in silico tools are depicted in Fig. 7.
Fig. 7.
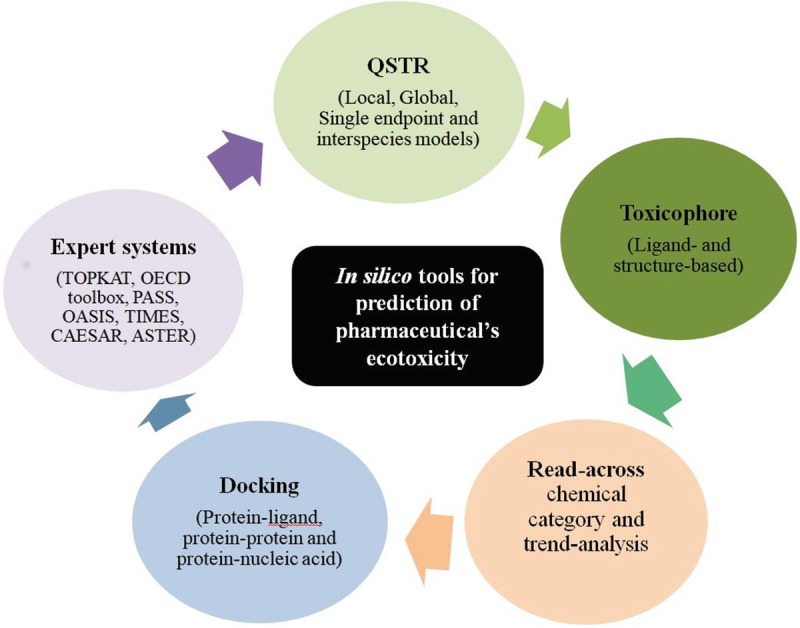
Most common in silico tools for the prediction of pharmaceuticals ecotoxicity
Among the available in silico predictive models for ecotoxicity, majority of the models are generated employing QSAR techniques, for toxicity prediction some time termed as quantitative structure–toxicity relationship (QSTR). Along with the typical QSAR/QSTR models, toxicophore or structural alerts, read-across, chemical analogue, trend analysis and docking approaches are employed in many successful prediction researches. The QSTR approach attempts to correlate structural/molecular properties (x 1, x 2, …, x n) with toxicity response (Y), for a set of molecules by means of statistical methods [107], generating simple mathematical relationship as follows:
| 1 |
The major objective of QSAR/QSTR modeling is to examine and recognize the influential factors for the measured activity/toxicity for a particular system, to have an insight of the mechanism and behavior of the studied system. This strategy generates a mathematical model which joins experimental measures with a set of chemical descriptors determined from the molecular structure for the studied compounds. The constructed model should possess good predictive abilities in order to predict the studied response for untested or future compounds. The factors leading the events in a biological system are depicted by a multitude of physicochemical descriptors [107]. From its initial days, the QSAR approach has come a long way. Along with the time, new and modified methods, algorithms and dimensions (1D to 7D) have been applied in QSAR studies are discussed elsewhere [36, 37]. Goodness-of-fit and prediction quality is two most important features for an acceptable and reliable QSAR model. The predictive quality of the QSAR model is checked through different validation statistics and metrics. Thus, validation of QSAR models is the major step along with defining the applicability domain for the prediction of untested and future compounds [108, 109].
Why In Silico Models Required?
The 3Rs concept signifies “Reduction,” “Replacement,” and “Refinement” regarding animal experimentation in scientific experiments. “Reduction” defines to the lessening the number of animals used to get precise results, “Replacement” corresponds to the implication of nonliving resources to substitute conscious living higher animals, and “Refinement” suggests turn down the severity or cruelty of inhuman methodologies applied to the experimental animals [110]. Thus to set up the 3Rs concept, in silico techniques are one of the best options available. The European Centre for the Validation of Alternative Methods (ECVAM) was established in the year 1991 that agrees with the principle of 3Rs.
The ban of animal experimentation by regulatory agencies and government organizations initiated the need of molecular modeling approach [111]. Council Directive 86/609/EEC on the approximation of Laws, Regulations, and Administrative provisions of the member states regarding the protection of animals used for experimental and other scientific purposes. The testing ban on the finished cosmetic products applies since 11 September 2004; the testing ban on ingredients or combination of ingredients applies since 11 March 2009. The marketing ban applies since 11 March 2009 for all human health effects with the exception of repeated-dose toxicity, reproductive toxicity, and toxicokinetics. For these specific health effects, the marketing ban applies since 11 March 2013, irrespective of the availability of alternative nonanimal tests [112].
Regulatory decision making through SARs and QSARs models for predicting aquatic toxicity, physicochemical parameters and environmental fate properties.
Filling data gaps for ecotoxicity due to pharmaceuticals hazards as acceptable toxicity data of pharmaceuticals to environment and human health is <5% [113]. In silico prediction has the proficiency to help out in the prioritization of pharmaceuticals for testing, and for predicting ecotoxicity to allow for classification. Computer models are a reliable source for toxicity predictions as they can be used as one of the significant sources for filling the missing data of ecotoxicity.
Understanding the real mechanism of action for each pharmaceutical for specific endpoints and environment compartment system. For many modeling approaches, it may be assumed that molecules fitting the similar QSAR models are acting by the same MOA [114].
Cost and time saving are two major reasons for the use of in silico approaches. Even a simple ecotoxicological assay may cost several thousand dollars. On the contrary, early toxicity prediction can save a good amount of time and money [115]. A schematic representation is provided in Fig. 8 showing the requirement of in silico modeling evaluating pharmaceuticals ecotoxicity .
Fig. 8.

The need of in silico modeling assessing the impact of pharmaceuticals ecotoxicity
Successful In Silico Models for Ecotoxicity Prediction of Pharmaceuticals
Sanderson et al. [116] provided a baseline to fill the screening data regarding API environmental toxicity utilizing the US EPA generic aquatic QSAR model ECOSAR for screening of more than 2800 pharmaceuticals and. The model can be successfully used to predict both acute and chronic aquatic toxicity. In another work, toxic potential of mixtures of the β-blockers and related metabolites are modeled for the phytotoxicity endpoint by Escher et al. [117]. For performing the modeling, they assumed two conditions; first, the metabolites lose their definite response and act as baseline toxicants and second, the metabolites expose the identical specific MOA like their parent drug. The authors accounted experimentally determined liposome–water partition ratios at pH 7 as independent variable for correlating with the response variable to make the QSAR analysis more reliable.
Sanderson and Thomsen [118] overestimated the toxicity for 70% of the 59 pharmaceuticals and more than 94% underestimated toxicity predictions by less than a factor of ten for the remaining 30% pharmaceuticals by ECOSAR v3.20. The authors have reported correlation coefficients ranging from 0.73 to 0.76 between all the modeled Log EC50 and Log K ow. The slopes of the Log EC50-Log K ow regression based on measured data from the US National Oceanic and Atmospheric Administration (NOAA) database for both fish and daphnia equal to −0.86 which suggested a narcotic MOA. In another study, acute toxicity was predicted (>92%) employing a QSAR model developed by Sanderson and Thomsen [45] suggesting a narcotic MOA of 275 pharmaceuticals. Their analysis suggested 68% of the pharmaceuticals have a nonspecific MOA based on model prediction error. The authors have also compared the measured effect data to the predicted effect concentrations utilizing ECOSAR regarding the predictability of ecotoxicity of pharmaceuticals and accurate hazard categorization relative to Global Harmonized System (GHS). Pharmaceuticals were predicted employing the model resulting in 71% algae, 74% daphnia, and 83% fish datasets that could be compared.
One of the first interspecies QSAR models for 77 pharmaceuticals’ ecotoxicity was reported by Kar and Roy [119] for Daphnia magna and fish endpoints. Analyzing the interspecies models, the authors have reported that the keto group and the  (aasC) fragment are predominantly accountable for higher toxicity of pharmaceuticals to D. magna. On the contrary, along with the keto group, structural fragments like X=C=X, R–C(=X)–X, and R–C≡X are principally responsible for fish toxicity. The interspecies models were also employed to predict fish toxicities of 59 pharmaceuticals and Daphnia toxicities of 30 pharmaceuticals when Daphnia and fish toxicity data were present, respectively.
(aasC) fragment are predominantly accountable for higher toxicity of pharmaceuticals to D. magna. On the contrary, along with the keto group, structural fragments like X=C=X, R–C(=X)–X, and R–C≡X are principally responsible for fish toxicity. The interspecies models were also employed to predict fish toxicities of 59 pharmaceuticals and Daphnia toxicities of 30 pharmaceuticals when Daphnia and fish toxicity data were present, respectively.
Christen et al. [120] developed VirtualTox Lab [121] for the prediction of pharmaceuticals’ effect in the aquatic system . The study guides to the conclusion that the MOA perception is most suitable for the classification of highly active compounds (HC). The authors also reported that modification could be performed by balancing this concept utilizing the QSAR model (VirtualTox Lab), whereas the fish plasma model appeared to be less appropriate due to the requisite of environmental concentration above 10 ng/L for the identification of a risk. The Virtual-ToxLab can support the MOA concept and can be advantageous to distinguish surplus targets of the pharmaceutical to assess the ecotoxicity.
Das et al. [122] reported interesting interspecies correlation models using rodent toxicity as dependent variable and fish, daphnia and algae toxicity data as independent variables separately for 194 pharmaceuticals. All interspecies extrapolation QSAR models were generated using multiple statistical tools. Explaining the models, the authors concluded that heteroatom count and charge distribution were noteworthy parameters of the rodent toxicity along with the atom level logP contributions of various structural fragments and a mixture of extended topochemical atom (ETA) indices reflecting electronic information and branching pattern of molecules. The authors also concluded that atom level logP contributions of dissimilar fragments, charge distribution, shape and ETA parameters were imperative in describing the daphnia and fish toxicities in the interspecies correlation models with algae toxicity. Interestingly, the toxicity of pharmaceuticals to rodents bears minimum interspecies correlation with other mentioned nonvertebrate and vertebrate toxicity endpoints.
De García et al. [123] performed the environmental risk assessment of 26 pharmaceuticals and personal care products (PPCPs) based on the ecotoxicity data tested through bioluminescence and respirometry assays. This was followed by classification and labeling of pharmaceuticals by the GHS using the US EPA ecological structure–activity relationship (ECOSAR™). The risk impact of these pharmaceuticals in WWTPs and in the aquatic environment was predicted following the criteria of the EMA. According to their two ecotoxicity tests, 65.4% of the PPCPs showed prominent toxicity to aquatic organisms. Pharmaceuticals like 1,4-benzoquinone, ciprofloxacin, acetaminophen, clofibrate, clarithromycin, omeprazole, ibuprofen, triclosan, and parabens showed risk threat for aquatic environments and/or for activated sludge of WWTPs according to the performed analysis.
Sangion and Gramatica proposed [39] a screening approach to evaluate the potential PBT of around 1200 pharmaceuticals employing two different QSAR models. The authors applied the Insubria-PBT-Index, a Multiple Linear Regression (MLR) QSAR model based on simple molecular descriptors, implemented in QSARINS software. An agreement of 86% was reported between the two models and a priority list of 35 pharmaceuticals, highlighted as potential PBTs by consensus, was suggested for additional experimental validation. The models can be useful in the hazard assessment, performing preliminary screening and prioritization of pharmaceuticals, mainly associated with the potential PBT behavior of the prioritized pharmaceuticals.
Externally validated QSAR models, specific to predict acute toxicity of APIs in three aquatic trophic levels endpoints, i.e., algae, Daphnia and two species of fish were developed using the QSARINS software by Sangion and Gramatica [124]. The developed MLR-ordinary least squares (MLR-OLS) models were developed with theoretical molecular descriptors computed through PaDEL-Descriptor software. The selections of descriptors was performed by Genetic Algorithm (GA). The generated models were employed further to predict acute toxicity for a large set of APIs without experimental data by Principal Component Analysis (PCA). Further, a trend was set by the combination of toxicities for all the studied organisms and the trend is termed as Aquatic Toxicity Index (ATI) which allowed the raking of pharmaceuticals according to their potential toxicity upon the complete aquatic environment. Not only that, the accuracy of the models was compared with the accuracy of the frequently used software ECOSAR, and the authors concluded that their models showed better performances.
Sangion and Gramatica [125] proposed quantitative activity-activity relationship (QAAR) models, implemented in QSARINS by theoretical molecular descriptors to enhance the quality and predictivity of the interspecies relationships between toxicity toward Daphnia magna and two fish species, Pimephales promelas and Oncorhynchus mykiss. The authors claimed that the developed invertebrate-fish interspecies models could reduce the composite experimental tests on upper trophic organisms and reduce animal experiments. They also illustrated that the Daphnia could serve as a surrogate for fish toxicity and the fish-fish intercorrelations could be used for evaluating toxicological data when sufficient information is unavailable.
Successful in silico models, especially QSAR models on ecotoxicity of pharmaceuticals were discussed in this section. There is no doubt that reported number of models is quite low compared to that of chemical toxicity models. One of the main reasons for this is the limited experimental data on ecotoxicity of pharmaceuticals. One has to understand that it is impossible to study toxicity of each pharmaceutical in different species in diverse compartments. Thus, QSAR models will provide the predicted values when experimental data are absent for specific pharmaceutical. On the other point, making of more interspecies models is the need of time to extrapolate toxicity data from one species to another.
Endpoints
To generate ecotoxicity data of pharmaceuticals, they should be assayed employing specific endpoints which are sometime called as test batteries. The evaluated data are rich source of information for making ecotoxicity database as well as for making in silico models and expert systems. Thus a clear understanding is essential about the ecotoxicity endpoints as they are important to make in silico models as well as for deeper understanding about the mode of toxicity of a specific drug for a definite endpoint. With extensive literature survey, we have enlisted most frequently employed endpoints by the scientist community in Table 4.
Table 4.
Toxicity endpoints for the modeling of pharmaceuticals ecotoxicity
| Endpoints | Species | Depiction | Implication |
|---|---|---|---|
| Algae |
Chlorella vulgaris Chlorella pyrenoidosa |
Unicellular fresh-water green microalgae comprises a major part of phytoplankton | Study the toxic action of organic compounds |
| Pseudokirchneriella subcapitata (Selenastrum capricornutum) | One of the prime producers of the aquatic ecosystem and ideal test organisms for toxicological studies. | Ecotoxicity is measured by growth rate inhibition of green algae P. subcapitata | |
| Scenedesmus obliquus | Common cosmopolitan green algae, often occurring as almost a pure culture in fresh water plankton. | Ability to grow in industrial wastewaters of different origins showing good adaptation ability and versatile microalgae as toxicity test endpoint | |
| Scenedesmus vacuolatus | Green algae of the Chlorophyceae. Colonial and nonmotile in nature. | Employed in the prediction of photoinduced toxicity of polycyclic aromatic hydrocarbons and ionic liquids (ILs) | |
| Bacterium | Escherichia coli | A Gram-negative, facultative anaerobic, rod-shaped bacterium of the genus Escherichia. | E. coli is used as a model organism in ecotoxicity modeling studies. For example, it is used to test cytotoxicity of metal oxide nanoparticles |
| Vibrio fischeri, Vibrio natriegens | Gram-negative rod-shaped bacterium having bioluminescent properties and found predominantly in symbiosis with various marine animals | Employed in the research of microbial bioluminescence, quorum sensing along with ecotoxicity testing for diverse range of organic chemicals and pharmaceuticals | |
| Bacillus | A genus of gram-positive, rod-shaped bacteria and member of the phylum Firmicutes | Chlorophenol toxicity is tested on bacillus species. | |
| Pseudomonas fluorescens | P. fluorescens has versatile metabolism. Generally found in the soil and in water. | Can be employed for modeling of antibiotics toxicity and resistance studies | |
| Crustaceans | Daphnia magna, Daphnia pulex, Daphnia ambigua, Daphnia melanica | Small planktonic crustacean and one of the small aquatic crustaceans commonly called water fleas. Most commonly employed species is D. magna. | Invertebrate species in aquatic food webs has been used as a representative test species for ecotoxicological evaluation of diverse organic chemicals using immobilization test |
| Thamnocephalus platyurus | A family of crustaceans with wide distribution including Western Australia and Southern Africa. | 24 h toxicity test employed for screening of pure compounds, effluents, sediments, surface and ground waters, wastewaters, and biotoxins | |
| Duckweed | Lemna minor | One form of aquatic vascular plant (duckweed) known as thallus, which floats on the surface of the water. | Used in modeling of phytotoxicity of ILs and growth inhibition test of duckweeds |
| Lemna gibba | Used in testing the phytotoxicity of pesticides and other environmental chemicals to higher plants. | ||
| Enzyme | Acetylcholinesterase | Catalyzes the hydrolysis of acetylcholinesters with a relative specificity for acetylcholine in autonomic nervous system function. | Enzyme inhibition data of the acetylcholinesterase from Electrophorus electricus, the AMP deaminase and the antioxidant enzyme system of mouse liver are important for toxicity prediction |
| Fish | Channel Catfish Ovary (CCO) | CCO is the cell line of choice for the propagation and diagnosis of Channel Catfish Virus (CCV). | Standard for diagnosing Channel Catfish Virus Disease (CCVD) in farm reared Channel Catfish. Prediction of ILs has also been performed |
| Zebrafish (Danio rerio) | A tropical freshwater fish belonging to the family Cyprinidae | Standardized under the OECD and employed to test chemicals and pharmaceuticals | |
| Fathead minnow (Pimephales promelas) | Pimephales promelas is the EPA recommended vertebrate species for freshwater chronic toxicity tests. | Studied to examine the effects of waste materials on the aquatic life. Effects induced by progestins are largely studied employing fathead minnow. | |
| Mammalian cells | Human keratinocyte cell line (HaCaT) | HaCaT cells are a spontaneously immortalized, human keratinocyte line. | Used for studies of skin biology and cytotoxicity assessment of metal oxide |
| CaCo-2 | Heterogeneous human epithelial colorectal adenocarcinoma cells. | Permeability coefficients across the cellular membranes of Caco-2 cells are generally employed for modeling | |
| HeLa | A prototypical cells of the human epithelium used in scientific research. | Derived from cervical cancer cells and largely employed for anticancer activity | |
| Prostate cancer cell line (PC3) | A human prostate cancer cell lines | Used in modeling of prostate cancer inhibitors | |
| Human malignant melanoma (Fem-X) | Derived from a lymph node metastasis of a melanoma patient | Modeling of anticancer drugs. | |
| HT-29 | A human colorectal adenocarcinoma cell line with epithelial morphology | Sensitive to the chemotherapeutic drugs used in standard treatment for colorectal cancer | |
| Rat cell line—IPC-81 | Promyelotic leukemia rat cell line IPC-81 | Employed in cytotoxicity assays of ILs | |
| Protozoa |
Tetrahymena thermophila Tetrahymena pyriformis |
Free-living unicellular ciliated protozoa | Commonly employed endpoint for the assessment of the environmental toxicity |
| Tadpoles | Bufo vulgaris formosus, Rana japonica | Larvae of the frogs, typical amphibious animals bridging the gap between aquatic and terrestrial animals. | Regularly implicated for toxicity testing purposes and risk assessments, have been recommended by the EU-TGD. |
| Yeast | Saccharomyces cerevisiae | Eukaryotic model organism, small in size, reproduction time quick and economic. | Important species for modeling of metal oxide nanoparticles |
Databases
The first condition to develop a reliable, accurate, and reproducible in silico model is to use good quality ecotoxicological data of pharmaceuticals and their metabolites in diverse environmental compartments with different concentration. A significant number of toxicity databases toward environment are accessible to public and the numbers are increasing with time. Although according to the seriousness of threat of hazard assessment, the available databases are countable with hand in respect to pharmaceuticals library. As the in silico models are need of the hour, so there must be expansion and transparency of ecotoxicity database regarding pharmaceuticals hazard and these must be accessible to the public at no cost. Publicly accessible toxicity databases describing environmental and human health hazards due to pharmaceuticals implicated in risk assessment, management and hazard characterization is illustrated in Table 5.
Table 5.
Representative list of publicly available databases related to the environmental toxicity due to pharmaceuticals
| Database | Country/organizations in charge | Website |
|---|---|---|
| ACToR | US EPA National Center for Computational Toxicology | http://actor.epa.gov/actor/faces/ACToRHome.jsp |
| BDSM | University of Louisville | http://systemsanalysis.louisville.edu/ |
| Cal/EPA | State of California EPA | http://www.oehha.ca.gov/risk/ChemicalDB/index.asp |
| CCRIS (Chemical carcinogenesis research information system) | US National Library of Medicine (NLM) | http://toxnet.nlm.nih.gov/cgi-bin/sis/htmlgen?CCRIS |
| CPDB (Carcinogenic potency database) | University of California, Berkeley | http://potency.berkeley.edu/ |
| Danish (Q)SAR | Danish EPA | http://ecbQSTR.jrc.ec.europa.eu/ |
| DEMETRA | EC-funded project | http://www.demetra-tox.net/ |
| DevTox | German industry and government | http://www.devtox.org |
| DSSTox (Distributed Structure-Searchable Toxicity database) | National Center for Computational Toxicology, US EPA | http://www.epa.gov/ncct/dsstox/index.html |
| ECOTOX | US EPA | http://cfpub.epa.gov/ecotox/ |
| ESIS (European Chemical Substances Information system) | Joint Research Centre of the European Commission | http://ecb.jrc.ec.europa.eu/esis/ |
| EXTOXNET (The EXtension TOXicology NETwork) | Cooperative effort of University of California-Davis, Oregon State University, Michigan State University, Cornell University, and the University of Idaho | http://extoxnet.orst.edu/ghindex.html |
| FDA Poisonous Plant Database | US FDA and Center for Food Safety and Applied Nutrition (CFSAN) | http://www.accessdata.fda.gov/scripts/plantox/index.cfm |
| GAC (Genetic Alterations in Cancer) | US National Institutes of Health (NIH) and National Institute of Environmental Health Sciences (NIEHS) | http://www.niehs.nih.gov/research/resources/databases/gac/index.cfm |
| Gene-Tox | US NLM | http://toxnet.nlm.nih.gov/cgi-bin/sis/htmlgen?GENETOX |
| HERA (Human and Environmental Risk Assessment) | A.I.S.E., the international Association for Soaps, Detergents and Maintenance Products official representative body of industries in Europe. | http://www.heraproject.com/RiskAssessment.cfm |
| Household Products Database | US Department of Health and Human Services (DHHS) | http://householdproducts.nlm.nih.gov/ |
| IARC Monograph (International Agency for Research on Cancer Monograph) | World Health Organization | http://monographs.iarc.fr/ |
| IRIS (Integrated Risk Information System) | National Center for Environmental Assessment (NCEA) and US EPA | http://cfpub.epa.gov/ncea/iris/index.cfm |
| ISSCAN | Istituto Superiore di Sanità, Italy, | http://www.iss.it/ampp/dati/cont.php?id=233&lang=1&tipo=7 |
| ITER (International Toxicity Estimates for Risk) | TERA (Toxicity Excellence for Risk Assessment) | http://www.tera.org/iter/ |
| IUCLID (International Uniform ChemicaL Information Database) | OECD, EU Biocides and EU REACH | http://iuclid.echa.europa.eu/ |
| JECDB | Japanese Ministry of Health, Labour and Welfare | http://dra4.nihs.go.jp/mhlw_data/jsp/SearchPageENG.jsp |
| JRC QSTR Database | European Commission, Joint Research Centre’s | http://ecb.jrc.ec.europa.eu/QSTR/background/ |
| KATE (KAshinhou Tool for Ecotoxicity) | Japanese National Institute for Environmental Studies (NIES), Ministry of the Environment (MoE), Government of Japan | http://kate.nies.go.jp |
| MRL (Minimal Risk Levels) | US DHHS and Agency for Toxic Substances and Disease Registry | http://www.atsdr.cdc.gov/mrls/index.html |
| NPIC | National Pesticide Information Center through Oregon State University and US EPA | 166.1 http://npic.orst.edu/ |
| NTP (National Toxicology Program) | US NIH/NIEHS | http://ntp.niehs.nih.gov/ |
| PAN Pesticide | Pesticide Action Network, North America | http://www.pesticideinfo.org/ |
| Pesticide Database | Toyohashi University of Technology, Japan | http://chrom.tutms.tut.ac.jp/JINNO/PESDATA/00alphabet.html |
| RITA (Registry of Industrial Toxicology Animal-data) | Fraunhofer Institute of Toxicology and Experimental Medicine (ITEM) Hannover | http://www.item.fraunhofer.de/reni/public/rita/index.php |
| TEXTRATOX | The University of Tennessee Institute of Agriculture | http://www.vet.utk.edu/TETRATOX/index.php |
| TOXNET | US National Library of Medicine (NLM) | http://toxnet.nlm.nih.gov/ |
| ToxRefDB | US EPA | http://www.epa.gov/ncct/toxrefdb/ |
| Toxtree | European Commission, Joint Research Centre | http://ecb.jrc.ec.europa.eu/QSTR/QSTR-tools/index.php?c=TOXTREE |
| TSCATS (Toxic Substances Control Act Test Submissions) | US EPA | https://toxplanet.com/tscats/ |
| USGS | US Geological Survey | http://137.227.231.90/data/acute/acute.html |
| 197.1 WikiPharma | Swedish research programme MistraPharma | www.wikipharma.org |
Software
Expert systems are expedient option for toxicity prediction over the traditional QSAR models as they permit ecotoxicity prediction with the input of structure of pharmaceuticals only by selecting endpoints and environment compartment. For speedy and economical prediction as well as to make risk management guidelines, regulatory authorities and industries are largely employing expert systems. To prioritize the ecotoxicity assessment of pharmaceuticals, the major aim is to distinguish between toxicologically active and inactive molecules. Again, as multiple mechanisms can lead to similar effects, therefore this complexity needs high quality predictive tools which are capable of differentiating diverse regions in the activity space. Expert systems can handle wide structural and mechanistic complexity regions compare to the local models. We have tried to summarize open access and commercially available expert systems to predict pharmaceuticals ecotoxicity in Table 6.
Table 6.
An illustrative list of available expert systems to predict diverse endpoints of environmental toxicity
| Expert system | Country/Organization | Endpoints and source of data | Website |
|---|---|---|---|
| ASTER | US EPA, NHEERL | Assessment Tools for the Evaluation of Risk (ASTER) designed to provide high quality data for discrete chemicals (databases like ECOTOX and EcoChem), and QSTR-based estimates | http://www.epa.gov/med/Prods_Pubs/aster.htm |
| CAESAR | EC funded project (Project no. 022674SSPI) | Computer Assisted Evaluation of industrial chemical Substances According to Regulations (CAESAR) generates reproducible toxicity models. Five endpoints considered are bioconcentration factor , skin sensitization, carcinogenicity, mutagenicity, developmental toxicity | http://www.caesar-project.eu/ |
| CATALOGIC | LMC University “As Zlatarov”, Bourgas, Bulgaria | Platform for models and databases related to the environment fate of chemicals such as abiotic and biotic degradation, bioaccumulation, and acute aquatic toxicity. | http://oasis-lmc.org/products/software/catalogic.aspx |
| DEREK | Harvard University Office of Technology Development | Deductive estimation of risk from existing Knowledge (DEREK) consist of 21 structural alerts for teratogenicity including four alerts and associated reasoning rules and examples for estrogenicity. | http://www.lhasalimited.org/index.php?cat=2&sub_cat=64 |
| DfW (Derek for Windows) and Meteor Nexus | Lhasa Limited | Covering 361 toxicological endpoints alerts with toxicophore. The skin sensitization knowledge base was developed in collaboration with Unilever in 1993 using its database of GPMT data for 294 chemicals. Version 9.0.0 contains 64 alerts for skin sensitization. | https://www.lhasalimited.org/ |
| ECOSAR | US EPA | Hazard assessment of environmentally occurring pharmaceuticals to fish, daphnia, and green algae using ECOlogical Structure–Activity Relationships (ECOSAR). | http://www.epa.gov/oppt/exposure/docs/episuitedl.htm |
| HazardExpert Pro | CompuDrug Inc. | Teratogenicty and reproductive toxicity | http://www.compudrug.com/ |
| MCASE/MC4PC | MultiCASE Inc. | Predictive models for blue gill, FHM, rainbow trout, and red killifish are available. 180 modules covering various areas of toxicology and pharmacology endpoints including skin sensitization, retinoids, developmental toxicity, FDA/TERIS developmental toxicity, developmental toxicants in FDA teratogenicity are available | http://www.multicase.com/products/products.htm |
| OASIS and TIMES (TIssue MEtabolism Simulator) | Laboratory of Mathematical Chemistry, LMC University “As Zlatarov”, Bourgas, Bulgaria | Uses the response-surface approach for modeling acute toxicity for two types of toxicochemical domains: noncovalent (reversible) acting chemicals and irreversible covalent bioreactive chemicals. Interspecies correlations for acute toxicity to 17 aquatic species, such as fish, snail, tadpole, hydrozoan, crustacean, insect larvae, and bacteria have been developed | http://oasis-lmc.org/products/software/times.aspx |
| OECD (Q)SAR Application Toolbox | OECD, Paris | A platform that allows for chemical information management, similarity searches, and toxicological profiling. | http://www.oecd.org/document/23/0,3343,en_2649_34379_33957015_1_1_1_1,00.html |
| ONCOLOGIC | US FDA/CDER | Predicts cancer-causing potential by applying the rules of SAR analysis, mimicking the decision logic of human experts, and incorporating knowledge of how chemicals cause cancer | http://www.epa.gov/oppt/sf/pubs/oncologic.htm |
| OSIRIS property explorer | Actelion Pharmaceuticals Ltd., Allschwil, Switzerland | Fragments developed from the analysis of 3570 compounds with reproductive effects listed in RTECS. | http://www.organic-chemistry.org/prog/peo/tox.html |
| PASS | Institute of Biomedical Chemistry of the Russian Academy of Medical Sciences, Moscow | Abortion inducer, alkylator, carcinogenic, DNA intercalator, DNA repair enzyme inhibitor, DNA synthesis inhibitor, DNA topoisomerase ATP hydrolyzing Inhibitor, DNA topoisomerase inhibitor, DOPA decarboxylase inhibitor, embryotoxic, estradiol 17β-dehydrogenase stimulant, ER modulator, estrone sulfatase inhibitor, estrone sulfotransferase stimulant, fertility enhancer, menopausal disorders treatment, mutagenic, uterine stimulant, and a collection of diverse set of receptor agonists and antagonists. | http://ibmc.p450.ru/PASS// |
| SARET | MRC “MEDTOXECO”, Department of General Hygiene, Russia and IBMC RAMS, Russia | Structure–Activity Relationships for Environmental Toxicology (SARET) model is designed for statistical analysis of data and calculation of unknown parameters of substances on the basis of QSARs. Provides the information necessary to evaluate the hazard of chemicals and to estimate their unknown characteristics. | http://www.ibmh.msk.ru |
| TERA | Russia | Tools for environmental risk assessment (TERA) contains information about assessment of multidomain risk, assessment of carcinogenic potency risk, prediction of lead concentrations in blood of fetus, children, adults, health risk connected with lead exposure and prediction of emission of chemical substances | http://www.tera.org/ |
| TerraQSTR-FHM | TerraBase Inc., Hamilton, Ontario, Canada | Stand-alone neural network -based program to compute the acute toxicity of organic chemicals to the FHM using a proprietary neural network algorithm | http://www.terrabase-inc.com |
| TOPKAT | BIOVIA | TOxicity Prediction by C(K)omputer Assisted Technology (TOPKAT) uses a range QSAR models for assessing acute toxicity to FHM and Daphnia. It comprises two sets of skin sensitization models. The TOPKAT modeling has been employed by the Danish EPA in their project to develop QSTR models for around 47,000 organic substances on the European Inventory of Existing Commercial chemical Substances (EINECS) | http://www.accelrys.com/products/topkat/ |
| Toxmatch | European Union Reference Laboratory for alternatives to animal testing (EURL-ECVAM) | Open-source computer program of Joint Research Centre (EC) that encodes several chemical similarity indices in order to facilitate the grouping of chemicals, thereby supporting the development of chemicals and the application of read-across between analogs | http://ecb.jrc.ec.europa.eu/QSTR/QSTR-tools/index.php?c=TOXMATCH |
Future Avenues
As the situation is very alarming already, there must be apposite future plans to tackle the ecotoxicity threat due to pharmaceuticals. Therefore, a set of plans must be addressed and followed for efficient risk assessment along with quick risk management in different compartments of environment which are again directly related to human health.
Along with pharmaceuticals used by humans, veterinary pharmaceuticals need to be monitored carefully.
The packaging system should be biodegradable and eco-friendly to minimize the packaging related hazards.
Dose of drugs should be small and drugs must be ineffective after expiry date to some extent considering its biological response.
Green chemistry principles should be followed for risk management of pharmaceuticals for quick and nuisance free degradability after its usage. In this perspective, advancement of synthesis and renewable feedstock are crucial issues for preparation of environment friendly pharmaceuticals [100]. Thus, “benign by design” criteria can be practiced which asking for easy degradability after application. This can lead to economic rewards in the long run and will fit into green pharmacy [126]. Only thing needed to remember is that pharmaceuticals should not lose its therapeutic action due to the introduction of green chemistry.
The toxicity of metabolites and mixtures of pharmaceuticals needs to be addressed more carefully as majority of drugs are combinations of two or more API and some of them are prodrugs in nature.
There is significant deficiency of data on the effects of long-term exposure in nontarget organisms, as well as how an uninterrupted exposure may affect a population is not explored till today.
Pharmaceuticals’ ecotoxicity databases need to be organized and classified in terms of endpoints, assay concentrations, environment compartments with different experimental conditions.
The expert system should be more equipped with applicability domain , conformal prediction and uncertainty issue for reliable prediction.
Conclusion
The vibrant characteristic of pharmaceuticals is that they have explicit mode of action and they are designed to exert specific response to biological system which differentiate them from other organic chemicals. Thus, once they are released into the environment in an altered form or in their real form, they affect the living organism of diverse compartments of environment which is the sufficient reason to monitor and assess the potential effects of pharmaceuticals to environment. Assessment of occurrence level of pharmaceuticals in diverse compartments is necessary as the observed amount highly varies from one compartment to another which makes the situation complex for environmentalists. On the other hand, most of the interactions between pharmaceuticals and natural stressors of aquatic and terrestrial communities are unexplained till date. Thus to evaluate the risk of waste hazards pose to wildlife, it has been recommended to utilize the toxicity data derived from mammals during the production stages of pharmaceuticals which may be helpful for future prediction. To quantify the ecotoxic concentration threshold of any pharmaceutical, calculation of the risk quotient (RQ) is very important. The RQ expresses the ratio between the predicted concentration in the environment (PEC) and the concentration at which no effect is expected (PNEC) [21].
The present chapter reviews the most common routes, sources and occurrence of pharmaceutical hazards along with their fate and effects in different environments. Apart from studies on individual pharmaceuticals, the need of risk assessment and management of their metabolites and mixture of pharmaceuticals are discussed. The role of government authorities and different policies regulated regarding identification of risk assessment and management must be implemented in a proper way with right direction. Conversely, insufficient ecotoxicity data related to a definite class of pharmaceuticals has slowed down the computational modeling to some point. So, properly documented database is the need of the hour for environmentalists. On the contrary, though a good number of in silico models, especially QSAR models are reported for toxicity prediction of chemicals hazards, but a few successful models exist for pharmaceutical hazards. Thus, ample number of models should be generated to get the ecotoxicity data for pharmaceuticals for diverse endpoints and environmental compartments. Expert systems have the ability to make fast and reliable predictions with a single click of mouse, which urges to develop more expert systems for a set of endpoints. There is no doubt that in silico methods cannot completely substitute experimental approaches, but they can be integrated for better understanding and quantification of pharmaceuticals’ ecotoxicity.
Acknowledgments
S.K. and J.L. wish to thank the National Science Foundation (NSF/CREST HRD-1547754, and NSF/RISE HRD-1547836) for financial support. K.R. is thankful to the UGC, New Delhi for financial assistance under the UPE II scheme.
Contributor Information
Orazio Nicolotti, Phone: +39390805442551, Email: orazio.nicolotti@uniba.it.
Kunal Roy, Email: kunal.roy@jadavpuruniversity.in.
References
- 1.Aherne G, English J, Marks V. The role of immunoassay in the analysis of microcontaminants in water samples. Ecotoxicol Environ Saf. 1985;9:79–83. doi: 10.1016/0147-6513(85)90037-5. [DOI] [PubMed] [Google Scholar]
- 2.Richardson M, Bowron J. The fate of pharmaceutical chemicals in the aquatic environment. J Pharm Pharmacol. 1985;37:1–12. doi: 10.1111/j.2042-7158.1985.tb04922.x. [DOI] [PubMed] [Google Scholar]
- 3.Santosa LHMLM, Araújoa AN, Fachinia A, et al. Ecotoxicological aspects related to the presence of pharmaceuticals in the aquatic environment. J Hazard Mater. 2010;175:45–95. doi: 10.1016/j.jhazmat.2009.10.100. [DOI] [PubMed] [Google Scholar]
- 4.Li WC. Occurrence, sources, and fate of pharmaceuticals in aquatic environment and soil. Environ Pollut. 2014;187:193–201. doi: 10.1016/j.envpol.2014.01.015. [DOI] [PubMed] [Google Scholar]
- 5.WHO (2011) The World Medicines Situation
- 6.Busfield J. Assessing the over use of medicines. Soc Sci Med. 2015;131:199–206. doi: 10.1016/j.socscimed.2014.10.061. [DOI] [PubMed] [Google Scholar]
- 7.IWW (2014) Pharmaceuticals in the environment: occurence, effects and options for action. Research project funded by German Federal Environment Agency (UBA) within the Environmental Research Plan No.371265408.
- 8.Roy K, Kar S. In silico models for ecotoxicity of pharmaceuticals. In: Benfenati E, editor. In silico methods for predicting drug toxicity. New York, NY: Springer; 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Taylor D, Senac T. Human pharmaceutical products in the environment – the “problem” in perspective. Chemosphere. 2014;115:95–99. doi: 10.1016/j.chemosphere.2014.01.011. [DOI] [PubMed] [Google Scholar]
- 10.Kümmerer K. Pharmaceuticals in the environment: sources, fate, effects and risks. Berlin: Springer Science & Business Media; 2013. [Google Scholar]
- 11.Hughes SR, Kay P, Brown LE. Global synthesis and critical evaluation of pharmaceutical datasets collected from river systems. Environ Sci Technol. 2013;47:661–677. doi: 10.1021/es3030148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Han GH, Hur HG, Kim SD. Ecotoxicological risk of pharmaceuticals from wastewater treatment plants in Korea: occurrence and toxicity to Daphnia magna. Environ Toxicol Chem. 2006;25:265–271. doi: 10.1897/05-193R.1. [DOI] [PubMed] [Google Scholar]
- 13.Fernandez C, Gonzalez-Doncel M, Pro J, et al. Occurrence of pharmaceutically active compounds in surface waters of the henares-jarama-tajo river system (Madrid, Spain) and a potential risk characterization. Sci Total Environ. 2010;408:543–551. doi: 10.1016/j.scitotenv.2009.10.009. [DOI] [PubMed] [Google Scholar]
- 14.Papageorgiou M, Kosma C, Lambropoulou D. Seasonal occurrence, removal, mass loading and environmental risk assessment of 55 pharmaceuticals and personal care products in a municipal wastewater treatment plant in Central Greece. Sci Total Environ. 2016;543:547–569. doi: 10.1016/j.scitotenv.2015.11.047. [DOI] [PubMed] [Google Scholar]
- 15.Oliveira TS, Murphy M, Mendola N, et al. Characterization of pharmaceuticals and personal care products in hospital effluent and waste water influent/effluent by direct-injection LC-MS-MS. Sci Total Environ. 2015;518:459–478. doi: 10.1016/j.scitotenv.2015.02.104. [DOI] [PubMed] [Google Scholar]
- 16.Rivas J, Encinas A, Beltran F, Grahan N. Application of advanced oxidation processes to doxycycline and norfloxacin removal from water. J Environ Sci Health A Tox Hazard Subst Environ Eng A. 2011;46:944–951. doi: 10.1080/10934529.2011.586249. [DOI] [PubMed] [Google Scholar]
- 17.Kolpin DW, Furlong ET, Meyer MT, et al. Pharmaceuticals, hormones, and other organic wastewater contaminants in U.S. streams, 1999-2000: a national reconnaissance. Environ Sci Technol. 2002;36:1202–1211. doi: 10.1021/es011055j. [DOI] [PubMed] [Google Scholar]
- 18.Halling-Sørensen B, Nors Nielsen S, Lanzky PF, et al. Occurrence, fate and effects of pharmaceutical substances in the environment-a review. Chemosphere. 1998;36(2):357–393. doi: 10.1016/S0045-6535(97)00354-8. [DOI] [PubMed] [Google Scholar]
- 19.Daughton CG, Ternes TA. Pharmaceuticals and personal care products in the environment: agents of subtle change? Environ Health Perspect. 1999;107:907–937. doi: 10.1289/ehp.99107s6907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Adler P, Steger-Hartmann T, Kalbfus W. Distribution of natural and synthetic estrogenic steroid hormones in water samples from southern and middle Germany. Acta Hydrochim Hydrobiol. 2001;29:227–241. doi: 10.1002/1521-401X(200111)29:4<227::AID-AHEH227>3.0.CO;2-R. [DOI] [Google Scholar]
- 21.Ternes T. Occurence of drugs in German sewage treatment plants and rivers. Water Res. 1998;32:3245–3260. doi: 10.1016/S0043-1354(98)00099-2. [DOI] [Google Scholar]
- 22.Ahrer W, Scherwenk E, Buchberger W. Occurence and fate of fluoroquinolone, macrolide, and sulphonamide antibiotics during wastewater treatment and in ambient waters in Switzerland. In: Daughton CG, Jones-Lepp T, editors. Pharmaceuticals and personal care products in the environment: scientific and regulatory issues. Symposium Series 791. Washington, DC: American Chemical Society; 2001. pp. 56–69. [Google Scholar]
- 23.Frick EA, Henderson AK, Moll DM, et al (2001) Presented at the Georgia Water Resources Conference, Athens, GA.
- 24.Tauber R. Quantitative analysis of pharmaceuticals in drinking water from ten Canadian cities. Winnipeg, MB: Enviro-Test Laboratories; 2003. [Google Scholar]
- 25.Seiler JP. Pharmacodynamic activity of drugs and ecotoxicology can the two be connected? Toxicol Lett. 2002;131:105–115. doi: 10.1016/S0378-4274(02)00045-0. [DOI] [PubMed] [Google Scholar]
- 26.EMEA . Guideline on the environmental impact assessment of medicinal products for human use (Report no. CPMP/SWP/4447/00) London: European Agency for the Evaluation of Medicinal Products; 2006. [Google Scholar]
- 27.FDA-CDER . Guidance for industry-environmental assessment of human drugs and biologics applications, Revision 1. Rockville, VA: FDA Center for Drug Evaluation and Research; 1998. [Google Scholar]
- 28.FDA: U.S. Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research (CDER), Center for Biologics Evaluation and Research (CBER) (1998) CMC 6 - Revision 1
- 29.European Commission, Directive 2006/121/EC of the European Parliament and of the Council of 18 December 2006 amending Council Directive 67/548/EEC on the approximation of laws, regulations and administrative provisions relating to the classification, packaging and labelling of dangerous substances in order to adapt it to Regulation (EC) No. 1907/2006 concerning the Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH) and establishing a European Chemicals Agency. Off. J. Eur. Union, L 396/850 of 30.12.2006, Office for Official Publications of the European Communities (OPOCE), Luxembourg
- 30.Directive 2004/27/EC of the European Parliament and of the Council of 31 March 2004 amending Directive 2001/83/EC on the Community code relating to medicinal products for human use. Official Journal L 136, 30/04/2004 pp. 34-57. 2004
- 31.Directive 2004/28/EC of the European Parliament and of the Council of 31 March 2004 amending Directive 2001/82/EC on the Community code relating to veterinary medicinal products. Official Journal L 136, 30/04/2004 pp. 58-84. 2004
- 32.DIRECTIVE 2013/39/EU OF THE EUROPEAN PARLIAMENT AND OF THE COUNCIL of 12 August 2013 amending Directives 2000/60/EC and 2008/105/EC as regards priority substances in the field of water policy. 2013
- 33.COMMISSION IMPLEMENTING DECISION (EU) 2015/495 of 20 March 2015 establishing a watch list of substances for Union-wide monitoring in the field of water policy pursuant to Directive 2008/105/EC of the European Parliament and of the Council. 2015
- 34.Barbosa MO, Moreira NFF, Ribeiro AR, et al. Occurrence and removal of organic micropollutants: an overview of the watch list of EU Decision 2015/495. Water Res. 2016;94:257–279. doi: 10.1016/j.watres.2016.02.047. [DOI] [PubMed] [Google Scholar]
- 35.Cassani S, Gramatica P. Identification of potential PBT behavior of personal care products by structural approaches. Sustain Chem Pharm. 2015;1:19–27. doi: 10.1016/j.scp.2015.10.002. [DOI] [Google Scholar]
- 36.Roy K, Kar S, Das RN. Understanding the Basics of QSAR for Applications in Pharmaceutical Sciences and Risk Assessment. San Diego, CA: Academic Press; 2015. [Google Scholar]
- 37.Roy K, Kar S, Das RN. A primer on QSAR/QSPR modeling: fundamental concepts (SpringerBriefs in Molecular Science) New York, NY: Springer; 2015. [Google Scholar]
- 38.Howard PH, Muir DCG. Identifying new persistent and bioaccumulative organics among chemicals in commerce II: pharmaceuticals. Environ Sci Technol. 2011;45:6938–6946. doi: 10.1021/es201196x. [DOI] [PubMed] [Google Scholar]
- 39.Sangion A, Gramatica P. PBT assessment and prioritization of contaminants of emerging concern: pharmaceuticals. Environ Res. 2016;147:297–306. doi: 10.1016/j.envres.2016.02.021. [DOI] [PubMed] [Google Scholar]
- 40.European Commission (2001) CSTEE. Discussion paper on environmental risk assessment of medical products for human use (non-geneticallymodified organisms (non-GMO) containing). CPMPpaperRAssessHumPharm12062001/D(01)
- 41.US EPA (2012) The ECOSAR (ECOlogical Structure Activity Relationship) Class Program.
- 42.US EPA (2015) ECOTOX user guide: ECOTOXicology database system. Version 4.0. http:/www.epa.gov/ecotox/. Accessed 4 July, 2017
- 43.Mendoza A, Acena J, Perez S, et al. Pharmaceuticals and iodinated contrast media in a hospital wastewater: a case study to analyse their presence and characterise their environmental risk and hazard. Environ Res. 2015;140:225–241. doi: 10.1016/j.envres.2015.04.003. [DOI] [PubMed] [Google Scholar]
- 44.Ortiz de García S, Pinto GP, García-Encina PA, et al. Ranking of concern, based on environmental indexes, for pharmaceutical and personal care products: an application to the Spanish case. J Environ Manage. 2013;129:384–397. doi: 10.1016/j.jenvman.2013.06.035. [DOI] [PubMed] [Google Scholar]
- 45.Sanderson H, Thomsen M. Comparative analysis of pharmaceuticals versus industrial chemicals acute aquatic toxicity classification according to the United Nations classification system for chemicals. Assessment of the (Q)SAR predictability of pharmaceuticals acute aquatic toxicity and their predominant acute toxicmode-of-action. Toxicol Lett. 2009;187:84–93. doi: 10.1016/j.toxlet.2009.02.003. [DOI] [PubMed] [Google Scholar]
- 46.Singh KP, Gupta S, Basant N. QSTR modeling for predicting aquatic toxicity of pharmacological active compounds in multiple test species for regulatory purpose. Chemosphere. 2015;120:680–689. doi: 10.1016/j.chemosphere.2014.10.025. [DOI] [PubMed] [Google Scholar]
- 47.Persson M, Sabelström E, Gunnarsson B. Handling of unused prescription drugs-knowledge, behaviour and attitude among Swedish people. Environ Int. 2009;35:771–774. doi: 10.1016/j.envint.2008.10.002. [DOI] [PubMed] [Google Scholar]
- 48.Li D, Yang M, Hu J, et al. Determination and fate of oxytetracycline and related compounds in oxytetracycline production wastewater and the receiving river. Environ Toxicol Chem. 2008;27:80–86. doi: 10.1897/07-080.1. [DOI] [PubMed] [Google Scholar]
- 49.José Gómez M, Petrovic M, Fernández-Alba AR, et al. Determination of pharmaceuticals of various therapeutic classes by solid-phase extraction and liquid chromatographye-tandem mass spectrometry analysis in hospital effluent wastewaters. J Chromatogr. 2006;1114:224–233. doi: 10.1016/j.chroma.2006.02.038. [DOI] [PubMed] [Google Scholar]
- 50.Ternes TA, Hirsch R. Occurrence and behavior of X-ray contrast media in sewage facilities and the aquatic environment. Environ Sci Technol. 2000;34:2741–2748. doi: 10.1021/es991118m. [DOI] [Google Scholar]
- 51.Serrano PH. Responsible use of antibiotics in aquaculture. fisheries technical paper 469. Rome: Food and Agriculture Organization of the United Nations (FAO); 2005. [Google Scholar]
- 52.Kreuzig R, Höltge S, Brunotte J, et al. Test plat studies on runoff of sulfonamides from manured soil after sprinkler irrigation. Environ Toxicol Chem. 2005;24:777–781. doi: 10.1897/04-019R.1. [DOI] [PubMed] [Google Scholar]
- 53.http://www.apsnet.org/online/feature/Antibiotics/. Accessed 4 July, 2017
- 54.http://www.esac.ua.ac.be/main.aspx?c=*ESAC2&n=1066l. Accessed 4 July, 2017
- 55.Ye Z, Weinberg HS, Meyer MT. Trace analysis of trimethoprim and sulfonamide, macrolide, quinolone, and tetracycline antibiotics in chlorinated drinking water using liquid chromatography electrospray tandem mass spectrometry. Anal Chem. 2007;79:1135–1144. doi: 10.1021/ac060972a. [DOI] [PubMed] [Google Scholar]
- 56.Hamscher G, Sczesny S, Höper H, et al. Determination of persistent tetracycline residues in soil fertilized with liquid manure by high-performance liquid chromatography with electrospray ionization tandem mass spectrometry. Anal Chem. 2002;74:1509–1518. doi: 10.1021/ac015588m. [DOI] [PubMed] [Google Scholar]
- 57.Kümmerer K, Alexy R, Hüttig J. Standardized tests fail to assess the effects of antibiotics on environmental bacteria. Water Res. 2004;38:2111–2116. doi: 10.1016/j.watres.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 58.Carucci A, Cappai G, Piredda M. Biodegradability and toxicity of pharmaceuticals in biological wastewater treatment plants. Environ Sci Health A Tox Hazard Subst Environ Eng. 2006;41:1831–1842. doi: 10.1080/10934520600779000. [DOI] [PubMed] [Google Scholar]
- 59.Hernando MD, DeVettori S, Martínez-Bueno MJ, et al. Toxicity evaluation with Vibrio fisheri test of organic chemicals used in aquaculture. Chemosphere. 2007;68:724–730. doi: 10.1016/j.chemosphere.2006.12.097. [DOI] [PubMed] [Google Scholar]
- 60.Brain RA, Johnson DJ, Richards SM. Microcosm evaluation of the effects of an eight pharmaceutical mixture to the aquatic macrophytes Lemna gibba and Myriophyllum sibiricum. Aquat Toxicol. 2004;70:23–40. doi: 10.1016/j.aquatox.2004.06.011. [DOI] [PubMed] [Google Scholar]
- 61.Hansen AJ, Jensen J, Krogh PH. Effects of the antibiotics oxytetracycline and tylosin on soil fauna. Chemosphere. 2000;40:751–757. doi: 10.1016/S0045-6535(99)00449-X. [DOI] [PubMed] [Google Scholar]
- 62.Holt D, Harvey D, Hurley R. Chloramphenicol toxicity. Adverse Drug React Toxicol Rev. 1993;12:83–95. [PubMed] [Google Scholar]
- 63.Harrison PF, Lederberg J. Antimicrobial Resistance, Issues and Options. Washington DC: National Academic Press; 1998. [PubMed] [Google Scholar]
- 64.Isidori M, Nardelli A, Pascarella L, et al. Toxic and genotoxic impact of fibrates and their photoproducts on non-target organism. Environ Int. 2007;33:635–641. doi: 10.1016/j.envint.2007.01.006. [DOI] [PubMed] [Google Scholar]
- 65.Triebskorn R, Casper H, Scheil V, et al. Ultrastructural effects of pharmaceuticals (carbamazepine, clofibric acid, metoprolol, diclofenac) in rainbow trout (Oncorhynchus mykiss) and common carp (Cyprinus carpio) Anal Bioanal Chem. 2007;387:1405–1416. doi: 10.1007/s00216-006-1033-x. [DOI] [PubMed] [Google Scholar]
- 66.Misra SK, Sharma AK, Mehdi H, et al. Effect of cholesterol and alphap- chlorophenoxyisobutyyrate on virulence in Acanthamoeba culbertsoni strain A-1 and C-7. Int J Parasitol. 1986;16:191–196. doi: 10.1016/0020-7519(86)90043-3. [DOI] [PubMed] [Google Scholar]
- 67.Moder M, Braun P, Lange F, et al. Determination of endocrine disrupting compounds and acidic drugs in water by coupling of derivatization, gas chromatography and negative-chemical ionization mass spectrometry. Clean. 2007;35:444–451. [Google Scholar]
- 68.Cleuvers M. Mixture toxicity of the anti-inflammatory drugs diclofenac, ibuprofen, naproxen and acetylsalicylic acid. Ecotoxicol Environ Saf. 2004;59:309–315. doi: 10.1016/S0147-6513(03)00141-6. [DOI] [PubMed] [Google Scholar]
- 69.Schwaiger J, Ferling H, Mallow U, et al. Toxic effects of the non-steroidal anti-inflammatory drug diclofenac: Part I: histopathological alterations and bioaccumulation in rainbow trout. Aquat Toxicol. 2004;68:141. doi: 10.1016/j.aquatox.2004.03.014. [DOI] [PubMed] [Google Scholar]
- 70.Huggett DB, Brooks BW, Peterson B, et al. Toxicity of select beta adrenergic receptor-blocking pharmaceuticals (β-blockers) on aquatic organisms. Arch Environ Contam Toxicol. 2002;43:229. doi: 10.1007/s00244-002-1182-7. [DOI] [PubMed] [Google Scholar]
- 71.Coppi A, Merali S, Eichinger D. The enteric parasite Entamoeba uses an autocrine catecholamine system during differentiation into the infectious cysts stage. J Biol Chem. 2002;277:8083–8090. doi: 10.1074/jbc.M111895200. [DOI] [PubMed] [Google Scholar]
- 72.Sanderson H, Brain RA, Johnson DJ, et al. Toxicity classification and evaluation of four pharmaceuticals classes: antibiotics, antineoplastics, cardiovascular, and sex hormones. Toxicology. 2004;203:27–40. doi: 10.1016/j.tox.2004.05.015. [DOI] [PubMed] [Google Scholar]
- 73.Henschel K-P, Wenzel A, Diedrich M, et al. Environmental hazard assessment of pharmaceuticals. Regul Toxicol Pharmacol. 1997;25:220–225. doi: 10.1006/rtph.1997.1102. [DOI] [PubMed] [Google Scholar]
- 74.Boroskova Z, Dvoroznakova E, Tomasovicova O, et al. The effect of cyclophosphamide on the cellular and humoral immune responses on experimental Toxocara canis infestation. Helminthologia. 2001;38:193–199. [Google Scholar]
- 75.Grujić S, Vasiljević T, Lauŝević M. Determination of multiple pharmaceutical classes in surface and ground waters by liquid chromatography-ion trap-tandem mass spectrometry. J Chromatogr A. 2009;1216:4989–5000. doi: 10.1016/j.chroma.2009.04.059. [DOI] [PubMed] [Google Scholar]
- 76.Ellenberger TE, Wright JE, Rosowsky A, et al. Wild-type and drugresistant Leishmania major hydrolyze methotrexate to N-10-methyl-4-deoxy-4-aminopteroate without accumulation of methotrexate polyglutamates. J Biol Chem. 1989;264:15960–15966. [PubMed] [Google Scholar]
- 77.Minagh E, Hernan R, O’Rourke K, et al. Aquatic ecotoxicity of the selective serotonin reuptake inhibitor sertraline hydrochloride in a battery of freshwater test species. Ecotoxicol Environ Saf. 2009;72:434–440. doi: 10.1016/j.ecoenv.2008.05.002. [DOI] [PubMed] [Google Scholar]
- 78.Crane M, Watts C, Boucard T. Chronic aquatic environmental risks from exposure to human pharmaceuticals. Sci Total Environ. 2006;367:23–41. doi: 10.1016/j.scitotenv.2006.04.010. [DOI] [PubMed] [Google Scholar]
- 79.Baqui A, Ansari JA. Investigations on the response to various stimuli of the microfilariae of Setaria cervi in white rats. Helminthologia. 1987;24:33–42. [Google Scholar]
- 80.Jones-Brando L, Torrey EF, Yolkem R. Drugs used in the treatment of schizophrenia and bipolar disorder inhibit the replication of Toxoplasma gondii. Schizophr Res. 2003;62:237–244. doi: 10.1016/S0920-9964(02)00357-2. [DOI] [PubMed] [Google Scholar]
- 81.Pawlowski S, van Aerle R, Tyler CR, et al. Effects of 17α-ethinylestradiol in a fathead minnow (Pimephales promelas) gonadal recrudescence assay. Ecotoxicol Environ Saf. 2004;57:330–345. doi: 10.1016/j.ecoenv.2003.07.019. [DOI] [PubMed] [Google Scholar]
- 82.Arcay L. Influence of sex hormones on the experimental infection produced by a strain of Leishmania mexicana amazonensis from Venezuela. Rev Latinoam Microbiol. 1985;27:195–207. [PubMed] [Google Scholar]
- 83.Wang R, Belosevic M. Estradiol increases susceptibility of goldfish to Trypanosoma danilewskyi. Dev Comp Immunol. 1994;18:377–387. doi: 10.1016/0145-305X(94)90003-5. [DOI] [PubMed] [Google Scholar]
- 84.Martín J, Camacho-Muñoz D, Santos JL, et al. Occurrence of pharmaceutical compounds in wastewater and sludge from wastewater treatment plants: removal and ecotoxicological impact of wastewater discharges and sludge disposal. J Hazard Mater. 2012;239-240:40–47. doi: 10.1016/j.jhazmat.2012.04.068. [DOI] [PubMed] [Google Scholar]
- 85.Boxall AB, Fogg LA, Baird DJ, et al. Targeted monitoring study for veterinary medicines in the environment. Bristol: Environment Agency; 2005. [Google Scholar]
- 86.Grønvold J, Svendsen TS, Kraglund HO, et al. Effect of the antiparasitic drugs fenbendazole and ivermectin on the soil nematode Pristionchus maupasi. Vet Parasitol. 2004;24:91–99. doi: 10.1016/j.vetpar.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 87.Svendsen TS, Hansen PE, Sommer C, et al. Life history characteristics of Lumbricus terrestris and effects of the veterinary antiparasitics compounds Ivermectin and fenbendazole. Soil Biol Biochem. 2005;37:927–936. doi: 10.1016/j.soilbio.2004.10.014. [DOI] [Google Scholar]
- 88.Sun Y, Diao X, Zhang Q, et al. Bioaccumulation and elimination of avermectin B1a in the earthworm (Eisenia fetida) Chemosphere. 2005;60:699–704. doi: 10.1016/j.chemosphere.2005.01.044. [DOI] [PubMed] [Google Scholar]
- 89.Singer AC, Johnson AC, Anderson PD. Reassessing the risks of Tamiflu use during a pandemic to the Lower Colorado River. Environ Health Perspect. 2008;116:A285–A286. doi: 10.1289/ehp.11407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Soderstrom H, Jarhult JD, Fick J, et al (2010) Levels of antivirals and antibiotics in the river Thames, UK, during the pandemic 2009. SETAC Europe Annual Meeting, Seville
- 91.http://www.biomedicalwastesolutions.com/medical-waste-disposal/. Accessed 4 July, 2017
- 92.Rappaport SM. Implications of the exposome for exposure science. J Expo Sci Environ Epidemiol. 2011;21:5–9. doi: 10.1038/jes.2010.50. [DOI] [PubMed] [Google Scholar]
- 93.NRC (National Research Council) Risk assessment in the federal government: managing the process. Washington, DC: National Academies Press; 1983. [PubMed] [Google Scholar]
- 94.NRC (National Research Council) Toxicity testing in the 21st century: a vision and a strategy. Washington, DC: National Academies Press; 2007. [Google Scholar]
- 95.Wignall JA, Shapiro AJ, Wright FA, et al. Standardizing benchmark dose calculations to improve science-based decisions in human health assessments. Environ Health Perspect. 2014;122:499–505. doi: 10.1289/ehp.1307539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wetmore BA, Wambaugh JF, Ferguson SS, et al. Integration of dosimetry, exposure, and high-throughput screening data in chemical toxicity assessment. Toxicol Sci. 2012;125:157–174. doi: 10.1093/toxsci/kfr254. [DOI] [PubMed] [Google Scholar]
- 97.Jones DP, Park Y, Ziegler TR. Nutritional metabolomics: progress in addressing complexity in diet and health. Annu Rev Nutr. 2012;32:183–202. doi: 10.1146/annurev-nutr-072610-145159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.van Gestel CA, Jonker M, Kammenga JE. Mixture toxicity: linking approaches from ecological and human toxicology. New York, NY: Taylor& Francis; 2010. [Google Scholar]
- 99.Presidential/Congressional Commission on Risk Assessment Risk Management (1997). Risk assessment and risk management in regulatory decision-making. Final Report. Vol. 2. Washington, DC:PCRARM. Available: http://www.riskworld.com. Accessed 4 July, 2017
- 100.Kümmerer K. Sustainable from the very beginning: rational design of molecules by life cycle engineering as an important approach for green pharmacy and green chemistry. Green Chem. 2007;9:899–907. doi: 10.1039/b618298b. [DOI] [Google Scholar]
- 101.Nowotny N, Epp B, Von Sonntag C. Quantification and modeling of the elimination behavior of ecologically problematic wastewater micropollutants by adsorption on powdered and granulated activated carbon. Environ Sci Technol. 2007;41:2050–2055. doi: 10.1021/es0618595. [DOI] [PubMed] [Google Scholar]
- 102.Fjodorova N, Novich M, Vrachko N, et al. Directions in QSAR modeling for regulatory uses in OECD member countries, EU and in Russia. J Environ Sci Health Part C. 2008;26:201–236. doi: 10.1080/10590500802135578. [DOI] [PubMed] [Google Scholar]
- 103.Knacker T, Duis K, Ternes T, et al. The EU-project ERAPharmsIncentives for the further development of guidance documents? Environ Sci Pollut Res. 2005;12:62–65. doi: 10.1065/espr2005.02.238. [DOI] [PubMed] [Google Scholar]
- 104.Cleuvers M. Aquatic ecotoxicology of selected pharmaceutical agents algal and acute Daphnia tests. Umweltwissenschaften und Schadstoff-Forschung. 2002;14:85–89. doi: 10.1065/uwsf2002.04.025. [DOI] [Google Scholar]
- 105.VICH (2004) Environmental impact assessment (EIAs) for veterinary medicinal products (VMPs)–Phase II, Draft Guidance, August, 2003, International Cooperation on Harmonization of the Technical Requirements for Registration of Veterinary Medicinal Products. Available at: http://vich.eudra.org/pdf/10_2003/g138_st4.pdf
- 106.http://www.nicnas.gov.au/. Accessed 4 July, 2017
- 107.Dearden JC. The history and development of quantitative structure-activity relationships (QSARs) Int J Quant Struct-Prop Relat. 2016;1:1–44. [Google Scholar]
- 108.Roy K, Kar S (2015) How to judge predictive quality of classification and regression based QSAR models? In: Haq ZU, Madura J (eds) Frontiers of computational chemistry. Bentham, pp 71–120
- 109.Roy K, Kar S. Importance of applicability domain of QSAR models. In: Roy K, editor. Quantitative structure-activity relationships in drug design, predictive toxicology, and risk assessment. Hershey PA: IGI Global; 2015. pp. 180–211. [Google Scholar]
- 110.http://ec.europa.eu/research/biosociety/pdf/anim_al_see_final_report.pdf. Accessed 4 July, 2017
- 111.Kar S, Roy K. Risk assessment for ecotoxicity of pharmaceuticals - an emerging issue. Expert Opin Drug Saf. 2012;11:235–274. doi: 10.1517/14740338.2012.644272. [DOI] [PubMed] [Google Scholar]
- 112.Kanter J (2013) E.U. Bans cosmetics with animal-tested ingredients. http://www.nytimes.com. Accessed 4 July, 2017
- 113.Cronin MTD, Livingstone DJ. Predicting chemical toxicity and fate. Washington DC: CRC press; 2004. [Google Scholar]
- 114.Schultz TW, Cronin MTD, Walker JD. Quantitative structure-activity relationships (QSARs) in toxicology: a historical perspective. J Mol Struct (THEOCHEM) 2003;622:1–22. doi: 10.1016/S0166-1280(02)00614-0. [DOI] [Google Scholar]
- 115.Kar S, Roy K. Predictive toxicology using QSAR: a perspective. J Indian Chem Soc. 2010;87:1455–1515. [Google Scholar]
- 116.Sanderson H, Johnson D, Reitsma T, et al. Ranking and prioritization of environmental risks of pharmaceuticals in surface waters. Reg Pharm Toxicol. 2004;39:158–183. doi: 10.1016/j.yrtph.2003.12.006. [DOI] [PubMed] [Google Scholar]
- 117.Escher BI, Bramaz N, Richter M, et al. Comparative ecotoxicological hazard assessment of beta-blockers and their human metabolites using a mode-of-action-based test battery and a QSTR approach. Environ Sci Technol. 2006;40:7402–7408. doi: 10.1021/es052572v. [DOI] [PubMed] [Google Scholar]
- 118.Sanderson H, Thomsen M. Ecotoxicological quantitative structure–activity relationships for pharmaceuticals. Bull Environ Contam Toxicol. 2007;79:331–335. doi: 10.1007/s00128-007-9249-9. [DOI] [PubMed] [Google Scholar]
- 119.Kar S, Roy K. First report on interspecies quantitative correlation of ecotoxicity of pharmaceuticals. Chemosphere. 2010;81:738–747. doi: 10.1016/j.chemosphere.2010.07.019. [DOI] [PubMed] [Google Scholar]
- 120.Christen V, Hickmann S, Rechenberg B, et al. Highly active human pharmaceuticals in aquatic systems: a concept for their identification based on their mode of action. Aquat Toxicol. 2010;96:167–181. doi: 10.1016/j.aquatox.2009.11.021. [DOI] [PubMed] [Google Scholar]
- 121.http://www.biograf.ch. Accessed 4 July, 2017
- 122.Das RN, Sanderson H, Mwambo AE, et al. Preliminary studies on model development for rodent toxicity and its interspecies correlation with aquatic toxicities of pharmaceuticals. Bull Environ Contam Toxicol. 2013;90:375–381. doi: 10.1007/s00128-012-0921-3. [DOI] [PubMed] [Google Scholar]
- 123.de García MSAO, Pinto GP, García-Encina PA, et al. Ecotoxicity and environmental risk assessment of pharmaceuticals and personal care products in aquatic environments and wastewater treatment plants. Ecotoxicology. 2014;23:1517–1533. doi: 10.1007/s10646-014-1293-8. [DOI] [PubMed] [Google Scholar]
- 124.Sangion A, Gramatica P. Hazard of pharmaceuticals for aquatic environment: prioritization by structural approaches and prediction of ecotoxicity. Environ Int. 2016;95:131–143. doi: 10.1016/j.envint.2016.08.008. [DOI] [PubMed] [Google Scholar]
- 125.Sangion A, Gramatica P. Ecotoxicity interspecies QAAR models from Daphnia toxicity of pharmaceuticals and personal care products. SAR QSAR Environ Res. 2016;27:781–798. doi: 10.1080/1062936X.2016.1233139. [DOI] [PubMed] [Google Scholar]
- 126.Kümmerer K. The presence of pharmaceuticals in the environment due to human use-present knowledge and future challenges. J Environ Manage. 2009;90:2354–2366. doi: 10.1016/j.jenvman.2009.01.023. [DOI] [PubMed] [Google Scholar]